Abstract
Osteoarthritis (OA) affects over 250 million people worldwide and despite various existing treatment strategies still has no cure. It is a multifactorial disease characterized by cartilage loss and low-grade synovial inflammation. Focusing on these two targets together could be the key to developing currently missing disease-modifying OA drugs (DMOADs). This review aims to discuss the latest cell-free techniques applied in cartilage tissue regeneration, since they can provide a more controllable approach to inflammation management than the cell-based ones. Scaffolds, extracellular vesicles, and nanocarriers can be used to suppress inflammation, but they can also act as immunomodulatory agents. This is consistent with the latest tissue engineering paradigm, postulating a moderate, controllable inflammatory reaction to be beneficial for tissue remodeling and successful regeneration.
Keywords: osteoarthritis, inflammation, synovitis, cell-free tissue engineering, extracellular vesicles, matrix-bound nanovesicles
1. Introduction
Osteoarthritis (OA) has long been [1,2,3] and is sometimes still [4,5] referred to as a non-inflammatory condition. Moreover, when studying “classic” inflammatory arthritides, such as rheumatoid arthritis (RA) or spondyloarthritis, researchers would often use tissue and biologic fluid samples from OA patients as negative (non-inflammatory) controls [6,7]. Although currently researchers agree that many aspects contribute to OA progression, including gender [8], it has long been regarded mainly as the consequence of cartilage wear and tear. Cartilage damage was reported to lead to joint biomechanics impairment resulting in further cartilage loss and joint deformity, while the inflammatory component was commonly underestimated.
Cartilage tissue itself, being avascular, cannot develop a classic immune response. However, when considering the joint as a whole, including synovium, ligaments, and subchondral bone, inflammation (synovitis) seems to play an important role in OA progression. There is evidence that synovitis is associated with increased OA severity [9,10], and therefore it is a promising target for currently missing disease-modifying OA drug (DMOADs) development. Most available OA management options, including lifestyle changes, pharmacotherapy, and surgery [11,12,13], also target joint inflammation to varying degrees.
Lifestyle modification is the basis of most chronic disease management and can be beneficial to compliant patients. A balanced diet and physical activity can postpone the OA onset or slow down its progression both by reducing the joint loading due to weight loss and decreasing the levels of adipokines, which are known to contribute to the inflammatory component of OA development [14,15]. Currently recommended pharmacological treatments, including non-steroidal anti-inflammatory drugs (NSAIDs) and corticosteroids, target inflammation and relieve pain [11], while the operative approaches aim to both reduce pain and improve joint functions [13]. However, one of the major drawbacks of all these strategies is that none of them address the problem of cartilage loss. Due to its low self-healing capacity, hyaline cartilage can only be repaired with involvement of cartilage-repair techniques, and various strategies, both scaffold-based and scaffold-free and cell-based and cell-free, are being proposed [16,17]. Unfortunately, most conventional cartilage tissue repair techniques focus on cartilage regeneration, while their effect on joint inflammation is rarely being discussed. Apparently, in order to fully address the problem of OA, a combination of cartilage repair techniques and strategies targeting inflammation should be considered.
This narrative review aims to summarize the evolving approaches which target both inflammation and cartilage damage to treat OA. We chose to focus on cell-free techniques and selected original research articles reporting the application of those in vivo. In addition, we aimed to promote the idea of immunomodulation as a potent tool for both inflammation management and successful regeneration.
2. Inflammation in an OA Joint
Homeostasis in a healthy joint is maintained by the synovial intima cells, namely type A macrophage-like synoviocytes, responsible for debris phagocytosis, and type B fibroblast-like synoviocytes, which produce synovial fluid components, including hyaluronan [18]. In an OA joint the products of the cartilage extracellular matrix (ECM) degradation (thoroughly reviewed elsewhere [7]) are bound to the pattern recognition receptors (PRRs) and recognized by the innate immune system as damage/danger-associated molecular patterns (DAMPs). Other groups of DAMPs in an OA joint include plasma proteins (e.g., α1 and α2 microglobulins, fibrinogen, vitamin D-binding protein), crystals of basic calcium phosphate, calcium pyrophosphate dihydrate, and uric acid [19], and so-called alarmins [6], including HMGB1 and the S100 family of proteins. There is evidence of some DAMPs’ ability to activate the complement system [19]. Some DAMPs, for example, crystals, rather bind to cytoplasmic PRRs (e.g., NLRP3), initiating inflammasomes activation [20,21]. However, it seems that most DAMPs activate Toll-like receptors (TLRs), a large membrane-bound family of PRRs. TLRs are reported to be expressed both in the cartilage [22], being upregulated in the lesion areas [23], and in the synovium [19]. When stimulated by DAMPs, they trigger catabolic pathways in chondrocytes [23] and proinflammatory factor production by macrophages and mast cells [19]. The proinflammatory mediators can promote matrix metalloproteinases (MMP) production, directly enhancing the catabolic processes in the cartilage. They can also stimulate angiogenesis [24], increasing the influx of plasma proteins [7], which also act as DAMPs. Thus, more DAMPs are attracted to the area. Moreover, proinflammatory cytokines can boost their own production: exposure to proinflammatory cytokines promotes proinflammatory (M1) macrophage polarization, which in turn stimulates proinflammatory cytokine synthesis [25]. Thus, multiple vicious circles of further cartilage damage are established.
Synovial macrophages are the key cells orchestrating the processes of inflammation and healing within the joint because of their capacity to exhibit different phenotypes ranging from proinflammatory (M1) to anti-inflammatory (M2). In an OA joint, macrophages are caught into a vicious circle, being constantly attracted to the site by the perpetuated cartilage degradation and proinflammatory cytokine production, infiltrating the synovium and impairing its functions, notably, synovial fluid component synthesis [26], although to a lesser extent than in RA [27]. Alterations in the OA synovial fluid contents influence its properties, for example, the lack of hyaluronan leads to its decreased viscosity and elasticity [28], resulting in less effective lubrication of the joint surfaces and promoting cartilage damage. Aiming to find a way to disrupt the vicious circle involving the synovial macrophages, some researchers study the consequences of their depletion when modeling OA. Local macrophage depletion using intra-articular injections of clodronate-loaded liposomes has demonstrated rather positive outcomes in murine models, such as reduced MMPs’ expression in the synovial tissue and decreased osteophyte formation [29,30,31]. On the contrary, systemic depletion of macrophages in CSF-1R-GFP+ macrophage Fas-induced apoptosis (MaFIA)-transgenic mice placed on a high-fat diet induced systemic inflammation. Moreover, it led to massive infiltration of CD3+ T cells and neutrophils in the synovium [32], although neutrophil infiltration is characteristic of RA [33] and is hardly ever observed in OA [27].
3. Existing Strategies for Targeting Inflammation and Cartilage Regeneration in OA
3.1. Inflammation
OA can affect people of any age. Accidental trauma, for example, joint ligament damage, can lead to reactive inflammation and post-traumatic OA development. Even if the articular cartilage itself has not been damaged, the inflammatory process in the joint can lead to the activation of catabolic pathways in the cartilage tissue [34].
However, OA is generally discussed in the context of older adults and elderly patients, and one cannot talk about these groups of patients without mentioning comorbidities. Unfortunately, oral NSAIDs and acetaminophen (paracetamol), traditionally used for pain management in OA, are associated with adverse side effects. Those include gastrointestinal [35] and cardiovascular [36] damage for NSAIDs, while paracetamol use may be associated with liver [37] and renal [38] damage. On the other hand, topical NSAIDs are considered a safer but still rather effective option and are strongly recommended for OA patients [12]; however, their use is not enough to reverse the OA progression. Efficacy of intra-articular injections of steroids or hyaluronic acid (HA) remains debatable [11,12]. Mixed results were obtained when targeting proinflammatory cytokines as well. For example, anakinra, a recombinant human IL-1 receptor antagonist protein, was reported to perform better than placebo in patients with severe knee injury [39], and at the same time in OA patients its effect was comparable with placebo [40]. AMG 108, a monoclonal antibody binding the IL-1 receptor, also showed moderate effectiveness in OA patients with minimal clinical benefit [41]. Similarly, anti-tumor necrosis factor alpha (TNF-α) monoclonal antibodies, such as adalimumab and infliximab, despite sporadic encouraging evidence [42,43], were reported to have limited effectiveness in hand OA patients [44,45].
Speaking of arthroscopic procedures, joint lavage seems to hold promise in reducing inflammation following the removal of debris from the joint cavity; however, evidence suggests that it does not provide any significant improvement either [46].
3.2. Cartilage Regeneration
Despite undeniable progress in the field, cartilage regeneration remains a challenge. There are multiple approaches to cartilage repair depending on the size of the defect, and unfortunately each of them has some drawbacks. For example, bone marrow stimulating techniques, such as subchondral drilling and microfracturing, lead to the formation of fibrocartilage, whose mechanical properties are inferior to those of hyaline cartilage [47]. The use of bone marrow stimulation technique with hydrogel implantation into the defect provides the appropriate environment for hyaline cartilage formation [48]. Localized co-delivery of agents inducing hyaline cartilage formation such as bone morphogenic protein 2 (BMP2) and vascular endothelial growth factor (VEGF) receptor antagonist [49] in a hydrogel has also been proposed.
Another group of approaches is based on autologous chondrocyte implantation (ACI), sometimes applied together with collagen-based scaffolds (matrix-induced ACI, or MACI), and is used for larger cartilage defects. These approaches are reported to result in repair with hyaline-like cartilage; however, those are expensive multi-stage procedures requiring long-term rehabilitation [50].
In contrast, autologous stem cell transplantation may be performed in one stage, has a shorter rehabilitation period, and is less expensive than ACI. However, this approach requires longer-term studies to be recommended as a first-line treatment [50].
Osteochondral autografts or allografts are used for the largest cartilage lesions [51]. The osteochondral autograft transfer system (OATS, also known as mosaiplasty) has demonstrated good clinical outcomes, but even though the grafts are harvested from low-bearing regions in the joint, donor-site morbidity cannot be fully avoided. On the other hand, allografts, taken from deceased donors, seem to solve the problem of donor-site morbidity, but despite consensual cartilage immune privilege, some histocompatibility concerns cannot be ignored [52].
4. Inflammation Management in Tissue Engineering
In tissue engineering inflammation is mostly regarded as an adverse effect and a challenge to overcome. All the components of the tissue engineering triad (i.e., biomaterials, cells, and biochemical factors) are therefore being discussed in the context of biocompatibility. Furthermore, various modifications promoting better engraftment as well as minimizing the undesired immune reactions are being proposed for the existing approaches.
