Abstract
Background
Critical culprit lesion locations (CCLL) such as left main (LM) and proximal left anterior descending (LAD) are associated with worse clinical outcome in myocardial infarction without cardiogenic shock (CS).
Aims
We aimed to assess whether CCLL identify a subgroup of patients with poorer prognosis when presenting with CS.
Methods
In the CULPRIT-SHOCK trial, a core laboratory reviewed all coronary angiograms to identify CCLL. A CCLL was defined as a culprit lesion with a >70% diameter stenosis of the LM, LM equivalent (>70% diameter stenosis of both proximal LAD and proximal circumflex), proximal LAD or last remaining vessel. We evaluated the primary study endpoint of the CULPRIT-SHOCK trial according to CCLL.
Results
A total of 269 (43%) out of 626 patients eligible for this analysis had a CCLL. Death or renal replacement therapy within 30 days, death within 30 days and death within one year were significantly higher in the CCLL than in the non-CCLL group (58.4% vs 43.4%, p<0.001, 55.8% vs 39.5%, p<0.001, 61.0% vs 44.5%, p<0.001, respectively). This was consistent after adjustment for baseline and angiographic characteristics. No interaction with the randomisation group (culprit lesion-only or immediate multivessel PCI) was found.
Conclusions
CCLL is frequent in CS and independently associated with worse clinical outcomes irrespective of the revascularisation strategy. Trial registration: www.clinicaltrials.gov NCT01927549
Introduction
Left main (LM) or proximal left anterior descending (LAD) are critical culprit lesion locations (CCLL) with impaired outcome in patients with myocardial infarction (MI)1,2,3,4. In cardiogenic shock (CS) complicating acute MI (AMI), the relationship between CCLL and outcome is controversial. In an analysis of the SHOCK trial5, a right coronary artery culprit was associated with superior survival in comparison to the LAD. In the IABP-SHOCK II trial6, a higher late mortality was observed in patients with distal culprit lesions, whereas no difference was observed for mortality with respect to the culprit lesion location itself. In patients with CS complicating AMI, the randomised trial entitled Culprit Lesion Only PCI versus Multi-vessel PCI in Cardiogenic Shock (CULPRIT-SHOCK)7,8 demonstrated that percutaneous coronary intervention (PCI) of the culprit lesion only, with the option of staged revascularisation of non-culprit lesions, was superior to immediate multivessel PCI with respect to a composite endpoint of death or renal replacement therapy at 30 days. The aim of this CULPRIT-SHOCK substudy was to assess whether CCLL identifies a group of patients with poorer prognosis independently of baseline and other angiographic characteristics.
Methods
This is a post hoc analysis of the CULPRIT-SHOCK trial, whose design and results have been described previously7,8,9. Briefly, the CULPRIT-SHOCK trial was a randomised, open-label study conducted at 83 European centres where 686 patients presenting with acute MI and multivessel coronary artery disease (CAD) complicated by CS were randomised, in a 1:1 ratio, to a strategy of culprit lesion-only PCI (with optional staged revascularisation) or immediate multivessel PCI between April 2013 and April 2017. Patients who initially underwent culprit lesion-only PCI had lower rates of death or renal replacement therapy at 30 days compared to patients who underwent immediate multivessel PCI. The investigation was approved by the ethics committee or institutional review board of each participating centre. The CULPRIT-SHOCK trial was supported by a grant agreement (602202) from the European Union Seventh Framework Program and by the German Heart Research Foundation and the German Cardiac Society.
STUDY POPULATION
All patients of the CULPRIT-SHOCK trial with a core laboratory identification of the culprit lesion were included. Patients with prior coronary artery bypass graft (CABG) were excluded. The choice was taken to exclude patients with a prior CABG to obtain a homogenous AMI-related CS population – culprit lesion location on native vessels only. Prior CABG is an independent predictor of adverse outcomes in AMI-related CS10,11,12. The population with AMI-related CS and prior CABG is older, has extensive CAD and more often suffers from heart failure, diabetes mellitus, hypertension and dyslipidaemia in comparison with those with no prior CABG, as shown in Vallabhajosyula’s 16-year cohort12. The mechanisms of AMI and CS in previous CABG recipients may be different in comparison to patients without prior CABG, with a high prevalence of acute occlusion of venous bypass grafts and pre-existing left ventricular dysfunction13. Furthermore, it is difficult to define a critical lesion location on CABG considering the great variety of CABG surgeries. Finally, only a minority of patients (33 out of 686 [4.8%]) had a prior CABG in the CULPRIT-SHOCK study.
ANGIOGRAPHIC CORE LABORATORY PROTOCOL
All coronary angiograms and PCI were independently reviewed at the core laboratory of the ACTION Study Group (Pitié-Salpêtrière Hospital, Paris, France). CCLL was defined as a culprit lesion with a >70% diameter stenosis of the LM or LM equivalent (defined as >70% diameter stenosis of both proximal LAD and proximal left circumflex), proximal LAD or last remaining vessel. The last remaining vessel was defined as a sole remaining artery with chronic occlusion of the two other territories. PCI is known to be risky in patients whose coronary circulation depends on a last remaining vessel14, and thus the last remaining vessel represents a critical lesion location. The objective was to determine whether CCLL were independently associated with short- and long-term outcomes. Outcomes of interest for this substudy were all-cause death or renal replacement therapy and all-cause death at 30 days and all-cause death at one year.
