Abstract
Glycoconjugates are major constituents of mammalian cells that are formed via covalent conjugation of carbohydrates to other biomolecules like proteins and lipids and often are expressed on the cell surfaces. Among the three major classes of glycoconjugates, proteoglycans and glycoproteins contain glycans linked to the protein backbone via amino acid residues such as Asn for N-linked glycans and Ser/Thr for O-linked glycans. In glycolipids, glycans are linked to a lipid component such as glycerol, polyisoprenyl pyrophosphate, fatty acid ester, or sphingolipid. Recently, glycoconjugates have become better structurally defined and biosynthetically understood, especially those associated with human diseases, and are accessible to new drug, diagnostic, and therapeutic developments. This review describes the status and new advances in the biological study and therapeutic application of natural and synthetic glycoconjugates, including proteoglycans, glycoproteins, and glycolipids. The scope, limitations, and novel methodologies in the synthesis and clinical development of glycoconjugates including vaccines, glyco-remodeled antibodies, glycan-based adjuvants, glycan-specific receptor-mediated drug delivery platforms, etc., and their future prospectus are discussed.
Graphical Abstract
1. INTRODUCTION
1.1. Glycoconjugates in Biological Systems
Carbohydrates are often attached to proteins or lipids to form glycoproteins or glycolipids on the cell surfaces.1–3 Because of their structural heterogeneity and functional complexity, the roles of glycans in biology have historically been underexplored compared with other biomolecules.4,5 Carbohydrates can be conjugated to proteins or lipids to form the adduct as glycoprotein bearing N-/O-linked phosphorylated glycans, glycosaminoglycans (GAGs), and glycosylphosphatidylinositol (GPI) anchors, or as glycolipid such as glycosphingolipids (GSLs) (Figure 1).3,5–8 Oligosaccharides are formed by linking one sugar unit to another via a glycosidic bond between the hydroxyl group of one sugar and the anomeric carbon of another in α or β stereochemistry that led to tremendous diversity.9 In addition, each monosaccharide can be linked at any of the several positions with another monosaccharide, allowing for a staggering variety in connectivity and branching. Finally, the glycans can be further modified, for example, through selective oxidation, acetylation, phosphorylation, and sulfation, which greatly expand the diversity and complexity, to generate biological information that is read out by receptors on other cells.10–12
Figure 1:
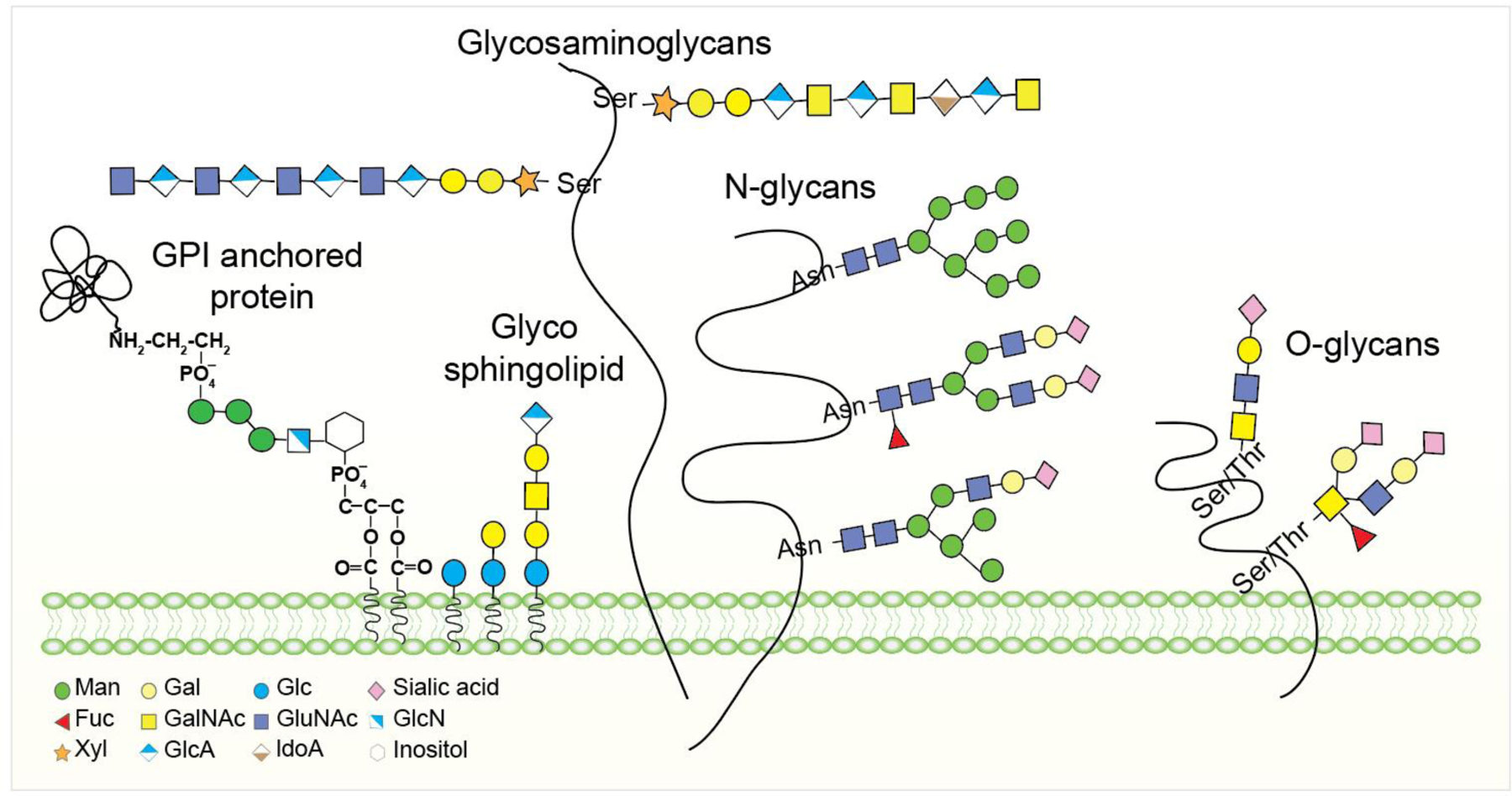
Cell surface glycoconjugates in living systems. Membrane carbohydrates are linked to glycolipids and glycoproteins. Proteoglycans have part of their amino acid sequences inserted among lipid chains.
Protein glycosylation begins in the endoplasmic reticulum (ER) and Golgi apparatus, while the final processing occurs in the cis-, medial-, and trans-Golgi compartments.8,13 During the synthesis of N-glycoprotein, an oligosaccharide is first assembled on dolichol pyrophosphate (Dol-PP) then transferred to the Asn residue of a Asn-X-Ser/Thr sequon where the X residue is not proline, or to the aromatic sequon (Phe/Trp-X-Asn-X-Ser/Thr).14 Next, the enzymatic processing of N-glycans by differentially expressed glycosidases and glycosyltransferases (GTs) occur in the ER and Golgi.13 This glycosylation process is very complex and cell-dependent, so the N-glycans of a mature glycoprotein expressed in different cells are very heterogeneous and have different patterns and compositions. The O-glycosylation process begins in the late ER or early Golgi that also produces heterogeneous glycoforms with an enormous structural complexity.15 Most of the O-glycoproteins carry glycans initiated by N-acetylglucosamine (GlcNAc) or N-acetyl galactosamine (GalNAc) linked to Ser or Thr residue.16,17 Mucins are the glycoproteins carrying numbers of O-GalNAc glycans, known as mucin-type O-glycans. The O-GalNAc glycans generally consist of GalNAc, galactose (Gal), GlcNAc, fucose (Fuc), and sialic acid (Neu5Ac), whereas mannose (Man), glucose (Glc), or xylose (Xyl) residue is not present.18 Sialic acid may be further modified by O-acetylation, and oxidation at the N-acetyl group to the glycolyl group, and sulfation can occur on Gal and GlcNAc residues.19 O-GalNAc glycans may vary in length from a single GalNAc to more than 20 sugar residues including blood group and other glycan epitopes.20
GAGs are O-linked glycans with huge structural diversity.21 Proteoglycans contain chains of GAGs linked to a serine residue of proteins through the glucuronic acid (GlcA) containing tetrasaccharide (GlcA)β1–3Galβ1–3Galβ1–4Xyl, except for keratan sulfate, which is linked to N- and O-glycans. GAGs are longer polysaccharides containing repeating units of the disaccharide with GalNAc or GlcNAc linked to GlcA or Gal.22 Based on the structure of the disaccharide, GAGs are subdivided into three types: (a) dermatan sulfate (DS) and chondroitin sulfate (CS) (GlcA-GalNAc), (b) heparin/heparan sulfate (HS) (GlcA-GlcNAc), and (c) keratan sulfate (KS) (Gal-GlcNAc).23 In DS and heparin/HS, GlcA can be present as the epimerized form of iduronic acid. The additional heterogeneity of GAGs arises from variable O-sulfations, including, for example, uniform sulfation in heparin or specific sulfation in heparin sulfate (HS). Deacetylation and N-sulfation of GlcNAc can also occur in heparin or HS.24
GSLs are a subclass of glycolipids, consisting of carbohydrate moieties linked to the 1-hydroxyl group of a ceramide backbone via a β-linkage.25 GSLs are ubiquitously embedded in the cell plasma membrane. The astonishing structural diversity of GSLs arises from linking hundreds of different glycan heads to tens of different ceramide chains, which are responsible for various biological activities, like regulation of cell growth, differentiation, and signaling.26 Among mammalian GSLs, 90% of GSLs are derived from glucosyl ceramide (GlcCer) and the rest are from galactosyl ceramide (GalCer). The GalCer series GSLs are composed of GalCer itself, 3-O-sulfate ester, sulfatide (sulfogalactosyl ceramide), and galabiosyl ceramide. The GlcCer series GSLs are further subclassified into gangliosides (GalNAc-β1,4-Gal), globosides (Gal-β1,4-Gal), lactosides (Gal-β1,3-GlcNAc-β1,3-Gal), and neolacto (Gal-β1,4-GlcNAc-β 1,3-Gal) series GSLs (Figure 2).27
Figure 2:
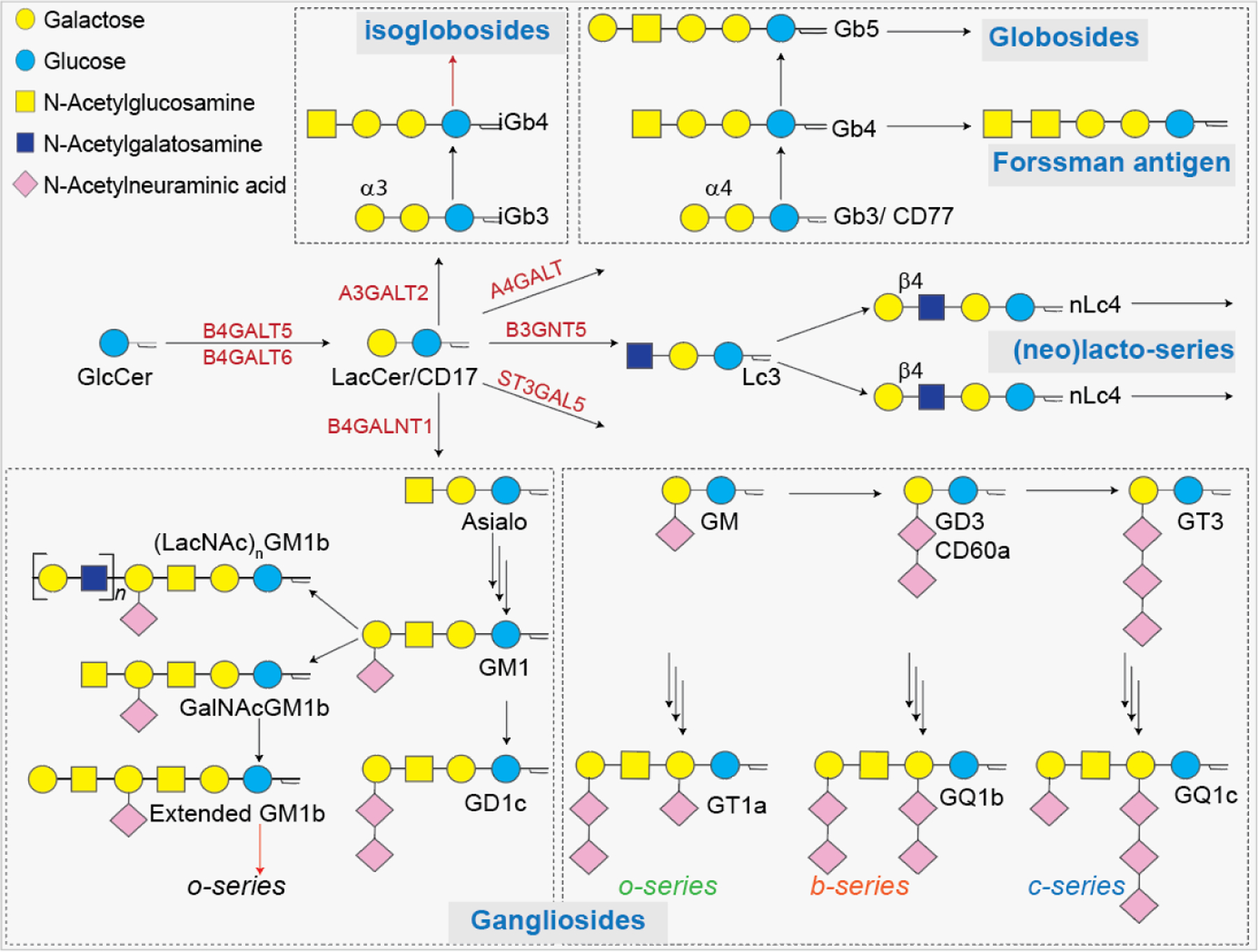
Schematic presentation of diverse types of GSLs. Major GSLs expressed in immune cells and proposed GSL biosynthetic pathways.
In the Golgi apparatus, various Golgi-resident GTs transfer a specific sugar residue to specific acceptors such as a ceramide the oligosaccharide on ceramide.26 Lactosyl ceramide (LacCer), which is the branching point for the synthesis of GlcCer series GSLs, is generated from GlcCer, after it is translocated to the luminal leaflet of Golgi by β1,4-galactosyl transferases 5/6 (B4GALT5/6).28 Once produced, the LacCer cannot be translocated back to the cytosolic phase of cell membranes. Galactose can be added to the 4-O or 3-O position of LacCer as α-galactoside, resulting in the synthesis of the globo-series Gb3 or isoglobo-series iGb3. For the synthesis of lacto-series GSLs, the enzyme β1,3-N-acetylglucosaminyltransferase 5 (B3GNT5) catalyzes the addition of GlcNAc to LacCer to form intermediate Lc3,29 whereas, in the case of the ganglio-series GSLs, β1,4-GalNAc transferase (B4GALNT1) catalyzes the addition of GalNAc residue to the hydroxy group of galactose on LacCer to form asialo-GM1. In the synthesis of o-, b-, and c-series gangliosides, GM3 is the key intermediate that is formed by α 2,3-sialyltransferase 5 (ST3GAL5) catalyzed addition of sialic acid to LacCer (Figure 2).30 The precursors such as Gb3, iGb3, Lc3, sialo-GM2, and GM3 are modified by various GTs to generate GSLs as asialo-, ganglio-, globo-, isoglobo-, lacto-, and neolacto-series. In the case of GalCer series GSLs, the precursor GalCer is transported to the Golgi complex where it can be sialylated to produce GM4 ganglioside or sulfated to produce sulfogalactolipids.25
Glycosylphosphatidylinositol (GPI) often links to a glycan and acts as an anchor for a variety of cell surface proteins31 as part of a post-translational protein modification process and is present in diverse eukaryotic species.32,33 The GPI anchor consists of a phosphoethanolamine moiety linked to the terminal mannose of a highly conserved core glycan Manα1–2Manα1–6Manα1–4GlcNH2α1–6myo-inositol, and the phospholipid tail (Figure 1).34 The GPI-anchored protein is linked to the amine group of the phosphoethanolamine moiety on the glycan core, while the phospholipid tail inserts into the cell membrane. The GPI anchored protein synthesized in the ER first moved to the lumen where the coupling of protein C-terminal to GPI anchor and insertion of phosphatidylinositol into the lipid membrane takes place.35,36 During the maturation process, the Man3 glycan on GPI anchored protein may undergo further glycosylations37 and involved in many functions such as protein transportation, signal transduction, cell adhesion, and protection.38
1.2. Biological Significance of Glycoconjugates
In eukaryotes, glycolipids and glycoproteins are anchored to the cell membrane, while proteoglycans cover the extracellular matrix that leads to the highly glycosylated environment essential for intracellular signaling.12,14,39 Therefore, cell surface glycans engage in numerous aspects of cell–cell interactions with self-molecules and invaded pathogens (Figure 3a).40,41
Figure 3:
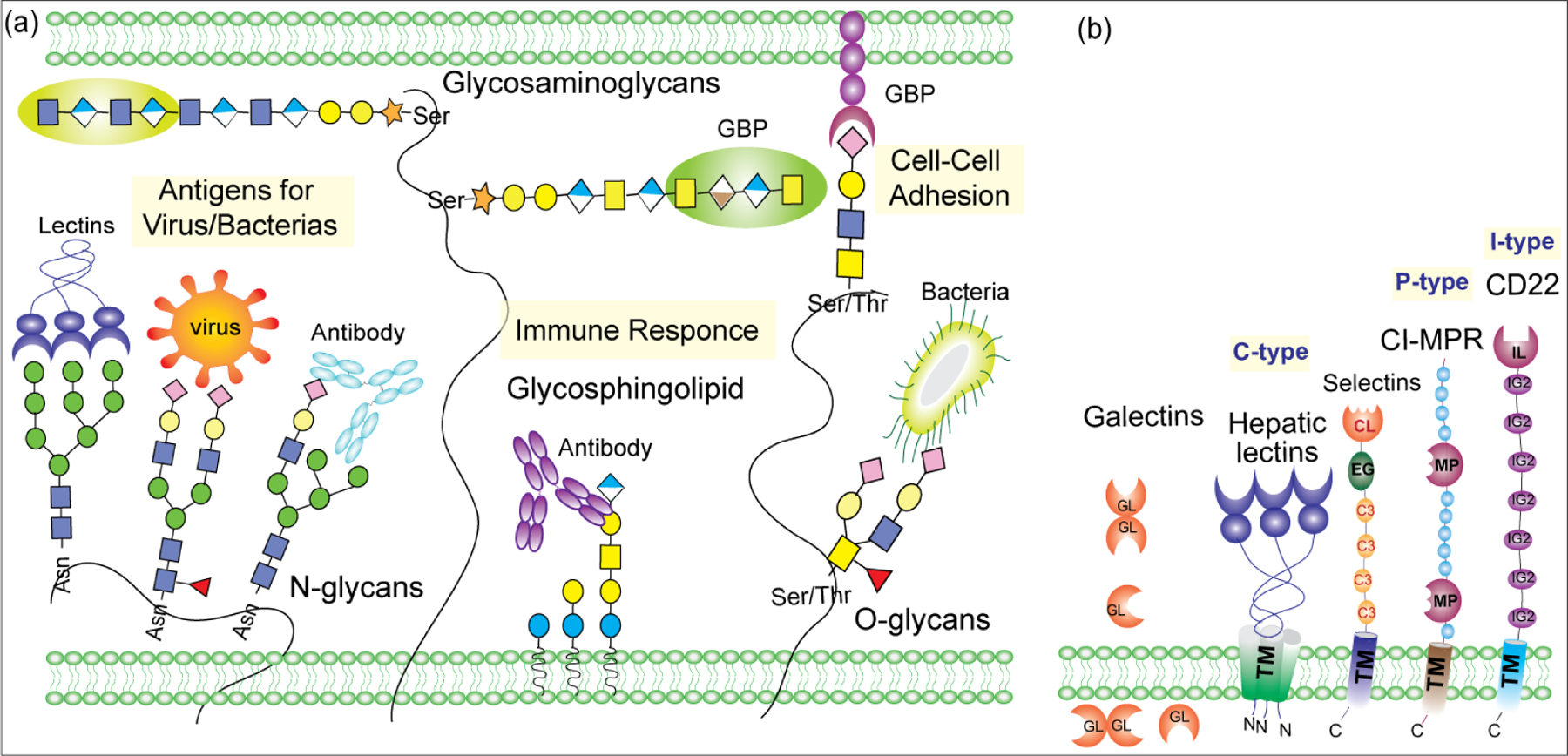
a) Role of glycoconjugates in biological processes. b) Schematic of overall structure of C-type, S-type, P-type, and I-type lectins. The number of domains accompanying the CRDs varies among family members.
Glycan-binding proteins (GBPs) are classified into: lectins and sulfated GAG-binding proteins.42 Animal lectins include the C-type, P-type, S-type, and I-type lectins, differing in the affinity of their carbohydrate recognition domain (CRDs) toward the glycan residues such as Man, Gal, GalNAc, GlcNAc, Fuc, and Neu5Ac. CRDs can exist as proteins in monomeric form or as a multidomain protein. GBPs containing specific CRDs that confers glycan-binding specificity often recognize complementary glycans on cell surfaces.43 The first discovered animal lectin, asialo-glycoprotein receptor (ASGPR), is involved in the rapid clearance of asialo-glycoproteins with exposed Gal residues by liver via a cell surface receptor that recognizes terminal Gal or GalNAc in β-linkage.44 Other examples of glycan-specific receptors involved in glycoprotein clearance include mannose 6-phosphate receptor (CI-MPR) and mannose receptors (MR or CD206) that removes glycoproteins with terminal Man, Fuc, and GlcNAc residue from the circulation.45
Upon glycan binding, the other functional domains on GBPs translate the binding into appropriate downstream signaling.43 Some of the classical examples include glycans and GBPs on the surfaces of immune cells involved in immune activation, deactivation, pathogen recognition, and regulation within a dynamic pathogen landscape.46,47 Extracellular carbohydrates and GBPs interact with certain molecules in the matrix or the adjacent glycan to regulate intracellular signaling.48 GBPs on the surface of immune cells are involved in modulation of leukocyte trafficking, pathogen recognition, antigen processing, and immune regulation.47,49 For example, surface glycoproteins and glycolipids on immune cells and GBPs and other molecules can help the immune system to sense environmental changes.50
Many receptors present on immune cells interact with glycan-containing molecules on pathogens51 such as bacterial lipopolysaccharides (LPS), capsular polysaccharides, mannans on fungal surfaces, and peptidoglycans. These glycan-based epitopes on microbial surfaces have been utilized in vaccine design.52,53
1.2.1. Role of Glycoconjugates in Immune Regulation.
In the adaptive immune system, the interaction of specific cell surface glycan ligands with extracellular or secreted proteins, like siglecs, galectins, selectins, CD43, and CD45, plays a critical role in B- and T-cell differentiation.54,55 The glycoproteins CD43 and CD45 that are highly expressed on the surface of B/T cells contain both O- and N-glycans which are important for the modulation of cell motility, downstream signaling, cell survival, and apoptosis.56,57 The interactions between CD43 or CD45 with their ligands are thus greatly influenced by the glycosylation pattern.
Galectins.
Galectins are carbohydrate-binding proteins that contain a conserved CRD specific for β-galactosides including galactose, lactose, poly lactosamine, and N-acetyl-lactosamine (LacNAc).58 Structurally, galectins have a conserved CRDs, with a six-stranded β-sheet that binds β-galactosides and a five-stranded β-sheet.59 Galectins in the extracellular domain recognize the galactosylated oligosaccharides in a bi- or multivalent manner, resulting in cross-linking of cell surface glycoconjugates and activation of programmed cell death, cytokine production, cell adhesion, and migration.60 The glycan binding specificities of galectins and their occurrence on immune cells are summarized in Table 1.61 Both intracellular and cell-surface galectins are associated with many cellular functions including cancer metastasis, immune response, and cell death.62–64 The binding affinity of galectins is dependent on glycan structure that regulates the signals induced by galectin binding. For example, terminal sialylation affects galectin binding, α2,6-sialic acid capping prevents binding to galectin-2, whereas galectin-1 binds α2, 3- but not α2,6-sialylated glycans, and galectin-3 binds to some glycans terminating with either α2,3- or α2,6-sialic acid.65 The functional role of galectins in regulating various biological activities has been used as a promising therapeutic option for treatment of inflammatory diseases and cancers. For example, the interaction between galectin-3 binding protein and galectin-1 is important for breast cancer metastasis, Galectin-8 N-domain binds α2,3-sialylated galactoside while the C-domain binds galactosides, and numerous galectin antagonists are currently under clinical evaluation.66–68 Galectin Therapeutics developed Belapectin (GR-MD-02), a new polysaccharide comprising of galacturonic acid, galactose, arabinose, rhamnose, and smaller amounts of other sugars, as an inhibitor of galectin-1 and −3 for treatment of NASH cirrhosis. In addition, Belapectin in combination with Keytruda is in advanced clinical trials for treating metastatic melanoma and head and neck cancer.69–72
Table 1:
Subsets of galectins and siglecs, their cell type expression and preferred natural glycan ligands.
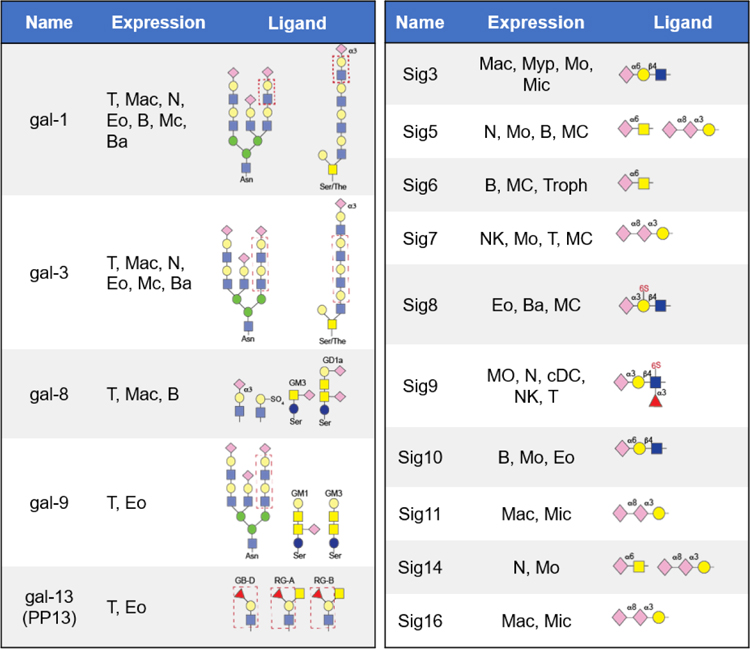
|
Abbreviations: B cells (B), basophils (Ba), dendritic cells (DC), eosinophils (Eo), macrophages (Mac), mast cells (MC), microglia (Mic), monocytes (Mo), natural killer cells (NK), neutrophils (N), and T cells (T).
Siglecs.
Sialic acid-binding immunoglobulin-like lectins (siglecs) are type 1 membrane proteins, displaying an amino-terminal V set IgG domain that binds to sialic acid-containing glycoproteins and glycolipids.73 Based on their sequence similarity, siglecs are classified into (a) sialoadhesin (siglec-1), CD22 (siglec-2), myelin-associated glycoprotein (MAG, siglec-4), and siglec-15, and (b) CD33-related siglecs such as CD33 (siglec-3), Siglecs-5–15 and –16. Most of the siglecs contain a tyrosine-based signaling motif, especially an immunoreceptor tyrosine inhibitory motif (ITIM), in their cytosolic domains that are implicated in endocytosis and cell siglaling.74 Because sialic acid-containing glycans are abundant in mammalian cells, siglecs, such as siglec-5, −7, −9, −10, and −15 act as immune check points on immune cells to differentiate self vs nonself signals and to avoid unwanted immune responses.46,75 Despite their common N-terminal V domain, each member of the siglec family represents defined specificity toward terminal sialic acid residues on glycoproteins or glycolipids (Table 1).75 Sialic acid can be linked via α2–3 or α2–6 linkage to an inner galactose residue, or via α2–8 or α2–9 linkage to an inner sialic acid residue. There are several possible elements in the sialylated glycoproteins that can be recognized by siglecs to induce biological responses consequently,73 including terminal sugar linkage, composition of oligosaccharide, and other modifications like sulfation or N-acetylation.
CD22 (siglec-2) is the most studied siglec on B cells that recognize α2,6-sialyted motifs to control B cell receptor (BCR) signaling following antigen binding.76,77 Regulation of BCR signaling is essential for maintaining self-tolerance. CD22 deficiency may cause autoimmune diseases and therefore offered an effective mean to autoimmune diseases.76 CD169 (also known as siglec 1) is an essential member of siglec family that binds to α2,3 sialylated N- and O-glycoproteins and glycolipids.78,79 CD169 receptor shows a low binding affinity toward monomeric sialic acid; hence, to have an effective interactions, its ligand must be heavily sialylated to form a multivalent sialoside.80 CD169 is a macrophage marker and plays a critical role in initiating antibacterial and antiviral immune responses and in development of autoimmune diseases.81 Human T cells generally lack siglecs, however, recent studies showed that, siglecs such as siglec-5, siglec-7, siglec-9, and siglec-10 are present and negatively control T cell functions.82,83
Cancer cells escape from the attack of macrophages by overexpressing antiphagocytic molecules called “do not eat me” signals, such as CD47/signal regulatory protein α (SIRPα), PD-L1/PD-1, and the β−2 macroglobulin subunit of the major histocompatibility class I complex (B2M)/leukocyte immunoglobulin like receptor B1 (LILRB1).84,85 Blocking the interactions between macrophage receptor and the tumor associated do not eat me molecule using antibodies has shown significant therapeutic prospect for cancer immunotherapy. Recently, the Weissman group demonstrated that tumor expressing CD24 stimulates macrophage mediated phagocytosis through interactions with siglec-10 specifically expressed on tumor associated macrophages (TAMs) in ovarian and breast cancer. In addition, elimination of CD24 or siglec-10 genetically or antibody mediated blocking of CD24-siglec 10 interaction drastically improved the phagocytosis of all CD24+-tumors, indicating that blockade of CD24-siglec-10 interaction has the potential for cancer treatment.86 Siglec-15 is mainly present on a subclass of myeloid cells that binds specifically to the sialyl-Tn glycan and is a promising target for osteoporosis treatment because of its involvement in osteoclast differentiation.87,88 Recent studies revealed the unexpected role of siglec-15 in microbial infection and the cancer microenvironment.89
Selectins.
The selectins are glycan-binding transmembrane glycoproteins found on the surface of endothelial cells, platelets, and leukocytes, that bind to sialylated, fucosylated glycan ligands and sometimes to a subset of heparan sulfateGAGs.90 This family of GBP comprises E-selectin, P-selectin, and L-selectin.91 Selectins are important for the trafficking of immune cells, T lymphocytes, and platelets.92 During inflammation, selectins on epithelial cells enable the initial attachment of leukocytes to epithelial cells from the bloodstream, which causes leukocyte movement along the endothelium via adhesive interactions referred to as leukocyte rolling. The absence of selectins or their ligands cause a significant health concerns like recurrent bacterial infections and progression. L-selectin is a 74–100 kDa glycoprotein mainly involved in the early stages of the adhesion cascade by mediating lymphocyte homing and adhesion to endothelial cells.93 Inflammatory stimulation of endothelial cells triggers overexpression of P-selectin on platelets and endothelial cells. In leukocyte rolling, P-selectin interacts with the P-selectin glycoprotein ligand-1 (PSGL-1) consisting of a sialyl Lewis x (sLex) glycan and a sulfate group on tyrosine expressed on all leukocytes, facilitate leukocyte rolling along the venular endothelium.94 E-Selectin is expressed only on endothelial cells activated by cytokines TNF-α and IL-1β. E-Selectin recognizes sialylated glycan ligands on glycoproteins, especially sLex expressed on specific immune cells. Adhesion of these cells to the acute and chronic inflammatory sites is associated with expression of E-selectin, suggesting the role of E-selectin in mediating immune cell recruitment to inflammatory sites.95 The inhibition of selectins has been investigated in a mice to model for the treatment of sickle cell disease. For example, an inhibitor of P- and L-selectin, GMI 1070, has been evaluated in clinical studies for the treatment of sickle cell anemia, and the result showed that GMI 1079 effectively suppressed vascular occlusion.96 Inclacumab is a fully human mAb that selectively targets P-selectin and was shown to reduce vaso-occlusive crisis in sickle cell disease.97 These examples support that these selectins are potential targets for the treatment of inflammatory diseases.
1.2.2. Glycoconjugates in Signal Transduction.
Binding of small protein ligands like hormones, cytokines, and growth factors to specific cell receptors triggers intracellular signaling events, which ultimately lead to the activation or inhibition of gene transcription.98 Most of these receptors are highly glycosylated membrane associated proteins, whereas their ligands are also glycoproteins.99 The glycans on the receptors affect their conformational flexibility and ligand binding. Receptor–ligand binding on a cell surface results in clustering of the receptor followed by phosphorylation of the cytoplasmic domain and activation of the downstream signaling events that control protein expression.100,99
Carbohydrates at extracellular domain are critical for many signaling pathways. For example, (1) the extracellular region of the Notch receptor carries many epidermal growth factor like repeating units which are glycosylated with O-fucose and O-glucose, as well as N-glycans. Activation of Notch receptors by Notch ligand regulates cell fate decisions in metazoa, however, disruption of O-fucose glycan leads to Notch signaling defects.101 (2) In the injured adult nervous system, myelin-associated glycoprotein (MAG) binds to receptors such as gangliosides (GD1a and GT1b) and GPI-anchored Nogo receptors (NgRs) on axons, forming signaling complexes that inhibit axonal outgrowth and limit functional recovery.102 (3) Some cell surface carbohydrates are important mediators of the signal transduction pathways for B-cell receptor activation and T-cell apoptosis.103,104 (4) Sialylation of EGFR inhibits EGFR dimerization and signaling associated with drug resistance.105 (5) O-GlcNAc addition to histone lysine methyl transferase EZH2 or MLL5 causes activation of this enzyme for methylation of histone and thereby leads to tumor suppression or cell lineage determination.106,107
1.2.3. Glycoconjugates in pathogenic infections.
Host–pathogen interactions (HPIs) during infection are highly complex regarding their mechanism and relation with the progression of infectious diseases. HPIs are facilitated via cell surface protein–protein interactions between hosts and pathogens. Recognition and attachment to specific cell surface carbohydrates is the first and critical step in viral entry.51 Cell surface carbohydrates are utilized by a wide variety of viruses as a receptor and most of these structures are negatively charged, such as sialic acid-containing glycans or polysulfated proteoglycans. One of such classical examples is hemagglutinin, an influenza glycoprotein, which binds to sialic acid-containing glycans on human airways epithelium to facilitate viral entry into the host. Upon replication, a large number of viral particles bud out from infected cells and are released by removal of surface sialic acid by sialidase and then infect another fresh target cell.108 The rational design of transition state analogues as sialidase inhibitors led to development of the anti-influenza drugs Relenza (GlaxoSmithKline) and Tamiflu (Genentech).109,110 These drugs inhibit the activity of sialidase so that the freshly budded virions do not disseminate from infected cells and thus stop further infection. In humans, α2,6-sialylated glycans are more predominant in the upper respiratory tract than the lower respiratory tract, whereas α2,3-sialylated glycans are dominant in avian species. The presence of both α2,6/2,3-sialylated glycans in swine makes them vulnerable to influenza viruses of human and avian origin.111,112 Glycan microarray profiling of influenza strains has provided a new understanding of the specificity of hemagglutinins, especially the specificity of binding toward the internal glycan beyond the sialyl galactose linkage.113,114 Different virus families and their preference for carbohydrate structures as receptors for entry are presented in Table 2. Most of the members of these virus families bind to glycoepitopes containing terminal sialic acids of sulfated glycan motifs of proteoglycan chains.115–119 Protein–glycan interactions also occur during the infection of many other pathogens.
Table 2.
Classification of major viruses targeting glycosylated receptors and their glycoepitopes.
| Virus family/subfamily | Virus type | Glycoepitope |
|---|---|---|
| Orthomyxoviridae | Influenza A virus | (α 2–3)-linked Neu5Ac: Avian virus; (α 2–6)-linked Neu5Ac: Human virus |
| Influenza B virus | (α 2–6)-linked Neu5Ac; (α 2–3)-linked Neu5Ac | |
| Influenza C virus | 9-O-acetyl Neu5Ac | |
| Flavivirus | Dengue virus | Heparan sulfate |
| Japanese encephalitis virus. | ||
| West Nile virus | ||
| Hepacivirus | Hepatitis C virus | Heparan sulfate |
| Adenoviridae | Adeno 37 | (α 2–3)-linked Neu5Ac |
| Adenovirus 2, 5 | Heparan sulfate | |
| Papillomavirus | Human papillomavirus types 11, 16, 33 | Heparan sulfate |
| Coronavirus | MERS-CoV | Heparan sulfate |
| SARS-CoV | ||
| SARS-CoV-2 |
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a member of the Coronaviridae family, which causes the COVID-19 pandemic.120,121 The SARS-CoV-2 S protein exists as a trimer on viral surface, and each monomer is composed of S1 and S2 subunits with a total of 1273 amino acids. The receptor binding domain (RND) in S1 is responsible for viral entry into the host cell by interaction with the angiotensin-converting enzyme 2 (ACE2) receptor, while fusion of viral and host cell membrane is mediated by the S2 subunit.122‘123 Recently, Clausen et al. showed that the cell surface heparan sulfate interacts with SARS-CoV-2 S protein and triggers a conformational change to favor the high affinity binding with ACE2 receptors on the host cell.124–126 To facilitate viral entry, engagement of both cell surface HS and ACE2 is critical, indicating that heparan sulfate acts as a coreceptor.124 The SARS-CoV-2 S protein contains 22 N-linked and at least two O-linked glycosites that are important for proper protein folding, binding to receptor, and escape from host’s immune response and antibody neutralization.127
Many bacterial lectins function as adhesins for attachment to host and invasion. For example, type 1 fimbrin d-mannose specific adhesin (FimH) from Escherichia coli (E. coli) preferentially binds to oligomannose type glycans on host cells in urinary tract infections.128 FimH has been shown to bind with the variability to the mannose structure, leading to different tissue tropism.128 A series of C1-modified α-mannosides have been rationally designed as FimH antagonists and tested in animal models as a potential UTI therapeutic.129 The Gram-negative bacterium Heliobacter pylori attaches to the heavily glycosylated human gastric mucosa and epithelial lining using various adhesins that specifically recognize Lewis B, sLeX, and Lewis A, and di-LacNAc structures, which are expressed in gastric epithelial cells as mucins within the mucosa.130–132 These adhesins may have a role in persistent H. pylori infection with a majority of hosts being asymptomatic; however, 1–3% of those infected develop gastric cancer.133 Alternatively, pathogenic bacteria can also interact with host lectins such as the C-type lectin, dendritic cell-specific ICAM-3-grabbing nonintegrin 1 (DC-SIGN), and the mannose-binding lectin that enable bacteria to adhere and enter host cells.134,135 For example, the Neisseria gonorrheae strain 1291 LOS expresses a terminal lacto-N-neotetraose structure on surface and binds to ASGPRs to mediate invasion of male primary urethral epithelial cells.136
Bacteria utilize a range of GTs and GHs to modify host glycoconjugates and to enable cell adhesion. Neuraminidases are the most widely studied enzymes regarding their varying specificities.137 Following influenza A virus infection, the viral neuraminidase acts on the sialylated viral receptors on host cells to cleave the terminal sialic acid residue and promote subsequent coinfection by Streptococcus pneumoniae.138 Glycoconjugates on host cells are also targeted by bacterial toxins. For example, Vibrio cholerae (cholera toxin) targets complex N-linked glycoproteins; Shigella dysenteriae and Shiga toxigenic E. coli (Shiga toxin) specifically binds to the trisaccharide motif of the Gb3 receptor, which is mainly present on endothelial cells in the brain, intestine, and B lymphocytes; the neurotoxin from Clostridium botulinum (botulinum toxin) binds to both the peptide and the N-linked glycan on the neural receptor SV2; and high affinity binding of Clostridium tetani (tetanus toxin) to neurons is mediated solely by gangliosides.139–143
1.2.4. Glycoconjugates in Cancers.
Glycosylation is utilized by cancer cells to evade immune clearance and move to metastatic sites such as lung, liver, and brain, etc.99 Altered glycosylation with increase in sialylation, core fucosylation, N-glycan branching, or mucin-type O-glycosylation, is the most common phenomenon observed in cancer cells.144 During cancer progression, the glycosylation pattern changes to truncated structures, such as the Tn antigen in O-glycans, or to abnormal structures, such as sLeX (Figure 4).145 Formation of such neoantigens in cancers facilitates their metastasis to other tissues or organs.146 The abnormal glycosylation pattern arises from the availability of GTs during the biosynthesis of glycoconjugates inside the cancer cell that catalyze the addition of extra sugar residues in the core or at the termini of N-linked and O-linked glycans.147 In addition, altered glycosylations are also associated with malignancy. GTs such as SiaT and FucT involved in the terminal glycosylation are often overexpressed in tumors to produce certain unusual glycans.148 Several tumor-associated carbohydrate antigens (TACAs) have been reported to have a high correlation with cancers, including sLeX, sialyl Lewis A(sLeA), Tn, sialyl-Tn (sTn), GM2, GD2, GD3, and Globo-H (Figure 5).149 sLeX is overexpressed in colorectal, breast, lung, and gastrointestinal carcinomas, sLeA in colorectal and pancreatic cancer, and Tn in breast and other cancers.150 Elevation of serum STn was found to correlate strongly with poor survival of patient with ovarian cancer.151 In human melanoma and neuroblastoma, gangliosides GD2, GM2, and GD3 are highly expressed.152 Globo-H was found in breast carcinoma cell line MCF-7, embryonal carcinoma 2102 cells, and breast, ovarian, stomach, oral, and prostate cancers.153 Taken together, TACAs are commonly found on cancer cells but absent on normal cells and have received more and more attention in the development of anticancer immunotherapies.154
Figure 4.
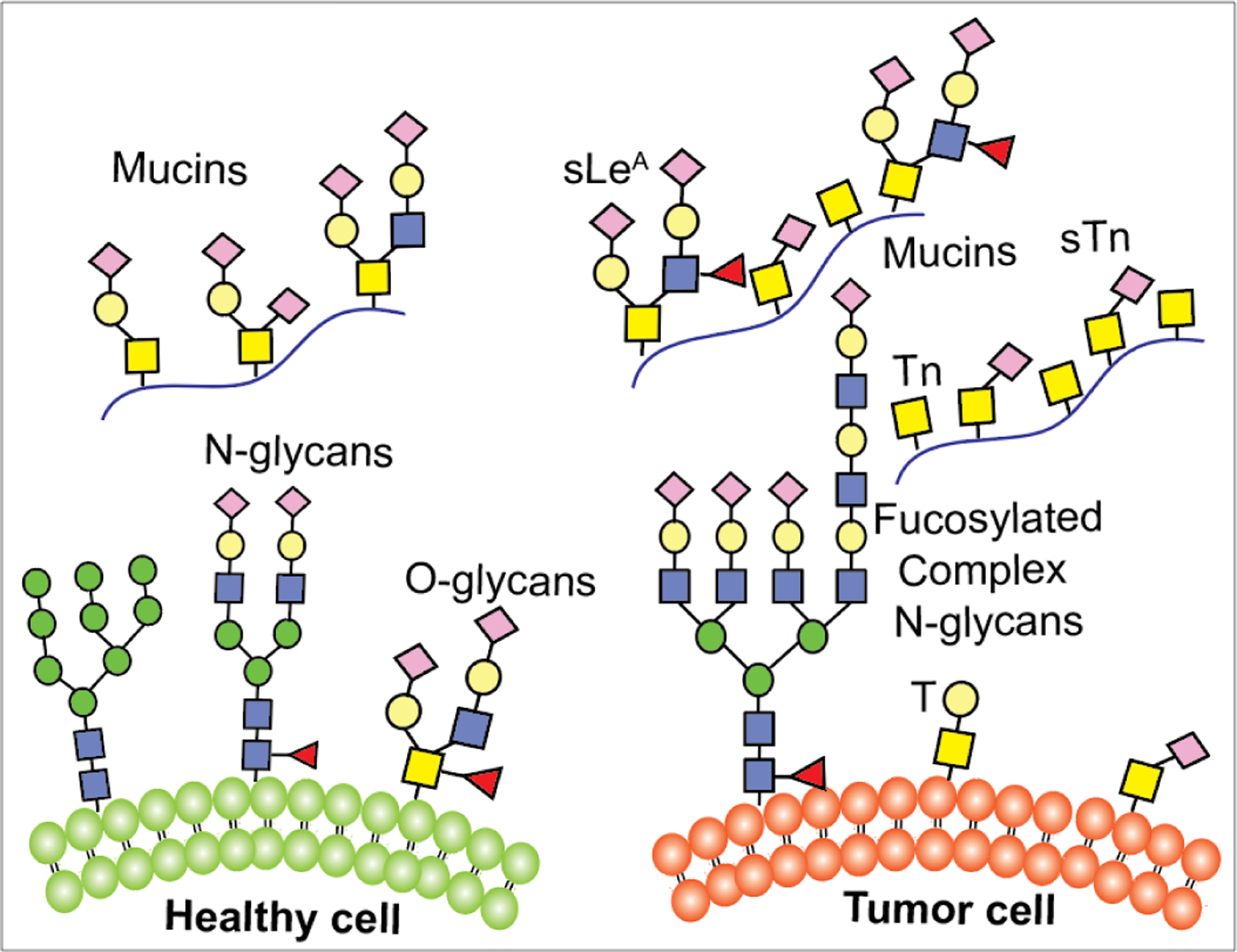
Cell surface glycans in healthy and diseased states.
Figure 5:
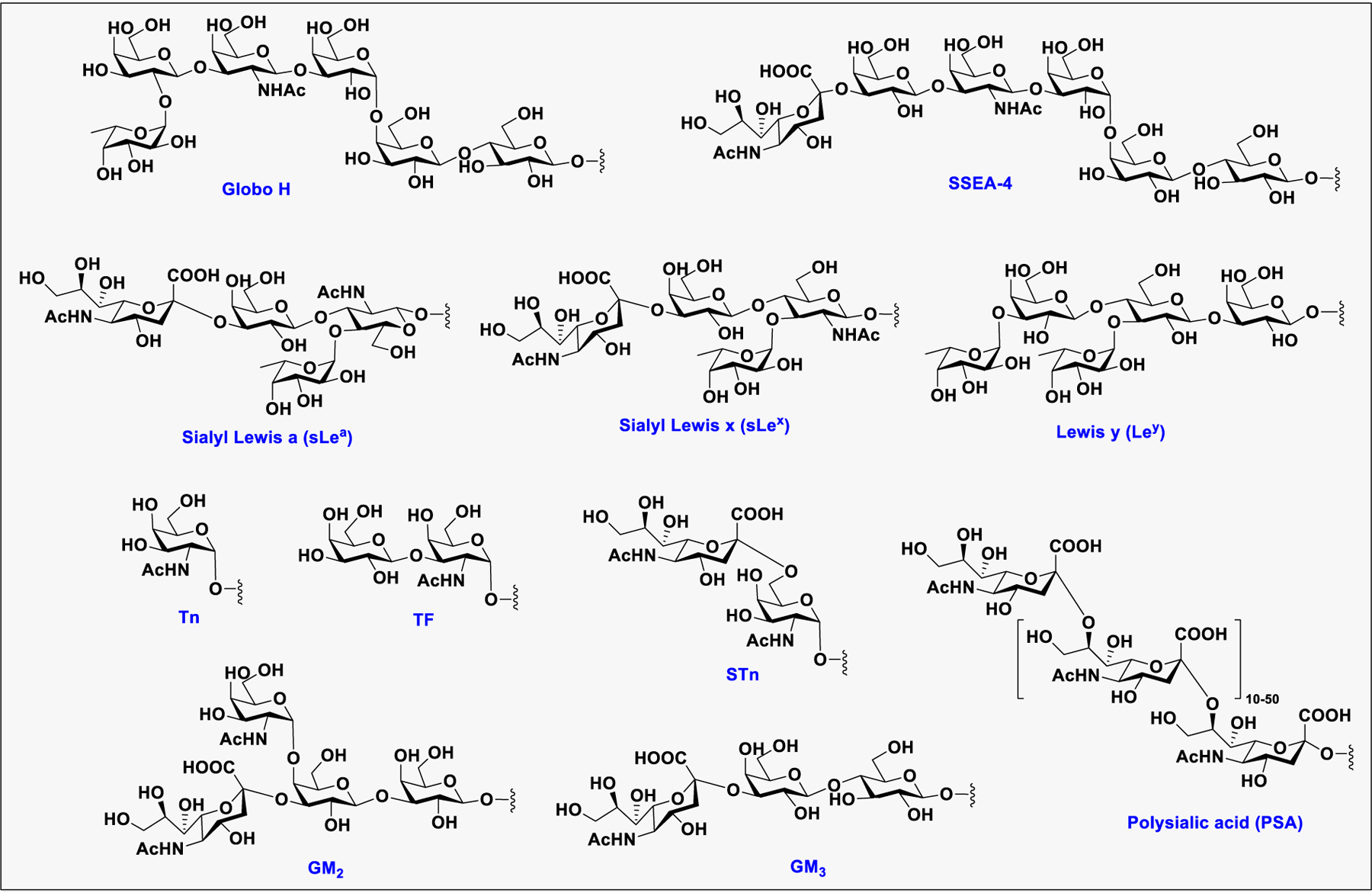
Structures of representative cancer associated glycans.
Another major characteristic of cancer cells is the overexpression of abnormal glycoproteins and glycolipids. For example, O-glycosylated mucins are often overproduced by epithelial tumors and therefore can be used as cancer markers for diagnosis and development of therapeutics.155 Increased levels of complex gangliosides including GD2, GD3, and fucosyl GM1 are also found on small-cell lung carcinomas, neuroblastomas, and melanomas.156
Recently, the globo-series GSLs, including Globo H, SSEA3, and SSEA4, are found exclusively on the cell surface of many cancers and correlate with tumor metastasis and progression.157,158 The enzyme β1,3-galactosyltransferase V (β3GalT5) is essential for the biosynthesis of globo-series GSLs, as it catalyzes the galactosylation of Gb4 to SSEA3, which is further modified to GloboH and SSEA4. Overexpression of β3GalT5 increases the expression level of surface SSEA-3 in breast cancer cells (Figure 6a).159 Globo H is synthesized from SSEA3 by fucosyltransferases 1 and 2 (FUT1 and FUT2)160 whereas SSEA4 is synthesized by β-galactoside α 2,3-sialyltransferase 2 (ST3Gal2).161 In breast cancer cells, the lipid moiety of globo-series GSLs interacts with caveolin-1 (CAV1) and focal adhesion kinase (FAK) to form a complex, which then interacts with AKT (protein kinase B) and receptor-interacting protein kinase (RIP), respectively (Figure 6b). The interaction between FAK and RIP prevents apoptosis triggered by the interaction between RIP and the Fas death domain (FADD) through the Fas-dependent pathway.158 Knockdown of β3GalT5 suppressed cell growth and induced cell apoptosis. However, knockdown of the enzymes for the synthesis of GH from SSEA3 (FUT1 and FUT2), or the enzyme for the synthesis of SSEA4 from SSEA3 (ST3Gal2) does not induce apoptosis in MDA-MB-231 cells.159 These studies indicated that β3GalT5 is the key enzyme to sustain survival of cancer cells, and this enzyme and all the three globo-series glycans could be the targets of cancer immunotherapy.
Figure 6:
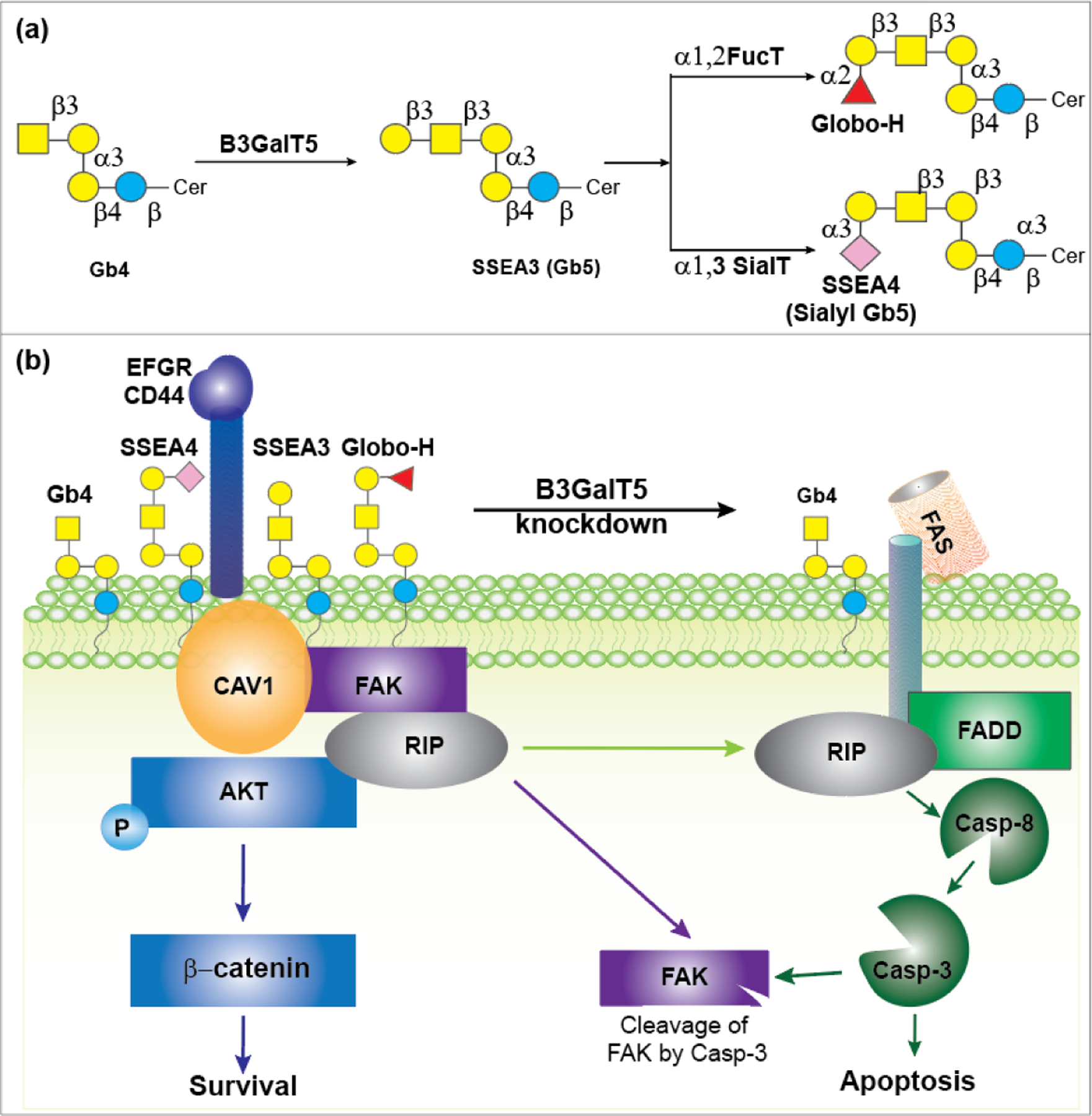
a) Biosynthetic pathway of SSEA3, GH, and SSEA4 involving B3GalT5. b) The key role of the B3GalT5 enzyme and the globo-series GSLs in the apoptosis and survival of breast carcinoma cells.
2. NATURALLY OCCURRING GLYCOCONJUGATES
2.1. Proteoglycans
2.1.1. Structure and Classification of Proteoglycans.
Proteoglycans (PGs) are a class of highly complex biomolecules composed of long linear chains of GAGs such as CS, DS, KS, heparin, and HS linked to a protein backbone at serine residues.39 Hyaluronan is the only GAG which is present as a noncovalently linked complex with protein. GAGs are linear, negatively charged complex polysaccharides composed of repeating disaccharide (GalNAc or GlcNH2 linked to d-glucuronic acid or l-iduronic acid) units with varying degrees of sulfation, and KS has the GlcNAc residue linked to the Gal–Gal unit (Figure 7).
Figure 7:
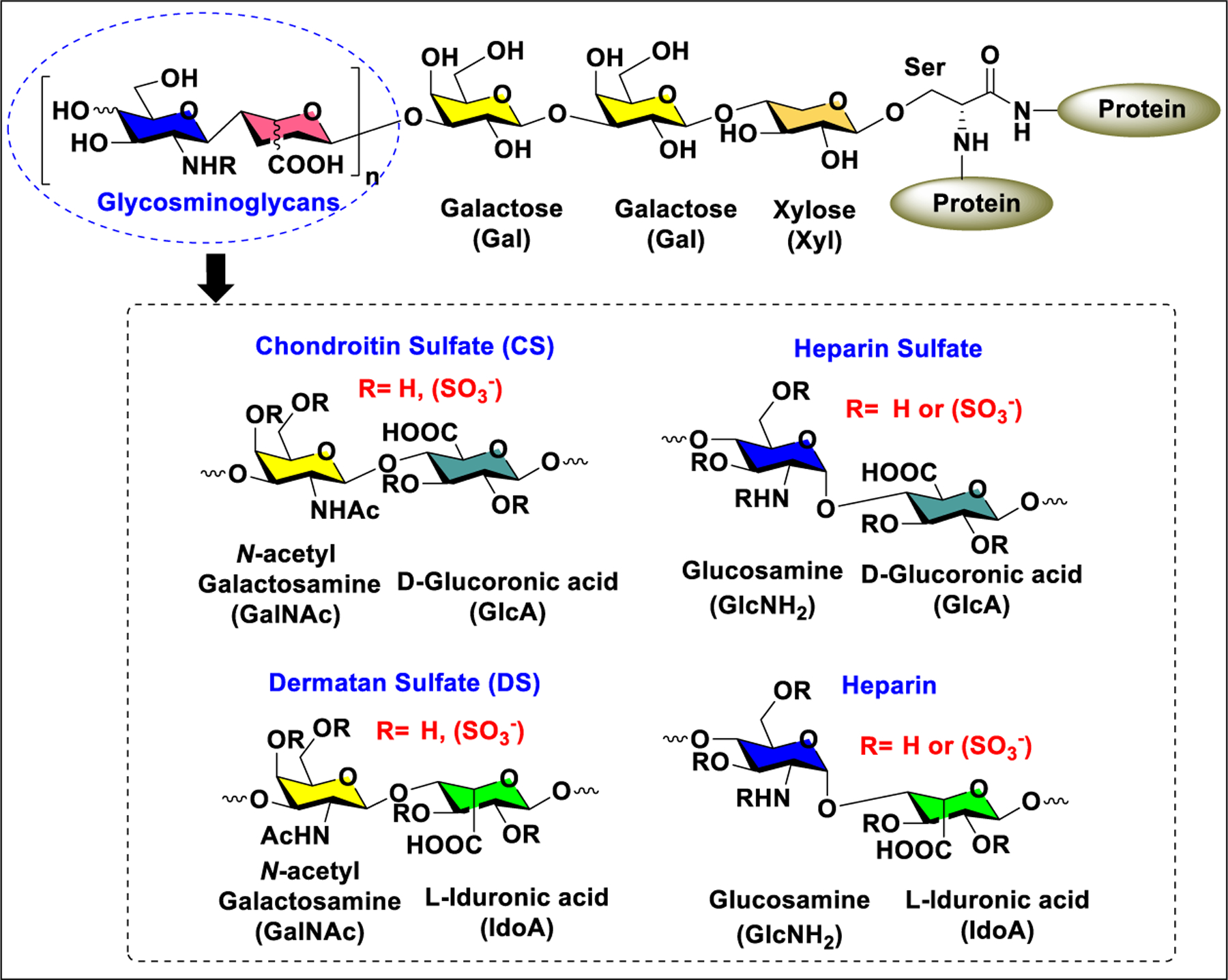
General structure of proteoglycans.
Depending on the cellular and subcellular localization, PGs are classified into extracellular and intracellular proteoglycans. Each type is again divided into subtypes based on composition, sequence homology, structure of core protein, and size.162 The only known intracellular PG is Serglycin, which contains heparin side chains. Mast cells use Serglycin to store mast cell specific proteases that are released upon inflammation.163 Out of 13 cell surface proteoglycans, seven have transmembrane domains and six are GPI-anchored proteoglycans. Syndecans and glypicans are two main families of extracellular PGs.164 Syndecans are hybrid PGs because their ectodomain are generally attached to HS and in some cases to CS. Glypicans are found on the cell surface or in the pericellular matrix. Glypicans attached to cell surface via protein core or a GPI anchor.165 Some examples of pericellular PGs include perlecan and agrin.162
2.1.2. Functions of Oroteoglycans.
The microheterogeneity of PGs forms the basis of their diverse cellular activities.162 In addition to their role as a structural element in tissue organization, PGs are also involved in cell signaling by interactions with cell surface signaling molecules.166 The catalogue of biological phenomenon in which PGs are involved is growing rapidly.
2.1.2.1. Cellular Gunctions.
PGs are implicated in a wide variety of biological events ranging from cell–cell, cell–extracellular matrix (ECM), to ligand–receptor interactions.167 Chondroitin sulfate PGs, a key element of the extracellular matrix in the central nervous system (CNS) involved in the development of CNS and prevention of neural damage.168 Chondroitin sulfates also promote or inhibit the neural growth by interactions with growth factors or transmembrane receptors, respectively.169 The interactions between CS and specific proteins are greatly affected by the degree of sulfation with CS GAG chains. Interestingly, the sulfation pattern of CS GAG chains changes during development of nervous system and in response to CNS injury.170 The role of HSPGs during the development of the mammalian CNS has also been documented.171,172
2.1.2.2. Signal Transduction.
Cell surface HSPGs such as syndecans and glypicans bind to several growth factors and other matrix associated molecules that are implicated in various signal transduction pathways and are important for cell proliferation. Syndecans, through their HS chains bind to numerous growth factors to dictate morphogen gradients during development. Along with their role as an endocytosis receptor for the uptake of exosomes, syndecans also act as coreceptors for many receptor tyrosine kinases and lipoproteins.173 Syndecan-1 has been shown to drive the clearance of triglyceride-rich lipoproteins from the liver or intestine.174 Glypicans, such as glypican-3 are critical for tumor growth and angiogenesis.175
2.2. Glycoproteins
2.2.1. N-Linked and O-Linked Glycoproteins.
Post-translational proteins glycosylation is important for the proper folding, stability, and intracellular trafficking of proteins. In addition, the carbohydrate domains of glycoproteins can directly interfere with a wide variety of physiological processes. Compared to other post-translational modifications like protein phosphorylation and methylation, the post-translational glycosylation is highly diverse and complex. The N-linked and O-linked glycans are the two types of most studied glycoforms in protein glycosylation.6
N-Glycans contain a common core pentasaccharide structure, Man3GlcNAc2, that linked to the Asn residue in the protein backbone through amide bond formation (Figure 8). There are three types of N-glycans, namely, high-mannose, hybrid-type, and complex-type glycans. High-mannose glycans (HMGs) have additional mannose sugars residues at both the α−3 and α−6 mannose sites, known as the “D1 and D2 arms”. HMGs are named according to number of mannose residues attached to the chitobiose core, for example, Man9GlcNAc2 contains nine mannoses attached to GlcNAc2. In complex glycans, the terminal mannose residues of the core pentasaccharide are substituted with differently linked GlcNAc residues to form “antennae.” Complex type glycans exist as bi-, tri-, and tetra-antennary forms depending on the number of antennae present on the core. Hybrid-type glycans are characterized as containing both high mannose type and complex type antennae on the core. The complex type antennae present in both hybrid and complex type glycans are extended with β(1→4)Gal linkage. Additional modifications such as repeating LacNAc (GlcNAc-β(1→4)Gal) units linked to terminal Gal via β(1→3) linkage, addition of a bisecting GlcNAc at the mannosyl core, and fucosyl residue on the innermost as well as outer GlcNAc or Gal residues are also possible. Complex glycans commonly terminate with sialic acid residues linked via either α2,3- or α2,6-linkages.176
Figure 8:
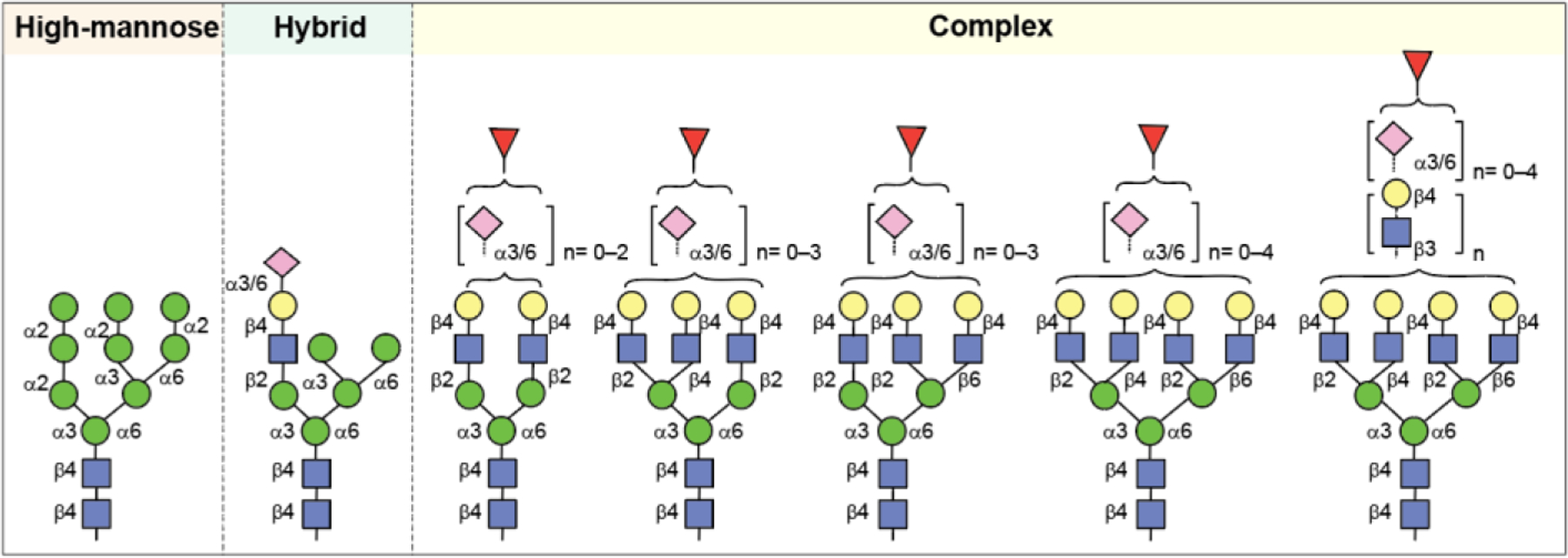
Representative structures of N-linked oligosaccharides.
O-Glycans are mostly linked to the side chains hydroxyl of Ser or Thr residue of proteins without any necessity of a consensus sequence. Other than Ser and Thr, Tyr, hydroxylysine, or hydroxyproline may also be the sites for O-linked glycosylation (Figure 9). The most commonly occurring O-linked glycans are the mucin-type, which contains a GalNAc residue at the reducing end that is linked to proteins. There are eight mucin-type core structures, however, further modifications to the core, such as sialylation, fucosylation, etc., make them highly heterogeneous.177 In general, O-linked glycans are comparatively less complex than N-glycans. The highly dense O-linked glycans on mucin resulted in cross-linked structures to form mucus.
Figure 9:
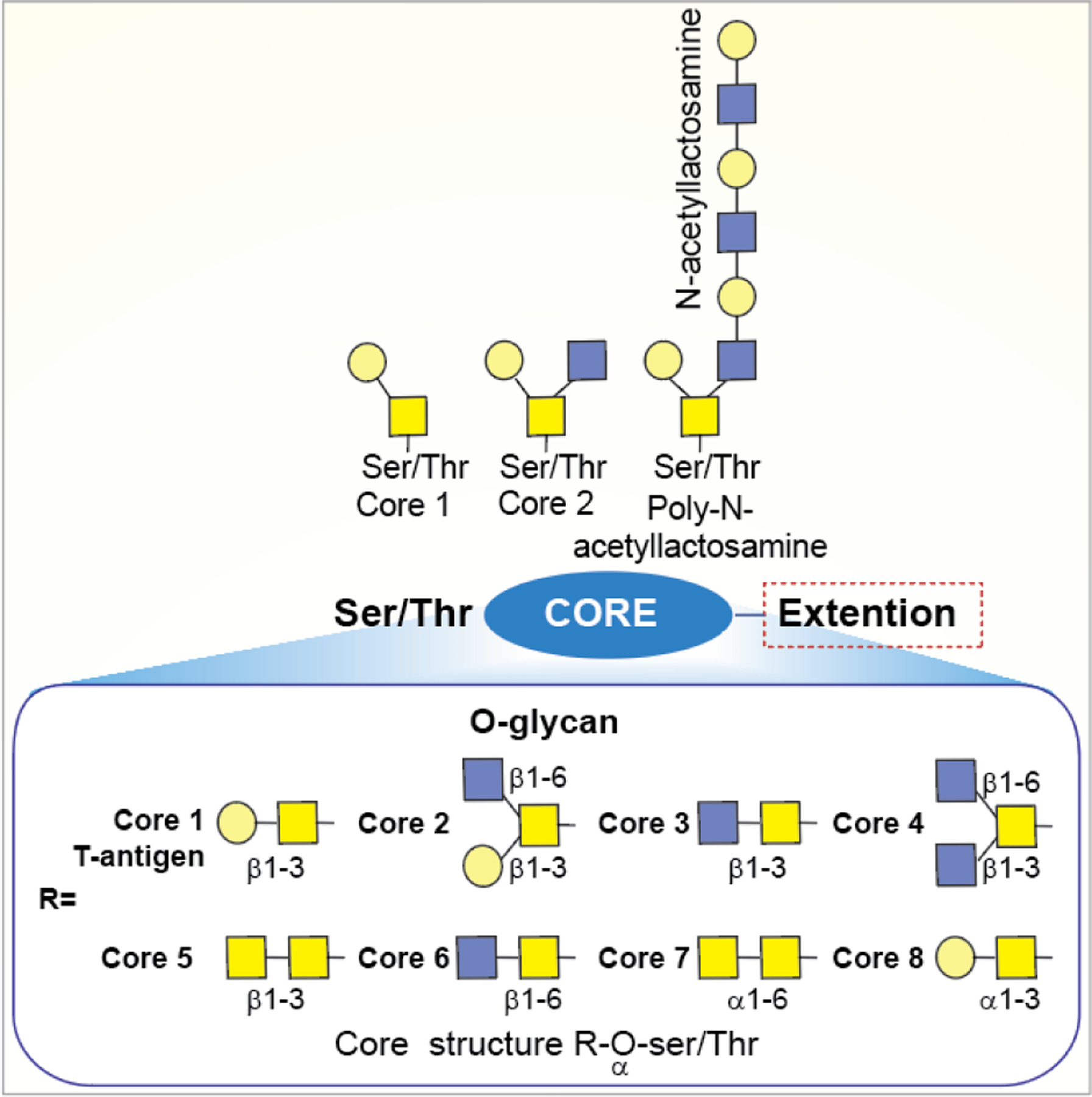
General structure of O-linked oligosaccharides.
