Abstract
Rationale:
Much of our understanding of the targets of IgE comes from studies of allergy, though little is known about the natural immunogenic targets seen following parasitic worm infections. The use of human monoclonal antibodies (mAbs) allow for an unbiased, comprehensive characterization of the immunodominant antigens targeted by IgE in conditions (e.g., allergy, helminth infection) associated with elevated levels of IgE.
Methods:
Using human hybridoma technology to immortalize IgE encoding B-cells from peripheral blood of subjects with filarial infections and elevated IgE, we generated naturally-occurring human IgE mAbs. B-cell cultures were screened in an unbiased manner for IgE production without regard to specificity. Isolated IgE mAbs then were tested for binding to Brugia malayi somatic extracts (BmA) using ImmunoCAP, immunoblot, and ELISA. Immunoprecipitation followed by mass spectrometry proteomics was used to identify helminth antigens that were then expressed in E. coli for IgE binding characterization.
Results:
We isolated 56 discrete IgE mAbs from 7 individuals with filarial infections. From these mAbs, we were able to definitively identify 19 filarial antigens. All IgE mAbs targeted filarial excreted/secretory (E/S) proteins, including a family of previously uncharacterized proteins. Interestingly, the transthyretin-related antigens acted as the dominant inducer of the filaria-specific IgE antibody response. These filaria-specific IgE mAbs were potent inducers of anaphylaxis when passively administered to hFcεRI-expressing mice.
Conclusions:
We have generated human hybridomas secreting naturally-occurring helminth-specific IgE mAbs from filarial-infected subjects. This work provides much needed insights into the ontogeny of the helminth-induced immune response/IgE antibody response.
Keywords: IgE, antibodies, monoclonal, helminth, parasite
CAPSULE SUMMARY
This is the first description of the dominant antigens targeted by the IgE antibody response during human parasitic worm infection.
INTRODUCTION
There is intense interest in the immunogenic triggers responsible for the rising epidemic of IgE-mediated allergic diseases; however, the ontogeny of the IgE antibody response in helminth infection has been poorly studied. While elevated serum levels of IgE antibodies are commonly found in tissue-invasive helminth infections, including the filarial nematodes that cause lymphatic filariasis, loiasis, and onchocerciasis1, there is limited understanding of antigenic triggers underlying this response and are opposing data published regarding the protective function of IgE. Epidemiological data supporting the protective function of IgE show that anti-parasite IgE responses are associated with a degree of immune-mediated protection in humans infected with hookworms2,3, Trichuris4, Ascaris5,6 and schistosomes7-13. However, much uncertainty exists as to the primary antigenic targets of IgE and its role in immune defense. Early serologic studies characterize IgE antibodies as ‘nonspecific’ (non-antigen specific), implying that IgE antibodies do not protect hosts in the course of parasitic infection 14. Others have focused on allergen-like proteins such as tropomyosin to explore overlapping IgE responses between helminth infection and allergic diseases, these helminthic allergen-like proteins are included in the allergens database 15-17. Still other studies report the presence of helminth-specific oligosaccharides and classical CCD (cross-reactive carbohydrate determinants) that are the targets for IgE antibodies in helminth infection, but there is relatively little evidence regarding the significance of IgE antibodies to these epitopes18,19. The overarching limitations of these studies stem from the difficulty of studying IgE using human sera, given the broad repertoire and exceedingly low concentration of antigen-specific IgE. An important new way to dissect the function of IgE in the context of human helminth infection is to create naturally occurring IgE monoclonal antibodies (mAbs). Our group has developed methods that allow for the generation of stable human hybridomas from the very rare population of helminth-specific IgE-encoding B cells in peripheral blood of infected human subjects. Here, we performed an unbiased study of IgE antibodies associated with filarial infection and identified several filarial antigens targeted by the IgE response, which have strong diagnostic and vaccine potential. Moreover, we suggest that IgE antibodies can act as an early detection system that can trigger a type one hypersensitivity response to constrain the parasite at a vulnerable early stage.
Filarial nematodes are threadlike worms with complex life cycles in which the adult worms reside in lymphatic or subcutaneous tissue of their host. These parasites are responsible for causing lymphatic filariasis (LF; Wuchereria bancrofti, Brugia malayi, Brugi timori), onchocericasis (O. volvulus) and/or loiasis (Loa loa). These chronic infections are responsible for an extraordinary degree of morbidity and collectively affect more than 180 million people worldwide. The canonical host immune response to these particular tissue-invasive helminths is of the T-helper 2 (Th2) type and involves the production of cytokines, interleukin (IL)-4, IL-5, IL-9, IL-10, and IL-13; the antibody isotypes immunoglobulin G1 (IgG1), IgG4, and IgE; and expanded populations of eosinophils, basophils, mast cells, type 2 innate lymphoid cells, and alternatively activated macrophages20. Interestingly, filarial infection in travelers and tropical pulmonary eosinophilia (TPE; an unusual syndrome seen in W. bancrofti and B. malayi infection) are associated with both extraordinarily elevated IgE levels and “allergic” symptomatology21. Thus, having insights into the antigens driving IgE and mediating pathology is of paramount interest.
METHODS
Data reporting
No statistical methods were used to predetermine sample size. Experiments were not randomized, and investigators were not blinded to allocation during experiments and outcome assessment.
Research subjects
We analyzed seven subjects with a clinical history of lymphatic filariasis, loiasis, or onchocerciasis, two diagnosed with TPE. Relevant clinical information is summarized in Supplementary Table 1. The study was approved by the Institutional Review Board of Vanderbilt University Medical Center (IRB 170308). Samples were obtained after written informed consent. Cells remained stored in liquid nitrogen until use.
Human hybridoma generation and variable gene sequencing
IgE-secreting human hybridomas were generated using methodology described previously22; please see supplemental methods for additional details. Purified monoclonal antibodies, expressed by large scale growth of human hybridomas in serum free culture medium, were then used for target identification, binding analysis, and anaphylaxis assessments. IgE antibody variable gene sequences were determined using RT-PCR amplification of total RNA extracted from 1 million clonal IgE-expressing human hybridoma cells, please see supplemental methods for additional information.
Parasite material
Adult parasites, infective larvae (L3), L4 larvae and microfilariae of B. malayi were obtained from the NIAID/NIH Filariasis Research Reagent Repository Center (FR3; Athens, GA; www.filariasiscenter.org). Additional adult B. malayi were purchased from TRS Labs (Athens, GA). D. immitis worms were also a source of filarial antigens: adult male and female D. immitis parasites were obtained from surgical removal in severe cases of heartworm infection in canines. Soluble antigen from adult B. malayi and D. immitis worms was prepared by grinding frozen worms to a fine powder in liquid nitrogen using a mortar and pestle (cryogenic grinding). The homogenized powder was suspended in phosphate buffer saline (PBS 0.05 M, pH 7.2) containing Halt protease inhibitor cocktail (Thermo Scientific, 78429) rocking for 1 h at 4°C. Proteins soluble in PBS were recovered by centrifugation (15,000 g) at 4°C for 30 min. Protein concentration was measured by absorbance at UV280. Filarial worm extracts were aliquoted and stored at −20°C until use. Extracts then were used in binding analysis as well as identification of target antigens using immunoprecipitation and mass spectrometry; please see supplemental methods.