STATISTICAL ANALYSIS
Categorical variables were described as proportion and compared with the chi-square test or Fisher’s exact test. Continuous variables were described as median (Q1; Q3) and compared using the Wilcoxon rank-sum test. Event rates were compared using the chi-square test, as previously published7,8. Kaplan-Meier curves were also used to show event rates over time, with classification according to CCLL, and compared using the log-rank test. Patients without an event were censored at 30 days or one year.
Multivariate logistic regression models were used to evaluate the independent association between CCLL and outcomes. In each model, CCLL was adjusted for baseline clinical and procedural characteristics possibly associated with outcomes in univariate analysis (p<0.2) (Supplementary Table 1).
Given that, in the CULPRIT-SHOCK trial, approximately 10% of randomised patients had crossover, sensitivity multivariable analyses adjusted on consistent covariates as well as the effective revascularisation procedure undergone by the patients were additionally performed.
Results are reported as adjusted odds ratio (aOR) with their 95% confidence interval (95% CI). A p-value <0.05 was considered significant. All statistical analyses were performed with SAS release 9.4 (SAS Institute Inc., Cary, NC, USA) statistical software.
Results
Of the 686 randomised patients with available informed consent, 33 patients were excluded because of prior CABG surgery, 8 patients had missing data concerning prior CABG status and 19 patients had no available core laboratory data. A total of 626 (91.3%) patients were finally included in this analysis, in whom a CCLL was found in 269 (43.0%) (Supplementary Figure 1). Thirty-eight patients (14.1%) had an LM lesion, 76 (28.3%) had an LM equivalent lesion, 148 (55%) had a proximal LAD lesion, and 7 (2.6%) had a last remaining vessel lesion. Baseline and procedural characteristics are presented in Table 1 and Table 2. Patients with a CCLL had more chronic total occlusions, higher pre-PCI SYNTAX score, lower post-PCI Thrombolysis In Myocardial Infarction (TIMI) flow grade (TFG) 3 and left ventricular ejection fraction (LVEF).
Table 1. Baseline characteristics.
| Total (n=626) | Critical location (n=269) | Non-critical location (n=357) | p-value | ||
| Age, years | 69 (60-78) | 70 (60-79) | 68 (60-77) | 0.20 | |
| Male sex | 477 (76.2%) | 213 (79.2%) | 264 (73.9%) | 0.13 | |
| Body mass index, kg/m² | 26.7 (24.5-29.4) | 26.8 (24.2-29.4) | 26.6 (24.7-29.4) | 0.40 | |
| Cardiovascular risk factors | Current smoking | 165/608 (27.1%) | 68/266 (25.6%) | 97/342 (28.4%) | 0.44 |
| Hypertension | 369/621 (59.4%) | 157/268 (58.6%) | 212/353 (60.1%) | 0.71 | |
| Hypercholesterolaemia | 200/619 (32.3%) | 88/268 (32.8%) | 112/351 (31.9%) | 0.81 | |
| Diabetes mellitus | 192/619 (31.0%) | 94/268 (35.1%) | 98/351 (27.9%) | 0.06 | |
| Prior myocardial infarction | 97/623 (15.6%) | 44/268 (16.4%) | 53/355 (14.9%) | 0.61 | |
| Prior stroke | 40/624 (6.4%) | 23/269 (8.6%) | 17/355 (4.8%) | 0.06 | |
| Prior peripheral artery disease | 70/625 (11.2%) | 39/269 (14.5%) | 31/356 (8.7%) | 0.02 | |
| Prior percutaneous coronary intervention | 105/623 (16.9%) | 41/268 (15.3%) | 64/355 (18.0%) | 0.37 | |
| Resuscitation before randomisation | 328/625 (52.5%) | 127/268 (47.4%) | 201/357 (56.3%) | 0.03 | |
| Fibrinolysis <24 hrs before randomisation | 32/624 (5.1%) | 12/268 (4.5%) | 20/356 (5.6%) | 0.52 | |
| ST-segment elevation myocardial infarction | 392/608 (64.5%) | 165/261 (63.2%) | 227/347 (65.4%) | 0.58 | |
| Left bundle branch block | 86/609 (14.1%) | 38/261 (14.6%) | 48/348 (13.8%) | 0.79 | |
| Heart rate, beats/min | 90.0 (72.0-107.0) | 96.0 (79.0-110.0) | 87.0 (67.0-104.0) | <0.001 | |
| Systolic blood pressure, mmHg | 100.0 (85.0-125.0) | 101.0 (84.0-125.0) | 100.0 (86.0-125.0) | 0.82 | |
| Diastolic blood pressure, mmHg | 60.0 (50.0-80.0) | 62.5 (50.0-80.0) | 60.0 (50.0-80.0) | 0.99 | |
| Mean blood pressure, mmHg | 76.0 (63.3-93.3) | 76.7 (63.3-93.3) | 75.7 (63.3-93.3) | 0.94 | |
| Arterial lactate >2.0 mmol/L | 403/610 (66.1%) | 173/265 (65.3%) | 230/345 (66.7%) | 0.72 | |
| Number of affected vessels | 1 | 5/626 (0.8%) | 1/269 (0.4%) | 4/357 (1.1%) | 0.32 |
| 2 | 232/626 (37.1%) | 93/269 (34.6%) | 139/357 (38.9%) | ||
| 3 | 389/626 (62.1%) | 175/269 (65.1%) | 214/357 (59.9%) | ||
| ≥1 chronic total occlusion* | 132/626 (21.1%) | 69/269 (25.7%) | 63/357 (17.6%) | 0.015 | |
| Pre-PCI SYNTAX score* | Low SYNTAX score | 261/617 (42.3%) | 66/263 (25.1%) | 195/354 (55.1%) | <0.001 |
| Intermediate SYNTAX score | 204/617 (33.1%) | 106/263 (40.3%) | 98/354 (27.7%) | ||
| High SYNTAX score | 152/617 (24.6%) | 91/263 (34.6%) | 61/354 (17.2%) | ||
| Left ventricular ejection fraction§ | 31.0 (25.0-40.0) | 30.0 (20.0-40.0) | 35.0 (28.0-45.0) | <0.001 | |
| *according to core laboratory data. § available in 238 patients. PCI: percutaneous coronary intervention; SYNTAX score: Synergy between PCI with Taxus drug-eluting stent and cardiac surgery score | |||||
Table 2. Procedural characteristics according to critical culprit lesion location.
