Abstract
Phylogenomic analyses routinely estimate species trees using methods that account for gene tree discordance. However, the most scalable species tree inference methods, which summarize independently inferred gene trees to obtain a species tree, are sensitive to hard-to-avoid errors introduced in the gene tree estimation step. This dilemma has created much debate on the merits of concatenation versus summary methods and practical obstacles to using summary methods more widely and to the exclusion of concatenation. The most successful attempt at making summary methods resilient to noisy gene trees has been contracting low support branches from the gene trees. Unfortunately, this approach requires arbitrary thresholds and poses new challenges. Here, we introduce threshold-free weighting schemes for the quartet-based species tree inference, the metric used in the popular method ASTRAL. By reducing the impact of quartets with low support or long terminal branches (or both), weighting provides stronger theoretical guarantees and better empirical performance than the unweighted ASTRAL. Our simulations show that weighting improves accuracy across many conditions and reduces the gap with concatenation in conditions with low gene tree discordance and high noise. On empirical data, weighting improves congruence with concatenation and increases support. Together, our results show that weighting, enabled by a new optimization algorithm we introduce, improves the utility of summary methods and can reduce the incongruence often observed across analytical pipelines.
Keywords: phylogenomics, ILS, summary methods, ASTRAL, gene tree estimation error
Introduction
Genome-wide data are increasingly available across the tree of life, giving researchers a chance to systematically resolve the evolutionary relationships among species (i.e., species trees) using phylogenomic data. A central promise of phylogenomics is that processes such as incomplete lineage sorting (ILS) that can cause discordance (Maddison 1997; Degnan and Rosenberg 2009) among evolutionary histories of different parts of the genome (i.e., gene trees) can be modeled (Edwards 2009). There has been much progress in developing the theory and methods for species tree inference in the presence of ILS (Mirarab et al. 2021) and other sources of discordance (Elworth et al. 2019; Smith and Hahn 2021). These phylogenomics approaches have also been widely and increasingly adopted in practice. Yet, substantial challenges remain. Analyses of real data using different methods often reveal incongruent results (Smith et al. 2015; Reddy et al. 2017; Shen et al. 2017; Walker et al. 2018; Gatesy et al. 2019), sparking debate about the cause. Meanwhile, simulation studies have revealed that the best choice of the method is data-dependent (e.g., Bayzid and Warnow 2013; Mirarab and Warnow 2015).
A major challenge in phylogenomics is that when we infer gene trees, often from relatively short sequences, the results tend to be highly error-prone (Patel 2013; Mirarab, Bayzid, et al. 2014; Springer and Gatesy 2016). Co-estimation of gene trees and species trees (Szöllõsi et al. 2014) is perhaps the most accurate approach to dealing with such noise (Leaché and Rannala 2011; Knowles et al. 2012). However, despite some progress (Ogilvie et al. 2017), these methods have remained limited in their scalability to even moderately large numbers of species. The approach that is far more scalable and is used often in the “summary” approach: first estimate gene trees from sequence data independently and then summarize them into a species tree by solving optimization problems that provide guarantees of statistical consistency if we allow ourselves to ignore the error in the input tree.
Many summary methods (e.g., Liu et al. 2009, 2010; Mossel and Roch 2010; Liu and Yu 2011; Vachaspati and Warnow 2015) were developed and proved statistically consistent under the multispecies coalescent (MSC) model (Takahata 1989) of ILS. Species trees inferred by these tools can be highly accurate even under high levels of ILS. Among the summary tools, ASTRAL (Mirarab, Reaz, et al. 2014) is among the most widely used and is integrated into other packages (Alanjary et al. 2019; Wang et al. 2020). ASTRAL simply seeks the species tree that maximizes the number of shared quartets (unrooted four-taxon subtrees) between gene trees and the species tree, an optimization problem that guarantees a statistically consistent estimator under the MSC model. The empirical accuracy and scalability of ASTRAL have compared favorably with other methods (e.g., Mirarab 2019). Moreover, it has now been shown that ASTRAL is also consistent and/or accurate under the gene duplication and loss (GDL) model (Legried et al. 2021; Yan et al. 2021), some horizontal gene transfer models (Davidson et al. 2015), and combined models of ILS and GDL (Markin and Eulenstein 2021), but not gene flow (Solís-Lemus et al. 2016). Zhang et al. (2020) have further adopted the quartet-based approach to multicopy inputs.
Nevertheless, all summary methods, ASTRAL included, have a shortcoming: inaccuracies in input gene trees can translate to errors in the output species tree (Patel 2013; DeGiorgio and Degnan 2014; Lanier and Knowles 2015; Huang and Knowles 2016; Molloy and Warnow 2018). In fact, Roch et al. (2019) proved that summary methods (and concatenation) are positively misleading under pathological examples even in the absence of much true gene tree discordance. These concerns are not just theoretical and can impact biological analyses. For example, on an order-level avian phylogenomic dataset (Jarvis et al. 2014), summary methods, including ASTRAL, produce species trees contradicting the well-established relationships when given input gene trees that have extremely low support (Bayzid et al. 2015), a condition that motivated Mirarab, Bayzid, et al. (2014) to bin multiple genes together. As an alternative, Zhang et al. (2018) showed that contracting very low-support branches before running ASTRAL-III can improve accuracy in simulations and on biological datasets such as the avian dataset. However, this form of reduction in species tree estimation error comes with caveats. Contracted branches may still include signals that will be lost. In particular, when contraction is overly aggressive (e.g., with moderately high thresholds such as 50% or 75%), filtering is often harmful. More pragmatically, the best choice of threshold is dataset dependent, and making a principled choice is challenging, if not impossible.
Threshold-free approaches for incorporating gene tree branch support into summary methods have also been proposed. Multilocus bootstrapping (MLBS) runs the summary method on the bootstrap replicates of gene trees, repeating the process many times to obtain several species trees, which are then combined using a consensus method (Seo 2008). MLBS can be understood as weighting inferences made from each gene by their uncertainty, and thus, a way to deal with noise. However, previous studies show that MLBS, in fact, reduces the accuracy compared with using maximum-likelihood (ML) trees (Mirarab et al. 2016). The related method of simply combining all bootstrap replicates into a single run of the summary method has also not been accurate (Mirarab, Reaz, et al. 2014). A plausible explanation is that bootstrap replicates have much higher rates of discordance and error than ML trees (Sayyari and Mirarab 2016), and thus, using them directly as input adds noise, even if it reveals uncertainty.
An alternative to using bootstrap trees is to use ML trees as input but explicitly weight gene tree branches (or their quartets) by their statistical support. We can generalize the moderately successful gene contraction approach, which effectively assigns weights of zero or one to quartets, to weight each quartet shared between an estimated gene tree and the proposed species tree according to the statistical support of the quartet resolution. Such an approach will free us from picking arbitrary contraction thresholds and may lead to better accuracy. However, weighting by branch support has not yet been incorporated into existing summary methods such as ASTRAL-III for several reasons. (1) Quartet weights must be implicitly calculated, as explicitly examining all quartets of species alone will take time. The existing general (e.g., Avni et al. 2015) and MSC-based weighted quartet methods (Yourdkhani and Rhodes 2020; Richards and Kubatko 2021) require weights explicitly calculated for every quartet, making them less scalable with . The reason ASTRAL-III can scale to a large number of species is that it optimizes a score defined over all quartets without explicitly enumerating them. Designing a scalable weighting method will require weights that can be implicitly computed based on examining gene tree branches. (2) It is difficult to design efficient algorithms to optimize a weighted score. Unless weights satisfy certain properties, it may not be possible to find an algorithm better than even for the much simpler problem of computing the total quartet weights of a gene tree. However, with favorable definitions of weights, these difficulties are not insurmountable.
Here, we introduce weighting schemes that avail themselves to efficient optimization with weights conveniently obtained from tree branch lengths (wASTRAL-bl), branch support values (wASTRAL-s), or both (wASTRAL-h). We introduce the weighted ASTRAL algorithm, an efficient method that, similarly to unweighted ASTRAL, optimizes a quartet score but is different in several ways: (1) Its optimization criterion weights each gene tree quartet. (2) Its optimization algorithm is entirely different from unweighted ASTRAL. Whereas the algorithm is more complex and slower in some cases, it scales much better (linearly instead of quadratically) as the number of genes () increases and handles missing data better. (3) Its software package is implemented from scratch and is in C++ instead of Java. We show that wASTRAL-h is superior to unweighted ASTRAL in terms of its theoretical guarantees under the MSC model and has better accuracy on simulated data in terms of its topology and branch support values. Moreover, wASTRAL-h is more accurate than concatenation performed using unpartitioned ML (CA-ML) in our simulations except when there is a large number of inaccurate gene trees or low levels of discordance, where CA-ML is slightly more accurate. Most interestingly, wASTRAL-h is more congruent than the unweighted ASTRAL with CA-ML on real datasets.
Result
Weighted ASTRAL Algorithm
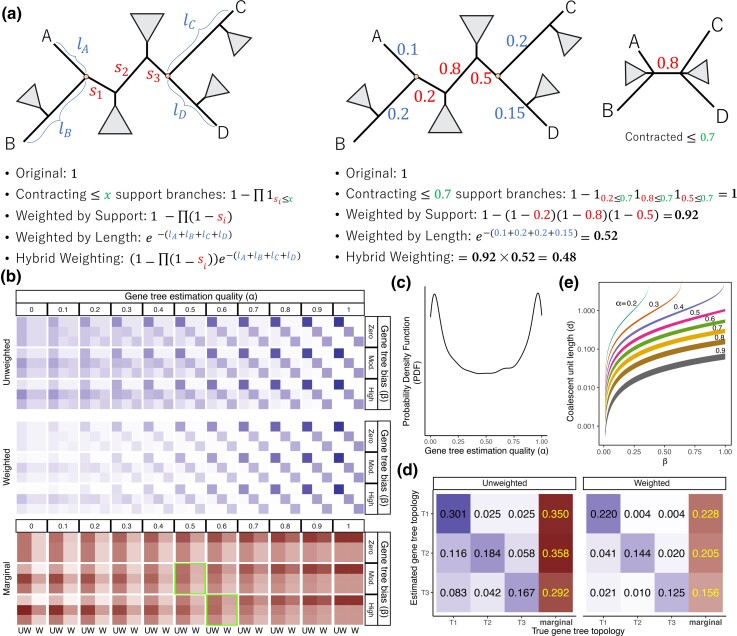
Unlike unweighted ASTRAL, where each (resolved) quartet in each gene tree contributes equally to the objective function, weighted ASTRAL assigns each quartet with a weight based on the support or lengths of branches corresponding to it. More specifically, we define three weighting schemes (fig. 1a).
Fig. 1.
