Abstract
In patients with human granulocytic ehrlichiosis (HGE), the HGE agent has been seen only in the peripheral blood granulocytes, which have a life span too short for ehrlichial proliferation. To determine if the HGE agent delays the apoptosis of human peripheral blood neutrophils for its advantage, peripheral blood granulocytes consisting mostly of neutrophils were incubated with freshly freed host cell-free HGE agent in vitro. The HGE agent induced a significant delay in morphological apoptosis and the cytoplasmic appearance of histone-associated DNA fragments in the granulocytes. This antiapoptotic effect was dose dependent. Although much weaker than the HGE agent freshly freed from the host cells, noninfectious purified HGE agent stored frozen and thawed also had antiapoptotic effect, which was lost with proteinase K treatment but not with periodate treatment. Treatment of neutrophils with a transglutaminase inhibitor, monodansylcadaverine, blocked the antiapoptotic effect of the HGE agent. Addition of oxytetracycline, however, did not prevent or reverse the antiapoptotic effect of the HGE agent. These results suggest that binding of a protein component(s) of the HGE agent to neutrophils and subsequent cross-linking and/or internalization of the receptor and ehrlichiae are required for antiapoptotic signaling, but ehrlichial protein synthesis and/or proliferation is not required. MG-132, a proteasome inhibitor, and cycloheximide accelerated the apoptosis of neutrophils and overrode the antiapoptotic effect of the HGE agent. Studies with specific inhibitors suggest that protein kinase A, NF-κB, and interleukin 1β are not involved in the antiapoptotic mechanism of the HGE agent.
Human granulocytic ehrlichiosis (HGE) is a newly recognized tick-borne zoonosis in the United States and Europe (3, 5, 6, 8, 9). HGE is an acute febrile systemic disease associated with hematologic abnormalities, such as thrombocytopenia and leukopenia, as well as increased serum aminotransferase activity. HGE may be fatal when patients are immunocompromised or antibiotic treatment is delayed. The etiologic agent, called the HGE agent, is a small gram-negative coccus closely related to previously known granulocytotropic Ehrlichia spp.—Ehrlichia phagocytophila, the agent of tick-borne fever in sheep and goats, and Ehrlichia equi, the agent of equine ehrlichiosis (9). These bacteria are incapable of extracellular survival and are seen to replicate in the cytoplasm of peripheral blood granulocytes. A small number of individually dispersed ehrlichiae are difficult to recognize under the light microscope. However, microcolonies, called morulae, that result from ehrlichial replication stain dark blue to purple with Romanowsky dye; they are large and have a characteristic morphology and thus can be recognized by the trained eye (34). All 12 patients initially reported in Minnesota and Wisconsin had detectable morulae in 1 to 41% of their peripheral blood granulocytes (5). In another study, intracytoplasmic morulae were seen in 3 of 12 patients in New York, and the frequency of infected granulocytes ranged from 0.3 to 6% (3). The majority of infected granulocytes seen were neutrophils. Mature neutrophils undergo rapid apoptosis (programmed cell death), with a half-life of several hours in vivo (11). Considering the low growth rate of cytoplasmic ehrlichiae and the important roles neutrophils play in host defense and acute inflammation, the life span of neutrophils is a critical determinant for ehrlichial survival and HGE pathogenesis. Recently, the HGE agent was reported to induce apoptosis in the human promyelocytic leukemia cell line HL-60 (17). However, no information is available on whether the HGE agent modulates apoptosis of human neutrophils, its natural host. In the present study, we examined whether the HGE agent alters constitutive apoptosis of human peripheral blood neutrophils in vitro. Furthermore, mechanisms by which apoptosis is delayed by the HGE agent were examined by using an HGE agent treated with various reagents and host cells inhibited in intracellular signaling pathways. This study, therefore, is helpful in understanding not only the intracellular survival strategy of the HGE agent but also the role of neutrophils in the pathogenesis of HGE.
MATERIALS AND METHODS
HGE agent and cell culture.
HGE isolate HZ (37) was cultivated in HL-60 cells in RPMI 1640 medium (GIBCO, Grand Island, N.Y.) supplemented with 5% fetal bovine serum (Atlanta Biologicals, Norcross, Ga.), 2 mM l-glutamine (GIBCO), 0.1 mM minimal essential medium-nonessential amino acid mixture (GIBCO), and 1 mM minimal essential medium-sodium pyruvate (GIBCO). When >90% of the cells were infected, as determined by examining cells stained with Diff-Quik (Baxter Scientific Products, Obetz, Ohio), the infected cells were sonicated and centrifuged at 500 × g for 5 min. The supernatant was centrifuged at 10,000 × g for 10 min, and the pellet, containing the host cell-free, viable HGE agent, was immediately used to infect human peripheral blood neutrophils or peripheral blood leukocytes (PBL). Because the HGE agent is small and multiplies as microcolonies, it is impractical to accurately count individual organisms. Therefore, the number of host cell-free ehrlichiae was estimated by using the following formula: number of ehrlichial organisms = total infected cell number × average number of morulae in an infected cell (typically five) × average number of ehrlichial organisms in a morula (typically 19) × percentage of ehrlichiae recovered as host cell free (typically 50% as determined by using metabolically [35S]methionine-labeled ehrlichiae [36]).
Purified HGE agent was prepared by brief sonication, differential centrifugation, and Sephacryl S-1000 chromatography and was kept frozen at −80°C until it was used, as previously described (35). Periodate-treated ehrlichiae were prepared by incubating purified HGE agent with 20 mM sodium periodate (Sigma Chemical Co., St. Louis, Mo.) in 50 mM sodium acetate buffer (pH 4.5) for 1 h at room temperature in the dark followed by incubation with 50 mM sodium borohydride (Sigma) in sterile phosphate-buffered saline (PBS; 2.7 mM KCl–1.8 mM KH2PO4–137 mM NaCl–10 mM NaH2PO4, pH 7.4) for 30 min at room temperature (21, 22). For proteinase K treatment, the purified HGE agent was incubated in 1 mg of proteinase K (GIBCO)/ml in distilled water at 60°C for 2 h. After incubation, 1 mM phenylmethylsulfonyl fluoride (Sigma) was added, the mixture was incubated for 10 min at 60°C, and then the ehrlichiae were washed three times in RPMI 1640 medium (21, 22).
Neutrophils were isolated from buffy coats from healthy donors (Ohio Red Cross, Columbus, Ohio). The buffy coat was overlaid on double layers of Histopaque 1077 and 1119 (Sigma) and centrifuged at 700 × g for 15 min. Neutrophils at the interface between the 1077 and 1119 were collected and washed twice with PBS followed by hemolysis in 0.83% ammonium chloride (Fisher Scientific, Fair Lawn, N.J.) for 5 min at room temperature. Of the total cells, >98% were neutrophils in morphology, as demonstrated by Diff-Quik staining, and >98% were viable by the trypan blue dye exclusion test. PBL were obtained from buffy coats by simply lysing erythrocytes as described above.
Treatment.