| Total (n=626) | Critical location (n=269) | Non-critical location (n=357) | p-value | ||
| Stent in culprit lesion | 597/626 (95.4%) | 250/269 (92.9%) | 347/357 (97.2%) | 0.01 | |
| Aspiration thrombectomy of culprit lesion | 92/626 (14.7%) | 42/269 (15.6%) | 50/357 (14.0%) | 0.57 | |
| TIMI flow grade before PCI of culprit lesion* | Pre-PCI TFG 0-1-2 | 420/622 (67.5%) | 189/267 (70.8%) | 231/355 (65.1%) | 0.13 |
| Pre-PCI TFG 3 | 202/622 (32.5%) | 78/267 (29.2%) | 124/355 (34.9%) | ||
| TIMI flow grade after PCI of culprit lesion * | Post-PCI TFG 0-1-2 | 131/602 (21.8%) | 69/254 (27.2%) | 62/348 (17.8%) | 0.006 |
| Post-PCI TFG 3 | 471/602 (78.2%) | 185/254 (72.8%) | 286/348 (82.2%) | ||
| Immediate PCI of non-culprit lesion | 327/626 (52.2%) | 137/269 (50.9%) | 190/357 (53.2%) | 0.57 | |
| Immediate complete revascularisation achieved | 284/626 (45.4%) | 118/269 (43.9%) | 166/357 (46.5%) | 0.51 | |
| Total dose of contrast material, mL | 220.0 (155.0-300.0) | 223.0 (165.0-300.0) | 215 (150.0-300.0) | 0.65 | |
| Total fluoroscopy duration, min | 15.1 (9.2-24.2) | 16.0 (9.5-25.3) | 15.0 (9.0-23.0) | 0.23 | |
| Staged PCI of non-culprit lesions | 61/626 (9.7%) | 21/269 (7.8%) | 40/357 (11.2%) | 0.16 | |
| Induced mild hypothermia | 206/624 (33%) | 76/269 (28.3%) | 130/355 (36.6%) | 0.03 | |
| Mechanical circulatory support | 174/626 (27.8%) | 99/269 (36.8%) | 75/357 (21.0%) | <0.001 | |
| Mechanical ventilation | 503/623 (80.7%) | 213/267 (79.8%) | 290/356 (81.5%) | 0.60 | |
| Duration of mechanical ventilation, days | 3.0 (1.0-8.0) | 2.0 (1.0-6.5) | 3.0 (1.0-8.0) | 0.048 | |
| Use of catecholamines | 559/623 (89.7%) | 240/267 (89.9%) | 319/356 (89.6%) | 0.91 | |
| Duration of catecholamines, days | 2.0 (1.0-5.0) | 2.0 (1.0-5.0) | 2.0 (1.0-5.0) | 0.44 | |
| Time to haemodynamic stabilisation, days | 3.0 (1.0-6.0) | 3.0 (1.0-7.0) | 3.0 (1.0-6.0) | 0.86 | |
| Duration of intensive care, days | 5.0 (2.0-11.0) | 5.0 (2.0-11.0) | 5.0 (2.0-11.0) | 0.47 | |
| Subsequent medications in patients who survived until hospital discharge | |||||
| Statin | 312/335 (93.1%) | 111/119 (93.3%) | 201/216 (93.1%) | 0.94 | |
| Beta-blocker | 306/335 (91.3%) | 111/119 (93.3%) | 195/216 (90.3%) | 0.35 | |
| ACE or ARB inhibitors | 293/335 (87.5%) | 106/119 (89.1%) | 187/216 (86.6%) | 0.51 | |
