Abstract
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)-induced disease (COVID-19) and Gaucher disease (GD) exhibit upregulation of complement 5a (C5a) and its C5aR1 receptor, and excess synthesis of glycosphingolipids that lead to increased infiltration and activation of innate and adaptive immune cells, resulting in massive generation of pro-inflammatory cytokines, chemokines and growth factors. This C5a–C5aR1–glycosphingolipid pathway- induced pro-inflammatory environment causes the tissue damage in COVID-19 and GD. Strikingly, pharmaceutically targeting the C5a–C5aR1 axis or the glycosphingolipid synthesis pathway led to a reduction in glycosphingolipid synthesis and innate and adaptive immune inflammation, and protection from the tissue destruction in both COVID-19 and GD. These results reveal a common involvement of the complement and glycosphingolipid systems driving immune inflammation and tissue damage in COVID-19 and GD, respectively. It is therefore expected that combined targeting of the complement and sphingolipid pathways could ameliorate the tissue destruction, organ failure, and death in patients at high-risk of developing severe cases of COVID-19.
Keywords: lipid, viral infection, rare-genetic disease, innate and adaptive immunity, inflammation
1. Introduction
Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2)-induced Disease (COVID-19) displays complement activation products (Table 1) and excess formation of sphingolipids [1,2,3,4]. Additionally, SARS-CoV-2 triggers infiltration and activation of several classes of innate and adaptive immune cells, as well as the abnormal production of pro-inflammatory cytokines, chemokines, and growth factors in COVID-19 (Table 1 and Table 2). Such SARS-CoV-2-induced immune inflammation affects multiple organs (i.e., lung, liver, spleen, cardiovascular system, and brain) and causes the development of moderate (e.g., high fever, shortness of breath, loss of taste and/or smell, sore throat, nausea, and vomiting) to severe (e.g., pneumonia, bronchitis, respiratory failure, lung damage and death) symptoms of COVID-19 [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23].
Table 1.
Immune cells and their effector inflammatory mediators in COVID-19.
| Coronavirus | Immune Cell Involvement |
Source | Changes in Complement Products, Cytokines and Chemokines |
References |
|---|---|---|---|---|
| SARS-CoV-2 | Leucocytes, PMNs PCs Endothelial cells |
Blood Sera Lungs |
C5a +++ C5aR1 +++ MAC +++ |
[24,25,26,27,28] |
| SARS-CoV-2 | Type-II pneumocytes Pulmonary cells Platelets |
Blood Sera Lungs |
C1q P+++ C3b-regulatory factor H (FH) P+++ C3 P+++ |
[26,29,30,31] |
| SARS-CoV-2 | Type-II pneumocytes PBMCs | Blood Sera Lung |
C3a P+++ C3aR P +++ C3b-CD46 P+++ |
[26,31,32,33,34] |
| SARS-CoV-2 | PBMCs | Blood Sera |
sC5b-9 P+++ | [27,35,36,37] |
| SARS-2 | Respiratory specimen cells Alveolar cells |
Blood Sera Lung |
C4d P+++ | [35,38] |
| SARS-CoV-2 | Respiratory specimen cells |
Blood Sera |
C3bBbP P+++ | [35] |
| SARS-CoV-2 | Respiratory specimen cells |
Blood Sera |
C3bc P+++ | [35] |
| SARS-CoV-2 | PBMCs Glomeruli Cardiac Microthrombi and Alveolar cells |
Blood Sera Lung Heart Kidney |
C5b-C9 P+++ | [36,37,38,39,40] |
| SARS-CoV-2 | Pulmonary cells | Lung | C1r P+++ | [29] |
| SARS-CoV-1 | Pulmonary cells | Lung | iC3b P+++ | [31] |
| SARS-CoV-1 | Pulmonary cells | Lung | C3c P++ | [31] |
| SARS-CoV-1 | Pulmonary cells | Lung | C3dg P+++ | [31] |
| MERS | MOs/Mɸs T cells mDCs pDCs PMNs |
Liver SPL Lung Kidney Heart Serum |
IL12 M+++ IL8 M+++ TNFα M+++ IL-6 M&P+++ IFNλ M+++ CXCL10 M&P+++ CCL2 M+++ CCL3 M+++ CCL5 M+++ IFNα M+ & P+++ IFNβ M+ & P+++ |
[41,42,43,44,45,46,47] |
| SARS-CoV-1 | Mos Mɸs DCs Cord blood cells |
Lung Blood |
IFNβ M+ IFNα M+ TNFα M+++ IFNλ M+++ IL8 M+++ TNFα M+++ IL6 M+++ CCL2 M+++ CCL3 M+++ CCL5 M+++ CXCL10 M+ |
[42,48,49,50] |
| SARS-CoV-2 | MOs Mɸs |
Blood | IL6 P+++ IL8 P+++ IL10 P+++ TNFα P+++ |
[51] |
| SARS-CoV-2 | CD8+ cells NK+ cells |
PBMCs | IL2 P+ TNFα P+ IFNγ P+++ Granzyme B P+++ |
[52,53] |
| SARS-CoV-2 | CD8+ cells CD4+ cells |
Liver Lung Heart |
Cytokines NR | [53] |
| SARS-CoV-2 | PMNs | Lung | Cytokines NR | [54] |
| MERS | CD4+ T cells CD8+ T cells |
Lymph Nodes Spleen Tonsils PBMCs |
Caspase-3 P+++ | [55] |
| SARS-CoV-1 | CD4+ cells CD8+ cells CD45RO+ and CD27+ cells |
PBMCs | IL2 P++ TNFα P++ IFNγ P+++ IL4 PNS CXCL10 PNS |
[56,57] |
| SARS-CoV-2 | CD4+ T cells CD8+ T cells Regulatory T cells |
PBMCs | CCR6 P+++ Perforin P+++ IL6 P+++ IL2 M+++ IL7 M+++ |
[51,58,59,60,61,62,63] |
| MERS | Epithelial cells | Lung | IL1β M+++ IL6 M+++ IL8 M+++ IFNα M+ CCL2 M+ CXCL10 M+ |
[64,65] |
| SARS-CoV-1 | Epithelial cells | Lung | TNFα M+++ IFNβ M+++ CXCL10 M+++ |
[64] |
COVID-19 (Coronavirus disease 2019), MERS-CoV (Middle East Respiratory Syndrome Coronavirus), SARS-CoV-1 (Severe Acute Respiratory Syndrome Coronavirus-1), SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2), AECs (Airway epithelial cells), Mɸs (Macrophages), DCs (Dendritic cells), PMNs (Polymorphonuclear cells), NK (Natural Killer cells), IFN (interferon), α (alpha), β (beta), γ(gamma), λ (lambda), IL (interleukin), TNF (tumor necrosis factor), CCL (Chemokine (C-C motif) ligand), CXCL (Chemokine (C-X-C motif) ligand), PBMCs (Peripheral Blood Mononuclear Cells), PCs (Pulmonary Cells), MAC (Membrane Attack Complex; MAC), C3a (Complement 3a), C3b (Complement 3b), C3aR (Complement 3a Receptor), C5a (Complement 5a), C5aR1 (C5a Receptor 1), sC5b-9 (soluble C5b-9; MAC); C4d (Complement 4d). C3bBbP (complement 3 b bound protease fragment), C3bc (Complement 3 bc), P (Protein expression level), M (mRNA expression level) + (low), ++ (moderate), and +++ (high), NS (not significant).
Increased levels of complement activation products have been linked to innate and adaptive immune cell activation and increased production of pro-inflammatory cytokines, chemokines, and growth factors in GD (Table 3 and Table 4). The excess tissue and cellular accumulation of glucosylceramides (GCs) and their subsequent roles in the induction of innate and adaptive immune inflammation significantly affect visceral organs (e.g., liver, spleen, lung, bone, and kidney) and the central nervous system (CNS), causing the development of GD manifestations characterized by anemia, thrombocytopenia, hypergammaglobulinemia, splenomegaly, hepatomegaly, respiratory distress, skeletal weakness, loss of neurons, and death [66,67,68,69,70,71,72,73,74,75,76].
Clinical evidence together with laboratory investigations suggest a common involvement of the C5–C5a–C5aR1 and glycosphingolipids (GSLs) pathways for activating innate and adaptive immune cells, including monocytes (MOs), macrophages (Mɸs) dendritic cells (DCs), polymorphonuclear cells (PMNs), and CD4+ T cells. Similarly, the abnormal production of pro-inflammatory cytokines was identified, including interferon-alpha (IFNα), IFN-gamma (IFNγ), tumor necrosis factor-alpha (TNFα), interleukin-1 (IL1), IL2, IL6, IL7, IL8, IL12, IL17), CCL (C-C motif ligand chemokines, e.g., CCL2, CCL3, and CCL5), CXCL (C-X-C motif ligand chemokines, e.g., CXCL9 and CXCL10), and growth factors (e.g., transforming growth factor-beta, TGFβ), granulocyte colony stimulating factor (GCSF), granulocyte-Mɸ colony stimulating factor (GMCSF) in COVID-19 and GD (Table 1, Table 2, Table 3 and Table 4). Such immune abnormalities affect multiple tissues and lead to the development of organ failure and/or death in both COVID-19 and GD patients [6,7,8,9,10,11,12,13,14,71,72,74,75,76,77].
Several vaccines and alternative drugs have been applied and/or proposed for treating COVID-19 and its complications (Table 5). Some of these treatments have been used off-label, though their efficacy is still debated. Studies have shown that pharmaceutical targeting of complement at the level of the C5–C5a–C5aR1 axis or at glucosylceramide synthase (GCS) inhibited the replication of SARS-CoV-2, and the SARS-CoV-2 or GC-induced immune inflammation and tissue destruction in COVID-19 and GD [78,79,80,81,82]. The study therefore provided updated information regarding the involvement of the complement-sphingolipid axis and the impact in propagating the immune inflammation and disease process of COVID-19 and GD. This information could be helpful to understand the disease mechanism of COVID-19 and to aid in the development of additional potential therapies to slow or reduce disease severity and death in COVID-19 patients.
1.1. COVID-19
COVID-19 is caused by infection with SARS-CoV-2, a member of the family of betacoronaviruses that also includes the SARS-CoV-1 and Middle East Respiratory Syndrome-CoV (MERS-CoV) [83,84]. SARS-CoV-2 is a large, enveloped, single-stranded positive-sense RNA virus with a genome size of about 30 kb. The 5′ end of the SARS-CoV-2 genome encodes two polyproteins termed PP1a and PP1ab, which are mutually called replicases. These polyproteins are classified with 16 non-structural proteins, including RNA-dependent RNA polymerase (RDRP), 3-chymotrypsin-like protease (3CLP), and papain-like protease (PLP). The 3′ end of the SARS-CoV-2 genome encodes four essential structural proteins: spike (S; essential for the viral entry into host cells), envelope (E; responsible for viral membrane twist and binding to the nucleocapsid), membrane (M; bind to the viral RNA genome and guarantee the conservation of the RNA in the shape of the beads-on-a-string) and the nucleocapsid (N; required for viral replication and pathogenesis of the disease). The 3′ end also encodes non-structural proteins, including PLP, 3CLP, RDRP, helicase, and the collection of accessory proteins, which affect the host-specific immune reactions [85,86,87]. The S protein is critical for the infectivity of SARS-CoV-2 and is cut by a host protease into the S1 and S2 subunits. The S1 subunit binds to angiotensin converting enzyme-2 (ACE2), which acts as a protease to cleave angiotensin II and also counteracts the effect of angiotensin II [88,89]. ACE2 is the cellular receptor for SARS-CoV-2 and is widely expressed in blood vessels, tongue, lung, adipose tissue, adrenal gland, heart, esophagus, lung, muscle, ovary [22,90,91,92] and eye tissues (i.e., conjunctiva, choroid, vascular endothelium, and nerves) [93,94,95,96,97].
The S2 subunit is activated by transmembrane protease serine-2 (TMPRSS2) associated with the host surface. These combined actions result in host-viral membrane fusion and SARS-CoV-2 entry into the host cells [98,99]. The viral RNA genome is released into the host cell cytoplasm, where it first uses the host translational machinery for the formation of the viral structural and accessory proteins [85,99]. The newly synthesized structural and accessory proteins are transferred through the endoplasmic reticulum and Golgi bodies followed by assembly of new virions in the growing Golgi vesicles [87]. Finally, similar to the MERS-CoV and SARS-CoV-1, the mature SARS-CoV-2 virions are exocytosed from the host cell into the surrounding environment to repeat the infection cycle. Infection results in activation of innate and adaptive immune cells and a massive generation of several pro-inflammatory mediators (Table 1 and Table 2) that instigate moderate to severe disease symptoms of COVID-19 [14,15,16,17,18,19,20,21,22,23]. The SARS-CoV-2-induced development of COVID-19 disease has been reported in people with immunocompromised and morbid conditions, such as sepsis, acute cardiac injury, heart failure, and multi-organ (e.g., liver, spleen, kidney, and brain) disease [100]. Epidemiological studies related to COVID-19 have found that males are slightly more prone to infection as compared to females, but both sexes experience severe forms of COVID-19 and death. Persons > 60 years old or with chronic diseases, such as type 2 diabetes and essential hypertension, were at higher risk for SARS-CoV-2-induced systemic inflammation leading to a severe form of COVID-19 [101,102,103].
Table 2.
Coronavirus-induced production of circulatory cytokines.
| Coronavirus | Source | Cytokines | Chemokines | Growth Factors | References |
|---|---|---|---|---|---|
| MERS | Sera | IFNα P+++ IL6 P+++ IL8 P+++ |
CCL5 P+++ CXCL10 P+++ |
[45,47,104] | |
| SARS-CoV-1 | Sera | IFNα P+++ IFNγ P+++ IL1 P+++ IL2 P+++ IL6 P+++ IL8 P+++ IL10 P+ IL12 P+++ |
CCL2 P+++ CXCL9 P+++ CXCL10 P+++ |
TGFβ P+++ | [104,105,106,107,108,109,110,111,112,113,114,115] |
| SARS-CoV-2 | Sera | IFNγ P+++ TNFα P+++ IL1b P+++ IL1RA P+++ IL2 P++ IL2R P++ IL4 P++ IL5 P++ IL6 P+++ IL7 P+++ IL8 P+++ IL9 P+++ IL10 NS IL12 P+++ |
CCL2 P+ CCL3 P+ CXCL10 P+ |
G-CSF P+++ GMCSF P+++ VEGF P+++ FGF P+++ PDGF P+++ |
[57,59,62,104,116,117,118,119,120] |
| SARS-CoV-2 | Sera | CCL2 P+++ | [115,120] | ||
| SARS-CoV-2 | Sera | CCL3 NS | [115] | ||
| SARS-CoV-2 | Sera | CXCL8 P+++ | [120] | ||
| SARS-CoV-2 | Sera | CXCL10 P++ | [115,120] |
MERS-CoV (Middle East Respiratory Syndrome Coronavirus), SARS-CoV-1 (Severe Acute Respiratory Syndrome Coronavirus-1), SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2), IFN (interferon), IL (interleukin), TNF (tumor necrosis factor), CCL (C-C motif ligand), CXCL (C-X-C motif ligand), TGF (transforming growth factor), GCSF (granulocyte colony stimulating factor), GMCSF (granulocyte-Mɸ colony stimulating factor), VEGF (vascular endothelial cell growth factor), FGF (fibroblast growth factor), PDGF (platelet-derived growth factor), α (alpha), β (beta), γ (gamma), λ (lambda), P (Protein expression level), M (mRNA expression level): + (low), ++ (moderate), and +++ (high), NS (not significant).
Table 3.
Immune cells involvement in GD.
| Mouse Model of GD | GD Patients | |||
|---|---|---|---|---|
| Immune Cells | Tissue Recruitment | References | Immune Cells | References |
| MOs | Blood +++ | [121] | Blood - | [122,123] |
| Mɸs | Blood +++, Liver +++, Spleen +++, Lung +++ |
[66,121,124,125] | Lymph node +++ | [126] |
| mDCs | Blood +++, Liver +++, Spleen +++, Lung +++ |
[66,121,124,125] | Blood - | [122,123,127,128] |
| pDCs | Blood - | [122,123,127] | ||
| PMNs | Blood +++, Liver +++, Spleen +++, Bone Marrow +++ |
[66,121] | ||
| CD4 + TCells | Liver +++, Spleen +++, Lung +++ | [66,124,125] | Blood +++ | [127,128] |
| CD8 + T Cells | Thymus +++, Spleen +++ |
[124,125] | Blood +++ | [128,129] |
| NK Cells | Blood - | [127,129] | ||
MOs (Monocytes), Mɸs (Macrophages), mDCs (myeloid dendritic cells), pDCs (plasmacytoid dendritic cells), PMNs (Polymorphonuclear cells), NK (Natural killer cells), Increased (+++) and decreased (-) tissue recruitment.
1.2. COVID-19 Vaccines and Alternative Treatments
Several vaccines have been developed against multiple strains of SARS-CoV-2 infection, including mRNA vaccines, viral vector vaccines, inactivated vaccines, and protein-based vaccines [130] (Table 5). mRNA vaccines have shown an efficacy ranging from 48 to >90% at 5–6 months’ follow-up post second vaccine dose [130]. Viral vector vaccines had an efficacy ranging from 65 to 91.6% (Sputnik), around 70% (in Brazil), or between 10 and 64% (South Africa), according to the different vaccine types and countries in which they were used [130]. Finally, inactivated and subunit vaccines, showed an efficacy ranging from 50 to 91.6%, again based on the different subtypes and the country in which they were employed [130]. SARS-CoV-2 continuously develops mutations (variants) and will continue to do so because relatively few people globally have been vaccinated [131]. These findings suggest that the variability in vaccine efficacy is linked to the dominant variant circulating in the country at the time.
Despite the high efficacy of different vaccines, multiple studies have shown the phenomenon of immunity waning occurring approximately 6 months after vaccine administration [132]. In addition, all of the vaccines can be associated with severe adverse events, namely severe anaphylaxis, myocarditis/pericarditis, lymphadenopathy, appendicitis, herpes-zoster infection, viral facial palsy, transverse myelitis, Guillain-Barré syndrome, encephalopathy, thromboembolism, and thrombosis-thrombocytopenia syndrome [130]. These severe adverse events are rare, and may vary significantly according to specific features of the target population such as age. For example, myocarditis is more frequently reported in males under the age of 30 when compared to other population categories [133].
Several drugs, mainly belonging to the classes of antivirals, antibiotics, immune-modulators, immune-suppressants, or anti-inflammatory molecules, have also been proposed for treating COVID-19 and its complications (Table 5). Some of these drugs have been used off-label and their efficacy is still debated. Lopinavir and ritonavir are two protease inhibitors used for the treatment of human immunodeficiency virus (HIV)-induced acquired immunodeficiency syndrome (AIDS) [134,135]. Their use in combination has been proposed for COVID-19 [135,136]. These drugs may also prevent the secondary immune burst without causing severe adverse events at early stages of COVID-19 and are therefore not recommended for COVID-19 at severe stages of the disease [137]. Among several repurposed drugs currently used in patients with COVID-19, the nucleoside analogs are a preferred class [138], with two drugs in particular: (1) the nucleotide precursor favipiravir, and (2) the nucleoside analogue remdesivir, which inhibit the RDRP [139,140]. A recent systematic review of the literature has shown that favipiravir may significantly improve the clinical condition of COVID-19 patients, although the results suggested no significant differences in the length of hospitalization and clinical recovery [138]. A combination of favipiravir with other therapies (e.g., tocilizumab) has shown more promising results in improving a patients’ clinical status [138]. Possible safety concerns for favipiravir include increased blood uric acid and teratogenicity, as the medication is secreted in semen and breast milk and has demonstrated toxicity in animal studies [141]. Remdesivir may help improve recovery and the clinical outcomes of hospitalized COVID-19 patients, although its effects in reducing mortality are still uncertain [142,143]. It should be administered in a hospital setting. Unfortunately, data on its possible toxicity are sparse [138,144].
Several immune-modulating agents have been proposed for treating COVID-19 with the aim of reducing SARS-CoV-2–induced cytokine signalling and increased inflammation (Table 5). Among the specific immune-modulator drugs, the most important are IFNγ, GMCSF, IL1 receptor antagonists, IL-6 receptor antagonists, Janus kinase (JAK) inhibitors, and monoclonal antibodies to TNFα and GMCSF [145,146,147,148,149,150]. Among the non-specific immune modulator drugs, the most important are intravenous immunoglobulins (IVIGs), corticosteroids, and IFNβ-1b or α-2b that have antiviral and immunomodulating properties [149,151,152]. Finally, among immune-modulators with mixed effects, the most important are statins, renin-angiotensin-aldosterone system (RAAS) inhibitors, angiotensin-converting-enzyme (ACE) inhibitors, angiotensin-receptor blockers, and macrolides [153,154]. All these drugs may be associated with side effects of variable immune system suppression and may result in opportunistic infections.
1.3. SARS-CoV-2 Reinfection
Several cases of SARS-CoV-2 reinfection after vaccination/first infection have been reported in association with waning of the immune response. While reinfection with viruses associated with systemic infections is uncommon, reinfection with viruses causing mucosal infections without viremia, such as respiratory syncytial virus, influenza, and coronavirus, is much more common [155]. In unvaccinated patients infected by coronaviruses, the mean time to reinfection ranged from 30 to 55 months, and 14% of patients infected had multiple reinfections with the same seasonal coronavirus strain [156]. In a more recent systematic review of the literature on more than 615,000 participants, reinfection was found to be an uncommon event and occurred in 0–1.1% [157]. The data from that review suggested that overall, naturally acquired SARS-CoV-2 immunity does not wane for about 10 months post-infection [157].