4.1. Biomaterials
Both natural and synthetic biomaterials used in tissue engineering have some strong advantages and some critical issues. For example, when assessing biomechanical properties or reproducibility, synthetic biomaterials, such as polycaprolactone (PCL), poly(glycolic acid) (PGA), polylactide (PLA), or poly(lactic-co-glycolic acid) (PLGA), are superior to natural biomaterials. On the other hand, when speaking about biocompatibility, natural biomaterials take precedence. It was reported that most synthetic polymers induce considerable inflammation in vivo [53,54], while natural biomaterials such as collagen [55] or silk [56,57,58,59] cause a significantly lower immune response. Still, synthetic polymers remain attractive substrates for tissue engineering and can be functionalized to enhance their biocompatibility. For example, magnesium hydroxide nanoparticles may help to neutralize pH changes by PLGA degradation acidic products, alleviating inflammation [60].
Hydrophilicity or hydrophobicity of the scaffold is another important characteristic affecting the protein adsorption and therefore the host immune response. Most synthetic polymers are hydrophobic, which correlates with high immunogenicity, but hydrophilic molecules such as polyethylene oxide (PEO) [61], polyethylene glycol (PEG) [61], or graphene oxide (GO) [62] can be used to modify their surface chemistry [63]. There is evidence that compound scaffolds consisting of both synthetic and natural biomaterials show rather good biocompatibility, for example, collagen from micronized porcine cartilage alleviated the inflammatory effect of a PLGA scaffold in a rat model [54]. However, the best available option in terms of biocompatibility remains decellularized ECM (dECM). Not only does dECM provide a perfect microenvironment for cells, it was also repeatedly reported to have immunomodulatory properties, including the influence on the macrophages, namely their polarization to the anti-inflammatory M2 phenotype [63,64,65,66]. Unsurprisingly, many researchers try to mimic ECM properties when designing biomaterials [67].
Some researchers propose scaffold-free approaches to avoid any possible adverse immune reactions to the biomaterials. Both chondrocyte-based [68] and synovial mesenchymal stem cells (MSCs)-based [69] cell sheets were demonstrated to promote good cartilage regeneration without any undesired inflammatory response in vivo. However, although considered “scaffold-free”, both constructs contained the ECM synthesized by the cells [70], and therefore these data may speak in favor of the use of ECM as well.
4.2. Cells
MSCs from different sources, the cell type the most extensively used in tissue engineering, are appreciated for their low immunogenicity and certain immunosuppressive capacity [71]. However, it was reported that in the process of differentiation in vivo their immunogenicity is induced due to MHC-I and MHC-II expression [72], and there is evidence that MSCs can cause a memory T-cell response in immunocompetent hosts [73].
MSCs’ therapeutic potential has long been discussed in the context of their differentiation capacity, i.e., ability to replace damaged tissues. However, a growing body of evidence suggests that MSCs exert their regenerative effect via paracrine activity [74]. Cytokines, chemokines, and, most importantly, extracellular vesicles (EVs) secreted by MSCs are now considered the key players in MSC-based therapies. EVs are heterogeneous membrane nanoparticles providing intercellular communication. Their research and use in various fields of regenerative medicine have been boosted over the past years, and they were demonstrated to possess all the advantages of MSCs without considerable drawbacks. For example, MSC-derived EVs exhibit no risk of tumor formation and demonstrate even lower immunogenicity than MSCs [75]. Moreover, they have lower storage demands and are therefore a promising agent for biomedical product development. Summing up, this makes cell-free approaches more and more prevalent in tissue engineering, including cartilage repair [76].
4.3. Biochemical Factors
Cytokines and growth factors control inflammation as well as cell proliferation, migration, and differentiation, thus orchestrating tissue remodeling and regeneration. Biochemical factors are naturally synthesized by the cellular component of tissue-engineered constructs: for example, MSCs are known to produce a variety of both growth factors and cytokines [77,78]. Moreover, cell cultures can be transfected or transduced so that the cells will secrete the desired bioactive molecules. In a study conducted by Holladay and colleagues, transfected rat bone-marrow-derived MSCs over-expressing an anti-inflammatory cytokine interleukin 10 (IL-10) demonstrated a good retention rate within collagen scaffolds in vivo for up to 7 days in comparison to unmodified cells, while the number of inflammatory cells during this period was decreased [79]. However, the authors reported that IL-10 modified the MSCs’ retention rate to reduce almost to the same level as unmodified cells by day 21, with an unexpected increase in inflammatory cell number on day 7, speculating that prolonged culturing might have altered the MSCs’ phenotype to become more immunogenic. Thus, given the difficulty to control implanted cells in vivo as well as the growing popularity of cell-free approaches, scaffold loading with bioactive factors has been developed. For example, in the same study, Holladay and colleagues proposed an alternative method where rat bone-marrow-derived MSCs were implanted in scaffolds loaded with IL-10 plasmid–polymer complexes, or “polyplexes”, allowing in vivo transfection [79]. This approach increased the MSCs’ retention rates for up to 21 days, which led to prolonged IL-10 release and decreased number of inflammatory cells. Although the idea of scaffold loading with bioactive molecules is not a new one [80], their delivery remains a challenge and ranges from chemical [81,82] or physical [83] incorporation to the use of micro- [84,85,86] or nanoparticles [87]. In this light, ECM as a natural source of bioactive molecules, such as VEGF [88], granulocyte-macrophage colony-stimulating factor (G-MCSF) [66], hepatocyte growth factor (HGF) [66,88], transforming growth factor-beta (TGFβ) [66,88], interleukin 3 (IL-3) [66], or interferon-gamma (INFγ) [66], is of great interest. Moreover, while state-of-the-art approaches suggest EVs’ integration into scaffolds as a powerful tool for cell signaling [89,90,91], ECM-based scaffolds are a natural source of matrix-bound nanovesicles (MBVs), a subgroup of EVs known for their unique immunomodulatory properties [92].
5. Cell-Free Approaches to Cartilage Repair: Targeting Inflammation
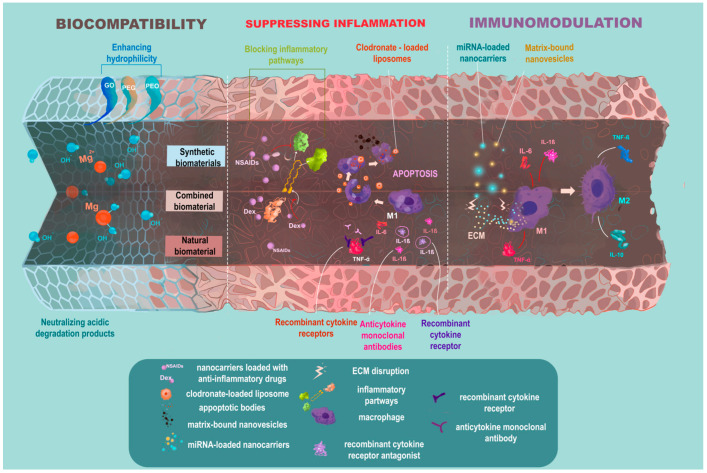
Until quite recently, blocking the inflammatory pathways in OA was expected to be the cure for the disease; however, both anti-cytokine therapy [39,40,41,42,43,44,45] and macrophage depletion in vivo [32] have shown modest results. At present, complete evasion of inflammation is not considered beneficial for the disease outcome [93]. On the contrary, it is generally agreed upon that a moderate, controlled inflammatory reaction is essential for tissue remodeling and successful regeneration [94,95]. Therefore, there is a trend of changing the paradigm of “immune-evasive” bioinert tissue constructs to “immune-interactive”, bioactive ones [96], and enhancement of their immunomodulatory potential (Figure 1).
Figure 1.
Evolution of the biocompatibility paradigm in tissue engineering. Excessive post-implantation inflammation is a common adverse effect in tissue engineering, and therefore biocompatibility is the cornerstone for constructs applied in tissue regeneration. Various strategies enhancing the construct’s biocompatibility have been proposed, including the use of natural biomaterials or functionalizing synthetic biomaterials in a way that would neutralize their degradation products or enhance their hydrophilicity. Yet, there is a trend of changing the paradigm of bioinert tissue constructs to bioactive ones. Thus, not only should they be biocompatible, but they should also be functionalized in a way that would target inflammation. Different strategies suppressing inflammation such as blocking inflammatory pathways or inducing inflammatory cells’ apoptosis have been proposed. However, state-of-the-art approaches suggest immunomodulation rather than suppressing inflammation for successful tissue remodeling and regeneration. In this light, miRNAs known for their unique ability to regulate multiple processes including macrophage polarization are of great interest as therapeutic agents.
Paradoxically, the quest for “tunable” inflammation might lead to reconsideration of cell-based therapies, including MSC-based ones. Despite promising results in various fields of medicine, one important issue concerning their applicability in addition to those discussed in previous sections is their unpredictable behavior in vivo [97,98]. Not only are MSCs reported to become more immunogenic in the process of differentiation, but also to exhibit diametrically opposite immunoregulatory properties depending on the microenvironment. There is evidence that MSCs’ immunosuppressive effect is exerted only in the case of strong inflammation, while low inflammatory signals reduce MSCs’ immunosuppressive capacities and can even lead to MSC-mediated immune system activation [99], which is critical in treating low-grade inflammatory diseases, such as OA. Although MSCs’ plasticity is of great interest and warrants further study, cell-free approaches seem more appropriate for development of controllable therapeutic approaches at the moment.
While host immune response remains the fundamental problem in tissue engineering, inflammatory microenvironment, such as in OA joint, makes tissue regeneration even more challenging: speaking of scaffolds, not only should they be biocompatible, but they should also be functionalized in a way that would target already existing inflammation [100]. Although clinical trials are lacking, inflammation-targeting scaffolds for treating cartilage defects have demonstrated promising results in vivo (Table 1).
Table 1.
