Abstract
Background
Electronic cigarettes (ECs) are handheld electronic vaping devices which produce an aerosol by heating an e‐liquid. Some people who smoke use ECs to stop or reduce smoking, although some organizations, advocacy groups and policymakers have discouraged this, citing lack of evidence of efficacy and safety. People who smoke, healthcare providers and regulators want to know if ECs can help people quit smoking, and if they are safe to use for this purpose. This is a review update conducted as part of a living systematic review.
Objectives
To examine the effectiveness, tolerability, and safety of using electronic cigarettes (ECs) to help people who smoke tobacco achieve long‐term smoking abstinence.
Search methods
We searched the Cochrane Tobacco Addiction Group's Specialized Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, and PsycINFO to 1 July 2022, and reference‐checked and contacted study authors.
Selection criteria
We included randomized controlled trials (RCTs) and randomized cross‐over trials, in which people who smoke were randomized to an EC or control condition. We also included uncontrolled intervention studies in which all participants received an EC intervention. Studies had to report abstinence from cigarettes at six months or longer or data on safety markers at one week or longer, or both.
Data collection and analysis
We followed standard Cochrane methods for screening and data extraction. Our primary outcome measures were abstinence from smoking after at least six months follow‐up, adverse events (AEs), and serious adverse events (SAEs). Secondary outcomes included the proportion of people still using study product (EC or pharmacotherapy) at six or more months after randomization or starting EC use, changes in carbon monoxide (CO), blood pressure (BP), heart rate, arterial oxygen saturation, lung function, and levels of carcinogens or toxicants, or both. We used a fixed‐effect Mantel‐Haenszel model to calculate risk ratios (RRs) with a 95% confidence interval (CI) for dichotomous outcomes. For continuous outcomes, we calculated mean differences. Where appropriate, we pooled data in meta‐analyses.
Main results
We included 78 completed studies, representing 22,052 participants, of which 40 were RCTs. Seventeen of the 78 included studies were new to this review update. Of the included studies, we rated ten (all but one contributing to our main comparisons) at low risk of bias overall, 50 at high risk overall (including all non‐randomized studies), and the remainder at unclear risk.
There was high certainty that quit rates were higher in people randomized to nicotine EC than in those randomized to nicotine replacement therapy (NRT) (RR 1.63, 95% CI 1.30 to 2.04; I2 = 10%; 6 studies, 2378 participants). In absolute terms, this might translate to an additional four quitters per 100 (95% CI 2 to 6). There was moderate‐certainty evidence (limited by imprecision) that the rate of occurrence of AEs was similar between groups (RR 1.02, 95% CI 0.88 to 1.19; I2 = 0%; 4 studies, 1702 participants). SAEs were rare, but there was insufficient evidence to determine whether rates differed between groups due to very serious imprecision (RR 1.12, 95% CI 0.82 to 1.52; I2 = 34%; 5 studies, 2411 participants).
There was moderate‐certainty evidence, limited by imprecision, that quit rates were higher in people randomized to nicotine EC than to non‐nicotine EC (RR 1.94, 95% CI 1.21 to 3.13; I2 = 0%; 5 studies, 1447 participants). In absolute terms, this might lead to an additional seven quitters per 100 (95% CI 2 to 16). There was moderate‐certainty evidence of no difference in the rate of AEs between these groups (RR 1.01, 95% CI 0.91 to 1.11; I2 = 0%; 5 studies, 1840 participants). There was insufficient evidence to determine whether rates of SAEs differed between groups, due to very serious imprecision (RR 1.00, 95% CI 0.56 to 1.79; I2 = 0%; 8 studies, 1272 participants).
Compared to behavioural support only/no support, quit rates were higher for participants randomized to nicotine EC (RR 2.66, 95% CI 1.52 to 4.65; I2 = 0%; 7 studies, 3126 participants). In absolute terms, this represents an additional two quitters per 100 (95% CI 1 to 3). However, this finding was of very low certainty, due to issues with imprecision and risk of bias. There was some evidence that (non‐serious) AEs were more common in people randomized to nicotine EC (RR 1.22, 95% CI 1.12 to 1.32; I2 = 41%, low certainty; 4 studies, 765 participants) and, again, insufficient evidence to determine whether rates of SAEs differed between groups (RR 1.03, 95% CI 0.54 to 1.97; I2 = 38%; 9 studies, 1993 participants).
Data from non‐randomized studies were consistent with RCT data. The most commonly reported AEs were throat/mouth irritation, headache, cough, and nausea, which tended to dissipate with continued EC use. Very few studies reported data on other outcomes or comparisons, hence evidence for these is limited, with CIs often encompassing clinically significant harm and benefit.
Authors' conclusions
There is high‐certainty evidence that ECs with nicotine increase quit rates compared to NRT and moderate‐certainty evidence that they increase quit rates compared to ECs without nicotine. Evidence comparing nicotine EC with usual care/no treatment also suggests benefit, but is less certain. More studies are needed to confirm the effect size. Confidence intervals were for the most part wide for data on AEs, SAEs and other safety markers, with no difference in AEs between nicotine and non‐nicotine ECs nor between nicotine ECs and NRT. Overall incidence of SAEs was low across all study arms. We did not detect evidence of serious harm from nicotine EC, but longest follow‐up was two years and the number of studies was small.
The main limitation of the evidence base remains imprecision due to the small number of RCTs, often with low event rates, but further RCTs are underway. To ensure the review continues to provide up‐to‐date information to decision‐makers, this review is a living systematic review. We run searches monthly, with the review updated when relevant new evidence becomes available. Please refer to the Cochrane Database of Systematic Reviews for the review's current status.
Plain language summary
Can electronic cigarettes help people stop smoking, and do they have any unwanted effects when used for this purpose?
What are electronic cigarettes?
Electronic cigarettes (e‐cigarettes) are handheld devices that work by heating a liquid that usually contains nicotine and flavourings. E‐cigarettes allow you to inhale nicotine in a vapour rather than smoke. Because they do not burn tobacco, e‐cigarettes do not expose users to the same levels of chemicals that can cause diseases in people who smoke conventional cigarettes.
Using an e‐cigarette is commonly known as 'vaping'. Many people use e‐cigarettes to help them to stop smoking tobacco. In this review we focus primarily on e‐cigarettes containing nicotine.
Why we did this Cochrane Review
Stopping smoking lowers your risk of lung cancer, heart attacks and many other diseases. Many people find it difficult to stop smoking. We wanted to find out if using e‐cigarettes could help people to stop smoking, and if people using them for this purpose experience any unwanted effects.
What did we do?
We searched for studies that looked at the use of e‐cigarettes to help people stop smoking.
We looked for randomized controlled trials, in which the treatments people received were decided at random. This type of study usually gives the most reliable evidence about the effects of a treatment. We also looked for studies in which everyone received an e‐cigarette treatment.
We were interested in finding out:
· how many people stopped smoking for at least six months; and · how many people had unwanted effects, reported on after at least one week of use.
Search date: We included evidence published up to 1st July 2022.
What we found
We found 78 studies which included 22,052 adults who smoked. The studies compared e‐cigarettes with:
· nicotine replacement therapy, such as patches or gum;
· varenicline (a medicine to help people stop smoking); · e‐cigarettes without nicotine;
· other types of nicotine‐containing e‐cigarettes (e.g. pod devices, newer devices); · behavioural support, such as advice or counselling; or · no support for stopping smoking.
Most studies took place in the USA (34 studies), the UK (16), and Italy (8).
What are the results of our review?
People are more likely to stop smoking for at least six months using nicotine e‐cigarettes than using nicotine replacement therapy (6 studies, 2378 people), or e‐cigarettes without nicotine (5 studies, 1447 people).
Nicotine e‐cigarettes may help more people to stop smoking than no support or behavioural support only (7 studies, 3126 people).
For every 100 people using nicotine e‐cigarettes to stop smoking, 8 to 12 might successfully stop, compared with only 6 of 100 people using nicotine‐replacement therapy, 7 of 100 using e‐cigarettes without nicotine, or 4 of 100 people having no support or behavioural support only.
We are uncertain if there is a difference between how many unwanted effects occur using nicotine e‐cigarettes compared with nicotine replacement therapy, no support or behavioural support only. There was some evidence that non‐serious unwanted effects were more common in groups receiving nicotine e‐cigarettes compared to no support or behavioural support only. Low numbers of unwanted effects, including serious unwanted effects, were reported in studies comparing nicotine e‐cigarettes to nicotine replacement therapy. There is probably no difference in how many non‐serious unwanted effects occur in people using nicotine e‐cigarettes compared to e‐cigarettes without nicotine.
The unwanted effects reported most often with nicotine e‐cigarettes were throat or mouth irritation, headache, cough and feeling sick. These effects reduced over time as people continued using nicotine e‐cigarettes.
How reliable are these results?
Our results are based on a few studies for most outcomes, and for some outcomes, the data varied widely.
We found evidence that nicotine e‐cigarettes help more people to stop smoking than nicotine replacement therapy. Nicotine e‐cigarettes probably help more people to stop smoking than e‐cigarettes without nicotine but more studies are still needed to confirm this.
Studies comparing nicotine e‐cigarettes with behavioural or no support also showed higher quit rates in people using nicotine e‐cigarettes, but provide less certain data because of issues with study design.
Most of our results for the unwanted effects could change when more evidence becomes available.
Key messages
Nicotine e‐cigarettes can help people to stop smoking for at least six months. Evidence shows they work better than nicotine replacement therapy, and probably better than e‐cigarettes without nicotine.
They may work better than no support, or behavioural support alone, and they may not be associated with serious unwanted effects.
However, we still need more evidence, particularly about the effects of newer types of e‐cigarettes that have better nicotine delivery than older types of e‐cigarettes, as better nicotine delivery might help more people quit smoking.
Summary of findings
Summary of findings 1. Nicotine EC compared to NRT for smoking cessation.
| Nicotine EC compared to NRT for smoking cessation | ||||||
| Patient or population: People who smoke Setting: New Zealand, UK, USA Intervention: Nicotine EC Comparison: NRT | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with NRT | Risk with Nicotine EC | |||||
| Smoking cessation at 6 months to 1 year Assessed with biochemical validation |
Study population | RR 1.63 (1.30 to 2.04) | 2378 (6 RCTs) | ⊕⊕⊕⊕ HIGH | ‐ | |
| 6 per 100 | 10 per 100 (8 to 12) | |||||
| Adverse events at 4 weeks to 6‐9 months Assessed by self‐report |
Study population | RR 1.02 (0.88 to 1.19) | 1702 (4 RCTs) | ⊕⊕⊕⊝ MODERATEa | ‐ | |
| 27 per 100 | 27 per 100 (24 to 32) | |||||
| Serious adverse events at 4 weeks to 1 year Assessed via self‐report and medical records |
Study population | RR 1.12 (0.82 to 1.52) | 2411 (5 RCTs) | ⊕⊕⊝⊝ LOWb | 2 studies reported no events; effect estimate based on the three studies in which events were reported | |
| 6 per 100 | 7 per 100 (5 to 9) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For cessation, the assumed risk in the control group is based on assumed quit rates for NRT assuming receipt of limited behavioural stop‐smoking support (as per Hartmann‐Boyce 2018a). The assumed risk for adverse events and serious adverse events is a weighted mean average of quit rates across control groups in contributing studies. CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded one level due to imprecision; CIs consistent with benefit and harm bDowngraded two levels due to imprecision; fewer than 300 events and CIs encompass clinically important harm and clinically important benefit
Summary of findings 2. Nicotine EC compared to non‐nicotine EC for smoking cessation.
| Nicotine EC compared to non‐nicotine EC for smoking cessation | ||||||
| Patient or population: People who smoke cigarettes Setting: Canada, Italy, New Zealand, UK, USA Intervention: Nicotine EC Comparison: Non‐nicotine EC | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with non‐nicotine EC | Risk with Nicotine EC | |||||
| Smoking cessation at 6‐12 months Assessed with biochemical validation |
Study population | RR 1.94 (1.21 to 3.13) | 1447 (5 RCTs) | ⊕⊕⊕⊝ MODERATEa,b | ‐ | |
| 7 per 100 | 14 per 100 (9 to 23) | |||||
| Adverse events at 1 week to 6 months Assessed via self‐report |
Study population | RR 1.01 (0.91 to 1.11) | 840 (5 RCTs) | ⊕⊕⊕⊝ MODERATEb | ‐ | |
| 9 per 100 | 9 per 100 (8 to 10) | |||||
| Serious adverse events at 1 week to 1 year Assessed via self‐report and medical records |
Study population | RR 1.00 (0.56 to 1.79) | 1272 (8 RCTs) | ⊕⊕⊝⊝ LOWc | 4 studies reported no events; effect estimate based on the 3 studies in which events were reported | |
| 3 per 100 | 3 per 100 (2 to 6) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For cessation, the assumed risk in the control group is based on receipt of moderate‐intensity behavioural stop‐smoking support. The assumed risk for adverse events and serious adverse events is a weighted mean average of quit rates across control groups in contributing studies. CI: Confidence interval; RCT: randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aNot downgraded for risk of bias. One of four studies considered high risk of bias; removing this study increased the direction of the effect in favour of the intervention. bDowngraded one level due to imprecision; < 300 events overall cDowngraded two levels due to imprecision: confidence intervals encompass clinically significant harm as well as clinically significant benefit.
Summary of findings 3. Nicotine EC compared to behavioural support only/no support for smoking cessation.
| Nicotine EC compared to behavioural support only/no support for smoking cessation | ||||||
| Patient or population: People who smoke Setting: Canada, Italy, UK, USA Intervention: Nicotine EC Comparison: Behavioural support only/no support | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with behavioural support only/no support | Risk with Nicotine EC | |||||
| Smoking cessation at 6 to 12 months Assessed using biochemical validation |
Study population | RR 2.66 (1.52 to 4.65) | 3126 (7 RCTs) | ⊕⊝⊝⊝ VERY LOWa,b | ‐ | |
| 1 per 100 | 3 per 100 (2 to 5) | |||||
| Adverse events at 12 weeks to 6 months Assessed via self‐report |
Study population | RR 1.22 (1.12 to 1.32) | 765 (4 RCTs) | ⊕⊕⊝⊝ LOWa | ‐ | |
| 66 per 100 | 80 per 100 (74 to 87) | |||||
| Serious adverse events at 4 weeks to 8 months Assessed via self‐report and medical records |
Study population | RR 1.03 (0.54 to 1.97) | 1993 (9 RCTs) | ⊕⊝⊝⊝ VERY LOWa,c | 5 of the 9 studies reported no SAEs; MA is based on pooled results from 4 studies. | |
| 2 per 100 | 2 per 100 (1 to 4) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). For cessation, the assumed risk in the control group is based on receipt of limited stop‐smoking support. The assumed risk for adverse events and serious adverse events is a weighted mean average of quit rates across control groups in contributing studies. CI: Confidence interval; MA: meta‐analysis; RCT: randomized controlled trial; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
aDowngraded two levels due to risk of bias. Due to lack of blinding and differential support between arms, judged to be at high risk of bias. bDowngraded one level due to imprecision; although confidence intervals are consistent with clinically important difference, event count is very low (< 100). cDowngraded two levels due to imprecision; confidence intervals incorporate clinically significant benefit and clinically significant harm.
Background
Throughout this review, we discuss (1) conventional cigarettes and (2) electronic cigarettes, defined as hand held and producing for inhalation an aerosol formed by heating an e‐liquid using a battery‐powered heating coil. In this review, all mention of smoking, smoking cessation, cigarette use, smoke intake, etc. concerns combustible tobacco cigarettes. When the text concerns electronic cigarettes we use the abbreviation 'ECs'. EC users are sometimes described as 'vapers', and EC use as 'vaping'. We refer to ECs that do not contain nicotine as non‐nicotine ECs; these can also be conceptualized as placebo ECs, but we are using the term non‐nicotine EC, as they can be conceptualized as an intervention in themselves. This review does not address the use of vaping devices to inhale substances other than nicotine, such as cannabis.
Description of the condition
Stopping smoking is associated with large health benefits. Despite most people who smoke wanting to quit, many find it difficult to succeed in the long term. Almost half who try to quit without support will not manage to stop for even a week, and fewer than five per cent remain abstinent at one year after quitting (Hughes 2004).
Behavioural support and medications such as nicotine patches or gum increase the chances of quitting through providing nicotine to help alleviate withdrawal symptoms, but even with this additional support, long‐term quit rates remain low (Cahill 2016; Hartmann‐Boyce 2018b; Hartmann‐Boyce 2019). One of the limitations of current treatments is that, apart from providing nicotine more slowly and at lower levels than smoking, none adequately addresses the sensory, behavioural and/or social aspects of smoking that ex‐smokers miss when they stop smoking (e.g. holding a cigarette in their hands, taking a puff, enjoyment of smoking, feeling part of a group). ECs may offer a way to overcome these limitations (Notley 2018b).
There is no doubt that people become dependent on tobacco, and find it difficult to stop smoking, primarily because of nicotine and its actions on the brain's reward system (Balfour 2004). However, developing dependence on tobacco smoking is a complex biopsychosocial process (Benowitz 2010; Rose 2006). Other tobacco chemicals, such as acetaldehyde and MAO inhibitors seem to potentiate effects of nicotine (Rose 2006). In addition, sensory and behavioural cues provide additional reinforcement of smoking behaviour (Rose 1993; Rose 2000) and may over time become almost as rewarding as nicotine. There are several lines of evidence to support this. Firstly, people who smoke appear to have a preference for cigarette smoke compared to other forms of nicotine delivery. This is partly related to the speed of nicotine delivery through smoke inhalation. However, even when nicotine is administered intravenously it does not provide the same level of satisfaction or reward as smoking (Rose 2000; Westman 1996). Secondly, the local sensory effects of smoking (e.g. the ‘scratch’ in the back of the throat) may be important for enjoyment and reward. Numbing the sensations of cigarette smoke by anaesthetizing the upper and lower respiratory tract leads to less enjoyment of smoking (Rose 1985). Conversely, products that mimic the sensory effects of smoking on the mouth and throat (such as citric acid, black pepper, and ascorbic acid) reduce craving and some withdrawal symptoms, at least in the short term (Levin 1993; Rose 1994; Westman 1995). Thirdly, very low nicotine content cigarettes (VLNCs), which have a very low content of nicotine (e.g. 0.08 mg instead of the normal 1 mg) and so have negligible or no central effects, have also been investigated for their role in aiding smoking cessation (Przulj 2013). Despite delivering low levels of nicotine, VLNCs are satisfying over the initial few days of abstinence from nicotine (Donny 2007; Donny 2015; Pickworth 1999; Rose 2000). They also reduce tobacco withdrawal symptoms, including urges to smoke and low mood (Barrett 2010; Donny 2009; McRobbie 2016; Perkins 2010; Rose 2000), and have been shown to improve long‐term continuous abstinence rates in one study (Walker 2012). Social aspects of smoking, such as feeling part of a like‐minded group, or including smoking behaviour as part of one's social identity are also elements of cigarette smoking that some people who smoke report to be drivers of cigarette use (Notley 2018a).
Considering the other factors that contribute to tobacco dependence, there is interest in developing smoking‐cessation products that would not only help relieve the unpleasant effects of nicotine withdrawal but would also act as an effective substitute for smoking behaviour and the rituals and sensations that accompany smoking, without the health risks associated with the inhalation of tobacco smoke. Until recently, the only pharmaceutical treatment available that had some of these characteristics was the nicotine inhalator. However, these do not have greater cessation efficacy than the other nicotine replacement therapy (NRT) products (Hajek 1999; Hartmann‐Boyce 2018a). This may in part be due to the considerable effort (e.g. 20 minutes of continuous puffing) needed to provide nicotine blood concentrations consistent with other NRTs (Schneider 2001). Adherence to correct use of the inhalator is low compared to other NRTs (Hajek 1999). It is therefore possible that any advantage of sensorimotor replacement is diminished by low nicotine delivery and limited similarities between inhalator use and sensations of smoking (Bullen 2010). A nicotine inhaler using pressurized air is approved as a smoking cessation aid in the UK. The nicotine delivery from this device is substantially lower than from cigarettes, and also lower than from the nicotine inhalator (Romeu 2020).
Description of the intervention
ECs are hand held and produce for inhalation an aerosol formed by heating an e‐liquid using a battery‐powered heating coil (E‐cigarette ontology 2021). The e‐liquid, usually comprising propylene glycol and glycerol, with or without nicotine and flavours, is stored in disposable or refillable cartridges or a reservoir or 'pod'. The commonly‐used term for this aerosol is vapour, which we use throughout the review. ECs are marketed as consumer products. Although routes are in place for licensing them as medicine or medical devices in some areas, no country yet has a licensed medicinal EC.
ECs provide sensations similar to smoking a cigarette. They provide taste and throat sensations that are closer to smoking than those provided by the nicotine inhalator (Barbeau 2013). The vapour that looks like tobacco smoke is only visible when the user exhales after drawing on the mouthpiece, not when the device is being held. In qualitative studies, users report a sense of shared identity with other users, similar to tobacco‐smoking identity, and also report pleasure and enjoyment of use, suggesting that ECs may be viewed less as medical cessation aids but rather as acceptable alternatives to tobacco smoking (Cox 2017; Notley 2018a).
There are many different brands and models of EC available. Variation exists both in the device ('product') and consumable (e‐liquid used). There is a wide variation in the composition of e‐liquids (nicotine content, flavours and other components) (Goniewicz 2012; Goniewicz 2014), with some users choosing to mix their own e‐liquids (Cox 2019b). Initial studies showed that early models of EC delivered very low amounts of nicotine to naïve users (Bullen 2010; Eissenberg 2010; Vansickel 2010). Later studies that have measured nicotine pharmacokinetics in both experienced and naïve EC users have found that some EC users can achieve blood nicotine levels similar to those achieved with smoking, albeit more slowly, and that their ability to do so often improves over time (Hajek 2015b; Vansickel 2012; Vansickel 2013; Yingst 2019a; Yingst 2019b).
Early on in their development, ECs looked like cigarettes and used disposable cartridges. These models were often called 'cig‐a‐likes'. The nicotine delivery from these products was low, and even the modern versions of EC devices that use pre‐filled cartridges, generally produced by the tobacco industry, for the most part have only low nicotine delivery (Hajek 2017). The later refillable, or 'tank', products have a larger battery and a transparent container that users fill with an e‐liquid of their choice, and usually provide faster and more efficient nicotine delivery, allow a wider choice of flavours and nicotine concentrations, and are typically used by experienced vapers who manage to switch to vaping completely (ASH 2019; Dawkins 2013b; Farsalinos 2014). Observational evidence suggests people who smoke are more likely to successfully quit using tank models than with cig‐a‐likes (Chen 2016; Hitchman 2015). Smaller 'pod' devices that use nicotine salt are also available (e.g. Juul). This nicotine formulation reduces irritant effects and allows the delivery of higher nicotine levels that closely mimic the pharmacokinetic profile of nicotine delivery from cigarettes, despite the low battery power of the devices (Hajek 2020). The EU Tobacco Products Directive (European Parliament 2014) does not allow sales of e‐liquids with nicotine content higher than 20 mg/mL, and so the US version of Juul (59 mg/nl nicotine) is not available within the EU (Huang 2019; Talih 2020). Most recently, there has been rapid growth in the use of small disposable devices (Tattan‐Birch 2022). These are available in a range of attractive flavours, generally have a high nicotine content, are low cost and have a closed system that is designed to be disposed of following use (approximately 200 puffs). According to ASH 2022, for adults in GB, tank style devices are the most popular. For youth, the ASH 2022 report disposables are now the most popular.
The different device types may differ significantly in their efficacy in helping people who smoke to quit, as they differ in delivery of nicotine. Nicotine itself, when delivered through mechanisms and doses similar to that delivered in traditional NRT, is not considered harmful (Hartmann‐Boyce 2018a). The safety profile of the different types of nicotine EC may be similar as they use the same constituents, although within the generic range of EC types, there is some evidence to suggest EC providing less nicotine may pose higher risks. This is because low‐nicotine delivery devices need to be puffed with higher intensity to provide users with the nicotine levels that they seek, and more intensive puffing is accompanied by increased inhalation of potential toxicants (Dawkins 2016; Dawkins 2018; Smets 2019). Throughout this review, we refer to a nicotine‐containing EC as ‘nicotine EC’ and to nicotine‐free EC as 'non‐nicotine EC', which can also be considered 'placebo EC'. The 'placebo' comparison is a test just of the nicotine effect and not of the potential sensorimotor or behavioural and social replacement that the EC may provide.
There is no one agreed classification system for EC devices, and product development has moved so quickly that the definitions used within trials of the devices tested may no longer necessarily be fit for purpose. In this review, the definitions used are based on those drawn from the included trials. We currently label three different types of EC as 'cartridges' for devices with disposable cartridges and ‐ typically, but not always ‐ low nicotine delivery (e.g. cig‐a‐likes); refillable ECs for devices that vapers fill with their own choice of e‐liquids; and pods for the small devices that commonly use nicotine salts. To date, there are no trials of disposable devices, so we do not include this category in the current review. We may review this categorization system in future versions of the review as new trials and devices emerge.
Why it is important to do this review
Since ECs appeared on the market in 2006, there has been a steady increase in their use. In the UK, the ASH 2022 surveys found 19.4% of the adult population had ever tried vaping, but only 8.3% were current vapers. EC use is most prevalent in current (22%) and former (14%) smokers (ASH 2022). Only 1.3% of never‐smokers report currently using ECs. Prevalence data from the USA in 2019 showed that 4.4% of adults were current EC users (Du 2020). Data from lower‐income countries suggest similar levels of EC use and awareness (Besaratinia 2019; Jiang 2016; Palipudi 2016).
Regulatory approaches being used for ECs currently vary widely, from no regulation to partial and complete bans (McNeill 2022). Within the USA, for example, the Food and Drug Administration (FDA) has classified EC as tobacco products and laws include prohibition of EC use indoors, requirement for retailers to have a license to sell, and prohibition of sales to minors. Laws prohibiting sales to minors apply nationwide, but other laws vary by state (Du 2020). The European Union includes ECs in their Tobacco Products Directive, except where therapeutic claims are made or in instances where they contain over 20 mg/nl of nicotine, when they will require medicines authorization (European Parliament 2014).
Categorical statements about the toxicity of ECs are not possible because of the large number of devices and liquids available and the frequent addition of new products to the market. In 2019, cases of severe lung injury associated with EC use were reported in the USA and, by February 2020, there were around 2800 hospitalized cases or deaths (CDC 2020). This illness was termed E‐cigarette or Vaping‐Associated Lung Injury (EVALI) and caused concern throughout the world (Hall 2020), and a negative change in people's perception of the risks of EC use compared to smoking (Tattan‐Birch 2020). These cases were somewhat at odds with data from trials and cohort studies, and it was later found that these injuries were related to use of tetrahydrocannabinol (THC)‐containing products adulterated with vitamin E acetate (Blount 2020; Hartnett 2020). Amongst those brands of nicotine EC that have been tested, levels of toxins have been found to be substantially lower than in cigarettes (Hajek 2014; McNeill 2022). Long‐term effects beyond 12 months are unclear, although based on what is known about liquid and vapour constituents and patterns of use, a report from the UK's Royal College of Physicians has concluded that using an EC is likely to be considerably safer than smoking (RCP 2016). The US National Academies of Sciences, Engineering, and Medicine (NASEM) concluded that ECs are likely to be far less harmful than continuing to smoke cigarettes, with the caveat that the long‐term health effects of e‐cigarette use are not yet known (NASEM 2018).
Despite general acknowledgement that EC use exposes the user to fewer toxicants and at lower levels than smoking cigarettes (McNeill 2021; McNeill 2022; NASEM 2018; RCP 2016), there remains some hesitancy in making these products available to people who smoke as a harm‐reduction tool or smoking‐cessation aid (e.g. McDonald 2020). Lack of quality control measures, possible harms of second‐hand EC vapour inhalation, concerns that the products may be a gateway to smoking initiation or nicotine dependence among nicotine‐naïve users or may prolong continued dual use of tobacco amongst cigarette smokers, concerns that ECs may undermine smoke‐free legislation if used in smoke‐free spaces, concerns about the involvement of the tobacco industry, and concerns that the long‐term effects of EC use on health are not yet known are often cited (McNeill 2022). A report from the US Preventive Services Taskforce concluded "that the current evidence is insufficient to assess the balance of benefits and harms of electronic cigarettes (e‐cigarettes) for tobacco cessation in adults" (USPFTS 2021). However, others suggest that potential benefits outweigh potential disadvantages (Farsalinos 2014; Hajek 2014; McNeill 2021; McNeill 2022; NASEM 2018; RCP 2016).
People who smoke, healthcare providers and regulators are interested to know if ECs can help smokers quit and if it is safe to use them to do so. In particular, healthcare providers have an urgent need to know what they should recommend to people to help them to stop smoking. The largest health gains are achieved from stopping smoking completely, as opposed to reducing cigarette consumption and, as such, this review focuses on the effectiveness of ECs in aiding complete smoking cessation.
This review was first published in 2014, and updated in 2016, 2020, 2021 and 2022.
Following the publication of the 2020 update of this review, we are maintaining it as a living systematic review (Brooker 2019). This means we are continually running searches and incorporating new evidence into the review. For more information about the living systematic review methods being used, see Appendix 1. A living systematic review approach is appropriate for this review, for three reasons. First, the review addresses an important public health issue: the role of ECs in enabling people who smoke to stop smoking, with potential for substantial ongoing individual and societal benefits, if effective. Secondly, there remains uncertainty in the existing evidence; more studies are needed to confirm the degree of benefit for different comparisons and product types, and there is considerable uncertainty about adverse events and other markers of safety. Thirdly, we are aware of multiple ongoing trials on this topic that are likely to have an important impact on the conclusions of the review.
Objectives
To examine the safety, tolerability and effectiveness of using electronic cigarettes (ECs) to help people who smoke tobacco achieve long‐term smoking abstinence.
Methods
Criteria for considering studies for this review
Types of studies
We include randomized controlled trials (RCTs) and randomized cross‐over trials in which people who smoke are randomized to ECs or to a control condition. RCTs are the best available primary evidence, but the continued paucity of RCTs in this area requires that we also include uncontrolled intervention studies in which all participants are given an EC intervention.
We include studies regardless of their publication status or language of publication.
Types of participants
People defined as currently smoking cigarettes at enrolment into the studies. Participants could be motivated or unmotivated to quit.
Types of interventions
Any type of EC or intervention intended to promote EC use for smoking cessation, including studies which did not measure smoking cessation but provided ECs with the instruction they be used as a complete substitute for cigarette use. ECs may or may not contain nicotine.
Types of comparators
We compare nicotine ECs with non‐nicotine ECs, ECs versus alternative smoking cessation aids, including NRT or no intervention, and ECs added to standard smoking cessation treatment (behavioural or pharmacological or both) with standard treatment alone.
Types of outcome measures
Primary outcomes
Cessation at the longest follow‐up point, at least six months from the start of the intervention, measured on an intention‐to‐treat basis using the strictest definition of abstinence, preferring biochemically‐validated results where reported
Number of participants reporting adverse events or serious adverse events at one week or longer (as defined by study authors)
Secondary outcomes
Number of people still using study product (EC or pharmacotherapy) at longest follow‐up (at least six months). Product could be that provided by the study, or could be the same product type but bought independently by the participant.
Changes in the following measures at longest follow‐up (one week or longer):
Carbon monoxide (CO), as measured through breath or blood
Blood pressure
Heart rate
Blood oxygen saturation
Lung function measures
Known toxins/carcinogens, as measured through blood or urine (toxicant names and abbreviations are listed in Appendix 2)
Studies had to report one of the primary or secondary outcomes above to be eligible for inclusion.
Search methods for identification of studies
Electronic searches
Searches are conducted monthly. This update includes results from searches conducted up to 1st July 2022:
Cochrane Tobacco Addiction Group Specialized Register (CRS‐Web)
Cochrane Central Register of Controlled Trials (CENTRAL 2022; Issue 6) via CRS‐Web
MEDLINE (OVID SP; 1st January 2004 to 1st July 2022)
Embase (OVID SP; 1st January 2004 to 1st July 2022)
PsycINFO (OVID SP; 1st January 2004 to 1st July 2022)
ClinicalTrials.gov (via CENTRAL 2022; Issue 6)
WHO International Clinical Trials Registry Platform (ICTRP: www.who.int/ictrp/en/, via CENTRAL 2022; Issue 6)
At the time of the search, the Register included the results of searches of MEDLINE (via OVID) to update 20220614; Embase (via OVID) to week 202224; PsycINFO (via OVID) to update 20220613. See the Tobacco Addiction Group website for full search strategies and a list of other resources searched.
For the first version of the review, we also searched CINAHL (EBSCO Host) (2004 to July 2014). We did not search this database from 2016 onwards, as it did not contribute additional search results to the first version of the review. The search terms were broad and included 'e‐cig$' OR 'elect$ cigar$' OR 'electronic nicotine'. The search for the 2016 update added the terms 'vape' or 'vaper' or 'vapers' or 'vaping'. The 2020 searches added further terms, including the MESH heading 'Electronic Nicotine Delivery Systems' and terms to limit by study design. All current search strategies are listed in Appendix 3. The previously‐used search strategy is shown in Appendix 4. The search date parameters of the original searches were limited to 2004 to the present, due to the fact that ECs were not available before 2004.
Searching other resources
We searched the reference lists of eligible studies found in the literature search and contacted authors of known trials and other published EC studies. We also searched abstracts from the Society for Research on Nicotine and Tobacco (SRNT) Annual Meetings.
Data collection and analysis
Selection of studies
Two review authors (for this update from: ARB, JHB, NL, AT) independently prescreened all titles and abstracts obtained from the search, using a screening checklist, and then independently screened full‐text versions of the potentially relevant papers for inclusion. We resolved any disagreements by discussion or with a third review author.
Data extraction and management
Two reviewers (for this update from: ARB, AT, CN, PB) extracted data from the included studies using a pre‐piloted data extraction form, and checked them against each other. We resolved any disagreements by discussion or with a third review author. We extracted data on:
Author
Date and place of publication
Study dates
Study design
Inclusion and exclusion criteria
Setting
Summary of study participant characteristics
Summary of intervention and control conditions
Number of participants in each arm
Smoking cessation outcomes
Type of biochemical validation (if any)
Adverse events (AEs), serious adverse events (SAEs), number of people still using study product, and relevant biomarkers
Continued EC use or pharmaceutical intervention (PI) use at longest follow‐up
Assessment time points
Study funding source
Author declarations of interest
Risk of bias in the domains specified below
Additional comments
We adopted a broad focus to detect a variety of adverse events.
One review author (JHB) then entered the data into Review Manager 2020 software for analyses, and another checked them (NL for this update).
Assessment of risk of bias in included studies
Two review authors (for this update from: ARB, AT, CN, PB) independently assessed the risks of bias for each included study, using the Cochrane risk of bias tool v1 (Higgins 2011). This approach uses a domain‐based evaluation that addresses seven different areas: random sequence generation; allocation concealment; blinding of participants and providers; blinding of outcome assessment; incomplete outcome data; selective outcome reporting; and other potential sources of bias. We assigned a grade (low, high, or unclear) for risk of bias for each domain. We resolved disagreements by discussion or by consulting a third review author.
Specific considerations about judgements for individual domains in this review are outlined below:
Random sequence generation/allocation concealment: We rated all non‐randomized studies at high risk in these domains;
Blinding of participants and personnel: We did not evaluate this domain for non‐randomized studies, as we considered it not to be applicable. For randomized studies which did not use blinding, we considered studies to be at low risk in this domain if the intervention was compared to an active control of similar intensity, as we judged performance bias to be unlikely in this circumstance. If studies were unblinded and the comparator group was a minimal‐intervention control or of lower intensity than the intervention group, we considered the study to be at high risk of bias in this domain;
Following standard methods of the Cochrane Tobacco Addiction Review Group, we considered studies to be at low risk of detection bias (blinding of outcome assessment) if our primary outcome was objectively measured or if the intensity of the intervention was similar between groups, or both. For studies where cessation was measured, our judgement was based on whether cessation was biochemically verified. Where cessation was not measured, we judged this domain based on adverse or serious adverse events;
Again following standard methods of the Cochrane Tobacco Addiction Group, we rated studies at high risk of attrition bias if loss to follow‐up was greater than 50% overall or if there was a difference in follow‐up rates of more than 20% between study arms.
We judged studies to be at high risk of bias overall if they were rated at high risk in at least one domain, and at low risk of bias overall if they were judged to be at low risk across all domains evaluated. We judged the remaining studies to be at unclear risk of bias overall.
Measures of treatment effect
We analyzed dichotomous data by calculating the risk ratio (RR). For cessation, we calculated the RR as ((number of events in intervention condition/intervention denominator)/(number of events in control condition/control denominator)) with a 95% confidence interval (CI), using data at the longest follow‐up period reported.
We analyzed continuous data (other measures of tobacco exposure) by comparing the difference between the mean change from baseline to follow‐up in the intervention and comparator groups, or by comparing absolute data at follow‐up where insufficient data were available on mean change. For outcomes other than cessation where data were reported at multiple time points, we used data at the longest follow‐up point at which ECs were still being provided or their use was encouraged.
Unit of analysis issues
In the case of trials with multiple arms, we do not combine data between arms unless this is the way it has been presented by study authors, or there is no evidence of difference between similar trial arms for the outcome of interest. We note in our analyses where this is the case.
For all but one study, the unit of assignment was the individual. Dawkins 2020 assigned condition based on homeless support service; this was a small pilot study with very few events and hence we judged clustering to have very little impact on our overall result. If larger cluster‐randomized trials are eligible in the future, we will assess whether study authors have adjusted for this clustering, and whether this had an impact on the overall result. When clustering appears to have had little impact on the results, we will use unadjusted quit‐rate data; however when clustering does appear to have an impact on results, we will adjust for this using the intraclass correlation (ICC).
For randomized cross‐over trials, we report results at the end of the first assignment period where available and where sufficiently long to meet our inclusion criteria for outcomes. All other outcomes from randomized cross‐over trials are reported narratively. We offer a narrative synthesis of data from non‐randomized studies and outcomes from comparative trials which aren't reported in sufficient data for meta‐analysis, using effect direction plots as described in the Cochrane Handbook where possible (Higgins 2021).
Dealing with missing data
For smoking cessation, we used a conservative approach, as is standard for the Cochrane Tobacco Addiction Group, treating participants with missing data as still smoking. We based the proportion of people affected by adverse events on the number of people available for follow‐up, and not the number randomized. For other outcomes, we use complete‐case data and do not attempt to impute missing values.
Assessment of heterogeneity
We assessed the clinical and methodological diversity between studies to guide our decision whether data should be pooled. We were also guided by the degree of statistical heterogeneity, assessed by calculating the I2 statistic (Higgins 2003), and considering a value greater than 50% as evidence of substantial heterogeneity. We did not present pooled results where I2 values exceeded 75%.
Assessment of reporting biases
Reporting bias can be assessed using funnel plots, where 10 or more RCTs contribute to an outcome. However, there was only one analysis with sufficient studies to support this approach.
Data synthesis
We provide a narrative summary of the included studies. Where appropriate, we have pooled data from these studies in meta‐analyses. For dichotomous data, we used a fixed‐effect Mantel‐Haenszel model to calculate the RR with a 95% confidence interval, in accord with the standard methods of the Cochrane Tobacco Addiction Group for cessation studies.
For continuous outcomes, we pooled mean differences (or standardized mean differences for studies using different measures for the same construct), using the inverse variance approach (also with a 95% CI).
Subgroup analysis and investigation of heterogeneity
We had planned to undertake subgroup analyses to investigate differences between studies, such as:
Intensity of behavioural support used;
Type of EC (cartridge; refillable; pod);
Instructions for EC use (e.g. study provision, length of provision, whether participants had a role in product choice);
Type of participants (e.g. experience of EC use).
However, there were too few studies to conduct such analyses. Should further studies become available in future, we will follow this approach. For continuous outcomes, we subgroup data based on whether absolute values or change scores were available. For adverse events, we subgroup data by length of follow‐up for descriptive purposes.
In the absence of sufficient data for subgroup analyses on EC type, in the text we specify the type of nicotine EC when reporting pooled results for cessation.
Sensitivity analysis
We conducted sensitivity analyses to detect whether pooled results were sensitive to the removal of studies judged to be at high risk of bias.
Summary of findings and assessment of the certainty of the evidence
Following standard Cochrane methodology, we created summary of findings tables for our three main comparisons using GRADEpro GDT: nicotine EC versus non‐nicotine EC; nicotine EC versus NRT; and nicotine EC versus behavioural support only/no support. We selected these comparisons a priori as being the most clinically relevant. In the summary of findings tables, we present data on our primary outcomes (cessation, adverse events, serious adverse events) for these main comparisons. Also following standard Cochrane methodology, we used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the certainty of the body of evidence for each outcome, and to draw conclusions about the certainty of evidence within the text of the review.
Results
Description of studies
Results of the search
For this update, our bibliographic database searches identified 2534 non‐duplicate records (Figure 1). We screened all records and retrieved the full‐text papers of 220 potentially relevant articles. After screening and checking the full‐text of 220 papers, we included 88 records, representing 17 new studies for this update (Bonafont Reyes 2022; Caponnetto 2021; Edmiston 2022; Hajek 2022; Kerr 2020; Kimber 2021; Morphett 2022a; Morphett 2022b; Morris 2022; Myers‐Smith 2022; NCT03492463; Okuyemi 2022; Pratt 2022; Skelton 2022; Tattan‐Birch 2022; Vickerman 2022; White 2021), 41 new articles linked to studies already identified, and 30 new references to ongoing studies (see Characteristics of ongoing studies). Secondary study reports, commentaries, and correspondence relating to included studies are linked to studies in the reference section. Figure 2, Figure 3, Figure 4, Figure 5 and Figure 6 present PRISMA flow charts for previous versions of this review.
1.
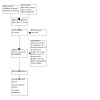
PRISMA diagram for 2022 update
2.
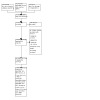
PRISMA diagram for 2021 update (Autumn update)
3.
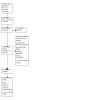
2021 update flow diagram (Spring update)
4.

2020 update flow diagram
5.

2016 update flow diagram
6.
2014 flow diagram
Included studies
In total, we include 78 studies, with 17 new included studies and 61 eligible included studies included in previous versions of the review. Key features of the included studies are summarized below. Further details on each included study can be found in the Characteristics of included studies tables.
Participants
The 78 included studies represented 22,052 participants. Thirty‐four studies were conducted in the USA, 16 were conducted in the UK, eight in Italy, five in Australia, four in Greece, two each in New Zealand and Canada, and one each in Belgium, Ireland, Poland, the Republic of Korea, South Africa, Switzerland, and Turkey. All studies were conducted in adults who smoke. Twenty‐two studies exclusively recruited participants who were not motivated to quit smoking, and 39 studies exclusively recruited participants motivated to quit; motivation was not specified for the other studies. Twenty‐nine studies were recruited from specific population groups; these included nine studies which recruited participants based on physical health condition (heart attack, cancer, HIV, periodontitis, awaiting surgery, smoking‐related chronic disease), five studies which recruited participants with serious mental illness, four studies which recruited participants in treatment or having recently completed treatment for alcohol or other drug use, and three studies in dual users of EC and conventional cigarettes. Two studies recruited people accessing homeless centres or using supported temporary accommodation. One study each recruited: people aged 55 or older, young adults, people who self‐identified as African‐American, pregnant women, and black and Latino participants.
Interventions and comparators
Three studies recruited dual users of combustible cigarettes and EC at baseline, and instructed them to continue using their own EC devices (Czoli 2019; Martinez 2021; Vickerman 2022); the remaining studies all provided some form of nicotine EC.
In two studies where nicotine ECs were provided on their own, nicotine levels were judged to be so low as to be clinically comparable to non‐nicotine EC (Lee 2019; Van Staden 2013); we include these studies in non‐nicotine EC comparisons. Ten studies compared nicotine EC with non‐nicotine EC, 22 studies compared nicotine EC to behavioural support only or to no support, and 17 studies compared nicotine EC to NRT. Five studies compared high‐ versus low‐nicotine EC devices (Caponnetto 2013a; Cobb 2021; Kimber 2021; Morris 2022; White 2021), three studies included comparisons based on flavours (Edmiston 2022; Morris 2022; White 2021), two studies directly compared device types (Kimber 2021; Yingst 2020), and two studies directly compared a freebase nicotine to a salt‐based nicotine device (Morris 2022; Russell 2021). Results from these studies are reported by comparison in Effects of interventions. Further details on the intervention and comparator groups (where applicable) for each study can be found in the Characteristics of included studies tables.
Where reported in the primary research publications, details of the devices tested can also be found in the Characteristics of included studies tables. Of the studies with sufficient data with which to judge, 30 used cartridge devices, 30 used refillable devices, four used both types, four used a pod device, and the remainder did not report device type.
Outcomes
Of the 78 included studies:
32 reported data on abstinence at six months or longer
55 reported data on adverse events
38 reported data on serious adverse events
46 reported data on carbon monoxide
11 reported data on heart rate
13 reported data on blood pressure
4 reported data on blood oxygen saturation
14 reported data on at least one known toxin/carcinogen
7 reported data on at least one measure of lung function
14 reported data on study product use at six months or longer
One study (Skelton 2022) measured safety outcomes but did not report them in the text available at time of writing (they may be forthcoming), hence this study currently does not contribute any data to this review.
Study types and funding
Forty studies were RCTs, 22 of which contributed to cessation analyses. Seven studies used randomized cross‐over designs, and the remainder were uncontrolled cohort studies. Of the 65 studies which reported funding information, 47 had no EC industry funding or support.
Excluded studies
We list 91 studies excluded at full‐text stage, along with reasons for exclusion, in the Characteristics of excluded studies table. The most common reason for exclusion was that studies were short‐term, following up participants for periods of less than one week.
Risk of bias in included studies
Overall, we judged ten studies (Bullen 2013; Cobb 2021; Eisenberg 2020; Hajek 2019; Hajek 2022; Kerr 2020; Lee 2018; Lee 2019; Martinez 2021; Myers‐Smith 2022) to be at low risk of bias, 18 to be at unclear risk, and the remaining 50 at high risk of bias (this includes the non‐randomized studies, which we deemed to be at high risk due to this lack of randomization).
Details of risk of bias judgements for each domain of each included study can be found in the Characteristics of included studies table. Figure 7 and Figure 8 illustrate judgements for each included study.
7.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
8.

Allocation
We judged 28 studies to be at high risk of selection bias; for the majority of cases this is because the study was not randomized. We rated a pilot cluster‐randomized trial to be at high risk as randomization was not carried out as intended for pragmatic reasons (Dawkins 2020). We judged 25 studies to be at low risk of selection bias, and the remainder to be at unclear risk as there was insufficient information with which to judge.
Blinding
Of the 40 studies assessed for these domains, we judged 28 to be at low risk for both performance and detection bias. We rated 19 to be at high risk for performance or detection bias, or both. In these studies, blinding was not used and different levels of support were provided; this alone or in conjunction with the outcome measures being used (subjective rather than objective measures) meant we thought there was a high risk of bias being introduced. We judged the rest to be at unclear risk, or ineligible for this domain due to single‐arm design.
Incomplete outcome data
We judged most studies (56 out of 78) to be at low risk of attrition bias. We rated nine studies with substantial loss to follow‐up at high risk of attrition bias. The remainder did not provide sufficient data on which to judge, and hence we judged them to be at unclear risk.
Selective reporting
Of the 78 studies, we considered that 40 were at low risk of reporting bias, as all prespecified or expected outcomes were reported. We rated eight as being at high risk, as data were not available as specified in the original protocols (note in some cases these are recent studies, and judgement on these may change as more publications emerge). We judged the rest to be at unclear risk, due to insufficient information with which to make a judgement.
Other potential sources of bias
We considered Ioakeimidis 2018 to be at high risk of other bias; data were from a conference poster and the associated abstract, and quit rates in the intervention arm differed between the two sources. Three further studies were considered to be at unclear risk in this domain.
Effects of interventions
See: Table 1; Table 2; Table 3
Data on our outcomes of interest are summarized below. Due to the volume of data available, some relevant information is hosted on a companion repository; these data are open‐access and can be found at https://doi.org/10.5287/bodleian:JbB1VNgDq. They are referred to below as supplemental tables. Forest plots are available through 'analysis' links; for some outcomes, benefit is plotted on the right, for others on the left. This is due to direction of effect, e.g. an increase in cessation is a benefit, whereas an increase in a carcinogen is not.
Direct comparisons between nicotine EC and other pharmacotherapies
Comparisons reported here include cartridge and refillable nicotine ECs versus NRT, and cartridge nicotine ECs versus varenicline. Only randomized controlled trials contributed data.
Cessation
Pooled data from six studies (2 cartridges, 3 refillable, 1 pod), five of which were rated at low risk of bias and the sixth as unclear, showed higher quit rates in people randomized to nicotine EC than to NRT (risk ratio (RR) 1.63, 95% confidence interval (CI) 1.30 to 2.04; I2 = 10%; 2378 participants; Analysis 1.1). One study included in this analysis, Hajek 2022, was conducted in pregnant women. There was no evidence of a subgroup difference between this study and studies in participants not selected on the basis of pregnancy (P = 0.90, I2 for subgroup differences = 0%). Follow‐up time was based on end of pregnancy, and our primary analysis included only those participants with follow‐up of at least six months. Results were not sensitive to including all participants followed‐up at end of pregnancy (RR 1.49, 95% CI 1.21 to 1.84, I2 = 0%; analysis not shown).
1.1. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 1: Smoking cessation
One study (Ioakeimidis 2018), available as a conference presentation only and considered at high risk of bias due to inconsistencies in the data reported and an unclear definition of abstinence, found lower quit rates in people allocated to nicotine EC (cartridge) compared to those allocated to varenicline (RR 0.31, 95% CI 0.11 to 0.82; 54 participants; Analysis 2.1).
2.1. Analysis.

Comparison 2: Nicotine EC versus varenicline, Outcome 1: Smoking cessation
Adverse events
Pooled data from four studies (all considered at low risk of bias) showed no evidence of a difference in the number of participants reporting adverse events (AEs) between nicotine EC and NRT arms (RR 1.02, 95% CI 0.88 to 1.19; I2 = 0%; 1702 participants; Analysis 1.2). Hajek 2019 and Bonafont Reyes 2022 did not contribute data to this analysis due to the way in which events were recorded. In Hajek 2019's prespecified adverse reactions of interest, nausea was more frequent in the NRT group, throat/mouth irritation was more frequent in the nicotine EC group, and there was little difference in other reactions (see Supplemental Table 1 for more detail). Bonafont Reyes 2022 recruited participants with COPD and reported "a trend towards decreased dyspnoea and COPD symptoms...in the EC arm compared to the NRT arm", but did not provide further detail.
1.2. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 2: Adverse events
In Ioakeimidis 2018, reports of sleep disorders were evenly distributed between groups, and nausea was more common in the varenicline arm than in the nicotine EC arm (see Supplemental Table 1 for more detail).
Serious adverse events
Five studies at low risk of bias comparing nicotine ECs with NRT provided data on SAEs. In some studies, no events occurred. Pooled results showed a small increased number of events in the nicotine EC arms, but with wide CIs incorporating no difference, as well as clinically significant harm and clinically significant benefit (RR 1.12, 95% CI 0.82 to 1.52; I2 = 34%; 2411 participants; Analysis 1.3). In Hajek 2022 (conducted in pregnant women), the authors also reported no evidence of a difference in birth outcomes overall. However, low birthweight (< 2500 g) was less frequent in the EC than NRT arm (14.8% vs. 9.6%; RR 0.65, 95% CI 0.47 to 0.90).
1.3. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 3: Serious adverse events
No SAEs occurred in Ioakeimidis 2018 (Analysis 2.2).
2.2. Analysis.

Comparison 2: Nicotine EC versus varenicline, Outcome 2: Serious adverse events
Carbon monoxide (CO)
Pooled data from three studies (Hatsukami 2020; Kerr 2020; Lee 2018; none considered at high risk of bias) comparing nicotine EC with NRT found that CO levels decreased more in those randomized to nicotine EC (MD −2.74 ppm, 95% CI −5.42 to ‐0.07; I2 = 3%; 191 participants; Analysis 1.4). A fourth, small study (Eisenhofer 2015; n = 11) was reported as a conference abstract and hence had limited data available. At three weeks, this study showed that both EC and NRT groups had "significantly reduced" CO, but between‐group differences were not reported.
1.4. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 4: Carbon monoxide (ppm)
Heart rate, blood pressure, and oxygen saturation
Pooled data from two studies (166 participants; one study judged to be at unclear risk of bias, one at low risk) showed no clear evidence of a clinically meaningful difference in heart rate (MD 0.53 bpm, 95% CI ‐1.76 to 2.83; I2 = 0%; Analysis 1.5), systolic blood pressure (MD ‐1.62, 95% CI ‐3.59 to 0.36; I2 = 0%; Analysis 1.6), or blood oxygen saturation (MD ‐0.14, 95% CI ‐0.59 to 0.30; I2 = 0%; Analysis 1.7), although confidence intervals were wide.
1.5. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 5: Heart rate (bpm)
1.6. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 6: Systolic blood pressure
1.7. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 7: Blood oxygen saturation
Toxicants
Only Hatsukami 2020 (unclear risk of bias, n = 111) contributed data for these outcomes. For PheT, CEMA, and AAMA (Analysis 1.12; Analysis 1.13; Analysis 1.14), point estimates favoured NRT but CIs included no difference. For 3‐HPMA, 2‐HPMA, and HMPMA, point estimates favoured EC but CIs included no difference (Analysis 1.8; Analysis 1.10; Analysis 1.11). There was no evidence of a difference for NNAL (nitrosamine 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐ butanol) but CIs were again wide (Analysis 1.9).
1.12. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 12: PheT (pmol/mg creatinine)
1.13. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 13: CEMA (pmol/mg creatinine)
1.14. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 14: AAMA (pmol/mg creatinine)
1.8. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 8: 3‐HPMA (pmol/mg creatinine)
1.10. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 10: 2‐HPMA (pmol/mg creatinine)
1.11. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 11: HMPMA (pmol/mg creatinine)
1.9. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 9: NNAL (pmol/mg creatinine))
Lung function
Lee 2018 and Kerr 2020 measured change in FEV1 (forced expiratory volume) and FEV1/FVC (forced vital capacity) (both low risk of bias; n = 81). High statistical heterogeneity (I2 = 89%) precluded pooling for FEV1 (Analysis 1.15); the point estimate for Lee 2018 favoured EC and for Kerr 2020 favoured NRT, but in both cases CIs also included no difference. There was no evidence of a difference for FEV1/FVC, but there was moderate unexplained statistical heterogeneity, and again CIs were wide (MD ‐0.16%, 95% CI ‐1.83 to ‐1.50; I2 = 51%; Analysis 1.16).
1.15. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 15: FEV1
1.16. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 16: FEV1/FVC (%)
Study product use
Five studies reported study product use at six months or longer, but statistical heterogeneity precluded pooling (I2 = 95%). Whereas Russell 2021 and Lee 2018 found no difference between EC and NRT arms, in the other three studies people in the EC arm were more likely to be continuing to use study product (EC) than those in the NRT arm (Analysis 1.18). A companion publication explored long‐term rates in more detail (Butler 2022).
1.18. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 18: Product use at 6+ months
Nicotine EC alone or versus control
Comparisons reported here include nicotine EC versus non‐nicotine EC, and nicotine EC compared to behavioural support only or to no support. In this section, we also reported results from studies in which all participants received nicotine EC (cohort studies and randomized studies which did not differ across arms in EC provision, device generation, or nicotine content).
Cessation
Randomized controlled trials
At six months or longer, quit rates were higher in nicotine EC groups than in comparator groups. Compared to EC without nicotine (placebo EC), pooled results showed nicotine EC produced higher quit rates (RR 1.94, 95% CI 1.21 to 3.13; I2 = 0%; 5 studies of cartridge devices, 1447 participants; Analysis 3.1). The effect size increased when we removed the one study at high risk of bias (Lucchiari 2020). The effect was more pronounced when comparing nicotine EC to behavioural support only or to no support (RR 2.66, 95% CI 1.52 to 4.65; I2 = 0%; 7 studies (4 refillable, 3 cartridge), 3126 participants; Analysis 4.1). As this involved unblinded comparisons with unequal levels of support, we judged all data contributing to this outcome to be at high risk of bias.
3.1. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 1: Smoking cessation
4.1. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 1: Smoking cessation
Pulvers 2020 (pod device) measured cessation at six months in the intervention group only, using self‐report. As they did not measure cessation at six months in the comparator group, we could not include these data in our meta‐analysis. At six months, 23 (24%) intervention participants were exclusively using EC and 10 (10.4%) reported using neither EC nor combustible cigarettes (making a combined quit rate of 34.4% in the intervention arm at six months).
Data from other studies
Nine studies provided all participants with nicotine EC and assessed abstinence at six months or longer (Table 4; 1 refillable, 6 cartridges, 1 pod, 1 not specified). The highest proportion of quitters was observed in Ely 2013 (cartridge), in which all participants (n = 48) used EC and 18 used additional pharmacotherapy: 44% of participants were abstinent at six months. The lowest quit rates were seen in two studies where participants were not motivated to quit at baseline: in Caponnetto 2013b, 14% of participants were abstinent at 12 months and, in Polosa 2011, 23% of participants were abstinent at six months, but this fell to 13% at 24 months (both studies used cartridge devices).
1. Summary of proportion of participants abstinent from smoking at 6+ months follow‐up: cohort studies of nicotine EC.
| Study | Motivated or unmotivated to quit smoking? | % abstinent | ||||
| Cohort studies | 6‐month | 12‐month | 18‐month | 24‐month | Notes | |
| Adriaens 2014a | Unmotivated to quit | 19.6% (10/51) | ‐ | ‐ | ‐ | Data from 8‐month follow‐up |
| Bell 2017 | "Willing to attempt to quit" | 26.6% (8/30) | ‐ | ‐ | ‐ | ‐ |
| Caponnetto 2013b | Unmotivated to quit | ‐ | 14% (2/14) | ‐ | ‐ | ‐ |
| Caponnetto 2021 | Unmotivated to quit | 35% (14/40) | ‐ | ‐ | ‐ | ‐ |
| Ely 2013b | Motivated to quit | 44% (21/48) | ‐ | ‐ | ‐ | ‐ |
| Pacifici 2015 | Unmotivated to quit | ‐ | 53% (18/34) | ‐ | ‐ | ‐ |
| Polosa 2011 | Unmotivated to quit | 23% (9/40) | ‐ | 15% (6/40) | 13% (5/40) | ‐ |
| Polosa 2014b | Unmotivated to quit | 36% (18/50) | ‐ | ‐ | ‐ | ‐ |
| Polosa 2015 | Not defined | 42% (30/71) | 41% (29/71) | ‐ | ‐ | ‐ |
aTechnically an RCT but observational for purposes of EC analysis bAll participants (N = 48) used an EC, but 16 also used bupropion and 2 used varenicline
Adverse events
Randomized controlled trials
Pooled data from five studies (none at high risk of bias) showed no evidence of a difference in the number of participants experiencing adverse events when comparing nicotine EC to non‐nicotine EC (RR 1.01, 95% CI 0.91 to 1.11; I2 = 0%; 840 participants; Analysis 3.2). When comparing nicotine EC to behavioural support only or to no support, more people in the groups randomized to nicotine EC reported experiencing adverse events (RR 1.22, 95% CI 1.12 to 1.32; I2 = 41%; 4 studies, 765 participants; Analysis 4.2). As this involved unblinded comparisons with unequal levels of support, we judged all data contributing to this outcome to be at high risk of bias.
3.2. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 2: Adverse events
4.2. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 2: Adverse events
A further ten randomized controlled trials provided adverse event or related data for this comparison, but could not be included in the meta‐analysis due to the way in which data were presented (see Supplemental Table 1). In the studies comparing nicotine EC to non‐nicotine EC, one found similar event rates across arms (Caponnetto 2013a), and two reported more events in the nicotine EC arms (Felicione 2019; Tseng 2016). In a further study comparing nicotine to non‐nicotine EC, events were reported by type, with an increase in some seen in the nicotine group and an increase in others seen in the non‐nicotine group (Lucchiari 2020). In the six studies comparing nicotine EC to behavioural support only or traditional cigarettes, Kumral 2016 found an increase in sinonasal symptoms in the group receiving nicotine EC compared to behavioural support only, and Ozga‐Hess 2019 found that throat irritation, cough, and dry mouth increased in the e‐cigarette group relative to the traditional cigarette group. By contrast, Pulvers 2020 found a reduction in respiratory symptoms in the e‐cigarettes compared to the traditional cigarettes group. Begh 2021 found an increase in throat irritation, palpitations and dizziness in the EC group, but decreases in cough, headache, nausea, dry mouth, shortness of breath, and stomach pain. Edmiston 2022 did not break down AEs by group but reported that three subjects experienced a non‐serious adverse event definitely related to study product. Pratt 2022 reported no statistically significant between‐group difference in AEs.
Data from other studies
Eighteen studies provided all participants with nicotine EC and assessed adverse events at one week or longer (see Supplemental Table 1). In the seven studies which tracked event rates over time, six showed adverse events reducing over time (Bell 2017; Caponnetto 2013b; Goniewicz 2017; Polosa 2011; Polosa 2014b; Pratt 2016). Hickling 2019 showed no change. The most commonly‐reported adverse events were throat/mouth irritation, headache, cough, and nausea.
Serious adverse events
Randomized controlled trials
Eight studies compared nicotine EC with non‐nicotine EC and reported data on SAEs; in four of these, no events occurred, so results could not contribute to the meta‐analysis, although they are included in the forest plots for descriptive purposes. In the four studies (three low risk of bias, one unclear) where events occurred, there was no evidence of a difference between groups, but CIs were wide (RR 1.00, 95% CI 0.56 to 1.79; 1272 participants; Analysis 3.3).
3.3. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 3: Serious adverse events
Nine studies compared nicotine EC with behavioural support only or no support and reported data on SAEs; in five of these, no events occurred. Pooled results from the four studies in which events occurred showed no clear evidence of a difference between arms, but CIs were wide (RR 1.03, 95% CI 0.54 to 1.97; I2 = 38%; 1993 participants; Analysis 4.3).
4.3. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 3: Serious adverse events
In a study in people experiencing homelessness (Dawkins 2020), SAEs were not reported, but authors reported that four to seven participants in the usual‐care arm and five to seven participants in the nicotine EC arm visited Accident & Emergency services at a hospital. The authors reported that these visits were unrelated to study treatment and were assessed to gather data for future economic evaluation. Further detail can be seen in Supplemental Table 2.
Data from other studies
Eight studies provided all participants with nicotine EC and reported SAEs at a week or longer (Supplemental Table 2.). In six of these (Bell 2017; Caponnetto 2013b; Caponnetto 2021; Humair 2014; Polosa 2011; Valentine 2018), authors reported that no SAEs occurred. In NCT02648178 (19 participants), one death occurred (no further detail provided). Hickling 2019 (50 participants) recruited participants from mental health settings; five SAEs were recorded during the study, all of which were psychiatric hospitalizations. None were considered related to study treatment.
Carbon monoxide
Randomized controlled trials
High statistical heterogeneity (I2 = 81%) precluded pooling CO data from the five trials (n = 511, none considered at high risk of bias) comparing nicotine EC with non‐nicotine EC (Analysis 3.4). Point estimates from three studies favoured nicotine EC and from two favoured non‐nicotine EC, but in all cases CIs were consistent with no clinically meaningful difference. Three further randomized studies measured CO levels in those assigned to nicotine EC and those assigned to non‐nicotine EC, but did not present data in a way that could be pooled: George 2019 did not compare data by group; Tseng 2016 reported no between‐group differences; and Meier 2017 found a slightly higher CO reading in those using nicotine EC, but the clinical and statistical significance of this difference was not clear (see Supplemental Table 3 for more detail). These data were from all study participants based on group randomized, not on subsequent EC or cigarette use.
3.4. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 4: Carbon monoxide (ppm)
Pooled data from 11 studies comparing nicotine EC to behavioural support alone or to no support resulted in a high I2 value (89%); thus, pooled results were not presented here (see Analysis 4.4 for individual study data). A funnel plot did not show asymmetry (Figure 9). Heterogeneity was primarily driven by magnitude rather than direction of effect, with results in 10 of 11 studies favouring nicotine EC. Three further trials reported data which could not be included in a meta‐analysis. Walele 2018 compared nicotine EC to cigarettes and found CO levels declined in the EC group and remained similar to baseline in the cigarette group. Czoli 2019 instructed baseline dual users to spend periods only using EC or only using traditional cigarettes; CO measured during sole EC use was lower than baseline and lower than during cigarette‐only periods. Further detail can be seen in Supplemental Table 3.
4.4. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 4: Carbon monoxide (ppm)
9.

Funnel plot for comparison 4.4
Data from other studies
Nineteen studies provided all participants with nicotine EC and reported data on CO at one week or longer. In the 18 studies that presented change over time, CO declined from baseline although, in Ikonomidis 2018, CO levels were equivalent to baseline again at 24 weeks and, in Polosa 2014b, a decline was observed in people who quit smoking or reduced cigarette consumption by at least half, but not in those who continued smoking at least half as many cigarettes as they had from baseline.
Heart rate
Randomized controlled trials
One RCT (Caponnetto 2013a, unclear risk of bias, n = 141) provided data on heart rate and compared nicotine EC with non‐nicotine EC; there was no evidence of a clinically significant between‐group difference (Analysis 3.5). This was comparable with findings from the one RCT (Hatsukami 2020, unclear risk of bias, n = 90) comparing nicotine EC with no pharmacotherapy, which also found no evidence of a clinically significant difference (Analysis 4.5).
3.5. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 5: Heart rate
4.5. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 5: Heart rate (bpm)
A further three RCTs provided data on heart rate which could not be used to calculate effect estimates. George 2019 compared nicotine to non‐nicotine EC and found no difference in heart rate between arms; Walele 2018 compared a nicotine EC with a traditional cigarette and reported "no clinically significant changes", and Cobb 2021 found decreases in both the EC and QuitSmart cigarette substitute groups, with the decrease being slightly greater in the latter group. See Supplemental Table 4 for further information.
Data from other studies
Six studies in which all participants received a nicotine EC also reported data on heart rate; for five, changes were minimal and directions of effect were mixed, and for Caponnetto 2021 (n = 40) the rate reduced by 9 bpm at 12 weeks (see Supplemental Table 4).
Blood pressure
Caponnetto 2013a found no evidence of a difference in the change in systolic blood pressure (BP) between nicotine EC and non‐nicotine EC arms (unclear risk of bias, 141 participants; Analysis 3.6). Three studies (2 at high risk of bias, 1 at unclear risk of bias) compared nicotine EC to behavioural support only and reported data on systolic blood pressure; there was a small difference favouring the EC arms (MD ‐2.3, 95% CI ‐3.9 to ‐0.7, I2 = 24%; 298 participants; Analysis 4.6). Three further RCTs measured change in blood pressure but presented results in such a way that they could not be pooled. George 2019 compared nicotine EC and non‐nicotine EC and combined data from both groups; BP declined over time. Compared to a QuitSmart cigarette substitute, Cobb 2021 found EC led to a greater reduction in BP. Walele 2018 found "no clinically significant changes" when comparing nicotine EC to a conventional cigarette at two weeks.
3.6. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 6: Systolic blood pressure
4.6. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 6: Systolic blood pressure
Five studies which provided nicotine EC to all participants reported change in blood pressure; results were clinically insignificant except for Caponnetto 2021 in which systolic BP reduced by 12 (from 134 to 122) at 12 weeks (see Supplemental Table 5 for further detail on all studies reporting this outcome).
Oxygen saturation
Hatsukami 2020 found no evidence of a difference in blood oxygen saturation when comparing nicotine EC to cigarettes (90 participants, Analysis 4.7). Van Staden 2013, a short‐term pre‐post study which measured outcomes after two weeks of EC use, found that people who smoked and switched to ECs had significant improvement in blood oxygen saturation (96.2% (SD 1.8) to 97.5% (SD 1.3); 1.3% increase, 95% CI 0.6 to 2.1; P = 0.002).
4.7. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 7: Blood oxygen saturation
Toxicants
Unless stated otherwise, all randomized controlled trials measuring these outcomes compared nicotine EC with no pharmacotherapy.
Two trials measured change in 3‐HPMA (one at high risk of bias). In both, the point estimate favoured the EC arm, but pooling was precluded due to difference in measurement methods (Analysis 4.8). Five further studies, in which all participants were given nicotine EC, measured 3‐HPMA; all found reductions over time (Supplemental Table 6).
4.8. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 8: 3‐HPMA (SMD)
Five trials measured change in NNAL (four at high risk of bias; Analysis 4.9). Three of the five studies found results favouring nicotine EC, but the final two indicated no difference; statistical heterogeneity was high (I2 = 96%), so pooled results were not presented. Pulvers 2018 and Morris 2022, which provided all participants with nicotine EC, found a reduction in NNAL over time and Czoli 2019, which was a cross‐over trial, found NNAL decreased when using nicotine EC compared to using traditional cigarettes (Supplemental Table 6). An additional two RCTs (one unclear and one low risk of bias) compared nicotine EC versus non‐nicotine EC and found no evidence of difference, with wide CIs and moderate statistical heterogeneity (‐0.02 pmol/mg creatinine, 95% CI ‐0.45 to 0.41; I2 = 54%; 363 participants; Analysis 3.10).
4.9. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 9: NNAL (SMD)
3.10. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 10: NNAL (pmol/mg creatinine)
One trial (n = 90, unclear risk of bias) found non‐statistically significant lower levels of 2‐HPMA, HMPMA, PhET and AAMA in nicotine EC arms compared to control (Analysis 4.10; Analysis 4.11; Analysis 4.12; Analysis 4.14). A further two studies in which all participants received nicotine EC found reductions in 2‐HPMA and AAMA measures over time (Supplemental Table 6). No difference was found in the one trial (n = 90, unclear risk of bias) evaluating CEMA (Analysis 4.13).
4.10. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 10: 2‐HPMA (pmol/mg creatinine)
4.11. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 11: HMPMA (pmol/mg creatinine)
4.12. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 12: PheT (pmol/mg creatinine)
4.14. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 14: AAMA (pmol/mg creatinine)
4.13. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 13: CEMA (pmol/mg creatinine)
One trial (n = 90, unclear risk of bias) found reductions in S‐PMA compared to control (Analysis 4.15); this was consistent with the two studies in which all participants received nicotine EC that measured S‐PMA, where levels declined over time (Supplemental Table 6).
4.15. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 15: S‐PMA (nanograms)
Of the 30 remaining measurements in single studies where all participants received a nicotine EC, 25 reduced over time and five increased (Supplemental Table 6).
Lung function
Caponnetto 2013a measured a number of lung function parameters. FeNO increased more in the nicotine EC than the non‐nicotine EC group (MD 2.35, 95% CI 1.78 to 2.92; 90 participants; Analysis 3.7). No difference was found between nicotine and non‐nicotine EC for FEV1 or FEV1/FVC (Analysis 3.8; Analysis 3.9).
3.7. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 7: FeNO (ppb)
3.8. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 8: FEV1 (l)
3.9. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 9: FEV1/FVC
Compared to behavioural support only/no support, pooled results from two studies (both high risk of bias) found improvements in FEV1 but with moderate statistical heterogeneity and CIs including no difference (SMD 0.15, 95% CI ‐0.01 to 0.31, I2 = 50%; 714 participants; Analysis 4.16). Data from one study at high risk of bias showed no difference in PEF (peak expiratory flow) 25‐75 (101 participants; Analysis 4.18). Pooled data from two studies (both high risk of bias) showed no difference in FEF (forced expiratory flow) 25‐75, with substantial levels of statistical heterogeneity (MD −0.06, 95% CI −0.18 to 0.06, I2 = 73%; 2 studies, 555 participants; Analysis 4.17). The one study (115 participants, high risk of bias) reporting FEV1/FVC favoured nicotine EC (Analysis 4.19).
4.16. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 16: FEV1 (SMD)
4.18. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 18: PEF 25‐75 (litres/minute)
4.17. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 17: FEF 25‐75 (litres/second))
4.19. Analysis.

Comparison 4: Nicotine EC versus behavioural support only/no support, Outcome 19: FEV1/FVC
Cobb 2021, which randomized participants to EC or the QuitSmart cigarette substitute, measured change in a number of lung function parameters: direction of effect was mixed across these, with no statistically or clinically significant between‐group differences at 12 weeks (Supplemental Table 7).
Two studies which provided all participants with nicotine EC measured change in lung function over time: Hickling 2019 found an increase in peak flow, and Oncken 2015 "no significant differences" in airway function (Supplemental Table 7).
Study product use
Three trials (all low risk), comparing nicotine EC with non‐nicotine EC, reported the number of participants still using EC at six months or longer. Slightly more participants were still using EC in the nicotine EC arms, but CIs were wide and included no difference (RR 1.15, 95% CI 0.94 to 1.41, I2 = 30%; 874 participants; Analysis 3.11). Data on this outcome from single‐arm studies can be found in a companion publication (Butler 2022).
3.11. Analysis.

Comparison 3: Nicotine EC versus non‐nicotine EC, Outcome 11: Product use at 6+ months
Direct comparisons between nicotine EC
Note, studies reported in this section are only those where participants were randomized to different nicotine EC conditions.
Comparisons based on nicotine dose
Six trials provided data comparing different doses of nicotine in EC (although other studies provided a range of doses, these were not randomly assigned). Only one study provided data on abstinence; in Cobb 2021 (low risk of bias), quit rates were higher in the higher‐dose arm but the 95% CI included no difference (RR 2.50, 95% CI 0.80 to 7.77, 260 participants, Analysis 5.1).
5.1. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 1: Smoking cessation
The three studies that provided data on adverse events and contributed to this comparison provided them in such a way that the studies could not be pooled. Kimber 2021 reported "no changes over time or differences between condition", and Pratt 2022 and Morris 2022 did not compare AEs by nicotine strength (see Supplemental Table 1).
In Caponnetto 2013a, no serious adverse events were reported in either arm; in Cobb 2021, there were more events in the higher‐dose arm but CIs were wide (RR 1.51, 95% CI 0.51 to 4.42, 239 participants, Analysis 5.2). In Morris 2022, no serious adverse events occurred (Supplemental Table 2).
5.2. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 2: Serious adverse events
Point estimates favoured EC and CIs excluded no difference for carbon monoxide and FEV1/FVC (Analysis 5.3; Analysis 5.9). There were no clinical or statistically significant differences between arms for heart rate, blood pressure, other lung function measures, or NNAL (Analysis 5.4; Analysis 5.5; Analysis 5.6; Analysis 5.7; Analysis 5.8; Analysis 5.10). More participants in the higher‐dose nicotine group were still using EC at six months or longer, but data were from one study and CIs were wide and included no difference (Analysis 5.11). In Yingst 2020 (cross‐over, comparing different doses and different devices), exhaled CO and reported nausea did not differ between devices; self‐reported dizziness was low overall but slightly higher in the higher‐dose arm. Further detail can be found in Supplemental Table 1 and Supplemental Table 3. Morris 2022 measured a range of toxicants but did not compare these based on nicotine level assignments (Supplemental Table 6).
5.3. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 3: Carbon monoxide (ppm)
5.9. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 9: FEV1/FVC
5.4. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 4: Heart rate
5.5. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 5: Systolic blood pressure
5.6. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 6: FeNO (ppb)
5.7. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 7: FEV1 (l)
5.8. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 8: FVC (l)
5.10. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 10: NNAL (pg/mg creatinine) at 24 weeks
5.11. Analysis.

Comparison 5: Higher versus lower nicotine content, Outcome 11: Product use at 6+ months
One further study, White 2021, also included comparisons based on nicotine levels (1.8% free‐base nicotine, designated by the authors as 'moderate', and 0.3% free‐base nicotine, designated by the authors as 'low'). This was a factorial trial (unpublished at the time of writing) which, in addition to e‐liquid nicotine content, also manipulated cigarette nicotine content and e‐liquid flavour availability. The authors reported no significant main effects for nicotine content on CO or CEMA, and no statistically significant interactions for these conditions. There also appear to have been no differences in proportions of people experiencing adverse events, but the study terminated early and was likely underpowered to detect differences.
Comparisons based on flavour
One study (Edmiston 2022, n = 300, high risk of bias) randomized participants to different flavours (tobacco versus menthol) and provided data in a way that could have been used to compute risk ratios, although no SAEs occurred in either arm (Analysis 6.1). NNAL and FEV1/FVC were lower in the tobacco flavour group but CIs were wide and included no difference (Analysis 6.2; Analysis 6.4). There was no evidence of a difference in FEV1 (Analysis 6.3). No other outcomes from this paper were eligible for inclusion in our review.
6.1. Analysis.

Comparison 6: Tobacco vs. menthol flavour, Outcome 1: Serious adverse events
6.2. Analysis.

Comparison 6: Tobacco vs. menthol flavour, Outcome 2: NNAL (ng/g)
6.4. Analysis.

Comparison 6: Tobacco vs. menthol flavour, Outcome 4: FEV1/FVC
6.3. Analysis.

Comparison 6: Tobacco vs. menthol flavour, Outcome 3: FEV1 (% predicted)
Morris 2022, a randomized cross‐over trial, tested the effect of 10 different flavours (as well as nicotine strengths and salt versus free‐base nicotine). Only their data on AE and SAE were eligible for inclusion in our review, but analyses were not reported by flavour (Supplemental Table 1; Supplemental Table 2).
White 2021 also contributed data to this comparison, with conditions being tobacco flavours only, or tobacco, fruit, dessert and mint flavours. No significant main effects or interactions were found for flavours on the outcomes relevant to this review, namely CO and CEMA, and no difference was discernable in occurrence of AEs. However, as noted above, the study terminated early and hence was underpowered to detect differences.
More information on flavour choices across the studies in this review can be found in a companion publication (Lindson 2022b).
Comparisons based on device type
Kimber 2021 (n = 50, high risk of bias) is the only study to directly compare device types (cartridge versus refillable). Outcomes eligible for this review were CO and AE. There was no difference between arms for CO, but CI were wide (Analysis 7.1). The authors reported "no changes over time or differences between condition" for AEs (see Supplemental Table 1).
7.1. Analysis.

