Abstract
Introduction
Juul is a leading electronic cigarette (e-cigarette) brand in the USA. By November 2019, Juul pre-emptively limited online and in-store sales of non-tobacco or menthol-flavoured pods ahead of impending flavour bans. Since this removal, sale of mango-flavoured Juul-compatible pods was introduced to the market by smaller companies. The aim of this study was to compare chemical constituents of original Juul mango pods with mango-flavoured Juul-compatible pods.
Methods
Juul and 16 brands of Juul-compatible mango-flavoured pods were purchased online in May 2018 (original Juul) and November 2019 (Juul-compatible), after Juul voluntarily removed their flavoured pods from the market. Liquid was extracted from pods and analysed using chromatography and mass spectrometry methods for nicotine concentration, solvent ratios, nicotine salt identification, as well as flavouring identification and quantitation.
Results
Juul-compatible pods had a significantly lower average nicotine concentration compared with original Juul pod (42.8±8.9 vs 57.2±0.9 mg/mL, p<0.0001). Nicotine benzoate was used in original Juul pod and all Juul-compatible pods. The propylene glycol to vegetable glycerin volumetric ratio of Juul-compatible pods averaged 55:45, while the original Juul pod was 35:65 (p<0.0001). Total number of flavouring chemicals detected was significantly higher in Juul-compatible pods as compared with Juul (p<0.0001). In Juul-compatible pods, average concentrations of benzyl alcohol (fruity flavouring) were 0.8±1.3 mg/mL, approximately 27 times higher than in original Juul pod (p<0.0001).
Conclusions
Adulterated Juul-compatible products may expose e-cigarette consumers to more chemical constituents at higher concentrations than previously found in the original product, despite similarity in product design.
Keywords: Electronic nicotine delivery devices, Nicotine, Non-cigarette tobacco products
WHAT IS ALREADY KNOWN ON THIS TOPIC
Flavoured electronic cigarettes (e-cigarettes) are appealing to youth.
Increased quantity and concentration of flavouring chemicals in e-cigarettes could negatively affect potential risk to users.
WHAT THIS STUDY ADDS
Comprehensive federal restrictions of non-tobacco flavours that reduce the appeal of flavoured vaping products to youth, while avoiding unintended negative impacts on adult e-cigarette users who have effectively transitioned from tobacco cigarettes, are needed.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Mango-flavoured knockoff Juul-compatible pods are chemically diverse products that have significantly more flavour chemicals and significantly less nicotine than original Juul pods.
Smaller manufacturers may respond to policy changes differently than their larger counterparts. This may lead to smaller manufacturers selling adulterated knockoffs, filling the gap left by larger companies’ compliance with new policy.
This study supports the need for comprehensive restrictions of non-tobacco flavours in e-cigarettes including sale of adulterated vaping products.
Background
Electronic cigarettes (e-cigarettes) have evolved since their introduction to the USA in 2007.1 The fourth generation of e-cigarette devices uses high-concentration nicotine salts with either disposable or refillable cartridges referred to as pods.2 Juul is one of the most popular pod brands in the USA.3 This popularity was largely driven by its ease of use as well as availability of assorted flavours, with mango the most popular flavour in 2018, as seen in the annual national youth tobacco survey.4 During this same time period, Juul was primarily advertising to younger users and was found guilty of this practice in North Carolina in late 2021.5 This resulted in one in eight US high school students reportedly vaping Juul in 2019.6
In response to youth-targeted marketing, the Food and Drug Administration (FDA) issued letters to Juul as well as other e-cigarette manufacturers in September 2018 requiring them to cease marketing to youth within 60 days.7 In November 2018, Juul announced that it planned to stop selling flavoured pods other than tobacco, menthol and mint in stores.8 In February 2020, the FDA used discretionary enforcement, informing manufacturers to remove unauthorised flavoured cartridges and pods, other than tobacco and menthol from the market. A survey of e-cigarette users aged 21+ years found a significant decrease in the use of mango Juul pods after Juul announced stopping selling of flavoured pods.9 The same study found that more than 10% of users aged 21+ years continued to use generic Juul-compatible pods largely driven by the availability of flavours.9 In one study that analysed nicotine and solvent concentrations in pods collected from high schools, it was determined that most contained concentrations not consistent with original Juul products.6 This study acknowledged that those pods could have been counterfeit or Juul-compatible.
