Abstract
Background/Aims:
A significant number of people with Alzheimer’s disease or related dementia diagnoses will be cared for in nursing homes near the end of life. Advance care planning (ACP), the process of eliciting and documenting patient-centered preferences for care, is considered essential to providing high quality care for this population. Nursing homes are currently required by regulations to offer ACP to residents and families, but no training requirements exist for nursing home staff and approaches to fulfilling this regulatory and ethical responsibility vary. As a result, residents may receive care inconsistent with their goals, such as unwanted hospitalizations. Pragmatic trials offer a way to develop and test ACP in real world settings to increase the likelihood of adoption of sustainable best practices.
Methods:
The “Aligning Patient Preferences – a Role Offering Alzheimer’s patients, Caregivers, and Healthcare providers Education and Support (APPROACHES)” project is designed to pragmatically test and evaluate a staff-led program in 137 nursing homes (68 = intervention, 69 = control) owned by two nursing home corporations. Existing nursing home staff receive standardized training and implement the ACP Specialist program under the supervision of a corporate lead. The primary trial outcome is the annual rate of hospital transfers (admissions and emergency department visits). Consistent with the spirit of a pragmatic trial, study outcomes rely on data already collected for quality improvement, clinical or billing purposes. Configurational analysis will also be performed to identify conditions associated with implementation.
Results:
Partnerships with large corporate companies enable the APPROACHES trial to rely on corporate infrastructure to roll out the intervention, with support for a corporate implementation lead who is charged with the initial introduction and ongoing support for nursing home based ACP Specialists. These internal champions connect the project with other company priorities and use strategies familiar to nursing home leaders for the initiation of other programs. Standardized data collection across nursing homes also supports the conduct of pragmatic trials in this setting.
Discussion:
Many interventions to improve care in nursing homes have failed to demonstrate an impact or, if successful, maintain impact over time. Pragmatic trials, designed to test interventions in real-world contexts that are evaluated through existing data sources collected routinely as part of clinical care, are well-suited for the nursing home environment. A robust program that increases access to ACP for nursing home residents has the potential to increase goal-concordant care and is expected to reduce hospital transfers. If successful, the ACP Specialist Program will be primed for rapid translation into nursing home practice to reduce unwanted, burdensome hospitalizations and improve quality of care for residents with dementia.
Keywords: Nursing facility, Alzheimer’s disease, palliative care, decision-making, end of life care
Background/Aims
Nursing homes are an important site of end-of-life care for people living with Alzheimer’s disease and related dementias (ADRD) and their families. The majority of residents in nursing homes have cognitive impairment, and half have a documented diagnosis of dementia.1,2 Overall, about 70% of people with ADRD will spend time near the end of their lives in nursing homes.3
Most nursing home residents with ADRD lack the capacity to make their own treatment decisions as their disease progresses, thus the responsibility for decision-making falls primarily to family members.4 Common treatment decisions include choices about resuscitation, feeding options, hospitalization, and treatment of infections. Family members are often asked by nursing home staff to make urgent treatment decisions with little preparation.5 Health care providers may be aware of the trajectory and complications of dementia, yet family members are often ill-prepared due to a lack of knowledge about the natural course of dementia.6–9 Lack of preparation and support to make care decisions have been found to be a source of stress for family caregivers and can lead to unwanted and burdensome treatments.10–12
Advance care planning (ACP) is the process of understanding and sharing values, goals of care and preferences for future medical treatments.13 ACP can reduce unwanted and burdensome care by supporting nursing home residents and family caregivers to identify treatment preferences in advance of a crisis. ACP includes helping residents and families make medical decisions, documenting these decisions, and ensuring that treatments provided are consistent with these preferences.14 Knowing and honoring patient and family preferences is requisite to providing patient-centered care. ACP reduces family caregiver stress and anxiety and increases both family and patient satisfaction with care.15 Documented preferences about hospitalization are strongly associated with reducing transfers of ADRD from the nursing home to the hospital.16–20 A recent systematic review identified 16 nursing home ACP clinical trials using a variety of approaches and found that ACP improves key patient-centered outcomes.21 However, these strategies are often not adopted into practice by nursing homes due to the complexities of the intervention and difficulties replicating across settings due to resources required.22 A lack of minimum training requirements and practice standards for nursing home staff contributes to high variability in counseling quality, inaccurate or incomplete documentation of preferences, and infrequent re-evaluation of prior decisions.6–8, 23–29 The regulatory standards only require that nursing homes “offer ACP” and have policies about ACP that are known by staff.30 The result is a gap between best practices developed through rigorous research and implementation in an environment of few resources and minimal incentives. ACP interventions need to be developed with these practical realities in mind to lower the barriers to translating evidence into practice.
Pragmatic clinical trials offer a potential solution to this persistent challenge. Pragmatic clinical trials are designed to test and evaluate interventions in real-world settings to accelerate the implementation of successful interventions.31–33 The pragmatic clinical trial ‘Aligning Patient Preferences – a Role Offering Alzheimer’s patients, Caregivers, and Healthcare providers Education and Support (APPROACHES)’ was funded by the National Institute on Aging in 2018 to test the effectiveness of practical strategies for integrating ACP into nursing homes for persons with ADRD. The intervention is based on a model using an ACP Specialist, developed as part of a demonstration project to reduce potentially avoidable hospitalizations of long-stay residents.34–35 It is anticipated that a pragmatic research approach, developed and conducted in partnership with nursing home health care systems, will yield greater impact on outcomes and will be more sustainable compared to previous interventions. The primary outcome is the annual rate of hospital transfers (admissions and emergency department visits) for persons with ADRD. Secondary outcomes are: 1) ACP preferences documentation; 2) hospice enrollment; 3) death in hospital for residents with ADRD. We hypothesize that the transfer rate will be lower in the intervention group than in the control group. We hypothesize that intervention residents will have fewer hospital transfers, a higher rate of ACP documentation of preferences for treatment limitations, greater use of hospice, and lower rates of dying in the hospital. There are few pragmatic trials in nursing homes as this focus is relatively new,36 so it is important to describe the methodology and design of these trials to support the growth in this critical area. The purpose of this report is to describe the APPROACHES design and methodology.
