Graphical abstract
Keywords: Intradermal vaccination, Chemical adjuvant, Physical adjuvant, Laser adjuvant, Radiofrequency adjuvant, Local reactogenicity
Abstract
The majority of vaccines have been delivered into the muscular tissue. Skin contains large amounts of antigen-presenting cells and has been recognized as a more immunogenic site for vaccine delivery. Intradermal delivery has been approved to improve influenza vaccine efficacy and spare influenza vaccine doses. In response to the recent monkeypox outbreak, intradermal delivery has been also approved to stretch the limited monkeypox vaccine doses to immunize more people at risk. Incorporation of vaccine adjuvants is promising to further increase intradermal vaccine efficacy and spare more vaccine doses. Yet, intradermal vaccination is associated with more significant local reactions than intramuscular vaccination. Thus, adjuvants suitable to boost intradermal vaccination need to have a good local safety without inducing overt local reactions. This review introduces currently approved adjuvants in licensed human vaccines and their relative reactogenicity for intradermal delivery and then introduces emerging chemical and physical adjuvants with a good local safety to boost intradermal vaccination. The rational to develop physical adjuvants, the types of physical adjuvants, and the unique advantages of physical adjuvants to boost intradermal vaccination are also introduced in this review.
1. Skin structure
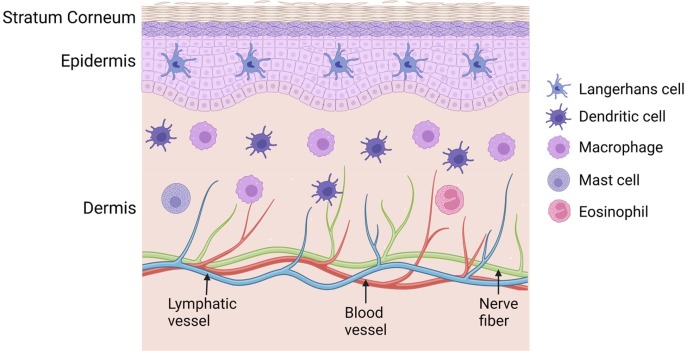
Skin covers the entire body with important physiological functions, such as prevention of heat and water loss and protection against environmental pathogen infection. Skin can be divided into 3 layers: stratum corneum (SC), epidermis, and dermis (Nguyen and Soulika, 2019). SC layer is situated at the outmost layer of the skin. SC layer is made of corneocytes, the terminally differentiated keratinocytes, and is highly lipophilic (Menon, 2002). SC layer plays a major barrier function of the skin (Menon, 2002). Epidermal layer is situated underneath the SC layer and is made primarily of keratinocytes (∼90%) with Langerhans cells (LCs, 3–5%) scattered in between to serve as immune sentinels (Deckers et al., 2018, Mestrallet et al., 2021). Epidermal layer also contains melanocytes to give skin a special color depending on its densities and melanin levels. Underneath the epidermal layer is the dermal layer that contains more anatomical structures than the SC and epidermal layers. Sweat glands and hair follicles are contained in the dermal layer together with blood and lymphatic vessel networks and nerve fibers (Nguyen and Soulika, 2019). Dermal layer also contains diverse types of cells that include fibroblasts and innate immune cells, such as dendritic cells (DCs), macrophages, mast cells, and eosinophils (Nguyen and Soulika, 2019). Interestingly, skin also contains a good number of T lymphocytes with the majority of them to be skin-resident memory T cells in both epidermal and dermal layers (Lafouresse and Groom, 2018). Skin-resident innate immune cells serve as the first line of defense against pathogen infection and also contribute to the induction of pathogen or vaccine-specific adaptive immunity. A simplified skin model with resident innate immune cells was shown in Fig. 1 .
Fig. 1.
Illustration of skin structures and resident innate immune cells Skin contains 3 major layers: stratum corneum, epidermis, and dermis. Epidermis contains Langerhans cells and dermis contains dendritic cells, macrophages, mast cells, and eosinophils as major innate immune cells.
2. Intradermal vaccination and benefits
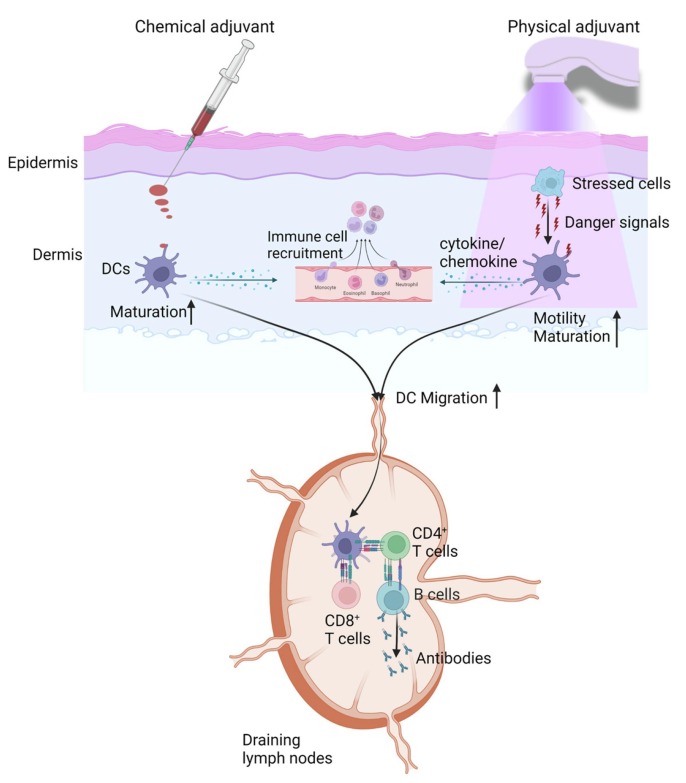
The majority of vaccines have been delivered into the muscular tissue due to the convenience of intramuscular injection with minimal training. A large number of studies in the last 2–3 decades found modification of vaccine administration from intramuscular route to intradermal route could induce more potent immune responses (Sticchi et al., 2010, Combadiere and Liard, 2011, Kim and Prausnitz, 2011). The more immunogenic intradermal vaccination can be largely ascribed to the presence of rich antigen-presenting cells, such as epidermal LCs and dermal DCs, in the skin rather than the muscle. Antigen-presenting cells (mainly dermal DCs) are responsible for the uptake and presentation of intradermal vaccine antigens on major histocompatibility complex (MHC) I or II molecules followed by migration to draining lymph nodes to elicit antigen-specific T and B cell responses. Intradermal vaccines may also activate local innate immune cells to secret cytokines and chemokines and recruit circulating innate immune cells, contributing to vaccine-induced local reactions.
Intradermal delivery is promising to improve vaccine efficacy and spare vaccine doses. A recent review study found intradermal delivery of influenza vaccines was typically more immunogenic than intramuscular or subcutaneous delivery in the elderly (Kennedy, 2022). Intradermal delivery of reduced doses of influenza vaccines (6 or 9 µg) was found to elicit comparable immune responses to full-dose (15 µg) intramuscular vaccination in young adult counterparts (Icardi et al., 2012, Belshe et al., 2004). Besides influenza vaccines, intradermal delivery is also effective to enhance immune responses induced by other vaccines, such as rabies vaccine and hepatitis B vaccine (Kyi et al., 2002, Verma et al., 2011). Due to the recent monkeypox outbreak and the shortage of monkeypox vaccines, the Food and Drug Administration (FDA) authorized intradermal delivery of monkeypox vaccines to stretch vaccine doses to immunize more people at risk (Brooks et al., 2022).
3. Limitations of intradermal vaccination
Although intradermal delivery improves vaccine immunogenicity, the degree of improvement is relatively weak. Intradermal delivery only spares influenza vaccine doses by 40–60%. Intradermal delivery in the elderly only increased seroconversion rates from 40% to 52.3% for H1N1 strain, from 49.1% to 61.3% for H3N2 strain, and from 56.4% to 61.3% for type B strain (Boonnak et al., 2017). Similar results were found in another study with a slight increase of seroconversion rates by intradermal delivery of influenza vaccines in the elderly (Arnou et al., 2009).
Incorporation of vaccine adjuvants is expected to further increase vaccine efficacy and spare more vaccine doses. Yet, adjuvanted intradermal vaccines face a risk to induce significant local reactions. Studies consistently found intradermal vaccine delivery without adjuvants induced more frequent and severe local adverse reactions than intramuscular vaccination (Skountzou et al., 2017, Chen and Wu, 2011). This can be explained by the residence of various types of innate immune cells in the skin (e.g., LCs, DCs, macrophages). These cells contribute to increased antigen uptake and more potent adaptive immunity and at the same time are responsible for local adverse reactions by synthesis and release of cytokines and chemokines and recruitment of peripheral neutrophils, monocytes, and eosinophils. Thus, adjuvants for intradermal vaccination need to have a better local safety than those used in intramuscular vaccination.
This review focuses on introducing adjuvants to boost intradermal vaccination against infectious disease although this route is also attractive to vaccination against other diseases, such as allergies and malignant tumors. This review also focuses on introducing adjuvants to boost intradermal protein and subunit vaccine-induced immune responses. In addition, vaccine/adjuvant formulations (e.g., polymer nanoparticles, liposomes) with adjuvant effects at least partly contributed by the co-delivery of vaccine/adjuvants were not included in this review.
4. Approved adjuvants and their intradermal reactogenicity
Vaccine adjuvants received increasing attention in the 21st century due to their critical roles in developing new and improved vaccines (Reed et al., 2013, McKee and Marrack, 2017, Nanishi et al., 2020, Petrovsky and Aguilar, 2004, Di Pasquale et al., 2015). The National Institute of Allergy and Infectious Diseases (NIAID) launched ‘Adjuvant Development Program’ in 2008 to support the screening, identification, and preclinical/clinical development of novel adjuvants. With increasing interests and investments in this field, the last two decades have seen the approval of five adjuvants for human use whereas the preceding 80 years had only two adjuvants approved for human use (Table 1 ) (Li et al., 2021c). Interestingly, all these adjuvants were approved for intramuscular delivery. Their tendency to induce local adverse reactions following intradermal delivery was predicted based on their local reactogenicity following intramuscular delivery if the intradermal reactogenicity data were not available. Local adverse reactions can be skin erythema, swelling, discoloration, or histological changes, such as tissue necrosis or cell deaths, or local inflammatory responses, such as cytokine and chemokine release and immune cell infiltration.
-
•
Alum adjuvant
Table 1.
