Abstract
Klebsiella pneumoniae is a notorious bacterium in clinical practice. Virulence, carbapenem‐resistance and their convergence among K. pneumoniae are extensively discussed in this article. Hypervirulent K. pneumoniae (HvKP) has spread from the Asian Pacific Rim to the world, inducing various invasive infections, such as pyogenic liver abscess, endophthalmitis, and meningitis. Furthermore, HvKP has acquired more and more drug resistance. Among multidrug‐resistant HvKP, hypervirulent carbapenem‐resistant K. pneumoniae (Hv‐CRKP), and carbapenem‐resistant hypervirulent K. pneumoniae (CR‐HvKP) are both devastating for their extreme drug resistance and virulence. The hypervirulence of HvKP is primarily attributed to hypercapsule, macromolecular exopolysaccharides, or excessive siderophores, although it has many other factors, for example, lipopolysaccharides, fimbriae, and porins. In contrast with classical determination of HvKP, that is, animal lethality test, molecular determination could be an optional and practical method after improvement. HvKP, including Hv‐CRKP and CR‐HvKP, has been progressing. R‐M and CRISPR‐Cas systems may play pivotal roles in such evolutions. Hv‐CRKP and CR‐HvKP, in particular the former, should be of severe concern due to their being more and more prevalent.
Factors involved in HvKP.
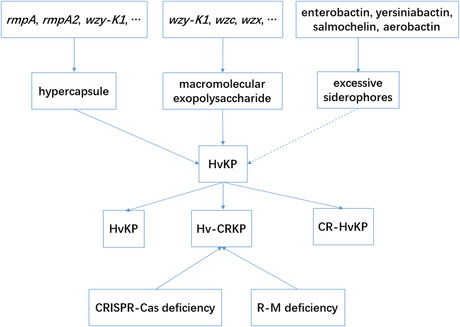
1. INTRODUCTION
Klebsiella pneumoniae (K. pneumoniae) is a gram‐negative and non‐motile bacterium and belongs to Enterobacteriaceae, which was first described by Carl Friedlander in 1882 as a bacterium and isolated from the lungs of patients who had died of pneumonia. K. pneumoniae is ubiquitously found in water, soil, humans, and animals and can colonize healthcare‐related sites. 1 , 2 K. pneumoniae could colonize a variety of sites in human body, for example, axilla, intestinal tract, and nasopharynx. 3 , 4 , 5 In the past decades, K. pneumoniae was of greater and greater concern worldwide, mainly due to its enhanced resistance and recently focused hypervirulence. 6 , 7 Despite SHV‐1 β‐lactamase, encoded on the chromosome and being intrinsic resistance, K. pneumoniae could antagonize antimicrobials though various pathways, for example, hydrolyzing enzymes, missing porins, efflux overexpression, topoisomerase, and lipopolysaccharide (LPS) modification. 8 Alarmingly, carbapenem‐resistant K. pneumoniae (CR‐KP) has reached a rate of over 30.0% among K. pneumoniae strains and brought formidable challenges in clinical practice. 9 In 2016, The World Health Organization (WHO) made a list of the most “critical” bacteria at a global level with an urgent need for new treatments, among which carbapenem‐resistant Enterobacteriaceae (CRE) was classified as a critical priority organism.
CR‐KP could come into existence via multiple mechanisms, that is, (i) production of carbapenemase; (ii) decreased expression or loss of outer membrane proteins with overexpression of extended‐spectrum β‐lactamases and AmpC cephalosporinases; and (iii) activation of efflux pumps. 10 Carbapenemases include K. pneumoniae carbapenemases, New Delhi metallo‐β‐lactamase, Verona integron‐encoded metallo‐β‐lactamase, imipenemase, and oxacillinase. 11 Disruption or deficiency of OmpK35 and OmpK36 could result in CR‐KP. 12 Overexpression of efflux pumps also confers carbapenem resistance. 13 While the reasons of CR‐KP differ remarkably worldwide, it is dominantly conferred by the mobile genetic elements harboring a variety of antibiotic‐resistance genes, for example, beta lactamase K. pneumoniae carbapenemases gene (bla KPC), New Delhi metallo‐β‐lactamase gene (bla NDM), and oxacillinase‐48 gene (bla OXA‐48). 14 , 15
Hypervirulent K. pneumoniae (HvKP), first recognized as a unique clinical pathogen in the 1980s in Taiwan, 16 is an entity of being more virulent than classical K. pneumoniae (cKP), which is determined by animal (mice, wax moth larvae) lethality tests, neutrophil assay, and so on. 17 , 18 , 19 cKP is often associated with nosocomial infections, for example, pneumonia, urinary tract infection (UTI), and bacteremia, among those at extremes of age or with underlying immunodeficiencies while HvKP often induces infections, such as pyogenic liver abscess (PLA), lung abscess, endophthalmitis, in otherwise healthy individuals (Table 1). The prevalence of HvKP varies in different regions, ranging from 12% to 45% in HvKP‐endemic areas. 20 , 21 , 22 , 23 , 24 Recent studies unveiled a convergent trend of CR‐KP and HvKP, 25 which needs our deeper insights. Here, we try to focus on the determinants of HvKP, detection, and its evolution.
TABLE 1.
Characteristics of cKP and HvKP
| Parameters | cKP | HvKP | Hv‐CRKP or CR‐HvKP | References |
|---|---|---|---|---|
| Typical infections | Pneumonia, UTI, bacteremia | PLA; lung, neck and kidney abscesses; endophthalmitis; necrotizing fasciitis; meningitis; pneumonia; cellulitis; myositis; septic arthritis; osteomyelitis | Combined infections by cKP and HvKP | 19, 26, 27, 28, 29, 30, 31, 32, 33 |
| Susceptible populations | Immunocompromised (diabetics, patients with malignancies or transplant, bedridden individuals) | Diabetics, otherwise healthy individuals | Combined populations | 19, 20, 28, 29, 34, 35, 36, 37 |
| Serotypes | K1‐K79 | Mostly K1 and K2, seldom K5 and K57 | K64, K47, K20, K2 and K20 | 1, 28, 29, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50 |
| Siderophores (positive rates, %) | Enterobactin (100), yersiniabactin (17–46), salmochelin (2–4), aerobactin (6) | Enterobactin (100), yersiniabactin (90), salmochelin (>90), aerobactin (>93) | Enterobactin (100), yersiniabactin (90), salmochelin (40), aerobactin (>93) | 40, 51, 52, 53, 54, 55, 56 |
| Geographical prevalence | Worldwide | Mostly the Asian Pacific Rim, the trend to the world | Mainly Asia, the trend to the world | 29, 30, 32, 38, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76 |
| Commonly acquired infection type | Primarily nosocomial | Community acquired | Often nosocomial and seldom community‐acquired | 29, 59, 77, 78, 79, 80 |
| Drug‐resistance | Frequent (for example ESBLs and carbapenemase‐producing) | Rare except penicillin‐resistance | Carbapenemase‐producing | 9, 19, 28, 29, 81, 82 |
Abbreviations: cKP, classical Klebsiella pneumoniae; CR‐HvKP, carbapenem‐resistant hypervirulent Klebsiella pneumoniae; ESBLs, extended‐spectrum β‐lactamases; HvKP, hypervirulent Klebsiella pneumoniae; Hv‐CRKP, hypervirulent carbapenem‐resistant Klebsiella pneumoniae; PLA, pyogenic liver abscess; UTI, urinary tract infection ; PLA, pyogenic liver abscess.
2. CONCEPTION AND CLASSIFICATION
cKP is a group of K. pneumoniae that lacks hypercapsule, macromolecular exopolysaccharide, or excessive siderophores, which incurs a high median lethality dose (LD50) and rarely induces diseases in otherwise healthy individuals (except UTI) regardless of its being multidrug‐resistant (MDR). cKP often causes infections, for example, pneumonia, UTI, bacteremia, or meningitis in immunocompromised individuals. 26 , 27 , 28 cKP brings a LD50 of >107 colony forming unit (CFU) in a BALB/c mouse pneumonia model. 29 By contrast, HvKP is another type of K. pneumoniae that harbors hypercapsule, macromolecular exopolysaccharide, or excessive siderophores, which incurs a lower LD50 and induces infections in both immunocompromised and otherwise healthy individuals. HvKP yields a LD50 of <102 CFU in a BALB/c mouse pneumonia model. 29 , 30 HvKP could be divided into two categories: drug‐sensitive and MDR. Among MDR‐HvKP, hypervirulent carbapenem‐resistant K. pneumoniae (Hv‐CRKP) and carbapenem‐resistant hypervirulent K. pneumoniae (CR‐HvKP) are both notorious for their super drug‐resistance and hypervirulence, the former being much more frequent than the latter. The characteristics of cKP and HvKP are listed in Table 1. 31
The basis of Hv‐CRKP is classical CR‐KP while CR‐HvKP evolves from HvKP (serotypes K1, K2, K5, K10, K20, K25, K27, and K57) acquiring carbapenem‐resistant plasmids. 69 , 83 As reported in the document, 62 521 K. pneumoniae strains were collected from GenBank as of May 13, 2020; Molecular combinations predicted 29 strains to be bla KPC‐positive and hypervirulent, of which 7 were CR‐HvKP and 22 were Hv‐CRKP; 94 and 165 strains were HvKP and bla KPC‐positive K. pneumoniae, respectively. As of May 31, 2021, 890 K. pneumoniae genomes from GenBank were analyzed, and 53 Hv‐CRKP and 17 CR‐HvKP strains were designated 84 ; 478 and 168 strains were CR‐KP and HvKP, respectively. Among another 530 clinical K. pneumoniae strains collected in Mainland China from January 2017 to February 2018, 28 and 6 were Hv‐CRKP and CR‐HvKP respectively 84 ; 227 and 171 were CR‐KP and HvKP, respectively, showing constituent ratios of 12.3% for Hv‐CRKP among CRKP and 3.5% for CR‐HvKP among HvKP. Such surveillance results suggest Hv‐CRKP is far more prevalent than CR‐HvKP.
3. DETERMINANTS
3.1. Hypercapsule
Capsule (Figure 1) is an essential layer of polysaccharide bound on the surface protein Wzi of K. pneumoniae, 1 , 53 , 85 , 86 the loss of which would render K. pneumoniae remarkably less virulent or nonvirulent. 30 , 85 , 87 , 88 To date, there are in total 79 serotypes for K. pneumoniae. 52 In contrast with cKP, HvKP could produce a hypercapsule, which contributes to hypervirulence. 52 , 65 A basic production and the serotype of capsule is controlled by a chrosomal operon, cps, which harbors a couple of genes, that is, wzi, wza, wzb, wzc, gnd, wca, cpsB, cpsG, and galF 89 , 90 (Table 2). Sequencing of wzi and wzc could be a shortcut to determine serotypes of K. pneumoniae instead of a traditional serological method. 18 , 55 , 91 More importantly, hypercapsule could be regulated by several specific virulence genes, that is, c‐rmpA, c‐rmpA2, p‐rmpA, p‐rmpA2, and wzy‐K1. Genes c‐rmpA, c‐rmpA2, and wzy‐K1 are both in chromosome while p‐rmpA and p‐rmpA2 are plasmid‐borne. 92 , 93 , 94 Each of the 5 virulence genes could result in hypercapsule. However, their different combinations may yield different production of capsule. c‐rmpA and c‐rmpA2 in the ICEKp genomic island exist only in serotype K1 K. pneumoniae with a positive rate of <50.0%. 95 HvKP shows positive rates of 55–100% for rmpA or rmpA2 while cKP rarely harbors. 21 , 95 RmpA, described in 1989, is a regulator of mucoid phenotype in pK100, 96 along with RmpB, another virulence plasmid‐encoded regulator. 97
FIGURE 1.
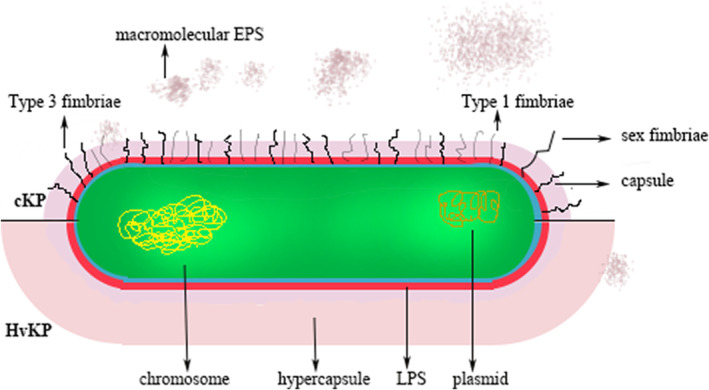
The schematic diagram of Klebsiella pneumoniae and the difference between cKP and HvKP
TABLE 2.
