Abstract
Background
Little is known regarding the dose‐response function in multidomain interventions for dementia prevention.
Method
The Multidomain Alzheimer Preventive Trial is a 3‐year randomized controlled trial comprising cognitive training, physical activity, nutrition, and omega‐3 polyunsaturated fatty acids for at‐risk older adults. The dose delivered (number of sessions attended) was modeled against global cognition, memory, and fluency in 749 participants. Interaction effects were assessed for age, sex, education, dementia score (CAIDE), frailty score, and apolipoprotein E (APOE) ε4 status.
Results
The dose‐response models were non‐linear functions indicating benefits up to about 12 to 14 training hours or 15 to 20 multidomain sessions followed by a plateau. Participants who benefited from a higher dose included women, younger participants, frail individuals, and those with lower education or lower risk of dementia.
Discussion
The non‐linear function indicates that a higher dose is not necessarily better in multidomain interventions. The optimal dose was about half of the potentially available sessions.
Keywords: cognitive aging, cognitive decline, cognitive training, dementia, dosage, dose‐response, intervention, multidomain, prevention
1. BACKGROUND
Epidemiological evidence indicates that modifiable lifestyle factors contribute to the risk of dementia and cognitive decline in older age. 1 As a result, large‐scale prevention studies have examined the role of multidomain preventive interventions to reduce cognitive decline in older adults who are at risk of dementia (eg, the World‐Wide FINGERS global initiative 2 ). These interventions have focused on modifiable risk factors, most often physical activity, cognitively stimulating activities, diet, and vascular risk factors. 3 , 4 , 5 , 6 , 7
The results published so far indicate variable effects on cognition. The Finnish Geriatric Interventions Study to Prevent Cognitive Impairment and Disability (FINGER), a 2‐year multidomain intervention targeting exercise, diet, cognitive training, and vascular monitoring, reported improved global cognition in participants randomized to the intervention relative to those receiving usual care 3 . Secondary analyses indicated positive effects on executive function and speed but not on memory processes, suggesting that cognitive components are not equally sensitive to these interventions. 3 The 3‐year Multidomain Alzheimer Preventive Trial (MAPT) reported neutral results in their primary analysis but a positive effect in individuals at risk of decline due to higher scores on the Cardiovascular Risk Factors, Aging and Incidence of Dementia (CAIDE) scale. 4 Both studies used multidomain interventions that spanned 2 to 3 years with variable adherence among participants (adherence defined as at least a 66% completion of the prescribed intervention). 8 Thus the dose delivered to individual participants may have influenced the magnitude of the effect observed, which may vary as a function of the cognitive domain measured or characteristics of participants.
Dose is a critical factor examined in pharmacological treatments, but little is known about dose effects for non‐pharmacological multidomain interventions, as well as the optimal dose or the mathematical function that relates dose to improvement. Determining the optimal dose is critical because multidomain interventions are complex to deliver and demanding for the participant. Interventions must be available in the person's community and involves commitment and motivation. A dose that is too high increases costs and may have a detrimental effect on participant motivation. In turn, administering a sub‐optimal dose is problematic for obvious reasons. Thus identifying how dose optimizes efficacy may impact the design of large prevention trials, and inform public recommendations and prevention programs that target at‐risk populations.
HIGHLIGHTS
The dose‐response function was examined in a multidomain intervention.
Optimal dose was 12 to 14 hours of training, or half the potentially available dose.
Frail people or those with lower cognitive reserve benefitted from a higher dose.
These data provide critical information to guide prevention interventions.
RESEARCH IN CONTEXT
Systematic Review: There is a lack of published data on the effect of dose on non‐pharmacological intervention outcomes.
Interpretation: The optimal dose for the intervention was found to be 12 to 14 hours of training, which was about half the dose potentially available to participants. Individual factors moderated the dose‐response function, indicating that different individuals benefited from different doses. The data provide critical information to guide the design of lifestyle prevention interventions.
Future Directions: Similar analyses in other studies will contribute to further delineate the best conditions for the efficacy of non‐pharmacological interventions. Future studies will be needed to determine whether the same dose is optimal when using dementia diagnosis as an end point rather than cognition.
Response to training is likely to vary with the dose delivered; however, because few data exist, it is critical to determine the effect of dose on the efficacy of multidomain interventions or on other non‐pharmacological interventions. Most assume that a linear function typically describes the dose‐response relationship in non‐pharmacological interventions, where additional dose linearly increases the effect and larger doses are best. However, the relationship between dose and response is probably more complex. 9 For instance, in a meta‐analysis of computerized training in older adults, Lampit et al. 10 reported an inverse U‐shaped parabolic function, where increasing the number of weekly sessions improved efficacy up to a certain point, after which an increased number of sessions became detrimental. In the Advanced Cognitive Training for Independent and Vital Elderly (ACTIVE) study, Edwards et al. 11 reported that the number of attentional training sessions attended was related linearly to the progression of dementia, but there was no dose effect for reasoning or memory when adjusting for risk factors. The study did not assess non‐linear models.
Dose effects should be examined in interaction with individual characteristics. The moderating effect of individual characteristics on the dose‐response relationship can be interpreted in relation to the magnification versus reserve perspective based on the observation that older adults differ in terms of their resources (see 12 for a discussion). The magnification perspective 13 predicts that healthier or more able older adults will benefit more from prolonged practice because they have higher brain plasticity, which magnifies interindividual differences. In turn, the reserve model predicts that training compensates for unfavorable initial conditions and that less able or less healthy individuals will benefit more from additional training, thereby reducing initial cognitive disadvantages. These two hypotheses will be examined here in relation to age, sex, education, dementia risk, and frailty.