Currently approved adjuvants and their intradermal reactogenicity.
| Adjuvants | Description | Year of approval | Vaccines | Th1/Th2 | Particle size | Intradermal reactogenicity |
|---|---|---|---|---|---|---|
| Alum | Aluminum salts | 1930s | Tetanus and diphtheria vaccines, etc. | Th2-dominant | 0.5–10 µm | +++ |
| MF59 | Squalene nanoemulsion (Novartis) | 1997 | Seasonal influenza vaccine | Th2-biased (weak Th1) | 160 nm | ++ |
| AS04 | MPL adsorbed on Alum adjuvant | 2009 | Human papillomavirusvaccine | Balanced Th1/Th2 | 0.5–10 µm | ++++ |
| AS03 | Squalene nanoemulsion (GlaxoSmithKline) | 2013 | Pre-pandemic H5N1 vaccine | Th2-biased (weak Th1) | 160 nm | ++ |
| AS01 | MPL/QS21 in liposome | 2015 | RTS,S malaria vaccine | Th1-biased (weak Th2) | 106 nm | ++++ |
| CpG 1018 | 22-mer oligonucleotide | 2017 | Hepatitis B vaccine | Th1-dominant | – | + |
| Matrix-M | Two distinctive nanoparticle formulations prepared from saponin fraction A and C | 2022 | Protein-based Covid-19 vaccine | Th1-biased (weak Th2) | 40 nm | ++ |
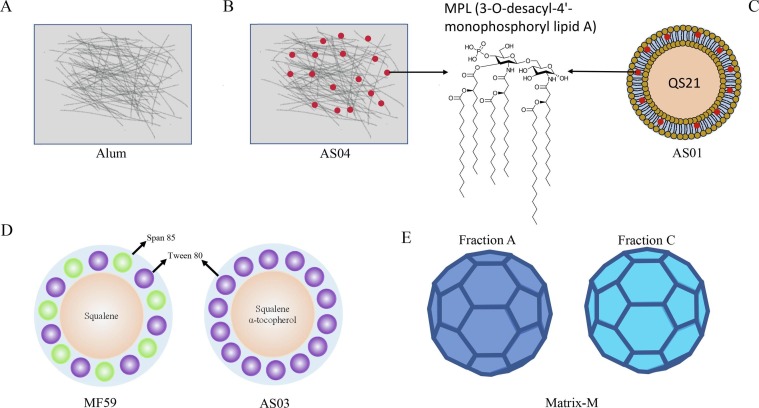
Alum adjuvant is an aluminum salt-based adjuvant. Clinical Alum adjuvant is mainly based on aluminum hydroxide that forms a broad range of micrometer structures (0.5–10 µm) due to aggregation of fibrous nanoparticles (Fig. 2 A) or aluminum phosphate that forms plate-like nanoparticles (Shardlow et al., 2016, HogenEsch et al., 2018). Alum adjuvant has been the most widely used adjuvant in the globe and it has been incorporated in a number of childhood vaccines, such as Tdap (tetanus, diphtheria, and pertussis), hepatitis B, haemophilus influenzae type b vaccines (Di Pasquale et al., 2015). Alum adjuvants mainly enhance T helper 2 (Th2)-biased antibody responses with weak ability to induce cell-mediated immune responses (HogenEsch et al., 2018, Marrack et al., 2009).
Fig. 2.
Illustration of currently approved particulate adjuvants A. Aluminum hydroxide adjuvant. B. AS04 adjuvant prepared by adsorption of MPL (3-O-desacyl-4′-monophosphoryl lipid A) on Alum hydroxide adjuvant. C. AS01 adjuvant prepared by encapsulation of MPL and QS21 in liposomes. D. Squalene nanoemulsion-based MF59 and AS03 adjuvant. E. Matrix-M adjuvant composed of two nanoparticle formulations made from two fractions of saponin extracts (fraction A and C).
Injection of alum adjuvant into the muscular tissue induced significant tissue stress and cell deaths (McKee et al., 2013; Marichal et al., 2011). Similar phenomenon was observed following intraperitoneal injection of alum adjuvants (Franchi and Nunez, 2008, Kool et al., 2008, Li et al., 1950). Intramuscular injection of Alum adjuvant induced significant cytokine/chemokine release and rapidly recruited neutrophils, monocytes, and eosinophils (Calabro et al., 2011, McKee et al., 2009, Lu and Hogenesch, 2013). Intradermal injection of Imject (an experimental alum adjuvant) in mouse skin was found to induce infiltration of a high density of inflammatory cells that lasted for at least four days (Chen and Wu, 2011). Due to the induction of significant tissue stress and inflammation, Alum adjuvants have a high risk to induce significant local reactions following intradermal delivery.
-
•
AS04 adjuvant
AS04 adjuvant is a combinatorial adjuvant prepared by adsorption of MPL (3-O-desacyl-4′-monophosphoryl lipid A) on Alum hydroxide adjuvant (Fig. 2B) (Nanishi et al., 2020, Laupeze et al., 2019). MPL is a toll-like receptor (TLR) 4 agonist (Dowling and Mansell, 2016). MPL/TLR4 binding activates myeloid differentiation primary response 88 (MyD88) and downstream signaling pathways, leading to nuclear factor-κB (NFκB) and activator protein (AP)-1 activation and proinflammatory cytokine gene expression (Takeda and Akira, 2005). AS04 was approved to boost human papillomavirus vaccine efficacy(Garcon et al., 2011). AS04-adjuvanted vaccines were found to induce higher levels of cytokines and chemokines at local injection site and recruit higher numbers of monocytes and DCs in the draining lymph nodes than Alum-adjuvanted vaccines (Laupeze et al., 2019). AS04-adjuvanted vaccines were found to induce more frequent local reactions, such as pain, redness, and swelling, than vaccines without adjuvant (Laupeze et al., 2019). Local adverse reactions were found to resolve in a few days (Laupeze et al., 2019). AS04 adjuvant was found to induce more balanced T helper 1 (Th1)/Th2 immune responses (Laupeze et al., 2019). Our prior study found intradermal injection of MPL/Alum adjuvant induced erythema and swelling accompanied with infiltration of large numbers of inflammatory cells in the dermal tissue of the skin (Chen and Wu, 2011). These data indicated the high risk of AS04 adjuvant to induce significant local reactions following intradermal delivery.
-
•
AS01 adjuvant
AS01 is a liposome formulation containing MPL and QS21, a saponin component purified from the soap bark tree (Quillaja saponaria) (Fig. 2C) (Laupeze et al., 2019). AS01 adjuvant has been approved to boost malaria RTS,S vaccine efficacy (Gosling and von Seidlein, 2016, Laurens, 2020). AS01 adjuvant was found to induce Th1-dominant immune responses with weak induction of Th2 responses (Coccia et al., 2017).
Rapid clearance of AS01 adjuvant was found from adjuvant-injected muscle with significantly reduced AS01 levels at 3 h and barely detectable AS01 levels at 24 h (Didierlaurent et al., 1950). AS01 induced a transient expression of cytokines that peaked within 24 h and subsided rapidly after (Didierlaurent et al., 1950). AS01 also induced rapid recruitment of neutrophils and monocytes into adjuvant-injected muscle (Didierlaurent et al., 1950). Considering AS01 contains MPL, local cytokine and chemokine stimulation is expected to be at least partially contributed by stimulation of TLR4 and downstream MyD88 signaling pathways. Studies found MPL and QS21 synergistically activated early interferon (IFN) γ production in lymph node-resident natural killer (NK) and CD8+ T cells, which was controlled by subcapsular macrophages via interleukin (IL)-18 secretion and supported by IL-12 (Coccia et al., 2017). The synergistic effects of MPL and QS21 and the strong induction of local inflammation suggested a high risk of AS01 adjuvant to induce significant local reactions after intradermal delivery.
-
•
MF59 and AS03 adjuvants
MF59 and AS03 are squalene-based oil-in-water nanoemulsion adjuvants (O’Hagan et al., 2013; Garcon et al., 2012, O'Hagan et al., 2013). Squalene is a natural triterpene oil existing as a biochemical intermediate in cholesterol synthesis in animals and humans (O'Hagan et al., 2013). The biodegradable nature of squalene makes it a safe component in adjuvant formulations. MF59 and AS03 are developed by Novartis and GlaxoSmithKline (GSK), respectively, with slightly different chemical compositions (Fig. 2D). MF59 contains squalene as the major oil-phase component, while AS03 contains squalene and α-tocopherol (vitamin E) in the oil phase (O’Hagan et al., 2013; Garcon et al., 2012, O'Hagan et al., 2013). α-tocopherol is also biodegradable with immune-modulating functions(Morel et al., 2011). Regarding surfactant, MF59 contains two surfactants (Tween 80 and Span 85), while AS03 only contains polysorbate 80 (Tween 80) (O’Hagan et al., 2013; Garcon et al., 2012, O'Hagan et al., 2013). MF59 was initially approved to boost seasonal influenza vaccine efficacy in the elderly in Europe (O'Hagan et al., 2013). MF59 was recently approved to enhance seasonal influenza vaccine (Fluad Quadrivalent) efficacy in the elderly and also the pre-pandemic H5N1 vaccine (Audenz) efficacy in the United States. AS03 was initially approved to enhance influenza pandemic 2009 H1N1 vaccine (Pandemrix) efficacy during the 2009 H1N1 influenza pandemic in Europe and later approved to enhance the pre-pandemic H5N1 vaccine efficacy in the United States (Pulendran and O'Hagan, 2021, Godeaux et al., 2015). MF59 and AS03 adjuvants were found to induce Th2-biased immune responses with weak induction of Th1 responses (Lee and Nguyen, 2015, Ko and Kang, 2018).
Intramuscular injection of MF59 adjuvant was found to induce tissue stress and cell deaths (Vono et al., 2013). Furthermore, MF59 was found to be cleared independent of antigens with a clearance half-life of 42 h in the muscle (Awate et al., 2013, Dupuis et al., 1999). Intramuscular injection of MF59 was found to more vigorously recruit neutrophils, monocytes, and eosinophils than intramuscular injection of Alum despite the same pattern of temporal recruitment (Calabro et al., 2011). MF59 induced a diverse pattern of cytokine and chemokine release as compared to Alum adjuvant after intramuscular injection (Calabro et al., 2011). Intradermal injection of MF59-like AddaVax adjuvant induced vigorous recruitment of neutrophils, monocytes, and eosinophils with significantly elevated neutrophil and monocyte levels 4 days after injection (Cao et al., 2018). Intradermal injection of AddaVax also induced prolonged expression of various cytokines and chemokines for at least 4 days (Cao et al., 2018). These data hinted a high risk of MF59 to induce significant local reactions after intradermal delivery.
Intramuscular injection of AS03 adjuvant was found to induce persistent cytokine expression (e.g., C–C motif chemokine ligand 2 (CCL2), IL-1β) for at least 7 days (Morel et al., 2011). AS03 induced more profound cytokine expression than Alum adjuvant (Morel et al., 2011). α-tocopherol in AS03 was found to significantly increase cytokine expression via activation of macrophages and monocytes (Morel et al., 2011). AS03 adjuvant was found to induce a rapid and transient downregulation of lipid metabolism, resulting in increased intracellular lipid levels and induction of endoplasmic reticulum (ER) stress and activation of unfolded protein response pathway (Givord et al., 2018). Furthermore, depletion of ER stress kinase sensor inositol-requiring transmembrane kinase endoribonuclease-1α (IRE1α) in myeloid cells decreased AS03-induced cytokine expression and the induction of high-affinity antibodies (Givord et al., 2018). The strong induction of local inflammatory cytokines and chemokines and ER stress hinted AS03 adjuvant might also induce significant local reactions following intradermal delivery.
-
•
Matrix-M adjuvant
Matrix-M adjuvant is made of two distinctive 40-nm particle formulations from two fractions of saponin extracts (fractions A and C) (Fig. 2E) and has been incorporated in the recently approved protein-based Novavax Covid-19 vaccine (NVX-CoV2373) for emergency use during the Covid-19 pandemic (Lovgren Bengtsson et al., 2011, Heath et al., 2021). Each nanoparticle formulation was made of saponin fraction A or C with cholesterol and phospholipids (Lovgren Bengtsson et al., 2011). Matrix-M adjuvant was found to induce Th1-biased immune responses (Lovgren Bengtsson et al., 2011).
Matrix-M-adjuvanted Covid-19 vaccine was found to induce more frequent local reactions than the placebo (first dose: 57.6% vs. 17.9%; second dose: 79.6% vs. 16.4%) (Heath et al., 2021). Most of the local adverse reactions were mild or moderate and resolved in 3 days (Heath et al., 2021). Matrix-M adjuvant was found to stimulate porcine monocytes and lymphocytes to express IL-1β and C-X-C motif chemokine ligand 8 (CXCL8) but not tumor necrosis factor α (TNFα) or IL-6 (Ahlberg et al., 2017)(. Intramuscular injection of Matrix-M adjuvant in pigs was found to induce acute inflammation characterized as infiltration of neutrophils, monocytes, lymphocytes (Ahlberg et al., 2012). Local adverse reactions also included hemorrhage in most of the pigs and necrosis in some pigs (Ahlberg et al., 2012). More frequent redness was found in Matrix-M group than saline control group (Ahlberg et al., 2012). Matrix-M adjuvant was found to significantly increase 384 gene expression and decrease 162 gene expression as compared to control (Ahlberg et al., 2012). Among the most up-regulated genes, 10 out of 23 was related to interferon-regulated genes (IRGs) (Ahlberg et al., 2012). Although no study so far explored the potency and safety of Matrix-M to boost intradermal vaccination, one study found intradermal delivery of QS-21 in liposomes (another saponin-based adjuvant) dose-dependently induced local adverse reactions, such as erythema, lump, and scab, in swine models (Poirier et al., 2017).