Methods
Overview
The implementation period will be 18 months (6-month ramp up and 12-month study time-period). All short and long-stay residents with ADRD with at least one Minimum Data Set assessment during the 12-month study time-period (September 2021 – August 2022) will be included in the analysis. In addition to the primary outcome analysis, configurational analysis will be conducted to assess the impact of implementation conditions on overall outcomes.44–46, 50
Nursing home selection
A total of 140 candidate nursing homes owned by the two corporate nursing home partners (Company A, N = 107; Company B, N = 33) were identified for participation. Two nursing homes were eliminated as they were the sole nursing home for the company in a state and one was eliminated because it did not meet our minimum size criteria (≥ 50 beds), leaving 97.8% (137/140) of nursing homes eligible for participation.
Random assignment
Randomization at the level of the nursing home was performed by study statisticians. We used purchased 2018 Minimum Data Set data from the Centers for Medicaid and Medicare Services to calculate the average number of hospital transfers per 1000 person-days alive for one quarter. Nursing homes were stratified based on corporate ownership and then grouped into low versus high hospitalization rates, using the median hospitalization rate for each nursing home company as the threshold value for dichotomization. Nursing homes were randomized to the intervention or control within strata at a 1-to-1 ratio. The stratification ensures comparability of the facilities in the two treatment arms. Randomization occurred at the nursing home level, meaning that all residents with ADRD in the intervention buildings were eligible for inclusion in the trial. See Table 1 for participating nursing home characteristics and Figure 1 for an overview of the randomization.
Table 1.
Characteristics of intervention and control nursing homes.
| Intervention | Control | Total | |
|---|---|---|---|
| Number of facilities | |||
| Company A, N | 52 | 52 | 104 |
| Company B, N | 16 | 17 | 33 |
| Total, N | 68 | 69 | 137 |
| Facility characteristics | |||
| Facility hospitalization rate, mean (SD)a | 3.5 (1.8) | 3.5 (1.9) | 3.5 (1.8) |
| Occupancy rate (proportion), mean (SD)c | 0.7 (0.1) | 0.7 (0.1) | 0.7 (0.1) |
| Number of certified beds, mean (SD)b | 102.7 (38.1) | 109.7 (33.1) | 106.2 (35.8) |
| Centers for Medicare and Medicaid Services Five-Star Rating, mean (SD),c | 2.8 (1.4) | 3.1 (1.4) | 2.9 (1.4) |
| Adjusted total nursing staff hours per resident per day, mean (SD)b | 3.5 (0.4) | 3.6 (0.6) | 3.5 (0.5) |
| Resident characteristics | |||
| Proportion of long-stay residents, mean (SD) | 0.4 (0.1) | 0.4 (0.1) | 0.4 (0.1) |
| Number of admissions, mean (SD) | 1,076.4 (813.5) | 1,151.7 (866.4) | 1,114.1 (838.8) |
| Number of admissions per bed, mean (SD) | 10.2 (6.5) | 10.0 (6.8) | 10.1 (6.6) |
| Number of residents, mean (SD) | 247.9 (154.5) | 260.2 (126.5) | 254.1 (140.9) |
| Proportion of residents with advanced dementia (ADRD), mean (SD) | 0.5 (0.2) | 0.5 (0.2) | 0.5 (0.2) |
| Proportion of patients with CFS score >= 2, mean (SD)d | 0.5 (0.1) | 0.5 (0.1) | 0.5 (0.1) |
| Proportion of residents with diagnosis of Alzheimer’s disease, mean (SD) | 0.1 (0.1) | 0.1 (0.1) | 0.1 (0.1) |
| Proportion of residents with diagnosis of dementia, mean (SD) | 0.3 (0.1) | 0.2 (0.1) | 0.3 (0.1) |
Facility hospitalization rate calculated as number of hospitalizations per 1000 resident days.
Data from May 2021, accessed June 2021. Publicly available CMS Provider Data https://data.cms.gov/provider-data/dataset/4pq5-n9py.
CMS Five-Star Rating System ranges from 1 (much below average) to 5 (much above average).
Cognitive Function Scale: 0–2 = intact/mild impairment; 3–4=moderate impairment; 5–6=severe impairment.2
Figure 1.
