Abstract
Major depressive disorder (MDD) is characterized by psychological and physiological manifestations contributing to the disease severity and outcome. In recent years, several lines of evidence have suggested that individuals with MDD have an elevated risk of age-related adverse outcomes across the lifespan. This review provided evidence of a significant overlap between the biological abnormalities in MDD and biological changes commonly observed during the aging process (i.e., hallmarks of biological aging). Based on such evidence, we formulate a mechanistic model showing how abnormalities in the hallmarks of biological aging can be a common denominator and mediate the elevated risk of age-related health outcomes commonly observed in MDD. Finally, we proposed a roadmap for novel studies to investigate the intersection between the biology of aging and MDD, including the use of geroscience-guided interventions, such as senolytics, to delay or improve major depression by targeting biological aging.
Keywords: Major depression, late-life depression, biology of aging, geroscience, cellular, senescence
Introduction
Major depressive disorder (MDD) is one of the most common mental disorders across the lifespan. Its prevalence varies in different populations, and recent estimates suggest a 12-month and lifetime prevalence of 10.4% and 20.6%, respectively(Hasin et al., 2018). In addition to its high prevalence, it also ranks among the five most disabling disorders worldwide (Whiteford et al., 2013).
The disability associated with MDD is not solely due to the burden of psychopathology. MDD is associated with a higher risk of cardiovascular, cerebrovascular disease, and metabolic disorders (Leung et al., 2012; Richmond-Rakerd et al., 2021). MDD across the lifespan is a major risk factor for Alzheimer’s disease and related dementia (ADRD), frailty, and decreased health span (Diniz et al., 2013; Richmond-Rakerd et al., 2022; Soysal et al., 2017). Finally, MDD increases the risk of all-cause and cardiovascular-related mortality (Diniz et al., 2014b; Leung et al., 2012). Notably, these are features commonly associated with advancing chronological aging, suggesting that individuals with MDD present with, or are at higher risk for, a premature aging phenotype.
Despite robust clinical and epidemiological evidence that associates MDD with premature aging phenotypes, the underlying mechanisms are not well-understood. In this review, we aim to summarize the current evidence suggesting that individuals with MDD across the lifespan present with cellular and molecular changes related to biological aging. We also aim to provide a novel conceptual, mechanistic framework by which MDD increases the risk of adverse health outcomes commonly associated with advanced aging.
The hallmarks of biological aging: Implications for major depressive disorder
Biological aging (BA) is a complex process involving interconnected changes in multiple biological pathways that ultimately lead to accumulating damage in cells and tissues. Detailed reviews about processes that drive BA and the current challenges of measuring it have been previously published (Ferrucci et al., 2020; López-Otín et al., 2013). Table 1 describes commonly accepted BA hallmarks and the evidence that such processes are affected in MDD. In this review, we will focus on studies that examined individuals with MDD. When data from human studies are lacking, we will review data from animal models of depression.
Table 1.
The hallmarks of biological aging10 and the evidence of its association with major depressive disorder.
| Hallmark of biological aging | Description | Evidence |
|---|---|---|
| Cellular senescence | Includes a state of persistent cell arrest due to telomere attrition, intracellular accumulation of lipids and glycoproteins (e.g., senescence-associated β-galactosidase, lipofuscin), telomere shortening, and activation of cell cycle control pathways (e.g., p16INK4A and p21 genes) | ↑↑↑ |
| Altered intercellular communication | Includes changes in circulating biomarkers (e.g., the SASP, inflammaging) that can act in a paracrine or endocrine fashion to convey, to different cells and tissues, information about the molecular state of a cell or tissue | ↑↑↑ |
| Mitochondrial dysfunction | Includes the abnormal and unchecked production of the reactive oxygen species (ROS), mtDNA damage, loss of mitochondrial integrity | ↑↑↑ |
| Deregulation of nutrient sensing | Includes the abnormal activation of the insulin and IGF-1 signaling (IIS) pathway and other nutrient sensing pathways (e.g., mTOR, AMPK, and SIRT1) | ↑↑ |
| Epigenetic alterations | Includes histone modification, chromatin remodeling, and other mechanisms that influence gene expression without alteration of DNA sequence (e.g., microRNAs) | ↑↑ |
| Genomic instability | Includes the accumulation of DNA damage (nuclear and mitochondrial DNA): base damage, double-strand breaks, base mismatching; and the failure of DNA repair mechanisms | ↑ |
| Loss of proteostasis | Includes the accumulation of unfolded, misfolded and aggregated proteins due to reduced capacity of protein stabilization and degradation | ↑ |
| Stem cell exhaustion | Includes the decline in the regenerative potential of tissues due to deficient proliferating capacity of stem cells | ↑ |
↑↑↑: strong evidence; ↑↑: moderate evidence; ↑: poor or lack of evidence.
Cellular Senescence
The accumulation of senescent cells has become one of the most widely recognized BA hallmarks. Cellular senescence is characterized by irreversible growth arrest that occurs when cells experience replicative exhaustion, oncogenic insults, cellular stress, or genomic instability (Campisi and d’Adda di Fagagna, 2007; Sharpless and Sherr, 2015). Senescent cells share common morpho-functional features, for example, enlarged cell size, accumulation of senescence-associated β-galactosidase, and lipofuscin in the cytoplasm (Gasek et al., 2021; Hernandez-Segura et al., 2018; Ogrodnik, 2021). They also show accumulation of DNA damage foci, condensed heterochromatin regions, telomere shortening, and overexpression of cell cycle regulator markers like p16 and p21 (Gasek et al., 2021; Hernandez-Segura et al., 2018; Ogrodnik, 2021). Other key characteristics of senescent cells are the incapacity to initiate pro-apoptotic pathways, relying on immune cells for tissue clearance, and changes in the cellular secretome (the senescence-associated secretory phenotype, SASP) (Deursen, 2014; Fafian-Labora and O’Loghlen, 2020).
Few studies have focused on the expression of cyclin-dependent kinases p16 and p21 in MDD. In a small post-mortem study, p21 expression in hippocampal neurons was significantly higher in older individuals with MDD than in the control group (Epp et al., 2013). This study also found that the use of antidepressants was associated with even higher p21 expression in the hippocampus. In another study, p16 mRNA expression in PBMCs was significantly higher in subjects with MDD and significantly correlated with the severity of depressive symptoms (Teyssier et al., 2012).
Telomere attrition
Telomeres are nucleoprotein complexes at the end of chromosomes, composed of TTAGGG repeats and a 3′- rich single-stranded overhang, and are critical to the stability and integrity of DNA (Lu et al., 2013). Telomeres are shortened after each cell cycle division, and after achieving a critical threshold, it signals the cells to stop further replication and to enter into a senescent state (i.e., replicative senescence) (Aubert, 2014).
Previous studies have demonstrated the association between telomere attrition and MDD. Community-based studies showed that individuals with MDD had shorter leukocyte telomere length (LTL) compared with never-depressed control subjects (Schaakxs et al., 2015; Verhoeven et al., 2018). Longer duration of depressive episodes, the severity of depressive symptoms, and the presence of medical comorbidity are features associated with shorter LTL in MDD (Mendes-Silva et al., 2021; Schutte and Malouff, 2015; Wolkowitz et al., 2011). Telomere attrition has also been associated with higher IL-6 and oxidative stress marker levels in MDD (Wolkowitz et al., 2011). Interestingly, one longitudinal study showed that changes in the course of depressive symptoms over six years were not associated with different rates of LTL attrition (Verhoeven et al., 2016). On the other hand, young individuals who experienced incident major depressive episodes (MDE) showed a greater LTL attrition than those without incident MDE, independent of baseline telomere length (Shalev et al., 2014), suggesting that telomere attrition may be a trait-dependent phenomenon in MDD. Despite their importance, there are several limitations in interpreting the findings showing significant telomere attrition in MDD. First, these studies focus on LTL analyses, and it is unclear how LTL is related to telomere length in brain cells. Second, neurons are terminally differentiated cells, and the functional role of neuronal telomeres is not clear. Interestingly, studies including post-mortem brain samples showed more significant telomere attrition in different brain cortical areas and oligodendrocytes in MDD (Mamdani et al., 2015; Szebeni et al., 2014), reinforcing the association between MDD and cellular senescence in the brain.
Altered intercellular communication
The concept of intercellular communication is very broad and can encompass almost any known physiological function. It is usually defined as the transfer of information from one cell to another through paracrine, autocrine, endocrine, or cell-to-cell contact signaling. Changes in intercellular communication processes are another common hallmark of BA and affect many overlapping pathways between aging and MDD.
Senescence-associated secretory phenotype (SASP)
One of the most important characteristics of cellular senescence is a shift in the cellular secretome profile, the senescence-associated secretory phenotype (SASP) (Fafian-Labora and O’Loghlen, 2020). Senescent cells accumulating in tissues secrete a myriad of pro-inflammatory cytokines, chemokines, extracellular matrix proteases, angiogenic factors, growth factors, cell cycle, and metabolic regulating factors (Basisty et al., 2020; Coppé et al., 2010; Coppé et al., 2008). If senescent cells are not effectively cleared, they can accumulate, driving the increase of SASP factors expression, and their release to the systemic circulation, leading to further deleterious effects on neighboring and distant cells and tissues (Young and Narita, 2009).
Since SASP factors comprise several signaling proteins associated with inflammatory control, tissue remodeling, cell growth, cell cycle control, and metabolic regulation, the analyses of a single or few markers may not reflect the complexity of the SASP. To address this issue, we developed and optimized a biomarker-composite index, the SASP index, to reflect the interrelated associations between distinct SASP factors into a single variable reflecting a systemic cellular senescence burden at a molecular level. We first showed that older adults with MDD expressed higher levels of different SASP factors and had higher SASP index scores than never-depressed age-matched older adults, suggesting an increasing cellular senescence burden in MDD (Diniz et al., 2017). Additional studies using independent cohorts confirmed our initial findings and demonstrated that a higher SASP index was also associated with global cognitive impairment, specifically with executive dysfunction and slower information processing speed (Diniz et al., 2021). We further expanded our findings to show that young and middle-aged adults with MDD had higher SASP index scores than never-depressed individuals (Diniz et al., 2019). Finally, in this study, we demonstrated the relevance of somatic health parameters to the accumulation of SASP among individuals with MDD since we found a strong association between higher SASP index, higher body mass index, and higher medical comorbidity burden.
Although our initial findings indicated a clear association between SASP factors, somatic parameters, and cognitive impairment in older adults with MDD, these data did not indicate if SASP factors were associated with structural changes in brain areas related to emotional and cognitive processing. Thus, we tested if the SASP index would negatively impact brain structural changes. We found that higher SASP index scores negatively affected brain health markers and were associated with reduced cortical thickness, lower fractional anisotropy, and higher mean diffusivity measures in older adults with major depressive disorder (Diniz et al., 2017; Mendes-Silva et al., 2019). These changes were more intense in brain areas related to executive function and episodic memory performance, providing mechanistic links between abnormal SASP factors and worse cognitive performance in older adults with MDD. Interestingly, these structural brain changes have also been related to neurodegenerative changes and Alzheimer’s disease pathology in older adults (Jack et al., 2014), reduced axonal integrity, and higher inflammatory activity in the brain (O’Donnell and Westin, 2011), providing potential mechanistic links between MDD and the risk of age-related neurodegenerative disorders.