The more frequent local adverse reactions following intramuscular immunization in human subjects, the strong local inflammatory responses and hemorrhage and necrosis in at least some pigs, and the dose-dependent induction of local adverse reactions following intradermal delivery of a closely related QS-21 adjuvant indicated a high risk of Matrix-M adjuvant to induce significant local reactions following intradermal delivery.
-
•
CpG 1018
CpG 1018 is an unmethylated CpG motif-containing oligonucleotide (Campbell, 2017). CpG 1018 activates TLR9 and MyD88 signaling pathways that lead to activation of various transcriptions factors, like NFκB, AP-1 and interferon regulator factor (IRF), and their controlled cytokine expression, such as TNFα, IL-1β, type I interferon, and IL-12 (Bode et al., 2011). CpG 1018 stimulate Th1-biased antibody responses and cell-mediated immune responses (Campbell, 2017, Bode et al., 2011). CpG 1018 has been incorporated in a recently approved hepatitis B vaccine (Campbell, 2017). CpG 1018-adjuvanted hepatitis B vaccine was found to elicit more potent immune responses than Alum-adjuvanted hepatitis B vaccine (Campbell, 2017). Different from species-specific CpG oligonucleotides, CpG 1018 has broad responsiveness in rodents, non-human primates, and humans and thus eliminates the needs to change CpG sequences in preclinical and clinical studies (Campbell, 2017). Intradermal injection of a murine-specific CpG was found to induce minimal local reactions with a low-level immune cell recruitment (Chen and Wu, 2011). CpG 1018 is expected to have low intradermal reactogenicity and is promising to safely boost intradermal vaccination.
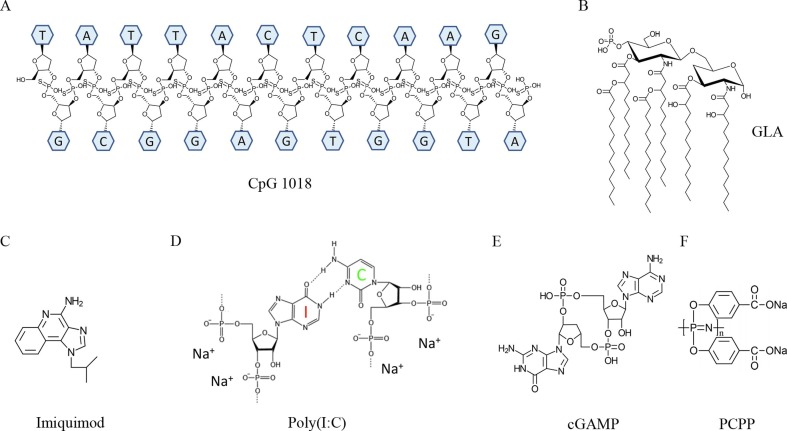
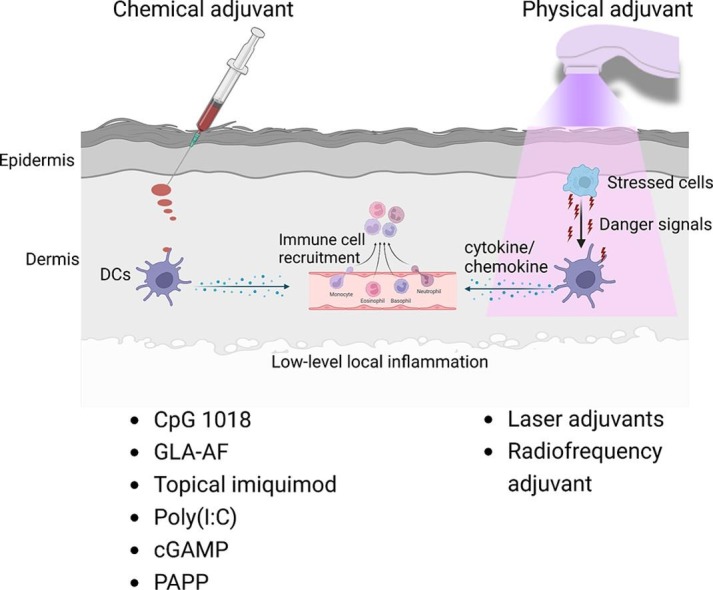
In summary, most of the approved adjuvants have a high risk to induce significant local reactions following intradermal delivery. Interestingly, chemical adjuvants with predicted high intradermal reactogenicity are particulates in nature (Table 1), which strongly activate local innate immune systems and induce significant local reactions. CpG 1018 may be an exception due to its induction of mild inflammation without overt local reactions (Fig. 3 A).
Fig. 3.
Chemical structures of safe adjuvants for intradermal vaccination A. CpG 1018. DNA backbone has phosphorothioate modification to improve adjuvant stability. B. Synthetic GLA with six 14-carbon side chains. C. Imiquimod. D. Poly(I:C). E. cGAMP. F. PCPP polymer.
5. Other chemical adjuvants for safe intradermal vaccination
Concerned about the high local reactogenicity of approved adjuvants, safety and efficacy of other chemical adjuvants were also explored to boost intradermal vaccination. Several of these adjuvants have advanced to clinical tests. Due to the risk of vaccine adjuvants to induce significant local reactions after intradermal delivery, this review didn’t include adjuvants without local safety data reported.
-
•
GLA-AF adjuvant
Several approved adjuvants, such as AS01 and AS04, contain MPL as a major component to boost vaccination. Although MPL has not been approved as a standalone adjuvant, we found intradermal injection of MPL induced mild inflammation with no overt local adverse reactions in murine models (Chen and Wu, 2011). Carter et al. evaluated a synthetic TLR4 agonist glucopyranosyl lipid adjuvant (GLA)-aqueous formulation (AF) (Fig. 3B) to boost intradermal H5N1 vaccination in ferrets and humans and found GLA-AF was critical for the intradermal H5N1 vaccine to induce single-shot protection in ferrets and elicit seroprotection in humans (Carter et al., 2018). Furthermore, GAL-AF-incorporated H5N1 vaccine induced no temperature spikes or body weight losses in guinea pigs and induced transient erythema with no other noticeable adverse reactions in human clinical studies (Carter et al., 2018). This study indicated the potency and safety of GLA-AF and possibly other TLR4 agonists for intradermal vaccination.
-
•
Topical imiquimod adjuvant
Topical imiquimod cream (5% Aldara) is approved by FDA to treat various medical conditions, such as actinic keratoses, superficial basal cell carcinoma, and external genital warts (Wagstaff and Perry, 2007). Imiquimod is a TLR7 agonist (Fig. 3C) and activates TLR7-MyD88 signaling pathways, leading to NFκB and IRF7 activation and proinflammatory cytokine and type I interferon gene expression (Hemmi et al., 2002). Interestingly, the vehicle of the topical imiquimod cream might also contribute to the observed therapeutic effects due to the induction of inflammasome activation, keratinocyte deaths, and IL-1 release in the absence of imiquimod (Walter et al., 2013). Topical application of imiquimod cream before intradermal influenza vaccination elicited higher seroconversion rates against vaccine viral strains and better seroconversion rates against non-vaccine viral strains in a double-blinded, randomized, and controlled clinical trial (Hung et al., 2016). The most common local adverse reactions were grade 1 redness and swelling, which were more frequent in topical imiquimod group despite the lack of statistically significant difference from other groups (Hung et al., 2016). Topical imiquimod cream followed by intradermal hepatitis B vaccination induced significantly higher seroprotection rates in inflammatory bowel disease (IBD) patients than topical aqueous cream followed by intramuscular vaccination (Ko et al., 2022). No significant difference in local adverse reactions was found between groups although topical imiquimod group induced more frequent itch and swelling (Ko et al., 2022). These results indicated topical imiquimod cream could significantly increase intradermal vaccine efficacy with an overall good local safety.
-
•
Poly(I:C) adjuvant
Polyinosinic-polycytidylic acid (Poly(I:C)) is a synthetic analogue of double-stranded RNA (dsRNA, Fig. 3D) and activates two distinct signaling pathways depending on locations (Coffman et al., 2010). Endosomal poly(I:C) activates TLR3 and induces IL-12 and type I IFN production, while cytosolic poly(I:C) activates retinoic acid-inducible gene I (RIG-I) and melanoma differentiation-associated protein 5 (MDA-5) and enhances type I IFN production (Coffman et al., 2010). Poly (I:C) has been also explored in intradermal or transdermal vaccination to induce potent immune responses against various types of infectious diseases. Weldon et al. found poly(I:C)-adjuvanted subunit influenza vaccine elicited similar immune responses and protection to subunit vaccine alone when coated on microneedle surface in murine models (Weldon et al., 2012). The lack of significant adjuvant effects could be related to the relatively low poly(I:C) dose (1 µg) used in this study (Weldon et al., 2012). In a different study, Bardel et al. found 25 µg poly(I:C) could significantly enhance intradermal HIV-1 gp140 or HSV-2 gD glycoprotein-induced systemic and mucosal antibody responses as well as protection in HSV-2 challenge studies without overt skin reactogenicity in murine models(Bardel et al., 2016). This study indicated the good safety and potency of intradermal poly(I:C)-adjuvanted HIV-1 gp140 or HSV-2 gD glycoprotein vaccine to induce potent mucosal immunity. Du et al. found encapsulation of diphtheria toxin (DT) and poly(I:C) into liposomes followed by hollow microneedle-based intradermal delivery could significantly enhance DT-specific IgG2a antibody responses when compared to hollow microneedle-based intradermal delivery of DT alone (Du et al., 2018). Interestingly, relatively low poly(I:C) dose (0.31 µg) was used in this study and no significant enhancement of total IgG or IgG1 antibody responses was observed (Du et al., 2018). Recently, poly(I:C) stabilized with poly-L-lysine was tested in a phase 1 clinical study to enhance HPV peptide vaccination in combination with photochemical internalization (PCI), a technology to facilitate the cytosol release of endocytosed antigens via a light-induced process to promote the induction of cell-mediated immune responses (Otterhaug et al., 2020). The overall approach was found to be safe in study subjects when the photosensitizer dose was below certain threshold although the potency of poly(I:C)-based adjuvant to enhance HPV peptide vaccination was not studied (Otterhaug et al., 2020). The above studies indicated good safety of poly(I:C) adjuvant to boost intradermal vaccination.
-
•
cGAMP adjuvant
2′3′-cyclic GMP-AMP (cGAMP, Fig. 3E) is a stimulator of intracellular stimulator of interferon genes (STING) pathway, leading to NFκB and IRF3 activation and proinflammatory cytokine and type I IFN gene expression (Li and Chen, 2018). cGAMP and its analogues, such as cyclic di-AMP (cdAMP) and cyclic di-GMP (cdGMP), have been explored as effective adjuvants to induce Th1 immune responses against infectious diseases and cancer (Hernandez-Franco et al., 2021, Wang et al., 2016). Intradermal administration of cGAMP together with influenza H5N1 or pandemic 2009 H1N1 vaccine was found to significantly enhance vaccine-induced immune responses without evoking significant local adverse reactions in murine and swine models (Wang et al., 2016). Furthermore, cGAMP was found to be a more potent adjuvant than CpG or MPL to boost intradermal H5N1 immunization in murine models (Wang et al., 2016). Recently, Hernandez-Franco et al. explored the safety and efficacy of cdAMP and a plant-based cationic α-D-glucan nanoparticles (Nano-11) to enhance intradermal ovalbumin (OVA) immunization in mice and pigs and found cdAMP-adjuvanted intradermal OVA immunization induced a transient discoloration 24 h after injection, which disappeared 14 days later, while Nano-11-adjuvanted intradermal OVA immunization induced no visible adverse reactions (Hernandez-Franco et al., 2021). These studies support STING agonists as safe adjuvants to boost intradermal vaccination.