The goal of this exploratory study was to use the data from the MAPT study to model the relationship between dose and cognitive improvement in a multidomain intervention and determine the optimal dose. Dose was modeled separately for global cognition, delayed memory recall, and executive function measured with verbal fluency. Global cognition was the primary outcome in the MAPT efficacy study. Delayed memory recall and verbal fluency were found to predict progression from mild cognitive impairment to dementia. 14 We then examined whether age, sex, education, dementia risk, frailty, and apolipoprotein E (APOE) ε4 status were related to differences in the dose‐response function and if the optimal dose varied as a function of individual differences. We then assessed whether individual variables supported a magnification or reserve perspective.
2. METHODS
2.1. Design
The MAPT trial was a 3‐year intervention multicenter randomized controlled trial with four parallel arms: multidomain intervention plus placebo, multidomain intervention plus polyunsaturated fatty acids, polyunsaturated fatty acids alone, and placebo alone. The initial study included 1680 participants, who were randomly assigned to one of the four conditions (1;1;1;1). The methods and design have been described in detail elsewhere 15 , 4 and registered in www.clinicaltrials.gov (NCT00672685). Cognitive status was assessed at baseline, at 6 months, and then annually at year 1, year 2, and year 3. Neuropsychologists conducting the evaluation were blinded to group assignment. Written consent was obtained from all participants. The study protocol was approved by the Toulouse Ethical Committee and authorized by the French health authority.
Because the present study evaluated the effect of dose for the multidomain intervention, we only used data from 749 participants randomized to this intervention (multidomain intervention plus placebo or multidomain intervention plus polyunsaturated fatty acids). We, therefore, summarized only the methodological elements related to the multidomain intervention and those related to the outcome analyses. Additional information on the other components (polyunsaturated fatty acids and placebo) and measures can be found in Vellas et al. 15 and Andrieu et al. 4
2.2. Participants
Participants were community‐dwelling older adults at risk of cognitive decline based on the presence of at least one of three frailty criteria: (1) memory complaint to their practitioner; (2) slow walking speed (ie <.08 m/s); or (3) limitation in one instrumental activity of daily living. Participants were excluded if they had received a diagnosis of dementia, obtained a score <24 on the Mini Mental State Examination (MMSE), or showed difficulties on basic activities of daily living. Participants’ characteristics are provided in Table 1. The full inclusion and exclusion criteria are described in Vellas et al. 15
TABLE 1.
Baseline characteristics of the study sample (N = 749), data on participation pattern, and relationship with total dose received measured with Spearman correlations (r value) for continuous variables and a t‐test for sex as a dichotomous variable
| Mean (SD) | Range | Correlation with dose or group difference (P values) | |
|---|---|---|---|
| Dose, sessions (SD) | 20.23 (6.61) | 1 – 28 | NA |
| Multidomain dose, sessions (SD) | 25.86 (9.26) | 1 – 37 | r = 0.988 (< .001**) |
| Intensive dose, sessions (SD) | 10.03 (2.52) | 1 – 12 | r = .0754 (< .001**) |
| Age, years (SD) | 75.15 (4.24) | 69 – 89 | r = ‐0.048 (.186) |
| Sex ratio: M/F | 272 /477 | NA | t = 0.844 (.718) |
| Education level, /5 (SD) | 3.51 (1.19) | 1–5 | r = 0.00 (.994) |
| APOE status: % ε4 carriers | 24.2% | NA | t = ‐0.688 (.439) |
| Frailty score, entry criteria met, /3 (SD); % frail (2‐3 entry criteria) | 1.22 (.49); 19% | 1 – 3 | r = ‐0.092 (. 012*) |
| Dementia risk, CAIDE score, /15 (SD) | 7.55 (2.01) | 4 – 14 | r = ‐0.033 (.38) |
| Cognitive outcomes at baseline | |||
| Global cognition score, composite z score (SD) | −.01 (.70) | −2.96 – 1.89 | r = 0.18 (< .001**) |
| Delayed memory score, /16 (SD) | 10.51 (2.90) | 0 – 16 | r = 0.107 (.003**) |
| Verbal fluency score (SD) | 19.82 (6.37) | 4 – 41 | r = 0.079 (.031*) |
| MMSE at baseline, /30 (SD) | 28.07 (1.59) | 24 – 30 | r = 0.171(< .001**) |
Note: Education level: 1: No diploma; 2: Primary school certificate; 3: Secondary education; 4: High school diploma; 5: University level.
Frailty score: 1 to 3 entry criteria met for frailty.
CAIDE = Cardiovascular Risk Factors, Aging and Incidence of Dementia.
Global cognition score = composite z score of (1) RL/RI, Free and Cued recall test; (2) 10 Mini‐Mental State Examination (MMSE) orientation items; (3) Digit Symbol Substitution Test score for the Wechsler Adult Intelligence Scale‐Revised; (4) Category Naming Test (2‐minute fluency score for the animal category).
Delayed memory score: delayed recall of the RL/RI Free and Cued Recall Test.
Verbal fluency score: COWAT = Controlled Oral Word Association Test.
Significant Spearman's correlation at P < .05.
Significant Spearman's correlation at P < .01.