1.4. Gaucher Disease
GD is a classic example of a lysosomal storage disease and has a worldwide incidence of approximately 1/40,000 to 1/60,000 [158]. GD is classified into Types 1, 2, and 3, which are all caused by pathogenic variants in GBA1 (in human)/Gba1 (in mouse) that lead to the functional disruption of the encoded lysosomal enzyme, acid β-glucosidase (β-D-glucosyl-N-acylsphingosine glucohydrolase, EC 4.2.1.25; GCase) and to excess tissue accumulation of glucosylceramides (GC) [23]. Type 1 GD mainly affects the liver, spleen, lung, bone, and kidney [77,159]. A significant proportion of patients with Type 1 GD also have CNS manifestations characterized by the mild brain inflammation [66,67,160,161,162]. Type 2 and Type 3 GD are the neuronopathic forms of GD (nGD), which are characterized by severe and chronic brain inflammation that leads to the loss of neurons and early death (e.g., <2 years in patients with Type 2 nGD and 10–40 years in patients with Type 3 nGD) [162,163,164,165,166,167,168,169,170,171,172,173,174,175]. The main neurological symptoms of Type 2 and 3 nGDs are characterized by selective degeneration of the cerebellar dentate nucleus and the dentato-rubro-thalamic pathway, generalized epilepsy and seizures, horizontal saccadic eye movements, ataxia, spasticity, oculomotor abnormalities, hypertonia of the neck muscles, extreme arching of the neck, bulbar signs, limb rigidity, occasional choreoathetoid movements, and progressive dementia [176,177,178,179,180,181,182,183,184,185,186,187,188,189]. Enzyme replacement therapy (e.g., imiglucerase, velaglucerase, or taliglucerase) and substrate reduction therapy (e.g., eliglustat and miglustat) are available to treat many of the visceral aspects of GD but have no impact on the CNS disease and are of limited benefit in the management of immune inflammation and disease complications in multiple organs such as the bones, lungs, and lymph nodes [70,190,191,192]. The development of alternative therapies, i.e., gene, substrate reduction, and enzyme replacement therapies, has been hampered by limitations in understanding disease pathogenesis, inability of therapies to fully cross the blood-brain barrier, and toxicity concerns due to procedural risks [159,193,194,195,196].
1.5. Complement Activation in COVID-19 and Gaucher Disease
The complement system comprises a group of liquid and cell membrane-associated proteins, which are mainly produced in the liver. However, certain brain cells, such as microglial cells, astrocytes, and neurons, are also involved in the direct synthesis of complement proteins [197,198,199]. Complement activation is a complex process, which largely occurs by the classical, alternative, and lectin pathways [200]. The classical pathway is activated by ligation of the IgG and/or IgM immune complexes (ICs) to their corresponding receptors and/or C1q on the cell surface. The alternative pathway is activated by binding of spontaneously activated C3 protein (C3b fragment) to host and non-host cell surfaces [201,202]. The lectin pathway is activated by the binding of the mannan-binding lectin (MBL) to mannose-containing carbohydrates or related ficolins to certain carbohydrates or acetylated structures [198,203,204]. Each of the complement activation pathways follows a series of reactions generating common key components known as C3 and C5 [205]. The downstream cleavage of C3 by the C3 convertases causes the formation of C3a and C3b [205]. Similarly, the downstream cleavage of C5 by the C5 convertases causes the formation of C5a and C5b [205]. C3a binds the C3aR receptor, and C5a binds C5aR1 and C5aR2 receptors [205]. C3b is a major opsonin that induces the tagging and phagocytic uptake of pathogens, and C5b initiates the terminal complement pathway, resulting in the formation of the membrane attack complex (MAC) composed of C5b, C6, C7, C8 and multiple C9 molecules [35,205,206,207,208]. Several activated components of the complement system are essential for controlling cellular and metabolic functions in both visceral organs and the CNS [209,210,211,212,213]. However, the abnormal activation and production of complement components such as C3, C3b, iC3b, CR3, C5, C5a, and MAC/C5b-9 can contribute to visceral and CNS tissue damage in several diseases including, but not limited to, allergic diseases, cardiovascular diseases, age-related macular degeneration, systemic lupus erythematosus, traumatic brain injury, stroke, neuromyelitis optica spectrum disorders, and neurodegenerative diseases such as amyotrophic lateral sclerosis, Alzheimer’s disease and Huntington disease [214,215,216,217,218,219,220,221,222,223,224,225,226,227,228,229,230,231,232].
Complement activation has been observed in H5N1 and H1N1 influenza virus infections [233]. The sera and lung tissues of the MERS-CoV-infected, hDPP4-transgenic (hDPP4-Tg) mouse model showed complement activation and increased production of C5a and C5b-9, as well as increased viral replication [234,235]. Furthermore, targeting C5aR with anti-C5aR antibody treatment in the MERS-CoV-infected hDPP4-Tg mouse model decreased viral replication and damage to lung and spleen tissue [234,235]. The lung of the mouse-adapted SARS-CoV animal model has also demonstrated complement activation and higher virus replication [31]. To investigate whether SARS-CoV triggered complement activation in the mouse model, C3-/- and background matched control wildtype (WT) mice were infected with SARS-CoV-2 and their lung tissues analyzed for immune inflammation. The data showed that C3-/- mice infected with SARS-CoV-2 were protected against the development of respiratory dysfunction and lung damage when compared to WT mice [31].
SARS-CoV-2 infected primary human airway epithelial cells also caused complement activation and increased production of C3a [236]. C3aR and C5aR targeting in cellular models also showed protection from lung tissue damage [236]. Lung and skin tissues and sera from patients with severe COVID-19 have shown increased complement activation and massive generation of C5a, C5b-C9, and C5aR1 in blood and pulmonary myeloid cells [209]. Studies have shown that SARS-CoV-2 induced hyperinflammation is linked to the development of acute respiratory distress syndrome (ARDS), systemic clotting, and a variety of cutaneous manifestations in patients with COVID-19 [80,81,82,237,238,239,240,241]. Strikingly, administration of C3 (AMY101) or C5 (eculizumab) targeting drugs have shown marked reduction in the SARS-CoV-2-induced development of severe disease symptoms and lung damage in COVID-19 patients [80,81,82]. These data suggest that complement activation and the resultant activation of the C5a–C5aR1 axis is critical for propagating the disease complications in patients with COVID-19.
Investigations into the mechanisms by which complement activation occurs in COVID-19 revealed (1) the receptor-binding domain of the spike protein of SARS-CoV-2 binds to specific IgG/IgM antibodies, (2) heparan sulfate binds to the spike protein of SARS-CoV-2, and (3) recognition of the spike and nucleocapsid proteins of SARS-CoV-2 by lectin pathway components subsequently triggers complement activation by the classical, alternative, and lectin pathways [32,35,242,243,244]. Mouse IgGs (IgG1, IgG2a/c, IgG2b, and IgG3) and their matching receptors (e.g., FcγRI, FcγRIIb, FcγRIII and FcγRIV) differ from the human IgGs (e.g., IgG1, IgG2, IgG3, and IgG 4) and their receptors (e.g., FcγRI, FcγRIIa, FcγRIIc, FcγRIIIa, FcγRIIb, and FcγRIIIb) [245]. Human or mouse FcγRI binds only to the monomeric IgG, but all the other FcγRs in the mouse (e.g., FcγRIIb, FcγRIII and FcγRIV) and human (e.g., FcγRIIa, FcγRIIb, FcγRIIIa and FcγRIIIb) can bind to IgG-ICs [245,246]. SARS-CoV-2 infection can result in the sustained development of memory B cells and long-lived bone marrow plasma B cells [247,248,249] and the production of IgG, IgM, and IgA antibodies to S, the RBD of S, and N proteins (Table 6). The SARS-CoV-2-specific IgA/IgM antibodies are present only for a short period of time, whereas the SARS-CoV-2-specific IgG antibodies persist for long periods of time, thereby providing protection against SARS-CoV-2 in patients with COVID-19 [250,251].
The crosslinking of IgG2a/IgG2b ICs to the FcγRIII/FcγRIV in mice and IgG1 IC crosslinking to the activating FcγR in humans cause optimal complement activation and C5a formation [23,252,253,254,255,256,257,258]. Analysis of IgG and their receptors revealed higher expression of the activating FcγR receptors [259] and increased production of SARS-CoV-2-specific IgG antibodies (IgG1 > IgG2 > IgG3) in patients with COVID-19 [23,251,260]. These findings suggest that the IgG isotypes developing in COVID-19 contribute to complement activation. We and others have reported higher levels of complement activation products (e.g., C1q, C3, C4b C5a, C3aR, C5aR1, and C-type lectins) in mouse models and patients with GD (Table 4). Studies on the Gba1 mutant mouse model, specimens from Type 1 GD patients, and induced pluripotent stem cell (iPSC)-derived Mɸs and lymph nodes from Type 2 and type 3 nGD patients have shown that activation of complement and the C5a–C5aR1 axis leads to tissue inflammation and organ failure in GD [126]. Further, we and others have shown that genetic deficiency of C5aR1 or pharmaceutical targeting to block this receptor in mouse models of GD and in human cell models resulted in marked reduction of immune inflammation and tissue damage (e.g., lung, liver, and spleen) [79,258]. Increased production of autoantibodies specific to GC, IgG2a/c and IgG2b are seen in the Gba19V/- mutant mouse model of GD. Also, high levels of GC-specific IgG1 autoantibodies and lower levels of IgG2 and IgG3 autoantibodies have been observed in GD patients [258]. Similarly, experimental mouse models of GD and GD patients have shown elevated levels of mouse/human specific FcγRIII and FcγRIV [258,261,262]. C1q-binding to IgG-IC drives a series of events resulting in the proteolytic cleavage of C4, C2, and C3 and eventually of C5 into C5a and C5b. Further, C5a can be generated locally in immune cells through an FcγR-dependent mechanism involving LAT phosphorylation and production of a C5-cleaving protease [215,252,253,254,255,256,257,263,264,265]. We have observed that massive GC accumulation drives the production of GC-specific IgG2a/c and IgG2b-ICs in Gba19V/- mice, as well as IgG1 and IgG3 ICs in GD patients to initiate complement activation through the classical pathway [258]. These findings suggest common involvement of complement activation and C5a–C5aR1 axis-mediated tissue inflammation in GD and COVID-19. Therefore, targeting complement at the level of the C5a–C5aR1 axis could control SARS-CoV-2-induced tissue inflammation in COVID-19 in addition to GD patients with COVID-19.
Table 4.
Immune cells and their effector inflammatory mediators in GD.
| Immunological Signature |
Mouse Model of GD | Human GD | ||
|---|---|---|---|---|
| Source | References | Source | References | |
| C1qα M+++ | Lung | [261] | ||
| C1qβ M+++ | Lung | [261] | Lymph node | [126] |
| C1qc M+++ | Lung | [261] | ||
| C3 M+++ | Lymph node | [126] | ||
| Clec4d M+++ Clec4n M+++ Clec5a M+++ |
Lung | [261] | ||
| Clec7 M+++ | Liver | [261] | ||
| MR P+ | Lymph node, Mɸs | [126] | ||
| C3aR1 M+++ | Lung | [261] | ||
| C4b P++ | Lymph node | [126] | ||
| C5a M/P+++ | Mɸs, DCs, Sera | [258] | Lymph node, CBE-induced GCase-targeted Mɸs, iPSCs-derived Mɸs of human Gaucher disease, Sera |
[79,126,258] |
| C5aR1 P+++ | Mɸs and DCs | [258] | Lymph node, Mɸs | [126,258] |
| IFNβ P+++ | Neurons | [266] | ||
| IFNγ P+++ | Anti-CD3 and CD28-stimulated liver, spleen, and lung derived CD4+ T cells, GC-stimulated liver, spleen, and lung-derived DCs, CD4+ T cells, sera, plasma, microglial cells | [66,267] | Sera/Plasma and GC-ICs stimulated CBE-induced GCase targeted Mɸs |
[66,67,267] |
| TNFα P+++ | Anti-CD3 and CD28-stimulated Liver, spleen, and lung-derived CD4+ T cells, GC-stimulated liver, spleen, and lung-derived DCs, CD4+ T cells, sera, plasma, microglial cells, whole brain | [66,267] | Sera/Plasma, GC-ICs stimulated CBE-induced GCase-targeted Mɸs, and iPSCs-derived Mɸs of human Gaucher disease |
[66,67,79,267] |
| IL1α M/P +++ | Sera/Plasma and whole brain | [258] | ||
| IL1β P+++ | Anti-CD3 and CD28-stimulated liver, spleen, and lung-derived CD4+ T cells, GC-stimulated liver, spleen, and lung-derived DCs, CD4+ T cells, sera, plasma, and whole brain | [66,258,267] | Sera/Plasma, GC-ICs stimulated CBE-induced GCase-targeted Mɸs |
[66,67,267] |
| IL6 M/P+++ | Anti-CD3 and CD28-stimulated liver, spleen, and lung- derived CD4+ T cells, GC-stimulated liver, spleen, and lung derived DCs, CD4+ T cells, sera, plasma, microglial cells, and whole brain |
[66,267] | Sera/Plasma and GC-ICs stimulated CBE-induced GCase-targeted Mɸs |
[66,67,267] |
| IL8 P++ | Sera | [127] | ||
| IL12 P+++ | Anti-CD3 and CD28-stimulated liver, spleen, and lung- derived CD4+ T cells, GC-stimulated liver, spleen, and lung derived DCs, CD4+ T cells, sera, and plasma |
[66,267] | Sera/Plasma and GC-ICs stimulated CBE-induced GCase-targeted Mɸs |
[66,67,267] |
| IL17 P+++ | Anti-CD3 and CD28-stimulated liver, spleen, and lung- derived CD4+ T cells, GC-stimulated liver, spleen, and lung-derived DCs, CD4+ T cells, β-GC 22:0 (βGL1-22)-stimulated type II natural killer+ CD19+ B cells, sera, and plasma |
[66,67,267,268] | Sera/Plasma, peripheral blood-derived MOs, β-GC 22:0 (βGL1-22)- stimulated type II natural killer cells, CD19+ B cells, and GC-ICs stimulated CBE-induced GCase- targeted Mɸs |
[66,67,267,268] |
| IL18 P+++ | Sera | Sera/Plasma | [127] | |
| IL21 P+++ | β-GC 22:0 (βGL1-22)-stimulated type II natural killer+ CD19+ B cell | [67,267,268] | Peripheral blood-derived Mos, β-GC 22:0 (βGL1-22)- stimulated type II natural killer cells, and CD19+ B cell |
[67,267,268] |
| IL23 P+++ | Anti-CD3 and CD28 stimulated liver, spleen, and lung-derived CD4+ T cells, GC-stimulated liver, spleen, and lung-derived DCs, CD4+ T cells Sera/Plasma |
[66,267] | Sera/Plasma and GC-ICs stimulated CBE-induced GCase-targeted Mɸs | [66,67,267] |
| CCL1 P+++ | Sera/Plasma | [258] | ||
| CCL2 M/P+++ | Sera/Plasma Lung tissues |
[161,258] | Sera/Plasma, Lymph node and Spleen tissues | [67,126,161] |
| CCL3 M/P+++ | Sara/Plasma Lung tissues |
[161,258] | Sera/Plasma | [67,161] |
| CCL4 P+++ | Sera/Plasma | [161,258] | Sera/Plasma | [67,161] |
| CCL5 P+++ | Sera/Plasma | [161,258] | Sera/Plasma | [67,161] |
| CCL6 M +++ | Lung tissues | [161] | ||
| CCL9 M +++ | Lung tissues | [161] | ||
| CCL17 M +++ | Lung tissues | [161] | ||
| CCL18 P+++ | Sera/Plasma | [161] | Sera/Plasma and Spleen tissues |
[67,161] |
| CCL22 M+++ | Lung tissues | [67,161] | ||
| CXCL1 M/P+++ | Sera/Plasma Lung tissues |
[161] | ||
| CXCL2 P+++ | Sera/Plasma | [161,258] | ||
| CXCL8 P+++ | Sera/Plasma | [161] | Sera/Plasma | [67,161] |
| CXCL9 M/P+++ | Sera/Plasma Lung macrophages |
[121,258] | ||
| CXCL10 P+++ | Sera/Plasma | [121,258] | ||
| CXCL11 P+++ | Sera/Plasma | [121,258] | ||
| CXCL12 M+++ | Lung tissues | [67,161] | ||
| CXCL13 P+++ | Sera/Plasma | [258] | ||
| TGFβ1 P+++ | Sera/Plasma | [67,161] | Lymph node | [126] |
| HGF P+++ | Sera/Plasma | [67,161] | ||
| MCSF P+++ | Sera/Plasma | [67,161,258] | Sera/Plasma | [67,161] |
| GCSF P+++ | Sera/Plasma | [67,161] | Sera/Plasma | [67,161] |
| GMCSF P+++ | Sera/Plasma | [67,161,258] | ||
GD (Gaucher disease), C1qa (complement component 1, q subcomponent, alpha polypeptide), C3 (complement 3), C5a (complement 5a), C5a receptor1 (C5aR1), Clec4a (C-type lectin domain family 4, member d), Clec4n (C-type lectin domain family 4, member n), Clec5a (C-type lectin domain family 5, member a), Clec7a (C-type lectin domain family 7, member a), MR (Mannose receptor), IFN (interferon), TNF (tumor necrosis factor), IL (interleukin), CCL (Chemokine, C-C motif ligand), CXCL (Chemokine, C-X-C motif ligand), TGF (transforming growth factor), HGF (hepatocyte growth factor), MCSF (Mɸ colony-stimulating factor), GCSF (granulocyte colony stimulating factor), GMCSF (granulocyte-Mɸ colony stimulating factor), CBE (Conduritol B epoxide), GCase (Glucocerebrosidase), Mɸs (macrophages), iPSCs (induced pluripotent stem cells), α (alpha), β (Beta), γ (gamma), P (Protein expression level), M (mRNA expression level) + (low), ++ (moderate), and +++ (high).
Table 5.
COVID-19 Vaccines and Alternative Treatments.
| Vaccines/Alternative Treatment | Treatment Efficacy | Effectiveness Time Period, Notes | Possible Side Effects | References |
|---|---|---|---|---|
| mRNA Vaccines | 48–>90% | 5–6 months | Severe anaphylaxis, myocarditis/pericarditis, lymphadenopathy, appendicitis, herpes-zoster infection, viral facial palsy, transverse myelitis, Guillain-Barré syndrome, encephalopathy, thromboembolism, and thrombosis-thrombocytopenia syndrome | [130,132,133] |
| Viral vector vaccines (Sputnik) | 65–91.6% | |||
| Inactivated Vaccines | 50–91.6% | |||
| Protein-based Vaccines | 90% (80% in adults over 65) |
|||
| Lopinavir and Ritonavir (protease inhibitor | No significant difference with the standard of care | NA | Could prevent the secondary immune burst without causing severe adverse events at early stages of COVID-19 and are therefore not recommended for COVID-19 at severe stages of disease | [135,136,137] |
| Favipiravir (Nucleoside precursor) | Improved the clinical condition of COVID-19 patients | No significant differences in hospitalization length and clinical recovery | Possible safety concerns for favipiravir include increased blood uric acid and teratogenicity, as the medication is secreted in semen and breast milk and has demonstrated toxicity in animal studies | [138,139,140,141] |
| Remdesivir (Nucleoside analog) | Improved recovery and clinical outcomes of hospitalized COVID-19 patients, though its effects in reducing mortality are still uncertain | Favorable influence on length of hospital stay but does not confer any mortality benefit | Nausea, hypokalemia, and headaches; mortality rate is not different from placebo | [138,139,140,142,143,144] |
| IFNγ | Rapid decline in SARS-CoV-2 load and a positive-to-negative viral culture conversion. Four patients recovered, and no signs of hyperinflammation were observed |
Administration within 5–6 days of symptom onset/diagnosis may improve outcomes in patients with SARS-CoV-2, particularly time to viral clearance and time to clinical improvement | Nausea, temporary digestion issues | [150] |
| Statins | Partially associated with altered mortality | Reduced in-hospital mortality, mainly in patients with coronary disease | 18% increased risk of severe COVID-19 infection | [269,270,271,272] |
| RAAS inhibitors | Partially associated with altered mortality | RAAS inhibitors should be continued in patients with COVID-19 | Does not negatively affect clinical course of COVID-19 in hypertensive patients |
[273,274] |
| ACE inhibitors (e.g., Lisinopril) | Efficiently treated COVID-19 vaccination- induced development of hyaluronic acid delayed inflammatory reaction | Reduced risk of COVID-19 positivity | Cough, hyperkalemia, fatigue, dizziness, headache, dysgeusia | [275,276] |
| IL-1α/β inhibitor (Anakinra) | Prevented severe respiratory failure of COVID-19 and decreased the mortality | Significantly reduced the risk of worse clinical outcome at day 28 |
Immunosuppression with anakinra may facilitate sepsis, necessitating continuous screening for bacterial superinfections |
[277] |
| IL-6R antagonists (tocilizumab and sarilumab) | Controlled the disease severity and improved survival in critically ill patients with COVID-19 | Significantly reduced the risk of worse clinical outcome at day 28 |
Bacterial, viral, fungal, and opportunistic infections | [278,279] |
| JAK inhibitors (ruxolitinib and baricitinib) | Decreased use of invasive mechanical ventilation; borderline impact on intensive care unit rates | JAK-inhibitors did not decrease length of hospitalization but exhibited a lower 28-day mortality rate |
Viral (herpes, influenza), fungal, mycobacterial infections; musculoskeletal and connective tissue disorders, embolism and thrombosis, neoplasms | [280,281,282,283] |
| Anti-TNFα antibody (adalimumab) | Not shown; no alterations in mortality or mechanical ventilation requirements |
No significant differences with the standard of care | Cutaneous swelling, pain, nose and throat sinus infections, headache, stomach pain, muscle pain |
[148,284] |
| GMCSF (sargramostim) |
Increased anti- viral antibody titers, lowered mean lung viral titers, and increased survival in SARS-CoV-2 infected human ACE2 transgenic mouse model of COVID-19 |
NA | Flu-like symptoms, leukocytosis and capillary leak syndrome, acute lung injury in rare cases | [285] |
| Anti-GMCSF monoclonal antibody (gimsilumab) | No improvement in mortality or other key clinical outcomes, i.e., pneumonia and evidence of systemic inflammation in patients with COVID-19 | All-cause mortality did not vary between gimsilumab and placebo at day 43 | Infections and sepsis, renal failure, infusion-related reactions | [286] |
| IVIGs | Improved the lung symptoms, cutaneous vasculitis, and acute encephalopathy in patients with COVID-19 | IVIGs reduced mortality and increased the hospitalization length in severe COVID-19 infection | Thromboembolic events | [287,288] |
| Corticosteroids | Reduce mortality in patients with COVID-19 | Acute trial | Hyperglycemia. No increase of neuromuscular weakness, gastrointestinal bleeding, or superinfections | [289] |
SARS-CoV2 (Severe Acute Respiratory Syndrome Coronavirus-2), COVID-19 (Coronavirus Disease 2019), mRNA (messenger ribonucleic acid), IFNγ (interferon gamma), RAAS (renin-angiotensin-aldosterone system), ACE (angiotensin-converting-enzyme), IL-1α/β (interleukin-1 alpha/beta), IL-6R (interleukin-6 receptor), JAK (janus kinase), TNFα (tumor necrosis factor-α), GMCSF (granulocyte-macrophage colony stimulating factors), and IVIGs (intravenous immunoglobulins), NA (not available).
