Abstract
In this paper, we present the development of the Altered States Database (ASDB), an open-science project based on a systematic literature review. The ASDB contains psychometric questionnaire data on subjective experiences of altered states of consciousness (ASC) induced by pharmacological and non-pharmacological methods. The systematic review follows the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Scientific journal articles were identified through PubMed and Web of Science. We included studies that examined ASC using the following validated questionnaires: Altered States of Consciousness Rating Scale (APZ, 5D-ASC, 11-ASC), Phenomenology of Consciousness Inventory (PCI), Hallucinogen Rating Scale (HRS), or Mystical Experience Questionnaire (MEQ30). The systematic review resulted in the inclusion of a total of 165 journal articles, whereof questionnaire data was extracted and is now available on the Open Science Framework (OSF) website (https://osf.io/8mbru) and on the ASDB website (http://alteredstatesdb.org), where questionnaire data can be easily retrieved and visualized. This data allows the calculation of comparable psychometric values of ASC experiences and of dose-response relationships of substances inducing ASC.
Subject terms: Translational research, Psychology, Medical research
| Measurement(s) | Psychometric questionnaire data |
| Technology Type(s) | Systematic literature review (PRISMA) |
| Sample Characteristic - Organism | Human |
Background & Summary
In recent years there has been a growing interest in the scientific study of consciousness, including the investigation of altered states of consciousness (ASC). ASC are mental states distinct from ordinary states of consciousness and can involve profound changes in subjective experiences, such as changes in perception of the external world, of one’s own feelings, sensations, and thoughts, an altered sense of space and time, the disintegration of self-consciousness (ego dissolution), hallucinations or the experience of unity1–5. What varieties of experiences occur under what circumstances gained interest among clinicians, empirical scientists, philosophers, and the public6–10. In addition, recently, an increasing number of clinical trials started to explore the potential therapeutic effects of psychedelic drugs which can induce profound ASC experiences11,12.
In the experimental study of consciousness, particular attention is paid to induced ASC that are reversible and of short-term duration. These can be induced by pharmacological methods such as psychedelics, stimulants, or narcotics and non-pharmacological methods such as meditation, sensory deprivation, or breathing techniques. There is growing interest in how these experiences can be mapped and taxonomized at a phenomenological level of description, how they relate to psychiatric disorders such as schizophrenia or mania, and how they can potentially be used therapeutically1.
To quantitatively assess the subjective experiences of ASC, standardized and validated questionnaires for retrospective assessments have been developed13,14. (For more information on the questionnaires and their validation see de Deus Pontual et al.15 and Schmidt & Majić13). The available psychometric tools can be used to quantify different aspects of ASC phenomena, allowing for direct comparisons between induction methods, individual subjective effects, and experimental designs1.
The Altered States Database (ASDB, http://alteredstatesdb.org) is an open-science project that aims to provide researchers and non-scientists with easy access to valuable information concerning ASC. Here we report on the upgrade of the ASDB to be in accordance with the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 Statement Guidelines16 for reporting systematic reviews and meta-analyses and to extend the existing database by including recently published data. Articles were searched in the PubMed and Web of Science databases. After screening and identifying relevant studies, significant data were extracted and made available in the Open Science Framework (OSF, https://osf.io/8mbru)17. Based on a comprehensive review of currently available psychometric data on ASC, this revised version of the ASDB allows for a direct comparison of the psychological effects of different induction substances and techniques1. Researchers conducting clinical trials on substances or nonpharmacological methods that induce ASC can use ASDB data to compare their results with previous studies, as well as with recreational settings and related field studies. In addition, pharmacological dose-response calculations are facilitated (see Hirschfeld and Schmidt18).
Methods
This article reports on the Altered States Database (ASDB; Website: http://alteredstatesdb.org), an open-science project containing extracted psychometric questionnaire data on altered states of consciousness from journal articles collected in a systematic literature review according to the PRISMA 2020 Statement Guidelines16.
Eligibility criteria
This systematic review included studies on ASC experiences that used one or more standardized questionnaires to quantitatively assess the subjectively experienced qualities of ASC. A list of standardized questionnaires can be found in Table 1. Studies using both pharmacological and nonpharmacological ASC-inducing methods were included. Studies on both healthy and clinical subjects were reviewed. Only primary research data were considered.
Table 1.
Psychometric questionnaires and their factors and scales.
| Questionnaire | Versions | Scales/Factors | Original publication |
|---|---|---|---|
| Altered States of Consciousness Rating Scale | APZ | (1) Oceanic Boundlessness, (2) Dread of Ego Dissolution, (3) Visionary Restructuralization | Dittrich, 197519, 198520, 19982 |
| 5D-ASC | (1) Oceanic Boundlessness, (2) Dread of Ego Dissolution, (3) Visionary Restructuralization, (4) Auditory Alterations, (5) Vigilance Reduction | Bodmer et al.21 Dittrich et al.22 | |
| 11-ASC | (1) Experience of Unity, (2) Spiritual Experience, (3) Blissful State, (4) Insightfulness, (5) Disembodiment, (6) Impaired Control and Cognition, (7) Anxiety, (8) Complex Imagery, (9) Elementary Imagery, (10) Audio-Visual Synesthesia, (11) Changed Meaning of Percepts | Studerus et al.3 | |
| Phenomenology of Consciousness Inventory | PCI | (1) Positive Affect, (a.) Joy, (b.) Sexual Excitement, (c.) Love, (2) Negative Affect, (a.) Anger, (b.) Sadness, (c.) Fear, (3) Altered Experience, (a.) Altered Body Image, (b.) Altered Time Sense, (c.) Altered Perception, (d.) Altered Meaning, (4) Visual Imagery, (a.) Amount, (b.) Vividness, (5) Attention, (a.) Direction, (b.) Absorption, (6) Self Awareness, (7) Altered State of Awareness, (8) Internal Dialogue, (9) Rationality, (10) Volitional Control, (11) Memory, (12) Arousal | Pekala,199123; Pekala R. J. and Levine R. L24. |
| Hallucinogen Rating Scale | HRS | (1) Somaesthesia, (2) Affect, (3) Perception, (4) Cognition, (5) Volition, (6) Intensity | Strassman et al.25 |
| Mystical Experience Questionnaire | MEQ30 | (1) Mystical, (2) Positive Mood, (3) Transcendence of time and space, (4) Ineffability | Pahnke, 196326, 196627; MacLean et al.28 |
Information sources and article searching
An electronic search for eligible studies was conducted on the following search engines and databases: MEDLINE via PubMed and ISI Web of Science.
Article identification using PubMed search
The PubMed search was conducted with the aim to collect journal articles which studied ASC experiences by using defined questionnaires.
The following substances that have been described to pharmacologically induce ASC were included in the PubMed search query:
2C-B, 4-FA, 4-Fluoroamphetamine, 5-MeO-DMT, Amanita muscaria, Amphetamine, Angel dust, Atropa belladonna, Ayahuasca, Buprenorphine, Cannabidiol, CBD, Cocaine, Dimethyltryptamine, DMT, Ergine, Ergotamine, Gamma hydroxybutyric acid, GHB, Hallucinogens, Hawaiian baby woodrose, Henbane, Heroin, Hyoscyamine, Ibogaine, Kava, Ketamine, Kratom, LSA, LSD, Lysergic acid amide, Lysergic acid diethylamide, Magic mushrooms, MDA, MDMA, Methamphetamine, Morning glory, Morphine, Myristicin, Nicotine, Nitrous oxide, Nutmeg, PCP, Peyote, Phencyclidine, Pituri, Psilocybin, Psychedelics, Salvinorin, San pedro, Tetrahydrocannabinol, THC, and Triazolam.
The following techniques reported to induce ASC non-pharmacologically were included in the PubMed search query:
Aikido, Alternate nostril breathing, Ananda marga, Ashtanga, Autogenic training, Binaural beats, Breathwork, Capoeira, Chanting, Dancing, Dehydration, Dream machine, Drumming, Electronic gaming machine, Fasting, Flicker light, Flotation tank, Ganzfeld, Hyperventilation, Hypnosis, Hypnotic, I-OBE, Isha shoonya, Kriya, Kundalini, Kung fu, Mantra, Martial arts, Meditation, Mind machine, Nidra, Perceptual deprivation, Poker machines, Pranayama, Progressive muscle relaxation, Qigong, Repetitive speech, Runner’s high, Sahaja, Sensory deprivation, Slot machines, Stroboscopic, Sufi whirling, Sweat lodge, Tai chi, Trance, Vipassana, Yoga, Yogic breathing, and Zen training.
The PubMed search query also included the following names of psychometric questionnaires for qualitative assessment of ASC experiences and their abbreviations:
Abnormal Mental States Questionnaire, APZ, Altered States of Consciousness Rating Scale, 5D-ASC, Hallucinogen Rating Scale, HRS, Mystical Experience Questionnaire, MEQ, and Phenomenology of Consciousness Inventory, PCI.
To further increase accuracy in identifying suitable journal articles and because questionnaire names or abbreviations could not be provided in the article titles or abstracts, the following “inclusion terms” were also added to the PubMed search query:
Phenomenology, Psychometric, Psychometry, Subjective effect*, Subjective experience*, and Subjectively perceived.
To exclude animal or in-vitro studies, as well as studies in which the above abbreviations were used in contexts other than the questionnaires or ASC-induction methods (e.g., “DMT” used for “dance movement therapy” instead of referring to the chemical substance), the PubMed search query also contained following “exclusion terms”:
Action on salt china, Active symptom control, Alprazolam, Ambulatory surgery center, Appropriate symbol communication, Aripiprazole, Ascorbate, Ascorbic acid, Azapropazone, Cat, Cats, Dance movement therapy, Dance movement therapy, Dance/movement training, Dexmedetomidine, Disease modifying therapy, Fisher’s lsd, Gene, Genes, Hazard ratios, Hepatorenal syndrome, Least significance difference, Mice, Mouse, Percutaneous cardiovascular procedures, Percutaneous coronary intervention, Percutaneous intervention, Post hoc lsd test, Post-hoc lsd test, Primate, Primates, Rat, Rats, Rodent, Rodents, Squamous cell, Stem cell, and Stromal cell.
