Abstract
Background and Aims:
Patients with NAFLD with significant hepatic fibrosis (Stage ≥ 2) are at increased risk of liver-related morbidity and are candidates for pharmacologic therapies. In this study, we compared the diagnostic accuracy of MEFIB (the combination of magnetic resonance elastography [MRE] and Fibrosis-4 [FIB-4]) and FAST (FibroScan–aspartate aminotransferase; combined liver stiffness measurement by vibration-controlled transient elastography, controlled attenuation parameter, and aspartate aminotransferase) for detecting significant fibrosis.
Approach and Results:
This prospective cohort study included 234 consecutive patients with NAFLD who underwent liver biopsy, MRE, and FibroScan at the University of California San Diego (UCSD cohort) and an independent cohort (N = 314) from Yokohama City University, Japan. The primary outcome was diagnostic accuracy for significant fibrosis (Stage ≥ 2). The proportions of significant fibrosis in the UCSD and Yokohama cohorts were 29.5% and 66.2%, respectively. Area under the receiver operating characteristic curve (95% CI) of MEFIB (0.860 [0.81–0.91]) was significantly higher than that of FAST (0.757 [0.69–0.82]) in the UCSD cohort (p = 0.005), with consistent results in the Yokohama cohort (AUROC, 0.899 [MEFIB] versus 0.724 [FAST]; p < 0.001). When used as the rule-in criteria (MEFIB, MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6; FAST ≥ 0.67), the positive predictive value for significant fibrosis was 91.2%–96.0% for MEFIB and 74.2%–89.2% for FAST. When used as the rule-out criteria (MEFIB, MRE < 3.3 kPa and FIB-4 < 1.6; FAST ≤ 0.35), the negative predictive value for significant fibrosis was 85.6%–92.8% for MEFIB and 57.8%–88.3% for FAST.
Conclusions:
MEFIB has higher diagnostic accuracy than FAST for significant fibrosis in NAFLD, and our results support the utility of a two-step strategy for detecting significant fibrosis in NAFLD.
INTRODUCTION
NAFLD is one of the main causes of chronic liver disease, and > 25% of the general population is affected by NAFLD worldwide.[1,2] Due to the increase of patients with diabetes mellitus and metabolic syndrome, the number of patients with NAFLD has been rising, resulting in NAFLD emerging as a major health problem.[3–5] A subset of patients with NAFLD has the progressive form called NASH, which may lead to cirrhosis, HCC, and liver failure. Therefore, the identification of patients with NAFLD with a high risk of disease progression is an unmet need in clinical practice.
Recent studies have demonstrated that liver fibrosis is the most important prognostic feature in NAFLD.[6,7] Liver biopsy is a gold standard method for diagnosing liver fibrosis, but liver biopsy has several limitations including its invasiveness, sampling error, and interobserver and intraobserver reproducibility.[8,9] As a result of the limitations of liver biopsy, several noninvasive methods have been developed and are in use in clinical practice. Noninvasive assessment for predicting hepatic fibrosis in NAFLD includes serum-based fibrosis markers, ultrasound-based methods, and MRI-based methods.[10,11]
Treatment for high-risk patients with NAFLD is in high demand; but there is no drug approved by the US Food and Drug Administration currently, and many clinical trials are ongoing. Patients with significant fibrosis (Stage ≥ 2) are candidates for clinical trial participation given the risk for poor prognosis. However, a high screening failure rate is a major problem of NASH clinical trials, and a non-invasive method for detecting candidates for clinical trials and reducing unnecessary liver biopsy is needed. The FibroScan–aspartate aminotransferase (FAST) score is derived from combined noninvasive measures of liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE), controlled attenuation parameter (CAP), and aspartate aminotransferase (AST); and the utility for detecting patients at risk of progressive NASH with significant activity and fibrosis has been reported.[12] Recently, we have demonstrated that the MEFIB index, combining Fibrosis-4 (FIB-4) as a serum-based marker and magnetic resonance elastography (MRE), may be used for noninvasive identification of patients with NAFLD and significant fibrosis.[13] To address these gaps in knowledge, we compared the diagnostic ability of MEFIB and FAST in a well-characterized cohort of adults with NAFLD and contemporaneous liver biopsy, LSM by MRE and VCTE, and CAP assessment at the University of California San Diego (UCSD). We then evaluated the diagnostic ability of MEFIB and FAST in a separate Yokohama (Japan) cohort.
PATIENTS AND METHODS
Study design
This is a prospective study that includes a well-characterized cohort with biopsy-proven NAFLD. This study includes 234 consecutive uniquely phenotyped adults who underwent liver biopsy with contemporaneous MRE, VCTE, and CAP assessment at the NAFLD Research Center, UCSD, from August 2014 through March 2021. Furthermore, a total of 314 consecutive patients with NAFLD who underwent liver biopsy with contemporaneous MRE, VCTE, and CAP assessment at Yokohama City University from March 2014 through April 2021 were also enrolled in the study as a geographically distinct cohort. All patients completed written informed consent prior to enrollment. The study was approved by the institutional review boards at both sites.
Inclusion and exclusion criteria
In the UCSD cohort, adults (≥ 18 years of age) with biopsy-proven NAFLD and written informed consent were included in the study. For this study, patients were included if LSM by MRE, LSM by VCTE, and CAP were measured contemporaneously with liver biopsy. Patients meeting any of the following criteria were excluded from the study: significant alcohol consumption (defined as ≥ 14 drinks/week for men or ≥ 7 drinks/week for women) within the previous 2-year period; underlying liver disease including hepatitis B, hepatitis C, hemochromatosis, Wilson’s disease, alpha-1 antitrypsin deficiency, glycogen storage disease, autoimmune hepatitis, and cholestatic or vascular liver disease; clinical or laboratory evidence of secondary causes or chronic conditions associated with hepatic steatosis including nutritional disorders, HIV infection, and use of steatogenic drugs such as amiodarone, glucocorticoids, methotrexate, l-asparaginase, and valproic acid; major systemic illnesses; decompensated liver disease (defined as Child-Pugh score > 7 points); contraindications to MRI including metallic implants, claustrophobia, and body circumference exceeding the imaging chamber capacity; pregnancy or attempting to become pregnant.
In the Yokohama cohort, adults (≥ 18 years of age) with biopsy-proven NAFLD and written informed consent were included in the study. For this study, patients were included if LSM by MRE, LSM by VCTE, and CAP were measured contemporaneously with liver biopsy. Patients with a history of excessive alcohol consumption (> 10 drinks for male and > 5 drinks for women per week); other liver diseases such as chronic hepatitis; or using drugs that are associated with fatty liver, weight reduction, renal disease, or thyroid disease were excluded. All subjects provided written consent prior to examination.
