Abstract
Background
Patients with serious respiratory illness and their caregivers suffer considerable burdens, and palliative care is a fundamental right for anyone who needs it. However, the overwhelming majority of patients do not receive timely palliative care before the end of life, despite robust evidence for improved outcomes.
Goals
This policy statement by the American Thoracic Society (ATS) and partnering societies advocates for improved integration of high-quality palliative care early in the care continuum for patients with serious respiratory illness and their caregivers and provides clinicians and policymakers with a framework to accomplish this.
Methods
An international and interprofessional expert committee, including patients and caregivers, achieved consensus across a diverse working group representing pulmonary–critical care, palliative care, bioethics, health law and policy, geriatrics, nursing, physiotherapy, social work, pharmacy, patient advocacy, psychology, and sociology.
Results
The committee developed fundamental values, principles, and policy recommendations for integrating palliative care in serious respiratory illness care across seven domains: 1) delivery models, 2) comprehensive symptom assessment and management, 3) advance care planning and goals of care discussions, 4) caregiver support, 5) health disparities, 6) mass casualty events and emergency preparedness, and 7) research priorities. The recommendations encourage timely integration of palliative care, promote innovative primary and secondary or specialist palliative care delivery models, and advocate for research and policy initiatives to improve the availability and quality of palliative care for patients and their caregivers.
Conclusions
This multisociety policy statement establishes a framework for early palliative care in serious respiratory illness and provides guidance for pulmonary–critical care clinicians and policymakers for its proactive integration.
Keywords: quality of life, caregivers, healthcare disparities, advance care planning, lung diseases
Contents
Introduction
Methods
-
Palliative Care Domains
-
I.
Delivery Models
-
II.
Comprehensive Symptom Assessment and Management
-
III.
Advance Care Planning and Goals of Care Discussions
-
IV.
Caregiver Support
-
V.
Health Disparities
-
VI.
Mass Casualty Events and Emergency Preparedness
-
VII.
Research Priorities
-
I.
Conclusions
Introduction
Palliative care is specialized medical care for people living with serious illness and focuses on providing relief of distressing symptoms and improving the quality of life (QOL) for both the patient and their family. Palliative care is distinct from hospice care, which focuses on care delivered at the very end of life, whereas the integration of palliative care is appropriate at any stage of a serious illness and is most beneficial when provided in conjunction with curative or disease-modifying treatments. Palliative care delivery is the responsibility of all clinicians caring for patients with serious respiratory illness. In 2008, the ATS (American Thoracic Society) published a policy statement on palliative care for patients with respiratory disease and critical illness (1). That policy statement described barriers to receiving comprehensive palliative care and called on the ATS to work with other professional societies to support palliative care, enhance education, and encourage funding of palliative care research to provide best practices. Since then, there has been significant growth in the field of palliative care (2), yet barriers to palliative care remain, especially outside of hospitals and in communities where patients with serious respiratory illness are living and receiving medical care.
This policy statement focuses on people living with serious respiratory illness (e.g., chronic obstructive pulmonary disease [COPD], interstitial lung disease [ILD], and lung cancer) who often have a chaotic end of life, seeking relief from debilitating symptoms such as breathlessness in acute care settings and receiving fragmented and burdensome care that may not be aligned with their values and wishes. Even in high medical resource settings, palliative care is underresourced, particularly in outpatient settings, and cannot meet the needs of patients and their families (3, 4). This is especially concerning in rural settings (4) and developing countries, where 86% of the global population lacks access to the highest degree of palliative care (5). Given the persistence of these known deficiencies for decades, coupled with the anticipated doubling of the burden of serious health-related suffering expected by 2060 (5), a concerted effort to increase the accessibility and quality of palliative care in pulmonary and critical care medicine is urgently needed.
There are several important definitions used by the committee in this policy statement (Table 1). There are three distinct levels of palliative care on the basis of clinician training, care complexity, and setting (primary, secondary or specialist, and tertiary palliative care) (9, 10) (Figure 1). In this statement, we also discuss serious respiratory illness (6) and the importance of caregivers who provide support for the medical needs or daily activities of patients. This policy statement is intended to build on previous ATS work to 1) encourage timely, integrated palliative care in respiratory illness, highlighting current evidence; 2) review and promote innovative primary and secondary or specialist palliative care delivery models; and 3) advocate for research and policy initiatives to improve the quality and availability of palliative care in pulmonary and critical care medicine. The committee used a values- and principles-based approach (Table 2) to derive recommendations designed to provide a framework and identify policy priorities for palliative care integration among patients with serious respiratory illness.
Table 1.
Policy Statement Definitions
| Serious respiratory illness (6) | A respiratory condition (e.g., chronic obstructive pulmonary disease, pulmonary hypertension, interstitial lung disease, lung cancer, etc.) that carries a high risk of mortality and symptom burden (e.g., dyspnea and depression) and either negatively impacts a person’s daily function or quality of life or negatively impacts their caregivers. |
| Hospice care | This care is not synonymous with palliative care. Hospice care is specialized care for people who are experiencing a life-limiting illness and their caregivers so that they may live as comfortably as possible. The hospice philosophy accepts death as the final stage of life but does not try to hasten or postpone death. Bereavement services and respite care (i.e., short-term relief) for families are a core component of this care. Settings can include inpatient units, community facilities, and in-home care or a combination of these on the basis of needs. In the United States, the Centers for Medicare and Medicaid Services provides a hospice care program for people with a life expectancy of 6 mo or less. |
| ACP | This is a process that supports persons “at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care. The goal of ACP is to help ensure that people receive medical care that is consistent with their values, goals, and preferences during serious and chronic illness” (7). |
| GOC discussions | This term is sometimes used in a narrow sense, specifically referring to discussions regarding resuscitation preferences or “code status” during hospitalizations. This policy statement employs a broader definition of this term, meaning any discussions and/or decisions regarding specific treatments, the intensity of care, and future planning for care, including values, goals, and preferences elicitations. GOC discussions are expected to occur across healthcare settings and are considered an important component of ACP. |
| Informal caregiver or caregiver | A person (e.g., partner, family member, or friend, among others) who provides unpaid support for the medical needs or daily activities of someone living with a serious respiratory illness. This committee acknowledges that this terminology frames these individuals only in terms of the patient and the patient’s needs and does not encompass the totality of caregiver responsibilities, contributions, or their own need for more individualized support and recognition. |
| Surrogate | A person designated to make decisions related to the health care of an individual in the event that he or she is unable to do so (i.e., the patient is incapacitated). This person could be designated in a legal document called an HCPA. The HCPA refers to both the legal document and the person designated to make healthcare decisions. In lieu of a legal document, there are state-specific guidelines for who is the legally recognized person able to make health decisions (e.g., spouse, adult child, etc.). Surrogate decisions are often guided by the substituted judgment principle, in which a surrogate should attempt to determine what the patient would have wanted under the given circumstances or what the patient would have decided if he or she were competent to choose (8). |
| Types of palliative care (9, 10) | |
| Primary | Care is provided by clinicians who are trained in basic tenets of palliative care but are not palliative care specialists (e.g., pulmonary and critical care clinicians, primary care clinicians, etc.). |
| Secondary | Care provided by trained palliative care specialists (e.g., nurses, physicians, advance practice providers, social workers, chaplains, etc.). This care is sometimes referred to as specialist palliative care. |
| Tertiary | Care provided by trained palliative care specialists at tertiary and quaternary medical centers and includes caring for the most complex patients. This may include care in dedicated palliative care units. |
Definition of abbreviations: ACP = advanced care planning; GOC = goals of care; HCPA = medical or healthcare power of attorney.
Figure 1.
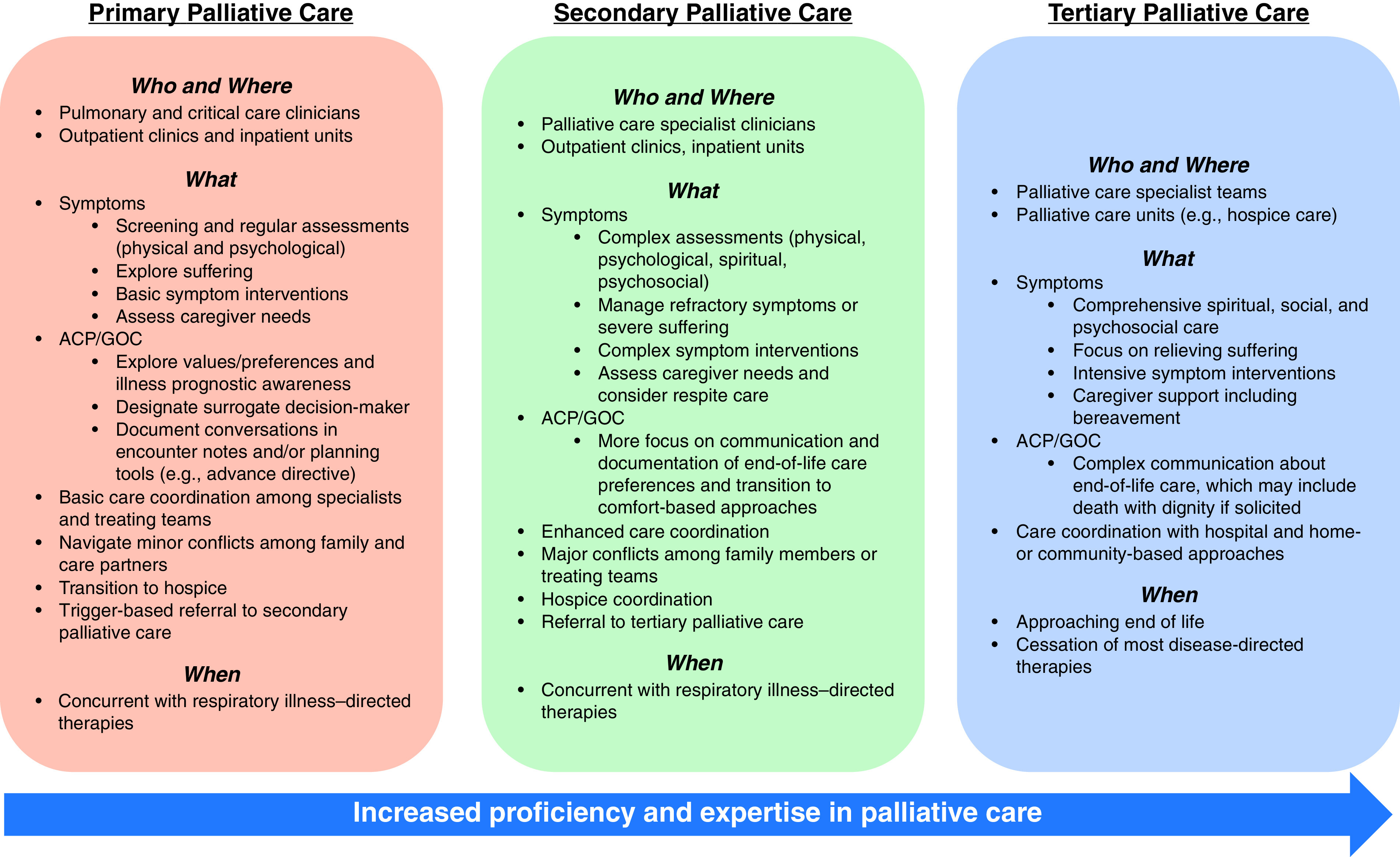
Levels of palliative care. This figure illustrates the who, where, what, and when of palliative care across three levels of increasing proficiency and expertise (primary, secondary or specialist, and tertiary palliative care). ACP = advanced care planning; GOC = goals of care.
Table 2.
Fundamental Values and Principles of Palliative Care in Serious Respiratory Illness
| 1. All patients with serious respiratory illness are eligible for palliative care on the basis of needs and should have equitable access, regardless of demographic characteristics (e.g., race/ethnicity, LGBTQIA+, age, sex, houselessness, and citizenship status), geographic area of residence, stage of illness, or insurance status. 2. Primary and secondary or specialist palliative care should be provided throughout the course of the illness when needs arise, integrated with illness-directed treatment, and should not be limited to patients in the final months or weeks of life. 3. Palliative care approaches include assessment and management of physical, psychosocial, ethical, and spiritual/existential domains; therefore, this care should be interprofessional and engage all available and relevant disciplines. 4. Palliative care communications should be provided in the preferred language/linguistic style of patients and their caregivers, partners, or family. 5. Pulmonary and critical care clinicians should prioritize the development and ongoing maintenance of primary palliative care knowledge and skills. 6. Pulmonary and critical care professional societies should encourage a basic degree of primary palliative care knowledge and skill development among its members, which may include approaches such as serious illness communication and symptom assessment and management. 7. Policies and payment models that provide resources to facilitate palliative care delivery, including the provision of primary palliative care, are essential. 8. Palliative care should be included in healthcare disaster and pandemic planning and preparedness activities as part of local, regional, state, and federal disaster planning efforts. 9. Patients with their informal caregivers, partners, or family together are the principal unit of care to be identified, acknowledged, and effectively supported. 10. Pulmonary and critical care and palliative care professional societies should support research, training, and professional education to improve the provision of palliative care among patients with serious respiratory illness. |
-
•
Recommendation 1: The spectrum of palliative care in serious respiratory illness begins with primary palliative care delivered by pulmonary and critical care clinicians that is integrated concomitantly with usual disease-modifying therapies and should be complemented by interprofessional secondary or specialist palliative care expertise when necessary.
-
•
Recommendation 2: Comprehensive, individualized assessment of symptoms and needs should occur at every routine clinical encounter.
-
•
Recommendation 3: Advance care planning should be an iterative and longitudinal process of communication that starts at the diagnosis of a serious respiratory illness and evolves with changes in patient health status, goals, and preferences.
-
•
Recommendation 4: Informal caregivers should be identified and incorporated as a part of the primary unit of care with patients; effective engagement is necessary to support both patients and informal caregivers throughout the illness journey, including respite and bereavement.
-
•
Recommendation 5: Comprehensive primary palliative care training of pulmonary–critical care clinicians is essential and can help mitigate disparities in access; this should begin early in professional training and includes elements such as palliative care terminology and communication, advance care planning, active listening, cultural awareness and sensitivity, and symptom assessment and management.
-
•
Recommendation 6: Emergency response planning and preparedness should include provisions for the delivery of palliative care in the context of a catastrophic event and/or resource limitation. The palliative care response must include both the existing and new vulnerable populations who will be most impacted by a catastrophic disaster and should consider eight critical elements of crisis palliative care provision: stuff, staff, space, systems, sedation, separation, communication, and equity.
-
•
Recommendation 7: Pulmonary–critical care and palliative care professional societies should create a Pulmonary Palliative Care Research Consortium to support collaborative research opportunities with associated funding mechanisms to advance pulmonary palliative care science.
Methods
A diverse international, interprofessional committee from relevant disciplines developed this policy statement using an iterative approach. Project co-chairs invited international experts to join the committee to ensure the full working group was diverse and represented a breadth of professionals (e.g., nurses, patients, caregivers, pharmacists, physicians, physiotherapists, social workers, and researchers), disciplines (e.g., pulmonary–critical care, palliative care, bioethics, law and policy, geriatrics, nursing, physiotherapy, social work, pharmacy, patient advocacy, psychology, and sociology), and specific content area expertise (e.g., advance care planning and informal caregivers). In addition to a core of ATS Behavioral Science and Health Services Research Assembly members, the co-chairs solicited representation from the following ATS groups: Patient Advisory Roundtable, Nursing Assembly, Health Policy Committee, Ethics and Conflict of Interest Committee, Critical Care Assembly, and Patient and Family Education Committee to ensure balanced advice and input from a variety of perspectives. To achieve the greatest potential impact in the field, representatives with expertise in serious respiratory illness from the following national stakeholder societies were also solicited: AAHPM (American Academy of Hospice and Palliative Medicine), CAPC (Center to Advance Palliative Care), HPNA (Hospice and Palliative Nurses Association), and SWHPN (Social Work Hospice and Palliative Care Network).
