Background:
To evaluate whether transcutaneous electrical acupoint stimulation (TEAS) decreases rates of perioperative neurocognitive disorders (PND) when used as an adjuvant method during perioperative period in geriatric patients since the new definition was released in 2018.
Methods:
Six databases [Chinese National Knowledge Infrastructure, VIP Database for Chinese Technical Periodicals, WanFang Database, PubMed, EMBASE, and Cochrane Library] were systematically searched. Data analysis was performed using RevMan 5.4.1 software (Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2020). Risk ratios (RR) with 95% confidence interval were calculated using a random effects model. Quality of evidence was assessed using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) approach.
Results:
13 randomized clinical trials (999 patients) in total were included. TEAS had positive effects on preventing the incidence of PND (RR: 0.43; 0.31, 0.61; P < .001; low certainty) [postoperative delirium within 7 days (RR: 0.39; 0.26, 0.59; P < .001), delayed neurocognitive recovery within 3 months (RR: 0.51; 0.33, 0.78; P = .002)]. TEAS could also improve the scores of the confusion assessment method (CAM) (Mean difference: −1.30; −2.14, −0.46; P = .003; low certainty). Limited evidence suggested that TEAS could reduce the serum levels of biochemical indicator (S100β) (SMD = −1.08, −1.67, –0.49, P < .001; I2 = 83%; very low certainty) as well as anesthetic requirements (remifentanil) (SMD: −1.58; −2.54, −0.63; P = .001; I2 = 87%; very low certainty). Subgroup analysis indicated that different protocols of TEAS had significant pooled benefits (TEAS used only in surgery and in combination with postoperative intervention) (RR: 0.45; 0.31, 0.63; P < .001). Acupoint combination (LI4 and PC6) in the TEAS group had more significantly advantages (RR: 0.34; 0.17, 0.67; P = .002). TEAS group had a lower incidence of PND in different surgery type (orthopedic surgery and abdominal surgery) (RR: 0.43; 0.30, 0.60; P < .001), as well as with different anesthetic modality (intravenous anesthesia and intravenous and inhalational combined anesthesia) (RR: 0.38; 0.23, 0.61; P < .001).
Conclusion:
In terms of clinical effectiveness, TEAS appeared to be beneficial for prophylaxis of PND during a relatively recent period, noting the limitations of the current evidence.
Keywords: cognitive dysfunction, meta-analysis, perioperative neurocognitive disorders, prevention, systematic review, transcutaneous electrical acupoint stimulation
1. Introduction
Cognitive impairment after anesthesia and surgery is a recognized clinical phenomenon, which is the most common complication experienced by older individuals as they have more predisposing risk factors.[1–4] A multicentre study (the International Study of Post-Operative Cognitive Dysfunction) confirmed unequivocally that risk of postoperative cognitive decline increases with age in people over 60 years of age.[5] The Perioperative Cognition Nomenclature Working Group, defined postoperative cognitive impairment as perioperative neurocognitive disorders (PND) in 2018.[6] Based on the new definition, PND included neurocognitive disorders (NCD) in the pre-operative period, postoperative delirium (POD), delayed neurocognitive recovery (DNR) diagnosed up to 30 days after the procedure, and postoperative neurocognitive disorder (post-operative NCD). The incidence of POD was 15% to 25% after major elective surgery and 50% after high-risk procedures, such as hip fracture repair and cardiac surgery.[7] The incidence of DNR was reported as about 19.2% 1 week after major non-cardiac surgery, while postoperative neurocognitive disorders (postoperative NCD) occurred at 7% at 3 months.[8] Patients with PND are exposed to the risk of longer hospitalization time, worse cognitive dysfunction, and death. An 8-year observational study found that the presence of cognitive dysfunction 3 months after noncardiac surgery was associated with increased mortality.[9] Furthermore, elderly patients with PND are 3 times more likely to experience permanent cognitive impairment or dementia.[10] With aging populations and improvements in survival, optimizing the perioperative management of elderly surgical patients to avoid harmful sequelae such as postoperative neurological complications is crucial.[11]
Current research indicates that the best treatment for PND is prevention, along with promptly identifying and treating any modifiable underlying cause.[12] The intervention methods for PND could be pharmaceutical or non-pharmaceutical. Despite many recent technological advances, the efficacy of currently available therapeutic remedies is limited.[13] Major research suggests that drugs are effective only in a small subset of aged patients and have debilitating side effects.[14] Transcutaneous electrical acupoint stimulation (TEAS) is a new acupuncture treatment that combines transcutaneous electrical nerve stimulation with acupoint stimulation. The TEAS works by placing electrodes on the surface of the acupoints and controlling the input pulse current to stimulate. As a non-pharmaceutical intervention, it has been widely used in clinical practice and has become an important part of perioperative management owing to the lack of drug-induced side effects and noninvasive. A considerable number of emerging trials now demonstrate the clinical efficacy of TEAS in PND. TEAS may exert neuroprotective effects to defer the pathological process of PND through multiple pathways.
So far, there are no comprehensive systematic reviews (SRs) assessing the effectiveness of TEAS for preventing the incidence of PND in geriatric patients. One of the meta-analysis[15] with a wide range of patient ages (>18 years) and various treatments (TEAS combined with dexmedetomidine or controlled hypotension) were included. Given that several studies which meet the new definition in 2018 have not been included in the published SRs, it is a strong necessity for us to conduct an updated SR and meta-analysis.
2. Method
This review was conducted and reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis statement guidelines.[16] We had registered the review in the international prospective register of SRs (PROSPERO, Center for Reviews and Dissemination, University of York, No. CRD42022325416).
2.1. Search strategy
An electronic search was completed on 3 English-language databases (PubMed, EMBASE, and Cochrane Library) and 3 Chinese-language databases (VIP Database for Chinese Technical Periodicals, China National Knowledge Infrastructure, and Wanfang) that include “gray literature,” such as unpublished studies and conference reports up to January 2022. We obtained copies of all papers that could be reports of randomized controlled trials (RCTs) or reviews of RCTs. We scanned the bibliographies of all papers retrieved for further reference. The search strategy consisted of 3 components: clinical condition [postoperative cognitive dysfunction, PND, POD], intervention (transcutaneous electrical acupoint stimulation, TEAS, transcutaneous acupoint electrical stimulation, TAES, acustimulation), and study type (randomized clinical trial). The specific search strategies are presented in Supplemental Digital Content, http://links.lww.com/MD/I155. Two authors independently screened the records of comprehensive searches by titles and abstracts, or full text as needed, to establish the eligibility of the studies. Articles published without restriction on race, gender, and language publication type (that is, either full article or abstract) were included if they were RCTs investigating the association of transcutaneous electrical acupoint stimulation with PND.
2.2. Inclusion criteria
Original RCTs are human studies published in full text and those for which we had full access to all original data. We included: participants aged 60 years or older, who undergo surgical treatments without limitation on the forms of anesthesia or operations to comprehensively evaluate the preventive effects of TEAS under different operations and anesthetic modalities; only trials on the utilization of TEAS at the perioperative period: before and/or during and/or after surgery; participants in the control groups were treated with a sham intervention, or with a blank control; and the primary outcome measures included incidence of PND. Based on a definition proposed in 2018, we included literature assessing POD within 7 days postoperatively or prior to hospital discharge, as measured by a validated tool or diagnostic criteria, for example, Diagnostic and Statistical Manual of Mental Disorders (DSM-5), the Confusion Assessment Method (CAM[17]) or the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU[18]), International Classification of Diseases-10 (ICD-10). And the research evaluated the incidence of DNR and postoperative NCD as defined and measured by the study authors (DNR within 30 days after operations, and postoperative NCD from 30 days to 12 months after surgeries). The secondary outcomes were broadly categorized into cognitive function [CAM or CAM-ICU scores, the Mini-Mental State Examination (MMSE scores[19]), biochemical indicators, and dosage of anesthetics.
2.3. Exclusion criteria
We excluded: non-RCTs, such as case studies or case series, reviews, and protocols for RCTs, or nonhuman (in vitro or animal) research, or duplicate publications; RCTs that evaluated TEAS used in combination with other anesthetic drugs during surgery; RCTs that treated patients with TEAS after diagnosis of PND; and the outcome data could not be extracted or used to analyze.
2.4. Data extraction
According to the predetermined criteria, 2 researchers screened each article independently and were blinded to the findings of the other reviewer in order to conduct a rigorous screening to collect qualified articles. In case of disagreement, they would discuss with each other or consult a third reviewer to reach a consensus. Data were extracted from these selected articles through the standardized data collection table, which included: necessary information such as first author, publication date, and country of origin; demographic characteristics of different groups of participants; intervention measures, including methods of the TEAS and the control groups such as time to use TEAS, selection of acupoints; the details of outcomes, including the incidence of PND, tools to assess the cognitive function, preoperative and postoperative scale scores and so on; and The conclusion of the study.
