Abstract
Approximately $20 billion of apple sales are generated annually in the United States. With an estimated 5 million tons produced yearly in the U.S. within the last decade, apple consumption is considered ubiquitous. Apples are comprised of bioactive constituents such as phytochemicals and prebiotics that may potentiate intestinal health and the gut microbiome. This study aimed to evaluate the effects of Empire apple juice, pomace, and pulp soluble extracts on intestinal functionality, morphology, and the microbiome in vivo (Gallus gallus). There were five treatment groups: non-injected (NI); 18 MΩ H2O (H2O); 6% apple juice (AJ); 6% apple pomace (APo); 6% apple pulp (APu). The eggs were treated by intra-amniotic administration of the samples on day 17 of incubation. After hatching, the blood, tissue, and cecum samples were collected for further analyses—including duodenal histomorphology, hepatic and duodenal mRNA expression, and cecal bacterial populations. Crypt depth was significantly (p < 0.5) shortest in AJ when compared to APo and APu. APo and APu soluble extracts significantly improved villi surface area compared to NI and H2O control groups. The highest count of Paneth cells per crypt was observed in APo as compared to all groups. In addition, the expression of brush border membrane micronutrient metabolism and functional proteins varied between treatments. Lastly, Lactobacillus cecal microbial populations increased significantly in the AJ group, while AJ, APu, and APu increased the abundance of Clostridium (p < 0.5). Ultimately, these results indicate the potential of Empire apple pomace to improve host intestinal health and the gut microbiome.
Keywords: apples, intra amniotic administration, gene expression, microbiome, intestinal morphology, Gallus gallus
1. Introduction
Apples (Malus domestica) are a well-established, domesticated fruit worldwide, with approximately 86 million metric tons produced annually [1]. On a fresh basis, apples are among the top fruit varieties grown in the United States—second only to grapes [2]. Fresh apple consumption is most common among consumers, however, 35% of the apples consumed are processed [3]. While processed apple products generally include jams, jellies, cider, vinegar, and dried products, most apples are processed for apple juice. There is a 75% juice extraction efficiency in the apple juice industry; thus, 25–30% of the fruit waste remains. Known as the pomace, this leftover fraction is a heterogeneous mixture of skin, flesh, seeds, stems, core, and calyx [4,5]. According to the U.S. Apple Association, approximately 33.4 million bushels (701.4 million kg) of apples were produced in 2021–2022 for juice and cider production [6]. Based on the juice extraction efficiency (75%), an estimated 175,350 metric tons of apple pomace were produced annually [4]. Apple waste is typically disposed of in landfills, leading to several damaging environmental effects, as disruption to the carbon:nitrogen ratio of soil can occur due to sugar content, organic acids, and microbial fermentation of apple pomace [7]. The high-water content of apple pomace is also an issue as it can cause water pollution. Given the complications of pomace disposal, various industries have taken advantage of the by-product as it is a rich source of nutrients such as carbohydrates, micronutrients, and phytochemicals [8]. Further utilization includes pectin extraction, production of enzymes and aroma compounds, cultivation of microbial strains and edible mushrooms, and incorporation into animal feed [5]. However, the burden remains to prevent the disposal of apple waste into landfills and, ultimately, avoid environmental pollution.
Apples contain health-promoting bioactive constituents. Quercetin derivatives (galactoside, glucoside, rhamnoside), catechin, gallic acid, phloretin, and chlorogenic acid are polyphenolic compounds that are reportedly found in apples [8]. Studies have reported the antioxidative [9,10,11,12,13], antiproliferative [11,14,15,16,17], and anti-inflammatory [18,19,20] potential of apple polyphenols. It is important to note that apple phenolic compounds differ in concentration throughout the fruit matrix. For example, while apple flesh and apple peels contain chlorogenic acid, the flesh contains higher concentrations [21,22]. Research has also shown apple peels to contain greater amounts of antioxidants and, therefore, greater antioxidant potential compared to apple flesh. In addition, interest in apple seeds as a source of polyphenolic compounds has been investigated and found to be a rich source of quercetin derivatives, phenolic acids, catechin, and phloridzin [23]. Another constituent with health-promoting properties within the apple is dietary fiber, specifically, the non-digestible soluble polysaccharide pectin. Historically utilized as a commercial thickening and gelling agent, pectin holds potential functional properties which may improve intestinal health. The resistance to gastric digestion enables pectin to reach the host gut and undergo fermentation by microbiota, and ultimately produces short-chain fatty acid (SCFA) metabolites [24,25,26,27]. Previous studies have shown SCFAs to be beneficial to the gut by promoting enteric epithelial cell proliferation, enhancing gut barrier function, enhancing micronutrient absorption, and favoring the growth of beneficial bacteria over potentially pathogenic bacteria [28,29,30,31,32].
This study aimed to evaluate the in vivo effects of apple juice, pomace, and pulp soluble extracts on intestinal morphology, functionality, and the microbiome using Gallus gallus as our model. The broiler chicken is an established model employed to evaluate the effects of plant-origin bioactives on intestinal health and the microbiome [33]. The broiler chicken model exhibits genetic homology, a complex gut microbiota, and notable microbial similarity at the phylum level to human gut microbiota [34].
2. Materials and Methods
2.1. Apple Preparation
Empire apples (Malus domestica) were harvested (>2 tons) during the fall of 2021 from multiple trees from the Cornell AgriTech Orchards and processed at the Cornell Food Venture Center Pilot Plant (Geneva, NY, USA). Before processing, Empire apples were destemmed and washed. Apple pulp was made by removing the core and seeds and dicing. The apple pieces were then freeze-dried (Max53, Millrock Technology, Kingston, NY, USA) for 24 h. The dried apple was then ground into a fine powder using a bench-scale processor (Robo Coue; Jackson, MI, USA) at 1500 RPM. Apple juice was made to typical industry standards. For apple juice, whole apples were ground in a hammer mill in a blade configuration. The pulp was then pressed using a pilot-scale hydraulic press (Orchard Equipment Co., Conway, MA, USA) at 1200–1400 PSI. The juice was commercially sterilized by hot-packing into PET bottles at 85 °C and keeping it hot for 2 min. Apple pomace was the resulting pomace from the juice pressing. After juice pressing, the pomace was freeze-dried for 24 h. The dried pomace was ground into a fine powder using a bench-scale grinder. The powders were vacuumed-sealed, and all samples were kept frozen until use.
2.2. Apple Analysis Sample Preparation
Apple samples were extracted under dark conditions utilizing absolute methanol and constant agitation for 2 h. The resulting slurry was centrifuged and decanted to acquire the supernatant. The subsequent isolate and washings were diluted to attain an extract (15% w/v) which was ultimately utilized for the further analysis below.
2.2.1. Polyphenol Analysis
The Folin-Ciocalteu method previously detailed by Waterhouse was utilized to quantify total polyphenol content (TPC) [35]. Essentially, the Folin-Ciocalteu reagent and the extract were allowed to incubate at room temperature. The sodium carbonate solution was used to quench the reaction and sample absorbance was measured immediately using a UV-visible spectrophotometer (Thermo Fisher; Waltham, MA, USA) at 765 nm. Therefore, TPC was calculated as gallic equivalents (GE) using a standard curve prepared under the same conditions.
2.2.2. Fibrous and Non-Fibrous Carbohydrate Analysis
According to AOAC 962.09, the non-fibrous carbohydrate analysis (NFC) was completed. Acid detergent fiber (ADF) and neutral detergent fiber (NDF) analyses were conducted according to AOAC 973.18. The analysis was performed by Dairy One Co-Op Inc. (Ithaca, NY, USA).
2.3. Extraction of Soluble Apple Contents
Apple powders and juice samples were dissolved and diluted in distilled water to create 6% concentrations. All apple samples were heated via water bath for 1 h at 60 °C, centrifuged (3500 RPM) for 10 min at room temperature, and the supernatant was collected only in the case of the pomace and pulp.
2.4. Animals and Design
Fertile Cornish-cross broiler chicken eggs (n = 55) were provided by a hatchery (Moyer’s chicks, Quakertown, PA, USA). All animal protocols were approved by Cornell University Institutional Animal Care and Use Committee (ethic approval code: 2020-0077). Apple extract powders and juice were diluted with 18 MΩ H2O to acquire the necessary concentration to maintain an osmolarity value of less than 320 Osm. On day 17 of embryonic incubation, viable eggs were weighed and randomly allocated into five groups (n = 12) with a similar weight frequency distribution. After identifying the amniotic fluid by candling, the treatment solution (1 mL) was injected with a 21-gauge needle. Subsequent to injection, the injection site was sealed with cellophane tape. Eggs were placed in hatching baskets for each treatment and equal representation at each incubator location. The five treatment groups consisted as follows: non-injected (NI); 18 MΩ H2O (H2O); 6% apple juice (AJ); 6% apple pomace (APo); and 6% apple pulp (APu). On day 21, exposure to CO2 was used to euthanize hatchlings, and the blood, pectoral muscle, liver, duodenum, and cecum were collected for further analysis.
