Abstract
Background
Spirometra infection is aneglected food‐ and waterborne disease with worldwide distribution.
Objectives
The present study aims to estimate the global prevalence of Spirometra species in snakes, frogs, dogs and cats.
Methods
Multiple databases (PubMed, Scopus, ProQuest, Web of Science and Google Scholar) were searched for relevant literatures published up to March 2022.
Results
Among 131 data sets (including 113 articles) that met the inclusion, 15 investigations reported Spirometra infection in snakes, 23 in frogs, 41 in dogs and 52 in cats. The pooled prevalence (95% confidence interval) in intermediate hosts and definitive hosts was found to be 0.313% and 0.089%, respectively. Based on continent, the infection was most prevalent in Asia for studies on snakes (0.696%) and frogs (0.181%), while Africa (0.224%) and Oceania (0.203%) were the regions with the highest pooled prevalence rates of the infection in dogs and cats, respectively. Among different diagnostic methods, the highest pooled prevalence was related to morphological method for studies on snakes, frog and cats with rate of 0.665%, 0.189% and 0.104%, respectively. Regarding studies on dogs, the highest pooled prevalence was observed for molecular technique (0.101%).
Conclusions
The results presented here revealed the importance of establishing a prevention and control measure focused on protection of aquaculture systems from being contaminated with faeces of dogs and cats, and raising awareness of parasitic zoonotic diseases to decrease the transmission risk.
Keywords: amphibians, canine, feline, reptiles, Spirometra, zoonosis
Spirometra species have been considered as neglected helminths in snakes, frogs, dogs and cats with a more recent growing interest in understanding its prevalence, means for prevention and better treatment, and the phylogenetic relationship of Spirometra in its various hosts. To build on our understanding of the epidemiology of Spirometra. The review and meta‐analysis in the submitted manuscript will contribute to filling this gap in knowledge.
1. INTRODUCTION
The worldwide‐distributed pseudophyllidean tapeworms of the genus Spirometra inhabit the intestine of canids, felids and other mammals (Kavana et al., 2015, 2014; Le et al., 2017). The life‐cycle involves the crustaceans from Cyclops genus as the first intermediate host, amphibians and reptiles, birds and mammals as the second intermediate hosts, carnivores such as dogs and cats as the final hosts and humans as accidental hosts (Kavana et al., 2014). The procercoid larvae develop in Cyclops sp., and the plerocercoids develop in amphibians or reptiles (Wiwanitkit, 2005; Yudhana et al., 2019). These plerocercoid larvae (sparganum) are the causative agents of human larval migrans syndromes called sparganosis (spirometrosis), a food and waterborne zoonotic disease, which was firstly described by Manson in 1882 (Anantaphruti et al., 2011; Li et al., 2011; Wang et al., 2014). The disease is endemic in East Asian countries, and has also been reported in populations from Europe, America, Africa and Australia (Wang et al., 2014). So far, there are more than 2000 cases of sparganosis that have been reported in humans worldwide (Kuchta et al., 2015, 2021). However, the number of human cases reported from Eastern and Southeastern Asia is outstanding (Zhang et al., 2020). Humans acquire the infection through drinking water containing procercoid larvae in copepods, consuming raw or undercooked flesh of frogs, snakes, birds and mammals (e.g. pigs) as well as using flesh of frogs or snakes with plerocercoids as poultices on the eyes or open wounds (Li et al., 2011; Q. Liu et al., 2015). The research on sparganosis concentrated more in Asia, where the raw flesh of snake or frog is consumed as a remedy due to the traditional misbelieve and the infection is a serious hazard for humans (MARTA Kołodziej‐Sobocińska et al., 2018; Kuchta et al., 2015). Plerocercoid larvae mostly affect the subcutaneous connective tissues, causing nodules. However, occasionally they invade muscles, the abdominal cavity, eyes, central nervous system, liver, lungs, heart and urinary system (Cui et al., 2011; Kim et al., 2020; Kuchta et al., 2015; Nithiuthai et al., 2004). The migration and proliferation of larvae may result in paralysis, and even death following serious pathologic damages (Oda et al., 2016). There are more than 64 nominal species of Spirometra tapeworms (Kuchta et al., 2021). However, only four of them including S. erinaceieuropaei, S. mansonoides, S. pretoriensis and S. theileri are recognised as valid species (Kuchta et al., 2021; Yamasaki et al., 2021). Epidemiological data are critical for successful application of preventive and control programs against Spirometra infection in animals and raises the awareness of the public health hazard caused by these helminthic parasites. In this regard, the present review and meta‐analysis was designed to estimate the pooled prevalence of Spirometra tapeworms in snakes, frogs, dogs, and cats in different geographic locations of the world through evaluating available scientific reports.
2. MATERIALS AND METHODS
2.1. Search strategy
This systematic review and meta‐analysis followed the Preferred Reporting Items for Systematic reviews and Meta‐Analyses (PRISMA) guidelines (Page et al., 2021). Relevant published articles on the prevalence of Spirometra in snakes, frogs, dogs, and cats were searched in the following electronic bibliographic databases: PubMed, Scopus, ProQuest, Web of Science and Google Scholar. A systematic search was carried out using the keywords described as follows: Spirometra, Sparganosis, Foodborne parasites, Foodborne Diseases, Intestinal helminth, worm, snakes, frogs, dogs, cats, prevalence, frequency, global, worldwide using AND and/or OR Boolean operators. The searching process, evaluation of titles and abstracts and review of the full‐texts were conducted by two independent authors. After removing duplicates and irrelevant records, the reference lists of obtained articles were screened for further studies that were not found in the database search.
2.2. Inclusion and exclusion criteria
Full‐text literatures were evaluated for eligibility, if they met the inclusion criteria described below: (1) peer‐reviewed original papers, (2) cross‐sectional studies reporting the prevalence of Spirometra in snakes, frogs, dogs, and cats, (3) having accessible full‐text and abstract in English, (4) having total sample size and exact number of positive cases and (5) articles published in English language up to March 2022. Review articles, case reports, case series, publications with non‐original data, letters, editorials and papers with unclear/undetermined results, as well as papers written in other languages were excluded. Moreover, those articles that reported Spirometra infection in humans and in animals other than dogs, cats, snakes and frogs were excluded from the analyses of the current study. A Microsoft Excel® version 2016 was used to separately collect the following information that was retrieved from each of the included articles: first author's name, publication year, country where the study was conducted, continent, country‐level income, sample size, number of positive samples, climate, average temperature, annual rainfall, humidity, diagnostic methods including morphological detection and molecular techniques and species of Spirometra (Table 1, Table 2).
TABLE 1.