2.2.2. Biosynthetic Pathway of N-Linked and O-Linked Glycoproteins.
The biosynthesis of N-linked glycoprotein begins with the synthesis of a dolichol-linked Glc3Man9GlcNAc2 precursor, the glycan of which is then transferred to the side chain of Asn in a consensus glycosylation sequon of Asn-X-Ser/Thr, catalyzed by oligosaccharyltransferase (OST).178 The terminal glucose residues of the oligosaccharide precursor are then digested by α-glucosidase-I and -II to form a monoglucosylated glycoform (Glc1Man9GlcNAc2), which is passed through the gate keeper calnexin/calreticulin chaperone and folded properly. The glycan on the properly folded glycoprotein is trimmed further to Man8GlcNAc2, which then exits the ER and enters the Golgi apparatus for further processing by Golgi-resident GHs and GTs to form hybrid-type or complex type glycoforms (Figure 10a).
Figure 10:
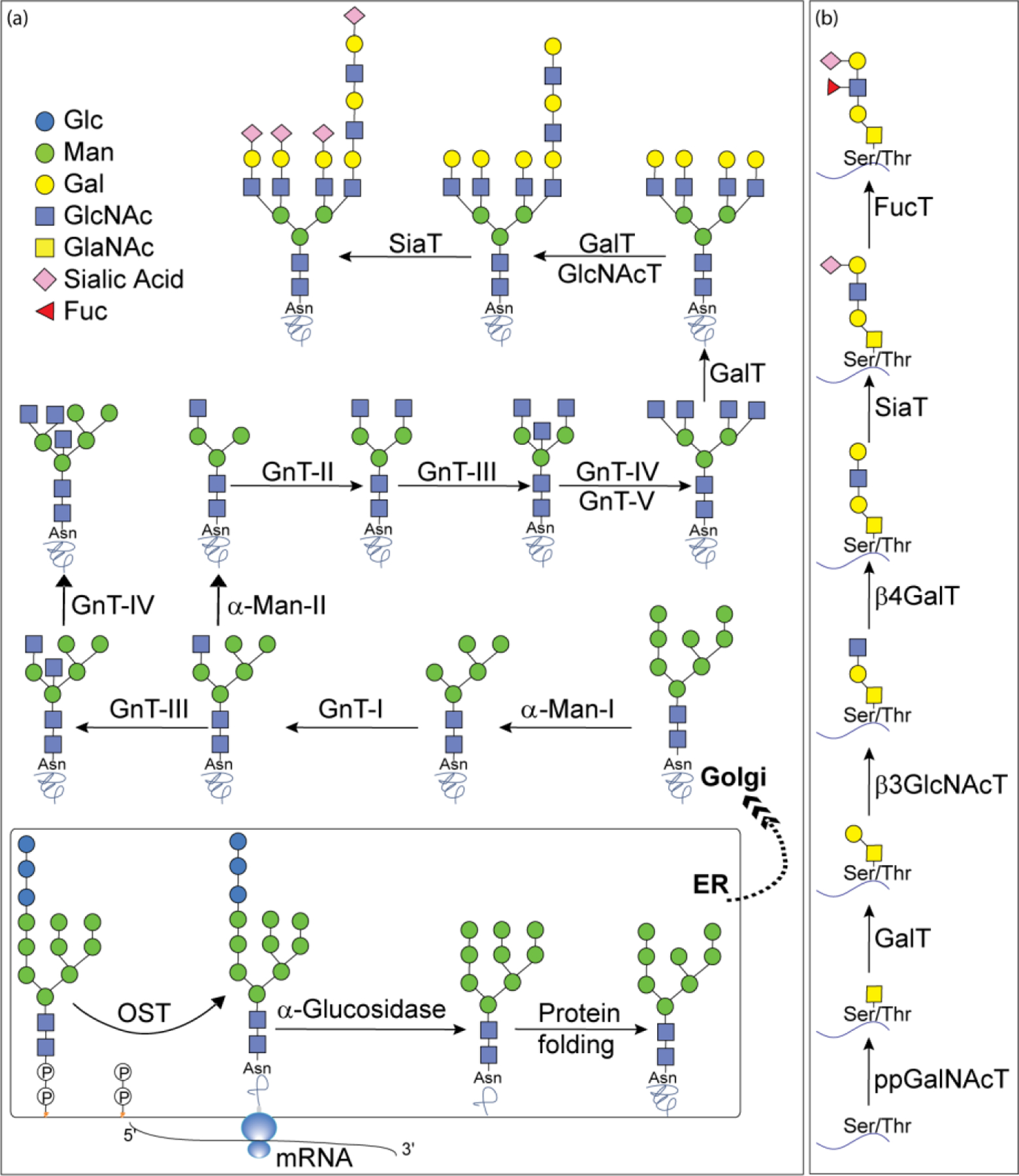
Biosynthetic pathway for A) N-linked; and B) O-linked glycoprotein synthesis.
The medial-Golgi mannosidase removes the terminal mannose residues of Man8GlcNAc2 to form Man5GlcNAc2, which is further acted upon by an GlcNAc transferase called GnTI (MGAT1) to add a GlcNAc residue via β−1,2 linkage to the C-2 position of the mannose residue at the α−1,3 arm of Man5GlcNAc2. Subsequently, the terminal α1–3Man, and α1–6Man residues from GlcNAcMan5GlcNAc2 are trimmed by α-mannosidase II to form GlcNAcMan3GlcNAc2. Upon removal of both mannose residues, GnTII (MGAT2) catalyzes addition of a second GlcNAc residue to the C-2 of the mannose at the α1–6 arm to give the main precursor for all biantennary complex-type N-glycans. Hybrid-type N-glycans are formed if the GlcNAcMan5GlcNAc2 glycan is not trimmed by α-mannosidase II and the intermediate GlcNAcMan5GlcNAc2 is further extended by β1,4-galactosyl and/or α2,3/2,6-sialyl transferase. Additional branches on biantennary complex glycans can be formed by addition of β−1,4 bisecting GlcNAc at C-4 of the core Man with GnTIII or by addition of β−1,4 GlcNAc at C-4 of α1–3Man with GnTIV or at C-6 of α1,6Man with GnTV to give tri- and tetra-antennary N-glycans.179 Further glycosylations with galactosyltransferases, sialyltransferases, and fucosyltransfersases change the N-glycans into highly diverse complex-type N-glycans, including glycans with core fucose and extended glycans with poly-LacNAc motifs.180
The O-linked glycoproteins are formed by addition of GlcNAc residue to the hydroxyl of Ser/Thr side chain (Figure 10b).177 At least 12 GalNAc transferases (ppGalNAcT) isozymes have been identified that initiate O-glycosylation of mucin glycoprotein. The synthesis of mucin-type glycans involves ppGalNAcT-catalyzed glycosylation in the presence of UDP-GalNAc as donor. Subsequent elongation and termination of O-linked glycans is conducted by several GTs.181 The expression and subcellular distribution of the various GTs determine the outcome of O-glycans which are often terminated with Gal, GlcNAc, GalNAc, Fuc, or Neu5Ac.
2.2.3. Glycoprotein Therapeutics.
It is well documented that glycosylation profile of therapeutic proteins significantly modulates production yield, stability, biological activity, immunogenicity, pharmacokinetics, and pharmacodynamics. Advances in the area of glycobiology has expedited the development of glycoprotein therapeutics including glycoconjugate vaccines, glyco-engineered monoclonal antibodies, antibody–drug conjugates(ADC), and other recombinant proteins for the treatment of life threatening diseases, including cancer, autoimmune diseases, etc.182
Erythropoietin (EPO) is a glycoprotein best known for its binding to the erythropoietin receptor to promote the maturation of erythroid progenitor cells to erythrocytes and initiate hemoglobin synthesis.183 Natural and recombinant forms of EPO were developed for the treatment of anemia caused after chemotherapy or for those with deficiency of erythropoietin. EPO contains 3–5 N-glycosylation sites which accommodate tri- and tetra-antennary complex type glycans terminating with galactose or sialic acid residues.184 Although the in vitro activity of the deglycosylated form of EPO is not significantly affected compared to native form, the in vivo activity is greatly reduced due to rapid clearance of poorly glycosylated EPO by filtration in the kidney.185 Galactose-terminated EPO is also rapidly up taken by ASGPR in hepatocytes and macrophages. Various approaches have been developed to incorporate fully sialylated tetra-antennary glycans to boost circulatory half-life and in vivo activity.186
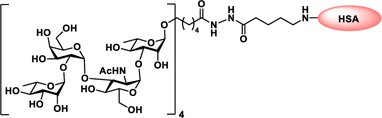
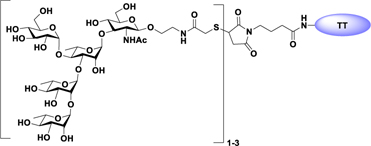
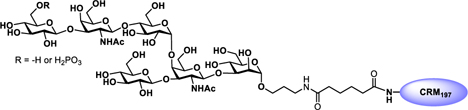
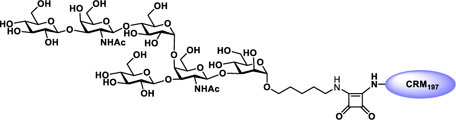
Glyconjugate vaccines are another important class of glycoprotein therapeutics that are part of routine vaccination schedules for protection against pathogenic infections.187 The semisynthetic Hib glycoconjugate vaccine, that is marketed in Cuba, has been very successful at preventing Haemophilus influenzae type b (Hib) infection.188 Pneumococcal conjugate vaccines have been formulated to cover more serotypes. The best-selling vaccine, Prevnar13 (Pfizer), is effective against the serotypes that are responsible for >70% of the invasive pneumococcal infections worldwide.189 Glycoconjugate vaccines against Neisseria meningitidis are also successful. Several conjugated CPS vaccines are available, for example, Menactra, Menveo, and Nimenrix against serogroups A, C, W, and Y, Meningitec, Menjugate, NeisVac-C against serogroup C, MenHibrix against serogroups C/Y, and MenAfriVac against serogroup A.190 At present several synthetic carbohydrate based vaccine are being developed against varieties of bacterial and viral infections.53
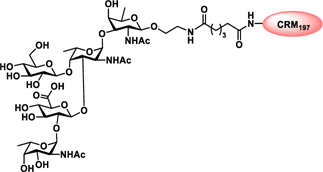
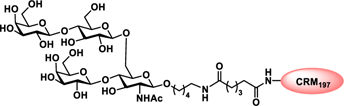
Vaccines containing the glycan of cancer associated gangliosides including GM2, GD2, and globoH have been advanced to late stage clinical trials.191 However, the progress of vaccines targeting mucin type sialyl-Tn (sialylα2–6GalNAcα-) antigen has seen slow in 20 years.192 A synthetic Globo H-KLH conjugate combined with QS-21 adjuvant is in phase 3 clinical trials for the treatment of triple negative breast cancer (NCT03562637) and a Globo H-DT conjugate with C34 adjuvant designed to induce a class switch and improve the IgG titer is in phase 2 trials for the treatment of multiple cancers (NCT02310464).
2.3. Glycolipids and Lipopolysaccharides
2.3.1. Structure of Lipopolysaccharides.
The glycolipid molecules commonly present on the surface of Gram-negative bacteria are lipopolysaccharides that are comprised of O-antigen, core oligosaccharide, and lipid A.193 LPSs are endotoxins that cause severe symptoms, such as high fever, diarrhea, blood pressure decrease, or septic shock and sometimes could result in death.194
Lipid A, the hydrophobic part of the LPS consists of GlcNH2 β−1→6GlcNH2 disaccharide with a phosphate group at the 1 and 4′ positions.195 The 2-amino and 3-hydroxyl groups of both glucosamine residues are linked to fatty acid chains (Figure 11). The number of lipid chains and their length depend on species but generally remained conserved. The fatty acids of lipid A on the LPS molecule are embedded in the cell membrane while the rest of the LPS molecule projects from the bacterial outer membrane. Upon lysis of the bacterial cell wall by the immune system, the infection may result in fever, diarrhea, and septic shock.196
Figure 11.
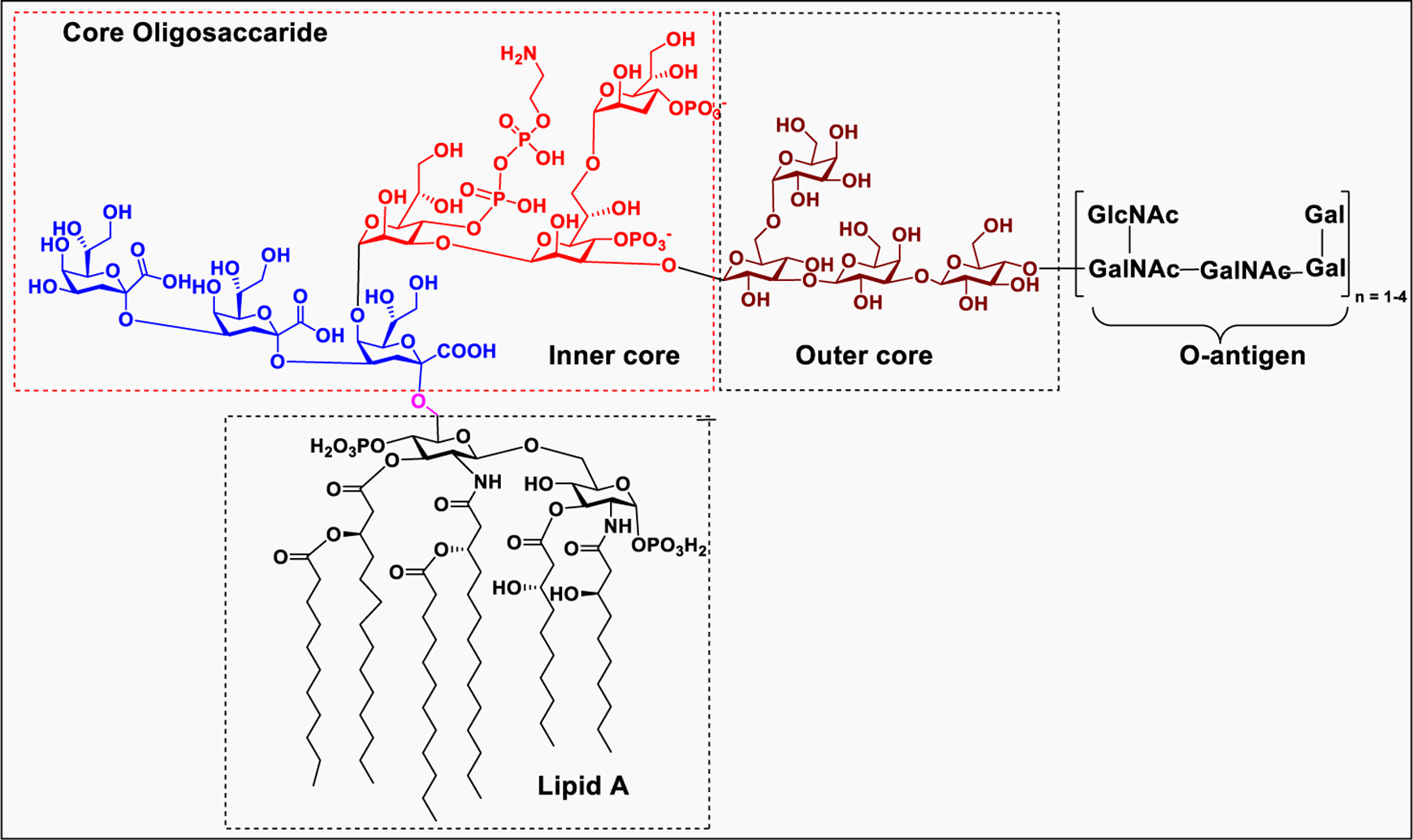
A general structure for bacterial lipopolysaccharides (LPS).
The core oligosaccharide consists of inner core and outer core that connect lipid A to the O-antigen.197 The inner core typically contains 3-deoxy-α-D-manno-octulosonic acid, also known as KDO, that attached directly to the 6-O position of the GlcNAc of lipid A. LPS typically contains one to four molecules of KDO, however, bacteria need at least one molecule of KDO for survival.198 The inner core KDO residue is modified with 2-amino ethyl phosphate or heptose monosaccharide. The outer core oligosaccharide is structurally more diverse, consisting of glucose, galactose, and GlcNAc.199 The O-antigen is the outermost part of LPS attached to the end of core oligosaccharide, and the structure varies among different strains but typically contains repeating chains of glycans with four to five sugar residues.200 The O-antigen component of LPS is much longer and highly complex and contains at least 20 different types of glycans residues which are not commonly found in nature.201 Among different domains of LPS, the O-antigen has high structural diversity compared to lipid A and core oligosaccharide.202
2.3.2. Functions of Lipopolysaccharides.
The LPS in the bacterial cell is strongly amphipathic in nature due to the hydrophobic lipid chain and the hydrophilic core oligosaccharide and O-antigen that set a permeability barrier for toxic molecules.203 The effectiveness of the barrier depends on how densely the LPS is packed within the cell membrane. The LPS of Gram-negative bacteria is termed as “endotoxin” because the immune response raised against LPS can be toxic to the host. The immune system has evolved to target the most conserved component of LPS, the lipid A. Because of the considerable structural diversity of lipid A among bacterial species, different LPS structures trigger different host immune responses.204,205 For example, hexa-acylated, bisphosphorylated lipid A of E. coli and Salmonella is highly immunogenic, compared to other forms of lipid A.206 Synthesis of the less immunogenic lipid A structures is used by some pathogens to evade the immune attack. Alternatively, some pathogens mask the most conserved domain of LPS with highly variable sugar chains in the O-antigen domain to escape from host immune response. In addition, the presence of the O-antigen not only protects bacteria from lysis but also contributes to pathogen evasion of immune cell-mediated phagocytosis.207
2.3.3. Biosynthesis of Lipopolysaccharides.
LPS on the bacterial outer membrane is essential for the pathogen to survive. Although the biosynthesis of LPS is well-defined and common in most of the Gram-negative bacteria, some pathogens possess variability in the core domain of LPS. The biosynthesis of LPS is initiated in the cytoplasm and periplasm and then moved to the plasma membranes.197 Biosynthesis of Kdo-Lipid A begins with the small building block, UDP-GlcNAc. During assembly of LPS, multiple enzymes work together sequentially to convert UDP-GlcNAc to disaccharide-1-P, Kdo2-lipid A, core oligosaccharide-lipid A, and O-antigen (Scheme 1).208,209
Scheme 1:
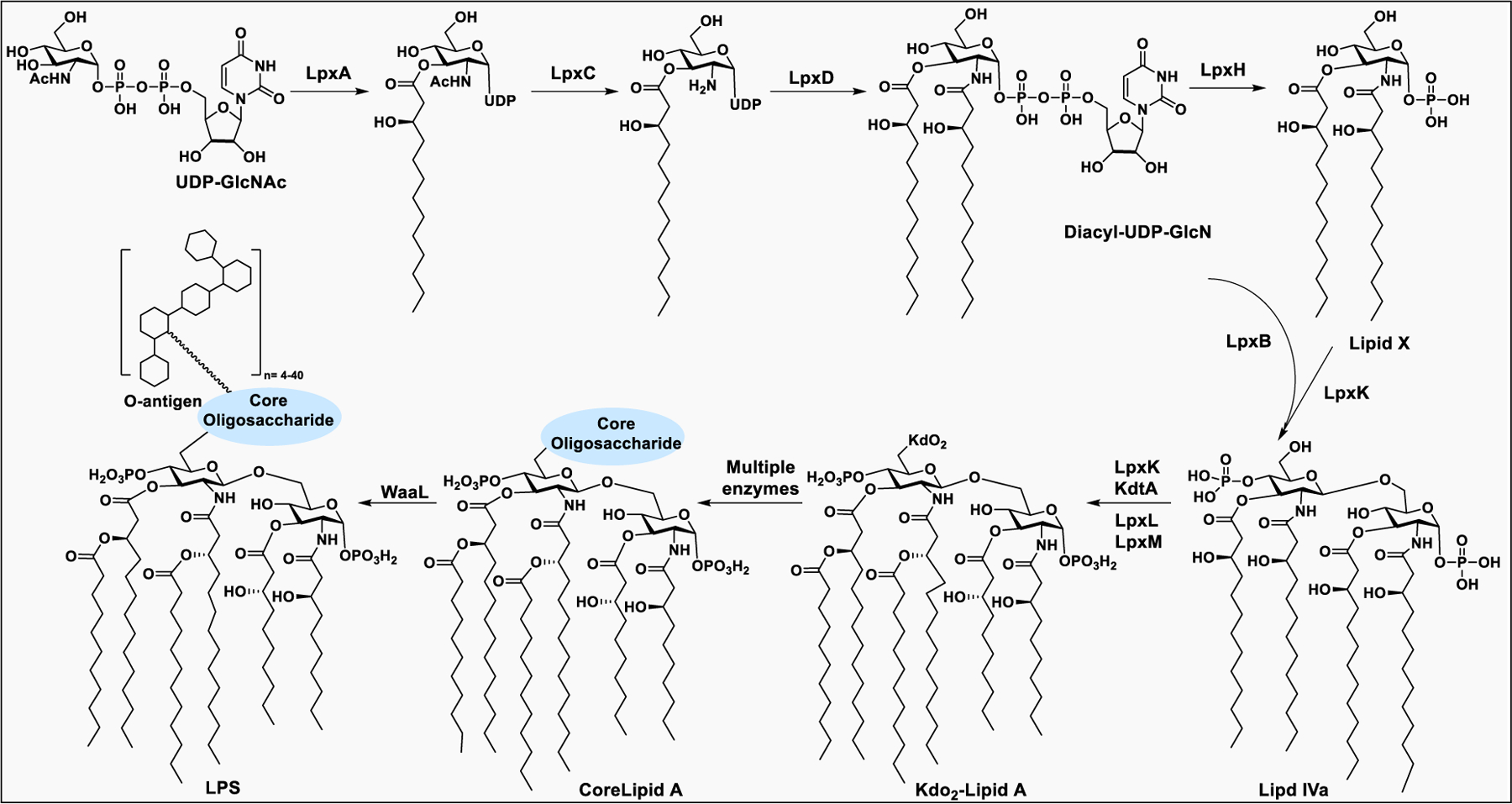
Biosynthetic pathway for the synthesis of bacterial LPS
The synthesis of lipid A takes place in the cytoplasm catalyzed by nine enzymes. The sugar nucleotide donor, UDP-GlcNAc, is processed to add fatty acid chains by three soluble enzymes, LpxA, LpxC, and LpxD. First, LpxA catalyzes addition of a lipid chain to the 3-O position of UDP-GlcNAc, followed by deacetylation of the 2-acetamino group of UDP-3-O-acyl-GlcNAc by LpxC. Next, LpxD introduces another lipid chain to the 2-amino group of glucosamine to form UDP-2,3-diacyl-GlcN. Next, LpXH cleaves the pyrophosphate bond of UDP-2,3-diacyl-GlcN to form the intermediate lipid X. Then, LpxB, an inverting glycosyltransferase, catalyzes the transfer of 2,3-diacyl-GlcN from UDP-2,3-diacyl-GlcN to lipid X, and releases UDP. In the subsequent steps of the pathway, LpxK catalyzes the ATP-dependent phosphorylation of the disaccharide-1-P intermediate to form lipid IVa.208
The core oligosaccharides are successively constructed on lipid A through the action of several membrane-associated glycosyltransferases, using sugar nucleotide donors. For the inner core, the enzyme KdtA catalyzes the incorporation of Kdo residues at the 6′-O positions of the distal GlcNAc of lipid A, using the sugar nucleotide CMP-Kdo as the donor.210 The resulting Kdo-lipid A is further acylated with fatty acids at the distal GlcNAc, catalyzed by LpxL and LpxM to form the hexa-acylated Kdo-lipid A.211
The assembly of the O-antigen takes place in the cytoplasm by GTs on membrane bound undecaprenyl phosphate. The O-antigen synthesis is not a stepwise addition of monosaccharides to the growing LPS,200 it is synthesized separately on a lipid carrier by the enzymes encoded by the rfb gene cluster and then transferred to the growing lipid A on the periplasmic face of the plasma membrane. The structural complexity of the O-antigens stems from variations in sugars, the sequences and linkages, and the substitution of monomers with either sugar or nonsugar residues. The structure of O-antigen may be linear or branched.212 During transportation from periplasm to inner membrane, the O-antigen is polymerized and connected to the core lipid A to form LPS213
2.3.4. Structural Modification of Lipopolysaccharides and Bacterial Virulence.
Gram-negative bacteria contain numerous genes to synthesize the various components of LPS and transport the whole complex to the cell surface.214 In addition, they also contain genes to change the composition of LPS, the O-antigen, the core oligosaccharide, and even the most conserved lipid A.206 Structural modifications in lipid A, which usually occur in the fatty acid chain as well as in the hydrophilic sugar head, help the pathogen evade the recognition by host innate immune responses. The two phosphate groups in lipid A impart a net negative charge that helps to bind positively charged cationic antimicrobial peptides (CAMPs); however, some pathogens have evolved to contain a less negative charge to evade the immune attack. Some bacteria either remove the phosphate groups at the 1- and 4′-positions or modify them with phosphoethanolamine, which helps increase resistance to CAMPs.200
LPSs are key activators of immune responses during pathogenic infections. In milder infections, immune activation helps the host clear the pathogen; however, in more severe infections, induction of a cytokine storm might result in septic shock.200 Lipid A is responsible for immune activation, however, some Gram-negative pathogens modify lipid A structure to evade human TLR 4.196 Some structural elements of Lipid A, particularly the phosphate group and fatty acyl chains, regulate TLR4 activation. For example, the E. coli lipid A, which is a potent immune activator consisting of two phosphate groups and six acyl chains with 12 or 14 carbons. In contrast, the highly infectious Francisella tularensis can produce LPS without the core-oligosaccharide and O-antigens.206,208
3. SYNTHETIC GLYCOCONJUGATES
3.1. Synthetic Glycoconjugates and Their Clinical Significance
In nature, glycoproteins are highly heterogeneous, i.e., various glycoforms are present at a given glycosylation site of the same peptide sequence. Therefore, to elucidate the underlying functions of carbohydrate in the context of glycoconjugates, a plethora of strategies and methods have been developed to prepare fully defined glycan structures and their conjugation to protein or lipid core. The synthesis of O- and N-glycoproteins was carried out through combined use of glycan and peptide synthesis or through ligation of synthetic glycopeptides or expressed proteins.215 Neoglycoproteins are often considered as the best starting point to study the effects of glycan composition on protein function.
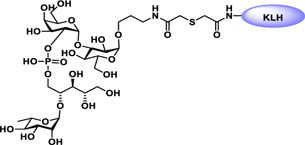
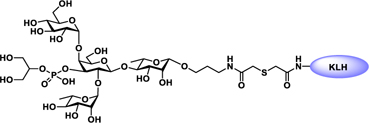
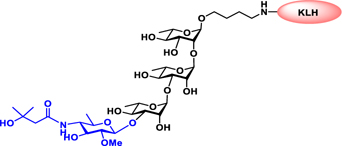
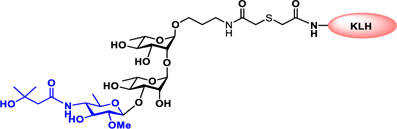
Pathogen-associated carbohydrate activates host immune responses during infection to induce cytokines and the production of antiglycan antibodies.216 However, carbohydrates are weak immunogens, therefore their conjugation to a carrier protein is often necessary to enhance the immunogenicity. Some commonly used modifications on glycans includes introduction of alkenes, thiols, or activated esters at the reducing end for attachment of glycan to protein surface. The conjugation reaction usually takes place between an activated glycan and the primary amine of lysine residues, carboxylates, or the thiol group of cysteine on proteins, or with unnatural modifications that are site specifically inserted into the protein.217 Alternatively, some cross-linkers have been developed that contain a difunctionalized spacer which is reactive against two different nucleophiles; for example, an amine from the glycan part and a thiol from the protein and vice versa.218 Carbohydrate-based vaccines using the carrier protein such as keyhole limpet hemocyanin (KLH), tetanus toxoid, or its nontoxic variant CRM197 to facilitate multivalent glycan presentation for glycan specific immune responses are under development for cancers and infectious diseases.53,191 Other than carrier proteins, several other platforms have been explored for synthesis of glycoconjugates such as ferritin, dendrimers, polymers, nanoparticles, and carbon nanotubes etc. Gold nanoparticles have been used for glycan conjugation to study carbohydrate–protein interactions and to enhance the binding affinity through multivalent glycan presentation on the nanoparticle surface.219
Conjugation of carbohydrate to lipids transforms glycans from being poorly immunogenic to being strong immune activators. Glycolipids play a dual role by interacting both with carbohydrate-binding and lipid-binding receptors, such as TLRs on immune cells, to induce an immune response.220 These interactions of glycolipids with immune cells could stimulate the immune system to exhibit adjuvant or modulation activities. The adjuvant activity of glycolipids has been demonstrated in synthetic vaccines through conjugation of immuno-active lipids to carbohydrate antigens.221
3.2. Methods of Glycoconjugate Synthesis
3.2.1. Proteoglycan Conjugates.
The therapeutic potential of proteoglycans and their GAGs for new treatments remain unexploited because of their complex structural organization and association with various biophysical processes. Because of sulfation, most of the GAGs exist as anionic molecules linked to a protein backbone. Being part of proteoglycans, GAGs also interact with other biomolecules through electrostatic, hydrophobic, and/or hydrogen bond interactions. Recently, GAGs grafted onto synthetic polymers, peptides, and nanoparticles have been explored for their therapeutic applications.
3.2.1.1. Glycosaminoglycan–Polymer Conjugates.
The proteoglycan, aggrecan, is composed of negatively charged CS linked to the core protein in a bottlebrush manner. Aggrecan is essential for connective tissue hydration; however, with age, enzymatic degradation of aggrecans affects its cellular synthesis and deficiency of this important biomolecule results in loss of tissue water retention. Recently, the Marcolongo group incorporated CS into a stable synthetic poly(acryloyl) backbone to mimic the naturally occurring aggrecan and serve as a tool for drug delivery and tissue enginering.222 In another study, CS and heparin were grafted onto a hyaluronan backbone that is functionalized with a hydrazide linker.223 The construct prepared through reductive amination chemistry allows insertion of different ratios of CS and heparin side chains on the hyaluronic acid (HA) core.224 These copolymers were used for delivery of fibroblast growth factor (FGF-2) to mesenchymal stem cells (MSCs). The synthetic GAG copolymers has been used to fine-tune the graft density of PG mimetics for developing biomaterials with desired biochemical properties. The potential of embryonic stem cells (ESCs) for the treatment of neurodegenerative diseases, such as Alzheimer’s disease (AD), multiple sclerosis (MS), and Parkinson’s disease (PD), sparked a great interest in developing strategies for the efficient differentiation of ESCs into neural cells.225 Because GAGs have been implicated in the regulation of ESCs differentiation,226 treatment with exogenous GAG-grafted biomolecules for specific differentiation of ESCs into neural cells, emerged as a promising strategy.227 Interactions of growth factors with their receptors are often facilitated by PGs side chains. Heparin and HS can form ternary complexes with FGF2 and the corresponding FGF receptors presented on mouse ESC membranes.228,229 Recently, Liu et al. used lipid-anchored synthetic GAG-mimicking glycopolymers (lipo-pSGF) for incorporation into the surface of ESCs to promote FGF signaling and ESC differentiation. The lipo-pSGF was found to bind efficiently to FGF-2 and enhanced the phosphorylation of ERK1/2, thereby promoting neural differentiation (Scheme 2a).230 In another related study, the Godula group reported fluorescently labeled synthetic neoproteoglycan conjugates to assess their affinity for FGF-2 using a microarray and the activation of ERK1/2. The synthetic neoproteoglycans were introduced into the plasma membrane of ESCs with deficient HS biosynthesis to study the possible mechanism involved in glycopolymer-mediated neural differentiation (Scheme 2b).231
Scheme 2:
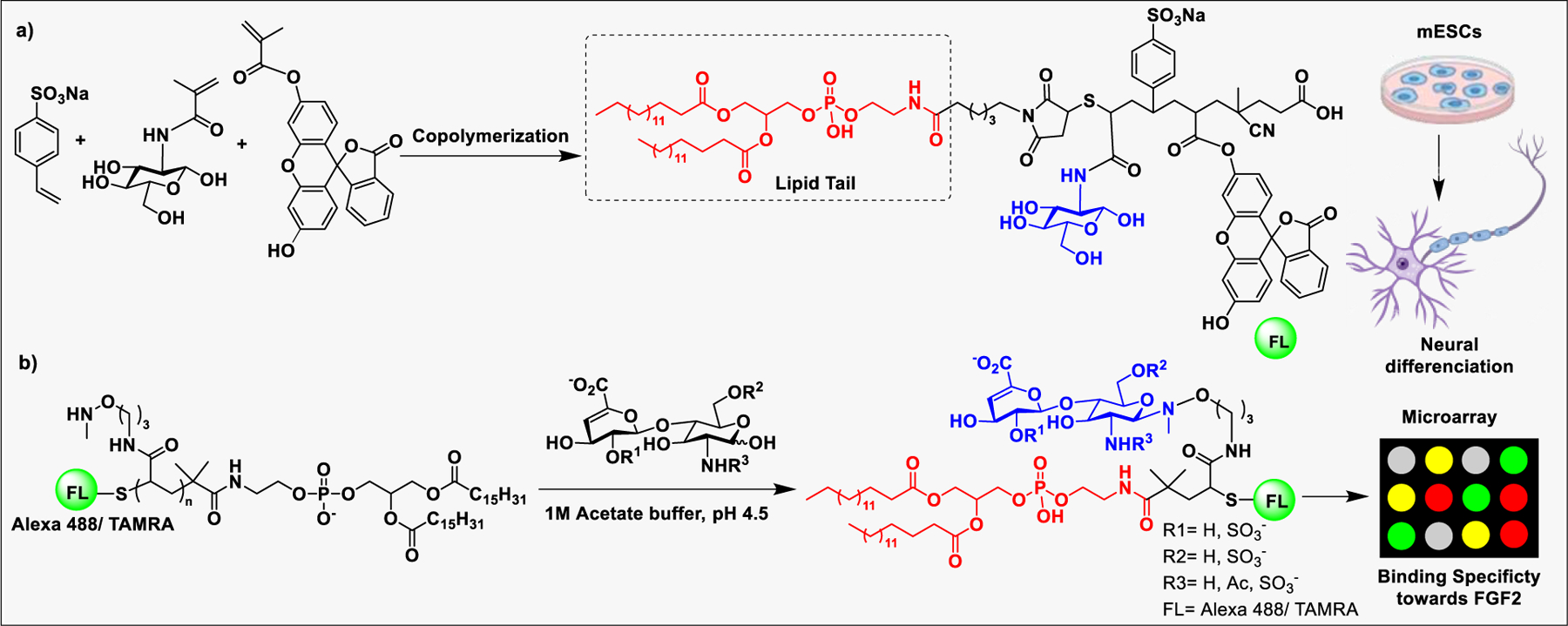
a) Synthesis of lipid-anchored GAG biomimetics and their incorporation into mouse ESC cell membranes for neural differentiation. b) Microarray screening of a library of fluorescently labeled neoproteoglycan conjugates for those binding to FGF2 and promoting neural differentiation.
3.2.1.2. Glycosaminoglycan–Peptide Conjugates.
Conjugation of polyethylene glycol (PEG) to a protein or peptide drug has been proven to be beneficial for improving the pharmacokinetics.232 PEGylation of peptides reduces renal extraction and degradation by proteolytic enzymes.233 However, PEG is not biodegradable, therefore, repeated administration resulted in cellular accumulation.234 In addition, PEGylated proteins or peptides often cause the production of anti-PEG antibodies.235 As an alternative to PEG, highly hydrophilic and biodegradable GAGs such as hyaluronan and heparosan (HPN) have been explored to improve the pharmacokinetics of protein or peptide drugs (Scheme 3).236 Recently, GAG conjugates of two antidiabetic peptide drugs, glucagon-like peptide-1 (GLP-1) and insulin, were shown to have much better half-life and blood-glucose lowering efficacy than unconjugated peptides after subcutaneous injection in mice.237,238 Various GAGs including chondroitin (CH) and heparosan (HPN) were conjugated to GLP-1 (at engineered Cys35) and insulin (at GlyA1, LysB29, or both) using a hydroxy succinimide-maleimide heterobifunctional linker and various arm lengths. Among the GAGs tested, conjugates containing CH and HPN provided the best-balanced profile of in vitro activity and circulation period in mice, suggesting that conjugation with GAGs is a promising strategy for improving the duration of peptide drugs.237,238
Scheme 3:
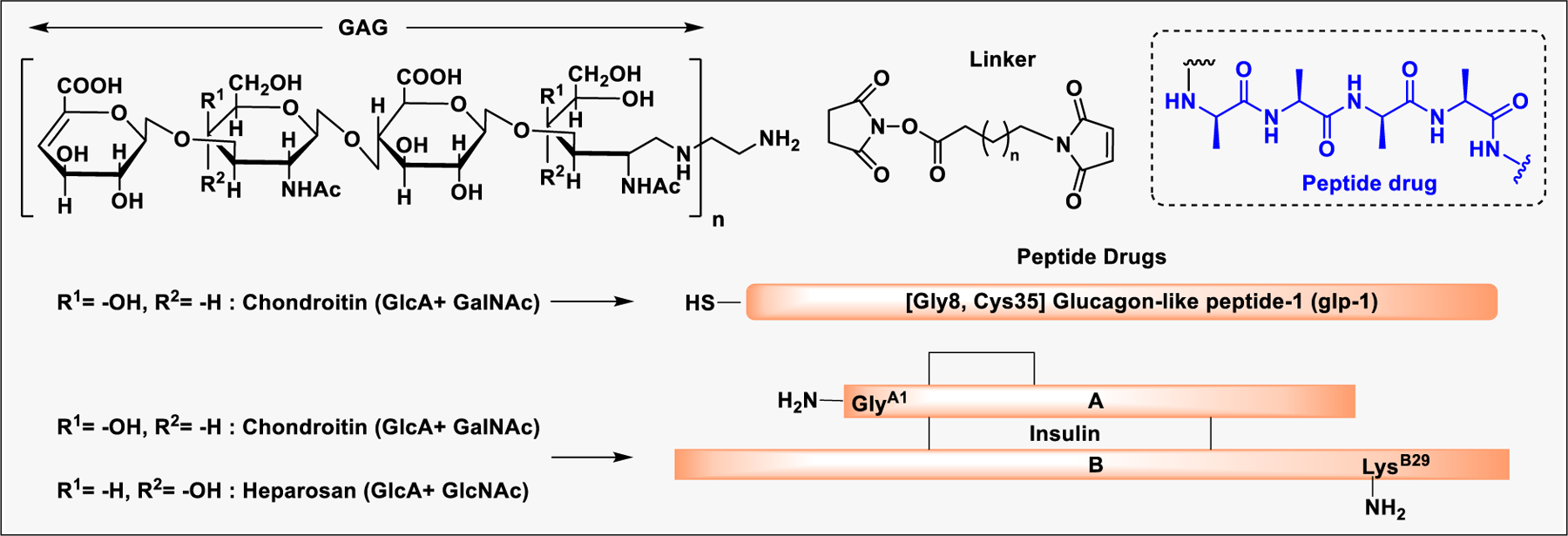
GAG conjugates of anti-diabetic peptide drugs, Glucagon-like peptide-1 (GLP-1) and Insulin, to improve pharmacokinetics
3.2.1.3. Glycosaminoglycan–Nanoparticle Conjugates for Targeted Delivery.
Functionalized nanoparticles have generated considerable attention in biomedical uses such as targeted drug delivery for cancer therapy and bioimaging.239 In particular, mesoporous silica nanoparticles (MSNPs) have been widely used as a carriers for anticancer drugs because of their biocompatibility, efficient surface functionalization, and chemical stability, etc.240 Several approaches have been explored for the targeted delivery of anticancer drugs to certain cancer cells. These strategies are relied on functionalization with specific ligands which can bind to the tumor-associated receptors to promote internalization via receptor-mediated endocytosis.241 In this context, HA has been used to target CD44 on solid tumors, on metastatic cancers, and cancer stem cells.242,243
HA-functionalized MSNPs incorporating doxorubicin (DOX) and a photosensitizer Chlorin e6 (Ce6) was used in photodynamic therapy (PDT) and chemotherapy to treat squamous cell carcinoma 7 (SCC7).244 The nanoconjugate (DOX/Ce6/HA-MSNP) binds CD44 ligand, and the whole complex undergoes endocytosis to exhibit photoinduced toxicity by forming highly reactive singlet oxygen (SO) in SCC7 cells. In other related studies, (i) doxorubicin loaded on HA-MSNPs functionalized with polyethyleneimine (PEI) has been used for targeted delivery with increased endosomal escape efficiency and controlled drug release;245 (ii) CD44-targeted HA nanoparticles were used to deliver siRNA into tumor cells;246 and (iii) HA-PEI/HA-PEG-based nanoparticles were also used to target CD44 on ovarian cancer for delivery of MDR1 siRNA in vivo to enhance drug potency and overcome MDRI-related multidrug resistance.(Figure 12).247
Figure 12.
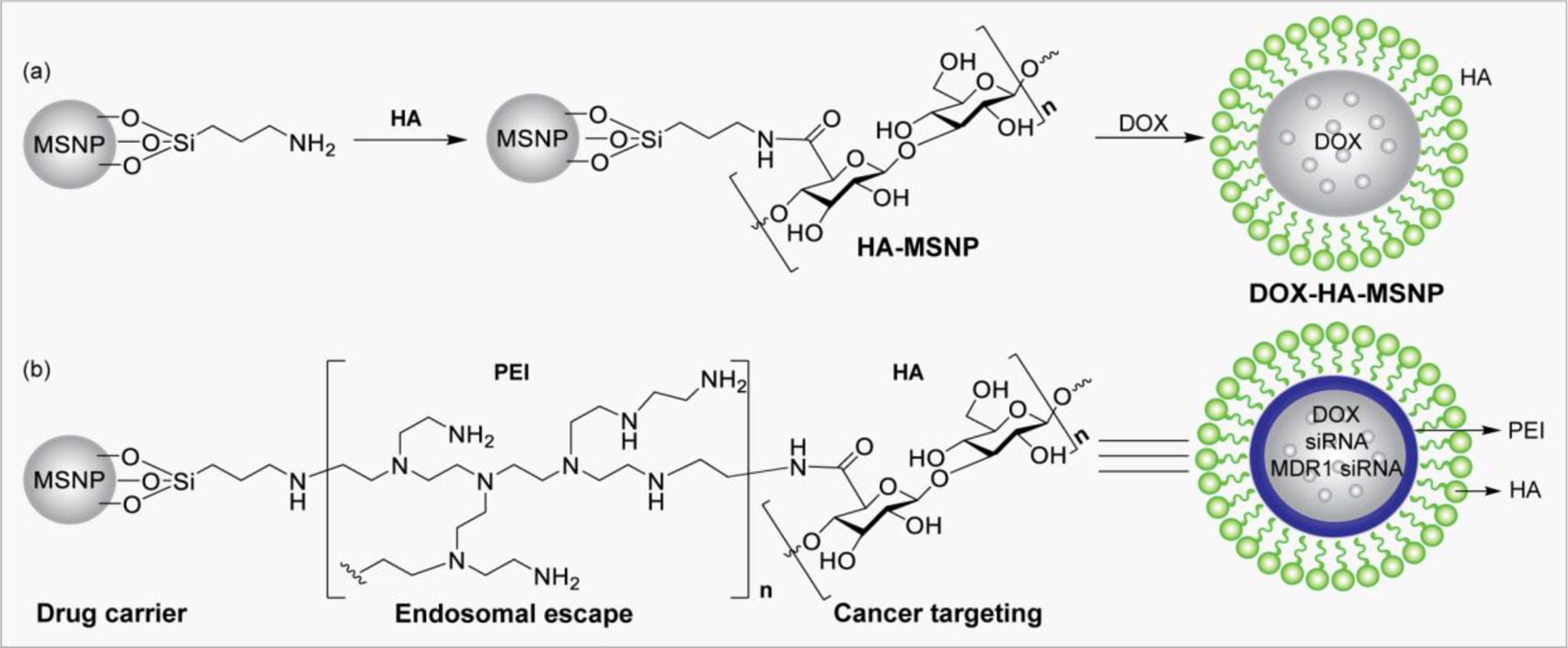
a) HA-functionalized MSNPs incorporating doxorubicin (DOX). b) CD44-targeted HA-PEI -based nanoparticle to deliver DOX, siRNA, and MDR1 siRNA.
Lipid nanoparticles (LNPs) have gained tremendous momentum as a versatile nanocarrier platform to deliver many hydrophobic or hydrophilic therapeutic agents. A variety of drug liposome formulation have been developed and clinically approved for therapeutic use, indicating the success of this delivery platform from concept to clinic.248,249 For example, doxorubicin formulated with LNP was approved for ovarian cancer.250 Epaxal is another early example that used a LNP as the protein antigen in a hepatitis vaccine.251 Many other liposome formulations have been approved, while numerous clinical trials are ongoing using liposomes for targeted delivery of anticancer, anti-inflammatory, antibiotic, antifungal, anesthetic, and other drugs and gene therapies.252
Liposomes consist of one or several lipid bilayers of phospholipids such as phosphatidylcholines, phosphatidyl ethanolamines, phosphatidylserines, and phosphatidylglycerols, and stabilizers such as cholesterol, ranging in size between 20 and ∼1000 nm.253 Hydrophilic drugs can be enclosed in the aqueous interior of liposomes, while hydrophobic drugs can be entrapped in the hydrophobic environment, making liposomes a versatile drug delivery system. The structure of liposome is dependent on the preparation method and liposome size is a critical parameter in determining the efficiency of drug encapsulation and half-life in circulation. The charge on the surface of lipid nanoparticle may be either positive or negative or zwitterionic based on the lipid headgroup that regulates the overall stability of the nanoparticles. Particles with neutral charge or low charge densities tend to aggregate over the time, whereas highly charged particles prevent aggregation.254
The important milestone in LNP-based drug delivery is evidenced by the recent development of COVID-19 mRNA vaccines by Pfizer/BioNTech and Moderna, which have shown notable effectiveness in disease prevention.255 The LNP formulation of mRNA encoding spike protein is delivered into host cells to produce spike protein as a foreign antigen and to elicits immune responses to the virus.252,256 The lipid nanoparticles of the two mRNA vaccines contain an ionizable lipid that is positively charged at low pH and is neutral at physiological pH to reduce toxicity and facilitate payload release.257,258 The LNPs also contain a PEGylated lipid to reduce the possibility of antibody association (opsonization) by serum proteins and clearance by phagocytes, thus conferring longer systemic circulation.259
3.2.2. Glycoprotein and Glycopeptide Synthesis.
Synthesis of glycopeptide or glycoprotein includes covalent attachment of a sugar residue or a glycan to an oligopeptide or protein. Glycopeptides and glycoproteins with precise glycan composition have been made by a variety of methods. Using the conventional solid-phase peptide synthesis (SPPS), the glycosylated amino acids can be coupled to the growing polypeptide chain; however, larger peptides are difficult to prepare because of accumulation of side products from incomplete reactions and epimerization that results in poor yield.260 As an alternative, the convergent coupling of a partially protected glycopeptide building unit to another short peptide has been used to overcome the issues with linear SPPS, but the stability of glycosidic bond remains a major problem in deprotection or release of the peptide from the solid support. Another straightforward strategy is direct coupling of the sugar residue to the aspartic acid side chain of polypeptide through amide linkage. Nevertheless, a key concern of this method is the low coupling efficiency of large oligosaccharides due to steric hindrance between glycans and peptide side chains.260–262
Nowadays, chemoselective ligation has become an incredibly attractive approach to make homogeneous glycopeptides or glycoproteins. This technique allows the efficient conjugation of reactive glycosyl donors with unprotected peptides, avoiding protecting group manipulations. So far, the most efficient chemical method for the synthesis of glycopeptides and glycoproteins is native chemical ligation (NCL).262 An alternative strategy is to combine the flexibility of chemical synthesis and the regio- and stereoselectivity of enzymatic synthesis. This chemoenzymatic method allows a convergent ligation of a preformed oligosaccharide to a polypeptide moiety, without the need for any protecting groups.260 Glycosyltransferases have been explored for stepwise addition of sugar residues on preformed glycopeptides using respective sugar nucleotide donors. In contrast, endoglycosidases, whose intrinsic activity is to cleave the β−1,4 linkage between two adjacent GlcNAc residues to release large intact oligosaccharide moieties from glycoproteins, were also shown to catalyze the transfer of various oligosaccharide building blocks in the form of oxazolines to the GlcNAc acceptor in a single step.261 A major disadvantage of this method is the nonselective reaction of oxazoline with protein, hydrolysis of the tranglycosylated product, and some of glycosidic linkages cannot be formed due to the lack of respective enzymes and the nucleotide sugars are expensive.
3.2.2.1. Native Chemical Ligation.
The concept of amide bond formation in the context of oligopeptide synthesis was first introduced by Kemp and co-workers via intramolecular aminoacyl transfer. In this strategy, a dibenzofuran scaffold was utilized to ligate a peptide bearing an electrophilic C-terminal activated ester with another peptide bearing a nucleophilic amino group at the N-terminal.263 Dibenzofuran moiety served as an auxiliary that facilitates the temporary formation of a disulfide linkage to bring the two peptides close enough for coupling with subsequent promotion of the O to N acyl transfer. Lastly, the auxiliary can be cleaved to afford the native peptide. Inspired by this strategy, several other scaffolds have been prepared for efficient peptide ligation to obtain large peptides or glycoproteins (Scheme 4a).264
Scheme 4:
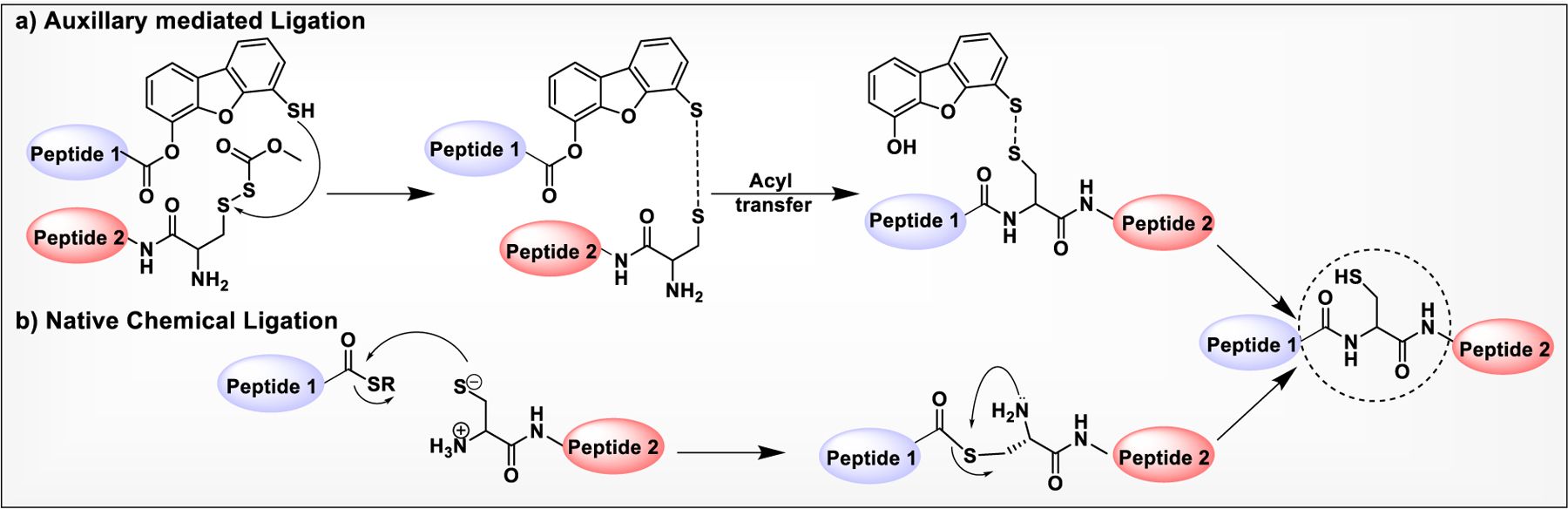
a) Dibenzofuran-Based Rigid Scaffold Mediated Ligation of Peptides. b) Mechanism of Native Chemical Ligation.
In 1994, the Kent group introduced NCL that allows the chemoselective ligation of an unprotected peptide component with formation of an amide bond at the ligation site.265 In this approach, a peptide with a preinstalled C-terminal thioester couples with an N-terminal cysteine residue of another peptide to undergo thiol/thioester exchange to form a thioester intermediate with the cysteine thiol (Scheme 4b). This thioester intermediate promotes nucleophilic attack of the α-amino group of cysteine on the ester carbonyl that results in highly favored intramolecular S to N acyl rearrangement, leading to an irreversible formation of the native peptide bond. The introduction of N-terminal cysteine and thioester functionalities during the synthesis of polypeptides by SPPS provided additional flexibilities for insertion of unnatural amino acids. However, synthesis of peptide precursors on large scale for peptides large than 50 residues become a tedious and costlier endeavor that limits the use of NCL to smaller proteins or peptides.
NCL has been used as a powerful method for the preparation of biologically significant glycoconjugates. The Bertozzi group reported the NCL-based total chemical synthesis of antibacterial glycopeptide, diptericin ε. Due to the lack of Cys residue in the primary sequence of diptericin, a G25C mutation was introduced at the connection site.266 In another early report, the same group employed NCL for the synthesis of a glycoprotein called lymphotactin (Lptn).267 Lymphotactin is an effective chemoattractant for T- and NK-cells, consisting of 93 amino acids and eight O-linked glycosites. In 2005, Unverzagt reported the first synthesis of an N-linked RNase B glycopeptide fragment having a complex-type glycan by NCL.268 The total synthesis of chemokine monocyte chemotactic protein-3 (MCP-3) containing 76 amino acid residues carrying a complex type N-glycan was first reported by Kajihara and co-workers, using a double NCL of three peptides with or without glycan.269 Later in 2012, the Danishefsky group reported the synthesis of two glycoforms of the α-subunit of human glycoprotein hormone (α-hGPH) bearing simple chitobiose units, as well as core-fucosylated, sialylated biantennary complex type N-linked dodecasaccharides.270 Following it, the total synthesis of homogeneous full-length β-hCG containing two chitobiose residues and four GalNAc moieties at the N- and O-glycosylation sites was reported by the same group in 2014 (Figure 13).271
Figure 13:
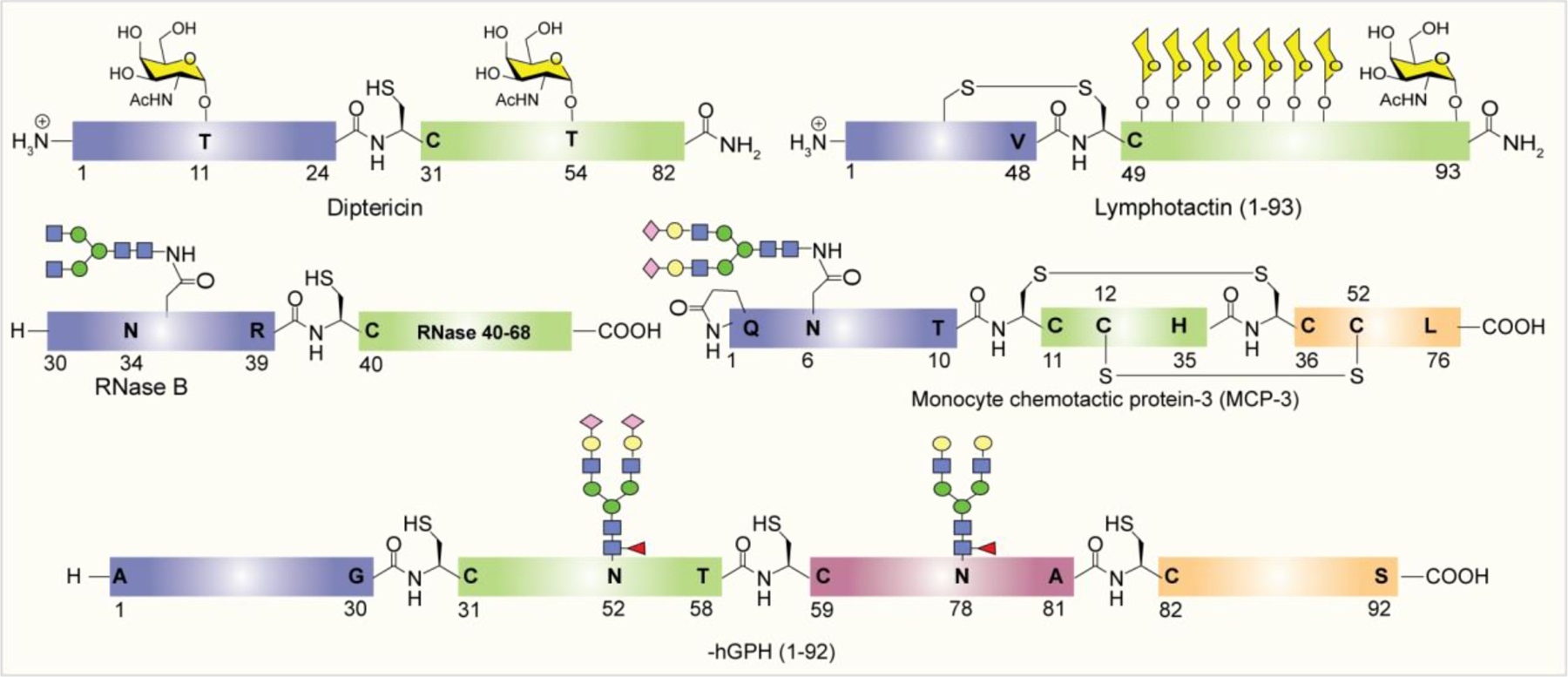
Examples of glycoproteins prepared by NCL.
Despite its potential, the use of NCL in protein synthesis is limited by the necessity of having a N-termini Cys residue of one of the coupling partners. Therefore, to find an alternative to the use of N-terminal Cys residue, highly innovative methodologies have emerged involving the use of N-terminal auxiliaries.272,273 Although auxiliary-mediated ligations improved ligation efficiencies in peptide couplings, these methods suffer from longer reaction time, side reactions such as hydrolysis and epimerization, and tedious auxiliary removal steps. This limits their application in the preparation of large proteins.274
3.2.2.2. Expressed Protein Ligation.
The power of NCL combined with expressed protein ligation (EPL) allows synthesis of larger targets where one of the two coupling partners is often produced by recombinant DNA technology. Several methodologies for the synthesis of coupling fragments have been reported.275 For example, the peptide fragment with N-terminal cysteine was produced in bacteria by inserting a protease cleavage site adjacent to cysteine residue, which can be cleaved by proteases such as factor Xa and tobacco etch virus protease (TEV protease) to form the peptide fragment with N-terminal cysteine for coupling with the peptide containing a C-terminal thioester.276–278 The thioester containing coupling partner can be prepared as an intein fusion protein, which upon intein-mediated acyl transfer generates the fragment with C-terminal thioester for coupling with the cysteine containing fragment (Scheme 5).
Scheme 5:
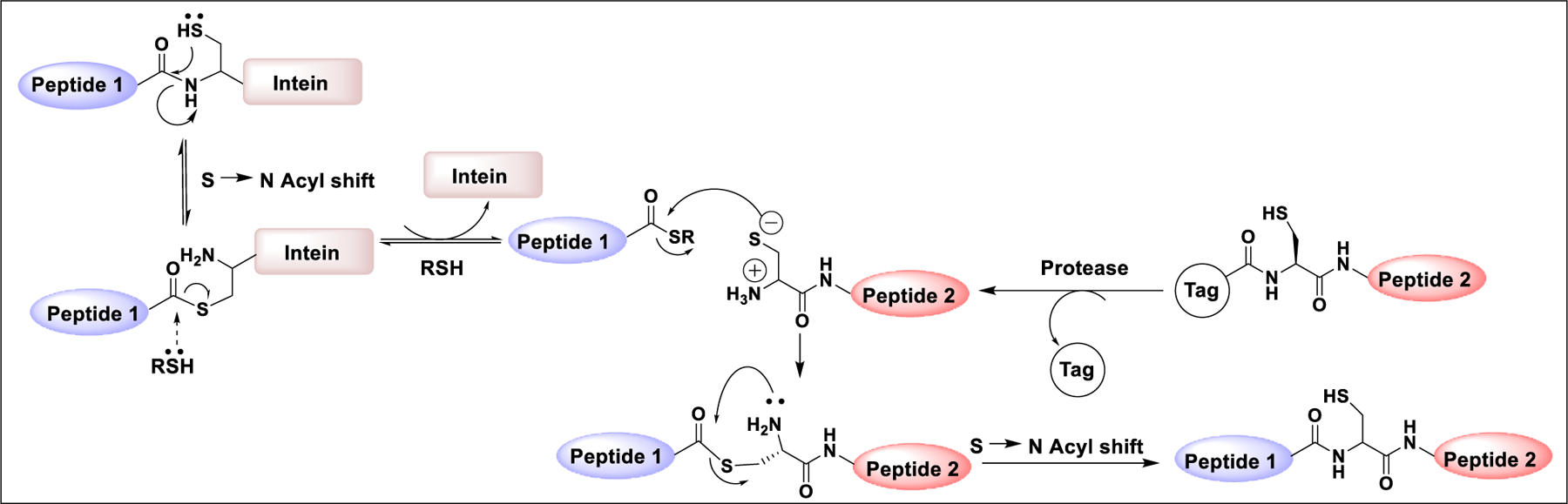
Mechanism of expressed protein ligation
The synthesis of a T-cell growth factor, interleukin-2 (IL-2), was reported by Tolbert et al.277 In this method, the C-terminal fragment containing the TEV protease recognition sequence (ENLYFQ) and a long C-terminal fragment of IL-2 (7–133) was expressed as a fusion protein. TEV protease mediated cleavage followed by NCL with a synthetic glycopeptide provided the homogeneous glycoform of full-length IL-2.277 The power of EPL was applied to the synthesis of maltose-binding protein (MBP) having homogeneous glycosylation.279 First, MBP was expressed in E. coli an intein fusion protein. Next, the thioester exchange reaction cleaves the intein from the protein, and the resulting thioester intermediate was then reacted in situ with a glycopeptide bearing an N-terminal cysteine, e.g., H-Cys-Asn(β-GlcNAc)-OH to give the thioester, which then went through an S–N acyl shift to furnish homogeneous MBP 26 bearing C-terminal glycosylation.279 Macmillan and Bertozzi reported a EPL-based synthesis of GlyCAM-1 containing two glycosylated mucin subunits at both termini.280 The two consecutive ligations of glycosylated N- and C-terminal fragments with internal nonglycosylated fragment afforded the full-length multi-GalNAc containing glycoprotein.281 EPL has also been utilized for preparation of full-length ribonuclease C and erythropoietin containing two complex type sialylated glycans (Figure 14).282–284
Figure 14:
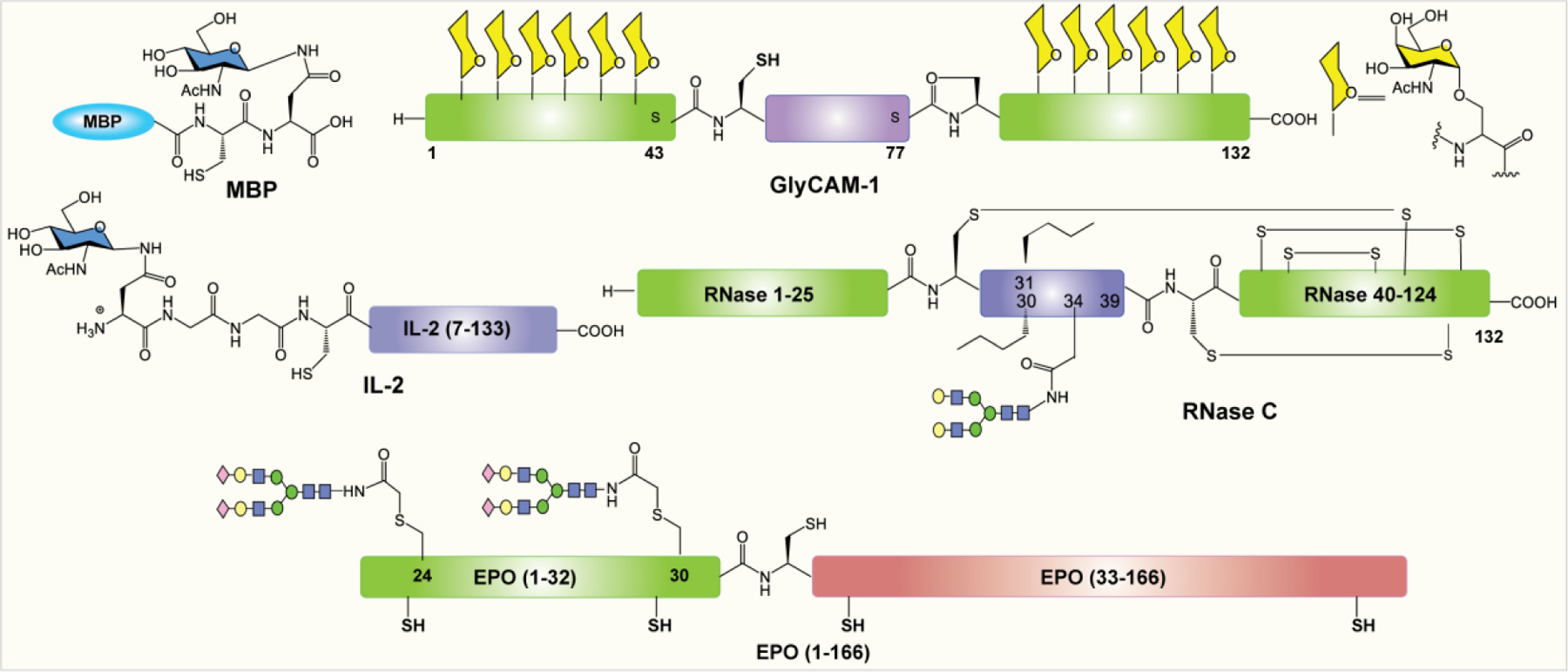
Structures of glycoproteins prepared by EPL-based methods.
Entry of SARS-CoV-2 is initiated by interaction with host ACE2 receptor via the receptor binding domain (RBD) of the spike protein, which is an important target for vaccine design.285–287 However, the heterogeneous glycan compositions of the RBD may affect the neutralization and evasion of immune response generated by RBD-based vaccines.127,288 To understand the impact of RBD glycosylation on infection and antibody neutralization is of great interest; however, the size, physical properties, high structural diversity, and complexity of the RBD glycoproteins represent a significant synthetic challenge. Recently, EPL was used for construction of glycosylated RBDs containing homogeneous N-linked glycans at N331, N343, and O-linked glycan at T323 (Scheme 6).289 The synthesis of homogeneous glycosylated RBDs was divided into two fragments: a synthetic glycopeptide RBD (319–360) fragment 1 with N-/O-glycans, which was functionalized with a C-terminal hydrazide; and a recombinant RBD fragment 2 (361–537) possessing an N-terminal Cys residue facilitating NCL. The glycopeptide fragment 1 (319–360) was prepared by NCL of fragment 3 (R319-L335) having desired glycosylation at sites T323 and N331 and fragment 4 (C336-N360) having biantennary complex type glycan at N343.