Policy statement development proceeded in four phases (Figure 2). In phase one (conceptualization), the project co-chairs and selected committee members reviewed existing relevant literature, including previous ATS policies and policies of other organizations (1, 11–13), to identify subtopic domains and prepare introductory materials across these domains. Next, in phase two (preparation), small group facilitators participated in teleconferences with co-chairs to review small group structure and function. In phase three (presentation, facilitation, and exploration), presentations and small group sessions were followed by full committee discussions over two half-day virtual meetings to begin developing consensus. Small group sessions and discussions were conducted using SWOT (Strengths, Weaknesses, Opportunities, and Threats) analysis (14), followed by loose variants of the nominal group technique and Delphi techniques (15) adapted from previous work in rheumatology (16). The purpose of this process was to work toward consensus in identifying key priorities and identifying the lack of consensus (“dissensus”) where it existed. In phase four (integration and compilation), additional rounds of Delphi techniques were used toward developing full committee consensus. The writing committee drafted the policy statement, with successive drafts reviewed by the full committee, who revised it in an iterative process. The resulting final draft was subject to review by the ATS Documents Editor, the ATS Board of Directors, and anonymous peer reviewers from cosponsoring societies, per ATS policy. Potential conflicts of interest were disclosed and managed in accordance with the policies and procedures of ATS.
Figure 2.
Multiphase iterative development of the policy statement. This figure illustrates the four phases of iterative development of the policy statement over multiple years through conceptualization (phase 1), preparation (phase 2), presentation, facilitation, and exploration (phase 3), and integration and compilation (phase 4).
Palliative Care Domains
I. Delivery Models
Palliative care has undergone a dramatic evolution since its inception in the 1960s, from primarily community-based care focused on patients approaching death toward a vision for palliative care integration concurrent with conventional disease-modifying therapies throughout the course of illness and across medical treatment settings (17, 18) (Figure 3). The benefits of secondary palliative care are unequivocal (19–22) and can be extrapolated to patients with serious respiratory illness. Today, the major delivery models of specialist palliative care include inpatient consultation teams, acute palliative care units, and ambulatory, home, or community-based outpatient services. Hybrid models also include integrated, interprofessional clinics where palliative care specialists are embedded within a pulmonary clinic (18, 23, 24). These hybrid models are often disease-focused (e.g., lung cancer) (24) or symptom-focused (e.g., breathlessness) (18, 25). Models also differ in their team structures, care processes, components, patient populations, and reimbursement. Financial incentives can influence delivery models, such as the recently introduced Centers for Medicare and Medicaid Services reimbursement codes for advance care planning (ACP) (26) and the Medicare Care Choices Model demonstration allowing concurrent curative and hospice care (27). Unfortunately, access to palliative care is limited (4, 5), particularly in outpatient settings, and the looming specialist palliative care workforce shortage will exacerbate the unmet palliative care needs of patients (3, 28). As a result, there is an increased emphasis on the holistic model referred to as primary palliative care, or the delivery of palliative care by all clinicians who care for patients with serious respiratory illness and their families (9, 10).
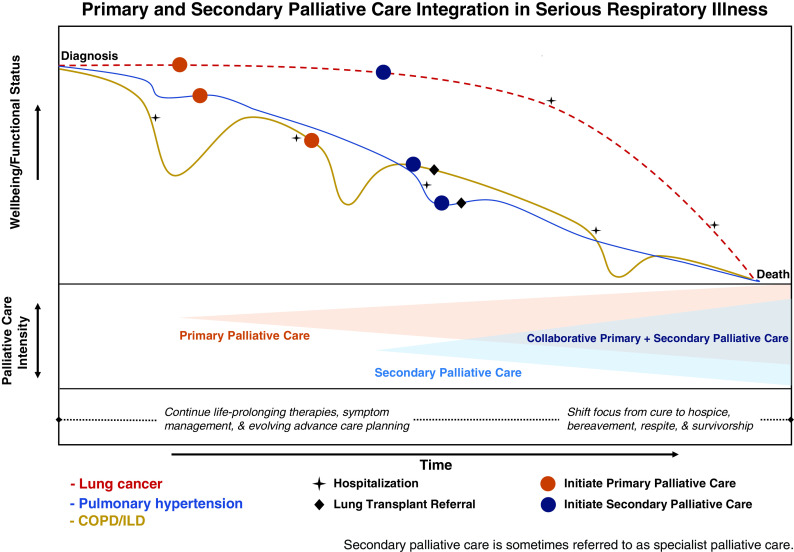
Figure 3.
Primary and secondary palliative care integration in serious respiratory illness. This figure has three panels. In the top panel, the x-axis denotes patient wellbeing and function, and the y-axis denotes time. The top panel illustrates the hypothetical integration of palliative care across illness trajectories of lung cancer (red dashed line), pulmonary hypertension (blue line), and chronic obstructive pulmonary disease/interstitial lung disease (COPD/ILD; gold line), each punctuated by declines in wellbeing at hospitalizations (stars) and potential lung transplant referral (diamonds). The integration of primary palliative care (orange circles) starts early (see Figure 4 for triggers), and the integration of secondary palliative care (blue circles) is added later, but well before the end of life. In the middle panel, the x-axis denotes palliative care intensity, and the y-axis denotes time. Primary palliative care increases in intensity after initiation (expanding orange triangle) as serious respiratory illness worsens, and secondary palliative care starts later and also increases in intensity but layers on top of secondary palliative care (expanding blue triangle). Through ongoing comanagement, a period of collaborative primary and secondary palliative care (overlapping triangles) should occur as illness severity worsens through the end of life. The bottom panel illustrates how palliative care may evolve across a continuum of serious respiratory illness. When illness is less severe, palliative care occurs concurrent with illness-directed therapies and then shifts focus from cure to end-of-life care (e.g., hospice and bereavement support) near and after death. This figure is adapted by permission from Iyer, AF. The Role of Palliative Care in COPD. Chest 2022;161: 1250–1262.
In addition to potentially improving access, primary palliative care has several other potential benefits as it can leverage longitudinal therapeutic patient–clinician relationships and trust, use a continuum of care approach gradually integrating palliative with usual medical care over time, and normalize early components of ACP such as identifying surrogate medical decision-makers (29–32). Therefore, all pulmonary–critical care clinicians should screen patients for symptoms and palliative care needs and know how to integrate core aspects of palliative care within usual care for patients with serious respiratory illness (11). Inherent in this model are established best practices facilitated by comprehensive palliative care education and requisite training that includes, but is not limited to, serious illness communication and comprehensive symptom assessment and management (33–35). Clinician competencies should be commensurate with the degree of specialization and relevant to the populations served (e.g., patients with COPD or ILD). To date, the benefits of a primary palliative care model for those with serious respiratory illness have not been well studied, with limited evaluation of nurse practitioners in oncology clinics (36, 37). Other key unanswered questions regarding primary palliative care models include the following: 1) discerning strategies for early identification of patients who may benefit from palliative care; 2) identifying potential referral triggers for secondary palliative care when needs exceed that of a frontline pulmonary–critical care clinician (Figure 4); and 3) developing the most effective ways to teach pulmonary–critical care clinicians and trainees (e.g., fellows and respiratory therapy students) through continuing palliative care education, comanagement delivery models (particularly for more complex needs), and health system-level policies to support implementation. Sources for palliative care education, training, and other resources are included in Table 3.
Figure 4.
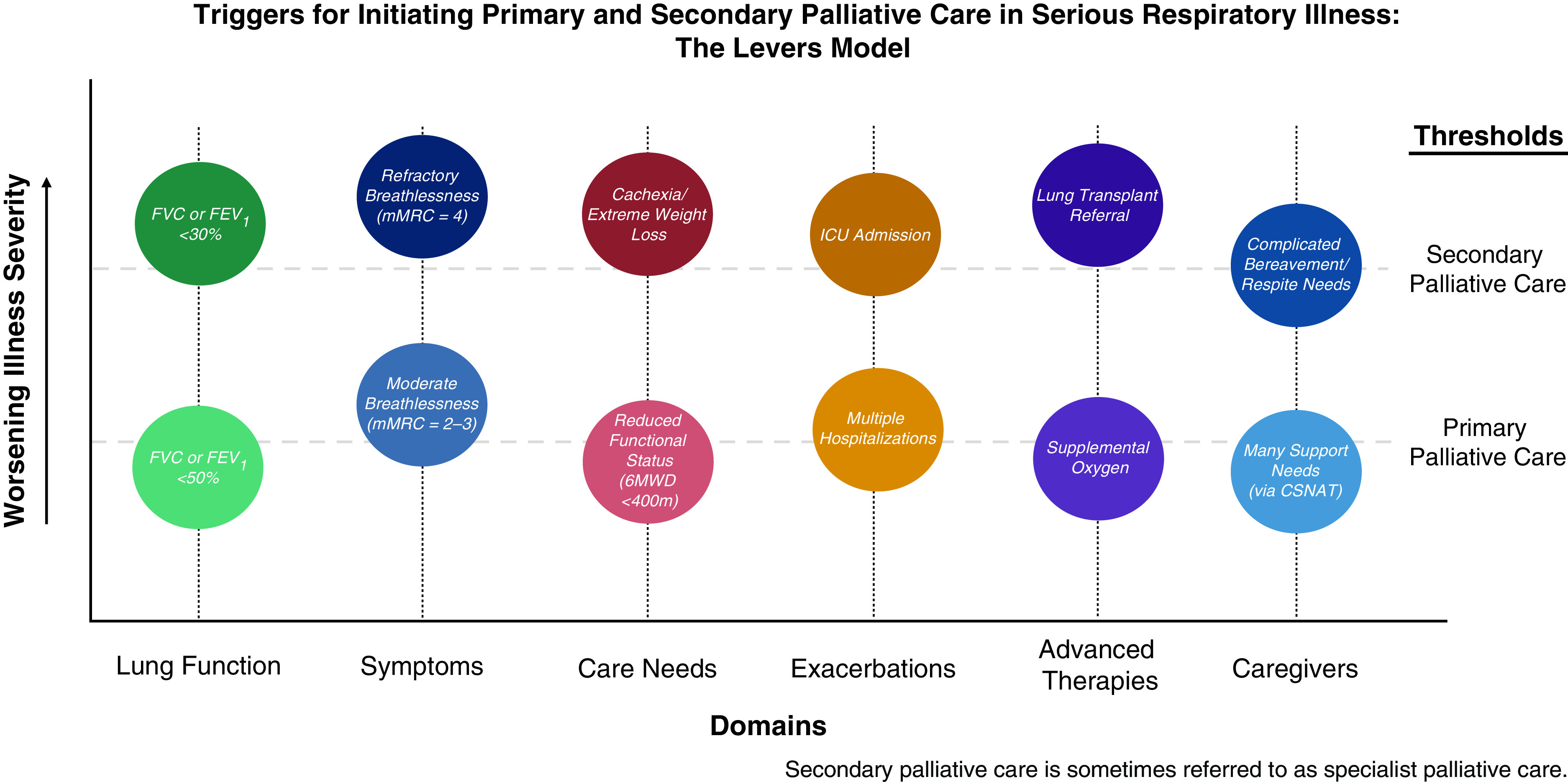
Triggers for initiating primary and secondary palliative care in serious respiratory illness: the levers model. Hypothetical triggers for primary and secondary palliative care occur across multiple domains: lung function, symptoms, care needs, exacerbations, advanced therapies, and caregivers. The x-axis denotes worsening illness severity, whereas the y-axis denotes multiple domains, each with a circle functioning as a lever that can move up or down, crossing a threshold for primary or secondary palliative care, illustrated by the dashed lines. Any one or multiple domains can cross a trigger threshold for consideration for primary or secondary palliative care initiation. For example, a person can trigger consideration for primary palliative care when their FEV1 drops below 50% predicted (lung function domain). Also, a person can trigger consideration for secondary palliative care when their breathlessness becomes severe on the Modified Medical Research Council (mMRC) (symptoms domain). 6MWD = 6-minute-walk distance; CSNAT = carer support needs assessment tool. This figure is adapted by permission from Iyer, AF. The Role of Palliative Care in COPD. Chest 2022;161: 1250–1262.
Table 3.
Sources for Palliative Care Education, Training, and Other Resources
| Organization | Content Focus | Website |
|---|---|---|
| American Academy of Hospice and Palliative Medicine | Professional organization for physicians specializing in hospice and palliative medicine, nurses, and other healthcare professionals. Provides education and advocacy. | http://aahpm.org/education/overview |
| American Thoracic Society | Medical society dedicated to accelerating the advancement of global respiratory health through multidisciplinary collaboration, education, and advocacy. | www.thoracic.org |
| California State University Shiley Haynes Institute for Palliative Care | Advance care planning and palliative care education courses for health professionals and organizations looking to develop their teams. | www.csupalliativecare.org |
| Cambia Palliative Care Center of Excellence at the University of Washington | Provides palliative care education and training for clinicians and conducts research and quality improvement projects to promote innovation in delivering value-based, patient-centered care to diverse populations across the lifespan. | https://cpcce.uw.edu/ |
| Center to Advance Palliative Care | Provides healthcare professionals and organizations with the training, tools, and technical assistance necessary to provide quality health care for people living with a serious illness. | www.capc.org |
| End-of-Life Nursing Education Consortium | National and international education initiative to improve palliative care through training of nurses and other healthcare providers. | www.aacnnursing.org/ELNEC |
| Fast Facts | Provide concise, practical, peer-reviewed, and evidence-based summaries on key palliative care topics important to clinicians and trainees caring for patients facing serious illness. | https://www.mypcnow.org/fast-facts/ |
| Get Palliative Care | Consumer-facing palliative care videos and information. | www.getpalliativecare.org |
| Harvard Medical School Center for Palliative Care | Provides palliative care education for physicians, nurses, social workers, pharmacists, and other healthcare professionals. | https://pallcare.hms.harvard.edu/ |
| Hospice and Palliative Nurses Association (HPNA) | National professional organization that represents the specialty of palliative nursing, which includes hospice and palliative nurses. HPNA supports the profession through education programs, research initiatives, and advocacy. | www.advancingexpertcare.org/HPNA |
| Prepare for your care | Patient-centered tools to help make medical decision-making easier for people and caregivers. | www.prepareforyourcare.org |
| Respecting Choices | Guides organizations and communities to integrate and disseminate evidence-based best practices that ensure individual preferences and decisions for health care are known and honored. | https://respectingchoices.org/ |
| Social Work Hospice & Palliative Care Network | Advocacy and professional development through education, evidence-informed best practice, social work–informed research, and specialty certification. | https://www.swhpn.org |
| VitalTalk | Evidence-based communication training for physicians and advanced practice providers working with seriously ill patients and their families. | https://www.vitaltalk.org |
The coronavirus disease (COVID-19) pandemic brought unprecedented changes to the delivery of healthcare, including the rapid implementation of telehealth and virtual technology (38). The potential value of telehealth in delivering palliative care is difficult to overlook, especially in partnership with community-based programs. Telehealth may be particularly advantageous in delivering palliative care to those with serious respiratory illness who may have mobility and transportation barriers and those in remote or resource-limited settings (39, 40). There also are legitimate concerns that technology could worsen inequities (41), and the unintended consequences of remote delivery of palliative care for patients with serious respiratory illness are largely unexplored (42). However, models of telehealth primary palliative care delivered in a collaborative fashion with rural, community nursing teams have been successful (43, 44).