ImmunoCAP, ELISA, and immunoblot analysis
Prototype ImmunoCAP B. malayi tests were developed for research use from a B. malayi somatic extract. Analytical characteristics of ImmunoCAP tests were determined and an accelerated stability study was performed. The test was used to screen reactivity of human IgE mAbs (at an approximate concentration of 1-10 μg/mL) against B. malayi. An antibody was considered positive if it bound to B. malayi in ImmunoCAP (>1.0 kUA/L). We note that the assigned cutoff for ImmunoCAP positivity is much higher than the standard cutoff for serum analysis, as we used excess IgE antibody and antigen concentration is the limiting factor. For a detailed description of ELISA and immunoblotting procedures used in the identification and characterization of IgE mAbs, please see supplemental methods.
Expression and analysis of recombinant antigens
Recombinant his-tagged filarial antigens were expressed in SHuffle® T7 competent E. coli bacteria. For detailed description of the gene sequences used for expression, please see supplemental methods. Recombinant protein was purified using TALON metal affinity resin, followed by desalting to remove imidazole and to exchange buffer to PBS. Purified proteins were assessed for purity and appropriate molecular weight by SDS–PAGE.
Human FcεRI–transgenic mouse anaphylaxis
Mouse studies were carried out in accordance with recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. Human FcεRI–transgenic mice [B6.Cg-Fcεr1αtm1Knt Tg(FCER1A)1Bhk/J] were purchased from The Jackson Laboratory (stock 010506), bred and genotyped. These mice carry 2 gene mutations: the human Fc fragment of IgE receptor α polypeptide (FCER1A) under control of the human FCER1A promoter and a mutation targeting Fcεr1αtm1Knt, blocking expression of murine FCER1A23. Human IgE can induce anaphylaxis in mice hemizygous for the transgene and homozygous for targeted deletion of mouse FcεRI. Transgenic mice were sensitized by i.p. injection of 100 μg total IgE and challenged by i.p. injection of 50 μg purified recombinant antigen. Changes in mouse core body temperature were monitored over 90 min using implanted temperature probes.
Statistics
For ELISA studies, mean relative fluorescence units were calculated using three independently performed assays performed in triplicate. The descriptive statistics mean ± SEM or mean ± SD were determined for continuous variables as noted. Statistical analysis was performed using Prism v9.0 (GraphPad) software. In mouse studies, the comparison of temperature-change curves was performed independently for each antigen challenge and at each time point using paired 2-tailed t test, assuming unequal variance (Microsoft Excel Office Professional Plus 2016). Time points with calculated P values less than 0.05 were labeled and considered significant. Error bars for mouse temperature measurements represent SEM.
RESULTS
IgE-Positive B Cells are Present in Blood of Subjects with Filariasis
To date, few studies have directly examined the B cells that produce IgE antibodies in humans22,24. Thus, we asked whether IgE-producing B cells are present in blood of subjects infected with filarial nematodes and, if so, could they be used to generate human IgE mAbs. To do so, we obtained frozen peripheral blood mononuclear cells (PBMC) from subjects with lymphatic filariasis, TPE, loiasis, or onchocerciasis. Using our previously published human hybridoma methodology22 (Supplemental Data Fig. 1a), we generated 56 IgE mAbs from B cells from 7 individuals without selection for antigenic specificity (See Table 1). Those observations indicate a frequency of IgE-expressing B cells in circulation of these subjects ranging from 6-14 cells per 10 million PBMCs, slightly lower than the number of IgE-expressing B cells we previously reported for subjects with allergic bronchopulmonary aspergillosis22.
Table 1.
Subject demographics and IgE encoding B cell frequencies
| Subject code |
Age | Sex | Diagnosis | Geographic location of infection |
Serum IgE IU/ml |
IgE B-cell frequency (per 107 PBMCs) |
IgE hybridomas generated |
|---|---|---|---|---|---|---|---|
| P1 | 61 | M | TPE | India | 4260 | 13.5 | 22 |
| P2 | 29 | M | TPE | India | 9810 | 8.1 | 1 |
| P3 | 21 | F | Loiasis | Cameroon | 20290 | 9.2 | 9 |
| P4 | 40 | M | Loiasis | Cameroon | 8520 | 10.9 | 12 |
| P5 | 61 | F | Loiasis | Cameroon | 11362 | 6.2 | 5 |
| P6 | 29 | M | Loiasis | Gabon | 7628 | 6.0 | 1 |
| P7 | 42 | M | Onchocerciasis | Sierra Leone | 8680 | 13.7 | 6 |
| P8 | 49 | F | TPE | India | 6140 | Serum only | Serum only |
| P9 | 27 | M | TPE | India | 7440 | Serum only | Serum only |
Subject helminth disease, geographic location of their infection, serum IgE titer, IgE B-cell frequency and IgE hybridomas yield are shown. IgE B cell frequencies are expressed as the number of IgE-positive cells per 10 million PBMCs. The total number of IgE-expressing human hybridomas generated for each subject is listed. No PBMCs, only sera, were used from subjects P8 & P9. TPE, tropical pulmonary eosinophilia.
Identification of Filaria-Specific IgE Antibodies
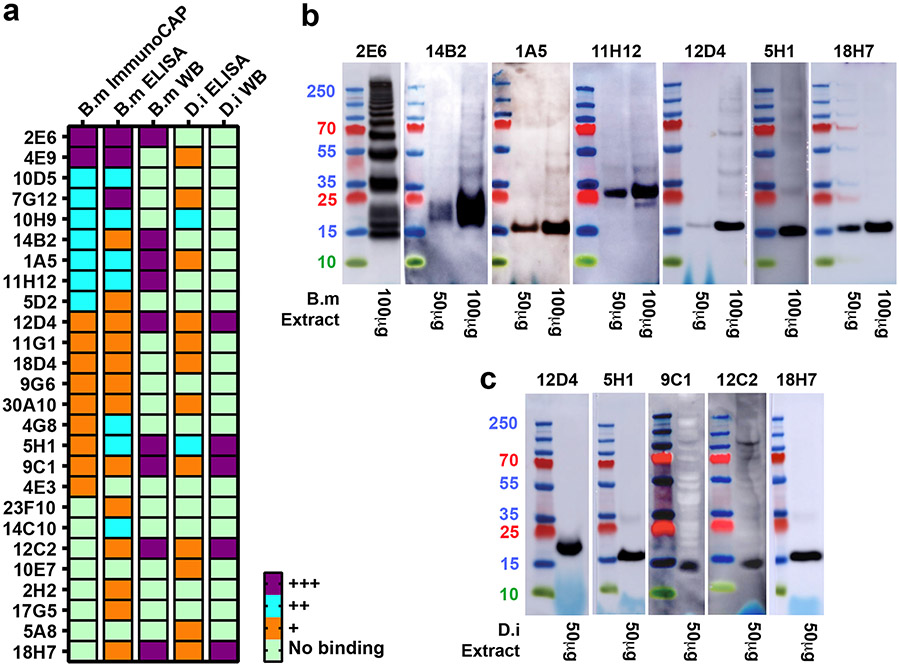
We next determined whether candidate IgE mAbs were directed against filarial antigens (Supplemental Data Fig. 1b). To do so we produced whole-worm somatic extracts from B. malayi or Dirofilaria immitis, which are responsible for filariasis in humans or dogs, respectively, and tested mAb reactivity against each extract using three complementary assays—immunoblotting, ELISA and B. malayi ImmunoCAP. Of the 56 human mAb candidates, 26 were positive in at least one screen and some in all three (Fig. 1a and Supplementary Table 1). Of the 26 antibodies with detectable binding, 9 (35%) were immunoblot-positive against B. malayi with 5 (19%)also capable of blotting D. immitis. Representative blot images showing different banding patterns observed for candidate mAbs are shown for B. malayi in Fig. 1b and D. immitis in Fig. 1c. MAb 2E6, which had the greatest positivity in B. malayi ImmunoCAP, also showed robust positivity on immunoblots and exhibited a ladder-like pattern ranging from ~14 kDa to >250 kDa. Interestingly, except for mAb 2E6, all bands detected by immunoblot analysis were <30 kDa in size.