Inflammation-targeting scaffolds for treating cartilage defects in vivo.
| Scaffold | Primary Agent Targeting Inflammation |
Animal Model | Defect Type | Treatment | Follow-Up | Effect on Inflammation |
Assessment Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| chitosan-based hydrogel with alginate-chitosan beads |
chitosan | rabbit | ACLT | single intra-articular injection 1 week after the defect formation |
6 weeks | reduced synovial inflammation |
H&E staining |
[101] |
| electrospun polylactic acid/gelatin-based scaffold functionalized with chondroitin sulfate |
chondroitin sulfate | rabbit | chondral defect (3 mm diameter and 4 mm depth) |
Immediate scaffold implantation |
12 weeks | iNOS
PGES in the joint tissues |
IHC staining |
[102] |
| atelocollagen-based hydrogel with polyacrylic acid and resveratrol | resveratrol | rabbit | chondral defect (4 mm diameter and 4 mm depth) |
immediate scaffold implantation |
2, 4, 6 weeks | IL-1β
MMP-13 COX-2 in the joint tissues |
qRT-PCR | [103] |
| silk/graphene oxide-based scaffold modified with tannic acid/Sr2+ coating |
tannic acid | rat | papain- induced OA |
intra-articular injection of the scaffold extract from day 10 every 5 days |
4 weeks | IL-6
IL-8 MMPs in the meniscus and cartilage tissue |
RT-PCR | [104] |
| catechol-modified gelatin and dopamine-modified oxidized hyaluronic acid-based hydrogel with Fe[3]+ and dendritic mesoporous organic silica nanoparticles |
dexamethasone | rat | osteochondral defect (3.5 mm diameter and 5 mm depth) | single intra-articular injection |
8 weeks | TNF-α
IL-6 in the joint tissues |
IHC staining |
[105] |
| alginate hydrogel supplied with rAAV- IGF-I | IGF-I | minipig | chondral defect (4 mm diameter) |
immediate scaffold implantation |
1 year | IL-1β
TNF-α in the joint tissues |
IHC staining |
[106] |
| poly (salicylic acid)-F127-poly (salicylic acid) and hyaluronic acid-3-hydroxyanthranilic acid-based hydrogel | 3-hydroxyanthranilic acid | rat | papain- induced OA |
intra-articular injections once per week |
3, 6 weeks | reduced synovial inflammation iNOS M1 M2 in the synovium |
H&E- staining IHC staining |
[107] |
ACLT—anterior cruciate ligament transection, COX-2—cyclooxygenase-2, H&E—hematoxylin and eosin, IGF-I—insulin-like growth factor 1, IHC—immunohistochemistry, iNOS—inducible nitric oxide synthase, M1—proinflammatory macrophages, M2—anti-inflammatory macrophages, PGES—prostaglandin E synthase, qRT-PCR—quantitative real-time polymerase chain reaction, rAAV—recombinant adeno-associated virus, Ref.—reference, RT-PCR—reverse transcription-polymerase chain reaction.
For example, a chitosan-based hydrogel with alginate-chitosan beads was reported to significantly reduce synovial inflammation in OA rabbit models for 6 weeks [101]. At the same time, an electrospun polylactic acid/gelatin-based scaffold functionalized with chondroitin sulfate was shown to downregulate inducible nitric oxide synthase (iNOS) and prostaglandin E synthase (PGES) in OA rabbit models for 12 weeks [102].
Known for their antioxidant properties, polyphenols such as tannins, curcumin, and resveratrol are extensively researched for treating multiple diseases, including OA [108]. Resveratrol was used by Wang and colleagues in the atelocollagen-based hydrogel system with polyacrylic acid, and significantly downregulated IL-1β, MMP-13, and cyclooxygenase-2 (COX-2) in OA rabbits for 6 weeks [103]. Li and colleagues have demonstrated that even an extract of a silk/graphene oxide-based scaffold modified with tannic acid/Sr2+ coating downregulated IL-6, IL-8, and MMPs in a model of papain-induced OA in rats for up to 4 weeks [104].
Dong and colleagues opted for dexamethasone as the primary inflammation-targeting agent [105]. They proposed a hydrogel based on catechol-modified gelatin and dopamine-modified oxidized hyaluronic acid and functionalized it with dexamethasone-loaded dendritic mesoporous organic silica nanoparticles. This system was reported to downregulate IL-6 and TNF-α in a rat OA model for 8 weeks [105].
Speaking about longer terms of follow-up, alginate hydrogel supplied with recombinant adeno-associated virus (rAAV)-associated IGF-I insulin-like growth factor 1 was reported to downregulate IL-1β and TNF-α in an OA model on minipigs for 1 year [106].
A recent study by Jia and colleagues was the first one to focus on macrophage polarization while treating OA with hydrogel in vivo [107]. The authors reported poly (salicylic acid)-F127-poly (salicylic acid) and hyaluronic acid-3-hydroxyanthranilic acid-based hydrogel not only to reduce synovial inflammation and downregulate iNOS in the synovium, but also to shift macrophage polarization towards the anti-inflammatory M2 phenotype.
EVs, used either to functionalize the scaffolds or alone, are of interest not only as a natural source of bioactive molecules but also as a unique vehicle for targeted therapies (Table 2). Their high biological activity is mainly determined by nucleic acids, namely miRNAs. Some estimates suggest that miRNAs potentially regulate more than 60% of human protein-coding genes [109].
Table 2.
Inflammation-targeting EVs for treating cartilage defects in vivo.
| EV Source | miRNA Studied | EV loading Method | Animal Model | Defect Type | Treatment | EV Dose | Follow-Up | Effect on Inflammation |
Assessment Method | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| hSMSCs | miR-26a-5p | - | rat | ACLT, MCLT, MMT, | intra-articular injections on day 7, 14, 21 |
1011 EVs/mL, 30 μL | 4 weeks | TNF-α
IL-10 in the joint tissues |
ELISA | [110] |
| hBMSCs | miR-26a-5p | lentiviral vector transduction |
rat | ACLT, meniscectomy |
intra-articular injections for 7 days post-surgery |
250 ng/5 µL | 8 weeks | reduced number of inflammatory cells IL-1β in serum |
H&E staining ELISA |
[111] |
| hBMSCs | miR-361-5p | EV electroporation | rat | ACLT | intra-articular injections for 7 days post-surgery |
250 ng/5 µL | 8 weeks | iNOS
MMP-3 MMP-13 IL-18 IL-6 TNF-α in the synovial tissues |
Western blot |
[112] |
| hSMSCs | miR-31 | lentiviral vector transduction |
mouse | ACLT, MCLT, MMT | intra-articular injection every 3 days for 4 weeks |
5 μL/mL | 12 weeks | IL-1β
IL-6 TNF-α in the synovial fluid |
ELISA | [113] |
| primary rat synovial fibroblasts |
miR-126-3p | cell culture transfection | rat | ACLT, MMR | intra-articular injection once per week from the 4th week post-surgery |
500 μg/mL, 40 μL | 10 weeks | reduced synovial inflammation IL-1β TNF-α in the cartilage |
H&E- staining IHC staining |
[114] |
| primary rat synovial fibroblasts | miR-214-3p | cell culture transfection | rat | ACLT, MMR | intra-articular injection once per week from the 4th week post-surgery |
not specified |
10 weeks | reduced synovial inflammation IL-1β TNF-α in the cartilage and synovium |
H&E- staining IHC staining |
[115] |
| mouse BMSCs | miR-3960 | cell culture transfection | mouse | MCLT, MMT | intra-articular injection once per week for 3 weeks |
100 μg/mL,10 μL | 7 weeks | IL-6
TNF-α in the serum |
ELISA | [116] |
ACLT—anterior cruciate ligament transection, H&E—hematoxylin and eosin, hBMSCs—human bone marrow mesenchymal stem cells, hSMSCs—human synovial mesenchymal stem cells, IHC—immunohistochemistry, iNOS—inducible nitric oxide synthase, MCLT—medial collateral ligament transection, MMR—medial meniscus resection, MMT—medial meniscus transection, Ref.—reference.
In order to study the precise effect of a particular miRNA, EVs can be additionally loaded with different miRNAs. Another approach suggests blocking or downregulating miRNAs of interest to assess their contribution to any particular process. Thus, downregulating miR-26a-5p in human SMSCs demonstrated that hSMSC-derived EVs carrying miR-26a-5p could downregulate TNF-α and upregulate IL-10 in a rat OA model for 4 weeks [110]. On the other hand, human bone marrow mesenchymal stem cell (hBMSC)-derived EVs loaded with miR-26a-5p via lentiviral vector transduction were reported to reduce inflammatory cell infiltration in the synovium of OA rats for 8 weeks [111].
Similarly, hBMSC-derived EVs loaded with miR-361-5p via electroporation downregulated iNOS, MMP-3, MMP-13, IL-18, IL-6, and TNF-α in OA rats’ synovial tissues for 8 weeks [112]. At the same time, miR-31-loaded EVs derived from lentiviral-vector-transduced culture of hSMSCs downregulated IL-1β, IL-6, and TNF-α expression in a murine OA model for 12 weeks [113]. While many researchers choose human MSCs as the EVs’ source for OA treatment, some groups opt for allogenic cells for in vivo studies. Zhou [114] and Lai [115] chose primary rat synovial fibroblasts as the EVs’ source for rat OA models. The groups studied miR-126-3p [114] and miR-214-3p-loading [115], and both reported reduced synovial inflammation and downregulation of IL-1β and TNF-α for 10 weeks. Ye and colleagues transfected murine BMSCs with miR-3960 and reported the derived EVs to downregulate IL-6 and TNF-α in the serum of OA mice [116].
Both scaffolds and EVs are a source of inspiration for designing different nanomaterials and nanocarriers for treating various pathologic conditions [117,118,119,120], including OA (Table 3).
Table 3.