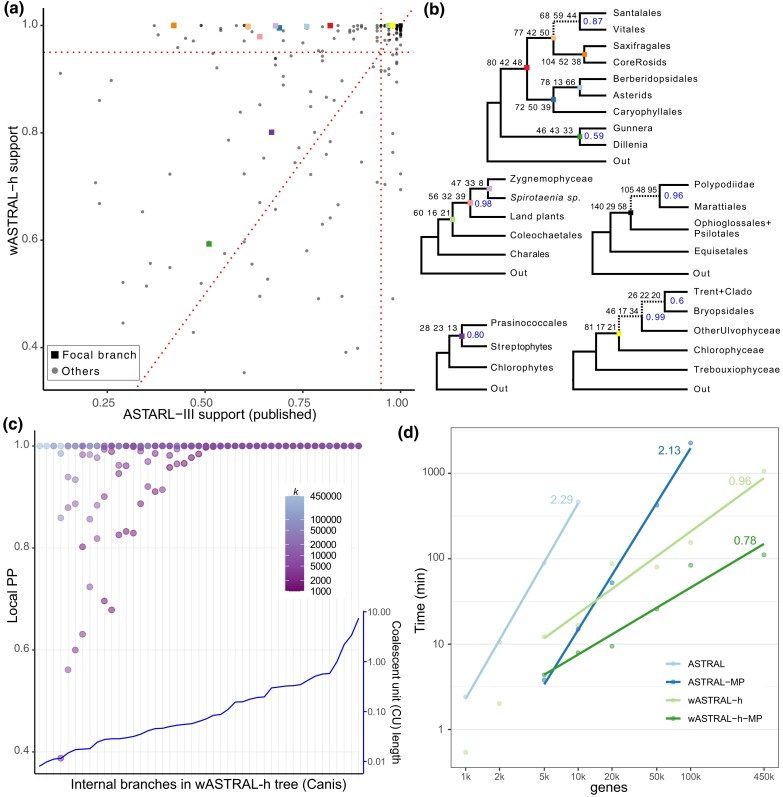
Weighted ASTRAL (wASTRAL) method. (a) Illustration of weighting methods. The generic formula and an example of weighting gene tree quartet . Trees are annotated with the support () of branches between anchors (hollow dots) and the substitution per site length of each leaf-to-anchor path (). (b–e) Impact of weighting under the MSC+Error+Support model for a quartet species tree. (b) Each element of each grid in the top and middle panels corresponds to a true (by column) and an estimated (by row) quartet gene tree topology. Diagonal elements correspond to no gene tree error. The first row/column represents the species tree topology. The second row/column corresponds to the topology toward which gene tree estimation is biased. The gene tree estimation quality ranges in . Gene tree estimation bias is set to zero, moderate (0.4), or high (0.6). Internal branch length is in coalescent units (CU). The color shades on the top panel show the joint probability of a true/estimated topology combination, which corresponds to the expected quartet scores in unweighted ASTRAL; the colors in the middle panel show the expected scores in wASTRAL-s. The row marginals of each grid are shown in the bottom panel: Each grid shows the expected score of each topology (rows) for unweighted ASTRAL (UW column) and weighted ASTRAL (W column). Note the reduced darkness of W columns as decreases, showing that poor gene trees contribute less. Two highlighted girds: the score is highest for the wrong (second row) topology without weights but is higher for the correct topology (first row) with weights. (c,d) In a toy example, we draw from the distribution (c) and show the joint () and marginal probabilities of topologies with and without weighting with moderate bias () and CU length. (e) For eight values of and every value of , the band shows the range of CU quartet internal branch lengths where unweighted ASTRAL is not consistent, but wASTRAL-s is.
Weighting by support extends the definition of branch support to a quartet. Let be the set of branches on the path between internal nodes of a quartet tree (also called anchors; see fig. 1a) and let denote the support of a branch . We define the support of the quartet as
which essentially assumes support values are probabilities of correctness and that branches are independent (both assumptions can be disputed). Given a set of gene trees where each internal branch has a support value, using this definition, we define the weight of each quartet of each gene tree to be its support. The goal is to improve the accuracy by down-weighting quartets with low support. Although we study this goal in our simulation and empirical analyses, we also provide theoretical results.
Making theoretical statements about estimated gene trees is difficult because we lack an accepted way of modeling gene tree estimation errors. To be able to interrogate theoretical properties of weighted ASTRAL, we propose a simple model of gene tree estimation error called MSC+Error (“Material and Methods”). In this model, for any true gene tree topology on a quartet , the estimated topology is drawn from a distribution that has two features: first, each gene has a gene-specific level of the signal, controlled by a parameter , and second, all genes can be adversarially biased toward any topology by an amount bounded by a parameter called . The joint distribution of true and estimated quartet gene trees in the most difficult case can be expressed as a function of and as well as , where is the coalescent unit (CU) length of the internal branch of the quartet (table 1 and fig. 1b). Under the MSC+Error model, the distribution of quartet gene tree topologies, written as a vector with the first element corresponding to the species tree, changes (in the worst case) from
Table 1.
Joint probabilities (δ) and weights (w) of estimated and true gene tree topologies under the MSC+Error+Support with the worst-case scenario when , , and for all genes; parameters are per quartet and per gene but we omit and superscript for brevity.
The estimated gene tree distribution matches the MSC model when and is uniformly random when . A choice of adds noise to the MSC probabilities, and any creates an adversarial bias toward the second topology (fig. 1b). Note that noise and bias parameters can change across genes and quartets, creating flexibility. However, quartets are considered independent.
Under the MSC+Error model, the unweighted ASTRAL is statistically consistent with estimated gene trees under limited choices of and . Assuming that the support of a quartet matches the estimated gene tree distribution, we can get our main result. Theorem 1 in “Material and Methods” proves that support-weighted ASTRAL (wASTRAL-s) is statistically consistent under a strictly larger super-set of and parameters than those of unweighted ASTRAL. Thus, there are levels of bias in gene tree estimation (e.g., due to long branch attraction) that, combined with low signal, render unweighted ASTRAL inconsistent (as shown by Roch et al. 2019) but keep wASTRAL-s consistent.
Examining the marginal probabilities and expected weights can illuminate the reason behind the advantage of wASTRAL-s (fig. 1b). First, gene trees with higher levels of noise (i.e., lower ) are down-weighted relative to gene trees with less noise (fig. 1b: note lighter colors as decreases). Thus, the correct topology benefits from summing weights over gene trees with different . For example, assume some genes have high noise, and others have low noise following the distribution shown in figure 1c. The less noisy genes will be up-weighted such that wASTRAL-s becomes consistent even when unweighted ASTRAL is not (fig. 1d). Second, unless gene trees are extremely noisy (i.e., very low ), wASTRAL-s down-weights the species tree topology less than the other two topologies; in extreme cases, we have scenarios (fig. 1b, bottom, highlighted boxes) where the species tree is dominant with weighted scores but not with unweighted scores. In fact, for fixed and , there exists a range of CU quartet internal branch lengths for which unweighted ASTRAL is not consistent but wASTRAL-s is (fig. 1e); note that for branch lengths below this range, neither method is consistent and for branch lengths above this range, both methods are consistent.
Weighting by length down-weights quartets with long terminal branches. Let be the sum of terminal branch lengths in the gene tree induced to a quartet provided in substitution-per-site units (SU). We assign as the weight of the quartet and offer two justifications. First, deeper coalescence events tend to generate longer terminal branch lengths; thus, gene trees that match the species tree are expected, on average, to have shorter branch lengths (see proof of Theorem 2). Thus, down-weighting gene tree quartets with long terminal branches is expected to down-weight genes that do not match the species tree. Doing so can provably provide a bigger gap between the score of the true species tree and alternatives, as shown in Theorem 2. Besides the connection to the MSC model, it has also been long appreciated that the so-called long quartets are harder to estimate correctly due to long branch attraction (Erdos et al. 1999; Snir et al. 2008). Many quartet-based methods focus their attention on the so-called short quartets (Warnow et al. 2001; Nelesen et al. 2012). Our weighting scheme seeks the same impact by down-weighting long quartets versus short quartets (fig. 1a).
Hybrid weighting combines both weighting schemes where each quartet is assigned with weight
This weighting scheme aims to combine the strengths of both weighting by support and weighting by length and to improve over both; we will empirically show that such improvements are obtained.
Although defining weighting schemes is easy, designing scalable algorithms to optimize the weighted quartet score is not. Adopting the existing ASTRAL-III algorithm to incorporate per-quartet weights is challenging for reasons elaborated in “Material and Methods.” A major contribution of this paper is designing a set of algorithms (supplementary Algorithm S1–S3, Supplementary Material online) to optimize the weighted quartet using a set of new techniques paired with a dynamic programming (DP) step similar to ASTRAL. We leave the detailed description of the algorithm to the “Optimization algorithm” section; see Theorems 3, 4, and 6 for correctness and Theorem 5 for the asymptotic running time being where is the average gene tree height.
Simulation Results
Comparison of Weighting Schemes
We start by comparing the accuracy of weighting schemes and branch support types on two simulated datasets (S100 and S200). Our default method for computing branch support, used unless otherwise specified, is approximate Bayesian supports from IQ-TREE (aBayes) normalized to range from 0 to 1.
S100
This dataset adopted from Zhang et al. (2018) has gene trees inferred from sequences with varying lengths resulting in various levels of gene tree error (see “Datasets”). In most cases, weighting by support (wASTRAL-s) produces species trees with higher accuracy than weighting by length (wASTRAL-bl), and the improvements are statistically significant (supplementary fig. S1, Supplementary Material online); according to a repeated-measure ANOVA test (see “Statistical Tests”). The improvement in accuracy varies with () and perhaps sequence length (). The accuracy of hybrid weighting (wASTRAL-h) on average is better than the accuracy of wASTRAL-s on all model conditions () and the improvement in accuracy may depend on () and sequence length (). With genes, wASTRAL-h is better than both support and length, showing that combining the two weightings makes wASTRAL-h more powerful.
On this dataset, bootstrap support computed using FastTree-2 is provided by Zhang et al. (2018). Thus, we also compute wASTRAL trees using bootstrap supports (wASTRAL-s* and wASTRAL-h*). For weighting by support, aBayes weighting is much better than bootstrap weighting (), but the gap in error significantly ( for both) shrinks as and sequence length increase (supplementary fig. S1, Supplementary Material online). For hybrid weighting, aBayes weighting is, on average, only slightly better than bootstrap weighting (the mean error increases across all conditions by only 0.2%).
S200
This 200-taxon dataset has species trees sampled under two birth rates (), which control whether speciations are dispersed at random or closer to the tips (supplementary fig. S3, Supplementary Material online), and tree heights, which control levels of ILS (see “Datasets”). On this dataset, bootstrapped gene trees are not available; instead, local SH-like support (Shimodaira and Hasegawa 1999) defined by FastTree-2 is available, which we use (wASTRAL-s* and wASTRAL-h*). Patterns of accuracy across wASTRAL versions are similar to S100 (supplementary fig. S4, Supplementary Material online) as wASTRAL-h is more accurate than wASTRAL-s on all model conditions (), and the improvements depend on (), ILS level (), and birth rate (). Using SH-like support with wASTRAL-h is, on average, worse than aBayes support, increasing the error by 9%.
Comparison of Topological Accuracy to Other Methods
We next compare wASTRAL-h, the most accurate version of wASTRAL, with other methods.
Impact of Gene Tree Estimation Error (S100 dataset)
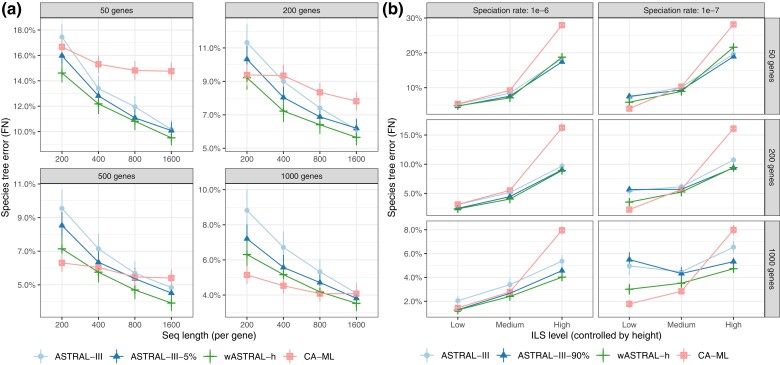
On the S100 dataset (fig. 2a and supplementary fig. S5, Supplementary Material online), wASTRAL-h is more robust to gene tree estimation error than ASTRAL-III, regardless of whether low bootstrap support (BS) branches (5%) are contracted. Whereas contracting low support branches improves the accuracy of ASTRAL-III, weighting improves accuracy even more in most conditions. For example, the average error with 1,000 gene trees obtained from 200 base-pair (bp) alignments goes down from 9% with ASTRAL-III to 7% after contracting 5% BS branches and 6% with wASTRAL-h. Although wASTRAL-h is better than ASTRAL-III in all conditions with or without contraction (), the difference in accuracy varies across sequence lengths ( without contraction and with contraction). Similar to wASTRAL-h, wASTRAL-h* has mean error lower than that of ASTRAL-III-5% in every condition ().
Fig. 2.
Species tree topological error on simulated datasets, measured using false negative (FN) rate. We compare weighted ASTRAL hybrid (wASTRAL-h) with ASTRAL-III using fully resolved and contracted gene trees and concatenation using ML (CA-ML). (a) Results on the S100 dataset with gene trees (boxes) and gene sequence length (-axis). Gene trees and CA-ML are both inferred using FastTree-2. ASTRAL-III-5% contracts branches with <5% BS. (b) Results on the S200 dataset with , rates of speciation and , and three ILS levels. Gene trees and CA-ML are both inferred using FastTree-2. ASTRAL-III-90% contracts branches with aBayes support <90%. Note the change in -axis scale across panels and refer to supplementary fig. S2, Supplementary Material online for a version where -axis is kept fixed. See supplementary figs. S5 and S6, Supplementary Material online for box plots.