. Main characteristics of the included studies reporting the prevalence of Spirometra in cats, dogs, frog and snakes
| No | Reference | Publication year | Country | Continent | Total samples | positive samples | Detection method |
|---|---|---|---|---|---|---|---|
| Cats | |||||||
| 1 | Read | 1948 | United States | North America | 14 | 2 | Morphological detection |
| 2 | Olsen et al. | 1976 | United States | North America | 82 | 8 | Morphological detection |
| 3 | Ryan et al. | 1976 | Australia | Oceania | 146 | 89 | Morphological detection |
| 4 | Gregory et al. | 1976 | Australia | Oceania | 107 | 61 | Morphological detection |
| 5 | Coman et al. | 1981 | Australia | Oceania | 327 | 107 | Morphological detection |
| 6 | Fujinami et al. | 1983 | Japan | Asia | 171 | 55 | Morphological detection |
| 7 | Poglayen et al. | 1985 | Italy | Europe | 116 | 1 | Morphological detection |
| 8 | Oikawa et al. | 1991 | Japan | Asia | 1064 | 166 | Morphological detection |
| 9 | Meloni et al. | 1993 | Australia | Oceania | 33 | 5 | Morphological detection |
| 10 | Huh et al. | 1993 | South Korea | Asia | 41 | 17 | Morphological detection |
| 11 | Milstein et al. | 1997 | Australia | Oceania | 39 | 3 | Morphological detection |
| 12 | Hata et al. | 2000 | Japan | Asia | 326 | 44 | Morphological detection |
| 13 | Mcglade et al. | 2003 | Australia | Oceania | 418 | 2 | Morphological detection |
| 14 | Scholz et al. | 2003 | Lao PDR | Asia | 55 | 7 | Morphological detection |
| 15 | Sohn and Chai | 2005 | South Korea | Asia | 438 | 181 | Morphological detection |
| 16 | Zibaei et al. | 2007 | Iran | Asia | 114 | 4 | Morphological detection |
| 17 | Palmer et al. | 2008 | Australia | Oceania | 1063 | 29 | Morphological detection |
| 18 | Yamamoto et al. | 2009 | Japan | Asia | 1079 | 90 | Morphological detection |
| 19 | Castro et al. | 2009 | Uruguay | South America | 4 | 3 | Morphological detection |
| 20 | Shin et al. | 2009 | South Korea | Asia | 4 | 4 | Morphological detection |
| 21 | Lucio‐Forster and Bowman | 2011 | United States | North America | 1322 | 5 | Morphological detection |
| 22 | Headley et al. | 2012 | British West Indies | North America | 55 | 10 | Morphological detection |
| 23 | Spada et al. | 2012 | Italy | Europe | 139 | 2 | Morphological detection |
| 24 | Al‐Obaidi | 2012 | Iraq | Asia | 55 | 13 | Morphological detection |
| 25 | Sabshin et al. | 2012 | United States | North America | 100 | 1 | Morphological detection |
| 26 | Borkataki et al. | 2013 | India | Asia | 100 | 8 | Morphological detection |
| 27 | Ramos et al. | 2013 | Brazil | South America | 146 | 6 | Morphological detection |
| 28 | Hoopes et al. | 2013 | Canada | North America | 635 | 1 | Morphological detection |
| 29 | Ngui et al. | 2014 | Malaysia | Asia | 105 | 2 | Morphological detection |
| 30 | Rojekittikhun et al. | 2014 | Thailand | Asia | 300 | 12 | Morphological detection |
| 31 | Zanzani et al. | 2014 | Italy | Europe | 156 | 2 | Morphological detection |
| 32 | Tun et al. | 2015 | Malaysia | Asia | 152 | 14 | Morphological detection |
| 33 | Fang et al. | 2015 | China | Asia | 39 | 13 | Morphological detection |
| 34 | Zanzani et al. | 2015 | Italy | Europe | 103 | 1 | Morphological detection |
| 35 | Rojekittikhun et al. | 2015 | Thailand | Asia | 100 | 7 | Morphological detection |
| 36 | Hong et al. | 2016 | China | Asia | 116 | 47 | Morphological detection |
| 37 | Pumidonming et al. | 2016 | Thailand | Asia | 180 | 36 | Morphological detection |
| 38 | Eslahi et al. | 2017 | Iran | Asia | 12 | 3 | Morphological detection |
| 39 | Marques et al. | 2017 | Brazil | South America | 339 | 1 | Morphological detection |
| 40 | Blasco et al. | 2017 | Spain | Europe | 423 | 1 | Morphological detection |
| 41 | Wyrosdick et al. | 2017 | United States | North America | 76 | 6 | Morphological detection |
| 42 | Sedionoto and Anamnart | 2018 | Thailand | Asia | 23 | 3 | Morphological detection |
| 43 | Salman et al. | 2018 | Japan | Asia | 351 | 1 | Morphological detection |
| 44 | Amoei et al. | 2018 | Iran | Asia | 42 | 1 | Morphological detection |
| 45 | Andersen et al. | 2018 | United States | North America | 482 | 16 | Morphological detection |
| 46 | Loftin et al. | 2019 | United States | North America | 56 | 5 | Morphological detection |
| 47 | Hoggard et al. | 2019 | United States | North America | 103 | 3 | Morphological detection |
| 48 | Traversa et al. | 2019 | Italy | Europe | 1000 | 3 | Morphological detection |
| 49 | Nagamori et al. | 2020 | United States | North America | 2586 | 2 | Morphological detection |
| 50 | Jitsamai et al. | 2021 | Thailand | Asia | 509 | 8 | Morphological detection |
| 51 | Tong et al. | 2021 | China | Asia | 135 | 1 | Morphological detection |
| 52 | Nath et al. | 2022 | Bangladesh | Asia | 50 | 19 | Morphological detection |
| Dogs | |||||||
| 1 | Olsen et al. | 1976 | United States | North America | 2 | 2 | Morphological detection |
| 2 | Cho et al. | 1981 | South Korea | Asia | 102 | 2 | Morphological detection |
| 3 | Dalimi and Mobedi | 1992 | Iran | Asia | 35 | 2 | Morphological detection |
| 4 | Meloni et al. | 1993 | Australia | Oceania | 182 | 4 | Morphological detection |
| 5 | Saeki et al. | 1997 | Japan | Asia | 916 | 1 | Morphological detection |
| 6 | Hee et al. | 1998 | South Korea | Asia | 304 | 1 | Morphological detection |
| 7 | Traub et al. | 2002 | India | Asia | 101 | 28 | Morphological detection |
| 8 | Asano et al. | 2004 | Japan | Asia | 772 | 8 | Morphological detection |
| 9 | Inpankaew et al. | 2007 | Thailand | Asia | 229 | 7 | Morphological detection |
| 10 | Palmer et al. | 2008 | Australia | Oceania | 1400 | 140 | Morphological detection |
| 11 | Yamamoto et al. | 2009 | Japan | Asia | 906 | 9 | Morphological detection |
| 12 | Lin et al. | 2010 | China | Asia | 31 | 6 | Morphological detection |
| 13 | Itoh et al. | 2011 | Japan | Asia | 2365 | 1 | Morphological detection |
| 14 | Cardoso et al. | 2013 | Portugal | Europe | 301 | 1 | Morphological detection |
| 15 | Schar et al. | 2014 | Cambodia | Asia | 94 | 20 | Morphological detection |
| 16 | Ngui et al. | 2014 | Malaysia | Asia | 105 | 8 | Morphological detection |
| 17 | Rojekittikhun et al. | 2014 | Thailand | Asia | 500 | 3 | Morphological detection |
| 18 | Tun et al. | 2015 | Malaysia | Asia | 227 | 8 | Morphological detection |
| 19 | Itoh et al. | 2015 | Japan | Asia | 573 | 2 | Morphological detection |
| 20 | Kavana et al. | 2015 | Tanzania | Africa | 59 | 17 | Morphological detection |
| 21 | Fang et al. | 2015 | China | Asia | 40 | 4 | Morphological detection |
| 22 | Bang et al. | 2015 | Vietnam | Asia | 414 | 28 | Morphological detection |
| 23 | Inpankaew et al. | 2015 | Cambodia | Asia | 50 | 9 | Morphological detection |
| 24 | Binod et al. | 2015 | India | Asia | 223 | 98 | Morphological detection |
| 25 | Hong et al. | 2016 | China | Asia | 229 | 63 | Morphological detection |
| 26 | Pumidonming et al. | 2016 | Thailand | Asia | 197 | 30 | Morphological detection |
| 27 | Harriott | 2016 | Australia | Oceania | 201 | 72 | Morphological detection |
| 28 | Eslahi et al. | 2017 | Iran | Asia | 27 | 2 | Morphological detection |
| 29 | Sato et al. | 2017 | Lao PDR | Asia | 34 | 15 | Molecular detection |
| 30 | Gillespie and Bradbury | 2017 | Australia | Oceania | 300 | 4 | Morphological detection |
| 31 | Binod et al. | 2018 | India | Asia | 223 | 1 | Morphological detection |
| 32 | Amouei et al. | 2018 | Iran | Asia | 42 | 1 | Morphological detection |
| 33 | Beiromvand et al. | 2018 | Iran | Asia | 167 | 1 | Molecular detection |
| 34 | Rusdi et al. | 2018 | Australia | Oceania | 141 | 5 | Molecular detection |
| 35 | Little et al. | 2019 | United States | North America | 1202 | 1 | Morphological detection |
| 36 | Nagamori et al. | 2020 | United States | North America | 7409 | 7 | Morphological detection |
| 37 | Stafford et al. | 2020 | United States | North America | 3006 | 2 | Morphological detection |
| 38 | Tong et al. | 2021 | China | Asia | 135 | 1 | Morphological detection |
| 39 | Mulinge et al. | 2021 | Kenya | Africa | 65 | 11 | Morphological detection |
| 40 | Sobotyk et al. | 2021 | United States | North America | 4692 | 2 | Morphological detection |
| 41 | Nath et al. | 2022 | Bangladesh | Asia | 100 | 62 | Morphological detection |
| Frogs | |||||||
| 1 | Ooi et al. | 2000 | Taiwan | Asia | 176 | 18 | Morphological detection |
| 2 | Berger et al. | 2009 | Australia | Oceania | 243 | 12 | Molecular detection |
| 3 | Mao et al. | 2009 | China | Asia | 818 | 131 | Morphological detection |
| 4 | WeiMin et al. | 2009 | China | Asia | 671 | 209 | Morphological detection |
| 5 | Liu et al. | 2010 | China | Asia | 292 | 59 | Molecular detection |
| 6 | Lin et al. | 2010 | China | Asia | 446 | 75 | Morphological detection |
| 7 | Young et al. | 2012 | China | Asia | 877 | 218 | Morphological detection |
| 8 | Deng et al. | 2012 | China | Asia | 1149 | 306 | Morphological detection |
| 9 | Zhang et al. | 2014 | China | Asia | 214 | 65 | Molecular detection |
| 10 | Nelli et al. | 2014 | Armenia | Europe | 22 | 4 | Morphological detection |
| 11 | Ruijia et al. | 2015 | China | Asia | 153 | 31 | Morphological detection |
| 12 | Wei et al. | 2015 | China | Asia | 3482 | 565 | Molecular detection |
| 13 | Borteiro et al. | 2015 | Uruguay | South America | 139 | 2 | Morphological detection |
| 14 | Hong et al. | 2016 | China | Asia | 1949 | 229 | Morphological detection |
| 15 | Zhang et al. | 2016 | China | Asia | 276 | 55 | Molecular detection |
| 16 | Wang et al. | 2018 | China | Asia | 511 | 50 | Molecular detection |
| 17 | Zhang et al. | 2020 | China | Asia | 386 | 19 | Molecular detection |
| 18 | Yudhana et al. | 2020 | Indonesia | Asia | 185 | 17 | Molecular detection |
| 19 | Chai et al. | 2020 | Myanmar | Asia | 20 | 15 | Morphological detection |
| 20 | Fu et al. | 2020 | China | Asia | 1556 | 201 | Morphological detection |
| 21 | Zhang et al. | 2020 | China | Asia | 4078 | 447 | Molecular detection |
| 22 | Zhang et al. | 2020 | China | Asia | 386 | 19 | Molecular detection |
| 23 | Fu et al. | 2022 | China | Asia | 1556 | 201 | Morphological detection |
| Snakes | |||||||
| 1 | WeiMin et al. | 2009 | China | Asia | 3 | 3 | Morphological detection |
| 2 | Wang et al. | 2011 | China | Asia | 1160 | 345 | Morphological detection |
| 3 | Zhang et al. | 2014 | China | Asia | 6 | 3 | Molecular detection |
| 4 | Wang et al. | 2014 | China | Asia | 456 | 251 | Morphological detection |
| 5 | Sargsyan et al. | 2014 | Armenia | Europe | 22 | 2 | Morphological detection |
| 6 | Pranashinta et al. | 2017 | Indonesia | Asia | 60 | 41 | Morphological detection |
| 7 | Kondzior et al. | 2018 | Poland | Europe | 59 | 2 | Molecular detection |
| 8 | Wang et al. | 2018 | China | Asia | 346 | 141 | Molecular detection |
| 9 | Lu et al. | 2018 | China | Asia | 30 | 26 | Molecular detection |
| 10 | Xiao et al. | 2019 | China | Asia | 149 | 55 | Molecular detection |
| 11 | Yudhana et al. | 2019 | Indonesia | Asia | 378 | 192 | Morphological detection |
| 12 | Liu et al. | 2020 | China | Asia | 375 | 344 | Molecular detection |
| 13 | Yudhana et al. | 2020 | Indonesia | Asia | 43 | 43 | Morphological detection |
| 14 | Yudhana et al. | 2020 | Indonesia | Asia | 37 | 21 | Morphological detection |
| 15 | Yudhana et al. | 2021 | Indonesia | Asia | 55 | 51 | Morphological detection |
TABLE 2.