Scheme 6:
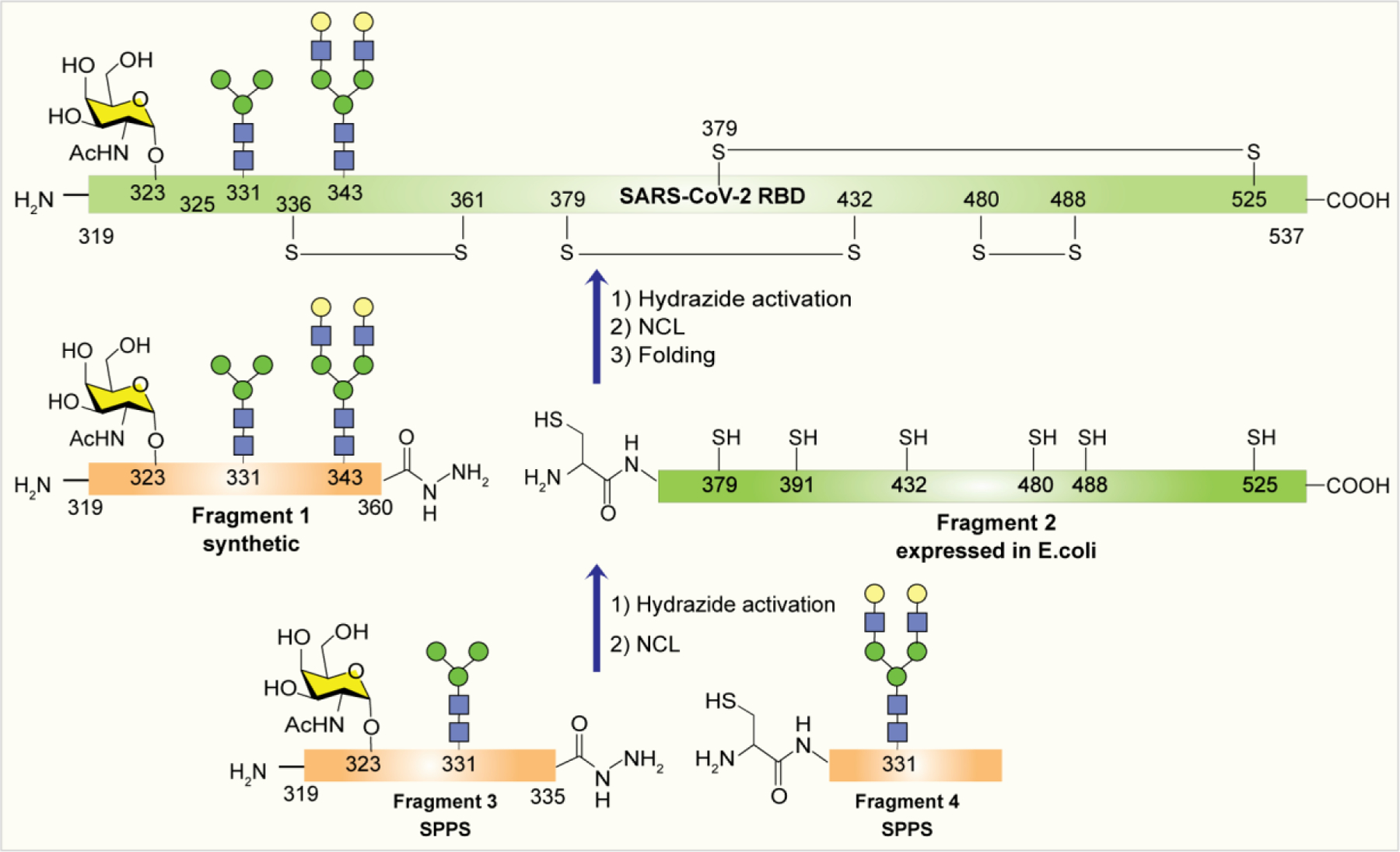
Semisynthetic preparation of SARS-CoV-2 RBD glycoforms using EPL.
3.2.2.3. Sugar-Assisted Ligation.
To eliminate the necessity for Cys-bearing peptides, the Wong group developed a sugar-assisted ligation (SAL) strategy for synthesis of cysteine-free β-O-linked and N-linked glycopeptides.290,291 SAL utilized the acetamido group at the 2-position of sugar to introduce a sulfhydryl group for transthioesterification with peptide thioester that favors S → N rearrangement at the ligation site. At last, the sugar can be regenerated by removal of the sulfhydryl group using hydrogenation (Scheme 7). This strategy was further extended with modified sugar analogues containing labels.292
Scheme 7:
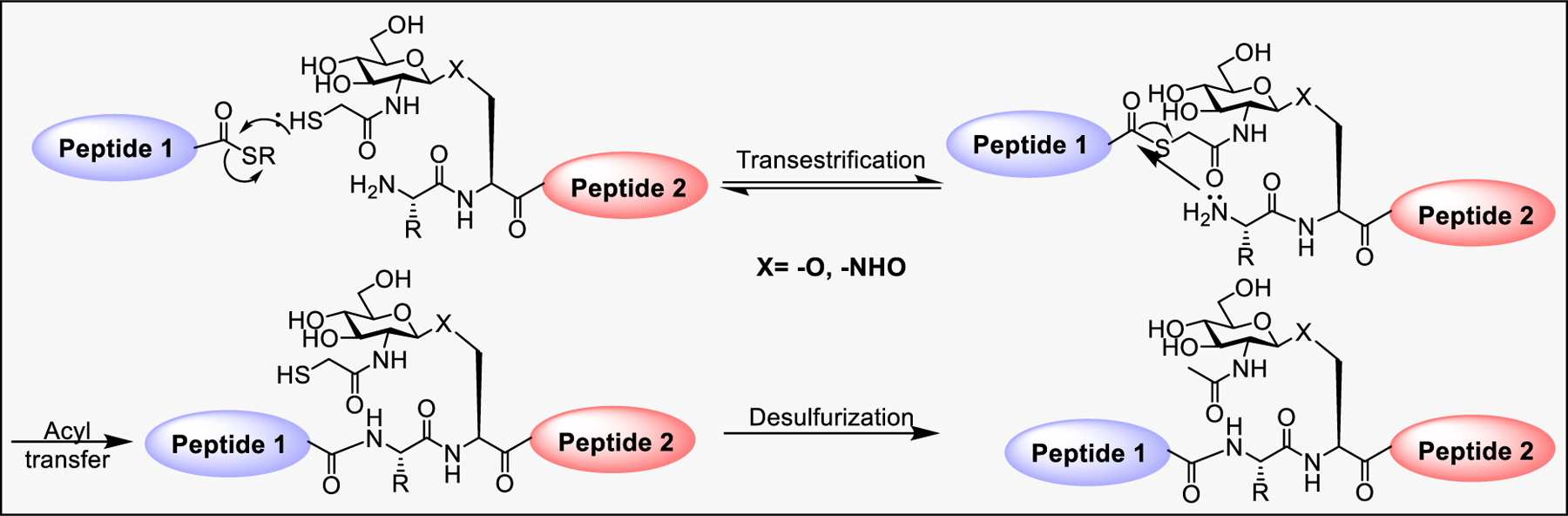
Mechanism of SAL
3.2.2.4. Enzymatic Glycoremodeling for Synthesis of Homogeneous Glycoproteins.
Glycosyltransferases have been used extensively for construction of a glycosidic bond.293 However, the use of GTs for large-scale production of glycoproteins is limited by their availability, strict substrate specificity, and use of expensive sugar nucleotide substrates such as UDP-Glc, UDP-GlcNAc, UDP-Gal, GDP-Man, GDP-Fuc, and CMP-sialic acid.294 However, one-pot in situ sugar nucleotide regeneration has been developed to overcome these limitations. Endoglycosidase catalyzed glycosylation of heterogeneous natural or recombinant glycoproteins is emerging as a powerful methodology for the production of proteins with homogeneous glycoforms.295 In a typical mechanism, endoglycosidase cleaves the β1,4-linkage between the two adjacent GlcNAc residues at the N-linked glycosylation site to form mono-GlcNAc containing peptide or protein. Next, the predefined N-glycan oxazoline can be transferred en bloc to the GlcNAc protein via glycosynthase-catalyzed transglycosylation (Scheme 8).296 The transglycosylation based glycoprotein synthesis is enabled by exploitation of donor substrates and the discovery of glycosynthases, mutant of endoglycosidases without product hydrolyzing activities. Notable examples that include Endo-M, Endo-A, Endo-D, Endo-S, Endo-S2, and Endo-F3 with broader acceptor substrate specificity have been transformed into glycosynthases through site-directed mutagenesis.261 Among those, EndoSN322A,297 EndoS2D184M,298 and EndoS2T138Q299 have been extensively used in the synthesis of complex glycoproteins, and glycopeptides such as glycosylated CD52, CMV, and pramlintide antigens and the homogeneous form of anticancer antibodies.
Scheme 8:
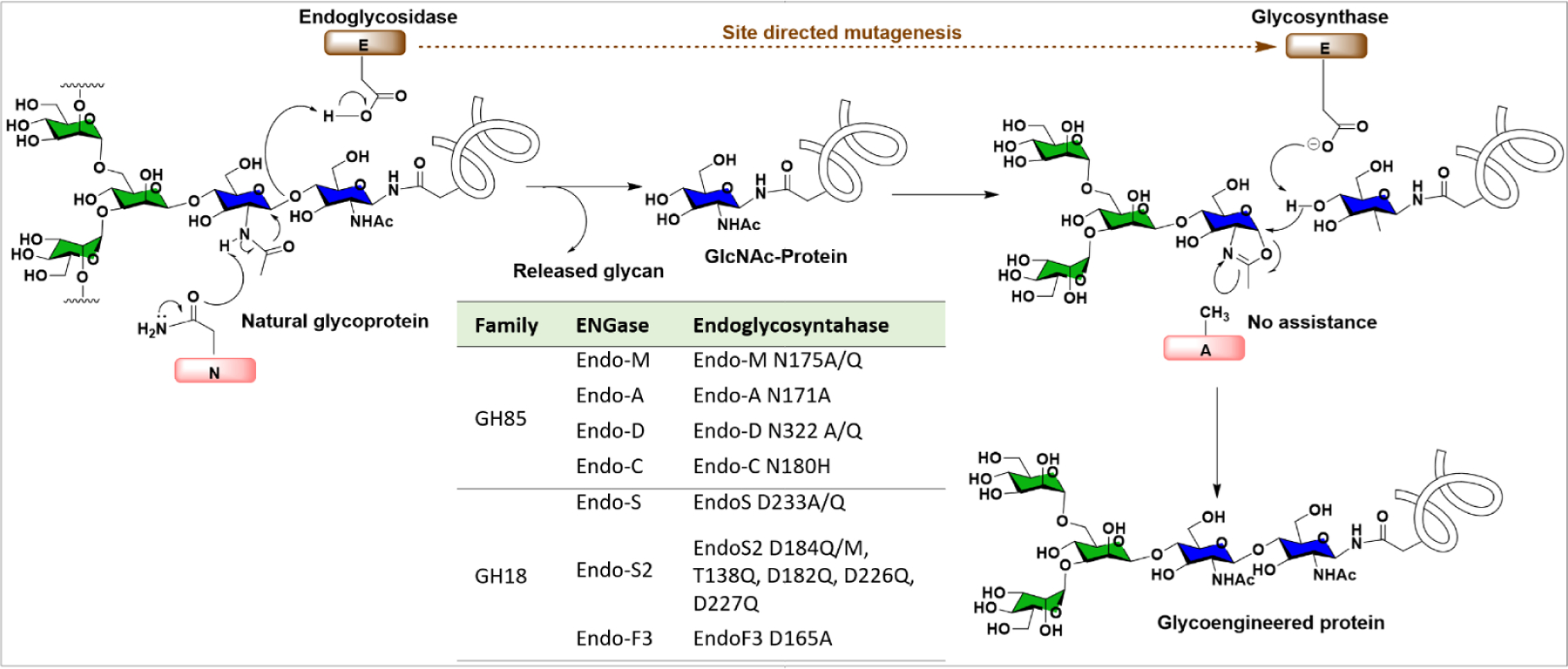
Catalytic mechanism of glycosidase and glycosynthase during glycoprotein glycoremodeling.
The synthesis of sperm-associated CD52 antigen carrying both N- and O-glycan at the predefined sites was accomplished by the Wang group.300 The GlcNAc-Asn and GalNAc-Thr building blocks were used to introduce GlcNAc and GalNAc residues at the desired sites using SPPS followed by transglycosylation of complex type glycan at the N-glycosite and glycosyl transferase mediated extension of GalNAc at the O-glycosite.300 In another related study, they also reported core-fucosylated bi- and triantennary complex glycoforms of CD52 (Figure 15).301,302 Several high-mannose glycopeptides derived from the human cytomegalovirus (CMV) tegument protein pp65 and incorporated with T-cell epitope have been prepared by chemo-enzymatic synthesis to target human antigen presenting cells (APCs).303 In addition, a Man3 and sialylated complex type N-glycan variant of the antidiabetic drug, pramlintide were generated by using the EndoA-E173H and EndoM-N175Q mutants for transglycosylation, and the resultant glycopeptides showed both in vitro and in vivo activity as amylin receptor agonists with blood glucose lowering activity.304
Figure 15:
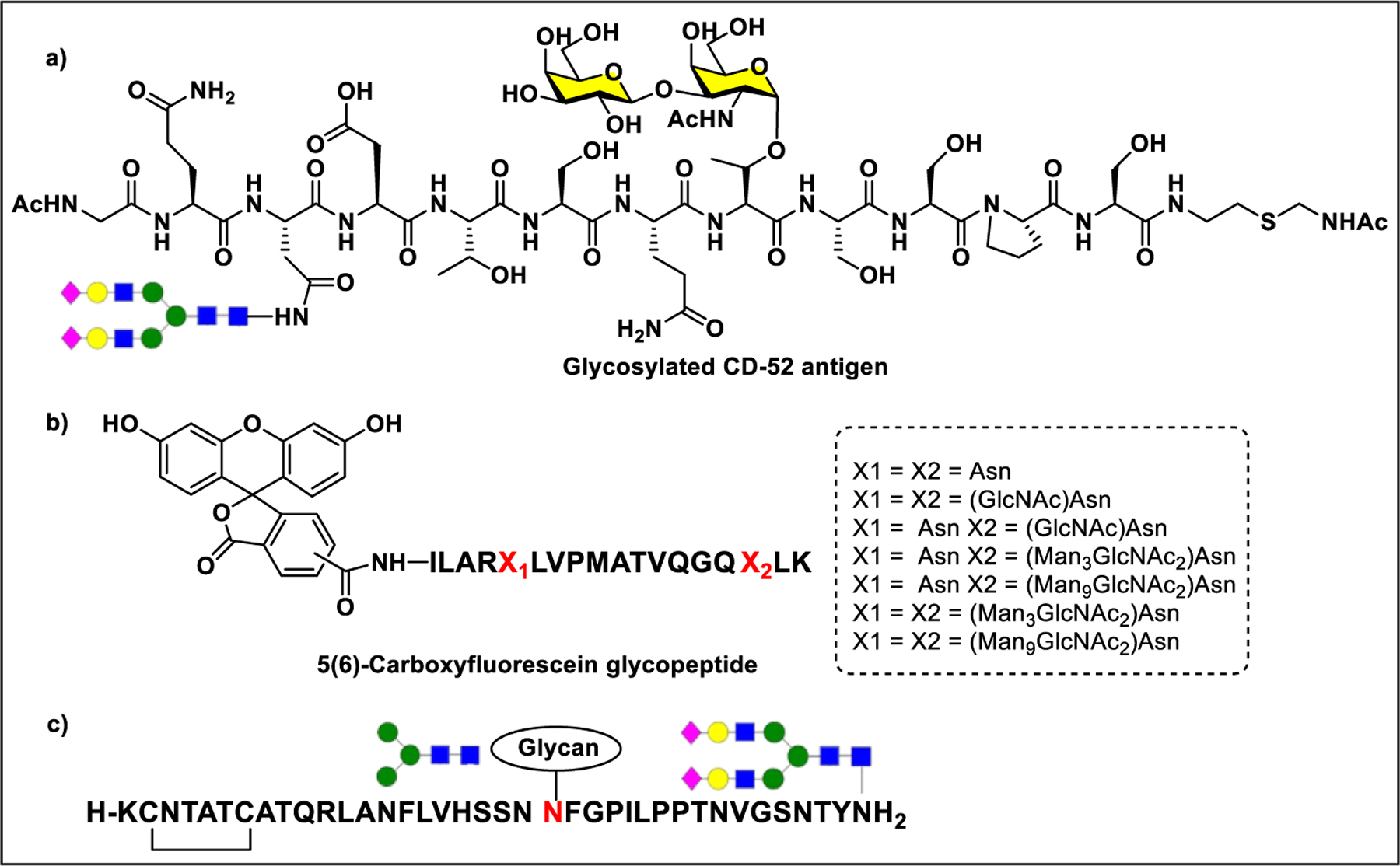
Synthesis of glycosylated peptides.
3.2.2.5. Pathway Engineering.
In recent years, various methods of cell line engineering have been developed to produce proteins with desired glycosylation patterns in diverse species such as mammals, plants, insect, yeast, and bacteria.305 Among the techniques used, knockin, knockout, and knockdown of certain genes of the enzymes involved in glycan biosynthesis, overexpression of enzymes of interest, and small molecule inhibitors that change the activities of glycosidases and GTs inside cells, have been used to remodel glycosylation of expressed proteins. Advances in gene editing, like the CRISPR technology, facilitated the process of cell glycoengineering.306,307 Chinese hamster ovary (CHO) cells, for example, have been used widely to produce proteins with altered glycosylation pattern.308
The highly conserved N297 glycosite in the IgG Fc region is heterogeneously glycosylated with biantennary complex type glycans, containing core fuc, terminal GlcNAc, Gal, Neu5Ac, and bisecting GlcNAc.309 The composition of the IgG Fc domain glycan regulates the differential engagement of FcRs. It has been demonstrated that the α−1,6 linked Fuc residue on the core GlcNAc at N297 is the major determinant of antibody-dependent cellular cytotoxicity (ADCC), and therefore removal of Fuc residue considerably improved the ADCC activity.310 Fucosyl transferase 8 (FUT8) catalyzes the α−1,6 linkage of Fuc to the core GlcNAc in the presence of GDP-fucose as substrate. Knocking out the FUT8 gene is an ideal strategy for recombinant production of IgGs with a low fucose content and improved ADCC. Yamane-Ohnuki et al. successfully demonstrated the production of anti-CD20 antibody from FUT8−/− CHO/DG44 cell lines, which showed enhanced binding to the FcγRIIIA and 100-fold improvement in the ADCC activity compared to the antibody from normal CHO/DG44 cells (Figure 16).311
Figure 16:
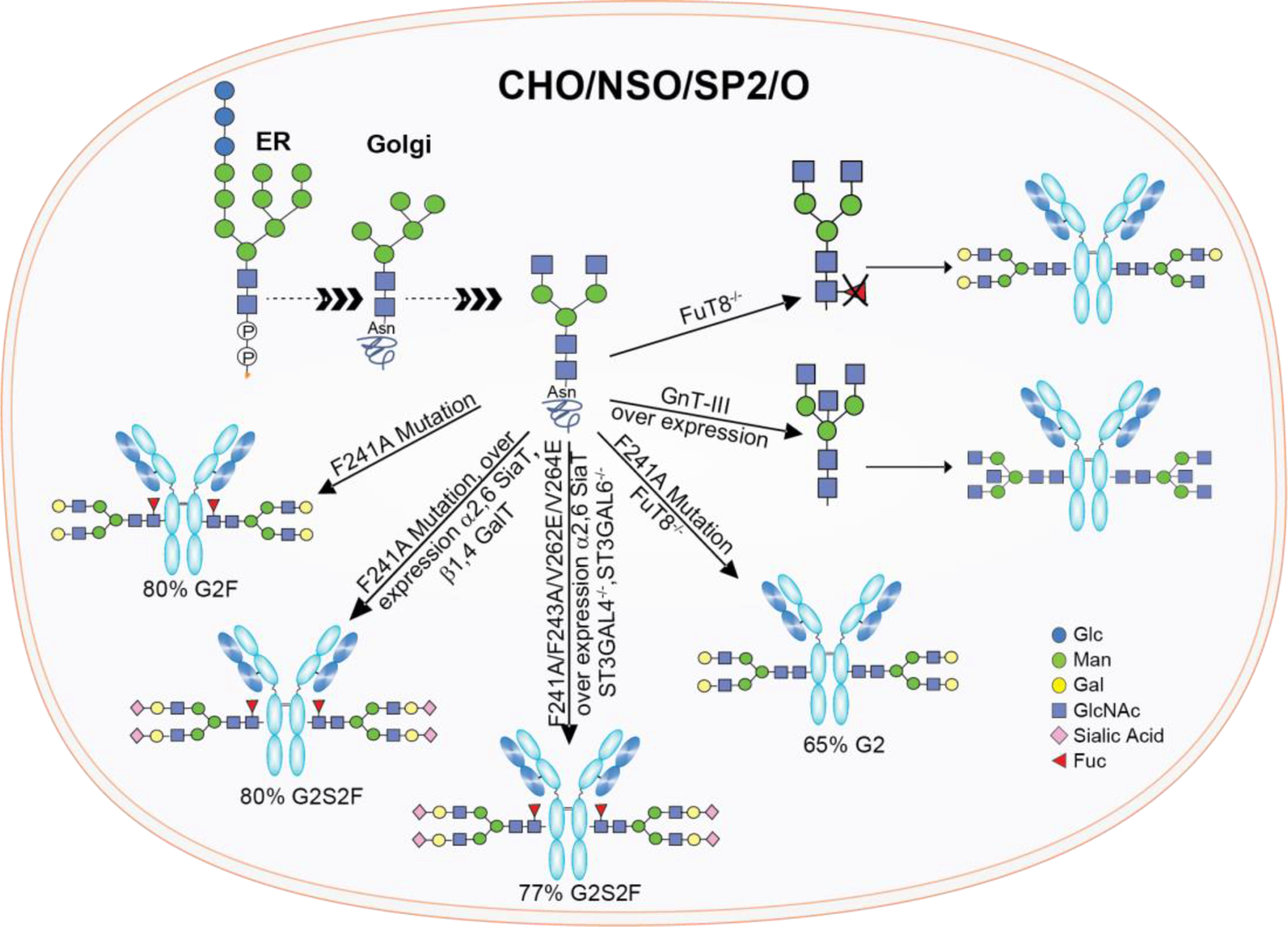
Strategies for cell line engineering to remodel protein glycosylation.
During glycan biosynthesis, N-acetylglucosaminyltransferase III (GnTIII) adds GlcNAc to Man residue through a β1,4-linkage.312 Addition of bisecting GlcNAc to the N-glycan attached to Asn297 of IgG could also regulate ADCC. Umana et al. constructed a GnTIII cDNA transfected CHO cell line to produce antibodies with increased bisecting GlcNAc content and ADCC activity,313 suggesting that bisecting GlcNAc has a positive impact on ADCC.
In addition to CHO cells, other mammalian cell lines such as NS0 and SP2/0 have been utilized for glycoengineering due to their easy handling and high-yield expression.314 Apart from these, plant, insect, yeast, and bacteria cells have also been used for protein engineering.315 Compared to mammalian cells, yeast expression systems are highly productive and cost-effective.316 The utility of methylotrophic yeast Pichia pastoris facilitated the production of proteins with high mannose-type glycans.317 Recently, bacterial expression systems emerged as a simple, quick, and cost-effective means for glycoengineering.318,319 To lower the risk of an immunogenic response to nonhuman glycans, various strategies used to humanize yeast and to modulate bacterial glycosylation have been explored. Hamilton et al. developed a humanized P. pastoris strain through knockout of four yeast genes and knock-in of 14 heterologous glycosylation genes to produce human like erythropoietin with increased sialylation.320 The Wong group developed a glycoengineered yeast strain to produce antibodies of high-mannose glycoforms followed by endoglycosidase mediated cleavage and transglycosylation in vitro to generate homogeneous antibodies.321 However, although there is a wide selection of nonmammalian expression systems available for glycoprotein production, none of them has been used routinely due to their complexity.
The N-glycan composition of IgG Fc domain contains mainly the G0F and G1F glycoforms with less amount of sialylation, wherein the Gal residues are critical for complement-dependent cytotoxicity (CDC) and sialic acid is associated with anti-inflammatory properties.322,323 Therefore, various strategies have been developed to remodel or to enrich the desired glycosylation pattern. The Jeffery’s lab attempted amino acid mutations in the Fc region of an antibody, including F241A, F243A, V264A, D265A, and R301A mutations, that were designed to provide access for GalT and SiaT to the glycosylation site, leading to more processed glycoforms.324 In another approach, hypergalactosylated antibodies were produced by knocking out genes ST3GAL4 and ST3GAL6 responsible for α 2,3-sialyl transferases.325 A single point mutation, F241A, in IgG-Fc resulted in production of IgG with 80% G2F and when further combined with a FUT8 knockout system, provides IgGs with 65% G2 glycoform (Figure 16).325 In addition to the point mutation F241A, overexpression of α2,6-SiaT and β1,4-GalT produced IgG with 80% sialylation, the majority of which is α2,6-sialylation.326 Further work by Betenbaugh and co-workers showed that antibodies with 77% α2,6-sialyation could be achieved by overexpressing α2,6-SiaT and knocking out ST3GAL4 and ST3GAL6 with CRISPR/Cas9 in combination with four-point mutations (F241A, F243A, V262E, V264E) (Figure 16).327
3.2.2.6. Glycan-Mediated Antibody–Drug Conjugation.
Antibody–drug conjugates (ADCs) are one of the fastest growing biopharmaceuticals which are produced by conjugation of cancer cell-specific monoclonal antibodies with highly potent cytotoxic drugs.328,329 To date, a total of 11 ADCs have been approved by the FDA as a targeted therapy for cancer treatment. One of the key components of ADCs is the linker, which is responsible for coupling the cytotoxic drug to the antibody to maintain ADC stability during the systemic circulation.330 The conjugation site on the antibody along with the chemical properties of the linker play important roles in the stability and PK/PD properties of the ADC and the therapeutic window. In early development, cytotoxic drugs were nonselectively conjugated to the exposed lysine or cysteine, from interchain disulfides after partial reduction, on the antibody, leading to formation of a heterogeneous mixture with a varied number of drugs attached to several possible sites.331,332 This methodology resulted in reduction in potency and safety and made optimization of the process and pharmacological properties significantly challenging. Moreover, nonspecific conjugation near the complementarity-determining region may reduce binding affinity and specificity.333 To develop ADCs with controlled conjugation with a higher therapeutic index, various methodologies have been explored to overcome the limitations of nonspecific conjugation.334,335 Some of the major strategies developed for site-specific conjugation include insertion of a specific amino acid, unnatural amino acid, short peptide, and glycan-based conjugation. The insertion of an unnatural amino acid in the antibody sequence allows site-specific conjugation of payload with a controlled drug–antibody ratio (DAR) for production of homogeneous ADCs.336
Glycan-based conjugation offers the unique advantage to couple the payload to the glycan N297 site in the CH2 domain rather than conjugation through amino acid residues. All IgGs have at least one conserved glycosylation site in the Fc domain.337 The biantennary complex type glycan present in the Fc domain can be used to generate the attachment site. For example, nonreducing end glycan residues, such as sialic acid, galactose, GlcNAc, mannose, and core fucose, have been explored to generate the conjugation site. The two major approaches to glycan-based conjugation include: (1) enzymatic deglycosylation of N-glycan to generate the acceptor, followed by enzymatic transfer of an unnatural sugar moiety containing a tag for conjugation, using a respective sugar nucleotide donor, and (2) endoglycosidase-mediated deglycosylation of IgG glycan leaving behind the innermost GlcNAc, followed by trans glycosylation with predefined oligosaccharide in the form of oxazoline, bearing the desired tag.
The fucose analogue 6-thiofucose was metabolically incorporated into anti-CD30 or anti-CD70 antibody for conjugation with maleimide containing monomethyl auristatin E (MMAE).338 The ADCs generated through thiofucose conjugation showed improved potency and homogeneity compared to the drug conjugate through hinge disulfides (Figure 17a). Qasba et al. discovered a mutant of galactosyltransferase, GalT(Y289L) that can transfer a modified GalNAc to GlcNAc acceptor.339,340 ADC generated using C-2 keto galactose conjugated to auristatin F showed excellent in vitro anticancer activity (Figure 17b).341 In another study, GlcNAc-IgG obtained by endoglycosidase-mediated trimming of Fc N-glycan was used to introduce azido-GalNAc to the core GlcNAc using GalT(Y289L).342 Payloads including MMAE, monomethyl auristatin F (MMAF), maytansine, or doxorubicin were efficiently conjugated to the azido-GalNAc with copper-free click chemistry to obtain homogeneous ADCs on gram scales (Figure 17c).342 The anti-HER2 ADCs generated by this method showed a better in vivo antitumor activity compared to the ADC prepared by conventional lysine conjugation.342
Figure 17:
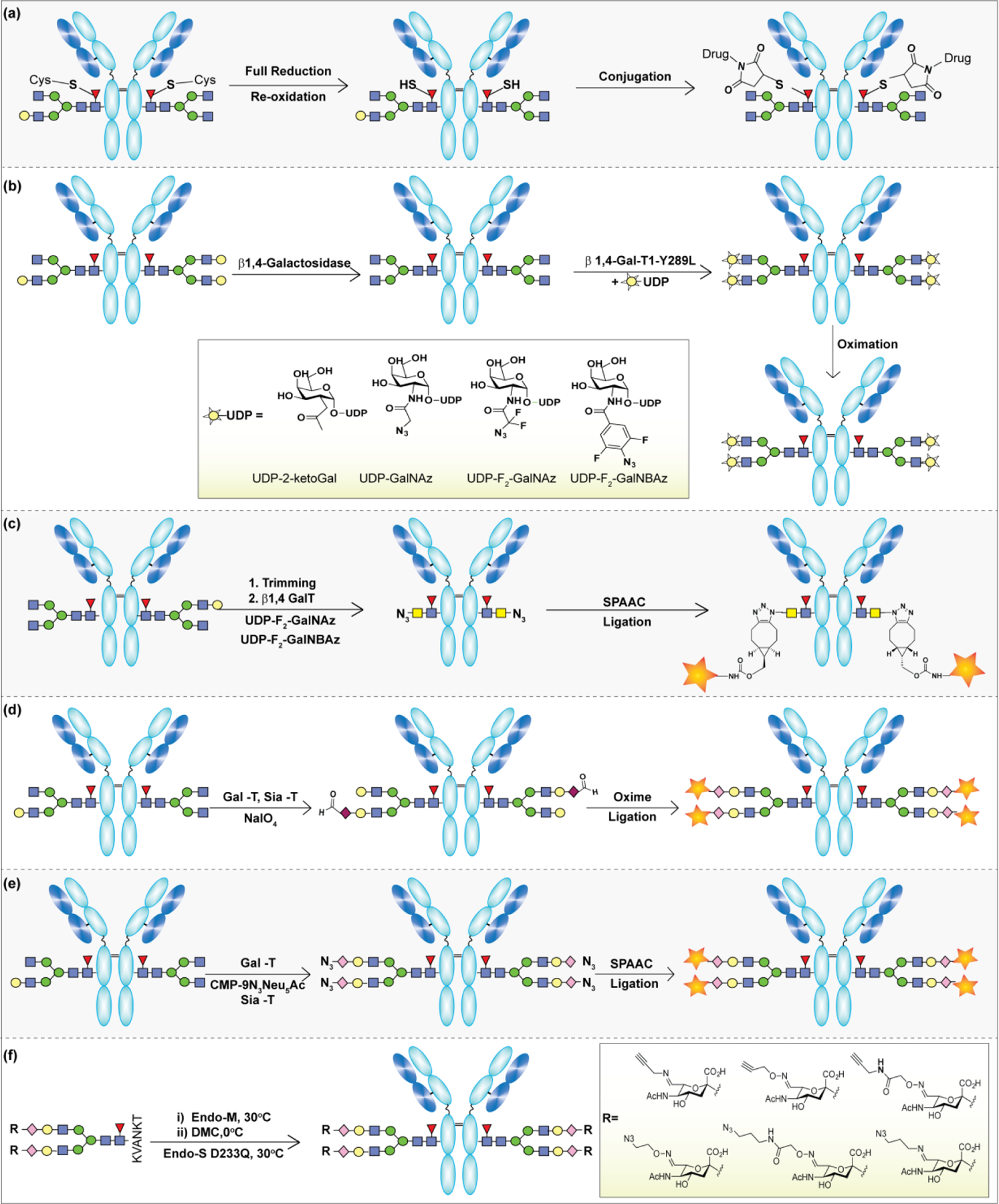
Strategies for glycan-based site-specific antibody-drug conjugation.
Recently, terminal sialic acid gathered considerable attention for site-specific conjugation to obtained highly defined ADCs and glycoproteins. The native glycan on the N297 site of Fc domain lacks terminal sialic acid residues, therefore, in vitro sialylation has been performed to transfer either azido-modified or natural sialic acid using CMP-Neu5Ac as a donor and a mixture of β1,4-Gal T and α2,6-Sial T to transfer Gal and Neu5Ac residues on to the native glycans of an antibody.343 The sialic acid residues could be oxidized under mild condition using periodates to yield aldehyde functionalized antibodies. The aldehyde or azido group on the sialic acid on IgG Fc glycan serves as a handle for conjugation via oxime ligation and copper-free click chemistry, respectively. Pan et al. reported a strategy in which an aldehyde group was introduced at the terminal Neu5Ac of the Fc glycan via periodate oxidation for conjugation to aminooxy functionalized cytotoxic drugs to achieve DAR ∼ 1.6 (Figure 17d).343 These glycoconjugated ADCs exhibited target-dependent anticancer activity and greater antitumor efficacy than the native antibody in a Her2-positive tumor xenograft model.343 In an alternative strategy, Haung et al. reported an Endo-S mutant that catalyzed the transglycosylation of Fuc-α1,6 GlcNAc-IgG with a sialylated complex type N-glycan oxazoline to obtain a homogeneous Fc-sialylated antibody. The oxidation of terminal Neu5Ac residues formed an aldehyde, which was then treated with a cytotoxin bearing aminooxy or hydrazide nucleophiles to generate an oxime or hydrazone-linked conjugate, respectively (Figure 17f).344 The Boons group introduced a C-9 azido group on the terminal sialic acid of anti-CD22 monoclonal antibody Fc glycan. The azido moieties were then selectively conjugated using strain promoted alkyne–azide cycloaddition chemistry (SPAAC) to biotin, FITC, and the cytotoxic drug, doxorubicin (Figure 17e).345
4. GLYCOCONJUGATE THERAPEUTICS
4.1. Glycoconjugate Vaccines
4.1.1. Antimicrobial Vaccines.
The capsular polysaccharides (CPS) on the surface of pathogens served as an immunogen for development of vaccines against a variety of infectious diseases, including meningitis and pneumonia.346,347 After the first pneumococcal capsular polysaccharide vaccine was developed in 1917,348 several other vaccines, such as the tetravalent meningococcal, the Haemophilus influenza type b and Samonella typhi Vi etc., were developed and routinely used in an immunization schedule.349 In polysaccharide vaccines, the APC are not involved in presenting the CPS to the B cells, therefore the CD4+ T-cells are not stimulated, resulting in a weak proliferation of B cells and production of IgM antibodies without affinity maturation.350,351 These limitations led to development of protein conjugate–polysaccharide vaccines.352,353
The first pneumococcal glycoconjugate vaccine, Prevnar (PCV7), consisting of seven serogroups, 4, 6B, 9V, 14, 18C, 19F, and 23F, and conjugated to the carrier protein, CRM197, was used against infections caused by S. pneumoniae for children younger than 2 years.354 To cover additional serotypes, six more serotypes were included in PCV7 (PCV7 + 1, 3, 5, 6B, 7F, and 19A) to develop PCV13, which was approved in the USA in 2010 for use in children.355 The pneumococcal and meningococcal infections are controllable by the existing vaccines; however, continuous monitoring of the vaccine serotype coverage is required to combat emerging lethal serotypes. A typical example, PCV13 has been broaden with additional S. pneumoniae serogroups to develop PCV15 and PCV20 vaccines, both of which are in phase 3 clinical trials.356
The majority of the licensed glycoconjugate antibacterial vaccines were developed by conjugation of polysaccharides isolated from microbial culture to immunogenic carrier protein.52
The main issues associated with manufacturing include glycan structural heterogeneity, complex purification, contamination with cell-based debris, and inconsistent protein conjugation steps.357 Synthetic glycoconjugate vaccines offer an attractive strategy to address the issues with traditional vaccine development and provide a safer and more effective alternative vaccine design. One of the significant discoveries in glycoconjugate vaccine research is the development of the first synthetic glycoconjugate Hib vaccine, Quimi-Hib, which is composed of synthetically produced antigen comprising seven repeating units of poly-3-β-d-ribosyl-(1→1)-d-ribitol-5-phosphate (PRP), conjugated to thiolated TT through thiol-maleimide coupling chemistry, was approved in Cuba for immunization of children against Haemophilus influenzae type b (Scheme 9).188 However, the approved Quimi-Hib vaccine contains a mixture of various length polysaccharides; therefore, to identify the minimum glyco-epitope length for effective vaccine design, the Seeberger group assessed various lengths of well-defined Hib oligosaccharides conjugated to DT-CRM197 in immunization studies. All of the vaccines were found to be immunogenic in a rabbit model, and it was revealed that the tetrameric conjugate is an excellent starting point for design of next generation Hib vaccines (Scheme 9).358
Scheme 9:
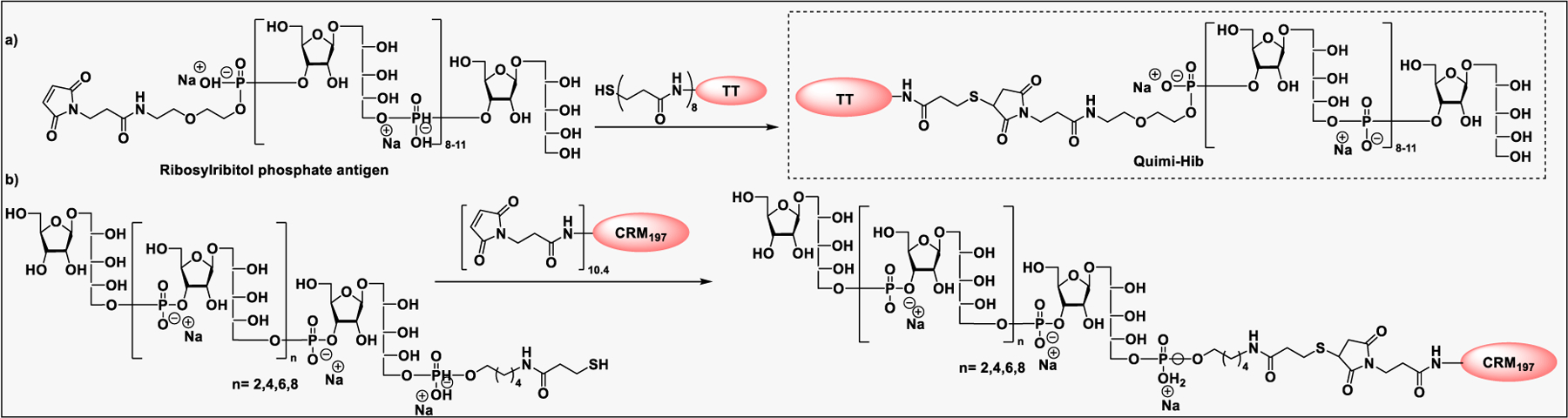
Synthesis of commercially available Haemophilus influenza vaccines, a) Quimi-Hib; and b) Synthetic Haemophilus influenza vaccines by the Seeberger group.
Neisseria meningitidis (N. meningitidis) is one of the causative agents of bacterial meningitidis worldwide among the population under the age of five years and in adolescents.359 Most of the lethal strains are surrounded by a polysaccharide capsule, which is used to differentiate 12 N. meningitidis serogroups. Six of them, A, B, C, W, X, and Y, cause the vast majority of infections in humans.360 Currently, there are three quadrivalent glycoconjugate vaccines available against serotypes A, C, Y, and W135.190 For example, Menveo (GSK), consisting of polysaccharide of serotypes A, C, W135, and Y conjugated to CRM197, Menectra (Sanofi), consisting of polysaccharide of serotypes A, C, W135, abd Y conjugated to DT, and Nimenirix (Pfizer), consisting of polysaccharide of serotypes A, C, W135, and Y conjugated to TT. Furthermore, there are three monovalent serogroup C (Menjugate (GSK), and Meningtec and NeisVac-C (Pfizer)) and one serogroup A (MenAfriVac from the Serum Institute of India) vaccines available for all age groups. Glycoconjugate vaccines against serogroup B are limited by the concern of autoimmunity caused by self-antigen, the vaccine generated from the outer membrane vesicle (OMV) without any glycan antigen was developed and approved in Cuba against serogroup B.361 Various strategies have also been developed to prepare homogeneous glycoconjugate vaccines against N. meningitidis based on the serotype-specific repeating units of meningococcal capsular polysaccharide (Figure 18).361
Figure 18:
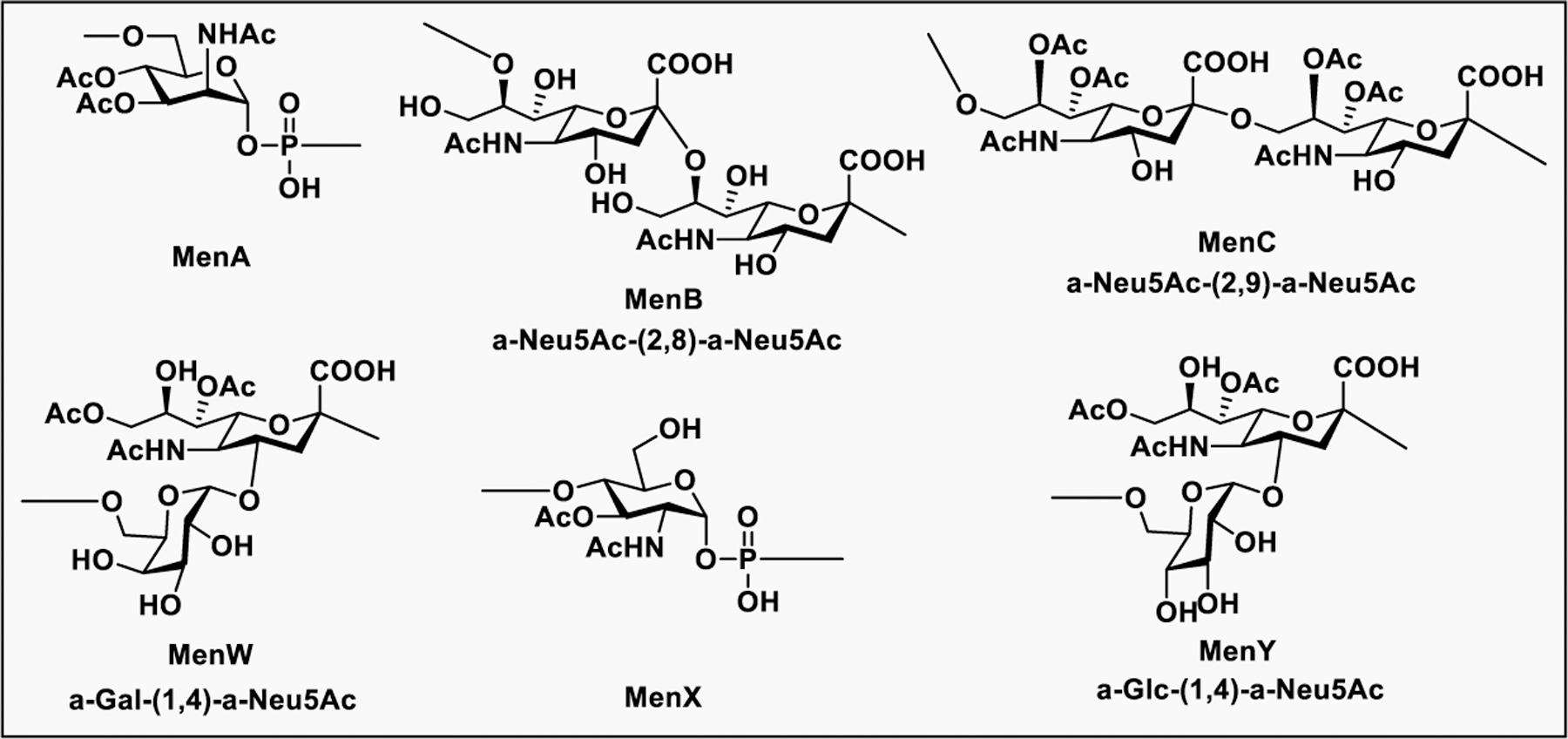
Chemical structures of meningococcal capsular polysaccharide repeating units from different serogroups.
Pozsgay et al. reported the synthesis of MenA CPS fragments and their conjugation to human serum albumin (HSA) via Diels alder cycloaddition to evaluate their antigenicity by reaction with meningococcal A antiserum (Scheme 10a).362,363 The glycoconjugate vaccine against MenA CPS that suffers from poor hydrolytic stability is a CPS consisting of repeating units of (1→6)-linked 2-acetamido-2-deoxy-α-D-mannopyranosyl phosphate.364 Therefore, replacement of the ring oxygen with a methylene group tends to be attractive strategy to enhance its stability. Accordingly, Gao et al. reported the carbocylic analogues of MenA CPS from monosaccharide to trisaccharide and their conjugation to CRM197 and HSA (Scheme 10b).365,366 Immunization of these glycoconjugates induced anti-MenA CPS specific IgG with in vitro antibacterial activity, although to a lesser extent than the isolated LPS conjugates to same carrier protein.366 In another study by Fallarini et al., HSA conjugates of MenA CPS were able to induce both in vitro T-cell proliferation and in vivo antigen specific IgG production (Scheme 10c).367
Scheme 10:
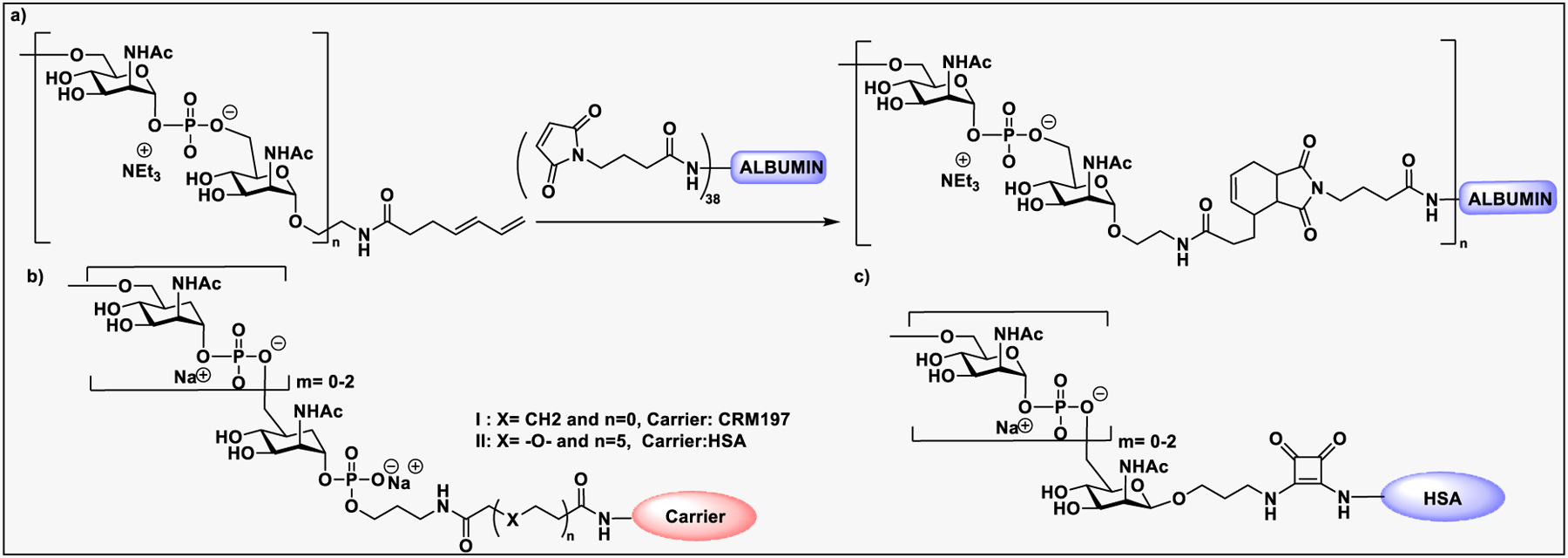
a) Conjugation of MenA CPS fragment to albumin. b) Synthetic 1-C phosphono and carbocyclic analogue MenA CPS fragments conjugated to CRM197. c) MenA CPS fragments conjugated to HSA.
The MenC polysaccharide α-(2,9)-linked polysialic acid with random acetylation at 7/8-positions has been used for vaccine development. Despite having the O-acetylated (OAc1) polysaccharides in the licensed MenC vaccine, some MenC strains contain di-O-acetylated (OAc2) polysaccharide which may interfere with immune responce.368 Liao et al. reported a series of nonacetylated α-(2,9)-oligosialic acids of various lengths ranging from a dimer to a pentamer,369 synthesized using methodology originally developed by the Wu group (Scheme 11a).370 The synthetic polysialic acid was conjugated to a carrier protein such as KLH or HSA for immunization in mice.369 The vaccine elicited robust T cell response in mice and the trimeric form was shown promising as an integral part of vaccines against MenC. Later in 2016, the same group reported a new type of fully synthetic vaccine conjugate composed of α2,9-linked di-, tri-, tetra-, and pentasialic acids as epitopes and the glycolipid monophosphoryl lipid A (MPLA) as adjuvant (Scheme 11b).371 Evaluation of vaccination in C57BL/6J mice showed robust immune responses comparable to the corresponding polysialic acid conjugates and adjuvant. The MenW CPS consists of a glycan repeating unit of [→6)-α-d-Galp-(1→4)-α-d-Neup5Ac(7/9OAc)-(2→]. Wang et al. disclosed the first iterative glycosylation and deprotection synthetic strategy for making MenW CPS oligosaccharides in various lengths from di- to decasaccharides to form the glycoconjugate with CRM197 (Scheme 11c).372 The immunological evaluation suggests that the tetra saccharide is the minimum saccharide length required to induce bactericidal antibodies.372
Scheme 11:
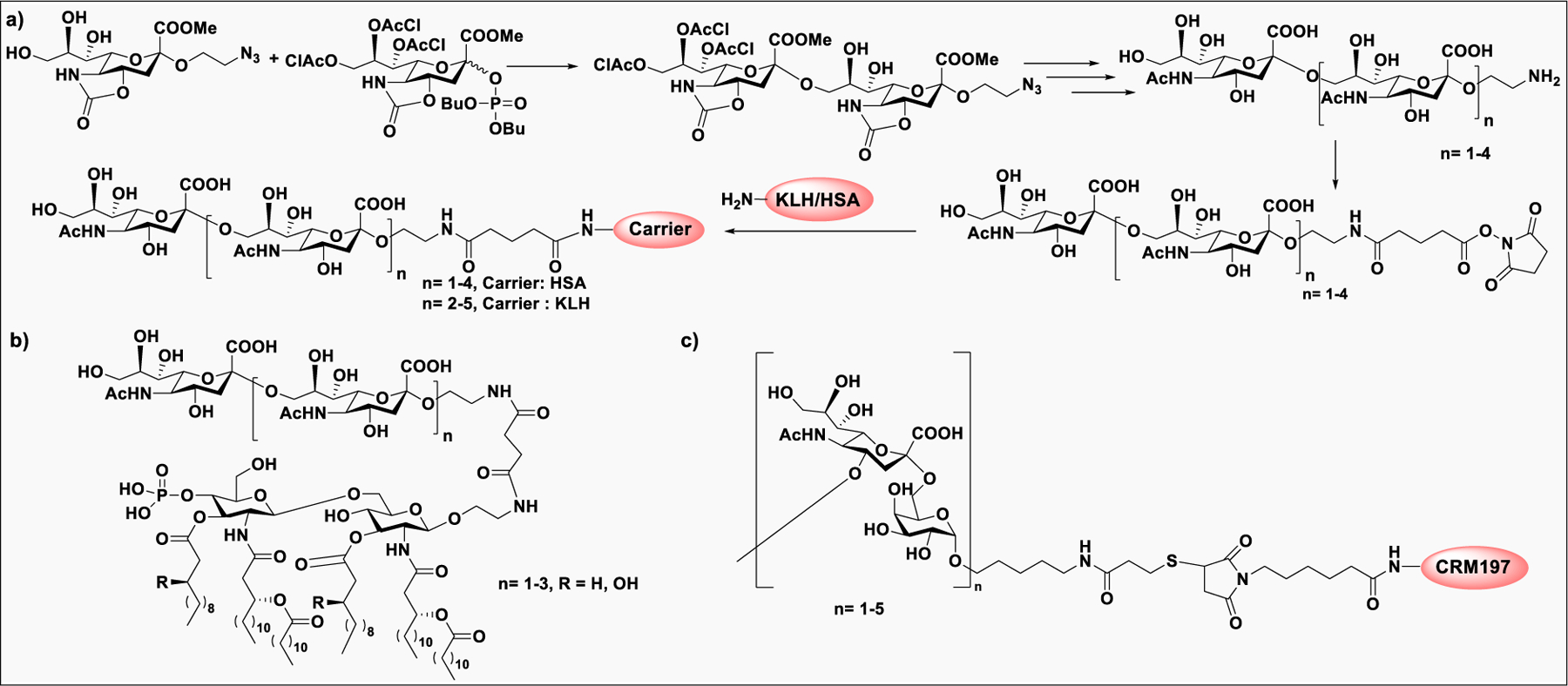
a) Convergent synthesis of MenC CPS polysialic acid antigen and its bioconjugation to carrier protein; b) Synthetic self-adjuvanted MenC polysialic acid conjugate vaccine; (c) Synthetic MenW CPS glycoconjugate vaccines.
N. meningitidis serotype X is considered to be a big health threat because currently available antimeningococcal vaccines do not cover the MenX capsular antigen. The CPS of MenX is a homopolymer of (1→4)-linked 2-acetamido-2-deoxy-α-d-glucopyranosyl phosphate residues. A synthetic glycoconjugate consisting of MenX CPS fragments conjugated to CRM197 was used to determine minimal length for immunogenic activity.373 Upon immunization, it was revealed that oligomers longer than three repeating units are essential to mimic the native CPS.373 In another similar study, a tetrameric unit of MenX CPS conjugated to tetanus toxoid were immunologically evaluated for the development of a potential MenX vaccine candidate (Scheme 12b).374 Recently, a longer length MenX CPS fragment was prepared by an enzyme-catalyzed on-column elongation procedure using UDP-GlcNAc as a nucleotide donor and conjugated to CRM197 for immunological study in a mouse model.375 The functional antibodies generated from the vaccine are similar to those generated from immunization with naturally occurring or enzymatically prepared MenX CPS conjugates (Scheme 12c).375
Scheme 12:
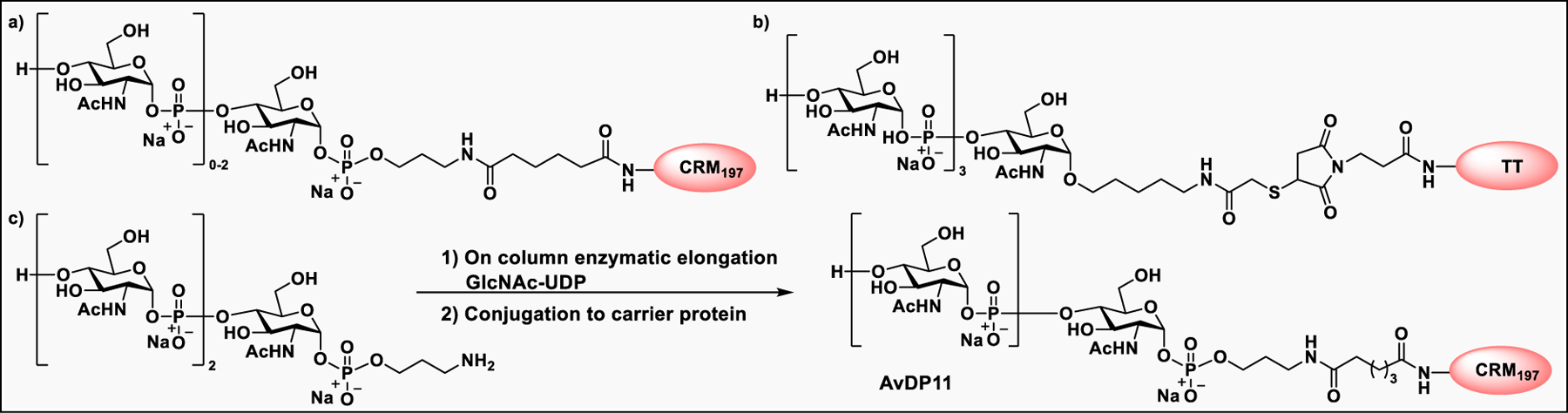
Synthesis of MenX CPS conjugate vaccines.
Pneumococcal infection is caused by the bacteria Streptococcus pneumoniae (S. pneumoniae). Depending on the structures of their polysaccharide capsules, 97 serotypes (ST) of S. pneumoniae were characterized, and 20 of them caused 90% of pneumococcal disease.376 Currently, there are two types of vaccine available; a pneumococcal polysaccharide vaccine, PPSV23 (Pneumovax23), and pneumococcal conjugate vaccines such as PCV10 (Synflorix) and PCV13 (Prevnar13).187 A 15-valent vaccine developed by Merck has lately finished late stage clinical trials and may be available in near future.377 The Seeberger group reported a synthetic glycoconjugate containing fragments of S. pneumoniae serotype 2 (ST2), 3 (ST3), and 8 (ST8) capsular polysaccharides (Scheme 13).189,378 Vaccination with neoglycoconjugates consisting of polysaccharide fragments conjugated to CRM197 elicited a robust T-cell-dependent B-cell response in mice.379
Scheme 13:
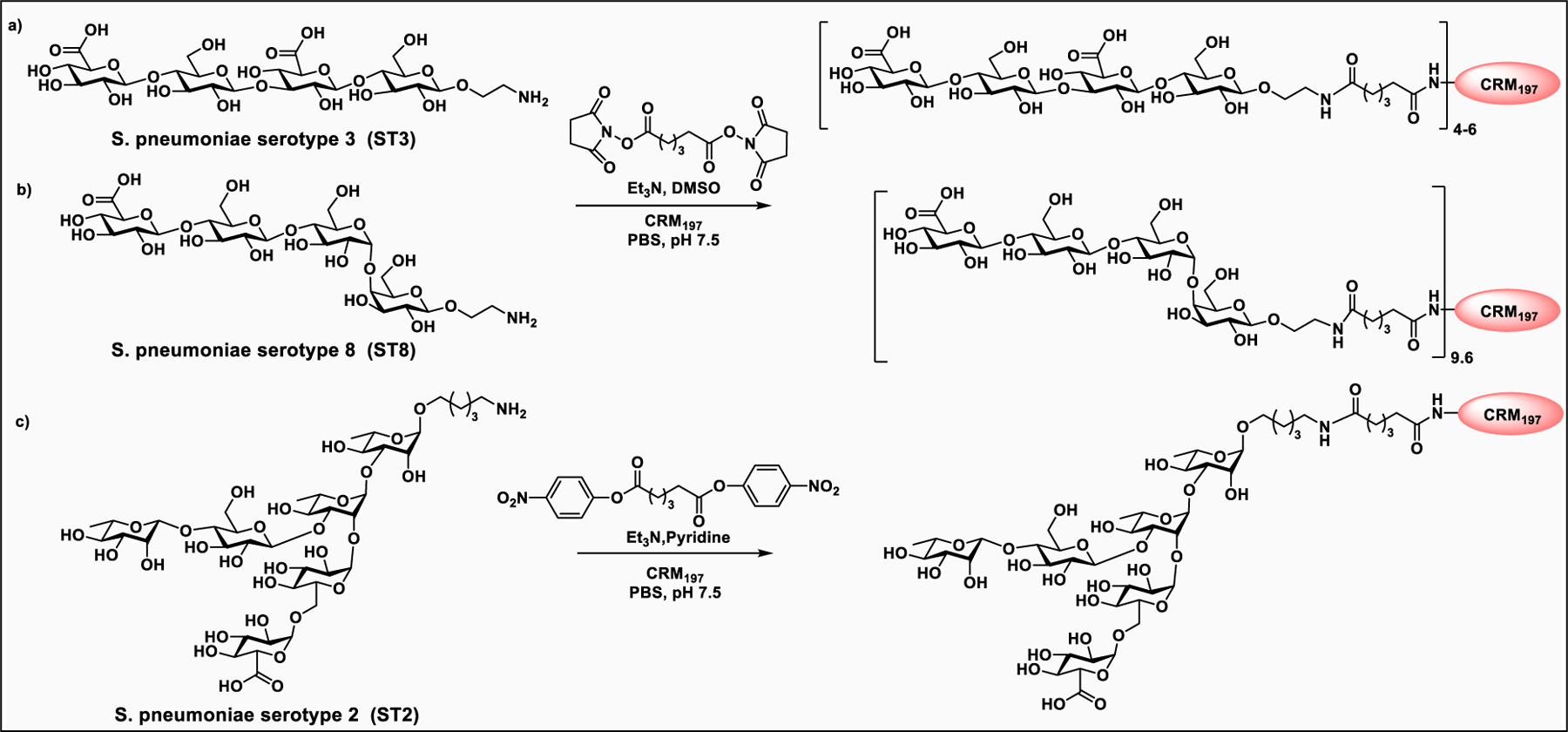
Synthesis of glycoconjugates based on S. pneumoniae serotype 2, 3, and 8 polysaccharides.
Some of the synthetic antibacterial and antifungal glycoconjugate vaccine candidates are summarized in Table 3.380,381
Table 3:
Synthetic antibacterial and antifungal glycoconjugate vaccine
The surface glycoproteins hemagglutinin and neuraminidase are the major determinants of immune response against influenza viruses,390 which are largely responsible for the outbreak of the influenza epidemic and pandemic.391 Currently, immunization with chicken egg-derived inactivated virus is the common method to control influenza infections. However, current vaccines are only effective against circulating strains that closely match the inoculating strain and must be updated yearly. Our group studied the effect of glycosylation on the structure, folding, and function of influenza hemagglutinin from different strains and subtypes and identified two (N27 and N142) from six N-glycosites on each hemagglutinin monomer which are essential for hemagglutinin folding and viral infection.392 The innermost GlcNAc residues were also found to be essential for folding and trimerization, and the epitopes covered by glycans are highly conserved. Using these findings, hemagglutinin-based universal influenza vaccines were developed with broadly protective immune responses, including the monoglycosylated hemagglutinin of consensus sequence,393,394 the egg-based virus with such monoglycosylated hemagglutinin on the surface,395 a chimeric hemagglutinin with consensus H5 as the head and consensus H1 as the stem, and the neuraminidase-defective attenuated virus.396 Immunization studies showed that the antibodies generated from hemagglutinin bearing only a GlcNAc residue at each glycosite showed better hemagglutinin stem selectivity, higher hemagglutinin inhibition, and broader neutralization activity against influenza subtypes than the antibodies elicited by fully glycosylated hemagglutinins. In addition, the highly conserved stem region of hemagglutinin was expressed in yeast and used as an immunogen for universal vaccine design.397 Upon immunization with C34 adjuvant, the monoglycosylated hemagglutinin stem protein induced cross-reactive antibody neutralization activities, strong ADCC-mediated protection as well as T-cell responses against various strains, and a broad protection in challenge studies. Recently, a chimeric vaccine with consensus avian influenza H5 sequence as globular head and consensus human influenza H1 sequence as stem was shown to elicit highly effective and broadly protective antibody, CD4+ and CD8+ T cell responses against influenza variants.398 Interestingly, the mono-GlcNAc chimeric vaccine induced a high titer of stem-specific antibodies with improved ADCC, better neutralizing potency and cross reactivity against H1, H3, H5, and H7 strains and subtypes, as compared to the fully glycosylated chimeric vaccine. Immunization studies of the chimeric vaccine adjuvanted with glycolipid C34, showed higher levels of IFN-γ and IL-4 secretion and production of more CD8+ T cells.
The SARS-CoV-2 S protein exists as a trimer and contains 22 N-linked and at least 2 O-linked glycosites per monomer.288 The S protein glycosylation is critical for protein folding and for evasion of the host immune attack, and thus affecting the vaccine efficacy. Recently, the impact of site-specific S protein glycosylation on viral infectivity was studied, and the result showed that lung epithelial cells derived S protein is more infective than from other cell lines.399 The work has led to the development of mono-GlcNAc-containing S protein (SMG) with better exposure of the conserved epitopes shielded by glycans as a candidate vaccine. The mice immunization studies with SMG suggested that glycodeletion is a simple and effective approach for development of a broadly protective COVID-19 vaccine. In another relevant study, the Wong group showed that the glycosite-deleted mRNA vaccine of S protein elicited robust antibody and CD8+ T cell responses with broader protection against diverse COVID-19 variants compared to the unmodified mRNA vaccine400
4.1.2. Anticancer Vaccines.
Glycan biosynthesis is a well-controlled phenomenon through the action of various enzymes involved in the pathway. Aberrant or unusual protein glycosylations, such as a change in glycosylation and carbohydrate composition is typically regulated by glycan processing enzymes, correlate with the development of diseases, most commonly cancer.149,401 Carbohydrates in the context of cancer, regulate tumor development, such as invasion, proliferation, and metastasis.402 Accordingly, changes in the glycosylation patterns were recognized as a biomarker in the progression of human cancers. Cancer immunotherapy recently emerged as a potential treatment because of its excellent target specificity and lesser side effects.403 However, most of the immunotherapeutic agents target tumor-specific surface proteins such as programmed death ligand-1 (PDL-1), cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) etc. Targeting the tumor-associated carbohydrate antigens (TACAs) presents an alternative way to develop immunotherapeutic agents and therapeutic vaccines.191 Depending on their structures, TACAs are subcategorized into globo series glycolipids, such as Globo H, SSEA4, and SSEA3; the gangliosides, such as GD2, GD3, GM2, GM3, and fucosyl GM1; the blood group antigens, such as Lewisx, Lewisy, sialyl Lewisx, and sialyl Lewisy; and the glycoproteins including Thomsennouveau (Tn), Thomsen–Friendreich (TF), and sialyl-Tn (STn).157,404–407 TACAs are distinctly overexpressed in a large number of tumors such as breast, prostate, colon, ovary, and lung cancer, melanoma, neuroblastoma, B-cell lymphoma, etc., but not on normal cells.404,406 TACAs on tumor cells are immunogenic, rendering them a potential target for vaccine development.191 However, isolation of these tumor antigens from natural sources is extremely challenging due to their structural heterogeneity. Therefore, advanced methodologies in the field of glycan synthesis such as one-pot synthesis, chemoenzymatic synthesis, and automated glycan synthesis have been utilized to produce well-defined cancer antigens for anticancer vaccines development.
In many instances, carbohydrates are not immunogenic. However, a large majority of anticancer vaccines are composed of TACAs covalently conjugated to an immunogenic protein or lipo (peptide) carrier to induce a T-cell response, which leads to the production of high affinity IgG antibodies against the tumor expressing the antigen and to induce the activation of long-lasting memory B-cell and cytotoxic T-cells.192,408,409 Until now, various linker strategies and conjugation chemistries have been explored and a series of glycoconjugate tumor vaccines have been generated using TACAs such as gangliosides, Lewis structure series, O-glycans and Globo series, and carrier proteins including bovine serum albumin (BSA), keyhole limpet hemocyanin (KLH), diphtheria toxoid (DT), tetanus toxoid (TT), ovalbumin (OVA), and MUC1 peptides.53,192,408,410–414
Depending on the type of glycan antigen and the components of immune activation, the glycoconjugate cancer vaccines are classified into monoepitope monocomponent, clustered monoepitope monocomponent, clustered monoepitope multicomponent, and multiepitope multicomponent vaccines. The monoepitope vaccines containing a single glycan epitope conjugated to a carrier protein are most extensively explored,415 and some of them, for example GM2-KLH416 and sTn-KLH417 vaccines, failed to achieve the desired end point and overall survival in phase 3 clinical trials.417 The immune response generated by monoepitope vaccines did not react well with carbohydrate antigen,418,419 particularly the mucin-type glycan antigen which often present in clusters on the tumor surface.420 These limitations opened the opportunities for design of vaccines presenting clusters of tumor antigens. Accordingly, a Tn(c)-KLH conjugate adjuvanted with QS-21 (Scheme 14) was evaluated in phase 1 clinical studies to show that the level of prostate specific antigen (PSA) in treated population was either reduced or remained stable.421 In another phase 1 clinical trial, a KLH conjugate of TF(c) in combination with QS-21 showed anticancer effects in patients with relapsed prostate cancer.422 Zhu et al. designed and synthesized a vaccine construct consisting of three alternating repeats of Gb3 and MUC5AC peptide marker to target ovarian carcinoma (Scheme 14).423
Scheme 14:
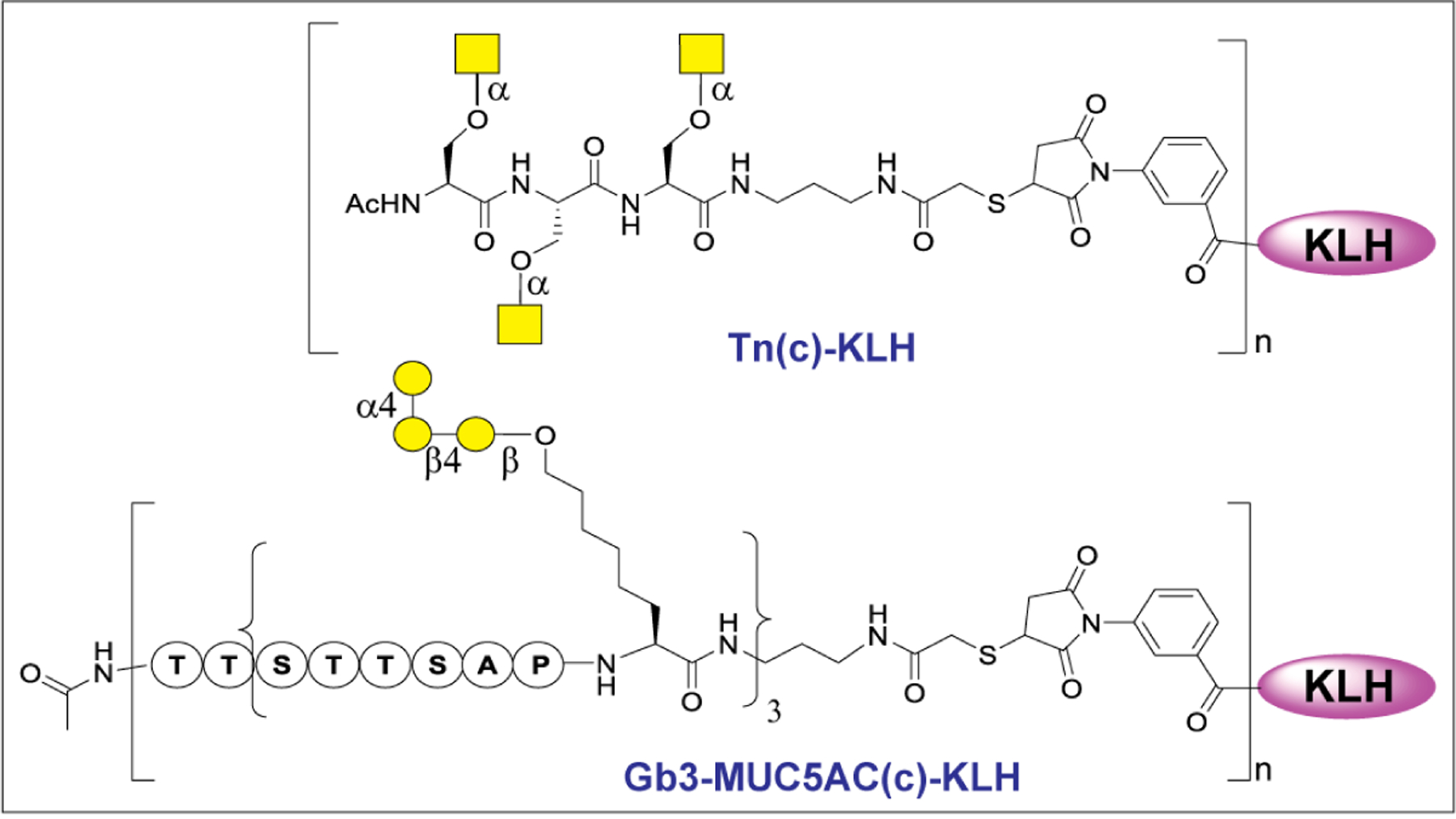
Structure of semisynthetic mono-epitope cnjugate vaccines.
