ABSTRACT
Consumption of nuts and seeds is associated with a range of health outcomes. Summarizing the best evidence on essential health outcomes from the consumption of nuts is essential to provide optimal recommendations. Our objective is to comprehensively assess health outcome associations related to the consumption of nuts and seeds, using a culinary definition including tree nuts and peanuts (registered in PROSPERO: CRD42021258300). Health outcomes of interest include cardiovascular disease, cancer, diabetes, obesity, respiratory disease, mortality, and their disease biomarkers. We present associations for high compared with low consumption, per serving, and dose–response relations. MEDLINE, Embase, Cochrane, and Epistemonikos were searched and screened for systematic reviews and meta-analyses. Evidence was extracted from 89 articles on the consumption of nuts and relevant health outcomes, including 23 articles with meta-analysis on disease and mortality, 66 articles on biomarkers for disease, and 9 articles on allergy/adverse outcomes. Intake of nuts was associated with reduced risk of cardiovascular diseases and related risk factors, with moderate quality of evidence. An intake of 28 g/d nuts compared with not eating nuts was associated with a 21% RR reduction of cardiovascular disease (including coronary heart disease incidence and mortality, atrial fibrillation, and stroke mortality), an 11% risk reduction of cancer deaths, and 22% reduction in all-cause mortality. Nut consumption was also inversely associated with mortality from respiratory diseases, infectious diseases, and diabetes; however, associations between nut consumption and diabetes incidence were mixed. Meta-analyses of trials on biomarkers for disease generally mirrored meta-analyses from observational studies on cardiovascular disease, cancers, and diabetes. Allergy and related adverse reactions to nuts were observed in 1–2% of adult populations, with substantial heterogeneity between studies. Overall, the current evidence supports dietary recommendations to consume a handful of nuts and seeds per day for people without allergies to these foods.
Keywords: nuts; cardiovascular diseases; neoplasms; type 2 diabetes mellitus; diet, food, nutrition; mortality; biomarkers
Statement of Significance: This umbrella review provides comprehensive and up-to-date evidence on nut consumption and the risk of cardiovascular disease, cancers, diabetes, and mortality. It also presents per serving and dose–response relations, and evidence on biomarkers for diseases.
Introduction
Nuts and seeds have been part of diets worldwide for millennia (1). Nuts and seeds are highly nutrient-dense dietary components, rich in macronutrients including MUFAs and PUFAs, proteins, and fibers (2, 3). They are also rich in vitamins and minerals, and a range of active metabolites such as phenolic acids, phytosterols, carotenoids, and polyphenolic compounds (2, 4–6). Some of the compounds present in nuts, including polyphenols, have been found to have antioxidant, antimicrobial, and antiproliferative properties (4, 7, 8). Nuts were utilized in ancient medicinal traditions, an example being Hippocrates’ description of almonds as a treatment for colds and coughs (1).
Nuts are botanically categorized as tree nuts and peanuts. Nuts have hard shells covering the seed, and examples of frequently consumed tree nuts include almonds, walnuts, hazelnuts, cashews, Brazil nuts, macadamias, and pistachios. Tree nuts and peanuts have many compositional/nutritional similarities, and even though peanuts are botanically classified as legumes, their culinary use is similar to tree nuts (9). Further, seeds such as sesame and sunflower are related food groups (10). Consumption of nuts and seeds varies between cultural settings, both in preferences for nut and seed types and the amounts consumed (11, 12), with higher consumption generally reported in Canada, some African countries, parts of Europe, and the Middle East, and lower intakes in South America.
Consumption of nuts and seeds has been inversely associated with the risks of cardiovascular disease, cancers, and respiratory diseases (13–18). Cardiovascular disease, cancer, respiratory diseases, diabetes, and neurodegenerative diseases are globally among the leading causes of death and life years lost (19–21), contributing to 32%/20%, 16%/13%, 11%/10%, 3%/2%, 5%/2%, and 2%/2% of deaths/life years lost from these outcomes, respectively. On the other hand, nut allergies and related reactions are potential unintended effects (22). Some compounds, such as phytates, might also reduce the bioavailability of some nutrients in the gastrointestinal tract (23). Nuts might also impact the microbiota, but the results are uncertain regarding whether they tend to have more prebiotic properties and stimulate the growth of nonpathogenic gut bacteria, or promote pathogenic bacteria (24, 25). To contribute to optimizing intake levels through diet recommendations, both positive and adverse effects need to be considered. Therefore, summarizing the best evidence on health outcomes from consumption of nuts and seeds is essential. Umbrella reviews have been conducted focusing on cardiovascular and metabolic outcomes (26–28). However, these do not cover all relevant morbidities, and many relevant meta-analyses have been published subsequently. Thus, a comprehensive update could give more precise estimates and balance various health outcomes.
This umbrella review provides a systematic and comprehensive overview of the evidence on the consumption of nuts and seeds and the associations with various diseases, including high compared with low consumption, per serving, and dose–response relations. We have used a culinary definition of nuts and seeds, thus including tree nuts, peanuts, and seeds, and presenting data on biomarkers for diseases as intermediate causal factors contributing to understanding the evidence.
Methods
To summarize the evidence from meta-analyses and systematic reviews on the consumption of nuts and relevant health outcomes such as cardiovascular disease, cancer, diabetes, obesity, respiratory disease, mortality, and their intermediate factors, we used an umbrella review framework (29, 30). The protocol for the study has been registered in PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42021258300).
Eligibility criteria
We evaluated meta-analyses and systematic reviews presenting analyses from cohorts and trials on the consumption of nuts and seeds and associations with incidence and mortality of different diseases and intermediary factors related to these diseases. For inclusion and exclusion criteria, see below. Studies with a cross-sectional design or only presenting regional estimates not representative of a general population were excluded. No search restrictions were imposed on the publication date or publication status. We excluded articles written in languages other than English, German, French, Norwegian, Danish, or Swedish.
Inclusion criteria
We included meta-analyses and systematic reviews presenting analyses from longitudinal observation studies (e.g., cohorts, nested case-control) and trials, in which the exposure was consumption of nuts and seeds (using a culinary definition). The comparators were high compared with low consumption, per serving, and dose–response relation between exposure and outcomes. The outcomes were cardiovascular diseases, cancer, diabetes and metabolic disease, respiratory, infectious, and other diseases, adverse effects including allergies, and mortality, as well as intermediary factors for these diseases. Included articles were published in English, German, French, Norwegian, Danish, or Swedish, and indexed in MEDLINE, Embase, Cochrane, and Epistemonikos from inception to May 27, 2021.
Exclusion criteria
We excluded nonsystematic reviews and studies not presenting results for nuts separately but only as part of a combined diet.
Types of outcome measures
Outcomes included were the following: coronary heart disease, coronary heart disease mortality, cardiovascular disease, cardiovascular disease mortality, cancer mortality, diabetes mellitus, diabetes mortality, obesity or overweight, metabolic syndrome, heart failure, stroke and subtypes including hemorrhagic stroke incidence, ischemic stroke incidence, stroke mortality, infectious disease and related mortality, kidney disease and related mortality, neuro-degenerative disease mortality, respiratory disease mortality, adverse effects including allergies and anaphylactic reactions, and all-cause mortality (Supplemental Table 1). We also assessed biomarkers for disease (intermediate factors), including blood lipids, cholesterols, endothelial function, blood pressure, body composition and weight, hunger and fullness, glucose and insulin, inflammation, and gut microbiota.
Information sources
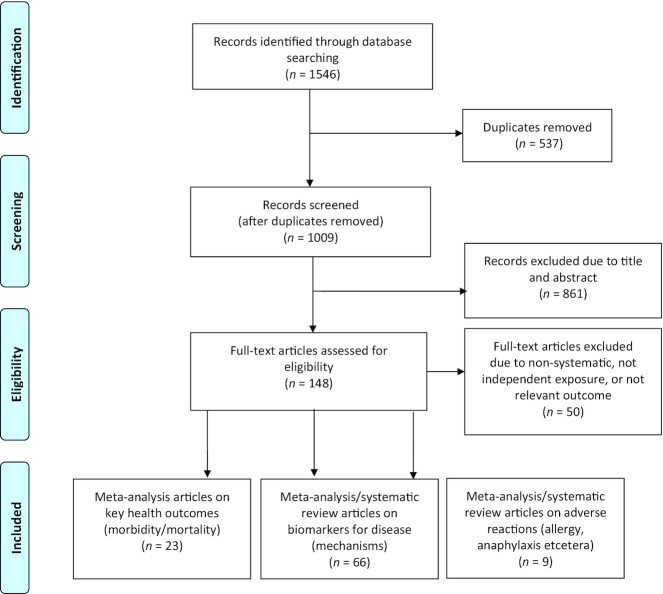
Overall, in collaboration with an experienced librarian, 1546 records were retrieved from the databases in MEDLINE, Embase, Cochrane, and Epistemonikos (also extracting through CINAHL, PsycINFO, LILACS, DARE, The Campbell Collaboration online library, JBI Database, and EPPI-Centre Evidence Library). The search period was from inception to May 27, 2021. After automatic deduplication in EndNote, 1009 records remained (see Figure 1 and details of search in Supplemental Material). No limits were applied for language or publication date. This systematic review has made efforts to adhere to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) criteria (31).
FIGURE 1.