The clearest patterns are observed when comparing wASTRAL-h and concatenation (CA-ML). Although increasing the sequence length (and hence reducing the gene tree error) consistently reduces the error of all ASTRAL variants, it has a more subdued impact on CA-ML. As a result, the relative accuracy significantly depends on () and gene sequence length () and the choice of the best method varies across conditions. Generally, wASTRAL-h tends to be more accurate than CA-ML under smaller and greater sequence lengths. With , wASTRAL-h is more accurate than CA-ML for all sequence lengths. With , CA-ML is better for smaller gene alignments, and wASTRAL-h is better for longer alignments, with the only conditions when CA-ML has noticeable improvements over wASTRAL-h corresponding to 200 bp genes.
Impact of ILS Level (S200 dataset)
On the S200 dataset that controls levels of ILS (see “Datasets”), overall, error rates of wASTRAL-h are lower than that of ASTRAL-III (fig. 2b and supplementary fig. S6, Supplementary Material online) and the improvements are significant (). The improvements of wASTRAL-h compared with ASTRAL-III increase with more gene trees () but appear to decrease with more ILS (). Although Mirarab and Warnow (2015) reported no improvement in accuracy when contracting branches with low SH-like support, contracting branches with aBayes support <90% (ASTRAL-III-90%) does improve accuracy in some conditions. Nevertheless, wASTRAL-h has yet lower error () overall. Also, improvements of wASTRAL-h are significantly larger for the birth rates, which tend to have earlier speciations (supplementary fig. S3, Supplementary Material online), than the rate ().
The comparison between wASTRAL-h and CA-ML significantly depends on several factors (birth rate: ; ILS: ; : ). Overall, CA-ML is less robust to ILS levels and is always worse than wASTRAL-h when ILS is high and in several cases when ILS is at the medium level. However, with low ILS, the comparison depends on the birth rate: with (more recent speciation), wASTRAL-h is better than CA-ML () while with (earlier speciation), CA-ML is better (). Thus, in some conditions with low ILS, wASTRAL-h has reduced but not eliminated the gap between ASTRAL-III and CA-ML. For example, given 1,000 gene trees and low ILS with birth rate, ASTRAL-III has 5% error, which is not helped by branch contraction, whereas wASTRAL-h has 3%, which is much closer to the 2% achieved by CA-ML. To summarize, wASTRAL-h retains and magnifies the advantages of ASTRAL-III over CA-ML for high ILS conditions and reduces or eliminates the advantages of CA-ML under medium and low ILS conditions.
Support Accuracy
We next test whether, by accounting for gene tree uncertainty, wASTRAL-h improves support values computed using the local posterior probability (localPP) measure (see “Branch Support”). We examine the calibration of support (i.e., whether the support matches the probability of correctness of a branch), its ability to distinguish correct and incorrect branches examined through ROC curves, and distributions of support (see “Evaluation Criteria”).
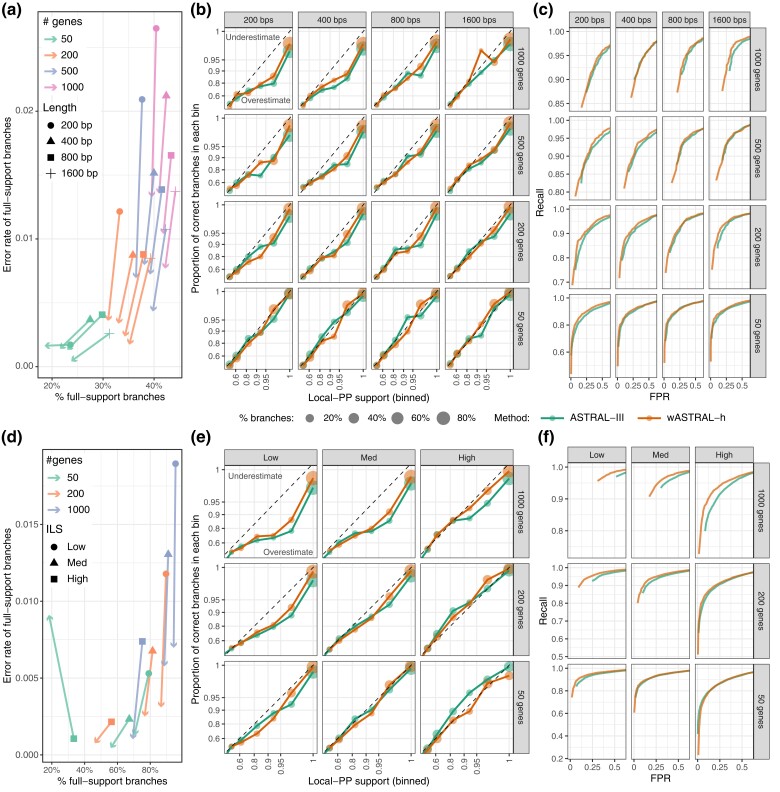
Although wASTRAL-h generally gives higher support values than ASTRAL-III (supplementary fig. S8, Supplementary Material online), it has fewer cases of highly supported incorrect branches, especially with higher and shorter sequences (fig. 3a). For both ASTRAL-III and wASTRAL-h, while increased support often leads to increased frequency of correctness (fig. 3b), support underestimation or overestimation can also be observed for certain sequence length and combinations. For example, wASTRAL-h has a tendency to overestimate for large values and short sequences. In terms of predictive power, for any desired false positive rate (FPR), the recall of wASTRAL-h is as good as or better than ASTRAL-III in all conditions (fig. 3c), though the improvements in ROC can be small. Moreover, in most conditions, the minimum FPR obtained by wASTRAL-h (e.g., at 1.0 support) is lower than the minimum FPR obtained by ASTRAL-III. Support values on the S200 dataset exhibit similar patterns to S100 (fig. 3d–f). The most notable difference is that when , wASTRAL-h has a clear advantage over ASTRAL-III in trading off precision and recall according to ROC curves (fig. 3f and supplementary fig. S10, Supplementary Material online). This advantage shrinks as decreases.
Fig. 3.
Support accuracy across (a–c) S100 dataset with and sequence length and (d–f) S200 dataset with and levels of ILS from low to high. (a,d) Change in 100% support branches. Each line shows the portion of full-support branches that are wrong (-axis) and the percentage of all branches that have full support (-axis) for wASTRAL-h (the arrowhead) and ASTRAL-III (other shapes). Arrows pointing downwards indicate less frequent errors in wASTRAL-h. (b,e) Support calibration. Branches are binned by their support, and for each bin, the percentage of branches that are correct are depicted versus the center of the bin. The dotted lines indicate ideal (calibrated) support. Top (bottom) triangle corresponds to the underestimation (overestimation) of support. (c,f) Receiver operating characteristic (ROC) curves where each dot corresponds to a contraction threshold, (“Evaluation Criteria”). See supplementary figs. S7–S12, Supplementary Material online.
Comparison of the Optimization Algorithms
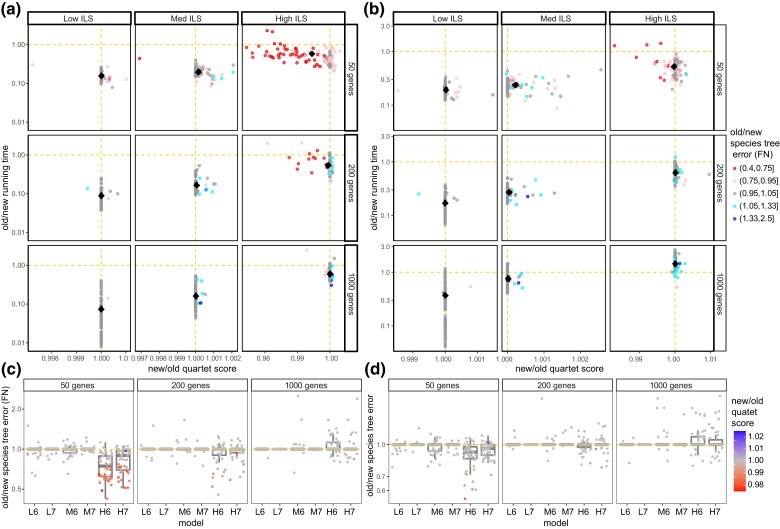
Assigning weights to quartets required a new optimization algorithm, which can also be used for unweighted optimization. We next study whether the new optimization algorithm (denoted as DAC) is as effective as that of ASTRAL-III (denoted as A3) when no weights are used.
Testing on the S200 dataset, without missing data, DAC is in most cases slower than the A3 (fig. 4aandc), a pattern that is pronounced with lower ILS levels. The change in relative running time with ILS levels is due to the dependence of the search space of A3 but not DAC on gene tree discordance levels (Zhang et al. 2018). In terms of accuracy, DAC and A3 are comparable for low and medium ILS levels (fig. 4c). However, in the high ILS case, A3 is clearly better with only 50 genes, slightly better with 200 genes, and perhaps slightly worse with 1,000 genes. Cases with reduced accuracy also have reduced quartet scores for the 50 genes scenario and high ILS (fig. 4a), showing that A3 is preferable with few gene trees. Thus, the improved accuracy of wASTRAL-h over ASTRAL-III is despite the fact that its DAC optimization algorithm is not always as effective as A3.
Fig. 4.
Comparison of the running time, quartet score, and species tree error (measured using FN) between the old DP-based (A3) and the new optimization algorithms (DAC), both run without weighting on the S200 dataset. (a and b) The ratio between the running time and quartet scores before (a) and after (b) randomly removing 5% of taxa from each gene tree; colors denote the ratio of species tree estimation error between the two methods. Note that the upper-right corner and blue color favor DAC. Results are separated by ILS levels from low to high and by . (c and d) The species tree topological error using the A3 algorithm divided by the DAC algorithm before (c) and after (d) randomly removing of 5% taxa from each gene tree with colors denoting the ratio of quartet scores. L6 to H7 indicate model conditions with low, medium, and high ILS with and rates.
These patterns change when we add low levels of missing data by randomly removing 5% of leaves in each gene tree (fig. 4bandd). DAC becomes closer to A3 in terms of running time in most cases and is even faster with high ILS and (fig. 4b). Regarding accuracy, A3 and DAC are comparable in low and medium ILS levels (fig. 4d). However, in the high ILS case, the error of A3 is slightly less, comparable, and slightly higher with 50, 200, and 1,000 genes, respectively. Substantial changes in accuracy are caused by changes in quartet scores (fig. 4b). Thus, DAC is competitive or better than the A3 in the presence of even low levels of missing data found to various degrees in biological datasets.
Biological Data
We next study seven biological datasets (“Datasets”). On the canis dataset, which was the only input with at least 5 h of running time for wASTRAL-h (supplementary table S2, Supplementary Material online), we also examine the running time.
OneKp
Overall, 47 out of 1,175 (4%) branches change between the published ASTRAL-III tree and our wASTRAL-h tree. Most of these branches had low support in the ASTRAL-III tree (mean: 62%, max: 99%) but not in the wASTRAL-h tree (supplementary fig. S13, Supplementary Material online). OneKP Initiative (2019) focused most of their attention on 20 branches, corresponding to nine major evolutionary events that have been historically hard to resolve (e.g., early Eudicot diversification). Among 47 branches that change in wASTRAL-h, four of them are among the 20 focal branches. Beyond topological changes, the support values tend to increase in wASTRAL-h (fig. 5a). In particular, all of the 20 focal branches that had less than full support in the ASTRAL-III tree have increased support in the wASTRAL-h tree, leaving only four with support below 0.95 (as opposed to 12 branches with ASTRAL-III).
Fig. 5.
Results on OneKp (a and b) and canis (c and d) datasets. (a) LocalPP support of all species tree branches shared between wASTRAL-h and the published ASTRAL-III. Focal branches (squares) with support less than 100% in one of the two trees are colored and labeled in panel b. (b) wASTRAL-h resolutions of focal branches that differ from ASTRAL-III in topology or support. Branch labels: total weights of all quartets around each branch for the three possible topologies computed using (7) with weights coming from (5); the species tree topology is shown first. Node labels: localPP support when not equal to 100%. Dashed: focal branches that differ from ASTRAL-III. (c) LocalPP of wASTRAL-h internal branches versus the number of genes for each branch found in the wASTRAL-h output tree with all gene trees as input (-axis). The inset with right -axis scale shows the internal branch lengths in coalescent units on ASTRAL-III tree, sorted from low to high. The leftmost three branches are found only with . (d) Log–log plot of total running time of ASTRAL-III and wASTRAL-h using both a single core (light colors) and 16 cores (dark colors) vs on the canis dataset for ranging from 1,000 to 450,000; slopes of fitted lines, which estimate asymptotic growth exponent, are labeled. All test cases are performed on a server with AMD EPYC 7742 CPUs.