These “exclusion terms” were chosen by manually scanning through the PubMed results of the search query without “exclusion terms” and identifying misleading uses of abbreviations etc.
The above search terms were combined with PubMed Boolean operators to detect articles describing one or more of the listed ASC-inducing methods, in addition to one or more questionnaires, or one or more “inclusion terms”, and exclude articles containing one or more “exclusion terms”. The PubMed search query also comprised the following filters:
publication date was constrained to range from 1975 to 2021 (as the first published questionnaire contained in this review is the “Abnormal Mental States Questionnaire”, which was published in 1975),
language filter was set to English.
To enhance the search depth, MeSH (Medical Subject Headings) terms were activated.
See Supplementary File 1 for the complete PubMed search query. The PubMed search was conducted on 2021-12-31.
Article identification using ISI Web of Science search
To identify further studies, references of questionnaires assessing the subjective experience of ASC were tracked using the ISI Web of Science search engine. Original publications of the questionnaires of Table 1 were identified in the ISI Web of Science search engine and forward citation tracking was undertaken. Review articles were excluded. The ISI Web of Science search was conducted on 2021-12-31.
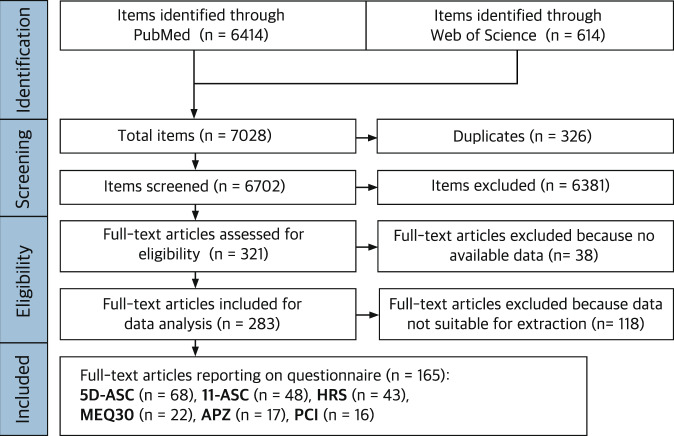
The process of item identification and screening is shown schematically as flow chart in Fig. 1. The PubMed search yielded 6414 items and the ISI Web of Science search yielded 614 items. The results from PubMed and ISI Web of Science were collected in the reference manager Zotero (https://www.zotero.org) and totaled 7028 items. Of these, 326 duplicates were detected and merged, leaving 6702 items eligible for the screening procedure.
Fig. 1.
PRISMA flowchart of systematic review describing the process of study search and selection.
Article screening
The study selection process comprised of a first screening step in which only the title and abstract of the articles were analyzed, and a second screening step, in which full-texts and questionnaire data of the articles were examined. The aim of the first screening step was to determine whether the articles collected matched our review interest. Items were excluded if the item type was “book”, “book section” or “video recording”, if the language was not English, if they reported on animal or in-vitro studies, if they were reviews or other secondary literature, if they used other types of questionnaires or if the articles were not accessible. In the first screening stage, 6381 items were excluded, and 321 items were included for the full-text screening. The aim of the second screening step was to assess whether included items contain accessible and extractable data on the questionnaire results generated during the described study. For data extraction, full texts containing data on questionnaire results were collected. The second screening step resulted in the exclusion of 38 full texts that did not contain accessible data and were therefore rejected, and in the inclusion of 283 journal articles for data analysis. Thereof 118 items were excluded due to unsuitable data reporting, for example, because only total questionnaire scores or only correlation measures were reported.
Data extraction
165 journal articles were included in the data extraction process, of which 68 contained data on the 5D-ASC, 48 on the 11-ASC, 43 on the HRS, 22 on the MEQ30, 17 on the APZ, and 16 on the PCI (note: several articles contain data from more than one questionnaire). The means and standard deviations of the responses to each of the factors and dimensions of the questionnaire described by the journal article were extracted. If only the standard error of the mean was given, it was converted to the standard deviation. When data were provided graphically only, they were extracted using WebPlotDigitizer v4.5.
Results of individual studies and statistical syntheses
This systematic literature review results in the inclusion of data from 165 journal articles reporting a total of 674 datasets (experiments); these contain a total of 4689 data points as group-level summary statistics for all the factors/dimensions of all questionnaires combined (i.e., counting the data points of each dimensions/factors of a questionnaire for all datasets); these, in turn, result from a total of 17792 measurements (number of applications of all questionnaires on individual study participants). An individual dataset was defined as any unique combination of experimental conditions and questionnaire to capture that a research article may contain multiple datasets (i.e., applications of different induction methods and/or dosages result in different datasets). Table 2 reports a summary of the amount of extracted data regarding different ASC induction methods. Table 3 provides references to the articles from which data were extracted, sorted according to the different questionnaires.
Table 2.
Summary of included data sorted according to ASC induction methods.
| Induction Method | Articles | Datasets | Number of Applications | Mean sample size per dataset ± SD | |
|---|---|---|---|---|---|
| Pharmacological | 2C-B (4-Bromo-2,5-Dimethoxyphenethylamine) | 2 | 2 | 51 | 25.5 ± 13.4 |
| 4-FA (4-Fluoroamphetamine) | 1 | 3 | 36 | 12 | |
| 5-MeO-DMT (5-Methoxy-N,N- Dimethyltryptamine) | 5 | 15 | 932 | 62.1 ± 79.7 | |
| DMT + MAO* Inhibitor (“Ayahuasca”) | 11 | 20 | 436 | 21.8 ± 18.3 | |
| DMT (N,N-Dimethyltryptamine) | 9 | 22 | 295 | 13.4 ± 8 | |
| DXM (Dextromethorphan) | 1 | 7 | 84 | 12 | |
| D-Amphetamine | 1 | 8 | 32 | 4 | |
| D-Methamphetamine | 1 | 1 | 8 | 8 | |
| Ergotamine | 1 | 3 | 51 | 17 | |
| Ibogaine | 1 | 1 | 27 | 27 | |
| Kambô (Giant Leaf Frog) | 1 | 4 | 22 | 22 | |
| Ketamine | 24 | 33 | 599 | 18.1 ± 7.3 | |
| LSD (Lysergic Acid Diethylamide) | 20 | 66 | 2494 | 37.8 ± 151.6 | |
| Mazindol | 1 | 1 | 10 | 10 | |
| MDA (3,4-Methylene dioxy amphetamine) | 1 | 3 | 36 | 12 | |
| MDE (3,4-Methylenedioxyethylamphetamine) | 2 | 2 | 22 | 11 ± 4.2 | |
| MDMA (3,4-Methylenedioxy methamphetamine, “Ecstasy”) | 20 | 45 | 815 | 18.1 ± 9 | |
| Mescaline (Peyote, San Pedro cacti) | 1 | 1 | 12 | 12 | |
| Psilocybin (“Magic Mushrooms”) | 35 | 112 | 1900 | 17 ± 12.2 | |
| Salvinorin-A (Salvia Divinorum) | 10 | 69 | 692 | 8.2 ± 10 | |
| THC (Tetrahydrocannabinol) | 1 | 1 | 19 | 19 | |
| Triazolam | 2 | 4 | 64 | 16 ± 4.6 | |
| Non-pharmacological | Chanting (religious) | 1 | 9 | 401 | 44.6 ± 27 |
| Drumming and Dancing | 2 | 3 | 187 | 62.3 ± 38.9 | |
| Flicker Light Stimulation | 1 | 6 | 144 | 24 | |
| Ganzfeld | 3 | 13 | 306 | 23.5 ± 5.2 | |
| Hetero-Hypnosis | 8 | 20 | 1059 | 53 ± 78.7 | |
| Kundalini Meditation | 1 | 1 | 12 | 12 | |
| Mind Machine | 1 | 2 | 60 | 30 | |
| Olfactory Epithelium Stimulus† | 1 | 1 | 12 | 12 | |
| Sweat Lodge | 1 | 2 | 110 | 55 | |
| Zen Meditation | 1 | 1 | 14 | 14 | |
This table contains, the sum of journal articles reporting on each of the identified induction method (“Articles”), the sum of experiments conducted (“Datasets”), the sum of all applications of the questionnaires on individual study participants (“Applications”), as well as the sample size per dataset given as mean ± standard deviation (“Mean sample size per dataset ± SD”).
*MAO: Monoamine oxidase †Olfactory Epithelium Stimulus is not an induction method itself, but it is investigated as the mechanism underlying breathing techniques.
Table 3.
