Abstract
Rationale:
Cannabidiol (CBD), the major non-psychoactive constituent of cannabis, has therapeutic potential for the treatment of anxiety. Most preclinical studies investigate only acute effects of CBD and only in males, yet the drug is most likely to be used over a sustained period in clinical practice.
Objectives:
The objectives of this study were to investigate the anxiolytic-like effect of CBD in female rats compared to males and to determine whether the responsiveness of females was influenced by the stage of the estrous cycle.
Methods:
We carried out experiments to compare the effect of CBD in male and female rats in the elevated plus maze (EPM) in response to acute and short-term (4 days) administration through a complete cycle in females.
Results:
Male and female rats behaved in a similar manner in the EPM, but females in the late diestrus (LD) phase exhibited more anxiety-like behavior than at other stages, the difference reaching statistical significance compared to proestrus stages. CBD produced anxiolytic-like effects in both sexes, but female rats were responsive only in LD and 10-fold lower dose than males. After sub-chronic (4 days) treatment, responsiveness to CBD was maintained in females in LD, but females in proestrus remained unresponsive to CBD treatment.
Conclusions:
We suggest that there are sex differences in the anxiolytic-like effects of CBD in rats that reflect different underlying mechanisms: based on literature data, gonadal hormone status linked to GABAA receptor expression in females, and 5-HT1A receptor activation in males.
Keywords: Cannabidiol, sex differences, proestrus, diestrus, defensive behavior, anxiety
Introduction
The rise in cannabis legalization and decriminalization has kindled interest in the therapeutic possibilities for using cannabis derivatives in psychiatry. The native plant Cannabis sativa contains two principal phytocannabinoids: delta (9)-tetrahydrocannabinol (THC) and cannabidiol (CBD). Of these, CBD, the major non-psychoactive constituent of cannabis, has therapeutic potential for the treatment of anxiety. Several recent reviews evidence the anxiolytic nature of CBD in humans, supported by data obtained in animal models (García-Gutiérrez et al., 2020; Lisboa et al., 2017; Melas et al., 2021; Wright et al., 2020). The excellent safety profile of CBD (Dos Santos et al., 2020; Iffland and Grotenhermen, 2017) makes it an attractive candidate for further development.
CBD has a complex and highly nonselective pharmacology, affecting many different neurotransmitter systems in addition to its actions at cannabinoid receptors (Cifelli et al., 2020; Murkar et al., 2021). Its anxiolytic effects are characterized by a bell-shaped dose-response curve (Linares et al., 2019), seen in both humans (Linares et al., 2019; Zuardi et al., 2017) and animals (Campos and Guimarães, 2008; Gomes et al., 2011; Guimarães et al., 1990). However, these findings are based on evidence gathered almost exclusively from male subjects. Although female participants were included in several recent studies with human subjects, data from males and females were pooled for analysis (Bolsoni et al., 2022; Zuardi et al, 2017). As far as we are aware, there has been no systematic investigation of sex differences in responsiveness to CBD in humans.
In animal studies, sex differences in the effects of cannabis and the cannabinoid THC have been reported, although the findings are equivocal (see Cooper and Craft, 2018, for a critical review). There is also evidence that gonadal hormones can modify the effects of THC (Cooper and Craft, 2018). Much less attention has been directed toward CBD, despite it being, alongside THC, the major constituent of cannabis that has therapeutic potential. In one recent study in female rats, anxiolytic-like effects in the elevated plus maze (EPM) were seen in response to intraperitoneal injection of 3 mg/kg CBD (Salviato et al., 2021). This is within the dose range known to be effective in similar studies in males (Genaro et al., 2017; Guimarães et al., 1994). On the other hand, CBD was without effect in the EPM in adult female mice (5–20 mg/kg), although 5 mg/kg significantly increased the percentage of time spent in the center of an open field arena in adolescents (Kasten et al., 2019). Lower doses were not tested in that study. Moreover, neither study considered the possible influence of the estrous cycle in females. This is an important omission since estrous cycle-linked effects have been reported for other anxiolytic agents (Devall et al., 2009; Machado-Figueiredo et al., 2019; Soares-Rachetti et al., 2016).
In view of the rising popularity of cannabis derivatives for medical purposes and the two-fold higher prevalence of anxiety-related disorders in women compared to men (Hantsoo and Epperson, 2017), an understanding of sex differences in responsiveness to CBD is overdue. To begin to address this shortfall in knowledge, we carried out experiments with the following objectives: firstly, to examine sex-related differences, and in females, the effect of the different phases of the estrous cycle on anxiety-like behaviors; secondly, to characterize the effect of CBD (0.3, 3, and 30 mg/kg, i.p.) in female rats compared to males and determine whether females’ responsiveness was influenced by the stage of the estrous cycle. In addition, since most preclinical studies have investigated only acute effects of CBD, whereas, in clinical practice, the drug is most likely to be used over a sustained period, we investigated the effects of both acute and short-term (daily for 4 days) administration of CBD in females.
Methods
Animals and housing
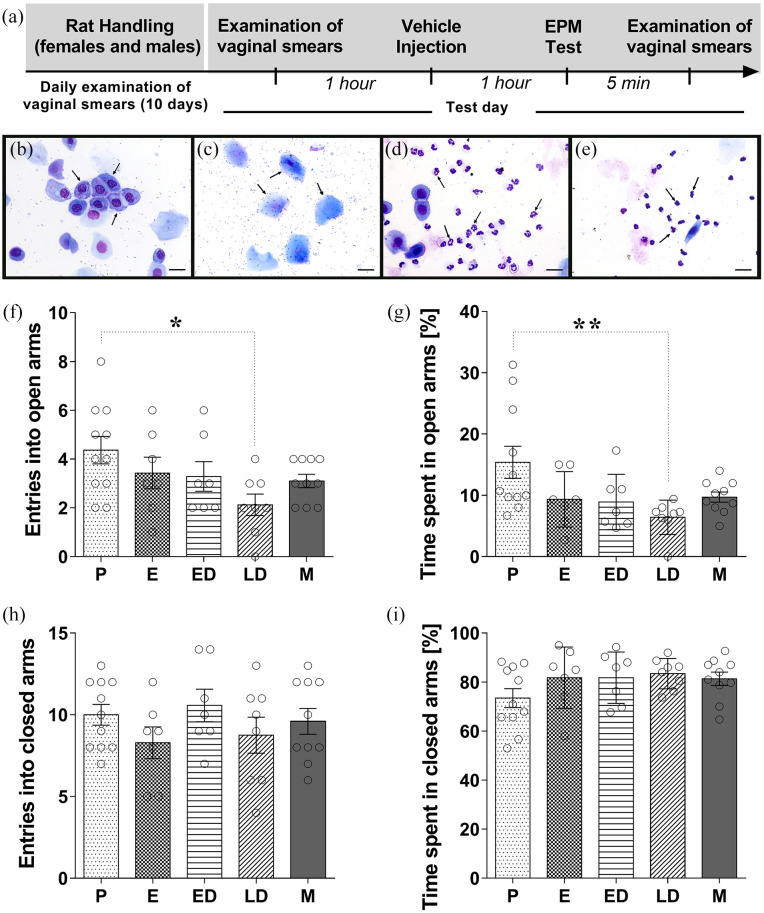
Male and female Wistar rats (University of São Paulo, Ribeirão Preto Campus, SP, Brazil) weighing 220–240 g were used. They were housed under a 12:12-h light/dark cycle starting at 7:00 AM and tested according to the University of São Paulo, Ribeirão Preto—Institutional Animal Care and Use Committee-approved protocols. The animals had access to food and water ad libitum and were assigned randomly to experimental and control groups. The first cohort of rats was used to examine whether the exploratory behaviors of male and female rats at different stages of the estrous cycle were differentially expressed in the EPM test after vehicle treatment, females (n = 24) and males (n = 6). The second cohort of naïve rats, females (n = 43) and males (n = 47), were used to investigate the effect of CBD administered acutely 1 h before the EPM test. We chose females during proestrus and late diestrus (LD) to further test the effect of CBD on behavior in the EPM because of the respective high and low levels of responding demonstrated in experiment 1. Males and females during proestrus and LD that received the vehicle in experiment 1 and experiment 2 were pooled together to constitute the vehicle group for both experiments. To investigate whether the estrous cycle-linked differences in responsiveness to CBD were maintained after sub-chronic treatment, a third cohort of naïve female rats (n = 34) received CBD (0.3 mg/kg) for 4 consecutive days, and these females were tested on the EPM 1 h after the last CBD injection when they were in the P or LD phase. Thus, CBD levels were maintained throughout a whole cycle. A fourth cohort of naïve rats, females (n = 85) and males (n = 41) were subjected to the open field test as a second paradigm to investigate the effect of CBD on exploratory behaviors and anxiety-like behaviors. The naïve rats in all the cohorts (males and females) were handled and daily examination of vaginal smears was performed to determine the estrous cycle phase in females. Figure 1 shows the experimental timeline and general features of experimental procedure on the test day.