Locations of virulence‐related genes in this study
| Genes | Related factor | Plasmid | Chromosome |
|---|---|---|---|
| wzy‐K1, wzx, wzc, wza, wzb, wzi, gnd, wca, cpsA, cpsB, cpsG, galF | Capsule/exopolysaccharide | √ | |
| allS | Allantoin | √ | |
| rmpA, rmpB, rmpA2 | Capsule | √ | √ |
| kvrA, kvrB; rcsA, rcsB | Capsule | √ | |
| p‐rmpA, p‐rmpA2 | Capsule | √ | |
| c‐rmpA, c‐rmpA2 | Capsule | √ | |
| entABCDEF | Enterobactin | entC/E/F | √ |
| fepABCDG | Enterobactin | √ | √ |
| irp, ybt, fyu | Yersiniabactin | √ | √ |
| iroABCDE | Salmochelin | √ | √ |
| iucABCD, iutC | Aerobactin | √ | √ |
| fim | Type 1 fimbria | fimC/D | √ |
| mrkABCD | Type 3 fimbria | √ | |
| wb, waa, lpx, uge, wabG | LPS | √ | |
| pks, clbA‐S | Colibactin | √ | √ |
| terZA‐Z, terWXY | Tellurite resistance | √ | |
| silS | Silver resistance | √ | |
| peg‐344 | Metabolite transporter | √ |
Abbreviations: LPS, lipopolysaccharide.
The expression of rmpA depends on RcsB, KvrA, and KvrB. 98 KvrA, KvrB, and RcsB contribute to capsule regulation through the control of the rmpA promoter and through additional mechanisms. K. pneumoniae strains with deletions of kvrA and kvrB are less virulent than wild type. 99 Genes c‐rmpA, c‐rmpA2, p‐rmpA, and p‐rmpA2 positively regulate the cps locus at the transcriptional level. 94 , 97 , 100 , 101 Gene wzy‐K1 was firstly termed as magA, which was discovered in 2004 102 and specific to serotype K1 K. pneumoniae. 103 , 104 , 105 Wzy is a polymerase present in the inner membrane, 106 which combines Wzx into “Wzx/Wzy pathway” 107 or “Wzx/Wzy secretion system” 108 and is imperative for polymerization of undecaprenyl diphosphate‐linked K‐antigen sugar moiety in the periplasmic region. Wzy polymerizes the repeating oligosaccharides following their being flipped by Wzx in a blockwise manner. 109 Wzy then releases the oligosaccharide moiety from its lipid carrier to the nascent polymer. 110 In addition, the regulation of capsule A (cpsA) and B (cpsB) genes can also result in hypercapsule. Glucose could also be a signal to increase capsule production. 94 , 111
Capsule could protect K. pneumoniae through the following strategies: inhibiting phagocytosis by hosts' immune cells, preventing activation of the early immune response, and hampering lysis by complement and antimicrobial peptides. 31 Capsule confers resistance to opsonophagocytosis in K. pneumoniae regardless of opsonins. 112 Capsule could also contribute to biofilm formation, 113 , 114 which is contrary to another report. 115 Capsule induces a defective immunological host response, for example, maturation of dendritic cells (DCs) and pro‐Th1 cytokine production by hampering bacterial binding and internalization. 116 The rate of phagocytosis by immune cells is inversely proportional to the amount of capsule on the bacterial cell surface. Capsule induces DCs maturation with upregulation of CD83, CD86, and toll‐like receptor (TL) and downregulation of CD14 and DC‐SIGN. Capsule also suppresses the host immunological responses by inducing lower cytokines TNF‐α, IFN‐γ, and IL‐6 in the early stages of lung infection, reverse in the late stage. 88 , 117 In contrast, capsule also induces persistently higher IL‐10 level, which down‐regulates the expression of pro‐inflammatory cytokines. 117 Klebsiella inhibits Rac1 activation; and inhibition of Rac1 activity triggers a NOD1‐mediated CYLD and MKP‐1 expression, which in turn attenuates IL‐1β‐induced IL‐8 secretion. Purified capsular polysaccharide (CPS) neither reduces IL‐1β‐induced IL‐8 secretion nor induces the expression of CYLD and MKP‐1, thereby indicating that CPS is necessary but not sufficient to attenuate inflammation. 118 More immune cells were recruited to lungs infected with acapsular than capsular K. pneumoniae strains. 88 Capsule could protect K. pneumoniae against human defensin‐mediated bactericidal activity, 119 attenuate the production of human defensins in vitro, 119 and enhance pneumonia in mouse models. 120 , 121 , 122 The hypercapsule would render HvKP more prominent defense in comparison with cKP.
CPS, but not LPS O side chain is a major complement resistance factor in K. pneumoniae isolates due to its modulating the deposition of C3 and protecting the microorganisms against human alveolar macrophage phagocytosis. 87 , 123 Anionic capsule, but not cationic or uncharged, blocked the bactericidal activity of antimicrobial peptides or proteins, for example, human neutrophil defensin 1, beta‐defensin 1, lactoferrin, and protamine sulfate, by binding them, thereby reducing the amount of peptides reaching K. pneumoniae surface. 124 , 125 Capsule both protects K. pneumoniae from the bactericidal action of defensins and impedes their expression via the expression of CYLD and MKP‐1. 119
3.2. Macromolecular exopolysaccharide
Despite of polysaccharides bound on Wzi, namely CPS, Enterobacteriaceae including K. pneumoniae could also synthesize and secret a variety of extracellular saccharides with low or high molecular weight, included in which are alginate, cellulose, colonic acid, curdlan, dextran, diutan, gellan, hyaluronic acid, levan, succinoglycan, welan, and xanthan. 126 Among such exopolysaccharides, colonic acid is closely related to HvKP for its macromolecular weight and ability to form biofilm, which is produced via “Wzx/Wzy pathway”. 127 , 128 , 129
Exopolysaccharides could be produced via 4 pathways: (1) the so‐called Wzx/Wzy‐dependent pathway; (2) the ATP‐binding cassette (ABC) transporter‐dependent pathway; (3) the synthase‐dependent pathway and (4) the extracellular synthesis by use of a single sucrase protein. 126 “Wzx/Wzy pathway” is closely related with HvKP, which per se includes a couple of virulence proteins, that is, Wzx, Wzy, Wzc, Wza and Wzb. Hypervirulence via “Wzx/Wzy pathway” is limited to serotype K1 K. pneumoniae strains. Wzy‐K1, wzx, and wzc but not wza and wzb are always positive simultaneously, suggesting they are one integrity to form hypervirulence; The following knockout of each of them resulted in non‐mucoviscous colonies by “string test” 44 and disappeared macromolecular exopolysaccharides by Periodic Acid Schiff stain. 102
Wzx is a flippase 109 and Wzc is an inner membrane tyrosine autokinase. Following the action of Wzx and Wzy, nascent K antigen is translocated onto the bacterial surface by synergetic action of Wza, Wzc, and Wzb. 130 Wzc and Wzb control the length and amount of K antigen. 131 , 132 Wzc and Wzb are cognate low molecular weight phosphotyrosine phosphatase. 133 Wzc belongs to polysaccharide copolymerse 2a subfamily, which is essential for the assembly of Group 1 capsule 1. 134 Wza is an outer membrane translocon that translocates the nascent capsular polysaccharides on the bacterial surface. 135 , 136 Wza octamerizes across the periplasmic and outer membrane regions. 137 , 138 Wza is widely distributed in various K. pneumoniae including HvKP and cKP, loss of which induces ineffective exporting CPS. 139 , 140 To date, the structures and exact functions of Wzx, Wzy, Wzc, Wza, and Wzb were primarily elucidated in Escherichia coli, which is a close member with K. pneumoniae in Enterobacteriaceae.
3.3. Excessive siderophores
Iron is an essential element for bacteria to strive during infection, which is restricted by the host, 141 a process called nutritional immune. 142 Iron supply could affect the proliferation of K. pneumoniae and consequent bacterial count, which is also a vital factor for virulence. The majority of iron is deposited in a bound manner in the host, for example, transferrin and ferritin, little being free. Therefore, to capture iron is a prerequisite for K. pneumoniae to survive and propagate. 143 K. pneumoniae could harbor 4 kinds of siderophores, that is, enterobactin, yersiniabactin, salmochelin, and aerobactin, which possess higher affinity than host transport proteins and can thus steal iron successfully from hosts' iron‐chelating proteins. 143 At least one siderophore is harbored by K. pneumoniae with the positive rate of enterobactin being 100.0%. The affinity of the 4 siderophores is not equal, ranging from aerobactin with the lowest to enterobactin with the highest. 144 , 145
Enterobactin is the basic iron uptake system in K. pneumoniae, 57 , 146 , 147 , 148 with its biosynthesis and transport being encoded by the chromosomal gene cluster entABCDEF and fepABCDG. 58 , 149 Enterobactin could be neutralized by the host‐secreted lipocalin‐2, 59 , 150 , 151 which has several antimicrobial capabilities and is secreted by many cell lineages, for example, neutrophils. Lipocalin‐2 is upregulated by the host in the respiratory tract infection of K. pneumoniae. 152 , 153 , 154 In addition, lipocalin‐2 also has proinflammatory effects, leading to neutrophil recruitment to the site of infection via IL‐8. 155 , 156 The presence of lipocalin‐2 aids in the clearance of K. pneumoniae with only one siderophore: enterobactin. 60
Yersiniabactin is another “basic” siderophore in K. pneumoniae with a positive rate of over 75.0%, 62 which originated from Yersinia and is also encoded by chromosome. Yersiniabactin is positive in 18% of cKP strains and 90% in HvKP. 58 , 60 irp gene cluster encodes proteins for yersiniabactin synthesis, ybt and fyu encode transporters for the secretion of enterobactin and ybtO encodes the uptake receptor of enterobactin. 58 , 59 , 157 During lung infection, yersiniabactin together with enterobactin is highly expressed and it is not inhibited by lipocalin‐2 in vivo. 59 , 60 , 143 Yersiniabactin alone is not capable of acquiring the iron for K. pneumoniae and lack of the other 3 siderophores would render K. pneumoniae not capable of disseminating from the lungs, 60 which may be the reason why the positive rate of enterobactin is 100.0%. In addition to lung infection, yersiniabactin may be important for K. pneumoniae to induce PLA. 58 , 158
Salmochelin is an additional siderophore in K. pneumoniae, which is per se a c‐glucosylated form of enterobactin. 159 , 160 The c‐glucosylation is carried out by iro gene cluster, that is, iroABCDE on either the chromosome or a plasmid. 58 IroN contributes to the transport of salmochelin carrying iron. 149 , 161 Salmochelin is not neutralized by lipocalin‐2 159 and induces K. pneumoniae colonization of the nasopharynx 155 and consequent pneumonia. Salmochelin is seldom present in cKP with a rate of 2–4% but usual in HvKP with a rate of > 90%. 57 , 58 , 60
Aerobactin is a citrate‐hydroxamate siderophore, which is rarely present in cKP with a rate of ~ 6% but common in HvKP rating over 90.0%. 46 , 57 , 61 , 146 Aerobactin is usually associated with a hypercapsule while K. pneumoniae with hypercapsule does not inevitably harbor aerobactin. 46 , 61 , 143 Aerobactin is controlled by the gene cluster iucABCD and its transport is determined by iutA, both of which are often present on the same pLVPK‐like plasmids carrying p‐rmpA. 58 , 93 , 97 , 162 , 163 Aerobactin is not neutralized by lipocalin‐2. Aerobactin is crucial for some HvKP causing lung infection 158 and it accounts for the majority of the total siderophores in HvKP. 164 Aerobactin, but not enterobactin, yersiniabactin, or salmochelin, is essential for successful infection by HvKP in pneumonic and subcutaneous mouse infection models. 158 , 164 However, in a mouse i.p. HvKP infection model, aerobactin along with yersiniabactin and salmochelin is seemingly redundant unless the 3 are all deleted. 58
In addition to the aforementioned 4 siderophores, other iron‐stealing systems may also support the pathogenesis of HvKP. K. pneumoniae NTUH‐K2044 also encodes ferrichrome, ferric, ferrous, and haem‐iron uptake systems. 58 Intriguingly, another study reported a CR‐HvKP strain with a positive string test and K2 ST14 types, 165 which lacked rmpA, rmpA2, and yersiniabactin, but harbored receptors (not biosynthetic genes) for aerobactin and salmochelin. It suggests curious capability of “stealing” siderophores from other species with their coexistence and the following transformation into being hypervirulent.
3.4. Other virulence factors
Apart from hypercapsule, exopolysaccharide, and excessive siderophores, which are all vital for HvKP, HvKP could also possess a couple of other virulence factors, such as fimbriae, LPS, colibactin, tellurite and silver resistance and allantoin metabolism.