Tumor cells usually express a mixture of carbohydrate antigens in different proportions at each stage during progression of the disease.424 Therefore, design of a multiantigen vaccine is a promising way to target a transformed tumor population. Danishefsky’s group addressed this issue by mixing several mono-epitopes to form a single vaccine for administration. In the phase II clinical study, the patients were coinjected with monovalent KLH conjugates of GM2, Globo H, Lewisy, TF(c), Tn(c), STn(c), and Tn-MUC1 mixed with adjuvant QS21 as a heptavalent vaccine.425 A strong immune response was elicited in the vaccinated patients against at least three antigens. The overall antibody titer against each of the antigens in the mixture was lower than administration of each vaccine individually, probably due to the overdose of highly immunogenic carrier protein and impaired the response against the TACAs.425 The similar findings were reported during coadministration of a mixture of GM2-KLH, Globo H-KLH, Ley-KLH, TF(c)-KLH, Tn(c)-KLH, sTn(c)-KLH, and glycosylated MUC1-KLH.426 Therefore, a multi-epitope monocomponent pentavalent vaccine consisting of prostate and breast cancer specific TACAs was prepared and studied.427 In earlier attempts, three different TACAs, such as Tn, Ley, and Globo H, were conjugated to KLH for immunization and the antigen specific IgM and IgG response was reported.427,428 Later, a highly elaborative multiepitope vaccine was prepared using Globo-H, STn, Tn, TF, and Ley antigens as amino acid building blocks and coupled together via peptide bonds (Figure 19).428 The glycopeptide cargo was then conjugated to KLH for immunological evaluation to demonstrate that the vaccine induced strong IgG responses against each of the antigens except Ley.429 Therefore, in the second generation vaccine, Ley was replaced with a clinically proven TACA, GM2.428,429 In preclinical evaluation, antigen specific antibodies were elicited that recognized the cancer cells overexpressing the respective antigens. The vaccine was safe in a phase I clinical study and elicited a strong IgG and IgM titer against at least three or more antigens in the study population.429
Figure 19:
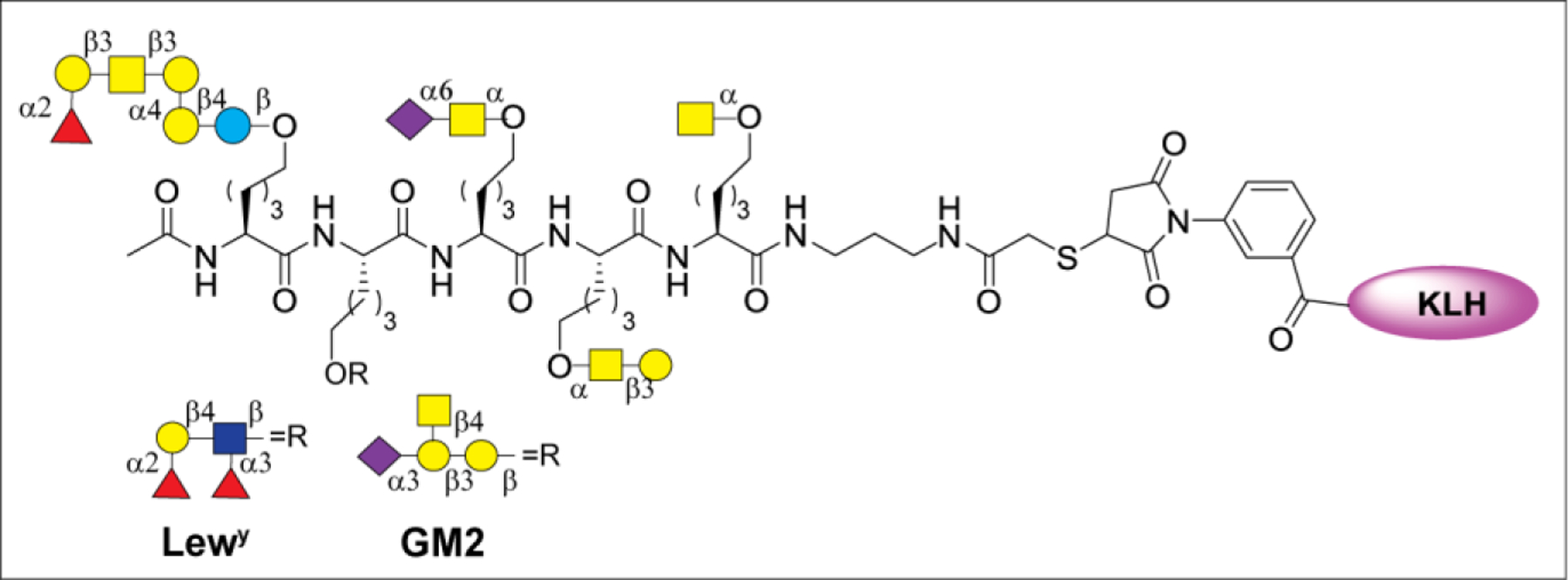
Pentavalent vaccine containing Globo H, STn, Tn, LeY or GM2 and TF conjugated to KLH.
Despite promising and encouraging preclinical results, most of the conjugate anticancer vaccines failed to enter advanced phase clinical studies.430 The major limitations include complicated conjugation steps, inconsistency in conjugation numbers, ambiguous nature of the glycoconjugate, and finally the strong immunogenicity of the carrier protein and immunodominant functional groups in the linkers that ultimately affect vaccine efficacy and reduce the desired antibody response.191,431 Fully synthetic homogeneous vaccines were pursued, containing a TACA conjugated to an adjuvant or other immunological epitope without the use of carrier protein. The first synthetic vaccine of such kind was developed by Toyokuni et al., in which a dimeric Tn antigen was conjugated to a TLR agonist such as the immunoactive lipopeptide, tripalmitoyl-S-glyceryl-cysteinyl serine (Pam3Cys) (Figure 20a).432 The vaccine, di-Tn-Pam3Cys, induced an antigen-specific IgG response without the carrier protein. In another study, a series of Pam3Cys-based conjugates of Ley antigens were prepared and studied for the impact of multivalent antigen presentation, conjugate structure, and adjuvant effect (Figure 20b).433
Figure 20.
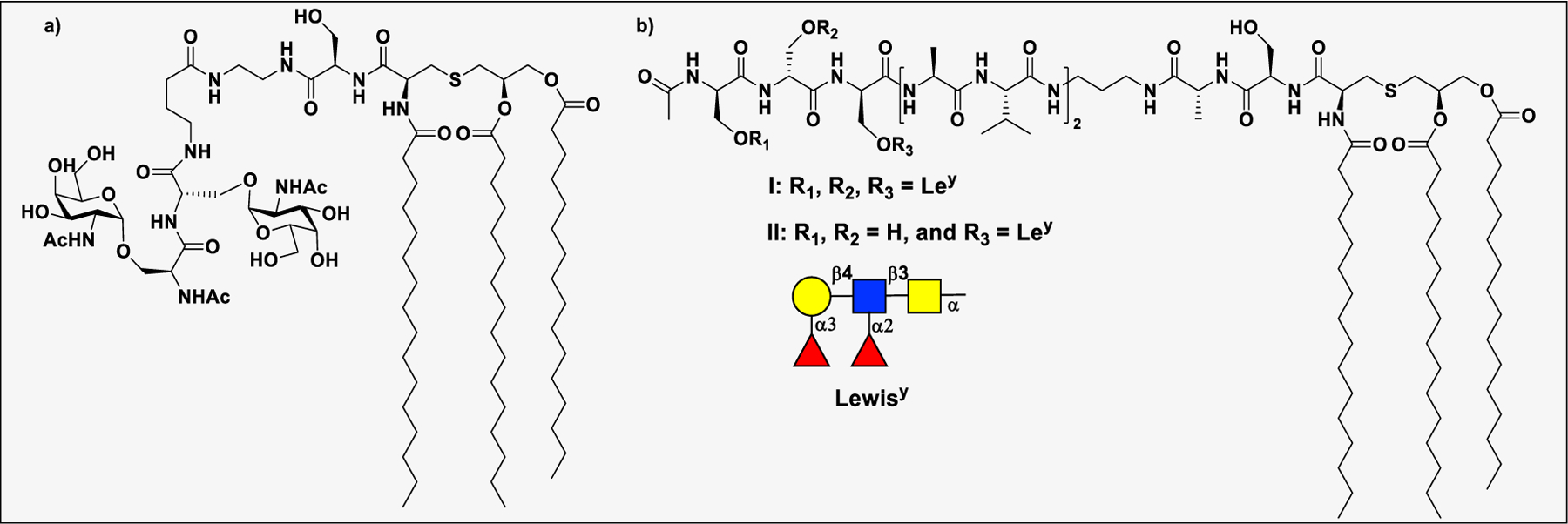
Fully synthetic two-component vaccines linked to Pam3Cys adjuvant, a toll-like agonist.
To induce antibody class switch and long-term memory B cells, the involvement of T cells is required for antibody affinity maturation in B cells. Accordingly, the two-component glycopeptide vaccines containing both B- and a T-cell epitopes were prepared and evaluated for their immune response. Lo-Man et al. reported a dendrimeric multiple antigenic glycopeptide (MAG) consisting of Tn antigen linked to a poliovirus (PV) CD4+ T cell epitope (Figure 21a).434,435 The elicited antibody cross reacted with murine and human tumor cell lines expressing the Tn antigen with antibody-dependent cell cytotoxicity.436
Figure 21:
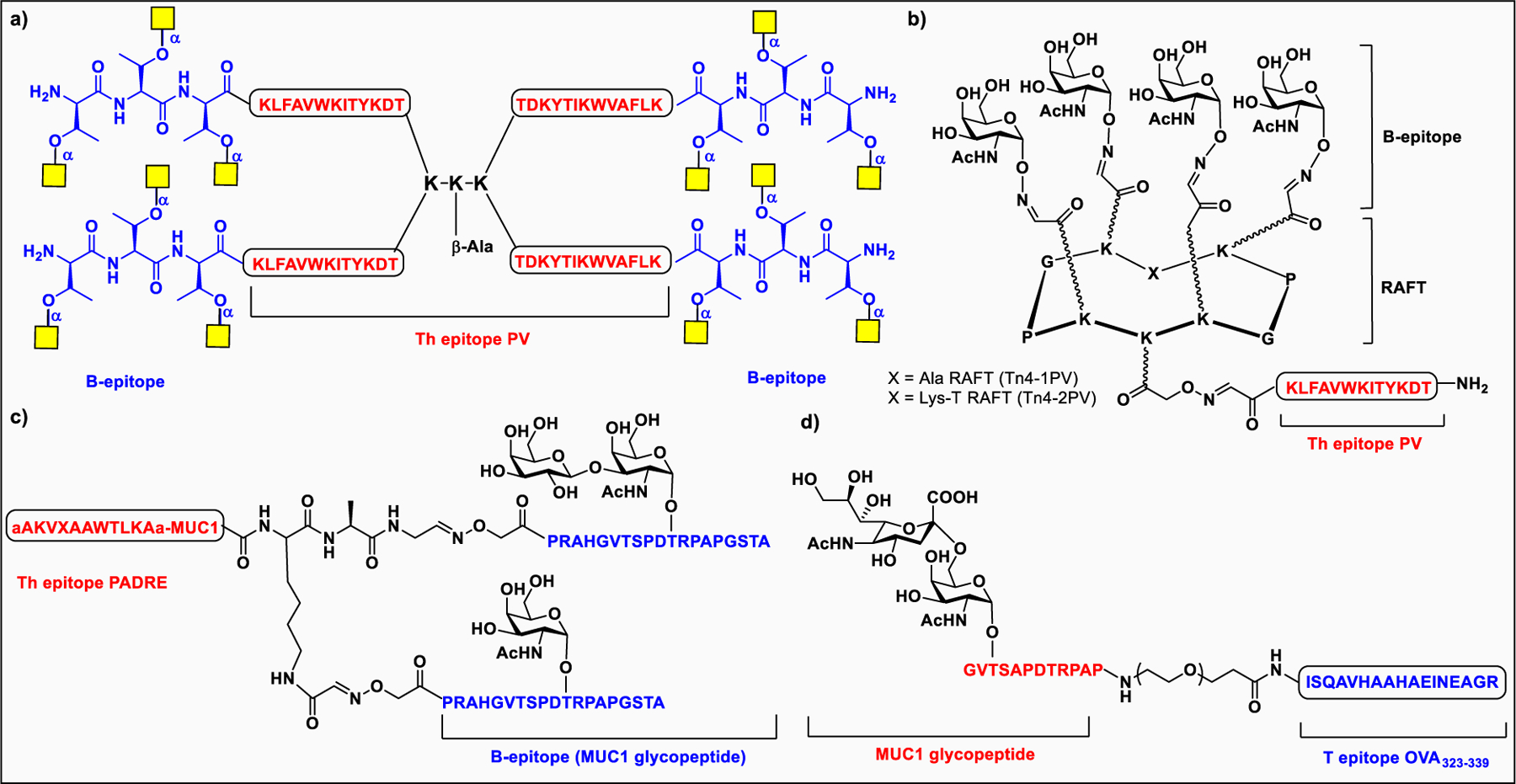
Two component synthetic vaccines. a) Th epitope PV conjugated Tn glycopeptides; b) Th epitope PV conjugated with RAFT cyclic peptide and tetravalent of Tn; c) Th epitope PADRE conjugated Tn and Tf-MUC1.
Dumy et al. designed regioselectively addressable functionalized templates (RAFTs) as new scaffolds to exhibit clustered Tn antigen (B-cell epitope) and the CD4+ helper T-cell peptide from the type 1 poliovirus (Figure 21b).437 The RAFT-Tn4–1PV and RAFT-Tn4–2PV vaccines upon mice immunization, elicited antibodies that recognize the native form of the Tn epitope expressed on human tumor cells. In another study, a universal T-helper epitope, nonnatural pan HLA DR-binding epitope (PADRE) peptide, was used to prepare a glycopeptide construct composed of PADRE and three tumor-related epitopes from the human mucin MUC1, a nonglycosylated repeat unit and two units glycosylated with the Tn and TF epitopes, respectively (Figure 21c).438 A robust antigen specific response was generated upon immunization.439 Kunz’s and co-workers conjugated sialyl-Tn glycopeptide antigen from the tandem repeat region of MUC1 to a TH-cell peptide epitope from ovalbumin (OVA323–339) via nonimmunogenic linker (Figure 21d).440 It has been proposed that after this vaccine is taken up by antigen-presenting cells (APCs) to present the ovalbumin T-cell epitope to the T-cell receptor (TCR), it would lead to the activation and differentiation of naive T cells followed by stimulation of B cells.440,441
Toll-like receptor ligands, for example tripalmitoyl-S-glyceryl cysteine peptides like Pam3CysSer(Lys)4, were used as immunostimulators by the Kunz group to construct a vaccine consisting of Pam3CSKKKK lipopeptide conjugated to tumor-associated MUC1 glycopeptides containing Tn, STn, and TF antigens (Figure 22a).442 Mice immunized with Pam3Cys–icosaglycopeptide conjugates in combination with complete Freund’s adjuvant (CFA) led to a specific humoral immune response; however, the antiserum titers were not as high as those from the corresponding MUC1 tetanus toxoid vaccine.442 A monophosphorylated derivative of Neisseria meningitidis lipid A was also used as a built-in adjuvant for the construction of structurally defined glycoconjugate vaccines (Figure 22b). The Guo group reported MPLA conjugates of modified GM3,221 STn,443 or Globo H444 and showed that MPLA derivatives with Globo H elicited high titers of antigen-specific IgG antibodies compared to the use of modified sTn and GM3 antigens without an external adjuvant.445
Figure 22.
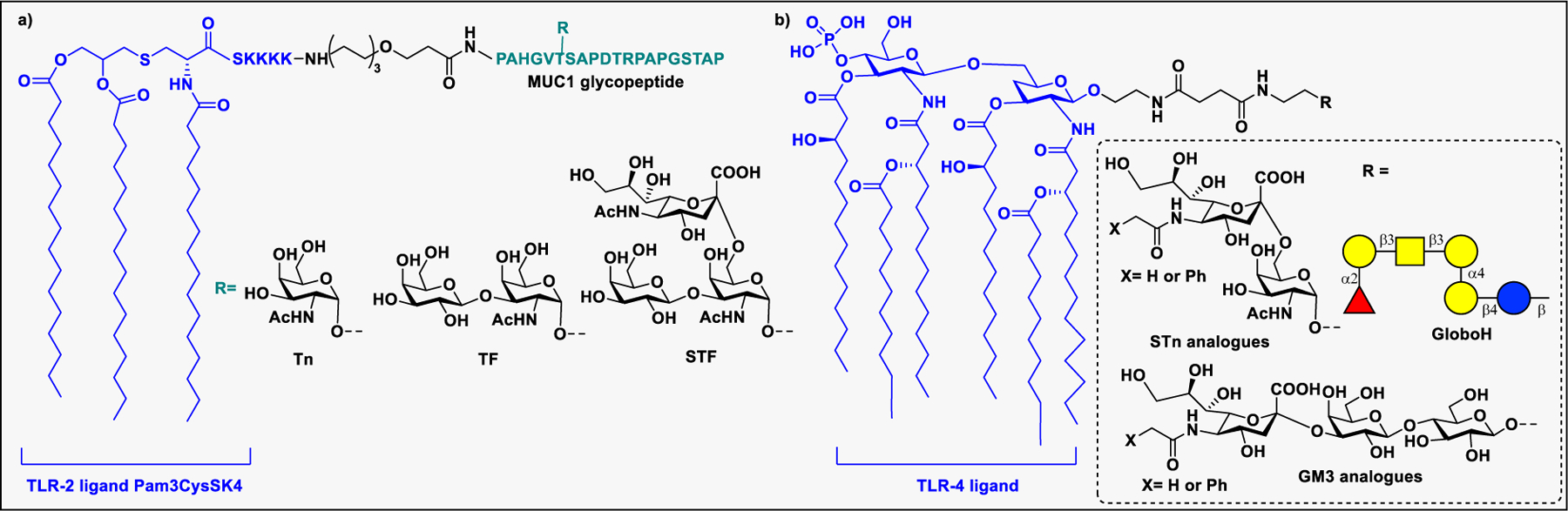
Two component synthetic vaccines. a) TLR2 ligand Pam3CSKKKK conjugated to MUC1 glycopeptides containing Tn, STn, and TF antigens. b) MPLA conjugates of modified GM3, STn, or Globo H.
The first examples of anticancer vaccines without the use of a carrier protein or another immune stimulant epitope were developed by the Andreana group by conjugation of Tn or STn antigen to zwitterionic polysaccharides (ZPSs) that can induce MHCII-mediated immune responses (Figure 23).446,447 The immunization of the vaccine with commercially available MPL-based adjuvant elicited a strong immune response and high titers of IgM/IgG antibodies that not only recognized STn-expressing cancer cells (MCF-7 and OVCAR-5) but also showed complement-dependent cellular cytotoxicity.447
Figure 23:
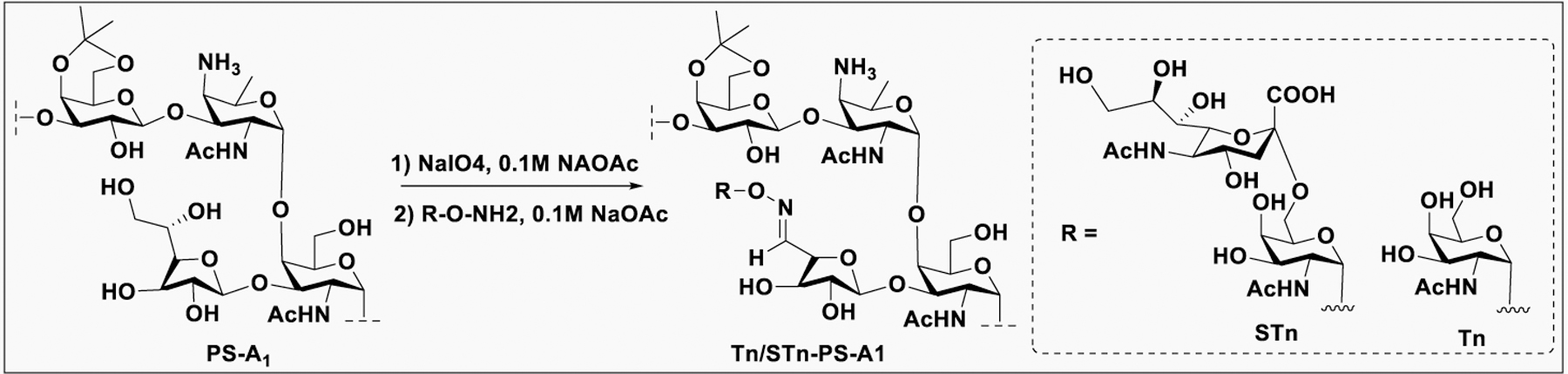
Preparation of Tn/STn-PS A1 from PS A1 and aminooxy TACA antigen.
The presence of a B-cell epitope (TACA), a Th cell epitope, and a built-in adjuvant in a single construct led to development of three- and four-component synthetic vaccines to induce strong and long-lasting IgG responses. Accordingly, Boon’s group developed a novel three-component vaccine consisting of a B cell epitope (tumor associated MUC1 glycopeptide), an adjuvant (Pam2CysSK4 or Pam3CysSK4), and a Th epitope (a mouse MHC class II restricted PV) (Figure 24a).448 The vaccine induced a highly robust IgG response that reacts with MUC1-expressing cancer cells without coadministration of external adjuvant.448 Later, Renaudet et al. prepared a four-component vaccine consisting of Tn antigen, PADRE, a CD8+ CTL peptide epitope (OVA257–264), and a built-in adjuvant (Pam3Cys) (Figure 24b).449 This vaccine elicited robust IgG/IgM, CD4+, and CD8+ T-cell responses against Tn antigen, PADRE, and OVA257–264, respectively, probably due to proper antigen processing and presentation. Immunization of the resultant vaccine reduced the tumor size in the challenge study.450 These studies demonstrate the potential of cancer vaccines with B cell, CD4+, and CD8+ T cell epitopes as self-adjuvants.
Figure 24.
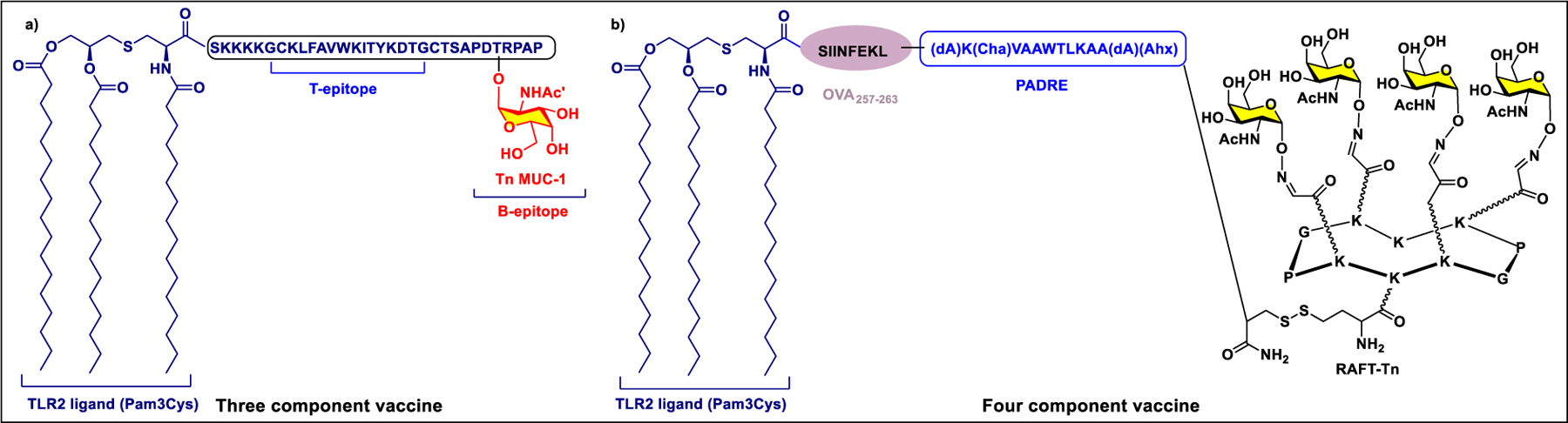
Examples of three- and four-component vaccines
Globo H, one of the most thoroughly studied TACAs, was originally isolated in the form of glycolipid from the breast cancer cell line, MCF-7.149 In addition to its abundance on ovarian, gastric, lung, prostate, pancreatic, endometrial, and liver cancers, GloboH is an important regulator in the tumor microenvironment, promoting tumor progression through several mechanisms, therefore representing an ideal target in cancer immunotherapy.149,451 Adagloxad simolenin (OBI-822), originated in the Danishfsky lab, is a vaccine developed by the Taiwanese biotech company, OBI Pharma, consisting of the Globo H epitope covalently linked to the immunostimulatory carrier protein KLH (Figure 25). Adjuvanted by the potent saponin-based adjuvant QS-21, the Globo H-KLH vaccine was well tolerated in two phase I studies in patients with metastatic breast or prostate cancer.452,453 OBI-822 is currently in a phase 3 clinical trial (GLORIA) for the treatment of patients with Globo H-positive, triple-negative breast cancer. The second-generation GloboH vaccine was developed in our lab by using a different combination of carrier proteins and adjuvants. Globo H conjugated to DT-CRM197 in combination with glycolipid adjuvant showed improved an immunological profile compared to the first-generation Globo H-KLH vaccine (Figure 25).454 Glycolipid C34 is a well-known ligand of the CD1d receptor present on DCs. Upon presentation of glycolipid by CD1d to the T-cell receptors on NK cells, activation of the NKT cells generated a selective Th1 response rather than Th2, and resulted in significant class switching to produce a robust IgG response against three globo-series epitopes: Globo H, SSEA4, and SSEA3.454
Figure 25:
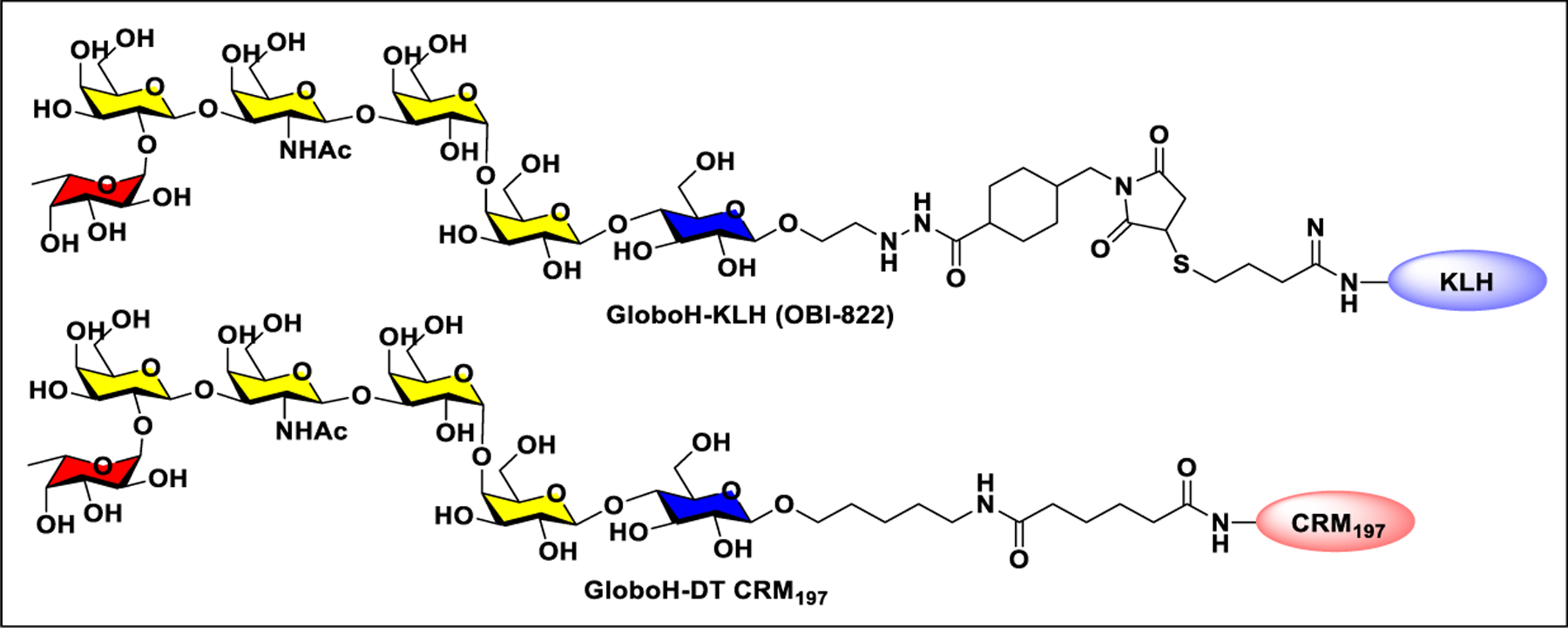
Structures of Globo H-KHL and Globo H-DT vaccines.
Because of the expression of TACAs on normal tissues or cells, some of the TACA-based vaccines faced major setbacks due to the self-tolerance by the immune system that resulted in poor immunogenicity. Unnatural modifications of TACAs have been used as a potential strategy to generate robust antibody responses that can cross react with native antigens on tumor cells (Figure 26). The Ye group introduced modifications on GM3 and STn antigens and then conjugated the modified antigens to protein carriers for vaccination.455,456 Mice immunization studies showed that the modified antigen–KLH conjugates elicited higher titers of anti-GM3/STn antibodies than the unmodified GM3/STn–KLH conjugates (Figure 26).455,456 More recently, the same group also reported several Globo H analogues with modification on the N-acyl group and conjugated to CRM197. The immunological studies of modified GloboH-CRM197 adjuvanted with glycolipid C34 showed that the fluorine-modified N-acyl Globo H conjugates induced higher titers of IgG antibodies that recognize and eliminate the cancer cells expressing the Globo H antigen (Figure 26).457 Another vaccine with azido modifications at the reducing and non-reducing ends of Globo H conjugated to CRM197 elicited stronger IgG response than the native Globo H vaccine that can cross react with Globo H+ cancer cells.458
Figure 26.
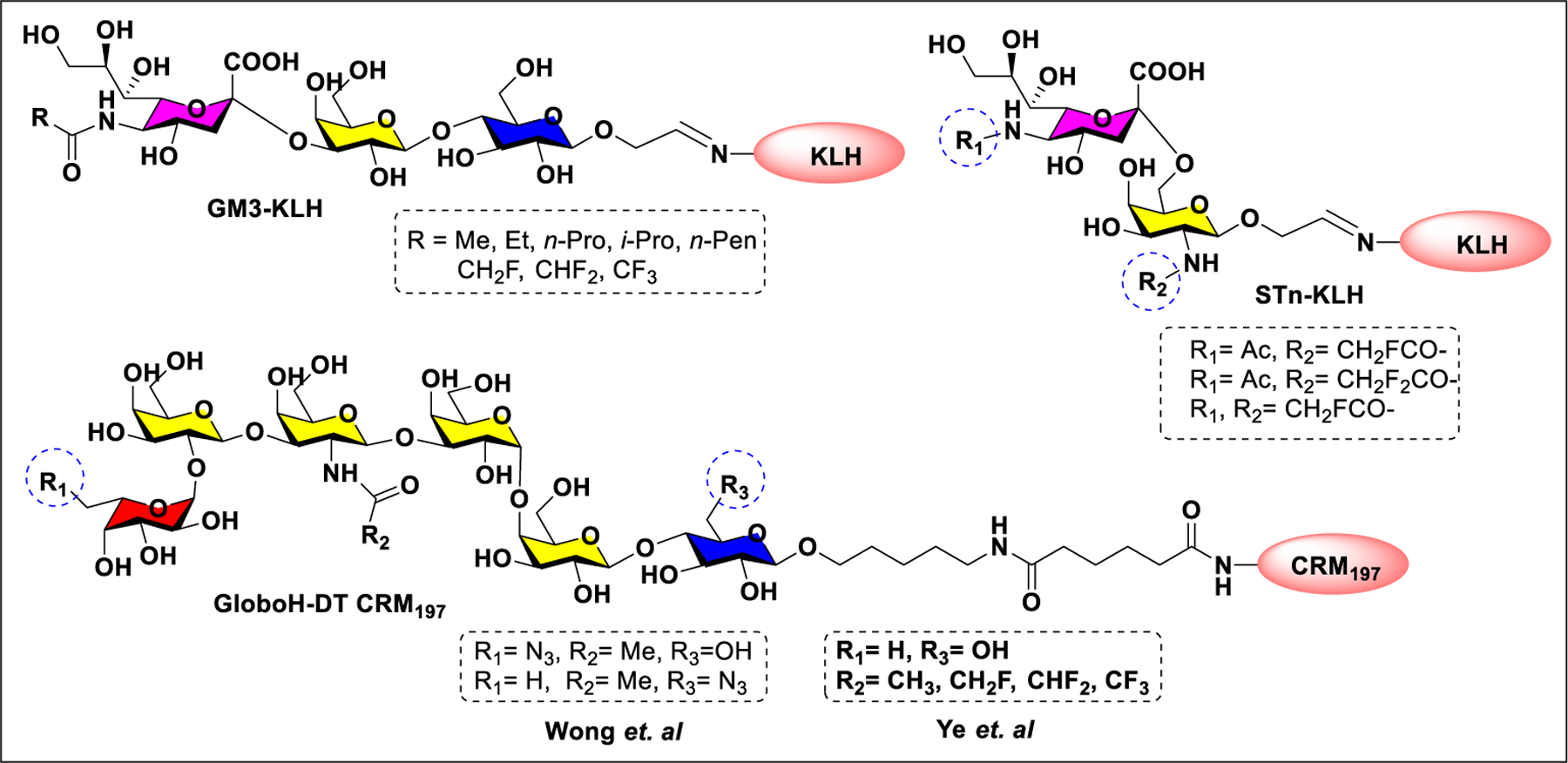
Structures of TACA modified vaccines.
4.1.3. Anti-HIV Vaccines.
The HIV-1 envelope glycoprotein is a trimer of the heterodimer consisting of receptor-binding gp120 and transmembrane gp41 subunits. The gp120 spike is heavily glycosylated by the host derived glycosylation machinery, where half of the gp120 mass comes from the glycan coat.459 There are approximately 25 glycosylation sites, of which ∼7–8 glycosites are in the V1/V2 and V3 variable loops, ∼4 are in inner domain and the others in the rest of outer domain of gp120.460 Glycosylation on gp120 is essential for proper folding, viral infectivity, and to help the virus escape from host immune attack.461 The outer domain of gp120 is the primary viral component first exposed to the immune system. However, dense glycosylation covers the large protein surface shielding the neutralization sensitive peptide epitopes from immune recognition.462,463 Nevertheless, the discovery of broadly neutralizing antibodies (bNAbs) that penetrate the glycan shield and bind to the epitope consisting of both protein and carbohydrate components offer exciting opportunities for development of glycoconjugate-based HIV-1 vaccine (Figure 27).464–468 First-generation bNAbs, such as 2G12 and b12, were reported to target a high mannose glycan patch on the gp120 outer domain and the CD4-binding site, respectively.469–471 Despite relatively weak coverage and potency, passive transfer of these antibodies provided protection against simian-HIV in rhesus macaques.469,472,473 Second-generation antibodies, including PG9, PG16, andPGT series antibodies, VRCO1, 35O22, VRC-PG05, and VRC-34.01, etc., were obtained by cloning antigen-specific antibodies from B-cells of HIV-1 positive donors.463,468 Structural and biophysical epitope mapping studies of these antibodies led to identification of several vulnerable targets on the viral spike protein. Most of these antibodies are in various phases of development for HIV-1 prevention and treatment.474 Synthetic carbohydrate-based immunogens, consisting of a glycan or glycopeptide mimic of epitopes recognized by the bNAbs conjugated to a protein carrier, are capable of inducing bNAbs.475
Figure 27:
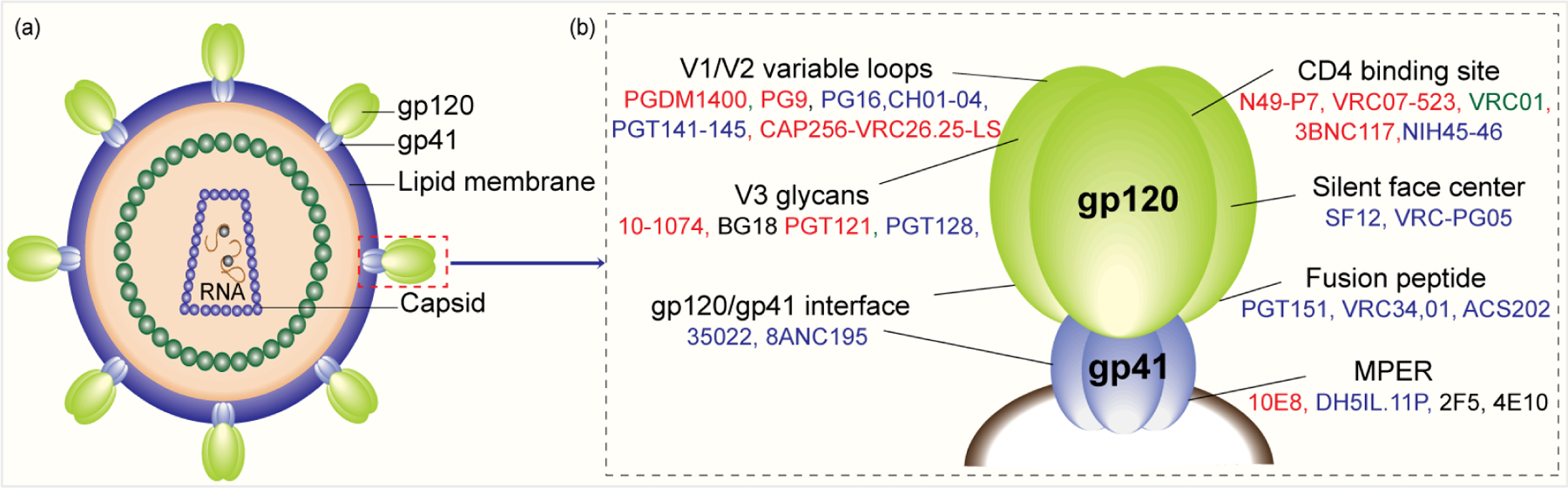
a) Schematic diagram of HIV-1. b) Epitopes of bNAbs on trimeric HIV-1 envelope spike glycoprotein. Antibodies in green font (phase 3), red (phase 1), and blue (preclinical).
The bNAb, 2G12, was the first anticarbohydrate antibody to target the high mannose oligosaccharide patch on gp120, particularly at glycosylation sites N295, N332, N386, and N392.476 The antibody was shown to bind Man9 and Man4 glycans where the terminal Man-α1,2-Man disaccharide was involved in binding.470 In an attempt to mimic the 2G12 epitope, the Wang group reported a cholic acid scaffold conjugated to three high-mannose type Man9GlcNAc2 glycans regioselectively (Figure 28a).477 The cluster was found to be 46-fold more effective than monovalent glycan in competition assays. In another study, the same group used galactose as a scaffold to attach Man9GlcNAc2 glycan, which was identified as the best 2G12 ligand, using thiol–maleimide coupling methods. The 2G12 antibody showed 70-fold higher affinity toward the tetravalent Man9 cluster than Man9GlcNAc2Asn (Figure 28b).478 The oligocluster was then conjugated to either a carrier protein (KLH) or a universal T-helper peptide for vaccination. The rabbit immunization studies showed a modest antiglycan IgG response, weak cross reactivity with gp120, and lack of neutralization activity.479
Figure 28:
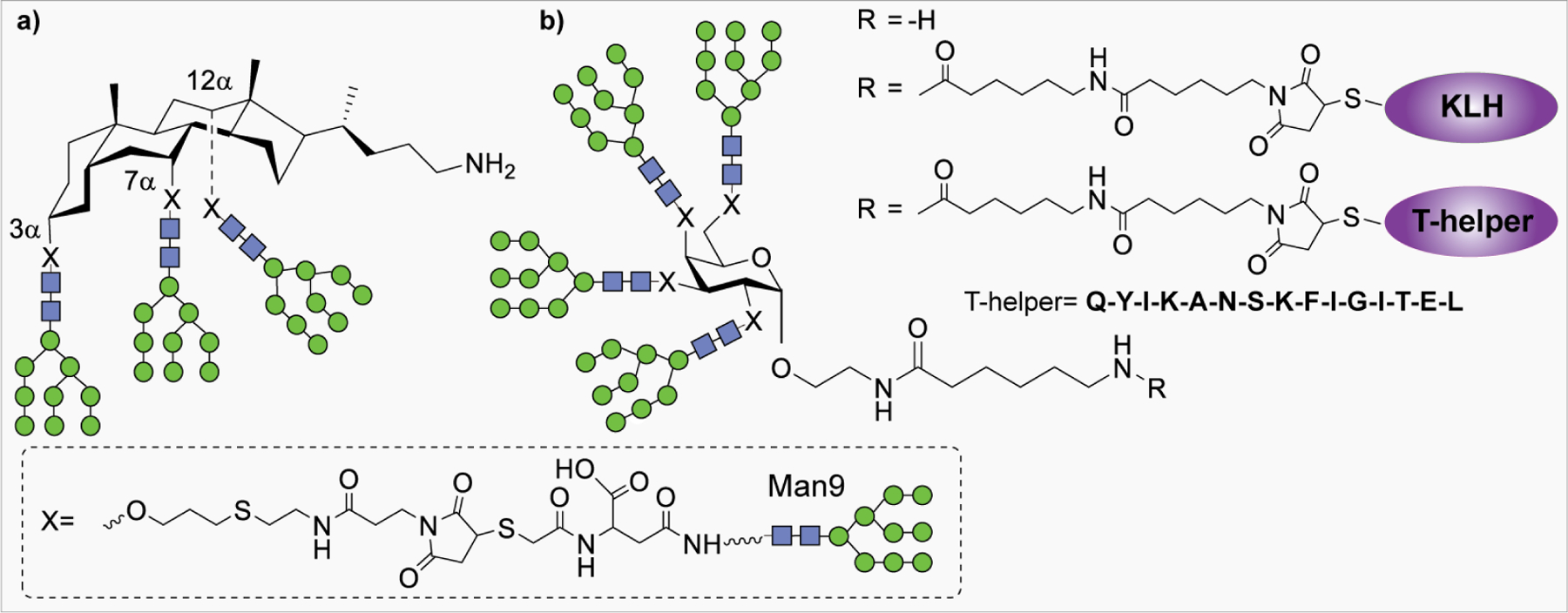
a) Structure of a cholic acid-based oligomannose cluster to mimic the 2G12 epitope; b) Tetravalent Man9GlcnAc2 scaffold conjugated to KLH and T-helper peptide for immunization.
The Wang group prepared another tetravalent cluster of Man4GlcNAc2, a D1 arm of Man9GlcNAc2, mounted on a cyclic peptide scaffold through Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition reaction (Figure 29a). Additionally, a fluoride was also introduced at 6-O position of the terminal mannose residue. The synthetic constructs were then coupled to T-helper peptide epitopes for 2G12 binding studies.480 In a similar study, the Danishfsky group designed a Man9GlcNAc2 cluster on a cyclic peptide scaffold containing 0–3 oligomannose units. The bivalent construct was then attached to outer membrane protein complex (OMPC), yielding almost 2000 glycopeptide copies per conjugate (Figure 29b).481,482 Immunological evaluation of the glycoconjugate in the presence of QS-21 adjuvant elicited a higher titer of antiglycan antibodies in guinea pigs and rhesus macaques. However, the antisera failed to cross-react with HIV gp160 and did not neutralize the different viral isolates.483 Our lab developed an AB3 type of dendrimeric scaffold to attach 3, 9, and 27 copies of synthetic Man4 and Man9 glycans and achieved an affinity for 2G12 that is comparable to that of gp120 for 2G12, suggesting that the synthetic glyco-dendrimer might be an ideal vaccine candidate.484 Constantino et al. exploited a flexible polyamidoamine (PAMAM) scaffold to attach four and eight copies of high mannose antigens such as Man4, Man6, and Man9 to improve avidity toward 2G12 (Figure 29c). Conjugates of synthetic glycodendrons with DT-CRM197 were prepared and formulated with MF59 adjuvant for immunization in mice and rabbits to elicit a strong antigen specific response; however, the antisera failed to recognize recombinant gp120.485 Burton and colleagues designed neoglycoconjugates consisting of a variable number of Man4 glycans on bovine serum albumin (BSA) (Figure 29d). Immunization of rabbits with BSA-(Man4)14 elicited Man4 specific antibodies but again failed to bind gp120.486
Figure 29:
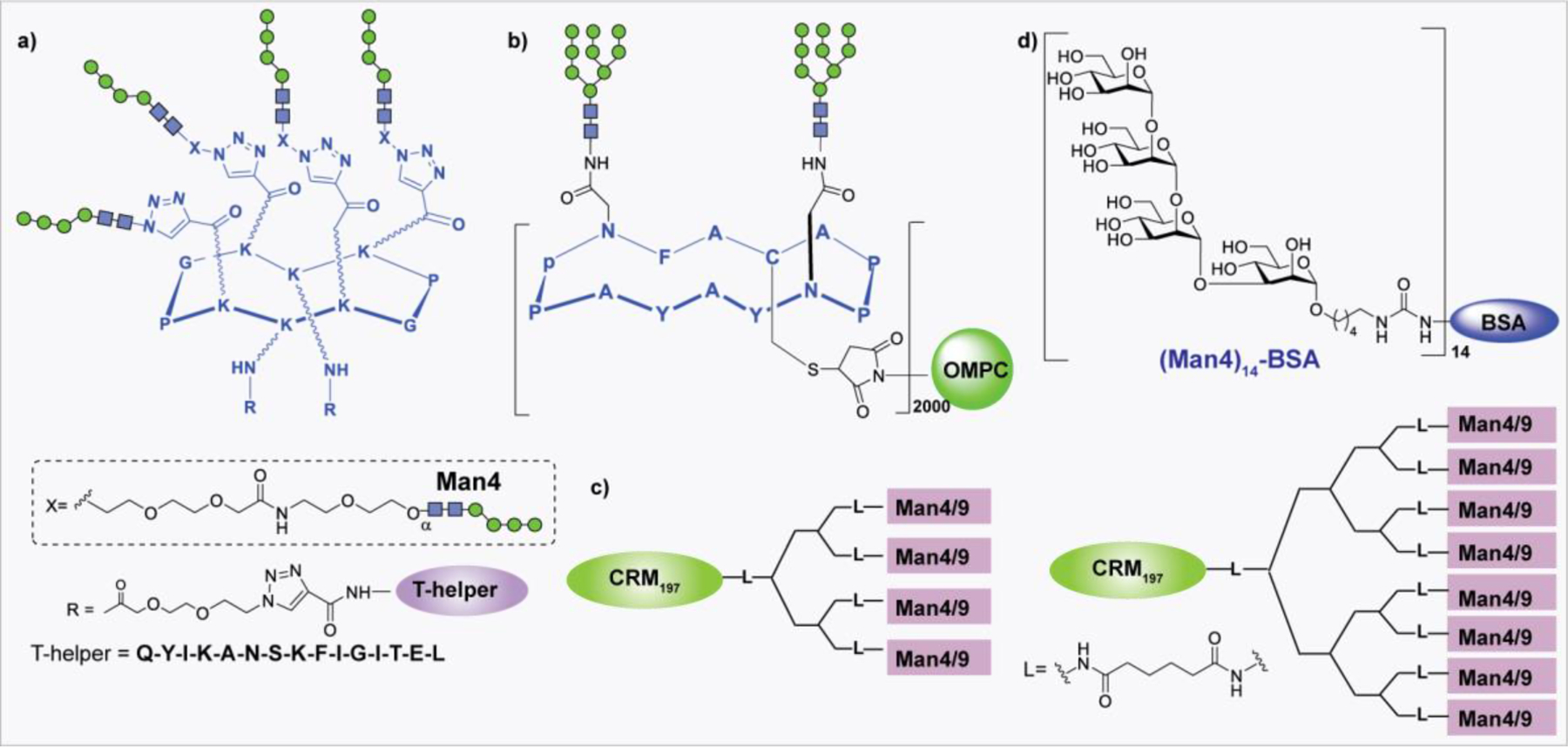
Examples of synthetic multivalent vaccines.
The icosahedral capsids of bacteriophage Qβ has also been utilized because of its safety, immunogenicity, and ability to create multivalently on its surface.487 The Qβ is recombinantly expressed in E. coli and self-assembles into virus-like particles. Finn et al. prepared Qβ -Man4 and Qβ-Man9 to better mimic the oligomannose clustering on gp120 (Figure 30a).488 Later, Davis reported unnatural analogues of mannose residues and incorporated them at the termini of Man4 glycan, which was then conjugated to bacteriophage Qβ. The nonself D1 arm mimics are better inhibitors of 2G12/gp120 binding than the natural form both as unconjugated and protein-conjugated forms.489 In both studies, immunization of rabbits with both Qβ-Man4/Man9 and Qβ-nonself D1 arm generated glycan-specific antibodies (Figure 30b).488,489 Nonetheless, these antibodies were not cross-reactive to native gp120 and did not show any HIV-1 neutralizing activity. Recently, Nguyen et al. designed highly antigenic 2G12-binding glycopeptides through in vitro selection.490 The trivalent Man9-glycopeptide conjugated to CRM197 was used for immunization of mice in combination with Adjuplex as an adjuvant (Figure 30c).491 Glycopeptide-specific antibodies were elicited with no detectable HIV-1 neutralizing activity. Interestingly, the immune response was raised against the core mannose residue.491
Figure 30:
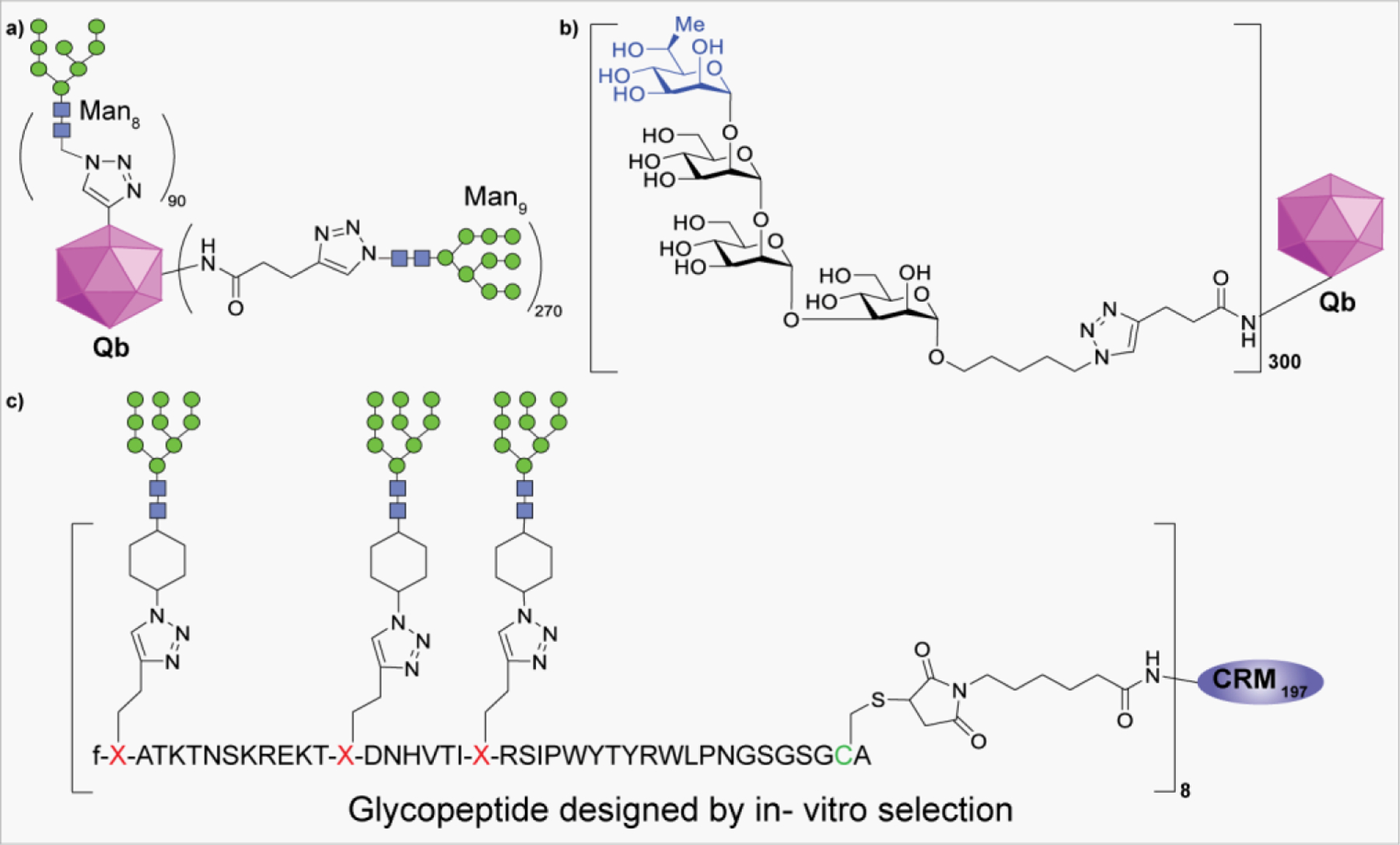
a) Bacteriophage Qβ conjugated to Man8 and Man9 glycans. b) Unnatural Man4 cluster on bacteriophage Qβ. c) Trivalent Man9-glycopeptide conjugated to the CRM197.
Since the discovery of new bNAbs from HIV positive patients, including for example, PG9, PG16, and PGT series antibodies, research in glycan-based vaccines has shifted the focus to identify the real ligands of such antibodies that recognize the glycan epitopes on gp120. Kwong et al. reported the structure of PG9 cocrystallized with a scaffolded HIV-1 V1/V2 loop to demonstrate that PG9 interacts with gp120 through the N-linked glycans at N160 and N156/N173 and a peptide strand.466 Based on this information, the Wang group reported a series of V1/V2 glycopeptides from two different HIV-1 strains (CAP45 and ZM109).492 The peptides were glycosylated with Man5GlcNAc2 and sialylated complex type glycans (SCT) at N160 and N156/N173 sites using transglycosylation of GlcNAc peptides with respective glycan oxazolines (Scheme 15a). Binding analysis studies of synthetic glycopeptides with PG9 suggest the necessity for Man5GlcNAc2 at N160 and additional SCT glycans at N156/N173 for high affinity PG9 recognition.492 Later, the Danishfsky group generated V1/V2 loop peptides from HIV-1 A244 strains containing Man5GlcNAc2, and a core chitobiose at the N160 and N156 sites.493 The glycosyl amine building blocks were coupled to peptide fragments through a Lansbury aspartylation followed by NCL of two peptide fragments to construct the glycosylated V1/V2 peptide (Scheme 15b). The surface plasmon resonance (SPR) binding analysis suggests the binding of PG9 to only Man5- and Man3-containing peptides.493
Scheme 15:
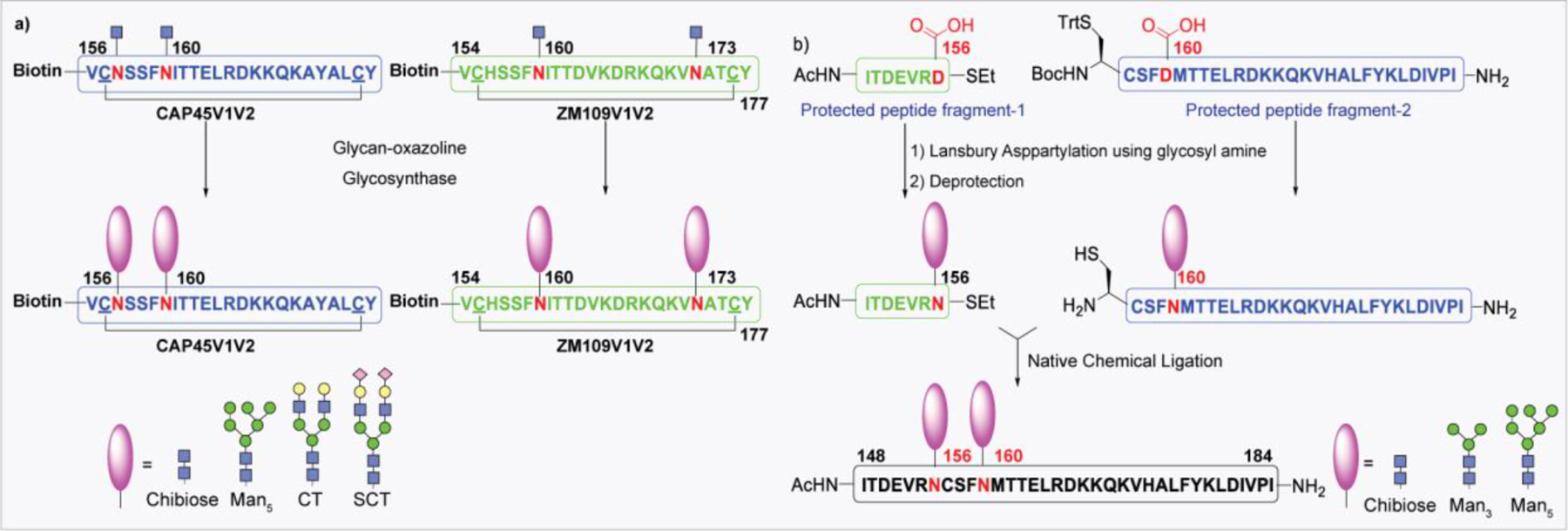
Synthetic glycopeptide mimics of the gp120 V1/V2-loop. Cyclic glycopeptides derived from stains (a) CAP45 and ZM109, and (c) strain A224 containing different N-glycans
The PGT series antibodies, such as PGT128 and PGT121, are highly potent and neutralize over 70% of globally circulating strains.464,494 Structural and biophysical studies showed that these antibodies recognize specific high mannose N-glycans (at N322 and N301) around the V3 loop and the conserved peptide domain in the V3 loop of gp120.464,495 To mimic the PGT antibodies epitopes, Wang et al. prepared V3 glycopeptide derived from the HIV-1 JR-FL strain containing Man9GlcNAc2 at the N332 site.496 In another related study, they also prepared V3 glycopeptide derived from the HIV-1 A224 strain, where the Man9GlcNAc2 glycan is located at the N334 site (Figure 31a,b).497 Synthetic glycopeptides were tested for their antigenicity by binding with bNAbs and showed that PGT128 is highly specific for high mannose glycan at N301/N332, antibody 10–1074 for high mannose glycan at N332, and PGT121 for sialylated complex type glycan at N301.498 Based on promising preliminary results, they prepared a three components vaccine by conjugation of V3 glycopeptides from both the HIV-1 strains (JR-FL and A224) to the T-helper epitope and the TLR2 agonist adjuvant. Immunization of rabbits with these synthetic immunogens elicited glycan-specific antibodies that were cross-reactive to various gp120s but failed to neutralize HIV-1 virions.497 Haynes et al. reported an HIV-1 JR-FL V3 glycopeptide fragment with two Man9GlcNAc2 glycans at N301 and N332 (Figure 31c) installed via Lansbury aspartylation.475 This HIV-1 JR-FL glycopeptide fragment containing two Man9GlcNAc2 glycans at N301 and N332 was used for immunization in rhesus macaques to report that the glycopeptide induced antibodies bound specifically to envelope glycoprotein but did not neutralize HIV-1.499
Figure 31:
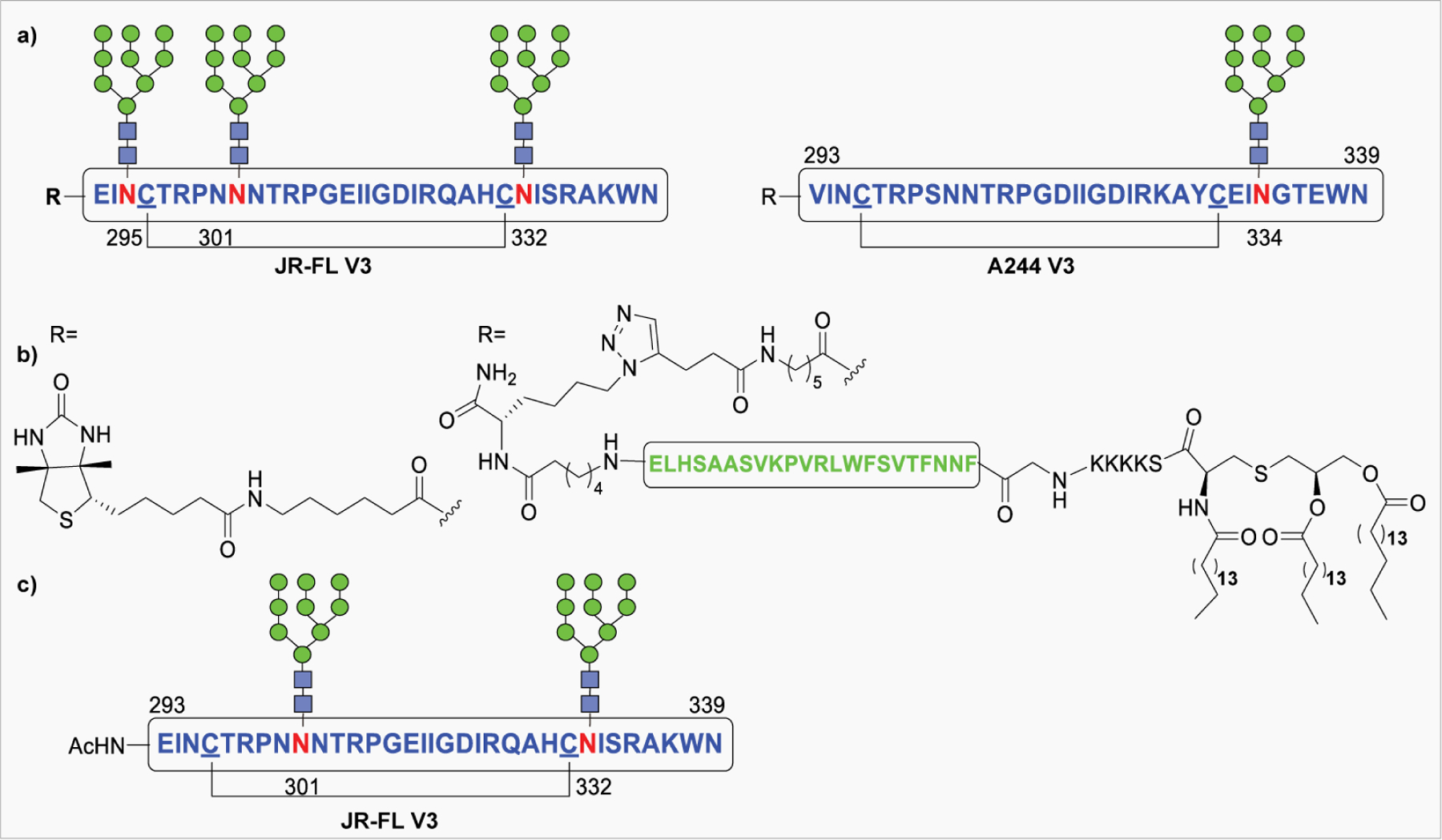
Synthetic V3-targeting bNAb epitope. a) glycopeptides from JR-FL (left) and A244 (right) strains. b) Structure of three-component vaccine bearing gp120 glycopeptide, T-helper epitope, and a TLR2 agonist adjuvant. c) JR-FL strain-derived V3 glycopeptide.
Recently, the Wang group prepared another three-component vaccine wherein the V3 glycopeptide derived from the HIV-1 JR-FL strain was linked together to create a trivalent presentation followed by conjugation to T-helper epitope and the Pam3CysSK4 (Figure 32).500 A stronger glycopeptide-specific antibody response was generated that cross-reacted with heterologous HIV-1 gp120s and gp140 trimers, but the antisera did not show HIV-1 neutralizing activity.500
Figure 32:
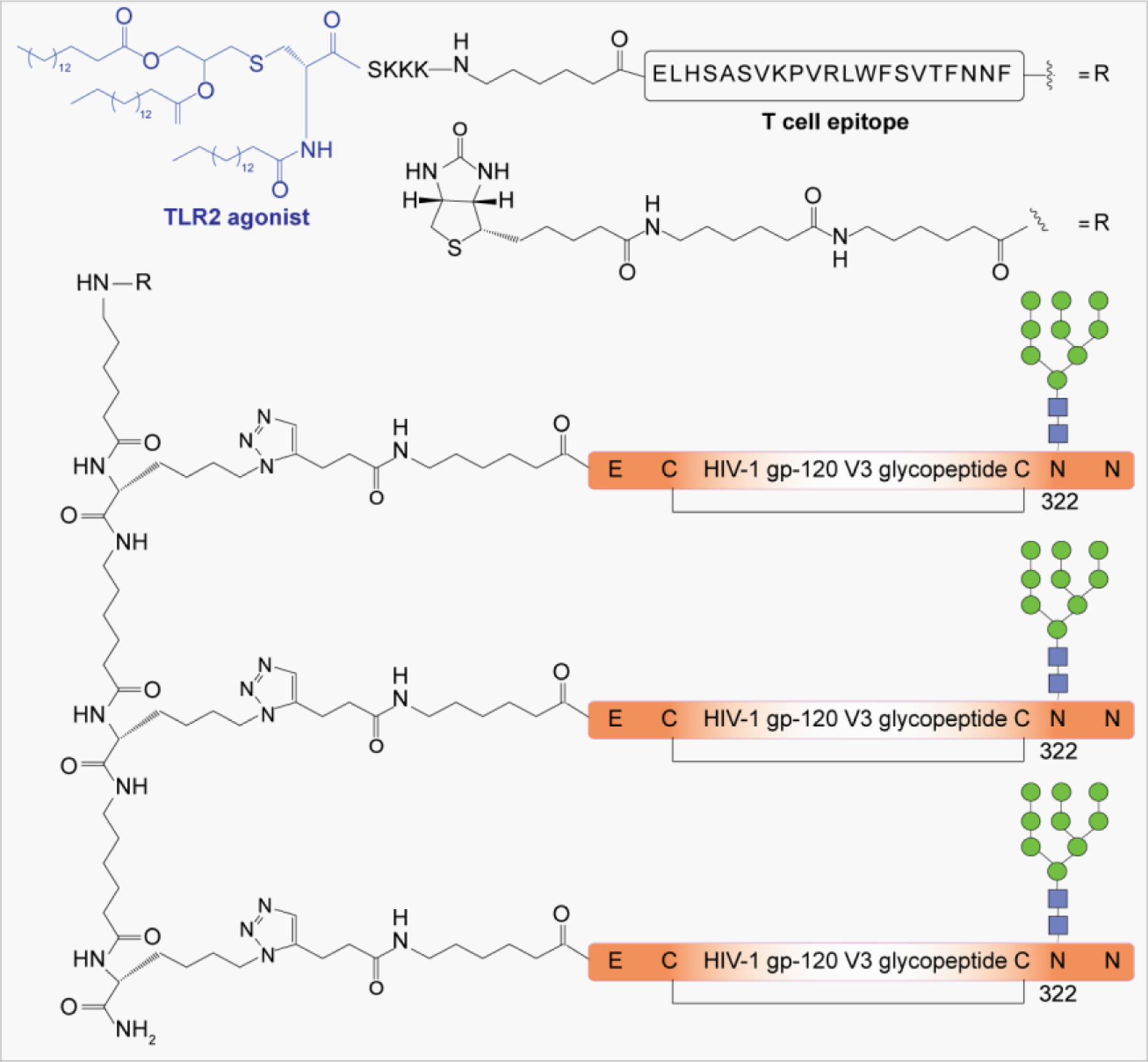
The three-component vaccine containing A244 derived V3 glycopeptide, a T-cell epitope and a TLR2 agonist adjuvant.