Inflammation-targeting nanocarriers for treating cartilage defects in vivo.
| Nanocarrier | Loaded Agents | Animal Model | Defect Type | Treatment | Follow-Up | Effect on Inflammation |
Assessment Method | Ref. |
|---|---|---|---|---|---|---|---|---|
| chitosan-modified molybdenum disulfide nanosheets |
dexamethasone | mouse | papain- induced OA |
intra-articular injections every 3 days; near-infrared light exposure |
4 weeks | IL-1β
IL-8 TNF-α in the synovium |
IHC staining | [121] |
| thermo-responsive chitosan oligosaccharide nanospheres conjugated with pluronic F127 grafting carboxyl group |
kartogenin diclofenac |
rat | ACLT, MM destabilization | intra-articular injections at weeks 7 and 10 |
8 weeks | COX-2
in the synovium |
ELISA | [122] |
| poly(d,l-lactide-co-glycolide) nanoparticles | diacerein | rat | MIA- induced OA |
single intra-articular injection |
9 weeks | IL-1
IL-6 TNF-α MMP-3 MMP-13 COX-2 ADAMTS-5 IL-4 IL-10 in the whole blood |
real-time PCR | [123] |
| hollow MnO2
nanoparticles modified with NH2-PEG-NH2 |
- | mouse | MM destabilization | intra-articular injections 3 times a week for 4 weeks |
8 weeks | IL-1β
IL-6 in the serum |
ELISA | [124] |
| polyhydroxylated fullerene C60 (fullerol) nanoparticles |
- | rat | MIA- induced OA |
single intra-venous injection |
3 weeks | reduced synovial inflammation |
H&E staining | [125] |
| polyethylenimine conjugated with chondrocyte-affinity peptide |
anti-Hif-2α siRNA | mouse | ACLT and MCL dissection | weekly intra-articular injections |
7 weeks | reduced synovial inflammation IL-1β in the synovial fluid |
H&E stainingELISA | [126] |
| methoxy poly(ethylene glycol)-b-poly (D,L-lactide) and PLGA-based nanoparticles | rebamipide | rat | MIA- induced OA |
single intra-articular injection |
4, 8 weeks | IL-1β
IL-6 TNF-α MMP-3 MMP-13 COX-2 in the whole blood |
real-time PCR | [127] |
| bilirubin grafted polylysine nanoparticles |
IgG, berberine | rat | ACLT | intra-articular injections on day 35, 40, 45, 50, 55, and 60 |
65 days | reduced synovial inflammation TNF-α M1/M2 ratio in the synovium |
H&E stainingIHC | [128] |
| nanoliposomes | resolvin D1 | mouse | MM destabilization | intra-articular injections at weeks 1, 4, and 8 |
3 months | M1/M2 ratio
in the synovium |
IHC | [129] |
ACLT—anterior cruciate ligament transection, anti-Hif-2α—hypoxia-inducible factor-2α, H&E—hematoxylin and eosin, IHC—immunohistochemistry, M1—proinflammatory macrophages, M2—anti-inflammatory macrophages, MCL—medial collateral ligament, MIA—monosodium iodoacetate, MM—medial meniscus, Ref.—reference.
For example, chitosan-modified molybdenum disulfide nanosheets loaded with dexamethasone were reported to downregulate IL-1β, IL-8, and TNF-α in a murine OA model for 4 weeks [121]. Chitosan was also used in designing thermo-responsive nanospheres in a study by Kang and colleagues [122]. Loaded with kartogenin and diclofenac, these nanospheres were reported to significantly decrease the levels of COX-2 in the synovium of OA rats for up to 8 weeks. At the same time Jung and colleagues reported poly(d,l-lactide-co-glycolide) nanoparticles loaded with diacerein to suppress the blood levels of a variety of proinflammatory cytokines in OA rats for up to 9 weeks after a single intra-articular injection [123]. Some nanocarriers act as therapeutic agents themselves without any extra loading: for example, hollow MnO2 nanoparticles modified with NH2-PEG-NH2 downregulated the levels of IL-1β and IL-6 in the serum of OA mice for 8 weeks [124]. While most researchers use intra-articular injections when administering EV-based or nanocarrier-based OA treatment, Pei and colleagues reported reduced synovial inflammation in OA rats for 3 weeks following an intra-venous injection of polyhydroxylated fullerol nanoparticles [125]. Reduced synovial inflammation as well as IL-1β downregulation in the synovial fluid of OA mice were also reported for polyethylenimine conjugated with chondrocyte-affinity peptide and loaded with anti-Hif-2α siRNA [126]. Kim and colleagues reported methoxy poly(ethylene glycol)-b-poly (D,L-lactide) and PLGA-based nanoparticles loaded with the cytoprotective drug rebamipide to reduce the blood levels of IL-1β, IL-6, TNF-α, MMP-3, MMP-13, and COX-2 in OA rats [127].
Two recent studies focused on macrophage polarization modulation via functionalized nanoparticles. Bilirubin grafted polylysine nanoparticles loaded with IgG and the anti-inflammatory agent berberine were reported to reduce synovial inflammation, downregulate TNF-α, and decrease the M1/M2 ratio in the synovium of OA rats [128]. Resolvin D1-loaded nanoliposomes were equally reported to reduce the M1/M2 ratio in a murine OA model [129].
6. Matrix-Bound Nanovesicles: A Promising Therapeutic Agent
EVs discussed in the previous section are exclusively liquid-phase vesicles, i.e., microvesicles or exosomes. However, MBVs bound to the ECM fibers can also serve as a source of regulatory miRNAs. It seems that there is no full overlap of liquid-phase EV and MBV functions due to the differences in their miRNA composition: for example, more than 50% of miRNAs were found to be differentially expressed in MBVs compared to liquid-phase EVs in mouse embryonic fibroblasts NIH 3T3 [130]. Inter alia, Hussey and colleagues reported miR-27a-5p to be upregulated in MBVs compared to liquid-phase EVs, which might contribute to their immunomodulatory potential: miR-27a-5p is known to exert negative regulation of NF-κB transcription factor activity, thus downregulating the proinflammatory IL-1β signaling pathway [131]. Moreover, being enriched with polyunsaturated fatty acids, MBVs seem to serve as a source of signaling lipid mediators, regulating inflammation-related pathways [130].
MBVs were reported to switch macrophages’ phenotype from proinflammatory M1 to anti-inflammatory M2 in vitro [92]. Furthermore, the fact that MBVs are embedded within the ECM as long as it is intact and are detached from the fibers ready for the cellular uptake in case of ECM disruption could be associated with their superior regenerative properties. Should this hypothesis be confirmed, it might mean no need for extra loading of MBVs with miRNAs when used as a therapeutic agent.
While there are no studies yet evaluating MBVs’ effect in OA animal models, MBVs were reported to mitigate both acute and chronic RA in vivo [132]. The authors reported MBVs’ therapeutic efficacy to be equal to that of methotrexate and associated this effect with modulation of local synovial macrophages.
ECM-based scaffolds and hydrogels successfully used in tissue engineering should probably contain MBVs, but to what extent MBVs contribute to their regenerative potential is yet to be confirmed. On the other hand, it was reported that short-term enzyme treatment of cartilage defects could improve the tissue-engineered constructs integration [133,134,135], which was associated with facilitated cell migration. However, enzyme treatment could also lead to the detachment and cellular uptake of MBVs, stimulating the healing of the tissue.
7. Conclusions
Multiple strategies for OA management have been proposed, but the cure is yet to be found. One of the latest concepts suggests that OA treatment should focus on both cartilage repair and immunomodulation. This dual targeting can be achieved via the use of functionalized biomaterials, native or engineered nanovesicles, or else the combination of all these techniques. In view of the foregoing, mimicking natural processes of regeneration but enhancing them with the help of tissue engineering approaches or nanotechnologies seems to be the winning strategy.
Acknowledgments
The authors deeply acknowledge the unique scientific facility of Transgenebank. The authors would also like to thank Viktoria Chernikovich for assisting in drawing Figure 1.
Abbreviations
ACI—autologous chondrocyte implantation; BMP2—bone morphogenic protein 2; COX-2—cyclooxygenase-2; DAMPs—damage/danger-associated molecular patterns; dECM—decellularized extracellular matrix; DMOADs—disease-modifying osteoarthritis drugs; ECM—extracellular matrix; EVs—extracellular vesicles; G-MCSF—granulocyte-macrophage colony-stimulating factor; GO—graphene oxide; HA—hyaluronic acid; HGF—hepatocyte growth factor; HMGB1—high-mobility group protein B1; HPMAm-lac—hydroxypropyl methacrylamide mono- and dilactate; IGF-I—insulin-like growth factor 1; IL—interleukin; INFγ—interferon gamma; iNOS—inducible nitric oxide synthase; MACI—matrix-induced autologous chondrocyte implantation; MBVs—matrix-bound nanovesicles; MMPs—matrix metalloproteinases; MSCs—mesenchymal stromal cells; NF-κB—nuclear factor kappa-light-chain-enhancer of activated B cells; NLRP3—NOD-, LRR-, and pyrin domain-containing protein 3; NSAIDs—non-steroidal anti-inflammatory drugs; OA—osteoarthritis; OATS—osteochondral autograft transfer system; PCL—polycaprolactone; PEG—polyethylene glycol; PEO—polyethylene oxide; PGA—poly(glycolic acid); PGES—prostaglandin E synthase; PLA—polylactide; PLGA—poly(lactic-co-glycolic acid); PRRs—pattern recognition receptors; RA—rheumatoid arthritis; rAAV—recombinant adeno-associated virus; TGFβ—transforming growth factor beta; TLRs—Toll-like receptors; TNF-α—tumor necrosis factor alpha; VEGF—vascular endothelial growth factor.