Fimbriae in KP are classified as type 1 and 3 with type 1 being thin, thread‐like protrusions and type 3 being helix‐like filaments. 31 Type 1 fimbriae, encoded by fim gene cluster, 31 are widely distributed in K. pneumoniae with a positive rate of over 90.0%, 166 , 167 which was over 95.0% in our study. 62 Type 3 fimbriae, encoded by mrkABCD gene cluster, 168 are “mannose insensitive” and therefore do not bind mannose. Type 1 fimbriae contribute to UTI 169 and biofilm formation, including on urinary catheters. 170 Type 3 fimbriae have been shown to bind extracellular matrix proteins such as type IV and V collagens 171 and contribute to biofilm formation. 170 , 172 Type 3 fimbriae are also over 95.0% positive in K. pneumoniae strains. 62
LPS, also termed as endotoxin, typically consists of an O antigen, a core oligosaccharide and lipid A, which are ubiquitous and encoded by wb, waa, and lpx gene clusters respectively. 173 , 174 , 175 , 176 LPS protects K. pneumoniae against humoral defenses and also serves as a strong immune activator. Lipid A is a potent ligand for TLR4, which leads to the production of cytokines and chemokines, followed by the recruiting and activating of neutrophils and macrophages. Lipid A protects K. pneumoniae against some cationic antimicrobial peptides. 177 , 178 K. pneumoniae strains with K1, K10, and K16 serotypes could mask their LPS, 179 which was also found in HvKP. 180 K. pneumoniae could also modify its LPS as unrecognizable by certain immune receptors. 177 For K. pneumoniae, LPS is the primary means of protection against complement, even in the presence of capsule. 179 The O antigen of LPS protects against C3 by binding C3b and abrogates pore formation. 179 , 181 , 182 , 183 uge, which encodes a UDP galacturonate 4‐epimerase, and wabG, which encodes a GalA transferase, are also involved in the production of LPS. 184 , 185
Colibactin, also termed as “genotoxin,” is a natural and genotoxic chemical compound, which is encoded by pks genomic island with a length of 54 Kb. 186 The pks island represents a total of 19 genes, that is, clbA to clbS. 186 The pks locus is usually present in a chromosomal integrative and conjugative element (ICE). 47 , 187 Colibactin could induce DNA double‐strand breaking, chromosome aberrations, and cell cycle arrest in the G2/M phase. 186 , 188 In addition, colibactin contributes to colonization and survival of K. pneumoniae 189 and the global spread of clonal group (CG) 23. 190 The pks‐related island could also encode numerous compounds 191 and contribute to the anti‐inflammatory, 192 antibiotic, 193 , 194 and analgesic effects 195 for colibactin‐producing K. pneumoniae. Prevalence of pks differs in different regions, ranging from 3.5% (5/141) in Europe to 25.6% (53/207) in Taiwan. 187 , 196 In particular, pks is highly prevalent in serotype K1 K. pneumoniae rating from 71.4% (35/41) and 78.8% (26/33). 187 , 197 Carriage of rmpA, iutC, and ybtA was significantly higher in the pks‐positive isolates than the pks‐negative isolates (95.5% vs. 13.2%, p < 0.001), which indicates the emerging pks genotoxic trait is associated with increasing HvKP strains. 197
Tellurite and silver resistance is encoded by terZA to Z, terWXY and silS, respectively, which may be important for systemic infections. 163 , 198 Such genes are in the virulence plasmid and not HvKP specific, 92 , 199 loss of which decreases tellurite and silver resistance in K. pneumoniae but did not affect virulence in a mouse pneumonia model. 199
Allantoin metabolism is one pathway for K. pneumoniae to obtain carbon and nitrogen from allantoin, 200 a degradation product from nucleic acids that some microbials can use as a source of nitrogen. 201 Allantoin metabolism is under control of all gene cluster 202 ; all gene cluster is enriched in strains associated with PLA versus commensal strains. 203 , 204 allS is present in K1 but no other serotype HvKP causing PLA with a rate of 100.0%, 46 which suggests an association with K1 serotype. However, if specimen types are of consideration, allS is not inevitably in line with K1 serotype, vice versa. The absence of allS does not reduce virulence. 46
Peg‐344 is a metabolite transporter encoded by HvKP virulence plasmid. 205 The exact function of peg‐344 needs to be elucidated. Peg‐344 could increase RNA abundance when K. pneumoniae is grown in human ascites, which suggests Peg‐344 may transport an unidentified growth factor present in ascites. 205 It may be per se a coincidence that peg‐344 could be a rather good indicator of HvKP because of its being in virulence plasmid.
4. ROLE OF RACE FACTOR
PLA is a typical infection caused by HvKP. PLA is endemic in East Asia, 69 , 75 , 83 , 206 which indicates a close relationship between certain races and HvKP. In North America, 78.3% of patients were of Asian origin, 63 who were with K. pneumoniae‐induced PLA. A surveillance showed high carriage rate of K. pneumoniae (21.1%) in Korean intestinal tract 207 ; among the strains, 23.0% were K1 serotype, predominant in PLA‐derived K. pneumoniae strains. 208 Chinese ethnicity is also a major factor predisposing to intestinal colonization by serotype K1/K2 K. pneumoniae isolates. 3 The colonization of HvKP in intestinal tract is a vital step for the formation of PLA. As carbapenem resistance is taken into consideration, CR‐HvKP and Hv‐CRKP are mainly distributed in healthcare settings 209 and presents no significant correlation with race.
On the contrary, the role of race factor is some limited. For instance, immigration to western countries decreased the carriage of K. pneumoniae (5.6% vs 24.1%, p = 0.024) in Korean intestinal tract, 207 which indicates the important role of environmental factors. 210
5. DETERMINATION
To determine a K. pneumoniae strain as HvKP, the golden standard should be animal tests, such as mouse lethality test, 15 Galleria mellonella lethality test. 18 , 19 , 25 Besides, serum killing assay is also an alternative method. 211 However, the aforementioned tests are cumbersome, sometimes confusing 212 and not ready to use for clinical laboratories. It is essential to find novel methods of determining HvKP for clinical practice.
“String test” was once used for HvKP. The formation of a viscous string >5 mm is considered positive when the colony is stretched out using a loop from a blood‐agar plate. 102 “String test” showed a sensitivity of 89% and a specificity of 91%, 92 which are inconsistent with another report. 213 Sequence types (ST) and serotypes are both not so specific for HvKP, but the virulence gene repertoire may be one optimal clue to choose markers. 54 , 65 , 81 , 214 Virulence genes in the virulence plasmids (for example, pK2044 and pLVPK) 47 , 93 , 215 are more accurate for defining HvKP than those in integrative and conjugative elements (ICE), 187 , 190 , 197 , 216 among which are genes encoding yersiniabactin and colibactin, not vital for hypervirulence. Nevertheless, iroB, iucA, peg‐344, rmpA, and rmpA2 in the virulence plasmids are all optimal biomarkers for defining HvKP, 92 among which iuc and/or either rmpA or rmpA2 would be the best combined markers. In the recent report of 5 HvKP strains, 25 iro, peg‐344, and rmpA were all negative in the relevant plasmid. With the recognition on molecular biomarkers progressing for defining HvKP, novel single or combined markers are likely to be designated in the near future.
On the other hand, hypervirulence of K. pneumoniae is restricted by series of virulence genes (Table 2), which means that the polymorphism and deletion of any of such genes may affect the eventual virulence. The lack of hypervirulent phenotype in virulence plasmid‐bearing CR‐KP strains was found to be due to the mutation's presence on rmpA and rmpA2 genes, which rendered them non‐functional, while some strains carrying wild type rmpA did not exhibit hypervirulent phenotype either suggesting that other factors might also contribute to the hypervirulence of CR‐KP. 217 A large proportion (58%) of CR‐KP strains in China mainland during 2014–2017 were found to harbor a virulence plasmid, while only 13% of such strains exhibited a hypervirulent phenotype by string test and neutrophil assay. 217 Therefore, the complexity of virulence in K. pneumoniae indicates the molecular combinations for hypervirulence need further investigations and optimizations.
6. EVOLUTION
Clonal group (CG) 23 is associated with K1 capsule and HvKP, which accounts for over 30% of (ST) for HvKP‐inducing PLA. 214 , 218 , 219 , 220 Another study showed over 80% of CG23 K. pneumoniae strains inducing PLA belong to CG23‐I, which emerged in ~1928 following acquisition of ICEKp10, and then disseminated globally 190 ; Ninety‐four of the 97 strains possessed plasmid‐borne iro, iuc, rmpA, and rmpA2. 190 The possible dates for the most recent common ancestors for the entire CG23 population, the CG23‐I sublineage, and the equine strains could be 1878, 1928, and 1972, respectively.
Plasmids pK2044 (224,152 bp) and pLVPK (219,385 bp) 93 , 215 are the 2 classical ones conferring hypervirulence in K. pneumoniae, 97 , 162 , 163 which is mediated through iuc, rmpA, and rmpA2 genes. pK2044 and pLVPK are highly similar. 220 , 221 Their descendants varied in the length, for example, 121 Kb, 90 Kb, 200 Kb, and 178 Kb. 25 , 47 , 220
The first KPC‐producing K. pneumoniae was found in a patient in a North Carolina Hospital in 1996, 222 while the first KPC‐2‐producing K. pneumoniae in China was found in Zhejiang Province in 2007. 223 Furthermore, the first Hv‐CRKP, showing K2 type and ST65 emerged in China in 2013. 15 Another recent study unveiled an outbreak of Hv‐CRKP with ST11 and a pLVPK‐like virulence plasmid pVir‐CR‐hvKP4 (178,154 bp). 25 Compared with pLVPK, pVir‐CR‐hvKP4 had a deletion of 41,231 bp fragment, which included the virulence genes rmpA and iro. Another report from Taiwan investigated a strain TVGHCRE225 with ST11 and a pVir plasmid (297,984 bp), a hybrid HvKp virulence plasmid. 224 38% of pVir shared 49% and 47% of identities with pK2044 and pLVPK, respectively, the remaining portion possessing 61% coverage with pPMK‐NDM, a resistance plasmid, at 99% identity.
MDR and extremely drug‐resistant (XDR) HvKP are concerning pathogens, which accounts for 7.4–15.0% among CR‐KP 225 albeit 57% (20/35) HvKP strains inducing bacteremia were concurrently CR‐KP in a 2016 investigation from China. 226 Hv‐CRKP and CR‐HvKP, the most notorious ones among MDR and XDR HvKP, are now seemingly emerging worldwide. 25 , 227 Such evolution may occur through 2 mechanisms. The first pathway is via HvKP acquiring a plasmid carrying drug resistance determinants 228 , 229 or by the insertion of resistance genes into virulence plasmid or chromosome harbored by HvKP. 23 , 230 The second pathway is via MDR/XDR cKP acquiring a pK2044‐ or pLVPK‐like virulence plasmid or integrated virulence genes into drug resistance plasmid. 25 The virulence plasmids themselves are usually non‐conjugative and therefore non‐self‐mobilizable. However, they could be mobilized or co‐transferred with the help of the self‐transferable incompatibility group F plasmids. 84 , 231 Due to the hypercapsule of HvKP itself, which could mask the fimbriae and hamper the conjugation, the second pathway may be more convenient. Hv‐CRKP is far more prevalent than CR‐HvKP. 84 ST11 accounted for over 70.0% among CR‐HvKP/Hv‐CRKP strains. 62 However, such overall assessment is lacking (Table 3). 7 Hv‐CRKP and CR‐HvKP are both still progressing, obtaining other resistance, for example, to colistin. 232
TABLE 3.
Characteristics of Hv‐CRKP and CR‐HvKP
| Classification | Location | ST | Serotype | Clinical context | Note | References |
|---|---|---|---|---|---|---|
| Hv‐CRKP | China | ST11 | K47 | Ventilator‐associated pneumonia | Few cases: 5 isolates; XDR; 178 Kb pLVPK‐like plasmid; One clone | 25 |
| Hv‐CRKP | China | ST11 | K47 | Retrospective study | One case; pLVPK‐like plasmid and 2 drug resistance plasmid; unique feature of 5 tandem copies of bla KPC‐2 | 233 |
| CR‐HvKP | USA | ST23 | K1 | UTI | One case; bla SHV‐36, fosA, oqxAB on chromosome; drug resistance plasmid with bla KPC‐2, bla TEM‐1A and truncated bla OXA‐9 | 227 |
| CR‐HvKP | China | ST23 | K1 | Sepsis | One case; pLVPK‐like plasmid with insertion of bla KPC‐2 and dfrA14 | 23, 234 |
| CR‐HvKP | China | ST36 | K62 | Bloodstream, burn wounds | One case; pLVPK‐like plasmid and drug resistance plasmid with bla KPC‐2, fosA, oqxAB | 229 |
| CR‐HvKP | China | ST86 | K2 | Burn wound | One case; bla KPC‐2 and bla NDM‐1; 215 Kb virulence plasmid | |
| CR‐HvKP | China | ST65 | K2 | Septicemia | One case; Encodes enterobactin and aerobactin but not yersiniabactin or kfu; bla SHV‐11, bla TEM‐53, ompK35/36 decreased expression | |
| CR‐HvKP | Canada | ST86 | KL2 | UTI | One case; Plasmid with bla KPC‐2 as well as bla SHV‐1 and fosA | 236 |
Abbreviations: CR‐HvKP, carbapenem‐resistant hypervirulent Klebsiella pneumoniae; Hv‐CRKP, hypervirulent carbapenem‐resistant Klebsiella pneumoniae; ST, sequence type; XDR, extreme drug resistance; UTI, urinary tract infection.