Clinical and laboratory data
The patient’s age, sex, height, weight, and alcohol in-take were recorded at entry into the study. Standard blood counts and biochemistry tests were obtained in all patients. The FIB-4 index was calculated according to the following formula: FIB-4 = age (years) × AST (IU/L)/platelets (109/L) × alanine aminotransferase (IU/L)1/2.[14]
Histological evaluation
All patients underwent a liver biopsy. An adequate liver specimen was defined as > 10 mm in length and/or having > 10 portal tracts in both sites. Histologic assessments of liver biopsies were systematically assessed by an experienced liver pathologist of each site blinded to clinical data. Biopsy results were scored using the NASH Clinical Research Network histologic scoring system.[15] Fibrosis was scored from 0 to 4, with stage 4 fibrosis defined as cirrhosis. Hepatic steatosis and lobular inflammation were scored from 0 to 3. Hepatocyte ballooning was scored from 0 to 2. Hepatic steatosis, lobular inflammation, and hepatocyte ballooning were combined to obtain the NAFLD activity score, ranging from 0 to 8. The median (interquartile range [IQR]) specimen length and number of portal tracts were 20 (16–25) mm and 14 (11–20), respectively, in the UCSD cohort and 17 (15–22) mm and 12 (10–17), respectively, in the Yokohama cohort.
MRE assessment and MEFIB criteria
LSM by MRE and a two-dimensional MRE protocol were performed using a 3.0T research scanner (GE Signa EXCITE HDxt; GE Healthcare, Waukesha, WI) as described at both sites.[16,17] The image analysts were blinded to all clinical and biochemical data. MEFIB was evaluated using MRE and FIB-4, and the rule-in and rule-out criteria for significant fibrosis were defined as follows: the rule-in criteria were MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6, and the rule-out criteria were MRE < 3.3 kPa and FIB-4 < 1.6, based on previous study.[13] The median (IQR) interval between liver biopsy and MRE assessment was 11 (−19 to 51) days in the UCSD cohort and 27 (−65 to 87) days in the Yokohama cohort.
FibroScan assessment and FAST score
LSM by VCTE and CAP were performed by a trained technician using the FibroScan 502 Touch model in the UCSD cohort and the FibroScan 502 Touch model or FibroScan 430 model in the Yokohama cohort (Echosens, Paris, France) according to described methods.[18] Technicians were blinded to clinical and histologic data. All patients were first scanned by applying the M probe (3.5 MHz), and rescanning using the XL probe (2.5 MHz) was performed only prompted by the automatic prove selection tool. At minimum, 10 measurements were made to obtain the median valid LSMs in kilopascals and the median valid CAP in decibels per meter. The FAST score was calculated according to the previous study, and FAST scores of ≥ 0.67 and ≤ 0.35 were used as the rule-in and rule-out criteria, respectively.[12] The median (IQR) interval between liver biopsy and FibroScan assessment was 5 (−19 to 40) days in the UCSD cohort and 17 (−37 to 57) days in the Yokohama cohort.
Primary outcome
The primary outcome of the study was defined as the diagnostic accuracy for significant fibrosis (fibrosis stage ≥ 2).
Statistical analysis
Patient characteristic between the UCSD cohort and the Yokohama cohort were compared using Fisher’s exact test or the Mann-Whitney U test. Receiver operating characteristic (ROC) curve analysis was performed for MRE, FIB-4, and FAST; and these factors were used in a continuous value. Similarly, ROC analyses were performed for MEFIB and FAST. MEFIB and FAST were examined using the rule-in and rule-out criteria. The Delong test was used to compare the area under the ROCs (AUROC). A two-tailed p ≤ 0.05 was considered statistically significant for all analyses. All analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Shimotsuke, Japan),[19] a graphical user interface for R, version 3.2.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Patient characteristics
A total of 234 biopsy-confirmed patients with NAFLD were enrolled in the UCSD cohort, and 314 biopsy-confirmed patients with NAFLD were enrolled in the Yokohama cohort. The baseline patient characteristics are shown in Table 1. The median (IQR) ages of the UCSD and Yokohama cohorts were 54 (42–63) years and 61 (51–71) years, respectively. The proportions of patients with significant fibrosis (fibrosis stage ≥ 2) were 29.5% (69/234) in the UCSD cohort and 66.2% (208/314) in the Yokohama cohort. Median (IQR) MRE, FIB-4, and FAST scores were 2.47 (2.1–3.3) kPa, 1.19 (0.8–1.8), and 0.38 (0.23–0.57), respectively, in the UCSD cohort and 4.01 (2.6–5.4) kPa, 1.87 (1.1–3.2), and 0.46 (0.26–0.64), respectively, in the Yokohama cohort. The proportion of significant fibrosis was higher in the Yokohama cohort than the UCSD cohort.
TABLE 1.
Patient characteristics
| UCSD (USA) cohort | Yokohama (Japan) cohort | ||
|---|---|---|---|
| (n = 234) | (n = 314) | p | |
| Age (years) | 54 (42–63) | 61 (51–71) | <0.001 |
| Gender, male (%) | 103 (44.0%) | 170 (31.4%) | 0.02 |
| BMI (kg/m2) | 31.5 (28–35) | 27.4 (25–31) | <0.001 |
| Diabetes mellitus, n (%) | 95 (40.6%) | 189 (60.2%) | <0.001 |
| AST (IU/L) | 35 (26–50) | 40 (29–57) | 0.01 |
| ALT (IU/L) | 48 (34–73) | 46 (30–70) | 0.1 |
| Platelets (109/L) | 230 (184–282) | 202 (150–241) | <0.001 |
| FIB-4 | 1.19 (0.8–1.8) | 1.87 (1.1–3.2) | <0.001 |
| FIB-4 < 1.6 | 167 (71.4%) | 137 (43.6%) | <0.001 |
| FIB-4 ≥ 1.6 | 67 (28.6%) | 177 (56.4%) | |
| MRI findings | |||
| Liver stiffness by MRE (kPa) | 2.47 (2.1–3.3) | 4.01 (2.6–5.4) | <0.001 |
| MRE < 3.3 kPa | 172 (73.5%) | 118 (37.6%) | <0.001 |
| MRE ≥ 3.3 kPa | 62 (26.5%) | 196 (62.4%) | |
| MEFIB index | |||
| MEFIB rule-in (MRE ≥ 3.3 kPa + FIB-4 ≥ 1.6) | 34 (14.5%) | 149 (47.5%) | <0.001 |
| MEFIB rule-out (MRE <3.3 kPa + FIB-4 < 1.6) | 139 (59.4%) | 90 (28.7%) | |
| MEFIB indeterminate | 61 (26.1%) | 75 (23.9%) | |
| FibroScan findings | |||
| Liver stiffness by VCTE (kPa) | 6.8 (5.0–10) | 9.8 (6.3–15) | <0.001 |
| CAP (dB/m) | 320 (285–354) | 291 (249–319) | <0.001 |
| Probe type | <0.001 | ||
| M probe | 136 (58.1%) | 276 (87.9%) | |
| XL probe | 98 (41.9%) | 38 (12.1%) | |
| FAST score | 0.38 (0.23–0.57) | 0.46 (0.26–0.64) | 0.005 |
| FAST ≥ 0.67 | 35 (15.0%) | 65 (20.7%) | 0.03 |
| FAST ≤ 0.35 | 111 (47.4%) | 116 (36.9%) | |
| FAST 0.36–0.66 (indeterminate) | 88 (37.6%) | 133 (42.4%) | |
| Histological findingsa | |||
| Fibrosis stage | <0.001 | ||
| 0 | 87 (37.2%) | 15 (4.8%) | |
| 1 | 78 (33.3%) | 91 (29.0%) | |
| 2 | 28 (12.0%) | 63 (20.1%) | |
| 3 | 24 (10.2%) | 100 (31.8%) | |
| 4 | 17 (7.3%) | 45 (14.3%) | |
| Steatosis grade | 0.7 | ||
| 0 | 16 (6.8%) | 15 (4.8%) | |
| 1 | 109 (46.6%) | 143 (45.5%) | |
| 2 | 79 (33.8%) | 111 (35.4%) | |
| 3 | 30 (12.8%) | 45 (14.3%) | |
| Lobular inflammation | 0.004 | ||
| 0 | 17 (7.3%) | 4 (1.3%) | |
| 1 | 136 (58.1%) | 185 (58.9%) | |
| 2 | 74 (31.6%) | 115 (36.6%) | |
| 3 | 7 (3.0%) | 10 (3.2%) | |
| Hepatocellular ballooning | <0.001 | ||
| 0 | 106 (45.3%) | 83 (26.4%) | |
| 1 | 107 (45.7%) | 195 (62.1%) | |
| 2 | 21 (9.0%) | 36 (11.5%) | |
| NAS | 4 (2–4) | 4 (3–5) | 0.007 |
Note: Data are shown as median (IQR). p value indicates difference between UCSD cohort and Yokohama cohort.