-
•
PCPP adjuvant
Microneedle has been an attractive technique to deliver vaccines into the skin (Prausnitz, 2017). Vaccines can be coated on the surface of metal or non-dissolvable polymer microneedles or encapsulated into dissolving polymer microneedle fabrications (Prausnitz, 2017). Considering microneedles usually induce comparable immune responses to intramuscular injection deliveries (Rouphael et al., 2017, Sullivan et al., 2010), safe adjuvants have been explored to boost microneedle-based intradermal vaccination. Andrianov et al. found poly[di(carboxylatophenoxy)phosphazene] (PCPP, Fig. 3F), a synthetic, water-soluble, and biodegradable polyphosphazene polymer with broad applications in drug delivery and tissue engineering, to be a safe adjuvant to boost microneedle-based intradermal hepatitis B vaccination in pigs (Andrianov et al., 2009). Furthermore, PCPP could serve as a key microneedle vaccine fabrication material to reduce the dependence on surfactants (Andrianov et al., 2009). Mildly red skin marks and no severe adverse reactions were observed in this study (Andrianov et al., 2009). Besides PCPP, a new polyphosphazene polymer (poly[di(sodium carboxylatoethylphenoxy)phosphazene] (PCEP)) was found to safely boost intradermal influenza H1N1 vaccination in piglets despite the lack of heterosubtypic protection against H3N2 viruses (Magiri et al., 2020).
In summary, chemical adjuvants with low intradermal reactogenicity are usually water-soluble or prepared as aqueous formulations to permit quick clearance from local delivery sites. These adjuvants are mostly based on pathogen-associated molecular patterns (PAMPs) or their synthetic analogues and activate pattern-recognition receptors (PRRs), such as TLR3, TLR4, TLR7, TLR9, and STING, to mediate their adjuvant effects without over activation of the innate immune systems. Although PCPP is not a PAMP, PCPP was found to directly activate immune cells through TLR3, TLR4 and TLR9 (Magiri et al., 2018). These studies indicated the safety of certain PRR agonists as intradermal adjuvants.
6. Physical adjuvants for safe intradermal vaccination
Instead of injection of chemical adjuvants, brief application of physical energies on skin surface prior to intradermal vaccine delivery has been also explored to boost intradermal vaccination.
-
•
Rational of development
Adjuvant development in the past was largely relied on empirical experience or trial and error approaches due to the lack of understanding on how adjuvant works, especially those non-PAMP-based adjuvants, such as Alum, MF59, and QS21 (Di Pasquale et al., 2015, Garcon and Di Pasquale, 2017). These adjuvants have no specific cellular receptors to mediate their adjuvant effects and thus direct activation of innate immune cells, such as DCs and macrophages, is not expected (Coffman et al., 2010, O'Hagan et al., 2012, Marciani, 2003). Instead, these adjuvants are more likely to stimulate tissue stress and cell deaths to release endogenous danger signals or damage-associated molecular patterns (DAMPs) to mediate their adjuvant effects. ‘Danger theory’ was proposed in 1990s by Matzinger and her colleagues to demonstrate that the immune systems were activated by danger signals released from stressed or damaged cells (Yatim et al., 2017, Kono and Rock, 2008). ‘Danger theory’ explained the induction of adaptive immunity in the absence of foreign pathogen invasion, such as transplantation rejection, certain chemotherapy-induced systemic activation of anti-tumor immunity (Yatim et al., 2017, Kono and Rock, 2008). Various types of DAMPs have been identified in the last 2–3 decades that include small chemicals, such as uric acid and ATP, and macromolecules, such as dsDNA, with the ability to modulate immune system function in line with the ‘danger theory’(Schaefer, 2014, Shi et al., 2003). These materials are sequestered from immune system recognition in physiological conditions and can be released under tissue stress or cell deaths to activate immune systems (Yatim et al., 2017, Kono and Rock, 2008, Schaefer, 2014). Alum adjuvant was found to stimulate uric acid release to activate NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome and Caspase 1 activation to partly mediate its proinflammatory responses (Kool et al., 2008). Alum adjuvant was found to also stimulate host DNA release to induce IgG1 antibody production and IgE class switching and also prolong DC and CD4+ T cell interactions (McKee et al., 2013; Marichal et al., 2011). MF59 adjuvant was found to stimulate ATP release to potentiate immune responses to vaccination (Vono et al., 2013). These advances hinted physical modalities that stimulated well-controlled tissue stress or cell deaths might induce endogenous danger signal release to boost vaccination.
-
•
Physical adjuvant types
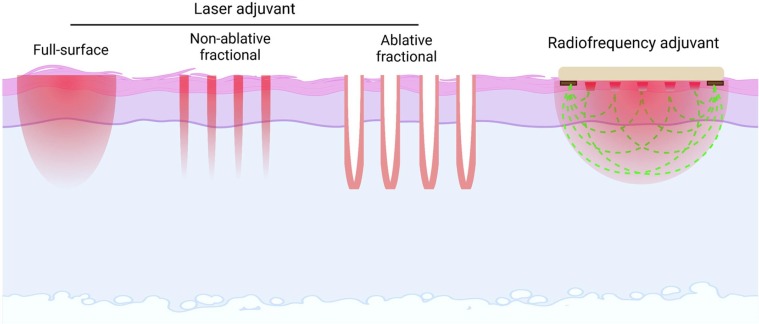
Two most studied physical energies for vaccine adjuvantation are laser and radiofrequency. The diverse types of laser adjuvants and radiofrequency adjuvant were summarized in Fig. 4 .
-
•
Laser adjuvants
Fig. 4.
Diverse types of physical adjuvants A brief illustration of different types of laser adjuvants (full-surface, non-ablative fractional, ablative fractional) and radiofrequency adjuvant.
Laser emits a narrow wavelength of light, which can be absorbed by specific tissue chromophores (Parker, 2007). Visible light is mainly absorbed by melanin and hemoglobin, while near-infrared light is mainly absorbed by tissue water (Parker, 2007). Laser has unique applications in aesthetics and medicine. Due to its ultrafine beams, high-energy laser has been used in bladeless laser-assisted in situ keratomileusis (LASIK) with minimal postoperative complications (Khalkhal et al., 2019). Laser has been also used in low-level laser therapy to stimulate wound healing and pain relief (Andrade Fdo et al., 2014, Huang et al., 2015). Laser has been also used in skin resurfacing, birthmark and wrinkle removal (Alexiades-Armenakas et al., 2008, Preissig et al., 2012).
Several lasers have been explored to boost intradermal vaccination. Onikienko et al. explored a copper vapor laser emitting two wavelengths of light (510 nm and 578 nm) to enhance vaccine-induced immune responses by induction of persistent local inflammation, resembling that of chemical adjuvants (Kashiwagi et al., 2014). We explored non-invasive Q-Switched Nd:YAG 532 nm laser treatment to enhance intradermal vaccine-induced immune responses and found the non-invasive laser treatment could significantly enhance model antigen OVA and influenza vaccine-induced immune responses in murine models without induction of visible or histological skin damages (Chen et al., 2010). Laser treatment induced little cytokine expression or immune cell recruitment (Chen et al., 2010). Further studies found the non-inflammatory laser treatment significantly enhanced migration of MHC II+ cells (e.g., macrophages and DCs) within the skin (Chen et al., 2010). Enhanced local motility of MHC II+ macrophages and DCs likely contributed to enhanced antigen uptake and augmented adaptive immunity. We further found the laser adjuvant could enhance DC-based anti-tumor immunotherapy by promotion of DC entry into the lymphatic vessels and migration to the draining lymph nodes (Chen et al., 2012b). The laser adjuvant was found to also enhance intradermal nicotine vaccine-induced anti-nicotine antibody production (Chen et al., 2012a).
Near-infrared lasers (NIR) at 1,064 nm were also explored to boost intradermal vaccination. Non-tissue damaging NIR treatment was found to transiently induce expression of a defined set of chemokines, such as CCL2 and CCL20, and increased DC concentration in both epidermis and dermis (Kashiwagi et al., 2013). The NIR laser treatment was found to significantly increase intradermal OVA and influenza vaccine-induced immune responses in murine models (Kashiwagi et al., 2013). Further studies found the NIR increased and activated Lang+ and CD11b- DC subsets in the skin and promoted DC migration to the draining lymph nodes to enhance vaccine-induced immune responses (Morse et al., 1064). Consistent to this study, CD103+ DC subsets in the skin was found to play a crucial role to the NIR adjuvant effects (Yokomizo et al., 2021). Pilot clinical studies explored the maximal tolerated dose of the NIR treatment in humans and found the NIR promoted migration of CD1a+ LCs and CD11c+ DCs in the dermis (Gelfand et al., 2019).
Different from the non-invasive full-surface laser treatment, none-ablative fractional laser (NAFL) was also explored to boost intradermal vaccination. NAFL emits high-energy micro-laser beams and creates microthermal zones in skin surface. Dying cells in microthermal zones recruited plasmacytoid DCs (pDCs), which could be activated by topical imiquimod to boost intradermal influenza vaccination (Wang et al., 2014). NAFL treatment followed by intradermal influenza vaccination and then topical imiquimod treatment elicited better immune responses than intradermal influenza vaccination in the presence of NAFL or topical imiquimod and comparable immune responses to intramuscular influenza vaccination in the presence of AddaVax adjuvant (Wang et al., 2014). Interestingly, the combinatorial NAFL/imiquimod-adjuvanted influenza vaccine showed a good local and systemic safety in murine and swine models (Wang et al., 2014). In another study, topical imiquimod followed by NAFL treatment and then intradermal vaccination of herpes peptide vaccine was found to induce the most potent vaccine-specific CD4+ and CD8+ T cell responses and confer the most significant protection against genital herpes virus infection in murine models as compared to intradermal herpes peptide vaccination in the presence of NAFL or topical imiquimod alone (Lopes et al., 2018). These studies indicated the order of NAFL, topical Imiquimod application, and intradermal vaccination was not critical to the high immunogenicity of this group except NAFL was administered before intradermal vaccine delivery to avoid potential heat inactivation of vaccine antigens. Further studies found the NAFL stimulated release of dsDNA from dying cells and activated STING and IRF pathway to mediate its adjuvant effects (Wang et al., 2015). NAFL was also found to safely enhance microneedle-based influenza vaccination in murine and swine models (Wang et al., 2015). More detailed classification of laser vaccine adjuvants can be found in other reviews (Kashiwagi et al., 2014; Kashiwagi, 2020, Maki et al., 2021).
Besides the use of laser solely as an adjuvant, we recently developed laser-based powder delivery system (LPD) for needle-free transcutaneous vaccine delivery and adjuvantation without the need of external adjuvants (Li et al., 2021b). LPD is based on ablative fractional laser (AFL) treatment to generate skin microchannels followed by topical application of powder vaccine-coated array patches to deliver vaccines into the skin via AFL-generated skin microchannels (Li et al., 2021b). We found the Ultrapulse CO2 AFL at 10 mJ energy and 10% coverage possessed potent adjuvant effects to boost intradermal OVA immunization (Li et al., 2021b). Furthermore, AFL-based powder delivery of influenza vaccine induced more potent antibody responses than intradermal injection of the same dose of influenza vaccine (Li et al., 2021b). LPD of influenza vaccine also conferred more significant protection than intradermal injection delivery following lethal viral challenges (Li et al., 2021b). The AFL treatment induced significant cytokine and chemokine release and recruited neutrophils, monocytes, and eosinophils into microchannel-surrounding tissues (Li et al., 2021b). Due to the ablation of a small fraction of the skin and the localized inflammatory responses in close proximity to skin microchannels, AFL-treated skin showed no overt adverse reactions and achieved complete recovery in days (Li et al., 2021b). The adjuvant effects of the AFL were believed to be due to the bystander photothermal effects. Due to long pulse duration of the laser, heat generated during laser ablation dissipated to surrounding tissues and caused thermal damage of a thin layer of microchannel-surrounding tissues. dsDNA was found to release from microthermal zones and induce cytokine gene expression (Li et al., 2021b). AFL was found to also activate NLRP3 inflammasome and Caspase 1, leading to active IL-1β release (Li et al., 2021b). Interestingly, dsDNA, NLRP3 inflammasome, and IL-1β were dispensable for laser adjuvant effects and rather MyD88 contributed to laser adjuvant effects (Li et al., 2021b). Different from photothermal stress, mechanical stress applied during microneedle-based vaccine delivery was found to also enhance vaccine-induced immune responses. Ng et al. found increasing microprojection array application energy to induce mechanical stress and cell deaths enhanced vaccine (coated on microneedle surface)-induced immune responses without incorporation of chemical adjuvants (Ng et al., 2019). This approach was found to induce gene expression associated with the TNFα and NF-κB signaling pathways (Ng et al., 2019). These pioneering studies support the development of self-adjuvanted vaccine delivery technologies for more immunogenic vaccination without needle injection or chemical adjuvants.
