Abstract
Areca nut (AN) is widely consumed all over the world, bringing great harm to human health and economy. Individuals with AN chewing are at high risk of cardiovascular disease and impaired immune system and metabolic system. Despite a growing number of studies having reported on the adverse effects brought by AN chewing, the exact mechanism of it is limited and the need for additional exploration remains. In recent years, the interaction between microorganisms, especially intestinal microorganism and host, has been extensively studied. AN chewing might disrupt the oral and intestinal microbiota communities through direct connect with the microbes it contains, altering PH, oxygen of oral and intestinal microenvironment, and disturbing the immune homeostasis. These mechanisms provide insights into the interplay between areca nut and host microbiota. Emerging studies have proposed that bidirectional interaction between polyphenols and intestinal microbes might play a potential role in the divergence of polyphenol, extracted from AN, among individuals with or without AN-induced cancer development and progression. Although some AN chewers have been aware of the harmful effects brought by AN, they cannot abolish this habit because of the addiction of AN. Increasing studies have tried to revealed that gut microbiota might influence the onset/development of addictive behaviors. Altogether, this review summarizes the possible reasons for the disturbance of host microbiota caused by areca nut chewing and clarifies the complex interaction between human microbiome and major constituents and the addiction and carcinogenicity of AN, tempting to provide novel insights into the development and utilization of it, and to control the adverse consequences caused by AN chewing.
Keywords: areca nut, polyphenol, arecoline, gut microbiota, addiction, carcinogenicity
1. Introduction
Approximately 10–20% of the world’s population use areca nut (AN) products in some form, with the most prevalent use in many regions in south Asia, south-east Asia, and the Asia Pacific region [1,2]. AN is also widely consumed, with or without tobacco, among the Australian, Canadian, European, and USA Indo-Asian immigrants [3]. AN has many clinical effects, such as dispelling nausea, improving cognitive performance, aiding digestion, inhibiting inflammation, fighting against parasitic infections and hypertension, and acting as an antidepressant as well as a substitute for cigarettes [4,5]. However, AN is the fourth most addictive substance used in the world, only surpassed by nicotine, alcohol, and caffeine [6,7]. AN has been identified as carcinogenic to humans (Group 1) by the International Agency for Cancer Research (IARC) [8]. There is much evidence that AN chewing is associated with oral, pharyngeal, and esophageal cancers [9,10,11]. It is also reported that AN chewing habit has detrimental effects on the metabolic system [12], increasing the risk of obesity [13], hyperglycemia [14], and, causing hypothyroidism [15] and vitamin D deficiency [16]. Chronic AN consumption also interferes with the immune system [17,18,19], causing suppression of T-cell activity and decreased release of cytokines [20]. AN usage also increases cardiovascular disease rate, including heart attack, coronary artery diseases [21,22] and paroxysmal supraventricular tachycardia (PSVT) [23]. Despite a growing number of studies that have reported the adverse effects brought by areca chewing, the exact mechanism remains to be explored. There are still about 600 million people, including children using betel around the world [6], causing great damage to human health and property.
As the second genome of the human body, the human microbiome has become an area of utmost interest, which is not a passive victim in many pathological processes, but a driver or stimulator in pathophysiological processes [24]. Microbiome refers to the community of microbes that reside in a defined environment, including bacteria, viruses, fungi, protozoa, along with their genes and genomes. The gastrointestinal tract is the most popular region for microbiota colonizing, followed by oral cavity [25]. Microbes residing in humans evolve with hosts and are susceptible to living habits, such as diet, tobacco, alcohol, and areca nut, which are causal factors of many disorders. Early studies implicated alterations in oral microbial composition in areca nut chewers with distinct oral premalignant lesions such as leukoplakia, erythroplakia, and submucous fibrosis [26,27], which contributes to oral cancers. Mei, et al. reported that AN seed polyphenol altered the composition of the gut microbiome [28]. In this review, we summarize the current understanding of the interaction of major constituents in areca nut and host microbiome and its involvement in the addiction and carcinogenesis of AN in an attempt to raise profound research questions that remain to be explored.
2. Microorganisms Contained in Areca Nut Alter Oral and Intestinal Microenvironment
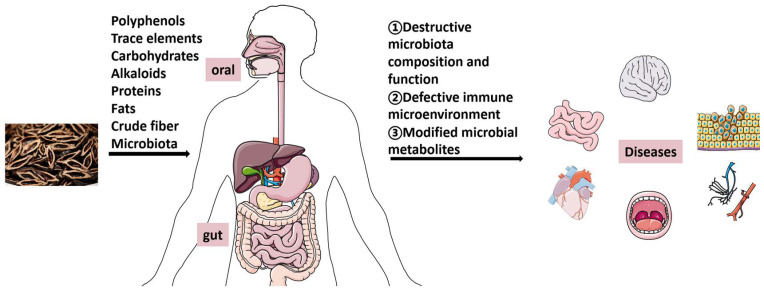
AN products are easily available and the quality is not controlled very strictly because of their low prices [29]. AN is used at distinct stages of maturity in natural state or after processing in many forms, bringing different microbes to host microbiome. A pilot study, evaluating 12 samples of areca nut-containing chewing substances, found that wet gutka preparations were contaminated by Escherichia coli and Enterobacteriacaea. High levels of fungal aflatoxin (range: 0.43–1.84 mg/kg), a proven carcinogen, were identified in all samples [30]. Massive studies demonstrated the adverse health impacts of areca nut on systemic pathophysiological changes that might lead to disease, and that they are associated with the chemistry; metabolism; and pharmacology of polyphenols, tannins, trace elements, and areca alkaloids, specifically presenting in AN [31]. However, few studies investigated the microbes in areca nut in recent years, and this may be incriminated as causative factors in AN-chewing-associated diseases. More studies are required to be conducted to explore the microbes that distinct AN products contain. AN chewing is known to impair the host immune system, presenting suppression of T-cell activity and decreased release of cytokines [32]. Disruption of the immune balance might induce changes in the composition and function of the host microorganism. Furthermore, it is reasonable that chronic exposure to areca nut chewing is likely to favor specific bacterial colonization via altering oxygen, PH, and acid production of oral cavity. Hernandez, et al. demonstrated that current chewers had significantly elevated levels of Streptococcus infantis and various levels of distinct taxa of the Actinomyces and Streptococcus genera [26]. Zhou, et al. explored the effects of the Fuzhuan brick tea supplemented with different concentrations of ANs on gut microbiota in mice, and found that influence on intestinal microbial structure increased as the concentration of AN increased [33]. Altogether, mechanisms of areca nut chewing to influence host microbiota are through direct connect with the microbes it contains, altering the PH and oxygen of the oral and intestinal microenvironment, or through disturbing the immune homeostasis, and these mechanisms provide insights into the interplay between areca nut and host microbiota (Figure 1).
Figure 1.
The major constituents of areca nut-induced dysbiosis of microbiome and its possible role in different diseases.
3. Major Constituents in Areca Nut
Areca nut, as a natural product, is composed of a variety of ingredients. Major constituents in AN include trace elements, polyphenols (flavanols and tannins), carbohydrates, alkaloids, proteins, fats, and crude fiber. Among them, the carcinogenic potential of AN is attributed to polyphenols and areca alkaloids [34,35]. The latter also contributes to addiction of AN [35]. Next, we reviewed the main components of areca nut that drives addiction and oncogenicity and highlighted the mechanisms by which microbiota and/or their microbial metabolites exert their action on the polyphenols and areca alkaloids of areca nut as promoters of the addictive and carcinogenic effects.
4. Carcinogens in Areca Nut
A large body of literature found that habitual AN chewing was tightly associated with the occurrence and development of oral, esophageal, and pharyngeal cancers [9,10,11,36,37]. In addition, many studies revealed that long-term AN usage increased the risk of hepatocellular carcinoma (HCC) [38]. Chao, et al. found that AN chewing was a significant factor for non-muscle-invasive bladder cancer recurrence [39]. In-vitro arrays showed that areca nut extract treatment enhanced migration and invasion of head and neck squamous cell carcinoma (HNSCC) cells by upregulating cyclooxygenase-2 (COX-2)/vimentin expression, which is associated with poor survival of HNSCC patients [40]. Studies have reviewed the potential carcinogenic mechanism of alkaloids, while few studies have been conducted to explore the oncogenicity of areca nut polyphenols.