After being washed twice, purified neutrophils or PBL were suspended at 106 cells/ml in RPMI 1640 medium. Uninfected HL-60 cells, prepared by brief sonication of the cell suspension at 106 cells/ml followed by differential centrifugation as previously described (21), and the purified HGE agent prepared as described above was added at a final concentration of 100 μg of protein/ml. Host cell-free HGE agent was added to purified neutrophils at a multiplicity of infection (MOI) of 100 immediately after the isolation of the HGE agent. Treated or infected neutrophil suspensions were seeded in 96-well flat-bottom plates (Becton Dickinson Co., Franklin Lakes, N.J.) at 200 μl/well in triplicate wells per assay point, and these samples were incubated at 37°C in a 5% CO2 atmosphere for ≤96 h. Cells were harvested every 8 h. To consider individual human variations, all experiments were independently repeated two or three times on different days using neutrophils derived from different donors and the freshly prepared host cell-free HGE agent each time. Donor cells were never mixed, and each donor neutrophil assay included positive and negative controls to ensure the quality of both neutrophil and HGE agent preparation.
For monodansylcadaverine (MDC) treatment, fresh human neutrophils were suspended in RPMI medium with or without 250 μM MDC, plated in triplicate at 2 × 106 cells/well in a 24-well plate, and incubated for 30 min at 37°C in 95% air–5% CO2. The freshly prepared host cell-free HGE agent in RPMI medium was added to each well, and the mixture was incubated for 2 h at 37°C. The cells were centrifuged at 500 × g for 5 min, treated with 2 mg of pronase/ml in sterile PBS for 5 min at 37°C to remove extracellular ehrlichiae, washed two times with RPMI medium, and replated in medium without MDC. In another experiment, cells were incubated continuously without removing MDC and the extracellular HGE agent. Oxytetracycline (10-μg/ml final concentration) was added to each well at 0 or 8 h after the addition of freshly prepared host cell-free HGE agent. The cells were incubated at 37°C and cytocentrifuged to determine the percent apoptosis and percent infected neutrophils.
H-89 (1 or 10 μM; BIOMOL Research Laboratories, Plymouth Meeting, Pa.), 50 μM genistein (Sigma), 100 μM MG-132 (BIOMOL), 100 μg of SN-50 (BIOMOL)/ml, 2 μg of cycloheximide (Sigma)/ml, and 50 ng of blocking monoclonal antibody to recombinant human interleukin 1β (IL-1β) (clone 8516.311; R & D Systems, Minneapolis, Minn.)/ml or 100 μM acetyl-Tyr-Val-Ala-Asp-chloromethyl ketone (YVAD-CMK) (Calbiochem, San Diego, Calif.) were added to triplicate wells at 0 h or 8 h (10 μM H-89 only) after the addition of HGE agent.
Microscopic determination of apoptosis.
Cell suspensions (80 μl) were centrifuged on a glass slide at 30 × g for 1 min with Cytospin 3 (Shandon Inc., Pittsburgh, Pa.). The cells were stained with Diff-Quik and examined microscopically at ×1,000. Apoptotic cells were determined based on their morphology, including densely condensed and homogeneous nuclei, loss of connection between the lobules of nuclei, and eosinophilic cytoplasm (Fig. 1). A total of 500 cells were scored from each well. The time required for 50% of the neutrophils to show morphological apoptosis (T50) was determined by plotting the percentage of apoptotic cells observed at each incubation time point.
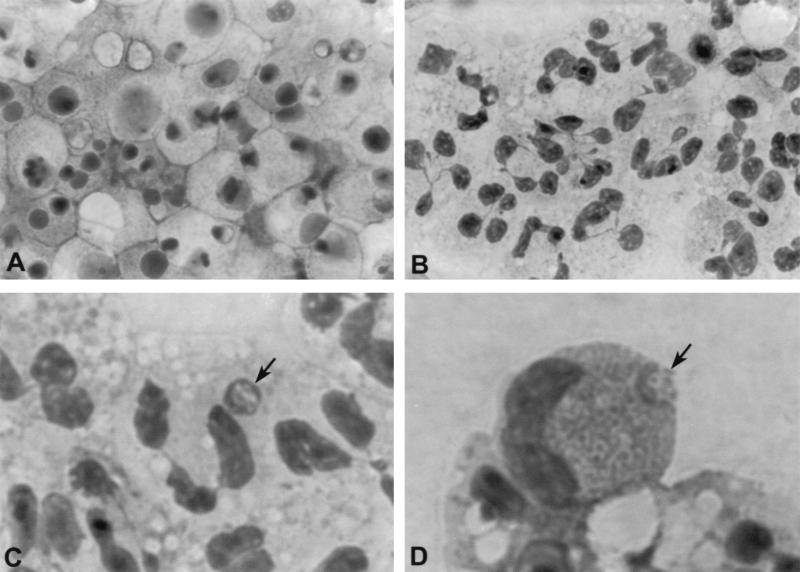
FIG. 1.
Morphological apoptosis of human peripheral blood neutrophils incubated in vitro for 24 h. (A) Uninfected neutrophils. (B) Neutrophils incubated with freshly prepared host cell-free HGE agent. (C) A morula (arrow) in a neutrophil incubated with freshly freed HGE agent for 24 h. (D) A morula (arrow) in an eosinophil incubated with freshly freed HGE agent for 24 h. Magnifications: A and B, ×630; C and D, ×1350.
Detection of histone-associated DNA fragments.
To detect the fragmentation of DNA in apoptotic neutrophils, histone-associated DNA fragments were examined by using a Cell Death Detection ELISA Plus kit (Boehringer Mannheim, Indianapolis, Ind.). This assay is based on the quantitative sandwich enzyme immunoassay principle, with mouse monoclonal antibodies directed against DNA and histones. Cell suspensions (80 μl) incubated in a 96-well plate for 8, 12, and 16 h were harvested and centrifuged at 200 × g for 10 min. The lysis buffer (200 μl) was added to the pellet and incubated at room temperature for 30 min. After centrifugation at 200 × g for 1 min, a sample of the supernatant was diluted 10-fold with lysis buffer, and 20 μl was applied in a well of the streptavidin-coated microtiter plate. An immunoreagent mixture (80 μl) containing anti-histone-biotin (4 μl), anti-DNA-peroxidase (4 μl), and incubation buffer (72 μl) was added to each well and incubated for 2 h at room temperature. After three washes with the incubation buffer, 100 μl of the substrate solution (2,2′-azino-di[3-ethylbenzthiazolin-sulfonate]) was added. The absorbances of samples at 405 nm and background at 490 nm were measured.
Statistical analysis.
Statistical significance compared with the addition of the medium alone as a control was determined by Student's t test with Sigmaplot version 4.0. A P value of <0.05 was considered significant.
RESULTS
Morphology.
As a first approach, we investigated whether the HGE agent could interfere with physiological apoptosis that occurs in mature neutrophils isolated from blood and cultured. The results of various treatment groups were compared using neutrophils prepared from a single donor for each experiment, and the experiments were repeated more than three times with neutrophils derived from different donors. Neutrophil apoptosis can be assessed by various parameters, including changes in cellular morphology. Apoptotic neutrophils have condensed and homogenous nuclei whose lobules have lost their connection in condensed eosinophilic cytoplasm (Fig. 1A). The percentage of apoptotic neutrophils by these criteria rapidly increased in untreated neutrophils after 8 h of incubation in vitro (Fig. 1A). Neutrophils treated with HL-60 cell lysate as a negative control had a rate of apoptosis similar to that without HL-60 cell lysate, indicating that HL-60 cell lysate (a fraction of which was present in the host cell-free HGE agent preparation because the HGE agent had been cultivated in HL-60 cells) does not influence in vitro apoptosis (Fig. 2). Almost 100% of untreated as well as HL-60 cell lysate-treated neutrophils were apoptotic after 24 h of incubation in vitro (Fig. 1 and 2). In contrast, most neutrophils incubated with freshly prepared host cell-free HGE agent for 24 h showed normal cytoplasm and lobulation of nuclei (Fig. 1B and 2). Of all neutrophils, >50% that were incubated with freshly freed HGE agent were not apoptotic for up to 48 h, and 10 to 20% of neutrophils were not apoptotic after 96 h of incubation. In these nonapoptotic cells, formation of typical ehrlichial morulae was observed after 24 h of incubation, although the neutrophils having morulae were approximately 5% of all neutrophils (Fig. 1C). Morulae were also observed in eosinophils present in the neutrophil preparation after 24 h of incubation (Fig. 1D). Eosinophil numbers varied widely among the blood donors, but they survived longer even after the apoptosis of a majority of neutrophils at 96 h, and morulae became larger in these cells.