Summary of included studies of ASC experiences.
| Induction Method | APZ (3D) | 5D-ASC | 11-ASC | HRS | MEQ30 | PCI | |
|---|---|---|---|---|---|---|---|
| Pharmacological | 2C-B (4-Bromo-2,5-Dimethoxyphenethylamine) | 29,30 | |||||
| 4-FA (4-Fluoroamphetamine) | 31 | 31 | 31 | ||||
| 5-MeO-DMT (5-Methoxy-N,N- Dimethyltryptamine) | 32,33 | 34 | 35–37 | ||||
| DMT + MAO* Inhibitor (“Ayahuasca”) | 38–40 | 41 | 41 | 38,39,42–50 | 44,51,52 | ||
| DMT (N,N-Dimethyltryptamine) | 53,54 | 55 | 25,56–59 | 51,55 | |||
| DXM (Dextromethorphan) | 60 | 60,61 | 60 | ||||
| D-Amphetamine | 62 | 62 | 42,43,63 | 62 | |||
| D-Methamphetamine | 64 | ||||||
| Ergotamine | 65 | ||||||
| Ibogaine | 66 | ||||||
| Kambô (Giant Leaf Frog) | 67 | 67 | 67 | 67 | |||
| Ketamine | 68 | 53,54,69–83 | 79,84,85 | 86–89 | 85 | ||
| LSD (Lysergic Acid Diethylamide) | 8,62,90–95 | 62,91,92,94,96–106 | 51,62,91,92,94,105 | ||||
| Mazindol | 107 | ||||||
| MDA (3,4-Methylene dioxy amphetamine) | 108,109 | 109 | |||||
| MDE (3,4-Methylenedioxyethylamphetamine) | 64,110 | ||||||
| MDMA (3,4-Methylenedioxy methamphetamine, “Ecstasy”) | 62,94,111–126 | 62,94,116–119,125 | 127 | 62,94 | |||
| Mescaline (Peyote, San Pedro cacti) | 128 | ||||||
| Psilocybin (“Magic Mushrooms”) | 64,129–131 | 65,79,83,132–146 | 60,79,141,147–159 | 60,129–131,136,137,157 | 35,51,60,136,137,152,154,160,161 | ||
| Salvinorin-A (Salvia Divinorum) | 162–165 | 162–171 | |||||
| THC (Tetrahydrocannabinol) | 172 | ||||||
| Triazolam | 61,87 | ||||||
| Non-pharmacological | Chanting (religious) | 173 | |||||
| Drumming and Dancing | 174 | 175 | |||||
| Flicker Light Stimulation | 176 | 176 | 176 | ||||
| Ganzfeld | 177,178 | 177,178 | 178,179 | ||||
| Hetero-Hypnosis | 180–187 | ||||||
| Kundalini Meditation | 188 | ||||||
| Mind Machine | 189 | ||||||
| Olfactory Epithelium Stimulus† | 190 | ||||||
| Sweat Lodge | 191 | ||||||
| Zen Meditation | 192 | ||||||
This table contains both pharmacological and non-pharmacological studies on ASC experiences, which were included in our systematic literature review and in the data extraction process. It categorizes studies according to which psychometric questionnaire was used to assess ASC experiences. Substances inducing ASC that do not have direct biological sources were named by the chemical formula; substances that are directly derived from biological sources were named by the active component and the species name. The street names of substances are in quotation marks. Three studies137,141,142 combined psilocybin administration with meditation but were included in this table only under the psilocybin category. One study158 investigated the effect of setting (physical and social environment during the ASC experience) on phenomenology without psilocybin being administered, yet it is included in this table under the psilocybin category because participants were led to believe that they were being administered psilocybin. We have not included substances in this table that were used as either active or passive controls in the cited studies (e.g., ketanserin, niacin, citalopram), while corresponding control-datasets are included in the ASDB.
*MAO: Monoamine oxidase. †Olfactory Epithelium Stimulus is not an induction method itself, but it is investigated as the mechanism underlying breathing techniques.
In total, 145 articles report on pharmacologically induced ASC and 20 report on non-pharmacologically induced ASC. The most common questionnaire to assess pharmacologically induced ASC experiences is the 5D-ASC, (65 articles, 90 datasets, 1792 applications), followed by the 11-ASC (43 articles, 144 datasets, 2321 applications), the HRS (43 articles, 128 datasets, 1804 applications), the MEQ30 (21 articles, 58 datasets, 5183 applications), the APZ (15 articles, 45 datasets, 551 applications) and lastly the PCI (1 article, 1 dataset, 22 applications). For non-pharmacologically induced ASC experiences, the most frequently used questionnaire is the PCI (15 articles, 32 datasets, 1450 applications), followed by the 11-ASC (4 articles, 7 datasets, 151 applications), the 5D-ASC (3 articles, 6 datasets, 133 applications), the APZ (2 articles, 4 datasets, 170 applications), and lastly the MEQ30 (1 article, 9 datasets, 401 applications).
Data Records
The results of the reported systematic literature research and the full report of extracted psychometric questionnaire data on ASC experiences are available on Open Science Framework (https://osf.io/8mbru, 10.17605/OSF.IO/8MBRU)17 in the folder “ASDB_v2.0_12-2021”. The psychometric questionnaire data is organized in one Microsoft Excel file per questionnaire. The data contained in the files are listed according to each individual application of the respective questionnaire. The data files are structured to fit a mySQL database structure as previously described1.
Following data columns are described:
number of subjects
control or experiment condition
questionnaire application time
reference of experience assessment
subjects’ health
questionnaire abbreviation
PubMed ID
DOI
main author
date published
reference text
abstract text
paper link
dosage quantity
dosage unit
info about induction
induction method
injection method
pharmacological or non-pharmacological study
laboratory or field study
psychometric data (with as many columns needed for the various factors and dimensions, each described by mean and standard deviation, of the corresponding questionnaire)
comments on additional information on the data extraction process such as the conversion of standard error of the mean to standard deviation.
In addition to the questionnaire data files, the OSF also contains a list of excluded studies, containing their PubMed ID, publication year, first author, and a comment on the reason for exclusion. In addition to the data availability on OSF, questionnaire data can also be retrieved and visualized on the ASDB website (http://alteredstatesdb.org), providing easy and direct access.
Technical Validation
To validate the search strategy, a comparison was made with the 105 journal articles already included in the previous version of the ASDB (last updated 2020-12-28). The references of the articles were retrieved from OSF (https://osf.io/8mbru)17. The comparison showed that all articles from the older version of the ASDB were covered by the current systematic literature review. To reduce the risk of bias in study identification and selection, the study selection process was performed independently by J.P. and J.D. and subsequently cross-checked. The data extraction process was performed by J.P., E.D., and J.D. and then cross-checked as well. Any discrepancies on study eligibility and data extraction were resolved by consensus. Reasons for exclusion are documented and can be obtained together with the overall data set. No automated tools were used in screening studies, other than detecting but not merging duplicate studies in Zotero. The selection of studies should not be prone to error, as we tried to include all available studies in this research area.
Supplementary information
Acknowledgements
We would like to thank Hendrik Berkemeyer for the data curation in the mySQL database used as backend implementation for the website and Hristofor Lukanov for his contributions to the web visualization of the data.
Author contributions
J.P. was responsible for the systematic literature review including the journal article identification and screening, and for the data extraction process; J.D. assisted on the journal article screening process, and E.D. and J.D. assisted with the data extraction process. E.D. and J.P. were responsible for the statistical description of the psychometric questionnaire data extracted from the journal articles. J.P. and T.T.S. were responsible for the manuscript writing. T.T.S. has initiated and conceptualized the project and was leading and organizing the work. C.C. contributed to conception and writing.
Funding
Open Access funding enabled and organized by Projekt DEAL.
Code availability
No custom code was used to generate, process, or analyse the data presented in the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-022-01822-4.
References
- 1.Schmidt, T. T. & Berkemeyer, H. The Altered States Database: Psychometric Data of Altered States of Consciousness. Frontiers in Psychology9, 10.3389/fpsyg.2018.01028 (2018). [DOI] [PMC free article] [PubMed]
- 2.Dittrich A. The standardized psychometric assessment of altered states of consciousness (ASCs) in humans. Pharmacopsychiatry. 1998;31(Suppl 2):80–84. doi: 10.1055/s-2007-979351. [DOI] [PubMed] [Google Scholar]
- 3.Studerus, E., Gamma, A. & Vollenweider, F. X. Psychometric Evaluation of the Altered States of Consciousness Rating Scale (OAV). PLoS ONE5, 10.1371/journal.pone.0012412 (2010). [DOI] [PMC free article] [PubMed]
- 4.Tagliazucchi E, et al. Increased Global Functional Connectivity Correlates with LSD-Induced Ego Dissolution. Current Biology. 2016;26:1043–1050. doi: 10.1016/j.cub.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 5.Wittmann, M., Giersch, A. & Berkovich‐Ohana, A. Altered states of consciousness: With special reference to time and the self. PsyCh Journal8, 10.1002/pchj.284 (2019). [DOI] [PubMed]
- 6.Carhart-Harris RL, et al. Neural correlates of the psychedelic state as determined by fMRI studies with psilocybin. Proceedings of the National Academy of Sciences. 2012;109:2138–2143. doi: 10.1073/pnas.1119598109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Muthukumaraswamy SD, et al. Broadband cortical desynchronization underlies the human psychedelic state. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2013;33:15171–15183. doi: 10.1523/JNEUROSCI.2063-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmid Y, et al. Acute Effects of Lysergic Acid Diethylamide in Healthy Subjects. Biological psychiatry. 2015;78:544–553. doi: 10.1016/j.biopsych.2014.11.015. [DOI] [PubMed] [Google Scholar]
- 9.Corlett PR, Frith CD, Fletcher PC. From drugs to deprivation: a Bayesian framework for understanding models of psychosis. Psychopharmacology. 2009;206:515–530. doi: 10.1007/s00213-009-1561-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Majić T, Schmidt TT, Gallinat J. Peak experiences and the afterglow phenomenon: When and how do therapeutic effects of hallucinogens depend on psychedelic experiences? J Psychopharmacol. 2015;29:241–253. doi: 10.1177/026988111456804011. [DOI] [PubMed] [Google Scholar]
- 11.Nayak, S. & Johnson, M. W. Psychedelics and Psychotherapy. Pharmacopsychiatry54, 10.1055/a-1312-7297 (2021). [DOI] [PubMed]
- 12.Reiff, C. M. et al. Psychedelics and Psychedelic-Assisted Psychotherapy. American Journal of Psychiatry177, 10.1176/appi.ajp.2019.19010035 (2020). [DOI] [PubMed]
- 13.Schmidt, T. T. & Majić, T. Empirische Untersuchung veränderter Bewusstseinszustände. in Handbuch Psychoaktive Substanzen, 10.1007/978-3-642-55214-4_65-1 (Springer Berlin Heidelberg, 2016).