While Juul has been shown to produce less free radicals and carbonyls than older generations of e-cigarettes,10 use of highly concentrated nicotine in Juul pods can result in increased risk of nicotine dependence in adolescents.11 Additionally, several flavouring compounds identified in Juul have been shown to cause epithelial cell and DNA damage in cell lines.12 With many Juul-compatible pods introduced on the market after original products had been withdrawn by Juul, there is a knowledge gap regarding potential differential risk from using Juul-compatible products.13 The aim of this study was to compare nicotine, solvents, nicotine salts and flavouring chemicals (flavourings) in original Juul with those used in Juul-compatible pods.
Materials and methods
Juul and Juul-compatible pods
A single pack of original mango Juul pods (n=4 pods) was purchased directly from the manufacturer’s website in February of 2018. One pack of each brand of mango-flavoured Juul-compatible pods was selected based on an observational study of Instagram and YouTube users in Fall 2019 (online supplemental table 1). One pack per each brand of mango Juul-compatible pods (n=16 brands, n=4–5 pods per pack) was purchased online from the retail site Ziip Stock in November 2019. Upon arrival to the laboratory, a single pod was randomly selected from each pack and e-liquid was manually removed from each pod. All extracted e-liquid was stored in Eppendorf tubes at 4°C in a dark place until analysis.
tc-2022-057476supp001.pdf (66.2KB, pdf)
Measurement of nicotine concentration and solvent ratio
Nicotine concentration and solvent ratios were determined using gas chromatography mass spectrometry (GC-MS) with an Agilent 7890B (GC) and 5977A (MS) using a modified version of a previous method.14 Calibration levels were prepared starting with 100:0 propylene glycol to vegetable glycerin (PG:VG) and 0.1 mg/mL nicotine, with decreasing ratios of PG to VG until 0:100 PG:VG and 74.1 mg/mL nicotine. Limits of quantitation (LOQs) for each compound were as follows: nicotine 0.1 mg/mL, PG 10 V/V and VG 15 V/V.
Analysis of flavourings, salts and other additives
Ingredients in e-liquids were identified using GC-MS as described previously.15 Quantitation of select flavours was conducted with GC quadrupole time-of-flight (GC/Q-TOF) using Agilent 7890B (GC) and 7250A (Q-TOF) and a fully validated method as described previously.16 Flavour calibration range was between 0.02 and 10.0 mg/mL. LOQs values can be found in online supplemental table 1.
Statistical analysis
Kruskal-Wallis non-parametric tests were performed using Prism GraphPad V.8.4.3 (San Diego, California, USA). Dunn’s multiple comparisons tests were also performed comparing the mean rank of Juul (control) with the mean rank of all other Juul-compatible pods. All samples were run at least in triplicate.
Results
Nicotine concentration
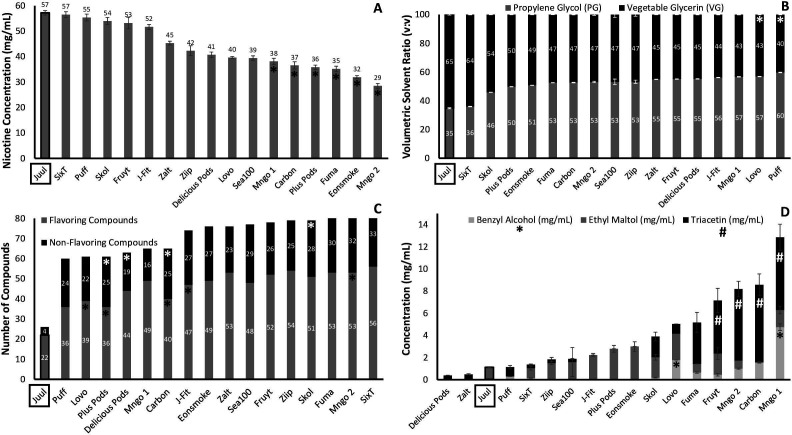
Nicotine concentrations of Juul-compatible pods varied greatly, with an average of 42.8±8.9 mg/mL, significantly lower than the original Juul mango pod that contained 57.2±0.9 mg/mL nicotine (p<0.0001, figure 1A). Over half of Juul-compatible pods (56.3%) had >20% difference between their labelled versus determined nicotine concentrations (online supplemental table 1). We found that Mngo 1, Carbon, Plus Pods, Fuma, Eonsmoke and Mngo 2 had significantly less nicotine (p<0.0443) than the original Juul pod (figure 1A).
