Abstract
Objectives
To quantify the prognostic effects of demographic and modifiable factors in streptococcal toxic shock syndrome (STSS).
Design
Systematic review and meta-analysis.
Data sources
MEDLINE, EMBASE and CINAHL from inception to 19 September 2022, along with citations of included studies.
Eligibility criteria
Pairs of reviewers independently screened potentially eligible studies of patients with Group A Streptococcus-induced STSS that quantified the association between at least one prognostic factor and outcome of interest.
Data extraction and synthesis
We performed random-effects meta-analysis after duplicate data extraction and risk of bias assessments. We rated the certainty of evidence using the Grading of Recommendations, Assessment, Development and Evaluation approach.
Results
One randomised trial and 40 observational studies were eligible (n=1918 patients). We found a statistically significant association between clindamycin treatment and mortality (n=144; OR 0.14, 95% CI 0.06 to 0.37), but the certainty of evidence was low. Within clindamycin-treated STSS patients, we found a statistically significant association between intravenous Ig treatment and mortality (n=188; OR 0.34, 95% CI 0.15 to 0.75), but the certainty of evidence was also low. The odds of mortality may increase in patients ≥65 years when compared with patients 18–64 years (n=396; OR 2.37, 95% CI 1.47 to 3.84), but the certainty of evidence was low. We are uncertain whether non-steroidal anti-inflammatory drugs increase the odds of mortality (n=50; OR 4.14, 95% CI 1.13 to 15.14; very low certainty). Results failed to show a significant association between any other prognostic factor and outcome combination (very low to low certainty evidence) and no studies quantified the association between a prognostic factor and morbidity post-infection in STSS survivors.
Conclusions
Treatment with clindamycin and within clindamycin-treated patients, IVIG, was each significantly associated with mortality, but the certainty of evidence was low. Future research should focus on morbidity post-infection in STSS survivors.
PROSPERO registration number
CRD42020166961.
Keywords: epidemiology, bacteriology, general medicine (see internal medicine)
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Strengths of this review include its systematic and explicit search of the literature, capture of a wide breadth of patient-important outcomes within and outside of critical care and the use of meta-analysis to increase statistical power in studying relationships between prognostic factors and outcomes in streptococcal toxic shock syndrome (STSS) patients.
These strengths directly address limitations of an existing narrative synthesis of STSS prognosis restricted to the critical care setting.
In the absence of large cohort studies and randomised trials, conclusions for STSS prognosis in this review are limited by very low to low certainty evidence.
A limitation of the evidence is the lack of long-term outcome data reported, including morbidity post-infection in STSS survivors.
Introduction
Streptococcal toxic shock syndrome (STSS) is an acute and severe life-threatening complication of predominantly invasive Group A Streptococcus (GAS) infections. STSS is relatively uncommon, but is fatal.1 Using US data from 2000 to 2004, the Centers for Diseases Control and Prevention estimated an annual incidence rate of 0.2 cases per 100 000 people and a fatality rate of 36%.2 STSS has important consequences for morbidity as well, in which patients may require radical surgical debridement, and patients with organ failure may have permanent respiratory and renal insufficiency.1
Although extensive multidisciplinary clinical management efforts by intensivists, infectious disease specialists and surgeons have curbed STSS all-cause mortality,1 data on the natural history of long-term sequelae in surviving patients, such as renal, respiratory and neuropsychiatric complications, are sparse.1–4 Published studies of prognostic and treatment factors for STSS have consistently focused on associations with all-cause mortality,5–14 with few reporting on outcomes capturing the morbidity post-infection in STSS survivors.7 12 Furthermore, a thorough and systematic review to corroborate this evidence is lacking. A narrative review of STSS was limited by the lack of a systematic or explicit search of the literature, it included studies that were only narratively synthesised, and the focus was limited to studies within a critical care setting.1
Understanding prognosis of STSS is important for patients, clinicians and healthcare decision makers. We conducted a systematic review to summarise the prognostic and treatment factors, and outcomes of STSS. We aimed to capture a wide breadth of patient-important outcomes with follow-up that included both short-term and long-term outcomes within and outside of critical care.
Materials and methods
We registered a protocol for the present systematic review and meta-analysis with PROSPERO (CRD42020166961).15 16 We report this systematic review and meta-analysis following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses and Meta-analyses of Observational Studies in Epidemiology checklists.17 18 Decisions regarding criteria for study inclusion, search methods for identification of studies, data collection, risk of bias, evaluation of the certainty of evidence and analysis were established a priori.
Search strategy and selection criteria
We searched MEDLINE (OVID interface, Epub Ahead of Print, In-Process & Other Non-Indexed Citations, 1946–19 September 2022), EMBASE (OVID interface, 1974–19 September 2022) and the Cumulative Index to Nursing And Allied Health Literature (CINAHL) from inception to 19 September 2022, with no restrictions on publication date. We applied search filters for randomised controlled trials and non-randomised studies (cohort, case–control and case series with at least two STSS patients),19 20 and tailored search strategies to each database. We restricted included studies to the English language to facilitate screening of full texts21 22 and searched citations of included studies to minimise the risk of failing to include relevant studies.
We included studies of randomised and non-randomised designs that reported the association of at least one prognostic factor of interest with at least one outcome of interest, and compared GAS-induced STSS patients with the prognostic factor of interest (ie, exposed) to GAS-induced STSS patients without the prognostic factor of interest (ie, unexposed). Studies of patients with microbiologically confirmed STSS, probable cases of STSS and patients with clinical evidence of STSS as defined by study authors and generally consistent with the below criteria were eligible.3 23 Clinical evidence of STSS included hypotension and at least two of the following: renal impairment, coagulopathy, liver function abnormality, acute respiratory distress syndrome, generalised erythematous macular rash (with desquamation), soft-tissue necrosis (including necrotising fasciitis, myositis or gangrene) or meningitis. Probable cases of STSS were defined as meeting clinical evidence with GAS isolated from a non-sterile site (eg, throat, sputum, superficial skin lesion) or antigen detected. Confirmed cases of STSS were defined as meeting clinical evidence with GAS isolated from a sterile site (eg, blood, cerebrospinal fluid, deep tissue specimen taken during surgery).3 23 Demographic, comorbidity, infection, modifiable and process variables were prognostic factors of interest. Informed by clinical expertise in the review team, we selected outcomes based on importance to patients. Further, we aimed to capture the long-term sequelae in patients surviving STSS.1 2 4 We chose the following outcomes of interest: (time to) mortality, hospital length of stay, intensive care unit (ICU) admission, ICU length of stay, mechanical ventilation, duration of mechanical ventilation, (time to) clinical cure/improvement or resolution of shock, change in Sequential Organ Failure Assessment (SOFA) score from baseline, functional status (eg, physical component summary score on the 36-item Short Form Health Survey) and health-related quality of life (HRQoL). We also extracted cost outcomes, which are relevant to hospital and patient payees.
We excluded case reports and conference abstracts, and studies in which the population was less than 80% GAS-induced STSS cases (ie, toxic shock syndrome of bacterial aetiologies other than GAS made up more than 20% of the study population). Because prognostic evidence in STSS patients is scarce,1 2 4 we did not apply any restrictions based on analytical method (eg, conducting an adjusted, multivariable analysis) or sample size.