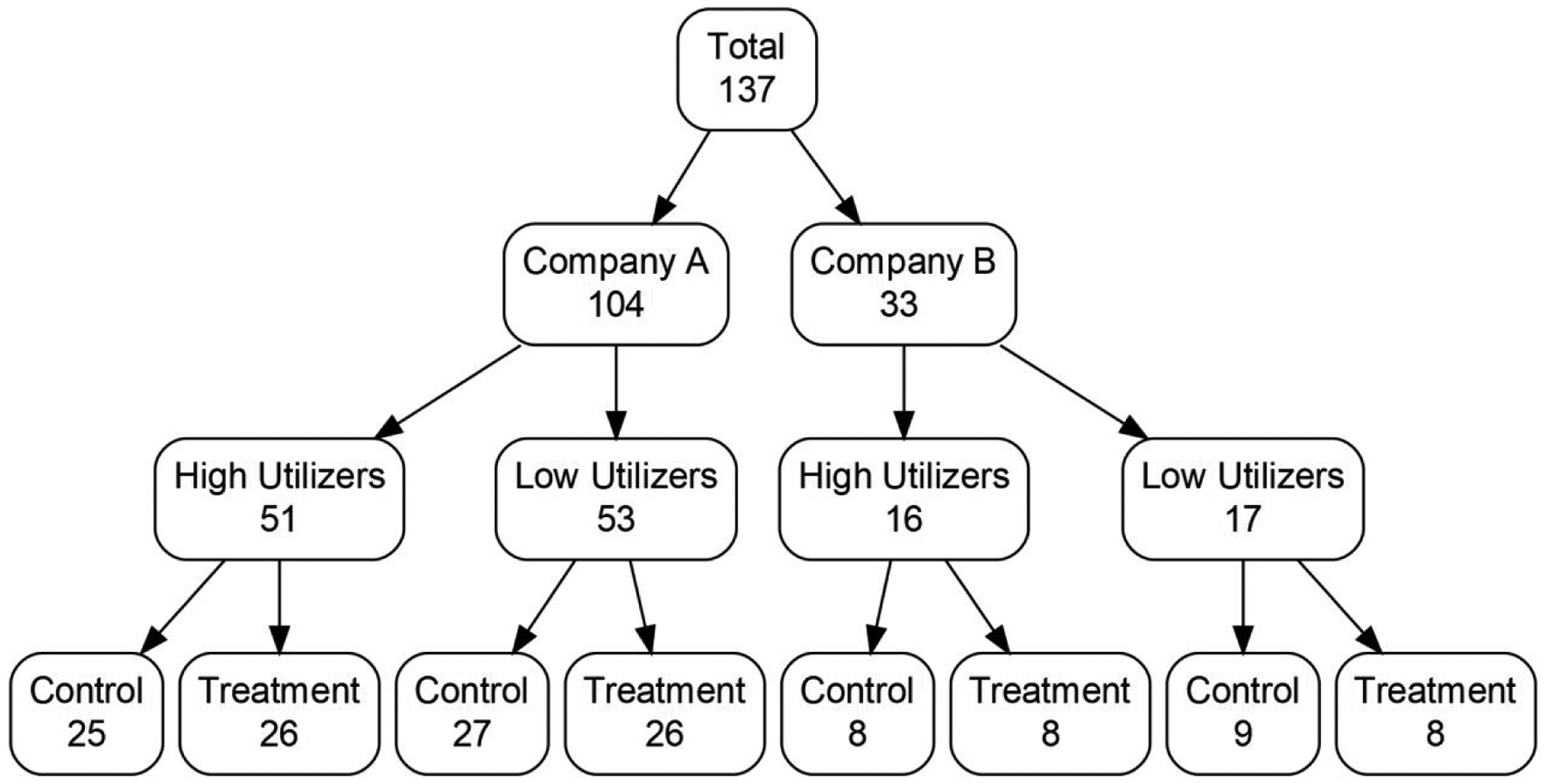
Stratification and randomization schema for participating nursing facilities.
Note: High and low utilizer groups defined by being above or below the median hospital utilization rate of each preceding group in the flow chart.
Nursing home recruitment
Each company has identified a Corporate Implementation Champion (with some salary support on the grant) to serve as the primary point of contact for the study team and lead program implementation. The Corporate Implementation Champions reach out to intervention nursing homes to share information about the program, work with the nursing home leadership to identify the nursing home-based Advance Care Planning (ACP) Specialist(s), and provide ongoing support. Since the program is being rolled out as a company initiative, nursing home participation in the ACP Specialist Program is expected.
Study population
Resident eligibility criteria include: a) short and long-stay residents residing in the nursing home during the 12-month study time-period; and b) ADRD as defined by a diagnosis of Alzheimer’s disease or dementia on the Minimum Data Set or a score of 2 or greater on the Cognitive Function Scale suggesting at least mild cognitive impairment.2 Residents are ineligible if they are enrolled in hospice at the start of the study time-period. The ACP Specialists are asked to prioritize facilitating ACP conversations with residents with ADRD and their surrogate decision-makers, but ACP Specialists may engage in ACP with other residents as well.
Intervention structure
The ACP Specialist Program intervention includes three components: 1) identification of a site-based ACP Specialist with dedicated time for this role; 2) structured on-line training for the ACP Specialists and key administrative leaders; and 3) tools to facilitate ACP clinical activity.
Identification of the ACP Specialist.
The ACP Specialist is typically a nurse, chaplain, or social services existing staff member who already engages residents in ACP discussions as part of their role. Additional ACP Specialists responsibilities include educating other staff, working with nursing home leaders in implementing policies and procedures to support ACP, communicating with physicians and other providers, and proactively engaging in ACP discussions with residents and families. Consistent with the principles of a pragmatic trial, instructions about how to implement the protocol role permit adaptation to site-specific workflows.31 For example, many nursing homes train more than one ACP Specialist within the building to share responsibilities and provide more coverage, e.g., during vacations or illnesses. There is also flexibility in baseline level of expertise required by practitioners delivering the intervention, so the ACP Specialist may be any staff person deemed qualified by the nursing home Executive Director to fulfill the role based on their experience with ACP, interpersonal skills, interest, and their ability to shift responsibilities to make time for the ACP Specialist role.
Structured online training.