Abbreviations: ALT, alanine aminotransferase; BMI, body mass index; NAS, NAFLD activity score.
NASH Clinical Research Network Scoring System is used.
Diagnostic accuracy of MRE, FIB-4, and FAST for significant fibrosis
Diagnostic accuracy of MRE, FIB-4, and FAST for detecting significant fibrosis (fibrosis stage ≥2) was compared as a continuous trait. In the UCSD cohort, AUROCs (95% CI) of MRE, FIB-4, and FAST were 0.937 (0.92–0.96), 0.818 (0.78–0.85), and 0.763 (0.72–0.80), respectively. The AUROC of MRE and FIB-4 was significantly higher than that of FAST (p < 0.001 for MRE, p = 0.02 for FIB-4; Figure 1A). Similarly, in the Yokohama cohort, AUROCs (95% CI) of MRE, FIB-4, and FAST were 0.923 (0.89–0.96), 0.828 (0.78–0.88), and 0.760 (0.70–0.82), respectively; and the AUROC of MRE and FIB-4 was also significantly higher than that of FAST (p < 0.001 for MRE and p = 0.03 for FIB-4; Figure 1B).
FIGURE 1.
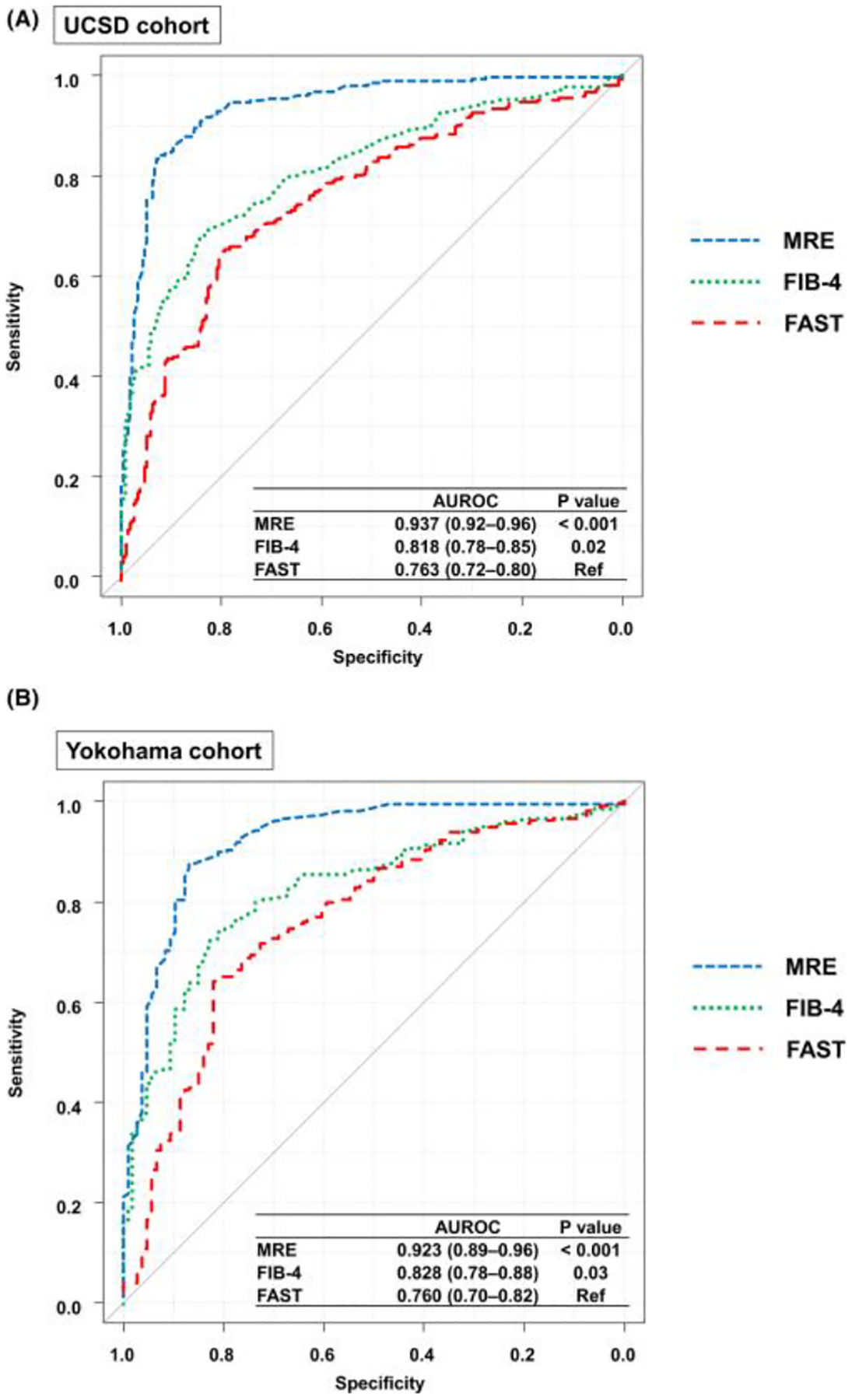
AUROCs of MRE, FIB-4, and FAST for significant fibrosis. (A) AUROCs of MRE, FIB-4, and FAST for significant fibrosis in the UCSD cohort. (B) AUROCs of MRE, FIB-4, and FAST for significant fibrosis in the Yokohama cohort
Diagnostic utility of the rule-in and rule-out criteria of MEFIB and FAST for significant fibrosis
MEFIB and FAST were divided into three categories using the rule-in and rule-out criteria for MEFIB and FAST as follows: the rule-in criteria (MEFIB, MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6; FAST, ≥ 0.67), the rule-out criteria (MEFIB, MRE < 3.3 kPa and FIB-4 < 1.6; FAST, ≤ 0.35).