Study selection for the umbrella analysis of health outcomes of nuts and seeds.
Search
The search included the following terms (≥1 of 1, 2, and 3): 1) nut, almond, Brazil nut, cashew nut, hazelnut, pecan, pistachio, walnut, peanut, macadamia, sesame, oilseed, hickory, seeds, pine seed, sunflower seed, chia, poppy seed, hemp seed, quinoa, pumpkin seed, or flaxseed; 2) intake, consumption, eat, or diet; and 3) systematic review or meta-analysis.
For further details on search, see Supplemental Material. The references were imported into EndNote X9.
Study selection
The 2 first authors (RB and TB) screened the imported references. The screening was done by reading the title, abstract, and assessing full-text articles assumed to be relevant. Two authors read all obtainable relevant articles in full text, and possible differences in assessment were discussed between the authors and resolved by consensus. None of the available meta-analyses reported on total cancer incidence, although some meta-analyses combined different cancer types in an overall analysis (32–35). None of the original studies included in these meta-analyses reported on total cancer incidence, only on the incidence of specific cancers, and because of this the results for different cancers combined were not considered reliable and were not used in the current analysis. Data values extracted were also double-checked.
Data collection process and data items
Data considered relevant were extracted into a Microsoft Excel table, and information was gathered on the first author, title, primary outcome(s), aims of the studies, conclusion, exposure (types of nuts/seeds), inclusion and exclusion criteria, design, type and number of studies, number of participants, number of cases/outcomes, outcome measures, heterogeneity, findings reported on high vs. low intake, findings on dose-response or per serving, and findings categorized otherwise (Supplemental Table 1). We used the data from the source published last for duplicate data identified. A total of 148 full-text articles were assessed in detail.
Risk of bias in individual studies and across studies
The risk of bias was assessed with the AMSTAR-2 tool [A MeaSurement Tool to Assess systematic Reviews (version 2)] (36). The quality of the reviews was categorized into high/moderate/low (e.g., AMSTAR-2: high). Details from the assessments are listed in Supplemental Table 2.
Analysis
Tables with extracted data from included studies were made. These data were summarized in figures visualizing the associations between nuts and various health outcomes for low compared with high, per serving, and dose–response. For per serving, when units other than per serving of 20–30 g/d were used, a conversion of the RR was estimated (for 4 servings of 28 g/wk: RR(7/4); for 1 serving per week: RR4; for servings of 20 g/d: RR1; for servings of 12 g/d: RR2). We present forest plots for the most comprehensive meta-analysis for each outcome measure (and similar emphasizing the most recent meta-analyses). The most comprehensive was defined as the relevant meta-analysis including the most relevant studies and having the most participants with the relevant outcomes. The forest plots include information on source/reference, the number of participants and cases, included studies, and heterogeneity. We also present data for nuts and subgroups of nuts and groups of outcomes, including:
Cardiovascular disease/coronary heart disease/stroke/heart failure/atrial fibrillation
All-cause mortality
Diabetes, diabetes mortality, and metabolic syndrome
Cancer mortality
Other morbidities including infectious disease mortality, kidney disease mortality, neurodegenerative disease mortality, respiratory disease mortality.
For dose–response, we present relevant data from meta-analyses, extracted values through the Web Plot Digitizer tool (https://apps.automeris.io/wpd/), and present these in the form of supplemental figures. Stata SE 17 (StataCorp LLC) was used for data analysis and graphical presentation.
Results
Twenty-three meta-analysis articles provided 190 outcome measures for disease and disease-related mortality (Supplemental Tables 1 and 3) (13–16, 32–35, 37–52). Most outcome measures were available for all-cause mortality, cancer mortality, and cardiovascular disease, with subcategories including related incidence and mortality, coronary heart disease, and stroke. Sixty-six full-text articles provided data on biomarkers for disease and disease mechanisms (53–118), and most of these were trials.
Cardiovascular disease
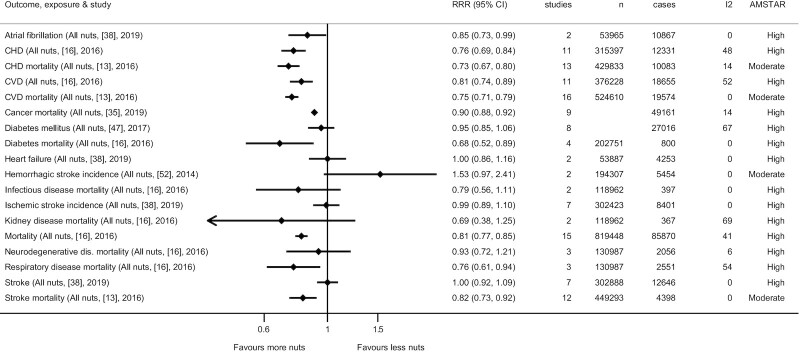
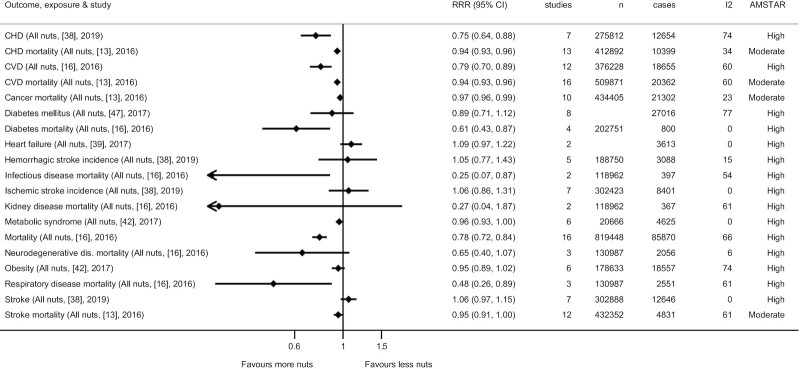
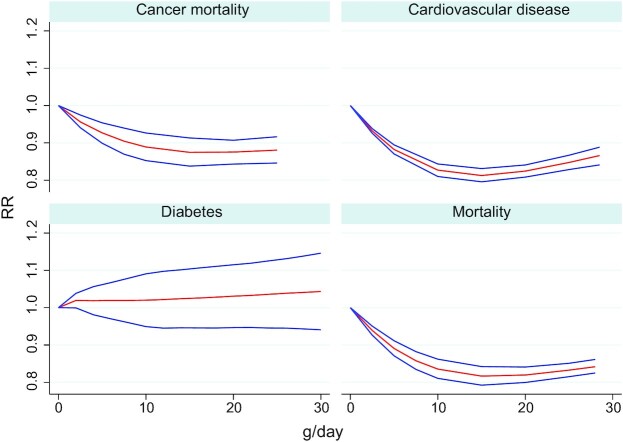
Meta-analyses indicate inverse associations between high compared with low consumption of nuts and cardiovascular diseases (Supplemental Figures 1–4; Figure 2) (16, 37–39, 43–45, 48–50, 52). Similar findings are also seen for coronary heart disease and related mortality. For per serving data, a daily intake of 28 g nuts is associated with an RR of 0.79 (95% CI: 0.70–0.89; I2: 60%; AMSTAR-2: high) for overall cardiovascular disease, 0.75 (95% CI: 0.64–0.88; I2: 74%; AMSTAR-2: high) for coronary heart disease, and 0.78 (95% CI: 0.73–0.83; I2: 60%; AMSTAR-2: moderate) for cardiovascular mortality (Figure 3). The associations were generally similar between the intake of tree nuts compared with peanuts and cardiovascular outcomes. An inverse association was observed between intake of peanuts and stroke, although this was nonsignificant for tree nuts. Dose–response associations between nut consumption and risk of cardiovascular diseases and coronary heart diseases suggest optimal intake levels of ∼15–20 g/d nuts and limited benefits in increasing intake beyond 1 serving of 28 g/d (Figure 4; Supplemental Figures 5–7). There were 376,228 participants and 18,655 cases in the overall cardiovascular disease analyses for all nuts.
FIGURE 2.
Summary of associations from the most comprehensive meta-analyses between high compared with low consumption of nuts and risk of various morbidities and mortalities. Reference number is listed in brackets and search year is listed within the parentheses.
FIGURE 3.
Summary of per serving associations from the most comprehensive meta-analyses between consumption of 28 g/d nuts and risk of various morbidities and mortalities. Reference number is listed in brackets and search year is listed within the parentheses.
FIGURE 4.
Summary of dose–response associations from the most comprehensive meta-analyses between consumption of nuts and risk of cancer mortality, cardiovascular disease, diabetes, and all-cause mortality. Red lines indicate the RR associations estimate whereas blue lines represent the CI of the RRs.
Mechanisms related to cardiovascular disease
Meta-analyses investigating the effects of nut and seed consumption on biomarkers for disease (intermediary outcomes) related to cardiovascular disease generally reported favorable effects on blood lipid profile (linked with reduced risk of diseases), particularly for total cholesterol, LDL, triglycerides, and apoB (56, 59, 62, 69–71, 75, 77, 79, 81, 85, 93, 99–101, 105) (Table 1). In contrast, some reported no significant biomarker change (66, 72, 73, 77, 81, 90, 109). Evidence on vascular endothelial function and blood pressure outcomes either indicated favorable (55, 63, 65, 66, 73, 77, 78, 86, 95, 108, 112, 116), or neutral/no significant biomarker change (55, 56, 62, 63, 65, 66, 69, 71–73, 79, 80, 85, 89, 109). However, the duration of many of these trials could have been too short to identify potential effects on changes in blood pressure and hypertension (60, 62, 63, 72, 73, 80, 89). Meta-analyses of observational studies reported an inverse association between consumption of nuts and risk of hypertension (41, 107, 118).