Sub‐group analysis of the prevalence of Spirometra based on included studies diagnostic method, country‐level income level, genus and species, climate, average temperature, annual rainfall, humidity and continent
| Heterogeneity** | |||||||
|---|---|---|---|---|---|---|---|
| Number of studies | Sample size | Number infected | Pooled prevalence (%) (95% CI) | I 2 | τ 2 | p Value* | |
| Variable (cat) | |||||||
| Diagnostic method | |||||||
| Morphological detection | 52 | 15,631 | 1131 | 0.104 (0.061 to 0.155) | 98 | 0.074 | <0.001 |
| Income level | |||||||
| High income | 36 | 13,349 | 987 | 0.115 (0.055 to 0.193) | 98 | 0.099 | <0.001 |
| Upper‐middle income | 9 | 1854 | 89 | 0.051 (0.017 to 0.104) | 92 | 0.014 | <0.001 |
| Lower‐middle income | 7 | 428 | 55 | 0.134 (0.039 to 0.273) | 87 | 0.030 | <0.001 |
| Genus and species | |||||||
| Spirometra erinaceieuropaei | 15 | 5295 | 866 | 0.268 (0.123 to 0.445) | 98 | 0.110 | <0.001 |
| Spirometra mansoni | 5 | 733 | 75 | 0.094 (0 to 0.314) | 96 | 0.049 | <0.001 |
| Spirometra mansonoides | 5 | 886 | 39 | 0.051 (0.001 to 0.164) | 84 | 0.020 | <0.001 |
| Spirometra spp. | 27 | 8717 | 151 | 0.049 (0.020 to 0.089) | 93 | 0.039 | <0.001 |
| Climate | |||||||
| Humid continental climate | 15 | 5594 | 311 | 0.142 (0.033 to 0.309) | 98 | 0.131 | <0.001 |
| Tropical savanna climate | 11 | 4153 | 441 | 0.113 (0.046 to 0.204) | 96 | 0.032 | <0.001 |
| Semi‐desert climate | 3 | 168 | 8 | 0.065 (0 to 0.433) | 63 | 0.026 | 0.070 |
| Tropical marine climate | 1 | 55 | 10 | 0.181 (0.092 to 0.293) | NA | NA | NA |
| Subarctic climate | 1 | 635 | 1 | 0.001 (0 to 0.006) | NA | NA | NA |
| Tropical monsoon climate | 2 | 155 | 15 | 0.097(0 to 0.513) | 0 | 0 | 0.350 |
| Humid subtropical climate | 9 | 2426 | 20 | 0.024 (0 to 0.114) | 73 | 0.064 | <0.001 |
| Oceanic climate | 8 | 2188 | 309 | 0.203 (0.026 to 0.487) | 99 | 0.105 | <0.001 |
| Tropical rainforest climate | 2 | 257 | 16 | 0.050 (0 to 0.930) | 86 | 0.011 | 0.007 |
| Average temperature | |||||||
| >20°C | 14 | 2169 | 146 | 0.091 (0.043 to 0.154) | 92 | 0.025 | <0.001 |
| 10–20°C | 37 | 12,827 | 984 | 0.115 (0.057 to 0.190) | 98 | 0.096 | <0.001 |
| <10 | 1 | 635 | 1 | 0.001 (0 to 0.006) | NA | NA | NA |
| Annual rainfall | |||||||
| >1500 mm | 3 | 307 | 35 | 0.128 (0 to 0.733) | 94 | 0.066 | <0.001 |
| 1001–1500 mm | 26 | 10,002 | 696 | 0.101 (0.045 to 0.174) | 98 | 0.071 | <0.001 |
| 401–1000 mm | 19 | 5099 | 379 | 0.105 (0.030 to 0.217) | 98 | 0.099 | <0.001 |
| <400 mm | 4 | 223 | 21 | 0.103 (0 to 0.357) | 85 | 0.031 | <0.001 |
| Humidity | |||||||
| >75 | 4 | 742 | 23 | 0.030 (0 to 0.115) | 89 | 0.010 | <0.001 |
| 40–75 | 44 | 14,666 | 1087 | 0.113 (0.063 to 0.175) | 98 | 0.083 | <0.001 |
| <40 | 4 | 223 | 21 | 0.103 (0 to 0.357) | 85 | 0.031 | <0.001 |
| Continent | |||||||
| Asia | 25 | 5561 | 756 | 0.154 (0.081 to 0.244) | 96 | 0.075 | <0.001 |
| Europe | 6 | 1937 | 10 | 0.005 (0.001 to 0.011) | 0 | 0 | 0.44 |
| North America | 11 | 5511 | 59 | 0.037 (0.009 to 0.081) | 92 | 0.017 | <0.001 |
| Oceania | 7 | 2133 | 296 | 0.203(0.026 to 0.487) | 99 | 0.105 | <0.001 |
| South America | 3 | 489 | 10 | 0.144 (0 to 1.000) | 91 | 0.235 | <0.001 |
| Variable (dog) | |||||||
| Diagnostic method | |||||||
| Morphological detection | 38 | 27,759 | 668 | 0.070 (0.032 to 0.120) | 98 | 0.070 | <0.001 |
| Molecular detection | 3 | 342 | 21 | 0.101 (0 to 0.854) | 95 | 0.112 | <0.001 |
| Income level | |||||||
| High income | 20 | 25,138 | 328 | 0.029 (0.002 to 0.087) | 98 | 0.081 | <0.001 |
| Upper‐middle income | 7 | 1329 | 60 | 0.065 (0.018 to 0.138) | 92 | 0.015 | <0.001 |
| Lower‐middle income | 13 | 1575 | 278 | 0.159 (0.061 to 0.291) | 97 | 0.066 | <0.001 |
| Low income | 1 | 59 | 17 | 0.288 (0.180 to 0.409) | NA | NA | NA |
| Genus and species | |||||||
| Spirometra erinaceieuropaei | 13 | 7997 | 266 | 0.045 (0.006 to 0.116) | 98 | 0.048 | <0.001 |
| Spirometra mansoni | 7 | 1413 | 105 | 0.141 (0 to 0.519) | 96 | 0.201 | <0.001 |
| Spirometra spp. | 21 | 18,691 | 318 | 0.080 (0.029 to 0.154) | 98 | 0.062 | <0.001 |
| Climate | |||||||
| Humid continental climate | 14 | 17,710 | 148 | 0.070 (0.006 to 0.195) | 97 | 0.111 | <0.001 |
| Tropical savannah climate | 10 | 6623 | 134 | 0.050(0 to 0.169) | 97 | 0.075 | <0.001 |
| Semi‐desert climate | 4 | 271 | 6 | 0.026 (0 to 0.092) | 49 | 0.004 | 0.11 |
| Tropical monsoon climate | 5 | 640 | 159 | 0.250 (0.034 to 0.577) | 98 | 0.070 | <0.001 |
| Humid subtropical climate | 1 | 301 | 1 | 0.003 (0 to 0.013) | NA | NA | NA |
| Oceanic climate | 5 | 2224 | 225 | 0.078 (0 to 0.273) | 97 | 0.044 | <0.001 |
| Tropical rainforest climate | 2 | 332 | 16 | 0.050 (0 to 0.510) | 57 | 0.002 | 0.12 |
| Average temperature | |||||||
| >20°C | 15 | 2621 | 345 | 0.162 (0.074 to 0.275) | 97 | 0.059 | <0.001 |
| 10–20°C | 26 | 25,480 | 344 | 0.034 (0.008 to 0.077) | 97 | 0.061 | <0.001 |
| Annual rainfall | |||||||
| >1500 mm | 4 | 846 | 106 | 0.157 (0 to 0.650) | 98 | 0.108 | <0.001 |
| 1001–1500 mm | 19 | 23,654 | 123 | 0.037 (0.002 to 0.109) | 94 | 0.094 | <0.001 |
| 401–1000 mm | 14 | 3330 | 454 | 0.129 (0.055 to 0.227) | 97 | 0.047 | <0.001 |
| <400 mm | 4 | 271 | 6 | 0.026 (0 to 0.092) | 49 | 0.004 | 0.11 |
| Humidity | |||||||
| >75 | 3 | 746 | 44 | 0.057 (0.015 to 0.123) | 48 | 0.001 | 0.14 |
| 40–75 | 33 | 27,019 | 628 | 0.078 (0.031 to 0.143) | 98 | 0.088 | <0.001 |
| <40 | 5 | 336 | 17 | 0.051 (0.003 to 0.152) | 83 | 0.013 | <0.001 |
| Continent | |||||||
| Asia | 28 | 9141 | 421 | 0.076 (0.034 to 0.133) | 97 | 0.055 | <0.001 |
| Europe | 1 | 301 | 1 | 0.003 (0 to 0.013) | NA | NA | NA |
| North America | 5 | 16,311 | 14 | 0.071 (0 to 0.755) | 80 | 0.377 | <0.001 |
| Oceania | 5 | 2224 | 225 | 0.078 (0 to 0.273) | 97 | 0.044 | <0.001 |
| Africa | 2 | 124 | 28 | 0.224 (0 to 0.971) | 60 | 0.005 | 0.11 |
| Variable (frog) | |||||||
