Abstract
Helicobacter pylori, the causative agent of gastritis and ulcer disease in humans, secretes a toxin called VacA (vacuolating cytotoxin) into culture supernatants. VacA was initially characterized and purified on the basis of its ability to induce the formation of intracellular vacuoles in tissue culture cells. H. pylori strains possessing different alleles of vacA differ in their ability to express active toxin. Those strains expressing higher toxin levels are correlated with more severe gastric disease. However, the specific role(s) played by VacA during the course of infection and disease is not clear. We have used a mouse model of H. pylori infection to begin to address this role. A null mutation of vacA compromises H. pylori in its ability to initially establish infection. If an infection by a vacA mutant is established, the bacterial load and degree of inflammation are similar to those associated with an isogenic wild-type strain. Thus, in this infection model, vacA plays a role in the initial colonization of the host, suggesting that strains of H. pylori expressing active alleles of vacA may be better adapted for host-to-host transmission.
Helicobacter pylori infection of the human stomach can result in a broad spectrum of disease outcomes ranging from mild gastritis to severe ulcers (8). Additionally, H. pylori is associated with two types of cancer: gastric lymphoid tissue-associated B-cell lymphoma (32, 34) and gastric adenocarcinoma (25). The disease outcome in each infected individual appears to be determined by a combination of host and bacterial factors. The genotypes of H. pylori clinical isolates vary in many genetic loci, including the presence or absence of a pathogenicity island (1, 5) and allelic variation of the vacuolating cytotoxin gene (vacA) (2) and genes encoding adhesion molecules such as BabA2, which binds the Lewisb fucosylated moiety found on human gastric tissue (16). Epidemiological studies suggest that strains expressing the pathogenicity island, those expressing high levels of VacA, and those expressing functional BabA2 correlate with more severe disease (8, 13).
VacA enters eukaryotic cells and exerts its action in the cytoplasm (9, 12). VacA recently was shown to form chloride-conducting channels in both artificial and cellular lipid bilayers (30). Additionally, cells exposed to VacA accumulate vesicles containing rab7, a cellular marker of the late endosome, and lgp110, a marker of lysosomes (21, 24). This VacA-induced alteration of intracellular membranes has been shown to disrupt normal lysosomal degradation of surface receptors in epithelial cells (28) and to interrupt antigen processing in immune cells (22). Comparison of vacA gene sequences among clinical isolates has revealed variability both in the coding region of the signal sequence and in the middle region of the functional protein. Certain alleles of the signal sequence correlate both with higher expression of active toxin and with more severe disease (2). Alleles of the middle region probably act in targeting and internalization of the toxin but do not affect toxin activity once it enters the host cell cytoplasm (23).
The mechanisms by which VacA contributes to infection and disease have remained elusive. Vacuolization of cells in human biopsy samples has been observed (4, 11), and oral administration of partially purified toxin to mice was shown to cause measurable epithelial damage (14). However, isogenic vacA mutants not only colonize but also cause indistinguishable degrees of gastritis in both gnotobiotic piglets (10) and Mongolian gerbils (33). These results, suggesting that vacA is not a virulence factor, contradict the human epidemiology data. This may reflect differences in the animal models relative to the human host or may indicate that VacA is not essential for the establishment or persistence of H. pylori infection. The latter conclusion is particularly unsatisfying since the presence of vacA seems to distinguish H. pylori from Helicobacter species that do not infect humans or interact intimately with the gastric epithelium in their natural hosts (19).
We decided to reexamine the role of VacA in an established mouse model of infection using H. pylori strain SS1 in C57BL/6NTac mice (20). In this model system, we found that isogenic vacA null mutants are severely defective in the ability to establish initial colonization of the host, which profoundly attenuates the virulence potential of these strains.
MATERIALS AND METHODS
Bacterial and cell culture.
The mouse-adapted H. pylori strain SS1 was used for these studies (20). H. pylori was grown on solid media on horse blood agar (HB) plates, containing 4% Columbia agar base (Oxoid), 5% defibrinated horse blood (HemoStat Labs), 0.2% β-cyclodextrin (Sigma), 10 μg of vancomycin (Sigma) per ml, 5 μg of cefsulodin (Sigma) per ml, 2.5 U of polymyxin B (Sigma) per ml, 50 μg of cycloheximide (Sigma) per ml, 5 μg of trimethoprim (Sigma) per ml, and 8 μg of amphotericin B (Sigma) per ml, under microaerobic conditions at 37°C. A microaerobic atmosphere was generated either by using a CampyGen sachet (Oxoid) in a gas pack jar or by incubating the culture in an incubator equilibrated with 10% CO2 and 90% air. For liquid culture, H. pylori was grown in brucella broth (Difco) containing 10% fetal bovine serum (Gibco/BRL) (BB10) with shaking in a microaerobic atmosphere. Escherichia coli growth and manipulations were performed as specified by standard laboratory protocols (3). AGS cells were grown in Dulbecco modified Eagle medium with high glucose, l-glutamine, sodium pyruvate, and pyridoxine hydrochloride (Gibco/BRL) supplemented with 10% fetal bovine serum.
Construction of vacA and cagA mutant strains and restored derivatives.