2.5. Study quality assessment
The methodological quality of each included study was assessed by 2 authors (LSY and JHL) using Cochrane Collaboration’s risk of bias tool (version 5.1.0[20]), which contains 7 specified domains: sequence generation; allocation concealment; blinding of patients, personnel, and outcome assessment; incomplete outcome data; selective reporting; and other sources bias. Each item was rated as “low,” “unclear” or “high” risk. The results of this assessment were summarized in both a “risk of bias” graph and a “risk of bias” summary. If some items were not described or were ambiguous, we would contact the corresponding authors to clarify the details of each bias.
We assessed the overall certainty of the evidence for each outcome using the Grading Recommendations Assessment, Development and Evaluation (GRADE) approach.[21] We used the Guideline Development Tool to formulate the Summary of Findings table. Any occurred conflicts for RoB or GRADE assessment were resolved by a third investigator (LW) referring to the original article and discussing them together.
2.6. Statistical analysis
For pooled data, summary test statistics including attributes data and variables data were separately calculated using the Mantel-Haenszel model and Inverse Variance model, which are the fixed effects model used in the Review Manager software version 5.4.1. The M-H model stratifies the study results and estimates the effect of each stratum. If the difference between the effect values of each stratum is not statistically significant, the results of each stratum can be combined by the M-H method to derive the overall effect value. The M-H method has better robustness when the sample size is relatively small. The inspection level for the pooled data was 2-sided, and P < .05 was regarded as statistically significant. The risk ratio (RR) and mean difference (MD) with a 95% confidence interval (CI) were employed to analyze the dichotomous and continuous data. And standardized mean differences (SMDs) have been used when different scales were applied to measure the same outcome.
We evaluated heterogeneity using both the I2 statistic and the P-value of the chi-square test of heterogeneity. If P ≤ .1 and I2 ≥ 50%, it indicates that the results of the studies are heterogeneous.[22] The decision to use random-effects or fixed-effects models was based on I2 quantification of heterogeneity, as well as variability in the clinical and methodological aspects of the studies, the number of studies available for pooling, and study sample sizes. We searched the source of heterogeneity and analyzed the sensitivity to assess the impact of a single study on the overall analysis. Publication bias was assessed by using the funnel plot or Egger’s test when there were more than 10 studies. We had planned to conduct subgroup analyses about different anesthetic modalities, types of surgery, and protocols of TEAS, such as acupoint selection, and the utilization of TEAS during the intraoperative or intraoperative and postoperative periods. If there was only 1 study in a subgroup, no analysis was done. We compiled a narrative review of trial results and characteristics where trials were unsuitable for meta-analysis.
3. Results
3.1. Literature search
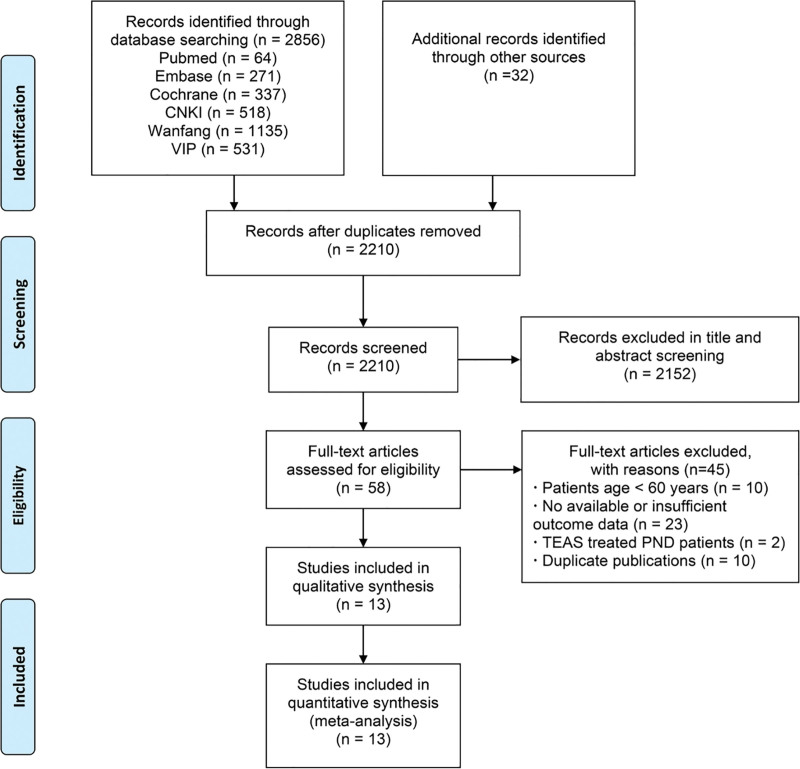
The initial search in PubMed, EMBASE, Cochrane library, and other databases identified 2856 reports and additional records identified 32 reports through other sources. Duplicates removal reduced the number of reports to 2210. Then, 2152 studies were further excluded after reviewing the title and abstracts. The full text of the remaining 58 studies was retrieved for evaluation, 45 out of the 58 studies were further excluded due to 1 or more of the following reasons: patients age < 60 years (n = 10); no available or insufficient outcome data could be extracted or used to analyze (n = 23); TEAS treated PND patients (n = 2); duplicate publications (n = 10). Reviewing the reference lists of the retrieved studies did not identify any new eligible studies. Finally, 13 RCTs were included in the present review.[23–35] A flow diagram illustrating the literature search and trial screening process was shown in Figure 1.
Figure 1.
Flow diagram of the study identification and selection process.
3.2. Characteristics of included studies
All trials were conducted in China in 2016, and 1 trial[24] was published in English. Only 2 trials[23,26] adopted new definition (PND). Seven studies recorded the incidence of POD, 8 studies recorded the incidence of DNR and postoperative NCD was recorded in 1 study. Detailed information on the study characteristics is presented in Table 1.
Table 1.
Characteristics of included studies.
| Author | Sex (M/F) | Surgery | Anesthesia method | TEAS group | Control group | Outcomes | |||
|---|---|---|---|---|---|---|---|---|---|
| Method | Acupoint | NO. | Method | NO. | |||||
| Qian, 2021 | 23/41 | Open spinal surgery | i | A 2/100 Hz ab, <10 mA | LI4 PC6 | 32 | ① | 32 | POD at postoperative 7 days (CAM) and DNR at 1 month (TICS-m). |
| Chang, 2021 | 50/35 | Total hip arthroplasty | iv | B 2/100 Hzab, 6–10 mA | HT7 LI4 PC6 | 44 | ① | 41 | POD at 1 day, 3 days, and 5 days postoperatively (CAM) and DNR at 1 month and postoperative NCD at 3 months (neuropsychologic test battery). |
| Wu et al, 2021 | 35/25 | Gastrointestinal cancer Operation | ii | B 2/100 Hzab, 1–30 mA, 0.25 ms | HT7 ST36 PC6 | 30 | ① | 30 | POD at postoperative days 1–7 (CAM). |
| Zhang, 2021 | 27/59 | hip fracture | v | A 2/100 Hzab, 1–7 mA | GV24 GV29 LI4 PC6 | 43 | ① | 43 | POD during the first 3 days after surgery (CAM). |
| Gao et al 2018 | 33/31 | Spine surgery | i | A 2/100 Hzab | LI4 PC6 | 32 | ① | 32 | The incidence of POD during the first 3 days after surgery (CAM-ICU). |
| Liu, 2019 | 82/38 | Radical mastectomy | i | D 2/100 Hzab, 6–10 mA | LI4 PC6 | 59 | ① | 59 | POD at postoperative days 1–3 (CAM-ICU). |
| Wang et al, 2016a | 34/26 | General anesthesia laparoscopic Gastric cancer radical surgery and rectal colon cancer radical surgery | i | A 2/60 Hzab, 8–12 mA | GV20 PC6 ST36 SP6 | 30 | ② | 30 | DNR at 1 day, 3 days, and 7 days postoperatively (MMSE). |
| Wang et al, 2016b | 36/24 | Artificial femoral head replacement | vi | F 2/60 Hzab, 8–12 mA | GV20 PC6# ST36* SP6* | 30 | ② | 30 | DNR at 1 day, 3 days, and 7 days postoperatively (MMSE). |
| Wu and Luo, 2020 | / | Elective noncardiac surgery | i | E 2/60 Hzab, 6 mA | LI4 PC6 | 42 | ① | 42 | DNR at 1 day, 3 days, and 7 days postoperatively (MoCA and MMSE). |
| Tan et al, 2017 | 41/29 | Cholecystectomy | / | G 2/100 Hzab, 0.2–0.6 ms, GV20, PC6: 3~4 mA; ST36, SP6: 6~8 mA | GV20 PC6 ST36 SP6 | 35 | ① | 35 | DNR at 3 days, and 7 days postoperatively (MMSE). |
| Wei et al, 2021 | 39/63 | Hip replacement | iii | C 100 Hzbc | HT7 PC6 | 50 | ① | 52 | POD during the first 3 days after surgery (CAM). |
| Tang and Qing, 2017 | 49/41 | Colorectal cancer surgery | i | A 2/100 Hzab | GV20 GV24 | 45 | ② | 45 | DNR at 1 day, 3 days, 5 days, and 7 days after surgery (MMSE). |
| Wang et al, 2021 | 24/26 | laparoscopic radical bladder cancer surgery | ii | H 2 Hzcd | GV20 PC6 ST36 SP6 | 27 | ② | 29 | DNR at 1 week (MMSE). |
i: Intravenous general anesthesia; ii: Intravenous and inhalational combined anesthesia; iii: Nerve block anesthesia was administered in the supine position, and after pain relief, intraspinal or general anesthesia was administered. For intravertebral anesthesia, combined spinal-epidural anesthesia was administered, and the anesthesia was maintained by intravenous and inhalational combined anesthesia; iv: Subarachnoid block anesthesia in combination with fascial iliac block; v: Combined spinal-epidural anesthesia in combination with fascial iliac block; vi: Combined spinal-epidural anesthesia.