Table 6.
Antibodies to SARS-CoV-2 in COVID-19 Patients.
| SARS-CoV-2 Protein | IgG and Isotypes Antibodies to SARS-CoV-2 Proteins |
IgM Antibodies to SARS-CoV-2 Proteins |
IgA Antibodies to SARS-CoV-2 Proteins |
|---|---|---|---|
| S (S1 and/or S2) | IgG++ [260,290,291,292,293,294,295,296,297,298,299,300,301,302,303] +++IgG1 > IgG2 > IgG3 [260] |
IgM+ [260,292,293,298,299,300,301,302] | IgA+ [260,295,299,301,302] |
| RBD | IgG++ [251,300] and IgG1+++ [251] | IgM++ [251] | |
| N | IgG+++ [251,290,291,296,300,303,304,305,306] | IgM+ [293,306] | |
| E | IgM+ [292] |
SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2), COVID-19 (Coronavirus Disease 2019), S (spike), E (envelope), N (nucleocapsid protein), RBD (spike receptor binding domain), IgG (immunoglobulin G), IgM (immunoglobulin M), IgA (immunoglobulin A), + (low production), ++ (moderate production), and +++ (massive production).
1.6. Complement Activation Is Linked to the Increased Synthesis of Glucosylceramide Synthase Enzymes and Excess Production of Sphingolipids in COVID-19 and GD
Several studies have shown that in vivo and ex vivo stimulation of immune cells with immune complexes (e.g., IgG and IgM ICs) and complement activation products (e.g., C3, C3a, C3b, and C5a) can cause excess secretion of lysosomal enzymes (Table 7). Sphingolipids are ubiquitous components of the plasma membrane of eukaryotic cells and are essential for controlling cell proliferation, survival, and death [4,307]. However, prolonged overabundance of sphingolipids is detrimental, as seen in several lysosomal storage disorders such as GD, Fabry disease, Tay-Sachs disease, Sandhoff disease, Krabbe disease, and Niemann–Pick disease [308]. GC is an important sphingolipid, representing the backbone of more than 450 structurally different glycosphingolipids. Activation of GCS, the enzyme that places a glucosyl moiety onto ceramide, is the first pathway-committed step in the production of more complex GSLs, such as lactosylceramide and gangliosides [309].
Increased formation of GSLs such as GM2-ganglioside and lactosylceramide, have been observed upon infection by Zika virus and hepatitis C virus, respectively [310,311]. Human cytomegalovirus infection also causes an excess formation of ceramide and GM2-ganglioside [312]. Dengue virus causes the increased production of ceramide and sphingomyelin [313]. Influenza virus infection has also demonstrated increased synthesis of GC and sphingomyelin [314,315]. Conversely, suppression of the biosynthesis of cellular GSLs results in the inhibition of maturation of influenza virus particles in vitro [316,317]. It was recently suggested that GSLs support SARS-CoV-2 replication and infection [318,319]. Elevated levels of 3-ketosphinganine (16-0, 18-0, 18-1, and 20-0), sphinganine, sphingosine, dihydrosphingosine, ceramides, dihydroceramides, sphinganine-1-phosphate (S1P), GA1, and GM3 have been observed in SARS-CoV-2 infection in vitro and in vivo in experimental mouse and human cells models as well as in the sera/plasma of COVID-19 patients [1,2,3,4]. Furthermore, drugs targeting GCS inhibited the replication of SARS-CoV-2 [78].
Pathogenic variants in GBA1/Gba1 and the resultant deficiency of acid-β glucosidase drive massive increases of GCs: 18-0, 20-0, 22-1, 22- 0, 24-1, and 24-0 in tissues (e.g., lung, liver, spleen, and brain) from mouse models and human patients with type 1, 2 and 3 GD [66,67,121,161,165,166,167,258,320,321,322,323,324,325,326]. Genetic deficiency or pharmaceutical targeting of C5aR1 in mouse models and human cellular models of GD (e.g., macrophages, and dendritic cells), showed marked reduction in the generation of GCS and GCs (e.g.,16-0, 18-0, 20-0, 22-1, 22- 0, 24-1, and 24-0) [79,258]. Importantly, studies have shown that a majority of GD patients treated with substrate reduction and/or enzyme replacement therapies and experienced SARS-CoV-2 infection only developed mild symptoms of COVID-19 and survived (Table 8). These findings suggest that the C5a–C5aR1-induced excess sphingolipid production propagates the disease in COVID-19 and GD. Thus, targeting the C5a–C5aR1 axis-mediated overproduction of GC could control SARS-CoV-2 replication and prevent development of severe disease in patients with COVID-19 and GD patients with COVID-19.
Table 7.
Immune cell stimulation with immune complexes or compliment activation products trigger excess release of several enzymes.
| Complement Activation Products | Cells | Enzyme Secretion | References |
|---|---|---|---|
| IgG and IgM-IC | PMNs R | Alkaline phosphatase, Acid phosphatase, and β -Glucuronidase |
[327] |
| BSA-anti-BSA | Mɸs R | β-glucuronidase | [328] |
| Rheumatoid factor complex (IgG/IgM) | Leuko H | β-glucuronidase | [329] |
| C3 | Mɸs M | β-glucuronidase | [330] |
| C3a | Mɸs M | β-glucuronidase, | [330] |
| C3b | Mɸs M | β-galactosidase, β-glucuronidase, N-acetyl-β-D-glucosaminidase |
[330,331] |
| C3d | PMN H | Elastase | [332] |
| C5a | PMN H | β-glucuronidase, Lysozyme | [333,334] |
IgG (immunoglobulin G), IgM (immunoglobulin M), IC (immune complex), C (complement), PMNs (polymorphonuclear cells), BSA (bovine serum albumin), Mɸs (macrophages), DCs (dendritic cells), Leuko (Leukocytes), M (mouse), R (rabbit), H (human).
Table 8.
Effect of COVID-19 in Patients with Gaucher Disease.
| GD Patients |
Treatment | GD Patients with COVID-19 | SARS-CoV-2 Positivity | Symptoms | References |
|---|---|---|---|---|---|
| 181 | SRT, ERT | 45/181 | 18 | Fever, dyspnea, cough, fatigue, chills, night sweats, taste and smell loss, chest pain, feeling of short breath, COVID-19 toes, extreme exhaustion, weakness, and no death | [335] |
| 8 | SRT, ERT | 7/8 | 2 | Fever, dyspnea, cough, fatigue, bilateral pneumonia, and multi-organ failure 50% death |
[336] |
| 550 | NR | 1 | 1 | Mild and short clinical course managed by quarantine for 14 days | [337] |
| 39 | NR | 39 | Hypertension, Type 2 diabetes mellitus, dyslipidemia, asthma or chronic obstructive pulmonary disease, chronic kidney disease, coronary artery disease, heart failure, cancer | [338] | |
| 1 | ERT | 1 | 1 | Fever, cough, severe neutropenia, and cavitation in the chest |
[339] |
| 1471 | ERT, SRT | 82 | 0 | Asymptomatic, mildly affected but 2/82 exhibited severe/critical infection |
[340] |
| 2 | ERT, Ambroxol |
0 | 0 |
Mood changes, cognitive and
motor deterioration |
[341] |
GD (Gaucher disease), COVID-19 (Coronavirus-2 Disease, 2019), SARS-CoV-2 (Severe Acute Respiratory Syndrome Coronavirus-2, SRT (Substrate Reduction Therapy), ERT (Enzyme Replacement Therapy), and NR (Not Reported).
1.7. Complement Activation Is Linked to the Increased Production of Chemokines, Growth Factors, Immune Cells Infiltration, and Pro-Inflammatory Cytokine Production in COVID-19 and GD
Complement activation products such as C5a are critical for generation of chemokines, growth factors, infiltration of innate and adaptive immune cells that lead to the production of pro-inflammatory cytokines and cause tissue destruction in many inflammatory diseases [200,263,264,342,343,344,345]. Findings from mouse models, cellular systems, and samples from patients with SARS-CoV-2-induced COVID-19 have shown massive generation of C5a, CCL chemokines (e.g., CCL2, CCL3, and CCL5), CXCL chemokines (e.g., CXCL9 and CXCL10), and growth factors, i.e., TGFβ, GCSF, GMCSF, VEGF, FGF, and PDGF (Table 1 and Table 2). Similar observation in mouse models, cellular systems, and human specimens from patients with GD have shown increased production of C5a and CCL chemokines (e.g., CCL1, CCL2, CCL3, CCL4, CCL5, CCL6, CCL9, CCL17, CCL18, and CCL22), CXCL chemokines (e.g., CXCL1, CXCL2, CXCL8, CXCL9, CXCL10, CXCL11, CXCL12, and CXCL13), and growth factors, i.e., TGFβ1, HGF, MCSF, GCSF, and GMCSF (Table 4).
Certain inflammatory conditions cause increased production of growth factors (e.g., GCSF, GMCSF, MCSF, HGF, VEGF, FGF, PDGF, and TGFβ1) and the indicated CCL and CXCL chemokines (i.e., CCL2/MCP1, CCL3/MIP1α, CCL4/MIP1β, CCL5/RANTES, CXCL1, CXCL2, CXCL9, CXCL10, and CXCL11), which account for the development and trafficking of immunological cells (e.g., MOs, Mɸs, DCs and the CD4+ and CD8+ T cells) from the peripheral blood and bone marrow to the sites of inflammation, where the generation of pro-inflammatory cytokines lead to tissue destruction [346,347]. A similar process is thought to occur with SARS-CoV-2 infection-induced complement activation, resulting in increased infiltration of several types of immune cells (i.e., MOs, Mɸs, DCs, PMNs, CD4+ T cells, CD8+ T cells), an overproduction of pro-inflammatory cytokines (e.g., IFN α, IFNγ, TNFα, IL1, IL2, IL6, IL7, IL8, IL12, IL17) and a decreased production of IFNα and IFNβ in COVID-19 patients (Table 1 and Table 2). In addition, the GC-induced complement activation, the increased infiltration of immunological cells (i.e., Mɸs, DCs, CD4+ T cells, CD8+ T cells) and the increased production of pro-inflammatory cytokines (i.e., IFNγ, TNFα, IL1α, IL1β, IL6, IL12, IL17,IL18, IL21, and IL23) were seen in mouse models and patients with GD (Table 3 and Table 4).
Sera and lung tissues of the MERS-CoV infected hDPP4-transgenic (hDPP4-Tg) mouse model showed increased concentrations of the C5a and C5b-9, as well as the overexpression of caspase-1 and IL1β. Furthermore, targeting C5aR1 by using anti-C5aR1 antibody treatment in the MERS-CoV-infected hDPP4-Tg mouse model caused decreased viral replication and reduced expression of IL1β and caspase-1, as well as the protection from cell death and tissue damage (e.g., lungs and spleen) [234,235]. A mouse-adapted SARS-CoV animal model showed complement activation is linked to the increased production of pro-inflammatory cytokines and chemokines, and the massive lung infiltration of inflammatory subsets of immune cells. C3-/- mice infected with SARS-CoV-2 had marked reduction in the production of IL1α, IL6, TNFα, CXCL2, GCSF, and decreased lung infiltration of inflammatory subsets of immune cells (e.g., MOs and PMNs), as well as the significant protection against the development of respiratory dysfunction and lung damage when compared to control WT mice.
SARS-CoV-2 infected primary human airway epithelial cells also caused increased production of C3a, IL1α, IL6, CCL2, and CCL5 [236]. C3aR and C5aR targeting in these mice and a cell model showed pronounced reduction in the development of immune inflammation and decreased lung tissue damage [236]. Furthermore, lung, skin, and sera specimens from patients with severe COVID-19 infection have shown massive generation of C5a, C5b-C9, and C5aR1 with myeloid cells infiltration and pro-inflammatory cytokines production (Table 1). Strikingly, administration of C3 (AMY101) and C5 targeting drugs (eculizumab) resulted in marked reduction of pro-inflammatory cytokines and improved lung function in SARS-CoV-2-induced COVID-19 [80,81,82]. Similarly, GC-induced activation of complement and the resultant production of C5a caused overproduction of chemokines and growth factors, leading to increased tissue recruitment of Mɸs, DCs, and T cells. The interaction of Mɸs, DCs, and T cells eventually led to the production of inflammatory cytokines in mouse models and patients with type 1 GD [66,67,121,161]. Additionally, massive generation of chemokines (e.g., CCL2, CCL3, and CCL5), growth factors (e.g., MCSF and TGFβ), activation of the microglial cells and astrocytes, and the immense generation of pro-inflammatory cytokines (e.g., IFNγ, TNFα, IL1α, IL1β, and IL6), reactive oxygen species (ROS), nitric oxide (NO), and their combined impact inducing neuronal loss and early death were observed in Gbaflox/flox; nestin-Cre mice, K14-lnl/lnl mice, 4L;C*, C57BL/6J-Gbatm1Nsb and the conduritol B epoxide-induced chemical model of nGD [167]. Type 2 and Type 3 human nGD display microglial cell activation and upregulation of pro-inflammatory cytokines (e.g., TNF α, IL1β, and IL6) that lead to the loss of neurons and to early death in patients with Type 2 and Type 3 nGD [167,172]. Furthermore, data from the Gba19V/- mouse model, conduritol B epoxide-induced GCase-targeted mouse model and human cells, and iPSCs-derived macrophages from Type 1, Type 2 and Type 3 nGD patients, showed that the C5a–C5aR1 axis causes overgeneration of chemokines and growth factors. This leads to the increased recruitment and/or activation of innate and adaptive immune cells in visceral organs and microglial cells and/or neurons in the brain that causes overproduction of pro-inflammatory cytokines, leading to the development of severe and chronic visceral and brain tissue inflammation in GD [126]. Further, we and others have shown that genetic deficiency or pharmaceutical targeting of C5aR1 in GD mouse models and human cell model of GD resulted in marked reduction in the production of CCL chemokines (e.g., CCL1, CCL2, CCL3, CCL4, CCL5, CCL6, CCL9, CCL12, CCL17, CCL18, CCL22), CXCL chemokines (e.g., CXCL1, CXCL2, CXCL8, CXCL9, CXCL19, CXCL11, and CXCL13), and growth factors (e.g., MCSF, GCSF, and GMCSF). In addition, a decreased tissue recruitment of immune cells (e.g., Mɸs, DCs, CD4+ T cells), marked reduction in generation of the pro-inflammatory cytokines (e.g., IFNγ, TNFα, IL1α, IL1β, IL6, IL12p40, IL12p70, IL17,IL18, IL21, and IL23) and protection of lung, liver, and spleen disruption was de,omnstrated [79,258]. These findings suggest that targeting the C5a–C5aR1 axis could control the SARS-CoV-2/GC-induced excess production of chemokines, tissue recruitment of immune cells, pro-inflammatory cytokines production and lessen the resultant tissue damage in COVID-19 and GD patients.
2. Discussion
SARS-CoV-2-induced increased production of complement activation products such as C5a, C5b-C9, and C5aR, which are linked to the increased tissue recruitment and activation of MOs, Mɸs, DCs, PMNs, CD4+ T cells, CD8+ T cells, and the extensive production of pro-inflammatory cytokines, chemokines, and growth factors (Table 1 and Table 2). In addition, excess synthesis of GCS and the corresponding glycosphingolipids have been observed in COVID-19 [1,2,3,4]. Moreover, drugs targeting complement activation at the C5a–C5aR1 stage in the complement pathway and drugs targeting GCS have shown marked reduction in the replication of SARS-CoV-2, as well as the SARS-CoV-2-mediated induction of innate and adaptive immune inflammation that leads to the tissue destruction and death in patients with COVID-19 [78,80,81,82,236].
Similarly, in vitro, ex vivo, and in vivo studies performed with the Gba1 mutant mouse models and for patients with GD have shown a link between the upregulation of C1q, C3, C4b, C5a, C5aR1 and the increased production of GC, excess recruitment and activation of innate and adaptive immune cells, and overproduction of pro-inflammatory cytokines, chemokines, and growth factors in many tissues in GD (Table 3 and Table 4). Studying the mechanistic connections between the complement-sphingolipid axis in the induction of tissue inflammation in GD, we and others have found that massive GC storage causes the development of GC-specific IgG autoantibodies that bind to GC and produce the GC-specific IgG-ICs (GC-ICs) [258,348,349]. Such ICs drive complement activation, local production of C5a, and the activation of C5aR1 in Gba1 mutant (Gba19V/-) mouse model and patients with GD [79,126,258]. The activation of the indicated C5a–C5aR1 axis causes the excess cellular production of GCS and GC, innate and adaptive immune cell activation, and the development of tissue inflammation in GD [79,126,258]. Furthermore, we and others have shown that genetic deficiency or pharmaceutical targeting of C5aR1 in mouse models and patients with GD reduces generation of GCS-glycosphingolipid, inhibits the production of pro-inflammatory cytokines, chemokines, and growth factors, tissue recruitment of innate and adaptive immune cells, and protects the lung, liver, and spleen tissues from damage [79,258].
Several vaccines and alternative treatments have shown poor efficacy and or immunity against SARS-CoV-2 (Table 5). Strikingly, agents targeting C5–C5a–C5aR1 or glycosphingolipid-lowering therapies have been linked to the disruption of the SARS-CoV-2 replication and the suppression of the SARS-CoV-2-induced activation of immune inflammation in COVID-19 [24,78,350,351]. However, the mechanism by which targeting C5–C5a–C5aR1 or GCS-glycosphingolipid pathways inhibit the viral replication, and the induction of immune inflammation remains unknown; thus, additional in vitro, ex vivo, and in vivo studies on different variants of SARS-CoV-2 (e.g., Alpha, Beta Delta, BA.4, and BA.5)-induced COVID-19 are required. Studies to test whether these SARS-CoV-2 variant-induced COVID-19 triggers the excess synthesis of GCS and the corresponding development of glycosphingolipids, glycosphingolipids-specific IgG antibodies, and complement activation in patients with COVID-19 and GD patients with COVID-19 are also needed. Furthermore, large scale experimental and clinical trials are critical to test whether combined targeting of complement at the level of C5a–C5aR1 and the GCS-glycosphingolipid pathway can affect the disease severity and/or death in patients with COVID-19 and in GD patients with COVID-19.
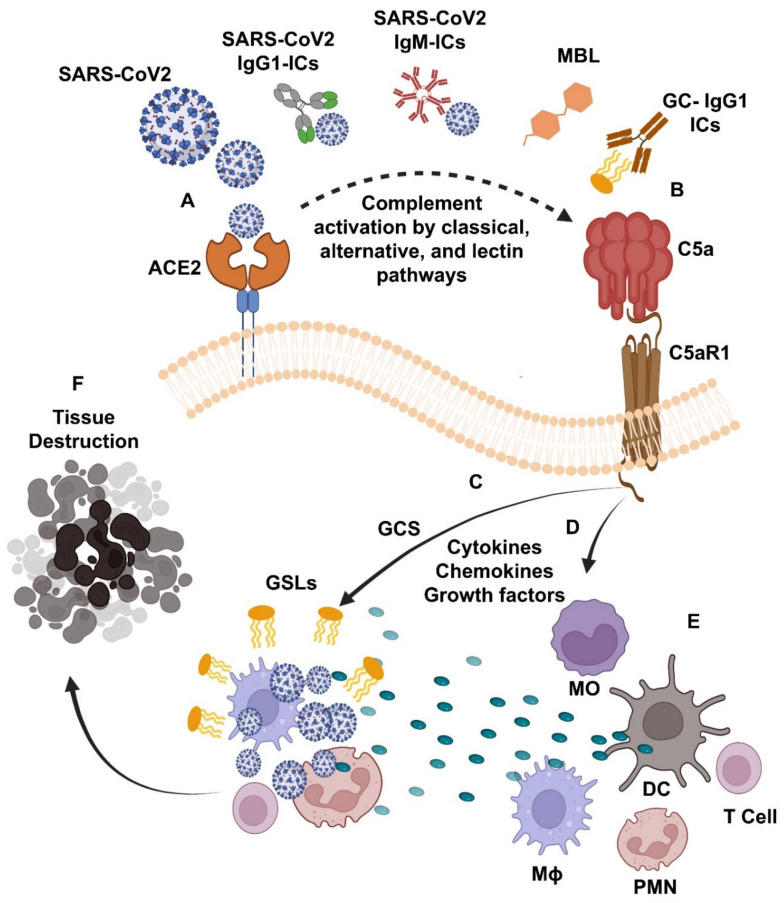
Figure 1 provides a summary of the complement–glycosphingolipid axis, as supported by study findings from patients and mouse models for COVID-19 and GD reviewed herein. It is suggested that SARS-CoV-2 infection triggers the development of SARS-CoV-2 IgM-ICs and the SARS-CoV-2 and/or GSL; GC-specific IgG1-ICs. The binding of such SARS-CoV-2 IgM-ICs to FcμR and SARS-CoV-2 IgG1-ICs/GC-specific IgG1-ICs to activating FcγR, and the direct binding of SARS-CoV-2 to mannose-binding lectin (MBL-MASP 1-2) lead to the massive production of C5a in patients with COVID-19 and GD patients with COVID-19 (Figure 1A,B). The interaction of such C5a to its C5aR1 receptor triggers GC synthase (GCS)-mediated excess synthesis of GSLs, which promotes the SARS-CoV-2 growth and replication (Figure 1C) and over production of the cytokines, chemokines, and growth factors listed in Table 1, Table 2, Table 3 and Table 4 and shown in Figure 1D. These pro-inflammatory mediators cause increased tissue infiltration and activation of several of the immune cells, i.e., MO, Mɸ, DC, PMN, and T cells, additional generation of pro-inflammatory cytokines (Figure 1E) and lead to the tissue damage in patients with COVID-19 and GD with COVID-19 (Figure 1F). Hence, targeting the C5– C5aR1–GCS glycosphingolipid pathway could protect the SARS-CoV-2-induced development of s severe form of the disease in patients with COVID-19 and GD patients with COVID-19. These findings also open up the exciting possibility of using drugs already available that effectively target the C5a–C5aR1 axis at the level of C5 (e.g., Eculizumab), which prevents C5 from being cleaved into C5a and C5b and is currently used for treating patients with Atypical hemolytic uremic syndrome and Paroxysmal Nocturnal Hemoglobinuria) [352,353]. Similarly, C5a-suppressing oral treatments (e.g., Avacopan, approved for the treatment for anti-PMN cytoplasmic antibody-associated vasculitis) and the pharmaceutical targeting of the GCS (e.g., Venglustat, which inhibits the synthesis of glycosphingolipids) can be used as potential approaches to control the tissue inflammation, organ failure, and death in patients with COVID-19 and GD patients with COVID-19 experiencing uncontrolled infections with different variants of SARS-CoV-2 (e.g., Alpha, Beta Delta, BA.4, and BA.5) [354,355].