Figure 1.
Exploratory behavior of male and female rats submitted to the EPM. (a) Timeline of the protocol for the experiments. Representative photomicrographs of each phase of estrous cycle proestrus (b), estrus (c), early diestrus (d), and late diestrus (e) with arrows to indicate the different cellular types: round-nucleated epithelial cells (b); larger, cornified cells (c); polymorphonuclear leucocytes with distinctly lobed nuclei (d); and polymorphonuclear leucocytes with clumped nucleus or disintegrating nuclei (e). Scale bars represent 20 µm for all. Behavior of male and female rats in the EPM during the 5-min exposure: (f) entries into the open arms and (g) percentage of time spent in the open arms, (h) entries into the closed arms, and (i) percentage of time spent in the closed arms of the maze. One-way ANOVA was performed, taking sex/estrous cycle as the main factor: females during proestrus (P), estrus (E), early diestrus (ED), late diestrus (LD), and male (M). The values are mean ± SEM. Tukey’s post hoc analysis, *p < 0.05; **p < 0.01. N = 7–11/group. EPM: elevated plus maze; ANOVA: analysis of variance.
Determination of stage of estrous cycle
The estrous cycle stage was determined by the histology of vaginal smears, collected as described by Soares-Rachetti et al. (2016). A vaginal smear was taken daily, starting 10 days before the behavioral tests, every morning between 9 h and 10 h to establish that the animals were spontaneously cycling (Machado-Figueiredo et al., 2019). In brief, an inoculation loop was sterilized in a flame, dipped in sterile water, and then gently inserted into the vagina to gather cells, which were smeared onto a glass slide. The smears were stained with a 2% methylene blue solution from Panótico LB Kit (Laborclin Produtos para Laboratórios Ltda, Pinhais, PR, Brazil). Proestrus (P) was characterized by nucleated epithelial cells, estrus (E) by cornified squamous cells, early diestrus (ED) by a preponderance of small nucleated leucocytes typically with lobed nuclei, and in LD, fewer leukocytes were present with clumped and or disintegrating nuclei (Hartman, 1944; Brack and Lovick, 2007). Experiments were performed on animals that had completed at least two regular cycles. Three observers confirmed the estrous phases, representative photomicrographs of each phase are illustrated in Figure 1(b) to (e). Handling stress associated with collection of vaginal smears is a potential source of variability that could influence behavior in females (Pompili et al, 2010). Rats were handled and smears were collected daily for at least 10 days prior to the experimental day. The reason for this was, firstly, to fully habituate the animals to the procedure and secondly, to establish that each rat was cycling normally, that is, progressing through two full 4–5 day cycles. In our hands, the rats do not show overt signs of being stressed and remain calm during the smear collection procedure. Even so, to further minimize the possibility of an acute stress-related effect, on the experimental day, a vaginal smearing was done in the home environment 1 h before the behavioral experiment started and cages were brought to the common staging area 1 h prior to testing in order to avoid generating stress in animals. An extra vaginal smear was taken following the completion of each experimental test to confirm the estrous cycle phase. Females that presented different phases before and after the behavioral testing were excluded from the analysis.
Drug
CBD (99.6% pure, kindly supplied by BSPG-Pharm, Sandwich, UK) was freshly dissolved in 2% TWEEN 80 (Sigma–Aldrich, St Louis, MO, USA) and saline (NaCl 0.9%) for intraperitoneal injections as previously described (Chaves et al., 2020; de Morais et al., 2018). The dose range tested (0.3–30 mg/kg, i.p.) was chosen based on results from our previous study on male rats (Genaro et al., 2017).
Elevated plus-maze
The EPM is a classic behavioral test to study anxiety-like behaviors in rodents (Borelli and Brandão, 2008; Handley and Mithani, 1984; Pellow et al., 1985). The apparatus comprised two open (50 × 10 cm) and two closed (50 × 10 × 40 cm) arms extending from a central platform (10 × 10 cm) elevated 50 cm above the floor. All testing was conducted during the light phase of the light/dark cycle, between 10:00 AM and 1:00 PM. The apparatus was located inside a room with constant background noise (50 dB). The animal behaviors were recorded by a video camera (Everfocus, Orange, CA, USA) positioned above the maze, and the signal was relayed to a monitor in another room via a closed-circuit TV camera. Luminosity at the open and closed arms level was 27 lx. The rats were placed individually in the center of the maze facing an open arm and allowed 5-min free exploration. Each rat was tested only once, and the apparatus was cleaned with 20% ethyl alcohol after each session.
The performance of each animal in the maze was scored by an observer blind to the experimental groups. The standard measurements recorded in each section of the maze (closed and open arms) comprised the frequency of open and closed arm entries (an arm entry or exit being defined as all four paws into or out an arm, respectively), total arm entries, and the percentage of time spent on the open and closed arms were calculated as percentage of the total time the rat spent on the maze [arm time % = 100 × (arm time/total time)]. In addition, the frequencies of the following ethological parameters were measured: (1) head-dipping: dipping of the head below the level of the maze floor; (2) end-arm exploration: the number of times the rat reached the end of an open arm; (3) stretched-attend postures: when the animal stretches to its full length with the forepaws (keeping the hind paws in the same place and turns back to the anterior position); and (4) rearing: vertical movements in any direction, including sniffing of maze walls. Most of these categories were defined previously following work with rats and mice (Blanchard et al., 1993; Borelli and Brandão, 2008; Rodgers and Johnson, 1995). In our initial experiment, males and separate groups of female rats at the four different stages of the cycle were tested on the EPM. These experiments revealed significant differences in responding between the P and LD stages. Thus, for subsequent experiments testing the effect of CBD, we compared rats only in P and LD.
Open field
A different cohort of animals was used for the open-field test. The open-field test is widely used to test rodents’ exploratory behavior and general activity (Crusio, 2001). The experimental protocol for the open-field test was based on Borelli et al. (2004) and de Oliveira et al. (2006). Locomotor activity and exploration were evaluated in an arena consisting of a circular enclosure made of transparent acrylic (60 cm in diameter, 50-cm height, floor divided into 12 sections). All testing was conducted during the light phase of the light/dark cycle, between 10:00 AM and 1:00 PM. Although rats are nocturnal, we chose to carry out experiments in their light phase in order to be able to make comparisons with other studies, the vast majority of which conducted experiments during the light phase. Each rat was placed in the middle of the arena and left for a 5-min period of free exploration. For the course of the session, the total number of crossings (number of floor sections traversed), total number of rearings (standing with the forepaws raised in the middle of the arena or against the walls), and the time animals spent in the center of the arena were evaluated. The videos were scored by a blinded observer. The experimental room condition and the apparatus cleaning procedure were similar to the EPM test.
Statistical analysis
All the graphs, calculations, and statistical analyses were performed using GraphPad Prism software version 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). For experiment 1: behavior in the EPM of males and females at different stages of the estrous cycle, statistical differences were determined by one-way analysis of variance (ANOVA) with estrous cycle phase (P, E, ED, and LD) and males as the factor with Tukey’s multiple comparisons test. For examining drug effects in experiments 2, 3, and 4, two-way ANOVA with Tukey’s post hoc test was used to analyze differences between groups (P, LD, and male) and treatments (vehicle and CBD). Also, one-way ANOVA was used to compare the treatment effect in each group (sex/estrous cycle: P, LD, and male) with Tukey’s multiple comparisons test in experiments 2. In experiment 3, we compared the effects of treatment at a specific stage of the estrous cycle. For this within-group comparison, Student’s t-test was applied to each phase (P and LD). Differences were considered significant if p < 0.05. The results are expressed as mean value ± SEM.
Results
Experiment 1—Behavior of male and female rats in the EPM
Figure 1(a) shows the experimental timeline of the experimental protocol. In order to examine whether the exploratory behaviors of male rats and female rats at different stages of the estrous cycle were differentially expressed in the EPM, a one-way ANOVA was performed, taking sex/estrous cycle stages as the main factor. Entries into the open arms and percentage of the total time spent in the open arms of the maze are considered as indices of anxiety-like behavior. This analysis showed significant effects for sex/estrous cycle phase upon the frequency of open arms entries (F(4,38) = 2.665; p < 0.05) and percentage of time spent in the open arms (F(4,38) = 3.629; p < 0.05). Tukey’s post hoc analysis revealed that the frequency of entries in the open arms of females in LD was lower (p < 0.05) compared to the P stage (Figure 1(f)). In addition, females in LD spent less time in the open arms compared to rats in P (Figure 1(g)). There was no effect for sex/estrous cycle upon the frequency of closed arms entries (F(4,38) = 0.980; p = 0.43) and percentage of time spent on the closed arms (F(4,38) = 1.434; p = 0.24) of the maze (Figure 1(h) and (I), respectively). The ethological parameters of risk assessment and exploration were also not significantly changed by injections of vehicle in male and female rats (see Table 1).