A: 30 minutes before anesthesia to the end of the surgical suture; B: 30 minutes before the start of anesthesia and at PM8:00 on the 1st, 2nd, and 3rd postoperative days, half an hour each time; C:30 min before surgery, 18:00 on the day of surgery, 18:00 on the first day after surgery, 18:00 on the second day after surgery, half an hour each time; D: After anesthesia until the end of the procedure; E: Lasting at the beginning of anesthesia until the end of surgery; F: TEAS group was continually treated before anesthesia to the end of the operation; G: Preoperative 1 day to 7 days postoperative in the morning, 30 minutes each time, 1 time a day; H: 10 minutes before induction of anesthesia until the end of the surgery.
a: 2 Hz (low-frequency) and the 100 Hz (high-frequency) exchange output; b: intensity of tolerable level; c: The current frequency is continuous wave; d: no mentioned of frequency.
LI4: Hegu; PC6: Neiguan; HT7: Shenmen; ST36: Zusanli; SP6: Sanyinjiao; GV20: Baihui; GV24: Shenting; GV29: Yintang. #: bilateral; *: healthy side. ①: Sham intervention; ②: No treatment.
CAM = confusion assessment method, CAM-ICU = confusion assessment method for the intensive care unit, DNR = delayed neurocognitive recovery, MMSE = mini-mental state examination, MoCA = montreal cognitive assessment, POD = postoperative delirium, postoperative NCD = postoperative neurocognitive disorder, TICS-m: telephone interview for cognitive status-modified.
In all trials, however, patients received TEAS on the surgery, before and/or after the procedure, and the details of the treatment were shown in the table. In 13 trials, the TEAS protocol and selection of acupuncture points were designed for the sole purpose of improving rates of PND, 2 trials[23,32] designed acupoints for improving sleep disorders in post-operative patients while observing the effect of TEAS on neurocognitive function. All the 13 RCTs selected fixed acupoint prescriptions with slightly different compositions. The acupoints that were used more than twice among the 13 trials include PC6 (12 times), LI4 (6 times), ST36 (5 times), GV20 (5 times), SP6 (4 times), and HT7 (3 times). The most frequently used acupoint combinations are PC6 + LI4, and GV20 + PC6 + ST36 + SP6. Nine of the trials used a sham control, of which 7 trials applied electrode pads to the acupoints without electrical stimulation. Group C in 1 study[33] was stimulated at the non-acupuncture point (4 cm medial to the same acupoint at the group TEAS). And 1 trial[27] designed the control group which reduced the current intensity to 1 mA when the patient first felt the current stimulus and other contents were the same as the observation group. In 4 trials,[28–31] the control group was given only drug anesthesia without assisting with TEAS during intraoperative anesthesia. According to the diagnosis of DNR and postoperative NCD, 6 trials,[23,27-30,28,29,34] were confirmed by performing baseline cognitive performance tests prior to surgery and comparing the cognitive status following surgery. However, 2 trials[26,31] did not clearly define the incidence of cognitive decline by using cognitive performance tests. In addition to the incidence of PND and scale scores, the levels of different kinds of laboratory indicators in serum at various postoperative time points were also measured by part of our included studies. The details were listed in Table 2. Eight RCTs,[25,26,29,30,32-35,33,34] reported adverse events (AEs), listed in Table 3. These included postoperative nausea and vomiting, postoperative pain, sinus bradycardia, hypotension, re-intubation of the trachea, postoperative wound infection, postoperative pneumonia and postoperative somnolence. None of the other RCTs reported whether AEs had occurred. We performed no subgroup analyses about AEs due to an insufficient number of trials.
Table 2.
Measurements of circulating IL-6, MMP-9, TNF-α, CRP, and S100β levels at different time points.
| Trials | IL-6 (pg/mL) | MMP-9 (ng/mL) | TNF-α (ug/mL) | S100β (pg/mL) | CRP (ug/L) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | T | C | |
| Wang, 2021 | NA | NA | NA | 64.63 ± 19.72a 168.22 ± 28.28c* | 64.90 ± 14.98 205.86 ± 21.04 | NA | ||||
| Chang, 2021 | NA | NA | NA | NA | 10.6 ± 16.5a | 9.1 ± 14.9 | ||||
| 70.7 ± 7.9f* | 85.6 ± 22.9 | |||||||||
| 91.7 ± 11.7h* | 102.9 ± 7.6 | |||||||||
| 39.7 ± 10.3i* | 64.9 ± 9.0 | |||||||||
| Tan, 2017 | NA | NA | NA | 129.4 ± 22.8a | 126.7 ± 19.1 | NA | ||||
| 161.5 ± 23.3h* | 175.2 ± 26.8 | |||||||||
| 136.7 ± 25.6j | 141.4 ± 23.5 | |||||||||
| Liu, 2019 | NA | NA | NA | 150 ± 50a | 140 ± 50 | NA | ||||
| 3070 ± 1260d* | 5270 ± 1350 | |||||||||
| 2270 ± 1170e* | 4860 ± 1630 | |||||||||
| 1070 ± 930f* | 3360 ± 1460 | |||||||||
| 970 ± 270g* | 2640 ± 660 | |||||||||
| Gao et al, 2018 | 35400000 ± 2900000a | 35900000 ± 2700000 | 3.0 ± 0.6a | 3.2 ± 0.5 | 19.9 ± 0.9a | 19.7 ± 0.8 | 52.8 ± 8.9a | 51.5 ± 6.3 | NA | |
| 52400000 ± 6600000b* | 56700000 ± 6500000 | 3.3 ± 0.7b | 3.5 ± 0.5 | 26.7 ± 5.7b* | 30.4 ± 5.6 | 59.9 ± 10.2b | 60.8 ± 7.5 | |||
| 58800000 ± 6100000c* | 62100000 ± 6700000 | 3.7 ± 0.7c* | 4.1 ± 0.6* | 33.4 ± 6.3c* | 38.4 ± 7.1 | 83.2 ± 12.5c* | 90.2 ± 13.1 | |||
| Zhang, 2021 | 32.96 ± 9.80a | 32.05 ± 12.40 | 5.75 ± 3.29a | 4.88 ± 2.29 | NA | 23.27 ± 5.6a | 24.50 ± 3.68 | 53540 ± 21660a | 56020 ± 25800 | |
| 56.78 ± 19.11c | 81.46 ± 21.41 | 5.81 ± 0.83c | 6.11 ± 2.80 | 52.46 ± 23.20c | 63.96 ± 10.23 | 74700 ± 8350c | 87070 ± 26870 | |||
| 74.16 ± 12.92f | 97.27 ± 14.11 | 7.22 ± 2.58f | 7.30 ± 0.78 | 56.60 ± 8.93f | 69.61 ± 12.69 | 97030 ± 9740f | 111160 ± 23400 | |||
C = control group, CRP = C-reactive protein, IL-6 = interleukin-6, MMP-9 = Matrix metalloproteinase-9, S100β = S100 calcium-binding protein β, TNF-α = tumor necrosis factor-alpha, T = TEAS group.
Compared with the Control group, P < .05; NA: not available.
: indicate preoperative, 30 minutes after skin incision, immediate postoperative period, 1 hour after surgery, 12 hours after surgery, 24 hours after surgery, 48 hours after surgery, 72 hours after surgery, 5 days after surgery, and 7 days after surgery, respectively.
Table 3.