Comparison 7: Refillable versus cartridge, Outcome 1: Exhaled CO
Nicotine salt versus free‐base nicotine
One study (Russell 2021, unclear risk of bias) contributed data to this comparison. Quit rates and study product use were both similar between arms (RR 1.25, 95% CI 0.85 to 1.83, n = 285; Analysis 8.1; and RR 1.07, 95% CI 0.82 to 1.41, n = 227; Analysis 8.2, respectively).
8.1. Analysis.

Comparison 8: Nicotine salt EC versus free‐base nicotine EC, Outcome 1: Smoking cessation
8.2. Analysis.

Comparison 8: Nicotine salt EC versus free‐base nicotine EC, Outcome 2: Product use at 6+ months
As described above, Morris 2022 also tested salt versus free‐base nicotine, but did not provide data broken down by these characteristics for our outcomes of interest (Supplemental Table 1, Supplemental Table 2).
Non‐nicotine EC
Although non‐nicotine ECs serve as a 'control group' in our primary analysis, due to their behavioural properties, they can also be considered an intervention in and of themselves. Comparisons included here are: non‐nicotine EC versus NRT; non‐nicotine EC versus usual care; and non‐nicotine EC as an adjunct to NRT. All contributing data were from randomized controlled trials. None of these studies reported data on change in CO, heart rate, blood pressure, oxygen saturation, toxicants, or lung function.
Cessation
When comparing non‐nicotine EC to behavioural support only, pooled results from two studies (n = 388) found higher quit rates in participants randomized to non‐nicotine EC, but the confidence interval included the possibility of no difference (RR 1.74, 95% CI 0.76 to 3.96; I2 = 0%; Analysis 9.1). When evaluating non‐nicotine EC as an adjunct to NRT, Walker 2020 also found higher quit rates in participants randomized to non‐nicotine EC, although again the confidence interval included no difference (Analysis 10.1).
9.1. Analysis.

Comparison 9: Non‐nicotine EC versus behavioural support only/no support, Outcome 1: Smoking cessation
10.1. Analysis.

Comparison 10: Non‐nicotine EC + NRT versus NRT, Outcome 1: Smoking cessation
Lee 2019 compared non‐nicotine EC with NRT; the point estimate favoured NRT, but the confidence interval included no difference (Analysis 11.1).
11.1. Analysis.

Comparison 11: Non‐nicotine EC versus NRT, Outcome 1: Smoking cessation
Adverse events
Eisenberg 2020 found a higher rate of adverse events in the non‐nicotine EC arm than in behavioural support only, with the confidence interval excluding no difference (Analysis 9.2). By contrast, Walker 2020 found fewer adverse events in participants receiving non‐nicotine EC + NRT compared to NRT alone, with the confidence interval excluding no difference (Analysis 10.2). Lee 2019 also found that fewer participants receiving non‐nicotine EC reported adverse events than those receiving NRT, with the confidence interval excluding no difference (Analysis 11.2).
9.2. Analysis.

Comparison 9: Non‐nicotine EC versus behavioural support only/no support, Outcome 2: Adverse events at 12 weeks
10.2. Analysis.

Comparison 10: Non‐nicotine EC + NRT versus NRT, Outcome 2: Adverse events
11.2. Analysis.

Comparison 11: Non‐nicotine EC versus NRT, Outcome 2: Adverse events
Serious adverse events
Eisenberg 2020 found a higher rate of SAEs in the non‐nicotine EC arm than in the behavioural support‐only arm, but confidence intervals were wide and incorporated clinically significant benefit and clinically significant harm (Analysis 9.3). In Walker 2020, more SAEs occurred in the group randomized to non‐nicotine EC + NRT than in the NRT‐alone group, but the confidence interval included no difference as well as the potential for a clinically significant difference in favour of the intervention (Analysis 10.3). No SAEs were reported in either arm of Lee 2019 (non‐nicotine EC versus NRT).
9.3. Analysis.

Comparison 9: Non‐nicotine EC versus behavioural support only/no support, Outcome 3: Serious adverse events at 24 weeks
10.3. Analysis.

Comparison 10: Non‐nicotine EC + NRT versus NRT, Outcome 3: Serious adverse events
Advice to use own EC to quit
Three studies did not provide EC but instead provided dual users with advice on how to use their EC to stop smoking. Czoli 2019 and Vickerman 2022 were short‐term studies and contributed data to supplementary tables only. In Martinez 2021, people receiving tailored self‐help material with information on how to use EC to quit smoking had marginally higher long‐term quit rates than those receiving self‐help material without EC advice (RR 1.04, 95% CI 0.89 to 1.22; 2321 participants; Analysis 12.1). The RR was higher and CIs excluded one when compared to an assessment‐only control group. At six months, 64% in the targeted booklet arm, 66% in the generic booklet arm, and 68% in the assessment‐only arm were still using EC.
12.1. Analysis.

Comparison 12: Advice to use e‐cigarettes compared to no advice to use e‐cigarettes, Outcome 1: Smoking cessation
Combination therapy
Nicotine EC and NRT
This section covers two comparisons: studies in which all arms received NRT and participants were randomized to nicotine EC or non‐nicotine EC, and studies in which all participants received NRT and one arm was randomized to nicotine EC in addition. All studies contributing data were randomized controlled trials. No studies in this group reported data on heart rate, blood pressure, oxygen, or toxicants.
Cessation
Two trials (both at high risk of bias, both testing refillable devices) in which all participants received NRT compared nicotine EC to non‐nicotine EC; pooled results favoured nicotine EC, with the CI excluding no difference (RR 1.77, 95% CI 1.07 to 2.94; I2 = 0%; 1039 participants; Analysis 13.1).
13.1. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 1: Smoking cessation
Three studies (two high risk of bias, one unclear risk; two refillable, one cartridge) also compared nicotine EC + NRT to NRT alone. Pooling results from all three studies resulted in high statistical heterogeneity precluding meta‐analysis (I2 = 83%). This heterogeneity was driven by the study of a cartridge device (Morphett 2022a, RR 1.00, 95% CI 0.64 to 1.55, 1712 participants); historically cartridge devices have had poorer nicotine delivery than refillables. Once this study was removed, heterogeneity disappeared (I2 = 0%), but only two studies remained. In these two studies, pooled results showed more people quit in the refillable nicotine EC + NRT arm than in the NRT alone arm (RR 3.53, 95% CI 1.93 to 6.44; 908 participants; Analysis 14.1). In two of these studies, participants in both groups received nicotine patches but, in Morphett 2022b, participants in the NRT only arm also received a short‐acting form of NRT.
14.1. Analysis.

Comparison 14: Nicotine EC + NRT versus NRT, Outcome 1: Smoking cessation
Adverse events
Three trials in which nicotine ECs were compared to non‐nicotine ECs reported data on AEs. Baldassarri 2018 reported results combined across groups but noted "no significant differences by treatment group" (Supplemental Table 1). Pooled data from the other two studies also showed no clear evidence of difference (RR 1.11, 95% CI 0.93 to 1.32, I2 = 0%; 677 participants; Analysis 13.2).
13.2. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 2: Adverse events
The three trials comparing nicotine EC + NRT to NRT alone that contributed data to this outcome were all at high risk of bias. Pooled results showed no evidence of a difference in AEs between arms, but with moderate statistical heterogeneity (RR 0.96, 95% CI 0.83 to 1.11, I2 = 64%; 1984 participants; Analysis 14.2).
14.2. Analysis.

Comparison 14: Nicotine EC + NRT versus NRT, Outcome 2: Adverse events
Serious adverse events
Pooled data from two studies (one high risk, one unclear) comparing nicotine EC with non‐nicotine EC as adjuncts to NRT showed fewer SAEs in the nicotine EC group than in the non‐nicotine EC group, but the CI included no difference (RR 0.66, 95% CI 0.38 to 1.14, I2 = 0%; 1069 participants; Analysis 13.3).
13.3. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 3: Serious adverse events
Four studies (all high risk of bias) provided data on SAEs and compared nicotine EC + NRT to NRT alone. The pooled estimate favoured the NRT‐alone group, but the CI was wide and included no difference (RR 1.26, 95% CI 0.46 to 3.42: I2 = 0; 2245 participants; Analysis 14.3).
14.3. Analysis.

Comparison 14: Nicotine EC + NRT versus NRT, Outcome 3: Serious adverse events
Carbon monoxide
Walker 2020 (which compared nicotine EC + NRT, non‐nicotine EC + NRT, and NRT alone) measured change in CO levels but did not report data in a way that could be pooled. CO declined over time, with the greatest reduction seen in the nicotine EC group (see Supplemental Table 3). Pooled data from two studies (one high risk of bias, one unclear) comparing nicotine and non‐nicotine EC as adjuncts to NRT found a slightly greater reduction in CO in the nicotine EC group, but the CI included no clear evidence of a difference (MD ‐1.73 ppm, 95% CI ‐4.44 to 0.98, I2 = 0%; 70 participants; Analysis 13.4) between groups.
13.4. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 4: Carbon monoxide (ppm)
Lung function
Baldassarri 2018, which compared nicotine EC to non‐nicotine EC, in which both groups received NRT, found no between‐group differences in FeNO, FEV1, or FVC (Analysis 13.5; Analysis 13.6; Analysis 13.7); confidence intervals were wide for all outcomes.
13.5. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 5: FeNO (ppb)
13.6. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 6: FEV1 (%)
13.7. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 7: FVC (%)
Study product use
In Walker 2020, at six months, 40% of the patches‐only arm (n = 52) were still using patches, and in the patches + nicotine EC group (n = 317), 22% were using patches only, 45% were using EC only, and 11% were using both patch and EC. In the patches + non‐nicotine EC group (n = 308), 29% were still using patches, 36% were using EC only, and 13% were using both patches and EC. In Baldassarri 2018, there was no difference between arms in product use, but only nine participants contributed data (Analysis 13.8).
13.8. Analysis.

Comparison 13: Nicotine EC + NRT versus non‐nicotine EC + NRT, Outcome 8: Study product use at 6+ months
Nicotine EC and varenicline
One study, Tattan‐Birch 2022 (high risk of bias, 92 participants), evaluated nicotine EC and varenicline compared to varenicline alone. The study terminated early due to varenicline supply issues (an international recall), and the only data eligible for inclusion in this review related to AEs and SAEs. There was no evidence of a difference in AEs, though CIs were wide (Analysis 15.1), and no SAEs occurred (Analysis 15.2).
15.1. Analysis.

Comparison 15: Nicotine EC + varenicline vs. varenicline, Outcome 1: Adverse events at 12 weeks
15.2. Analysis.

Comparison 15: Nicotine EC + varenicline vs. varenicline, Outcome 2: Serious adverse events at 12 weeks
Discussion
Summary of main results
This update includes a further 17 studies published since the last version. Our three main comparisons, nicotine EC compared to NRT, nicotine EC compared to non‐nicotine EC, and nicotine EC compared to behavioural support only/no support still show increased quit rates in people assigned to nicotine EC arms; this is now high‐certainty for the comparison with NRT, moderate‐certainty for the comparison with non‐nicotine EC, and very low‐certainty for the comparison with behavioural support only/no support (Table 1; Table 2; Table 3). In absolute terms, pooled data suggest an additional two to six people for every 100 would quit smoking with nicotine EC compared to NRT, an additional two to sixteen people for every 100 would quit smoking with nicotine EC compared to non‐nicotine EC, and an additional one to four people for every 100 would quit smoking with nicotine EC compared to behavioural support only or no support for smoking cessation. Most data come from studies of cartridge and refillable devices.
There remains moderate certainty of no difference in rates of adverse events in nicotine EC compared to non‐nicotine EC, and there is now also moderate‐certainty evidence of no difference in rates of adverse events in nicotine EC compared to NRT. Evidence on adverse events (AEs) and serious adverse events (SAEs) was of low to very low certainty across all other comparisons, due to a paucity of data. Many of the studies which measured SAEs reported no such events in either study arm. For nicotine EC compared to non‐nicotine EC, pooled data suggest no difference in the number of people experiencing AEs or SAEs. Conversely, data from comparisons between nicotine EC and behavioural support alone or no support suggest an additional 14 people per 100 assigned to nicotine EC may experience AEs, but with no difference in SAEs; this evidence was of low and very low certainty, respectively. As with AEs from other smoking cessation treatments (e.g. NRT, Hartmann‐Boyce 2018a), AEs in these studies typically related to irritation at site (e.g. dry mouth, cough) and resolved over time. No studies in any of the different comparison conditions detected serious harms considered to be related to EC use. No authors explicitly identified SAEs as attributable to treatment, but few studies reported detail on this.
Beyond AEs and SAEs, we consider data on a range of safety‐ and health‐related outcomes, including carbon monoxide and other toxins, lung function, blood pressure, pulse, and oxygen levels. Data on all of these outcome measures were limited; for most outcomes within most comparisons, only one or two studies currently contribute data. A companion paper provides more data on the measured toxicants, analysing studies based on actual use of ECs and combustible cigarettes (Hartmann‐Boyce 2022). Consistent with findings from this review, the companion paper found that most measured toxicants were lower in people exclusively using EC than those exclusively smoking or those both smoking and using EC. Most measured toxicants were lower in people using both EC and smoking compared to smoking only.
In this update, we also have more data from studies testing nicotine EC as adjuncts to other stop‐smoking treatments. As with the previous update, pooled data from two studies in which all participants received NRT showed that nicotine EC led to higher quit rates than non‐nicotine EC, but we judged both studies to be at high risk of bias, meaning the effect remains uncertain. Three studies now compare nicotine EC + NRT to NRT alone. Pooling results from all three studies resulted in high statistical heterogeneity precluding meta‐analysis, but this heterogeneity was driven by the one study of a cartridge device. When restricting the analyses to refillable devices, heterogeneity disappeared (I2 = 0%), and results showed more people quit in the nicotine EC + NRT arm than in the NRT alone arm. These results should also be treated with caution as one of the two studies was judged to be at high risk of bias, but they do suggest that this is an area where further research is warranted. It is well‐established that combining short and long‐acting forms of NRT ('combined NRT') leads to greater success than single‐form NRT (Lindson 2019) but, of note, one of the studies showing a benefit of nicotine EC in this comparison compared nicotine EC + patch to short‐acting NRT + patch, suggesting it is not just the 'combined NRT' effect that is driving increased effectiveness.
We also included data on the proportion of participants still using study product (EC or pharmacotherapy) at six months or longer. We introduced this new outcome in our last update after feedback from readers and key stakeholders. There remains no clear evidence of a between‐group difference for this outcome, which is also now explored further in a companion publication (Butler 2022).
Overall completeness and applicability of evidence
This field of research and EC devices themselves continue to evolve rapidly. This is the third update conducted as part of our 'living systematic review' approach, with which we will proceed until at least the end of 2022, meaning we can continue to rapidly incorporate new evidence (see Appendix 1). This is important, as all but two of our analyses currently demonstrate imprecision.
This update incorporates data from 1 June 2021 to 1 July 2022. Subsequent monthly searches will keep this review current. Although studies predominantly came from the USA and UK, overall this review covers data from 14 countries. Geographical range in studies may be particularly important in this area, due to the marked differences in EC regulation between countries; for example, studies conducted in countries that limit nicotine dose in EC or allow only certain EC devices to be tested may observe less pronounced effects on quitting. This review includes studies in some under‐researched populations, including people not motivated to quit smoking, people with substance misuse disorders, people with serious mental health conditions, and people experiencing homelessness. Quit rates in these groups are traditionally lower, which may make it more difficult to detect effects of interventions. However, it could be that these groups may particularly stand to benefit from EC if they are effective because, in absolute terms, conventional cessation methods are often not as effective for them.
As well as the rapid pace of research in this field, evolutions in EC technology pose a challenge when considering the applicability of our evidence to the present. We had downgraded the certainty of our data in the 2016 update, as the devices tested in the trials were first‐generation 'cig‐a‐like' devices which did not deliver nicotine well, meaning the studies may have yielded more conservative estimates than would be seen with newer models, as newer devices and models have tended towards improved nicotine delivery. Nicotine delivery is also relevant to the comparator NRT arms tested; use of both a shorter‐ and a longer‐acting form of NRT show the highest success, and it is important that, where possible, this be the comparator chosen for such trials (Lindson 2019). We no longer downgrade the evidence on this basis as studies with newer device types are now included, although there will always be a time lag between current devices and the research evidence available. Within our primary comparisons, none of the analyses of our primary outcomes signified substantial levels of statistical heterogeneity, despite the fact that different devices were used in the included studies. However, this could be because confidence intervals were wide for individual studies, and does not rule out clinically significant differences in effects between EC types. As further data emerge, we hope to be able to formally test for differences in subgroup analyses, and in head‐to‐head comparisons of different device types. As of this 2022 update, we continue to have only one study of a pod device contributing to our cessation analysis (Russell 2021, abstract only). No studies tested newer disposable devices, which data show are growing in popularity (Tattan‐Birch 2022). There also continues to be little evidence on the impact of different devices, flavours, and nicotine delivery profiles when directly compared to one another. A companion paper explores available data on flavours in more detail (Lindson 2022b).
The adverse effects described in both the RCT and cohort studies continue to look similar, regardless of the brand of EC used or nicotine content, with placebo and nicotine‐containing ECs showing similar numbers and types of adverse events in direct comparisons. They also reflect what is reported in survey data (Dawkins 2013b; Etter 2011).
The structure of our analyses follows standard practice of the Cochrane Tobacco Addiction Group, i.e. evaluating outcomes on an intention‐to‐treat basis, meaning our pooled results represent the effect of offering an EC intervention. This is different from evaluating the per protocol effect, or the effect only in those who use the EC to quit smoking entirely, or continue to smoke whilst also using EC. Although pragmatic and hopefully of use to those designing and delivering interventions, we acknowledge that our intention‐to‐treat approach limits the ability to use the data presented here to draw conclusions about biomarkers in subgroups of participants based on subsequent EC use/smoking profiles. A new companion publication attempts to address this deficit (Hartmann‐Boyce 2022).
Certainty of the evidence
We consider the certainty of the evidence below as it relates to primary outcomes for our three main comparisons: nicotine EC versus NRT; nicotine EC versus non‐nicotine EC; nicotine EC versus behavioural support only/no support (Table 1; Table 2; Table 3). The certainty of evidence for all other comparisons and outcomes should be considered very low due to a paucity of data and issues with risk of bias.
Our summary of findings tables and assessments of certainty are based on the evidence from randomized controlled trials (RCTs). The cohort studies that we include are all deemed to have high risks of bias, which is inherent in the study design. Data presented from these studies need to be interpreted with caution. However, data from cohort studies were reassuringly consistent with data from RCTs.
Risk of bias did not impact on the certainty of evidence for comparisons between nicotine and non‐nicotine EC, or between nicotine EC and NRT. For the latter, we judged all three studies to be at low risk of bias overall. For the former, removing the one study at high risk of bias increased the effect estimate for our efficacy outcome. Risk of bias decreased our certainty in the effect estimates for our nicotine EC versus behavioural support only/no support comparison as, due to the nature of the comparison, blinding was not possible and different levels of support could lead to bias. All but two of our main comparisons were downgraded for imprecision, due to wide confidence intervals and few events. Other than risk of bias and imprecision, we identified no other issues which decreased the certainty of the primary outcomes for our main comparisons. Due to the small number of studies contributing to individual analyses, we were unable to formally test for publication bias and cannot rule this out.
Cessation
All three comparisons found effect estimates favouring nicotine EC for smoking cessation. For nicotine EC versus NRT, we now judge the evidence to be of high certainty, meaning we are now very confident that the true effect lies close to the estimate of the effect. For nicotine EC versus non‐nicotine EC, we continue to judge the evidence to be of moderate certainty, meaning we think the true effect is likely to be close to the estimate of effect. For nicotine EC versus behavioural support only/no support, we judged the evidence to be of very low certainty, meaning we have very little confidence in the effect estimate. Another way to look at this, however, is to consider that nicotine EC versus non‐nicotine EC comparisons isolate the effect of nicotine as provided by an EC, and nicotine EC versus NRT comparisons isolate the effect of the sensorimotor elements provided by an EC. Given that both of these comparisons find a benefit of nicotine EC for smoking cessation, it might logically follow that the comparison between nicotine EC and behavioural support only/no support would find a benefit in favour of nicotine EC, since this comparison would capture both pharmacological and sensorimotor mechanisms of effect. This increases our confidence in the effect of nicotine EC when compared to behavioural support alone or to no support. Nicotine replacement therapy has also been shown to be more effective than behavioural support alone, further supporting the likelihood that nicotine EC would be more effective than behavioural support alone (Hartmann‐Boyce 2018a).
Adverse and serious adverse events
We now have moderate‐certainty evidence of no difference in adverse events for nicotine EC compared to NRT as well as to non‐nicotine EC. For all other outcomes in this category, evidence is of low or very low certainty. Imprecision remains a key issue for these outcomes, and particularly for SAEs. None of the analyses signalled serious harm, nor did complementary data from cohort studies but, unlike our cessation analyses, many of the confidence intervals encompassed the possibility of both clinically significant harm and clinically significant benefit. This uncertainty should reduce as more studies become available.
Potential biases in the review process
We consider the review process we used to be robust. For outcome assessment, we followed the standard methods used for Cochrane Tobacco Addiction Review Group cessation reviews. Our search strategy included the Cochrane Tobacco Addiction Group Specialized Register and we were able to capture a number of ongoing studies. However, there may be unpublished data that our searches did not uncover. We also considered participants lost to follow‐up as continuing to smoke, which is standard practice in this field. There are concerns that frequently updating meta‐analyses can lead to issues with multiple testing; we followed Cochrane guidance in conducting this living systematic review and hence do not adjust for multiple testing (Brooker 2019).
Four of our review authors are authors of the included studies. These authors were not involved in the decisions about inclusion of their studies, or in risk of bias assessment for these studies.
Agreements and disagreements with other studies or reviews
This Cochrane Review aligns with but updates the conclusions of the 2018 U.S. National Academies of Science, Engineering, and Medicine Consensus Study Report, Public Health Consequences of E‐cigarettes (NASEM 2018), which reviewed literature published through August 2017 to address the question, “Do e‐cigarettes help smokers quit smoking combustible tobacco cigarettes?”. Focusing on RCTs and existing systematic reviews, it used a prespecified Level of Evidence framework to develop conclusions. The report’s overall conclusion was that there was “limited evidence that e‐cigarettes may be effective aids to promote smoking cessation.” Based on the RCTs available, it concluded that there was “moderate evidence” that e‐cigarettes containing nicotine were more effective for cessation than e‐cigarettes without nicotine, but “insufficient evidence” about the effectiveness of e‐cigarettes compared to no treatment or to FDA‐approved smoking cessation treatments. Our review contradicts this latter point, as we now find high‐certainty evidence of benefit when comparing nicotine EC with NRT; this is due to the inclusion of studies published after NASEM 2018. Reviews from the Office for Health Improvements and Disparities (formerly Public Health England) conclude that, compared to their 2018 review, there is now stronger evidence that nicotine vaping products are effective for smoking cessation (McNeill 2021, McNeill 2022).
Findings are also broadly consistent with those from other recent reviews, with some exceptions. Amato 2020 did not evaluate effectiveness and focused only on safety; consistent with our review, they found very low‐ to moderate‐certainty evidence on a range of possible adverse effects, with the most frequently reported being cough, dry mouth, shortness of breath, irritation of the mouth and throat, and headache. Consistent with our review, the studies reviewed by McNeill 2022 showed that, compared to combustible cigarettes, using ECs led to a substantial reduction in biomarkers of toxicant exposure associated with cigarette smoking; Wilson 2021 also agrees with this finding. Akiyama 2021 reviewed biomarker findings from clinical studies and also concluded that the use of EC could lead to a significant reduction in exposure to harmful substances compared to traditional cigarettes; this is again consistent with findings from our review. A systematic review of 22 studies found that several carcinogens with a known link to bladder cancer were present in the urine of EC users and recommended further study on the urological safety of ECs (Bjurlin 2021). We will continue to gather information on biomarkers of harm.
Martinez‐Morata 2021 reviewed blood pressure findings and concluded that EC may result in short‐term elevations, but that more data are needed; our review also lacks sufficient data to draw any conclusions about blood pressure at one week or longer. A scoping review by Gugala 2022 looked at the pulmonary health effects of EC and found an association between EC use and negative pulmonary symptoms. EC use resulted in worse outcomes than nonsmoking, but resulted in improved outcomes when compared with combustible cigarette use or dual use of combustible cigarettes and EC. The review by McNeill 2022 found acute and short to medium exposure to most potential respiratory toxicants from ECs to be significantly lower than combustible cigarettes, with substantial reductions in some biomarkers. For the respiratory toxicants assessed at long‐term exposure, evidence was moderate. McNeill 2022 found moderate evidence that exposure to most respiratory toxicants from ECs was similar to non‐use of tobacco or nicotine products. Banks 2022 focused on the absolute risks of EC; in this review, we are interested in both their absolute and relative risks in comparison to smoking.
Zhang 2021 conducted a rapid review; while their pooled analysis also suggested that EC increased quit rates compared to NRT or non‐nicotine EC, they judged the evidence to be of low certainty according to GRADE, driven by imprecision and inconsistency. Zhang 2021 combined studies with NRT comparators and those with non‐nicotine EC comparators in the same analysis and found moderate statistical heterogeneity; we evaluated these two comparisons separately and did not find evidence of statistical heterogeneity. We now include more studies than Zhang 2021 and have no longer downgraded our finding for nicotine EC compared to NRT based on imprecision. Patnode 2021 reviewed evidence on tobacco cessation interventions for the US Preventive Services Task Force (USPFTS 2021). The authors stated that none of their included EC trials suggested higher rates of serious adverse events; this is in line with our analyses. However, they reported that findings across EC trials were inconsistent for effectiveness, with some finding statistically significant evidence of benefit and some finding no statistically significant difference. They did not conduct statistical meta‐analyses and included five trials, all of which are included in our cessation meta‐analyses. None of our cessation meta‐analyses, which include these trials, detected levels of heterogeneity beyond what would be expected from chance alone. Wang 2021 reviewed data both from observational studies and from randomized controlled trials; in the trials, e‐cigarettes were associated with increased smoking cessation (as with our review). In observational studies, ECs were not associated with increased smoking cessation. As discussed in Methods, although we included non‐randomized studies in which an EC intervention is provided in this review, we did not include observational studies in which no EC intervention is provided, due to known issues with confounding.
Chan 2021, Grabovac 2021 and Vanderkam 2022 also reviewed evidence from randomized controlled trials and found higher quit rates in people assigned to nicotine EC than to NRT or non‐nicotine EC, although Grabovac 2021 noted that evidence was less clear at longer follow‐up when comparing nicotine EC to counselling alone. Pound 2021 compared only nicotine EC with NRT; their pooled estimate showed a higher quit rate with nicotine EC (RR 1.42) but 95% CIs were wide and included the possibility of no difference. They included two studies in their comparison that we do not: one which measured cessation at less than six months and hence was not eligible for inclusion in our cessation analysis, and one in which the nicotine level was so low that we classify the study as non‐nicotine (Lee 2019). The latter introduced statistical heterogeneity to their pooled results. We also include additional studies not available at the time of their analyses.
A network meta‐analysis, with searches up‐to‐date until February 2019, used direct and indirect evidence to compare the effectiveness and safety of ECs to placebo, bupropion, NRT and varenicline (Thomas 2022). The evidence was imprecise, however, there was evidence of a benefit of ECs with a nicotine level of 15 mg over placebo. The effect estimate also suggested a benefit of ECs with a 10 mg nicotine level, but the credibility interval indicated the possibility of both benefit and harm. Similarly, when EC was compared with individual pharmacotherapies, the direction of effect was in favour of ECs, however, imprecision means further evidence may change the interpretation of the effect. The safety data for ECs was inconclusive. A second network meta‐analysis also suffered from imprecision when comparing EC and NRT, though CIs were consistent with our results (Quigley 2021). A component network analysis is currently underway that will update this evidence and investigate additional components of the interventions and studies (Lindson 2022a).
Reviews of ECs for policymaking are often broader in scope than our review, which focuses exclusively on their role in supporting smoking cessation in people who smoke. Outside of smoking cessation, there remain unanswered questions about the impact of EC availability and use on young people; we will be evaluating this in a separate review.
Authors' conclusions
Implications for practice.
Evidence suggesting nicotine EC can aid in smoking cessation is consistent across several comparisons. There is now high‐certainty evidence that EC with nicotine increases quit rates at six months or longer compared to NRT, and there remains moderate‐certainty evidence that EC with nicotine increases quit rates at six months or longer compared to non‐nicotine EC. There is very low‐certainty evidence (limited by risk of bias as well as imprecision) that EC with nicotine increases quit rates compared to behavioural support alone or to no support.
Issues with risk of bias, few studies, and differences between studies preclude strong conclusions regarding the effect of nicotine EC when added to NRT, but the data available suggest a benefit.
None of the included studies (short‐ to midterm, up to two years) detected serious adverse events considered possibly related to EC use. The most commonly‐reported adverse effects are throat/mouth irritation, headache, cough, and nausea, which tend to dissipate with continued use. In some studies, reduced toxin concentrations and biomarkers of harm were observed in people who smoked and switched to vaping, consistent with reductions seen in people who stopped smoking without EC.
Implications for research.
Further randomized controlled trials of nicotine EC are needed, following up participants at six months or longer. Studies with active comparators (i.e. comparing nicotine EC to frontline smoking cessation pharmacotherapies) are likely to be of particular use to decision‐makers, as are those testing EC as an adjunct to existing stop‐smoking pharmacotherapies. All studies (including uncontrolled intervention cohort studies) should aim to assess the safety profile of EC for as long as possible (the current review only includes data up to two years), and ideally be powered to detect differences in safety outcomes, including adverse events and serious adverse events. Safety results should be presented in both absolute and relative risk terms (in comparison to the risks of continuing to smoke tobacco).
Studies should offer recent devices with good nicotine delivery to participants to be most representative of what will be on the market at the time results are released. Studies should also monitor and collect data on participants switching use of other devices during trials, and use of different flavours and nicotine strengths. Protocols and statistical analysis plans should be registered in advance and openly available.
Further RCTs need to be adequately powered. Further trials of pod and newer disposable devices would be of particular value, as would RCTs providing ECs in a way that would be used in real‐world settings (e.g. taking into account individual preferences for strengths and flavours of e‐liquids and even EC devices, and also allowing for changes in preferences over time). Further studies directly comparing nicotine ECs based on characteristics including nicotine content and delivery, flavour, and device type, and reporting outcomes including cessation at six months or longer, would also be particularly useful.
Further reviews, using the best available methods, need to be conducted to evaluate the possible relationships between EC use and availability and youth uptake of EC and conventional cigarettes.
What's new
| Date | Event | Description |
|---|---|---|
| 15 March 2023 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st March 2023. In addition to the studies identified from August 2022 to February 2023, we found one new reference linked to a previously identified study. We will incorporate this into the review as part of a future update. We have also fixed a typo in the plain language summary. For future monthly search results, please see 'Monthly search results' via the following link: https://www.cebm.ox.ac.uk/research/electronic-cigarettes-for-smoking-cessation-cochrane-living-systematic-review-1. |
History
Protocol first published: Issue 11, 2012 Review first published: Issue 12, 2014
| Date | Event | Description |
|---|---|---|
| 4 February 2023 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st February 2023. In addition to the studies identified from August 2022 to January 2023, we found one new included study, one new ongoing study and 2 linked references. We will incorporate these into the review as part of a future update. The DOI for the 1 new included study (Kanobe 2022) is: https://doi.org/10.1038/s41598-022-25054-z. |
| 5 January 2023 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 3rd January 2023. In addition to the studies identified from August 2022 to December 2022, we found one new ongoing study. We will incorporate these into the review as part of a future update. In addition, some minor corrections were made to the Characteristics of Included Studies table for Hajek 2022 based on a published correction to the study's primary manuscript (https://doi.org/10.1038/s41591-022-02099-1). |
| 12 December 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st December 2022. In addition to the studies identified from August 2022 to November 2022, we found one new ongoing study and 3 records linked to previously identified studies. We will incorporate these into the review as part of a future update. |
| 25 November 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st November 2022. We found no new eligible references. As part of this amendment we also updated the citation for additional reference Lindson 2022b, and corrected a slight error in wording in the Discussion section. |
| 19 October 2022 | New citation required and conclusions have changed | Certainty changes for some of the primary outcomes. |
| 19 October 2022 | New search has been performed | 17 new included studies. Incorporates evidence up to the 1st July 2022. |
| 7 October 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st October 2022. In addition to the studies identified from June 2021 to September 2022, we found one new included study, 3 new ongoing studies and 1 record linked to a previously identified study. The DOI for the 1 new included study is: Klonizakis 2022 (https://doi.org/10.1186/s12916-022-02451-9). We will incorporate these into the review as part of a future update. |
| 27 September 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st September 2022. In addition to the studies identified from June 2021 to August 2022, we found two records linked to previously identified studies. We will incorporate these into the review as part of a future update. |
| 17 August 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st August 2022. In addition to the studies identified from June 2021 to July 2022, we found two new included studies, 1 new ongoing study and 3 records linked to previously identified studies. The DOIs for the 2 new included studies are: Coffey 2020 (DOI: 10.1177/1757913920912436) and Price 2022 (DOI: https://doi.org/10.1186/s12889-022-13711-x). We will incorporate these into the review as part of a future update. |
| 8 July 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st July 2022. In addition to the studies identified from June 2021 to June 2022, we found four new included studies, 1 new ongoing study and 8 records linked to previously identified studies. The DOIs for 3 of the new included studies are: Edmiston 2022 (DOI: 10.1093/ntr/ntac029); Tattan‐Birch 2022 (DOI: 10.1093/ntr/ntac149) and Morphett 2022a (DOI: 10.1093/ntr/ntab266). The fourth new included study was presented at SRNT 2022 (abstract reference: SYM17‐4). We will incorporate these into the review as part of a future update. |
| 15 June 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st June 2022. In addition to the studies identified from June 2021 to May 2022, we found three new included studies (all previously listed as ongoing studies) and 2 records linked to a previously identified study. The DOIs for the new included studies are: Hajek 2022 (https://doi.org/10.1038/s41591-022-01808-0); Bonafont Reyes 2022 (https://doi.org/10.1111/jgs.17755) and Vickerman 2022 (https://doi.org/10.1093/ntr/ntac129). We will incorporate these into the review as part of a future update. |
| 6 May 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st May 2022. In addition to the studies identified from June 2021 to April 2022, we found two new included studies (previously listed as ongoing studies), 3 new ongoing studies and 2 records linked to previously identified studies. The DOIs for the new included studies are: Skelton 2022 (doi: 10.1016/j.addbeh.2022.107328); Pratt 2022 (doi: 10.1093/ntr/ntac082). We will incorporate these into the review as part of a future update. |
| 6 April 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st April 2022. In addition to the studies identified from June 2021 to March 2022, we found 4 new ongoing studies. We will incorporate these into the review as part of a future update. |
| 7 March 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st March 2022. In addition to the studies identified from June 2021 to February 2022, we found 1 record linked to a study already identified as ongoing. We will incorporate these into the review as part of a future update. |
| 11 February 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st February 2022. In addition to the studies identified from June 2021 to January 2022, we found 2 ongoing studies and 2 records linked to studies already included in the review. We will incorporate these into the review as part of a future update. |
| 12 January 2022 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st January 2022. In addition to the studies identified from June to December 2021, we found 4 ongoing studies and 1 record linked to a study already included in the review. We will incorporate these into the review as part of a future update.[Enter text here] |
| 16 December 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date: 1st December 2021. In addition to the studies identified from June to November 2021, we found six new included studies, 15 ongoing studies and 18 records linked to studies already included in the review. The DOI or trial IDs for the new included studies are: NCT02433015; NCT03111537; NCT03185546; NCT03358953; Caponnetto 2021 (DOI: 10.1093/ntr/ntab005); Lum 2021 (DOI: 10.1016/j.addbeh.2021.107097). We will incorporate these into the review as part of a future update. |
| 3 November 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st November 2021. In addition to the studies identified from June to October 2021, we found one new included study. The DOI for the new included study (Okuyemi 2021) is 10.1093/ntr/ntab212. We will incorporate these into the review as part of a future update. |
| 20 October 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st October 2021. In addition to the studies identified from June to September 2021, we found one new included study two reports linked to studies already in the review, and one new ongoing. The DOI for the new included study (Morris 2021) is https://doi.org/10.1007/s11739-021-02813-w. We will incorporate these into the review as part of a future update. |
| 16 September 2021 | Amended | Change made to correct data; SAE data from Cobb 2021 moved from comparison with NRT to comparison with no‐nicotine EC. No changes to conclusions. |
| 6 September 2021 | New search has been performed | This is a Living Systematic Review. We run and screen searches monthly. Last search update 1st September 2021. We found no new studies for inclusion this month; however results from searches carried out from June to August 2021 will be incorporated into a future update of the review. |
| 6 September 2021 | New citation required and conclusions have changed | New secondary outcome added (continued product use), first study of pod device contributing data to cessation meta‐analysis added, two new comparisons added (nicotine salt EC versus freebase nicotine EC; advice on how to quit smoking using EC versus no EC advice). Conclusions for primary outcomes remain largely unchanged. |
| 6 September 2021 | New search has been performed | Updated with five new included studies. Incorporates evidence up to 1 May 2021. |
| 5 August 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 2nd August 2021. In addition to the studies identified from March to July 2021, we found two new ongoing studies and one report linked to a study already in the review. We will incorporate these into the review as part of a future update. |
| 7 July 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st July 2021. In addition to the studies identified from March to June 2021, we found two new included studies and two reports linked to studies already in the review. DOIs for the two new included studies are as follows: Myers‐Smith 2021: https://doi.org/10.1111/add.15628 & Kimber 2021: 10.1016/j.addbeh.2021.106909. We will incorporate these into the review as part of a future update. |
| 9 June 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st June 2021. In addition to the studies identified from March to May 2021, we found one report linked to a study already in the review, one ongoing study, and one potentially new study that we are looking into further. We will incorporate these into the review as part of a future update. As part of this new update we will also include a new outcome ‐ proportion of people still using e‐cigarettes or other pharmacotherapy at longest follow‐up. |
| 12 May 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 4th May 2021. In addition to the studies identified from March and April 2021, we found four new ongoing studies. We will incorporate these into the review as part of a future update. |
| 15 April 2021 | New citation required and conclusions have changed | 6 new included studies added (Czoli 2019; Ikonomidis 2020a; Ozga‐Hess 2019; Pulvers 2020; Scheibein 2020; Yingst 2020), certainty in finding of no difference in adverse events between nicotine EC and non‐nicotine EC updated to moderate (from low). First study of pod EC device included. |
| 15 April 2021 | New search has been performed | Updated with six new included studies and new data from one previously included study. Most recent search 1 Feb 2021. |
| 1 April 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st April 2021. In addition to the studies identified from March 2021 we found two new ongoing studies and one paper linked to a study already included in the review. We will incorporate these into the review as part of a future update. |
| 17 March 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st March 2021. Studies identified in March are not included in this version of the review, but will be incorporated into a subsequent version. We found four new included studies, five new ongoing studies and five papers linked to studies already included in the review. The four new included studies were all conference abstracts; three of which were identified from the SRNT 2021 abstract book (SYM2A, SYM2B, PH‐353; www.srnt.org/page/2021_Meeting). The fourth is available here: dx.doi.org/10.1016/j.drugalcdep.2015.07.1091. |
| 4 February 2021 | Amended | This is a Living Systematic Review. We run and screen searches monthly. Last search date 1st February 2021. In addition to the studies identified from our December 2020 and January 2021 searches we found one paper linked to a study already included in the review (Lucchiari 2020), and have preliminary results from a study listed as ongoing (Begh 2021). We will incorporate this paper and data into the review as part of a future update. |
| 20 January 2021 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 4th January 2021. In addition to the studies identified from our December 2020 searches we found four new completed studies, one new ongoing study and one paper linked to a study already included in the review. These studies and papers will be incorporated into the review at the next update. DOIs for the four new included studies are as follows: Ozga‐Hess et al. 2019: 10.1016/j.addbeh.2019.106105; Pulvers et al. 2020: 10.1001/jamanetworkopen.2020.26324; Scheibein 2020: 10.1186/s12954-020-00406-y; Yingst et al. 2020: 10.1080/09540121.2019.1687835 |
| 15 December 2020 | Amended | This is a Living Systematic Review. Searches are run and screened monthly. Last search date 1st December 2020. Searches found 3 new completed studies, 11 new ongoing studies and 9 papers linked to studies already included in the review. These studies and papers will be incorporated into the review at the next update. DOIs for the three new included studies are as follows: Czoli et al:10.1093/ntr/nty174;Bonevski et al: 10.1093/ntr/ntaa143;Eisenberg et al: 10.1001/jama.2020.18889. |
| 20 July 2020 | New citation required and conclusions have changed | Strength of evidence increased for existing comparisons; new comparisons added |
| 20 July 2020 | New search has been performed | New searches run January 2020. 35 new studies added. Living systematic review protocol incorporated |
| 14 December 2016 | Amended | Clarification on outcome data from Adriaens ‐ no changes to conclusions |
| 23 June 2016 | New search has been performed | Update search run January 2016, 11 new included studies added. Reduction removed as outcome, now covered in Harm Reduction review. |
| 23 June 2016 | New citation required but conclusions have not changed | 11 new included studies added; no changes to conclusions. |
Acknowledgements
This update and the two previous updates (April 2021, and October 2020) were supported through a Tobacco Advisory Group Cancer Research UK Project Grant. The 2020 update was also supported by the Cochrane Incentives Award Scheme and the University of Oxford Returning Carer's Fund, as well as through core infrastructure funding from the National Institute for Health Research (NIHR) for the Cochrane Tobacco Addiction Review Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS) or the Department of Health.
We thank Dr Jonathan Livingstone‐Banks for running monthly searches. We thank Phoebe Barnett (PB) for help with data extraction on the 2022 update.
We would like to thank Assistant Professor Stephen R. Baldassarri, Drs Pasquale Caponnetto, Fabio Cibella and Professor Riccardo Polosa, Professor Matthew Carpenter, Bruce R. Lindgren and Professor Dorothy Hatsukami, Assistant Professor Tracy Smith, Assistant Professor Susan Veldheer, Lauren Hickling, Stephanie K. Bell, Associate Professor Coral Gartner, Professor Billie Bonevski, Karolien Adriaens, Dr Sharon Cox, Professor Lynne E. Dawkins, Professor Mark Eisenberg, Andrea Hebert‐Losier, Kris Filion, Dr Ignatios Ikonomidis, Dr Kim Pulvers, Florian Scheibein, Dr Markos Klonizakis, Dr Scott Sherman, Dr Kylie Morphett, Dr Ivan Berlin, Marzena Orzol, Dr Katie Myers Smith, Christine Czoli, Dr Caroline Cobb, Dr Jonathan Foulds, Professor Thomas Brandon, Dr Eleanor Leavens, Dr Michael Arnold, Dr Paul Bateman, Dr Konstantinos Katogiannis, Dr Christopher Russell, Dr Francesca Pesola, Professor Christian Delles, Dr Danièle Kerr and Cassidy White for providing additional data or information. We would like to thank Dr Sharon Cox, Senior Research Fellow at University College London and Professor Lynne E. Dawkins, Centre for Addictive Behaviours Research, London South Bank University, for performing peer review for this update, and Dr Debbie Robson for performing editorial review for this update.
Anne Lethaby copyedited the 2022 update.
Appendices
Appendix 1. Protocol for living systematic review
Justification for ‘Living Review’ status
Living systematic reviews (LSRs) offer a new approach to updating reviews, in which the review is continually updated by incorporating relevant new evidence as it becomes available (Brooker 2019). Previous versions of this Cochrane Review of electronic cigarettes (ECs) for smoking cessation have informed policy worldwide (Hartmann‐Boyce 2016; McRobbie 2014). This update has found high degrees of uncertainty (low‐ and very low‐certainty evidence) for most outcomes, due to the small number of included randomized controlled trials, and the resulting imprecision in effect estimates. This means that some conclusions are likely to change substantially as new evidence emerges.
On average, Cochrane Reviews are updated every three to four years. For EC, where the evidence base is rapidly evolving, this schedule impedes the ability of the review to provide the most up‐to‐date evidence to decision‐makers. As EC use, availability, and design changes, policymakers are frequently drawing on this review to inform decisions, so it is imperative that it is up‐to‐date to ensure decisions are being made on the basis of the entirety of the evidence. Regular updates have the potential to strengthen the existing conclusions of the review or to change conclusions where conflicting evidence or evidence on new outcomes emerges (e.g. comparisons between EC and other interventions; longer‐term safety data).
Objective of the change to ‘Living Review’ status
To implement approved Cochrane LSR methods to provide an up‐to‐date, accessible, engaging and unbiased review of the evidence on the effect and safety of using EC to quit smoking.
LSR methodological considerations
The methods outlined below are specific to maintaining this review of Electronic cigarettes for smoking cessation as an LSR on the Cochrane Library. These methods will be ‘active’ immediately upon publication of this update. Core review methods, such as the criteria for considering studies in the review and assessment of risks of bias, are unchanged and are detailed in the main body of the review. Below we outline the methods for which specific considerations apply as a result of the change to ‘living’ status.
Search methods for identification of studies
We will conduct database searches monthly, beginning December 2020. These searches will be of the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, Embase, PsycINFO, and clinical trial registries, as detailed in the main body of the review. The funders of this LSR – Cancer Research UK (CRUK) ‐ already run monthly searches of the EC evidence and so we will work alongside their health information officer to ensure that we are identifying all the relevant literature with our searches. We will review our search strategies on an ongoing basis every 12 months, as indexing terms and keywords may change, and new search filters may be published. Such changes will be managed by input from experienced information specialists.
Selection of studies
We will immediately screen any new citations retrieved by the monthly searches using Covidence, undertaking dual screening of title and abstract, and then full text, by independent review authors. Where we find multiple citations of the same study we will group them into one study record with a single study ID. One review author (AB) will contact corresponding authors of potentially relevant ongoing studies as they are identified and ask them to advise when results are available, or to share early or unpublished data. Based on the information and projected time scales shared, we will contact corresponding authors on an ongoing basis to retrieve new evidence as it becomes available.
Data synthesis
Whenever we identify new studies relevant to the review, we will extract the relevant data and assess risks of bias as detailed in the main body of the review. We will highlight availability of this new evidence on both the Cochrane Library and on our own dedicated website. We will incorporate the new data into meta‐analyses and tables in the Revman (Review Manager 2020) and supplementary data files, and carry out GRADE assessments (GRADEpro GDT). We will conduct a full update of the review (full incorporation and interpretation of all new data within the review and re‐publishing) when the accumulating evidence leads to changes in any one of:
The direction of effect or clinical significance of the findings for one or more outcomes;
The certainty (e.g. GRADE rating) of one or more outcomes;
The availability of studies investigating new settings, populations, interventions, comparisons or outcomes.
Formal sequential meta‐analysis approaches will not be used for updated meta‐analyses, in line with Cochrane guidance for LSRs.
Future updates of review methods
The LSR approach acknowledges that reviews may cease to need to be ‘living’ over time, as the review findings become stable, or the question is no longer a priority for decision‐makers (Brooker 2019). Eighteen months into this review’s ‘living’ status (March 2022) we will evaluate the LSR approach, including the likely benefits of and challenges to continuing this methodology for this evidence base, and whether such an approach remains warranted. If the evidence is high certainty for all outcomes and all comparisons at that point, meaning further studies are judged very unlikely to impact the effect estimate, we would consider ceasing living mode for this review. If, as is more likely, some or all outcomes are not yet certain, we will facilitate discussions within the author team and Cochrane, as well as engaging with a wider PPI panel and key decision‐makers, e.g. policymakers, in order to determine next steps. If the decision is made to continue in living mode, we will review, and if necessary revise, the living review methods described in this Appendix before continuing.
Appendix 2. Toxins/carcinogen names and abbreviations
| Abbreviation | Name |
| ‐ | 1‐Hydroxyfluorene |
| ‐ | 1‐Hydroxyphenanthrene |
| ‐ | 1‐Hydroxypyrene |
| 2‐HPMA | 2‐hydroxypropylmercapturic acid |
| ‐ | 2‐Hydroxyfluorene |
| ‐ | 2‐Hydroxyphenanthrene |
| ‐ | 2‐Naphthol |
| ‐ | 3‐, 4‐Hydroxyphenanthrenes |
| 3‐HPMA | 3‐hydroxypropylmercapturic acid |
| ‐ | 3‐Hydroxyfluorene |
| AAMA | N‐acetyl‐S‐(carbamoylethyl)‐L‐cysteine (synonym: 2‐carbamoylethylmercapturic acid) |
| CEMA/CNEMA | 2‐cyanoethylmercapturic acid; referred to as 'acrylonitrile' in Pulvers 2018 |
| ‐ | Formic acid |
| HEMA | 2‐hydroxyethylmercapturic acid |
| HMPMA/HPMMA | 3‐hydroxy‐1‐methyl propylmercapturic acid |
| MHBMA | 2‐hydroxy‐3‐buten‐1‐ylmercapturic acid |
| MMA | N‐nitrosodimethyamine |
| NNAL | 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol |
| PheT | Phenanthrene tetraol |
| PMA | phenylmercapturic acid; referred to as 'benzene' in Pulvers 2018 |
| S‐PMA | S‐phenylmercapturic acid |
Appendix 3. Search strategies ‐ 2020 update onwards
Ovid databases (MEDLINE, Embase, PsycINFO)
1. exp case control studies/ or exp cohort studies/ or Case control.tw. or (cohort adj (study or studies)).tw. or Cohort analy$.tw. or (Follow up adj (study or studies)).tw. or (observational adj (study or studies)).tw. or Longitudinal.tw.
2. (e‐cig$ or ecig$ or electr$ cigar$ or electronic nicotine).mp. or (vape or vapes or vaporizer or vapourizer or vaporiser or vapouriser or vaper or vapers or vaping).ti,ab. or exp Electronic Nicotine Delivery Systems/
3. (randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.
4. exp animals/ not human/
5. 3 not 4
6. 2 and 5
7. 1 and 2
8. 6 or 7
9. smoking cessation.mp. or exp Smoking Cessation/
10. tobacco cessation.mp. or "Tobacco‐Use‐Cessation"/
11. (nicotine dependence or tobacco dependence).mp.
12. exp Smoking/th
13. "Tobacco‐Use‐Disorder"/
14. Smoking reduction/ or Smoking reduction.mp.
15. exp Pipe smoking/ or exp Tobacco smoking/ or exp Tobacco Products/
16. ((quit$ or stop$ or ceas$ or giv$ or abstain* or abstinen*) adj5 (smoking or smoke* or tobacco)).ti,ab.
17. exp Tobacco/ or exp Nicotine/
18. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17
19. 8 and 18
CRS‐Web databases (CTAG SPecialised Register, CENTRAL)
1. (e‐cig* or ecig* or electr* cigar* or electronic nicotine):ti,ab,KY,MH,EMT,KW,XKY,EH,KY
2. (vape or vapes or vaporizer or vapourizer or vaporiser or vapouriser or vaper or vapers or vaping):ti,ab,KY,MH,EMT,KW,XKY,EH,KY
3. MESH DESCRIPTOR Electronic Nicotine Delivery Systems EXPLODE ALL
4. #3 OR #2 OR #1
Appendix 4. MEDLINE search strategy ‐ pre‐2020
e‐cig$.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
electr$ cigar$.mp.
electronic nicotine.mp.
(vape or vaper or vapers or vaping).ti,ab.
1 OR 2 OR 3 OR 4
Identical terms used for other databases.
Line 4 added to search strategy for 2016 update.
Data and analyses
Comparison 1. Nicotine EC versus NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Smoking cessation | 6 | 2378 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.30, 2.04] |
| 1.1.1 Not selected on pregnancy | 5 | 2059 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.29, 2.04] |
| 1.1.2 Pregnant population | 1 | 319 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.45, 6.97] |
| 1.2 Adverse events | 4 | 1702 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.88, 1.19] |
| 1.2.1 4 weeks | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.31, 1.73] |
| 1.2.2 6 months | 2 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.82, 1.24] |
| 1.2.3 3 months after end of pregnancy | 1 | 1110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.84, 1.31] |
| 1.3 Serious adverse events | 5 | 2411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.82, 1.52] |
| 1.3.1 4 weeks | 1 | 29 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 1.3.2 6 months | 2 | 563 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.81, 2.88] |
| 1.3.3 1 year | 1 | 698 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.77, 2.41] |
| 1.3.4 3 months after end of pregnancy | 1 | 1121 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.52, 1.31] |
| 1.4 Carbon monoxide (ppm) | 3 | 191 | Mean Difference (IV, Fixed, 95% CI) | ‐2.74 [‐5.42, ‐0.07] |
| 1.4.1 Absolute values at follow‐up | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐1.87 [‐5.15, 1.41] |
| 1.4.2 Change from baseline | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐4.47 [‐9.09, 0.15] |
| 1.5 Heart rate (bpm) | 2 | 166 | Mean Difference (IV, Fixed, 95% CI) | 0.53 [‐1.76, 2.83] |
| 1.5.1 Absolute values at follow‐up | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | ‐0.74 [‐5.17, 3.69] |
| 1.5.2 Change from baseline | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐1.69, 3.69] |
| 1.6 Systolic blood pressure | 2 | 166 | Mean Difference (IV, Fixed, 95% CI) | ‐1.62 [‐3.59, 0.36] |
| 1.6.1 Absolute values at follow‐up | 1 | 111 | Mean Difference (IV, Fixed, 95% CI) | 1.00 [‐4.54, 6.54] |
| 1.6.2 Change from baseline | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐2.00 [‐4.11, 0.11] |
| 1.7 Blood oxygen saturation | 2 | 165 | Mean Difference (IV, Fixed, 95% CI) | ‐0.14 [‐0.59, 0.30] |
| 1.7.1 Absolute values at follow‐up | 1 | 110 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.72, 0.32] |
| 1.7.2 Change from baseline | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | 0.00 [‐0.83, 0.83] |
| 1.8 3‐HPMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.8.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9 NNAL (pmol/mg creatinine)) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.9.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10 2‐HPMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.10.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11 HMPMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.11.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12 PheT (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.12.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.13 CEMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.13.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14 AAMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.14.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.15 FEV1 | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.15.1 Change from baseline | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.16 FEV1/FVC (%) | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.83, 1.50] |
| 1.16.1 Change from baseline | 2 | 81 | Mean Difference (IV, Fixed, 95% CI) | ‐0.16 [‐1.83, 1.50] |
| 1.17 PEF (L/min) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.17.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.18 Product use at 6+ months | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
1.17. Analysis.

Comparison 1: Nicotine EC versus NRT, Outcome 17: PEF (L/min)
Comparison 2. Nicotine EC versus varenicline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.2 Serious adverse events | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 2.2.1 12 weeks | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 3. Nicotine EC versus non‐nicotine EC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Smoking cessation | 5 | 1447 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.94 [1.21, 3.13] |
| 3.2 Adverse events | 5 | 840 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.91, 1.11] |
| 3.2.1 1 week | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.50 [0.27, 8.19] |
| 3.2.2 8 weeks | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.38, 3.66] |
| 3.2.3 12 weeks | 1 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.94, 1.08] |
| 3.2.4 6 months | 2 | 513 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.71, 1.34] |
| 3.3 Serious adverse events | 8 | 1272 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.56, 1.79] |
| 3.3.1 1 week | 1 | 48 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3.2 4 weeks | 1 | 74 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.3.3 8 weeks | 1 | 24 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.50 [0.16, 78.19] |
| 3.3.4 6 months | 4 | 1009 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.52, 1.72] |
| 3.3.5 1 year | 1 | 117 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 3.4 Carbon monoxide (ppm) | 5 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.4.2 Absolute values at follow‐up | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5 Heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.5.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6 Systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.6.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7 FeNO (ppb) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.7.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.8 FEV1 (l) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.8.1 Absolute values at follow‐up | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.9 FEV1/FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.9.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.10 NNAL (pmol/mg creatinine) | 2 | 363 | Mean Difference (IV, Fixed, 95% CI) | ‐0.02 [‐0.45, 0.41] |
| 3.10.1 Change from baseline | 1 | 148 | Mean Difference (IV, Fixed, 95% CI) | 15.27 [‐4.98, 35.52] |
| 3.10.2 Absolute values at follow‐up | 1 | 215 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.46, 0.40] |
| 3.11 Product use at 6+ months | 3 | 874 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.94, 1.41] |
Comparison 4. Nicotine EC versus behavioural support only/no support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Smoking cessation | 7 | 3126 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.52, 4.65] |
| 4.2 Adverse events | 4 | 765 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.12, 1.32] |
| 4.2.1 12 weeks | 2 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.11, 1.30] |
| 4.2.2 16 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.67, 2.07] |
| 4.2.3 6 months | 1 | 58 | Risk Ratio (M‐H, Fixed, 95% CI) | 11.00 [0.64, 190.26] |
| 4.3 Serious adverse events | 9 | 1993 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.54, 1.97] |
| 4.3.1 4 to 6 weeks | 2 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3.2 8 weeks | 1 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.06, 1.35] |
| 4.3.3 12 weeks | 2 | 858 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.69 [0.21, 66.17] |
| 4.3.4 16 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 4.3.5 6 months | 2 | 307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.16, 3.10] |
| 4.3.6 8 months | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [0.68, 4.70] |
| 4.4 Carbon monoxide (ppm) | 11 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4.1 Change from baseline | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.4.2 Absolute values at follow‐up | 9 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5 Heart rate (bpm) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.5.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.6 Systolic blood pressure | 3 | 298 | Mean Difference (IV, Fixed, 95% CI) | ‐2.30 [‐3.91, ‐0.69] |
| 4.6.1 Change from baseline | 1 | 168 | Mean Difference (IV, Fixed, 95% CI) | ‐2.68 [‐4.38, ‐0.98] |
| 4.6.2 Absolute values at follow‐up | 2 | 130 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐3.95, 6.18] |
| 4.7 Blood oxygen saturation | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.7.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8 3‐HPMA (SMD) | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8.1 Absolute values at follow‐up | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.8.2 Change from baseline | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9 NNAL (SMD) | 5 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9.1 Absolute values at follow‐up | 2 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.9.2 Change from baseline | 3 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10 2‐HPMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.10.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11 HMPMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.11.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.12 PheT (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.12.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.13 CEMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.13.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.14 AAMA (pmol/mg creatinine) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.14.1 Absolute values at follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.15 S‐PMA (nanograms) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.15.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.16 FEV1 (SMD) | 2 | 714 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.01, 0.31] |
| 4.16.1 Change from baseline | 2 | 714 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.15 [‐0.01, 0.31] |
| 4.17 FEF 25‐75 (litres/second)) | 2 | 555 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 4.17.1 Change from baseline | 2 | 555 | Mean Difference (IV, Fixed, 95% CI) | ‐0.06 [‐0.18, 0.06] |
| 4.18 PEF 25‐75 (litres/minute) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.18.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.19 FEV1/FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.19.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 5. Higher versus lower nicotine content.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2 Serious adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2.1 1 year | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.2.2 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 5.3 Carbon monoxide (ppm) | 3 | 348 | Mean Difference (IV, Fixed, 95% CI) | ‐0.92 [‐1.71, ‐0.13] |
| 5.3.1 Change from baseline | 2 | 309 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.70, ‐0.10] |
| 5.3.2 Absolute values at follow‐up | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.66 [‐6.65, 3.33] |
| 5.4 Heart rate | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.4.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5 Systolic blood pressure | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.5.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6 FeNO (ppb) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.6.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.7 FEV1 (l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.7.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.8 FVC (l) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.8.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.9 FEV1/FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.9.1 12 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.10 NNAL (pg/mg creatinine) at 24 weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5.11 Product use at 6+ months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 6. Tobacco vs. menthol flavour.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 6.1 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6.2 NNAL (ng/g) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.2.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3 FEV1 (% predicted) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.3.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.4 FEV1/FVC | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.4.1 Change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 7. Refillable versus cartridge.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 7.1 Exhaled CO | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 8. Nicotine salt EC versus free‐base nicotine EC.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 8.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.2 Product use at 6+ months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 9. Non‐nicotine EC versus behavioural support only/no support.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 9.1 Smoking cessation | 2 | 388 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.76, 3.96] |
| 9.2 Adverse events at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.3 Serious adverse events at 24 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 10. Non‐nicotine EC + NRT versus NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 10.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3 Serious adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.3.1 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 11. Non‐nicotine EC versus NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 11.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.2 Adverse events | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.2.1 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.3 Serious adverse events | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 11.3.1 6 months | 1 | 132 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
11.3. Analysis.

Comparison 11: Non‐nicotine EC versus NRT, Outcome 3: Serious adverse events
Comparison 12. Advice to use e‐cigarettes compared to no advice to use e‐cigarettes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 12.1 Smoking cessation | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.2 Adverse events at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 12.3 Serious adverse events at 3 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
12.2. Analysis.

Comparison 12: Advice to use e‐cigarettes compared to no advice to use e‐cigarettes, Outcome 2: Adverse events at 3 months
12.3. Analysis.