Using a systematic review software, Rayyan,24 following training and calibration exercises, pairs of reviewers independently screened all titles and abstracts, followed by full-texts of records that were identified as potentially eligible. When necessary, consensus was reached through discussion between the review pair, and arbitration by a senior coinvestigator in the absence of consensus.
Data analysis
For each eligible study, pairs of reviewers extracted data independently using a standardised, pilot-tested data extraction form. Reviewers collected information on study characteristics (study design as defined by study authors, sample size, country), patient characteristics (age, sex), disease characteristics (confirmed vs probable STSS, presence of necrotising fasciitis), prognostic factors and outcomes of interest (means or medians and measures of variability for continuous outcomes and the proportion of participants who experienced an event for dichotomous outcomes). If multiple time points were reported for outcomes of interest, we extracted all time points. To minimise risk of confounding associated with prognostic effect estimates on dichotomous outcomes in non-randomised studies, we preferentially extracted adjusted ORs and corresponding 95% CIs over proportions when both were reported. We used the proportions to calculate crude ORs when no adjusted ORs were provided. Reviewers resolved discrepancies by discussion and, when necessary, with adjudication by a senior coinvestigator.
Following training and calibration exercises, reviewers, independently and in duplicate, used the Quality in Prognosis Studies (QUIPS) tool to rate each prognostic factor and outcome combination at low, moderate or high risk of bias. Based on prespecified sets of questions, we assessed risk of bias across the following domains: participation, attrition, prognostic factor measurement, outcome measurement, confounding and statistical analysis and reporting.25 For studies addressing more than one prognostic factor and outcome combination, we reported the highest risk of bias rating among the prognostic factor and outcome combinations within a study for each domain. In addition to assessing risk of bias at the domain level as outlined in the QUIPS tool, we applied the following rules to assess risk of bias overall at the study level. We rated overall study risk of bias as low if the study was prospective and five or more domains were assessed as low risk of bias, and high if two or more domains were assessed as high risk of bias. All other studies were rated as moderate risk of bias overall. Due to high risk of selection bias and residual confounding, we rated all case series as high risk of bias overall. Reviewer pairs resolved discrepancies by discussion and, when needed, with adjudication by a senior coinvestigator.
Pairs of reviewers used the Grading of Recommendations, Assessment, Development and Evaluation (GRADE) approach to independently assess the certainty of prognostic evidence for each meta-analysed outcome. Criteria for rating the certainty for each prognostic factor and outcome as high, moderate, low or very low, included considerations of risk of bias, inconsistency, indirectness, size and precision of the association and publication bias.26 27 Judgements of imprecision for this systematic review were made using a minimally contextualised approach. This approach considers whether CIs include the null effect. Further, the terminology used to report GRADE ratings (eg, low certainty evidence) is based on published GRADE guidance.28 29 The online supplemental file presents the detailed guidance we developed to facilitate the certainty of the evidence assessment in this review. To facilitate interpretation of the results in which the summary measure was an OR, we used the median event rate in the reference group of studies reporting proportions to calculate baseline risks and subsequently calculated absolute effects. GRADE evidence summaries (Summary of Findings tables) were generated in the MAGIC Authoring and Publication Platform (www.magicapp.org).
bmjopen-2022-063023supp001.pdf (14.8MB, pdf)
When at least two included studies reported on the same prognostic factor and outcome in patients with GAS-induced STSS, we conducted DerSimonian and Laird random-effects meta-analyses using the metafor package in R V.4.0.4 (R Studio, Boston, Massachusetts, USA).30 We summarised the effects of prognostic factors on dichotomous outcomes using ORs and corresponding 95% CIs, and on continuous outcomes using mean differences and corresponding 95% CIs. For prognostic factor and dichotomous outcome combinations in which every patient in the reference arm experienced the outcome, we summarised the effects by directly calculating risk differences and corresponding 95% CIs. We set the criterion for statistical significance at alpha=0.05. Visual inspection of forest plots and the chi-square test were performed to evaluate heterogeneity. We interpreted an I2 statistic value of 0%–40%, 30%–60%, 50%–90% or 75%–100% as not likely important, moderate, substantial or considerable heterogeneity, respectively.31 If an I2 statistic value was within a range of overlapping values (eg, 80%), we would interpret heterogeneity as more important (eg, considerable instead of substantial) if the meta-analysis contained few studies, we observed inconsistent magnitudes and directions of summary estimates on visual inspection of the forest plots, or the χ2 test was significant.31 For meta-analyses of continuous outcomes, we imputed means and SD for studies reporting medians and IQR, respectively.32 33
Patient-level data from case series were aggregated when possible to enable comparative analysis via meta-analysis. We planned to perform a regression analysis for each study for which age was reported at the patient level to generate a study and age category (0–17 years old vs 18–64 years old vs 65 years old or older) specific OR that could be used in meta-analysis when a study had at least 10 observations for continuous outcomes and 10 events for dichotomous outcomes; however, no study met the sample size or event number requirements. Further, scarcity and variability of data precluded our plan to narratively synthesise the evidence from included studies for which meta-analysis of a prognostic factor and outcome combination was not possible.
The analysis plan included performing subgroup analyses of STSS patients treated with clindamycin versus no clindamycin, STSS patients with necrotising fasciitis versus without necrotising fasciitis, age (0–17 years old vs 18–64 years old vs 65 years old or older), sex (male vs female) and risk of bias (high vs moderate vs low) when at least two studies were present for each subgroup. Because select meta-analyses were limited by small numbers of events, we performed a post hoc sensitivity analysis using the Peto method for meta-analysis, which is recommended for meta-analysis of rare events,34 and compared the results to those from the DerSimonian and Laird method we applied in this review.
Patient and public involvment
It was not appropriate or possible to involve patients or the public in the design, or conduct, or reporting, or dissemination plans of our research.
Results
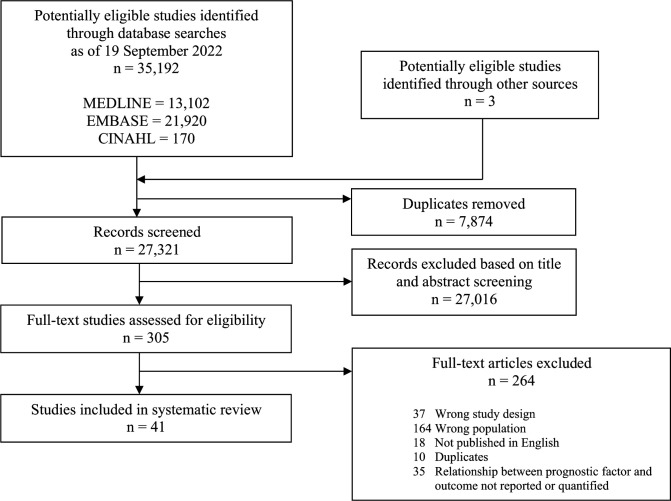
After screening 27 321 titles and abstracts, and 305 full texts, 41 studies that reported on the association between at least one prognostic factor and outcome of interest in STSS patients proved eligible (figure 1). All but one study (40/41, 98%) were non-randomised. Eligible studies were published between 1989 and 2021, ranged in sample size from 2 to 476, included 1918 STSS patients in total and were conducted in 22 different countries, most commonly in the USA (15/41, 37%).
Figure 1.