Despite the evidence that SASP factors were associated with MDD across the lifespan, the prognostic significance of the SASP factors was unclear. In a recent study, we examined data from a large clinical trial of older adults with MDD and found that a higher SASP index significantly predicted lower treatment remission rates after adjusting for well-established predictors of treatment remission in this population (Diniz et al., 2022). Interestingly, none of the individual SASP factors included in the SASP index significantly predicted treatment remission in this population. Our findings suggest that the SASP index is more informative than its individual components in evaluating complex clinical and biological phenomena, in line with recent reports showing that a global phenotypic aging measure is better than its individual components for tracking longitudinal aging changes (Belsky et al., 2022; Belsky et al., 2015).
“Inflammaging”
Inflammaging is a chronic, sterile, low-grade inflammation state throughout the body that can lead to tissue dysfunction, disease pathology, and poor disease outcomes (Ferrucci and Fabbri, 2018). Although reviewed separately, this pro-inflammatory secretory profile can be viewed as part of the SASP.
The inflammatory response and its resolution are tightly regulated processes in which the balance between pro- and anti-inflammatory systems is essential to elicit the appropriate cellular responses and resolution of the inflammatory process. In aging, however, this balance is gradually disrupted with a gradual preponderance of pro-inflammatory over anti-inflammatory markers (i.e., inflammaging) (Ferrucci and Fabbri, 2018; Franceschi et al., 2007; Franceschi et al., 2018).
In the past decades, a large bulk of evidence has demonstrated the association between MDD across the lifespan, abnormal levels of inflammatory markers, and changes in the immune response. For example, a meta-analysis showed that specific sets of cytokines preferentially upregulated in depressed individuals, including IL-6, IL-10, sIL-2R, TNF-α, IL-1β (Kohler et al., 2017). Another study suggests that inflammation correlates with depressive episode severity and that the levels of inflammatory markers decrease after depressive episode recovery but do not reach the levels observed in never-depressed individuals (Dahl et al., 2014). Interestingly, high inflammatory markers such as IL-6 and CRP are specifically associated with physical and cognitive decline (Frank et al., 2021). Finally, low-grade inflammation has also been associated with poor treatment and health outcomes in MDD (Chamberlain et al., 2019; Strawbridge et al., 2015).
Deregulated nutrient sensing
Aging is accompanied by significant changes in the regulation of nutrient-sensing processes in cells and tissues. Changes include increased insulin resistance, impaired growth hormone-insulin-like growth factor 1 (IGF-1) signaling, and an imbalance between catabolic and anabolic processes (López-Otín et al., 2013). Systemic consequences of changes in nutrient sensing cascades include the increased incidence of metabolic disorders (e.g., diabetes, obesity), sarcopenia, osteoporosis, and frailty in older adults (Farr and Almeida, 2018).
Several clinical and epidemiological studies have demonstrated a potential link between MDD and deregulated nutrient sensing. Individuals with MDD have a higher prevalence and incidence of diabetes, obesity, and other metabolic disorders (Ghanei Gheshlagh et al., 2016). MDD has also been associated with a higher risk of sarcopenia and low muscle strength in middle-aged adults (Hayashi et al., 2019). Low grip strength was also an independent predictor of prevalent and incident clinically significant depressive symptoms in older adults in community-based cohort studies (Brooks et al., 2018; Carvalho et al., 2021).
Additional evidence of the association between major depression and deregulated nutrient sensing comes from studies that evaluated the markers of insulin resistance and components of the insulin signaling cascade system. The HOMA-IR (Homeostatic Model Assessment of Insulin Resistance) is a proxy measure of insulin resistance used in clinical practice (Tahapary et al., 2022). A meta-analysis of 18 cross-sectional studies, including more than 25,000 individuals, showed a significant association between insulin resistance and major depression (Kan et al., 2013). Insulin resistance, measured by the HOMA-IR, was independently associated with the severity of depressive symptoms and mediated the association between depressive symptoms and metabolic syndrome in a large population-based cohort of Mexican-American older adults (Diniz et al., 2018a). Our group also demonstrated that older adults with MDD have lower adiponectin levels, an insulin-sensitizing hormone secreted by adipocytes, indicating higher insulin resistance in this sample (Diniz et al., 2012).
Sirtuins are important regulators of the insulin signaling cascade, improving insulin sensitivity and glucose and fatty acid metabolism (Nogueiras et al., 2012). Sirtuins are a family of conserved nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase and mono-ADP-ribosyl transferase (Sirt1–7). Their activity is regulated by the bioavailability of NAD+, and they are involved in a broad range of cellular functions, including metabolic control, mitochondrial homeostasis, DNA repair, inflammation, autophagy, and apoptosis (Pardo and Boriek, 2020).
Reduced sirtuins activity has been implicated in accelerated aging processes and age-related medical disorders (Hall et al., 2013). Case-control studies and a genome-wide association study (GWAS), including mainly Chinese participants, showed a significant association between SIRT gene polymorphisms and MDD (consortium, 2015; Kishi et al., 2010; Liu et al., 2019). However, these findings have not been replicated in recent mega GWAS analyses, including more diverse samples (Howard et al., 2019). Individuals with MDD present with a significant, state-dependent reduction of mRNA levels of SIRT1, 2, and 6 in the blood (Abe et al., 2011; McGrory et al., 2018). A large study, including two independent cohorts in China, showed a significant reduction of the SIRT1 gene expression in the amygdala post-mortem among individuals with a history of MDD (Liu et al., 2019).
Mitochondrial dysfunction
Mitochondrial dysfunction closely correlates to cellular and organismal aging and has been implicated in many diseases like depression, diabetes, and Alzheimer’s disease (AD) (Caruso et al., 2019; Lopez-Otin et al., 2016; Wang et al., 2020). Mitochondrial dysfunction during aging can occur at different levels, including defects in the biogenesis, electron transport chain, and mitochondria clearance(Webb and Sideris, 2020). Moreover, mitochondrial dysfunction directly impacts other hallmarks of aging, like deregulated nutrient sensing, genomic instability, and loss of proteostasis (Lopez-Otin et al., 2016).
The number of mitochondrial DNA copies (mtDNA-cn) is an indirect marker of mitochondrial function, where lower mtDNA-cn indicates more intense mitochondria dysfunction(Castellani et al., 2020). However, data from studies including young adults with MDD are conflicting, with studies showing a significant decrease in mtDNA-cn in MDD (Chang et al., 2015; Edwards et al., 2016), while other studies, including larger sample sizes, did not confirm the associations between MDD and mtDNA-cn (Lindqvist et al., 2018; Verhoeven et al., 2018).
Oxidative stress (OS) is a sub-product of mitochondrial function, and elevated ROS production is another marker of mitochondrial dysfunction that, if unchecked, can lead to mitochondrial, cellular, and DNA damage (Valko et al., 2007). Young and middle-aged individuals with MDD have consistently shown higher levels of OS markers (e.g., TBARS, 8-OH-DNA, protein carbonyl content) and lower levels of antioxidants (e.g., glutathione peroxidase, glutathione transferase) (Black et al., 2015). Studies focusing on older adults also showed significantly higher lipid peroxidation markers (Pomara et al., 2012) and an imbalance between oxidative stress vs. anti-oxidative stress markers in MDD (Diniz et al., 2018b) compared to non-depressed controls. Moreover, increased oxidative stress has been associated with poor response to antidepressant treatment (Lindqvist et al., 2017).
Another consequence of elevated OS is the oxidation and fragmentation of mitochondrial DNA (mtDNA). Fragmented mtDNA can escape to the cell cytosol and extracellular fluids such as the blood (i.e., circulating cell-free mtDNA, ccf-mtDNA), and ccf-mtDNA is a promising biomarker for aging, tissue damage, and cellular stress in different conditions (Kananen et al., 2022). Young adults with MDD had significantly higher ccf-mtDNA levels than non-depressed controls (Kageyama et al., 2018; Lindqvist et al., 2018). Interestingly, individuals who did not respond to antidepressant treatment with SSRIs showed a significant increase in ccf-mtDNA levels, while those who responded to treatment showed no significant changes in ccf-mtDNA levels after treatment (Lindqvist et al., 2018). Higher ccf-mtDNA has been associated with more severe depressive episodes and suicidal behaviors(Lindqvist et al., 2016). Our group investigated the association between ccf-mtDNA and MDD in older adults and found significantly higher ccf-mtDNA levels compared to never-depressed control individuals, suggesting more intense mitochondrial dysfunction in this population (Goncalves et al., 2021). Interestingly, we also found that ccf-mtDNA was significantly correlated with higher levels of IL-6, a master regulator of inflammatory response and a major component of the SASP. In a follow-up study, we also demonstrated that older adults with comorbid MDD and frailty presented significantly higher ccf-mtDNA levels (Ampo et al., 2022).
Epigenetic alterations
Epigenetic alterations to the genome are an important form of control of gene expression without modifying the DNA sequence. They include DNA and histone modifications (e.g., methylation and acetylation) and post-translational control of gene expression by microRNA and other non-coding RNAs (López-Otín et al., 2013). Increasing DNA methylation rate is a consistent feature of advanced chronological aging, and several studies have developed algorithms to determine biological aging based on genome-wide methylation data (Belsky et al., 2022; Rutledge et al., 2022). Individuals with MDD have higher global DNA methylation rates than healthy comparison subjects (Li et al., 2019). Interestingly, some genes with higher DNA methylation rates have also been affected by chronological aging, e.g., the BDNF gene (Dell’Osso et al., 2014; Januar et al., 2015). Another recent large-scale study also showed a significant correlation of gene methylation changes in the blood and in brain areas that have been involved in the MDD (e.g., anterior and rostral prefrontal cortex) (Aberg et al., 2020). Epigenome-wide association studies also show that differentially methylated genes in MDD are involved in age-related biological processes, like the control of metabolic processes and proteostasis (e.g., insulin receptor signaling, mTOR signaling biological pathways) (Zhu et al., 2019), inflammatory response (e.g., p38 MAPK pathways) (Cordova-Palomera et al., 2015), or genes that show age-dependent changes in mRNA expression (McKinney et al., 2019).