The diagnostic utility of MEFIB and FAST for significant fibrosis (rule-in, rule-out, and indeterminate) was investigated. The AUROCs (95% CI) of MEFIB and FAST in the UCSD cohort were 0.860 (0.81–0.91) and 0.757 (0.69–0.82), respectively, and the AUROC of MEFIB was significantly higher than that of FAST (p = 0.005; Table 2). Similarly, the AUROCs (95% CI) of MEFIB and FAST in the Yokohama cohort were 0.899 (0.86–0.94) and 0.724 (0.67–0.78), respectively, and the AUROC of MEFIB was also significantly higher than that of FAST (p < 0.001; Table 2). Next, the diagnostic ability of the rule-in and out criteria for significant fibrosis was investigated. In the UCSD cohort, 34 patients (14.5%, 34/234) fulfilled the MEFIB rule-in criteria, and the positive predictive value (PPV) for significant fibrosis in these patients was 91.2% (31/34) (Table 2). Furthermore, 139 patients (59.4%, 139/234) fulfilled the MEFIB rule-out criteria, and the negative predictive value (NPV) for significant fibrosis (PPV for fibrosis stage 0 or 1) was 92.8% (129/139). On the other hand, the PPV of the FAST rule-in criteria for significant fibrosis was 74.2%, and the NPV of the FAST rule-out criteria was 88.3%. The PPV and the NPV of the MEFIB rule-in and rule-out criteria were superior than those of FAST. When the MEFIB criteria were used in the UCSD cohort, 73.9% (173/234) of patients fulfilled the rule-in or rule-out criteria, and the PPV among these patients was 92.5% (160/173).
TABLE 2.
Diagnostic ability of the MEFIB and FAST rule-in and rule-out criteria for significant fibrosis
| Rule-in diagnostic ability for significant fibrosis | Indeterminate | Rule-out diagnostic ability for significant fibrosis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AUROC | p | Patient number (%) | PPV | Sensitivity | Specificity | patient no. (%) | Patient no. (%) | NPV | Sensitivity | Specificity | |
| UCSD cohort (n = 234) | |||||||||||
| MEFIB | 0.860 (0.81–0.91) | 0.005 | 34 (14.5%) | 91.2% (31/34) | 44.9% (31/69) | 98.2% (162/165) | 61 (26.1%) | 139 (59.4%) | 92.8% (129/139) | 85.5% (59/69) | 78.2% (129/165) |
| FAST | 0.757 (0.69–0.82) | Ref | 35 (15.0%) | 74.2% (26/35) | 37.7% (26/69) | 94.5% (156/165) | 88 (37.6%) | 111 (47.4%) | 88.3% (98/111) | 81.2% (56/69) | 59.4% (98/165) |
| Yokohama cohort (n = 314) | |||||||||||
| MEFIB | 0.899 (0.86–0.94) | <0.001 | 149 (47.5%) | 95.6% (143/149) | 68.9% (143/208) | 94.3% (100/106) | 75 (23.9%) | 90 (28.7%) | 85.6% (77/90) | 93.8% (195/208) | 72.6% (77/106) |
| FAST | 0.724 (0.67–0.78) | Ref | 65 (20.7%) | 89.2% (58/65) | 27.9% (58/208) | 93.4% (99/106) | 133 (42.4%) | 116 (36.9%) | 57.8% (67/116) | 76.4% (159/208) | 63.2% (67/106) |
| Combined cohort (n = 548) | |||||||||||
| MEFIB | 0.899 (0.87–0.92) | <0.001 | 183 (33.4%) | 95.1% (174/183) | 62.8% (174/277) | 96.7% (262/271) | 136 (24.8%) | 229 (41.8%) | 90.0% (206/229) | 91.7% (254/277) | 76.0% (206/271) |
| FAST | 0.729 (0.69–0.77) | Ref | 100 (18.2%) | 84.0% (84/100) | 30.3% (84/277) | 94.1% (255/271) | 221 (40.3%) | 227 (41.4%) | 72.7% (165/227) | 77.6% (215/277) | 60.9% (165/271) |
Note: The rule-in and rule-out criteria of MEFIB are defined as MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6 and MRE < 3.3 kPa and FIB-4 < 1.6, respectively. The rule-in and rule-out criteria of FAST are defined as FAST ≥ 0.67 and FAST ≤ 0.35, respectively. AUROCs of MEFIB and FAST are investigated using trichomotized data by the rule-in and out criteria.
In the Yokohama cohort, 149 (47.5%, 149/314) patients fulfilled the MEFIB rule-in criteria, and the PPV for significant fibrosis in these patients was 96.0% (143/149). For the MEFIB rule-out criteria, 90 (28.7%, 90/314) patients fulfilled the MEFIB rule-out criteria, and the NPV for significant fibrosis was 85.6% (77/90). The PPV and the NPV of the MEFIB rule-in and rule-out criteria were superior than those of FAST (PPV of the rule-in criteria, 89.2%; NPV of the rule-out criteria, 57.8%). When the MEFIB criteria were used in the Yokohama cohort, 76.1% (239/314) of patients fulfilled the MEFIB rule-in or rule-out criteria, and the PPV among these patients was 92.1% (220/239).
In the combined cohort, 33.4% of patients fulfilled the rule-in criteria (MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6), and the PPV for significant fibrosis among these patients was 95.1%. Furthermore, 41.8% of patients fulfilled the rule-out criteria (MRE < 3.3 kPa and FIB-4 < 1.6), and the NPV for significant fibrosis (PPV for fibrosis stage 0 or 1) was 90.0%. Indeterminate patients by the MEFIB and FAST criteria were 24.8% and 40.3%, respectively.