2.3. Intervention
A multidomain intervention with 43 sessions on cognition, physical activity, and nutrition was provided in person to small groups (six to eight participants). First, 12 sessions were provided during a 2‐month intensive phase (two sessions per week for the first month, and one per week for the second month), which were multidomain sessions that included 60 minutes of cognitive training, and 45 minutes of advice on physical activity and nutrition (except for three sessions that did not include nutrition). Starting on the third month, participants had access to thirty‐one 90‐minute monthly sessions. Fourteen of those monthly sessions provided cognitive training, six involved physical activity advice, three provided nutritional advice, six provided advice on general health, and two were multidomain (see detailed schedule in Supplementary Material). Cognitive training included both reasoning and memory training. Reasoning training involved teaching strategies to find the pattern underlying a series of elements or actions. 16 Memory training involved teaching mnemonics based on semantic elaboration or mental imagery to help memorize verbal material. The reasoning and memory training were adapted from the ACTIVE 17 , 18 and Training Method for an Optimal Memory/Méthode d'Intervention pour une Mémoire Optimale (MEMO) program, respectively. 19 , 20 , 21 The physical activity sessions provided advice and support to complete a home exercise training program based on current recommendations. The nutrition sessions provided recommendations for a healthy diet based on the French Nutrition and Health Program for older adults. 22
Dose was measured as the number of cognitive training sessions attended (defined as participants who were present at the start of the session; max = 28), because only cognitive training included active interventions and not solely advice. We also examined multidomain dose, which was defined as the number of total sessions attended (max = 43).
2.4. Cognitive outcomes
Three measures were used to assess dose effect. First, we analyzed dose response in the global cognition score, which was a composite Z‐score combining four cognitive scores: the free and total recall of the rappel libre/rappel indicé test (RL/RI, Free and Cued Recall Test), 23 10 MMSE orientation items, the Digit Symbol Substitution Test score for the Wechsler Adult Intelligence Scale‐Revised, 24 and the Category Naming Test 25 (ie, the 2‐minute fluency score for the animal category). In addition, dose was analyzed in the delayed memory score of the RL/RI memory test and the Controlled Oral Word Association Test (COWAT) score, which is a test of lexical verbal fluency. 25
2.5. Analyses
A three‐step analysis, following the residuals approach, was used to measure the dose effect: (1) We first used polynomial regression analyses to determine which model was the best fit to describe cognitive performance of the participants over time; (2) we then derived the residuals of these models: raw score minus expected score at each time (M6, M12, M24, and M36) found using the significant models in Step 1. (3) Next we used mixed‐model analyses to assess the effect of the dose on these residuals. We used the total dose as the main independent variable and tested which model best described the effect of the dose on the residuals. Time was entered as repeated effects with a heterogeneous autoregressive covariance matrix (ARH1). The intercept and participant's slopes were entered as random effects, with a variance components correlation matrix, while controlling for age, education, sex, initial cognitive performance (baseline MMSE score), and APOE ε4 status. Optimal dose was defined as the point at which the significant polynomial reached the first critical point (the maximum for a quadratic function (f(x) = ax2 + bx + c; maximum = ‐b/2a) and the first of the two critical points for a cubic function (f(x) = ax3 + bx2 + cx + d; critical points = ). Because the beta between the two critical points in the cubic functions was small, it was interpreted as a plateau. The analysis was first done using cognitive training sessions as a dose proxy and then using multidomain dose as a dose proxy.
To determine the effect of individual factors, we assessed the presence of an interaction between the dose‐effect models using cognitive training sessions as a proxy, and using age, sex, education, dementia risk score based on the CAIDE, frailty score based on the number of inclusion criteria met at study entry, and APOE ε4 status (see Supplementary Material for the interactions using multidomain dose as a proxy). In the case of a significant interaction, dose models were assessed individually in the groups separated by the median for age (74 years) and CAIDE score (7), sex, education level (levels 1, 2, and 3 vs levels 4 and 5), entry criteria for frailty (one vs two or three entry criteria), and presence of an APOE allele. We also examined group differences (t‐tests for independent samples) before and after the optimal dose, defined in the main model for each cognitive domain. This determined whether group differences (in terms of effect size) remained present after the optimal dose.
Because the intervention was delivered in two stages, which included a 2‐month intensive period followed by distributed sessions, we re‐ran the main models excluding the 73 participants, who completed only the 2‐month intensive period. This allowed us to address whether delivering intensity makes a difference.
Analyses were done using IBM SPSS Statistics 26 software.
3. RESULTS
Table 1 presents the means, standard deviations, and range for the dose, multidomain dose, and intensive dose delivered, and the scores on the individual characteristics included in the interaction models, the scores obtained on the three cognitive outcomes, and the MMSE score. The table also presents the relationship between individual variables and dose delivered.
The dose‐response distribution for the main effect and interaction models of dose can be found in Figures 1, 2 and 3 for the global cognition score, delayed recall, and verbal fluency, respectively, and in Figure 4 for the multidomain dose. The main results are summarized in Table 2.
FIGURE 1.
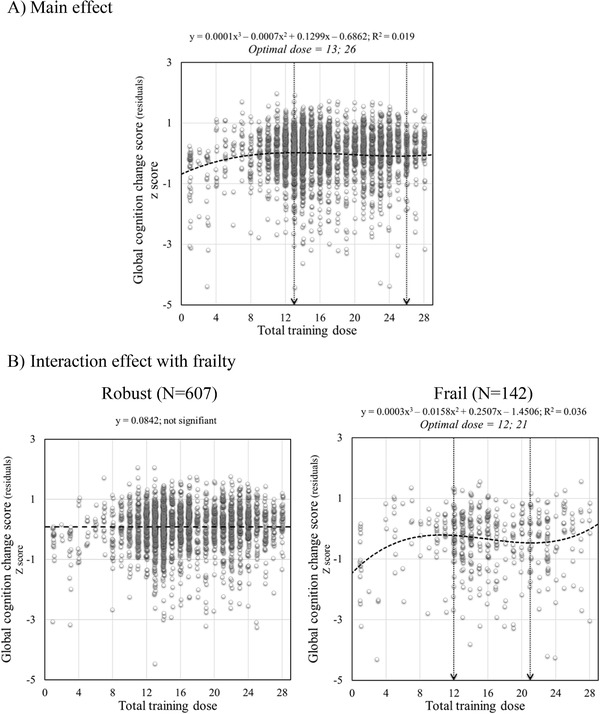
Scatter plots of total training dose on global cognition change score: (A) main effect and (B) interaction with frailty. For illustrational purposes, robust and frail are plotted separately. Circles represent individual participants residuals for each time model. The equation for each model is provided with y representing the residual and x representing the dose when it is significant. The arrow represents inflection points
FIGURE 2.