TABLE 1.
List of biomarkers for various disease and intermediate mechanisms for various morbidities from systematic reviews and meta-analyses including cardiovascular, diabetes and weight, and other outcomes1
| Favorable | Neutral | Unfavorable | |
|---|---|---|---|
| Blood lipids | |||
| HDLs | (72) | (59), (85), (62), (69), (70), (71), (73), (77), (81), (90), (93), (99), (100), (101), (105), (66), (75), (109), (56) | (79) |
| LDLs | (62), (69), (70), (81), (93), (99), (100), (105), (75), (79), (56) | (71), (73), (77), (90), (101), (72), (66), (109) | — |
| Triglycerides | (59), (85), (62), (69), (70), (71), (77), (81), (93), (75), (56) | (59), (73), (81), (90), (99), (100), (101), (105), (72), (66), (79), (109) | — |
| Total cholesterol | (59), (62), (69), (70), (71), (81), (81), (93), (99), (100), (101), (105), (75), (79), (56) | (73), (77), (81), (90), (72), (66, 109) | — |
| Lipoprotein A | (106), (70) | — | — |
| ApoA | — | (62), (79) | — |
| ApoB | (62), (69), (79) | — | — |
| Endothelial function | |||
| Brachial artery diameter | (65) | — | — |
| Flow-mediated dilatation | (116), (86), (95), (108) | (85), (62), (65), (56) | — |
| Blood pressure | |||
| Systolic blood pressure (trials) | (55), (73), (78), (112), (66), (77) | (63), (69), (71), (80), (89), (72), (79), (109), (62), (85) | — |
| Diastolic blood pressure (trials) | (63), (78), (112), (77) | (85), (62), (69), (71), (73), (80), (89), (55), (72), (79), (109), (66) | — |
| Hypertension (observational) | (107), (41), (118) | — | — |
| Body composition and weight | |||
| Body composition | — | (74) | — |
| Body weight | (120), (121), (122), (123), (68, 87), (124) | (125), (124), (68), (115), (64), (74) | — |
| BMI | (123), (115), (124) | (121), (124), (68), (64), (74) | — |
| Energy intake | (125) | — | — |
| Fat mass | (68), (121) | (124), (68) | — |
| Overweight/obesity risk | (120), (121) | — | — |
| Waist circumference | (120), (124) | (60), (121), (68), (115), (64), (74) | — |
| Hunger and fullness | |||
| Fullness | — | (125) | — |
| Hunger | (125) | — | — |
| Leptin | (84) | — | — |
| Glucose and insulin | |||
| Fasting blood glucose | (60), (97), (98), (60), (123), (88) | (74) | — |
| Glycemic control | (88), (114), (123) | — | — |
| Insulin sensitivity | (98) | — | — |
| Fasting plasma insulin | (98) | (97) | — |
| Adiponectin | — | (84), (94) | — |
| HOMA-IR | (123), (88) | (74) | — |
| HbA1c | (97), (98), (88) | — | |
| Glycemic indices | (92) | (74), (92) | — |
| Inflammation | |||
| C-reactive protein | (58), (102) | (84), (95), (102), (103), (113) | — |
| TNF-α | (58), (102) | (84), (95) | — |
| IL-6, IL-10 | (58) | (84), (95), (102) | — |
| Vascular, intercellular, andendothelial-leukocyte celladhesion proteins 1 (VCAM-1,ICAM-1, E-selectin) | (58) | (95) | — |
| Antioxidant defense system | (67), (129) | — | — |
| Gut microbiota | |||
| Fecal microbiota | — | (24), (25) | — |
| Cognitive function | |||
| Cognitive performance | (54) | (110) | — |
Studies are categorized based on biomarker change [favorable/reduced disease risk, neutral (no significant change), or unfavorable/increased risk], and listed by reference number. HbA1c, glycated hemoglobin; ICAM-1, intercellular adhesion molecule 1; VCAM-1, vascular cell adhesion molecule 1.
Diabetes, obesity, and metabolic disease
Meta-analyses reported mixed associations between high compared with low consumption of nuts and diabetes, obesity, and metabolic disease (15, 34, 37, 42, 43, 47, 51) (Supplemental Figures 8 and 9). For per serving data, a daily intake of 28 g nuts was associated with an RR of 0.89 (95% CI: 0.71–1.12; I2: 77%; AMSTAR-2: high) for diabetes mellitus type 2, and 0.61 (95% CI: 0.43–0.87; I2: 0%; AMSTAR-2: high) for diabetes-related mortality. For obesity there was a nonsignificant association (RR: 0.81; 95% CI: 0.62–1.07; I2: 74%; AMSTAR-2: moderate), whereas when assessing obesity/overweight, a significant association (RR: 0.89; 95% CI: 0.83–0.94; I2: 0%; AMSTAR-2: moderate) was observed. There were no significant nonlinear dose–response associations between consumption of nuts and diabetes (Supplemental Figure 10). There were 27,016 cases in the analysis of nut consumption and type 2 diabetes incidence.
Neither tree nuts nor peanuts separately were significantly associated with diabetes mortality. Most meta-analyses were adjusted for BMI, and these results might have been overadjusted. When not adjusting for BMI, an association between diabetes and nut consumption was seen, with an RR of 0.80 (95% CI: 0.69–0.94; I2: 51%, 2 large cohorts; AMSTAR-2: high), which could indicate that weight reduction might be a potential effect mediator for a potential effect on diabetes incidence (119).
Mechanisms related to diabetes, obesity, and metabolic disease
Trials and cohort studies have shown that diets enriched with nuts do not increase body weight, BMI, or waist circumference (60, 64, 68, 74, 87, 115, 120–125), with a tendency to a slight reduction in all of these. Overall, nuts and seeds showed a favorable trend in improving fasting blood glucose concentrations, glycemic control, and insulin sensitivity (60, 74, 88, 92, 97, 98, 114, 123). Furthermore, nuts have been found to contribute positively to satiety and reducing hunger (125), which might be one of the reasons studies have not found nuts to be linked with obesity (115, 126).
Cancer
Meta-analyses indicated substantial inverse associations between high compared with low consumption of nuts and cancer-related mortality (16, 32–35) (Supplemental Figures 11 and 12). For per serving data, a daily intake of 28 g/d nuts was associated with an RR of 0.89 (95% CI: 0.83–0.94; I2: 23%; AMSTAR-2: moderate) for cancer mortality. For a meta-analysis on cancer mortality, 1 serving of 28 g/d was associated with the lowest risk of cancer mortality (Figure 4; Supplemental Figure 13). There were 49,161 cancer mortality cases in the overall analysis for all nuts.
There was a tendency toward stronger associations between tree nuts and cancer mortality than for peanuts. There is more uncertainty regarding the association between nut consumption and specific cancers. However, inverse associations were reported between nut consumption and endometrial, colon, pancreatic, gastric, and lung cancers, with less clear associations for rectal, esophageal, liver, endometrial, prostate, and breast cancer (35, 127, 128).
Mechanisms related to cancer
Trials assessing inflammatory outcomes from nut consumption have generally found a slightly favorable or neutral change in inflammatory markers such as C-reactive protein, ILs, TNF-α, cell adhesion molecules, and antioxidant defense system (58, 67, 84, 95, 102, 103, 113, 129–132). Further, insulin sensitivity, glycemic control, and obesity (see above) might also be relevant for cancers.
All-cause mortality and other cause-specific mortality
Meta-analyses have shown substantial inverse associations between nut intake and all-cause mortality (Supplemental Figures 14 and 15) (13, 14, 16, 33, 40, 43, 45, 46). A daily intake of 28 g/d nuts was associated with an RR of 0.78 (95% CI: 0.72–0.84; I2: 66%; AMSTAR-2: high), with the dose–response curves plateauing from ∼20 g/d (Supplemental Figure 16). There were 819,448 participants and 85,870 deaths in the mortality analyses. There were no clear differences in mortality outcomes between tree nuts and peanuts.
Relating to other cause-specific mortality, there were also observed inverse associations between nut consumption and mortality from respiratory disease (RR: 0.48; 95% CI: 0.26–0.89; I2: 61%; AMSTAR-2: high), and infectious disease (RR: 0.25; 95% CI: 0.07–0.87; I2: 54%; AMSTAR-2: high) (16) (Supplemental Figures 17 and 18). A nonsignificant association was observed for neurodegenerative disease mortality (RR: 0.65; 95% CI: 0.40–1.07; P = 0.086; I2: 6%; AMSTAR-2: high). No significant association was seen for kidney-related disease mortality. For these 4 outcomes, 2551/397/367/2056 deaths were included in each analysis.