Figure 1.
Legend. Complement-Glycosphingolipid Axis leads to the tissue inflammation in patients with Coronavirus Disease 2019 (COVID-19) and Gaucher Disease (GD). The interaction of Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) with its cellular receptor (e.g., angiotensin converting enzyme-2; ACE2) triggers the massive generation of complement 5a (C5a) by SARS-CoV-2 and/or glycosphingolipid (GSL; glucosylceramide; GC)-specific immunoglobulin G1 immune complexes (SARS-CoV-2/GSL-IgG1-ICs)-mediated classical, SARS-CoV-2-IgM-ICs-mediated alternative, and crosslinking of SARS-CoV-2 mannose-binding lectin (MBL)-mediated lectin pathway of complement activation (A,B). The interaction of C5a and its C5aR1 receptor triggers glucosylceramide synthase (GCS)-mediated excess synthesis of GSLs, which ameliorates SARS-CoV-2 replication (C), as well as the overproduction of cytokines, chemokines and growth factors (D). These pro-inflammatory mediators cause excess tissue recruitment and activation of innate and adaptive immune cells, i.e., polymorphonuclear cell (PMN), monocytes (MO), macrophage (Mɸ), dendritic cell (DC), T cells, and the resulting excess production of pro-inflammatory cytokines (E; and listed in Table 1, Table 2, Table 3 and Table 4) and tissue destruction in patients with COVID-19 and GD patients with COVID-19 (F). Accordingly, the combined targeting of C5a–C5aR1 and/or GSL synthesis pathway could stop the SARS-CoV-2-mediated tissue inflammation and organ damage in patients with COVID-19 and GD patients with COVID-19.
From a clinical standpoint, this review emphasizes the possible role for medications targeting the complement-glycosphingolipid pathway as an additional anti-inflammatory therapeutic strategy to ameliorate disease progression in COVID-19 patients, patients at high-risk of developing COVID-19 or COVID-19 reinfection, and GD patients with COVID-19 disease. In sum, these immune strategies may be useful in patients for whom treatments and vaccines cannot be easily used, for example, patients at risk of developing severe, treatment-related adverse events, those with vaccine-related serious adverse events, or in patients with reinfection due to the immunity waning phenomenon.
Acknowledgments
Manoj Kumar Pandey is supported by a grant from the Alexion Pharmaceuticals (Boston, Massachusetts, USA), Michael J. Fox Foundation (New YORK, NY, USA) and Orphan Disease Center, University of Pennsylvania (Philadelphia, PA, USA). Authors would like to acknowledge Krista Johnson and Melissa Lasaro (Alexion Pharmaceuticals, Boston, Massachusetts, USA) for critical review of the manuscript. Authors would also like to acknowledge the BioRender.com (Licensed to CCHMC) for creating the figure, Charles D. Loftice for laboratory support, and Kenya N. Mays for secretarial help.
Author Contributions
V.S.T., A.F.M., R.R., L.M. and M.A.M. have prepared the tables. A.M.S., D.R.P. and R.J.H. have schemed the tables and performed the critical review of the manuscript. R.R. has schemed and created the figure and D.R.P. completed the final editing. M.K.P. contributed to the conception, design, collection, extensive assessment of the original literature, scheming the figures, as well as the writing, interpretation, final proof reading, and acceptance of the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
Author affirms that that this manuscript was completed in absence of any commercial or financial interactions that could be interpreted as a potential conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Wu D., Shu T., Yang X., Song J.-X., Zhang M., Yao C., Liu W., Huang M., Yu Y., Yang Q., et al. Plasma metabolomic and lipidomic alterations associated with COVID-19. Natl. Sci. Rev. 2020;7:1157–1168. doi: 10.1093/nsr/nwaa086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vitner E.B., Avraham R., Politi B., Melamed S., Israely T. Elevation in sphingolipid upon SARS-CoV-2 infection: Possible implications for COVID-19 pathology. Life Sci. Alliance. 2021;5:e202101168. doi: 10.26508/lsa.202101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khodadoust M.M. Inferring a causal relationship between ceramide levels and COVID-19 respiratory distress. Sci. Rep. 2021;11:20866. doi: 10.1038/s41598-021-00286-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torretta E., Garziano M., Poliseno M., Capitanio D., Biasin M., Santantonio T.A., Clerici M., Caputo S.L., Trabattoni D., Gelfi C. Severity of COVID-19 Patients Predicted by Serum Sphingolipids Signature. Int. J. Mol. Sci. 2021;22:10198. doi: 10.3390/ijms221910198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Russo F.P., Burra P., Zanetto A. COVID-19 and liver disease: Where are we now? Nat. Rev. Gastroenterol. Hepatol. 2022;19:277–278. doi: 10.1038/s41575-022-00607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tahtabasi M., Hosbul T., Karaman E., Akın Y., Konukoglu O., Sahiner F. Does COVID-19 cause an increase in spleen dimensions? Possible effects of immune activation, hematopoietic suppression and microthrombosis. Clin. Imaging. 2021;79:104–109. doi: 10.1016/j.clinimag.2021.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Suwaidi A.S., Alakasheh B.J., Al-Ozaibi L.S. Splenic Infarction in a COVID-19 Patient without Respiratory Symptoms. Dubai Med. J. 2022;5:74–77. doi: 10.1159/000521207. [DOI] [Google Scholar]
- 8.Shaukat I., Khan R., Diwakar L., Kemp T., Bodasing N. Atraumatic splenic rupture due to COVID-19 infection. Clin. Infect. Pract. 2020;10:100042. doi: 10.1016/j.clinpr.2020.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie Y., Xu E., Bowe B., Al-Aly Z. Long-term cardiovascular outcomes of COVID-19. Nat. Med. 2022;28:583–590. doi: 10.1038/s41591-022-01689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abbasi J. Even Mild COVID-19 May Change the Brain. JAMA. 2022;327:1321–1322. doi: 10.1001/jama.2022.4507. [DOI] [PubMed] [Google Scholar]
- 11.Douaud G., Lee S., Alfaro-Almagro F., Arthofer C., Wang C., McCarthy P., Lange F., Andersson J.L.R., Griffanti L., Duff E., et al. SARS-CoV-2 is associated with changes in brain structure in UK Biobank. Nature. 2022;604:697–707. doi: 10.1038/s41586-022-04569-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haj E.M., Altintas E., Chapelet G., Kapogiannis D., Gallouj K. High depression and anxiety in people with Alzheimer’s disease living in retirement homes during the COVID-19 crisis. Psychiatry Res. 2020;291:113294. doi: 10.1016/j.psychres.2020.113294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lou J.J., Movassaghi M., Gordy D., Olson M.G., Zhang T., Khurana M.S., Chen Z., Perez-Rosendahl M., Thammachantha S., Singer E.J., et al. Neuropathology of COVID-19 (neuro-COVID): Clinicopathological update. Free Neuropathol. 2021;2:2. doi: 10.17879/freeneuropathology-2021-2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hui D.S., Azhar E.I., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., McHugh T.D., Memish Z.A., Drosten C., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. IJID Off. Publ. Int. Soc. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo Y.-R., Cao Q.-D., Hong Z.-S., Tan Y.-Y., Chen S.-D., Jin H.-J., Tan K.-S., Wang D.-Y., Yan Y. The origin, transmission and clinical therapies on coronavirus disease 2019 (COVID-19) outbreak—An update on the status. Mil. Med. Res. 2020;7:11. doi: 10.1186/s40779-020-00240-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luk H.K.H., Li X., Fung J., Lau S.K.P., Woo P.C.Y. Molecular epidemiology, evolution and phylogeny of SARS coronavirus. Infect. Genet. Evol. 2019;71:21–30. doi: 10.1016/j.meegid.2019.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramadan N., Shaib H. Middle East respiratory syndrome coronavirus (MERS-CoV): A review. Germs. 2019;9:35–42. doi: 10.18683/germs.2019.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Menachery V.D., Yount B.L., Jr., Debbink K., Agnihothram S., Gralinski L.E., Plante J.A., Graham R.L., Scobey T., Ge X.-Y., Donaldson E.F., et al. A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence. Nat. Med. 2015;21:1508–1513. doi: 10.1038/nm.3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ge X.-Y., Li J.-L., Yang X.-L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C., et al. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hemida M.G., Ali A.M., Alnaeem A. The Middle East respiratory syndrome coronavirus (MERS-CoV) nucleic acids detected in the saliva and conjunctiva of some naturally infected dromedary camels in Saudi Arabia-2019. Zoonoses Public Health. 2021;68:353–357. doi: 10.1111/zph.12816. [DOI] [PubMed] [Google Scholar]
- 21.Hemida M.G., Alhammadi M., Almathen F., Alnaeem A. Lack of detection of the Middle East respiratory syndrome coronavirus (MERS-CoV) nucleic acids in some Hyalomma dromedarii infesting some Camelus dromedary naturally infected with MERS-CoV. BMC Res. Notes. 2021;14:96. doi: 10.1186/s13104-021-05496-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benito-Pascual B., Gegúndez A.J., Díaz-Valle D., Arriola-Villalobos P., Carreño E., Culebras E., Rodríguez-Avial I., Benitez-Del-Castillo J.M. Panuveitis and Optic Neuritis as a Possible Initial Presentation of the Novel Coronavirus Disease 2019 (COVID-19) Ocul. Immunol. Inflamm. 2020;28:922–925. doi: 10.1080/09273948.2020.1792512. [DOI] [PubMed] [Google Scholar]
- 23.Pandey M.K. Pre-existing humoral immune comebacks control the development of the severe form of coronavirus disease 2019 in Gaucher patients. Clin. Transl. Discov. 2022;2:e96. doi: 10.1002/ctd2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carvelli J., Demaria O., Vély F., Batista L., Benmansour N.C., Fares J., Carpentier S., Thibult M.-L., Morel A., Remark R., et al. Association of COVID-19 inflammation with activation of the C5a–C5aR1 axis. Nature. 2020;588:146–150. doi: 10.1038/s41586-020-2600-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L., Sahu S.K., Cano M., Kuppuswamy V., Bajwa J., McPhatter J., Pine A., Meizlish M.L., Goshua G., Chang C.H., et al. Increased complement activation is a distinctive feature of severe SARS-CoV-2 infection. Sci. Immunol. 2021;6:1–21. doi: 10.1126/sciimmunol.abh2259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Afzali B., Noris M., Lambrecht B.N., Kemper C. The state of complement in COVID-19. Nat. Rev. Immunol. 2021;22:77–84. doi: 10.1038/s41577-021-00665-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cugno M., Meroni P.L., Gualtierotti R., Griffini S., Grovetti E., Torri A., Panigada M., Aliberti S., Blasi F., Tedesco F., et al. Complement activation in patients with COVID-19: A novel therapeutic target. J. Allergy Clin. Immunol. 2020;146:215–217. doi: 10.1016/j.jaci.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sinkovits G., Mező B., Réti M., Müller V., Iványi Z., Gál J., Gopcsa L., Reményi P., Szathmáry B., Lakatos B., et al. Complement Overactivation and Consumption Predicts In-Hospital Mortality in SARS-CoV-2 Infection. Front. Immunol. 2021;12:663187. doi: 10.3389/fimmu.2021.663187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rockx B., Baas T., Zornetzer G.A., Haagmans B., Sheahan T., Frieman M., Dyer M.D., Teal T.H., Proll S., Brand J.V.D., et al. Early Upregulation of Acute Respiratory Distress Syndrome-Associated Cytokines Promotes Lethal Disease in an Aged-Mouse Model of Severe Acute Respiratory Syndrome Coronavirus Infection. J. Virol. 2009;83:7062–7074. doi: 10.1128/JVI.00127-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perico L., Morigi M., Galbusera M., Pezzotta A., Gastoldi S., Imberti B., Perna A., Ruggenenti P., Donadelli R., Benigni A., et al. SARS-CoV-2 Spike Protein 1 Activates Microvascular Endothelial Cells and Complement System Leading to Platelet Aggregation. Front. Immunol. 2022;13:827146. doi: 10.3389/fimmu.2022.827146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gralinski L.E., Sheahan T.P., Morrison T.E., Menachery V., Jensen K., Leist S.R., Whitmore A., Heise M.T., Baric R.S. Complement Activation Contributes to Severe Acute Respiratory Syndrome Coronavirus Pathogenesis. mBio. 2018;9:e01753-18. doi: 10.1128/mBio.01753-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali Y.M., Ferrari M., Lynch N.J., Yaseen S., Dudler T., Gragerov S., Demopulos G., Heeney J.L., Schwaeble W.J. Lectin Pathway Mediates Complement Activation by SARS-CoV-2 Proteins. Front. Immunol. 2021;12:714511. doi: 10.3389/fimmu.2021.714511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ip W.K.E., Chan K.H., Law H.K.-W., Tso G.H.W., Kong E.K.P., Wong H.S.W., To Y.F., Yung R.W.H., Chow E.Y., Au K.L., et al. Mannose-Binding Lectin in Severe Acute Respiratory Syndrome Coronavirus Infection. J. Infect. Dis. 2005;191:1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen B., Yi X., Sun Y., Bi X., Du J., Zhang C., Quan S., Zhang F., Sun R., Qian L., et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell. 2020;182:59–72.e15. doi: 10.1016/j.cell.2020.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Holter J.C., Pischke S.E., de Boer E., Lind A., Jenum S., Holten A.R., Tonby K., Barratt-Due A., Sokolova M., Schjalm C., et al. Systemic complement activation is associated with respiratory failure in COVID-19 hospitalized patients. Proc. Natl. Acad. Sci. USA. 2020;117:25018–25025. doi: 10.1073/pnas.2010540117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Syrimi E., Fennell E., Richter A., Vrljicak P., Stark R., Ott S., Murray P.G., Al-Abadi E., Chikermane A., Dawson P., et al. The immune landscape of SARS-CoV-2-associated Multisystem Inflammatory Syndrome in Children (MIS-C) from acute disease to recovery. iScience. 2021;24:103215. doi: 10.1016/j.isci.2021.103215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romanova E.S., Vasilyev V.V., Startseva G., Karev V., Rybakova M.G., Platonov P.G. Cause of death based on systematic post-mortem studies in patients with positive SARS-CoV-2 tissue PCR during the COVID-19 pandemic. J. Intern. Med. 2021;290:655–665. doi: 10.1111/joim.13300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Magro C., Mulvey J.J., Berlin D., Nuovo G., Salvatore S., Harp J., Baxter-Stoltzfus A., Laurence J. Complement associated microvascular injury and thrombosis in the pathogenesis of severe COVID-19 infection: A report of five cases. Transl. Res. 2020;220:1–13. doi: 10.1016/j.trsl.2020.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pfister F., Vonbrunn E., Ries T., Jäck H.-M., Überla K., Lochnit G., Sheriff A., Herrmann M., Büttner-Herold M., Amann K., et al. Complement Activation in Kidneys of Patients With COVID-19. Front. Immunol. 2020;11:594849. doi: 10.3389/fimmu.2020.594849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect. Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Coleman C.M., Sisk J.M., Halasz G., Zhong J., Beck S.E., Matthews K.L., Venkataraman T., Rajagopalan S., Kyratsous C.A., Frieman M.B. CD8+ T Cells and Macrophages Regulate Pathogenesis in a Mouse Model of Middle East Respiratory Syndrome. J. Virol. 2017;91:1–22. doi: 10.1128/JVI.01825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tynell J., Westenius V., Rönkkö E., Munster V., Melén K., Österlund P., Julkunen I. Middle East respiratory syndrome coronavirus shows poor replication but significant induction of antiviral responses in human monocyte-derived macrophages and dendritic cells. J. Gen. Virol. 2016;97:344–355. doi: 10.1099/jgv.0.000351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou J., Chu H., Li C., Wong B.H.-Y., Cheng Z.-S., Poon V.K.-M., Sun T., Lau C.C.-Y., Wong K.K.-Y., Chan J.Y.-W., et al. Active Replication of Middle East Respiratory Syndrome Coronavirus and Aberrant Induction of Inflammatory Cytokines and Chemokines in Human Macrophages: Implications for Pathogenesis. J. Infect. Dis. 2013;209:1331–1342. doi: 10.1093/infdis/jit504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ng D.L., Hosani A.F., Keating M.K., Gerber S.I., Jones T.L., Metcalfe M.G., Tong S., Tao Y., Alami N.N., Haynes L.M., et al. Clinicopathologic, Immunohistochemical, and Ultrastructural Findings of a Fatal Case of Middle East Respiratory Syndrome Coronavirus Infection in the United Arab Emirates, April 2014. Am. J. Pathol. 2016;186:652–658. doi: 10.1016/j.ajpath.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Min C.-K., Cheon S., Ha N.-Y., Sohn K.M., Kim Y., Aigerim A., Shin H.M., Choi J.-Y., Inn K.-S., Kim J.H., et al. Comparative and kinetic analysis of viral shedding and immunological responses in MERS patients representing a broad spectrum of disease severity. Sci. Rep. 2016;6:25359. doi: 10.1038/srep25359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scheuplein V.A., Seifried J., Malczyk A.H., Miller L., Höcker L., Vergara-Alert J., Dolnik O., Zielecki F., Becker B., Spreitzer I., et al. High Secretion of Interferons by Human Plasmacytoid Dendritic Cells upon Recognition of Middle East Respiratory Syndrome Coronavirus. J. Virol. 2015;89:3859–3869. doi: 10.1128/JVI.03607-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim E.S., Choe P.G., Park W.B., Oh H.S., Kim E.J., Nam E.Y., Na S.H., Kim M., Song K.-H., Bang J.H., et al. Clinical Progression and Cytokine Profiles of Middle East Respiratory Syndrome Coronavirus Infection. J. Korean Med. Sci. 2016;31:1717–1725. doi: 10.3346/jkms.2016.31.11.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Law H.K.-W., Cheung C.Y., Ng H.Y., Sia S.F., Chan Y.O., Luk W., Nicholls J.M., Peiris J.S.M., Lau Y.L. Chemokine up-regulation in SARS-coronavirus–infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cheung C.Y., Poon L., Ng I.H.Y., Luk W., Sia S.-F., Wu M.H.S., Chan K.-H., Yuen K.-Y., Gordon S., Guan Y., et al. Cytokine Responses in Severe Acute Respiratory Syndrome Coronavirus-Infected Macrophages In Vitro: Possible Relevance to Pathogenesis. J. Virol. 2005;79:7819–7826. doi: 10.1128/JVI.79.12.7819-7826.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spiegel M., Schneider K., Weber F., Weidmann M., Hufert F.T. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J. Gen. Virol. 2006;87:1953–1960. doi: 10.1099/vir.0.81624-0. [DOI] [PubMed] [Google Scholar]
- 51.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., et al. Dysregulation of Immune Response in Patients with Coronavirus 2019 (COVID-19) in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zheng M., Gao Y., Wang G., Song G., Liu S., Sun D., Xu Y., Tian Z. Functional exhaustion of antiviral lymphocytes in COVID-19 patients. Cell Mol. Immunol. 2020;17:533–535. doi: 10.1038/s41423-020-0402-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fox S.E., Akmatbekov A., Harbert J.L., Li G., Brown J.Q., Heide R.S.V. Pulmonary and cardiac pathology in African American patients with COVID-19: An autopsy series from New Orleans. Lancet Respir. Med. 2020;8:681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartigue J., Crawford J.M., Daßler-Plenker J., Guerci P., Huynh C., Knight J.S., et al. Targeting potential drivers of COVID-19: Neutrophil extracellular traps. J. Exp. Med. 2020;217:e20200652. doi: 10.1084/jem.20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chu H., Zhou J., Wong B.H.-Y., Li C., Chan J.F.-W., Cheng Z.-S., Yang D., Wang D., Lee A.C.-Y., Li C., et al. Middle East Respiratory Syndrome Coronavirus Efficiently Infects Human Primary T Lymphocytes and Activates the Extrinsic and Intrinsic Apoptosis Pathways. J. Infect. Dis. 2015;213:904–914. doi: 10.1093/infdis/jiv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Fan Y.-Y., Huang Z.-T., Li L., Wu M.-H., Yu T., Koup R.A., Bailer R.T., Wu C.-Y. Characterization of SARS-CoV-specific memory T cells from recovered individuals 4 years after infection. Arch. Virol. 2009;154:1093–1099. doi: 10.1007/s00705-009-0409-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Li C.K.-F., Wu H., Yan H., Ma S., Wang L., Zhang M., Tang X., Temperton N., Weiss R.A., Brenchley J.M., et al. T Cell Responses to Whole SARS Coronavirus in Humans. J. Immunol. 2008;181:5490–5500. doi: 10.4049/jimmunol.181.8.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C., Liu S., Zhao P., Liu H., Zhu L., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S., Huang H., Zhang L., Zhou X., Du C., et al. Risk Factors Associated With Acute Respiratory Distress Syndrome and Death in Patients With Coronavirus Disease 2019 Pneumonia in Wuhan, China. JAMA Intern. Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Diao B., Wang C., Tan Y., Chen X., Liu Y., Ning L., Chen L., Li M., Liu Y., Wang G., et al. Reduction and Functional Exhaustion of T Cells in Patients with Coronavirus Disease 2019 (COVID-19) Front. Immunol. 2020;11:827. doi: 10.3389/fimmu.2020.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., Wang B., Xiang H., Cheng Z., Xiong Y., et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus—Infected Pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., et al. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214:108393. doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bai Y., Yao L., Wei T., Tian F., Jin D.Y., Chen L., Wang M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA. 2020;323:1406–1407. doi: 10.1001/jama.2020.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lau S.K.P., Lau C.C.Y., Chan K.-H., Li C.P.Y., Chen H., Jin D.-Y., Chan J.F.W., Woo P.C.Y., Yuen K.-Y. Delayed induction of proinflammatory cytokines and suppression of innate antiviral response by the novel Middle East respiratory syndrome coronavirus: Implications for pathogenesis and treatment. J. Gen. Virol. 2013;94:2679–2690. doi: 10.1099/vir.0.055533-0. [DOI] [PubMed] [Google Scholar]