Design of the optimal immunogen for induction of bNAbs is the principal challenge in the development of HIV-1 vaccine. The discovery of bNAbs that recognize glycan-dependent regions on the HIV-1 Env protein, such as the high-mannose cluster centered at N332 (2G12) and the variable loops V1, V2 (PG9, PG16), and V3 (PGT-series) offered valuable information to the immunogen design. Among the synthetic glycoconjugate constructs studied to date, some can elicit an antigen-specific immune response and can cross react with HIV-1 gp120, but the induction of an effective HIV-1 protective response remains elusive.
4.1.4. Processing of Carbohydrate Antigen in Glycoconjugate Vaccines.
APCs such as B cells, macrophages, and DCs, process and present antigens by major histocompatibility complex (MHC) molecules to T cells. As professional APCs, DCs are expert in the activation of naive CD8+ and CD4+ T cells by presenting antigens through MHCI and MHCII, respectively, and by providing cytokines and costimulatory stimuli.501 DCs can uptake antigen through endocytic mechanisms, including phagocytosis, macropinocytosis, clathrin-mediated endocytosis, and trogocytosis. After uptake, protein antigens will be transported through early endosomes to proteosome, where it is processed into peptides by cathepsins. The processed peptides will be loaded on MHCII and presented to CD4+ T cells.502 Meanwhile, through cross-presentation, exogenous protein antigens can be transported into the cytosol, where they are processed into peptides by the proteasome. Synthetic glycopeptides or glycopeptides from glycoprotein processed by DCs can bind to MHCI or MHCII molecules, and the complexes are presented to CD8+ or CD4+ T cells, respectively.503 Under normal conditions, resting DCs present endogenous peptide to maintain peripheral tolerance.504 DCs are activated upon receiving invaded signals through innate pattern-recognition receptors (PRRs). Mature DCs present foreign antigens, increase surface levels of costimulatory molecules, and secrete cytokines to stimulate adaptive immune responses.505
4.2. Glyco-engineered Therapeutic Antibodies
With over 60 antibody drugs available for use, monoclonal antibodies (mAbs) are one of the most successful class of biopharmaceuticals that are being used for treatment of life-threatening diseases such as cancers and autoimmune disorders.506 Certain types of mAbs, for example anticancer antibodies (rituximab, trastuzumab, and alemtuzumab), act by immune mediated effector functions such as ADCC and CDC. ADCC is a mechanism by which antibodies recruit immune cells to kill target cells through interaction of their Fc-domain with Fcγ receptors (FcγRs) on immune cells such as dendritic cells and macrophages.507,508 CDC is triggered when the IgG Fc domain interacts with complement component 1q (C1q), resulting in activation of complement cascade and lysis of target cells.509 Typically, the N297 glycosite in the IgG Fc domain is heterogeneously glycosylated,510 with more than 30 different glycoforms, usually the biantennary complex type glycans bearing O-2 terminal galactose and mostly core fucosylated, being characterized.511,512 Recent studies demonstrated that the glycan structure on the IgG Fc-domain can differentially affect the biological activity and efficacy of therapeutic antibodies.513,514 For example, core Fucose at Fc-glycan significantly lowered the binding affinity to FcγIIIA receptor that led to reduction in ADCC.315,337 Moreover, the glycans bearing terminal α2,6-sialic acid has been implicated in anti-inflammatory activities of IVIG in animal models.515 The high mannose glycan at N394 of the human IgE Fc domain is essential for initiation of anaphylaxis.516 Therefore, the Fc-mediated effector functions of mAbs could be controlled by modulating Fc–FcγRs interactions through optimizing the structure of the N-glycan present on the IgG Fc-domain.
4.2.1. In Vivo Glycoengineering.
Recombinantly produced therapeutic antibodies are highly heterogeneous in their Fc-glycosylation.517 Thus, different strategies have been developed to produce highly defined homogeneous glycoforms or to enrich specific glycoforms for functional studies and improvement of therapeutic efficacy. In vivo alteration of the glycosylation pathway in host expression systems has been used to produce antibodies, with lack of core fucose, increase in galactose, and sialic acid content, etc.518 Although glycan processing enzymes are well organized in the biosynthetic pathway, competition of the enzymes for acceptor substrates is the main factor causing the glycan microheterogeneity on expressed proteins. The addition of bisecting GlcNAc on core mannose residues of Fc N-glycan via overexpression of GnTIII to restrict the downstream action of α1,6-fucosyl transferases to reduce core fucosylation313 formed the basis of GlycoMab technology originally developed by Glycart Biotechnology (acquired by Roche).519 This platform is used for development of the anti-CD20 antibody, Obinutuzumab, which was approved for the treatment of patients with previously untreatable chronic lymphocytic leukemia.520 Although bisecting GlcNAc inhibits core fucosylation, it is unable to block the glycosylation completely. Therefore, disabling the α1,6-fucosyl transferases activity encoded by the FUT8 gene has emerged as a powerful strategy.311,521 Potelligent Technology developed by Kyowa Hakko Kirin is one of the most successful platforms for production of 100% nonfucosylated antibodies using FUT8−/− CHO cells.522 The anti-CCR4 antibody, Mogamulizumab, developed using this technology is the first glycoengineered antibody approved for treatment of relapsed/refractory cutaneous T-cell lymphoma.523,524 This platform has been used to produce several glycoengineered antibodies for clinical trials (Table 4). In fucosylation, the GDP-fucose is an essential substrate for α1,6-fucosyl transferases to transfer the fucose residue. GDP mannose 4,6-dehydratase (GMD) is involved in the biosynthesis of GDP-fucose from GDP-glucose. Therefore, targeting GMD to interfere with GDP-fucose biosynthesis using GMD knock out CHO cells produced 100% nonfucosylated antibodies.525,526 In addition, introduction of heterologous GDP-6-deoxy-D-xylo-4-hexulose reductase (RMD), an enzyme that essential for biosynthesis of GDP-D-rhamnose could also inhibit GMD activity to reduce the GDP-fucose level and ultimately lower the core fucosylation.527 This technology termed as GlymaxX was developed by ProBiogen.
Table 4.
List of antibodies produced from cell line-based glycoengineering platforms.
| Technology | Company | mAb | Target | Status | ref |
|---|---|---|---|---|---|
|
Potteligent Technology FUT8−/− CHO Cells |
Mogamulizumab | CCR4 | Approved in 2018 | 523,545 | |
| Kyowa Hakko | BIW-8962 | GM2 | In clinical trials | 546 | |
| Kirin (KHK) | KHK-2823 | CD123 | In clinical trials | 547 | |
| MDX-1342 | CD19 | In clinical trials | 548 | ||
| KHK and Amgen | KHK-4083 | OXO-40 | In clinical trials | 549 | |
| KHK and MedImmune | Benralizumab | Anti-IL-5Ra | Approved in 2017 | 550 | |
| MEDI-551 | CD19 | In clinical trials | 551 | ||
| KHK and Teva | KHK-2804 | Anti-tumor specific Antigen | In clinical trials | 552 | |
| KHK and Life Science Pharma | Ecromeximab | GD3 | In clinical trials | 553 | |
|
GlycoMab Technology GnTIII overexpressed CHO cells |
Glycart-Roche | Obinutuzumab | CD-20 | Approved in 2013 | 313,554,555 |
| GA-201 | EGFR | In clinical trials | 556 | ||
|
GlycoExpress Technology Glycoengineered human cell lines |
Glycotope | Lumretuzumab | Anti-HER3 | In clinical trials | 557 |
| PankoMab | Anti-TA-MUC1 | In clinical trials | 558 | ||
| TrasGEX | Anti-HER2 | In clinical trials | 559 | ||
| CetuGEX | Anti-EGFR | In clinical trials | 560 | ||
|
CHOptimax Technology In-vitro glycoengineering |
CHO Pharma Inc. | CHO-H01 | Anti-CD20 | In clinical trials | NCT03221348 |
|
SEA Technology Fucose analogues to inhibit core fucosylation |
Seattle Genetics | SEA-CD40 | Anti-CD40 | In clinical trials | 540,542 |
The recombinant glycoproteins produced from mammalian cell lines such as CHO, BHK, or SP2/O can be heterogeneous and sometimes contain nonhuman glycans. For example, the glycoproteins from CHO cells contain nonhuman N-glycolylneuraminic acid (Neu5Gc). In addition, CHO cells produce glycoprotein with α2,3-linked instead of α2,6-linked Neu5Ac. Moreover, NS0 and SP2/0 mouse myeloma or baby hamster kidney (BHK) cells generate glycoproteins with Gal-α1,3-Gal (α-Gal epitope) that are recognized as antigenic epitopes by humans.528,529 To overcome these limitations, Glycotope developed GlycoExpress technology that offers a toolbox of glycoengineered human cell lines optimized for production of antibodies with the desired glycosylation to improve therapeutic potency.530,531 The GlycoExpress technology allows efficient control of production of glycoproteins with or without core fucose, α2,3/2,6 sialylated or nonsialylated, digalactosylated (G2), or nongalactosylated (G0) glycans. For therapeutic efficacy, glycoproteins require a homogeneous and humanized glycosylation profile which may not be achievable using commonly used expression systems due to complex glycan biosynthetic pathway. To produce homogeneous glycoproteins, Meuris and co-workers developed the GlycoDelete technique, in which the GnT-I−/− mutant of human embryonic kidney 293S cells [293SGnTI (−) cells] was produced to convert the N-glycans into hybrid and complex types.532,533
Other than mammalian expression systems, eukaryotic systems have been used for glycoengineering to produce nonfucosylated antibodies. Yeast-based expression systems gained considerable attention as an alternative to mammalian expression systems due to their high expression yields, less contamination with human viruses, and low production costs.321,534 However, the recombinant proteins produced by yeast expression systems generally cause immunogenic reactions due to presence of hypermannosylated glycans.317 Therefore, various technologies have been developed to humanize the yeast expression systems. For example, Jacobs and colleagues have successfully produced humanized glycoproteins by altering the N-glycosylation pathway of the methylotrophic yeast Pichia pastoris.535,536 In this GlycoSwitch Technology, they knocked out the α1,6-mannosyltransferase OCH1 gene involved in hypermannosylation and introduced various glycosyl transferases genes such as GnT-I, mannosidase II, and GnT-II to produce humanized glycoproteins.536,537 In another similar attempt, GlycoFi Inc. (acquired by Merck) also used P. pastoris, knocking out four genes and introducing 14 genes to produce a glycoprotein with 90% homogeneous human-like glycans.320,538 EPO produced using GlycoFi technology showed a remarkable improvement in potency and serum half-life compared to EPO produced using a wild-type expression system.539
In contrast to gene manipulation techniques, modified sugar analogues have been used as inhibitors of certain glycan biosynthesis pathway. For example, kifunensine, an α1, 2-mannosidase inhibitor that prevents the trimming of α1,2-mannose residues from high-mannose glycans before they are processed to complex types, resulted in the production of polymannosylated glycoproteins. Okeley et al. reported fucose analogues such as 2-fluorofucose and 5-alkynefucose for the production of nonfucosylated antibodies by selectively blocking the GDP-fucose biosynthetic pathway responsible for endogenous synthesis of fucosylated glycans.540 An anti-CD40 antibody, SEA-CD40, produced in CHO cells fed with fucose analogues, exhibits enhanced ADCC activity and is currently in phase 1 clinical studies.541,542 Later, 6,6,6-trifluorofucose and its 1-phosphonate analogue were reported as potent GMD inhibitors for the production of low fucose content antibodies expressed in CHO cells543 and from murine hybridoma cell lines.544
4.2.2. In Vitro Glycoengineering.
In-vitro Fc-glycoengineering of IgGs is an emerging technology to produce homogeneous antibodies with improved effector functions and for site-specific ADCs.561,562 The in vitro glycan remodeling of antibodies, using glycosidase to trim the terminal sugars or GTs to attach the glycan residues at the nonreducing end, has been employed on an intact antibody.562 This method is independent of the production cell line and, therefore, has been used for making glycovariants of therapeutic proteins in milligram to kilogram quantities with relatively minimal production effort. The glycan profile of mAbs produced by GnTIII overexpressing CHO cells has been shown to have an increase in terminal galactose and a decrease in core fucose, and as a result, a significant increase in ADCC activity was observed.313 In vitro glycoengineering has been used for the addition of bisecting GlcNAc to the degalactosyated Fc N-glycan of Rituxan and Herceptin, using recombinant rat GnTIII to obtain the modified mAbs with 31–85% GlcNAc content (Scheme 16).563 The modified mAbs with >80% bisecting GlcNAc content showed a 10-fold enhancement in ADCC activity with minimal effect on CDC. However, degalactosylation resulted in a 50% reduction in CDC compared to variably galactosylated control antibodies. In another study, scientists at Roche used GTs, such as α2,6-SiaT and β1,4-GalT, and activated sugars (UDP-Gal, CMP-Neu5Ac) to edit the terminal glycosylation of Fc glycan of an intact IgG1 against a receptor of the EGFR family, expressed in CHO cells.564 This method allows production of well-defined antibodies in adequate quantities in less amount of time than cell line engineering. Recently, Washburn et al. reported a robust and scalable enzymatic approach for in vitro sialylation of IVIG.515 The tetra-Fc-sialylated IVIG showed a 10-fold enhancement in anti-inflammatory activity in animal models. Despite significant developments, the success of this strategy relies on the IgG Fc N-glycan composition, and the efficiencies of the respective glycosidases and GTs used in the reaction sequence.
Scheme 16:
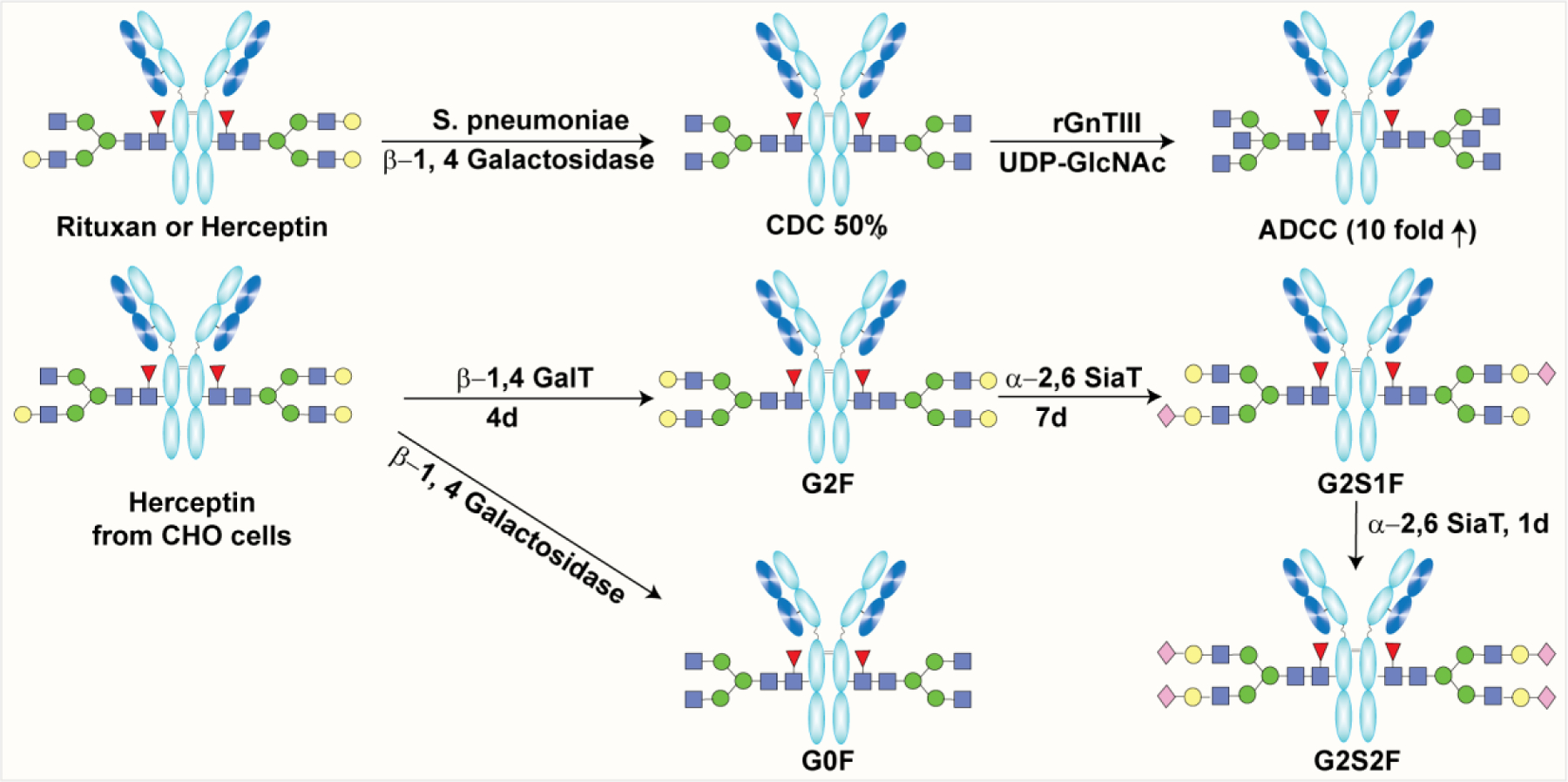
Methods for in-vitro glycoengineering. a) Preparation of IgGs with bisecting GlcNAc G0F glycan; b) Preparation of different glycovariants of Herceptin on bulk scale by roche.
Another highly convergent two-step in vitro glycoengineering strategy is based on endoglycosidase-mediated removal of the highly heterogeneous glycan mixture at the desired glycosylation site, leaving behind the core GlcNAc on protein backbone. The predefined N-glycan is then transferred en bloc to GlcNAc catalyzed by glycosynthase to produce a homogeneous glycoprotein.297,565 The field picked up momentum when the Wang group reported two novel mutants of endoglycosidase S (EndoS) from Streptococcus pyogenes, namely, EndoS D233A, and D233Q with better transglycosylation activity and lower hydrolyzing activity, for glycosylation of GlcNAc- or core-fucosylated GlcNAc-containing antibodies.297 The discovery of these glycosynthase mutants led to the preparation of antibodies with desired glycoforms for functional studies.322,566–568 Our group used this platform for glycoengineering the anticancer antibodies, Rituxan and Herceptin, to identify the α2,6-sialylated biantennary complex type glycan as the optimized glycoform for enhancement of ADCC and CDC.566 Kurogochi et al. used Herceptin, having nonfucosylated pauci-mannose, high-mannose, and complex type glycans produced in transgenic silkworm cocoons. Glycoengineering using ENG’ases and their mutants (such as EndoSD233Q) provided a library of glycovariants of Herceptin to study the effect of Fc glycosylation on antibody effector functions.567 The Davis group used in vitro glycoremodeling of Herceptin to incorporate modified Neu5Ac with a chemical reporter in the Fc glycan for fluorescent imaging and ADC development.568
The potential of EndoS and its glycosynthase mutants for protein engineering is limited by their strict substrate specificity typically toward biantennary complex type glycans.569,570 Later, EndoS2, a glycosidase from the same family of EndoS, was reported to show much broader specificity toward high mannose, hybrid, and complex type glycans.571 The systematic site-directed mutagenesis studies on EndoS2 by the Wang group identified residue D184, which is homologous to the EndoS D233, to prepare a series of mutants with excellent transglycosylation and diminished hydrolytic activity.298 Wong and co-workers identified several novel sites on EndoS2, including T138, D182, D226, T227, etc., to prepare the mutants that can transglycosylate all high mannose, hybrid, bi-, and triantennary complex type glycans (Scheme 17).299 Other novel glycosynthase mutants (D165A and D165Q) of EndoF3 from the bacterial GH18 family were capable of transferring triantennary complex type glycans onto core-fucosylated IgG.301 Apart from sugar oxazolines, sialyglycopeptide has been used as a substrate for transglycosylation to prepare homogeneous IgG glycoform.572
Scheme 17:
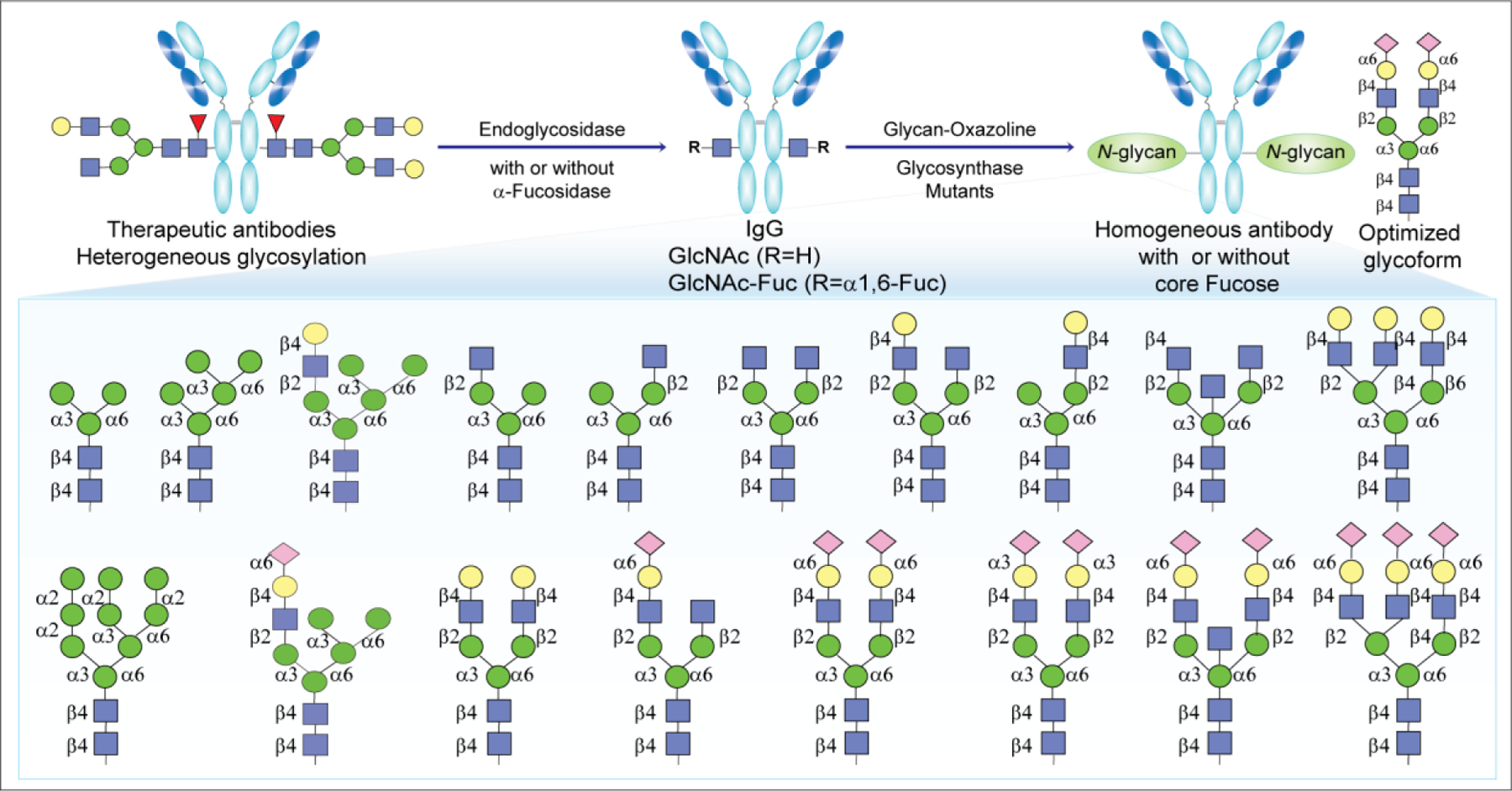
Endoglycosidase mediated in-vitro glycoengineering. Structures of various N-glycans used for SAR studies to identify α 2,6-sialylated biantennary complex type as an optimized glycan for effector functions.
In addition to glycosylation in the Fc region, some antibodies are also glycosylated in the Fab region, which may be critical for the recognition of target and the half-life of antibodies. Nearly 20% of IVIG are N-glycosylated in the Fab region. Recently, an interesting study published by Wang and co-workers used a chemoenzymatic approach to manipulate Fc and Fab glycosylation of the therapeutic anti-EGFR antibody, Cetuximab.565 Using three different endoglycosidases (Endo-S, Endo-S2, and Endo-F3), their glycosynthase mutants and α1,6-fucosidase, the authors remodeled immunogenic N-glycans to sialylated complex type N-glycans and fully nonfucosylated galactosylated N-glycan at the Fc domain. The modified Cetuximab showed better affinity for the FcγIIIa receptor and significantly improved ADCC.565
Success of the in vitro glycoengineering platforms led to the identification of clinical candidates for cancer immunotherapy (Table 4).308,573 The first glyco-engineered Rituxan (CHO-H01) developed from CHOptimax technology by the Taiwanese Biotech company, CHO Pharma Inc., is in phase 2a clinical trials in Taiwan for the treatment of refractory or relapsed follicular lymphoma (ClinicalTrials.gov Identifier: NCT03221348). Moreover, GlycoT Therapeutics, a University of Maryland spin-off, is developing chemo-enzymatic glyco-engineering technologies to produce therapeutic antibodies or glycoproteins with well-defined glycoforms with unique properties.
4.3. Glycan-Based Adjuvants
Advancement in vaccine development technologies provided insights into their immunological mechanism for prevention of infectious diseases and in cancer immunotherapy. Vaccines contain both molecularly defined antigen and immune activators, called adjuvants, to induce antigen-specific immune response. Compared to traditional whole-pathogen vaccines, the synthetic subunit vaccines are precisely designed for their efficacy and safety. However, the subunit vaccines are less immunogenic and therefore required adjuvants to enhance antigen-specific B and T cell responses.191 Alum has been the most widely used adjuvant used in approved vaccines.574,575 Cervarix, a combination of alum with the TLR-4 ligand MPLA, was approved for use in a vaccine against human papillomavirus (HPV) infections576,577 Vaccines adjuvanted with aluminum are not very immunogenic and only elicit antibody-mediated Th2 immune responses. MF59 is a potent oil-in-water emulsion used in anti-influenza vaccine with an excellent safety profile. MF59 is composed of squalene mixed with surfactants Tween 80 and Span 85 to significantly enhance the potency and breadth against the viral strains that are not included in the vaccine.578,579 Despite its potency, MF59 may not be suitable for every vaccine because of its poor induction of Th1 responses. Nevertheless, another Th1 immunoactivator, CpG, has been added to the MF59 to boost the immune response and the Th2 to Th1 shift.580,581 The adjuvant system, AS01, is a liposome-based combination of 3-O-desacyl-4′-monophosphoryl lipid A (MPL) and the saponin QS-21. AS01 has been used in the vaccine formulations targeting malaria and herpes zoster, for enhancing antigen-specific immune responses.576,582
4.3.1. Monophosphoryl Lipid A (MPLA).
Because of their excellent biocompatibility and great safety profile, carbohydrate-containing adjuvants have been preclinically and clinically evaluated in human vaccines.583 Lipid A is the most bioactive component of LPS from Gram-negative bacteria, and it is mainly responsible for triggering a strong immune response through interactions specifically with TLRs.197 The discoveries of different TLRs and their role as signaling receptors for lipid A facilitated the thorough investigation of TLR recognition that led to a better understanding of immune response.584,585 The number, structure, and location of fatty acid chains and the degree of phosphorylation determine the immunological and endotoxic activity of lipid A.586
In the 1970, Edgar Ribi removed the 1-O phosphate group of lipid A disaccharide from Samonella minnesota R595 through selective hydrolysis to prepare MPLA, a mixture of hexa-acylated diglucosamines, without polysaccharide side chains, and one phosphoryl group (Scheme 18).587 Compared to parent LPS, MPLA was at most 0.1% toxic, which had no impact on its immunostimulatory activity.587–589 Ribi Immunochemicals, a commercial supplier of the MPLA adjuvant system (a formulation of MPLA, trehalose, and oil), was acquired by Corixa and then by GSK Biologicals. Later, Myers et al. reported that removal of the 3-O-position fatty acid chain further reduced the pyrogenic properties without substantially affecting the adjuvant properties.590,591 The resulting MPL, which is isolated and structurally derivatized from the LPS of S. minnesota R595, was shown to be a safe and potent adjuvant.592 Currently, MPLA is actively used by GSK Biologicals in several vaccine formulations, such as AS01, AS02, and AS04. The AS04 adjuvant system has been used in clinically approved vaccines such as FENDrix against hepatitis B virus and Cervarix against HPV.576,577 Combinations of MPL adjuvant and QS21 (AS15, AS02, and AS01) have been developed to generate effective immune response against infectious diseases and cancers.582,593
Scheme 18:
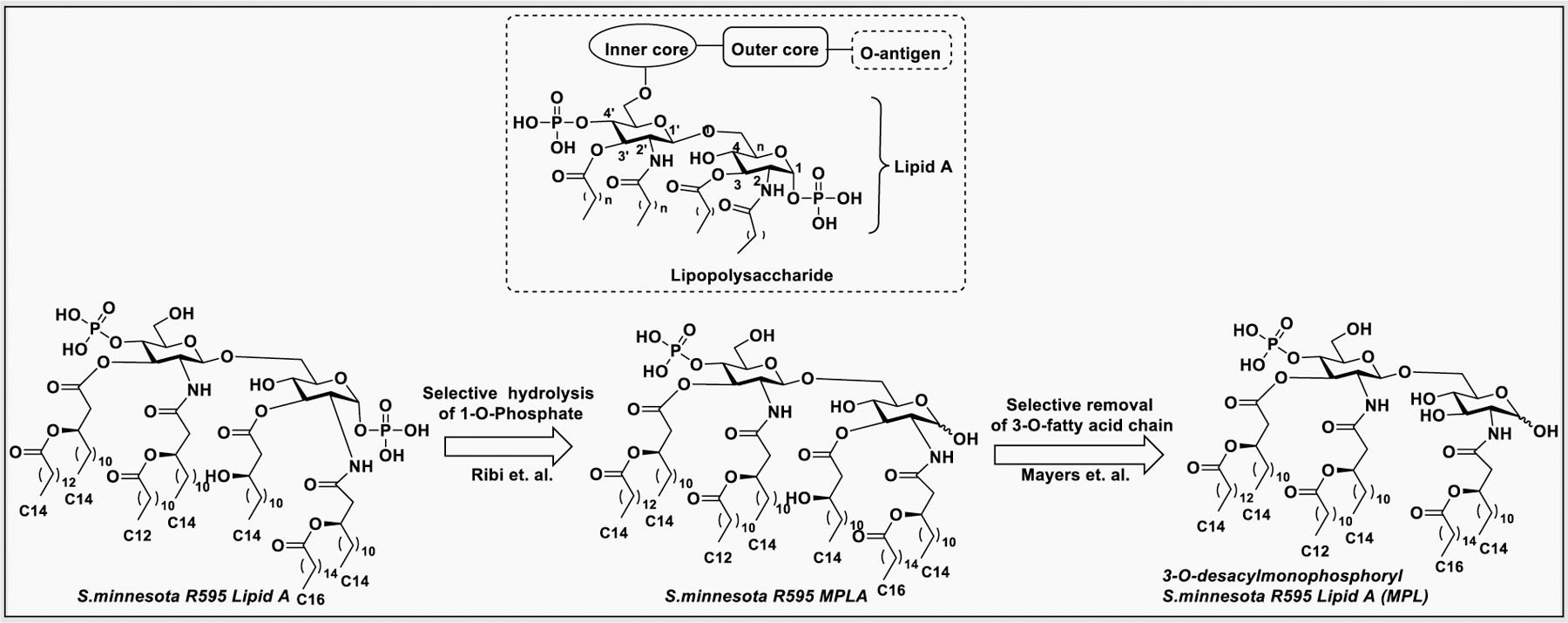
General structure of LPS. Structure of natural lipid A from Salmonella minnesota. Structures of chemically modified monophosphoryl lipid and 3-O-desacylmonophosphoryl from S. minnesota R595 Lipid A.
The majority of studies have demonstrated that the adjuvant activity of MPL is its ability to activate antigen presenting cells, such as DCs and macrophages, leading to induction of cytokines.594,595 Upon activation, these cells phagocyte, process, and present the vaccine antigen to T lymphocytes. In addition, MPL either directly or indirectly stimulates the induction of Th1.596 This capacity of MPL to stimulate the cytokine cascades necessary for the induction of cellular immunity make it an effective adjuvant by itself.585
Despite their clinical success, the MPLAs isolated from bacteria are highly heterogeneous in their structural integrity due to incomplete and nonspecific hydrolysis, which affect their biological activity. Extensive SAR studies with modifications to the carbohydrate part, phosphorylation, and the number of fatty acid chains of various lengths provided insights into their structure, toxicity, and adjuvant activities.597–599 In 1999, Johnson et al. modified the side chain lipids of MPL with different lengths of fatty acid chains to show that the chain length of fatty acid is the main determinant for adjuvant activity in human peripheral monocytes (Figure 33a).600 Later, Jiang et al. reported the study of modifying the reducing end sugar of MPLA with 3-O-substituted analogues and showed that there was no effect on the adjuvant activity as compared to natural lipid A (Figure 33b).601
Figure 33:
a) MPL derivatives with various length fatty acid side chains. b) MPLA derivatives bearing varied 3-O-substitution.
Corsaro et al. reported novel analogues of E. coli MPLA, including those with introduction of methyl phosphate, modification of 6-OH to aldehyde or substituted amides, and alteration of lipid pattern in addition to 6-O modifications (Figure 34).602 Preliminary in vitro immunostimulatory evaluation suggests that some of these novel derivatives, particularly those with modification at the 6-O position, induce stronger TNF-α secretion than MPL.
Figure 34:
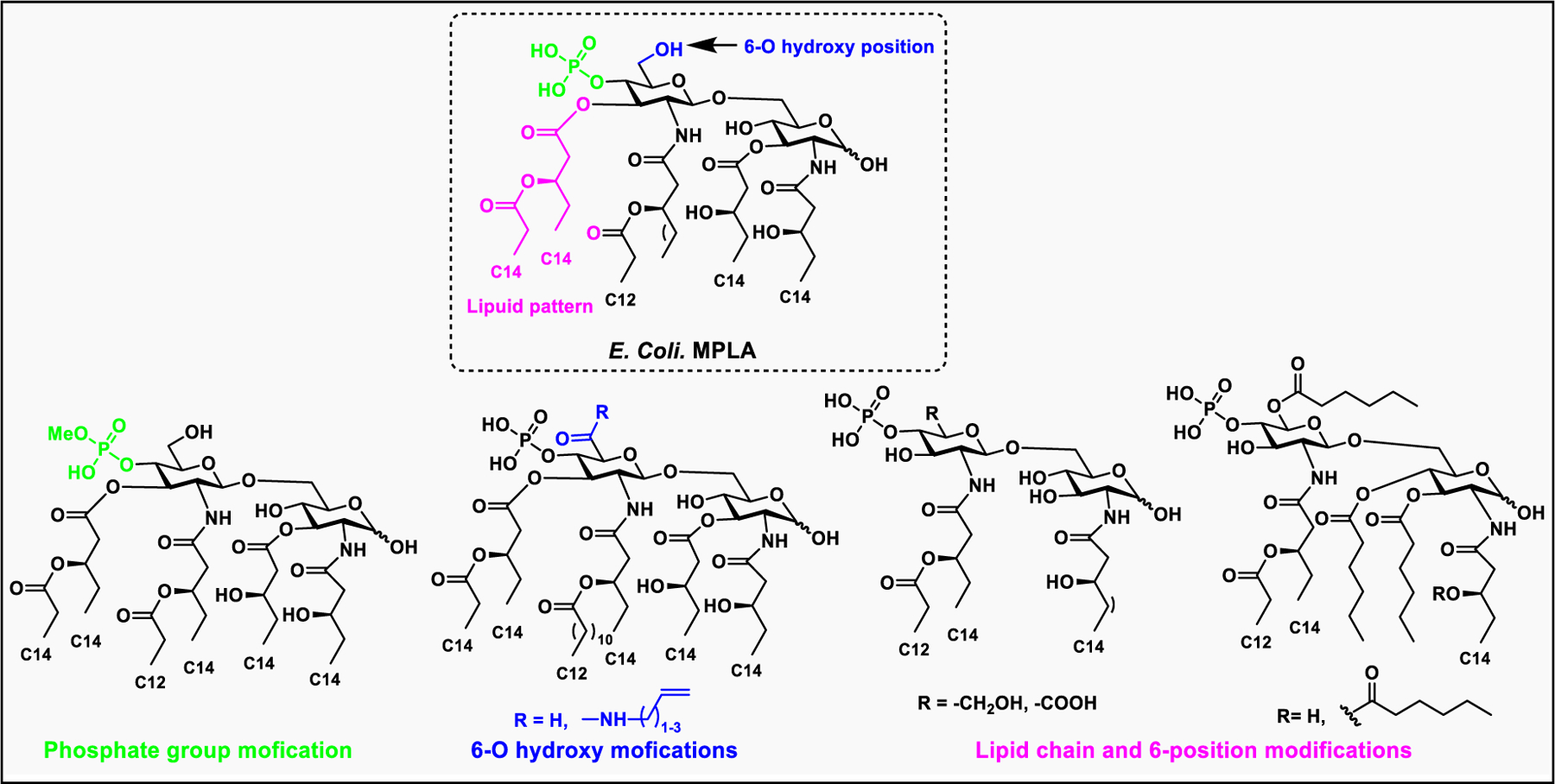
Structure of E. coli MPLA and its semisynthetic modifications in lipid pattern, phosphate group, and at the 6-O hydroxy position.
Because of its strong immunostimulatory activities and nontoxic nature, MPLA has been utilized as a built-in adjuvant in combination with synthetic vaccines against cancers and HIV-1 in preclinical studies.191 In this context, MPLA has been utilized as carrier and a built-in adjuvant for synthetic vaccines against cancers and HIV-1 in preclinical studies. The covalent attachment of MPLA to various TACAs, including GM3, GM3 derivative (GM3NPhAc), STnNPhAc, Globo H, and α2,9-sialylated di-, tri-, tetra-, and penta-sialic acid showed more robust immune response than conjugation with protein carriers.221,371,443,603,604
4.3.2. Saponin-Based Adjuvant QS-21.
QS21 is a purified plant extract derived from the soapbark tree Quillaja saponaria Molina.605 Structurally, QS-21 consists of four domains: a central quillaic acid triterpene backbone (black), a branched trisaccharide domain (blue) that is linked to the triterpene through glucuronic acid, a tetra saccharide domain (pink), and an acyl chain domain (red) which is linked to the triterpene through a hydrolytic labile ester linkage (Figure 35). QS-21 is a 65:35% mixture of two isomers, namely QS-21-apiose and QS-21-xylose, based on the structure of terminal sugar of the linear tetrasaccharide.606
Figure 35:
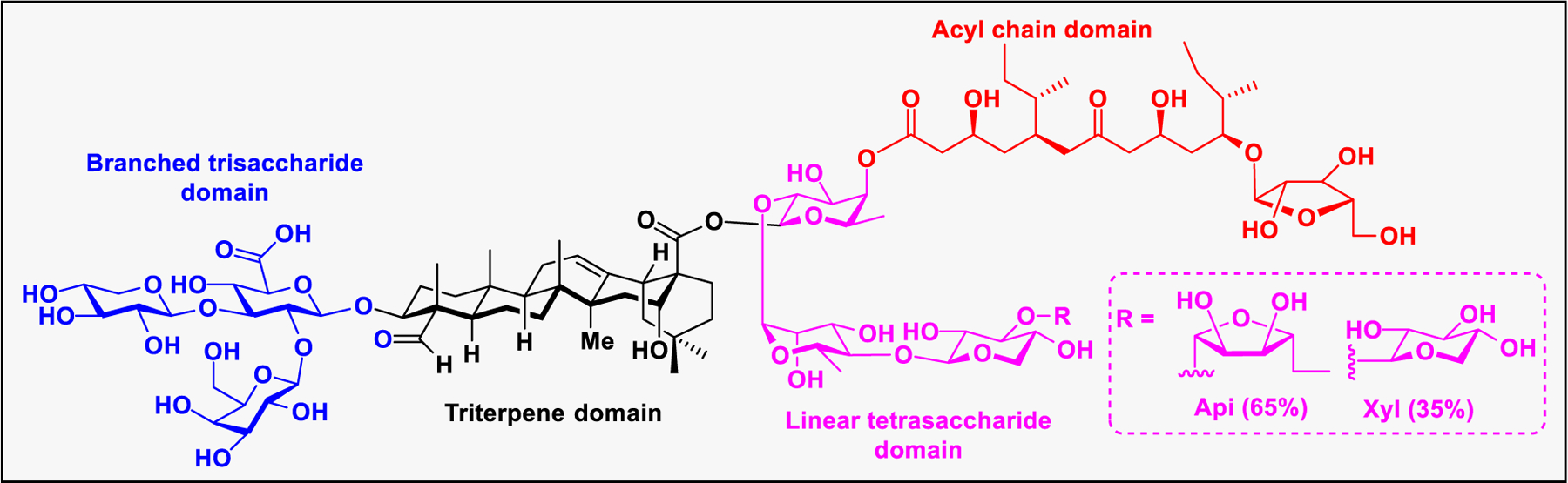
Structures of QS-21 and its four domains.
QS-21 stimulates both Th1 and Th2 immune responses and activate the production of antigen-specific cytotoxic CD8+ T-cells.607,608 The immune stimulatory activities of QS-21 led to the development of its use as adjuvant, either alone or in combination (AS01, AS02) in numerous clinical studies of vaccines against different cancers 607 and infectious diseases.580,609 Recently, QS-21 containing the AS01 adjuvant has been approved in the vaccines against malaria (Mosquirix)610 and shingles (Shingrix)611 from GSK.
Despite promising adjuvant activity and extensive clinical studies, the use of QS-21 alone as an adjuvant is limited by inherent issues, such as availability, toxicity, and the spontaneous hydrolysis of the acyl chain ester linkage.612 In addition, the mechanism behind the mode of action of QS-21 is poorly understood, which further hinders the rational design of potent adjuvant. Therefore, to address the limitations and to identify the functional groups that are crucial for its immunostimulatory properties, diverse semisynthetic QS-21 analogues were synthesized to establish the structure–activity relationships (SAR).598,607,613 One of the strategies to modulate QS-21 efficacy is through the chemical derivatization of naturally derived saponin. For example, Kensil et al. prepared QS-21 derivatives through modifications at the carboxylic group on the glucuronic acid and the aldehyde on the triterpene.614 Immunogenicity evaluation of the antigen, and ovalbumin (OVA) in C57BLl6 mice in the presence of QS-21 derivatives suggests that the modifications at the −COOH retained adjuvant activity, however, the modifications at the aldehyde did not show adjuvant activity for antibody production, suggesting that aldehyde functionality is involved in immune stimulation.614 In a separate study, Marciani et al. reported the synthesis of the saponin adjuvant, GPI-0100 from Quillaia saponaria bark extract, by hydrolysis of the acyl chain followed by conjugation of dodecyl amine to the carboxylic acid of glucuronic acid via amide bond formation (Scheme 19a). The deacylated QS-21 induced a Th2 response but not a Th1 or cytotoxic T lymphocyte response, whereas the amidated variant, GPI-0100, restored the Th1 immunity and induced CD8+ T-cell responses.615 The Michalek group reported the chemical synthesis of structurally defined QS-21616 and QS-17/18617-based adjuvant candidates. Among the QS-21-based analogues, the xylose substituted derivatives showed effective adjuvant activity and maintain a robust antibody response.616 The synthetic QS-17/18 analogue IV showed an adjuvant activity similar to GPI-0100, with enhanced Th-1/Th-2 immune responses (Scheme 19b).617 The same group recently synthesized two analogues of QS-7, differing in the acetylation at the 3- and 4-O positions of the quillaic acid C28 fucose residue. The acetylated analogues potentiated a mixed Th1/Th2 antigen-specific immune response, whereas nonacetylated analogues only induced a Th2-biased immunity, suggesting that 3- and/or 4-O acetyl groups of the fucose are crucial for the adjuvant activity of the QS-7 analogues.618
Scheme 19:
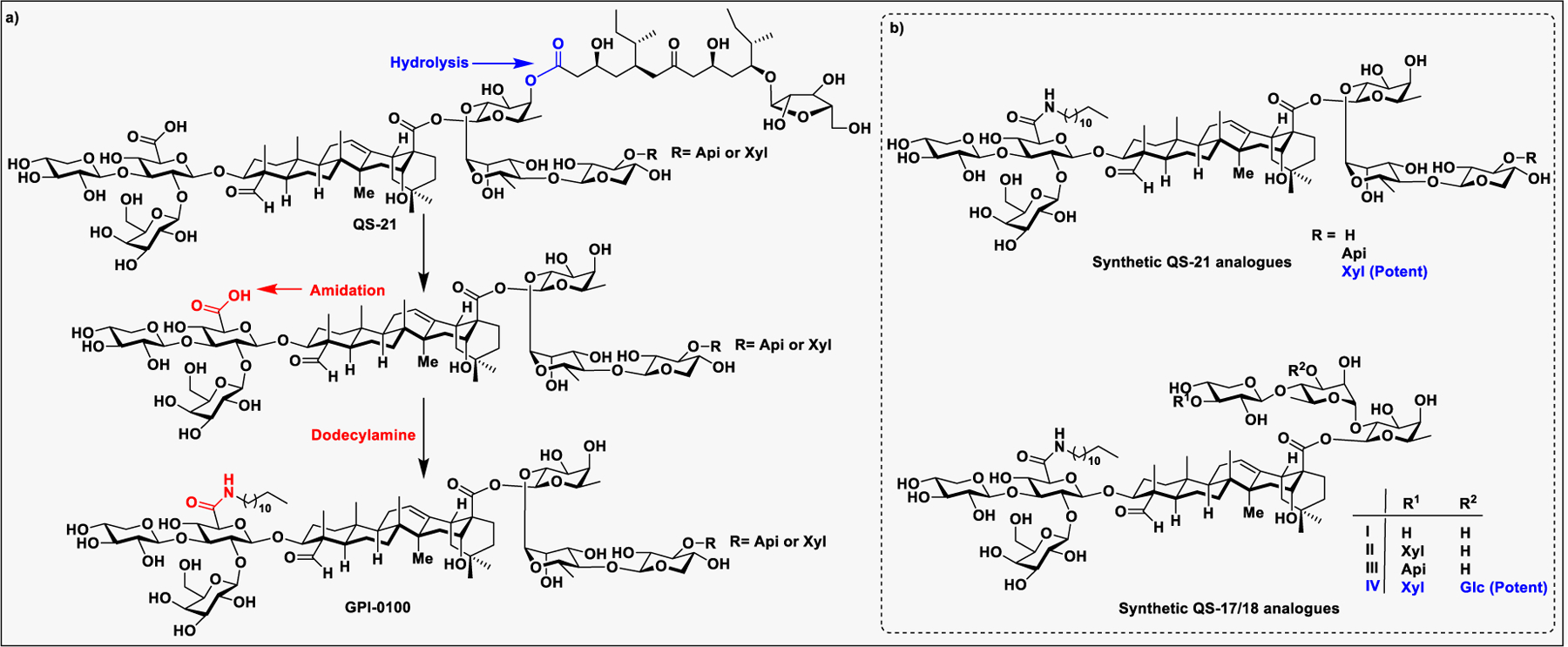
a) Synthesis of GPI-0100 from deacylated Quillaja saponins. b) Synthetic analogues of QS-21 and QS-17/18.
Gin and co-workers pioneered the development of novel glycosylation strategies for convergent synthesis of homogeneous and pure samples of saponin isomers including QS-21Api,619,620 QS-21Xyl,621 and QS-7Api.622 Gin’s group also reported the design, synthesis, and adjuvant evaluation of QS-21 with stable amide bearing acyl chains.623 These novel, non-natural analogues exhibited no impairment in adjuvant activity but differ in toxicity profile (Table 5). The simplified acyl chain analogue [SQS-0102] showed unacceptable toxicity, while the [SQS-0103] was found to be safe but lost aqueous solubility.623 The carboxyacyl variant [SQS-0–0-4–5] showed improved water solubility and excellent adjuvant properties when combined with anti-MUC1 and anti-KLH. In addition, the low toxicity of this variant made it into a promising lead for further modification.613 Accordingly, truncations in the linear tetrasaccharide domain of [SQS-0–0-4–5] provided respective tri-, di-, and monosaccharide variants. Immunoadjuvant activity evaluation revealed that the trisaccharide derivative [SQS-0–0-5–5] is equipotent to QS-21, whereas the di- [SQS-0–0-6–5] and monosaccharides variants [SQS-0–0-9–5] resulted in higher toxicity and reduced potency. These results demonstrated that the end sugar of linear tetrasaccharide is tolerable for modification.613 Later, to evaluate the role of the central glycosyl linkage in adjuvant activity, analogues with variable linker lengths, and stereochemistry were prepared and evaluated. These derivatives showed conformational dependent in vivo adjuvant activity.624 Next, saponin variants which contain iodobenzoic acid612 and aldehyde tucaresol625 on the acyl chain, with or without a branched trisaccharide domain were prepared to understand the effect of branched trisaccharide in the adjuvant mechanism. The new analogues were safe, highly potent, and induced both Th1 and Th2 responses, suggesting that the entire branched trisaccharide may be dispensable for adjuvant activity. The aldehyde on the triterpene was proposed to be important for Th1 immunity, however, the Gin group showed that the adjuvant activity was affected by the C4-aldehyde when measured with IgG responses in mice.612 The extensive SAR established on QS-21 and its semisynthetic analogues by the Gin group that led to the discovery of QS variants with enhanced adjuvant properties were described recently.609,613
Table 5.
Structural modifications to the QS-21 by the Gin group.
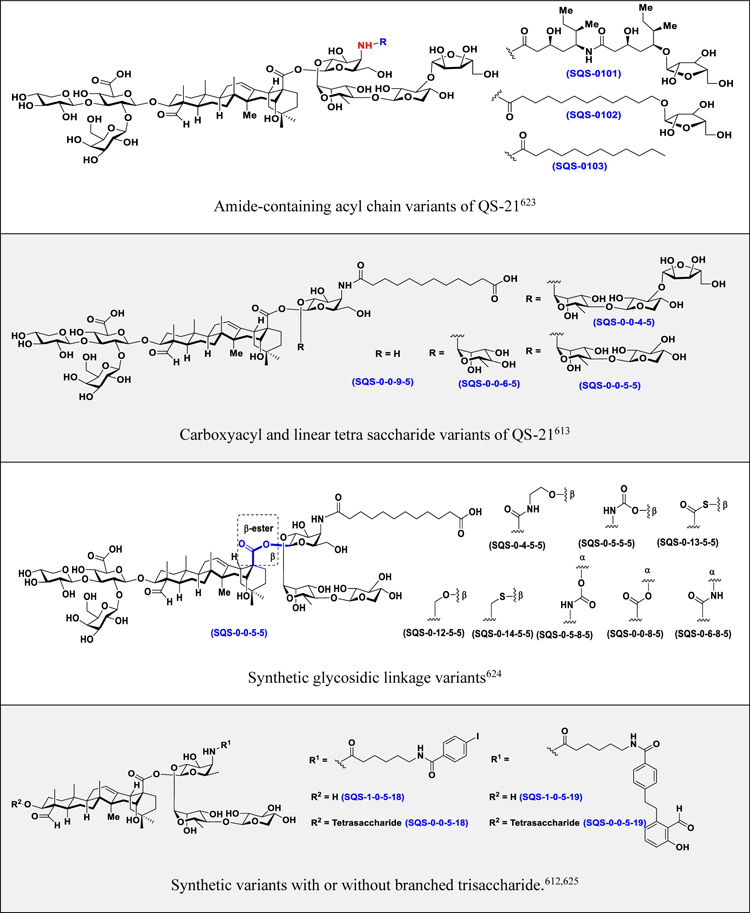
|
4.3.3. α-Galactosyl Ceramide-Derived Adjuvants.
Invariant natural killer T (iNKT) cells are a type of cytotoxic T cells that are activated to secrete Th1 and Th2 cytokines upon interaction of the T cell receptors with a glycolipid presented by CD1d on dendritic cells. Activation of NKT cells triggers regulation of immune responses against pathogens, autoantigens, and cancers.626,627 NKT cells are classified into invariant NKT cells (iNKT) and type II NKT cells based on phenotype and the types of secreted cytokines.628 Both subtypes get activated upon binding to the glycolipid antigen.629,630 Many glycolipids from bacterial or human origins have been identified as activators of NKT cells.631 KRN7000, a synthetic analogue of α-galactosyl ceramide (α-GalCer) isolated from the marine sponge Agelas mauritianus, is a potent antitumor agent in a variety of experimental and spontaneous tumor metastasis models.630,632 KRN7000 was originally developed by Kirin as a potent stimulator of NKT cells.633 It consists of a galactose head which is linked to ceramide through an α-O-glycosidic linkage. The crystal structure of Cd1d–glycolipid complex suggested that the fatty acid chain occupied the CD1d binding groove, while the sugar head was exposed to the surface for interaction with receptors on iNKT cells (Figure 36).634–636 The formation of CD1d–glycolipid complex activates iNKT cells that led to a massive secretion of IL-4 and IFN-γ cytokines with increased cytotoxic activity (Figure 36).637,638 In addition to activating other cell types such as T-, B-, NK-, and DCs, the secreted cytokines would also induce the Th1 response with antitumor, antiviral, and adjuvant activities or the Th2 response, which is correlated with autoimmune diseases.639,640,649
Figure 36:
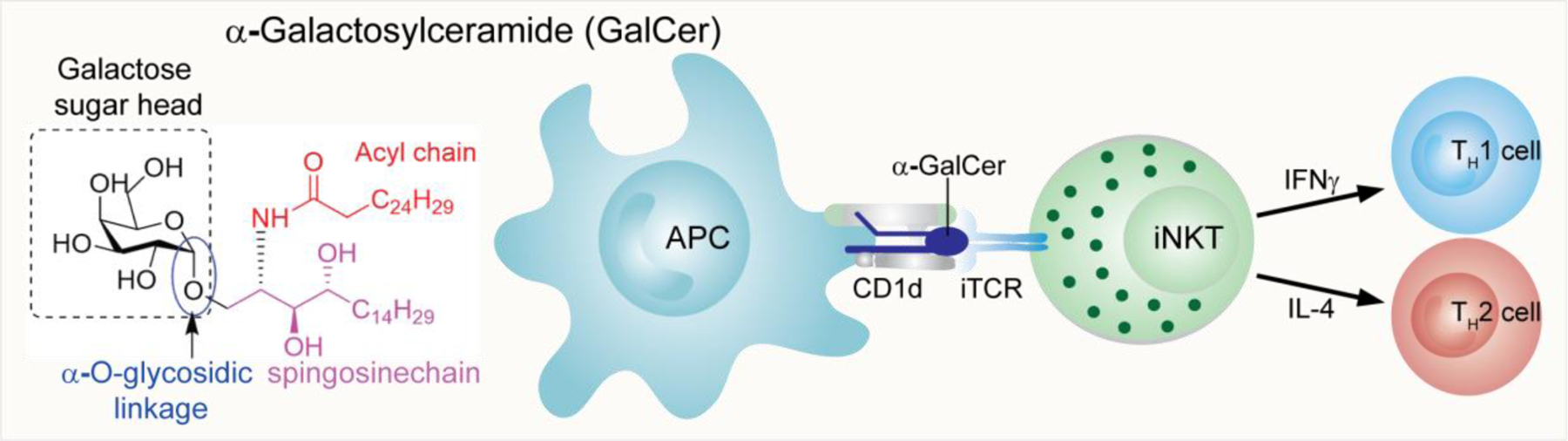
Structure of α-GalCer, a synthetic analogue of a glycolipid isolated from the marine sponge Agelas mauritianus. b) Mechanism of NKT cell activation.
The structure of α-GalCer-CD1d binary complex revealed the hydrophobic interactions between a region residing the A′ and F′ pockets of Cd1d and the lipid chain of α-GalCer.634,636 Therefore, changes in the lipid chain length was thought to affect the affinity of TCR toward the glycolipid–CD1d complex and ultimately, iNKT cell activation.641 Miyamoto et al. reported an α-GalCer analogue with a shorter Phyto sphingosine chain, known as OCH, that stimulates iNKT cells to secrete higher amounts of IL-4 than IFN-γ.642,643 Modifications in the acyl chain resulted in the discovery of 7DW8–5, a phenyl glycolipid having a fluorophenyl substituted C10 fatty acid chain,644 which exhibited potent adjuvant activity in vaccines against HIV, malaria, and influenza vaccines and induced a robust CD8+ T-cell response in nonhuman primates (Figure 37).644–646 In addition, several 4-(4-fluorophenoxy)phenyl undecanoyl analogues of α-GalCer with different sugar heads (Gal and Glc) were prepared647,648 and shown to stimulate both murine and human iNKT cells and provide antipathogen protection as well as potent adjuvant effect when combined with Globo-H based anticancer vaccines.454,649
Figure 37:
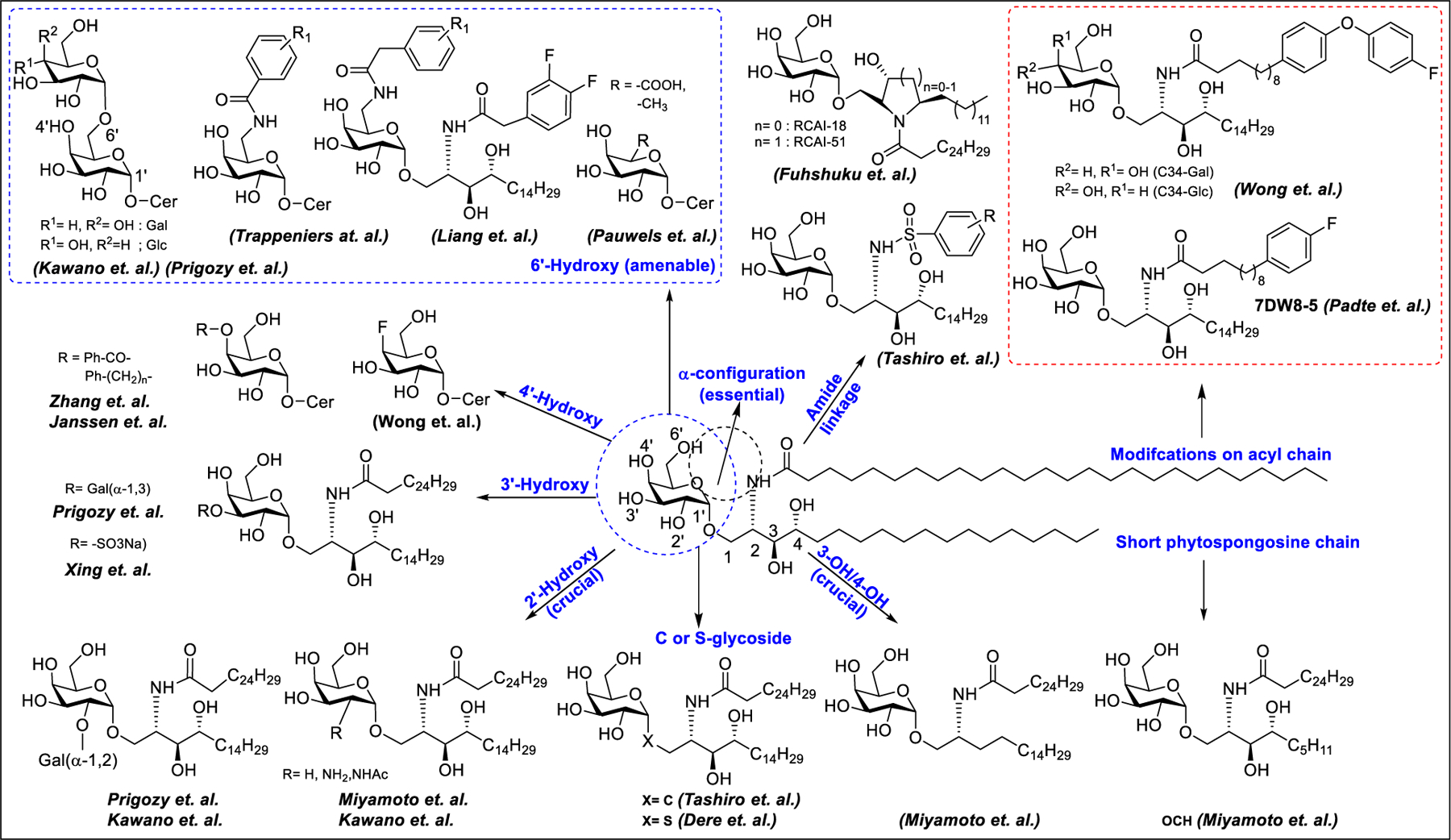
Structural modifications of α-GalCer for SAR studies
Modifications on the sugar head of α-GalCer revealed that the α-anomeric conformation of the galactose moiety is essential for adjuvant activity as activation with both α-GalCer and α-GlcCer resulted in stimulation of NTK cells, but β-GalCer showed no proliferative responses.630 The α-anomeric configuration of the inner sugar is also found to be important in disaccharide sugar heads. For example, α-linked ceramides of Gal-α1,6Gal, Gal-α1,6Glc, Gal-α1,3Gal, and Gal-α1,2Gal could stimulate NKT cells when the inner sugar is either Glc or Gal despite the configuration of the outer sugar moiety, whereas Gal-β1,4Gal-β-Cer could not.630,650 There was no difference in the stimulatory activity of α-GalCer and α-GlcCer, indicating that the 4-OH configuration of sugar appears not to be essential; however, α-ManCer showed no stimulatory activity, suggesting the importance of the 2’-OH of inner sugar, probably for the TCR contact site of the glycolipid.626,630 The necessity of the 2′-OH position was further confirmed by the modifications such as H, azido, amino, NHAc, methoxy, etc., that led to reduction in adjuvant activity.598,630,642 Because the 4′-OH is not as sensitive to substitution as the 2′-OH position, Zhang et al. reported novel aromatic 4′-O substituted analogues that induced comparable Th1/Th2 responses,651 while benzyl-modified 4-O variants by Janssen et al. promoted Th1-biased immunity (Figure 37).652 Sulfation at 3′-O positions have also been reported to stimulate Th1 immune responses.653
According to the crystal structure of TCR/α-GalCer/CD1d ternary complex, the 6′-O position of Gal is pointed toward solvent and therefore not affecting the binding between the other complexes626 Accordingly, modifications such as α1,6-Gal, α1,6-Glc,630 and a small fluorophore654 at the 6′-O position of Gal retained the activity to stimulate NKT cells. The carboxylic acid and methyl substitution at the 6-O-position of Gal resulted in induction of both Th1 and Th2 responses.655 In addition, the 6′-modified substituted phenyl amide analogues with an intact phytoceramide tail made by Trappeniers et al. activated the secretion of Th1 cytokines and showed potent immunogenicity.656 In another related study, Liang et al. reported the study of analogues where substituted phenyl acetamide was introduced at 6-position of Gal that resulted in higher IFN-γ/IL-4 secretion than α-GalCer in vitro (Figure 37).657 Recently, addition of an acyl chain at the 6′-position of Gal resulted in stimulation of iNKT cells to induce a Th2 response. Interestingly, α-GalCer-6′-(1-naphthyl) urea (NU-α-GalCer) and α-GalCer-6′-(pyridin-4-yl) carbamate analogues with modification at the 6′-O position of Gal elicited Th1 response and reduced lung metastasis in the B16 melanoma model.658,659
The C-glycoside is an analogue of α-GalCer where the glycosidic oxygen is replaced with the methylene group to show enhanced Th1 responses.660 Thioglycosides661 showed no adjuvant activity in mice but induced Th1 responses in humans.662 Removal of the 3-OH and 4-OH on the Phyto sphingosine polar portion produced nonstimulatory analogues, revealing that both hydroxy groups are important for NKT cell activation.642 Moreover, modification at the 4-OH, such as a 4-deoxy-4,4-difluoro analogue, induced CD1d-dependent TCR activation of NKT cells.663 X-ray crystallographic studies revealed that the amide NH of phytosphingosine chain interacts with the α2 loop of mouse Cd 1d through hydrogen bond.664 The α-GalCer derivatives with inverted NH stereochemistry showed a reduced stimulation of mouse iNKT but not human iNKT.665 Replacement of the amide group with a cyclic ring system such as azetidine (RCAI-18) or pyrrolidine (RCAI-51) resulted in the induction of a slightly lower level of cytokines for RCAI-18, whereas RCAI-51 was not active to stimulate murine iNKT cells.666 Other modifications, such as replacement of the amide with an ester and methyl substitution at the amide nitrogen of α-GalCer, reduced cytokine secretion by iNKT cells.598,641
4.4. Glycan-Mediated Targeted Delivery of Oligonucleotide Therapeutics
With 14 approved products in the markets and over 100 in the clinical pipeline, oligonucleotides are emerging as a class of therapeutics with extraordinary potential for treating a wide range of rare diseases.667,668 Oligonucleotide drugs are designed based on Watson–Crick base pairing and the sequence of the RNA associated with the disease. Representative oligonucleotide-based therapeutics include small interfering RNA (siRNA) that targets and degrades disease-causing mRNA through RNA-induced silencing complex (RISC) mediated RNA interference and antisense oligonucleotide (ASO) that binds complementary mRNA and induces sequence-specific cleavage of the RNA by endonuclease RNase H. Approval of several oligonucleotide-based drugs generated tremendous involvement by major pharmaceuticals as well academic laboratories; however, wide acceptance of oligonucleotides as a drug is limited by several properties such as large molecular weight and negatively charged phosphate backbone that restrict free uptakes of siRNA drugs by cells in the absence of any delivery agent. In addition, naked siRNAs are instantly cleaved by RNases, rapidly eliminated from circulation by kidneys and absorbed by liver, contributed to the poor drug-like properties of siRNAs..669 Although chemical modifications to the internucleotide linkages and the ribose sugar were introduced to improve serum stability, protein binding, and potency, and to lower immunogenicity, these modifications were still not enough to pass the lipid bilayer.670 Lipid nanoparticles have been used extensively for delivery of siRNA drugs to limit enzymatic degradation and to enhance endosomal escape.668
ASGPR was discovered by Gilbert Ashwell and Anatol Morell in 1965. They demonstrated that asialylated ceruloplasmin was rapidly cleared from the circulation and fully recovered in liver within 5–10 min, however degalactosylation of ceruloplasmin reduced the clearance, implicated the important of terminal galactose residues for recognition by ASGPR.671–673 The structure of carbohydrate ligand (GalNAc > Gal), ligand valency (4 = 3 > 2 > 1), and the spatial ligand arrangement play important roles in binding and, ultimately, clearance from the circulatory system.674,675
Building on decades of work on ASGPR mediated delivery, scientists at Alnylam Pharmaceuticals conjugated a trimer of GalNAc to siRNA and showed that the conjugates had drastically improved RNAi activity in liver hepatocytes in vivo.676,677 Multivalent GalNAc moieties avidly bind to the ASGPR, therefore, the GalNAc–oligonucleotide conjugates were used for targeted liver delivery. Hepatocytes contains ∼500 000 copies of ASGPR at surface of which 5–10% are present at a time during the recycling process.678 Cell surface ASGPR interacts with the GalNAc-siRNA in the presence of Ca2+ at pH > 6 in clathrin-coated pits on the plasma membrane, which then internalize the ligands via clathrin dependent receptor-mediated endocytosis.679 Following complete internalization, acidification during endosomal maturation dissociates the GalNAc-siRNA from the ASGPR, which then recycles back to the plasma membrane of the hepatocyte. At last, glycosidases in the endosome cleaves the tri-GalNAc ligands from siRNA and degrades the linker part within 4 h (Figure 38).680
Figure 38:
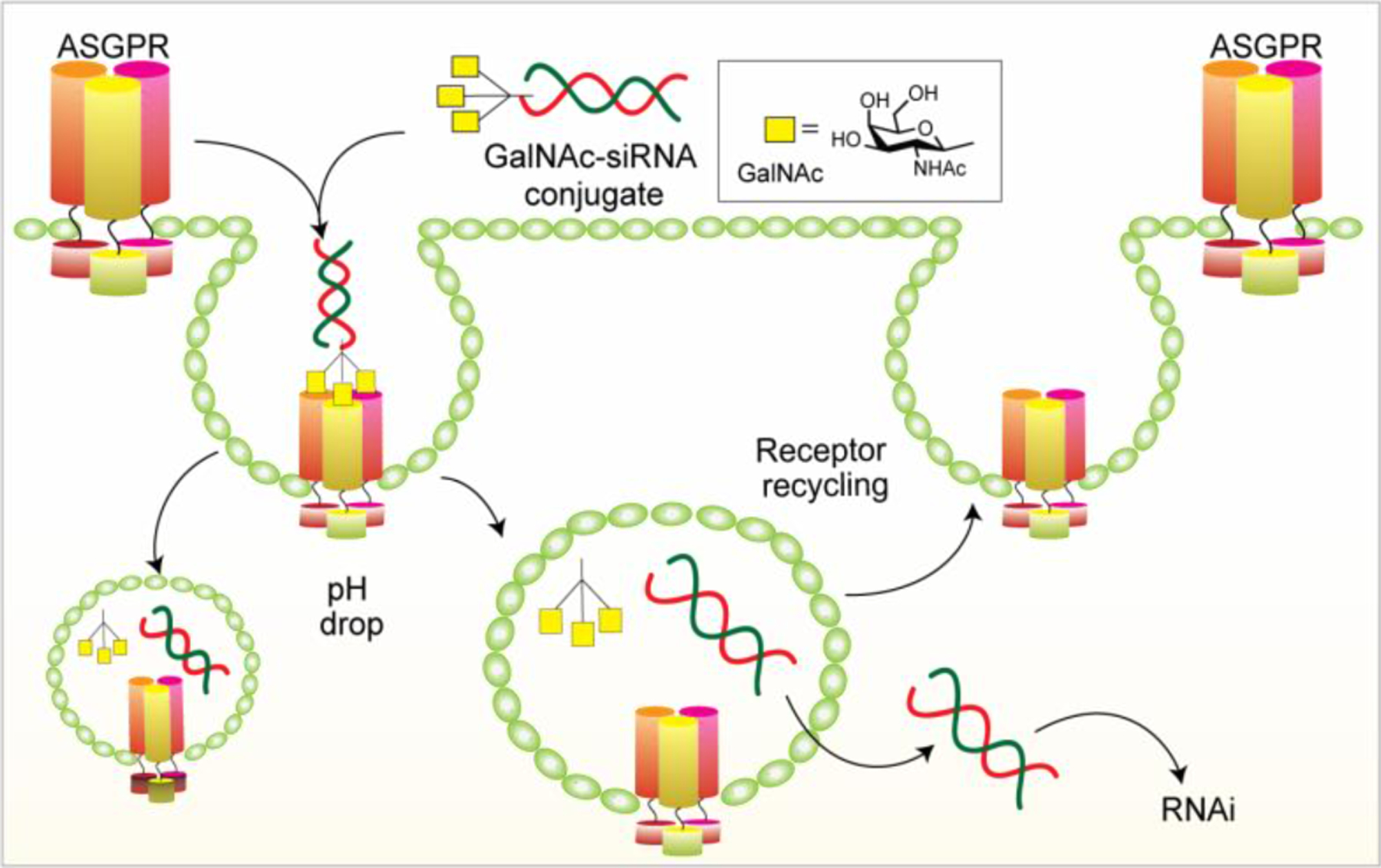
Targeting the ASGPR for delivery of GalNAc-siRNA to hepatocytes.