II. Comprehensive Symptom Assessment and Management
Measurement is a fundamental principle of illness management. As Edwards Deming noted, “if you can’t measure it, you can’t improve it”. Interprofessional approaches to symptom assessment and management in serious respiratory illnesses offer opportunities for more effective collaboration and coordination of care across disciplines. This is vital as patients with serious respiratory illness can experience significant symptom burden (45–48); however, symptoms often go undetected by clinicians (49–51) or are underreported by patients during routine clinical encounters (52). Detailed patient-reported symptom assessments can increase rates of symptom discussions between patients and clinicians (53–55), intensify symptom management by clinicians (53, 56, 57), and improve patient symptom control and health outcomes such as QOL (58–60). Comprehensive, individualized symptom assessments help determine the true impact of an illness on a patient’s life, including limitations of activity, psychological manifestations of disease, missed work and economic hardships, and effects on family routines and overall wellbeing, among others. Accurate symptom assessment is also essential for consideration of additional therapeutic approaches and prognostication (61–66).
Individualized, evidence-based symptom assessments should occur routinely to assess change and identify new symptoms. Important symptoms to assess among patients with serious respiratory illness include chronic dyspnea or breathlessness, episodes of dyspnea crisis, cough, pain, fatigue, sleep disturbances/insomnia, psychological and spiritual distress or suffering (e.g., anxiety, panic attacks, depression, existential angst, and sense of meaning), and weight loss/anorexia (47, 67–69) (Table 4). These symptoms may present in isolation or as symptom clusters, which are a nonrandom distribution of symptoms suggesting a common etiology or pathway (131–134). Pain–insomnia–fatigue and dyspnea–cough are commonly recognized clusters (135). In addition, social assessments should be conducted periodically, including patient relationships with their partners or caregivers with attention to caregiver support and strain, as there is a significant correlation between patient and caregiver health status (20, 25, 136–138).
Table 4.
Symptom Assessment Instruments
| Symptom | Tool | Brief Description | Validation |
|---|---|---|---|
| Health-related quality of life | Chronic Respiratory Questionnaire | 25 items rated on 7-point Likert scale. Focused on the last 2 wk. Available in interviewer or self-administered versions with optional dyspnea domain. Licensed. |
Originally designed and validated for patients with COPD (70). Patients with COPD before and after pulmonary rehabilitation (71). Patients with ILD before and after pulmonary rehabilitation (72). Patients with COPD and ILD (73). |
| King’s Brief Interstitial Lung Disease | 15 items measuring three domains (breathlessness and activities, chest symptoms, and psychological). Focused on last 2 wk, one question with no time period. Licensed. |
Designed and validated for patients with ILD (74). Updated MCID in patients with ILD (75). |
|
| St George’s Respiratory Questionnaire | 50 items measuring symptoms impacts. Focus on 1, 3, or 12-mo recall. Free to use for noncommercial entities. |
Patients with COPD before and after pulmonary rehabilitation (71). Patients with ILD before and after pulmonary rehabilitation (72). |
|
| Short-form 36-item Survey | 36 items rated on Likert scales measuring 8 concepts, broadly categorized into mental and physical component scores. Focus on the past 4 wk and the current. Free to use. |
Patients with ILD before and after pulmonary rehab (72). Male patients with COPD (76). Partially responsive to pulmonary rehabilitation in male and female patients with COPD (77). |
|
| Clinical COPD Questionnaire | 10 items rated on 7-point Likert scale. Focuses on the past week. Free to use. |
Patients with COPD before and after pulmonary rehabilitation (71). | |
| Euro-QOL 5D | 6 questions, allow for monitoring of changes in self-reported health status through time or in response to treatment. Free to use for noncommercial entities. |
Patients with COPD (78). | |
| Living with Idiopathic Fibrosis | 44-item questionnaire specific for patients living with IPF. Licensed. |
Patients with IPF (79). | |
| COPD Assessment Test | 8 items rated on a 0–5 scale of severity. Self-administered. Licensed. |
Developed and validated in patients with COPD (80). Responsive to pulmonary rehabilitation in patients with COPD (81). Validated in patients with ILD (82). |
|
| Breathlessness | Modified Medical Research Council Dyspnea Scale | Single item assessing breathlessness severity on a scale of 0-4. Free to use. |
Predicts degrees of physical activity in patients with COPD (83). Comparison with CAT in patients with COPD (84). Patients with ILD (85). |
| Visual Analog Dyspnea Scale | A single-item rating of breathlessness along a visual line. Has the same limitations as the NRS—single domains measured only. Free to use. |
Validated in patients with COPD and asthma against the Borg exertion scale (86). Validation in patients with ILD, including MCID (87). |
|
| Numeric Rating Scale | A single-item rating of 0 to 10. Modeled from the NRS for pain. Only measures one domain but can be used for multiple separate symptom questions (e.g., breathlessness intensity and breathlessness unpleasantness questions). Free to use. |
Validated in patients with COPD with high correlation against VADS (88). Patients with COPD and ILD (73). |
|
| Dyspnea-12 | 12 items rated on a scale of 0 to 3 severity. Focuses on “these days”. Free to use. |
Developed and validated in patients with COPD, ILD, and chronic heart failure (89). Validated in patients with ILD (90). |
|
| Respiratory Distress Observation Scale | 8 items rated on a scale of 0 to 2 severity assessing the intensity and distress of dyspnea among patients unable to self-report. Free to use. |
Developed and validated in patients with a pulmonary or cardiovascular disorder and postoperative pain and healthy volunteers (91, 92). | |
| Cough | Leicester Cough Questionnaire | 19 items rated on 7-point Likert scale across three domains (physical, psychological, and social), measuring the impact of cough. Self-administered. Free to use. |
Designed and validated for chronic cough patients (93). Validated for patients with COPD (94). Validation in patients with ILD (85). |
| Cough Symptom Score | 2 items rated on a 0–5 scale assessing cough symptoms during daytime and nighttime. No information on copyright. |
Design and validation in chronic cough patients (95). | |
| Cough and Sputum Assessment Questionnaire | 20 items measuring 4 domains (cough and sputum symptoms and respective impact). Focused on past 7 d. Self-administered. Licensed. |
Development and validation in COPD and bronchitis (96). Responsive to exacerbations in COPD (97). Usage in ILD (98). |
|
| Pain | Pain Sensitivity Questionnaire | 17 items rated from 0 to 10. Self-administered No information on copyright. |
Review of validation in COPD (99). |
| Geriatric Pain Measure | 24 items scored as yes/no or on a scale of 0–10. Measures multiple dimensions of pain and can be self-administered. Free to use. |
Review of validation in COPD (99). | |
| Brief Pain Inventory | Short form consists of 9 items. Self-administered. Measures average, least, and worse pain intensity. Free to use. |
Validation in patients with ILD (100). Validation in COPD (101). Validation in COPD during exacerbations (102). |
|
| Short-form McGill Pain Questionnaire | Short form consists of 2 subscales, 11 sensory items, and 4 affective. Rated on a scale of 0–3. Licensed. |
Validation in patients with ILD (100). Validation in COPD during exacerbations (102). |
|
| Fatigue | Functional Assessment of Chronic Illness Therapy scale for fatigue |
Short version consists of 13 items modified for COPD. Free to use for noncommercial entities. |
Development and validation of COPD version (103). |
| Fatigue Severity Scale | 9 items rated on 7-point Likert scale. Free to use for noncommercial entities. |
Validated in COPD (104). Before and after pulmonary rehab in general advanced respiratory illness (105). |
|
| Manchester COPD-Fatigue Scale | 27 items measuring three dimensions (physical, cognitive, and psychosocial). No information on copyright. |
Developed and validated in COPD (106). | |
| Sleep | Pittsburgh Sleep Quality Index | 10 items measuring 7 subscores, self-administered. Focused on last month. Free to use on request. |
Validation in COPD against SGRQ QOL score (107). Validation in COPD (108). Moderate correlation with CAT score in COPD (109). Associated with poor sleep quality in ILD (110). |
| Berlin Questionnaire | 10 items measuring three categories. Free to use on request. |
Validation in COPD against SGRQ QOL score (107). Did NOT predict obstructive sleep apnea in patients with COPD (111) or ILD (112). |
|
| Epworth Sleepiness Scale | 8 items rated on 0–3 scale, self-administered. Licensed. |
Validation in COPD against SGRQ QOL score (107). Did NOT predict obstructive sleep apnea in patients with COPD (111). Associated with poor sleep quality in ILD (110). |
|
| Psychological wellbeing | Hospital and Anxiety Depression Scale | 14 items rated on a 4-point scale, 7 assess depression, and 7 assess anxiety. Licensed. |
Validated in COPD, including MCID (113). Correlation between dyspnea and comorbidities in patients with ILD (114). |
| Patient Health Questionnaire-9 | 9 items rated on the Likert Scale, assesses depression severity. Free to use. |
Usage in COPD (115, 116). | |
| Geriatric Depression Scale | Long-form is 30 items. Short form consists of 15 items measured as yes/no responses. Free to use. |
Correlated with life event stress in patients with COPD (117). Responsive to pulmonary rehab in ILD (118). |
|
| Beck Depression Inventory II | 21 items rated on a 4-point scale of severity, focuses on 1–2 weeks. Self-administered. Licensed. |
Validated in patients with COPD when question 21 was excluded (119). Not responsive to antifibrotic treatment in ILD (120). |
|
| Weight loss and anorexia | Malnutrition Universal Screen Tool | 3-item assessment combining BMI, unplanned weight loss, and acute illness effect to assess malnutrition risk. Clinician-assessed. Free to use. |
Usage in COPD (121). |
| General palliative care needs | Needs at the End of Life Screening Tool | 13-item scale adapted for use by family members of patients in the ICU that samples from all eight domains of palliative care quality. No information on copyright. | Original version for use by patients with cancer (122, 123). Version adapted for use by family members of patients in the ICU (124, 125). |
| Palliative Care Outcomes Scale/Integrated Palliative Care Outcome Scale | 10-item scales; POS and IPOS contain two similar measures, one completed by staff, the other by patients to assess: physical, emotional, psychological, spiritual, and provision of information and support dimensions. Licensed. | Usage in patients with serious respiratory illness (e.g., lung cancer, ILD, and COPD) in diverse settings (25, 126, 127). | |
| Edmonton Symptom Assessment Scale | 9-item scale developed for use in assessing the symptoms of patients receiving palliative care. Free to use. | Usage in COPD (128, 129) and lung cancer (130). |
Definition of abbreviations: BMI = body mass index; CAT = COPD assessment test; COPD = chronic obstructive pulmonary disease; ILD = interstitial lung disease; IPF = idiopathic fibrosis; IPOS = integrated palliative care outcome scale; MCID = minimally clinical important difference; NRS = Numeric Rating Scale; POS = palliative care outcome scale; SGRQ QOL = St. George’s Respiratory Questionnaire quality of life; VADS = visual analog dyspnea scale.
Central to symptom management in several serious respiratory illnesses is pulmonary rehabilitation; for people with COPD and ILD, it is an effective intervention for breathlessness, fatigue, and emotional symptoms (139, 140). This and other nonpharmacologic treatment options may complement disease-directed pharmacologic therapies such as inhalers, nebulizers, mucus clearance medications, immunosuppressants, and antifibrotics, among others (66). Although not exhaustive, some of the more common pharmacologic and nonpharmacologic therapies are listed (Table 5). Low-dose opioids are the mainstay of refractory breathlessness treatment in patients with advanced cancer; however, they are often underused in those with serious respiratory illness because of concerns they may reduce respiratory drive and hasten death. However, the potential for serious opioid-related side effects may be overstated, as a recent trial found that low-dose oral sustained-release morphine improved health status in patients with COPD without causing hypercapnia or other serious adverse effects (163). Additional advanced palliative therapies may be considered in select patients if the potential benefits outweigh the risks (e.g., endobronchial valves in COPD, palliative radiation, or stents in lung cancer).
Table 5.
Complementary Pharmacologic and Nonpharmacologic Symptom Management Therapies in Serious Respiratory Illness*
| Symptom | Nonpharmacologic | Pharmacologic |
|---|---|---|
| Dyspnea or breathlessness | Acupuncture/acupressure/massage, noninvasive positive pressure ventilation, supplemental oxygen therapy, breathing or relaxation techniques (e.g., pursed lip breathing, breathing‐relaxation training), fan therapy (141–146) | Opioids (141–144) |
| Cough | Chest physiotherapy, speech therapy, breathing exercises (147–149) | Opioids, neuromodulators, cromoglicic acid/sodium cromoglycate, central antitussive agents, GABA analogs (150–153) |
| Pain | Acupuncture/acupressure/massage, music therapy, surgical options (e.g., surgical debulking and vertebroplasty) (154–157) | Opioids, antidepressants, anticonvulsants, muscle relaxants, anxiolytics, corticosteroids, topical analgesics (158–160) |
| Psychological distress (e.g., anxiety and depression) | Cognitive-behavioral therapy, counseling and other support, coping skills training, mindfulness-based stress reduction, music therapy (161, 162) | SSRI or tricyclic antidepressants, anxiolytics, ketamine, methylphenidate (161, 162) |
Definition of abbreviations: GABA = γ-aminobutyric acid; SSRI = selective serotonin reuptake inhibitor.
These approaches may be considered complementary to evidence-based and disease-directed pharmacologic and nonpharmacologic therapies (e.g., inhalers, nebulizers, mucus clearance and immunosuppressive medications, antifibrotics, and pulmonary rehabilitation, among others).
Clinicians should encourage self-care maintenance among patients with serious respiratory illness as it is an essential treatment adjunct that can improve QOL, reduce respiratory-related hospital admissions, and decrease dyspnea (164). According to the middle-range theory of self-care in chronic illness, interventions may focus on one of three related dimensions: self-care maintenance, monitoring, and management (165). Self-management of breathlessness among patients with serious respiratory illness should be a focus as chronic breathlessness causes significant panic, distress, and desperation that significantly limits daily activities and social life (166). Among patients with serious respiratory illness, components of self-care interventions include recognition and treatment of exacerbations, physical activity and exercise behaviors, and education and tools to self-manage symptoms such as breathlessness (164, 167). For instance, clinicians can help patients and caregivers in developing an action plan for breathlessness with instructions for worsening symptoms, including crises. These action plans may include home spirometry monitoring, starting an oral corticosteroid, temporarily limiting strenuous activities, avoiding tobacco products (if applicable), practicing breathing–relaxation training, receiving massage therapy, or notifying their clinicians (168, 169). Patients and their caregivers can become adept at preventing, controlling, and managing the physical, psychological, and social consequences of respiratory illness, and clinicians should encourage self-care management activities while acknowledging their potential limitations.
III. Advance Care Planning and Goals of Care Discussions
ACP is a process that supports persons “at any age or stage of health in understanding and sharing their personal values, life goals, and preferences regarding future medical care. The goal of advance care planning is to help ensure that people receive medical care that is consistent with their values, goals, and preferences during serious and chronic illness” (7). Goals of care (GOC) discussions may help ensure that care is consistent with patient preferences, and these discussions can be framed around specific medical interventions (e.g., intubation and mechanical ventilation). However, this committee considered a broader definition of GOC discussions as an important component of the ACP process in developing a better understanding of patient values and preferences and does not distinguish these as separate constructs. The ethical principle of respect for patient autonomy underpins the ACP process and autonomous decisions as those that are made intentionally and with substantial understanding and freedom from controlling influences (170). ACP positively impacts the quality of end-of-life care (171, 172) and can support patients, surrogates, and clinicians by avoiding crisis-oriented decision-making at the very end of life that may be inconsistent with patient goals and increase the burden and stress for surrogates (173–176). This is particularly relevant for patients with serious respiratory illness who are at elevated risk of losing the ability to communicate because of unexpected acute deterioration and the need for decision-making after acute respiratory failure (175, 177–180). As a result, pulmonary-critical care clinicians are well-positioned to be leaders in conducting ACP discussions among patients with serious illness and their families.