Figure 1.
Comparison of e-liquid characteristics of mango Juul and Juul-compatible pods. Error bars represent SD. Juul e-liquid was significantly different compared with average Juul-compatible e-liquid for: nicotine concentration (A), volumetric solvent ratio (B), number of compounds (C) and concentrations of flavouring (D), p<0.0001 for all. * and # indicate significance (p<0.05) as compared with Juul using Dunn's multiple comparison. (D) * indicates significance for benzyl alcohol and # indicates significance for triacetin. No significance was found for ethyl maltol using multiple comparisons.
Solvent volumetric ratio
The PG:VG volumetric ratio of the Juul-compatible pods was about 55:45, while the original Juul mango pod contained a volumetric ratio of 35:65 (figure 1B). Overall, Juul-compatible pods contained significantly less VG than original Juul (p<0.0001, figure 1B). We found that Lovo and Puff had significantly less VG (p<0.0002) than the original Juul pod (figure 1B).
Identified flavouring chemicals, nicotine salts and other additives
On average, Juul-compatible pods contained significantly more flavouring chemicals than original Juul pod: 48±7 vs 22±0 (p<0.0001, figure 1C). We also detected significantly more non-flavouring chemicals including minor tobacco alkaloids like nicotyrine and anabasine in Juul-compatible pods (26±4) than in the Juul pod (4±0) (p<0.0001, figure 1C). We observed a significant increase in total number of chemicals in Juul-compatible pods (73±15) as compared with original Juul pod (26±0) (p<0.0001, figure 1C). We found that Plus Pods, Delicious Pods, Carbon and Skol had significantly more flavouring chemicals (p≤0.0308) than the original Juul pod (figure 1C). We also found that Lovo, Plus Pods, Carbon, J-Fit and Mngo 2 had significantly more non-flavouring chemicals (p≤0.0462) than the original Juul pod (figure 1C), and Skol, Lovo, Plus Pods, Carbon and Mngo 2 had significantly more total chemicals (p≤0.0308) than the original Juul pod (online supplemental table 2). Benzoic acid (used to create nicotine benzoate) was found in all Juul and all Juul-compatible pods (online supplemental table 2). Lactic acid (nicotine lactate) was also found only in Mngo 1. See online supplemental table 2 for a complete list of detected chemicals in all products.
tc-2022-057476supp002.pdf (76.6KB, pdf)
Measured concentrations of flavouring chemicals
Of the 20 flavouring chemicals that we attempted to quantify, only 5 had concentrations above LOQs. Quantifiable flavourings included: benzyl alcohol (fruity), ethyl maltol (sweetener), furaneol (caramellic), L-menthol (coolant) and triacetin (creamy) (online supplemental table 1). Among those five flavourings, on average higher concentrations were found in Juul-compatible pods as compared with Juul pod (online supplemental table 1, p<0.0001). Notably, triacetin (creamy) and benzyl alcohol (fruity) were both detected in 81.3% (13) Juul-compatible pods at an average concentration of 2.6±2.7 and 0.8±1.3 mg/mL, respectively. Those two flavourings, were below LOQ for triacetin and 0.03±0.00 mg/ml for benzyl alcohol in the original Juul pod (figure 1D). Average concentrations of ethyl maltol (sweetener) were 1.8±0.7 mg/mL in 75.0% Juul-compatible pods and 1.1±0.1 mg/mL in the original Juul pod (figure 1D). We found triacetin (creamy) concentrations that were significantly higher in Fruyt, Mngo 2, Carbon and Mngo 1 as compared with original Juul (figure 1D, p≤0.0385). Benzyl alcohol (fruity) concentrations were significantly higher in Lovo and Mngo 1 as compared with original Juul (figure 1D, p≤0.0355). Although ethyl maltol (sweetener) was present in more than half (56.3% (9)) of Juul-compatible pods, its concentration was not significantly higher than in original Juul (figure 1D).
Discussion
This study investigated chemical ingredients of Juul and Juul-compatible pod products. Our results demonstrate that Juul-compatible pods use the same nicotine salt as Juul and contain lower nicotine concentrations. However, more than half of purchased products were mislabeled as they contained at least 20% less nicotine than listed (online supplemental table 1). As a result, pod users may adjust puffing behaviour to compensate for lower nicotine concentrations found in Juul-compatible pods as shown in a previous study.17 These potential changes in vaping patterns may result in consumers using Juul-compatible products more intensively than original Juul (eg, puffing more frequently), thus increasing exposure to flavouring compounds.