- 14.Pekala, R. J. & Cardeña, E. Methodological issues in the study of altered states of consciousness and anomalous experiences. American Psychological Associationarieties, 10.1037/10371-002 (2000).
- 15.de Deus Pontual, A. A., Senhorini, H. G., Corradi-Webster, C. M., Tófoli, L. F. & Daldegan-Bueno, D. Systematic Review of Psychometric Instruments Used in Research with Psychedelics. J. Psychoactive Drugs 1–10, 10.1080/02791072.2022.2079108 (2022). [DOI] [PubMed]
- 16.Page, M. J. et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ, 10.1136/bmj.n71 (2021). [DOI] [PMC free article] [PubMed]
- 17.Schmidt, TT. & Prugger, J. The Altered States Database (ASDB), OSF, 10.17605/OSF.IO/8MBRU (2022).
- 18.Hirschfeld T, Schmidt TT. Dose–response relationships of psilocybin-induced subjective experiences in humans. J Psychopharmacol. 2021;35:384–397. doi: 10.1177/0269881121992676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dittrich, A. Zusammenstellung eines Fragebogens (APZ) zur Erfassung abnormer psychischer Zustände [Construction of a questionnaire (APZ) for assessing abnormal mental states]. Z Klin Psychol Psychiatr Psychother 12–20.
- 20.Dittrich, A. Ätiologie-unabhängige Strukturen veränderter Wachbewußtseinszustände: Ergebnisse empirischer Untersuchungen über Halluzinogene I. und II.Ordnung, sensorische Deprivation, hypnagoge Zustände, hypnotische Verfahren sowie Reizüberflutung; 119 Tabellen. (Enke, 1985).
- 21.Bodmer I, Dittrich A, Lamparter D. Aussergewöhnliche Bewusstseinszustände-Ihre gemeinsame Struktur und Messung [Altered states of consciousness-Their common structure and assessment] Welten des Bewusstseins. Bd. 1994;3:45–58. [Google Scholar]
- 22.Dittrich, A., Lamparter, D. & Maurer, M. 5D-ABZ: Fragebogen zur Erfassung Aussergewöhnlicher Bewusstseinszustände. Eine kurze Einführung [5D-ASC: Questionnaire for the Assessment of Altered States of Consciousness. A Short Introduction]. Zürich: PSIN PLUS Publications (2006).
- 23.Pekala, R. J. Quantifying Consciousness. Springer US, 10.1007/978-1-4899-0629-8 (1991).
- 24.Pekala RJ, Levine RL. Quantifying states of consciousness via an empirical-phenomenological approach. Imagination. Cognition and Personality. 1982;2:51–71. doi: 10.2190/2D3H-CKP8-DYNH-4KV3. [DOI] [Google Scholar]
- 25.Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R. Dose-response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Archives of general psychiatry. 1994;51:98–108. doi: 10.1001/archpsyc.1994.03950020022002. [DOI] [PubMed] [Google Scholar]
- 26.Pahnke, W. N. Drugs and mysticism: An analysis of the relationship between psychedelic drugs and the mystical consciousness. Harvard University Press (1963).
- 27.Pahnke, W. N. Drugs and Mysticism. International Journal of Parapsychology 295–314 (1966).
- 28.MacLean KA, Leoutsakos J-MS, Johnson MW, Griffiths RR. Factor Analysis of the Mystical Experience Questionnaire: A Study of Experiences Occasioned by the Hallucinogen Psilocybin. Journal for the scientific study of religion. 2012;51:721–737. doi: 10.1111/j.1468-5906.2012.01685.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caudevilla-Gálligo F, et al. 4-Bromo-2,5-dimethoxyphenethylamine (2C-B): presence in the recreational drug market in Spain, pattern of use and subjective effects. Journal of Psychopharmacology (Oxford, England) 2012;26:1026–1035. doi: 10.1177/0269881111431752. [DOI] [PubMed] [Google Scholar]
- 30.Papaseit E, et al. Acute Pharmacological Effects of 2C-B in Humans: An Observational Study. Frontiers in Pharmacology. 2018;9:206. doi: 10.3389/fphar.2018.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuypers KPC, et al. A First-in-Man Study with 4-Fluoroamphetamine Demonstrates it Produces a Mild Psychedelic State. J Psychoactive Drugs. 2019;51:225–235. doi: 10.1080/02791072.2019.1569286. [DOI] [PubMed] [Google Scholar]
- 32.Reckweg J, et al. A Phase 1, Dose-Ranging Study to Assess Safety and Psychoactive Effects of a Vaporized 5-Methoxy-N, N-Dimethyltryptamine Formulation (GH001) in Healthy Volunteers. Front Pharmacol. 2021;12:760671. doi: 10.3389/fphar.2021.760671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Uthaug, M. V. et al. Prospective examination of synthetic 5-methoxy-N,N-dimethyltryptamine inhalation: effects on salivary IL-6, cortisol levels, affect, and non-judgment. Psychopharmacology (Berl)237, 773–785, 10.1007/s00213-019-05414-w (2020). [DOI] [PMC free article] [PubMed]
- 34.Uthaug MV, et al. A single inhalation of vapor from dried toad secretion containing 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) in a naturalistic setting is related to sustained enhancement of satisfaction with life, mindfulness-related capacities, and a decrement of psychopathological symptoms. Psychopharmacology. 2019;236:2653–2666. doi: 10.1007/s00213-019-05236-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barsuglia J, et al. Intensity of Mystical Experiences Occasioned by 5-MeO-DMT and Comparison With a Prior Psilocybin Study. Frontiers in psychology. 2018;9:2459. doi: 10.3389/fpsyg.2018.02459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davis AK, Barsuglia JP, Lancelotta R, Grant RM, Renn E. The epidemiology of 5-methoxy- N, N-dimethyltryptamine (5-MeO-DMT) use: Benefits, consequences, patterns of use, subjective effects, and reasons for consumption. Journal of psychopharmacology (Oxford, England) 2018;32:779–792. doi: 10.1177/0269881118769063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davis AK, So S, Lancelotta R, Barsuglia JP, Griffiths RR. 5-methoxy-N,N-dimethyltryptamine (5-MeO-DMT) used in a naturalistic group setting is associated with unintended improvements in depression and anxiety. The American journal of drug and alcohol abuse. 2019;45:161–169. doi: 10.1080/00952990.2018.1545024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Riba J, Rodríguez-Fornells A, Barbanoj M. Effects of ayahuasca on sensory and sensorimotor gating in humans as measured by P50 suppression and prepulse inhibition of the startle reflex, respectively. Psychopharmacology. 2002;165:18–28. doi: 10.1007/s00213-002-1237-5. [DOI] [PubMed] [Google Scholar]
- 39.Valle M, et al. Inhibition of alpha oscillations through serotonin-2A receptor activation underlies the visual effects of ayahuasca in humans. Eur Neuropsychopharmacol. 2016;26:1161–1175. doi: 10.1016/j.euroneuro.2016.03.012. [DOI] [PubMed] [Google Scholar]
- 40.Alonso, J. F., Romero, S., Mañanas, M. À. & Riba, J. Serotonergic Psychedelics Temporarily Modify Information Transfer in Humans. International Journal of Neuropsychopharmacology18, 10.1093/ijnp/pyv039 (2015). [DOI] [PMC free article] [PubMed]
- 41.Uthaug M, et al. A placebo-controlled study of the effects of ayahuasca, set and setting on mental health of participants in ayahuasca group retreats. Psychopharmacology. 2021;238:1899–1910. doi: 10.1007/s00213-021-05817-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Barbanoj MJ, et al. Daytime Ayahuasca administration modulates REM and slow-wave sleep in healthy volunteers. Psychopharmacology. 2008;196:315–326. doi: 10.1007/s00213-007-0963-0. [DOI] [PubMed] [Google Scholar]
- 43.Dos Santos RG, et al. Autonomic, neuroendocrine, and immunological effects of ayahuasca: a comparative study with d-amphetamine. Journal of clinical psychopharmacology. 2011;31:717–726. doi: 10.1097/JCP.0b013e31823607f6. [DOI] [PubMed] [Google Scholar]
- 44.Palhano-Fontes F, et al. Rapid antidepressant effects of the psychedelic ayahuasca in treatment-resistant depression: a randomized placebo-controlled trial. Psychol Med. 2019;49:655–663. doi: 10.1017/S0033291718001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pasquini L, Palhano-Fontes F, Araujo DB. Subacute effects of the psychedelic ayahuasca on the salience and default mode networks. J Psychopharmacol. 2020;34:623–635. doi: 10.1177/0269881120909409. [DOI] [PubMed] [Google Scholar]
- 46.Riba J, et al. Subjective effects and tolerability of the South American psychoactive beverage Ayahuasca in healthy volunteers. Psychopharmacology. 2001;154:85–95. doi: 10.1007/s002130000606. [DOI] [PubMed] [Google Scholar]
- 47.Riba J, et al. Topographic pharmaco-EEG mapping of the effects of the South American psychoactive beverage ayahuasca in healthy volunteers: Topographic pharmaco-EEG of ayahuasca. British Journal of Clinical Pharmacology. 2002;53:613–628. doi: 10.1046/j.1365-2125.2002.01609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Riba J, et al. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion, and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306:73–83. doi: 10.1124/jpet.103.049882. [DOI] [PubMed] [Google Scholar]
- 49.Riba J, et al. Increased frontal and paralimbic activation following ayahuasca, the pan-amazonian inebriant. Psychopharmacology. 2006;186:93–98. doi: 10.1007/s00213-006-0358-7. [DOI] [PubMed] [Google Scholar]
- 50.Grob CS, et al. Human Psychopharmacology of Hoasca, A Plant Hallucinogen Used in Ritual Context in Brazil. The Journal of Nervous and Mental Disease. 1996;184:86–94. doi: 10.1097/00005053-199602000-00004. [DOI] [PubMed] [Google Scholar]