Author Contributions
M.P. drafted the manuscript, created the picture, and prepared the tables with primary editing and revision support from A.S. and N.K.; S.R.-L. and F.L. critically revised the manuscript; D.T., A.L. and P.T. coordinated the manuscript preparation; X.-J.L. coordinated the entire work and revised the final draft. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was financially supported by the Ministry of Science and Higher Education of the Russian Federation under the grant agreement No. 075-15-2021-951 (13.2251.21.0022).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Oleksyszyn J., Augustine A.J. Plasminogen modulation of IL-1-stimulated degradation in bovine and human articular cartilage explants. The role of the endogenous inhibitors: PAI-1,α 2-antiplasmin,α 1-PI,α 2-macroglobulin and TIMP. Agents Actions. 1996;45:464–472. doi: 10.1007/BF02252318. [DOI] [PubMed] [Google Scholar]
- 2.Pascual E., Jovaní V. Synovial fluid analysis. Best Pract. Res. Clin. Rheumatol. 2005;19:371–386. doi: 10.1016/j.berh.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Gerwin N., Hops C., Lucke A. Intraarticular drug delivery in osteoarthritis. Adv. Drug Deliv. Rev. 2006;58:226–242. doi: 10.1016/j.addr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- 4.Brown J.M., Mantoku A., Sabokbar A., Oppermann U., Hassan A.B., Kudo A., Athanasou N. Periostin expression in neoplastic and non-neoplastic diseases of bone and joint. Clin. Sarcoma Res. 2018;8:1–8. doi: 10.1186/s13569-018-0105-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou L., Gu M., Ma X., Wen L., Zhang B., Lin Y., Pan J. Long non-coding RNA PCAT-1 regulates apoptosis of chondrocytes in osteoarthritis by sponging miR-27b-3p. J. Bone Miner. Metab. 2021;39:139–147. doi: 10.1007/s00774-020-01128-8. [DOI] [PubMed] [Google Scholar]
- 6.Bosch M.H.V.D. Inflammation in osteoarthritis: Is it time to dampen the alarm(in) in this debilitating disease? Clin. Exp. Immunol. 2019;195:153–166. doi: 10.1111/cei.13237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sokolove J., Lepus C.M. Role of inflammation in the pathogenesis of osteoarthritis: Latest findings and interpretations. Ther. Adv. Musculoskelet. Dis. 2013;5:77–94. doi: 10.1177/1759720X12467868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peshkova M., Lychagin A., Lipina M., Di Matteo B., Anzillotti G., Ronzoni F., Kosheleva N., Shpichka A., Royuk V., Fomin V., et al. Gender-Related Aspects in Osteoarthritis Development and Progression: A Review. Int. J. Mol. Sci. 2022;23:2767. doi: 10.3390/ijms23052767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ayral X., Pickering E., Woodworth T., Mackillop N., Dougados M. Synovitis: A potential predictive factor of structural progression of medial tibiofemoral knee osteoarthritis–results of a 1 year longitudinal arthroscopic study in 422 patients. Osteoarthr. Cartil. 2005;13:361–367. doi: 10.1016/j.joca.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 10.Krasnokutsky S., Belitskaya-Lévy I., Bencardino J., Samuels J., Attur M., Regatte R., Rosenthal P., Greenberg J., Schweitzer M., Abramson S.B., et al. Quantitative magnetic resonance imaging evidence of synovial proliferation is associated with radiographic severity of knee osteoarthritis. Arthritis Rheum. 2011;63:2983–2991. doi: 10.1002/art.30471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bannuru R.R., Osani M.C., Vaysbrot E.E., Arden N.K., Bennell K., Bierma-Zeinstra S.M.A., Kraus V.B., Lohmander L.S., Abbott J.H., Bhandari M., et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthr. Cartil. 2019;27:1578–1589. doi: 10.1016/j.joca.2019.06.011. [DOI] [PubMed] [Google Scholar]
- 12.Cao P., Li Y., Tang Y., Ding C., Hunter D.J. Pharmacotherapy for knee osteoarthritis: Current and emerging therapies. Expert Opin. Pharmacother. 2020;21:797–809. doi: 10.1080/14656566.2020.1732924. [DOI] [PubMed] [Google Scholar]
- 13.Robinson P.D., McEwan J., Adukia V., Prabhakar M. Osteoarthritis and arthroplasty of the hip and knee. Br. J. Hosp. Med. 2018;79:C54–C59. doi: 10.12968/hmed.2018.79.4.C54. [DOI] [PubMed] [Google Scholar]
- 14.Xie C., Chen Q. Adipokines: New Therapeutic Target for Osteoarthritis? Curr. Rheumatol. Rep. 2019;21:71. doi: 10.1007/s11926-019-0868-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tu C., He J., Wu B., Wang W., Li Z. An extensive review regarding the adipokines in the pathogenesis and progression of osteoarthritis. Cytokine. 2019;113:1–12. doi: 10.1016/j.cyto.2018.06.019. [DOI] [PubMed] [Google Scholar]
- 16.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015;11:21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Z., Le H., Wang Y., Liu H., Li Z., Yang X., Wang C., Ding J., Chen X. Instructive cartilage regeneration modalities with advanced therapeutic implantations under abnormal conditions. Bioact. Mater. 2021;11:317–338. doi: 10.1016/j.bioactmat.2021.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwanaga T., Shikichi M., Kitamura H., Yanase H., Nozawa-Inoue K. Morphology and Functional Roles of Synoviocytes in the Joint. Arch. Histol. Cytol. 2000;63:17–31. doi: 10.1679/aohc.63.17. [DOI] [PubMed] [Google Scholar]
- 19.Robinson W.H., Lepus C.M., Wang Q., Raghu H., Mao R., Lindstrom T.M., Sokolove J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016;12:580–592. doi: 10.1038/nrrheum.2016.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pazár B., Ea H.-K., Narayan S., Kolly L., Bagnoud N., Chobaz V., Roger T., Lioté F., So A., Busso N. Basic Calcium Phosphate Crystals Induce Monocyte/Macrophage IL-1β Secretion through the NLRP3 Inflammasome In Vitro. J. Immunol. 2011;186:2495–2502. doi: 10.4049/jimmunol.1001284. [DOI] [PubMed] [Google Scholar]
- 21.Denoble A.E., Huffman K.M., Stabler T.V., Kelly S.J., Hershfield M.S., McDaniel G.E., Coleman R.E., Kraus V.B. Uric acid is a danger signal of increasing risk for osteoarthritis through inflammasome activation. Proc. Natl. Acad. Sci. USA. 2011;108:2088–2093. doi: 10.1073/pnas.1012743108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sillat T., Barreto G., Clarijs P., Soininen A., Ainola M., Pajarinen J., Korhonen M., Konttinen Y.T., Sakalyte R., Hukkanen M., et al. Toll-like receptors in human chondrocytes and osteoarthritic cartilage. Acta Orthop. 2013;84:585–592. doi: 10.3109/17453674.2013.854666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H.A., Cho M.-L., Choi H.Y., Yoon C.S., Jhun J.Y., Oh H.J., Kim H.-Y. The catabolic pathway mediated by Toll-like receptors in human osteoarthritic chondrocytes. Arthritis Rheum. 2006;54:2152–2163. doi: 10.1002/art.21951. [DOI] [PubMed] [Google Scholar]
- 24.Berenbaum F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!) Osteoarthr. Cartil. 2013;21:16–21. doi: 10.1016/j.joca.2012.11.012. [DOI] [PubMed] [Google Scholar]
- 25.Kennedy A., Fearon U., Veale D.J., Godson C. Macrophages in Synovial Inflammation. Front. Immunol. 2011;2:52. doi: 10.3389/fimmu.2011.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandes T.L., Gomoll A.H., Lattermann C., Hernandez A.J., Bueno D.F., Amano M.T. Macrophage: A Potential Target on Cartilage Regeneration. Front. Immunol. 2020;11:111. doi: 10.3389/fimmu.2020.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Lange-Brokaar B., Ioan-Facsinay A., van Osch G., Zuurmond A.-M., Schoones J., Toes R., Huizinga T., Kloppenburg M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012;20:1484–1499. doi: 10.1016/j.joca.2012.08.027. [DOI] [PubMed] [Google Scholar]
- 28.Vincent H.K., Percival S.S., Conrad B.P., Seay A.N., Montero C., Vincent K.R. Hyaluronic Acid (HA) Viscosupplementation on Synovial Fluid Inflammation in Knee Osteoarthritis: A Pilot Study. Open Orthop. J. 2013;7:378–384. doi: 10.2174/1874325001307010378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Lent P.L.E.M., Blom A.B., Van Der Kraan P., Holthuysen A.E.M., Vitters E., Van Rooijen N., Smeets R.L., Nabbe K.C.A.M., Berg W.B.V.D. Crucial role of synovial lining macrophages in the promotion of transforming growth factor?-mediated osteophyte formation. Arthritis Rheum. 2004;50:103–111. doi: 10.1002/art.11422. [DOI] [PubMed] [Google Scholar]
- 30.Blom A.B., van Lent P.L., Holthuysen A.E., van der Kraan P.M., Roth J., van Rooijen N., Berg W.B.V.D. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004;12:627–635. doi: 10.1016/j.joca.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 31.Blom A.B., van Lent P.L., Libregts S., Holthuysen A.E., van der Kraan P.M., van Rooijen N., Berg W.B.V.D. Crucial role of macrophages in matrix metalloproteinase–mediated cartilage destruction during experimental osteoarthritis: Involvement of matrix metalloproteinase 3. Arthritis Rheum. 2007;56:147–157. doi: 10.1002/art.22337. [DOI] [PubMed] [Google Scholar]
- 32.Wu C.-L., McNeill J., Goon K., Little D., Kimmerling K., Huebner J., Kraus V., Guilak F. Conditional Macrophage Depletion Increases Inflammation and Does Not Inhibit the Development of Osteoarthritis in Obese Macrophage Fas-Induced Apoptosis-Transgenic Mice. Arthritis Rheumatol. 2017;69:1772–1783. doi: 10.1002/art.40161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright H., Moots R.J., Edwards S.W. The multifactorial role of neutrophils in rheumatoid arthritis. Nat. Rev. Rheumatol. 2014;10:593–601. doi: 10.1038/nrrheum.2014.80. [DOI] [PubMed] [Google Scholar]