On average, Juul-compatible pods contained more than double the number of flavouring chemicals as original Juul, as well as almost four times the average concentration of those flavouring compounds. As previous studies have shown, some flavour categories (eg, creamy/buttery, strawberry) can have a significant impact on toxicity in immortalised cell lines18 19; however, more work is needed to examine respiratory effects of specific flavourings (eg, butyric acid, furaneol). While many flavourings were identified in this study (online supplemental table 2), several including ethyl maltol, benzyl alcohol and triacetin were found in most pods tested including both Juul and Juul-compatible pods. These flavourings, also identified in a previous Juul study,20 when tested individually, were shown to have cytotoxic effect on cell lines.21 Comprehensive restrictions on specific flavouring chemicals rather than broad categories (eg, tobacco) may better assist in harm prevention.
By removing mango-flavoured Juul pods from the market, a void was created and filled by smaller competitors allowing continued sale of flavoured Juul-compatible pods. At the time of this study, Juul was one of the most popular devices used among youth,22 driven by its easy concealability,23 high nicotine content13 and availability of flavourings.24 These same characteristics also make Juul an appealing device for adult tobacco cigarette users to switch to.25 While comprehensive regulation restricting access to flavoured vaping products could prevent further youth initiation, potential unintended consequences of such regulatory approaches, such as impeding combustible adult users from switching to less harmful vaping products, need to be recognised, monitored and mitigated. For example, enhanced regulation of online sales requiring age verification that is not easily bypassed as well as enforcement of regulation on small online retailers who are not following current regulation is needed. Of the 16 mango Juul-compatible pods purchased in November of 2019, 2 months prior to the FDA’s enforcement on flavoured pods, all 16 were still available for sale as of March 2022. Since the deadline for marketing applications of e-cigarette products was 9 September 2020, without authorisation, these products remain on the market illegally. The only mango product examined in this study that is currently not available is original Juul.
This study has several limitations that should be noted. First, a single pack of Juul and Juul-compatible pods was purchased for each brand, limiting generalisability within brands. Second, this study only determined the chemical composition of select mango-flavoured Juul and Juul-compatible pods. Comprehensive toxicity studies are needed to fully understand the long-term health effects of all flavour chemicals present in e-cigarettes included in this study.
In conclusion, implementation of comprehensive flavoured e-cigarette policy intended to reduce vaping rates among youth should also consider unintended consequences including sale of adulterated replacement products. Such regulations should also take into consideration accessibility of vaping products to adult smokers who switched completely from combustible products.
Footnotes
Contributors: MLG contributed to the conception of the work. LGD, MKP, NJL and MLG contributed to data analysis. LGD, MKP, NJL and MLG drafted the manuscript. LGD and MKP ran all experiments. All authors approved the final version of the manuscript. MLG has full access to all study data and takes responsibility for the integrity of the data and accuracy of the data analysis.
Funding: Research reported here was supported by the National Cancer Institute of the National Institutes of Health (NIH) and the Food and Drug Administration (FDA) Center for Tobacco Products under Award Number U54CA228110.
Disclaimer: The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA.
Competing interests: MLG reports grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking-cessation drugs. Other authors declare no conflict of interest.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1. CDC . Surgeon General’s Advisory on E-cigarette Use Among Youth. 2018.
- 2. Williams R. The rise of disposable JUUL-type e-cigarette devices. Tob Control 2020;29:e134–5. 10.1136/tobaccocontrol-2019-055379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Huang J, Duan Z, Kwok J, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tob Control 2019;28:146–51. 10.1136/tobaccocontrol-2018-054382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Morean ME, Bold KW, Kong G, et al. High school students' use of JUUL pod flavors before and after JUUL implemented voluntary sales restrictions on certain flavors in 2018. PLoS One 2020;15:e0243368. 10.1371/journal.pone.0243368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ahmed N. Attorney General Stein Reaches Agreement with JUUL for $40 Million and Drastic Business Changes 2021.
- 6. Wang TW, Neff LJ, Park-Lee E, et al. E-cigarette Use Among Middle and High School Students - United States, 2020. MMWR Morb Mortal Wkly Rep 2020;69:1310–2. 10.15585/mmwr.mm6937e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. FDA . Request letter to JUUL Labs, Inc; 2018.