Integration and conjugation (Figure 2) are two primary means of acquiring foreign nucleic acids. tRNA sites are often targets for integration and 73% of K. pneumoniae strains had an ICE inserted into one or more of the four asparagine tRNA genes. 216 ICEKp1, 76 Kb long in NTUH‐2044, was more prevalent in HvKP than in cKP: 38/42 vs 5/32. 237 Later, a more widely conserved ICEKp (KPHPI208‐GM1 also designated ICEKp10) was described to possess 8 genomic modules, 187 which is ubiquitous in CG23 HvKP strains. 47 ICEKp10, the most common ICE, was found in 77% of ST23, 40% of ST258, and in 25 other STs strains. 238 However, ICEKp10 is poorly conserved in non‐CG23 HvKP. The comparative analysis of 97 CG23 genomes showed the 81 members of sublineage CG23‐I had acquired ICEKp10 containing genes that encode yersiniabactin and colibactin. 190 , 238 ICEKp is widely distributed in both cKP and HvKP, which bore 14 variants. 238 ICE could also carry other virulence genes apart from those encoding yersiniabactin and colibactin. ICEKp1 encodes RmpA and salmochelin. 237 In addition, the integration of other ICE or genomic islands is also a commonplace. 216 , 239 The yersiniabactin carried by ICE is more beneficial in cKP than in HvKP. 60 , 158 Colibactin contributes to colonization, mucosal invasion, and/or dissemination of K. pneumoniae. 189 Furthermore, the other factors carried by ICEKp or other genomic islands may prove to play critical roles in various settings.
FIGURE 2.
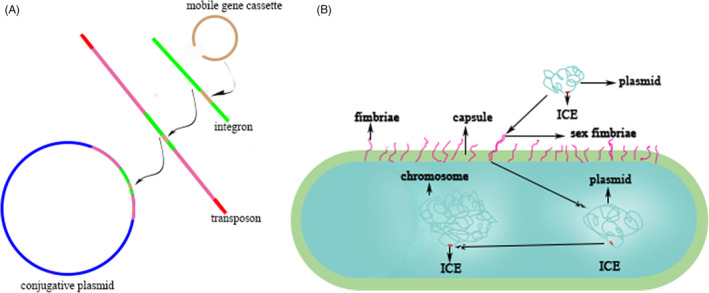
The formation and transfer of drug resistance or virulence elements. (A) the formation of drug resistance or virulence elements. (B) the transfer of drug resistance or virulence elements
The clustered regularly interspaced short palindromic repeats (CRISPR) and CRISPR associated proteins (Cas), restriction and modification (R‐M) are the two primary immune systems in bacteria, which are both responsible for surveillance of nucleic acids and may play salient roles in horizontal gene transfer (HGT) of drug resistance or virulence genes. 240 CRISPR‐Cas system could cut exogenous DNA by targeting proto‐spacers. 241 , 242 , 243 Recent studies 244 , 245 confirmed that CG258 K. pneumoniae strains lack type I‐E CRISPR‐Cas system and another investigation suggested such absence is associated with the dissemination of IncF epidemic drug resistance plasmids in CG258. 246 Furthermore, CG258 K. pneumoniae strains also has an impaired R‐M system. 247 Type I R‐M system consists of HsDR (slicing), HsdM (methylating) and HsdS (targeting), 240 , 248 which could hamper invasion of foreign DNA via methyltransferase and restriction endonuclease. 248 In addition, CRISPR‐Cas and R‐M systems could cooperate to combat exogenous nucleic acids. 249 , 250 Therefore, lack of CRISPR‐Cas and R‐M renders CG258 more propense to acquire outer plasmids carrying drug resistance or virulence genes, which is called HGT. Armed with extreme drug resistance and virulence, CR‐HvKP and Hv‐CRKP strains can then survive better in both community and healthcare settings and cause consequent both vertical transfer and HGT, forming a vicious cycle. KPC(+) ST11 CR‐KP accounted for 11 mobile genetic element clusters with type A and F sharing 20.83% and 54.76%, respectively, 251 showing an evident combination of HGT and vertical transfer which is likely present in Hv‐CRKP.
7. CONCLUSIONS
Klebsiella pneumoniae was first described in the late 19th century, which was more and more documented for its hypervirulence and drug resistance. Hypervirulence of K. pneumoniae is primarily dependent on some factors, that is, hypercapsule, macromolecular exopolysaccharide, or excessive siderophores albeit K. pneumoniae harbors numerous virulence genes. Molecular determination of HvKP is a practical pathway in contrast with the traditional laborious lethality tests while further improvement is needed. HvKP, including Hv‐CRKP and CR‐HvKP, could bring great challenges worldwide in clinical practice in the future due to their extreme virulence and drug resistance. The impaired immune systems (CRISPR‐Cas and R‐M) could enhance such trend.
As confirmed now and speculated in the future, Hv‐CRKP is far more prevalent than CR‐HvKP. They would not bring more and more novel virulence, but present more and more drug resistance, for example, polymyxin resistance. Their targeted options are rather limited. More researches are needed to be done to fight against it.
AUTHOR CONTRIBUTIONS
Piaopiao Dai and Dakang Hu conceived of and wrote the review, which was revised by Dakang Hu.
FUNDING INFORMATION
The study is supported by Zhejiang Medical and Health Research Program (2023KY1326).
CONFLICT OF INTEREST
None declared.
Dai P, Hu D. The making of hypervirulent Klebsiella pneumoniae . J Clin Lab Anal. 2022;36:e24743. doi: 10.1002/jcla.24743
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
REFERENCES
- 1. Podschun R, Ullmann U. Klebsiella spp. as nosocomial pathogens: epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin Microbiol Rev. 1998;11(4):589‐603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Podschun R, Pietsch S, Höller C, Ullmann U. Incidence of Klebsiella species in surface waters and their expression of virulence factors. Appl Environ Microbiol. 2001;67(7):3325‐3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lin YT, Siu LK, Lin JC, et al. Seroepidemiology of Klebsiella pneumoniae colonizing the intestinal tract of healthy Chinese and overseas Chinese adults in Asian countries. BMC Microbiol. 2012;12:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lima AB, de Oliveira Leão LS, Oliveira LS, Pimenta FC. Nasopharyngeal Gram‐Negative bacilli colonization in brazilian children attending day‐care centers. Braz J Microbiol. 2010;41(1):24‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kloos WE, Musselwhite MS. Distribution and persistence of Staphylococcus and Micrococcus species and other aerobic bacteria on human skin. Appl Microbiol. 1975;30(3):381‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. De Oliveira DMP, Forde BM, Kidd TJ, et al. Antimicrobial resistance in ESKAPE pathogens. Clin Microbiol Rev. 2020;33(3):e00181‐199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choby JE, Howard‐Anderson J, Weiss DS. Hypervirulent Klebsiella pneumoniae – clinical and molecular perspectives. J Intern Med. 2020;287(3):283‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hennequin C, Robin F. Correlation between antimicrobial resistance and virulence in Klebsiella pneumoniae . Eur J Clin Microbiol Infect Dis. 2016;35(3):333‐341. [DOI] [PubMed] [Google Scholar]
- 9. Effah CY, Sun T, Liu S, Wu Y. Klebsiella pneumoniae: an increasing threat to public health. Ann Clin Microbiol Antimicrob. 2020;19(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lan P, Jiang Y, Zhou J, Yu Y. A global perspective on the convergence of hypervirulence and carbapenem resistance in Klebsiella pneumoniae . J Glob Antimicrob Resist. 2021;25:26‐34. [DOI] [PubMed] [Google Scholar]
- 11. Lai CC, Yu WL. Klebsiella pneumoniae harboring carbapenemase genes in taiwan: its evolution over 20 years, 1998‐2019. Int J Antimicrob Agents. 2021;58(1):106354. [DOI] [PubMed] [Google Scholar]
- 12. Kaczmarek FM, Dib‐Hajj F, Shang W, Gootz TD. High‐level carbapenem resistance in a Klebsiella pneumoniae clinical isolate is due to the combination of bla(ACT‐1) beta‐lactamase production, porin OmpK35/36 insertional inactivation, and down‐regulation of the phosphate transport porin phoe. Antimicrob Agents Chemother. 2006;50(10):3396‐3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li XZ, Plesiat P, Nikaido H. The challenge of efflux‐mediated antibiotic resistance in Gram‐negative bacteria. Clin Microbiol Rev. 2015;28(2):337‐418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee CR, Lee JH, Park KS, Kim YB, Jeong BC, Lee SH. Global dissemination of carbapenemase‐producing Klebsiella pneumoniae: epidemiology, genetic context, treatment options, and detection methods. Front Microbiol. 2016;7:895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang Y, Zeng J, Liu W, et al. Emergence of a hypervirulent carbapenem‐resistant Klebsiella pneumoniae isolate from clinical infections in China. J Infect. 2015;71(5):553‐560. [DOI] [PubMed] [Google Scholar]
- 16. Liu YC, Cheng DL, Lin CL. Klebsiella pneumoniae liver abscess associated with septic endophthalmitis. Arch Intern Med. 1986;146(10):1913‐1916. [PubMed] [Google Scholar]
- 17. Deleo FR, Chen L, Porcella SF, et al. Molecular dissection of the evolution of carbapenem‐resistant multilocus sequence type 258 Klebsiella pneumoniae . Proc Natl Acad Sci USA. 2014;111(13):4988‐4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Diago‐Navarro E, Chen L, Passet V, et al. Carbapenem‐resistant Klebsiella pneumoniae exhibit variability in capsular polysaccharide and capsule associated virulence traits. J Infect Dis. 2014;210(5):803‐813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. McLaughlin MM, Advincula MR, Malczynski M, Barajas G, Qi C, Scheetz MH. Quantifying the clinical virulence of Klebsiella pneumoniae producing carbapenemase Klebsiella pneumoniae with a Galleria mellonella model and a pilot study to translate to patient outcomes. BMC Infect Dis. 2014;14:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lan Y, Zhou M, Jian Z, Yan Q, Wang S, Liu W. Prevalence of pks gene cluster and characteristics of Klebsiella pneumoniae‐induced bloodstream infections. J Clin Lab Anal. 2019;33(4):e22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Li W, Sun G, Yu Y, et al. Increasing occurrence of antimicrobial‐resistant hypervirulent (hypermucoviscous) Klebsiella pneumoniae isolates in China. Clin Infect Dis. 2014;58(2):225‐232. [DOI] [PubMed] [Google Scholar]
- 22. Liu Z, Gu Y, Li X, et al. Identification and characterization of NDM‐1‐producing hypervirulent (Hypermucoviscous) Klebsiella pneumoniae in China. Ann Lab Med. 2019;39(2):167‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang R, Lin D, Chan EWC, Gu D, Chen GX, Chen S. Emergence of carbapenem‐resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother. 2016;60(1):709‐711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Liu C, Shi J, Guo J. High prevalence of hypervirulent Klebsiella pneumoniae infection in the genetic background of elderly patients in two teaching hospitals in China. Infect Drug Resist. 2018;11:1031‐1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gu D, Dong N, Zheng Z, et al. A fatal outbreak of ST11 carbapenem‐resistant hypervirulent Klebsiella pneumoniae in a Chinese hospital: a molecular epidemiological study. Lancet Infect Dis. 2018;18(1):37‐46. [DOI] [PubMed] [Google Scholar]
- 26. Wang Y, Zhang Q, Jin Y, Jin X, Yu J, Wang K. Epidemiology and antimicrobial susceptibility profiles of extended‐spectrum beta‐lactamase‐producing Klebsiella pneumoniae and Escherichiacoli in China. Braz J Microbiol. 2019;50(3):669‐675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Meatherall BL, Gregson D, Ross T, Pitout JDD, Laupland KB. Incidence, risk factors, and outcomes of Klebsiella pneumoniae bacteremia. Am J Med. 2009;122(9):866‐873. [DOI] [PubMed] [Google Scholar]
- 28. Korvick JA, Hackett AK, Yu VL, Muder RR. Klebsiella pneumonia in the modern era: clinicoradiographic correlations. South Med J. 1991;84(2):200‐204. [DOI] [PubMed] [Google Scholar]
- 29. Fodah RA, Scott JB, Tam HH, et al. Correlation of Klebsiella pneumoniae comparative genetic analyses with virulence profiles in a murine respiratory disease model. PLoS One. 2014;9(9):e107394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lawlor MS, Hsu J, Rick PD, Miller VL. Identification of Klebsiella pneumoniae virulence determinants using an intranasal infection model. Mol Microbiol. 2005;58(4):1054‐1073. [DOI] [PubMed] [Google Scholar]
- 31. Paczosa MK, Mecsas J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol Mol Biol Rev. 2016;80(3):629‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patel PK, Russo TA, Karchmer AW. Hypervirulent Klebsiella pneumoniae . Open Forum Infect Dis. 2014;1(1):ofu028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ko WC, Paterson DL, Sagnimeni AJ, et al. Community‐acquired Klebsiella pneumoniae bacteremia: global differences in clinical patterns. Emerg Infect Dis. 2002;8(2):160‐166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang Q, Jia X, Zhou M, et al. Emergence of ST11‐K47 and ST11‐K64 hypervirulent carbapenem‐resistant Klebsiella pneumoniae in bacterial liver abscesses from China: a molecular, biological, and epidemiological study. Emerg Microbes Infect. 2020;9(1):320‐331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Xu M, Fu Y, Fang Y, et al. High prevalence of KPC‐2‐producing hypervirulent Klebsiella pneumoniae causing meningitis in Eastern China. Infect Drug Resist. 2019;12:641‐653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Monié M, Drieux L, Nzili B, et al. Klebsiella pneumoniae necrotizing fasciitis of the leg in an elderly French woman. Clin Interv Aging. 2014;9:1171‐1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ku YH, Chuang YC, Yu WL. Clinical spectrum and molecular characteristics of Klebsiella pneumoniae causing community‐acquired extrahepatic abscess. J Microbiol Immunol Infect. 2008;41(4):311‐317. [PubMed] [Google Scholar]