PRISMA study flow diagram. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Table 1 describes the characteristics of included studies reporting on the association of at least one prognostic factor and outcome of interest. The online supplemental data includes additional study characteristics for each study. Of the 41 included studies, 29 (71%) reported on demographic prognostic factors of interest, 5 (12%) medical history of being immunocompromised, 11 (27%) early disease characteristics and 16 (39%) treatment. Of the dichotomous outcomes, mortality was most commonly reported (36/41, 88%), followed by ICU admission (10/41, 24%), clinical cure or improvement (8/41, 20%) and need for mechanical ventilation (6/41, 15%). Few studies reported on hospital (3/41, 7%) and ICU length of stay (2/41, 5%). Two studies reported on time to mortality in days7 35; however, only one reported sufficient data precluding meta-analysis.7 One study each reported on cost,14 change in SOFA score,7 time to clinical cure/improvement or resolution of shock7 and duration of mechanical ventilation10 precluding meta-analysis for these continuous outcomes. No studies quantified the association between a prognostic factor and functional status or HRQoL outcomes. A multivariable analysis was conducted in two (5%) of the included studies.10 11 A total of 19 of the 41 studies were cohort studies (authors reported on at least one comparative analysis), 19 were case series (authors did not report a comparative analysis) and 2 were case–control studies. To meta-analyse the data, we aggregated the data from the individual patients the case series reported on. Further, we pooled the one randomised study7 with non-randomised studies in meta-analyses and included patients receiving intravenous or intramuscular IVIG from one non-randomised study.36
Table 1.
Study characteristics.
| Characteristics | (41 studies, 1918 patients) |
| Range of publication year | 1989–2021 |
| Median (IQR) no of patients | 11 (5–29) |
| Geographical region | |
| North America | 19 (46) |
| Europe | 14 (34) |
| Central/South America | 0 (0) |
| Asia | 4 (10) |
| Other | 4 (10) |
| Study design | |
| Randomised trial | 1 (2) |
| Cohort | 19 (46) |
| Case-control | 2 (5) |
| Case-series | 19 (46) |
| Case definition | |
| Probable STSS patients | 115 (6) |
| Confirmed STSS patients | 227 (12) |
| Prognostic factor type | |
| Demographic | 29 (71) |
| Medical history | 5 (12) |
| Early disease | 11 (27) |
| Treatment | 16 (39) |
Values are numbers (percentages) of studies unless stated otherwise. Medical history included prognostic variable: immunocompromised. Early disease included prognostic variables: necrotising fasciitis, acute renal failure.
STSS, streptococcal toxic shock syndrome.
The online supplemental file 1 includes the forest plots depicting the studies included in the meta-analysis of each prognostic factor-outcome combination. It also includes the list of studies reporting on prognostic factor-outcome combinations of interest that were not eligible for any meta-analysis, along with the reasons for exclusion from meta-analysis.
Risk of bias in included studies
Online supplemental file presents the risk of bias assessment of the 41 included studies. The majority of studies were rated as high risk of bias overall owing to residual confounding and lack of adjustment for confounding in statistical analyses (37/41, 90%).2 5 6 10 35–67 Three studies were rated at moderate risk of bias overall7 14 68 and one at low risk of bias overall.11
Prognostic factors for mortality
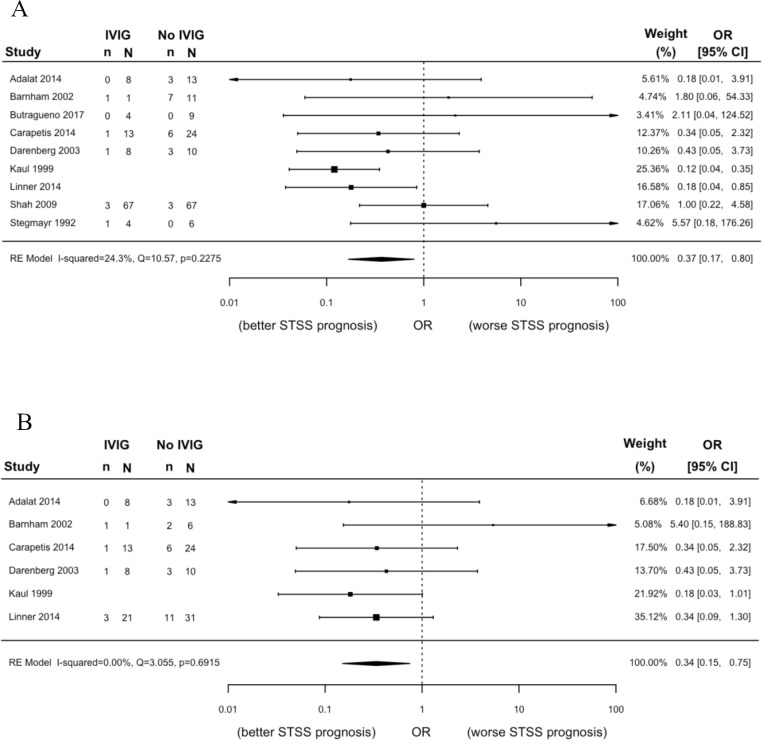
Eleven prognostic factors from 32 studies including 1343 patients were eligible for analysis (table 2, online supplemental data). We found a statistically significant association between clindamycin treatment and mortality (n=144; OR 0.14, 95% CI 0.06 to 0.37), but the certainty of evidence was low. Within clindamycin-treated STSS patients, we found a statistically significant association between intravenous Ig treatment and mortality (figure 2; n=188; OR 0.34, 95% CI 0.15 to 0.75), but the certainty of evidence was also low. We are uncertain whether IVIG treatment reduces the odds of mortality in all STSS patients regardless of concurrent clindamycin treatment (figure 2; n=365, OR 0.37, 95% CI 0.17 to 0.80; very low certainty of evidence). The odds of mortality may increase in patients ≥65 years when compared with patients 18–64 years (n=396; OR 2.37, 95% CI 1.47 to 3.84), but the certainty of evidence was low. We are less certain whether the same is true for patients ≥65 years compared with patients <18 years (n=136, OR 10.66, 95% CI 1.28 to 88.57; very low certainty of evidence). We are also uncertain whether non-steroidal anti-inflammatory drugs (NSAIDs) increase the odds of mortality (n=50, OR 4.14, 95% CI 1.13 to 15.14; very low certainty of evidence). Very low certainty evidence failed to show a significant association with any other prognostic factor and mortality in STSS patients: male versus female (n=80, OR 0.95, 95% CI 0.36 to 2.52), patients <18 years vs patients 18 to 64 years (n=694, OR 0.54, 95% CI 0.15 to 1.94), immunocompromised versus not immunocompromised (n=33, OR 1.65, 95% CI 0.33 to 8.26), necrotiszing fasciitis versus no necrotising fasciitis (n=840, OR 0.81, 95% CI 0.51 to 1.29), acute renal failure versus no acute renal failure (n=91, OR 2.50, 95% CI 0.97 to 6.42), haemodialysis versus no haemodialysis (n=42, OR 1.94, 95% CI 0.22 to 16.99) and any antibiotic versus no antibiotic (n=19, OR 0.48, 95% CI 0.05 to 4.76).
Table 2.