4.1. Contradictory Role of Polyphenols in Cancers
Evidence is emerging on the toxicity of the dietary polyphenols extracted by areca nut [41]. Major polyphenols found in AN are tannins, catechins, flavonoids, safrole, and eugenol, among which, tannins, safrole, eugenol, and catechins have been proven to be carcinogens. Many experimental and preclinical studies proposed that polyphenol and tannin fractions of AN had a relevant role in BQ-induced cancer development and progression, mainly attributed to their immunomodulatory properties [42,43]. For example, reactive oxygen species (ROS) produced during the autoxidation of BN polyphenols in the saliva of chewing BQ is crucial in the initiation and promotion of oral cancer [44]. Also, incidences of esophageal cancer have been reported to be associated with consumption of tannins-rich foods such as BN, and carcinogenic activity of tannins might be related to components associated with tannins rather than tannins themselves, suggesting a potential role of gut microbiota in tannins carcinogenicity. Epidemiological studies found that a polyphenol-rich diet protected against cancer, as well as many short-term assays revealed that AN polyphenols and tannins were not mutagenic and, in fact, even had antimutagenic effects. Some literature reported that polyphenols favored the generation of ROS [45]. Contrasting this, AN polyphenols were reported to be able to form conjugates with carcinogens, to trap nitrite and ROS [46,47]. The prominent polyphenols in AN and their contradictory activities are summarized in Table 1. And reported major classes of polyphenols extracted from other plants are also showed in Table 2.
Table 1.
Prominent polyphenols in AN and their activities.
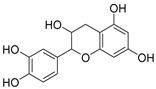
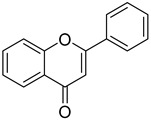
| Formulas | Polyphenols | Activity | Gut Microbiota-Relevant | Reference |
|---|---|---|---|---|
|
Catechin | Antimicrobial, antioxidant, anti-cancer and carcinogenic activity | Yes | [48,49] |
|
Flavonoids | Anti-inflammatory, antioxidant and anti-cancer effects, anti-bacteria and anti-virus | Yes | [9,50] |
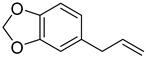
|
Safrole | Anti-cancer and antioxidant effects | Yes | [51] |
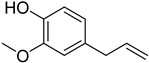
|
Eugenol | Anti-bacteria and antihypertensive effect | Yes | [52] |
Table 2.
Other reported major classes of polyphenols extracted from plants and their activities.
| Polyphenols | Representative Plant | Activity | Gut Microbiota-Dependent | Reference |
|---|---|---|---|---|
| Resveratrol | Reynoutria japonica Houtt. and Vitis | Antimicrobial, antioxidant, and anti-inflammatory activity | Yes | [53] |
| Quercetin | Fagopyrum esculentum Moench. | Anti-virus, anti-bacterial, anti-cancer, and cardiovascular-protective effect | Yes | [54] |
| Catechin | Green tea | Anti-cancer, anti-virus, anti-fungi, anti-bacterial, and cardiovascular-protective effect | Yes | [55] |
| Puerarin | Pueraria lobata | Anti-oxidant, anti-inflammatory, antihypertensive, and neuroprotective activity | Yes | [56] |
| Anthocyanidin | Elderberries | Anti-oxidant, anti-mutagenic, and anti-proliferative properties | Yes | [57] |
| Tannic acid | Fruit | Anti-oxidant and anti-bacterial effect | Yes | [58] |
The underlying mechanism of these observed dual, and apparently contradictory, functions of AN polyphenols and tannins in the process of BQ-induced carcinogenesis remains to be explored.
4.2. Bidirectional Interaction between Polyphenols and Intestinal Microbes
Polyphenols have an extremely low oral bioavailability and are almost unchanged when reaching the colon, and most of them are intensively catabolized by gut microbiota to a wide variety of new chemical structures that are often more active and better absorbed than the original phenolic compound passing hardly into the systemic blood circulation [59,60,61], and in turn, polyphenols could modulate oral and gut microbiota composition in host homeostasis and diseases. Several human and animal studies reported that polyphenols can elevate butyrate-producing bacterial and probiotics such as Lactobacillus and Bifidobacterium, while inhibiting opportunistic pathogenic or proinflammatory microbes. For example, bound polyphenol from foxtail millet bran can inhibit colitis-associated carcinogenesis by remodeling gut microbiota in a mice model [62]. The two-way interplay between microbial communities and polyphenols in the intestine is important for the latter to exert anticancer effects and might be the underlying mechanism for their contradictory and dual effects. Emerging studies have reported that diet polyphenols exert anti-obesity [63,64,65,66], anti-inflammatory, and anti-oxidant effects via modulating gut microbiota. For instance, Ho, et al. found that Heterogeneity in gut microbiota drive polyphenol metabolism that influences α-synuclein misfolding and toxicity [67]. Mei, et al. revealed that areca nut (areca catechu L.) seed polyphenol could ameliorate osteoporosis via altering gut microbiota to increase lysozyme expression and controlled the inflammatory reaction in estrogen-deficient rats [28]. Studies have shown that AN could supplement polyphenols such as chlorogenic acid, (+)-catechin, (−)-epigallocatechin gallate, (−)-gallocatechin gallate, rutin, and theaflavin, which could greatly reduce high-fat diet-induced adverse effects, via easing food stagnation, eliminating indigestion, enhancing gastrointestinal motility, and regulating the activity of related enzymes [68,69]. Meanwhile, studies have revealed that AN could increase the risk of obesity and hyperglycemia. Gut microbes have the potential to explain these two opposite effects induced by AN. Despite few studies having explored the role of gut microbes in the carcinogenicity of polyphenols, there have been several studies linking gut microbes to cancer development and treatment [70,71,72,73]. For example, intestinal fusobacterium nucleatum promotes colorectal cancer development and facilitates tumor metastasis and chemoresistance to 5-fluorouracil via its immunosuppressive effects [74,75,76]. Also, gut microbiota regulates the activity, efficacy, and toxicity of chemotherapy agents, such as gemcitabine [77], cyclophosphamide [78,79], irinotecan [80,81,82], and cisplatin [83,84,85]. Further research is required to clarify whether areca nut chewing changes the composition and function of intestinal microbes or whether intestinal microbes metabolize areca nut into carcinogens.
In recent years, there has been increasing evidence that the aryl hydrocarbon receptor (AhR) plays a major role in tumorigenesis and makes the AhR an interesting pharmacological target in cancer treatment [86,87,88,89]. Polyphenols, especially flavonoids, major constituents of AN, are the largest class of natural AhR ligands that are available for humans and animals [90,91]. Mounting evidence demonstrated that flavonoids, exhibiting AhR agonist and/or antagonist activity, are widely used for the regulation of the intestinal immune system and tumor treatment [92,93,94,95,96]. Dietary flavonoids are absorbed in the intestine, and the intestinal microbiota, which is deeply involved in the metabolism of them, originated from foods [97], and in turn, acting as AhR ligands, are able to regulate intestinal microbiota composition and intestinal immunity [98]. For example, tryptophan, a reported AhR ligand, could be metabolized by the certain bacterial strain, Lactobacillus bulgaricus OLL11816, to AhR-activating indoles that have shown AhR-activating potential [99,100]. However, whether flavonoids are dietary, generated by the host, or through bacterial metabolism has not been exactly established and requires further investigation.
In conclusion, few studies have reported the gut microbiome profiles in AN chewers, but some studies have shown oral microbiota composition alteration might mirror oral cancer progression in AN chewers [26,27]. However, whether microbial changes are involved in areca nut-induced oral carcinogenesis is only speculative. Further research is required to discern the clinical significance of an altered oral microbiota and the mechanisms of oral cancer development in areca nut chewers. Additional studies are necessary to clarify the precise metabolic intermediates of AN by gut microbiota or the single agent responsible for AN toxicity.
