Abstract
Alzheimer’s disease and related dementias is the collective term for a progressive neurodegenerative disease for which there is presently no cure. This paper focuses on two symptoms of the disease, sleep disturbances and depression, and discusses how light can be used as a non-pharmacological intervention to mitigate their negative effects. Bright days and dark nights are needed for health and well-being, but the present components of the built environment, especially those places where older adults spend most of their days, are too dimly illuminated during the day and too bright at night. To be effective light needs to be correctly specified, implemented, and measured. Yet without the appropriate specification and measurement of the stimulus, researchers will not be able to successfully demonstrate positive results in the field, nor will lighting designers and specifiers have the confidence to implement lighting solutions for promoting better sleep and mood in this population.
Keywords: Alzheimer’s disease and related dementias, circadian rhythms, pharmacological and non-pharmacological therapies, lighting interventions, sleep, depression
1. Background
Alzheimer’s disease (AD) is the most common form of dementia, which is neurodegenerative syndrome initially characterized by memory loss and cognitive impairment. Dementia is a progressive, degenerative disease of the brain and the strongest risk factor is age. By 2030, it is estimated that about 73 million older persons will live in the U.S. 1 Furthermore, the older population is living longer; persons reaching age 65 have an average life expectancy of an additional 19.5 years.2 There is no known cure for the disease, and there are very few effective treatments. Almost 6 million Americans have Alzheimer’s disease and related dementias (ADRD), and it is projected that the number will more than double by 2050.3 More than 7 out of 10 people with ADRD live at home and are provided unpaid care by over 16 million family members, friends, or neighbours.3 In global terms, the World Health Organization estimates that there 50 million persons living with dementia (PLWD) worldwide, with the addition of nearly 10 million new diagnoses annually, 4 and cites ADRD as the seventh leading cause of death in 2019. 5
As the disease progresses, the early short-term memory symptoms are followed by the impairment of language skills, visual-spatial orientation (e.g., navigation, maintaining balance), and abstract thought, as well as various behavioural and psychological symptoms (depression, sleep disturbances psychosis, aggression, etc), culminating in the widespread destruction of brain neurons and their connections, extensive brain damage, and death. 6 Currently, because there is no cure for AD/ADRD, therapies are generally targeted to the mitigation of symptoms associated with the disease. This paper focuses on two symptoms, sleep disturbances and depression, and discusses how light can be used as a non-pharmacological intervention to mitigate the negative effects of sleep disturbances and depression in this population.
1.1. Circadian rhythms and the sleep/wake cycle
The world rotates around its axis and as a result, all creatures on earth experience 24-hour cycles of light and dark. Living organisms have adapted to this daily rotation of the earth by developing biological rhythms that repeat at an approximate 24-hour interval. These are called circadian rhythms (Latin: circa, about; dies, a day). It is widely known that, in mammals, circadian rhythms are regulated by an internal biological clock located in the suprachiasmatic nuclei (SCN)7 of the hypothalamus in the brain.8 The SCN in humans have a natural period that is slightly greater than 24 hours and external cues reset and synchronize the SCN daily, ensuring that the body’s behavioural and physiological rhythms are in synchrony with the daily rhythms in its environment. Light–dark cycles are the main synchronizer of the SCN to the solar day and set the timing of our biological clock so that we “do the right things at the right times” to ensure survival.
The sleep/wake cycle is a circadian rhythm that, in entrained individuals, is partially regulated by the biological clock. The regulation of sleep and waking has been broadly conceptualized as a two-process model involving interaction between a homeostatic process, which maintains stability by counteracting opposing forces or influences, and a process driven by the biological clock. 9, 10 The homeostatic process, known variously as Process S or simply “sleep debt,” is a natural tendency toward sleep that accumulates during our waking hours and declines while we sleep. This tendency is counteracted to maintain daytime wakefulness by an alerting signal that is regulated by the circadian system, called Process C. Experiments using animals whose circadian rhythms have been disrupted by surgical lesions on the SCN have shown that Process S is not affected, suggesting that the two processes are independently regulated despite whatever “crosstalk” might occur between them. 11
1.2. Lighting characteristics affecting the circadian system
There are five important characteristics of light for both the human visual and circadian systems: quantity, spectrum, timing, duration, and distribution. Lighting characteristics for vision are, however, quite different from those that are most effective for the circadian system.12, 13 In brief, while the threshold for activating the circadian system is not yet firmly established, the quantity of polychromatic white light necessary to activate the circadian system (measured through melatonin suppression or phase shift) is significantly greater than the amount that activates the visual system. The spectral sensitivity of the circadian system peaks at short wavelengths,14, 15 while the visual system is most sensitive to the middle wavelength portion of the visible spectrum.
Operation of the visual system responds well to light stimulus at any time of the day or night. However, depending on the timing of light exposure, light can phase advance or phase delay the biological clock.16 In addition, while the visual system responds to a light stimulus very quickly (< 1 second), the duration of light exposure needed to affect the circadian system can take much longer. For example, to achieve measurable melatonin suppression from exposure to a moderate amount of light in young adults, the duration of light exposure needed is at least 5–10 minutes.17, 18 For the visual system, it is well known that spatial light distribution is critical (e.g., when reading black letters on white paper), while the exact impact of spatial distribution on the circadian system is still under investigation. In fact, research has shown that light reaching the lower retina is more effective in suppressing melatonin than light reaching the upper retina, 19 while other studies have shown that it is the nasal part that is more effective. 20, 21
One’s short-term history of light exposure also affects the circadian system’s sensitivity to light. For example, the higher the exposure to outdoor light during the day (e.g., 4 hours per day for one week), the lower the sensitivity of the circadian system to light at night, as measured by nocturnal melatonin suppression.22 Importantly, accounting for total light exposures during waking hours 23 is necessary for predicting shifts in circadian phase.
2. Current light therapy approaches
2.1. ADRD and sleep disturbances: pharmacological and non-pharmacological therapies
Of the estimated 5.8 million people in the United States living with ADRD 3, at least one-third experience difficulty sleeping 24, 25 and approximately two-thirds of their estimated 18.5 million unpaid caregivers report sleep disturbances themselves. 3, 26, 27 The precipitating factor for institutionalization of those with ADRD is often a disturbed sleep–wake cycle that leads them to remain awake at night, causing stress and fatigue for their families and caregivers. This behaviour continues in nursing home environments, where residents with daytime agitation behaviour also tend to sleep poorly at night and nap during the day 28.
Sleep disturbance is not just a symptom of ADRD.29, 30 Chronic sleep disturbance increases ADRD risk,31 as signs of sleep disturbances may appear before mild cognitive impairment (MCI) or ADRD manifest;32 lower ADRD risk is associated with better sleep quality;33 and treatment of sleep disturbance associated with sleep apnoea delays MCI.34 Other research directly links sleep deficits to ADRD pathology (both accumulation of amyloid beta protein (Aβ, which forms in abnormal clusters between nerve cells in the brain) and development of tauopathy (damage to the protein tau, which is vital for nerve cell health), suggesting a bidirectional relationship between sleep and ADRD pathology. For example, sleep disruption in rodents and drosophila leads to Aβ and tau accumulation in the brain 29, 35–38 and, conversely, increased cortical Aβ leads to fragmented sleep. 35, 36 In patients with ADRD and MCI, as well as in cognitively normal older adults, poor sleep correlates with the severity of Aβ and tau pathology,39–43 and recent studies show selective associations between slow oscillations (SOs) 44 and sleep spindles 45 (which both play a key role in memory consolidation) and ADRD pathology.41, 46 Aβ clearance through the glymphatic system occurs during sleep,38 providing a mechanism linking sleep disturbance and accumulation of brain Aβ. Crucially, sleep is a potentially modifiable factor that can be enhanced to counteract memory decline and the continued progression to ADRD.