Summary of findings for prognostic factor—outcome meta-analyses
| Prognostic factor | No of patients (studies) | OR (95% CI) | Absolute effect estimates | GRADE: certainty of the evidence | |
| Risk without prognostic factor | Risk with prognostic factor | ||||
| Mortality | |||||
| Demographic | |||||
| Male versus female | 80 (13) | 0.95 (0.36 to 2.52) | 250 per 1000 | 241 per 1000 | Very low |
| −9 (−143 to 207) | Due to very serious risk of bias and imprecision | ||||
| <18 vs 18–64 years | 694 (5) | 0.54 (0.15 to 1.94) | 234 per 1000 | 142 per 1000 | Very low |
| −92 (−190 to 138) | Due to very serious risk of bias and imprecision, and serious inconsistency | ||||
| ≥65 vs <18 years | 136 (2) | 10.66 (1.28 to 88.57)* | 50 per 1000 | 359 per 1000 | Very low |
| 309 (13 to 773) | Due to very serious risk of bias and serious imprecision | ||||
| ≥65 vs 18−64 years | 396 (2) | 2.37 (1.47 to 3.84)* | 193 per 1000 | 362 per 1000 | Low |
| 169 (67 to 286) | Due to very serious risk of bias | ||||
| Medical history | |||||
| Immunocompromised versus not Immunocompromised | 33 (4) | 1.65 (0.33 to 8.26) | 438 per 1000 | 563 per 1000 | Very low |
| 125 (−233 to 428) | Due to very serious risk of bias and imprecision | ||||
| Early disease | |||||
| Acute renal failure versus no acute renal failure | 91 (4) | 2.50 (0.97 to 6.42) | NA per 1000 | NA per 1000 | Very low |
| 140 (−60 to 330) | Due to very serious risk of bias and imprecision | ||||
| Necrotising fasciitis versus no necrotising fasciitis | 840 (10) | 0.81 (0.51 to 1.29) | 347 per 1000 | 301 per 1000 | Very low |
| −46 (−134 to 60) | Due to very serious risk of bias and imprecision | ||||
| Treatment | |||||
| IVIG versus no IVIG (all STSS patients) | 365 (9) | 0.37 (0.17 to 0.80)* | 231 per 1000 | 100 per 1000 | Very low |
| −131 (−182 to −37) | Due to very serious risk of bias and serious imprecision | ||||
| IVIG versus no IVIG (subset of STSS patients treated with clindamycin) | 188 (6) | 0.34 (0.15 to 0.75)* | 300 per 1000 | 127 per 1000 | Low |
| −173 (−240 to −57) | Due to serious risk of bias and imprecision | ||||
| Any antibiotic versus no antibiotic | 19 (3) | 0.48 (0.05 to 4.76) | NA per 1000 | NA per 1000 | Very low |
| −120 (−490 to 260) | Due to very serious risk of bias and imprecision | ||||
| Clindamycin versus no clindamycin antibiotic | 144 (4) | 0.14 (0.06 to 0.37)* | 800 per 1000 | 359 per 1000 | Low |
| −441 (−606 to −203) | Due to serious risk of bias and imprecision | ||||
| Haemodialysis versus no haemodialysis | 42 (4) | 1.94 (0.22 to 16.99) | 107 per 1000 | 189 per 1000 | Very low |
| 82 (−81 to 564) | Due to very serious risk of bias and imprecision | ||||
| NSAIDs vs no NSAIDs | 50 (4) | 4.14 (1.13 to 15.14)* | 100 per 1000 | 315 per 1000 | Very low |
| 215 (12 to 527) | Due to very serious risk of bias and serious imprecision | ||||
| ICU admission | |||||
| Demographic | |||||
| Male vs female | 19 (3) | 2.87 (0.29 to 28.27) | NA per 1000 | NA per 1000 | Very low |
| 150 (−160 to 450) | Due to very serious risk of bias and imprecision | ||||
| Early disease | |||||
| Necrotising fasciitis vs no necrotising fasciitis | 28 (3) | 0.74 (0.12 to 4.48) | 900 per 1000 | 869 per 1000 | Very low |
| −31 (−381 to 76) | Due to very serious risk of bias and imprecision | ||||
| Treatment | |||||
| IVIG versus no IVIG (all STSS patients) | 156 (3) | 1.09 (0.43 to 2.77) | 833 per 1000 | 845 per 1000 | Very low |
| 12 (−151 to 100) | Due to very serious risk of bias and imprecision | ||||
| Any antibiotic versus no antibiotic | 14 (2) | 4.60 (0.29 to 72.89) | 500 per 1000 | 821 per 1000 | Very low |
| 321 (−275 to 486) | Due to very serious risk of bias and imprecision | ||||
| Haemodialysis versus no haemodialysis | 13 (2) | 3.25 (0.21 to 50.35) | 875 per 1000 | 958 per 1000 | Very low |
| 83 (−280 to 122) | Due to very serious risk of bias and imprecision | ||||
| NSAIDs versus no NSAIDs | 15 (2) | 0.86 (0.06 to 12.48) | NA per 1000 | NA per 1000 | Very low |
| −10 (−430 to 400) | Due to very serious risk of bias and imprecision | ||||
| Clinical cure or improvement | |||||
| Demographic | |||||
| Male versus female | 23 (4) | 3.33 (0.47 to 23.59) | 875 per 1000 | 959 per 1000 | Very low |
| 84 (−108 to 119) | Due to very serious risk of bias and imprecision | ||||
| Early disease | |||||
| Necrotising fasciitis versus no necrotising fasciitis | 24 (2) | 0.34 (0.02 to 5.20) | 950 per 1000 | 866 per 1000 | Very low |
| −84 (−675 to 40) | Due to very serious risk of bias and serious imprecision | ||||
| Treatment | |||||
| IVIG versus no IVIG (in all STSS patients) | 23 (2) | 0.27 (0.02 to 3.76) | NA per 1000 | NA per 1000 | Very low |
| −100 (−350 to 140) | Due to very serious risk of bias and imprecision | ||||
| Haemodialysis versus no haemodialysis | 26 (3) | 1.43 (0.15 to 14.08) | NA per 1000 | NA per 1000 | Very low |
| 50 (−240 to 340) | Due to very serious risk of bias and imprecision | ||||
| Need for mechanical ventilation | |||||
| Demographic | |||||
| Male versus female | 21 (3) | 2.09 (0.32 to 13.74) | NA per 1000 | NA per 1000 | Very low |
| 120 (−200 to 440) | Due to very serious risk of bias and imprecision | ||||
| Early disease | |||||
| Acute renal failure versus no acute renal failure | 20 (2) | 1.14 (0.17 to 7.82) | 750 per 1000 | 774 per 1000 | Very low |
| 24 (−412 to 209) | Due to very serious risk of bias and imprecision | ||||
| Necrotising fasciitis versus no necrotising fasciitis | 31 (3) | 3.75 (0.47 to 29.81) | 700 per 1000 | 897 per 1000 | Very low |
| 197 (−177 to 286) | Due to very serious risk of bias and imprecision | ||||
| Treatment | |||||
| IVIG versus no IVIG (in all STSS patients) | 157 (3) | 2.22 (0.78 to 6.32) | 333 per 1000 | 526 per 1000 | Very low |
| 193 (−53 to 426) | Due to very serious risk of bias and imprecision | ||||
| Haemodialysis vs no haemodialysis | 26 (3) | 2.05 (0.39 to 10.70) | 500 per 1000 | 672 per 1000 | Very low |
| 172 (−219 to 415) | Due to very serious risk of bias and imprecision | ||||
| Duration of hospitalisation | |||||
| Treatment | |||||
| IVIG versus no IVIG (all STSS patients) | 201 (3) | NA | NA per 1000 | NA per 1000 | Low |
| On average, 5.51 fewer days (17.64 fewer to 6.62 more) | Due to serious risk of bias and imprecision | ||||
| Duration of ICU stay | |||||
| Treatment | |||||
| IVIG versus no IVIG (all STSS patients) | 131 (2) | NA | NA per 1000 | NA per 1000 | Very low |
| On average, 3.80 more days (3.62 fewer to 11.23 more) | Due to very serious risk of bias and serious imprecision | ||||
*Statistical evidence of an association.