Comparison 12: Advice to use e‐cigarettes compared to no advice to use e‐cigarettes, Outcome 3: Serious adverse events at 3 months
Comparison 13. Nicotine EC + NRT versus non‐nicotine EC + NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 13.1 Smoking cessation | 2 | 1039 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.77 [1.07, 2.94] |
| 13.2 Adverse events | 2 | 677 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.93, 1.32] |
| 13.2.1 8 weeks | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.78, 1.99] |
| 13.2.2 12 weeks | 1 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.90, 1.31] |
| 13.3 Serious adverse events | 2 | 1069 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.38, 1.14] |
| 13.3.1 8 weeks | 1 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.11, 3.34] |
| 13.3.2 6 months | 1 | 999 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.37, 1.19] |
| 13.4 Carbon monoxide (ppm) | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐1.73 [‐4.44, 0.98] |
| 13.4.1 change from baseline | 1 | 25 | Mean Difference (IV, Fixed, 95% CI) | ‐1.40 [‐4.26, 1.46] |
| 13.4.2 absolute values at follow‐up | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐4.60 [‐13.02, 3.82] |
| 13.5 FeNO (ppb) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.5.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.6 FEV1 (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.6.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.7 FVC (%) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.7.1 6 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.8 Study product use at 6+ months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 14. Nicotine EC + NRT versus NRT.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 14.1 Smoking cessation | 2 | 980 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [1.93, 6.44] |
| 14.2 Adverse events | 3 | 1984 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.83, 1.11] |
| 14.2.1 12 weeks | 2 | 421 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.69, 1.11] |
| 14.2.2 7 months | 1 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.82, 1.19] |
| 14.3 Serious adverse events | 4 | 2245 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.46, 3.42] |
| 14.3.1 5 weeks | 1 | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
| 14.3.2 12 weeks | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.00 [0.13, 70.30] |
| 14.3.3 6 months | 1 | 625 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.39, 3.27] |
| 14.3.4 7 months | 1 | 1563 | Risk Ratio (M‐H, Fixed, 95% CI) | Not estimable |
Comparison 15. Nicotine EC + varenicline vs. varenicline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 15.1 Adverse events at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15.2 Serious adverse events at 12 weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adriaens 2014.
| Study characteristics | ||
| Methods | Design: 3‐armed RCT; with all participants then assigned to nicotine EC (treated as cohort in this review) Recruitment: Advertisement on university website, flyers on university campuses, emails to personnel and advertisement in local newspaper Setting: Community and laboratory, Belgium Study start date/end date: Not stated |
|
| Participants | Total N: 48 provided data Randomized to: EC1 16; EC2 17; control 17 Inclusion criteria: smoke ≥ 3 yrs; ≥ 10 cpd; not intending to quit in the near future but willing to try a less unhealthy alternative. Exclusion criteria: diabetes; severe allergies; asthma or other respiratory diseases; psychiatric problems; dependence on chemicals other than nicotine; pregnancy; breastfeeding; hypertension; CV disease; currently using any kind of smoking cessation therapy; prior use of EC. 56% women, mean age 44, mean cpd 19, mean FTCD 5.79, all unwilling to quit with no baseline EC use |
|
| Interventions |
EC: Refillable Intervention: 2 intervention groups (EC1 and EC2) provided with EC and instructed to use EC or smoke ad libitum (EC1 group provided with Joyetech eGO‐C, EC2 group provided with Kanger T2‐CC) and provided guidance on EC use. For both types, provided 30 mL bottles of tobacco‐flavoured e‐liquid (Dekang “Turkish Blend”), containing 18 mg/mL of nicotine. 4 bottles at baseline replenished at 4 weeks, keep any remaining after 8 weeks Control: 6 bottles for 2 months at week 8 (half offered EC1, half offered EC2); no guidance on use |
|
| Outcomes | 3 lab sessions over 2 months (weeks 1, 4 and 8), plus online questionnaires, further follow‐up at 3 and 6 m after last lab session. Cessation: measured but definition not provided, validated with eCO 5 ppm or less Adverse events and biomarkers: eCO, salivary cotinine measured during lab sessions. Also collected craving and withdrawal symptoms via lab sessions, “benefits and complaints”, mood, EC usage |
|
| Study funding | "No external funding for this study was obtained. Electronic cigarettes and e‐liquids were purchased at E‐cig4U (`t Rond 10, 4285 DE Woudrichem, The Netherlands; http://www.e‐cig4u.nl/) with balances of previous research funds obtained by Frank Baeyens." | |
| Author declarations | The authors declare no conflict of interest. | |
| Notes | Randomization was for short‐term outcomes only. Additional data provided from authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomization was performed by using a randomization tool available on the website www.randomizer.org |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unblinded but as this review only includes data on objective measurements and not cessation judged unlikely to affect outcomes |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unblinded but as this review only includes data on objective measurements and not cessation judged unlikely to affect outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 out of 48 completed follow‐up (11/16 in EC1 group, 12/17 in EC2 group, 13/17 in control group) |
| Selective reporting (reporting bias) | Unclear risk | Outcome reporting somewhat non‐traditional; for example, collecting complaints but not explicitly adverse events, and incidence of AEs not reported. Unable to find prospectively‐registered protocol |
Baldassarri 2018.
| Study characteristics | ||
| Methods | Design: Randomized parallel‐assignment double‐blind trial Recruitment: outpatient pulmonary and primary care clinics, Tobacco Treatment Service, referrals from medical providers Setting: Hospital outpatient and primary care clinics, USA Study start date: October 2014; Study end date: June 2014 |
|
| Participants | Total N: 40 N per arm: Non‐Nicotine: 20; Nicotine EC: 20 Inclusion criteria: ≥ 18 years; ≥ 1 cpd; willing to quit smoking Exclusion criteria: unstable psychiatric or medical conditions requiring hospitalization within the past 4 months; acute coronary syndromes or stroke within the past 30 days; history of allergic reactions to adhesives; women who were pregnant, nursing, or not practicing effective contraception; current use of an EC for the purpose of stopping tobacco cigarette smoking. Women: 52.5%; Mean age: 53 Mean cpd: 17 Mean FTND: 5.9; motivated to quit E cigarette use at baseline: Not reported |
|
| Interventions |
EC: Refillable Both groups received standard care (8 weeks nicotine patch and counselling) and were randomized to nicotine EC or non‐nicotine EC. EC: eGO style EC (650 mAh battery, EVOD clearomizer, 3.7 V, 1.8 Ω single bottom coil), provided with e‐liquid purchased from an online vape shop (0 mg/mL or 24 mg/mL nicotine strength, 70/30 propylene glycol/vegetable glycerin, tobacco flavour); Instructed to use it as needed as a substitute for tobacco to try to satisfy cravings to smoke. If the patch alone proved adequate to prevent withdrawal and smoking cravings, the participant was advised not to use the EC. Additional EC devices, replacement coils, and liquid were provided as needed for the first 8 weeks of the study |
|
| Outcomes | Questionnaires and CO measurements taken at baseline, treatment visits at week 2, 4, 6, 8 and follow‐up at week 24 Cessation: 7‐day point prevalence abstinence, eCO ≤ 6 ppm Adverse events and biomarkers: Side effects were measured although it is unclear whether a questionnaire with prespecified symptoms was used Spirometry and FeNO at baseline and 6‐month follow‐up Other outcomes: Change in reported number of cpd at weeks 8 and 24; Change in per cent predicted FEV1 and FVC from baseline to week 24, and EC use patterns |
|
| Study funding | "Funding for this study was provided by the Yale School of Medicine, Section of Pulmonary, Critical Care, and Sleep Medicine and the National Heart, Lung, and Blood Institute grant T32HL007778. NHLBI had no role in the study design, collection, analysis, or interpretation of the data, writing the manuscript, or the decision to submit the paper for publication." | |
| Author declarations | "Dr. Toll received a grant from Pfizer for medicine only for a research study, and he receives funding as an expert witness in litigation filed against the tobacco industry. Dr. Chupp received grants from NIH, Genetech, Glaxo Smith Kline, Astra Zeneca/Medimmune and Boston Scientific. He received consulting/speaking fees from Genetech, Astra Zeneca/Medimmune, Mannkind, and Boston Scientific. There are no other conflicts of interest for the remaining authors." | |
| Notes | New for 2020 update. Study listed as ongoing study NCT02498145 in 2016 review update Additional data provided from authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomized using a random number generator with 1:1 blocked randomization (block size n= 8).” |
| Allocation concealment (selection bias) | Unclear risk | Both groups received standard care (nicotine patch and counselling) and were randomized to: nicotine EC or non‐nicotine EC (no further detail given) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Treatment assignment was blinded to both the investigators and participants” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CO biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: “The study had a modest loss to follow‐up (20%) at week 24.” Number lost to follow‐up in each group is not reported in the paper Week 24 retention rate: Nicotine EC group: 19/20 (95%); Non‐nicotine EC group: 13/20 (65%); > 20% difference between groups |
| Selective reporting (reporting bias) | Low risk | Outcomes reported align with those listed in the clinicaltrials.gov record. (registered 2015; prior to study completion in 2016) |
Begh 2021.
| Study characteristics | ||
| Methods | Individually randomized, blinded, 2‐arm trial Setting: 39 general practices, England Recruitment: Primary care registries |
|
| Participants | 325 (164 intervention; 161 intervention) 47.4% female. Mean age 57.8. Mean cpd 20.1. Mean FTCD 4.2 Inclusion criteria: Current smoker ≥ 10 ppm for exhaled CO and smokes a minimum of 8 cigarettes/8 grams of tobacco per day (including pipe, cigars or tobacco roll‐ups) with no intention of stopping immediately or seeking cessation support. Diagnosed with 1 or more of the following chronic conditions: ischaemic heart disease, peripheral vascular disease, hypertension, diabetes mellitus (Type 1 and Type 2), stroke, asthma, COPD, chronic kidney disease, depression, schizophrenia, bipolar disorder or other psychoses. Informed consent. ≥ 18 years Exclusion criteria: GP believes that switching to EC would not benefit the patient, given their current medical condition; currently using EC, NRT or other cessation therapies (e.g. bupropion, nortriptyline or varenicline); plans to stop smoking before or at the annual review; currently enrolled in another smoking‐related study or other study where the aims of the studies are incompatible; cannot consent due to mental incapacity; pregnancy, breastfeeding |
|
| Interventions | EC type: refillable Control: No additional support beyond standard care. Intervention: practitioners gave brief advice about EC and offered participants a free EC for the purpose of switching from smoking to vaping. The instruction was to reduce their smoking. If the offer was accepted, participants received: a starter pack containing an Aspire PockeX all‐in‐one e‐cigarette, 2 x 0.6 ohm coils and 1 x 1.2 ohm coil, 3 nicotine e‐liquids in 18 mg/mL (blueberry, menthol) and 12 mg/mL (mixed fruit) strengths and an accompanying practical support booklet developed by the study team. The practical support booklet contained information on how to set up the device, correct ways to vape, common issues with use and a list of local vape shops. It included motivational support to reinforce practitioners’ advice about EC, including the benefits of cutting down on cigarettes through e‐cigarette use and addressing perceived risks and concerns. It included links to a study‐dedicated website with video demonstrations on how to use EC and testimonials. Participants could opt into receiving an introductory telephone call from an experienced vaper in the first week of receiving their EC, to guide them on technical aspects of EC use (not behavioural support). Thereafter, participants could contact the vaper by telephone for up to 2 months after receiving their kit. All: Practitioners offered routine smoking cessation support to all participants. Although this varied across practices, standard care typically involved brief advice about stopping smoking and assistance to do so either by referral to the NHS stop smoking services or offer of pharmacotherapy. If the participant declined standard care, they were randomized by the practitioner to either the intervention or control arm. In the control arm, participants received no further support beyond standard care |
|
| Outcomes | 0 months, consultation visit, 2 months post‐consultation, 8 months post‐consultation "Patients attended four visits at their GP practice: a baseline visit, a therapeutic visit (‘annual review’) with their GP or nurse and two follow‐up visits two months and eight months post‐consultation." Primary outcomes: • 7‐day PPA from smoked tobacco at 2 months, defined as complete self‐reported abstinence from smoking – not even a puff – in the past 7 days, accompanied by a salivary anabasine concentration of < 1 ng/ml If there are technical issues with the analysis of saliva samples (e.g. if there is not enough saliva present in the sample for anabasine analysis), we will use exhaled CO as verification of abstinence (CO < 10 ppm) (Deviation from SAP: CO used due to imprecision of values for anabasine) • Reduction in cigarette consumption at 2 months, defined as at least a 50% reduction in self‐reported cigarettes per day on each of the last 7n days at 2 months compared with baseline consumption, accompanied by evidence of reduced smoke intake indicated by a CO measurement lower than baseline Secondary outcomes: • 7‐day PPA measured at 8 months, biochemically confirmed by an exhaled CO of < 10 ppm • 6‐month prolonged abstinence using the Russell standard criteria, defined as smoking < 5 cigarettes between 2‐ and 8‐month follow‐ups, confirmed by an anabasine concentration of < 1 ng/ml at 2 months and an exhaled CO concentration of < 1 ng/ml at 8 months if CO measurement unavailable) • Mean change in salivary anabasine concentration and CO from baseline to 2 months. • Percentage reduction in self‐reported cigarettes per day from baseline to 2 months; and from baseline to 8 months. SAEs & AEs reported. AEs: throat/mouth irritation; cough; headache; palpitations; nausea; dry mouth; dizziness; shortness of breath; stomach pain |
|
| Study funding | NIHR Postdoctoral Fellowship and NIHR School for Primary Care Research funded randomized controlled trial | |
| Author declarations | All authors declare no competing interests | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised to intervention or control with a 1:1 allocation ratio. A randomisation list was generated by the trial statistician using the current version of Stata and validated by a second statistician within the Primary Care Clinical Trials Unit (PC‐CTU). The randomisation was stratified by practice and used varying block sizes to ensure allocation concealment." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation list was passed to someone independent of the trial who created the randomisation envelopes. The trial statisticians were blinded to the treatment allocation during analyses" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Due to the nature of the trial, GPs and practice nurses were aware of the participant’s treatment allocation to ensure that the correct intervention was given. Therefore, practitioners who delivered the intervention could not be blinded to treatment. While participants knew whether they had been offered support to cut down by using an e‐cigarette or not by their GP or nurse, the participant was not informed that the study investigated this specifically and therefore were in some respects blind to allocation. Groups not matched for face‐to‐face contact time |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “7‐day point‐prevalence abstinence from smoked tobacco at two months, defined as complete self‐reported abstinence from smoking – not even a puff – in the past seven days, accompanied by a salivary anabasine concentration of <1ng/ml” or exhaled CO as verification of abstinence (CO <10 ppm)." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 8 months: Control 144/161; Intervention 148/164 |
| Selective reporting (reporting bias) | Low risk | Used CO above anabasine, but reported both (Deviation from SAP: CO used due to imprecision of values for anabasine) All predefined outcomes listed in the published protocol and clinical trial register are reported |
Bell 2017.
| Study characteristics | ||
| Methods | Design: Pragmatic, uncontrolled, mixed‐methods trial Recruitment: Targeted settings for people with HIV Setting: Community, Brisbane, Australia Study start date: 21 February 2017; Study end date: 26 October 2017 |
|
| Participants | Total N: 30 Inclusion criteria: diagnosis of HIV; ≥ 18 years; ≥ 5 cpd at the time of enrolment into the trial; smoking ≥12 months; willing to attempt to quit tobacco smoking after study enrolment. Exclusion criteria: participating in a smoking‐cessation programmed; pregnancy or breastfeeding; experienced chest pain, or another cardiovascular event or procedure in the last month; being treated with oxygen therapy. Inclusion based on specific population characteristic: People living with HIV 29 participants identified as male, and 1 participant did not identify as male or female; Mean age: 42; Mean cpd: 18 EC use at baseline: 46.7% (n = 14) Never tried; 50% (n = 15) Tried, never used for an extended period; 3.3% (n = 1) Used on a regularly (weekly) basis Willing to attempt to quit |
|
| Interventions |
EC: Refillable Single‐arm study. Print materials to help quit smoking. Provided booklet with instructions on how to use, store and handle EC; copies of device user manuals. Given Innokin Endura T18® vaporiser kit, Innokin Endura T22® vaporiser kit, 4 spare coils, 1 wall charger, 10 x 10‐mL bottles of Nicophar® 12 mg nicotine e‐liquid. Supplies to last 12 weeks |
|
| Outcomes | Weeks 1, 4, 8, 12, 24; Self‐report and semistructured interviews Cessation: 7 days point prevalence at weeks 4, 8, 12 and 24. Continuous abstinence at weeks 12 and 24. No biochemical validation Adverse events Other outcomes: Acceptability and use of trial products; Number of quit attempts |
|
| Study funding | "This work was supported by the HIV Foundation Queensland. The funder will play no role in the analysis and interpretation of results. All trial products were purchased and the suppliers have no involvement in the conduct of the trial or the interpretation or reporting of the results." | |
| Author declarations | "No other authors declare conflicts of interest. Mark Boyd has received research grant funding (paid to the institution) from AbbVie, Gilead and Merck and received honoraria for participation in HIV Advisory Boards and for the preparation and delivery of educational materials from AbbVie, Boehringer‐Ingelheim, Bristol Myers Squibb, Gilead, Janssen‐Cilag, Merck and ViiV Healthcare." | |
| Notes | Additional data provided from authors. New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled study |
| Allocation concealment (selection bias) | High risk | Uncontrolled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “At Week 24, 26 of the 30 participants who enrolled in the study were followed up.” (confirmed by authors) |
| Selective reporting (reporting bias) | Low risk | Study not published at time of data extraction, but study protocol published |
Bonafont Reyes 2022.
| Study characteristics | ||
| Methods | Design: 'A mixed methods study’ Recruitment: We recruited patients with COPD, aged 21 to 75, listed as current smokers in the NYU Langone Health electronic health record by phone, mail, and MyChart. Participants: patients with COPD. Setting: NYU, USA Study start date: Not reported |
|
| Participants | Tota N: 48 Inclusion criteria: moderate COPD (based on the COPD Assessment Test score (CAT)); interested in quitting Exclusion criteria: not reported Female 54%. Mean age 60 (SD 8.2) E‐cigarette use at baseline: Not reported Motivated to quit: Yes |
|
| Interventions | EC: no detail reported Arm 1 EC Arm 2 NRT Both groups: Over 12 weeks, participants received 5 counselling sessions and were asked about their COPD symptoms, CC use, EC use, and nicotine withdrawal symptoms. We used Ecological Momentary Assessment (four text messages/day) to assess current EC/NRT and CC use. |
|
| Outcomes | 12 weeks Combustible cigarette use measured. Dyspnoea COPD symptoms. Mixed methods study assessing the relationship between race/ethnicity and switching from CC to EC; evaluated whether it is mediated by social norms, risk perception, and overall opinions of CC and EC |
|
| Study funding | Not reported | |
| Author declarations | Not reported | |
| Notes | Student presentation New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail: “We randomized participants to EC or nicotine replacement therapy (NRT) for switching from CC.” |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Two active interventions. No detail |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Still collecting data. Outcome data not reported. Numbers not reported |
| Selective reporting (reporting bias) | Unclear risk | Still collecting data |
Bullen 2013.
| Study characteristics | ||
| Methods | Design: 3 parallel groups RCT Recruitment: People who smoke recruited from the community, via newspaper advertisements Setting: Research Unit, New Zealand Study start date: 6 September 2011; Study end date: 5 July 2013 |
|
| Participants | Total N: 657. 289 nicotine EC (NEC), 295 patch, 73 non‐nicotine EC (PEC) Inclusion criteria: ≥ 18 years; smoked 10 or more cpd over past year; wanted to stop smoking Exclusion criteria: pregnancy and breastfeeding; sing cessation medicines or using other support to quit; heart attack, stroke, severe angina in the last 2 weeks; poorly‐controlled medical disorder; allergies, other chemical dependence. 62% women, mean age 42, ⅓ NZ Maori, smoking 18 cpd, mean FTND score 5.5 Motivated to quit E cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like Randomized to NEC, PATCH or PEC use for 13 weeks (from 1 week prior to TQD)
All participants referred to Quitline and received an invitation to access phone‐ or text‐based support. This was accessed by < 10% |
|
| Outcomes | Sustained (≤ 5 cigarettes allowed) validated (exhaled breath CO < 10 ppm) abstinence at 6 months ≥ 50% self‐reported reduction in baseline cigarettes at 6 months Participants reporting any adverse events Proportion of AEs that were serious Proportion of unrelated AEs |
|
| Study funding | Health Research Council of New Zealand | |
| Author declarations | "We declare that we have received no support from any companies for the submitted work and have no non‐financial interests that might be relevant to the submitted work. ML, via his company Health New Zealand, previously did research funded by Ruyan (an e‐cigarette manufacturer). CB and HM have done research on Ruyan e‐cigarettes funded by Health New Zealand, independently of Ruyan. HM has received honoraria for speaking at research symposia, has received benefits in kind and travel support from, and has provided consultancy to, the manufacturers of smoking cessation drugs. NW has provided consultancy to the manufacturers of smoking cessation drugs, received honoraria for speaking at a research meeting and received benefits in kind and travel support from a manufacturer of smoking cessation drugs. JW has provided consultancy to the manufacturers of smoking cessation medications." | |
| Notes | Accessed support: NEC: 115/289; PATCH: 106/295; PEC: 26/73 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerized block randomization |
| Allocation concealment (selection bias) | Low risk | Computerized via study statistician |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | NEC and PEC were blind to treatment condition in relation to one another. No blinding for NEC/PEC vs PATCH conditions, but as NEC and PATCH were both active treatments performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | LTFU 22% (all considered to be smoking). Patch group had a higher LTFU and withdrawal than EC (loss to follow‐up 17% NEC, 27% patches, 22% PEC). However, minimal difference in per‐protocol and ITT analyses |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Caponnetto 2013a.
| Study characteristics | ||
| Methods | Design: 3‐arm double‐blind randomized controlled trial: EC with 7.2 mg nicotine for 12 weeks; same for 6 weeks followed by 5.2 mg for 6 weeks: EC with no nicotine for 12 weeks Recruitment: Newspaper advertisements Setting: Outpatient clinic, Italy Study start date: April 2010; Study end date:April 2012 |
|
| Participants | Total N: 300 Inclusion criteria: smoked ≥ 10 cpd for past 5 years; age 18‐70; in good health; not currently or intending to quit smoking in the next 30 days. Exclusion criteria: symptomatic cardiovascular or respiratory disease; regular psychotropic medicine use; current or past history of alcohol abuse; use of smokeless tobacco or NRT; pregnancy or breastfeeding. 36% women, mean age 44 (SD 12.5), mean cpd 20 (IQR: 15‐25) Not currently or intending to quit smoking in the next 30 days E cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like EC presented as a healthier alternative to tobacco smoke and could be freely used, ad libitum (up to 4 cartridges a day) for 12 weeks, as a tobacco substitute EC used: 'Categoria' (model 401) with disposable cartridges
Baseline visit and up to 7 follow‐up visits to receive more cartridges, hand‐in diaries, measure CO and vital signs |
|
| Outcomes | Abstinence at 12 months (complete self‐reported abstinence from tobacco smoking since previous visit at 6 months, confirmed with CO < 7 ppm at 12 months) ≥ 50% reduction in baseline cigarettes at 12 months Recorded AEs thought to be related to tobacco smoking and EC at baseline and at each study visit (7 follow‐up visits over 12 weeks, plus at 24 and 52 weeks) |
|
| Study funding | "This research was supported by a grant‐in‐aid from Lega Italiana AntiFumo. The study sponsor had no involvement in the study design, collection, analysis, and interpretation of data, the writing of the manuscript or the decision to submit the manuscript for publication. RP and PC are currently funded by the University of Catania, Italy. The e‐cigarette supplier had no involvement in the study design, collection, analysis, and interpretation of data, the writing of the manuscript or the decision to submit the manuscript for publication." | |
| Author declarations | "RP has received lecture fees and research funding from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has served as a consultant for Pfizer and Arbi Group Srl, the distributor of the CategoriaTM e‐Cigarette. The other authors have no relevant conflict of interest to declare in relation to this work." | |
| Notes | Additional data provided from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, block size 15 (5:5:5 ratio) |
| Allocation concealment (selection bias) | Low risk | Randomization carried out by pharmacy, who did not have direct contact with the participants |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Quote: “Blinding was ensured by the identical external appearance of the cartridges. The hospital pharmacy was in charge of randomization and packaging of the cigarettes” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 211 (70.3%) and 183 (61%) attended 6‐ and 12‐month follow‐up (at 12 m, 35% lost in 7.2 group; 37% lost in 5.4 group; 45% lost in no‐nicotine group) |
| Selective reporting (reporting bias) | Unclear risk | Unclear if original intention was to combine groups A+B or not. In sample size calculation they compared A+B with C, but results are not always reported in this way |
Caponnetto 2013b.
| Study characteristics | ||
| Methods | Design: Prospective cohort Recruitment and setting: Inpatients at a psychiatric institution in Italy Study start date/end date: Not specified |
|
| Participants | Total N: 14 Inclusion criteria: smoked ≥ 20 cpd for at least the past 10 years; diagnosis of schizophrenia. Exclusion criteria: alcohol and illicit drug use; recent myocardial infarction; angina pectoris; high blood pressure (BP > 140 mmHg systolic or 90 mmHg diastolic, or both); diabetes mellitus; severe allergies; poorly‐controlled asthma or other airway diseases; inclusion based on specific population characteristic: Diagnosis of schizophrenia 57% women, mean age 44.6 (SD 12.5), mean pack years smoked 28.8 (SD 12.9) Motivated to quit: Not specified E cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like Seen at baseline, given EC ('Categoria' brand) with an initial 4‐week supply of 7.4 mg nicotine cartridges. Instructed to use ad libitum up to 4 cartridges a day. EC cartridges supplied at months 1, 2, and 3 No instruction on cessation or reduction was provided. |
|
| Outcomes | Follow‐up at 1, 2, 3, 6 and 12 months where cigarette consumption, CO, AEs and positive and negative symptoms of schizophrenia were measured Sustained reduction of ≥ 50% for at least 30 days at 12 months 30‐day point prevalence CO‐validated abstinence at 12 months Adverse events |
|
| Study funding | "We wish to thank Arbi Group Srl (Milano, Italy) for the free supplies of “Categoria” e‐cigarette kits and nicotine cartridges as well as their support. We would also like to thank LIAF (Lega Italiana AntiFumo) for the collaboration." | |
| Author declarations | "Pasquale Caponnetto, Roberta Auditore, Cristina Russo and Giorgio Carlo Cappello declare no conflict of interest. Riccardo Polosa has received lecture fees and research funding from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has served as a consultant for Pfizer and Arbi Group Srl (Milano, Italy), the distributor of the CategoriaTM e‐cigarette." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort; no randomization |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 0/14 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Caponnetto 2021.
| Study characteristics | ||
| Methods | Design: single‐arm pilot study Recruitment: Authors wrote to physicians, psychiatrists, and other health care providers to inform them about the study. Flyers were posted within and outside of the Smoking Cessation Center of Catania University (Centro per la Prevenzione e Cura del Tabagismo — CPCT), at the Policlinico Vittorio Emanuele. Participants were recruited from Catania outpatient psychiatric clinics by researchers of CPCT. Clinicians from outpatient psychiatric clinics identified suitable participants and drew their attention to the study flyers. Setting: Catania, Italy Study start date: 2017. End date not stated. Recruitment September 2017 to October 2017 |
|
| Participants | Total N: 40 (single‐arm) All participants: individuals with schizophrenia spectrum disorders who smoke cigarettes Inclusion criteria: Adults attending psychiatric outpatient clinics in Catania who smoked 20 or more cigarettes daily were included. Able to meet the criteria for a schizophrenia spectrum disorder diagnosis without evidence of current exacerbation of illness Exclusion criteria: Pregnancy, breastfeeding, myocardial infarction or angina pectoris within the past 3 months, current poorly controlled asthma or chronic obstructive pulmonary disease. Female 35%. Mean age 48.3 (SD 12.1). Mean CPD 28 (SD 9). Mean FTND 8.3 (SD 1.8). E‐cigarette use at baseline: 12 (30%) had used EC either regularly or had tried. Motivated to quit: No |
|
| Interventions | EC: pod JUUL pod e‐cigarette, PAX Labs, a closed pod e‐cigarette product. The pod contains 0.7 mL of e‐liquid and up to 5% nicotine by weight. At the BL visit, participants were given a free e‐cigarette starter kit containing one JUUL device with a charger and 5% nicotine pods, Virginia tobacco flavour with instructions on how to charge, activate, and use the e‐cigarette. A 4‐week supply of pods equivalent to their current cigarette smoking behaviour, according to the manufacturer’s guidelines, was supplied to each participant (one pod for every packet of 20 cigarettes; mean 128, minimum 80, and maximum 200). Support: Eligible participants were invited to use a JUUL e‐cigarette for at least 12 weeks and were followed up prospectively for 24 weeks. Participants received a 4‐week supply of pods on three occasions, BL, week 4 (study visit 2), and week 8 (study visit 3). Participants were informed that the product was potentially less harmful than combustible cigarettes and could be used as a cigarette substitute as much as they liked. Limited behavioural support was provided as part of the intervention and included behaviour substitution of combustible cigarettes with e‐cigarettes and self‐monitoring of combustible cigarette consumption through the use of study diaries. Phone contact at week 2, 6 & 10. Participants attended a total of five study visits. |
|
| Outcomes | Baseline, week 4 (study visit 2), week 8 (study visit 3), 12 weeks. 24‐week FU. Cessation: CO Reduction: CPD AEs: dry cough, headache, throat irritation Other outcomes: vital signs (BP and HR), weight, and mental health, subjective effects (e.g. satisfied, no aversion), acceptability |
|
| Study funding | The e‐cigarettes used in the study were donated by the manufacturer, PAX Labs (on June 13, 2017 the company became known as JUUL Labs). Acknowledgements: The authors wish also to thank PAX Labs (on June 13, 2017 the company became known as JUUL Labs) for the free supplies of JUUL e‐cigarette kits and pods. At the time the research was conducted, JUUL Labs were not part owned by Altria, a tobacco company. PAX Labs agreed also to supply pods for a further 3 months after the end of the pilot to participants who expressed a wish to continue using as JUUL was not available in Italy when this study was conducted and not currently available at the 5% nicotine strength. No separate funding was secured for the study. Altria Group (formerly Philip Morris Companies) acquired a 35% stake in JUUL Labs on December 20, 2018, but the study was completed before Altria invested in JUUL. |
|
| Author declarations | MM is fixed‐term researcher at Centro per la Prevenzione e Cura del Tabagismo, University of Catania. JD is full‐time employee of City University of New York (United States). JK is full‐time employee of Weill Medical College of Cornell University, New York (United States). RP is full‐time employee of the University of Catania, Italy. In relation to his work in the area of tobacco control and respiratory diseases, RP has received lecture fees and research funding from Pfizer, Inc., GlaxoSmithKline plc, CV Therapeutics, NeuroSearch A/S, Sandoz, MSD, Boehringer Ingelheim, Novartis, Duska Therapeutics, and Forest Laboratories. He has also served as a consultant for Pfizer, Inc., Global Health Alliance for treatment of tobacco dependence, CV Therapeutics, NeuroSearch A/S, Boehringer Ingelheim, Duska Therapeutics, Forest Laboratories, ECITA (Electronic Cigarette Industry Trade Association, in the United Kingdom), Health Diplomat (consulting company that delivers solutions to global health problems with special emphasis on harm minimization), and Pharmacielo. RP was awarded an Investigator‐Initiated Study award programme established by Philip Morris International in 2017, but subsequently resigned from the role of Principal Investigator in 2018, before the trial began. Lecture fees from a number of European EC industry and trade associations (including Fédération Interprofessionnelle de la VAPE in France and Federazione Italiana Esercenti Svapo Elettronico in Italy) were directly donated to vaper advocacy no‐profit organizations. RP is the Founder of the Center of Excellence for the acceleration of Harm Reduction at the University of Catania (CoEHAR), which has received a grant from Foundation for a Smoke Free World to develop and carry out eight research projects. RP is also currently involved in the following pro bono activities: scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti Smoking League) and Chair of the European Technical Committee for standardization on Requirements and test methods for emissions of electronic cigarettes (CEN/TC 437; WG4). PC is paid by the University of Catania as an external part‐time researcher and adjunct professor of clinical, addiction, and general psychology. He has been affiliated to the CoEHAR since December 2019 in a pro bono role. He is coauthor of a protocol paper supported by an Investigator‐Initiated Study award programme established by Philip Morris International in 2017. The other authors have no conflict of interests to declare. | |
| Notes | New to 2022 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Single‐arm, open‐label |
| Allocation concealment (selection bias) | High risk | Single‐arm, open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: 37 (92.5%) participants completed all study visits and attended their follow‐up visit. |
| Selective reporting (reporting bias) | Unclear risk | No detail |
Carpenter 2017.
| Study characteristics | ||
| Methods | Design: Randomized parallel‐assignment open‐label trial Recruitment: Recruitment from local urban community in southeastern USA, using various media outlets Setting: Community, southeastern USA Study start date: November 2014; Study end date: May 2016 |
|
| Participants | Total N: 68 N per arm: Control group: 22; ENDS group: 46 (split into 2 non‐randomized groups: BluCig 16 mg: 25; BluCig 24 mg: 21) Inclusion criteria:
Exclusion criteria:
Women: 59.7%; Mean age: 42.2; Mean cpd: 15.3; Heaviness of smoking (0‐6): 2.9 EC use: Control: 9%; ENDS 16 mg group: 4%; ENDS 24mg group: 33% Motivation to quit smoking in next month (0 – 10): Control: 4.0; ENDS 16 mg: 5.0; ENDS 24 mg: 4.4 |
|
| Interventions |
EC: Cig‐a‐like Intervention: At study start, choice of tobacco or menthol flavour Blu Starter Pack EC, with 16 mg/mL nicotine. Midway through study, the manufacturer of Blu altered the product and discontinued availability of the device, replaced with BluPlusþ, with 24 mg/mL nicotine. 3‐week sampling period, given up to 7 cartridges at each of 3 weekly visits. Instructions on usage "kept minimal to preserve naturalistic intent." The study team suggested that ENDS could be used "as you wish, to cut down or quit smoking, help manage smoking restrictions, or both." Control: own brand of cigarettes |
|
| Outcomes | Weeks 2, 3, 4, 8, 12 and 16 Carbon monoxide, NNAL Other outcomes: cessation (< 6 months), product evaluation, EMA |
|
| Study funding | "Support was provided by NIH R21 DA037407 (to M.J. Carpenter), P01 CA200512 (to K.M. Cummings, M.J. Carpenter, and M.L. Goniewicz), UL1 TR001450, and P30 CA138313. M.L. Goniewicz's laboratory is supported via P30 CA016056. B.W. Heckman is supported via K12 DA031794 and K23 DA041616. T.L. Wagener's effort is partially supported by the Oklahoma Tobacco Research Center, which is funded by the Oklahoma Tobacco Settlement Endowment Trust." | |
| Author declarations | "M.L. Goniewicz is a consultant/advisory board member for Johnson & Johnson. K.M. Cummings reports receiving a commercial research grant from and is a consultant/advisory board member for Pfizer Inc., and has provided expert witness testimony for various plaintiffs in lawsuits involving cigarette manufacturers. No potential conflicts of interest were disclosed by the other authors." | |
| Notes | New for 2020 update. Listed as ongoing study NCT02357173 in 2016 review update. Additional data provided from authors In all, 25 participants (54%) received the Blu Starter Pack (16 mg), and 21 participants (46%) received BluPlusþ (24 mg); no switches were made within participants. Note: this is not included in our analysis of higher v lower as assignment to nicotine dose was not done at random; 24 mg and 16 mg merged in our main analysis |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization to group was stratified by motivation to quit in the next 30 days (0–6 vs. 7–10 on a VAS scale) but proportioned 2:1 (ENDS:control) to increase precision estimates for e‐cigarette uptake and usage.” |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and includes non‐active control |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | CO biochemically verified but abstinence not used as outcome in this review, so rated based on adverse event reporting. Self‐report, no blinding of participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Retention rate: Week 4: Control:19/22 (86%); ENDS 16 mg: 23/25 (92%); ENDS 24 mg: 20/21 (95%) Week 16: Control: 16/22 (73%); ENDS 16 mg: 19/25 (76%); ENDS 24 mg: 15/21 (71%) |
| Selective reporting (reporting bias) | Unclear risk | Not specified |
| Other bias | Low risk | Midway through the study, the manufacturer of Blu altered the product and discontinued availability of the device, replaced with BluPlusþ, with 24 mg/mL nicotine, again offered in both tobacco and menthol flavourings, and with improved battery duration (4‐watt battery for both devices). In all, 25 participants (54%) received the Blu Starter Pack (16 mg), and 21 participants (46%) received BluPlusþ (24 mg); no switches were made within participants. The change in product (IRB approved) allowed us the unexpected opportunity to assess what impact, if any, the change in product design had on study outcomes. Note that the manufacturer, style of device, and packaging did not change, nor did our messaging to participants. The only difference was the strength of product. Thus, trial outcomes are reported across 3 groups: control versus 16 mg versus 24 mg ENDS. We have not rated this as high risk of bias as our analyses do not compare on nicotine strength and both nicotine arms are combined in our main analysis |
Cobb 2021.
| Study characteristics | ||
| Methods | Design: Randomized parallel‐assignment double‐blind trial Setting: USA (Penn State Medical Center in Hershey, Pennsylvania (n=300). Virginia Commonwealth University in Richmond, Virginia (n=220) Recruitment: Community advertisements Study start date: June 2015; Study end date: June 2018. |
|
| Participants | Total N: 520 (though Veldheer paper only reports 263) N per arm: 130 per arm Inclusion criteria: age 21‐65; smoke > 9 cigarettes per day ; smoke regular filtered cigarettes or machine‐rolled cigarettes with a filter; CO measurement > 9 ppm at baseline; not planning to quit in the next 6 mths; interested in reducing cigarette consumption; no serious quit attempt in last mth, or use of FDA‐approved smoking cessation medication (varenicline, bupropion (used specifically as a quitting aid), patch, gum, lozenge, inhaler, and nasal spray). Exclusion criteria: unstable or significant medical condition in the past 12 mths (recent heart attack or some other heart conditions, stroke, severe angina including high blood pressure if systolic > 159 or diastolic > 99 observed during screening); immune system disorders, respiratory diseases (exacerbations of asthma or COPD, require oxygen, require oral prednisone), kidney (dialysis) or liver diseases (cirrhosis), or any medical disorder/medication; use of any non‐cigarette nicotine delivery product (pipe, cigar, dip, chew, snus, hookah, e‐cigs, strips, sticks) in the past 7 days; uncontrolled mental illness or substance abuse or inpatient treatment for these in the past 6 months; difficulty providing blood samples; no surgery requiring general anaesthesia in the past 6 weeks; use of EC for ≥5 in the past 28 days or any use in the past 7 days; use of marijuana or any illicit drug/prescription drugs for non‐medical use daily/almost daily, or weekly in the past 3 mths per NIDA Quick Screen; hand‐rolled, roll‐your‐own cigarettes; allergy to propylene glycol /vegetable glycerin; pregnancy/breastfeeding. 58% women; mean age 47; mean cpd 18; mean FTND: Not specified Motivated to quit: Interested in reducing cigarette intake but not planning to quit in next 6 months EC use at baseline: None |
|
| Interventions |
EC: Cartridge For 24 weeks: 1) Cigarette substitute: QuitSmart cigarette substitute ‐ plastic tube looks like a real cigarette, designed to provide the same draw resistance as a smoker's usual cigarette. No drug delivery. 2 cigarette substitutes and a product manual are provided to participants following randomization and replacement products are provided throughout the intervention period (24 weeks). At baseline, associated user manual, research staff explain how to use product. Reduction goal to 50% at weeks 0 and 1, 75% at weeks 2 and 4, continue reducing onwards from there 2) EC with no nicotine: EGO e‐cigarette. Cartomizers containing 0 mg/mL nicotine provided throughout the intervention period (24 weeks) Associated user manual, research staff explain how to use product. 3) As (2) but 8 mg/mL nicotine 4) As (2) but 36 mg/mL nicotine |
|
| Outcomes | 0, 1, 2, 4, 8, 12, 16, 20, 24, 28, and 36 weeks. Provision of the condition‐specific product lasted for 24 weeks (intervention period). There was a 12‐week follow‐up period after the intervention period for each condition (36 weeks). NNAL collected at baseline 4, 12 & 24 weeks Cessation: (a) intent‐to‐treat, self‐reported 7‐day point prevalence cigarette abstinence (PPA), biochemically confirmed by exhaled CO<10ppm (7‐day PPA) for each visit up to 24 weeks after randomization (last visit of randomized phase of the trial), with those not attending visits counted as smoking. Additional outcomes included (b) self‐reported 28 or more days of cigarette abstinence at week 24 (biochemically validated by exhaled CO < 10 ppm at weeks 20 and 24), (c) the number (%) of participants in each group who reported at least one full day without smoking a cigarette (no biochemical verification), from week 1 to week 24, and (d) the total number of days on which participants self‐reported being abstinent from cigarettes from week 1 to week 24.
Other outcomes measured
|
|
| Study funding | This research was supported by grants P50DA036105 and U54DA036105 from the National Institute on Drug Abuse of the National Institutes of Health and the Center for Tobacco Products of the US Food and Drug Administration. Data collection was supported by UL1TR002649 at Virginia Commonwealth University and by UL1TR002014 at Penn State University from the National Center for Advancing Translational Sciences of the National Institutes of Health. The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the US Food and Drug Administration. | |
| Author declarations | COC reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. JF reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study, and grants, personal fees, and non‐financial support from Pfizer, outside of the submitted work. AAL reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. JMY reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. LK reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. SV reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study. CB has previously undertaken trials of electronic cigarettes for smoking cessation (with electronic cigarettes purchased from an online retailer [NZVAPOR], electronic cigarette liquid for one trial purchased from Nicopharm, Australia, and nicotine patches supplied by the New Zealand Government via their contract with Novartis [Sydney, Australia]). Neither NZVAPOR nor Nicopharm have links with the tobacco industry. None of these parties had any role in the design, conduct, analysis, or interpretation of the trial findings, or writing of this publication. TE reports grants from National Institute on Drug Abuse and US Food and Drug Administration, during the conduct of the study, and is a paid consultant in litigation against the tobacco industry and also the electronic cigarette industry and is named on one patent for a device that measures the puffing behaviour of electronic cigarette users and on another patent for a smartphone application that determines electronic cigarette device and liquid characteristics. M‐SY reports grants from the National Institute on Drug Abuse and the US Food and Drug Administration, during the conduct of the study. | |
| Notes | Study listed as ongoing study Lopez 2016 in the 2016 review update and as Veldheer 2019 in 2020 and April 2021 updates Cessation data from Foulds which is pre‐print only |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study statistician (M‐SY) prepared site‐specific randomisation lists using the sample function in R version 3.2.0 (blocks of eight). These lists were uploaded onto a study‐specific website that interfaced with the data collection and management system (REDCap)." |
| Allocation concealment (selection bias) | Low risk | “Once a participant has been confirmed eligible for randomization, a computer procedure will assign the participant to the next condition on the list automatically.” 'Only unmasked researchers at each site with no participant contact accessed their list to prepare cartomisers for dispensing.’ |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded for non‐EC arms but given similar level of support/product, so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded for non‐EC arms but given similar level of support/product, so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "188 (36%) of 520 participants were lost to follow‐up by week 24; attrition did not differ by group (39 [30%] of 130 in the cigarette substitute group, 56 [43%] of 130 in the ENDS with 0 mg/mL nicotine group, 49 [38%] of 130 in the ENDS with 8 mg/mL nicotine group, and 44 [34%] of 130 in the ENDS with 36 mg/mL nicotine group; P = 0·15)." |
| Selective reporting (reporting bias) | Low risk | All specified outcomes available or being written up |
Czoli 2019.
| Study characteristics | ||
| Methods | Design: Nonblinded within‐participants cross‐over Recruitment: advertisements placed in newspapers, online, and in local vape shops, and received CAD 295 for participating in the study Setting: Kitchener−Waterloo and Toronto, Ontario, Canada Study start date: September 2015. Study end date: NR |
|
| Participants | Total N: 48 29.2% female; mean age 35.9 (SD 11.7); mean cpd NR; dual EC users at baseline; not motivated to quit Inclusion criteria: > 18+ years; dual users of tobacco cigarettes and EC. Exclusion criteria: serious intentions to quit smoking in the next 6 months; tobacco products, NRT, any smoking cessation medications, participation counselling programs for smoking cessation in the past 7 days; serious cardiac health issues; heart attack or stroke within the last 3 months; cancer within the last year; asthma, chronic obstructive pulmonary disease, a seizure disorder, or any life‐threatening medical conditions with a prognosis of ≤ a year; history of psychosis, schizophrenia, bipolar disorder, or suicidal thoughts. |
|
| Interventions |
EC: own choice (mainly tank) 3 consecutive 7‐day periods in which the use of tobacco cigarettes and e‐cigarettes was experimentally manipulated 4 study conditions: Dual use (e‐cigarette and tobacco cigarette); Tobacco cigarette; E‐cigarette; No product use Virtually all dual users reported using tank systems (92%) and e‐cigarettes with nicotine (94%) To control for order effects, participants were randomly assigned to 1 of 2 condition orders, A or B Following the baseline condition of dual use: Group A participants switched to E‐cigarette use, then to Tobacco cigarette use, and finally to No product use Group B participants switched to Tobacco cigarette use, then to E‐cigarette use, and finally to No product use |
|
| Outcomes | Baseline (visit 1) and after each of the 7‐day periods (visit 2 (week 1), visit 3 (week 2), visit 4 (week 3)) Carbon monoxide Urinary concentration of cotinine Urinary concentrations of 1‐hydroxypyrene (1‐HOP) and 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL) |
|
| Study funding | This research was supported by an Ontario Ministry of Health and LongTerm Care Health System Research Fund grant (#06697 awarded to DH). Additional support was provided by the Canadian Institutes of Health Research (CIHR), the Vanier Canada Graduate Scholarship (CDC), a CIHR and Public Health Agency of Canada, Applied Public Health Chair (DH), and an Ontario Institute for Cancer Research Investigator Award (GTF) | |
| Author declarations | MLG reports grants from and served as an advisory board member to pharmaceutical companies that manufacture smoking cessation drugs. DH has provided paid expert testimony in tobacco litigation on behalf of governments and class‐action plaintiffs on issues related to tobacco product science and regulation. The other authors have no competing interests to declare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details of randomization method given |
| Allocation concealment (selection bias) | High risk | No blinding |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All followed up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
Dawkins 2020.
| Study characteristics | ||
| Methods | Design: Prospective cohort 4‐center pragmatic cluster feasibility trial Recruitment: At homeless centres Setting: 4 homeless centres in the UK Study start date: 1 October 2018; Study end date: 31 March 2020 |
|
| Participants | Total N: 80 N per arm: EC 48; UC 32 Inclusion criteria: adults (≥ 18 years) who smoke accessing homeless support services on a regular basis and also known to staff; daily smokers; smoking status was also biochemically verified by exhaled CO breath. Exclusion criteria: non‐smokers, or using another smoking cessation aid; pregnancy, or unable to consent, e.g. currently intoxicated or unable to speak English; not well known to centre staff. Inclusion based on specific population characteristic: people accessing homeless centres 35% women; mean age 42.7; mean cpd 20; mean FTND: FTCD 5.51 Motivated to quit: “varied considerably; large majority expressed a desire to quit smoking in the near future” EC use at baseline: Not specified |
|
| Interventions |
EC: Refillable Usual care: Written information on quitting smoking (adapted from NHS Choices); signposting to the local stop‐smoking service (SSS) by center staff Intervention: as usual care, plus refillable EC provided once with e‐liquid provided 1 x wk for 4 weeks, Aspire PockeX (tank style), choice of 3 flavors (fruit, menthol, tobacco) and 2 nicotine strengths (12 mg/mL or 18 mg/mL). Written info for EC use and support from center staff, who met once a week to provide e‐liquid and troubleshoot EC use |
|
| Outcomes | Weeks: 4, 12, 24; Clinic visits and self‐report Cessation: CO‐validated sustained at 24 weeks Adverse events and biomarkers: Self‐reported negative effects in EC arm only – each participant asked to rate on scale so cannot meta‐analyse; exhaled CO; unintended consequences Other outcomes measured: Qualitative process evaluation; costs; self‐reported positive and negative affects; recruitment rates; retention; EC/other tobacco/nicotine product use at study end; HRQoL; healthcare service utilization; other drug use/dependence; unintended consequences |
|
| Study funding | This study is funded by the National Institute for Health Research Public Health (project reference: 17/44/29) | |
| Author declarations | SC, AF, JL, CB, AT, DR, IU, LB, SP have no competing interests. PH has received research grant from and provided consultancy to Pfizer. LD has provided consultancy for the pharmaceutical industry relating to the development of smoking cessation products | |
| Notes | New for 2021 update. Authors provided information prior to peer review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Intention was to randomize but were unable to due to practical constraints Quote: “Thus the actual allocation of centres to each arm was a pragmatic decision based on centre readiness and staff/researcher availability though we balance potential confounders and differences in environment by ensuring each cluster (EC and UC) contained one day centre and one residential unit.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Participants joined after cluster randomisation… Allocation was concealed to participants until after the baseline assessment.” Comment: But unclear if allocation was concealed for those recruiting, and allocation would have been known to new participants |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and different levels of support between arms, so performance bias cannot be ruled out |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Cessation (primary outcome) biochemically‐validated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 13/48 (27.1%) lost to follow‐up in the intervention arm and 20/32 (62.5%) lost to follow‐up in the control arm at 24 weeks |
| Selective reporting (reporting bias) | Low risk | All anticipated outcomes reported |
Edmiston 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Subjects were recruited to 10 clinical sites via site databases and using IRB‐approved radio and print ads. Setting: USA Study start date: January 17 2017. Study end date: November 6 2018 |
|
| Participants | Total N: 450 EC Test group 1: classic (tobacco) = 150 EC Test group 2: menthol = 150 Control = 150 Inclusion criteria: smoked ≥ 10 years, smoked an average of ≥ 10 manufactured cigarettes pd for 12 mths. Willing and able to replace their cigarettes for 12 weeks with the assigned test e‐Vapor product. Age 30 to 65 yrs Exclusion criteria: health condition that would jeopardize the safety of the subject or impact the validity of the study results; currently taking medication for depression, asthma or diabetes For a full list of inclusion and exclusion criteria, see publication. Female 51%. Mean age 44.4 (SD 9.73). Mean CPD 17.6 (SD 4.95) |
|
| Interventions | EC: cartridge Arm 1 Experimental: Test 1 EC classic (tobacco) Exclusive ad libitum use of test product MarkTen Bold Classic (test product 1) 4.0% nicotine by weight without use of any other type of tobacco/nicotine containing product, for the entire duration of study participation. This replaced test e‐Vapor Product NuMark LLC, MarkTen® XL Bold CLASSIC* (as no longer sold). Arm 2 Experimental: Test 2 EC menthol Exclusive ad libitum use of test products MarkTen Bold Menthol (test product 2) 4.0% nicotine by weight Arm 1 and 2 subjects were to completely replace their cigarettes with the test EVPs. Subjects had 7 days to switch to EVPs prior to clinic visits. Arm 3 No Intervention: Control Assigned to continue smoking their own brand cigarettes under ad libitum conditions |
|
| Outcomes | Baseline, weeks 1, 3, 6, 9, and 12 Baseline weeks 1, 6 & 12 (blood & urine). Weeks 1, 3, 6, 9, and 12 (eCO) Lung function was assessed at screening, baseline, at week 12 of study 1, [and at weeks 18 and 24 of study 2]: FEV1, percent of predicted FEV1, FVC, percent of predicted FVC, FEV1/FVC, and percent of predicted FEV1/FVC, forced expiratory flow at 25%–75% (FEF). NNAL urinary total 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol and its glucuronides (ng/g creatinine), WBC, COHb, HDLC Adverse events (AEs) and medications were recorded and monitored throughout the study. |
|
| Study funding | This study was funded by Altria Client Services LLC. [Altria is the parent company of Philip Morris USA (producer of Marlboro cigarettes), John Middleton, Inc., U.S. Smokeless Tobacco Company, Inc., and Philip Morris Capital Corporation.] |
|
| Author declarations | All authors were employees of Altria Client Services LLC at the time of the study. | |
| Notes | Paper reported two studies; only study one was eligible. New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised – no further information in paper or supplementary materials. |
| Allocation concealment (selection bias) | Unclear risk | No detail provided |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No detail provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 50% attrition |
| Selective reporting (reporting bias) | Low risk | Outcomes reported |
Eisenberg 2020.
| Study characteristics | ||
| Methods | Design: 3‐arm RCT Recruitment: Community Setting: Canada Study start date: November 2016. Study end date: September 2019. |
|
| Participants | Total N: 376; Nicotine e‐cigarettes = 128; Non‐nicotine e‐cigarettes = 127; Counselling (control) = 121 47% female; mean age 52.66; mean cpd 21; mean FTND 6 (SD 2). Motivated to quit ‐ Yes Inclusion criteria: active smoker, ≥10 CPD for past year; ≥ 18 years; motivated to quit according to the Motivation To Stop Scale (MTSS) (level 5 or higher); provide informed consent in English or French; available for follow‐up (1 year). Exclusion criteria: medical condition with a prognosis < 1 year; current or recent cancer (≤ 1 yr in remission); pregnancy/breastfeeding; current/ recent use any pharmacotherapy or behavioural therapy for smoking cessation (e.g. NRT, bupropion, varenicline, or counselling); any EC use (nicotine/non‐nicotine) in past 60 days, or ever use of any EC ≥ 7 days consecutively; psychosis, schizophrenia, or bipolar disorder; ≤ 1 mth following myocardial infarction, life‐threatening arrhythmia, severe or worsening angina pectoris, or cerebral vascular accident; illegal drug use past yr (excluding marijuana); planned use of tobacco products other than conventional cigarettes (e.g. cigarillos, cigars, snuff, shisha, etc.) or marijuana during the study period. |
|
| Interventions |
EC: Cig‐a‐like Nicotine e‐cigarettes plus counselling: 12 weeks of e‐cigarettes. Rechargeable base with prefilled, disposable, tobacco‐flavoured liquid cartridges (15 or 0 mg nicotine/mL), which were produced specifically for use in clinical studies (purchased from NJOY Inc, Scottsdale, Arizona). 21 cartridges at baseline with additional cartridges supplied as needed. Nicotine and non‐nicotine e‐cigarettes were identical in appearance. Instructed to be used as desired. No schedule for e‐cigarette tapering, but participants were aware that they would return their e‐cigarettes after 12 weeks Participants received individual smoking cessation and relapse prevention counselling (minimum 30 minutes at baseline, 10 minutes during telephone follow‐ups, and 15‐20 minutes at clinic visits). Individualized quit plans Non‐nicotine e‐cigarettes plus counselling: As above with 0 mg nicotine/mL in liquid cartridge Counselling (control): Participants received individual smoking cessation and relapse prevention counselling (minimum 30 minutes at baseline, 10 minutes during telephone follow‐ups, and 15‐20 minutes at clinic visits). Individualized quit plans |
|
| Outcomes | Follow‐up was conducted by telephone at weeks 1, 2, 8, and 18, and at clinic visits at weeks 4, 12, 24, and 52 Self‐reported smoking (7‐day recall), adherence, and adverse events (AEs) were assessed during follow‐up contacts Biochemically‐validated 7‐day point prevalence smoking abstinence at 4, 12 and 24 weeks, defined as self‐reported abstinence in the past 7 days with exhaled carbon monoxide < 11 ppm At baseline: cpd; FTND; Glover‐Nilsson Smoking Behavioral Questionnaire (to assess behavioural dependence on smoking); and Beck Depression Inventory II (BDI‐II; to assess depressive symptoms) |
|
| Study funding | This trial was funded by the Canadian Institutes of Health Research (CIHR; funding reference No. 133727 and 155969). Both nicotine e‐cigarettes and non‐nicotine e‐cigarettes were purchased from NJOY Inc (Scottsdale, Arizona) | |
| Author declarations | Dr Eisenberg reported receiving educational grants from Pfizer Inc for providing continuing medical education in cardiology. Dr Wilderman reported receiving financial compensation from Pfizer Inc for his involvement in a smoking cessation study using varenicline. Dr Filion reported receiving salary support from the Fonds de Recherche du Quebec, a William Dawson Scholar award from McGill University, and personal fees from Institut National D’excellence en Santé et Services Sociaux. No other disclosures were reported | |
| Notes | New cessation and adverse event data for 2021 update. Previously listed as NCT02417467 (included with SAE data only) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Eligible participants were randomized via an online central randomization system. The system used a computer‐generated randomization list containing permuted blocks of 6 and 9, stratified by centre |
| Allocation concealment (selection bias) | Low risk | Participants, investigators, and study personnel were blinded to nicotine content in the e‐cigarette groups |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, investigators, and study personnel were blinded to nicotine content in the e‐cigarette groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, investigators, and study personnel were blinded to nicotine content in the e‐cigarette groups |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low numbers lost to follow‐up, treated as ITT |
| Selective reporting (reporting bias) | Low risk | Due to a prolonged and unforeseen delay in e‐cigarette manufacturing, enrolment was paused on 27 September 2019, and then terminated on 14 November 2019. Given reduced power, the timing of the primary endpoint was changed from 52 weeks to 12 weeks on 04 December 2019. No 12‐month follow‐up but this was for manufacturing reasons and was reported |
Eisenhofer 2015.
| Study characteristics | ||
| Methods | Design: RCT Setting: USA |
|
| Participants | 11 EC = 4; NRT = 7 Veterans who meet DSM criteria for tobacco use disorder 18% female. Mean age 52.6. Mean cpd 26.4. Mean ftnd 7.5. 64% African American |
|
| Interventions | EC type: cartridge Arm 1: Electronic cigarettes 16 mg cartridge. Arm 2: NRT. Participants attended thrice‐weekly visits during the first 2 weeks (week 1‐“baseline” with participants smoking ad libitum) and attended five visits during the third week (week 3‐“efficacy” with participants smoking as little as possible while using NRT or E‐cigs) |
|
| Outcomes | Self‐reports of cigarettes smoked in last 24 hours, confirmed by breath CO levels and salivary cotinine | |
| Study funding | This work was conducted at and supported by resources at the MEDVAMC, including a MEDVAMC Research Enhancement Seed Grant | |
| Author declarations | NS | |
| Notes | Study information extracted from conference abstract only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Veterans were randomized to either NRT (16mg patch;N=7) or E‐cigs (16mgcartridge;N=4).” No further information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No blinding but active interventions provided to both arms |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "CO levels and salivary cotinine were recorded during each visit.' 'Self‐reports of cigarettes smoked in last 24 h, and this was confirmed by significant reductions of breath CO levels by NRT (t = 3.7, P = 0.01) and E‐cigs (t = 3.9, P = .03)." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial record available to determine whether all prespecified outcomes are reported |
Ely 2013.
| Study characteristics | ||
| Methods | Design: Prospective cohort Recruitment: Letter sent to family practice patients who currently smoked Setting: Single family practice, Colorado USA Study start date: 14 April 2013; Study end date: Not specified |
|
| Participants | Letters sent to 640 patients, 48 chose to participate and 44 completed the programme, 4 were lost to follow‐up Inclusion criteria:
Exclusion criteria:
Of the 44 participants, 66% women, all non‐Hispanic/white, aged 20‐75 (30% were age 51‐60), 57% had a high school education or less Motivated to quit: Want to quit or switch from tobacco cigarettes to ECs E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like The 6‐month smoking cessation programme was based on The '5 A's' model and transtheoretical model. Options for treatment were discussed with each participant at the start of the programme. All used an EC, with 16 using bupropion and 2 using varenicline as well Participants were provided with written information on “blu cig” and “smoke tip” ECs, about cost, availability, nicotine dosage options |
|
| Outcomes | Phone follow‐ups at 2 weeks, 1 month, 3 months, and 6 months At completion of programme (using ITT) Abstinence from smoking and EC use Abstinence from smoking but not EC use ≥ 50% reduction of baseline cigarette consumption (still using ECs) |
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/48 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
| Other bias | Unclear risk | No definition of abstinence provided Not clear if 'completed programme' was at 6 months. |
Felicione 2019.
| Study characteristics | ||
| Methods | Design: Double‐blind RCT Recruitment: People who smoke were recruited from an outpatient opioid‐maintenance clinic in West Virginia, USA Setting: Outpatient opioid‐maintenance clinic in West Virginia, USA Study start date/Study end date: Not reported |
|
| Participants | Total N: 25; N per arm: Placebo (non‐nicotine): 11; Active (18 mg/mL nicotine): 14 Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: People who smoke who were currently receiving a buprenorphine/naloxone combination in sublingual form, and had maintained sobriety from opioids and all other illicit substances for at least 90 consecutive days as verified via urinalysis 73.0% women; mean age 32.5; mean cpd 22; mean FTND 5.8 Motivated to quit: Quit ladder Score (range 1‐10): 5.6 average |
|
| Interventions |
EC: Refillable Compared nicotine (18 mg/mL) to non‐nicotine EC. Second‐generation EC consisted of the eGo‐T battery (900mAh, 3.3 V constant output) (Joyetech; Irvine, CA) and the Kanger mini Protank‐II, 1.5 ml Pyrex glass tank with a drip tip and atomizer head coils (KangerTech; China), choice between tobacco (n = 15) and menthol (n = 10) flavoured liquid (2‐week supply). Participants were then trained in EC device operation, including assembly, liquid filling, manual battery operation, and cleaning/storage. Practised puffing on EC in the presence of a team member, and asked questions if needed. Participants instructed to use their ECIG ad libitum every day for 2 weeks |
|
| Outcomes | Baseline (day 1), 14 days, 28 days for clinic measures. Data also collected via text‐messages over 2‐week intervention period Withdrawal/side effects: Every evening during the 2‐week intervention period, participants rated a variety of effects possibly experienced as a result of nicotine/tobacco withdrawal and/or use of the ECIG: nausea, dizziness, throat irritation/soreness, cough, dry mouth, headache, shortness of breath, irritability/frustration/anger, craving/urge to smoke, and other. Each item was rated on a continuous scale that ranged from 0 (not at all) to 100 (extremely) Expired air CO Other outcomes: Self‐reported cigarette and EC use; readiness to quit at day 1, 14 and 28 |
|
| Study funding | Not reported | |
| Author declarations | Not reported | |
| Notes | New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Using a mixed factorial, simple randomization, double‐blind study design, participants were assigned to one of two ECIG conditions…” (No further details given) |
| Allocation concealment (selection bias) | Unclear risk | No details on allocation given. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: “double‐blind study design”, no further detail given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: “double‐blind study design”, no further details given. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “…80.6% completed the two‐week intervention (n=14 active; n=11 placebo), and 70.9% also completed the follow‐up session (n=13 active; n=9 placebo).” Active follow‐up completion rate: 13/14 = 93%; Placebo follow‐up completion rate: 9/11= 82% N.B. 6 participants were disqualified post‐randomization: Quote: “Of those individuals who were screened for the study, 93.9% were enrolled (n = 18 active; n = 13 placebo); two individuals who were ineligible provided an expired air CO level < 10 ppm. Six of the enrolled participants (n = 4 active and n = 2 placebo; n = 5 tobacco flavour and n = 1 menthol flavour) were disqualified for responding to 7 or fewer days of text messages.” |
| Selective reporting (reporting bias) | Unclear risk | All measures listed were reported: Self‐reported cigarette use, text message‐based cigarette use, e‐cig use, expired air CO, readiness to quit ladder, withdrawal/side effect; No study protocol or clinical trial record available to confirm all intended outcome measures were reported |
George 2019.
| Study characteristics | ||
| Methods | Design: Prospective, randomized controlled trial with a parallel, non‐randomized preference cohort Recruitment: Participants were recruited from local advertisements, smoking cessation databases, and visits to local businesses, as well as via the Scottish Primary Care Research Network Setting: Single tertiary research centre, UK Study start date: August 2016; Study end date: July 2018 |
|
| Participants | Total N: 114 in “final evaluable dataset” (145 recruited into the trial) N per arm: Tobacco cigarettes (TC): 40; EC nicotine (16 mg): 37; EC‐Nicotine‐free: 37 Inclusion criteria: ≥ 18 years of age who had smoked ≥ 15 cigarettes/day for at least 2 years; free from established CV disease, diabetes, and chronic kidney disease (& not on medication for those conditions); willing to stop tobacco cigarettes for period of study if required & not to use ECif required. Exclusion criteria: pregnant /breastfeeding; not abstaining from sex or using effective contraception; medication for CVD; history of CVD (excluding hypertension), diabetes, active malignance or chronic renal disease; nut allergy; participation in another clinical trial (other than observational trials and registries) with an investigational product and/or intervention within 30 days before visit 1. 65.4% women; mean age 46.9; mean cpd 18.7 Motivated to quit: TC group: No; EC nicotine (16 mg): Yes; EC‐Nicotine‐free: Yes. |
|
| Interventions |
EC: Cig‐a‐like EC nicotine (16 mg) arm: EC containing 16 mg nicotine (Vapourlites Starter Kit with XR5 16 mg nicotine cartomizer; Vapourlites, Peterlee, United Kingdom) EC‐Nicotine‐free arm: Nicotine‐free EC plus nicotine flavoring (Vapourlites Starter Kit with 0 mg nicotine cartomizer) (non‐randomized) TC arm: continued their usual daily smoking habits and did not use EC for the 4‐week period of the trial |
|
| Outcomes | Week 4 Adverse events and biomarkers: BP, heart rate, adverse events Other outcomes measured: Endothelial function, oxidized low‐density lipoprotein, high‐sensitivity C‐reactive protein, tissue plasminogen activator, and platelet activation inhibitor‐1 |
|
| Study funding | "The VESUVIUS (Vascular Effects of Regular Cigarettes Versus Electronic Cigarette Use) trial was funded by the British Heart Foundation (grant PG/15/64/31681); and supported by Immunoassay Biomarker Core Laboratory, University of Dundee, the Tayside Medical Sciences Centre, and the NHS Tayside Smoking Cessation Service. The funder had no role in the study design, data collection, data analysis, data interpretation, writing of the report, or in the decision to submit for publication." | |
| Author declarations | "Dr. Donnan has received research grants from AbbVie, Shire, and Gilead Sciences. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose." | |
| Notes | New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Consented participants who were willing to quit smoking were randomized to one of the EC arms in a 1:1 fashion using a centrally controlled web‐based good clinical practices– compliant randomization system to either: 1) EC containing 16 mg nicotine; or 2) nicotine‐free EC plus nicotine flavoring because it was considered by the institutional ethics committee as ethically unacceptable to randomize those who were willing to quit smoking into a smoking arm. Those unwilling to consider quitting smoking continued in the parallel preference TC cohort |
| Allocation concealment (selection bias) | Low risk | Central randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not specified |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not blinded and AE/SAE data are self‐report only. For other outcomes, low risk as objectively measured: Quote: “Patients fasted overnight and measurements were conducted at baseline and 1 month according to the International Brachial Artery Reactivity Task Force guidelines (19) by a single operator (M.H.) blinded to study allocation at a single site.” “Pulse wave velocity and augmentation index were measured at baseline and 1 month by a single operator (M.H.) blinded to study allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number randomized not provided per group. Quote: “A total of 145 patients were recruited into the trial (Figure 3). A final number of 114 patients (40 TC, 37 EC‐nicotine, 37 EC‐nicotine‐free) completed both visits.” |
| Selective reporting (reporting bias) | Low risk | Clinical trial record lists: Change in FMD; Change in oxidized LDL; Change in PAI‐1; Change in hs‐CRP; Change in Pulse Wave Velocity; Change in tPA; Change in Augmentation Index@75bpm All reported in the paper |
Goniewicz 2017.
| Study characteristics | ||
| Methods | Design: Longitudinal within‐subjects observational Recruitment: Advertisements in the media, the internet, posted advertisements in clinics and offices, and by word of mouth Setting: University, Poland Study start date: March 2011; Study end date: June 2011 |
|
| Participants | Total N: 22 started out and 2 dropped out in the first week due to an adverse event (nausea) and inability to commit to clinic visits. This resulted in analytic sample of 20 Inclusion criteria:
Exclusion criteria:
60% women; mean age 31; mean cpd 16; mean FTND 3.9 Motivated to quit: At the time of screening, 95% of participants (n = 19) reported planning to quit smoking, with 80% (n = 16) reporting that they have made at least 1 quit attempt prior to involvement in the study E cigarette use at baseline: Not reported |
|
| Interventions |
EC: Cig‐a‐like Pen‐style M201 e‐cigarettes for 2 weeks, with an automatically‐operated battery with an output power of 4.6 Volts (280 mAh) and the heating element resistance of 3.6 – 3.8 Ohms. At baseline, provided with EC (M201 Mild, Poland) with 20 tobacco‐flavoured cartridges a week containing 11.0 ± 1.5 mg of nicotine in a mixture of propylene glycol and vegetable glycerin (50:50). Encouraged to substitute their regular cigarettes with the e‐cigarette for 2 weeks and refrain from smoking |
|
| Outcomes | Day 7, Day 14 Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | “This work was supported by the Ministry of Science and Higher Education of Poland (grant number N N404 025638). Instrumentation and analytical chemistry at UCSF was supported by the National Institutes of Health, P30 DA012393 and S10 RR026437. The study sponsor had no involvement in the study design, collection, analysis, and interpretation of data, the writing of the manuscript or the decision to submit the manuscript for publication.” | |
| Author declarations | "MLG was a faculty member of the Medical University of Silesia, Poland during the study. He received a research grant from Pfizer, a pharmaceutical company that markets smoking cessation medications. MLG and NLB have been consultants to pharmaceutical companies that market smoking cessation medications. NLB has been an expert witness in litigation against tobacco companies. The other authors declare no potential conflicts of interest." | |
| Notes | New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts – 1 for nausea, 1 could not complete clinic visits. Analysis based on 20 completers |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Guillaumier 2018.
| Study characteristics | ||
| Methods | Design: Pragmatic, open‐label, single‐centre, 2‐arm randomized controlled trial Recruitment: Withdrawal service in Melbourne, Australia Setting: Substance use disorder treatment setting, and following discharge, community setting, Melbourne, Australia Study start date: 1 August 2017; Study end date: April 2019. |
|
| Participants | Total N: 100 N per arm: EC intervention = 50; NRT Control = 50 Inclusion criteria: ≥ 18 years; tobacco smoker on entering the residential service; capacity to consent and able to understand the participant materials. Exclusion criteria: used an END containing nicotine in the past month; pregnancy / breastfeeding; currently enrolled in another study; scheduled to be transferred to a long‐term rehabilitation unit following discharge from the residential withdrawal unit. Inclusion based on specific population characteristic: Participants were discharged from a smoke‐free alcohol or other drugs (AOD) residential withdrawal service. 32% women; mean age 40.9; mean cpd 21 Motivated to quit: Median (SD) = 7.3 (2.4) of 1 to 10 scale with 10 "highly motivated" |
|
| Interventions |
EC: Refillable. Up to an hours training session, information pack. Innokin Endura T22 starter kit and refill liquid (Nicophar). 4‐week supply of liquid nicotine, with further supplies of liquid nicotine mailed twice at 4‐ week intervals. Dosing schedule of e‐liquid dependent nicotine dependence score: high‐nicotine‐dependence category assigned initial 4‐week e‐liquid supply (total 8 × 10 ml bottles) consisting of: 2 × 10 ml bottles of 18 mg e‐liquid and 6 × 10 ml bottles of 12 mg e‐liquid. The second and third batches = 8 × 10 ml bottles of 12 mg e‐liquid only. Participants scoring in the moderate‐ and low‐dependence categories: three 4‐week supplies of 8 × 10 ml bottles of 12 mg e‐liquid. Participants given 1‐week supply of nicotine patches for use while getting used to the EC. NRT control: Information pack, 12 weeks NRT on the same schedule as for ENDs. 4‐week supply of patches plus a nicotine spray and inhaler, followed by refills including patches plus inhaler, gum and lozenges. Both groups received proactive referral to quitline counselling (call‐back service), which provides calls at pre‐discharge and on days 1, 3, 7, 14 and 28 post‐discharge, with an emphasis on relapse prevention. Counsellors trained on the use of ENDs. |
|
| Outcomes | Week 6, 12; self‐report. Adverse events collected Other outcomes measured:
|
|
| Study funding | "The study is supported by a VicHealth Innovation Research Grant (2016–0096). AG is supported by a post‐doctoral fellowship from the Heart Foundation. ALB is supported by an Australian National Health and Medical Research Council (NHMRC) senior research fellowship and a Faculty of Health and Medicine, University of Newcastle Gladys M Brawn senior research fellowship. BB is supported by an Australian NHMRC career development fellowship (GNT1063206) and a Faculty of Health and Medicine, University of Newcastle Gladys M Brawn career development fellowship." "This study was supported by a VicHealth Innovation Research Grant (2016‐0096)." |
|
| Author declarations | "The authors declare that they have no competing interests." "None to declare." |
|
| Notes | New for 2020 update; additional data originally provided by authors and subsequently published | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Upon completing the baseline survey, participants were randomised 1:1 to an intervention via a computer‐sequenced 4–6 block randomisation embedded in the tablet device software.” |
| Allocation concealment (selection bias) | Low risk | Quote: “At the end of the baseline survey, participants will be randomised 1:1 to an intervention via a computer‐sequenced 4–6 block randomisation embedded in the iPad.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Participants were informed of their intervention group by the RA and provided with a training session of up to one hour.” “Due to the nature of the intervention, neither participants nor staff can be blinded to allocation. However, the data safety monitoring committee and the statistician responsible for the data analysis will be blinded.” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | No biochemical validation, self‐report data |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “At 6 and 12‐weeks, 63 participants (63%) and 50 participants (50%) were followed up, respectively. While slightly higher retention rates were evidence in the VNP group at 6‐weeks (68% vs 58% in NRT group; P = 0.30); there were no differences between groups at 12‐weeks (25 re‐contacted in both arms; i.e. 50%).” |
| Selective reporting (reporting bias) | Low risk | Unpublished findings provided by authors report on all outcomes mentioned in the protocol |
Hajek 2015a.
| Study characteristics | ||
| Methods | Design: Prospective cohort, intervention provided Recruitment: People who smoke attending stop‐smoking service Study start date: March 2014; Study end date: March 2015 Setting: Stop‐smoking service, London, UK |
|
| Participants | Total N: 100 (69 of whom accepted offer of EC) Inclusion criteria:
38% women (those who accepted) 55% women (those who declined), mean age 41, mean cpd 14, all motivated to quit. EC use at baseline not specified but some who declined EC offer had used EC in the past Motivated to quit: Yes E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like and refillable EC: offered to all people who smoke joining service; offered choice of ‘cig‐a‐like’ (Gamucci, 1.6% or 2.2% nicotine per ml) product or tank model (EVOD, 1.8%; later replaced with Aspire product due to leakage issues). 69% of those offered received an EC on TQD Medication: Offered stop‐smoking medications including NRT and varenicline as in standard protocol. Of EC users 33% opted to also use NRT, 29% varenicline, 38% nothing Support: weekly, as in standard protocol |
|
| Outcomes | Adverse events collected throughout, method for collection unclear Also collected: 4‐week biochemically‐validated abstinence, participant feedback, cost |
|
| Study funding | "The pilot study was sponsored by City of London Corporation." | |
| Author declarations | "Peter Hajek received research funds from and provided consultancy to manufacturers of smoking cessation medications. The remaining authors have no conflicts of interest to declare." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 26% lost in EC group, dropout rate in EC decliners not reported. Reasons for dropout not stated |
| Selective reporting (reporting bias) | Unclear risk | Unclear which outcomes authors set out to collect, no protocol available |
Hajek 2019.
| Study characteristics | ||
| Methods | Design: Multicentre pragmatic randomized controlled trial to examine the efficacy of e‐cigarettes compared with nicotine replacement therapy Recruitment: participants attending UK stop‐smoking service and via social media Setting: U.K. National Health Service stop‐smoking services Study start date: 1 April 2015; Study end date: 31 March 2018 |
|
| Participants | Total N: 886 N per arm: EC: 439; NRT: 447 Inclusion criteria:
Exclusion criteria:
48% women; median age 41; median cpd 15 ; mean FTND 4.6; 41.5% reported past use of ECs Motivated to quit: Not reported |
|
| Interventions |
EC: Refillable NRT: Informed of range of NRT products and selected preferred product, encouraged to use combination. Participants free to switch products. Supplies provided for up to 3 months EC: Starter pack (1 Kit, Aspire UK) provided along with 30 ml bottle of Tobacco Royale flavour e‐liquid, concentration 18 mg/mL. Participants showed how to use and asked to purchase future e‐liquid online or from local vape shops and to buy different EC device if the 1 provided did not meet their needs. Enouraged to experiment with e‐liquids of different strengths and flavors. If unable to obtain own supply, provided with further 10‐ml bottle (not proactively offered). Oral and written info on how to operate EC Both arms received multi‐session behavioural support as per UK stop‐smoking service practice (one‐to‐one sessions weekly with local clinicians, exhaled CO monitored for at least 4 weeks post‐TQD); signed behavioural contract not to use other therapy for at least 4 weeks |