| Diagnostic method | |||||||
| Morphological detection | 13 | 9532 | 1640 | 0.189 (0.105 to 0.290) | 96 | 0.037 | <0.001 |
| Molecular detection | 10 | 10,053 | 1308 | 0.121 (0.070 to 0.183) | 95 | 0.014 | <0.001 |
| Income level | |||||||
| High income | 20 | 19,358 | 2912 | 0.143 (0.104 to 0.187) | 96 | 0.015 | <0.001 |
| Upper‐middle income | 1 | 22 | 4 | 0.181 (0.052 to 0.365) | NA | NA | NA |
| Lower‐middle income | 2 | 205 | 32 | 0.385 (0 to 1.000) | 97 | 0.260 | <0.001 |
| Genus and species | |||||||
| Spirometra erinaceieuropaei | 14 | 13,077 | 1777 | 0.130 (0.089 to 0.177) | 95 | 0.011 | <0.001 |
| Spirometra mansoni | 6 | 4793 | 953 | 0.202 (0.135 to 0.280) | 96 | 0.006 | <0.001 |
| Spirometra spp. | 3 | 1715 | 218 | 0.232 (0 to 1.000) | 97 | 0.218 | <0.001 |
| Climate | |||||||
| Humid continental climate | 18 | 18,822 | 2884 | 0.163 (0.125 to 0.204) | 96 | 0.010 | <0.001 |
| Tropical monsoon climate | 1 | 20 | 15 | 0.750 (0.542 to 0.910) | NA | NA | NA |
| Humid subtropical climate | 2 | 315 | 20 | 0.049 (0 to 0.998) | 92 | 0.018 | <0.001 |
| Oceanic climate | 1 | 243 | 12 | 0.049 (0.025 to 0.080) | NA | NA | NA |
| Tropical rainforest climate | 1 | 185 | 17 | 0.091 (0.054 to 0.137) | NA | NA | NA |
| Average temperature | |||||||
| >20°C | 3 | 381 | 50 | 0.273 (0 to 1.000) | 95 | 0.165 | <0.001 |
| 10–20°C | 20 | 19,204 | 2898 | 0.146 (0.107 to 0.190) | 96 | 0.015 | <0.001 |
| Annual rainfall | |||||||
| >1500 mm | 2 | 361 | 35 | 0.096 (0.041 to 0.172) | 0 | <0 | 0.74 |
| 401–1000 mm | 20 | 19,202 | 2909 | 0.162 (0.105 to 0.229) | 96 | 0.031 | <0.001 |
| <400 mm | 1 | 22 | 4 | 0.181 (0.052 to 0.365) | NA | NA | NA |
| Humidity | |||||||
| 40–75 | 23 | 19,585 | 2948 | 0.156 (0.107 to 0.213) | 96 | 0.027 | <0.001 |
| Continent | |||||||
| Asia | 20 | 19,181 | 2930 | 0.181 (0.052 to 0.365) | 96 | 0.025 | <0.001 |
| Europe | 1 | 22 | 4 | 0.172 (0.119 to 0.232) | NA | NA | NA |
| Oceania | 1 | 243 | 12 | 0.049 (0.025 to 0.080) | NA | NA | NA |
| South America | 1 | 139 | 2 | 0.014 (0.001 to 0.040) | NA | NA | NA |
| Variable (snake) | |||||||
| Diagnostic method | |||||||
| Morphological detection | 9 | 2214 | 949 | 0.665 (0.349 to 0.915) | 97 | 0.165 | <0.001 |
| Molecular detection | 6 | 965 | 571 | 0.513 (0.132 to 0.884) | 98 | 0.155 | <0.001 |
| Income level | |||||||
| High income | 9 | 2584 | 1170 | 0.550 (0.252 to 0.830) | 98 | 0.150 | <0.001 |
| Upper‐middle income | 1 | 22 | 2 | 0.090 (0.009 to 0.242) | NA | NA | NA |
| Lower‐middle income | 5 | 573 | 348 | 0.790 (0.402 to 0.995) | 96 | 0.102 | <0.001 |
| Genus and species | |||||||
| Spirometra erinaceieuropaei | 8 | 2147 | 918 | 0.424 (0.142 to 0.736) | 98 | 0.141 | <0.001 |
| Spirometra mansoni | 1 | 3 | 3 | 1.000 (0.712 to 1.000) | NA | NA | NA |
| Spirometra spp. | 6 | 1029 | 599 | 0.752 (0.438 to 0.963) | 96 | 0.092 | <0.001 |
| Climate | |||||||
| Humid continental climate | 9 | 2547 | 1170 | 0.565 (0.284 to 0.824) | 98 | 0.129 | <0.001 |
| Oceanic climate | 1 | 59 | 2 | 0.033 (0.003 to 0.094) | NA | NA | NA |
| Tropical rainforest climate | 5 | 573 | 348 | 0.790(0.402 to 0.995) | 96 | 0.102 | <0.001 |
| Average temperature | |||||||
| >20°C | 5 | 573 | 348 | 0.790 (0.402 to 0.995) | 96 | 0.102 | <0.001 |
| 10–20°C | 9 | 2547 | 1170 | 0.565 (0.284 to 0.824) | 98 | 0.129 | <0.001 |
| <10°C | 1 | 59 | 2 | 0.033 (0.003 to 0.094) | NA | NA | NA |
| Annual rainfall | |||||||
| >1500 mm | 5 | 573 | 348 | 0.790 (0.402 to 0.995) | 96 | 0.102 | <0.001 |
| 401–1000 mm | 9 | 2584 | 1170 | 0.550 (0.252 to 0.830) | 98 | 0.150 | <0.001 |
| <400 mm | 1 | 22 | 2 | 0.090 (0.009 to 0.242) | NA | NA | NA |
| Humidity | |||||||
| >75 | 1 | 59 | 2 | 0.033 (0.003 to 0.094) | NA | NA | NA |
| 40–75 | 14 | 3120 | 1518 | 0.653 (0.444 to 0.835) | 98 | 0.125 | <0.001 |
| Continent | |||||||
| Asia | 13 | 3098 | 1516 | 0.696 (0.502 to 0.859) | 98 | 0.100 | <0.001 |
| Europe | 2 | 81 | 4 | 0.049 (0 to 0.656) | 0 | 0.002 | 0.33 |
Data analysis was conducted using chi‐square tests.
Heterogeneity between studies was evaluated using Cochrane's Q test and the I2 statistic.
2.3. Quality assessment
A Newcastle–Ottawa Scale was implemented for quality assessment of the included studies (Supplementary Table S1) (Modesti et al., 2016). Scoring was based on three following items: selection (maximum of 5 stars), comparability (maximum of 2 stars) and outcome (maximum of 3 stars) (Badri et al., 2022; Eslahi et al., 2021; Eslahi et al., 2022; Mirzadeh et al., 2021)
2.4. Data synthesis and statistical analysis
The pooled prevalence of Spirometra in snakes, frogs, dogs and cats reported globally was calculated with 95% confidence interval. Meta‐regression analysis was conducted to evaluate the impact of average temperature, and year of publication on prevalence. Egger's test and Begg's test were applied to specify the possible publication bias. Moreover, publication bias was assessed by the Luis Furuya‐Kanamori (LFK) index and Doi plot (Barendregt & Doi, 2016). An LFK index within the range of ±1, ±2 and outside ±2 was inferred as symmetrical, slightly/minor asymmetrical and significantly/major asymmetrical, respectively, where symmetrical index indicates the absence of publication bias. Freeman‐Tukey double arcsine transformation for the random‐effects model (based on the inverse variance approach for measuring weight) was used to compute the pooled prevalence estimates. Cochrane's Q test and inconsistency index (I 2 statistics) was used to assess the magnitude of heterogeneity among included studies, with I 2 values of <25%, 25–75% and >75% were taken as low, moderate and high heterogeneity, respectively. A p‐value less than 0.05 was set as statistically significant. All statistical analyses were conducted using the meta‐package of R (version 3.6.1, R Foundation for Statistical Computing, Vienna, Austria) (Team, 2020).