The ΔvacA::aphA3 (ΔV) derivative of SS1 was made by transforming SS1 with 2 μg of genomic DNA prepared from strain 342sΔV (provided by Marta Marchetti), using natural transformation (http://www.metazoa.com/UPL3244). 342sΔV contains the Campylobacter coli aphA3 gene, conferring kanamycin resistance, inserted at nucleotide 1392 (amino acid 296) of the vacA coding sequence (31). Kanamycin-resistant colonies were isolated on HB plates containing kanamycin (25 μg/ml). The ΔcagA::aphA3 derivative of SS1 was made by transforming SS1 with 5 μg of pCagKan as described above. pCagKan was made by subcloning the C. coli aphA3 gene from pILL550 (17) into the NdeI site at position 1053 of the cagA gene in pBSCagA (7).
To make an independent vacA mutant in SS1, we first amplified the entire coding region of vacA from strain NCTC 11638 (accession number U07145) (26) using PCR with primers VN (CGCTTTGATGGACACCCCACA) and VC (GCGATCTGGCATGATAAG) in reaction mixtures containing 4 μM (each) primers and 20 ng of NCTC 11638 genomic DNA. The resulting reaction product was gel purified and cloned into the TopoXL vector using the TopoXL kit (Invitrogen) as specified by the manufacturer. Plasmids from two resulting clones, TV2 and TV7, that contained the expected 4-kb inserts were sequenced to confirm they contained the expected gene. DNA sequencing revealed that both clones contained several mutations. However, the three mutations in pTV7 were confined to a SspI fragment which contained no mutations in the pTV2 clone. Therefore, the SspI fragment from pTV2 was subcloned into pTV7. Sequencing revealed that the new clone, pTV2/7, contained the entire vacA coding region with no mutations. pVacKanSacB was made by subcloning an XhoI-SmaI fragment from pKSFII (6) containing the aphA3 gene, conferring kanamycin resistance, and the sacB gene, conferring sucrose sensitivity, into pTV2/7 that had been digested with SspI, removing the DNA between positions 1332 and 2340 of the coding sequence. SS1 was then transformed with 10 μg of pVacKanSacB using natural transformation, and kanamycin-resistant clones were selected as described above. Several of the resulting colonies were screened for sucrose sensitivity (from the sacB gene) by plating on HB plates containing 6% sucrose. One kanamycin-resistant, sucrose-sensitive clone VS3 was further analyzed. PCR amplification with primers flanking the SspI sites (VS4 [GATAAACACTTCAAAAGT] and VS5 [TTAGCTGTATGAGCATCC]) and within the aphA3 gene (Aph3out [GGCGTATAACATAGTAGCTAC]) were used to confirm that a single double crossover event had occurred.
The vacA locus was then restored to the wild-type sequence by transforming VS3 with pTV2/7 and selecting sucrose-resistant colonies. Clone 3.1 was additionally found to be kanamycin sensitive, suggesting a double crossover, and again PCR was used to confirm the double crossover as described above.
Immunoblot detection of VacA.
The presence of the VacA protein in bacterial extracts was determined by resuspending half of a blue inoculating loop (Nunc) full of plate-grown bacteria in 2× sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer (3). This sample was fractionated by SDS-PAGE (10% polyacrylamide), and the proteins were transferred to a Hybond-P membrane (Amersham). The resulting blot was incubated with rabbit anti-VacA polyclonal serum (1:10,000) (provided by Antonello Covacci [31] in TBS-T (50 mM Tris [pH 7.4], 150 mM NaCl, 0.05% Tween 20) followed by a secondary anti-rabbit immunoglobulin G antibody conjugated to horseradish peroxidase (Amersham) at the same dilution. The immunoreactive bands were visualized using the ECL-Plus detection system as specified by the manufacturer (Amersham).
Mouse infections.
Bacteria were inoculated from frozen stocks onto HB plates. After 2 to 5 days of growth, bacteria harvested from the plates were used to inoculate liquid cultures. Cultures were grown with shaking under microaerobic conditions overnight to an optical density at 600 nm (OD600) between 0.3 and 1. Cultures were assessed by light microscopy for potential contamination, spiral morphology, and motility. Bacteria were diluted to 1 × 108 to 9 × 108 bacteria per ml, assuming 3 × 108 bacteria/OD600 unit, in BB10 unless otherwise indicated. Female C57BL/6NTac mice (6 to 8 weeks old) were infected by oral gavage with 1 ml of bacterial suspension. The mice were housed in an Association for the Assessment and Accreditation of Laboratory Animal Care-accredited facility in microisolator caging and provided with standard chow and water ad libitum. All manipulations were approved by the institutional Animal Care and Use Committee. Mice were euthanized at the indicated intervals by inhalation of CO2. The glandular stomach was removed, separated from the forestomach, and cut along the lesser curvature. Any ingested food in the stomach was removed with forceps, and the stomach was bisected with a clean scalpel blade by cutting along the greater curvature. One half of the stomach was transferred to an Eppendorf tube containing 0.5 ml of BB10, weighed, and homogenized with a tissue homogenizer pestle (VWR). Serial dilutions of the homogenate were plated onto HB plates to determine the numbers of both wild-type bacteria and mutant bacteria or onto HB plates supplemented with 25 μg of kanamycin per ml to detect vacA mutant bacteria only. The percentage of wild-type bacteria was determined using the following equation: percent wild type = 100 − [(CFU Kanr/CFU plain) × 100]. The remaining half of the stomach was fixed in 10% neutral buffered formalin (Fisher Scientific) and processed using standard methods. Tissue sections to be examined by light microscopy were stained with hematoxylin and eosin or with a silver stain (Warthin-Starry method).