|
| Outcomes | Weeks 4, 26 and 52 Cessation: Sustained and biochemically‐validated CO < 8 ppm Adverse events and biomarkers: “adverse reactions”: presence or absence of nausea, sleep disturbance and throat and mouth irritation, and respiratory symptoms (presence or absence of shortness of breath, wheezing, coughing and phlegm), death Other outcomes measured:
|
|
| Study funding | “Supported by the National Institute for Health Research (NIHR) Health Technology Assessment Programme (project number, 12/167/135) and by a grant (A16893) from the Cancer Research UK Prevention Trials Unit.” | |
| Author declarations | From ICJME disclosure forms: “Miss Natalie Bisal has nothing to disclose. Dr. Dawkins reports personal fees from Johnson & Johnson, outside the submitted work; Dr. Goniewicz reports personal fees from Johnson and Johnson, outside the submitted work; Dr. Hajek reports grants and personal fees from Pfizer, outside the submitted work; Ms. Li reports grants from NCCHTA, during the conduct of the study; Dr. McRobbie reports grants from NIHR HTA programme, during the conduct of the study; personal fees from Pfizer, personal fees from Johnson & Johnson, outside the submitted work; Dr. Myers Smith has nothing to disclose. Dr. Parrott has nothing to disclose. Dr. Pesola has nothing to disclose. Mrs Anna Phillips‐Waller has nothing to disclose. Dr. Przulj reports grants from Pfizer, outside the submitted work; Dr. Ross has nothing to disclose. Dr. Sasieni has nothing to disclose. Ms. Wu has nothing to disclose." | |
| Notes | New for 2020 update, listed as ongoing study ISRCTN60477608 in 2016 review update Note higher use of allocated product at 12 m in intervention group compared to control group: “Among participants with 1‐year abstinence, 80% (63 of 79) were using e‐cigarettes at 52 weeks in the e‐cigarette group and 9% (4 of 44) were using nicotine replacement in the nicotine‐replacement group.” |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization took place on the quit date to limit differential dropout. Randomization sequences (1:1 ratio in permuted blocks of 20, stratified according to trial site) were generated with the use of a pseudorandom number generator in Stata software and were embedded into an application that only revealed the next treatment assignment once a participant had been entered into the database.” |
| Allocation concealment (selection bias) | Low risk | Refer to 'Random sequence generation'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but as both arms contained active interventions performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At 12 months: EC Arm: 356/439 NRT Arm: 342/447 |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Hajek 2022.
| Study characteristics | ||
| Methods | Design: RCT multicentre Participants: Pregnant smokers (12 to 24 weeks gestation) who smoke daily and are interested in stopping smoking Setting: Maternity services across the UK. 23 hospital sites across England and one National Health Service Stop Smoking Service in Scotland Recruitment: Recruitment was managed by research midwives in England and by the Stop Smoking Service in Scotland. Participants were identified from patient records and sent study information and invitation letters or invited via telephone, email or text; approached in person when attending antenatal hospital appointments; referred by community midwives or stop‐smoking advisors; or self‐referred via posters advertising the study at the sites’ antenatal clinics. Study start date: 01/05/2017. Study end date: 26/11/2020 |
|
| Participants | Total N: 1140 EC arm: 571 NRT arm: (nicotine patches) 569 Inclusion criteria: 12 to 24 weeks pregnant, daily smoker, wants help with stopping smoking. Willing to be randomised to use either NRT or EC and agreeing to use only the allocated stop‐smoking product for at least the first 4 weeks of their quit attempt. Exclusion criteria: Allergy to nicotine skin patches. Current daily use of NRT or e‐cigarettes, and serious medical problems or high‐risk pregnancy Inclusion based on specific population characteristic: Pregnant women Female 100%. Mean age 27. Mean CPD 10 E‐cigarette use at baseline: No Motivated to quit: Yes |
|
| Interventions | EC: Refillable Arm 1: EC Participants were sent an EU Tobacco Product Directive‐compliant refillable e‐cigarette starter kit (One Kit by the UK E‐cig Store), together with two 10 mL bottles of tobacco‐flavoured e‐cigarette liquid (1.8% nicotine; 70% propylene glycol and 30% vegetable glycerol), a pack of five replacement coils, and an instruction leaflet (Supplementary Data, Appendix 5). Further supplies of e‐cigarette liquid were posted on request for up to 8 weeks. A lower strength e‐cigarette liquid (1.1%) and e‐cigarette liquid with fruit flavour were available as alternatives. Participants were encouraged to source e‐cigarette liquids of the strength and flavour they liked, as well as different e‐cigarette devices, and arrange their own supplies after 8 weeks if needed. The cost of the kit provided by the study was £22.75 and the cost of e‐cigarette liquid was up to £24 for an 8‐week supply. Products used during the initial 4 weeks (n = 344) # * N (%): Refillable e‐cigarettes 324 (94.2%); Cig‐a‐like 1 (0.3%); Cartridge/Pod 1 (0.3%); Information missing 18 (5.2%). Nicotine strength N (%): 0 mg/mL 7 (2.0); 1‐10 mg/mL 47 (13.7); 11‐20 mg/mL 199 (57.9); Information missing 91 (26.5) Arm 2: NRT ‐ Nicotine patches Participants were sent an initial 2‐week supply of Nicorette Invisi 15 mg 16 h nicotine patches with manufacturer instruction leaflets and instructed to apply patches every day upon waking, and remove them before bedtime. Further supplies were posted on request for up to 8 weeks. A lower strength patch (10 mg 16 h) was available as an alternative. Participants were encouraged to access further supplies themselves via their general practitioner or local Stop Smoking Service. This could be patches and/or other NRT products such as nicotine chewing gum, inhalator or mouth spray, to use in addition to the patch alone if needed. In the United Kingdom, pregnant women who smoke receive NRT free of charge. Behavioural support was given that accompanied both study arms. Participants received six phone calls from stop‐smoking advisors who followed the practice of the UK Stop Smoking Service 61. |
|
| Outcomes | Baseline, weeks 1‐4 after target quit date (TQD) (phone call), end of pregnancy (EOP) at least 6 months (saliva & CO), 3 mths post‐partum (phone call) Cessation: saliva samples & carbon monoxide readings collected AEs & SAEs Continued use of study product Flavours EC nicotine strength |
|
| Study funding | The study was funded by the National Institute of Health Research, Health Technology Programme (ref. no. 15/57/85). The funder had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or the decision to submit the manuscript for publication. For part of the trial F.P. was supported by Cancer Research UK (grant no. C8162/A25356). | |
| Author declarations | P.H. provided consultancy to and received research funding from Pfizer. D.P. received research funding from Pfizer. H.M. has received honoraria for speaking at smoking cessation educational events and sitting on an advisory board organized by Pfizer. All other authors have no competing interests. | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An independent statistician developed the randomization sequence using permuted block randomization with a block size of at least 6 and a maximum of 12". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization list was accessible only to the independent statistician, on a secure server. Researchers conducting randomization used the database application to inform the participants of their study arm allocation. Researchers conducting follow‐up calls were blind to treatment allocation until the follow‐up contact was made". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants received equally intensive interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Researchers conducting follow‐up calls were blind to treatment allocation until the follow‐up contact was made. Once contact was made and the trial application was opened, condition‐specific questions were visible on the computer screen. The trial statistician was blind to participant allocation until the analysis of the primary and secondary outcomes was complete. This was achieved by extracting and importing into Stata only the baseline characteristics, study arm and smoking status variables in the first stage of the analysis. Variables coding treatment adherence and product use were extracted only after the primary and secondary outcome analyses were completed". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | EC arm 515/571. NRT arm 495/569 |
| Selective reporting (reporting bias) | Low risk | Analysis prespecified |
Halpern 2018.
| Study characteristics | ||
| Methods | Design: Randomized clinical trial Recruitment: Eligible participants were employees and their spouses at 54 companies that used Vitality wellness programs Setting: Online resources via workplace setting (54 companies), USA Study start date: First phase of recruitment October 2014, second phase November 2015 (to meet recruitment target); Study end date: 20 April 2017 |
|
| Participants | Total N: 6006 N per arm: Usual care: 813; Free e‐cigarettes: 1199; Free cessation aids: 1588; Reward incentives plus free cessation aids: 1198; Redeemable deposit plus free cessation aids: 1208. Inclusion criteria:
Exclusion criteria:
51.1% women; median age 44; median cpd 10 Ecig use at baseline: 10.7% current use; 23.1% past but not current use; 39.7% never used ECs Motivated to quit: Unselected sample (total sample): 9.2% no plan to quit; 61.6% want to quit later; 27.7% want to quit/need help |
|
| Interventions |
EC: Cig‐a‐like a) Usual care: Standardized Vitality programme aimed at promoting tobacco cessation. This programme includes existing employee benefits for quitting and the use of text/email messages to encourage tobacco cessation b) as (a), plus free EC: Free NJOY e‐cigarettes (including battery sticks, a USB charger, and up to 20 chambers with 1.0 to 1.5% nicotine per week in participants’ chosen flavors). Use of all products was free until 6 months after the quit date c) as (b) plus access to free NRT, bupropion or varenicline d) as (c) plus incentives across 6 m for testing negative for tobacco use e) as (c) plus provide money at start and lose money from this fund if they do not test negative across 6 m |
|
| Outcomes | Months 1, 3, 6 and 12 Cessation: Sustained smoking abstinence for 6 months, biochemical validation (urine cotinine, anabasine and blood carboxyhemoglobin) Other outcomes measured: Costs |
|
| Study funding | "Supported by a grant from the Vitality Institute to the University of Pennsylvania Center for Health Incentives and Behavioral Economics." | |
| Author declarations | "Disclosure forms provided by the authors are available with the full text of this article at NEJM.org. Check these and: Dr. Troxel reports other from VAL Health, outside the submitted work. Dr. Volpp reports grants and personal fees from CVS Health, personal fees from VAL Health, grants from Humana, grants from Merck, grants from Weight Watchers, grants from Hawaii Medical Services Association, grants from Oscar Health Insurance, outside the submitted work. All of the other authors state that they have nothing to disclose." | |
| Notes | New for 2020 update. Study listed as ongoing study NCT02328794 in 2016 review update Only arms (a) and (b) included in our analyses. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and different amounts of support given to each group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | At 12 months very low numbers completed biochemical validation. Submitted a sample n = CG:1, free e‐cigs;4, free cessation:5, rewards: 14, deposits:16 |
| Selective reporting (reporting bias) | Low risk | Expected outcomes reported and checked with trial registration |
Hatsukami 2020.
| Study characteristics | ||
| Methods | Design: randomized trial Recruitment: Media advertisements Setting: Clinic visits in community, USA Study start date: 25 November 2014; Study end date: 2 December 2018 |
|
| Participants | Total N: 264 N per arm: Usual brand: 36; AD‐E: 76; CS‐E: 76; CS‐NRT: 76. Inclusion criteria: ≥ 18 years of age; smoking ≥ 5 cpd for the past year with a breath CO ≥10 ppm or NicAlert test = level 6 if CO < 10 ppm; instable physical and mental health. Exclusion criteria: serious quit attempt in the past 3 mths; recent (< 3 months) alcohol or drug abuse problems; regular use of other nicotine or tobacco products (e.g. > 9 days per month to minimize confounding effects of these products on biomarker outcomes); planning to quit smoking in the next 3 mths; chronic conditions affecting results of biomarker analyses (e.g., liver disease); using NRT or other cessation medications; pregnancy/breastfeeding. 49% women; mean age 45.2; mean cpd 15.2; mean FTND 3.4 E cigarette use at baseline: Not reported Motivated to quit: Initially uninterested |
|
| Interventions |
EC: Cig‐a‐like, but the only cig‐a‐like product with high nicotine content Usual brand arm: Purchased their own usual brand of cigarettes; at end of clinical trial phase (week 8), offered ECs or NRT for up to 8 weeks, with a choice of product and no specific instructions for use EC AD‐E arm: Use EC whenever you like instead of a cigarette; can smoke as many or as few cigarettes as you want EC CS‐E arm: Complete substitution with e‐cigarettes (i.e. “you will stop smoking cigarettes and use only e‐cigarettes”) The primary e‐cigarette product was Vuse Solo (4.8% nicotine, manufactured by RJ Reynolds, Inc). Initially a choice of Blu cigarettes (cartridge‐based system, marketed previously by Lorillard) and Fin (prefilled tanks system, manufactured by Fin Branding Group) was offered; but because Vuse attained the highest market share during the early phase of the study, switched exclusively to Vuse. Participants could choose 1 of 4 flavors: tobacco, mint, menthol, and berry. Participants were provided 7 cartridges a week with the option of returning to the clinic before their next visit to obtain additional cartridges if needed. All products provided free to the participants. All unused products and used EC cartridges were collected at each visit CS‐NRT arm: Complete substitution with 4 mg nicotine gum or lozenge, with the participant choosing what product they would like to use (i.e. “you will stop smoking cigarettes and use only nicotine gum or lozenge”). The 4 mg was down‐titrated to 2 mg if adverse side effects were experienced. Nicotine gum came in mint, cinnamon, and fruit flavors, while the nicotine lozenge was mint or cherry flavors. All these products were provided free to the participants and unused products were collected at each visit Behavioural support: CS‐E arm and CS‐NRT arm: received brief counselling on how to avoid smoking cigarettes |
|
| Outcomes | 2‐week baseline period (weeks −1 and 0); Week 1, 2, 3, 4, 6 and 8 Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | "supported by grants U19CA157345 from the National Cancer Institute (DKH/PS), UL1 TR000062 and UL1 TR002494 from the National Center for Advancing Translational Science of the National Institutes of Health, and T32 DA007097 from the National Institute of Drug Abuse (EM). The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies" | |
| Author declarations | "RJC is a member of the FDA Tobacco Products Scientific Advisory Committee. PGS serves or has served as an expert witness in tobacco company litigation on behalf of plaintiffs" | |
| Notes | New for 2020 update. AD‐E arm not included in this review Additional data provided from authors. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not blinded and some interventions contained different levels of support |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not blinded but all relevant outcomes for our analyses were objective |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “There was a significant difference in dropout rates across groups following study entry (P = 0.04), with the highest dropout rates observed in the complete substitution groups, particularly in the NRT group…” AD‐E: Week 1 = 73/76; Week 2 = 73/76; Week 4 = 69/76; Week 6 = 66/76; Week 8 = 65/76 = 85% CS‐E: Week 1 =69/76; Week 2 = 67/76; Week 4 = 66/76; Week 6 = 61/76; Week 8 = 58/76 = 69.7% CS‐NRT: Week 1 =72/76; Week 2 = 65/76; Week 4 = 60/76; Week 6 = 57/76; Week 8 = 53/76 = 69.7% UB: Week 1 = 35/36; Week 2 = 35/36; Week 4 = 33/36; Week 6 = 33/36; Week 8 = 32/36 = 88.8% |
| Selective reporting (reporting bias) | Low risk | Table in supplementary section describes that heart rate, blood pressure and oxygen levels were measured, but findings not reported in paper; however, provided by authors upon request |
Hickling 2019.
| Study characteristics | ||
| Methods | Design: Single‐group assignment – pre‐test post‐test pilot study Recruitment: Participants were referred from community mental health teams within the South London and Maudsley NHS Foundation Trust. Setting: Healthcare setting, UK. Study start date: 24 September 2014; Study end date: 2 May 2017 |
|
| Participants | Total N: 50 Inclusion criteria: 18–70 years; daily smoker (unwilling to quit soon); exhaled CO ≥ 5 ppm; established clinical diagnosis of schizophreniform, schizophrenia, schizoaffective disorder or bipolar disorder, or attending an early detection service in a high‐risk state. Exclusion criteria: use of EC ≥ 2 occasions in past 30 days; intention to quit smoking in the next 30 days; medication use that may reduce smoking (including, bupropion, nicotine replacement therapies, acamprosate, varenicline, baclofen, clonidine, naltrexone, buprenorphine, nortriptyline, disulfiram and anti‐seizure medications); hospitalisation/change in dose of psychotropic medication(s) in the last 30 days; unstable physical health in the past 3 mths; previous serious stomach ulcer and/or phaeochromocytoma; severe heartburn, stroke, unstable kidney/liver disease, an uncontrolled overactive thyroid gland past 3 mths; meet the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM‐IV) criteria for illicit/alcohol drug dependency; contraindications to nicotine; asthma; suicidal ideation/suicide attempt in the past mth; pregnancy Inclusion based on specific population characteristic: People who smoke tobacco with a psychotic disorder (established clinical diagnosis of schizophreniform, schizophrenia, schizoaffective disorder or bipolar disorder, or attending an early detection service in a high‐risk state) 24% women; mean age 38.96; mean cpd 17.94; mean FTND not reported Motivated to quit: “unwilling to quit soon” E‐cigarette use at baseline: Must not have used e‐cigarettes on more than 2 occasions in the past 30 days |
|
| Interventions |
EC: Cig‐a‐like Participants provided with free tobacco‐flavoured NJOY traditional bold disposable e‐cigarette (4.5% nicotine) in an "amount equivalent to 150% of their daily tobacco use (as recommended by the manufacturer)" for 6 weeks. Participants were instructed in the use EC; not required to stop smoking tobacco, but were encouraged to replace it with EC as much as possible. Followed up at 4 weeks and encouraged to continue EC use, informed about EC types and where these could be purchased |
|
| Outcomes | Weeks 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 24 Self‐reported and biochemical validation Cessation: Tobacco use, as measured by the Time Line Follow Back. Tobacco cigarette use was also indexed weekly by measuring exhaled CO levels with a Smokerlyzer ED50 CO meter (Bedfont Instruments, UK) Adverse events and biomarkers:
In a subsample of participants (N = 8), 3‐hydroxypropylmercapturic acid (3‐HPMA, a measure of the toxicant acrolein) and formic acid were measured at baseline and week 6. These participants were chosen as their tobacco intake had decreased by more than 50% in this period. The measurement of 3‐HPMA and formic acid was also performed by validated LC‐MS/MS assays Other outcomes measured:
|
|
| Study funding | "This work was funded by the Maudsley Charity (grant number 715); and supported by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London." | |
| Author declarations | "R.P‐I. has received honoraria and speaker support from Lundbeck. L.D. has provided consultancy for the pharmaceutical industry (Johnson & Johnson 2015, 2017) and acted as an expert witness for an e‐cigarette patent infringement case (Porzio, Bromberg & Newman Attorneys at Law, 2015). Between 2011 and 2013, she conducted research for several independent electronic cigarette companies (Totally Wicked, SKYCIGS and E‐Lites) for which the University of East London received funds. The e‐cigarette companies involved had no input into the design, conduct or write up of these projects and she has not received any funds from e‐cigarette companies in the last 4 years. She has no links with, and has not received any funds from, the tobacco industry, although two e‐cigarette companies that she worked with in 2013 were subsequently acquired by the tobacco industry (SKYCIGs and E‐Lites). L.H., T.R., K‐V.S., J.M., A.M. and P.M. have no conflicts of interest." | |
| Notes | Study listed as ongoing study NCT02212041 in the 2016 review update Additional data provided from authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled study |
| Allocation concealment (selection bias) | High risk | Uncontrolled study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up: Week 6: 46/50; Week 10: 42/50; Week 24: 40/50 |
| Selective reporting (reporting bias) | Low risk | Report all outcomes listed on clinical trials.gov [http://clinical trials.gov] except NNAL. Authors confirmed that they had intended to test for NNAL but had major issues with the assays |
Holliday 2019.
| Study characteristics | ||
| Methods | Design: Pilot RCT Recruitment: Recruited via the Newcastle Dental Hospital and by primary care practitioners working in the north‐east England region Setting: Dental clinical research facility (DCRF), located in the Newcastle Dental Hospital, Newcastle upon Tyne, UK. Study start date: 20 September 2016; Study end date: 31 July 2018 |
|
| Participants | Total N: 80 N per arm: Intervention group: 40; Control group: 40 Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: Periodontitis 52.5% women; mean age 44.36; mean cpd 17.4; mean FTND 5 Motivated to quit: Not selected on motivation and not reported E‐cigarette use at baseline: Not currently using an e‐cigarette, or not having used 1 for more than 2 days in the last 30 days |
|
| Interventions |
EC: Refillable All participants given standard stop‐smoking advice (10‐15 minutes in duration) and offer of referral to stop‐smoking services Intervention: given EC starter kit (Vype eTank clearomizer) and brief training on its use by a dentist. Provided with an approximately 2‐week supply of e‐liquid (20 ml) with a choice of flavour (Blended Tobacco, Crisp Mint, Dark Cherry and Vpure (flavourless)) and nicotine strength (0 mg/mL, 6 mg/mL, 12 mg/mL, 18 mg/mL) and information on where to buy more. EC intervention delivered directly following the standard stop‐smoking advice and was expected to be 10‐15 minutes in duration Control group: no further intervention |
|
| Outcomes | Months 1 and 6; Self‐report and biochemical validation of smoking status Cessation: Rates of continuous eCO‐verified smoking abstinence at 6 months were calculated following the Russell Standard (RS6) Adverse events and biomarkers: expired air CO, adverse events monitored at each study visit Other outcomes measured:
|
|
| Study funding | "Richard Holliday is funded by a National Institute for Health Research Doctoral Research Fellowship (DRF‐2015‐08‐077). This paper presents independent research funded by the National Institute for Health Research (NIHR). The views expressed are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care." | |
| Author declarations | "The authors declare that they have no competing interests." | |
| Notes | New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization was performed using a secure password‐protected web‐based system |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation allocation schedule will be generated by a statistician with no other involvement in the study to achieve concealment of allocation." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Nature of study precluded blinding; different levels of support across intervention arms |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition < 50% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes are reported |
Humair 2014.
| Study characteristics | ||
| Methods | Design: Prospective cohort Recruitment: People attending an outpatient clinic Setting: University hospital outpatient clinic, Switzerland Study start date/end date: Not specified |
|
| Participants | Total N: 17 Inclusion criteria:
Inclusion based on specific population characteristic: No Mean 23 cpd, 82% had a psychiatric illness Motivated to quit: Yes E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like Offered an EC with nicotine 59% also reported using NRT or varenicline in addition to EC |
|
| Outcomes | Smoking cessation and reduction by at least 30% at 12 months (self‐report) Adverse events No significant side effects |
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | Abstract only, hence little detail available Not clear if EC was provided by clinic or if participants had to buy their own |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers lost to follow‐up not reported |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Ikonomidis 2018.
| Study characteristics | ||
| Methods | Design: (acute phase) Randomized cross‐over assignment (outcomes measured within hours of the intervention and hence do not meet the criteria of 1 week or more); chronic phase: non‐randomized, single‐group assignment Recruitment: Hospital smoking cessation unit Setting: Hospital smoking‐cessation unit, Greece Study start date: 31 January 2017; Study end date: Estimated completion date: December 2021 |
|
| Participants | Total N: 90 Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: No 54% women; mean age 50.2; mean cpd 23.4; mean FTND: Not reported Motivated to quit: Yes – recruited from smoking cessation unit E‐cigarette use at baseline: Not reported |
|
| Interventions |
EC: not clear E cigarette details: In the chronic phase, all 70 participants were instructed to replace their conventional cigarettes (con‐cig) with an e‐cig containing nicotine (12 mg/dL (e‐cig fluid with nicotine concentration of 12 mg/mL (propylene glycol 74.3%, glycerin 20%, flavoring 4.5%, nicotine 1.2%))) for 1 month |
|
| Outcomes | 1 month; Self‐report and objective measures Cessation: Self‐report cessation at 1 month. CO measured at 1 month. Cessation data not used as < 6 months Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | This study was supported by a grant from the Hellenic Cardiology Society and Hellenic Society of Lipidiology and Atherosclerosis. | |
| Author declarations | None | |
| Notes | New for 2020 update. Acute phase of trial not relevant for the review as very short‐term outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded and differential levels of support given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Objective measures used for all outcomes reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 70 participants and 20 controls recruited – no dropout |
| Selective reporting (reporting bias) | Unclear risk | NCT record states that chronic endothelial integrity, platelet aggregation and high‐shear stress‐dependent platelet function would be assessed but is not reported in this research letter – however study estimated completion date is December 2021, so perhaps data not ready for publication or limited capacity in the research letter – not the primary publication |
| Other bias | Unclear risk | Few details – written as commentary. Trial registration suggests this is an ongoing study |
Ikonomidis 2020a.
| Study characteristics | ||
| Methods | RCT Recruitment: Smoking cessation clinic of second cardiology department of National and Kapodistrian University of Athens, Attikon General Hospital Setting: Hospital smoking‐cessation unit, Greece Study start date: NS |
|
| Participants | N = 40; Arm 1 E‐cigarette n = 20; Arm 2 conventional tobacco cigarette n = 20 80% female; mean age 44.8 (SD 11.3); mean cpd: 25.8 (C‐cig 25.5 SD 9.3. E‐cig: 26.2 SD 9.1) Inclusion criteria: smokers without cardiovascular disease, who used to smoke 25.8 ± 9.2 conventional cigarettes per day of their choice Exclusion criteria: abnormal renal function; hepatic failure (bilirubin > 2 mg/dl); active malignancy; people treated with drugs that affect platelet function; history of coronary artery disease or peripheral artery disease; history of cardiomyopathy; thrombocytopenia (PLTs < 100 × 109 /L); anaemia (HCT < 28%); alcohol or drug abuse; age < 21 years; pregnancy; risk factors for cardiovascular disease |
|
| Interventions |
EC: Refillable E‐cig: second‐generation e‐cig device and popular in Greek Market e‐liquid (NOBACCO eGo Epsilon BDC 1100, eGo battery, 1100 mAh, operating at 3.9 V ‐ propylene glycol 74.3%, glycerin 20%, flavoring 4.5%, nicotine 1.2%/12 mg/mL) |
|
| Outcomes | Baseline, 4 months: Exhaled CO concentration; blood pressure Also, cpd; Ppatelet function by Platelet Function Analyzer PFA‐100 and Light Transmission Aggregometry; Pulse wave velocity; Plasma malondialdehyde levels as oxidative stress index |
|
| Study funding | "There was no funding for this study" | |
| Author declarations | "The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers as reproduced from the online randomization software www.graphpad.com/quickcalcs/index |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not possible |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Only CO outcomes used here, which are objectively measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All followed up (confirmed via contact with authors) |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial record available to confirm whether all prespecified criteria were reported |
Ikonomidis 2020b.
| Study characteristics | ||
| Methods | Design: RCT Setting: Smoking Cessation Clinic of Second Cardiology Department of National and Kapodistrian University of Athens, Attikon General Hospital, Greece |
|
| Participants | 40 Inclusion criteria: current smokers without cardiovascular disease |
|
| Interventions | EC type: NS Conventional cigarette (conv‐cig) or an electronic cigarette (e‐cig) with nicotine concentration 12 mg/dL for 1 month |
|
| Outcomes | a) Perfused boundary region (PBR) of the sublingual arterial micro vessels (range 5 – 25 micrometers), a marker inversely related with glycocalyx thickness b) Pulse wave velocity (PWV), central systolic blood pressure (cSBP) and augmentation index (AIx) c) Platelet function by 2 different methods, namely the novel Platelet Function Analyzer PFA‐100 and the traditional Light Transmission Aggregometry (LTA) d) Exhaled CO level (ppm) as a smoking status marker e) Plasma malondialdehyde (MDA) levels, as an oxidative stress burden index |
|
| Study funding | Funding Acknowledgement: Type of funding source: None | |
| Author declarations | NS | |
| Notes | Information extracted from a conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail given. Quote: "40 current smokers (mean age 48 years±5) without cardiovascular disease were randomized to smoke either a conventional cigarette (conv‐cig) or an electronic cigarette (e‐cig) (electronic cigarette fluid with nicotine concentration of 12 mg/dL) for one month." |
| Allocation concealment (selection bias) | Unclear risk | NS |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | NS |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | NS |
| Selective reporting (reporting bias) | High risk | Results reported in summary form, not fully, with only some significant changes reported. Information taken from a conference abstract only |
Ioakeimidis 2018.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Recruitment: Not specified Setting: Hospital, Greece. Study start date/Study end date: Not specified |
|
| Participants | Total N: 54 N per arm: Arm 1: 27; Arm 2: 27 Inclusion criteria: ≥ 10 cpd; motivation to quit; hospitalized with acute coronary syndrome (ACS); ≥ 18 years Exclusion criteria: prior EC use; history of neuropsychiatric disorders; prior varenicline use or use of SC pharmacotherapy at time of ACS; cardiogenic shock or renal impairment; hepatic impairment prior to ACS; excessive alcohol use or current use of marijuana or non‐cigarette tobacco products Inclusion based on specific population characteristic: People who have experienced acute coronary syndrome 65% women; mean age 52; mean cpd 21; mean FTND 5.6 Motivated to quit: Yes E‐cigarette use at baseline: No prior EC use |
|
| Interventions |
EC: information on whether cig‐a‐like or refillable not provided Both arms given "low intensity counselling" Intervention 1: 12‐week use of EC 12 mg/mL nicotine Intervention 2: 12‐week varenicline |
|
| Outcomes | Weeks: 4, 12, 24 Cessation: 7‐day PP at 24 weeks, self‐report Adverse events and biomarkers: Unclear how these were reported. Abstract says no SAEs, poster implies this may have just been CV or neuropsychiatric SAEs. Abstract says nothing about AEs but nausea and sleeping disorders given in table in poster. Implies (S)AEs collected during treatment period only Other outcomes measured: Not specified |
|
| Study funding | Not reported | |
| Author declarations | Not reported | |
| Notes | New for 2020 update. Abstract and poster only; limited data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not specified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not specified but equal amounts of contact and support between arms so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only but equal amounts of contact between arms, no reason to suspect differential misreport |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Unclear risk | Abstract/poster only so not able to judge |
| Other bias | High risk | Abstract and poster only. Two different figures presented for quit rate in EC arm (no difference in those presented in varenicline arm) between abstract and poster. Poster percentage aligns with figure, so using that (16.5%) as opposed to abstract figure (32.5%). Contacted authors but no reply. Calculated n quit based on percentages but unclear what denominators were; EC calculates back to whole number for EC but not for varenicline |
Kerr 2020.
| Study characteristics | ||
| Methods | Design: RCT. Pragmatic, two‐armed, single‐centre, randomised controlled pilot study Recruitment: Community. Recruitment from NHSGGC Smokefree Services. GP surgeries Secondary care QEUH, GRI, New Victoria Hospital and Glasgow Dental School Advertised on NHSGGC staff payslips, local newspapers, community magazines and on Gumtree. The British Heart Foundation, University of Glasgow and NHSGGC released a joint press release, which generated media coverage in four newspapers. Social media. Public engagement “pop up stands”, were held collaboratively with smokefree services and the VAPOUR study team in the entrance foyer of the QEUH and the at the Celtic Football Club Healthy Hoops Event. Setting: Scotland, UK Study start date: 1 December 2015. Study end date: 13 July 2018 |
|
| Participants | Total N: 55 EC arm 28. NRT arm 27 Inclusion criteria: Aged 18 to 65 yrs; habitual tobacco smokers (smoking on average 1‐15 tobacco cigarettes pd > 6 mths); willing to quit tobacco smoking; with either the use of nicotine replacement patches or an EC with nicotine‐containing e‐liquid, in addition to engaging with NHSGGC Community Smokefree Service's 12‐wk behavioural support programme. No established history of cardiovascular disease Exclusion criteria: Pregnant or breastfeeding; had used an EC or nicotine replacement patch in the last 3 mths; were allergic to the active substances in either of the nicotine replacement products; history of illicit drug use, major depressive illness or other psychiatric conditions, peripheral arterial occlusive disease (PAOD), COPD, renal impairment (eGFR < 45 mL/min), uncontrolled hypertension (BP ≥ 165/95 mmHg), or CVD. Female 43.7%. Mean age 44.2. Mean CPD 15. Mean FTND 7. E‐cigarette use at baseline: not reported Motivated to quit: participants were willing to quit. |
|
| Interventions | EC: Refillable EC arm E‐cigarette starter pack contained two commercially available second generation e‐cigarette devices with charging devices, 11 replacement atomisers & 12 x 10 bottles of nicotine containing e‐liquid, tobacco flavoured e‐liquid, 18 mg/mL nicotine. Each e‐cigarette device consisted of a 1300 mAh variable voltage rechargeable battery, a tank and an atomiser, and the charging device comprised of USB e‐c‐cigarette charger and USB mains adapter (SmokeMax; Groove Trading Ltd, 194 Glasgow UK), and written instructions. At baseline, oral and written information was given on how to operate the e‐cigarette, and for the duration of the study participants were asked only to use the study e‐cigarette and e‐liquid they were provided with. NRT arm: NRT Nicotine replacement patches, 12‐week reducing nicotine regimen (21 mg, 14 mg, 7 mg) of Nicotinell® Patches Weekly supply of nicotine replacement patches. If required, participants were also permitted to use additional other licensed nicotine replacement products (gum, lozenges, nasal spray, inhalers and micro‐tabs), in combination with the nicotine replacement patches. Both arms: All participants received 12 weeks of behavioural support provided by NHSGGC Smokefree Community Services. Following the baseline visit, participants were asked to define their “quit date”. |
|
| Outcomes | Baseline and 12 weeks CO confirmed smoking cessation Secondary outcomes: cardiovascular function (heart rate SBP, DBP), lung function, weight |
|
| Study funding | Grant from British Heart Foundation (Centre of Research Excellence Award, reference number RE/13/5/30177) | |
| Author declarations | There were no conflicts of interest with the tobacco industry. | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a secure website (Sealed Envelope Ltd, 2016), participants were randomised in a 1:1 fashion, to either the e‐cigarettes combined with behavioural support or nicotine replacement patch group combined with behavioural support. Using a permuted block design with a computer random number generator, block randomisation with block sizes of 4, 6 and 8 was used to reduce bias and achieve balance in the allocation of participants to treatment arms". |
| Allocation concealment (selection bias) | Low risk | Quote: “Secure website (Sealed Envelope Ltd, 2016) participants randomised in a 1:1 fashion, ……Using a permuted block design with a computer random number generator, etc.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded, but as both arms contained active interventions, performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | BP, heart rate and oxygen saturation measured. Abstinence at 12 weeks, self‐report was CO validated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 31/55 (56.3%) EC arm 18/28; NRT arm 14/27 |
| Selective reporting (reporting bias) | Low risk | Reported outcomes ‘per protocol’ |
Kimber 2021.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Participants were screened via phone interviews. Setting: East London, UK Study start date: Participants recruited between December 2015 and December 2016 |
|
| Participants | Total: N = 50 people who smoked combustible cigarettes and were e‐cigarette naive. Initial sample (N = 70) attended first session; all analyses were conducted on the N = 50 who returned for their 2nd and 3rd session. Cig‐a‐likes: N = 11 Tank18: N = 20 Tank6: N = 19 Inclusion criteria: Smoke daily ≥ 5 cigarettes, have smoked for ≥ 1 year, not currently using an EC, willing to abstain 1 hr before the start of the session and willing to make a quit attempt Exclusion criteria: < 18 years, not fluent in English, pregnancy or breastfeeding, or a known neurobiological or heart condition 64% women; mean age 29.5 (SD 9.31); mean CPD 13.09 (SD 6.66), mean FTND 4.14 (SD 2.45) Motivated to quit: ‘Willing to make a quit attempt’ E‐cigarette use at baseline: No |
|
| Interventions | EC: cartridge and refillable Arm 1: cig‐a‐like (18 mg/mL) The ‘Blu’ (n = 13) and ‘TECC Go e‐cigarette’ models (n = 11) were used for the Cig‐a‐like condition, due to issues of leakages with the latter. Non‐adjustable battery power output and could be recharged, a supply of spare disposable cartridges were provided. Arm 2: a tank model containing 18 mg/mL (Tank18) Arm 3: a tank model containing 6 mg/mL (Tank6) For both conditions Tank18 and Tank6 the ‘Totally Wicked mini curve’ was mounted with a 2 mL capacity tank which housed a standard atomiser of 1.5 ohm resistance. E‐liquid ingredients composition and flavours were kept consistent across all conditions using the same ratio of propylene glycol and vegetable glycerin (PG/VG: 50/50) and tobacco flavour. Intervention. All 3 groups participants vaped 20 min ad libitum in 3 separate sessions (baseline, 1 and 2 weeks post‐baseline). Ahead of their baseline session, participants were instructed to abstain from smoking for an hour. Rated their craving and withdrawal symptoms (at the beginning and end of the session), before receiving instructions on how to use their EC and to vape ad libitum for 20 mins Positive and adverse effects were measured at the end of the last puff. All vaping sessions were video‐recorded. At the end of each session, participants were given the EC and were instructed to keep a record of the number of cigarettes smoked at the end of each day until their next and subsequent sessions. Each participant was provided with a weekly supply of either, 60–80 mL of e‐liquid in refill bottles for those in the tank conditions, or 15 cartridges for those in the cig‐a‐like condition at the end of each testing session. The session was repeated the following week, then one week later. Participants were asked to keep the device and encouraged to try and replace as many tobacco cigarettes as they could with the use of their EC. |
|
| Outcomes | Baseline, week 1, week 2 CO was measured at baseline, wk 1 and wk 2. Self‐reported CPD. Adverse events. Puff duration, puff number, inter‐puff intervals (IPI). Cigarette dependence, craving, withdrawal, and subjective effects |
|
| Study funding | This work was funded by the University of East London through a PhD studentship award. The funder had no role in the study design, collection, analysis or interpretation of data, writing the manuscript and the decision to submit the manuscript for publication. | |
| Author declarations | CK and KS have no conflicts of interest to declare. LD has provided consultancy for the pharmaceutical industry relating to the development of smoking cessation products. | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomly allocated (using SPSS). |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention arms received equally intensive interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CO measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was a > 20% difference in FU between arms at 2 weeks. Overall 50/70 = 71.43%. Cig‐a‐like 11/24 = 45%. Tank6 19/23 = 82.61%. Tank18 20/23 = 86.96% |
| Selective reporting (reporting bias) | High risk | Reported on AEs but excluded those who had reported AEs (as they did not complete all sessions (due to AEs) |
Kumral 2016.
| Study characteristics | ||
| Methods | Design: Prospective randomized clinical trial Recruitment: All patients admitted to a smoking cessation clinic at the Department of Otorhinolaryngology‐Head and Neck Surgery, Okmeydanı Training and Research hospital Setting: Smoking cessation clinic, Turkey Study start date: March 2013; Study end date: November 2013 |
|
| Participants | Total N: 98 but analysis excludes 16 from intervention and 10 from control who did not stop smoking; thus 72 analyzed N per arm: EC: 58 (42 ana lysed); Non‐EC 40 (30 ana lysed) Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: No 44% women; mean age 36; mean cpd and mean FTND not specified Motivated to quit: “All patients were willing to quit smoking” E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Unclear EC arm: “used EC to quit smoking” – allowed to select brand and flavour, used “medium density” liquid (11‐12 mg/mL) (no further detail given) Non‐EC arm: Received cognitive behavioural therapy (no further detail given) |
|
| Outcomes | 3 Months Sino‐nasal outcome test (SNOT‐22) via self‐administered questionnaire, to evaluate changes in subjective symptoms. Saccharin transit test to evaluate nasal mucociliary clearance (MCC) function which authors state is “an important defence mechanism” |
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Patients participating in the study were randomly divided into two groups; EC smokers (group 1) and non‐EC smokers (group 2).” No further detail provided |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. The trial is described as single‐blinded and outcome assessors were blinded. No placebo used |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported outcome data, participants not blinded and unequal amounts of support between arms |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout rate not clear. Only ana lysed people who quit |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Lee 2018.
| Study characteristics | ||
| Methods | Randomized parallel‐assignment double‐blind pilot trial Setting: San Francisco Veterans Affairs Medical Center (SFVAMC), USA Recruitment: veterans awaiting surgery Recruitment: In VA hospital presenting for surgery Study start date: August 2015; Study end date: May 2016 |
|
| Participants | Total N: 50 N per arm: NRT: 30; END: 20 Inclusion criteria: presented to the anaesthesia preoperative clinic for elective surgery 3 or more days before surgery; currently smoked ≥ 2 CPD, having smoked at least once in the last 7 days. Exclusion criteria: exclusively used other forms of tobacco or marijuana only; pregnancy /breastfeeding; unstable cardiac condition; currently using smoking cessation pharmacotherapy; already enrolled in a smoking cessation trial; using EC on a daily basis. Inclusion based on specific population characteristic: Patients awaiting elective surgery 10% women; mean age 54; mean cpd 14; mean FTND 3.3 Motivated to quit: Not specified E‐cigarette use at baseline: Not specified but excluded daily users |
|
| Interventions |
EC: Cig‐a‐like Both groups receive: i) referral to the California Smokers' Helpline, ii) brief advice lasting less than 2 minutes, iii) a brochure from the ASA about quitting smoking before surgery EC arm: 6‐week supply of NJOY e‐cigarettes (disposable, first generation). Instructed to use Bold (4.5%) ad lib for 3 weeks, then Gold (2.4%) ad lib for 2 weeks and then study (0%) ad lib for final week. Number of ECs issued corresponded to baseline cpd, assuming 1 EC = 10 cigarettes. Asked to refrain from the use of all study products at the end of 6 weeks NRT arm: 5‐week Nicoderm CQ patches, 1 week placebo patches. Dose based on cpd at baseline: ≥ 10 cpd, 21 mg/day for 3 weeks, 14 mg/day for 1 week, 7 mg/day for 1 week, 0 mg/day for 1 week. < 10 cpd at baseline: 14 mg/day for 3 weeks, 7 mg/day for 2 weeks, 0 mg/day for 1 week |
|
| Outcomes | 30 Days (phone), 8 Weeks (in person), 6 Months (phone) Cessation: 7‐day PP at 30 days (not validated), 8 weeks (CO‐validated), 6 months (not validated). Smoking cessation for at least 48 hours on day of surgery (CO‐validated) Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | “This work was funded by internal UCSF Department of Anesthesia and Perioperative Care funds (San Francisco, California, United States of America) and the UCSF Resource Allocation Program grant, administered by the Helen Diller Family Comprehensive Cancer Center developmental funds from the National Cancer Institute Cancer Center Support Grant (P30 CA 82103‐16). E‐cigarettes were purchased from NJOY using these funds. NJOY had no involvement in the design, execution, or analysis of the study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.” | |
| Author declarations | “The authors declare there are no competing interests” | |
| Notes | 3 NRT participants used EC, 2 EC participants used nicotine patch Study listed as ongoing study NCT02482233 in the 2016 review update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was computer‐generated, with randomly permuted block sizes of 3 or 6, in a 2:1 ratio using the ralloc program” |
| Allocation concealment (selection bias) | Low risk | Quote: “Allocation was concealed by consecutively numbered, sealed, opaque envelopes” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded but both interventions active with equal amounts of support so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only at 6 months and participants not blinded to condition, but similar level of support given to both groups so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 NRT and 1 ENDs loss to follow‐up at 6 months |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Lee 2019.
| Study characteristics | ||
| Methods | Design: Randomized controlled trial Recruitment: Recruited from motor company. Setting: Motor company, medical office in Korea Study start date: 5 January 2012; Study end date: 31 August 2012 |
|
| Participants | Total N: 150 N per arm: EC: 75; NRT: 75 Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: No 0% women; mean age 42.3; mean cpd: Not reported, 1.01 packs per day; mean FTND 4.05 Motivated to quit: Yes, or to reduce E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Refillable Both arms received 50 mins education session on smoking cessation and use of smoking cessation aids in medical office (no further detail given). Asked to return to medical office every 4 weeks (to 24 weeks?) for “evaluation and counselling by an independent health practitioner” Arm 1: 50‐min education sessions on smoking cessation and the use of smoking‐cessation aids, instructed to visit the medical office each month for evaluation and counselling by a health practitioner who was unaffiliated with the study. Participants supplied with eGo‐CTM EC (nicotine 0.01 mg/mL) from Ovale in 12‐wk supply Arm 2: As (1) but instead of EC given 2 mg nicotine gum in 12‐wk supply |
|
| Outcomes | 12, 24 weeks (in person) Cessation: continuous abstinence from 9‐24 weeks, exhaled CO < 10 ppm, negative urine cotinine Adverse events and biomarkers: Yes but just note ‘adverse events’ Other outcomes measured: 7‐day PPA, cigarette reduction |
|
| Study funding | “none” | |
| Author declarations | “none declared” | |
| Notes | Study listed as ongoing study KCT0001277 in the 2016 review update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer‐generated randomization sequence with a block size of 2” |
| Allocation concealment (selection bias) | Low risk | Quote: “The enrolment and assignment of all subjects were performed by a clinical research coordinator not involved in the study” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Not blinded but both interventions active with equal amounts of support, so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants not blinded but results biochemically validated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 61/75 NRT and 71/75 EC FU at 24 weeks |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported |
Lucchiari 2020.
| Study characteristics | ||
| Methods | Design: Randomized parallel‐assignment double‐blind trial Recruitment: Participants enrolled in lung cancer‐screening programme Setting: Early lung cancer detection programme (Cosmos II) at European Institute of Oncology, Italy Study start date: September 2014; Study end date: January 2016 |
|
| Participants | Total N: 210 N per arm: 70 participants per arm Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: 55 years of age or older 37% women; mean age 62.8; mean cpd 19.38; mean FTND 4.37 Motivated to quit: yes E‐cigarette use at baseline: Excluded people who smoke who had ever regularly used e‐cigarettes for more than 1 week alone or in combination with tobacco cigarettes |
|
| Interventions |
EC: Cig‐a‐like Both arms received “low intensity counseling” – phone at week 1, 4, 8 and 12, approx. 10 mins each Nicotine EC arm: e‐cigarette kit and 12 10‐mL liquid cartridges (8 mg/mL nicotine concentration). During the first week, participants could use the e‐cigarette ad libitum. At the end of the first week, asked to use only EC for the next 11 weeks Nicotine‐free EC (placebo) arm: Nicotine‐free EC – same as above but with nicotine‐free EC |
|
| Outcomes | Months 3, 6 and 12 (but only 3‐ and 6‐month data available) Cessation: Continuous abstinence for previous month, CO ≤ 7 ppm Adverse events and biomarkers: FOR EC ARMS ONLY:
Other outcomes measured:
|
|
| Study funding | This study was supported by a grant from Fondazione Umberto Veronesi (FUV) | |
| Author declarations | The authors declare no conflicts of interest | |
| Notes | Listed as ongoing study Lucchiari 2016 (NCT02422914) in 2016 review; new for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A randomization list using a permuted block design (40 blocks of 6 subjects randomly assigned to 1 of the 3 treatment arms) had been previously prepared by independent personnel.” |
| Allocation concealment (selection bias) | Low risk | Double‐blind, active and placebo e‐cigarettes labelled by independent personnel, researcher and participants blind |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “double blind” for nicotine vs no nicotine EC but limited info given; however, as similar levels of support across arms performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Approx. 73% followed up in each group at 6 months, very little difference between groups |
| Selective reporting (reporting bias) | High risk | Paper states data also collected at 12 m but this is not presented and unclear why. Paper states CO collected but data not presented |
Martinez 2021.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Participants were recruited throughout the USA through Facebook and multimedia advertisements (newspapers, radio, TV, e‐cigarette forums, and so on) for a study measuring attitudes and behaviours about cigarettes and e‐cigarettes Setting: USA Study start date: March 31 2015. Study end date June 30 2019 |
|
| Participants | 2896 dual users of nicotine EC and combustible tobacco cigarettes Assessment only n = 575; generic smoking cessation booklets n = 1154; targeted booklets n = 1167. 37% female. Mean age 29.9 Mean cpd: 1 – 10 1663 (57%); 11 – 20 972 (34%); > 20 259 (9%). Mean ftnd 3.6. E‐cigarette use at baseline Inclusion criteria: age 18 years or older, smoked 1 or more combustible cigarettes per week over the preceding year, used e‐cigarettes 1 or more times per week over the preceding month, not currently enrolled in a face‐to‐face smoking cessation programme, and able to speak and read English. The original inclusion criteria required daily smoking. However, early in the trial, it became apparent that many dual users were skipping smoking on some days. Therefore, to better reflect the dual‐using population, we amended the use frequency criteria to equate them for smoking and vaping at 1 or more uses per week. The protocol was amended on 25 September 2016. We had recruited 652 participants up to that date. Participants were not necessarily seeking treatment or motivated to quit smoking or vaping. Participation was limited to 1 individual per street address. Participants gave oral informed consent |
|
| Interventions | EC type: n/a Assessment only (n = 575) Generic smoking cessation self‐help booklets previously shown to be efficacious in smokers (n = 1154) (an introductory Stop smoking for good brochure, 10 x Stop smoking for good didactic booklets, and 9 How I quit smoking pamphlets); Booklets specifically targeting dual users (n = 1167, (If you vape: a guide to quitting smoking), which included an introductory If you vape brochure, a series of 10 x If you vape: guide to quitting smoking booklets, and 9 x My story pamphlets). Participants assigned to the GENERIC or eTARGET groups were sent the intervention materials by post, with the option of also receiving them electronically |
|
| Outcomes | 7‐day PPA at each assessment point. Sustained abstinence: 30‐day and 90‐day PPA Breath CO and saliva samples (for cotinine analysis) were collected at the 12‐ and 24‐month follow‐up points for participants who reported abstinence and resided within 100 miles of the home institution Cut‐offs of 8 ppm for CO and 10 ng/ml for cotinine were used to determine abstinence. The disconfirmation rates from this sample were used to estimate adjusted smoking rates for the full sample Full follow‐up assessments: 6, 12, 18, and 24 months. Abbreviated assessments:3, 9, 15, and 21 months after baseline |
|
| Study funding | This work was supported by the National Institute on Drug Abuse of the NIH (R01DA037961). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH This work has also been supported in part by the Biostatistics and Bioinformatics Shared Resource and the Participant Research, Interventions, and Measures Resource at the H Lee Moffitt Cancer Center and Research Institute, a National Cancer Institute designated Comprehensive Cancer Center (P30CA76292) |
|
| Author declarations | Quote: "THB has received research support from the US National Institutes of Health (NIH), the American Cancer Society, the Florida Department of Health, and Pfizer; has collaborated on funded research with Voxiva, Optum, and the University of East Anglia (Norwich, UK); spent sabbatical at the Trimbos Institute and Utrecht University (Utrecht, Netherlands); is on the advisory board of, and holds restricted stock in, Hava Health, which is developing a pharmaceutical grade electronic nicotine delivery system for smoking cessation; participated in a Best Brains Exchange for Health Canada, providing advice on e‐cigarette policy; and consulted for the Australian Government Solicitor regarding plain tobacco packaging. UM has received research support from the NIH and the Galician Plan of Research, Innovation, and Growth (Spain); and has received funding from the Barrie Foundation to receive predoctoral training at the University of Newcastle (Callaghan, NSW, Australia). VNS has received research support from the NIH and the Florida Department of Health. SKS has received research support from the NIH, the American Cancer Society, the Florida Department of Health, and Pfizer. DJD has received research support from the NIH, the American Cancer Society, and the Florida Department of Health; and has provided paid expert testimony in litigation against tobacco companies. MMB has received funding from the NIH, the Florida Department of Health, the US Department of Veterans Affairs, the US Centers for Disease Control and Prevention, the National Science Foundation, and the US Department of Housing & Urban Development; and has received research support from Gilead Sciences, Florida Blue Foundation, Bristol Myers Squibb Foundation, Merck Foundation, Maine Cancer Foundation, and Pfizer. PTH has received research support from the NIH, US Food and Drug Administration (FDA), and Virginia Foundation for Healthy Youth. TE conducts research supported by the National Institute on Drug Abuse of the NIH and the Center for Tobacco Products of the FDA; is a paid consultant in litigation against the tobacco industry and the electronic cigarette industry; is named on one patent for a device that measures the puffing behaviour of electronic cigarette users and on another patent for a smartphone app that determines electronic cigarette device and liquid characteristics; owns shares in a variety of mutual funds, the exact stock makeup of which he has no control, and owns shares in three publicly traded companies, none of which are in any way related to the tobacco industry, the electronic cigarette industry, or any other aspect of this work; and has served as a special government employee of the US Government in the context of his service on the FDA’s Tobacco Products Scientific Advisory Committee and the Department of Health and Human Services Secretary’s Advisory Committee on Human Research Protection. CRB has received research support from the New Zealand Ministry of Health, the Health Research Council of New Zealand, CureKids Foundation, Heart Foundation, Health Promotion Agency, and Auckland Council and Sanitarium; collaborates on funded research with Newcastle University (Australia) through a grant from the Australian National Health and Medical Research Council, with Zhejiang University (Hangzhou, China) and Kunming University (Yunnan, China) on an Education New Zealand Tripartite grant, and with the University of Malaya (Kuala Lumpur, Malaysia) on a University of Malaya Grand Challenges grant; received funding from Pfizer Australasia for a survey of the impact of COVID‐19 on health workers in low‐income and middle‐income countries and from Johnson & Johnson Japan for consultancy on smoking cessation medication; and was a consultant to Moffit Cancer Center on this study through an NIH grant. LRM and KOB declare no competing interests. The employees of Moffitt Cancer Center—UM, VNS, SKS, DJD, LRM, KOB, MMB, and THB—are eligible for sharing of any revenue that might be generated by products developed during their employment, including the intervention used in this study." | |
| Notes | Quote: "Participants were compensated US$10–20 for the first eight assessments and $40 for the final one, and they were eligible for $40–60 bonuses for completing at least seven assessments. Participants returning assessments within 1 week were sent inexpensive appreciation gifts.’ Appendix: ‘Participants were not aware in advance of the interview that they would be asked for biosamples, and new informed consent was obtained at that time. Participants received $20 for completing a biochemical verification interview and $15 for providing biosamples." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used computer generated randomisation with balanced permuted blocks (block size 10, with 2‐4‐4 ratio) to allocate participants to assessment only (ASSESS group), generic smoking cessation self‐help booklets (GENERIC group), or booklets targeting dual users (eTARGET group)." |
| Allocation concealment (selection bias) | Low risk | Computer‐generated (see above) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Tailored versus generic booklet judged low risk as similar intensity; this is the comparison used in the meta‐analysis. Tailored versus no support would be high risk due to differential levels of support provided and no blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | As above, tailored versus generic similar intensity so judged to be low risk of differential misreport (self‐reported cessation only) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All > 50% at 24 months ASSES: 361/575*100 = 62.8% GENERIC: 619/1154*100 = 53.6% eTARGET: 642/1167*100 = 55% |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported in the trial register reported in the publication |
Martner 2019.
| Study characteristics | ||
| Methods | Design: A non‐concurrent multiple baseline across participants design. Three phases were included: Baseline, EC, and EC + CM. Half the participants received the EC phase following baseline; the other half received EC + CM following baseline Recruitment: Community Setting: Set‐up meetings occurred at the University of Florida Behavioral Health and Technology Research Clinic, USA Study start date/Study end date: Not specified. |
|
| Participants | Total N: 12 Inclusion criteria: 18‐65 yrs old; smoked ≥ 2 years; smoked ≥ 8 cpd on average; smoked in the past 24 hours; expressed a desire to quit smoking (yes/no); reliable access to the internet and a computer or smartphone; breath CO ≥ 10 ppm at set‐up. Exclusion criteria: current or previous medical condition that would pose an increased risk to participation; use of benzodiazepines, cocaine, or opiates in the previous 6 months; smoke marijuana more than twice a month; exposed to elevated CO levels (e.g. spouse smokes in house); pregnant or expected to become pregnant in the next 6 months. 58.3% women; mean age 37.5; mean cpd 16.25; mean FTND 5 Motivated to quit: Expressed a desire to quit smoking. E‐cigarette use at baseline: 3 participants never tried an EC prior to the study; 2 owned an EC but quit using it more than a month prior to the study; remaining 7 had tried an EC more than a year prior to the study but never owned one |
|
| Interventions |
EC: Refillable All participants provided with smokio electronic cigarettes (second‐generation ECs) and V2 e‐liquid with a concentration of 24 mg/mL (2.4%) of nicotine. Researchers provided participants with a copy of the National Cancer Institute’s brochure Clearing the Air (smokefree. gov [http://smokefree. gov]). Then researchers and participants read through a manual that described the study procedures, and showed participants how to use the software to measure CO and how to use the EC Participants initially received EC without contingency for a period of 14 days following the quit attempt. If participants failed to reduce CO levels during this phase, they received contingency management in addition to EC |
|
| Outcomes | 4 weeks Adverse events and biomarkers: Adverse events collected in 4‐day smoking behaviour questionnaires; eCO Other outcomes measured: acceptability and use of EC; overall experience of study |
|
| Study funding | "The study was supported in part by crowd‐sourced funding enabled by Experiment.com. Preparation of this paper was supported in part by Grant P30DA029926." | |
| Author declarations | "The authors declare no conflicts of interest." | |
| Notes | N of 1 (within‐participants randomized design, not between groups). New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided. |
| Selective reporting (reporting bias) | Unclear risk | AEs measured in behavioural change questionnaire but not reported |
McRobbie 2015.
| Study characteristics | ||
| Methods | Design: Prospective cohort Recruitment: advertisements in free London newspapers Setting: Smokers' clinic, East London, UK Study start date: February 2013; Study end date: September 2013 |
|
| Participants | Total N: 40 Inclusion criteria:
Exclusion criteria:
45% women, mean age 47 (SD 12), mean cpd 19 (SD 10), mean FTND 5.2 (SD 2.8), 65% in full‐time employment Motivated to quit: Yes E‐cigarette use at baseline: Excluded those who had used EC for more than 1 week in the past |
|
| Interventions |
EC: Cig‐a‐like Participants attended baseline session 1 week prior to their TQD. On the TQD, participants were provided with an EC (Green Smoke, 1st generation device, 2.4% nicotine cartridges). 2 cartridges a day were supplied initially, with the supply adjusted to actual use later. Attended 4 weekly follow‐up sessions and received standard behavioural support |
|
| Outcomes | Cigarette consumption and CO readings collected at each session. Urine sample for cotinine and 3‐HPMA analysis collected at baseline and 4 weeks post‐TQD Change in urinary 3‐HPMA (ng/mg creatinine) at 4 weeks Change in urinary cotinine (ng/mg creatinine) at 4 weeks Change in CO at 4 weeks |
|
| Study funding | "This study was funded by a grant given to P. Hajek, H. McRobbie, and M.L.Goniewicz from the UK Medicines and Healthcare Products Regulatory Agency (MHRA). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact." | |
| Author declarations | "H. McRobbie is Clincal Director at The Dragon Institute; reports receiving commercial research grant from Pfizer; and has received speakers bureau honoraria from Johnson&Johnson and Pfizer. M.L. Goniewicz reports receiving commercial research grant from Pfizer. P. Hajek has received speakers bureau honoraria from and is a consultant/advisory board member for the manufacturers of stop‐smoking medications. No potential conflicts of interest were disclosed by the other authors." | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 7/40 participants were lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All predefined outcomes reported |
Meier 2017.
| Study characteristics | ||
| Methods | Design: Randomized cross‐over trial (e‐cig vs placebo) Recruitment: via local media outlets Setting: Community, USA Study start date/Study end date: Not specified. |
|
| Participants | Total N: 24 Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: No 25% women; mean age 48.5; mean cpd 16.3; FTND not reported Motivated to quit: No (eligibility criteria was to not want to quit in next 30 days) E‐cigarette use at baseline: 8/24 (33%) had previously tried an EC, avg 9.4 months since last use, avg length of use 3.6 days |
|
| Interventions |
EC: Cig‐a‐like
Smoked “as usual” for 1 week followed by 2 weeks of either placebo or active 1st generation EC BluCig starter kit with up to 7 cartridges (prefilled, with either active 16 mg or 0 mg nicotine solution) Participants were instructed “this e‐cig may or may not contain nicotine; we ask that you try it at least once, but use it however you like; smoke regular cigarettes as you wish.” Shown how to charge the device and sampled the product during the visit. Provided a handout on how to use the product (e.g., switching cartridges) and general information about ECs |
|
| Outcomes | 1 week in each condition, in person Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | “..supported by grants P01 CA138389, P30 CA138313 (Hollings Cancer Center Support Grant) from the National Cancer Institute of the National Institutes of Health and UL1 TR000062 from the National Center for Advancing Translational Science of the National Institutes of Health. BWH was supported by K12DA031794” | |
| Author declarations | “KMC has received grant funding from the Pfizer, Inc., to study the impact of a hospital‐based tobacco cessation intervention. He also receives funding as an expert witness in litigation filed against the tobacco industry. We have no other declarations of interests to declare” | |
| Notes | New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Participants were randomized to receive either an active or placebo EC first”, no further information provided. |
| Allocation concealment (selection bias) | Unclear risk | Refer to 'Random sequence generation'. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Participants and research staff conducting sessions were blinded to dose. All cartridges were pre‐loaded by the manufacturer. Labeling was removed by a research team member not involved in participant contact to mask placebo versus active ECs. We restricted flavour options to regular tobacco flavour or menthol to most closely match usual cigarette brand flavour profile and reduce unwanted variance in product” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Participants and research staff conducting sessions were blinded to dose. All cartridges were pre‐loaded by the manufacturer. Labeling was removed by a research team member not involved in participant contact to mask placebo versus active ECs. We restricted flavour options to regular tobacco flavour or menthol to most closely match usual cigarette brand flavour profile and reduce unwanted variance in product” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not specified |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Morphett 2022a.
| Study characteristics | ||
| Methods | Design: RCT Setting: Australia Recruitment: commercial market research panel and supplemental recruitment by study researchers. An invitation to participate was available to 46 857 I‐view panel members, including those with no smoking status recorded. In addition, advertisements to the general public were used to recruit additional participants Study start date: 2014 |
|
| Participants | Total: N 1712 (Table 1 full sample 1562) Arm A [Standard cessation advice & NRT (NRT short‐term)] flow diagram 368 (324 received intervention) Arm B [Quit or substitute advice and NRT: advice to use NRT as a longer‐term substitute for smoking if required to maintain smoking cessation] (flow diagram 671 (620 received intervention) Arm C [Quit or substitute advice and NRT and/or e‐cigarettes] flow diagram 673 (619 received intervention) Inclusion criteria: Current daily smoking (at least 6 cpd). Agree to try samples of nicotine products. 18+ years. Exclusion criteria: Currently being treated for serious cardiovascular disease, cancer, taking regular medication for mental health condition, uncontrolled high blood pressure, stomach ulcer, kidney or liver disease, overactive thyroid or adrenal gland cancer. Use insulin for diabetes. Asthma or chronic throat disease. Pregnant or planning to become pregnant/breastfeeding Female 64%. Mean age 46.7 (SD 12.3). Mean CPD 18.2 (SD8.7) E‐cigarette use at baseline? 28% had previously tried an EC. Motivated to quit? Yes. 58% want to quit a lot. |
|
| Interventions | EC type: cartridge Arm C only: disposable e‐cigarette available in two strengths (free‐base nicotine 3.0% and 4.5%). A rechargeable version of the same brand with replaceable cartridges (3.0% v/v and 4.5% v/v) was substituted when the disposables were discontinued by the manufacturer (September 2014). The e‐cigarettes contained only nicotine, vegetable glycerin, and water, and were unflavoured. a) Arm A (usual care smoking cessation practice in Australia) comprising quit with NRT. Factsheet explaining relative harm of NRT compared to smoking, free sample of NRT, participant chooses preferences, has the intervention free for 3 weeks then offered at subsidized rate for further 6 months. The NRT products included nicotine gum, lozenges, inhalator and mouth spray. Lozenges and gum were offered at two strengths. b) Arm B As (a), but with additional information provided: advice to quit or substitute with NRT c) Arm C as (a), but additional information on electronic cigarettes and emphasis on cessation, and may select electronic cigarettes as well as NRT |
|
| Outcomes | Baseline, 7 months and 12 months, self‐report
|
|
| Study funding | Funding was from the National Health and Medical Research Council, Australia (#GNT1020123). The e‐cigarettes supplied in this trial were Vype brand, and were purchased from the manufacturer Nicoventures Trading Ltd., a UK‐based company that was a division of British American Tobacco. The other nicotine products were purchased in Australia from various distributors. Participant recruitment and survey data collection was managed by I‐View Social Research. None of these entities had any role in study design, data collection, data analysis, data interpretation, or writing of this paper. | |
| Author declarations | No authors have received financial support for the submitted work from any companies with a financial interest in the products under investigation. C.B. has undertaken consultancy for J&J Japan, a manufacturer of nicotine replacement therapy. N.W. and C.B. have completed a smoking cessation trial in which cytisine was supplied by Achieve Life Sciences, and a smoking cessation trial in which varenicline and matching placebo were supplied by Pfizer under their investigator‐initiated research programme. N.W. and C.B. have previously undertaken two trials of e‐cigarettes for smoking cessation (with e‐cigarettes purchased from a NZ e‐cigarette online retailer [NZVAPOR, https://www.nzvapor.com/], e‐liquid for one trial purchased from NicoPharm, Australia and nicotine patches supplied by the NZ government via their contract with Novartis [Sydney, Australia]). Neither NZVAPOR nor NicoPharm have links with the tobacco industry. None of the authors' spouses, partners, or children have financial relationships that may be relevant to the submitted work. All authors have no non‐financial interests that may be relevant to the submitted work. | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After completing the baseline survey, participants were block randomized in a 1:2:2 ratio to one of the three conditions by a computer generated random number sequence". |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants and researchers were not blind to the allocated treatment, however, participants were not advised that there were different treatment conditions". |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants and researchers were not blind to the allocated treatment, however, participants were not advised that there were different treatment conditions". |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "72.5% of participants who received the allocated intervention in Condition A completed the 7‐month survey, compared with 74.4% of Condition B and 72.9% of Condition C" At 12 mths: Arm A 233/324; Arm B 457/671; Arm C 448/673 |
| Selective reporting (reporting bias) | Low risk | Specified outcomes reported |
Morphett 2022b.
| Study characteristics | ||
| Methods | Design: RCT, pragmatic, randomized, partial cross‐over Setting: Australia Recruitment: Not stated Study start date: recruited in 2018‐2019 |
|
| Participants | Total: N 355 Arm A: 181 Arm B: 174 Inclusion criteria: Diagnosed with/ treatment for HIV or Hepatitis C (HCV) or receiving opioid substitution therapy (OST). Diagnosed with or receiving treatment for priority health conditions in the past 12 months. Referral to Quitline counselling and smoking cessation support programme (standard care) but has not begun quit attempt. 18+ years; currently smoke 10+ cigarettes per day; willing to make a quit attempt Exclusion criteria: Already started quit attempt (i.e. post‐quit day) or enrolled in another smoking cessation clinical trial or using varenicline or bupropion or used a nicotine vaporizer product in the last 30 days. Health reason (e.g. CVD, terminal illness, recent hospitalization for mental health reason, pregnancy) |
|
| Interventions | EC type: refillable Arm 1) Referral to Quitline telephone smoking cessation counselling + Nicotine patches (15 mg/16‐hr) delivered at baseline + refillable nicotine vaporizer device (2 x kits) + nicotine vaporising liquid (in high and low strength ‐ high strength: nicotine 1.8%; low strength: nicotine 0.6%). 1 patch to be applied daily to skin for up to 84 days. The vaporizer with nicotine liquid is to be used as needed up to 3.5 mL per day to treat withdrawal symptoms for up to 2 years (concurrently with patches for the first 84 days) to assist smoking cessation and relapse prevention. Participants start on high‐strength nicotine liquid and may decrease their dose to low strength to assist with dose reduction prior to stopping use of the vaporizer. Arm 2) Referral to Quitline telephone smoking cessation counselling + Nicotine patches (15 mg/16‐hr) + participant’s choice of either nicotine gum or nicotine lozenges (up to 800 x 4 mg pieces to be used up to 8 per day) delivered at baseline. Between 6‐9 months post‐baseline ‐ participants in Arm 2 who are smoking (either failed to quit or relapsed) will be offered: refillable nicotine vaporizer (2 x kits) + nicotine vaporizing liquid (in high and low strength ‐ high strength: nicotine 1.8% ; low strength: nicotine 0.6%) to make a second quit attempt. Participants will have until 2 years from baseline to use the vaporizer for smoking cessation and relapse prevention. Arm B participants who were smoking at 6 months were offered the NVP intervention (NVPs as second‐line therapy). Switched over to EC intervention, called Arm C |
|
| Outcomes | Baseline 6 mths, 12 mths, 24 mths Primary outcomes: Continuous abstinence from smoking from weeks 12 to 26 assessed at 26 weeks from baseline by self‐report (bio‐confirmed) Secondary outcomes: Continuous abstinence from smoking AEs at 12 weeks and 26 weeks Abstinence is assessed through study‐specific survey questions in Module CS. Combustible Smoking Questions – Urine specimens will be batch‐tested for anabasine and cotinine at 6‐, 12‐ and 21‐month time points. |
|
| Study funding | Not reported | |
| Author declarations | Not reported | |
| Notes | Abstract only New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Intervention arms both received interventions. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants that self‐report abstinence from smoking will be asked for a urine specimen for bio‐confirmation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No detail in conference abstract |
| Selective reporting (reporting bias) | Unclear risk | Conference abstract. Outcomes may be reported more fully at a later date. |
Morris 2022.
| Study characteristics | ||
| Methods | Design: Randomised cross‐over, open‐label, two‐part study Recruitment: Study participants were recruited from areas surrounding the study sites (Celerion, Lincoln, NE; Frontage, Secaucus, NJ) using standard advertising methods and were compensated for their participation in the study. Setting: 2 clinical research centres USA (Celerion, Lincoln, NE; Frontage, Secaucus, NJ) Subjects were confined to the respective clinics for the full duration of the study. Study start date: November 2019. Study end date: January 2020 |
|
| Participants | Total N: 79 (single‐arm) Inclusion criteria: Smoking an average of > 10 manufactured combustible cigarettes pd for at least 12 mths Exclusion criteria: Relevant illness history; presence of clinically significant mental or physical health conditions; high blood pressure; acute illnesses (e.g. upper respiratory infection, viral infection); relevant medication use; use of prescription smoking cessation treatments, anti‐diabetic or insulin drugs or medications known to interact with Cytochrome P450 2A6; body mass index (BMI) > 40 kg/m2 or < 18 kg/m2; allergy to propylene glycol or glycerin; planning to quit smoking during the study; pregnancy/breastfeeding; urine screen for alcohol or drugs of use Participants were between 21‐65 years. CPD 'at least 10' cpd. Percentage women not reported Motivated to quit: No E‐cigarette use at baseline: No |
|
| Interventions | EC: Pod Myblu™ two‐piece closed system comprised of a rechargeable 350 mAh battery and disposable pod containing an e‐liquid Sixteen commercial disposable liquid pod variants; different flavours; 5 different strengths (12, 24, 25, 36, 40 mg/mL); 2 forms of nicotine: nicotine salt or free‐base nicotine The e‐liquid mixtures consisted of VG, PG, nicotine and a proprietary blend of favours; pods contained 1.5 mL of e‐liquid, equating to approximately 200 puffs under standardized machine puffing conditions. The ENDS were charged and assembled for the participants and product information sheets provided. On each study day, fresh pods and a fully charged device were provided. All participants received training from clinic staff on how to operate their ENDS and to ensure compliance in the clinic; all participants used their products under the supervision of suitably qualified staff. |
|
| Outcomes | Baseline (‐2 day), day 9, day 14 15 biomarkers of exposure (BoE) to selected harmful and potentially harmful constituents (HPHCs) associated with tobacco smoking AEs |
|
| Study funding | This work was funded entirely by Fontem US LLC, a subsidiary of Imperial Brands PLC. | |
| Author declarations | This work was funded by Fontem US LLC, a subsidiary of Imperial Brands PLC, and manufacturers of the myblu™ products used in this study. This work was performed by Imperial Brands PLC on behalf of Fontem US LLC as a service provider. Work was contracted to Celerion, who conducted the study and analysed the data. At the time of the study and/or writing, PM, SM, FC, TV, XC, MS, JT, NC and GOC were employees of Imperial Brands PLC. | |
| Notes | Part 1 study data used only New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Study randomized, though for our analyses we treat as single‐arm. Quote from protocol: “Subjects who complete the study screening assessments and meet all the eligibility criteria and are randomized will be assigned a unique randomization identification number on Day ‐1 for Part 1 and on Day 10 for Part 2, and will receive study products according to the randomization scheme generated by Celerion”. |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Seventy‐two subjects completed the study and met the conditions for inclusion in the data analysis (out of a total of 79 recruited).” |
| Selective reporting (reporting bias) | High risk | FEV1 & FVC clinical trial registry outcomes not included in paper |
Myers‐Smith 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Clients of Queen Mary University of London's community stop‐smoking service who did not manage to stop smoking with routine treatment were invited to take part. Also recruited eligible smokers seeking help with quitting via social media. Setting: Queen Mary University of London, community stop‐smoking service. Study start date: April 2017 to August 2018 |
|
| Participants | Total: N = 135 N per arm: E‐cigarette 68; NRT 67 Inclusion criteria: History of failed quit attempts using stop‐smoking medications and/or stop‐smoking services. Willing to use their allocated harm‐reduction strategy for at least 4 weeks. 18+ years Exclusion criteria: Currently using EC or any stop‐smoking products. Strong preference to use or not to use NRT or EC. Pregnancy or breastfeeding Women: 49%; Mean age 40; Median CPD 15 (IQR 10); Median FTND: 5 EC arm, 4 NRT arm; motivated to quit E cigarette use at baseline: percentage tried EC earlier: 31% EC arm, 49% NRT arm |
|
| Interventions | EC: Refillable EC Arm. EC starter pack and instructions to purchase further e‐liquids of flavour and strength of their choice (voucher for up to £40). Participants paid for further supplies themselves. They were encouraged to try e‐liquids of different strengths and flavours if the initial purchase did not meet their needs. Up to 8 weeks supply. Minimal behaviour support NRT Arm: NRT of choice. The choice of products included nicotine patch, chewing gum, nasal spray, microtab, inhalator, and mouth spray. Up to 8 weeks supply. At the baseline visit, participants selected an NRT product or product combination. Minimal behavioural support Both groups: 2 face‐to‐face sessions (baseline & week 1). Baseline: Participants selected products of their choice and received instructions on how to obtain them. Week 1: Smokers bring allocated product to the session, receive advice on use, test and start product use. Commitment to not using unallocated products for the next four weeks. Those wishing to stop smoking altogether were asked to set a target quit date (TQD) . Participants received phone calls one and four weeks later to monitor product use and smoking status and to provide brief support. |
|
| Outcomes | Baseline (week 0), week 1, week 4, week 24 Primary outcome measure: Cigarette consumption per day, assessed by self‐report in the follow‐up survey created for the purpose of the study at 1, 4 and 24 weeks post‐quit date/preparation date. Those who report ≥ 50% smoking reduction will be validated with a CO reading in the clinic. Secondary outcome measures: 1. Use of allocated harm reduction strategies. 2. Strategy ratings. 3. Changes in smoking behaviour. 4. Proportion of people still using allocated strategy at 6 months. All measured by the follow‐up survey created for the purpose of the study at 1, 4 and 24 weeks post‐quit date/preparation date. AEs |
|
| Study funding | The study was funded by a Tobacco Advisory Group project grant, Cancer Research UK (C6815/A20503) | |
| Author declarations | PH and HM have received research funding from and provided consultancy to Pfiizer, a manufacturer of stop‐smoking medications. DP has received research funding from Pfizer. All other authors had no conflicts to declare. | |