Figure 1. Characterization of IgE mAbs isolated from peripheral blood of subjects with filarial infection.
a, Heatmap representing IgE binding to B. malayi ImmunoCAP (considered positive if >1 kUA/L) and B. malayi and D. immitis binding in ELISA (− no binding detected; + binding 3-10 times background; ++ binding 10-100 times background; +++ binding >100 times background) and immunoblot (WB) (− no binding; +++ presence of a clear band). MAbs are ordered by ImmunoCAP binding value. Data are representative of at least two independent experiments. b, Immunoblot analysis of IgE mAb binding to protein in somatic extracts from B. malayi. c, Analysis as in (b) using D. immitis extracts. For (b) and (c) only immunoblot-positive antibodies are shown.
Of note, of the 26 filaria-specific IgE mAbs, 15 were obtained from the two subjects with TPE (15 of their 23 mAbs, 65%). Because W. bancrofti (which is responsible for 90% of LF infections), Loa loa and Onchocerca volvulus cannot be maintained in conventional mouse strains, we could not screen our IgE mAbs against these filarial somatic extracts and thus could not identify Wuchereria-specific, Onchocerca-specific, or Loa-specific IgE mAbs in our panel. Of the 26 filaria-specific IgE mAbs, only 13 showed cross-reactivity towards dog heartworm (Supplementary Table 1). Taken together this suggests that the IgE antibody response is highly specific to antigens of the infecting filarial worm, though, at the protein level there is a high (>90%) average sequence identity across the filarial species that are pathogenic for humans25.
Given reports that IgE from patients with parasitic infection cross-react with common allergens18,19, we asked whether our 26 candidate antibodies obtained from filaria-infected subjects cross-reacted with common allergen proteins. To do so we analyzed all 26 using an ImmunoCAP ISAC allergen microarray containing 112 purified allergens. We observed no significant reactivity of anti-filarial IgE to any allergen tested (Supplemental Data Fig. 2), suggesting that the IgE antibody response induced during filarial infection are highly filarial-specific.
The Antigenic Landscape of the Anti-filaria IgE Antibody Response
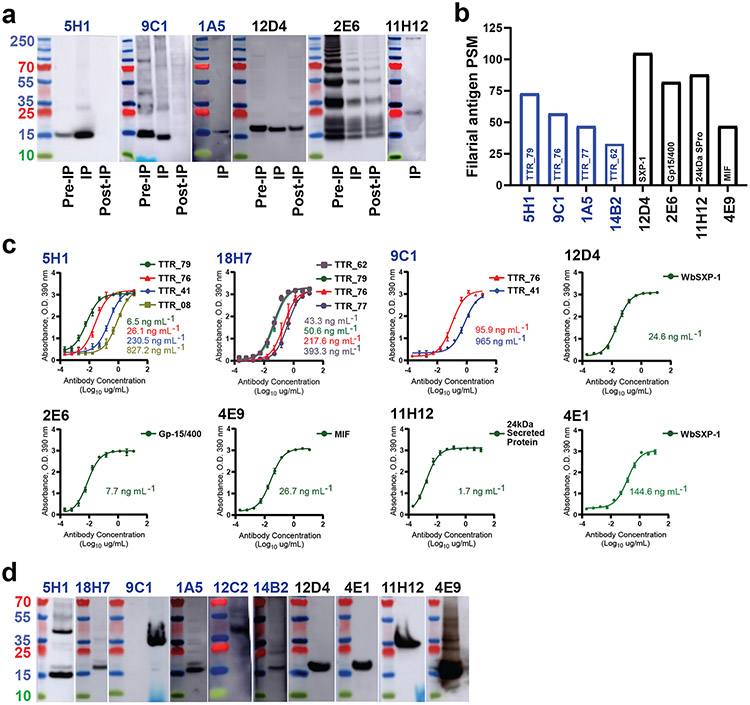
To assess antibody specificity, we focused on the 26 antibodies that showed reactivity to a filarial worm extract in multiple assays, as described above. To identify antigen targets, we independently immobilized the 26 candidate mAbs to magnetic beads and performed immunoprecipitation (IP) of B. malayi and D. immitis somatic extracts and subsequent mass spectrometry analysis. Immunoprecipitates with human IgE mAbs specific for irrelevant antigens from mold and peanut (mAbs 21E2, 16A8) served as specificity controls. IP was followed by immunoblotting to confirm antigen enrichment, as can be seen by examples shown in Fig. 2a. In some cases, such as with mAb 5H1 and 9C1, filarial antigen was at such a low concentration that IP resulted in complete depletion of antigen. In other cases, such as with mAb 2E6, a very high concentration of the filarial antigen resulted in saturation, as antigen can be seen in the post-IP material.
Figure 2. Identification of filarial antigens targeted by IgE mAbs.
a, Analysis of specificity of each IgE mAb for its target antigen by IP followed by immunoblot. A filarial worm somatic extract was immunoprecipitated with IgE mAbs. Whole somatic extract (Pre-IP), IP product and extract after IP (Post-IP) were then blotted with the same IgE mAb. Only immunoblot-positive antibodies are shown. b, Shown is the number of peptide spectrum matches (PSMs) identified for each antigen by mass spectrometry analysis. c, Specificity validation of each IgE mAb for its target by ELISA. Data obtained in triplicate are shown as the mean ± SEM and are representative of three experiments. Calculated EC50 values are shown on the graph. d, Specificity validation of each IgE mAb for its target by immunoblot analysis.