FIG. 2.
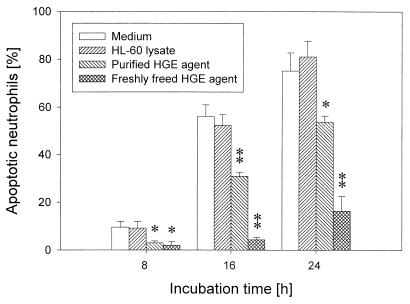
Time course of morphological apoptosis of peripheral blood neutrophils incubated in vitro with freshly prepared host cell-free or purified HGE agent. Incubation with freshly freed or purified HGE agent in vitro significantly (∗, P < 0.05; ∗∗, P < 0.01) delayed apoptosis of neutrophils compared with medium alone or HL-60 cell lysate control (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from more than three independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
The population of morphologically apoptotic cells increased in a time-dependent manner (Fig. 2). The T50 of neutrophils incubated with freshly freed HGE agent was 45.0 ± 9.8 h (n = 3), which was significantly longer than that of uninfected neutrophils, 12.2 ± 2.5 h (n = 3). The T50 of neutrophils incubated with HL-60 lysate did not significantly differ from that of untreated neutrophils (Fig. 2). The antigenic and molecular characteristics of the purified HGE agent preparation were described previously (20, 45, 46). Ehrlichiae lose their infectivity within 3 h after becoming extracellular (31). The purified HGE agent was noninfectious due to being host cell free for a long period of time and to the freezing and thawing procedure, but it consistently had a weak antiapoptotic effect (Fig. 2 to 5), indicating ehrlichial infection is not essential for the antiapoptotic effect. The T50 of neutrophils incubated with the purified HGE agent was 16.8 ± 4.1 h (n = 3). All figures show the results of independent experiments performed on different days with different pairs of donor cells and freshly prepared host cell-free HGE agent.
FIG. 5.
Morphological apoptosis of human neutrophils incubated in vitro with proteinase K- or periodate-treated purified HGE agent. Treatment of purified HGE agent with proteinase K completely eliminated the antiapoptotic effect of the purified HGE agent. However, periodate treatment did not change the antiapoptotic effect of the purified HGE agent. The results were significantly (∗, P < 0.05; ∗∗, P < 0.01) different from those with medium alone (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from three independent experiments performed, each with neutrophils derived from different donors.
Detection of cytoplasmic histone-associated DNA fragments.
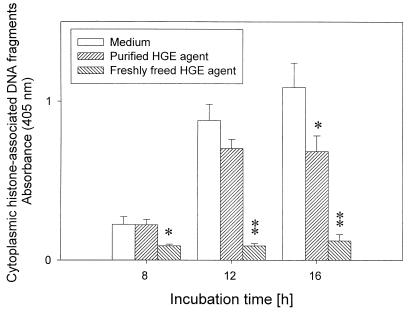
Internucleosomal DNA fragmentation in apoptosis appears as detectable histone-associated DNA fragments in the cytoplasm of apoptotic cells, after enrichment of mono- and oligonucleosomes in the cytoplasm of the apoptotic cells caused by DNA degradation occurring several hours before plasma membrane breakdown (15). Because morphological apoptotic changes in uninfected neutrophils were observed in >50% of untreated neutrophils after ∼12 h of incubation, we examined the numbers of these DNA fragments after 8 and 16 h of incubation (Fig. 3). Untreated cells revealed rapid increases in cytoplasmic histone-associated DNA fragments starting at 12 h of incubation, which were paralleled by an increase in morphologically apoptotic cells (Fig. 2). DNA fragments of neutrophils infected with freshly prepared host cell-free HGE agent did not increase up to a 16-h incubation period. The purified HGE agent had a weak but significant inhibitory effect on apoptosis at 16 h postincubation (Fig. 3). Chromatin fragmentation by internucleosomal endonuclease during apoptosis was also examined by the conventional agarose gel electrophoresis of DNA extracted from neutrophils. Although the typical “ladder patterns” were detectable in control neutrophils, but not in HGE agent-infected neutrophils, at more than 24 h of incubation, the assay was not quantitative and was less sensitive than the detection of cytoplasmic histone-associated DNA fragments (data not shown).
FIG. 3.
Enzyme-linked immunosorbent assay quantitation of histone-associated DNA fragments in the cytoplasm of human neutrophils incubated in vitro with freshly prepared host cell-free or purified HGE agent. Neutrophils incubated with freshly freed or purified HGE agent had significantly (∗, P < 0.05; ∗∗, P < 0.01) increased cytoplasmic histone-associated DNA fragments compared with neutrophils incubated with medium alone (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from three independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
Dose response.
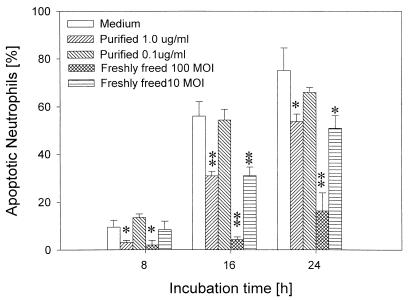
The inhibitory effects on apoptosis of purified and freshly prepared host cell-free HGE agents were dose dependent (Fig. 4). The purified HGE agent at 1 μg of protein/ml significantly inhibited the morphological apoptosis of neutrophils, but at <0.1 μg/ml there was no effect. The level of inhibitory effect with freshly freed HGE agent at an MOI of 10 was comparable to that of 1 μg of protein/ml of the purified HGE agent.
FIG. 4.
Morphological apoptosis of human neutrophils incubated in vitro with different dosages of freshly prepared host cell-free or purified HGE agent. Freshly freed HGE agent added at an MOI of 100 or 10 or the purified HGE agent added at 1 μg of protein/ml significantly (∗, P < 0.05; ∗∗, P < 0.01) delayed apoptosis of neutrophils compared with medium alone (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from three independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
Treatment of HGE agent with proteinase K or periodate.
To examine the requirement for ehrlichial protein(s) or carbohydrate(s) in the antiapoptotic effect, the purified HGE agent was treated with 1 mg of proteinase K/ml or 20 mM sodium periodate. The HGE agent treated with proteinase K completely lost its inhibitory effect, whereas periodate treatment had no effect (Fig. 5). The results indicates that ehrlichial proteins are required for antiapoptotic effect.
Effects of MDC and oxytetracycline.