-
•
Radiofrequency adjuvant
Radiofrequencies (RFs) are alternating electromagnetic waves. RFs at medium–high frequencies (0.3–10 MHz) generate tissue heating with broad applications in aesthetics and medicine (Lolis and Goldberg, 2012, Tatli et al., 2012, Goldberg and Gazelle, 2001). Due to its thermal heating effects, RFs have been used to ablate cancerous tissues in tumor therapy or induce neo-collagen synthesis in skin resurfacing (Lolis and Goldberg, 2012, Alexiades-Armenakas et al., 2008). In pursuit of alternative physical energies to boost intradermal vaccination, we explored the potential adjuvant effects of a bipolar RF treatment of a small area of the skin prior to intradermal vaccine delivery. RF treatment was controlled to induce significant thermal heating but not skin damage (Cao et al., 2018). We found the non-invasive RF treatment induced transient, low-level local inflammation, while chemical adjuvants, such as Alum, AddaVax, and MPL, induced persistent and more intense local inflammation (Cao et al., 2018). RF treatment was found to significantly enhance antigen uptake in dermal CD103+ and CD11b- DC subsets and promoted dermal DC migration to the draining lymph nodes (Cao et al., 2018). In addition, RF treatment significantly increased conventional DC and pDC maturation in the draining lymph nodes (Cao et al., 2018). RF treatment significantly increased OVA-induced antibody responses with adjuvant effects comparable to AddaVax and also augmented OVA-specific cellular immune responses with adjuvant effects comparable to CpG adjuvant (Cao et al., 2018). In addition, RFA was found to similarly enhance influenza pandemic 2009 H1N1 vaccine-induced antibody responses and protection to AddaVax-adjuvanted vaccine at 0.3 µg vaccine dose and more significantly enhance influenza pandemic 2009 H1N1 vaccine-induced antibody responses and protection than AddaVax-adjuvanted vaccine at 0.06 µg vaccine dose(Cao et al., 2018). The superior RFA effects at low vaccine doses hinted its excellent dose-sparing effects. In support, we found RFA could aid nanograms of H3N2 vaccine to induce potent immune responses and protection in murine models (Li et al., 2022a). Considering the prior laser adjuvant effects were primarily compared with the relatively weak Alum adjuvant, RFA represented the first physical adjuvant that showed comparable adjuvant effects to the widely used chemical adjuvant (MF59-like AddaVax) to boost influenza vaccination.
Due to the ability of RFA to aid protein or subunit vaccines to induce cell-mediated immune responses, we further explored the ability of RFA to aid in development of recombinant nucleoprotein (NP) and matrix protein 1 (M1)-based universal T cell vaccine. Universal influenza T cell vaccines mainly target conserved viral intracellular antigens, such as NP and M1 (Cargnelutti et al., 2013). Adjuvants provide a convenient strategy to aid NP and M1 to induce CD8+ T cell responses. We found the RFA could enhance intradermal NP and M1-induced CD8+ T cell responses (Li et al., 2021a). Intradermal NP/M1 immunization in the presence of RFA conferred significant protection against lethal viral challenges in murine models (Li et al., 2021a). RFA showed a similar adjuvant effect to AddaVax in boosting NP/M1 immunization (Li et al., 2021a). RFA was recently found to also broaden influenza vaccine-induced immune responses against heterologous viruses (Li et al., 2022a). The cross-protective immunity was likely to be due to its induction of cross-protective cytotoxic T lymphocytes (Li et al., 2022a).
RFA was also effective to boost intradermal H5N1 immunization in both male and female mice (Li et al., 2022b). Intradermal H5N1 vaccine in the presence of RFA (RFA/ID) induced significantly higher serum hemagglutination inhibition (HI) titer and IgG antibody titer than intradermal vaccine alone (ID) (Li et al., 2022b). After lethal viral challenges, RFA/ID but not ID group showed significantly reduced lung viral titer (Li et al., 2022b). Interestingly, significantly higher TNFα and IFNγ-secreting CD4+ and CD8+ T cells were found in the lung after challenge in RFA/ID group as compared to ID group. RFA/ID group also conferred significant protection against body weight loss. All mice survived in RFA/ID group regardless of gender, while partial mice survived in ID group (25% female mice and 75% male mice). Interestingly, weaker RFA effects were observed to boost H5N1 vaccination when compared to AddaVax adjuvant in this study.
Comparative tissue proteomics was recently used to identify uniquely expressed proteins following RFA treatment. We found RFA induced 14 uniquely expressed proteins when compared to chemical adjuvant treatment (MPL, AddaVax, Alum, MPL/Alum) (Li et al., 2023). Among the uniquely expressed proteins, heat shock protein 70 (HSP70) played a crucial role in RFA effects (Li et al., 2023). In support, significantly reduced RFA effects to boost OVA and pdm09 vaccination were found in HSP70 knockout than in wild-type mice (Li et al., 2023). Interestingly, this study found RFA induced the least change of local tissue proteome, while AddaVax adjuvant induced the most change of local tissue proteome (Li et al., 2023). The least change of local tissue proteome by RFA was in line with its induction of transient and low-level local inflammation (Cao et al., 2018).
-
•
Advantages of physical adjuvants
Physical adjuvants briefly apply physical energies on skin surface followed by intradermal vaccine delivery to enhance vaccine-induced immune responses (Fig. 5 ). Physical adjuvants are mainly delivered by medical devices and can be kept at room temperature without cold-chain storage. This differs from chemical adjuvants, which are usually kept together with vaccines and requite cold-chain storage. Physical adjuvant devices can be used repeatedly and the cost of each application can be similar to chemical adjuvants. Physical adjuvants do not need to be mixed with vaccines and thus have no need to change or modify vaccine manufacturing or administration processes. Physical adjuvants are less likely to induce significant systemic or long-term side effects, which can be a concern for chemical adjuvants. This is because physical adjuvant effects are mainly limited to the treatment site, while chemical adjuvants can migrate to draining lymph nodes and even reach the systemic circulation to activate immune systems broadly. Physical adjuvants induce well-controlled tissue stress (e.g., thermal) and permit quick skin recovery without lasting effects. Physical adjuvants often induce transient, low-level local inflammation, while chemical adjuvants often induce persistent and more intense local inflammation. Physical adjuvants mainly modify local environment to increase DC motility, induce endogenous danger signal release to enhance antigen uptake and DC maturation, or promote DC migration to draining lymph nodes to enhance vaccine-induced immune responses (Fig. 5). Physical adjuvant effects can be as potent as chemical adjuvants with appropriate development.
Fig. 5.
Different action mechanisms of chemical and physical adjuvants in intradermal vaccination. Chemical adjuvants after intradermal delivery directly activate DCs by binding to PRRs to induce DC maturation, induce DCs to release cytokines/chemokines, recruit innate immune cells, or promote DC migration to draining lymph nodes to enhance vaccine-induced immune responses. Physical adjuvants deliver laser or radiofrequency energies and induce tissue stress to release danger signals to stimulate DC maturation, induce DCs to release cytokines/chemokines, and recruit innate immune cells. Physical adjuvants can also increase DC motility within the dermal tissue and promote DC migration to draining lymph nodes to enhance vaccine-induced immune responses.
7. Expert commentary
Skin has been recognized as a highly immunogenic site for vaccine delivery. Intradermal vaccine delivery often induces more potent immune responses than intramuscular delivery and holds a great promise to increase vaccine efficacy or spare vaccine doses. In fact, several vaccines have been licensed for intradermal delivery that include influenza vaccine, rabies vaccine, and the recent monkeypox vaccine. Yet, intradermal vaccination faces a risk to induce significant local reactions. The majority of the currently approved adjuvants are not suitable for intradermal delivery due to their high risk to induce significant local reactions. Novel adjuvants are demanded to safely boost intradermal vaccination without overt skin reactions.
Recent studies identified several chemical adjuvants with a potential to safely boost intradermal vaccination that include CpG 1018, GLA-AF, topical imiquimod, poly(I:C), cGAMP, and PCPP. CpG 1018 has been licensed to boost intramuscular hepatitis B vaccination, while GLA-AF and topical imiquimod have been tested in clinical trials to boost intradermal influenza vaccination. These adjuvants can be readily tested to boost intradermal vaccination due to their established safety profiles in humans. Poly(I:C) has been explored in clinical trials to boost anti-tumor immunity following intratumoral, intramuscular, or subcutaneous injections (De Waele et al., 2021, Rodriguez-Ruiz et al., 2018). The overall good tolerability supports evaluation of poly(I:C) to boost intradermal vaccination. The benefits of adjuvanted intradermal vaccination can be further increase of vaccine efficacy, sparing more vaccine doses, and potential induction of cross-protective immunity. Influenza vaccines can be good targets to test adjuvanted intradermal vaccination to address vaccine mismatch issues (Belongia and McLean, 2019, Xie et al., 2015), which lower vaccine efficacy significantly, or tackle the annual influenza vaccine manufacturing burdens by sparing vaccine doses. Benefit/risk ratios need to be carefully evaluated in approval of adjuvanted intradermal vaccines for general public use.
Besides chemical adjuvants, different types of physical adjuvants (laser, radiofrequency) have been explored to safely boost intradermal vaccination. Physical adjuvants represent a relatively new adjuvant type that takes advantage of physical energies to induce tissue stress to boost intradermal vaccination. Although their potential to increase human vaccine efficacy remains to be explored, the recent identification of key roles of endogenous danger signals in chemical adjuvant effects (e.g., Alum, MF59) supports the development of physical adjuvants to induce tissue stress and release of endogenous danger signals to boost vaccination. The physical adjuvant concept and their safety and potency to boost intradermal vaccination have been recently studied in preclinical animal models. These studies support good safety and potency of physical adjuvants to boost intradermal vaccination. Despite these promising results, the development of physical adjuvants towards human use faces several challenges. First, physical adjuvants are device-based. Currently, there are no guidelines in place to instruct the development and approval of physical adjuvants. Second, physical adjuvant development needs collaborative efforts of device manufacturers and vaccine companies and also needs to bring in regulatory agencies in adjuvant development and medical device approval. The involvement of multiple parties may bring new challenges to physical adjuvant development. Third, intradermal vaccination with the current physical adjuvants require two steps: physical adjuvant treatment and intradermal vaccine delivery. The two-step vaccination process takes more time than chemical adjuvant-incorporated vaccination and the acceptance of the novel type of vaccination by the general public remains to be explored.
Adjuvant development received tremendous attention in the 21st century. Significant advances have been made in approval of new adjuvants for human use and elucidation of underlying mechanisms of vaccine adjuvants. All the efforts are expected to facilitate the development of safe chemical and physical adjuvants to boost intradermal vaccination.
CRediT authorship contribution statement
Xinyuan Chen: Conceptualization, Writing - original draft, Writing - review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work is supported by the National Institutes of Health grants AI139473 and AI156510 (to X.Y.C.).
Data availability
No data was used for the research described in the article.