ACP conversations may include the creation of documents such as advance directives, living wills, assignment of a healthcare proxy, and POLST (Portable/Physician Orders for Life-Sustaining Treatment) that are readily available to the healthcare team. These forms should be reviewed and updated by patients on a regular basis as health or conditions change. However, completion of these documents without considering the quality of the preceding patient–clinician communication should never be used as a quality metric. In addition, ACP documents have limited ability to address all potential health scenarios; thus, healthcare proxies should be prepared to make substituted judgments (181–184) and, ideally, with patient consent, should be included as active participants in ACP discussions as healthcare decisions can be overwhelming.
ACP consists of structured communication and documentation that are woven into the regular process of care provided to patients and their families and should begin in the early stages of respiratory illness (Figure 5). This early and iterative approach may help to normalize these conversations and is a key recommendation of the 2014 Institute of Medicine, Dying in America Report (185). Early initiation of ACP also is consistent with a recent consensus definition that states that ACP should support persons at any age or stage of health (7). In addition, early ACP can strengthen patient–clinician relationships and provides an opportunity to introduce the concept of palliative care (186–188). Respecting Choices is one successful model intended to normalize ACP communication using a stepped approach in which the early stages of the process may only focus on asking patients to identify a medical decision-making surrogate (189).
Figure 5.
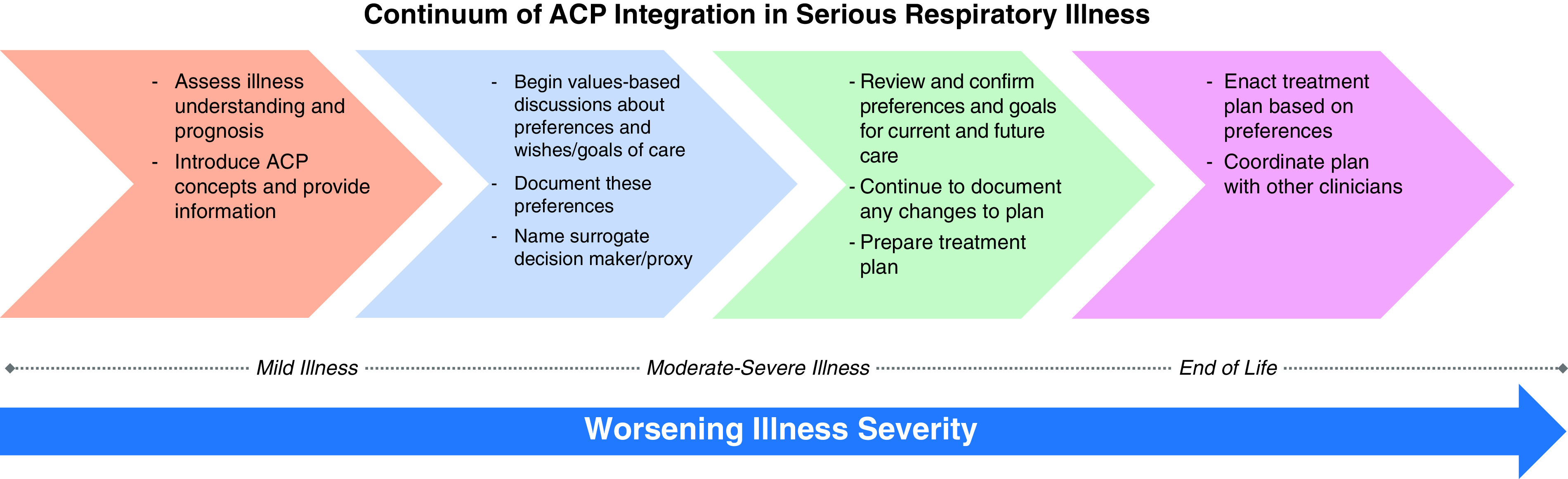
Continuum of advance care planning integration in serious respiratory illness. The continuum of ACP in serious respiratory illness is a longitudinal process of communication that begins soon after illness diagnosis (stage 1: first arrow) and increases in intensity on the basis of patient illness trajectory (stages 2–4). This process should be free of coercion, and clinicians should be alert to signs of patient distress or discomfort. Patient-designated surrogates or healthcare proxies should be included across all stages with patient permission. ACP = advance care planning.
Improving uptake of ACP is not without significant challenges (190–192), including the need for clinicians to acquire specialized knowledge and skills through feedback from ACP facilitators and/or serious illness communication experts, as well as ongoing opportunities to practice these skills. Unfortunately, a lack of knowledge or formal training in ACP persists among pulmonary–critical care clinicians (193–195) despite evidence that training and education have positive effects on clinician knowledge, attitude, and skills in conducting ACP (196). For these reasons, completion of serious illness communication and ACP training should be required of all pulmonary–critical care clinicians and trainees as a core competency. This training has been endorsed by representatives of the ATS, the American College of Chest Physicians, the Society of Critical Care Medicine, and the Association of Pulmonary and Critical Care Medicine Program Directors as Entrustable Professional Activities (197).
Despite the potential importance of ACP, it is worth noting emerging concerns about the limited evidence supporting the effectiveness of ACP in achieving goal-concordant care or in improving family member perceptions of quality of care (190, 198). These findings may be because of the inability to plan for or envision the myriad of potential health situations that may arise over the course of an illness and the inability to foresee the emotional and psychological challenges that can occur during in-the-moment decision-making (190, 198). Some dissensus existed within the committee regarding the importance and future role of ACP in serious respiratory illness, given concerns about its effectiveness and the focus within the ACP literature on the creation of documents (e.g., advance directives). These concerns reinforce the committee's consensus recommendations regarding ACP for 1) early identification of healthcare proxies to enable them to better prepare for in-the-moment decision-making using substituted judgment; and 2) a shift in focus away from document completion as the goal of ACP to ensuring high-quality serious illness communication occurs instead.
IV. Caregiver Support
Informal caregivers or caregivers (e.g., family members, partners, and friends in unpaid roles) provide essential support to patients living with serious respiratory illness (199) which can be rewarding and provide caregivers a sense of accomplishment (200). Caregivers are alternatively referred to as “carers”, “care partners”, or sometimes “supporters”. The caring role can also have negative impacts on the caregiver(s), and both patients and caregivers report a range of unmet support needs throughout the illness course (199, 201, 202). These can include a need for disease education, learning how to use respiratory equipment (e.g., oxygen delivery devices), information about financial assistance, and help navigating the healthcare system. Supporting the caregiver helps to sustain caregiving, thereby supporting the patient (See Box 1).
Box 1. A Patient and Caregiver Perspective
Patient Voice:
Lung disease is awful. When you receive the bad news that you have a serious or terminal lung disease, you freeze, and you hear nothing else. Then you hope that your clinician or team of clinicians can help guide you and your family through the process of making tough treatment decisions. Decisions that reflect “who you are” and “how you want to live”. Sadly, this happens all too seldom. Even clinicians with communication training can get it wrong when the conversation begins with an advance directive checklist and questions about end-of-life decisions; you turn off, and you lose trust. Consequently, you miss out on a lot of support that can help down the road. Clinicians need to first understand who a patient is as a person, what we are experiencing, and what we want and need now and in the future before explaining how palliative care services can benefit us and our loved ones. Invite us and our caregivers to be active participants in the team to set the ground rules for future interactions. We tend to be receptive to receiving support if we believe we are being taken seriously and respected, which gains trust. Once trust is established, it makes it much easier to introduce palliative care and end-of-life issues. It is critical that all clinicians are educated to communicate with patients and our families using standard approaches, and hopefully, this can ensure we receive high-quality palliative care. It doesn’t matter which member of the team introduces palliative care; getting help for the patient and caregiver is the goal.
Caregiver Voice:
We were told by my husband’s doctor we had 1–2 years when he was diagnosed with pulmonary fibrosis: we got 21 months and made every minute count. Every day we said aloud, “I love you”. Although we had family and friends to help, the majority of care fell on me as a spouse. Each day was a regimented, at times exhausting, schedule. No one discussed palliative care with us. From life’s experiences and a little medical knowledge, I was able to ask the right questions to know what he really wanted at life’s end. Visiting nurses started only 3 months before he passed away; hospice entered 10 days before his death. Both assured me I had been correctly caring for him all those months by myself. What a relief to my heart. Our journey would have been so much easier if palliative care was offered as soon as we were told his illness was terminal. Caregivers (spouses, children, family members, and friends) need to be included and acknowledged at every medical visit, including when palliative care is introduced. We need to know what the palliative care team can offer us and what they can’t provide (like care during a crisis in the middle of the night). We need to be asked about our loved ones—we see and know things clinicians don’t. Importantly, we need to be offered support and services for our own needs, too, including a support network.
The first step in supporting caregivers is identifying them (199). Many patients and caregivers do not recognize labels such as “caregiver” or “carer” and can confuse these labels with formal (i.e., paid) caregivers. Caregivers see themselves in relational terms (e.g., spouse or partner, child, or friend), and patients may not accept the idea of being “cared for” and so reject the label. It is, therefore, better to reframe identifying questions to patients as “who gives you help and support at home?” (199, 201). Clinicians can thus be instrumental in helping caregivers access crucial support services and financial benefits. Clinicians should also acknowledge that there can be multiple caregivers performing various roles, and these caregivers may be geographically distant and can change throughout the lifespan of the patient. Therefore, ongoing identification of caregivers should be revisited during follow-up clinical encounters.
Condition-specific needs related to caregivers of patients with serious respiratory illness exist (201). For instance, patients being evaluated for lung transplantation and those on the transplant waitlist are required to have an identified caregiver present at transplant evaluation and through posttransplant. Incorporating an assessment of caregiver needs early in the lung transplant process may be useful in identifying caregivers who need more support from the transplant team and/or a palliative care consult in the posttransplant period (202). Respiratory illnesses also often require technical equipment (e.g., mechanical ventilator and oxygen delivery device), and palliative care may include helping caregivers adjust to being responsible for this equipment (203, 204).
Caregiver support is everyone’s responsibility. The caregiver should be acknowledged by the clinician and, at a minimum, asked how he/she is doing (directly or via the patient if the caregiver is not present). Evidence-based approaches (e.g., CSNAT-1 [Carer Support Needs Assessment Tool Intervention]) (205, 206) allow caregiver support needs to be comprehensively identified and addressed. Exploring caregiver needs jointly with the patient can raise patient awareness of caregiver needs, but opportunities to talk about needs separately from the patient should also be provided. As needs change, conversations should be revisited along the illness trajectory, including into bereavement. It can sometimes take a clinician to identify caregiver needs, as caregivers themselves may not recognize them (i.e., being overprotective of the patient). Timely/early provision of information on support options can reinforce the idea that caregivers also need to care for themselves.
Clinicians should take a person-centered, caregiver-led approach to meeting needs and identifying solutions. Conversations around support needs are enough to make caregivers believe they are more supported. Other times provision of information or education, resource signposting (Table 6) or referral is required (e.g., support groups, advocacy organizations, psychological support, or wellbeing services). Caregivers should be kept informed and involved in decision-making (with the permission of the patient). Technology can facilitate this for geographically distant caregivers (e.g., telephone, video conferencing, or Caring Bridge [207]), although technical skills and preferential barriers may exist.
Table 6.
Resources for Caregivers
Definition of abbreviation: COPD = chronic obstructive pulmonary disease.
V. Health Disparities
Given the evidence base for the benefits of palliative care in serious respiratory illness, it is imperative that palliative care services are equitably distributed, of similar quality, and meet the needs of all eligible people. Access to palliative care is a fundamental right regardless of citizenship status, sex, race/ethnicity, houselessness, LGBTQIA+ status, rurality of home residence, or insurance status. However, evidence demonstrates that social, cultural, and economic factors continue to limit access (208–210). Striking disparities in access exist among socially disadvantaged populations (211) as well as rural communities (4, 212). Inequitable access may be worse internationally, especially in low- and middle-income countries, as only 14% of the global population has access to the highest degree of palliative care, which outside of the United States and Canada is concentrated in Western Europe and Australia (5). Synonymous with inadequate access to palliative care, racial and ethnic disparities in opioid treatment and accessibility for pain exist in many communities (213–220). This is particularly meaningful as respiratory illness disproportionately affects individuals from disadvantaged populations (221), and the lowest socioeconomic groups are up to 14 times more likely to be afflicted than the highest group (222).
Even in similarly-resourced settings, disparities in the quality of palliative care are reported, particularly regarding clinician communication (223–226). Breakdowns in communication can result in more intense care at the end of life, increased in-hospital deaths, and increased financial burden and complicated bereavement for families (227). Ineffective communication also contributes to reduced knowledge and completion rates of advance care planning among African Americans, Hispanics, and Asians (228–233). Ensuring equitable access to advance care planning among disadvantaged populations is important; however, these efforts should emphasize efforts to determine patient readiness, avoidance of coercion, and development of a better understanding of cultural beliefs and practices about and attitudes toward end-of-life care choices enabling individualized approaches. Underlying ineffective communication may be a result of a lack of cultural awareness, such as proper language/linguistics regarding end-of-life issues among clinicians, which likely perpetuates the ongoing lack of trust among some groups (234). Both the National Quality Forum and National Consensus Project for Quality Palliative Care identify the provision of culturally sensitive care as one of eight core domains of high-quality palliative care (235). Successful palliative care interventions to reduce disparities have included strategies to enhance cultural awareness among clinicians and improve health literacy among patients (231, 236–239). In addition, efforts to diversify the palliative care workforce, including healthcare professionals, social workers, spiritual care workers, and community health workers, are long overdue and will require innovative solutions. Comprehensive training (e.g., palliative care terminology and communication; active listening; and cultural traditions, awareness, and sensitivity) of pulmonary–critical care clinicians is essential in the provision of primary palliative care. (Table 7)
Table 7.
Communication Considerations and Strategies among Disadvantaged Groups
| Theme | Rationale | Implementation |
|---|---|---|
| Attitudes toward ACP documentation | Patients who belong to some ethnic groups are less likely to engage in ACP because of cultural or religious beliefs or distrust of the medical system as a result of historical mistreatment of certain communities (240–242). | Offer referral to a clinician of a similar ethnic/cultural background as some patients may be more comfortable because of historical or structural discrimination. Patients and families may also be encouraged to derive support from their individual systems of faith or consult their faith leaders for additional guidance. Clinicians can use electronic medical record templates to document detailed ACP discussions (allowing patients to edit content through secured portals); printed copies can be shared with patients. |
| Family support in decision-making | There are important differences in cultural orientation toward individualism or communalism as values of how individuals express their autonomy (243). Therefore, clinicians may encounter meaningful differences in family involvement in serious illness decision-making by race and ethnicity. | If patients are amenable, clinicians should recommend family or informal caregiver inclusion in serious illness discussions. Clinicians should inquire how and to what extent patients want their families involved. |
| Lower health literacy | Clarify potential discordant understanding of the diagnosis or clinical situation during discussions. Discordance affects patient and family concerns and understanding of their needs at all stages of serious respiratory illness. | At the outset of all discussions, ask patients and families to describe their understanding of the situation, diagnosis, or trajectory of illness. |
| Non-English speakers | Tone of voice, facial expressions, body language and gestures, and eye contact are all forms of nonverbal communication that are interpreted by patients and families (244–246). Ensure patient and family understanding when interpreters are used (247, 248). | Be cognizant of nonverbal communication. Unless you are a native speaker, using translators as professionally trained interpreters can be effective cultural mediators (249). |
| Refugees, immigrants, and undocumented citizens | On the basis of responses, clinicians may be able to provide patients and families access to local or institutional resources to address needs. | Ask questions about patient and family backgrounds that may inform decision-making (e.g., historical and political context of their lives, place of birth, experiences with poverty, experience with discrimination, and degree of integration within their ethnic community). |
| Prognostication | Some cultures believe that discussing death hastens the process and/or have taboos against talking about death (243, 250–252). | Refrain from making assumptions about the degree of information patients and families want. Ask patients and families directly who they want to receive information, how they want it conveyed, and how much information they want to know. Given the potential for prognostic uncertainty at the end of life, providing categorical time estimates such as “days”, “weeks”, or “months” without specifying a number may facilitate a shared awareness (253, 254). |
Definition of abbreviation: ACP = advanced care planning.