| Notes | Participants invited for CO readings at 4 weeks and 6 mths received £10 in compensation for their time and travel at both visits. New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation sequences (1:1 ratio in permuted blocks of 20) were produced by an independent statistician using computer generated randomisation codes.“ |
| Allocation concealment (selection bias) | Low risk | Quote:“Codes were sealed in opaque envelopes and marked with a unique randomisation number. Study staff allocated randomisation numbers sequentially. Staff opened the next envelope and entered the allocation onto the clinical record form (CRF) and randomisation log.” |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Data analysis was completed blind by an independent statistician.” Both arms active intervention |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Data analysis was completed blind by an independent statistician.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “Follow‐up rates were 85% and 88% at 4 weeks and 88% and 70% at 6 months in the EC and NRT group, respectively.” |
| Selective reporting (reporting bias) | Low risk | Outcomes registered were all reported. |
NCT02648178.
| Study characteristics | ||
| Methods | Setting: Medical centre, USA Recruitment: People with cancer Design: Non‐randomized single‐group assignment trial Recruitment: Clinical settings, including outpatient clinics and the infusion suite Study start date: June 2016; Study end date: May 2018 |
|
| Participants | Total N: 19 Inclusion criteria: histological or cytological diagnosis of aerodigestive tract cancers or bladder cancer within the past 5 years (≥ 1 tobacco‐related malignancy is allowed); AJCC stages I‐IV; daily smoking (≥10 CPD for 10 years) and breath CO2 ≥ 8 ppm; does not wish to quit smoking now (anyone wishing to quit smoking will be referred for smoking cessation counselling through the WRJ VAMC or DHMC programme); may be receiving anti‐cancer agents; age ≥ 18+ years. Exclusion criteria: cancer surgery or radiation planned in the next 9 weeks; actively trying to quit smoking, or planning to in the next 30 days.; any EC use in the past 30 days; pregnant or trying to get pregnant. Inclusion based on specific population characteristic: Patients with stage I‐IV aerodigestive tract cancers or bladder cancer who smoke daily 42.1% women; mean age: not reported ‐categories 18‐65 years: N = 9, > 65 years: N = 10; cpd and FTND: not reported. Motivated to quit: No (inclusion criterion) E‐cigarette use at baseline: Not specified but EC use within 30 days is an exclusion criterion |
|
| Interventions |
EC: Cig‐a‐like and refillable Instructed on use of EC, and given a supply that is "approximately equivalent to their current nicotine intake". Given Halo Triton EC (leak‐proof refillable tank system) or Halo G6 leak proof prefilled cartomizers. Began participants with 18 mg/mL and moved nicotine content up or down based on participant preference. Choice of flavors, provided for 9 weeks |
|
| Outcomes | Weeks 3, 6, 9, 12. Self‐report at clinic visits Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | Not reported – data extracted from clinical trial registry record | |
| Author declarations | Not reported – data extracted from clinical trial registry record | |
| Notes | Study listed as ongoing study in the 2016 review update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized, single‐group assignment |
| Allocation concealment (selection bias) | High risk | Not randomized, single‐group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 19 enrolled; 10 participants followed up at 12 weeks |
| Selective reporting (reporting bias) | Unclear risk | The following measures were not reported: exhaled carbon dioxide; urine propylene glycol; urine nicotine, cotinine, NNAL and 1‐ hydroxy naphthalene (1‐HOP), and Timeline Follow‐Back Questionnaire (TLFB). Data at 6, 12 months also not reported |
NCT02918630.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Clinics Setting: SMI clinics, USA Study start date: October 2016; Study end date: August 2017 |
|
| Participants | Total N: 7 N per arm: NRT: 4; EC+NRT 3 Inclusion criteria: diagnosed with schizophrenia (or other SMI, not clear); be in stable medical condition (DSM‐V); report smoking ≥ 10 tobacco cigarettes/day; breath CO ≥ 10 ppm; report wanting to reduce their cigarette smoking; stable living situation. Exclusion criteria: pregnant or breastfeeding; report wanting to quit smoking in the immediate future; test positive for illicit drugs except THC; any illness, medical condition, or use of medications, which in the opinion of the study physicians would preclude safe or successful completion of the study, or both. Inclusion based on specific population characteristic: Yes ‐ SMI (schizophrenia and schizoaffective disorder, bipolar disorder, or PTSD) 43% women; mean age 48.3; mean cpd: NR; mean FTND: NR Motivated to quit: Wanted to quit or reduce their cigarette smoking but did not want to quit in the immediate future (this was an exclusion criterion) NB – trial registry states wanted to reduce and protocol states wanted to quit or reduce as inclusion criteria E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Refillable Both arms received a nicotine patch 21 mg for 4 weeks EC + NRT: 4 weeks: 1) a 3.3 V, 1000 mAh battery; and 2) a 1.5 Ohm, dual‐coil cartomizer (SmokTech; Shenzhen, China). Nicotine concentrations 36 mg/mL. Verbal and written instructions on how to use and maintain the e‐cigarettes at Week 1 visit NRT arm: NRT only |
|
| Outcomes | 5 weeks Cessation: n/a but “change in smoking” Adverse events and biomarkers: Breath CO, COPD‐related symptoms, EC side effects (e‐cig side effects questionnaire), AEs, SAEs Other outcomes measured: Urinary cotinine, cpd, tobacco dependence, craving, withdrawal symptoms, desire to quit, confidence to quit, EC dependence, EC use, satisfaction with EC, nicotine dependence, schizophrenia symptoms (brief psychiatric rating scale), cognitive domains associated with schizophrenia (MATRICS consensus cognitive battery), changes in positive symptoms of schizophrenia (scale for the assessment of positive symptoms), changes in negative schizophrenia symptoms (scale for the assessment of negative symptoms), suicide ideation (Columbia Suicide Severity Rating Scale) |
|
| Study funding | Not reported | |
| Author declarations | Not reported | |
| Notes | New for 2020 update. Information from clinical trials gov [http://clinical trials gov] registry and unpublished protocol; discrepancies between the two in terms of trial methods. Feasibility for future NIH grant application. Intended to recruit 20 participants but only 7 started and completed | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | “double‐blind” but “open‐label” elsewhere, no further info given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | High risk | Schizophrenia and COPD outcomes not reported. |
| Other bias | Unclear risk | Some discrepancies between clinical trials record and protocol linked to from record, including when NRT started and inclusion criteria (just schizophrenia or all SMI). Target sample size was 20 but only 7 people recruited |
NCT03492463.
| Study characteristics | ||
| Methods | RCT Initially the study design included placebo patch control conditions, but due to limitations in budget and period of support, enrolment in these arms was discontinued. All participants currently received active nicotine patches. Setting: USA |
|
| Participants | Total: N 94 Female 59.6%. Mean age 47.2 (SD 10.3) Inclusion criteria: Smoke an average of > 10 cpd and for > 1 cumulative year; expired CO > 10 ppm; body weight of > 110 lbs (50 kg) & ≤ 300 lbs (136 kg). Potential subjects of childbearing potential must agree to use acceptable contraception. Potential subjects must agree to avoid participation in any other nicotine‐related modification strategy outside of this protocol. Exclusion criteria: Seeking treatment for nicotine dependence. Hypertension, hypotension, CVD or other health conditions, illegal drug use. Pregnancy/breastfeeding. See NCT record for full exclusion criteria. |
|
| Interventions | EC: 'e‐cigarettes containing nicotine' All participants will be asked to switch from combustible cigarette use to use of the study EC for eight weeks. Arm 1. Nicotine EC + nicotine patches Arm 2. Non‐nicotine EC + nicotine patches Arm 3. Nicotine EC + placebo patches Arm 4. Non‐nicotine EC + placebo patches |
|
| Outcomes | Week 8 Expired air carbon monoxide (CO) to assess recent smoking. Cigarette use. EC use. Total urinary 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL). AEs. |
|
| Study funding | National Institute of Drug Abuse (NIDA) | |
| Author declarations | Nor reported | |
| Notes | Results posted 28 June 2022 New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available |
| Allocation concealment (selection bias) | Unclear risk | No information available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Placebo‐controlled but no detail on the nature of placebos (trial registry only) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Placebo‐controlled but no detail on the nature of placebos (trial registry only) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Overall attrition was less than 50% and similar between arms. |
| Selective reporting (reporting bias) | Unclear risk | Partial results available in trial registry to date |
Nides 2014.
| Study characteristics | ||
| Methods | Design: Open‐label non‐comparative study Recruitment: Study site database and community advertisements Setting: Clinical Trials Unit, USA Study start date: April 2013; Study end date: 10 July 2013 |
|
| Participants | Total N: 29 Inclusion criteria: age 18‐65 yrs; good health; BMI 18‐35; smoking 10+ cpd; CO > 10 ppm Exclusion criteria: pregnancy or breastfeeding; other drug dependency; use of any psychiatric or opioid medications; EC within the previous 14 days; use of NRT in last 30 days' want to reduce or quit smoking within the next 30 days. Exclusion criterion: EC within the previous 14 days; use of NRT in last 30 days 44% women; mean age 43; mean cpd 20.1; mean FTND 4.5 Motivated to quit: no E‐cigarette use at baseline |
|
| Interventions |
EC: Cig‐a‐like Participants attended 3 clinic visits at 1‐week intervals Visit 1: Baseline Visit 2: Provided with 1st generation type ‐ 'NJOY® King Bold' (NJOY, Inc. Scottsdale, AZ), with 26 mg nicotine. Used ad libitum for 20 minutes in the clinic, then ad libitum use over the next week. Recorded use of regular cigarettes and puffs on EC Visit 3: Participants abstained from all sources of nicotine for 12 hours prior to visit |
|
| Outcomes | Adverse events | |
| Study funding | Funding for this study was provided by NJOY, Inc., Scottsdale, AZ | |
| Author declarations | Dr Nides has received compensation from NJOY, Inc. and GlaxoSmithKline. Dr Leischow has received compensation from GlaxoSmithKline, Pfizer, and Cypress Bioscience. Mr Simmons and Ms Bhatter have no conflict of interest to report | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 participants dropped out between visits 1 and 2 |
| Selective reporting (reporting bias) | Low risk | Planned comparisons reported |
Okuyemi 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: The study identified African‐American current cigarette smokers using the electronic health record in the University of Minnesota Fairview Health system. The authors sent recruitment letters that provided a brief description of the study and invited recipients who were interested in the study to contact study staff. Participants were also invited to refer contacts outside their family to the study. Setting: Study visits were conducted in the Delaware Clinical Research Unit (DCRU) through the Clinical and Translational Science Institute (CTSI) at the University of Minnesota, USA. Study start date: June 2018. Study end date September 2019. (NCT record: Start date November 15 2016. End date March 8 2019). |
|
| Participants | Total N: 234 Nicotine EC arm: 118 Non‐nicotine EC arm: 116 Inclusion criteria: 1) Self‐identification as African‐American or black, 2) smoked ⩾ 5 cigarettes daily for the past yr, smoking status confirmed by expired CO ≥ 5 ppm or positive NicAlert screen, 3) willingness to use EC, 4) 18‐79 years Exclusion criteria: 1) Recent unstable or untreated psychiatric diagnosis including substance abuse (DSM‐IV criteria), 2) EC use in the past 30 days, 3) planning to quit smoking in the next 30 days, 4) pregnancy or nursing, 5) CO was < 5 ppm and no cotinine detected in the urine Female: 43.9%. Mean age 50.8 (SD 11.2). Mean CPD 11.5 (SD 6.0) Motivated to quit: No E‐cigarette use at baseline: No |
|
| Interventions | EC: Refillable EC with 24 mg of nicotine added. Nicotine EC rechargeable Halo G6 brand 2.4% nicotine (24 mg, equivalent to the nicotine content of combustible cigarettes) EC ‐ No‐nicotine EC rechargeable Halo G6 brand 0% nicotine (0 mg) For both groups: A free Halo G6 brand rechargeable EC starter kit with the accessories including the charger, batteries, and a 2‐week supply of liquid cartridges. The Halo G6 device was 3.3‐4.2 (average 3.7) volts. The prefilled cartomizers coil resistance was 2.2‐2.8 ohms. At the wk‐2 visit, participants received an additional 4‐wk supply of cartridges. Participants were given oral and written instructions about how to use the products. Ad lib for 6 weeks. All participants were provided with EC by the study; menthol and non‐menthol flavoured EC cartridges were available. Participants could purchase their own if needed after 6 weeks. Participants were compensated for their time and transportation: $40 at baseline, $40 at week 2, $50 at week 6, and $20 at week 12, for a maximum of $150 over 12 weeks. |
|
| Outcomes | Baseline, 2, 6, and 12 weeks (all visits were in‐person except week 12 which was a telephone survey) Biomarkers at baseline and 6 weeks. Urinary biomarkers (NNAL, NNK) and total nicotine equivalents (TNE, total nicotine + total cotinine + total 3‐hydroxycotinine + nicotine N‐oxide). Expired carbon monoxide (CO) Combustible cigarettes self‐reported number of cigarettes smoked per day (and baseline). EC use (Penn State EC Dependence Index) EC dependence (10‐item Penn State EC Dependence Index) Nicotine withdrawal symptoms (baseline, week 2, 6, 12) (modified Minnesota Nicotine Withdrawal (MNWS) Data collection was self‐administered and collected on electronic tablets. |
|
| Study funding | ClearWay Minnesota Grant Award #RC‐2014‐0009 | |
| Author declarations | The authors declared that they had no competing interests. | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: ”To ensure balance in the number of intervention assignments between the study groups, randomization was blocked (block size unknown to staff or investigators) by nicotine versus no nicotine e‐cigarettes.” No other information given |
| Allocation concealment (selection bias) | Unclear risk | No information given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Single‐blind. Interventions equally intensive |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biomarkers measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nicotine EC arm: 109/118 Non‐nicotine EC arm: 106/116 |
| Selective reporting (reporting bias) | Low risk | Reported outcomes in NCT record |
Oncken 2015.
| Study characteristics | ||
| Methods | Design: Randomized cross‐over study Recruitment: Newspaper advertisements, radio announcements, and from local general medicine practices Setting: Lab‐based study, Connecticut, USA Study start date: October 2012; Study end date: June 2015 |
|
| Participants | Total N: 27 Inclusion criteria: non‐treatment‐seeking people who smoke who were willing to try EC for 2 weeks and abstain from conventional cigarette smoking; 18–55 years of age who smoked at least 10 cpd. Exclusion criteria: pregnant; previous myocardial infarction or stroke; uncontrolled hypertension (blood pressure (BP) > 160/100); insulin‐dependent diabetes; COPD or current asthma; known allergy to propylene glycol. 45% women; mean age 42; 70% white; 15% Hispanic, 15% black; mean cpd 16; 45% had tried EC at baseline, 50% smoked menthol cigarettes Motivated to quit: No E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like Prescribed Joye eGo‐C (www.joyetech.com) and e‐Juice (18 mg/mL nicotine) procured from American eLiquid (www.americanliquid.com) Cross‐over study between menthol‐flavoured and non‐menthol tobacco‐flavoured EC. Requested not to smoke their regular cigarettes during study period, but most (60%) reported intermittently smoking cigarettes during study |
|
| Outcomes | Follow‐up at 1 wk and 2 weeks BP, heart rate, body plethysmography, static lung volumes and airways resistance (Raw) and specific conductance (sGaw) – taken at lab visits after abstaining from EC for at least 2 hrs, then taken again after inhaling EC and repeated 5 mins later Adverse events also reported but method for measuring not stated Also measured nicotine concentrations, rates of cigarette and EC use |
|
| Study funding | This project was supported by Academic Enhancement funds from the Department of Medicine at the University of Connecticut Health Center (to CO) and the Clinical Research Center at the University of Connecticut Health Center | |
| Author declarations | CO is currently receiving study medication (nicotine inhaler and placebo) from Pfizer pharmaceuticals for an NIH funded of nicotine inhaler for smoking cessation during pregnancy | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated; Quote: "Subjects were then randomly assigned to use the menthol or plain e‐cigarette cartridge for one week, switching to the other cartridge for the second week" |
| Allocation concealment (selection bias) | Unclear risk | No detail given |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | No detail given on blinding but equal levels of support between arms, so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Some subjective outcomes but equal levels of support between arms so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20/27 followed up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Ozga‐Hess 2019.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Cigarette smokers were recruited from the community via fliers, online postings, and word of mouth Setting: Morgantown, West Virginia, USA Study start date: Not reported. Study end date: Not reported |
|
| Participants | Total N: 60; E‐cigarette plus own brand = 30. Own brand cigarette (control) = 30 38.3% female; mean age completers 35.1 (SD 11) (N = 34) non‐completers 36.8 (SD 12.9) (N = 26); mean cpd completers 16.7 (SD 4.9), non‐completers 19.6 (SD 6.1); mean FTND completers 5.3 (SD 1.8), non‐completers 5.9 (SD 1.9) Inclusion criteria: 18 to 60 years; smoking ≥ 10 cigarettes per day for ≥ 1 year; exhaled air carbon monoxide (CO) level of ≥ 10 ppm (Micro+™ basic monitor; CoVita; Haddonfield, NJ); contemplation or Preparation Stage of Change (indicating interest in a quit attempt within the next 1‐6 months). Exclusion criteria: reported chronic health or psychiatric conditions; past month use of marijuana ≥ 5 days; past month use of any other illicit drugs, or regular use of ECIGs or other tobacco products (i.e. ≥ 1 day per week); individuals in the Precontemplation (no interest in quitting) or Action (actively trying to quit) Stage of Change; pregnancy/breastfeeding. |
|
| Interventions |
EC: Refillable E‐cigarette (18 mg/mL ) plus own brand cigarette. Kanger mini Protank‐II, which is a 1.5 ml Pyrex glass tank with a drip tip and atomizer head coils (KangerTech; China), and a 3.3 V constant output, 900 mAh, eGo‐T battery (Joyetech; Irvine, CA). The liquid (The Vapor Room, Sky Vapors LLC, Frostburg, MD) was labelled as 70% propylene glycol and 30% vegetable glycerin, with a nicotine concentration requested of 18 mg/mL. Participants could choose tobacco, menthol or wild berry flavour and could switch between sessions. Ad libitum use for 4 weeks Own brand cigarette ad libitum use for 4 weeks |
|
| Outcomes | Daily for salivary cotinine samples. Daily self‐monitoring device to log e‐cigarette and cigarette use. Collected used cigarette filters Weekly CO breath test Attended the laboratory weekly for assessments (Days 8, 15, 22, and 29). Then completed a follow‐up visit 1‐month post‐intervention self‐reported withdrawal symptoms Reported experience of specific symptoms rated using a visual analog scale with a range from 0 (not at all) to 100 (extremely). e.g. craving, irritability, dry mouth, throat irritation, and cough |
|
| Study funding | Financial support provided to MDB and GAD by WVU Senate Grant for Research, and to GAD, MDB, and NAT by Cooperative Agreement Number 1‐U48‐DP‐005004 from the Centers for Disease Control and Prevention (CDC) to the West Virginia Prevention Research Center. Support provided to NJF and JEOH by the National Institute of General Medical Sciences (NIGMS T32 GM081741). Additional support provided by WV Tobacco Cessation QuitLine | |
| Author declarations | Author SGF has consulted for various pharmaceutical companies on matters relating to smoking cessation. All other authors declare that they have no conflicts of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Using a simple randomized design" Comment: not adequately explained |
| Allocation concealment (selection bias) | Unclear risk | Not adequately described in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and investigators were not blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40% retention, but no difference between groups |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Pacifici 2015.
| Study characteristics | ||
| Methods | Design: Uncontrolled pre‐post pilot study Recruitment: Word of mouth Setting: Hospital‐based smoking cessation clinic, Italy Study start date/end date: Not specified |
|
| Participants | Total N: 34 Inclusion criteria: adults who smoke, unwilling to quit smoking tobacco cigarettes and who have never tried a quit‐smoking protocol or have refused any smoking cessation treatment, or both Exclusion criteria: none stated Inclusion based on specific population characteristic: No 47.1% women, mean age 40.6, mean cpd 21.5 no EC use at baseline, not motivated to quit |
|
| Interventions |
EC: Refillable Participants were given commercially‐available EC (AVATAR device, Battery 550 mAh/3.9 V, W: 7.8, cartomizer with 2, 2 ohm resistance, tank capacity 1.5 mL, temperature of the aerosol: 55/65 degrees), 2 different chargers for each EC and PUFFIT e‐liquids with nicotine content matching the individual nicotine daily intake and tobacco and/or other flavors freely chosen by each participant W1: nicotine‐free e‐liquid W2&3: Own EC with personal nicotine dosage, encouraged to use as substitute for traditional cigarettes W4: Encouraged to forego all traditional cigarettes Throughout: assistance at any time of day from centre staff with any EC‐related problem, plus follow‐up group sessions and smartphone messaging application Behavioural support: Multi‐component medically‐assisted training programme with monitoring of nicotine intake as a biomarker of correct EC use, including Information about general working principles, safety and risks of EC, together with medically‐assisted face‐to‐face training on how to correctly use the device to absorb nicotine vapor |
|
| Outcomes | Follow‐up at 1, 4 and 8 m Cessation (measure not defined) Adverse events Exhaled CO, COT, 3‐HCOT concentration cpd |
|
| Study funding | The authors thank Renata Solimini, Adele Minutillo, Emilia Marchei and Maria Concetta Rotolo for their technical assistance. This work was supported by the Department of Therapeutic Research and Medicines Evaluation Istituto Superiore di Sanità, Roma, Italy | |
| Author declarations | The authors declare no conflict of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not controlled |
| Allocation concealment (selection bias) | High risk | Not controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants followed up |
| Selective reporting (reporting bias) | High risk | AEs measured but not reported |
Polosa 2011.
| Study characteristics | ||
| Methods | Design: Prospective cohort Recruitment: Advertisments in local hospital in Catania, Italy Setting: not specified Study start date: February 2010; Study end date: June 2010 |
|
| Participants | Total N: 40, hospital staff Inclusion criteria: healthy people who smoke; 18‐60 years; smoking ≥ 15 cpd for ≥ past 10 years, and not wanting to quit smoking at any time in the next 30 days. Exclusion criteria: history of alcohol and illicit drug use; psychiatric illness; recent myocardial infarction; angina pectoris; high blood pressure (BP > 140 mmHg systolic or 90 mmHg diastolic, or both); diabetes mellitus; severe allergies; poorly‐controlled asthma or other airways diseases. 35% women, mean age 42.9 (SD 8.8), median cpd 25 (IQR 20‐30), median FTND 6.0 (IQR 6‐8) Motivated to quit: No E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like Seen at baseline, given EC ('Categoria' brand) with an initial 4‐week supply of 7.4 mg nicotine cartridges. Instructed to use ad libitum up to 4 cartridges per day. EC cartridges supplied at months 1, 2, and 3 No instruction on cessation or reduction was provided |
|
| Outcomes | Follow‐up at 1, 2, 3, 6, 18 and 24 months where cigarette consumption, CO, and AEs were measured, incl. 30‐day PP CO‐validated abstinence at 6 months and CO‐validated abstinence at 18 and 24 months (not otherwise defined) Adverse events |
|
| Study funding | "We wish to thank Arbi Group Srl (Milano, Italy) for the free supplies of ‘Categoria’ e‐Cigarette kits and nicotine cartridges as well as their support. We would also like to thank the study participants for all their time and effort and LIAF (Lega Italiana AntiFumo) for the collaboration" | |
| Author declarations | "None of the authors have any competing interests to declare, but RP has received lecture fees from Pfizer and, from Feb 2011, he has been serving as a consultant for Arbi Group Srl.Arbi Group Srl (Milano, Italy), the manufacturer of the e‐Cigarette supplied the product, and unrestricted technical and customer support. They were not involved in the study design, running of the study or analysis and presentation of the data" | |
| Notes | Smoking cessation services provided to those who spontaneously asked for assistance with quitting. These participants were excluded from the study protocol | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 13/40 were lost to follow‐up, but used ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Polosa 2014b.
| Study characteristics | ||
| Methods | Design: Prospective cohort study Recruitment: Volunteers, leaflets, cessation service kiosk in hospital Setting: Smoking cessation clinic, Italy Study start date: January 2013; Study end date: November 2013 |
|
| Participants | Total N: 50 Inclusion criteria: healthy people who smoke; 18–60 years; smoking ≥ 15 conventional cpd ≥10 years; unwilling to quit. Exclusion criteria: none stated 40% women, mean age 41, mean cpd 25, mean FTND 6.0 No EC use at baseline, not motivated to quit |
|
| Interventions |
EC: Refillable 2nd generation devices (personal vaporisers ‐ PVs): EGO/CE4 model, filled with tobacco aroma e‐Liquid containing 9 mg/mL nicotine; instructed to use the study products ad libitum (up to a maximum of 5 ml/day; i.e. half vial) Behavioural support: Participants were instructed how to charge, fill, activate and use the EC. Key troubleshooting was addressed and phone numbers were supplied for assistance. “No emphasis on encouragement, motivation and reward for the smoking cessation‐related efforts were provided during the study.” |
|
| Outcomes | 4, 8, 12 and 24 weeks 30‐day PP verified by CO ≤ 10 ppm Adverse events Cpd, exhaled CO, reduction rates, product usage, and opinions of the EC products |
|
| Study funding | "The authors wish to thank FlavourArt (Oleggio, NO, Italy; www.flavourart.it). Authors wish to thank LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti Smoking League) for supporting this research" | |
| Author declarations | "RP has received lecture fees and research funding from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has also served as a consultant for Pfizer and Arbi Group Srl, an Italian distributor of e‐Cigarettes. RP is currently scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti Smoking League). PC, MM, JBM, and CR have no relevant competing interest to declare in relation to this work" |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not controlled |
| Allocation concealment (selection bias) | High risk | Not controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 76% followed up, ITT analysis used, no significant differences in baseline characteristics between completers and those lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Polosa 2015.
| Study characteristics | ||
| Methods | Design: Prospective cohort Recruitment: Professional retail staff in participating vape shops Setting: 7 vape shops in Catania province, Italy Study start date/end date: Not specified |
|
| Participants | Total N: 71 Inclusion criteria: adults who smoke (≥ 18 years); making first purchase at participating vape shop (definition of smoker not stated). Exclusion criteria: none stated 38% women, mean age 41.7, mean cpd 24.9, mean FTND 5 No EC use at baseline |
|
| Interventions |
EC: Refillable Instructed how to charge, fill, activate and use EC; key troubleshooting advice provided; phone number available for technical support “Encouraged to use these products in anticipation of reducing the number of cig/day smoked” |
|
| Outcomes | 6 and 12 m follow‐up 30‐day PPA via self‐report Details of product purchase Sustained 50% and 80% reduction in cpd from baseline |
|
| Study funding | Authors wish to thank the local participating Vape Shops and LIAF, Lega Italiana Anti Fumo (Italian acronym for the Italian Anti‐Smoking League) for supporting this research | |
| Author declarations | Riccardo Polosa has received lecture fees and research funding from Pfizer and GlaxoSmithKline, manufacturers of stop smoking medications. He has also served as a consultant for Pfizer and Arbi Group Srl, an Italian distributor of e‐Cigarettes. Riccardo Polosa is currently scientific advisor for LIAF, Lega Italiana Anti Fumo (Italian acronym for Italian Anti‐Smoking League). Jacques Le‐Houezec is a consultant for Johnson & Johnson France, a manufacturer of nicotine replacement therapy, and was reimbursed for travel and accommodation to present at a conference in Shenzhen (China) organized by the e‐cig manufacturer association (CECMOL). Pasquale Caponnetto and Fabio Cibella have no relevant conflict of interest to declare in relation to this work | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not controlled |
| Allocation concealment (selection bias) | High risk | Not controlled |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 69% follow‐up at 12 m. Participants lost to follow‐up considered as continuing smokers |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Pratt 2016.
| Study characteristics | ||
| Methods | Design: Observational study – uncontrolled experimental study Recruitment: community mental health centre through self‐referral and clinician referrals Setting: community mental health centre (USA) Study start date: October 2013; Study end date: June 2014 |
|
| Participants | Total N: 19 (21 originally recruited, however 2 participants did not return for any weekly visits so 19 analyzed) Inclusion criteria:
Exclusion criteria:
Inclusion based on specific population characteristic: Psychiatrically stable, in‐treatment, people who smoke with a schizophrenia spectrum disorder or bipolar disorder 68% women; mean age 42; mean cpd: Only cigarettes per week reported: 192 (SD = 159.3). This would be an average of 27 cpd; mean FTND 5.5 Motivated to quit: “None of the participants was actively engaged in a quit attempt during the study” E‐cigarette use at baseline: E‐cig use was an exclusion criterion |
|
| Interventions |
EC: Cig‐a‐like E‐cigarette details: (NJOY brand) based on each participant's level of use of combustible tobacco. Each e‐cigarette cartridge was approximately equivalent to 2 packs of combustible cigarettes. Trained research interviewers instructed participants on the proper use of e‐cigarettes |
|
| Outcomes | Week 1, 2, 3, 4 Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | “Financial support to purchase the e‐cigarettes and pay small stipends to the participants in this unfunded pilot study came from Dr. Mary Brunette's discretionary reserve account.” | |
| Author declarations | “All authors declare that they have no conflicts of interest” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 dropouts (9.5%) failed to return to clinic. Analysis based on 19 participants |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Pratt 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Clinician referrals, posters/brochures and mailings. After eligibility confirmation, potential participants were invited for an informational meeting, and interested individuals returned to review the consent form and provide written informed consent. Setting: Two urban mental health agencies (Kentucky and Massachusetts) serving primarily Medicaid beneficiaries with SMI. USA Study start date: March 1 2017. Study end date: January 31 2021 |
|
| Participants | Total: N = 240 EC = 120 Assessment only = 120 Inclusion criteria: 1) Diagnosis of schizophrenia,schizoaffective disorder, or bipolar disorder, 2) enrolled in services at the research site for a minimum of 3 months, 3) regular smoker (approx. 10 cigarettes for the past 5 years) with a history of at least 1 quit attempt, 4) 18+ years Exclusion criteria: 1) Regular use of EC in the past month, 2) current interest/plan to quit smoking, 3) regular use of NRT or bupropion or varenicline to quit smoking, 4) use of emergency room or hospitalization for psychiatric reasons in the past 30 days, 5) pregnancy, 6) psychiatric instability (hospitalization in the past mth), 7) active substance use disorder Female 47.9%. Mean age 45.9 (SD 11.9). Mean CPD 18.7. Mean FTND 6.9 (SD 1.5). E‐cigarette use at baseline: No Motivated to quit: No |
|
| Interventions | EC: Cartridge Arm 1: EC The Study Coordinator provided participants with a 2‐week supply of e‐cigarettes (EC) and instructions on their safe use. Per product packaging, each disposable EC provided up to 300 puffs, roughly the equivalent of 20 cigarettes. Participants were given the opportunity to practice using EC before leaving the appointment to ensure proper use. The Study Coordinator also provided brief information on safety (e.g. keeping EC out of the reach of children) and they gave participants additional 2‐week supplies at 2, 4, and 6 weeks. The EC arm was provided with 8 weeks of free ECs based on self‐report of regular tobacco use. Participants assigned in this arm were asked to switch combustible tobacco with ECs. The appeal of EC and health impacts were measured, but the authors were not targeting quitting combustible tobacco or reducing craving. Arm 2: Assessment only (no intervention) EC was given at final FU visit. Following randomization, Study Coordinators provided participants with appointments for follow‐up study visits, asked them to refrain from using ECs, and reminded participants that they would receive a 4‐week supply of ECs at the final follow‐up visit. |
|
| Outcomes | Baseline, 2, 4, 6, 8, 13 and 26 weeks Breath CO was measured by the blinded Research Interviewers at each visit using the Smokerlyzer Breath Carbon Monoxide Monitor (Bedfont Scientific) as a biologic measure of toxin exposure. CO, CPD, nicotine dependence, EC use (EC count) In NCT record but not reported in paper: change in cancer related toxin, 4‐(Methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol or NNAL [time frame: baseline, 4 weeks, 8 weeks, 13 weeks, 26 weeks], urine NNAL analysis AEs: Cough, itchy throat, bad throat |
|
| Study funding | The study was funded by the National Institute on Drug Abuse (NIDA, 1R01DA041416) in the United States. | |
| Author declarations | None declared | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Unblinded Study Coordinator randomly assigned participants within site using an automated program that stratified by diagnosis (schizophrenia vs. bipolar disorder) and amount of daily smoking (> 20 vs. ≤ 20 cigarettes), in blocks of four to assure balance between arms (1:1 ratio).” |
| Allocation concealment (selection bias) | Low risk | As above, automated programme |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Interventions of different intensity. EC vs assessment only (control) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinded Research Interviewers” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 240 randomized participants. Among those participants, 210 (87.5%) were assessed at 8 weeks, and 214 (89.2%) were assessed at 26 weeks. |
| Selective reporting (reporting bias) | Unclear risk | Some outcomes not reported here |
Pulvers 2018.
| Study characteristics | ||
| Methods | Design: Observational uncontrolled experimental study Recruitment: Community Setting: Visits took place in University labs, USA Study start date: January 2015; Study end date: April 2015 |
|
| Participants | Total N: 40 Inclusion criteria: ≥ 18 years; cigarette smoking ≥4 days of the past 30 days for at least 1 year; never using EC regularly (less than 25 lifetime uses); not having used EC on ≥3 of the past 30 days; willing to switch from smoking regular cigarettes to ECs; fluency in English; regular access to a telephone and transportation to attend appointments; willing to abstain from using marijuana during the study. Exclusion criteria: any use of other tobacco products (OTPs) including smokeless tobacco, cigarillos, pipes, cigars, hand‐rolled cigarettes, and hookah in the past 30 days; currently in a smoking cessation programme or another clinical trial; past 30 day use of NRT or medication which aids smoking cessation including bupropion, clonidine, nortriptyline, or varenicline; uncontrolled asthma, severe allergies, or diabetes mellitus; taking prescription medication for emotional distress, depression, or other psychological problems; current dependence on a substance other than nicotine; presence of any cardiovascular or pulmonary illnesses in the past 6 months; pregnancy. Inclusion based on specific population characteristic: No 27% women; mean age 30.08; mean cpd 8.76; FTND not reported Motivated to quit: over half either did not intend to quit at all or did not intend to quit in the next 6 months 22/40 (55%) E‐cigarette use at baseline: Inclusion criteria included the following:
|
|
| Interventions |
EC: Refillable 2nd generation EC starter kit with 2 e‐Go C batteries (3.7 volts/650 MaH), a USB connection cord, an AC adapter, and a carrying case, and a supply of Saturn V4i atomizers (2.4 ohms) filled with liquid in their preferred flavour (28 atomizers total; 2/day). Provided 24 mg/mL dosage vegetable glycerin liquid in a tester sample to all participants. Those who reported the 24 mg was too strong were provided 12 mg/mL dosage liquid. The first session included brief education, training, action planning for making a complete switch to EC. A referral to the California Smokers’ Helpline was made at the final visit (week 4). |
|
| Outcomes | 3 lab visits (baseline, week 2, and week 4) and 2 phone visits (week 1 and week 3). Biological samples were taken at all 3 in‐person visits (baseline, week 2, and week 4). However, due to budgetary restrictions, only the baseline and week 4 biological data were analyzed Adverse events and biomarkers:
Other outcomes measured: Cotinine, change in tobacco consumption (cpd using TLFB interview), change in frequency of EC use, change in nicotine dependence and attitudes/behaviour, change in 30‐day nicotine exposure |
|
| Study funding | “This study was funded by the University of Minnesota (JSA), P30 DA012393 (NLB), P50 CA180890 (NLB), and California State University San Marcos (KP).” | |
| Author declarations | “Benowitz is a consultant to pharmaceutical companies that market smoking cessation medications and has been an expert witness in litigation against tobacco companies. The other authors have no conflicts of interest.” | |
| Notes | New for 2020 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Not randomized |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 37/40 provided follow‐up data |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
Pulvers 2020.
| Study characteristics | ||
| Methods | Design: RCT. Unblinded. 2:1 ratio Recruitment: Participants were recruited from the San Diego, California, and Kansas City, Missouri and Kansas, metropolitan areas Setting: USA Study start date: May 2018. Study end date: May 2019 |
|
| Participants | Total N = 186; Electronic‐cigarettes = 125. Own brand cigarette = 61 40.3% female; mean age 43.3 (SD 12.5); mean cpd 12.1 (SD 7.2). E‐cigarettes use at baseline: 0.05 (0.3%) Inclusion criteria: > 21 years of age; smoked cigarettes on > 25 of past 30 days; smoked > 5 cpd on days smoked; smoked cigarettes > 6 months; carbon monoxide > 5 PPM at baseline; systolic BP of < 160 mmHg and diastolic BP of < 105 mmHg at baseline; Hispanic/Latino or African‐American/Black; fluent in English or Spanish; willing to switch from smoking cigarettes to ECs for 6 weeks; regular access to telephone; transportation to attend appointments (KC Only). Exclusion criteria: primary use of other tobacco products or equal use of cigarettes and other tobacco products; EC use on > 4 of the past 30 days; currently in a smoking cessation programme or another clinical trial; use of NRT or medication which aids smoking cessation in the past 30 days; hospitalization for a psychiatric issue in the past 30 days; heart‐related event in the past 30 days (e.g. heart attack, stroke, severe angina (i.e. chest pain), ischaemic heart disease, and vascular disease); uncontrolled blood pressure; planning to move out of study centres (San Diego or Kansas City) in the next 6 wks; another person in the household enrolled in the study; pregnancy / breastfeeding; unstable mental status or health status. |
|
| Interventions |
EC: pod Electronic‐cigarettes: JUUL (5% nicotine); Choice of flavors (Menthol, Mango, Cool Mint, Virginia Tobacco); Given 1 pod per pack of cigarettes; Given a 2‐week supply at baseline and then a further 4‐week supply at week‐2 visit. At each follow‐up appointment (week 1, telephone call; week 2, in‐person visit; and week 4, telephone call), barriers and benefits of switching to e‐cigarette were discussed and action planning for exclusive switching was revisited. Compensated on a schedule of USD 20 at baseline, USD 40 at week 2 and USD 60 at week 6 Own brand cigarettes: Compensated on a schedule of USD 20 at baseline, USD 40 at week 2 and USD 60 at week 6 |
|
| Outcomes | Baseline, week 2 and week 6. Telephone survey at 6 months Change in past 7‐day combustible cigarette use measured by 7‐day timeline follow‐back interview 30‐day point prevalence at 6 months (EC group only)
Lung function; Pulmonary function test of small airway disease that is most sensitive to effects of cigarette smoking; mean mid‐expiratory phase of forced expiratory (FEF 25%‐75%); respiratory symptoms as measured with the American Thoracic Society Questionnaire (scores range from 0‐32, with higher scores indicating greater respiratory symptoms) Blood pressure Adverse events: respiratory symptoms |
|
| Study funding | Drs Pulvers and Nollen and Ms Rice were supported by grant No. 5SC3GM122628 from the National Institutes of Health (NIH). Drs Schmid and Ahluwalia were supported in part by grant No. P20GM130414, from the NIH‐funded Center of Biomedical Research Excellence (COBRE). Dr Schmid was partially supported by Institutional Development Award No. U54GM115677 from the National Institute of General Medical Sciences of the NIH, which funds Advance Clinical and Translational Research (Advance‐CTR) | |
| Author declarations | Dr Schmid reported serving as a consultant for legal firms representing Eli Lilly, Boehringer‐Ingelheim, and Gilead outside the submitted work. Dr Benowitz reported receiving personal fees from Pfizer and Achieve Life Sciences and serving as a consultant to pharmaceutical companies that market smoking cessation medications and as an expert witness in litigation against tobacco companies outside the submitted work. Dr Ahluwalia reported receiving personal fees from Lucy Goods outside the submitted work. No other disclosures were reported. | |
| Notes | Additional data provided by authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence was generated with an Excel (Microsoft) random number formula applied to each site (2:1 ratio) |
| Allocation concealment (selection bias) | Low risk | Allocation was placed into sealed individual envelopes labelled with participant identification numbers for each site, retrieved from a locked cabinet monitored by the project manager, and opened individually following consent of each participant |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Carbon monoxide validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | E‐cig: 115/126 OB: 54/61 |
| Selective reporting (reporting bias) | Low risk | Per protocol reporting |
Russell 2021.
| Study characteristics | ||
| Methods | Design: RCT Setting: London, UK |
|
| Participants | 426, 53% M NRT = 141; Myblu plus NSPs group = 145; Myblu plus FBNPs group = 140 Inclusion criteria: Established daily cigarette smokers aged 18 years and older were recruited in London, UK |
|
| Interventions | EC type: pod 3 arms: NRT; mybluTM containing nicotine salt e‐liquid pods (NSPs); myblu plus freebase nicotine e‐liquid pods (FBNPs). NRT: Over‐the‐counter nicotine replacement therapies (NRTs). Free for 3 months Myblu plus NSPs group: A closed system pod e‐vapour product (mybluTM) containing nicotine salt e‐liquid pods (NSPs). Free for 3 months Myblu plus FBNPs group: A closed system pod e‐vapour product (mybluTM) containing freebase nicotine e‐liquid pods (FBNPs). Free for 3 months Participants of both myblu arms were given a primary device, a backup device, and reimbursement for retail purchases of up to 12 e‐liquid pods (6 packs of x2 pods) per month for 3 months. Participants were encouraged to use their assigned e‐vapour product and to choose and change flavours and nicotine concentrations of their assigned e‐liquid pods as they wished |
|
| Outcomes | Online surveys administered at study enrolment and then at 1, 2, 3 and 6‐months post‐enrolment assessed self‐reported past 30‐day consumption of conventional cigarettes and use of NRTs and assigned e‐vapour products Self‐reported 6‐month past 30‐day cigarette abstinence rate Reduction in smoking |
|
| Study funding | E‐cigarette/Alternative nicotine products Industry | |
| Author declarations | NS | |
| Notes | Conference abstract | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unclear if participants were blinded (conference abstract only) but all participants received active interventions so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Self‐report only but all participants received active interventions so differential misreport judged unlikely |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The 6‐month retention rate was 85.8% in the NRT group, 85.5% in the myblu plus NSPs group, and 73.6% in the myblu plus FBNPs group |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial record available to determine whether all prespecified outcomes are reported |
Scheibein 2020.
| Study characteristics | ||
| Methods | Design: Non‐randomized single‐arm Recruitment: From supported temporary accommodation (STA) service STA project workers and support staff identified potential study participants who smoked and wished to quit Setting: Dublin Simon Community, Ireland Study start date: Recruitment February 2019 (overall trial start date March 2018). Study end date: June 2019 |
|
| Participants | Total N: 23 but only report baseline for the 9 that completed the study. % female 8.7% (2/23) at baseline, (22.2% 2/9) completed and reported; mean age 43.89 (SD 7.36); mean cpd 25.22 (SD 7.77); mean FTND 7.89 (SD 1.2); mean CO 21.89 (SD 14.4 corresp) E‐cigarettes use at baseline: no Motivated to quit: yes Inclusion criteria: > 5 CO ppm (carbon monoxide); active smoking status; expressed intention to quit using ENDS‐device. Exclusion criteria: self‐reported pregnancy; exhibition of florid psychotic or substance use‐related symptoms which could have affected ability to consent. |
|
| Interventions |
EC: Refillable Electronic‐cigarette: Endura T22e Electronic Nicotine Delivery System and 2 10 ml bottles of fluid strengths (0, 6, 11, 18 and 20 mg/mL) and flavours ('Purple Berry', 'Ice Menthol', 'Regular Blend' and 'American Tobacco') |
|
| Outcomes | Baseline (‘week 1’), week 4, week 8, week 12: CO, adverse events Also number of cigarettes smoked; Fagerström Test Scores |
|
| Study funding | This study was completed as part of a Tobacco Harm Reduction Scholarship funded by Knowledge Action Change | |
| Author declarations | FS was a recipient of a Tobacco Harm Reduction Scholarship provided by Knowledge Action Change. He is currently the recipient of an Enhanced Scholarship from the same organization. AM and KM acted as mentors for both the Tobacco Harm Reduction Scholarship and Enhanced Scholarship. AM is an associate of New Nicotine Alliance. KM is a recipient of a grant from the Foundation for a Smoke Free World. JW declares no interests. WR declares no interests |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Only 1 arm |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not randomized |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Self‐reported adverse events |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9/23 completed. Reason was many people moved away so not linked to unacceptability of the study. Incomplete paperwork to enable to be followed |
| Selective reporting (reporting bias) | Unclear risk | Protocol published afterwards |
Skelton 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Clients of AOD (alcohol or drug) centre. Informed of study and given PIS. Interested clients were telephoned by RA. Setting: Two AOD clinical programmes, an opiate agonist treatment (i.e. methadone or buprenorphine) programme and cannabis clinic (behavioural treatment for cannabis misuse and cannabis use disorder) located within one local health district service in New South Wales, Australia Study start date: April 2018. Study end date: July 2019 |
|
| Participants | Total N: 66 [67 in flow diagram] EC abrupt CC cessation = 30 [flow diagram 32] EC gradual CC cessation = 30 [flow diagram 35] Inclusion criteria: 1) Client of participating HNELHD Alcohol Or Drug (AOD) programme, 2) 18+ years, 3) daily tobacco smoker, 4) interested in making a serious quit attempt in the next 30 days, 5) has not used an END containing nicotine in past month Exclusion criteria: Pregnancy or breastfeeding Female 26.9%. Mean age 42.3 (SD 8). Mean CPD 22 (SD 14.2) Motivated to quit: Yes E‐cigarette use at baseline: No |
|
| Interventions | EC: Refillable For both arms: All study participants received T22 and T18 starter kits (both Innokin Endura®). The T22 kit had a 1.5 O atomizer, 2000 mAh battery, and 4.1 mL tank. The T18 kit had a 1.5 O atomizer, 1000mAh battery, and 2.5 mL tank. Both kits included an additional atomizer and micro USB cable. An additional five atomizers were provided with the starter kits as replacements. A prescription for 12 mg/10 mL nicotine e‐liquid was provided for all study participants. Participants received a total of 24 bottles (8 bottles per month). At weeks 3 and 7, participants were provided with their next supply of e‐liquid nicotine by either post or face‐to‐face at the AOD programme for which they were recruited. Training day to learn how to use the VNP devices Arm 1: EC abrupt CC cessation Arm 2: EC gradual CC cessation At their training day, participants were provided with a personalized gradual cessation schedule based on the baseline number of cigarettes smoked per day recorded in the baseline survey. Participants were told their quit date was four weeks’ hence. Participants were instructed to reduce the number of cigarettes smoked by 25% at week 1, 50% at week 2, 75% at week 3, and 100% at week 4. Safety was monitored: All participants were briefly contacted to complete safety check‐ins by telephone at weeks one, three, five, seven, and 10 following their training day. |
|
| Outcomes | Baseline. Training day. 6 weeks post‐training day. 12 weeks post‐training day Continuous abstinence Seven‐day point prevalence abstinence, biochemically verified by CO breath test: ≤ 8 ppm Feasibility outcomes – acceptability, quit type preference, adherence, CPD Safety: Not reported |
|
| Study funding | We acknowledge HMRI and Hunter New England Local Health District for the present funding, as well as the participating trial sites staff who notified present clients about the study. | |
| Author declarations | The authors declared that they had no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper. | |
| Notes | Safety data not reported. No outcomes to extract New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The randomisation sequence (1:1 in blocks of 4 or 6, stratified by AOD program) was generated by the study statistician using SAS software”. |
| Allocation concealment (selection bias) | Low risk | Quote: "generated by the study statistician using SAS software and were uploaded into REDcap" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Both arms received the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “investigators will be blinded for outcome assessments”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Arm 1: 25/31. Arm 2: 27/35 |
| Selective reporting (reporting bias) | High risk | Not all protocol defined outcomes reported |
Smith 2020.
| Study characteristics | ||
| Methods | Design: Double‐blind randomized controlled trial Recruitment: Recruited from the local area via advertising on Craigs List social media Setting: Laboratory and electronic diaries, USA Study start date/Study end date: Not specified. |
|
| Participants | Total N: 30 N per arm: PG/VG ratio 70/30 = NR; PG/VG ratio 50/50 = NR; PG/VG ratio 0/100 = NR Inclusion criteria: adults age ≥ 18 years who have been smoking at least 5 cigarettes daily for the past year (expired CO > 8); usual brand is non‐menthol; use of ENDS on 5 or fewer lifetime occasions; regular use of e‐mail or smartphone ownership with capacity to receive SMS text and internet access (necessary for electronic diaries). Exclusion criteria: unwilling to use ENDS / EC as part of the trial; use of smokeless, hookah, or tobacco products other than cigarettes ≥ 10 days in the past 30 days; pregnancy / breastfeeding; recent history of cardiovascular distress in the last 3 months (arrhythmia, heart attack, stroke, uncontrolled hypertension); current use of cessation medications; another household member currently enrolled in the study. 30% women; mean age 43.7; mean cpd 18.5; mean FTND 5.4 Motivated to quit: Not specified E‐cigarette use at baseline: Participants had used an e‐cigarette an average of 1.6 times in their life, and no one reported use in the last 30 days |
|
| Interventions |
EC: Cig‐a‐like EC provided for 1 week. All aspects of the ENDS device and e‐liquid were held constant between groups with the exception of PG/VG ratio: PG/VG ratio 70/30; PG/VG ratio 50/50; PG/VG ratio 0/100. Ego‐T 1100 mAh battery and disposable cartomizers (510 Smoketech, 1.5‐Ω dual coil). E‐liquid was tobacco‐flavoured (Classic Tobacco, American E‐liquid) and contained 18 mg/mL nicotine |
|
| Outcomes | 1 week; 2 lab visits pre and post and participant diaries Adverse events and biomarkers: Participants provided a CO sample at each visit Other outcomes measured: cpd, ENDS puffs |
|
| Study funding | Funding for this project was provided by pilot funding from the National Cancer Institute (P01CA200512 to K.M.C.). Salary support provided by the National Institute on Drug Abuse (K12DA031794 to T.T.S., K23DA041616 to B.W.H.) | |
| Author declarations | M.J.C. has received consulting honoraria from Pfizer. K.M.C. has received payment as a consultant to Pfizer, Inc., for service on an external advisory panel to assess ways to improve smoking cessation delivery in health care settings. He also has served as paid expert witness in litigation filed against the tobacco industry | |
| Notes | Additional data provided from authors. New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “At the conclusion of the lab visit, participants were randomized and assigned to take home one of the three e‐liquids to use at home for a 1‐week sampling period (10 participants/ratio).” Quote: “Participants were randomly assigned to receive one e‐liquid to take home for 1 week.” (no further detail given) |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “PG/VG ratio was blinded from participant and staff members who conducted experimental sessions.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Number of participants at follow‐up not reported, but this may be due to the 1‐week follow‐up and it seems that all participants (excluding 1 participant who was not randomized) were followed up |
| Selective reporting (reporting bias) | Unclear risk | No protocol. Few details for CO measurements, just percentage change for each group, but mean CO data provided by author on request |
Stein 2016.
| Study characteristics | ||
| Methods | Design: Non‐controlled open‐label experimental study Recruitment: A flyer posted at a large methadone maintenance treatment programme Setting: Methadone maintenance treatment programme, USA Study start date: April 2015; Study end date: Not specified |
|
| Participants | Total N: 12 Inclusion criteria: current moderate or heavy cigarette use (10+ cpd for at least 12 mths prior to enrolment); current MMT for at least 3 months; ready to make a smoking quit attempt in the next 14 days; plan to remain on MMT for at least 12 weeks. Exclusion criteria: used EC on ≥ 2 of the past 30 days; currently used medications that may reduce smoking (bupropion, varenicline, NRT); had unstable medical or psychiatric conditions (past‐month suicidal ideation or past‐year suicide attempt, hospitalization for myocardial infarction or stroke in the prior 3 months); had regular use of marijuana (self‐report or positive urine drug test). Inclusion based on specific population characteristic: People receiving MMT for opoid use disorder 50% women; mean age 45.9; mean cpd 17.8; mean FTND: Not reported Motivated to quit: yes E‐cigarette use at baseline: Had not used e‐cigarettes for more than 2 of the past 30 days |
|
| Interventions |
EC: Cig‐a‐like 2 week supply of NJOY e‐cigarettes at week 1 (quit day), consisting of 5 packs of NJOY e‐cigarettes (15 in total). Participants could request an additional 5 pack (20 in total) for the following 2‐week study period, if they ran out before a study visit. Participants instructed to use EC exclusively for a total of 6 weeks (end of treatment). They were referred to the state telephone QuitLine for supportive counselling at the quit‐day visit (week 1) |
|
| Outcomes | Participants quit and received e‐cigs at week 1. Assessments were carried out at week 3, 5, 7 and 9 Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | “MDS is a recipient of National Institute on Drug Abuse Award K24 DA000512. This award funded the project described here.” | |
| Author declarations | “None declared.” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization |
| Allocation concealment (selection bias) | High risk | No randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: “One individual dropped out after week 3 and did not return; another completed all follow‐up assessments except week 7.” |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Strasser 2016.
| Study characteristics | ||
| Methods | Design: Randomized, factorial trial (Participants were randomized to one of the 5 brands of e‐cigarettes – although only 4 brands analyzed) Recruitment: Media ads Setting: Recruitment from the community, study took place at University, USA. Study start date/Study end date: Not specified. |
|
| Participants | Total N: Analysis based on 24 (28 originally recruited, but the first 4 participants enrolled experienced malfunctioning NJOY e‐cigs and withdrew – the project was removed from the market before the 5th participant was randomized) N per arm: blu: 6; Green Smoke: 6; V2: 6; White Cloud: 6 Inclusion criteria: age 18 to 65 and self‐reported smoking at least 10 cigarettes per day. Exclusion criteria: use of other tobacco or nicotine‐containing products, including e‐cigarettes (no more than 3 previous episodes of use and not currently using); current diagnosis or evidence of substance abuse or dependence or major depression; current or history of psychotic or bipolar disorder; history of suicide attempt; history of cancer or cardiovascular disease; uncontrolled hypertension; use of smoking cessation medications; current plans to try to quit smoking; pregnancy or lactation. Inclusion based on specific population characteristic: Not applicable 29% women; mean age 43.3; mean cpd 17; mean FTND 3.7 Motivated to quit: Participants had no current plans to try to quit smoking (eligibility criterion) E‐cigarette use at baseline: No more than 3 previous episodes of use and not currently using (eligibility criterion) |
|
| Interventions |
EC: Cig‐a‐like All participants received nicotine EC and were instructed to use them exclusively for 9 days The 5 brands selected, including brand reported nicotine levels, were: (1) NJOY (18mg nicotine) – this brand was discontinued and not analyzed as the e‐cigs provided malfunctioned; (2) V2, 18 mg nicotine; (3) Green Smoke, 18.9‐20.7 mg nicotine; (4) blu, 20‐24 mg nicotine; and (5) White Cloud, 23‐24 mg nicotine. Each brand advertised the delivery of the same level of nicotine (appropriate for about a pack/day smoker), provided the standard tobacco flavour (no other flavors made available), and used a disposable cigarette‐like device |
|
| Outcomes | Day 10 is the only testing point of interest for us but participants were also tested at days 1 and 5 Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding | “National Cancer Institute (NCI) of the National Institutes of Health (NIH) and FDA Center for Tobacco Products (CTP) under Award Number P50CA179546, as well as grants from the National Cancer Institute (P50 CA143187, P30 CA16520, and P30 DA12393)” | |
| Author declarations | “Dr Benowitz has served on scientific advisory boards for Pfizer and GlaxoSmithKline related to smoking cessation medications and has been an expert witness in litigation against tobacco companies. Dr Schnoll receives medication and placebo free of charge from Pfizer and has provided consultation to Pfizer and GlaxoSmithKline. These companies had no involvement in this study. Dr Strasser has received funding through the Pfizer GRAND programme, an independent peer‐reviewed grant programme funded through Pfizer (2008‐2011); all investigators have received funding from the United States National Institutes of Health” | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Although participants were randomized to different brands of EC, no description on how randomization was carried out |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No description of whether groups were blind to other conditions, but given similar levels of support between arms, so performance bias judged unlikely |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Unclear whether any blinding took place, some outcomes were measured using objective measures and there was no difference in contact between arms |
| Incomplete outcome data (attrition bias) All outcomes | High risk | For blu, Green Smoke, and V2 groups, 83% of participants completed the 10‐day study; only 33% of participants randomized to White Cloud completed the 10‐day study; meaning loss to follow‐up was considerably higher in this group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Tattan‐Birch 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Stop‐smoking services in England, free to access for smokers trying to quit. Services recruited participants and delivered the intervention during one‐to‐one in‐person counselling sessions with trained stop‐smoking advisors. Setting: 6 stop‐smoking services, England, UK Study start date: April 2019. Study end date: November 2021 |
|
| Participants | Total: N 92 Arm 1: E‐cigarette‐varenicline group 48 Arm 2: Varenicline‐only group 44 Inclusion criteria: 1) Aged 18+ years, 2) smoker, 3) attending SSS one‐to‐one specialist support in London LAs, 4) firm target quit date, 5) elect to use varenicline to support quit attempt, 6) willing to try e‐cigarettes Exclusion criteria: Pregnancy or breastfeeding Female 51%. Mean age 43.9 (SD 13.1) E‐cigarette use at baseline: No Motivated to quit: Yes |
|
| Interventions | EC refillable Arm 1: EC + varenicline EC: a nicotine e‐cigarette starter‐kit, Aspire PockeX e‐cigarette e‐liquid to last for approximately four weeks, and an information booklet. Participants could choose a total of eight 10 mL e‐liquid bottles (from Aspire or Totally Wicked) in any combination from a selection of three flavours (fruit, menthol, and tobacco) and three nicotine concentrations (6, 12 and 18 mg/mL). Participants were encouraged to buy further bottles from local vape shops. Varenicline: same for both arms. Prescribed the standard 12‐week course of varenicline, starting approximately two weeks prior to their target quit date. They were advised to take one 0.5 mg pill daily for the first three days, then two 0.5 mg pills daily for days four to seven, and finally two 1 mg pills daily for the remaining 11 weeks. As this was a pragmatic trial, participants were not asked to avoid using e‐cigarettes. Behavioural support: weekly or fortnightly support until 12 weeks after their quit date. Behavioural support aimed to minimise participants’ motivation to smoke, maximise their motivation to remain abstinent, and guide their use of pharmacotherapy. Arm 2: Varenicline only Same as Arm 1 for varenicline and behavioural support |
|
| Outcomes | Baseline, 9‐12 weeks Smoking abstinence, self‐reported between weeks 9‐12 from the target quit date and validated by an expired air CO concentration of below 10 ppm at week 12 Adherence to varenicline During each session, advisors recorded smoking status, exhaled CO, adherence, adverse events, and respiratory symptoms using existing software (QuitManager or PharmOutcomes). |
|
| Study funding | This project was funded by the Global Research Awards for Nicotine Dependence (GRAND) unrestricted research grant programme supported by Pfizer. Additional funding was provided by Cancer Research UK (PRCRPG‐Nov21\100002). All authors are members of the UK Centre for Tobacco and Alcohol Studies (UKCTAS), funded under the auspices of the UK Clinical Research Collaboration (MR/K023195/1). | |
| Author declarations | LS has received a research grant and honoraria for a talk and travel expenses from manufacturers of smoking cessation medications (Pfizer and Johnson & Johnson). JB has received unrestricted research funding from Pfizer to study smoking cessation. RW has received travel funds and hospitality from, and undertaken research and consultancy for, pharmaceutical companies that manufacture or research products aimed at helping smokers to stop. The other authors have no conflicts of interest to declare. None of the authors have ever received personal fees or research funding of any kind from electronic cigarette or tobacco companies. | |
| Notes | “The trial was stopped early due to COVID‐19 restrictions and a varenicline recall (92/1266 participants used).” “The evidence is tentative because our sample size was smaller than planned — caused by Coronavirus Disease 2019 (COVID‐19) restrictions and a manufacturing recall. This meant our effect estimates were imprecise, and additional evidence is needed to confirm that providing e‐cigarettes and varenicline together helps more people remain abstinent than varenicline alone.” New to 2022 update |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomised [by] 1:1 ratio in blocks of 6 or 8 participants, stratified by service, using a computer‐generated random sequence with allocation concealed within opaque envelopes. Due to the nature of the intervention, participants and advisors could not be blinded to treatment assignment." |
| Allocation concealment (selection bias) | Low risk | Quote: "with allocation concealed within opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | 2 active interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CO–validated smoking abstinence at 6 months following the target quit‐date was measured. Trial stopped at 12 weeks. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | EC + varenicline: 26/48 Varenicline only: 20/44 |
| Selective reporting (reporting bias) | Low risk | Preregistered and all expected outcomes reported (some outcomes not reported but that was judged due to early termination) |
Tseng 2016.
| Study characteristics | ||
| Methods | Design: 2‐arm; double‐blind placebo‐controlled RCT Recruitment: Advertisements placed in Craigslist as well as flyers distributed on the street and placed in New York City venues with details for how to contact study staff. Setting: Community, USA Study start date: July 2014 – 2015 (month unclear); Study end date: Not specified |
|
| Participants | Total N: 99 (100 were randomized but 1 participant randomized to the control arm was found to be ineligible between randomization and baseline) N per arm: Nicotine EC: 50; Placebo EC: 49 Inclusion criteria: age 21–35; daily smoker; smoked ≧ 10 cigarettes a day (verified by a CO level of ≥ 8 ppm); interested in reducing cigarette consumption; able to provide consent; had a cell phone and was willing/able to receive text messages and counselling on their cell phone; willing to use an EC for 3 weeks Exclusion criteria: pregnant and/or breastfeeding; history of asthma, other airways diseases, or heart disease; currently using smoking cessation medications (including other forms of NRT, bupropion, or varenicline), or enrolled in a smoking cessation programme or another cessation trial; use of EC in the past 14 days or any other tobacco products (pipe, cigar, cigarillos, snuff, chewing tobacco, rolling tobacco, or hookah/shisha) in the past 30 days; moderate to severe drug use disorder defined as a score of ≥ 5 on the Drug Abuse Screening Test‐10 and/or a hazardous or active alcohol use disorder defined as at least 7 for men and at least 5 for women on the Alcohol Use Disorders Identification. Inclusion based on specific population characteristic: Young adults 32.3% women; mean age 28.43; mean cpd 14.33; FTND not measured but time to first cigarette was measured categorically. The mode category was 6‐30 mins (39/99; 41.5%) Smoking behavioural dependence scale (11 items): mode category ‘Moderate’ (51/99; 51.5%) Motivated to quit: Readiness to quit (1 – 10 scale, 1 – 8 apply to current people who smoke): 5.57 ± 1.49 E‐cigarette use at baseline: No use of e‐cigs in past 14 days (eligibility criterion) |
|
| Interventions |
EC: Cig‐a‐like E‐cigarette details: 3 weeks of disposable 4.5% nicotine NJOY, King Bold (NJOY, Inc, Scottsdale, AZ) which resemble conventional cigarettes. NJOY also manufactured the non‐nicotine placebo EC. Both nicotine and placebo ECs were tobacco‐flavoured. The products were purchased by the investigators and provided to the participants free of charge Other stop‐smoking pharmacotherapies: None Behavioural support: Prior to receiving the ECs, participants were required to complete a 20‐ to 30‐minute telephone counselling session with a trained tobacco cessation Counsellor. The purpose of the telephone counselling was to review current smoking patterns and offer behavioural and environmental change strategies. These included specific smoking reduction options, such as eliminating cigarettes at work and in the home, carrying only those cigarettes needed for that day, dropping cigarettes associated with less intense triggers first, avoiding smoking triggers, and other strategies to manage urges. 18 participants were asked to reduce the number of cigarettes smoked daily by at least 50% of the total number of cigarettes smoked per day at baseline. To mimic real‐life EC use, minimum EC use instruction was provided. Participants were encouraged to replace cigarettes with as much or as little use of an EC as needed in order to reduce nicotine withdrawal symptoms |
|
| Outcomes | Week 1, 3 Cessation: Not applicable Adverse events and biomarkers: adverse events and symptoms related to EC use Other outcomes measured:
|
|
| Study funding | “This work was supported by the National Center for Advancing Translational Sciences at the National Institutes of Health (grant number UL1TR000038).” | |
| Author declarations | “None declared” | |
| Notes | Study listed as ongoing study NCT02628964 in the 2016 review update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “computer generated” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “…was concealed from research assistants. Blinding of the allocation of nicotine or placebo EC was ensured by the identical appearance of the ECs”. However, not enough information given on how allocation was concealed at the point of randomization |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Blinding of the allocation of nicotine or placebo EC was ensured by the identical appearance of the ECs” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Blinding of the allocation of nicotine or placebo EC was ensured by the identical appearance of the ECs” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nicotine EC lost to follow‐up: 10/50; Placebo EC lost to follow‐up: 10/49 |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Valentine 2018.
| Study characteristics | ||
| Methods | Design: Open‐label prospective cohort study Recruitment: Recruited from within the Department of Veterans Affairs (VA) Connecticut Healthcare System by word of mouth Setting: Receiving psychiatric services from Department of Veterans Affairs healthcare system, USA Study start date/Study end date: Not specified. |
|
| Participants | Total N: 50 (sample analyzed for primary outcomes on week 1 completers – N = 43) Inclusion criteria: no immediate intention to stop smoking; smoking history of ≥5 CPD for the past year. Exclusion criteria: current untreated medical or psychiatric or substance use disorders, or both, as determined by a review of the veteran’s electronic medical record; current use of nicotine replacement or other cessation pharmacotherapies; use of e‐cigarettes or smokeless tobacco products for more than 2 of the past 30 days. Inclusion based on specific population characteristic: Military veteran people who smoke who had no immediate intention to stop smoking and were currently receiving psychiatric services from the Department of Veterans Affairs healthcare system. 7% women; mean age 56.9; mean cpd 16.6; mean FTND 4.9 Motivated to quit: Had no immediate intention to stop smoking E‐cigarette use at baseline: E‐cigarettes or smokeless tobacco products may have been used for less than 2 of the past 30 days |
|
| Interventions |
EC: Refillable All given eVic Supreme (Joyetech), "a commercial, variable‐power, tank‐type device". 6.5 mL tank (Delta 23, Joyetech) and a C3 triple coil atomizer head (Joyetech) with a total resistance of 1.8 ohms. Participants could choose flavour (menthol or tobacco) and nicotine concentration (12 or 24 mg/mL). Participants taught how to use EC, with additional materials dispensed as needed. Participants were informed that they could use the study e‐cigarette or regular tobacco cigarettes, or both, ad libitum during study participation |
|
| Outcomes | Week 1, 2, 3, 4, 8 (Weekly lab visits and 1 month follow‐up) Adverse events and biomarkers: Alveolar (breath) CO levels (ppm) Other outcomes measured:
|
|
| Study funding | "This research was supported by the New England Mental Illness Research, Education and Clinical Center and the U.S. Department of Veterans Affairs. Statistical analyses, biochemical assays, and analyses of e‐cigarette solutions were supported by the Administrative and Laboratory cores of P50DA036151 (Yale TCORS) from the National Institutes of Health and the U.S. Food and Drug Administration Center for Tobacco Products. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or of the U.S. Food and Drug Administration." | |
| Author declarations | "Ralitza Gueorguivea, PhD, discloses consulting fees for Palo Alto Health Sciences and Mathematica Policy Research and a provisional patent submission by Yale University: Chekroud, A. M., Gueorguieva, R., & Krystal, K. H. “Treatment Selection for Major Depressive Disorder” (filing date June 3, 2016, USPTO docket number Y0087.70116US00). The authors report no other financial relationships with commercial interests." | |
| Notes | New for 2020 update. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Uncontrolled cohort study |
| Allocation concealment (selection bias) | High risk | Uncontrolled cohort study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow‐up: 31/50 at week 8 |
| Selective reporting (reporting bias) | Unclear risk | No protocol or clinical trial record. |
Van Staden 2013.
| Study characteristics | ||
| Methods | Design: Single‐group within‐subject design Recruitment: Participants from a military hospital in South Africa Setting: South Africa Study start date/ end date: Not specified |
|
| Participants | Total N: 15, mean age 38 years, smoked 20 cpd (range 10‐30), for an average of 17 years (range 5‐27) Total N: 13 completed the study (5 women) Inclusion criteria: adults who smoke daily, ≥ 10 cpd. Exclusion criteria: history of lung disease. Inclusion based on specific population characteristic: No Motivated to quit: Not specified E‐cigarette use at baseline: Not specified |
|
| Interventions |
EC: Cig‐a‐like Participants were asked to use an EC only for 2 weeks (i.e. no cigarettes) EC: 'Twisp eGo' cartridge 0.8 ml containing 0.0144 mg of nicotine |
|
| Outcomes | The following measurements were taken at baseline and 2‐week follow‐up:
|
|
| Study funding | "We are grateful for the sponsorship of the eGo e‐cigarette packs by Twisp and also for the valuable advice and laboratory assistance given by Col. (Dr) J Lubbe, Chemical Pathologist, 1 Military Hospital, Pretoria with regard to the measurement of the cotinine levels. We also wish to acknowledge Professor Martin Veller for his insightful contributions during the preparation of this manuscript and also Dr Richard van Zyl‐Smith for his assistance and review." | |
| Author declarations | "The sponsor of the Twisp e‐cigarette had no role in the design and conduction; the collection, analysis and interpretation of the study; or in the preparation, review or approval of the manuscript." | |
| Notes | Dropouts (N = 2) were due to illness (headache and fever) and undertaking a military course associated with high stress and exposure to others smoking, making it difficult to abstain from cigarettes The paper states that the EC cartridge contained 0.8 ml of solution with 0.0144 mg of nicotine. This would be an unusually low concentration of nicotine and we have assumed an error in units where milligrams should have been grams (0.0144 grams of nicotine would make the concentration 18 mg/mL) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospective cohort |
| Allocation concealment (selection bias) | High risk | Not randomized |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/15 lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unable to determine prespecified outcomes |
Vickerman 2022.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: Participants were recruited from incoming callers to the Oklahoma Tobacco Helpline (OTH) between November 2018 and March 2020 who were smoking and had used e‐cigarettes in the past 30 days. The OTH is a free, public health resource offering tobacco cessation services via phone, web, text, and/ or print materials, as well as NRT Setting: Study start date: |
|
| Participants | Total: N 110 55 in arm 1: enhanced e‐cigarette coaching (EEC) 54 in arm 2: quitline treatment‐as‐usual (TAU) Dual users of EC and combustible cigarettes Inclusion criteria: 1) Requested quitline coaching services & enrolled in 5‐call Oklahoma Tobacco Helpline (OKHL) programme, smoked at ⩾ 1 cigarette per day (CPD), 2) using EC or planning to use EC, 3) willing to quit cigarettes, 4) consented to receive automated phone outreach via the quitline, 5) Android smartphone, regular access to email, 6) 18+ years Exclusion criteria: 1) schizophrenia, heart attack, stroke or heart condition in past 2 wks, 2) taking varenicline or bupropion, 3) pregnancy Female 61.5%. Mean age 40.6 (SD 13.5). Mean CPD 19.2 (SD 11.3) E‐cigarette use at baseline: Yes Motivated to quit: Yes |
|
| Interventions | No EC intervention. Advice only Arm 1: Enhanced e‐cigarette coaching (EEC) [Quitline (NRT available) + EC advice (no EC intervention)] The EEC protocol added four intervention components to standard evidence‐based quitline treatment: EC education, a shared decision‐making model (SDM) for quit plan development (offering selection of NRT, EC, both NRT & EC, or no nicotine replacement aid; in these conversations, coaches discussed EC as a quitting tool similar to NRT and as an alternative form of nicotine replacement), behavioural support tailored to the selected quit plan, and a requirement for coaches to assess and address EC use on every call. Arm 2: Quitline treatment‐as‐usual (TAU) (NRT available) The TAU protocol included recommending participants stop both cigarettes and e‐cigarettes on their quit date when they start NRT. Both arms: Standard benefits available through the OTH (2–8 weeks of patch, gum, and/or lozenge) The quitline intervention, focused on 5 key strategies for quitting: committing to a quit date, coping with urges, using medications effectively, disposing of tobacco paraphernalia, and utilizing social support. The 5 coaching calls for the 2 groups were delivered over approx. 2 mths, with call 1 intended to take approx. 20–25 mins, and later calls to take approx. 15 mins. Participants downloaded a smartphone app to complete daily questionnaires on product use for 12 wks. Participants received $20 for completing the baseline survey and up to $110 for daily questionnaires. |
|
| Outcomes | Cessation 3 mths (CO confirmed) AEs Continued EC use |
|
| Study funding | This work was supported by the National Institute on Drug Abuse (R21DA042960 to KAV) and used the InsightTM mHealth Platform and Android smartphone app, which was supported by the National Cancer Institute Cancer Center Support Grant P30CA225520 awarded to the University of Oklahoma Stephenson Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or Optum. | |
| Author declarations | KAV, KMC, LNM, JMH, and KAW are employees of Optum, the provider of quitline services for the Oklahoma Helpline in this study. THB is on the Advisory Board of Hava Health, Inc., a start‐up (with no connections to the tobacco industry) that is developing a therapeutic e‐cigarette. All other authors declared no conflicts of interest. | |
| Notes | New to 2022 update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants were then randomized to receive quitline treatment as usual (TAU) or EEC, using blocked randomization stratified by gender (male vs female; other response options for gender were not included for the quitline at the time of this trial, but have since been added) with an allocation ratio of 1:1. The quitline participant record software accessed a randomization table to automatically assign a participant to a group after a coach clicked a randomize button". |
| Allocation concealment (selection bias) | Low risk | As above |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No detail |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CO measured |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | EEC: 34/55 (46) TAU: 32/54 (50) |
| Selective reporting (reporting bias) | Low risk | Outcomes from NCT record reported |
Wadia 2016.
| Study characteristics | ||
| Methods | Design: Uncontrolled experimental study Recruitment: Dental hospital staff were recruited – not specified how Setting: Dental hospital, UK Study start date: April 2015; Study end date: December 2015 |
|
| Participants | Total N: 20 (18 of the 20 attended the reassessment visit) Inclusion criteria: 18‐65 years; systemically healthy; smoked ≥ 10 CPD for at least 5 years; ≥ 24 natural teeth (excluding third molars) and had no probing pocket depths over 4 mm at any site; did not wish to quit. Exclusion criteria: systemic condition known to exacerbate or modulate periodontitis (for example, diabetes); antibiotics in the previous 3 months; anti‐inflammatory drugs or other medication likely to affect the periodontal tissues were taken routinely; pregnancy/breastfeeding Percentage of women in study, age, cpd and FTND: not specified. Motivated to quit: enrolled people who smoke who did not intend to quit smoking, but were prepared to attempt to substitute smoking with the use of e‐cigarettes for 2 weeks E‐cigarette use at baseline: not specified |
|
| Interventions |
EC: Refillable Participants provided with a blu PROTM e‐cigarette kit (Electric Tobacconist®), an extra bottle of blu PRO Tobacco™ e‐Liquid (Electric Tobacconist) and written instructions. The e‐Liquid was Classic Tobacco‐flavoured and contained 18 mg of nicotine (medium strength). The participants agreed to substitute their regular smoking habits with the use of e‐cigarettes for 2 weeks. They were asked to make a note of any cigarette smoking during the 2 weeks if complete abstinence was unsuccessful |
|
| Outcomes | 2 weeks Adverse events and biomarkers: adverse effects Other outcomes measured:
|
|
| Study funding | Not specified | |
| Author declarations | Not specified | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No randomization |
| Allocation concealment (selection bias) | High risk | No randomization |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
Walele 2018.
| Study characteristics | ||
| Methods | Design: RCT (short‐term, Cravo 2016) followed by cohort study (Walele 2018) in which all participants were given nicotine EC Recruitment: Community Setting: 2 centres in the UK (Covance Clinical Research Unit Ltd, Leeds and Simbec Research Ltd, Wales) Study start date: December 2013; Study end date: December 2016 |
|
| Participants | 420 participants Inclusion criteria differ per study phase Cravo 2016 (short‐term RCT), inclusion: 21‐65 years; BMI 18‐35 kg/m2; 5‐30 CPD for ≥ 1 year (self‐reported); in good health (determined by medical history, a physical examination, a 12‐lead ECG, lung function tests and clinical laboratory evaluations); people who smoke (urinary cotinine ≥ 3 and exhaled CO ≥ 6 ppm). Additional criteria for Walele 2018 (participants from Cravo 2016): Participants assessed by PI as being compliant in Cravo 2016 (e.g. having attended outpatient visits and having been compliant with study procedures). Participants had to be willing to use the study product as the only nicotine‐containing product for the duration of the study, and, as deemed by PI, had to have no clinically significant abnormalities in 12‐lead electrocardiogram, vital signs, spirometry and clinical laboratory assessments in the preceding study In addition, participants who were assigned to the conventional cigarette (CC arm) in Cravo 2016 had to be established people who smoke CCs, which was assessed by urinary cotinine levels (a score of 3 and above on a NicAlert™ test strip was considered positive), eCO levels (a readout > 6 ppm was considered positive) and by review of a smoking history questionnaire Exclusion criteria: Cravo 2016: Use of NRT, snuff or chewing tobacco in 14 days previous, or intended to use during study; trying to stop smoking or considering quitting; clinically‐significant illness or disorder, history of drug or alcohol abuse within 2 years prior to study start; woman of “childbearing potential” unwilling to use “acceptable contraceptive measure” during study. Walele 2018 (participants from Cravo 2016): people who had taken or received any form of NRT, snuff or chewing tobacco during the previous study or intended to use it during this study; relevant illness history; history of drug or alcohol abuse; lung function test or vital signs considered unsuitable; trying to stop smoking; women who are pregnant, or unwilling to use acceptable contraceptive method for the duration of the study. Cravo 2016 Total N: 419 randomized, 408 analyzed (excludes 11 who were excluded prior to any product use) N per arm: EVP: 306; Control: 102 45% women; mean age 34.6; Mean cpd: most 11‐20 cpd (56% int, 62% control); Mean FTND: most moderate (57% int, 54% cont) Motivated to quit: No E‐cigarette use at baseline: Not excluded based on prior EC use Walele 2018 Total N: 209 (147 pre‐EVP group; 62 pre‐CC group) 45% women; mean age 36.6; mean cpd 2.6 (data from figure): Not reported; FTND: Not reported Motivated to quit: As reported for Cravo 2016 E‐cigarette use at baseline: Not reported |