Determination of ID50.
To determine the 50% infective dose (ID50), mice were infected with serial dilutions of bacteria as described above. The inoculum was diluted and plated to determine the actual bacterial dose. After 1 month the mice were sacrificed and the stomachs were cultured as above to determine the number of animals infected at each dose. The Reed-Muench calculation was then used to determine the ID50 (27).
Mixed infection in vitro.
Bacteria were resuspended in BB10 after 1 day of growth on plates, and the OD600 was determined. A mixture containing approximately 3 × 107 of both VS3 (vacA::apha3:sacB) and 3.1 (vacA) bacteria were inoculated into 5 ml of BB10 for the 0.5-day time point. A parallel 5-ml culture was made by diluting this culture 10-fold for the 1-day time point. For subsequent time points, 3 × 106 bacteria from that day's culture were diluted into 5 ml of fresh BB10 and grown for 24 h. All cultures were grown with shaking in a microaerobic atmosphere. At 0, 0.5, 1, 2, 3, 4, and 5 days, the cultures were plated on both plain plates and plates supplemented with kanamycin to distinguish VS3 (Kanr) from 3.1 (Kans). The percentage of wild-type bacteria was determined using the above equation.
RESULTS
Wild-type H. pylori outcompete vacA mutant bacteria in the mouse stomach.
To assess the role of VacA in colonization, we made a vacA mutant derivative (ΔV) of the mouse-adapted H. pylori strain, SS1 (20), by natural transformation with genomic DNA from H. pylori strain 342sΔV, which contained the aphA3 gene from C. coli, conferring kanamycin resistance, inserted into the vacA coding region and selection of a kanamycin-resistant clone. First we tested the infecting potential of a high dose (9 × 108 CFU) of wild-type and mutant bacteria alone and in a coinfection experiment with a 50:50 mixture of the two strains. In the mixed infection, we could distinguish mutant bacteria from wild-type bacteria on the basis of the kanamycin resistance gene used to generate the vacA mutation. Each strain could infect mice, and the infections resulted in a similar bacterial load after 1 month (Table 1). In the mixed infection, however, only wild-type bacteria were recovered. We repeated this experiment using both 50:50 and 90:10 ratios of mutant to wild-type bacteria. In both cases, we could recover only wild-type bacteria from infected animals (Table 2).
TABLE 1.
Colonization of wild-type and vacA::aphA3 H. pylori in mice after 1 month alone and in competition
| Inputa | Wt of stomach (g)b | CFU of wild-type + mutantc at dilution of:
|
CFU of mutantc at dilution of:
|
Log CFU/g | ||||
|---|---|---|---|---|---|---|---|---|
| 10−1 | 10−2 | 10−3 | 10−1 | 10−2 | 10−3 | |||
| Medium | 0.1 | 0 | 0 | 0 | NCe | |||
| WT | 0.128 | TMCd | 556 | 51 | 5.62 | |||
| WT | 0.076 | TMC | 936 | 56 | 6.03 | |||
| WT | 0.081 | TMC | 946 | 89 | 6.36 | |||
| ΔV | 0.111 | 0 | 0 | 0 | NC | |||
| ΔV | 0.093 | TMC | 1,054 | 87 | 6.01 | |||
| ΔV | 0.093 | TMC | 519 | 58 | 5.77 | |||
| WT + ΔV | 0.088 | TMC | 1,024 | 99 | 0 | 0 | 0 | 6.06 |
| WT + ΔV | 0.1 | TMC | 360 | 94 | 0 | 0 | 0 | 5.92 |
| WT + ΔV | 0.089 | TMC | 964 | 103 | 0 | 0 | 0 | 6.05 |
Individual mice were infected with medium or 9 × 108 CFU of SS1 (WT) or SS1vacA::aphA3 (ΔV).
Weight of the stomach from each mouse.
After 1 month of infection, the mice were sacrificed and the stomach from each mouse was homogenized and plated at the indicated dilution on HB plates, to determine the CFU of both wild-type and ΔV mutant bacteria or H.B plates containing 25 μg of kanamycin to determine the CFU of ΔV mutant bacteria.
TMC, too many to count.
NC, not colonized.
TABLE 2.
Colonization of 50:50 and 90:10 vacA::aphA3 mutant–wild-type mixtures in mice
| Input and ratioa | Log (CFU/g) of wild-type plus mutantb | Log (CFU/g) of mutantb |
|---|---|---|
| WT +ΔV (50:50) | 5.8 | NC |
| WT +ΔV (50:50) | 6.3 | NC |
| WT +ΔV (50:50) | NCc | |
| WT +ΔV (50:50) | 5.2 | NC |
| WT +ΔV (90:10) | 5.7 | NC |
| WT +ΔV (90:10) | NC | |
| WT +ΔV (90:10) | 6.4 | NC |
| WT +ΔV (90:10) | 5.9 | NC |
Individual mice were infected with 2.5 × 107 bacteria each of strains SS1 (WT) and SS1vacA::aphA (ΔV) (50:50) or with 4.5 × 107 bacteria of strains SS1 (WT) and 5 × 106 bacteria of strain SS1vacA::aphA3 (ΔV) (90:10).