GRADE, Grading of Recommendations, Assessment, Development and Evaluation; ICU, intensive care unit; NA, not available; NSAIDs, non-steroidal anti-inflammatory drugs; STSS, streptococcal toxic shock syndrome.
Figure 2.
Meta-analyses comparing IVIG treatment to no IVIG treatment for the outcome mortality in (A) all STSS patients; and (B) the subset of STSS patients treated with clindamycin. Please note proportions are blank for study rows where we meta-analysed adjusted ORs instead of crude proportions. IVIG, intravenous Ig; STSS, streptococcal toxic shock syndrome.
Prognostic factors for ICU admission
Six prognostic factors from eight studies including 174 patients were eligible for analysis (table 2, online supplemental data). We found no statistical evidence of an association between any prognostic factor and ICU admission: male versus female sex (n=19, OR 2.87, 95% CI 0.29 to 28.27), necrotising fasciitis versus no necrotising fasciitis (n=28, OR 0.74, 95% CI 0.12 to 4.48), haemodialysis versus no haemodialysis (n=13, OR 3.25, 95% CI 0.21 to 50.35), NSAIDs versus no NSAIDs (n=15, OR 0.86, 95% CI 0.06 to 12.48), IVIG treatment versus no IVIG treatment (n=156, OR 1.09, 95% CI 0.43 to 2.77) and antibiotic treatment versus no antibiotic treatment (n=14, OR 4.60, 95% CI 0.29 to 72.98). The certainty of all ICU admission evidence was very low due to very serious risk of bias and imprecision.
Prognostic factors for clinical cure or improvement
Four prognostic factors from six studies including 38 STSS patients were eligible for analysis (table 2, online supplemental data). We found no statistical evidence of an association between any prognostic factor and clinical cure or improvement: male versus female sex (n=23, OR 3.33, 95% CI 0.47 to 23.59), necrotising fasciitis versus no necrotising fasciitis (n=24, OR 0.34, 95% CI 0.02 to 5.20), haemodialysis versus no haemodialysis (n=26, OR 1.43, 95% CI 0.15 to 14.08) and IVIG treatment versus no IVIG treatment (n=23, OR 0.27, 95% CI 0.02 to 3.76). The certainty of all clinical cure or improvement evidence was very low due to very serious risk of bias and serious or very serious imprecision.
Prognostic factors for mechanical ventilation
Five prognostic factors from six studies including 170 STSS patients were eligible for analysis (table 2, online supplemental data). We found no statistical evidence of an association between any prognostic factor and need for mechanical ventilation: male versus female sex (n=21, OR 2.09, 95% CI 0.32 to 13.74), necrotising fasciitis versus no necrotising fasciitis (n=31, OR 3.75, 95% CI 0.47 to 29.81), acute renal failure versus no acute renal failure (n=20, OR 1.14, 95% CI 0.17 to 7.82), haemodialysis versus no haemodialysis (n=26, OR 2.05, 95% CI 0.39 to 10.70) and IVIG treatment versus no IVIG treatment (n=157, OR 2.22, 95% CI 0.78 to 6.32). The certainty of all need for mechanical ventilation evidence was very low due to very serious risk of bias and imprecision.
Prognostics factors for hospital length of stay
One prognostic factor from three studies including 201 STSS patients was eligible for analysis (table 2, online supplemental data). Low certainty evidence—due to serious risk of bias and imprecision—provides no support for an association between IVIG treatment and hospital length of stay, when compared with no IVIG treatment (n=201, MD −5.51 days, 95% CI −17.64 to 6.62).
Prognostic factors for ICU length of stay
One prognostic factor from two studies including 131 STSS patients was eligible for analysis (table 2, online supplemental data). We are uncertain if IVIG treatment compared with no IVIG treatment is associated with ICU length of stay (n=131, MD 3.80 days, 95% CI −3.62 to 11.23; very low certainty evidence due to very serious risk of bias and serious imprecision).
Subgroup and sensitivity analysis
Sparsity of data precluded our planned subgroup analyses by clindamycin treatment, presence of necrotising fasciitis and sex (ie, each subgroup did not have at least two studies). We collapsed the low and moderate risk of bias categories to allow for subgroup analysis by risk of bias (low or moderate vs high). The prognostic factor-outcome combinations for which there was sufficient evidence for subgroup analysis by age or modified risk of bias level were IVIG-mortality and sex-mortality. We found no statistical evidence that the association between IVIG and mortality differed between low or moderate and high risk of bias studies in all STSS patients (p=0.884) and clindamycin-treated STSS patients (p=0.867) or between studies with STSS patients <18 years and patients 18–64 years (p=0.328). We also found no statistical evidence that the association between sex and mortality differed between studies with patients <18 years and patients 18–64 years (p=0.666). Because results were consistent across Peto, and DerSimonian and Laird methods, our post hoc sensitivity analysis applying the Peto method supported our main results.
Discussion
This systematic review and meta-analysis provides a comprehensive overview of the prognostic evidence for STSS. Prognostic factors for which there was a statistically significant association with mortality in STSS patients were age, clindamycin treatment, IVIG treatment and NSAIDs treatment. Patients ≥65 years compared with patients 18–64 years may have increased odds of mortality (low certainty of evidence); however, we are uncertain if the same is true for patients ≥65 years compared with patients <18 years (very low certainty of evidence). We are also uncertain whether NSAIDs increase the odds of mortality (very low certainty of evidence). Low certainty evidence suggests the odds of mortality may be reduced by treatment with clindamycin and within clindamycin-treated patients, IVIG. We are highly uncertain whether IVIG reduces mortality in all STSS patients, regardless of clindamycin treatment (very low certainty of evidence). Results failed to show a significant association between all other meta-analysed prognostic factors and outcomes (table 2). The certainty of STSS prognostic evidence was low or very low due to serious or very serious risk of bias and imprecision concerns.