Allergy and adverse reactions to nuts and seeds
Nine meta-analyses and systematic review articles provided data on allergies and adverse reactions. Using the gold standard diagnostic methods of peanut allergy, the prevalence ranged between 0% to 2.8%, with heterogeneity between age groups and settings (9, 133–135) (Supplemental Table 4). Food challenge tests indicated the following age-specific prevalence of allergies to tree nuts: 0–6 y: 0.03–0.2%; 6–18 y: 0.2–2.3%; and adults: 0.4–1.4% (136). Challenge-proven data are sparse for non-European countries. Anaphylactic reactions were rare, but among these peanut seems to be the leading food allergen (22), and can be life-threatening if not handled promptly and correctly. Among individuals with peanut allergy, 1 to 6 anaphylaxis events are estimated per 2500 patients exposed to low-dose nut protein (137). Consumption of cashew nuts is also a relatively common cause of anaphylactic reactions, often with cross-reactions to pistachio nuts (138). The prevalence of allergy to sesame seeds was estimated as 0.1–0.2% (139).
Discussion
An intake of 28 g/d nuts compared with not eating nuts was associated with a 21% RR reduction for cardiovascular disease (including coronary heart disease incidence and mortality, atrial fibrillation, and stroke mortality), 11% risk reduction for cancer deaths, and 22% reduction for all-cause mortality. Nut consumption was also associated with a reduced risk of mortality from respiratory diseases, infectious diseases, and diabetes; however, associations between nut consumption and diabetes incidence were mixed. Generally, these associations seem to be relatively similar for different nuts, including different tree nuts and peanuts. Meta-analyses of trials on biomarkers for disease (intermediate factors) generally mirrored meta-analyses from observational studies on cardiovascular disease, cancer mortality, and diabetes. Dose–response relations suggest optimal intake levels of 15–40 g/d with generally limited benefits in increasing intake beyond 28 g/d.
We observed mixed associations between consumption of nuts and diabetes incidence or diabetes mortality. It is possible that a potential inverse association between consumption of nuts and diabetes incidence could largely be mediated by body weight, and that meta-analyses adjusting for BMI might have overadjusted analyses masking associations between consumption of nuts and diabetes incidence (119). This assumption is supported by studies that generally showed weaker associations between nuts and diabetes incidence when adjusting for BMI (119). Consumption of a handful of nuts per day is unlikely to contribute to overweight and obesity based on the current evidence (126). Around half of the meta-analyses conducted indicated slightly favorable effects of nuts on body weight and fat mass.
Allergies for nuts are reported in ∼1–2%, with substantial heterogeneity between populations (134). Allergies to seeds are relatively uncommon (139). Severe allergic reactions, and particularly anaphylactic reactions, can be life-threatening if not handled promptly and correctly (9, 138). However, many reactions are also milder cross-reactions (140). Roasting generally reduces the allergenicity of some nut allergies (e.g., hazelnut and almonds) (140). Because avoidance of known allergens is the cornerstone for people with allergies, labeling of food to ensure transparency is essential (22). Legislation for allergen disclosure generally reflects allergens commonly responsible for food anaphylaxis (133). Some nuts, such as Brazil nuts, are more prone to contain potentially harmful fungal toxins (such as aflatoxin) when stored after inadequate drying (23). The presence of such toxins can generally be limited by regulations in the processing and distribution of nuts (23).
The current evidence strongly supports nut consumption as part of a healthy but also sustainable diet, in terms of greenhouse gas emissions, land and energy use, and potential for acidification and eutrophication (141–146). Furthermore, an increased intake of nuts to ≥20 g/d could have averted 4.4 million deaths in North and South America, Europe, Southeast Asia, and the Western Pacific (16). This is estimated from probable reductions in premature deaths related to cardiovascular disease and cancers and possible reductions in mortality from respiratory disease and diabetes. Some systematic reviews have further suggested that nut consumption is positively associated with cognitive function tests (54, 147), and nuts might have a role both in child development and in slowing some age-related cognitive decline. For children, less evidence is available relating to the effect of nut consumption on disease patterns, but the studies generally show some similarities in trends to what is presented for adults (148). Intake amounts can be adapted to the individual's age, and the youngest children generally need less energy. Recommending an intake of a handful of nuts per day will to some degree take this into consideration because hand size will also grow relatively parallel with body size.
This study has several strengths and some limitations. This is the most comprehensive umbrella review conducted on nut and seed consumption and its associations with disease and mortality outcomes. For many of the included outcomes, no umbrella reviews are presently available, and for the remainder, many studies were published subsequent to the relevant umbrella reviews. We have included both disease and mortality outcomes, and biomarkers for disease as intermediate outcomes with mechanistic studies to better identify causal effects. We have strived to adhere to the PRISMA criteria (31). Still, some data might have been missed due to inadequate indexing in MEDLINE and Embase, or titles and abstracts not indicating the articles to be relevant. The former is more common for older studies, but these are probably few. Some trials have included nuts as a component of a complex intervention (83, 149). For several of the interventions with several components that can contribute to the outcomes of interest, the duration of these trials might also have been too short to achieve relevant effects on many of these chronic diseases (83, 150). Relating to cancer, one might argue against assessing all cancers combined because cancers are heterogeneous. On the other hand, cancers generally share a range of mechanisms, and assessing all cancers separately increases the risk of random variability errors. In meta-analyses on cancer incidence, there might have been studies including data on assessing cancer-related mortality (mixing different outcomes). Thus, the validity of the cancer incidence is uncertain, and some studies have questioned whether nut intake is associated with cancer incidence (150, 151). However, there is more agreement on the inverse associations between nut intake and cancer mortality. There are also many studies on diet patterns that include nuts but do not assess the effect of nuts and other food groups individually. Our assessment omitted these because it is difficult to ascribe effects to separate food groups. Finally, inadequately described study methods, such as lacking specification, might have been the cause for the rejection of otherwise relevant studies. Double controlling has contributed to preventing mistakes, and when there is room for different interpretations, these have been discussed among the authors.
Conclusion
Intake of nuts is inversely associated with the risk of cardiovascular diseases. This is mirrored with experimental studies on biomarkers for cardiovascular disease, with the overall quality of evidence considered moderate. Compared with not eating nuts, a handful of nuts per day is associated with a risk reduction of cardiovascular disease and mortality by a fifth, and cancer deaths by a tenth. Nut consumption is also associated with a substantial reduction in mortality risk from respiratory diseases, infectious diseases, and diabetes; however, associations between nut consumption and diabetes incidence are mixed and might be explained by adiposity differences. Meta-analyses of trials on intermediate factors of other chronic diseases also generally mirror meta-analyses from observational studies on cardiovascular, cancer, metabolic, and infectious diseases.
Hence, the current evidence supports dietary recommendations to consume a handful of nuts and seeds per day for people without allergies to these foods. Different types of nuts and seeds seem to have broadly similar benefits.
Supplementary Material
Acknowledgements
We thank the librarian Elisabeth Ebner who assisted us with the search.
The authors’ responsibilities were as follows—all authors: contributed to conceiving and designing the study; RB, TB, MB: led screening and data extractions; LTF: led the analysis, supervised the study, and drafted the first manuscript; and all authors: read and approved the final manuscript, had full access to the data, and take responsibility for the integrity of the data and the accuracy of the data analysis.
Notes
The authors were funded by their respective institutions. The study had no additional funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1–18, Supplemental Tables 1–4, and Supplemental Material are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Contributor Information
Rajiv Balakrishna, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway.
Tonje Bjørnerud, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway.
Mitra Bemanian, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Department of Addiction Medicine, Haukeland University Hospital, Bergen, Norway.
Dagfinn Aune, Department of Epidemiology and Biostatistics, School of Public Health, Imperial College London, London, United Kingdom; Department of Nutrition, Oslo New University College, Oslo, Norway; Department of Endocrinology, Morbid Obesity and Preventive Medicine, Oslo University Hospital, Oslo, Norway; Unit of Cardiovascular and Nutritional Epidemiology, Institute of Environmental Medicine, Karolinska Institute, Stockholm, Sweden.
Lars T Fadnes, Department of Global Public Health and Primary Care, University of Bergen, Bergen, Norway; Department of Addiction Medicine, Haukeland University Hospital, Bergen, Norway.