- 51.Griffiths RR, Hurwitz ES, Davis AK, Johnson MW, Jesse R. Survey of subjective ‘God encounter experiences’: Comparisons among naturally occurring experiences and those occasioned by the classic psychedelics psilocybin, LSD, ayahuasca, or DMT. PloS one. 2019;14:e0214377. doi: 10.1371/journal.pone.0214377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ruffell SGD, et al. Ceremonial Ayahuasca in Amazonian Retreats-Mental Health and Epigenetic Outcomes From a Six-Month Naturalistic Study. Frontiers in Psychiatry. 2021;12:687615. doi: 10.3389/fpsyt.2021.687615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Daumann J, et al. Pharmacological modulation of the neural basis underlying inhibition of return (IOR) in the human 5-HT2A agonist and NMDA antagonist model of psychosis. Psychopharmacology. 2008;200:573–583. doi: 10.1007/s00213-008-1237-1. [DOI] [PubMed] [Google Scholar]
- 54.Gouzoulis-Mayfrank E, et al. Psychological Effects of (S)-Ketamine and N,N-Dimethyltryptamine (DMT): A Double-Blind, Cross-Over Study in Healthy Volunteers. Pharmacopsychiatry. 2005;38:301–311. doi: 10.1055/s-2005-916185. [DOI] [PubMed] [Google Scholar]
- 55.Pallavicini C, et al. Neural and subjective effects of inhaled N,N-dimethyltryptamine in natural settings. Journal of psychopharmacology (Oxford, England) 2021;35:406–420. doi: 10.1177/0269881120981384. [DOI] [PubMed] [Google Scholar]
- 56.Riba J, Rodríguez-Fornells A, Strassman RJ, Barbanoj MJ. Psychometric assessment of the Hallucinogen Rating Scale. Drug and Alcohol Dependence. 2001;62:215–223. doi: 10.1016/S0376-8716(00)00175-7. [DOI] [PubMed] [Google Scholar]
- 57.Riba J, McIlhenny EH, Bouso JC, Barker SA. Metabolism and urinary disposition of N,N-dimethyltryptamine after oral and smoked administration: a comparative study. Drug testing and analysis. 2015;7:401–406. doi: 10.1002/dta.1685. [DOI] [PubMed] [Google Scholar]
- 58.Schenberg EE, et al. Acute Biphasic Effects of Ayahuasca. PLoS One. 2015;10:e0137202. doi: 10.1371/journal.pone.0137202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strassman, R. J., Qualls, C. R. & Berg, L. M. Differential tolerance to biological and subjective effects of four closely spaced doses of N,N-dimethyltryptamine in humans. Biol Psychiatry39, 784–795, 10.1016/0006-3223(95)00200-6 (1996). [DOI] [PubMed]
- 60.Carbonaro TM, Johnson MW, Hurwitz E, Griffiths RR. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology (Berl) 2018;235:521–534. doi: 10.1007/s00213-017-4769-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reissig CJ, et al. High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology. 2012;223:1–15. doi: 10.1007/s00213-012-2680-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Holze F, et al. Distinct acute effects of LSD, MDMA, and D-amphetamine in healthy subjects. Neuropsychopharmacology. 2020;45:462–471. doi: 10.1038/s41386-019-0569-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tibbo P, et al. A single photon emission computed tomography scan study of striatal dopamine D2 receptor binding with 123I-epidepride in patients with schizophrenia and controls. Journal of psychiatry & neuroscience: JPN. 1997;22:39–45. [PMC free article] [PubMed] [Google Scholar]
- 64.Gouzoulis-Mayfrank E, et al. Psychopathological, neuroendocrine and autonomic effects of 3,4-methylenedioxyethylamphetamine (MDE), psilocybin and d -methamphetamine in healthy volunteers. Psychopharmacology. 1999;142:41–50. doi: 10.1007/s002130050860. [DOI] [PubMed] [Google Scholar]
- 65.Pokorny T, Preller KH, Kraehenmann R, Vollenweider FX. Modulatory effect of the 5-HT1A agonist buspirone and the mixed non-hallucinogenic 5-HT1A/2A agonist ergotamine on psilocybin-induced psychedelic experience. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2016;26:756–766. doi: 10.1016/j.euroneuro.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 66.Heink A, Katsikas S, Lange-Altman T. Examination of the Phenomenology of the Ibogaine Treatment Experience: Role of Altered States of Consciousness and Psychedelic Experiences. J Psychoactive Drugs. 2017;49:201–208. doi: 10.1080/02791072.2017.1290855. [DOI] [PubMed] [Google Scholar]
- 67.Schmidt TT, Reiche S, Hage CLC, Bermpohl F, Majić T. Acute and subacute psychoactive effects of Kambô, the secretion of the Amazonian Giant Maki Frog (Phyllomedusa bicolor): retrospective reports. Scientific reports. 2020;10:21544. doi: 10.1038/s41598-020-78527-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vollenweider FX, Vontobel P, Oye I, Hell D, Leenders KL. Effects of (S)-ketamine on striatal dopamine: a [C-11]raclopride PET study of a model psychosis in humans. Journal of Psychiatric Research. 2000;34:35–43. doi: 10.1016/S0022-3956(99)00031-X. [DOI] [PubMed] [Google Scholar]
- 69.Curic S, et al. Reduced auditory evoked gamma-band response and schizophrenia-like clinical symptoms under subanesthetic ketamine. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2019;44:1239–1246. doi: 10.1038/s41386-019-0328-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Höflich, A. et al. Ketamine-Induced Modulation of the Thalamo-Cortical Network in Healthy Volunteers As a Model for Schizophrenia. Int J Neuropsychopharmacol18, 10.1093/ijnp/pyv040 (2015). [DOI] [PMC free article] [PubMed]
- 71.Lehmann M, et al. Effects of ketamine on brain function during metacognition of episodic memory. Neuroscience of consciousness. 2021;2021:niaa028. doi: 10.1093/nc/niaa028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mueller, F. et al. Pharmacological fMRI: Effects of subanesthetic ketamine on resting-state functional connectivity in the default mode network, salience network, dorsal attention network and executive control network. Neuroimage Clin19, 745–757, 10.1016/j.nicl.2018.05.037 (2018). [DOI] [PMC free article] [PubMed]
- 73.Musso F, et al. Ketamine effects on brain function — Simultaneous fMRI/EEG during a visual oddball task. NeuroImage. 2011;58:508–525. doi: 10.1016/j.neuroimage.2011.06.045. [DOI] [PubMed] [Google Scholar]
- 74.Northoff G, et al. NMDA hypofunction in the posterior cingulate as a model for schizophrenia: an exploratory ketamine administration study in fMRI. Schizophrenia research. 2005;72:235–248. doi: 10.1016/j.schres.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 75.Passie T, et al. Effects of different subanaesthetic doses of (S)-ketamine on psychopathology and binocular depth inversion in man. J Psychopharmacol. 2003;17:51–56. doi: 10.1177/0269881103017001698. [DOI] [PubMed] [Google Scholar]
- 76.Passie T, Karst M, Wiese B, Emrich HM, Schneider U. Effects of different subanesthetic doses of (S)-ketamine on neuropsychology, psychopathology, and state of consciousness in man. Neuropsychobiology. 2005;51:226–233. doi: 10.1159/000085724. [DOI] [PubMed] [Google Scholar]
- 77.Scheidegger M, et al. Effects of ketamine on cognition-emotion interaction in the brain. Neuroimage. 2016;124:8–15. doi: 10.1016/j.neuroimage.2015.08.070. [DOI] [PubMed] [Google Scholar]
- 78.Scheidegger M, et al. Ketamine administration reduces amygdalo-hippocampal reactivity to emotional stimulation. Human brain mapping. 2016;37:1941–1952. doi: 10.1002/hbm.23148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Schmidt A, et al. Mismatch Negativity Encoding of Prediction Errors Predicts S-ketamine-Induced Cognitive Impairments. Neuropsychopharmacol. 2012;37:865–875. doi: 10.1038/npp.2011.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sprenger T, et al. Imaging Pain Modulation by Subanesthetic S-(+)-Ketamine. Anesthesia & Analgesia. 2006;103:729–737. doi: 10.1213/01.ane.0000231635.14872.40. [DOI] [PubMed] [Google Scholar]
- 81.Thiebes, S. et al. Alterations in interhemispheric gamma-band connectivity are related to the emergence of auditory verbal hallucinations in healthy subjects during NMDA-receptor blockade. Neuropsychopharmacology43, 1608–1615, 10.1038/s41386-018-0014-z (2018). [DOI] [PMC free article] [PubMed]
- 82.Thiebes S, et al. Glutamatergic deficit and schizophrenia-like negative symptoms: new evidence from ketamine-induced mismatch negativity alterations in healthy male humans. J Psychiatry Neurosci. 2017;42:273–283. doi: 10.1503/jpn.160187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Umbricht D, Koller R, Vollenweider FX, Schmid L. Mismatch negativity predicts psychotic experiences induced by nmda receptor antagonist in healthy volunteers. Biological Psychiatry. 2002;51:400–406. doi: 10.1016/S0006-3223(01)01242-2. [DOI] [PubMed] [Google Scholar]
- 84.Curic S, et al. Ketamine Alters Functional Gamma and Theta Resting-State Connectivity in Healthy Humans: Implications for Schizophrenia Treatment Targeting the Glutamate System. Frontiers in psychiatry. 2021;12:671007. doi: 10.3389/fpsyt.2021.671007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Vlisides, P. E. et al. Subanaesthetic ketamine and altered states of consciousness in humans. Br J Anaesth121, 249–259, 10.1016/j.bja.2018.03.011 (2018). [DOI] [PMC free article] [PubMed]