- 65.Yen Y.-T., Liao F., Hsiao C.-H., Kao C.-L., Chen Y.-C., Wu-Hsieh B.A. Modeling the Early Events of Severe Acute Respiratory Syndrome Coronavirus Infection In Vitro. J. Virol. 2006;80:2684–2693. doi: 10.1128/JVI.80.6.2684-2693.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pandey M.K., Rani R., Zhang W., Setchell K., Grabowski G.A. Immunological cell type characterization and Th1–Th17 cytokine production in a mouse model of Gaucher disease. Mol. Genet. Metab. 2012;106:310–322. doi: 10.1016/j.ymgme.2012.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandey M.K., Grabowski G.A. Immunological Cells and Functions in Gaucher Disease. Crit. Rev. Oncog. 2013;18:197–220. doi: 10.1615/CritRevOncog.2013004503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gigis I., Pitsilos C., Samoladas E., Pavlopoulos C., Hytiroglou P., Ditsios K., Papadopoulos P. Gaucher Disease: An Unusual Cause of Knee Pain. JAAOS Glob. Res. Rev. 2022;6:1–7. doi: 10.5435/JAAOSGlobal-D-21-00243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rosenbloom B.E., Weinreb N.J. Gaucher Disease: A Comprehensive Review. Crit. Rev. Oncog. 2013;18:163–175. doi: 10.1615/CritRevOncog.2013006060. [DOI] [PubMed] [Google Scholar]
- 70.Dandana A., Khelifa B.S., Chahed H., Miled A., Ferchichi S. Gaucher Disease: Clinical, Biological and Therapeutic Aspects. Pathobiology. 2016;83:13–23. doi: 10.1159/000440865. [DOI] [PubMed] [Google Scholar]
- 71.Carubbi F., Cappellini M.D., Fargion S., Fracanzani A.L., Nascimbeni F. Liver involvement in Gaucher disease: A practical review for the hepatologist and the gastroenterologist. Dig. Liv. Dis. 2020;52:368–373. doi: 10.1016/j.dld.2020.01.004. [DOI] [PubMed] [Google Scholar]
- 72.Arévalo N.B., Lamaizon C.M., Cavieres V.A., Burgos P.V., Álvarez A.R., Yañez M.J., Zanlungo S. Neuronopathic Gaucher disease: Beyond lysosomal dysfunction. Front. Mol. Neurosci. 2022;15:934820. doi: 10.3389/fnmol.2022.934820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stirnemann J., Belmatoug N., Camou F., Serratrice C., Froissart R., Caillaud C., Levade T., Astudillo L., Serratrice J., Brassier A., et al. A Review of Gaucher Disease Pathophysiology, Clinical Presentation and Treatments. Int. J. Mol. Sci. 2017;18:441. doi: 10.3390/ijms18020441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Motta I., Splenomegaly Gaucher Group. Consonni D., Stroppiano M., Benedetto C., Cassinerio E., Tappino B., Ranalli P., Borin L., Facchini L., et al. Predicting the probability of Gaucher disease in subjects with splenomegaly and thrombocytopenia. Sci. Rep. 2021;11:2594. doi: 10.1038/s41598-021-82296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee F.-S., Yen H.-J., Niu D.-M., Hung G.-Y., Lee C.-Y., Yeh Y.-C., Chen P.C.-H., Chang S.-K., Yang C.-F. Allogeneic hematopoietic stem cell transplantation for treating severe lung involvement in Gaucher disease. Mol. Genet. Metab. Rep. 2020;25:100652. doi: 10.1016/j.ymgmr.2020.100652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mauhin W., Brassier A., London J., Subran B., Zeggane A., Besset Q., Jammal C., Montardi C., Mellot C., Strauss C., et al. Manifestations pulmonaires des maladies héréditaires du métabolisme. Rev. Mal. Respir. 2022;39:758–777. doi: 10.1016/j.rmr.2022.09.002. [DOI] [PubMed] [Google Scholar]
- 77.Grabowski G.A., Antommaria A.H., Kolodny E.H., Mistry P.K. Gaucher disease: Basic and translational science needs for more complete therapy and management. Mol. Genet. Metab. 2020;132:59–75. doi: 10.1016/j.ymgme.2020.12.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vitner E.B., Achdout H., Avraham R., Politi B., Cherry L., Tamir H., Yahalom-Ronen Y., Paran N., Melamed S., Erez N., et al. Glucosylceramide synthase inhibitors prevent replication of SARS-CoV-2 and influenza virus. J. Biol. Chem. 2021;296:100470. doi: 10.1016/j.jbc.2021.100470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Serfecz J.C., Saadin A., Santiago C.P., Zhang Y., Bentzen S.M., Vogel S.N., Feldman R.A. C5a Activates a Pro-Inflammatory Gene Expression Profile in Human Gaucher iPSC-Derived Macrophages. Int. J. Mol. Sci. 2021;22:9912. doi: 10.3390/ijms22189912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Laurence J., Mulvey J.J., Seshadri M., Racanelli A., Harp J., Schenck E.J., Zappetti D., Horn E.M., Magro C.M. Anti-complement C5 therapy with eculizumab in three cases of critical COVID-19. Clin. Immunol. 2020;219:108555. doi: 10.1016/j.clim.2020.108555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lo M.W., Kemper C., Woodruff T.M. COVID-19: Complement, Coagulation, and Collateral Damage. J. Immunol. 2020;205:1488–1495. doi: 10.4049/jimmunol.2000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mastellos D.C., da Silva B.G.P., Fonseca B.A., Fonseca N.P., Auxiliadora-Martins M., Mastaglio S., Ruggeri A., Sironi M., Radermacher P., Chrysanthopoulou A., et al. Complement C3 vs. C5 inhibition in severe COVID-19: Early clinical findings reveal differential biological efficacy. Clin. Immunol. 2020;220:108598. doi: 10.1016/j.clim.2020.108598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.ElFiky A.A., Mahdy S.M., Elshemey W.M. Quantitative structure-activity relationship and molecular docking revealed a potency of anti-hepatitis C virus drugs against human corona viruses. J. Med. Virol. 2017;89:1040–1047. doi: 10.1002/jmv.24736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chan J.F., Lau S.K., To K.K., Cheng V.C., Woo P.C., Yuen K.Y. Middle East Respiratory Syndrome Coronavirus: Another Zoonotic Betacoronavirus Causing SARS-Like Disease. Clin. Microbiol. Rev. 2015;28:465–522. doi: 10.1128/CMR.00102-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cui J., Li F., Shi Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fehr A.R., Perlman S. Coronaviruses: An overview of their replication and pathogenesis. Methods Mol. Biol. 2015;1282:1–23. doi: 10.1007/978-1-4939-2438-7_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Boehm M., Nabel E.G. Angiotensin-Converting Enzyme 2—A New Cardiac Regulator. N. Engl. J. Med. 2002;347:1795–1797. doi: 10.1056/NEJMcibr022472. [DOI] [PubMed] [Google Scholar]
- 89.Crackower M.A., Sarao R., Oudit G.Y., Yagil C., Kozieradzki I., Scanga S.E., Oliveira-Dos-Santos A.J., Costa D.J., Zhang L., Pei Y., et al. Angiotensin-converting enzyme 2 is an essential regulator of heart function. Nature. 2002;417:822–828. doi: 10.1038/nature00786. [DOI] [PubMed] [Google Scholar]
- 90.Marinho P.M., Marcos A.A.A., Romano A.C., Nascimento H., Belfort R. Retinal findings in patients with COVID-19. Lancet. 2020;395:1610. doi: 10.1016/S0140-6736(20)31014-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhou P., Yang X.-L., Wang X.-G., Hu B., Zhang L., Zhang W., Si H.-R., Zhu Y., Li B., Huang C.-L., et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chen J., Jiang Q., Xia X., Liu K., Yu Z., Tao W., Gong W., Han J.J. Individual variation of the SARS-CoV-2 receptor ACE2 gene expression and regulation. Aging Cell. 2020;19:e13168. doi: 10.1111/acel.13168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Seah I., Agrawal R. Can the Coronavirus Disease 2019 (COVID-19) Affect the Eyes? A Review of Coronaviruses and Ocular Implications in Humans and Animals. Ocul. Immunol. Inflamm. 2020;28:391–395. doi: 10.1080/09273948.2020.1738501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cardona G.C., Pájaro L.D.Q., Marzola I.D.Q., Villegas Y.R., Salazar L.R.M. Neurotropism of SARS-CoV 2: Mechanisms and manifestations. J. Neurol. Sci. 2020;412:116824. doi: 10.1016/j.jns.2020.116824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lu C.-W., Liu X.-F., Jia Z.-F. 2019-nCoV transmission through the ocular surface must not be ignored. Lancet. 2020;395:e39. doi: 10.1016/S0140-6736(20)30313-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Seah I., Su X., Lingam G. Revisiting the dangers of the coronavirus in the ophthalmology practice. Eye. 2020;34:1155–1157. doi: 10.1038/s41433-020-0790-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cheema M., Aghazadeh H., Nazarali S., Ting A., Hodges J., McFarlane A., Kanji J.N., Zelyas N., Damji K.F., Solarte C. Keratoconjunctivitis as the initial medical presentation of the novel coronavirus disease 2019 (COVID-19) Can. J. Ophthalmol. 2020;55:e125–e129. doi: 10.1016/j.jcjo.2020.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.-H., Nitsche A., et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e278. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mokhtari T., Hassani F., Ghaffari N., Ebrahimi B., Yarahmadi A., Hassanzadeh G. COVID-19 and multiorgan failure: A narrative review on potential mechanisms. Histochem. J. 2020;51:613–628. doi: 10.1007/s10735-020-09915-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Qu C.Q., He J.X., Liu L., Shan H., Lei C.L., Hui D.S.C., et al. Clinical Characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Camporota L., Cronin J.N., Busana M., Gattinoni L., Formenti F. Pathophysiology of coronavirus-19 disease acute lung injury. Curr. Opin. Crit. Care. 2022;28:9–16. doi: 10.1097/MCC.0000000000000911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., et al. Relationships among lymphocyte subsets, cytokines, and the pulmonary inflammation index in coronavirus (COVID-19) infected patients. Br. J. Haematol. 2020;189:428–437. doi: 10.1111/bjh.16659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zawawi A., Naser A.Y., Alwafi H., Minshawi F. Profile of Circulatory Cytokines and Chemokines in Human Coronaviruses: A Systematic Review and Meta-Analysis. Front. Immunol. 2021;12:666223. doi: 10.3389/fimmu.2021.666223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Chien J.-Y., Hsueh P.-R., Cheng W.-C., Yu C.-J., Yang P.-C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11:715–722. doi: 10.1111/j.1440-1843.2006.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wang C.-H., Liu C.-Y., Wan Y.-L., Chou C.-L., Huang K.-H., Lin H.-C., Lin S.-M., Lin T.-Y., Chung K.F., Kuo-Hsiung H. Persistence of lung inflammation and lung cytokines with high-resolution CT abnormalities during recovery from SARS. Respir. Res. 2005;6:42. doi: 10.1186/1465-9921-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wong C.K., Lam C.W.K., Wu A.K.L., Ip W.K., Lee N.L.S., Chan I.H.S., Lit L.C.W., Hui D.S.C., Chan M.H.M., Chung S.S.C., et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zhang Y., Li J., Zhan Y., Wu L., Yu X., Zhang W., Ye L., Xu S., Sun R., Wang Y., et al. Analysis of Serum Cytokines in Patients with Severe Acute Respiratory Syndrome. Infect. Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cameron M.J., Bermejo-Martin J.F., Danesh A., Muller M.P., Kelvin D.J. Human immunopathogenesis of severe acute respiratory syndrome (SARS) Virus Res. 2008;133:13–19. doi: 10.1016/j.virusres.2007.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cameron M.J., Ran L., Xu L., Danesh A., Bermejo-Martin J.F., Cameron C.M., Muller M.P., Gold W.L., Richardson S.E., Poutanen S.M., et al. Interferon-Mediated Immunopathological Events Are Associated with Atypical Innate and Adaptive Immune Responses in Patients with Severe Acute Respiratory Syndrome. J. Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang K.-J., Su I.-J., Theron M., Wu Y.-C., Lai S.-K., Liu C.-C., Lei H.-Y. An interferon-?-related cytokine storm in SARS patients. J. Med. Virol. 2004;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Theron M., Huang K.-J., Chen Y.-W., Liu C.-C., Lei H.-Y. A probable role for IFN-γ in the development of a lung immunopathology in SARS. Cytokine. 2005;32:30–38. doi: 10.1016/j.cyto.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kim J.S., Lee J.Y., Yang J.W., Lee K.H., Effenberger M., Szpirt W., Kronbichler A., Shin J.I. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11:316–329. doi: 10.7150/thno.49713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chen Y., Wang J., Liu C., Su L., Zhang D., Fan J., Yang Y., Xiao M., Xie J., Xu Y., et al. IP-10 and MCP-1 as biomarkers associated with disease severity of COVID-19. Mol. Med. 2020;26:97. doi: 10.1186/s10020-020-00230-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Conti P., Gallenga C.E., Tete G., Caraffa A., Ronconi G., Younes A., Toniato E., Ross R., Kritas S.K. How to reduce the likelihood of coronavirus-19 (CoV-19 or SARS-CoV-2) infection and lung inflammation mediated by IL-1. J. Biol. Regul. Homeost. Agents. 2020;34:333–338. doi: 10.23812/Editorial-Conti-2. [DOI] [PubMed] [Google Scholar]
- 118.Ruan Q., Yang K., Wang W., Jiang L., Song J. Clinical predictors of mortality due to COVID-19 based on an analysis of data of 150 patients from Wuhan, China. Intensive Care Med. 2020;46:846–848. doi: 10.1007/s00134-020-05991-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McElvaney O.J., McEvoy N.L., McElvaney O.F., Carroll T.P., Murphy M.P., Dunlea D.M., Choileain N.O., Clarke J., O’Connor E., Hogan G., et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am. J. Respir. Crit. Care Med. 2020;202:812–821. doi: 10.1164/rccm.202005-1583OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Pandey M.K., Jabre N.A., Xu Y.-H., Zhang W., Setchell K.D., Grabowski G.A. Gaucher disease: Chemotactic factors and immunological cell invasion in a mouse model. Mol. Genet. Metab. 2014;111:163–171. doi: 10.1016/j.ymgme.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 122.Zahran A.M., Saad K., Abdallah A.-E.M., Gad E.F., Abdel-Raheem Y.F., Zahran Z.A.M., Abdelsalam E.M.N., Elhoufey A., Alruwaili T., Mahmoud K.H., et al. Dendritic cells and monocyte subsets in children with Gaucher disease. Pediatr. Res. 2021;90:664–669. doi: 10.1038/s41390-020-01300-w. [DOI] [PubMed] [Google Scholar]
- 123.Micheva I., Marinakis T., Repa C., Kouraklis-Symeonidis A., Vlacha V., Anagnostopoulos N., Zoumbos N. Dendritic cells in patients with type I Gaucher disease are decreased in number but functionally normal. Blood Cells Mol. Dis. 2006;36:298–307. doi: 10.1016/j.bcmd.2005.12.029. [DOI] [PubMed] [Google Scholar]
- 124.Magnusen A.F., Rani R., McKay M.A., Hatton S.L., Nyamajenjere T.C., Magnusen D.N.A., Köhl J., Grabowski G.A., Pandey M.K. C-X-C Motif Chemokine Ligand 9 and Its CXCR3 Receptor Are the Salt and Pepper for T Cells Trafficking in a Mouse Model of Gaucher Disease. Int. J. Mol. Sci. 2021;22:12712. doi: 10.3390/ijms222312712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mistry P.K., Liu J., Yang M., Nottoli T., McGrath J., Jain D., Zhang K., Keutzer J., Chuang W.-L., Mehal W.Z., et al. Glucocerebrosidase gene-deficient mouse recapitulates Gaucher disease displaying cellular and molecular dysregulation beyond the macrophage. Proc. Natl. Acad. Sci. USA. 2010;107:19473–19478. doi: 10.1073/pnas.1003308107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim N.E., Do H., Jeong H., Kim T., Heo S.H., Kim Y., Cheon C.K., Lee Y., Choi Y., Choi I.H., et al. Identification of a novel therapeutic target underlying atypical manifestation of Gaucher disease. Clin. Transl. Med. 2022;12:e862. doi: 10.1002/ctm2.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Braudeau C., Graveleau J., Rimbert M., Néel A., Hamidou M., Grosbois B., Besançon A., Giraudet S., Terrien C., Josien R., et al. Altered innate function of plasmacytoid dendritic cells restored by enzyme replacement therapy in Gaucher disease. Blood Cells Mol. Dis. 2013;50:281–288. doi: 10.1016/j.bcmd.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 128.Sønder S.U., Limgala R.P., Ivanova M.M., Ioanou C., Plassmeyer M., Marti G.E., Alpan O., Goker-Alpan O. Persistent immune alterations and comorbidities in splenectomized patients with Gaucher disease. Blood Cells Mol. Dis. 2016;59:8–15. doi: 10.1016/j.bcmd.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 129.Zahran A.M., Saad K., Elsayh K.I., Abdou M.A.A., Abo-Elgheet A.M., Eloseily E.M., Khalaf S.M., Sror S., Ahmad F.-A., Elhoufey A., et al. Upregulation of Cytotoxic T-cells in pediatric patients with Gaucher disease. Sci. Rep. 2022;12:4977. doi: 10.1038/s41598-022-08843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fiolet T., Kherabi Y., MacDonald C.-J., Ghosn J., Peiffer-Smadja N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2021;28:202–221. doi: 10.1016/j.cmi.2021.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rubin R. COVID-19 Vaccines vs Variants—Determining How Much Immunity Is Enough. JAMA. 2021;325:1241. doi: 10.1001/jama.2021.3370. [DOI] [PubMed] [Google Scholar]
- 132.Barouch D.H., Stephenson K.E., Sadoff J., Yu J., Chang A., Gebre M., McMahan K., Liu J., Chandarshekar A., Patel S., et al. Durable Humoral and Cellular Immune Responses Following Ad26.COV2.S Vaccination for COVID-19. N. Engl. J. Med. 2021;385:951–953. doi: 10.1056/NEJMc2108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., Broder K.R., Gee J., Weintraub E., Shimabukuro T., et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices—United States, June. MMWR Morb. Mortal. Wkly. Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chandwani A., Shuter J. Lopinavir/ritonavir in the treatment of HIV-1 infection: A review. Ther. Clin. Risk Manag. 2008;4:1023–1033. doi: 10.2147/tcrm.s3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Magro P., Zanella I., Pescarolo M., Castelli F., Quiros-Roldan E. Lopinavir/ritonavir: Repurposing an old drug for HIV infection in COVID-19 treatment. Biomed. J. 2020;44:43–53. doi: 10.1016/j.bj.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lim J., Jeon S., Shin H.-Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.-W., Kang Y.M., Lee B., Park S.-J. Case of the Index Patient Who Caused Tertiary Transmission of Coronavirus Disease 2019 in Korea: The Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Pneumonia Monitored by Quantitative RT-PCR. J. Korean Med. Sci. 2020;35:e79. doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rehman S.U., Rehman S.U., Yoo H.H. COVID-19 challenges and its therapeutics. Biomed. Pharmacother. 2021;142:112015. doi: 10.1016/j.biopha.2021.112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Qomara W.F., Primanissa D.N., Amalia S.H., Purwadi F.V., Zakiyah N. Effectiveness of Remdesivir, Lopinavir/Ritonavir, and Favipiravir for COVID-19 Treatment: A Systematic Review. Int. J. Gen. Med. 2021;14:8557–8571. doi: 10.2147/IJGM.S332458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gordon C.J., Tchesnokov E.P., Woolner E., Perry J.K., Feng J.Y., Porter D.P., Götte M. Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 2020;295:6785–6797. doi: 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kokic G., Hillen H.S., Tegunov D., Dienemann C., Seitz F., Schmitzova J., Farnung L., Siewert A., Höbartner C., Cramer P. Mechanism of SARS-CoV-2 polymerase stalling by remdesivir. Nat. Commun. 2021;12:279. doi: 10.1038/s41467-020-20542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pilkington V., Pepperrell T., Hill A. A review of the safety of favipiravir—a potential treatment in the COVID-19 pandemic? J. Virus Erad. 2020;6:45–51. doi: 10.1016/S2055-6640(20)30016-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mahajan L., Singh A. Gifty Clinical outcomes of using remdesivir in patients with moderate to severe COVID-19: A prospective randomised study. Indian J. Anaesth. 2021;65:S41–S46. doi: 10.4103/ija.IJA_149_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bistrovic P., Manola S., Lucijanic M. Bradycardia during remdesivir treatment might be associated with improved survival in patients with COVID-19: A retrospective cohort study on 473 patients from a tertiary centre. Postgrad. Med. J. 2021;98:501–502. doi: 10.1136/postgradmedj-2021-141079. [DOI] [PubMed] [Google Scholar]