5. Addiction in Areca Nut
5.1. Areca Alkaloids(Arecoline)
Although some AN chewers have been aware of the harmful effects brought by AN, they cannot abolish this habit because AN quid chewing is able to produce a sense of well-being, euphoria, warm sensation of the body, salivation, palpitation, sweating and heightened alertness, combat against hunger, and increase capacity and stamina to work by its numerous central nervous system effects [101]. It has been reported that the addictive property of AN is prominently due to its alkaloids [35]. Further, various levels of areca alkaloids could potentially contribute to variations in addictive potential in AN [102,103]. Major alkaloids found in AN are arecoline, arecaidine, guvacoline, and guvacine. Major alkaloids found in AN and their activities are showed in Table 3. Among these, arecoline has deep brain penetration to exert its numerous parasympathetic and muscarinic effects, which is responsible for the addiction and habitual use of AN. Mechanistically, arecoline acts on nicotinic acetylcholine receptor (nAChR), which partly accounts for the addiction and habitual use of AN [104,105], while the present data suggests a role also played by sympathetic activation [99]. These studies demonstrated further that the effects of arecoline reached the maximal within 4–6 min and high levels of arecoline are present in the oral cavity even 10 min after the onset of AN chewing, suggesting that active compounds released from areca nut chewing are absorbed mainly in the oral cavity, most probably through the mucous membrane [106].
Table 3.
Major alkaloids found in AN and their activities.
| Formulas | Alkaloids | Activity | Reference |
|---|---|---|---|
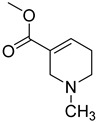
|
Arecoline | Effects on nervous, cardiovascular, endocrine and digestive system; anti-parasitic effects; carcinogenic; and genotoxic | [90] |
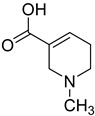
|
Arecaidine | Effects on nervous and endocrine system | [107] |
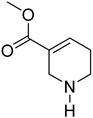
|
Guvacoline | Effects on nervous system, anti-inflammatory, and anti-cancer activity | [91] |
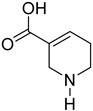
|
Guvacine | Effects on nervous system and anti-cancer activity | [108] |
5.2. Microbiota-Gut-Brain Axis: A Potential Regulator of AN Addiction
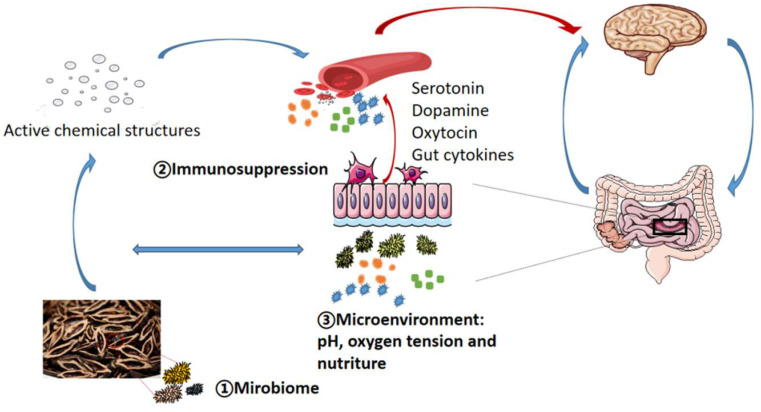
In addition, the proposal of microbiota–gut–brain axis provides a novel insight into clarifying the addictive mechanism of AN (Figure 2). Gut cytokines are known to activate the vagus nerve that constitutes the main axis transferring gut microbiota information to the brain, while the latter induces pro-rewarding effects in nucleus accumbens [109]. An expanding body of evidence supports that gut microbiota modifications and/or manipulations may also play a crucial role in the manifestation of specific behavioral responses regulated by neuroendocrine pathways. The gut microbiota and their metabolites influence neuroendocrine function through several routes, including the vagus nerve; immune activation with production of immune mediators; and production of neurotransmitters, short chain fatty acids (SCFAs), and tryptophan, to modify host behaviors relevant to stress, addiction, cognition, eating, and sexual and social behavior [110]. Xu, et al. examined the composition and diversity of intestinal microbiota in patients with substance use disorders (SUDs) and in healthy controls (HCs). The results showed that the abundances of Thauera, Paracoccus, and Prevotella are significantly higher in SUDs compared to HCs [111]. Further, gut microbiota is related to excessive alcohol consumption and induces altered striatal dopamine receptor expression in a compulsive alcohol-seeking model [112]. Mounting studies have shown that cocaine and alcoholism addiction is associated with changes in the composition of the gut microbiota [113,114,115,116,117,118].
Figure 2.
The possible mechanisms of AN-chewing-induced microbial dysbiosis and the role of gut microbiota in areca nut metabolism, as well as the direct and indirect interaction among gut microbiota, neuroendocrinology, and addiction.
Importantly, emerging studies suggested that the gut microbiota might influence the onset/development of addictive behaviors. The role of gut microbes in alcohol dependence has been increasingly reported. Oral administration of nonabsorbable antibiotics reduces the voluntary alcohol intake in alcohol-preferring animals [109]. Individuals presented with an increased intestinal permeability and a dysbiosis might show a more severe profile of alcohol dependence than other non-dysbiosis controls [119]. Also, gut microbiota dysbiosis during chronic alcohol exposure is closely correlated with alcohol-induced neuropsychic behaviors and BDNF/Gabra1 expression [120]. The above studies provide a new perspective for understanding underlying mechanisms in alcohol addiction. Further, gut microbiota is capable of modulating alcohol withdrawal-induced anxiety in mice [121], and altering sociability and depression by inducing β-hydroxybutyrate metabolism changes in alcohol use disorder [122]. Peterson, et al. found sex-dependent associations between addiction-related behaviors and the gut microbiota composition in outbred rats [123].
Alteration of the gut microbiota in mice also drives the behavioral response to cocaine. Animals with reduced gut bacteria, by treating with prolonged treatment with non-absorbable antibiotics, showed an enhanced sensitivity to cocaine reward and enhanced sensitivity to the locomotor-sensitizing effects of repeated cocaine administration [44]. Lee, et al. showed that the gut microbiota causally mediated reward and sensory responses related to regimen-selective morphine dependence. Depleting the gut microbiota via antibiotic treatment recapitulated neuroinflammation and sequelae, including reduced opioid analgesic potency and impaired cocaine reward following intermittent morphine treatment [124]. Chronic depletion of gut microbiota also affects other behavioral and neurochemical consequences in the rat [125]. For example, Burokas, et al. showed that manipulating microbiota, thus targeting the microbiota–gut–brain axis, had anxiolytic and antidepressant-like effects and reversed the impact of chronic stress in mice [126]. In addition, oral administration of heat-killed lactobacilli can alter the social behavior of healthy mice [127]. The above findings provided direct evidence of the link between gut microbiota, neuroendocrinology, and addiction or other behavioral responses, highlighting the key role of the gut microbiota in the formation and treatment of drug dependence, and may provide new treatment strategies for using novel medicine targeting gut microbiota to treat drug addiction. In addition, gut microbiota might directly influence the development or onset of addiction via modulating the oxytocin, serotonin, and dopamine levels and function [110]. While the underling mechanism is required to further investigate, in order to assess which other neuroendocrine pathways are involved in addiction and in which cases the gut microbiota plays a causal role. The relationship between microbes and areca nut addiction also remains to be explored.
6. Conclusions and Future Perspectives
Altogether, more microbiome research remains to be conducted in the coming years in an attempt to better characterize the role of human microbiome in carcinogenicity and addiction of areca nut. AN chewing is known to be a risk factor for several diseases, it could influence the human microbiome directly and indirectly via introducing its own microbiota, suppressing the immune system, changing the local microenvironment, or other potential mechanisms. The exact explanation of how AN chewing affects the microbiome still requires further exploration.
The property of carcinogenicity and addiction in AN brings great harm to people’s health; there is no adequate explanation for it currently. However, it is known that the carcinogenicity of areca nut is mainly polyphenols and arecoline, and the latter is significantly associated with areca nut addiction. Polyphenols, originating from various natural products, have been reported to have both carcinogenic and anticancer effects, with a two-way interaction with gut microbiota. Most polyphenols are intensively catabolized by gut microbiota to a wide variety of new chemical structures, with physiological and biochemical effects. Further, polyphenols could change the gut microbiota composition and function. Establishing an adequate mechanism of how gut microbes and polyphenols interplay might help us clarify dual, contradictory property of polyphenols. Recently, the notion of microbiota–gut–brain axis provides a novel insight into clarifying addictive mechanisms of AN. Several studies have reported a significant alteration in individuals of alcohol and drug addiction. It has long been recognized that gut cytokines can activate the vagus nerve, and the latter induces pro-rewarding effects in nucleus accumbens. In addition, mounting studies suggest that the gut microbiota might directly or indirectly influence to some extent the onset/development of addictive behaviors. Despite the findings for the interaction of AN chewing addiction and gut microbiota being scarce, reported studies provide some evidence of the link among gut microbiota, neuroendocrinology, and addiction. Further investigation is required in order to assess in which cases the gut microbiota plays a causal role in AN addiction and which other neuroendocrine pathways are involved. More studies integrating metagenomics, transcriptomics, and metabolomics with clinical results are required to gain more insight into the hugely complex network of the AN chewing-microbiome-host phenotype; in addition, finding a novel intervention target to solve the carcinogenicity and addiction of areca nut is important.