Previous results in our laboratory have shown that internalization and infection of Ehrlichia risticii in P388D1 cells and of Ehrlichia chaffeensis and the HGE agent in THP-1 cells (7, 26, 31, 36) was inhibited by the reversible transglutaminase inhibitor MDC. Transglutaminase catalyzes the formation of ɛ-(γ-glutamyl)-lysine between protein molecules by coupling amines and diamines to the γ-carboxyl residue of glutamine (23). Receptor-mediated endocytosis of various ligands has been shown to be inhibited by transglutaminase inhibitors (23), suggesting an involvement of transglutaminase and receptor-mediated endocytosis in ehrlichial uptake. Therefore, we utilized MDC to determine whether internalization of the HGE agent is required to prevent apoptosis. MDC blocked the antiapoptotic effect of the host cell-free HGE agent: the means and standard deviations of the percentage of apoptotic neutrophils were 49.9 ± 4.4 for the medium control, 54.9 ± 1.8 for the medium-plus-MDC control, 25.5 ± 0.8 with the host cell-free HGE agent, and 45.1 ± 3.4 with the host cell-free HGE agent plus MDC (n = 3) when MDC and the extracellular HGE agent were removed after 2 h of incubation and continuously incubated for 24 h. When MDC and the extracellular HGE agent were removed at 2 h, the means and standard deviations of the percentage of infected cells at 24 h were 0.9 ± 0.4 with MDC treatment and 6.2 ± 0.2 (n = 3) without MDC, indicating that MDC blocked internalization of the HGE agent. Similar results were obtained when MDC and the extracellular HGE agent were not removed at 2 h and were kept throughout the incubation period. These results suggest that clustering and/or internalization of the ehrlichiae and their receptors is required for the inhibition of apoptosis.
To evaluate whether new protein synthesis or intracellular proliferation of ehrlichiae is required to inhibit neutrophil apoptosis, 10 μg of oxytetracycline/ml was added to the cell suspension at 0 or 8 h after the addition of freshly prepared host cell-free HGE agent. This concentration of oxytetracycline completely inhibited the proliferation of the HGE agent but had no effect on apoptosis of neutrophils in either the presence or absence of the HGE agent (Fig. 6 [data at 0 h after addition is shown]). Oxytetracycline (100 μg/ml) added at 8 h also did not have any influence on the inhibition of apoptosis by the HGE agent. Ehrlichial morulae were not seen in these neutrophils treated with oxytetracycline after up to 48 h of incubation. The result suggests that ehrlichial new protein synthesis and/or intracellular proliferation is not required for inducing or maintaining the inhibition of neutrophil apoptosis.
FIG. 6.
Effect of oxytetracycline on morphological apoptosis of human neutrophils incubated with freshly prepared host cell-free HGE agent. Oxytetracycline (OTC) added at 0 h did not block the inhibition of apoptosis of neutrophils by the HGE agent (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from three independent experiments performed, each with neutrophils derived from different donors and freshly prepared HGE agent.
Effects of H-89.
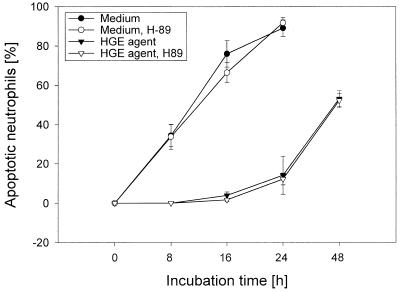
The cyclic AMP (cAMP) analog 8-CTP-cAMP was shown to delay the spontaneous and cycloheximide- or anti-FAS-induced apoptosis of human neutrophils in vitro (32). cAMP-elevating agents—prostaglandins and the phosphodiesterase type IV inhibitor RO 20-1724—were shown to inhibit neutrophil apoptosis, and treatment of human peripheral blood neutrophils with 1 μM H-89, a selective inhibitor of protein kinase A (10), prevented prostaglandin E2- and RO 20-1724-induced inhibition of cell apoptosis (30). To investigate whether the host cell protein kinase A is involved in the inhibition of apoptosis of neutrophils by the HGE agent, H-89 (1 μM at 0 h or 10 μM at 8 h postinfection) was added. The apoptosis of neutrophils in the presence or absence of the viable HGE agent did not change with this concentration of H-89 (Fig. 7). A concentration of H-89 of >10 μM present for >48 h was toxic to neutrophils. The result suggests that delayed in vitro apoptosis of neutrophils by the HGE agent is not mediated by protein kinase A activation.
FIG. 7.
Effect of a protein kinase A inhibitor, H-89, on morphological apoptosis of human neutrophils incubated with freshly prepared host cell-free HGE agent. Treatment with 1 μM H-89 added at 0 h had no influence on morphological apoptosis of neutrophils in the presence or absence of the freshly freed HGE agent (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from three independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
Effects of genistein, MG-132, and SN-50.
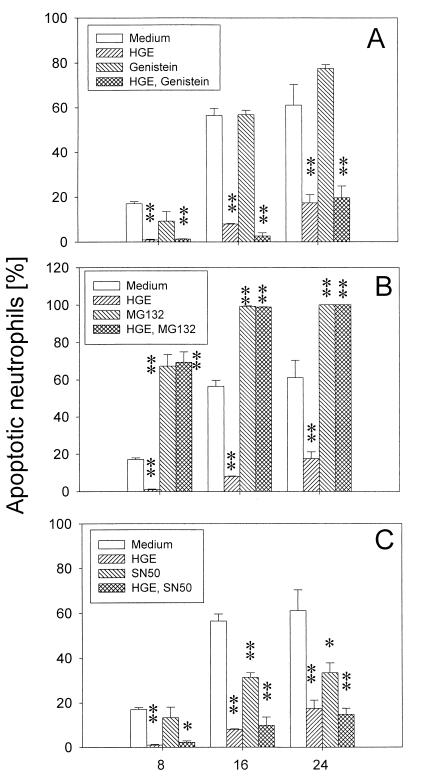
Lipopolysaccharide (LPS)- or granulocyte-macrophage colony-stimulating factor (GM-CSF)-induced delay in the spontaneous apoptosis of human neutrophils was reported to be blocked by a tyrosine kinase inhibitor, herbimycin A, or by pyrrolidine dithiocarbamate (40). Both herbimycin A and pyrrolidine dithiocarbamate inhibit NF-κB activation in human granulocytes in response to LPS (40). To investigate whether NF-κB activation was involved in the delayed apoptosis of neutrophils by the HGE agent, effects of 50 μM genistein, a tyrosine kinase inhibitor (4); 100 mM MG-132, a cell-permeable peptide-aldehyde protease inhibitor that blocks NF-κB activation via its effect on the proteasome (18); and 100 μg of SN-50/ml, a cell-permeable inhibitory peptide of nuclear translocation of NF-κB (24), were examined. Genistein had no effect on apoptosis of neutrophils in the absence or presence of the HGE agent (Fig. 8A). MG-132 accelerated the apoptosis of neutrophils regardless of the presence of the HGE agent. Of both infected and uninfected neutrophils, ∼100% became morphologically apoptotic after 16 h of incubation in vitro. This suggests that degradation of some host proteins by proteasomes is required for the inhibition of the apoptotic process in both normal and HGE agent-infected neutrophils (Fig. 8B). On the other hand, 100 μg of SN-50/ml slightly delayed apoptosis of uninfected neutrophils, but the percentage of morphologically apoptotic neutrophils infected with the HGE agent was the same whether SN-50 was present in the medium or not (Fig. 8C). These results suggest that NF-κB is not involved in delaying apoptosis of neutrophils by the HGE agent.
FIG. 8.