- 86.Bowdle, T. A. et al. Psychedelic effects of ketamine in healthy volunteers: relationship to steady-state plasma concentrations. Anesthesiology88, 82–88, 10.1097/00000542-199801000-00015 (1998). [DOI] [PubMed]
- 87.Carter, L. P., Kleykamp, B. A., Griffiths, R. R. & Mintzer, M. Z. Cognitive effects of intramuscular ketamine and oral triazolam in healthy volunteers. Psychopharmacology (Berl)226, 53–63, 10.1007/s00213-012-2883-x (2013). [DOI] [PMC free article] [PubMed]
- 88.Krupitsky E, et al. Ketamine psychotherapy for heroin addiction: immediate effects and two-year follow-up. Journal of Substance Abuse Treatment. 2002;23:273–283. doi: 10.1016/S0740-5472(02)00275-1. [DOI] [PubMed] [Google Scholar]
- 89.Lofwall MR, Griffiths RR, Mintzer MZ. Cognitive and subjective acute dose effects of intramuscular ketamine in healthy adults. Experimental and clinical psychopharmacology. 2006;14:439–449. doi: 10.1037/1064-1297.14.4.439. [DOI] [PubMed] [Google Scholar]
- 90.Family N, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of low dose lysergic acid diethylamide (LSD) in healthy older volunteers. Psychopharmacology. 2020;237:841–853. doi: 10.1007/s00213-019-05417-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Holze F, et al. Acute dose-dependent effects of lysergic acid diethylamide in a double-blind placebo-controlled study in healthy subjects. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2021;46:537–544. doi: 10.1038/s41386-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liechti ME, Dolder PC, Schmid Y. Alterations of consciousness and mystical-type experiences after acute LSD in humans. Psychopharmacology (Berl) 2017;234:1499–1510. doi: 10.1007/s00213-016-4453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Müller F, et al. Increased thalamic resting-state connectivity as a core driver of LSD-induced hallucinations. Acta Psychiatr Scand. 2017;136:648–657. doi: 10.1111/acps.12818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schmid Y, Gasser P, Oehen P, Liechti ME. Acute subjective effects in LSD- and MDMA-assisted psychotherapy. Journal of psychopharmacology (Oxford, England) 2021;35:362–374. doi: 10.1177/0269881120959604. [DOI] [PubMed] [Google Scholar]
- 95.Grumann C, et al. Pharmacokinetics and subjective effects of 1P-LSD in humans after oral and intravenous administration. Drug testing and analysis. 2020;12:1144–1153. doi: 10.1002/dta.2821. [DOI] [PubMed] [Google Scholar]
- 96.Bershad, A. K. et al. Preliminary Report on the Effects of a Low Dose of LSD on Resting-State Amygdala Functional Connectivity. Biol Psychiatry Cogn Neurosci Neuroimaging5, 461–467, 10.1016/j.bpsc.2019.12.007 (2020). [DOI] [PMC free article] [PubMed]
- 97.Carhart-Harris RL, et al. The paradoxical psychological effects of lysergic acid diethylamide (LSD. Psychol Med. 2016;46:1379–1390. doi: 10.1017/S0033291715002901. [DOI] [PubMed] [Google Scholar]
- 98.Carhart-Harris RL, et al. Neural correlates of the LSD experience revealed by multimodal neuroimaging. Proc Natl Acad Sci USA. 2016;113:4853–4858. doi: 10.1073/pnas.1518377113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hutten NRPW, et al. Mood and cognition after administration of low LSD doses in healthy volunteers: A placebo controlled dose-effect finding study. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2020;41:81–91. doi: 10.1016/j.euroneuro.2020.10.002. [DOI] [PubMed] [Google Scholar]
- 100.Kraehenmann R, et al. LSD Increases Primary Process Thinking via Serotonin 2A Receptor Activation. Front Pharmacol. 2017;8:814. doi: 10.3389/fphar.2017.00814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Murray, C. H. et al. Low doses of LSD reduce broadband oscillatory power and modulate event-related potentials in healthy adults. Psychopharmacology (Berl), 10.1007/s00213-021-05991-9 (2021). [DOI] [PMC free article] [PubMed]
- 102.Preller, K. H. et al. Changes in global and thalamic brain connectivity in LSD-induced altered states of consciousness are attributable to the 5-HT2A receptor. Elife7, 10.7554/eLife.35082 (2018). [DOI] [PMC free article] [PubMed]
- 103.Preller, K. H. et al. Role of the 5-HT2A Receptor in Self- and Other-Initiated Social Interaction in Lysergic Acid Diethylamide-Induced States: A Pharmacological fMRI Study. J Neurosci38, 3603–3611, 10.1523/JNEUROSCI.1939-17.2018 (2018). [DOI] [PMC free article] [PubMed]
- 104.Preller KH, et al. The Fabric of Meaning and Subjective Effects in LSD-Induced States Depend on Serotonin 2A Receptor Activation. Curr Biol. 2017;27:451–457. doi: 10.1016/j.cub.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 105.Wießner, I. et al. LSD, madness and healing: Mystical experiences as possible link between psychosis model and therapy model. Psychological medicine 1–15, 10.1017/S0033291721002531 (2021). [DOI] [PubMed]
- 106.Bershad AK, Schepers ST, Bremmer MP, Lee R, de Wit H. Acute Subjective and Behavioral Effects of Microdoses of Lysergic Acid Diethylamide in Healthy Human Volunteers. Biological psychiatry. 2019;86:792–800. doi: 10.1016/j.biopsych.2019.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kimura Y, et al. Measurement of psychological state changes at low dopamine transporter occupancy following a clinical dose of mazindol. Psychopharmacology (Berl) 2017;234:323–328. doi: 10.1007/s00213-016-4464-x. [DOI] [PubMed] [Google Scholar]
- 108.Baggott MJ, et al. Investigating the Mechanisms of Hallucinogen-Induced Visions Using 3,4-Methylenedioxyamphetamine (MDA): A Randomized Controlled Trial in Humans. PLoS ONE. 2010;5:e14074. doi: 10.1371/journal.pone.0014074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Baggott MJ, et al. Effects of the Psychedelic Amphetamine MDA (3,4-Methylenedioxyamphetamine) in Healthy Volunteers. Journal of Psychoactive Drugs. 2019;51:108–117. doi: 10.1080/02791072.2019.1593560. [DOI] [PubMed] [Google Scholar]
- 110.Hermle L, Spitzer M, Borchardt D, Kovar KA, Gouzoulis E. Psychological effects of MDE in normal subjects. Are entactogens a new class of psychoactive agents? Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 1993;8:171–176. doi: 10.1038/npp.1993.19. [DOI] [PubMed] [Google Scholar]
- 111.Dolder PC, Müller F, Schmid Y, Borgwardt SJ, Liechti ME. Direct comparison of the acute subjective, emotional, autonomic, and endocrine effects of MDMA, methylphenidate, and modafinil in healthy subjects. Psychopharmacology (Berl) 2018;235:467–479. doi: 10.1007/s00213-017-4650-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Frei E, et al. Localization of MDMA-induced brain activity in healthy volunteers using low resolution brain electromagnetic tomography (LORETA) Hum. Brain Mapp. 2001;14:152–165. doi: 10.1002/hbm.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hasler F, Studerus E, Lindner K, Ludewig S, Vollenweider F. Investigation of serotonin-1A receptor function in the human psychopharmacology of MDMA. J Psychopharmacol. 2009;23:923–935. doi: 10.1177/0269881108094650. [DOI] [PubMed] [Google Scholar]
- 114.Gamma A. 3,4-Methylenedioxymethamphetamine (MDMA) Modulates Cortical and Limbic Brain Activity as Measured by [H215O]-PET in Healthy Humans. Neuropsychopharmacology. 2000;23:388–395. doi: 10.1016/S0893-133X(00)00130-5. [DOI] [PubMed] [Google Scholar]
- 115.Hysek C, et al. Carvedilol inhibits the cardiostimulant and thermogenic effects of MDMA in humans. Br J Pharmacol. 2012;166:2277–2288. doi: 10.1111/j.1476-5381.2012.01936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hysek CM, et al. The Norepinephrine Transporter Inhibitor Reboxetine Reduces Stimulant Effects of MDMA (“Ecstasy”) in Humans. Clin Pharmacol Ther. 2011;90:246–255. doi: 10.1038/clpt.2011.78. [DOI] [PubMed] [Google Scholar]
- 117.Hysek CM, et al. Duloxetine inhibits effects of MDMA (‘ecstasy’) in vitro and in humans in a randomized placebo-controlled laboratory study. PLoS One. 2012;7:e36476. doi: 10.1371/journal.pone.0036476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hysek CM, et al. Effects of the α2-adrenergic agonist clonidine on the pharmacodynamics and pharmacokinetics of 3,4-methylenedioxymethamphetamine in healthy volunteers. J Pharmacol Exp Ther. 2012;340:286–294. doi: 10.1124/jpet.111.188425. [DOI] [PubMed] [Google Scholar]
- 119.Hysek CM, et al. α1-Adrenergic receptors contribute to the acute effects of 3,4-methylenedioxymethamphetamine in humans. J Clin Psychopharmacol. 2013;33:658–666. doi: 10.1097/JCP.0b013e3182979d32. [DOI] [PubMed] [Google Scholar]
- 120.Hysek CM, et al. Pharmacokinetic and pharmacodynamic effects of methylphenidate and MDMA administered alone or in combination. International Journal of Neuropsychopharmacology. 2014;17:371–381. doi: 10.1017/S1461145713001132. [DOI] [PubMed] [Google Scholar]
- 121.Liechti, M. E. & Vollenweider, F. X. Acute psychological and physiological effects of MDMA (‘Ecstasy’) after haloperidol pretreatment in healthy humans. Eur Neuropsychopharmacol10, 289–295, 10.1016/S0924-977X(00)00086-9 (2000). [DOI] [PubMed]