Author Contributions
Writing—Original draft preparation, L.C., L.K. and S.C.; Writing—Reviewing and Editing, F.Y., X.L. and W.Z.; Visualization, F.Y., X.L. and W.Z.; Supervision, F.Y., X.L. and W.Z.; Funding acquisition, F.Y., X.L. and W.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by 2021YFA1301200 from National Key R&D Program of China, Grant No. 81874329 and 82073945 by National Natural Science Foundation of China, B2013-097 from Scientific Research Project of Hunan Provincial Health Commission, and 2018SK50907 from Sci-ence and Technology Innovation Program of Hunan Province.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nilesh C., Himanshu A.G. A Qualitative Study of Perceptions and Practices Related to Areca Nut Use Among Adolescents in Mumbai, India. Nicotine Tob. Res. 2021;23:1793–1800. doi: 10.1093/ntr/ntab067. [DOI] [PubMed] [Google Scholar]
- 2.Hongmei H., Ting W. Occurrence of areca alkaloids in wastewater of major Chinese cities. Sci. Total Environ. 2021;783:146961. doi: 10.1016/j.scitotenv.2021.146961. [DOI] [PubMed] [Google Scholar]
- 3.Nidhi S., Rona P. Perceptions and Practices of General Practitioners towards Oral Cancer and Emerging Risk Factors among Indian Immigrants in Australia: A Qualitative Study. Int. J. Environ. Res. Public Health. 2021;18:11111. doi: 10.3390/ijerph182111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strickland S.S. Anthropological perspectives on use of the areca nut. Addict. Biol. 2002;7:85–97. doi: 10.1080/13556210120091446. [DOI] [PubMed] [Google Scholar]
- 5.Liu F.L., Chen C.L., Lai C.C., Lee C.C., Chang D.M. Arecoline suppresses RANKL-induced osteoclast differentiation in vitro and attenuates LPS-induced bone loss in vivo. Phytomedicine. 2020;69:153195. doi: 10.1016/j.phymed.2020.153195. [DOI] [PubMed] [Google Scholar]
- 6.Benegal V., Rajkumar R.P., Muralidharan K. Does areca nut use lead to dependence? Drug Alcohol Depend. 2008;97:114–121. doi: 10.1016/j.drugalcdep.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Pandya S., Chaudhary A.K., Singh M., Singh M., Mehrotra R. Correlation of histopathological diagnosis with habits and clinical findings in oral submucous fibrosis. Head Neck Oncol. 2009;1:10. doi: 10.1186/1758-3284-1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza S.S., Shafique K., Vart P., Arain M.I. Areca nut chewing and dependency syndrome: Is the dependence comparable to smoking? a cross sectional study. Subst. Abuse Treat. Prev. Policy. 2011;6:23. doi: 10.1186/1747-597X-6-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nurmeen A., Hamad A. Evaluation of cytotoxicity of areca nut and its commercial products on normal human gingival fibroblast and oral squamous cell carcinoma cell lines. J. Hazard. Mater. 2021;403:123872. doi: 10.1016/j.jhazmat.2020.123872. [DOI] [PubMed] [Google Scholar]
- 10.Gupta S., Gupta R., Sinha D.N., Mehrotra R. Relationship between type of smokeless tobacco & risk of cancer: A systematic review. Indian J. Med. Res. 2018;148:56–76. doi: 10.4103/ijmr.IJMR_2023_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shehryar N.K., Arif J., Raza T.H. APrevalence of pain in oral cancer: A retrospective study. Oral Dis. 2021;27:1806–1812. doi: 10.1111/odi.13701. [DOI] [PubMed] [Google Scholar]
- 12.Javed F., Al-Hezaimi K., Warnakulasuriya S. Areca-nut chewing habit is a significant risk factor for metabolic syndrome: A systematic review. J. Nutr. Health Aging. 2012;16:445–448. doi: 10.1007/s12603-011-0353-5. [DOI] [PubMed] [Google Scholar]
- 13.Yen A.M., Chiu Y.H., Chen L.S., Wu H.M., Huang C.C., Boucher B.J., Chen T.H. A population-based study of the association between betel-quid chewing and the metabolic syndrome in men. Am. J. Clin. Nutr. 2006;83:1153–1160. doi: 10.1093/ajcn/83.5.1153. [DOI] [PubMed] [Google Scholar]
- 14.Hsu H.F., Tsou T.C., Chao H.R., Shy C.G., Kuo Y.T., Tsai F.Y., Yeh S.C., Ko Y.C. Effects of arecoline on adipogenesis, lipolysis, and glucose uptake of adipocytes-A possible role of betel-quid chewing in metabolic syndrome. Toxicol. Appl. Pharmacol. 2010;245:370–377. doi: 10.1016/j.taap.2010.04.008. [DOI] [PubMed] [Google Scholar]
- 15.Dasgupta R., Chatterji U., Nag T.C., Chaudhuri-Sengupta S., Nag D., Maiti B.R. Ultrastructural and hormonal modulations of the thyroid gland following arecoline treatment in albino mice. Mol. Cell. Endocrinol. 2010;319:1–7. doi: 10.1016/j.mce.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 16.Ogunkolade W.B., Boucher B.J., Bustin S.A., Burrin J.M., Noonan K., Mannan N., Hitman G.A. Vitamin D metabolism in peripheral blood mononuclear cells is influenced by chewing “betel nut” (Areca catechu) and vitamin D status. J. Clin. Endocrinol. Metab. 2006;91:2612–2617. doi: 10.1210/jc.2005-2750. [DOI] [PubMed] [Google Scholar]
- 17.Yen C.Y., Chiang W.F., Liu S.Y., Cheng P.C., Lee S.Y., Hong W.Z., Lin P.Y., Liu Y.C. Long-term stimulation of areca nut components results in increased chemoresistance through elevated autophagic activity. J. Oral Pathol. Med. 2014;43:91–96. doi: 10.1111/jop.12102. [DOI] [PubMed] [Google Scholar]
- 18.Hu S., Chen W.C., Hwang G.S., Chen S.T., Kuo S.B., Chen Y., Idova G., Wang S.W. Changes in plasma steroids and cytokines levels in betel chewing patients in Taiwan. Steroids. 2016;111:134–138. doi: 10.1016/j.steroids.2016.03.011. [DOI] [PubMed] [Google Scholar]
- 19.Li K., Wang C., Zhao Z., Wu Z., Wu Z., Tian X., Xiao Y., Li Z., Wang Y. A comparison for the effects of raw, smoked, and smoked and brined areca nut extracts on the immune and inflammatory responses in the Kunming mice. J. Food Biochem. 2020;44:e13319. doi: 10.1111/jfbc.13319. [DOI] [PubMed] [Google Scholar]
- 20.Garg A., Chaturvedi P., Gupta P.C. A review of the systemic adverse effects of areca nut or betel nut. Indian J. Med. Paediatr. Oncol. 2014;35:3–9. doi: 10.4103/0971-5851.133702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Choudhury M.D., Chetia P., Choudhury K.D., Talukdar A.D., Datta-Choudhari M. Atherogenic effect of Arecoline: A computational study. Bioinformation. 2012;8:229–232. doi: 10.6026/97320630008229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khan M.S., Bawany F.I., Ahmed M.U., Hussain M., Khan A., Lashari M.N. Betel nut usage is a major risk factor for coronary artery disease. Glob. J. Health. Sci. 2013;6:189–195. doi: 10.5539/gjhs.v6n2p189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiang W.T., Yang C.C., Deng J.F., Bullard M. Cardiac arrhythmia and betel nut chewing--is there a causal effect? Vet. Hum. Toxicol. 1998;40:287–289. [PubMed] [Google Scholar]
- 24.Owyang C., Wu G.D. The gut microbiome in health and disease. Gastroenterology. 2014;146:1433–1436. doi: 10.1053/j.gastro.2014.03.032. [DOI] [PubMed] [Google Scholar]