2.5. Blood Analysis
Blood was collected from the heart using micro-hematocrit heparinized capillary tubes (Fisher Scientific Waltham, MA, USA). Blood glucose concentrations were determined using the Accu-Chek® blood glucose monitor following the manufacturer’s protocol.
2.6. Pectoral Glycogen
The pectoral muscle was collected on the day of the hatch. Glycogen analysis was completed as previously described [36,37,38]. Briefly, 20 mg of the sample was homogenized in 8% perchloric acid and centrifuged at 12,000 rpm (4 °C) for 15 min. The supernatant was removed, and 1.0 mL of petroleum ether was added. Following mixing, the petroleum ether fraction was discarded, and the remaining sample layer was transferred to a new container with the color reagent (300 µL). Samples were read in an ELISA reader at 450 nm, and glycogen content was analyzed based on the standard curve. Total glycogen content in the pectoral sample was identified as the product of multiplying tissue weight by the amount of glycogen per 1 g of wet tissue.
2.7. Total RNA Extraction from Duodenum and Liver Tissue Samples
Total RNA was extracted from 30 mg of duodenal and liver tissue samples (n = 5) as previously described [39,40,41,42,43]. Briefly, the Qiagen Rneasy Mini Kit (Rneasy Mini Kit, Qiagen Inc., Valencia, CA, USA) was used. All protocols were carried out according to the manufacturer and under Rnase-free conditions. RNA was quantified by absorbance at A 260/280. The integrity of 18S ribosomal rRNA was verified by 1.5% agarose gel electrophoresis, followed by ethidium bromide staining. RNA was stored at −80 °C until further use.
2.8. Real-Time Polymerase Chain Reaction (RT-PCR)
cDNA was created from the extracted RNA by a 20 µL reverse transcriptase (RT) reaction. To complete the reaction, the BioRad C1000 touch thermocycler using the Improm-II Reverse Transcriptase Kit (Catalog #A1250; Promega, Madison, WI, USA) was utilized. The cDNA concentration was measured by Nanodrop (Thermo Fisher Scientific, Waltham, MA, USA) at an absorbance of 260 nm and 280 nm using an extinction coefficient of 33 (for single-stranded DNA). Genomic DNA contamination was assessed by a real-time RT-PCR assay for the reference gene samples.
The RT-PCR primers were designed based on relevant gene sequences from the GenBank database using the Real-Time Primer Design Tool software (IDT DNA, Coralvilla, IA, USA), as detailed previously [44]. Table 1 indicates the primer sequences used in accordance with iron, zinc, and vitamin A metabolism, immune response, and brush border membrane functionality. The reference gene used was the Gallus gallus primer 18S rRNA. BLAST searches against the genomic National Center for Biotechnology Information (NCBI) database were applied to verify primer specificity.
Table 1.
DNA primer sequences used in this study.
| Analyte | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Base Pair | GI Identifier |
|---|---|---|---|---|
| Iron Metabolism | ||||
| DcytB | CATGTGCATTCTCTTCCAAAGTC | CTCCTTGGTGACCGCATTAT | 103 | 20380692 |
| DMT1 | TTGATTCAGAGCCTCCCATTAG | GCGAGGAGTAGGCTTGTATTT | 101 | 206597489 |
| Ferroportin | CTCAGCAATCACTGGCATCA | ACTGGGCAACTCCAGAAATAAG | 98 | 423984 |
| Hepcidin | AGACGACAATGCAGACTAACC | CTGCAGCAATCCCACATTTC | 132 | SAMN08056490 |
| Zinc Metabolism | ||||
| ZnT1 | GGTAACAGAGCTGCCTTAACT | GGTAACAGAGCTGCCTTAACT | 105 | 54109718 |
| ZnT7 | GGAAGATGTCAGGATGGTTCA | CGAAGGACAAATTGAGGCAAAG | 87 | 56555152 |
| ZIP4 | TCTCCTTAGCAGACAATTGAG | GTGACAAACAAGTAGGCGAAAC | 95 | 107050877 |
| ZIP1 | TGCCTCAGTTTCCCTCAC | GGCTCTTAAGGGCACTTCT | 144 | 121112053 |
| Vitamin A Metabolism | ||||
| CRBP2 | GGCTACATGGTTGCACTAGACA | AACCACCCGGTTATCGAGTC | 195 | NM_001277417.1 |
| LRAT | GATTTTGCCTATGGCGGCAG | TTGTCGGTCTGGAAGCTGAC | 197 | XM_420371.7 |
| RBP4 | TGCCACCAACACAGAACTCTC | CTTTGAAGCTGCTCACACGG | 149 | NM_205238.2 |
| STRA6 | GTGCGCTGAACTTTGTCTGC | TTCTTCCTGCTCCCGACCT | 116 | NM_001293202.2 |
| Inflammatory Response | ||||
| NF-κB | CACAGCTGGAGGGAAGTAAAT | TTGAGTAAGGAAGTGAGGTTGAG | 100 | 2130627 |
| TNF-α | GACAGCCTATGCCAACAAGTA | TTACAGGAAGGGCAACTCATC | 109 | 53854909 |
| IL6 | ACCTCATCCTCCGAGACTTTA | GCACTGAAACTCCTGGTCTT | 105 | 302315692 |
| Brush Border Membrane Functionality | ||||
| VDAC2 | CAGCACTCGCTTTGGAATTG | GTGTAACCCACTCCAACTAGAC | 99 | 395498 |
| OCLN | GTCTGTGGGTTCCTCATCGT | GTTCTTCACCCACTCCTCCA | 124 | 396026 |
| SI | CCAGCAATGCCAGCATATTG | CGGTTTCTCCTTACCACTTCTT | 95 | 2246388 |
| MUC6 | CCAACTTGCAGTGTTCCAAAG | CTGACAGTGTAGAGCAAGTACAG | 106 | XM_015286750.1 |
| 18S rRNA | GCAAGACGAACTAAAGCGAAAG | TCGGAACTACGACGGTATCT | 100 | 7262899 |
DcytB, Duodenal cytochrome B; DMT1, Divalent metal transport 1; ZnT1, Zinc transporter 1; ZnT7, Zinc transporter 7; CRBP2, Cellular retinol-binding protein 2; LRAT, Lecithin; Retinol Acyltransferase; RBP4, Retinol binding protein 4; STRA6, Stimulated by Retinoic aid 6; NF-kB, Nuclear factor kappa beta; TNF-α, Tumor necrosis factor-alpha; IL6, Interleukin 6; VDAC2, Voltage-dependent anion channel 2; OCLN, Occludin; SI, Sucrase isomaltase; MUC6, Mucin 6.
2.9. Microbial Samples and Intestinal Contents DNA Isolation
Cecum samples were weighed and placed in sterile tubes containing PBS. Subsequently, the samples were vortexed with sterile glass beads for 3 min. All protocols were completed as previously described [42,45,46,47,48,49].
2.10. Primer Design and PCR Amplification of Bacterial 16S rRNA
Bifidobacterium, Lactobacillus, Escherichia coli, Clostridium, Klebsiella, and L. plantarum primers were used. 16S rRNA was the universal primer and internal standard. Therefore, the proportions of each bacterial group are presented. PCR products were applied to 1.5% agarose gel with ethidium bromide stain and quantified with Gel-Pro analyzer version 3.0 (Media Cybernetics LP, Rockville, MD, USA).
2.11. Histomorphological Examination
On the day of the hatch, proximal duodenal samples were collected. Subsequently, the samples were soaked in 4% (v/v) buffered formaldehyde, dehydrated, cleared, and embedded in paraffin. Several sections were cut with a 5 µm thickness and placed on glass slides. Intestinal sections were then deparaffinized in xylene and rehydrated in a series of graded alcohol. Ultimately, the slides were stained with Alcian Blue/Periodic acid-Schiff and investigated by light microscopy using EPIX XCAP software (Standard version, Olympus, Waltham, MA, USA). The following features were measured in the duodenum: villus surface area, crypt depth, villus and crypt goblet diameter, crypt goblet cell number and type, and Paneth cell number and diameter within the crypt, as previously described [42,43,49,50,51]. Per treatment group, five biological samples (n = 5) (four segments each) were analyzed. Ten randomly selected villi and crypts were measured and analyzed, and cell measurements and counts were completed in ten randomly selected villi or crypts per segment. The following equation was utilized to calculate the villus surface area:
| (1) |
in which VW is the mean of three villus width measurements, and VL is the villus length.