Adverse events of included trials.
| Trials | Postoperative Nausea and Vomiting* | Postoperative Pain | Sinus bradycardia* | Hypotension* | Re-intubation of the trachea* | Postoperative wound infection* | Postoperative pneumonia* | Postoperative somnolence* | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T | C | T | C | T | C | T | C | T | C | T | C | T | C | T | C | |
| Qian, 2021 | 4/32 | 12/32 | NA | NA | NA | NA | 2/32 | 4/32 | NA | NA | ||||||
| Wang et al,2016a | NA | 2.6 ± 0.8 | 2.8 ± 1 | NA | NA | NA | NA | NA | NA | |||||||
| Wang et al,2016b | NA | 2.3 ± 1 | 2.5 ± 1 | NA | NA | NA | NA | NA | NA | |||||||
| Wu and Luo, 2020 | 4/42 | 5/42 | NA | NA | NA | NA | NA | NA | NA | |||||||
| Wu et al, 2021 | 1/30 | 6/30 | NA | NA | NA | NA | NA | NA | 2/30 | 9/30 | ||||||
| Zhang, 2021 | 4/43 | 2/43 | 4/43* | 4/43* | NA | NA | NA | NA | 3/43 | 3/43 | NA | |||||
| Wei et al, 2021 | NA | 3.54 ± 0.9a# | 3.5 ± 0.96a# | NA | NA | NA | NA | NA | NA | |||||||
| 2.74 ± 0.77b# | 2.67 ± 0.81b# | |||||||||||||||
| 2.2 ± 0.75c# | 2.15 ± 0.84c# | |||||||||||||||
| Liu, 2019 | NA | NA | 14/59 | 15/59 | 13/59 | 11/59 | 1/59 | 2/59 | NA | NA | NA | |||||
a: The VAS pain score at 6 h postoperatively.
b: The VAS pain score at 24 h postoperatively.
c: The VAS pain score at 48 h postoperatively.
*: Values are numbers: (n/N).
#: The means and standard deviations for the scores of Visual Analogue Scale: ().
3.3. Risk of bias
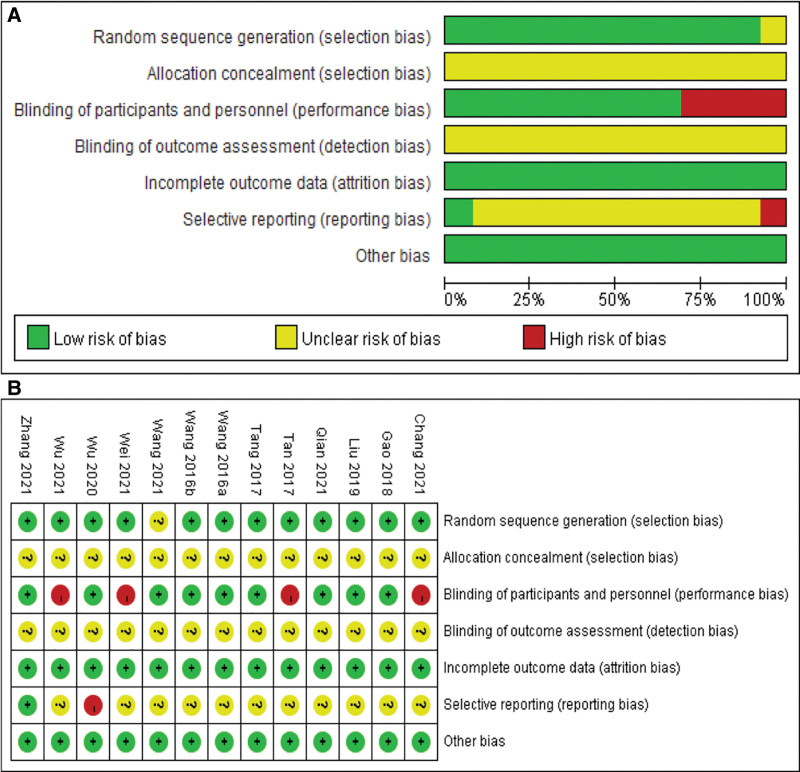
The methodological and reporting quality of the RCTs were generally unsatisfactory. Twelve trials (92.31%) described the method for generating the random allocation sequence by using a random number table, and 1 trial[35] used stratified block randomization method. However, another 1 trial[31] was assigned as “unclear risk” because the articles only mentioned “randomization” and the corresponding authors did not provide further details. All included studies did not clearly describe the proper way to complete allocation concealment. However, the randomization appeared to be successful in creating similar groups, as there was baseline similarity between the 2 groups. In judging the adequacy of blinding, only 9 of the trials were assigned a judgment of “Low risk.” All studies (100%) reported insufficiently whether personnel responsible for outcome assessment were blinded to the intervention. Expected follow-up outcomes were not reported in 1 trial,[33] therefore, we judged this study to have high risk of selective outcome reporting bias. Most of the studies did not report clinical trials registration or prospectively published study protocols except for 1,[35] so selective reporting was difficult to judge. Almost all the trials had no information on other risks of bias (Fig. 2).
Figure 2.
Assessment of ROB using the Cochrane tool. (A) ROB graph and (B) ROB summary. ROB = risk of bias.
3.4. Synthesis of the results
3.4.1. The primary outcomes
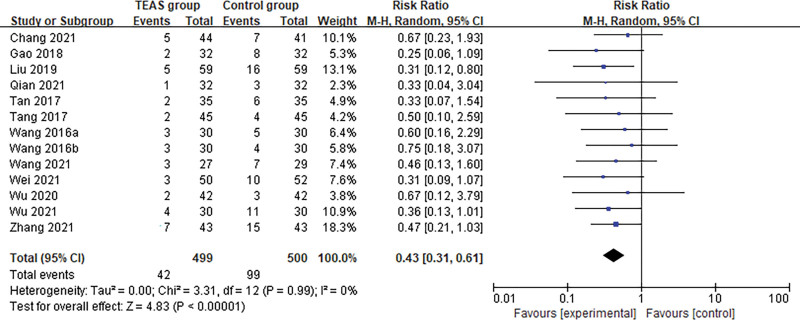
Thirteen trials including 999 patients undergoing surgery reported the outcome of incidence of PND between the TEAS group (n = 499) and the control group (sham intervention or no intervention, n = 500). After synthesizing the data, the results showed that TEAS significantly reduced the incidence of PND in senile patients after surgery compared with the control group (Random effects; RR: 0.43; 95% confidence interval [95% CI]: 0.31, 0.61; P < .001; I2 = 0%). (Fig. 3)
Figure 3.
Meta-analysis and forest plot for the incidence of PND. PND = perioperative neurocognitive disorders.
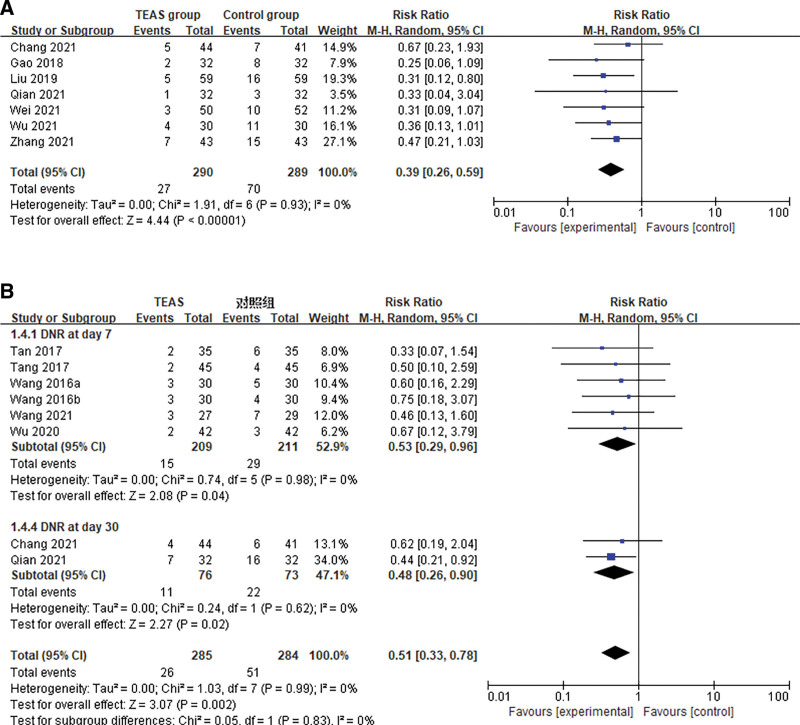
Seven trials including 579 patients undergoing surgery reported the outcome of incidence of POD between the TEAS group (n = 290) and the sham intervention group (n = 289). After synthesizing the data, the results showed that TEAS significantly reduced the incidence of POD in senile patients after surgery compared with the control group (Random effects; RR: 0.39; 95% CI: 0.26, 0.59; P < .001; I2 = 0%). (Fig. 4A)
Figure 4.
Meta-analysis and forest plot for the incidence of the different subtypes of the PND. (A) POD and (B) DNR. DNR = delayed neurocognitive recovery, PND = perioperative neurocognitive disorders.
Eight trials based on data from 569 (TEAS group, n = 285; control group, n = 284) patients showed that the use of TEAS did significantly prevent DNR after major surgeries compared to that of control treatment (Random effects; RR: 0.51; 95% CI: 0.33, 0.78; P = .002; I2 = 0%), without significant between-study heterogeneity (I2 = 0%, P = .99). We found significantly benefits of TEAS in reducing the incidence of DNR at both postoperative day 7 and day 30 (RR: 0.53; 95% CI: 0.29, 0.96; RR: 0.48; 95% CI: 0.26, 0.90) (Fig. 4B). And 1 trial reported that no statistical difference was observed between the TEAS group and control group after 3 months (P > .05). Due to the limited number of articles, the incidence of postoperative NCD was not analyzed for this.