Study investigators developed an evidence-based, narrated online training program to provide foundational information about ACP as well as specific instructions about the ACP Specialist Role. The training is based on an intervention developed for a prior demonstration project.34, 35, 37 The APPROACHES training was reviewed and revised based on feedback from members of the research team, study consultants, nursing home corporate champions, and nursing home staff who participated in the pilot. The training modules were uploaded into each corporate partners’ learning management system and assigned to staff identified as the ACP Specialists. The training consists of an initial “Launch” model, 7 modules that provide deeper instruction on facilitating ACP and implementing the ACP Specialist role, and a booster module (see Supplemental Table 1). Training was also developed for administrators, directors of nursing, medical directors, and corporate leaders to summarize the program and emphasize the need for their support and buy-in. This module was made available in each learning management system as well as through a link to an online narrated video version of the same content for ease of access.
ACP Specialist tools.
Each ACP Specialist was provided with a binder as well as an electronic file containing information including the following: program background; ACP Specialist position description; ACP Specialist forms to support their work (e.g., a worksheet for exploring existing policies and procedures, and ACP flow-chart, discussion guide, follow-up letter for families); resources for staff (e.g., informational sheets covering common topics such as artificial nutrition); and resources for residents and families (e.g., information about cardiopulmonary resuscitation); an ACP Facilitation Guide; and a tool to track efforts. Additionally, a standardized ACP documentation form was created to capture work activity, including attempts, conversations, goals of care, treatment preferences, and narrative summary of the conversation (see Figure 1). This documentation template is built into each company’s electronic health record system.
Intervention implementation
Components of the intervention implementation include leadership engagement, monthly assignment lists to systematically identify residents to approach, and monthly reporting of outcomes at the nursing home level.
Leadership engagement.
Leadership engagement is essential to intervention implementation. The corporate champion initially met with the leadership of each nursing home to introduce the program and ask the leader to identify ACP Specialists and support their work. The expectation is that 20% of a full-time employee total per nursing home will be dedicated to the program. Nursing home leadership are asked to support the work of the ACP Specialists by offloading other responsibilities to allow time for ACP conversations.
Monthly assignments.
Monthly assignment lists are generated to identify residents who will be approached about engaging in ACP conversations. Although nursing home practices vary, these lists are primarily generated using the list of residents due for a quarterly care planning meeting. The ACP Specialists are instructed to identify a list of 10 residents each month and to prioritize residents with ADRD in creating this list. Other clinical triggers may include a readmission, a significant change in condition, or when there is an opportunity to engage family who is typically unavailable.
Monthly reporting.
The ACP Specialist will report monthly status updates about their ACP discussions as part of the monthly nursing home Quality Assurance meeting.
Implementation monitoring
Researchers generate monthly reports for each nursing home by company to identify the number of ACP attempts and discussions documented. This information includes the number of residents who required assistance to make ACP decisions as a proxy for tracking the number of residents with a diagnosis of ADRD. These reports are shared with the corporate champions and corporate stakeholders to monitor progress. Corporate implementation champions maintain a spreadsheet with information about the ACP Specialists in each nursing home including roles and turnover to support regular meetings with ACP Specialists in each nursing home, via phone or in person, to troubleshoot issues and reinforce key aspects of implementation. The champions report on nursing home-level implementation experience during monthly individual and joint meetings with the project team.
Control condition
The control condition is a usual care condition. Since the ACP Specialist Program is being introduced as a company initiative, some control nursing homes are aware of the program but understand that it is not being rolled out in their building yet. Control nursing homes do not receive access to the ACP Specialist Training and do not have access to study tools or materials, including the electronic health record documentation template. The goal of this pragmatic trial is to compare the intervention to “real world” conditions.
Data sources
We use four data sources: 1) ACP Specialist personnel tracking data provided by the corporate implementation champions; 2) Electronic health record data provided directly from our nursing home corporate partners on a monthly basis; 3) Minimum Data Set data; 4) Medicare claims data. Final Minimum Data Set and Medicare claims data will not be available until after the intervention is completed; electronic health record data will be used in final analyses in addition to providing ongoing feedback during implementation. All study data are transferred using HIPAA compliant, encrypted processes for storage on Indiana University School of Medicine Department of Biostatistics servers accessible only to members of the study analysis team. Once data are securely transferred, linked to Minimum Data Set and Medicare claims data, and verified, the resident identifiers will be replaced with a new alphanumeric ID that will allow analysts to link data.
ACP Specialist personnel tracking.
The corporate champions each maintain a spreadsheet to monitor staffing related issues including the identity of the specialists in each building, dates of training, training status, and turnover.
Electronic health record.
Each nursing home corporate partner has a robust electronic health record used to document and support clinical care. Two companies use PointClickCare, and the third uses MatrixCare. Minor adaptations were made to the electronic health record to support standardization and documentation of ACP outcomes, including whether there is an ACP attempt or discussion. (See Figure 2).
Figure 2.



Advance Care Planning Documentation Template for Electronic Health Record
Minimum Data Set 3.0.
All nursing homes that receive federal funding are required to collect Minimum Data Set 3.0 resident assessment data at the time of admission, quarterly, annually, and when a resident experiences a significant change of condition or discharge from the nursing home (including hospitalization).38,39 Minimum Data Set data includes information about patient demographics, diagnoses, cognitive functioning, and functional status. Minimum Data Set data from 2017 was initially used to identify hospitalization rates for Companies #1 and #2 to assist with the randomization. Minimum Data Set data will also be obtained from Centers for Medicare and Medicaid Services approximately 9 months after the end of the intervention period, which is the earliest it is available. This data will be used to describe participating residents and aggregate resident data will be used to describe nursing homes enrolled in APPROACHES.
Medicare claims data.
Claims data, purchased from Centers for Medicare and Medicaid Services, contains information about billing claims filed for health care services provided to Medicare beneficiaries. This will contain information from inpatient services, skilled nursing home stays, and hospice use about dates of service and procedures.