2.12. Statistical Analysis
In this paper, values are portrayed as the mean values ± standard error means. Experimental treatments and controls for the intra-amniotic administration were assigned with approximately equal weight distribution. Tested parameters were analyzed for normal distribution and equal variance through a Shapiro-Wilk test. If the test was accepted, a one-way analysis for variance (ANOVA) was utilized. ANOVA was used to analyze the results, followed by a Duncan post-hoc test to determine significance based on p-values (p < 0.05). For all statistical evaluations, software SPSS version 20.0 was utilized.
3. Results
3.1. Apple Polyphenol and Fiber Content
Empire apple pulp had the highest (p < 0.05) TPC content, followed by apple pomace (APo) and apple juice (AJ) (Table 2). Apple pomace had the greatest acid detergent fiber (ADF) and neutral detergent fiber (NDF) compared to apple pulp and apple juice. Lastly, non-fiber carbohydrate content was greatest in the apple pulp.
Table 2.
Total polyphenolic (mg/g GAE) and fiber content of apple products.
| Sample | TPC (mg/g GAE) | ADF (%/DM) | NDF (%/DM) | NFC (%/DM) |
|---|---|---|---|---|
| Pomace | 0.834 ± 0.059 b | 22.6 | 25.6 | 62 |
| Juice | 0.300 ± 0.029 c | NA | NA | NA |
| Pulp | 1.57 ± 0.074 a | 6.7 | 7.9 | 85.4 |
Values are the means ± SEM, a–c Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test. ADF: cellulose, lignin, and insoluble minerals; NDF: cellulose, lignin, insoluble minerals, and hemicellulose; NFC: sugars, starches, organic acids, and pectin.; GAE: gallic acid equivalents; DM: dry matter; NA: not applicable.
3.2. Body Weight, Blood Glucose, and Glycogen Content
No significant differences were observed between the treatment and control groups for body weight, blood glucose, and glycogen content (p < 0.05, Table 3).
Table 3.
Effect of the intra-amniotic administration of apple fraction soluble extracts on body weight (g), blood glucose (mg/dL), and glycogen (mg/g).
| Treatment Group | Body Weight (g) | Blood Glucose (mg/dL) | Glycogen (mg/g) |
|---|---|---|---|
| NI | 40.83 ± 1.24 a | 254.11 ± 23.83 a | 0.396 ± 0.101 a |
| H2O | 38.29 ± 4.31 a | 234.4 ± 11.16 a | 0.294 ± 0.093 a |
| AJ | 40 ± 0.99 a | 225.5 ± 11.43 a | 0.431 ± 0.092 a |
| APo | 35.7 ± 0.67 a | 230.56 ± 21.28 a | 0.271 ± 0.054 a |
| APu | 36.44 ± 0.85 a | 205.5 ± 32.05 a | 0.442 ± 0.077 a |
Values are the means ± SEM, n = 5. a Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test.
3.3. Duodenal and Hepatic Gene Expression of Related Proteins
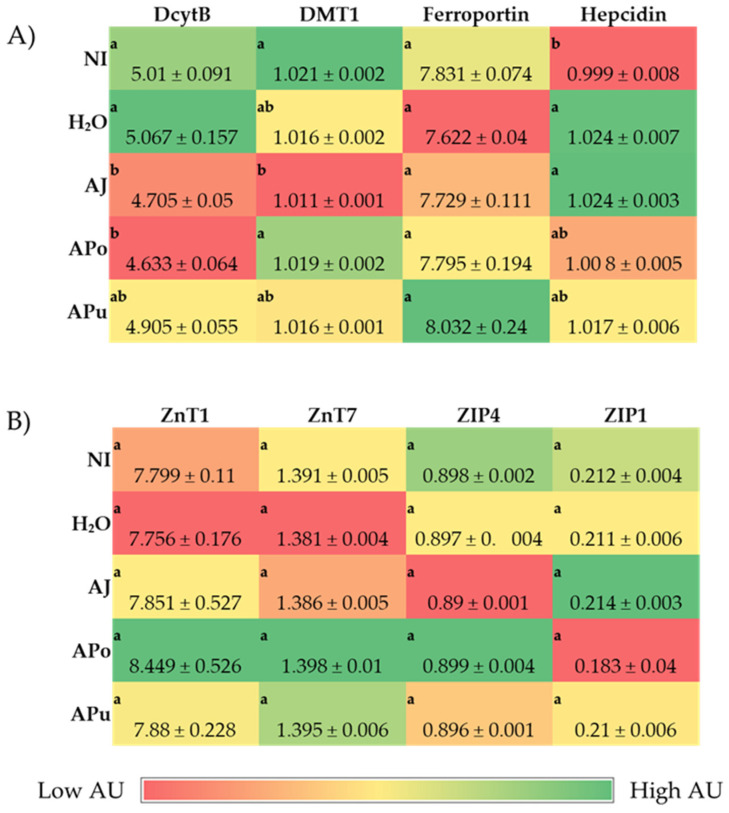
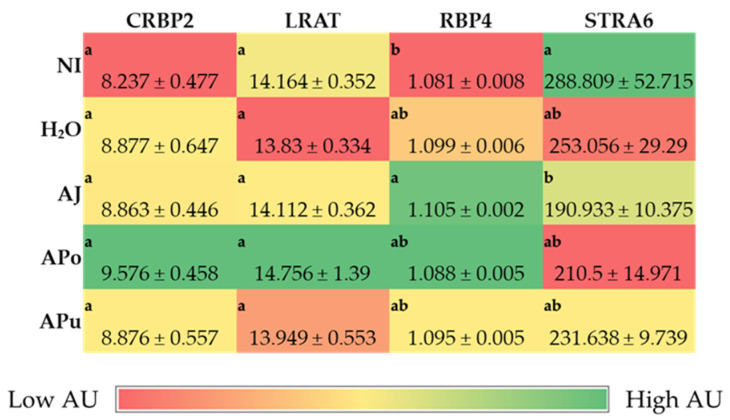
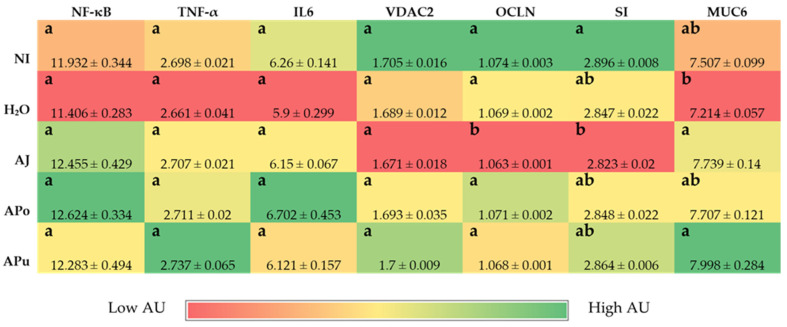
Figure 1, Figure 2 and Figure 3 depict the gene expression of proteins relevant to micronutrients (iron, zinc, and vitamin A) metabolism, immune response, and functionality.
Figure 1.
Effect of intra-amniotic administration of apple fraction soluble extracts on iron (A) and zinc (B) intestinal and liver (Hepcidin) gene expression. Values are the means ± SEM, n = 5. a, b Per gene, treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test. DcytB, Duodenal cytochrome B; DMT1, Divalent metal transporter 1; Zinc transporters: ZnT1, ZnT7, ZIP4, ZIP1; AU, Arbitrary units.
Figure 2.
Effect of intra-amniotic administration of apple fraction soluble extracts on vitamin A intestinal (CRBP2 and LRAT) gene expression and hepatic (RBP4 and STRA6) metabolic proteins. Values are the means ± SEM, n = 5. a, b Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test. CRBP2, Cellular retinol-binding protein 2; LRAT, Lecithin: Retinol Acyltransferase; RBP4, Retinol binding protein 4; STRA6, Stimulated by Retinoic acid 6; AU, Arbitrary units.
Figure 3.
Effect of intra-amniotic administration of apple fraction soluble extracts on inflammatory and functional intestinal protein gene expression. Values are the means ± SEM, n = 5. a, b Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test. NF-kB, Nuclear factor kappa beta; TNF-α, Tumor necrosis factor-alpha; IL6, Interleukin 6, VDAC2, Voltage-dependent anion channel 2; OCLN, Occludin; SI, Sucrase isomaltase; MUC6, Mucin 6; AU, Arbitrary units.