Diagnostic ability for fibrosis stages 2 and 3 (except fibrosis stage 4)
Diagnostic accuracy of MEFIB, FAST, MRE, and VCTE for fibrosis stages 2–3 (versus fibrosis stages 0–1) after excepting patients with fibrosis stage 4 was investigated in the combined cohort (n = 486, 44.2% [215/486] of patients were fibrosis stages 2 and 3). MEFIB and FAST were divided into three categories using the rule-in and rule-out criteria for MEFIB and FAST. MRE and VCTE were dichotomized using thresholds of 3.34 kPa and 7.8 kPa determined by the Youden index. AUROCs (95% CI) of MEFIB, FAST, MRE, and VCTE for fibrosis stage 2–3 were 0.880 (0.85–0.91), 0.715 (0.67–0.76), 0.863 (0.83–0.89), and 0.771 (0.73–0.81), respectively (Figure 2 and Table 3); and the AUROC of MEFIB was significantly higher than those of FAST (p < 0.001) and VCTE (p < 0.001). When using the rule-in criteria of MEFIB and FAST and thresholds of MRE ≥ 3.34 kPa and VCTE ≥ 7.8 kPa, the PPVs for fibrosis stages 2–3 were 93.0% in MEFIB, 78.7% in FAST, 89.6% in MRE, and 71.9% in VCTE; and the PPV was higher in MEFIB (Table 3). The sensitivity of MEFIB was 55.3% (119/215), and a false negative was observed in 96 patients with significant fibrosis. Among these false-negative patients, 54, 20, and 22 patients were misdiagnosed by FIB-4 < 1.6, MRE < 3.3 kPa, and both FIB-4 < 1.6 and MRE < 3.3 kPa, respectively.
FIGURE 2.
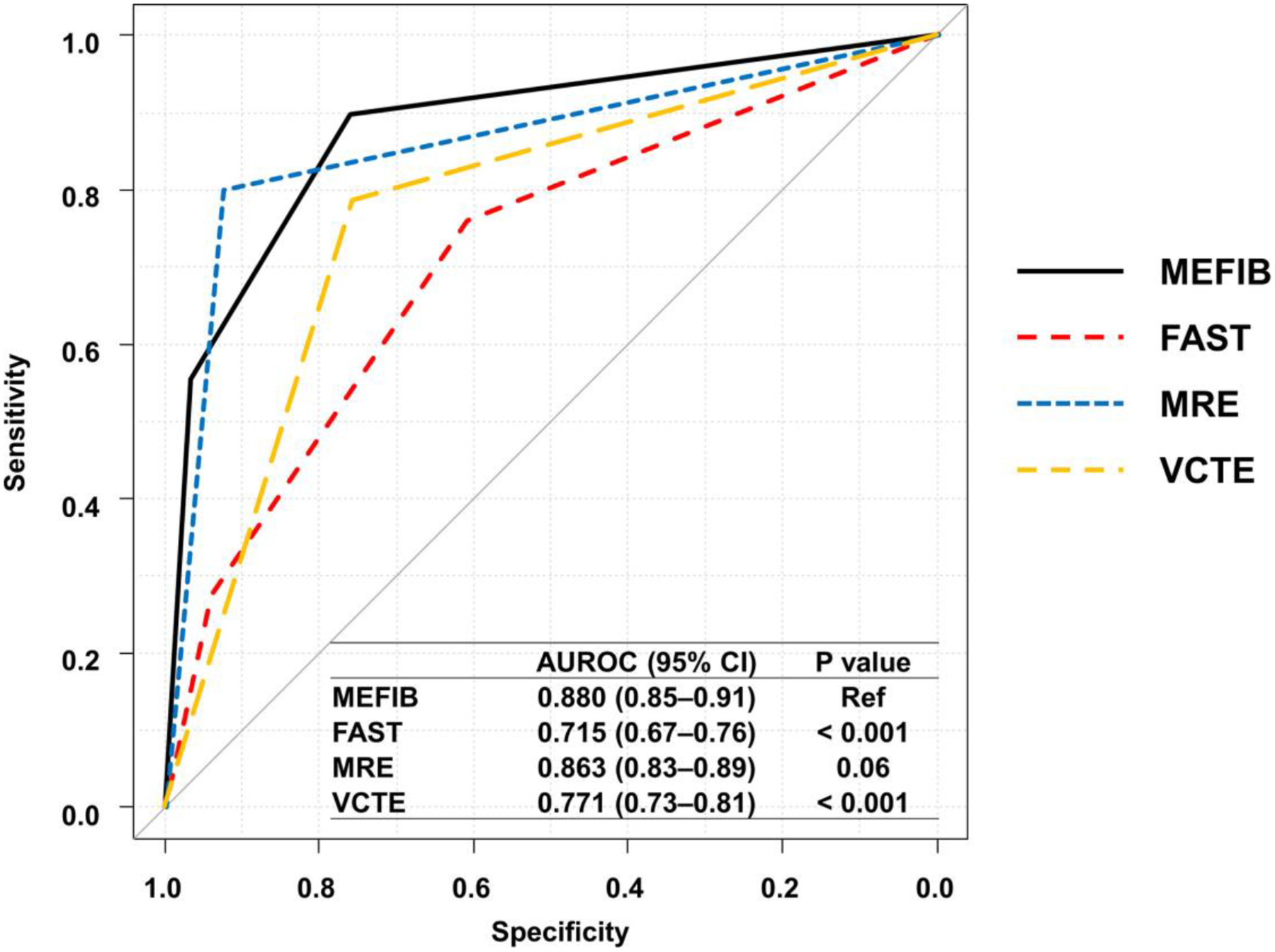
AUROCs of MEFIB, FAST, MRE, and VCTE for fibrosis stages 2 and 3 (except patients with fibrosis stage 4). Diagnostic accuracy of MEFIB, FAST, MRE, and VCTE for fibrosis stages 2–3 (versus fibrosis stage 0–1) after excepting patients with fibrosis stage 4 was investigated in the combined cohort. MEFIB and FAST were divided into three categories using the rule-in criteria (MEFIB, MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6, FAST ≥ 0.67) and the rule-out criteria (MEFIB, MRE < 3.3 kPa and FIB-4 < 1.6, FAST ≤ 0.35). MRE and VCTE were dichotomized using thresholds of 3.34 and 7.8 kPa determined by the Youden index
TABLE 3.
Diagnostic ability for fibrosis stages 2 and 3 (except fibrosis stage 4) in the combined cohort
| Rule-in diagnostic ability for fibrosis stages 2 and 3 | |||||
|---|---|---|---|---|---|
| AUROC (95% CI) | p | PPV | Sensitivity | Specificity | |
| MEFIB | 0.880 (0.85–0.91) | Ref | 93.0% (119/128) | 55.3% (119/215) | 96.7% (262/271) |
| FAST | 0.715 (0.67–0.76) | <0.001 | 78.7% (59/75) | 27.4% (59/215) | 94.1% (255/271) |
| MRE | 0.863 (0.83–0.89) | 0.06 | 89.6% (172/192) | 80.0% (172/215) | 92.6% (251/271) |
| VCTE | 0.771 (0.73–0.81) | <0.001 | 71.9% (169/235) | 75.6% (169/215) | 75.6% (205/271) |
Note: Patients with fibrosis stage 4 are excluded. The rule-in and rule-out criteria of MEFIB are defined as MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6 and MRE < 3.3 kPa and FIB-4 < 1.6, respectively. The rule-in and rule-out criteria of FAST are defined as FAST ≥ 0.67 and FAST ≤ 0.35, respectively. AUROCs of MEFIB and FAST are investigated using trichomotized data by the rule-in and out criteria. AUROCs of MRE and VCTE are investigated using dichotomized data by thresholds of 3.34 and 7.8 kPa determined by the Youden index, respectively.