Effect of genistein, MG-132, and SN-50 on delayed apoptosis of neutrophils incubated with freshly prepared host cell-free HGE agent. (A) Treatment of neutrophils with 50 μM genistein had no effect on the apoptosis of neutrophils in the absence or presence of the HGE agent. (B) MG-132 (100 mM) significantly (∗, P < 0.05; ∗∗, P < 0.01 compared with medium alone) accelerated the apoptosis of neutrophils regardless of the presence of the HGE agent. (C) SN-50 (100 μg/ml) slightly (∗, P < 0.05 compared to medium alone) delayed apoptosis of uninfected neutrophils but not that of infected neutrophils (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from two independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
Effects of inhibitors of the IL-1β pathway.
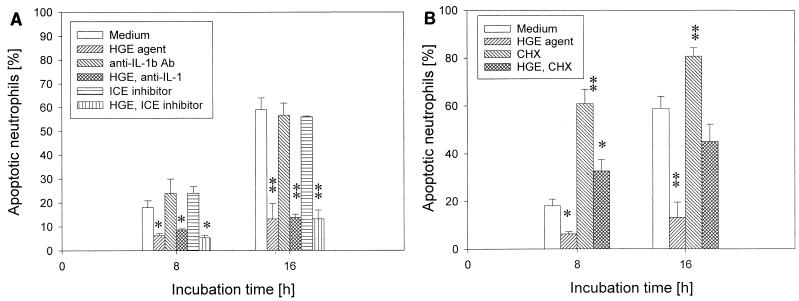
Our results showed that the HGE agent induces IL-1β expression in PBL and neutrophils (H.-Y. Kim and Y. Rikihisa, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. D/B-129, p. 234, 1999). Two different proinflammatory stimuli, LPS and GM-CSF, upregulate the expression of IL-1β-converting enzyme, also known as caspase-1, and delay the apoptosis of neutrophils. The delay is blocked by blocking antibody to IL-1β or a caspase-1 inhibitor (42). Therefore, we examined whether endogenous IL-1β generated by neutrophils in response to the HGE agent or caspase-1 is involved in inhibition of neutrophil apoptosis by the HGE agent by adding blocking anti-human IL-1β antibody or an irreversible tetrapeptide inhibitor of caspase-1, YVAD-CMK (39), to the assay system. There was no change in the apoptosis of neutrophils regardless of the presence of the HGE agent (Fig. 9A).
FIG. 9.
Effect of inhibitors of the IL-1β pathway or cycloheximide on delayed apoptosis of neutrophils incubated with freshly prepared host cell-free HGE agent. (A) There was no change in the inhibition of neutrophil apoptosis by the HGE agent after adding 50 ng of blocking anti-human IL-1β antibody/ml or 100 μM YVAD-CMK (caspase-1 inhibitor) to the assay system. (B) Cycloheximide (CHX) (2 μg/ml), a eukaryotic protein synthesis inhibitor, accelerated apoptosis regardless of whether the HGE agent was present or not (n = 3 assays using neutrophils derived from a single donor). ∗, P < 0.05; ∗∗, P < 0.01 compared with medium alone. A representative experiment is shown from two independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
Cycloheximide, a eukaryotic protein synthesis inhibitor, is known to enhance the apoptosis of neutrophils in vitro (32). Cycloheximide has no effect on NF-κB activation but blocks the antiapoptotic effect of LPS and GM-CSF by inhibiting IL-1β and caspase-1 upregulation (42). Cycloheximide treatment accelerated apoptosis regardless of the presence of the HGE agent (Fig. 9B).
Effect of HGE agent on apoptosis of neutrophils in PBL.
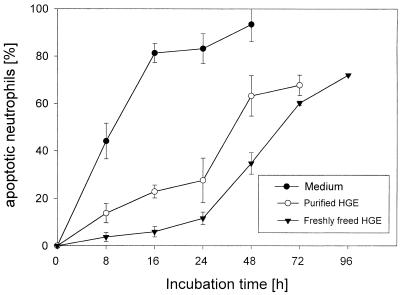
Because other leukocytes, such as monocytes and lymphocytes, which coexist in the blood influence neutrophil apoptosis through cytokines and cell-cell interactions (11), we examined whether the coexistence of other cell types in the blood influences the inhibition of neutrophil apoptosis by the HGE agent in vitro. Freshly prepared host cell-free HGE agent was more effective in inhibiting apoptosis of neutrophils in PBL, which consisted of ∼60% neutrophils, than in purified neutrophils (Fig. 10). The purified HGE agent was less effective than host cell-free HGE agent but was more effective in inhibiting apoptosis of neutrophils in PBL than in purified neutrophils. The rate of apoptosis of uninfected neutrophils was, however, similar in PBL and purified neutrophil preparation.
FIG. 10.
Effect of the HGE agent on apoptosis of neutrophils in PBL. Freshly prepared host cell-free HGE agent was effective in inhibiting apoptosis of neutrophils in PBL. The purified HGE agent was less effective than freshly freed HGE agent but was more effective in inhibiting apoptosis of neutrophils in PBL than in purified neutrophil preparations (n = 3 assays using neutrophils derived from a single donor). A representative experiment is shown from more than three independent experiments performed, each with neutrophils derived from different donors and freshly prepared host cell-free HGE agent.
DISCUSSION
The host of the HGE agent is the neutrophil, a suicidal effector cell equipped with the most powerful antibacterial armamentarium. Since ehrlichiae die if they remain extracellular, the HGE agent must enter neutrophils. Once it is intracellular, the HGE agent has solved the problem of lysosomal destruction by inducing the formation of a unique membrane-bound niche, a parasitophorus vacuole which does not fuse with lysosomes (28). However, normal neutrophils survive for a limited time in the peripheral blood. Unless the neutrophil's life span is extended, the intracellular HGE agent will die together with the host cell before having the chance to proliferate. The present study revealed that the HGE agent delays the apoptosis of human peripheral blood neutrophils sufficiently to allow intracellular proliferation of the HGE agent in vitro, resulting in a significant morula formation in 24 to 48 h of incubation at a level comparable to those seen in patients with HGE (3, 5). The actual percentage of infected cells may be greater, since by using immunolabeling and flow cytometry we previously found that almost 100% of P388D1 cells take up E. risticii at low levels at 3 h postincubation (26), although by Diff-Quik staining this low level of intracellular ehrlichiae is not apparent (31).
Although small, condensed, elementary-body-like and large, light, reticular body-like ehrlichiae have been seen (34), a chlamydia-like developmental cycle or eclipse stage has not been demonstrated in ehrlichiae. However, when we follow the time course of in vitro ehrlichia infection in a leukemia cell line, during the first day of culture we can seldom see infected cells (lag phase). After day 2 to 3 days of culture, logarithmic growth occurs, and 100% of the cells are infected with a large number of organisms by day 5 to 7 of culture, after which the cells are lysed (31). Since even with the delay the apoptosis of infected neutrophils takes place prior to complete ehrlichial proliferation and host cell lysis, the HGE agent in neutrophils must be horizontally transmitted to the next generation of neutrophils prior to host cell apoptosis in order to survive. This may be one reason why heavily infected neutrophils are rarely seen in patients. In the present study, nearly all neutrophils could be prevented from undergoing rapid apoptosis when stimulated with the host cell-free HGE agent at an MOI of 100. Delaying apoptosis of all neutrophils is advantageous for the HGE agent, because it gives more time for the HGE agent to survive and replicate inside neutrophils and to enhance the chance of its intercellular spreading. How the HGE agent spreads from infected to uninfected cells is unknown. Infected neutrophils were rarely seen filled with the HGE agent and/or lysed. Spreading of monocytic ehrlichiae can occur without lysis of the infected host cells (36), probably by ehrlichial exocytosis from the infected cells followed by endocytosis of freed ehrlichiae by other cells (36). The HGE agent may be transmitted by a similar mechanism from infected to uninfected neutrophils after brief intracellular proliferation.