3. RESULTS
3.1. Search results and study selection
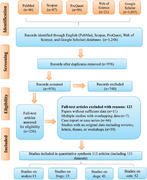
The initial database search identified 1248 articles including 46 from PubMed, 87 from Scopus, 59 from ProQuest, 21 from Web of Science and 1035 from Google Scholar, of which 59 duplicates were removed. Of 976 records screened, 740 articles excluded, as they did not meet the inclusion criteria. Of 236 full‐text articles assessed for eligibility, 125 articles were excluded with the following reasons: papers without sufficient data (n = 11), multiple studies with overlapping data (n = 7), case report or case series (n = 66), studies with no original data including reviews, letters, theses or workshops (n = 39). Finally, 113 articles (including 131 data sets) were included in the current systematic review and meta‐analysis (Figure 1).
FIGURE 1.

Flow diagram of the study design process
3.2. Prevalence in intermediate hosts (snakes and frogs)
For snake hosts, a total of 15 studies (3179 cases) were analysed, of which 1520 harboured Spirometra parasites spargana (Table 2). Global pooled prevalence rate for snakes was 0.6048% (95%CI: 0.3819–0.8068%) (Figure 2). According to the species of the parasite, the estimated pooled prevalence was as follows: 1.000% (95%CI: 0.712–1.000%) for S. mansoni, 0.752% (95%CI: 0.438–0.963%) for Spirometra spp. and 0.424% (95%CI: 0.142–0.736%) for S. erinaceieuropaei (Table 2). The highest prevalence was found in Asia (0.696, 95%CI: 0.502–0.859). The analyses based on different climates and climatic parameters revealed that the infection was most prevalent in regions with tropical rainforest climate (0.790, 95%CI: 0.402–0.995), average temperature of >20°C (0.790, 95%CI: 0.402–0.995), annual rainfall of >1500 mm (0.790, 95%CI: 0.402–0.995) and humidity of 40–75% (0.653, 95%CI: 0.444–0.835). The pooled prevalence rate with regard to the income level was the highest for lower‐middle income countries (0.790, 95%CI: 0.402–0.995) (Table 2).
FIGURE 2.
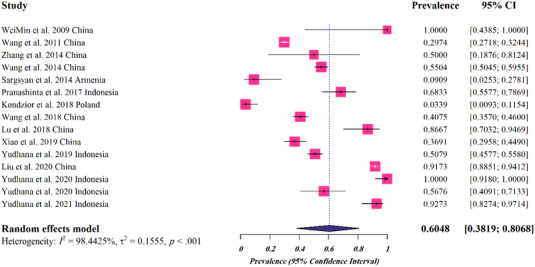
Forest plots for random‐effects meta‐analysis of Spirometra in snakes
For frog hosts, a total of 23 studies (19,585 cases) were analysed, of which 2948 found to be infected with Spirometra parasites spargana, giving a global pooled prevalence of 0.1565% (95%CI: 0.1072–0.2131%) (Table 2 and Figure 3). Regarding the species of the parasite, the estimated pooled prevalence was as follows: 0.232% (95%CI: 0–1.000%) for Spirometra spp., 0.202% (95%CI: 0.135–0.280%) for S. mansoni and 0.130% (95%CI: 0.089–0.177) for S. erinaceieuropaei (Table 2). The highest prevalence rate was related to Asia (0.181, 95%CI: 0.052–0.365). The analyses based on different climates and climatic parameters revealed that the infection was most prevalent in regions with Tropical monsoon climate (0.750, 95%CI: 0.542–0.910), average temperature of >20°C (0.273, 95%CI: 0–1.000), annual rainfall of <400 mm (0.181, 95%CI: 0.052–0.365) and humidity of 40–75% (0.156, 95%CI: 0.107–0.213). The pooled prevalence rate with regard to the income level was highest for lower‐middle income countries (0.385, 95%CI: 0–1.000) (Table 2).
FIGURE 3.

Forest plots for random‐effects meta‐analysis of Spirometra in frogs
3.3. Prevalence in definitive hosts (dogs and cats)
For dog hosts, a total of 41 studies (28,101 cases) were analysed, of which 689 harboured Spirometra parasites, giving a pooled prevalence of 0.0723% (95%CI: 0.0351–0.1215%) (Table 2 and Figure 4). Regarding the species of the parasite, the estimated pooled prevalence was as follows: 0.141% (95%CI: 0–0.519%) for S. mansoni, 0.080% (95%CI: 0.029–0.154%) for Spirometra spp. and 0.045% (95%CI: 0.006–0.116%) for S. erinaceieuropaei (Table 2). The highest prevalence rate was related to Africa (0.224%, 95%CI: 0–0.971%). The analyses based on different climates and climatic parameters revealed that the infection was most prevalent in regions with Tropical monsoon climate (0.250%, 95%CI: 0.034–0.577%), average temperature of >20°C (0.162%, 95%CI: 0.074–0.275%), annual rainfall of >1500 mm (0.157%, 95%CI: 0–0.650%) and humidity of 40–75% (0.078%, 95%CI: 0.031–0.143%). The pooled prevalence rate with regard to the income level was highest for low‐income countries (0.288%, 95%CI: 0.180–0.409%) (Table 2).
FIGURE 4.

Forest plots for random‐effects meta‐analysis of Spirometra in dogs
For cat hosts, a total of 52 studies (15,631 cases) were analysed, of which 1131 were infected with Spirometra parasites, giving a pooled prevalence of 0.1040% (95%CI: 0.0619–0.1555%) (Table 2 and Figure 5). Based on the species of the parasite, the estimated pooled prevalence was as follows: 0.268% (95%CI: 0.123–0.445%) for S. erinaceieuropaei, 0.094% (95%CI: 0–0.314%) for S. mansoni, 0.051% (95%CI: 0.001–0.164%) for S. mansonoides, 0.049% (95%CI: 0.020–0.089%) for Spirometra spp. (Table 2). The highest prevalence rate was related to Oceania (0.203%, 95%CI: 0.026–0.487%). The analyses with regard to different climates and climatic parameters revealed that the infection was most prevalent in regions with Oceanic climate (0.203%, 95%CI: 0.026–0.487%), average temperature of 10–20°C (0.115%, 95%CI: 0.057–0.190%), annual rainfall of >1500 mm (0.128%, 95%CI: 0–0.733%) and humidity of 40–75% (0.113%, 95%CI: 0.063–0.175%). The pooled prevalence rate with regard to the income level was highest for lower‐middle income countries (0.134%, 95%CI: 0.039–0.273%) (Table 2).
FIGURE 5.
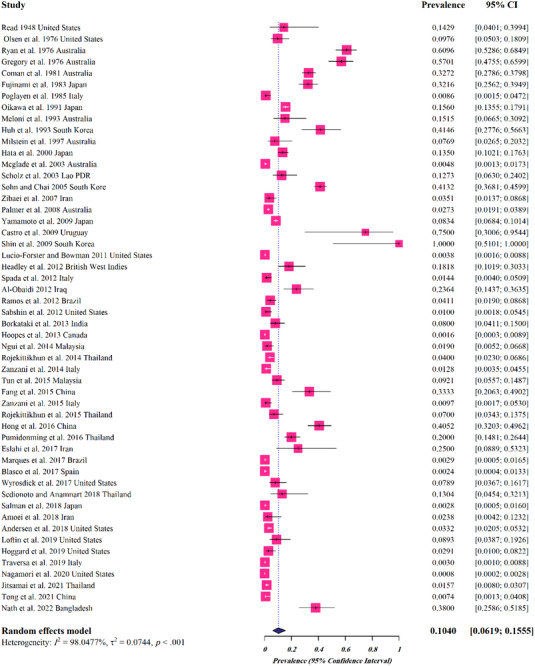
Forest plots for random‐effects meta‐analysis of Spirometra in cats
3.4. Prevalence based on diagnostic methods
There were two diagnostic procedures used for the detection of adult and spargana of Spirometra spp. in included studies (Table 2). All studies on intermediate hosts (snakes and frogs) were conducted on carcasses, while studies on definitive hosts (dogs and cats) were performed on stool specimens or carcasses. Totally 112 studies used morphological detection method and 19 studies used molecular detection method. According to the diagnostic method, the highest pooled prevalence was related to morphological method for studies on snakes, frog and cats with rate of 0.665% (95%CI: 0.349–0.915%), 0.189% (95%CI: 0.105–0.290%) and 0.104% (95%CI: 0.061–0.155%), respectively. However, all studies on cats were performed via morphological method (Table 2). Regarding studies on dogs, the highest pooled prevalence was observed for molecular technique (0.101%, 95%CI: 0–0.854%) (Table 2).