DISCUSSION
Main findings
In this study, we demonstrated that the diagnostic accuracy of MEFIB for significant fibrosis (fibrosis stage ≥ 2) was higher than that of FAST, and these remained statistically and clinically significant in an independent racially and geographically diverse validation cohort from Japan. Using the MEFIB rule-in criteria (MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6), the PPV for significant fibrosis was 95.1%. Furthermore, using the MEFIB rule-out criteria, the NPV for significant fibrosis (PPV for fibrosis stage 0 or 1) was 90.0%. Because patients with significant fibrosis are at high risk of poor prognosis and are candidates for NASH treatment clinical trials, MEFIB is useful for detecting these high-risk patients and may be used as a noninvasive screening method.
Context with published literature
Liver fibrosis is the most important prognostic factor in NAFLD, and mortality risk increases for patients with significant fibrosis (fibrosis stage ≥ 2).[6] Therefore, detecting patients with significant fibrosis is an unmet clinical need. Because liver biopsy has several limitations, several noninvasive methods including serum markers, ultrasound-based modalities, and MRI-based modalities have been developed; and such noninvasive methods have been reported to detect these high-risk patients.[8,9,11]
FibroScan is the first approved ultrasound-based modality, and LSM and CAP by FibroScan are associated with fibrosis stage and steatosis grade.[20] To further improve the diagnostic accuracy for liver fibrosis, one strategy is to combine noninvasive markers. The FAST score is a marker combining LSM by VCTE, CAP, and AST; and high diagnostic accuracy for NASH with significant activity and fibrosis was reported. The diagnostic accuracy increased by combining these factors compared to using each factor alone. Furthermore, the FAST score is not affected by probe selection and has high reproducibility.[21,22] Therefore, FAST may be a suitable method for screening patients with NAFLD who are candidates for NASH treatment clinical trials.
MRE is another noninvasive modality for measuring liver stiffness. LSM by MRE is associated with fibrosis stage, and furthermore, LSM by MRE is also associated with HCC development, liver failure, and prognosis.[23–25] Although VCTE and MRE are used as noninvasive methods for LSM, MRE has higher diagnostic accuracy than VCTE for detecting liver fibrosis.[26,27] Based on these results, MRE is permitted for use in inclusion criteria and as an endpoint in early-phase clinical trials instead of liver biopsy.[17,28] FIB-4 is a serum-based fibrosis marker, and it is also associated with fibrosis and prognosis.[29–31] Recently, we demonstrated that the diagnostic accuracy for significant fibrosis increased by combining MRE and FIB-4 (MEFIB) versus MRE alone.[13]
Although MEFIB and FAST are thought to be effective methods for detecting patients with NAFLD and significant fibrosis, there is no head-to-head comparison between MEFIB and FAST in assessing the comparative utility of these noninvasive tests in clinical risk stratification in NAFLD. Because the diagnostic accuracy of MRE is higher than that of VCTE for detecting liver fibrosis, we hypothesized that MEFIB would have a higher diagnostic accuracy than the FAST score. In this study, we demonstrated that the diagnostic accuracy of MEFIB for significant fibrosis is higher than that of FAST, and more importantly it has superior PPV and NPV than FAST. This study provides evidence regarding MEFIB, and MEFIB may be suitable for detecting patients with significant fibrosis who are candidates for clinical trials when available.
Strengths and limitations
The strengths of the study are that it enrolled prospectively recruited well-characterized patients with NAFLD in two geographically distinct cohorts. Consecutive patients who underwent a liver biopsy, MRE, VCTE, and CAP were enrolled in the study. The proportions of patients with significant fibrosis were different between the two cohorts (29.5% in the UCSD cohort and 66.2% in the Yokohama cohort), and the utility of MEFIB for significant fibrosis was confirmed in both cohorts. This indicates that MEFIB could be used in another cohort, but the utility of MEFIB in a cohort with a low proportion of patients with significant fibrosis should be validated in a future study.
Future implication
In this study, we demonstrated that MEFIB (MRE + FIB-4) is useful for detecting patients with NAFLD and significant fibrosis (fibrosis stage ≥ 2), more so than FAST. Regarding enrollment in NASH clinical trials, a high screening failure rate is a major problem. To mitigate the failure rate, a two-step screening strategy is recommended.[32,33] In a primary care center, a readily and widely available blood-based test is suitable, and FIB-4 could be applied for the first screening. Because FIB-4 < 1.3 has a high NPV for significant fibrosis, patients with FIB-4 < 1.3 need no further examination, and patients with FIB-4 ≥ 1.3 may be referred for further assessment; this may be refined further by targeting especially those with a FIB-4 ≥ 1.6 who may need additional elastographic examination at a referral care center.[31] As a second step in those with a FIB-4 ≥ 1.6, MRE could be applied based on the present study results. Patients with the MEFIB rule-in criteria (MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6) have a high PPV for significant fibrosis, and they are likely good candidates for clinical trials. MRE alone also demonstrated high diagnostic accuracy for significant fibrosis. However, PPV for significant fibrosis increased by combining MRE and FIB-4 rather than using MRE alone. Because a high screening failure rate is a major problem in the enrollment of NASH clinical trials, the two-step strategy (combining FIB-4 and MRE) may be more suitable than MRE alone to reduce the screening failure rate in NASH clinical trials that use MRI-based assessment. Furthermore, a recent study showed that a two-step strategy contributes to reducing health care costs,[34] and MEFIB may contribute to reducing health care costs by reducing excessive MRE examinations. Therefore, MEFIB, a combination of FIB-4 and MRE, appears to be a suitable tool for the two-step screening strategy for studies and centers that are using MRI-based assessment. For centers where MRE is not available, FAST may still be used and remains an important alternative, although it has a much lower PPV and NPV. The two-step screening strategy using MEFIB and the diagnostic ability of MEFIB for significant fibrosis are summarized in Figure 3.
FIGURE 3.