3.4.2. The secondary outcomes
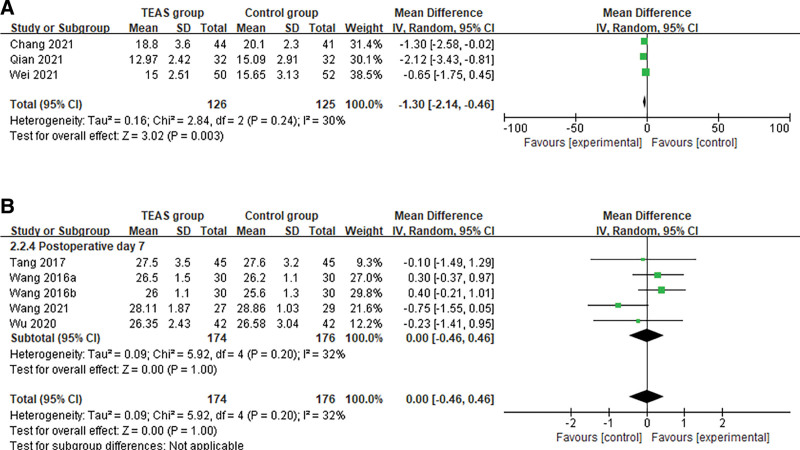
Only 3 trials provided clear data use of the CAM scale during the postoperative period. The main results, including heterogeneity tests, effect models adopted accordingly, and the pooled MDs with their 95% CI: and the P value of this meta-analysis were presented in Figure 5A, suggesting that heterogeneity was not apparent. It is showed that the results with continuous data focusing on CAM scores were credible within 7 days after the surgery. Using the random-effects model, the pooled MD for the CAM scores within 7 days was −1.30 (95% CI: −2.14, −0.46, Z = 3.02; P = .003; I2 = 30%), indicating that in terms of CAM scores within 7 days postoperatively, TEAS would improve the scores of cognitive function in the elderly patients. A total of 5 trials were included in the analysis, we found no statistically significant pooled benefits of TEAS relative to either control, for the clinical MMSE scores within 3 months (MD 0.00, 95% CI: −0.46, 0.46; P = 1.00), and the overall statistical heterogeneity was no substantial (I2 = 32%) (Fig. 5B). This may be partly because the assessment of cognitive function is highly subjective. Only 1 trial[23] included long-term follow-up time points, and this trial did find both significant difference between TEAS group and sham group in the MMSE scores of postoperative elderly patients at 1 and 3 months (P < .05). Despite its contributions, the study has several limitations that should be acknowledged.
Figure 5.
Meta-analysis and forest plot for cognitive function scores. (A) CAM within 7 days. (B) MMSE within 3 months. CAM = confusion assessment method, MMSE = mini-mental state examination.
A total of 4 studies from 324 (TEAS group, n = 161; control group, n = 163) patients which measured the level of S100β respectively in controls and in TEAS groups before treatment and at the end of the operations. Using the random-effects model, we found statistically significant effects of utilization of TEAS intervention on S100β (SMD = −1.08, 95% CI: −1.67, –0.49, P < .001) [see Fig. S1A, Supplemental Digital Content, http://links.lww.com/MD/I156, which illustrates the meta-analysis and forest plot for biochemical indicator (S100β)]. In addition, we found significant pooled benefits of TEAS used to reduce remifentanil requirements (Random effects; SMD: −0.99; 95% CI: −1.74, −0.25; P < .001) over propofol (P = .37) among 3 trials (see Fig. S1B, Supplemental Digital Content, http://links.lww.com/MD/I156, which illustrates the meta-analysis and forest plot for dosage of anesthetics). However, there were high heterogeneity in the studies that measured changes in S100β levels (I2 = 83%) and anesthetic requirements (I2 = 91%).
3.5. Subgroup analysis
Most of the intervention groups in the studies (n = 7, 53.85%) were given TEAS starting 30 minutes before induction of anesthesia and continuing until the end of the procedure. Meanwhile, the most of parameters (n = 9, 69.23%),[23-26,28-30,33,35] were set as follows: a frequency of sparse and dense waves of 2/100 Hz within the max-tolerance intensity of each patient. Most of the intervention groups (n = 12, 92.31%) used TEAS during surgeries, and 3 of them used TEAS both intraoperative and postoperative period. We observed the efficacy of 2 kinds of TEAS intervention: the treatment groups underwent TEAS during the surgery; the treatment groups also underwent TEAS in the post-operative period. A total of twelve trials for the prevention of PND were included in the analysis, 9 trials analyzed the efficacy of utilization of TEAS during the surgery and 3 trials also used TEAS after the operations. We found significant pooled benefits of TEAS used in 2 ways to reduce the incidence of PND within 7 days postoperatively (Random effects; RR: 0.45; 95% CI: 0.31, 0.63; P < .001; I2 = 0%) (see Fig. S2A, Supplemental Digital Content, http://links.lww.com/MD/I157, which illustrates the meta-analysis and forest plot for the different protocols of TEAS). However, due to the small sample size, further confirmation is still needed. All RCTs selected fixed acupoint prescriptions with slightly different compositions. In the subgroup analysis of acupoint selection, we observed the efficacy of 2 kinds of acupoint combinations used more than 2 times in the included studies. Our meta-analysis showed that acupoint combination (LI4 and PC6) in the TEAS group had more significantly advantages in preventing PND (Random effects; RR: 0.34; 95% CI: 0.17, 0.67; P = .002; I2 = 0%) than GV20, PC6, ST36 and SP6 (P = .06) (see Fig. S2B, Supplemental Digital Content, http://links.lww.com/MD/I157, which illustrates the meta-analysis and forest plot for the different choice of acupoints).
We also performed subgroup analyses according to the surgery type and anesthetic modality. The results were shown in Supplemental Digital Content. Figure S3A and B, Supplemental Digital Content, http://links.lww.com/MD/I158. Six trials[23,24,26,30,32,35] reported outcomes following orthopedic surgery and 5 trials[25,27–29,31,33] following abdominal surgery of TEAS compared with control. The results of the heterogeneity test showed that the heterogeneity of the included studies was small (P = .99, I2 = 0%). The meta-analysis of the pooled data showed patients in the TEAS group had had a lower incidence of PND than those in the control group both orthopedic surgery and abdominal surgery (Random effects; RR: 0.43; 95% CI: 0.30, 0.60; P < .001). Five trials were performed under general anesthesia while 2 trials were performed under intravenous and inhalational combined anesthesia. There was no significant heterogeneity of the overall result (P = .87, I2 = 0%). The meta-analysis of pooled data showed that the incidence of PND was lower in patients in the TEAS group than in the control group regardless of the perioperative use of intravenous anesthesia and intravenous and inhalational combined anesthesia (Random effects; RR: 0.38; 95% CI: 0.23, 0.61; P < .001).
3.6. Sensitivity analysis and publication bias
Owing to the significant heterogeneity in biochemical indicator (S100β) and intraoperative dose of anesthetics, we conducted a sensitivity analysis to explore the source of heterogeneity by removing 1 study in each turn, and to examine the stability of the main outcome by excluding poor-quality trials with high risks of bias. We found that the 2 results were relatively robust, and the data was not overly influenced by any 1 study (Tables 4 and 5). The funnel plot (Figure S4, Supplemental Digital Content, http://links.lww.com/MD/I159) showed small study effects, with the intervention effects estimated in smaller studies showing more benefit than the effects estimated in larger studies. No obvious publication bias was observed from the roughly symmetrical shapes of these funnel plots.
Table 4.
Sensitivity analysis for the serum levels of biochemical indicators (S100β).
| Deletion | OR (95% CI) | I2 statistic (%) |
|---|---|---|
| Zhang, 2021 | OR = −1.24, 95% CI [−1.96, −0.52] | 84 |
| Wang, 2021 | OR = −0.96, 95% CI [−1.69, −0.23] | 87 |
| Liu, 2019 | OR = −0.86, 95% CI [−1.40, −0.32] | 71 |
| Gao et al, 2018 | OR = −1.26, 95% CI [−1.94, −0.58] | 84 |
Table 5.
Sensitivity analysis for dosage of anesthetics.
| Deletion | OR (95% CI) | I2 statistic (%) | |
|---|---|---|---|
| Remifentanil | Gao et al, 2018 | OR = −0.68, 95% CI [−1.29, −0.08] | 84 |
| Wang, 2021 | OR = −1.01, 95% CI [−1.92, −0.09] | 93 | |
| Wu et al, 2021 | OR = −0.95, 95% CI [−1.86, −0.05] | 92 | |
| Propofol | Gao et al, 2018 | OR = −1.02, 95% CI [−1.95, −0.09] | 93 |
| Wang, 2021 | OR = −1.29, 95% CI [−1.86, −0.72] | 81 | |
| Wu et al, 2021 | OR = −1.02, 95% CI [−1.95, −0.10] | 93 | |
3.7. Certainty of evidence
Tables S1 and S2, Supplemental Digital Content, http://links.lww.com/MD/I160 show the summary of findings for all outcomes, including the certainty of evidence. However, all trials of TEAS were of only very low to low quality. We downgraded the certainties by 2 levels, either due to the use of small single studies (substantial imprecision) or high levels of inconsistency (substantial heterogeneity).