Strengths of this review include its systematic and explicit search of the literature, capture of a wide breadth of patient-important outcomes within and outside of critical care and the use of meta-analysis to increase statistical power in studying relationships between prognostic factors and outcomes in STSS patients. These strengths directly address limitations of a narrative synthesis of STSS prognosis restricted to the critical care setting.1
In the absence of large cohort studies and randomised trials, conclusions for STSS prognosis in this review are limited by very low to low certainty evidence. The majority of included studies were non-randomised (40/41, 98%) and small (median sample size was 11 patients), introducing bias from residual confounding and imprecision around pooled summary estimates. Small numbers of events further contributed to the imprecision around summary estimates and limited the interpretation of our findings. With few participants and events, minor changes in the data can cause major changes in the results. In such instances, results can be exaggerated by the presentation of relative effect estimates only. To minimise the risk of misinterpreting results from the inclusion of small studies in our meta-analyses, we calculated an absolute effect estimate for each relative effect estimate (table 2). Further, despite expecting small studies to be more heterogeneous than large studies, we did not find statistical evidence of heterogeneity in any of our 33 meta-analyses and in interpreting the I2 statistic value, we found not likely important heterogeneity in all but one meta-analysis.69 Creation of an international registry of STSS patients may improve the credibility of prognostic evidence for STSS and facilitate the conduct of high-quality cohort studies. Although we meta-analysed adjusted ORs from included studies when possible, almost all included studies reported crude data (39/41, 95%), precluding adjustment for important confounders. A limitation of the evidence is the lack of long-term outcome data reported. For example, no studies quantified associations between prognostic factors and functional status or HRQoL outcomes post-infection in STSS survivors. Given the high morbidity associated with STSS,70 future research in STSS prognosis should quantify these patient-important outcomes, facilitating future meta-analyses and providing further insights into STSS prognosis.
Our finding that IVIG treatment may reduce the odds of mortality in STSS patients who receive clindamycin treatment is consistent with a systematic review and meta-analysis of IVIG treatment in clindamycin-treated STSS patients, which found statistical evidence of a decreased risk of mortality in IVIG-treated and clindamycin-treated STSS patients when compared with only clindamycin-treated STSS patients.70 For this question relevant to clindamycin-treated STSS patients, our meta-analysis included one additional non-randomised study, whose small sample size and imprecision contributed to an overall point estimate of greater magnitude.35 Our findings suggest that treatment regimens of IVIG in adjunct to clindamycin and clindamycin alone may significantly improve STSS prognosis. We found a significant association between a regimen of IVIG regardless of clindamycin treatment and mortality; however, due to very serious risk of bias and serious imprecision, and thus very low certainty evidence, we cannot rule out the possibility that clindamycin treatment may be necessary for STSS patients to benefit from IVIG treatment. Further, only one study reported on IVIG treatment in STSS patients that were not also treated with clindamycin36; therefore, our planned subgroup analysis to test if the beneficial effect of IVIG is modified in the absence of clindamycin was precluded. Based on very low certainty evidence, our finding that NSAID treatment is significantly associated with mortality in STSS patients can be explained by clinical and basic science literature, which suggests non-selective NSAIDs mask early signs and symptoms of GAS infection, such as fever, subsequently delaying time to antibiotic treatment—a risk factor for severe sepsis and shock, and mortality.71 72
After analysing 30 different prognostic factor and outcome combinations, we found that clindamycin treatment was significantly associated with an improved STSS prognosis. Further, we found that IVIG treatment may reduce the odds of mortality in STSS patients who receive clindamycin treatment, but we are uncertain if this is true for all STSS patients, regardless of clindamycin treatment. Although these findings support the use of IVIG as an adjunctive treatment in clindamycin-treated STSS patients, the certainty of evidence was low due to serious risk of bias and imprecision. Age equal to or older than 65 years and treatment with NSAIDs were significantly associated with a worse STSS prognosis. Results from very low to low certainty evidence failed to show a significant association between any other factors of interest and STSS prognosis.
Supplementary Material
Footnotes
Contributors: All authors attest they meet the ICMJE criteria for authorship. All authors have made substantial contributions to the following: (1) the conception and design of the study (JJB, LT, DM, ML), acquisition of data (JJB, ZE, PR, CKLL), analysis and interpretation of data (JJB, ZE, PR, CKLL, ML); (2) drafting the article and revising it critically for important intellectual content (JJB, LT, DM, ML), (3) final approval of the version to be submitted (JJB, ZE, PR, CKLL, LT, DM, ML). The manuscript’s guarantor (ML) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned have been explained.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: ML declares grants or contracts from the WHO, consulting fees from AVIR Pharma, and participating on data safety monitoring or advisory boards for Paladin Labs and Sunovion Pharmaceuticals.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Data extracted from individual studies are available upon reasonable request to the corresponding author. All other data relevant to the study are included in the article or uploaded as online supplemental information.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
.
References
- 1. Schmitz M, Roux X, Huttner B, et al. Streptococcal toxic shock syndrome in the intensive care unit. Ann Intensive Care 2018;8:88. 10.1186/s13613-018-0438-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. O'Loughlin RE, Roberson A, Cieslak PR, et al. The epidemiology of invasive group A streptococcal infection and potential vaccine implications: United States, 2000-2004. Clin Infect Dis 2007;45:853–62. 10.1086/521264 [DOI] [PubMed] [Google Scholar]
- 3. Canada Go. Group A streptococcal diseases: for health professionals, 2019. Available: https://www.canada.ca/en/public-health/services/diseases/group-a-streptococcal-diseases/health-professionals.html
- 4. Davies HD, McGeer A, Schwartz B, et al. Invasive group A streptococcal infections in Ontario, Canada. N Engl J Med Overseas Ed 1996;335:547–54. 10.1056/NEJM199608223350803 [DOI] [PubMed] [Google Scholar]
- 5. Adalat S, Dawson T, Hackett SJ, et al. Toxic shock syndrome surveillance in UK children. Arch Dis Child 2014;99:1078–82. 10.1136/archdischild-2013-304741 [DOI] [PubMed] [Google Scholar]
- 6. Carapetis JR, Jacoby P, Carville K, et al. Effectiveness of clindamycin and intravenous immunoglobulin, and risk of disease in contacts, in invasive group A streptococcal infections. Clin Infect Dis 2014;59:358–65. 10.1093/cid/ciu304 [DOI] [PubMed] [Google Scholar]