Data elements
Consistent with the principles of pragmatic clinical trials,31 study outcomes will rely on data that is captured in existing data sources. This data includes nursing home characteristics (bed size, rural/urban location) and resident characteristics (demographics, cognitive status, and functional status). A list of data collection measures and sources are listed in Table 2.
Table 2.
Data elements and sources of data.
| Data Element | Purpose | Source | |||
|---|---|---|---|---|---|
| Tracking | EHR | MDS | Medicare | ||
| Nursing Home Level | |||||
| ACP Specialist staffing | Configurational Analysis | x | |||
| Nursing Home Names and Location | Randomization | ||||
| Hospitalization ratea | Randomization | x | |||
| Physician practice presenceb | Randomization | ||||
| Number of certified beds | Inclusion/Exclusion Criteria | x | |||
| Patient-level | |||||
| Demographics | Covariates | x | x | ||
| Functional Status | Target sub-population, identification, covariate | x | |||
| Cognitive Functioning Scale Score2 | Target sub-population, identification, covariate | x | |||
| Diagnosis of Dementia | Target sub-population, identification, covariate | x | |||
| Hospital Transfersc | 1° outcome | x | x | ||
| ACP Preference Documentationd | 2° outcome | x | |||
| Hospice use | 2° outcome | x | x | ||
| Location of Death | 2° outcome | x | |||
Minimum Data Set data provided by Companies A & B
Presence of national physician group practice with co-occurring palliative care intervention - Company 3 only
Annual number of hospital admissions/emergency department visits
Orders reflecting preferences including Do Not Resuscitate, Do Not Hospitalize, Do Not Intubate, No Feeding Tube; POLST Paradigm forms; Goals of Care
MDS = Minimum Data Set
Masking
Members of the study team are not masked in order to support the implementation of the program in collaboration with the corporate champions, in accordance to accepted practice of pragmatic trials.41
Regulatory considerations
This study was reviewed and approved by the Indiana University Institutional Review Board as an expedited study. Specifically, the study was approved under category 5 (research conducted using materials collected for non-research purposes) to address the use of electronic health record data to study the effect of an educational intervention developed by the APPROACHES team. The study was granted a waiver of informed consent.41 Resident and family caregiver refusals to participate in ACP discussions offered by ACP Specialists are honored.
The study is overseen by a Data Safety and Monitoring Board consisting of three external members, including a statistician, physician, and long-term care expert. Board members were reviewed and approved by the National Institute on Aging. Significant distress by residents or family members in the intervention nursing homes during an ACP conversation was defined as a potential adverse event. Such events may be the manifestation of an extreme negative emotional reaction during a facilitated ACP discussion with the ACP Specialist or complaining to nursing home leadership. Due to the sensitive nature of the material, tearing up, crying, expressions of emotion, or early termination of the conversation can be expected during ACP and are not deemed to be adverse events. ACP is the standard of care in nursing homes. The risk of potential distress caused by exposure to the ACP Specialist Program is no greater than the risk of distress from experiencing these conversations in routine clinical care. It was determined that there are no potential consequences of APPROACHES that could be considered serious adverse events.
Results
The primary outcome is the annual rate of hospital transfers among residents with ADRD at 137 nursing homes based on hospital transfers, including emergency department visits and admissions, as determined by Medicare fee-for-service claims data and discharge to hospital from the Minimum Data Set. Secondary outcomes will be identified from Medicare claims data and/or the electronic health record, and include: 1) Hospice enrollment during the 12 month study time period; 2) Proportion of residents who died in the hospital among all enrolled residents who died during the 12 month study time period; and 3) the proportion of ADRD residents with specific documented orders reflecting preferences for life-sustaining treatment (e.g., do not resuscitate/full code orders, do not hospitalize orders, no tube-feeding orders, and/or do not intubate orders), POLST (orders about code status, medical interventions, and the use of artificial nutrition) in nursing homes located in states where POLST paradigm programs are available.
Statistical analysis
Analysis will be carried out in an intention-to-treat framework, which gives a pragmatic estimate of the intervention benefit.41 The intention-to-treat analysis will use data from all eligible residents with ADRD in randomized nursing homes, regardless of the level of nursing homes’ adherence to the study protocol and resident attrition.
We will compare the annual rate of hospital transfers (hospitalizations and emergency department visits) in residents with ADRD between the intervention vs. control nursing homes over 12 months (Primary trial outcome). We hypothesize that hospital transfers/person-days alive will be lower among residents with ADRD in intervention nursing homes. Counts of transfer events will be analyzed using Poisson regression.42 With the cluster randomized control trial design, we will consider a mixed-effects Poisson regression model, in which residents from the same nursing home share a common nursing home-specific intercept; this random intercept helps to accommodate the within-nursing home correlations among hospitalization counts from residents in the same nursing home. The model can be written generically as , where Yij is the event count of the jth resident in the ith nursing home, ACPi is a binary intervention indicator, α is the regression coefficient representing the treatment effect, β0 is the intercept, where exp(β0)can be viewed as the mean event when all independent variables are set to zero; Ui~N(0,σ2) is the random nursing home effect, and is a vector of resident characteristics (age, gender, comorbidities, etc.) and nursing home characteristics (bed size, urban/rural location, etc). This will also include select quality of care/quality of life indicators from the Minimum Data Set 3.0 and Centers for Medicare and Medicaid Services nursing home compare star ratings, including overall star rating and long-stay quality of care clinical metrics. We will also include the stratification variables (corporate ownership and dichotomized rates of hospitalization) as well as any unbalanced resident characteristics in the main analytical model to ensure the validity of the inference.52 From the fitted model, we can ascertain the incident rate ratio associated with the intervention, . Importantly, an offset parameter can be added to account for the unequal lengths of observation periods among study participants. As a result, death and early dropout can be appropriately accommodated. We will consider using negative binomial regression models, with or without zero inflation, if extra-Poisson variability exists.