The initial work on GalNAc-conjugated oligonucleotides focused on using a trivalent cluster with the presentation distance between sugars thought to be optimal at 15–20 Å between each GalNAc.681 A trivalent cluster was linked to the oligonucleotide either by postsynthesis conjugation (e.g., amide coupling, phosphonamidite coupling, or click chemistry) or by coupling the cluster to the solid support prior to the oligonucleotide synthesis. Great improvements in GalNAc-siRNA conjugates, including the siRNA sequence used, backbone modifications, and GalNAc presentation, facilitated the GalNAc-conjugated oligonucleotides-based drug development.681,682
At the beginning, Alnylam Pharmaceuticals used the Tri-GalNAc ligands conjugated to the sense strand of siRNA via a (3R,5S)-3-hydroxy-5-hydroxymethylpyrrolidine moiety.676,677 The siRNA backbone was modified by introducing 2′-F or 2′-OMe substitutions on ribose sugar and with two phosphorothioate linkages at the 3′ of the guide strand to enhance stability and binding affinity toward the target gene. Later, siRNAs are further modified, resulting in a significant enhancement in stability, pharmacokinetics, and higher potency than first-generation conjugates.683 This platform technology paves the way for Alnylam Pharmaceuticals to generate a promising pipeline of oligonucleotide medicines for cardiometabolic and liver diseases.679,684 Recently, the U.S. FDA has approved Vutrisiran (Amvuttra, Alnylam Pharmaceuticals) for the treatment of transthyretin-mediated (ATTR) amyloidosis.. Vutrisiran Sodium is a transthyretin (TTR) targeting siRNA conjugated to Tri-GalNAc.
Arrowhead Pharmaceuticals developed a dynamic polyconjugates (DPCs) platform using GalNAc as a targeting ligand. DPCs are composed of a polymer which is linked to a PEG to inhibit membrane interactions of polymer, a targeting ligand for delivery to specific cell type, and the siRNA, which is active pharmaceutical ingredient (API).685 Targeting ligand guides the cargo to the cell of interest and the DPC-siRNA complex is taken up into the endosome, where the low pH cause protonation of polymer that led to endosomal rupture and release of siRNA into cell cytoplasm. For the first-generation DPC technology, conjugation of siRNA to the polymer backbone was done though disulfide linkage. In the second-generation DPC.2 technology, the siRNA is not attached to the polymer but instead conjugated to targeting ligand and coinjected with DPC.686 By employing the second-generation platform, Arrowhead Pharmaceuticals has established an attractive siRNA therapeutics pipeline.679,684,687 GalXC is another RNAi technology platform developed by Dicerna Pharmaceuticals.684 In this platform, GalNAc monomer is covalently attached to the four nucleotides in the extended region of dicer-substrate siRNAs. Data from clinical and preclinical studies suggested that GalXC platform offered higher potency, excellent targeting specificity, long duration of action, and high therapeutic index.679,684
4.5. Targeted Protein Degradation via Carbohydrate-Specific Lysosomal Targeting Receptors
Recently, targeted protein degradation became a potential tool to eliminate disease causing proteins. In the past, many tools have been developed to degrade proteins by exploiting the natural protein homeostasis machinery available in our body.688 For example, PROTAC or the proteolysis-targeting chimera composed of a protein of interest (POI)-binding component attached to an E3 ligase-binding component using a suitable linker. PROTACs degrade proteins by catalyzing the K48 polyubiquitination of the POI, thereby marking it for degradation by the proteasome.689 While PROTACs have been around for a while, researchers have recently been digging into the possibilities of targeted protein degradation, and lysosome targeting chimeras (LYTACs), first reported by the Bertozzi group at Stanford University, was used to recruit proteins to lysosome-shuttling receptors located at the cell surface (Figure 39) for degradation.
Figure 39:
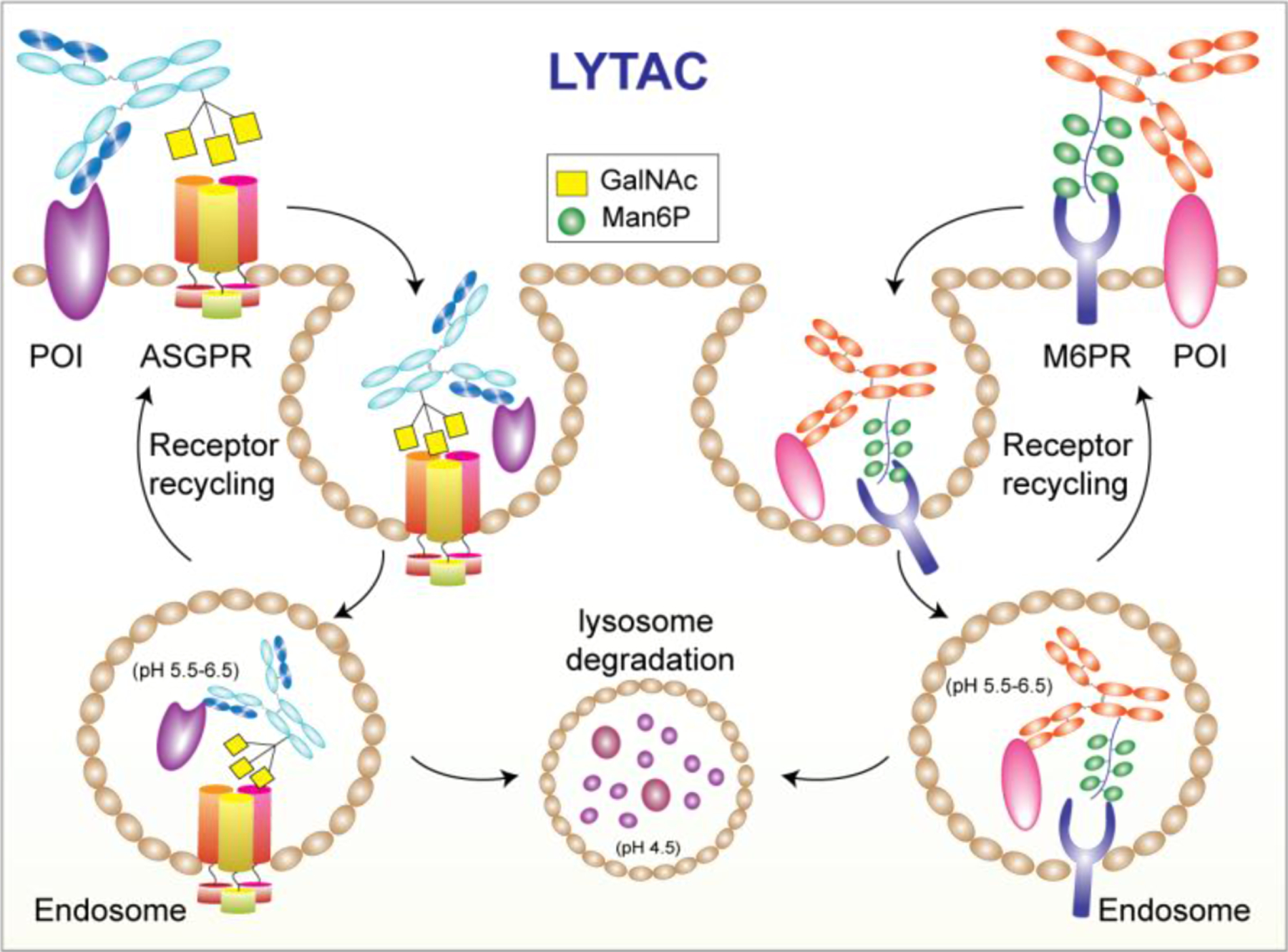
LYTAC technology for targeted degradation of cell membrane and extracellular proteins.
The first-generation LYTAC consists of a POI-binding element attached to a synthetic glycopeptide ligand, an agonist of the cation-independent mannose-6-phosphate receptor (CI-M6PR). LYTACs employing antibodies as POI-binders have also been made, with one of them successfully degrading the neurodegenerative disease-causing protein: apolipoprotein E4 (ApoE4). LYTACS targeting membrane-associated proteins like EGFR, CD71, and PD-L1 has also been constructed.690 The second-generation LYTAC exploits liver-specific ASGPR using the tri-GalNAc-LYTAC conjugate to showcase the more efficient uptake than the M6Pn-LYTAC in an HCC cell line, owing to the surface abundance of ASGPR compared to CI-M6PR in hepatocytes.691
Because of their high molecular weight compared to PROTACs, delivery of LYTACs is particularly challenging. In vivo studies of antibody-LYTACs showed a rapid clearance phase post injection, however, between 6 and 72 h, only a moderate decrease in serum concentration was observed.690 Nonetheless, LYTACs appear to be a promising new strategy for the targeted degradation of extracellular proteins and membrane-associated proteins. The liver-specific GalNAc-LYTAC has opened the possibility of synthesizing more cell type-specific LYTACs.692
SUMMARY
Carbohydrates are a fundamental part of human health and defects in glycosylation are a main contributor to human disease. Significant developments in glycobiology made this field more tractable and understandable. The implications of dysregulated glycosylation in disease progression, from immune evasion to cognition, led to considerable progress in targeting glycans for therapeutic applications. In fact, glycobiology has already been started serving human health with several approved drugs, and some are currently in clinic studies.
Naturally isolated capsular polysaccharides as well as their conjugates with carrier proteins have been used in vaccine formulation for the treatment of various infectious diseases. However, naturally isolated polysaccharides are limited by their heterogeneous composition and difficulty in characterization. Consequently, vaccines with superior safety profile and clearly defined oligosaccharide structures with high manufacturing reproducibility are leading the carbohydrate-based vaccine research. The carbohydrates aberrantly expressed by malignant cells present a window of opportunity for development of glycoconjugate vaccines which can elicit T cell-dependent and long-term immune responses to eliminate tumor cells. Novel vaccine designs including multicomponent epitopes, vaccines with built-in adjuvants, and vaccines with highly immunogenic tumor antigens have fueled the clinical pipeline and increased the success of anticancer vaccine development.
For anticancer antibodies that work via ADCC, the fine structure of Fc glycans is crucial for safety and therapeutic potency. Different glycoforms have been shown to modulate the ADCC and CDC activities, and the pharmacodynamic and pharmacokinetic behaviors of the therapeutic antibodies, whereas other Fc glycan composition may become immunogenic. In vitro glycoengineering approaches using endoglycosidase-based glycosynthases facilitated the development of homogeneous IgG glycoforms with improved clinical outcome as next-generation therapeutic antibodies. In addition, the glycoengineering or synthetic glycobiology methods developed to produce humanized or human glycoconjugates from species ranging from bacteria to yeast and higher eukaryotes have opened new opportunities for discovery research and translational innovation.
ACKNOWLEDGMENTS
We are grateful to the National Institutes of Health (AI130227) and the National Science Foundation (CHE-1954031) for financial support of work described in this review.
ABBREVIATIONS
- ACE2
angiotensin-converting enzyme 2
- ADCC
antibody-dependent cellular cytotoxicity
- ADCs
antibody drug conjugates
- APCs
antigen presenting cells
- ASGRs
asialoglycoprotein receptors
- B3GNT5
N-acetylglucosaminyltransferase 5
- B4GALNT1
β 1,4 GalNAc transferase
- BHK
baby hamster kidney
- bNAbs
broadly neutralizing antibodies
- BSA
bovine serum albumin
- CAMPs
cationic antimicrobial peptides
- CDC
complement-dependent cytotoxicity
- CHO cells
Chinese hamster ovary cells
- CPS
capsular polysaccharide
- CRD
carbohydrate-recognition domain
- DAR
drug–antibody ratio
- DC-SIGN
dendritic cell-specific ICAM-3-grabbing non-integrin 1
- DT
diphtheria toxoid
- Endo
endoglycosidase
- EPL
expressed protein ligation
- ER
endoplasmic reticulum
- FcγRs
Fcγ receptors
- FGF2
fibroblast growth factor 2
- FITC
fluorescein isothiocyanate
- FUT8
fucosyl transferase 8
- GAGs
glycosaminoglycans
- GBPs
glycan-binding proteins
- G-M CSF
granulocyte–macrophage colony stimulating factor
- GMD
GDP mannose 4,6 dehydratase
- GnTIII
N-acetylglucosaminyltransferase III
- GPI
glycosylphosphatidylinositols
- GSLs
glycosphingolipids
- GT
glycosyl transferase
- H. influenza
Haemophilus influenza
- HPI
host–pathogen interactions
- HSA
human serum albumin
- IFNg
interferon g
- iNKT cells
invariant natural killer T cells
- KdO
3-deoxy-D-manno-octulosonic acid
- KLH
keyhole limpet hemocyanin
- LPS
lipopolysaccharides
- MAG
myelin-associated glycoprotein
- MAG
multiple antigenic glycopeptide
- MERS
Middle East respiratory syndrome
- MPLA
monophosphoryl lipid A
- MSCs
mesenchymal stem cells
- MSNPs
mesoporous silica nanoparticles
- NCL
native chemical ligation
- Neu5Gc
glycolylneuraminic acid
- NKT cells
natural killer T cells
- OVA
ovalbumin
- Pam3Cys
tripalmitoyl-S-glyceryl-cysteinyl serine
- PAMAM
polyamidoamine
- PEG
polyethylene glycol
- PG
proteoglycans
- RAFT
regioselectively addressable functionalized templates
- RBD
receptor binding domain
- SAL
sugar assisted ligation
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- Siglecs
sialic-acid-binding immunoglobulin-like lectins
- SPPS
solid-phase peptide synthesis
- ST3GAL5
α 2,3-sialyltransferase 5
- TACA
tumor associated carbohydrate antigens
- TCR
T-cell receptor
- TLRs
Toll-like receptors
- TNFα
tumor necrosis factor α
- TT
tetanus toxoid
- UDP
uridine diphosphate
- ZPSs
zwitterionic polysaccharides
- α-GalCer
α-galactosylceramide
Biographies
Sachin S. Shivatare obtained his Ph.D. in Carbohydrate Chemistry (2013) from Academia Sinica, Taiwan, under the supervision of Prof. Chi-Huey Wong and Prof. Chung-Yi Wu. His doctoral thesis focused on development of chemoenzymatic synthesis of HIV-1 gp120 related complex carbohydrates, the development of glycan microarrays, and glycoconjugate vaccines. In 2013, he joined CHO Pharma Inc., Taiwan, as a senior scientist where he worked on the glycoengineering of therapeutic antibodies and the development of carbohydrate-based therapeutics. Later in 2019, he joined Prof. Wong’s lab at The Scripps Research Institute, California, as a staff scientist. Currently, he is working at Amgen Inc., California, as a Senior Scientist. His research interest is development of biotherapeutic agents, such as antibody–drug conjugates, and glycoengineered antibodies with improved therapeutic potencies, development of technologies for oligonucleotide drug delivery.
Vidya S. Shivatare obtained her Ph.D. in Chemistry (2015) from Academia Sinica, Taiwan. In 2015, she joined Prof. Wong’s lab at Genomics Research Center, Academia Sinica, as a postdoctoral associate. Currently, she is working at The Scripps Research Institute, California, as a postdoctoral associate. Her research interest is in vitro glycoremodeling of antibodies for study of effector functions and therapeutic applications.
Chi-Huey Wong is currently the Scripps Family Chair Professor of Chemistry at the Scripps Research Institute. He also holds a joint appointment as Distinguished Professor at the Genomics Research Center of Academia Sinica, Taiwan. His research interests include the development of new methods to study biological glycosylation and related disease progression.
Footnotes
The authors declare no competing financial interest.
Contributor Information
Sachin S. Shivatare, Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037, United States.
Vidya S. Shivatare, Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037, United States
Chi-Huey Wong, Department of Chemistry, The Scripps Research Institute, La Jolla, California 92037, United States; Genomics Research Center, Academia Sinica, Taipei 115, Taiwan.
REFERENCES
- (1).Varki A Biological Roles of Glycans. Glycobiology 2017, 27 (1), 3–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Broussard AC; Boyce M Life is Sweet: the Cell Biology of Glycoconjugates. Mol. Biol. Cell 2019, 30 (5), 525–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Schjoldager KT; Narimatsu Y; Joshi HJ; Clausen H Global View of Human Protein Glycosylation Pathways and Functions. Nat. Rev. Mol. Cell Biol 2020, 21 (12), 729–749. [DOI] [PubMed] [Google Scholar]
- (4).Ratner DM; Adams EW; Disney MD; Seeberger PH Tools for Glycomics: Mapping Interactions of Carbohydrates in Biological Systems. Chembiochem 2004, 5 (10), 1375–1383. [DOI] [PubMed] [Google Scholar]
- (5).Reily C; Stewart TJ; Renfrow MB; Novak J Glycosylation in Health and Disease. Nat. Rev. Nephrol 2019, 15 (6), 346–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Spiro RG Protein Glycosylation: Nature, Distribution, Enzymatic Formation, and Disease Implications of Glycopeptide Bonds. Glycobiology 2002, 12 (4), 43R–56R. [DOI] [PubMed] [Google Scholar]
- (7).Eichler J Protein Glycosylation. Curr. Biol 2019, 29 (7), R229–R231. [DOI] [PubMed] [Google Scholar]
- (8).Bieberich E Synthesis, Processing, and Function of N-glycans in N-glycoproteins. Adv. Neurobiol 2014, 9, 47–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Nicolaou KC; Mitchell HJ Adventures in Carbohydrate Chemistry: New Synthetic Technologies, Chemical Synthesis, Molecular Design, and Chemical Biology. Angew. Chem., Int. Ed 2001, 40 (9), 1576–1624. [PubMed] [Google Scholar]
- (10).Davis BG Recent Developments in Glycoconjugates. J. Chem. Soc., Perkin Trans. 1 1999, 1999, 3215–3237. [Google Scholar]
- (11).Gamblin DP; Scanlan EM; Davis BG Glycoprotein Synthesis: An Update. Chem. Rev 2009, 109 (1), 131–163. [DOI] [PubMed] [Google Scholar]
- (12).Ohtsubo K; Marth JD Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126 (5), 855–867. [DOI] [PubMed] [Google Scholar]
- (13).Fisher P; Thomas-Oates J; Wood AJ; Ungar D The N-Glycosylation Processing Potential of the Mammalian Golgi Apparatus. Front. Cell Dev. Biol 2019, 7, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Hirata T; Kizuka Y N-Glycosylation. Adv. Exp. Med. Biol 2021, 1325, 3–24. [DOI] [PubMed] [Google Scholar]
- (15).Carraway KL; Hull SR O-Glycosylation Pathway for Mucin-Type Glycoproteins. BioEssays 1989, 10 (4), 117–121. [DOI] [PubMed] [Google Scholar]
- (16).Hanisch FG O-Glycosylation of the Mucin Type. Biol. Chem. Biol. Chem 2001, 382, 143–149. [DOI] [PubMed] [Google Scholar]
- (17).Hang HC; Bertozzi CR The Chemistry and Biology of Mucin-Type O-Linked Glycosylation. Bioorg. Med. Chem 2005, 13 (17), 5021–5034. [DOI] [PubMed] [Google Scholar]
- (18).Gill DJ; Clausen H; Bard F Location, Location, Location: New Insights into O-Galnac Protein Glycosylation. Trends Cell Biol 2011, 21 (3), 149–158. [DOI] [PubMed] [Google Scholar]
- (19).Tian E; Ten Hagen KG Recent Insights into the Biological Roles of Mucin-Type O-Glycosylation. Glycoconj. J 2009, 26 (3), 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Marcos NT; Pinho S; Grandela C; Cruz A; Samyn-Petit B; Harduin-Lepers A; Almeida R; Silva F; Morais V; Costa J; et al. Role of the Human ST6GalNAc-I and ST6GalNAc-II in the Synthesis of the Cancer-Associated Sialyl-Tn Antigen. Cancer. Res 2004, 64 (19), 7050–7057. [DOI] [PubMed] [Google Scholar]
- (21).Theocharis AD; Skandalis SS; Gialeli C; Karamanos NK Extracellular Matrix Structure. Adv. Drug Deliv Rev 2016, 97, 4–27. [DOI] [PubMed] [Google Scholar]
- (22).Karamanos NK; Piperigkou Z; Theocharis AD; Watanabe H; Franchi M; Baud S; Brézillon S; Götte M; Passi A; Vigetti D; et al. Proteoglycan Chemical Diversity Drives Multifunctional Cell Regulation and Therapeutics. Chem. Rev 2018, 118 (18), 9152–9232. [DOI] [PubMed] [Google Scholar]
- (23).Afratis N; Gialeli C; Nikitovic D; Tsegenidis T; Karousou E; Theocharis AD; Pavão MS; Tzanakakis GN; Karamanos NK Glycosaminoglycans: Key Players in Cancer Cell Biology and Treatment. FEBS J 2012, 279 (7), 1177–1197. [DOI] [PubMed] [Google Scholar]
- (24).Mende M; Bednarek C; Wawryszyn M; Sauter P; Biskup MB; Schepers U; Bräse S Chemical Synthesis of Glycosaminoglycans. Chem. Rev 2016, 116 (14), 8193–8255. [DOI] [PubMed] [Google Scholar]
- (25).Zhang X; Kiechle FL Review: Glycosphingolipids in Health and Disease. Ann. Clin. Lab. Sci 2004, 34, 3–13. [PubMed] [Google Scholar]
- (26).Zhang T; de Waard AA; Wuhrer M; Spaapen RM The Role of Glycosphingolipids in Immune Cell Functions. Front. Immunol 2019, 10, 90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Lingwood CA Glycosphingolipid Functions. Cold Spring Harb Perspect Biol 2011, 3 (7), a004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Jennemann R; Gröne HJ Cell-Specific in Vivo Functions of Glycosphingolipids: Lessons From Genetic Deletions of Enzymes Involved in Glycosphingolipid Synthesis. Prog. Lipid Res 2013, 52 (2), 231–248. [DOI] [PubMed] [Google Scholar]
- (29).Ichikawa S; Hirabayashi Y Glucosylceramide Synthase and Glycosphingolipid Synthesis. Trends Cell Biol 1998, 8 (5), 198–202. [DOI] [PubMed] [Google Scholar]
- (30).Erdmann M; Wipfler D; Merling A; Cao Y; Claus C; Kniep B; Sadick H; Bergler W; Vlasak R; Schwartz-Albiez R Differential Surface Expression and Possible Function of 9-O- and 7-O-Acetylated GD3 (CD60 b and c) During Activation and Apoptosis of Human Tonsillar B and T Lymphocytes. Glycoconj. J 2006, 23 (9), 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Paulick MG; Bertozzi CR The Glycosylphosphatidylinositol Anchor: a Complex Membrane-Anchoring Structure for Proteins. Biochemistry 2008, 47 (27), 6991–7000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).McConville MJ; Menon AK Recent Developments in the Cell Biology and Biochemistry of Glycosylphosphatidylinositol Lipids (review). Mol. Membr. Biol 2000, 17 (1), 1–16. [DOI] [PubMed] [Google Scholar]
- (33).Tsai YH; Liu X; Seeberger PH Chemical Biology of Glycosylphosphatidylinositol Anchors. Angew. Chem., Int. Ed 2012, 51 (46), 11438–11456. [DOI] [PubMed] [Google Scholar]
- (34).Nakano M; Sabido-Bozo S; Okazaki K; Aguilera-Romero A; Rodriguez-Gallardo S; Cortes-Gomez A; Lopez S; Ikeda A; Funato K; Muniz M Structural Analysis of the GPI Glycan. PloS one 2021, 16 (9), No. e0257435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Kinoshita T; Fujita M Biosynthesis of GPI-Anchored Proteins: Special Emphasis on GPI Lipid Remodeling. J. Lipid Res 2016, 57 (1), 6–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Liu YS; Fujita M Mammalian GPI-Anchor Modifications and the Enzymes Involved. Biochem. Soc. Trans 2020, 48 (3), 1129–1138. [DOI] [PubMed] [Google Scholar]
- (37).Kinoshita T Biochemistry Of Glycosylphosphatidylinositol (GPI) Anchored Proteins. Seikagaku 2014, 86 (5), 626–636. [PubMed] [Google Scholar]
- (38).Kinoshita T; Fujita M Thematic Review Series: Glycosylphosphatidylinositol (GPI) Anchors: Biochemistry and Cell Biology Biosynthesis of GPI-Anchored Proteins: Special Emphasis on GPI Lipid Remodeling. J. Lipid Res 2016, 57 (1), 6–24.26563290 [Google Scholar]
- (39).Wang ST; Neo BH; Betts RJ Glycosaminoglycans: Sweet as Sugar Targets for Topical Skin Anti-Aging. Clin. Cosmet. Investig. Dermatol 2021, 14, 1227–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Cohen M Notable Aspects of Glycan-Protein Interactions. Biomolecules 2015, 5 (3), 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Raman R; Tharakaraman K; Sasisekharan V; Sasisekharan R Glycan-Protein Interactions in Viral Pathogenesis. Curr. Opin. Struct. Biol 2016, 40, 153–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (42).Krautter F; Iqbal AJ Glycans and Glycan-Binding Proteins as Regulators and Potential Targets in Leukocyte Recruitment. Front. Cell Dev. Biol 2021, 9, 624082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (43).Schnaar RL Glycans and Glycan-Binding Proteins in Immune Regulation: A Concise Introduction to Glycobiology for the Allergist. J. Allergy Clin. Immun 2015, 135 (3), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).D’Souza AA; Devarajan PV Asialoglycoprotein Receptor Mediated Hepatocyte Targeting - Strategies and Applications. J. Controlled Release 2015, 203, 126–139. [DOI] [PubMed] [Google Scholar]
- (45).Kang J-Y; Shin KK; Kim HH; Min J-K; Ji ES; Kim JY; Kwon O; Oh D-B Lysosomal Targeting Enhancement by Conjugation of Glycopeptides Containing Mannose-6-phosphate Glycans Derived From Glyco-engineered Yeast. Sci. Rep 2018, 8, 8730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Bochner BS; Zimmermann N Role of Siglecs and Related Glycan-Binding Proteins in Immune Responses and Immunoregulation. J. Allergy Clin. Immun 2015, 135 (3), 598–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Schnaar RL Glycans and Glycan-Binding Proteins in Immune Regulation: A Concise Introduction to Glycobiology for the Allergist. J. Allergy Clin. Immun 2015, 135 (3), 609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Cohen M; Varki A The Sialome-Far More Than the Sum of Its Parts. Omics A J. Integr Biol 2010, 14 (4), 455–464. [DOI] [PubMed] [Google Scholar]
- (49).Lenza MP; Oyenarte I; Diercks T; Quintana JI; Gimeno A; Coelho H; Diniz A; Peccati F; Delgado S; Bosch A; et al. Structural Characterization of N-Linked Glycans in the Receptor Binding Domain of the SARS-CoV-2 Spike Protein and Their Interactions with Human Lectins. Angew. Chem., Int. Ed 2020, 59 (52), 23763–23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Johnson JL; Jones MB; Ryan SO; Cobb BA The Regulatory Power of Glycans and Their Binding Partners in Immunity. Trends Immunol 2013, 34 (6), 290–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Lin B; Qing X; Liao J; Zhuo K Role of Protein Glycosylation in Host-Pathogen Interaction. Cells 2020, 9 (4), 1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Jones C Vaccines Based on the Cell Surface Carbohydrates of Pathogenic Bacteria. Anais Acad. Bras. Ciências 2005, 77 (2), 293–324. [DOI] [PubMed] [Google Scholar]
- (53).Mettu R; Chen CY; Wu CY Synthetic Carbohydrate-Based Vaccines: Challenges and Opportunities. J. Biomed. Sci 2020, 27, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Wolfert MA; Boons GJ Adaptive Immune Activation: Glycosylation Does Matter. Nat. Chem. Biol 2013, 9 (12), 776–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Schnaar RL Glycobiology Simplified: Diverse Roles of Glycan Recognition in Inflammation. J. Leukoc. Biol 2016, 99 (6), 825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (56).Clark MC; Baum LG T Cells Modulate Glycans on CD43 and CD45 During Development and Activation, Signal Regulation, and Survival. Ann. N.Y. Acad. Sci 2012, 1253, 58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Justement LB The Role of the Protein Tyrosine Phosphatase CD45 in Regulation of B Lymphocyte Activation. Int. Rev. Immunol 2001, 20 (6), 713–738. [DOI] [PubMed] [Google Scholar]
- (58).Thiemann S; Baum LG Galectins and Immune Responses-Just How Do They Do Those Things They Do? Annu. Rev. Immunol 2016, 34, 243–264. [DOI] [PubMed] [Google Scholar]
- (59).Leffler H; Carlsson S; Hedlund M; Qian Y; Poirier F Introduction to Galectins. Glycoconj. J 2002, 19 (7–9), 433–440. [DOI] [PubMed] [Google Scholar]
- (60).Rubinstein N; Ilarregui JM; Toscano MA; Rabinovich GA The Role of Galectins in the Initiation, Amplification and Resolution of the Inflammatory Response. Tissue Antigens 2004, 64 (1), 1–12. [DOI] [PubMed] [Google Scholar]
- (61).Blois SM; Dveksler G; Vasta GR; Freitag N; Blanchard V; Barrientos G Pregnancy Galectinology: Insights Into a Complex Network of Glycan Binding Proteins. Front. Immunol 2019, 10, 1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (62).Liu FT; Patterson RJ; Wang JL Intracellular Functions of Galectins. Biochim. Biophys. Acta 2002, 1572 (2–3), 263–273. [DOI] [PubMed] [Google Scholar]
- (63).Blaser C; Kaufmann M; Müller C; Zimmermann C; Wells V; Mallucci L; Pircher H Beta-Galactoside-Binding Protein Secreted by Activated T Cells Inhibits Antigen-Induced Proliferation of T Cells. Eur. J. Immunol 1998, 28 (8), 2311–2319. [DOI] [PubMed] [Google Scholar]
- (64).Perillo NL; Pace KE; Seilhamer JJ; Baum LG Apoptosis of T Cells Mediated by Galectin-1. Nature 1995, 378 (6558), 736–739. [DOI] [PubMed] [Google Scholar]
- (65).Stowell SR; Arthur CM; Mehta P; Slanina KA; Blixt O; Leffler H; Smith DF; Cummings RD Galectin-1, −2, and −3 Exhibit Differential Recognition of Sialylated Glycans and Blood Group Antigens. J. Biol. Chem 2008, 283 (15), 10109–10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Lin TW; Chang HT; Chen CH; Chen CH; Lin SW; Hsu TL; Wong CH Galectin-3 Binding Protein and Galectin-1 Interaction in Breast Cancer Cell Aggregation and Metastasis. J. Am. Chem. Soc 2015, 137 (30), 9685–9693. [DOI] [PubMed] [Google Scholar]
- (67).Ideo H; Matsuzaka T; Nonaka T; Seko A; Yamashita K Galectin-8-N-Domain Recognition Mechanism for Sialylated and Sulfated Glycans. J. Biol. Chem 2011, 286 (13), 11346–11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Dings RPM; Miller MC; Griffin RJ; Mayo KH Galectins as Molecular Targets for Therapeutic Intervention. Int. J. Mol. Sci 2018, 19, 905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Miller MC; Nesmelova IV; Platt D; Klyosov A; Mayo KH The Carbohydrate-Binding Domain on Galectin-1 is More Extensive for a Complex Glycan Than for Simple Saccharides: Implications for Galectin-Glycan Interactions at the Cell Surface. Biochem. J 2009, 421 (2), 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Sturgill ER; Rolig AS; Linch SN; Mick C; Kasiewicz MJ; Sun Z; Traber PG; Shlevin H; Redmond WL Galectin-3 Inhibition With Belapectin Combined With Anti-OX40 Therapy Reprograms the Tumor Microenvironment to Favor Anti-Tumor Immunity. Oncoimmunology 2021, 10, 1892265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Curti BD; Koguchi Y; Leidner RS; Rolig AS; Sturgill ER; Sun Z; Wu Y; Rajamanickam V; Bernard B; Hilgart-Martiszus I Enhancing Clinical and Immunological Effects of Anti-PD-1 With Belapectin, a Galectin-3 Inhibitor. J. Immunother. Cancer 2021, 9, e002371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Al Attar A; Antaramian A; Noureddin M Review of Galectin-3 Inhibitors in the Treatment of Nonalcoholic Steatohepatitis. Expert Rev. Clin. Pharmacol 2021, 14 (4), 457. [DOI] [PubMed] [Google Scholar]
- (73).Crocker PR; Paulson JC; Varki A Siglecs and Their Roles in the Immune System. Nat. Rev. Immunol 2007, 7 (4), 255–266. [DOI] [PubMed] [Google Scholar]
- (74).Lenza MP; Atxabal U; Oyenarte I; Jiménez-Barbero J; Ereño-Orbea J Current Status on Therapeutic Molecules Targeting Siglec Receptors. Cells 2020, 9 (12), 2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (75).Duan S; Paulson JC Siglecs as Immune Cell Checkpoints in Disease. Annu. Rev. Immunol 2020, 38, 365–395. [DOI] [PubMed] [Google Scholar]
- (76).Clark EA; Giltiay NV CD22: A Regulator of Innate and Adaptive B Cell Responses and Autoimmunity. Front. Immunol 2018, 9, 2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).O’Keefe TL; Williams GT; Batista FD; Neuberger MS Deficiency in CD22, a B Cell-Specific Inhibitory Receptor, is Sufficient to Predispose to Development of High Affinity Autoantibodies. J. Exp. Med 1999, 189 (8), 1307–1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Sewald X; Ladinsky MS; Uchil PD; Beloor J; Pi R; Herrmann C; Motamedi N; Murooka TT; Brehm MA; Greiner DL; et al. Retroviruses Use CD169-Mediated Trans-Infection of Permissive Lymphocytes to Establish Infection. Science 2015, 350 (6260), 563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (79).Asano K; Takahashi N; Ushiki M; Monya M; Aihara F; Kuboki E; Moriyama S; Iida M; Kitamura H; Qiu CH; et al. Intestinal CD169(+) Macrophages Initiate Mucosal Inflammation by Secreting CCL8 That Recruits Inflammatory Monocytes. Nat. Commun 2015, 6, 7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Fraschilla I; Pillai S Viewing Siglecs Through the Lens of Tumor Immunology. Immunol. Rev 2017, 276 (1), 178–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Eakin AJ; Bustard MJ; McGeough CM; Ahmed T; Bjourson AJ; Gibson DS Siglec-1 and −2 as Potential Biomarkers in Autoimmune Disease. Proteomics Clin Appl 2016, 10 (6), 635–644. [DOI] [PubMed] [Google Scholar]
- (82).Smith BAH; Bertozzi CR The Clinical Impact of Glycobiology: Targeting Selectins, Siglecs and Mammalian Glycans. Nat. Rev. Drug Discovery 2021, 20 (3), 217–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Ikehara Y; Ikehara SK; Paulson JC Negative Regulation of T Cell Receptor Signaling by Siglec-7 (p70/AIRM) and Siglec-9. J. Biol. Chem 2004, 279 (41), 43117–43125. [DOI] [PubMed] [Google Scholar]
- (84).Gordon SR; Maute RL; Dulken BW; Hutter G; George BM; McCracken MN; Gupta R; Tsai JM; Sinha R; Corey D; et al. PD-1 Expression by Tumour-Associated Macrophages Inhibits Phagocytosis and Tumour Immunity. Nature 2017, 545 (7655), 495–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Barkal AA; Weiskopf K; Kao KS; Gordon SR; Rosental B; Yiu YY; George BM; Markovic M; Ring NG; Tsai JM; et al. Engagement of MHC Class I by the Inhibitory Receptor LILRB1 Suppresses Macrophages and is a Target of Cancer Immunotherapy. Nat. Immunol 2018, 19 (1), 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Barkal AA; Brewer RE; Markovic M; Kowarsky M; Barkal SA; Zaro BW; Krishnan V; Hatakeyama J; Dorigo O; Barkal LJ; et al. CD24 Signalling Through Macrophage Siglec-10 is a Target for Cancer Immunotherapy. Nature 2019, 572 (7769), 392–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (87).Sun J; Lu Q; Sanmamed MF; Wang J Siglec-15 as an Emerging Target for Next-Generation Cancer Immunotherapy. Clin. Cancer Res 2021, 27 (3), 680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Angata T Siglec-15: a Potential Regulator of Osteoporosis, Cancer, and Infectious Diseases. J. Biomed. Sci 2020, 27, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Wang J; Sun J; Liu LN; Flies DB; Nie X; Toki M; Zhang J; Song C; Zarr M; Zhou X; et al. Siglec-15 as an Immune Suppressor and Potential Target for Normalization Cancer Immunotherapy. Nat. Med 2019, 25 (4), 656–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (90).Lasky LA Selectin-Carbohydrate Interactions and the Initiation of the Inflammatory Response. Annu. Rev. Biochem 1995, 64, 113–139. [DOI] [PubMed] [Google Scholar]
- (91).Norgard-Sumnicht K; Varki A Endothelial Heparan Sulfate Proteoglycans That Bind to L-Selectin Have Glucosamine Residues With Unsubstituted Amino Groups. J. Biol. Chem 1995, 270 (20), 12012–12024. [DOI] [PubMed] [Google Scholar]
- (92).Ley K The Role of Selectins in Inflammation and Disease. Trends Mol. Med 2003, 9 (6), 263–268. [DOI] [PubMed] [Google Scholar]
- (93).Ivetic A; Hoskins Green HL; Hart SJ L-Selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol 2019, 10, 1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Lorant DE; Topham MK; Whatley RE; McEver RP; McIntyre TM; Prescott SM; Zimmerman GA Inflammatory Roles of P-Selectin. J. Clin. Investig 1993, 92 (2), 559–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (95).Silva M; Videira PA; Sackstein R E-Selectin Ligands in the Human Mononuclear Phagocyte System: Implications for Infection, Inflammation, and Immunotherapy. Front. Immunol 2018, 8, 1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Wun T; Styles L; DeCastro L; Telen MJ; Kuypers F; Cheung A; Kramer W; Flanner H; Rhee S; Magnani JL; et al. Phase 1 Study of the E-Selectin Inhibitor GMI 1070 in Patients With Sickle Cell Anemia. PloS one 2014, 9 (7), No. e101301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Stähli BE; Gebhard C; Duchatelle V; Cournoyer D; Petroni T; Tanguay JF; Robb S; Mann J; Guertin MC; Wright RS Effects of the P-Selectin Antagonist Inclacumabon Myocardial Damage After Percutaneous Coronary Intervention According to Timing of Infusion: Insights From the SELECT-ACS Trial. J. Am. Heart Assoc 2016, 5, 004255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Gao Y; Luan X; Melamed J; Brockhausen I Role of Glycans on Key Cell Surface Receptors That Regulate Cell Proliferation and Cell Death. Cells 2021, 10 (5), 1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (99).Ferreira IG; Pucci M; Venturi G; Malagolini N; Chiricolo M; Dall’Olio F Glycosylation as a Main Regulator of Growth and Death Factor Receptors Signaling. Int. J. Mol. Sci 2018, 19, 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Koch S; Tugues S; Li X; Gualandi L; Claesson-Welsh L Signal Transduction by Vascular Endothelial Growth Factor Receptors. Biochem. J 2011, 437 (2), 169. [DOI] [PubMed] [Google Scholar]
- (101).Stanley P Regulation of Notch Signaling by Glycosylation. Curr. Opin. Struct. Biol 2007, 17 (5), 530–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (102).Mehta NR; Lopez PH; Vyas AA; Schnaar RL Gangliosides and Nogo Receptors Independently Mediate Myelin-Associated Glycoprotein Inhibition of Neurite Outgrowth in Different Nerve Cells. J. Biol. Chem 2007, 282 (38), 27875–27886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (103).Grewal PK; Boton M; Ramirez K; Collins BE; Saito A; Green RS; Ohtsubo K; Chui D; Marth JD ST6Gal-I restrains CD22-dependent Antigen Receptor Endocytosis and Shp-1 Recruitment in Normal and Pathogenic Immune Signaling. Mol. Cell. Biol 2006, 26 (13), 4970–4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Van Dyken SJ; Green RS; Marth JD Structural and Mechanistic Features of Protein O glycosylation Linked to CD8+ T-cell Apoptosis. Mol. Cell. Biol 2007, 27 (3), 1096–1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Yen H-Y; Liu Y-C; Chen N-Y; Tsai C-F; Wang Y-T; Chen Y-J; Hsu T-L; Yang P-C; Wong C-H Effect of Sialylation on EGFR Phosphorylation and Resistance to Tyrosine Kinase Inhibition. Proc. Natl. Acad. Sci. U.S.A 2015, 112 (22), 6955–6960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (106).Fujiki R; Chikanishi T; Hashiba W; Ito H; Takada I; Roeder RG; Kitagawa H; Kato S GlcNAcylation of a Histone Methyltransferase in Retinoic-Acid-Induced Granulopoiesis. Nature 2009, 459 (7245), 455–459. [DOI] [PubMed] [Google Scholar]
- (107).Chu CS; Lo PW; Yeh YH; Hsu PH; Peng SH; Teng YC; Kang ML; Wong CH; Juan LJ O-GlcNAcylation Regulates EZH2 Protein Stability and Function. Proc. Natl. Acad. Sci. U.S.A 2014, 111 (4), 1355–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (108).Gottschalk A The Influenza Virus Neuraminidase. Nature 1958, 181 (4606), 377–378. [DOI] [PubMed] [Google Scholar]
- (109).von Itzstein M; Wu WY; Kok GB; Pegg MS; Dyason JC; Jin B; Van Phan T; Smythe ML; White HF; Oliver SW; et al. Rational Design of Potent Sialidase-Based Inhibitors of Influenza Virus Replication. Nature 1993, 363 (6428), 418–423. [DOI] [PubMed] [Google Scholar]
- (110).von Itzstein M The War Against Influenza: Discovery and Development of Sialidase Inhibitors. Nat. Rev. Drug Discovery 2007, 6 (12), 967–974. [DOI] [PubMed] [Google Scholar]
- (111).Long JS; Mistry B; Haslam SM; Barclay WS Host and Viral Determinants of Influenza A Virus Species Specificity. Nat. Rev. Microbiol 2019, 17 (2), 67–81. [DOI] [PubMed] [Google Scholar]
- (112).Rumschlag-Booms E; Rong L Influenza A Virus Entry: Implications in Virulence and Future Therapeutics. Adv. Virol 2013, 2013, 121924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Zhao N; Martin BE; Yang C-K; Luo F; Wan X-F Association Analyses of Large-Scale Glycan Microarray Data Reveal Novel Host-Specific Substructures in Influenza A Virus Binding Glycans. Sci. Rep 2015, 5, 15778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (114).Stevens J; Blixt O; Paulson JC; Wilson IA Glycan Microarray Technologies: Tools to Survey Host Specificity of Influenza Viruses. Nat. Rev. Microbiol 2006, 4 (11), 857–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (115).Olofsson S; Bergström T Glycoconjugate Glycans as Viral Receptors. Ann. Med 2005, 37 (3), 154–172. [DOI] [PubMed] [Google Scholar]
- (116).Cagno V; Tseligka ED; Jones ST; Tapparel C Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Koehler M; Delguste M; Sieben C; Gillet L; Alsteens D Initial Step of Virus Entry: Virion Binding to Cell-Surface Glycans. Annu. Rev. Virol 2020, 7 (1), 143–165. [DOI] [PubMed] [Google Scholar]
- (118).Stencel-Baerenwald JE; Reiss K; Reiter DM; Stehle T; Dermody TS The Sweet Spot: Defining Virus-Sialic Acid Interactions. Nat. Rev. Microbiol 2014, 12 (11), 739–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (119).Milewska A; Zarebski M; Nowak P; Stozek K; Potempa J; Pyrc K Human Coronavirus NL63 Utilizes Heparan Sulfate Proteoglycans for Attachment to Target Cells. J. Virol 2014, 88 (22), 13221–13231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Wu F; Zhao S; Yu B; Chen YM; Wang W; Song ZG; Hu Y; Tao ZW; Tian JH; Pei YY; et al. A New Coronavirus Associated With Human Respiratory Disease in China. Nature 2020, 579 (7798), 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (121).Gates B Responding to Covid-19 - A Once-in-a-Century Pandemic? N. Engl. J. Med 2020, 382 (18), 1677–1679. [DOI] [PubMed] [Google Scholar]
- (122).Huang Y; Yang C; Xu X.-f.; Xu W; Liu S.-w. Structural and Functional Properties of SARS-CoV-2 Spike Protein: Potential Antivirus Drug Development for COVID-19. Acta Pharmacol. Sin 2020, 41 (9), 1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Xia X Domains and Functions of Spike Protein in Sars-Cov-2 in the Context of Vaccine Design. Viruses 2021, 13 (1), 109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Clausen TM; Sandoval DR; Spliid CB; Pihl J; Perrett HR; Painter CD; Narayanan A; Majowicz SA; Kwong EM; McVicar RN; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183 (4), 1043–1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Kalra RS; Kandimalla R Engaging the Spikes: Heparan Sulfate Facilitates SARS-CoV-2 Spike Protein Binding to ACE2 and Potentiates Viral Infection. Signal Transduct. Target. Ther 2021, 6, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Kim SY; Jin W; Sood A; Montgomery DW; Grant OC; Fuster MM; Fu L; Dordick JS; Woods RJ; Zhang F; et al. Characterization of Heparin and Severe Acute Respiratory Syndrome-Related Coronavirus 2 (SARS-CoV-2) Spike Glycoprotein Binding Interactions. Antiviral Res 2020, 181, 104873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Watanabe Y; Allen JD; Wrapp D; McLellan JS; Crispin M Site-Specific Glycan Analysis of the SARS-CoV-2 Spike. Science 2020, 369 (6501), 330–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Sokurenko EV; Chesnokova V; Dykhuizen DE; Ofek I; Wu XR; Krogfelt KA; Struve C; Schembri MA; Hasty DL Pathogenic Adaptation of Escherichia coli by Natural Variation of the FimH Adhesin. Proc. Natl. Acad. Sci. U.S.A 1998, 95 (15), 8922–8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Totsika M; Kostakioti M; Hannan TJ; Upton M; Beatson SA; Janetka JW; Hultgren SJ; Schembri MA A FimH Inhibitor Prevents Acute Bladder Infection and Treats Chronic Cystitis Caused by Multidrug-Resistant Uropathogenic Escherichia coli ST131. J. Infect. Dis 2013, 208 (6), 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Ilver D; Arnqvist A; Ogren J; Frick IM; Kersulyte D; Incecik ET; Berg DE; Covacci A; Engstrand L; Borén T Helicobacter pylori Adhesin Binding Fucosylated Histo-Blood Group Antigens Revealed by Retagging. Science 1998, 279 (5349), 373–377. [DOI] [PubMed] [Google Scholar]
- (131).Mahdavi J; Sondén B; Hurtig M; Olfat FO; Forsberg L; Roche N; Angstrom J; Larsson T; Teneberg S; Karlsson KA; et al. Helicobacter pylori SabA Adhesin in Persistent Infection and Chronic Inflammation. Science 2002, 297 (5581), 573–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Rossez Y; Gosset P; Boneca IG; Magalhães A; Ecobichon C; Reis CA; Cieniewski-Bernard C; Joncquel Chevalier Curt M; Léonard R; Maes E; et al. The lacdiNAc-Specific Adhesin LabA Mediates Adhesion of Helicobacter pylori to Human Gastric Mucosa. J. Infect. Dis 2014, 210 (8), 1286–1295. [DOI] [PubMed] [Google Scholar]
- (133).Magalhães A; Marcos-Pinto R; Nairn AV; Dela Rosa M; Ferreira RM; Junqueira-Neto S; Freitas; Gomes J; Oliveira P; Santos MR; et al. Helicobacter pylori Chronic Infection and Mucosal Inflammation Switches the Human Gastric Glycosylation Pathways. Biochim. Biophys. Acta 2015, 1852 (9), 1928–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).van Kooyk Y; Geijtenbeek TB DC-SIGN: Escape Mechanism for Pathogens. Nat. Rev. Immunol 2003, 3 (9), 697–709. [DOI] [PubMed] [Google Scholar]
- (135).Poole J; Day CJ; von Itzstein M; Paton JC; Jennings MP Glycointeractions in Bacterial Pathogenesis. Nat. Rev. Microbiol 2018, 16 (7), 440–452. [DOI] [PubMed] [Google Scholar]
- (136).Harvey HA; Jennings MP; Campbell CA; Williams R; Apicella MA Receptor-Mediated Endocytosis of Neisseria gonorrhoeae into Primary Human Urethral Epithelial Cells: The Role of the Asialoglycoprotein Receptor. Mol. Microbiol 2001, 42 (3), 659–672. [DOI] [PubMed] [Google Scholar]
- (137).Juge N; Tailford L; Owen CD Sialidases From Gut Bacteria: A Mini-Review. Biochem. Soc. Trans 2016, 44 (1), 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Morris DE; Cleary DW; Clarke SC Secondary Bacterial Infections Associated with Influenza Pandemics. Front. Microbiol 2017, 8, 1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (139).De S; Kaus K; Sinclair S; Case BC; Olson R Structural Basis of Mammalian Glycan Targeting by Vibrio cholerae Cytolysin and Biofilm Proteins. PLoS Pathog 2018, 14 (2), No. e1006841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (140).Maria Cherian R; Gaunitz S; Nilsson A; Liu J; Karlsson NG; Holgersson J Shiga-Like Toxin Binds With High Avidity to Multivalent O-Linked Blood Group P1 Determinants on Mucin-Type Fusion Proteins. Glycobiology 2014, 24 (1), 26–38. [DOI] [PubMed] [Google Scholar]
- (141).Yao G; Zhang S; Mahrhold S; Lam KH; Stern D; Bagramyan K; Perry K; Kalkum M; Rummel A; Dong M; et al. N-Linked Glycosylation of SV2 is Required for Binding and Uptake of Botulinum Neurotoxin A. Nat. Struct. Mol. Biol 2016, 23 (7), 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (142).Kulkarni AA; Weiss AA; Iyer SS Glycan-Based High-Affinity Ligands for Toxins and Pathogen Receptors. Med. Res. Rev 2010, 30 (2), 327–393. [DOI] [PubMed] [Google Scholar]
- (143).Chen C; Fu Z; Kim JJ; Barbieri JT; Baldwin MR Gangliosides as High Affinity Receptors for Tetanus neurotoxin. J. Biol. Chem 2009, 284 (39), 26569–26577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Holmes EH; Ostrander GK; Clausen H; Graem N Oncofetal Expression of Lex Carbohydrate Antigens in Human Colonic Adenocarcinomas. Regulation Through Type 2 Core Chain Synthesis Rather Than Fucosylation. J. Biol. Chem 1987, 262 (23), 11331–11338. [PubMed] [Google Scholar]
- (145).Pinho SS; Reis CA Glycosylation in Cancer: Mechanisms and Clinical Implications. Nat. Rev. Cancer 2015, 15 (9), 540–555. [DOI] [PubMed] [Google Scholar]
- (146).Oliveira-Ferrer L; Legler K; Milde-Langosch K Role of Protein Glycosylation in Cancer Metastasis. Semin. Cancer Biol 2017, 44, 141–152. [DOI] [PubMed] [Google Scholar]
- (147).Sell S Cancer-Associated Carbohydrates Identified by Monoclonal Antibodies. Hum. Pathol 1990, 21 (10), 1003–1019. [DOI] [PubMed] [Google Scholar]
- (148).Taylor-Papadimitriou J; Epenetos AA Exploiting Altered Glycosylation Patterns in Cancer: Progress and Challenges in Diagnosis and Therapy. Trends Biotechnol 1994, 12 (6), 227–233. [DOI] [PubMed] [Google Scholar]
- (149).Hakomori S Tumor-associated Carbohydrate Antigens Defining Tumor Malignancy: Basis for Development of Anti-Cancer Vaccines. Adv. Exp. Med. Biol 2001, 491, 369–402. [DOI] [PubMed] [Google Scholar]
- (150).Symington FW; Hedges DL; Hakomori S Glycolipid Antigens of Human Polymorphonuclear Neutrophils and the Inducible HL-60 Myeloid Leukemia Line. J. Immunol 1985, 134, 2498–2506. [PubMed] [Google Scholar]
- (151).Koprowski H; Herlyn M; Steplewski Z; Sears HF Specific Antigen in Serum of Patients With Colon Carcinoma. Science 1981, 212 (4490), 53–55. [DOI] [PubMed] [Google Scholar]
- (152).Watanabe T; Pukel CS; Takeyama H; Lloyd KO; Shiku H; Li LT; Travassos LR; Oettgen HF; Old LJ Human Melanoma Antigen AH is an Autoantigenic Ganglioside Related to GD2. J. Exp. Med 1982, 156 (6), 1884–1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (153).Menard S; Tagliabue E; Canevari S; Fossati G; Colnaghi MI Generation of Monoclonal Antibodies Reacting With Normal and Cancer Cells of Human Breast. Cancer. Res 1983, 43, 1295–1300. [PubMed] [Google Scholar]
- (154).Orntoft TF; Vestergaard EM Clinical Aspects of Altered Glycosylation of Glycoproteins in Cancer. Electrophoresis 1999, 20 (2), 362–371. [DOI] [PubMed] [Google Scholar]
- (155).Hollingsworth MA; Swanson BJ Mucins in Cancer: Protection and Control of the Cell Surface. Nat. Rev. Cancer 2004, 4 (1), 45–60. [DOI] [PubMed] [Google Scholar]
- (156).Hakomori S Traveling for the Glycosphingolipid Path. Glycoconj. J 2000, 17 (7–9), 627–647. [DOI] [PubMed] [Google Scholar]
- (157).Chang WW; Lee CH; Lee P; Lin J; Hsu CW; Hung JT; Lin JJ; Yu JC; Shao LE; Yu J; et al. Expression of Globo H and SSEA3 in Breast Cancer Stem Cells and the Involvement of Fucosyl Transferases 1 and 2 in Globo H Synthesis. Proc. Natl. Acad. Sci. U.S.A 2008, 105 (33), 11667–11672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Chuang PK; Hsiao M; Hsu TL; Chang CF; Wu CY; Chen BR; Huang HW; Liao KS; Chen CC; Chen CL; et al. Signaling Pathway of Globo-Series Glycosphingolipids and β1,3-Galactosyltransferase V (β3GalT5) in Breast Cancer. Proc. Natl. Acad. Sci. U.S.A 2019, 116 (9), 3518–3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Cheung SK; Chuang PK; Huang HW; Hwang-Verslues WW; Cho CH; Yang WB; Shen CN; Hsiao M; Hsu TL; Chang CF; et al. Stage-Specific Embryonic Antigen-3 (SSEA-3) and β3GalT5 are Cancer Specific and Significant Markers for Breast Cancer Stem Cells. Proc. Natl. Acad. Sci. U.S.A 2016, 113 (4), 960–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Rajan VP; Larsen RD; Ajmera S; Ernst LK; Lowe JB A cloned Human DNA Restriction Fragment Determines Expression of a GDP-L-Fucose: Beta-D-Galactoside 2-Alpha-L-Fucosyltransferase in Transfected Cells. Evidence for Isolation and Transfer of the Human H Blood Group Locus. J. Biol. Chem 1989, 264 (19), 11158–11167. [PubMed] [Google Scholar]
- (161).Saito S; Aoki H; Ito A; Ueno S; Wada T; Mitsuzuka K; Satoh M; Arai Y; Miyagi T Human Alpha 2,3-Sialyltransferase (ST3Gal II) is a Stage-Specific Embryonic Antigen-4 Synthase. J. Biol. Chem 2003, 278 (29), 26474–26479. [DOI] [PubMed] [Google Scholar]
- (162).Io zzo RV; Schaefer L Proteoglycan Form and Function: A Comprehensive Nomenclature of Proteoglycans. Matrix Biol 2015, 42, 11–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Douaiher J; Succar J; Lancerotto L; Gurish MF; Orgill DP; Hamilton MJ; Krilis SA; Stevens RL Development of Mast Cells and Importance of Their Tryptase and Chymase Serine Proteases in Inflammation and Wound Healing. Adv. Immunol 2014, 122, 211–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Bernfield M; Götte M; Park PW; Reizes O; Fitzgerald ML; Lincecum J; Zako M Functions of Cell Surface Heparan Sulfate Proteoglycans. Annu. Rev. Biochem 1999, 68, 729–777. [DOI] [PubMed] [Google Scholar]
- (165).Filmus J; Capurro M; Rast J Glypicans. Genome Biol 2008, 9 (5), 224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Couchman JR Transmembrane Signaling Proteoglycans. Annu. Rev. Cell Dev. Biol 2010, 26, 89–114. [DOI] [PubMed] [Google Scholar]
- (167).Höök M; Woods A; Johansson S; Kjellén L; Couchman JR Functions of Proteoglycans at the Cell Surface. Ciba Found. Symp 1986, 124, 143–157. [DOI] [PubMed] [Google Scholar]
- (168).Galtrey CM; Fawcett JW The Role of Chondroitin Sulfate Proteoglycans in Regeneration and Plasticity in the Central Nervous System. Brain Res. Rev 2007, 54 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- (169).Gu WL; Lu PH Chondroitin Sulfate Proteoglycans in Neural Development and Regeneration. Sheng Li Ke Xue Jin Zhan 2007, 38, 101–105. [PubMed] [Google Scholar]
- (170).Miller GM; Hsieh-Wilson LC Sugar-Dependent Modulation of Neuronal Development, Regeneration, and Plasticity By Chondroitin Sulfate Proteoglycans. Exp. Neurol 2015, 274, 115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Yamaguchi Y Heparan Sulfate Proteoglycans in the Nervous System: Their Diverse Roles in Neurogenesis, Axon Guidance, and Synaptogenesis. Semin. Cell Dev. Biol 2001, 12 (2), 99–106. [DOI] [PubMed] [Google Scholar]
- (172).Sanderson RD Heparan Sulfate Proteoglycans in Invasion and Metastasis. Semin. Cell Dev. Biol 2001, 12 (2), 89–98. [DOI] [PubMed] [Google Scholar]
- (173).Lambaerts K; Wilcox-Adelman SA; Zimmermann P The Signaling Mechanisms of Syndecan Heparan Sulfate Proteoglycans. Curr. Opin. Cell Biol 2009, 21 (5), 662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Stanford KI; Bishop JR; Foley EM; Gonzales JC; Niesman IR; Witztum JL; Esko JD Syndecan-1 is the Primary Heparan Sulfate Proteoglycan Mediating Hepatic Clearance of Triglyceride-Rich Lipoproteins in Mice. J. Clin. Invest 2009, 119, 3236–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Filmus J Glypicans in Growth Control and Cancer. Glycobiology 2001, 11, 19R–23R. [DOI] [PubMed] [Google Scholar]
- (176).Mohanty S; Chaudhary BP; Zoetewey D Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase. Biomolecules 2020, 10, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (177).Van den Steen P; Rudd PM; Dwek RA; Opdenakker G Concepts and Principles of O-Linked Glycosylation. Crit. Rev. Biochem. Mol. Biol 1998, 33 (3), 151–208. [DOI] [PubMed] [Google Scholar]
- (178).Aebi M N-Linked Protein Glycosylation in the ER. Biochim. Biophys. Acta 2013, 1833 (11), 2430–2437. [DOI] [PubMed] [Google Scholar]
- (179).Stanley P Golgi Glycosylation. Cold Spring Harb Perspect Biol 2011, 3 (4), a005199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Bieberich E Synthesis, Processing, and Function of N-glycans in N-glycoproteins. Adv. Neurobiol 2014, 9, 47–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Peter-Katalinic J Methods in Enzymology: O-Glycosylation of Proteins. Methods Enzymol 2005, 405, 139–171. [DOI] [PubMed] [Google Scholar]
- (182).Solá RJ; Griebenow K Glycosylation of Therapeutic Proteins: an Effective Strategy to Optimize Efficacy. BioDrugs 2010, 24 (1), 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (183).Elliott S; Pham E; Macdougall IC Erythropoietins: a Common Mechanism of Action. Exp. Hematol 2008, 36 (12), 1573–1584. [DOI] [PubMed] [Google Scholar]
- (184).Solá RJ; Griebenow K Effects of Glycosylation on the Stability of Protein Pharmaceuticals. J. Pharm. Sci 2009, 98 (4), 1223–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (185).Dordal MS; Wang FF; Goldwasser E The Role of Carbohydrate in Erythropoietin Action. Endocrinology 1985, 116 (6), 2293–2299. [DOI] [PubMed] [Google Scholar]
- (186).Yang Q; An Y; Zhu S; Zhang R; Loke CM; Cipollo JF; Wang L-X Glycan Remodeling of Human Erythropoietin (EPO) Through Combined Mammalian Cell Engineering and Chemoenzymatic Transglycosylation. ACS Chem. Biol 2017, 12 (6), 1665–1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (187).Feldman C; Anderson R Review: Current and New Generation Pneumococcal Vaccines. J. Infect 2014, 69, 309–325. [DOI] [PubMed] [Google Scholar]
- (188).Verez-Bencomo V; Fernández-Santana V; Hardy E; Toledo ME; Rodríguez MC; Heynngnezz L; Rodriguez A; Baly A; Herrera L; Izquierdo M; et al. A Synthetic Conjugate Polysaccharide Vaccine Against Haemophilus Influenzae Type b. Science 2004, 305 (5683), 522–525. [DOI] [PubMed] [Google Scholar]
- (189).Kaplonek P; Khan N; Reppe K; Schumann B; Emmadi M; Lisboa MP; Xu FF; Calow ADJ; Parameswarappa SG; Witzenrath M; et al. Improving Vaccines Against Streptococcus pneumoniae Using Synthetic Glycans. Proc. Natl. Acad. Sci. U.S.A 2018, 115 (52), 13353–13358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (190).McCarthy PC; Sharyan A; Sheikhi Moghaddam L Meningococcal Vaccines: Current Status and Emerging Strategies. Vaccines (Basel) 2018, 6, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (191).Lang S; Huang X Carbohydrate Conjugates in Vaccine Developments. Front. Chem 2020, 8, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (192).Jin KT; Lan HR; Chen XY; Wang SB; Ying XJ; Lin Y; Mou XZ Recent Advances in Carbohydrate-Based Cancer Vaccines. Biotechnol. Lett 2019, 41 (6–7), 641–650. [DOI] [PubMed] [Google Scholar]
- (193).Steimle A; Autenrieth IB; Frick J-S Structure and Function: Lipid A Modifications in Commensals and Pathogens. International J. Med. Microbiol 2016, 306 (5), 290–301. [DOI] [PubMed] [Google Scholar]
- (194).Silhavy TJ; Kahne D; Walker S The Bacterial Cell Envelope. Cold Spring Harb. Perspect. Biol 2010, 2 (5), a000414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (195).Li Y; Shi F; Li Y; Wang X Structure and Function of Lipopolysaccharide Lipid A in Bacteria-a Review. Wei Sheng Wu Xue Bao 2008, 48, 844–849. [PubMed] [Google Scholar]
- (196).Wang X; Quinn PJ Endotoxins: Lipopolysaccharides of Gram-Negative Bacteria. Subcell. Biochem 2010, 53, 3–25. [DOI] [PubMed] [Google Scholar]
- (197).Raetz CR; Whitfield C Lipopolysaccharide Endotoxins. Annu. Rev. Biochem 2002, 71, 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (198).Heinrichs DE; Yethon JA; Whitfield C Molecular Basis for Structural Diversity in the Core Regions of the Lipopolysaccharides of Escherichia coli and Salmonella Enterica. Mol. Microbiol 1998, 30 (2), 221–232. [DOI] [PubMed] [Google Scholar]
- (199).Silipo A; Molinaro A The Diversity of the Core Oligosaccharide in Lipopolysaccharides. Endotoxins: Structure, Function and Recognition; Subcellular Biochemistry; Springer Netherlands: Dordrecht, 2010; Vol. 53, pp 69–99. [DOI] [PubMed] [Google Scholar]
- (200).Raetz CRH; Whitfield C Lipopolysaccharide Endotoxins. Annu. Rev. Biochem 2002, 71, 635–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (201).DebRoy C; Fratamico PM; Yan X; Baranzoni G; Liu Y; Needleman DS; Tebbs R; O’Connell CD; Allred A; Swimley M; et al. Comparison of O-Antigen Gene Clusters of All O-Serogroups of Escherichia coli and Proposal for Adopting a New Nomenclature for O-Typing. PloS one 2016, 11 (1), No. e0147434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (202).Huszczynski SM; Lam JS; Khursigara CM The Role of Pseudomonas aeruginosa Lipopolysaccharide in Bacterial Pathogenesis and Physiology. Pathogens 2020, 9 (1), 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (203).Nikaido H Molecular Basis of Bacterial Outer Membrane Permeability Revisited. Microbiol. Mol. Biol. Rev 2003, 67 (4), 593–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (204).Needham BD; Trent MS Fortifying the Barrier: the Impact of Lipid A Remodelling on Bacterial Pathogenesis. Nat. Rev. Microbiol 2013, 11 (7), 467–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (205).Scott AJ; Oyler BL; Goodlett DR; Ernst RK Lipid A Structural Modifications in Extreme Conditions and Identification of Unique Modifying Enzymes to Define the Toll-Like Receptor 4 Structure-Activity Relationship. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2017, 1862 (11), 1439–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (206).Bertani B; Ruiz N Function and Biogenesis of Lipopolysaccharides. EcoSal Plus 2018, 8, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (207).Saldías MS; Ortega X; Valvano MA Burkholderia cenocepacia O Antigen Lipopolysaccharide Prevents Phagocytosis by Macrophages and Adhesion to Epithelial Cells. J. Med. Microbiol 2009, 58, 1542–1548. [DOI] [PubMed] [Google Scholar]
- (208).Wang X; Quinn PJ Lipopolysaccharide: Biosynthetic Pathway and Structure Modification. Prog. Lipid Res 2010, 49 (2), 97–107. [DOI] [PubMed] [Google Scholar]
- (209).Williams AH; Raetz CR Structural Basis for the Acyl Chain Selectivity and Mechanism of UDP-N-Acetylglucosamine Acyltransferase. Proc. Natl. Acad. Sci. U.S.A 2007, 104 (34), 13543–13550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (210).Brozek KA; Hosaka K; Robertson AD; Raetz CR Biosynthesis of Lipopolysaccharide in Escherichia coli. Cytoplasmic Enzymes That Attach 3-deoxy-D-manno-octulosonic acid to Lipid A. J. Biol. Chem 1989, 264 (12), 6956–6966. [PubMed] [Google Scholar]
- (211).Brozek KA; Raetz CR Biosynthesis of Lipid A in Escherichia coli. Acyl Carrier Protein-Dependent Incorporation of Laurate and Myristate. J. Biol. Chem 1990, 265 (26), 15410–15417. [PubMed] [Google Scholar]
- (212).Kalynych S; Morona R; Cygler M Progress in Understanding the Assembly Process of Bacterial O-Antigen. FEMS Microbiol. Rev 2014, 38 (5), 1048–1065. [DOI] [PubMed] [Google Scholar]
- (213).Abeyrathne PD; Daniels C; Poon KK; Matewish MJ; Lam JS Functional Characterization of WaaL, a Ligase Associated With Linking O-Antigen Polysaccharide to the Core of Pseudomonas aeruginosa Lipopolysaccharide. J. Bacteriol 2005, 187 (9), 3002–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (214).Shrivastava R; Chng S-S Lipid Trafficking Across the Gram-Negative Cell Envelope. J. Biol. Chem 2019, 294 (39), 14175–14185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (215).Wang LX; Amin MN Chemical and Chemoenzymatic Synthesis of Glycoproteins for Deciphering Functions. Chem. Biol 2014, 21 (1), 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (216).van Kooyk Y; Rabinovich GA Protein-Glycan Interactions in the Control of Innate and Adaptive Immune Responses. Nat. Immunol 2008, 9 (6), 593–601. [DOI] [PubMed] [Google Scholar]
- (217).Sarkar B; Jayaraman N Glycoconjugations of Biomolecules by Chemical Methods. Front. Chem 2020, 8, 570185–570185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (218).Kalia J; Raines RT Advances in Bioconjugation. Curr. Org. Chem 2010, 14 (2), 138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (219).Müller C; Despras G; Lindhorst TK Organizing Multivalency in Carbohydrate Recognition. Chem. Soc. Rev 2016, 45 (11), 3275–3302. [DOI] [PubMed] [Google Scholar]