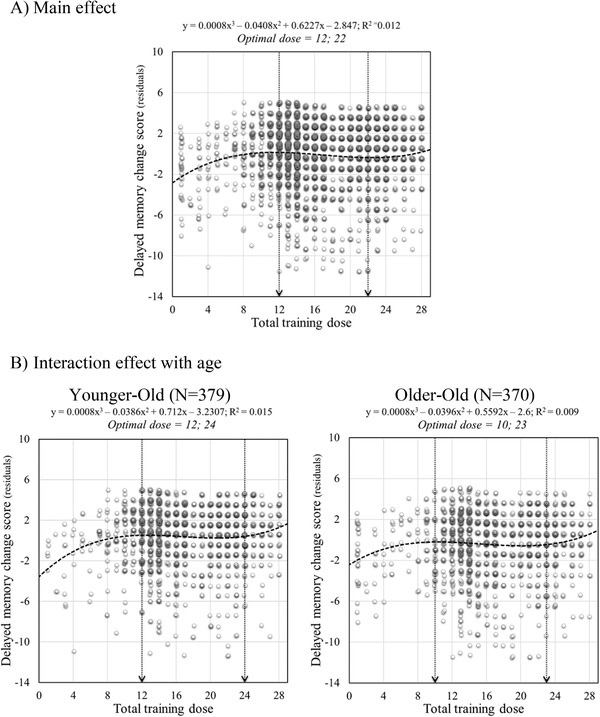
Scatter plots of total training dose effect on delayed memory change score: (A) main model and (B) interaction with age. For illustrational purposes young‐old and old‐old defined with a median‐split (74 years old) are plotted separately. Circles represent individual participant residuals for each time model. The equation for each model is provided with y representing the residual and x representing the dose. The arrow represents inflection points
FIGURE 3.
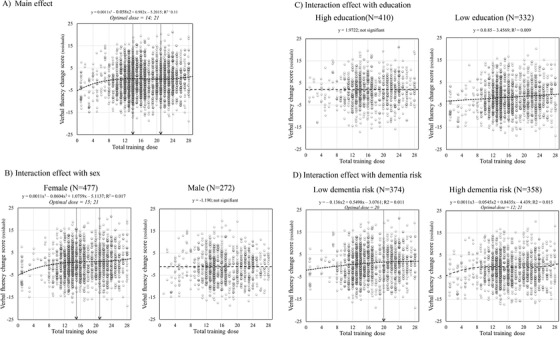
Scatter plots of total training dose effect on the verbal fluency change score: (A) Main effect and interactions with sex (B), education (C), and dementia risk score (D). For illustrational purposes Female‐Male, high vs low education level (levels 4 and 5 vs levels 1, 2, and 3), and low vs high dementia risk score (Cardiovascular Risk Factors, Aging and Incidence of Dementi( CAIDE) score smaller or equal to 7 vs larger than 7) are plotted separately. Circles represent individual participant residuals for each time model. The equation for each model is provided with y representing the residual and x representing the dose when it is significant. The arrow represents inflection points
FIGURE 4.
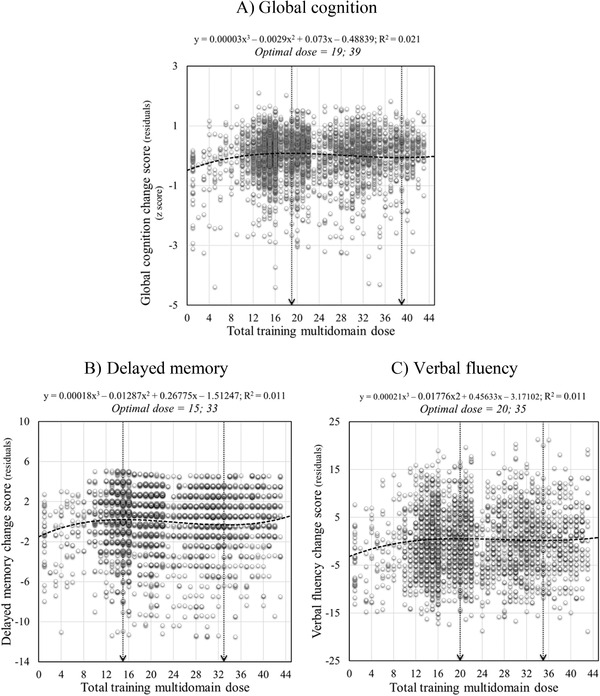
Scatter plots of total multidomain training dose main models on change scores (residuals) (A) global cognition, (B) delayed memory, and (C) verbal fluency. Circles represent individual participant residuals for each time model. The equation for each model is provided with y representing the residual and x representing the dose. The arrow represents inflection points
TABLE 2.