3.5. Meta regression analysis
Heterogeneity was noted for the year of publication and average temperature. Accordingly, the results of the test were significant for the year of publication for studies on cats (slop = 0.0069, p < 0.0062), and average temperature for studies on dogs (slop = 0.0149, p < 0.0172) (Supplementary Figure S1).
3.6. Publication bias
Asymmetry of the funnel plot indicates that publication bias was present in studies on cats (Egger's test: t = 3.31, p = 0.0017, and Begg's test: p = 0.0043) and dogs (Egger's test: t = 5.30, p = 0.0001, and Begg's test: p = 0.0003). There was no statistical publication bias for studies in snakes and frogs (Supplementary Figure S2A1–B4). Furthermore, asymmetrical Doi plots suggest presence of publication bias for prevalence in snakes, dogs and cats. Accordingly, there was major asymmetry for snakes (LFK index = 2.93), dogs (LFK index = 5.32) and cats (LFK index = 3.57). In contrast, there was no asymmetry for prevalence in frogs (LFK index = 0.92) (Supplementary Figure S2C1–C4).
A QGIS3 software (version 3.1) was used to provide a map representing the global prevalence of Spirometra in snakes, frogs, dogs and cats in different geographical regions of the world based on included studies (Figure 6)
FIGURE 6.
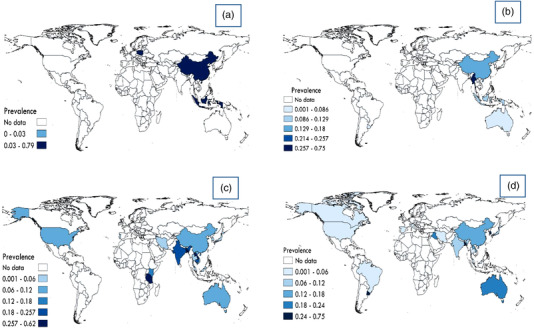
Prevalence of Spirometra in (a) snakes, (b) frogs, (c) dogs and (d) cats in different geographical regions of the world based on the included studies
4. DISCUSSION
The current systematic review and meta‐analysis aims to estimate the global prevalence of Spirometra infection in snakes, frogs, dogs and cats. The overall prevalence of Spirometra in intermediate hosts and definitive hosts was found to be 0.313% and 0.089%, respectively. Among intermediate hosts analysed in this study, Spirometra infection was more prevalent in Asia with higher rate in snakes (0.6048%) than in frogs. Occurrence of infection with plerocercoid in snakes is more probably caused by ingestion of infected frogs than acquisition of the procercoid (Oda et al., 2016). In reptile hosts subcutaneous tissues and muscles are the frequent sites involved with infective larvae of the parasite (Mendoza‐Roldan et al., 2020). Snakes are the most common reptiles to be an intermediate host for Spirometra, a best‐known reptile‐borne zoonotic tapeworm. The raw or undercooked snakes meat for consumption or for purposes such as zootherapeutic remedies are regarded as a rout for transmission of sparganosis (Magnino et al., 2009; Mendoza‐Roldan et al., 2020).
One of the causes for the growth in reports of foodborne diseases in recent years has been the rising demand for animal protein, as well as, exotic and raw foods resulted in the expansion of some farming systems (wildlife farming) in emerging or underdeveloped nations where health monitoring may be inadequately managed (Broglia & Kapel, 2011; Xiao et al., 2021).
The complex life cycle of Spirometra parasites gives them the opportunity to be acquired not only via consumption of raw wild animal products (e.g. snakes and frogs infected with plerocercoids), but also by drinking contaminated water containing infected copepods (water‐borne route) (Yudhana et al., 2021).
It has been documented that the consumption of wild meat (bushmeat) has a direct relationship with poverty and low‐income communities, where is a lack of sanitary drinking water sources, sanitation and hygiene (Badri et al., 2022; Eslahi et al., 2021; Kouassi et al., 2019; Maleki et al., 2020; Prüss‐Ustün et al., 2014; Van Velden et al., 2020). Also, water hygienic interventions in low‐ and low‐middle‐income settings, which place less emphasis on limiting animal faeces exposure in water sources, help to maintain parasitic remains and provide the water‐borne cycle (Delahoy et al., 2018).
In Southeast Asian countries over the last decades, there has been a growing interest in snakes, and despite the negative impact on wildlife, snake farming and the international trade of snakes have emerged as significant phenomena. It is mostly visible in the Chinese population relative to the involvement of snakes in their dietary habits (Aust et al., 2017; Xiao et al., 2019).
Furthermore, sushi and sashimi as popular dishes prepared from the meat of frogs and snakes are the major sources of human sparganosis in Southeast Asia, where there is still a lack of awareness relating to the risk of this infection (Nawa et al., 2005).
Among the definitive hosts we analysed in the current study, cats have a higher infection rate (0.1040%) than dogs. This finding suggests that cats are the potential sources of maintenance for tapeworms of the genus Spirometra. They have a significant role in environmental contamination, transmission of many microbial pathogens and can serve as reservoirs for several parasites of both public and wildlife health importance (Hernandez et al., 2018; Jeon et al., 2018; Taghipour et al., 2021). Cats are predators of a broad variety of prey, including amphibians and reptiles. Thus, it is believed that the prevalence of Spirometra parasites is impacted by host diets and the access of definitive hosts to infected intermediate hosts (Hernandez et al., 2018).
The identification of both larval stage and adults of Spirometra species is through morphological and molecular approaches (Jeon et al., 2018). The morphological identification of Spirometra tapeworms to the species level is based on taxonomic differences (Badri et al., 2017). Recent advances of molecular techniques promote species identification for both adults and larvae. These techniques that rely on DNA sequencing of the whole mitochondrial COI (cytochrome c oxidase subunit I) gene are considered as the only approach to precisely specify the species (Kuchta et al., 2021). However, it is dependent on the availability of gene sequences and the accuracy of data, especially in the case that parasites are lacerated in the host's cadaver due to the road‐killing incidences (Eslahi et al., 2021; Tang et al., 2017).
Asia was the most prevalent region for Spirometra infection in snakes and frogs, whereas Africa and Oceania had been shown to have the highest pooled prevalence rates in dogs and cats, respectively. The geographical variation found for the prevalence emphasises that the infection risk is different in each region. Spirometra tapeworms have a broad host spectrum and they have been reported in domesticated and wild animals from different geographical regions all over the world (except Antarctica) (Bagrade et al., 2021). Although, this infection is mostly observed in tropical and sub‐tropical areas with the highest prevalence in South‐east Asia and East Africa (Farrar et al., 2013; L. N. Liu et al., 2015). This statement is in consistent with our analyses suggesting that oceanic and tropical climates present the highest prevalence for the infection.
The persistence of the heteroxenous life cycle and survival of Spirometra are affected by environmental factors including physiochemical conditions (pH, pCO2, O2, viscosity) and temperature (Muller & Wakelin, 2002). Moreover, humid areas with abundant river networks offer an optimal condition for development of Spirometra parasites (Marta Kołodziej‐Sobocińska et al., 2019). However, plerocercoids are able to tolerate stress conditions, such as diverse range of pH. As well, they have ability to survive in various vertebrate hosts even cold‐blooded ones, except fish (Kavana, 2015; Muller & Wakelin, 2002).
Given that the information on distinct morphological traits of both adults and plerocercoids are limited and there were lack of molecular diagnostics at the time of the study, most reports cannot identify Spirometra to the species level. Regardless of the fact that several species of the parasite have been identified, the taxonomy of Spirometra tapeworms is still unclear and needs to be more clarified (Bagrade et al., 2021).
The results of the present systematic review and meta‐analysis should be interpreted cautiously referring to some of the limitations. First, our analyses may have been affected by publication bias, as the result of absence or the low number of published studies from some geographic regions. Second, there were several single case reports of Spirometra species other than those included in the current study. Another point is the fact that this study was limited to publications in English. Finally, there were small‐study effects in some studies we included in our analyses attributable to (1) small sample size, (2) sampling bias relating to the nature of sample collection from wildlife over several years and (3) lack of a sensitive diagnostic technique. Despite these limitations, this study provides the most comprehensive estimates of the prevalence of Spirometra infection in snakes, frogs, dogs and cats from a global perspective.
5. CONCLUSION
The findings of the current systematic review and meta‐analysis indicate the relatively significant burden and current status of Spirometra infection in snakes, frogs, dogs and cats in different parts of the world and highlight the importance of conducting investigations in more geographical regions. Furthermore, our results showed that this infection may represent a significant risk for public health, especially in low‐ and lower‐middle‐income countries and in regions with oceanic and tropical climates. Paying attention to preventive strategies such as protection of aquaculture systems from being contaminated with faeces of dogs and cats, development of precise diagnostic approaches for foodborne parasitic infection during preparation, distribution, and selling stages, improving public education regarding the hazard of consuming reptile and amphibian products. Moreover, breaking the life cycle of the parasites and decreasing the burden of infective larvae in the aquatic environment play a key role in a large‐scale control measure in endemic regions.