References
- 1. Casas-Agustench P, Salas-Huetos A, Salas-Salvado J. Mediterranean nuts: origins, ancient medicinal benefits and symbolism. Public Health Nutr. 2011;14(12A):2296–301. [DOI] [PubMed] [Google Scholar]
- 2. Bolling BW, Chen CY, McKay DL, Blumberg JB. Tree nut phytochemicals: composition, antioxidant capacity, bioactivity, impact factors. A systematic review of almonds, Brazils, cashews, hazelnuts, macadamias, pecans, pine nuts, pistachios and walnuts. Nutr Res Rev. 2011;24(2):244–75. [DOI] [PubMed] [Google Scholar]
- 3. Ros E, Mataix J. Fatty acid composition of nuts – implications for cardiovascular health. Br J Nutr. 2006;96(Suppl 2):S29–35. [DOI] [PubMed] [Google Scholar]
- 4. Ros E, Izquierdo-Pulido M, Sala-Vila A. Beneficial effects of walnut consumption on human health: role of micronutrients. Curr Opin Clin Nutr Metab Care. 2018;21(6):498–504. [DOI] [PubMed] [Google Scholar]
- 5. Terzo S, Baldassano S, Caldara GF, Ferrantelli V, Lo Dico G, Mule Fet al. Health benefits of pistachios consumption. Nat Prod Res. 2019;33(5):715–26. [DOI] [PubMed] [Google Scholar]
- 6. Alasalvar C, Salvado JS, Ros E. Bioactives and health benefits of nuts and dried fruits. Food Chem. 2020;314:126192. [DOI] [PubMed] [Google Scholar]
- 7. Baptista A, Gonçalves RV, Bressan J, Pelúzio M. Antioxidant and antimicrobial activities of crude extracts and fractions of cashew (Anacardium occidentale L.), cajui (Anacardium microcarpum), and pequi (Caryocar brasiliense C.): a systematic review. Oxid Med Cell Longev. 2018;2018:3753562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim Y, Keogh JB, Clifton PM. Benefits of nut consumption on insulin resistance and cardiovascular risk factors: multiple potential mechanisms of actions. Nutrients. 2017;9(11):1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McWilliam V, Koplin J, Lodge C, Tang M, Dharmage S, Allen K. The prevalence of tree nut allergy: a systematic review. Curr Allergy Asthma Rep. 2015;15(9):54. [DOI] [PubMed] [Google Scholar]
- 10. Cardoso CA, Oliveira GMM, Gouveia LAV, Moreira ASB, Rosa G. The effect of dietary intake of sesame (Sesamumindicum L.) derivatives related to the lipid profile and blood pressure: a systematic review. Crit Rev Food Sci Nutr. 2018;58(1):116–25. [DOI] [PubMed] [Google Scholar]
- 11. de Souza RJ, Dehghan M, Mente A, Bangdiwala SI, Ahmed SH, Alhabib KFet al. Association of nut intake with risk factors, cardiovascular disease, and mortality in 16 countries from 5 continents: analysis from the Prospective Urban and Rural Epidemiology (PURE) study. Am J Clin Nutr. 2020;112(1):208–19. [DOI] [PubMed] [Google Scholar]
- 12. Nuts and dried fruits statistical yearbook [Internet]. International Nut and Dried Fruit Foundation; 2019; [cited May 17, 2021]. Available from: https://www.nutfruit.org/files/tech/1587539172_INC_Statistical_Yearbook_2019-2020.pdf.
- 13. Chen GC, Zhang R, Martínez-González MA, Zhang ZL, Bonaccio M, van Dam RMet al. Nut consumption in relation to all-cause and cause-specific mortality: a meta-analysis 18 prospective studies. Food Funct. 2017;8(11):3893–905. [DOI] [PubMed] [Google Scholar]
- 14. Grosso G, Yang J, Marventano S, Micek A, Galvano F, Kales SN. Nut consumption on all-cause, cardiovascular, and cancer mortality risk: a systematic review and meta-analysis of epidemiologic studies. Am J Clin Nutr. 2015;101(4):783–93. [DOI] [PubMed] [Google Scholar]
- 15. Becerra-Tomás N, Paz-Graniel I, Hernández-Alonso P, Jenkins DJA, Kendall CWC, Sievenpiper JLet al. Nut consumption and type 2 diabetes risk: a systematic review and meta-analysis of observational studies. Am J Clin Nutr. 2021;113:960–71. [DOI] [PubMed] [Google Scholar]
- 16. Aune D, Keum N, Giovannucci E, Fadnes LT, Boffetta P, Greenwood DCet al. Nut consumption and risk of cardiovascular disease, total cancer, all-cause and cause-specific mortality: a systematic review and dose-response meta-analysis of prospective studies. BMC Med. 2016;14(1):207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Souza RGM, Schincaglia RM, Pimentel GD, Mota JF. Nuts and human health outcomes: a systematic review. Nutrients. 2017;9(12):1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Schwingshackl L, Hoffmann G, Missbach B, Stelmach-Mardas M, Boeing H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Design. 2017;23(7):1016–27. [DOI] [PubMed] [Google Scholar]
- 19. Institute for Health Metrics and Evaluation (IHME). GBD compare; Viz Hub [Internet]. University of Washington [cited August 13, 2020]. Available from: http://vizhub.healthdata.org/gbd-compare .
- 20. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey Met al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet. 2018;392(10159):2052–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. GBD 2017 Causes of Death Collaborators . Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Versluis A, Knulst AC, Kruizinga AG, Michelsen A, Houben GF, Baumert JLet al. Frequency, severity and causes of unexpected allergic reactions to food: a systematic literature review. Clin Exp Allergy. 2015;45(2):347–67. [DOI] [PubMed] [Google Scholar]
- 23. Cardoso BR, Duarte GBS, Reis BZ, Cozzolino SMF. Brazil nuts: nutritional composition, health benefits and safety aspects. Food Res Int. 2017;100(2):9–18. [DOI] [PubMed] [Google Scholar]
- 24. Creedon AC, Hung ES, Berry SE, Whelan K. Nuts and their effect on gut microbiota, gut function and symptoms in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients. 2020;12(8):2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fitzgerald E, Lambert K, Stanford J, Neale EP. The effect of nut consumption (tree nuts and peanuts) on the gut microbiota of humans: a systematic review. Br J Nutr. 2021;125(5):508–20. [DOI] [PubMed] [Google Scholar]
- 26. Kim Y, Keogh J, Clifton PM. Nuts and cardio-metabolic disease: a review of meta-analyses. Nutrients. 2018;10(12):1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kwok CS, Gulati M, Michos ED, Potts J, Wu P, Watson Let al. Dietary components and risk of cardiovascular disease and all-cause mortality: a review of evidence from meta-analyses. Eur J Prev Cardiol. 2019;26(13):2047487319843667. [DOI] [PubMed] [Google Scholar]
- 28. Schwingshackl L, Hoffmann G, Missbach B, Stelmach-Mardas M, Boeing H. An umbrella review of nuts intake and risk of cardiovascular disease. Curr Pharm Des. 2017;23(7):1016–27. [DOI] [PubMed] [Google Scholar]
- 29. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. Int J Evid Based Healthc. 2015;13(3):132–40. [DOI] [PubMed] [Google Scholar]
- 30. Fusar-Poli P, Radua J. Ten simple rules for conducting umbrella reviews. Evid Based Ment Health. 2018;21(3):95–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPAet al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7):e1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Long J, Ji Z, Yuan P, Long T, Liu K, Li Jet al. Nut consumption and risk of cancer: a meta-analysis of prospective studies. Cancer Epidemiol Biomarkers Prev. 2020;29(3):565–73. [DOI] [PubMed] [Google Scholar]
- 33. Naghshi S, Sadeghian M, Nasiri M, Mobarak S, Asadi M, Sadeghi O. Association of total nut, tree nut, peanut, and peanut butter consumption with cancer incidence and mortality: a comprehensive systematic review and dose-response meta-analysis of observational studies. Adv Nutr. 2021;12(3):793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73(7):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang D, Dai C, Zhou L, Li Y, Liu K, Deng YJet al. Meta-analysis of the association between nut consumption and the risks of cancer incidence and cancer-specific mortality. Aging (Albany NY). 2020;12(11):10772–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran Jet al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Afshin A, Micha R, Khatibzadeh S, Mozaffarian D. Consumption of nuts and legumes and risk of incident ischemic heart disease, stroke, and diabetes: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Becerra-Tomás N, Paz-Graniel I, Kendall CWC, Kahleova H, Rahelić D, Sievenpiper JLet al. Nut consumption and incidence of cardiovascular diseases and cardiovascular disease mortality: a meta-analysis of prospective cohort studies. Nutr Rev. 2019;77(10):691–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal Ket al. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr. 2019;59(7):1071–90. [DOI] [PubMed] [Google Scholar]
- 40. van den Brandt PA, Schouten LJ. Relationship of tree nut, peanut and peanut butter intake with total and cause-specific mortality: a cohort study and meta-analysis. Int J Epidemiol. 2015;44(3):1038–49. [DOI] [PubMed] [Google Scholar]
- 41. Guo K, Zhou Z, Jiang Y, Li W, Li Y. Meta-analysis of prospective studies on the effects of nut consumption on hypertension and type 2 diabetes mellitus. J Diabetes. 2015;7(2):202–12. [DOI] [PubMed] [Google Scholar]