Summary of main findings and interpretation in terms of the magnification and reserve models
| OUTCOME | Group | Function | Optimal dose | Plateau | Magnification /Reserve |
|---|---|---|---|---|---|
| Global cognition | |||||
| Dose effect | All | Cubic | 13 | 13 to 24 | |
| Frailty × Dose interaction | Robust | NS | N/A | N/A | Reserve |
| Frail | Cubic | 12 | 12 to 21 | ||
| Delayed memory recall | |||||
| Dose effect | All | Cubic | 12 | 12 to 22 | |
| Age × Dose interaction | Younger‐Old | Cubic | 12 | 12 to 24 | Magnification |
| Older‐Old | Cubic | 10 | 10 to 23 | ||
| Verbal fluen | |||||
| Dose effect | All | Cubic | 14 | 14 to 21 | |
| Sex × Dose interaction | Female | Cubic | 15 | 15 to 21 | N/A |
| Male | NS | N/A | N/A | ||
| Education × Dose Interaction | High education | NS | N/A | N/A | Reserve |
| Low education | Linear | N/A | N/A | ||
| Dementia risk × Dose interaction | Low dementia risk | Quadratic | 20 | N/A | Magnification |
| High dementia risk | Cubic | 12 | 12 to 21 |
Note: An effect is interpreted as reflecting magnification when participants with higher initial cognitive performance benefit more from intervention, hence magnifying the initial difference. An effect is interpreted as reflecting reserve when participants with lower initial cognitive performance benefit more from intervention, hence reducing the initial difference.
Frailty status: Robust: 1 inclusion criteria; Frail: 2 or 3 inclusion criteria.
Education level: Lower education: 1 to 3; higher education: 4 and 5.
Dementia risk: median split with CAIDE score = 7
Abbreviation: CAIDE, Cardiovascular Risk Factors, Aging and Incidence of Dementia.
The effect of the dose for the global cognition score followed a cubic function (f(x) = 0.0001x3 – 0.0075x2 + 0.1299x – 0.6862; f(x) = 0.0003x3 – 0.0029x2 + 0.07312x – 0.48839 for multidomain doses) with an optimal dose of 13 sessions followed by a plateau between sessions 13 and 26 (Figure 1A), and 19 sessions and a plateau until session 39 when considering multidomain doses (Figure 4). There was a significant interaction with the frailty index. When separating participants as a function of frailty (see Figure 1B), we found no dose effect in robust individuals, but a significant cubic function for frail ones, with an optimal dose of 12 sessions and a plateau between sessions 12 and 21. Global cognition was lower in frail than robust participants for smaller doses and increased at medium doses. The group difference was no longer present at larger doses (frailty score x dose interaction, F = 52.05, P < .01; robust > frail for doses ≤13, t = 4.52, P < .05 [Cohen's D = 0.44], for doses 14–24, t = 7.4, P < .05 [Cohen's D = 0.52], and for doses >24, t = 1.41, P .17).
The effect of the dose on the residuals for delayed recall followed a cubic function (f(x) = 0.0008x3 – 0.0408x2 + 0.6227x – 2.847; f(x) = 0.00018x3 – 0.01287x2 + 0.26775x – 1.51247 for multidomain doses) with an optimal dose of 12 sessions, followed by a plateau between 12 and 22 sessions (Figure 2A), and 15 sessions and a plateau until session 33 when considering multidomain doses (Figure 4). There was a significant interaction with age. A cubic function described the dose‐response relationship in both younger‐old and older‐old participants (Figure 2B), but old‐old individuals reached their plateau earlier than younger‐old, who continued to improve over a few additional sessions (10 instead of 12). The performance of younger‐old individuals was higher than older‐old people overall, with a small increase of the age effect with increasing dose (age x dose interaction, F = 4.21, P < .05; YO > OO for doses ≤12, t = 2.76; P < .05 [Cohen's D = 0.24], for doses 13–22, t = 5.05; P < .05 [Cohen's D = 0.26], and for doses >22, t = 2.9; P < .05 [Cohen's D = 0.27].
A cubic function best described the effect of dose for verbal fluency (f(x) = 0.0011x3 – 0.058x2 + 0.982x – 5.2015; f(x) = 0.00021x3 – 0.0177x2 + 0.45633x – 3.17102 for multidomain doses) with an optimal dose of 14 sessions followed by a plateau between 14 and 21 sessions (Figure 3A), and 20 sessions and a plateau until session 35 when considering multidomain doses (Figure 4). For verbal fluency, there were significant interactions between dose and sex, education, and CAIDE score.
When examining the interaction with sex (See Figure 3B), we found no significant effect of dose in men, but a significant cubic trend in women, with an optimal dose of 15 sessions and a plateau between the sessions 15 and 21. Women's performance was higher than men's overall, and the difference was larger as dose increased (sex x dose interaction, F = 5.52, P < .05; W > M for doses ≤ 14, t = 3.14; P < .05 [Cohen's D = 0.19], doses 15–21, t = 5.1; P < .05 [Cohen's D = 0.36], and doses >21, t = 4.2; P < .05 [Cohen's D = 0.35]).
When examining the interaction with education (Figure 3C), we found that the effect of dose was not significant in the high education group, but there was a significant linear function in those with lower education. Performance of the high education group was better than the lower education group, but the difference gradually decreased as dose increased (education x dose interaction, F = 4.59, P < .05; HE > LE for doses 1–14, t = ‐6.983, p < .05 [Cohen's D = 0.52], doses 15–21, t = 4.96; P < .05 [Cohen's D = 0.42], and doses >21, t = 3.48; P < .05 [Cohen's D = 0.36].