Similarly, appropriate generalized linear mixed effects models will be used for the analysis of secondary outcomes. Binary and continuous outcomes will be analyzed using logistic (logit link function) and linear regression (identity link function) models, respectively. Specifically, we will compare the secondary outcomes between ADRD residents in intervention vs. control nursing homes over 12 months. We hypothesis that ADRD residents in the intervention vs. control nursing homes will have: i. Greater ACP documentation of preferences to limit treatments; ii. Greater hospice enrollment; and iii. Lower rates of dying in the hospital. All analysis will be implemented using SAS. P values less than 0.05 will be considered statistically significant.
Statistical power and sample size requirements
Power calculations.
We determined the sample size to ensure the study has adequate power for the primary hypothesis on hospital transfer rates. With 137 nursing homes (at least 68 per treatment arm) and an average of 149 residents per nursing home, we will have 80% power to detect an incidence rate ratio of 0.78 by using a Poisson regression model with 0.05 level of significance, assuming the coefficients of variation cluster event rates are 0.5 for both treatment groups based on estimates using Minimum Data Set data.48,49 Such an incidence rate ratio value implies that the hospitalization rate in the intervention is only 78% of that in the control group, i.e., a 22% reduction. For example, if the rate of hospitalization is 1.2 per resident year in the control group, and 0.94 per resident year in the intervention group, we will have 80% chance to detect the difference. In Table 3, we present additional scenarios of the control and intervention group hospitalization rates that can be detected with 80% power.
Table 3.
The effect sizes, absolute rate difference, and incidence rate ratio of hospitalization rates among long-stay residents with ADRD (annual number of hospitalizations) that are detectable with 80% power, at 0.05 significance level.
| Rate in Control group | Rate in Intervention group | Event rate difference | Incidence rate ratio |
|---|---|---|---|
| 0.6 | 0.47 | −0.13 | 0.78 |
| 0.7 | 0.55 | −0.15 | 0.78 |
| 0.8 | 0.62 | −0.18 | 0.78 |
| 0.9 | 0.70 | −0.20 | 0.78 |
| 1.0 | 0.78 | −0.22 | 0.78 |
| 1.1 | 0.86 | −0.24 | 0.78 |
| 1.2 | 0.94 | −0.26 | 0.78 |
| 1.3 | 1.01 | −0.29 | 0.78 |
| 1.4 | 1.09 | −0.31 | 0.78 |
Configurational analysis
Implementation of interventions in complex settings may succeed or fail based on a number of conditions. We will conduct a configurational analysis using existing data in order to understand the implementation conditions associated with higher uptake of the intervention. Configurational analysis is a mathematical approach to looking across a range of variables to identify what combination of conditions are needed in order to achieve the desired outcome.44–46,50 We will focus on conditions associated with higher rates of ACP documentation as a reflection of successful implementation. In order to identify variables for inclusion in this analysis and remain consistent with the principals of pragmatic research, we will use the Contextual Framework for Implementation Outcomes to identify available data about the outer setting (e.g., rural versus urban setting) inner setting (e.g., staff turnover), intervention (e.g., length of conversation, goals of care) and people involved (e.g,- ACP Specialist is a nurse vs. social services).51 The results of this additional analysis will strengthen our ability to provide insights into the specific conditions associated with successful implementation of a new program in nursing homes.
Conclusion
A robust program that increases access to ACP for nursing home residents has the potential to increase goal-concordant care and is expected to reduce hospital transfers. Many interventions to improve care in nursing homes have failed to demonstrate an impact or, if successful, maintain impact over time. Pragmatic trials, designed to test interventions in real-world contexts, are well-suited for the nursing home environment. The APPROACHES trial relies on corporate infrastructure to roll out the intervention, with support for a central corporate implementation champion who is charged with the initial introduction and ongoing support for nursing homes. These internal champions are able to connect the project with other company priorities and use strategies familiar to nursing home leaders for the initiation of other programs. Existing standardized data collection across nursing homes also supports the conduct of pragmatic trials in this setting. All nursing homes are required to collect the Minimum Data Set on all residents at regular intervals. Further, nursing home residents in the United States are nearly all covered by Medicare and thus claims data can be accessed to understand medical treatments and care trajectories. A majority of nursing homes do use electronic health records. Partnering with large nursing home chains that have consistent documentation across nursing homes and are willing to embed new clinical encounter forms into their systems allows the project team to monitor implementation during the project.
Recognizing real-world conditions, we designed this pragmatic trial to be flexible. Flexibility was particularly needed due to the devastating impact of the COVID-19 pandemic on nursing homes. We remained in close communication with our corporate implementation champions throughout the pandemic and made several adaptations to our timeline, training plan, and launch of the trial. The initial plan required ACP Specialists to complete seven educational modules representing 4–5 hours of content before beginning ACP conversations, followed by an overview booster module six months after the first conversation. Our corporate partners were focused on preparation and response to the COVID-19 pandemic, hampering the implementation of any new program that was perceived as “burdensome.” They all acknowledged, however, that understanding goals of care of nursing home residents was even more critical given the infectious disease threat. To respond to these realities, the training was adapted so that nursing home staff were only required to complete a modified version of the booster module before they could begin conversations. This new module was named the “launch” module and contained focused direction about the ACP discussion guide. ACP Specialists were required to complete the remaining modules over the following month. Configurational analysis will support the identification of implementation conditions associated with successful outcomes to enhance learning about implementation of clinical programs in nursing homes.