3.3.1. Iron and Zinc-Related Proteins
DcytB expression in treatment groups AJ and Apo were downregulated (p < 0.05) compared to the NI and H2O controls (Figure 1). AJ reduced (p < 0.05) gene expression of DMT1 relative to the NI control, but no difference was observed relative to the H2O control. The apple groups did not alter (p < 0.05) the gene expression of ferroportin compared to the NI and H2O groups. Hepcidin, an iron-related protein located in the liver, was upregulated by the AJ treatment relative to the NI control. APo and APu were not significantly altered compared to NI and H2O controls.
The administration of apple juice, pomace, and pulp extractions did not alter (p < 0.05) the gene expression of zinc-related proteins at the brush border membrane relative to the NI and H2O controls.
3.3.2. Vitamin A-Related Proteins
No significant differences (p < 0.05) were observed in the gene expression of CRBP2 and LRAT between the treatment and control groups (Figure 2). AJ had the greatest (p < 0.05) expression of RBP4 compared to the NI control, which had the lowest (p < 0.05) expression of RBP4, however, there were no differences (p < 0.05) between the H2O control and the apple treatment groups.
3.3.3. Inflammatory and Functionality-Related Proteins
The gene expression of proteins related to inflammation—NF-κB, TNF-α, and IL6—was not significantly different (p < 0.05) between the control and experimental groups (Figure 3). Further, gene expression of functional proteins VDAC2, SI, and MUC6 were not significantly (p < 0.05) different between treatment and control groups. However, the gene expression of OCLN was significantly (p < 0.05) lowered in the AJ group compared to all other treatment groups.
3.4. Morphometric Analysis
Groups APo and APu have significantly (p < 0.05) greater villi surface area compared to both control and experimental groups (Table 4). Crypt depth is significantly shortest (p < 0.05) in APu, APo, and AJ, respectively, related to both controls.
Table 4.
Effect of the intra-amniotic administration of apple fraction soluble extracts on villi surface area and crypt depth.
| Treatment Group | Villi Surface Area (µm2) | Crypt Depth (µm) |
|---|---|---|
| NI | 16,458.04 ± 771.84 ᵇ | 22.1 ± 0.81 ᵃ |
| H2O | 16,101.54 ± 383.07 ᵇ | 21.93 ± 0.72 ᵃ |
| AJ | 17,470.91 ± 444.08 ᵇ | 14.45 ± 0.54 ᶜ |
| APo | 23,116.65 ± 509.84 ᵃ | 16.85 ± 0.79 ᵇ |
| APu | 23,520.69 ± 739.04 ᵃ | 17.38 ± 0.71 ᵇ |
Values are the means ± SEM, n = 5. a–c Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test.
The intra-amniotic administration of AJ increased (p < 0.05) goblet cell diameter within the villi relative to the other experimental groups and H2O control, but not the NI control (Table 5). Goblet cell diameter within the crypts was significantly (p < 0.05) lower in the APo group. Further, the count of goblet cells within the intestinal crypts was highest (p < 0.05) in APu relative to the other treatment and control groups. The abundance of acidic goblet cells per unit area was more significant (p < 0.05) in the APu treatment compared to the other apple fraction treatments and NI and H2O controls. Neutral goblet cell abundance was significantly (p < 0.05) lowered in all apple fraction treatment groups relative to the H2O control but was similar to the NI control. Mixed goblet cells were most numerous (p < 0.05) in APu relative to the APo, NI, and H2O groups.
Table 5.
Effect of the intra-amniotic administration of apple fraction soluble extracts on villi and crypt goblet cells.
| Treatment Group | Villi Goblet Diameter (µm) | Crypt Goblet Diameter (µm) | Crypt Goblet Cell Number | Crypt Goblet Cell Number | ||
|---|---|---|---|---|---|---|
| Acidic | Neutral | Mixed | ||||
| NI | 3.48 ± 0.07 a | 3.01 ± 0.05 a | 7.01 ± 0.24 c | 5.79 ± 0.2 c | 0.02 ± 0.02 b | 1.21 ± 0.13 c |
| H2O | 3.17 ± 0.06 b | 2.89 ± 0.05 a | 8.55 ± 0.32 b | 6.92 ± 0.27 b | 0.13 ± 0.03 a | 1.51 ± 0.12 bc |
| AJ | 3.55 ± 0.07 a | 2.88 ± 0.05 a | 7.51 ± 0.26 c | 5.81 ± 0.22 c | 0.02 ± 0.01 b | 1.69 ± 0.11 ab |
| APo | 3.17 ± 0.06 b | 2.71 ± 0.06 b | 8.36 ± 0.27 b | 6.75 ± 0.23 b | 0.01 ± 0.01 b | 1.60 ± 0.10 b |
| APu | 3.08 ± 0.08 b | 2.84 ± 0.05 a | 9.14 ± 0.31 a | 7.35 ± 0.26 a | 0.00 ± 0.00 b | 1.79 ± 0.11 a |
Values are the means ± SEM, n = 5. a–c Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test.
With regard to the morphometric measurements of Paneth cells within the crypt, APo and APu groups had the greatest (p < 0.05) crypt Paneth cell count per unit area, respectively (Table 6). The H2O group had the lowest (p < 0.05) observed Paneth cell count per unit area relative to all other groups, yet had the greatest Paneth cell diameter.
Table 6.
Effect of the intra-amniotic administration of apple fraction soluble extracts on Paneth cells.
| Treatment Group | Crypt Paneth Cell Number | Paneth Cell Diameter (µm) |
|---|---|---|
| NI | 1.22 ± 0.03 c | 1.37 ± 0.02 c |
| H2O | 1.04 ± 0.01 ᵈ | 1.5 ± 0.02 ᵃ |
| AJ | 1.31 ± 0.04 bc | 1.45 ± 0.02 ᵃᵇ |
| APo | 1.44 ± 0.04 ᵃ | 1.43 ± 0.02 b |
| APu | 1.38 ± 0.04 b | 1.45 ± 0.02 ᵃᵇ |
Values are the means ± SEM, n = 5. a–d Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test.
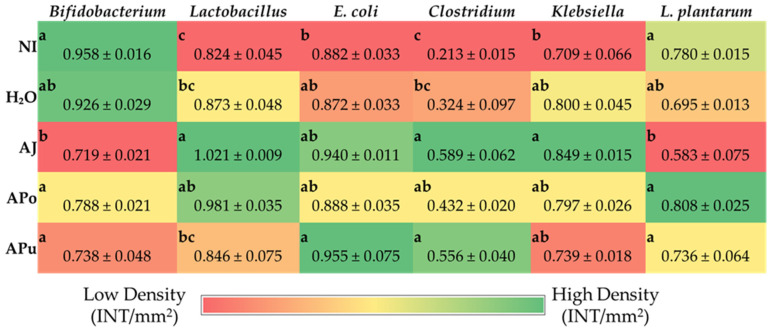
3.5. Microbial Analysis
The AJ group reduced the relative abundance of Bifidobacterium (p < 0.05) compared to the control and experimental groups (Figure 4). Furthermore, the greatest increase (p < 0.05) of Lactobacillus abundance relative to NI and water-only control groups occurred in the AJ group, while APo also increased (p < 0.05) Lactobacillus abundance relative only to the water injection control. All apple treatment groups have an increased (p < 0.05) abundance of Clostridium relative to the NI control, whereas only AJ and APu abundance is significantly higher than both controls. No differences were observed between the apple treatments relative to all controls for Klebsiella and L. plantarum microbial abundance.
Figure 4.
Effect of intra-amniotic administration of apple fraction soluble extracts on genera- and species-level bacterial populations from lower intestine contents on the day of hatch. Values are the means ± SEM, n = 5. a–c Treatment groups not indicated by the same letter in the same column are significantly different (p < 0.05) by Duncan’s post-hoc test.
4. Discussion
The Empire apple variety is a cross between McIntosh (Malus domestica “McIntosh”) and Red Delicious (Malus domestica “Red Delicious”) cultivars and is native to New York state [52]. According to the United States Apple Association, Empire apples are among the top-produced apples in the nation [6,53]. Here, we have investigated the effects of Empire apple juice (AJ), pomace (APo), and pulp (APu) extracts via intra-amniotic administration on micronutrient absorption, intestinal immune response, gut morphology, and cecal bacterial populations. To our knowledge, this is the first study to examine such physiological effects of the Empire apple cultivar.
Body weight, blood glucose, and glycogen content (Table 3) did not differ throughout the treatment and control groups. As apples contain select macro- and micronutrients, consumption of this fruit and weight gain prevention have been studied in previous animal trials [54]. While Cho et al. (2013) reported apple pomace and juice supplementation reduced (p < 0.05) body weight gain in Sprague-Dawley rats [55], and Samout et al. (2016) found apple pectin to exert anti-obesity effects in Wistar rats [56], changes in body weight did not occur in our study. We hypothesize that this may be the case as treatment groups were a single dose in a naïve system, while the aforementioned studies utilized overweight models over a prolonged period.