Aging clocks, brain age, and major depressive disorder
Recently, a large body of work has identified aging clocks based on different biomarkers. Ideally, these aging clocks should be easy to measure and calculate, reflect critical features of the biology of aging, be well correlated with chronological aging, and predict adverse, age-related health outcomes (Ferrucci et al., 2020; Hamczyk et al., 2020; Rutledge et al., 2022). For example, Han and colleagues, using data from a large community-based study, showed that subjects with MDD had significantly higher epigenetic aging than never-depressed subjects, with a significant dose-effect with increasing symptom severity in the overall sample and replicated in an independent dataset of post-mortem brain sample (Han et al., 2018). Another study used the GrimAge epigenetic clock to estimate the biological age, and individuals with MDD showed a biological age acceleration compared to non-depressed controls (Protsenko et al., 2021). A more recent study, using data from a large population-based cohort of young adults, showed a higher burden of psychopathology, including depressive symptoms and other internalizing disorders, was independently associated with a faster DNA-methylation pace of aging between ages 26 and 45 years (Wertz et al., 2021). One key aspect of the biology of aging is that different organs and tissues age at different paces, and a systemic measure of biological aging may not reflect the aging process of the brain (Nie et al., 2022; Schaum et al., 2020). Thus, brain-specific biological aging clocks are needed to address if MDD was associated with accelerated brain aging (Cole et al., 2019). An early study using structural brain MRI to estimate the “brain age” showed that young and middle-aged adults with MDD had four years more estimated brain age (i.e., brain age gap) than their chronological age (Koutsouleris et al., 2014). In this work, the brain regions that were mostly predictive of age mapped to subcortical and periventricular, orbitofrontal, cerebellar, limbic, cingulate, and perisylvian regions. A study using data from the Enhancing Neuro Imaging Genetics through Meta-Analysis (ENIGMA) Consortium showed that participants with MDD had higher predicted brain age than individuals without a psychiatric diagnosis (predicted brain age difference of +1.08 (SE 0.22)), though with a small effect size (Cohen’s d=0.14, 95% CI: 0.08–0.20)) (Han et al., 2021a). In this study, the brain areas that showed the highest predicted age difference were the paralimbic, temporal, and orbitofrontal areas. Another epidemiologic study showed that subjects with MDD had higher predicted brain age than health controls (+2.78 years, Cohen’s d=0.25, 95% CI −0.10–0.60) (Han et al., 2021b). In a multivariable regression model, the severity of somatic depressive symptoms was the only variable significantly associated with higher predicted brain age in MDD. In contrast, the current use of antidepressant was associated with lower predicted brain age, suggesting a potential protective effect of antidepressant use against accelerated brain aging in MDD. Interestingly, predicted brain age was not significantly correlated with different omics-based clocks (epigenetic, transcriptomic, and metabolomic) and telomere length (with Pearson r in the range of −0.03–0.15), and showed a significant negative association age proteomic clock (r = −0.24, p = 0.02) in this cohort. Finally, a recent meta-analysis confirmed the association between MDD and accelerated brain aging and showed that the average estimated brain age gap was 1.12 years [0.41; 1.83] (Ballester et al., 2022). A recent study using deep learning statistical methods (i.e., DeepBrainNet) obtained robust brain-age estimates across the lifespan and better fit than other commonly used machine learning methods using minimally processed brain MRI scans (Bashyam et al., 2020). This study also showed that subjects with different neuropsychiatric disorders, including MDD, showed an accelerated brain aging pattern across the lifespan.
Interestingly, chronological age may significantly moderate the association between MDD and the brain age gap since older adults have a more significant brain age gap than younger adults (Ballester et al., 2021). This finding suggests there may be a non-linear accumulation of age-related abnormalities in the hallmarks of biological aging processes among individuals with MDD that reflects on more significant brain age gap estimates among older individuals with MDD.
Genomic Instability
Genomic instability is driven by various factors, including environmental stressors, DNA damage and replication errors, telomere attrition, and genetic mutations (López-Otín et al., 2013). Genomic instability and damage increase with age, activating DNA damage response (DDR) mechanisms to repair and avoid further DNA damage (Yousefzadeh et al., 2021). If DDR is ineffective, several cellular fates can ensue, including apoptosis, cell cycle arrest, and cellular senescence (Schumacher et al., 2021). Individuals with MDD across the lifespan have evidence of increased levels of oxidative DNA and RNA damage (Chang et al., 2015; Czarny et al., 2015; Vieira et al., 2021). Also, individuals with MDD have more DNA double-stranded breaks following oxidative damage and reduced ability to perform DNA repair (Czarny et al., 2015).
Loss of proteostasis
Proteostasis is essential for maintaining cellular function, particularly following exposure to cell stress (Kaushik and Cuervo, 2015; López-Otín et al., 2013). When proteins are exposed to excessive stress, e.g., heat, pH disruption, oxidative stress, a tightly regulated network of other proteins, such as heat-shock proteins and chaperones, proteasomes, and lysosomes work to stabilize and correct the misfolded protein or degrade it (Hetz and Saxena, 2017; Pomatto et al., 2018). These cellular responses prevent the aggregation and proteotoxicity that plays a significant role in the pathogenesis of several neurodegenerative diseases such as Alzheimer’s disease and Parkinson’s disease (Haass and Selkoe, 2007).
Very few studies have addressed abnormalities in proteostasis control in MDD. A previous study from our group identified that proteins related to biological pathways involved in controlling protein stability, dimerization activity, protein localization, and protein complex binding are abnormally regulated in older adults with MDD(Diniz et al., 2016). A post-mortem study showed the excessive activation of the unfolded protein response (UPR) in MDD (Kowalczyk et al., 2021). Finally, another study showed that abnormalities in proteostasis control are strongly associated with more significant cognitive impairment in older adults with MDD (Diniz et al., 2015).
Stem cell exhaustion
Stem cells are essential to maintaining tissue homeostasis and regenerative responsiveness to injuries owed to their unique ability to self-renew and differentiate into mature tissue-specific subtypes (Oh et al., 2014). Since they persist throughout life, stem cells are particularly susceptible to an age-associated decline in number, proliferative, and differentiation capacity. Intrinsic, e.g., DNA damage, mitochondrial dysfunction, and extrinsic stressors, e.g., reactive oxygen species, can impair a tissue-specific stem cell niche environment, leading to the accumulation of cellular damage and induction of senescence over time (Duscher et al., 2014; Oh et al., 2014). A functional consequence is a depletion of fully functional stem cell populations, diminishing tissue repair and regenerative capacity, which are essential for proper organ functioning and survival.
Evidence supporting a clear mechanistic link between stem cell exhaustion and MDD is lacking, especially in humans. However, studies with animal models of depression have shown decreased neurogenesis in which neural stem cells (NSC) and neural progenitor cells (NPC) show limited ability to proliferate and differentiate into new neurons in mice (Berger et al., 2020; de Andrade et al., 2013; Pham et al., 2003). Interestingly, many different factors compromising the ability of neural stem cells to proliferate and differentiate into functional neurons have been associated with the pathophysiology of depression. For example, neurotrophic factors such as brain-derived neurotrophic factor (BDNF) and the nerve growth factor (NGF), which play an essential role in NSC niche maintenance and cellular differentiation (Numakawa et al., 2017), have consistently shown reduced levels in individuals with MDD across the lifespan. They have also been associated with worse treatment response and cognitive impairment in MDD (Diniz et al., 2014a; Molendijk et al., 2014). Despite the evidence from animal models suggesting that MDD is linked with changes in NSC biology, current methodological challenges to quantifying and evaluating the function of NSC in humans in vivo hinder the ability to understand their role in the pathophysiology of MDD.
A few studies evaluated the impact of MDD on circulating progenitor cells in humans, focusing on endothelial progenitor cells (EPC). A study including middle-aged individuals with MDD showed a significant reduction in the circulating levels of mature (CD34+/VEGFR2+) and immature (CD34+/CD133+/VEGFR2+) EPCs (Dome et al., 2009). These results were replicated in another study that showed the link between lower EPC count and higher inflammatory cytokine levels (Yang et al., 2011). The authors hypothesized that the lower EPC count in MDD could represent a mechanistic link between endothelial dysfunction, vascular disease, and the development of major depressive episodes, a condition known as vascular depression (Aizenstein et al., 2016). However, a recent study including a younger cohort of individuals with MDD did not find a significant difference between depressed and non-depressed individuals (Liou et al., 2021).
An integrative model linking abnormalities in biological aging and MDD
As reviewed above, robust evidence suggests that many pathophysiological abnormalities observed in MDD are in tandem with abnormalities in processes associated with the BA hallmarks. However, these studies have not been consistently framed from a life-course developmental perspective nor the biology of aging or geroscience perspectives. The geroscience framework suggests that identifying and targeting biological processes related to biological aging can ideally prevent or delay the onset or progression of multiple chronic diseases and adverse age-related health outcomes typically observed in older adults (Kennedy et al., 2014).
Within the geroscience framework, the association between abnormalities in biological hallmarks of aging and MDD can be viewed from two main perspectives. First, they can be viewed as having a causal role in MDD. Although there is evidence that high levels of inflammatory markers, mostly CRP and IL-6, or immunoinflammatory challenges (e.g., IFN-γ treatment for hepatitis C) are associated with the development of depressive symptoms and MDD, these findings are not universal (Au et al., 2015; Beratis et al., 2005; Verduijn et al., 2015). There is no evidence that drugs with potent anti-inflammatory or immunomodulatory effects can single-handedly treat a major depressive episode in the general population (Köhler et al., 2014). Other biological aging hallmarks, like telomere attrition over time, are not consistently associated with changes in depressive symptoms severity or trajectory (Verhoeven et al., 2016), despite a robust cross-sectional association between them. There is even less evidence to indicate that any hallmark of biological aging is causally linked with MDD from human studies. To that end, if abnormalities in the hallmarks of biological aging were causal to MDD, we would expect an abrupt increase in the incidence and prevalence of MDD among older adults, but this is not supported by epidemiological studies (Buchtemann et al., 2012). In fact, the incidence and prevalence of MDD peak in young adulthood and decrease with older age. Therefore, the literature evidence so far does not provide evidence for the perspective that abnormalities in the hallmarks of biological aging are antecedent to MDD onset and can be causally linked with MDD.
An alternative perspective is that the abnormalities in the hallmarks of biological aging are secondary to the emergence of MDD across the lifespan. Independent of the etiological factors that lead to MDD in an individual, its presence triggers a myriad of downstream, deleterious abnormalities in several biological processes, many of them related to the hallmarks of biological aging. The persistence of these biological abnormalities over time can lead to gradual changes in cellular response mechanisms in the brain and the peripheral tissues, creating a state of heightened biological vulnerability and reduced resilience demonstrated via a diminished capacity to deal with novel or ongoing endogenous and exogenous systemic or brain-specific stressors, which can be independent of the primary MDD etiopathological mechanisms. Furthermore, if these biological abnormalities remain unchallenged or are not promptly resolved (e.g., due to the persistence of MDD, recurrent depressive episodes, and treatment resistance, among others), they can accumulate over time, leading to a positive feedback loop, decreasing systemic resilience and increasing the biological vulnerability to endogenous and exogenous stressors, in a process called homeoestenosis or reduced adaptive homeostasis capacity (Davies, 2016; Epel, 2020; Khan et al., 2017).