- 144.Al-Abdouh A., Bizanti A., Barbarawi M., Jabri A., Kumar A., Fashanu O.E., Khan S.U., Zhao D., Antar A.A., Michos E.D. Remdesivir for the treatment of COVID-19: A systematic review and meta-analysis of randomized controlled trials. Contemp. Clin. Trials. 2021;101:106272. doi: 10.1016/j.cct.2021.106272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kyriazopoulou E., Poulakou G., Milionis H., Metallidis S., Adamis G., Tsiakos K., Fragkou A., Rapti A., Damoulari C., Fantoni M., et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021;27:1752–1760. doi: 10.1038/s41591-021-01499-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kaly L., Rosner I. Tocilizumab—A novel therapy for non-organ-specific autoimmune diseases. Best Pract. Res. Clin. Rheumatol. 2012;26:157–165. doi: 10.1016/j.berh.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 147.Rizk J.G., Kalantar-Zadeh K., Mehra M.R., Lavie C.J., Rizk Y., Forthal D.N. Pharmaco-Immunomodulatory Therapy in COVID-19. Drugs. 2020;80:1267–1292. doi: 10.1007/s40265-020-01367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Herold S., Hoegner K., Vadász I., Gessler T., Wilhelm J., Mayer K., Morty R.E., Walmrath H.-D., Seeger W., Lohmeyer J. Inhaled Granulocyte/Macrophage Colony–Stimulating Factor as Treatment of Pneumonia-associated Acute Respiratory Distress Syndrome. Am. J. Respir. Crit. Care Med. 2014;189:609–611. doi: 10.1164/rccm.201311-2041LE. [DOI] [PubMed] [Google Scholar]
- 150.Van Laarhoven A., Kurver L., Overheul G.J., Kooistra E.J., Abdo W.F., van Crevel R., Duivenvoorden R., Kox M., Oever J.T., Schouten J., et al. Interferon gamma immunotherapy in five critically ill COVID-19 patients with impaired cellular immunity: A case series. Med. 2021;2:1163–1170.e2. doi: 10.1016/j.medj.2021.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Villar J., Ferrando C., Martínez D., Ambrós A., Muñoz T., Soler J.A., Aguilar G., Alba F., González-Higueras E., Conesa L.A., et al. Dexamethasone treatment for the acute respiratory distress syndrome: A multicentre, randomised controlled trial. Lancet Respir. Med. 2020;8:267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 152.Chen L., Shi M., Deng Q., Liu W., Li Q., Ye P., Yu X., Zhang B., Xu Y., Li X., et al. A multi-center randomized prospective study on the treatment of infant bronchiolitis with interferon α1b nebulization. PLoS ONE. 2020;15:e0228391. doi: 10.1371/journal.pone.0228391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Dashti-Khavidaki S., Khalili H. Considerations for Statin Therapy in Patients with COVID-19. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2020;40:484–486. doi: 10.1002/phar.2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Chang Y., Wei W. Angiotensin II in inflammation, immunity and rheumatoid arthritis. Clin. Exp. Immunol. 2015;179:137–145. doi: 10.1111/cei.12467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Cohen J.I., Burbelo P.D. Reinfection With SARS-CoV-2: Implications for Vaccines. Clin. Infect. Dis. 2020;73:e4223–e4228. doi: 10.1093/cid/ciaa1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Galanti M., Shaman J. Direct Observation of Repeated Infections with Endemic Coronaviruses. J. Infect. Dis. 2020;223:409–415. doi: 10.1093/infdis/jiaa392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Murchu E.O., Byrne P., Carty P.G., Gascun D.C., Keogan M., O’Neill M., Harrington P., Ryan M. Quantifying the risk of SARS-CoV-2 reinfection over time. Rev. Med. Virol. 2021;32:e2260. doi: 10.1002/rmv.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nguyen Y., Stirnemann J., Belmatoug N. La maladie de Gaucher: Quand y penser? Revue Méd. Interne. 2019;40:313–322. doi: 10.1016/j.revmed.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 159.Grabowski G.A., Mistry P.K. Therapies for lysosomal storage diseases: Principles, practice, and prospects for refinements based on evolving science. Mol. Genet. Metab. 2022;137:81–91. doi: 10.1016/j.ymgme.2022.07.014. [DOI] [PubMed] [Google Scholar]
- 160.Xu Y.H., Quinn B., Witte D., Grabowski G.A. Viable mouse models of acid beta-glucosidase deficiency: The defect in Gaucher disease. Am. J. Pathol. 2003;163:2093–2101. doi: 10.1016/S0002-9440(10)63566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Pandey M.K., Grabowski G.A. Advances in Gaucher Disease: Basic and Clinical Perspectives 78–93. Future Medicine Ltd.; London, UK: 2013. [Google Scholar]
- 162.Koprivica V., Stone D.L., Park J.K., Callahan M., Frisch A., Cohen I.J., Tayebi N., Sidransky E. Analysis and Classification of 304 Mutant Alleles in Patients with Type 1 and Type 3 Gaucher Disease. Am. J. Hum. Genet. 2000;66:1777–1786. doi: 10.1086/302925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sun Y., Liou B., Ran H., Skelton M.R., Williams M.T., Vorhees C.V., Kitatani K., Hannun Y.A., Witte D.P., Xu Y.-H., et al. Neuronopathic Gaucher disease in the mouse: Viable combined selective saposin C deficiency and mutant glucocerebrosidase (V394L) mice with glucosylsphingosine and glucosylceramide accumulation and progressive neurological deficits. Hum. Mol. Genet. 2010;19:1088–1097. doi: 10.1093/hmg/ddp580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Wong K., Sidransky E., Verma A., Mixon T., Sandberg G.D., Wakefield L.K., Morrison A., Lwin A., Colegial C., Allman J.M., et al. Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol. Genet. Metab. 2004;82:192–207. doi: 10.1016/j.ymgme.2004.04.011. [DOI] [PubMed] [Google Scholar]
- 165.Vitner E.B., Farfel-Becker T., Eilam R., Biton I., Futerman A. Contribution of brain inflammation to neuronal cell death in neuronopathic forms of Gaucher’s disease. Brain. 2012;135:1724–1735. doi: 10.1093/brain/aws095. [DOI] [PubMed] [Google Scholar]
- 166.Vitner E.B., Dekel H., Zigdon H., Shachar T., Farfel-Becker T., Eilam R., Karlsson S., Futerman A.H. Altered expression and distribution of cathepsins in neuronopathic forms of Gaucher disease and in other sphingolipidoses. Hum. Mol. Genet. 2010;19:3583–3590. doi: 10.1093/hmg/ddq273. [DOI] [PubMed] [Google Scholar]
- 167.Dasgupta N., Xu Y.-H., Li R., Peng Y., Pandey M.K., Tinch S.L., Liou B., Inskeep V., Zhang W., Setchell K.D., et al. Neuronopathic Gaucher disease: Dysregulated mRNAs and miRNAs in brain pathogenesis and effects of pharmacologic chaperone treatment in a mouse model. Hum. Mol. Genet. 2015;24:7031–7048. doi: 10.1093/hmg/ddv404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Orvisky E., Park J., Parker A., Walker J., Martin B., Stubblefield B., Uyama E., Tayebi N., Sidransky E. The identification of eight novel glucocerebrosidase (GBA) mutations in patients with Gaucher disease. Hum. Mutat. 2002;19:458–459. doi: 10.1002/humu.9024. [DOI] [PubMed] [Google Scholar]
- 169.Conradi N.G., Sourander P., Nilsson O., Svennerholm L., Erikson A. Neuropathology of the Norrbottnian type of Gaucher disease. Acta Neuropathol. 1984;65:99–109. doi: 10.1007/BF00690463. [DOI] [PubMed] [Google Scholar]
- 170.Gegg M.E., Burke D., Heales S.J.R., Cooper J.M., Hardy J., Wood N.W., Schapira A.H.V. Glucocerebrosidase deficiency in substantia nigra of parkinson disease brains. Ann. Neurol. 2012;72:455–463. doi: 10.1002/ana.23614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Murphy K.E., Gysbers A.M., Abbott S.K., Tayebi N., Kim W.S., Sidransky E., Cooper A., Garner B., Halliday G.M. Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Pt 3Brain. 2014;137:834–848. doi: 10.1093/brain/awt367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Farfel-Becker T., Vitner E.B., Kelly S.L., Bame J.R., Duan J., Shinder V., Merrill A.H., Jr., Dobrenis K., Futerman A.H. Neuronal accumulation of glucosylceramide in a mouse model of neuronopathic Gaucher disease leads to neurodegeneration. Hum. Mol. Genet. 2013;23:843–854. doi: 10.1093/hmg/ddt468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mignot C., Doummar D., Maire I., Villemeur D.T.B. Type 2 Gaucher disease: 15 new cases and review of the literature. Brain Dev. 2006;28:39–48. doi: 10.1016/j.braindev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 174.Gupta N., Oppenheim I., Kauvar E., Tayebi N., Sidransky E. Type 2 Gaucher disease: Phenotypic variation and genotypic heterogeneity. Blood Cells Mol. Dis. 2011;46:75–84. doi: 10.1016/j.bcmd.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Harris C., Taylor D., Vellodi A. Ocular Motor Abnormalities in Gaucher Disease. Neuropediatrics. 1999;30:289–293. doi: 10.1055/s-2007-973507. [DOI] [PubMed] [Google Scholar]
- 176.Cogan D.G., Chu F.C., Reingold D., Barranger J. Ocular Motor Signs in Some Metabolic Diseases. Arch. Ophthalmol. 1981;99:1802–1808. doi: 10.1001/archopht.1981.03930020676010. [DOI] [PubMed] [Google Scholar]
- 177.Sidransky E., Tsujl S., Stubblefield B.K., Gurrie J., FitzGibbon E.J., Glnns E.I. Gaudier patients with oculomotor abnormalities do not have a unique genotype. Clin. Genet. 2008;41:1–5. doi: 10.1111/j.1399-0004.1992.tb03618.x. [DOI] [PubMed] [Google Scholar]
- 178.Patterson M.C., Horowitz M., Abel R.B., Currie J.N., Yu K.-T., Kaneski C., Higgins J.J., O’Neill R.R., Fedio P., Pikus A., et al. Isolated horizontal supranuclear gaze palsy as a marker of severe systemic involvement in Gaucher’s disease. Neurology. 1993;43:1993. doi: 10.1212/WNL.43.10.1993. [DOI] [PubMed] [Google Scholar]
- 179.King J.O. Progressive myoclonic epilepsy due to Gaucher’s disease in an adult. J. Neurol. Neurosurg. Psychiatry. 1975;38:849–854. doi: 10.1136/jnnp.38.9.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Winkelman M.D., Banker B.Q., Victor M., Moser H.W. Non-infantile neuronopathic Gaucher’s disease: A clinicopathologic study. Neurology. 1983;33:994. doi: 10.1212/WNL.33.8.994. [DOI] [PubMed] [Google Scholar]
- 181.Neil J.F., Glew R.H., Peters S.P. Familial Psychosis and Diverse Neurologic Abnormalities in Adult-Onset Gaucher’s Disease. Arch. Neurol. 1979;36:95–99. doi: 10.1001/archneur.1979.00500380065007. [DOI] [PubMed] [Google Scholar]
- 182.Yoshikawa H., Fueki N., Sasaki M., Sakuragawa N. Uncoupling of blood flow and oxygen metabolism in the cerebellum in type 3 Gaucher disease. Brain Dev. 1991;13:190–192. doi: 10.1016/S0387-7604(12)80029-5. [DOI] [PubMed] [Google Scholar]
- 183.Seeman P., Hoppner J., Lakner V., Liebisch I., Grau G., Rolfs A., Finckh U. Two new missense mutations in a non-Jewish Caucasian family with type 3 Gaucher disease. Neurology. 1996;46:1102–1107. doi: 10.1212/WNL.46.4.1102. [DOI] [PubMed] [Google Scholar]
- 184.Grover W.D., Tucker S.H., Wenger D.A. Clinical variation in 2 related children with neuronopathic Gaucher disease. Ann. Neurol. 1978;3:281–283. doi: 10.1002/ana.410030316. [DOI] [PubMed] [Google Scholar]
- 185.Conradi N., Kyllerman M., Percy A.K., Svennerholm L. Late-infantile Gaucher disease in a child with myoclonus and bulbar signs: Neuropathological and neurochemical findings. Acta Neuropathol. 1991;82:152–157. doi: 10.1007/BF00293959. [DOI] [PubMed] [Google Scholar]
- 186.Dobbelaere D., Sukno S., Defoort-Dhellemmes S., Lamblin M.-D., Largillière C. Neurological outcome of a patient with Gaucher disease type III treated by enzymatic replacement therapy. J. Inherit. Metab. Dis. 1998;21:74–76. doi: 10.1023/A:1005319632539. [DOI] [PubMed] [Google Scholar]
- 187.Verghese J., Goldberg R.F., Desnick R.J., Grace M.E., Goldman J.E., Lee S.C., Dickson D.W., Rapin I. Myoclonus from selective dentate nucleus degeneration in type 3 Gaucher disease. Arch. Neurol. 2000;57:389–395. doi: 10.1001/archneur.57.3.389. [DOI] [PubMed] [Google Scholar]
- 188.Erikson A. Gaucher disease-Norrbottnian type (III) Acta Paediatr. 1986;75:1–42. doi: 10.1111/j.1651-2227.1986.tb14936.x. [DOI] [PubMed] [Google Scholar]
- 189.Park J.K., Orvisky E., Tayebi N., Kaneski C., Lamarca M.E., Stubblefield B.K., Martin B.M., Schiffmann R., Sidransky E. Myoclonic Epilepsy in Gaucher Disease: Genotype-Phenotype Insights from a Rare Patient Subgroup. Pediatr. Res. 2003;53:387–395. doi: 10.1203/01.PDR.0000049515.79882.94. [DOI] [PubMed] [Google Scholar]
- 190.Limgala R.P., Ioanou C., Plassmeyer M., Ryherd M., Kozhaya L., Austin L., Abidoglu C., Unutmaz D., Alpan O., Goker-Alpan O. Time of Initiating Enzyme Replacement Therapy Affects Immune Abnormalities and Disease Severity in Patients with Gaucher Disease. PLoS ONE. 2016;11:e0168135. doi: 10.1371/journal.pone.0168135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Zahran A.M., Youssef M.A.M., Shafik E.A., Zahran Z.A.M., El-Badawy O., Elgheet A.M.A., Elsayh K.I. Downregulation of B regulatory cells and upregulation of T helper 1 cells in children with Gaucher disease undergoing enzyme replacement therapy. Immunol. Res. 2020;68:73–80. doi: 10.1007/s12026-020-09129-5. [DOI] [PubMed] [Google Scholar]
- 192.Kishnani P.S., Dickson P.I., Muldowney L., Lee J.J., Rosenberg A., Abichandani R., Bluestone J.A., Burton B.K., Dewey M., Freitas A., et al. Immune response to enzyme replacement therapies in lysosomal storage diseases and the role of immune tolerance induction. Mol. Genet. Metab. 2016;117:66–83. doi: 10.1016/j.ymgme.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 193.Mistry P.K., Lopez G., Schiffmann R., Barton N.W., Weinreb N.J., Sidransky E. Gaucher disease: Progress and ongoing challenges. Mol. Genet. Metab. 2017;120:8–21. doi: 10.1016/j.ymgme.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Ohashi T. Molecular diagnosis and gene therapy for Gaucher disease. Nihon Rinsho Jpn. J. Clin. Med. 1993;51:2300–2307. [PubMed] [Google Scholar]
- 195.Bennett L.L., Fellner C. Pharmacotherapy of Gaucher Disease: Current and Future Options. PTA Peer Rev. J. Formul. Manag. 2018;43:274–309. [PMC free article] [PubMed] [Google Scholar]
- 196.Silva A.K.A., Sagné C., Gazeau F., Abasolo I. Enzyme replacement therapy: Current challenges and drug delivery prospects via extracellular vesicles. Rare Dis. Orphan Drugs J. 2022;1:13. doi: 10.20517/rdodj.2022.09. [DOI] [Google Scholar]
- 197.Veerhuis R., Nielsen H.M., Tenner A.J. Complement in the brain. Mol. Immunol. 2011;48:1592–1603. doi: 10.1016/j.molimm.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Bajic G., Degn S.E., Thiel S., Andersen G.R. Complement activation, regulation, and molecular basis for complement-related diseases. EMBO J. 2015;34:2735–2757. doi: 10.15252/embj.201591881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Magdalon J., Mansur F., Silva A.L.T.E., Goes D.V.A., Reiner O., Sertié A.L. Complement System in Brain Architecture and Neurodevelopmental Disorders. Front. Neurosci. 2020;14:23. doi: 10.3389/fnins.2020.00023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ricklin D., Hajishengallis G., Yang K., Lambris J.D. Complement: A key system for immune surveillance and homeostasis. Nat. Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yan C., Gao H. New insights for C5a and C5a receptors in sepsis. Front. Immunol. 2012;3:368. doi: 10.3389/fimmu.2012.00368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Law S.K., Levine R.P. Interaction between the third complement protein and cell surface macromolecules. Proc. Natl. Acad. Sci. USA. 1977;74:2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hein E., Garred P. In: Immune Responses to Biosurfaces. Lambris J.D., Ekdahl K.N., Ricklin D., Nilsson B., editors. Springer International Publishing; Cham, Switzerland: 2015. pp. 77–92. [Google Scholar]
- 204.Sørensen R., Thiel S., Jensenius J.C. Mannan-binding-lectin-associated serine proteases, characteristics and disease associations. Springer Semin. Immunopathol. 2005;27:299–319. doi: 10.1007/s00281-005-0006-z. [DOI] [PubMed] [Google Scholar]
- 205.Schartz N.D., Tenner A.J. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J. Neuroinflamm. 2020;17:354. doi: 10.1186/s12974-020-02024-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Morgan B.P., Harris C.L. Complement, a target for therapy in inflammatory and degenerative diseases. Nat. Rev. Drug Discov. 2015;14:857–877. doi: 10.1038/nrd4657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Orsini F., Blasio D.D., Zangari R., Zanier E.R., de Simoni M.G., Orsini F., Blasio D.D., Zangari R., Zanier E.R., de Simoni M.G. Versatility of the complement system in neuroinflammation, neurodegeneration and brain homeostasis. Front. Cell Neurosci. 2014;8:380. doi: 10.3389/fncel.2014.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kolev M., Friec L.G., Kemper C. Complement—Tapping into new sites and effector systems. Nat. Rev. Immunol. 2014;14:811–820. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- 209.Cedzyński M., Thielens N.M., Mollnes T.E., Vorup-Jensen T. Editorial: The Role of Complement in Health and Disease. Front. Immunol. 2019;10:1869. doi: 10.3389/fimmu.2019.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Stevens B., Allen N.J., Vazquez L.E., Howell G.R., Christopherson K.S., Nouri N., Micheva K.D., Mehalow A.K., Huberman A.D., Stafford B., et al. The Classical Complement Cascade Mediates CNS Synapse Elimination. Cell. 2007;131:1164–1178. doi: 10.1016/j.cell.2007.10.036. [DOI] [PubMed] [Google Scholar]
- 211.Schafer D.P., Lehrman E.K., Kautzman A.G., Koyama R., Mardinly A.R., Yamasaki R., Ransohoff R.M., Greenberg M.E., Barres B.A., Stevens B. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron. 2012;74:691–705. doi: 10.1016/j.neuron.2012.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Hou L., Bao X., Zang C., Yang H., Sun F., Che Y., Wu X., Li S., Zhang D., Wang Q. Integrin CD11b mediates α-synuclein-induced activation of NADPH oxidase through a Rho-dependent pathway. Redox Biol. 2017;14:600–608. doi: 10.1016/j.redox.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Stephan A.H., Barres B.A., Stevens B. The Complement System: An Unexpected Role in Synaptic Pruning During Development and Disease. Annu. Rev. Neurosci. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- 214.Kaartinen K., Safa A., Kotha S., Ratti G., Meri S. Complement dysregulation in glomerulonephritis. Semin. Immunol. 2019;45:101331. doi: 10.1016/j.smim.2019.101331. [DOI] [PubMed] [Google Scholar]
- 215.Köhl J., Baelder R., Lewkowich I.P., Pandey M.K., Hawlisch H., Wang L., Best J., Herman N.S., Sproles A.A., Zwirner J., et al. A regulatory role for the C5a anaphylatoxin in type 2 immunity in asthma. J. Clin. Investig. 2006;116:783–796. doi: 10.1172/JCI26582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Fritsche L.G., Lauer N., Hartmann A., Stippa S., Keilhauer C.N., Oppermann M., Pandey M.K., Köhl J., Zipfel P.F., Weber B.H., et al. An imbalance of human complement regulatory proteins CFHR1, CFHR3 and factor H influences risk for age-related macular degeneration (AMD) Hum. Mol. Genet. 2010;19:4694–4704. doi: 10.1093/hmg/ddq399. [DOI] [PubMed] [Google Scholar]