- 7. Darenberg J, Ihendyane N, Sjölin J, et al. Intravenous immunoglobulin G therapy in streptococcal toxic shock syndrome: a European randomized, double-blind, placebo-controlled trial. Clin Infect Dis 2003;37:333–40. 10.1086/376630 [DOI] [PubMed] [Google Scholar]
- 8. Kadri SS, Swihart BJ, Bonne SL, et al. Impact of intravenous immunoglobulin on survival in necrotizing fasciitis with Vasopressor-Dependent shock: a propensity score-matched analysis from 130 US hospitals. Clin Infect Dis 2017;64:877–85. 10.1093/cid/ciw871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kaul R, McGeer A, Low DE, et al. Population-based surveillance for group A streptococcal necrotizing fasciitis: clinical features, prognostic indicators, and microbiologic analysis of seventy-seven cases. Ontario group A streptococcal study. Am J Med 1997;103:18–24. 10.1016/S0002-9343(97)00160-5 [DOI] [PubMed] [Google Scholar]
- 10. Kaul R, McGeer A, Norrby-Teglund A, et al. Intravenous immunoglobulin therapy for streptococcal toxic shock syndrome--a comparative observational study. The Canadian Streptococcal Study Group. Clin Infect Dis 1999;28:800–7. 10.1086/515199 [DOI] [PubMed] [Google Scholar]
- 11. Linnér A, Darenberg J, Sjölin J, et al. Clinical efficacy of polyspecific intravenous immunoglobulin therapy in patients with streptococcal toxic shock syndrome: a comparative observational study. Clin Infect Dis 2014;59:851–7. 10.1093/cid/ciu449 [DOI] [PubMed] [Google Scholar]
- 12. Madsen MB, Hjortrup PB, Hansen MB, et al. Immunoglobulin G for patients with necrotising soft tissue infection (instinct): a randomised, blinded, placebo-controlled trial. Intensive Care Med 2017;43:1585–93. 10.1007/s00134-017-4786-0 [DOI] [PubMed] [Google Scholar]
- 13. Mehta S, McGeer A, Low DE, et al. Morbidity and mortality of patients with invasive group A streptococcal infections admitted to the ICU. Chest 2006;130:1679–86. 10.1016/S0012-3692(15)50887-8 [DOI] [PubMed] [Google Scholar]
- 14. Shah SS, Hall M, Srivastava R, et al. Intravenous immunoglobulin in children with streptococcal toxic shock syndrome. Clin Infect Dis 2009;49:1369–76. 10.1086/606048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. York., C.f.R.a.D.U.o . PROSPERO International prospective register of systematic reviews. Available: http://www.crd.york.ac.uk/PROSPERO/2017
- 16.et alJessica Bartoszko ZE, Mertz D, Thabane L. Natural history and prognosis of streptococcal toxic shock syndrome: systematic review and meta-analysis, 2020. Available: https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42020166961 [DOI] [PMC free article] [PubMed]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. 10.1136/bmj.b2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 2000;283:2008–12. 10.1001/jama.283.15.2008 [DOI] [PubMed] [Google Scholar]
- 19. Practice BB . Study design search filters. Available: https://bestpractice.bmj.com/info/toolkit/learn-ebm/study-design-search-filters/
- 20. Sterne JAC, Sutton AJ, Ioannidis JPA, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011;343:d4002. 10.1136/bmj.d4002 [DOI] [PubMed] [Google Scholar]
- 21. Morrison A, Polisena J, Husereau D, et al. The effect of English-language restriction on systematic review-based meta-analyses: a systematic review of empirical studies. Int J Technol Assess Health Care 2012;28:138–44. 10.1017/S0266462312000086 [DOI] [PubMed] [Google Scholar]
- 22. Moher D, Pham B, Klassen TP, et al. What contributions do languages other than English make on the results of meta-analyses? J Clin Epidemiol 2000;53:964–72. 10.1016/S0895-4356(00)00188-8 [DOI] [PubMed] [Google Scholar]
- 23. Canada Go. National case definition: invasive group A streptococcal disease, 2008. [Google Scholar]
- 24. Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan-a web and mobile APP for systematic reviews. Syst Rev 2016;5:210. 10.1186/s13643-016-0384-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hayden JA, van der Windt DA, Cartwright JL, et al. Assessing bias in studies of prognostic factors. Ann Intern Med 2013;158:280–6. 10.7326/0003-4819-158-4-201302190-00009 [DOI] [PubMed] [Google Scholar]
- 26. Guyatt G, Oxman AD, Akl EA, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol 2011;64:383–94. 10.1016/j.jclinepi.2010.04.026 [DOI] [PubMed] [Google Scholar]
- 27. Huguet A, Hayden JA, Stinson J, et al. Judging the quality of evidence in reviews of prognostic factor research: adapting the grade framework. Syst Rev 2013;2:71. 10.1186/2046-4053-2-71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Cochrane style manual: common terms and terminology. Available: https://community.cochrane.org/style-manual/names-common-terms-and-terminology/common-terms-and-terminology
- 29. Guyatt RSaG. What is grade? BMJ Best Practice. [Google Scholar]
- 30. Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software 2010;1:2010. [Google Scholar]
- 31. Higgins JP GS. Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. handbook.cochrane.org [Google Scholar]
- 32. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005;5:13. 10.1186/1471-2288-5-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014;14:135. 10.1186/1471-2288-14-135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Efthimiou O. Practical guide to the meta-analysis of rare events. Evid Based Ment Health 2018;21:72–6. 10.1136/eb-2018-102911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Barnham MRD, Weightman NC, Anderson AW, et al. Streptococcal toxic shock syndrome: a description of 14 cases from North Yorkshire, UK. Clin Microbiol Infect 2002;8:174–81. 10.1046/j.1469-0691.2002.00396.x [DOI] [PubMed] [Google Scholar]
- 36. Stegmayr B, Björck S, Holm S, et al. Septic shock induced by group A streptococcal infection: clinical and therapeutic aspects. Scand J Infect Dis 1992;24:589–97. 10.3109/00365549209054644 [DOI] [PubMed] [Google Scholar]
- 37. Abuhammour W, Hasan RA, Unuvar E. Group A beta-hemolytic streptococcal bacteremia. Indian J Pediatr 2004;71:915–9. 10.1007/BF02830836 [DOI] [PubMed] [Google Scholar]
- 38. Al-ajmi JA, Hill P, O' Boyle C, et al. Group A Streptococcus toxic shock syndrome: an outbreak report and review of the literature. J Infect Public Health 2012;5:388–93. 10.1016/j.jiph.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 39. Bernaldo de Quirós JC, Moreno S, Cercenado E, et al. Group A streptococcal bacteremia. A 10-year prospective study. Medicine 1997;76:238–48. 10.1097/00005792-199707000-00002 [DOI] [PubMed] [Google Scholar]
- 40. Brogan TV, Nizet V, Waldhausen JH, et al. Group A streptococcal necrotizing fasciitis complicating primary varicella: a series of fourteen patients. Pediatr Infect Dis J 1995;14:588–94. 10.1097/00006454-199507000-00007 [DOI] [PubMed] [Google Scholar]
- 41. Butragueño Laiseca L, García Morín M, Barredo Valderrama E, et al. Toxic shock syndrome in a paediatric intensive care unit over the last 15 years. Anales de Pediatría 2017;87:111–3. 10.1016/j.anpede.2016.10.013 [DOI] [PubMed] [Google Scholar]
- 42. Carapetis J, Robins-Browne R, Martin D, et al. Increasing severity of invasive group A streptococcal disease in Australia: clinical and molecular epidemiological features and identification of a new virulent M-nontypeable clone. Clin Infect Dis 1995;21:1220–7. 10.1093/clinids/21.5.1220 [DOI] [PubMed] [Google Scholar]
- 43. Cimolai N, Trombley C, Adderley RJ, et al. Invasive Streptococcus pyogenes infections in children. Can J Public Health 1992;83:230–3. [PubMed] [Google Scholar]