References
- Ahlberg V., Hjertner B., Wallgren P., Hellman S., Lovgren Bengtsson K., Fossum C. Innate immune responses induced by the saponin adjuvant Matrix-M in specific pathogen free pigs. Vet. Res. 2017;48(1):30. doi: 10.1186/s13567-017-0437-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahlberg V., Lovgren Bengtsson K., Wallgren P., Fossum C. Global transcriptional response to ISCOM-Matrix adjuvant at the site of administration and in the draining lymph node early after intramuscular injection in pigs. Dev. Comp. Immunol. 2012;38(1):17–26. doi: 10.1016/j.dci.2012.03.005. [DOI] [PubMed] [Google Scholar]
- Alexiades-Armenakas M., Dover J.S., Arndt K.A. Unipolar versus bipolar radiofrequency treatment of rhytides and laxity using a mobile painless delivery method. Lasers Surg. Med. 2008;40(7):446–453. doi: 10.1002/lsm.20667. [DOI] [PubMed] [Google Scholar]
- Alexiades-Armenakas M.R., Dover J.S., Arndt K.A. The spectrum of laser skin resurfacing: nonablative, fractional, and ablative laser resurfacing. J. Am. Acad. Dermatol. 2008;58(5):719–737. doi: 10.1016/j.jaad.2008.01.003. quiz 738-40. [DOI] [PubMed] [Google Scholar]
- Andrade Fdo S., Clark R.M., Ferreira M.L. Effects of low-level laser therapy on wound healing. Rev. Col. Bras Cir. 2014;41(2):129–133. doi: 10.1590/s0100-69912014000200010. [DOI] [PubMed] [Google Scholar]
- Andrianov A.K., DeCollibus D.P., Gillis H.A., Kha H.H., Marin A., Prausnitz M.R., Babiuk L.A., Townsend H., Mutwiri G. Poly[di(carboxylatophenoxy)phosphazene] is a potent adjuvant for intradermal immunization. Proc. Natl. Acad. Sci. U.S.A. 2009;106(45):18936–18941. doi: 10.1073/pnas.0908842106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnou R., Icardi G., De Decker M., Ambrozaitis A., Kazek M.P., Weber F., Van Damme P. Intradermal influenza vaccine for older adults: a randomized controlled multicenter phase III study. Vaccine. 2009;27(52):7304–7312. doi: 10.1016/j.vaccine.2009.10.033. [DOI] [PubMed] [Google Scholar]
- Awate S., Babiuk L.A., Mutwiri G. Mechanisms of action of adjuvants. Front. Immunol. 2013;4:114. doi: 10.3389/fimmu.2013.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardel E., Doucet-Ladeveze R., Mathieu C., Harandi A.M., Dubois B., Kaiserlian D. Intradermal immunisation using the TLR3-ligand Poly (I:C) as adjuvant induces mucosal antibody responses and protects against genital HSV-2 infection. NPJ Vacc. 2016;1:16010. doi: 10.1038/npjvaccines.2016.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belongia E.A., McLean H.Q. Influenza vaccine effectiveness: defining the H3N2 problem. Clin. Infect. Diseases: Off. Publ. Infect. Diseases Soc. Am. 2019;69(10):1817–1823. doi: 10.1093/cid/ciz411. [DOI] [PubMed] [Google Scholar]
- Belshe R.B., Newman F.K., Cannon J., Duane C., Treanor J., Van Hoecke C., Howe B.J., Dubin G. Serum antibody responses after intradermal vaccination against influenza. N. Engl. J. Med. 2004;351(22):2286–2294. doi: 10.1056/NEJMoa043555. [DOI] [PubMed] [Google Scholar]
- Bode C., Zhao G., Steinhagen F., Kinjo T., Klinman D.M. CpG DNA as a vaccine adjuvant. Exp. Rev. Vacc. 2011;10(4):499–511. doi: 10.1586/erv.10.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonnak K., Dhitavat J., Thantamnu N., Kosoltanapiwat N., Auayporn M., Jiang L., Puthavathana P., Pitisuttithum P. Immune responses to intradermal and intramuscular inactivated influenza vaccine among older age group. Vaccine. 2017;35(52):7339–7346. doi: 10.1016/j.vaccine.2017.10.106. [DOI] [PubMed] [Google Scholar]
- Brooks J.T., Marks P., Goldstein R.H., Walensky R.P. Intradermal vaccination for monkeypox - benefits for individual and public health. N. Engl. J. Med. 2022;387(13):1151–1153. doi: 10.1056/NEJMp2211311. [DOI] [PubMed] [Google Scholar]
- Calabro S., Tortoli M., Baudner B.C., Pacitto A., Cortese M., O'Hagan D.T., De G.E., Seubert A., Wack A. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- Campbell J.D. Development of the CpG Adjuvant 1018: a case study. Methods Mol. Biol. 2017;1494:15–27. doi: 10.1007/978-1-4939-6445-1_2. [DOI] [PubMed] [Google Scholar]
- Cao Y., Zhu X., Hossen M.N., Kakar P., Zhao Y., Chen X. Augmentation of vaccine-induced humoral and cellular immunity by a physical radiofrequency adjuvant. Nat. Commun. 2018;9(1):3695. doi: 10.1038/s41467-018-06151-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargnelutti D.E., Sanchez M.V., Mattion N.M., Scodeller E.A. Development of a universal CTL-based vaccine for influenza. Bioengineered. 2013;4(6):374–378. doi: 10.4161/bioe.23573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter D., van Hoeven N., Baldwin S., Levin Y., Kochba E., Magill A., Charland N., Landry N., Nu K., Frevol A., Ashman J., Sagawa Z.K., Beckmann A.M., Reed S.G. The adjuvant GLA-AF enhances human intradermal vaccine responses. Sci. Adv. 2018;4(9):eaas9930. doi: 10.1126/sciadv.aas9930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Kim P., Farinelli B., Doukas A., Yun S.H., Gelfand J.A., Anderson R.R., Wu M.X. A novel laser vaccine adjuvant increases the motility of antigen presenting cells. PLoS.One. 2010;5(10):e13776. doi: 10.1371/journal.pone.0013776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Pravetoni M., Bhayana B., Pentel P.R., Wu M.X. High immunogenicity of nicotine vaccines obtained by intradermal delivery with safe adjuvants. Vaccine. 2012;31(1):159–164. doi: 10.1016/j.vaccine.2012.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Wu M.X. Laser vaccine adjuvant for cutaneous immunization. Exp. Rev. Vacc. 2011;10(10):1397–1403. doi: 10.1586/erv.11.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Zeng Q., Wu M.X. Improved efficacy of dendritic cell-based immunotherapy by cutaneous laser illumination. Clinical cancer research : an official journal of the American Association for. Cancer Res. 2012;18(8):2240–2249. doi: 10.1158/1078-0432.CCR-11-2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coccia M., Collignon C., Herve C., Chalon A., Welsby I., Detienne S., van Helden M.J., Dutta S., Genito C.J., Waters N.C., Deun K.V., Smilde A.K., Berg R., Franco D., Bourguignon P., Morel S., Garcon N., Lambrecht B.N., Goriely S., Most R.V., Didierlaurent A.M. Cellular and molecular synergy in AS01-adjuvanted vaccines results in an early IFNgamma response promoting vaccine immunogenicity. NPJ Vacc. 2017;2:25. doi: 10.1038/s41541-017-0027-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffman R.L., Sher A., Seder R.A. Vaccine adjuvants: putting innate immunity to work. Immunity. 2010;33(4):492–503. doi: 10.1016/j.immuni.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combadiere B., Liard C. Transcutaneous and intradermal vaccination. Hum. Vaccin. 2011;7(8):811–827. doi: 10.4161/hv.7.8.16274. [DOI] [PubMed] [Google Scholar]
- De Waele J., Verhezen T., van der Heijden S., Berneman Z.N., Peeters M., Lardon F., Wouters A., Smits E. A systematic review on poly(I:C) and poly-ICLC in glioblastoma: adjuvants coordinating the unlocking of immunotherapy. J. Exp. Clin. Cancer Res. 2021;40(1):213. doi: 10.1186/s13046-021-02017-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers J., Hammad H., Hoste E. Langerhans cells: sensing the environment in health and disease. Front. Immunol. 2018;9:93. doi: 10.3389/fimmu.2018.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Pasquale A., Preiss S., Tavares Da Silva F., Garcon N. Vaccine adjuvants: from 1920 to 2015 and beyond. Vaccines. 2015;3(2):320–343. doi: 10.3390/vaccines3020320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent A.M., Collignon C., Bourguignon P., Wouters S., Fierens K., Fochesato M., Dendouga N., Langlet C., Malissen B., Lambrecht B.N., Garcon N., Van Mechelen M., Morel S. Enhancement of adaptive immunity by the human vaccine adjuvant AS01 depends on activated dendritic cells. J. Immunol. (Baltimore, Md. : 1950) 2014;193(4):1920–1930. doi: 10.4049/jimmunol.1400948. [DOI] [PubMed] [Google Scholar]
- Dowling J.K., Mansell A. Toll-like receptors: the swiss army knife of immunity and vaccine development. Clin. Transl. Immunol. 2016;5(5):e85. doi: 10.1038/cti.2016.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du G., Leone M., Romeijn S., Kersten G., Jiskoot W., Bouwstra J.A. Immunogenicity of diphtheria toxoid and poly(I:C) loaded cationic liposomes after hollow microneedle-mediated intradermal injection in mice. Int. J. Pharm. 2018;547(1–2):250–257. doi: 10.1016/j.ijpharm.2018.06.001. [DOI] [PubMed] [Google Scholar]
- Dupuis M., McDonald D.M., Ott G. Distribution of adjuvant MF59 and antigen gD2 after intramuscular injection in mice. Vaccine. 1999;18(5–6):434–439. doi: 10.1016/s0264-410x(99)00263-7. [DOI] [PubMed] [Google Scholar]
- Franchi L., Nunez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur. J. Immunol. 2008;38(8):2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcon N., Di Pasquale A. From discovery to licensure, the Adjuvant System story. Hum. Vaccin. Immunother. 2017;13(1):19–33. doi: 10.1080/21645515.2016.1225635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcon N., Morel S., Didierlaurent A., Descamps D., Wettendorff M., Van Mechelen M. Development of an AS04-adjuvanted HPV vaccine with the adjuvant system approach. BioDrugs. 2011;25(4):217–226. doi: 10.2165/11591760-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Garcon N., Vaughn D.W., Didierlaurent A.M. Development and evaluation of AS03, an Adjuvant System containing alpha-tocopherol and squalene in an oil-in-water emulsion. Exp. Rev. Vacc. 2012;11(3):349–366. doi: 10.1586/erv.11.192. [DOI] [PubMed] [Google Scholar]
- Gelfand J.A., Nazarian R.M., Kashiwagi S., Brauns T., Martin B., Kimizuka Y., Korek S., Botvinick E., Elkins K., Thomas L., Locascio J., Parry B., Kelly K.M., Poznansky M.C. A pilot clinical trial of a near-infrared laser vaccine adjuvant: safety, tolerability, and cutaneous immune cell trafficking. FASEB J. 2019;33(2):3074–3081. doi: 10.1096/fj.201801095R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Givord C., Welsby I., Detienne S., Thomas S., Assabban A., Lima Silva V., Molle C., Gineste R., Vermeersch M., Perez-Morga D., Leo O., Collignon C., Didierlaurent A.M., Goriely S. Activation of the endoplasmic reticulum stress sensor IRE1alpha by the vaccine adjuvant AS03 contributes to its immunostimulatory properties. NPJ Vacc. 2018;3:20. doi: 10.1038/s41541-018-0058-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godeaux O., Izurieta P., Madariaga M., Drame M., Li P., Vaughn D.W. Immunogenicity and safety of AS03A-adjuvanted H5N1 influenza vaccine prepared from bulk antigen after stockpiling for 4 years. Vaccine. 2015;33(18):2189–2195. doi: 10.1016/j.vaccine.2014.07.062. [DOI] [PubMed] [Google Scholar]
- Goldberg S.N., Gazelle G.S. Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepatogastroenterology. 2001;48(38):359–367. [PubMed] [Google Scholar]