- 8. discontinued JUULpods flavors.
- 9. Reilly SM, Bitzer ZT, Goel R, et al. Free radical, carbonyl, and nicotine levels produced by Juul electronic cigarettes. Nicotine Tob Res 2019;21:1274–8. 10.1093/ntr/nty221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boykan R, Goniewicz ML, Messina CR. Evidence of nicotine dependence in adolescents who use Juul and similar pod devices. Int J Environ Res Public Health 2019;16:2135. 10.3390/ijerph16122135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Muthumalage T, Lamb T, Friedman MR, et al. E-Cigarette flavored pods induce inflammation, epithelial barrier dysfunction, and DNA damage in lung epithelial cells and monocytes. Sci Rep 2019;9:1–11. 10.1038/s41598-019-51643-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jackler RK, Ramamurthi D. Nicotine arms race: JUUL and the high-nicotine product market. Tob Control 2019;28:623–8. 10.1136/tobaccocontrol-2018-054796 [DOI] [PubMed] [Google Scholar]
- 13. Cox S, Leigh NJ, Vanderbush TS, et al. An exploration into "do-it-yourself" (DIY) e-liquid mixing: Users' motivations, practices and product laboratory analysis. Addict Behav Rep 2019;9:100151. 10.1016/j.abrep.2018.100151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Page MK, Block AC, Santiago AL. Changes in product labeling practices and the use of flavoring chemical additives in Vaping products after implementation of statewide flavor restriction. Tobacco Control 2022;31:s224–30. 10.1136/tobaccocontrol-2022-057469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Page MK, Goniewicz ML. New analytical method for quantifying flavoring chemicals of potential respiratory health risk concerns in e-cigarette liquids. Front Chem 2021;9:763940. 10.3389/fchem.2021.763940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Dawkins LE, Kimber CF, Doig M, et al. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology 2016;233:2933–41. 10.1007/s00213-016-4338-2 [DOI] [PubMed] [Google Scholar]
- 17. Behar RZ, Wang Y, Talbot P. Comparing the cytotoxicity of electronic cigarette fluids, aerosols and solvents. Tob Control 2018;27:325–33. 10.1136/tobaccocontrol-2016-053472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Leigh NJ, Lawton RI, Hershberger PA, et al. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ends). Tob Control 2016;25:ii81–7. 10.1136/tobaccocontrol-2016-053205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Omaiye EE, McWhirter KJ, Luo W, et al. High-nicotine electronic cigarette products: toxicity of JUUL fluids and aerosols correlates strongly with nicotine and some flavor chemical concentrations. Chem Res Toxicol 2019;32:1058–69. 10.1021/acs.chemrestox.8b00381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Behar RZ, Luo W, McWhirter KJ, et al. Analytical and toxicological evaluation of flavor chemicals in electronic cigarette refill fluids. Sci Rep 2018;8:1–11. 10.1038/s41598-018-25575-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hammond D, Wackowski OA, Reid JL, et al. Use of JUUL e-cigarettes among youth in the United States. Nicotine Tob Res 2020;22:827–32. 10.1093/ntr/nty237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ramamurthi D, Chau C, Jackler RK. JUUL and other stealth vaporisers: hiding the habit from parents and teachers. Tob Control 2019;28:610–6. 10.1136/tobaccocontrol-2018-054455 [DOI] [PubMed] [Google Scholar]
- 23. Leventhal AM, Miech R, Barrington-Trimis J, et al. Flavors of e-cigarettes used by youths in the United States. JAMA 2019;322:2132–4. 10.1001/jama.2019.17968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Goldenson NI, Shiffman S, Hatcher C, et al. Switching away from cigarettes across 12 months among adult smokers purchasing the JUUL system. Am J Health Behav 2021;45:443–63. 10.5993/AJHB.45.3.4 [DOI] [PubMed] [Google Scholar]
- 25. Yingst JM, Bordner CR, Hobkirk AL, et al. Response to Flavored Cartridge/Pod-Based Product Ban among Adult JUUL Users: “You Get Nicotine However You Can Get It”. Int J Environ Res Public Health 2021;18:207. 10.3390/ijerph18010207 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
tc-2022-057476supp001.pdf (66.2KB, pdf)
tc-2022-057476supp002.pdf (76.6KB, pdf)