Overall, 16 IgE mAbs were able to immunoprecipitate a filarial antigen; we identified 14 unique antigens (Supplemental Table 1). MAbs 1A5, 5H1, 9C1 and 14B2 precipitated transthyretin-related protein (Fig. 2b), a 16 kDa nematode-secreted protein and member of the “(TTR)-like” family of proteins. Proteins of this family are reportedly prominent components of the secretome of several parasites including B. malayi26, D. immitis27, T. colubriformis28, and C. elegans29. Their function is largely unknown, but a TTR protein from the plant parasite M. javanica reportedly interferes with the host immune response and promotes parasitism30. MAb 12D4 precipitated WbSXP-1 (Fig. 2b), a 14 kDa nematode-specific secreted protein (also known as Wb14 antigen) previously reported as a target of IgG4 antibody31 and an antigen used in multiple diagnostic tests32,33. WbSXP-1 homologues are present in Anisakis34 and have been pursued as vaccine candidates against Ascaris35. MAb 11H12 precipitated a 24 kDa nematode-specific secreted protein with no known function (24 kDa SPro) (Fig. 2b). Although there were no NCBI sequences reported for the W. bancrofti homologue of this protein, a similar protein in D. immitis named 22–24 kDa excretory-secretory 22U protein has been reported36. MAb 4E9 precipitated Macrophage Migration Inhibitory Factor (MIF) (Fig. 2b), a worm homologue of human MIF, a proinflammatory cytokine37. Filarial MIF functions in immunoregulation and possibly pathogenesis via attracting host monocytes/macrophages to modify host immune responses and promote parasite survival. Finally, mAb 2E6, which showed the most intense signal in all screening assays, precipitated the polyprotein ladder-like protein or gp15/400 (Fig. 1a, b, 2a), a ~400 kDa nematode polyprotein allergen/antigen (NPA-1) with 20 tandemly arranged repeat subunits of 132 amino acid residues. B. malayi gp15/400 protein is associated with the worm surface and distributed in all tissues of the parasite38. The presence of gp15/400 protein in the B. malayi secretome was confirmed by immunoblot of culture media used to keep worms alive for 7 days (Supplemental Data Fig. 3). While antibody 2E6 is specific for B. malayi and W. bancrofti gp15/400, it did not show cross-reactivity with D. immitis gp15/400.
To confirm IgE mAb specificity, we expressed recombinant forms of 19 filarial antigens (SXP, 24 kDa SPro, MIF, gp15/400, and 15 different TTR proteins) in bacteria using the W. bancrofti sequence and tested binding by ELISA (Fig. 2c). If IgE mAbs showed positivity in an immunoblot of somatic extract, we also performed immunoblotting (Fig. 2d). When we were able to express and purify antigen protein in sufficient quantities, we calculated the EC50 for each antibody (Fig. 2c, Table 2). EC50 measurements ranged from single ng/mL, such as with mAb 11H12, to nearly μg/mL concentrations. Antibodies 5H1 and 18H7 showed a range of EC50 binding values to different TTR proteins, demonstrating their varied breadth of cross-reactivity within this protein family (Fig. 2c). Another interesting feature of TTR protein-specific mAbs is their varied tendency to bind TTR dimers (see Fig. 2d). MAb 9C1 binds preferentially to dimeric TTR, 18H7 prefers monomeric TTR, and 5H1 binds equally to both.
Table 2.
Filaria-reactive human IgE mAb target proteins
| IgE mAb |
Subject code |
ImmunoCAP reactivity (kUA/L) |
Binding to filarial worm extract |
EC50 (ng/mL) |
Mass spectrometry |
Antigen name |
Sequence ID | |
|---|---|---|---|---|---|---|---|---|
| ELISA | Immunoblot | |||||||
| 12D4 | P3 | 5.3 | + | +++ | 24.6 | WbSXP-1 | Antigen Wb14 | AF063940.1 |
| 4E1 | P3 | 0.5 | − | +++ | 144.6 | WbSXP-1 | Antigen Wb14 | AF063940.1 |
| 9C1 | P3 | 1 | + | +++ | 95.9 | TTR-76 | ? | VDM14676.1 |
| 12C2 | P3 | 0.3 | + | +++ | 95.9 | TTR-76 | ? | VDM14676 .1 |
| 5H1 | P1 | 1.4 | ++ | +++ | 6.5 | TTR-79 | ? | EJW78979 .1 |
| 18H7 | P1 | 0 | + | +++ | 43.3 | TTR-79, TTR-62 | ? | EJW86262.1, EJW78979.1 |
| 1A5 | P1 | 14.2 | ++ | +++ | ND | TTR-77 | ? | VDM13477.1 |
| 10D5 | P5 | 31 | ++ | − | ND | TTR-32 | ? | VDM07632.1 |
| 11G1 | P2 | 4.8 | + | − | ND | TTR-90 | ? | VDM14190.1 |
| 10H9 | P1 | 28.6 | ++ | − | ND | TTR-04 | ? | EJW80404.1 |
| 14B2 | P1 | 23.9 | + | +++ | ND | TTR-61 | ? | EJW84161.1 |
| 30A10 | P4 | 2.5 | + | − | ND | TTR-16 | ? | EJW86116.1 |
| 18D4 | P1 | 3.5 | + | − | ND | TTR-08 | ? | VDM13708.1 |
| 2E6 | P1 | 465.7 | +++ | +++ | 7.7 | Ladder protein, gp15/400 | Nematode polyprotein allergen-1 (NPA-1) | AAG31482.1 |
| 4E9 | P3 | 57.5 | +++ | − | 26.7 | Macrophage Migration Inhibitory Factor (MIF) | MIF−1 | EJW88743.1 |
| 11H12 | P1 | 12 | ++ | +++ | 1.7 | 24kDa secreted protein | Alt-1, P22 | VIO90327.1 |
The most prominent antigen(s) are listed for TTR-specific IgE mAbs (such as 5H1, 9C1 and 18H7) that cross-react with multiple members of the TTR family. ND: not determined due to low expression of target proteins.
We next tested the 30 IgE mAbs from the original 56 that had not tested positive in reactivity tests against each recombinant antigen to ensure that no antibody was missed due to its specificity for W. bancrofti. MAb 4E1, for example, was found specific for WbSXP-1 but negative in all primary extract screens. Unlike mAb 12D4, 4E1 is highly specific to W. bancrofti and does not cross-react with B. malayi or D. immitis SXP-1 proteins (Supplemental Data Fig. 4). Finally, two mAbs, 5D2 and 7G12, were positive by ELISA and ImmunoCAP but negative in IP analysis (Supplemental Table 1). MAb 5D2 and 4G8 showed weak binding to several allergen proteins based on the ImmunoCAP ISAC assay (Supplemental Data Fig. 2), suggesting poly-reactivity to a carbohydrate epitope. Overall, these results indicate that the humoral immune response to filarial infection targets a restricted set of filarial-specific E/S proteins.
TTR Proteins are Immunodominant Filarial Antigens with Diagnostic Potential
TTR proteins identified here as target antigens of multiple filaria-specific IgE mAbs have not been previously reported as filarial immunogens. Thus, we asked whether our panel of filaria-specific IgE included mAbs specific to other TTR proteins. To do so, we first assembled a phylogenetic tree using WbTTR protein sequences (total of 25 encoded by W. bancrofti) collected from NCBI (Supplemental Data Fig. 5). TTR family proteins shared a low degree of identity, ranging from 18.1% to 70.1% when compared in pairwise fashion (Supplemental Data Fig. 5b). Since this family of proteins has not been well studied, the functions of which remain unknown, we are unable to speculates as to the purpose for the large number and tremendous variability of the TTR proteins excreted by filarial helminths.