- (220).Li Q; Guo Z Recent Advances in Toll Like Receptor-Targeting Glycoconjugate Vaccines. Molecules 2018, 23 (7), 1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (221).Wang Q; Zhou Z; Tang S; Guo Z Carbohydrate-Monophosphoryl Lipid a Conjugates are Fully Synthetic Self-Adjuvanting Cancer Vaccines Eliciting Robust Immune Responses in the Mouse. ACS Chem. Biol 2012, 7 (1), 235–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (222).Prudnikova K; Lightfoot Vidal SE; Sarkar S; Yu T; Yucha RW; Ganesh N; Penn LS; Han L; Schauer CL; Vresilovic EJ; et al. Aggrecan-Like Biomimetic Proteoglycans (Bpgs) Composed of Natural Chondroitin Sulfate Bristles Grafted onto a Poly(acrylic acid) Core for Molecular Engineering of the Extracellular Matrix. Acta Biomater 2018, 75, 93–104. [DOI] [PubMed] [Google Scholar]
- (223).Miller T; Goude MC; McDevitt TC; Temenoff JS Molecular Engineering of Glycosaminoglycan Chemistry for Biomolecule Delivery. Acta Biomater 2014, 10 (4), 1705–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (224).Place LW; Kelly SM; Kipper MJ Synthesis and Characterization of Proteoglycan-Mimetic Graft Copolymers With Tunable Glycosaminoglycan Density. Biomacromolecules 2014, 15 (10), 3772–3780. [DOI] [PubMed] [Google Scholar]
- (225).Mimeault M; Hauke R; Batra SK Stem Cells: a Revolution in Therapeutics-Recent Advances in Stem Cell Biology and Their Therapeutic Applications in Regenerative Medicine and Cancer Therapies. Clin. Pharmacol. Ther 2007, 82 (3), 252–264. [DOI] [PubMed] [Google Scholar]
- (226).Nurcombe V; Ford MD; Wildschut JA; Bartlett PF Developmental Regulation of Neural Response to FGF-1 and FGF-2 by Heparan Sulfate Proteoglycan. Science 1993, 260 (5104), 103–106. [DOI] [PubMed] [Google Scholar]
- (227).Ding K; Wang Y; Wang H; Yuan L; Tan M; Shi X; Lyu Z; Liu Y; Chen H 6-O-Sulfated Chitosan Promoting the Neural Differentiation of Mouse Embryonic Stem Cells. ACS Appl. Mater. Interfaces 2014, 6 (22), 20043–20050. [DOI] [PubMed] [Google Scholar]
- (228).Ornitz DM FGFs, Heparan Sulfate and FGFRs: Complex Interactions Essential for Development. BioEssays 2000, 22 (2), 108–112. [DOI] [PubMed] [Google Scholar]
- (229).Yayon A; Klagsbrun M; Esko JD; Leder P; Ornitz DM Cell Surface, Heparin-Like Molecules are Required for Binding of Basic Fibroblast Growth Factor to Its High Affinity Receptor. Cell 1991, 64 (4), 841–848. [DOI] [PubMed] [Google Scholar]
- (230).Liu Q; Lyu Z; Yu Y; Zhao Z-A; Hu S; Yuan L; Chen G; Chen H Synthetic Glycopolymers for Highly Efficient Differentiation of Embryonic Stem Cells into Neurons: Lipo- or Not? ACS Appl. Mater. Interfaces 2017, 9 (13), 11518–11527. [DOI] [PubMed] [Google Scholar]
- (231).Huang ML; Smith RAA; Trieger GW; Godula K Glycocalyx Remodeling With Proteoglycan Mimetics Promotes Neural Specification in Embryonic Stem Cells. J. Am. Chem. Soc 2014, 136 (30), 10565–10568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (232).Veronese FM; Pasut G PEGylation, Successful Approach to Drug Delivery. Drug Discovery Today 2005, 10 (21), 1451–1458. [DOI] [PubMed] [Google Scholar]
- (233).Turecek PL; Bossard MJ; Schoetens F; Ivens IA PEGylation of Biopharmaceuticals: A Review of Chemistry and Nonclinical Safety Information of Approved Drugs. J. Pharm. Sci 2016, 105 (2), 460–475. [DOI] [PubMed] [Google Scholar]
- (234).Zhang F; Liu MR; Wan HT Discussion About Several Potential Drawbacks of PEGylated Therapeutic Proteins. Biol. Pharm. Bull 2014, 37 (3), 335–339. [DOI] [PubMed] [Google Scholar]
- (235).Schellekens H; Hennink WE; Brinks V The Immunogenicity of Polyethylene Glycol: Facts and Fiction. Pharm. Res 2013, 30 (7), 1729–1734. [DOI] [PubMed] [Google Scholar]
- (236).Lee F; Chung JE; Kurisawa M An injectable Hyaluronic Acid-Tyramine Hydrogel System for Protein Delivery. J. Controlled Release 2009, 134 (3), 186–193. [DOI] [PubMed] [Google Scholar]
- (237).Fukushima M; Hirayama T; Ichikawa M; Kitazawa I; Kojima K; Sakai T; Takatsu Y; Ohtaki T Glycosaminoglycan-Conjugated Insulin Derivatives Suitable for Once-Daily Formulations. ACS Omega 2019, 4 (3), 5517–5525. [Google Scholar]
- (238).Ichikawa M; Hirayama T; Fukushima M; Kitazawa I; Kojima K; Sakai T; Takatsu Y; Ohtaki T Glycosaminoglycan Conjugation for Improving the Duration of Therapeutic Action of Glucagon-Like Peptide-1. ACS Omega 2018, 3 (5), 5346–5354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (239).Wang EC; Wang AZ Nanoparticles and Their Applications in Cell and Molecular Biology. Integr. Biol. (Camb) 2014, 6 (1), 9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (240).Yang Y; Yu C Advances in Silica Based Nanoparticles for Targeted Cancer Therapy. Nanomedicine 2016, 12 (2), 317–332. [DOI] [PubMed] [Google Scholar]
- (241).Tarn D; Ashley CE; Xue M; Carnes EC; Zink JI; Brinker CJ Mesoporous Silica Nanoparticle Nanocarriers: Bio-functionality and Biocompatibility. Acc. Chem. Res 2013, 46 (3), 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (242).Misra S; Heldin P; Hascall VC; Karamanos NK; Skandalis SS; Markwald RR; Ghatak S Hyaluronan-CD44 Interactions as Potential Targets for cancer Therapy. FEBS J 2011, 278 (9), 1429–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (243).Yoon HY; Koo H; Choi KY; Lee SJ; Kim K; Kwon IC; Leary JF; Park K; Yuk SH; Park JH; et al. Tumor-Targeting Hyaluronic Acid Nanoparticles for Photodynamic Imaging and Therapy. Biomaterials 2012, 33 (15), 3980–3989. [DOI] [PubMed] [Google Scholar]
- (244).Park S; Park H; Jeong S; Yi BG; Park K; Key J Hyaluronic Acid-Conjugated Mesoporous Silica Nanoparticles Loaded With Dual Anticancer Agents for Chemophotodynamic Cancer Therapy. J. Nanomater 2019, 2019, 3481397. [Google Scholar]
- (245).Meng H; Liong M; Xia T; Li Z; Ji Z; Zink JI; Nel AE Engineered Design of Mesoporous Silica Nanoparticles to Deliver Doxorubicin and P-Glycoprotein siRNA to Overcome Drug Resistance in a Cancer Cell Line. ACS Nano 2010, 4 (8), 4539–4550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (246).Ganesh S; Iyer AK; Morrissey DV; Amiji MM Hyaluronic Acid Based Self-Assembling Nanosystems for CD44 Target Mediated siRNA Delivery to Solid Tumors. Biomaterials 2013, 34 (13), 3489–3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (247).Yang X; lyer AK; Singh A; Choy E; Hornicek FJ; Amiji MM; Duan Z MDR1 siRNA Loaded Hyaluronic Acid-Based CD44 Targeted Nanoparticle Systems Circumvent Paclitaxel Resistance in Ovarian Cancer. Sci. Rep 2015, 5, 8509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (248).Mishra V; Bansal KK; Verma A; Yadav N; Thakur S; Sudhakar K; Rosenholm JM Solid Lipid Nanoparticles: Emerging Colloidal Nano Drug Delivery Systems. Pharmaceutics 2018, 10 (4), 191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (249).Paliwal R; Paliwal SR; Kenwat R; Kurmi BD; Sahu MK Solid Lipid Nanoparticles: a Review on Recent Perspectives and Patents. Expert Opin Ther. Pat 2020, 30 (3), 179–194. [DOI] [PubMed] [Google Scholar]
- (250).Working PK; Dayan AD Pharmacological-Toxicological Expert Report. CAELYX. (Stealth liposomal doxorubicin HCl). Hum. Exp. Toxicol 1996, 15, 751–785. [PubMed] [Google Scholar]
- (251).Bulbake U; Doppalapudi S; Kommineni N; Khan W Liposomal Formulations in Clinical Use: An Updated Review. Pharmaceutics 2017, 9, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (252).Tenchov R; Bird R; Curtze AE; Zhou Q Lipid Nanoparticles-From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [DOI] [PubMed] [Google Scholar]
- (253).Akbarzadeh A; Rezaei-Sadabady R; Davaran S; Joo SW; Zarghami N; Hanifehpour Y; Samiei M; Kouhi M; Nejati-Koshki K Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett 2013, 8, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (254).Smith MC; Crist RM; Clogston JD; McNeil SE Zeta Potential: a Case Study of Cationic, Anionic, and Neutral Liposomes. Anal. Bioanal. Chem 2017, 409 (24), 5779–5787. [DOI] [PubMed] [Google Scholar]
- (255).DeFrancesco L Whither COVID-19 Vaccines? Nat. Biotechnol 2020, 38 (10), 1132–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (256).Li Y; Tenchov R; Smoot J; Liu C; Watkins S; Zhou Q A Comprehensive Review of the Global Efforts on COVID-19 Vaccine Development. ACS Cent. Sci 2021, 7 (4), 512–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (257).Yanez Arteta M; Kjellman T; Bartesaghi S; Wallin S; Wu X; Kvist AJ; Dabkowska A; Székely N; Radulescu A; Bergenholtz J; LIndfors L Successful Reprogramming of Cellular Protein Production Through mRNA Delivered by Functionalized Lipid Nanoparticles. Proc. Natl. Acad. Sci. U.S.A 2018, 115, E3351–E3360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (258).Sabnis S; Kumarasinghe ES; Salerno T; Mihai C; Ketova T; Senn JJ; Lynn A; Bulychev A; McFadyen I; Chan J; et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol. Ther 2018, 26 (6), 1509–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (259).Schoenmaker L; Witzigmann D; Kulkarni JA; Verbeke R; Kersten G; Jiskoot W; Crommelin DJA mRNA-Lipid Nanoparticle COVID-19 Vaccines: Structure and Stability. Int. J. Pharm 2021, 601, 120586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (260).Liu L; Bennett CS; Wong CH Advances in Glycoprotein Synthesis. Chem. Commun. (Camb) 2006, 1, 21–33. [DOI] [PubMed] [Google Scholar]
- (261).Li C; Wang LX Chemoenzymatic Methods for the Synthesis of Glycoproteins. Chem. Rev 2018, 118 (17), 8359–8413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (262).Bennett CS; Wong CH Chemoenzymatic Approaches to Glycoprotein Synthesis. Chem. Soc. Rev 2007, 36 (8), 1227–1238. [DOI] [PubMed] [Google Scholar]
- (263).Kemp DS; Kerkman DJ; Leung S-L; Hanson G Intramolecular O, N-acyl transfer Via Cyclic Intermediates of Nine and Twelve Members. Models for Extensions of the Amine Capture Strategy for Peptide Synthesis. J. Org. Chem 1981, 46 (3), 490–498. [Google Scholar]
- (264).Brik A; Wong CH Sugar-Assisted Ligation for the Synthesis of Glycopeptides. Eur. J. Chem 2007, 13 (20), 5670–5675. [DOI] [PubMed] [Google Scholar]
- (265).Dawson PE; Muir TW; Clark-Lewis I; Kent SB Synthesis of Proteins by Native Chemical Ligation. Science 1994, 266 (5186), 776–779. [DOI] [PubMed] [Google Scholar]
- (266).Shin Y; Winans KA; Backes BJ; Kent SBH; Ellman JA; Bertozzi CR Fmoc-Based Synthesis of Peptide-αThioesters: Application to the Total Chemical Synthesis of a Glycoprotein by Native Chemical Ligation. J. Am. Chem. Soc 1999, 121 (50), 11684–11689. [Google Scholar]
- (267).Marcaurelle LA; Mizoue LS; Wilken J; Oldham L; Kent SB; Handel TM; Bertozzi CR Chemical Synthesis of Lymphotactin: a Glycosylated Chemokine With a C-Terminal Mucin-Like Domain. Chem.—Eur. J 2001, 7, 1129–1132. [DOI] [PubMed] [Google Scholar]
- (268).Mezzato S; Schaffrath M; Unverzagt C An Orthogonal Double-Linker Resin Facilitates the Efficient Solid-Phase Synthesis of Complex-Type N-Glycopeptide Thioesters Suitable for Native Chemical Ligation. Angew. Chem., Int. Ed. Engl 2005, 44 (11), 1650–1654. [DOI] [PubMed] [Google Scholar]
- (269).Yamamoto N; Tanabe Y; Okamoto R; Dawson PE; Kajihara Y Chemical Synthesis of a Glycoprotein Having an Intact Human Complex-Type Sialyloligosaccharide under the Boc and Fmoc Synthetic Strategies. J. Am. Chem. Soc 2008, 130 (2), 501–510. [DOI] [PubMed] [Google Scholar]
- (270).Aussedat B; Fasching B; Johnston E; Sane N; Nagorny P; Danishefsky SJ Total Synthesis of the α-Subunit of Human Glycoprotein Hormones: Toward Fully Synthetic Homogeneous Human Follicle-Stimulating Hormone. J. Am. Chem. Soc 2012, 134, 3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (271).Fernández-Tejada A; Vadola PA; Danishefsky SJ Chemical Synthesis of the β-Subunit of Human Luteinizing (hLH) and Chorionic Gonadotropin (hCG) Glycoprotein Hormones. J. Am. Chem. Soc 2014, 136 (23), 8450–8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (272).Macmillan D Evolving Strategies for Protein Synthesis Converge on Native Chemical Ligation. Angew. Chem., Int. Ed. Engl 2006, 45 (46), 7668–7672. [DOI] [PubMed] [Google Scholar]
- (273).Offer J Native Chemical Ligation With Nalpha Acyl Transfer Auxiliaries. Biopolymers 2010, 94 (4), 530–541. [DOI] [PubMed] [Google Scholar]
- (274).Kulkarni SS; Sayers J; Premdjee B; Payne RJ Rapid and Efficient Protein Synthesis Through Expansion of the Native Chemical Ligation Concept. Nat. Rev. Chem 2018, 2, 0122. [Google Scholar]
- (275).Payne RJ; Wong CH Advances in Chemical Ligation Strategies for the Synthesis of Glycopeptides and Glycoproteins. Chem. Commun. (Camb) 2010, 46 (1), 21–43. [DOI] [PubMed] [Google Scholar]
- (276).Erlanson DA; Chytil M; Verdine GL The Leucine Zipper Domain Controls the Orientation of AP-1 in the NFAT.AP-1.DNA Complex. Chem. Biol 1996, 3 (12), 981–991. [DOI] [PubMed] [Google Scholar]
- (277).Tolbert TJ; Franke D; Wong CH A New Strategy for Glycoprotein Synthesis: Ligation of Synthetic Glycopeptides With Truncated Proteins Expressed in E. coli as TEV Protease Cleavable Fusion Protein. Bioorg. Med. Chem 2005, 13 (3), 909–915. [DOI] [PubMed] [Google Scholar]
- (278).Muralidharan V; Muir TW Protein Ligation: an Enabling Technology for the Biophysical Analysis of Proteins. Nat. Methods 2006, 3 (6), 429–438. [DOI] [PubMed] [Google Scholar]
- (279).Tolbert TJ; Wong C-H Intein-Mediated Synthesis of Proteins Containing Carbohydrates and Other Molecular Probes. J. Am. Chem. Soc 2000, 122 (23), 5421–5428. [Google Scholar]
- (280).Macmillan D; Bertozzi CR New Directions in Glycoprotein Engineering. Tetrahedron 2000, 56 (48), 9515–9525. [Google Scholar]
- (281).Macmillan D; Bertozzi CR Modular Assembly of Glycoproteins: Towards the Synthesis of GlyCAM-1 by Using Expressed Protein Ligation. Angew. Chem., Int. Ed. Engl 2004, 43 (11), 1355–1359. [DOI] [PubMed] [Google Scholar]
- (282).Piontek C; Ring P; Harjes O; Heinlein C; Mezzato S; Lombana N; Pöhner C; Püttner M; Varón Silva D; Martin A; et al. Semisynthesis of a Homogeneous Glycoprotein Enzyme: Ribonuclease C: Part 1. Angew. Chem., Int. Ed. Engl 2009, 48 (11), 1936–1940. [DOI] [PubMed] [Google Scholar]
- (283).Piontek C; Varón Silva D; Heinlein C; Pöhner C; Mezzato S; Ring P; Martin A; Schmid FX; Unverzagt C Semisynthesis of a Homogeneous Glycoprotein Enzyme: Ribonuclease C: Part 2. Angew. Chem., Int. Ed. Engl 2009, 48 (11), 1941–1945. [DOI] [PubMed] [Google Scholar]
- (284).Hirano K; Macmillan D; Tezuka K; Tsuji T; Kajihara Y Design and Synthesis of a Homogeneous Erythropoietin Analogue With Two Human Complex-Type Sialyloligosaccharides: Combined Use of Chemical and Bacterial Protein Expression Methods. Angew. Chem., Int. Ed. Engl 2009, 48 (50), 9557–9560. [DOI] [PubMed] [Google Scholar]
- (285).Lu R; Zhao X; Li J; Niu P; Yang B; Wu H; Wang W; Song H; Huang B; Zhu N; et al. Genomic Characterisation and Epidemiology of 2019 Novel Coronavirus: Implications for Virus Origins and Receptor Binding. Lancet 2020, 395 (10224), 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (286).Yan R; Zhang Y; Li Y; Xia L; Guo Y; Zhou Q Structural Basis for the Recognition of SARS-Cov-2 by Full-Length Human ACE2. Science 2020, 367 (6485), 1444–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (287).Law JLM; Logan M; Joyce MA; Landi A; Hockman D; Crawford K; Johnson J; LaChance G; Saffran HA; Shields J; et al. SARS-COV-2 Recombinant Receptor-Binding-Domain (RBD) Induces Neutralizing Antibodies Against Variant Strains of SARS-CoV-2 and SARS-CoV-1. Vaccine 2021, 39 (40), 5769–5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (288).Watanabe Y; Berndsen ZT; Raghwani J; Seabright GE; Allen JD; Pybus OG; McLellan JS; Wilson IA; Bowden TA; Ward AB; Crispin M Vulnerabilities in Coronavirus Glycan Shields Despite Extensive Glycosylation. Nat. Commun 2020, 11, 2688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (289).Ye F; Zhao J; Xu P; Liu X; Yu J; Shangguan W; Liu J; Luo X; Li C; Ying T; et al. Synthetic Homogeneous Glycoforms of the SARS-CoV-2 Spike Receptor-Binding Domain Reveals Different Binding Profiles of Monoclonal Antibodies. Angew. Chem., Int. Ed. Engl 2021, 60 (23), 12904–12910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (290).Brik A; Yang Y-Y; Ficht S; Wong C-H Sugar-Assisted Glycopeptide Ligation. J. Am. Chem. Soc 2006, 128 (17), 5626–5627. [DOI] [PubMed] [Google Scholar]
- (291).Brik A; Ficht S; Yang Y-Y; Wong C-H Sugar-Assisted Ligation of N-Linked Glycopeptides With Broad Sequence Tolerance at the Ligation Junction. J. Am. Chem. Soc 2006, 128, 15026–15033. [DOI] [PubMed] [Google Scholar]
- (292).Yang YY; Ficht S; Brik A; Wong CH Sugar-Assisted Ligation in Glycoprotein Synthesis. J. Am. Chem. Soc 2007, 129 (24), 7690–7701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (293).Schmaltz RM; Hanson SR; Wong CH Enzymes in the Synthesis of Glycoconjugates. Chem. Rev 2011, 111 (7), 4259–4307. [DOI] [PubMed] [Google Scholar]
- (294).Hancock SM; Vaughan MD; Withers SG Engineering of Glycosidases and Glycosyltransferases. Curr. Opin. Chem. Biol 2006, 10 (5), 509–519. [DOI] [PubMed] [Google Scholar]
- (295).Rich JR; Withers SG Emerging Methods for the Production of Homogeneous Human Glycoproteins. Nat. Chem. Biol 2009, 5 (4), 206–215. [DOI] [PubMed] [Google Scholar]
- (296).Wang LX; Lomino JV Emerging Technologies for making Glycan-Defined Glycoproteins. ACS Chem. Biol 2012, 7 (1), 110–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (297).Huang W; Giddens J; Fan SQ; Toonstra C; Wang LX Chemoenzymatic Glycoengineering of Intact IgG Antibodies for Gain of Functions. J. Am. Chem. Soc 2012, 134 (29), 12308–12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (298).Li T; Tong X; Yang Q; Giddens JP; Wang LX Glycosynthase Mutants of Endoglycosidase S2 Show Potent Transglycosylation Activity and Remarkably Relaxed Substrate Specificity for Antibody Glycosylation Remodeling. J. Biol. Chem 2016, 291 (32), 16508–16518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (299).Shivatare SS; Huang LY; Zeng YF; Liao JY; You TH; Wang SY; Cheng T; Chiu CW; Chao P; Chen LT; et al. Development of Glycosynthases With Broad Glycan Specificity for the Efficient Glyco-Remodeling of Antibodies. Chem. Commun. (Camb) 2018, 54 (48), 6161–6164. [DOI] [PubMed] [Google Scholar]
- (300).Huang W; Zhang X; Ju T; Cummings RD; Wang LX Expeditious chemoenzymatic Synthesis of CD52 Glycopeptide Antigens. Org. Biomol. Chem 2010, 8 (22), 5224–5233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (301).Giddens JP; Lomino JV; Amin MN; Wang LX Endo-F3 Glycosynthase Mutants Enable Chemoenzymatic Synthesis of Core-fucosylated Triantennary Complex Type Glycopeptides and Glycoproteins. J. Biol. Chem 2016, 291 (17), 9356–9370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (302).Huang W; Li J; Wang LX Unusual Transglycosylation Activity of Flavobacterium meningosepticum Endoglycosidases Enables Convergent Chemoenzymatic Synthesis of Core Fucosylated Complex N-Glycopeptides. Chembiochem 2011, 12 (6), 932–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (303).McIntosh JD; Brimble MA; Brooks AES; Dunbar PR; Kowalczyk R; Tomabechi Y; Fairbanks AJ Convergent Chemo-Enzymatic Synthesis of Mannosylated Glycopeptides; Targeting of Putative Vaccine Candidates to Antigen Presenting Cells. Chem. Sci 2015, 6 (8), 4636–4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (304).Tomabechi Y; Krippner G; Rendle PM; Squire MA; Fairbanks AJ Glycosylation of Pramlintide: Synthetic Glycopeptides That Display in Vitro and in Vivo Activities as Amylin Receptor Agonists. Chem.—Eur. J 2013, 19, 15084–15088 [DOI] [PubMed] [Google Scholar]
- (305).Ma B; Guan X; Li Y; Shang S; Li J; Tan Z Protein Glycoengineering: An Approach for Improving Protein Properties. Front. Chem 2020, 8, 622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (306).Karottki KJC; Hefzi H; Xiong K; Shamie I; Hansen AH; Li S; Pedersen LE; Li S; Lee JS; Lee GM; et al. Awakening Dormant Glycosyltransferases in CHO Cells With CRISPRa. Biotechnol. Bioeng 2020, 117 (2), 593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (307).Sergeeva D; Camacho-Zaragoza JM; Lee JS; Kildegaard HF CRISPR/Cas9 as a Genome Editing Tool for Targeted Gene Integration in CHO Cells. Methods Mol. Biol 2019, 1961, 213–232. [DOI] [PubMed] [Google Scholar]
- (308).Wang Q; Chung CY; Chough S; Betenbaugh MJ Antibody Glycoengineering Strategies in Mammalian Cells. Biotechnol. Bioeng 2018, 115 (6), 1378–1393. [DOI] [PubMed] [Google Scholar]
- (309).Reusch D; Tejada ML Fc Glycans of Therapeutic Antibodies as Critical Quality Attributes. Glycobiology 2015, 25 (12), 1325–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (310).Shields RL; Lai J; Keck R; O’Connell LY; Hong K; Meng YG; Weikert SH; Presta LG Lack of Fucose on Human IgG1 N-Linked Oligosaccharide Improves Binding to Human Fcgamma RIII and Antibody-Dependent Cellular Toxicity. J. Biol. Chem 2002, 277 (30), 26733–26740. [DOI] [PubMed] [Google Scholar]
- (311).Yamane-Ohnuki N; Kinoshita S; Inoue-Urakubo M; Kusunoki M; Iida S; Nakano R; Wakitani M; Niwa R; Sakurada M; Uchida K; et al. Establishment of FUT8 Knockout Chinese Hamster Ovary Cells: an Ideal Host Cell Line for Producing Completely Defucosylated Antibodies With Enhanced Antibody-Dependent Cellular Cytotoxicity. Biotechnol. Bioeng 2004, 87 (5), 614–622. [DOI] [PubMed] [Google Scholar]
- (312).Davies J; Jiang L; Pan LZ; LaBarre MJ; Anderson D; Reff M Expression of GnTIII in a Recombinant Anti-CD20 CHO Production Cell Line: Expression of Antibodies With Altered Glycoforms Leads to an Increase in ADCC Through Higher Affinity for FC gamma RIII. Biotechnol. Bioeng 2001, 74 (4), 288–294. [PubMed] [Google Scholar]
- (313).Umaña P; Jean-Mairet J; Moudry R; Amstutz H; Bailey JE Engineered Glycoforms of an Antineuroblastoma IgG1 With Optimized Antibody-Dependent Cellular Cytotoxic Activity. Nat. Biotechnol 1999, 17 (2), 176–180. [DOI] [PubMed] [Google Scholar]
- (314).Lifely MR; Hale C; Boyce S; Keen MJ; Phillips J Glycosylation and Biological Activity of CAMPATH-1H Expressed in Different Cell Lines and Grown Under Different Culture Conditions. Glycobiology 1995, 5 (8), 813–822. [DOI] [PubMed] [Google Scholar]
- (315).Dicker M; Strasser R Using Glyco-engineering to Produce Therapeutic Proteins. Expert Opin. Biol. Ther 2015, 15 (10), 1501–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (316).De Wachter C; Van Landuyt L; Callewaert N Engineering of Yeast Glycoprotein Expression. Adv. Biochem. Eng. Biotechnol 2021, 175, 93. [DOI] [PubMed] [Google Scholar]
- (317).Chiba Y; Suzuki M; Yoshida S; Yoshida A; Ikenaga H; Takeuchi M; Jigami Y; Ichishima E Production of Human Compatible High Mannose-Type (Man5GlcNAc2) Sugar Chains in Saccharomyces cerevisiae. J. Biol. Chem 1998, 273 (41), 26298–26309. [DOI] [PubMed] [Google Scholar]
- (318).Rosano GL; Ceccarelli EA Recombinant Protein Expression in Escherichia coli: Advances and Challenges. Front. Microbiol 2014, 5, 172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (319).Rosano GL; Morales ES; Ceccarelli EA New Tools for Recombinant Protein Production in Escherichia coli: A 5-Year Update. Protein sci 2019, 28 (8), 1412–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (320).Hamilton SR; Bobrowicz P; Bobrowicz B; Davidson RC; Li H; Mitchell T; Nett JH; Rausch S; Stadheim TA; Wischnewski H; et al. Production of Complex Human Glycoproteins in Yeast. Science 2003, 301 (5637), 1244–1246. [DOI] [PubMed] [Google Scholar]
- (321).Liu CP; Tsai TI; Cheng T; Shivatare VS; Wu CY; Wu CY; Wong CH Glycoengineering of Antibody (Herceptin) Through Yeast Expression and in vitro Enzymatic Glycosylation. Proc. Natl. Acad. Sci. U.S.A 2018, 115 (4), 720–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (322).Quast I; Keller CW; Maurer MA; Giddens JP; Tackenberg B; Wang LX; Münz C; Nimmerjahn F; Dalakas MC; Lünemann JD Sialylation of IgG Fc Domain Impairs Complement-Dependent Cytotoxicity. J. Clin. Investig 2015, 125 (11), 4160–4170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (323).Anthony RM; Ravetch JV A Novel Role for the IgG Fc Glycan: the Anti-Inflammatory Activity of Sialylated IgG Fcs. J. Clin. Immunol 2010, 30, 9–14. [DOI] [PubMed] [Google Scholar]
- (324).Lund J; Takahashi N; Pound JD; Goodall M; Jefferis R Multiple Interactions of IgG With Its Core Oligosaccharide Can Modulate Recognition by Complement and Human Fc Gamma Receptor I and Influence the Synthesis of Its Oligosaccharide Chains. J. Immunol 1996, 157, 4963–4969. [PubMed] [Google Scholar]
- (325).Chung CY; Wang Q; Yang S; Ponce SA; Kirsch BJ; Zhang H; Betenbaugh MJ Combinatorial Genome and Protein Engineering Yields Monoclonal Antibodies With Hypergalactosylation From CHO Cells. Biotechnol. Bioeng 2017, 114 (12), 2848–2856. [DOI] [PubMed] [Google Scholar]
- (326).Raymond C; Robotham A; Spearman M; Butler M; Kelly J; Durocher Y Production of α2,6-Sialylated IgG1 in CHO Cells. MAbs 2015, 7 (3), 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (327).Chung CY; Wang Q; Yang S; Yin B; Zhang H; Betenbaugh M Integrated Genome and Protein Editing Swaps α−2,6 Sialylation for α−2,3 Sialic Acid on Recombinant Antibodies From CHO. Biotechnol. J 2017, 12 (2), 1600502. [DOI] [PubMed] [Google Scholar]
- (328).Khongorzul P; Ling CJ; Khan FU; Ihsan AU; Zhang J Antibody-Drug Conjugates: A Comprehensive Review. Mol. Cancer. Res 2020, 18 (1), 3–19. [DOI] [PubMed] [Google Scholar]
- (329).Drago JZ; Modi S; Chandarlapaty S Unlocking the Potential of Antibody-Drug Conjugates for Cancer Therapy. Nat. Rev. Clin. Oncol 2021, 18 (6), 327–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (330).Zhao P; Zhang Y; Li W; Jeanty C; Xiang G; Dong Y Recent Advances of Antibody Drug Conjugates for Clinical Applications. Acta Pharm. Sin. B 2020, 10 (9), 1589–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (331).Stephan JP; Kozak KR; Wong WL Challenges in Developing Bioanalytical Assays for Characterization of Antibody-Drug Conjugates. Bioanalysis 2011, 3 (6), 677–700. [DOI] [PubMed] [Google Scholar]
- (332).Wakankar AA; Feeney MB; Rivera J; Chen Y; Kim M; Sharma VK; Wang YJ Physicochemical Stability of the Antibody-Drug Conjugate Trastuzumab-DM1: Changes Due to Modification and Conjugation Processes. Bioconjugate Chem 2010, 21 (9), 1588–1595. [DOI] [PubMed] [Google Scholar]
- (333).Panowski S; Bhakta S; Raab H; Polakis P; Junutula JR Site-Specific Antibody Drug Conjugates for Cancer Therapy. MAbs 2014, 6 (1), 34–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (334).Zhou Q Site-Specific Antibody Conjugation for ADC and Beyond. Biomedicines 2017, 5, 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (335).Sochaj AM; Swiderska KW; Otlewski J Current Methods for the Synthesis of Homogeneous Antibody-Drug Conjugates. Biotechnol. Adv 2015, 33, 775–784. [DOI] [PubMed] [Google Scholar]
- (336).Schumacher D; Hackenberger CPR; Leonhardt H; Helma J Current Status: Site-Specific Antibody Drug Conjugates. J. Clin. Immunol 2016, 36, 100–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (337).Kiyoshi M; Tsumoto K; Ishii-Watabe A; Caaveiro JMM Glycosylation of IgG-Fc: a Molecular Perspective. Int. Immunol 2017, 29 (7), 311–317. [DOI] [PubMed] [Google Scholar]
- (338).Okeley NM; Toki BE; Zhang X; Jeffrey SC; Burke PJ; Alley SC; Senter PD Metabolic Engineering of Monoclonal Antibody Carbohydrates for Antibody-Drug Conjugation. Bioconjugate Chem 2013, 24 (10), 1650–1655. [DOI] [PubMed] [Google Scholar]
- (339).Ramakrishnan B; Qasba PK Structure-Based Design of Beta 1,4-Galactosyltransferase I (beta 4Gal-T1) With Equally Efficient N-Acetylgalactosaminyltransferase Activity: Point Mutation Broadens Beta 4Gal-T1 Donor Specificity. J. Biol. Chem 2002, 277 (23), 20833–20839. [DOI] [PubMed] [Google Scholar]
- (340).Ramakrishnan B; Boeggeman E; Pasek M; Qasba PK Bioconjugation Using Mutant Glycosyltransferases for the Site-Specific Labeling of Biomolecules With Sugars Carrying Chemical Handles. Methods Mol. Biol 2011, 751, 281–296. [DOI] [PubMed] [Google Scholar]
- (341).Zhu Z; Ramakrishnan B; Li J; Wang Y; Feng Y; Prabakaran P; Colantonio S; Dyba MA; Qasba PK; Dimitrov DS Site-Specific Antibody-Drug Conjugation Through an Engineered Glycotransferase and a Chemically Reactive Sugar. MAbs 2014, 6 (5), 1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (342).van Geel R; Wijdeven MA; Heesbeen R; Verkade JM; Wasiel AA; van Berkel SS; van Delft FL Chemoenzymatic Conjugation of Toxic Payloads to the Globally Conserved N-Glycan of Native mAbs. Provides Homogeneous and Highly Efficacious Antibody-Drug Conjugates. Bioconjugate Chem 2015, 26 (11), 2233–2242. [DOI] [PubMed] [Google Scholar]
- (343).Zhou Q; Stefano JE; Manning C; Kyazike J; Chen B; Gianolio DA; Park A; Busch M; Bird J; Zheng X; et al. Site-Specific Antibody-Drug Conjugation Through Glycoengineering. Bioconjugate Chem 2014, 25 (3), 510–520. [DOI] [PubMed] [Google Scholar]
- (344).Tang F; Wang LX; Huang W Chemoenzymatic Synthesis of Glycoengineered IgG Antibodies and Glycosite-Specific Antibody-Drug Conjugates. Nat. Protoc 2017, 12 (8), 1702–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (345).Li X; Fang T; Boons GJ Preparation of Well-Defined Antibody-Drug Conjugates Through Glycan Remodeling and Strain-Promoted Azide-Alkyne Cycloadditions. Angew. Chem., Int. Ed 2014, 53 (28), 7179–7182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (346).Heidelberger M; Avery OT The Soluble Specific Substance of Pneumococcus. J. Exp. Med 1923, 38 (1), 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (347).Heidelberger M; Dilapi MM; Siegel M; Walter AW Persistence of Antibodies in Human Subjects Injected With Pneumococcal Polysaccharides. J. Immunol 1950, 65, 535–541. [PubMed] [Google Scholar]
- (348).Dochez AR; Avery OT The Elaboration of Specific Soluble Substance by Pneumococcus During Growth. J. Exp. Med 1917, 26 (4), 477–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (349).Poolman JT Expanding the Role of Bacterial Vaccines into Life-Course Vaccination Strategies and Prevention of Antimicrobial-Resistant Infections. NPJ Vaccines 2020, 5, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (350).Snapper CM; Mond JJ A Model for Induction of T Cell-Independent Humoral Immunity in Response to Polysaccharide Antigens. J. Immunol 1996, 157, 2229–2233. [PubMed] [Google Scholar]
- (351).Cobb BA; Wang Q; Tzianabos AO; Kasper DL Polysaccharide Processing and Presentation by the MHCII Pathway. Cell 2004, 117 (5), 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (352).Avery OT; Goebel WF Chemo-Immunological Studies on Conjugated Carbohydrate-Proteins: V. The Immunological Specifity of an Antigen Prepared by Combining the Capsular Polysaccharide of Type III Pneumococcus With Foreign Protein. J. Exp. Med 1931, 54 (3), 437–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (353).Avery OT; Goebel WF Chemo-Immunological Studies on Conjugated Carbohydrate-Proteins: II. Immunological Specificity of Synthetic Sugar-Protein Antigens. J. Exp. Med 1929, 50 (4), 533–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (354).Oosterhuis-Kafeja F; Beutels P; Van Damme P Immunogenicity, Efficacy, Safety and Effectiveness of Pneumococcal Conjugate Vaccines (1998–2006). Vaccine 2007, 25 (12), 2194–2212. [DOI] [PubMed] [Google Scholar]
- (355).Nunes MC; Madhi SA Review on the Immunogenicity and Safety of PCV-13 in Infants and Toddlers. Expert Rev. Vaccines 2011, 10 (7), 951–980. [DOI] [PubMed] [Google Scholar]
- (356).Oligbu G Higher Valent Pneumococcal Conjugate Vaccines: is it a Roller Coaster? AIMS Public Health 2020, 7 (1), 29–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (357).Costantino P; Rappuoli R; Berti F The Design of Semi-Synthetic and Synthetic Glycoconjugate Vaccines. Expert Opin. Drug Discovery 2011, 6 (10), 1045–1066. [DOI] [PubMed] [Google Scholar]
- (358).Baek JY; Geissner A; Rathwell DCK; Meierhofer D; Pereira CL; Seeberger PH A Modular Synthetic Route to Size-Defined Immunogenic Haemophilus Influenzae B Antigens is Key to the Identification of an Octasaccharide Lead Vaccine Candidate. Chem. Sci 2018, 9 (5), 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (359).Rosenstein NE; Perkins BA; Stephens DS; Popovic T; Hughes JM Meningococcal Disease. N. Engl. J. Med 2001, 344 (18), 1378–1388. [DOI] [PubMed] [Google Scholar]
- (360).Harrison OB; Claus H; Jiang Y; Bennett JS; Bratcher HB; Jolley KA; Corton C; Care R; Poolman JT; Zollinger WD; et al. Description and Nomenclature of Neisseria meningitidis Capsule Locus. Emerg. Infect. Dis 2013, 19 (4), 566–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (361).Crum-Cianflone N; Sullivan E Meningococcal Vaccinations. Infect. Dis. Ther 2016, 5 (2), 89–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (362).Berkin A; Coxon B; Pozsgay V Towards a Synthetic Glycoconjugate Vaccine Against Neisseria meningitidis A. Chem.—Eur. J 2002, 8, 4424–4433. [DOI] [PubMed] [Google Scholar]
- (363).Slättegård R; Teodorovic P; Kinfe HH; Ravenscroft N; Gammon DW; Oscarson S Synthesis of Structures Corresponding to the Capsular Polysaccharide of Neisseria meningitidis Group A. Org. Biomol. Chem 2005, 3, 3782–3787. [DOI] [PubMed] [Google Scholar]
- (364).Berti F; Romano MR; Micoli F; Pinto V; Cappelletti E; Gavini M; Proietti D; Pluschke G; MacLennan CA; Costantino P Relative Stability of Meningococcal Serogroup A and X Polysaccharides. Vaccine 2012, 30 (45), 6409–6415. [DOI] [PubMed] [Google Scholar]
- (365).Gao Q; Zaccaria C; Tontini M; Poletti L; Costantino P; Lay L Synthesis and Preliminary Biological Evaluation of Carba Analogues From Neisseria meningitidis A Capsular Polysaccharide. Org. Biomol. Chem 2012, 10 (33), 6673–6681. [DOI] [PubMed] [Google Scholar]
- (366).Gao Q; Tontini M; Brogioni G; Nilo A; Filippini S; Harfouche C; Polito L; Romano MR; Costantino P; Berti F; et al. Immunoactivity of Protein Conjugates of Carba Analogues From Neisseria meningitidis a Capsular Polysaccharide. ACS Chem. Biol 2013, 8 (11), 2561–2567. [DOI] [PubMed] [Google Scholar]
- (367).Fallarini S; Buzzi B; Giovarruscio S; Polito L; Brogioni G; Tontini M; Berti F; Adamo R; Lay L; Lombardi G A Synthetic Disaccharide Analogue From Neisseria meningitidis A Capsular Polysaccharide Stimulates Immune Cell Responses and Induces Immunoglobulin G (IgG) Production in Mice When Protein-Conjugated. ACS Infect. Dis 2015, 1 (10), 487–496. [DOI] [PubMed] [Google Scholar]
- (368).Richmond P; Borrow R; Findlow J; Martin S; Thornton C; Cartwright K; Miller E Evaluation of De-O-Acetylated Meningococcal C Polysaccharide-Tetanus Toxoid Conjugate Vaccine in Infancy: Reactogenicity, Immunogenicity, Immunologic Priming, and Bactericidal Activity Against O-Acetylated and De-O-Acetylated Serogroup C Strains. Infect. Immun. Infect. Immun 2001, 69 (4), 2378–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (369).Liao G; Zhou Z; Guo Z Synthesis and Immunological Study of α−2,9-Oligosialic acid Conjugates as Anti-Group C Meningitis Vaccines. Chem. Commun. (Camb) 2015, 51 (47), 9647–9650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (370).Chu KC; Ren CT; Lu CP; Hsu CH; Sun TH; Han JL; Pal B; Chao TA; Lin YF; Wu SH; et al. Efficient and Stereoselective Synthesis of α(2→9) Oligosialic acids: From Monomers to Dodecamers. Angew. Chem., Int. Ed 2011, 50 (40), 9391–9395. [DOI] [PubMed] [Google Scholar]
- (371).Liao G; Zhou Z; Suryawanshi S; Mondal MA; Guo Z Fully Synthetic Self-Adjuvanting α−2,9-Oligosialic Acid Based Conjugate Vaccines Against Group C Meningitis. ACS Cent. Sci 2016, 2 (4), 210–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (372).Wang CH; Li ST; Lin TL; Cheng YY; Sun TH; Wang JT; Cheng TJ; Mong KK; Wong CH; Wu CY Synthesis of Neisseria meningitidis Serogroup W135 Capsular Oligosaccharides for Immunogenicity Comparison and Vaccine Development. Angew. Chem., Int. Ed 2013, 52 (35), 9157–9161. [DOI] [PubMed] [Google Scholar]
- (373).Morelli L; Cancogni D; Tontini M; Nilo A; Filippini S; Costantino P; Romano MR; Berti F; Adamo R; Lay L Synthesis and Immunological Evaluation of Protein Conjugates of Neisseria meningitidis X Capsular Polysaccharide Fragments. Beilstein J. Org. Chem 2014, 10, 2367–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (374).Harale KR; Dumare NB; Singh D; Misra AK; Chhikara MK Synthesis of a Tetrasaccharide and Its Glycoconjugate Corresponding to the Capsular Polysaccharide of Neisseria meningitidis Serogroup X and Its Immunochemical Studies. RSC adv 2015, 5, 41332–41340. [Google Scholar]
- (375).Oldrini D; Fiebig T; Romano MR; Proietti D; Berger M; Tontini M; De Ricco R; Santini L; Morelli L; Lay L; et al. Combined Chemical Synthesis and Tailored Enzymatic Elongation Provide Fully Synthetic and Conjugation-Ready Neisseria meningitidis Serogroup X Vaccine Antigens. ACS Chem. Biol 2018, 13 (4), 984–994. [DOI] [PubMed] [Google Scholar]
- (376).Geno KA; Gilbert GL; Song JY; Skovsted IC; Klugman KP; Jones C; Konradsen HB; Nahm MH Pneumococcal Capsules and Their Types: Past, Present, and Future. Clin. Microbiol. Rev 2015, 28 (3), 871–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (377).Rupp R; Hurley D; Grayson S; Li J; Nolan K; McFetridge RD; Hartzel J; Abeygunawardana C; Winters M; Pujar H; et al. A Dose Ranging Study of 2 Different Formulations of 15-Valent Pneumococcal Conjugate Vaccine (PCV15) in Healthy Infants. Hum. Vaccin. Immunother 2019, 15 (3), 549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (378).Seeberger PH Discovery of Semi- and Fully-Synthetic Carbohydrate Vaccines Against Bacterial Infections Using a Medicinal Chemistry Approach. Chem. Rev 2021, 121 (7), 3598–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (379).Anish C; Schumann B; Pereira CL; Seeberger PH Chemical Biology Approaches to Designing Defined Carbohydrate Vaccines. Chem. Biol 2014, 21 (1), 38–50. [DOI] [PubMed] [Google Scholar]
- (380).Gening ML; Kurbatova EA; Nifantiev NE Synthetic Analogs of Streptococcus pneumoniae Capsular Polysaccharides and Immunogenic Activities of Glycoconjugates. Russ. J. Bioorg. Chem 2021, 47 (1), 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (381).Lisboa MP; Khan N; Martin C; Xu FF; Reppe K; Geissner A; Govindan S; Witzenrath M; Pereira CL; Seeberger PH Semisynthetic Glycoconjugate Vaccine Candidate Against Streptococcus pneumoniae Serotype 5. Proc. Natl. Acad. Sci. U.S.A 2017, 114 (42), 11063–11068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (382).Jansen WT; Hogenboom S; Thijssen MJ; Kamerling JP; Vliegenthart JF; Verhoef J; Snippe H; Verheul AF Synthetic 6B Di-, Tri-, and Tetrasaccharide-Protein Conjugates Contain Pneumococcal Type 6A and 6B Common and 6B-Specific Epitopes That Elicit Protective Antibodies in Mice. Infect. Immun 2001, 69 (2), 787–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (383).van Steijn AM; Kamerling JP; Vliegenthart JF Synthesis of a Spacer-Containing Repeating Unit of the Capsular Polysaccharide of Streptococcus pneumoniae Type 23F. Carbohydr. Res 1991, 211 (2), 261–277. [DOI] [PubMed] [Google Scholar]
- (384).Pozsgay V; Chu C; Pannell L; Wolfe J; Robbins JB; Schneerson R Protein Conjugates of Synthetic Saccharides Elicit Higher Levels of Serum IgG Lipopolysaccharide Antibodies in Mice Than Do Those of the O-Specific Polysaccharide From Shigella dysenteriae Type 1. Proc. Natl. Acad. Sci. U.S.A 1999, 96 (9), 5194–5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (385).Phalipon A; Tanguy M; Grandjean C; Guerreiro C; Bélot F; Cohen D; Sansonetti PJ; Mulard LA A Synthetic Carbohydrate-Protein Conjugate Vaccine Candidate Against Shigella flexneri 2a Infection. J. Immunol 2009, 182 (4), 2241–2247. [DOI] [PubMed] [Google Scholar]
- (386).Tamborrini M; Werz DB; Frey J; Pluschke G; Seeberger PH Anti-Carbohydrate Antibodies for the Detection of Anthrax Spores. Angew. Chem., Int. Ed 2006, 45 (39), 6581–6582. [DOI] [PubMed] [Google Scholar]
- (387).Mehta AS; Saile E; Zhong W; Buskas T; Carlson R; Kannenberg E; Reed Y; Quinn CP; Boons GJ Synthesis and Antigenic Analysis of the BclA Glycoprotein Oligosaccharide From the Bacillus anthracis Exosporium. Chem.—Eur. J 2006, 12, 9136–9149. [DOI] [PubMed] [Google Scholar]
- (388).Adamo R; Romano MR; Berti F; Leuzzi R; Tontini M; Danieli E; Cappelletti E; Cakici OS; Swennen E; Pinto V; et al. Phosphorylation of the Synthetic Hexasaccharide Repeating Unit is Essential for the Induction of Antibodies to Clostridium difficile PSII Cell Wall Polysaccharide. ACS Chem. Biol 2012, 7 (8), 1420–1428. [DOI] [PubMed] [Google Scholar]
- (389).Oberli MA; Hecht ML; Bindschädler P; Adibekian A; Adam T; Seeberger PH A Possible Oligosaccharide-Conjugate Vaccine Candidate for Clostridium difficile is Antigenic and Immunogenic. Chem. Biol 2011, 18 (5), 580–588. [DOI] [PubMed] [Google Scholar]
- (390).Wu NC; Wilson IA Influenza Hemagglutinin Structures and Antibody Recognition. Cold Spring Harb. Perspect. Med 2020, 10 (8), a038778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (391).Al Khatib HA; Al Thani AA; Gallouzi I; Yassine HM Epidemiological and Genetic Characterization of pH1N1 and H3N2 Influenza Viruses Circulated in MENA Region During 2009–2017. BMC Infect. Dis 2019, 19, 314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (392).Wu CY; Lin CW; Tsai TI; Lee CD; Chuang HY; Chen JB; Tsai MH; Chen BR; Lo PW; Liu CP; et al. Influenza A Surface Glycosylation and Vaccine Design. Proc. Natl. Acad. Sci. U.S.A 2017, 114 (2), 280–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (393).Chen JR; Yu YH; Tseng YC; Chiang WL; Chiang MF; Ko YA; Chiu YK; Ma HH; Wu CY; Jan JT; et al. Vaccination of Monoglycosylated Hemagglutinin Induces Cross-Strain Protection Against Influenza Virus Infections. Proc. Natl. Acad. Sci. U.S.A 2014, 111 (7), 2476–2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (394).Wang CC; Chen JR; Tseng YC; Hsu CH; Hung YF; Chen SW; Chen CM; Khoo KH; Cheng TJ; Cheng YS; et al. Glycans on Influenza Hemagglutinin Affect Receptor Binding and Immune Response. Proc. Natl. Acad. Sci. U.S.A 2009, 106 (43), 18137–18142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (395).Tseng YC; Wu CY; Liu ML; Chen TH; Chiang WL; Yu YH; Jan JT; Lin KI; Wong CH; Ma C Egg-Based Influenza Split Virus Vaccine With Monoglycosylation Induces Cross-Strain Protection Against Influenza Virus Infections. Proc. Natl. Acad. Sci. U.S.A 2019, 116 (10), 4200–4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (396).Wu CY; Chuang HY; Wong CH Influenza Virus Neuraminidase Regulates Host CD8(+) T-Cell Response in Mice. Commun. Biol 2020, 3, 748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (397).Wang SC; Liao HY; Zhang JY; Cheng TR; Wong CH Development of a Universal Influenza Vaccine Using Hemagglutinin Stem Protein Produced From Pichia pastoris. Virology 2019, 526, 125–137. [DOI] [PubMed] [Google Scholar]
- (398).Liao HY; Wang SC; Ko YA; Lin KI; Ma C; Cheng TR; Wong CH Chimeric Hemagglutinin Vaccine Elicits Broadly Protective CD4 and CD8 T Cell Responses Against Multiple Influenza Strains and Subtypes. Proc. Natl. Acad. Sci. U.S.A 2020, 117 (30), 17757–17763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (399).Huang HY; Liao HY; Chen X; Wang SW; Cheng CW; Shahed-Al-Mahmud M; Liu YM; Mohapatra A; Chen TH; Lo JM; et al. Vaccination with SARS-CoV-2 Spike Protein Lacking Glycan Shields Elicits Enhanced Protective Responses in Animal Models. Sci. Transl. Med 2022, 14, No. eabm0899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (400).Wu CY; Cheng CW; Kung CC; Liao KS; Jan JT; Ma C; Wong CH Glycosite-Deleted mRNA of SARS-CoV-2 Spike Protein as a Broad-Spectrum Vaccine. Proc. Natl. Acad. Sci. U.S.A 2022, 119, No. e2119995119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (401).Glavey SV; Huynh D; Reagan MR; Manier S; Moschetta M; Kawano Y; Roccaro AM; Ghobrial IM; Joshi L; O’Dwyer ME The Cancer Glycome: Carbohydrates as Mediators of Metastasis. Blood Rev 2015, 29 (4), 269–279. [DOI] [PubMed] [Google Scholar]
- (402).Nardy AF; Freire-de-Lima L; Freire-de-Lima CG; Morrot A The Sweet Side of Immune Evasion: Role of Glycans in the Mechanisms of Cancer Progression. Front. Oncol 2016, 6, 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (403).Schietinger A; Philip M; Schreiber H Specificity in Cancer Immunotherapy. Semin. Immunol 2008, 20 (5), 276–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (404).Hakomori S Antigen Structure and Genetic Basis of Histo-Blood Groups A, B and O: Their Changes Associated With Human Cancer. Biochim. Biophys. Acta 1999, 1473 (1), 247–266. [DOI] [PubMed] [Google Scholar]
- (405).Hattrup CL; Gendler SJ Structure and Function of the Cell Surface (tethered) Mucins. Annu. Rev. Physiol 2008, 70, 431–457. [DOI] [PubMed] [Google Scholar]
- (406).Heimburg-Molinaro J; Lum M; Vijay G; Jain M; Almogren A; Rittenhouse-Olson K Cancer Vaccines and Carbohydrate Epitopes. Vaccine 2011, 29 (48), 8802–8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (407).Hamilton WB; Helling F; Lloyd KO; Livingston PO Ganglioside Expression on Human Malignant Melanoma Assessed by Quantitative Immune Thin-Layer Chromatography. Int. J. Cancer 1993, 53 (4), 566–573. [DOI] [PubMed] [Google Scholar]
- (408).Hevey R; Ling CC Recent Advances in Developing Synthetic Carbohydrate-Based Vaccines for Cancer Immunotherapies. Future Med. Chem 2012, 4 (4), 545–584. [DOI] [PubMed] [Google Scholar]
- (409).Zhu J; Warren JD; Danishefsky SJ Synthetic Carbohydrate-Based Anticancer Vaccines: the Memorial Sloan-Kettering Experience. Expert Rev. Vaccines 2009, 8 (10), 1399–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (410).Fernández-Tejada A; Cañada FJ; Jiménez-Barbero J Recent Developments in Synthetic Carbohydrate-Based Diagnostics, Vaccines, and Therapeutics. Chem.—Eur. J 2015, 21, 10616–10628. [DOI] [PubMed] [Google Scholar]
- (411).Hossain F; Andreana PR Developments in Carbohydrate-Based Cancer Therapeutics. Pharmaceuticals (Basel) 2019, 12 (2), 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (412).Huo CX; Ye XS Recent Advance in Carbohydrate-Based Cancer Vaccines. Acta Pharm. Sin 2012, 47 (3), 261–270. [PubMed] [Google Scholar]
- (413).Liu CC; Ye XS Carbohydrate-Based Cancer Vaccines: Target Cancer With Sugar Bullets. Glycoconj. J 2012, 29 (5–6), 259–271. [DOI] [PubMed] [Google Scholar]
- (414).Yin Z; Huang X Recent Development in Carbohydrate Based Anti-cancer Vaccines. J. Carbohydr. Chem 2012, 31 (3), 143–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (415).Franco A Glycoconjugates as Vaccines for Cancer Immunotherapy: Clinical Trials and Future Directions. Anticancer Agents Med. Chem 2008, 8 (1), 86–91. [DOI] [PubMed] [Google Scholar]
- (416).Chapman PB; Morrissey DM; Panageas KS; Hamilton WB; Zhan C; Destro AN; Williams L; Israel RJ; Livingston PO Induction of Antibodies Against GM2 Ganglioside by Immunizing Melanoma Patients Using GM2-Keyhole Limpet Hemocyanin + QS21 Vaccine: a Dose-Response Study. Clin. Cancer Res 2000, 6, 874–879. [PubMed] [Google Scholar]
- (417).Holmberg LA; Sandmaier BM Vaccination With Theratope (STn-KLH) as Treatment for Breast Cancer. Expert Rev. Vaccines 2004, 3 (6), 655–663. [DOI] [PubMed] [Google Scholar]
- (418).Zhang S; Walberg LA; Ogata S; Itzkowitz SH; Koganty RR; Reddish M; Gandhi SS; Longenecker BM; Lloyd KO; Livingston PO Immune Sera and Monoclonal Antibodies Define Two Configurations for the Sialyl Tn Tumor Antigen. Cancer. Res 1995, 55, 3364–3368. [PubMed] [Google Scholar]
- (419).Adluri S; Helling F; Ogata S; Zhang S; Itzkowitz SH; Lloyd KO; Livingston PO Immunogenicity of Synthetic TF-KLH (Keyhole Limpet Hemocyanin) and sTn-KLH Conjugates in Colorectal Carcinoma Patients. Cancer Immunol. Immunother 1995, 41 (3), 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (420).Carlstedt I; Davies JR Glycoconjugates Facing the Outside World. Biochem. Soc. Trans 1997, 25 (1), 214–219. [DOI] [PubMed] [Google Scholar]
- (421).Slovin SF; Ragupathi G; Musselli C; Olkiewicz K; Verbel D; Kuduk SD; Schwarz JB; Sames D; Danishefsky S; Livingston PO; et al. Fully Synthetic Carbohydrate-Based Vaccines in Biochemically Relapsed Prostate Cancer: clinical Trial Results With Alpha-N-Acetylgalactosamine-O-Serine/Threonine Conjugate Vaccine. J. Clin. Oncol 2003, 21 (23), 4292–4298. [DOI] [PubMed] [Google Scholar]
- (422).Slovin SF; Ragupathi G; Musselli C; Fernandez C; Diani M; Verbel D; Danishefsky S; Livingston P; Scher HI Thomsen-Friedenreich (TF) Antigen as a Target for Prostate Cancer Vaccine: Clinical Trial Results With TF Cluster (c)-KLH Plus QS21 Conjugate Vaccine in Patients With Biochemically Relapsed Prostate Cancer. Cancer Immunol. Immunother 2005, 54 (7), 694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (423).Zhu J; Wan Q; Ragupathi G; George CM; Livingston PO; Danishefsky SJ Biologics Through Chemistry: Total Synthesis of a Proposed Dual-acting Vaccine Targeting Ovarian Cancer by Orchestration of Oligosaccharide and Polypeptide Domains. J. Am. Chem. Soc 2009, 131 (11), 4151–4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (424).Livingston P The Unfulfilled Promise of Melanoma Vaccines. Clin. Cancer Res 2001, 7, 1837–1838. [PubMed] [Google Scholar]
- (425).Ragupathi G; Cappello S; Yi SS; Canter D; Spassova M; Bornmann WG; Danishefsky SJ; Livingston PO Comparison of Antibody Titers After Immunization With Monovalent or Tetravalent KLH Conjugate Vaccines. Vaccine 2002, 20 (7–8), 1030–1038. [DOI] [PubMed] [Google Scholar]
- (426).Ragupathi G; Koide F; Sathyan N; Kagan E; Spassova M; Bornmann W; Gregor P; Reis CA; Clausen H; Danishefsky SJ; et al. A Preclinical Study Comparing Approaches for Augmenting the Immunogenicity of a Heptavalent KLH-Conjugate Vaccine Against Epithelial Cancers. Cancer Immunol. Immunother 2003, 52 (10), 608–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (427).Allen JR; Harris CR; Danishefsky SJ Pursuit of Optimal Carbohydrate-Based Anticancer Vaccines: Preparation of a Multiantigenic Unimolecular Glycopeptide Containing the Tn, MBr1, and Lewis(y) Antigens. J. Am. Chem. Soc 2001, 123 (9), 1890–1897. [DOI] [PubMed] [Google Scholar]
- (428).Keding SJ; Danishefsky SJ Prospects for Total Synthesis: a Vision for a Totally Synthetic Vaccine Targeting Epithelial Tumors. Proc. Natl. Acad. Sci. U.S.A 2004, 101 (33), 11937–11942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (429).Ragupathi G; Koide F; Livingston PO; Cho YS; Endo A; Wan Q; Spassova MK; Keding SJ; Allen J; Ouerfelli O; et al. Preparation and Evaluation of Unimolecular Pentavalent and Hexavalent Antigenic Constructs Targeting Prostate and Breast Cancer: a Synthetic Route to Anticancer Vaccine Candidates. J. Am. Chem. Soc 2006, 128 (8), 2715–2725. [DOI] [PubMed] [Google Scholar]
- (430).Roy R New Trends in Carbohydrate-Based Vaccines. Drug Discovery Today Technol 2004, 1 (3), 327–336. [DOI] [PubMed] [Google Scholar]
- (431).Schutze MP; Leclerc C; Jolivet M; Audibert F; Chedid L Carrier-Induced Epitopic Suppression, a Major Issue for Future Synthetic Vaccines. J. Immunol 1985, 135, 2319–2322. [PubMed] [Google Scholar]
- (432).Toyokuni T; Hakomori S; Singhal AK Synthetic Carbohydrate Vaccines: Synthesis and Immunogenicity of Tn Antigen Conjugates. Bioorg. Med. Chem 1994, 2 (11), 1119–1132. [DOI] [PubMed] [Google Scholar]
- (433).Kudryashov V; Glunz PW; Williams LJ; Hintermann S; Danishefsky SJ; Lloyd KO Toward Optimized Carbohydrate-Based Anticancer Vaccines: Epitope Clustering, Carrier Structure, and Adjuvant All Influence Antibody Responses to Lewis(y) Conjugates in Mice. Proc. Natl. Acad. Sci. U.S.A 2001, 98 (6), 3264–3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (434).Lo-Man R; Vichier-Guerre S; Bay S; Dériaud E; Cantacuzene D; Leclerc C Anti-Tumor Immunity Provided by a Synthetic Multiple Antigenic Glycopeptide Displaying a Tri-Tn Glycotope. J. Immunol 2001, 166 (4), 2849–2854. [DOI] [PubMed] [Google Scholar]
- (435).Vichier-Guerre S; Lo-Man R; Bay S; Deriaud E; Nakada H; Leclerc C; Cantacuzene D Short Synthetic Glycopeptides Successfully Induce Antibody Responses to Carcinoma-Associated Tn Antigen. J. Pept. Res 2000, 55 (2), 173–180. [DOI] [PubMed] [Google Scholar]
- (436).Lo-Man R; Vichier-Guerre S; Perraut R; Dériaud E; Huteau V; BenMohamed L; Diop OM; Livingston PO; Bay S; Leclerc C A Fully Synthetic Therapeutic Vaccine Candidate Targeting Carcinoma-Associated Tn Carbohydrate Antigen Induces Tumor-Specific Antibodies in Nonhuman Primates. Cancer. Res 2004, 64 (14), 4987–4994. [DOI] [PubMed] [Google Scholar]
- (437).Grigalevicius S; Chierici S; Renaudet O; Lo-Man R; Dériaud E; Leclerc C; Dumy P Chemoselective Assembly and Immunological Evaluation of Multiepitopic Glycoconjugates Bearing Clustered Tn Antigen as Synthetic Anticancer Vaccines. Bioconjugate Chem 2005, 16 (5), 1149–1159. [DOI] [PubMed] [Google Scholar]
- (438).Cremer GA; Bureaud N; Piller V; Kunz H; Piller F; Delmas AF Synthesis and Biological Evaluation of a Multiantigenic Tn/TF-Containing Glycopeptide Mimic of the Tumor-Related MUC1 Glycoprotein. ChemMedChem 2006, 1 (9), 965–968. [DOI] [PubMed] [Google Scholar]
- (439).Alexander J; del Guercio MF; Maewal A; Qiao L; Fikes J; Chesnut RW; Paulson J; Bundle DR; DeFrees S; Sette A Linear PADRE T Helper Epitope and Carbohydrate B Cell Epitope Conjugates Induce Specific High Titer IgG Antibody Responses. J. Immunol 2000, 164 (3), 1625–1633. [DOI] [PubMed] [Google Scholar]
- (440).Dziadek S; Hobel A; Schmitt E; Kunz H A Fully Synthetic Vaccine Consisting of a Tumor-Associated Glycopeptide Antigen and a T-cell Epitope for the Induction of a Highly Specific Humoral Immune Response. Angew. Chem., Int. Ed 2005, 44 (46), 7630–7635. [DOI] [PubMed] [Google Scholar]
- (441).Westerlind U; Hobel A; Gaidzik N; Schmitt E; Kunz H Synthetic Vaccines Consisting of Tumor-Associated MUC1 Glycopeptide Antigens and a T-Cell Epitope for the Induction of a Highly Specific Humoral Immune Response. Angew. Chem., Int. Ed 2008, 47 (39), 7551–7556. [DOI] [PubMed] [Google Scholar]
- (442).Kaiser A; Gaidzik N; Becker T; Menge C; Groh K; Cai H; Li YM; Gerlitzki B; Schmitt E; Kunz H Fully Synthetic Vaccines Consisting of Tumor-Associated MUC1 Glycopeptides and a Lipopeptide Ligand of the Toll-Like Receptor 2. Angew. Chem., Int. Ed 2010, 49 (21), 3688–3692. [DOI] [PubMed] [Google Scholar]
- (443).Zhou Z; Mondal M; Liao G; Guo Z Synthesis and Evaluation Of Monophosphoryl Lipid A Derivatives as Fully Synthetic Self-Adjuvanting Glycoconjugate Cancer Vaccine Carriers. Org. Biomol. Chem 2014, 12 (20), 3238–3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (444).Zhou Z; Liao G; Mandal SS; Suryawanshi S; Guo Z A Fully Synthetic Self-Adjuvanting Globo H-Based Vaccine Elicited Strong T Cell-Mediated Antitumor Immunity. Chem. Sci 2015, 6 (12), 7112–7121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (445).Zhou Z; Mandal SS; Liao G; Guo J; Guo Z Synthesis and Evaluation of GM2-Monophosphoryl Lipid A Conjugate as a Fully Synthetic Self-Adjuvant Cancer Vaccine. Sci. Rep 2017, 7, 11403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (446).De Silva RA; Wang Q; Chidley T; Appulage DK; Andreana PR Immunological Response From an Entirely Carbohydrate Antigen: Design of Synthetic Vaccines Based on Tn-PS A1 Conjugates. J. Am. Chem. Soc 2009, 131 (28), 9622–9623. [DOI] [PubMed] [Google Scholar]
- (447).Shi M; Kleski KA; Trabbic KR; Bourgault JP; Andreana PR Sialyl-Tn Polysaccharide A1 as an Entirely Carbohydrate Immunogen: Synthesis and Immunological Evaluation. J. Am. Chem. Soc 2016, 138 (43), 14264–14272. [DOI] [PubMed] [Google Scholar]
- (448).Ingale S; Wolfert MA; Gaekwad J; Buskas T; Boons GJ Robust Immune Responses Elicited by a Fully Synthetic Three-Component Vaccine. Nat. Chem. Biol 2007, 3 (10), 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (449).Renaudet O; BenMohamed L; Dasgupta G; Bettahi I; Dumy P Towards a Self-Adjuvanting Multivalent B and T Cell Epitope Containing Synthetic Glycolipopeptide Cancer Vaccine. ChemMedChem 2008, 3 (5), 737–741. [DOI] [PubMed] [Google Scholar]
- (450).Bettahi I; Dasgupta G; Renaudet O; Chentoufi AA; Zhang X; Carpenter D; Yoon S; Dumy P; BenMohamed L Antitumor Activity of a Self-Adjuvanting Glyco-Lipopeptide Vaccine Bearing B Cell, CD4+ and CD8+ T Cell Epitopes. Cancer Immunol. Immunother 2009, 58 (2), 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (451).Danishefsky SJ; Shue YK; Chang MN; Wong CH Development of Globo-H Cancer Vaccine. Acc. Chem. Res 2015, 48 (3), 643–652. [DOI] [PubMed] [Google Scholar]
- (452).O’Cearbhaill RE; Deng W; Chen LM; Lucci JA 3rd; Behbakht K; Spirtos NM; Muller CY; Benigno BB; Powell MA; Berry E; et al. A Phase II Randomized, Double-Blind Trial of a Polyvalent Vaccine-KLH Conjugate (NSC 748933 IND# 14384) + OPT-821 Versus OPT-821 in Patients with Epithelial Ovarian, Fallopian Tube, or Peritoneal Cancer Who are in Second or Third Complete Remission: An NRG Oncology/GOG Study. Gynecol. Oncol 2019, 155 (3), 393–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (453).Huang CS; Yu AL; Tseng LM; Chow LWC; Hou MF; Hurvitz SA; Schwab RBJLM; Chang HK; Chang HT; et al. Globo H-KLH Vaccine Adagloxad simolenin (OBI-822)/OBI-821 in Patients With Metastatic Breast Cancer: Phase II Randomized, Placebo-Controlled Study. J. Immunother. Cancer 2020, 8 (2), No. e000342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (454).Huang YL; Hung JT; Cheung SK; Lee HY; Chu KC; Li ST; Lin YC; Ren CT; Cheng TJ; Hsu TL; et al. Carbohydrate-Based Vaccines with a Glycolipid Adjuvant for Breast Cancer. Proc. Natl. Acad. Sci. U.S.A 2013, 110 (7), 2517–2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (455).Zheng XJ; Yang F; Zheng M; Huo CX; Zhang Y; Ye XS Improvement of the Immune Efficacy of Carbohydrate Vaccines by Chemical Modification on the GM3 Antigen. Org. Biomol. Chem 2015, 13 (22), 6399–6406. [DOI] [PubMed] [Google Scholar]
- (456).Yang F; Zheng XJ; Huo CX; Wang Y; Zhang Y; Ye XS Enhancement of the Immunogenicity of Synthetic Carbohydrate Vaccines by Chemical Modifications of STn Antigen. ACS Chem. Biol 2011, 6 (3), 252–259. [DOI] [PubMed] [Google Scholar]
- (457).Zhai C; Zheng XJ; Song C; Ye XS Synthesis and Immunological Evaluation of N-Acyl Modified Globo H Derivatives as Anticancer Vaccine Candidates. RSC Med. Chem 2021, 12 (7), 1239–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (458).Lee HY; Chen CY; Tsai TI; Li ST; Lin KH; Cheng YY; Ren CT; Cheng TJ; Wu CY; Wong CH Immunogenicity Study of Globo H Analogues With Modification at the Reducing or Nonreducing End of the Tumor Antigen. J. Am. Chem. Soc 2014, 136 (48), 16844–16853. [DOI] [PubMed] [Google Scholar]