- Gosling R., von Seidlein L. The future of the RTS, S/AS01 malaria vaccine: an alternative development plan. PLoS Med. 2016;13(4):e1001994. doi: 10.1371/journal.pmed.1001994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., Chadwick D.R., Clark R., Cosgrove C., Galloway J., Goodman A.L., Heer A., Higham A., Iyengar S., Jamal A., Jeanes C., Kalra P.A., Kyriakidou C., McAuley D.F., Meyrick A., Minassian A.M., Minton J., Moore P., Munsoor I., Nicholls H., Osanlou O., Packham J., Pretswell C.H., San Francisco Ramos A., Saralaya D., Sheridan R.P., Smith R., Soiza R.L., Swift P.A., Thomson E.C., Turner J., Viljoen M.E., Albert G., Cho I., Dubovsky F., Glenn G., Rivers J., Robertson A., Smith K., Toback S. V.S.G. nCo, Safety and Efficacy of NVX-CoV2373 Covid-19 Vaccine. New Engl. J. Med. 2021;385(13):1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H., Kaisho T., Takeuchi O., Sato S., Sanjo H., Hoshino K., Horiuchi T., Tomizawa H., Takeda K., Akira S. Small anti-viral compounds activate immune cells via the TLR7 MyD88-dependent signaling pathway. Nat. Immunol. 2002;3(2):196–200. doi: 10.1038/ni758. [DOI] [PubMed] [Google Scholar]
- Hernandez-Franco J.F., Mosley Y.C., Franco J., Ragland D., Yao Y., HogenEsch H. Effective and safe stimulation of humoral and cell-mediated immunity by intradermal immunization with a cyclic dinucleotide/nanoparticle combination adjuvant. J. Immunol. 2021;206(4):700–711. doi: 10.4049/jimmunol.2000703. Baltimore, Md. : 1950. [DOI] [PubMed] [Google Scholar]
- HogenEsch H., O'Hagan D.T., Fox C.B. Optimizing the utilization of aluminum adjuvants in vaccines: you might just get what you want. NPJ Vacc. 2018;3:51. doi: 10.1038/s41541-018-0089-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Z., Ma J., Chen J., Shen B., Pei F., Kraus V.B. The effectiveness of low-level laser therapy for nonspecific chronic low back pain: a systematic review and meta-analysis. Arthrit. Res. Ther. 2015;17:360. doi: 10.1186/s13075-015-0882-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung I.F., Zhang A.J., To K.K., Chan J.F., Li P., Wong T.L., Zhang R., Chan T.C., Chan B.C., Wai H.H., Chan L.W., Fong H.P., Hui R.K., Kong K.L., Leung A.C., Ngan A.H., Tsang L.W., Yeung A.P., Yiu G.C., Yung W., Lau J.Y., Chen H., Chan K.H., Yuen K.Y. Topical imiquimod before intradermal trivalent influenza vaccine for protection against heterologous non-vaccine and antigenically drifted viruses: a single-centre, double-blind, randomised, controlled phase 2b/3 trial. Lancet Infect. Dis. 2016;16(2):209–218. doi: 10.1016/S1473-3099(15)00354-0. [DOI] [PubMed] [Google Scholar]
- Icardi G., Orsi A., Ceravolo A., Ansaldi F. Current evidence on intradermal influenza vaccines administered by Soluvia licensed micro injection system. Hum. Vaccin. Immunother. 2012;8(1):67–75. doi: 10.4161/hv.8.1.18419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S. Laser adjuvant for vaccination. FASEB J. 2020;34(3):3485–3500. doi: 10.1096/fj.201902164R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S., Brauns T., Gelfand J., Poznansky M.C. Laser vaccine adjuvants. History, progress, and potential. Hum. Vaccin. Immunother. 2014;10(7):1892–1907. doi: 10.4161/hv.28840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashiwagi S., Yuan J., Forbes B., Hibert M.L., Lee E.L., Whicher L., Goudie C., Yang Y., Chen T., Edelblute B., Collette B., Edington L., Trussler J., Nezivar J., Leblanc P., Bronson R., Tsukada K., Suematsu M., Dover J., Brauns T., Gelfand J., Poznansky M.C. Near-infrared laser adjuvant for influenza vaccine. PLoS.One. 2013;8(12):e82899. doi: 10.1371/journal.pone.0082899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy H.Q.Q.R.B. Enhancing immunogenicity of influenza vaccine in the elderly through intradermal vaccination: a literature analysis. Viruses. 2022;14 doi: 10.3390/v14112438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalkhal E., Rezaei-Tavirani M., Zali M.R., Akbari Z. The evaluation of laser application in surgery: a review article. J. Lasers Med. Sci. 2019;10(Suppl 1):S104–S111. doi: 10.15171/jlms.2019.S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.C., Prausnitz M.R. Enabling skin vaccination using new delivery technologies. Drug Deliv. Transl. Res. 2011;1(1):7–12. doi: 10.1007/s13346-010-0005-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko E.J., Kang S.M. Immunology and efficacy of MF59-adjuvanted vaccines. Hum. Vaccin. Immunother. 2018;14(12):3041–3045. doi: 10.1080/21645515.2018.1495301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K.L., Lam Y.F., Cheung K.S., Hung I.F., Leung W.K. Clinical trial: intra dermal hepatitis B vaccination with topical imiquimod versus intra muscular hepatitis B vaccination in patients with inflammatory bowel disease. Aliment. Pharmacol. Ther. 2022;56(2):301–309. doi: 10.1111/apt.16970. [DOI] [PubMed] [Google Scholar]
- Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8(4):279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kool M., Soullie T., van Nimwegen M., Willart M.A., Muskens F., Jung S., Hoogsteden H.C., Hammad H., Lambrecht B.N. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J. Exp. Med. 2008;205(4):869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyi K.P., Oo K.M., Htun M.M., Tun W.M., Aye K.K., Oo S.S., Lwin K.O., Nyunt S. Clinical trial of the intradermal administration of hepatitis B vaccine produced at the Department of Medical Research, Myanmar. Vaccine. 2002;20(11–12):1649–1652. doi: 10.1016/s0264-410x(01)00468-6. [DOI] [PubMed] [Google Scholar]
- Lafouresse F., Groom J.R. A task force against local inflammation and cancer: lymphocyte trafficking to and within the skin. Front. Immunol. 2018;9:2454. doi: 10.3389/fimmu.2018.02454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laupeze B., Herve C., Di Pasquale A., Tavares Da Silva F. Adjuvant Systems for vaccines: 13years of post-licensure experience in diverse populations have progressed the way adjuvanted vaccine safety is investigated and understood. Vaccine. 2019;37(38):5670–5680. doi: 10.1016/j.vaccine.2019.07.098. [DOI] [PubMed] [Google Scholar]
- Laurens M.B. RTS, S/AS01 vaccine (Mosquirix): an overview. Hum. Vaccin. Immunother. 2020;16(3):480–489. doi: 10.1080/21645515.2019.1669415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Nguyen M.T. Recent advances of vaccine adjuvants for infectious diseases. Immune network. 2015;15(2):51–57. doi: 10.4110/in.2015.15.2.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H., Willingham S.B., Ting J.P., Re F. Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Chen Z.J. The cGAS-cGAMP-STING pathway connects DNA damage to inflammation, senescence, and cancer. J. Exp. Med. 2018;215(5):1287–1299. doi: 10.1084/jem.20180139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li Z., Zhao Y., Chen X. Potentiation of recombinant NP and M1-induced cellular immune responses and protection by physical radiofrequency adjuvant. Vaccines. 2021;9(12) doi: 10.3390/vaccines9121382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Cao Y., Li Y., Zhao Y., Chen X. Vaccine delivery alerts innate immune systems for more immunogenic vaccination. JCI Insight. 2021;6(7) doi: 10.1172/jci.insight.144627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Zhao Y., Li Y., Chen X. Adjuvantation of influenza vaccines to induce cross-protective immunity. Vaccines. 2021;9(2) doi: 10.3390/vaccines9020075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Kang X., Kim K.H., Zhao Y., Li Y., Kang S.M., Chen X. Effective adjuvantation of nanograms of influenza vaccine and induction of cross-protective immunity by physical radiofrequency adjuvant. Sci. Rep. 2022;12(1):21249. doi: 10.1038/s41598-022-25605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Kim K.H., Bhatnagar N., Park B.R., Jeeva S., Jung Y.J., Raha J., Kang S.M., Chen X. Physical radiofrequency adjuvant enhances immune responses to influenza H5N1 vaccination. FASEB J. 2022;36(3):e22182. doi: 10.1096/fj.202101703R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Li Z., Chen X. Comparative tissue proteomics reveals unique action mechanisms of vaccine adjuvants. iScience. 2023;26(1) doi: 10.1016/j.isci.2022.105800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lolis M.S., Goldberg D.J. Radiofrequency in cosmetic dermatology: a review. Dermatol. Surg.: Off. Publ. Am. Soc. Dermatol. Surg. et al. 2012;38:1765–1776. doi: 10.1111/j.1524-4725.2012.02547.x. [DOI] [PubMed] [Google Scholar]
- Lopes P.P., Todorov G., Pham T.T., Nesburn A.B., Bahraoui E., BenMohamed L. Laser adjuvant-assisted peptide vaccine promotes skin mobilization of dendritic cells and enhances protective CD8(+) TEM and TRM cell responses against herpesvirus infection and disease. J. Virol. 2018;92(8) doi: 10.1128/JVI.02156-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovgren Bengtsson K., Morein B., A.d. Osterhaus, ISCOM technology-based Matrix M adjuvant: success in future vaccines relies on formulation. Exp. Rev. Vacc. 2011;10(4):401–403. doi: 10.1586/erv.11.25. [DOI] [PubMed] [Google Scholar]
- Lu F., Hogenesch H. Kinetics of the inflammatory response following intramuscular injection of aluminum adjuvant. Vaccine. 2013;31(37):3979–3986. doi: 10.1016/j.vaccine.2013.05.107. [DOI] [PubMed] [Google Scholar]
- Magiri R., Mutwiri G., Wilson H.L. Recent advances in experimental polyphosphazene adjuvants and their mechanisms of action. Cell Tissue Res. 2018;374(3):465–471. doi: 10.1007/s00441-018-2929-4. [DOI] [PubMed] [Google Scholar]
- Magiri R.B., Lai K.J., Mutwiri G.K., Wilson H.L. Experimental PCEP-adjuvanted swine influenza H1N1 vaccine induced strong immune responses but did not protect piglets against heterologous H3N2 virus challenge. Vaccines. 2020;8(2) doi: 10.3390/vaccines8020235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maki Y., Kashiwagi S., Kimizuka Y. Laser vaccine adjuvants: Light-augmented immune responses. Vaccine. 2021;39(46):6805–6812. doi: 10.1016/j.vaccine.2021.09.042. [DOI] [PubMed] [Google Scholar]
- Marciani D.J. Vaccine adjuvants: role and mechanisms of action in vaccine immunogenicity. Drug Discov. Today. 2003;8(20):934–943. doi: 10.1016/s1359-6446(03)02864-2. [DOI] [PubMed] [Google Scholar]
- Marichal T., Ohata K., Bedoret D., Mesnil C., Sabatel C., Kobiyama K., Lekeux P., Coban C., Akira S., Ishii K.J., Bureau F., Desmet C.J. DNA released from dying host cells mediates aluminum adjuvant activity. Nat. Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- Marrack P., McKee A.S., Munks M.W. Towards an understanding of the adjuvant action of aluminium. Nat. Rev. Immunol. 2009;9(4):287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.S., Burchill M.A., Munks M.W., Jin L., Kappler J.W., Friedman R.S., Jacobelli J., Marrack P. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. PNAS. 2013;110(12):E1122–E1131. doi: 10.1073/pnas.1300392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.S., Marrack P. Old and new adjuvants. Curr. Opin. Immunol. 2017;47:44–51. doi: 10.1016/j.coi.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee A.S., Munks M.W., MacLeod M.K., Fleenor C.J., Van R.N., Kappler J.W., Marrack P. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 2009;183(7):4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon G.K. New insights into skin structure: scratching the surface. Adv. Drug Deliv. Rev. 2002;54(Suppl 1):S3–S. doi: 10.1016/s0169-409x(02)00121-7. [DOI] [PubMed] [Google Scholar]