After 1 month of infection, the mice were sacrificed and half of the stomach from each mouse was homogenized and plated on HB plates, to determine the CFU of both wild-type and ΔV mutant bacteria or on HB plates containing 25 μg of kanamycin per ml to determine the CFU of ΔV mutant bacteria.
NC, not colonized.
The vacA mutant has a 320-fold-higher ID50 than the wild-type strain dose.
We next tested both the wild-type and vacA mutant (ΔV) strains to determine the precise ID50. In this experiment, we infected mice with 10-fold serially diluted bacteria and determined the number of animals colonized at each dose. The Reed-Muench calculation was used to determine the number of bacteria required to obtain colonization of 50% of the animals (27). Table 3 shows that the ID50 for the wild-type strain was less than 5 × 105 bacteria while the vacA mutant had an ID50 of 1.6 × 108. This is at least a 320-fold difference.
TABLE 3.
ID50 determination for wild-type, vacA::aphA3, and cagA::aphA3 strains in mice
| Dilutiona (CFU/ml) | No. of mice after 1 mob:
|
Total no. infectedc | Total no. uninfectedd | % Infectede | |
|---|---|---|---|---|---|
| Infected | Uninfected | ||||
| Wild type | |||||
| 5 × 108 | 5 | 0 | 18 | 0 | 100 |
| 5 × 107 | 5 | 0 | 13 | 0 | 100 |
| 5 × 106 | 5 | 0 | 8 | 0 | 100 |
| 5 × 105 | 3 | 2 | 3 | 2 | 60 |
| ΔvacA::aphA3 | |||||
| 5 × 108 | 3 | 1 | 4 | 1 | 80 |
| 5 × 107 | 1 | 3 | 1 | 4 | 20 |
| 5 × 106 | 0 | 4 | 0 | 8 | 0 |
| 5 × 105 | 0 | 4 | 0 | 12 | 0 |
| ΔcagA::aphA3 | |||||
| 7.9 × 108 | 5 | 0 | 18 | 0 | 100 |
| 7.9 × 107 | 5 | 0 | 13 | 0 | 100 |
| 7.9 × 106 | 5 | 0 | 8 | 0 | 100 |
| 7.9 × 105 | 3 | 2 | 3 | 2 | 60 |
| 7.9 × 104 | 0 | 5 | 0 | 7 | 0 |
Mice were inoculated with wild-type SS1 bacteria or mutant derivatives of SS1 at the indicated dilutions. Reed-Muench ID50 calculations were <5 × 105 for wild type, 1.6 × 108 for Δvac::aphA3, and 5.4 × 105 for ΔcagA::aphA3.
The number of mice infected or uninfected at each dilution after 1 month.
Cumulative total of mice infected from the lowest dilution.
Cumulative total of mice uninfected from the highest dilution.
Percentage of total mice infected.
To rule out possible effects of the presence of the aphA3 gene, which confers kanamycin resistance in the mutant strain, we determined the ID50 of another mutant, generated by insertion of the aphA3 gene into its coding region, the cagA gene, shown previously to colonize animal models of infection (33). We also observed good colonization of the cagA mutant both alone and in competition with wild-type bacteria (data not shown). The ID50 of the cagA mutant was 5.4 × 105, similar to that of the wild-type bacteria. We also determined the localization and density of infection by staining histological sections of infected animals with the Warthin-Starry stain to visualize the bacteria. In animals infected with both the wild-type and vacA mutant bacteria both strains were localized primarily in the stomach antrum, with some bacteria being found in the cardia at the junction of the forestomach and the stomach. The density of infection observed by histological examination appeared similar for the two strains, consistent with the culture results. Examination of the stomachs with hematoxylin and eosin staining revealed no appreciable inflammation after 1 month of infection with either wild-type or mutant bacteria.
Restoration of the mutant gene and the colonization phenotype.
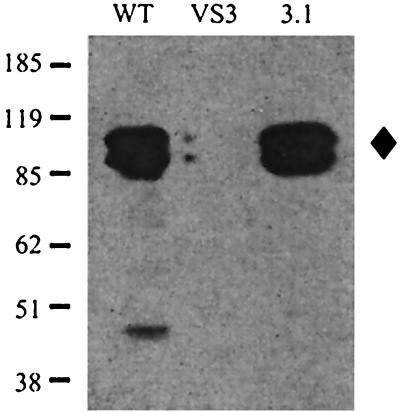
The vacA mutant used in the above studies was generated by transformation of chromosomal DNA from another H. pylori strain that contained a kanamycin resistance cassette inserted in the vacA gene. This led to the formal possibility that the phenotype we observed was not due to the insertion at the vacA locus but was due to some other strain difference acquired during the genetic transformation. To rule out this possibility, we made an independent vacA mutant derivative of SS1 by generating a plasmid containing the vacA gene with a 1.1-kb internal deletion, into which was inserted a genetic cassette carrying the aphA3 gene (kanamycin resistance) and the sacB gene (sucrose sensitivity). This plasmid was used to transform SS1. The resulting transformants were selected by resistance to kanamycin, and all were sensitive to sucrose. The insertion of one clone, VS3, was confirmed by PCR to result from a double crossover at the vacA locus, and this clone was used for further studies. VS3 was then restored to the wild-type sequence at the vacA locus by transformation with a second plasmid containing the full-length vacA gene. Of 30 sucrose-resistant sucrose colonies, 29 were kanamycin sensitive, indicating that the mutated allele had been replaced by the wild-type allele due to a double crossover at the vacA locus. This was confirmed by PCR, and one clone, 3.1, was used for further analysis. The full-length vacA gene had proved difficult to clone in E. coli, and many of the derivatives we isolated showed point mutations or small deletions. Therefore, we used Western blotting of whole-cell extracts of the wild type, a new mutant derivative, VS3, and the reconstituted wild-type vacA strain, 3.1, to show that both the starting strain and the restored strain expressed full length (94-kDa) VacA protein while mutant strain VS3 had no expression of VacA (Fig. 1).