The reduced adaptive homeostatic capacity due to the accumulation of biological aging abnormalities in MDD can manifest clinically by the observed risk of age-related adverse health outcomes (e.g., higher rates of multimorbidity, frailty, cognitive impairment, and dementia) or even MDD-specific phenomena like increased rates of recurrence and relapse, worse treatment response, the severity of depressive symptoms and associated disability. Within this perspective, the abnormalities on the hallmarks of biological aging are factors contributing to and mechanistically linked to the emergence of a premature aging phenotype in MDD across the lifespan.
MDD, behavioral and social processes, and biology of aging:
This review has focused on the intersection between the MDD and the abnormalities in hallmarks of biological aging. However, we do acknowledge other possible theoretical frameworks by which MDD may contribute to a premature aging phenotype, specially via behavioral and social processes. When MDD occurs at its peak prevalence during young adulthood, the illness derails the healthy transition from adolescence to adulthood, interferes with educational attainment, diminishes work life, income, and wealth, reduces social connectedness, increases social isolation, and disrupts health behaviors necessary for healthy aging, such as physical activity. All of these are known as social hallmarks of accelerated aging (Crimmins, 2020). Moreover, many of these social and behavioral processes have been associated with abnormalities in hallmarks of biological aging, in particular an elevated pro-inflammatory status, independently of the presence of MDD or other psychiatric disorders (Ahmadian et al., 2020; Lam et al., 2021; Shankar et al., 2011; Vingeliene et al., 2019). Therefore, testing how social and behavioral processes can modify the associations between MDD and the biological hallmarks of aging will bring greater explanatory precision and translational power.
Future directions
The conceptualization of many biological changes observed in MDD from a geroscience perspective is novel and brings many new unanswered questions. First, is there a hierarchy of changes among the hallmarks of biological aging, acting in a cascade fashion? How do these changes impact and interact with each other? Are abnormalities in one hallmark of biological aging linked to specific adverse MDD outcomes, or are they broadly associated with any adverse outcomes in MDD? Are elevated aging hallmarks specific to MDD, or are they shared by other mental disorders that peak in early adulthood and are commonly comorbid with MDD(Caspi et al., 2020)? This set of questions highlights the importance of well-powered studies, including the simultaneous measurement of multiple hallmarks of biological aging, depressive symptoms, and diverse health outcomes relevant to aging in a diverse study sample followed longitudinally with repeated measures for an empirical lifespan approach.
Many studies of the hallmarks of aging reviewed here have reported on older adults, but because MDD incidence peaks in early adulthood and declines in older adulthood, more studies should assess the hallmarks of aging in young-adult MDD patients. Studies of national register medical record datasets(Plana-Ripoll et al., 2022; Richmond-Rakerd et al., 2022; Richmond-Rakerd et al., 2021) can also be helpful by documenting to what extent patients diagnosed with MDD tend to be the same patients later diagnosed with age-related diseases and whether MDD poses a specific risk for diseases with known inflammatory etiology versus equivalent risk for all age-related diseases.
Second, should interventions focusing on the modulation of abnormalities on hallmarks of biological aging improve mental health treatment outcomes or mitigate the risk of long-term adverse events in this population? In fact, physical exercise significantly improves cellular senescence markers(Englund et al., 2021; Valente et al., 2021) and has shown a significant benefit as an adjuvant for MDD treatment and prevention(Bellon et al., 2021; Lee et al., 2021). Lithium carbonate, a drug long used for treating recurrent and treatment-resistant depressive episodes, has also shown a potential effect on clearing senescent cells, improving cellular senescence markers, and modulating SASP factors(Fang et al., 2022; Squassina et al., 2016; Viel et al., 2020). The development of senolytic drugs, which can clear senescence cells(Kirkland and Tchkonia, 2020; Partridge et al., 2020), also offers a promise that their use as adjuvants to antidepressant treatment can ameliorate the abnormalities in the hallmarks of biological aging in MDD, improving treatment response rates and reducing the risk of age-related adverse health outcomes in this population. Finally, it will be important to include measures of hallmarks of aging and outcomes in randomized clinical trials of MDD and other psychiatric disorders to evaluate if and how currently available psychiatric treatment can impact age-related biological processes and related adverse health outcomes.
Third, are there moderators of the association between the abnormalities in the hallmarks of biological aging and adverse outcomes in MDD? To respond to this question, studies need to address whether potential demographic (e.g., chronological age), biological (e.g., biological sex, gut microbiota), and social determinants of health (e.g., educational status, race, socio-economic status, social isolation) moderate or mediate the association between MDD, abnormalities in the hallmarks of biological aging, and adverse health outcomes.
All of the above issues will need to be addressed, as will growing heterogeneity between individuals in terms of MDD, related clinical conditions, and rate of biological aging in late life, to develop a precision geroscience approach to MDD. Such an approach will shed light on how MDD (and probably other psychiatric disorders) negatively affect the hallmarks of biological aging, both in the brain and systemically, lead to a premature aging phenotype in individuals with a history of MDD across the lifespan, and help identify novel therapeutic targets and approaches to mitigate the long-term disability and adverse health outcomes associated with MDD.
Figure 1:

Hallmarks of biological aging in MDD.
Major depressive disorder can show changes in many different hallmarks of biological aging.
Figure 2:
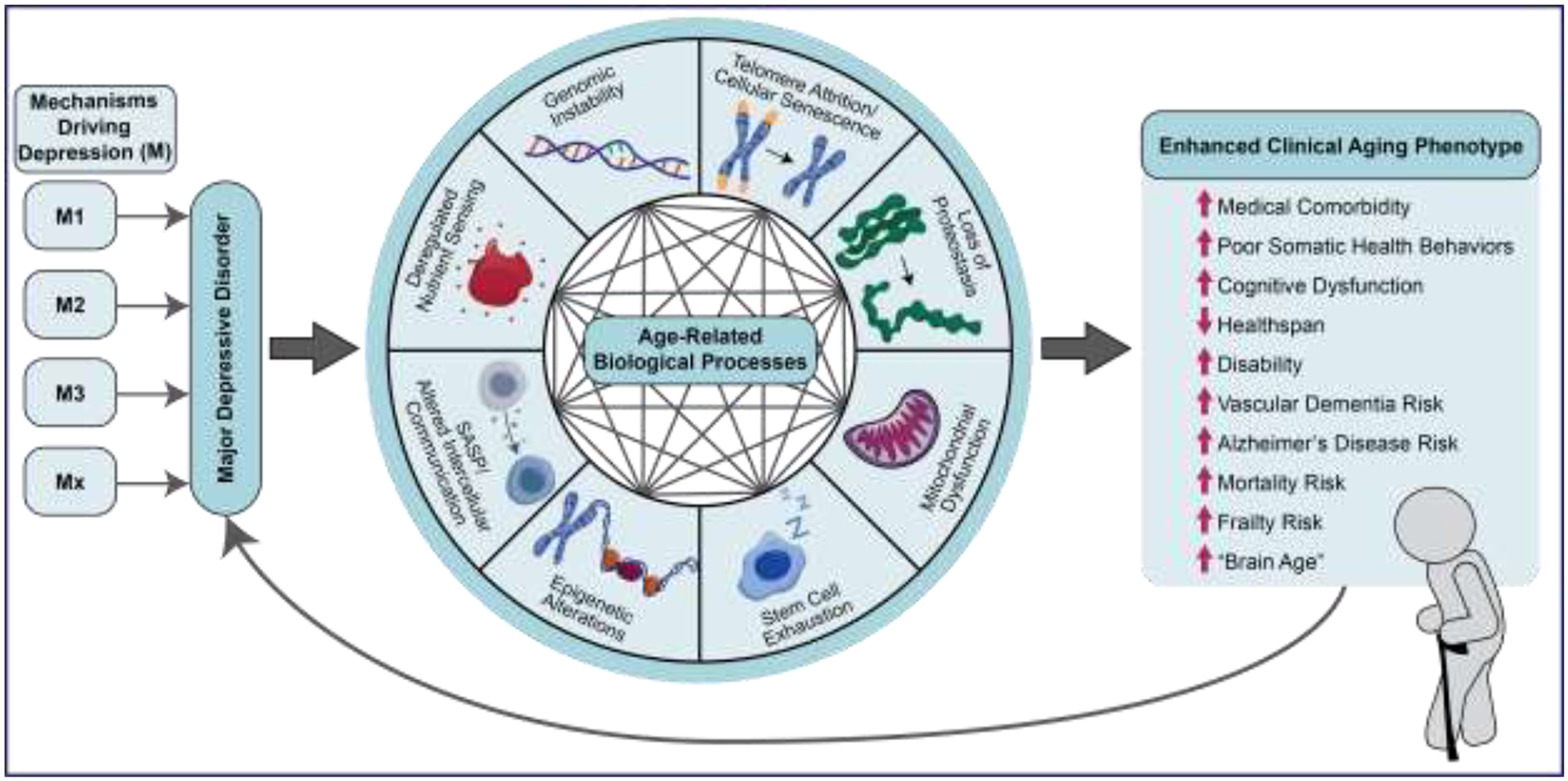
Mechanistic model linking the hallmarks of biological aging with adverse health outcomes in MDD.
Major depressive disorder (MDD) triggers abnormalities in many hallmarks of biological aging which can be independent of the primary etiological mechanisms of the MDD. It is not clear how MDD triggers the dysregulation of different hallmarks of biological or what is the cascade or hierarchy of changes. However, once these biological processes are activated, they may work in concert and interact among themselves leading to a self-perpetuating deleterious cycle of damage accumulation and diminished resilience to endogenous and exogenous insults (i.e., homeoestenosis). If this deleterious cycle remains unchecked, it can manifest clinically as different adverse health outcomes commonly observed among individuals with MDD.
Highlights.
There is robust clinical and epidemiological evidence that major depressive disorder (MDD) is associated with elevated risk of adverse age-related health outcomes.
Individuals with MDD show significant biological abnormalities in biological processes related to accelerated biological aging (i.e., hallmarks of biological aging).
The abnormalities in the hallmarks of biological aging in MDD can be a mechanistic link for the elevated risk of adverse health outcomes observed in MDD.
Geroscience-guided approaches can provide novel avenues for the development of interventions to improve depressive symptoms and mitigate the risk of adverse health outcomes in MDD.
Funding sources:
This work was supported by NIH grants R01MH115953, R01MH118311 (Dr. Diniz); R01AG73207 (Dr. Moffitt); P30AG067988 (Dr. Kuchel).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: All authors have no conflict of interest to report.