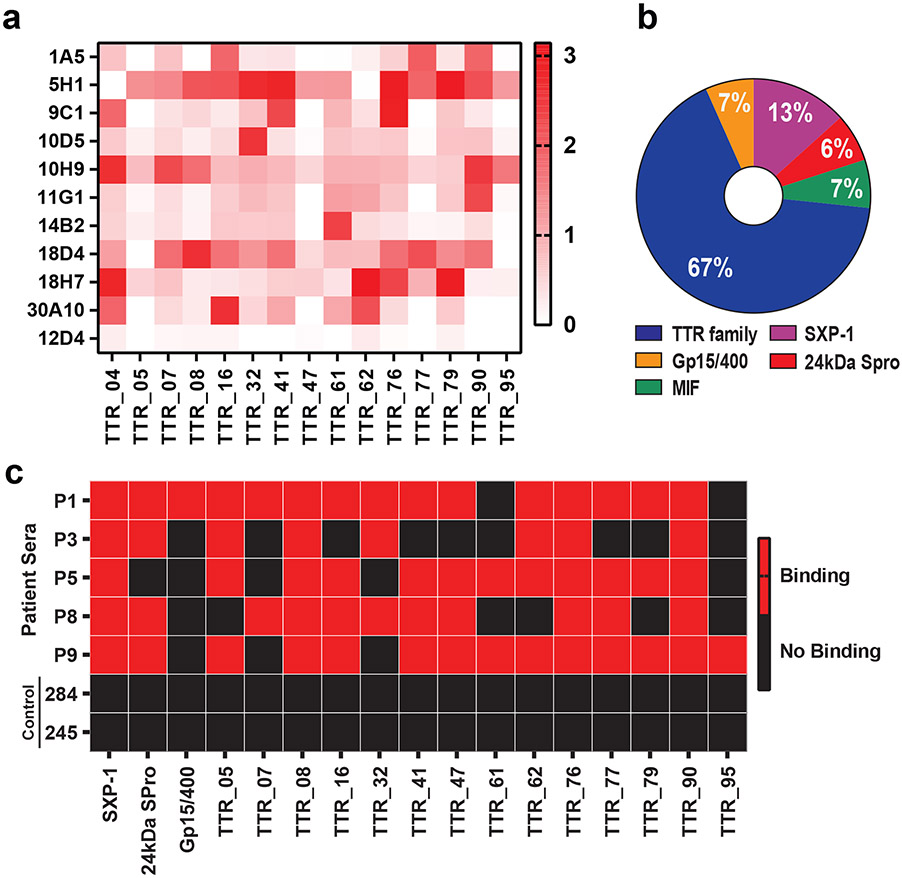
Next, we chose 15 TTR proteins as representatives of the family and expressed each as a his-tagged fusion protein for IgE mAb testing in ELISA and immunoblotting. Binding analysis of the 15 TTR proteins with 10 IgE mAbs revealed that some antibodies were highly specific to one TTR protein (Fig. 3a), while others showed varying degrees of cross-reactivity across different TTR protein family members (Fig. 3a, Supplemental Data Fig. 6). Of note, two IgE mAbs, 5H1 and 18H7, broadly cross-reacted with 13 different TTR proteins despite minimal amino acid conservation (Fig 2c, 3a Supplemental Data Fig. 5b, 6, 7). Antibodies targeting TTR protein antigens made up the greatest proportion of our panel of filaria-specific antibodies (Fig. 3b). Overall, these results indicate that TTR family proteins are the most dominant filarial antigens targeted by the human IgE antibody response (Fig. 3b, Table 2).
Figure 3. TTR family proteins are the prominent filarial antigens targeted by IgE antibodies and have diagnostic potential.
a, ELISA analysis depicting TTR-specific antibody cross-reactivity profile to multiple TTR proteins. MAb 12D4, a human IgE mAb specific for WbSXP-1, served as negative control. OD, optical density. b, Proportion of filaria-specific antibodies binding different antigens. c, Binary heatmap showing presence (red) or absence (black) of antibody in indicated patient sera against filarial antigens tested in immunoblot analysis. Two serum samples (284 and 245) from respective mold- and shellfish-allergic patients served as negative controls.
We next tested sera of subjects with filarial infection for the presence of filaria-specific IgE antibodies using immunoblotting of 17 recombinant filarial antigens. We chose 3 subjects (P1, P3, P5) from whom IgE mAbs were generated from their PBMCs in this study, and 2 subjects (P8, P9) in which no PBMCs were used and no IgE mAbs were developed, to assess each for the presence of specific antibodies. Immunoblotting not only verified the presence of antibodies with the same specificity as those of our IgE mAbs in the serum of subjects from whom mAbs were developed, but also confirmed presence of antibodies with similar specificities in other filarial worm infected subjects. Each subject sample showed a distinct pattern of reactivity predominantly to TTR family proteins (Fig. 3c, Supplemental Data Fig. 8), confirming that antigens identified here could be used to develop species-specific diagnostics.
Filaria-Specific IgE mAbs Exhibit a High Degree of Somatic Hypermutation (SHM)
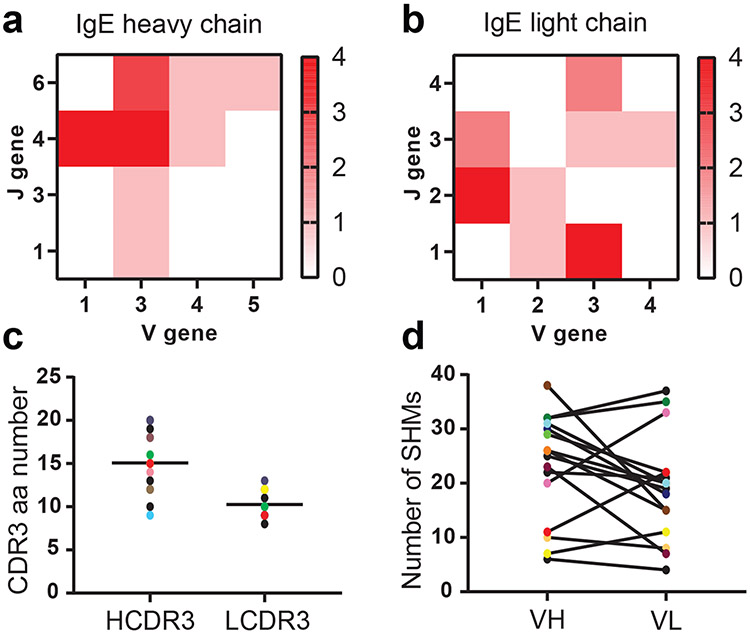
To evaluate sequence diversity and degree of somatic hypermutation, we performed sequence analysis of 16 filaria-specific IgE hybridoma clones (Fig. 4). That analysis revealed 16 unique Vh–Jh–CDRH3–Vl–Jl–CDRL3 clonotypes (Supplementary Data Fig. 9). Filaria-specific IgE antibodies varied widely in their antibody variable region (VH and VL) germline gene usage, with no statistically significant over-representation of any germline gene (Fig. 4a). They also varied in the lengths of VH and VL complementarity-determining region 3 (CDR3), but relative to other isotypes, IgE B cells show similar length distributions of CDR3 amino acids in the heavy and light chains39 (Fig. 4b, c). Filaria-specific IgE mAbs exhibited a high degree of SHM in VH and VL genes (Fig. 4d), which contained an average of 23 and 19 SHMs, respectively. No meaningful correlation was observed between VH and VL mutation frequencies. The high degree of SHM of filaria-specific antibodies suggests a prolonged and ongoing humoral immune response to antigens.