| Aspirin | 330/335 (98.5%) | 117/119 (98.3%) | 213/216 (98.6%) | 1.00 | |
| Clopidogrel | 145/335 (43.3%) | 56/119 (47.1%) | 89/216 (41.2%) | 0.30 | |
| Prasugrel | 120/335 (35.8%) | 35/119 (29.4%) | 85/216 (39.4%) | 0.07 | |
| Ticagrelor | 133/335 (39.7%) | 48/119 (40.3%) | 85/216 (39.4%) | 0.86 | |
| *according to core laboratory data. ACE: angiotensin-converting enzyme; ARB: angiotensin II receptor blocker; PCI: percutaneous coronary intervention; TFG: TIMI flow grade; TIMI: Thrombolysis In Myocardial Infarction | |||||
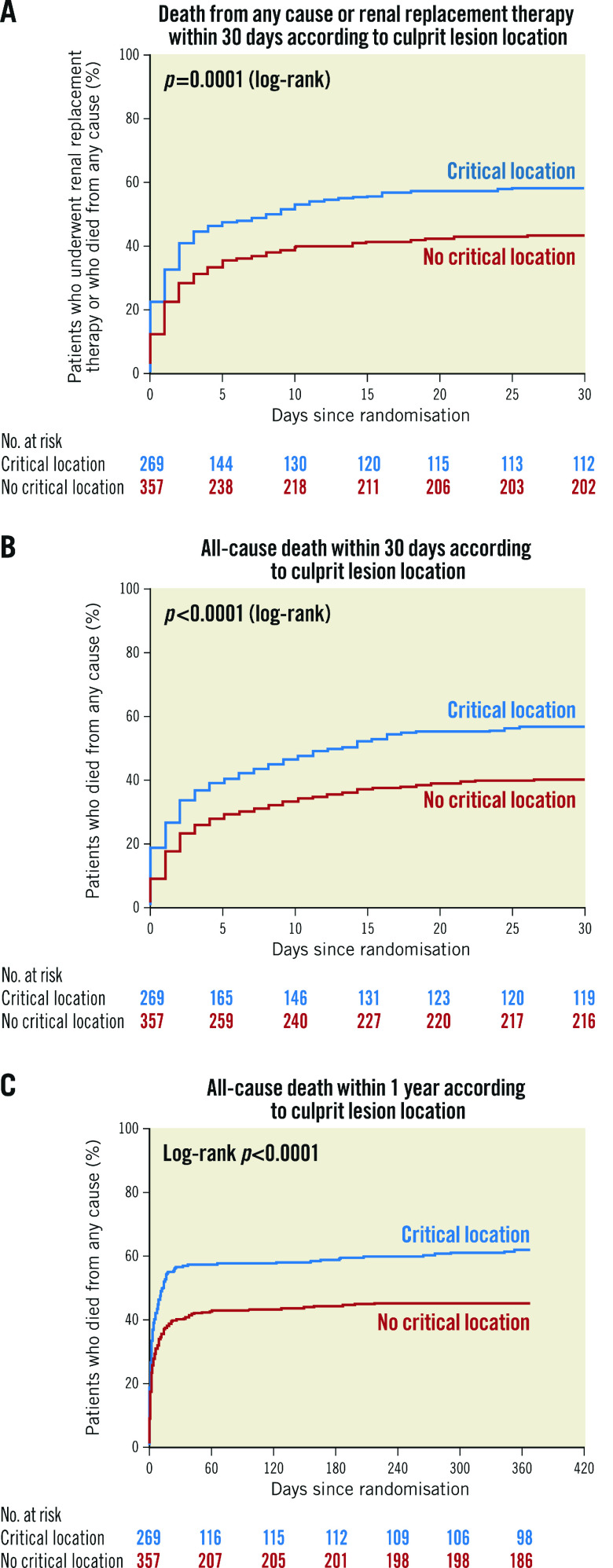
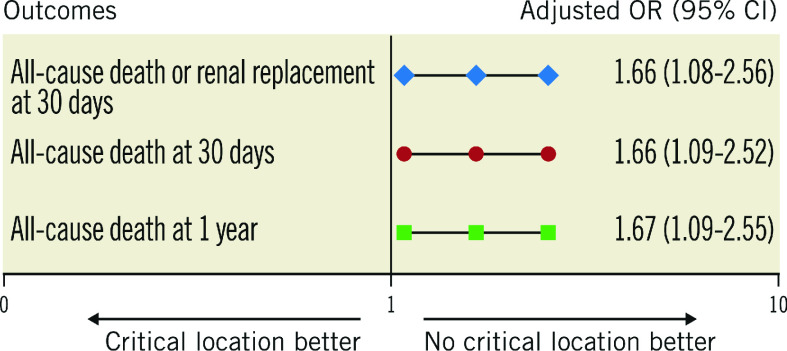
In the CCLL group, death or renal replacement therapy within 30 days, all-cause death within 30 days and death within one year were significantly higher than in the non-CCLL group (157 [58.4%] vs 155 [43.4%], p<0.001, 150 [55.8%] vs 141 [39.5%], p<0.001, 164 [61.0%] vs 159 [44.5%], p<0.001, respectively) (Table 3, Figure 1A-Figure 1C). These significant differences were consistent after adjustment (aOR=1.66 [1.08; 2.56], p=0.021, aOR=1.66 [1.09-2.52], p=0.019, and aOR=1.67 [1.09-2.55], p=0.018, respectively) (Figure 2). Results remained consistent in the sensitivity analysis adjusted for the revascularisation strategy (Supplementary Figure 2). Of note, no interaction with the randomisation group (culprit lesion-only or immediate multivessel PCI) was found for all outcomes (p=0.56, p=0.49 and p=0.11, respectively).
Table 3. Early and late outcomes according to critical culprit lesion location.
| Critical location (n=269) | Non-critical location (n=357) | Unadjusted OR (95% CI) | p-value | Adjusted OR (95% CI) | p-value | |
| 30-day outcomes | ||||||
| All-cause death or renal replacement therapy* | 157 (58.4%) | 155 (43.4%) | 1.83 (1.33-2.52) | <0.001 | 1.66 (1.08-2.56) | 0.021 |
| All-cause death** | 150 (55.8%) | 141 (39.5%) | 1.93 (1.40-2.66) | <0.001 | 1.66 (1.09-2.52) | 0.019 |
| 1-year outcomes | ||||||
| All-cause death§ | 164 (61.0%) | 159 (44.5%) | 1.95 (1.41-2.68) | <0.001 | 1.67 (1.09-2.55) | 0.018 |
| *N=548 patients, covariates of adjustment are detailed in Supplementary Table 1. **N=565 patients, covariates of adjustment are detailed in Supplementary Table 1. § N=565 patients, covariates of adjustment are detailed in Supplementary Table 1. | ||||||
Figure 1.