Table 1.
Experiment 1—Ethological parameters measured in female and male rats submitted to the elevated plus maze test.
| Behavior | P | E | ED | LD | M | F (3,38) | p |
|---|---|---|---|---|---|---|---|
| SAP | 10.00 ± 2.33 | 12.00 ± 1.79 | 14.29 ± 0.97 | 14.75 ± 1.60 | 9.9 ± 1.57 | 1.54 | 0.21 |
| HD | 14.91 ± 2.49 | 9.43 ± 1.49 | 12.29 ± 1.71 | 10.75 ± 2.23 | 13.6 ± 1.14 | 1.23 | 0.32 |
| RE | 14.18 ± 0.95 | 16.14 ± 1.26 | 17.14 ± 2.71 | 15.75 ± 2.48 | 16.3 ± 2.38 | 0.32 | 0.86 |
| EAE | 2.90 ± 0.41 | 1.57 ± 0.53 | 2.00 ± 0.62 | 1.25 ± 0.59 | 1.7 ± 0.34 | 1.96 | 0.12 |
The values are mean ± SEM. One-way ANOVA showed that there was not a statistically significant effect of the groups (P, E, ED, LD, and M) upon the ethological parameters of risk assessment and exploration in female and male rats. P: proestrus; E: estrus; ED: early diestrus; LD: late diestrus, M: male; SAP: stretched-attend postures; HD: head-dipping; RE: rearings; EAE: end-arm exploration; ANOVA: analysis of variance.
Experiment 2—Effect of CBD administered acutely before the EPM test in male rats and females in proestrus and LD
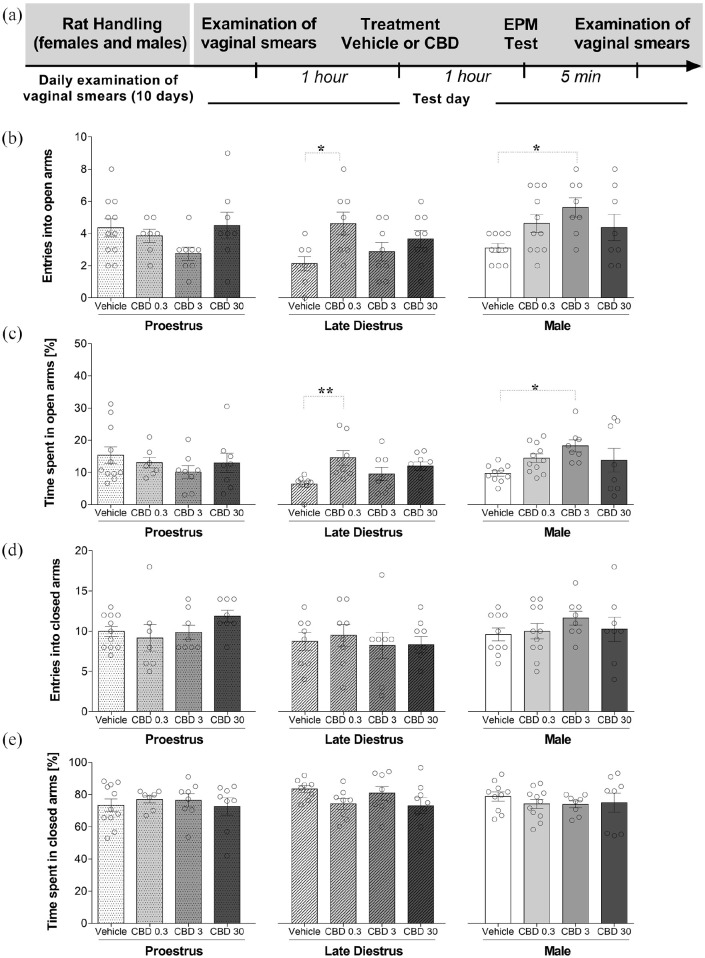
We chose females during P and LD to further test the effect of CBD on behavior in the EPM because of the respective high and low levels of responding demonstrated in experiment 1. Figure 2(a) shows the experimental timeline of the experimental protocol. In terms of the number of entries in the open arms, two-way ANOVA showed that there was a statistically significant effect of the groups (sex/estrous cycle) (F(2,92) = 3.695; p = 0.029) and interaction between factor (F(6,92) = 3.079; p = 0.009), but not of the treatments (F(3,92) = 2.539; p = 0.061). Tukey’s post hoc analysis revealed a main group (sex/estrous cycle) effect between females in LD and males (p < 0.05). We followed up this result by determining whether there were effects of the treatment for each group (sex/estrous cycle). One-way ANOVA was used to compare the treatment effect in each group (sex/estrous cycle). One-way ANOVA showed that there was not a statistically significant effect of the treatment (vehicle, CBD 0.3, 3, and 30 mg/kg) upon the frequency of open arms entries in females tested during P (F(3,30) = 1.781; p = 0.172). However, statistical analyses revealed significant treatment effects in females during LD (F(3,29) = 3.451; p = 0.029) and males (F(3,33) = 3.272; p = 0.033). Tukey’s multiple comparisons test showed that CBD 0.3 mg/kg treatment increased (p = 0.01) entries into the open arms of female rats in the LD phase compared with the vehicle-treated group (Figure 2(b)). Also, CBD at the dose of 3 mg/kg increased (p = 0.01) entries into the open arms of males compared with vehicle-treated group (Figure 2(b)).
Figure 2.
Effects of CBD on the exploratory behavior of female (during proestrus and late diestrus) and male rats submitted to the EPM. Each animal received an intraperitoneal injection 1 h before the test (vehicle or CBD 0.3, 3, or 30 mg/kg). (a) Timeline of the protocol for the experiments, (b) the number of entries into the open arms and (c) percentage of time spent in the open arms, and (d) the number of entries into closed arms, and (e) percentage of time spent in the closed arms of the maze. The data are expressed as the mean ± SEM. One-way ANOVA was used to compare the treatment effect in each group (sex/estrous cycle) followed by Tukey’s post hoc test. *p < 0.05, compared to control group. N = 7–11/group. ANOVA: analysis of variance; CBD: cannabidiol; EPM: elevated plus maze.
Time spent on the open arms was calculated as percentage of the total test time on the maze. A two-way ANOVA revealed that there was a statistically significant interaction between factors on the percentage of total time in the open arms (F(6,92) = 2.654; p = 0.020). However, there was no overall effect of either group (F(2,92) = 2.721; p = 0.071) or treatment (F(3,92) = 1.525; p = 0.213), so no multiple comparison was performed. A one-way ANOVA was performed to compare the effect of treatment on the percentage of time spent in the open arms in each group (sex/estrous cycle). A one-way ANOVA revealed that, in females during P, there was not a statistically significant difference in the percentage of total time spent in the open arms among the mean values for the different treatment groups (F(3,30) = 0.789; p = 0.510). However, statistical analyses revealed significant differences of treatment effects in females during LD (F(3,29) = 4.032; p = 0.016) and males (F(3,33) = 3.040; p = 0.042). Tukey’s multiple comparisons test showed that CBD 0.3 mg/kg treatment increased (p = 0.007) the percentage of time spent in the open arms of adult female rats in LD phase compared with vehicle group (Figure 2(c)). Also, CBD at the dose of 3 mg/kg increased (p = 0.014) the percentage of time spent in the open arms of males compared with vehicle group (Figure 2(c)).
For closed arms data, two-way ANOVA showed that there was not a statistically significant effect of the groups (sex/estrous cycle) (F(2,92) = 2.744; p = 0.070), treatments (F(3,92) = 0.267; p = 0.849], and interaction between factors: (F(6,92) = 0.827; p = 0.551) on the number of entries into the closed arms. Also, two-way ANOVA showed that there was not a statistically significant effect of the groups (sex/estrous cycle) (F(2,92) = 0.650; p = 0.524, treatments (F(3,92) = 0.974; p = 0.408), and interaction between factors (F(6,92) = 0.689; p = 0.659) on the percentage of time spent in the closed arms (Figure 2(d) and (e)). A one-way ANOVA also revealed that there was not a statistically significant difference in the number of entries and the percentage of time in the closed arms among the mean values for the different treatment groups in females and males (p > 0.05). Similarly, CBD administered acutely in male rats and females in P and LD did not significantly change the other ethological parameters measured in the EPM (see Table 2).