- 34.Punzi L., Galozzi P., Luisetto R., Favero M., Ramonda R., Oliviero F., Scanu A. Post-traumatic arthritis: Overview on pathogenic mechanisms and role of inflammation. RMD Open. 2016;2:e000279. doi: 10.1136/rmdopen-2016-000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sostres C., Gargallo C.J., Lanas A. Nonsteroidal anti-inflammatory drugs and upper and lower gastrointestinal mucosal damage. Arthritis Res. Ther. 2013;15((Suppl. S3)):S3–S8. doi: 10.1186/ar4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bally M., Dendukuri N., Rich B., Nadeau L., Helin-Salmivaara A., Garbe E., Brophy J. Risk of acute myocardial infarction with NSAIDs in real world use: Bayesian meta-analysis of individual patient data. BMJ. 2017;357:j1909. doi: 10.1136/bmj.j1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Popiolek I., Hydzik P., Jagielski P., Zrodlowska M., Mystek K., Porebski G. Risk Factors for Hepatotoxicity Due to Paracetamol Overdose in Adults. Medicina. 2021;57:752. doi: 10.3390/medicina57080752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts E., Nunes V.D., Buckner S., Latchem S., Constanti M., Miller P., Doherty M., Zhang W., Birrell F., Porcheret M., et al. Paracetamol: Not as safe as we thought? A systematic literature review of observational studies. Ann. Rheum. Dis. 2016;75:552–559. doi: 10.1136/annrheumdis-2014-206914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kraus V., Birmingham J., Stabler T., Feng S., Taylor D., Moorman C., Garrett W., Toth A. Effects of intraarticular IL1-Ra for acute anterior cruciate ligament knee injury: A randomized controlled pilot trial ( NCT00332254) Osteoarthr. Cartil. 2012;20:271–278. doi: 10.1016/j.joca.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 40.Chevalier X., Goupille P., Beaulieu A.D., Burch F.X., Bensen W.G., Conrozier T., Loeuille D., Kivitz A.J., Silver D., Appleton B.E. Intraarticular injection of anakinra in osteoarthritis of the knee: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Rheum. 2009;61:344–352. doi: 10.1002/art.24096. [DOI] [PubMed] [Google Scholar]
- 41.Cohen S.B., Proudman S., Kivitz A.J., Burch F.X., Donohue J.P., Burstein D., Sun Y.-N., Banfield C., Vincent M.S., Ni L., et al. A randomized, double-blind study of AMG 108 (a fully human monoclonal antibody to IL-1R1) in patients with osteoarthritis of the knee. Arthritis Res. Ther. 2011;13:R125. doi: 10.1186/ar3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verbruggen A., Wittoek R., Cruyssen B.V., Elewaut D. Tumour necrosis factor blockade for the treatment of erosive osteoarthritis of the interphalangeal finger joints: A double blind, randomised trial on structure modification. Ann. Rheum. Dis. 2012;71:891–898. doi: 10.1136/ard.2011.149849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maksymowych W.P., Russell A.S., Chiu P., Yan A., Jones N., Clare T., Lambert R.G. Targeting tumour necrosis factor alleviates signs and symptoms of inflammatory osteoarthritis of the knee. Arthritis Res. Ther. 2012;14:R206. doi: 10.1186/ar4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fioravanti A., Fabbroni M., Cerase A., Galeazzi M. Treatment of erosive osteoarthritis of the hands by intra-articular infliximab injections: A pilot study. Rheumatol. Int. 2009;29:961–965. doi: 10.1007/s00296-009-0872-0. [DOI] [PubMed] [Google Scholar]
- 45.Chevalier X., Ravaud P., Maheu E., Baron G., Rialland A., Vergnaud P., Roux C., Maugars Y., Mulleman D., Lukas C., et al. Adalimumab in patients with hand osteoarthritis refractory to analgesics and NSAIDs: A randomised, multicentre, double-blind, placebo-controlled trial. Ann. Rheum. Dis. 2015;74:1697–1705. doi: 10.1136/annrheumdis-2014-205348. [DOI] [PubMed] [Google Scholar]
- 46.Avouac J., Vicaut E., Bardin T., Richette P. Efficacy of joint lavage in knee osteoarthritis: Meta-analysis of randomized controlled studies. Rheumatology. 2010;49:334–340. doi: 10.1093/rheumatology/kep382. [DOI] [PubMed] [Google Scholar]
- 47.Gao L., Goebel L.K.H., Orth P., Cucchiarini M., Madry H. Subchondral drilling for articular cartilage repair: A systematic review of translational research. Dis. Model. Mech. 2018;11:dmm034280. doi: 10.1242/dmm.034280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schreiner M.M., Raudner M., Szomolanyi P., Ohel K., Ben-Zur L., Juras V., Mlynarik V., Windhager R., Trattnig S. Chondral and Osteochondral Femoral Cartilage Lesions Treated with GelrinC: Significant Improvement of Radiological Outcome Over Time and Zonal Variation of the Repair Tissue Based on T2 Mapping at 24 Months. Cartilage. 2021;13((Suppl. S1)):604S–616S. doi: 10.1177/1947603520926702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Murphy M.P., Koepke L.S., Lopez M.T., Tong X., Ambrosi T.H., Gulati G.S., Marecic O., Wang Y., Ransom R.C., Hoover M.Y., et al. Articular cartilage regeneration by activated skeletal stem cells. Nat. Med. 2020;26:1583–1592. doi: 10.1038/s41591-020-1013-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chimutengwende-Gordon M., Donaldson J., Bentley G. Current solutions for the treatment of chronic articular cartilage defects in the knee. EFORT Open Rev. 2020;5:156–163. doi: 10.1302/2058-5241.5.190031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hevesi M., Jacob G., Shimomura K., Ando W., Nakamura N., Krych A.J. Current hip cartilage regeneration/repair modalities: A scoping review of biologics and surgery. Int. Orthop. 2021;45:319–333. doi: 10.1007/s00264-020-04789-2. [DOI] [PubMed] [Google Scholar]
- 52.Yang F., Zhang Y., Liu B., Cao M., Yang J., Tian F., Yang P., Qin K., Zhao D. Basic fibroblast growth factor and agarose gel promote the ability of immune privilege of allogeneic cartilage transplantation in rats. J. Orthop. Transl. 2019;22:73–80. doi: 10.1016/j.jot.2019.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Asawa Y., Sakamoto T., Komura M., Watanabe M., Nishizawa S., Takazawa Y., Takato T., Hoshi K. Early Stage Foreign Body Reaction against Biodegradable Polymer Scaffolds Affects Tissue Regeneration during the Autologous Transplantation of Tissue-Engineered Cartilage in the Canine Model. Cell Transplant. 2012;21:1431–1442. doi: 10.3727/096368912X640574. [DOI] [PubMed] [Google Scholar]
- 54.Kim S., Jang J.E., Lee J.H., Khang G. Composite scaffold of micronized porcine cartilage/poly(lactic-co-glycolic acid) enhances anti-inflammatory effect. Mater. Sci. Eng. C. 2018;88:46–52. doi: 10.1016/j.msec.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 55.van Putten S.M., Ploeger D.T., Popa E.R., Bank R.A. Macrophage phenotypes in the collagen-induced foreign body reaction in rats. Acta Biomater. 2013;9:6502–6510. doi: 10.1016/j.actbio.2013.01.022. [DOI] [PubMed] [Google Scholar]
- 56.Meinel L., Hofmann S., Karageorgiou V., Kirker-Head C., McCool J., Gronowicz G., Zichner L., Langer R., Vunjak-Novakovic G., Kaplan D.L. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005;26:147–155. doi: 10.1016/j.biomaterials.2004.02.047. [DOI] [PubMed] [Google Scholar]
- 57.Aramwit P., Kanokpanont S., De-Eknamkul W., Srichana T. Monitoring of inflammatory mediators induced by silk sericin. J. Biosci. Bioeng. 2009;107:556–561. doi: 10.1016/j.jbiosc.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 58.Zhang Y., Wu C., Luo T., Li S., Cheng X., Miron R.J. Synthesis and inflammatory response of a novel silk fibroin scaffold containing BMP7 adenovirus for bone regeneration. Bone. 2012;51:704–713. doi: 10.1016/j.bone.2012.06.029. [DOI] [PubMed] [Google Scholar]
- 59.Qi C., Liu J., Jin Y., Xu L., Wang G., Wang Z., Wang L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials. 2018;163:89–104. doi: 10.1016/j.biomaterials.2018.02.016. [DOI] [PubMed] [Google Scholar]
- 60.Park K.-S., Kim B.-J., Lih E., Park W., Lee S.-H., Joung Y.K., Han D.K. Versatile effects of magnesium hydroxide nanoparticles in PLGA scaffold–mediated chondrogenesis. Acta Biomater. 2018;73:204–216. doi: 10.1016/j.actbio.2018.04.022. [DOI] [PubMed] [Google Scholar]
- 61.Drury J.L., Mooney D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials. 2003;24:4337–4351. doi: 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- 62.Aidun A., Firoozabady A.S., Moharrami M., Ahmadi A., Haghighipour N., Bonakdar S., Faghihi S. Graphene oxide incorporated polycaprolactone/chitosan/collagen electrospun scaffold: Enhanced osteogenic properties for bone tissue engineering. Artif. Organs. 2019;43:E264–E281. doi: 10.1111/aor.13474. [DOI] [PubMed] [Google Scholar]
- 63.Wei F., Liu S., Chen M., Tian G., Zha K., Yang Z., Jiang S., Li M., Sui X., Chen Z., et al. Host Response to Biomaterials for Cartilage Tissue Engineering: Key to Remodeling. Front. Bioeng. Biotechnol. 2021;9:664592. doi: 10.3389/fbioe.2021.664592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fishman J.M., Lowdell M.W., Urbani L., Ansari T., Burns A.J., Turmaine M., North J., Sibbons P., Seifalian A.M., Wood K.J., et al. Immunomodulatory effect of a decellularized skeletal muscle scaffold in a discordant xenotransplantation model. Proc. Natl. Acad. Sci. USA. 2013;110:14360–14365. doi: 10.1073/pnas.1213228110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.He C., Yang Z., Jin Y., Qi X., Chu J., Deng X. ADM Scaffolds Generate a Pro-regenerative Microenvironment During Full-Thickness Cutaneous Wound Healing Through M2 Macrophage Polarization via Lamtor1. Front. Physiol. 2018;9:657. doi: 10.3389/fphys.2018.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Londono R., Badylak S.F. Biologic Scaffolds for Regenerative Medicine: Mechanisms of In vivo Remodeling. Ann. Biomed. Eng. 2015;43:577–592. doi: 10.1007/s10439-014-1103-8. [DOI] [PubMed] [Google Scholar]
- 67.He W., Bai J., Chen X., Suo D., Wang S., Guo Q., Yin W., Geng D., Wang M., Pan G., et al. Reversible dougong structured receptor–ligand recognition for building dynamic extracellular matrix mimics. Proc. Natl. Acad. Sci. USA. 2022;119:e2117221119. doi: 10.1073/pnas.2117221119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang F., Hu Y., He D., Zhou G., Ellis E. Scaffold-free cartilage cell sheet combined with bone-phase BMSCs-scaffold regenerate osteochondral construct in mini-pig model. Am. J. Transl. Res. 2018;10:2997–3010. [PMC free article] [PubMed] [Google Scholar]