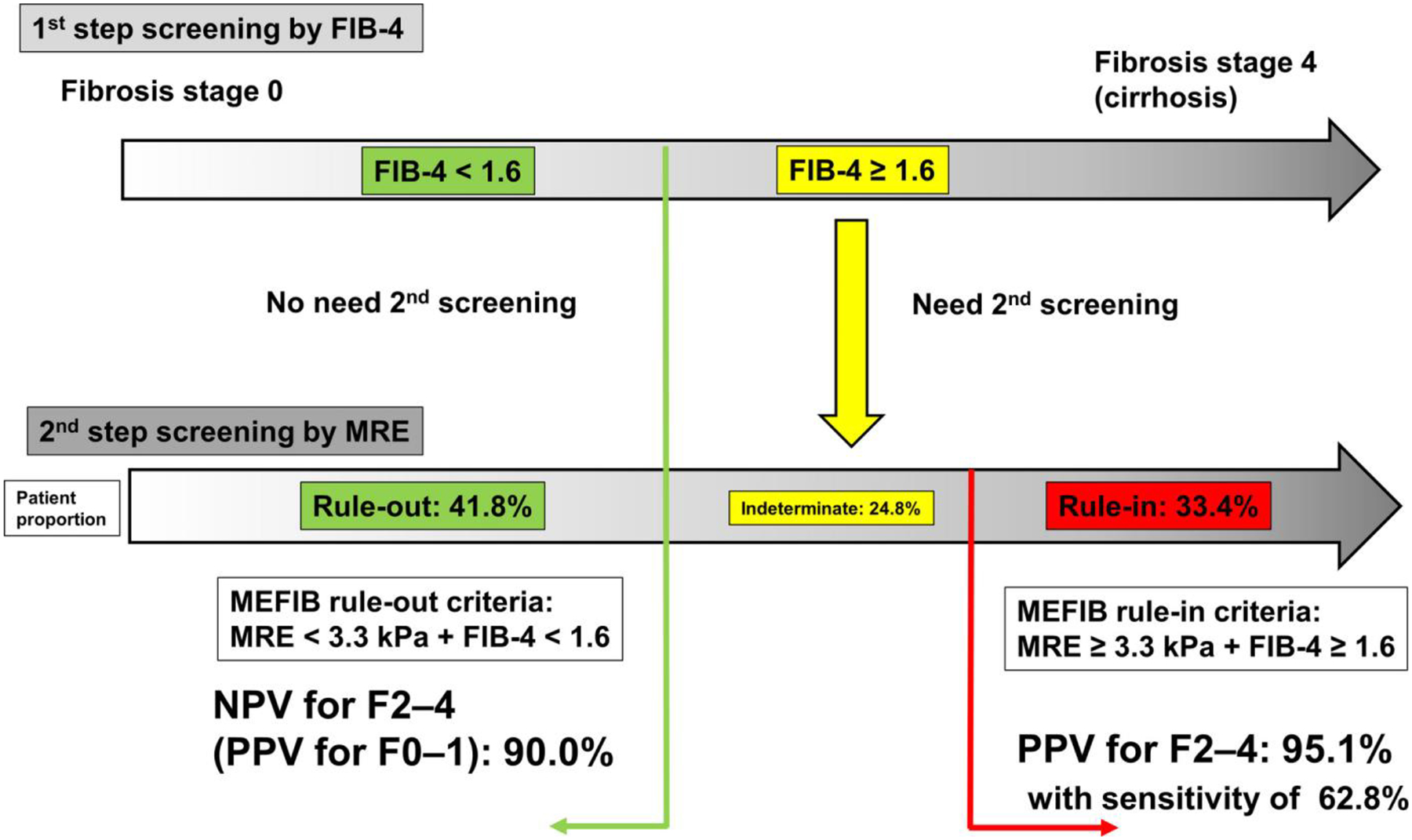
A two-step screening strategy for significant fibrosis by MEFIB and the summary of the diagnostic ability of MEFIB. As a first screening step in a primary care center, FIB-4 could be applied for the first screening as a readily available blood-based test. Patients with FIB-4 < 1.6 need no further examination, and patients with FIB-4 ≥ 1.6 need a further examination at a referral care center. As a second step, MRE could be applied. Patients with the MEFIB rule-in criteria (MRE ≥ 3.3 kPa and FIB-4 ≥ 1.6) have a high PPV for significant fibrosis (95%) and are good candidates for clinical trials. Furthermore, patients with the MEFIB rule-out criteria (MRE < 3.3 kPa and FIB-4 < 1.6) have a high NPV (90%) for significant fibrosis (PPV for nonsignificant fibrosis), and these patients could be followed up by a repeat blood-based test (FIB-4)
In conclusion, MEFIB has higher diagnostic accuracy than FAST for patients with significant fibrosis who are at high risk of poor prognosis and candidates for clinical trials, and our results support the utility of a two-step strategy for detecting significant fibrosis in adults with NAFLD.
Funding information
Supported by the National Institute of Environmental Health Sciences (5P42ES010337), the National Center for Advancing Translational Sciences (5UL1TR001442), the Department of Defense’s Peer-Reviewed Cancer Research Program (W81XWH-18-2-0026), the National Institute of Diabetes and Digestive and Kidney Diseases (U01DK061734, R01DK106419, R01DK121378, R01DK124318, P30DK120515), the National Heart, Lung, and Blood Institute (P01HL147835), and the National Institute on Alcohol Abuse and Alcoholism (U01AA029019, all to R. L.) and by the Uehara Memorial Foundation (201940021, to N. T.).
CONFLICT OF INTEREST
Dr. Loomba consults for, advises, and received grants from Boehringer-Ingelheim, Bristol-Myers Squibb, Cirius, Eli Lilly, Galmed, Gilead, Janssen, Merck, NGM, Pfizer, Prometheus, and Siemens. He consults for and advises Anylam/Regeneron, Arrowhead, AstraZeneca, Bird Rock Bio, Celgene, CohBar, Conatus, Gemphire, Glympse bio, GNI, GRI Bio, Inipharm, Intercept, Ionis, Metacrine, Novartis, Novo Nordisk, Promethera, Sanofi, and Viking. He received grants from Allergan, Galectin, GE, Genfit, Intercept, Grail, Madrigal, NuSirt, and pH Pharma. He is cofounder of Liponexus, Inc. Dr. Sirlin received grants from GE, Siemens, Philips, Bayer, Gilead; personal consultation fees from Blade, Boehringer, and Epigenomics; consultation under the auspices of the university to AMRA, BMS, Exact Sciences, GE Digital, and IBM-Watson; lab service agreements from Enanta, Gilead, ICON, Intercept, Nusirt, Shire, Synageva, and Takeda. Dr. Nakajima received grants from Astellas, Kowa, EA pharma, Mochida, Mylan EPD, Bioferumin, and Aska. Dr. Yoneda received grants from Kowa.