4. Discussion
4.1. Summary of main results
In this systematic review, 13 RCTs (999 patients) in total were included for meta-analysis. In general, the results of this meta-analysis revealed that TEAS, as a low-cost, available, safe and convenient intervention method, can effectively prevent the cognitive impairment in the perioperative period. The merged data indicated that TEAS is more beneficial for preventing the incidence of PND (POD within postoperative 7 days, DNR within postoperative 30 days) in elderly patients (>60-year-old) compared with the sham or blank controls. It is shown that TEAS also improves the scores of the cognitive function (CAM) of elderly patients after the surgery. Furthermore, subgroup analysis showed that more favorable results were also observed when TEAS was used intraoperatively only than in combination with postoperative intervention. We found that acupoint combination (LI4 and PC6) in the TEAS group had more significantly advantages in preventing PND (P = .002; I2 = 0%) than GV20, PC6, ST36 and SP6. TEAS used in both orthopedic surgery and abdominal surgery were beneficial in reducing the incidence of PND compared with controls. Similar results were seen with a different anesthetic modality (intravenous general anesthesia and intravenous and inhalational combined anesthesia). However, due to the high heterogeneity, limited evidence suggested that TEAS could reduce the serum levels of biochemical indicator (S100β) as well as anesthetic requirements (remifentanil and propofol). Sensitivity analysis using the leave-one-out approach indicated the findings are robust and not dependent on any 1 study. In addition, no publication bias was detected. Our findings indicate that TEAS could reduce the incidence of PND, especially within 7 days after surgery, supporting the use of TEAS as adjuvant therapy in preventing PND. Confidence in these results is still limited due to the poor risk of bias and overall quality of all included studies. However, included studies suggest TEAS has a good safety profile for the elderly during the perioperative period, and may be considered as a therapeutic option, particularly there are no effective drugs to be used in treatment and prevention of PND in geriatric patients.
4.2. Applicability for clinical practice and implication for future RCTs
Since classical acupuncture is highly dependent on the skill of the acupuncturist and some people are afraid of the painful sensation of puncturing, TEAS is becoming a popular trend because of its noninvasive nature, adjustable strength, frequency and easy quantification in the clinic. And TEAS is widely used in the perioperative period, and some studies have confirmed that TEAS has gastrointestinal regulation,[36] circulatory improvement,[37] immune enhancement,[38,39] anti-inflammatory,[40] and stress-reduction effects,[41] which would lead to a shorter time of convalescence and hospitalization, improving the quality of life of patients. In terms of the prevention of PND in elderly patients, as the first comprehensive systematic review and meta-analysis after the new definition in 2018, the results may have important implications for clinicians and researchers.
In the present review, different clinical groups used different protocols like various traditional acupoints locations, different intervention periods and waves, frequencies and intensities for the prevention of PND. The selection of acupoints is an important factor in the TEAS treatment. Based on the recommendations of the expert consensus survey,[42] acupoint combination should be performed in accordance with syndrome differentiation and personal characteristics. However, all trials in recent and our review selected fixed acupoint prescriptions. As the main method for PND is prevention, most researchers preferred to use TEAS 30 minutes before anesthesia to the end of the surgical suture. Our subgroup analysis suggested that intraoperative use of TEAS may be more effective and is not restricted to surgery type and anesthetic modality. However, most of the trials included in our review have only observed that the use of TEAS can achieve prevention of PND in the early postoperative period, which lead the insufficient evidence for its preventive effect on distant postoperative NCD. And the retention time of TEAS also depends on the time of surgery and was not well documented in 14 included trials. In addition, parameter settings: a frequency of sparse and dense waves of 2/100 HZ within the max-tolerance intensity of each patient can be regarded as a reference for clinicians and researchers. It is critical that future trials should seek to determine the optimal protocol during the perioperative period. However, the time to assess cognitive function and the tools used to evaluate the severity of cognitive dysfunction were different among our included 13 studies, and the definition of PND also varied. The main determinants of the incidence of PND are the type of cognitive performance tests, time of postoperative assessments, and specificity and sensibility of the cognitive tests.[72] In general, it is mandatory to train the team on the basic features of cognitive dysfunction as well as the features of any tools that will be used.[44] Future authors could improve study quality and comparability through optimal timing of assessment, the use of commonly used cognitive tests, the application of appropriate diagnostic rules, and detailed reporting of the methods used.
4.3. How the intervention might work
PND results from a complex interaction of different factors.[43] This complexity greatly increases the difficulties in the prevention and treatment. The latest guidelines and reviews[44–47] presented the consensus statements for preoperative, intraoperative, and postoperative risk factors for PND, such as preoperative cognitive impairment, older age, surgery type, inappropriate depth of anesthesia, poor pain control, anemia, and infections.[48–51] Apart from this, few studies comprehensively described risk factors for late PND. Early PND was likely a predictor of late PND,[52] therefore, it is particularly important to prevent early PND. The underlying mechanisms of the condition is still unknown, numerous studies have shown that TEAS, as adjunctive therapy, can significantly reduce anesthetic requirements,[53] and current theories related to the pathogenesis of PND include a central inflammatory response,[54,55] reduced function of the central cholinergic system,[56,57] synaptic dysfunction,[58,59] abnormal protein function,[60,61] disturbed microflora of the gut,[62] etc. Among them, the central inflammatory response mechanism is the most important and has received wide attention. Early studies have shown that TEAS can reduce central and peripheral inflammatory responses, inhibit microglia activation,[63,64] restrain the neuronal apoptosis, and execute an important cerebral protective role.[65] Meanwhile, our systematic review found that the use of TEAS during surgery can reduce the inflammatory reaction perioperatively (Table 2). However, it is difficult to perform a comprehensive meta-analysis because of the different types of inflammatory factors observed in each included trials, as well as the inconsistent timing of blood sampling. The present meta-analysis only found that the levels of S100β during the immediate post-operative period were attenuated by the use of TEAS in elderly patients undergoing surgeries, although the evidence level was downgraded to very low due to statistical heterogeneity. To better investigate this potential mechanism, we strongly recommend the serum levels of inflammatory factors and postoperative complications be measured in the terms of uniform timing and methods.
4.4. Strengths and limitations
The strengths of this review include the pooled risk ratios that are highly significant and clinically important; fairly consistent effect sizes across trials; homogeneity of the TEAS protocols; and overall high validity of the trials, as well as robustness of the results to sensitivity analyses on the effects of study validity variables.
Our review is based on a new definition of surgery-related cognitive decline published in 2018, thus providing the most comprehensive update on the effectiveness of TEAS used in perioperative geriatric patients in this area. The terminology of cognitive change in 2018[6] could offer a framework for understanding the impact of an esthesia and surgery on outcomes for the elderly in order to promote cross-specialty communication, aid clinical management of patients, and further high-quality research. Currently, a meta-analysis and review that meet the new definition is still lacking, so we performed qualitative and quantitative analysis according to the available data. Notably, we included the largest number of RCTs for the most comprehensive analysis of POD, which have been limitations of previous trials and SRs. In addition, a pre-registered protocol and a comprehensive literature search including unpublished sources, duplicate and independent screening and data abstraction, which all improve the credibility and generalizability of our findings. And GRADE assessment of certainty of evidence was utilized. In the meantime, we carefully developed the more comprehensive inclusion criteria for the present review to aim for higher homogeneity and thus reproducibility. First, the effect of anesthesia on the incidence of PND has still been inconsistent and controversial.[66–68] We therefore chose patients who undergo surgical treatments without limitation on the type of operation and anesthetic modality. Indeed, several studies have now shown that the incidence of PND is similar for different surgeries.[69–71] Third, we considered these RCTs to be fundamentally different, in terms of the direction of the research. That is, when TEAS combined with other anesthetic drugs such as dexmedetomidine is used intraoperative and the control group was given an anesthetic drug alone on top of the basic anesthesia, the primary purpose is to evaluate the role of the combination of the 2 without highlighting the role of TEAS.
There were also several potential limitations. There was little clinical expected heterogeneity in several aspects of the included trials, such as precise terms, outcome measurement timings and definitions, and other unpredictable factors during the perioperative period such as fluid therapy, analgesia types, etc. This information was not universally available, so we were still unable to clearly identify the source of some of the heterogeneity. Due to details relating to sequence generation, allocation concealment and selective outcome reporting were lacking, none of the studies were free from bias. The GRADE approach is prone to downgrade research that is not double-blinded, which is challenging in studies of manual therapies. The necessity to blind participants, however, is arguable when the intervention is performed intraoperatively. It seems unlikely that the patient will know intraoperatively whether he is receiving a TEAS intervention or not. Although the quality of evidence is limited, the role of TEAS remains a means of prevention of PND worthy of further investigation, considering that even specific selective a2-adrenaline receptor agonist (i.e., dexmedetomidine) and monitoring technologies (i.e., electroencephalography), there are still no meta-analyses[73,74] evaluating these methods of preventing PND using GRADE approach. And then, the long-term such as 1 year after surgery results are unknown, because the observation time of the included studies for PND was mostly within 1 week after surgery. Future trials with larger sample sizes and longer follow-up are needed to draw firmer conclusions. Although it is challenging, trialists should attempt to use standardized endpoints (for both efficacy and adverse effects) and consider aspects relating to the cost and acceptability of interventions. These data are needed to enable the best synthesis of the evidence for making recommendations and informing clinical practice.