The intra-amniotic administration of apple juice and pomace extracts reduced the gene expression of DcytB reductase relative to the controls (Figure 1A). Located in the proximal duodenum, DcytB functions to reduce ferric dietary iron to the bioavailable form (Fe2+) for uptake into the enterocyte [57]. No significant changes occurred in the remaining iron metabolism proteins (DMT1, Ferroportin, and Hepcidin). Nonetheless, the reduced expression of DcytB potentially suggests an improvement in iron absorption into the enterocyte [58,59]. Shah et al. (2003) reported that apple juice enhances iron bioavailability in American children of 3 to 6 years of age [60]. The soluble apple fractions did not enhance zinc transporter proteins and vitamin A metabolism proteins, yet these results revealed no adverse effects of a single-dose administration. We expected to observe an anti-inflammatory effect of the apple treatments due to the naturally occurring phenolics in apples (represented in Table 2), which possess antioxidative properties (e.g., chlorogenic acid, quercetin glycosides, catechin) [21,22,23]. However, Figure 3 reveals no effect of the apple treatments on inflammatory cytokine expression (NF-κB, TNF-α, IL6). Given the reported anti-inflammatory potential of apples [10,18,19,20], the lack of agreement with this study’s results can be attributed to the short exposure time and concentrations administered. Conversely, our results reveal that apple pomace extract did not stimulate a negative intestinal immune response. Apple seeds are known to generate toxic cyanogenic glycosides upon grinding and have been a factor of concern when upscaling apple pomace for consumption. In a recent study, using Fisher rats, Ravn-Haren et al. investigated the effects of a different cultivar (Shampion) apple pomace with and without seeds [61]. It was reported that apple pomace, regardless of seed content, did not elevate alanine aminotransferase, a liver toxicity biomarker [61].
Intestinal barrier integrity is vital to gastrointestinal functionality and health, and tight junction proteins play a crucial role in maintaining the luminal structure [62,63]. Located in luminal epithelial cells, tight junctions, such as claudin and occludin regulate the permeability of ions, water, and macronutrients [64,65,66]. Expression of the tight junction protein occludin (OCLN) was significantly reduced by apple juice administration (Figure 3), which suggests an increase in epithelial permeability. Apple juice is known to naturally contain high amounts of simple sugars such as glucose and fructose [67,68]. High-sugar diets have reportedly increased intestinal barrier permeability, although the direct mechanism is unclear [69]. One proposed mechanism is through the intestinal microbiome, as diets rich in simple sugars have been linked to disrupting the balance of gut microbes, causing dysbiosis [69]. Dysbiosis can be characterized by an increase in the Firmicutes/Bacteroidetes ratio [69,70,71]. AJ increased the abundance of Clostridium and Klebsiella, as depicted in Figure 4. The Clostridium genus comprises commensal bacteria within the Firmicutes phylum that can exert pathogenic effects under dysbiosis conditions. Essentially, the overgrowth of opportunistic species within the Clostridium and Klebsiella may lead to the degradation of the intestinal barrier [72,73,74] or render severe infection [75]. Conversely, while the beneficial bacteria Bifidobacterium decreased abundance in AJ, Lactobacillus increased abundance. Lactobacillus is a lactic acid-producing bacteria within the Firmicutes phylum; thus, our results suggest a possible selective stimulation of Firmicute proliferation by AJ.
Figure 4 reveals the increased abundance of Clostridium within APo and APu relative to the NI control. According to our results in Figure 3 and Table 4, Table 5 and Table 6, it is possible that the Clostridium genera exerted a beneficial effect as an induced effect on intestinal permeability and inflammatory cytokine expression was not observed. Therefore, we hypothesize that valuable species of Clostridia were increased. Clostridium is an SCFA-producing genus that has been reported to grow in abundance through pectin fermentation [76,77,78]. APo and APu had the greatest amount of non-fiber carbohydrates (including pectin) (Table 2), villi surface area (Table 4), and acidic goblet cells within the crypt (Table 5). A previous study reported that apple-derived pectin-fed rats increased Clostridium species abundance four-fold, whilst also increasing butyrate levels [79]. Butyrate is a short-chain fatty acid produced upon carbohydrate fermentation by Clostridium that can lower intestinal pH [78], which could explain the increased count of acidic goblet cells for APo and APu. Dufourny et al. (2021) previously assessed the effects of apple pomace on intestinal morphology and microbiota in weaned piglets. They found the pomace to increase Clostridia abundance and duodenal and ileal villi length [80]. Our results agree with the significant findings of this study. This further establishes the role of apple pomace to modulate Clostridia groups in both animal models which leads to improvements in gut health and intestinal homeostasis.
Shortened crypt depth was observed in all apple treatment groups (Table 4). Shortened crypt depths are morphological evidence for improved intestinal health as it suggests a slower intestinal epithelial cell turnover rate, allowing sufficient time for enterocytes to differentiate and function at capacity [81,82,83]. Within intestinal crypts, goblet cells play a role in maintaining the gut epithelial layer as they secrete mucus and mucin glycoproteins that function as a protective layer along the intestinal lumen [84]. A previous study found apple polysaccharide isolated from Fuji apple pomace to stimulate the enhancement of gut epithelial integrity by goblet cell autophagy [85]. Our study observed that APu and APo had the greatest crypt goblet cell count per unit area, respectively. In addition, APu had a significantly lower crypt goblet cell diameter. This finding likely suggests lower mucus content since intestinal crypt goblet cells only secrete mucus upon stimulation [84] Our findings suggest that the Empire apple has a level of effect on goblet cells located in the crypt. However, further studies should be completed to elucidate the potential impact. Moreover, Paneth cells are in the small intestinal crypts and function in the gut immunological response—secreting antimicrobial peptides and immunomodulating proteins to maintain intestinal homeostasis [86,87,88]. Crypt Paneth cell count per unit area was greatest (p < 0.05) in APo, APu, and AJ, respectively, compared to H2O control (Table 6). Yet, the Paneth cell diameter of APo treatment was similar (p > 0.05) to APu and AJ, and lower (p > 0.05) than the H2O control. Based on these observations and a recent review that summarized the impacts of dietary fiber on host gastrointestinal immune response, it seems that the Empire apple may stimulate Paneth cell function and improve intestinal immune response [89].
5. Conclusions
The intra-amniotic administration of Empire apple soluble extracts from various fractions was completed in this study. The data suggests that each apple fraction can alter duodenal brush border membrane functionality, morphology, and the cecal microbial populations. More specifically, the potential health benefits of apple pomace are revealed in this study, evident by reducing iron metabolism protein gene expression (DcytB), increasing villi surface area and decreasing crypt depth, increasing Paneth cell count per intestinal crypts, and increasing potentially beneficial gut bacteria (Clostridium spp.). Additional long-term studies should be completed to further establish potential health benefits.
Author Contributions
Conceptualization, O.I.P.-Z. and E.T.; Methodology, N.K., N.A., V.S., O.I.P.-Z. and E.T.; Investigation, C.J., N.A., V.S. and N.K.; Data curation, C.J., N.A., V.S. and N.K.; Writing—original draft preparation, C.J. and E.T.; Writing—review and editing, V.S., N.K., O.I.P.-Z. and E.T.; Supervision, O.I.P.-Z. and E.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Animal protocol used in this study was conducted according to the guidelines of the Declaration of Helsinki and were approved by the Cornell University Institutional Animal Care and Use committee by the ethic approval code: 2020-0077.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research was partially funded by the National Institute of Food and Agriculture, U.S. Department of Agriculture Federal Capacity Funds Multistate Project (NC1023).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.FAO Apple Production Worldwide from 2010 to 2020 (in Million Metric Tons) [(accessed on 1 June 2022)]. Available online: https://www.statista.com/statistics/961248/production-of-apples-worldwide/
- 2.US Department of Agriculture Economic Research Service Leading Fruits in the United States in 2018, Based on Production Volume (in 1000 Tons) [(accessed on 9 June 2022)]. Available online: https://www.statista.com/statistics/631886/leading-fruits-united-states-based-on-production-quantity/
- 3.Nicklas T.A., O’Neil C.E., Fulgoni V.L. Consumption of Various Forms of Apples Is Associated with a Better Nutrient Intake and Improved Nutrient Adequacy in Diets of Children: National Health and Nutrition Examination Survey 2003–2010. Food Nutr. Res. 2015;59:25948. doi: 10.3402/fnr.v59.25948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lyu F., Luiz S.F., Azeredo D.R.P., Cruz A.G., Ajlouni S., Ranadheera C.S. Apple Pomace as a Functional and Healthy Ingredient in Food Products: A Review. Processes. 2020;8:319. doi: 10.3390/pr8030319. [DOI] [Google Scholar]
- 5.Vendruscolo F., Albuquerque P.M., Streit F., Esposito E., Ninow J.L. Apple Pomace: A Versatile Substrate for Biotechnological Applications. Crit. Rev. Biotechnol. 2008;28:1–12. doi: 10.1080/07388550801913840. [DOI] [PubMed] [Google Scholar]
- 6.U.S. Apple Association US Apple Industry Outlook 2021. 2021. [(accessed on 22 October 2022)]. Available online: https://usapple.org/wp-content/uploads/2021/08/USAppleIndustryOutlook2021.pdf.