|
| Interventions |
EC: Cig‐a‐like Cravo 2016 EC: EVP prototype (2.0% nicotine), developed by Fontem Ventures B.V. (Amsterdam, the Netherlands). Instructed to only use EVP for study period. It consisted of a rechargeable battery (voltage range of 3.0e4.2 V), an atomiser and a capsule (small cartridge) containing e‐liquid. The capsules were replaceable and the battery and atomiser were reusable. Could choose between two different e‐liquids, which differed solely in their flavour: a menthol‐flavoured e‐liquid with 2.0% nicotine (2.7 mg nicotine/capsule) and a tobacco‐flavoured e‐liquid with 2.0% nicotine (2.7 mg nicotine/capsule) Control: Used their own usual conventional cigarette brand Walele 2018 E‐cigarette details: Commercially available Puritane™ (closed system EVP) consists of a lithium‐ion rechargeable battery and a replaceable cartomiser comprising of an e‐liquid reservoir pre‐filled by the manufacturer, a heating element and a mouthpiece; 1.6% nicotine (16 mg/g) Available in tobacco or menthol. 2 weeks before baseline, participants had a familiarization session with Puritane™, where they could see and try the EVP |
|
| Outcomes |
Cravo 2016: Weeks 1, 2, 4, 6, 8, 10 and 12 Walele 2018: starting on the last day of the previous trial): Months 1, 2, 3, 6, 9, 12, 15, 18, 21 and 24 Study centre visits for assessments Adverse events and biomarkers:
Other outcomes measured:
|
|
| Study funding |
Cravo 2016 "This work was funded and supported by Fontem Ventures B.V. Imperial Brands plc is the parent company of Fontem Ventures B.V. the manufacturer of the EVP prototype used in this study" Walele 2018 "This work was funded and supported by Fontem Ventures B.V. Imperial Brands Group plc is the parent company of Fontem Ventures B.V., the manufacturer of the EVP used in this study" |
|
| Author declarations |
Cravo 2016 "Dr. Cravo has nothing to disclose. Mrs Martin reports personal fees from Fontem Ventures B.V. during the conduct of the study; personal fees from Tobacco and pharmaceutical industries outside the submitted work. Dr. Sharma reports other from Fontem Ventures B.V. during the conduct of the study. Dr. Bush reports other from Fontem Ventures B.V. during the conduct of the study. Mrs Savioz reports personal fees from Fontem Ventures B.V. during the conduct of the study; personal fees from Tobacco and pharmaceutical industries outside the submitted work. Mr Craige has nothing to disclose. Mr Walele has nothing to disclose." Walele 2018 (copied from Transparency documents) "Dr. Koch reports other from Fontem Ventures B.V., during the conduct of the study; Dr. Martin reports personal fees from Fontem Ventures B.V., during the conduct of the study; personal fees from Tobacco and pharmaceutical industries, outside the submitted work; Dr. O'Connell has nothing to disclose. Dr. Bush reports other from Fontem Ventures B.V., during the conduct of the study; Dr. Savioz reports personal fees from Fontem Ventures B.V., during the conduct of the study; personal fees from Tobacco and pharmaceutical industries, outside the submitted work; Dr. Walele has nothing to disclose." |
|
| Notes | Sponsor: Imperial Tobacco Group PLC Study listed as ongoing studies NCT02029196 and NCT02143310 in 2016 review update. Treated as single study in this review due to including the same participants, and no time lag between studies "The same subjects who participated in our previous clinical trial (ClinicalTrials.gov, #NCT02029196) conducted in the same centres, with another EVP (Cravo et al., 2016), were invited to participate the study by Walele 2018. All volunteering subjects were assigned to switch to using Puritane™, a closed system EVP, for two years, starting on the last day of the previous trial (End of Study [EoS] visit), which corresponded to the baseline visit of Walele 2018." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomisation was performed using an Interactive Web Response System (IWRS; Almac Clinical Technologies)” |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was performed using an Interactive Web Response System (IWRS; Almac Clinical Technologies)” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label, no blinding, differential levels of support/product use so performance bias cannot be ruled out |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label, no blinding, with differential levels of support/product use and subjective outcomes |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Cravo: 286/306 int (4.5% ltfp) and 101/102 (1% ltfp) control completed study but all who received product included in analysis. In EVP group, 14 withdrew consent, 2 experienced AEs, 1 death, 3 “other”. CC group 1 AE Walele 2018: High 209/387 enrolled for study Walele 2018. A total of 102 participants (48.8%; EVP: 75/145 (51%); CC: 27/61 (43.5%) completed the study |
| Selective reporting (reporting bias) | Low risk | Cravo 2016: Low All anticipated outcomes reported (study registered prior to study completion) Walele 2018: Low All anticipated outcomes reported (study registered prior to study completion) |
Walker 2020.
| Study characteristics | ||
| Methods | Design: RCT Recruitment: National media advertising Setting: Community based, New Zealand Study start date: Recruitment between March 2016; Study end date: Aug 2018 |
|
| Participants | N per arm: Patches‐only group: 125; Patches plus nicotine e‐cigarette group: 500; Patches plus nicotine‐free e‐cigarette group: 499 Inclusion criteria: living in New Zealand; 18 years or older; smoked tobacco (amount not specified); motivated to quit in the next 2 weeks; able to provide verbal consent; prepared to use any of the trial treatments; access to a telephone. Exclusion criteria: pregnancy or breastfeeding; used an EC for smoking cessation for ≥ 1 week anytime in the past year; currently using smoking cessation medication; enrolled in another cessation programme or study; history of severe allergies; poorly‐controlled asthma; cardiovascular event in the 2 weeks before enrolment; only 1 participant per household was permitted. 69% women; mean age 41.6; mean cpd 17.3; mean FTND 5.2 Motivated to quit: yes E‐cigarette use at baseline: Not reported but use of an e‐cigarette for smoking cessation for more than 1 week anytime in the past year was an exclusion criterion |
|
| Interventions |
EC: Refillable Moderate‐intensity behavioural support was available for all participants immediately after randomization, then once a week for 6 weeks. This support consisted of 10 – 15 mins of withdrawal‐oriented behavioural support and advice on using their allocated treatment, delivered proactively over the phone by researchers who had received standardized training in delivery of such support. Assigned to: 1) Nicotine patch for 14 weeks including 2‐week prequit. 21 mg, 24‐hr nicotine patch (Habitrol) 2) Nicotine patch and nicotine‐free EC for 14 weeks. As 1, plus 14‐week supply at no cost. A 2nd generation eVOD (Kangertech, Shenzhen GuangDong, China) starter kit, with a choice of 1 of 2 tobacco e‐liquid flavors. Advised to start using the e‐cigarette 2 weeks before their quit date, as and when necessary or desired, and in accordance with the manufacturer’s written instructions, to become familiar with its use Participants were instructed to stop smoking from their quit date and continue with their allocated treatment for 12 weeks (ad libitum use of the e‐cigarette), irrespective of any lapses to smoking 3) Nicotine patch and nicotine EC for 14 weeks. As above, but 18 mg/mL nicotine |
|
| Outcomes | Quit date, 1, 3, 6 and 12 months Continuous abstinence at 6 months with CO validation Adverse events and biomarkers: Known side‐effects associated with e‐cigarette use and nicotine patch use; SAEs Other outcomes measured:
|
|
| Study funding | Funding: Health Research Council of New Zealand. "The sponsor of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication." | |
| Author declarations | NW, CB, MV, GL, ML, and VP report grants from the Health Research Council of New Zealand, during the conduct of the study. NW, CB, MV, and VP report grants from Pfizer, outside of the submitted work. GL chairs the organization End Smoking New Zealand, which advocates for harm reduction approaches to tobacco control. E‐cigarettes were purchased from a New Zealand e‐cigarette online retailer (NZVAPOR, https://www.nzvapor.com/), e‐liquid was purchased from Nicopharm, Australia (https://www.nicopharm.com.au/), and nicotine patches were supplied by the New Zealand Government via their contract with Novartis (Sydney, Australia). NZVAPOR also provided, at no cost to participants, on‐line and phone support regarding use of the e‐cigarettes. Neither NZVAPOR nor Nicopharm have links with the tobacco industry. None of the above parties had any role in the design, conduct, analysis, or interpretation of the trial findings, or writing of this publication. | |
| Notes | Study listed as ongoing study NCT02521662 in the 2016 review update | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence |
| Allocation concealment (selection bias) | Low risk | Quote: “We ensured allocation concealment because the statistician who generated the random allocation was not the person randomising participants.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: “Participants and researchers collecting outcome data were masked to the nicotine content of the e‐liquid” but those allocated to patch only would be aware they did not have an E‐cigarette Quote: “Third, while we attempted to minimise detection bias by masking the nicotine content of the e‐liquid, we were only 30% successful, and thus some bias in favour of nicotine e‐cigarettes could have occurred.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Biochemical validation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | < 50% lost to follow‐up, similar rates of attrition between groups (within 20%) |
| Selective reporting (reporting bias) | Unclear risk | CO‐verified abstinence at 12 months stated as a secondary outcome but data are not reported in the main text. However, state in the appendix that too few people in each group were followed up to 12 months (36/1124) so no data are presented for this time point |
White 2021.
| Study characteristics | ||
| Methods | Design: RCT (2 x 2 x 2 factorial design) Recruitment: Setting: Winston‐Salem, NC, Philadelphia, PA and the respective surrounding areas, USA Study start date: August 6 2018. Study end date: March 13 2020 |
|
| Participants | Total study N: 50 50 were randomized, 32 completed study. Inclusion criteria: Self‐reported smoking 5‐50 cpd CO and urinary cotinine confirmed Reported vaping on at least 2 separate occasions, without an allergic or otherwise adverse experience 18+ years Exclusion criteria: Self‐reporting frequent use of e‐cigarettes (> 15 days in the past month) Female 40%. Mean age 41.6 (SD 11.7). Mean CPD 22.8 (SD 10.5). Mean FTDN 5.7 (SD 1.8) E‐cigarette use at baseline? 30% Motivated to quit: NR |
|
| Interventions | EC type: refillable EC: Halo Triton (3.7 V battery; 650 mAh) vape pen and compatible Triton 2.4 mL refillable tanks with 2.2‐2.4 ohm coils. E‐liquid, sourced from Syndicate Distribution (Westchase, Florida, US) in 10 mL bottles; had a base of 70% propylene glycol and 30% vegetable glycerin Combustible cigarettes: Participants received Spectrum research cigarettes matched to their usual brand cigarette menthol preference. All received study cigarettes and a vape pen with e‐liquid to use for 12 weeks. Study cigarettes (very low nicotine content (0.4 mg/g of tobacco)) Study cigarettes (normal nicotine content (15.8 mg/g of tobacco; double‐blind)) Vape pen ‐ E‐liquids contained low nicotine (0.3% free‐base nicotine) levels of nicotine Vape pen ‐ E‐liquids contained moderate nicotine (1.8% free‐base nicotine; open‐label) Flavours: 3 tobacco flavours (robust tobacco, light tobacco, tobacco/menthol blend), mint, fruit and dessert flavour options (mixed berry, watermelon, berry, cookies and crème, chocolate, caramel, spearmint, peppermint, and menthol) Arm 1: Normal Nicotine Content (NNC) cigarette + moderate nicotine e‐liquid (1.8% free‐base nicotine) + tobacco flavours Arm 2: NNC cigarette + low nicotine e‐liquid (0.3% free‐base nicotine) + tobacco flavours(0.3% free‐base nicotine) Arm 3: NNC cigarette + moderate nicotine e‐liquid + variety flavours Arm 4: NNC cigarette + low nicotine e‐liquid + variety flavours Arm 5: Very Low Nicotine Content (VLNC) cigarette + moderate nicotine e‐liquid + tobacco flavour Arm 6: VLNC cigarette + low nicotine e‐liquid + tobacco flavours Arm 7: VLNC cigarette + moderate nicotine e‐liquid + variety flavours Arm 8: VLNC cigarette + low nicotine e‐liquid + variety flavours Participants could be compensated up to $1070 for completing all study procedures. |
|
| Outcomes | Week 1, 2, 3, 4, 6, 8, 10, 12 CPD during week 12 was the primary outcome. CO up to 12 weeks. Measure of cigarette smoke exposure using data on expired breath CO. CO (ppm) was captured using Covita Smokelyzer devices and standard exhalation procedures. TCD N‐acetyl‐S‐(2‐cyanoethyl) cysteine (CEMA), the mercapturic acid metabolite of acrylonitrile, which serves as a biomarker of cigarette smoke exposure Anatabine levels from the spot urine samples to assess whether participants assigned to VLNC cigarettes adhered to only using VLNC cigarettes Total nicotine equivalents |
|
| Study funding | The research reported in this paper was funded by National Institute on Drug Abuse and the U.S. Food and Drug Administration (FDA) Center for Tobacco Products (U54DA031659, ECD & DKH). Research reported in this publication was supported by National Institutes of Health (NIH) grant P30 CA77598 utilizing the Biostatistics and Bioinformatics Core shared resource of the Masonic Cancer Center, University of Minnesota and by the National Center for Advancing Translational Sciences of the National Institutes of Health Award Number UL1TR0002494. Author support also included NIDA U54DA036105 (COC) and NIDA R36DA054481 (CMW). The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the FDA. | |
| Author declarations | Pre‐print. NR | |
| Notes | Study recruitment ended early due to concerns about pending regulations and the availability/relevance of the study vaping products. New to 2022 review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detail on how randomisation was carried out |
| Allocation concealment (selection bias) | Unclear risk | No detail |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Cigarette nicotine content was masked to participants and researchers. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | CO measured |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 50% dropout in 2 arms At FU: 4/5; 2/6; 4/5; 7/10; 4/7; 5/6; 2/5; 4/6 |
| Selective reporting (reporting bias) | High risk | Outcomes not all reported |
Yingst 2020.
| Study characteristics | ||
| Methods | Design: Cross‐over study Recruitment: Participants were recruited from people living with HIV/AIDS (PLWHA) (who smoked) seeking care at the Penn State Health HIV Comprehensive Care Program Setting: USA Study start date:Not reported |
|
| Participants | Total N: 17; 41.2% female; mean age 49.1 (SD 8.8); mean cpd 16.9 (SD 7.9); mean CO 22.4 (13.1) E‐cigarettes use at baseline: not reported Motivated to quit: No Inclusion criteria: adult (age ≥ 18 years); smokers (≥ 10 cigarettes daily); not planning to quit smoking; documented history of a positive HIV status. Exclusion criteria: not reported |
|
| Interventions |
EC: Cig‐a‐like; Refillable Cig‐a‐like device (Blu), nicotine concentration 24 mg/mL. Propylene glycol/ vegetable glycerin ratio 70/30. Nicotine delivery 4.56 ng/ml after 20 puffs in 10 minutes Button‐operated device (eGO), nicotine concentration 36 mg/mL. Propylene glycol/ vegetable glycerin ratio 70/30. Nicotine delivery 6.9 ng/ml after 10 puffs in 5 minutes (refillable) |
|
| Outcomes | Visits: baseline, day 7, day 14, day 21 CO measured (day 0, 7, 14, 21); adverse events (nausea, dizziness) Also: Number of tobacco cigarettes smoked per day (self‐report); EC puffs per day (self‐report) |
|
| Study funding | This study was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number P50DA036107 and the Center for Tobacco Products of the U.S. Food and Drug Administration. JY is also funded by the Penn State Cancer Institute (PSCI) and TE is also supported by U54DA036105. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Food and Drug Administration | |
| Author declarations | JF has done paid consulting for pharmaceutical companies involved in producing smoking cessation medications, including GSK, Pfizer, Novartis, J&J, and Cypress Bioscience. TE is a paid consultant in litigation against the tobacco industry and the electronic cigarette industry and is named on a patent application for a device that measures the puffing behaviour of electronic cigarette users | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used 2 ENDS in a random order – not enough information |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Unable to blind, but interventions judged equally intensive |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Objective outcome‐ CO monitoring (CO < 10 ppm) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear what outcomes were prespecified |
AE: adverse event AOD: alcohol or other drugs BL: baseline BMI: body mass index BoE: biomarkers of exposure BP: blood pressure CAT: COPD assessment tool CC: combustible cigarette CO: carbon monoxide COHB: carboxyhaemoglobin COPD: chronic obstructive pulmonary disease CPD: cigarettes per day COT: cotinine cpd: cigarettes per day CRF: cardiovascular risk factors CVD: cardiovascular disease DBP: diastolic blood pressure DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders IV EC: electronic cigarette eCO: expired carbon monoxide EEC: enhanced e‐cigarette coaching eGFR: estimated glomerular filtration rate ENDS: electronic nicotine delivery system EOP: end of pregnancy EVP: electronic vaping product FEF: forced expiratory flow FEV1: forced expiratory volume FTCD: Fagerström Test for Cigarette Dependence FTND: Fagerström Test for Nicotine Dependence FU: follow up FVC: forced vital capacity HCV: hepatitis C HDLC: high‐density lipoprotein HIV: human immunodeficiency virus HR: heart rate HRQoL: health‐related quality of life IPI: inter‐puff intervals IQR: interquartile range ITT: intention‐to‐treat LTFU: lost to follow‐up MMT: methadone maintenance treatment MNWS: modified Minnesota Nicotine Withdrawl NEC: nicotine electronic cigarette NNAL: carcinogen found in tobacco smoke (4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol) NNC: non nicotine cigarette NNK: nicotine‐derived nitrosamine ketone NR: not reported NRT: nicotine replacement therapy NVP: nicotine vaping product OST: opioid substitution therapy OTH: Oklahoma Tobacco Helpline PAOD: peripheral arterial occlusive disease pd: per day PEC: placebo electronic cigarette PG: propylene glycol PIS: patient information sheet PP(A): point prevalence (abstinence) ppm: parts per million RA: research assistant SAE: serious adverse event SBP: systolic blood pressure SD: standard deviation SDM: standardised mean SMI: serious mental illness SSS: stop smoking services TAU: treatment as usual TCD: transcranial doppler TNE: total nicotine equivalents TQD: target quit date UC: usual care USB: universal serial bus VG: vegetable glycerine VLNC: very low nicotine content VNP: vaporized nicotine products WBC: white blood cells
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adkison 2013 | Although this study uses a prospective cohort design, no data on EC use were collected at baseline, with EC use data only being available at follow‐up |
| Al‐Delaimy 2015 | Observational study with no intervention provided ‐ included in previous versions, but excluded from 2020 |
| Anonymous 2019 | Commentary of included study (not primary study) |
| Battista 2013 | Short‐term EC use only |
| Bianco 2019 | Ineligible intervention |
| Biener 2015 | Cohort study, but EC use evaluated retrospectively only |
| Biondi‐Zoccai 2019 | Less than 1 week follow‐up |
| Biondi‐Zoccai 2020 | Acute EC use only |
| Borderud 2014 | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Brose 2015 | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Brown 2014a | Cross‐sectional survey |
| Bullen 2010 | Short‐term EC use only |
| Bullen 2018 | Withdrawn trial registry |
| Caponnetto 2019 | Ineligible intervention |
| Cavarretta 2019 | Less than 1 week follow‐up |
| Chaumont 2018 | Less than 1 week follow‐up |
| Chaumont 2019 | Ineligible intervention |
| Chausse 2015 | Ineligible study design |
| Choi 2014 | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Chorti 2012 | Short‐term EC use only |
| Collins 2019 | Ineligible intervention |
| Cook 2019 | Commentary of included study (not primary study) |
| Cox 2019a | Short‐term abstinence only (< 6 months) |
| Czogala 2012 | Short‐term EC use only |
| D'Ruiz 2017 | Less than 1 week follow‐up |
| Dawkins 2012 | Short‐term EC use only |
| Dawkins 2013a | Short‐term EC use only |
| Dawkins 2014 | Short‐term EC use only |
| Douptcheva 2013 | Longitudinal study, but no data are reported for smoking cessation or reduction or for adverse events |
| Dutra 2014 | Cross‐sectional survey |
| Eissenberg 2010 | Short‐term EC use only |
| Etter 2014 | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Farsalinos 2012 | Short‐term EC use only |
| Farsalinos 2013a | Included people that had already stopped smoking conventional cigarettes |
| Farsalinos 2013b | Short‐term EC use only |
| Farsalinos 2013c | Short‐term EC use only |
| Farsalinos 2013d | Short‐term EC use only |
| Flouris 2012 | Short‐term EC use only |
| Flouris 2013 | Short‐term EC use only |
| Gmel 2016 | Cohort study, but EC use only evaluated retrospectively |
| Gottlieb 2019 | Commentary of included study (not primary study) |
| Grana 2014b | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| James 2016 | Follow‐up at 12 weeks, AE data not collected |
| Kasza 2013 | Longitudinal study, but no data are reported for smoking cessation or reduction or for adverse events |
| Kouretas 2012 | Short‐term EC use only |
| Kousta 2019 | Commentary of included study (not primary study) |
| Lechner 2015 | Less than 1 week follow‐up |
| Lee 2014 | Cross‐sectional survey |
| Manzoli 2015 | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Marini 2014 | Short‐term EC use only |
| Mayor 2019 | Commentary of included study (not primary study) |
| Miura 2015 | Tests a device which is not an EC |
| NCT02487953 | Withdrawn trial registry |
| NCT02527980 | No intervention: observation only |
| NCT03036644 | Less than 1 week follow‐up |
| NCT03575468 | Ineligible intervention |
| NCT04107779 | Less than 1 week follow‐up |
| Nolan 2016 | Short‐term abstinence only (< 6 months) |
| NTR6224 | Study terminated early; no usable results. Previously listed as ongoing |
| Palamidas 2014 | Short‐term EC use only |
| Pearson 2012 | Longitudinal study, but no data are reported for smoking cessation or reduction or for adverse events |
| Pokhrel 2013 | Cross‐sectional survey |
| Polosa 2014a | Observational study with no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Popova 2013 | Cross‐sectional survey |
| Prochaska 2014 | RCT but no EC intervention provided ‐ included in previous versions, but excluded from 2020 |
| Russo 2018 | Ineligible study design |
| Schober 2014 | Short‐term EC use only |
| Siegel 2011 | Retrospective survey of 222 EC users that responded to a survey sent to 5000 new users of the 'Blu' EC. Likely to be a self‐selected sample |
| Song 2020 | Ineligible patient population |
| St.Helen 2020 | Ineligible intervention |
| Stein 2019 | Commentary of included study (not primary study) |
| Stower 2019 | Ineligible study design |
| Tsikrika 2014 | Short‐term EC use only |
| Tucker 2018 | Short‐term abstinence only (< 6 months) |
| Tzatzarakis 2013 | Short‐term EC use only |
| Vakali 2014 | Short‐term EC use only |
| Valentine 2016 | Less than 1 week follow‐up |
| Van Heel 2017 | Ineligible study design |
| Vansickel 2010 | Short‐term EC use only |
| Vansickel 2012 | Short‐term EC use only |
| Vansickel 2013 | Short‐term EC use only |
| Vardavas 2012 | Short‐term EC use only |
| Vickerman 2013 | Cross‐sectional survey |
| Voos 2019 | Less than 1 week follow‐up |
| Voos 2020 | Ineligible study design |
| Wagener 2014 | EC use for up to 1 week, but does not report on any adverse events |
| Walele 2016a | RCT but follow‐up too short |
| Walele 2016b | RCT but follow‐up too short |
| Yan 2015 | Ineligible study design |
| Yuki 2017 | Less than 1 week follow‐up |
| Zhang 2019 | Commentary of included study (not primary study) |
EC: electronic cigarette
Characteristics of ongoing studies [ordered by study ID]
ACTRN12617001324303.
| Study name | Vaporised nicotine products versus oral forms of nicotine replacement therapy (NRT) products for tobacco smoking cessation among low‐socioeconomic status (low‐SES) people who smoke |
| Methods | Parallel, single‐blinded, randomized controlled trial Setting: Australia Recruitment: Not stated |
| Participants | Target sample size: 868 Inclusion criteria:
The term “current smoker” in this trial will refer to those who use either factory‐made or roll‐own cigarettes. Exclusion criteria:
People will also be excluded if they report any of the following medical conditions in the previous 3 months: serious chronic lung diseases, arrhythmia, heart attack, stroke, or severe angina |
| Interventions | Vaporised nicotine product (VNP) arm:
Oral nicotine replacement therapy (NRT) arm:
|
| Outcomes | Primary outcome: Carbon monoxide‐verified six‐month continuous abstinence (smoking not more than 5 cigarettes) from the quit date (8 months from baseline) Secondary outcomes measured at 2‐week and 6‐week check‐in calls and 8‐month follow‐up
Weekly text message surveys and check‐in calls 2 weeks and 6 weeks into the treatment period. These check‐in calls will also assess smoking status, short‐term outcomes, and adverse events at these time points. |
| Starting date | Anticipated start date: 30 April 2019 |
| Contact information | Richard P Mattick, r.mattick@unsw.edu.au Alexandra Aiken, a.aiken@unsw.edu.au |
| Notes |
ACTRN12619001787178.
| Study name | Project NEAT: NicotinE As Treatment for tobacco smoking following discharge from residential withdrawal services |
| Methods | RCT Project NEAT: A randomized controlled trial to examine the efficacy of vaporised nicotine products and telephone quitline support compared with nicotine replacement therapy and telephone quitline support when used following discharge from residential withdrawal services Setting: Australia (New South Wales, Queensland, Victoria) Recruitment 4 hospital sites: Belmont Hospital, Belmont; St Vincent's Hospital, Darlinghurst; Turning Point Drug and Alcohol Centre, Richmond; Royal Brisbane & Womens Hospital, Herston |
| Participants | Target sample size: 926 Inclusion criteria: • Aged 18 or over • Daily tobacco smoker (10 or more cigarettes) on entering withdrawal unit • Accessing treatment from participating services • Want to quit smoking in the next 30 days • Has capacity to consent and able to understand the participant materials and follow the study instructions and procedure (e.g. sufficient English language ability and not too unwell as judged by medical staff) Exclusion criteria: • Pregnant or breastfeeding • Enrolled in another study • Scheduled to be transferred to a long‐term residential rehabilitation service following discharge from the withdrawal unit • Used VNP (containing nicotine) in the last 30 days • Currently engaged in Quitline’s call‐back services • No ready access to a phone |
| Interventions | Condition one: Vaporised nicotine products and Quitline Condition two: Current best practice treatment for tobacco smoking (combination Nicotine Replacement Therapy and Quitline) |
| Outcomes | 9 months after inpatient withdrawal unit discharge: Self‐reported 7 months continuous abstinence from tobacco smoking Biochemically‐verified 7‐month continuous abstinence from tobacco smoking 3 and 9 months after inpatient withdrawal unit discharge: 30‐day point prevalence abstinence 7‐day point prevalence abstinence Abstinence from all nicotine/tobacco products |
| Starting date | Anticipated enrolment: 19 December 2019. Anticipated date last data collection: 19 September 2022 |
| Contact information | Prof Billie Bonevski, Billie.Bonevski@newcastle.edu.au |
| Notes | Funding: National Health and Medical Research Council (grant number: G1800272), Canberra ACT 2601 |
ACTRN12621000076875.
| Study name | Vaporised nicotine products versus nicotine replacement therapy for tobacco smoking cessation among low‐socioeconomic status smokers: a randomised controlled trial |
| Methods | Design: RCT |
| Participants | Inclusion criteria: Participants can be included if they meet the following criteria: • willing to allow the research team and study clinician to access their data for quality assurance and to maintain the integrity of the trial • 18 years of age or older • receiving a government pension or allowance (proxy for low SES) • are a current daily smoker • interested in quitting smoking and using the study products • willing to make a quit attempt in the next 2 weeks • have a mobile phone that can receive text messages • available for follow‐up over a 7‐month period • agree to use the allocated study product and refrain from using another quit‐smoking medication whilst using the study products • willing to receive daily quit‐support text messages during the treatment period (with the option to opt out during the study) • speak English and can provide consent • willing to allow the research team to share the collected contact detail |
| Interventions | Vaporised nicotine product (VNP) devices (1 tank device and 1 pod device) for 8 weeks plus 5‐week Text Message behavioural quit Support (TMS) with the option to opt out at any stage if desired. Participants will receive a mix of quit‐smoking support text messages with content including information on how to use the study products; coping with nicotine withdrawal symptoms; study progress updates; and motivational ‘feel good’ messages. A mix of text, emojis and links to resources such as videos, websites and Graphics Interchange Format (GIF) images, will be used throughout the TMS programme to promote engagement with the programme. Each device will be charged using the provided USB charger and wall adaptor. A replacement battery and replacement coils (5 pieces per pack) will also be provided. The VNP tank device used is the Innokin Endura T18 Personal Vaporizer, which has a refillable 2.5 mL tank for the e‐liquid (18 mg/mL nicotine). 3 e‐liquid flavours will be provided: tobacco, menthol and a fruit flavour. The study will have 3 e‐liquid suppliers to guarantee ongoing supply throughout the study: Lumo Liquid in 10 mL bottles; VAPO e‐liquid in 30 mL bottles; and DashVapes e‐liquid in 30 mL bottles. All e‐liquids are 18 mg/mL in strength. Lumo Liquid ingredients are as follows (w/w): tobacco flavouring (1.19%), nicotine (1.60%), vegetable glycerine (24.56%), propylene glycol (73.24%); menthol flavouring (4.83%), nicotine (1.60%), vegetable glycerine (22.99%), propylene glycol (71.18%); strawberry flavouring (0.63%), nicotine (1.60%), vegetable glycerine (33.00%), propylene glycol (71.00%). VAPO e‐liquid additional flavour ingredients are as follows (w/w): tobacco flavouring (25.88%), nicotine (17.25%), vegetable glycerine (36.53%), propylene glycol (20.34). |
| Outcomes | PRIMARY OUTCOME: CO‐verified 6‐month continuous abstinence at 7‐month follow‐up. Continuous 6‐month abstinence will be defined as having remained quit for 6 months (having smoked no more than 5 cigarettes in that time), and a CO level of ≤ 5 ppm. Depending on the participant's indicated preference, the CO breath test will be self‐administered using an using a hand‐held iCO™ Smokerlyzer® (using provided instructions), or administered by a trained researcher using a hand‐held iCO™ Micro+™ Smokerlyzer® with a disposable, one‐use mouthpiece. Both devices are non‐invasive and require the participant to blow air into the device for 15 seconds to measure their CO level. An exhaled CO level of ≤ 5 ppm will be considered abstinent.
The final follow‐up interview will occur 7 months after the baseline interview completion date SECONDARY OUTCOME: Change in financial stress (assessed using Index of Financial Stress). |
| Starting date | |
| Contact information | |
| Notes |
ACTRN12621000148875.
| Study name | HARMONY: HARM reduction for Opiates, Nicotine and You |
| Methods | Design: randomized single‐blinded parallel‐group trial |
| Participants | INCLUSION CRITERIA: • Provide written, informed consent to participate in the study • Aged 18 to 65 years • Be accessing opioid agonist treatment from a participating service • Current daily tobacco smokers on self‐report • Want to quit or cut down their tobacco smoking • Be willing and able to comply with requirements of study (including having access to a phone) |
| Interventions | Comparison of a 12‐week course of liquid nicotine delivered via Vaporised Nicotine Product (VNP) to best practice Nicotine Replacement Therapy (NRT) Condition 1: VNP (Innokin Endura T18‐II starter kit) • Device loaded with one bottle of 12 mg/mL e‐liquid • An additional seven (7) bottles of 12 mg/mL e‐liquid • A brief information session on how to use the VNP • A VNP information pack including safe storage of e‐liquid nicotine and disposal • 1‐week supply of nicotine patches • Training on the use of NRT patches • Where possible, ensure that participant uses the VNP before leaving and leaves wearing an NRT patch. Adherence will be measured via questionnaires. In addition to the VNP, liquid nicotine and NRT patches, participants will be shown the New Zealand website vapingfacts.health.nz/ and encouraged to visit the site as an online resource throughout the trial. Participants will also receive training in the forms of brief videos, information pamphlets, user manuals and interactive discussions with research staff. |
| Outcomes | PRIMARY OUTCOME: Self‐reported 7‐day PPA from tobacco smoking assessed in the following dichotomous question “In the last 7 days, have you smoked a cigarette, even a puff?" [Week 12] SECONDARY OUTCOME: A cost consequence study setting out detailed comparative costs of treatments ‐ from the perspective of healthcare provider and primary and secondary outcomes of the VNP and NRT Adverse events recorded Biochemically‐verified PPA: This will be measured via a CO monitor breath test for participants who self‐report 7‐day PPA at end of treatment [Week 12] Changes in nicotine craving and withdrawal symptoms |
| Starting date | |
| Contact information | |
| Notes |
Berlin 2019.
| Study name | Randomized, placebo‐controlled, double‐blind, double‐dummy, multicentre trial comparing electronic cigarettes with nicotine to varenicline and to electronic cigarettes without nicotine: the ECSMOKE trial protocol |
| Methods | 3‐arm randomized, placebo‐controlled, multicentre, double‐blind, double‐dummy, parallel‐group phase III type trial Setting: Smoking cessation clinics of both academic and community hospitals Recruitment is either local (a) directly by the centres or centralized (b) using a web page and a centralized study‐specific phone number and email address
|
| Participants | Estimated enrolment: 650 participants Inclusion criteria: people who smoke, ≥ 10 CPD (factory‐made or roll‐your‐own) in the past year; aged 18–70; motivated to quit, defined as a score > 5 on a visual rating scale ranging from 0 (not motivated at all) to 10 (extremely motivated); informed consent; understanding and speaking French; women of childbearing age can be included if they use an effective contraceptive method: either hormonal contraception or an intrauterine device started at least 1 month before the first research visit; individual affiliated to a health insurance system; previous failure of NRT for smoking cessation Exclusion criteria: any unstable disease condition within the last 3 months defined by the investigator as major change in symptoms or treatments, such as recent myocardial infarction, unstable or worsening angina, severe cardiac arrhythmia, unstable or uncontrolled arterial hypertension, recent stroke, cerebrovascular disease, obliterative peripheral arterial disease, cardiac insufficiency, diabetes, hyperthyroidism, pheochromocytoma, severe hepatic insufficiency, history of seizures, severe depression, COPD; any life‐threatening condition with life expectancy of < 3 months; alcohol use disorder defined as a score ≥ 10 on the Alcohol Use Disorders Identification Test (AUDIT)‐C questionnaire; abuse of or dependence on illegal drugs in the last 6 months, revealed by medical history; regular use of tobacco products other than cigarettes; current or previous (last 6 months) use of EC; pregnancy/breastfeeding; protected adults; current or past 3 months participation in another interventional research; current or past 3 months use of smoking cessation medication such as varenicline, bupropion, NRTs; known lactose intolerance (placebo tablets contain lactose); hypersensitivity to the active substance or to any of the excipients; known severe renal failure |
| Interventions | A) EC without nicotine (ECwoN) plus placebo tablets of varenicline (0.50 mg) administered by oral route: placebo condition B) EC with nicotine (ECwN) plus placebo tablets of varenicline: ECwN condition. V C) Reference: ECwoN plus 0.5 mg varenicline tablets: varenicline condition. Varenicline administered according to the marketing authorization E‐cigarette details:
Behavioural support:
Treatment duration: 1 week + 3 months |
| Outcomes | Week 2, 4, 8, 10, 12, 24 after target quit day Primary outcome:
Secondary outcomes:
|
| Starting date | 17 October 2018 |
| Contact information | Ivan Berlin, ivan.berlin@aphp.fr |
| Notes |
Caponnetto 2014.
| Study name | Smoking cessation and reduction In schizophrenia (the SCARIS study) |
| Methods | 3‐arm prospective 12‐m randomized controlled trial investigating efficacy and safety of EC Setting: psychiatric and smoking cessation centres, Italy Recruitment: local newspapers and radio/television advertisements |
| Participants | 153 participants Inclusion criteria
Exclusion criteria
|
| Interventions | 12‐wk supply of:
|
| Outcomes | Follow‐up visits at 4, 8, 12, 24 and 52 weeks Outcome measures:
|
| Starting date | September 2014 |
| Contact information | Pasquale Caponnetto, p.caponnetto@unict.it |
| Notes |
Caponnetto 2020.
| Study name | Non‐inferiority trial comparing cigarette consumption, adoption rates, acceptability, tolerability, and tobacco harm reduction potential in smokers switching to Heated Tobacco Products or electronic cigarettes: study protocol for a randomized controlled trial |
| Methods | RCT 12 weeks |
| Participants | 220 healthy people who smoke tobacco cigarettes |
| Interventions | Arm 1 ‐ Heated Tobacco Products (HTPs) Arm 2 ‐ E‐cigarettes (ECs) |
| Outcomes | 12‐week study. Follow‐up 24 weeks Biochemically‐verified self‐reported continuous abstinence at 12 weeks from the previous visit Secondary outcomes will include: smoking reduction from baseline, adoption rates and product acceptability, tolerability, changes in step test values and in the level of selected biomarkers of exposure in exhaled breath (i.e. eCO) and in spot urine samples A follow‐up visit at 24 weeks to review product usage and smoking behaviour under naturalistic condition of use |
| Starting date | Recruitment May 2019; enrolment is expected to be completed in November 2019. Results to be reported in 2020 |
| Contact information | Pasquale Caponnetto, p.caponnetto@unict.it |
| Notes | Funded by Philip Morris New to 2022 update |
Cox 2022.
| Study name | E‐cigarettes vs usual care for smoking cessation when offered at homeless centres |
| Methods | RCT. A multicentre cluster‐randomized controlled trial Setting: 32 centres across six areas in Great Britain: Scotland; Wales; London; South‐East England; South‐West England and East England |
| Participants | Estimated enrolment: 480 participants Inclusion criteria: Currently accessing homeless centre services and actively engaging with the service; > 18 years; self‐reported daily smoking, then biochemically verified Exclusion criteria: Never‐ and ex‐smokers; currently using a smoking cessation aid; unable to provide written consent; not known to centre staff; allergic to any of the e‐liquid ingredients (EC arm only); pregnant |
| Interventions | EC: refillable Cluster rather than individual randomization will be used. Arm 1: Planned intervention Delivery of the EC intervention will be as per our feasibility study. Centre staff will provide EC arm participants with a tank‐style refillable EC starter kit (e.g. the PockeX as used in our feasibility study or similar model determined via our PPI work), a choice of nicotine strength e‐liquids (12 mg/mL & 18 mg/mL) and flavours (tobacco, menthol or fruit) and an EC fact‐sheet. E‐liquids (five 10 mL bottles per week) will be supplied for four weeks at weekly intervals by centre staff. Arm 2: Control/comparator group Control arm will be usual care (UC). This will include very brief advice (VBA) to quit (in the form of an ‘NHS choices’ leaflet adapted for this population as used in our feasibility study) and signposting to the local SSS. All participants (intervention and control) will be offered a £15 Love2Shop gift card (which cannot be used for tobacco or alcohol purchases) for each follow‐up appointment attended. |
| Outcomes | Baseline, 4, 12 and 24 weeks Primary outcome measure Current primary outcome measure as of 27/07/2022: Sustained CO‐validated smoking cessation at 24 weeks using the Russell Standard for cessation trials and intention‐to‐treat analysis (i.e. no more than 5 cigarettes since 2 weeks from baseline, validated by expired CO < 8 ppm. Secondary outcome measures Current secondary outcome measure as of 27/07/2022: 1. Smoking reduction at 24 weeks 2. 7‐day point prevalence quit rates at 4, 12 and 24 weeks self‐reported and validated by expired CO < 8 ppm. 3. Changes in the frequency of risky smoking practices (e.g. sharing cigarettes, smoking discarded cigarettes) 4. Cost‐effectiveness of the intervention measured using a service use questionnaire and the EQ‐5D‐5L (4, 12, 24 weeks) 5. Fidelity of intervention implementation 6. Mechanisms of change measured quantitatively via questions (e.g. attitudes and perceptions of e‐cigs) 7. Contextual influences and sustainability telephone interviews |
| Starting date | Start date 23 April 2021. Estimated study end date: 31 August 2024 |
| Contact information | Lynne Dawkins, dawkinl3@lsbu.ac.uk |
| Notes | New to 2022 update |
El‐Khoury 2021.
| Study name | Preference‐based tools for smoking cessation among disadvantaged smokers, a pragmatic randomised controlled trial (STOP) |
| Methods | RCT France |
| Participants | Estimated enrolment 528 Inclusion criteria: daily smokers (≥ 5 cigarettes/day); low socioeconomic position; available for at least 4 appointments over a 6‐month period; affiliation to or benefiting from social security or state medical support Exclusion criteria: individuals who do not speak French; major citizens protected by law, adults unable to express their consent; pregnant women; regular smokers who vape daily (at least once a day) |
| Interventions | EC: type not stated Arm 1: The STOP intervention Assisting smokers with low socioeconomic position in their smoking cessation attempt. Routine care and adapted advice supplemented with a free delivery of any or several type(s) of nicotine replacement therapy (NRT) (patches, inhalers, gum, tablets, etc.) and/or an e‐cigarette + e‐liquid, based on the smokers' preference and choice Arm 2: Standard care Participants randomised to the standard care group will be given standard care in assisting their smoking cessation attempt, but without free delivery of NRT or e‐cigarettes. Standard care includes motivational interviewing, advice to quit and prescription for NRTs. |
| Outcomes | Smoking abstinence at 6 months after inclusion Total number of days of abstinence at 6 months Smoking abstinence at 1 and 3 months after inclusion Number of relapses; CPD; proportion of participants who have significantly reduced daily smoking |
| Starting date | |
| Contact information | |
| Notes | New to 2022 update |
Elling 2021.
| Study name | Effects of providing tailored information about e‐cigarettes in a web‐based smoking cessation intervention: protocol for a randomized controlled trial |
| Methods | RCT |
| Participants | Estimated enrolment 687 Inclusion criteria were that participants are at least 18 years old, have sufficient command of the Dutch language, have necessary internet literacy to use the intervention, have smoked tobacco in the past 7 days, and are motivated to quit tobacco smoking within 5 years. |
| Interventions | Digital computer‐tailored smoking cessation intervention. Arm 1: intervention condition Participants in the intervention condition will receive tailored information on e‐cigarettes based on 5 items. The computer‐tailored intervention will be based on the I‐Change model. Arm 2: control condition The control condition will not receive tailored information about e‐cigarettes. |
| Outcomes | Baseline, 6 mths Number of tobacco cigarettes smoked in the past 7 days; average number of tobacco cigarettes smoked per day; 7‐day point prevalence tobacco abstinence; 7‐day point prevalence e‐cigarette abstinence Smoking cessation methods; determinants of decision‐making; process evaluation |
| Starting date | |
| Contact information | Jan Mathis Elling, m.elling@maastrichtuniversity.nl |
| Notes | New to 2022 update |
ISRCTN13158982.
| Study name | Enhancing dental health advice |
| Methods | RCT Newcastle Clinical Trials Unit, UK |
| Participants | Estimated enrolment: 1460 1460 adult regular tobacco smokers, with 455 of those with periodontitis going into a subgroup for additional examination and analysis Inclusion criteria: A basic periodontal examination completed within the last 3 months; ≥ 18 years; current smoker Periodontitis subgroup: Minimum of 16 natural teeth; diagnosis of periodontitis stage II (or greater) Exclusion criteria: Pregnant or currently breastfeeding; enrolled in another interventional research trial; used quit‐smoking aid or reduce/quit alcohol; phaeocromocytoma, uncontrolled hyperthyroidism, extensive dermatitis/skin disorder; hypersensitivity to nicotine or any component of the study products; taking: clozapine, olanzapine, theophylline or aminophylline |
| Interventions | EC type not specified. The starter kit will include ten, 10 mL bottles of e‐liquid with different flavours and nicotine concentrations. Condition: Smoking cessation in dental patients with or without gum disease Arm 1: Control arm: Very Brief Advice (VBA) 1. VBA is usual care for smokers in dental settings usually following the 3As: Ask, Advise, Act technique. This will signpost participants to a GP, pharmacy or stop‐smoking service (SSS). 2. Participants in the control group will be free to use NRT or ECs as they wish but these will not be provided by the dental professional. 3. Conducted at baseline visit, only a 5‐minute intervention 4. Patients will be followed up for up to 12 months from baseline. Arm 2: Nicotine Replacement Therapy (NRT) 1. If a participant is randomised to a NRT arm, a trained dental professional will provide a single‐visit behavioural support intervention including the offer of NRT. 2. 12‐week course of combination NRT (patch plus faster acting form such as chewing gum or lozenge), in line with current recommendations 3. Duration will be 12 weeks if a participant wants to continue NRT after initial 4‐week supply. 4. Participants will be followed up for up to 12 months from baseline. Arm 3: E‐cigarette (EC) Receive the same behavioural intervention as the NRT group along with EC starter kit. The starter kit will include ten, 10 mL bottles of e‐liquid with a choice of one of four packages of flavour and nicotine concentrations. 2. Participants will be expected to source their own supply of e‐liquid after the initial supply and advice will be given as to where to source suitable MHRA registered products. 3. Duration will vary depending on use of EC. 4. Participants will be followed up for up to 12 months from baseline. |
| Outcomes | Baseline to 12 months. Primary outcome measure: Biochemically verified smoking abstinence 6 months after randomization using a carbon monoxide monitor Secondary outcome measures: 1. Continuous biochemically verified smoking abstinence is measured using exhaled Carbon Monoxide (eCO) at 12 months. 2. Nicotine dependence is measured using Fagerstrom Test for Nicotine Dependence (FTND) at baseline and 6 months 3. Cigarette withdrawal symptoms are measured using Mood and Physical Symptoms Scale (MPSS) at baseline and 6 months. 4. Quality of life related to oral health 5. Oral health is measured using number of teeth at baseline and 6 months. 6. Health economic evaluation For periodontitis subgroup only: Periodontal health is measured. See study for more detail. |
| Starting date | Study start date February 2022. Estimated completion date: March 2025 |
| Contact information | Dr Richard Holliday, richard.holliday@newcastle.ac.uk |
| Notes | New to 2022 update |
ISRCTN17691451.
| Study name | ESCAPE: E‐cigarettes for smoking cessation and reduction in people with mental illness |
| Methods | RCT Setting: Hospitals, UK. West Park Hospital; Bradford District Care NHS Foundation Trust; Sheffield Clinical Commissioning Group |
| Participants | The target sample size for the randomized controlled feasibility trial is 72, with 36 participants allocated to each group. Inclusion criteria: receiving treatment for a mental illness; > 18 years; smoker; willing to attempt to quit Exclusion criteria: inpatient admission in the last 3 mths according to their health care record; smokers using EC; participating in other smoking cessation trials; being treated for comorbid drug or alcohol problems; Alzheimer’s disease or dementia; pregnancy or breastfeeding |
| Interventions | EC: Aspire PockeX electronic cigarette The intervention consists of an e‐cigarette starter kit containing a third generation e‐cigarette (Aspire PockeX), a four‐week supply of e‐liquid (a choice of flavours and concentrations will be offered) and an information leaflet. Intervention delivery will take place at a previously scheduled appointment with a clinician. The control condition is usual care. |
| Outcomes | Baseline, 2‐4 weeks, 1 month Primary outcome measure: 1. Feasibility and acceptability outcomes: the primary feasibility outcome measures in the feasibility trial will be consenting rate and recruitment frequency. 2. Clinical (smoking‐related) outcomes: continuous abstinence assessed at 1 month will be defined as not having smoked in the two weeks prior to follow‐up, verified by a CO reading below 10 ppm, in keeping with the standard measure used in Stop‐Smoking Services. Secondary outcome measures: 1. Feasibility and acceptability outcomes Characteristics of ‘usual care’ in different locations will be also noted, recording two interactions of patients with CMHTs or GPs at each site at baseline and using short pro‐forma with control group participants at 1‐month follow‐up. 2. Clinical (smoking‐related) outcomes: Self‐reported abstinence 2‐4 weeks from enrolment or target quit date (whichever is later) will be recorded at 1‐month follow‐up. The change in cigarette consumption (and reduction in exhaled breath CO reading) from baseline to 1‐month follow‐up will be calculated in both intervention and control group participants. 3. Clinical (mental health‐related) outcomes 4. Cost effectiveness 5. Serious adverse events (SAE) and adverse events (AE) |
| Starting date | Start date 29 September 2021. Trial end date: 30 November 2022 |
| Contact information | Professor Lion Shahab, lion.shahab@ucl.ac.uk
Dr Elena Ratschen, elena.ratschen@york.ac.uk Dr Anna‐Marie Marshall, a.marshall@york.ac.uk |
| Notes | New to 2022 update |
ISRCTN61193406.
| Study name | Do e‐cigarettes help smokers quit when not accompanied by intensive behavioural support? A multi‐center randomized controlled trial |
| Methods | RCT Setting: UK Multicentre. Participants will be recruited mainly from hospitals and GP practices across the UK by the Clinical Research Network. The study is being organized by Queen Mary University of London (QMUL). Researchers from QMUL will provide the study treatment and conduct follow‐up calls. |
| Participants | 1170 people who smoke tobacco cigarettes Inclusion criteria:
Exclusion criteria:
|
| Interventions | 1. Control: NHS Quit Now programme (QN) 2. E‐cigarette starter pack with no ongoing support (EC) 3. EC starter pack with helpline support (EC+) The study will aim to use a refillable EC that is similar to the type used in a previous EC trial (One Kit ‐ Innokin, UK Ecig Store), and one that is compliant with UK regulations, and not produced by a tobacco company. |
| Outcomes | Follow‐up at 4 weeks, 6 months and 12 months. CO at 6 and 12 months Primary outcome measure: Sustained smoking cessation at 6 months post‐TQD. This is measured by asking participants if they have smoked since their TQD at the 6‐month follow‐up. To be counted as a 'quitter', participants must report smoking no more than 5 cigarettes since 2 weeks post‐TQD with no smoking in the previous week, validated by carbon monoxide (CO) reading of < 8 ppm. Participants lost to follow‐up will be counted as smokers. Secondary outcome measures:
|
| Starting date | Overall trial start date: 01 September 2020 Trial end date: 31 May 2024 Not yet recruiting. Last edited 12 August 2020 |
| Contact information | Dr Katie Myers Smith, katie.smith@qmul.ac.uk |
| Notes |
Klonizakis 2017.
| Study name | Smokers making a quit attempt using e‐cigarettes with or without nicotine or prescription nicotine replacement therapy: impact on cardiovascular function (ISME‐NRT) ‐ a study protocol |
| Methods | Pragmatic, 3‐group, randomized, assessor‐blinded, single‐centre trial Setting: Centre for Sport and Exercise Science (CSES) of Sheffield Hallam University, UK Recruitment: From the community in the wider Sheffield area will be by: i) low‐cost newspaper and post‐office advertisement, ii) posters in local pharmacies, libraries, mosques, churches, and clubs, iii) social media or search engine advertisement (Facebook, Google ads) iv) notices in newsletters or participation in outreach events of community organizations (such as Sheffield U3A and AGE UK), iv) a study website, and v) out‐reach events in local ethnic community centres or places of worship |
| Participants | Estimated enrolment: 258 participants (86 participants arm) Inclusion Criteria:
Exclusion Criteria:
|
| Interventions |
|
| Outcomes | Follow‐up: Within 3 days of “quit date”, 3 and 6 months past quit date Outcome measures:
|
| Starting date | 24 April 2017 |
| Contact information | Markos Klonizakis, m.klonizakis@shu.ac.uk |
| Notes |
Krysinski 2021.
| Study name | International randomized controlled trial evaluating metabolic syndrome in type 2 diabetic cigarette smokers following switching to combustion‐free nicotine delivery systems: the DIASMOKE protocol |
| Methods | Design: RCT, multicentre Italy |
| Participants | 576 People with type 2 diabetes who smoke cigarettes Participants will be at least 23 years old and of any gender Inclusion criteria: Participants will be required to satisfy all of the following criteria at the screening visit, unless otherwise stated: • Participants will be: 1.1. over 23 years of age • T2DM patients will have: 2.1. body mass index (BMI) between 17.6 and 32.0 kg/m2, inclusive; 2.2. body weight exceeding 50 kg (men) or 40 kg women; 2.3 6.5 < HbA1C < 10; 3.2. completion of proforma (CRF); 3.3. lab assessment as outlined in the CRF
Exclusion criteria: Participants will be excluded at the screening visit based on the following criteria:
|
| Interventions | EC Arm A (control): tobacco cigarettes (continuing smoking their own tobacco cigarette brand) (will be offered referral to smoking cessation programmes) Arm B (intervention): switching to using combustion‐free nicotine delivery systems (C‐F NDS) At screening and prior to enrolment, all patients will be offered a locally‐available free smoking cessation programme as per local guidelines. Those who express the intention of booking for the cessation programme together with those who, at screening, are planning to quit smoking in the next 6 months, will not be recruited into the study. Patients taking part in the study will be informed that they are free to quit smoking and withdraw from the study at any time. Any person who decides to quit smoking will be directed to local stop‐smoking services. |
| Outcomes | Time frame: 3 months, 6 months, 1 year and 2 years Change in metabolic syndrome prevalence Change in plasma glucose Change in triglycerides Change in high‐density lipoprotein (HDL) Change in waist circumference (Primary outcome measures include fasting plasma glucose, blood pressure, triglycerides, high‐density lipoprotein and waist circumference, while secondary outcome measures feature absolute change in the sum of the individual factors of MetS and change in each individual factor of MetS measured at each study time point) |
| Starting date | Participant recruitment will start in February 2021 and is expected to be completed by December 2021. Results reported between 2023 and 2024 From: NCT04231838 (updated Jan 2022): Actual start date: 17 September 27 2021. Estimated study completion March 31 2026 |
| Contact information | Daniela Saitta, PhD, daniela.saitta@eclatrbc.it Riccardo Polosa, PhD, polosa@unict.it |
| Notes |
Murray 2020.
| Study name | Yorkshire Enhanced Stop Smoking (YESS) study: a protocol for a randomized controlled trial to evaluate the effect of adding a personalized smoking cessation intervention to a lung cancer screening programme |
| Methods | RCT Setting: Yorkshire, UK |
| Participants | Anticipated recruitment: 1040 people who smoke tobacco cigarettes Participants are aged 55–80, registered with a general practitioner (GP) in the Leeds Clinical Commissioning Group area and registered as a current or ex‐smoker in primary care databases Inclusion criteria:
Exclusion criteria:
|
| Interventions | Arm 1: enhanced, personalized smoking cessation (SC) support package, including CT scan images. SC support over 4 weeks comprising behavioural support, pharmacotherapy and/or a commercially available e‐cigarette Arm 2: continued standard best practice |
| Outcomes | Follow‐up contact will be requested at 4 weeks, 3 months and 12 months, with a 2‐week window to accommodate participant availability. The primary objective is to measure 7‐day point prevalent carbon monoxide (CO)‐validated SC after 3 months. Secondary outcomes include CO‐validated cessation at 4 weeks and 12 months, self‐reported continuous cessation at 4 weeks, 3 months and 12 months, attempts to quit smoking and changes in psychological variables, including perceived risk of lung cancer, motivation to quit smoking tobacco, confidence and efficacy beliefs (self and response) at all follow‐up points. |
| Starting date | January 2019 and December 2020 with follow‐up data collection ending December 2021 |
| Contact information | Professor Rachael L Murray; rachael.murray@nottingham. ac.uk |
| Notes |
NCT01842828.
| Study name | Spain‐UK‐Czech E‐cigarette Study (SUKCES) |
| Methods | Randomized controlled trial, open‐label pilot study Setting: smoking cessation clinics in London, Madrid and Prague Recuitment: via smoking cessation clinics |
| Participants | 220 people who smoke, seeking help to quit Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes |
|
| Starting date | December 2013 |
| Contact information | Peter Hajek, p.hajek@qmul.ac.uk |
| Notes |
NCT01989923.
| Study name | Smoking cessation in women with gynaecological conditions |
| Methods | Randomized controlled trial, open‐label feasibility study Setting: hospital clinic, USA Recruitment: in clinic |
| Participants | 30 women who smoke with cervical dysplasia Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Both groups receive identical cessation counselling. |
| Outcomes | At 6 and 12 weeks via survey:
|
| Starting date | June 2013 |
| Contact information | Laura A Beebe, laura-beebe@ouhsc.edu |
| Notes |
NCT02004171.
| Study name | Electronic cigarettes or nicotine inhaler for smoking cessation |
| Methods | Randomized controlled trial, open‐label safety/efficacy study Setting and recruitment not specified, USA |
| Participants | 40 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | 4 weeks:
|
| Outcomes | Over 4 weeks:
|
| Starting date | December 2013 |
| Contact information | Barney Vaughan, vaughan@nyspi.columbia.edu |
| Notes |
NCT02124187.
| Study name | Smoking cessation and reduction in depression (SCARID) |
| Methods | 3‐arm prospective 12‐m randomized controlled trial investigating efficacy and safety of ECs |
| Participants | 129 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | 12‐wk supply of:
|
| Outcomes | Follow‐up visits at 4, 8, 12, 24 and 52 weeks Outcome measures:
|
| Starting date | February 2015 |
| Contact information | Pasquale Caponnetto NCT02124187,%20SCARID,%20Smoking%20Cessation%20And%20Reduction%20in%20Depression" type="EXTERNAL">p.caponnetto@unict.it |
| Notes |
NCT02398487.
| Study name | Head‐to‐head comparison of personal vaporizers versus cig‐a‐like: prospective 6‐month randomized control design study (VAPECIG 2) |
| Methods | Randomized parallel‐assignment open‐label trial Setting: Italy, community |
| Participants | Estimated enrolment: 200 Inclusion criteria:
Exclude if:
|
| Interventions | Comparison between 2 types of EC; 'personal vaporizers' and 'cig‐a‐like' |
| Outcomes | 24 weeks:
|
| Starting date | October 2014 |
| Contact information | Riccardo Polosa |
| Notes |
NCT02590393.
| Study name | The role of nicotine and non‐nicotine alkaloids in e‐cigarette use and dependence |
| Methods | Randomized parallel‐assignment double‐blind trial Setting: Smoking research clinic, USA Recruitment: volunteers |
| Participants | Estimated enrolment: 375 Inclusion criteria:
Exclude if: multiple, related to baseline health status |
| Interventions |
|
| Outcomes | Primary:
Secondary:
|
| Starting date | May 2016 |
| Contact information | Jed Rose |
| Notes | "This is not a smoking cessation study; people who smoke will not be asked to quit smoking, and e‐cigarettes will not be used as a medical device or therapy." |
NCT03113136.
| Study name | Examination of low wattage and high wattage e‐cigarettes (SWITCH) |
| Methods | RCT, randomized interventional clinical trial |
| Participants | Estimated enrolment: 453 participants Inclusion criteria: no quit attempt in the prior 3 mths and no plan to quit in the next 3 months; smoke ≥ 5 cigarettes per day for the past year; minimal interest in switching to an alternative product; never purchased or regularly used a tank system, mechanical mod, or advanced personal vaporizer EC, though previous use of cig‐a‐like devices will be allowed Exclusion criteria: unstable or significant medical condition such as respiratory, kidney, or liver disease; unstable or significant psychiatric conditions; history of cardiac event or distress within the past 3 months; and currently pregnant, planning to become pregnant, or breastfeeding; < 18 years |
| Interventions | Active comparator: Low wattage e‐cigarette device. The low wattage e‐cigarette device will be provided to the participant and they will be instructed to vape ad libitum for 12 weeks and then be assessed at 6 months and 12 months for continued use of device. Active comparator: High wattage e‐cigarette device. The high wattage e‐cigarette device will be provided to the participant and they will be instructed to vape ad libitum for 12 weeks and then be assessed at 6 months and 12 months for continued use of device. Active comparator: Usual brand cigarette. The usual brand of cigarettes will be provided to the participant and they will be instructed to smoke ad libitum for the duration of the study. |
| Outcomes | Baseline, week 1, week 4, week 8, week 12, week 26, and week 52 Complete change from conventional cigarettes Exhaled carbon monoxide of ≤ 10 ppm Secondary outcomes: EC dependence; EC preference; biomarkers of exposure NNAL; NNN; TNE; nicotine metabolite ratio; nickel and other relevant metals; cadmium and other relevant metals; lead and other relevant metals; 8‐iso‐PGF2a; PGEM; q‐PADDA |
| Starting date | June 29, 2017. Estimated study completion date: August 31 2022 |
| Contact information | Theodore Wagener, PhD 405‐271‐8001 ext 44350, theodore‐wagener@ouhsc.edu |
| Notes | New to 2022 update |
NCT03249428.
| Study name | E‐cigarette inner city RCT A community‐based participatory action pragmatic randomized controlled trial using electronic‐cigarette for tobacco dependence in the inner city population with a holistic approach |
| Methods | RCT. Randomized cross‐over assignment. Multicentre 3‐yr multicentre pragmatic Randomized Controlled Trial (RCT) to compare effectiveness of nicotine e‐cigarettes (e‐cigs) (with counselling) with peer‐led PROMPT strategy (nicotine replacement therapy (NRT) and counselling) for tobacco dependence in the inner city population. Setting: Setting: 1) The Bridge Engagement Centre, Ottawa, adjacent to the largest homeless shelter downtown; and 2) Nicotine Dependence Clinic, Center for Addiction and Mental Health, catering for Toronto's inner city population downtown. Canada |
| Participants | Estimated enrolment: 200 Toronto and Ottawa inner city homeless/at‐risk for homelessness participants using poly‐substances Inclusion criteria: using poly‐substances within the past year, excluding marijuana or alcohol; ≥ 16 yrs; living in Ottawa or Toronto over the past 3 mths Exclusion criteria: consent declined; planning on accessing addictions treatment (inpatient drug rehabilitation) in Ottawa or Toronto and hence will be unavailable for follow‐up; currently or recently (in the past 30 days) enrolled in any other smoking cessation programme or have used/is using any EC (nicotine or non‐nicotine) in the past 60 days; terminal illness with a life expectancy of less than 3 months |
| Interventions | EC: cig‐a‐like NJOY Recharge Active comparator: Nicotine replacement therapy & one‐on‐one counselling Standard NRT such as the nicotine patch, gum, inhaler, and/or lozenge as per participant liking and clinical indication deemed necessary by the expert smoking cessation nurse. Counseling will include a number of approaches such as reviewing smoking history, development and revision of a reduced or quit plan, encouragement of self‐monitoring, review of triggers and challenges, and coping skills. Active comparator: Electronic nicotine delivery systems (EC) & one‐on‐one counselling EC with nicotine. Counseling will include a number of approaches such as reviewing smoking history, development and revision of a reduced or quit plan, encouragement of self‐monitoring, review of triggers and challenges, and coping skills. |
| Outcomes | 12, 26 and 52 weeks Quality of life (QOL) measured by questionnaire (EQ‐5D‐5L scores) at 26 weeks Smoking prevalence: biochemically validated 7‐day point prevalence smoking abstinence |
| Starting date | Estimated study start date Sept 2019. Estimated primary completion date delayed |
| Contact information | Smita Pakhale, MD 613‐737‐8899 ext 79979 spakhale@ohri.ca Sadia Jama, MSc 613‐518‐7172 sajama@ohri.ca |
| Notes | Contacted authors July 2022; study delayed New to 2022 update |
NCT03277495.
| Study name | Predictors and consequences of combustible cigarette smokers' switch to standardized research e‐cigarettes |
| Methods | RCT. Randomized parallel assignment Setting; USA |
| Participants | Estimated enrolment 120 participants Nicotine EC = 60; placebo EC = 60 Inclusion criteria: ≥ 21 years; ≥ 7 cpd ≥ 1 yr; breath CO ≥ 10 ppm; interested in reducing combustible cigarette use; willing to try EC; attend in‐person assessments for 5 mths; English‐speaking; women who are of childbearing age cannot be pregnant and must agree to use an approved form of birth control during the study. Exclusion criteria: current use of any smoking cessation medication or participation in a smoking cessation programme or study; daily EC use; pregnancy; no 2 members of the same household may participate in this study. |
| Interventions | EC: Standardized Research E‐Cigarette (SREC) Participants will be stratified by sex and use of menthol cigarettes and randomly assigned with a 1:1 allocation ratio to one of two conditions: 1) Active comparator: nicotine SREC. The liquid in the e‐cigarette refills contains nicotine and comes in the following flavours: tobacco, menthol, blueberry, and watermelon. 2) Placebo comparator: placebo SREC The liquid in the e‐cigarette refills does not contain nicotine and comes in the following flavours: tobacco, menthol, blueberry, and watermelon. |
| Outcomes | 3, 4, 5‐13, 14, 18 weeks Combustible cigarette use Abstinence from combustible cigarettes (defined as no cigarette smoking in the past 7 days) The total number of cigarettes smoked in the 7 days prior to the last assessment CO level. BP. Heart rate. Weight. Self‐report of respiratory symptoms. Fagerstrom Test for Nicotine Dependence |
| Starting date | Estimated starting date June 2022. Estimated completion date: August 2023 |
| Contact information | Kathleen Diviak, PhD 312‐996‐2327 kdiviak@uic.edu |
| Notes | New to 2022 update |
NCT03589989.
| Study name | The ESTxENDS Trial‐Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E‐cigarette) as an aid for smoking cessation. (ESTxENDS) |
| Methods | Randomized, parallel‐assignment, open‐label trial Setting: Switzerland Recruitment: Not specified |
| Participants | Estimated enrolment: 1172
Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcome: Continuous smoking abstinence at 6 months post‐quit date measured by:
Seconday outcomes:
|
| Starting date | 16 July 2018 |
| Contact information | Reto Auer, reto.auer@biham.unibe.ch Anna Schöni, anna.schoeni@biham.unibe.ch |
| Notes | Linked trials: NCT03603340; NCT03603353; NCT03612336; NCT03612375; NCT03612453; NCT03612544; NCT03632421; NCT03938298 |
NCT03625986.
| Study name | Does switching to nicotine containing electronic cigarettes reduce health tisk markers |
| Methods | RCT. Prospective parallel‐group randomized double‐blind, placebo‐controlled study Setting: Penn State Milton S. Hershey Medical Center, USA |
| Participants | Estimated enrolment: 240 Inclusion criteria: age 21 to 70 years; smoke regular, filtered cigarettes or machine‐rolled cigarettes with a filter ≥ 5 cpd for ≥ 12 months (CO ≥ 6 ppm at baseline visit); no serious quit attempt in prior month; willing to stop cigarette consumption and switch to an EC and to attend regular visits over a 7‐week period Exclusion criteria: unstable or significant medical condition such as COPD, kidney disease, or liver disease in the past 12 months or severe immune system disorders, uncontrolled mental illness or substance abuse or use of illicit drug/prescription, history of a seizure or seizure medication. Use of any non‐cigarette nicotine delivery product in the past 7 days (including EC); use of hand‐rolled, roll‐your‐own cigarettes; allergy to propylene glycol or vegetable glycerin; pregnancy or breastfeeding |
| Interventions | EC: Pod The electronic cigarette (e‐cig) used in this study will be the Standardized Research Electronic Cigarette (SREC). The SREC product is a pod‐based device and comprises a replaceable pre‐filled liquid reservoir ("pod") and a rechargeable power supply unit. Arm 1. Experimental: Nicotine‐containing electronic cigarette The experimental group will be provided with and encouraged to use a Standardized Research Electronic Cigarette (SREC) with liquid containing 58 mg/mL nicotine for the duration of 6 weeks. Arm 2. Placebo comparator: Non‐nicotine electronic cigarette The placebo group will be provided with and encouraged to use a Standardized Research Electronic Cigarette (SREC) with liquid containing 0 mg/mL nicotine for the duration of 6 weeks. |
| Outcomes | 3 weeks, 6 weeks, 10 weeks (phone) 3 weeks and 6 weeks after switching NNAL, FEV1, CO, plasma cotinine concentration, Fagerstrom Test for Nicotine Dependence mean total score, cpd, abstinence from cigarettes and other tobacco (not including e‐cigs) CO < 6 ppm, total score on Minnesota Nicotine Withdrawal Scale, EC use days, self‐reported abstinence |
| Starting date | Actual start date April 22 2022. Estimated study completion date: December 2023. |
| Contact information | Jessica Yingst, DrPH 7175314637, jyingst@phs.psu.edu Nicolle Krebs, MS 7175315673, nkrebs@pennstatehealth.psu.edu |
| Notes | New to 2022 update |
NCT03700112.
| Study name | An open‐label, randomized cross‐over study comparing nicotine pharmacokinetics of seven electronic cigarette products and one traditional cigarette across two delivery (10 puff and ad‐libitum) conditions, in healthy adult smokers |
| Methods | Open‐label, randomized cross‐over trial Setting and recruitment not specified, New Zealand |
| Participants | Estimated enrolment: 24 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Day 48 Outcomes:
|
| Starting date | 7 December 2018 |
| Contact information | Study director: Concetta Carbonaro Responsible party: Juul Labs, Inc. |
| Notes |
NCT03962660.
| Study name | Harm reduction for tobacco smoking with support of tobacco‐replacing electronic nicotine delivery systems (HaRTS‐TRENDS) |
| Methods | Parallel, randomized controlled trial Setting: USA Recruitment: from prominent Housing First programmes serving chronically homeless people who are often affected by multiple psychiatric, medical and substance‐use disorders. The proposed sample will be recruited from a highly vulnerable and marginalized population in a tight‐knit urban community. |
| Participants | Estimated enrolment: 94 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcomes, measured across the 12‐month follow‐up:
Secondary outcomes, measured across the 12‐month follow‐up:
Other outcomes:
|
| Starting date | 9 May 2019 |
| Contact information | Tatiana M Ubay, tatiubay@uw.edu |
| Notes |
NCT04003805.
| Study name | Biomarkers of exposure and effect in standardized research e‐cigarette (SREC) users |
| Methods | Design: RCT Setting: USA |
| Participants | Estimated enrolment: 125 Inclusion criteria:
Exclusion criteria:
For a full list see NCT record. |
| Interventions | EC: Standardized Research E‐cigarette (SREC) Arm 1: Experimental: Switching from Smoking Cigarettes to E (SREC) The device operates at a single output voltage (3.30 ± 0.05 V) and uses sealed disposable 3 mL cartridges with tobacco‐flavoured e‐liquid (~350 puffs/cartridge). The concentration of nicotine in e‐liquid is 15 mg/mL, and the vehicle composition is 50:50 propylene glycol and glycerin. The device uses a battery that can be recharged via a micro USB port. Arm 2: Experimental: Switching from smoking cigarettes to nicotine mini‐lozenge We will use commercially available nicotine mini‐lozenges containing 2 or 4 mg nicotine/lozenge (Nicorette, manufactured by GlaxoSmithKline). Dose will be determined per instructions on the package (e.g. if smoking within 30 minutes upon awakening, then 4 mg dose will be prescribed). |
| Outcomes | 1 year 4 & 8 weeks for formaldehyde‐DNA adducts & oxidative DNA adduct 8‐oxo‐dG in DNA Biomarkers: TNE, NNAL, NNN, PneT, mercapturic acids HMPMA, 2‐HPMA, 3‐HPMA, formaldehyde‐DNA adducts, oxidative DNA adduct 8‐oxo‐dG in DNA, NNN and nornicotine, HPB‐releasing DNA adducts cpd, product use (EC & nicotine lozenges), CC avoidance |
| Starting date | Actual start date: May 11 2022. Estimated study completion date: January 15 2023 |
| Contact information | Hanna Vanderloo, RN, MSN 612.624.4983, hannav@umn.edu |
| Notes | New to 2022 update |
NCT04058717.
| Study name | Low nicotine cigarettes plus electronic cigarettes |
| Methods | RCT: Randomized parallel‐group assignment, 2 X 2 factorial design Setting: USA |
| Participants | Estimated enrolment 240 participants Inclusion criteria:
Exclusion criteria:
For a full list see NCT record. |
| Interventions | EC: type of EC not reported Arm 1 Experimental: NNC cigarettes + high nicotine containing e‐cigarette. Participants are provided with normal nicotine content (NNC) cigarettes (11.6 mg nicotine/cigarette) plus e‐cigarette with high nicotine e‐liquid. Arm 2 Experimental: NNC cigarettes + zero nicotine containing e‐cigarette. Participants are provided with normal nicotine content (NNC) cigarettes (11.6 mg nicotine/cigarette) plus e‐cigarette with zero nicotine e‐liquid. Arm 3 Experimental: VLNC cigarettes + high nicotine containing e‐cigarette. Participants are provided with very low nicotine content (VLNC) cigarettes (0.2 mg nicotine/cigarette) plus e‐cigarette with high nicotine e‐liquid. Arm 4 Experimental: VLNC cigarettes + zero nicotine containing e‐cigarette. Participants are provided with very low nicotine content (VLNC) cigarettes (0.2 mg nicotine/cigarette) plus e‐cigarette with zero nicotine e‐liquid. |
| Outcomes | 4, 8, 12 and 16 weeks Urinary NNAL (4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol) Exhaled carbon monoxide Kessler‐6 score measure of serious psychological distress Penn State Cigarette Dependence Index Penn State Electronic Cigarette Dependence Index Cigarette abstinence. No cigarette use in the past 7 days and exhaled carbon monoxide < 6 ppm |
| Starting date | Actual start date: June 1 2021. Estimated completion date: November 30, 2023 |
| Contact information | Nicolle Krebs, MS 717‐531‐5673, nkrebs@pennstatehealth.psu.edu Jonathan Foulds, PhD 717‐531‐3504, jfoulds@psu.edu |
| Notes | New to 2022 update |
NCT04063267.
| Study name | Electronic cigarettes as a harm reduction strategy in individuals with substance use disorder |
| Methods | Parallel‐group, randomized trial Recruitment/setting: Not specified |
| Participants | Estimated enrolment: 240 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
E‐cigarettes arm:
Nicotine Replacement Therapy arm:
|
| Outcomes | Proportion of participants who achieve 50% reduction in cpd at 3 weeks |
| Starting date | 15 September 2019 |
| Contact information | NYU Langone Health, Scott.Sherman@nyulangone.org |
| Notes |
NCT04084210.
| Study name | Impact of alternative nicotine‐delivery products on combustible cigarette use |
| Methods | RCT Setting: USA |
| Participants | 180
Inclusion criteria:
Exclusion criteria:
|
| Interventions | EC: pod Juul Electronic cigarette The Juul e‐cigarette pods contain 0.7 mL nicotine by volume/5% nicotine by weight. Active nicotine patches, with dosing based on the package insert (> 10 cigs/day = 21 mg patch and < 11 cigs/day = 14 mg patches) Placebo patch containing no nicotine Arm 1: Active comparator: Juul + active patch in wk 1 and placebo patch in wk 2 Participants will be given Juul e‐cigarettes for four weeks; in switch week 1, participants also will use active nicotine patches; in switch week 2, participants will use placebo patches. Arm 2: Active comparator: Juul + placebo patch in wk 1 and active patch in wk 2 Participants will be given Juul e‐cigarettes for four weeks; in switch week 1, participants also will use placebo patches; in switch week 2, participants will use active nicotine patches. Arm 3: Active comparator: VLNC + active patch in wk 1 and placebo patch in wk 2 Participants will be given very low nicotine cigarettes (VLNCs) for four weeks; in switch week 1, participants also will use active nicotine patches; in switch week 2, participants will use placebo patches. Arm 4: Active comparator: VLNC + placebo patch in wk 1 and active patch in wk 2 Participants will be given very low nicotine cigarettes (VLNCs) for four weeks; in switch week 1, participants also will use placebo patches; in switch week 2, participants will use active nicotine patches. Arm 5: No product + active patch in wk 1 and placebo patch in wk 2 Participants will be given no alternative nicotine delivery products but in switch week 1, participants will use active nicotine patches; in switch week 2, participants will use placebo patches. Arm 6: No product + placebo patch in wk 1 and active patch in wk 2 Participants will be given no alternative nicotine delivery products for two weeks but in switch week 1 participants will use placebo patches; in switch week 2, participants will use active nicotine patches. |
| Outcomes | Weeks 1 to 4 Weeks 1 through 4, participants will use a smartphone to record, in the moment, each time they use their own cigarettes or any alternative product. Primary outcome: Number of conventional cigarettes smoked during each switch week Secondary outcome: Number of VLNCs or Juul pods used during each switch week |
| Starting date | Start date: September 9 2020. Study completion date: May 23 2022 |
| Contact information | Megan E Piper, PhD, University of Wisconsin, Madison |
| Notes | New to 2022 update |
NCT04090879.
| Study name | Low nicotine content cigarettes in vulnerable populations: affective disorders |
| Methods | RCT Setting: USA |
| Participants | Estimated enrolment 232 participants Inclusion criteria
Exclusion criteria
|
| Interventions | EC: Not stated 'e‐cigarette...commercially available device' Either normal nicotine content cigarettes (15.8 mg/g) or reduced nicotine content cigarettes (0.4 mg/g) 1) Altering the nicotine content of the tobacco research cigarette E‐cigarettes 1) Altering the availability of e‐cigarettes 2) Altering option to personalize the e‐liquid in the e‐cig condition Use assigned product for 16 weeks Arm 1: RC 1 (Research Cigarettes) only. Either normal nicotine content cigarettes (15.8 mg/g) or reduced nicotine content cigarettes (0.4 mg/g) Arm 2: RC 2 only Arm 3: Research Cigarettes #2 plus e‐cigarettes #1 (participants receive tobacco flavour only). E Arm 4: Research Cigarettes #2 plus e‐cigarettes #2 (participants can choose among varying flavours) |
| Outcomes | 16 weeks Total cpd, cigarette demand assessed by behavioural economics‐based purchase tasks, craving, withdrawal, psychiatric symptoms, breath carbon monoxide (CO), biomarkers of tobacco toxicant exposure, brain function and structure, and airway inflammation (fractional nitric oxide concentration in exhaled breath [FeNO]) |
| Starting date | Study start date: September 18 2019. Estimated completion date: July 2023 |
| Contact information | Shirley Plucinski 9788752361, shirley.plucinski@uvm.edu |
| Notes | New to 2022 update |
NCT04092101.
| Study name | Low nicotine content cigarettes in vulnerable populations: opioid use disorder |
| Methods | RCT Recruitment: Daily smokers who are receiving methadone or buprenorphine treatment will be recruited at University of Vermont and Johns Hopkins University. Setting: University of Vermont and Johns Hopkins University, USA |
| Participants | Estimated enrolment: 310 Inclusion criteria:
|
| Interventions | EC: type not specified, 'normal nicotine content cigarette and ... a reduced nicotine content cigarette' Cigarettes with varying nicotine content E‐Cigarettes Arm 1 Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette. Research Cigarettes #1 Arm 2: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette. Research Cigarettes #2. Arm 3: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette. E‐Cigarettes. 1) Altering the availability of e‐cigarettes; 2) Altering option to personalize the e‐liquid in the e‐cig condition Research Cigarettes #2 plus E‐cigarettes #1 Arm 4: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette E‐Cigarettes. 1) Altering the availability of e‐cigarettes; 2) Altering option to personalize the e‐liquid in the e‐cig condition. Research Cigarettes #2 plus E‐cigarettes #2 |
| Outcomes | 16 weeks Outcome measures include total cpd, cigarette demand assessed by behavioural economics‐based purchase tasks, craving, withdrawal, psychiatric symptoms, breath carbon monoxide (CO), biomarkers of tobacco toxicant exposure, brain function and structure, and airway inflammation (fractional nitric oxide concentration in exhaled breath [FeNO]) |
| Starting date | Start date: September 24 2019. Estimated completion date: July 2023 |
| Contact information | Shirley Plucinski 9788752361, shirley.plucinski@uvm.edu |
| Notes | New to 2022 update |
NCT04092387.
| Study name | Low nicotine content cigarettes in vulnerable populations: women of reproductive age |
| Methods | RCT Setting: Johns Hopkins University and the University of Vermont, USA |
| Participants | Estimated enrolment 246 Inclusion criteria:
|
| Interventions | EC: type not specified, 'normal nicotine content cigarette and ... a reduced nicotine content cigarette' Cigarettes with varying nicotine content E‐Cigarettes Arm 1: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette. Research Cigarettes #1 Arm 2: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette. Research Cigarettes #2 Arm 3: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette E‐Cigarettes. 1) Altering the availability of e‐cigarettes; 2) Altering option to personalize the e‐liquid in the e‐cig condition Research Cigarettes #2 plus E‐cigarettes #1 Arm 4: Cigarettes with varying nicotine content. Altering the nicotine content of the tobacco research cigarette E‐Cigarettes. 1) Altering the availability of e‐cigarettes; 2) Altering option to personalize the e‐liquid in the e‐cig condition. Research Cigarettes #2 plus E‐cigarettes #2 |
| Outcomes | 16 weeks Participants will be asked to use only their assigned study products for 16 weeks. Outcome measures include total cpd, cigarette demand assessed by behavioural economics‐based purchase tasks, craving, withdrawal, psychiatric symptoms, breath carbon monoxide (CO), biomarkers of tobacco toxicant exposure, brain function and structure, and airway inflammation (fractional nitric oxide concentration in exhaled breath [FeNO]) |
| Starting date | Start date: September 18 2019. Estimated study completion date: July 2023. |
| Contact information | Catherine Markesich 8026569981, cmarkesi@uvm.edu |
| Notes | New to 2022 update |
NCT04218708.
| Study name | Electronic cigarettes as a harm reduction strategy among people living with HIV/AIDS |
| Methods | RCT Setting: NYU Langone Health, USA |
| Participants | Estimated enrolment 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions | EC: Pod. NIDA Standardized Research E‐cigarettes (SREC) (15 mg/mL nicotine in tobacco flavour) Arm 1: counselling + nicotine replacement therapies NRT A research assistant (RA) trained in motivational interviewing and qualitative methods will support the PI to deliver counselling sessions and conduct interviews. Briefly, during each visit, with help of the RA, participants will provide exhaled CO and saliva cotinine test, and complete surveys in REDCAP using a tablet, allowing programmed logic checks and skip patterns to minimize burden. The RA will also deliver brief motivational counselling tailored to the participant's readiness to quit and arm in the study (NRT). Participants will also receive their NRT to last them to the following visit based on their baseline smoking. Arm 2: Counseling + Standardized Research E‐cigarettes (SREC) Participants in the SREC arm to practice using the SREC and RA to give them instructions to return with their SREC and used refill tanks on every visit. A research assistant (RA) trained in motivational interviewing and qualitative methods will support the PI to deliver counselling sessions and conduct interviews. Briefly, during each visit, with help of the RA, participants will provide exhaled CO and saliva cotinine test, and complete surveys in REDCAP using a tablet, allowing programmed logic checks and skip patterns to minimize burden. The RA will also deliver brief motivational counselling tailored to the participant's readiness to quit and arm in the study (SREC). Participants will also receive their SREC to last them to the following visit based on their baseline smoking. |