Previous observations with other granulocytotropic ehrlichiae support our observation. In vitro incubation of peripheral blood granulocytes from dogs experimentally infected with Ehrlichia ewingii or heparinized whole blood from sheep experimentally infected with E. phagocytophila results in an increase in the proportion of infected neutrophils and the number of morulae in infected cells even after 2 to 4 days or 24 h, respectively (43, 44), suggesting that these infected granulocytes survive for a longer period in vitro to allow for the growth of ehrlichiae. Our result was the opposite of a previous report (17). This difference might be due to a difference in host cells, since the previous study used an immortalized leukemia cell line, HL-60.
As has been seen in patients' blood (3), we found that eosinophils become infected with the HGE agent and survive much longer than neutrophils in vitro. Eosinophils display a similar capacity to undergo constitutive apoptosis when aged in vitro, but this process is much slower than that observed for the neutrophil and is differentially regulated; for example, it is stimulated rather than inhibited by corticosteroids (25). Eosinophils, therefore, may potentially serve as a reservoir for the increasing numbers of HGE agents for longer periods of time than do neutrophils, in order to infect circulating neutrophils. Several cytokines, such as granulocyte CSF (1), GM-CSF, IL-1β (13, 42), IL-2 (11, 33), gamma interferon (IFN-γ) (13), and IL-8 (19), are reported to delay apoptosis of neutrophils in vitro, but IL-6, tumor necrosis factor alpha (TNF)-α, and IL-10 are reported to accelerate neutrophil apoptosis (2, 11, 40). Although significant levels of TNF-α and IL-6 were generated by PBL in response to the HGE agent in vitro (H.-Y. Kim and Y. Rikihisa, Abstr. 99th Gen. Meet. Am. Soc. Microbiol. 1999, abstr. D/B-129, p. 234, 1999), in the present study neutrophils in PBL, which may be closer to the in vivo situation, survived as long as purified neutrophils in vitro, and the effect of the HGE agent in delaying apoptosis of neutrophils is greater in the presence of other leukocytes, indicating that the influence of the HGE agent is reproducible and the presence of other leukocyte populations does not override or cancel the influence.
Unlike E. coli, which accelerates the apoptosis of neutrophils (41), it is becoming clear that several intracellular microorganisms delay apoptosis of host cells. Detailed signaling pathways for the inhibition of apoptosis, however, are not yet known for any of these agents. Rickettsia rickettsii (12) activates NF-κB to inhibit the apoptotic process of the host endothelial cells. NF-κB is expressed in human neutrophils, and inhibition of an inducible form of NF-κB is linked to the induction of neutrophil apoptosis (40). Our present study, however, suggests that NF-κB activation is not involved in delaying apoptosis of neutrophils by the HGE agent. Mycobacterium tuberculosis, which has a longer doubling time than the HGE agent, at low numbers delays apoptosis of human monocytes in vitro (16). The inhibition is partially neutralized with anti-TNF-α antibodies, suggesting that TNF-α partially mediates the antiapoptotic effect of M. tuberculosis. Unlike monocytes, TNF-α is known to induce rapid apoptosis of human peripheral blood neutrophils in vitro (11, 40). An intracellular infection by the protozoan parasite Toxoplasma gondii counteracts apoptosis of murine lymphoma cell lines induced by several kinds of stimuli, such as Fas ligation, granzyme B, γ- or UV irradiation, and calcium ionophores (29), suggesting that a mechanism common to many apoptotic pathways is involved. In contrast to the HGE agent, protection against apoptosis by T. gondii requires the continued presence of live organisms and ongoing protein synthesis (29). The intracellular protozoan parasite Leishmania donovani or treatment with lipophosphoglycan, the major surface molecule of the Leishmania promastigote, also inhibits mouse bone marrow macrophage apoptosis induced by removal of macrophage CSF in vitro (27). Although exogenous TNF-α inhibits apoptosis in this assay system and Leishmania infection induces TNF-α secretion, the inhibition was not restored by anti-TNF-α-neutralizing antibodies. As far as we know, the HGE agent is the first infectious agent known to delay apoptosis of neutrophils, and the antiapoptotic mechanism of the HGE agent appears to be different from the mechanisms of other intracellular microorganisms. Therefore, the HGE agent may serve as a new tool for analysis of the apoptotic mechanism of neutrophils.
Because the noninfectious purified HGE agent had an antiapoptotic effect and because inhibition of ehrlichial protein synthesis or proliferation did not prevent or turn off the antiapoptotic signal, infection per se is not essential for delaying apoptosis. However, the protein residue of the HGE agent, rather than carbohydrates, is required for the inhibition. When monocytic ehrlichiae are treated with trypsin, a milder proteolytic enzyme than proteinase K, ehrlichial binding and subsequent internalization in host cells is prevented (26). In addition, our MDC study showed that ehrlichial internalization and/or receptor cross-linking is required for apoptosis inhibition. The inhibitory effect of the HGE agent on apoptosis was dose dependent. These results suggest that initial occupation and cross-linking of host cell receptors by preformed proteins of the HGE agent may be sufficient to trigger the antiapoptotic signal. The reason the freshly prepared host cell-free HGE agent had stronger antiapoptotic activity than the purified HGE agent may be that the structural or conformational integrity present in the freshly prepared host cell-free HGE agent, which is lost in the purified HGE agent, is required for effective cross-linking of receptors or internalization.
E. chaffeensis, upon binding to THP-1 cells, increases protein kinase A activity 25-fold within 30 min and inhibits tyrosine phosphorylation of Jak-1 and -2 and Stat1α in response to IFN-γ. This inhibition does not require the internalization of E. chaffeensis in THP-1 cells or the carbohydrate residue of the organism, but binding of the protein of E. chaffeensis to the host cells is required (22). It appears that these conditions are similar to those required for delaying apoptosis by the HGE agent. However, we did not find the involvement of protein kinase A activation in apoptosis delay by the HGE agent. Similarly, inhibition of basal protein kinase A activity by 25 μM H-89 has no influence on (does not accelerate) spontaneous or cycloheximide- or anti-Fas-induced neutrophil apoptosis (32), although conditions that raise intracellular cAMP are shown to delay spontaneous neutrophil apoptosis and inhibit apoptosis induced by cycloheximide or anti-Fas (30, 32). Additionally, because host cell interactions with E. chaffeensis and the HGE agent differ in several respects, such as the intracellular compartments they occupy (28) and upregulation of host transferrin receptor mRNA (7) and cytokine mRNA expression (21; Kim and Rikihisa, Abstr. 99th Gen. Meet. Am. Soc. Microbiol), host cell receptors and intracellular signaling pathways may be different. The antiapoptotic mechanism may share the signaling pathway with dexamethasone-induced apoptosis, because suppression of apoptosis by both dexamethasone and the HGE agent is abolished by cotreatment with cycloheximide (14).