- 7.Gołębiewska E., Kalinowska M., Yildiz G. Sustainable Use of Apple Pomace (AP) in Different Industrial Sectors. Materials. 2022;15:1788. doi: 10.3390/ma15051788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Patocka J., Bhardwaj K., Klimova B., Nepovimova E., Wu Q., Landi M., Kuca K., Valis M., Wu W. Malus Domestica: A Review on Nutritional Features, Chemical Composition, Traditional and Medicinal Value. Plants. 2020;9:1408. doi: 10.3390/plants9111408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fernandes P.A.R., Ferreira S.S., Bastos R., Ferreira I., Crus M.T., Pinto A., Coelho E., Passos C.P., Coimbra M.A., Cardoso S.M., et al. Apple Pomace Extract as a Sustainable Food Ingredient. Antioxidants. 2019;8:189. doi: 10.3390/antiox8060189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zielinska D., Laparra-Llopis J.M., Zielinski H., Szawara-Nowak D., Gimenez-Bastida J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients. 2019;11:1173. doi: 10.3390/nu11051173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wolfe K., Wu X., Liu R.H. Antioxidant Activity of Apple Peels. J. Agric. Food Chem. 2003;51:609–614. doi: 10.1021/jf020782a. [DOI] [PubMed] [Google Scholar]
- 12.Vieira F.G.K., Borges G.D.S.C., Copetti C., Di Pietro P.F., da Costa Nunes E., Fett R. Phenolic Compounds and Antioxidant Activity of the Apple Flesh and Peel of Eleven Cultivars Grown in Brazil. Sci. Hortic. 2011;128:261–266. doi: 10.1016/j.scienta.2011.01.032. [DOI] [Google Scholar]
- 13.Wojdylo A., Oszmianski J., Laskowski P. Polyphenolic Compounds and Antioxidant Activity of New and Old Apple Varieties. J. Agric. Food Chem. 2008;56:6520–6530. doi: 10.1021/jf800510j. [DOI] [PubMed] [Google Scholar]
- 14.Reagan-Shaw S., Eggert D., Mukhtar H., Ahmad N. Antiproliferative Effects of Apple Peel Extract against Cancer Cells. Nutr. Cancer. 2010;62:517–524. doi: 10.1080/01635580903441253. [DOI] [PubMed] [Google Scholar]
- 15.Luo J., Zhang P., Li S., Shah N.P. Antioxidant, Antibacterial, and Antiproliferative Activities of Free and Bound Phenolics from Peel and Flesh of Fuji Apple. J. Food Sci. 2016;81:M1735–M1742. doi: 10.1111/1750-3841.13353. [DOI] [PubMed] [Google Scholar]
- 16.Li C.X., Zhao X.H., Zuo W.F., Zhang T.L., Zhang Z.Y., Chen X.S. Phytochemical Profiles, Antioxidant, and Antiproliferative Activities of Red-Fleshed Apple as Affected by in Vitro Digestion. J. Food Sci. 2020;85:2952–2959. doi: 10.1111/1750-3841.15358. [DOI] [PubMed] [Google Scholar]
- 17.He X., Liu R.H. Phytochemicals of Apple Peels: Isolation, Structure Elucidation, and Their Antiproliferative and Antioxidant Activities. J. Agric. Food Chem. 2008;56:9905–9910. doi: 10.1021/jf8015255. [DOI] [PubMed] [Google Scholar]
- 18.Andre C.M., Greenwood J.M., Walker E.G., Rassam M., Sullivan M., Evers D., Perry N.B., Laing W.A. Anti-Inflammatory Procyanidins and Triterpenes in 109 Apple Varieties. J. Agric. Food Chem. 2012;60:10546–10554. doi: 10.1021/jf302809k. [DOI] [PubMed] [Google Scholar]
- 19.Jung M., Triebel S., Anke T., Richling E., Erkel G. Influence of Apple Polyphenols on Inflammatory Gene Expression. Mol. Nutr. Food Res. 2009;53:1263–1280. doi: 10.1002/mnfr.200800575. [DOI] [PubMed] [Google Scholar]
- 20.Carrasco-Pozo C., Speisky H., Brunser O., Pastene E., Gotteland M. Apple Peel Polyphenols Protect against Gastrointestinal Mucosa Alterations Induced by Indomethacin in Rats. J. Agric. Food Chem. 2011;59:6459–6466. doi: 10.1021/jf200553s. [DOI] [PubMed] [Google Scholar]
- 21.Kschonsek J., Wolfram T., Stockl A., Bohm V. Polyphenolic Compounds Analysis of Old and New Apple Cultivars and Contribution of Polyphenolic Profile to the in Vitro Antioxidant Capacity. Antioxidants. 2018;7:20. doi: 10.3390/antiox7010020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyer J., Liu R.H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004;3:5. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fidelis M., de Moura C., Kabbas T., Pap N., Mattila P., Mäkinen S., Putnik P., Kovačević D.B., Tian Y., Yang B., et al. Fruit Seeds as Sources of Bioactive Compounds: Sustainable Production of High Value-Added Ingredients from by-Products within Circular Economy. Molecules. 2019;24:3854. doi: 10.3390/molecules24213854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fu X., Liu Z., Zhu C., Mou H., Kong Q. Nondigestible Carbohydrates, Butyrate, and Butyrate-Producing Bacteria. Crit. Rev. Food Sci. Nutr. 2019;59:S130–S152. doi: 10.1080/10408398.2018.1542587. [DOI] [PubMed] [Google Scholar]
- 25.Elshahed M.S., Miron A., Aprotosoaie A.C., Farag M.A. Pectin in Diet: Interactions with the Human Microbiome, Role in Gut Homeostasis, and Nutrient-Drug Interactions. Carbohydr. Polym. 2021;255:117388. doi: 10.1016/j.carbpol.2020.117388. [DOI] [PubMed] [Google Scholar]
- 26.Koh A., de Vadder F., Kovatcheva-Datchary P., Bäckhed F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell. 2016;165:1332–1345. doi: 10.1016/j.cell.2016.05.041. [DOI] [PubMed] [Google Scholar]
- 27.Macfarlane G.T., Macfarlane S. Bacteria, Colonic Fermentation, and Gastrointestinal Health. J. AOAC Int. 2012;95:50–60. doi: 10.5740/jaoacint.SGE_Macfarlane. [DOI] [PubMed] [Google Scholar]
- 28.Campbell J.M., Fahey G.C., Jr., Wolf B.W. Selected Indigestible Oligosaccharides Affect Large Bowel Mass, Cecal and Fecal Short-Chain Fatty Acids, PH, and Microflora in Rats. J. Nutr. 1997;127:130–136. doi: 10.1093/jn/127.1.130. [DOI] [PubMed] [Google Scholar]