Significantly, all four focal branches that change from ASTRAL-III to wASTRAL-h become consistent with CA-ML, whereas the ASTRAL-III tree was inconsistent with CA-ML. At the base of eudicots, Vitales (grapes) becomes sister to Santalales in wASTRAL-h tree with moderate support (0.87), which is consistent with CA-ML (fig. 5b). Two branches in the so-called TUC clade also change: ASTRAL-III breaks down the class Ulvophyceae by uniting Bryopsidales with Chlorophyceae while wASTRAL-h recovers Ulvophyceae as sister to Chlorophyceae, which is the traditional resolution and is in agreement with CA-ML. Finally, the early diversification of ferns differs between CA-ML and ASTRAL-III but is identical between CA-ML and wASTRAL-h. Thus, wASTRAL-h makes coalescent analyses more congruent with CA-ML for the focal branches.
Canis
On the canis dataset of Gopalakrishnan et al. (2018) that spans a relatively shallow time scale (many branches are among populations of the same species), the majority of branches of the ASTRAL-III tree are shorter than 0.1 CU (fig. 5c). Despite that, due to the large numbers of genes used, both wASTRAL-h and ASTRAL-III produce species trees with at least 99% support on all branches (supplementary fig. S14, Supplementary Material online). The ASTRAL-MP tree (on 100k gene trees) is identical to the published consensus tree, while the wASTRAL-h tree (on 450k gene tees) differs from it in only one branch (i.e., placement of the Egyptian dogs).
The linear running time scaling of wASTRAL-h with respect to enables us to analyze randomly sampled subsets of 1,000–450,000 genes (fig. 5c). The shortest branches need very many genes to achieve universal full support. Using fewer genes (even as many as 100,000) always leaves at least one branch with less than 99% support. Since many of the shortest branches are within species, a tree-like model of evolution is likely insufficient for such branches (Gopalakrishnan et al. 2018). Longer branches, which are mostly across species, do not require large numbers of genes to reach high support; the 21 longest branches have at least 99% support with as few as 1,000 gene trees. Furthermore, wASTRAL-h is more scalable compared with ASTRAL-III with respect to the number of genes (fig. 5d). As Theorem 3 predicts, the running time of wASTRAL-h scales almost linearly with , while ASTRAL-III scales close to quadratically (fig. 5d and supplementary fig. S15, Supplementary Material online). ASTRAL-III fails to finish for within h, and ASTRAL-MP with 16 cores takes more than h for . By contrast, wASTRAL-h finishes on within h and h with one and 16 cores, respectively. Even when , ASTRAL-III takes more than wASTRAL-h due to the high levels of gene tree discordance and abundance of missing data, both of which increase the running time of ASTRAL-III but not wASTRAL-h.
Avian
On the avian dataset, the wASTRAL-h tree fully agrees with the ASTRAL-III trees after contracting low support branches and is very similar to original trees published by Jarvis et al. (2014) based on CA-ML (only five branches differ) and statistical binning (only two branches differ). This is in contrast to the ASTRAL-III tree without contraction from Zhang et al. (2018), which is in conflict with strong results from the literature and other methods. Moreover, all but one branch in the wASTRAL-h tree has higher or equal support compared with ASTRAL-III with any thresholds of contraction (supplementary fig. S16, Supplementary Material online). Interestingly, the only branch that experiences a reduction in support, the placement of Caprimulgimorphae as sister to Telluraves (core land-birds), is a branch that disagrees with both the published CA-ML and statistical binning trees. Finally, four branches with 99–100% support in wASTRAL-h are found by all coalescent-based methods (wASTRAL-h, ASTRAL-III and binned MP-EST) but not CA-ML, possibly pointing to a consistent signal that can be recovered only using coalescent-based analyses.
Cetaceans
The wASTRAL-h tree (supplementary fig. S17, Supplementary Material online) is similar to ASTRAL-multi and CA-ML trees reported by McGowen et al. (2020) with only a few differences (three branches to ASTRAL-multi and four to CA-ML). Interestingly, wASTRAL-h agrees with CA-ML and earlier studies (McGowen et al. 2009) and disagrees with ASTRAL-multi tree on the position of the Lissodelphis with high support (though the placement has low support in the ASTRAL-multi). On the other hand, both wASTRAL-h and ASTRAL-III break the monophyly of the genus Tursiops as T. truncatus moves away from T. aduncus and Stenella with high support. The question of the monophyly of Tursiops, supported by morphology, has been answered differently in two recent analyses and remains likely (Moura et al. 2020) but uncertain due to evidence for gene flow (Guo et al. 2022). Close to Tursiops is also the placement of the two Stenella clymene individuals, which is a known hybrid species evolved from S. longirostris and S. coeruleoalba. Interestingly, the two S. clymene individuals are placed apart, one as sister to S. longirostris and the other at the most recent common ancestor of S. longirostris and S. coeruleoalba. This placement is in contrast to CA-ML, which puts both individuals as sister to S. longirostris. Beyond Delphininae, two branches, the placements of Orcinus orca and Neophocaena phocaenoides, disagree with both ASTRAL-multi and CA-ML, but both branches have very low support in wASTRAL-h and cannot be trusted. These two are among 11 species where McGowen et al. (2009) used data from existing genomes and transcriptomes instead of their own targeted capture, and it is possible that differences in the analytical pipeline may have caused the low support in wASTRAL-h.
Insect Datasets
On all three insect datasets, the differences between wASTRAL-h and ASTRAL-III are minimal and strictly limited to branches with low support. On the Nomiinae dataset, there is no conflict among highly supported branches. wASTRAL-h and ASTRAL-III differ in only one low support branch, and both trees differ from CA-ML in two low support branches (supplementary fig. S18, Supplementary Material online). On the Lepidoptera dataset, only seven out of 200 branches differ between wASTRAL-h and ASTRAL-III, and all of these branches have support below 75% (supplementary fig. S19, Supplementary Material online). Across the tree, wASTRAL-h has slightly more branches with support above 95% than ASTRAL-III (173 vs. 169). On the Papilionidae datasets, wASTRAL-h tree and ASTRAL-III tree share the same topology, and all branches in both trees have high (99%) support (supplementary fig. S20, Supplementary Material online).
Discussion
We introduced a family of new weighting schemes for quartet-based species tree estimation, including weighting quartets by terminal branch length (wASTRAL-bl), internal branch support (wASTRAL-s), or both (wASTRAL-h). We saw that the combined method (wASTRAL-h) has the best accuracy among the three and dominates unweighted ASTRAL in terms of accuracy. We next further comment on more subtle patterns observed in the data and end by pointing out directions for future research.
Further Observations Based on the Results
The choice between CA-ML and summary methods has been a long-standing debate (Giarla and Esselstyn 2015; Leaché et al. 2015; Simmons and Gatesy 2015; Edwards et al. 2016; Meiklejohn et al. 2016). Although CA-ML is inconsistent under MSC (Roch and Steel 2015), the most careful simulation studies have found that the best method depends on the dataset: CA-ML has been more accurate when gene discordance is low and gene signal is limited, and summary methods have been more accurate when discordance is high. Other factors such as deep versus shallow radiations, changes in evolutionary rates across genes, heterotachy, and the number of genes may also matter. Since we cannot reliably predict the superior method in practice, studies often report both types of analyses. We saw that weighting reduced (but did not fully eliminate) the gap between CA-ML and unweighted ASTRAL in conditions with lower ILS or heightened gene tree error (fig. 2). Overall, our results point to wASTRAL-h being a reasonable, if not always optimal, choice regardless of the condition. Consistent with simulations, on real datasets, we observed that wASTRAL-h eliminates many of the differences between unweighted ASTRAL and CA-ML. Thus, using wASTRAL-h can help reduce the long-standing challenge of getting incongruent results from different analyses.
In our simulations, wASTRAL-h matched or improved on ASTRAL-III in all model conditions in terms of accuracy, leaving no clear incentive to prefer ASTRAL-III in this regard. Contracting low support branches improved ASTRAL-III trees, but the weighting is more accurate than contracting and does not require hard-to-tune (Bossert et al. 2021) thresholds. Interestingly, the improvements, which were modest in many conditions but substantial in others, appeared more pronounced as the number of genes increased. We speculate the reason is that with more genes, not only the noise in the frequency of observed quartet topologies reduces, but also, the quartet weights become less noisy. Thus, having more genes benefits all wASTRAL versions in two ways (less topological noise and better weights), only one of which is enjoyed by unweighted ASTRAL.
Although topological improvements of wASTRAL-h over ASTRAL-III were marginal in many cases, the improvements in support were dramatic. The percentage of full support branches that were wrong was reduced in wASTRAL-h by half or more in most conditions (fig. 3aandd), rendering the full support branches more reliable. This increase in precision did not come at the cost of lowering support. Both real and simulated datasets (e.g., supplementary figs. S8 and S11, Supplementary Material online) saw increased support with wASTRAL. Two aspects of how we compute support have changed (“Branch Support”). One is the handling of missing data (see eq. (8)); it can be easily shown that, all else being equal, this change will decrease the localPP. Thus, the increase has to be due to the second change, which is the incorporation of weights. Since localPP support is a function of discordance, the increased support is empirical evidence that down-weighted gene tree quartets tend to be those that are more incongruent with the output species tree.
Branch support used as input by wASTRAL-s and wASTRAL-h can be computed in numerous ways with vastly different computational requirements. One practical question is whether one method should be preferred and, if so, which? We tested three ways of computing support on simulated data and noticed that IQ-TREE’s aBayes has the best accuracy, closely followed by bootstrapping (supplementary fig. S1, Supplementary Material online). In contrast, SH-like support was noticeably less effective. IQ-TREE’s aBayes is a local measure of support (i.e., computed for the nearest neighbor interchanges around a branch), and a local notion of support is consistent with how we interpret branch support (i.e., as independent, leading to a product). Moreover, computing local support is much faster than bootstrapping. Thus, while bootstrapping is a good option in terms of accuracy, IQ-TREE’s aBayes support can be used to build an accurate and efficient pipeline. Nevertheless, note that in the presence of rouge taxa that move widely across a gene tree, local measures of support may provide high support for most branches, whereas global support can result in low support for many branches, effectively down-weighting that gene. In such situations, global support may be more robust.
Limits and Future Work
We examined statistical consistency of the wASTRAL-s optimization problem, when solved exactly, given estimated gene trees under our MSC+Error+Support model. Although this model is general, our assumptions about support values and independence of quartets are strong, and support estimation methods do not necessarily fulfill them (e.g., see debates in Felsenstein and Kishino 1993; Hillis and Bull 1993; Susko 2009). Thus, the proofs of consistency should be taken more as a theoretical justification of the weighting approach rather than a prediction of behavior on real data. Support values that over or underestimate branch supports (compared with our assumptions) may or may not lead to inconsistency of the method, as our assumptions are sufficient but not necessary. Future work can seek more forgiving conditions for support that retain consistency, or conversely, conditions where the method is misleading.
We only studied the statistical consistency of wASTRAL-s and wASTRAL-bl under the MSC and MSC+Error+Support models, respectively, and left the treatment of wASTRAL-h to future work. Even more intriguing is whether any flavor of wASTRAL (which can take multi-individual/multicopy trees as input) is statistically consistent under combined models of GDL and ILS, as ASTRAL-multi is (Hill et al. 2020; Markin and Eulenstein 2021). This question is particularly important for datasets where assumptions of MSC are violated. For example, on the OneKP dataset, examining the relative support for the three topologies around each branch (fig. 5b) reveals that the quartet frequencies do not always follow the MSC expectations (one high frequency and two equal low frequencies). We believe weighting by support will continue to be beneficial for models of GDL. However, it is unclear whether weighting by branch length is profitable when gene tree discordance is due to GDL and especially horizontal transfer; thus, we caution the use of branch length when these processes are suspected. Finally, future work can incorporate weighting in the ASTRAL-Pro (Zhang et al. 2020) algorithm that natively supports paralogy.