5. Conclusion
Results of the present review and meta-analysis suggest that the utilization of TEAS during the perioperative period will prevent the incidence of PND in geriatric patients. However, TEAS interventions lack data support in terms of long-term cognitive function. Findings from this review should be interpreted with caution, taking into consideration the biases identified and limited quality of evidence of the included trials. Further researchers need to resolve the low quality of studies and investigate the efficacy of different application regimens of TEAS.
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
Conceptualization: Shuying Li, Hailun Jiang.
Data curation: Shuying Li, Hailun Jiang, Wei Liu.
Formal analysis: Yu Yin, Hailun Jiang.
Funding acquisition: Yuzheng Du, Qi Zhao, Yi Zhang, Chen Li.
Investigation: Hailun Jiang, Chunsheng Yin.
Methodology: Shuying Li, Hailun Jiang, Wei Liu, Chen Li.
Software: Yu Yin, Hao Chen, Qi Zhao.
Validation: Shuying Li, Yu Yin, Yi Zhang.
Writing – original draft: Shuying Li, Hailun Jiang, Wei Liu.
Writing – review & editing: Shuying Li, Hailun Jiang, Yuzheng Du.
All authors read and approved the final manuscript.
Supplementary Material
Abbreviations:
- 95% CI =
- 95% confidence interval
- AEs =
- adverse events
- DNR =
- delayed neurocognitive recovery
- MD =
- mean difference
- PND =
- perioperative neurocognitive disorders
- POD =
- postoperative delirium
- postoperative NCD =
- postoperative neurocognitive disorders
- RCTs =
- randomized controlled trials
- RR =
- risk ratio
- SRs =
- systematic reviews
- TEAS =
- transcutaneous electrical acupoint stimulation
Supplemental Digital Content is available for this article.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
This work was supported by grants from the National Key Research and Development Program (NO. 2018YFC1706004), National Key Research and Development Program (NO. 2019YFC0840709), and Tianjin Science and Technology Project (NO. 18PTLCSY00060).
Authors declare that there are no conflicts of interest regarding the publication of this paper. Statement of human and animal rights: This article does not contain any studies with human participants or animals performed by any of the authors.
Patient and public involvement: There was no patient and public involvement in present meta-analysis. An ethical approval is not necessary for a meta-analysis.
How to cite this article: Li S, Jiang H, Liu W, Yin Y, Yin C, Chen H, Du Y, Zhao Q, Zhang Y, Li C. Transcutaneous electrical acupoint stimulation for the prevention of perioperative neurocognitive disorders in geriatric patients: A systematic review and meta-analysis of randomized controlled trials. Medicine 2022;101:50(e32329).
Contributor Information
Shuying Li, Email: lichen741206@sina.com.
Hailun Jiang, Email: woqumaibocai@163.com.
Wei Liu, Email: junebomer@hotmail.com.
Yu Yin, Email: yihaixiaosheng@163.com.
Chunsheng Yin, Email: yihaixiaosheng@163.com.
Hao Chen, Email: how7302@163.com.
Qi Zhao, Email: houhaowen2@163.com.
Yi Zhang, Email: zhangyi790707@163.com.
Chen Li, Email: lichen741206@sina.com.
References
- [1].Daiello LA, Racine AM, Yun GR, et al. Postoperative delirium and postoperative cognitive dysfunction: overlap and divergence. Anesthesiology. 2019;131:477–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Gustafson Y, Berggren D, Brännström B, et al. Acute confusional states in elderly patients treated for femoral neck fracture. J Am Geriatr Soc. 1988;36:525–30. [DOI] [PubMed] [Google Scholar]
- [3].Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].O’Keeffe ST, Ní CA. Postoperative delirium in the elderly. Br J Anaesth. 1994;73:673–87. [DOI] [PubMed] [Google Scholar]
- [5].Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term postoperative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International study of post-operative cognitive dysfunction. Lancet. 1998;351:857–61. [DOI] [PubMed] [Google Scholar]
- [6].Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Br J Anaesth. 2018;121:1005–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Marcantonio ER. Postoperative delirium: A 76-year-old woman with delirium following surgery. JAMA. 2012;308:73–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Johnson T, Monk T, Rasmussen LS, et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96:1351–7. [DOI] [PubMed] [Google Scholar]
- [9].Steinmetz J, Christensen KB, Lund T, et al. Long-term consequences of postoperative cognitive dysfunction. Anesthesiology. 2009;110:548–55. [DOI] [PubMed] [Google Scholar]
- [10].Sprung J, Roberts RO, Weingarten TN, et al. Postoperative delirium in elderly patients is associated with subsequent cognitive impairment. Br J Anaesth. 2017;119:316–23. [DOI] [PubMed] [Google Scholar]
- [11].Alam A, Hana Z, Jin Z, et al. Surgery, neuroinflammation and cognitive impairment. EBioMedicine. 2018;37:547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Young J, Murthy L, Westby M, et al. Prevention, and management of delirium: summary of NICE guidance. BMJ. 2010;341:c3704. [DOI] [PubMed] [Google Scholar]
- [13].Kalisvaart KJ, de Jonghe JF, Bogaards MJ, et al. Haloperidol prophylaxis for elderly hip-surgery patients at risk for delirium: a randomized placebo-controlled study. J Am Geriatr Soc. 2005;53:1658–66. [DOI] [PubMed] [Google Scholar]
- [14].Kotekar N, Shenkar A, Nagaraj R. Postoperative cognitive dysfunction - current preventive strategies. Clin Interv Aging. 2018;13:2267–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang T, Ou L, Chen Z, et al. Transcutaneous electrical acupoint stimulation for the prevention of postoperative cognitive dysfunction: a systematic review and meta-analysis. Front Med. 2021;8:756366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Inouye SK, van Dyck CH, Alessi CA, et al. Clarifying confusion: The confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–8. [DOI] [PubMed] [Google Scholar]
- [18].Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10. [DOI] [PubMed] [Google Scholar]
- [19].Folstein MF, Folstein SE, McHugh PR. “Mini-mental state.” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–98. [DOI] [PubMed] [Google Scholar]
- [20].Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Cumpston M, Li T, Page MJ, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019;10:D142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Chang M. Z. Effect of percutaneous acupoint electrical stimulation on postoperative fatigue and neurocognitive disorders inelderly patients with sleep disorder undergoing total hiparthroplasty. Dalian Medical University; 2021. [Google Scholar]
- [24].Gao F, Zhang Q, Li Y, et al. Transcutaneous electrical acupoint stimulation for prevention of postoperative delirium in geriatric patients with silent lacunar infarction: a preliminary study. Clin Interv Aging. 2018;13:2127–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Liu W. Effects of transcutaneous acupoint electrical stimulation on postoperative delirium and early rehabilitation of elderly patients undergoing radical mastectomy. J Basic Clin Oncol. 2019;32:400–4. [Google Scholar]
- [26].Qian XW. Effect of TEAS on perioperative neurocognitive disorders in elderly patients undergoing open spine surgery. Beijing Univ Chin Med. 2021. [Google Scholar]
- [27].Tan XH. Effect of TEAS on cognitive function and serum S100 beta protein in senile patients after cholecystectomy. J Clin Acupunc Moxib. 2017;33:9–12. [Google Scholar]
- [28].Tang Y, Qing MJ. Effect of transcutaneous electrical acupoint stimulation on postoperative cognitive function in elderly patients with colorectal cancer. J Mod Med Health. 2017;33:3623–5. [Google Scholar]
- [29].Wang DD, Ma TT, Zhou C, et al. Clinical observation of transcutaneous acupoint electrical stimulation on cognitive dysfunction in elderly patients after general anesthesia abdominal surgery. Zhejiang J Int Trad Chin West Med. 2016a;26:732–5. [Google Scholar]
- [30].Wang DD, Peng CB, Ma TT, et al. Effect of transcutaneous acupoint electrical stimulation on postoperative cognitive function in elderly patients with artificial femoral head replacement. Chin Arch Trad Chin Med. 2016b;34:431–3. [Google Scholar]
- [31].Wang HD. The effect of TEAS and dexmedetomidine on postoperative cognitive dysfunction in elderly. Shanxi Univ Chin Med. 2021. [Google Scholar]
- [32].Wei L, Luo W, Huang J, et al. The effect of transcutaneous electric acupointstimulation of Shenmen and Neiguan Pointson sleep quality and postoperative delirium in elderly patients undergoing hip replacement. Int J Anesthesiol Resusc. 2021;42:1056–60. [Google Scholar]