- 122.Liechti ME, Baumann C, Gamma A, Vollenweider FX. Acute psychological effects of 3,4-methylenedioxymethamphetamine (MDMA, ‘Ecstasy’) are attenuated by the serotonin uptake inhibitor citalopram. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2000;22:513–521. doi: 10.1016/S0893-133X(99)00148-7. [DOI] [PubMed] [Google Scholar]
- 123.Liechti M. Psychological and Physiological Effects of MDMA (“Ecstasy”) after Pretreatment with the 5-HT2 Antagonist Ketanserin in Healthy Humans. Neuropsychopharmacology. 2000;23:396–404. doi: 10.1016/S0893-133X(00)00126-3. [DOI] [PubMed] [Google Scholar]
- 124.Liechti ME, Gamma A, Vollenweider FX. Gender differences in the subjective effects of MDMA. Psychopharmacology. 2001;154:161–168. doi: 10.1007/s002130000648. [DOI] [PubMed] [Google Scholar]
- 125.Schmid Y, et al. Differential effects of MDMA and methylphenidate on social cognition. J Psychopharmacol. 2014;28:847–856. doi: 10.1177/0269881114542454. [DOI] [PubMed] [Google Scholar]
- 126.Vollenweider FX, Liechti ME, Paulus MP. MDMA affects both error-rate dependent and independent aspects of decision-making in a two-choice prediction task. J Psychopharmacol. 2005;19:366–374. doi: 10.1177/0269881105053287. [DOI] [PubMed] [Google Scholar]
- 127.Bouso JC, Doblin R, Farré M, Alcázar MÁ, Gómez-Jarabo G. MDMA-Assisted Psychotherapy Using Low Doses in a Small Sample of Women with Chronic Posttraumatic Stress Disorder. Journal of Psychoactive Drugs. 2008;40:225–236. doi: 10.1080/02791072.2008.10400637. [DOI] [PubMed] [Google Scholar]
- 128.Hermle L, et al. Mescaline-induced psychopathological, neuropsychological, and neurometabolic effects in normal subjects: experimental psychosis as a tool for psychiatric research. Biological psychiatry. 1992;32:976–991. doi: 10.1016/0006-3223(92)90059-9. [DOI] [PubMed] [Google Scholar]
- 129.Bravermanová A, et al. Psilocybin disrupts sensory and higher order cognitive processing but not pre-attentive cognitive processing-study on P300 and mismatch negativity in healthy volunteers. Psychopharmacology (Berl) 2018;235:491–503. doi: 10.1007/s00213-017-4807-2. [DOI] [PubMed] [Google Scholar]
- 130.Griffiths RR, Richards WA, McCann U, Jesse R. Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology. 2006;187:268–83. doi: 10.1007/s00213-006-0457-5. [DOI] [PubMed] [Google Scholar]
- 131.Griffiths, R. R. et al. Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology (Berl)218, 649–665, 10.1007/s00213-011-2358-5 (2011). [DOI] [PMC free article] [PubMed]
- 132.Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F. The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacology. 2013;225:227–239. doi: 10.1007/s00213-012-2811-0. [DOI] [PubMed] [Google Scholar]
- 133.Bogenschutz MP, et al. Psilocybin-assisted treatment for alcohol dependence: a proof-of-concept study. J Psychopharmacol. 2015;29:289–299. doi: 10.1177/0269881114565144. [DOI] [PubMed] [Google Scholar]
- 134.Carhart-Harris RL, et al. The administration of psilocybin to healthy, hallucinogen-experienced volunteers in a mock-functional magnetic resonance imaging environment: a preliminary investigation of tolerability. J Psychopharmacol. 2011;25:1562–1567. doi: 10.1177/0269881110367445. [DOI] [PubMed] [Google Scholar]
- 135.Carter OL, et al. Modulating the Rate and Rhythmicity of Perceptual Rivalry Alternations with the Mixed 5-HT2A and 5-HT1A Agonist Psilocybin. Neuropsychopharmacol. 2005;30:1154–1162. doi: 10.1038/sj.npp.1300621. [DOI] [PubMed] [Google Scholar]
- 136.Griffiths RR, et al. Psilocybin produces substantial and sustained decreases in depression and anxiety in patients with life-threatening cancer: A randomized double-blind trial. J Psychopharmacol. 2016;30:1181–1197. doi: 10.1177/0269881116675513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Griffiths RR, et al. Psilocybin-occasioned mystical-type experience in combination with meditation and other spiritual practices produces enduring positive changes in psychological functioning and in trait measures of prosocial attitudes and behaviors. J Psychopharmacol. 2018;32:49–69. doi: 10.1177/0269881117731279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grob CS, et al. Pilot Study of Psilocybin Treatment for Anxiety in Patients With Advanced-Stage Cancer. Arch Gen Psychiatry. 2011;68:71. doi: 10.1001/archgenpsychiatry.2010.116. [DOI] [PubMed] [Google Scholar]
- 139.Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX. Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology. 2004;172:145–156. doi: 10.1007/s00213-003-1640-6. [DOI] [PubMed] [Google Scholar]
- 140.Quednow BB, Kometer M, Geyer MA, Vollenweider FX. Psilocybin-Induced Deficits in Automatic and Controlled Inhibition are Attenuated by Ketanserin in Healthy Human Volunteers. Neuropsychopharmacol. 2012;37:630–640. doi: 10.1038/npp.2011.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Smigielski, L. et al. Characterization and prediction of acute and sustained response to psychedelic psilocybin in a mindfulness group retreat. Sci Rep9, 14914, 10.1038/s41598-019-50612-3 (2019). [DOI] [PMC free article] [PubMed]
- 142.Smigielski L, Scheidegger M, Kometer M, Vollenweider FX. Psilocybin-assisted mindfulness training modulates self-consciousness and brain default mode network connectivity with lasting effects. Neuroimage. 2019;196:207–215. doi: 10.1016/j.neuroimage.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 143.Vollenweider FX, Csomor PA, Knappe B, Geyer MA, Quednow BB. The Effects of the Preferential 5-HT2A Agonist Psilocybin on Prepulse Inhibition of Startle in Healthy Human Volunteers Depend on Interstimulus Interval. Neuropsychopharmacol. 2007;32:1876–1887. doi: 10.1038/sj.npp.1301324. [DOI] [PubMed] [Google Scholar]
- 144.Wittmann M, et al. Effects of psilocybin on time perception and temporal control of behaviour in humans. J Psychopharmacol. 2007;21:50–64. doi: 10.1177/0269881106065859. [DOI] [PubMed] [Google Scholar]
- 145.Carter OL, et al. Using Psilocybin to Investigate the Relationship between Attention, Working Memory, and the Serotonin 1A and 2A Receptors. Journal of Cognitive Neuroscience. 2005;17:1497–1508. doi: 10.1162/089892905774597191. [DOI] [PubMed] [Google Scholar]
- 146.Carter OL, et al. Psilocybin links binocular rivalry switch rate to attention and subjective arousal levels in humans. Psychopharmacology. 2007;195:415–424. doi: 10.1007/s00213-007-0930-9. [DOI] [PubMed] [Google Scholar]
- 147.Bernasconi F, et al. Spatiotemporal brain dynamics of emotional face processing modulations induced by the serotonin 1A/2A receptor agonist psilocybin. Cereb Cortex. 2014;24:3221–3231. doi: 10.1093/cercor/bht178. [DOI] [PubMed] [Google Scholar]
- 148.Carhart-Harris RL, et al. Psilocybin with psychological support for treatment-resistant depression: six-month follow-up. Psychopharmacology. 2018;235:399–408. doi: 10.1007/s00213-017-4771-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Kometer M, et al. Psilocybin biases facial recognition, goal-directed behavior, and mood state toward positive relative to negative emotions through different serotonergic subreceptors. Biol Psychiatry. 2012;72:898–906. doi: 10.1016/j.biopsych.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 150.Lewis, C. R., Preller, K. H., Braden, B. B., Riecken, C. & Vollenweider, F. X. Rostral Anterior Cingulate Thickness Predicts the Emotional Psilocybin Experience. Biomedicines8, 10.3390/biomedicines8020034 (2020). [DOI] [PMC free article] [PubMed]
- 151.Lewis CR, et al. Two dose investigation of the 5-HT-agonist psilocybin on relative and global cerebral blood flow. NeuroImage. 2017;159:70–78. doi: 10.1016/j.neuroimage.2017.07.020. [DOI] [PubMed] [Google Scholar]
- 152.Madsen MK, et al. Psychedelic effects of psilocybin correlate with serotonin 2A receptor occupancy and plasma psilocin levels. Neuropsychopharmacology. 2019;44:1328–1334. doi: 10.1038/s41386-019-0324-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Madsen, M. K. et al. A single psilocybin dose is associated with long-term increased mindfulness, preceded by a proportional change in neocortical 5-HT2A receptor binding. Eur Neuropsychopharmacol33, 71–80, 10.1016/j.euroneuro.2020.02.001 (2020). [DOI] [PubMed]
- 154.Madsen MK, et al. Psilocybin-induced changes in brain network integrity and segregation correlate with plasma psilocin level and psychedelic experience. European neuropsychopharmacology: the journal of the European College of Neuropsychopharmacology. 2021;50:121–132. doi: 10.1016/j.euroneuro.2021.06.001. [DOI] [PubMed] [Google Scholar]
- 155.Preller KH, et al. Effects of serotonin 2A/1A receptor stimulation on social exclusion processing. Proc Natl Acad Sci USA. 2016;113:5119–5124. doi: 10.1073/pnas.1524187113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pokorny T, Preller KH, Kometer M, Dziobek I, Vollenweider FX. Effect of Psilocybin on Empathy and Moral Decision-Making. Int J Neuropsychopharmacol. 2017;20:747–757. doi: 10.1093/ijnp/pyx047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Moreno, F. A. safety tolerability and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J Clin Psychiatry, 10.4088/jcp.v67n1110 (2006). [DOI] [PubMed]