Kaplan-Meier curves. A) Death from any cause or renal replacement therapy within 30 days according to critical culprit lesion location. B) All-cause death within 30 days according to critical culprit lesion location. C) All-cause death within one year according to critical culprit lesion location.
Figure 2.
Early and late outcomes according to critical culprit lesion location (covariates of adjustment are detailed in Supplementary Table 1).
No significant difference was found between each CCLL (LM, LM equivalent, proximal LAD, last remaining vessel) on all outcomes (p=0.88, 0.89 and 0.71 for death or renal replacement therapy within 30 days, death within 30 days and death within one year, respectively) (Supplementary Figure 3).
Discussion
Our results indicate that patients with AMI-related cardiogenic shock and a critical anatomic location of the culprit lesion have worse clinical outcomes than patients without such a critical location. They identify patients at higher risk of short- and long-term mortality, even after adjustment for confounding clinical and procedural characteristics.
In this core laboratory analysis, CCLL was defined as a culprit lesion with a >70% diameter stenosis of the LM, LM equivalent, proximal LAD or last remaining vessel. This anatomical marker is a strong determinant of a higher mortality compared with infarction in other vascular territories14,15,16,17,18. Thus, an LM equivalent lesion location should have an equivalent prognosis.
In contrast to the IABP-SHOCK II randomised trial and registry sub-analysis that shows no impact of culprit vessel type but an excess of mortality in case of distal culprit lesion location6, we found that patients with a CCLL had worse short- and long-term outcomes. A methodological difference between the IABP-SHOCK II study and the present study has to be mentioned. In the IABP-SHOCK II study, no comparison of mortality among the LM, proximal LAD and LM equivalent was provided. The increase in one-year mortality among patients with distal culprit lesions in this previous study was explained by higher rates of diabetes mellitus and known renal insufficiency in those patients. In the IABP-SHOCK II trial, the rates of post-PCI TFG 3 did not differ in patients with proximal or distal culprit lesions (81 vs 79%). The clinical impact of CCLL found in our study might not be related to the lower rate of post-PCI TFG 3 found in CCLL versus non-CCLL patients (73 vs 82%, p=0.006). Indeed, our results remain consistent after adjustment on post-PCI TFG; this implies that CCLL is a significant and independent predictor of worse outcome, information immediately available at the time of the coronary angiogram. Our results are consistent with other studies19,20,21,22,23 showing higher mortality rates for patients with an LM culprit lesion.
Patients with a proximal culprit lesion location may have a larger area at risk and a larger infarct size. The benefit of PCI would be expected to be more rapidly significant. Our results suggest that the initial excess risk associated with the critical location of the culprit lesion is not fully neutralised by revascularisation, and that these patients continue to be at higher risk of mortality after percutaneous revascularisation. In our study, LVEF was lower in the critical location group than in the non-critical location group (30.0% [20.0-40.0] vs 35.0% [28.0-45.0], p<0.001). Since no cardiac magnetic resonance imaging was performed in this vulnerable population, no additional data on myocardial salvage index were available.
Post-procedural TIMI flow is a well-known independent prognostic factor in patients with MI and CS undergoing PCI21,24, whereas baseline SYNTAX score and residual SYNTAX score have no or limited added prognostic value over clinical assessment and risk scores25. In our analysis, the critical anatomic location increased the risk of death at 30 days and one year (aOR=1.66 and 1.67, respectively) independently of other reperfusion parameters of poor outcome such as post-PCI TIMI flow and independently of the extent of coronary disease as measured by the SYNTAX score also included in our model. An overall score to predict mortality based on clinical settings readily available at the time of CS diagnosis has been proposed26, which provides good discrimination of mortality risk in infarct-related CS. This kind of score, however, is mainly useful for clinical trials and not easy to use or widely used in clinical practice, whereas the CCLL is a simple and strong predictor of adverse outcome available in early CS management.
Limitations
Some limitations regarding this substudy of a randomised controlled trial have to be mentioned. First, only 626 patients (91.3%) out of 686 in the original study were eligible for this core laboratory sub-analysis. Second, the choice was made to exclude patients with a prior bypass graft (n=33, 4.8%). However, they represent a limited number of patients in our series and in real life. Finally, LVEF data were available for only 238 (38.0%) out of 626 patients and were therefore not accounted for in multivariate analysis. Other known predictors of poor outcome such as “arterial lactate >6 mmol/L” would have been relevant but there was also too much missing information (missing data: 36.3%). However, “arterial lactate >2 mmol/L” was included in the multivariate analysis, as well as “mechanical circulatory support”, “mechanical ventilation” and “catecholamine therapy”, which are known predictors of poor outcome and clinically relevant.
Conclusions
Among multivessel disease patients with acute myocardial infarction-related cardiogenic shock, critical culprit lesion location (LM, LM equivalent, proximal LAD or last remaining vessel) is independently associated with 30-day and one-year mortality. Critical culprit lesion location is a major prognostic marker, immediately available in multivessel disease patients with myocardial infarction-related cardiogenic shock.
Impact on daily practice
Critical culprit lesion location (left main, left main equivalent, proximal left anterior descending or last remaining vessel) in patients with infarct-related cardiogenic shock and multivessel disease is frequent, immediately available and an independent prognostic marker of adverse outcome.
Supplementary data
Variables associated with outcomes in univariate analysis.
Flow chart.
Early and late outcomes according to critical culprit lesion location adjusted for revascularisation strategy.
Early and late outcomes according to individual critical culprit lesion location.