Table 2.
Experiment 2—Effect of CBD administered acutely in females (proestrus and late diestrus) and male rats on ethological parameters measured in the elevated plus maze test.
| Proestrus | ||||||
|---|---|---|---|---|---|---|
| Behavior | Vehicle | CBD 0.3 | CBD 3 | CBD 30 | F (3,30) | p |
| SAP | 10.00 ± 2.33 | 9.71 ± 1.43 | 13.00 ± 1.61 | 8.13 ± 0.79 | 1.15 | 0.35 |
| HD | 14.91 ± 2.49 | 15.14 ± 1.91 | 12.75 ± 0.94 | 11.13 ± 1.73 | 0.86 | 0.47 |
| RE | 14.18 ± 0.95 | 15.00 ± 1.53 | 17.25 ± 1.89 | 16.88 ± 1.76 | 0.36 | 0.39 |
| EAE | 2.90 ± 0.41 | 1.71 ± 0.57 | 1.50 ± 0.38 | 2.62 ± 0.75 | 1.72 | 0.18 |
| Late diestrus | ||||||
| Behavior | Vehicle | CBD 0.3 | CBD 3 | CBD 30 | F (3,29) | p |
| SAP | 9.88 ± 1.84 | 9.00 ± 1.57 | 11.00 ± 1.77 | 14.11 ± 1.40 | 1.94 | 0.15 |
| HD | 10.75 ± 2.23 | 14.38 ± 1.71 | 10.75 ± 1.93 | 10.56 ± 1.68 | 0.92 | 0.44 |
| RE | 15.75 ± 2.48 | 14.38 ± 2.14 | 9.75 ± 2.21 | 11.89 ± 1.54 | 1.58 | 0.21 |
| EAE | 1.25 ± 0.59 | 2.37 ± 0.65 | 1.62 ± 0.56 | 2.33 ± 0.44 | 0.96 | 0.42 |
| Male | ||||||
| Behavior | Vehicle | CBD 0.3 | CBD 3 | CBD 30 | F (3,33) | p |
| SAP | 9.90 ± 1.57 | 8.18 ± 1.64 | 12.88 ± 2.14 | 12.25 ± 1.93 | 1.48 | 0.24 |
| HD | 13.60 ± 1.14 | 15.91 ± 1.46 | 17.38 ± 1.28 | 12.25 ± 2.65 | 1.75 | 0.18 |
| RE | 16.30 ± 2.38 | 19.09 ± 1.67 | 16.50 ± 1.64 | 17.50 ± 1.88 | 0.47 | 0.70 |
| EAE | 1.70 ± 0.34 | 2.73 ± 0.56 | 3.12 ± 0.35 | 1.88 ± 0.77 | 1.62 | 0.20 |
The values are mean ± SEM. One-way ANOVA showed that there was not a statistically significant effect of the treatment (vehicle and CBD 0.3, 3, and 30 mg/kg) upon the ethological parameters of risk assessment and exploration in female and male rats. SAP: stretched-attend postures; HD: head-dipping; RE: rearings; EAE: end-arm exploration; ANOVA: analysis of variance; CBD: cannabidiol.
Experiment 3—Effect of sub-chronic treatment with CBD before the EPM test in female rats
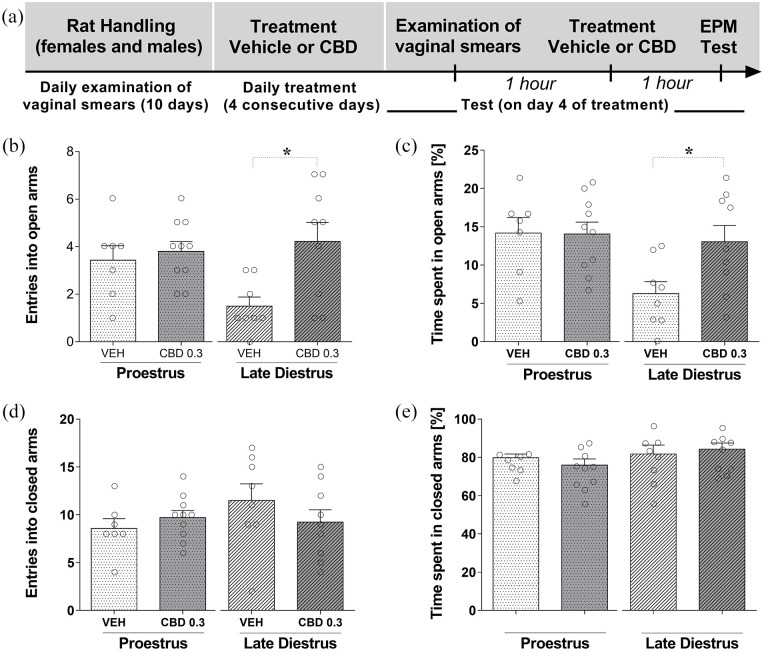
Figure 3(a) shows the experimental timeline of the experimental protocol. Sub-chronic treatment for 4 consecutive days with the lower dose of CBD (0.3 mg/kg) was investigated in females during P and LD. Two-way ANOVA showed that there was a statistically significant effect of the treatment on the number of entries into the open arms (F(1,30) = 7.063; p = 0.012), but not of the group (P and LD) (F(1,30) = 1.675; p = 0.205) and no interaction between factors (F(1,30) = 4.078; p = 0.052). Subsequently, a Student’s t-test was used to compare sub-chronic treatment effects in each group (P and LD). The Student’s t-test indicated that there was a significant difference between females that received CBD 0.3 mg/kg compared to the vehicle group during LD (t15 = 2.965; p = 0.010), but not in females during P (t15 = 0.522; p = 0.610] (Figure 3(b)).
Figure 3.
CBD effects after subchronic treatment on behavior of females rats (during proestrus and late diestrus) in the EPM. Each animal was treated for 4 consecutive days with one injection of vehicle or CBD (0.3 mg/kg). (a) Timeline of the protocol for the experiments, (b) the number of entries into the open and (c) percentage of time spent in the open arms, and (d) the number of entries into closed arms and (e) percentage of time spent in the closed arms of the maze. The data are expressed as the mean ± SEM. Student’s t-test was used to compare treatment effects in each group (P and LD). *p < 0.05, compared to control group. N = 7–10/group. CBD: cannabidiol; EPM: elevated plus maze.
Similar statistical results were obtained upon the percentage of time in the open arms. Two-way ANOVA showed that there was a statistically significant effect of the groups (P and LD) (F(1,30) = 5.851; p = 0.022), but not of the treatment (F(1,30) = 3.202; p = 0.083) on the percentage of time in the open arms, and no interaction between factors (F(1,30) = 3.490; p = 0.071). The Student’s t-test indicated that there was a significant difference between females that received CBD 0.3 mg/kg compared to the vehicle group during LD (t15 = 2.498; p = 0.025), but not females during P (t15 = 0.058; p = 0.954) (Figure 3(c)).
For closed arms data, two-way ANOVA showed there was not a statistically significant effect the treatments (F(1,30) = 0.851; p = 0.216), groups (P and LD): (F(1,30) = 0.981; p = 0.330), and no interaction between factors (F(1,30) = 1.896; p = 0.179) on the number of entries in the closed arms (Figure 3(d)). Similar statistical results were obtained upon the percentage of time spent in the closed arms, there was not a statistically significant effect the treatments (F(1,30) = 0.035; p = 0.853), groups (P and LD): (F(1,30) = 2.182; p = 0.150), and no interaction between factors (F(1,30) = 0.844; p = 0.365) on the percentage of time spent in the closed arms (Figure 3(e)). The Student’s t-test also revealed that, there was not a statistically significant difference in the number of entries and the percentage of time in the closed arms (p > 0.05) after CBD sub-chronic treatment compared to vehicle group in females during P and LD.