- 38. Prokesch BC, TeKippe M, Kim J, Raj P, TeKippe EME, Greenberg DE. Primary osteomyelitis caused by hypervirulent Klebsiella pneumoniae . Lancet Infect Dis. 2016;16(9):e190‐e195. [DOI] [PubMed] [Google Scholar]
- 39. Chang CM, Ko WC, Lee HC, Chen YM, Chuang YC. Klebsiella pneumoniae psoas abscess: predominance in diabetic patients and grave prognosis in gas‐forming cases. J Microbiol Immunol Infect. 2001;34(3):201‐206. [PubMed] [Google Scholar]
- 40. Tsay RW, Siu LK, Fung CP, Chang FY. Characteristics of bacteremia between community‐acquired and nosocomial Klebsiella pneumoniae infection: risk factor for mortality and the impact of capsular serotypes as a herald for community‐acquired infection. Arch Intern Med. 2002;162(9):1021‐1027. [DOI] [PubMed] [Google Scholar]
- 41. Cheng DL, Liu YC, Yen MY, Liu CY, Wang RS. Septic metastatic lesions of pyogenic liver abscess. Their association with Klebsiella pneumoniae bacteremia in diabetic patients. Arch Intern Med. 1991;151(8):1557‐1559. [PubMed] [Google Scholar]
- 42. Yang PC, Luh KT, Lee YC, et al. Lung abscesses: US examination and US‐guided transthoracic aspiration. Radiology. 1991;180(1):171‐175. [DOI] [PubMed] [Google Scholar]
- 43. Wang JL, Chen KY, Fang CT, Hsueh PR, Yang PC, Chang SC. Changing bacteriology of adult community‐acquired lung abscess in Taiwan: Klebsiella pneumoniae versus anaerobes. Clin Infect Dis. 2005;40(7):915‐922. [DOI] [PubMed] [Google Scholar]
- 44. Shon AS, Bajwa RP, Russo TA. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae: a new and dangerous breed. Virulence. 2013;4(2):107‐118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mizuta K, Ohta M, Mori M, Hasegawa T, Nakashima I, Kato N. Virulence for mice of Klebsiella strains belonging to the O1 group: relationship to their capsular (K) types. Infect Immun. 1983;40(1):56‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Yu WL, Ko WC, Cheng KC, Lee CC, Lai CC, Chuang YC. Comparison of prevalence of virulence factors for Klebsiella pneumoniae liver abscesses between isolates with capsular K1/K2 and non‐K1/K2 serotypes. Diagn Microbiol Infect Dis. 2008;62(1):1‐6. [DOI] [PubMed] [Google Scholar]
- 47. Struve C, Roe CC, Stegger M, et al. Mapping the evolution of hypervirulent Klebsiella pneumoniae . MBio. 2015;6(4):e00630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gomez‐Simmonds A, Uhlemann AC. Clinical implications of genomic adaptation and evolution of carbapenem‐resistant Klebsiella pneumoniae . J Infect Dis. 2017;215(suppl_1):S18‐S27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bialek‐Davenet S, Criscuolo A, Ailloud F, et al. Genomic definition of hypervirulent and multidrug‐resistant Klebsiella pneumoniae clonal groups. Emerg Infect Dis. 2014;20(11):1812‐1820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fung CP, Chang FY, Lee SC, et al. A global emerging disease of Klebsiella pneumoniae liver abscess: is serotype K1 an important factor for complicated endophthalmitis? Gut. 2002;50(3):420‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Lee IR, Molton JS, Wyres KL, et al. Differential host susceptibility and bacterial virulence factors driving Klebsiella liver abscess in an ethnically diverse population. Sci Rep. 2016;6:29316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yeh KM, Kurup A, Siu LK, et al. Capsular serotype K1 or K2, rather than magA and rmpA, is a major virulence determinant for Klebsiella pneumoniae liver abscess in Singapore and Taiwan. J Clin Microbiol. 2007;45(2):466‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rahn A, Beis K, Naismith JH, Whitfield C. A novel outer membrane protein, Wzi, is involved in surface assembly of the Escherichia coli K30 group 1 capsule. J Bacteriol. 2003;185(19):5882‐5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Holt KE, Wertheim H, Zadoks RN, et al. Genomic analysis of diversity, population structure, virulence, and antimicrobial resistance in Klebsiella pneumoniae, an urgent threat to public health. Proc Natl Acad Sci U S A. 2015;112(27):E3574‐E3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pan YJ, Fang HC, Yang HC, et al. Capsular polysaccharide synthesis regions in Klebsiella pneumoniae serotype K57 and a new capsular serotype. J Clin Microbiol. 2008;46(7):2231‐2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Follador R, Heinz E, Wyres KL, et al. The diversity of Klebsiella pneumoniae surface polysaccharides. Microb Genom. 2016;2(8):e000073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. el Fertas‐Aissani R, Messai Y, Alouache S, Bakour R. Virulence profiles and antibiotic susceptibility patterns of Klebsiella pneumoniae strains isolated from different clinical specimens. Pathol Biol (Paris). 2013;61(5):209‐216. [DOI] [PubMed] [Google Scholar]
- 58. Hsieh PF, Lin TL, Lee CZ, Tsai SF, Wang JT. Serum‐induced iron‐acquisition systems and TonB contribute to virulence in Klebsiella pneumoniae causing primary pyogenic liver abscess. J Infect Dis. 2008;197(12):1717‐1727. [DOI] [PubMed] [Google Scholar]
- 59. Lawlor MS, O'Connor C, Miller VL. Yersiniabactin is a virulence factor for Klebsiella pneumoniae during pulmonary infection. Infect Immun. 2007;75(3):1463‐1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Bachman MA, Oyler JE, Burns SH, et al. Klebsiella pneumoniae yersiniabactin promotes respiratory tract infection through evasion of lipocalin 2. Infect Immun. 2011;79(8):3309‐3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Vernet V, Philippon A, Madoulet C, Vistelle R, Jaussaud R, Chippaux C. Virulence factors (aerobactin and mucoid phenotype) in Klebsiella pneumoniae and Escherichia coli blood culture isolates. FEMS Microbiol Lett. 1995;130(1):51‐57. [DOI] [PubMed] [Google Scholar]
- 62. Hu D, Li Y, Ren P, et al. Molecular epidemiology of hypervirulent carbapenemase‐producing Klebsiella pneumoniae . Front Cell Infect Microbiol. 2021;11:661218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Nadasy KA, Domiati‐Saad R, Tribble MA. Invasive Klebsiella pneumoniae syndrome in North America. Clin Infect Dis. 2007;45(3):e25‐e28. [DOI] [PubMed] [Google Scholar]
- 64. Paterson DL, Hujer KM, Hujer AM, et al. Extended‐spectrum beta‐lactamases in Klebsiella pneumoniae bloodstream isolates from seven countries: dominance and widespread prevalence of SHV‐ and CTX‐M‐type beta‐lactamases. Antimicrob Agents Chemother. 2003;47(11):3554‐3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Yu WL, Ko WC, Cheng KC, et al. Association between rmpA and magA genes and clinical syndromes caused by Klebsiella pneumoniae in Taiwan. Clin Infect Dis. 2006;42(10):1351‐1358. [DOI] [PubMed] [Google Scholar]
- 66. McCabe R, Lambert L, Frazee B. Invasive Klebsiella pneumoniae infections, California, USA. Emerg Infect Dis. 2010;16(9):1490‐1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kashani AH, Eliott D. The emergence of Klebsiella pneumoniae endogenous endophthalmitis in the USA: basic and clinical advances. J Ophthalmic Inflamm Infect. 2013;3(1):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Mgbemena O, Serota DP, Kumar S, Wozniak JE, Weiss DS, Kempker RR. Peculiar purulence: Hypervirulent Klebsiella pneumoniae causing pyomyositis. Int J Infect Dis. 2017;65:90‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Siu LK, Yeh KM, Lin JC, Fung CP, Chang FY. Klebsiella pneumoniae liver abscess: a new invasive syndrome. Lancet Infect Dis. 2012;12(11):881‐887. [DOI] [PubMed] [Google Scholar]
- 70. Lederman ER, Crum NF. Pyogenic liver abscess with a focus on Klebsiella pneumoniae as a primary pathogen: an emerging disease with unique clinical characteristics. Am J Gastroenterol. 2005;100(2):322‐331. [DOI] [PubMed] [Google Scholar]
- 71. Odouard C, Ong D, Shah PR, et al. Rising trends of endogenous Klebsiella pneumoniae endophthalmitis in Australia. Clin Exp Ophthalmol. 2017;45(2):135‐142. [DOI] [PubMed] [Google Scholar]
- 72. Sturm E, Tai A, Lin B, et al. Bilateral osteomyelitis and liver abscess caused by hypervirulent Klebsiella pneumoniae‐ a rare clinical manifestation (case report). BMC Infect Dis. 2018;18(1):380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Decré D, Verdet C, Emirian A, et al. Emerging severe and fatal infections due to Klebsiella pneumoniae in two university hospitals in France. J Clin Microbiol. 2011;49(8):3012‐3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Sobirk SK, Struve C, Jacobsson SG. Primary Klebsiella pneumoniae Liver Abscess with Metastatic Spread to Lung and Eye, a North‐European Case Report of an Emerging Syndrome. Open Microbiol J. 2010;4:5‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Moore R, O'Shea D, Geoghegan T, Mallon PWG, Sheehan G. Community‐acquired Klebsiella pneumoniae liver abscess: an emerging infection in Ireland and Europe. Infection. 2013;41(3):681‐686. [DOI] [PubMed] [Google Scholar]
- 76. Shankar C, Veeraraghavan B, Nabarro LEB, Ravi R, Ragupathi NKD, Rupali P. Whole genome analysis of hypervirulent Klebsiella pneumoniae isolates from community and hospital acquired bloodstream infection. BMC Microbiol. 2018;18(1):6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Rafat C, Messika J, Barnaud G, et al. Hypervirulent Klebsiella pneumoniae, a 5‐year study in a French ICU. J Med Microbiol. 2018;67(8):1083‐1089. [DOI] [PubMed] [Google Scholar]
- 78. Turton JF, Payne Z, Coward A, et al. Virulence genes in isolates of Klebsiella pneumoniae from the UK during 2016, including among carbapenemase gene‐positive hypervirulent K1‐ST23 and 'non‐hypervirulent' types ST147, ST15 and ST383. J Med Microbiol. 2018;67(1):118‐128. [DOI] [PubMed] [Google Scholar]
- 79. Remya P, Shanthi M, Sekar U. Occurrence and characterization of hyperviscous K1 and K2 serotype in Klebsiella pneumoniae . J Lab Physicians. 2018;10(3):283‐288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Russo TA, Shon AS, Beanan JM, et al. Hypervirulent K. pneumoniae secretes more and more active iron‐acquisition molecules than "classical" K. pneumoniae thereby enhancing its virulence. PLoS One. 2011;6(10):e26734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Turton JF, Payne Z, Micah K, Turton JA. Capsular type K54, clonal group 29 and virulence plasmids: an analysis of K54 and non‐K54 closely related isolates of Klebsiella pneumoniae . Epidemiol Infect. 2018;146(14):1813‐1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Rossi B, Gasperini ML, Leflon‐Guibout V, et al. Hypervirulent Klebsiella pneumoniae in Cryptogenic Liver Abscesses, Paris. France Emerg Infect Dis. 2018;24(2):221‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chen YC, Lin CH, Chang SN, Shi ZY. Epidemiology and clinical outcome of pyogenic liver abscess: an analysis from the National Health Insurance Research Database of Taiwan, 2000‐2011. J Microbiol Immunol Infect. 2016;49(5):646‐653. [DOI] [PubMed] [Google Scholar]
- 84. Tian D, Liu X, Chen W, et al. Prevalence of hypervirulent and carbapenem‐resistant Klebsiella pneumoniae under divergent evolutionary patterns. Emerg Microbes Infect. 2022;11(1):1936‐1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Lawlor MS, Handley SA, Miller VL. Comparison of the host responses to wild‐type and cpsB mutant Klebsiella pneumoniae infections. Infect Immun. 2006;74(9):5402‐5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Bushell SR, Mainprize IL, Wear MA, Lou H, Whitfield C, Naismith JH. Wzi is an outer membrane lectin that underpins group 1 capsule assembly in Escherichia coli . Structure. 2013;21(5):844‐853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cortés G, Borrell N, de Astorza B, Gómez C, Sauleda J, Albertí S. Molecular analysis of the contribution of the capsular polysaccharide and the lipopolysaccharide O side chain to the virulence of Klebsiella pneumoniae in a murine model of pneumonia. Infect Immun. 2002;70(5):2583‐2590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Yamaguchi K. Role of bacterial capsule in local and systemic inflammatory responses of mice during pulmonary infection with Klebsiella pneumoniae . J Med Microbiol. 2000;49(11):1003‐1010. [DOI] [PubMed] [Google Scholar]