VI. Mass Casualty Events and Emergency Preparedness
Natural disasters and catastrophic mass casualty events, such as COVID-19 or influenza pandemics, are increasing in frequency and severity, resulting in widespread human, material, economic, and environmental impacts yielding thousands of victims whose needs overwhelm local and regional healthcare systems, personnel, and resources (255). These events generally fall into two categories: 1) ‘‘big bang’’ single incidents with immediate or sudden impact, such as airplane or train crashes, which yield large numbers of casualties at the outset of the event with few added over time; and 2) ‘‘rising tide’’ incidents that start with few casualties such as pandemics or widespread, ongoing exposures to chemical, biological, and nuclear agents yielding a gradual increase in the number of people affected over time, rising to catastrophic numbers with prolonged impact and necessitating a more prolonged response (256). Such conditions necessitate deploying scarce resources in a manner that is different from usual care, ranging from conventional to contingency to crisis standards of care.
The COVID-19 pandemic has highlighted increasing palliative care needs across healthcare systems (257). As illustrated by the pandemic, crisis palliative care focuses on three domains: 1) management of symptoms; 2) discussion of patient wishes, expectations, and values; and 3) supporting families of those with life-limiting injury or illness (209). At a minimum, palliative care in catastrophic disasters should include aggressive and appropriate treatment of pain and other symptoms and support and comfort for patients, their families, and their clinicians (256–260). Although the primary goal of a coordinated response to a mass casualty event is to maximize the number of lives saved, a comprehensive response should also seek to minimize the suffering of those who may not survive (256). As healthcare clinicians, there is always something to offer, even at the end of life. A framework for providing palliative care during a catastrophic event is summarized in Table 8.
Table 8.
A Framework for Planning a Palliative Care Response during a Catastrophic Event on the Basis of the U.S. Task Force on Mass Casualty Critical Care Guidelines (258)
| Critical elements | Description |
|---|---|
| Stuff | A medication stockpile (“palliative symptom management kits”) should be created for long-term use by all facilities, paramedics, and other healthcare professionals. Adequate PPE should be available to facilitate safe care delivery. |
| Staff | Response plans should include all healthcare professionals with palliative care training as well as those who can educate others in palliative care. |
| Space | Usage of nearby locations or specialized wards may be necessary to ensure a peaceful and quiet environment for dying patients. |
| Systems | Triage systems and virtual care models can be used to protect clinicians from the risk of infection while also increasing their efficiency. |
| Sedation | Sedation can offer comfort to patients whose symptoms are not responding to standard comfort medications. |
| Separation | Patients can use video calling and other technologies to connect with family members to lessen the sense of separation they may experience because of isolation measures. In-person visitation should be considered if all parties are willing to accept risks and appropriate PPE is available. |
| Communication | Some patients may not want to undergo life-saving measures, so an understanding of patient wishes is critical. It is important to maintain open communication with patients and their families. |
| Equity | All patients should have access to palliative care, regardless of status as a marginalized group, such as those with disabilities, trauma, or poverty. |
Definition of abbreviation: PPE = personal protective equipment.
VII. Research Priorities
Currently, there are numerous challenges to delivering palliative care to the right patient at the right time. In fact, it is unclear who ideally should receive palliative care, from whom it should be delivered, and what exactly the appropriate intervention(s) should be. Patient and caregiver populations for palliative care research studies should be identified on the basis of needs for, and the likelihood of benefit from, palliative care services. Palliative care trigger tools (i.e., tools that indicate the need or suggest the timing of referral) are available for specific patient populations, such as those admitted to an intensive care unit (ICU) (261). However, given that palliative care triggers may not be associated with palliative care needs (125), measurement tools to differentiate patients who could benefit from primary vs secondary palliative care further upstream in the illness trajectory (i.e., in ambulatory settings) would be helpful. Palliative care outcome measures should expand beyond healthcare system/usage outcomes (262, 263) as palliative care services often include complex interventions such as ACP and symptom management (264). Therefore, multiple outcomes (e.g., patient- and family-centered measures) and implementation outcomes like adoption and sustainability and the use of newer study designs such as MOST (multiphase optimization strategy) (265) are needed to examine which specific component(s) of the intervention are effective, including measuring specific impacts proximal to the intervention and potential harms (20). Research is needed to identify when to initiate palliative care to achieve maximal benefit for patients and families for specific chronic respiratory illnesses (e.g., COPD) (48). Effective telehealth-based palliative care delivery models that include sustainable payment models (266) are critical to reaching patients living in rural and/or underserved communities.
Developing and testing primary palliative care training interventions that are practical, scalable, and embedded within existing clinical settings is an additional priority. The critical shortage of palliative care specialists (267) necessitates pulmonary–critical care clinician competency in fundamental palliative care skills. More work is also needed to develop models with integrated or comanagement of primary and secondary palliative care in ways that are acceptable, sensible, and efficient (268). Application of novel methodologies and study designs are needed to improve the quality of palliative care evidence in pulmonary and critical care medicine. Examples include quasi-experimental/natural experiments, adaptive designs, hybrid and pragmatic trials, and mixed methods research. Pulmonary palliative care interventions that have been conducted in ICU settings can guide this work; however, further research is urgently needed for seriously ill patients and their caregivers managed in pulmonary outpatient clinics. Lastly, it is critical to include people of color, non-English speakers, and members of rural communities in future studies to ensure a better understanding of their unique palliative care needs (269). Expanding funding for the cultural and linguistic adaptation of effective palliative care interventions to diverse populations offers a high-value opportunity to broaden the reach of palliative care.
The “culture problem” of integrating palliative care into pulmonary and critical care medicine is a challenge that is not unique to the field. Despite recommendations from professional societies to offer palliative care to patients with advanced cancer, the adoption of palliative care is variable depending on the service and setting (22, 270). Therefore, trials are needed to test the effectiveness of efficacious interventions with primary aims focusing on the outcomes of implementation science, such as clinician adoption of the intervention and sustainability in different practice settings. A clearer framework could help the field guide and successfully implement palliative care while enhancing its scalability. Palliative care interventions in the ICU and pulmonary outpatient settings appear promising, but more research is needed to understand the full range of patient, family, and healthcare system impacts (271).
Technological advancements, including the leveraging of electronic health record systems for useful clinical tasks and the use of methods such as artificial intelligence and machine learning, are likely to accelerate the conduct of future clinical research. Examples include patient identification for study criteria and outcomes collection. Technology in the form of telehealth can be used to support clinician education, patient self-management, and improved care delivery and disparities. A number of self-directed mobile app-based interventions targeting psychological distress and physical symptoms are also available (272–274). Lastly, researchers from around the globe can meet and collaborate without traveling by using available technology to access data and conduct collaborative research to accelerate innovation.
Other important research priorities include characterizing the impact of clinicians engaging in early and regular GOC discussions on “in-the-moment decision-making” and goal concordant care (191, 275). The palliative care field is making progress in developing measures of goal concordance (276) and applying them in research studies. Approaches toward managing common symptoms in respiratory illnesses, such as acute breathlessness, cough, and fatigue, require further investigation to improve the efficacy of patient and caregiver self-care management as well as their QOL. For example, the effectiveness of specific opioid formulations and routes as well as target illnesses has not been established. Furthermore, the effects in daily life of supplemental oxygen in patients without hypoxemia and the use of heliox on dyspnea and functional ability are unknown (277). In addition, studies on the effects of nonpharmacological approaches to symptom management, such as graded exercise therapy for fatigue, are important next steps to tailor care. Lastly, caregiver research is a priority as the demand for informal caregiver involvement in patient care increases while the caregiver population ages. Developing and evaluating dyad-targeted palliative care interventions for patients and caregivers living with serious respiratory illnesses is important as they may improve QOL, and this research can inform state and federal policy initiatives on caregiver support.
In summary, to comprehensively address the research priorities identified by the members of this committee (Table 9), it is imperative to engage a broad group of partners that includes patients, families, caregivers, policymakers, and funders (e.g., National Institutes of Health, Patient-Centered Outcomes Research Institute, and foundations) to help prioritize topics and ensure adequate funding (264, 265).
Table 9.
Research Priorities
| Categories | Examples |
|---|---|
| Study methodology | |
| Measurement | Develop and test novel outcome measures (e.g., patient- and family-centered measures); better define populations who should receive palliative care and the timing of initiation of palliative care |
| Implementation | Determine how best to operationalize interventions among the seriously ill, using novel study designs when appropriate to evaluate, develop, and test implementation outcomes such as acceptability, adoption, penetration, and sustainability |
| Content areas | |
| Patient identification tools | Measurement tools and the use of machine learning to differentiate patients who could benefit from various degrees of palliative care (e.g., primary vs. secondary palliative care) along the illness trajectory (i.e., in ambulatory settings) |
| Delivery models | Primary palliative care; primary plus secondary palliative care integration; multiprovider and nonclinician integration; technology intervention such as leveraging electronic health records and telehealth interventions |
| Symptom management | Patient and caregiver self-management strategies; studies identifying best practices in pharmaceutical and nonpharmaceutical management of common symptoms in respiratory illnesses (e.g., acute breathlessness, cough, and fatigue) |
| ACP and decision-making | Improving ACP and “in-the-moment” decision-making; identifying proxies; examining the outcomes of longitudinal goals-of-care discussions |
| Caregivers | Dyad-targeted palliative care interventions |
| Disparities | Interventions to increase workforce diversity; interventions tailored to different cultures and languages and rural populations |
Definition of abbreviation: ACP = advanced care planning.
Conclusions
Patients with serious respiratory illness and their caregivers suffer from significant physical, emotional, spiritual, and financial burdens and are in need of early palliative care to help guide treatment planning. Ideally, primary palliative care should be initiated by pulmonary–critical care clinicians early in the care continuum seamlessly alongside disease-directed treatments. Secondary palliative care has an important complementary role in supporting primary palliative care by providing interprofessional expertise as needed. Given the robust evidence-based support for palliative care in improving QOL among patients with serious illness, the provision of this care is a fundamental right for any patient and/or caregiver who may benefit. This multisociety policy statement presents essential palliative care recommendations, values, and guiding principles among patients with serious respiratory illness and is designed to provide guidance for clinicians and to inform policy initiatives.
Acknowledgments
This policy statement was prepared by an ad hoc subcommittee of the American Thoracic Society, American Academy of Hospice and Palliative Medicine, Hospice and Palliative Nurses Association, and Social Work Hospice and Palliative Care Network.
Members of the subcommittee are as follows:
Donald R. Sullivan, M.D., M.A., M.C.R.1,2* (Co-Chair)
Anand S. Iyer, M.D., M.S.P.H.3,4,5 (Co-Chair)
Susan Enguidanos, Ph.D., M.P.H.6‡ (Co-Chair)
Lynn F. Reinke, Ph.D., A.N.P.-B.C.7‡ (Co-Chair)
Margaret L. Campbell, Ph.D., R.N.8§
Katherine R. Courtright, M.D., M.S.H.P.9‡
Christopher E. Cox, M.D., M.P.H.10‡
Morag Farquhar, M.Sc., Ph.D.11‡
Tracy K. Fasolino, Ph.D., F.N.P.-B.C., A.C.H.P.N.12§
Patricia M. Fogelman, D.N.P.13§
Larry Gershon14*‖
Thayer Gershon, M.A.14,15*‖
Christiane Hartog, M.D.16§
Daisy J. A. Janssen, M.D., Ph.D.17,18‡
Erin K. Kross, M.D.19,20‡
Kathleen O. Lindell, Ph.D., R.N.21‡
Judy Luther14*‖
Matthew Maddocks, M.C.S.P., Ph.D.22‡
Mary Lynn McPherson, Pharm.D., M.A., M.D.E., B.C.P.S., C.P.E.23,24‡
Diane E. Meier, M.D.25,26*
Richard A. Mularski, M.D., M.S.H.S., M.C.R.1,27,28,29‡
Judith E. Nelson, M.D., J.D.30,31§
Elliot Rabinowitz, M.D.32,33§
Cynda H. Rushton, Ph.D., M.S.N., R.N.34,35,36§
Danetta H. Sloan, Ph.D., M.S.W., M.A.37§
Natasha Smallwood, B.Med.Sci., B.M.B.S., M.Sc., Ph.D.38,39‡
J. Daryl Thornton, M.D., M.P.H.40,41,42‡
Alison E. Turnbull, D.V.M., M.P.H., Ph.D.43,44‡
Anne M. Wilkinson, Ph.D., M.A.45,46‡
*Presenter.
‡Small group facilitator.
§Participant.
‖PAR representative.
1Division of Pulmonary, Critical Care and Sleep Medicine and 29Center for Ethics in Health Care, Oregon Health and Science University, Portland, Oregon; 2Center to Improve Veteran Involvement in Care, Veterans Affairs-Portland Health Care System, Portland, Oregon; 3Division of Pulmonary, Allergy, and Critical Care Medicine, 4Center for Palliative and Supportive Care, Division of Gerontology, Geriatrics, and Palliative Care, Department of Medicine, and 5School of Nursing, University of Alabama at Birmingham, Birmingham, Alabama; 6Leonard Davis School of Gerontology, University of Southern California, Los Angeles, California; 7College of Nursing, University of Utah, Salt Lake City, Utah; 8College of Nursing, Wayne State University, Detroit, Michigan; 9Palliative and Advanced Illness Research Center, Perelman School of Medicine, University of Pennsylvania, Philadelphia, Pennsylvania; 10Division of Pulmonary and Critical Care Medicine, Duke University, Durham, North Carolina; 11School of Health Sciences, University of East Anglia, Norwich, United Kingdom; 12School of Nursing, Clemson University, Clemson, South Carolina; 13Guthrie Robert Packer Hospital, Sayre, Pennsylvania; 14American Thoracic Society Patient Advisory Roundtable, New York, New York; 15Stanford Children’s Hospital, Palo Alto, California; 16Department of Anesthesiology and Intensive Care, Charité Universitätsmedizin Berlin, Klinik Bavaria, Kreischa, Germany; 17Department of Health Services Research, Care and Public Health Research Institute, Faculty of Health Medicine and Life Sciences, Maastricht University, Maastricht, the Netherlands; 18Department of Research and Development, Ciro, Horn, the Netherlands; 19Division of Pulmonary, Critical Care and Sleep Medicine, University of Washington, Seattle, Washington; 20Cambia Palliative Care Center of Excellence, University of Washington School of Medicine, Seattle, Washington; 21College of Nursing and Medicine, Medical University of South Carolina, Charleston, South Carolina; 22Cicely Saunders Institute of Palliative Care, Policy and Rehabilitation, King’s College London, London, United Kingdom; 23Department of Pharmacy Practice and Science, School of Pharmacy, and 24Center to Advance Chronic Pain Research, University of Maryland, Baltimore, Maryland; 25Center to Advance Palliative Care, New York, New York; 26Department of Geriatrics and Palliative Medicine, Icahn School of Medicine, Mount Sinai, New York, New York; 27Kaiser Permanente Center for Health Research, Portland, Oregon; 28Kaiser Permanente Bernard J. Tyson School of Medicine, Pasadena, California; 30Supportive Care Service, joint appointment in Critical Care, Memorial Sloan Kettering Cancer Center, New York, New York; 31Weill Cornell Medical College, Cornell University, New York, New York; 32Division of Pulmonary Medicine, Boston Children’s Hospital, Boston, Massachusetts; 33Department of Psychosocial Oncology and Palliative Care, Dana-Farber Cancer Institute, Harvard Medical School, Cambridge, Massachusetts; 34Clinical Ethics, Berman Institute of Bioethics, 36School of Nursing, 41Center for Reducing Health Disparities, 42Population Health Research Institute, 43Division of Pulmonary and Critical Care Medicine, Department of Medicine, and 44Department of Epidemiology, Johns Hopkins University, Baltimore, Maryland; 35University of Technology of Sydney, Sydney, Australia; 37Department of Health, Behavior, and Society, Johns Hopkins Bloomberg School of Public Health, Baltimore, Maryland; 38Department of Respiratory and Sleep Medicine, Alfred Hospital, Melbourne, Australia; 39Central Clinical School, Monash University, Melbourne, Australia; 40Division of Pulmonary, Critical Care, and Sleep Medicine, The MetroHealth Campus of Case Western Reserve University, Cleveland, Ohio; 45Edith Cowan University, Joondalup, Australia; and 46School of Nursing and Midwifery, Perth, Australia.