References
- Abe N, Uchida S, Otsuki K, Hobara T, Yamagata H, Higuchi F, Shibata T, Watanabe Y, 2011. Altered sirtuin deacetylase gene expression in patients with a mood disorder. J Psychiatr Res 45, 1106–1112. [DOI] [PubMed] [Google Scholar]
- Aberg KA, Dean B, Shabalin AA, Chan RF, Han LKM, Zhao M, van Grootheest G, Xie LY, Milaneschi Y, Clark SL, Turecki G, Penninx B, van den Oord E, 2020. Methylome-wide association findings for major depressive disorder overlap in blood and brain and replicate in independent brain samples. Mol Psychiatry 25, 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadian AJ, Lin JE, Neylan TC, Woolley JD, O’Donovan A, Cohen BE, 2020. Social integration and inflammation in individuals with and without posttraumatic stress disorder. Brain Behav Immun 89, 168–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizenstein HJ, Baskys A, Boldrini M, Butters MA, Diniz BS, Jaiswal MK, Jellinger KA, Kruglov LS, Meshandin IA, Mijajlovic MD, Niklewski G, Pospos S, Raju K, Richter K, Steffens DC, Taylor WD, Tene O, 2016. Vascular depression consensus report - a critical update. BMC Med 14, 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ampo E, Mendes-Silva AP, Goncalves V, Bartley JM, Kuchel GA, Diniz BS, 2022. Increased Levels of Circulating Cell-Free mtDNA in the Plasma of Subjects With Late-Life Depression and Frailty: A Preliminary Study. Am J Geriatr Psychiatry 30, 332–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au B, Smith KJ, Gariépy G, Schmitz N, 2015. The longitudinal associations between C-reactive protein and depressive symptoms: evidence from the English Longitudinal Study of Ageing (ELSA). International Journal of Geriatric Psychiatry 30, 976–984. [DOI] [PubMed] [Google Scholar]
- Aubert G, 2014. Chapter Four - Telomere Dynamics and Aging, in: Calado RT (Ed.), Progress in Molecular Biology and Translational Science. Academic Press, pp. 89–111. [DOI] [PubMed] [Google Scholar]
- Ballester PL, Romano MT, de Azevedo Cardoso T, Hassel S, Strother SC, Kennedy SH, Frey BN, 2022. Brain age in mood and psychotic disorders: a systematic review and meta-analysis. Acta Psychiatr Scand 145, 42–55. [DOI] [PubMed] [Google Scholar]
- Ballester PL, Suh JS, Nogovitsyn N, Hassel S, Strother SC, Arnott SR, Minuzzi L, Sassi RB, Lam RW, Milev R, Muller DJ, Taylor VH, Kennedy SH, Frey BN, Team C-BI, 2021. Accelerated brain aging in major depressive disorder and antidepressant treatment response: A CAN-BIND report. Neuroimage Clin 32, 102864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashyam VM, Erus G, Doshi J, Habes M, Nasrallah IM, Truelove-Hill M, Srinivasan D, Mamourian L, Pomponio R, Fan Y, Launer LJ, Masters CL, Maruff P, Zhuo C, Völzke H, Johnson SC, Fripp J, Koutsouleris N, Satterthwaite TD, Wolf D, Gur RE, Gur RC, Morris J, Albert MS, Grabe HJ, Resnick S, Bryan RN, Wolk DA, Shou H, Davatzikos C, on behalf of the ISTAGING Consortium, t.P.A.s.d.C., ADNI,, studies, C., 2020. MRI signatures of brain age and disease over the lifespan based on a deep brain network and 14 468 individuals worldwide. Brain 143, 2312–2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basisty N, Kale A, Jeon OH, Kuehnemann C, Payne T, Rao C, Holtz A, Shah S, Sharma V, Ferrucci L, Campisi J, Schilling B, 2020. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol 18, e3000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellon JA, Conejo-Ceron S, Sanchez-Calderon A, Rodriguez-Martin B, Bellon D, Rodriguez-Sanchez E, Mendive JM, Ara I, Moreno-Peral P, 2021. Effectiveness of exercise-based interventions in reducing depressive symptoms in people without clinical depression: systematic review and meta-analysis of randomised controlled trials. Br J Psychiatry 219, 578–587. [DOI] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Corcoran DL, Sugden K, Poulton R, Arseneault L, Baccarelli A, Chamarti K, Gao X, Hannon E, Harrington HL, Houts R, Kothari M, Kwon D, Mill J, Schwartz J, Vokonas P, Wang C, Williams BS, Moffitt TE, 2022. DunedinPACE, a DNA methylation biomarker of the pace of aging. Elife 11, e73420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky DW, Caspi A, Houts R, Cohen HJ, Corcoran DL, Danese A, Harrington H, Israel S, Levine ME, Schaefer JD, Sugden K, Williams B, Yashin AI, Poulton R, Moffitt TE, 2015. Quantification of biological aging in young adults. Proc Natl Acad Sci U S A 112, E4104–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beratis S, Katrivanou A, Georgiou S, Monastirli A, Pasmatzi E, Gourzis P, Tsambaos D, 2005. Major depression and risk of depressive symptomatology associated with short-term and low-dose interferon-alpha treatment. J Psychosom Res 58, 15–18. [DOI] [PubMed] [Google Scholar]
- Berger T, Lee H, Young AH, Aarsland D, Thuret S, 2020. Adult Hippocampal Neurogenesis in Major Depressive Disorder and Alzheimer’s Disease. Trends Mol Med 26, 803–818. [DOI] [PubMed] [Google Scholar]
- Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BW, 2015. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51, 164–175. [DOI] [PubMed] [Google Scholar]
- Brooks JM, Titus AJ, Bruce ML, Orzechowski NM, Mackenzie TA, Bartels SJ, Batsis JA, 2018. Depression and Handgrip Strength Among U.S. Adults Aged 60 Years and Older from NHANES 2011–2014. J Nutr Health Aging 22, 938–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtemann D, Luppa M, Bramesfeld A, Riedel-Heller S, 2012. Incidence of late-life depression: a systematic review. J Affect Disord 142, 172–179. [DOI] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F, 2007. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol 8, 729–740. [DOI] [PubMed] [Google Scholar]
- Caruso G, Benatti C, Blom JMC, Caraci F, Tascedda F, 2019. The Many Faces of Mitochondrial Dysfunction in Depression: From Pathology to Treatment. Front Pharmacol 10, 995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AF, Maes M, Solmi M, Brunoni AR, Lange S, Husain MI, Kurdyak P, Rehm J, Koyanagi A, 2021. Is dynapenia associated with the onset and persistence of depressive and anxiety symptoms among older adults? Findings from the Irish longitudinal study on ageing. Aging Ment Health 25, 468–475. [DOI] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Ambler A, Danese A, Elliott ML, Hariri A, Harrington H, Hogan S, Poulton R, Ramrakha S, Rasmussen LJH, Reuben A, Richmond-Rakerd L, Sugden K, Wertz J, Williams BS, Moffitt TE, 2020. Longitudinal Assessment of Mental Health Disorders and Comorbidities Across 4 Decades Among Participants in the Dunedin Birth Cohort Study. JAMA Netw Open 3, e203221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani CA, Longchamps RJ, Sun J, Guallar E, Arking DE, 2020. Thinking outside the nucleus: Mitochondrial DNA copy number in health and disease. Mitochondrion 53, 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamberlain SR, Cavanagh J, de Boer P, Mondelli V, Jones DNC, Drevets WC, Cowen PJ, Harrison NA, Pointon L, Pariante CM, Bullmore ET, 2019. Treatment-resistant depression and peripheral C-reactive protein. Br J Psychiatry 214, 11–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CC, Jou SH, Lin TT, Lai TJ, Liu CS, 2015. Mitochondria DNA change and oxidative damage in clinically stable patients with major depressive disorder. PLoS One 10, e0125855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole JH, Marioni RE, Harris SE, Deary IJ, 2019. Brain age and other bodily ‘ages’: implications for neuropsychiatry. Mol Psychiatry 24, 266–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- consortium C, 2015. Sparse whole-genome sequencing identifies two loci for major depressive disorder. Nature 523, 588–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé J-P, Patil CK, Rodier F, Krtolica A, Beauséjour CM, Parrinello S, Hodgson GJ, Chin K, Desprez P-Y, Campisi J, 2010. A Human-Like Senescence-Associated Secretory Phenotype Is Conserved in Mouse Cells Dependent on Physiological Oxygen. PLoS ONE 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppé JP, Patil CK, Rodier F, Sun Y, Muñoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J, 2008. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol 6, 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordova-Palomera A, Fatjo-Vilas M, Gasto C, Navarro V, Krebs MO, Fananas L, 2015. Genome-wide methylation study on depression: differential methylation and variable methylation in monozygotic twins. Transl Psychiatry 5, e557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crimmins EM, 2020. Social hallmarks of aging: Suggestions for geroscience research. Ageing Research Reviews 63, 101136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czarny P, Kwiatkowski D, Kacperska D, Kawczynska D, Talarowska M, Orzechowska A, Bielecka-Kowalska A, Szemraj J, Galecki P, Sliwinski T, 2015. Elevated level of DNA damage and impaired repair of oxidative DNA damage in patients with recurrent depressive disorder. Med Sci Monit 21, 412–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J, Ormstad H, Aass HC, Malt UF, Bendz LT, Sandvik L, Brundin L, Andreassen OA, 2014. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45, 77–86. [DOI] [PubMed] [Google Scholar]
- Davies KJ, 2016. Adaptive homeostasis. Mol Aspects Med 49, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade JS, Cespedes IC, Abrao RO, Dos Santos TB, Diniz L, Britto LR, Spadari-Bratfisch RC, Ortolani D, Melo-Thomas L, da Silva RC, Viana MB, 2013. Chronic unpredictable mild stress alters an anxiety-related defensive response, Fos immunoreactivity and hippocampal adult neurogenesis. Behav Brain Res 250, 81–90. [DOI] [PubMed] [Google Scholar]
- Dell’Osso B, D’Addario C, Carlotta Palazzo M, Benatti B, Camuri G, Galimberti D, Fenoglio C, Scarpini E, Di Francesco A, Maccarrone M, Altamura AC, 2014. Epigenetic modulation of BDNF gene: differences in DNA methylation between unipolar and bipolar patients. J Affect Disord 166, 330–333. [DOI] [PubMed] [Google Scholar]