Figure 4. IgH and IgL gene sequence analysis of filaria-specific IgE antibodies.
Sixteen filaria-specific IgE antibodies were found to have unique sequences and were analyzed for genetic features. a, Heatmap showing the total number of unique sequences with corresponding V and J genes. b, CDR3 amino acid length distribution for heavy and light chains with indicated mean. c, IgH and IgL CDR3 amino acid number was determined using the ImMunoGeneTics (IMGT) database. d, Number of SHMs in the VH and VL chain of filaria-specific mAb genes.
Filaria-Specific IgE Antibodies Induce Anaphylaxis in Mice
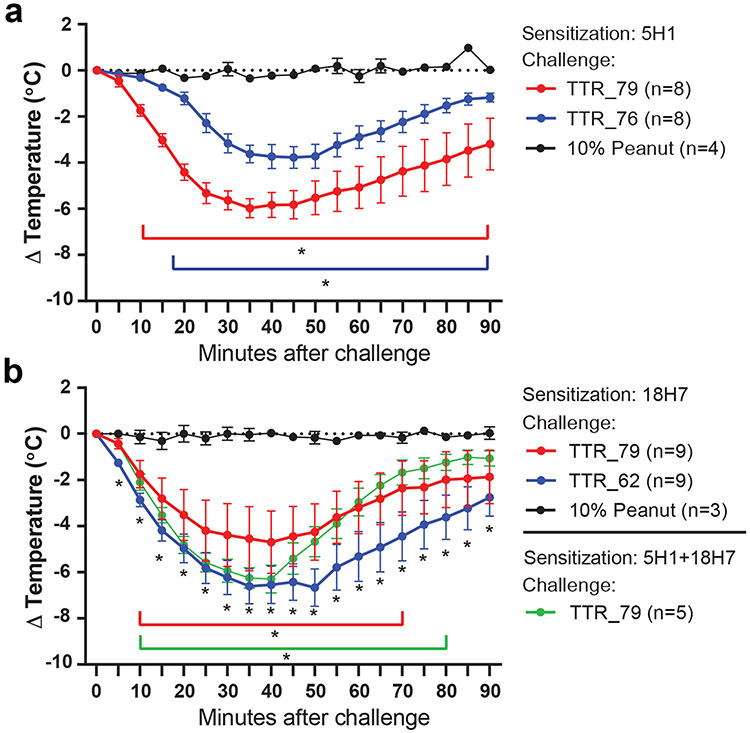
We next assessed function of two filaria-specific IgE mAbs, 5H1 and 18H7, using a human FcεRIα–transgenic mouse model of passive systemic anaphylaxis. We chose these two IgE mAbs because of their ability to bind TTR protein dimers, which could in theory allow cross-linking of FcεRI to trigger mast cell degranulation. Thus, we first sensitized mice with TTR–specific IgE mAbs 5H1, 18H7, both, or with injection of an isotype control antibody. Three days later mice were challenged with single intraperitoneal injection with recombinant TTR proteins (50 μg of filarial antigen TTR_76, TTR_79, or TTR_62) or with control peanut allergen extract. All mice injected with recombinant TTRs, whether sensitized with mAbs 5H1, 18H7, or both, exhibited a significant drop in temperature of up to 6°C, reaching the nadir within 35 minutes of challenge, while animals receiving peanut allergen extract showed no temperature change (Fig. 5a, b). This response is indicative of anaphylaxis40. Of note, we observed no additive or synergistic effects in mice injected with both 5H1 and 18H7 IgE mAbs (Fig. 5b).
Figure 5. Filarial antigens induce anaphylaxis in IgE mAb-sensitized human FcεRIα–transgenic mice.
Mice were sensitized with a 100 μg i.p. injection of human IgE mAb. Three days later, mice were injected i.p. with 50 μg of filarial antigens or a control allergen (10% peanut extract). Body temperature over 90 minutes was monitored using an implanted temperature probe. a, Mice were sensitized with a 100 μg i.p. injection of human IgE mAb 5H1 and challenged with TTR_76, TTR_79, or 10% peanut extract. b, Mice were sensitized with 5H1 or 18H7 or both and challenged with TTR_79, TTR_62, or 10% peanut extract. Change in body temperature of experimental compared to control allergen groups at each time point using a paired 2-tailed t test assuming unequal variance. Time points with calculated P values <0.05 are highlighted by an asterisk. Data are means ± SEM of each experimental group. The number of mice (n) for each experimental group is shown.
DISCUSSION
Despite our deep understanding of the major allergen proteins responsible for induction of allergic diseases, little is known about immunogenic triggers of the IgE antibody response in the defense against helminth infection. We adopted an unbiased approach to investigate specificities and functions of IgE antibodies produced in the context of filarial worm infection. Of the 56 human mAbs studied, 26 showed binding to B. malayi and/or D. immitis worm somatic extracts and none of them showed significant cross-reactivity toward commonly known allergens. We identified 14 unique filarial antigens targeted by 16 filaria-specific IgE mAbs and report here for the first time the “(TTR)-like” family of proteins as being potent filarial immunogens. It is remarkable that members of this family of proteins do not share much homology, yet of the 10 TTR-specific IgE mAbs two broadly cross-react with 13 different TTR proteins. As two thirds of our filaria-specific antibodies are targeting TTR proteins, we find this family to contain the immunodominant antigens inducing IgE antibodies in response to filarial infections. Results from our studies also show that IgE antibodies specific for filarial antigens identified here (WbSXP-1, TTRs, 24kDa secreted protein, and NPA-1) are naturally produced during infection and can be identified within the serum of subjects. These findings further confirm the unbiasedness of our study in identifying the most prominent filarial antigens and proposes a sensitive species-specific diagnostic approach.
We report that all filarial antigens targeted by IgE antibodies in our analysis were nematode-specific and E/S proteins. Our in vivo data shows that filaria-specific IgE antibodies induce a robust type one hypersensitivity response. Adult filarial worms copulate within their host and produce microfilaria, but new infections are required to increase the number of adult worms. Stopping new larvae from maturing after entering the skin via mosquito bite would prevent an increase in the number of worms living within the host. Taken together, this suggests that evolutionarily the IgE antibody response developed to enable prompt recognition of invading worms at the larval stage as they penetrate the epithelial barrier. We hereby propose that IgE antibodies produced in response to parasitic infections function to prevent establishment of new infections at the epithelial site of entry. Mast cell activation occurring through IgE cross-linking associated with excreted worm antigens allows immediate detection, prompting eosinophil and basophil infiltration and targeting the worm when it is most vulnerable. This “gatekeeper” function of IgE was initially proposed and studied for its effect on microbial toxin neutralization41-43, but also appears to be the specific role it plays in helminth immunity. Future experiments will further evaluate this role directly in defending against helminth infection by passively sensitizing hFcεRI mice with functional human IgE mAbs described here and challenging with living B. malayi infective stage larvae. Given the impact of helminthic diseases in developing countries, it is of central importance to identify key helminth immunogens that can inform work toward improved diagnostics and the host/parasite interface. In addition to providing insights into the ontogeny of the IgE response in helminth infection, findings reported here have implications for rational design of helminth vaccines providing IgE-mediated pathology can be circumvented.