- 42. Li H, Li X, Yuan S, Jin Y, Lu J. Nut consumption and risk of metabolic syndrome and overweight/obesity: a meta-analysis of prospective cohort studies and randomized trials. Nutr Metab. 2018;15(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu Met al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):256–69. [DOI] [PubMed] [Google Scholar]
- 44. Ma L, Wang F, Guo W, Yang H, Liu Y, Zhang W. Nut consumption and the risk of coronary artery disease: a dose-response meta-analysis of 13 prospective studies. Thromb Res. 2014;134(4):790–4. [DOI] [PubMed] [Google Scholar]
- 45. Mayhew AJ, de Souza RJ, Meyre D, Anand SS, Mente A. A systematic review and meta-analysis of nut consumption and incident risk of CVD and all-cause mortality. Br J Nutr. 2016;115(2):212–25. [DOI] [PubMed] [Google Scholar]
- 46. Schwingshackl L, Schwedhelm C, Hoffmann G, Lampousi AM, Knüppel S, Iqbal Ket al. Food groups and risk of all-cause mortality: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2017;105(6):1462–73. [DOI] [PubMed] [Google Scholar]
- 47. Schwingshackl L, Hoffmann G, Lampousi AM, Knüppel S, Iqbal K, Schwedhelm Cet al. Food groups and risk of type 2 diabetes mellitus: a systematic review and meta-analysis of prospective studies. Eur J Epidemiol. 2017;32(5):363–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Shao C, Tang H, Zhao W, He J. Nut intake and stroke risk: a dose-response meta-analysis of prospective cohort studies. Sci Rep. 2016;6:30394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Shi ZQ, Tang JJ, Wu H, Xie CY, He ZZ. Consumption of nuts and legumes and risk of stroke: a meta-analysis of prospective cohort studies. Nutr Metab Cardiovasc Dis. 2014;24(12):1262–71. [DOI] [PubMed] [Google Scholar]
- 50. Weng YQ, Yao J, Guo ML, Qin QJ, Li P. Association between nut consumption and coronary heart disease: a meta-analysis. Coron Artery Dis. 2016;27(3):227–32. [DOI] [PubMed] [Google Scholar]
- 51. Zhang Y, Zhang DZ. Relationship between nut consumption and metabolic syndrome: a meta-analysis of observational studies. J Am Coll Nutr. 2019;38(6):499–505. [DOI] [PubMed] [Google Scholar]
- 52. Zhang Z, Xu G, Wei Y, Zhu W, Liu X. Nut consumption and risk of stroke. Eur J Epidemiol. 2015;30(3):189–96. [DOI] [PubMed] [Google Scholar]
- 53. Altamimi M, Zidan S, Badrasawi M. Effect of tree nuts consumption on serum lipid profile in hyperlipidemic individuals: a systematic review. Nutr Metab Insights. 2020;13:1178638820926521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arias-Fernández L, López García E, Struijk EA, Rodríguez Artalejo F, Lana Pérez A. [Nut consumption and cognitive function: a systematic review]. Nutricion Hospitalaria. 2019;36(5):1179–88. [DOI] [PubMed] [Google Scholar]
- 55. Asbaghi O, Hadi A, Campbell MS, Venkatakrishnan K, Ghaedi E. Effects of pistachios on anthropometric indices, inflammatory markers, endothelial function, and blood pressure in adults: a systematic review and meta-analysis of randomized controlled trials. Br J Nutr. 2021;126(5):718–29. [DOI] [PubMed] [Google Scholar]
- 56. Asbaghi O, Moodi V, Hadi A, Eslampour E, Shirinbakhshmasoleh M, Ghaedi Eet al. The effect of almond intake on lipid profile: a systematic review and meta-analysis of randomized controlled trials. Food Funct. 2021;12:1882–96. [DOI] [PubMed] [Google Scholar]
- 57. Askari G, Rouhani MH, Ghaedi E, Ghavami A, Nouri M, Mohammadi H. Effect of Nigella sativa (black seed) supplementation on glycemic control: a systematic review and meta-analysis of clinical trials. Phytother Res. 2019;33(5):1341–52. [DOI] [PubMed] [Google Scholar]
- 58. Askarpour M, Karimi M, Hadi A, Ghaedi E, Symonds ME, Miraghajani Met al. Effect of flaxseed supplementation on markers of inflammation and endothelial function: a systematic review and meta-analysis. Cytokine. 2020;126:154922. [DOI] [PubMed] [Google Scholar]
- 59. Banel DK, Hu FB. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: a meta-analysis and systematic review. Am J Clin Nutr. 2009;90(1):56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Blanco Mejia S, Kendall CW, Viguiliouk E, Augustin LS, Ha V, Cozma AIet al. Effect of tree nuts on metabolic syndrome criteria: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2014;4(7):e004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. De Lira C, Jimenez-Cruz A, Bacardi-Gascon M. Effectiveness of long-term consumption of nuts, seeds and seeds' oil on glucose and lipid levels in people with type 2 diabetes: systematic review. Obesity. 2011;19:S225. [Google Scholar]
- 62. Del Gobbo LC, Falk MC, Feldman R, Lewis K, Mozaffarian D. Effects of tree nuts on blood lipids, apolipoproteins, and blood pressure: systematic review, meta-analysis, and dose-response of 61 controlled intervention trials. Am J Clin Nutr–56. 2015;102(6): 1347–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Eslampour E, Asbaghi O, Hadi A, Abedi S, Ghaedi E, Ghaedi Eet al. The effect of almond intake on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020;50:102399. [DOI] [PubMed] [Google Scholar]
- 64. Flores-Mateo G, Rojas-Rueda D, Basora J, Ros E, Salas-Salvadó J. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr. 2013;97(6):1346–55. [DOI] [PubMed] [Google Scholar]
- 65. Fogacci F, Cicero AFG, Derosa G, Rizzo M, Veronesi M, Borghi C. Effect of pistachio on brachial artery diameter and flow-mediated dilatation: a systematic review and meta-analysis of randomized, controlled-feeding clinical studies. Crit Rev Food Sci Nutr. 2019;59(2):328–35. [DOI] [PubMed] [Google Scholar]
- 66. Ghanavati M, Ghanavati M, Rahmani J, Hosseinabadi SM, Clark CCT, Rahimlou M. Pistachios and cardiometabolic risk factors: a systematic review and meta-analysis of randomized controlled clinical trials. Complement Ther Med. 2020;52:102513. [DOI] [PubMed] [Google Scholar]
- 67. Gouveia L de A, Cardoso CA, de Oliveira GM, Rosa G, Moreira AS. Effects of the intake of sesame seeds (Sesamum indicum L.) and derivatives on oxidative stress: a systematic review. J Med Food. 2016;19(4):337–45. [DOI] [PubMed] [Google Scholar]
- 68. Guarneiri LL, Cooper JA. Intake of nuts or nut products does not lead to weight gain, independent of dietary substitution instructions: a systematic review and meta-analysis of randomized trials. Adv Nutr. 2021;12(2):384–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Guasch-Ferré M, Li J, Hu FB, Salas-Salvadó J, Tobias DK. Effects of walnut consumption on blood lipids and other cardiovascular risk factors: an updated meta-analysis and systematic review of controlled trials. Am J Clin Nutr. 2018;108(1):174–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Hadi A, Askarpour M, Salamat S, Ghaedi E, Symonds ME, Miraghajani M. Effect of flaxseed supplementation on lipid profile: an updated systematic review and dose-response meta-analysis of sixty-two randomized controlled trials. Pharmacol Res. 2020;152:104622. [DOI] [PubMed] [Google Scholar]
- 71. Huang Y, Zheng S, Wang T, Yang X, Luo Q, Li H. Effect of oral nut supplementation on endothelium-dependent vasodilation – a meta-analysis. Vasa. 2018;47(3):203–7. [DOI] [PubMed] [Google Scholar]
- 72. Jafari Azad B, Daneshzad E, Azadbakht L. Peanut and cardiovascular disease risk factors: a systematic review and meta-analysis. Crit Rev Food Sci Nutr. 2020;60(7):1123–40. [DOI] [PubMed] [Google Scholar]
- 73. Jalali M, Karamizadeh M, Jalali M, Karamizadeh M, Zare M, Akbarzadeh Met al. The effects of cashew nut intake on lipid profile and blood pressure: a systematic review and meta-analysis of randomized controlled trials. Complement Ther Med. 2020;50:102387. [DOI] [PubMed] [Google Scholar]
- 74. Jamshidi S, Moradi Y, Nameni G, Mohsenpour MA, Vafa M. Effects of cashew nut consumption on body composition and glycemic indices: a meta-analysis and systematic review of randomized controlled trials. Diabetes Metab Syndr. 2021;15(2):605–13. [DOI] [PubMed] [Google Scholar]
- 75. Karimian J, Abedi S, Shirinbakhshmasoleh M, Moodi F, Moodi V, Ghavami A. The effects of quinoa seed supplementation on cardiovascular risk factors: a systematic review and meta-analysis of controlled clinical trials. Phytother Res. 2021;35(4):1688–96. [DOI] [PubMed] [Google Scholar]
- 76. Kendall C, Mejia SB, Viguiliouk E, Augustin L, Ha V, Cozma Aet al. Tree nuts improve criteria of the metabolic syndrome: a systematic review and meta-analysis of randomized controlled dietary trials. FASEB J. 2014;28(S1):1025.6. doi: 10.1096/fasebj.28.1_supplement.1025.6. [DOI] [Google Scholar]
- 77. Khalesi S, Irwin C, Schubert M. Flaxseed consumption may reduce blood pressure: a systematic review and meta-analysis of controlled trials. J Nutr. 2015;145(4):758–65. [DOI] [PubMed] [Google Scholar]
- 78. Khosravi-Boroujeni H, Nikbakht E, Natanelov E, Khalesi S. Can sesame consumption improve blood pressure? A systematic review and meta-analysis of controlled trials. J Sci Food Agric. 2017;97(10):3087–94. [DOI] [PubMed] [Google Scholar]