Several mechanisms have been postulated for the prevalence of sleep disorders in ADRD, the foremost being a reduction in neuronal receptiveness to environmental cues of light and dark due to the degeneration of the optic nerve and retinal ganglion cells, 47, 48 the loss of functionality of the SCN, 49, 50 and the exacerbation of the preceding two factors by inadequate exposure to light compared to healthy elderly controls. 51, 52 Moreover, changes to the aging eye, such as reduced pupil size (senile miosis) and lens thickening, result in a reduction of retinal illumination by as much as two-thirds in older persons compared to young adults. 53 In terms of reduced exposure in older adults, research has demonstrated that middle-aged adults receive approximately 58 minutes of bright light per day54 while older adults in assisted-living facilities receive bright light for only 35 minutes per day.55 Adults in nursing homes see as little as 2 minutes per day. 52, 56 Given the reduced social activity and more sedentary lifestyle of those with ADRD, those living at home in the community are likely to receive just as little light as those in nursing homes. This exposure amount may now be even more reduced due to the COVID-19 pandemic because older adults are a high-risk population recommended to stay home.
2.1.1. Pharmacological therapy for sleep disturbances in ADRD patients
Although widely used, little is known about the safety and effectiveness of medications for treating chronic sleep disturbances in ADRD patients. The risks of sleep-inducing medications for older people who are cognitively impaired are considerable and include increased risk for falls and fractures, increased confusion and worsened memory, overdosing, and a decline in the ability to care for oneself. Moreover, there are ongoing variable costs for prescriptions, medications and the medical supervision of their administration, and the possible unintended consequences for negative side effects due to combinations with other medications. The treatment benefits of using sleep medications in older individuals with ADRD may not outweigh the potential risks.
2.1.2. Non-pharmacological therapy for sleep disturbances in ADRD patients
Light therapy has shown great promise as a nonpharmacological treatment to help regulate sleep in persons with ADRD. Studies have demonstrated that daytime light exposure can consolidate and increase night-time sleep efficiency, while increasing daytime wakefulness and reducing evening agitation. 57–60 Table 1 summarizes the most prominent studies. As shown in Table 1 and discussed below, the results in the literature are mixed, despite the solid science behind the effect of light on human circadian system.
Table 1.
Selected projects using light therapy to improve sleep/wake patterns and depression in older adults with ADRD. All illuminance values for the interventions, when the plane of measurement was specified by the authors, were obtained on the vertical plane.
| Study | Population | Intervention | Outcomes/conclusions |
|---|---|---|---|
| Van Someren et al. 1997 61 | Older adult inpatients with ADRD in a psychogeriatric ward | All-day bright light (790–2190 lx) while in living rooms for 4 weeks | • Increased interdaily stability (IS); stronger coupling of rhythms to environmental cues • Decreased intradaily variability (IV); indicates less fragmentation of rhythm • No change in relative amplitude (RA) of rhythm |
| Satlin et al. 1992 62 | Older hospitalized adults with AD | Daily evening (19:00–21:00) bright light (1500–2000 lx) for 1 week | • Increase in RA; indicates greater stability • Improvement in IV • No change in IS • Decreased night-time activity and sundowning symptoms |
| Lyketsos et al. 1999 63 | Institutionalized older adults with AD and agitated behaviors | Morning light (10,000 lx) 1 hour per day, 4 weeks | • Increased night-time sleep time • No significant effects on behaviour or mood |
| Ancoli-Israel et al. 2003 64 | Older adults with mixed types of dementia residing in nursing homes | Morning (07:30–11:30) or evening (17:30–19:30) light (2500 lx), 10 days | • Both groups had more consolidated sleep at night • No effects on total sleep time nor wake times |
| Alessi et al. 2005 65 | Nursing home residents with abnormal sleep-wake patterns | Daily (between 08:00 and 20:00) sunlight (>10,000 lx) for 30 minutes, 5 days | • Decreased duration of night-time awakenings • Decreased daytime sleeping • Increased participation in social activities and conversation |
| Dowling et al. 2005 66 | Nursing home residents with AD | Morning (09:30–10:30) vs. afternoon (15:30–16:30) bright light (≥2500 lux), 10 weeks | • No significant differences in actigraphy measures of night-time sleep or daytime wake • Significantly more stable rest-activity acrophase over the treatment period for both experimental groups |
| Sloane et al. 2007 67 | Older adults with ADRD in inpatient and residential care | Morning (07:00–11:00), evening (16:00–20:00), and all-day (07:00–20:00) bright light (2500 lux), 3 weeks | • Night-time sleep increased in the morning and all-day light groups • Morning light advanced phase and evening light delayed phase • Greater improvement among those with more severe dementia |
| Hickman et al. 2007 68 | Older adults with ADRD in long-term care | Morning (07:00–11:00), evening (16:00–20:00), and all-day (07:00–20:00) bright light (2535–2638 lx), 3 weeks | • Results suggest that bright light therapy does not consistently improve depressive symptoms in PLWD • Morning light had the greatest effects on both sexes • After morning light exposure, depressive symptoms were lower for women than for men |
| Riemersma-van der Lek et al. 2008 69 | Older adults in 12 assisted care facilities (87% with ADRD) | All-day (09:00–18:00) bright light (± 1000 lx) and evening oral melatonin (2.5 mg), either alone or in combination, 15 months | • Light alone reduced cognitive deficits and depressive symptoms, lessened the increase of functional limitations, and increased sleep duration • Oral melatonin alone shortened sleep onset latency and increased sleep duration, but adversely affected caregiver ratings of withdrawn behaviour and mood • Combined light and melatonin treatment improved sleep efficiency and nocturnal restlessness (suggesting that the adverse impact of melatonin on mood could be counteracted by light) |
| Sloane et al. 2015 70 | Older adults with ADRD and caregivers living at home | All-day (waking to 18:00) blue-white fluorescent (13,000 K) and LED light box (peak wavelength ≅ 470 nm) light (300–400 lx), 6 weeks | • No change in objective or subjective sleep quality of PLWD; authors suggested null findings due to lack of sufficient exposures, as shown by the circadian light meter • Improvement in caregivers’ sleep quality and role strain scores |
| Konis et al. 2018 71 | Older adults with ADRD living in dementia care communities | Morning (08:00–10:00) exposure to daylit (mean illuminance = 159.3 lx) perimeter room for socializing, 12 weeks vs ambient electrical lighting (mean illuminance = 42.3 lx) control | • Intervention group showed significantly lower depression scores compared to nonsignificant increased scores for control group • Intervention notably successful among subjects with probable major depression, reaching mean scores below threshold for depression at the end of the trial |
PLWD: persons living with dementia
2.2. ADRD and depression: pharmacological and non-pharmacological therapies
Depression is estimated to affect 30–50% of those with ADRD.72 Population studies, which are less susceptible to referral bias, estimate that 20–24% of persons with ADRD experienced major depression in the prior month, and after 18 months the incidence of depressive symptoms was about 18%. Depression in persons with ADRD contributes to earlier placement in assisted living facilities and nursing homes, reduces quality of life and impairs activities of daily living, increases likelihood of aggression and suicide, and increases caregivers’ depression and burden.72 Current treatment relies heavily on antidepressants, which have been demonstrated to have limited efficacy and associations with a number of side effects (see Section 2.2.1).