- 89. Pan YJ, Lin TL, Chen YH, et al. Capsular types of Klebsiella pneumoniae revisited by wzc sequencing. PLoS One. 2013;8(12):e80670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Arakawa Y, Wacharotayankun R, Nagatsuka T, Ito H, Kato N, Ohta M. Genomic organization of the Klebsiella pneumoniae cps region responsible for serotype K2 capsular polysaccharide synthesis in the virulent strain Chedid. J Bacteriol. 1995;177(7):1788‐1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Brisse S, Passet V, Haugaard AB, et al. wzi Gene sequencing, a rapid method for determination of capsular type for Klebsiella strains. J Clin Microbiol. 2013;51(12):4073‐4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Russo TA, Olson R, Fang CT, et al. Identification of biomarkers for differentiation of hypervirulent Klebsiella pneumoniae from classical K. pneumoniae . J Clin Microbiol. 2018;56(9):e00776‐793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Chen YT, Chang HY, Lai YC, Pan CC, Tsai SF, Peng HL. Sequencing and analysis of the large virulence plasmid pLVPK of Klebsiella pneumoniae CG43. Gene. 2004;337:189‐198. [DOI] [PubMed] [Google Scholar]
- 94. Lai YC, Peng HL, Chang HY. RmpA2, an activator of capsule biosynthesis in Klebsiella pneumoniae CG43, regulates K2 cps gene expression at the transcriptional level. J Bacteriol. 2003;185(3):788‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hsu CR, Lin TL, Chen YC, Chou HC, Wang JT. The role of Klebsiella pneumoniae rmpA in capsular polysaccharide synthesis and virulence revisited. Microbiology (Reading). 2011;157(Pt 12):3446‐3457. [DOI] [PubMed] [Google Scholar]
- 96. Nassif X, Fournier JM, Arondel J, Sansonetti PJ. Mucoid phenotype of Klebsiella pneumoniae is a plasmid‐encoded virulence factor. Infect Immun. 1989;57(2):546‐552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nassif X, Honoré N, Vasselon T, Cole ST, Sansonetti PJ. Positive control of colanic acid synthesis in Escherichia coli by rmpA and rmpB, two virulence‐plasmid genes of Klebsiella pneumoniae . Mol Microbiol. 1989;3(10):1349‐1359. [DOI] [PubMed] [Google Scholar]
- 98. Walker KA, Miner TA, Palacios M, et al. A Klebsiella pneumoniae regulatory mutant has reduced capsule expression but retains hypermucoviscosity. MBio. 2019;10(2):e00089‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Palacios M, Miner TA, Frederick DR, et al. Identification of two regulators of virulence that are conserved in Klebsiella pneumoniae classical and hypervirulent strains. MBio. 2018;9(4):e01443‐1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Arakawa Y, Ohta M, Wacharotayankun R, et al. Biosynthesis of Klebsiella K2 capsular polysaccharide in Escherichia coli HB101 requires the functions of rmpA and the chromosomal cps gene cluster of the virulent strain Klebsiella pneumoniae Chedid (O1:K2). Infect Immun. 1991;59(6):2043‐2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Wacharotayankun R, Arakawa Y, Ohta M, et al. Enhancement of extracapsular polysaccharide synthesis in Klebsiella pneumoniae by RmpA2, which shows homology to NtrC and FixJ. Infect Immun. 1993;61(8):3164‐3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Fang CT, Chuang YP, Shun CT, Chang SC, Wang JT. A novel virulence gene in Klebsiella pneumoniae strains causing primary liver abscess and septic metastatic complications. J Exp Med. 2004;199(5):697‐705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Struve C, Bojer M, Nielsen EM, Hansen DS, Krogfelt KA. Investigation of the putative virulence gene magA in a worldwide collection of 495 Klebsiella isolates: magA is restricted to the gene cluster of Klebsiella pneumoniae capsule serotype K1. J Med Microbiol. 2005;54(Pt 11):1111‐1113. [DOI] [PubMed] [Google Scholar]
- 104. Yeh KM, Chang FY, Fung CP, Lin JC, Siu LK. magA is not a specific virulence gene for Klebsiella pneumoniae strains causing liver abscess but is part of the capsular polysaccharide gene cluster of K. pneumoniae serotype K1. J Med Microbiol. 2006;55(Pt 6):803‐804. [DOI] [PubMed] [Google Scholar]
- 105. Fang CT, Lai SY, Yi WC, Hsueh PR, Liu KL. The function of wzy_K1 (magA), the serotype K1 polymerase gene in Klebsiella pneumoniae cps gene cluster. J Infect Dis. 2010;201(8):1268‐1269. [DOI] [PubMed] [Google Scholar]
- 106. Whitfield C. Glycan chain‐length control. Nat Chem Biol. 2010;6(6):403‐404. [DOI] [PubMed] [Google Scholar]
- 107. Schmid J, Sieber V. Enzymatic transformations involved in the biosynthesis of microbial exo‐polysaccharides based on the assembly of repeat units. Chembiochem. 2015;16(8):1141‐1147. [DOI] [PubMed] [Google Scholar]
- 108. Whitney JC, Howell PL. Synthase‐dependent exopolysaccharide secretion in Gram‐negative bacteria. Trends Microbiol. 2013;21(2):63‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Whitfield C. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli . Annu Rev Biochem. 2006;75:39‐68. [DOI] [PubMed] [Google Scholar]
- 110. Schild S, Lamprecht AK, Reidl J. Molecular and functional characterization of O antigen transfer in Vibrio cholerae. J Biol Chem. 2005;280(27):25936‐25947. [DOI] [PubMed] [Google Scholar]
- 111. Lin CT, Chen YC, Jinn TR, Wu CC, Hong YM, Wu WH. Role of the cAMP‐dependent carbon catabolite repression in capsular polysaccharide biosynthesis in Klebsiella pneumoniae . PLoS One. 2013;8(2):e54430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Domenico P, Salo RJ, Cross AS, Cunha BA. Polysaccharide capsule‐mediated resistance to opsonophagocytosis in Klebsiella pneumoniae . Infect Immun. 1994;62(10):4495‐4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Dzul SP, Thornton MM, Hohne DN, et al. Contribution of the Klebsiella pneumoniae capsule to bacterial aggregate and biofilm microstructures. Appl Environ Microbiol. 2011;77(5):1777‐1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Wu MC, Lin TL, Hsieh PF, Yang HC, Wang JT. Isolation of genes involved in biofilm formation of a Klebsiella pneumoniae strain causing pyogenic liver abscess. PLoS One. 2011;6(8):e23500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Huang TW, Lam I, Chang HY, Tsai SF, Palsson BO, Charusanti P. Capsule deletion via a lambda‐Red knockout system perturbs biofilm formation and fimbriae expression in Klebsiella pneumoniae MGH 78578. BMC Res Notes. 2014;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Evrard B, Balestrino D, Dosgilbert A, et al. Roles of capsule and lipopolysaccharide O antigen in interactions of human monocyte‐derived dendritic cells and Klebsiella pneumoniae . Infect Immun. 2010;78(1):210‐219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Yoshida K, Matsumoto T, Tateda K, Uchida K, Tsujimoto S, Yamaguchi K. Induction of interleukin‐10 and down‐regulation of cytokine production by Klebsiella pneumoniae capsule in mice with pulmonary infection. J Med Microbiol. 2001;50(5):456‐461. [DOI] [PubMed] [Google Scholar]
- 118. Regueiro V, Moranta D, Frank CG, et al. Klebsiella pneumoniae subverts the activation of inflammatory responses in a NOD1‐dependent manner. Cell Microbiol. 2011;13(1):135‐153. [DOI] [PubMed] [Google Scholar]
- 119. Moranta D, Regueiro V́, March C, et al. Klebsiella pneumoniae capsule polysaccharide impedes the expression of beta‐defensins by airway epithelial cells. Infect Immun. 2010;78(3):1135‐1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Pan YJ, Lin TL, Hsu CR, Wang JT. Use of a Dictyostelium model for isolation of genetic loci associated with phagocytosis and virulence in Klebsiella pneumoniae . Infect Immun. 2011;79(3):997‐1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. March C, Cano V, Moranta D, et al. Role of bacterial surface structures on the interaction of Klebsiella pneumoniae with phagocytes. PLoS One. 2013;8(2):e56847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. de Astorza B, Cortés G, Crespí C, Saus C, Rojo J́Ḿ, Albertí Ś. C3 promotes clearance of Klebsiella pneumoniae by A549 epithelial cells. Infect Immun. 2004;72(3):1767‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Alvarez D, Merino S, Tomás JM, Benedí VJ, Albertí S. Capsular polysaccharide is a major complement resistance factor in lipopolysaccharide O side chain‐deficient Klebsiella pneumoniae clinical isolates. Infect Immun. 2000;68(2):953‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Campos MA, Vargas MA, Regueiro V́, Llompart CM, Albertí Ś, Bengoechea J́A. Capsule polysaccharide mediates bacterial resistance to antimicrobial peptides. Infect Immun. 2004;72(12):7107‐7114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Llobet E, Tomas JM, Bengoechea JA. Capsule polysaccharide is a bacterial decoy for antimicrobial peptides. Microbiology. 2008;154(Pt 12):3877‐3886. [DOI] [PubMed] [Google Scholar]
- 126. Schmid J, Sieber V, Rehm B. Bacterial exopolysaccharides: biosynthesis pathways and engineering strategies. Front Microbiol. 2015;6:496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Goebel WF. Colanic acid. Proc Natl Acad Sci U S A. 1963;49:464‐471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stevenson G, Andrianopoulos K, Hobbs M, Reeves PR. Organization of the Escherichia coli K‐12 gene cluster responsible for production of the extracellular polysaccharide colanic acid. J Bacteriol. 1996;178(16):4885‐4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Rehm BH. Bacterial polymers: biosynthesis, modifications and applications. Nat Rev Microbiol. 2010;8(8):578‐592. [DOI] [PubMed] [Google Scholar]
- 130. Cress BF, Englaender JA, He W, Kasper D, Linhardt RJ, Koffas MAG. Masquerading microbial pathogens: capsular polysaccharides mimic host‐tissue molecules. FEMS Microbiol Rev. 2014;38(4):660‐697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Morona R, Van Den Bosch L, Daniels C. Evaluation of Wzz/MPA1/MPA2 proteins based on the presence of coiled‐coil regions. Microbiology. 2000;146(Pt 1):1‐4. [DOI] [PubMed] [Google Scholar]
- 132. Obadia B, Lacour S, Doublet P, Baubichon‐Cortay H, Cozzone AJ, Grangeasse C. Influence of tyrosine‐kinase Wzc activity on colanic acid production in Escherichia coli K12 cells. J Mol Biol. 2007;367(1):42‐53. [DOI] [PubMed] [Google Scholar]
- 133. Standish AJ, Morona R. The role of bacterial protein tyrosine phosphatases in the regulation of the biosynthesis of secreted polysaccharides. Antioxid Redox Signal. 2014;20(14):2274‐2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in gram‐negative bacteria. Microbiol Mol Biol Rev. 2009;73(1):155‐177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Beis K, Collins RF, Ford RC, Kamis AB, Whitfield C, Naismith JH. Three‐dimensional structure of Wza, the protein required for translocation of group 1 capsular polysaccharide across the outer membrane of Escherichia coli . J Biol Chem. 2004;279(27):28227‐28232. [DOI] [PubMed] [Google Scholar]
- 136. Hagelueken G, Ingledew W J, Huang H, et al. PELDOR spectroscopy distance fingerprinting of the octameric outer‐membrane protein Wza from Escherichia coli . Angew Chem Int Ed Engl. 2009;48(16):2904‐2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Drummelsmith J, Whitfield C. Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J. 2000;19(1):57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Ford RC, Brunkan‐LaMontagne AL, Collins RF, et al. Structure‐function relationships of the outer membrane translocon Wza investigated by cryo‐electron microscopy and mutagenesis. J Struct Biol. 2009;166(2):172‐182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Nesper J, Hill CMD, Paiment A, et al. Translocation of group 1 capsular polysaccharide in Escherichia coli serotype K30. Structural and functional analysis of the outer membrane lipoprotein Wza. J Biol Chem. 2003;278(50):49763‐49772. [DOI] [PubMed] [Google Scholar]
- 140. Dong C, Beis K, Nesper J, et al. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature. 2006;444(7116):226‐229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Bullen JJ, Rogers HJ, Griffiths E. Iron binding proteins and infection. Br J Haematol. 1972;23(4):389‐392. [DOI] [PubMed] [Google Scholar]
- 142. Palmer LD, Skaar EP. Transition metals and virulence in bacteria. Annu Rev Genet. 2016;50:67‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Miethke M, Marahiel MA. Siderophore‐based iron acquisition and pathogen control. Microbiol Mol Biol Rev. 2007;71(3):413‐451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Brock JH, Williams PH, Licéaga J, Wooldridge KG. Relative availability of transferrin‐bound iron and cell‐derived iron to aerobactin‐producing and enterochelin‐producing strains of Escherichia coli and to other microorganisms. Infect Immun. 1991;59(9):3185‐3190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Perry RD, Balbo PB, Jones HA, Fetherston JD, DeMoll E. Yersiniabactin from Yersinia pestis: biochemical characterization of the siderophore and its role in iron transport and regulation. Microbiology. 1999;145(Pt 5):1181‐1190. [DOI] [PubMed] [Google Scholar]