- 217.Mahajan S.D., Parikh N.U., Woodruff T.M., Jarvis J.N., Lopez M., Hennon T., Cunningham P., Quigg R.J., Schwartz S.A., Alexander J.J. C5a alters blood-brain barrier integrity in a human in vitro model of systemic lupus erythematosus. Immunology. 2015;146:130–143. doi: 10.1111/imm.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Jacob A., Hack B., Chen P., Quigg R.J., Alexander J.J. C5a/CD88 signaling alters blood-brain barrier integrity in lupus through nuclear factor-κB. J. Neurochem. 2011;119:1041–1051. doi: 10.1111/j.1471-4159.2011.07490.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Jacob A., Hack B., Chiang E., Garcia J.G.N., Quigg R.J., Alexander J.J. C5a alters blood-brain barrier integrity in experimental lupus. FASEB J. 2010;24:1682–1688. doi: 10.1096/fj.09-138834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Flierl M.A., Stahel P.F., Rittirsch D., Huber-Lang M., Niederbichler A.D., Hoesel L.M., Touban B.M., Morgan S.J., Smith W.R., Ward P.A., et al. Inhibition of complement C5a prevents breakdown of the blood-brain barrier and pituitary dysfunction in experimental sepsis. Crit. Care. 2009;13:R12. doi: 10.1186/cc7710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Landlinger C., Oberleitner L., Gruber P., Noiges B., Yatsyk K., Santic R., Mandler M., Staffler G. Active immunization against complement factor C5a: A new therapeutic approach for Alzheimer’s disease. J. Neuroinflamm. 2015;12:150. doi: 10.1186/s12974-015-0369-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Woodruff T.M., Crane J.W., Proctor L.M., Buller K.M., Shek A.B., Vos D.K., Pollitt S., Williams H.M., Shiels I.A., Monk P.N., et al. Therapeutic activity of C5a receptor antagonists in a rat model of neurodegeneration. FASEB J. 2006;20:1407–1417. doi: 10.1096/fj.05-5814com. [DOI] [PubMed] [Google Scholar]
- 223.Farkas I., Takahashi M., Fukuda A., Yamamoto N., Akatsu H., Baranyi L., Tateyama H., Yamamoto T., Okada N., Okada H. Complement C5a Receptor-Mediated Signaling May Be Involved in Neurodegeneration in Alzheimer’s Disease. J. Immunol. 2003;170:5764–5771. doi: 10.4049/jimmunol.170.11.5764. [DOI] [PubMed] [Google Scholar]
- 224.Yuan B., Fu F., Huang S., Lin C., Yang G., Ma K., Shi H., Yang Z. C5a/C5aR Pathway Plays a Vital Role in Brain Inflammatory Injury via Initiating Fgl-2 in Intracerebral Hemorrhage. Mol. Neurobiol. 2016;54:6187–6197. doi: 10.1007/s12035-016-0141-7. [DOI] [PubMed] [Google Scholar]
- 225.Kim G.H., Mocco J., Hahn D.K., Kellner C.P., Komotar R.J., Ducruet A.F., Mack W.J., Connolly E.S. Protective Effect of C5a Receptor Inhibition after Murine Reperfused Stroke. Neurosurgery. 2008;63:122–126. doi: 10.1227/01.NEU.0000335079.70222.8D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee J.D., Kumar V., Fung J.N.T., Ruitenberg M.J., Noakes P.G., Woodruff T.M. Pharmacological inhibition of complement C5a-C5a1 receptor signalling ameliorates disease pathology in the hSOD1G93A mouse model of amyotrophic lateral sclerosis. J. Cereb. Blood Flow Metab. 2017;174:689–699. doi: 10.1111/bph.13730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Piatek P., Domowicz M., Lewkowicz N., Przygodzka P., Matysiak M., Dzitko K., Lewkowicz P. C5a-Preactivated Neutrophils Are Critical for Autoimmune-Induced Astrocyte Dysregulation in Neuromyelitis Optica Spectrum Disorder. Front. Immunol. 2018;9:1694. doi: 10.3389/fimmu.2018.01694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.McGeer P.L., McGeer E.G. The possible role of complement activation in Alzheimer disease. Trends Mol. Med. 2002;8:519–523. doi: 10.1016/S1471-4914(02)02422-X. [DOI] [PubMed] [Google Scholar]
- 229.Shen Y., Meri S. Yin and Yang: Complement activation and regulation in Alzheimer’s disease. Prog. Neurobiol. 2003;70:463–472. doi: 10.1016/j.pneurobio.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 230.Yamada T., McGeer P.L., McGeer E.G. Lewy bodies in Parkinson’s disease are recognized by antibodies to complement proteins. Acta Neuropathol. 1992;84:100–104. doi: 10.1007/BF00427222. [DOI] [PubMed] [Google Scholar]
- 231.Iseki E., Marui W., Akiyama H., Uéda K., Kosaka K. Degeneration process of Lewy bodies in the brains of patients with dementia with Lewy bodies using α-synuclein-immunohistochemistry. Neurosci. Lett. 2000;286:69–73. doi: 10.1016/S0304-3940(00)01090-9. [DOI] [PubMed] [Google Scholar]
- 232.Tan S.M., Snelson M., Østergaard J.A., Coughlan M.T. The Complement Pathway: New Insights into Immunometabolic Signaling in Diabetic Kidney Disease. Antioxid. Redox Signal. 2022;37:781–801. doi: 10.1089/ars.2021.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.O’Brien K.B., Morrison T.E., Dundore D.Y., Heise M.T., Schultz-Cherry S. A Protective Role for Complement C3 Protein during Pandemic 2009 H1N1 and H5N1 Influenza A Virus Infection. PLoS ONE. 2011;6:e17377. doi: 10.1371/journal.pone.0017377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Jiang Y., Zhao G., Song N., Li P., Chen Y., Guo Y., Li J., Du L., Jiang S., Guo R., et al. Blockade of the C5a-C5aR axis alleviates lung damage in hDPP4-transgenic mice infected with MERS-CoV. Emerg. Microbes Infect. 2018;7:77. doi: 10.1038/s41426-018-0063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jiang Y., Li J., Teng Y., Sun H., Tian G., He L., Li P., Chen Y., Guo Y., Li J., et al. Complement Receptor C5aR1 Inhibition Reduces Pyroptosis in hDPP4-Transgenic Mice Infected with MERS-CoV. Viruses. 2019;11:39. doi: 10.3390/v11010039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Posch W., Vosper J., Noureen A., Zaderer V., Witting C., Bertacchi G., Gstir R., Filipek P.A., Bonn G.K., Huber L.A., et al. C5aR inhibition of nonimmune cells suppresses inflammation and maintains epithelial integrity in SARS-CoV-2–infected primary human airway epithelia. J. Allergy Clin. Immunol. 2021;147:2083–2097.e6. doi: 10.1016/j.jaci.2021.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Conway E.M., Pryzdial E.L.G. Is the COVID-19 thrombotic catastrophe complement-connected? J. Thromb. Haemost. 2020;18:2812–2822. doi: 10.1111/jth.15050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Fletcher-Sandersjöö A., Bellander B.-M. Is COVID-19 associated thrombosis caused by overactivation of the complement cascade? A literature review. Thromb. Res. 2020;194:36–41. doi: 10.1016/j.thromres.2020.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Boussier J., Yatim N., Marchal A., Hadjadj J., Charbit B., Sissy E.C., Carlier N., Pène F., Mouthon L., Tharaux P.-L., et al. Severe COVID-19 is associated with hyperactivation of the alternative complement pathway. J. Allergy Clin. Immunol. 2022;149:550–556.e2. doi: 10.1016/j.jaci.2021.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Santiesteban-Lores L.E., Amamura T.A., da Silva T.F., Midon L.M., Carneiro M.C., Isaac L., Bavia L. A double edged-sword-The Complement System during SARS-CoV-2 infection. Life Sci. 2021;272:119245. doi: 10.1016/j.lfs.2021.119245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Droesch C., Do M.H., DeSancho M., Lee E.-J., Magro C., Harp J. Livedoid and Purpuric Skin Eruptions Associated With Coagulopathy in Severe COVID-19. JAMA Dermatol. 2020;156:1022. doi: 10.1001/jamadermatol.2020.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Yu J., Gerber G.F., Chen H., Yuan X., Chaturvedi S., Braunstein E.M., Brodsky R.A. Complement dysregulation is associated with severe COVID-19 illness. Haematologica. 2021;107:1095–1105. doi: 10.3324/haematol.2021.279155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Yu J., Yuan X., Chen H., Chaturvedi S., Braunstein E.M., Brodsky R.A. Direct activation of the alternative complement pathway by SARS-CoV-2 spike proteins is blocked by factor D inhibition. Blood. 2020;136:2080–2089. doi: 10.1182/blood.2020008248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Satyam A., Tsokos M.G., Brook O.R., Hecht J.L., Moulton V.R., Tsokos G.C. Activation of classical and alternative complement pathways in the pathogenesis of lung injury in COVID-19. Clin. Immunol. 2021;226:108716. doi: 10.1016/j.clim.2021.108716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Pandey M.K. The Role of Alpha-Synuclein Autoantibodies in the Induction of Brain Inflammation and Neurodegeneration in Aged Humans. Front. Aging Neurosci. 2022;14:1–16. doi: 10.3389/fnagi.2022.902191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Nimmerjahn F., Ravetch J.V. Fcγ Receptors: Old Friends and New Family Members. Immunity. 2006;24:19–28. doi: 10.1016/j.immuni.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 247.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 248.Gaebler C., Wang Z., Lorenzi J.C.C., Muecksch F., Finkin S., Tokuyama M., Cho A., Jankovic M., Schaefer-Babajew D., Oliveira T.Y., et al. Evolution of antibody immunity to SARS-CoV-2. Nature. 2021;591:639–644. doi: 10.1038/s41586-021-03207-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Turner J.S., Kim W., Kalaidina E., Goss C.W., Rauseo A.M., Schmitz A.J., Hansen L., Haile A., Klebert M.K., Pusic I., et al. SARS-CoV-2 infection induces long-lived bone marrow plasma cells in humans. Nature. 2021;595:421–425. doi: 10.1038/s41586-021-03647-4. [DOI] [PubMed] [Google Scholar]
- 250.Nguyen-Contant P., Embong A.K., Kanagaiah P., Chaves F.A., Yang H., Branche A.R., Topham D.J., Sangster M.Y. S Protein-Reactive IgG and Memory B Cell Production after Human SARS-CoV-2 Infection Includes Broad Reactivity to the S2 Subunit. mBio. 2020;11:e01991-20. doi: 10.1128/mBio.01991-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Hartley G.E., Edwards E.S.J., Aui P.M., Varese N., Stojanovic S., McMahon J., Peleg A.Y., Boo I., Drummer H.E., Hogarth P.M., et al. Rapid Generation of Durable B Cell Memory to SARS-CoV-2 Spike and Nucleocapsid Proteins in COVID-19 and Convalescence. Sci. Immunol. 2020;5:eabf8891. doi: 10.1126/sciimmunol.abf8891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Williams J.W., Tjota M.Y., Sperling A.I. The Contribution of Allergen-Specific IgG to the Development of Th2-Mediated Airway Inflammation. J. Allergy. 2012;2012:236075. doi: 10.1155/2012/236075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Karsten C.M., Köhl J. The immunoglobulin, IgG Fc receptor and complement triangle in autoimmune diseases. Immunobiology. 2012;217:1067–1079. doi: 10.1016/j.imbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 254.Nimmerjahn F., Bruhns P., Horiuchi K., Ravetch J.V. FcγRIV: A Novel FcR with Distinct IgG Subclass Specificity. Immunity. 2005;23:41–51. doi: 10.1016/j.immuni.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 255.Seino J., Eveleigh P., Warnaar S., van Haarlem L.J.M., van Es L.A., Daha M.R. Activation of human complement by mouse and mouse/human chimeric monoclonal antibodies. Clin. Exp. Immunol. 1993;94:291–296. doi: 10.1111/j.1365-2249.1993.tb03446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Syed S.N., Konrad S., Wiege K., Nieswandt B., Nimmerjahn F., Schmidt R.E., Gessner J.E. Both FcγRIV and FcγRIII are essential receptors mediating type II and type III autoimmune responses via FcRγ-LAT-dependent generation of C5a. Eur. J. Immunol. 2009;39:3343–3356. doi: 10.1002/eji.200939884. [DOI] [PubMed] [Google Scholar]
- 257.Pandey M.K. Molecular Basis for Downregulation of C5a-Mediated Inflammation by IgG1 Immune Complexes in Allergy and Asthma. Curr. Allergy Asthma Rep. 2013;13:596–606. doi: 10.1007/s11882-013-0387-3. [DOI] [PubMed] [Google Scholar]
- 258.Pandey M.K., Burrow T.A., Rani R., Martin L.J., Witte D., Setchell K.D., Mckay M.A., Magnusen A.F., Zhang W., Liou B., et al. Complement drives glucosylceramide accumulation and tissue inflammation in Gaucher disease. Nature. 2017;543:108–112. doi: 10.1038/nature21368. [DOI] [PubMed] [Google Scholar]
- 259.Schäfer A., Muecksch F., Lorenzi J.C., Leist S.R., Cipolla M., Bournazos S., Schmidt F., Maison R.M., Gazumyan A., Martinez D.R., et al. Antibody potency, effector function, and combinations in protection and therapy for SARS-CoV-2 infection in vivo. J. Exp. Med. 2020;218:1–14. doi: 10.1084/jem.20201993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Moncunill G., Mayor A., Santano R., Jiménez A., Vidal M., Tortajada M., Sanz S., Méndez S., Llupià A., Aguilar R., et al. SARS-CoV-2 Seroprevalence and Antibody Kinetics Among Health Care Workers in a Spanish Hospital After 3 Months of Follow-up. J. Infect. Dis. 2020;223:62–71. doi: 10.1093/infdis/jiaa696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dasgupta N., Xu Y.-H., Oh S., Sun Y., Jia L., Keddache M., Grabowski G.A. Gaucher Disease: Transcriptome Analyses Using Microarray or mRNA Sequencing in a Gba1 Mutant Mouse Model Treated with Velaglucerase alfa or Imiglucerase. PLoS ONE. 2013;8:e74912. doi: 10.1371/journal.pone.0074912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xu Y.-H., Jia L., Quinn B., Zamzow M., Stringer K., Aronow B., Sun Y., Zhang W., Setchell K., Grabowski G.A. Global gene expression profile progression in Gaucher disease mouse models. BMC Genom. 2011;12:20. doi: 10.1186/1471-2164-12-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Guo R.-F., Ward P.A. Role of C5a in Inflammatory Responses. Annu. Rev. Immunol. 2005;23:821–852. doi: 10.1146/annurev.immunol.23.021704.115835. [DOI] [PubMed] [Google Scholar]
- 264.Zhang X., Köhl J. A complex role for complement in allergic asthma. Expert Rev. Clin. Immunol. 2010;6:269–277. doi: 10.1586/eci.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Nimmerjahn F., Ravetch J.V. Fcγ receptors as regulators of immune responses. Nat. Rev. Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 266.Vitner E.B., Farfel-Becker T., Ferreira N.S., Leshkowitz D., Sharma P., Lang K.S., Futerman A.H. Induction of the type I interferon response in neurological forms of Gaucher disease. J. Neuroinflamm. 2016;13:104. doi: 10.1186/s12974-016-0570-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Ozaki K., Spolski R., Ettinger R., Kim H.-P., Wang G., Qi C.-F., Hwu P., Shaffer D.J., Akilesh S., Roopenian D.C., et al. Regulation of B Cell Differentiation and Plasma Cell Generation by IL-21, a Novel Inducer of Blimp-1 and Bcl-6. J. Immunol. 2004;173:5361–5371. doi: 10.4049/jimmunol.173.9.5361. [DOI] [PubMed] [Google Scholar]
- 268.Nair S., Boddupalli C.S., Verma R., Liu J., Yang R., Pastores G.M., Mistry P.K., Dhodapkar M.V. Type II NKT-TFH cells against Gaucher lipids regulate B-cell immunity and inflammation. Blood. 2015;125:1256–1271. doi: 10.1182/blood-2014-09-600270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Ayeh S.K., Abbey E.J., Khalifa B.A.A., Nudotor R.D., Osei A.D., Chidambaram V., Osuji N., Khan S., Salia E.L., Oduwole M.O., et al. Statins use and COVID-19 outcomes in hospitalized patients. PLoS ONE. 2021;16:e0256899. doi: 10.1371/journal.pone.0256899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Lohia P., Kapur S., Benjaram S., Mir T. Association between antecedent statin use and severe disease outcomes in COVID-19: A retrospective study with propensity score matching. J. Clin. Lipidol. 2021;15:451–459. doi: 10.1016/j.jacl.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Gupta A., Madhavan M.V., Poterucha T.J., DeFilippis E.M., Hennessey J.A., Redfors B., Eckhardt C., Bikdeli B., Platt J., Nalbandian A., et al. Association between antecedent statin use and decreased mortality in hospitalized patients with COVID-19. Nat. Commun. 2021;12:1325. doi: 10.1038/s41467-021-21553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lohia P., Kapur S., Benjaram S., Cantor Z., Mahabadi N., Mir T., Badr M.S. Statins and clinical outcomes in hospitalized COVID-19 patients with and without Diabetes Mellitus: A retrospective cohort study with propensity score matching. Cardiovasc. Diabetol. 2021;20:140. doi: 10.1186/s12933-021-01336-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Felice C., Nardin C., Tanna D.G.L., Grossi U., Bernardi E., Scaldaferri L., Romagnoli M., Tonon L., Cavasin P., Novello S., et al. Use of RAAS Inhibitors and Risk of Clinical Deterioration in COVID-19: Results From an Italian Cohort of 133 Hypertensives. Am. J. Hypertens. 2020;33:944–948. doi: 10.1093/ajh/hpaa096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Aflaki M., Flannery A., Ferreira J.P., Cheng M.P., Kronfli N., Marelli A., Zannad F., Brophy J., Afillalo J., Huynh T., et al. Management of Renin-Angiotensin-Aldosterone System blockade in patients admitted to hospital with confirmed coronavirus disease (COVID-19) infection (The McGill RAAS-COVID-19): A structured summary of a study protocol for a randomized controlled trial. Trials. 2021;22:115. doi: 10.1186/s13063-021-05080-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Hallaj S., Ghorbani A., Aghdas S.A.M., Mirza-Aghazadeh-Attari M., Sevbitov A., Hashemi V., Hallaj T., Jadidi-Niaragh F. Angiotensin-converting enzyme as a new immunologic target for the new SARS-CoV-2. Immunol. Cell Biol. 2020;99:192–205. doi: 10.1111/imcb.12396. [DOI] [PubMed] [Google Scholar]
- 276.Michon A. ACE inhibitors—An effective treatment for hyaluronic acid soft tissue filler delayed inflammatory reaction following COVID-19 vaccination. J. Cosmet. Dermatol. 2022;21:1369–1370. doi: 10.1111/jocd.14826. [DOI] [PubMed] [Google Scholar]
- 277.Kaps L., Labenz C., Grimm D., Schwarting A., Galle P.R., Schreiner O. Treatment of cytokine storm syndrome with IL-1 receptor antagonist anakinra in a patient with ARDS caused by COVID-19 infection: A case report. Clin. Case Rep. 2020;8:2989–2993. doi: 10.1002/ccr3.3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Gordon A.C., Mouncey P.R., Al-Beidh F., Rowan K.M., Nichol A.D., Arabi Y.M., Annane D., Beane A., Bentum-Puijk V.W., Berry L.R., et al. Interleukin-6 Receptor Antagonists in Critically Ill Patients with COVID-19. N. Engl. J. Med. 2021;384:1491–1502. doi: 10.1056/nejmoa2100433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Amer M., Kamel A.M., Bawazeer M., Maghrabi K., Butt A., Dahhan T., Kseibi E., Khurshid S.M., Abujazar M., Alghunaim R., et al. Clinical characteristics and outcomes of critically ill mechanically ventilated COVID-19 patients receiving interleukin-6 receptor antagonists and corticosteroid therapy: A preliminary report from a multinational registry. Eur. J. Med. Res. 2021;26:117. doi: 10.1186/s40001-021-00591-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Lan S.-H., Wang C.-K., Chang S.-P., Lu L.-C., Hung S.-H., Lin W.-T. Janus kinase inhibitors for hospitalized patients with COVID-19: A meta-analysis of randomized controlled trials. Expert Rev. Anti Infect. Ther. 2021;20:773–779. doi: 10.1080/14787210.2022.2004120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Lin Z., Niu J., Xu Y., Qin L., Ding J., Zhou L. Clinical efficacy and adverse events of baricitinib treatment for coronavirus disease-2019 (COVID-19): A systematic review and meta-analysis. J. Med. Virol. 2021;94:1523–1534. doi: 10.1002/jmv.27482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Chen C.-X., Wang J.-J., Li H., Yuan L.-T., Gale R.P., Liang Y. JAK-inhibitors for coronavirus disease-2019 (COVID-19): A meta-analysis. Leukemia. 2021;35:2616–2620. doi: 10.1038/s41375-021-01266-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Chen C.-Y., Chen W.-C., Hsu C.-K., Chao C.-M., Lai C.-C. Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: A systematic review and meta-analysis of randomized controlled trials. Int. Immunopharmacol. 2021;99:108027. doi: 10.1016/j.intimp.2021.108027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Fakharian A., Barati S., Mirenayat M., Rezaei M., Haseli S., Torkaman P., Yousefian S., Dastan A., Jamaati H., Dastan F. Evaluation of adalimumab effects in managing severe cases of COVID-19: A randomized controlled trial. Int. Immunopharmacol. 2021;99:107961. doi: 10.1016/j.intimp.2021.107961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Bonaventura A., Vecchié A., Wang T.S., Lee E., Cremer P.C., Carey B., Rajendram P., Hudock K.M., Korbee L., Van Tassell B.W., et al. Targeting GM-CSF in COVID-19 Pneumonia: Rationale and Strategies. Front Immunol. 2020;11:1625. doi: 10.3389/fimmu.2020.01625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Criner G.J., Lang F.M., Gottlieb R.L., Mathews K.S., Wang T.S., Rice T.W., Madduri D., Bellam S., Jeanfreau R., Case A.H., et al. Anti-Granulocyte–Macrophage Colony–Stimulating Factor Monoclonal Antibody Gimsilumab for COVID-19 Pneumonia: A Randomized, Double-Blind, Placebo-controlled Trial. Am. J. Respir. Crit. Care Med. 2022;205:1290–1299. doi: 10.1164/rccm.202108-1859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Zavattaro E., Cammarata E., Tarantino V., Soddu D., Gironi L.C., Savoia P. Successful treatment of a bullous vasculitis with intravenous immunoglobulins in a COVID-19 patient. Dermatol. Ther. 2021;34:e14853. doi: 10.1111/dth.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Huo S., Ferse C., Bösl F., Reincke S.M., Enghard P., Hinrichs C., Treskatsch S., Angermair S., Eckardt K.-U., Audebert H.J., et al. Intravenous immunoglobulins for treatment of severe COVID-19-related acute encephalopathy. J. Neurol. 2022;269:4013–4020. doi: 10.1007/s00415-022-11152-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chaudhuri D., Sasaki K., Karkar A., Sharif S., Lewis K., Mammen M.J., Alexander P., Ye Z., Lozano L.E.C., Munch M.W., et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: A systematic review and meta-analysis. Intensive Care Med. 2021;47:521–537. doi: 10.1007/s00134-021-06394-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Kazachinskaia E., Chepurnov A., Shcherbakov D., Kononova Y., Saroyan T., Gulyaeva M., Shanshin D., Romanova V., Khripko O., Voevoda M., et al. IgG Study of Blood Sera of Patients with COVID-19. Pathogens. 2021;10:1421. doi: 10.3390/pathogens10111421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Li K., Huang B., Wu M., Zhong A., Li L., Cai Y., Wang Z., Wu L., Zhu M., Li J., et al. Dynamic changes in anti-SARS-CoV-2 antibodies during SARS-CoV-2 infection and recovery from COVID-19. Nat. Commun. 2020;11:6044. doi: 10.1038/s41467-020-19943-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ozturk T., Howell C., Benameur K., Ramonell R.P., Cashman K., Pirmohammed S., Bassit L., Roback J., Marconi V.C., Schinazi R.F., et al. Cross-sectional IgM and IgG profiles in SARS-CoV-2 infection. medRxiv. 2020:1–21. doi: 10.1101/2020.05.10.20097535. [DOI] [Google Scholar]