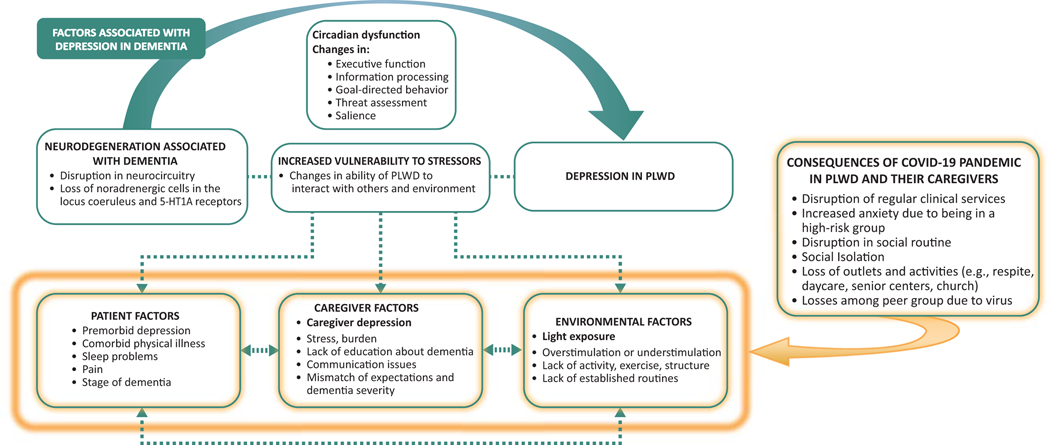
While the likely major vulnerability to depression in those with ADRD is not the psychological consequence of having the disease—but rather, results from the neurodegeneration associated with dementia itself—persons with ADRD, caregivers, and environmental factors often act as co-factors to trigger and exacerbate depression. 73 Figure 1 depicts the factors associated with development of depression in dementia. 73, 74
Figure 1.
Factors associated with the development of depression in dementia (adapted from Kales et al. 73,74). PLWD: persons living with dementia.
The COVID-19 pandemic is forcing older adults, especially those with underlying disease such as ADRD, to remain home, increasing isolation due to visitation restrictions and closure of activities and outlets, most likely worsening their depressive symptoms. Research on how the pandemic has affected depression in ADRD patients will undoubtedly be published in the near future.
Previous research using national data conducted by Kales and colleagues has found significantly higher rates of inpatient (medical and psychiatric) utilization among individuals with comorbid depression and ADRD compared to those with depression or dementia alone.75 A subsequent prospective study found that those with ADRD and comorbid depression had significantly higher rates of nursing home placement at one-year follow-up than those with depression or dementia alone.76 In that study, subjects with comorbid depression and ADRD had baseline dementia stage and cognitive function assessments similar to those with dementia alone, but showed significantly higher levels of functional impairment. Functional impairment was significantly correlated with one-year nursing home placement.
2.2.1. Pharmacological Therapy for Depression in ADRD Patients
As mentioned earlier, the treatment of depression in persons living with dementia (PLWD) relies heavily on antidepressants, with these agents prescribed to over 25% of PLWD in the community77 and 40% of those in long-term care. 78 Despite the ubiquity of their use, efficacy data from rigorous trials of antidepressants for depression in dementia have largely been negative.74, 79 Antidepressant side effects are numerous, including increased risk for nausea/vomiting, headaches, sleep changes, diarrhoea, tremor, falls, prolonged cardiac QT interval (an electrocardiographic indicator of ventricular depolarization and repolarization 80), and hyponatremia (low blood sodium).74 Moreover, these side effects increase caregiver burden. Given the prevalence of depression in dementia, its impact on outcomes for PLWD and their family caregivers, and the poor risk/benefit of antidepressant treatment, new modalities for treating depression in ADRD are essential and critically needed. One logical place to start would be to modify the factors depicted in Figure 1 that act as triggers for depression in PLWD who have pre-existing vulnerability due to neurodegenerative changes. Importantly, these triggers can be modifiable via nonpharmacological strategies (see Section 2.2.2). Light is an environmental factor that is associated with depression and which is eminently modifiable via ecological adjustments.
2.2.2. Non-pharmacological therapy for depression in ADRD patients
The underlying causes of behavioural and psychological symptoms of dementia (BPSD) like depression are myriad and complex as depicted in Figure 1.74 Because of the complex causes of BPSD, a “one size fits all” solution does not exist. While non-pharmacologic approaches offer significant benefit for both patients and their caregivers81 and are recommended as first-line by multiple expert groups,82, 83 they are infrequently used in real-world care settings.74 A major reason for lack of uptake is the absence of training among first-line providers, who may not be comfortable implementing non-pharmacologic approaches themselves, and are even less equipped to train caregivers.
Light therapy has been extensively used to treat seasonal affective disorder (SAD) and its efficacy is well known. Light therapy to treat major depression is less studied, but a recent meta-analysis reviewed 9 trials that met inclusion criteria. 84 After employing the more conservative random-effects model, the overall model showed a significant reduction of depressive symptoms after bright light administration (SMD=−0.62, p <0.001, I2=37%). In particular, the authors concluded that bright light appears to be efficacious when administered for 2–5 weeks (SMD = −0.78, p <0.001, I2=0%), and as monotherapy (SMD=−0.71, p <0.001, I2=18%) rather than in combination with other therapies.84 Two other meta-analyses 85, 86 reported that the efficacy of light therapy for treating depression is still questionable; however, both reviews strongly recommended the use of light therapy as an adjunct to anti-depressants, considering its minimal side effects and that a significant portion of the patients studied indeed showed clinical improvement in depressive symptoms.
In another study, those admitted to a psychiatric ward due to various types of depression had shorter lengths of stay if they stayed in brightly lit rooms (500 lx) compared to dimly lit rooms (200 lx) (the plane of measurement was not specified by the authors). 87 In a 12-week study conducted in nursing homes, 71ADRD patients who received natural light treatment (sitting within 3 meters of a window between 08:00–10:00) experienced significant improvement in depression as measured by the Cornell Scale for Depression in Dementia (CSDD). This study did not, however, measure dose and did not bring subjects outdoors, which may have reduced the actual number of light exposures they received. Table 1 summarizes the findings of the studies looking at using light to treat depression in persons with ADRD.
2.3. Drawbacks of current light therapy approaches
Not all studies to date, however, have shown a positive effect of light on sleep disturbances or rest-activity rhythms in those with dementia.88,89 Examining studies that used a variety of light therapy approaches, a 2014 Cochrane review90 found insufficient evidence for justifying the use of light therapy to improve sleep and behaviour in those with ADRD. Most current approaches to light therapy for reducing sleep disturbances and depressive symptoms in older adults do not consider the complete 24-hour light-dark pattern they experience, nor do they integrate light (and dark) treatment into a practical delivery system, thus compromising their therapeutic value.91 None of the studies included in the Cochrane review controlled or measured actual light doses received at subjects’ eyes during the various interventions, and pooling data from studies that were lacking in this regard may have affected the review’s outcomes. If light is not carefully designed to reach the patient’s eyes, the effect is reduced. The only formalized recommendations of light treatment are those used for treating symptoms of SAD; namely 10,000 lx of white light for 30 minutes or 2500 lx of the same white light for 2 hours (delivered via light box). Clinicians often find that patients have difficulty complying with recommendations because high light levels of white light can be very uncomfortable, resulting in squinting and gaze aversion; these compliance issues are even more salient in ADRD.
3. Improving light therapy approaches: a case study
3.1. Specification of the stimulus
Rea et al. developed a method to quantify and predict how spectral and absolute characteristics of a light source impact the circadian system using two proposed metrics: circadian light (CLA) and circadian stimulus (CS). CLA reflects the spectral sensitivity of the human circadian system and CS reflects the absolute sensitivity of the human circadian system. CS is, therefore, a measure of the effectiveness of the retinal light stimulus for the human circadian system, as measured by acute melatonin suppression, from threshold (CS=0.1) to saturation (CS=0.7). To synchronize the biological clock to the 24-hour day, the LRC has shown that a light source delivering a CS ≥ 0.3 at the retina (vertical illuminance) for at least 2 hours during the day (preferably in the morning) improves sleep and mood in various populations. 57, 92–94 Furthermore, providing a CS < 0.1, starting at least 2 hours before desired bedtime will help avoid circadian disruption. 95–97 To determine CS, the spectral power distribution (SPD) and how much light will reach the eye are needed. For more information about CS, see various publications by Rea and colleagues. 98–101
Using these two metrics, Figueiro et al proposed and tested in the field, a 24-hour lighting scheme to deliver a robust light/dark pattern to older adults, including those with ADRD. According to the proposed lighting scheme, a CS of 0.3 should be delivered at eye level during the day and a CS<0.1 should be delivered in the evening. It should be noted that other metrics are being proposed and no standardized luminous efficiency function has been developed for circadian system response. This should not, however, preclude designers from implementing better lighting in environments occupied by older people.