AUTHOR CONTRIBUTIONS
MB, LMMDC, MFH, and AVE contributed to study design. AK, LZ, FB and PM searched for primary publications, screened and appraised primary studies. MB and AVE extracted the data and wrote the study manuscript. MO contributed to data analysis. All authors read the manuscript and participated in the preparation of the final version of the manuscript.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interests.
ETHICAL APPROVAL
None required.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.932.
Supporting information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
We thank members of the Metabolic Diseases Research Center, Research Institute for Prevention of Non‐Communicable Diseases, Qazvin, Iran for their assistance with this project. This work was supported by Metabolic Diseases Research Center, Research Institute for Prevention of Non‐Communicable Diseases, Qazvin, Iran under the contract no. IR.QUMS.REC.1401.043.
Badri, M. , Olfatifar, M. , KarimiPourSaryazdi, A. , Zaki, L. , Madeira de Carvalho, L. M. , Fasihi Harandi, M. , Barikbin, F. , Madani, P. , & Vafae Eslahi, A. (2022). The global prevalence of Spirometra parasites in snakes, frogs, dogs, and cats: A systematic review and meta‐analysis. Veterinary Medicine and Science, 8, 2785–2805. 10.1002/vms3.932
Milad Badri and Leila Zaki contributed equally to this work.
Contributor Information
Meysam Olfatifar, Email: Ol.meysam92@gmail.com.
Aida Vafae Eslahi, Email: Vafaeeslahia@yahoo.com.
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.
REFERENCES
- Anantaphruti, M. T. , Nawa, Y. , & Vanvanitchai, Y. (2011). Human sparganosis in Thailand: An overview. Acta Tropica, 118(3), 171–176. [DOI] [PubMed] [Google Scholar]
- Aust, P. W. , Van Tri, N. , Natusch, D. J. D. , & Alexander, G. J. (2017). Asian snake farms: Conservation curse or sustainable enterprise? Oryx, 51(3), 498–505. [Google Scholar]
- Badri, M. , Eslahi, A. V. , Majidiani, H. , & Pirestani, M. (2017). Spirometra erinaceieuropaei in a wildcat (Felis silvestris) in Iran. Veterinary Parasitology: Regional Studies and Reports, 10, 58–61. 10.1016/j.vprsr.2017.08.004 [DOI] [PubMed] [Google Scholar]
- Badri, M. , Olfatifar, M. , Karim, M. R. , Modirian, E. , Houshmand, E. , Abdoli, A. , Nikoonejad, A. , Sotoodeh, S. , Zargar, A. , & Samimi, R. (2022). Global prevalence of intestinal protozoan contamination in vegetables and fruits: A systematic review and meta‐analysis. Food Control, 133, 108656. 10.1016/j.foodcont.2021.108656 [DOI] [Google Scholar]
- Badri, M. , Olfatifar, M. , Wandra, T. , Budke, C. M. , Mahmoudi, R. , Abdoli, A. , Hajialilo, E. , Pestehchian, N. , Ghaffarifar, F. , & Foroutan, M. (2022). The prevalence of human trichuriasis in Asia: A systematic review and meta‐analysis. Parasitology Research, 1–10. 10.1007/s00436-021-07365-8 [DOI] [PubMed] [Google Scholar]
- Bagrade, G. , Králová‐Hromadová, I. , Bazsalovicsová, E. , Radačovská, A. , & Kołodziej‐Sobocińska, M. (2021). The first records of Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidae), a causative agent of human sparganosis, in Latvian wildlife. Parasitology Research, 120(1), 365–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barendregt, J. J. , & Doi, S. A. (2016). MetaXL user guide. Version, 4, 2011–2016.
- Broglia, A. , & Kapel, C. (2011). Changing dietary habits in a changing world: Emerging drivers for the transmission of foodborne parasitic zoonoses. Veterinary Parasitology, 182(1), 2–13. [DOI] [PubMed] [Google Scholar]
- Cui, J. , Li, N. , Wang, Z. Q. , Jiang, P. , & Lin, X. M. (2011). Serodiagnosis of experimental sparganum infections of mice and human sparganosis by ELISA using ES antigens of Spirometra mansoni spargana. Parasitology Research, 108(6), 1551–1556. [DOI] [PubMed] [Google Scholar]
- Delahoy, M. J. , Wodnik, B. , McAliley, L. , Penakalapati, G. , Swarthout, J. , Freeman, M. C. , & Levy, K. (2018). Pathogens transmitted in animal feces in low‐and middle‐income countries. International Journal of Hygiene and Environmental Health, 221(4), 661–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslahi, A. V. , Hashemipour, S. , Olfatifar, M. , Houshmand, E. , Hajialilo, E. , Mahmoudi, R. , Badri, M. , & Ketzis, J. K. (2022). Global prevalence and epidemiology of Strongyloides stercoralis in dogs: A systematic review and meta‐analysis. Parasites & Vectors, 15(1), 1–13. 10.1186/s13071-021-05135-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eslahi, A. V. , Mowlavi, G. , Houshmand, E. , Pirestani, M. , Majidiani, H. , Nahavandi, K. H. , Johkool, M. G. , & Badri, M. (2021). Occurrence of Dioctophyme renale (Goeze, 1782) in road‐killed canids of Iran and its public health implication. Veterinary Parasitology: Regional Studies and Reports, 24, p.100568. 10.1016/j.vprsr.2021.100568 [DOI] [PubMed] [Google Scholar]
- Eslahi, A. V. , Olfatifar, M. , Houshmand, E. , Johkool, M. G. , Zibaei, M. , Foroutan, M. , Hosseini, H. , & Badri, M. (2021). Prevalence of Strongyloides stercoralis in the immunocompetent and immunocompromised individuals in Iran: A systematic review and meta‐analysis. Transactions of the Royal Society of Tropical Medicine and Hygiene, 116, 87–99. 10.1093/trstmh/trab104 [DOI] [PubMed] [Google Scholar]
- Eslahi, A. V. , Olfatifar, M. , Karim, M. R. , AbuOdeh, R. , Modirian, E. , Houshmand, E. , Abdoli, A. , Samimi, R. , Sotoodeh, S. , & Mahmoudi, R. (2021). Global incidence of helminthic contamination of vegetables, cucurbits and fruits: A systematic review and meta‐analysis. Food Control, 133, 108582. 10.1016/j.foodcont.2021.108582 [DOI] [Google Scholar]
- Farrar, J. , Hotez, P. J. , Junghanss, T. , Kang, G. , Lalloo, D. , & White, N. J. (2013). Manson's tropical diseases E‐book. Elsevier health sciences. [Google Scholar]
- Hernandez, S. M. , Loyd, K. A. T. , Newton, A. N. , Carswell, B. L. , & Abernathy, K. J. (2018). The use of point‐of‐view cameras (Kittycams) to quantify predation by colony cats (Felis catus) on wildlife. Wildlife Research, 45(4), 357–365. [Google Scholar]
- Jeon, H.‐K. , Park, H. , Lee, D. , Choe, S. , & Eom, K. S. (2018). Spirometra decipiens (Cestoda: Diphyllobothriidae) collected in a heavily infected stray cat from the Republic of Korea. The Korean Journal of Parasitology, 56(1), 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavana, N. J. , Kassuku, A. A. , & Kasanga, C. J. (2015). Incubation of Spirometra eggs at laboratory conditions by Modified Harada‐Mori method. Huria: Journal of the Open University of Tanzania, 19(1), 29–36. [Google Scholar]
- Kavana, N. J. , Lim, L. H. S. , & Ambu, S. (2014). The life‐cycle of Spirometra species from Peninsular Malaysia. Tropical Biomedicine, 31, 487–495. [PubMed] [Google Scholar]
- Kavana, N. J. (2015). Studies on spirometra species isolate from Minjingu and seroprevalence of human sparganosis in selected Districts of Manyara and Arusha regions, Tanzania. Sokoine University of Agriculture. 41.73.194.142. [Google Scholar]
- Kim, B.‐M. , Kim, D. J. , Chang, M.‐Y. , Kim, Y. J. , Kim, J. H. , & You, J. K. (2020). Axillary sparganosis, changes in ultrasound images over six months: A case report. Radiology Case Reports, 15(3), 177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kołodziej‐Sobocińska, M. , & Miniuk, M. (2018). Sparganosis neglected zoonosis and its reservoir in wildlife. Medycyna Weterynaryjna, 74(4), 224–227. [Google Scholar]
- Kołodziej‐Sobocińska, M. , Stojak, J. , Kondzior, E. , Ruczyńska, I. , & Wójcik, J. M. (2019). Genetic diversity of two mitochondrial DNA genes in Spirometra erinaceieuropaei (Cestoda: Diphyllobothridae) from Poland. Journal of Zoological Systematics and Evolutionary Research, 57(4), 764–777. [Google Scholar]
- Kouassi, J. A. K. , Normand, E. , Koné, I. , & Boesch, C. (2019). Bushmeat consumption and environmental awareness in rural households: A case study around Ta National Park, Côte d'Ivoire. Oryx, 53(2), 293–299. [Google Scholar]
- Kuchta, R. , Kołodziej‐Sobocińska, M. , Brabec, J. , Młocicki, D. , Sałamatin, R. , & Scholz, T. (2021). Sparganosis (Spirometra) in Europe in the molecular era. Clinical Infectious Diseases, 72(5), 882–890. [DOI] [PubMed] [Google Scholar]
- Kuchta, R. , Scholz, T. , Brabec, J. , & Narduzzi‐Wicht, B. (2015). Diphyllobothrium, Diplogonoporus and Spirometra . Biology of foodborne parasites. Section III important foodborne parasites. P28. eBook ISBN9780429095238.