FIG. 1.
Western blot of whole-cell extracts probed with anti-VacA antibodies. WT, wild-type strain SS1; VS3, vacA::aphA3:sacB; 3.1, restored vacA mutant. The positions of molecular mass markers in kilodaltons are indicated on the left, and the position of the 94-kDa VacA protein is shown by a diamond on the right.
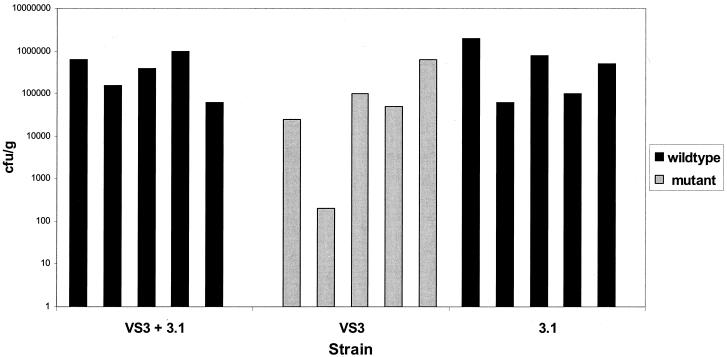
We tested the infectious potential of VS3 and 3.1, both alone and in competition, compared to wild-type bacteria. We could distinguish the vacA mutant, VS3, from the strain restored to wild type, 3.1, because VS3 contains a kanamycin resistance cassette, allowing selection on kanamycin-containing plates. After 1 month of infection, both strains were able to infect mice. The bacterial loads in the stomachs of mice infected with each strain were similar (VS3, 1.6 × 105 ± 2.7 × 105 CFU/g; 3.1, 6.9 × 105 ± 7.9 × 105 CFU/g). However, as above, only the restored wild-type bacteria could be recovered from a mixed infection (Fig. 2). Thus, strain 3.1, containing a reconstituted vacA gene, was indistinguishable from the wild type in its capacity to colonize animals and to outgrow a vacA mutant strain.
FIG. 2.
Colonization of C57BL/6NTac mice 1 month after infection of with 2 × 108 CFU of vacA mutant strain (VS3), restored wild-type strain (3.1), or a 50:50 mixture of the two strains. The number of restored wild-type (black bars) or vacA mutant (gray bars) bacteria recovered from each animal stomach is shown. Each bar represents one mouse. In the mixed infection, no mutant bacteria could be recovered from any of the five mice.
Kinetics of mixed infection.
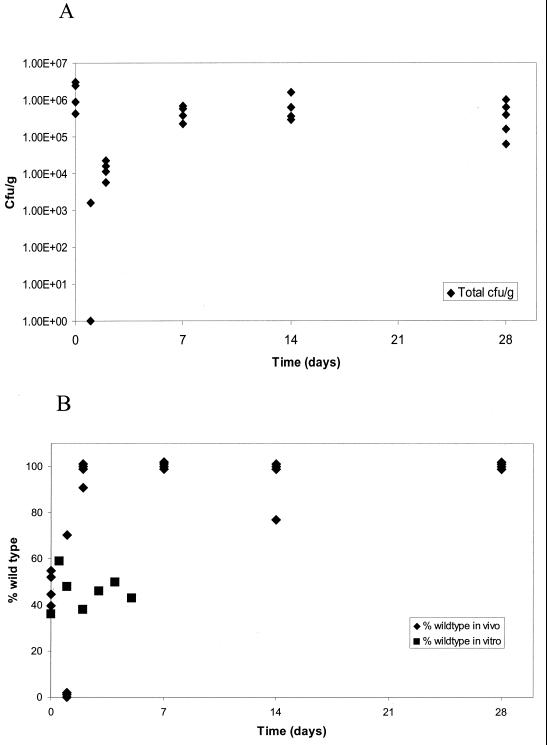
To determine when the vacA mutant bacteria were disappearing from the infection, we infected 24 mice with a 50:50 mixture of vacA mutant (VS3) and wild-type (3.1) bacteria. Four mice were sacrificed on each of days 0, 1, 2, 7, 14, and 28, and the viable counts of both strains were determined. The initial inoculum contained 2 × 108 bacteria of each strain. Immediately after infection, approximately 106 total organisms could be recovered (Fig. 3A). After 1 day, bacteria could be recovered from only one mouse, which had 1,600 CFU/g. On day 2, approximately 2 × 104 organisms were recovered from all four animals. By day 7, the bacterial load reached approximately 5 × 105 CFU/g for all four animals and remained at this level for the 14- and 28-day time points. We also determined the percentage of wild-type and mutant bacteria in each of the animals (Fig. 3B). At the time zero, approximately equal numbers of wild-type and mutant bacteria were recovered (the average percent wild type was 48%). The single infected animal at the 1-day time point had 70% wild-type bacteria and only 30% mutant bacteria. By 2 days, three mice were infected with 100% wild-type bacteria while one mouse had 9% mutant bacteria and 91% wild-type bacteria. At the later time points, all the mice were infected with only wild-type bacteria, except for one mouse at the 14-day time point, in which 33% of the bacteria recovered carried the vacA mutation.