The new wASTRAL software can optimize the unweighted quartet score using the new optimization algorithm (DAC) instead of the old algorithm (A3) of ASTRAL-III. In our simulations, DAC tended to be as accurate or more accurate than A3 in the presence of missing data (fig. 4b), but slower and less accurate without missing data. Our simulation results had no missing data, showing that the improved accuracy of wASTRAL-h was due to a better optimization objective, not a better optimization algorithm. Based on our experiments, we recommend that for datasets with substantial missing data, the new wASTRAL software package should be used instead of the ASTRAL-III package even for optimizing the unweighted quartet score. Moreover, similar to A3, DAC is also a heuristic method addressing an NP-hard problem. Just as the speed and accuracy of unweighted ASTRAL changed substantially through tweaks to the heuristics from ASTRAL-I to ASTRAL-III, we anticipate that future work can further increase our accuracy, speed, or both. Unweighted ASTRAL is also finely optimized for CPU, GPU, and vectorization (Yin et al. 2019). Currently, the wASTRAL software is only trivially parallelized for CPU, and future work can further optimize the code and implement GPU parallelization.
Our simulations, like any other, lacked some of the complexities of real biological data (Philippe et al. 2017; Springer and Gatesy 2018). We did not include recombination, horizontal transfer, gene flow, hidden paralogy, alignment error, mistaken homology, violations of the model of sequence evolution, or missing data. It can be hoped that weighting helps alleviate the effects of some of these other sources of error as well. However, since many of these can lead to high support for the wrong trees, there is no guarantee that weighting would not leave these misleading signals intact or even amplified. Methods for simulating many of these effects are available and can be used in future studies to compare wASTRAL with both CA-ML and unweighted ASTRAL. A related promising avenue for future research is exploring other ways of weighting quartets. For example, future work can incorporate homology and alignment quality metrics into the weighting schemes. The weights could also reflect other factors, such as evidence of heterotachy impacting gene trees (Braun et al. 2019) and deviations from stationarity (Jeffroy et al. 2006). Even more ambitious approaches could be imagined where biases in support estimation could be predicted using machine learning (Suvorov et al. 2020). In designing and testing such weighting schemes, one must remember that not every weighting method will allow fast optimization using DP.
Finally, several features of ASTRAL-III are missing from wASTRAL, but future work can address this limitation. Currently, wASTRAL-h does not output branch lengths since the natural branch lengths that it could compute would be in a hard to interpret unit (e.g., ). Future work can examine ways to compute branch lengths in substitution or coalescent units. Other missing features left to future work are the test of polytomy (Sayyari and Mirarab 2018), integration with visualization tools such as DiscoVista (Sayyari et al. 2018), and completion of gene trees with respect to each other. Nevertheless, the most valuable features of ASTRAL-III, including handling multi-individual datasets, handling polytomies, and outputting branch support, are all supported.
Material and Methods
Common Notations and Background
Let be a set of species. Let us suppose that we are given a set of input binary gene trees with . For each tree , let its leaf set be and its edge set be . For each branch , we let note its length. For a species set , let denotes restricted to . We refer to a set of four species as a quartet and define as the set of all quartets in . We define when and has topology ; otherwise we define . For nodes and of a gene tree , we let denote the set of branches on the path between and and let . For a quartet , we denote , for and being nodes of corresponding to the internal nodes (called the anchors) of ; that is, in case that has topology , anchors are the only node on and on .
We assume each true gene tree is generated from the true species tree under the MSC model. Branch lengths of are in coalescent units (CUs). For each quartet with topology in the species tree, let where is the CU length of the internal branch of the quartet. Under MSC, for each true gene tree , the following holds (Degnan 2013): and . The input set is a set of estimated gene trees, not true gene trees. In practice, these gene trees are estimated from sequence data using methods such as ML with branch lengths given in the substitution-per-site units (SU). Moreover, input gene trees are furnished with support values: maps each edge of to a support value in .
Theoretical Results: Improved Consistency and Sample Complexity
For a given species tree topology , we define its score against gene tree set as
| (1) |
where is a function mapping a quartet of to a number. In unweighted ASTRAL, for any ,
| (2) |
In this paper, we introduce three new ways of defining . Weighting by support sets:
| (3) |
Weighting by branch length uses
| (4) |
Finally, the hybrid weighting scheme combines weighting by support and weighting by length and uses
| (5) |
We study hybrid weighting only empirically but provide theoretical justifications for weighting by support (for estimated gene tree topologies) and weighting by length (for true gene tree topologies).
Weighting by Support
Genes have varying levels of signal, and hence gene tree estimation error, and estimated gene trees can also be biased toward a specific topology due to factors such as long branch attraction. When bias goes against the species tree topology, unweighted ASTRAL can be positively misleading (Roch et al. 2019). It is reasonable to assume that gene trees with lower signals have lower support regardless of bias. By down-weighting those genes, wASTRAL-s can rescue consistency. To formalize this intuition, we introduce a model of gene tree error that allows us to make a more formal statement, showing that wASTRAL-s is consistent under some conditions where unweighted ASTRAL is not.
MSC+Error+Support Model
We assume each input estimated gene tree is a draw from a distribution that depends on the true gene tree . For each quartet and each gene , let denote a parameter controlling the quality of the estimated quartet gene tree . We assume is independently drawn from the topology of and we let the expected value and variance of across genes be denoted by and . For each true gene tree topology, with probability , we simply set the estimated gene tree to the true topology. With probability , we choose among the three topologies with probabilities . When these numbers are equal, there is no bias in gene tree estimation, and unweighted ASTRAL remains consistent (easy to prove). However, in our model, we allow systematic bias toward any topology. Let be the maximum bias toward or away any topology across genes. Under this model, the joint probability of true and estimated gene trees would follow the inequalities laid out in table 2. For example, in the worst case, where , , and , the joint distribution of true and estimated gene trees is given in table 1 and depicted in figure 1b.
Table 2.
Joint probabilities (δ) and weights (w) of estimated and true gene tree topologies under the MSC+Error+Support will follow the inequalities shown here. We omit and superscript for brevity.
We assume that for each quartet, the quartet support defined using (3) matches the probability of that topology being observed given the true gene tree. Thus, with our model for estimated gene tree distributions, the support of the quartet topology is if it matches the true tree and if it does not, leading to expected topology weights given in tables 1 and 2.
We now state our main results. Proofs of all results are given in Appendix Proofs.
Proposition 1.
For each estimated gene tree , and .
For consistency of unweighted ASTRAL and wASTRAL-s, we need and , respectively. Figure 1e depicts the RHS of equations of Proposition 1, solving for and setting to zero (which is the worst-case for wASTRAL-s). It shows that wASTRAL-h is consistent for a larger set of species tree CU branch lengths, even in absence of any variation in gene tree quality. We next state this observation formally.
Theorem 1.
Given estimated gene trees furnished with support generated under MSC+Error+Support model, there exist conditions where (3) guarantee a statistically consistent estimator of but (2) does not, and the reverse is not true.
Weighting by length
Our next result shows that using the length-based weighting function (4) leads to a larger gap than unweighted ASTRAL between the expected score of the true species tree and the alternative trees and thus has better sample complexity. Shekhar et al. (2017) has established that the number of gene trees required by unweighted ASTRAL to recover the species tree scales with as where is the CU length of the shortest species tree branch. Following that paper, we focus on the regime with gene trees and show a constant factor improvement in sample complexity. All theoretical results in this section assume that an input gene tree matches the true gene tree in topology.
The improved sample complexity essentially follows from the fact that under the MSC model, gene trees that match the species tree have shorter CU terminal branch lengths on average because discordance is caused by deep coalescence. However, a theoretical difficulty is that input gene trees have SU branch lengths instead of CU length. Thus, we need a model to translate CU lengths in to SU lengths in , capturing the effects of change in mutation rates and population sizes. We examine two such models.
Naive Model
We start with a simple choice akin to a strict clock. Under this naive model, all branches of are scaled from branches of using a fixed multiplier .
Variable Rate Model
Let branches of the species tree be broken into segments of arbitrary length (supplementary fig. S21, Supplementary Material online). For each gene tree , a species tree in SU units is drawn from a fixed distribution (which does not change with ). matches in topology. The length of each segment in is scaled from the length of its corresponding segment in using a multiplier . The set of all multipliers can be jointly drawn from any distribution as long as for each segment , . Segments in can be used to divide into segments defined at the same points along each branch (supplementary fig. S21, Supplementary Material online). The gene tree is obtained from by multiplying the CU length of each of its segments by the multiplier assigned to that segment in . Because segments have different multipliers (even though they have the same expectation), gene tree deviates from ultrametricity. Because multipliers are drawn separately for each gene, deviations from ultrametricity happen in different ways across different genes.
We now state the results. Let and . Then,
Proposition 2.
For a true quartet species tree with topology and input gene trees generated under the naive model with any multiplier , let be the distance between anchors of . As , given gene trees, we have and
Similarly, under the variable rates model and assuming limited variance of rates across genes, we prove
Proposition 3.
For a true quartet species tree with topology and input gene trees generated under the variable rate model, let be the distance between anchors of and be the total length of all other branches. Assume that for every branch segment , the variance of its multiplier is bounded above: where . As , given gene trees, we have and
These propositions lead us to the main result.
Theorem 2.
Under the conditions of Proposition 2 or Proposition 3,
Optimization Algorithm
The objective of our optimization task is to find maximizing given in (1) for one of the functions (2)–(5). For a species tree , let denote the set of tripartitions corresponding to the internal nodes of . For a tripartition corresponding to an internal node in and a gene tree , let be the total score of all shared quartets of and that anchor at . Then,
and .
ASTRAL-III uses a traversal of gene trees to compute with weight function (2) without enumerating all quartets. At each gene tree node, the total number of shared quartets between that node and is computed using simple combinatorics. When quartets are weighted differently using weight functions (3)–(5), computing the aggregated weights of quartets around a node becomes more difficult as simple combinatorial equations become unavailable in the general case. Thus, we cannot simply use the same algorithm as ASTRAL-III and instead propose a new algorithm. In its simplest form (called the base version), the algorithm works as follows.
Starting from an empty tree, add each species to the tree one-by-one with a random order to obtain a full tree (see the “Placement Algorithm” section and supplementary Algorithm S1, Supplementary Material online). The algorithm also computes and stores tripartition scores for all tripartitions of the output tree.
Repeat the previous step for rounds; by default (see details under “Placement Algorithm”).
Combine results of the rounds using a final DP step, which reuses the tripartition weights computed in step 1; each internal node of the output is constrained to be in at least one of the greedy trees (see “Dynamic Programming” section and supplementary Algorithm S2, Supplementary Material online).
What makes this approach possible is step 1: a new algorithm that allows each addition to an existing tree to be performed optimally and efficiently. Importantly, while the base algorithm is a greedy heuristic, as Theorem 4 and the remark afterward will show, it retains the statistical consistency properties proved in Theorems 1 and 2. The running time of the base algorithm scales with in the worst case (Proposition 4) and is better with respect to but worse with respect to compared with ASTRAL-III, which is in the worst case and roughly in practice. Thus, we also propose a divide-and-conquer (DAC) algorithm for that uses the base algorithm on subsets of size (see the “DAC Algorithm” section and supplementary Algorithm S3, Supplementary Material online). This strategy improves the running time to under some assumptions, as detailed below under Theorem 5. The DAC algorithm also retains the statistical consistency guarantees (Theorem 6). We next detail each algorithmic component mentioned above.
Placement Algorithm
Mai and Mirarab (2022) use the idea pioneered by Brodal et al. (2013) to design a quasi-linear algorithm to find the optimal placement of a species on a backbone tree that minimizes its quartet distance to a set of reference trees (e.g., gene trees). This algorithm traverses a binary (or multifurcating) species tree in a top-down manner and colors species using three (or more) colors, , , and . When entering any node of the species tree, all species under are already colored and all other species are colored . At this point, the smaller child of is recolored with . The recoloring is done one species at a time; for each species, the path from the associated leaf in each gene tree to the root is visited, and several counters assigned to each gene tree node are updated. These counters enable calculating the score for placing the query on each species tree branch. After this recoloring is done and before moving from to any of its children , the sister of is colored , and if is the smaller child of , then is changed back to . This algorithm performs only species recoloring steps due to the smaller-child trick, which recolors the larger child of each node less often than the smaller child. Moreover, by representing each gene tree using an -height tree called HDT adopted from Brodal et al. (2013), it ensures each recoloring takes time.