Footnotes
This official policy statement of the American Thoracic Society (ATS), American Academy of Hospice and Palliative Medicine (AAHPM), Hospice and Palliative Nurses Association (HPNA), and Social Work Hospice and Palliative Care Network (SWHPN) was approved by the ATS, AAHPM, HPNA, and SWHPN May 2022
An Executive Summary of this document is available at http://www.atsjournals.org/doi/abs/10.1164/rccm.202207-1262ST
Dedication: This document is dedicated to ATS past-president J. Randall Curtis, M.D., M.P.H., the father of palliative care in pulmonary and critical care medicine, whose fierce leadership and tireless efforts in critical care medicine, research, and policy on behalf of his patients and their families inspires us to continue his legacy. Randy’s generosity, humility, enthusiasm, compassion, and courage, as well as his sage advice and mentorship, have guided the careers (and sometimes lives) of many members of this committee and we are forever indebted to him. Most importantly, his example reminds us to prioritize the things we love.
Subcommittee Disclosures: D.R.S. received research support from the Sojourns Scholar Leadership Program Award of the Cambia Health Foundation. A.S.I. served as a consultant for AstraZeneca; and received research support from National Institute on Aging and National Institutes of Health. L.F.R. served on a data safety and monitoring board for Hospice & Palliative Nursing Association Board of Directors; served as a speaker for UpToDate; received travel support from University of Utah, College of Nursing, Professional Development Funds; and received research support from Department of Veterans Affairs Health Services. C.E.C. received research and travel support from National Institutes of Health; and served on the data safety and monitoring board for National Institute on Aging. C.H. served as the elected leader of the ethics section of the European Society of Intensive Care Medicine; and received research support from Innovations Fund of the German Federal Joint Committee and European Society of Intensive Care Medicine. D.J.A.J. served as a speaker for Abbott, Boehringer Ingelheim, and Chiesi; served on a data safety and monitoring board for BETTER-B and Wolfson Palliative Care Research Centre Hull U.K.; and received research support from the Netherlands Organization for Health Research and Development and Stichting Astmabestriiding. K.O.L. received research support from U.S. Department of Health and Human Services, National Institutes of Health, and National Institute of Nursing Research; received travel support from the University of Pittsburgh, and Medical University of South Carolina; and holds a patent for A Program of SUPPORTÔ. M.M. received research support from National Institute for Health and Care Research U.K. R.A.M. served as a consultant for eThera; and received research support from and served on an advisory committee for GlaxoSmithKline. E.R. served as a speaker for and received travel support from North American Cystic Fibrosis Conference. N.S. served on the advisory board for Thoracic Society of Australia and New Zealand, and Lung Foundation of Australia; and served as a speaker for Boehringer Ingelheim, GlaxoSmithKline, and Thoracic Society of Australia and New Zealand. J.D.T. served on the data safety and monitoring board for Lift Study; and received research support from National Institutes of Health. A.E.T. served as a consultant for Medical Science Affiliates; and received research support from NHLBI. S.E., M.L.C., K.R.C., M.F., T.K.F., P.M.F., L.G., T.G., E.K.K., J.L., M.L.M., D.E.M., J.E.N., C.H.R., D.H.S., and A.M.W., reported no commercial or relevant noncommercial interests from ineligible companies.
References
- 1. Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, et al. ATS End-of-Life Care Task Force An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med . 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 2.Growth of palliative care in U.S 2021https://www.capc.org/documents/download/1031/.
- 3. Kamal AH, Bull JH, Swetz KM, Wolf SP, Shanafelt TD, Myers ER. Future of the palliative care workforce: preview to an impending crisis. Am J Med . 2017;130:113–114. doi: 10.1016/j.amjmed.2016.08.046. [DOI] [PubMed] [Google Scholar]
- 4.Morrison RS, Meier DE.America’s care of serious illness: a state-by-state report card on access to palliative care in our nation’s hospitals. New York: Center to Advance Palliative Care; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clark D, Baur N, Clelland D, Garralda E, López-Fidalgo J, Connor S, et al. Mapping levels of palliative care development in 198 countries: the situation in 2017. J Pain Symptom Manage . 2020;59:794–807.e4. doi: 10.1016/j.jpainsymman.2019.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kelley AS, Bollens-Lund E. Identifying the population with serious illness: the “denominator” challenge. J Palliat Med . 2018;21:S7–S16. doi: 10.1089/jpm.2017.0548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sudore RL, Lum HD, You JJ, Hanson LC, Meier DE, Pantilat SZ, et al. Defining advance care planning for adults: a consensus definition from a multidisciplinary Delphi panel. J Pain Symptom Manage . 2017;53:821–832.e1. doi: 10.1016/j.jpainsymman.2016.12.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bailey S. Decision making in health care: limitations of the substituted judgment principle. Nurs Ethics . 2002;9:483–493. doi: 10.1191/0969733002ne538oa. [DOI] [PubMed] [Google Scholar]
- 9. von Gunten CF. Secondary and tertiary palliative care in US hospitals. JAMA . 2002;287:875–881. doi: 10.1001/jama.287.7.875. [DOI] [PubMed] [Google Scholar]
- 10. Weissman DE, Meier DE. Identifying patients in need of a palliative care assessment in the hospital setting: a consensus report from the Center to Advance Palliative Care. J Palliat Med . 2011;14:17–23. doi: 10.1089/jpm.2010.0347. [DOI] [PubMed] [Google Scholar]
- 11.National Consensus Project for quality palliative care. 4th edition. Richmond, VA: National Coalition for Hospice and Palliative Care; 2018. Clinical Practice Guidelines for quality palliative care.https://www.nationalcoalitionhpc.org/ncp [DOI] [PubMed] [Google Scholar]
- 12. Dans M, Kutner JS, Agarwal R, Baker JN, Bauman JR, Beck AC, et al. NCCN guidelines insights: palliative care, version 2.2021. J Natl Compr Canc Netw . 2021;19:780–788. doi: 10.6004/jnccn.2021.0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferrell BR, Twaddle ML, Melnick A, Meier DE. National Consensus Project clinical practice guidelines for quality palliative care guidelines, 4th edition. J Palliat Med . 2018;21:1684–1689. doi: 10.1089/jpm.2018.0431. [DOI] [PubMed] [Google Scholar]
- 14.Teoli D, Sanvictores T, An J. SWOT analysis. Treasure Island, FL: StatPearls; 2022. [PubMed] [Google Scholar]
- 15. McMillan SS, King M, Tully MP. How to use the nominal group and Delphi techniques. Int J Clin Pharm . 2016;38:655–662. doi: 10.1007/s11096-016-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Humphrey-Murto S, Crew R, Shea B, Bartlett SJ, March L, Tugwell P, et al. Consensus building in OMERACT: recommendations for use of the Delphi for core outcome set development. J Rheumatol . 2019;46:1041–1046. doi: 10.3899/jrheum.181094. [DOI] [PubMed] [Google Scholar]
- 17. Saunders C. The evolution of palliative care. J R Soc Med . 2001;94:430–432. doi: 10.1177/014107680109400904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brighton LJ, Miller S, Farquhar M, Booth S, Yi D, Gao W, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax . 2019;74:270–281. doi: 10.1136/thoraxjnl-2018-211589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haun MW, Estel S, Rucker G, Friederich HC, Villalobos M, Thomas M, et al. Early palliative care for adults with advanced cancer. Cochrane Database Syst Rev . 2017;6:CD011129. doi: 10.1002/14651858.CD011129.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kavalieratos D, Corbelli J, Zhang D, Dionne-Odom JN, Ernecoff NC, Hanmer J, et al. Association between palliative care and patient and caregiver outcomes: a systematic review and meta-analysis. JAMA . 2016;316:2104–2114. doi: 10.1001/jama.2016.16840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gaertner J, Siemens W, Meerpohl JJ, Antes G, Meffert C, Xander C, et al. Effect of specialist palliative care services on quality of life in adults with advanced incurable illness in hospital, hospice, or community settings: systematic review and meta-analysis. BMJ . 2017;357:j2925. doi: 10.1136/bmj.j2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sullivan DR, Chan B, Lapidus JA, Ganzini L, Hansen L, Carney PA, et al. Association of early palliative care use with survival and place of death among patients with advanced lung cancer receiving care in the Veterans Health Administration. JAMA Oncol . 2019;5:1702–1709. doi: 10.1001/jamaoncol.2019.3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Maddocks M, Lovell N, Booth S, Man WD, Higginson IJ. Palliative care and management of troublesome symptoms for people with chronic obstructive pulmonary disease. Lancet . 2017;390:988–1002. doi: 10.1016/S0140-6736(17)32127-X. [DOI] [PubMed] [Google Scholar]
- 24. Temel JS, Greer JA, Muzikansky A, Gallagher ER, Admane S, Jackson VA, et al. Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med . 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 25. Higginson IJ, Bausewein C, Reilly CC, Gao W, Gysels M, Dzingina M, et al. An integrated palliative and respiratory care service for patients with advanced disease and refractory breathlessness: a randomised controlled trial. Lancet Respir Med . 2014;2:979–987. doi: 10.1016/S2213-2600(14)70226-7. [DOI] [PubMed] [Google Scholar]
- 26. Belanger E, Loomer L, Teno JM, Mitchell SL, Adhikari D, Gozalo PL. Early utilization patterns of the new Medicare procedure codes for advance care planning. JAMA Intern Med . 2019;179:829–830. doi: 10.1001/jamainternmed.2018.8615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Harrison KL, Connor SR. First Medicare demonstration of concurrent provision of curative and hospice services for end-of-life care. Am J Public Health . 2016;106:1405–1408. doi: 10.2105/AJPH.2016.303238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Limited IM. Washington: D.C.: Association of American Medical Colleges; The complexities of physician supply and demand: projections from 2019 to 2034; p. 2021. [Google Scholar]
- 29. Quill TE, Abernethy AP. Generalist plus specialist palliative care--creating a more sustainable model. N Engl J Med . 2013;368:1173–1175. doi: 10.1056/NEJMp1215620. [DOI] [PubMed] [Google Scholar]
- 30. Schenker Y, Arnold R. The next era of palliative care. JAMA . 2015;314:1565–1566. doi: 10.1001/jama.2015.11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ray A, Najmi A, Sadasivam B. Integrating palliative care with primary care: a synergistic mix. J Family Med Prim Care . 2019;8:3074–3075. doi: 10.4103/jfmpc.jfmpc_519_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Raina SK, Kumar R, Gupta RK. A primary care-based patient-centric palliative care model. J Family Med Prim Care . 2019;8:1519–1522. doi: 10.4103/jfmpc.jfmpc_391_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bernacki RE, Block SD, American College of Physicians High-Value Care Task Force Communication about serious illness care goals: a review and synthesis of best practices. JAMA Intern Med . 2014;174:1994–2003. doi: 10.1001/jamainternmed.2014.5271. [DOI] [PubMed] [Google Scholar]
- 34. Tulsky JA. Beyond advance directives: importance of communication skills at the end of life. JAMA . 2005;294:359–365. doi: 10.1001/jama.294.3.359. [DOI] [PubMed] [Google Scholar]
- 35. Weiner JS, Cole SA. Three principles to improve clinician communication for advance care planning: overcoming emotional, cognitive, and skill barriers. J Palliat Med . 2004;7:817–829. doi: 10.1089/jpm.2004.7.817. [DOI] [PubMed] [Google Scholar]
- 36. McCorkle R, Jeon S, Ercolano E, Lazenby M, Reid A, Davies M, et al. An advanced practice nurse coordinated multidisciplinary intervention for patients with late-stage cancer: a cluster randomized trial. J Palliat Med . 2015;18:962–969. doi: 10.1089/jpm.2015.0113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dyar S, Lesperance M, Shannon R, Sloan J, Colon-Otero G. A nurse practitioner-directed intervention improves the quality of life of patients with metastatic cancer: results of a randomized pilot study. J Palliat Med . 2012;15:890–895. doi: 10.1089/jpm.2012.0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Dosaj A, Thiyagarajan D, Ter Haar C, Cheng J, George J, Wheatley C, et al. Rapid implementation of telehealth services during the COVID-19 pandemic. Telemed J E Health . 2021;27:116–120. doi: 10.1089/tmj.2020.0219. [DOI] [PubMed] [Google Scholar]
- 39. Lurie N, Carr BG. The role of telehealth in the medical response to disasters. JAMA Intern Med . 2018;178:745–746. doi: 10.1001/jamainternmed.2018.1314. [DOI] [PubMed] [Google Scholar]
- 40. Evans R, Stone D, Elwyn G. Organizing palliative care for rural populations: a systematic review of the evidence. Fam Pract . 2003;20:304–310. doi: 10.1093/fampra/cmg312. [DOI] [PubMed] [Google Scholar]
- 41. Jaffe DH, Lee L, Huynh S, Haskell TP. Health inequalities in the use of telehealth in the United States in the lens of COVID-19. Popul Health Manag . 2020;23:368–377. doi: 10.1089/pop.2020.0186. [DOI] [PubMed] [Google Scholar]
- 42. Hancock S, Preston N, Jones H, Gadoud A. Telehealth in palliative care is being described but not evaluated: a systematic review. BMC Palliat Care . 2019;18:114. doi: 10.1186/s12904-019-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. White C, McIlfatrick S, Dunwoody L, Watson M. Supporting and improving community health services-a prospective evaluation of ECHO technology in community palliative care nursing teams. BMJ Support Palliat Care . 2019;9:202–208. doi: 10.1136/bmjspcare-2015-000935. [DOI] [PubMed] [Google Scholar]
- 44. Arora S, Smith T, Snead J, Zalud-Cerrato S, Marr L, Watson M, et al. Project ECHO: an effective means of increasing palliative care capacity. Am J Manag Care . 2017;23:SP267–SP271. [PubMed] [Google Scholar]
- 45. Schroedl CJ, Yount SE, Szmuilowicz E, Hutchison PJ, Rosenberg SR, Kalhan R. A qualitative study of unmet healthcare needs in chronic obstructive pulmonary disease. A potential role for specialist palliative care? Ann Am Thorac Soc . 2014;11:1433–1438. doi: 10.1513/AnnalsATS.201404-155BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Figueiredo D, Gabriel R, Jácome C, Cruz J, Marques A. Caring for relatives with chronic obstructive pulmonary disease: how does the disease severity impact on family carers? Aging Ment Health . 2014;18:385–393. doi: 10.1080/13607863.2013.837146. [DOI] [PubMed] [Google Scholar]