- 25.The Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hernandez B.Y., Zhu X., Goodman M.T., Gatewood R., Mendiola P., Quinata K., Paulino Y.C. Betel nut chewing, oral premalignant lesions, and the oral microbiome. PLoS ONE. 2017;12:e0172196. doi: 10.1371/journal.pone.0172196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhong X., Lu Q., Zhang Q., He Y., Wei W., Wang Y. Oral microbiota alteration associated with oral cancer and areca chewing. Oral Dis. 2021;27:226–239. doi: 10.1111/odi.13545. [DOI] [PubMed] [Google Scholar]
- 28.Mei F., Meng K., Gu Z., Yun Y., Zhang W., Zhang C., Zhong Q., Pan F., Shen X., Xia G., et al. Arecanut (Areca catechu L.) Seed Polyphenol-Ameliorated Osteoporosis by Altering Gut Microbiome via LYZ and the Immune System in Estrogen-Deficient Rats. J. Agric. Food Chem. 2021;69:246–258. doi: 10.1021/acs.jafc.0c06671. [DOI] [PubMed] [Google Scholar]
- 29.Chande M., Suba K. Tackling the Use of Supari (Areca Nut) and Smokeless Tobacco Products in the South Asian Community in the United Kingdom. Dent. Update. 2016;43:442–447. doi: 10.12968/denu.2016.43.5.442. [DOI] [PubMed] [Google Scholar]
- 30.Sulaiman A., Zubairi H., Irfan S., Ghias K. Microbiological safety of areca nut-containing, ready-to-eat chewing substances common among Pakistani paediatric population: A pilot study. J. Pak. Med. Assoc. 2019;69:450–454. [PubMed] [Google Scholar]
- 31.Gupta A.K., Tulsyan S., Thakur N., Sharma V., Sinha D.N., Mehrotra R. Chemistry, metabolism and pharmacology of carcinogenic alkaloids present in areca nut and factors affecting their concentration. Regul. Toxicol. Pharmacol. 2020;110:104548. doi: 10.1016/j.yrtph.2019.104548. [DOI] [PubMed] [Google Scholar]
- 32.Faouzi M., Neupane R.P., Yang J., Williams P., Penner R. Areca nut extracts mobilize calcium and release pro-inflammatory cytokines from various immune cells. Sci. Rep. 2018;8:1075. doi: 10.1038/s41598-017-18996-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou M.X., Tian X., Wu Z.Q., Li K., Li Z.J. Fuzhuan brick tea supplemented with areca nuts: Effects on serum and gut microbiota in mice. J. Food Biochem. 2021;45:e13737. doi: 10.1111/jfbc.13737. [DOI] [PubMed] [Google Scholar]
- 34.Anand R., Dhingra C., Prasad S., Menon I. Betel nut chewing and its deleterious effects on oral cavity. J. Cancer Res. Ther. 2014;10:499–505. doi: 10.4103/0973-1482.137958. [DOI] [PubMed] [Google Scholar]
- 35.Volgin A.D., Bashirzade A., Amstislavskaya T.G., Yakovlev O.A., Demin K.A., Ho Y.J., Wang D., Shevyrin V.A., Alpyshov E.T., Wappler-Guzzetta E.A., et al. DARK Classics in Chemical Neuroscience: Arecoline. ACS Chem. Neurosci. 2019;10:2176–2185. doi: 10.1021/acschemneuro.8b00711. [DOI] [PubMed] [Google Scholar]
- 36.Su S., Chien M., Lin C., Chen M., Yang S. RAGE gene polymorphism and environmental factor in the risk of oral cancer. J. Dent. Res. 2015;94:403–411. doi: 10.1177/0022034514566215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mehrtash H., Duncan K., Parascandola M., David A., Gritz E.R., Gupta P.C., Mehrotra R., Nordin A.S.A., Pearlman P.C., Warnakulasuriya S., et al. Defining a global research and policy agenda for betel quid and areca nut. Lancet Oncol. 2017;18:e767–e775. doi: 10.1016/S1470-2045(17)30460-6. [DOI] [PubMed] [Google Scholar]
- 38.Tsai J.F., Jeng J.E., Chuang L.Y., Ho M.S., Ko Y.C., Lin Z.Y., Hsieh M.Y., Chen S.C., Chuang W.L., Wang L.Y., et al. Habitual betel quid chewing and risk for hepatocellular carcinoma complicating cirrhosis. Medicine. 2004;83:176–187. doi: 10.1097/01.md.0000126971.80227.a4. [DOI] [PubMed] [Google Scholar]
- 39.Cao J., Xu R., Zhao X., Zhong Z., Zhang L., Zhu X., Wu S.W., Ai K. Areca Nut Chewing and an Impaired Estimated Glomerular Filtration Rate as Significant Risk Factors for Non-Muscle-Invasive Bladder Cancer Recurrence. Sci. Rep. 2016;6:29466. doi: 10.1038/srep29466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tseng Y.H., Chang K.W., Yang C.C., Liu C.J., Kao S.Y., Liu T.Y., Lin S.C. Association between areca-stimulated vimentin expression and the progression of head and neck cancers. Head Neck. 2012;34:245–253. doi: 10.1002/hed.21726. [DOI] [PubMed] [Google Scholar]
- 41.Jeng J.H., Hahn L.J., Lu F.J., Wang Y.J., Kuo M.Y. Eugenol triggers different pathobiological effects on human oral mucosal fibroblasts. J. Dent. Res. 1994;73:1050–1055. doi: 10.1177/00220345940730050601. [DOI] [PubMed] [Google Scholar]
- 42.Kumpawat K., Deb S., Ray S., Chatterjee A. Genotoxic effect of raw betel-nut extract in relation to endogenous glutathione levels and its mechanism of action in mammalian cells. Mutat. Res. 2003;538:1–12. doi: 10.1016/S1383-5718(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 43.Yadav P., Banerjee A., Boruah N., Singh C.S., Chatterjee P., Mukherjee S., Dakhar H., Nongrum H.B., Bhattacharjee A., Chatterjee A. Glutathione S-transferasesP1 AA (105Ile) allele increases oral cancer risk, interacts strongly with c-Jun Kinase and weakly detoxifies areca-nut metabolites. Sci. Rep. 2020;10:6032. doi: 10.1038/s41598-020-63034-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kiraly D.D., Walker D.M., Calipari E.S., Labonte B., Issler O., Pena C.J., Ribeiro E.A., Russo S.J., Nestler E.J. Alterations of the Host Microbiome Affect Behavioral Responses to Cocaine. Sci. Rep. 2016;6:35455. doi: 10.1038/srep35455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nair U.J., Friesen M., Richard I., MacLennan R., Thomas S., Bartsch H. Effect of lime composition on the formation of reactive oxygen species from areca nut extract in vitro. Carcinogenesis. 1990;11:2145–2148. doi: 10.1093/carcin/11.12.2145. [DOI] [PubMed] [Google Scholar]
- 46.Nagabhushan M., Bhide S.V. Anti-mutagenicity of catechin against environmental mutagens. Mutagenesis. 1988;3:293–296. doi: 10.1093/mutage/3.4.293. [DOI] [PubMed] [Google Scholar]
- 47.Azuine M.A., Bhide S.V. Protective single/combined treatment with betel leaf and turmeric against methyl (acetoxymethyl) nitrosamine-induced hamster oral carcinogenesis. Int. J. Cancer. 1992;51:412–415. doi: 10.1002/ijc.2910510313. [DOI] [PubMed] [Google Scholar]
- 48.Jurga B., Dalia M.K. The Role of Catechins in Cellular Responses to Oxidative Stress. Molecules. 2018;20:965. doi: 10.3390/molecules23040965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang P.L., Chi C.W., Liu T.Y. Areca nut procyanidins ameliorate streptozocin-induced hyperglycemia by regulating gluconeogenesis. Food Chem. Toxicol. 2013;55:137–143. doi: 10.1016/j.fct.2012.12.057. [DOI] [PubMed] [Google Scholar]
- 50.Daneel F., Jannie P., Desmond S., Larry A.W. Circular dichroic properties of flavan-3,4-diols. J. Nat. Prod. 2004;67:174–178. doi: 10.1021/np030318f. [DOI] [PubMed] [Google Scholar]
- 51.Hu L., Wu F., He J., Zhong L., Song Y., Shao H. Cytotoxicity of safrole in HepaRG cells: Studies on the role of CYP1A2-mediated ortho-quinone metabolic activation. Xenobiotica. 2019;49:1504–1515. doi: 10.1080/00498254.2019.1590882. [DOI] [PubMed] [Google Scholar]
- 52.Taleuzzaman M., Jain P., Verma R., Iqbal Z., Mirza M.A. Eugenol as a Potential Drug Candidate: A Review. Curr. Top. Med. Chem. 2021;21:1804–1815. doi: 10.2174/1568026621666210701141433. [DOI] [PubMed] [Google Scholar]