3.2. Delivery of the stimulus
Although light must be delivered as vertical illuminance (EV) in order to reach the back of the eye and thus be effective for promoting circadian entrainment, conventional light sources are generally mounted in ceilings as recessed downlights that tend to deliver high levels of horizontal illuminance (EH) and low EV/EH ratios.102 In other words, downward-directed light that predominantly emanates from the ceiling illuminates the work plane rather than the generally vertical plane of the retina. This is particularly the case for ADRD patients, who tend to spend their days seated at tables with their gaze directed downward. Field observations of this behaviour have led Figueiro and colleagues to develop various innovative methods (e.g. self-luminous light tables, 58 custom-built supplementary floor lamps, light boxes, and tabletop luminaires 60) for delivering the stimulus to the eyes of ADRD patients living at home 103 and in long-term care facilities. 94
3.3. Measurement of the stimulus
The Daysimeter is a personal circadian light exposure meter developed to account for the fact that all of the commercial light meters were calibrated to measure photopic illuminance, not circadian-effective light. The spectral sensitivity of the photosensors is calibrated to yield a spectral response function closely matching that of the human circadian system; it also provides accurate measurements of illuminance (in lux). The photosensor package has a cosine spatial response characteristic that closely matches the acceptance angles of light entering the eye. The device has a dynamic range of approximately five orders of magnitude with an absolute sensitivity near 1 lx. The Daysimeter can be deployed in the field to gather light data for up to 30 days. It provides light data in units of circadian weighted irradiance CLA, which is used to calculate circadian stimulus (CS) values. The Daysimeter 104, 105 can be used as pendant or pin and has been worn by various populations in the field, 103, 106–109 including those with ADRD. 57
3.4. Testing a lighting scheme in the field
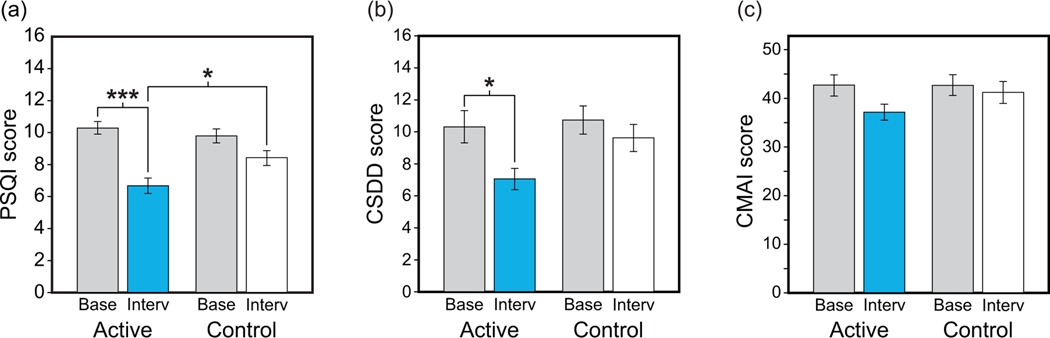
Sixty nursing home subjects who met the study’s inclusion and exclusion criteria were enrolled and 46 (30 females; mean age 85.1 years [SD 7.1]) completed in the study. All had been diagnosed with moderate or late-stage AD/ADRD. 94 As shown in Figure 2a, mean Pennsylvania Sleep Quality Index (PSQI) 110 scores were reduced significantly after 4 weeks of the active tailored lighting intervention (TLI) (p < 0.001) compared to the baseline lighting condition. The reduction in PSQI scores observed for the active TLI was significantly greater than that for the control intervention (p = 0.03). Similar results were observed with Cornell Scale for Depression in Dementia (CSDD) 111 scores (p = 0.04). Although a similar trend was observed for Cohen-Mansfield Agitation Index (CMAI) 112 scores, the results were not statistically significant.
Figure. 2.
Effects of the short-term TLI on subjective measures of sleep quality (a), depression (b), and agitation (c) (*p <0.05, ***p <0.001). Base: baseline; CMAI: Cohen-Mansfield Agitation Index; CSDD: Cornell Scale for Depression in Dementia; Interv: intervention; PSQI: Pittsburgh Sleep Quality Index.
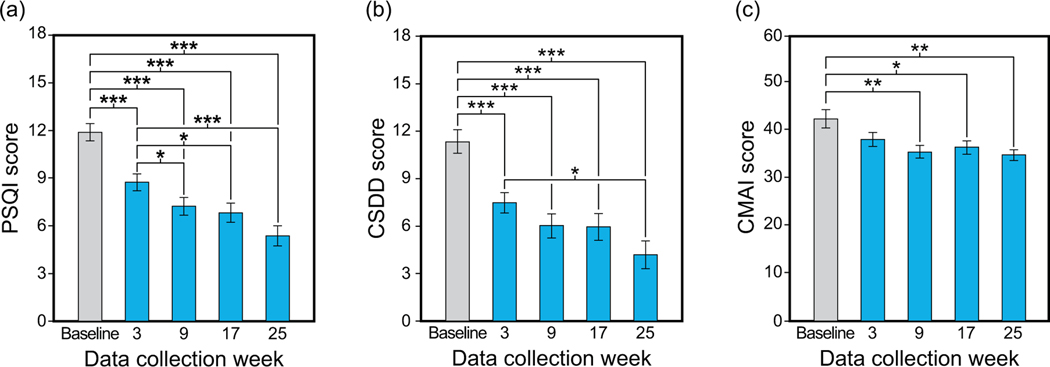
In study’s arm that tested the long-term effects of the TLI on sleep and behaviour of older adults with AD/ADRD, we successfully recruited 80 subjects for the 25-week protocol. Fifty-one subjects were enrolled and 47 subjects (27 females; mean age 85.3 years [SD 7.1] completed at least 1 week of the TLI and baseline lighting condition. As shown in Figure 3, the PSQI, CSDD and CMAI scores also showed a significant (p <0.05) continuous decline (i.e., improvement) with treatment over the course of the study. 60
Figure 3.
Effects of the long-term TLI on subjective measures of sleep quality (a), depression (b), and agitation (c) (* p < 0.05; ** p < 0.01; ***p <0.001). Adapted from Figures 2 and 3 in Figueiro et al. 60 (* p <0.05; **p <0.01; ***p <0.001). CMAI: Cohen-Mansfield Agitation Index; CSDD: Cornell Scale for Depression in Dementia; Interv: intervention; PSQI: Pittsburgh Sleep Quality Index.
4. Discussion
Light can have a significant impact on the lives of those suffering from sleep disturbances and depression, such as people living with ADRD and their caregivers. To be effective, however, light needs to be correctly specified, implemented, and measured. Without the appropriate specification and measurement of the stimulus, researchers will not be able to successfully demonstrate positive results in the field, nor will lighting designers and specifiers have the confidence to implement lighting solutions for promoting better sleep and mood in this population.
As noted above (see Section 2.1), age-related changes to the eye, as well as changes to the central nervous system, can have significant negative impacts on day-to-day functioning, general health, well-being, and the visual and non-visual systems’ responses to light. Lighting systems for elders should be designed to counteract these conditions, creating an environment with sufficiently high levels of ambient and task lighting while avoiding glare. For older adults in long-term care facilities, designers should consider using a 24-hour lighting scheme in common areas where residents are more likely to spend their waking hours. It is also important to tailor the scheme to the application. In an assisted-living setting, for example, a dual lighting scheme can be implemented between residents’ personal rooms and common areas, such as dining rooms or activity rooms. Similarly, nursing homes can employ a dual lighting scheme between day rooms and dining rooms. If power density requirements are in place, supplementary personal blue (λmax = 450–470 nm) LED luminaires can be integrated into the design. In cases where compliance with facility lighting is not an issue, such as healthy older adults living at home, ambient light levels can be set at an EV of 80–100 lx (or an EH of about 200–300 lx) from a 3000–4100 K light source, supplemented by a personal lighting device providing an EV of about 30 lx at the cornea from a blue (λmax = 450–470 nm) for at least 2 hours in the morning.