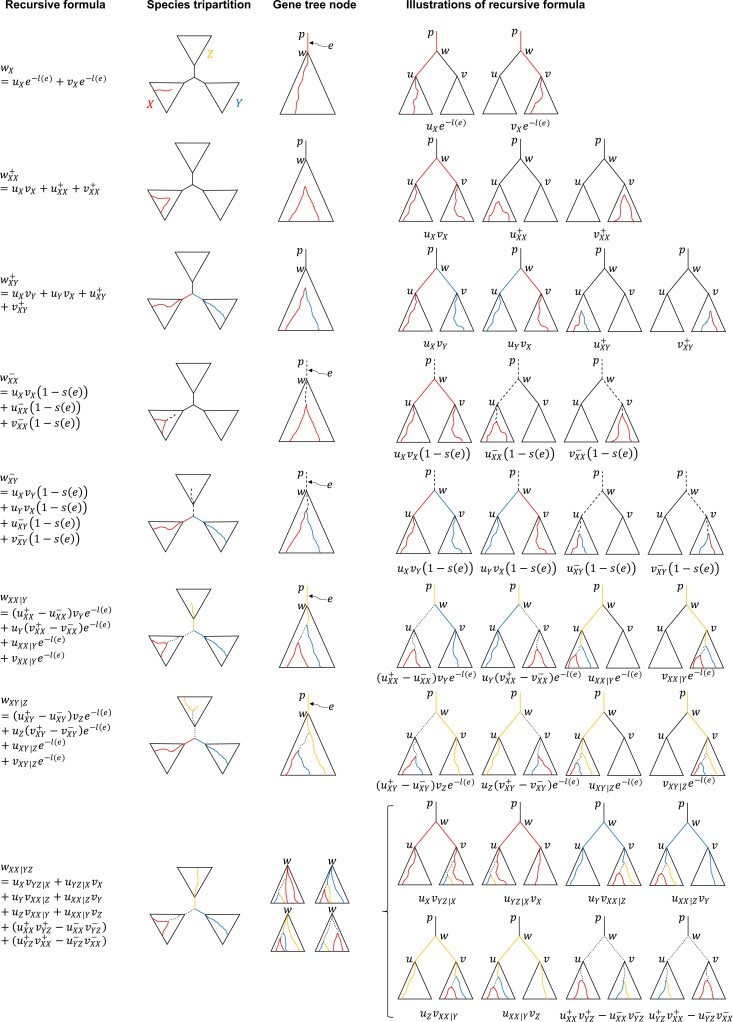
We build on the idea by Mai and Mirarab (2022) and adapt it to optimally solve the weighted quartet score placement problem (supplementary Algorithm S1, Supplementary Material online), changing it in three substantial ways. (1) We have created a new set of counters that enable us to compute the total weighted quartet score of all tripartitions resulting from all possible placements of the query. These counters essentially count the total weight of all the quartets with the same most recent common ancestor (MRCA) using recursive equations shown in figure 6 and supplementary table S1, Supplementary Material online. The derivation of these counters is the heart of the algorithm but is too complex to detail here. We leave a full description to Proof of Theorem 3. (2) At each node , we also recolor the query species as , , and and recompute the counters; this allows us to compute the quartet score for all tripartitions resulting from all placements of the query. (3) Since our counters are more complex than Mai and Mirarab (2022), we use input gene trees instead of HDTs, which would be hard to implement. As a result, the cost of a leaf recoloring in our algorithm is where is the average height of gene trees instead of had we used HDTs. Note that for sufficiently balanced gene trees, and are similar. We next prove that this algorithm finds the optimal solution.
Fig. 6.
Recursive definitions of Counters. For a species tree tripartition (), and a gene tree node , we compute the total hybrid weight of all quartets anchored at the species tripartition and with as the MRCA on the gene tree. Each solid colored path is weighed by the negative exponent of its length; each dashed path is weighted by one minus its support; each dotted path is weighted by its support. See also supplementary table S1, Supplementary Material online.
Theorem 3.
Let be a species tree, be a species not in , be the set of possible species tree topologies by placing onto , and be the output of Algorithm S1 online. Then, .
Although each individual placement is optimal, the greedy search is not guaranteed to find the optimal tree. We run the greedy search times each of which produces a full tree . Empirically, we found to have minimal impact on the accuracy, but small improvements in the quartet score are observed for up to rounds in outlier cases (supplementary fig. S22, Supplementary Material online). Based on these results, we set (which the user can adjust) using a dynamic heuristic: (1) start with rounds and perform the DP algorithm to get an optimal score; (2) run another rounds and perform DP using bipartitions from all previous rounds; (3) repeat step (2) until no improvement to the optimal score is obtained or step (2) has been repeated five times.
Dynamic Programming
In each greedy search, we add the tripartitions of each and their weights to a lookup table . The DP step computes an optimal species tree restricted to the tripartitions of (supplementary Algorithm S2, Supplementary Material online). The DP algorithm proceeds almost identically to ASTRAL-III, with one difference: Although the search space in ASTRAL-III is the set of bipartitions found in all of the trees, here, the search space is the set of all tripartitions. With this change, we do not need to compute weight scores for any tripartition as those are precomputed and stored in in the placement step.
Proposition 4.
The time complexity of Algorithm S2 online is .
Since for balanced trees and for caterpillar trees, the time complexity of supplementary Algorithm S2, Supplementary Material online is when gene trees are roughly balanced and when they are not. Note that because the counters are linearly related to counters of children of a node, in theory, the HDT structure can be adopted in our algorithm leading to a worst-case complexity. Since adopting HDT would add much more complexity for (potentially) little gain, we do not pursue it further.
Supplementary Algorithm S2, Supplementary Material online is not guaranteed to find the optimal solution. However, a positive theoretical result ensures that this lack of optimality does not impede the statistical consistency of the solution
Theorem 4.
If there exists a species tree topology satisfying that for each quartet subtree ,
(6) then the output of Algorithm S2 online will be .
Remark 1.
For a binary true species tree , as , satisfies the condition of Theorem 4 with an arbitrarily high probability for wASTRAL-s under the assumptions of Theorem 1 and for wASTRAL-bl under the assumptions of Theorem 2. To see this point, note that due to the consistency of the estimator, for a quartet to achieve a high probability a certain must be sufficient. Setting and using a union bound, it is easy to see that is enough to achieve the probability of correctness for all quartets. Thus, by Theorem 4, Algorithm S2 online is a statistically consistent estimator of the species tree under the assumptions of Theorems 1 and 2. We conjecture that wASTRAL-h can also be proved statistically consistent under assumptions similar to Theorem 1 for topology and support and Theorem 2 for branch length.
DAC Algorithm
The DAC procedure (supplementary Algorithm S3, Supplementary Material online) first computes a backbone tree on fewer species, adds all the remaining species onto the backbone tree, and then locally refines the topology around the backbone branch.
To compute a backbone tree , we randomly select leaves and apply the Algorithm S2 online with rounds of placements to get a backbone tree with species.
For the remaining species, we independently find their optimal placement on using the Algorithm S1 online. We group species placed on the same branch together to obtain clusters.
For each cluster corresponding to a branch , we sequentially place species in onto using the Algorithm S1 online and remove any “orphan” species that are not placed on or its derived branches; the result is called .
All trees in induce the same scaffold tree on their shared taxa. Thus, they can be easily merged into a uniquely defined tree .
If orphan species exist, at the end, we place them onto using the Algorithm S2 online.
The potential for orphan taxa makes it harder to establish the time complexity of the DAC algorithm theoretically, but a result can be proved.
Theorem 5.
When the inequality condition in Theorem 4 is satisfied, then the time complexity of the DAC algorithm is with arbitrarily high probability.
Similar to the base algorithm, the DAC algorithm retains statistical consistency.
Theorem 6.
Under the conditions of Theorem 4, the DAC Algorithm S3 online will output .
Remark 2.
Under assumptions of Theorem 1 for wASTRAL-s and Theorem 2 for wASTRAL-bl, Algorithm S3 online gives a statistically consistent estimator of the species trees.
Branch Support
We adopt the quartet-based metric introduced by Sayyari and Mirarab (2016) used for measuring branch support. This metric essentially quantifies the probability of the true quartet score around a species tree branch being more than given the observed quartet topologies assuming that gene trees are fully independent, but the quartets around the branch are fully dependent. The original metric gives all gene trees with at least one quartet around a branch of interest an equal weight of one. In wASTRAL-h, we instead weight each gene tree by the total support of all three topologies and normalize the counts. Removing an internal branch of the species tree and its four adjacent branches defines a quadripartition of species , and we assume is the bipartition defined by . Note that any quartet has the same internal branch as . Let denote the subset of gene trees with at least one element from each of , , , and . We define , the normalized quartet count for branch , as
| (7) |
The quartet counts for and are similarly defined and are denoted by and . This form of normalization models the observation that gene trees with higher weights also have higher variance in their weights. Using the method of Sayyari and Mirarab (2016), we set the localPP support to , where , is the beta function, is the regularized incomplete beta function, and is birth rate in the Yule prior distribution (default: ).
When all weights are set to 1, as in ASTRAL-III, the new definition is identical to the original one in the absence of missing data but can be different with missing data. Let be the number of quartets around the branch of interest present in a gene tree ; let be the number of those quartets that are compatible with . Then, the old definition sets , while the new definitions uses
| (8) |
The two definitions are identical only when all values are the same, which is the case when there is no missing data but can also happen in other scenarios. When patterns of missing data are different, the old calculations made sure all genes had equal weights (each gene has ). In the new definition, since each gene is weighted differently in wASTRAL, to begin with, we also allow genes to have a different total vote depending on their patterns of missing data. In the new formula, each gene votes (contributes to ) proportionally to the number of quartets they have around a branch.
Datasets
Simulated Data
S100 Simulated dataset by Zhang et al. (2018), includes 100 ingroups and one outgroup and is simulated using SimPhy (Mallo et al. 2016) with 50 replicates. The species trees are simulated under the birth-only process with birth rate , a fixed haploid population size of , and the number of generations sampled from a log-normal distribution with mean . true gene trees are simulated under the MSC model. The ILS level substantially varies across replicates, with a mean of 0.46 when measured by the average normalized Robinson and Foulds (1981) (RF) distance between the true species trees and true gene trees. Gene alignments of length bps are simulated using Indelible (Fletcher and Yang 2009) under the GTR model after assigning SU gene tree branch lengths that deviate from the clock using rate multipliers. Gene trees are reconstructed under the GTR+ model using FastTree-2 (Price et al. 2010). The gene tree estimation error, measured by the FN rate between the true gene trees and the estimated gene trees, is for lengths . The original publication has made BS obtained from replicates run using FastTree-2 available for each gene tree.
S200 Simulated dataset by Mirarab and Warnow (2015) includes 200 ingroup species and an outgroup. Its species trees are generated under two different birth rates each with 50 replicates and three different ILS levels, low (10%), medium (35%), and high (70%), controlled by max tree heights , , generations, respectively. The sequence length of each gene is uniformly drawn between 300 and 1500 bps, resulting in a wide range of gene tree estimation errors across replicates (mean: 25%, 31%, and 47%, for low, medium, and high ILS). Gene trees are estimated using FastTree-2, but because bootstrap replicates are not available, we compute aBayes support using IQ-TREE with fixed topologies.
By default, we compute branch length and support using IQ-TREE (v 1.6.12) aBayes option (--abayes) under GTR+ model. As each support value is between and , we normalize support value to so that the minimum is . To run wASTRAL (which currently takes only binary trees as input), we randomly resolve polytomies in input trees with length and support set to , which is equivalent to a polytomy for wASTRAL-s and -h.
ASTRAL-III version 5.7.4 is used throughout. ASTRAL-III-5% (S100 dataset) denotes running ASTRAL-III on gene trees with low BS branches (<5%) contracted. The 5% threshold is used because Zhang et al. (2018) found it to have the best accuracy overall. On the S200 dataset, because BS is not available, we instead rely on IQ-TREE aBayes support, which tends to be much higher than BS. Thus, we contract branches with support below a 0.90 threshold with aBayes, denoted as ASTRAL-III-90%.
CA-ML is performed using unpartitioned ML. On the S200 dataset, CA-ML was available from the original study (where they used FastTree-2 as the ML method) and is used here. On S100, we ran CA-ML using FastTree-2. Thus, on both datasets, the same tool is used for gene tree estimation and CA-ML, ensuring the comparisons are fair.