Experiment 4—Effect of CBD on behavior in the open-field test
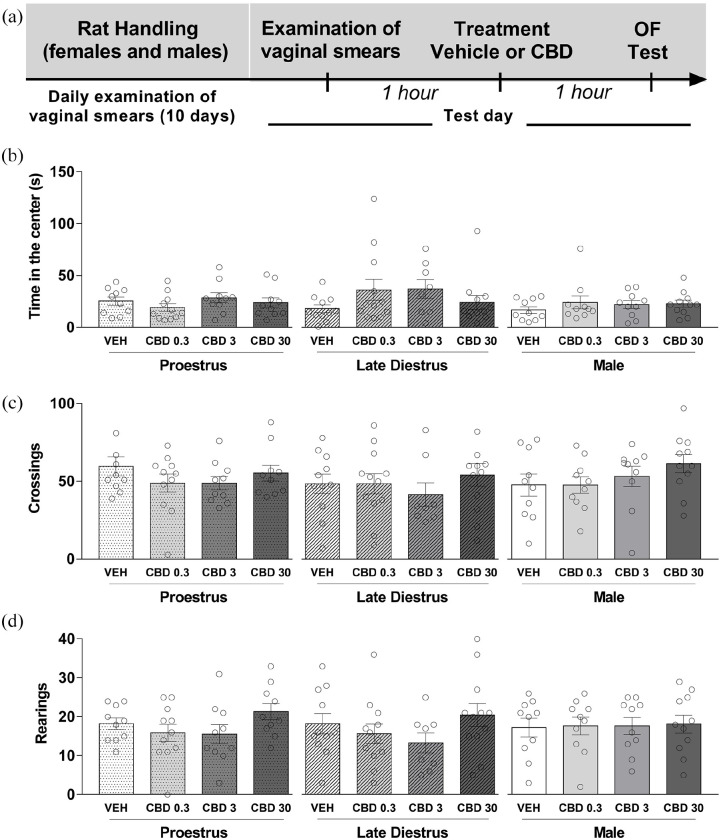
We examined the effects of acute treatment with CBD (0.3, 3, and 30 mg/kg) on the general activity in an arena in males and in females during P and LD. Figure 4(a) shows the experimental timeline of the experimental protocol. The systemic injection of vehicle or CBD 1 h before the test session did not induce significant effects among the groups in the open-field test. The two-way ANOVA showed that there was not a statistically significant effect of the treatment (vehicle, CBD 0.3, 3, and 30 mg/kg) (F(3,113) = 1.28; p > 0.05], group (sex/estrous cycle) (F(2,113) = 1.66; p > 0.05) and no interaction between the factors F(6,113) = 1.00; p > 0.05) upon the time spent in the center of the arena (Figure 4(b)). Similar statistical results were obtained upon the total number of crossings (treatment: (F(3,113) = 1.38; p > 0.05); sex/estrous cycle: (F(2,113) = 0.76; p > 0.05); and interaction: (F(6,113) = 0.54; p > 0.05)) and total number of rearings (treatment: F(3,113) = 2.00; p > 0.05; sex/estrous cycle: F(2,113) = 0.15; p > 0.05; and interaction: F(6,113) = 0.46; p > 0.05) (Figure 4(c) and (d), respectively). A one-way ANOVA also revealed that there was not a statistically significant difference in the behavioral responses investigated in the open field (p > 0.05) among the mean values for the different treatment groups in females and males.
Figure 4.
CBD did not affect the behavior of male and female rats in the open-field test. Each animal was injected 1 h before the test either with vehicle or CBD 0.3, 3, or 30 mg/kg. (a) Timeline of the protocol for the experiments, (b) time spent in the center of the open field, (c) the total number of crossings, and (d) the total number of rearings in the (OF). The data are expressed as the mean ± SEM. There was not a statistically significant difference in the behavioral responses investigated in the open field (p > 0.05) among the mean values for the different treatment groups in females and males. N = 8–12/group. CBD: cannabidiol; OF: open field.
Discussion
The EPM is arguably the most widely used test of anxiety-related behavior in rodents (Walf and Frye, 2007). Although the vast majority of studies have used only males, attention is starting to be directed toward females and to consider the influence of the estrous cycle. In our experiments, male and female rats behaved similarly in the EPM, with no sex-related differences. Also, the entire ethological analysis, including defensive coping strategies, such as direct exploration and risk assessment, was not significantly changed by injections of vehicle or CBD in males and females tested in the experiments 1 and 2. These results are in line with many studies that failed to detect sex differences in behavior on the EPM or in other experimental paradigms (Albrechet-Souza et al., 2020; Caraalho et al., 2021; Chari et al., 2020; Elliott et al., 2004; Pohl et al., 2007; Soares-Cunha et al., 2018; Yang et al., 2019). However, it should be noted that other studies reported less anxiety-like behavior in female rats compared to males in the EPM (Domonkos et al., 2017; Imhof et al., 1993; Johnston and File, 1991; Knight et al., 2021).
The first aim of the present study was to examine sex-related differences and, in females, the effect of the different phases of the estrous cycle on anxiety-like behaviors in the EPM. In our study, differences emerged within the female cohort in behavior in the EPM but not in the open field, depending on the estrous cycle stage. In the open field, the time spent in the center, an index of anxiety-like behavior did not differ between the LD and P stages. However, in the EPM, animals in the LD phase exhibited more anxiety-like behavior (fewer entries and shorter time spent in the open arms of the EPM) than at other stages, the difference reaching statistical significance between the LD and P stages. The difference could not be attributed to variations in motor behavior since closed arm entries and the number of crossings and rearings in the open-field test were similar in all groups. Similarly, there was no difference between the sexes in locomotor activity in the open field, in line with earlier studies (Scholl et al., 2019). However, others have reported higher innate locomotor activity in the open field in females compared to males (Aguilar et al., 2003; Bishnoi et al., 2021; Börchers et al., 2022; Lehmann et al., 1999; Seliger 1977).
In line with our findings, most previous studies in females that have considered estrous cycle reported more time spent in the open arms of the maze in proestrus/estrus compared to diestrus, although it is worth highlighting that a significant minority (approximately one-third) of such studies failed to see any estrous cycle-related difference (Lovick and Zangrossi, 2021, for a recent review). It is difficult to explain the lack of consensus, but subtle methodological and experimenter differences are likely to be the major contributory factors (see Lovick and Zangrossi, 2021, for discussion on this point). Although the findings are equivocal, sex differences in the effects of cannabis and the cannabinoid THC have been reported (see Cooper and Craft, 2018, for a critical review). There is also evidence that gonadal hormones can modify the effects of THC (Cooper and Craft, 2018). Despite its therapeutic potential, much less attention has been directed toward CBD. In our study, CBD produced anxiolytic-like effects in the EPM in males and female rats. The response was characterized by a bell-shaped dose-response relationship, in agreement with other single-sex studies (Guimarães et al., 1994; Salviato et al., 2021). By comparing the responsiveness of males and females in the same study, we found not only that the responsiveness of females was dependent on the estrous cycle stage but also that the optimal dose for females in the responsive LD stage (0.3 mg/kg) was 10-fold lower than for males (3 mg/kg). In contrast, females in the P phase were unaffected by CBD. The failure of CBD to influence activity of females in P is interesting as it suggests that the hormonal profile of the brain in LD favors CBD. However, given the bell-shaped drug-response curve, it will be important to test rats in P phase with even lower doses (i.e., < 0.3 mg/kg) to confirm a lack of effect.
In our study, CBD did not produce anxiolytic-like effects in the open-field test assessed by the time spent in the center of the apparatus. However, in mice, higher doses of CBD (5 mg/kg) outside the range used in the present study increased the time spent in the center of the open field in adult males, while 20 mg/kg decreased the time spent in the center in females (Kasten et al., 2019). Lower doses were not tested in that study, nor was the potential influence of the estrous cycle on females evaluated. These findings might reflect species differences. However, given the diverse mechanisms of action of CBD (see below), it is possible that high doses of CBD may elicit anxiolytic or anxiogenic-like effects by mechanisms that are pharmacologically distinct from those engaged by lower doses of the drug. Indeed, Campos and Guimarães (2009) demonstrated that in the dorsolateral periaqueductal grey (dlPAG), a key area for mediating escape behavior in the EPM, high doses of CBD activate the transient receptor potential vanilloid type 1 (TRPV1) receptor to facilitate glutamate neurotransmission and promote anxiogenic-like behavior, which offsets the 5-HT1A receptor-mediated anxiolytic effects evoked at lower doses of CBD (Campos and Guimaraes, 2008).