- 79. Lee-Bravatti MA, Wang J, Avendano EE, King L, Johnson EJ, Raman G. Almond consumption and risk factors for cardiovascular disease: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10(6):1076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Li J, Jiang B, Santos HO, Santos D, Singh A, Wang L. Effects of walnut intake on blood pressure: a systematic review and meta-analysis of randomized controlled trials. Phytother Res. 2020;34(11):2921–31. [DOI] [PubMed] [Google Scholar]
- 81. Liu K, Hui S, Wang B, Kaliannan K, Guo X, Liang L. Comparative effects of different types of tree nut consumption on blood lipids: a network meta-analysis of clinical trials. Am J Clin Nutr. 2020;111(1):219–27. [DOI] [PubMed] [Google Scholar]
- 82. Mahboobi S. The effect of cashew nut on cardiovascular risk factors and blood pressure: a systematic review and meta-analysis. Curr Dev Nutr. 2019;3(Suppl 1):nzz031. [Google Scholar]
- 83. Martin N, Germanò R, Hartley L, Adler AJ, Rees K. Nut consumption for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2015;9(9):CD011583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Mazidi M, Rezaie P, Ferns GA, Gao HK. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein (CRP): a systematic review and meta-analysis of randomized controlled clinical trials. Medicine. 2016;95(44):e5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Mejia SB, Kendall CW, Viguiliouk E, Augustin LS, Ha V, Cozma AIet al. Tree nut consumption on metabolic syndrome criteria: a systematic review and meta-analysis of randomized controlled trials. BMJ Open. 2014;4(7):e004660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Mohammadi-Sartang M, Bellissimo N, Totosy de Zepetnek JO, Bazyar H, Mahmoodi M, Mazloom Z. Effects of walnuts consumption on vascular endothelial function in humans: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr ESPEN. 2018;28:52–8. [DOI] [PubMed] [Google Scholar]
- 87. Mohammadi-Sartang M, Mazloom Z, Raeisi-Dehkordi H, Barati-Boldaji R, Bellissimo N, Totosy de Zepetnek JO. The effect of flaxseed supplementation on body weight and body composition: a systematic review and meta-analysis of 45 randomized placebo-controlled trials. Obes Rev. 2017;18(9):1096–107. [DOI] [PubMed] [Google Scholar]
- 88. Mohammadi-Sartang M, Sohrabi Z, Barati-Boldaji R, Raeisi-Dehkordi H, Mazloom Z. Flaxseed supplementation on glucose control and insulin sensitivity: a systematic review and meta-analysis of 25 randomized, placebo-controlled trials. Nutr Rev. 2018;76(2):125–39. [DOI] [PubMed] [Google Scholar]
- 89. Mohammadifard N, Salehi-Abarghouei A, Salas-Salvadó J, Guasch-Ferré M, Humphries K, Sarrafzadegan N. The effect of tree nut, peanut, and soy nut consumption on blood pressure: a systematic review and meta-analysis of randomized controlled clinical trials. Am J Clin Nutr. 2015;101(5):966–82. [DOI] [PubMed] [Google Scholar]
- 90. Morvaridzadeh M, Sepidarkish M, Farsi F, Akbari A, Mostafai R, Omidi Aet al. Effect of cashew nut on lipid profile: a systematic review and meta-analysis. Complement Med Res. 2020:27(5):348–56. [DOI] [PubMed] [Google Scholar]
- 91. Mukuddem-Petersen J, Oosthuizen W, Jerling JC. A systematic review of the effects of nuts on blood lipid profiles in humans. J Nutr. 2005;135(9):2082–9. [DOI] [PubMed] [Google Scholar]
- 92. Muley A, Fernandez R, Ellwood L, Muley P, Shah M. Effect of tree nuts on glycemic outcomes in adults with type 2 diabetes mellitus: a systematic review. JBI Evid Synth. 2020;19(5):966–1002. [DOI] [PubMed] [Google Scholar]
- 93. Musa-Veloso K, Paulionis L, Poon T, Lee HY. The effects of almond consumption on fasting blood lipid levels: a systematic review and meta-analysis of randomised controlled trials. J Nutr Sci. 2016;5:e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Neale EP, Guan V, Tapsell LC, Probst YC. Effect of walnut consumption on markers of blood glucose control: a systematic review and meta-analysis. Br J Nutr. 2020;124(7):1–33. [DOI] [PubMed] [Google Scholar]
- 95. Neale EP, Tapsell LC, Guan V, Batterham MJ. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(11):e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Neelakantan N, Narayanan M, de Souza RJ, van Dam RM. Effect of fenugreek (Trigonella foenum-graecum L.) intake on glycemia: a meta-analysis of clinical trials. Nutr J. 2014;13(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Nowrouzi-Sohrabi P, Sisakht M, Hassanipour S, Daryabeygi-Khotbehsara R, Savardashtaki A, Fathalipour Met al. The effectiveness of pistachio on glycemic control and insulin sensitivity in patients with type 2 diabetes, prediabetes and metabolic syndrome: a systematic review and meta-analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):1589–95. [DOI] [PubMed] [Google Scholar]
- 98. Ntzouvani A, Antonopoulou S, Nomikos T. Effects of nut and seed consumption on markers of glucose metabolism in adults with prediabetes: a systematic review of randomised controlled trials. Br J Nutr. 2019;122(4):361–75. [DOI] [PubMed] [Google Scholar]
- 99. Pan A, Yu D, Demark-Wahnefried W, Franco OH, Lin X. Meta-analysis of the effects of flaxseed interventions on blood lipids. Am J Clin Nutr. 2009;90(2):288–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Perna S, Giacosa A, Bonitta G, Bologna C, Isu A, Guido Det al. Effects of hazelnut consumption on blood lipids and body weight: a systematic review and Bayesian meta-analysis. Nutrients. 2016;8(12):747–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Phung OJ, Makanji SS, White CM, Coleman CI. Almonds have a neutral effect on serum lipid profiles: a meta-analysis of randomized trials. J Am Diet Assoc. 2009;109(5):865–73. [DOI] [PubMed] [Google Scholar]
- 102. Rahimlou M, Jahromi NB, Hasanyani N, Ahmadi AR. Effects of flaxseed interventions on circulating inflammatory biomarkers: a systematic review and meta-analysis of randomized controlled trials. Adv Nutr. 2019;10(6):1108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ren GY, Chen CY, Chen GC, Chen WG, Pan A, Pan CWet al. Effect of flaxseed intervention on inflammatory marker C-reactive protein: a systematic review and meta-analysis of randomized controlled trials. Nutrients. 2016;8(3):136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ribeiro PVM, da Silva A, de Almeida AP, Hermsdorff HHM, Alfenas RCG. Effect of chronic consumption of pistachios (Pistacia vera L.) on glucose metabolism in pre-diabetics and type 2 diabetics: a systematic review. Crit Rev Food Sci Nutr. 2019;59(7):1115–23. [DOI] [PubMed] [Google Scholar]
- 105. Sabaté J, Oda K, Ros E. Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Arch Intern Med. 2010;170(9):821–7. [DOI] [PubMed] [Google Scholar]
- 106. Sahebkar A, Katsiki N, Ward N, Reiner Ž. Flaxseed supplementation reduces plasma lipoprotein(a) levels: a meta-analysis. Altern Ther Health Med. 2021;27(3):50–3. [PubMed] [Google Scholar]
- 107. Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Iqbal K, Andriolo Vet al. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr. 2017;8(6):793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Smeets E, Mensink RP, Joris PJ. Effects of tree nut and groundnut consumption compared with those of L-arginine supplementation on fasting and postprandial flow-mediated vasodilation: meta-analysis of human randomized controlled trials. Clin Nutr. 2021;40(4):1699–1710. [DOI] [PubMed] [Google Scholar]
- 109. Teoh SL, Lai NM, Vanichkulpitak P, Vuksan V, Ho H, Chaiyakunapruk N. Clinical evidence on dietary supplementation with chia seed (Salvia hispanica L.): a systematic review and meta-analysis. Nutr Rev. 2018;76(4):219–42. [DOI] [PubMed] [Google Scholar]
- 110. Theodore LE, Kellow NJ, McNeil EA, Close EO, Coad EG, Cardoso BR. Nut consumption for cognitive performance: a systematic review. Adv Nutr. 2021;12(3):777–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Tindall AM, Johnston EA, Kris-Etherton PM, Petersen KS. The effect of nuts on markers of glycemic control: a systematic review and meta-analysis of randomized controlled trials. Am J Clin Nutr. 2019;109(2):297–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ursoniu S, Sahebkar A, Andrica F, Serban C, Banach M, Lipid and Blood Pressure Meta-analysis Collaboration (LBPMC) Group. Effects of flaxseed supplements on blood pressure: a systematic review and meta-analysis of controlled clinical trial. Clin Nutr. 2016;35(3):615–25. [DOI] [PubMed] [Google Scholar]
- 113. Ursoniu S, Sahebkar A, Serban MC, Pinzaru I, Dehelean C, Noveanu Let al. A systematic review and meta-analysis of clinical trials investigating the effects of flaxseed supplementation on plasma C-reactive protein concentrations. Arch Med Sci. 2019;15(1):12–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Viguiliouk E, Kendall CW, Blanco Mejia S, Cozma AI, Ha V, Mirrahimi Aet al. Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PloS One. 2014;9(7):e103376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Xia K, Yang T, An LY, Lin YY, Qi YX, Chen XZet al. The relationship between pistachio (Pistacia vera L.) intake and adiposity: a systematic review and meta-analysis of randomized controlled trials. Medicine. 2020;99(34):e21136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Xiao Y, Huang W, Peng C, Zhang J, Wong C, Kim JHet al. Effect of nut consumption on vascular endothelial function: a systematic review and meta-analysis of randomized controlled trials. Clin Nutr. 2018;37(3):831–9. [DOI] [PubMed] [Google Scholar]