Targeting a high CS (see Section 3.1) of 0.4 during the day, assuming losses from absorbed or scattered light through the room occupants’ crystalline lens, will ensure the delivery of an effective CS dose (i.e. ≥ 0.3). Given that the higher light levels attendant with higher CS levels can result in excessive glare, however, discomfort glare can be reduced by choosing luminaires whose distribution provides an EV/EH ratio > 0.6.
The light levels recommended for the circadian system should also be sufficient for older adults to perform daily visual tasks. More localized light will be needed to perform tasks that require the discernment of fine detail (e.g., reading prescription bottle labels, food preparation). Designers and specifiers should still provide lighting that delivers:
High, glare-free illumination for the task without a direct or reflected view of the light source
Soft shadows throughout the space
Balanced illuminance levels
High-quality color rendering.
Although the human circadian system’s spectral sensitivity is still a topic of debate in the field, this should not overshadow the positive results demonstrated in field studies. As long as there are no adverse effects on visual performance, elders in any setting should experience bright days and dark nights to promote their health and well-being. The present components of the built environment, especially those places where older adults spend most of their days, are too dimly illuminated during the day and too bright at night. We, as a community, do not need to agree on a metric per se, but certainly we can agree that a more robust pattern of light and dark needs to be experienced in the built environment. And, as exemplified above, designers can already implement some of these effective solutions in the field.
Acknowledgments
This research was funded by the National Institute on Aging (grant # R01AG034157). The following manufacturers are acknowledged for their provision of in-kind lighting products: GE Current, a Daintree company; GE Lighting; Ketra; and OSRAM Sylvania; Sharp Corporation. Neither the funding agency nor the in-kind contributors had any role in the design, methods, data analysis, or preparation of the manuscript. The authors would like to acknowledge Bridget Bradley, Mike Kalsher, Madison Laks, Sharon Lesage, Martin Overington, Howard Ohlhous, David Pedler, Charles Roohan, Levent Sahin, Gregory Ward, and Savana Wemette for their technical support in data collection and analyses.
References
- 1.U.S. Census Bureau. 2017 National Population Projections Tables: Main Series Washington, DC: U.S. Department of Commerce; 2017. Available from: https://www.census.gov/data/tables/2017/demo/popproj/2017-summary-tables.html. Accessed 19 February 2021 [Google Scholar]
- 2.Administration for Community Living. 2018 Profile of Older Americans. Washington, D.C.: U.S. Department of Health and Human Services. Accessed 18 April 2018. [Google Scholar]
- 3.Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–87. doi: 10.1016/j.jalz.2019.01.010 [DOI] [PubMed] [Google Scholar]
- 4.World Health Organization. Dementia Geneva: World Health Organization; 2020. Available from: https://www.who.int/news-room/fact-sheets/detail/dementia. Accessed 19 February 2021. [Google Scholar]
- 5.World Health Organization. Global Health Estimates: Life expectancy and leading causes of death and disability Geneva: World Health Organization; 2021. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates. Accessed 19 February 2021. [Google Scholar]
- 6.Bondi MW, Edmonds EC, Salmon DP. Alzheimer’s disease: Past, present, and future. Journal of the International Neuropsychological Society. 2017;23(9–10):818–31. doi: 10.1017/s135561771700100x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheer FA, van Doornen LJ, Buijs RM. Light and diurnal cycle affect human heart rate: possible role for the circadian pacemaker. J Biol Rhythms. 1999;14(3):202–12. doi: 10.1177/074873099129000614 [DOI] [PubMed] [Google Scholar]
- 8.Klein DC, Moore RY, Reppert SM. Suprachiasmatic nucleus: the mind’s clock. New York, NY: Oxford University Press; 1991. [Google Scholar]
- 9.Borbély AA, Achermann P. Sleep homeostasis and models of sleep regulation. J Biol Rhythms. 1999;14(6):557–68. doi: 10.1177/074873099129000894 [DOI] [PubMed] [Google Scholar]
- 10.Borbély AA, Daan S, Wirz-Justice A, Deboer T. The two-process model of sleep regulation: A reappraisal. J Sleep Res. 2016;25(2):131–43. doi: 10.1111/jsr.12371 [DOI] [PubMed] [Google Scholar]
- 11.Franken P, Dijk D-J. Circadian clock genes and sleep homeostasis. Eur J Neurosci. 2009;29(9):1820–9. doi: 10.1111/j.1460-9568.2009.06723.x [DOI] [PubMed] [Google Scholar]
- 12.Rea MS. IESNA Lighting Handbook: Reference and Application. 9th ed. New York, NY: Illuminating Engineering Society of North America; 2000. [Google Scholar]
- 13.Rea MS, Figueiro MG, Bullough JD. Circadian photobiology: An emerging framework for lighting practice and research. Lighting Res Technol. 2002;34(3):177–87. doi: 10.1191/1365782802lt057oa [DOI] [Google Scholar]
- 14.Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, et al. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21(16):6405–12. doi: 10.1523/JNEUROSCI.21-16-06405.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: Evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–7. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol. 1997;273(5 PT 2):R1800–9. doi: 10.1152/ajpregu.1997.273.5.r1800 [DOI] [PubMed] [Google Scholar]
- 17.Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295(5557):1070–3. doi: 10.1126/science.1067262 [DOI] [PubMed] [Google Scholar]
- 18.McIntyre IM, Norman TR, Burrows GD, Armstrong SM. Human melatonin suppression by light is intensity dependent. J Pineal Res. 1989;6(2):149–56. doi: 10.1111/j.1600-079X.1989.tb00412.x [DOI] [PubMed] [Google Scholar]
- 19.Glickman G, Hanifin JP, Rollag MD, Wang J, Cooper H, Brainard GC. Inferior retinal light exposure is more effective than superior retinal exposure in suppressing melatonin in humans. J Biol Rhythms. 2003;18(1):71–9. doi: 10.1177/0748730402239678 [DOI] [PubMed] [Google Scholar]
- 20.Rüger M, Gordijn MC, Beersma DG, de Vries B, Daan S. Nasal versus temporal illumination of the human retina: Effects on core body temperature, melatonin, and circadian phase. J Biol Rhythms. 2005;20(1):60–70. doi: 10.1177/0748730404270539 [DOI] [PubMed] [Google Scholar]
- 21.Visser EK, Beersma DG, Daan S. Melatonin suppression by light in humans is maximal when the nasal part of the retina is illuminated. J Biol Rhythms. 1999;14(2):116–21. doi: 10.1177/074873099129000498 [DOI] [PubMed] [Google Scholar]
- 22.Hébert M, Martin SK, Lee C, Eastman CI. The effects of prior light history on the suppression of melatonin by light in humans. J Pineal Res. 2002;33(4):198–203. doi: 10.1034/j.1600-079X.2002.01885.x [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woelders T, Beersma DGM, Gordijn MCM, Hut RA, Wams EJ. Daily light exposure patterns reveal phase and period of the human circadian clock. J Biol Rhythms. 2017;32(3):274–86. doi: 10.1177/0748730417696787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vitiello MV, Bliwise DL, Prinz PN. Sleep in Alzheimer’s disease and the sundown syndrome. Neurology. 1992;42(7 Suppl 6):89–93. doi: [PubMed] [Google Scholar]