After sub-chronic treatment, the anxiolytic-like effect of CBD in the EPM was retained in females in LD, but females in P remained unresponsive, as we expected. This raises the possibility that CBD at low doses acts acutely through mechanisms that are triggered only during the LD phase in females. CBD is known to interact with multiple neurotransmitter systems within the brain. In addition to CB1 and CB2 cannabinoid receptors, it also has actions at 5-HT1A receptors, TRPV1 receptor, and GABAergic systems (Bakas et al., 2017; Cifelli et al., 2020; Kaplan et al., 2017; Osborne et al., 2019; Pretzsch et al., 2019; Vitale et al., 2021). Of particular relevance to the present study is the finding that CBD acts as a positive allosteric modulator at extrasynaptic GABAA receptors with the α4βδ subunit configuration (Bakas et al., 2017). Interestingly, the neuroactive metabolite of progesterone allopregnanolone (ALLO) has a similar action (Paul and Purdy, 1992). During LD, the rapid decline in the secretion of progesterone and hence ALLO triggers increased expression of extrasynaptic α4βδ GABAA receptors in circuitry involved in mediating anxiety-like behavior (Griffiths and Lovick, 2005a, 2005b; Gulinello et al., 2001, 2003; Lambert et al., 2003; Smith et al., 1998). The consequent increase in neuronal excitability (Brack and Lovick, 2007) is thought to contribute to raised levels of anxiety that become apparent during the LD phase (Brack and Lovick, 2007; Devall et al., 2009; Maguire and Mody, 2007; Smith et al., 1998). It is possible that the administration of CBD in LD substitutes for the actions of the neurosteroid as ALLO concentration is declining, thereby preventing the withdrawal effect and disturbance in GABA signaling and increased anxiety-like behavior that usually characterizes the LD phase. These changes are followed by activation of the hypothalamic-pituitary-adrenal axis; in rodents, it has been widely shown that fear/anxiety increases plasma corticosterone levels (File et al., 1993, 1994; de Oliveira et al., 2017; Reis et al., 2022). Interestingly, studies showed that females have higher levels of stress-induced corticosterone than males, both in basal conditions and in response to a variety of stress conditions (Albrechet-Souza et al., 2020; Babb et al., 2013; Kalil et al., 2014; Kant et al., 1983; Oyola and Handa, 2017). It is important to note that several factors can affect plasma corticosterone concentrations across the estrous cycle, including the transition period for ovarian hormonal secretion, regularity of the estrous cycle, and the phases of circadian rhythm (Arikawe et al., 2021; Atkinson and Waddell, 1997; Gong et al., 2015). In the present study, we did not investigate the plasma levels of corticosterone and ovarian hormones; therefore, future studies aimed at evaluating the influence of CBD treatment on behavioral and endocrine responses will provide additional evidence for sex-related differences in response to drug treatment.
In male rats, a 10-fold higher dose of CBD (3 mg/kg) was required to produce anxiolytic-like effects in the EPM compared to females. This dose is similar to that reported to be effective in comparable studies using males (Campos and Guimarães, 2008; Campos et al., 2012). In males, the acute anxiolytic-like effects of CBD in the EPM have been shown to involve 5-HT1A receptor activation (Campos and Guimarães, 2008). 5-HT1A receptor activation is also involved in mediating the anti-aggressive effects of CBD in mice (Hartmann et al., 2019). At present, there is no comparable data from studies in females. However, a tendency for lower 5HT1A receptor binding potential in women compared to men (Moses-Kolko et al., 2011) together with estrogen-driven downregulation of activity and expression of 5HT1A autoreceptors reported in rats (Osterlund and Hurd, 1998) would suggest that serotonergic effects of CBD may be less important in females. It is also worth noting that notwithstanding the findings in rats, adult mice of either sex appeared unresponsive to CBD in the EPM (5–20 mg/kg, Kasten et al., 2019), suggesting there may be a species difference in responsiveness to CBD.
Our data indicate that sex and hormone-dependent processes may contribute to differences in CBD’s anxiolytic-like effects between males and females. Females were responsive to CBD only in their LD phase when they became much more sensitive to CBD than males. However, a limitation of our study is that not all stages of the estrous cycle were tested, and it will be important in future studies to include females in all stages of their cycle. It is also important that future studies repeat the experiments using another experimental paradigm for anxiety-like behavior. The exact mechanism of action responsible for these varying effects of CBD observed between males and females remains unknown but may involve different neurotransmitter systems. Sex differences in drug responses may affect drug safety and effectiveness, emphasizing the importance of including both sexes and discriminating between the estrous phases. A significant proportion of women develop adverse emotional symptoms during the late luteal (premenstrual) phase of their cycle, when progesterone secretion is falling rapidly (Dennerstein et al., 2011). The present study in rats suggests that administration of CBD, during the premenstrual period in women, may have therapeutic potential for minimizing the development of symptoms of emotional distress during the premenstrual period. In rodents, the effects of CBD have been shown to depend on the dose, the strain, the administration time course (acute vs chronic), and the route of administration (García-Gutiérrez et al., 2020). Moreover, our present findings indicate a 10-fold increase in sensitivity to CBD compared to males shown by female rats in the LD phase, which is similar to the late luteal (premenstrual) phase in women. Further work will be needed to translate findings in rodents to humans, but based on the current data obtained in rats, allometric scaling to estimate starting doses for clinical trials (Nair and Jacob, 2016) suggests that the effective dose in women could be considerably lower than that reported from studies of anxiolytic effects of CBD in men (Peng et al., 2022; Wright et al., 2020).
Acknowledgments
The authors are grateful to DH Elias-Filho for their expert technical assistance.
Footnotes
Author contributions: KG and ARO conceptualized and guided the project design. DF conducted behavioral experiments and contributed to conception, drafting, and revising the manuscript. MCC, WAP, MLB, AWZ, JAC, ARO, and TAL contributed with their academic and scientific expertise, physical infrastructure, and supply support. KG and TAL wrote the manuscript.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: JAC is a member of the International Advisory Board of the Australian Centre for Cannabinoid Clinical and Research Excellence (ACRE)—National Health and Medical Research Council (NHMRC). JAC has received travel support to attend scientific meetings and personal consultation fees from BSPG-Pharm. JAC is a coinventor of the patent “Fluorinated CBD compounds, compositions and uses thereof. Pub. No.: WO/2014/108899. International Application No.: PCT/IL2014/050023,” Def. US number Reg. 62193296; July 29, 2015; INPI on August 19, 2015 (BR1120150164927; Mechoulam R, Zuardi AW, Kapczinski F, Hallak JEC, Guimarães FS, Crippa JA, Breuer A). Universidade de São Paulo (USP) has licensed this patent to Phytecs Pharm (USP Resolution No. 15.1.130002.1.1) and has an agreement with Prati-Donaduzzi to “develop a pharmaceutical product containing synthetic CBD and prove its safety and therapeutic efficacy in the treatment of epilepsy, schizophrenia, Parkinson’s disease, and anxiety disorders.” JAC is a coinventor of the patent “Cannabinoid-containing oral pharmaceutical composition, method for preparing and using same,” INPI on September 16, 2016 (BR 112018005423-2). The other authors declare that they have no conflicts of interest. JAC is a consultant and/or has received speaker fees and/or sits on the advisory board and/or receives research funding from Janssen-Cilag, Torrent Pharm, Prati-Donaduzzi, PurMed Global, and BSPG Pharm over the past 3 years.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: DF was supported by undergraduate Programme Grant—Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Master Fellowship—Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). AWZ and JAC are recipients of fellowship awards from CNPq. ARO and MCC are recipients of CAPES Finance Code 001. TAL was a Visiting Researcher in Brazil supported by Fundação de Amparo à Pesquisa—FAPESP (grant number 2019/05240-6). In the course of literature research and assembly of the manuscript, KG received a NARSAD Young Investigator Grant 2019 | The Brain & Behavior Research Foundation.