- 29.Venegas D.P., de La Fuente M.K., Landskron G., González M.J., Quera R., Dijkstra G., Harmsen H.J.M., Faber K.N., Hermoso M.A. Short Chain Fatty Acids (SCFAs)Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019;10:277. doi: 10.3389/fimmu.2019.00277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Markowiak-Kopeć P., Śliżewska K. The Effect of Probiotics on the Production of Short-Chain Fatty Acids by Human Intestinal Microbiome. Nutrients. 2020;12:1107. doi: 10.3390/nu12041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander C., Swanson K.S., Fahey G.C., Jr., Garleb K.A. Perspective: Physiologic Importance of Short-Chain Fatty Acids from Nondigestible Carbohydrtae Fermentation. Adv. Nutr. 2019;10:576–589. doi: 10.1093/advances/nmz004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pearce S.C., Weber G.J., van Sambeek D.M., Soares J.W., Racicot K., Breault D.T. Intestinal Enteroids Recapitulate the Effects of Short-Chain Fatty Acids on the Intestinal Epithelium. PLoS ONE. 2020;15:e0230231. doi: 10.1371/journal.pone.0230231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hou T., Tako E. The in Ovo Feeding Administration (Gallus Gallus)—An Emerging in Vivo Approach to Assess Bioactive Compounds with Potential Nutritional Benefits. Nutrients. 2018;10:418. doi: 10.3390/nu10040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yegani M., Korver D.R. Factors Affecting Intestinal Health in Poultry. Poult. Sci. 2008;87:2052–2063. doi: 10.3382/ps.2008-00091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Waterhouse A.L. Determination of Total Phenolics. Curr. Protoc. Food Anal. Chem. 2002;6:11.1.1–11.1.8. doi: 10.1002/0471142913.FAI0101S06. [DOI] [Google Scholar]
- 36.Kornasio R., Halevy O., Kedar O., Uni Z. Effect of in Ovo Feeding and Its Interaction with Timing of First Feed on Glycogen Reserves, Muscle Growth, and Body Weight. Poult. Sci. 2011;90:1467–1477. doi: 10.3382/ps.2010-01080. [DOI] [PubMed] [Google Scholar]
- 37.Uni Z., Ferket P.R., Tako E., Kedar O. In Ovo Feeding Improves Energy Status of Late-Term Chicken Embryos. Poult. Sci. 2005;84:764–770. doi: 10.1093/ps/84.5.764. [DOI] [PubMed] [Google Scholar]
- 38.Dreiling C.E., Brown D.E., Casale L., Kelly L. Muscle Glycogen: Comparison of Iodine Binding and Enzyme Digestion Assays and Application to Meat Samples. Meat Sci. 1987;20:167–177. doi: 10.1016/0309-1740(87)90009-X. [DOI] [PubMed] [Google Scholar]
- 39.Tako E., Glahn R.P. Intra-Amniotic Administration and Dietary Inulin Affect the Iron Status and Intestinal Functionality of Iron-Deficient Broiler Chickens. Poult. Sci. 2012;91:1361–1370. doi: 10.3382/ps.2011-01864. [DOI] [PubMed] [Google Scholar]
- 40.Tako E., Beebe S., Reed S., Hart J., Glahn R.P. Polyphenolic Compounds Appear to Limit the Nutritional Benefit of Biofortified Higher Iron Black Bean (Phaseolus vulgaris L.) Nutr. J. 2014;13:28. doi: 10.1186/1475-2891-13-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Agrizzi Verediano T., Stampini Duarte Martino H., Kolba N., Fu Y., Cristina Dias Paes M., Tako E. Black Corn (Zea mays L.) Soluble Extract Showed Anti-Inflammatory Effects and Improved the Intestinal Barrier Integrity in Vivo (Gallus Gallus) Food Res. Int. 2022;157:111227. doi: 10.1016/j.foodres.2022.111227. [DOI] [PubMed] [Google Scholar]
- 42.Agarwal N., Kolba N., Jung Y., Cheng J., Tako E. Saffron (Crocus sativus L.) Flower Water Extract Disrupts the Cecal Microbiome, Brush Border Membrane Functionality, and Morphology in Vivo (Gallus Gallus) Nutrients. 2022;14:220. doi: 10.3390/nu14010220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.da Silva B.P., Kolba N., Martino H.S.D., Hart J., Tako E. Soluble Extracts from Chia Seed (Salvia hispanica L.) Affect Brush Border Membrane Functionality, Morphology and Intestinal Bacterial Populations in Vivo (Gallus Gallus) Nutrients. 2019;11:2457. doi: 10.3390/nu11102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Agarwal N., Kolba N., Khen N., Even C., Turjeman S., Koren O., Tako E. Quinoa Soluble Fiber and Quercetin Alter the Composition of the Gut Microbiome and Improve Brush Border Membrane Morphology In Vivo (Gallus Gallus) Nutrients. 2022;14:448. doi: 10.3390/nu14030448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X., Kolba N., Liang J., Tako E. Aleterations in Gut Microflora Populations and Brush Border Functionality Following Intra-Amniotic Administration (Gallus Gallus) of Wheat Bran Prebiotic Extracts. Food Funct. 2019;10:4834–4843. doi: 10.1039/C9FO00836E. [DOI] [PubMed] [Google Scholar]
- 46.Tako E., Glahn R.P., Welch R.M., Lei X., Yasuda K., Miller D.D. Dietary Inulin Affects the Expression of Intestinal Enterocyte Iron Transporters, Receptors and Storage Protein and Alters the Microbiota in the Pig Intestine. Br. J. Nutr. 2008;99:472–480. doi: 10.1017/S0007114507825128. [DOI] [PubMed] [Google Scholar]
- 47.Gomes M.J.C., Martino H.S.D., Kolba N., Cheng J., Agarwal N., de Moura Rocha M., Tako E. Zinc Biofortified Cowpea (Vigna unguiculata L. Walp.) Soluble Extracts Modulate Assessed Cecal Bacterial Populations and Gut Morphology In Vivo (Gallus Gallus) Front. Biosci.-Landmark. 2022;27:140. doi: 10.31083/j.fbl2705140. [DOI] [PubMed] [Google Scholar]
- 48.Dias D.M., Kolba N., Hart J.J., Ma M., Sha S.T., Lakshmanan N., Nutti M.R., Martino H.S.D., Glahn R.P., Tako E. Soluble Extracts from Carioca Beans (Phaseolus vulgaris L.) Affect the Gut Microbiota and Iron Related Brush Border Membrane Protein Expression in Vivo (Gallus Gallus) Food Res. Int. 2019;123:172–180. doi: 10.1016/j.foodres.2019.04.060. [DOI] [PubMed] [Google Scholar]
- 49.Martino H.S.D., Kolba N., Tako E. Yacon (Smallanthus sonchifolius) Flour Soluble Extract Improve Intestinal Bacterial Populations, Brush Border Membrane Functionality and Morphology in Vivo (Gallus Gallus) Food Res. Int. 2020;137:109705. doi: 10.1016/j.foodres.2020.109705. [DOI] [PubMed] [Google Scholar]
- 50.Tako E., Ferket P.R., Uni Z. Changes in Chicken Intestinal Zinc Exporter MRNA Expression and Small Intestinal Functionality Following Intra-Amniotic Zinc-Methionine Administration. J. Nutr. Biochem. 2005;16:339–346. doi: 10.1016/j.jnutbio.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Uni Z., Noy Y., Sklan D. Posthatch Development of Small Intestinal Function in the Poult. Poult. Sci. 1999;78:215–222. doi: 10.1093/ps/78.2.215. [DOI] [PubMed] [Google Scholar]
- 52.New York Apple Association Apples from New York: Varieties-Empire. [(accessed on 17 July 2022)]. Available online: https://www.applesfromny.com/varieties/empire/
- 53.US Apple Association U.S. Apple Association: Apple Varieties. [(accessed on 12 July 2022)]. Available online: https://usapple.org/apple-varieties.