| Outcomes | Week 1, 2, 4, 6, 8, 12 Change in cigarettes per day (cpd). Smoking reduction will be measured by a combination of self‐report, text message data and changes in CO and saliva cotinine between baseline and end of treatment. Assessing differences in nicotine withdrawal symptoms Assessing differences in E‐cigarette dependency Assessing differences in nicotine use Assessing differences in use of substance use Assessing differences in side effects associated with e‐cigarette use |
| Starting date | Study start date: June 17 2021. Estimated study completion date: December 2022 |
| Contact information | Omar El Shahawy, MD 1‐646‐501‐2587, omar.elshahawy@nyulangone.org |
| Notes | New to 2022 update |
NCT04238832.
| Study name | Impact of non‐cigarette tobacco product formulation on reinforcement value and use in current smokers Short title: Salt‐based e‐cigarette |
| Methods | RCT Setting: USA, South Carolina |
| Participants | 30 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Salt‐base nicotine Free‐base nicotine |
| Outcomes | Most preferred product [time frame: Lab visit 2, occurring approximately 1 week after the initial screening/baseline visit] Participants complete a preference assessment in which they choose between the salt liquid, free‐base liquid, or a traditional cigarette in a series of trials. The outcome of this assessment is the product chosen most often by each participant. Cigarettes per day [time frame: Week 2 of study] The average number of cigarettes smoked per day during the 1 week sampling period Biomarkers (i.e. expired CO, cotinine) will corroborate self‐reported indices of use. |
| Starting date | 23 June 2020. Estimated completion: August 2021 |
| Contact information | Tracy Smith, smithtra@musc.edu |
| Notes |
NCT04452175.
| Study name | Official title: Cigarette consumption after switchinG to high or low Nicotine strENght E‐cigaretteS In Smokers with schizophrenia spectrum disorders: a 12‐month randomized, double‐blind multicentre trial Brief title: Cigarette consumption after switchinG to high or low nicotine strENght E‐cigaretteS In Smokers with schizophrenia (GENESIS) |
| Methods | RCT Multicentre: Italy, Russia, Ukraine, UK Collaborators:
|
| Participants | Estimated enrolment: 260 Inclusion criteria:
Exclusion criteria:
|
| Interventions |
|
| Outcomes | Primary outcomes:
Secondary outcomes
|
| Starting date | Actual start date Oct 30 2021. Estimated study completion: February 2023 (NCT record update posted on May 23 2022) |
| Contact information | Pasquale Caponnetto, p.caponnetto@unict.it |
| Notes |
NCT04465318.
| Study name | Electronic cigarettes as a harm reduction strategy among patients with COPD |
| Methods | RCT Setting: USA |
| Participants | Estimated enrolment 120 Inclusion criteria:
Exclusion criteria:
|
| Interventions | EC: NJOY daily e‐cigarettes Arm 1: EC + Counseling E‐cigarette (EC). NJOY daily e‐cigarettes are self‐contained and non‐refillable. Each DAILY provides approximately 300 puffs, comparable to a full pack of cigarettes. Behavioural: Smoking harm reduction counselling sessions Counseling will cover health education, social support issues, and motivational enhancement to improve self‐efficacy while addressing other aspects know to contribute to smoking among people with COPD (e.g. tips on dealing with depression) Arm 2: Nicotine Replacement Therapy (NRT) Participants in the NRT arm will receive 21 mg nicotine patch (for those with cpd >= 20) or 14 mg nicotine patch (for those with cpd < 20) + 4 mg nicotine gum. cpd stands for cigarettes per day. Behavioural: Smoking harm reduction counselling sessions as for Arm 1 |
| Outcomes | 12 weeks Primary outcome measures: Number of participants who achieve 50% reduction in cigarettes per day (cpd) Average change in score on the mMRC Dyspnoea Scale Secondary outcome measures: Number of participants who reported satisfaction with use of ECs Number of participants who reported additional use of tobacco products and/or marijuana . Change in score of COPD Assessment Test (CAT) Change in score of Clinical COPD Questionnaire (CCQ) |
| Starting date | Start date: November 9 2020. Estimated study completion date: March 31 2023 |
| Contact information | Scott E. Sherman, MD, MPH NYU Langone Health Elizabeth Stevens, PhD, MPH NYU Langone Health |
| Notes | New to 2022 update |
NCT04521647.
| Study name | Effects of menthol in e‐cigarettes on smoking behaviors |
| Methods | Randomized cross‐over Setting: Connecticut Mental Health Center, USA |
| Participants | Estimated enrolment 85 Inclusion criteria: ≥ 21 years, use combustible cigarettes Exclusion Criteria: None |
| Interventions | EC: type not stated Arm 1: menthol flavour. Participants will receive 5% nicotine in an EC. Participants will receive 2 nicotine concentrations via EC. Each exposure will be 10 3‐sec puffs and ad libitum use. Arm 2: tobacco flavour. Participants will receive 5% nicotine in an EC. Participants will receive 2 nicotine concentrations via EC. Each exposure will be 10 3‐sec puffs and ad libitum use. |
| Outcomes | Baseline and 2, 5, 15, 30, 45, 60, 90, 120, and 180 minutes after nicotine exposure (plasma nicotine levels), 2 weeks (CO), 3 weeks, 5 weeks (BP and heart rate) Primary outcomes: Cigarette craving; plasma nicotine levels; carbon monoxide Secondary outcomes: EC craving; irritation/harshness; liking of EC; coolness; nicotine withdrawal; stimulation; EC use; cigarette use. Other outcomes measured: heart rate, blood pressure, pulse oximetry |
| Starting date | Study start date: November 1 2020. Estimated completion date: July 2025 |
| Contact information | Asti Jackson, PhD 4752414904, asti.jackson@yale.edu |
| Notes | New to 2022 update |
NCT04649645.
| Study name | International randomized controlled trial evaluating changes in oral health in smokers after switching to combustion‐free nicotine delivery systems (SMILE) |
| Methods | RCT Setting: multicentre: Italy, Moldova, Poland, UK and Indonesia |
| Participants | Estimated enrolment 606 participants Inclusion criteria:
For Arms A and B, participants have to be:
For Arm C, participants have to be:
Exclusion criteria:
|
| Interventions | Standard arm (Arm A): own tobacco cigarette brand Intervention arm (Arm B): combustion‐free nicotine delivery system (C‐F NDS) Control arm (Arm C): no smoking or use of any nicotine/tobacco products |
| Outcomes | Oral health parameters and teeth appearance, comparing short‐ and long‐term impact on periodontal health between smokers continuing with conventional cigarette smoking, those switching to combustion‐free nicotine delivery systems (C‐F NDS), and never‐smokers over 18 months |
| Starting date | Not yet recruiting (last updated February 2021) Estimated study start date: Feb 2021. Primary completion date: Feb 2023. Completion April 2023 |
| Contact information | Principal investigator: Antonio Pacino, DDS, Addendo srl, Catania, Italy, info@addendo.net |
| Notes |
NCT04708106.
| Study name | Characterization of product use in smokers switching from cigarettes to a RELX electronic nicotine delivery system Setting: USA Study start date: 15 October 2020. Estimated completion date: April 2021 |
| Methods | Design: RCT, multicentre, open‐label, parallel‐cohort study |
| Participants | Estimated 200 Inclusion criteria:
Exclusion criteria:
|
| Interventions | RELX ENDS tobacco flavour ad libitum use of the RELX ENDS tobacco flavour product RELX ENDS menthol flavour ad libitum use of the RELX ENDS menthol product Ad libitum use of the RELX ENDS tobacco and menthol flavour products |
| Outcomes | Primary outcomes: Weekly RELX ENDS product use; time frame: 56 days. Self‐reported number of RELX ENDS pods started each week Daily number of cigarettes smoked; time frame: 56 days. Self‐reported number of cigarettes smoked daily by study week Number of puffs from the RELX ENDS each day; time frame: 56 days. Self‐reported number of puffs from the RELX ENDS daily by study week (0, < 100, ≥ 100 per day) Secondary outcomes: Biomarkers of exposure measured in blood; time frame: baseline, day 28, and day 56; change in carbon monoxide concentration in the blood Biomarkers of tobacco exposure measured in urine; time frame: baseline, day 28, and day 56; change in creatinine‐adjusted NNAL, NNN, 3‐HPMA, CEMA, HMPMA, S‐PMA, HEMA, 1‐OHP, o‐toluidine, nicotine equivalents, and propylene glycol excreted in urine Subjective effects as measured by the Penn State [Electronic] Cigarette Dependence Index (PS[E]CDI); time frame: baseline, day 14, day 28, day 42, and day 56. Change in product dependence as measured by the PSCDI/PS[E]CDI total score. Total scores may range for 0 to 20, with higher levels of dependence associated with higher scores. Subjective effects as measured by the Cough Questionnaire; time frame: baseline, day 14, day 28, day 42, and day 56. Change in self‐reported cough symptoms as measured by responses to the Cough Questionnaire Subjective effects as measured by the Questionnaire of Smoking Urges‐Brief (QSU‐Brief); time frame: baseline, day 14, day 28, day 42, and day 56. Change in smoking urge as measured by the QSU‐Brief factor 1 and factor 2 scores. Questionnaire responses are measured on a Likert scale range of 1 [not at all] to 7 [extremely]. Subjective effects as measured by the Minnesota Tobacco Withdrawal Scale‐Revised (MTWS‐R); time frame: baseline, day 14, day 28, day 42, and day 56. Change in withdrawal symptoms as measured by the MTWS‐R total score, which includes the DSM‐5 and craving items from the Minnesota Tobacco Withdrawal Scale. Questionnaire responses are measured on a Likert scale range of 0 [none] to 4 [severe]). Subjective effects as measured by the Modified Product Evaluation Scale (mPES); time frame: baseline, day 14, day 28, day 42, and day 56. Change in product assessments as measured by mPES satisfaction, psychological reward, aversion, and relief subscale scores. Questionnaire responses are measured on a Likert scale range of 1 [not at all] to 7 [extremely]. Subjective effects as measured by the Future Intent to Use Questionnaire; time frame: baseline, day 14, day 28, day 42, and day 56. Change in future intent to use cigarettes and ENDS products as measured by responses to the Future Intent to Use Questionnaire Questionnaire responses are measured on a Likert scale range of 1 [extremely unlikely] to 7 [extremely likely] Subjective Effects as measured by the Health Effects Perceptions Questionnaire; time frame: baseline and day 56. Harmful and addictiveness perceptions as measured by responses to the Health Effects Perceptions Questionnaire Puff topography ‐ number of puffs; time frame: baseline, day 28, and day 56. Change in the number of puffs during a 1‐hour puff topography session Puff topography ‐ puff duration; time frame: baseline, day 28, and day 56. Change in puff duration during a 1‐hour puff topography session Puff topography ‐ puff volume. time frame: baseline, day 28, and day 56. Change in puff volume during a 1‐hour puff topography session Puff topography ‐ peak puff flow rate; time frame: baseline, day 28, and day 56; change in peak puff flow rate during a 1‐hour puff topography session Puff topography ‐ average flow rate; time frame: baseline, day 28, and day 56. Change in average flow rate during a 1‐hour puff topography session Puff topography ‐ inter‐puff interval; time frame: baseline, day 28, and day 56. Change in inter‐puff interval during a 1‐hour puff topography session RELX ENDS product use; time frame: day 28 and day 56; change in pod weight during a 1‐hour topography session Incidence of product‐use emergent adverse events [safety and tolerability]; time frame: 56 days Incidence of product‐use emergent adverse events |
| Starting date | |
| Contact information | |
| Notes |
NCT04709471.
| Study name | E‐cigarette nicotine study |
| Methods | Design: RCT. Parallel‐group assignment Setting: USA Start date: 20 January 2021. Estimated completion date: September 2021 |
| Participants | Estimated: 75 Eligibility criteria include at least 21 years old, use e‐cigarettes and tobacco cigarettes regularly, not planning to quit in the near future, and not pregnant, breastfeeding or planning to become pregnant or breastfeed in the next 2 months Additional criteria will be evaluated to assess for eligibility. |
| Interventions | Experimental: Switch to low nicotine e‐cigarettes: switch to e‐cigarettes containing 60% of baseline e‐cigarette nicotine content. Device: Juul e‐cigarette. Participants will switch to Juul pods containing less nicotine. Experimental: Reduce number of e‐cigarette pods: reduce e‐cigarette use to 60% of baseline number of pods per week Behavioural: Reduction: participants will reduce the number of Juul pods that they use No Intervention: Use e‐cigarettes as usual: continue using nicotine e‐cigarettes as usual |
| Outcomes | Primary outcome measure: Feasibility; time frame: Baseline and the 4‐week reduction period. The investigators will assess compliance with study e‐cigarettes and compare the percentage of non‐study e‐cigarette use between conditions to determine which behaviour‐changing strategy is more feasible. Combustible cigarette smoking; time frame: Baseline and the 4‐week reduction period. The investigators will compare change in number of cigarettes per day between conditions. Cigarette dependence; time frame: Baseline and the 4‐week reduction period. The investigators will compare change in cigarette dependence between conditions using the PATH dependence measure. E‐cigarette dependence: time frame: Baseline and the 4‐week reduction period. The investigators will compare change in e‐cigarette dependence between conditions using the PATH dependence measure. Secondary outcome measure: Cigarette demand; time frame: Baseline and the 4‐week reduction period. The investigators will compare change in cigarette demand using the Brief Assessment of Cigarette Demand task. E‐cigarette demand; time frame: Baseline and the 4‐week reduction period. The investigators will compare change in e‐cigarette demand using a version of the Brief Assessment of Cigarette Demand task adapted for e‐cigarettes. |
| Starting date | 20 January 2021. Estimated study completion date: September 2021 |
| Contact information | Elias M Klemperer, PhD802‐656‐1641, elias.klemperer@med.uvm.edu |
| Notes |
NCT04725656.
| Study name | Concentration Impact Nicotine Salt (CINS) |
| Methods | Design: RCT |
| Participants | Estimated enrolment: 312 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Active comparator: Active arm, low concentration (18 mg/mL) nicotine salt e‐liquids. Procedure: Smoking cessation counselling: smoking cessation counselling at baseline, week 1, week 2 and week 4
Other: Open system vape device and nicotine salt e‐liquids; ad libitum use of nicotine salt e‐liquids during 3 months Active comparator: Active arm, high concentration (59 mg/mL) nicotine salt e‐liquids. Procedure: Smoking cessation counselling: smoking cessation counselling at baseline, week 1, week 2 and week 4 Other: Open system vape device and nicotine salt e‐liquids; ad libitum use of nicotine salt e‐liquids during 3 months Control group: Receive only smoking cessation counselling. Procedure: Smoking cessation counselling: smoking cessation counselling at baseline, week 1, week 2 and week 4 |
| Outcomes | Primary outcome: 7‐day point prevalence tobacco abstinence (in terms of non‐inferiority); time frame: 1 month Defined as no smoking, i.e. "not a puff", self‐reported and confirmed by exhaled carbon monoxide (< 10 ppm) and urinary anabasine levels (< 3 ng/mL) when using low vs. high nicotine salt concentration e‐liquids Volume of e‐liquid used (in terms of superiority); time frame: 1 month; volume of e‐liquid used when using low vs. high nicotine salt concentration e‐liquids Secondary outcome: 7‐day point prevalence tobacco abstinence (in terms of non‐inferiority); time frame: 1 month Defined as no smoking, i.e. "not a puff", self‐reported and confirmed by exhaled carbon monoxide (< 10 ppm) and urinary anabasine levels (< 3 ng/mL) when using low vs. high nicotine salt concentration e‐liquids Volume of e‐liquid used (in terms of superiority); time frame: 1 month; volume of e‐liquid used when using low vs. high nicotine salt concentration e‐liquids Liking/rating of trial product (active arms); time frame: 1 and 3 months. Questions regarding helpfulness in refraining from smoking, how satisfying and how good the e‐cigarette tastes compared to the tobacco cigarettes, if they would recommend the assigned trial product to another smoker, and any potential practical problems they might have with the handling Respiratory symptoms; time frame: up to 12 months; checklist with specific questions regarding shortness of breath, wheezing, cough or phlegm Adverse events; time frame: up to 12 months; checklist with specific questions regarding presence or absence of nausea, sleep disturbance, throat/mouth irritation or other Total nicotine amount vaped; time frame: 1 and 3 months Total volume of e‐liquid consumed; time frame: 1 and 3 months |
| Starting date | Estimated start date: 01 September 2021. Estimated primary completion date: 30 December 2022. Estimated study completion date: 30 June 2023 |
| Contact information | Evangelia Liakoni, MD0041316325461, evangelia.liakoni@insel.ch |
| Notes |
NCT04854616.
| Study name | Cessation of Smoking Trial in the Emergency Department (CoSTED) |
| Methods | RCT |
| Participants | People attending the Emergency Department who smoke |
| Interventions | Behavioural: CoSTED intervention: Brief smoking cessation advice, the provision of an e‐cigarette starter kit and training in its use, and referral to stop smoking services No Intervention: Treatment‐as‐usual. Signposting to NHS smoking cessation services through provision of written information about local services |
| Outcomes | Baseline, 1, 3 & 6 mths Continuous smoking abstinence, 6 months after randomization, CO confirmed Smoking status 1, 3 & 6 months after randomization. Abstinence prevalence, CO validated (≥ 8 ppm) Number of cpd; number of times using an EC per day; self‐reported dry cough; mouth or throat irritation; use of GP services; use of smoking cessation services. Quality of Life questionnaire. Adverse events (6 months) |
| Starting date | 4 January 2022 |
| Contact information | Ian Pope MD, i.pope@uea.ac.uk Caitlin Notley PhD, c.notley@uea.ac.uk |
| Notes | New to 2022 update |
NCT04946825.
| Study name | Quit smoking study for people who use e‐cigarettes. A randomized controlled trial of smoking cessation treatment for young adult dual users of combustible and electronic cigarettes |
| Methods | RCT. Randomized factorial assignment Setting: Community. University of Vermont, USA |
| Participants | Estimated enrolment 390 Inclusion criteria: young adult; smokes tobacco cigarettes; uses EC; interested in quitting tobacco Exclusion criteria: pregnancy or breastfeeding; ≥ 1 contraindications for NRT |
| Interventions | EC: type not stated NRT: patch and lozenge A) NRT plus text messages to quit CCs only, B) NRT plus text messages to quit CCs and ECs simultaneously, C) text messages alone to quit CCs only, or D) text messages alone to quit CCs and ECs simultaneously. |
| Outcomes | Baseline, 3 mths, 6 mths CO confirmed 7‐day point‐prevalence abstinence at the end of treatment (i.e. 3 months after randomization) CO biochemically confirmed prolonged 30‐day abstinence, 3‐month follow‐up (i.e. end of treatment) and 6‐month follow‐up (3 months after the end of treatment) Self‐reported abstinence, 7 days, 30 days Attempts to quit combustible cigarettes (CC), cpd, CC dependence |
| Starting date | Study start date: June 27 2021. Estimated study completion: August 2023 |
| Contact information | Elias Klemperer, PhD 8026561641, elias.klemperer@med.uvm.edu Shaun Meyers, BA 8026568681, shaun.meyers@uvm.edu |
| Notes | New to 2022 update |
NCT05023096.
| Study name | Potential effects of electronic nicotine delivery system flavor regulations on African American menthol smokers (RVA Flavors) |
| Methods | RCT Virginia Commonwealth University, USA |
| Participants | Estimated enrolment: 210 Inclusion criteria: 21+ years; identify as Black/African‐American (single or multi‐race); used ≥ 5 cigarettes per day for ≥ 1 year (biochemically confirmed); regular cigarette brand menthol or mint flavoured; EC use in the past 30 days; no intent to quit smoking in the next 6 months; previous quit attempt using evidence‐based method; mobile phone, willing to receive calls/text Exclusion criteria: unwilling to use EC; report other tobacco use > 10 days in past 30 other than combustible cigarettes; unstable or significant medical condition in the past 12 months; > 15 days of marijuana or other illegal drug use in the past 30 days; pregnancy/breastfeeding |
| Interventions | EC: type not stated Arm 1: Menthol + tobacco. Both menthol and tobacco flavoured liquids for EC are available to choose from. Arm 2: Tobacco ‐ only tobacco flavoured liquid is available for EC. Arm 3: Unflavoured ‐ only unflavoured liquid is available for EC. Participants are instructed to smoke their usual brand of menthol cigarettes normally for 7 days and avoid using any other tobacco products. After this baseline week, participants are randomized to 1 of 3 EC flavour conditions, all contain 5% nicotine (menthol + tobacco, tobacco, unflavoured) with equal probability and provided with a supply of their condition‐specific EC and asked to use it in place of their usual menthol cigarettes for the next 6 weeks. |
| Outcomes | Week 1, week 6 Change in: average daily cigarette use; carbon monoxide exposure; urinary NNAL; urinary proplyne glycol exposure; average daily ENDS use Willingness to substitute from cigarettes to EC (ENDS); measure of substitution for condition‐specific tobacco products will be assessed using drug purchase tasks. Choices made during this task are not reinforced. Willingness to pay for ENDS (week 6); willingness to pay for condition‐specific tobacco products will be assessed using drug purchase tasks. Choices made during this task are not reinforced. |
| Starting date | Study start date: April 14 2022. Estimated primary completion date: June 2024 |
| Contact information | Andrew J. Barnes, PhD 804‐827‐4361, abarnes3@vcu.edu Caroline O. Cobb, PhD, cobbco@vcu.edu |
| Notes | New to 2022 update |
NCT05144542.
| Study name | Risk and benefits of electronic cigarettes to older smokers at high risk for lung cancer |
| Methods | RCT Setting: M. D. Anderson Cancer Center, Texas, USA |
| Participants | Estimated enrolment: 330 Inclusion criteria: Meeting National Comprehensive Cancer Network (NCCN) guideline for lung cancer screening; daily or non‐daily smoker; interested in trying ECs to change CC smoking behaviour; willing and able to complete two spirometry sessions Exclusion criteria: Used ECs on more than 2 days in the past 30 days; meet criteria for current major depressive disorder (MDD) or suicidality; report more than once weekly of tobacco products other than CCs during the past 30 days; ever diagnosis of lung cancer, have uncontrolled or unstable medical condition; spirometry forced expiratory volume in 1 second (FEV1) percentage reading < 50; pregnancy/breastfeeding |
| Interventions | EC: type not specified GROUP A: Participants smoke their usual brand of cigarettes for 26 weeks. Participants use smartphone to answer questions about nicotine cravings and mood, and log daily smoking activity every day for up to 182 days. Participants complete questionnaires over 50 minutes and undergo collection of urine sample at 1, 6, 12, and 26 weeks, and collection of blood samples at 6, 12, and 26 weeks. Participants may also undergo measurement of CO levels at 1, 6, 12, and 26 weeks. GROUP B: Participants vape EC for 26 weeks. Participants use smartphone to answer questions about nicotine cravings and mood, and log daily smoking activity every day for up to 182 days. Participants complete questionnaires over 50 mins and undergo collection of urine sample at 1, 6, 12, and 26 weeks, and collection of blood samples at 6, 12, and 26 weeks. Participants may also undergo measurement of CO levels at 1, 6, 12, and 26 weeks. |
| Outcomes | 1, 6, 12, and 26 weeks, and collection of blood samples at 6, 12, and 26 weeks Primary outcome measure: Cigarettes per day, diary data of combustible cigarette use over last 24 hours Secondary outcome measures: High‐sensitivity C‐reactive protein (hs‐CRP); white blood cells (WBC); 8‐epi prostaglandin F2 alpha (8‐epi‐PGF2a). All from blood draws at weeks 0, 6, 12, and 26 |
| Starting date | Start date: March 7 2022. Estimated completion date: April 30 2025 |
| Contact information | Jason Robinson, PHD 713‐792‐0919, jdrobinson@mdanderson.org |
| Notes | New to 2022 update |
NCT05199480.
| Study name | Understanding the impact of cartridge‐based electronic cigarettes and generated aerosols on cardiopulmonary health |
| Methods | RCT Virginia Commonwealth University, USA |
| Participants | Estimated enrolment: 57 Inclusion criteria: E‐cigarette group: ≥ 21 yrs; used EC (≥ 3 times/week for ≥ 3 months) Non‐e‐cigarette group: ≥ 21 yrs Exclusion criteria: Use of cigarettes for 15 days or more in the past 60 days; use of other tobacco products (cigars, hookah, smokeless) weekly or more frequently in the past 60 days; use of marijuana or any illicit or prescription drugs for non‐medical use weekly or more frequently in the past 60 days; allergy to propylene glycol or vegetable glycerin; evidence of cardiovascular, pulmonary, renal, hepatic, metabolic, or cerebral diseases; disorder or use of medication that affects cardiopulmonary health; pregnancy/breastfeeding |
| Interventions | EC: commercially available cartridge‐based EC device Arm 1: E‐cigarette liquid type 1 A commercially available cartridge‐based device with tobacco flavoured liquid. Participants will be instructed to use at least one study product daily in place of their own e‐cigarettes during the intervention period. Arm 2: E‐cigarettes liquid type 2 A commercially available cartridge‐based device with tobacco flavoured liquid. Participants will be instructed to use at least one study product daily in place of their own e‐cigarettes during the intervention period. Arm 3: No e‐cigarettes. No e‐cigarette use |
| Outcomes | Baseline, 2 weeks Change in peak oxygen consumption (VO2 peak) Change in expiratory volume Change in skeletal muscle O2 utilization Change in maximal microvascular dilation |
| Starting date | Study start date: January 10 2022. Estimated completion date: May 2023 |
| Contact information | Paula Rodriguez Miguelez, PhD804‐396‐4498, prodriguezmig@vcu.edu |
| Notes | New to 2022 update |
NCT05205811.
| Study name | A randomized controlled trial to determine the effects of combination zonisamide and bupropion on switching to an electronic cigarette |
| Methods | RCT Rose Research Center, USA |
| Participants | Estimated enrolment: 180 Inclusion criteria: 21 to 65 yrs; ≥ 10 commercially available cigarettes per day, for the last 12 mths (CO reading ≥ 10 ppm); interested in switching to an EC; smartphone with text message and data capabilities Exclusion criteria: unhealthy or cannot participate in the study for any reason; PHQ‐9 score greater than 9, or a score greater than 0 on item #9; plans to use an FDA‐approved smoking cessation product; high blood pressure, coronary heart disease, structural cardiac disease; BMI ≤ 15.0 kg/m2 or > 40.0 kg/m2; depression, anxiety, or nicotine withdrawal within 30 days of screening, or during the study, taking antidepressants, psychoactive medications or medications that prolong QTc For full list see NCT record |
| Interventions | EC: JUUL Zonisamide Bupropion Arm 1: Combination zonisamide and bupropion with EC After the first week of EC use (JUUL), participants will be given bupropion (150 mg each morning for days 1‐3, then 300 mg daily) with zonisamide (100 mg daily). The combination of zonisamide and bupropion use will continue for 7 weeks of treatment, and EC use will continue until the end of the study (an additional 4 weeks). EC for ad libitum use for 2 weeks prior to complete switch day and for an additional 10 weeks Arm 2: Bupropion with EC After the first week of EC use (JUUL), participants will be given bupropion (150 mg each morning for days 1‐3, then 300 mg daily) with placebo zonisamide. The combination of placebo and bupropion use will continue for 7 weeks of treatment, and e‐cigarette use will continue until the end of the study (an additional 4 weeks). EC for ad libitum use for 2 weeks prior to complete switch day and for an additional 10 weeks Arm 3: Placebo with EC After the first week of EC use (JUUL), participants will be given placebo bupropion with placebo zonisamide. The combination of these placebos will continue for 7 weeks of treatment, and EC use will continue until the end of the study (an additional 4 weeks). EC for ad libitum use for 2 weeks prior to complete switch day and for an additional 10 weeks |
| Outcomes | Baseline, week 8, week 12, 6 mths Complete switching from combustible cigarettes to JUUL EC as measured by: exhaled carbon monoxide (CO); change in total urinary 4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol (NNAL); change in self‐report of daily cigarette and EC use Seven‐day point abstinence at 6 months post‐switch, assessed by self‐report and confirmed by exhaled CO < 5 ppm. Change in smoking withdrawal symptoms. Change in rewarding and aversive effects of smoking and EC use AEs; SAEs |
| Starting date | Start date: December 14 2021. Estimated completion date: August 31 2023 |
| Contact information | Derek Mercedes 704‐350‐2999, derek.mercedes@roseresearchcenter.com |
| Notes | New to 2022 update |
NCT05206435.
| Study name | Methadone‐maintained smokers switching to e‐cigarettes (SHINE) |
| Methods | RCT Butler Hospital, Providence, Rhode Island, United States, 02906 |
| Participants | Estimated enrolment: 240 Inclusion criteria:
Exclusion criteria:
|
| Interventions | EC: type not stated Arm 1: Electronic cigarettes. Participants in this arm are randomized to receive electronic cigarettes for the 6‐week study period. Electronic cigarettes are provided to replace tobacco cigarettes. Arm 2: Nicotine lozenges. Participants in this arm are randomized to receive nicotine lozenges for the 6‐week study period. Nicotine lozenges are provided to replace tobacco cigarettes. |
| Outcomes | Baseline, 6 weeks Nicotine exposure (urine) Lung functioning: FVC (changes in Forced Vital Capacity, spirometry); FEV1 (changes in Forced Expiratory Volume (FEV ‐ during the first second), spirometry). FEV1/FVC Smoking behaviour and experiences (self‐report) For complete switchers: Nicotine exposure; lung functioning (FVC, FEV1, FEV1/FVC); smoking behaviour and experiences |
| Starting date | Study start date: March 31 2022. Estimated study completion date: June 30 2024. |
| Contact information | Michael Stein, MD 401‐455‐6200, michael_stein@brown.edu Ana Abrantes, PhD 401‐455‐6200, ana_abrantes@brown.edu |
| Notes | New to 2022 update |
NCT05257629.
| Study name | Aggressive smoking cessation therapy post‐acute coronary syndrome (ASAP) trial |
| Methods | RCT Setting Hospital . Jewish General Hospital, USA |
| Participants | Estimated enrolment: 798 Inclusion criteria: Currently hospitalized (or at time of discharge) for ACS. Defined as follows: MI, defined by positive troponin T, troponin I, or CK‐MB levels (as defined by institution‐specific cut‐offs). For definition see NCT record. CC user; motivated to quit smoking according to the Motivation To Stop Scale (MTSS) (≥ level 5); ≥ 18 years Exclusion criteria: Use of any of the following in the 30 days prior to ACS admission: i. Pharmacotherapy (e.g. NRTs, bupropion, or varenicline) for smoking cessation; ii. Nicotine or non‐nicotine e‐cigarettes; iii. Psychotropic medications (e.g. mood stabilizers, antipsychotics, prescribed opiates and sedatives); iv. Other anti‐craving medication (e.g. naltrexone, acamprosate) with the potential to alter substance‐seeking behaviours Pregnancy/breastfeeding For a full list see NCT record. |
| Interventions | EC: participants choice Arm 1: Combination therapy arm (varenicline and nicotine EC plus counselling) Patients in the combination therapy arm will be supplied funds and instructions for the purchase of EC and cartridges/pods upon hospital discharge and at the week 4 and 12 clinic visits. As with standard NRTs such as the gum, inhaler, and lozenge, we expect smokers will self‐regulate administration according to their withdrawal symptoms. Use will be monitored via self‐report for telephone follow‐ups. At clinic visits, patients will be asked to bring their EC, used and unused cartridges/pods, and purchasing receipts. Patients will be advised regarding the signs and symptoms of nicotine toxicity and of an allergic reaction. Arm 2: Varenicline plus counselling All patients will begin varenicline in‐hospital upon randomization. For the first 3 days, patients will take a 0.5 mg tablet once a day. They will then take a 0.5 mg tablet twice a day for the following 4 days, and one 1 mg tablet twice a day from day 8 onward for the remainder of the 12‐week treatment. Use will be monitored via self‐report for telephone follow‐ups and return of all unused tablets at the end of the treatment period. Should a patient experience severe side effects (such as headache, nausea, vomiting, dizziness, dyspepsia, fatigue, insomnia, abnormal dreams, constipation, or flatulence) on day 8 onward, the varenicline dose should be reduced from 1 mg twice daily to 0.5 mg twice daily prior to study medication discontinuation. |
| Outcomes | 1, 2, 8, 18, 24 weeks week 4, week 12, and week 52 Number of participants with: 7‐day point prevalence smoking abstinence (biochemically‐validated); continuous smoking abstinence; prolonged smoking abstinence; change in daily cigarette consumption; ≥ 50% reduction in daily cigarette consumption; point prevalent abstinence or ≥ 50% reduction in daily cigarette consumption at 24 weeks Frequency of Adverse Events (AEs) or SAEs Spirometry measurements (subset) at all other clinic visits (FVC, FEV1, and FEV1/FVC) O2 cost diagram and COPD Assessment Test (subset) at all other clinic visits Number of patients averaging ≥ 1 pill of varenicline/day |
| Starting date | Estimated start date: June 1 2022. Estimated completion date: March 7 2027 |
| Contact information | Carole Bohbot 514‐340‐8222 ext 22790. ASAP.Trial@ladydavis.ca, carole.bohbot@ladydavis.ca |
| Notes | New to 2022 update |
NCT05278065.
| Study name | Complimentary electronic cigarettes for harm reduction among adult smokers with asthma (SWAP) |
| Methods | RCT Setting & recruitment: Participants will be adults from the local community with persistent asthma symptoms who are regular combustible cigarette smokers and do not also regularly use ENDS. The study will recruit 30 non‐treatment seeking participants using flyers, advertisements, a website triaging visitors to the Center for Alcohol and Addiction Studies, and through targeted recruitment at community immunology clinic partners at Rhode Island Hospital, USA. |
| Participants | Estimated enrolment 30 Inclusion criteria:
Exclusion criteria:
|
| Interventions | EC: 4th generation & disposable cartridges Arm 1: Electronic cigarette Participants in this experimental condition will be provided with a 4th generation EC device and disposable cartridges. Participants will be provided with EC and 5% nicotine e‐liquid cartridges for 8 weeks and encouraged at weekly assessments to use the EC any time they would normally smoke. Participants will be able to choose commercially available e‐liquid flavours (tobacco) at each weekly assessment. Arm 2: Smoking‐as‐usual Participants in this assessment‐only condition will continue smoking‐as‐usual. |
| Outcomes | Baseline, week 8, week 16. Eight weekly visits to complete follow‐up assessments cpd EC use Asthma symptoms Pulmonary functioning, FEV, FVC, FEF25‐75, PEF. CO. Level of exhaled CO assessed with Smokerlyzer NNAL Cotinine Interleukin‐6 (IL‐6) Tumour necrosis factor alpha (TNF‐a) Chemokine ligand 9 (CXCL9) Matrix metallopeptidase 9 (MMP9) |
| Starting date | Start date: May 1 2022. Estimated study completion date: May 2023 |
| Contact information | Alexander W Sokolovsky, PhD 4018636629, alexander_sokolovsky@brown.edu Mary Ellen Fernandez, BA 4018635521, mary_fernandez@brown.edu |
| Notes | New to 2022 update |
NCT05311085.
| Study name | Cytisine and e‐cigarettes with supportive text‐messaging for smoking cessation (Cess@Tion) |
| Methods | RCT Setting: Community University of Auckland, New Zealand |
| Participants | Estimated enrolment: 800 Inclusion criteria: Daily smokers who live in New Zealand; motivated to quit smoking within the next two weeks & willing to use cytisine or an EC or both products; ≥ 18 years Exclusion criteria: another person in their household currently enrolled in the study; pregnancy/breastfeeding; using smoking cessation medication (including EC daily for the last month); hypersensitivity to cytisine or nicotine EC; health condition e.g. renal impairment; tuberculosis; myocardial infarction, stroke, or severe angina, high BP, seizures; strong preference to use or not to use cytisine and/or EC in their quit attempt For a full list see NCT record. |
| Interventions | EC: Pod device. Nicotine strength: 30 mg/mL (3%). Flavour: Tobacco. Brand name: UpOx Cytisine Arm 1: Monotherapy (Cytisine only) 12 weeks of cytisine: Participants allocated cytisine will be instructed to follow the manufacturer's 25‐day dosing regimen, then follow a maintenance dose of cytisine from day 26 to week 12. Participants will also receive 6 mths of text‐based smoking cessation support. Cytisine. Brand name: Tabex. Standard dosing of:
Followed by a maintenance dose of cystine from day 26 to week 12 (one tablet every six hours: two tablets/day) Arm 2: Monotherapy (Nicotine EC only) 12 weeks of a nicotine EC. Participants will also receive six months of text‐based smoking cessation support. Arm 3: Combination therapy (Cytisine plus a nicotine EC) 12 weeks of cytisine (as above) and 12 weeks of a nicotine EC. Participants will also receive six months of text‐based smoking cessation support. |
| Outcomes | Baseline, 3, 6 and 12 months post‐quit date Primary outcome: Proportion of participants with verified continuous smoking abstinence CO confirmed Self‐reported continuous smoking abstinence; self‐reported 7‐day point prevalence smoking abstinence; change from baseline in the number of cigarettes smoked per day; health‐related quality of life; cystine compliance; use of allocated treatment by participants; frequency of EC use, number of pods used; treatment switching; dual use; AEs; number of text‐based behavioural support messages received by participants; marginal cost per quitter |
| Starting date | Study start date: May 6 2022. Estimated primary completion date: February 2024 |
| Contact information | Natalie Walker, PhD 64‐9‐923‐9884, n.walker@auckland.ac.nz Chris Bullen, PhD MBChB 64‐9‐923‐4730, c.bullen@auckland.ac.nz |
| Notes | New to 2022 update |
NCT05327439.
| Study name | Using alternative nicotine delivery systems (ANDS) to reduce harm for low SES cigarette smokers. (Tri‐PEC study) |
| Methods | RCT Center for Alcohol and Addiction Studies, Brown University School of Public Health, USA |
| Participants | Estimated enrolment: 45 participants Inclusion criteria: household income < 250% federal poverty level (FPL); past 6 mths daily smoking of ≥ 5 cigarettes/day (exhaled CO ≥ 6 ppm at baseline); willingness to substitute combustible cigarettes for EC or NPs; aged 21+ years Exclusion criteria: intention to quit smoking during the next 30 days or current or past 30 day engagement in smoking cessation; current use of EC or NP ≥ 4 days per month or self‐report of primarily using tobacco products that are not combustible cigarettes; hospitalization for a psychiatric issue in the past 30 days or visible instability; heart‐related event in the past 30 days; pregnancy Note: Cannabis use will be assessed but not excluded. For full list see NCT record. |
| Interventions | EC: 4th generation electronic cigarette device and disposable cartridges (5% nicotine e‐liquid cartridges) Nicotine pouch, 4 mg Arm 1: Electronic cigarette Participants in this experimental condition will be provided with a 4th generation EC device and disposable cartridges. Participants will be provided with EC and 5% nicotine e‐liquid cartridges for 8 weeks and encouraged at in‐person and phone assessments to use the EC any time they would normally smoke. Participants will be able to choose one of two e‐liquid flavours (tobacco, menthol) at baseline. Arm 2: Nicotine pouch Participants in this experimental condition will be provided with nicotine pouches. Participants will be provided with 4 mg nicotine pouches for 8 weeks and encouraged at in‐person and phone assessments to use the nicotine pouches any time they would normally smoke. Participants will be able to choose one of two nicotine pouch flavours (tobacco, mint) at baseline. Arm 3: No Intervention: Smoking‐as‐usual Participants in this assessment‐only condition will continue smoking‐as‐usual. |
| Outcomes | Baseline, 8 weeks Change in cigarettes per day from baseline to week 8. Within and between‐group difference in past week average cigarettes per day assessed using timeline follow‐back (TLFB) Change in cigarette dependence from baseline to week 8 Cigarette abstinence at week 8. Past week any‐use of cigarettes assessed using timeline follow‐back (TLFB) Change in carbon monoxide; cotinine; NNAL; 8‐isoprostane from baseline to week 8 Feasibility and acceptability |
| Starting date | Start date: March 23 2022. Estimated completion date: December 30 2022 |
| Contact information | Jasjit S Ahluwalia, MD 401‐863‐6654, jasjit_ahluwalia@brown.edu, Brown University, USA |
| Notes | New to 2022 update |
8‐iso‐PGF2a: an isoprostane 1‐OHP: 1‐hydroxypyrene ACS: acute coronary syndrome AE: adverse event AHR: airway hyperresponsiveness AUD: alcohol use disorder AUDIT: AUDIT‐C checklist terminology for alcohol dependence BMI: body mass index BP: blood pressure CAL: clinical attachment loss CAR: continuous abstinence rate CAT: Computerized Adaptive Testing OR Computer‐Aided Tomography CC: combustible cigarette CCQ: Clinical COPD Questionnaire CEMA: 2‐cyanoethylmercapturic acid C‐F NDS: combustion‐free nicotine delivery systems CK‐MB: Creatine kinase, heart specific isoenzyme CMHT: Community Mental Health Team CO: carbon monoxide COPD: chronic obstructive pulmonary disease COVID: COVID‐19, disease caused by SARS‐CoV‐2 cpd: cigarettes per day CRF: cardiovascular risk factors CT: Computed tomography CVD: cardiovascular disease CXCL9: CSCL9 (chemokine ligand 9) DESC: DESC refers to a supportive housing project (see NCT03962660) DNA: Deoxyribonucleic Acid DSM‐IV/5: Diagnostic and Statistical Manual of Mental Disorders‐IV EC: electronic cigarette eCO: expired carbon monoxide ECG: electrocardiogram ECwN: electronic cigarette with nicotine ECwoN: electronic cigarette without nicotine ENDS: electronic nicotine delivery system EQ‐5D‐5L: EuroQol 5 Dimension 5 Level FDA: Food and Drug Administration FEF: forced expiratory flow FeNO: fractional exhaled nitric oxide FEV1: forced expiratory volume FPL: federal poverty level FSH: follicle‐stimulating hormone FTND: Fagerström Test for Nicotine Dependence FVC: forced vital capacity GIF: graphics interchange format GP: General Practitioner (Dr) HaRTS‐TRENDS: (trial name) Harm reduction for tobacco smoking with HbA1c: haemoglobin A1C, glycosylated haemoglobin HBsAg: hepatitis B surface antigen HCV: hepatitis C HDL: high‐density lipoprotein HEMA: 2‐hydroxyethylmercapturic acid HIV: human immunodeficiency virus HMPMA: 3‐hydroxy‐1‐methyl propylmercapturic acid HPB: Health Promotion Board HPMA: hydroxypropylmercapturic acid hs‐CRP: high‐sensitivity C‐reactive protein HTP: hydroxytryptophan ICD‐10: International Classification of Diseases, Tenth Edition ICF: International Classification of Functioning IL‐6: Interleukin 6 LDCT: low‐dose computed tomography LHC: lung health check mCEQ: modified Cigarette Evaluation Questionnaire MDD: major depressive disorder MetS: metabolic syndrome MHRA: Medicines and Healthcare products Regulatory Agency MI: myocardial infarction MMP9: matrix metallopeptidase 9 mMRC: modified Medical Research Council mPES: multi‐Parameter Evidence Synthesis MPSS: mood and physical symptoms scale MTSS: Motivation To Stop Scale MTWS‐R: Minnesota Tobacco Withdrawal Scale‐R (15 items) NHS: National Health Service NIDA: National Institute on Drug Abuse NNC: non nicotine cigarette NCCN: National Comprehensive Cancer Network NNAL: carcinogen found in tobacco smoke (4‐(methylnitrosamino)‐1‐(3‐pyridyl)‐1‐butanol) NNN: N'‐nitrosonornicotine NRT: nicotine replacement therapy OUD: opioid use disorder PANSS: Mean Positive and Negative Syndrome Scale PATH: Population Assessment of Tobacco and Health PEF: peak expiratory flow PG: propylene glycol PGEM: a stable metabolite of prostaglandin E2 (biomarker of inflammation) PHQ‐9: Patient Health Questionnaire 9 PI: principal investigator PK: pharmacokinetic PneT: PheT phenanthrene tetraol PP(A): point prevalence (abstinence) PROMPT: Community‐Based Participatory Tobacco Dependence Strategy (PROMPT Project) PS[E]CDI: Penn State Electronic Cigarette Dependence Index (e‐cigarette dependence measure) QN: NHS quit now programme QOL: quality of life q‐PADDA: primer anchored DNA damage detection assay QSU‐Brief: Questionnaire of Smoking Urges QTC: QT interval (time it takes for the electrical system to fire an impulse through the ventricles and then recharge) RA: research assistant RC: research cigarettes RCT: randomised controlled trial REDCAP: Research Electronic Data Capture (web application for surveys) SABA: short‐acting β2‐agonists SAE: serious adverse event SC: e‐salivary cotinine SCP: smoking cessation program SES: socioeconomic status S‐PMA: S‐phenylmercapturic acid SREC: standardized research e‐cigarette SRMH: self‐rated mental health SSS: stop smoking services T2DM: type 2 diabetes TC: tobacco cigarette THP: tobacco heating products TLFB: timeline followback TMS: transcranial magnetic stimulation TNE: total nicotine equivalents TNF‐a: tumour necrosis factor alpha TQD: target quit date UC: usual care USB: universal serial bus V: volts VBA: very brief advice VLNC: very low nicotine content VNP: vaporized nicotine products VO2: oxygen consumption WBC: white blood cell wk: week YLST: Yorkshire Lung Screening Trial yr: year
Differences between protocol and review
The protocol did not specify a minimum follow‐up period for data on adverse events. As of the 2016 update, we have changed the Methods section to clarify that we will exclude follow‐up data at less than a week.
The original version of this review included reduction as a secondary outcome. The 2016 update removed reduction as an outcome, to bring the review into line with other reviews of cessation treatments produced by the Cochrane Tobacco Addiction Group and to prevent substantial overlap with the update of the Group's review of interventions for harm reduction.
As prespecified in the 2016 update, in the 2020 update, we excluded non‐intervention studies. In the 2020 update, we also added in an appendix with a protocol setting out our plans to convert this review into a living systematic review in the future.
As specified in an amendment in June 2021, we now include a new secondary outcome: number of people still using study product (EC or pharmacotherapy) at longest follow‐up (6+ months).
For the next update of this review, we will change over from fixed to random effects meta‐analyses in accordance with evolving guidance in this space.
Contributions of authors
All authors contributed to the writing of this review. For this update, JHB, NL, RB, AT and ARB screened studies or extracted data, or both. JHB and ARB entered data for analysis.
Sources of support
Internal sources
-
Queen Mary University of London, UK
provides salary, office space and library resources for HM and PH
-
The University of Auckland, New Zealand
provides salary, office space and library resources for CB
-
University of Oxford, UK
Support from Returning Carers' Fund
-
University of Oxford, UK
Public Policy Challenge Fund
External sources
-
NIHR, UK
Infrastructure award for Cochrane Tobacco Addiction Group and Cochrane Incentive Award
-
Cancer Research UK, UK
Cancer Research UK project award funding to support living systematic review
Declarations of interest
RB holds an NIHR grant, but this did not directly fund this current work. She is principal investigator of an ongoing study listed in this review.
CB was principal investigator on the ASCEND e‐cigarette trial reported in the Cochrane Review and a co‐investigator on the ASCEND II trial and several other studies included in the review. CB has provided consultancy for J&J KK (Japan) on NRT products. CB reports research grants from the Health Research Council of NZ, the Heart Foundation of NZ and the NZ Ministry of Health. He has recently led a project funded by Pfizer (NZ) on chronic disease management.
ARB's work on this review has been supported by Cancer Research UK Project Award funding. This is not deemed a conflict of interest.
TF has no known conflicts of interest.
PH provided consultancy for and received research funding from Pfizer, a manufacturer of stop‐smoking medications. He was principal investigator on one of the trials included in this review and co‐investigator on other relevant studies.
JHB has received support for this work from the Cochrane Review Support Programme and the University of Oxford's Returning Carer's Fund. Neither of these are deemed conflicts of interest.
NL has received payment for lectures on systematic review methodology, and has been an applicant on project funding to carry out priority setting and systematic reviews in the area of tobacco control (NIHR funded). None of this is deemed a conflict of interest.
HM has no known conflicts of interest.
CN has no known conflicts of interest.
NAR has received royalties from UpToDate, Inc., for chapters on electronic cigarettes and occasional fees from academic hospitals or professional medical societies for lectures on smoking cessation that include discussion of electronic cigarettes. NAR was a member of the committee that produced the 2018 National Academies of Science, Engineering, and Medicine's Consensus Study Report on the Public Health Benefits of E‐cigarettes. She was unpaid for this work. Outside the topic of e‐cigarettes, NAR is a consultant for Achieve LifeSciences, which is developing an investigational smoking cessation medication for FDA approval (cytisine) and her institution (MGH) receives a grant from the company as a site for a clinical trial testing the safety and efficacy of cytisine. NAR holds grants from NIH for research work.
AT's work on this review has been supported by the Cochrane Review Support Programme and the University of Oxford's Returning Carer's Fund. Neither of these are deemed conflicts of interest.
TT has no known conflicts of interest.
Joint first author
Edited (no change to conclusions)
References
References to studies included in this review
Adriaens 2014 {published data only}
- Adriaens K, Van Gucht D, Declerck P, Baeyens F. Effectiveness of the electronic cigarette: an eight-week Flemish study with six-month follow-up on smoking reduction, craving and experienced benefits and complaints. International Journal of Environmental Research and Public Health 2014;11(11):11220-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Baldassarri 2018 {published data only}
- Baldassarri SR, Bernstein SL, Chupp GL, Slade MD, Fucito LM, Toll BA. Electronic cigarettes for adults with tobacco dependence enrolled in a tobacco treatment program: a pilot study. Addictive Behaviors May 2018;80:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassarri SR, Bernstein SL, Chupp GL, Toll BA . Electronic Cigarettes for Adults with Tobacco Dependence Undergoing a Tobacco Treatment Program: a Pilot Prospective Study, . American Journal of Respiratory and Critical Care Medicine, Meeting Abstracts 2016;193:A6511. [Google Scholar]
- NCT02498145. Short term effects of electronic cigarettes in tobacco dependent adults. clinicaltrials.gov/show/NCT02498145 (accessed 17 February 2016).
Begh 2021 {published data only (unpublished sought but not used)}
- Albury C, Barnes R, Ferrey A, Coleman T, Gilbert H, Naughton F, et al. The old and familiar meets the new and unknown: patient and clinician perceptions on e-cigarettes for smoking reduction in UK general practice, a qualitative interview study.. Addiction 2021 2022 May;117(5):1427-1437. [DOI: 10.1111/add.15760] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begh R, Bateman P, Williams N, Grabey J, Stevens R. Examining the effectiveness of general practitioner and nurse promotion of electronic cigarettes versus standard care for smoking reduction and abstinence in hardcore smokers with smoking-related chronic disease: a randomised controlled trial. Statistical Analysis Report, Version 2.0 2021:1-56. [DOI] [PMC free article] [PubMed]
- Begh R, Coleman T, Yardley L, Barnes R, Naughton F, Gilbert H, et al. Examining the effectiveness of general practitioner and nurse promotion of electronic cigarettes versus standard care for smoking reduction and abstinence in hardcore smokers with smoking-related chronic disease: protocol for a randomised controlled trial. Trials 2019;20(1):659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begh R. Training general practitioners and nurses to deliver brief advice about e-cigarettes for smoking reduction: lessons learned from a randomised controlled trial. In: SRNT E-Conference, SY3-04 . 2021.
- ISRCTN59404712. GP/nurse promotion of e-cigarettes in supporting reduced smoking and cessation in smokers. www.isrctn.com/ISRCTN59404712 (first received 28 November 2017).
Bell 2017 {unpublished data only}
- ACTRN12616001641482. Tobacco harm reduction with vaporised nicotine (THRiVe): feasibility study. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616001641482 (first received 28 November 2016).
- Bell S, Dean J, Gilks C, Boyd MA, Fitzgerald L, Mutch A, et al. Tobacco harm reduction with vaporised nicotine (THRiVe): the study protocol of an uncontrolled feasibility study of novel nicotine replacement products among people living with HIV who smoke. International Journal of Environmental Research and Public Health 2017;14(7):799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bonafont Reyes 2022 {published data only}
- Bonafont Reyes BV, Stevens E, Nicholson A, Lei L, Vojjala M, Sherman S . Understanding racial and ethnic differences in switching from combustible cigarettes to e-cigarettes in COPD patients. Journal of the American Geriatrics Society, Annual Scientific Meeting 2022;170(S1):S1-S345. [Google Scholar]
Bullen 2013 {published data only}
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes and smoking cessation: a quandary? - Authors' reply. Lancet 2014;383(9915):408-9. [DOI] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J, et al. Electronic cigarettes for smoking cessation: a randomised controlled trial. Lancet 2013;382(9905):1629-37. [DOI] [PubMed] [Google Scholar]
- Bullen C, Howe C, Laugesen M, McRobbie H, Parag V, Williman J. Do electronic cigarettes help smokers quit? Results from a randomised controlled trial [Abstract]. In: European Respiratory Society Annual Congress, 2013 September 7 - 11, Barcelona, Spain. Vol. 42. 2013:215s-[P1047].
- Bullen C, Williman J, Howe C, Laugesen M, McRobbie H, Parag V, et al. Study protocol for a randomised controlled trial of electronic cigarettes versus nicotine patch for smoking cessation. BMC Public Health 2013;13:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien B, Knight-West O, Walker N, Parag V, Bullen C. E-cigarettes versus NRT for smoking reduction or cessation in people with mental illness: secondary analysis of data from the ASCEND trial. Tobacco Induced Diseases 2015;13(1):5. [DOI] [PMC free article] [PubMed]
Caponnetto 2013a {published data only}
- Campagna D, Cibella F, Caponnetto P, Amaradio MD, Caruso M, Morjaria JB, et al. Changes in breathomics from a 1-year randomized smoking cessation trial of electronic cigarettes. European Journal of Clinical Investigation 2016;46(8):698-706. [DOI] [PubMed] [Google Scholar]
- Caponnetto P, Campagna D, Cibella F, Morjaria JB, Caruso M, Russo C, et al. EffiCiency and Safety of an eLectronic cigAreTte (ECLAT) as tobacco cigarettes substitute: a prospective 12-month randomized control design study. PlOS One 2013;8(6):e66317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibella F, Campagna D, Caponnetto P, Amaradio MD, Caruso M, Russo C, et al. Lung function and respiratory symptoms in a randomized smoking cessation trial of electronic cigarettes. Clinical Science 2016;130(21):1929–37. [DOI] [PubMed] [Google Scholar]
- Farsalinos K, Cibella F, Caponnetto P, Campagna D, Morjaria JB, Battaglia E, et al. Effect of continuous smoking reduction and abstinence on blood pressure and heart rate in smokers switching to electronic cigarettes. Internal and Emergency Medicine 2016;11(1):85-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01164072. Efficacy and safety of an electronic nicotine delivery device (e-cigarette). clinicaltrials.gov/ct2/show/NCT01164072 (first received 16 July 2010).
- NCT01188239. A Structured Protocol to Evaluate Efficacy and Safety of a Popular Electronic Nicotine Delivery Device (E-Cigarette) Efficacy and Safety of a Popular Electronic Nicotine Delivery Device (E-Cigarette). https://clinicaltrials.gov/ct2/show/NCT01188239.
- NCT01194583. Efficacy and safety of an electronic nicotine delivery device (e-cigarette) without nicotine cartridges. clinicaltrials.gov/ct2/show/NCT01194583 (first received 3 September 2010).
- Russo C, Cibella F, Caponnetto P, Campagna D, Maglia M, Frazzetto E, et al. Evaluation of post cessation weight gain in a 1-year randomized smoking cessation trial of electronic cigarettes. Scientific Reports 2016;6:18763. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caponnetto 2013b {published data only}
- Caponnetto P, Auditore R, Russo C, Cappello GC, Polosa R. Impact of an electronic cigarette on smoking reduction and cessation in schizophrenic smokers: a prospective 12-month pilot study. International Journal of Environmental Research and Public Health 2013;10(2):446-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minutolo G, Caponnetto P, Auditore R, Russo C, Polosa R. Management of smoking reduction and cessation in inpatients with schizophrenia: Impact of electronic cigarettes. European Neuropsychopharmacology 2013;23:S581-2. [Google Scholar]
Caponnetto 2021 {published data only}
- Caponnetto P, DiPiazza J, Kim J, Maglia M, Polosa R. A single-arm, open-label pilot, and feasibility study of a high nicotine strength e-cigarette intervention for smoking cessation or reduction for people with schizophrenia spectrum disorders who smoke cigarettes. Nicotine & Tobacco Research 2021;23(7):1113-22. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carpenter 2017 {published data only}
- Carpenter MJ, Heckman BW, Wahlquist AE, Wagener TL, Goniewicz ML, Gray KM, et al. A naturalistic, randomized pilot trial of e-cigarettes: uptake, exposure, and behavioral effects. Cancer Epidemiology, Biomarkers & Prevention 2017;26(12):1795-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02357173. A trial of e-cigarettes: natural uptake, patterns and impact of use. clinicaltrials.gov/show/NCT02357173 (accessed 17 February 2016).
- Smith TT, Wahlquist AE, Heckman BW, Cummings KM, Carpenter MJ. Impact of e-cigarette sampling on cigarette dependence and reinforcement value. Nicotine & Tobacco Research 2020;22(2):297-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cobb 2021 {published and unpublished data}
- Cobb CO, Foulds J, Yen M-S, Veldheer S, Lopez AA, Yingst JM et al. Effect of an electronic nicotine delivery system with 0, 8, or 36 mg/mL liquid nicotine versus a cigarette substitute on tobacco-related toxicant exposure: a four-arm, parallel-group, randomised, controlled trial. Lancet Respiratory Medicine April 2021;9(8):840-50. [DOI: org/10.1016/ S2213-2600(21)00022-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J, Cobb C, Yen M-S, Veldheer S, Brosnan P, Yingst J et al . Effect of electronic nicotine delivery systems on cigarette abstinence in smokers with no plans to quit: exploratory analysis of a randomized placebo-controlled trial. Nicotine Tobacco Research 2022 Jun 15;24(7):955-961. [DOI: 10.1093/ntr/ntab247] [DOI] [PMC free article] [PubMed]
- Lopez AA, Cobb CO, Yingst JM, Veldheer S, Hrabovsky S, Yen MS, et al. A transdisciplinary model to inform randomized clinical trial methods for electronic cigarette evaluation. BMC Public Health 2016;16(1):217. [PMCID:: PMC4778292] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02342795. Randomized controlled trial methods for novel tobacco products evaluation. clinicaltrials.gov/show/NCT02342795 (accessed 17 February 2016).
- Veldheer S, Yingst J, Midya V, Hummer B, Lester C, Krebs N, . Pulmonary and other health effects of electronic cigarette use among adult smokers participating in a randomized controlled smoking reduction trial. Addictive Behaviors 2019;91:95-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst J, Foulds J, Veldheer S, Cobb CO, Yen M, Hrabovsky S, et al. Measurement of electronic cigarette frequency of use among smokers participating in a randomized controlled trial. Nicotine & Tobacco Research 2020;22(5):699-704. [NCT02342795] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yingst J, Wang X, Anderson A, Breland A, Soule E, Barnes A et al. Evaluation of nicotine dependence among smokers using electronic cigarettes to reduce cigarette smoke, . In: Society for Research on Nicotine and Tobacco, Annual Meeting, Baltimore USA. March 2022. [DOI] [PMC free article] [PubMed]
- Yingst J, Wang X, Lopez AA, Breland A, Soule E, Barnes A et al. Randomized Control Trial Methods Workgroup of the Center for the Study of Tobacco Products. Changes in nicotine dependence among smokers using electronic cigarettes to reduce cigarette smoking in a randomized controlled trial. Nicotine Tobacco Research 2022 Jun 25;Epub ahead of print. [DOI: 10.1093/ntr/ntac153] [DOI] [PMC free article] [PubMed] [Google Scholar]
Czoli 2019 {published and unpublished data}
- Czoli CD, Fong GT, Goniewicz ML, Hammond D. Biomarkers of exposure among "dual users" of tobacco cigarettes and electronic cigarettes in Canada. Nicotine and Tobacco Research 2019;21(9):1259-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawkins 2020 {published and unpublished data}
- Dawkins L, Bauld L, Ford A, Robson D, Hajek P, Parrott S, et al. A cluster feasibility trial to explore the uptake and use of e-cigarettes versus usual care offered to smokers attending homeless centres in Great Britain. PLOS One 2020;15(10):e0240968. [ISRCTN14140672] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN14140672. Exploring the use and uptake of e-cigarettes for homeless smokers. www.isrctn.com/ISRCTN14140672 (first received 7 November 2018).
Edmiston 2022 {published data only}
- Edmiston JS, Webb KM, Wang J, Oliveri D, Liang Q, Sarkar M. Biomarkers of exposure and biomarkers of potential harm in adult smokers who switch to e-vapor products relative to cigarette smoking in a 24-week, randomized, clinical trial. Nicotine & Tobacco Research 2022;24(7):1047–54. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eisenberg 2020 {published data only}
- Eisenberg MJ, Hébert-Losier M, Windle SB, Greenspoon T, Brandys T, Fülöp T. Effect of e-cigarettes plus counseling vs counseling alone on smoking cessation: a randomized clinical trial. JAMA 2020;324(18):1844-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert-Losier A, Filion KB, Windle SB, Eisenberg MJ. A randomized controlled trial evaluating the efficacy of e-cigarette use for smoking cessation in the general population: E3 trial design. Canadian Journal of Cardiology Open 2020;2(3):168-75. [NCT02417467] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02417467. Evaluating the efficacy of e-cigarette use for smoking cessation (E3) trial. clinicaltrials.gov/show/NCT02417467 (accessed 17 February 2016).
Eisenhofer 2015 {published data only}
- Eisenhofer J, Makanjuola T, Martinez V, Thompson-Lake DG, Rodgman C, DeBrule DS, et al. Efficacy of electronic cigarettes for smoking cessation in veterans. Drug and Alcohol Dependence 2015;156:e63-e64. [Google Scholar]
Ely 2013 {published data only}
- Ely J. Evaluation of the use of electric cigarettes in a rural smoking cessation program. Available online at: digitalunc.coalliance.org/fedora/repository/cogru:4161 [no longer available] 2013 (accessed 1st November 2014).
Felicione 2019 {published data only}
- Felicione NJ, Enlow P, Elswick D, Long D, Sullivan CR, Blank MD. A pilot investigation of the effect of electronic cigarettes on smoking behavior among opioid-dependent smokers. Addictive Behaviors 2019;91:45-50. [DOI] [PubMed] [Google Scholar]
George 2019 {published data only}
- George J, Hussain M, Vadiveloo T, Ireland S, Hopkinson P, Struthers AD, et al. Cardiovascular effects of switching from tobacco cigarettes to electronic cigarettes. Journal of the American College of Cardiology 2019;74(25):3112-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02878421. Vascular EffectS of regUlar cigarettes Versus electronIc cigarette USe (VESUVIUS). clinicaltrials.gov/ct2/show/NCT02878421 (first received 25 August 2016).
Goniewicz 2017 {published data only}
- Goniewicz ML, Gawron M, Smith DM, Peng M, Jacob P 3rd, Benowitz NL. Exposure to nicotine and selected toxicants in cigarette smokers who switched to electronic cigarettes: a longitudinal within-subjects observational study. Nicotine & Tobacco Research 2017;19(2):160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guillaumier 2018 {unpublished data only}
- ACTRN12617000849392. The QuitNic Study: a pilot study of electronic nicotine devices for smoking cessation with drug and alcohol clients. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=372198 (first received 8 June 2017).
- Bonevski B, Manning V, Wynne O, Gartner C, Borland R, Baker A, et al. QuitNic: A pilot randomised controlled trial comparing nicotine vaping products with nicotine replacement therapy for smoking cessation following residential detoxification. Nicotine and Tobacco Research 2021;23(3):462-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillaumier A, Manning V, Wynne O, Gartner C, Borland R, Baker AL, et al. Electronic nicotine devices to aid smoking cessation by alcohol- and drug-dependent clients: protocol for a pilot randomised controlled trial. Trials 2018;19:415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2015a {published data only}
- Hajek P, Corbin L, Ladmore D, Spearing E. Adding e-cigarettes to specialist stop-smoking treatment: City of London pilot project. Journal of Addiction Research & Therapy 2015;6(244):(online ahead of print). [DOI: 10.4172/2155-6105.1000244] [DOI] [Google Scholar]
Hajek 2019 {published and unpublished data}ISRCTN60477608
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Myers Smith K, Bisal N, et al. A randomized trial of e-cigarettes versus nicotine-replacement therapy. New England Journal of Medicine 2019;380(7):629-37. [DOI] [PubMed] [Google Scholar]
- Hajek P, Phillips-Waller A, Przulj D, Pesola F, Smith KM, Bisal N, et al. E-cigarettes compared with nicotine replacement therapy within the UK Stop Smoking Services: the TEC RCT. Health Technology Assessment 2019;23(43):1-82. [ISRCTN60477608] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN60477608. The efficacy of e-cigarettes compared with nicotine replacement therapy, when used within the UK stop smoking service. www.isrctn.com/ISRCTN60477608 2014 (first received 2 April 2015).
- Li J, Hajek P, Pesola F, Wu Q, Phillips-Waller A, Przulj D, et al. Cost-effectiveness of e-cigarettes compared with nicotine replacement therapy in stop smoking services in England (TEC study): a randomized controlled trial. Addiction 2020;115(3):507-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2022 {published and unpublished data}
- Ford A, Uny I, Lowes J, Naughton F, Cooper S, Coleman T. A qualitative study of factors influencing adherence among pregnant women taking part in a trial of e-cigarettes for smoking cessation. International Journal of Environmental Research and Public Health 2021;18(2):430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajek P, Przulj D, Pesola F, Griffiths C, Walton R, McRobbie H, et al. Electronic cigarettes versus nicotine patches for smoking cessation in pregnancy: a randomized controlled trial. Nature Medicine 2022;28:958-64. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN62025374. Helping pregnant smokers quit: a multi-centre study of electronic cigarettes and nicotine patches. www.isrctn.com/ISRCTN62025374 (first received March 21 2017). [DOI: ]
Halpern 2018 {published data only}
- Halpern SD, Harhay MO, Saulsgiver K, Brophy C, Troxel AB, Volpp KG. A pragmatic trial of e-cigarettes, incentives, and drugs for smoking cessation. New England Journal of Medicine 2018;378(24):2302-10. [DOI] [PubMed] [Google Scholar]
- Harhay MO, Troxel AB, Brophy C, Saulsgiver K, Volpp KG, Halpern SD, . Financial incentives promote smoking cessation directly, not by increasing use of cessation aids. Annala of the American Thoracic Society 2019 February;16(2):280-282. [DOI: 10.1513/AnnalsATS.201808-574RL] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02328794. Randomized clinical trial to reduce harm from tobacco. clinicaltrials.gov/show/NCT02357173 (accessed 17 February 2016).
Hatsukami 2020 {published and unpublished data}
- Hatsukami D, Meier E, Lindgren BR, Anderson A, Reisinger S, Norton K, et al. A randomized clinical trial examining the effects of instructions for electronic cigarette use on smoking-related behaviors, and biomarkers of exposure. Nicotine & Tobacco Research 2020;22(9):1524-32. [DOI: 10.1093/ntr/ntz233] [DOI] [PMC free article] [PubMed]
- NCT03111537. Methods Project 4: Clinical Trial - Amended (COMET). https://clinicaltrials.gov/ct2/show/NCT03111537 (Accessed 10 September 2022).
Hickling 2019 {published data only}
- Hickling LM, Perez-Iglesias R, McNeill A, Dawkins L, Moxham J, Ruffell T, et al. A pre-post pilot study of electronic cigarettes to reduce smoking in people with severe mental illness. Psychological Medicine 2019;49(6):1033-40. [DOI] [PubMed] [Google Scholar]
- NCT02212041. Acceptability, patterns of use and safety of electronic cigarette in people with mental illness: a pilot study. clinicaltrials.gov/show/NCT02212041 (accessed 17 February 2016).
Holliday 2019 {published data only}
- Holliday et al. E-cigarettes for Smoking Cessation in Patients with Periodontitis: Pilot RCT. In: IADR International Association for Dental Research. 2019.
- Holliday R, Preshaw PM, Ryan V, Sniehotta FF, McDonald S, Bauld L, et al. A feasibility study with embedded pilot randomised controlled trial and process evaluation of electronic cigarettes for smoking cessation in patients with periodontitis. Pilot and Feasibility Studies 2019;5:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN17731903. Feasibility study of e-cigarettes in periodontitis. www.isrctn.com/ISRCTN17731903 (first received 27 September 2016).
Humair 2014 {published data only}
- Humair J-P, Tango R. Can e-cigarette help patients to reduce or stop smoking in primary care practice? Journal of General Internal Medicine 2014;29:S480. [Google Scholar]
Ikonomidis 2018 {published data only}
- Ikonomidis I, Vlastos D, Kourea K, Kostelli G, Varoudi M, Pavlidis G, et al. Electronic cigarette smoking increases arterial stiffness and oxidative stress to a lesser extent than a single conventional cigarette: an acute and chronic study. Circulation 2018;137(3):303-6. [DOI] [PubMed] [Google Scholar]
- NCT03039920. The effects of electronic cigarette smoking on the arterial wall and endothelial glycocalyx properties of smokers. clinicaltrials.gov/ct2/show/NCT03039920 (first received 1 February 2017).
Ikonomidis 2020a {published data only}
- Ikonomidis I, Katogiannis K, Kostelli G, Kourea K, Kyriakou E, Kypraiou A, et al. Effects of electronic cigarette on platelet and vascular function after four months of use. Food and Chemical Toxicology 2020;141:111389. [DOI] [PubMed] [Google Scholar]
Ikonomidis 2020b {published and unpublished data}
- Ikonomidis I, Katogiannis K, Kostelli G, Kourea K, Kyriakou E, Kypraiou A, et al. Effects of electronic cigarette on platelet and vascular function after one month of use. In: European Heart Journal 2020; Conference: European Society of Cardiology Congress, ESC 2020. Suppl 2 edition. Vol. 41. 2020:2359.
Ioakeimidis 2018 {published data only}
- Ioakeimidis N, Vlachopoulos C, Georgakopoulos C, Abdelrasoul M, Skliros N, Katsi V, et al. Smoking cessation rates with varenicline and electronic cigarettes in relapsed smokers with a history of acute coronary syndrome. European Heart Journal 2018;39(Suppl_1):242. [Google Scholar]
Kerr 2020 {published and unpublished data}
- Kerr D. Chapter 5: A randomised controlled trial investigating the cardiovascular effects of e-cigaretttes in comparison to nicotine replacement patches [personal communication]. Studies into the Cardiovascular and Respiratory Effects of Electronic Cigarettes (PhD thesis), email to: A Butler 27 September 2020.
- NCT03358953. The cardiovascular impacts of electronic cigarettes in comparison to the use of nicotine replacement patches (VAPOUR). clinicaltrials.gov/ct2/show/NCT03358953 (first received December 2 2017 ).
Kimber 2021 {published data only}
- Kimber CF, Soar K, Dawkins LE . Changes in puffing topography and subjective effects over a 2-week period in e-cigarette naive smokers: effects of device type and nicotine concentrations. Addictive Behaviors 2021;118:106909. [DOI: 10.1016/j.addbeh.2021.106909] [DOI] [PubMed] [Google Scholar]
Kumral 2016 {published data only}
- Kumral TL, Saltürk Z, Yildirim G, Uyar Y, Berkiten G, Atar Y, et al. How does electronic cigarette smoking affect sinonasal symptoms and nasal mucociliary clearance? B-ENT 2016;12(1):17-21. [PubMed] [Google Scholar]
Lee 2018 {published data only}
- Lee SM, Tenney R, Wallace A, Arjojmandi M. The end perioperative smoking pilot study: a randomized trial comparing e-cigarettes versus nicotine patch. Canadian Journal of Anesthesia 2017;64(1 Supplement 1):S48-S49. [Google Scholar]
- Lee SM, Tenney R, Wallace A, Arjomandi M. Exploring the teachable moment: facilitators and barriers to perioperative smoking cessation, a qualitative study,. Canadian Journal of Anesthesia 2017; Conference: 2017 Annual Meeting of the Canadian Anesthesiologists' Society, Canada. 61;1 Supplement:S46-S47. [Google Scholar]
- Lee SM, Tenney R, Wallace AW, Arjomandi M. E-cigarettes versus nicotine patches for perioperative smoking cessation: a pilot randomized trial. PeerJ 2018;6(9):e5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02482233. A pilot randomized controlled clinical trial - "Electronic nicotine delivery device (e-cigarette) for perioperative smoking cessation in veterans". clinicaltrials.gov/show/NCT02482233 (accessed 17 February 2016).
Lee 2019 {published data only}
- KCT0001277. Effect of an electronic cigarette for smoking reduction and cessation in Korean male smokers: a randomized, controlled study. KCT0001277 2014 (accessed 15 August 2016). [DOI] [PubMed]
- Lee SH, Ahn SH, Cheong YS. Effect of electronic cigarettes on smoking reduction and cessation in Korean male smokers: a randomized controlled study. Journal of the American Board of Family Medicine 2019;32(4):567-74. [DOI] [PubMed] [Google Scholar]
Lucchiari 2020 {published data only}
- Lucchiari C, Masiero M, Mazzocco K, Veronesi G, Maisonneauve P, Jemos C, et al. Benefits of e-cigarettes in smoking reduction and in pulmonary health among chronic smokers undergoing a lung cancer screening program at 6 months. Addictive Behaviours 2020;103:106222. [DOI] [PubMed] [Google Scholar]
- Lucchiari C, Masiero M, Veronesi G, Maisonneuve P, Spina S, Jemos C, et al. Benefits of e-cigarettes among heavy smokers undergoing a lung cancer screening program: randomized controlled trial protocol. JMIR Research Protocols 2016;5(1):e21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiero M, Lucchiari C, Mazzocco K, Veronesi G, Maisonneuve P, Jemos C, et al. E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine and Tobacco Research 2019;21(1):119-126. [NCT02422914] [DOI] [PubMed] [Google Scholar]
- Masiero M, Lucchiari C, Mazzocco K, Veronesi G, Maisonneuve P Jemos C, et al. Corrigendum: E-cigarettes may support smokers with high smoking-related risk awareness to stop smoking in the short run: preliminary results by randomized controlled trial. Nicotine and Tobacco Research 2020;22(4):594-5. [NCT02422914] [DOI] [PubMed] [Google Scholar]
- NCT02422914. Benefits of tobacco free cigarette among heavy smokers undergoing a lung cancer screening program: a randomized controlled study. clinicaltrials.gov/show/NCT02422914 (accessed 17 February 2016).
- Pravettoni G, Masiero M, Lucchiari C, Maisenneuve P, Mazzocco K, Veronesi G. The role of electronic cigarettes in smoking cessation among heavy smokers undergoing a lung cancer screening program: preliminary results of a randomized controlled study. Psycho-oncology 2016;25:72. [DOI: ] [Google Scholar]
Martinez 2021 {published data only}
- Byrne M, Simmons V, Martinez U, Sutton S, Brandon K, Brandon T . Cost effectiveness of a smoking cessation intervention for dual users of combustible and electronic cigarettes . In: Society for Research on Nicotine and Tobacco, Annual Meeting, Baltimore USA. March 2022.
- Gore LR, Sutton SK, Martinez U, Brandon KO, Simmons VN, Brandon TH. Mediators of initial abstinence for an extended self help smoking cessation intervention with dual users of combustible and electronic cigarettes. In: Society for Research on Nicotine and Tobacco, Annual Meeting, Baltimore USA. Vol. PS3-7. March 2022.
- Martinez U, Simmons VN, Brandon KO, Quinn GP, Brandon TH. Examining smoking and vaping behaviors, expectancies, and cessation outcomes between bisexual and heterosexual individuals. Behavioral Medicine Online May 2022;25:1-10. [DOI: 10.1080/08964289.2022.2077295] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez U, Simmons VN, Sutton SK, Drobes DJ, Meltzer LR, Brandon KO, et al. Targeted smoking cessation for dual users of combustible and electronic cigarettes: a randomised controlled trial. Lancet Public Health 2021;6(7):e500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer LR, Simmons VN, Sutton SK, Drobes DJ, Quinn GP, Meade CD, et al. A randomized controlled trial of a smoking cessation self-help intervention for dual users of tobacco cigarettes and e-cigarettes: intervention development and research design. Contemporary Clinical Trials 2017;60:56-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT02416011 . Smoking cessation self-help for dual users of tobacco cigarettes and e-cigarettes. clinicaltrials.gov/ct2/show/NCT02416011 (first received 18 August 2020).
- Sutton SK, Brandon KO, Harrell PT, Martínez Ú, Simmons VN, Gore LR et al. Identifying prospective subpopulations of combustible and electronic cigarette dual users in the United States via finite mixture modeling. Addiction 2022 Sep;117(9):2493-2503. [DOI: 10.1111/add.15906] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martner 2019 {published data only (unpublished sought but not used)}
- Martner SG, Dallery J. Technology-based contingency management and e-cigarettes during the initial weeks of a smoking quit attempt. Journal of Applied Behavior Analysis 2019;52(4):928-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
McRobbie 2015 {published data only}
- McRobbie H, Goniewicz M, Phillips A, Myers-Smith K, West O, Hajek P. Effects of the use of electronic cigarettes with and without concurrent smoking on acrolein delivery POS4-33. In: Society for Research on Nicotine and Tobacco, 20th Annual Meeting, Seattle, Washington. 2014:13. [www.srnt.org/conferences/2014/pdf/SRNT_2014_Rapids_C.pdf]
- McRobbie H, Phillips A, Goniewicz ML, Smith KM, Knight-West O, Przulj D, et al. Effects of switching to electronic cigarettes with and without concurrent smoking on exposure to nicotine, carbon monoxide, and acrolein. Cancer Prevention Research (Philadelphia, Pa.) 2015;8(9):873-8. [DOI] [PubMed] [Google Scholar]
- NCT01714778. Toxins and Delivery in E-cigarette USers (TADEUS). clinicaltrials.gov/ct2/show/NCT01714778 (first received 26 October 2012).
Meier 2017 {published data only}
- Meier E, Wahlquist AE, Heckman BW, Cummings KM, Froeliger B, Carpenter MJ. A pilot randomized crossover trial of electronic cigarette sampling among smokers. Nicotine & Tobacco Research 2017;19(2):176-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morphett 2022a {unpublished data only}
- ACTRN12612001210864. Can using nicotine as a long-term substitute enhance smoking cessation over using it only as a cessation aid? [An open-label randomised pragmatic policy trial examining effectiveness of short-term use of Nicotine Replacement Therapy (NRT) vs short- or long-term use of NRT vs short- or long-term use of NRT or electronic nicotine delivery systems for smoking cessation in cigarette smokers.]. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12612001210864 (first received November 15 2012).
- Fraser D, Borland R, Gartner C. Protocol for a randomised pragmatic policy trial of nicotine products for quitting or long-term substitution in smokers. BMC Public Health 2015;15:1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morphett K, Fraser D, Borland R, Hall W, Walker N, Bullen C, et al. A pragmatic randomized comparative trial of e-cigarettes and other nicotine products for quitting or long-term substitution in smokers. Nicotine & Tobacco Research 2022;24(7):1079-88. [DOI: doi: 10.1093/ntr/ntab266] [DOI] [PubMed] [Google Scholar]
Morphett 2022b {published and unpublished data}
- ACTRN12618000408280. Cessation And Relapse Prevention (CARP) trial: nicotine vaporisers compared to standard nicotine replacement therapy for smoking cessation among people with co-morbidities. www.anzctr.org.au/ACTRN12618000408280.aspx (first received 21 March 2018).
- Morphett K, Boyd M, Puljevic C, Gilks C, Bonevski B, Borland R, et al. Do nicotine vaping products increase quitting among priority population groups over standard smoking cessation therapy? A pragmatic randomised partial cross-over trial. In: Society for Research on Nicotine and Tobacco (SRNT), Annual Meeting 2022. Vol. SYM17-4. 2022.
Morris 2022 {published data only}
- Morris P, McDermott S, Chapman F, Verron T, Cahours X, Stevenson M, et al. Reductions in biomarkers of exposure to selected harmful and potentially harmful constituents following exclusive and partial switching from combustible cigarettes to myblu TM electronic nicotine delivery systems (ENDS). Internal & Emergency Medicine 2022;17(2):397-410. [DOI: 10.1007/s11739-021-02813-w] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04429932. A study to evaluate nicotine uptake and biomarkers in adult smokers using mybluTM electronic cigarettes. clinicaltrials.gov/ct2/show/NCT04429932 (first received June 7 2021).
- NCT04430634 . A study to evaluate nicotine uptake and biomarkers in smokers using mybluTM electronic cigarettes [An open-label, randomized, crossover study to assess nicotine uptake, tobacco-related biomarkers of exposure, biomarkers of potential harm, and puff topography with use of mybluTM electronic cigarettes in adult smokers]. clinicaltrials.gov/ct2/show/NCT04430634 (first received June 7 2021).
Myers‐Smith 2022 {published data only}
- ISRCTN13288677. Can electronic cigarettes and nicotine replacement treatment help reduce smoking in smokers who struggle to quit? www.isrctn.com/ISRCTN13288677 (first received 17 March 2017).
- Myers Smith K, Phillips-Waller A, Pesola F, McRobbie H, Przulj D, Orzol M, et al. E-cigarettes versus nicotine replacement treatment as harm reduction interventions for smokers who find quitting difficult: randomised controlled trial. Addiction 2022;117(1):224-33. [DOI: ] [DOI] [PubMed] [Google Scholar]
NCT02648178 {published data only}
- NCT02648178. Evaluation of appeal and impact of e-cigarettes among chronic smokers with smoking-related cancers. clinicaltrials.gov/show/NCT02648178 (accessed 17 February 2016).
NCT02918630 {published data only}
- NCT02918630. E-cigarettes to promote smoking reduction among individuals with schizophrenia. clinicaltrials.gov/ct2/show/NCT02918630 (first received 5 July 2018).
NCT03492463 {published data only}
- NCT03492463. The role of nicotine dose and route of delivery in affecting adoption of e-cigarettes and reducing exposure to toxic combustion products (ENDS-Switch). clinicaltrials.gov/ct2/show/study/NCT03492463 (first received April 10 2018).
Nides 2014 {published data only}
- NCT01898169. Evaluation of short-term safety and use patterns of an electronic nicotine delivery system. clinicaltrials.gov/ct2/show/NCT01898169 (first received 12 July 2013).
- Nides MA, Leischow SJ, Bhatter M, Simmons M. Nicotine blood levels and short-term smoking reduction with an electronic nicotine delivery system. American Journal of Health Behavior 2014;38(2):265-74. [DOI] [PubMed] [Google Scholar]
Okuyemi 2022 {published data only}
- NCT03084315. Changes in biomarkers associated with use of electronic cigarettes [Changes in biomarkers associated with use of electronic cigarettes among African American menthol and non-menthol smokers]. clinicaltrials.gov/ct2/show/NCT03084315 (first received March 20 2017).
- Okuyemi KS, Ojo-Fati O, Aremu TO, Friedrichsen CS, Grude L, Oyenuga M, et al. A randomized trial of nicotine vs. no-nicotine e-cigarettes among African American smokers: changes in smoking and tobacco biomarkers. Nicotine & Tobacco Research 2022;24(4):555-63. [DOI: ] [DOI] [PubMed] [Google Scholar]
Oncken 2015 {published data only}
- NCT01775787. Effects of Electronic Cigarettes on Nicotine Concentrations (ECIG). clinicaltrials.gov/ct2/show/NCT01775787 (first received 25 January 2013).
- Oncken CA, Litt MD, McLaughlin LD, Burki NA. Nicotine concentrations with electronic cigarette use: effects of sex and flavor. Nicotine & Tobacco Research 2015;17(4):473-8. [DOI] [PubMed] [Google Scholar]
- Swedeh MA, Oncken C, Burki NK. Acute effects of electronic cigarettes on airway function in human subjects. In: American Thoracic Society International Conference Abstracts. Vol. 189. 2014:A4089.
Ozga‐Hess 2019 {published data only}
- Ozga-Hess JE, Felicione NJ, Ferguson SG, Dino G, Elswick D, Whitworth C, et al. Piloting a clinical laboratory method to evaluate the influence of potential modified risk tobacco products on smokers' quit-related motivation, choice, and behaviour. Addictive Behaviors 2019;99:106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pacifici 2015 {published data only}
- Pacifici R, Pichini S, Graziano S, Pellegrini M, Massaro G, Beatrice F. Successful nicotine intake in medical assisted use of e-cigarettes: a pilot study. International Journal of Environmental Research and Public Health 2015;12(7):7638-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polosa 2011 {published data only}
- NCT01195597. Smoking cessation and reduction with an electronic nicotine delivery device (ENDD). clinicaltrials.gov/ct2/show/NCT01195597 (first received 6 September 2010).