FIG. 3.
Kinetics of mixed infection with a 50:50 mixture (2 × 108 CFU each) of vacA mutant (VS3) and restored wild-type (3.1) strains in vivo and in vitro. (A) Total number of bacteria recovered from the stomachs of each of four mice on days 0, 1, 2, 7, 14, and 28 after infection. (B) Percentage of wild-type bacteria recovered at each time point during infection of mice (diamonds) or during growth in vitro (squares).
In a parallel experiment, we checked the growth of a similar mixed culture in vitro at 0, 0.5, 1, 2, 3, 4 and 5 days. At all time points, the culture consisted of approximately 50% wild-type and 50% mutant bacteria (Fig. 3B). This is in stark contrast to the in vivo experiment, where the mutant bacteria represented only 30% of the population after 1 day and were essentially absent after 2 days. Thus, the vacA mutation seems to confer a specific disadvantage for colonization rather than growth.
DISCUSSION
In contrast to previous studies (10, 33), we have shown that two independent mutations in the vacA gene of H. pylori strain SS1 resulted in bacteria that could not survive in mice in the presence of competing wild-type bacteria. The fact that a biological effect could be revealed only in a competition experiment might suggest a subtle virulence phenotype for this gene. However, the vacA mutant had an ID50 fully 2 log units higher than that of the wild-type bacteria. The reasons why no role for vacA could be demonstrated in previous studies may reflect differences in the animal models used. Alternatively, the lack of phenotype may have resulted from the general practice of using very high inocula for animal infections, usually several log units above the ID50, and multiple rounds of infection. Interestingly, by using a competition experiment, we were able to see a phenotype, even at a dose above the ID50 for the mutant strain. This should provide an improved protocol for testing new potential virulence genes.
We were able to localize the defect of the vacA mutant to a role in colonization by determining the kinetics of infection during coinfection with the wild-type bacteria. After an initial inoculation of an equal mixture of 2 × 108 mutant and wild-type organisms, the challenged animals cleared most of the bacteria. On day 1, few viable or cultivatable organisms could be detected. However, by day 2, significant numbers of bacteria were detected, and this number increased on day 7, after which the bacterial load remained relatively constant over 1 month of observation. Examination of the genotype of the bacteria recovered from the mouse stomach revealed that a significant proportion of the mutant bacteria were present only immediately after inoculation. By day 2 postinfection, generally only wild-type bacteria could be recovered from the stomach. That 2 of 16 mice showed a small percentage of mutant bacteria may reflect the residual ability of this mutant to establish infection on its own or, since VacA is a secreted protein, trans-complementation from nearby wild-type bacteria.
The mechanism by which VacA facilitates colonization is not known; however, VacA host cell toxicity has been well documented. This toxicity may stimulate host cell turnover, presenting a new cell type to which H. pylori can adhere. We examined the ability of the vacA mutant to adhere to a gastric epithelial cell line but found no obvious differences (data not shown). Further studies with primary gastric epithelial cells may shed more light on this hypothesis. Alternatively, VacA may cause tissue damage that alters the local environment by releasing nutrients or altering the local pH, allowing H. pylori to survive. Local or global hypochlorhydria in the stomach has been postulated to have a significant impact on H. pylori colonization and to play a role in the expression of disease. Prolonged H. pylori infection can induce the host to produce autoantibodies against the Lewisx antigen found on parietal cells, resulting in loss of this acid-secreting cell type (15, 29). In the mouse model, inhibitors of acid secretion can change the distribution of H. pylori from a localized infection of the antrum to colonization of the entire stomach (18). It is possible that VacA plays a role in the local inhibition of acid secretion due to cell damage, which then facilitates the ability of H. pylori to establish infection.
VacA may increase the ability of H. pylori to be transmitted from host to host. There is considerable sequence variation in the vacA gene in different clinical isolates. These different alleles vary in VacA expression levels. Epidemiological studies show that strains expressing high levels of the vacA correlate more highly with ulcer disease. This could result from direct effects of the toxin or could occur because VacA promotes higher levels of sustained colonization in the stomach, possibly in a precise anatomic region of the stomach. The presence of low-expressing alleles could reflect a delicate balance between high levels of the toxin allowing successful transmission but also causing too much damage to the host.
The mouse model of H. pylori infection appears to reflect primarily the capacity to colonize animals and does not accurately reflect the inflammatory response seen during natural human infection. Hence, we may be assessing only one aspect of the contribution of VacA to H. pylori virulence. Nevertheless, our findings do present an interesting and unexpected facet of the effect of VacA on the biology of H. pylori infection and disease.
ACKNOWLEDGMENTS
We thank Antonello Covacci, Marta Marchetti, and Rino Rappuoli for strains, plasmids, antibodies, advice, and helpful discussions; Denise Monack for help with statistical analysis; and Denise Monack and Lalita Ramakrishnan for critical review of the manuscript.