ORCID iDs: Milene C Carvalho  https://orcid.org/0000-0002-5760-2999
https://orcid.org/0000-0002-5760-2999
José A Crippa  https://orcid.org/0000-0001-9520-6746
https://orcid.org/0000-0001-9520-6746
Thelma A Lovick  https://orcid.org/0000-0002-7329-4607
https://orcid.org/0000-0002-7329-4607
Karina Genaro  https://orcid.org/0000-0001-6450-5778
https://orcid.org/0000-0001-6450-5778
References
- Aguilar R, Gil L, Gray JA, et al. (2003) Fearfulness and sex in F2 Roman rats: males display more fear though both sexes share the same fearfulness traits. Physiol Behav 78: 723–732. [DOI] [PubMed] [Google Scholar]
- Albrechet-Souza L, Schratz CL, Gilpin NW. (2020) Sex differences in traumatic stress reactivity in rats with and without a history of alcohol drinking. Biol Sex Differ 11: 2–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arikawe AP, Rorato RC, Gomes N, et al. (2021) Hormonal and neural responses to restraint stress in an animal model of perimenopause in female rats. J Neuroendocrinol 33: e12976. [DOI] [PubMed] [Google Scholar]
- Atkinson HC, Waddell BJ. (1997) Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology 138: 3842–38428. [DOI] [PubMed] [Google Scholar]
- Bakas T, van Nieuwenhuijzen PS, Devenish SO, et al. (2017) The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol Res 119: 358–370. [DOI] [PubMed] [Google Scholar]
- Bishnoi IR, Ossenkopp KP, Kavaliers M. (2021) Sex and age differences in locomotor and anxiety-like behaviors in rats: from adolescence to adulthood. Dev Psychobiol 63: 496–511. [DOI] [PubMed] [Google Scholar]
- Blanchard RJ, Magee LK, Veniegas R, et al. (1993) Alcohol and anxiety: ethopharmacological approaches. Prog Neuropsychopharmacol Biol Psychiatry 17: 171–182. [DOI] [PubMed] [Google Scholar]
- Babb JA, Masini CV, Day HE, et al. (2013) Sex differences in activated corticotropin-releasing factor neurons within stress-related neurocircuitry and hypothalamic-pituitary-adrenocortical axis hormones following restraint in rats. Neuroscience 234: 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolsoni LM, Crippa JAS, Hallak JEC, et al. (2022) The anxiolytic effect of cannabidiol depends on the nature of the trauma when patients with post-traumatic stress disorder recall their trigger event. Braz J Psychiatry 44: 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börchers S, Krieger JP, Asker M, et al. (2022) Commonly-used rodent tests of anxiety-like behavior lack predictive validity for human sex differences. Psychoneuroendocrinology 141: 105733. [DOI] [PubMed] [Google Scholar]
- Borelli KG, Nobre MJ, Brandão ML, et al. (2004) Effects of acute and chronic fluoxetine and diazepam on freezing behavior induced by electrical stimulation of dorsolateral and lateral columns of the periaqueductal gray matter. Pharmacol Biochem Behav 77: 557–566. [DOI] [PubMed] [Google Scholar]
- Borelli KG, Brandão ML. (2008) Effects of ovine CRF injections into the dorsomedial, dorsolateral and lateral columns of the periaqueductal gray: a functional role for the dorsomedial column. Horm Behav 53: 40–50. [DOI] [PubMed] [Google Scholar]
- Brack KE, Lovick TA. (2007) Neuronal excitability in the periaqueductal grey matter during the estrous cycle in female Wistar rats. Neuroscience 144: 325–35. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. (2008) Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats. Psychopharmacology (Berl) 199: 223–230. [DOI] [PubMed] [Google Scholar]
- Campos AC, Guimarães FS. (2009) Evidence for a potential role for TRPV1 receptors in the dorsolateral periaqueductal gray in the attenuation of the anxiolytic effects of cannabinoids Prog Neuropsychopharmacol Biol Psychiatry 33: 1517–1521. [DOI] [PubMed] [Google Scholar]
- Campos AC, Moreira FA, Gomes FV, et al. (2012) Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos Trans R Soc Lond B Biol Sci 367: 3364–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho MC, Genaro K, Leite-Panissi CR, et al. (2021) Influence of estrous cycle stage on acquisition and expression of fear conditioning in female rats. Physiol Behav 234: 113372. [DOI] [PubMed] [Google Scholar]
- Chari T, Griswold S, Andrews NA, et al. (2020) The stage of the estrus cycle is critical for interpretation of female mouse social interaction behavior. Front Behav Neurosci 14: 113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves YC, Genaro K, Stern CA, et al. (2020) Two-weeks treatment with cannabidiol improves biophysical and behavioral deficits associated with experimental type-1 diabetes. Neurosci Lett 729: 135020. [DOI] [PubMed] [Google Scholar]
- Cifelli P, Ruffolo G, De Felice E, et al. (2020) Phytocannabinoids in neurological diseases: could they restore a physiological GABAergic transmission?. Int J Mol Sci 21: 723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Craft RM. (2018) Sex-dependent effects of cannabis and cannabinoids: a translational perspective. Neuropsychopharmacology 43: 34–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crusio WE. (2001) Genetic dissection of mouse exploratory behaviour. Behav Brain Res 125: 127–132. [DOI] [PubMed] [Google Scholar]
- de Morais H, Chaves YC, Waltrick APF, et al. (2018) Sub-chronic treatment with cannabidiol but not with URB597 induced a mild antidepressant-like effect in diabetic rats. Neurosci Lett 682: 62–68. [DOI] [PubMed] [Google Scholar]
- de Oliveira AR, Reimer AE, Brandão ML. (2006) Dopamine D2 receptor mechanisms in the expression of conditioned fear. Pharmacol Biochem Behav 84: 102–111. [DOI] [PubMed] [Google Scholar]
- de Oliveira AR, Reimer AE, Reis FM, et al. (2017) Dopamine D2-like receptors modulate freezing response, but not the activation of HPA axis, during the expression of conditioned fear. Exp Brain Res 235: 429–436. [DOI] [PubMed] [Google Scholar]
- Dennerstein L, Lehert P, Heinemann K. (2011) Global epidemiological study of variation of premenstrual symptoms with age and sociodemographic factors. Menopause Int 17: 96–101. [DOI] [PubMed] [Google Scholar]
- Devall AJ, Liu ZW, Lovick TA. (2009) Hyperalgesia in the setting of anxiety: sex differences and effects of the oestrous cycle in Wistar rats. Psychoneuroendocrinology 34: 587–596. [DOI] [PubMed] [Google Scholar]
- Domonkos E, Borbélyová V, Csongová M, et al. (2017) Sex differences and sex hormones in anxiety-like behavior of aging rats. Horm Behav 93: 159-165. [DOI] [PubMed] [Google Scholar]
- Dos Santos RF, Guimarães FS, Crippa JAS, et al. (2020) Serious adverse effects of cannabidiol (CBD): a review of randomized controlled trials. Expert Opin Drug Metab Toxicol 16: 517–526. [DOI] [PubMed] [Google Scholar]
- Elliott BM, Faraday MM, Phillips JM, et al. (2004) Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav 77: 21–28. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Sanders FL, et al. (1993) Dissociation between behavioral and corticosterone responses on repeated exposures to cat odor. Physiol Behav 54: 1109–11. [DOI] [PubMed] [Google Scholar]
- File SE, Zangrossi H, Jr, Sanders FL, et al. (1994) Raised corticosterone in the rat after exposure to the elevated plus-maze. Psychopharmacology (Berl) 113: 543–546. [DOI] [PubMed] [Google Scholar]
- García-Gutiérrez MS, Navarrete F, Gasparyan A, et al. (2020) Cannabidiol: a potential new alternative for the treatment of anxiety, depression, and psychotic disorders. Biomolecules 10: 1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genaro K, Fabris D, Arantes AL, et al. (2017) Cannabidiol is a potential therapeutic for the affective-motivational dimension of incision pain in rats. Front Pharmacol 8: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes FV, Casarotto PC, Resstel LBM, et al. (2011) Facilitation of CB1 receptor-mediated neurotransmission decreases marble burying behavior in mice. Prog Neuropsychopharmacoly Biol Psychiatry 35: 434–438. [DOI] [PubMed] [Google Scholar]
- Gong S, Miao YL, Jiao GZ, et al. (2015) Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS One 20 10: e0117503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. (2005. a) Withdrawal from progesterone increases expression of α4β1δ GABAA receptor subunits in neurons in the periaqueductal gray matter in female Wistar rats. J Comp Neurol 486: 89–97. [DOI] [PubMed] [Google Scholar]
- Griffiths JL, Lovick TA. (2005. b) GABAergic neurones in the rat periaqueductal grey matter express α4β1δ GABAA receptor subunits: plasticity during the oestrous cycle. Neuroscience 136: 457–466. [DOI] [PubMed] [Google Scholar]
- Guimarães FS, Chiaretti TM, Graeff FG, et al. (1990) Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl) 100: 558–559. [DOI] [PubMed] [Google Scholar]
- Guimarães FS, De Aguiar JC, Mechoulam R, et al. (1994) Anxiolytic effect of cannabidiol derivatives in the elevated plus-maze. Gen Pharmacol 25: 161–164. [DOI] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, et al. (2001) Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety in the female rat. Brain Res 910: 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Orman R, Smith SS. (2003) Sex differences in anxiety, sensorimotor gating and expression of the alpha4 subunit of the GABAA receptor in the amygdala after progesterone withdrawal. Eur J Neurosci 17: 641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handley SL, Mithani S. (1984) Effects of alpha-adrenoceptor agonists and antagonists in a maze-exploration model of ‘fear’-motivated behaviour. Naunyn Schmiedebergs Arch Pharmacol 327: 1–5. [DOI] [PubMed] [Google Scholar]