- 158.Olson JA, Suissa-Rocheleau L, Lifshitz M, Raz A, Veissière SPL. Tripping on nothing: placebo psychedelics and contextual factors. Psychopharmacology (Berl) 2020;237:1371–1382. doi: 10.1007/s00213-020-05464-5. [DOI] [PubMed] [Google Scholar]
- 159.Smigielski L, et al. P300-mediated modulations in self-other processing under psychedelic psilocybin are related to connectedness and changed meaning: A window into the self-other overlap. Hum Brain Mapp. 2020;41:4982–4996. doi: 10.1002/hbm.25174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Nicholas CR, et al. High dose psilocybin is associated with positive subjective effects in healthy volunteers. J Psychopharmacol. 2018;32:770–778. doi: 10.1177/0269881118780713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ross S, et al. Rapid and sustained symptom reduction following psilocybin treatment for anxiety and depression in patients with life-threatening cancer: a randomized controlled trial. Journal of Psychopharmacology. 2016;30:1165–1180. doi: 10.1177/0269881116675512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.González D, Riba J, Bouso JC, Gómez-Jarabo G, Barbanoj MJ. Pattern of use and subjective effects of Salvia divinorum among recreational users. Drug and alcohol dependence. 2006;85:157–162. doi: 10.1016/j.drugalcdep.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 163.MacLean KA, Johnson MW, Reissig CJ, Prisinzano TE, Griffiths RR. Dose-related effects of salvinorin A in humans: dissociative, hallucinogenic, and memory effects. Psychopharmacology (Berl) 2013;226:381–392. doi: 10.1007/s00213-012-2912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Maqueda, A. E. et al. Salvinorin-A Induces Intense Dissociative Effects, Blocking External Sensory Perception and Modulating Interoception and Sense of Body Ownership in Humans. Int J Neuropsychopharmacol18, pyv065, 10.1093/ijnp/pyv065 (2015). [DOI] [PMC free article] [PubMed]
- 165.Maqueda AE, et al. Naltrexone but not ketanserin antagonizes the subjective, cardiovascular and neuroendocrine effects of salvinorin-A in humans. International Journal of Neuropsychopharmacology. 2016;19:1–13. doi: 10.1093/ijnp/pyw016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Addy PH. Acute and post-acute behavioral and psychological effects of salvinorin A in humans. Psychopharmacology. 2012;220:195–204. doi: 10.1007/s00213-011-2470-6. [DOI] [PubMed] [Google Scholar]
- 167.Albertson DN, Grubbs LE. Subjective Effects of Salvia Divinorum: LSD- or Marijuana-like? Journal of Psychoactive Drugs. 2009;41:213–217. doi: 10.1080/02791072.2009.10400531. [DOI] [PubMed] [Google Scholar]
- 168.Johnson MW, MacLean KA, Reissig CJ, Prisinzano TE, Griffiths RR. Human psychopharmacology and dose-effects of salvinorin A, a kappa opioid agonist hallucinogen present in the plant Salvia divinorum. Drug and Alcohol Dependence. 2011;115:150–155. doi: 10.1016/j.drugalcdep.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Karam A, et al. Abuse and Effects of Salvia divinorum in a Sample of Patients Hospitalized for Substance Dependence. Community Ment Health J. 2019;55:702–708. doi: 10.1007/s10597-018-0347-4. [DOI] [PubMed] [Google Scholar]
- 170.Ona G, et al. The Kappa Opioid Receptor and the Sleep of Reason: Cortico-Subcortical Imbalance Following Salvinorin-A. Int J Neuropsychopharmacol. 2022;25:54–63. doi: 10.1093/ijnp/pyab063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ranganathan M, et al. Dose-Related Behavioral, Subjective, Endocrine, and Psychophysiological Effects of the κ Opioid Agonist Salvinorin A in Humans. Biological Psychiatry. 2012;72:871–879. doi: 10.1016/j.biopsych.2012.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zaytseva Y, et al. Cannabis-induced altered states of consciousness are associated with specific dynamic brain connectivity states. Journal of psychopharmacology (Oxford, England) 2019;33:811–821. doi: 10.1177/0269881119849814. [DOI] [PubMed] [Google Scholar]
- 173.Perry G, Polito V, Thompson WF. Rhythmic Chanting and Mystical States across Traditions. Brain Sciences. 2021;11:101. doi: 10.3390/brainsci11010101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Huels ER, et al. Neural Correlates of the Shamanic State of Consciousness. Frontiers in Human Neuroscience. 2021;15:610466. doi: 10.3389/fnhum.2021.610466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Maurer RLS, Kumar VK, Woodside L, Pekala RJ. Phenomenological experience in response to monotonous drumming and hypnotizability. The American journal of clinical hypnosis. 1997;40:130–145. doi: 10.1080/00029157.1997.10403417. [DOI] [PubMed] [Google Scholar]
- 176.Bartossek MT, Kemmerer J, Schmidt TT. Altered states phenomena induced by visual flicker light stimulation. PloS one. 2021;16:e0253779. doi: 10.1371/journal.pone.0253779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Schmidt, T. T., Jagannathan, N., Ljubljanac, M., Xavier, A. & Nierhaus, T. The multimodal Ganzfeld-induced altered state of consciousness induces decreased thalamo-cortical coupling. Sci Rep10, 18686, 10.1038/s41598-020-75019-3 (2020). [DOI] [PMC free article] [PubMed]
- 178.Schmidt TT, Prein JC. The Ganzfeld experience-A stably inducible altered state of consciousness: Effects of different auditory homogenizations. Psych J. 2019;8:66–81. doi: 10.1002/pchj.262. [DOI] [PubMed] [Google Scholar]
- 179.Kübel SL, Fiedler H, Wittmann M. Red visual stimulation in the Ganzfeld leads to a relative overestimation of duration compared to green. PsyCh journal. 2021;10:5–19. doi: 10.1002/pchj.395. [DOI] [PubMed] [Google Scholar]
- 180.Facco E, et al. The Neurophenomenology of Out-of-Body Experiences Induced by Hypnotic Suggestions. International Journal of Clinical and Experimental Hypnosis. 2019;67:39–68. doi: 10.1080/00207144.2019.1553762. [DOI] [PubMed] [Google Scholar]
- 181.Kasos E, Kasos K, Kolto A, Józsa E, Varga K. Phenomenological Experiences during Active-Alert Hypnosis: Comparison of Hypnotist and Subject. The International journal of clinical and experimental hypnosis. 2020;68:451–465. doi: 10.1080/00207144.2020.1802733. [DOI] [PubMed] [Google Scholar]
- 182.Pekala, R. J. et al. Positive affect, negative affect, and negative effects during a phenomenological hypnotic assessment within a substance abuse population. Int J Clin Exp Hypn57, 64–93, 10.1080/00207140802463674 (2009). [DOI] [PubMed]
- 183.Pekala RJ. Operationalizing trance II: clinical application using a psychophenomenological approach. The American journal of clinical hypnosis. 2002;44:241–255. doi: 10.1080/00029157.2002.10403484. [DOI] [PubMed] [Google Scholar]
- 184.Pekala RJ, Forbes EJ. Types of hypnotically (un)susceptible individuals as a function of phenomenological experience: towards a typology of hypnotic types. The American journal of clinical hypnosis. 1997;39:212–224. doi: 10.1080/00029157.1997.10403386. [DOI] [PubMed] [Google Scholar]
- 185.Pekala RJ, Kumar VK. Predicting hypnotic susceptibility via a self-report instrument: a replication. The American journal of clinical hypnosis. 1987;30:57–65. doi: 10.1080/00029157.1987.10402723. [DOI] [PubMed] [Google Scholar]
- 186.Pekala RJ, et al. Hypnotism as a Function of Trance State Effects, Expectancy, and Suggestibility: An Italian Replication. The International journal of clinical and experimental hypnosis. 2017;65:210–240. doi: 10.1080/00207144.2017.1276365. [DOI] [PubMed] [Google Scholar]
- 187.Varga K, Kekecs Z, Myhre PS, Józsa E. A Neutral Control Condition for Hypnosis Experiments: ‘Wiki’ Text. The International journal of clinical and experimental hypnosis. 2017;65:429–451. doi: 10.1080/00207144.2017.1348833. [DOI] [PubMed] [Google Scholar]
- 188.Venkatesh S, Raju TR, Shivani Y, Tompkins G, Meti BL. A study of structure of phenomenology of consciousness in meditative and non-meditative states. Indian journal of physiology and pharmacology. 1997;41:149–153. [PubMed] [Google Scholar]
- 189.Walach H, Käseberg E. Mind machines: a controlled study on the effects of electromagnetic and optic-acoustic stimulation on general well-being, electrodermal activity, and exceptional psychological experiences. Behav Med. 1998;24:107–114. doi: 10.1080/08964289809596388. [DOI] [PubMed] [Google Scholar]
- 190.Piarulli A, et al. Ultra-slow mechanical stimulation of olfactory epithelium modulates consciousness by slowing cerebral rhythms in humans. Scientific reports. 2018;8:6581. doi: 10.1038/s41598-018-24924-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Polito V, Langdon R, Brown J. The experience of altered states of consciousness in shamanic ritual: The role of pre-existing beliefs and affective factors. Consciousness and Cognition. 2010;19:918–925. doi: 10.1016/j.concog.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 192.Johnson M. A Randomized Study of a Novel Zen Dialogue Method for Producing Spiritual and Well-Being Enhancement: Implications for End-of-Life Care. J Holist Nurs. 2011;29:201–210. doi: 10.1177/0898010110391265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No custom code was used to generate, process, or analyse the data presented in the manuscript.