- 53.Rauf A., Imran M., Butt M.S., Nadeem M., Peters D.G., Mubarak M.S. Resveratrol as an anti-cancer agent: A review. Crit. Rev. Food Sci. Nutr. 2018;58:1428–1447. doi: 10.1080/10408398.2016.1263597. [DOI] [PubMed] [Google Scholar]
- 54.Gan R.Y., Li H.B., Sui Z.Q., Corke H. Absorption, metabolism, anti-cancer effect and molecular targets of epigallocatechin gallate (EGCG): An updated review. Crit. Rev. Food Sci. Nutr. 2018;58:924–941. doi: 10.1080/10408398.2016.1231168. [DOI] [PubMed] [Google Scholar]
- 55.Singh P., Arif Y., Bajguz A., Hayat S. The role of quercetin in plants. Plant Physiol. Biochem. 2021;166:10–19. doi: 10.1016/j.plaphy.2021.05.023. [DOI] [PubMed] [Google Scholar]
- 56.Zhou Y.X., Zhang H., Peng C. Puerarin: A review of pharmacological effects. Phytother. Res. 2014;28:961–975. doi: 10.1002/ptr.5083. [DOI] [PubMed] [Google Scholar]
- 57.Lee Y.M., Yoon Y., Yoon H., Park H.M., Song S., Yeum K.J. Dietary Anthocyanins against Obesity and Inflammation. Nutrients. 2017;9:1089. doi: 10.3390/nu9101089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shim G., Ko S., Park J.Y., Suh J.H., Le Q.V., Kim D., Kim Y.B., Im G.H., Kim H.N., Choe Y.S., et al. Tannic acid-functionalized boron nitride nanosheets for theranostics. J. Control Release. 2020;327:616–626. doi: 10.1016/j.jconrel.2020.09.009. [DOI] [PubMed] [Google Scholar]
- 59.Ozdal T., Sela D.A., Xiao J., Boyacioglu D., Chen F., Capanoglu E. The Reciprocal Interactions between Polyphenols and Gut Microbiota and Effects on Bioaccessibility. Nutrients. 2016;8:78. doi: 10.3390/nu8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stevens J.F., Maier C.S. The Chemistry of Gut Microbial Metabolism of Polyphenols. Phytochem. Rev. 2016;15:425–444. doi: 10.1007/s11101-016-9459-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Williamson G., Clifford M.N. Role of the small intestine, colon and microbiota in determining the metabolic fate of polyphenols. Biochem. Pharmacol. 2017;139:24–39. doi: 10.1016/j.bcp.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 62.Yang R., Shan S., Zhang C., Shi J., Li H., Li Z. Inhibitory Effects of Bound Polyphenol from Foxtail Millet Bran on Colitis-Associated Carcinogenesis by the Restoration of Gut Microbiota in a Mice Model. J. Agric. Food Chem. 2020;68:3506–3517. doi: 10.1021/acs.jafc.0c00370. [DOI] [PubMed] [Google Scholar]
- 63.Jiao X., Wang Y., Lin Y., Lang Y., Li E., Zhang X., Zhang Q., Feng Y., Meng X., Li B. Blueberry polyphenols extract as a potential prebiotic with anti-obesity effects on C57BL/6 J mice by modulating the gut microbiota. J. Nutr. Biochem. 2019;64:88–100. doi: 10.1016/j.jnutbio.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 64.Li X.W., Chen H.P., He Y.Y., Chen W.L., Chen J.W., Gao L., Hu H.Y., Wang J. Effects of Rich-Polyphenols Extract of Dendrobium loddigesii on Anti-Diabetic, Anti-Inflammatory, Anti-Oxidant, and Gut Microbiota Modulation in db/db Mice. Molecules. 2018;23:3245. doi: 10.3390/molecules23123245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao L., Zhang Q., Ma W., Tian F., Shen H., Zhou M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017;8:4644–4656. doi: 10.1039/C7FO01383C. [DOI] [PubMed] [Google Scholar]
- 66.Zhao H., Cheng N., Zhou W., Chen S., Wang Q., Gao H., Xue X., Wu L., Cao W. Honey Polyphenols Ameliorate DSS-Induced Ulcerative Colitis via Modulating Gut Microbiota in Rats. Mol. Nutr. Food Res. 2019;63:e1900638. doi: 10.1002/mnfr.201900638. [DOI] [PubMed] [Google Scholar]
- 67.Ho L., Zhao D., Ono K., Ruan K., Mogno I., Tsuji M., Carry E., Brathwaite J., Sims S., Frolinger T., et al. Heterogeneity in gut microbiota drive polyphenol metabolism that influences alpha-synuclein misfolding and toxicity. J. Nutr. Biochem. 2019;64:170–181. doi: 10.1016/j.jnutbio.2018.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song Z., Revelo X., Shao W., Tian L., Zeng K., Lei H., Sun H.S., Woo M., Winer D., Jin T. Dietary Curcumin Intervention Targets Mouse White Adipose Tissue Inflammation and Brown Adipose Tissue UCP1 Expression. Obesity. 2018;26:547–558. doi: 10.1002/oby.22110. [DOI] [PubMed] [Google Scholar]
- 69.Wang L., Zeng B., Liu Z., Liao Z., Zhong Q., Gu L., Wei H., Fang X. Green Tea Polyphenols Modulate Colonic Microbiota Diversity and Lipid Metabolism in High-Fat Diet Treated HFA Mice. J. Food Sci. 2018;83:864–873. doi: 10.1111/1750-3841.14058. [DOI] [PubMed] [Google Scholar]
- 70.Wong S.H., Yu J. Gut microbiota in colorectal cancer: Mechanisms of action and clinical applications. Nat. Rev. Gastroenterol. Hepatol. 2019;16:690–704. doi: 10.1038/s41575-019-0209-8. [DOI] [PubMed] [Google Scholar]
- 71.Wu X., Zhang T., Chen X., Ji G., Zhang F. Microbiota transplantation: Targeting cancer treatment. Cancer Lett. 2019;452:144–151. doi: 10.1016/j.canlet.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 72.Gori S., Inno A., Belluomini L., Bocus P., Bisoffi Z., Russo A., Arcaro G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Crit. Rev. Oncol. Hematol. 2019;143:139–147. doi: 10.1016/j.critrevonc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- 73.Wei M.Y., Shi S., Liang C., Meng Q.C., Hua J., Zhang Y.Y., Liu J., Zhang B., Xu J., Yu X.J. The microbiota and microbiome in pancreatic cancer: More influential than expected. Mol. Cancer. 2019;18:97. doi: 10.1186/s12943-019-1008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Guo S., Chen J., Chen F., Zeng Q., Liu W.L., Zhang G. Exosomes derived from Fusobacterium nucleatum-infected colorectal cancer cells facilitate tumour metastasis by selectively carrying miR-1246/92b-3p/27a-3p and CXCL. Gut. 2020;71:e1–e3. doi: 10.1136/gutjnl-2020-321187. [DOI] [PubMed] [Google Scholar]
- 75.Chen S., Su T., Zhang Y., Lee A., He J., Ge Q., Wang L., Si J., Zhuo W., Wang L. Fusobacterium nucleatum promotes colorectal cancer metastasis by modulating KRT7-AS/KRT. Gut Microbes. 2020;11:511–525. doi: 10.1080/19490976.2019.1695494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang S., Yang Y., Weng W., Guo B., Cai G., Ma Y., Cai S. Fusobacterium nucleatum promotes chemoresistance to 5-fluorouracil by upregulation of BIRC3 expression in colorectal cancer. J. Exp. Clin. Cancer Res. 2019;38:14. doi: 10.1186/s13046-018-0985-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geller L.T., Barzily-Rokni M., Danino T., Jonas O.H., Shental N., Nejman D., Gavert N., Zwang Y., Cooper Z.A., Shee K., et al. Potential role of intratumor bacteria in mediating tumor resistance to the chemotherapeutic drug gemcitabine. Science. 2017;357:1156–1160. doi: 10.1126/science.aah5043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Viaud S., Saccheri F., Mignot G., Yamazaki T., Daillere R., Hannani D., Enot D.P., Pfirschke C., Engblom C., Pittet M.J., et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–976. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Daillere R., Vetizou M., Waldschmitt N., Yamazaki T., Isnard C., Poirier-Colame V., Duong C.P.M., Flament C., Lepage P., Roberti M.P., et al. Enterococcus hirae and Barnesiella intestinihominis Facilitate Cyclophosphamide-Induced Therapeutic Immunomodulatory Effects. Immunity. 2016;45:931–943. doi: 10.1016/j.immuni.2016.09.009. [DOI] [PubMed] [Google Scholar]