- [33].Wu HY, Gao H, Mi ZH, et al. Effect of transcutaneous electrical acupoint stimulation on postoperative delirium in frail elderly patients. Chin J Anesthesiol. 2021;41:723–6. [Google Scholar]
- [34].Wu YJ, Luo HL. Effect of electrical acupoint stimulation on postoperative cognitive dysfunction after total intravenous anesthesia. Shanghai J Acupunc Moxib. 2020;39:1161–5. [Google Scholar]
- [35].Zhang YL. Effect of transcutaneous electrical acupoint stimulation on postoperative delirium in elderly patients with hip fracture. Guangzhou Univ Chin Med. 2021. [Google Scholar]
- [36].Gao W, Li W, Yan Y, et al. Transcutaneous electrical acupoint stimulation applied in lower limbs decreases the incidence of paralytic ileus after colorectal surgery: a multicenter randomized controlled trial. Surgery. 2021;170:1618–26. [DOI] [PubMed] [Google Scholar]
- [37].Chen Y, Gong Y, Huai X, et al. Effects of transcutaneous electrical acupuncture point stimulation on peripheral capillary oxygen saturation in elderly patients undergoing colonoscopy with sedation: a prospective randomized controlled trial. Acupunct Med. 2021;39:292–8. [DOI] [PubMed] [Google Scholar]
- [38].Chen WT, Wei JF, Wang L, et al. Effects of perioperative transcutaneous electrical acupoint stimulation on monocytic HLA-DR expression in patients undergoing coronary artery bypass grafting with cardiopulmonary bypass: study protocol for a double-blind randomized controlled trial. Trials. 2019;20:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Ao L, Shi J, Bai Y, et al. Effects of transcutaneous electrical acupoint stimulation on perioperative immune function and postoperative analgesia in patients undergoing radical mastectomy: a randomized controlled trial. Exp Ther Med. 2021;21:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Mo Y, Chen S, Yang L, et al. The effect of transcutaneous electrical acupoint stimulation on inflammatory response in patients undergoing limb Ischemia-Reperfusion. Mediators Inflamm. 2017;2017:8369737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bai WY, Yang YC, Teng XF, et al. Effects of transcutaneous electrical acupoint stimulation on the stress response during extubation after general anesthesia in elderly patients undergoing elective supratentorial craniotomy: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2018;30:337–46. [DOI] [PubMed] [Google Scholar]
- [42].Su XT, Wang LQ, Li JL, et al. Acupuncture therapy for cognitive impairment: a delphi expert consensus survey. Front Aging Neurosci. 2020;12:596081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Olotu C. Postoperative neurocognitive disorders. Curr Opin Anaesthesiol. 2020;33:101–8. [DOI] [PubMed] [Google Scholar]
- [44].Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol. 2017;34:192–214. [DOI] [PubMed] [Google Scholar]
- [45].Borozdina A, Qeva E, Cinicola M, et al. Perioperative cognitive evaluation. Curr Opin Anaesthesiol. 2018;31:756–61. [DOI] [PubMed] [Google Scholar]
- [46].Hughes CG, Boncyk CS, Culley DJ, et al. American society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg. 2020;130:1572–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Oh ST, Park JY. Postoperative delirium. Korean J Anesthesiol. 2019;72:4–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Gan J, Tu Q, Miao S, et al. Effects of oxycodone applied for patient-controlled analgesia on postoperative cognitive function in elderly patients undergoing total hip arthroplasty: a randomized controlled clinical trial. Aging Clin Exp Res. 2020;32:329–37. [DOI] [PubMed] [Google Scholar]
- [49].Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275:852–7. [PubMed] [Google Scholar]
- [50].Lynch EP, Lazor MA, Gellis JE, et al. The impact of postoperative pain on the development of postoperative delirium. Anesth Analg. 1998;86:781–5. [DOI] [PubMed] [Google Scholar]
- [51].Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth. 2013;110(Suppl 1):i98–105. [DOI] [PubMed] [Google Scholar]
- [52].Damuleviciene G, Lesauskaite V, Macijauskiene J. [Postoperative cognitive dysfunction of older surgical patients]. Medicina. 2010;46:169–75. [PubMed] [Google Scholar]
- [53].Greif R, Laciny S, Mokhtarani M, et al. Transcutaneous electrical stimulation of an auricular acupuncture point decreases anesthetic requirement. Anesthesiology. 2002;96:306–12. [DOI] [PubMed] [Google Scholar]
- [54].Saxena S, Lai IK, Li R, et al. Neuroinflammation is a putative target for the prevention and treatment of perioperative neurocognitive disorders. Br Med Bull. 2019;130:125–35. [DOI] [PubMed] [Google Scholar]
- [55].Subramaniyan S, Terrando N. Neuroinflammation and perioperative neurocognitive disorders. Anesth Analg. 2019;128:781–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kalb A, von Haefen C, Sifringer M, et al. Acetylcholinesterase inhibitors reduce neuroinflammation and -degeneration in the cortex and hippocampus of a surgery stress rat model. PLoS One. 2013;8:e62679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Xiong L, Duan L, Xu W, et al. Nerve growth factor metabolic dysfunction contributes to sevoflurane-induced cholinergic degeneration and cognitive impairments. Brain Res. 2019;1707:107–16. [DOI] [PubMed] [Google Scholar]
- [58].Orser BA, Wang DS. GABAA receptor theory of perioperative neurocognitive disorders. Anesthesiology. 2019;130:618–9. [DOI] [PubMed] [Google Scholar]
- [59].Shui M, Sun Y, Lin D, et al. Anomalous levels of CD47/Signal regulatory protein alpha in the hippocampus lead to excess microglial engulfment in mouse model of perioperative neurocognitive disorders. Front Neurosci. 2022;16:788675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ballweg T, White M, Parker M, et al. Association between plasma tau and postoperative delirium incidence and severity: a prospective observational study. Br J Anaesth. 2021;126:458–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Lage C, González-Suárez A, Alcalde-Hierro MP, et al. Major surgery affects memory in individuals with cerebral amyloid-β pathology. J Alzheimers Dis. 2021;79:863–74. [DOI] [PubMed] [Google Scholar]
- [62].Xu X, Hu Y, Yan E, et al. Perioperative neurocognitive dysfunction: Thinking from the gut? Aging. 2020;12:15797–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Feng X, Valdearcos M, Uchida Y, et al. Microglia mediate postoperative hippocampal inflammation and cognitive decline in mice. JCI Insight. 2017;2:e91229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Wang DS, Terrando N, Orser BA. Targeting microglia to mitigate perioperative neurocognitive disorders. Br J Anaesth. 2020;125:229–32. [DOI] [PubMed] [Google Scholar]
- [65].Yuan S, Zhang X, Bo Y, et al. The effects of electroacupuncture treatment on the postoperative cognitive function in aged rats with acute myocardial ischemia-reperfusion. Brain Res. 2014;1593:19–29. [DOI] [PubMed] [Google Scholar]
- [66].Cohendy R, Brougere A, Cuvillon P. Anaesthesia in the older patient. Curr Opin Clin Nutr Metab Care. 2005;8:17–21. [DOI] [PubMed] [Google Scholar]
- [67].Sprung J, Schulte PJ, Knopman DS, et al. Cognitive function after surgery with regional or general anesthesia: A population-based study. Alzheimers Dement. 2019;15:1243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gao Y, Liu L, Zhao B, et al. Effect of general and non-general anesthesia on postoperative cognitive dysfunction. J Coll Physicians Surg Pak. 2020;30:407–11. [DOI] [PubMed] [Google Scholar]
- [69].Evered LA, Silbert BS. Postoperative cognitive dysfunction and noncardiac surgery. Anesth Analg. 2018;127:496–505. [DOI] [PubMed] [Google Scholar]
- [70].Evered L, Scott DA, Silbert B, et al. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112:1179–85. [DOI] [PubMed] [Google Scholar]
- [71].Selnes OA, Grega MA, Borowicz LJ, et al. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. Ann Thorac Surg. 2003;75:13771384–841386. [DOI] [PubMed] [Google Scholar]
- [72].van Harten AE, Scheeren TW, Absalom AR. A review of postoperative cognitive dysfunction and neuroinflammation associated with cardiac surgery and anaesthesia. Anaesthesia. 2012;67:280–93. [DOI] [PubMed] [Google Scholar]
- [73].Ding L, Chen DX, Li Q. Effects of electroencephalography and regional cerebral oxygen saturation monitoring on perioperative neurocognitive disorders: a systematic review and meta-analysis. BMC Anesthesiol. 2020;20:254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Bi X, Wei J, Zhang X. Effects of dexmedetomidine on neurocognitive disturbance after elective non-cardiac surgery in senile patients: a systematic review and meta-analysis. J Int Med Res. 2021;49:3000605211014294. [DOI] [PMC free article] [PubMed] [Google Scholar]