Acknowledgments
Funding
The CULPRIT-SHOCK trial was supported by a grant agreement (602202) from the European Union Seventh Framework Program and by the German Heart Research Foundation and the German Cardiac Society. The current substudy was led by the ACTION Study Group at the Institute of Cardiology of Pitié-Salpêtrière Hospital (www.action-coeur.org).
Conflict of interest statement
E. Vicaut reports receiving personal fees from Eli Lilly; consultancy from Pfizer, Sanofi, LFB, Abbott, Fresenius, Medtronic, and Hexacath; being a member of the data safety monitoring board for CERC; lecture fees from Novartis, and grants from Boehringer and Sanofi. G. Montalescot has received research grants or consulting fees from Abbott, Amgen, Actelion, American College of Cardiology Foundation, AstraZeneca, Axis-Santé, Bayer, Boston Scientific, Boehringer Ingelheim, Bristol-Myers Squibb, Beth Israel Deaconess Medical, Brigham Women’s Hospital, China Heart House, Daiichi Sankyo, Idorsia, Elsevier, Europa, Fédération Française de Cardiologie, ICAN, Lead-Up, Medtronic, Menarini, MSD, Novo Nordisk, Partners, Pfizer, Quantum Genomics, Sanofi, Servier, and WebMD. U. Zeymer has received research grants or consulting fees from Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi Sankyo, Ferrer, Idorsia, The Medicines Company, Medtronic, MSD, Pfizer, Quantum Genomics, Sanofi, Servier, and WebMD. M. Zeitouni has received research grants from Institut Servier and Federation Française de Cardiologie. The other authors have no conflicts of interest to declare.
Abbreviations
- ACE
angiotensin-converting enzyme
- AMI
acute myocardial infarction
- aOR
adjusted odds ratio
- ARB
angiotensin II receptor blocker
- CABG
coronary artery bypass graft
- CAD
coronary artery disease
- CCLL
critical culprit lesion location(s)
- CS
cardiogenic shock
- LAD
left anterior descending
- LM
left main
- LVEF
left ventricular ejection fraction
- PCI
percutaneous coronary intervention
- SYNTAX
Synergy between PCI with Taxus drug-eluting stent and cardiac surgery
- TIMI
Thrombolysis In Myocardial Infarction
Contributor Information
Marie Hauguel-Moreau, Sorbonne Université, ACTION Study Group, INSERM UMRS 1166, Institut de Cardiologie (AP-HP), Paris, France.
Olvier Barthélémy, Sorbonne Université, ACTION Study Group, INSERM UMRS 1166, Institut de Cardiologie (AP-HP), Paris, France.
Serdar Farhan, The Zena and Michael A. Wiener Cardiovascular Institute, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Kurt Huber, 3rd Department of Medicine, Cardiology and Intensive Care Medicine, Wilhelminen hospital and Sigmund Freund University, Medical School, Vienna, Austria.
Stéphanie Rouanet, Statistician Unit, statEthic, Levallois-Perret, France.
Michel Zeitouni, Sorbonne Université, ACTION Study Group, INSERM UMRS 1166, Institut de Cardiologie (AP-HP), Paris, France.
Paul Guedeney, Sorbonne Université, ACTION Study Group, INSERM UMRS 1166, Institut de Cardiologie (AP-HP), Paris, France.
Georges Hage, Sorbonne Université, ACTION Study Group, INSERM UMRS 1166, Institut de Cardiologie (AP-HP), Paris, France.
Eric Vicaut, ACTION Study Group, Unité de Recherche Clinique, Hôpital Lariboisière (Ap-HP), Paris, France.
Uwe Zeymer, Heart Centre Ludwigshafen, Department of Cardiology, Ludwigshafen am Rhein, Germany.
Steffen Desch, Heart Centre Leipzig at University of Leipzig and Leipzig Heart Institute, Leipzig, Germany.
Holger Thiele, Heart Centre Leipzig at University of Leipzig and Leipzig Heart Institute, Leipzig, Germany.
Gilles Montalescot, Sorbonne Université, ACTION Study Group, INSERM UMRS 1166, Institut de Cardiologie (AP-HP), Paris, France.