- Mestrallet G., Rouas-Freiss N., LeMaoult J., Fortunel N.O., Martin M.T. Skin immunity and tolerance: focus on epidermal keratinocytes expressing HLA-G. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.772516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel S., Didierlaurent A., Bourguignon P., Delhaye S., Baras B., Jacob V., Planty C., Elouahabi A., Harvengt P., Carlsen H., Kielland A., Chomez P., Garcon N., Van Mechelen M. Adjuvant System AS03 containing alpha-tocopherol modulates innate immune response and leads to improved adaptive immunity. Vaccine. 2011;29(13):2461–2473. doi: 10.1016/j.vaccine.2011.01.011. [DOI] [PubMed] [Google Scholar]
- Morse K., Kimizuka Y., Chan M.P.K., Shibata M., Shimaoka Y., Takeuchi S., Forbes B., Nirschl C., Li B., Zeng Y., Bronson R.T., Katagiri W., Shigeta A., Sirbulescu R.F., Chen H., Tan R.Y.Y., Tsukada K., Brauns T., Gelfand J., Sluder A., Locascio J.J., Poznansky M.C., Anandasabapathy N., Kashiwagi S. Near-infrared 1064 nm laser modulates migratory dendritic cells to augment the immune response to intradermal influenza vaccine. J. Immunol. 2017;199(4):1319–1332. doi: 10.4049/jimmunol.1601873. (Baltimore, Md. : 1950) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanishi E., Dowling D.J., Levy O. Toward precision adjuvants: optimizing science and safety. Curr. Opin. Pediatr. 2020;32(1):125–138. doi: 10.1097/MOP.0000000000000868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng H.I., Tuong Z.K., Fernando G.J.P., Depelsenaire A.C.I., Meliga S.C., Frazer I.H., Kendall M.A.F. Microprojection arrays applied to skin generate mechanical stress, induce an inflammatory transcriptome and cell death, and improve vaccine-induced immune responses. NPJ Vacc. 2019;4:41. doi: 10.1038/s41541-019-0134-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen A.V., Soulika A.M. The dynamics of the skin's immune system. Int. J. Mol. Sci. 2019;20(8) doi: 10.3390/ijms20081811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Hagan D.T., Ott G.S., De G.E., Seubert A. The mechanism of action of M. Vaccine. 2012;30(29):4341–4348. doi: 10.1016/j.vaccine.2011.09.061. [DOI] [PubMed] [Google Scholar]
- O'Hagan D.T., Ott G.S., Nest G.V., Rappuoli R., Giudice G.D. The history of MF59((R)) adjuvant: a phoenix that arose from the ashes. Exp. Rev. Vacc. 2013;12(1):13–30. doi: 10.1586/erv.12.140. [DOI] [PubMed] [Google Scholar]
- O’Hagan D.T., Tsai T.F., Brito L.A. Emulsion based vaccine adjuvants. Hum. Vaccin. Immunother. 2013;9(8):1698–1700. [Google Scholar]
- Otterhaug T., Janetzki S., Welters M.J.P., Hakerud M., Nedberg A.G., Edwards V.T., Boekestijn S., Loof N.M., Selbo P.K., Olivecrona H., van der Burg S.H., Hogset A. Photochemical internalization enhanced vaccination is safe, and gives promising cellular immune responses to an HPV peptide-based vaccine in a Phase I clinical study in healthy volunteers. Front. Immunol. 2020;11 doi: 10.3389/fimmu.2020.576756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. Verifiable CPD paper: laser-tissue interaction. Br. Dent. J. 2007;202(2):73–81. doi: 10.1038/bdj.2007.24. [DOI] [PubMed] [Google Scholar]
- Petrovsky N., Aguilar J.C. Vaccine adjuvants: current state and future trends. Immunol. Cell Biol. 2004;82(5):488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]
- Poirier D., Renaud F., Dewar V., Strodiot L., Wauters F., Janimak J., Shimada T., Nomura T., Kabata K., Kuruma K., Kusano T., Sakai M., Nagasaki H., Oyamada T. Hepatitis B surface antigen incorporated in dissolvable microneedle array patch is antigenic and thermostable. Biomaterials. 2017;145:256–265. doi: 10.1016/j.biomaterials.2017.08.038. [DOI] [PubMed] [Google Scholar]
- Prausnitz M.R. Engineering microneedle patches for vaccination and drug delivery to skin. Annu. Rev. Chem. Biomol. Eng. 2017;8:177–200. doi: 10.1146/annurev-chembioeng-060816-101514. [DOI] [PubMed] [Google Scholar]
- Preissig J., Hamilton K., Markus R. Current laser resurfacing technologies: a review that delves beneath the surface. Semin. Plast. Surg. 2012;26(3):109–116. doi: 10.1055/s-0032-1329413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulendran B., O'Hagan S.A.P.D.T. Emerging concepts in the science of vaccine adjuvants. Nat. Rev. Drug Disc. 2021;20(6):454–475. doi: 10.1038/s41573-021-00163-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed S.G., Orr M.T., Fox C.B. Key roles of adjuvants in modern vaccines. Nat. Med. 2013;19(12):1597–1608. doi: 10.1038/nm.3409. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ruiz M.E., Perez-Gracia J.L., Rodriguez I., Alfaro C., Onate C., Perez G., Gil-Bazo I., Benito A., Inoges S., Lopez-Diaz de Cerio A., Ponz-Sarvise M., Resano L., Berraondo P., Barbes B., Martin-Algarra S., Gurpide A., Sanmamed M.F., de Andrea C., Salazar A.M., Melero I. Combined immunotherapy encompassing intratumoral poly-ICLC, dendritic-cell vaccination and radiotherapy in advanced cancer patients. Ann. Oncol. 2018;29(5):1312–1319. doi: 10.1093/annonc/mdy089. [DOI] [PubMed] [Google Scholar]
- Rouphael N.G., Paine M., Mosley R., Henry S., McAllister D.V., Kalluri H., Pewin W., Frew P.M., Yu T., Thornburg N.J., Kabbani S., Lai L., Vassilieva E.V., Skountzou I., Compans R.W., Mulligan M.J., Prausnitz M.R. The safety, immunogenicity, and acceptability of inactivated influenza vaccine delivered by microneedle patch (TIV-MNP 2015): a randomised, partly blinded, placebo-controlled, phase 1 trial. Lancet (London, England) 2017;390(10095):649–658. doi: 10.1016/S0140-6736(17)30575-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer L. Complexity of danger: the diverse nature of damage-associated molecular patterns. J. Biol. Chem. 2014;289(51):35237–35245. doi: 10.1074/jbc.R114.619304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shardlow E., Mold M., Exley C. From stock bottle to vaccine: elucidating the particle size distributions of aluminum adjuvants using dynamic light scattering. Front. Chem. 2016;4:48. doi: 10.3389/fchem.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Evans J.E., Rock K.L. Molecular identification of a danger signal that alerts the immune system to dying cells. Nature. 2003;425(6957):516–521. doi: 10.1038/nature01991. [DOI] [PubMed] [Google Scholar]
- Skountzou, I., Brock, N., Lelutiu, N., Compans, R.W., 2017. Chapter 20 - Adjuvants for skin vaccination. Editor(s): Virgil E.J.C. Schijns, Derek T. O'Hagan, Immunopotentiators in Modern Vaccines (Second Edition). Academic Press, pp. 399–419.
- Sticchi L., Alberti M., Alicino C., Crovari P. The intradermal vaccination: past experiences and current perspectives. J. Prev. Med. Hyg. 2010;51(1):7–14. [PubMed] [Google Scholar]
- Sullivan S.P., Koutsonanos D.G., Del Pilar Martin M., Lee J.W., Zarnitsyn V., Choi S.O., Murthy N., Compans R.W., Skountzou I., Prausnitz M.R. Dissolving polymer microneedle patches for influenza vaccination. Nat. Med. 2010;16(8):915–920. doi: 10.1038/nm.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K., Akira S. Toll-like receptors in innate immunity. Int. Immunol. 2005;17(1):1–14. doi: 10.1093/intimm/dxh186. [DOI] [PubMed] [Google Scholar]
- Tatli S., Tapan U., Morrison P.R., Silverman S.G. Radiofrequency ablation: technique and clinical applications. Diagn. Interv. Radiol. 2012;18(5):508–516. doi: 10.4261/1305-3825.DIR.5168-11.1. [DOI] [PubMed] [Google Scholar]
- Verma R., Khanna P., Prinja S., Rajput M. Intra-dermal administration of rabies vaccines in developing countries: at an affordable cost. Hum. Vaccin. 2011;7(7):792–794. doi: 10.4161/hv.7.7.15410. [DOI] [PubMed] [Google Scholar]
- Vono M., Taccone M., Caccin P., Gallotta M., Donvito G., Falzoni S., Palmieri E., Pallaoro M., Rappuoli R., Di Virgilio F., De Gregorio E., Montecucco C., Seubert A. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc. Nat. Acad. Sci. U.S.A. 2013;110(52):21095–21100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff A.J., Perry C.M. Topical imiquimod: a review of its use in the management of anogenital warts, actinic keratoses, basal cell carcinoma and other skin lesions. Drugs. 2007;67(15):2187–2210. doi: 10.2165/00003495-200767150-00006. [DOI] [PubMed] [Google Scholar]
- Walter A., Schafer M., Cecconi V., Matter C., Urosevic-Maiwald M., Belloni B., Schonewolf N., Dummer R., Bloch W., Werner S., Beer H.D., Knuth A., van den Broek M. Aldara activates TLR7-independent immune defence. Nat. Commun. 2013;4:1560. doi: 10.1038/ncomms2566. [DOI] [PubMed] [Google Scholar]
- Wang J., Li B., Wu M.X. Effective and lesion-free cutaneous influenza vaccination. Proc. Natl. Acad. Sci. U.S.A. 2015;112(16):5005–5010. doi: 10.1073/pnas.1500408112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Li P., Wu M.X. Natural STING agonist as an “Ideal” adjuvant for cutaneous vaccination. J. Invest. Dermatol. 2016;136(11):2183–2191. doi: 10.1016/j.jid.2016.05.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Shah D., Chen X., Anderson R.R., Wu M.X. A micro-sterile inflammation array as an adjuvant for influenza vaccines. Nat. Commun. 2014;5:4447. doi: 10.1038/ncomms5447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weldon W.C., Zarnitsyn V.G., Esser E.S., Taherbhai M.T., Koutsonanos D.G., Vassilieva E.V., Skountzou I., Prausnitz M.R., Compans R.W. Effect of adjuvants on responses to skin immunization by microneedles coated with influenza subunit vaccine. PLoS One. 2012;7(7):e41501. doi: 10.1371/journal.pone.0041501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie H., Wan X.F., Ye Z., Plant E.P., Zhao Y., Xu Y., Li X., Finch C., Zhao N., Kawano T., Zoueva O., Chiang M.J., Jing X., Lin Z., Zhang A., Zhu Y. H3N2 mismatch of 2014–15 Northern hemisphere influenza vaccines and head-to-head comparison between human and ferret antisera derived antigenic maps. Sci. Rep. 2015;5:15279. doi: 10.1038/srep15279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yatim N., Cullen S., Albert M.L. Dying cells actively regulate adaptive immune responses. Nat. Rev. Immunol. 2017;17(4):262–275. doi: 10.1038/nri.2017.9. [DOI] [PubMed] [Google Scholar]
- Yokomizo S., Katagiri W., Maki Y., Sano T., Inoue K., Fukushi M., Atochin D.N., Kushibiki T., Kawana A., Kimizuka Y., Kashiwagi S. Brief exposure of skin to near-infrared laser augments early vaccine responses. Nanophotonics. 2021;10(12):3187–3197. doi: 10.1515/nanoph-2021-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.