- Le, A. T. , Do, L.‐Q. T. , Nguyen, H.‐B. T. , Nguyen, H.‐N. T. , & Do, A. N. (2017). Case report: The first case of human infection by adult of Spirometra erinaceieuropaei in Vietnam. BMC Infectious Diseases, 17(1), 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, M.‐W. , Song, H.‐Q. , Li, C. , Lin, H.‐Y. , Xie, W.‐T. , Lin, R.‐Q. , & Zhu, X.‐Q. (2011). Sparganosis in mainland China. International Journal of Infectious Diseases, 15(3), e154–e156. [DOI] [PubMed] [Google Scholar]
- Liu, L. N. , Wang, Z. Q. , Zhang, X. , Jiang, P. , Qi, X. , Liu, R. D. , Zhang, Z. F. , & Cui, J. (2015). Characterization of Spirometra erinaceieuropaei plerocercoid cysteine protease and potential application for serodiagnosis of sparganosis. PLoS Neglected Tropical Diseases, 9(6), e0003807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, Q. , Li, M.‐W. , Wang, Z.‐D. , Zhao, G.‐H. , & Zhu, X.‐Q. (2015). Human sparganosis, a neglected food borne zoonosis. The Lancet Infectious Diseases, 15(10), 1226–1235. [DOI] [PubMed] [Google Scholar]
- Magnino, S. , Colin, P. , Dei‐Cas, E. , Madsen, M. , McLauchlin, J. , Nöckler, K. , Maradona, M. P. , Tsigarida, E. , Vanopdenbosch, E. , & Van Peteghem, C. (2009). Biological risks associated with consumption of reptile products. International Journal of Food Microbiology, 134(3), 163–175. [DOI] [PubMed] [Google Scholar]
- Maleki, B. , Dalimi, A. , Majidiani, H. , Badri, M. , Gorgipour, M. , & Khorshidi, A. (2020). Parasitic infections of wild boars (Sus scrofa) in Iran: A literature review. Infectious Disorders‐Drug Targets (Formerly Current Drug Targets‐Infectious Disorders), 20(5), 585–597. [DOI] [PubMed] [Google Scholar]
- Mendoza‐Roldan, J. A. , Modry, D. , & Otranto, D. (2020). Zoonotic parasites of reptiles: A crawling threat. Trends in Parasitology, 36(8), 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirzadeh, M. , Olfatifar, M. , Eslahi, A. V. , Abdoli, A. , Houshmand, E. , Majidiani, H. , Johkool, M. G. , Askari, S. , Hashemipour, S. , & Badri, M. (2021). Global prevalence of Trichomonas vaginalis among female sex workers: A systematic review and meta‐analysis. Parasitology Research, 120(7), 2311–2322. [DOI] [PubMed] [Google Scholar]
- Modesti, P. A. , Reboldi, G. , Cappuccio, F. P. , Agyemang, C. , Remuzzi, G. , Rapi, S. , Perruolo, E. , & Parati, G. (2016). Panethnic differences in blood pressure in Europe: A systematic review and meta‐analysis. PloS One, 11(1), e0147601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller, R. , & Wakelin, D. (2002). Worms and human disease . CABi.
- Nawa, Y. , Hatz, C. , & Blum, J. (2005). Sushi delights and parasites: The risk of fishborne and foodborne parasitic zoonoses in Asia. Clinical Infectious Diseases, 41(9), 1297–1303. 10.1086/496920 [DOI] [PubMed] [Google Scholar]
- Nithiuthai, S. , Anantaphruti, M. T. , Waikagul, J. , & Gajadhar, A. (2004). Waterborne zoonotic helminthiases. Veterinary Parasitology, 126(1–2), 167–193. [DOI] [PubMed] [Google Scholar]
- Oda, F. H. , Borteiro, C. , da Graca, R. J. , Tavares, L. E. R. , Crampet, A. , Guerra, V. , Lima, F. S. , Bellay, S. , Karling, L. C. , & Castro, O. (2016). Parasitism by larval tapeworms genus Spirometra in South American amphibians and reptiles: New records from Brazil and Uruguay, and a review of current knowledge in the region. Acta Tropica, 164, 150–164. [DOI] [PubMed] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , Shamseer, L. , Tetzlaff, J. M. , & Moher, D. (2021). Updating guidance for reporting systematic reviews: Development of the PRISMA 2020 statement. Journal of Clinical Epidemiology, 134, 103–112. [DOI] [PubMed] [Google Scholar]
- Prüss‐Ustün, A. , Bartram, J. , Clasen, T. , jr Colford , J. M., Cumming, O. , Curtis, V. , Bonjour, S. , Dangour, A. D. , De France, J. , & Fewtrell, L. (2014). Burden of disease from inadequate water, sanitation and hygiene in low‐and middle‐income settings: A retrospective analysis of data from 145 countries. Tropical Medicine & International Health, 19(8), 894–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghipour, A. , Khazaei, S. , Ghodsian, S. , Shajarizadeh, M. , Olfatifar, M. , Foroutan, M. , Eslahi, A. V. , Tsiami, A. , Badri, M. , & Karanis, P. (2021). Global prevalence of Cryptosporidium spp. in cats: A systematic review and meta‐analysis. Research in Veterinary Science, 137, 77–85. [DOI] [PubMed] [Google Scholar]
- Tang, T. H. C. , Wong, S. S. Y. , Lai, C. K. C. , Poon, R. W. S. , Chan, H. S. Y. , Wu, T. C. , Cheung, Y.‐F. , Poon, T.‐L. , Tsang, Y.‐P. , & Tang, W.‐L. (2017). Molecular identification of Spirometra erinaceieuropaei tapeworm in cases of human sparganosis, Hong Kong. Emerging Infectious Diseases, 23(4), 665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Team, R. C. (2020). R Core Team R. R: A Language and Environment for Statistical Computing R Foundation for Statistical Computing, Vienna, Austria. ISBN 3‐900051‐07‐0. Available at http://www.R‐project.org/ [Google Scholar]
- Van Velden, J. L. , Wilson, K. , Lindsey, P. A. , McCallum, H. , Moyo, B. H. Z. , & Biggs, D. (2020). Bushmeat hunting and consumption is a pervasive issue in African savannahs: Insights from four protected areas in Malawi. Biodiversity and Conservation, 29(4), 1443–1464. [Google Scholar]
- Wang, F. , Li, W. , Hua, L. , Gong, S. , Xiao, J. , Hou, F. , Ge, Y. , & Yang, G. (2014). Spirometra (Pseudophyllidea, Diphyllobothriidae) severely infecting wild‐caught snakes from food markets in Guangzhou and Shenzhen, Guangdong, China: Implications for public health. The Scientific World Journal, 2014, 874014. 10.1155/2014/874014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiwanitkit, V. (2005). A review of human sparganosis in Thailand. International Journal of Infectious Diseases, 9(6), 312–316. [DOI] [PubMed] [Google Scholar]
- Xiao, L. , Lu, Z. , Li, X. , Zhao, X. , & Li, B. V. (2021). Why do we need a wildlife consumption ban in China? Current Biology, 31(4), R168–R172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao, X. , Qi, R. , Han, H.‐J. , Liu, J.‐W. , Qin, X.‐R. , Fang, L.‐Z. , Zhou, C.‐M. , Gong, X.‐Q. , Lei, S.‐C. , & Yu, X.‐J. (2019). Molecular identification and phylogenetic analysis of Cryptosporidium, Hepatozoon and Spirometra in snakes from central China. International Journal for Parasitology: Parasites and Wildlife, 10, 274–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki, H. , Sanpool, O. , Rodpai, R. , Sadaow, L. , Laummaunwai, P. , Un, M. , Thanchomnang, T. , Laymanivong, S. , Aung, W. P. P. , & Intapan, P. M. (2021). Spirometra species from Asia: Genetic diversity and taxonomic challenges. Parasitology International, 80, 102181. [DOI] [PubMed] [Google Scholar]
- Yudhana, A. , Praja, R. N. , & Kartikasari, A. M. (2021). Sparganosis (Spirometra spp.) in Asian Water Monitor (Varanus salvator): A medical implications for veterinarians, breeders, and consumers. Veterinary World, 14(9), 2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yudhana, A. , Praja, R. N. , & Supriyanto, A. (2019). The medical relevance of Spirometra tapeworm infection in Indonesian Bronzeback snakes (Dendrelaphis pictus): A neglected zoonotic disease. Veterinary World, 12(6), 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X. , Hong, X. , Liu, S. N. , Jiang, P. , Zhao, S. C. , Sun, C. X. , Wang, Z. Q. , & Cui, J. (2020). Large‐scale survey of a neglected agent of sparganosis Spirometra erinaceieuropaei (Cestoda: Diphyllobothriidae) in wild frogs in China. PLoS Neglected Tropical Diseases, 14(2), e0008019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary material.