This work was supported by a postdoctoral fellowship from the Jane Coffin Childs memorial fund for medical research to N.S. and by NIH grant AI38459.
REFERENCES
- 1.Akopyants N S, Clifton S W, Kersulyte D, Crabtree J, Youree B E, Reece C A, Bukanov N O, Drazek E S, Roe B A, Berg D E. Analyses of the cag pathogenicity island of Helicobacter pylori. Mol Microbiol. 1998;28:37–53. doi: 10.1046/j.1365-2958.1998.00770.x. [DOI] [PubMed] [Google Scholar]
- 2.Atherton J C, Cao P, Peek R M, Jr, Tummuru M K, Blaser M J, Cover T L. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J Biol Chem. 1995;270:17771–17777. doi: 10.1074/jbc.270.30.17771. [DOI] [PubMed] [Google Scholar]
- 3.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Short protocols in molecular biology. 3rd ed. New York, N.Y: John Wiley & Sons, Inc.; 1997. [Google Scholar]
- 4.Bertram T A, Murray P D, Morgan D R, Jerdak G, Yang P, Czinn S. Gastritis associated with infection by Helicobacter pylori in humans: geographical differences. Scand J Gastroenterol Suppl. 1991;181:1–8. doi: 10.3109/00365529109093201. [DOI] [PubMed] [Google Scholar]
- 5.Censini S, Lange C, Xiang Z, Crabtee J E, Ghiara P, Borodovsky M, Rappuoli R, Covacci A. cag, a pathogenicity island of Helicobacter pylori, encodes type 1-specific and disease-associated virulence factors. Proc Natl Acad Sci USA. 1996;93:14648–14653. doi: 10.1073/pnas.93.25.14648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Copass M, Grandi G, Rappuoli R. Introduction of unmarked mutations in the Helicobacter pylori vacA gene with a sucrose sensitivity marker. Infect Immun. 1997;65:1949–1952. doi: 10.1128/iai.65.5.1949-1952.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covacci A, Censini S, Bugnoli M, Petracca R, Burroni D, Macchia G, Massone A, Papini E, Xiang Z, Figura N, Rappuoli R. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci USA. 1993;90:5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Covacci A, Telford J L, Del Giudice G, Parsonnet J, Rappuoli R. Helicobacter pylori virulence and genetic geography. Science. 1999;284:1328–1333. doi: 10.1126/science.284.5418.1328. [DOI] [PubMed] [Google Scholar]
- 9.deBernard M, Arico B, Papini E, Rizzuto R, Grandi G, Rappuoli R, Montecucco C. Helicobacter pylori toxin VacA induces vacuole formation by acting in the cell cytosol. Mol Microbiol. 1997;26:665–674. doi: 10.1046/j.1365-2958.1997.5881952.x. [DOI] [PubMed] [Google Scholar]
- 10.Eaton K A, Cover T L, Tummutu M K R, Blaser M J, Krakowka S. Role of vacoulating cytotoxin in gastritis due to Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1997;65:3462–3464. doi: 10.1128/iai.65.8.3462-3464.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Shoura S M. Helicobacter pylori. I. Ultrastructural sequences of adherence, attachment, and penetration into the gastric mucosa. Ultrastruct Pathol. 1995;19:323–333. doi: 10.3109/01913129509064237. [DOI] [PubMed] [Google Scholar]
- 12.Garner J A, Cover T L. Binding and internalization of the Helicobacter pylori vacuolating cytotoxin by epithelial cells. Infect Immun. 1996;64:4197–4203. doi: 10.1128/iai.64.10.4197-4203.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerhard M, Lehn N, Neumayer N, Boren T, Rad R, Schepp W, Miehlke S, Classen M, Prinz C. Clinical relevance of the Helicobacter pylori gene for blood-group antigen-binding adhesin. Proc Natl Acad Sci USA. 1999;96:12778–12783. doi: 10.1073/pnas.96.22.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghiara P, Marchetti M, Blaser M J, Tummuru M K, Cover T L, Segal E D, Tompkins L S, Rappuoli R. Role of the Helicobacter pylori virulence factors vacuolating cytotoxin, CagA, and urease in a mouse model of disease. Infect Immun. 1995;63:4154–4160. doi: 10.1128/iai.63.10.4154-4160.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guruge J L, Falk P G, Lorenz R G, Dans M, Wirth H-P, Blaser M J, Berg D E, Gordon J I. Epithelial attachment alters the outcome of Helicobacter pylori infection. Proc Natl Acad Sci USA. 1998;95:3925–3930. doi: 10.1073/pnas.95.7.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilver D, Arnqvist A, Ogren J, Frick I M, Kersulyte D, Incecik E T, Berg D E, Covacci A, Engstrand L, Borén T. Helicobacter pylori adhesin binding fucosylated histo-blood group antigens revealed by retagging. Science. 1998;279:373–377. doi: 10.1126/science.279.5349.373. [DOI] [PubMed] [Google Scholar]