- Polosa R, Caponnetto P, Morjaria JB, Papale G, Campagna D, Russo C. Effect of an electronic nicotine delivery device (e-cigarette) on smoking reduction and cessation: a prospective 6-month pilot study. BMC Public Health 2011;11:786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polosa R, Morjaria JB, Caponnetto P, Campagna D, Russo C, Alamo A, et al. Effectiveness and tolerability of electronic cigarette in real-life: a 24-month prospective observational study. Internal and Emergency Medicine 2014;9(5):537-46. [DOI] [PubMed] [Google Scholar]
Polosa 2014b {published data only}
- NCT02124200. High Cessation Rates in Smokers Using Personal Vaporizers (VAPECIG). clinicaltrials.gov/ct2/show/NCT02124200 (first received 28 April 2014).
- Polosa R, Caponnetto P, Maglia M, Morjaria JB, Russo C. Success rates with nicotine personal vaporizers: a prospective 6-month pilot study of smokers not intending to quit. BMC Public Health 2014;14:1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polosa 2015 {published data only}
- Polosa R, Caponnetto P, Cibella F, Le-Houezec J. Quit and smoking reduction rates in vape shop consumers: a prospective 12-month survey. International Journal of Environmental Research and Public Health 2015;12(4):3428-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pratt 2016 {published data only}
- Pratt SI, Sargent J, Daniels L, Santos MM, Brunette M. Appeal of electronic cigarettes in smokers with serious mental illness. Addictive Behaviors 2016;59:30-4. [DOI] [PubMed] [Google Scholar]
Pratt 2022 {published and unpublished data}
- NCT03050853. The appeal and impact of e-cigarettes in smokers with SMI. clinicaltrials.gov/ct2/show/NCT03050853 (first received February 13 2017).
- Pratt SI, Brunette MF, Ferron JC, Santos M, Sargent J, Xie H. E-cigarette provision to promote switching in cigarette smokers with serious mental illness - a randomized trial. Nicotine & Tobacco Research 2022;6(24):1405-12. [DOI: doi: 10.1093/ntr/ntac082] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pulvers 2018 {published data only}
- Pulvers K, Emami AS, Nollen NL, Romero DR, Strong DR, Benowitz NL, et al. Tobacco consumption and toxicant exposure of cigarette smokers using electronic cigarettes. Nicotine & Tobacco Research 2018;20(2):206-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pulvers 2020 {published and unpublished data}
- Arnold M, Nollen N, Mayo M, Ahluwalia J, Leavens E, Zhang G et al. Dual use of cigarettes and e-cigarettes in African American and LatinX smokers: Secondary analyses from a randomised controlled e-cigarettes switching trial. In: Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meeting. February 24-27 2021 virtual:POD4-2 p31. [DOI] [PMC free article] [PubMed]
- Arnold MJ, Nollen NL, Mayo MS, Ahluwalia JS, Leavens EL, Zhang G, Rice M, et al. Harm reduction associated with dual use of cigarettes and e-cigarettes in Black and Latino smokers: secondary analyses from a randomized controlled e-cigarette switching trial. Nicotine & tobacco research 2021;23(11 2021 Oct 7;23(11):192-1876. [DOI: 10.1093/ntr/ntab069] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leavens ELS, Nollen N, Ahluwalia J, Mayo M, Rice M, Brett E, et al. Changes in dependence, withdrawal, and craving among adult smokers who switch to nicotine salt pod-based e-cigarettes. In: Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meeting. February 24-27 2021 virtual:POD53-1 p96, C-6 p116. [DOI] [PMC free article] [PubMed]
- Leavens ELS, Nollen N, Ahluwalia JS, Mayo MS, Rice M, Brett EI et al. Changes in dependence, withdrawal, and craving among adult smokers who switch to nicotine salt pod-based e-cigarettes. Addiction 2021;117(1):207-215. [DOI: 10.1111/add.15597] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03511001. Effects Among Smokers Who Use and Do Not Use E-Cigarettes. https://clinicaltrials.gov/ct2/show/NCT03511001.
- Nollen N, Lambert L, Leavens E, Ahluwalia J, Rice M, Pulvers K. Does menthol flavoring help black and LatinX adult menthol combustible cigarette smokers switch to e-cigarettes?: secondary analyses from a randomized clinical trial. In: Society for Research on Nicotine and Tobacco, Annual Meeting, Baltimore USA. March 2022.
- Nollen NL, Leavens ELS, Ahluwalia JS, Rice M, Mayo MS, Pulvers K. Menthol versus non-menthol flavouring and switching to e-cigarettes in black and Latinx adult menthol combustible cigarette smokers: secondary analyses from a randomised clinical trial. Tobacco Control Online 29 March 2022;EPub. [DOI: 10.1136/tobaccocontrol-2021-057180] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulvers K, Nollen NL, Rice M, Schmid CH, Qu K, Benowitz NL, et al. Effect of pod e-cigarettes vs cigarettes on carcinogen exposure among African American and Latinx smokers: a randomized clinical trial. JAMA Netw0rk Open 2020;3(11):e2026324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice M, Nollen N, Woodcock A, Benowitz N, Ahluwalia J, Pulvers K. Effect of marijuana use on smokers switching to e-cigarettes in a randomised control trial. In: Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meeting. February 24-27 2021 virtual:SRNT C-44, p125. [DOI] [PMC free article] [PubMed]
- Rice M, Nollen NL, Ahluwalia JS, Benowitz N, Woodcock A, Pulvers K. Effects of Marijuana Use on Smokers Switching to E-cigarettes in a Randomized Clinical Trial. Nicotine Tob Res. 2022 Jun 15;24(7):994-1002. [DOI: 10.1093/ntr/ntac008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubenstein D, Sokolovsky A, Aston E, Nollen N, Schmid C, Pulvers K et al. Predictors of smoking reduction among African American and LatinX smokers in a randomised controlled trial of Juul e-cigarettes. In: Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meeting. Vol. C-56 . February 24-27 2021 virtual:129. [DOI] [PMC free article] [PubMed]
- Rubenstein D, Sokolovsky AW, Aston ER, Nollen NL, Schmid CH, Rice M, et al . Predictors of smoking reduction among African American and Latinx smokers in a randomized controlled trial of JUUL e-cigarettes. Addictive Behaviors 2021 November;122:107037. [DOI: 10.1016/j.addbeh.2021.107037] [DOI] [PMC free article] [PubMed] [Google Scholar]
Russell 2021 {published and unpublished data}
- Russell C, McKeganey N, Katsampouris E, Satchwell A, Haseen F. A randomised community-based trial of a closed-system pod e-vapour product and nicotine replacement therapy for cigarette abstinence and reduction. Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meeting February 24-27 2021 virtual:PH-353 p230.
Scheibein 2020 {published and unpublished data}
- Scheibein F, McGirr K, Morrison A, Roche, Wells JS. An exploratory non-randomized study of a 3-month electronic nicotine delivery system (ENDS) intervention with people accessing a homeless supported temporary accommodation service (STA) in Ireland. Harm Reduction Journal 2020;17(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Skelton 2022 {published data only}
- ACTRN12617000905369. Smoking reduction interventions for smoking cessation. ncbi.nlm.nih.gov/pmc/articles/PMC6953262/ (first received 9 June 2017). [DOI: 10.1002/14651858.CD013183.pub2] [DOI]
- Lum A, Skelton E, Robinson M, Guillaumier A, Wynne O, Gartner C, et al. Barriers and facilitators to using vaporised nicotine products as smoking cessation aids among people receiving treatment for substance use disorder. Addictive Behaviors 2021;124:107097. [DOI: 10.1016/j.addbeh.2021.107097] [DOI] [PubMed] [Google Scholar]
- Skelton E, Lum A, Robinson M, Dunlop A, Guillaumier A, Baker A, et al. A pilot randomised controlled trial of abrupt versus gradual smoking cessation in combination with vaporised nicotine products for people receiving alcohol and other drug treatment. Addictive Behaviors 2022;131:107328. [DOI: doi: 10.1016/j.addbeh.2022.107328] [DOI] [PubMed] [Google Scholar]
Smith 2020 {published and unpublished data}
- Smith TT, Heckman BW, Wahlquist AE, Cummings KM, Carpenter MJ. The impact of e-liquid propylene glycol and vegetable glycerin ratio on ratings of subjective effects, reinforcement value, and use in current smokers. Nicotine & Tobacco Research 2020;22(5):791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2016 {published data only}
- Stein MD, Caviness C, Grimone K, Audet D, Anderson BJ, Bailey GL. An open trial of electronic cigarettes for smoking cessation among methadone-maintained smokers. Nicotine & Tobacco Research 2016;18(5):1157-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Strasser 2016 {published data only}
- Strasser AA, Souprountchouk V, Kaufmann A, Blazekovic S, Leone F, Benowitz NL, et al. Nicotine replacement, topography, and smoking phenotypes of e-cigarettes. Tobacco Regulatory Science 2016;2(4):352-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tattan‐Birch 2022 {published data only}
- Tattan-Birch H, Kock L, Brown J, Beard E, Bauld L, West R, et al. E-cigarettes to augment stop smoking in-person support and treatment with varenicline (E-ASSIST): a pragmatic randomised controlled trial. Nicotine & Tobacco Research June 2022 Epub ahead of print. [DOI: doi: 10.1093/ntr/ntac149] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tseng 2016 {published data only}
- Guttentag A, Tuo-Yen T, Shelley D, Thomas K. Analyzing Trajectories of Acute Cigarette Reduction Post-Introduction of an E-Cigarette Using Ecological Momentary Assessment Data. International Journal of Environmental Research and Public Health 2022;19(12):7452. [DOI: 10.3390/ijerph19127452] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttentag A. Do e-cigarettes "Work" for smokers as a step on the way to cessation? An analysis of the temporal and spatial correlates of switching in a harm reduction trial among adult smokers. Dissertation Abstracts International: Section B: The Sciences and Engineering 2021;82:8-B. [Google Scholar]
- NCT02628964. Assessing the use of electronic cigarettes (e-cigarettes) as a harm reduction strategy. clinicaltrials.gov/show/NCT02628964 (accessed 17 February 2016).
- Tseng TY, Ostroff JS, Campo A, Gerard M, Kirchner T, Rotrosen J, et al. A randomized trial comparing the effect of nicotine versus placebo electronic cigarettes on smoking reduction among young adult smokers. Nicotine & Tobacco Research 2016;18(10):1937-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
Valentine 2018 {published data only}
- Valentine GW, Hefner K, Jatlow PI, Rosenheck RA, Gueorguieva R, Sofuoglu M. Impact of e-cigarettes on smoking and related outcomes in veteran smokers with psychiatric comorbidity. Journal of Dual Diagnosis 2018;14(1):2-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Staden 2013 {published data only}
- Van Staden SR, Groenewald M, Engelbrecht R, Becker PJ, Hazelhurst LT. Carboxyhaemoglobin levels, health and lifestyle perceptions in smokers converting from tobacco cigarettes to electronic cigarettes. South African Medical Journal 2013;103(11):865-8. [DOI] [PubMed] [Google Scholar]
Vickerman 2022 {published data only}
- NCT03575468. Enhanced e-cigarette coaching intervention for dual users of cigarettes and e-cigarettes. clinicaltrials.gov/ct2/show/NCT03575468 (first received 2 July 2018).
- Vickerman KA, Carpenter KM, Miles LN, Hsu JM, Watt KA, Brandon TH, et al. A randomized pilot of a tailored smoking cessation quitline intervention for individuals who smoke and vape. Nicotine & Tobacco Research 2022;24(11):1811-20. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickerman KA, Carpenter KM, Watt K, Miles L, Hsu J, Brandon T, et al. A behavioral smoking cessation intervention for Quitline callers who also use e-cigarettes. In: Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meeting; February 24-27 2021 (virtual). 2021:POD14-1 p50.
Wadia 2016 {published data only}
- Wadia R, Booth V, Yap HF, Moyes DL. A pilot study of the gingival response when smokers switch from smoking to vaping. British Dental Journal 2016;221(11):722-6. [DOI] [PubMed] [Google Scholar]
Walele 2018 {published data only}
- Cravo AS, Bush J, Sharma G, Savioz R, Martin C, Craige S, et al. A randomised, parallel group study to evaluate the safety profile of an electronic vapour product over 12 weeks. Regulatory Toxicology and Pharmacology 2016;81:S1-S14. [DOI] [PubMed] [Google Scholar]
- NCT02029196. A randomised, parallel group, multi-centre study to evaluate the safety profile of the ITG EVP G1 product. clinicaltrials.gov/show/NCT02029196 (accessed 16 July 2014).
- NCT02143310. A multi-centre study to evaluate the safety of use of electronic vapour products for two years. clinicaltrials.gov/show/NCT02143310 (accessed 16 July 2014).
- Walele T, Bush J, Koch A, Savioz R, Martin C, O'Connell G. Evaluation of the safety profile of an electronic vapour product used for two years by smokers in a real-life setting. Regulatory Toxicology and Pharmacology 2018;92:226-38. [DOI] [PubMed] [Google Scholar]
Walker 2020 {published data only}
- NCT02521662. A randomised-controlled clinical trial to evaluate the effectiveness and safety of combining nicotine patches with e-cigarettes (with and without nicotine) plus behavioural support, on smoking abstinence. clinicaltrials.gov/show/NCT02521662 (accessed 17 February 2016).
- Walker N, Parag V, Verbiest M, Laking G, Laugesen M, Bullen C. Nicotine patches used in combination with e-cigarettes (with and without nicotine) for smoking cessation: a pragmatic, randomised trial. Lancet. Respiratory Medicine 2020;8(1):54-64. [DOI] [PubMed] [Google Scholar]
- Walker N, Verbiest M, Kurdziel T, Laking G, Laugesen M, Parag V, et al. Effectiveness and safety of nicotine patches combined with e-cigarettes (with and without nicotine) for smoking cessation: study protocol for a randomised controlled trial. BMJ Open 2019;9(2):e023659. [DOI] [PMC free article] [PubMed] [Google Scholar]
White 2021 {published and unpublished data}
- NCT03185546. Project 2 cigarette and e-cigarette nicotine content and e-liquid flavors. clinicaltrials.gov/ct2/show/NCT03185546 (first received May 14 2021).
- White CM, Denlinger-Apte RL, Tessier KM, Koopmeiners JS, Hatsukami DK, Strasser AA. Cigarette nicotine reduction in the presence of an alternative: investigating how cigarette nicotine content and e-liquid content affect smoking. In: Society for Research on Nicotine and Tobacco (SRNT) 2021 Annual Meetingl February 24-27 2021 (virtual). 2021:SYM2C p2-3.
- White CM, Tessier KM, Koopmeiners JS, Denlinger-Apte RL, Cobb CO, Lane T, et al. Preliminary evidence on cigarette nicotine reduction with concurrent access to an e-cigarette: manipulating cigarette nicotine content, e-liquid nicotine content, and e-liquid flavor availability. Preventive Medicine Online 19 August 2022;EPub. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yingst 2020 {published data only}
- Yingst J, Foulds J, Zurlo J, Steinberg MB, Eissenberg T, Du P. Acceptability of electronic nicotine delivery systems (ENDS) among HIV positive smokers. AIDS Care 2020;32(10):1224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Adkison 2013 {published data only}
- Adkison SE, O'Connor RJ, Bansal-Travers M, Hyland A, Borland R, Yong HH, et al. Electronic nicotine delivery systems: international tobacco control four-country survey. American Journal of Preventive Medicine 2013;44(3):207-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Delaimy 2015 {published data only}
- Al-Delaimy WK, Myers MG, Leas EC, Strong DR, Hofstetter CR. E-cigarette use in the past and quitting behavior in the future: a population-based study. American Journal of Public Health 2015;105(6):1213-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donzelli A. E-cigarettes may impair ability to quit, but other explanations are possible. American Journal of Public Health 2015;105(11):e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2019 {published data only}
- Anonymous. E-cigarettes best other cessation tools. Cancer Discovery 2019;9(4):OF3. [DOI] [PubMed] [Google Scholar]
Battista 2013 {published data only}
- Battista L, Di Iorio M, Tancredi M, Acconcia MC, Torromeo C, Barilla F, et al. Cardiovascular effects of electronic cigarettes. Circulation 2013;128:A16755. [Google Scholar]
Bianco 2019 {published data only}
- Bianco CL, Pratt SI, Ferron JC, Brunette MF. Electronic cigarette use during a randomized trial of interventions for smoking cessation among Medicaid beneficiaries with mental illness. Journal of Dual Diagnosis 2019;15(3):184-91. [DOI] [PubMed] [Google Scholar]
Biener 2015 {published data only}
- Biener L, Hargraves JL. A longitudinal study of electronic cigarette use among a population-based sample of adult smokers: association with smoking cessation and motivation to quit. Nicotine & Tobacco Research 2015;17(2):127-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Biondi‐Zoccai 2019 {published data only}
- Biondi-Zoccai G, Sciarretta S, Bullen C, Nocella C, Violi F, Loffredo L, et al. Acute effects of heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: the Sapienza University of Rome-Vascular Assessment of Proatherosclerotic Effects of Smoking ( SUR - VAPES ) 2 randomized trial. Journal of the American Heart Association 2019;8(6):e010455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03301129. Role of Traditional Cigarettes, Electronic and IQOS Cigarettes on Oxidative Stress. https://clinicaltrials.gov/ct2/show/NCT03301129.
Biondi‐Zoccai 2020 {published data only}
- Biondi-Zoccai G, Carnevale R, Vitali M, Tritapepe L, Martinelli O, Macrina F, et al. A randomized trial comparing the acute coronary, systemic, and environmental effects of electronic vaping cigarettes versus heat-not-burn cigarettes in smokers of combustible cigarettes undergoing invasive coronary assessment: rationale and design of the SUR-VAPES 3 trial. Minerva Cardioangiologica 2020;68(6):548-55. [DOI] [PubMed] [Google Scholar]
Borderud 2014 {published data only}
- Barton MK. Electronic cigarettes did not help patients with cancer stop smoking. CA: a cancer journal for clinicians 2015;65(2):85-6. [DOI] [PubMed] [Google Scholar]
- Borderud SP, Li Y, Burkhalter JE, Sheffer CE, Ostroff JS. Electronic cigarette use among patients with cancer: characteristics of electronic cigarette users and their smoking cessation outcomes. Cancer 2014;120(22):3527-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillon M. Electronic cigarettes might not help cancer patients quit smoking. Journal of the National Cancer Institute 2015;107(1):496. [DOI] [PubMed] [Google Scholar]
Brose 2015 {published data only}
- Brose LS, Hitchman SC, Brown J, West R, McNeill A. Is the use of electronic cigarettes while smoking associated with smoking cessation attempts, cessation and reduced cigarette consumption? A survey with a 1-year follow-up. Addiction 2015;110(7):1160-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, West R, Beard E, Michie S, Shahab L, McNeill A. Prevalence and characteristics of e-cigarette users in Great Britain: Findings from a general population survey of smokers. Addictive Behaviors 2014;39(6):1120-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e-cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine & Tobacco Research 2015;17(10):1187-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brown 2014a {published data only}
- Brown J, Beard E, Kotz D, Michie S, West R. Real-world effectiveness of e-cigarettes when used to aid smoking cessation: a cross-sectional population study. Addiction 2014;109(9):1531-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bullen 2010 {published data only}
- Bullen C, McRobbie H, Thornley S, Glover M, Lin R, Laugesen M. Effect of an electronic nicotine delivery device (e cigarette) on desire to smoke and withdrawal, user preferences and nicotine delivery: randomised cross-over trial. Tobacco Control 2010;19(2):98-103. [DOI] [PubMed] [Google Scholar]
Bullen 2018 {published data only}
- ACTRN12616001355460. Varenicline alone or with nicotine e-cigarettes for smoking cessation in people with mental illness and drug and/or alcohol dependence. Australian New Zealand Clinical Trials Registry (ACTRN).
- Bullen C, Verbiest M, Galea-Singer S, Kurdziel T, Laking G, Newcombe D, et al. The effectiveness and safety of combining varenicline with nicotine e-cigarettes for smoking cessation in people with mental illnesses and addictions: study protocol for a randomised-controlled trial. BMC Public Health 2018;18(1):596. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caponnetto 2019 {published data only}
- Caponnetto P, Maglia M, Polosa R. Efficacy of smoking cessation with varenicline plus counselling for e-cigarettes users (VAREVAPE): a protocol for a randomized controlled trial. Contemporary Clinical Trials Communications 2019;15:100412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cavarretta 2019 {published data only}
- Cavarretta E, Sciarretta S, Nocella C, Peruzzi M, Marullo AG, Loffredo L, et al. Subjective smoking satisfaction between heat-not-burn, electronic vaping, and traditional tobacco combustion cigarettes: a sub-analysis of the SUR-VAPES 2 trial. European Journal of Preventive Cardiology 2019;26(Supplement 1):S114. [Google Scholar]
Chaumont 2018 {published data only}
- Chaumont M, Becker B, Zaher W, Culie A, Deprez G, Melot C, et al. Differential Effects of E-Cigarette on Microvascular Endothelial Function, Arterial Stiffness and Oxidative Stress: a Randomized Crossover Trial. Scientific reports 2018;8(1):10378. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chaumont 2019 {published data only}
- Chaumont M, El Channan M, Bernard A, Lesage A, Deprez G, Van Muylem A, et al. Short-term high wattage e-cigarette cessation improves cardiorespiratory outcomes in regular users: a randomized crossover trial. Journal of Hypertension 2019;Conference: 29th European Meeting on Hypertension and Cardiovascular Protection, ESH 2019. Italy. 37(Supplement 1):e8-9. [Google Scholar]
Chausse 2015 {published data only}
- Chausse P, Naughton G, Dutheil F. Electronic cigarettes: the resistance value of the heating filament could be the key to lung toxicity. Chest 2015;148(1):e29-30. [DOI] [PubMed] [Google Scholar]
Choi 2014 {published data only}
- Choi K, Forster JL. Beliefs and experimentation with electronic cigarettes: a prospective analysis among young adults. American Journal of Preventive Medicine 2014;6(2):175-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K Forster JL. Authors' Response to: Context on use is needed before public health recommendations are made about e-cigarettes. Americal Journal of Preventive Medicine 2014;46(6):e58-59. [DOI] [PubMed] [Google Scholar]
Chorti 2012 {published data only}
- Chorti M, Poulianiti K, Jamurtas A, Kostikas K, Tzatzarakis M, Vynias D, et al. Effects of active and passive electronic and tobacco cigarette smoking on lung function (P08-02). Toxicology Letters 2012;211:S64. [Google Scholar]
Collins 2019 {published data only}
- Collins SE, Nelson LA, Stanton J, Mayberry N, Ubay T, Taylor EM, et al. Harm reduction treatment for smoking (HaRT-S): findings from a single-arm pilot study with smokers experiencing chronic homelessness. Substance Abuse 2019;40(2):229-39. [DOI] [PubMed] [Google Scholar]
Cook 2019 {published data only}
- Cook R, Davidson P, Martin R. E-cigarettes helped more smokers quit than nicotine replacement therapy. BMJ 2019;365:l2036. [DOI] [PubMed] [Google Scholar]
Cox 2019a {published data only}
- Cox S, Dawkins L, Doshi J, Cameron J. Effects of e-cigarettes versus nicotine replacement therapy on short-term smoking abstinence when delivered at a community pharmacy. Addictive Behaviors Reports 2019;10:100202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czogala 2012 {published data only}
- Czogala J, Cholewinski M, Kutek A, Zielinska-Danch W. Evaluation of changes in hemodynamic parameters after the use of electronic nicotine delivery systems among regular cigarette smokers. Przeglad Lekarski 2012;69(10):841-5. [PubMed] [Google Scholar]
D'Ruiz 2017 {published data only}
- D'Ruiz CD, O'Connell G, Graff DW, Yan XS. Measurement of cardiovascular and pulmonary function endpoints and other physiological effects following partial or complete substitution of cigarettes with electronic cigarettes in adult smokers. Regulatory Toxicology and Pharmacology 2017;87:36-53. [DOI] [PubMed] [Google Scholar]
Dawkins 2012 {published data only}
- Dawkins L, Turner J, Hasna S, Soar K. The electronic-cigarette: effects on desire to smoke, withdrawal symptoms and cognition. Addictive Behaviors 2012;37(8):970-3. [DOI] [PubMed] [Google Scholar]
Dawkins 2013a {published data only}
- Dawkins L, Turner J, Crowe E. Nicotine derived from the electronic cigarette improves time-based prospective memory in abstinent smokers. Psychopharmacology 2013;227(3):377-84. [DOI] [PubMed] [Google Scholar]
Dawkins 2014 {published data only}
- Dawkins L, Corcoran O. Acute electronic cigarette use: nicotine delivery and subjective effects in regular users. Psychopharmacology 2014;231(2):401-7. [DOI] [PubMed] [Google Scholar]
Douptcheva 2013 {published data only}
- Douptcheva N, Gmel G, Studer J, Deline S, Etter JF. Use of electronic cigarettes among young Swiss men. Journal of Epidemiology & Community Health 2013;67(12):1075-6. [DOI] [PubMed] [Google Scholar]
Dutra 2014 {published data only}
- Dutra LM, Glantz SA. Electronic cigarettes and conventional cigarette use among U.S. adolescents: a cross-sectional study. JAMA Pediatrics 2014;168(7):610-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eissenberg 2010 {published data only}
- Eissenberg T. Electronic nicotine delivery devices: ineffective nicotine delivery and craving suppression after acute administration. Tobacco Control 2010;19(1):87-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Etter 2014 {published data only}
- Etter JF, Bullen C. A longitudinal study of electronic cigarette users. Addictive Behaviors 2014;39(2):491-4. [DOI] [PubMed] [Google Scholar]
Farsalinos 2012 {published data only}
- Farsalinos K, Tsiapras D, Kyrzopoulos S, Savvopoulou M, Avramidou E, Vasilopoulou D, et al. Acute effects of using an electronic nicotine-delivery device (e-cigarette) on myocardial function: comparison with the effects of regular cigarettes. European Heart Journal 2012;33:203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Acute effects of using an electronic nicotine-delivery device (electronic cigarette) on myocardial function: comparison with the effects of regular cigarettes. BMC Cardiovascular Disorders 2014;14:78. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farsalinos 2013a {published data only}
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Voudris V. Evaluating nicotine levels selection and patterns of electronic cigarette use in a group of "vapers" who had achieved complete substitution of smoking. Substance Abuse: Research and Treatment 2013;7:139-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farsalinos 2013b {published data only}
- Farsalinos KE, Romagna G, Tsiapras D, Kyrzopoulos S, Spyrou A, Voudris V. Impact of flavour variability on electronic cigarette use experience: an internet survey. International Journal of Environmental Research & Public Health 2013;10(12):7272-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Farsalinos 2013c {published data only}
- Farsalinos K, Tsiapras D, Kyrzopoulos S, Stefopoulos C, Spyrou A, Tsakalou M, et al. Immediate effects of electronic cigarette use on coronary circulation and blood carboxyhemoglobin levels: comparison with cigarette smoking. European Heart Journal 2013;34(Suppl 1):13. [Google Scholar]
Farsalinos 2013d {published data only}
- Farsalinos K, Tsiapras D, Kyrzopoulos S, Spyrou A, Stefopoulos C, Romagna G, et al. Effects of electronic cigarette use on the elastic properties of the ascending aorta in healthy subjects: comparison with the effects of tobacco cigarettes. European Heart Journal Cardiovascular Imaging 2013;14(Suppl 2):ii203. [Google Scholar]
Flouris 2012 {published data only}
- Flouris AD, Poulianiti KP, Chorti MS, Jamurtas AZ, Kouretas D, Owolabi EO, et al. Acute effects of electronic and tobacco cigarette smoking on complete blood count. Food and Chemical Toxicology 2012;50(10):3600-3. [DOI] [PubMed] [Google Scholar]
Flouris 2013 {published data only}
- Flouris AD, Chorti MS, Poulianiti KP, Jamurtas AZ, Kostikas K, Tzatzarakis MN, et al. Acute impact of active and passive electronic cigarette smoking on serum cotinine and lung function. Inhalation Toxicology 2013;25(2):91-101. [DOI] [PubMed] [Google Scholar]
Gmel 2016 {published data only}
- Gmel G, Baggio S, Mohler-Kuo M, Daeppen JB, Studer J. E-cigarette use in young Swiss men: is vaping an effective way of reducing or quitting smoking? Swiss Medical Weekly 2016;146:w14271. [DOI] [PubMed] [Google Scholar]
Gottlieb 2019 {published data only}
- Gottlieb MA. E-cigarettes versus nicotine-replacement therapy for smoking cessation. New England Journal of Medicine 2019;380(20):1974. [DOI] [PubMed] [Google Scholar]
Grana 2014b {published data only}
- Grana RA, Popova L, Ling PM. A longitudinal analysis of electronic cigarette use and smoking cessation. JAMA Internal Medicine 2014;174(5):812-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
James 2016 {published data only}
- James SA, Meier EM, Wagener TL, Smith KM, Neas BR, Beebe LA. E-Cigarettes for immediate smoking substitution in women diagnosed with cervical dysplasia and associated disorders. International Journal of Environmental Health Research 2016;13(3):E288. [DOI: 10.3390/ijerph13030288] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01989923. Smoking Cessation in Women With Gynecological Conditions. https://clinicaltrials.gov/ct2/show/NCT01989923 (Accessed 9 September 2022).
Kasza 2013 {published data only}
- Kasza KA, Bansal-Travers M, O'Connor RJ, Compton WM, Kettermann A, Borek N, et al. Cigarette smokers' use of unconventional tobacco products and associations with quitting activity: findings from the ITC-4 U.S. cohort. Nicotine & Tobacco Research 2013;16(6):672-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kouretas 2012 {published data only}
- Kouretas D, Poulianiti K, Chorti M, Jamurtas A, Kostikas K, Tzatzarakis M, et al. Effects of electronic cigarette and tobacco cigarette smoking on complete blood count (P08-03). Toxicology Letters 2012;211:S64. [DOI] [PubMed] [Google Scholar]
Kousta 2019 {published data only}
- Kousta S. E-cigarettes for smoking cessation. Nature Human Behaviour 2019;3:322. [DOI] [PubMed] [Google Scholar]
Lechner 2015 {published data only}
- Lechner WV, Meier E, Wiener JL, Grant DM, Gilmore J, Judah MR, et al. The comparative efficacy of first- versus second-generation electronic cigarettes in reducing symptoms of nicotine withdrawal. Addiction 2015;110(5):862-7. [DOI] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee S, Grana RA, Glantz SA. Electronic cigarette use among Korean adolescents: a cross-sectional study of market penetration, dual use, and relationship to quit attempts and former smoking. Journal of Adolescent Health 2014;54(6):684-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Manzoli 2015 {published data only}
- Manzoli L, Flacco ME, Fiore M, La Vecchia C, Marzuillo C, Gualano MR, et al. Electronic cigarettes efficacy and safety at 12 months: cohort study. PLOS One 2015;10(6):e0129443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoli L, La Vecchia C, Flacco ME, Capasso L, Simonetti V, Boccia S, et al. Multicentric cohort study on the long-term efficacy and safety of electronic cigarettes: study design and methodology. BMC Public Health 2013;13(1):883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01785537. The efficacy and safety of electronic cigarettes: a 5-year follow-up study. clinicaltrials.gov/ct2/show/NCT01785537 (first received 7 February 2013).
Marini 2014 {published data only}
- Marini S, Buonanno G, Stabile L, Ficco G. Short-term effects of electronic and tobacco cigarettes on exhaled nitric oxide. Toxicology and Applied Pharmacology 2014;278(1):9-15. [DOI] [PubMed] [Google Scholar]
Mayor 2019 {published data only}
- Mayor S. E-cigarettes help twice as many smokers quit as nicotine replacement therapy, trial finds. BMJ 2019;364:l473. [DOI] [PubMed] [Google Scholar]
Miura 2015 {published data only}
- Miura N, Yuki D, Minami N, Kakehi A, Futama Y. A study to investigate changes in the levels of biomarkers of exposure to selected cigarette smoke constituents in Japanese adult male smokers who switched to a non-combustion inhaler type of tobacco product. Regulatory Toxicology and Pharmacology 2015;71(3):498-506. [DOI] [PubMed] [Google Scholar]
NCT02487953 {published data only}
- NCT02487953. Electronic Nicotine Delivery Systems (ENDS) as a smoking cessation treatment. clinicaltrials.gov/ct2/show/NCT02487953 (first received 2 July 2015).
NCT02527980 {published data only}
- NCT02527980. E-cigarettes: dynamic patterns of use and health effects. clinicaltrials.gov/show/NCT02527980 (first received August 19 2015).
NCT03036644 {published data only}
- NCT03036644. Comparison of electronic cigarettes and tobacco cigarettes on cardiovascular function and oxidative stress. clinicaltrials.gov/ct2/show/NCT03036644 (first received 30 January 2017).
NCT03575468 {published data only}
- NCT03575468. Enhanced E-cigarette Coaching Intervention for Dual Users of Cigarettes and E-cigarettes. https://clinicaltrials.gov/ct2/show/NCT03575468 (first received 2 July 2018).
NCT04107779 {published data only}
- NCT04107779. Changes in biomarkers of cigarette smoke exposure after switching either exclusively or partly to JUUL ENDS. clinicaltrials.gov/ct2/show/NCT04107779 (first received 27 September 2019).
Nolan 2016 {published data only}
- Nolan M, Leischow S, Croghan I, Kadimpati S, Hanson A, Schroeder D, et al. Feasibility of electronic nicotine delivery systems in surgical patients. Nicotine & Tobacco Research 2016;18(8):1757-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
NTR6224 {published data only}
- NTR6224. Electronic cigarettes: an intervention for dual-users. www.who.int/trialsearch/Trial2.aspx?TrialID=NTR6224 (first received 12 January 2017).
Palamidas 2014 {published data only}
- Palamidas A, Gennimata SA, Kaltsakas G, Tsikrika S, Vakali S, Gratziou C, et al. Acute effect of an e-cigarette with and without nicotine on lung function. Tobacco Induced Diseases 2014;12(Suppl 1):A34. [Google Scholar]
Pearson 2012 {published data only}
- Pearson JL, Richardson A, Niaura RS, Vallone DM, Abrams DB. E-cigarette awareness, use, and harm perceptions in US adults. American Journal of Public Health 2012;102(9):1758-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pokhrel 2013 {published data only}
- Pokhrel P, Fagan P, Little MA, Kawamoto CT, Herzog TA. Smokers who try e-cigarettes to quit smoking: findings from a multiethnic study in Hawaii. American Journal of Public Health 2013;103(9):e57-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polosa 2014a {published data only}
- Polosa R, Morjaria J, Caponnetto P, Caruso M, Strano S, Battaglia E, et al. Effect of smoking abstinence and reduction in asthmatic smokers switching to electronic cigarettes: evidence for harm reversal. International Journal of Environmental Research & Public Health 2014;11(5):4965-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Popova 2013 {published data only}
- Ling PM, Popova L. Novel "Tobacco" product use and association with smoking cessation: a national study. Journal of General Internal Medicine 2012;27(2 Suppl):S254. [Google Scholar]
- Popova L, Ling PM. Alternative tobacco product use and smoking cessation: a national study. American Journal of Public Health 2013;103(5):923-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Prochaska 2014 {published data only}
- Prochaska JJ, Grana RA. E-cigarette use among smokers with serious mental illness. PLOS One 2014;9(11):e113013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Russo 2018 {published data only}
- Russo C, Cibella F, Mondati E, Caponnetto P, Frazzetto E, Caruso M, et al. Lack of substantial post-cessation weight increase in electronic cigarettes users. International Journal of Environmental Research and Public Health 2018;15(4):581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schober 2014 {published data only}
- Schober W, Szendrei K, Matzen W, Osiander-Fuchs H, Heitmann D, Schettgen T, et al. Use of electronic cigarettes (e-cigarettes) impairs indoor air quality and increases FeNO levels of e-cigarette consumers. International Journal of Hygiene and Environmental Health 2014;217(6):628-37. [DOI] [PubMed] [Google Scholar]
Siegel 2011 {published data only}
- Siegel MB, Tanwar KL, Wood KS. Electronic cigarettes as a smoking-cessation tool: results from an online survey. American Journal of Preventive Medicine 2011;40(4):472-5. [DOI] [PubMed] [Google Scholar]
Song 2020 {published data only}
- NCT02596685. Effects of Electronic Cigarette Use on the Lungs. https://www.clinicaltrials.gov/ct2/show/NCT02596685 (Accessed 8 September 2022).
- Song M-A, Reisinger SA, Freudenheim JL, Brasky TM, Mathe EA, McElroy JP, et al. Effects of electronic cigarette constituents on the human lung: a pilot clinical trial. Cancer Prevention Research 2020;13(2):145-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
St.Helen 2020 {published data only}
- StHelen G, Nardone N, Addo N, Dempsey D, Havel C, Jacob P, et al. Differences in nicotine intake and effects from electronic and combustible cigarettes among dual users. Addiction 2020;115(4):757-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stein 2019 {published data only}
- Stein JH, Korcarz CE. E-cigarettes versus nicotine-replacement therapy for smoking cessation. New England Journal of Medicine 2019;380(20):1973-4. [DOI] [PubMed] [Google Scholar]
Stower 2019 {published data only}
- Stower H. E-cigarettes to help smoking cessation. Nature Medicine 2019;25(3):358. [DOI] [PubMed] [Google Scholar]
Tsikrika 2014 {published data only}
- Tsikrika S, Vakali S, Gennimata SA, Palamidas A, Kaltsakas G, Koulouris N, et al. Short term use of an e-cig: influence on clinical symptoms, vital signs and eCO levels. Tobacco Induced Diseases 2014;12(Suppl 1):A30. [Google Scholar]
Tucker 2018 {published data only}
- Tucker MR, Laugesen M, Bullen C, Grace RC. Predicting short-term uptake of electronic cigarettes: effects of nicotine, subjective effects, and simulated demand. Nicotine & Tobacco Research 2018;20(10):1265-71. [DOI] [PubMed] [Google Scholar]
Tzatzarakis 2013 {published data only}
- Tzatzarakis MN, Tsitoglou KI, Chorti MS, Poulianiti KP, Jamurtas AZ, Koutedakis Y, et al. Acute and short term impact of active and passive tobacco and electronic cigarette smoking on inflammatory markers. Toxicology Letters 2013;221(Suppl):S86. [Google Scholar]
Vakali 2014 {published data only}
- Vakali S, Tsikrika S, Gennimata SA, Kaltsakas G, Palamidas A, Koulouris N, et al. E-cigarette acute effect on symptoms and airway inflammation: comparison of nicotine with a non-nicotine cigarette. Tobacco Induced Diseases 2014;12(Suppl 1):A35. [Google Scholar]
Valentine 2016 {published data only}
- Valentine GW, Jatlow PI, Coffman M, Nadim H, Gueorguieva R, Sofuoglu M. The effects of alcohol-containing e-cigarettes on young adult smokers. Drug and Alcohol Dependence 2016;159:272-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Heel 2017 {published data only}
- Van Heel M, Van Gucht D, Vanbrabant K, Baeyens F. The importance of conditioned stimuli in cigarette and e-cigarette craving reduction by e-cigarettes. International Journal of Environmental Research and Public Health 2017;14(2):193. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vansickel 2010 {published data only}
- Vansickel AR, Cobb CO, Weaver MF, Eissenberg TE. A clinical laboratory model for evaluating the acute effects of electronic "cigarettes": nicotine delivery profile and cardiovascular and subjective effects. Cancer Epidemiology, Biomarkers & Prevention 2010;19(8):1945-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vansickel 2012 {published data only}
- Vansickel AR, Weaver MF, Eissenberg T. Clinical laboratory assessment of the abuse liability of an electronic cigarette. Addiction 2012;107(8):1493-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vansickel 2013 {published data only}
- Vansickel AR, Eissenberg T. Electronic cigarettes: effective nicotine delivery after acute administration. Nicotine & Tobacco Research 2013;15(1):267-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vardavas 2012 {published data only}
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, Behrakis PK. Short-term pulmonary effects of using an electronic cigarette: Impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 2012;141(6):1400-6. [DOI] [PubMed] [Google Scholar]
Vickerman 2013 {published data only}
- Vickerman KA, Carpenter KM, Altman T, Nash CM, Zbikowski SM. Use of electronic cigarettes among state tobacco cessation quitline callers. Nicotine & Tobacco Research 2013;15(10):1787-91. [DOI] [PubMed] [Google Scholar]
Voos 2019 {published data only}
- Voos N, Kaiser L, Mahoney MC, Bradizza CM, Kozlowski LT, Benowitz NL, et al. Randomized within-subject trial to evaluate smokers' initial perceptions, subjective effects and nicotine delivery across six vaporized nicotine products. Addiction 2019;114(7):1236-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
Voos 2020 {published data only}
- Voos N, Smith D, Kaiser L, Mahoney MC, Bradizza CM, Kozlowski LT, et al. Effect of e-cigarette flavors on nicotine delivery and puffing topography: results from a randomized clinical trial of daily smokers. Psychopharmacology 2020;237(2):491-502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagener 2014 {published data only}
- Wagener TL, Meier E, Hale JJ, Oliver ER, Warner ML, Driskill LM, et al. Pilot investigation of changes in readiness and confidence to quit smoking after e-cigarette experimentation and 1 week of use. Nicotine & Tobacco Research 2014;16(1):108-14. [DOI] [PubMed] [Google Scholar]
Walele 2016a {published data only}
- Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part A: Pharmacokinetics. Regulatory Toxicity 2016;74:187-92. [DOI] [PubMed] [Google Scholar]
Walele 2016b {published data only}
- Walele T, Sharma G, Savioz R, Martin C, Williams J. A randomised, crossover study on an electronic vapour product, a nicotine inhalator and a conventional cigarette. Part B: Safety and subjective effects. Regulatory Toxicity 2016;74:193-9. [DOI] [PubMed] [Google Scholar]
Yan 2015 {published data only}
- Yan XS, D'Ruiz C. Effects of using electronic cigarettes on nicotine delivery and cardiovascular function in comparison with regular cigarettes. Regulatory Toxicology and Pharmacology 2015;71(1):24-34. [DOI] [PubMed] [Google Scholar]
Yuki 2017 {published data only}
- Yuki D, Sakaguchi C, Kikuchi A, Futamura Y. Pharmacokinetics of nicotine following the controlled use of a prototype novel tobacco vapor product. Regulatory Toxicology and Pharmacology 2017;87(2):30-5. [DOI] [PubMed] [Google Scholar]
Zhang 2019 {published data only}
- Zhang Y, Upson D. E-cigarettes versus nicotine-replacement therapy for smoking cessation. New England Journal of Medicine 2019;380(20):1973. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12617001324303 {published data only}
- ACTRN12617001324303. Vaporised nicotine products versus oral forms of nicotine replacement therapy (NRT) products for tobacco smoking cessation among low-socioeconomic status (low-SES) smokers. anzctr.org.au/ACTRN12617001324303.aspx (first received 15 September 2017).
ACTRN12619001787178 {published data only}
- ACTRN12619001787178. Project NEAT: nicotinE as treatment for tobacco smoking following discharge from residential withdrawal services. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12619001787178 (first received 18 November 2019).
ACTRN12621000076875 {published data only}
- ACTRN12621000076875. Vaporised nicotine products versus nicotine replacement therapy for tobacco smoking cessation among low-socioeconomic status smokers: a randomised controlled trial. trialsearch.who.int/Trial2.aspx?TrialID=ACTRN12621000076875 (first received January 29 2021). [CENTRAL: www.cochranelibrary.com/es/central/doi/10.1002/central/CN-02240824/full]
ACTRN12621000148875 {published data only}
- ACTRN12621000148875. HARMONY: harm reduction for opiates, nicotine and you. www.who.int/trialsearch/Trial2.aspx?TrialID=ACTRN12621000148875 (first received February 12 2021). [CENTRAL: www.cochranelibrary.com/central/doi/10.1002/central/CN-02240640/full]
Berlin 2019 {published data only}
- Berlin I, Dautzenberg B, Lehmann B, Palmyre J, Liégey E, De Rycke Y, et al. Randomised, placebo-controlled, double-blind, double-dummy, multicentre trial comparing electronic cigarettes with nicotine to varenicline and to electronic cigarettes without nicotine: the ECSMOKE trial protocol. BMJ Open 2019;9(5):e028832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03630614. Randomized trial of electronic cigarettes with or without nicotine in smoking cessation (ECSMOKE). clinicaltrials.gov/ct2/show/NCT03630614 (first received 15 August 2018).
Caponnetto 2014 {published data only}
- Caponnetto P, Polosa R, Auditore R, Minutolo G, Signorelli M, Maglia M, et al. Smoking cessation and reduction in schizophrenia (SCARIS) with e-cigarette: study protocol for a randomized control trial. Trials [electronic resource] 2014;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT01979796. Antismoking effects of electronic cigarettes in subjects with schizophrenia and their potential influence on cognitive functioning: design of a randomized trial. Smoking Cessation And Reduction In Schizophrenia (The SCARIS Study). clinicaltrials.gov/show/NCT01979796 (accessed 16 July 2014).
Caponnetto 2020 {published data only}
- Caponnetto P, Caruso M, Maglia M, Emma R, Saitta D, Busa B, et al. Non-inferiority trial comparing cigarette consumption, adoption rates, acceptability, tolerability, and tobacco harm reduction potential in smokers switching to Heated Tobacco Products or electronic cigarettes: study protocol for a randomized controlled trial. Contemporary Clinical Trials Communications 2020;8:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03569748 . Heated tobacco products vs electronic cigarettes. clinicaltrials.gov/ct2/show/NCT03569748 (first received June 25 2021).
Cox 2022 {published data only}
- Cox S, Bauld L, Brown R, Carlise M, Ford A, Hajek P. Evaluating the effectiveness of e-cigarettes compared with usual care for smoking cessation when offered to smokers at homeless centres: protocol for a multi-centre cluster-randomized controlled trial in Great Britain. Addiction 2022;117(7):2096-2107. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- ISRCTN18566874. E-cigarettes vs usual care for smoking cessation when offered at homeless centres. isrctn.com/ISRCTN18566874 (Accessed 8 September 2022) (first received October 12 2021). [DOI: ]
El‐Khoury 2021 {published data only}
- El-Khoury F, El Aarbaoui T, Heron M, Hejblum G, Metadieu B, Le Faou AL, et al. Smoking cessation using preference-based tools among socially disadvantaged smokers: study protocol for a pragmatic, multicentre randomised controlled trial. BMJ Open 2021;11(6):e048859. [DOI: 10.1136/bmjopen-2021-048859] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heron M, Le Faou AL, Ibanez G, Metadieu B, Melchior M, El-Khoury L, et al . Smoking cessation using preference-based tools: a mixed method pilot study of a novel intervention among smokers with low socioeconomic position.. Addiction Science & Clinical Practice 2021;16(1):43. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT04654585. Preference-based tools for smoking cessation among disadvantaged smokers, a pragmatic randomised controlled trial [Sevrage tabagique à l'aide des outils dédiés selon la préférence, un essai randomisé contrôlé pragmatique]. clinicaltrials.gov/ct2/show/NCT04654585 (first received December 4 2020).
Elling 2021 {published data only}
- Elling JM, Crutzen R, Talhout R, DeVries H. Effects of providing tailored information about e-cigarettes in a web-based smoking cessation intervention: protocol for a randomised control trial ,. Journal of Medical Internet Research, Research Protocols 2021;10(5):e27088. [DOI: 10.2196/27088] [DOI] [PMC free article] [PubMed] [Google Scholar]
ISRCTN13158982 {published data only}
- ISRCTN13158982. Enhancing dental health advice. isrctn.com/ISRCTN13158982 (first received May 10 2022). [DOI: ]
ISRCTN17691451 {published data only}
- ISRCTN17691451. ESCAPE: E-cigarettes for smoking cessation and reduction in people with mental illness [E-cigarettes for smoking cessation and reduction in people with mental illness - a randomised pilot feasibility trial]. isrctn.com/ISRCTN17691451 (first received September 30 2021). [DOI: ]
ISRCTN61193406 {published data only}
- ISRCTN61193406. Do e-cigarettes help smokers quit when not accompanied by intensive behavioural support? who.int/trialsearch/Trial2.aspx?TrialID=ISRCTN61193406 2020 (first received 11 August 2020).
Klonizakis 2017 {published data only}
- Jones G, McIntosh E, Brose LS, Klonizakis M. Participant experiences of a quit smoking attempt through either Nicotine Replacement Therapy (NRT) methods or the use of an e-cigarette. Journal of Addiction Medicine 2022;16(3):272-7. [DOI] [PubMed] [Google Scholar]
- Klonizakis M, Crank H, Gumber A, Brose LS. Smokers making a quit attempt using e-cigarettes with or without nicotine or prescription nicotine replacement therapy: impact on cardiovascular function (ISME-NRT) - a study protocol. BMC Public Health 2017;17(1):293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klonizakis M, Gumber A, McIntosh E, Brose LS. Short-term cardiovascular effects of e-cigarettes in adults making a stop-smoking attempt: a randomized controlled trial. Biology 2021;10(11):1208. [DOI: 10.3390/biology10111208] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT03061253. E-cigarettes and cardiovascular function (ISME-NRT). clinicaltrials.gov/ct2/show/NCT03061253 (first received 23 February 2017).
Krysinski 2021 {published data only}
- Krysinski A, Russo C, John S, Belsey JD, Campagna D, Caponnetto P, et al. International randomised controlled trial evaluating metabolic syndrome in type 2 diabetic cigarette smokers following switching to combustion-free nicotine delivery systems: the DIASMOKE protocol. BMJ Open 2021;11(4):e045396. [DOI: 10.1136/bmjopen-2020-045396] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- NCT04231838. Metabolic syndrome in diabetic smokers using cigarettes & combustion-free nicotine delivery systems (DIASMOKE). clinicaltrials.gov/ct2/show/NCT04231838 (first received January 18 2020).
Murray 2020 {published data only}
- Murray RL, Brain K, Britton J, Quinn-Scoggins HD, Lewis S, McCutchan GM, et al. Yorkshire Enhanced Stop Smoking (YESS) study: a protocol for a randomised controlled trial to evaluate the effect of adding a personalised smoking cessation intervention to a lung cancer screening programme. BMJ Open 2020;10(9):e037086. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01842828 {published data only}
- NCT01842828. E-cigarettes as an addition to multi-component treatment for tobacco dependence: a pilot study. clinicaltrials.gov/show/NCT01842828 (first received 16 July 2014).
NCT01989923 {published data only}
- NCT01989923. Immediate smoking cessation for patients at risk for cervical dysplasia, cervical cancer and lower genital tract dysplasia and cancer - a feasibility study comparing nicotine replacement therapy with the electronic nicotine delivery system. clinicaltrials.gov/show/NCT01989923 (first received November 21 2013).
NCT02004171 {published data only}
- NCT02004171. Electronic nicotine delivery devices (ENDDs) or nicotine inhaler for smoking cessation. clinicaltrials.gov/show/NCT02004171 (first received December 2013).
NCT02124187 {published data only}
- NCT02124187. Smoking cessation and reduction in depression (SCARID). clinicaltrials.gov/show/NCT02124187 (first received April 28 2014).
NCT02398487 {published data only}
- NCT02398487. Personal Vaporizer vs Cigalike (VAPECIG 2) [Head-to-head comparison of personal vaporizers versus cigalike: prospective 6-month randomized control design study]. clinicaltrials.gov/show/NCT02398487 (first received March 25 2015).
NCT02590393 {published data only}
- NCT02590393. The role of nicotine and non-nicotine alkaloids in e-cigarette use and dependence. clinicaltrials.gov/show/NCT02590393 (first received 17 February 2016).
NCT03113136 {published data only}
- NCT03113136. Examination of low wattage and high wattage e-cigarettes (SWITCH). clinicaltrials.gov/ct2/show/study/NCT03113136 (first received April 13 2017).
NCT03249428 {published data only}
- NCT03249428. E-cigarette inner city RCT [A community-based participatory action pragmatic randomized controlled trial using electronic-cigarette for tobacco dependence in the inner city population with a holistic approach]. clinicaltrials.gov/ct2/show/NCT03249428 (first received August 15 2017).
NCT03277495 {published data only}
- NCT03277495. Predictors and consequences of combustible cigarette smokers' switch to standardized research e-cigarettes. clinicaltrials.gov/ct2/show/NCT03277495 (first received September 11 2017).
NCT03589989 {published data only}
- NCT03589989. The ESTxENDS trial - electronic nicotine delivery systems (ENDS/vaporizer/e-cigarette) as an aid for smoking cessation (ESTxENDS). clinicaltrials.gov/ct2/show/record/NCT03589989 (first received 18 July 2018).
- NCT03603340. The ESTxENDS trial - effects of using Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) on depression (ESTxENDS). clinicaltrials.gov/show/NCT03603340 (first received 27 July 2018).
- NCT03603353. The ESTxENDS trial - effects of using Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) on sleep quality (ESTxENDS). clinicaltrials.gov/show/NCT03603353 (first received 27 July 2018).
- NCT03612336. The ESTxENDS trial - metabolic effects of using Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) (ESTxENDS). clinicaltrials.gov/show/NCT03612336 (first received 2 August 2018).
- NCT03612375. ESTxENDS trial - oxidative stress induced by Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) measured in urine (ESTxENDS). clinicaltrials.gov/show/NCT03612375 (first received 2 August 2018).
- NCT03612453. ESTxENDS Trial - oxidative stress induced by Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) measured in EBC (ESTxENDS). clinicaltrials.gov/show/NCT03612453 (first received 2 August 2018).
- NCT03612544. The ESTxENDS trial - toxins from using Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) (ESTxENDS). clinicaltrials.gov/show/NCT03612544 (first received 2 August 2018).
- NCT03632421. The ESTxENDS trial - effects of using Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) on respiratory symptoms (ESTxENDS). clinicaltrials.gov/show/NCT03632421 (first received 15 August 2018).
- NCT03938298. The ESTxENDS trial: pulmonary function substudy (PulmENDS). clinicaltrials.gov/ct2/show/nct03938298 (first received 6 May 2019).
- NCT04236791. The ESTxENDS trial - electronic nicotine delivery systems as an aid for smoking cessation - extension of follow-up. clinicaltrials.gov/show/NCT04236791 2020 (first received 22 January 2020).
- NCT04244773. ESTxENDS trial: MN substudy - micronuclei in buccal epithelium, a surrogate measure of future cancer risk, induced by Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig). clinicaltrials.gov/show/NCT04244773 (first received 28 January 2020).
- NCT04617444. The ESTxENDS trial - effects of using Electronic Nicotine Delivery Systems (ENDS/Vaporizer/E-cig) on olfactory function. clinicaltrials.gov/show/NCT04617444 (first received 05 November 2020).
NCT03625986 {published data only}
- NCT03625986. Does switching to nicotine containing electronic cigarettes reduce health risk markers. clinicaltrials.gov/ct2/show/NCT03625986 (first received August 10 2018).
NCT03700112 {published data only}
- NCT03700112. Clinical study comparing 7 ENDS products and 1 combustible cigarette using 2 delivery methods. clinicaltrials.gov/ct2/show/NCT03700112 (first received 7 December 2018).
NCT03962660 {published data only}
- NCT03962660. Harm Reduction for Tobacco Smoking with support of Tobacco-Replacing Electronic Nicotine Delivery Systems (HaRTS-TRENDS). clinicaltrials.gov/ct2/show/record/NCT03962660 (first received 24 May 2019).
NCT04003805 {published data only}
- NCT04003805. Biomarkers of exposure and effect in standardized research e-cigarette (SREC) users. clinicaltrials.gov/ct2/show/NCT04003805 (first received July 1 2019).
NCT04058717 {published data only}
- NCT04058717. Low nicotine cigarettes plus electronic cigarettes [Randomized trial of low nicotine cigarettes plus electronic cigarettes in smokers]. clinicaltrials.gov/ct2/show/NCT04058717 (first received August 15 2019).
NCT04063267 {published data only}
- NCT04063267. Electronic cigarettes as a harm reduction strategy in individuals with substance use disorder. clinicaltrials.gov/ct2/show/NCT04063267 (first received 21 August 2019).
NCT04084210 {published data only}
- NCT04084210. Impact of alternative nicotine-delivery products on combustible cigarette use [Understanding the real-world impact of the use of three alternate nicotine-delivery products on combustible cigarette use]. clinicaltrials.gov/ct2/show/NCT04084210 (first received September 10 2019).
NCT04090879 {published data only}
- DeAtley T, Harrison A, Camacho B, Cassidy R, Kuo C, Higgins ST, et al. Subjective experiences, contexts, and risk perceptions of very low content cigarettes and electronic cigarettes among people with affective disorders who smoke. In: Society for Research on Nicotine and Tobacco Annual Meeting, 2022 March; Baltimore USA. 2022.
- NCT04090879. Low nicotine content cigarettes in vulnerable populations: affective disorders. clinicaltrials.gov/ct2/show/NCT04090879 (first received September 16 2019).
NCT04092101 {published data only}
- NCT04092101 . Low nicotine content cigarettes in vulnerable populations: opioid use disorder. clinicaltrials.gov/ct2/show/NCT04092101 (first received September 17 2019).
NCT04092387 {published data only}
- NCT04092387. Low nicotine content cigarettes in vulnerable populations: women of reproductive age. clinicaltrials.gov/ct2/show/NCT04092387 (first received September 17 2019).
NCT04218708 {published data only}
- NCT04218708. Electronic cigarettes as a harm reduction strategy among people living with HIV/AIDS. clinicaltrials.gov/ct2/show/NCT04218708 (first received January 6 2020).
NCT04238832 {published data only}
- NCT04238832. Salt-based e-cigarette and IQOS Study. clinicaltrials.gov/show/NCT04238832 (first received 18 January 2020).
NCT04452175 {published data only}
- NCT04452175. Cigarette consumption after switchinG to high or low nicotine strENght E-cigaretteS In Smokers With Schizophrenia (GENESIS). clinicaltrials.gov/show/NCT04452175 (first received 30 June 2020).
NCT04465318 {published data only}
- NCT04465318. Electronic cigarettes as a harm reduction strategy among patients with COPD. clinicaltrials.gov/ct2/show/NCT04465318 (first received July 10 2020).
- Stevens ER, Lei L, Cleland CM, Berger KM, Sherman SE, Vojjala M. Electronic cigarettes as a harm reduction strategy among patients with COPD: protocol for an open-label two arm randomized controlled pilot trial. Addiction Science & Clinical Practice 2022;17(1):2. [DOI: 10.1186/s13722-021-00284-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT04521647 {published data only}
- NCT04521647. Effects of menthol in e-cigarettes on smoking behaviors. clinicaltrials.gov/ct2/show/NCT04521647 (first received August 20 2020).
NCT04649645 {published data only}
- NCT04649645. International randomized controlled trial evaluating changes in oral health in smokers after switching to combustion-free nicotine delivery systems. clinicaltrials.gov/show/NCT04649645 (first received 02 December 2020).
NCT04708106 {published data only}
- NCT04708106. Characterization of product use in smokers switching from cigarettes to a RELX electronic nicotine delivery system. clinicaltrials.gov/ct2/show/NCT04708106 (first posted 13 January 2021).
NCT04709471 {published data only}
- NCT04709471. E-cigarette nicotine study. clinicaltrials.gov/ct2/show/NCT04709471 (first posted 14 January 2021).
NCT04725656 {published data only}
- NCT04725656. Concentration Impact Nicotine Salt (CINS). clinicaltrials.gov/ct2/show/NCT04725656 (first posted January 27 2021).
NCT04854616 {published data only}
- NCT04854616. Cessation of Smoking Trial in the Emergency Department (CoSTED). clinicaltrials.gov/ct2/show/NCT04854616 (date first received April 22 2021).
NCT04946825 {published data only}
- NCT04946825. Quit smoking study for people who use e-cigarettes [ A randomized controlled trial of smoking cessation treatment for young adult dual users of combustible and electronic cigarettes]. clinicaltrials.gov/ct2/show/NCT04946825 (first received July 1 2021).
NCT05023096 {published data only}
- NCT05023096. Potential effects of electronic nicotine delivery system flavor regulations on African American menthol smokers (RVA flavors) [Effects of electronic nicotine delivery system flavor regulations on tobacco behavior, toxicity, and abuse liability among African American menthol smokers]. clinicaltrials.gov/ct2/show/NCT05023096 (first received August 26 2021).
NCT05144542 {published data only}
- NCT05144542. Risk and benefits of electronic cigarettes to older smokers at high risk for lung cancer. www.clinicaltrials.gov/ct2/show/NCT05144542 (first received December 3 2021).
NCT05199480 {published data only}
- NCT05199480. Understanding the impact of cartridge-based electronic cigarettes and generated aerosols on cardiopulmonary health. clinicaltrials.gov/ct2/show/NCT05199480 (first received January 20 2022).
NCT05205811 {published data only}
- NCT05205811. The effects of combination zonisamide and bupropion on switching to an electronic cigarette [A randomized controlled trial to determine the effects of combination zonisamide and bupropion on switching to an electronic cigarette]. clinicaltrials.gov/ct2/show/NCT05205811 (first received January 25 2022).
NCT05206435 {published data only}
- NCT05206435. Methadone-maintained smokers switching to e-cigarettes (SHINE). clinicaltrials.gov/ct2/show/NCT05206435 (first received January 25 2022).
NCT05257629 {published data only}
- NCT05257629. Aggressive smoking cessation therapy post-acute coronary syndrome (ASAP) trial. www.clinicaltrials.gov/ct2/show/NCT05257629 (first received February 25 2022).
NCT05278065 {published data only}
- NCT05278065. E-cigarettes for harm reduction in adult with asthma (SWAP) [Complimentary electronic cigarettes for harm reduction among adult smokers with asthma]. clinicaltrials.gov/ct2/show/NCT05278065 (first received March 14 2022).
NCT05311085 {published data only}
- NCT05311085. Cytisine and e-cigarettes with supportive text-messaging for smoking cessation (Cess@Tion). clinicaltrials.gov/ct2/show/NCT05311085 (first received April 5 2022).
NCT05327439 {published data only}
- NCT05327439. Using alternative nicotine delivery systems (ANDS) to reduce harm for low SES cigarette smokers (Tri-PEC study). clinicaltrials.gov/ct2/show/NCT05327439 (first received April 14 2022).
Additional references
Akiyama 2021
- Akiyama Y, Sherwood N. Systematic review of biomarker findings from clinical studies of electronic cigarettes and heated tobacco products. Toxicology Reports 2021;8:282-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Amato 2020
- Amato L, Cruciani F, Solimini R, Barca A, Pacifici R, Davoli M. Effects of electronic cigarettes on health: a systematic review of the available evidence. Recenti Progressi in Medicina 2020;111(1):30-43. [DOI] [PubMed] [Google Scholar]
ASH 2019
- Action on Smoking and Health. Use of electronic cigarettes (vapourisers) among adults in Great Britain. ash.org.uk/information-and-resources/fact-sheets/statistical/use-of-e-cigarettes-among-adults-in-great-britain-2019/ (accessed 21 July 2020).
ASH 2022
- Action on Smoking and Health. Use of e-cigarettes (vapes) among adults in Great Britain. ash.org.uk/resources/view/use-of-e-cigarettes-among-adults-in-great-britain-2021 (accessed 23 September 2022).
Balfour 2004
- Balfour D. The neurobiology of tobacco dependence: a preclinical perspective on the role of dopamine projections to the nucleus. Nicotine & Tobacco Research 2004;6(6):899-912. [DOI] [PubMed] [Google Scholar]
Banks 2022
- Banks E, Yazidjoglou A, Brown S, Nguyen M, Martin M, Beckwith K. Electronic Cigarettes and Health Outcomes: Systematic Review of Global Evidence. National Centre for Epidemiology and Population Health, ANU, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barbeau 2013
- Barbeau AM, Burda J, Siegel M. Perceived efficacy of e-cigarettes versus nicotine replacement therapy among successful e-cigarette users: a qualitative approach. Addiction: Science & Clinical Practice 2013;8(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrett 2010
- Barrett SP. The effects of nicotine, denicotinized tobacco, and nicotine-containing tobacco on cigarette craving, withdrawal, and self-administration in male and female smokers. Behavorial Pharmacology 2010;21(2):144-52. [DOI] [PubMed] [Google Scholar]
Benowitz 2010
- Benowitz NL. Nicotine addiction. New England Journal of Medicine 2010;362(24):2295-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
Besaratinia 2019
- Besaratinia A, Tommasi S. Vaping: a growing global health concern. EClinicalMedicine 2019;17:100208. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bjurlin 2021
- Bjurlin MA, Matulewicz RS, Roberts TR, Dearing BA, Schatz D, Sherman S, et al. Carcinogen biomarkers in the urine of electronic cigarette users and implications for the development of bladder cancer: a systematic review. European Urology Oncology 2021;4(5):766-83. [DOI] [PubMed] [Google Scholar]
Blount 2020
- Blount BC, Karwowski MP, Shields PG, Morel-Espinosa M, Valentin-Blasini L, Gardner M, et al. Vitamin E acetate in bronchoalveolar-lavage fluid associated with EVALI. New England Journal of Medicine 2020;382(8):697-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooker 2019
- Brooker J, Synnot A, McDonald S, Elliott J, Turner T, et al. Guidance for the production and publication of Cochrane living systematic reviews: Cochrane Reviews in living mode;December 2019. community.cochrane.org/sites/default/files/uploads/inline-files/Transform/201912_LSR_Revised_Guidance.pdf (accessed 30 July 2020).
Butler 2022
- Butler AR, Lindson N, Fanshawe TR, Theodoulou A, Begh R, Hajek P, et al. Longer-term use of electronic cigarettes when provided as a stop smoking aid: systematic review with meta-analyses. Preventive Medicine 2022;EPub. [DOI: 10.1016/j.ypmed.2022.107182] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cahill 2016
- Cahill K, Lindson-Hawley N, Thomas K, Fanshawe TR, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 5. Art. No: CD006103. [DOI: 10.1002/14651858.CD006103.pub7] [DOI] [PMC free article] [PubMed] [Google Scholar]
CDC 2020
- Centres for Disease Control and Prevention. Outbreak of lung injury associated with the use of e-cigarette, or vaping, products. www.cdc.gov/tobacco/basic_information/e-cigarettes/severe-lung-disease.html#latest-outbreak-information Feb 2020 (accessed 12 Aug 2020).
Chan 2021
- Chan GCK, Stepanovic D, Lim C, Sun T, Shanmuga Anandan AS, Connor JP, et al. A systematic review of randomised controlled trials and network meta-analysis of e-cigarettes for smoking cessation. Addictive Behaviours 2021;119:106912. [DOI: 10.1016/j.addbeh.2021.106912] [DOI] [PubMed] [Google Scholar]
Chen 2016
- Chen C, Zhuang YL, Zhu SH. E-cigarette design preference and smoking cessation: a U.S. population study. American Journal of Preventive Medicine 2016;51(3):356-63. [DOI: 10.1016/j.amepre.2016.02.002] [DOI] [PMC free article] [PubMed]
Covidence [Computer program]
- Covidence. Version accessed 30 July 2020. Melbourne, Australia: Veritas Health Innovation, 2020. Available at covidence.org.
Cox 2017
- Cox S, Jakes S. Nicotine and e-cigarettes: rethinking addiction in the context of reduced harm. International Journal of Drug Policy 2017;44:84-5. [DOI] [PubMed] [Google Scholar]
Cox 2019b
- Cox S, Leigh NJ, Vanderbush TS, Choo E, Goniewicz ML, Dawkins L. An exploration into "do-it-yourself" (DIY) e-liquid mixing: sers' motivations, practices and product laboratory analysis. Addictive Behaviors Reports 2019;9:100151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dawkins 2013b
- Dawkins L, Turner J, Roberts A, Soar K. 'Vaping' profiles and preferences: an online survey of electronic cigarette users. Addiction 2013;108(6):1115-25. [DOI] [PubMed] [Google Scholar]
Dawkins 2016
- Dawkins LE, Kimber CF, Doig M, Feyerabend C, Corcoran O. Self-titration by experienced e-cigarette users: blood nicotine delivery and subjective effects. Psychopharmacology (Berl) 2016;233(14-16):2933-41. [DOI: 10.1007/s00213-016-4338-2] [DOI] [PubMed] [Google Scholar]
Dawkins 2018
- Dawkins L, Cox S, Goniewicz M, McRobbie H, Kimber C, Doig M, et al. 'Real-world' compensatory behaviour with low nicotine concentration e-liquid: subjective effects and nicotine, acrolein and formaldehyde exposure. Addiction 2018;113(10):1874-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donny 2007
- Donny EC, Houtsmuller E, Stitzer ML. Smoking in the absence of nicotine: behavioral, subjective and physiological effects over 11 days. Addiction 2007;102(2):324-34. [DOI] [PubMed] [Google Scholar]
Donny 2009
- Donny EC, Jones M. Prolonged exposure to denicotinized cigarettes with or without transdermal nicotine. Drug and Alcohol Dependence 2009;104(1-2):23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Donny 2015
- Donny EC, Denlinger RL, Tidey JW, Koopmeiners JS, Benowitz NL, Vandrey RG, et al. Randomized trial of reduced-nicotine standards for cigarettes. New England Journal of Medicine 2015;373(14):1340-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Du 2020
- Du Y, Liu B, Xu G, Rong S, Sun Y, Wu Y, et al. Association of electronic cigarette regulations with electronic cigarette use among adults in the United States. JAMA Network Open 2020;3(1):e1920255. [DOI] [PMC free article] [PubMed] [Google Scholar]
E‐cigarette ontology 2021
- Anonymous. E-cigarette v1. www.qeios.com/read/0WX80U (accessed prior to 3 November 2022). [URL: www.qeios.com/read/0WX80U]
Etter 2011
- Etter JF, Bullen C. Electronic cigarette: users profile, utilization, satisfaction and perceived efficacy. Addiction 2011;106(11):2017-28. [DOI] [PubMed] [Google Scholar]
European Parliament 2014
- European Parliament and Council of the European Union. Directive 2014/40/EU of the European Parliament and of the Council of 3 April 2014 on the approximation of the laws, regulations and administrative provisions of the Member States concerning the manufacture, presentation and sale of tobacco and related products and repealing Directive 2001/37/EC. EUR-Lex - 32014L0040 - EN - EUR-Lex (europa.eu) 2014 (accessed 25th November 2014).
Farsalinos 2014
- Farsalinos KE, Polosa R. Safety evaluation and risk assessment of electronic cigarettes as tobacco cigarette substitutes: a systematic review. Therapeutic Advances in Drug Safety 2014;5(2):67-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Goniewicz 2012
- Goniewicz ML, Kuma T, Gawron M, Knysak J, Kosmider L. Nicotine levels in electronic cigarettes. Nicotine & Tobacco Research 2012;15(1):158-66. [DOI] [PubMed] [Google Scholar]
Goniewicz 2014
- Goniewicz ML, Hajek P, McRobbie H. Nicotine content of electronic cigarettes, its release in vapour and its consistency across batches: regulatory implications. Addiction 2014;109(3):500-7. [DOI] [PubMed] [Google Scholar]
Grabovac 2021
- Grabovac I, Oberndorfer M, Fischer J, Wiesinger W, Haider S, Dorner TE. Effectiveness of electronic cigarettes in smoking cessation: a systematic review and meta-analysis. Nicotine & Tobacco Research 2021;23(4):625-34. [DOI: 10.1093/ntr/ntaa181] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADEpro GDT. Version accessed 30 July 2020. Hamilton (ON): McMaster University (developed by Evidence Prime), 2020. Available at gradepro.org.
Gugala 2022
- Gugala E, Okoh CM, Ghosh S, Moczygemba LR. Pulmonary health effects of electronic cigarettes: a scoping review. Health Promotion Practice 2022;23(3):388-96. [DOI] [PubMed] [Google Scholar]
Hajek 1999
- Hajek P, West R, Foulds J, Nilsson F, Burrows S, Meadow A. Randomized comparative trial of nicotine polacrilex, a transdermal patch, nasal spray, and an inhaler. Archives of Internal Medicine 1999;159(17):2033-8. [DOI] [PubMed] [Google Scholar]
Hajek 2014
- Hajek P, Etter J-F, Benowitz N, Eissenberg T, McRobbie H. Electronic cigarettes: review of use, content, safety, effects on smokers and potential for harm and benefit. Addiction 2014;109(11):1801-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2015b
- Hajek P, Goniewicz ML, Phillips A, Myers Smith K, West O, McRobbie H. Nicotine intake from electronic cigarettes on initial use and after 4 weeks of regular use. Nicotine & Tobacco Research 2015;17(2):175-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2017
- Hajek P, Przulj D, Phillips A, Anderson R, McRobbie H. Nicotine delivery to users from cigarettes and from different types of e-cigarettes. Psychopharmacology (Berl) 2017;234(5):773-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hajek 2020
- Hajek P, Pittaccio K, Pesola F, Myers Smith K, Phillips-Waller A, Przulj D. Nicotine delivery and users' reactions to Juul compared with cigarettes and other e-cigarette products. Addiction 2020;115(6):1141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hall 2020
Hartmann‐Boyce 2018a
- Hartmann-Boyce J, Chepkin SC, Ye W, Bullen C, Lancaster T. Nicotine replacement therapy versus control for smoking cessation. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No: CD000146. [DOI: 10.1002/14651858.CD000146.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2018b
- Hartmann-Boyce J, Fanshawe TR, Lindson N, Livingstone-Banks J, Ordonez-Mena J, Aveyard P. Behavioural interventions for smoking cessation: an overview and network meta-analysis. Cochrane Database of Systematic Reviews 2018, Issue 12. Art. No: CD013229. [DOI: 10.1002/14651858.CD013229] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2019
- Hartmann-Boyce J, Hong B, Livingstone-Banks J, Wheat H, Fanshawe TR. Additional behavioural support as an adjunct to pharmacotherapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 6. Art. No: CD009670. [DOI: 10.1002/14651858.CD009670.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2022
- Hartmann-Boyce J, Butler AR, Theodoulou A, Onakpoya IJ, Hajek P, Bullen C, et al. Biomarkers of potential harm in people switching from smoking tobacco to exclusive e-cigarette use, dual use, or abstinence: secondary analysis of Cochrane systematic review of trials of e-cigarettes for smoking cessation. Addiction 2022:1-7. [DOI: 10.1111/add.16063] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartnett 2020
- Hartnett KP, Kite-Powell A, Patel MT, Haag BL, Sheppard MJ, Dias TP, et al. Syndromic surveillance for e-cigarette, or vaping, product use-associated lung injury. New England Journal of Medicine 2020;382(8):766-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2021
- Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al (editors). Cochrane Handbook for Systematic Reviews of Interventions version 6.2 (updated February 2021). The Cochrane Collaboration, 2021. Available from www.training.cochrane.org/handbook.
Hitchman 2015
- Hitchman SC, Brose LS, Brown J, Robson D, McNeill A. Associations between e-cigarette type, frequency of use, and quitting smoking: findings from a longitudinal online panel survey in Great Britain. Nicotine & Tobacco Research 2015;17(10):1187-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2019
- Huang J, Duan Z, Kwok J, Binns S, Vera LE, Kim Y, et al. Vaping versus JUULing: how the extraordinary growth and marketing of JUUL transformed the US retail e-cigarette market. Tobacco Control 2019;28(2):146-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2004
- Hughes JR, Keely J, Naud S. Shape of the relapse curve and long-term abstinence among untreated smokers. Addiction 2004;99(1):29-38. [DOI] [PubMed] [Google Scholar]
Jiang 2016
- Jiang N, Chen J, Wang MP, McGhee SM, Kwong AC, Lai VW, et al. Electronic cigarette awareness and use among adults in Hong Kong. Addictive Behaviors 2016;52:34-8. [DOI] [PubMed] [Google Scholar]
Levin 1993
- Levin ED, Behm F, Carnahan E, LeClair R, Shipley R, Rose JE. Clinical trials using ascorbic acid aerosol to aid smoking cessation. Drug and Alcohol Dependence 1993;33(3):211-23. [DOI] [PubMed] [Google Scholar]
Lindson 2019
- Lindson N, Chepkin SC, Ye W, Fanshawe TR, Bullen C, Hartmann-Boyce J. Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation. Cochrane Database of Systematic Reviews 2019, Issue 4. Art. No: CD013308. [DOI: 10.1002/14651858.CD013308] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindson 2022a
- Lindson N, Theodoulou A, Livingstone-Banks J, Aveyard P, Fanshawe TR, Ordóñez-Mena JM, et al. Pharmacological and electronic cigarette interventions for smoking cessation in adults: component network meta‐analyses. Cochrane Database of Systematic Reviews 2022, Issue 3. Art. No: CD015226. [DOI: 10.1002/14651858.CD015226] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lindson 2022b
- Lindson N, Butler AR, Liber A, Levy D, Barnett P, Theodoulou A, et al. An exploration of flavours in studies of e-cigarettes for smoking cessation: secondary analyses of a systematic review with meta-analyses. Addiction 2022;online first:Nov 18. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Martinez‐Morata 2021
- Martinez-Morata I, Sanchez TR, Shimbo D, Navas-Acien A. Electronic cigarette use and blood pressure endpoints: a systematic review. Current Hypertension Reports 2021;23(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
McDonald 2020
- McDonald CF, Jones S, Beckert L, Bonevski B, Buchanan T, Bozier J, et al. Electronic cigarettes: a position statement from the Thoracic Society of Australia and New Zealand. Respirology 2020 [Epub ahead of print]. [DOI] [PMC free article] [PubMed]
McNeill 2021
- McNeill A, Brose LS, Calder R, Bauld L, Robson D. Vaping in England: an evidence update including vaping for smoking cessation, February 2021: a report commissioned by Public Health England London, UK: Public Health England. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/962221/Vaping_in_England_evidence_update_February_2021.pdf (accessed prior to 3 November 2022).
McNeill 2022
- McNeill A, Simonavičius E, Brose LS, Taylor E, East K, Zuikova E, et al. Nicotine vaping in England: an evidence update including health risks and perceptions. A report commissioned by the Office for Health Improvement and Disparities. London: Office for Health Improvement and Disparities. assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1107701/Nicotine-vaping-in-England-2022-report.pdf (accessed prior to 3 November 2022).
McRobbie 2016
- McRobbie H, Przulj D, Smith KM, Cornwall D. Complementing the standard multicomponent treatment for smokers with denicotinized cigarettes: a randomized trial. Nicotine & Tobacco Research 2016;18(5):1134-41. [DOI] [PubMed] [Google Scholar]
NASEM 2018
- National Academies of Sciences, Engineering, and Medicine, Health and Medicine Division, Board on Population Health and Public Health Practice. Public Health Consequences of E-Cigarettes. Washington (DC): National Academies Press (US), 2018. [Google Scholar]
Notley 2018a
- Notley C, Colllins R. Redefining smoking relapse as recovered social identity - secondary qualitative analysis of relapse narratives. Journal of Substance Use 2018;23(6):660-6. [Google Scholar]
Notley 2018b
- Notley C, Ward E, Dawkins L, Holland R. The unique contribution of e-cigarettes for tobacco harm reduction in supporting smoking relapse prevention. Harm Reduct J 2018;15(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Palipudi 2016
- Palipudi KM, Mbulo L, Morton J, Bunnell R, Blutcher-Nelson G, Kosen S, et al. Awareness and current use of electronic cigarettes in Indonesia, Malaysia, Qatar, and Greece: findings from 2011–2013 global adult tobacco surveys. Nicotine & Tobacco Research 2016;18(4):501-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Patnode 2021
- Patnode CD, Henderson JT, Coppola EL, Melnikow J, Durbin S, Thomas RG. Interventions for tobacco cessation in adults, including pregnant persons: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2021;325(3):280-98. [DOI] [PubMed] [Google Scholar]
Perkins 2010
- Perkins KA, Karelitz JL, Conklin CA, Sayette MA, Giedgowd GE. Acute negative affect relief from smoking depends on the affect situation and measure but not on nicotine. Biological Psychology 2010;67(8):707-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pickworth 1999
- Pickworth WB, Fant RV, Nelson RA, Rohrer MS, Henningfield JE. Pharmacodynamic effects of new de-nicotinized cigarettes. Nicotine & Tobacco Research 1999;1(4):357-64. [DOI] [PubMed] [Google Scholar]
Pound 2021
- Pound CM, Zhang JZ, Kodua AT, Sampson M. Smoking cessation in individuals who use vaping as compared with traditional nicotine replacement therapies: a systematic review and meta-analysis. BMJ Open 2021;11(2):e044222. [DOI: 10.1136/bmjopen-2020-044222] [DOI] [PMC free article] [PubMed] [Google Scholar]
Przulj 2013
- Przulj D, McRobbie H, Hajek P. The effect of sensorimotor replacement on smoking cessation and craving. Open Addiction Journal 2013;5:41-50. [Google Scholar]
Quigley 2021
- Quigley JM, Walsh C, Lee C, Long J, Kennelly H, McCarthy A, et al. Efficacy and safety of electronic cigarettes as a smoking cessation intervention: a systematic review and network meta-analysis. Tobacco Prevention & Cessation 2021;7(69):1-14. [DOI: 10.18332/tpc/143077] [DOI] [PMC free article] [PubMed] [Google Scholar]
RCP 2016
- Tobacco Advisory Group of the Royal College of Physicians. Nicotine Without Smoke—Tobacco Harm Reduction. London: Royal College of Physicians, 2016. [Google Scholar]
Review Manager 2020 [Computer program]
- Review Manager 5 (RevMan 5). Version 5.4. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2020.
Romeu 2020
- Romeu ER. Voke® nicotine inhaler for smoking cessation and reduction. ntag.nhs.uk/docs/app/NTAG-Apprasial-Voke-nicotine-inhalator-Feb-2020-final-for-web.pdf 2020.
Rose 1985
- Rose JE, Tashkin DP, Ertle A, Zinser MC, Lafer R. Sensory blockade of smoking satisfaction. Pharmacology Biochemistry & Behavior 1985;23(2):289-93. [DOI] [PubMed] [Google Scholar]
Rose 1993
- Rose JE, Behm FM, Levin ED. Role of nicotine dose and sensory cues in the regulation of smoke intake. Pharmacology Biochemistry & Behavior 1993;44(4):891-900. [DOI] [PubMed] [Google Scholar]
Rose 1994
- Rose JE, Behm FM. Inhalation of vapor from black pepper extract reduces smoking withdrawal symptoms. Drug and Alcohol Dependence 1994;34(3):225-9. [DOI] [PubMed] [Google Scholar]
Rose 2000
- Rose JE, Behm FM, Westman EC, Johnson M. Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacology Biochemistry & Behavior 2000;67(1):71-81. [DOI] [PubMed] [Google Scholar]
Rose 2006
- Rose JE. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 2006;184(3-4):274-85. [DOI] [PubMed] [Google Scholar]
Schneider 2001
- Schneider NG, Olmstead RE, Franzon MA, Lunell E. The nicotine inhaler: clinical pharmacokinetics and comparison with other nicotine treatments. Clinical Pharmcokinetics 2001;40(9):661-84. [DOI] [PubMed] [Google Scholar]
Smets 2019
- Smets J, Baeyens F, Chaumont M, Adriaens K, Van Gucht D. When less is more: vaping low-nicotine vs. high-nicotine e-liquid is compensated by increased wattage and higher liquid consumption. International Journal of Environmental Research and Public Health 2019;16(5):723. [DOI] [PMC free article] [PubMed] [Google Scholar]
Talih 2020
- Talih S, Salman R, El-Hage R, Karam E, Salam S, Karaoghlanian N, et al. A comparison of the electrical characteristics, liquid composition, and toxicant emissions of JUUL USA and JUUL UK e-cigarettes. Scientific Reports 2020;10(1):7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tattan‐Birch 2020
- Tattan-Birch H, Brown J, Shahab L, Jackson SE. Association of the US outbreak of vaping-associated lung injury with perceived harm of e-cigarettes compared with cigarettes. JAMA Network Open 2020;3(6):e206981. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tattan‐Birch 2022
- Tattan-Birch H, Jackson SE, Kock L, Dockrell M, Brown J. Rapid growth in disposable e-cigarette vaping among young adults in Great Britain from 2021 to 2022: a repeat cross-sectional survey. Addiction 2022:1-5. [DOI: 10.1111/add.16044] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2022
- Thomas KH, Dalili MN, López‐López JA, Keeney E, Phillippo DM, Munafò MR, et al . Comparative clinical effectiveness and safety of tobacco cessation pharmacotherapies and electronic cigarettes: a systematic review and network meta‐analysis of randomized controlled trials. Addiction 2022;117(4):861-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
USPFTS 2021
Vanderkam 2022
- Vanderkam P, Bonneau A, Kinouani S, Dzeraviashka P, Castera P, Besnier M, et al. Duration of the effectiveness of nicotine electronic cigarettes on smoking cessation and reduction: systematic review and meta-analysis. Front Psychiatry 2022;4(13):915946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 2012
- Walker N, Howe C, Bullen C, Grigg M, Glover M, McRobbie H, et al. The combined effect of very low nicotine content cigarettes, used as an adjunct to usual Quitline care (nicotine replacement therapy and behavioural support), on smoking cessation: a randomized controlled trial. Addiction 2012;107(10):1857-67. [DOI] [PubMed] [Google Scholar]
Wang 2021
- Wang RJ, Bhadriraju S, Glantz SA. E-cigarette use and adult cigarette smoking cessation: a meta-analysis. American Journal of Public Health 2021;111:230-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Westman 1995
- Westman EC, Behm FM, Rose JE. Airway sensory replacement combined with nicotine replacement for smoking cessation. A randomized, placebo-controlled trial using a citric acid inhaler. Chest 1995;107(5):1358-64. [DOI] [PubMed] [Google Scholar]
Westman 1996
- Westman EC, Behm FM, Rose JE. Dissociating the nicotine and airway sensory effects of smoking. Pharmacology Biochemistry & Behavior 1996;53(2):309-15. [DOI] [PubMed] [Google Scholar]
Wilson 2021
- Wilson N, Summers JA, Ait Ouakrim D, Hoek J, Edwards R, Blakely T. Improving on estimates of the potential relative harm to health from using modern ENDS (vaping) compared to tobacco smoking. BMC Public Health 2021;21(1):2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yingst 2019a
- Yingst JM, Foulds J, Veldheer S, Hrabovsky S, Trushin N, Eissenberg TT, et al. Nicotine absorption during electronic cigarette use among regular users. PLOS One 2019;14(7):e0220300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yingst 2019b
- Yingst JM, Hrabovsky S, Hobkirk A, Trushin N, Richie JP Jr, Foulds J. Nicotine absorption profile among regular users of a pod-based electronic nicotine delivery system. JAMA Network Open 2019;2(11):e1915494. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zhang 2021
- Zhang YY, Bu FL, Dong F, Wang JH, Zhu SJ, Zhang XW, et al. The effect of e-cigarettes on smoking cessation and cigarette smoking initiation: an evidence-based rapid review and meta-analysis. Tobacco Induced Diseases 2021;19:04. [DOI: 10.18332/tid/131624] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hartmann‐Boyce 2016
- Hartmann-Boyce J, McRobbie H, Bullen C, Begh R, Stead LF, Hajek P. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2016, Issue 9. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2020
- Hartmann-Boyce J, McRobbie H, Lindson N, Bullen C, Begh R, Theodoulou A, et al. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2020, Issue 10. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartmann‐Boyce 2021
- Hartmann-Boyce J, McRobbie H, Butler AR, Lindson N, Bullen C, Begh R, et al. Electronic cigarettes for smoking cessation. Cochrane Database of Systematic Reviews 2021, Issue 4. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
McRobbie 2012
- McRobbie H, Bullen C, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews 2012, Issue 11. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216] [DOI] [PubMed] [Google Scholar]
McRobbie 2014
- McRobbie H, Bullen C, Hartmann-Boyce J, Hajek P. Electronic cigarettes for smoking cessation and reduction. Cochrane Database of Systematic Reviews 2014, Issue 12. Art. No: CD010216. [DOI: 10.1002/14651858.CD010216.pub2] [DOI] [PubMed] [Google Scholar]