Abbreviations:
- AST
aspartate aminotransferase
- AUROC
area under the ROC
- CAP
controlled attenuation parameter
- FAST
FibroScan-AST
- FIB-4
Fibrosis-4
- IQR
interquartile range
- LSM
liver stiffness measurement
- MEFIB
FIB-4 combined with MRE
- MRE
magnetic resonance elastography
- NPV
negative predictive value
- PPV
positive predictive value
- ROC
receiver operating characteristic
- UCSD
University of California San Diego
- VCTE
vibration-controlled transient elastography
REFERENCES
- 1.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. [DOI] [PubMed] [Google Scholar]
- 3.Li J, Zou B, Yeo YH, Feng Y, Xie X, Lee DH, et al. Prevalence, incidence, and outcome of non-alcoholic fatty liver disease in Asia, 1999–2019: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2019;4:389–98. [DOI] [PubMed] [Google Scholar]
- 4.Tampi RP, Wong V-S, Wong G-H, Shu S-T, Chan H-Y, Fung J, et al. Modelling the economic and clinical burden of non-alcoholic steatohepatitis in east Asia: data from Hong Kong. Hepatol Res. 2020;50:1024–31. [DOI] [PubMed] [Google Scholar]
- 5.Terai S, Buchanan-Hughes A, Ng A, Lee I-H, Hasegawa K. Comorbidities and healthcare costs and resource use of patients with nonalcoholic fatty liver disease (NAFLD) and non-alcoholic steatohepatitis (NASH) in the Japan medical data vision database. J Gastroenterol. 2021;56:274–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dulai PS, Singh S, Patel J, Soni M, Prokop LJ, Younossi Z, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–54. [DOI] [PubMed] [Google Scholar]
- 8.Kuwashiro T, Takahashi H, Hyogo H, Ogawa Y, Imajo K, Yoneda M, et al. Discordant pathological diagnosis of non-alcoholic fatty liver disease: a prospective multicenter study. JGH Open. 2020;4:497–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davison BA, Harrison SA, Cotter G, Alkhouri N, Sanyal A, Edwards C, et al. Suboptimal reliability of liver biopsy evaluation has implications for randomized clinical trials. J Hepatol. 2020;73:1322–32. [DOI] [PubMed] [Google Scholar]
- 10.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut. 2020;69:1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tapper EB, Loomba R. Noninvasive imaging biomarker assessment of liver fibrosis by elastography in NAFLD. Nat Rev Gastroenterol Hepatol. 2018;15:274–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Newsome PN, Sasso M, Deeks JJ, Paredes A, Boursier J, Chan W-K, et al. FibroScan-AST (FAST) score for the non-invasive identification of patients with non-alcoholic steatohepatitis with significant activity and fibrosis: a prospective derivation and global validation study. Lancet Gastroenterol Hepatol. 2020;5:362–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jung J, Loomba RR, Imajo K, Madamba E, Gandhi S, Bettencourt R, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut. 2021;70:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–25. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. [DOI] [PubMed] [Google Scholar]
- 16.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven non-alcoholic fatty liver disease. Gastroenterology. 2017;152:598–607.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial). Hepatology. 2015;61:1239–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caussy C, Brissot J, Singh S, Bassirian S, Hernandez C, Bettencourt R, et al. Prospective, same-day, direct comparison of controlled attenuation parameter with the M vs the XL probe in patients with nonalcoholic fatty liver disease, using magnetic resonance imaging-proton density fat fraction as the standard. Clin Gastroenterol Hepatol. 2020;18:1842–50.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kanda Y. Investigation of the freely available easy-to-use software “EZR” for medical statistics. Bone Marrow Transplant. 2013;48:452–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yoneda M, Honda Y, Nogami A, Imajo K, Nakajima A. Advances in ultrasound elastography for nonalcoholic fatty liver disease. J Med Ultrason. 2001;2020(47):521–33. [DOI] [PubMed] [Google Scholar]
- 21.Oeda S, Takahashi H, Imajo K, Seko Y, Kobayashi T, Ogawa Y, et al. Diagnostic accuracy of FibroScan-AST score to identify non-alcoholic steatohepatitis with significant activity and fibrosis in Japanese patients with non-alcoholic fatty liver disease: comparison between M and XL probes. Hepatol Res. 2020;50:831–9. [DOI] [PubMed] [Google Scholar]
- 22.Hirooka M, Koizumi Y, Yano R, Sunago K, Watanabe T, Yoshida O, et al. Validation of the FibroScan-AST score by vibration-controlled transient and B-mode ultrasound elastography. Hepatol Res. 2021;20:13646. [DOI] [PubMed] [Google Scholar]
- 23.Han MAT, Vipani A, Noureddin N, Ramirez K, Gornbein J, Saouaf R, et al. MR elastography-based liver fibrosis correlates with liver events in nonalcoholic fatty liver patients: a multicenter study. Liver Int. 2020;40:2242–51. [DOI] [PubMed] [Google Scholar]
- 24.Gidener T, Ahmed OT, Larson JJ, Mara KC, Therneau TM, Venkatesh SK, et al. Liver stiffness by magnetic resonance elastography predicts future cirrhosis, decompensation, and death in NAFLD. Clin Gastroenterol Hepatol. 2020;30:31374–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamaki N, Higuchi M, Kurosaki M, Loomba R, Izumi N; MRCH Liver Study Group. Risk difference of liver-related and cardiovascular events by liver fibrosis status in non-alcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2021;16:S1542–3565(21)00752–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu C, Caussy C, Imajo K, Chen J, Singh S, Kaulback K, et al. Magnetic resonance vs transient elastography analysis of patients with nonalcoholic fatty liver disease: a systematic review and pooled analysis of individual participants. Clin Gastroenterol Hepatol. 2019;17:630–7.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–37.e7. [DOI] [PubMed] [Google Scholar]
- 28.Loomba R, Morgan E, Watts L, Xia S, Hannan LA, Geary RS, et al. Novel antisense inhibition of diacylglycerol O-acyltransferase 2 for treatment of non-alcoholic fatty liver disease: a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2020;5:829–38. [DOI] [PubMed] [Google Scholar]
- 29.Yoneda M, Imajo K, Eguchi Y, Fujii H, Sumida Y, Hyogo H, et al. Noninvasive scoring systems in patients with nonalcoholic fatty liver disease with normal alanine aminotransferase levels. J Gastroenterol. 2013;48:1051–60. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y, Kurosaki M, Tamaki N, Yasui Y, Hosokawa T, Tsuchiya K, et al. Non-alcoholic fatty liver disease fibrosis score and FIB-4 scoring system could identify patients at risk of systemic complications. Hepatol Res. 2015;45:667–75. [DOI] [PubMed] [Google Scholar]
- 31.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ, et al. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Castera L, Friedrich-Rust M, Loomba R. Noninvasive assessment of liver disease in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156:1264–81.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoneda M, Imajo K, Nakajima A. Non-invasive diagnosis of nonalcoholic fatty liver disease. Am J Gastroenterol. 2018;113:1409–11. [DOI] [PubMed] [Google Scholar]
- 34.Vilar-Gomez E, Lou Z, Kong N, Vuppalanchi R, Imperiale TF, Chalasani N, et al. Cost effectiveness of different strategies for detecting cirrhosis in patients with nonalcoholic fatty liver disease based on united states health care system. Clin Gastroenterol Hepatol. 2020;18:2305–14.e12. [DOI] [PubMed] [Google Scholar]