- 25.Vitiello MV, Borson S. Sleep disturbances in patients with Alzheimer’s disease: Epidemiology,pathophysiology and treatment. CNS Drugs. 2001;15(10):777–96. doi: 10.2165/00023210-200115100-00004 [DOI] [PubMed] [Google Scholar]
- 26.McCurry SM, Teri L. Sleep Disturbance in Elderly Caregivers of Dementia Patients. Clinical Gerontologist. 1996;16(2):51–66. doi: 10.1300/J018v16n02_05 [DOI] [Google Scholar]
- 27.McCurry SM, Gibbons LE, Logsdon RG, Vitiello MV, Teri L. Insomnia in caregivers of persons with dementia: Who is at risk and what can be done about it? Sleep Med Clin. 2009;4(4):519–26. doi: 10.1016/j.jsmc.2009.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cohen-Mansfield J, Billig N, Lipson S, Rosenthal AS, Pawlson LG. Medical correlates of agitation in nursing home residents. Gerontology. 1990;36(3):150–8. doi: 10.1159/000213191 [DOI] [PubMed] [Google Scholar]
- 29.Cedernaes J, Osorio RS, Varga AW, Kam K, Schioöth HB, Benedict C. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer’s disease. Sleep Med Rev. 2017;31:102–11. doi: 10.1016/j.smrv.2016.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mander BA, Winer JR, Jagust WJ, Walker MP. Sleep: A Novel mechanistic pathway, biomarker, and treatment target in the pathology of Alzheimer’s disease? Trends Neurosci. 2016;39(8):552–66. doi: 10.1016/j.tins.2016.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yaffe K, Falvey CM, Hoang T. Connections between sleep and cognition in older adults. Lancet Neurol. 2014;13(10):1017–28. doi: 10.1016/S1474-4422(14)70172-3 [DOI] [PubMed] [Google Scholar]
- 32.Lauriola M, Esposito R, Delli Pizzi S, de Zambotti M, Londrillo F, Kramer JH, et al. Sleep changes without medial temporal lobe or brain cortical changes in community-dwelling individuals with subjective cognitive decline. Alzheimers Dement. 2016;13(7):783–91. doi: 10.1016/j.jalz.2016.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lim AS, Kowgier M, Yu L, Buchman AS, Bennett DA. Sleep Fragmentation and the risk of incident Alzheimer’s disease and cognitive decline in older persons. Sleep. 2013;36(7):1027–32. doi: 10.5665/sleep.2802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84(19):1964–671. doi: 10.1212/WNL.0000000000001566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kang J-E, Lim MM, Bateman RJ, Lee JJ, Smyth LP, Cirrito JR, et al. Amyloid-β dynamics are regulated by orexin and the sleep-wake cycle. Science. 2009;326(5955):1005–7. doi: 10.1126/science.1180962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roh JH, Huang Y, Bero AW, Kasten T, Stewart FR, Bateman RJ, et al. Disruption of the sleep-wake cycle and diurnal fluctuation of beta-amyloid in mice with Alzheimer’s disease pathology. Sci Transl Med. 2012;4(150):150ra22. doi: 10.1126/scitranslmed.3004291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tabuchi M, Lone SR, Liu S, Liu Q, Zhang J, Spira AP, et al. Sleep interacts with abeta to modulate intrinsic neuronal excitability. Curr Biol. 2015;25(6):702–12. doi: 10.1016/j.cub.2015.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie L, Kang H, Xu Q, Chen MJ, Liao Y, Thiyagarajan M, et al. Sleep drives metabolite clearance from the adult brain. Science. 2013;342(6156):373–7. doi: 10.1126/science.1241224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branger P, Arenaza-Urquijo EM, Tomadesso C, Mezenge F, Andre C, de Flores R, et al. Relationships between sleep quality and brain volume, metabolism, and amyloid deposition in late adulthood. Neurobiol Aging. 2016;41:107–14. doi: 10.1016/j.neurobiolaging.2016.02.009 [DOI] [PubMed] [Google Scholar]
- 40.Liguori C, Romigi A, Nuccetelli M, Zannino S, Sancesario G, Martorana A, et al. Orexinergic system dysregulation, sleep impairment, and cognitive decline in Alzheimer disease. JAMA Neurology. 2014;71(12):1498–505. doi: 10.1001/jamaneurol.2014.2510 [DOI] [PubMed] [Google Scholar]
- 41.Mander BA, Marks SM, Vogel JW, Rao V, Lu B, Saletin JM, et al. β-amyloid disrupts human NREM slow waves and related hippocampus-dependent memory consolidation. Nat Neurosci. 2015;18(7):1051–7. doi: 10.1038/nn.4035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Spira AP, Gamaldo AA, An Y, Wu MN, Simonsick EM, Bilgel M, et al. Self-reported sleep and beta-amyloid deposition in community-dwelling older adults. JAMA Neurology. 2013;70(12):1537–43. doi: 10.1001/jamaneurol.2013.4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sprecher KE, Bendlin BB, Racine AM, Okonkwo OC, Christian BT, Koscik RL, et al. Amyloid burden is associated with self-reported sleep in nondemented late middle-aged adults. Neurobiol Aging. 2015;36(9):2568–76. doi: 10.1016/j.neurobiolaging.2015.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marshall L, Helgadóttir H, Mölle M, Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444(7119):610–3. doi: 10.1038/nature05278 [DOI] [PubMed] [Google Scholar]
- 45.Schabus M, Gruber G, Parapatics S, Sauter C, Klösch G, Anderer P, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–85. doi: 10.1093/sleep/27.7.1479 [DOI] [PubMed] [Google Scholar]
- 46.Sharma RA, Kam K, Parekh A, Uribe-Cano S, Tweardy S, Bubu OM, et al. Reduced spindle frequency and density in stage 2 NREM sleep is associated with increased CSF p-Tau in cognitively normal elderly. Sleep. 2017;40(Issue suppl_1):A281-A. doi: 10.1093/sleepj/zsx050.757 [DOI] [Google Scholar]
- 47.Hinton DR, Sadun AA, Blanks JC, Miller CA. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med. 1986;315(8):485–7. doi: 10.1056/NEJM198608213150804 [DOI] [PubMed] [Google Scholar]
- 48.Katz B, Rimmer S, Iragui V, Katzman R. Abnormal pattern electroretinogram in Alzheimer’s disease: Evidence for retinal ganglion cell degeneration? Ann Neurol. 1989;26(2):221–5. doi: 10.1002/ana.410260207 [DOI] [PubMed] [Google Scholar]
- 49.Moore RY. The organization of the human circadian timing system. In: Hofman MA, Mimiran M, Ravid R, Van Leeuwen FW, editors. Prog Brain Res. 93. Amsterdam, The Netherlands: Elsevier; 1992. p. 101–17. [PubMed] [Google Scholar]
- 50.Swaab DF, Fliers E, Partiman TS. The suprachiasmatic nucleus of the human brain in relation to sex, age and senile dementia. Brain research. 1985;342(1):37–44. doi: 10.1016/0006-8993(85)91350-2 [DOI] [PubMed] [Google Scholar]
- 51.Campbell SS, Kripke DF, Gillin JC, Hrubovcak JC. Exposure to light in healthy elderly subjects and Alzheimer’s patients. Physiol Behav. 1988;42(2):141–4. doi: 10.1016/0031-9384(88)90289-2 [DOI] [PubMed] [Google Scholar]
- 52.Ancoli-Israel S, Kripke DF. Now I lay me down to sleep: The problem of sleep fragmentation in elderly and demented residents of nursing homes. Bull Clin Neurosci. 1989;54:127–32. [Google Scholar]
- 53.Weale RA. Retinal illumination and age. Transactions of the Illuminating Engineering Society of New York. 1961;26(2_IEStrans):95–100. doi: 10.1177/147715356102600204 [DOI] [Google Scholar]
- 54.Espiritu RC, Kripke DF, Ancoli-Israel S, Mowen MA, Mason WJ, Fell RL, et al. Low illumination experienced by San Diego adults: Association with atypical depressive symptoms. Biol Psychiatry. 1994;35(6):403–7. doi: 10.1016/0006-3223(94)90007-8 [DOI] [PubMed] [Google Scholar]
- 55.Sanchez R, Ge Y, Zee P. A comparison of the strength of external zeitgeber in young and older adults. Sleep Res. 1993;22:416–22. [Google Scholar]