The HGE agent might have LPS, since it belongs to the gram-negative bacteria. E. coli LPS is reported to delay apoptosis of neutrophils in vitro (38, 42). Inhibition of apoptosis of neutrophils by bacterial LPS is mediated by induced pro-IL-1β and caspase-1 through protein tyrosine phosphorylation-dependent activation of NF-κB (38, 42). IL-1β is known to delay apoptosis of neutrophils (42), and IL-1β was also induced in neutrophils exposed to the HGE agent (Kim and Rikihisa, Abstr. 99th Gen. Meet. Am. Soc. Microbiol.). However, IL-1β and NF-κB do not appear to be involved in inhibition of apoptosis by the HGE agent. This suggests that the HGE agent either lacks LPS or the structure and biological activity of ehrlichial LPS is distinct from those of E. coli LPS.
Delayed apoptosis of neutrophils may help ehrlichial proliferation and prolong proinflammatory cytokine generation, making patients more ill and thus prone to hospitalization. In agreement with this speculation, Bakken et al. reported a higher percentage of neutrophils in the PBL of hospitalized than nonhospitalized HGE patients (6). Elucidation of an ehrlichial factor(s) and the signaling pathway in the neutrophils that inhibit apoptosis would be important in understanding the pathogenesis of HGE. The HGE agent or its protein components may also serve as a tool in analyzing the fundamental apoptotic mechanisms of neutrophils.
ACKNOWLEDGMENTS
This research was supported by grant RO1AI30010 from the National Institutes of Health. K. Yoshiie was supported by a fellowship from The Japan Health Sciences Foundation.
REFERENCES
- 1.Adachi S, Kubota M, Lin Y W, Okuda A, Matsubara K, Wakazono Y, Hirota H, Kuwakado K, Akiyama Y. In vivo administration of granulocyte colony-stimulating factor promotes neutrophil survival in vitro. Eur J Haematol. 1994;53:129–134. doi: 10.1111/j.1600-0609.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- 2.Afford S C, Pongracz J, Stockley R A, Crocker J, Burnett D. The induction by human interleukin-6 of apoptosis in the promonocytic cell line U937 and human neutrophils. J Biol Chem. 1992;267:21612–21616. [PubMed] [Google Scholar]
- 3.Aguero-Rosenfeld M E, Horowitz H W, Wormser G P, McKenna D F, Nowakowski J, Munoz J, Dumler J S. Human granulocytic ehrlichiosis: a case series from a medical center in New York State. Ann Intern Med. 1996;125:904–908. doi: 10.7326/0003-4819-125-11-199612010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Akiyama T, Ogawara H. Use and specificity of genistein as inhibitor of protein-tyrosine kinases. Methods Enzymol. 1991;201:362–370. doi: 10.1016/0076-6879(91)01032-w. [DOI] [PubMed] [Google Scholar]
- 5.Bakken J S, Dumler J S, Chen S M, Eckman M R, van Etta L L, Walker D H. Human granulocytic ehrlichiosis in the upper Midwest United States. a new species emerging? JAMA. 1994;272:212–218. [PubMed] [Google Scholar]
- 6.Bakken J S, Krueth J, Wilson-Nordskog C, Tilden R L, Asanovich K, Dumler J S. Clinical and laboratory characteristics of human granulocytic ehrlichiosis. JAMA. 1996;275:199–205. [PubMed] [Google Scholar]
- 7.Barnewall R, Ohashi N, Rikihisa Y. Ehrlichia chaffeensis and E. sennetsu, but not the Human granulocytic ehrlichiosis agent, colocalize with transferrin receptor and up-regulate transferrin receptor mRNA by activating iron-responsive protein 1. Infect Immun. 1999;67:2258–2265. doi: 10.1128/iai.67.5.2258-2265.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brouqui P. Ehrlichiosis in Europe. In: Raoult D, Brouqui P, editors. Rickettsiae and rickettsial diseases at the turn of the third millennium. Paris, France: Elsevier; 1999. pp. 220–232. [Google Scholar]
- 9.Chen S M, Dumler J S, Bakken J S, Walker D H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J Clin Microbiol. 1994;32:589–595. doi: 10.1128/jcm.32.3.589-595.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino) ethyl] 5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 11.Christa H E, Homburg B, Roos D. Apoptosis of neutrophils. Curr Opin Hematol. 1996;3:94–99. doi: 10.1097/00062752-199603010-00014. [DOI] [PubMed] [Google Scholar]
- 12.Clifton D R, Goss R A, Sahni S K, Antwerp D V, Baggs R B, Marder V J, Silverman D J, Sporn L A. NF-κB-dependent inhibition of apoptosis is essential for host cell survival during Rickettsia rickettsii infection. Proc Natl Acad Sci USA. 1998;95:4646–4651. doi: 10.1073/pnas.95.8.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colotta F, Re F, Polentarutti N, Sozzani S, Mantovani A. Modulation of granulocyte survival and programmed cell death by cytokines and bacterial products. Blood. 1992;80:2012–2020. [PubMed] [Google Scholar]
- 14.Cox G, Gauldie J, Jordana M. Dexamethasone-induced suppression of apoptosis in human neutrophils requires continuous stimulation of new protein synthesis. J Leukoc Biol. 1992;61:224–230. doi: 10.1002/jlb.61.2.224. [DOI] [PubMed] [Google Scholar]
- 15.Duke R C, Cohen J J. IL-2 addiction: withdrawal of growth factor activates a suicide program in dependent T cells. Lymphokine Res. 1986;5:289–299. [PubMed] [Google Scholar]
- 16.Durrbaum-Landmann I, Gercken J, Flad H-D, Ernst M. Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect Immun. 1996;64:5384–5389. doi: 10.1128/iai.64.12.5384-5389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hsieh T-C, Aguero-Rosenfeld M E, Wu J M, Ng C, Papanikolaou N A, Varde S A, Schwartz I, Pizzolo J G, Melamed M, Horowitz H W, Nadelman R B, Wormser G P. Cellular changes and induction of apoptosis in human promyelocytic HL-60 cells infected with the agent of human granulocytic ehrlichiosis (HGE) Biochem Biophys Res Commun. 1997;232:298–303. doi: 10.1006/bbrc.1997.6276. [DOI] [PubMed] [Google Scholar]
- 18.Jensen T J, Loo M A, Pind S, Williams D B, Goldberg A L, Riordan J R. Multiple proteolytic systems, including the proteasome, contribute to CFTR processing. Cell. 1995;83:129–135. doi: 10.1016/0092-8674(95)90241-4. [DOI] [PubMed] [Google Scholar]
- 19.Kettritz R, Gaido M L, Haller H, Luft F C, Jennette C J, Falk R J. Interleukin-8 delays spontaneous and tumor necrosis factor-α-mediated apoptosis of human neutrophils. Kidney Int. 1998;53:84–91. doi: 10.1046/j.1523-1755.1998.00741.x. [DOI] [PubMed] [Google Scholar]