- 146. Podschun R, Sievers D, Fischer A, Ullmann U. Serotypes, hemagglutinins, siderophore synthesis, and serum resistance of Klebsiella isolates causing human urinary tract infections. J Infect Dis. 1993;168(6):1415‐1421. [DOI] [PubMed] [Google Scholar]
- 147. Tarkkanen AM, Allen BL, Williams PH, et al. Fimbriation, capsulation, and iron‐scavenging systems of Klebsiella strains associated with human urinary tract infection. Infect Immun. 1992;60(3):1187‐1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Koczura R, Kaznowski A. Occurrence of the Yersinia high‐pathogenicity Island and iron uptake systems in clinical isolates of Klebsiella pneumoniae . Microb Pathog. 2003;35(5):197‐202. [DOI] [PubMed] [Google Scholar]
- 149. Muller SI, Valdebenito M, Hantke K. Salmochelin, the long‐overlooked catecholate siderophore of Salmonella. Biometals. 2009;22(4):691‐695. [DOI] [PubMed] [Google Scholar]
- 150. Lai YC, Peng HL, Chang HY. Identification of genes induced in vivo during Klebsiella pneumoniae CG43 infection. Infect Immun. 2001;69(11):7140‐7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Goetz DH, Holmes MA, Borregaard N, Bluhm ME, Raymond KN, Strong RK. The neutrophil lipocalin NGAL is a bacteriostatic agent that interferes with siderophore‐mediated iron acquisition. Mol Cell. 2002;10(5):1033‐1043. [DOI] [PubMed] [Google Scholar]
- 152. Chan YR, Liu JS, Pociask DA, et al. Lipocalin 2 is required for pulmonary host defense against Klebsiella infection. J Immunol. 2009;182(8):4947‐4956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Nelson AL, Barasch JM, Bunte RM, Weiser JN. Bacterial colonization of nasal mucosa induces expression of siderocalin, an iron‐sequestering component of innate immunity. Cell Microbiol. 2005;7(10):1404‐1417. [DOI] [PubMed] [Google Scholar]
- 154. Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase‐associated lipocalin from humans. Genomics. 1997;45(1):17‐23. [DOI] [PubMed] [Google Scholar]
- 155. Bachman MA, Miller VL, Weiser JN. Mucosal lipocalin 2 has pro‐inflammatory and iron‐sequestering effects in response to bacterial enterobactin. PLoS Pathog. 2009;5(10):e1000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Wasserman SI, Soter NA, Center DM, Austen KF. Cold urticaria. Recognition and characterization of a neutrophil chemotactic factor which appears in serum during experimental cold challenge. J Clin Invest. 1977;60(1):189‐196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Bach S, de Almeida A, Carniel E. The Yersinia high‐pathogenicity Island is present in different members of the family Enterobacteriaceae. FEMS Microbiol Lett. 2000;183(2):289‐294. [DOI] [PubMed] [Google Scholar]
- 158. Russo TA, Olson R, MacDonald U, Beanan J, Davidson BA. Aerobactin, but not yersiniabactin, salmochelin, or enterobactin, enables the growth/survival of hypervirulent (hypermucoviscous) Klebsiella pneumoniae ex vivo and in vivo. Infect Immun. 2015;83(8):3325‐3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Fischbach MA, Lin H, Zhou L, et al. The pathogen‐associated iroA gene cluster mediates bacterial evasion of lipocalin 2. Proc Natl Acad Sci U S A. 2006;103(44):16502‐16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Fischbach MA, Lin H, Liu DR, Walsh CT. In vitro characterization of IroB, a pathogen‐associated C‐glycosyltransferase. Proc Natl Acad Sci U S A. 2005;102(3):571‐576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Hantke K, Nicholson G, Rabsch W, Winkelmann G. Salmochelins, siderophores of Salmonella enterica and uropathogenic Escherichia coli strains, are recognized by the outer membrane receptor IroN. Proc Natl Acad Sci U S A. 2003;100(7):3677‐3682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Nassif X, Sansonetti PJ. Correlation of the virulence of Klebsiella pneumoniae K1 and K2 with the presence of a plasmid encoding aerobactin. Infect Immun. 1986;54(3):603‐608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Tang HL, Chiang MK, Liou WJ, et al. Correlation between Klebsiella pneumoniae carrying pLVPK‐derived loci and abscess formation. Eur J Clin Microbiol Infect Dis. 2010;29(6):689‐698. [DOI] [PubMed] [Google Scholar]
- 164. Russo TA, Olson R, MacDonald U, et al. Aerobactin mediates virulence and accounts for increased siderophore production under iron‐limiting conditions by hypervirulent (hypermucoviscous) Klebsiella pneumoniae . Infect Immun. 2014;82(6):2356‐2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Krapp F, Morris AR, Ozer EA, Hauser AR. Virulence characteristics of carbapenem‐resistant Klebsiella pneumoniae strains from patients with necrotizing skin and soft tissue infections. Sci Rep. 2017;7(1):13533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Stahlhut SG, Tchesnokova V, Struve C, et al. Comparative structure‐function analysis of mannose‐specific FimH adhesins from Klebsiella pneumoniae and Escherichia coli . J Bacteriol. 2009;191(21):6592‐6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Klemm P, Schembri MA. Fimbrial surface display systems in bacteria: from vaccines to random libraries. Microbiology. 2000;146(Pt 12):3025‐3032. [DOI] [PubMed] [Google Scholar]
- 168. Tarkkanen AM, Westerlund‐Wikström B, Erkkilä L, Korhonen TK. Immunohistological localization of the MrkD adhesin in the type 3 fimbriae of Klebsiella pneumoniae . Infect Immun. 1998;66(5):2356‐2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Struve C, Bojer M, Krogfelt KA. Identification of a conserved chromosomal region encoding Klebsiella pneumoniae type 1 and type 3 fimbriae and assessment of the role of fimbriae in pathogenicity. Infect Immun. 2009;77(11):5016‐5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170. Stahlhut SG, Struve C, Krogfelt KA, Reisner A. Biofilm formation of Klebsiella pneumoniae on urethral catheters requires either type 1 or type 3 fimbriae. FEMS Immunol Med Microbiol. 2012;65(2):350‐359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Sebghati TA, Korhonen TK, Hornick DB, Clegg S. Characterization of the type 3 fimbrial adhesins of Klebsiella strains. Infect Immun. 1998;66(6):2887‐2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Schroll C, Barken KB, Krogfelt KA, Struve C. Role of type 1 and type 3 fimbriae in Klebsiella pneumoniae biofilm formation. BMC Microbiol. 2010;10:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. de Majumdar S, Yu J, Fookes M, et al. Elucidation of the RamA regulon in Klebsiella pneumoniae reveals a role in LPS regulation. PLoS Pathog. 2015;11(1):e1004627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Frirdich E, Whitfield C. Lipopolysaccharide inner core oligosaccharide structure and outer membrane stability in human pathogens belonging to the Enterobacteriaceae. J Endotoxin Res. 2005;11(3):133‐144. [DOI] [PubMed] [Google Scholar]
- 175. Merino S, Altarriba M, Izquierdo L, Nogueras ḾḾ, Regué M, Tomás JM. Cloning and sequencing of the Klebsiella pneumoniae O5 wb gene cluster and its role in pathogenesis. Infect Immun. 2000;68(5):2435‐2440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Raetz CR, Guan Z, Ingram BO, et al. Discovery of new biosynthetic pathways: the lipid A story. J Lipid Res. 2009;50(Suppl):S103‐S108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Llobet E, Martínez‐Moliner V, Moranta D, et al. Deciphering tissue‐induced Klebsiella pneumoniae lipid A structure. Proc Natl Acad Sci U S A. 2015;112(46):E6369‐E6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Clements A, Tull D, Jenney AW, et al. Secondary acylation of Klebsiella pneumoniae lipopolysaccharide contributes to sensitivity to antibacterial peptides. J Biol Chem. 2007;282(21):15569‐15577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Merino S, Camprubí S, Albertí S, Benedí VJ, Tomás JM. Mechanisms of Klebsiella pneumoniae resistance to complement‐mediated killing. Infect Immun. 1992;60(6):2529‐2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Wu MF, Yang CY, Lin TL, et al. Humoral immunity against capsule polysaccharide protects the host from magA+ Klebsiella pneumoniae‐induced lethal disease by evading Toll‐like receptor 4 signaling. Infect Immun. 2009;77(2):615‐621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181. Merle NS, Noe R, Halbwachs‐Mecarelli L, Fremeaux‐Bacchi V, Roumenina LT. Complement system part II: role in immunity. Front Immunol. 2015;6:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Albertí S, Alvarez D, Merino S, et al. Analysis of complement C3 deposition and degradation on Klebsiella pneumoniae . Infect Immun. 1996;64(11):4726‐4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Shankar‐Sinha S, Valencia GA, Janes BK, et al. The Klebsiella pneumoniae O antigen contributes to bacteremia and lethality during murine pneumonia. Infect Immun. 2004;72(3):1423‐1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Regué M, Hita B, Piqué N, et al. A gene, uge, is essential for Klebsiella pneumoniae virulence. Infect Immun. 2004;72(1):54‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185. Izquierdo L, Coderch Ń, Piqué N, et al. The Klebsiella pneumoniae wabG gene: role in biosynthesis of the core lipopolysaccharide and virulence. J Bacteriol. 2003;185(24):7213‐7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Nougayrède JP, Homburg S, Taieb F, et al. Escherichia coli induces DNA double‐strand breaks in eukaryotic cells. Science. 2006;313(5788):848‐851. [DOI] [PubMed] [Google Scholar]
- 187. Lai YC, Lin AC, Chiang MK, et al. Genotoxic Klebsiella pneumoniae in Taiwan. PLoS One. 2014;9(5):e96292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Cuevas‐Ramos G, Petit CR, Marcq I, Boury M, Oswald E, Nougayrède JP. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc Natl Acad Sci U S A. 2010;107(25):11537‐11542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Lu MC, Chen YT, Chiang MK, et al. Colibactin contributes to the hypervirulence of pks(+) K1 CC23 Klebsiella pneumoniae in mouse meningitis infections. Front Cell Infect Microbiol. 2017;7:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Lam MMC, Wyres KL, Duchêne S, et al. Population genomics of hypervirulent Klebsiella pneumoniae clonal‐group 23 reveals early emergence and rapid global dissemination. Nat Commun. 2018;9(1):2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Fischbach MA, Walsh CT. Assembly‐line enzymology for polyketide and nonribosomal Peptide antibiotics: logic, machinery, and mechanisms. Chem Rev. 2006;106(8):3468‐3496. [DOI] [PubMed] [Google Scholar]
- 192. Olier M, Marcq I, Salvador‐Cartier C, et al. Genotoxicity of Escherichia coli Nissle 1917 strain cannot be dissociated from its probiotic activity. Gut Microbes. 2012;3(6):501‐509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Faïs T, Cougnoux A, Dalmasso G, Laurent F, Delmas J, Bonnet R. Antibiotic activity of Escherichia coli against multiresistant Staphylococcus aureus . Antimicrob Agents Chemother. 2016;60(11):6986‐6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Vizcaino MI, Engel P, Trautman E, Crawford JM. Comparative metabolomics and structural characterizations illuminate colibactin pathway‐dependent small molecules. J Am Chem Soc. 2014;136(26):9244‐9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Pérez‐Berezo T, Pujo J, Martin P, et al. Identification of an analgesic lipopeptide produced by the probiotic Escherichia coli strain Nissle 1917. Nat Commun. 2017;8(1):1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Putze J, Hennequin C, Nougayrède JP, et al. Genetic structure and distribution of the colibactin genomic Island among members of the family Enterobacteriaceae. Infect Immun. 2009;77(11):4696‐4703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Chen YT, Lai YC, Tan MC, et al. Prevalence and characteristics of pks genotoxin gene cluster‐positive clinical Klebsiella pneumoniae isolates in Taiwan. Sci Rep. 2017;7:43120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Passet V, Brisse S. Association of tellurite resistance with hypervirulent clonal groups of Klebsiella pneumoniae . J Clin Microbiol. 2015;53(4):1380‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Martin RM, Cao J, Wu W, et al. Identification of pathogenicity‐associated loci in Klebsiella pneumoniae from hospitalized patients. mSystems. 2018;3(3):e00015‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Vogels GD, Van der Drift C. Degradation of purines and pyrimidines by microorganisms. Bacteriol Rev. 1976;40(2):403‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. French JB, Neau DB, Ealick SE. Characterization of the structure and function of Klebsiella pneumoniae allantoin racemase. J Mol Biol. 2011;410(3):447‐460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Chou HC, Lee CZ, Ma LC, Fang CT, Chang SC, Wang JT. Isolation of a chromosomal region of Klebsiella pneumoniae associated with allantoin metabolism and liver infection. Infect Immun. 2004;72(7):3783‐3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Yu WL, Fung CP, Ko WC, Cheng KC, Lee CC, Chuang YC. Polymerase chain reaction analysis for detecting capsule serotypes K1 and K2 of Klebsiella pneumoniae causing abscesses of the liver and other sites. J Infect Dis. 2007;195(8):1235‐1236. author reply 1236. [DOI] [PubMed] [Google Scholar]