- 56.Weldemichael DA, Grossberg GT. Circadian rhythm disturbances in patients with Alzheimer’s disease: A review. Int J Alzheimers Dis. 2010;2010(September 2):716453. doi: 10.4061/2010/716453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Figueiro MG, Plitnick BA, Lok A, Jones GE, Higgins P, Hornick TR, et al. Tailored lighting intervention improves measures of sleep, depression, and agitation in persons with Alzheimer’s disease and related dementia living in long-term care facilities. Clin Interv Aging. 2014;9:1527–37. doi: 10.2147/CIA.S68557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Figueiro M, Plitnick B, Rea M. Research Note: A self-luminous light table for persons with Alzheimer’s disease. Lighting Res Technol. 2016;48(2):253–9. doi: 10.1177/1477153515603881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Figueiro MG. Light, sleep and circadian rhythms in older adults with Alzheimer’s disease and related dementias. Neurodegenerative Disease Management. 2017;7(2):119–45. doi: 10.2217/nmt-2016-0060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Figueiro MG, Sahin L, Kalsher M, Plitnick B, Rea MS. Long-term, all-day exposure to circadian-effective light improves sleep, mood, and behavior in persons with dementia. J Alzheimers Dis Rep. 2020;4(1):297–312. doi: 10.3233/ADR-200212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Someren EJW, Kessler A, Mirmiran M, Swaab DF. Indirect bright light improves circadian rest-activity rhythm disturbances in demented patients. Biol Psychiatry. 1997;41(9):955–63. doi: 10.1016/S0006-3223(97)89928-3 [DOI] [PubMed] [Google Scholar]
- 62.Satlin A, Volicer L, Ross V, Herz L, Campbell S. Bright light treatment for behavioral and sleep disturbances in patients with Alzheimer’s disease. American Journal of Psychiatry. 1992;149(8):1028–32. doi: 10.1176/ajp.149.8.1028 [DOI] [PubMed] [Google Scholar]
- 63.Lyketsos CG, Lindell Veiel L, Baker A, Steele C. A randomized, controlled trial of bright light therapy for agitated behaviors in dementia patients residing in long-term care. International Journal of Geriatric Psychiatry. 1999;14(7):520–5. doi: [DOI] [PubMed] [Google Scholar]
- 64.Ancoli-Israel S, Gehrman P, Martin JL, Shochat T, Marler M, Corey-Bloom J, et al. Increased light exposure consolidates sleep and strengthens circadian rhythms in severe Alzheimer’s disease patients. Behav Sleep Med. 2003;1(1):22–36. doi: [DOI] [PubMed] [Google Scholar]
- 65.Alessi CA, Martin JL, Webber AP, Cynthia Kim E, Harker JO, Josephson KR. Randomized, controlled trial of a nonpharmacological intervention to improve abnormal sleep/wake patterns in nursing home residents. Journal of the American Geriatrics Society. 2005;53(5):803–10. doi: 10.1111/j.1532-5415.2005.53251.x [DOI] [PubMed] [Google Scholar]
- 66.Dowling GA, Mastick J, Hubbard EM, Luxenberg JS, Burr RL. Effect of timed bright light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. International Journal of Geriatric Psychiatry. 2005;20(8):738–43. doi: 10.1002/gps.1352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sloane PD, Williams CS, Mitchell CM, Preisser JS, Wood W, Barrick AL, et al. High-intensity environmental light in dementia: Effect on sleep and activity. Journal of the American Geriatrics Society. 2007;55(10):1524–33. doi: 10.1111/j.1532-5415.2007.01358.x [DOI] [PubMed] [Google Scholar]
- 68.Hickman SE, Barrick AL, Williams CS, Zimmerman S, Connell BR, Preisser JS, et al. The effect of ambient bright light therapy on depressive symptoms in persons with dementia. Journal of the American Geriatrics Society. 2007;55(11):1817–24. doi: 10.1111/j.1532-5415.2007.01428.x [DOI] [PubMed] [Google Scholar]
- 69.Riemersma-van der Lek RF, Swaab DF, Twisk J, Hol EM, Hoogendijk WJ, Van Someren EJW. Effect of bright light and melatonin on cognitive and noncognitive function in elderly residents of group care facilities: A randomized controlled trial. JAMA. 2008;299(22):2642–55. doi: 10.1001/jama.299.22.2642 [DOI] [PubMed] [Google Scholar]
- 70.Sloane PD, Figueiro M, Garg S, Cohen LW, Reed D, Williams CS, et al. Effect of home-based light treatment on persons with dementia and their caregivers. Lighting Res Technol. 2015;47(2):161–76. doi: 10.1177/1477153513517255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Konis K, Mack WJ, Schneider EL. Pilot study to examine the effects of indoor daylight exposure on depression and other neuropsychiatric symptoms in people living with dementia in long-term care communities. Clin Interv Aging. 2018;13:1071–7. doi: 10.2147/CIA.S165224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee HB, Lyketsos CG. Depression in Alzheimer’s disease: Heterogeneity and related issues. Biol Psychiatry. 2003;54(3):353–62. doi: 10.1016/s0006-3223(03)00543-2 [DOI] [PubMed] [Google Scholar]
- 73.Kales HC, Gitlin LN, Lyketsos CG. When less is more, but still not enough: Why focusing on limiting antipsychotics in people with dementia is the wrong policy imperative. Journal of the American Medical Directors Association. 2019;20(9):1074–9. doi: 10.1016/j.jamda.2019.05.022 [DOI] [PubMed] [Google Scholar]
- 74.Kales HC, Gitlin LN, Lyketsos CG. State of the Art Review: Assessment and management of behavioral and psychological symptoms of dementia. British Medical Journal. 2015;350:h369. doi: 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kales HC, Blow FC, Copeland LA, Bingham RC, Kammerer EE, Mellow AM. Health care utilization by older patients with coexisting dementia and depression. American Journal of Psychiatry. 1999;156(4):550–6. doi: 10.1176/ajp.156.4.550 [DOI] [PubMed] [Google Scholar]
- 76.Kales HC, Chen P, Blow FC, Welsh DE, Mellow AM. Rates of clinical depression diagnosis, functional impairment, and nursing home placement in coexisting dementia and depression. American Journal of Geriatric Psychiatry. 2005;13(6):441–9. doi: 10.1176/appi.ajgp.13.6.441 [DOI] [PubMed] [Google Scholar]
- 77.Kales HC, Zivin K, Kim HM, Valenstein M, Chiang C, Ignacio RV, et al. Trends in antipsychotic use in dementia 1999–2007. Arch Gen Psychiatry. 2011;68(2):190–7. doi: 10.1001/archgenpsychiatry.2010.200 [DOI] [PubMed] [Google Scholar]
- 78.Kales HC, Chiang C, Maust DT. Impact of the CMS National Partnership to Improve Dementia Care on use of antipsychotics and other psychotropics in long-term care in the United States. Alzheimers Dement. 2017;13(7):P1557. doi: 10.1016/j.jalz.2017.07.702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Livingston G, Sommerlad A, Orgeta V, Costafreda SG, Huntley J, Ames D, et al. Dementia prevention, intervention, and care. The Lancet. 2017;390(10113):2673–734. doi: 10.1016/S0140-6736(17)31363-6 [DOI] [PubMed] [Google Scholar]
- 80.Al-Khatib SM, LaPointe NMA, Kramer JM, Califf RM. What clinicians should know about the QT interval. JAMA. 2003;289(16):2120–7. doi: 10.1001/jama.289.16.2120 [DOI] [PubMed] [Google Scholar]
- 81.Brodaty H, Arasaratnam C. Meta-analysis of nonpharmacological interventions for neuropsychiatric symptoms of dementia. American Journal of Psychiatry. 2012;169(9):946–53. doi: 10.1176/appi.ajp.2012.11101529 [DOI] [PubMed] [Google Scholar]
- 82.American Psychiatric Association. Five things physicians and patients should question Philadelphia, PA: ABIM Foundation; 2013. updated April 22, 2015. Available from: https://www.choosingwisely.org/societies/american-psychiatric-association/. Accessed January 15, 2021 [Google Scholar]