Supplementary Material
KEY MESSEGES.
Despite it becoming common knowledge that the IgE antibody response is necessary for protection from parasitic worm infections there is limited understanding of antigenic triggers underlying this response and the natural function of IgE.
All filarial antigens targeted by IgE antibodies in our analysis were nematode-specific and excreted proteins.
Our data suggest that IgE antibodies enable prompt immune recognition of invading worms at the barrier of entry.
ACKNOWLEDGEMENTS
We would like to thank Digital Proteomics LLC for help with MS bioinformatics. This work was supported by research grants from NIH/National Institute of Allergy and Infectious Disease K08AI103038, R01AI130459, R21AI123307 (to SAS), Vanderbilt University CTSA UL1TR000445, and the Schlumberger Foundation Faculty for the Future Fellowship Award (to AH). This work was also supported in part by the Division of Intramural Research, National Institutes of Allergy and Infectious Diseases (TBN). Reagents were provided by the NIH/NIAID Filariasis Research Reagent Resource Center for distribution through BEI Resources, NIAID, NIH. We also thank all study volunteers at the NIH. Finally, we would also like to thank Dr. Elise Lamar for her exceptional editing of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
ABBREVIATIONS
- BmA
Brugia malayi somatic extracts
- MAb
Monoclonal Antibody
- E/S proteins
Excreted secretory proteins
- LF
Lymphatic filariasis
- CCD
Cross-reactive carbohydrate determinants
- Th2
T-helper 2
- TPE
Tropical pulmonary eosinophilia
- PBMC
Peripheral blood mononuclear cells
- IP
Immunoprecipitation
- MS
Mass spectrometry
- SHM
Somatic hypermutation
- TTR
Transthyretin-related
- MIF
Migration inhibitory factor
- NPA
Nematode polyprotein antigen
- EC50
Half maximal effective concentration
- PSM
Peptide spectrum matches
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author conflicts of interest:
Scott A. Smith is an inventor on a patent (US 10,908,168) describing a method to make IgE antibodies, including those related to this work.
RESOURCE AVAILABILITY
All IgE monoclonal antibodies generated in this study are available from the lead contact with a completed materials transfer agreement.
REFERENCES
- 1.Jarrett E & Bazin H. Elevation of total serum IgE in rats following helminth parasite infection. Nature 1974;251:613–614. [DOI] [PubMed] [Google Scholar]
- 2.Bethony J, Loukas A, Smout M, Brooker S, Mendez S, Plieskatt J, et al. Antibodies against a secreted protein from hookworm larvae reduce the intensity of hookworm infection in humans and vaccinated laboratory animals. FASEB J 2005;19:1743–1745. [DOI] [PubMed] [Google Scholar]
- 3.Pritchard DI, Quinnell RJ, & EA Walsh. Immunity in humans to Necator Americanus: IgE, parasite weight and fecundity. Parasite Immunol 1995;17:71–75. [DOI] [PubMed] [Google Scholar]
- 4.Faulkner H, Turner J, Kamgno J, Pion SD, Boussinesq M, Bradley JE. Age- and infection intensity-dependent cytokine and antibody production in human trichuriasis: The importance of IgE. J Infect Dis 2002;185:665–72. [DOI] [PubMed] [Google Scholar]
- 5.Turner JD, Faulkner H, Kamgno J, Kennedy MW, Behnke J, Boussinesq M, et al. Allergen-specific IgE and IgG4 are markers of resistance and susceptibility in a human intestinal nematode infection. Microbes Infect 2005;7:990–96. [DOI] [PubMed] [Google Scholar]
- 6.McSharry C, Xia Y, Holland CV & Kennedy MW. Natural immunity to Ascaris lumbricoides associated with immunoglobulin E antibody to ABA-1 allergen and inflammation indicators in children. Infect Immun 1999;67:484–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzsimmons CM, Jones FM, Pinot de Moira A, Protasio AV, Khalife J, Dickinson HA, et al. Progressive cross-reactivity in IgE responses: An explanation for the slow development of human immunity to schistosomiasis? Infect Immun 2012;80:4264–4270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pinot de Moira A, Fulford AJ, Kabatereine NB, Ouma JH, Booth M, Dunne DW. Analysis of complex patterns of human exposure and immunity to Schistosomiasis mansoni: The influence of age, sex, ethnicity and IgE. PLoS Neg Trop Dis 2010;4:e820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jiz M, Friedman JF, Leenstra T, Jarilla B, Pablo A, Langdon G, et al. Immunoglobulin E (IgE) responses to paramyosin predict resistance to reinfection with Schistosoma japonicum and are attenuated by IgG4. Infect Immun 2009;77:2051–2058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunne DW, Butterworth AE, Fulford AJ, Kariuki HC, Langley JG, Ouma JH, et al. Immunity after treatment of human schistosomiasis: Association between IgE antibodies to adult worm antigens and resistance to reinfection. Eur J Immunol 1992;22:1483–1494. [DOI] [PubMed] [Google Scholar]
- 11.Dunne DW, Webster M, Smith P, Langley JG, Richardson BA, Fulford AJ, et al. The isolation of a 22 kDa band after SDS-PAGE of Schistosoma mansoni adult worms and its use to demonstrate that IgE responses against the antigen(s) it contains are associated with human resistance to reinfection. Parasite Immunol 1997;19:79–89. [DOI] [PubMed] [Google Scholar]
- 12.Hagan P, Blumenthal UJ, Dunn D, Simpson AJ & Wilkins HA. Human IgE, IgG4 and resistance to reinfection with Schistosoma haematobium. Nature 1991; 349:243–245. [DOI] [PubMed] [Google Scholar]
- 13.Rihet P, Demeure CE, Bourgois A, Praia A & Dessein AJ. Evidence for an association between human resistance to Schistosoma mansoni and high anti-larval IgE levels. Eur J Immunol 1991;21:2679–2686. [DOI] [PubMed] [Google Scholar]
- 14.Jarrett E, Mackenzie S & Bennich H. Parasite-induced ‘nonspecific’ IgE does not protect against allergic reactions. Nature 1980;283:302–303. [DOI] [PubMed] [Google Scholar]
- 15.Sereda MJ, Hartmann S & Lucius R. Helminths and allergy: The example of tropomyosin. Trends Parasitol 2008;24:272–278. [DOI] [PubMed] [Google Scholar]
- 16.Gazzinelli-Guimaraes PH, Bennuru S, de Queiroz Prado R, Ricciardi A, Sciurba J, Kupritz J, et al. House dust mite sensitization drives cross-reactive immune responses to homologous helminth proteins. PLoS Pathog. 2021;17:e1009337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santiago HC, Bennuru B, Boyd A, Eberhard M & Nutman TB. Structural and immunologic cross-reactivity among filarial and mite tropomyosin: Implications for the hygiene hypothesis. J Allergy Clin Immunol 2011;127:479–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Homann A, Schramm G, Jappe U. Glycans and glycan-specific IgE in clinical and molecular allergology: sensitization, diagnostics, and clinical symptoms. J Allergy Clin Immunol 2017;140:356–368. [DOI] [PubMed] [Google Scholar]