- Deursen J.M.v., 2014. The role of senescent cells in ageing. Nature 509, 439–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Butters MA, Albert SM, Dew MA, Reynolds CF 3rd, 2013. Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 202, 329–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Fisher-Hoch S, McCormick J, 2018a. The association between insulin resistance, metabolic variables, and depressive symptoms in Mexican-American elderly: A population-based study. Int J Geriatr Psychiatry 33, e294–e299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Lin CW, Sibille E, Tseng G, Lotrich F, Aizenstein HJ, Reynolds CF, Butters MA, 2016. Circulating biosignatures of late-life depression (LLD): Towards a comprehensive, data-driven approach to understanding LLD pathophysiology. J Psychiatr Res 82, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Mendes-Silva AP, Silva LB, Bertola L, Vieira MC, Ferreira JD, Nicolau M, Bristot G, da Rosa ED, Teixeira AL, Kapczinski F, 2018b. Oxidative stress markers imbalance in late-life depression. J Psychiatr Res 102, 29–33. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Mulsant BH, Reynolds CF III, Blumberger DM, Karp JF, Butters MA, Mendes-Silva AP, Vieira EL, Tseng G, Lenze EJ, 2022. Association of Molecular Senescence Markers in Late-Life Depression With Clinical Characteristics and Treatment Outcome. JAMA Network Open 5, e2219678–e2219678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Reynolds CF 3rd, Begley A, Dew MA, Anderson SJ, Lotrich F, Erickson KI, Lopez O, Aizenstein H, Sibille EL, Butters MA, 2014a. Brain-derived neurotrophic factor levels in late-life depression and comorbid mild cognitive impairment: a longitudinal study. J Psychiatr Res 49, 96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Reynolds CF 3rd, Butters MA, Dew MA, Firmo JO, Lima-Costa MF, Castro-Costa E, 2014b. The effect of gender, age, and symptom severity in late-life depression on the risk of all-cause mortality: the Bambui Cohort Study of Aging. Depress Anxiety 31, 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Reynolds CF 3rd, Sibille E, Lin CW, Tseng G, Lotrich F, Aizenstein HJ, Butters MA, 2017. Enhanced Molecular Aging in Late-Life Depression: the Senescent-Associated Secretory Phenotype. Am J Geriatr Psychiatry 25, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Reynolds Iii CF, Sibille E, Bot M, Penninx B, 2019. Major depression and enhanced molecular senescence abnormalities in young and middle-aged adults. Transl Psychiatry 9, 198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Sibille E, Ding Y, Tseng G, Aizenstein HJ, Lotrich F, Becker JT, Lopez OL, Lotze MT, Klunk WE, Reynolds CF, Butters MA, 2015. Plasma biosignature and brain pathology related to persistent cognitive impairment in late-life depression. Mol Psychiatry 20, 594–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz BS, Teixeira AL, Campos AC, Miranda AS, Rocha NP, Talib LL, Gattaz WF, Forlenza OV, 2012. Reduced serum levels of adiponectin in elderly patients with major depression. Journal of psychiatric research 46, 1081–1085. [DOI] [PubMed] [Google Scholar]
- Diniz BS, Vieira EM, Mendes-Silva AP, Bowie CR, Butters MA, Fischer CE, Flint A, Herrmann N, Kennedy J, Lanctôt KL, Mah L, Pollock BG, Mulsant BH, Rajji TK, 2021. Mild cognitive impairment and major depressive disorder are associated with molecular senescence abnormalities in older adults. Alzheimers Dement (N Y) 7, e12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dome P, Teleki Z, Rihmer Z, Peter L, Dobos J, Kenessey I, Tovari J, Timar J, Paku S, Kovacs G, Dome B, 2009. Circulating endothelial progenitor cells and depression: a possible novel link between heart and soul. Mol Psychiatry 14, 523–531. [DOI] [PubMed] [Google Scholar]
- Duscher D, Rennert RC, Januszyk M, Anghel E, Maan ZN, Whittam AJ, Perez MG, Kosaraju R, Hu MS, Walmsley GG, Atashroo D, Khong S, Butte AJ, Gurtner GC, 2014. Aging disrupts cell subpopulation dynamics and diminishes the function of mesenchymal stem cells. Sci Reports 4, 7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AC, Aggen SH, Cai N, Bigdeli TB, Peterson RE, Docherty AR, Webb BT, Bacanu SA, Flint J, Kendler KS, 2016. Chronicity of Depression and Molecular Markers in a Large Sample of Han Chinese Women. Depress Anxiety 33, 1048–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Englund DA, Sakamoto AE, Fritsche CM, Heeren AA, Zhang X, Kotajarvi BR, Lecy DR, Yousefzadeh MJ, Schafer MJ, White TA, Atkinson EJ, LeBrasseur NK, 2021. Exercise reduces circulating biomarkers of cellular senescence in humans. Aging Cell 20, e13415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, 2020. The geroscience agenda: Toxic stress, hormetic stress, and the rate of aging. Ageing Res Rev 63, 101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epp JR, Beasley CL, Galea LA, 2013. Increased hippocampal neurogenesis and p21 expression in depression: dependent on antidepressants, sex, age, and antipsychotic exposure. Neuropsychopharmacology 38, 2297–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fafian-Labora JA, O’Loghlen A, 2020. Classical and Nonclassical Intercellular Communication in Senescence and Ageing. Trends Cell Biol 30, 628–639. [DOI] [PubMed] [Google Scholar]
- Fang Y, Chen B, Liu Z, Gong AY, Gunning WT, Ge Y, Malhotra D, Gohara AF, Dworkin LD, Gong R, 2022. Age-related GSK3beta overexpression drives podocyte senescence and glomerular aging. J Clin Invest 132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr JN, Almeida M, 2018. The Spectrum of Fundamental Basic Science Discoveries Contributing to Organismal Aging. J Bone Miner Res 33, 1568–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Fabbri E, 2018. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol 15, 505–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, Gonzalez-Freire M, Fabbri E, Simonsick E, Tanaka T, Moore Z, Salimi S, Sierra F, de Cabo R, 2020. Measuring biological aging in humans: A quest. Aging Cell 19, e13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S, 2007. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev 128, 92–105. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Zaikin A, Gordleeva S, Ivanchenko M, Bonifazi F, Storci G, Bonafè M, 2018. Inflammaging 2018: An update and a model. Semin Immunol 40, 1–5. [DOI] [PubMed] [Google Scholar]
- Frank P, Jokela M, Batty GD, Cadar D, Steptoe A, Kivimaki M, 2021. Association Between Systemic Inflammation and Individual Symptoms of Depression: A Pooled Analysis of 15 Population-Based Cohort Studies. Am J Psychiatry 178, 1107–1118. [DOI] [PubMed] [Google Scholar]
- Gasek NS, Kuchel GA, Kirkland JL, Xu M, 2021. Strategies for targeting senescent cells in human disease. Nature Aging 1, 870–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghanei Gheshlagh R, Parizad N, Sayehmiri K, 2016. The Relationship Between Depression and Metabolic Syndrome: Systematic Review and Meta-Analysis Study. Iran Red Crescent Med J 18, e26523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goncalves VF, Mendes-Silva AP, Koyama E, Vieira E, Kennedy JL, Diniz B, 2021. Increased levels of circulating cell-free mtDNA in plasma of late life depression subjects. J Psychiatr Res 139, 25–29. [DOI] [PubMed] [Google Scholar]
- Haass C, Selkoe DJ, 2007. Soluble protein oligomers in neurodegeneration: lessons from the Alzheimer’s amyloid beta-peptide. Nat Rev Mol Cell Biol 8, 101–112. [DOI] [PubMed] [Google Scholar]
- Hall JA, Dominy JE, Lee Y, Puigserver P, 2013. The sirtuin family’s role in aging and age-associated pathologies. J Clin Invest 123, 973–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamczyk MR, Nevado RM, Barettino A, Fuster V, Andres V, 2020. Biological Versus Chronological Aging: JACC Focus Seminar. J Am Coll Cardiol 75, 919–930. [DOI] [PubMed] [Google Scholar]
- Han LKM, Aghajani M, Clark SL, Chan RF, Hattab MW, Shabalin AA, Zhao M, Kumar G, Xie LY, Jansen R, Milaneschi Y, Dean B, Aberg KA, van den Oord E, Penninx B, 2018. Epigenetic Aging in Major Depressive Disorder. Am J Psychiatry 175, 774–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LKM, Dinga R, Hahn T, Ching CRK, Eyler LT, Aftanas L, Aghajani M, Aleman A, Baune BT, Berger K, Brak I, Filho GB, Carballedo A, Connolly CG, Couvy-Duchesne B, Cullen KR, Dannlowski U, Davey CG, Dima D, Duran FLS, Enneking V, Filimonova E, Frenzel S, Frodl T, Fu CHY, Godlewska BR, Gotlib IH, Grabe HJ, Groenewold NA, Grotegerd D, Gruber O, Hall GB, Harrison BJ, Hatton SN, Hermesdorf M, Hickie IB, Ho TC, Hosten N, Jansen A, Kahler C, Kircher T, Klimes-Dougan B, Kramer B, Krug A, Lagopoulos J, Leenings R, MacMaster FP, MacQueen G, McIntosh A, McLellan Q, McMahon KL, Medland SE, Mueller BA, Mwangi B, Osipov E, Portella MJ, Pozzi E, Reneman L, Repple J, Rosa PGP, Sacchet MD, Samann PG, Schnell K, Schrantee A, Simulionyte E, Soares JC, Sommer J, Stein DJ, Steinstrater O, Strike LT, Thomopoulos SI, van Tol MJ, Veer IM, Vermeiren R, Walter H, van der Wee NJA, van der Werff SJA, Whalley H, Winter NR, Wittfeld K, Wright MJ, Wu MJ, Volzke H, Yang TT, Zannias V, de Zubicaray GI, Zunta-Soares GB, Abe C, Alda M, Andreassen OA, Boen E, Bonnin CM, Canales-Rodriguez EJ, Cannon D, Caseras X, Chaim-Avancini TM, Elvsashagen T, Favre P, Foley SF, Fullerton JM, Goikolea JM, Haarman BCM, Hajek T, Henry C, Houenou J, Howells FM, Ingvar M, Kuplicki R, Lafer B, Landen M, Machado-Vieira R, Malt UF, McDonald C, Mitchell PB, Nabulsi L, Otaduy MCG, Overs BJ, Polosan M, Pomarol-Clotet E, Radua J, Rive MM, Roberts G, Ruhe HG, Salvador R, Sarro S, Satterthwaite TD, Savitz J, Schene AH, Schofield PR, Serpa MH, Sim K, Soeiro-de-Souza MG, Sutherland AN, Temmingh HS, Timmons GM, Uhlmann A, Vieta E, Wolf DH, Zanetti MV, Jahanshad N, Thompson PM, Veltman DJ, Penninx B, Marquand AF, Cole JH, Schmaal L, 2021a. Brain aging in major depressive disorder: results from the ENIGMA major depressive disorder working group. Mol Psychiatry 26, 5124–5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han LKM, Schnack HG, Brouwer RM, Veltman DJ, van der Wee NJA, van Tol MJ, Aghajani M, Penninx B, 2021b. Contributing factors to advanced brain aging in depression and anxiety disorders. Transl Psychiatry 11, 402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Sarvet AL, Meyers JL, Saha TD, Ruan WJ, Stohl M, Grant BF, 2018. Epidemiology of Adult DSM-5 Major Depressive Disorder and Its Specifiers in the United States. JAMA Psychiatry 75, 336–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T, Umegaki H, Makino T, Cheng XW, Shimada H, Kuzuya M, 2019. Association between sarcopenia and depressive mood in urban-dwelling older adults: A cross-sectional study. Geriatr Gerontol Int 19, 508–512. [DOI] [PubMed] [Google Scholar]