- 44. Cook A, Janse S, Watson JR, et al. Manifestations of Toxic Shock Syndrome in Children, Columbus, Ohio, USA, 2010-20171 . Emerg Infect Dis 2020;26:1077–83. 10.3201/eid2606.190783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cowan MR, Primm PA, Scott SM, et al. Serious group A beta-hemolytic streptococcal infections complicating varicella. Ann Emerg Med 1994;23:818–22. 10.1016/S0196-0644(94)70320-5 [DOI] [PubMed] [Google Scholar]
- 46. Crum NF, Hale BR, Judd SE, et al. A case series of group A Streptococcus necrotizing fasciitis in military trainees. Mil Med 2004;169:373–5. 10.7205/MILMED.169.5.373 [DOI] [PubMed] [Google Scholar]
- 47. Dahl PR, Perniciaro C, Holmkvist KA, et al. Fulminant group A streptococcal necrotizing fasciitis: clinical and pathologic findings in 7 patients. J Am Acad Dermatol 2002;47:489–92. 10.1067/mjd.2002.120536 [DOI] [PubMed] [Google Scholar]
- 48. Donaldson PM, Naylor B, Lowe JW, et al. Rapidly fatal necrotising fasciitis caused by Streptococcus pyogenes. J Clin Pathol 1993;46:617–20. 10.1136/jcp.46.7.617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Erdem G, Abe L, Kanenaka RY, et al. Characterization of a community cluster of group A streptococcal invasive disease in Maui, Hawaii. Pediatr Infect Dis J 2004;23:677–9. 10.1097/01.inf.0000130956.47691.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Eriksson BK, Andersson J, Holm SE, et al. Invasive group A streptococcal infections: T1M1 isolates expressing pyrogenic exotoxins A and B in combination with selective lack of toxin-neutralizing antibodies are associated with increased risk of streptococcal toxic shock syndrome. J Infect Dis 1999;180:410–8. 10.1086/314872 [DOI] [PubMed] [Google Scholar]
- 51. Forni AL, Kaplan EL, Schlievert PM, et al. Clinical and microbiological characteristics of severe group A Streptococcus infections and streptococcal toxic shock syndrome. Clin Infect Dis 1995;21:333–40. 10.1093/clinids/21.2.333 [DOI] [PubMed] [Google Scholar]
- 52. Fronhoffs S, Luyken J, Steuer K, et al. The effect of C1-esterase inhibitor in definite and suspected streptococcal toxic shock syndrome. Report of seven patients. Intensive Care Med 2000;26:1566–70. 10.1007/s001340000654 [DOI] [PubMed] [Google Scholar]
- 53. Hasegawa T, Hashikawa S-N, Nakamura T, et al. Factors determining prognosis in streptococcal toxic shock-like syndrome: results of a nationwide investigation in Japan. Microbes Infect 2004;6:1073–7. 10.1016/j.micinf.2004.06.001 [DOI] [PubMed] [Google Scholar]
- 54. Hayata E, Nakata M, Hasegawa J, et al. Nationwide study of mortality and survival in pregnancy-related streptococcal toxic shock syndrome. J Obstet Gynaecol Res 2021;47:928–34. 10.1111/jog.14619 [DOI] [PubMed] [Google Scholar]
- 55. Huang YC, Hsueh PR, Lin TY, et al. A family cluster of streptococcal toxic shock syndrome in children: clinical implication and epidemiological investigation. Pediatrics 2001;107:1181–3. 10.1542/peds.107.5.1181 [DOI] [PubMed] [Google Scholar]
- 56. Kansal RG, McGeer A, Low DE, et al. Inverse relation between disease severity and expression of the streptococcal cysteine protease, SpeB, among clonal M1T1 isolates recovered from invasive group A streptococcal infection cases. Infect Immun 2000;68:6362–9. 10.1128/IAI.68.11.6362-6369.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Linder KA, Alkhouli L, Ramesh M, et al. Effect of underlying immune compromise on the manifestations and outcomes of group A streptococcal bacteremia. J Infect 2017;74:450–5. 10.1016/j.jinf.2017.02.006 [DOI] [PubMed] [Google Scholar]
- 58. Luca-Harari B, Darenberg J, Neal S, et al. Clinical and microbiological characteristics of severe Streptococcus pyogenes disease in Europe. J Clin Microbiol 2009;47:1155–65. 10.1128/JCM.02155-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nelson GE, Pondo T, Toews K-A, et al. Epidemiology of invasive group A streptococcal infections in the United States, 2005-2012. Clin Infect Dis 2016;63:478–86. 10.1093/cid/ciw248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Safar A, Lennon D, Stewart J, et al. Invasive group A streptococcal infection and vaccine implications, Auckland, New Zealand. Emerg Infect Dis 2011;17:983–9. 10.3201/eid/1706.100804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Schwartz B, Elliott JA, Butler JC, et al. Clusters of invasive group A streptococcal infections in family, Hospital, and nursing home settings. Clin Infect Dis 1992;15:277–84. 10.1093/clinids/15.2.277 [DOI] [PubMed] [Google Scholar]
- 62. Sriskandan S, Cohen J. Kallikrein-Kinin system activation in streptococcal toxic shock syndrome. Clin Infect Dis 2000;30:961–2. 10.1086/313827 [DOI] [PubMed] [Google Scholar]
- 63. Stockmann C, Ampofo K, Hersh AL, et al. Evolving epidemiologic characteristics of invasive group A streptococcal disease in Utah, 2002-2010. Clin Infect Dis 2012;55:479–87. 10.1093/cid/cis422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tagini F, Aubert B, Troillet N, et al. Importance of whole genome sequencing for the assessment of outbreaks in diagnostic laboratories: analysis of a case series of invasive Streptococcus pyogenes infections. Eur J Clin Microbiol Infect Dis 2017;36:1173–80. 10.1007/s10096-017-2905-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zachariadou L, Stathi A, Tassios PT, et al. Differences in the epidemiology between paediatric and adult invasive Streptococcus pyogenes infections. Epidemiol Infect 2014;142:512–9. 10.1017/S0950268813001386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Stevens DL, Tanner MH, Winship J, et al. Severe group A streptococcal infections associated with a toxic shock-like syndrome and scarlet fever toxin A. N Engl J Med 1989;321:1–7. 10.1056/NEJM198907063210101 [DOI] [PubMed] [Google Scholar]
- 67. Torimitsu S, Abe H, Makino Y, et al. Streptococcal toxic shock syndrome with fatal outcome: report on four forensic autopsy cases. Leg Med 2021;50:101851. 10.1016/j.legalmed.2021.101851 [DOI] [PubMed] [Google Scholar]
- 68. Page AV, Kotb M, McGeer A, et al. Systemic dysregulation of angiopoietin-1/2 in streptococcal toxic shock syndrome. Clin Infect Dis 2011;52:e157–61. 10.1093/cid/cir125 [DOI] [PubMed] [Google Scholar]
- 69. IntHout J, Ioannidis JPA, Borm GF, et al. Small studies are more heterogeneous than large ones: a meta-meta-analysis. J Clin Epidemiol 2015;68:860–9. 10.1016/j.jclinepi.2015.03.017 [DOI] [PubMed] [Google Scholar]
- 70. Parks T, Wilson C, Curtis N, et al. Polyspecific intravenous immunoglobulin in Clindamycin-treated patients with streptococcal toxic shock syndrome: a systematic review and meta-analysis. Clin Infect Dis 2018;67:1434–6. 10.1093/cid/ciy401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Legras A, Giraudeau B, Jonville-Bera A-P, et al. A multicentre case-control study of nonsteroidal anti-inflammatory drugs as a risk factor for severe sepsis and septic shock. Crit Care 2009;13:R43. 10.1186/cc7766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bryant AE, Bayer CR, Aldape MJ, et al. The roles of injury and nonsteroidal anti-inflammatory drugs in the development and outcomes of severe group A streptococcal soft tissue infections. Curr Opin Infect Dis 2015;28:231–9. 10.1097/QCO.0000000000000160 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2022-063023supp001.pdf (14.8MB, pdf)
Data Availability Statement
Data are available on reasonable request. Data extracted from individual studies are available upon reasonable request to the corresponding author. All other data relevant to the study are included in the article or uploaded as online supplemental information.