Biological Datasets
Seven biological datasets were used.
OneKP (OneKP Initiative 2019) dataset includes 1,178 species spanning the plant tree of life obtained using transcriptomics. The original study has inferred 410 gene trees from amino acid alignments of putative single-copy genes using RAxML with BS (which we use), an ASTRAL-III species tree, and CA-ML using RAxML.
Canis (Gopalakrishnan et al. 2018) dataset includes 48 genomes across genus Canis with taxon sampling that allows reconstruction at both species and population levels. Loci with roughly 10 kbp lengths were selected across the genome at random, leading to 449,450 gene trees. Since ASTRAL-II could not handle this size, the original study partitioned the gene tree into 100 subsets and inferred one ASTRAL-II species tree per subset and published a consensus of those trees. We used wASTRAL-h to analyze all the available gene trees, which the original paper estimated using FastTree-2; we were also able to analyze up to 100,000 gene trees using ASTRAL-MP (Yin et al. 2019) (within 48 h). Due to the large number of genes, we simply use the provided SH-like FastTree-2 support instead of re-estimating support.
Avian (Jarvis et al. 2014) dataset includes 48 species designed to resolve the order-level avian relationships, which experience extremely high levels of gene tree discordance potentially due to a rapid radiation. The authors studied three data types: concatenation of exons per gene (exons), concatenation of introns per gene (introns), and ultraconserved elements (UCEs). Here, we analyze all 14,446 input gene trees (8,251 exons, 2,516 introns, and 3,679 UCEs) with bootstrap-annotated branches available from the original study. The main challenge on this dataset is the low gene tree resolution, which led to the development of the statistical binning method (Mirarab, Bayzid, et al. 2014). Without binning, the analyses of all 14,446 loci resulted in species trees that were clearly wrong. More recently, species tree inferred from ASTRAL-III without dealing with gene tree error also resulted in incorrect species trees (Zhang et al. 2018); however, contracting low support branches (e.g., 3, 5, and 10%) appeared to solve the problem.
Cetaceans (McGowen et al. 2020) dataset includes targeted-captured exonic data for 100 individuals from 77 cetacean species and 12 outgroups. The original study estimated gene trees using RAxML under the GTRCAT model but without support for 3,191 protein-coding genes. We computed Bayesian local supports and branch lengths for fixed gene tree topologies using IQ-TREE and reanalyzed the dataset using wASTRAL-h. We compare the results with two trees produced by the original study: a CA-ML tree and an ASTRAL-multi tree that forces individuals of the same species to be grouped together.
Insect Datasets
We also tested three insect datasets, in each case, using available gene trees. (1) a 32-taxon collection of 853 RAxML gene trees with BSs obtained from alignments of ultraconserved elements focused on the bee subfamily Nomiinae and particularly genus Pseudapis (Bossert et al. 2021), (2) a 203-taxon set of 1,930 RAxML gene trees with BS obtained from transcriptomic alignments focused on Lepidoptera (butterflies and moths) (Kawahara et al. 2019), and (3) a 61-taxon dataset of the Papilionidae (swallowtail butterflies) with 6,407 IQ-TREE gene trees with supports that we computed using aBayes (Allio et al. 2020) and obtained from amino-acid alignments of orthologous protein-coding genes.
Evaluation Criteria
To compare topological accuracy, we use the FN rate, which is the fraction of bipartitions of the true species tree recovered by an estimated tree. Since the true species tree and the reconstructed species tree are both binary, false-negative rate, false-positive rate, and normalized RF are all the same. For measuring the accuracy of support, we use three methods with different goals.
Calibration plots ask if support values perfectly indicate correctness (i.e., are calibrated). We break support values into these bins: , , , , , and (note that 1 means anything rounded to 1 by the tool). For each bin, we compute the average accuracy of branches with support in that range and plot it versus the midpoint of the boundaries of that bin. On such plots, points above (below) diagonal indicate underestimation (overestimation) of branch support. Even when support is not calibrated, it can be useful if higher support correlates with correctness; for example, if all support values are uniformly increased or decreased (say, divided by two), it can still be perfectly correlated with support. To measure this aspect, we use ROC curves. For a large number of thresholds between 0 and 1, we contract all branches with support below that threshold. Then, ROC depicts recall, which is the faction of correct branches that are kept, versus FPR, which is the fraction of incorrect branches that are kept. Note that the ROC curve remains the same with any monotonic transformation of support values assuming an infinite number of thresholds. We also plot the empirical cumulative density function (ECDF) of correct and incorrect branches. We expect higher support for correct branches than for incorrect branches; thus, the accuracy can be judged by the gap between ECDF curves of correct and incorrect branches.
Statistical Tests
We perform repeated measures ANOVA tests between two species tree reconstruction methods to test the significance of topological accuracy differences and whether the gap in accuracy depends on simulation model parameters. We limit the data to only the two methods being compared, and for each experimental condition, we use replicates as the repeated measures (i.e., the error term). We perform double-sided ANOVA tests on reconstruction methods vs. experimental conditions and report -values for the difference between methods and the impact of other variables on that difference.
Supplementary Material
Acknowledgment
This work was supported by National Science Foundation (NSF) grant III-1845967 and National Institute of health (NIH) grant R35GM142725. Computations were performed on the San Diego Supercomputer Center (SDSC) through XSEDE allocations, which is supported by the NSF grant ACI-1053575.
Contributor Information
Chao Zhang, Bioinformatics and Systems Biology, UC San Diego, La Jolla, CA, USA.
Siavash Mirarab, Department of Electrical and Computer Engineering, UC San Diego, La Jolla, CA, USA.
Supplementary Material
Supplementary data are available at Molecular Biology and Evolution online.
Data Availability
The wASTRAL software is available publicly at https://github.com/chaoszhang/ASTER. Data used here are available at https://github.com/chaoszhang/Weighted-ASTRAL.data.
References
- Alanjary M, Steinke K, Ziemert N. 2019. AutoMLST: an automated web server for generating multi-locus species trees highlighting natural product potential. Nucleic Acids Res. 47(W1):W276–W282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allio R, Scornavacca C, Nabholz B, Clamens A-L, Sperling FA, Condamine FL. 2020. Whole genome shotgun phylogenomics resolves the pattern and timing of swallowtail butterfly evolution. Syst Biol. 69(1):38–60. [DOI] [PubMed] [Google Scholar]
- Avni E, Cohen R, Snir S. 2015. Weighted quartets phylogenetics. Syst Biol. 64(2):233–242. [DOI] [PubMed] [Google Scholar]
- Bayzid MS, Mirarab S, Boussau B, Warnow T. 2015. Weighted statistical binning: enabling statistically consistent genome-scale phylogenetic analyses. PLoS ONE 10(6):e0129183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayzid MS, Warnow T. 2013. Naive binning improves phylogenomic analyses. Bioinformatics 29(18):2277–2284. [DOI] [PubMed] [Google Scholar]
- Bossert S, Murray EA, Pauly A, Chernyshov K, Brady SG, Danforth BN. 2021. Gene tree estimation error with ultraconserved elements: an empirical study on pseudapis bees. Syst Biol. 70(4):803–821. [DOI] [PubMed] [Google Scholar]
- Braun EL, Cracraft J, Houde P. 2019. Resolving the avian tree of life from top to bottom: the promise and potential boundaries of the phylogenomic era. In: Avian genomics in ecology and evolution. Cham: Springer International Publishing. p. 151–210.
- Brodal GS, Fagerberg R, Mailund T, Pedersen CNS, Sand A. 2013. Efficient algorithms for computing the triplet and quartet distance between trees of arbitrary degree. In: Proceedings of the Twenty-Fourth Annual ACM-SIAM Symposium on Discrete Algorithms. Philadelphia, PA: Society for Industrial and Applied Mathematics. p. 1814–1832.
- Davidson R, Vachaspati P, Mirarab S, Warnow T. 2015. Phylogenomic species tree estimation in the presence of incomplete lineage sorting and horizontal gene transfer. BMC Genom. 16(Suppl 10):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio M, Degnan JH. 2014. Robustness to divergence time underestimation when inferring species trees from estimated gene trees. Syst Biol. 63(1):66–82. [DOI] [PubMed] [Google Scholar]
- Degnan JH. 2013. Anomalous unrooted gene trees. Syst Biol. 62(4):574–590. [DOI] [PubMed] [Google Scholar]
- Degnan JH, Rosenberg NA. 2009. Gene tree discordance, phylogenetic inference and the multispecies coalescent. Trends Ecol Evol. 24(6):332–340. [DOI] [PubMed] [Google Scholar]
- Edwards SV. 2009. Is a new and general theory of molecular systematics emerging? Evolution 63(1):1–19. [DOI] [PubMed] [Google Scholar]
- Edwards SV, Xi Z, Janke A, Faircloth BC, McCormack JE, Glenn TC, Zhong B, Wu S, Lemmon EM, Lemmon AR, et al. . 2016. Implementing and testing the multispecies coalescent model: a valuable paradigm for phylogenomics. Mol Phylogenet Evol. 94:447–462. [DOI] [PubMed] [Google Scholar]
- Elworth RAL, Ogilvie HA, Zhu J, Nakhleh L. 2019. Advances in computational methods for phylogenetic networks in the presence of hybridization. p. 317–360.
- Erdos P, Steel M, Szekely L, Warnow T. 1999. A few logs suffice to build (almost) all trees: part II. Theor Comput Sci. 221(1–2):77–118. [Google Scholar]
- Felsenstein J, Kishino H. 1993. Is there something wrong with the bootstrap on phylogenies? A reply to Hillis and Bull. Syst Biol. 42(2):193–200. [Google Scholar]
- Fletcher W, Yang Z. 2009. INDELible: a flexible simulator of biological sequence evolution. Mol Biol Evol. 26(8):1879–1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatesy J, Sloan DB, Warren JM, Baker RH, Simmons MP, Springer MS. 2019. Partitioned coalescence support reveals biases in species-tree methods and detects gene trees that determine phylogenomic conflicts. Mol Phylogenet Evol. 139:106539. [DOI] [PubMed] [Google Scholar]
- Giarla TC, Esselstyn JA. 2015. The challenges of resolving a rapid, recent radiation: empirical and simulated phylogenomics of Philippine shrews. Syst Biol. 64(5):727–740. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan S, Sinding MHS, Ramos-Madrigal J, Niemann J, Samaniego Castruita JA, Vieira FG, Carøe Christian, de Manuel Montero Marc, Kuderna Lukas, Serres Aitor, et al. . 2018. Interspecific gene flow shaped the evolution of the genus Canis. Curr Biol. 28(21):3441–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo W, Sun D, Cao Y, Xiao L, Huang X, Ren W, Xu S, Yang G. 2022. Extensive interspecific gene flow shaped complex evolutionary history and underestimated species diversity in rapidly radiated dolphins. J Mamm Evol. 29(2):353–367. [Google Scholar]
- Hill M, Legried B, Roch S. 2020. Species tree estimation under joint modeling of coalescence and duplication: sample complexity of quartet methods.