- 17.Labigne-Roussel A, Harel J, Tompkins L. Gene transfer from Escherichia coli to Campylobacter species: development of shuttle vectors for genetic analysis of Campylobacter jejuni. J Bacteriol. 1987;169:5320–5323. doi: 10.1128/jb.169.11.5320-5323.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee A, Dixon M F, Danon S J, Kuipers E, Megraud F, Larsson H, Mellgard B. Local acid production and Helicobacter pylori: a unifying hypothesis of gastroduodenal disease. Eur J Gastroenterol Hepatol. 1995;7:461–465. [PubMed] [Google Scholar]
- 19.Lee A, O'Rourke J. Gastric bacteria other than Helicobacter pylori. Gastroenterol Clin North Am. 1993;22:21–42. [PubMed] [Google Scholar]
- 20.Lee A, O'Rourke J, De Ungria M C, Robertson B, Daskalopoulos G, Dixon M F. A standardized mouse model of Helicobacter pylori infection: Introducing the Sydney strain. Gastroenterology. 1997;112:1386–1397. doi: 10.1016/s0016-5085(97)70155-0. [DOI] [PubMed] [Google Scholar]
- 21.Molinari M, Galli C, Norais N, Telford J L, Rappuoli R, Luzio J P, Monticucco C. Vacuoles induced by Helicobacter pylori toxin contain both late endosomal and lysosomal markers. J Biol Chem. 1997;272:25339–25344. doi: 10.1074/jbc.272.40.25339. [DOI] [PubMed] [Google Scholar]
- 22.Molinari M, Salio M, Galli C, Norais N, Rappuoli R, Lanzavecchia A, Monticucco C. Selective inhibition of Ii-dependent antigen presentation by Helicobacter pylori toxin VacA. J Exp Med. 1998;187:135–140. doi: 10.1084/jem.187.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pagliaccia C, deBernard M, Lupetti P, Ji X, Burroni D, Cover T L, Papini E, Rappuoli R, Telford J L, Reyrat J-M. The m2 form of the Helicobacter pylori cytotoxin has cell type-specific vacuolating activity. Proc Natl Acad Sci USA. 1998;95:10212–10217. doi: 10.1073/pnas.95.17.10212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papini E, Bernard M D, Milia E, Bugnoli M, Zerial M, Rappuoli R, Montecucco C. Cellular vacuoles induced by Helicobacter pylori originate from late endosomal compartments. Proc Natl Acad Sci USA. 1994;91:9720–9724. doi: 10.1073/pnas.91.21.9720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parsonnet J, Friedman G D, Vandersteen D P, Chang Y, Vogelman J H, Orentreich N, Sibley R K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991;325:1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- 26.Phadnis S H, Ilver D, Janzon L, Normark S, Westblom T U. Pathological significance and molecular characterization of the vacuolating toxin gene of Helicobacter pylori. Infect Immun. 1994;62:1557–1565. doi: 10.1128/iai.62.5.1557-1565.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reed L J, Muench H. A simple method of estimating fifty per cent endpoints. Am J Hyg. 1938;27:493–497. [Google Scholar]
- 28.Satin B, Norais N, Telford J, Rappuoli R, Murgia M, Montecucco C, Papini E. Effect of Helicobacter pylori vacuolating toxin on maturation and extracellular release of procathepsin D and on epidermal growth factor degradation. J Biol Chem. 1997;272:25022–25028. doi: 10.1074/jbc.272.40.25022. [DOI] [PubMed] [Google Scholar]
- 29.Syder A J, Guruge J L, Li Q, Hu Y, Oleksiewicz C M, Lorenz R G, Karam S M, Falk P G, Gordon J I. Helicobacter pylori attaches to NeuAcalpha2,3Galbeta1,4 glycoconjugates produced in the stomach of transgenic mice lacking pariental cells. Mol Cell. 1999;3:263–274. doi: 10.1016/s1097-2765(00)80454-2. [DOI] [PubMed] [Google Scholar]
- 30.Szabo I, Brutsche S, Tombola F, Moschiono M, Satin B, Telford J L, Rappuoli R, Montecucco C, Papini E, Zoratti M. Formation of anion-selective channels in the cell plasma membrane by the toxin VacA of Helicobacter pylori is required for its biological activity. EMBO J. 1999;18:5517–5527. doi: 10.1093/emboj/18.20.5517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Telford J L, Ghiara P, Dell'Orco M, Comanducci M, Burroni D, Bugnoli M, Tecce M F, Censini S, Covacci A, Xiang Z, Papini E, Montecucco C, Parente L, Rappuoli R. Gene structure of Helicobacter pylori cytotoxin and evidence of its key role in gastric disease. J Exp Med. 1994;179:1653–1658. doi: 10.1084/jem.179.5.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.von Herbay A, Schreiter H, Rudi J. Simultaneous gastric adenocarcinoma and MALT-type lymphoma in Helicobacter pylori infection. Virchows Arch. 1995;427:445–450. doi: 10.1007/BF00199395. [DOI] [PubMed] [Google Scholar]
- 33.Wirth H P, Beins M H, Yang M, Tham K T, Blaser M J. Experimental infection of Mongolian gerbils with wild-type and mutant Helicobacter pylori strains. Infect Immun. 1998;66:4856–4866. doi: 10.1128/iai.66.10.4856-4866.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wotherspoon A C, Doglioni C, Diss T C, Pan L, Moschini A, de Boni M, Isaacson P G. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet. 1993;342:575–577. doi: 10.1016/0140-6736(93)91409-f. [DOI] [PubMed] [Google Scholar]