- Hernandez-Segura A, Nehme J, Demaria M, 2018. Hallmarks of Cellular Senescence. Trends in Cell Biology 28, 436–453. [DOI] [PubMed] [Google Scholar]
- Hetz C, Saxena S, 2017. ER stress and the unfolded protein response in neurodegeneration. Nature Reviews Neurology 13, 477–491. [DOI] [PubMed] [Google Scholar]
- Howard DM, Adams MJ, Clarke TK, Hafferty JD, Gibson J, Shirali M, Coleman JRI, Hagenaars SP, Ward J, Wigmore EM, Alloza C, Shen X, Barbu MC, Xu EY, Whalley HC, Marioni RE, Porteous DJ, Davies G, Deary IJ, Hemani G, Berger K, Teismann H, Rawal R, Arolt V, Baune BT, Dannlowski U, Domschke K, Tian C, Hinds DA, andMe Research T, Major Depressive Disorder Working Group of the Psychiatric Genomics, C., Trzaskowski M, Byrne EM, Ripke S, Smith DJ, Sullivan PF, Wray NR, Breen G, Lewis CM, McIntosh AM, 2019. Genome-wide meta-analysis of depression identifies 102 independent variants and highlights the importance of the prefrontal brain regions. Nat Neurosci 22, 343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR Jr., Wiste HJ, Weigand SD, Rocca WA, Knopman DS, Mielke MM, Lowe VJ, Senjem ML, Gunter JL, Preboske GM, Pankratz VS, Vemuri P, Petersen RC, 2014. Age-specific population frequencies of cerebral beta-amyloidosis and neurodegeneration among people with normal cognitive function aged 50–89 years: a cross-sectional study. Lancet Neurol 13, 997–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Januar V, Ancelin ML, Ritchie K, Saffery R, Ryan J, 2015. BDNF promoter methylation and genetic variation in late-life depression. Transl Psychiatry 5, e619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama Y, Kasahara T, Kato M, Sakai S, Deguchi Y, Tani M, Kuroda K, Hattori K, Yoshida S, Goto Y, Kinoshita T, Inoue K, Kato T, 2018. The relationship between circulating mitochondrial DNA and inflammatory cytokines in patients with major depression. J Affect Disord 233, 15–20. [DOI] [PubMed] [Google Scholar]
- Kan C, Silva N, Golden SH, Rajala U, Timonen M, Stahl D, Ismail K, 2013. A systematic review and meta-analysis of the association between depression and insulin resistance. Diabetes Care 36, 480–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kananen L, Hurme M, Burkle A, Moreno-Villanueva M, Bernhardt J, Debacq-Chainiaux F, Grubeck-Loebenstein B, Malavolta M, Basso A, Piacenza F, Collino S, Gonos ES, Sikora E, Gradinaru D, Jansen E, Dolle MET, Salmon M, Stuetz W, Weber D, Grune T, Breusing N, Simm A, Capri M, Franceschi C, Slagboom E, Talbot D, Libert C, Raitanen J, Koskinen S, Harkanen T, Stenholm S, Ala-Korpela M, Lehtimaki T, Raitakari OT, Ukkola O, Kahonen M, Jylha M, Jylhava J, 2022. Circulating cell-free DNA in health and disease - the relationship to health behaviours, ageing phenotypes and metabolomics. Geroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushik S, Cuervo AM, 2015. Proteostasis and aging. Nat Med 21, 1406–1415. [DOI] [PubMed] [Google Scholar]
- Kennedy BK, Berger SL, Brunet A, Campisi J, Cuervo AM, Epel ES, Franceschi C, Lithgow GJ, Morimoto RI, Pessin JE, Rando TA, Richardson A, Schadt EE, Wyss-Coray T, Sierra F, 2014. Geroscience: linking aging to chronic disease. Cell 159, 709–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SS, Singer BD, Vaughan DE, 2017. Molecular and physiological manifestations and measurement of aging in humans. Aging Cell 16, 624–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland JL, Tchkonia T, 2020. Senolytic drugs: from discovery to translation. J Intern Med 288, 518–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Yoshimura R, Kitajima T, Okochi T, Okumura T, Tsunoka T, Yamanouchi Y, Kinoshita Y, Kawashima K, Fukuo Y, Naitoh H, Umene-Nakano W, Inada T, Nakamura J, Ozaki N, Iwata N, 2010. SIRT1 gene is associated with major depressive disorder in the Japanese population. J Affect Disord 126, 167–173. [DOI] [PubMed] [Google Scholar]
- Kohler CA, Freitas TH, Maes M, de Andrade NQ, Liu CS, Fernandes BS, Stubbs B, Solmi M, Veronese N, Herrmann N, Raison CL, Miller BJ, Lanctot KL, Carvalho AF, 2017. Peripheral cytokine and chemokine alterations in depression: a meta-analysis of 82 studies. Acta Psychiatr Scand 135, 373–387. [DOI] [PubMed] [Google Scholar]
- Köhler O, Benros ME, Nordentoft M, Farkouh ME, Iyengar RL, Mors O, Krogh J, 2014. Effect of Anti-inflammatory Treatment on Depression, Depressive Symptoms, and Adverse Effects: A Systematic Review and Meta-analysis of Randomized Clinical Trials. JAMA Psychiatry 71, 1381–1391. [DOI] [PubMed] [Google Scholar]
- Koutsouleris N, Davatzikos C, Borgwardt S, Gaser C, Bottlender R, Frodl T, Falkai P, Riecher-Rossler A, Moller HJ, Reiser M, Pantelis C, Meisenzahl E, 2014. Accelerated brain aging in schizophrenia and beyond: a neuroanatomical marker of psychiatric disorders. Schizophr Bull 40, 1140–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk M, Kowalczyk E, Kwiatkowski P, Lopusiewicz L, Talarowska M, Sienkiewicz M, 2021. Cellular Response to Unfolded Proteins in Depression. Life (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam PH, Chiang JJ, Chen E, Miller GE, 2021. Race, socioeconomic status, and low-grade inflammatory biomarkers across the lifecourse: A pooled analysis of seven studies. Psychoneuroendocrinology 123, 104917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Gierc M, Vila-Rodriguez F, Puterman E, Faulkner G, 2021. Efficacy of exercise combined with standard treatment for depression compared to standard treatment alone: A systematic review and meta-analysis of randomized controlled trials. J Affect Disord 295, 1494–1511. [DOI] [PubMed] [Google Scholar]
- Leung YW, Flora DB, Gravely S, Irvine J, Carney RM, Grace SL, 2012. The impact of premorbid and postmorbid depression onset on mortality and cardiac morbidity among patients with coronary heart disease: meta-analysis. Psychosom Med 74, 786–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, D’Arcy C, Li X, Zhang T, Joober R, Meng X, 2019. What do DNA methylation studies tell us about depression? A systematic review. Transl Psychiatry 9, 68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Dhabhar FS, James SJ, Hough CM, Jain FA, Bersani FS, Reus VI, Verhoeven JE, Epel ES, Mahan L, Rosser R, Wolkowitz OM, Mellon SH, 2017. Oxidative stress, inflammation and treatment response in major depression. Psychoneuroendocrinology 76, 197–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Fernstrom J, Grudet C, Ljunggren L, Traskman-Bendz L, Ohlsson L, Westrin A, 2016. Increased plasma levels of circulating cell-free mitochondrial DNA in suicide attempters: associations with HPA-axis hyperactivity. Transl Psychiatry 6, e971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Wolkowitz OM, Picard M, Ohlsson L, Bersani FS, Fernstrom J, Westrin A, Hough CM, Lin J, Reus VI, Epel ES, Mellon SH, 2018. Circulating cell-free mitochondrial DNA, but not leukocyte mitochondrial DNA copy number, is elevated in major depressive disorder. Neuropsychopharmacology 43, 1557–1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou YJ, Chen MH, Hsu JW, Huang KL, Huang PH, Bai YM, 2021. Associations between increased circulating endothelial progenitor cell levels and anxiety/depressive severity, cognitive deficit and function disability among patients with major depressive disorder. Sci Rep 11, 18221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Yan H, Zhou D, Cai X, Zhang Y, Li S, Li H, Li S, Zhou DS, Li X, Zhang C, Sun Y, Dai JP, Zhong J, Yao YG, Luo XJ, Fang Y, Zhang D, Ma Y, Yue W, Li M, Xiao X, 2019. The depression GWAS risk allele predicts smaller cerebellar gray matter volume and reduced SIRT1 mRNA expression in Chinese population. Transl Psychiatry 9, 333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, 2013. The hallmarks of aging. Cell 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, Galluzzi L, Freije JMP, Madeo F, Kroemer G, 2016. Metabolic Control of Longevity. Cell 166, 802–821. [DOI] [PubMed] [Google Scholar]
- Lu W, Zhang Y, Liu D, Songyang Z, Wan M, 2013. Telomeres—structure, function, and regulation. Experimental Cell Research 319, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamdani F, Rollins B, Morgan L, Myers RM, Barchas JD, Schatzberg AF, Watson SJ, Akil H, Potkin SG, Bunney WE, Vawter MP, Sequeira PA, 2015. Variable telomere length across post-mortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl Psychiatry 5, e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrory CL, Ryan KM, Kolshus E, Finnegan M, McLoughlin DM, 2018. Peripheral blood SIRT1 mRNA levels in depression and treatment with electroconvulsive therapy. Eur Neuropsychopharmacol 28, 1015–1023. [DOI] [PubMed] [Google Scholar]
- McKinney BC, Lin CW, Rahman T, Oh H, Lewis DA, Tseng G, Sibille E, 2019. DNA methylation in the human frontal cortex reveals a putative mechanism for age-by-disease interactions. Transl Psychiatry 9, 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Silva AP, Mwangi B, Aizenstein H, Reynolds CF 3rd, Butters MA, Diniz BS, 2019. Molecular Senescence Is Associated With White Matter Microstructural Damage in Late-Life Depression. Am J Geriatr Psychiatry 27, 1414–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes-Silva AP, Vieira ELM, Xavier G, Barroso LSS, Bertola L, Martins EAR, Brietzke EM, Belangero SIN, Diniz BS, 2021. Telomere shortening in late-life depression: A potential marker of depression severity. Brain Behav 11, e2255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM, 2014. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N=9484). Mol Psychiatry 19, 791–800. [DOI] [PubMed] [Google Scholar]