- (459).Rathore U; Saha P; Kesavardhana S; Kumar AA; Datta R; Devanarayanan S; Das R; Mascola JR; Varadarajan R Glycosylation of the Core of the HIV-1 Envelope Subunit Protein GP120 is Not Required for Native Trimer Formation or Viral Infectivity. J. Biol. Chem 2017, 292 (24), 10197–10219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (460).Panico M; Bouché L; Binet D; O’Connor MJ; Rahman D; Pang PC; Canis K; North SJ; Desrosiers RC; Chertova E; et al. Mapping the Complete Glycoproteome of Virion-Derived HIV-1 GP120 Provides Insights into Broadly Neutralizing Antibody Binding. Sci. Rep 2016, 6, 32956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (461).Doores KJ The HIV Glycan Shield as a Target for Broadly Neutralizing Antibodies. FEBS J 2015, 282 (24), 4679–4691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (462).Qi Y; Jo S; Im W Roles of Glycans in Interactions Between GP120 and HIV Broadly Neutralizing Antibodies. Glycobiology 2016, 26, 251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (463).Liu Y; Cao W; Sun M; Li T Broadly Neutralizing Antibodies for HIV-1: Efficacies, Challenges and Opportunities. Emerg. Microbes. Infect 2020, 9 (1), 194–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (464).Pejchal R; Doores KJ; Walker LM; Khayat R; Huang PS; Wang SK; Stanfield RL; Julien JP; Ramos A; Crispin M; et al. A Potent and Broad Neutralizing Antibody Recognizes and Penetrates the HIV Glycan Shield. Science 2011, 334 (6059), 1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (465).Pancera M; Shahzad-Ul-Hussan S; Doria-Rose NA; McLellan JS; Bailer RT; Dai K; Loesgen S; Louder MK; Staupe RP; Yang Y; et al. Structural Basis for Diverse N-Glycan Recognition by HIV-1-Neutralizing V1-V2-Directed Antibody PG16. Nat. Struct. Mol. Biol 2013, 20 (7), 804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (466).McLellan JS; Pancera M; Carrico C; Gorman J; Julien JP; Khayat R; Louder R; Pejchal R; Sastry M; Dai K; et al. Structure of HIV-1 GP120 V1/V2 Domain with Broadly Neutralizing Antibody PG9. Nature 2011, 480 (7377), 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (467).Zhou T; Xu K Structural Features of Broadly Neutralizing Antibodies and Rational Design of Vaccine. Adv. Exp. Med. Biol 2018, 1075, 73–95. [DOI] [PubMed] [Google Scholar]
- (468).Sok D; Burton DR Recent Progress in Broadly Neutralizing Antibodies to HIV. Nat. Immunol 2018, 19 (11), 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (469).Trkola A; Purtscher M; Muster T; Ballaun C; Buchacher A; Sullivan N; Srinivasan K; Sodroski J; Moore JP; Katinger H Human Monoclonal Antibody 2G12 Defines a Distinctive Neutralization Epitope on the GP120 Glycoprotein of Human Immunodeficiency Virus Type 1. J. Virol 1996, 70 (2), 1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (470).Scanlan CN; Pantophlet R; Wormald MR; Ollmann Saphire E; Stanfield R; Wilson IA; Katinger H; Dwek RA; Rudd PM; Burton DR The Broadly Neutralizing Anti-Human Immunodeficiency Virus Type 1 Antibody 2G12 Recognizes a Cluster of Alpha1->2 Mannose Residues on the Outer Face of GP120. J. Virol 2002, 76 (14), 7306–7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (471).Zhou T; Xu L; Dey B; Hessell AJ; Van Ryk D; Xiang SH; Yang X; Zhang MY; Zwick MB; Arthos J; et al. Structural Definition of a Conserved Neutralization Epitope on HIV-1 GP120. Nature 2007, 445 (7129), 732–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (472).Mascola JR; Stiegler G; VanCott TC; Katinger H; Carpenter CB; Hanson CE; Beary H; Hayes D; Frankel SS; Birx DL; et al. Protection of Macaques Against Vaginal Transmission of a Pathogenic HIV-1/SIV Chimeric Virus by Passive Infusion of Neutralizing Antibodies. Nat. Med 2000, 6 (2), 207–210. [DOI] [PubMed] [Google Scholar]
- (473).Hessell AJ; Rakasz EG; Poignard P; Hangartner L; Landucci G; Forthal DN; Koff WC; Watkins DI; Burton DR Broadly Neutralizing Human Anti-HIV Antibody 2G12 is Effective in Protection Against Mucosal SHIV Challenge Even at Low Serum Neutralizing Titers. PLoS Pathog 2009, 5 (5), No. e1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (474).Mahomed S; Garrett N; Baxter C; Abdool Karim Q; Abdool Karim SS Clinical Trials of Broadly Neutralizing Monoclonal Antibodies for Human Immunodeficiency Virus Prevention: A Review. J. Infect. Dis 2021, 223 (3), 370–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (475).Alam SM; Aussedat B; Vohra Y; Meyerhoff RR; Cale EM; Walkowicz WE; Radakovich NA; Anasti K; Armand L; Parks R; et al. Mimicry of an HIV Broadly Neutralizing Antibody Epitope With a Synthetic Glycopeptide. Sci. Transl. Med 2017, 9, No. eaai7521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (476).Sanders RW; Venturi M; Schiffner L; Kalyanaraman R; Katinger H; Lloyd KO; Kwong PD; Moore JP The Mannose-Dependent Epitope for Neutralizing Antibody 2G12 on Human Immunodeficiency Virus Type 1 Glycoprotein GP120. J. Virol 2002, 76 (14), 7293–7305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (477).Li H; Wang LX Design and Synthesis of a Template-Assembled Oligomannose Cluster as an Epitope Mimic for Human HIV-Neutralizing Antibody 2G12. Org. Biomol. Chem 2004, 2 (4), 483–488. [DOI] [PubMed] [Google Scholar]
- (478).Wang LX; Ni J; Singh S; Li H Binding of High-Mannose-Type Oligosaccharides and Synthetic Oligomannose Clusters to Human Antibody 2G12: Implications for HIV-1 Vaccine Design. Chem. Biol 2004, 11, 127–134. [DOI] [PubMed] [Google Scholar]
- (479).Ni J; Song H; Wang Y; Stamatos NM; Wang LX Toward a Carbohydrate-Based HIV-1 Vaccine: Synthesis and Immunological Studies of Oligomannose-Containing Glycoconjugates. Bioconjugate Chem 2006, 17 (2), 493–500. [DOI] [PubMed] [Google Scholar]
- (480).Wang J; Li H; Zou G; Wang LX Novel Template-Assembled Oligosaccharide Clusters as Epitope Mimics for HIV-Neutralizing Antibody 2G12. Design, Synthesis, and Antibody Binding Study. Org. Biomol. Chem 2007, 5 (10), 1529–1540. [DOI] [PubMed] [Google Scholar]
- (481).Dudkin VY; Orlova M; Geng X; Mandal M; Olson WC; Danishefsky SJ Toward Fully Synthetic Carbohydrate-Based HIV Antigen Design: on the Critical Role of Bivalency. J. Am. Chem. Soc 2004, 126 (31), 9560–9562. [DOI] [PubMed] [Google Scholar]
- (482).Krauss IJ; Joyce JG; Finnefrock AC; Song HC; Dudkin VY; Geng X; Warren JD; Chastain M; Shiver JW; Danishefsky SJ Fully Synthetic Carbohydrate HIV Antigens Designed on the Logic of the 2G12 Antibody. J. Am. Chem. Soc 2007, 129 (36), 11042–11044. [DOI] [PubMed] [Google Scholar]
- (483).Joyce JG; Krauss IJ; Song HC; Opalka DW; Grimm KM; Nahas DD; Esser MT; Hrin R; Feng M; Dudkin VY; et al. An Oligosaccharide-Based HIV-1 2G12 Mimotope Vaccine Induces Carbohydrate-Specific Antibodies That Fail to Neutralize HIV-1 Virions. Proc. Natl. Acad. Sci. U.S.A 2008, 105 (41), 15684–15689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (484).Wang SK; Liang PH; Astronomo RD; Hsu TL; Hsieh SL; Burton DR; Wong CH Targeting the Carbohydrates on HIV-1: Interaction of oligomannose Dendrons With Human Monoclonal Antibody 2G12 and DC-SIGN. Proc. Natl. Acad. Sci. U.S.A 2008, 105 (10), 3690–3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (485).Kabanova A; Adamo R; Proietti D; Berti F; Tontini M; Rappuoli R; Costantino P Preparation, Characterization and Immunogenicity of HIV-1 Related High-Mannose Oligosaccharides-CRM197 Glycoconjugates. Glycoconj. J 2010, 27 (5), 501–513. [DOI] [PubMed] [Google Scholar]
- (486).Astronomo RD; Lee HK; Scanlan CN; Pantophlet R; Huang CY; Wilson IA; Blixt O; Dwek RA; Wong CH; Burton DR A Glycoconjugate Antigen Based on the Recognition Motif of a Broadly Neutralizing Human Immunodeficiency Virus Antibody, 2G12, is Immunogenic But Elicits Antibodies Unable to Bind to the Self Glycans of GP120. J. Virol 2008, 82 (13), 6359–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (487).Kozlovska TM; Cielens I; Vasiljeva I; Strelnikova A; Kazaks A; Dislers A; Dreilina D; Ose V; Gusars I; Pumpens P RNA Phage Q Beta Coat Protein as a Carrier for Foreign Epitopes. Intervirology 2004, 39 (1–2), 9–15. [DOI] [PubMed] [Google Scholar]
- (488).Astronomo RD; Kaltgrad E; Udit AK; Wang SK; Doores KJ; Huang CY; Pantophlet R; Paulson JC; Wong CH; Finn MG; et al. Defining Criteria for Oligomannose Immunogens for HIV Using Icosahedral Virus Capsid Scaffolds. Chem. Biol 2010, 17 (4), 357–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (489).Doores KJ; Fulton Z; Hong V; Patel MK; Scanlan CN; Wormald MR; Finn MG; Burton DR; Wilson IA; Davis BG A Nonself Sugar Mimic of the HIV Glycan Shield Shows Enhanced Antigenicity. Proc. Natl. Acad. Sci. U.S.A 2010, 107 (40), 17107–17112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (490).Bailey JK; Nguyen DN; Horiya S; Krauss IJ Synthesis of Multivalent Glycopeptide Conjugates that Mimic an HIV Epitope. Tetrahedron 2016, 72 (40), 6091–6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (491).Nguyen DN; Xu B; Stanfield RL; Bailey JK; Horiya S; Temme JS; Leon DR; LaBranche CC; Montefiori DC; Costello CE; et al. Oligomannose Glycopeptide Conjugates Elicit Antibodies Targeting the Glycan Core Rather Than Its Extremities. ACS Cent. Sci 2019, 5 (2), 237–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (492).Amin MN; McLellan JS; Huang W; Orwenyo J; Burton DR; Koff WC; Kwong PD; Wang LX Synthetic Glycopeptides Reveal the Glycan Specificity of HIV-Neutralizing Antibodies. Nat. Chem. Biol 2013, 9 (8), 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (493).Aussedat B; Vohra Y; Park PK; Fernández-Tejada A; Alam SM; Dennison SM; Jaeger FH; Anasti K; Stewart S; Blinn JH; et al. Chemical Synthesis of Highly Congested GP120 V1V2 N-Glycopeptide Antigens for Potential HIV-1-Directed Vaccines. J. Am. Chem. Soc 2013, 135 (35), 13113–13120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (494).Walker LM; Huber M; Doores KJ; Falkowska E; Pejchal R; Julien JP; Wang SK; Ramos A; Chan-Hui PY; Moyle M; et al. Broad Neutralization Coverage of HIV by Multiple Highly Potent Antibodies. Nature 2011, 477 (7365), 466–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (495).Mouquet H; Scharf L; Euler Z; Liu Y; Eden C; Scheid JF; Halper-Stromberg A; Gnanapragasam PN; Spencer DI; Seaman MS; et al. Complex-type N-Glycan Recognition by Potent Broadly Neutralizing HIV Antibodies. Proc. Natl. Acad. Sci. U.S.A 2012, 109, E3268–E3277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (496).Orwenyo J; Cai H; Giddens J; Amin MN; Toonstra C; Wang LX Systematic Synthesis and Binding Study of HIV V3 Glycopeptides Reveal the Fine Epitopes of Several Broadly Neutralizing Antibodies. ACS Chem. Biol 2017, 12 (6), 1566–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (497).Cai H; Zhang RS; Orwenyo J; Giddens J; Yang Q; LaBranche CC; Montefiori DC; Wang LX Synthetic HIV V3 Glycopeptide Immunogen Carrying a N334 N-Glycan Induces Glycan-Dependent Antibodies With Promiscuous Site Recognition. J. Med. Chem 2018, 61 (22), 10116–10125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (498).Cai H; Orwenyo J; Giddens JP; Yang Q; Zhang R; LaBranche CC; Montefiori DC; Wang LX Synthetic Three-Component HIV-1 V3 Glycopeptide Immunogens Induce Glycan-Dependent Antibody Responses. Cell. Chem. Biol 2017, 24 (12), 1513–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (499).Francica JR; Laga R; Lynn GM; Muzíková G; Androvic L; Aussedat B; Walkowicz WE; Padhan K; Ramirez-Valdez RA; Parks R; et al. Star Nanoparticles Delivering HIV-1 Peptide Minimal Immunogens Elicit Near-Native Envelope Antibody Responses in Nonhuman Primates. PLoS bio 2019, 17 (6), No. e3000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (500).Cai H; Zhang R; Orwenyo J; Giddens J; Yang Q; LaBranche CC; Montefiori DC; Wang LX Multivalent Antigen Presentation Enhances the Immunogenicity of a Synthetic Three-Component HIV-1 V3 Glycopeptide Vaccine. ACS Cent. Sci 2018, 4 (5), 582–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (501).den Haan JMM; Arens R; van Zelm MC The Activation of the Adaptive Immune System: Cross-Talk Between Antigen-Presenting Cells, T Cells and B Cells. Immunol. Lett 2014, 162, 103–112. [DOI] [PubMed] [Google Scholar]
- (502).ten Broeke T; Wubbolts R; Stoorvogel W MHC Class II Antigen Presentation by Dendritic Cells Regulated Through Endosomal Sorting. Cold Spring Harb. Perspect. Biol 2013, 5 (12), a016873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (503).Joffre OP; Segura E; Savina A; Amigorena S Cross-Presentation by Dendritic Cells. Nat. Rev. Immunol 2012, 12 (8), 557–569. [DOI] [PubMed] [Google Scholar]
- (504).Steinman RM; Hawiger D; Nussenzweig MC Tolerogenic Dendritic Cells. Annu. Rev. Immunol 2003, 21, 685–711. [DOI] [PubMed] [Google Scholar]
- (505).Joffre O; Nolte MA; Spörri R; Reis e Sousa C Inflammatory Signals in Dendritic Cell Activation and the Induction of Adaptive Immunity. Immunol. Rev 2009, 227, 234–247. [DOI] [PubMed] [Google Scholar]
- (506).Kaplon H; Reichert JM Antibodies to Watch in 2021. MAbs 2021, 13, 1860476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (507).Quast I; Peschke B; Lünemann JD Regulation of Antibody Effector Functions Through IgG Fc N-Glycosylation. Cell. Mol. Life Sci 2017, 74 (5), 837–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (508).Raju TS Terminal Sugars of Fc Glycans Influence Antibody Effector Functions of IgGs. Curr. Opin. Immunol 2008, 20 (4), 471–478. [DOI] [PubMed] [Google Scholar]
- (509).Bournazos S; Wang TT; Dahan R; Maamary J; Ravetch JV Signaling by Antibodies: Recent Progress. Annu. Rev. Immunol 2017, 35, 285–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (510).Wormald MR; Rudd PM; Harvey DJ; Chang SC; Scragg IG; Dwek RA Variations in Oligosaccharide-Protein Interactions in Immunoglobulin G Determine the Site-Specific Glycosylation Profiles and Modulate the Dynamic Motion of the Fc Oligosaccharides. Biochemistry 1997, 36 (6), 1370–1380. [DOI] [PubMed] [Google Scholar]
- (511).Takahashi N; Nakagawa H; Fujikawa K; Kawamura Y; Tomiya N Three-Dimensional Elution Mapping of Pyridylaminated N-Linked Neutral and Sialyl Oligosaccharides. Analytical biochemistry 1995, 226 (1), 139–146. [DOI] [PubMed] [Google Scholar]
- (512).Jefferis R Glycosylation of Recombinant Antibody Therapeutics. Biotechnol. Prog 2005, 21 (1), 11–16. [DOI] [PubMed] [Google Scholar]
- (513).Jefferis R Recombinant Antibody Therapeutics: the Impact of Glycosylation on Mechanisms of Action. Trends in pharmacological sciences 2009, 30 (7), 356–362. [DOI] [PubMed] [Google Scholar]
- (514).Liu L Antibody Glycosylation and Its Impact on the Pharmacokinetics and Pharmacodynamics of Monoclonal Antibodies and Fc-Fusion Proteins. J. Pharm. Sci 2015, 104 (6), 1866–1884. [DOI] [PubMed] [Google Scholar]
- (515).Washburn N; Schwab I; Ortiz D; Bhatnagar N; Lansing JC; Medeiros A; Tyler S; Mekala D; Cochran E; Sarvaiya H; et al. Controlled Tetra-Fc Sialylation of IVIg Results in a Drug Candidate With Consistent Enhanced Anti-Inflammatory Activity. Proc. Natl. Acad. Sci. U.S.A 2015, 112, E1297–E1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (516).Shade KT; Platzer B; Washburn N; Mani V; Bartsch YC; Conroy M; Pagan JD; Bosques C; Mempel TR; Fiebiger E; et al. A Single Glycan on IgE is Indispensable for Initiation of Anaphylaxis. J. Exp. Med 2015, 212 (4), 457–467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (517).Zauner G; Selman MH; Bondt A; Rombouts Y; Blank D; Deelder AM; Wuhrer M Glycoproteomic Analysis of Antibodies. Mol. Cell. Proteomics 2013, 12 (4), 856–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (518).Jefferis R Glycosylation as a Strategy to Improve Antibody-Based Therapeutics. Nat. Rev. Drug Discovery 2009, 8 (3), 226–234. [DOI] [PubMed] [Google Scholar]
- (519).Ferrara C; Brünker P; Suter T; Moser S; Püntener U; Umaña P Modulation of Therapeutic Antibody Effector Functions by Glycosylation Engineering: Influence of Golgi Enzyme Localization Domain and Co-expression of Heterologous Beta1, 4-N-Acetylglucosaminyltransferase III and Golgi Alpha-mannosidase II. Biotechnol. Bioeng 2006, 93 (5), 851–861. [DOI] [PubMed] [Google Scholar]
- (520).Ratner M Genentech’s Glyco-engineered Antibody to Succeed Rituxan. Nat. Biotechnol 2014, 32 (1), 6–7. [DOI] [PubMed] [Google Scholar]
- (521).Yamane-Ohnuki N; Satoh M Production of Therapeutic Antibodies With Controlled Fucosylation. MAbs 2009, 1 (3), 230–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (522).Matsushita T Engineered Therapeutic Antibodies With Enhanced Effector Functions: Clinical Application of the Potelligent® Technology. Korean journal of hematology 2011, 46 (3), 148–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (523).Beck A; Reichert JM Marketing Approval of Mogamulizumab: a Triumph for Glyco-engineering. MAbs 2012, 4 (4), 419–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (524).Subramaniam JM; Whiteside G; McKeage K; Croxtall JC Mogamulizumab: First Global Approval. Drugs 2012, 72 (9), 1293–1298. [DOI] [PubMed] [Google Scholar]
- (525).Kanda Y; Imai-Nishiya H; Kuni-Kamochi R; Mori K; Inoue M; Kitajima-Miyama K; Okazaki A; Iida S; Shitara K; Satoh M Establishment of a GDP-mannose 4,6-dehydratase (GMD) Knockout Host Cell Line: a New Strategy for Generating Completely Non-Fucosylated Recombinant Therapeutics. J. Biotechnol 2007, 130 (3), 300–310. [DOI] [PubMed] [Google Scholar]
- (526).Imai-Nishiya H; Mori K; Inoue M; Wakitani M; Iida S; Shitara K; Satoh M Double Knockdown of Alpha1,6-Fucosyltransferase (FUT8) and GDP-Mannose 4,6-dehydratase (GMD) in Antibody-Producing Cells: a New Strategy for Generating Fully Non-Fucosylated Therapeutic Antibodies With Enhanced ADCC. BMC biotechnology 2007, 7, 84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (527).von Horsten HH; Ogorek C; Blanchard V; Demmler C; Giese C; Winkler K; Kaup M; Berger M; Jordan I; Sandig V Production of Non-Fucosylated Antibodies by Co-expression of Heterologous GDP-6-deoxy-D-lyxo-4-hexulose Reductase. Glycobiology 2010, 20 (12), 1607–1618. [DOI] [PubMed] [Google Scholar]
- (528).Chung CH; Mirakhur B; Chan E; Le QT; Berlin J; Morse M; Murphy BA; Satinover SM; Hosen J; Mauro D; et al. Cetuximab-Induced Anaphylaxis and IgE Specific for Galactose-alpha-1,3-galactose. N. Engl. J. Med 2008, 358 (11), 1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (529).Noguchi A; Mukuria CJ; Suzuki E; Naiki M Immunogenicity of N-Glycolylneuraminic Acid-containing Carbohydrate Chains of Recombinant Human Erythropoietin Expressed in Chinese Hamster Ovary Cells. J. Biochem 1995, 117 (1), 59–62. [DOI] [PubMed] [Google Scholar]
- (530).Oriol R; Mollicone R; Cailleau A; Balanzino L; Breton C Divergent Evolution of Fucosyltransferase Genes From Vertebrates, Invertebrates, and Bacteria. Glycobiology 1999, 9 (4), 323–334. [DOI] [PubMed] [Google Scholar]
- (531).Nadeem T; Khan MA; Ijaz B; Ahmed N; Rahman ZU; Latif MS; Ali Q; Rana MA Glycosylation of Recombinant Anticancer Therapeutics in Different Expression Systems With Emerging Technologies. Cancer. Res 2018, 78 (11), 2787–2798. [DOI] [PubMed] [Google Scholar]
- (532).Meuris L; Santens F; Elson G; Festjens N; Boone M; Dos Santos A; Devos S; Rousseau F; Plets E; Houthuys E; et al. GlycoDelete Engineering of Mammalian Cells Simplifies N-Glycosylation of Recombinant Proteins. Nat. Biotechnol 2014, 32 (5), 485–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (533).Reeves PJ; Callewaert N; Contreras R; Khorana HG Structure and Function in Rhodopsin: High-Level Expression of Rhodopsin With Restricted and Homogeneous N-Glycosylation by a Tetracycline-Inducible N-Acetylglucosaminyltransferase I-Negative HEK293S Stable Mammalian Cell Line. Proc. Natl. Acad. Sci. U.S.A 2002, 99 (21), 13419–13424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (534).Nielsen J Production of Biopharmaceutical Proteins by Yeast: Advances Through Metabolic Engineering. Bioengineered 2013, 4 (4), 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (535).Wildt S; Gerngross TU The Humanization of N-Glycosylation Pathways in Yeast. Nat. Rev. Microbiol 2005, 3 (2), 119–128. [DOI] [PubMed] [Google Scholar]
- (536).Jacobs PP; Geysens S; Vervecken W; Contreras R; Callewaert N Engineering Complex-Type N-Glycosylation in Pichia pastoris Using GlycoSwitch Technology. Nat. Protoc 2009, 4 (1), 58–70. [DOI] [PubMed] [Google Scholar]
- (537).Vervecken W; Kaigorodov V; Callewaert N; Geysens S; De Vusser K; Contreras R In Vivo Synthesis of Mammalian-Like, Hybrid-Type N-Glycans in Pichia pastoris. Appl. Environ. Microbiol 2004, 70 (5), 2639–2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (538).Beck A; Cochet O; Wurch T GlycoFi’s Technology to Control the Glycosylation of Recombinant Therapeutic Proteins. Expert Opin. Drug Discovery 2010, 5 (1), 95–111. [DOI] [PubMed] [Google Scholar]
- (539).Hamilton SR; Gerngross TU Glycosylation Engineering in Yeast: the Advent of Fully Humanized Yeast. Curr. Opin. Biotechnol 2007, 18 (5), 387–392. [DOI] [PubMed] [Google Scholar]
- (540).Okeley NM; Alley SC; Anderson ME; Boursalian TE; Burke PJ; Emmerton KM; Jeffrey SC; Klussman K; Law CL; Sussman D; et al. Development of Orally Active Inhibitors of Protein and Cellular Fucosylation. Proc. Natl. Acad. Sci. U.S.A 2013, 110 (14), 5404–5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (541).Djureinovic D; Wang M; Kluger HM Agonistic CD40 Antibodies in Cancer Treatment. Cancers 2021, 13 (6), 1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (542).Coveler AL; Bajor DL; Masood A; Yilmaz E; Shields AF; Javle MM; Paluri RK; Vaccaro GM; Zalupski M; Grilley-Olson JE; et al. Phase I Study of SEA-CD40, Gemcitabine, Nabpaclitaxel, and Pembrolizumab in Patients with metastatic Pancreatic Ductal Adenocarcinoma (PDAC). J. Clin. Oncol 2020, 38, TPS4671. [Google Scholar]
- (543).Allen JG; Mujacic M; Frohn MJ; Pickrell AJ; Kodama P; Bagal D; San Miguel T; Sickmier EA; Osgood S; Swietlow A; et al. Facile Modulation of Antibody Fucosylation with Small Molecule Fucostatin Inhibitors and Cocrystal Structure With GDP-Mannose 4,6-Dehydratase. ACS Chem. Biol 2016, 11 (10), 2734–2743. [DOI] [PubMed] [Google Scholar]
- (544).McKenzie NC; Scott NE; John A; White JM; Goddard-Borger ED Synthesis and Use of 6,6,6-trifluoro-L-Fucose to Block Core-Fucosylation in Hybridoma Cell Lines. Carbohydr. Res 2018, 465, 4–9. [DOI] [PubMed] [Google Scholar]
- (545).Ishida T; Joh T; Uike N; Yamamoto K; Utsunomiya A; Yoshida S; Saburi Y; Miyamoto T; Takemoto S; Suzushima H; et al. Defucosylated Anti-CCR4Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: a Multicenter Phase II Study. J. Clin. Oncol 2012, 30 (8), 837–842. [DOI] [PubMed] [Google Scholar]
- (546).Baz RC; Zonder JA; Gasparetto C; Reu FJ; Strout V Phase I Study of Anti-GM2 Ganglioside Monoclonal Antibody BIW-8962 as Monotherapy in Patients With Previously Treated Multiple Myeloma. Oncol. Ther 2016, 4 (2), 287–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (547).Espinoza-Gutarra MR; Green SD; Zeidner JF; Konig H CD123-Targeted Therapy in Acute Myeloid Leukemia. Expert Rev. Hematol 2021, 14 (6), 561–576. [DOI] [PubMed] [Google Scholar]
- (548).Cardarelli PM; Rao-Naik C; Chen S; Huang H; Pham A; Moldovan-Loomis MC; Pan C; Preston B; Passmore D; Liu J; et al. A Nonfucosylated Human Antibody to CD19 With Potent B-Cell Depletive Activity for Therapy of B-Cell Malignancies. Cancer Immunol. Immunother 2010, 59 (2), 257–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (549).Kierkus J; Pesegova M; Klopocka M; Brankovic M; Kasai N; Efuni S; Kong J; Nakajima Y; Jordan C; Matsui T; et al. Randomized, Ascending Dose, Phase 2 Study of KHK4083, an Anti-OX40 Monoclonal Antibody, in Moderately Active Ulcerative Colitis. Crohn’s & Colitis 360 2020, 2, otaa049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (550).Dávila González I; Moreno Benítez F; Quirce S Benralizumab: A New Approach for the Treatment of Severe Eosinophilic Asthma. J. Investig. Allergol. Clin. Immunol 2019, 29 (2), 84–93. [DOI] [PubMed] [Google Scholar]
- (551).Agius MA; Klodowska-Duda G; Maciejowski M; Potemkowski A; Li J; Patra K; Wesley J; Madani S; Barron G; Katz E; et al. Safety and Tolerability of Inebilizumab (MEDI-551), an Anti-CD19 Monoclonal Antibody, in Patients With Relapsing Forms of Multiple Sclerosis: Results From a Phase 1 Randomised, placebo-Controlled, Escalating Intravenous and Subcutaneous Dose Study. Mult Scler 2019, 25 (2), 235–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (552).Mita MM; Nemunaitis J; Grilley-Olson J; El-Rayes B; Bekaii-Saab T; Harvey RD; Marshall J; Zhang X; Strout V Phase 1 Study of CEP-37250/KHK2804, a Tumor-Specific Antiglycoconjugate Monoclonal Antibody, in Patients with Advanced Solid Tumors.. Phase 1 Study of CEP-37250/KHK2804, a Tumor-specific Antiglycoconjugate Monoclonal Antibody, in Patients With Advanced Solid Tumors. Target. Oncol 2016, 11 (6), 807–814. [DOI] [PubMed] [Google Scholar]
- (553).Tarhini AA; Moschos SJ; Lin Y; Lin HM; Sander C; Yin Y; Venhaus R; Gajewski TF; Kirkwood JM Safety and Efficacy of the Antiganglioside GD3 Antibody Ecromeximab (KW2871) Combined with High-Dose Interferon-α2b in Patients With Metastatic Melanoma. Melanoma Res 2017, 27 (4), 342–350. [DOI] [PubMed] [Google Scholar]
- (554).Goede V; Fischer K; Busch R; Engelke A; Eichhorst B; Wendtner CM; Chagorova T; de la Serna J; Dilhuydy MS; Illmer T; et al. Obinutuzumab Plus Chlorambucil in Patients With CLL and Coexisting Conditions. N. Engl. J. Med 2014, 370 (12), 1101–1110. [DOI] [PubMed] [Google Scholar]
- (555).Sehn LH; Chua N; Mayer J; Dueck G; Trněný M; Bouabdallah K; Fowler N; Delwail V; Press O; Salles G; et al. Obinutuzumab plus Bendamustine Versus Bendamustine Monotherapy in Patients With Rituximab-Refractory Indolent Non-Hodgkin Lymphoma (GADOLIN): a Randomised, controlled, Open-Label, Multicentre, Phase 3 Trial. Lancet Oncol 2016, 17 (8), 1081–1093. [DOI] [PubMed] [Google Scholar]
- (556).Paz-Ares LG; Gomez-Roca C; Delord JP; Cervantes A; Markman B; Corral J; Soria JC; Bergé Y; Roda D; Russell-Yarde F; et al. Phase I Pharmacokinetic and Pharmacodynamic Dose-Escalation Study of RG7160 (GA201), the First Glycoengineered Monoclonal Antibody Against the Epidermal Growth Factor Receptor, in Patients with Advanced Solid Tumors. J. Clin. Oncol 2011, 29 (28), 3783–3790. [DOI] [PubMed] [Google Scholar]
- (557).Meulendijks D; Jacob W; Martinez-Garcia M; Taus A; Lolkema MP; Voest EE; Langenberg MH; Fleitas Kanonnikoff T; Cervantes A; De Jonge MJ; et al. First-in-Human Phase I Study of Lumretuzumab, a Glycoengineered Humanized Anti-HER3Monoclonal Antibody, in Patients With Metastatic or Advanced HER3-Positive Solid Tumors. Clin. Cancer Res 2016, 22 (4), 877–885. [DOI] [PubMed] [Google Scholar]
- (558).Fiedler W; DeDosso S; Cresta S; Weidmann J; Tessari A; Salzberg M; Dietrich B; Baumeister H; Goletz S; Gianni L; et al. A Phase I Study of PankoMab-GEX, a Humanised Glyco-Optimised Monoclonal Antibody to a Novel Tumour-Specific MUC1 Glycopeptide Epitope in Patients with Advanced Carcinomas. Eur. J. Cancer 2016, 63, 55–63. [DOI] [PubMed] [Google Scholar]
- (559).Fiedler W; Stoeger H; Perotti A; Gastl G; Weidmann J; Dietrich B; Baumeister H; Danielczyk A; Goletz S; Salzberg M; et al. Phase I Study of TrasGEX, a Glyco-Optimised Anti-HER2Monoclonal Antibody, in Patients with HER2-Positive Solid Tumours. ESMO open 2018, 3 (4), No. e000381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (560).Fiedler W; Sessa C; Gianni L; Cresta S; Schulze-Bergkamen H; Weidmann J; De Dosso SD; Tessari A; Salzberg MO; Baumeister H; et al. First-in-Human Phase I Study of CetuGEX, a Novel Anti-EGFR Monoclonal Antibody (mAb) With Optimized Glycosylation and Antibody Dependent Cellular Cytotoxicity. J. Clin. Oncol 2013, 31, 3008. [Google Scholar]
- (561).Li W; Zhu Z; Chen W; Feng Y; Dimitrov DS Crystallizable Fragment Glycoengineering for Therapeutic Antibodies Development. Front. Immunol 2017, 8, 1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (562).Wang LX; Tong X; Li C; Giddens JP; Li T Glycoengineering of Antibodies for Modulating Functions. Annu. Rev. Biochem 2019, 88, 433–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (563).Hodoniczky J; Zheng YZ; James DC Control of Recombinant Monoclonal Antibody Effector Functions by Fc N-Glycan Remodeling in Vitro. Biotechnol. Prog 2005, 21 (6), 1644–1652. [DOI] [PubMed] [Google Scholar]
- (564).Thomann M; Schlothauer T; Dashivets T; Malik S; Avenal C; Bulau P; Rüger, P.; Reusch, D. In Vitro Glycoengineering of IgG1 and Its Effect on Fc Receptor Binding and ADCC Activity. PloS one 2015, 10 (8), No. e0134949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (565).Giddens JP; Lomino JV; DiLillo DJ; Ravetch JV; Wang LX Site-Selective Chemoenzymatic Glycoengineering of Fab and Fc Glycans of a Therapeutic Antibody. Proc. Natl. Acad. Sci. U.S.A 2018, 115 (47), 12023–12027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (566).Lin CW; Tsai MH; Li ST; Tsai TI; Chu KC; Liu YC; Lai MY; Wu CY; Tseng YC; Shivatare SS; et al. A Common Glycan Structure on Immunoglobulin G for Enhancement of Effector Functions. Proc. Natl. Acad. Sci. U.S.A 2015, 112 (34), 10611–10616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (567).Kurogochi M; Mori M; Osumi K; Tojino M; Sugawara S; Takashima S; Hirose Y; Tsukimura W; Mizuno M; Amano J; et al. Glycoengineered Monoclonal Antibodies With Homogeneous Glycan (M3, G0, G2, and A2) Using a Chemoenzymatic Approach Have Different Affinities for FcγRIIIa and Variable Antibody-Dependent Cellular Cytotoxicity Activities. PloS one 2015, 10 (7), No. e0132848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (568).Parsons TB; Struwe WB; Gault J; Yamamoto K; Taylor TA; Raj R; Wals K; Mohammed S; Robinson CV; Benesch JL; et al. Optimal Synthetic Glycosylation of a Therapeutic Antibody. Angew. Chem., Int. Ed 2016, 55 (7), 2361–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (569).Collin M; Olsén A EndoS, a Novel Secreted Protein From Streptococcus pyogenes With Endoglycosidase Activity on Human IgG. EMBO journal 2001, 20 (12), 3046–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (570).Sjögren J; Cosgrave EF; Allhorn M; Nordgren M; Björk S; Olsson F; Fredriksson S; Collin M EndoS and EndoS2 Hydrolyze Fc-Glycans on Therapeutic Antibodies With Different Glycoform Selectivity and Can be Used for Rapid Quantification of High-Mannose Glycans. Glycobiology 2015, 25 (10), 1053–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (571).Sjögren J; Struwe WB; Cosgrave EF; Rudd PM; Stervander M; Allhorn M; Hollands A; Nizet V; Collin M EndoS2 is a Unique and Conserved Enzyme of Serotype M49 Group A Streptococcus that Hydrolyses N-Linked Glycans on IgG and α1-acid Glycoprotein. Biochem. J 2013, 455 (1), 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (572).Iwamoto M; Sekiguchi Y; Nakamura K; Kawaguchi Y; Honda T; Hasegawa J Generation of Efficient Mutants of Endoglycosidase From Streptococcus pyogenes and Their Application in a Novel One-Pot Transglycosylation Reaction for Antibody Modification. PloS one 2018, 13 (2), No. e0193534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (573).Gupta SK; Shukla P Glycosylation Control Technologies for Recombinant Therapeutic Proteins. Appl. Microbiol. Biotechnol 2018, 102 (24), 10457–10468. [DOI] [PubMed] [Google Scholar]
- (574).Kool M; Fierens K; Lambrecht BN Alum Adjuvant: Some of the Tricks of the Oldest Adjuvant. J. Med. Microbiol 2012, 61, 927–934. [DOI] [PubMed] [Google Scholar]
- (575).Oleszycka E; Lavelle EC Immunomodulatory Properties of the Vaccine Adjuvant Alum. Curr. Opin. Immunol 2014, 28, 1–5. [DOI] [PubMed] [Google Scholar]
- (576).Garçon N; Chomez P; Van Mechelen M GlaxoSmithKline Adjuvant Systems in Vaccines: Concepts, Achievements and Perspectives. Expert Rev. Vaccines 2007, 6 (5), 723–739. [DOI] [PubMed] [Google Scholar]
- (577).Kundi M New Hepatitis B Vaccine Formulated With an Improved Adjuvant System. Expert Rev. Vaccines 2007, 6 (2), 133–140. [DOI] [PubMed] [Google Scholar]
- (578).Ko EJ; Kang SM Immunology and Efficacy of MF59-Adjuvanted Vaccines. Hum. Vaccin. Immunother 2018, 14 (12), 3041–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (579).O’Hagan DT MF59 is a Safe and Potent Vaccine Adjuvant that Enhances Protection Against Influenza Virus Infection. Expert Rev. Vaccines 2007, 6 (5), 699–710. [DOI] [PubMed] [Google Scholar]
- (580).Del Giudice G; Rappuoli R; Didierlaurent AM Correlates of Adjuvanticity: A Review on Adjuvants in Licensed Vaccines. Semin. Immunol 2018, 39, 14–21. [DOI] [PubMed] [Google Scholar]
- (581).Yang M; Yan Y; Fang M; Wan M; Wu X; Zhang X; Zhao T; Wei H; Song D; Wang L; et al. MF59 Formulated with CpG ODN as a Potent Adjuvant of Recombinant HSP65-MUC1 for Inducing Anti-MUC1+ Tumor Immunity in Mice. Int. Immunopharmacol 2012, 13 (4), 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (582).Garçon N; Van Mechelen M Recent Clinical Experience With Vaccines Using MPL- and QS-21-Containing Adjuvant Systems. Expert Rev. Vaccines 2011, 10 (4), 471–486. [DOI] [PubMed] [Google Scholar]
- (583).Petrovsky N; Cooper PD Carbohydrate-Based Immune Adjuvants. Expert Rev. Vaccines 2011, 10 (4), 523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (584).Mazgaeen L; Gurung P Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci 2020, 21, 379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (585).Kieser KJ; Kagan JC Multi-Receptor Detection of Individual Bacterial Products by the Innate Immune System. Nat. Rev. Immunol 2017, 17 (6), 376–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (586).Bohannon JK; Hernandez A; Enkhbaatar P; Adams WL; Sherwood ER The Immunobiology of Toll-Like Receptor 4 Agonists: From Endotoxin Tolerance to Immunoadjuvants. Shock 2013, 40 (6), 451–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (587).Qureshi N; Takayama K; Ribi E Purification and Structural Determination of Nontoxic Lipid A Obtained From the Lipopolysaccharide of Salmonella typhimurium. J. Biol. Chem 1982, 257 (19), 11808–11815. [PubMed] [Google Scholar]
- (588).Casella CR; Mitchell TC Putting Endotoxin to Work for us: Monophosphoryl Lipid A as a Safe and Effective Vaccine Adjuvant. Cell. Mol. Life Sci 2008, 65 (20), 3231–3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (589).Erridge C; Bennett-Guerrero E; Poxton IR Structure and Function of Lipopolysaccharides. Microbes Infect 2002, 4 (8), 837–851. [DOI] [PubMed] [Google Scholar]
- (590).Hagen SR; Thompson JD; Snyder DS; Myers KR Analysis of a Monophosphoryl Lipid A Immunostimulant Preparation From Salmonella minnesota R595 by High-Performance Liquid Chromatography. J. Chromatogr. A 1997, 767 (1–2), 53–61. [DOI] [PubMed] [Google Scholar]
- (591).Ulrich JT; Myers KR Monophosphoryl Lipid A as an Adjuvant. Past Experiences and New Directions. Pharm. Biotechnol 1995, 6, 495–524. [PubMed] [Google Scholar]
- (592).Stoute JA; Slaoui M; Heppner DG; Momin P; Kester KE; Desmons P; Wellde BT; Garçon, N.; Krzych, U.; Marchand, M. A Preliminary Evaluation of a Recombinant Circumsporozoite Protein Vaccine Against Plasmodium falciparum Malaria. RTS,S Malaria Vaccine Evaluation Group. N. Engl. J. Med 1997, 336, 86–91. [DOI] [PubMed] [Google Scholar]
- (593).Cluff CW Monophosphoryl Lipid A (MPL) as an Adjuvant for Anti-Cancer Vaccines: Clinical Results. Adv. Exp. Med. Biol 2009, 667, 111–123. [DOI] [PubMed] [Google Scholar]
- (594).Cochet F; Peri F The Role of Carbohydrates in the Lipopolysaccharide (LPS)/Toll-Like Receptor 4 (TLR4) Signalling. Int. J. Mol. Sci 2017, 18, 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (595).Park BS; Lee JO Recognition of Lipopolysaccharide Pattern by TLR4 Complexes. Exp. Mol. Med 2013, 45 (12), No. e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (596).Akira S; Takeda K; Kaisho T Toll-Like Receptors: Critical Proteins Linking Innate and Acquired Immunity. Nat. Immunol 2001, 2 (8), 675–680. [DOI] [PubMed] [Google Scholar]
- (597).Gao J; Guo Z Progress in the Synthesis and Biological Evaluation of Lipid A and Its Derivatives. Med. Res. Rev 2018, 38 (2), 556–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (598).Pifferi C; Fuentes R; Fernández-Tejada A Natural and Synthetic Carbohydrate-Based Vaccine Adjuvants and Their Mechanisms of Action. Nat. Rev. Chem 2021, 5 (3), 197–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (599).Molinaro A; Holst O; Di Lorenzo F; Callaghan M; Nurisso A; D’Errico G; Zamyatina A; Peri F; Berisio R; Jerala R; et al. Chemistry of Lipid A: at the Heart of Innate Immunity. Chem.—Eur. J 2015, 21, 500–519. [DOI] [PubMed] [Google Scholar]
- (600).Johnson DA; Keegan DS; Sowell CG; Livesay MT; Johnson CL; Taubner LM; Harris A; Myers KR; Thompson JD; Gustafson GL; et al. 3-O-Desacyl Monophosphoryl Lipid A Derivatives: Synthesis and Immunostimulant Activities. J. Med. Chem 1999, 42 (22), 4640–4649. [DOI] [PubMed] [Google Scholar]
- (601).Jiang ZH; Budzynski WA; Qiu D; Yalamati D; Koganty RR Monophosphoryl Lipid A Analogues With Varying 3-O-Substitution: Synthesis and Potent Adjuvant Activity. Carbohydr. Res 2007, 342 (6), 784–796. [DOI] [PubMed] [Google Scholar]
- (602).D’Alonzo D; Cipolletti M; Tarantino G; Ziaco M; Pieretti G; Iadonisi A; Palumbo G; Alfano A; Giuliano M; De Rosa M; et al. A Semisynthetic Approach to New Immunoadjuvant Candidates: Site-Selective Chemical Manipulation of Escherichia coli Monophosphoryl Lipid A. Chem.—Eur. J 2016, 22, 11053–11063. [DOI] [PubMed] [Google Scholar]
- (603).Wang Q; Xue J; Guo Z Synthesis of a Monophosphoryl Lipid A Derivative and Its Conjugation to a Modified form of a Tumor-Associated Carbohydrate Antigen GM3. Chem. Commun. (Camb) 2009, 37, 5536–5537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (604).Wu J; Guo Z Improving the Antigenicity of sTn Antigen by Modification of Its Sialic Acid Residue for Development of Glycoconjugate Cancer Vaccines. Bioconjugate Chem 2006, 17 (6), 1537–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (605).Kensil CR; Patel U; Lennick M; Marciani D Separation and Characterization of Saponins With Adjuvant Activity From Quillaja saponaria Molina cortex. J. Immunol 1991, 146, 431–437. [PubMed] [Google Scholar]
- (606).Soltysik S; Bedore DA; Kensil CR Adjuvant Activity of QS-21 Isomers. Ann. N.Y. Acad. Sci 1993, 690, 392–395. [DOI] [PubMed] [Google Scholar]
- (607).Ragupathi G; Gardner JR; Livingston PO; Gin DY Natural and Synthetic Saponin Adjuvant QS-21 for Vaccines Against Cancer. Expert Rev. Vaccines 2011, 10 (4), 463–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (608).Kensil CR Saponins as Vaccine Adjuvants. Crit. Rev. Ther. Drug Carr 1996, 13, 1–55. [PubMed] [Google Scholar]
- (609).Hu J; Qiu L; Wang X; Zou X; Lu M; Yin J Carbohydrate-Based Vaccine Adjuvants - Discovery and Development. Expert Opin. Drug Discovery 2015, 10 (10), 1133–1144. [DOI] [PubMed] [Google Scholar]
- (610).Laurens MB RTS,S/AS01 Vaccine (Mosquirix): An Overview. Hum. Vaccin. Immunother 2020, 16 (3), 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (611).Bharucha T; Ming D; Breuer J A Critical Appraisal of ‘Shingrix’, a Novel Herpes Zoster Subunit Vaccine (HZ/Su or GSK1437173A) for Varicella Zoster Virus. Hum. Vaccin. Immunother 2017, 13 (8), 1789–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (612).Fernandez-Tejada A; Chea EK; George C; Pillarsetty N; Gardner JR; Livingston PO; Ragupathi G; Lewis JS; Tan DS; Gin DY Development of a Minimal Saponin Vaccine Adjuvant Based on QS-21. Nat. Chem 2014, 6 (7), 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (613).Fernández-Tejada A; Tan DS; Gin DY Development of Improved Vaccine Adjuvants Based on the Saponin Natural Product QS-21 Through Chemical Synthesis. Acc. Chem. Res 2016, 49 (9), 1741–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (614).Soltysik S; Wu JY; Recchia J; Wheeler DA; Newman MJ; Coughlin RT; Kensil CR Structure/Function Studies of QS-21 Adjuvant: Assessment of Triterpene Aldehyde and Glucuronic Acid Roles in Adjuvant Function. Vaccine 1995, 13 (15), 1403–1410. [DOI] [PubMed] [Google Scholar]
- (615).Marciani DJ; Press JB; Reynolds RC; Pathak AK; Pathak V; Gundy LE; Farmer JT; Koratich MS; May RD Development of Semisynthetic Triterpenoid Saponin Derivatives With Immune Stimulating Activity. Vaccine 2000, 18 (27), 3141–3151. [DOI] [PubMed] [Google Scholar]
- (616).Wang P; Dai Q; Thogaripally P; Zhang P; Michalek SM Synthesis of QS-21-Based Immunoadjuvants. J. Org. Chem 2013, 78 (22), 11525–11534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (617).Wang P; Škalamera Đ; Sui X; Zhang P; Michalek SM Synthesis and Evaluation of a QS-17/18-Based Vaccine Adjuvant. J. Med. Chem 2019, 62 (3), 1669–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (618).Wang P; Škalamera Đ; Sui X; Zhang P; Michalek SM Synthesis and Evaluation of QS-7-Based Vaccine Adjuvants. ACS Infect. Dis 2019, 5 (6), 974–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (619).Wang P; Kim YJ; Navarro-Villalobos M; Rohde BD; Gin DY Synthesis of the Potent Immunostimulatory Adjuvant QS-21A. J. Am. Chem. Soc 2005, 127 (10), 3256–3257. [DOI] [PubMed] [Google Scholar]
- (620).Kim YJ; Wang P; Navarro-Villalobos M; Rohde BD; Derryberry J; Gin DY Synthetic Studies of Complex Immunostimulants From Quillaja saponaria: Synthesis of the Potent Clinical Immunoadjuvant QS-21Aapi. J. Am. Chem. Soc 2006, 128 (36), 11906–11915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (621).Deng K; Adams MM; Damani P; Livingston PO; Ragupathi G; Gin DY Synthesis of QS-21-Xylose: Establishment of the Immunopotentiating Activity of Synthetic QS-21 Adjuvant With a Melanoma Vaccine. Angew. Chem., Int. Ed 2008, 47 (34), 6395–6398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (622).Deng K; Adams MM; Gin DY Synthesis and Structure Verification of the Vaccine Adjuvant QS-7-Api. Synthetic Access to Homogeneous Quillaja saponaria Immunostimulants. J. Am. Chem. Soc 2008, 130 (18), 5860–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (623).Adams MM; Damani P; Perl NR; Won A; Hong F; Livingston PO; Ragupathi G; Gin DY Design and Synthesis of Potent Quillaja saponin Vaccine Adjuvants. J. Am. Chem. Soc 2010, 132 (6), 1939–1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (624).Walkowicz WE; Fernández-Tejada A; George C; Corzana F; Jiménez-Barbero J; Ragupathi G; Tan DS; Gin DY Quillaja Saponin Variants With Central Glycosidic Linkage Modifications Exhibit Distinct Conformations and Adjuvant Activities. Chem. Sci 2016, 7 (3), 2371–2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (625).Fernández-Tejada A; Chea EK; George C; Gardner JR; Livingston PO; Ragupathi G; Tan DS; Gin DY Design, Synthesis, and Immunologic Evaluation of Vaccine Adjuvant Conjugates Based on QS-21 and Tucaresol. Bioorg. Med. Chem 2014, 22 (21), 5917–5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (626).Borg NA; Wun KS; Kjer-Nielsen L; Wilce MC; Pellicci DG; Koh R; Besra GS; Bharadwaj M; Godfrey DI; McCluskey J; et al. CD1d-Lipid-Antigen Recognition by the Semi-Invariant NKT T-Cell Receptor. Nature 2007, 448 (7149), 44–49. [DOI] [PubMed] [Google Scholar]
- (627).Mallevaey T; Selvanantham T Strategy of Lipid Recognition by Invariant Natural Killer T Cells: ‘One for All and All for One’. Immunology 2012, 136 (3), 273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (628).Renukaradhya GJ; Khan MA; Vieira M; Du W; Gervay-Hague J; Brutkiewicz RR Type I NKT Cells Protect (and Type II NKT Cells Suppress) the Host’s Innate Antitumor Immune Response to a B-Cell Lymphoma. Blood 2008, 111 (12), 5637–5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (629).Brossay L; Chioda M; Burdin N; Koezuka Y; Casorati G; Dellabona P; Kronenberg M CD1d-Mediated Recognition of an Alpha-Galactosylceramide by Natural Killer T Cells is Highly Conserved Through Mammalian Evolution. J. Exp. Med 1998, 188 (8), 1521–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (630).Kawano T; Cui J; Koezuka Y; Toura I; Kaneko Y; Motoki K; Ueno H; Nakagawa R; Sato H; Kondo E; et al. CD1d-Restricted and TCR-Mediated Activation of Valpha14 NKT Cells by Glyco-sylceramides. Science 1997, 278 (5343), 1626–1629. [DOI] [PubMed] [Google Scholar]
- (631).Spada FM; Koezuka Y; Porcelli SA CD1d-Restricted Recognition of Synthetic Glycolipid Antigens by Human Natural Killer T Cells. J. Exp. Med 1998, 188 (8), 1529–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (632).Kobayashi E; Motoki K; Uchida T; Fukushima H; Koezuka Y KRN7000, a Novel Immunomodulator, and Its Antitumor Activities. Oncol Res 1995, 7, 529–534. [PubMed] [Google Scholar]
- (633).Morita M; Motoki K; Akimoto K; Natori T; Sakai T; Sawa E; Yamaji K; Koezuka Y; Kobayashi E; Fukushima H Structure-Activity Relationship of Alpha-Galactosylceramides Against B16-Bearing Mice. J. Med. Chem 1995, 38 (12), 2176–2187. [DOI] [PubMed] [Google Scholar]
- (634).Koch M; Stronge VS; Shepherd D; Gadola SD; Mathew B; Ritter G; Fersht AR; Besra GS; Schmidt RR; Jones EY; et al. The Crystal Structure of Human CD1d With and Without Alpha-Galactosylceramide. Nat. Immunol 2005, 6 (8), 819–826. [DOI] [PubMed] [Google Scholar]
- (635).McCarthy C; Shepherd D; Fleire S; Stronge VS; Koch M; Illarionov PA; Bossi G; Salio M; Denkberg G; Reddington F; et al. The Length of Lipids Bound to Human CD1d Molecules Modulates the Affinity of NKT Cell TCR and the Threshold of NKT Cell Activation. J. Exp. Med 2007, 204 (5), 1131–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (636).Zeng Z; Castaño AR; Segelke BW; Stura EA; Peterson PA; Wilson IA Crystal Structure of Mouse CD1: An MHC-Like Fold With a Large Hydrophobic Binding Groove. Science 1997, 277 (5324), 339–345. [DOI] [PubMed] [Google Scholar]
- (637).Moody DB; Zajonc DM; Wilson IA Anatomy of CD1-Lipid Antigen Complexes. Nat. Rev. Immunol 2005, 5 (5), 387–399. [DOI] [PubMed] [Google Scholar]
- (638).Matsuda JL; Naidenko OV; Gapin L; Nakayama T; Taniguchi M; Wang CR; Koezuka Y; Kronenberg M Tracking the Response of Natural Killer T Cells to a Glycolipid Antigen Using CD1d Tetramers. J. Exp. Med 2000, 192 (5), 741–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (639).Carnaud C; Lee D; Donnars O; Park SH; Beavis A; Koezuka Y; Bendelac A Cutting Edge: Cross-Talk Between Cells of the Innate Immune System: NKT Cells Rapidly Activate NK Cells. J. Immunol 1999, 163, 4647–4650. [PubMed] [Google Scholar]
- (640).Fujii S; Shimizu K; Smith C; Bonifaz L; Steinman RM Activation of Natural Killer T Cells by Alpha-Galactosylceramide Rapidly Induces the Full Maturation of Dendritic Cells in Vivo and Thereby Acts as an Adjuvant for Combined CD4 and CD8 T Cell Immunity to a Coadministered Protein. J. Exp. Med 2003, 198 (2), 267–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (641).Hung JT; Huang JR; Yu AL Tailored Design of NKT-Stimulatory Glycolipids for Polarization of Immune Responses. J. Biomed. Sci 2017, 24, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (642).Miyamoto K; Miyake S; Yamamura T A Synthetic Glycolipid Prevents Autoimmune Encephalomyelitis by Inducing TH2 Bias of Natural Killer T Cells. Nature 2001, 413 (6855), 531–534. [DOI] [PubMed] [Google Scholar]
- (643).Oki S; Chiba A; Yamamura T; Miyake S The Clinical Implication and Molecular Mechanism of Preferential IL-4 Production by Modified Glycolipid-Stimulated NKT Cells. J. Clin. Investig 2004, 113 (11), 1631–1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (644).Padte NN; Boente-Carrera M; Andrews CD; McManus J; Grasperge BF; Gettie A; Coelho-dos-Reis JG; Li X; Wu D; Bruder JT; et al. A Glycolipid Adjuvant, 7DW8–5, Enhances CD8+ T Cell Responses Induced by an Adenovirus-Vectored Malaria Vaccine in Non-Human Primates. PloS one 2013, 8 (10), No. e78407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (645).Li X; Fujio M; Imamura M; Wu D; Vasan S; Wong CH; Ho DD; Tsuji M Design of a Potent CD1d-Binding NKT Cell Ligand as a Vaccine Adjuvant. Proc. Natl. Acad. Sci. U.S.A 2010, 107 (29), 13010–13015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (646).Feng H; Nakajima N; Wu L; Yamashita M; Lopes TJS; Tsuji M; Hasegawa H; Watanabe T; Kawaoka Y A Glycolipid Adjuvant, 7DW8–5, Enhances the Protective Immune Response to the Current Split Influenza Vaccine in Mice. Front. Microbiol 2019, 10, 2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (647).Lin KH; Liang JJ; Huang WI; Lin-Chu SY; Su CY; Lee YL; Jan JT; Lin YL; Cheng YS; Wong CH In Vivo Protection Provided by a Synthetic New Alpha-Galactosyl Ceramide Analog Against Bacterial and viral Infections in Murine Models. Antimicrob. Agents Chemother 2010, 54 (10), 4129–4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (648).Wu TN; Lin KH; Chang YJ; Huang JR; Cheng JY; Yu AL; Wong CH Avidity of CD1d-Ligand-Receptor Ternary Complex Contributes to T-Helper 1 (Th1) Polarization and Anticancer Efficacy. Proc. Natl. Acad. Sci. U.S.A 2011, 108 (42), 17275–17280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (649).Wu TN; Lin KH; Wu YT; Huang JR; Hung JT; Wu JC; Chen CY; Chu KC; Lin NH; Yu AL; et al. Phenyl Glycolipids with Different Glycosyl Groups Exhibit Marked Differences in Murine and Human iNKT Cell Activation. ACS Chem. Biol 2016, 11 (12), 3431–3441. [DOI] [PubMed] [Google Scholar]
- (650).Prigozy TI; Naidenko O; Qasba P; Elewaut D; Brossay L; Khurana A; Natori T; Koezuka Y; Kulkarni A; Kronenberg M Glycolipid Antigen Processing for Presentation by CD1d Molecules. Science 2001, 291 (5504), 664–667. [DOI] [PubMed] [Google Scholar]
- (651).Zhang W; Xia C; Nadas J; Chen W; Gu L; Wang PG Introduction of Aromatic Group on 4’-OH of α-GalCer Manipulated NKT Cell Cytokine Production. Bioorg. Med. Chem 2011, 19 (8), 2767–2776. [DOI] [PubMed] [Google Scholar]
- (652).Janssens J; Decruy T; Venken K; Seki T; Krols S; Van der Eycken J; Tsuji M; Elewaut D; Van Calenbergh S Efficient Divergent Synthesis of New Immunostimulant 4″-Modified α-Galactosylceramide Analogues. ACS Med. Chem. Lett 2017, 8 (6), 642–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (653).Xing GW; Wu D; Poles MA; Horowitz A; Tsuji M; Ho DD; Wong CH Synthesis and Human NKT Cell Stimulating Properties of 3-O-Sulfo-Alpha/Beta-Galactosylceramides. Bioorg. Med. Chem 2005, 13 (8), 2907–2916. [DOI] [PubMed] [Google Scholar]
- (654).Zhou XT; Forestier C; Goff RD; Li C; Teyton L; Bendelac A; Savage PB Synthesis and NKT Cell Stimulating Properties of Fluorophore- and Biotin-Appended 6″-Amino-6″-Deoxy-Galactosylceramides. Org. Lett 2002, 4 (8), 1267–1270. [DOI] [PubMed] [Google Scholar]
- (655).Pauwels N; Aspeslagh S; Vanhoenacker G; Sandra K; Yu ED; Zajonc DM; Elewaut D; Linclau B; Van Calenbergh S Divergent Synthetic Approach to 6’’-Modified α-GalCer Analogues. Org. Biomol. Chem 2011, 9 (24), 8413–8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (656).Trappeniers M; Van Beneden K; Decruy T; Hillaert U; Linclau B; Elewaut D; Van Calenbergh S 6’-Derivatised Alpha-GalCer Analogues Capable of Inducing Strong CD1d-Mediated Th1-Biased NKT Cell Responses in Mice. J. Am. Chem. Soc 2008, 130, 16468–16469. [DOI] [PubMed] [Google Scholar]
- (657).Hsieh MH; Hung JT; Liw YW; Lu YJ; Wong CH; Yu AL; Liang PH Synthesis and Evaluation of Acyl-Chain- and Galactose-6’’-Modified Analogues of α-GalCer for NKT Cell Activation. Chembiochem 2012, 13 (11), 1689–1697. [DOI] [PubMed] [Google Scholar]
- (658).Aspeslagh S; Li Y; Yu ED; Pauwels N; Trappeniers M; Girardi E; Decruy T; Van Beneden K; Venken K; Drennan M; et al. Galactose-Modified iNKT Cell Agonists Stabilized by an Induced Fit of CD1d Prevent Tumour Metastasis. EMBO J 2011, 30 (11), 2294–2305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (659).Aspeslagh S; Nemčovič M; Pauwels N; Venken K; Wang J; Van Calenbergh S; Zajonc DM; Elewaut D Enhanced TCR Footprint by a Novel Glycolipid Increases NKT-Dependent Tumor Protection. J. Immunol 2013, 191, 2916–2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (660).Franck RW; Tsuji M Alpha-c-Galactosylceramides: Synthesis and Immunology. Acc. Chem. Res 2006, 39 (10), 692–701. [DOI] [PubMed] [Google Scholar]
- (661).Dere RT; Zhu X The First Synthesis of a Thioglycoside Analogue of the Immunostimulant KRN7000. Org. Lett 2008, 10 (20), 4641–4644. [DOI] [PubMed] [Google Scholar]
- (662).Hogan AE; O’Reilly V; Dunne MR; Dere RT; Zeng SG; O’Brien C; Amu S; Fallon PG; Exley MA; O’Farrelly C; et al. Activation of Human Invariant Natural Killer T Cells With a Thioglycoside Analogue of α-Galactosylceramide. Clin. Immunol 2011, 140 (2), 196–207. [DOI] [PubMed] [Google Scholar]
- (663).Leung L; Tomassi C; Van Beneden K; Decruy T; Elewaut D; Elliott T; Al-Shamkhani A; Ottensmeier C; Van Calenbergh S; Werner J; et al. Synthesis and in Vivo Evaluation of 4-deoxy-4,4-difluoro-KRN7000. Org. Lett 2008, 10 (20), 4433–4436. [DOI] [PubMed] [Google Scholar]
- (664).Zajonc DM; Cantu C 3rd; Mattner J; Zhou D; Savage PB; Bendelac A; Wilson IA; Teyton L Structure and Function of a Potent Agonist for the Semi-Invariant Natural Killer T Cell Receptor. Nat. Immunol 2005, 6 (8), 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (665).Park JJ; Lee JH; Ghosh SC; Bricard G; Venkataswamy MM; Porcelli SA; Chung SK Synthesis of All Stereoisomers of KRN7000, the CD1d-Binding NKT Cell Ligand. Bioorg. Med. Chem. lett 2008, 18 (14), 3906–3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (666).Fuhshuku K; Hongo N; Tashiro T; Masuda Y; Nakagawa R; Seino K; Taniguchi M; Mori K RCAI-8, 9, 18, 19, and 49–52, Conformationally Restricted Analogues of KRN7000 With an Azetidine or a Pyrrolidine Ring: Their Synthesis and Bioactivity for Mouse Natural Killer T Cells to Produce Cytokines. Bioorg. Med. Chem 2008, 16 (2), 950–964. [DOI] [PubMed] [Google Scholar]
- (667).Smith CIE; Zain R Therapeutic Oligonucleotides: State of the Art. Annu. Rev. Pharmacol. Toxicol 2019, 59, 605–630. [DOI] [PubMed] [Google Scholar]
- (668).Hammond SM; Aartsma-Rus A; Alves S; Borgos SE; Buijsen RAM; Collin RWJ; Covello G; Denti MA; Desviat LR; Echevarría L; et al. Delivery of Oligonucleotide-Based Therapeutics: Challenges and Opportunities. EMBO Mol. Med 2021, 13, No. e13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (669).Dowdy SF Overcoming Cellular Barriers for RNA Therapeutics. Nat. Biotechnol 2017, 35 (3), 222–229. [DOI] [PubMed] [Google Scholar]
- (670).Khvorova A; Watts JK The Chemical Evolution of Oligonucleotide Therapies of Clinical Utility. Nat. Biotechnol 2017, 35 (3), 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (671).Grewal PK The Ashwell-Morell Receptor. Methods Enzymol 2010, 479, 223–241. [DOI] [PubMed] [Google Scholar]
- (672).Morell AG; Van den Hamer CJ; Scheinberg IH; Ashwell G Physical and Chemical Studies on Ceruloplasmin. IV. Preparation of Radioactive, Sialic Acid-Free Ceruloplasmin Labeled With Tritium on Terminal D-Galactose Residues. J. Biol. Chem 1966, 241 (16), 3745–3749. [PubMed] [Google Scholar]
- (673).Morell AG; Irvine RA; Sternlieb I; Scheinberg IH; Ashwell G Physical and Chemical Studies on Ceruloplasmin. V. Metabolic Studies on Sialic Acid-Free Ceruloplasmin in Vivo. J. Biol. Chem 1968, 243 (1), 155–159. [PubMed] [Google Scholar]
- (674).Van Den Hamer CJ; Morell AG; Scheinberg IH; Hickman J; Ashwell G Physical and Chemical Studies on Ceruloplasmin. IX. The Role of Galactosyl Residues in the Clearance of Ceruloplasmin From the Circulation. J. Biol. Chem 1970, 245 (17), 4397–4402. [PubMed] [Google Scholar]
- (675).Meier M; Bider MD; Malashkevich VN; Spiess M; Burkhard P Crystal Structure of the Carbohydrate Recognition Domain of the H1 Subunit of the Asialoglycoprotein Receptor. J. Mol. Biol 2000, 300 (4), 857–865. [DOI] [PubMed] [Google Scholar]
- (676).Nair JK; Willoughby JL; Chan A; Charisse K; Alam MR; Wang Q; Hoekstra M; Kandasamy P; Kel’in AV; Milstein S; et al. Multivalent N-acetylgalactosamine-Conjugated siRNA Localizes in Hepatocytes and Elicits Robust RNAi-Mediated Gene Silencing. J. Am. Chem. Soc 2014, 136 (49), 16958–16961. [DOI] [PubMed] [Google Scholar]
- (677).Rajeev KG; Nair JK; Jayaraman M; Charisse K; Taneja N; O’Shea J; Willoughby JL; Yucius K; Nguyen T; Shulga-Morskaya S; et al. Hepatocyte-Specific Delivery of siRNAs Conjugated to Novel Non-Nucleosidic Trivalent N-Acetylgalactosamine Elicits Robust Gene Silencing in Vivo. Chembiochem 2015, 16 (6), 903–908. [DOI] [PubMed] [Google Scholar]
- (678).D’Souza AA; Devarajan PV Asialoglycoprotein Receptor Mediated Hepatocyte Targeting - Strategies and Applications. J. Controlled Release 2015, 203, 126–139. [DOI] [PubMed] [Google Scholar]
- (679).Cui H; Zhu X; Li S; Wang P; Fang J Liver-Targeted Delivery of Oligonucleotides With N-Acetylgalactosamine Conjugation. ACS Omega 2021, 6 (25), 16259–16265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (680).Schwartz AL; Fridovich SE; Lodish HF Kinetics of Internalization and Recycling of the Asialoglycoprotein Receptor in a Hepatoma Cell Line. J. Biol. Chem 1982, 257 (8), 4230–4237. [PubMed] [Google Scholar]
- (681).Debacker AJ; Voutila J; Catley M; Blakey D; Habib N Delivery of Oligonucleotides to the Liver With GalNAc: From Research to Registered Therapeutic Drug. Mol. Ther 2020, 28 (8), 1759–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (682).Springer AD; Dowdy SF GalNAc-siRNA Conjugates: Leading the Way for Delivery of RNAi Therapeutics. Nucleic Acid Ther 2018, 28 (3), 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (683).Parmar R; Willoughby JL; Liu J; Foster DJ; Brigham B; Theile CS; Charisse K; Akinc A; Guidry E; Pei Y; et al. 5′-(E)-Vinylphosphonate: A Stable Phosphate Mimic Can Improve the RNAi Activity of siRNA-GalNAc Conjugates. Chembiochem 2016, 17 (11), 985–989. [DOI] [PubMed] [Google Scholar]
- (684).Huang Y Preclinical and Clinical Advances of GalNAc-Decorated Nucleic Acid Therapeutics. Mol. Ther. Nucleic Acids 2017, 6, 116–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (685).Rozema DB; Lewis DL; Wakefield DH; Wong SC; Klein JJ; Roesch PL; Bertin SL; Reppen TW; Chu Q; Blokhin AV; et al. Dynamic PolyConjugates for Targeted in Vivo Delivery of siRNA to Hepatocytes. Proc. Natl. Acad. Sci. U.S.A 2007, 104 (32), 12982–12987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (686).Wong SC; Klein JJ; Hamilton HL; Chu Q; Frey CL; Trubetskoy VS; Hegge J; Wakefield D; Rozema DB; Lewis DL Co-injection of a Targeted, Reversibly Masked Endosomolytic Polymer Dramatically Improves the Efficacy of Cholesterol-Conjugated Small Interfering RNAs in Vivo. Nucleic Acid Ther 2012, 22 (6), 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (687).van den Berg F; Limani SW; Mnyandu N; Maepa MB; Ely A; Arbuthnot P Advances with RNAi-Based Therapy for Hepatitis B Virus Infection. Viruses 2020, 12 (8), 851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (688).Schapira M; Calabrese MF; Bullock AN; Crews CM Targeted Protein Degradation: Expanding the Toolbox. Nat. Rev. Drug Discovery 2019, 18 (12), 949–963. [DOI] [PubMed] [Google Scholar]
- (689).Li K; Crews CM PROTACs: Past, Present and Future. Chem. Soc. Rev 2022, 51 (12), 5214–5236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (690).Banik SM; Pedram K; Wisnovsky S; Ahn G; Riley NM; Bertozzi CR Lysosome-Targeting Chimaeras for Degradation of Extracellular Proteins. Nature 2020, 584, 291–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (691).Ahn G; Banik SM; Miller CL; Riley NM; Cochran JR; Bertozzi CR LYTACs that Engage the Asialoglycoprotein Receptor for Targeted Protein Degradation. Nat. Chem. Biol 2021, 17 (9), 937–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (692).Whitworth C; Ciulli A New Class of Molecule Targets Proteins Outside Cells for Degradation. Nature 2020, 584 (7820), 193–194. [DOI] [PubMed] [Google Scholar]