- 80.Wallace B.D., Wang H., Lane K.T., Scott J.E., Orans J., Koo J.S., Venkatesh M., Jobin C., Yeh L.A., Mani S., et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bhatt A.P., Pellock S.J., Biernat K.A., Walton W.G., Wallace B.D., Creekmore B.C., Letertre M.M., Swann J.R., Wilson I.D., Roques J.R., et al. Targeted inhibition of gut bacterial beta-glucuronidase activity enhances anticancer drug efficacy. Proc. Natl. Acad. Sci. USA. 2020;117:7374–7381. doi: 10.1073/pnas.1918095117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Y., Sun L., Chen S., Guo S., Yue T., Hou Q., Feng M., Xu H., Liu Y., Wang P., et al. The administration of Escherichia coli Nissle 1917 ameliorates irinotecan-induced intestinal barrier dysfunction and gut microbial dysbiosis in mice. Life Sci. 2019;231:116529. doi: 10.1016/j.lfs.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Wu C.H., Ko J.L., Liao J.M., Huang S.S., Lin M.Y., Lee L.H., Chang L.Y., Ou C.C. D-methionine alleviates cisplatin-induced mucositis by restoring the gut microbiota structure and improving intestinal inflammation. Ther. Adv. Med. Oncol. 2019;11:1758835918821021. doi: 10.1177/1758835918821021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perales-Puchalt A., Perez-Sanz J., Payne K.K., Svoronos N., Allegrezza M.J., Chaurio R.A., Anadon C., Calmette J., Biswas S., Mine J.A., et al. Frontline Science: Microbiota reconstitution restores intestinal integrity after cisplatin therapy. J. Leukoc. Biol. 2018;103:799–805. doi: 10.1002/JLB.5HI1117-446RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lee T.H., Park D., Kim Y.J., Lee I., Kim S., Oh C.T., Kim J.K., Jo S.K. Lactobacillus salivarius BP121 prevents cisplatininduced acute kidney injury by inhibition of uremic toxins such as indoxyl sulfate and pcresol sulfate via alleviating dysbiosis. Int. J. Mol. Med. 2020;45:1130–1140. doi: 10.3892/ijmm.2020.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gluschnaider U., Hidas G., Cojocaru G., Yutkin V., Ben-Neriah Y., Pikarsky E. beta-TrCP inhibition reduces prostate cancer cell growth via upregulation of the aryl hydrocarbon receptor. PLoS ONE. 2010;5:e9060. doi: 10.1371/journal.pone.0009060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Portal-Nunez S., Shankavaram U.T., Rao M., Datrice N., Atay S., Aparicio M., Camphausen K.A., Fernández-Salguero P.M., Chang H., Lin P., et al. Aryl hydrocarbon receptor-induced adrenomedullin mediates cigarette smoke carcinogenicity in humans and mice. Cancer Res. 2012;72:5790–5800. doi: 10.1158/0008-5472.CAN-12-0818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang S., Lei P., Liu X., Li X., Walker K., Kotha L., Rowlands C., Safe S. The aryl hydrocarbon receptor as a target for estrogen receptor-negative breast cancer chemotherapy. Endocr. Relat. Cancer. 2009;16:835–844. doi: 10.1677/ERC-09-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ambolet-Camoit A., Bui L.C., Pierre S., Chevallier A., Marchand A., Coumoul X., Garlatti M., Andreau K., Barouki R., Aggerbeck M. 2,3,7,8-tetrachlorodibenzo-p-dioxin counteracts the p53 response to a genotoxicant by upregulating expression of the metastasis marker agr2 in the hepatocarcinoma cell line HepG. Toxicol. Sci. 2010;115:501–512. doi: 10.1093/toxsci/kfq082. [DOI] [PubMed] [Google Scholar]
- 90.Flaveny C.A., Murray I.A., Chiaro C.R., Perdew G.H. Ligand selectivity and gene regulation by the human aryl hydrocarbon receptor in transgenic mice. Mol. Pharmacol. 2009;75:1412–1420. doi: 10.1124/mol.109.054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhang S., Qin C., Safe S.H. Flavonoids as aryl hydrocarbon receptor agonists/antagonists: Effects of structure and cell context. Environ. Health Perspect. 2003;111:1877–1882. doi: 10.1289/ehp.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Xue Z., Li D., Yu W., Zhang Q., Hou X., He Y., Kou X. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017;8:1414–1437. doi: 10.1039/C6FO01810F. [DOI] [PubMed] [Google Scholar]
- 93.Shinde R., McGaha T.L. The Aryl Hydrocarbon Receptor: Connecting Immunity to the Microenvironment. Trends Immunol. 2018;39:1005–1020. doi: 10.1016/j.it.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Roman A.C., Carvajal-Gonzalez J.M., Merino J.M., Mulero-Navarro S., Fernandez-Salguero P.M. The aryl hydrocarbon receptor in the crossroad of signalling networks with therapeutic value. Pharmacol. Ther. 2018;185:50–63. doi: 10.1016/j.pharmthera.2017.12.003. [DOI] [PubMed] [Google Scholar]
- 95.Chen A.Y., Chen Y.C. A review of the dietary flavonoid, kaempferol on human health and cancer chemoprevention. Food Chem. 2013;138:2099–2107. doi: 10.1016/j.foodchem.2012.11.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Polozhentsev S.D., Reiza V.A., Malinskii D.M., Lebedev M.F. [Clinico-social aspects of arterial hypertension in the population of the Karelian ASSR] Sov. Zdravookhr. 1989;1:50–53. [PubMed] [Google Scholar]
- 97.Murota K., Nakamura Y., Uehara M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018;82:600–610. doi: 10.1080/09168451.2018.1444467. [DOI] [PubMed] [Google Scholar]
- 98.Schanz O., Chijiiwa R., Cengiz S.C., Majlesain Y., Weighardt H., Takeyama H., Förster I. Dietary AhR Ligands Regulate AhRR Expression in Intestinal Immune Cells and Intestinal Microbiota Composition. Int. J. Mol. Sci. 2020;21:3189. doi: 10.3390/ijms21093189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Takamura T., Harama D., Fukumoto S., Nakamura Y., Shimokawa N., Ishimaru K., Ikegami S., Makino S., Kitamura M., Nakao A. Lactobacillus bulgaricus OLL1181 activates the aryl hydrocarbon receptor pathway and inhibits colitis. Immunol. Cell Biol. 2011;89:817–822. doi: 10.1038/icb.2010.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cervantes-Barragan L., Chai J.N., Tianero M.D., Di Luccia B., Ahern P.P., Merriman J., Cortez V.S., Caparon M.G., Donia M.S., Gilfillan S., et al. Lactobacillus reuteri induces gut intraepithelial CD4(+)CD8alphaalpha(+) T cells. Science. 2017;357:806–810. doi: 10.1126/science.aah5825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shah G., Chaturvedi P., Vaishampayan S. Arecanut as an emerging etiology of oral cancers in India. Indian J. Med. Paediatr. Oncol. 2012;33:71–79. doi: 10.4103/0971-5851.99726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Liu Y.J., Peng W., Hu M.B., Xu M., Wu C.J. The pharmacology, toxicology and potential applications of arecoline: A review. Pharm. Biol. 2016;54:2753–2760. doi: 10.3109/13880209.2016.1160251. [DOI] [PubMed] [Google Scholar]