- 117. Yang L, Zhu H, Guo Z, Qi S, Fang T, Santos HOet al. Walnut intake may increase circulating adiponectin and leptin levels but does not improve glycemic biomarkers: a systematic review and meta-analysis of randomized clinical trials. Complement Ther Med. 2020;52:102505. [DOI] [PubMed] [Google Scholar]
- 118. Zhou D, Yu H, He F, Reilly KH, Zhang J, Li Set al. Nut consumption in relation to cardiovascular disease risk and type 2 diabetes: a systematic review and meta-analysis of prospective studies. Am J Clin Nutr. 2014;100(1):270–7. [DOI] [PubMed] [Google Scholar]
- 119. Luo C, Zhang Y, Ding Y, Shan Z, Chen S, Yu Met al. Nut consumption and risk of type 2 diabetes, cardiovascular disease, and all-cause mortality: a systematic review and meta-analysis. Am J Clin Nutr. 2014;100(1):256–69. [DOI] [PubMed] [Google Scholar]
- 120. Eslami O, Shidfar F, Dehnad A. Inverse association of long-term nut consumption with weight gain and risk of overweight/obesity: a systematic review. Nutr Res. 2019;68:1–8. [DOI] [PubMed] [Google Scholar]
- 121. Eslampour E, Moodi V, Asbaghi O, Ghaedi E, Shirinbakhshmasoleh M, Hadi Aet al. The effect of almond intake on anthropometric indices: a systematic review and meta-analysis. Food Funct. 2020;11(9):7340–55. [DOI] [PubMed] [Google Scholar]
- 122. Fogelholm M, Anderssen S, Gunnarsdottir I, Lahti-Koski M. Dietary macronutrients and food consumption as determinants of long-term weight change in adult populations: a systematic literature review. Food Nutr Res. [Internet] 2012;56. doi: 10.3402/fnr.v56i0.19103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Hajiahmadi S, Khosravi M, Hosseinzadeh E, Hosseinzadeh M. Flaxseed and its products improve glycemic control: a systematic review and meta-analysis. Obes Med. 2021;22:100311. [Google Scholar]
- 124. Fang Z, Dang M, Zhang W, Wang Y, Kord-Varkaneh H, Nazary-Vannani Aet al. Effects of walnut intake on anthropometric characteristics: a systematic review and dose-response meta-analysis of randomized controlled trials. Complement Ther Med. 2020;50:102395. [DOI] [PubMed] [Google Scholar]
- 125. Akhlaghi M, Ghobadi S, Zare M, Foshati S. Effect of nuts on energy intake, hunger, and fullness, a systematic review and meta-analysis of randomized clinical trials. Crit Rev Food Sci Nutr. 2020;60(1):84–93. [DOI] [PubMed] [Google Scholar]
- 126. Flores-Mateo G, Rojas-Rueda D, Basora J, Ros E, Salas-Salvadó J. Nut intake and adiposity: meta-analysis of clinical trials. Am J Clin Nutr. 2013;97(6):1346–55. [DOI] [PubMed] [Google Scholar]
- 127. Wu L, Wang Z, Zhu J, Murad AL, Prokop LJ, Murad MH. Nut consumption and risk of cancer and type 2 diabetes: a systematic review and meta-analysis. Nutr Rev. 2015;73(7):409–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Schwingshackl L, Schwedhelm C, Hoffmann G, Knüppel S, Laure Preterre A, Iqbal Ket al. Food groups and risk of colorectal cancer. Int J Cancer. 2018;142(9):1748–58. [DOI] [PubMed] [Google Scholar]
- 129. Silveira BKS, da Silva A, Hermsdorff HHM, Bressan J. Effect of chronic consumption of nuts on oxidative stress: a systematic review of clinical trials. Crit Rev Food Sci Nutr. 2022;62(3):726–37. [DOI] [PubMed] [Google Scholar]
- 130. Mazidi M, Rezaie P, Ferns GA, Gao HK. Impact of different types of tree nut, peanut, and soy nut consumption on serum C-reactive protein (CRP): a systematic review and meta-analysis of randomized controlled clinical trials. Medicine (Baltimore). 2016;95(44):e5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Neale EP, Tepcell LC, Guan V, Batterham MJ. The effect of nut consumption on markers of inflammation and endothelial function: a systematic review and meta-analysis of randomised controlled trials. BMJ Open. 2017;7(11):e016863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Xiao Y, Xia J, Ke Y, Cheng J, Yuan J, Wu Set al. Effects of nut consumption on selected inflammatory markers: a systematic review and meta-analysis of randomized controlled trials. Nutrition. 2018;54:129–43. [DOI] [PubMed] [Google Scholar]
- 133. Conrado AB, Patel N, Turner PJ. Global patterns in anaphylaxis due to specific foods: a systematic review. J Allergy Clin Immunol. 2021;148(6):1515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Nwaru BI, Hickstein L, Panesar SS, Roberts G, Muraro A, Sheikh A. Prevalence of common food allergies in Europe: a systematic review and meta-analysis. Allergy. 2014;69(8):992–1007. [DOI] [PubMed] [Google Scholar]
- 135. Rona RJ, Keil T, Summers C, Gislason D, Zuidmeer L, Sodergren Eet al. The prevalence of food allergy: a meta-analysis. J Allergy Clin Immunol. 2007;120(3):638–46. [DOI] [PubMed] [Google Scholar]
- 136. Zuidmeer L, Goldhahn K, Rona RJ, Gislason D, Madsen C, Summers Cet al. The prevalence of plant food allergies: a systematic review. J Allergy Clin Immunol. 2008;121(5):1210–8.e4. [DOI] [PubMed] [Google Scholar]
- 137. Patel N, Adelman DC, Anagnostou K, Baumert JL, Blom WM, Campbell DEet al. Using data from food challenges to inform management of food-allergic consumers: a systematic review with individual participant data meta-analysis. J Allergy Clin Immunol. 2021;147(6):2249–62.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. van der Valk JP, Dubois AE., Gerth van Wijk R, Wichers HJ, de Jong NW. Systematic review on cashew nut allergy. Allergy. 2014;69(6):692–8. [DOI] [PubMed] [Google Scholar]
- 139. Patel A, Bahna SL. Hypersensitivities to sesame and other common edible seeds. Allergy. 2016;71(10):1405–13. [DOI] [PubMed] [Google Scholar]
- 140. Masthoff LJ, Hoff R, Verhoeckx KC, van Os-Medendorp H, Michelsen-Huisman A, Baumert JLet al. A systematic review of the effect of thermal processing on the allergenicity of tree nuts. Allergy. 2013;68(8):983–93. [DOI] [PubMed] [Google Scholar]
- 141. Barre T, Perignon M, Gazan R, Vieux F, Micard V, Amiot MJet al. Integrating nutrient bioavailability and co-production links when identifying sustainable diets: how low should we reduce meat consumption?. PloS One. 2018;13(2):e0191767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Bertoluci G, Masset G, Gomy C, Mottet J, Darmon N. How to build a standardized country-specific environmental food database for nutritional epidemiology studies. PLoS One. 2016;11(4):e0150617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. van Dooren C, Tyszler M, Kramer GFH, Aiking H. Combining low price, low climate impact and high nutritional value in one shopping basket through diet optimization by linear programming. Sustainability. 2015;7:12837–55. [Google Scholar]
- 144. Fischer CG, Garnett T. Plates, pyramids and planets. Developments in national healthy and sustainable dietary guidelines: a state of play assessment. Food and Agriculture Organization of the United Nations;2016. [Google Scholar]
- 145. Willett W, Rockstrom J, Loken B, Springmann M, Lang T, Vermeulen Set al. Food in the Anthropocene: the EAT-Lancet Commission on healthy diets from sustainable food systems. Lancet. 2019;393(10170):447–92. [DOI] [PubMed] [Google Scholar]
- 146. Vanham D, Mekonnen MM, Hoekstra AY. Treenuts and groundnuts in the EAT-Lancet reference diet: concerns regarding sustainable water use. Glob Food Sec. 2020;24:100357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Solfrizzi V, Custodero C, Lozupone M, Imbimbo BP, Valiani V, Agosti Pet al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer's disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis. 2017;59(3):815–49. [DOI] [PubMed] [Google Scholar]
- 148. Mead LC, Hill AM, Carter S, Coates AM. The effect of nut consumption on diet quality, cardiometabolic and gastrointestinal health in children: a systematic review of randomized controlled trials. Int J Environ Res Public Health. 2021;18(2):454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149. Abdelhamid AS, Martin N, Bridges C, Brainard JS, Wang X, Brown TJet al. Polyunsaturated fatty acids for the primary and secondary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2018;7(7):CD012345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Nieuwenhuis L, van den Brandt PA. Nut and peanut butter consumption and the risk of total cancer: a prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2020;29(10):2100–4. [DOI] [PubMed] [Google Scholar]
- 151. Fang Z, Wu Y, Li Y, Zhang X, Willett WC, Eliassen AHet al. Association of nut consumption with risk of total cancer and 5 specific cancers: evidence from 3 large prospective cohort studies. Am J Clin Nutr. 2021;114(6):1925–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.