The APPROACHES intervention is built on an evidence base that ACP supports better care delivery for nursing home residents. This pragmatic intervention and trial leverages corporate structures and existing data sources to implement and evaluate an ACP program in a real-world environment. APPROACHES can serve as a model for others with similar goals to improve care in this setting. Future trials will benefit from an approach that includes leadership buy-in and engagement, a commitment for dedicated effort to support the intervention at the facility level, clear performance expectations, and regular reporting to monitor implementation.
Supplementary Material
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Aging [grant number: R33AG057463]. Clinicaltrials.gov number: NCT03323502
Footnotes
Declaration of conflicting interests
The authors declare that there are no conflicts of interest.
References
- 1.Centers for Disease Control. Alzheimer’s Disease Atlanta, GA: U.S. Department of Health & Human Services; 2016. [Available from: https://www.cdc.gov/nchs/fastats/alzheimers.htm. [Google Scholar]
- 2.Thomas KS, Dosa D, Wysocki A, et al. The Minimum Data Set 3.0 Cognitive Function Scale. Med Care 2017;55(9):e68–e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mitchell SL, Teno JM, Miller SC, et al. A national study of the location of death for older persons with dementia. J Am Geriatr Soc 2005;53(2):299–305. [DOI] [PubMed] [Google Scholar]
- 4.Robinson L, Dickinson C, Rousseau N, et al. A systematic review of the effectiveness of advance care planning interventions for people with cognitive impairment and dementia. Age Ageing 2012;41(2):263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Towsley GL, Hirschman KB and Madden C. Conversations about End of Life: Perspectives of Nursing Home Residents, Family, and Staff. J Palliat Med 2015;18(5):421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teno JM, Clarridge BR, Casey V, et al. Family perspectives on end-of-life care at the last place of care. JAMA 2004;291(1):88–93. [DOI] [PubMed] [Google Scholar]
- 7.Engel SE, Kiely DK and Mitchell SL. Satisfaction with End-of-Life Care for Nursing Home Residents with Advanced Dementia. J Am Geriatr Soc 2006;54(10):1567–1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Givens JL, Kiely DK, Carey K, et al. Healthcare proxies of nursing home residents with advanced dementia: Decisions they confront and their satisfaction with Decision-Making. J Am Geriatr Soc 2009;57(7):1149–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Caron CD, Griffith J and Arcand M. Decision making at the end of life in dementia: How family caregivers perceive their interactions with health care providers in long-term-care settings. J Appl Gerontol 2005;24(3):231–247. [Google Scholar]
- 10.Givens JL, Selby K, Goldfeld KS, et al. Hospital transfers of nursing home residents with advanced dementia. J Am Geriatr Soc 2012;60(5):905–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosemond C, Hanson LC and Zimmerman S. Goals of Care or Goals of Trust? How Family Members Perceive Goals for Dying Nursing Home Residents. J Palliat Med 2017;20(4):360–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMahan RD, Tellez I and Sudore RL. Deconstructing the Complexities of Advance Care Planning Outcomes: What Do We Know and Where Do We Go? A Scoping Review. J Am Geriatr Soc 2021;69(1):234–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sudore RL, Lum HD, You JJ, et al. Defining Advance Care Planning for Adults: A Consensus Definition From a Multidisciplinary Delphi Panel. J Pain Symptom Manag 2017;53(5):821–832 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Consensus Project for Quality Palliative Care. Clinical practice guidelines for quality palliative care: National Consensus Project for Quality Palliative Care; 2009.
- 15.Detering KM, Hancock AD, Reade MC, et al. The impact of advance care planning on end of life care in elderly patients: randomised controlled trial. BMJ 2010;340:c1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nicholas LH, Langa KM, Iwashyna TJ, et al. Regional variation in the association between advance directives and end-of-life Medicare expenditures. JAMA 2011;306(13):1447–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grabowski DC, Stewart KA, Broderick SM, et al. Predictors of nursing home hospitalization: a review of the literature. Med Care Res Rev 2008;65(1):3–39. [DOI] [PubMed] [Google Scholar]
- 18.Buchanan JL, Murkofsky RL, O’malley AJ, et al. Nursing home capabilities and decisions to hospitalize: a survey of medical directors and directors of nursing. J Am Geriatr Soc 2006;54(3):458–465. [DOI] [PubMed] [Google Scholar]
- 19.Hickman SE, Nelson CA, Perrin NA, et al. A comparison of methods to communicate treatment preferences in nursing facilities: traditional practices versus the physician orders for life-sustaining treatment program. J Am Geriatr Soc 2010;58(7):1241–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hammes BJ, Rooney BL, Gundrum JD, et al. The POLST program: a retrospective review of the demographics of use and outcomes in one community where advance directives are prevalent. J Palliat Med 2012;15(1):77–85. [DOI] [PubMed] [Google Scholar]
- 21.Flo E, Husebo BS, Bruusgaard P, et al. A review of the implementation and research strategies of advance care planning in nursing homes. BMC Geriatr 2016;16:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrison RS, Chichin E, Carter J, et al. The effect of a social work intervention to enhance advance care planning documentation in the nursing home. J Am Geriatr Soc 2005;53(2):290–294. [DOI] [PubMed] [Google Scholar]