- 69.Koizumi K., Ebina K., Hart D.A., Hirao M., Noguchi T., Sugita N., Yasui Y., Chijimatsu R., Yoshikawa H., Nakamura N. Synovial mesenchymal stem cells from osteo- or rheumatoid arthritis joints exhibit good potential for cartilage repair using a scaffold-free tissue engineering approach. Osteoarthr. Cartil. 2016;24:1413–1422. doi: 10.1016/j.joca.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Efremov Y.M., Zurina I.M., Presniakova V.S., Kosheleva N.V., Butnaru D.V., Svistunov A.A., Rochev Y.A., Timashev P.S. Mechanical properties of cell sheets and spheroids: The link between single cells and complex tissues. Biophys. Rev. 2021;13:541–561. doi: 10.1007/s12551-021-00821-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu L.-H., Zhang T.-T., Li Y., Yan H.-J., Qi H., Li F.-R. Immunogenicity of allogeneic mesenchymal stem cells transplanted via different routes in diabetic rats. Cell. Mol. Immunol. 2014;12:444–455. doi: 10.1038/cmi.2014.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang X.-P., Sun Z., Miyagi Y., Kinkaid H.M., Zhang L., Weisel R.D., Li R.-K. Differentiation of Allogeneic Mesenchymal Stem Cells Induces Immunogenicity and Limits Their Long-Term Benefits for Myocardial Repair. Circulation. 2010;122:2419–2429. doi: 10.1161/CIRCULATIONAHA.110.955971. [DOI] [PubMed] [Google Scholar]
- 73.Nauta A.J., Westerhuis G., Kruisselbrink A.B., Lurvink E.G.A., Willemze R., Fibbe W.E. Donor-derived mesenchymal stem cells are immunogenic in an allogeneic host and stimulate donor graft rejection in a nonmyeloablative setting. Blood. 2006;108:2114–2120. doi: 10.1182/blood-2005-11-011650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kusuma G., Li A., Zhu D., McDonald H., Chambers D., Frith J., Lim R. Engineering mesenchymal stem cell paracrine activity with 3D culture. Cytotherapy. 2020;22:S51. doi: 10.1016/j.jcyt.2020.03.064. [DOI] [Google Scholar]
- 75.Yin K., Wang S., Zhao R.C. Exosomes from mesenchymal stem/stromal cells: A new therapeutic paradigm. Biomark. Res. 2019;7:1–8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen P., Zheng L., Wang Y., Tao M., Xie Z., Xia C., Gu C., Chen J., Qiu P., Mei S., et al. Desktop-stereolithography 3D printing of a radially oriented extracellular matrix/mesenchymal stem cell exosome bioink for osteochondral defect regeneration. Theranostics. 2019;9:2439–2459. doi: 10.7150/thno.31017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Leuning D.G., Beijer N., du Fossé N., Vermeulen S., Lievers E., Van Kooten C., Rabelink T., De Boer J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018;8:7716. doi: 10.1038/s41598-018-25700-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Eom Y.W., Oh J.-E., Lee J.I., Baik S.K., Rhee K.-J., Shin H.C., Kim Y.M., Ahn C.M., Kong J.H., Kim H.S., et al. The role of growth factors in maintenance of stemness in bone marrow-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2014;445:16–22. doi: 10.1016/j.bbrc.2014.01.084. [DOI] [PubMed] [Google Scholar]
- 79.Holladay C., Power K., Sefton M., O’Brien T., Gallagher W., Pandit A. Functionalized Scaffold-mediated Interleukin 10 Gene Delivery Significantly Improves Survival Rates of Stem Cells In Vivo. Mol. Ther. 2011;19:969–978. doi: 10.1038/mt.2010.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee S.J. Cytokine delivery and tissue engineering. Yonsei Med. J. 2000;41:704–719. doi: 10.3349/ymj.2000.41.6.704. [DOI] [PubMed] [Google Scholar]
- 81.Sridhar B.V., Doyle N.R., Randolph M.A., Anseth K.S. Covalently tethered TGF- β 1 with encapsulated chondrocytes in a PEG hydrogel system enhances extracellular matrix production. J. Biomed. Mater. Res. Part A. 2014;102:4464–4472. doi: 10.1002/jbm.a.35115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cavalli E., Levinson C., Hertl M., Broguiere N., Brück O., Mustjoki S., Gerstenberg A., Weber D., Salzmann G., Steinwachs M., et al. Characterization of polydactyly chondrocytes and their use in cartilage engineering. Sci. Rep. 2019;9:4275. doi: 10.1038/s41598-019-40575-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhou T., Li X., Li G., Tian T., Lin S., Shi S., Liao J., Cai X., Lin Y. Injectable and thermosensitive TGF-β1-loaded PCEC hydrogel system for in vivo cartilage repair. Sci. Rep. 2017;7:10553. doi: 10.1038/s41598-017-11322-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bian L., Zhai D.Y., Tous E., Rai R., Mauck R.L., Burdick J.A. Enhanced MSC chondrogenesis following delivery of TGF-β3 from alginate microspheres within hyaluronic acid hydrogels in vitro and in vivo. Biomaterials. 2011;32:6425–6434. doi: 10.1016/j.biomaterials.2011.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo X., Park H., Young S., Kretlow J.D., Beucken J.J.V.D., Baggett L.S., Tabata Y., Kasper F.K., Mikos A.G., Jansen J.A. Repair of osteochondral defects with biodegradable hydrogel composites encapsulating marrow mesenchymal stem cells in a rabbit model. Acta Biomater. 2010;6:39–47. doi: 10.1016/j.actbio.2009.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Elisseeff J., McIntosh W., Fu K., Blunk T., Langer R. Controlled-release of IGF-I and TGF-β1 in a photopolymerizing hydrogel for cartilage tissue engineering. J. Orthop. Res. 2001;19:1098–1104. doi: 10.1016/S0736-0266(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 87.Wei P., Xu Y., Gu Y., Yao Q., Li J., Wang L. IGF-1-releasing PLGA nanoparticles modified 3D printed PCL scaffolds for cartilage tissue engineering. Drug Deliv. 2020;27:1106–1114. doi: 10.1080/10717544.2020.1797239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boyd D.F., Thomas P.G. Towards integrating extracellular matrix and immunological pathways. Cytokine. 2017;98:79–86. doi: 10.1016/j.cyto.2017.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yan H.-C., Yu T.-T., Li J., Qiao Y.-Q., Wang L.-C., Zhang T., Li Q., Zhou Y.-H., Liu D.-W. The Delivery of Extracellular Vesicles Loaded in Biomaterial Scaffolds for Bone Regeneration. Front. Bioeng. Biotechnol. 2020;8:1015. doi: 10.3389/fbioe.2020.01015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kang M., Lee C.-S., Lee M. Bioactive Scaffolds Integrated with Liposomal or Extracellular Vesicles for Bone Regeneration. Bioengineering. 2021;8:137. doi: 10.3390/bioengineering8100137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu Y., Ma Y., Zhang J., Yuan Y., Wang J. Exosomes: A Novel Therapeutic Agent for Cartilage and Bone Tissue Regeneration. Dose-Response. 2019;17:1559325819892702. doi: 10.1177/1559325819892702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Huleihel L., Hussey G.S., Naranjo J.D., Zhang L., Dziki J.L., Turner N.J., Stolz D.B., Badylak S.F. Matrix-bound nanovesicles within ECM bioscaffolds. Sci. Adv. 2016;2:e1600502. doi: 10.1126/sciadv.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Padmanabhan J., Kyriakides T.R. Nanomaterials, Inflammation, and Tissue Engineering. WIREs Nanomed. Nanobiotechnol. 2015;7:355–370. doi: 10.1002/wnan.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Jiang B., Liao R. The Paradoxical Role of Inflammation in Cardiac Repair and Regeneration. J. Cardiovasc. Transl. Res. 2010;3:410–416. doi: 10.1007/s12265-010-9193-7. [DOI] [PubMed] [Google Scholar]
- 95.Crupi A., Costa A., Tarnok A., Melzer S., Teodori L. Inflammation in tissue engineering: The Janus between engraftment and rejection. Eur. J. Immunol. 2015;45:3222–3236. doi: 10.1002/eji.201545818. [DOI] [PubMed] [Google Scholar]
- 96.Vasconcelos D.P., Águas A.P., Barbosa M.A., Pelegrín P., Barbosa J.N. The inflammasome in host response to biomaterials: Bridging inflammation and tissue regeneration. Acta Biomater. 2019;83:1–12. doi: 10.1016/j.actbio.2018.09.056. [DOI] [PubMed] [Google Scholar]
- 97.Fierabracci A., Del Fattore A., Muraca M., Delfino D.V., Muraca M. The Use of Mesenchymal Stem Cells for the Treatment of Autoimmunity: From Animals Models to Human Disease. Curr. Drug Targets. 2016;17:229–238. doi: 10.2174/1389450116666150722140633. [DOI] [PubMed] [Google Scholar]
- 98.Levy O., Kuai R., Siren E.M.J., Bhere D., Milton Y., Nissar N., De Biasio M., Heinelt M., Reeve B., Abdi R., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020;6:eaba6884. doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xu J., Chen J., Li W., Lian W., Huang J., Lai B., Li L., Huang Z. Additive Therapeutic Effects of Mesenchymal Stem Cells and IL-37 for Systemic Lupus Erythematosus. J. Am. Soc. Nephrol. 2020;31:54–65. doi: 10.1681/ASN.2019050545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y., Pizzute T., Pei M. Anti-Inflammatory Strategies in Cartilage Repair. Tissue Eng. Part B Rev. 2014;20:655–668. doi: 10.1089/ten.teb.2014.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oprenyeszk F., Chausson M., Maquet V., Dubuc J.-E., Henrotin Y. Protective effect of a new biomaterial against the development of experimental osteoarthritis lesions in rabbit: A pilot study evaluating the intra-articular injection of alginate-chitosan beads dispersed in an hydrogel. Osteoarthr. Cartil. 2013;21:1099–1107. doi: 10.1016/j.joca.2013.04.017. [DOI] [PubMed] [Google Scholar]
- 102.Chen S., Chen W., Chen Y., Mo X., Fan C. Chondroitin sulfate modified 3D porous electrospun nanofiber scaffolds promote cartilage regeneration. Mater. Sci. Eng. C. 2021;118:111312. doi: 10.1016/j.msec.2020.111312. [DOI] [PubMed] [Google Scholar]
- 103.Wang W., Sun L., Zhang P., Song J., Liu W. An anti-inflammatory cell-free collagen/resveratrol scaffold for repairing osteochondral defects in rabbits. Acta Biomater. 2014;10:4983–4995. doi: 10.1016/j.actbio.2014.08.022. [DOI] [PubMed] [Google Scholar]