When examining the interaction with the CAIDE score (See Figure 3D), we found that the effect of dose followed a quadratic trend, and performance improved gradually up to about 20 sessions in the group with lower dementia risk. In the group with a higher CAIDE score, the effect of dose followed a cubic trend, with an optimal dose of 12 sessions, followed by a plateau between 12 and 21 sessions. Performance of individuals with a lower CAIDE score was higher than that of higher CAIDE score participants for both lower and higher doses, but the difference was larger for those with medium and higher doses (CAIDE score x Dose interaction, F = 5.77, P < .05, LDR > HDR for doses ≤14, t = 4.78; P < .05 [Cohen's D = 0.31], doses 15–21, t = 7.83, P < .05 [Cohen's D = 0.58], and doses >21, t = 7.4, P < .05 [Cohen's D = 0.64]).
There was no significant interaction effect for APOE carrying status for all outcomes. When excluding participants who completed only the intensive phase (n = 73), only the effect of the dose for the delayed recall score remained significant. It followed a cubic function (f(x) = 0.0007x3 – 0.0331x2 + 0.4713 – 1.8798), with an optimal dose of 11 sessions followed by a plateau between 11 and 21 sessions.
4. DISCUSSION
Our study indicated a non‐linear dose‐response function in a multidomain intervention when examining cognition as an outcome. The function indicated a rapid increase up to about 12 to 14 sessions of training followed by a long plateau and a second very small increase around the 21st session/hour. The effect cumulated at 15 to 20 sessions when considering multidomain dose, with a second very small increase around the 35th session.
A very similar pattern was found irrespective of the cognitive process measured and type of dose proxy. However, there was variation related to individual characteristics, which were found to have an impact on the dose‐response function and optimal dose. Overall, younger participants, women, and those with a lower dementia risk score, higher frailty score, and lower education benefited more from increased dose than their comparison groups and as a result, reached their plateaus later. The case of frailty is particularly striking: the group difference on global cognition decreased with larger doses and it was no longer observed following doses higher than 21 hours. For frail individuals, the optimal dose may therefore be higher than the 12 to 14 hours identified for other participants. This finding highlights the potential of personalizing dose on the basis of frailty parameters, and suggests that larger doses should be provided to frail individuals to optimize effect. Differences in plateaus clearly arise from individual characteristics, which stresses the importance of individualizing interventions in future approaches.
The interaction effect between dose and individual characteristics can be interpreted within the magnification or reserve models. The magnification model proposes that interventions will be more beneficial to healthier, younger, and more apt individuals, and that as a result, interventions will increase inter‐individual differences. We found results indicative of a magnification effect in older participants and those with more dementia risk factors. Although all groups benefited from increasing dose, younger and healthier individuals continued to have an advantage from additional doses. As a result, initial group differences were magnified. In contrast, a reserve effect was found in more frail or less‐educated participants because they benefited from additional training experience, which was not the case for their more robust and educated counterparts. We found an interaction with sex, where dose increased women's initial advantage in verbal fluency.
One intriguing result is that we found no dose effect on verbal fluency for males, robust individuals, and people with high education. Examination of the dose‐response function suggests that verbal fluency does not benefit from the training in these individuals. In fact, verbal fluency was the measure for which we found the most inter‐individual differences in terms of the dose‐response relationship. It might be because it is a multi‐determined task reflecting multiple disease‐responsive mechanisms. 26
Another intriguing aspect of our findings is that for most of our dose‐response models, a long plateau was followed by a very short and small second benefit increase just before the end of training. It is not known whether this increase in cognitive benefits would have continued with even larger doses, as it occurs at the very end of dose distribution. Alternatively, this ultimate increase could be due to initial profile differences between highly compliant participants and those completing fewer doses, as they were slightly more robust and had better cognition. However, this cannot completely explain the phenomenon, as frail individuals benefited more from increasing dose and not the reverse. The effect of cognition on dose was of small magnitude but could partly account for some of the results.
These findings need to be replicated with other large data sets in light of some of the limitations of this study, which include our particular definition of dose, the exploratory nature of the analyses, and the absence of an analysis of site/provider interactions. In addition, our methods of measuring frailty differed from typical frailty indices. Future research is needed to assess whether finer‐grain units may increase our understanding of treatment efficacy. It would also be interesting for future studies to model dose effects for individual components, such as nutrition or physical activity.
These findings have important implications for future studies and designing prevention approaches for the general public. First, our results indicated that the optimal dose is about 12 to 14 sessions when using cognitive training and 15 to 20 sessions when using multidomain sessions as a dose proxy. In both cases, optimal dose corresponded to about half of the training provided. Hence, more is not necessarily better. It is important to note, however, that a second increase was observed at the end of the dose distribution. It could be argued that there is a low cost‐benefit ratio of increasing the dose to this level because the benefit was small. However, the plateau may have been driven by factors such as individual characteristics, type of training, or limited follow‐up. The study identified that the mathematical function and optimal dose varied depending on the characteristics of the target group. In particular, frailer individuals benefited from higher doses, which is important for policy decisions. Finally, it was found that increased dose reduced the initial detrimental effect of lower education and frailty.
CONFLICT OF INTEREST
Sylvie Belleville is a consultant on dementia prevention for Lucilab Inc.
Bruno vellas is investigator and consultant for roche, biogen, Lilly, Esai and taurx, outside the scope of the present paper.