- 47. Carvajalino S, Reigada C, Johnson MJ, Dzingina M, Bajwah S. Symptom prevalence of patients with fibrotic interstitial lung disease: a systematic literature review. BMC Pulm Med . 2018;18:78. doi: 10.1186/s12890-018-0651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Iyer AS, Dionne-Odom JN, Ford SM, Crump Tims SL, Sockwell ED, Ivankova NV, et al. A formative evaluation of patient and family caregiver perspectives on early palliative care in chronic obstructive pulmonary disease across disease severity. Ann Am Thorac Soc . 2019;16:1024–1033. doi: 10.1513/AnnalsATS.201902-112OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Fromme EK, Eilers KM, Mori M, Hsieh YC, Beer TM. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the quality-of-life questionnaire C30. J Clin Oncol . 2004;22:3485–3490. doi: 10.1200/JCO.2004.03.025. [DOI] [PubMed] [Google Scholar]
- 50. Laugsand EA, Sprangers MA, Bjordal K, Skorpen F, Kaasa S, Klepstad P. Health care providers underestimate symptom intensities of cancer patients: a multicenter European study. Health Qual Life Outcomes . 2010;8:104. doi: 10.1186/1477-7525-8-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fisch MJ, Lee JW, Weiss M, Wagner LI, Chang VT, Cella D, et al. Prospective, observational study of pain and analgesic prescribing in medical oncology outpatients with breast, colorectal, lung, or prostate cancer. J Clin Oncol . 2012;30:1980–1988. doi: 10.1200/JCO.2011.39.2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Langsetmo L, Platt RW, Ernst P, Bourbeau J. Underreporting exacerbation of chronic obstructive pulmonary disease in a longitudinal cohort. Am J Respir Crit Care Med . 2008;177:396–401. doi: 10.1164/rccm.200708-1290OC. [DOI] [PubMed] [Google Scholar]
- 53. Seow H, Sussman J, Martelli-Reid L, Pond G, Bainbridge D. Do high symptom scores trigger clinical actions? An audit after implementing electronic symptom screening. J Oncol Pract . 2012;8:e142–e148. doi: 10.1200/JOP.2011.000525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kotronoulas G, Kearney N, Maguire R, Harrow A, Di Domenico D, Croy S, et al. What is the value of the routine use of patient-reported outcome measures toward improvement of patient outcomes, processes of care, and health service outcomes in cancer care? A systematic review of controlled trials. J Clin Oncol . 2014;32:1480–1501. doi: 10.1200/JCO.2013.53.5948. [DOI] [PubMed] [Google Scholar]
- 55. Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health-related quality-of-life assessments and patient-physician communication: a randomized controlled trial. JAMA . 2002;288:3027–3034. doi: 10.1001/jama.288.23.3027. [DOI] [PubMed] [Google Scholar]
- 56. Santana MJ, Feeny D, Johnson JA, McAlister FA, Kim D, Weinkauf J, et al. Assessing the use of health-related quality of life measures in the routine clinical care of lung-transplant patients. Qual Life Res . 2010;19:371–379. doi: 10.1007/s11136-010-9599-3. [DOI] [PubMed] [Google Scholar]
- 57. Kroenke K, Krebs EE, Wu J, Yu Z, Chumbler NR, Bair MJ. Telecare collaborative management of chronic pain in primary care: a randomized clinical trial. JAMA . 2014;312:240–248. doi: 10.1001/jama.2014.7689. [DOI] [PubMed] [Google Scholar]
- 58. Basch E, Deal AM, Kris MG, Scher HI, Hudis CA, Sabbatini P, et al. Symptom monitoring with patient-reported outcomes during routine cancer treatment: a randomized controlled trial. J Clin Oncol . 2016;34:557–565. doi: 10.1200/JCO.2015.63.0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Cleeland CS, Wang XS, Shi Q, Mendoza TR, Wright SL, Berry MD, et al. Automated symptom alerts reduce postoperative symptom severity after cancer surgery: a randomized controlled clinical trial. J Clin Oncol . 2011;29:994–1000. doi: 10.1200/JCO.2010.29.8315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gilbert JE, Howell D, King S, Sawka C, Hughes E, Angus H, et al. Quality improvement in cancer symptom assessment and control: the provincial palliative care integration project (PPCIP) J Pain Symptom Manage . 2012;43:663–678. doi: 10.1016/j.jpainsymman.2011.04.028. [DOI] [PubMed] [Google Scholar]
- 61. Singh D, Agusti A, Anzueto A, Barnes PJ, Bourbeau J, Celli BR, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur Respir J . 2019;53:1900164. doi: 10.1183/13993003.00164-2019. [DOI] [PubMed] [Google Scholar]
- 62. Çolak Y, Afzal S. Prognostic significance of chronic respiratory symptoms in individuals with normal spirometry. Eur Respir J . 2010;55:1902226. doi: 10.1183/13993003.02226-2019. [DOI] [PubMed] [Google Scholar]
- 63. Galiè N, Hoeper MM, Humbert M, Torbicki A, Vachiery JL, Barbera JA, et al. ESC Committee for Practice Guidelines (CPG) Guidelines for the diagnosis and treatment of pulmonary hypertension: the task force for the diagnosis and treatment of pulmonary hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT) Eur Heart J . 2009;30:2493–2537. doi: 10.1093/eurheartj/ehp297. [DOI] [PubMed] [Google Scholar]
- 64. Temel JS, Pirl WF, Lynch TJ. Comprehensive symptom management in patients with advanced-stage non-small-cell lung cancer. Clin Lung Cancer . 2006;7:241–249. doi: 10.3816/CLC.2006.n.001. [DOI] [PubMed] [Google Scholar]
- 65. Miravitlles M, Ribera A. Understanding the impact of symptoms on the burden of COPD. Respir Res . 2017;18:67. doi: 10.1186/s12931-017-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 rReport. GOLD executive summary. Am J Respir Crit Care Med . 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 67. Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease, and renal disease. J Pain Symptom Manage . 2006;31:58–69. doi: 10.1016/j.jpainsymman.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 68. Mularski RA, Reinke LF, Carrieri-Kohlman V, Fischer MD, Campbell ML, Rocker G, et al. ATS Ad Hoc Committee on Palliative Management of Dyspnea Crisis An official American Thoracic Society workshop report: assessment and palliative management of dyspnea crisis. Ann Am Thorac Soc . 2013;10:S98–S106. doi: 10.1513/AnnalsATS.201306-169ST. [DOI] [PubMed] [Google Scholar]
- 69. Sullivan DR, Forsberg CW, Ganzini L, Au DH, Gould MK, Provenzale D, et al. Longitudinal changes in depression symptoms and survival among patients with lung cancer: a national cohort assessment. J Clin Oncol . 2016;34:3984–3991. doi: 10.1200/JCO.2016.66.8459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Guyatt GH, Berman LB, Townsend M, Pugsley SO, Chambers LW. A measure of quality of life for clinical trials in chronic lung disease. Thorax . 1987;42:773–778. doi: 10.1136/thx.42.10.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kon SS, Dilaver D, Mittal M, Nolan CM, Clark AL, Canavan JL, et al. The clinical COPD questionnaire: response to pulmonary rehabilitation and minimal clinically important difference. Thorax . 2014;69:793–798. doi: 10.1136/thoraxjnl-2013-204119. [DOI] [PubMed] [Google Scholar]
- 72. Chang JA, Curtis JR, Patrick DL, Raghu G. Assessment of health-related quality of life in patients with interstitial lung disease. Chest . 1999;116:1175–1182. doi: 10.1378/chest.116.5.1175. [DOI] [PubMed] [Google Scholar]
- 73. Lovell N, Etkind SN, Bajwah S, Maddocks M, Higginson IJ. To what extent do the NRS and CRQ capture change in patients’ experience of breathlessness in advanced disease? Findings from a mixed-methods double-blind randomized feasibility trial. J Pain Symptom Manage . 2019;58:369–381.e7. doi: 10.1016/j.jpainsymman.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 74. Patel A, Siegert R, Brignall K, Gordon P, Steer S, Desai SR, et al. The development and validation of the King’s brief interstitial lung disease (K-BILD) health status questionnaire. Thorax . 2012;67:804–810. doi: 10.1136/thoraxjnl-2012-201581. [DOI] [PubMed] [Google Scholar]
- 75. Sinha A, Patel A, Siegert R, Bajwah S, Maher TM, Renzoni E, et al. The King’s brief interstitial lung disease (KBILD) questionnaire: an updated minimal clinically important difference. BMJ Open Respir Res . 2019;6:e000363. doi: 10.1136/bmjresp-2018-000363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Mahler DA, Mackowiak JI. Evaluation of the short-form 36-item questionnaire to measure health-related quality of life in patients with COPD. Chest . 1995;107:1585–1589. doi: 10.1378/chest.107.6.1585. [DOI] [PubMed] [Google Scholar]
- 77. Limsuwat C, McClellan R, Amiri HM, Nugent K. Pulmonary rehabilitation improves only some domains of health-related quality of life measured by the short form-36 questionnaire. Ann Thorac Med . 2014;9:144–148. doi: 10.4103/1817-1737.134068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Nolan CM, Longworth L, Lord J, Canavan JL, Jones SE, Kon SS, et al. The EQ-5D-5L health status questionnaire in COPD: validity, responsiveness and minimum important difference. Thorax . 2016;71:493–500. doi: 10.1136/thoraxjnl-2015-207782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Swigris JJ, Andrae DA, Churney T, Johnson N, Scholand MB, White ES, et al. Development and initial validation analyses of the living with idiopathic pulmonary fibrosis questionnaire. Am J Respir Crit Care Med . 2020;202:1689–1697. doi: 10.1164/rccm.202002-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD assessment test. Eur Respir J . 2009;34:648. doi: 10.1183/09031936.00102509. [DOI] [PubMed] [Google Scholar]
- 81. Dodd JW, Hogg L, Nolan J, Jefford H, Grant A, Lord VM, et al. The COPD assessment test (CAT): response to pulmonary rehabilitation. A multicentre, prospective study. Thorax . 2011;66:425. doi: 10.1136/thx.2010.156372. [DOI] [PubMed] [Google Scholar]
- 82. Nagata K, Tomii K, Hayashi M, Otsuka K, Tachikawa R, Otsuka K. Evaluation of the COPD assessment test (CAT) for measuring health-related quality of life in patients with interstitial lung disease. Eur Respir J . 2011;38:3748. [Google Scholar]
- 83. Hayata A, Minakata Y, Matsunaga K, Nakanishi M, Yamamoto N. Differences in physical activity according to mMRC grade in patients with COPD. Int J Chron Obstruct Pulmon Dis . 2016;11:2203–2208. doi: 10.2147/COPD.S109694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Cheng S-L, Lin C-H, Wang C-C, Chan M-C, Hsu J-Y, Hang L-W, et al. Taiwan Clinical Trial Consortium for Respiratory Disease (TCORE) Comparison between COPD assessment test (CAT) and modified Medical Research Council (mMRC) dyspnea scores for evaluation of clinical symptoms, comorbidities, and medical resources utilization in COPD patients. J Formos Med Assoc . 2019;118:429–435. doi: 10.1016/j.jfma.2018.06.018. [DOI] [PubMed] [Google Scholar]
- 85. Yuan X-Y, Zhang H, Huang L-R, Zhang F, Sheng X-W, Cui A. Evaluation of health-related quality of life and the related factors in a group of Chinese patients with interstitial lung diseases. PLoS One . 2020;15:e0236346. doi: 10.1371/journal.pone.0236346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Gift AG. Validation of a vertical visual analogue scale as a measure of clinical dyspnea. Rehabil Nurs . 1989;14:323–325. doi: 10.1002/j.2048-7940.1989.tb01129.x. [DOI] [PubMed] [Google Scholar]
- 87. Yates H, Adamali HI, Maskell N, Barratt S, Sharp C. Visual analogue scales for interstitial lung disease: a prospective validation study. QJM . 2018;111:531–539. doi: 10.1093/qjmed/hcy102. [DOI] [PubMed] [Google Scholar]
- 88. Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care . 1998;7:200–204. [PubMed] [Google Scholar]
- 89. Yorke J, Moosavi SH, Shuldham C, Jones PW. Quantification of dyspnoea using descriptors: development and initial testing of the dyspnoea-12. Thorax . 2010;65:21. doi: 10.1136/thx.2009.118521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Yorke J, Swigris J, Russell A-M, Moosavi SH, Ng Man Kwong G, Longshaw M, et al. Dyspnea-12 is a valid and reliable measure of breathlessness in patients with interstitial lung disease. Chest . 2011;139:159–164. doi: 10.1378/chest.10-0693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Campbell ML, Templin T, Walch J. A respiratory distress observation scale for patients unable to self-report dyspnea. J Palliat Med . 2010;13:285–290. doi: 10.1089/jpm.2009.0229. [DOI] [PubMed] [Google Scholar]
- 92. Campbell ML. Psychometric testing of a respiratory distress observation scale. J Palliat Med . 2008;11:44–50. doi: 10.1089/jpm.2007.0090. [DOI] [PubMed] [Google Scholar]
- 93. Birring SS, Prudon B, Carr AJ, Singh SJ, Morgan MDL, Pavord ID. Development of a symptom-specific health status measure for patients with chronic cough: Leicester cough questionnaire (LCQ) Thorax . 2003;58:339–343. doi: 10.1136/thorax.58.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Berkhof FF, Boom LN, ten Hertog NE, Uil SM, Kerstjens HA, van den Berg JW. The validity and precision of the Leicester cough questionnaire in COPD patients with chronic cough. Health Qual Life Outcomes . 2012;10:4. doi: 10.1186/1477-7525-10-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hsu JY, Stone RA, Logan-Sinclair RB, Worsdell M, Busst CM, Chung KF. Coughing frequency in patients with persistent cough: assessment using a 24-hour ambulatory recorder. Eur Respir J . 1994;7:1246–1253. doi: 10.1183/09031936.94.07071246. [DOI] [PubMed] [Google Scholar]
- 96. Crawford B, Monz B, Hohlfeld J, Roche N, Rubin B, Magnussen H, et al. Development and validation of a cough and sputum assessment questionnaire. Respir Med . 2008;102:1545–1555. doi: 10.1016/j.rmed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 97. Monz BU, Sachs P, McDonald J, Crawford B, Nivens MC, Tetzlaff K. Responsiveness of the cough and sputum assessment questionnaire in exacerbations of COPD and chronic bronchitis. Respir Med . 2010;104:534–541. doi: 10.1016/j.rmed.2009.10.026. [DOI] [PubMed] [Google Scholar]
- 98. O’Brien EC, Hellkamp AS, Neely ML, Swaminathan A, Bender S, Snyder LD, et al. IPF-PRO Registry investigators Disease severity and quality of life in patients with idiopathic pulmonary fibrosis: a cross-sectional analysis of the IPF-PRO registry. Chest . 2020;157:1188–1198. doi: 10.1016/j.chest.2019.11.042. [DOI] [PubMed] [Google Scholar]
- 99. Johnson AM, Smith SM. A review of general pain measurement tools and instruments for consideration of use in COPD clinical practice. Int J Chron Obstruct Pulmon Dis . 2017;12:923–929. doi: 10.2147/COPD.S119889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Shen Q, Guo T, Song M, Guo W, Zhang Y, Duan W, et al. Pain is a common problem in patients with ILD. Respir Res . 2020;21:297. doi: 10.1186/s12931-020-01564-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Chen YW, HajGhanbari B, Road JD, Coxson HO, Camp PG, Reid WD. Reliability and validity of the brief pain inventory in individuals with chronic obstructive pulmonary disease. Eur J Pain . 2018;22:1718–1726. doi: 10.1002/ejp.1258. [DOI] [PubMed] [Google Scholar]
- 102. Maignan M, Chauny J-M, Daoust R, Duc L, Mabiala-Makele P, Collomb-Muret R, et al. Pain during exacerbation of chronic obstructive pulmonary disease: a prospective cohort study. PLoS One . 2019;14:e0217370. doi: 10.1371/journal.pone.0217370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Al-shair K, Muellerova H, Yorke J, Rennard SI, Wouters EF, Hanania NA, et al. ECLIPSE investigators Examining fatigue in COPD: development, validity, and reliability of a modified version of FACIT-F scale. Health Qual Life Outcomes . 2012;10:100. doi: 10.1186/1477-7525-10-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Inal-Ince D, Savci S, Saglam M, Calik E, Arikan H, Bosnak-Guclu M, et al. Fatigue and multidimensional disease severity in chronic obstructive pulmonary disease. Multidiscip Respir Med . 2010;5:162–167. doi: 10.1186/2049-6958-5-3-162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Talwar A, Sahni S, John S, Verma S, Cárdenas-Garcia J, Kohn N. Effects of pulmonary rehabilitation on fatigue severity scale in patients with lung disease. Pneumonol Alergol Pol . 2014;82:534–540. doi: 10.5603/PiAP.2014.0070. [DOI] [PubMed] [Google Scholar]