- 23.Mitchell SL, Teno JM, Kiely DK, et al. The clinical course of advanced dementia. N Engl J Med 2009;361(16):1529–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mitchell SL, Kiely DK and Hamel MB. Dying with advanced dementia in the nursing home. Arch Intern Med. 2004;164(3):321–6. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell SL, Teno JM, Intrator O, et al. Decisions to forgo hospitalization in advanced dementia: a nationwide study. J Am Geriatr Soc 2007;55(3):432–438. [DOI] [PubMed] [Google Scholar]
- 26.Morrison RS and Siu AL. Survival in end-stage dementia following acute illness. JAMA 2000;284(1):47–52. [DOI] [PubMed] [Google Scholar]
- 27.Ahronheim JC, Morrison RS, Baskin SA, et al. Treatment of the dying in the acute care hospital: advanced dementia and metastatic cancer. Arch Intern Med 1996;156(18):2094–2100. [PubMed] [Google Scholar]
- 28.Teno JM, Mitchell SL, Kuo SK, et al. Decision-Making and Outcomes of Feeding Tube Insertion: A Five-State Study. J Am Geriatr Soc 2011;59(5):881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hanson LC, Carey TS, Caprio AJ, et al. Improving decision-making for feeding options in advanced dementia: a randomized, controlled trial. J Am Geriatr Soc 2011;59(11):2009–2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Teno JM, Branco KJ, Mor V, et al. Changes in Advance Care Planning in Nursing Homes Before and After the Patient Self-Determination Act: Report of a 10-State Survey. J Am Geriatr Soc 1997;45(8):939–944. [DOI] [PubMed] [Google Scholar]
- 31.Thorpe KE, Zwarenstein M, Oxman AD, et al. A pragmatic-explanatory continuum indicator summary (PRECIS): a tool to help trial designers. J Clin Epidemiol 2009;62(5):464–475. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell SL, Mor V, Harrison J, McCarthy EP. Embedded Pragmatic Trials in Dementia Care: Realizing the Vision of the NIA IMPACT Collaboratory. J Am Geriatr Soc 2020;68 (Suppl 2):S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baier RR, Jutkowitz E, Mitchell SL, et al. Readiness assessment for pragmatic trials (RAPT): a model to assess the readiness of an intervention for testing in a pragmatic trial. BMC Med Res Methodol 2019;19(1):156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hickman SE, Unroe KT, Ersek M, et al. Systematic Advance Care Planning and Potentially Avoidable Hospitalizations of Nursing Facility Residents. J Am Geriatr Soc 2019;67(8):1649–1655. [DOI] [PubMed] [Google Scholar]
- 35.Hickman SE, Unroe KT, Ersek MT, et al. An Interim Analysis of an Advance Care Planning Intervention in the Nursing Home Setting. J Am Geriatr Soc 2016;64(11):2385–2392. [DOI] [PubMed] [Google Scholar]
- 36.Mitchell SL, Volandes AE, Gutman R, et al. Advance Care Planning Video Intervention Among Long-Stay Nursing Home Residents: A Pragmatic Cluster Randomized Clinical Trial. JAMA Intern Med 2020;180(8):1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Unroe KT, Nazir A, Holtz LR, et al. The Optimizing Patient Transfers, Impacting Medical Quality, and Improving Symptoms:Transforming Institutional Care approach: preliminary data from the implementation of a Centers for Medicare and Medicaid Services nursing facility demonstration project. J Am Geriatr Soc 2015;63(1):165–169. [DOI] [PubMed] [Google Scholar]
- 38.Mor V, Angelelli J, Jones R, et al. Inter-rater reliability of nursing home quality indicators in the U.S. BMC Health Serv Res 2003;3(1):20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morris JN, Hawes C, Fries BE, et al. Designing the national resident assessment instrument for nursing homes. Gerontologist 1990;30(3):293–307. [DOI] [PubMed] [Google Scholar]
- 40.McKinney RE Jr, Beskow LM, Ford DE, et al. Use of altered informed consent in pragmatic clinical research. Clin Trials 2015;12(5):494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hollis S and Campbell F. What is meant by intention to treat analysis? Survey of published randomised controlled trials. BMJ 1999;319(7211):670–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cameron AC and Trivedi PK. Regression analysis of count data: Cambridge University Press; 2013. [Google Scholar]
- 43.Kiely DK, Givens JL, Shaffer ML, et al. Hospice use and outcomes in nursing home residents with advanced dementia. J Am Geriatr Soc 2010;58(12):2284–2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baumgartner M and Epple R. A coincidence analysis of a causal chain: The Swiss minaret vote. Sociological Methods & Research 2014;43:280–312. [Google Scholar]
- 45.Baumgartner M and Thiem A. Identifying complex causal dependencies in configurational data with coincidence analysis. R Journal 2015;7:176–184. [Google Scholar]
- 46.Cragun D, Pal T, Vadaparampil ST, et al. Qualitative comparative analysis: A hybrid method for identifying factors associated with program effectiveness. J Mix Methods Res 2016;10:251–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Christian JB, Brouwer ES, Girman CJ, et al. Masking in Pragmatic Trials: Who, What, and When to Blind. Ther Innov Regul Sci 2020;54(2):431–436. [DOI] [PubMed] [Google Scholar]
- 48.Hayes R, Bennett S. Simple sample size calculation for cluster-randomized trials. Int J Epidemiol1999;28:319–26. [DOI] [PubMed] [Google Scholar]
- 49.Rutterford C, Copas A and Eldridge S. Methods for sample size determination in cluster randomized trials. Int J Epidemiol 2015;44(3):1051–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kane H, Lewis MA, Williams PA, et al. Using qualitative comparative analysis to understand and quantify translation and implementation. Transl Behav Med 2014;4(2):201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Damschroder LJ, Aron DC, Keith RE, et al. Fostering implementation of health services research findings into practice: a consolidated framework for advancing implementation science. Implement Sci 2009;4(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kahan BC and Morris TP. Reporting and analysis of trials using stratified randomisation in leading medical journals: review and reanalysis. BMJ 2012;345:e5840. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