Supporting information
SUPPORTING INFORMATION
SUPPORTING INFORMATION
ACKNOWLEDGMENT
The authors thank René‐Pier Filiou for her help in preparing the manuscript. In addition, we thank Annie Webb for the English‐language revision of the manuscript. The MAPT study was financially supported by grants from the Gérontopôle of Toulouse, the French Ministry of Health (PHRC 2008, 2009), Pierre Fabre Research Institute (manufacturer of the omega‐3 supplement), ExonHit Therapeutics SA, and Avid Radiopharmaceuticals Inc. The promotion of this study was supported by the University Hospital Center of Toulouse. The data sharing activity was supported by the Association Monegasque pour la Recherche sur la maladie d'Alzheimer (AMPA) and the unité mixte de recherche (UMR) 1027 Unit Institut National de la Santé et de la Recherche Médicale (INSERM)‐University of Toulouse III. The present work was supported by Natural Science and Engineering Research Council of Canada (NSERC) and Canadian Institutes of Health Research (CIHR) Foundation grants, and by a Canada Research Chair on Cognitive Neuroscience of Aging and Brain Plasticity to Belleville.
APPENDIX A. Collaborator: MAPT Study Group
Principal investigator: Bruno Vellas (Toulouse); Coordination: Sophie Guyonnet; Project leader: Isabelle Carrié; CRA: Lauréane Brigitte; Investigators: Catherine Faisant, Françoise Lala, Julien Delrieu, Hélène Villars; Psychologists: Emeline Combrouze, Carole Badufle, and Audrey Zueras; Methodology, statistical analysis and data management: Sandrine Andrieu, Christelle Cantet, and Christophe Morin; Multidomain group: Gabor Abellan Van Kan, Charlotte Dupuy, Yves Rolland (physical and nutritional components), Céline Caillaud, Pierre‐Jean Ousset (cognitive component), and Françoise Lala (preventive consultation). The cognitive component was designed in collaboration with Sherry Willis from the University of Seattle, and Sylvie Belleville, Brigitte Gilbert, and Francine Fontaine from the University of Montreal.
Co‐Investigators in associated centers: Jean‐François Dartigues, Isabelle Marcet, Fleur Delva, Alexandra Foubert, and Sandrine Cerda (Bordeaux); Marie‐Noëlle‐Cuffi and Corinne Costes (Castres); Olivier Rouaud, Patrick Manckoundia, Valérie Quipourt, Sophie Marilier, and Evelyne Franon (Dijon); Lawrence Bories, Marie‐Laure Pader, Marie‐France Basset, Bruno Lapoujade, Valérie Faure, Michael Li Yung Tong, Christine Malick‐Loiseau, and Evelyne Cazaban‐Campistron (Foix); Françoise Desclaux and Colette Blatge (Lavaur); Thierry Dantoine, Cécile Laubarie‐Mouret, Isabelle Saulnier, Jean‐Pierre Clément, Marie‐Agnès Picat, Laurence Bernard‐Bourzeix, Stéphanie Willebois, Iléana Désormais, and Noëlle Cardinaud (Limoges); Marc Bonnefoy, Pierre Livet, Pascale Rebaudet, Claire Gédéon, Catherine Burdet, and Flavien Terracol (Lyon); Alain Pesce, Stéphanie Roth, Sylvie Chaillou, and Sandrine Louchart (Monaco); Kristel Sudres, Nicolas Lebrun, and Nadège Barro‐Belaygues (Montauban); Jacques Touchon, Karim Bennys, Audrey Gabelle, Aurélia Romano, Lynda Touati, Cécilia Marelli, and Cécile Pays (Montpellier); Philippe Robert, Franck Le Duff, Claire Gervais, and Sébastien Gonfrier (Nice); Yannick Gasnier, Serge Bordes, Danièle Begorre, Christian Carpuat, Khaled Khales, Jean‐François Lefebvre, Samira Misbah El Idrissi, Pierre Skolil, and Jean‐Pierre Salles (Tarbes).
MRI group: Carole Dufouil (Bordeaux); Stéphane Lehéricy, Marie Chupin, Jean‐François Mangin, and Ali Bouhayia (Paris); Michèle Allard (Bordeaux); Frédéric Ricolfi (Dijon); Dominique Dubois (Foix); Marie‐Paule BonceourMartel (Limoges); François Cotton (Lyon); Alain Bonafé (Montpellier); Stéphane Chanalet (Nice); Françoise Hugon (Tarbes); Fabrice Bonneville, Christophe Cognard, and François Chollet (Toulouse).
PET scans group: Pierre Payoux, Thierry Voisin, Julien Delrieu, Sophie Peiffer, and Anne Hitzel, (Toulouse); Michèle Allard (Bordeaux); Michel Zanca (Montpellier); Jacques Monteil (Limoges); Jacques Darcourt (Nice).
Medico‐economics group: Laurent Molinier, Hélène Derumeaux, and Nadège Costa (Toulouse).
Biological sample collection: Bertrand Perret, Claire Vinel, and Sylvie Caspar‐Bauguil (Toulouse).
Safety management: Pascale Olivier‐Abbal.
DSA Group: Sandrine Andrieu, Christelle Cantet, and Nicola Coley.