- 204. Compain F, Babosan A, Brisse S, et al. Multiplex PCR for detection of seven virulence factors and K1/K2 capsular serotypes of Klebsiella pneumoniae . J Clin Microbiol. 2014;52(12):4377‐4380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Bulger J, MacDonald U, Olson R, Beanan J, Russo TA. Metabolite transporter PEG344 is required for full virulence of hypervirulent Klebsiella pneumoniae strain hvKP1 after pulmonary but not subcutaneous challenge. Infect Immun. 2017;85(10):e00093‐109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Tian LT, Yao K, Zhang XY, et al. Liver abscesses in adult patients with and without diabetes mellitus: an analysis of the clinical characteristics, features of the causative pathogens, outcomes and predictors of fatality: a report based on a large population, retrospective study in China. Clin Microbiol Infect. 2012;18(9):E314‐E330. [DOI] [PubMed] [Google Scholar]
- 207. Chung DR, Lee H, Park MH, et al. Fecal carriage of serotype K1 Klebsiella pneumoniae ST23 strains closely related to liver abscess isolates in Koreans living in Korea. Eur J Clin Microbiol Infect Dis. 2012;31(4):481‐486. [DOI] [PubMed] [Google Scholar]
- 208. Qu TT, Zhou JC, Jiang Y, et al. Clinical and microbiological characteristics of Klebsiella pneumoniae liver abscess in East China. BMC Infect Dis. 2015;15:161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Liao W, Liu Y, Zhang W. Virulence evolution, molecular mechanisms of resistance and prevalence of ST11 carbapenem‐resistant Klebsiella pneumoniae in China: A review over the last 10 years. J Glob Antimicrob Resist. 2020;23:174‐180. [DOI] [PubMed] [Google Scholar]
- 210. Harada S, Tateda K, Mitsui H, et al. Familial spread of a virulent clone of Klebsiella pneumoniae causing primary liver abscess. J Clin Microbiol. 2011;49(6):2354‐2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Lin JC, Koh TH, Lee N, et al. Genotypes and virulence in serotype K2 Klebsiella pneumoniae from liver abscess and non‐infectious carriers in Hong Kong, Singapore and Taiwan. Gut Pathog. 2014;6:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Li G, Shi J, Zhao Y, et al. Identification of hypervirulent Klebsiella pneumoniae isolates using the string test in combination with Galleria mellonella infectivity. Eur J Clin Microbiol Infect Dis. 2020;39:1673‐1679. [DOI] [PubMed] [Google Scholar]
- 213. Lin YC, Lu MC, Tang HL, et al. Assessment of hypermucoviscosity as a virulence factor for experimental Klebsiella pneumoniae infections: comparative virulence analysis with hypermucoviscosity‐negative strain. BMC Microbiol. 2011;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Brisse S, Fevre C, Passet V, et al. Virulent clones of Klebsiella pneumoniae: identification and evolutionary scenario based on genomic and phenotypic characterization. PLoS One. 2009;4(3):e4982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215. Wu KM, Li LH, Yan JJ, et al. Genome sequencing and comparative analysis of Klebsiella pneumoniae NTUH‐K2044, a strain causing liver abscess and meningitis. J Bacteriol. 2009;191(14):4492‐4501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Marcoleta AE, Berríos‐Pastén C, Nuñez G, Monasterio O, Lagos R. Klebsiella pneumoniae asparagine tDNAs are integration hotspots for different genomic islands encoding microcin E492 production determinants and other putative virulence factors present in hypervirulent strains. Front Microbiol. 2016;7:849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Yang X, Sun Q, Li J, et al. Molecular epidemiology of carbapenem‐resistant hypervirulent Klebsiella pneumoniae in China. Emerg Microbes Infect. 2022;11(1):841‐849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Siu LK, Fung CP, Chang FY, et al. Molecular typing and virulence analysis of serotype K1 Klebsiella pneumoniae strains isolated from liver abscess patients and stool samples from noninfectious subjects in Hong Kong, Singapore, and Taiwan. J Clin Microbiol. 2011;49(11):3761‐3765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Turton JF, Englender H, Gabriel SN, Turton SE, Kaufmann ME, Pitt TL. Genetically similar isolates of Klebsiella pneumoniae serotype K1 causing liver abscesses in three continents. J Med Microbiol. 2007;56(Pt 5):593‐597. [DOI] [PubMed] [Google Scholar]
- 220. Ye M, Tu J, Jiang J, et al. Clinical and genomic analysis of liver abscess‐causing Klebsiella pneumoniae identifies new liver abscess‐associated virulence genes. Front Cell Infect Microbiol. 2016;6:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Ramirez MS, Traglia GM, Lin DL, Tran T, Tolmasky ME. Plasmid‐mediated antibiotic resistance and virulence in gram‐negatives: the Klebsiella pneumoniae paradigm. Microbiol Spectr. 2014;2(5):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Yigit H, Queenan AM, Anderson GJ, et al. Novel carbapenem‐hydrolyzing beta‐lactamase, KPC‐1, from a carbapenem‐resistant strain of Klebsiella pneumoniae . Antimicrob Agents Chemother. 2001;45(4):1151‐1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Wei ZQ, du XX, Yu YS, Shen P, Chen YG, Li LJ. Plasmid‐mediated KPC‐2 in a Klebsiella pneumoniae isolate from China. Antimicrob Agents Chemother. 2007;51(2):763‐765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Huang YH, Chou SH, Liang SW, et al. Emergence of an XDR and carbapenemase‐producing hypervirulent Klebsiella pneumoniae strain in Taiwan. J Antimicrob Chemother. 2018;73(8):2039‐2046. [DOI] [PubMed] [Google Scholar]
- 225. Lee CR, Lee JH, Park KS, et al. Antimicrobial resistance of hypervirulent Klebsiella pneumoniae: epidemiology, hypervirulence‐associated determinants, and resistance mechanisms. Front Cell Infect Microbiol. 2017;7:483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226. Li J, Ren J, Wang W, et al. Risk factors and clinical outcomes of hypervirulent Klebsiella pneumoniae induced bloodstream infections. Eur J Clin Microbiol Infect Dis. 2018;37(4):679‐689. [DOI] [PubMed] [Google Scholar]
- 227. Karlsson M, Stanton RA, Ansari U, et al. Identification of a carbapenemase‐producing hypervirulent Klebsiella pneumoniae isolate in the United States. Antimicrob Agents Chemother. 2019;63(7):e00519‐537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Wei DD, Wan LG, Deng Q, Liu Y. Emergence of KPC‐producing Klebsiella pneumoniae hypervirulent clone of capsular serotype K1 that belongs to sequence type 11 in Mainland China. Diagn Microbiol Infect Dis. 2016;85(2):192‐194. [DOI] [PubMed] [Google Scholar]
- 229. Feng Y, Lu Y, Yao Z, Zong Z. Carbapenem‐resistant hypervirulent Klebsiella pneumoniae of sequence type 36. Antimicrob Agents Chemother. 2018;62(7):e02644‐2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Fu L, Tang L, Wang S, et al. Co‐location of the blaKPC‐2, blaCTX‐M‐65, rmtB and virulence relevant factors in an IncFII plasmid from a hypermucoviscous Klebsiella pneumoniae isolate. Microb Pathog. 2018;124:301‐304. [DOI] [PubMed] [Google Scholar]
- 231. Xu Y, Zhang J, Wang M, et al. Mobilization of the nonconjugative virulence plasmid from hypervirulent Klebsiella pneumoniae . Genome Med. 2021;13(1):119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Liu X, Wu Y, Zhu Y, et al. Emergence of colistin‐resistant hypervirulent Klebsiella pneumoniae (CoR‐HvKp) in China. Emerg Microbes Infect. 2022;11(1):648‐661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Dong N, Liu L, Zhang R, et al. An IncR plasmid harbored by a hypervirulent carbapenem‐resistant Klebsiella pneumoniae strain possesses five tandem repeats of the bla KPC‐2::NTEKPC‐Id fragment. Antimicrob Agents Chemother. 2019;63(3):e01775‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Dong N, Lin D, Zhang R, Chan EWC, Chen S. Carriage of blaKPC‐2 by a virulence plasmid in hypervirulent Klebsiella pneumoniae . J Antimicrob Chemother. 2018;73(12):3317‐3321. [DOI] [PubMed] [Google Scholar]
- 235. Liu Y, Long D, Xiang TX, et al. Whole genome assembly and functional portrait of hypervirulent extensively drug‐resistant NDM‐1 and KPC‐2 co‐producing Klebsiella pneumoniae of capsular serotype K2 and ST86. J Antimicrob Chemother. 2019;74(5):1233‐1240. [DOI] [PubMed] [Google Scholar]
- 236. Mataseje LF, Boyd DA, Mulvey MR, Longtin Y. Two hypervirulent Klebsiella pneumoniae isolates producing a bla KPC‐2 carbapenemase from a Canadian patient. Antimicrob Agents Chemother. 2019;63(7):e00517‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Lin TL, Lee CZ, Hsieh PF, Tsai SF, Wang JT. Characterization of integrative and conjugative element ICEKp1‐associated genomic heterogeneity in a Klebsiella pneumoniae strain isolated from a primary liver abscess. J Bacteriol. 2008;190(2):515‐526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Lam MMC, Wick RR, Wyres KL, et al. Genetic diversity, mobilisation and spread of the yersiniabactin‐encoding mobile element ICEKp in Klebsiella pneumoniae populations. Microb Genom. 2018;4(9):e000196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Lery LM, Frangeul L, Tomas A, et al. Comparative analysis of Klebsiella pneumoniae genomes identifies a phospholipase D family protein as a novel virulence factor. BMC Biol. 2014;12:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Goldberg GW, Marraffini LA. Resistance and tolerance to foreign elements by prokaryotic immune systems – curating the genome. Nat Rev Immunol. 2015;15(11):717‐724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Chen L, Chavda KD, Melano RG, et al. Complete sequence of a bla(KPC‐2)‐harboring IncFII(K1) plasmid from a Klebsiella pneumoniae sequence type 258 strain. Antimicrob Agents Chemother. 2013;57(3):1542‐1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Hullahalli K, Rodrigues M, Nguyen UT, Palmer K. An attenuated CRISPR‐Cas system in Enterococcus faecalis permits DNA acquisition. MBio. 2018;9(3):e00414‐431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Sampson TR, Saroj SD, Llewellyn AC, Tzeng YL, Weiss DS. A CRISPR/Cas system mediates bacterial innate immune evasion and virulence. Nature. 2013;497(7448):254‐257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Mackow NA, Shen J, Adnan M, Khan AS, Fries BC, Diago‐Navarro E. CRISPR‐Cas influences the acquisition of antibiotic resistance in Klebsiella pneumoniae . PLoS One. 2019;14(11):e0225131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Wyres KL, Wick RR, Judd LM, et al. Distinct evolutionary dynamics of horizontal gene transfer in drug resistant and virulent clones of Klebsiella pneumoniae . PLoS Genet. 2019;15(4):e1008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Tang Y, Fu P, Zhou Y, et al. Absence of the type I‐E CRISPR‐Cas system in Klebsiella pneumoniae clonal complex 258 is associated with dissemination of IncF epidemic resistance plasmids in this clonal complex. J Antimicrob Chemother. 2020;75:890‐895. [DOI] [PubMed] [Google Scholar]
- 247. Zhou Y, Tian D, Tang Y, et al. High‐risk KPC‐producing Klebsiella pneumoniae lack type I R‐M systems. Int J Antimicrob Agents. 2020;56(2):106050. [DOI] [PubMed] [Google Scholar]
- 248. Murray NE. Type I restriction systems: sophisticated molecular machines (a legacy of Bertani and Weigle). Microbiol Mol Biol Rev. 2000;64(2):412‐434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Dupuis ME, Villion M, Magadán AH, Moineau S. CRISPR‐Cas and restriction‐modification systems are compatible and increase phage resistance. Nat Commun. 2013;4:2087. [DOI] [PubMed] [Google Scholar]
- 250. Price VJ, Huo W, Sharifi A, Palmer KL. CRISPR‐Cas and restriction‐modification act additively against conjugative antibiotic resistance plasmid transfer in Enterococcus faecalis . mSphere. 2016;1(3):e00064‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Fu P, Tang Y, Li G, Yu L, Wang Y, Jiang X. Pandemic spread of blaKPC‐2 among Klebsiella pneumoniae ST11 in China is associated with horizontal transfer mediated by IncFII‐like plasmids. Int J Antimicrob Agents. 2019;54(2):117‐124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.