- 103.Jain V., Garg A., Parascandola M., Chaturvedi P., Khariwala S.S., Stepanov I. Analysis of Alkaloids in Areca Nut-Containing Products by Liquid Chromatography-Tandem Mass Spectrometry. J. Agric. Food Chem. 2017;65:1977–1983. doi: 10.1021/acs.jafc.6b05140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chu N.S. Neurological aspects of areca and betel chewing. Addict. Biol. 2002;7:111–114. doi: 10.1080/13556210120091473. [DOI] [PubMed] [Google Scholar]
- 105.Papke R.L., Horenstein N.A., Stokes C. Nicotinic Activity of Arecoline, the Psychoactive Element of “Betel Nuts”, Suggests a Basis for Habitual Use and Anti-Inflammatory Activity. PLoS ONE. 2015;10:e0140907. doi: 10.1371/journal.pone.0140907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Venkatesh D., Puranik R.S., Vanaki S.S., Puranik S.R. Study of salivary arecoline in areca nut chewers. J. Oral Maxillofac. Pathol. 2018;22:446. doi: 10.4103/jomfp.JOMFP_143_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kilian J., Millard M., Ozenil M., Krause D., Ghaderi K., Holzer W., Urban E., Spreitzer H., Wadsak W., Hacker M., et al. Synthesis, Biological Evaluation, and Docking Studies of Antagonistic Hydroxylated Arecaidine Esters Targeting mAChRs. Molecules. 2022;27:3173. doi: 10.3390/molecules27103173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chattopadhyay A., Ray J.G. Molecular Pathology of Malignant Transformation of Oral Submucous Fibrosis. J. Environ. Pathol. Toxicol. Oncol. 2016;35:193–205. doi: 10.1615/JEnvironPatholToxicolOncol.2016014024. [DOI] [PubMed] [Google Scholar]
- 109.Ezquer F., Quintanilla M.E., Moya-Flores F., Morales P., Munita J.M., Olivares B., Landskron G., Hermoso M.A., Ezquer M., Herrera-Marschitz M., et al. Innate gut microbiota predisposes to high alcohol consumption. Addict. Biol. 2021;26:e13018. doi: 10.1111/adb.13018. [DOI] [PubMed] [Google Scholar]
- 110.Cussotto S., Sandhu K.V., Dinan T.G., Cryan J.F. The Neuroendocrinology of the Microbiota-Gut-Brain Axis: A Behavioural Perspective. Front. Neuroendocrinol. 2018;51:80–101. doi: 10.1016/j.yfrne.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 111.Xu Y., Xie Z., Wang H., Shen Z., Guo Y., Gao Y., Chen X., Wu Q., Li X., Wang K. Bacterial Diversity of Intestinal Microbiota in Patients with Substance Use Disorders Revealed by 16S rRNA Gene Deep Sequencing. Sci. Rep. 2017;7:3628. doi: 10.1038/s41598-017-03706-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jadhav K.S., Peterson V.L., Halfon O., Ahern G., Fouhy F., Stanton C., Dinan T.G., Cryan J.F., Boutrel B. Gut microbiome correlates with altered striatal dopamine receptor expression in a model of compulsive alcohol seeking. Neuropharmacology. 2018;141:249–259. doi: 10.1016/j.neuropharm.2018.08.026. [DOI] [PubMed] [Google Scholar]
- 113.Zuo Z., Fan H., Tang X.D., Chen Y.M., Xun L.T., Li Y., Song Z.J., Zhai H.Q. Effect of different treatments and alcohol addiction on gut microbiota in minimal hepatic encephalopathy patients. Exp. Ther. Med. 2017;14:4887–4895. doi: 10.3892/etm.2017.5141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peterson V.L., Jury N.J., Cabrera-Rubio R., Draper L.A., Crispie F., Cotter P.D., Dinan T.G., Holmes A., Cryan J.F. Drunk bugs: Chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav. Brain Res. 2017;323:172–176. doi: 10.1016/j.bbr.2017.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Volpe G.E., Ward H., Mwamburi M., Dinh D., Bhalchandra S., Wanke C., alKane A.V. Associations of cocaine use and HIV infection with the intestinal microbiota, microbial translocation, and inflammation. J. Stud. Alcohol Drugs. 2014;75:347–357. doi: 10.15288/jsad.2014.75.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mutlu E.A., Gillevet P.M., Rangwala H., Sikaroodi M., Naqvi A., Engen P.A., Kwasny M., Lau C.K., Keshavarzian A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Gastrointest. Liver Physiol. 2012;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang G., Liu Q., Guo L., Zeng H., Ding C., Zhang W. Gut Microbiota and Relevant Metabolites Analysis in Alcohol Dependent Mice. Front. Microbiol. 2018;9:1874. doi: 10.3389/fmicb.2018.01874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Scorza C., Piccini C., Martinez Busi M., Abin Carriquiry J.A., Zunino P. Alterations in the Gut Microbiota of Rats Chronically Exposed to Volatilized Cocaine and Its Active Adulterants Caffeine and Phenacetin. Neurotox. Res. 2019;35:111–121. doi: 10.1007/s12640-018-9936-9. [DOI] [PubMed] [Google Scholar]
- 119.de Timary P., Leclercq S., Starkel P., Delzenne N. A dysbiotic subpopulation of alcohol-dependent subjects. Gut. Microbes. 2015;6:388–391. doi: 10.1080/19490976.2015.1107696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Xu Z., Wang C., Dong X., Hu T., Wang L., Zhao W., Zhu S., Li G., Hu Y., Gao Q., et al. Chronic alcohol exposure induced gut microbiota dysbiosis and its correlations with neuropsychic behaviors and brain BDNF/Gabra1 changes in mice. Biofactors. 2019;45:187–199. doi: 10.1002/biof.1469. [DOI] [PubMed] [Google Scholar]
- 121.Xiao H.W., Ge C., Feng G.X., Li Y., Luo D., Dong J.L., Li H., Wang H., Cui M., Fan S.J. Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 2018;287:23–30. doi: 10.1016/j.toxlet.2018.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Leclercq S., Le Roy T., Furgiuele S., Coste V., Bindels L.B., Leyrolle Q., Neyrinck A.M., Quoilin C., Amadieu C., Petit G., et al. Gut Microbiota-Induced Changes in beta-Hydroxybutyrate Metabolism Are Linked to Altered Sociability and Depression in Alcohol Use Disorder. Cell Rep. 2020;33:108238. doi: 10.1016/j.celrep.2020.108238. [DOI] [PubMed] [Google Scholar]
- 123.Peterson V.L., Richards J.B., Meyer P.J., Cabrera-Rubio R., Tripi J.A., King C.P., Polesskaya O., Baud A., Chitre A.S., Bastiaanssen T.F., et al. Sex-dependent associations between addiction-related behaviors and the microbiome in outbred rats. EBioMedicine. 2020;55:102769. doi: 10.1016/j.ebiom.2020.102769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lee K., Vuong H.E., Nusbaum D.J., Hsiao E.Y., Evans C.J., Taylor A.M.W. The gut microbiota mediates reward and sensory responses associated with regimen-selective morphine dependence. Neuropsychopharmacology. 2018;43:2606–2614. doi: 10.1038/s41386-018-0211-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Hoban A.E., Moloney R.D., Golubeva A.V., McVey Neufeld K.A., O’Sullivan O., Patterson E., Stanton C., Dinan T.G., Clarke G., Cryan J.F. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience. 2016;339:463–477. doi: 10.1016/j.neuroscience.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 126.Burokas A., Arboleya S., Moloney R.D., Peterson V.L., Murphy K., Clarke G., Stanton C., Dinan T.G., Cryan J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry. 2017;82:472–487. doi: 10.1016/j.biopsych.2016.12.031. [DOI] [PubMed] [Google Scholar]
- 127.Warda A.K., Rea K., Fitzgerald P., Hueston C., Gonzalez-Tortuero E., Dinan T.G., Hill C. Heat-killed lactobacilli alter both microbiota composition and behaviour. Behav. Brain. Res. 2019;362:213–223. doi: 10.1016/j.bbr.2018.12.047. [DOI] [PubMed] [Google Scholar]