- 83.American Geriatrics Society. Ten things clinicians and patients should question Philadelphia, PA: ABIM Foundation; 2013. updated April 23, 2015. Available from: https://www.choosingwisely.org/wp-content/uploads/2015/02/AGS-Choosing-Wisely-List.pdf. Accessed January 15, 2021 [Google Scholar]
- 84.Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. Journal of Affective Disorders. 2016;198:64–71. doi: 10.1016/j.jad.2016.03.016 [DOI] [PubMed] [Google Scholar]
- 85.Perera S, Eisen R, Bhatt M, Bhatnagar N, de Souza R, Thabane L, et al. Light therapy for non-seasonal depression: Systematic review and meta-analysis. BJPsych Open. 2016;2(2):116–26. doi: 10.1192/bjpo.bp.115.001610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Even C, Schröder CM, Friedman S, Rouillon F. Efficacy of light therapy in nonseasonal depression: A systematic review. Journal of Affective Disorders. 2008;108(1–2):11–23. doi: 10.1016/j.jad.2007.09.008 [DOI] [PubMed] [Google Scholar]
- 87.Beauchemin KM, Hays P. Sunny hospital rooms expedite recovery from severe and refractory depressions. Journal of Affective Disorders. 1996;40(1):49–51. doi: 10.1016/0165-0327(96)00040-7 [DOI] [PubMed] [Google Scholar]
- 88.Ancoli-Israel S, Martin JL, Kripke DF, Marler M, Klauber MR. Effect of light treatment on sleep and circadian rhythms in demented nursing home patients. Journal of the American Geriatrics Society. 2002;50(2):282–9. doi: 10.1046/j.1532-5415.2002.50060.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dowling GA, Burr RL, Van Someren EJ, Hubbard EM, Luxenberg JS, Mastick J, et al. Melatonin and bright-light treatment for rest-activity disruption in institutionalized patients with Alzheimer’s disease. Journal of the American Geriatrics Society. 2008;56(2):239–46. doi: 10.1111/j.1532-5415.2007.01543.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Forbes D, Blake CM, Thiessen EJ, Peacock S, Hawranik P. Light therapy for improving cognition, activities of daily living, sleep, challenging behaviour, and psychiatric disturbances in dementia. Cochrane Database Syst Rev. 2014;2:CD003946. doi: 10.1002/14651858.CD003946.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Montgomery P, Dennis J. Bright light therapy for sleep problems in adults aged 60+. Cochrane Database Syst Rev. 2002(2):CD003403. doi: 10.1002/14651858.CD003403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Figueiro MG, Steverson B, Heerwagen J, Kampschroer K, Hunter CM, Gonzales K, et al. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3(3):204–15. doi: 10.1016/j.sleh.2017.03.005 [DOI] [PubMed] [Google Scholar]
- 93.Figueiro MG, Kalsher M, Steverson BC, Heerwagen J, Kampschroer K, Rea MS. Circadian-effective light and its impact on alertness in office workers. Lighting Res Technol. 2019;51(2):171–83. doi: 10.1177/1477153517750006 [DOI] [Google Scholar]
- 94.Figueiro MG, Plitnick B, Roohan C, Sahin L, Kalsher M, Rea MS. Effects of a tailored lighting intervention on sleep quality, rest–activity, mood, and behavior in older adults with Alzheimer’s disease and related dementias: A randomized clinical trial. J Clin Sleep Med. 2019;15(12):1757–67. doi: 10.5664/jcsm.8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Figueiro MG, Nagare R, Price LLA. Non-visual effects of light: How to use light to promote circadian entrainment and elicit alertness. Lighting Res Technol. 2018;50(1):38–62. doi: 10.1177/1477153517721598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Figueiro MG. A proposed 24 h lighting scheme for older adults. Lighting Res Technol. 2008;40(2):153–60. doi: 10.1177/1477153507087299 [DOI] [Google Scholar]
- 97.Figueiro MG. An overview of the effects of light on human circadian rhythms: Implications for new light sources and lighting systems design. Journal of Light & Visual Environment. 2013;37(2&3):51–61. doi: 10.2150/jlve.IEIJ130000503 [DOI] [Google Scholar]
- 98.Rea MS, Figueiro MG, Bullough JD, Bierman A. A model of phototransduction by the human circadian system. Brain Res Rev. 2005;50(2):213–28. doi: 10.1016/j.brainresrev.2005.07.002 [DOI] [PubMed] [Google Scholar]
- 99.Rea MS, Figueiro MG, Bierman A, Hamner R. Modelling the spectral sensitivity of the human circadian system. Lighting Res Technol. 2012;44(4):386–96. doi: 10.1177/1477153511430474 [DOI] [Google Scholar]
- 100.Rea MS, Figueiro MG. Light as a circadian stimulus for architectural lighting. Lighting Res Technol. 2018;50(4):497–510. doi: 10.1177/1477153516682368 [DOI] [Google Scholar]
- 101.Rea MS, Nagare R, Figueiro MG. Modeling circadian phototransduction: Retinal neurophysiology and neuroanatomy. Front Neurosci. 2021;14:1467. doi: 10.3389/fnins.2020.615305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jarboe C, Snyder J, Figueiro MG. The effectiveness of light-emitting diode lighting for providing circadian stimulus in office spaces while minimizing energy use. Lighting Res Technol. 2019;52(2):167–88. doi: 10.1177/1477153519834604 [DOI] [Google Scholar]
- 103.Figueiro MG, Hunter CM, Higgins PA, Hornick TR, Jones GE, Plitnick B, et al. Tailored lighting intervention for persons with dementia and caregivers living at home. Sleep Health. 2015;1(4):322–30. doi: 10.1016/j.sleh.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bierman A, Klein TR, Rea MS. The Daysimeter: A device for measuring optical radiation as a stimulus for the human circadian system. Meas Sci Technol. 2005;16:2292–9. doi: 10.1088/0957-0233/16/11/023 [DOI] [Google Scholar]
- 105.Figueiro MG, Hamner R, Bierman A, Rea MS. Comparisons of three practical field devices used to measure personal light exposures and activity levels. Lighting Res Technol. 2013;45(4):421–34. doi: 10.1177/1477153512450453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Higgins PA, Hornick TR, Figueiro MG. Rest-activity and light exposure patterns in the home setting: a methodological case study. American journal of Alzheimer’s disease and other dementias. 2010;25(4):353–61. doi: 10.1177/1533317510363467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Figueiro MG, Brons JA, Plitnick B, Donlan B, Leslie RP, Rea MS. Measuring circadian light and its impact on adolescents. Lighting Res Technol. 2011;43(2):201–15. doi: 10.1177/1477153510382853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Figueiro MG. Individually tailored light intervention through closed eyelids to promote circadian alignment and sleep health. Sleep Health. 2015;1(1):75–82. doi: 10.1016/j.sleh.2014.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Figueiro MG, Steverson B, Heerwagen J, Yucel R, Roohan C, Sahin L, et al. Light, entrainment and alertness: A case study in offices. Lighting Res Technol. 2020;52(6):736–50. doi: 10.1177/1477153519885157 [DOI] [Google Scholar]
- 110.Buysse DJ, Reynolds CF, Monk TH, Hoch CC, Yeager AL, Kupfer DJ. Quantification of subjective sleep quality in healthy elderly men and women using the Pittsburgh Sleep Quality Index (PSQI). Sleep. 1991;14(4):331–8. doi: 10.1093/sleep/14.4.331 [DOI] [PubMed] [Google Scholar]
- 111.Alexopolous GS, Abrams RC, Young RC, Shamoian CA. Cornell Scale for Depression in Dementia. Biol Psychiatry. 1988;23(3):271–84. doi: 10.1016/0006-3223(88)90038-8 [DOI] [PubMed] [Google Scholar]
- 112.Cohen-Mansfield J, Marx MS, Rosenthal AS. A description of agitation in a nursing home. Journal of Gerontology. 1989;44(3):M77–M84. doi: 10.1093/geronj/44.3.m77 [DOI] [PubMed] [Google Scholar]