- 104.Li Y., Chen M., Yan J., Zhou W., Gao S., Liu S., Li Q., Zheng Y., Cheng Y., Guo Q. Tannic acid/Sr2+-coated silk/graphene oxide-based meniscus scaffold with anti-inflammatory and anti-ROS functions for cartilage protection and delaying osteoarthritis. Acta Biomater. 2021;126:119–131. doi: 10.1016/j.actbio.2021.02.046. [DOI] [PubMed] [Google Scholar]
- 105.Dong X., Li C., Zhang M., Zhao Y., Zhao Z., Li W., Zhang X. Multifunctional injectable hydrogel for effective promotion of cartilage regeneration and protection against osteoarthritis: Combined chondroinductive, antioxidative and anti-inflammatory strategy. Sci. Technol. Adv. Mater. 2022;23:361–375. doi: 10.1080/14686996.2022.2076568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Maihöfer J., Madry H., Rey-Rico A., Venkatesan J.K., Goebel L., Schmitt G., Speicher-Mentges S., Cai X., Meng W., Zurakowski D., et al. Hydrogel-Guided, rAAV-Mediated IGF-I Overexpression Enables Long-Term Cartilage Repair and Protection against Perifocal Osteoarthritis in a Large-Animal Full-Thickness Chondral Defect Model at One Year In Vivo. Adv. Mater. 2021;33:2008451. doi: 10.1002/adma.202008451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jia X., Ma J., Chen X., Li W., Zhou X., Lei B., Zhao X., Mao Y. Immunoregulation and anti-metalloproteinase bioactive injectable polysalicylate matrixgel for efficiently treating osteoarthritis. Mater. Today Bio. 2022;15:100277. doi: 10.1016/j.mtbio.2022.100277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ansari M.Y., Ahmad N., Haqqi T.M. Oxidative stress and inflammation in osteoarthritis pathogenesis: Role of polyphenols. Biomed. Pharmacother. 2020;129:110452. doi: 10.1016/j.biopha.2020.110452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sayed D., Abdellatif M. MicroRNAs in Development and Disease. Physiol. Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 110.Lu L., Wang J., Fan A., Wang P., Chen R., Lu L., Yin F. Synovial mesenchymal stem cell-derived extracellular vesicles containing microRN555A-26a-5p ameliorate cartilage damage of osteoarthritis. J. Gene Med. 2021;23:e3379. doi: 10.1002/jgm.3379. [DOI] [PubMed] [Google Scholar]
- 111.Jin Z., Ren J., Qi S. Human bone mesenchymal stem cells-derived exosomes overexpressing microRNA-26a-5p alleviate osteoarthritis via down-regulation of PTGS2. Int. Immunopharmacol. 2020;78:105946. doi: 10.1016/j.intimp.2019.105946. [DOI] [PubMed] [Google Scholar]
- 112.Tao Y., Zhou J., Wang Z., Tao H., Bai J., Ge G., Li W., Zhang W., Hao Y., Yang X., et al. Human bone mesenchymal stem cells-derived exosomal miRNA-361-5p alleviates osteoarthritis by downregulating DDX20 and inactivating the NF-κB signaling pathway. Bioorganic Chem. 2021;113:104978. doi: 10.1016/j.bioorg.2021.104978. [DOI] [PubMed] [Google Scholar]
- 113.Wang K., Li F., Yuan Y., Shan L., Cui Y., Qu J., Lian F. Synovial Mesenchymal Stem Cell-Derived EV-Packaged miR-31 Downregulates Histone Demethylase KDM2A to Prevent Knee Osteoarthritis. Mol. Ther. Nucleic Acids. 2020;22:1078–1091. doi: 10.1016/j.omtn.2020.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhou Y., Ming J., Li Y., Li B., Deng M., Ma Y., Chen Z., Zhang Y., Li J., Liu S. Exosomes derived from miR-126-3p-overexpressing synovial fibroblasts suppress chondrocyte inflammation and cartilage degradation in a rat model of osteoarthritis. Cell Death Discov. 2021;7:37. doi: 10.1038/s41420-021-00418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lai C., Liao B., Peng S., Fang P., Bao N., Zhang L. Synovial fibroblast-miR-214-3p-derived exosomes inhibit inflammation and degeneration of cartilage tissues of osteoarthritis rats. Mol. Cell. Biochem. 2022;1:1–13. doi: 10.1007/s11010-022-04535-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ye P., Mi Z., Wei D., Gao P., Ma M., Yang H. miR-3960 from Mesenchymal Stem Cell-Derived Extracellular Vesicles Inactivates SDC1/Wnt/β-Catenin Axis to Relieve Chondrocyte Injury in Osteoarthritis by Targeting PHLDA2. Stem Cells Int. 2022;2022:9455152. doi: 10.1155/2022/9455152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhao Y., Zhang Z., Pan Z., Liu Y. Advanced bioactive nanomaterials for biomedical applications. Exploration. 2021;1:20210089. doi: 10.1002/EXP.20210089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma J., Wu C. Bioactive inorganic particles-based biomaterials for skin tissue engineering. Exploration. 2022 doi: 10.1002/EXP.20210083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhou J., Zhang Z., Joseph J., Zhang X., Ferdows B.E., Patel D.N., Chen W., Banfi G., Molinaro R., Cosco D., et al. Biomaterials and nanomedicine for bone regeneration: Progress and future prospects. Exploration. 2021;1:20210011. doi: 10.1002/EXP.20210011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mijanović O., Pylaev T., Nikitkina A., Artyukhova M., Branković A., Peshkova M., Bikmulina P., Turk B., Bolevich S., Avetisov S., et al. Tissue Engineering Meets Nanotechnology: Molecular Mechanism Modulations in Cornea Regeneration. Micromachines. 2021;12:1336. doi: 10.3390/mi12111336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhao Y., Wei C., Chen X., Liu J., Yu Q., Liu Y., Liu J. Drug Delivery System Based on Near-Infrared Light-Responsive Molybdenum Disulfide Nanosheets Controls the High-Efficiency Release of Dexamethasone To Inhibit Inflammation and Treat Osteoarthritis. ACS Appl. Mater. Interfaces. 2019;11:11587–11601. doi: 10.1021/acsami.8b20372. [DOI] [PubMed] [Google Scholar]
- 122.Kang M.-L., Kim J.-E., Im G.-I. Thermoresponsive nanospheres with independent dual drug release profiles for the treatment of osteoarthritis. Acta Biomater. 2016;39:65–78. doi: 10.1016/j.actbio.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 123.Jung J.H., Kim S.E., Kim H.-J., Park K., Song G.G., Choi S.J. A comparative pilot study of oral diacerein and locally treated diacerein-loaded nanoparticles in a model of osteoarthritis. Int. J. Pharm. 2020;581:119249. doi: 10.1016/j.ijpharm.2020.119249. [DOI] [PubMed] [Google Scholar]
- 124.Chen L., Tiwari S.R., Zhang Y., Zhang J., Sun Y. Facile Synthesis of Hollow MnO2 Nanoparticles for Reactive Oxygen Species Scavenging in Osteoarthritis. ACS Biomater. Sci. Eng. 2021;7:1686–1692. doi: 10.1021/acsbiomaterials.1c00005. [DOI] [PubMed] [Google Scholar]
- 125.Pei Y., Cui F., Du X., Shang G., Xiao W., Yang X., Cui Q. Antioxidative nanofullerol inhibits macrophage activation and development of osteoarthritis in rats. Int. J. Nanomed. 2019;14:4145–4155. doi: 10.2147/IJN.S202466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pi Y., Zhang X., Shao Z., Zhao F., Hu X., Ao Y. Intra-articular delivery of anti-Hif-2α siRNA by chondrocyte-homing nanoparticles to prevent cartilage degeneration in arthritic mice. Gene Ther. 2015;22:439–448. doi: 10.1038/gt.2015.16. [DOI] [PubMed] [Google Scholar]
- 127.Kim S.E., Choi S.J., Park K., Kim H.-J., Song G.G., Jung J.H. Intra-Articular Injection of Rebamipide-Loaded Nanoparticles Attenuate Disease Progression and Joint Destruction in Osteoarthritis Rat Model: A Pilot Study. Cartilage. 2022;13:19476035211069250. doi: 10.1177/19476035211069250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kou L., Huang H., Tang Y., Sun M., Li Y., Wu J., Zheng S., Zhao X., Chen D., Luo Z., et al. Opsonized nanoparticles target and regulate macrophage polarization for osteoarthritis therapy: A trapping strategy. J. Control. Release. 2022;347:237–255. doi: 10.1016/j.jconrel.2022.04.037. [DOI] [PubMed] [Google Scholar]
- 129.Dravid A.A., Dhanabalan K.M., Agarwal S., Agarwal R. Resolvin D1 -loaded nanoliposomes promote M2 macrophage polarization and are effective in the treatment of osteoarthritis. Bioeng. Transl. Med. 2022;7:e10281. doi: 10.1002/btm2.10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hussey G.S., Molina C.P., Cramer M.C., Tyurina Y.Y., Tyurin V.A., Lee Y.C., El-Mossier S.O., Murdock M.H., Timashev P.S., Kagan V.E., et al. Lipidomics and RNA sequencing reveal a novel subpopulation of nanovesicle within extracellular matrix biomaterials. Sci. Adv. 2020;6:eaay4361. doi: 10.1126/sciadv.aay4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Romay M.C., Che N., Becker S.N., Pouldar D., Hagopian R., Xiao X., Lusis A.J., Berliner J.A., Civelek M. Regulation of NF-κB signaling by oxidized glycerophospholipid and IL-1β induced miRs-21-3p and -27a-5p in human aortic endothelial cells. J. Lipid Res. 2015;56:38–50. doi: 10.1194/jlr.M052670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Crum R.J., Hall K., Molina C.P., Hussey G.S., Graham E., Li H., Badylak S.F. Immunomodulatory matrix-bound nanovesicles mitigate acute and chronic pristane-induced rheumatoid arthritis. npj Regen. Med. 2022;7:13. doi: 10.1038/s41536-022-00208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seol D., Yu Y., Choe H., Jang K., Brouillette M.J., Zheng H., Lim T.-H., Buckwalter J.A., Martin J.A. Effect of Short-Term Enzymatic Treatment on Cell Migration and Cartilage Regeneration: In Vitro Organ Culture of Bovine Articular Cartilage. Tissue Eng. Part A. 2014;20:1807–1814. doi: 10.1089/ten.tea.2013.0444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Qu F., Lin J.-M.G., Esterhai J.L., Fisher M.B., Mauck R.L. Biomaterial-mediated delivery of degradative enzymes to improve meniscus integration and repair. Acta Biomater. 2013;9:6393–6402. doi: 10.1016/j.actbio.2013.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bravenboer J.V.D.B., Der Maur C.D.I., Bos P.K., Feenstra L., Verhaar J.A.N., Weinans H., Van Osch G.J.V.M. Improved cartilage integration and interfacial strength after enzymatic treatment in a cartilage transplantation model. Arthritis Res. Ther. 2004;6:R469–R476. doi: 10.1186/ar1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.