- 19.Platts-Mills TA, Hilger C, Jappe U, van Hage M, Gadermaier G, Spillner E, Lidholm J, et al. Carbohydrate epitopes currently recognized as targets for IgE antibodies. Allergy 2021;76:2383–2394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nutman TB. Looking beyond the induction of Th2 responses to explain immunomodulation by helminths. Parasite Immunol 2015;37:304–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottesen EA & Nutman TB. Tropical pulmonary eosinophilia. Annu Rev Med 1992;43:417–424. [DOI] [PubMed] [Google Scholar]
- 22.Wurth MA, Hadadianpour A, Horvath DJ, Daniel J, Bogdan O, Goleniewska K, et al. Human IgE mAbs define variability in commercial aspergillus extract allergen composition. JCI Insight 2018;3:e123387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dombrowicz D, Brini AT, Flamand V, Hicks E, Snouwaert JN, Kinet JP, et al. Anaphylaxis mediated through a humanized high affinity IgE receptor. J Immunol 1996;157:1645–1651. [PubMed] [Google Scholar]
- 24.Croote D, Darmanis S, Nadeau KC & Quake SR. High-affinity allergen-specific human antibodies cloned from single IgE B cell transcriptomes. Science 2018;362:1306–1309. [DOI] [PubMed] [Google Scholar]
- 25.Hewitson JP, Harcus YM, Curwen RS, Dowle AA, Atmadja AK, Ashton PD, et al. The secretome of the filarial parasite, Brugia malayi: Proteomic profile of adult excretory-secretory products. Mol Biochem Parasitol 2008;160:8–21. [DOI] [PubMed] [Google Scholar]
- 26.International Helminth Genomes Consortium. Comparative genomics of the major parasitic worms. Nat Genet 2019;51:163–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Geary J, Satti M, Moreno Y, Madrill N, Whitten D, Headley SA, et al. First analysis of the secretome of the canine heartworm, Dirofilaria immitis. Parasit Vectors 2012;10:5–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantacessi C, Mitreva M, Campbell BE, Hall RS, Young ND, Jex AR, et al. First transcriptomic analysis of the economically important parasitic nematode, Trichostrongylus colubriformis, using a next-generation sequencing approach. Infect Genet Evol 2010;10:1199–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sonnhammer EL & Durbin R. Analysis of protein domain families in Caenorhabditis elegans. Genomics 1997;42:200–216. [DOI] [PubMed] [Google Scholar]
- 30.Lin B, Zhuo K, Chen S, Hu L, Sun L, Wang X, et al. A novel nematode effector suppresses plant immunity by activating host reactive oxygen species-scavenging system. New Phytol 2016;209:1159–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dissanayake S, Xu M & Piessens WF. A cloned antigen for serological diagnosis of Wuchereria bancrofti microfilaremia with daytime blood samples. Mol Biochem Parasitol 1992;56:269–277. [DOI] [PubMed] [Google Scholar]
- 32.Rao KV, Eswaran M, Ravi V, Gnanasekhar B, Narayanan RB, Kaliraj P, et al. The Wuchereria bancrofti orthologue of Brugia malayi SXP1 and the diagnosis of bancroftian filariasis. Mol. Biochem. Parasitol 2000;107:71–80. [DOI] [PubMed] [Google Scholar]
- 33.Rahman AR, Hwen-Yee C & Noordin R. Pan LF-ELISA using BmR1 and BmSXP recombinant antigens for detection of lymphatic filariasis. Filaria J 2007;6:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.García-Mayoral MF, Treviño MA, Pérez-Piñar T, Caballero ML, Knaute T, Umpierrez A, et al. Relationships between IgE/IgG4 epitopes, structure and function in Anisakis simplex Ani s 5, a member of the SXP/RAL-2 protein family. PLoS Neg Trop Dis 2014;8:e2735–e2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuji N, Suzuki K, Kasuga-Aoki H, Isobe T, Arakawa T, Matsumoto Y. Mice Intranasally immunized with a recombinant 16-kilodalton antigen from roundworm Ascaris parasites are protected against larval migration of Ascaris suum. Infect Immun 2003;71:5314–5323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Frank GR, Wisnewski N, Brandt KS, Carter CR, Jennings NS, Selkirk ME. Molecular cloning of the 22-24 kDa excretory-secretory 22U protein of Dirofilaria immitis and other filarial nematode parasites. Mol Biochem Parasitol 1999;98:297–302. [DOI] [PubMed] [Google Scholar]
- 37.Pastrana DV, Raghavan N, FitzGerald P, Eisinger SW, Metz C, Bucala R, et al. Filarial nematode parasites secrete a homologue of the human cytokine macrophage migration inhibitory factor. Infect Immun 1998;66:5955–5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tweedie S, Paxton WA, Ingram L, Maizels RM, McReynolds LA, Selkirk ME. Brugia pahangi and Brugia malayi: A surface-associated glycoprotein (Gp15/400) is composed of multiple tandemly repeated units and processed from a 400-KDa precursor. Exp Parasitol 1993;76:156–164. [DOI] [PubMed] [Google Scholar]
- 39.Soto C, Bombardi RG, Branchizio A, Rose N, Matta P, Sevy AM, et al. High frequency of shared clonotypes in human B cell receptor repertoires. Nature 2019;566:398–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Osterfeld H, Ahrens R, Strait R, Finkelman FD, Renauld JC, Hogan SP. Differential roles for IL- 9/IL-9 receptor α-chain pathway in systemic and oral antigen-induced anaphylaxis. J Allergy Clin Immunol 2010;125:496–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinberg P, Ishizaka K & Norman PS. Possible role of IgE-mediated reaction in immunity. J Allergy Clin Immunol 1974;54:359–366. [Google Scholar]
- 42.Ishizaka K, Ishizaka T, Sugahara T, Maruyama S Studies on the relationship between toxin anaphylaxis and antitoxic immunity. III. Significance of allergic reaction on the neutralization of toxin injected intracutaneously. Jpn J Med Sci Biol 1957;10:343–361. [DOI] [PubMed] [Google Scholar]
- 43.Friedemann U Dynamics and mechanism of immunity reactions in vivo. Bacteriol Rev 1947;11:275–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SA & Crowe JE Jr. Use of human hybridoma technology to isolate human monoclonal antibodies. Microbiol Spectr 2015;3:0027–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Smith SA, Zhou Y, Olivarez NP, Broadwater AH, de Silva AM, Crowe JE Jr. Persistence of circulating memory B cell clones with potential for dengue virus disease enhancement for decades following infection. J Virol 2012;86:2665–2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith K, Garman L, Wrammert J, Zheng NY, Capra JD, Ahmed R, et al. Rapid generation of fully human monoclonal antibodies specific to a vaccinating antigen. Nature Protocols 2009;4:372–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lobstein J, Emrich CA, Jeans C, Faulkner M, Riggs P, Berkmen M. SHuffle, a novel Escherichia coli protein expression strain capable of correctly folding disulfide bonded proteins in its cytoplasm. Microb Cell Fact 2012;8:11–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.