Belleville S, Cloutier S, Mellah S, et al. Is more always better? Dose effect in a multidomain intervention in older adults at risk of dementia. Alzheimer's Dement. 2022;18:2140–2150. 10.1002/alz.12544
REFERENCES
- 1. Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396:413‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kivipelto M, Mangialasche F, Snyder HM, et al. World‐Wide FINGERS Network: a global approach to risk reduction and prevention of dementia. Alzheimers Dement. 2020;16:1078‐1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet. 2015;385:2255‐2263. [DOI] [PubMed] [Google Scholar]
- 4. Andrieu S, Guyonnet S, Coley N, et al. Effect of long‐term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo‐controlled trial. Lancet Neurol. 2017;16:377‐389. [DOI] [PubMed] [Google Scholar]
- 5. Anstey KJ, Bahar‐Fuchs A, Herath P, et al. Body brain life: a randomized controlled trial of an online dementia risk reduction intervention in middle‐aged adults at risk of Alzheimer's disease. Alzheimers Dement. 2015;1:72‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Richard E, Moll van Charante EP, Hoevenaar‐Blom MP, et al. Healthy ageing through internet counselling in the elderly (HATICE): a multinational, randomised controlled trial. Lancet Digit Heal. 2019;1:e424‐34. [DOI] [PubMed] [Google Scholar]
- 7. Moll van Charante EP, Richard E, Eurelings LS, et al. Effectiveness of a 6‐year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster‐randomised controlled trial. Lancet. 2016;388:797‐805. [DOI] [PubMed] [Google Scholar]
- 8. Coley N, Ngandu T, Lehtisalo J, et al. Adherence to multidomain interventions for dementia prevention: data from the FINGER and MAPT trials. Alzheimers Dement. 2019;15:729‐741. [DOI] [PubMed] [Google Scholar]
- 9. Lampit A, Hallock H, Moss R, et al. The timecourse of global cognitive gains from supervised computer‐assisted cognitive training: a randomised, active‐controlled trial in elderly with multiple dementia risk factors. J Prev Alzheimer's Dis. 2014;1:33‐39. [DOI] [PubMed] [Google Scholar]
- 10. Lampit A, Hallock H, Valenzuela M. Computerized cognitive training in cognitively healthy older adults: a systematic review and meta‐analysis of effect modifiers. PLoS Med. 2014;11:e1001756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Edwards JD, Xu H, Clark DO, Guey LT, Ross LA, Unverzagt FW. Speed of processing training results in lower risk of dementia. Alzheimers Dement. 2017;3:603‐611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Willis SL, Belleville S. Cognitive training in later adulthood. Handbook of the Psychology of Aging (Eighth Edition), Elsevier (pp. 219‐243). 2016. [Google Scholar]
- 13. Lövdén M, Brehmer Y, Li SC, Lindenberger U. Training‐induced compensation versus magnification of individual differences in memory performance. Front Hum Neurosci. 2012;6:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Belleville S, Fouquet C, Hudon C, Zomahoun HTV, Croteau J. Neuropsychological measures that predict progression from mild cognitive impairment to Alzheimer's type dementia in older adults: a systematic review and meta‐analysis. Neuropsychol Rev. 2017;27:328‐353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vellas B, Carrie I, Gillette‐Guyonnet S, et al. MAPT study: a multidomain approach for preventing Alzheimer's disease: design and baseline data. J Prev Alzheimer's Dis. 2014;1:13‐22. [PMC free article] [PubMed] [Google Scholar]
- 16. Willis SL, Schaie KW. Training the elderly on the ability factors of spatial orientation and inductive reasoning. Psychol Aging. 1986;1:239‐247. [DOI] [PubMed] [Google Scholar]
- 17. Jobe JB, Smith DM, Ball K, et al. ACTIVE: a cognitive intervention trial to promote independence in older adults. Control Clin Trials. 2001;22:453‐479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willis SL, Tennstedt SL, Marsiske M, et al. Long‐term effects of cognitive training on everyday functional outcomes in older adults. J Am Med Assoc. 2006;296:2804‐2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Belleville S, Gilbert B, Fontaine F, Gagnon L, Ménard E, Gauthier S. Improvement of episodic memory in persons with mild cognitive impairment and healthy older adults: evidence from a cognitive intervention program. Dement Geriatr Cogn Disord. 2006;22:486‐499. [DOI] [PubMed] [Google Scholar]
- 20. Belleville S, Hudon C, Bier N, et al. MEMO+: efficacy, durability and effect of cognitive training and psychosocial intervention in individuals with mild cognitive impairment. J Am Geriatr Soc. 2018;66:655‐633. [DOI] [PubMed] [Google Scholar]
- 21. Bier N, Grenier S, Brodeur C, et al. Measuring the impact of cognitive and psychosocial interventions in persons with mild cognitive impairment with a randomized single‐blind controlled trial: rationale and design of the MEMO+ study. Int Psychogeriatrics. 2015;27:511‐525. [DOI] [PubMed] [Google Scholar]
- 22. Hercberg S, Chat‐Yung S, Chaulia M. The French National Nutrition and Health Program: 2001‐2006‐2010. Int J Public Health. 2008;53:68‐77. [DOI] [PubMed] [Google Scholar]
- 23. Van der Linden, M , Coyette, F , Poitrenaud, J , Kalafat, M , Calicis, F , Wyns, C … Membres du GREMEM . L’épreuve de rappel libre/rappel indicé à 16 items (RL/RI‐16). In Van der Linden M., Adam S., Agniel A., & Membres du GRENEM (Eds.), L'évaluation des troubles de la mémoire: présentation de quatre tests de mémoire épisodique avec leur étalonnage(pp. 25‐47). 2004. Marseille: Sola. [Google Scholar]
- 24. Wechsler D. Scale‐Revised WAI. Psychological Corp; 1981. [Google Scholar]
- 25. Cardebat D, Doyon B, Puel M, Goulet P, Joanette Y. [Formal and semantic lexical evocation in normal subjects. Performance and dynamics of production as a function of sex, age and educational level]. Acta Neurol Belg. 1990;90:207‐217. [PubMed] [Google Scholar]
- 26. Amieva H, Jacqmin‐Gadda H, Orgogozo J‐M, et al. The 9 year cognitive decline before dementia of the Alzheimer type: a prospective population‐based study. Brain. 2005;128:1093‐1101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPORTING INFORMATION
SUPPORTING INFORMATION