- Hillis DM, Bull JJ. 1993. An empirical test of bootstrapping as a method for assessing confidence in phylogenetic analysis. Syst Biol. 42(2):182–192. [Google Scholar]
- Huang H, Knowles LL. 2016. Unforeseen consequences of excluding missing data from next-generation sequences: simulation study of RAD sequences. Syst Biol. 65(3):357–365. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Mirarab S, Aberer AJ, Li B, Houde P, Li C, Ho SYW, Faircloth BC, Nabholz B, Howard JT, et al. . 2014. Whole-genome analyses resolve early branches in the tree of life of modern birds. Science 346(6215):1320–1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffroy O, Brinkmann H, Delsuc F, Philippe H. 2006. Phylogenomics: the beginning of incongruence? Trends Genet. 22(4):225–231. [DOI] [PubMed] [Google Scholar]
- Kawahara AY, Plotkin D, Espeland M, Meusemann K, Toussaint EFA, Donath A, Gimnich F, Frandsen PB, Zwick A, dos Reis M, et al. . 2019. Phylogenomics reveals the evolutionary timing and pattern of butterflies and moths. Proc Natl Acad Sci U S A. 116(45):22657–22663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles LL, Lanier HC, Klimov PB, He Q. 2012. Full modeling versus summarizing gene-tree uncertainty: method choice and species-tree accuracy. Mol Phylogenet Evol. 65(2):501–509. [DOI] [PubMed] [Google Scholar]
- Lanier HC, Knowles LL. 2015. Applying species-tree analyses to deep phylogenetic histories: challenges and potential suggested from a survey of empirical phylogenetic studies. Mol Phylogenet Evol. 83:191–199. [DOI] [PubMed] [Google Scholar]
- Leaché AD, Banbury BL, Felsenstein J, de Oca A-M, Stamatakis A. 2015. Short tree, long tree, right tree, wrong tree: new acquisition bias corrections for inferring SNP phylogenies. Syst Biol. 64(6):1032–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaché AD, Rannala B. 2011. The accuracy of species tree estimation under simulation: a comparison of methods. Syst Biol. 60(2):126–137. [DOI] [PubMed] [Google Scholar]
- Legried B, Molloy EK, Warnow T, Roch S. 2021. Polynomial-time statistical estimation of species trees under gene duplication and loss. J Comput Biol. 28(5):452–468. [DOI] [PubMed] [Google Scholar]
- Liu L, Yu L. 2011. Estimating species trees from unrooted gene trees. Syst Biol. 60(5):661–667. [DOI] [PubMed] [Google Scholar]
- Liu L, Yu L, Edwards SV. 2010. A maximum pseudo-likelihood approach for estimating species trees under the coalescent model. BMC Evol Biol. 10(1):302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Yu L, Pearl DK, Edwards SV. 2009. Estimating species phylogenies using coalescence times among sequences. Syst Biol. 58(5):468–477. [DOI] [PubMed] [Google Scholar]
- Maddison WP. 1997. Gene trees in species trees. Syst Biol. 46(3):523–536. [Google Scholar]
- Mai U, Mirarab S. 2022. Completing gene trees without species trees in sub-quadratic time. Bioinformatics 38(6):1532–1541. [DOI] [PubMed] [Google Scholar]
- Mallo D, De Oliveira Martins L, Posada D. 2016. SimPhy: phylogenomic simulation of gene, locus, and species trees. Syst Biol. 65(2):334–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin A, Eulenstein O. 2021. Quartet-based inference is statistically consistent under the unified duplication-loss-coalescence model. Bioinformatics, 37:4064–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowen MR, Spaulding M, Gatesy J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Mol Phylogenet Evol. 53(3):891–906. [DOI] [PubMed] [Google Scholar]
- McGowen MR, Tsagkogeorga G, Álvarez-Carretero S, dos Reis M, Struebig M, Deaville R, Jepson PD, Jarman S, Polanowski A, Morin PA, et al. . 2020. Phylogenomic resolution of the cetacean tree of life using target sequence capture. Syst Biol. 69(3):479–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiklejohn KA, Faircloth BC, Glenn TC, Kimball RT, Braun EL. 2016. Analysis of a rapid evolutionary radiation using ultraconserved elements: evidence for a bias in some multispecies coalescent methods. Syst Biol. 65(4):612–627. [DOI] [PubMed] [Google Scholar]
- Mirarab S. 2019. Species Tree Estimation Using ASTRAL: Practical Considerations. Arxiv preprint, 1904.03826.
- Mirarab S, Bayzid MS, Boussau B, Warnow T. 2014. Statistical binning enables an accurate coalescent-based estimation of the avian tree. Science 346(6215):1250463–1250463. [DOI] [PubMed] [Google Scholar]
- Mirarab S, Bayzid MS, Warnow T. 2016. Evaluating summary methods for multilocus species tree estimation in the presence of incomplete lineage sorting. Syst Biol. 65(3):366–380. [DOI] [PubMed] [Google Scholar]
- Mirarab S, Nakhleh L, Warnow T. 2021. Multispecies coalescent: theory and applications in phylogenetics. Annu Rev Ecol Evol Syst. 52(1):247–268. [Google Scholar]
- Mirarab S, Reaz R, Bayzid MdS, Zimmermann T, Swenson MS, Warnow T. 2014. ASTRAL: genome-scale coalescent-based species tree estimation. Bioinformatics 30(17):i541–i548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirarab S, Warnow T. 2015. ASTRAL-II: coalescent-based species tree estimation with many hundreds of taxa and thousands of genes. Bioinformatics 31(12):i44–i52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molloy EK, Warnow T. 2018. To include or not to include: the impact of gene filtering on species tree estimation methods. Syst Biol. 67(2):285–303. [DOI] [PubMed] [Google Scholar]
- Mossel E, Roch S. 2010. Incomplete lineage sorting: consistent phylogeny estimation from multiple loci. IEEE/ACM Trans Comput Biol Bioinform. 7(1):166–171. [DOI] [PubMed] [Google Scholar]
- Moura AE, Shreves K, Pilot M, Andrews KR, Moore DM, Kishida T, Möller L, Natoli A, Gaspari S, McGowen M, et al. . 2020. Phylogenomics of the genus Tursiops and closely related Delphininae reveals extensive reticulation among lineages and provides inference about eco-evolutionary drivers. Mol Phylogenet Evol. 146:106756. [DOI] [PubMed] [Google Scholar]
- Nelesen S, Liu K, Wang L-S, Linder CR, Warnow T. 2012. DACTAL: divide-and-conquer trees (almost) without alignments. Bioinformatics 28(12):i274–i282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogilvie HA, Bouckaert RR, Drummond AJ. 2017. StarBEAST2 brings faster species tree inference and accurate estimates of substitution rates. Mol Biol Evol. 34(8):2101–2114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OneKP Initiative OTPT. 2019. One thousand plant transcriptomes and the phylogenomics of green plants. Nature 574(7780):679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S. 2013. Error in phylogenetic estimation for bushes in the tree of life. J Phylogenetics Evol Biol. 01(02):110. [Google Scholar]
- Philippe H, de Vienne DM, Ranwez V, Roure B, Baurain D, Delsuc F. 2017. Pitfalls in supermatrix phylogenomics. Eur J Taxon. 283:1–25. [Google Scholar]
- Price MN, Dehal PS, Arkin AP. 2010. FastTree-2: approximately maximum-likelihood trees for large alignments. PLoS ONE 5(3):e9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy S, Kimball RT, Pandey A, Hosner PA, Braun MJ, Hackett SJ, Han K-L, Harshman J, Huddleston CJ, Kingston S, et al. . 2017. Why do phylogenomic data sets yield conflicting trees? Data type influences the avian tree of life more than taxon sampling. Syst Biol. 66(5):857–879. [DOI] [PubMed] [Google Scholar]
- Richards A, Kubatko L. 2021. Bayesian-weighted triplet and quartet methods for species tree inference. Bull Math Biol. 83(9):93. [DOI] [PubMed] [Google Scholar]
- Robinson D, Foulds L. 1981. Comparison of phylogenetic trees. Math Biosci. 53(1-2):131–147. [Google Scholar]
- Roch S, Nute M, Warnow T. 2019. Long-branch attraction in species tree estimation: inconsistency of partitioned likelihood and topology-based summary methods. Syst Biol. 68(2):281–297. [DOI] [PubMed] [Google Scholar]
- Roch S, Steel M. 2015. Likelihood-based tree reconstruction on a concatenation of aligned sequence data sets can be statistically inconsistent. Theor Popul Biol. 100:56–62. [DOI] [PubMed] [Google Scholar]
- Sayyari E, Mirarab S. 2016. Fast coalescent-based computation of local branch support from quartet frequencies. Mol Biol Evol. 33(7):1654–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyari E, Mirarab S. 2018. Testing for polytomies in phylogenetic species trees using quartet frequencies. Genes. 9(3):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayyari E, Whitfield JB, Mirarab S. 2018. DiscoVista: interpretable visualizations of gene tree discordance. Mol Phylogenet Evol. 122:110–115. [DOI] [PubMed] [Google Scholar]
- Seo T-K. 2008. Calculating bootstrap probabilities of phylogeny using multilocus sequence data. Mol Biol Evol. 25(5):960–971. [DOI] [PubMed] [Google Scholar]
- Shekhar S, Roch S, Mirarab S. 2017. Species tree estimation using ASTRAL: how many genes are enough? IEEE/ACM Trans Comput Biol Bioinform. 15(5):1738–1747. [DOI] [PubMed] [Google Scholar]
- Shen X-X, Hittinger CT, Rokas A. 2017. Contentious relationships in phylogenomic studies can be driven by a handful of genes. Nat Ecol Evol. 1(5):0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimodaira H, Hasegawa M. 1999. Multiple comparisons of log-likelihoods with applications to phylogenetic inference. Mol Biol Evol. 16:1114–1116. [Google Scholar]
- Simmons MP, Gatesy J. 2015. Coalescence vs. concatenation: sophisticated analyses vs. first principles applied to rooting the angiosperms. Mol Phylogenet Evol. 91:98–122. [DOI] [PubMed] [Google Scholar]
- Smith ML, Hahn MW. 2021. New approaches for inferring phylogenies in the presence of paralogs. Trends Genet. 37(2):174–187. [DOI] [PubMed] [Google Scholar]
- Smith SA, Moore MJ, Brown JW, Yang Y. 2015. Analysis of phylogenomic datasets reveals conflict, concordance, and gene duplications with examples from animals and plants. BMC Evol Biol. 15(1):150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snir S, Warnow T, Rao S. 2008. Short quartet puzzling: a new quartet-based phylogeny reconstruction algorithm. J Comput Biol. 15(1):91–103. [DOI] [PubMed] [Google Scholar]
- Solís-Lemus C, Yang M, Ané C. 2016. Inconsistency of species tree methods under gene flow. Syst Biol. 65(5):843–851. [DOI] [PubMed] [Google Scholar]
- Springer MS, Gatesy J. 2016. The gene tree delusion. Mol Phylogenet Evol. 94(Part A):1–33. [DOI] [PubMed] [Google Scholar]
- Springer MS, Gatesy J. 2018. On the importance of homology in the age of phylogenomics. Syst Biodivers. 16(3):210–228. [Google Scholar]
- Susko E. 2009. Bootstrap support is not first-order correct. Syst Biol. 58(2):211–223. [DOI] [PubMed] [Google Scholar]
- Suvorov A, Hochuli J, Schrider DR. 2020. Accurate inference of tree topologies from multiple sequence alignments using deep learning. Syst Biol. 69(2):221–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szöllõsi GJ, Tannier E, Daubin V, Boussau B. 2014. The inference of gene trees with species trees. Syst Biol. 64(1):e42–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahata N. 1989. Gene genealogy in three related populations: consistency probability between gene and population trees. Genetics. 122(4):957–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vachaspati P, Warnow T. 2015. ASTRID: accurate species trees from internode distances. BMC Genom. 16(Suppl 10):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker JF, Brown JW, Smith SA. 2018. Analyzing contentious relationships and outlier genes in phylogenomics. Syst Biol. 67(5):916–924. [DOI] [PubMed] [Google Scholar]
- Wang LG, Lam TTY, Xu S, Dai Z, Zhou L, Feng T, Guo P, Dunn CW, Jones BR, Bradley T, et al. . 2020. Treeio: an R package for phylogenetic tree input and output with richly annotated and associated data. Mol Biol Evol. 37(2):599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warnow T, Moret BME, John KS. 2001. Absolute convergence: true trees from short sequences. In: Proceedings of the Annual ACM-SIAM Symposium on Discrete Algorithms.
- Yan Z, Smith ML, Du P, Hahn MW, Nakhleh L. 2021. Species tree inference methods intended to deal with incomplete lineage sorting are robust to the presence of paralogs. Syst Biol. 71(2):367–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin J, Zhang C, Mirarab S. 2019. ASTRAL-MP: scaling ASTRAL to very large datasets using randomization and parallelization. Bioinformatics 35(20):3961–3969. [DOI] [PubMed] [Google Scholar]
- Yourdkhani S, Rhodes JA. 2020. Inferring metric trees from weighted quartets via an intertaxon distance. Bull Math Biol. 82(7):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Rabiee M, Sayyari E, Mirarab S. 2018. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinform. 19(S6):153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Scornavacca C, Molloy EK, Mirarab S. 2020. ASTRAL-Pro: quartet-based species-tree inference despite paralogy. Mol Biol Evol. 37(11):3292–3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The wASTRAL software is available publicly at https://github.com/chaoszhang/ASTER. Data used here are available at https://github.com/chaoszhang/Weighted-ASTRAL.data.