- 293.Sun B., Feng Y., Mo X., Zheng P., Wang Q., Li P., Peng P., Liu X., Chen Z., Huang H., et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020;9:940–948. doi: 10.1080/22221751.2020.1762515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Li Y., Lai D.-Y., Lei Q., Xu Z.-W., Wang F., Hou H., Chen L., Wu J., Ren Y., Ma M.-L., et al. Systematic evaluation of IgG responses to SARS-CoV-2 spike protein-derived peptides for monitoring COVID-19 patients. Cell Mol. Immunol. 2021;18:621–631. doi: 10.1038/s41423-020-00612-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Beavis K.G., Matushek S.M., Abeleda A.P.F., Bethel C., Hunt C., Gillen S., Moran A., Tesic V. Evaluation of the EUROIMMUN Anti-SARS-CoV-2 ELISA Assay for detection of IgA and IgG antibodies. J. Clin. Virol. 2020;129:104468. doi: 10.1016/j.jcv.2020.104468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Wang H., Wiredja D., Yang L., Bulterys P.L., Costales C., Röltgen K., Manalac J., Yee J., Zehnder J., Shi R.Z., et al. Case-Control Study of Individuals with Discrepant Nucleocapsid and Spike Protein SARS-CoV-2 IgG Results. Clin. Chem. 2021;67:977–986. doi: 10.1093/clinchem/hvab045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Nakano Y., Kurano M., Morita Y., Shimura T., Yokoyama R., Qian C., Xia F., He F., Kishi Y., Okada J., et al. Time course of the sensitivity and specificity of anti-SARS-CoV-2 IgM and IgG antibodies for symptomatic COVID-19 in Japan. Sci. Rep. 2021;11:2776. doi: 10.1038/s41598-021-82428-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Liu X., Wang J., Xu X., Liao G., Chen Y., Hu C.-H. Patterns of IgG and IgM antibody response in COVID-19 patients. Emerg. Microbes Infect. 2020;9:1269–1274. doi: 10.1080/22221751.2020.1773324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Meinberger D., Koch M., Roth A., Hermes G., Stemler J., Cornely O.A., Streichert T., Klatt A.R. Analysis of IgM, IgA, and IgG isotype antibodies Directed against SARS-CoV-2 spike glycoprotein and ORF8 in the course of COVID-19. Sci. Rep. 2021;11:8920. doi: 10.1038/s41598-021-88356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Zhou C., Bu G., Sun Y., Ren C., Qu M., Gao Y., Zhu Y., Wang L., Sun L., Liu Y. Evaluation of serum IgM and IgG antibodies in COVID-19 patients by enzyme linked immunosorbent assay. J. Med. Virol. 2020;93:2857–2866. doi: 10.1002/jmv.26741. [DOI] [PubMed] [Google Scholar]
- 301.Isho B., Abe K.T., Zuo M., Jamal A.J., Rathod B., Wang J.H., Li Z., Chao G., Rojas O.L., Bang Y.M., et al. Persistence of serum and saliva antibody responses to SARS-CoV-2 spike antigens in COVID-19 patients. Sci. Immunol. 2020;5:eabe5511. doi: 10.1126/sciimmunol.abe5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Sterlin D., Mathian A., Miyara M., Mohr A., Anna F., Claër L., Quentric P., Fadlallah J., Devilliers H., Ghillani P., et al. IgA dominates the early neutralizing antibody response to SARS-CoV-2. Sci. Transl. Med. 2021;13:eabd2223. doi: 10.1126/scitranslmed.abd2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Shah J., Liu S., Potula H.-H., Bhargava P., Cruz I., Force D., Bazerbashi A., Ramasamy R. IgG and IgM antibody formation to spike and nucleocapsid proteins in COVID-19 characterized by multiplex immunoblot assays. BMC Infect. Dis. 2021;21:325. doi: 10.1186/s12879-021-06031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Batra M., Tian R., Zhang C., Clarence E., Sacher C.S., Miranda J.N., Fuente D.L.J.R.O., Mathew M., Green D., Patel S., et al. Role of IgG against N-protein of SARS-CoV2 in COVID-19 clinical outcomes. Sci. Rep. 2021;11:3455. doi: 10.1038/s41598-021-83108-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Johnson M., Wagstaffe H.R., Gilmour K.C., Mai A.L., Lewis J., Hunt A., Sirr J., Bengt C., Grandjean L., Goldblatt D. Evaluation of a novel multiplexed assay for determining IgG levels and functional activity to SARS-CoV-2. J. Clin. Virol. 2020;130:104572. doi: 10.1016/j.jcv.2020.104572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Zhang Z., Wang X., Wei X., Zheng S.W., Lenhart B.J., Xu P., Li J., Pan J., Albrecht H., Liu C. Multiplex quantitative detection of SARS-CoV-2 specific IgG and IgM antibodies based on DNA-assisted nanopore sensing. Biosens. Bioelectron. 2021;181:113134. doi: 10.1016/j.bios.2021.113134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Maceyka M., Payne S.G., Milstien S., Spiegel S. Sphingosine kinase, sphingosine-1-phosphate, and apoptosis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2002;1585:193–201. doi: 10.1016/S1388-1981(02)00341-4. [DOI] [PubMed] [Google Scholar]
- 308.Platt F.M. Sphingolipid lysosomal storage disorders. Nature. 2014;510:68–75. doi: 10.1038/nature13476. [DOI] [PubMed] [Google Scholar]
- 309.Messner M.C., Cabot M.C. Glucosylceramide in Humans. Adv. Exp. Med. Biol. 2010;688:156–164. doi: 10.1007/978-1-4419-6741-1_11. [DOI] [PubMed] [Google Scholar]
- 310.Melo C.F.O.R., de Oliveira D.N., Lima E.D.O., Guerreiro T.M., Esteves C.Z., Beck R.M., Padilla M.A., Milanez G.P., Arns C.W., Proenca-Modena J.L., et al. A Lipidomics Approach in the Characterization of Zika-Infected Mosquito Cells: Potential Targets for Breaking the Transmission Cycle. PLoS ONE. 2016;11:e0164377. doi: 10.1371/journal.pone.0164377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Khan I., Katikaneni D.S., Han Q., Sanchez-Felipe L., Hanada K., Ambrose R.L., Mackenzie J.M., Konan K.V. Modulation of Hepatitis C Virus Genome Replication by Glycosphingolipids and Four-Phosphate Adaptor Protein. J. Virol. 2014;88:12276–12295. doi: 10.1128/JVI.00970-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Low H., Mukhamedova N., Cui H.L., McSharry B.P., Avdic S., Hoang A., Ditiatkovski M., Liu Y., Fu Y., Meikle P.J., et al. Cytomegalovirus Restructures Lipid Rafts via a US28/CDC42-Mediated Pathway, Enhancing Cholesterol Efflux from Host Cells. Cell Rep. 2016;16:186–200. doi: 10.1016/j.celrep.2016.05.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Chotiwan N., Andre B.G., Sanchez-Vargas I., Islam M.N., Grabowski J.M., Hopf-Jannasch A., Gough E., Nakayasu E., Blair C.D., Belisle J.T., et al. Dynamic remodeling of lipids coincides with dengue virus replication in the midgut of Aedes aegypti mosquitoes. PLOS Pathog. 2018;14:e1006853. doi: 10.1371/journal.ppat.1006853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Tanner L.B., Chng C., Guan X.L., Lei Z., Rozen S.G., Wenk M.R. Lipidomics identifies a requirement for peroxisomal function during influenza virus replication. J. Lipid Res. 2014;55:1357–1365. doi: 10.1194/jlr.M049148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Achdout H., Manaster I., Mandelboim O. Influenza Virus Infection Augments NK Cell Inhibition through Reorganization of Major Histocompatibility Complex Class I Proteins. J. Virol. 2008;82:8030–8037. doi: 10.1128/JVI.00870-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hidari K.I.P.J., Suzuki Y., Suzuki T. Suppression of the Biosynthesis of Cellular Sphingolipids Results in the Inhibition of the Maturation of Influenza Virus Particles in MDCK Cells. Biol. Pharm. Bull. 2006;29:1575–1579. doi: 10.1248/bpb.29.1575. [DOI] [PubMed] [Google Scholar]
- 317.Drews K., Calgi M.P., Harrison W.C., Drews C.M., Costa-Pinheiro P., Shaw J.J.P., Jobe K.A., Nelson E.A., Han J.D., Fox T., et al. Glucosylceramidase Maintains Influenza Virus Infection by Regulating Endocytosis. J. Virol. 2019;93:1–20. doi: 10.1128/JVI.00017-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Carpinteiro A., Edwards M.J., Hoffmann M., Kochs G., Gripp B., Weigang S., Adams C., Carpinteiro E., Gulbins A., Keitsch S., et al. Pharmacological Inhibition of Acid Sphingomyelinase Prevents Uptake of SARS-CoV-2 by Epithelial Cells. Cell Rep. Med. 2020;1:100142. doi: 10.1016/j.xcrm.2020.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Törnquist K., Asghar M.Y., Srinivasan V., Korhonen L., Lindholm D. Sphingolipids as modulators of SARS-CoV-2 infection. Front. Cell Dev. Biol. 2021;9:1574. doi: 10.3389/fcell.2021.689854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Hong B.Y., Kim E.Y., Jung S.-C. Upregulation of Proinflammatory Cytokines in the Fetal Brain of the Gaucher Mouse. J. Korean Med. Sci. 2006;21:733–738. doi: 10.3346/jkms.2006.21.4.733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321.Kim E.Y., Hong B.Y., Go S.H., Lee B., Jung S.-C. Downregulation of neurotrophic factors in the brain of a mouse model of Gaucher disease: Implications for neuronal loss in Gaucher disease. Exp. Mol. Med. 2006;38:348–356. doi: 10.1038/emm.2006.41. [DOI] [PubMed] [Google Scholar]
- 322.Tybulewicz V.L.J., Tremblay M.L., Marca L., Willemsen R., Stubblefield B.K., Winfield S., Zablocka B., Sidransky E., Martin B.M., Huang S.P., et al. Animal model of Gaucher’s disease from targeted disruption of the mouse glucocerebrosidase gene. Nature. 1992;357:407–410. doi: 10.1038/357407a0. [DOI] [PubMed] [Google Scholar]
- 323.Vitner E.B., Salomon R., Farfel-Becker T., Meshcheriakova A., Ali M., Klein A.D., Platt F.M., Cox T.M., Futerman A.H. RIPK3 as a potential therapeutic target for Gaucher’s disease. Nat. Med. 2014;20:204–208. doi: 10.1038/nm.3449. [DOI] [PubMed] [Google Scholar]
- 324.Vardi A., Zigdon H., Meshcheriakova A., Klein A.D., Yaacobi C., Eilam R., Kenwood B.M., Rahim A.A., Massaro G., Merrill A.H., et al. Delineating pathological pathways in a chemically induced mouse model of Gaucher disease. J. Pathol. 2016;239:496–509. doi: 10.1002/path.4751. [DOI] [PubMed] [Google Scholar]
- 325.Kanfer J.N., Legler G., Sullivan J., Raghavan S.S., Mumford R.A. The Gaucher mouse. Biochem. Biophys. Res. Commun. 1975;67:85–90. doi: 10.1016/0006-291X(75)90286-7. [DOI] [PubMed] [Google Scholar]
- 326.Enquist I.B., Bianco C.L., Ooka A., Nilsson E., Månsson J.-E., Ehinger M., Richter J., Brady R.O., Kirik D., Karlsson S. Murine models of acute neuronopathic Gaucher disease. Proc. Natl. Acad. Sci. USA. 2007;104:17483–17488. doi: 10.1073/pnas.0708086104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Lucisano Y.M., Mantovani B. The role of complement in the stimulation of lysosomal enzyme release by polymorphonuclear leucocytes induced by immune complexes of IgG and of IgM. Immunology. 1988;65:171–175. [PMC free article] [PubMed] [Google Scholar]
- 328.Cardella C.J., Davies P., Allison A.C. Immune Complexes induce Selective Release of Lysosomal Hydrolases from Macrophages. Nature. 1974;247:46–48. doi: 10.1038/247046a0. [DOI] [PubMed] [Google Scholar]
- 329.Zurier R.B., Hoffstein S., Weissmann G. Mechanisms of Lysosomal Enzyme Release from Human Leukocytes. J. Cell Biol. 1973;58:27–41. doi: 10.1083/jcb.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Schorlemmer H.-U., Davies P., Allison A.C. Ability of activated complement components to induce lysosomal enzyme release from macrophages. Nature. 1976;261:48–49. doi: 10.1038/261048a0. [DOI] [PubMed] [Google Scholar]
- 331.Schorlemmer H.U., Allison A.C. Effects of activated complement components on enzyme secretion by macrophages. Immunology. 1976;31:781–788. [PMC free article] [PubMed] [Google Scholar]
- 332.Knudsen F., Pedersen J.O., Juhl O., Nielsen A.H., Jersild C. Complement and leukocytes during cardiopulmonary bypass: Effects on plasma C3d and C5a, leukocyte count, release of granulocyte elastase and granulocyte chemotaxis. J. Cardiothorac. Anesth. 1988;2:164–170. doi: 10.1016/0888-6296(88)90266-9. [DOI] [PubMed] [Google Scholar]
- 333.Goldstein I.M., Hoffstein S.T., Weissmann G. Influence of divalent cations upon complement-mediated enzyme release from human polymorphonuclear leukocytes. J. Immunol. 1975;115:665–670. [PubMed] [Google Scholar]
- 334.Goldstein I., Hoffstein S., Gallin J., Weissmann G. Mechanisms of Lysosomal Enzyme Release from Human Leukocytes: Microtubule Assembly and Membrane Fusion Induced by a Component of Complement. Proc. Natl. Acad. Sci. USA. 1973;70:2916–2920. doi: 10.1073/pnas.70.10.2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Fierro L., Nesheiwat N., Naik H., Narayanan P., Mistry P.K., Balwani M. Gaucher disease and SARS-CoV-2 infection: Experience from 181 patients in New York. Mol. Genet. Metab. 2020;132:44–48. doi: 10.1016/j.ymgme.2020.12.288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Andrade-Campos M., Escuder-Azuara B., de Frutos L.L., Gonzalo I.S., Giraldo P. Direct and indirect effects of the SARS-CoV-2 pandemic on Gaucher Disease patients in Spain: Time to reconsider home-based therapies? Blood Cells Mol. Dis. 2020;85:102478. doi: 10.1016/j.bcmd.2020.102478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Zimran A., Szer J., Revel-Vilk S. Impact of Gaucher disease on COVID-19. Intern. Med. J. 2020;50:894–895. doi: 10.1111/imj.14894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Demirci I., Demir T., Dagdelen S., Haymana C., Tasci I., Atmaca A., Ertugrul D., Ata N., Sahin M., Salman S., et al. No association of Gaucher Disease with COVID-19-related outcomes: A nationwide cohort study. Intern. Med. J. 2021;52:379–385. doi: 10.1111/imj.15673. [DOI] [PubMed] [Google Scholar]
- 339.Khalili M., Baeis M.G., Saneifard H., Ghanaie R.M., Shamsian B.S. Pediatric with Gaucher disease and COVID-19: Case report of uncommon manifestation of COVID-19 in chest Ct. Vis. J. Emerg. Med. 2021;22:100966. doi: 10.1016/j.visj.2021.100966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Hamiel U., Kurolap A., Cohen I.J., Ruhrman-Shahar N., Hershkovitz T., Niederau C., Zimran A., Revel-Vilk S., Istaiti M., Cappellini M.D., et al. Experts’ views on COVID-19 vaccination and the impact of the pandemic on patients with Gaucher disease. Br. J. Haematol. 2021;195:e135–e137. doi: 10.1111/bjh.17693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Kristal E., Pode-Shakked B., Hazan G., Banne E., Ling G., David O., Shany E., Raas-Rothschild A., Anikster Y., Kneller K., et al. The effects of the COVID-19 pandemic on patients with lysosomal storage disorders in Israel. Orphanet J. Rare Dis. 2021;16:379. doi: 10.1186/s13023-021-02007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Humbles A.A., Lu B., Nilsson C.A., Lilly C., Israel E., Fujiwara Y., Gerard N.P., Gerard C. A role for the C3a anaphylatoxin receptor in the effector phase of asthma. Nature. 2000;406:998–1001. doi: 10.1038/35023175. [DOI] [PubMed] [Google Scholar]
- 343.Krug N., Tschernig T., Erpenbeck V.J., Hohlfeld J.M., Köhl J. Complement Factors C3a and C5a Are Increased in Bronchoalveolar Lavage Fluid after Segmental Allergen Provocation in Subjects with Asthma. Am. J. Respir. Crit. Care Med. 2001;164:1841–1843. doi: 10.1164/ajrccm.164.10.2010096. [DOI] [PubMed] [Google Scholar]
- 344.Eglite S., Plüss K., Dahinden C.A. Requirements for C5a Receptor-Mediated IL-4 and IL-13 Production and Leukotriene C4 Generation in Human Basophils. J. Immunol. 2000;165:2183–2189. doi: 10.4049/jimmunol.165.4.2183. [DOI] [PubMed] [Google Scholar]
- 345.Woolhiser M. Activation of human mast cells by aggregated IgG through FcγRI: Additive effects of C3a. Clin. Immunol. 2004;110:172–180. doi: 10.1016/j.clim.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 346.Ueda Y., Yang K., Foster S.J., Kondo M., Kelsoe G. Inflammation Controls B Lymphopoiesis by Regulating Chemokine CXCL12 Expression. J. Exp. Med. 2004;199:47–58. doi: 10.1084/jem.20031104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Nagaoka H., Gonzalez-Aseguinolaza G., Tsuji M., Nussenzweig M.C. Immunization and Infection Change the Number of Recombination Activating Gene (Rag)-Expressing B Cells in the Periphery by Altering Immature Lymphocyte Production. J. Exp. Med. 2000;191:2113–2120. doi: 10.1084/jem.191.12.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pandey M.K., Grabowski G.A., Köhl J. An unexpected player in Gaucher disease: The multiple roles of complement in disease development. Semin. Immunol. 2018;37:30–42. doi: 10.1016/j.smim.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 349.Nair S., Branagan A.R., Liu J., Boddupalli C.S., Mistry P.K., Dhodapkar M.V. Clonal Immunoglobulin against Lysolipids in the Origin of Myeloma. N. Engl. J. Med. 2016;374:555–561. doi: 10.1056/NEJMoa1508808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350.Risitano A.M., Mastellos D.C., Huber-Lang M., Yancopoulou D., Garlanda C., Ciceri F., Lambris J.D. Complement as a target in COVID-19? Nat. Rev. Immunol. 2020;20:343–344. doi: 10.1038/s41577-020-0320-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Java A., Apicelli A.J., Liszewski M.K., Coler-Reilly A., Atkinson J.P., Kim A.H., Kulkarni H.S. The complement system in COVID-19: Friend and foe? JCI Insight. 2020;5:e140711. doi: 10.1172/jci.insight.140711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Lazo-Langner A., Chin-Yee I., Al-Ani F. Eculizumab in the management of paroxysmal nocturnal hemoglobinuria: Patient selection and special considerations. Ther. Clin. Risk Manag. 2016;12:1161–1170. doi: 10.2147/TCRM.S96720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Wong E.K., Kavanagh D. Anticomplement C5 therapy with eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria and atypical hemolytic uremic syndrome. Transl. Res. 2015;165:306–320. doi: 10.1016/j.trsl.2014.10.010. [DOI] [PubMed] [Google Scholar]
- 354.Cao Y., Yisimayi A., Jian F., Song W., Xiao T., Wang L., Du S., Wang J., Li Q., Chen X., et al. BA.2.12.1, BA.4 and BA.5 escape antibodies elicited by Omicron infection. Nature. 2022;608:593–602. doi: 10.1038/s41586-022-04980-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Chemaitelly H., Nagelkerke N., Ayoub H.H., Coyle P., Tang P., Yassine H.M., Al-Khatib H.A., Smatti M.K., Hasan M.R., Al-Kanaani Z., et al. Duration of immune protection of SARS-CoV-2 natural infection against reinfection. J. Travel Med. 2022;30:taac109. doi: 10.1093/jtm/taac109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.