- 54.Asgary S., Rastqar A., Keshvari M. Weight Loss Associated With Consumption of Apples: A Review. J. Am. Coll. Nutr. 2018;37:627–639. doi: 10.1080/07315724.2018.1447411. [DOI] [PubMed] [Google Scholar]
- 55.Cho K.-D., Han C.-K., Lee B.-H. Loss of Body Weight and Fat and Improved Lipid Profiles in Obese Rats Fed Apple Pomace or Apple Juice Concentrate. J. Med. Food. 2013;16:823–830. doi: 10.1089/jmf.2013.2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Samout N., Bouzenna H., Dhibi S., Ncib S., ElFeki A., Hfaiedh N. Therapeutic Effect of Apple Pectin in Obese Rats. Biomed. Pharmacother. 2016;83:1233–1238. doi: 10.1016/j.biopha.2016.08.038. [DOI] [PubMed] [Google Scholar]
- 57.McKie A.T. The Role of Dcytb in Iron Metabolism: An Update. Biochem. Soc. Trans. 2008;36:1239–1241. doi: 10.1042/BST0361239. [DOI] [PubMed] [Google Scholar]
- 58.Lane D., Bae D.-H., Merlot A., Sahni S., Richardson D. Duodenal Cytochrome b (Dcytb) in Iron Metabolism: An Update on Function and Regulation. Nutrients. 2015;7:2274–2296. doi: 10.3390/nu7042274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Warkentin T., Kolba N., Tako E. Low Phytate Peas (Pisum sativum L.) Improve Iron Status, Gut Microbiome, and Brush Border Membrane Functionality in Vivo (Gallus Gallus) Nutrients. 2020;12:2563. doi: 10.3390/nu12092563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shah M., Griffin I.J., Lifschitz C.H., Abrams S.A. Effect of Orange and Apple Juices on Iron Absorption in Children. Arch. Pediatr. Adolesc. Med. 2003;157:1232–1236. doi: 10.1001/archpedi.157.12.1232. [DOI] [PubMed] [Google Scholar]
- 61.Ravn-Haren G., Krath B.N., Markowski J., Poulsen M., Hansen M., Kolodziejczyk K., Kosmala M., Dragsted L.O. Apple Pomace Improves Gut Health in Fisher Rats Independent of Seed Content. Food. Funct. 2018;9:2931–2941. doi: 10.1039/C7FO01932G. [DOI] [PubMed] [Google Scholar]
- 62.Lee B., Moon K.M., Kim C.Y. Tight Junction in the Intestinal Epithelium: Its Association with Diseases and Regulation by Phytochemicals. J. Immunol. Res. 2018;2018:1–11. doi: 10.1155/2018/2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Suzuki T. Regulation of the Intestinal Barrier by Nutrients: The Role of Tight Junctions. Anim. Sci. J. 2020;91:e13357. doi: 10.1111/asj.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki T. Regulation of Intestinal Epithelial Permeability by Tight Junctions. Cell Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2: Novel Integral Membrane Proteins Localizing at Tight Junctions with No Sequence Similarity to Occludin. J. Cell Iology. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: A Novel Integral Membrane Protein Localizing at Tight Junctions. J. Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ma C., Sun Z., Chen C., Zhang L., Zhu S. Simultaneous Separation and Determination of Fructose, Sorbitol, Glucose and Sucrose in Fruits by Hplc-Elsd. Food. Chem. 2014;145:784–788. doi: 10.1016/j.foodchem.2013.08.135. [DOI] [PubMed] [Google Scholar]
- 68.Yang S., Meng Z., Li Y., Chen R., Yang Y., Zhao Z. Evaluation of Physiological Characteristics, Soluble Sugars, Organic Acids and Volatile Compounds in “Orin” Apples (Malus domestica) at Different Ripening Stages. Molecules. 2021;26:807. doi: 10.3390/molecules26040807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bischoff S.C., Kaden-Volynets V., Filipe Rosa L., Guseva D., Seethaler B. Regulation of the Gut Barrier by Carbohydrates from Diet–Underlying Mechanisms and Possible Clinical Implications. Int. J. Med. Microbiol. 2021;311:151499. doi: 10.1016/j.ijmm.2021.151499. [DOI] [PubMed] [Google Scholar]
- 70.Volynets V., Louis S., Pretz D., Lang L., Ostaff M.J., Wehkamp J., Bischoff S.C. Intestinal Barrier Function and the Gut Microbiome Are Differentially Affected in Mice Fed a Western-Style Diet or Drinking Water Supplemented with Fructose. J. Nutr. 2017;147:770–780. doi: 10.3945/jn.116.242859. [DOI] [PubMed] [Google Scholar]
- 71.Laffin M., Fedorak R., Zalasky A., Park H., Gill A., Agarwal A., Keshteli A., Hotte N., Madsen K.L. A High-Sugar Diet Rapidly Enhances Susceptibility to Colitis via Depletion of Luminal Short-Chain Fatty Acids in Mice. Sci. Rep. 2019;9:1–11. doi: 10.1038/s41598-019-48749-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Goldstein J., Morris W.E., Loidl C.F., Tironi-Farinatti C., McClane B.A., Uzal F.A., Fernandez Miyakawa M.E. Clostridium Perfringens Epsilon Toxin Increases the Small Intestinal Permeability in Mice and Rats. PLoS ONE. 2009;4:e7065. doi: 10.1371/journal.pone.0007065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lopetuso L.R., Scaldaferri F., Petito V., Gasbarrini A. Commensal Clostridia: Leading Players in the Maintenance of Gut Homeostasis. Gut Pathog. 2013;5:23. doi: 10.1186/1757-4749-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moore R., Pothoulakis C., LaMont J.T., Carlson S., Madara J.L.C. Difficile Toxin A Increases Intestinal Permeability and Induces Cl- Secretion. Am. J. Physiol. 1990;259:G165–G172. doi: 10.1152/ajpgi.1990.259.2.G165. [DOI] [PubMed] [Google Scholar]
- 75.Paczosa M.K., Mecsas J. Klebsiella Pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016;80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Bang S.-J., Kim G., Lim M.Y., Song E.-J., Jung D.-H., Kum J.-S., Nam Y.-D., Park C.-S., Seo D.-H. The Influence of in Vitro Pectin Fermentation on the Human Fecal Microbiome. AMB Express. 2018;8:10–1186. doi: 10.1186/s13568-018-0629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Larsen N., Bussolo de Souza C., Krych L., Barbosa Cahu T., Wiese M., Kot W., Meyer Hansen K., Blennow A., Venema K., Jespersen L. Potential of Pectins to Beneficially Modulate the Gut Microbiota Depends on Their Structural Properties. Front. Microbiol. 2019;10:223. doi: 10.3389/fmicb.2019.00223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guo P., Zhang K., Ma X., He P. Clostridium Species as Probiotics: Potentials and Challenges. J. Anim. Sci. Biotechnol. 2020;11:24. doi: 10.1186/s40104-019-0402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Licht T.R., Hansen M., Bergström A., Poulsen M., Krath B.N., Markowski J., Dragsted L.O., Wilcks A. Effects of Apples and Specific Apple Components on the Cecal Environment of Conventional Rats: Role of Apple Pectin. BMC Microbiol. 2010;10:13. doi: 10.1186/1471-2180-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dufourny S., Antoine N., Pitchugina E., Delcenserie V., Godbout S., Douny C., Scippo M.-L., Froidmont E., Rondia P., Wavreille J., et al. Apple Pomace and Performance, Intestinal Morphology and Microbiota of Weaned Piglets—A Weaning Strategy for Gut Health? Microorganisms. 2021;9:572. doi: 10.3390/microorganisms9030572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Laudadio V., Passantino L., Perillo A., Lopresti G., Passantino A., Khan R.U., Tufarelli V. Productive Performance and Histological Features of Intestinal Mucosa of Broiler Chickens Fed Different Dietary Protein Levels. Poult. Sci. 2012;91:265–270. doi: 10.3382/ps.2011-01675. [DOI] [PubMed] [Google Scholar]
- 82.Pluske J.R., Thompson M.J., Atwood C.S., Bird P.H., Williams I.H., Hartmann P.E. Maintenance of Villus Height and Crypt Depth, and Enhancement of Disaccharide Digestion and Monosaccharide Absorption, in Piglets Fed on Cows’ Whole Milk after Weaning. Br. J. Nutr. 1996;76:409–422. doi: 10.1079/BJN19960046. [DOI] [PubMed] [Google Scholar]
- 83.Oliveira M.C., Rodrigues E.A., Marques R.H., Gravena R.A., Guandolini G.C., Moraes V.M.B. Performance and Morphology of Intestinal Mucosa of Broilers Fed Mannan-Oligosaccharides and Enzymes [Desempenho e Morfologia Da Mucosa Intestinal de Frangos de Corte Alimentados Com Mananoligossacarídeos e Enzimas] Arq. Bras. Med. Vet. Zootec. 2008;60:442–448. doi: 10.1590/S0102-09352008000200025. [DOI] [Google Scholar]
- 84.Birchenough G.M.H., Johansson M.E.V., Gustafsson J.K., Bergström J.H., Hansson G.C. New Developments in Goblet Cell Mucus Secretion and Function. Mucosal. Immunol. 2015;8:712–719. doi: 10.1038/mi.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang S., Li Q., Zang Y., Zhao Y., Liu N., Wang Y., Xu X., Liu L., Mei Q. Apple Polysaccharide Inhibits Microbial Dysbiosis and Chronic Inflammation and Modulates Gut Permeability in HFD-Fed Rats. Int. J. Biol. Macromol. 2017;99:282–292. doi: 10.1016/j.ijbiomac.2017.02.074. [DOI] [PubMed] [Google Scholar]
- 86.Lueschow S.R., McElroy S.J. The Paneth Cell: The Curator and Defender of the Immature Small Intestine. Front. Immunol. 2020;11:587. doi: 10.3389/fimmu.2020.00587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Salzman N.H., Bevins C.L. Dysbiosis-A Consequence of Paneth Cell Dysfunction. Semin. Immunol. 2013;25:334–341. doi: 10.1016/j.smim.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 88.Bevins C., Salzman N. Paneth Cells, Antimicrobial Peptides and Maintenance of Intestinal Homeostasis. Nat. Rev. Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 89.Beukema M., Faas M., de Vos P. The Effects of Different Dietary Fiber Pectin Structures on the Gastrointestinal Immune Barrier: Impact via Gut Microbiota and Direct Effects on Immune Cells. Exp. Mol. Med. 2020;52:1364–1376. doi: 10.1038/s12276-020-0449-2. [DOI] [PMC free article] [PubMed] [Google Scholar]