- Nie C, Li Y, Li R, Yan Y, Zhang D, Li T, Li Z, Sun Y, Zhen H, Ding J, Wan Z, Gong J, Shi Y, Huang Z, Wu Y, Cai K, Zong Y, Wang Z, Wang R, Jian M, Jin X, Wang J, Yang H, Han JJ, Zhang X, Franceschi C, Kennedy BK, Xu X, 2022. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep 38, 110459. [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Habegger KM, Chaudhary N, Finan B, Banks AS, Dietrich MO, Horvath TL, Sinclair DA, Pfluger PT, Tschop MH, 2012. Sirtuin 1 and sirtuin 3: physiological modulators of metabolism. Physiol Rev 92, 1479–1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numakawa T, Odaka H, Adachi N, 2017. Actions of Brain-Derived Neurotrophic Factor and Glucocorticoid Stress in Neurogenesis. Int J Mol Sci 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell LJ, Westin CF, 2011. An introduction to diffusion tensor image analysis. Neurosurg Clin N Am 22, 185–196, viii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogrodnik M, 2021. Cellular aging beyond cellular senescence: Markers of senescence prior to cell cycle arrest in vitro and in vivo. Aging Cell 20, e13338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh J, Lee YD, Wagers AJ, 2014. Stem cell aging: mechanisms, regulators and therapeutic opportunities. Nat Med 20, 870–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo PS, Boriek AM, 2020. SIRT1 Regulation in Ageing and Obesity. Mech Ageing Dev 188, 111249. [DOI] [PubMed] [Google Scholar]
- Partridge L, Fuentealba M, Kennedy BK, 2020. The quest to slow ageing through drug discovery. Nat Rev Drug Discov 19, 513–532. [DOI] [PubMed] [Google Scholar]
- Pham K, Nacher J, Hof PR, McEwen BS, 2003. Repeated restraint stress suppresses neurogenesis and induces biphasic PSA-NCAM expression in the adult rat dentate gyrus. Eur J Neurosci 17, 879–886. [DOI] [PubMed] [Google Scholar]
- Plana-Ripoll O, Momen NC, McGrath JJ, Wimberley T, Brikell I, Schendel D, Thygesen M, Weye N, Pedersen CB, Mors O, Mortensen PB, Dalsgaard S, 2022. Temporal changes in sex- and age-specific incidence profiles of mental disorders-A nationwide study from 1970 to 2016. Acta Psychiatr Scand 145, 604–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, Sidtis JJ, Wisniewski TM, Mehta PD, Pratico D, 2012. Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. American Journal of Psychiatry 169, 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomatto LCD, Sun PY, Davies KJA, 2018. To Adapt or not to Adapt: Consequences of Declining Adaptive Homeostasis and Proteostasis with Age. Mechanisms of Ageing and Development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protsenko E, Yang R, Nier B, Reus V, Hammamieh R, Rampersaud R, Wu GWY, Hough CM, Epel E, Prather AA, Jett M, Gautam A, Mellon SH, Wolkowitz OM, 2021. “GrimAge,” an epigenetic predictor of mortality, is accelerated in major depressive disorder. Transl Psychiatry 11, 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, D’Souza S, Milne BJ, Caspi A, Moffitt TE, 2022. Longitudinal Associations of Mental Disorders With Dementia: 30-Year Analysis of 1.7 Million New Zealand Citizens. JAMA Psychiatry 79, 333–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond-Rakerd LS, D’Souza S, Milne BJ, Caspi A, Moffitt TE, 2021. Longitudinal Associations of Mental Disorders With Physical Diseases and Mortality Among 2.3 Million New Zealand Citizens. JAMA Network Open 4, e2033448–e2033448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge J, Oh H, Wyss-Coray T, 2022. Measuring biological age using omics data. Nature Reviews Genetics. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaakxs R, Verhoeven JE, Oude Voshaar RC, Comijs HC, Penninx B, 2015. Leukocyte telomere length and late-life depression. Am J Geriatr Psychiatry 23, 423–432. [DOI] [PubMed] [Google Scholar]
- Schaum N, Lehallier B, Hahn O, Palovics R, Hosseinzadeh S, Lee SE, Sit R, Lee DP, Losada PM, Zardeneta ME, Fehlmann T, Webber JT, McGeever A, Calcuttawala K, Zhang H, Berdnik D, Mathur V, Tan W, Zee A, Tan M, Tabula Muris C, Pisco AO, Karkanias J, Neff NF, Keller A, Darmanis S, Quake SR, Wyss-Coray T, 2020. Ageing hallmarks exhibit organ-specific temporal signatures. Nature 583, 596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher B, Pothof J, Vijg J, Hoeijmakers JHJ, 2021. The central role of DNA damage in the ageing process. Nature 592, 695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM, 2015. The association between depression and leukocyte telomere length: a meta-analysis. Depress Anxiety 32, 229–238. [DOI] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, Goldman-Mellor S, Harrington HL, Houts RM, Israel S, Poulton R, Robertson SP, Sugden K, Williams B, Caspi A, 2014. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol Psychiatry 19, 1163–1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A, McMunn A, Banks J, Steptoe A, 2011. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol 30, 377–385. [DOI] [PubMed] [Google Scholar]
- Sharpless NE, Sherr CJ, 2015. Forging a signature of in vivo senescence. Nat Rev Cancer 15, 397–408. [DOI] [PubMed] [Google Scholar]
- Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, Solmi M, Schofield P, Koyanagi A, Tseng PT, Lin PY, Chu CS, Cosco TD, Cesari M, Carvalho AF, Stubbs B, 2017. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res Rev 36, 78–87. [DOI] [PubMed] [Google Scholar]
- Squassina A, Pisanu C, Congiu D, Caria P, Frau D, Niola P, Melis C, Baggiani G, Lopez JP, Cruceanu C, Turecki G, Severino G, Bocchetta A, Vanni R, Chillotti C, Del Zompo M, 2016. Leukocyte telomere length positively correlates with duration of lithium treatment in bipolar disorder patients. Eur Neuropsychopharmacol 26, 1241–1247. [DOI] [PubMed] [Google Scholar]
- Strawbridge R, Arnone D, Danese A, Papadopoulos A, Herane Vives A, Cleare AJ, 2015. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol 25, 1532–1543. [DOI] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri T, Chandley MJ, Crawford JD, Stockmeier CA, Ordway GA, 2014. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int J Neuropsychopharmacol 17, 1579–1589. [DOI] [PubMed] [Google Scholar]
- Tahapary DL, Pratisthita LB, Fitri NA, Marcella C, Wafa S, Kurniawan F, Rizka A, Tarigan TJE, Harbuwono DS, Purnamasari D, Soewondo P, 2022. Challenges in the diagnosis of insulin resistance: Focusing on the role of HOMA-IR and Tryglyceride/glucose index. Diabetes Metab Syndr 16, 102581. [DOI] [PubMed] [Google Scholar]
- Teyssier JR, Chauvet-Gelinier JC, Ragot S, Bonin B, 2012. Up-regulation of leucocytes genes implicated in telomere dysfunction and cellular senescence correlates with depression and anxiety severity scores. PLoS One 7, e49677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente C, Andrade R, Alvarez L, Rebelo-Marques A, Stamatakis E, Espregueira-Mendes J, 2021. Effect of physical activity and exercise on telomere length: Systematic review with meta-analysis. J Am Geriatr Soc 69, 3285–3300. [DOI] [PubMed] [Google Scholar]
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J, 2007. Free radicals and antioxidants in normal physiological functions and human disease. The international journal of biochemistry & cell biology 39, 44–84. [DOI] [PubMed] [Google Scholar]
- Verduijn J, Milaneschi Y, Schoevers RA, Hemert A.M.v., Beekman ATF, Penninx B, 2015. Pathophysiology of major depressive disorder: mechanisms involved in etiology are not associated with clinical progression. Translational Psychiatry 5, e649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verhoeven JE, Revesz D, Picard M, Epel EE, Wolkowitz OM, Matthews KA, Penninx B, Puterman E, 2018. Depression, telomeres and mitochondrial DNA: between- and within-person associations from a 10-year longitudinal study. Mol Psychiatry 23, 850–857. [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, van Oppen P, Révész D, Wolkowitz OM, Penninx BW, 2016. Depressive and Anxiety Disorders Showing Robust, but Non-Dynamic, 6-Year Longitudinal Association With Short Leukocyte Telomere Length. Am J Psychiatry 173, 617–624. [DOI] [PubMed] [Google Scholar]
- Vieira EL, Mendes-Silva AP, Ferreira JD, Bertola L, Barroso L, Vieira M, Teixeira AL, Diniz BS, 2021. Oxidative DNA damage is increased in older adults with a major depressive episode: A preliminary study. J Affect Disord 279, 106–110. [DOI] [PubMed] [Google Scholar]
- Viel T, Chinta S, Rane A, Chamoli M, Buck H, Andersen J, 2020. Microdose lithium reduces cellular senescence in human astrocytes - a potential pharmacotherapy for COVID-19? Aging (Albany NY) 12, 10035–10040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vingeliene S, Hiyoshi A, Lentjes M, Fall K, Montgomery S, 2019. Longitudinal analysis of loneliness and inflammation at older ages: English longitudinal study of ageing. Psychoneuroendocrinology 110, 104421. [DOI] [PubMed] [Google Scholar]
- Wang W, Zhao F, Ma X, Perry G, Zhu X, 2020. Mitochondria dysfunction in the pathogenesis of Alzheimer’s disease: recent advances. Mol Neurodegener 15, 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb M, Sideris DP, 2020. Intimate Relations-Mitochondria and Ageing. Int J Mol Sci 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz J, Caspi A, Ambler A, Broadbent J, Hancox RJ, Harrington H, Hogan S, Houts RM, Leung JH, Poulton R, Purdy SC, Ramrakha S, Rasmussen LJH, Richmond-Rakerd LS, Thorne PR, Wilson GA, Moffitt TE, 2021. Association of History of Psychopathology With Accelerated Aging at Midlife. JAMA psychiatry 78, 530–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, Charlson FJ, Norman RE, Flaxman AD, Johns N, Burstein R, Murray CJ, Vos T, 2013. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 382, 1575–1586. [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, Su Y, Reus VI, Rosser R, Burke HM, Kupferman E, Compagnone M, Nelson JC, Blackburn EH, 2011. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress--preliminary findings. PLoS One 6, e17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Ruan LM, Ye HH, Cui HB, Mu QT, Lou YR, Ji YX, Li WZ, Sun DH, Chen XB, 2011. Depression is associated with lower circulating endothelial progenitor cells and increased inflammatory markers. Acta Neuropsychiatr 23, 235–240. [DOI] [PubMed] [Google Scholar]
- Young ARJ, Narita M, 2009. SASP reflects senescence. Embo Rep 10, 228–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousefzadeh M, Henpita C, Vyas R, Soto-Palma C, Robbins P, Niedernhofer L, 2021. DNA damage—how and why we age? Elife 10, e62852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Strachan E, Fowler E, Bacus T, Roy-Byrne P, Zhao J, 2019. Genome-wide profiling of DNA methylome and transcriptome in peripheral blood monocytes for major depression: A Monozygotic Discordant Twin Study. Transl Psychiatry 9, 215. [DOI] [PMC free article] [PubMed] [Google Scholar]