- Hantsoo L, Epperson CN. (2017) Anxiety disorders among women: a female lifespan approach. Focus (Am Psychiatr Publ) 15: 162–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman CG. (1944) Some new observations on the vaginal smear of the rat. Yale J Biol Med 17: 99–112. [PMC free article] [PubMed] [Google Scholar]
- Hartmann A, Lisboa SF, Buzolin Sonego A, et al. (2019) Cannabidiol attenuates aggressive behavior induced by social isolation in mice: involvement of 5-HT1A and CB1 receptors. Prog Neuropsychopharmacol Biol Psychiatry 94: 109637. [DOI] [PubMed] [Google Scholar]
- Iffland K, Grotenhermen F. (2017) An update on safety and side effects of cannabidiol: a review of clinical data and relevant animal studies. Cannabis Cannabinoid Res 2: 139–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imhof JT, Coelho ZM, Schmitt ML, et al. (1993) Influence of gender and age on performance of rats in the elevated plus maze apparatus. Behav Brain Res 56: 177–180. [DOI] [PubMed] [Google Scholar]
- Johnston AL, File SE. (1991) Sex differences in animal tests of anxiety. Physiol Behav 49: 245–250. [DOI] [PubMed] [Google Scholar]
- Kalil B, Leite CM, Carvalho-Lima M, et al. (2014) Role of sex steroids in progesterone and corticosterone response to acute restraint stress in rats: sex differences. Stress 16: 452–460. [DOI] [PubMed] [Google Scholar]
- Kant GJ, Lenox RH, Bunnell BN, et al. (1983) Comparison of stress response in male and female rats: pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology 8: 421–428. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Stella N, Catterall WA, et al. (2017) Cannabidiol attenuates seizures and social deficits in a mouse model of Dravet syndrome. Proc Natl Acad Sci U S A 114: 11229–11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasten CR, Zhang Y, Boehm SL., II (2019) Acute cannabinoids produce robust anxiety-like and locomotor effects in mice, but long-term consequences are age- and sex-dependent. Front Behav Neurosci 13: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight P, Chellian R, Wilson R, et al. (2021) Sex differences in the elevated plus-maze test and large open field test in adult Wistar rats. Pharmacol Biochem Behav 204: 173168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert JJ, Belelli D, Peden DR, et al. (2003) Neurosteroid modulation of GABAA receptors. Prog Neurobiol 71: 67–80. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Pryce CR, Bettschen D, et al. (1999) The maternal separation paradigm and adult emotionality and cognition in male and female Wistar rats. Pharmacol Biochem Behav 64: 705–715. [DOI] [PubMed] [Google Scholar]
- Linares IM, Zuardi AW, Pereira LC, et al. (2019) Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz J Psychiatry 41: 9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisboa SF, Gomes FV, Terzian ALB, et al. (2017) The endocannabinoid system and anxiety. Vitam Horm 103: 193–279. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Zangrossi H., Jr (2021) Effect of estrous cycle on behavior of females in rodent tests of anxiety. Front Psychiatry 12: 711065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Figueiredo R, de Carvalho MC, Brandão ML, et al. (2019) Short-term, low-dose fluoxetine prevents oestrous cycle-linked increase in anxiety-like behaviour in female rats. J Psychopharmacol 33: 548–557. [DOI] [PubMed] [Google Scholar]
- Maguire J, Mody I. (2007) Neurosteroid synthesis-mediated regulation of GABA(A) receptors: relevance to the ovarian cycle and stress. J Neurosci 27: 2155–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melas PA, Scherma M, Fratta W, et al. (2021) Cannabidiol as a potential treatment for anxiety and mood disorders: molecular targets and epigenetic insights from preclinical research. Int J Mol Sci 22: 1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Shan N, et al. (2011) Age, sex, and reproductive hormone effects on brain serotonin-1A and serotonin-2A receptor binding in a healthy population. Neuropsychopharmacology 36: 2729–2740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murkar A, De Koninck J, Merali Z. (2021) Cannabinoids: revealing their complexity and role in central networks of fear and anxiety. Neurosci Biobehav Rev 131: 30–46. [DOI] [PubMed] [Google Scholar]
- Nair AB, Jacob S. (2016) A simple practice guide for dose conversion between animals and human. J Basic Clin Phar 7: 27–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyola MG, Handa RJ. (2017) Hypothalamic-pituitary-adrenal and hypothalamic-pituitary-gonadal axes: sex differences in regulation of stress responsivity. Stress 20: 476–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne AL, Solowij N, Babic I, et al. (2019) Effect of cannabidiol on endocannabinoid, glutamatergic and GABAergic signalling markers in male offspring of a maternal immune activation (poly I:C) model relevant to schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry 95: 109666. [DOI] [PubMed] [Google Scholar]
- Osterlund MK, Hurd YL. (1998) Acute 17 beta-estradiol treatment down-regulates serotonin 5HT1A receptor mRNA expression in the limbic system of female rats. Brain Res Mol Brain Res 55: 169–72. [DOI] [PubMed] [Google Scholar]
- Paul SM, Purdy RH. (1992) Neuroactive steroids. FASEB J 6: 2311–2322. [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, et al. (1985) Validation of open: closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J Neurosci Methods 14: 149–167. [DOI] [PubMed] [Google Scholar]
- Peng J, Fan M, An C, et al. (2022) A narrative review of molecular mechanism and therapeutic effect of cannabidiol (CBD). Basic Clin Pharmacol Toxicol 130: 439–456. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, et al. (2007) Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety-and depression-like behaviors in adulthood: the importance of stressor type and gender. Behav Neurosci 121: 462–474. [DOI] [PubMed] [Google Scholar]
- Pompili A, Tomaz C, Arnone B, et al. (2010) Working and reference memory across the estrous cycle of rat: a long-term study in gonadally intact females. Behav Brain Res 213: 10–18. [DOI] [PubMed] [Google Scholar]
- Pretzsch CM, Freyberg J, Voinescu B, et al. (2019) Effects of cannabidiol on brain excitation and inhibition systems; a randomised placebo-controlled single dose trial during magnetic resonance spectroscopy in adults with and without autism spectrum disorder. Neuropsychopharmacology 44: 1398–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis FMCV, Novaes LS, Dos Santos NB, et al. (2022) Predator fear memory depends on glucocorticoid receptors and protein synthesis in the basolateral amygdala and ventral hippocampus. Psychoneuroendocrinology 41: 105757. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Johnson NJT. (1995) Factor analysis of spatiotemporal and ethological measures in the murine elevated plus-maze test of anxiety. Pharmacol Biochem Behav 52: 297–303. [DOI] [PubMed] [Google Scholar]
- Salviato BZ, Raymundi AM, da Silva TR, et al. (2021) Female but not male rats show biphasic effects of low doses of Δ9-tetrahydrocannabinol on anxiety: can cannabidiol interfere with these effects?. Neuropharmacology 196: 108684. [DOI] [PubMed] [Google Scholar]
- Seliger DL. (1977) Effects of age, sex, and brightness of field on open-field behaviors of rats. Percept Mot Skills 45: 1059–1067. [DOI] [PubMed] [Google Scholar]
- Scholl JL, Afzal A, Fox LC, et al. (2019) Sex differences in anxiety-like behaviors in rats. Physiol Behav 211: 112670. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, et al. (1998) Withdrawal from 3alpha-OH-5alpha-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the kinetics of hippocampal GABAA-gated current and increases GABAA receptor alpha 4 subunit in association with increased anxiety. J Neurosci 18: 5275–5284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Cunha C, Coimbra B, Borges S, et al. (2018) Mild prenatal stress causes emotional and brain structural modifications in rats of both sexes. Front Behav Neurosci 12: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares-Rachetti V, Pinto IAS, Santos RO, et al. (2016) Short term, low dose fluoxetine blocks estrous cycle-linked chances in responsiveness to diazepam in female rats. J Psychopharmacol 30: 1062–1068. [DOI] [PubMed] [Google Scholar]
- Vitale RM, Iannotti FA, Amodeo P. (2021) The (poly)pharmacology of cannabidiol in neurological and neuropsychiatric disorders: molecular mechanisms and targets. Int J Mol Sci 22: 4876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walf AA, Frye CA. (2007) The use of the elevated plus maze as an assay of anxiety-related behavior in rodents. Nat Protoc 2: 322–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright M, Di Ciano P, Brands B. (2020) Use of cannabidiol for the treatment of anxiety: a short synthesis of preclinical and clinical evidence. Cannabis Cannabinoid Res 5: 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Sun H, Wu Y, et al. (2019) Long-lasting sex-specific effects based on emotion-and cognition-related behavioral assessment of adult rats after post-traumatic stress disorder from different lengths of maternal separation. Front Psychiatry 10: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuardi AW, Rodrigues NP, Silva AL, et al. (2017) Inverted U-shaped dose-response curve of the anxiolytic effect of cannabidiol during public speaking in real life. Front Pharmacol 8: 259. [DOI] [PMC free article] [PubMed] [Google Scholar]