- 20.Kim H-Y, Rikihisa Y. Characterization of monoclonal antibodies against the major outer membrane protein of human granulocytic ehrlichiosis agent. J Clin Microbiol. 1998;36:3278–3284. doi: 10.1128/jcm.36.11.3278-3284.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee E H, Rikihisa Y. Absence of tumor necrosis factor-α, interleukin-6 (IL-6), and granulocyte-macrophage colony-stimulating factor expression but presence of IL-1β, IL-8, and IL-10 expression in human monocytes exposed to viable or killed Ehrlichia chaffeensis. Infect Immun. 1996;64:4211–4219. doi: 10.1128/iai.64.10.4211-4219.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee E H, Rikihisa Y. Protein kinase A-mediated inhibition of gamma interferon-induced tyrosine phosphorylation of Janus kinases and latent cytoplasmic transcription factors in human monocytes by Ehrlichia chaffeensis. Infect Immun. 1998;66:2514–2520. doi: 10.1128/iai.66.6.2514-2520.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levitzki A, Willingham M, Pastan I. Evidence for participation of transglutaminase in receptor-mediated endocytosis. Proc Natl Acad Sci USA. 1980;77:2706–2710. doi: 10.1073/pnas.77.5.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin Y, Yao S, Veach R A, Torgerson T R, Hawiger J. Inhibition of nuclear translocation of transcription factor NF-κB by a synthetic peptide containing a cell membrane-permeable motif and nuclear localization sequence. J Biol Chem. 1995;270:14255–14258. doi: 10.1074/jbc.270.24.14255. [DOI] [PubMed] [Google Scholar]
- 25.Meagher L C, Cousin J M, Seckl J R, Haslett C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J Immunol. 1996;156:4422–4428. [PubMed] [Google Scholar]
- 26.Messick J B, Rikihisa Y. Characterization of Ehrlichia risticii binding, internalization, and growth in host cells by flow cytometry. Infect Immun. 1993;61:3803–3810. doi: 10.1128/iai.61.9.3803-3810.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moore K J, Matlashewski G. Intracellular infection by Leishmania donovani inhibits macrophage apoptosis. J Immunol. 1994;152:2930–2937. [PubMed] [Google Scholar]
- 28.Mott J, Barnewall R, Rikihisa Y. Human granulocytic ehrlichiosis agent and Ehrlichia chaffeensis reside in different cytoplasmic compartments in HL-60 cells. Infect Immun. 1999;67:1368–1378. doi: 10.1128/iai.67.3.1368-1378.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nash P B, Purner M B, Leon R P, Clarke P, Duke R C, Curiel T J. Toxoplasma gondii-infected cells are resistant to multiple inducers of apoptosis. J Immunol. 1998;160:1824–1830. [PubMed] [Google Scholar]
- 30.Ottonello L, Gonella R, Dapino P, Sacchetti C, Dallegri F. Prostaglandin E2 inhibits apoptosis in human neutrophilic polymorphonuclear leukocytes: role of intracellular cyclic AMP levels. Exp Hematol. 1998;26:895–902. [PubMed] [Google Scholar]
- 31.Park J, Rikihisa Y. Inhibition of Ehrlichia risticii infection in murine peritoneal macrophages by gamma interferon, a calcium ionophore, and concanavalin A. Infect Immun. 1991;59:3418–3423. doi: 10.1128/iai.59.10.3418-3423.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parvathenani L K, Buescher S, Chacon-Cruz E, Beebe S J. Type I cAMP-dependent protein kinase delays apoptosis in human neutrophils at a site upstream of caspase-3. J Biol Chem. 1998;273:6736–6743. doi: 10.1074/jbc.273.12.6736. [DOI] [PubMed] [Google Scholar]
- 33.Pericle F, Liu J H, Diaz J I, Blanchard D K, Wei S, Forni G, Djeu J Y. Interleukin-2 prevention of apoptosis in human neutrophils. Eur J Immunol. 1994;24:440–444. doi: 10.1002/eji.1830240226. [DOI] [PubMed] [Google Scholar]
- 34.Rikihisa Y. The tribe Ehrlichieae and ehrlichial diseases. Clin Microbiol Rev. 1991;4:286–308. doi: 10.1128/cmr.4.3.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rikihisa Y. Cross-reacting antigens between Neorickettsia helminthoeca and Ehrlichia spp. shown by immunofluorescence and Western immunoblotting. J Clin Microbiol. 1991;29:2024–2029. doi: 10.1128/jcm.29.9.2024-2029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rikihisa Y, Zhang Y, Park J. Inhibition of infection of macrophages with Ehrlichia risticii by cytochalasins, monodansylcadaverine, and taxol. Infect Immun. 1994;62:5126–5132. doi: 10.1128/iai.62.11.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rikihisa Y, Zhi N, Wormser G, Wen B, Horowitz H W, Hechemy K E. Direct isolation and cultivation of human granulocytic ehrlichia from a human patient. J Infect Dis. 1997;175:210–213. doi: 10.1093/infdis/175.1.210. [DOI] [PubMed] [Google Scholar]
- 38.Sweeney J F, Nguyen P K, Omann G M, Hinshaw D B. Lipopolysaccharide protects polymorphonuclear leukocytes from apoptosis via tyrosine phosphorylation-dependent signal transduction pathways. J Surg Res. 1998;74:64–70. doi: 10.1006/jsre.1997.5193. [DOI] [PubMed] [Google Scholar]
- 39.Thornberry N, Bull H G, Calaycay J R, Chapman K T, Howard A D, Kostural M J, Miller D K, Molineaux S M, Weidner J R, Aununs J, Elliston K O, Ayala J M, Casano F J, Chin J, Ding G J-F, Egger L A, Gaffney E P, Limjuco G, Palyha O C, Raju S M, Rolando A M, Sally J P, Yamin T-T, Lee T D, Shively J E, MacCross M, Mumford R A, Schmidt J A, Tocci M J. A novel heterodimeric cysteine protease is required for interleukin-1β processing in monocytes. Nature. 1992;356:768–774. doi: 10.1038/356768a0. [DOI] [PubMed] [Google Scholar]
- 40.Ward C, Chilvers E R, Lawson M F, Pryde J G, Fujihara S, Farrow S N, Haslett C, Rossi A G. NF-κB activation is a critical regulator of human granulocyte apoptosis in vitro. J Biol Chem. 1999;274:4309–4318. doi: 10.1074/jbc.274.7.4309. [DOI] [PubMed] [Google Scholar]
- 41.Watson R W G, Redmond H P, Wang J H, Condron C, Bouchier-Hays D. Neutrophils undergo apoptosis following ingestion of Escherichia coli. J Immunol. 1996;156:3986–3992. [PubMed] [Google Scholar]
- 42.William R, Watson G, Rotstein O D, Parodo J, Bitar R, Marshall J C. The IL-1β-converting enzyme (caspase-1) inhibits apoptosis of inflammatory neutrophils through activation of IL-1β. J Immunol. 1998;161:957–962. [PubMed] [Google Scholar]
- 43.Winjum N, Riley L K. In vitro proliferation of a canine granulocytic Ehrlichia. Vet Microbiol. 1993;34:355–362. doi: 10.1016/0378-1135(93)90060-k. [DOI] [PubMed] [Google Scholar]
- 44.Woldehiwet Z, Scott G R. Stages in the development of Cytoectes phagocytophila, the causative agent of tick-borne fever. Comp Pathol. 1982;92:469–474. doi: 10.1016/0021-9975(82)90033-0. [DOI] [PubMed] [Google Scholar]
- 45.Zhi N, Ohashi N, Rikihisa Y. Multiple p44 genes encoding major outer membrane proteins are expressed in the human granulocytic ehrlichiosis agent. J Biol Chem. 1999;274:17828–17836. doi: 10.1074/jbc.274.25.17828. [DOI] [PubMed] [Google Scholar]
- 46.Zhi N, Rikihisa Y, Kim H-Y, Wormser G P, Horowitz H W. Comparison of major antigenic proteins of six strains of human granulocytic ehrlichiosis agents by Western immunoblot analysis. J Clin Microbiol. 1997;35:2606–2611. doi: 10.1128/jcm.35.10.2606-2611.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]