References
- Karha J, Murphy SA, Kirtane AJ, de Lemos JA, Aroesty JM, Cannon CP, Antman EM, Braunwald E, Gibson CM TIMI Study Group. Evaluation of the association of proximal coronary culprit artery lesion location with clinical outcomes in acute myocardial infarction. Am J Cardiol. 2003;92:913‑8. doi: 10.1016/s0002-9149(03)00969-x. [DOI] [PubMed] [Google Scholar]
- Harjai KJ, Mehta RH, Stone GW, Boura JA, Grines L, Brodie BR, Cox DA, O'Neill WW, Grines CL Primary Angioplasty In Myocardial Infarction (PAMI) Investigators. Does proximal location of culprit lesion confer worse prognosis in patients undergoing primary percutaneous coronary intervention for ST elevation myocardial infarction? J Interv Cardiol. 2006;19:285–94. doi: 10.1111/j.1540-8183.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- Stone PH, Raabe DS, Jaffe AS, Gustafson N, Muller JE, Turi ZG, Rutherford JD, Poole WK, Passamani E, Willerson JT, et al. Prognostic significance of location and type of myocardial infarction: independent adverse outcome associated with anterior location. J Am Coll Cardiol. 1988;11:453‑63. doi: 10.1016/0735-1097(88)91517-3. [DOI] [PubMed] [Google Scholar]
- Hands ME, Lloyd BL, Robinson JS, de Klerk N, Thompson PL. Prognostic significance of electrocardiographic site of infarction after correction for enzymatic size of infarction. Circulation. 1986;73:885‑91. doi: 10.1161/01.CIR.73.5.885. [DOI] [PubMed] [Google Scholar]
- Webb JG, Lowe AM, Sanborn TA, White HD, Sleeper LA, Carere RG, Buller CE, Wong SC, Boland J, Džavík V, Porway M, Pate G, Bergman G, Hochman JS SHOCK Investigators. Percutaneous coronary intervention for cardiogenic shock in the SHOCK trial. J Am Coll Cardiol. 2003;42:1380–6. doi: 10.1016/s0735-1097(03)01050-7. [DOI] [PubMed] [Google Scholar]
- Fuernau G, Fengler K, Desch S, Eitel I, Neumann FJ, Olbrich HG, de Waha A, de Waha S, Richardt G, Hennersdorf M, Empen K, Hambrecht R, Jung C, Böhm M, Pöss J, Strasser RH, Schneider S, Ouarrak T, Schuler G, Werdan K, Zeymer U, Thiele H. Culprit lesion location and outcome in patients with cardiogenic shock complicating myocardial infarction: a substudy of the IABP-SHOCK II-trial. Clin Res Cardiol. 2016;105:1030‑41. doi: 10.1007/s00392-016-1017-6. [DOI] [PubMed] [Google Scholar]
- Thiele H, Akin I, Sandri M, Fuernau G, de Waha S, Meyer-Saraei R, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Savonitto S, Torremante P, Vrints C, Schneider S, Desch S, Zeymer U CULPRIT-SHOCK Investigators. PCI Strategies in Patients with Acute Myocardial Infarction and Cardiogenic Shock. N Engl J Med. 2017;377:2419–32. doi: 10.1056/NEJMoa1710261. [DOI] [PubMed] [Google Scholar]
- Thiele H, Akin I, Sandri M, de Waha-Thiele S, Meyer-Saraei R, Fuernau G, Eitel I, Nordbeck P, Geisler T, Landmesser U, Skurk C, Fach A, Jobs A, Lapp H, Piek JJ, Noc M, Goslar T, Felix SB, Maier LS, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Barthelemy O, Huber K, Windecker S, Hunziker L, Savonitto S, Torremante P, Vrints C, Schneider S, Zeymer U, Desch S CULPRIT-SHOCK Investigators. One-Year Outcomes after PCI Strategies in Cardiogenic Shock. N Engl J Med. 2018;379:1699–710. doi: 10.1056/NEJMoa1808788. [DOI] [PubMed] [Google Scholar]
- Thiele H, Desch S, Piek JJ, Stepinska J, Oldroyd K, Serpytis P, Montalescot G, Noc M, Huber K, Fuernau G, de Waha S, Meyer-Saraei R, Schneider S, Windecker S, Savonitto S, Briggs A, Torremante P, Vrints C, Schuler G, Ceglarek U, Thiery J, Zeymer U CULPRIT-SHOCK Investigators. Multivessel versus culprit lesion only percutaneous revascularization plus potential staged revascularization in patients with acute myocardial infarction complicated by cardiogenic shock: Design and rationale of CULPRIT-SHOCK trial. Am Heart J. 2016;172:160–9. doi: 10.1016/j.ahj.2015.11.006. [DOI] [PubMed] [Google Scholar]
- Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625–34. doi: 10.1056/NEJM199908263410901. [DOI] [PubMed] [Google Scholar]
- Thiele H, Zeymer U, Neumann FJ, Ferenc M, Olbrich HG, Hausleiter J, Richardt G, Hennersdorf M, Empen K, Fuernau G, Desch S, Eitel I, Hambrecht R, Fuhrmann J, Böhm M, Ebelt H, Schneider S, Schuler G, Werdan K IABP-SHOCK II Trial Investigators. Intraaortic balloon support for myocardial infarction with cardiogenic shock. N Engl J Med. 2012;367:1287–96. doi: 10.1056/NEJMoa1208410. [DOI] [PubMed] [Google Scholar]
- Vallabhajosyula S, Kumar V, Vallabhajosyula S, Subramaniam AV, Patlolla SH, Verghese D, Ya'Qoub L, Stulak JM, Sandhu GS, Prasad A, Holmes DR Jr, Barsness GW. Acute myocardial infarction-cardiogenic shock in patients with prior coronary artery bypass grafting: A 16-year national cohort analysis of temporal trends, management and outcomes. Int J Cardiol. 2020;310:9–15. doi: 10.1016/j.ijcard.2020.02.033. [DOI] [PubMed] [Google Scholar]
- Lupi A, Schaffer A, Persampieri S. Acute myocardial infarction presenting with cardiogenic shock in patients with previous coronary artery bypass graft: neglected disease or end-stage condition? Int J Cardiol. 2020;310:25‑6. doi: 10.1016/j.ijcard.2020.03.047. [DOI] [PubMed] [Google Scholar]
- Tavano D, Corbett S, Airoldi F, Montorfano M, Carlino M, Godino C, Colombo A. Percutaneous coronary intervention in patients with a single remaining vessel. Am J Cardiol. 2007;99:470‑1. doi: 10.1016/j.amjcard.2006.08.059. [DOI] [PubMed] [Google Scholar]
- Tomey MI, Mehran R, Brener SJ, Maehara A, Witzenbichler B, Dizon JM, El-Omar M, Xu K, Gibson CM, Stone GW. Sex, adverse cardiac events, and infarct size in anterior myocardial infarction: an analysis of intracoronary abciximab and aspiration thrombectomy in patients with large anterior myocardial infarction (INFUSE-AMI). Am Heart J. 2015;169:86‑93. doi: 10.1016/j.ahj.2014.06.019. [DOI] [PubMed] [Google Scholar]
- Califf RM, Pieper KS, Lee KL, Van de Werf F, Simes RJ, Armstrong PW, Topol EJ. Prediction of 1-year survival after thrombolysis for acute myocardial infarction in the global utilization of streptokinase and TPA for occluded coronary arteries trial. Circulation. 2000;101:2231‑8. doi: 10.1161/01.CIR.101.19.2231. [DOI] [PubMed] [Google Scholar]
- De Luca G, Suryapranata H, Thomas K, van’t Hof AW, de Boer MJ, Hoorntje JC, Zijlstra F. Outcome in patients treated with primary angioplasty for acute myocardial infarction due to left main coronary artery occlusion. Am J Cardiol. 2003;91:235‑8. doi: 10.1016/s0002-9149(02)03115-6. [DOI] [PubMed] [Google Scholar]
- Grines CL, Browne KF, Marco J, Rothbaum D, Stone GW, O’Keefe J, Overlie P, Donohue B, Chelliah N, Timmis GC, et al. A comparison of immediate angioplasty with thrombolytic therapy for acute myocardial infarction. The Primary Angioplasty in Myocardial Infarction Study Group. N Engl J Med. 1993;328:673–9. doi: 10.1056/NEJM199303113281001. [DOI] [PubMed] [Google Scholar]
- Montalescot G, Brieger D, Eagle KA, Anderson FA, Fitzgerald G, Lee MS, Steg PG, Avezum A, Goodman SG, Gore JM GRACE Investigators. Unprotected left main revascularization in patients with acute coronary syndromes. Eur Heart J. 2009;30:2308–17. doi: 10.1093/eurheartj/ehp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overtchouk P, Pascal J, Lebreton G, Hulot JS, Luyt CE, Combes A, Kerneis M, Silvain J, Barthelemy O, Leprince P, Bréchot N, Montalescot G, Collet JP. Outcome after revascularisation of acute myocardial infarction with cardiogenic shock on extracorporeal life support. EuroIntervention. 2018;13:e2160–8. doi: 10.4244/EIJ-D-17-01014. [DOI] [PubMed] [Google Scholar]
- Auffret V, Cottin Y, Leurent G, Gilard M, Beer JC, Zabalawi A, Chagué F, Filippi E, Brunet D, Hacot JP, Brunel P, Mejri M, Lorgis L, Rouault G, Druelles P, Cornily JC, Didier R, Bot E, Boulanger B, Coudert I, Loirat A, Bedossa M, Boulmier D, Maza M, Le Guellec M, Puri R, Zeller M, Le Breton H ORBI and RICO Working Groups. Predicting the development of in-hospital cardiogenic shock in patients with ST-segment elevation myocardial infarction treated by primary percutaneous coronary intervention: the ORBI risk score. Eur Heart J. 2018;39:2090–102. doi: 10.1093/eurheartj/ehy127. [DOI] [PubMed] [Google Scholar]
- Trzeciak P, Gierlotka M, Gasior M, Lekston A, Wilczek K, Słonka G, Kalarus Z, Zembala M, Hudzik B, Polonski L. Mortality of patients with ST-segment elevation myocardial infarction and cardiogenic shock treated by PCI is correlated to the infarct-related artery--results from the PL-ACS Registry. Int J Cardiol. 2013;166:193‑7. doi: 10.1016/j.ijcard.2011.10.100. [DOI] [PubMed] [Google Scholar]
- Wong SC, Sanborn T, Sleeper LA, Mejnartowicz S, Antonelli TA, Lange R, French JK, Bergman V, LeJemtel T, Hochman JS. Angiographic findings and clinical correlates in patients with cardiogenic shock complicating acute myocardial infarction: a report from the SHOCK Trial Registry. SHould we emergently revascularize Occluded Coronaries for cardiogenic shocK? J Am Coll Cardiol. 2000;36:1077‑83. doi: 10.1016/s0735-1097(00)00873-1. [DOI] [PubMed] [Google Scholar]
- Mehta RH, Ou FS, Peterson ED, Shaw RE, Hillegass WB Jr, Rumsfeld JS, Roe MT American College of Cardiology-National Cardiovascular Database Registry Investigators. Clinical significance of post-procedural TIMI flow in patients with cardiogenic shock undergoing primary percutaneous coronary intervention. JACC Cardiovasc Interv. 2009;2:56–64. doi: 10.1016/j.jcin.2008.10.006. [DOI] [PubMed] [Google Scholar]
- Javanainen T, Sans-Roselló J, Harjola VP, Nieminen MS, Lassus J, Sionis A, Varpula M, Jurkko R. Prognostic impact of baseline and residual SYNTAX scores in cardiogenic shock. Catheter Cardiovasc Interv. 2019;93:1‑8. doi: 10.1002/ccd.27716. [DOI] [PubMed] [Google Scholar]
- Sleeper LA, Reynolds HR, White HD, Webb JG, Džavík V, Hochman JS. A severity scoring system for risk assessment of patients with cardiogenic shock: a report from the SHOCK Trial and Registry. Am Heart J. 2010;160:443‑50. doi: 10.1016/j.ahj.2010.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Variables associated with outcomes in univariate analysis.
Flow chart.
Early and late outcomes according to critical culprit lesion location adjusted for revascularisation strategy.
Early and late outcomes according to individual critical culprit lesion location.