- 106. Al-shair K, Kolsum U, Berry P, Smith J, Caress A, Singh D, et al. Development, dimensions, reliability and validity of the novel Manchester COPD fatigue scale. Thorax . 2009;64:950–955. doi: 10.1136/thx.2009.118109. [DOI] [PubMed] [Google Scholar]
- 107. Zohal MA, Yazdi Z, Kazemifar AM, Mahjoob P, Ziaeeha M. Sleep quality and quality of life in COPD patients with and without suspected obstructive sleep apnea. Sleep Disord . 2014;2014:508372. doi: 10.1155/2014/508372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. De S. Subjective assessment of quality of sleep in chronic obstructive pulmonary disease patient and its relationship with associated depression. Lung India . 2012;29:332–335. doi: 10.4103/0970-2113.102808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Vukoja M, Kopitovic I, Milicic D, Dabovic D, Maksimovic O, Ilic M. Sleep quality in patients with asthma and COPD. Eur Respir J . 2015;46:PA4587. doi: 10.1111/crj.12528. [DOI] [PubMed] [Google Scholar]
- 110. Cho J-G, Teoh A, Roberts M, Wheatley J. The prevalence of poor sleep quality and its associated factors in patients with interstitial lung disease: a cross-sectional analysis. ERJ Open Res . 2019;5:00062–02019. doi: 10.1183/23120541.00062-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Faria AC, da Costa CH, Rufino R. Sleep apnea clinical score, Berlin questionnaire, or Epworth sleepiness scale: which is the best obstructive sleep apnea predictor in patients with COPD? Int J Gen Med . 2015;8:275–281. doi: 10.2147/IJGM.S86479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Lee JH, Park CS, Song JW. Obstructive sleep apnea in patients with interstitial lung disease: prevalence and predictive factors. PLoS One . 2020;15:e0239963. doi: 10.1371/journal.pone.0239963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Puhan MA, Frey M, Büchi S, Schünemann HJ. The minimal important difference of the hospital anxiety and depression scale in patients with chronic obstructive pulmonary disease. Health Qual Life Outcomes . 2008;6:46. doi: 10.1186/1477-7525-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Holland AE, Fiore JF, Jr, Bell EC, Goh N, Westall G, Symons K, et al. Dyspnoea and comorbidity contribute to anxiety and depression in interstitial lung disease. Respirology . 2014;19:1215–1221. doi: 10.1111/resp.12360. [DOI] [PubMed] [Google Scholar]
- 115. von Siemens SM, Jörres RA, Behr J, Alter P, Lutter J, Lucke T, et al. COSYCONET study group Effect of COPD severity and comorbidities on the result of the PHQ-9 tool for the diagnosis of depression: results from the COSYCONET cohort study. Respir Res . 2019;20:30. doi: 10.1186/s12931-019-0997-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Schuler M, Strohmayer M, Mühlig S, Schwaighofer B, Wittmann M, Faller H, et al. Assessment of depression before and after inpatient rehabilitation in COPD patients: psychometric properties of the German version of the patient health questionnaire (PHQ-9/PHQ-2) J Affect Disord . 2018;232:268–275. doi: 10.1016/j.jad.2018.02.037. [DOI] [PubMed] [Google Scholar]
- 117. Lu Y, Nyunt MSZ, Gwee X, Feng L, Feng L, Kua EH, et al. Life event stress and chronic obstructive pulmonary disease (COPD): associations with mental well-being and quality of life in a population-based study. BMJ Open . 2012;2:e001674. doi: 10.1136/bmjopen-2012-001674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ryerson CJ, Cayou C, Topp F, Hilling L, Camp PG, Wilcox PG, et al. Pulmonary rehabilitation improves long-term outcomes in interstitial lung disease: a prospective cohort study. Respir Med . 2014;108:203–210. doi: 10.1016/j.rmed.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 119. Phan T, Carter O, Adams C, Waterer G, Chung LP, Hawkins M, et al. Discriminant validity of the hospital anxiety and depression scale, Beck depression inventory (II) and Beck anxiety inventory to confirmed clinical diagnosis of depression and anxiety in patients with chronic obstructive pulmonary disease. Chron Respir Dis . 2016;13:220–228. doi: 10.1177/1479972316634604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Tzouvelekis A, Karampitsakos T, Kourtidou S, Bouros E, Tzilas V, Katsaras M, et al. Impact of depression on patients with idiopathic pulmonary fibrosis. Front Med (Lausanne) . 2020;7:29. doi: 10.3389/fmed.2020.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Collins PF, Elia M, Kurukulaaratchy RJ, Stratton RJ. The influence of deprivation on malnutrition risk in outpatients with chronic obstructive pulmonary disease (COPD) Clin Nutr . 2018;37:144–148. doi: 10.1016/j.clnu.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 122. Emanuel LL, Alpert HR, Emanuel EE. Concise screening questions for clinical assessments of terminal care: the needs near the end-of-life care screening tool. J Palliat Med . 2001;4:465–474. doi: 10.1089/109662101753381601. [DOI] [PubMed] [Google Scholar]
- 123. Scandrett KG, Reitschuler-Cross EB, Nelson L, Sanger JA, Feigon M, Boyd E, et al. Feasibility and effectiveness of the NEST13+ as a screening tool for advanced illness care needs. J Palliat Med . 2010;13:161–169. doi: 10.1089/jpm.2009.0170. [DOI] [PubMed] [Google Scholar]
- 124. Cox CE, Jones DM, Reagan W, Key MD, Chow V, McFarlin J, et al. Palliative care planner: a pilot study to evaluate acceptability and usability of an electronic health records system-integrated, needs-targeted app platform. Ann Am Thorac Soc . 2018;15:59–68. doi: 10.1513/AnnalsATS.201706-500OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Cox CE, Ashana DC, Haines KL, Casarett D, Olsen MK, Parish A, et al. Assessment of clinical palliative care trigger status vs actual needs among critically ill patients and their family members. JAMA Netw Open . 2022;5:e2144093. doi: 10.1001/jamanetworkopen.2021.44093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med . 2010;13:1109–1118. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 127. Malik FA, Gysels M, Higginson IJ. Living with breathlessness: a survey of caregivers of breathless patients with lung cancer or heart failure. Palliat Med . 2013;27:647–656. doi: 10.1177/0269216313488812. [DOI] [PubMed] [Google Scholar]
- 128. Meharry R, Anderson D. Assessing symptom burden in patients with advanced COPD – Is CAT enough? Eur Respir J . 2016;48:PA3731. [Google Scholar]
- 129. Rantala HA, Leivo-Korpela S, Lehtimaki L, Lehto JT. Assessing symptom burden and depression in subjects with chronic respiratory insufficiency. J Palliat Care . 2021;37:134–141. doi: 10.1177/08258597211049592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. McGee SF, Zhang T, Jonker H, Laurie SA, Goss G, Nicholas G, et al. The impact of baseline Edmonton symptom assessment scale scores on treatment and survival in patients with advanced non-small-cell lung cancer. Clin Lung Cancer . 2018;19:e91–e99. doi: 10.1016/j.cllc.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 131. Kim HJ, McGuire DB, Tulman L, Barsevick AM. Symptom clusters: concept analysis and clinical implications for cancer nursing. Cancer Nurs . 2005;28:270–282, quiz 283–284. doi: 10.1097/00002820-200507000-00005. [DOI] [PubMed] [Google Scholar]
- 132. Miaskowski C. Symptom clusters: establishing the link between clinical practice and symptom management research. Support Care Cancer . 2006;14:792–794. doi: 10.1007/s00520-006-0038-5. [DOI] [PubMed] [Google Scholar]
- 133. Jenkins BA, Athilingam P, Jenkins RA. Symptom clusters in chronic obstructive pulmonary disease: a systematic review. Appl Nurs Res . 2019;45:23–29. doi: 10.1016/j.apnr.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 134. Seppälä S, Rajala K, Lehto JT, Sutinen E, Mäkitalo L, Kautiainen H, et al. Factor analysis identifies three separate symptom clusters in idiopathic pulmonary fibrosis. ERJ Open Res . 2020;6:00347–2020. doi: 10.1183/23120541.00347-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Kirkova J, Aktas A, Walsh D, Davis MP. Cancer symptom clusters: clinical and research methodology. J Palliat Med . 2011;14:1149–1166. doi: 10.1089/jpm.2010.0507. [DOI] [PubMed] [Google Scholar]
- 136. Seamark DA, Blake SD, Seamark CJ, Halpin DM. Living with severe chronic obstructive pulmonary disease (COPD): perceptions of patients and their carers. An interpretative phenomenological analysis. Palliat Med . 2004;18:619–625. doi: 10.1191/0269216304pm928oa. [DOI] [PubMed] [Google Scholar]
- 137. Farquhar MC, Prevost AT, McCrone P, Brafman-Price B, Bentley A, Higginson IJ, et al. Is a specialist breathlessness service more effective and cost-effective for patients with advanced cancer and their carers than standard care? Findings of a mixed-method randomised controlled trial. BMC Med . 2014;12:194. doi: 10.1186/s12916-014-0194-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Sloan DH, BrintzenhofeSzoc K, Mistretta E, Cheng MJ, Berger A. The influence of relationships on the meaning-making process: patients’ perspectives. Ann Palliat Med . 2017;6:220–226. doi: 10.21037/apm.2017.06.10. [DOI] [PubMed] [Google Scholar]
- 139. McCarthy B, Casey D, Devane D, Murphy K, Murphy E, Lacasse Y. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2015;(2):CD003793. doi: 10.1002/14651858.CD003793.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Dowman L, Hill CJ, Holland AE. Pulmonary rehabilitation for interstitial lung disease. Cochrane Database Syst Rev . 2014;(10):CD006322. doi: 10.1002/14651858.CD006322.pub3. [DOI] [PubMed] [Google Scholar]
- 141. Marciniuk DD, Goodridge D, Hernandez P, Rocker G, Balter M, Bailey P, et al. Canadian Thoracic Society COPD Committee Dyspnea Expert Working Group Managing dyspnea in patients with advanced chronic obstructive pulmonary disease: a Canadian Thoracic Society clinical practice guideline. Can Respir J . 2011;18:69–78. doi: 10.1155/2011/745047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. American Thoracic Society Committee on Dyspnea An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med . 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Bailey CD, Wagland R, Dabbour R, Caress A, Smith J, Molassiotis A. An integrative review of systematic reviews related to the management of breathlessness in respiratory illnesses. BMC Pulm Med . 2010;10:63. doi: 10.1186/1471-2466-10-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Hui D, Maddocks M, Johnson MJ, Ekström M, Simon ST, Ogliari AC, et al. ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org Management of breathlessness in patients with cancer: ESMO clinical practice guidelines†. ESMO Open . 2020;5:e001038. doi: 10.1136/esmoopen-2020-001038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Khor YH, Dudley KA, Herman D, Jacobs SS, Lederer DJ, Krishnan JA, et al. Summary for clinicians: clinical practice guideline on home oxygen therapy for adults with chronic lung disease. Ann Am Thorac Soc . 2021;18:1444–1449. doi: 10.1513/AnnalsATS.202102-165CME. [DOI] [PubMed] [Google Scholar]
- 146. Booth S, Farquhar M, Gysels M, Bausewein C, Higginson IJ. The impact of a breathlessness intervention service (BIS) on the lives of patients with intractable dyspnea: a qualitative phase 1 study. Palliat Support Care . 2006;4:287–293. doi: 10.1017/s1478951506060366. [DOI] [PubMed] [Google Scholar]
- 147. Molassiotis A, Bryan G, Caress A, Bailey C, Smith J. Pharmacological and non-pharmacological interventions for cough in adults with respiratory and non-respiratory diseases: a systematic review of the literature. Respir Med . 2010;104:934–944. doi: 10.1016/j.rmed.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 148. Gibson PG, Vertigan AE. Speech pathology for chronic cough: a new approach. Pulm Pharmacol Ther . 2009;22:159–162. doi: 10.1016/j.pupt.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 149. Chamberlain S, Birring SS, Garrod R. Nonpharmacological interventions for refractory chronic cough patients: systematic review. Lung . 2014;192:75–85. doi: 10.1007/s00408-013-9508-y. [DOI] [PubMed] [Google Scholar]
- 150. Morice AH, Millqvist E, Bieksiene K, Birring SS, Dicpinigaitis P, Domingo Ribas C, et al. ERS guidelines on the diagnosis and treatment of chronic cough in adults and children. Eur Respir J . 2020;55:1901136. doi: 10.1183/13993003.01136-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wee B, Browning J, Adams A, Benson D, Howard P, Klepping G, et al. Management of chronic cough in patients receiving palliative care: review of evidence and recommendations by a task group of the Association for Palliative Medicine of Great Britain and Ireland. Palliat Med . 2012;26:780–787. doi: 10.1177/0269216311423793. [DOI] [PubMed] [Google Scholar]
- 152. Molassiotis A, Bailey C, Caress A, Tan JY. Interventions for cough in cancer. Cochrane Database Syst Rev . 2015;5:CD007881. doi: 10.1002/14651858.CD007881.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Gibson P, Wang G, McGarvey L, Vertigan AE, Altman KW, Birring SS, CHEST Expert Cough Panel Treatment of unexplained chronic cough: CHEST guideline and expert panel report. Chest . 2016;149:27–44. doi: 10.1378/chest.15-1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Chen YW, Wang HH. The effectiveness of acupressure on relieving pain: a systematic review. Pain Manag Nurs . 2014;15:539–550. doi: 10.1016/j.pmn.2012.12.005. [DOI] [PubMed] [Google Scholar]
- 155. Nahin RL, Boineau R, Khalsa PS, Stussman BJ, Weber WJ. Evidence-based evaluation of complementary health approaches for pain management in the United States. Mayo Clin Proc . 2016;91:1292–1306. doi: 10.1016/j.mayocp.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Tick H, Nielsen A, Pelletier KR, Bonakdar R, Simmons S, Glick R, et al. Pain Task Force of the Academic Consortium for Integrative Medicine and Health Evidence-based nonpharmacologic strategies for comprehensive pain care: the consortium pain task force white paper. Explore (NY) . 2018;14:177–211. doi: 10.1016/j.explore.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 157. Bradt J, Dileo C, Magill L, Teague A. Music interventions for improving psychological and physical outcomes in cancer patients. Cochrane Database Syst Rev . 2016:CD006911. doi: 10.1002/14651858.CD006911.pub3. [DOI] [PubMed] [Google Scholar]
- 158.Chou R, Wagner J, Ahmed AY, Blazina I, Brodt E, Buckley DI, et al. Treatments for acute pain: a systematic review. Comparative effectiveness review no. 240. Rockville, MD: Agency for Healthcare Research and Quality; 2020. [PubMed] [Google Scholar]
- 159. Fine PG. Treatment guidelines for the pharmacological management of pain in older persons. Pain Med . 2012;13:S57–S66. doi: 10.1111/j.1526-4637.2011.01307.x. [DOI] [PubMed] [Google Scholar]
- 160. Chapman EJ, Edwards Z, Boland JW, Maddocks M, Fettes L, Malia C, et al. Practice review: evidence-based and effective management of pain in patients with advanced cancer. Palliat Med . 2020;34:444–453. doi: 10.1177/0269216319896955. [DOI] [PubMed] [Google Scholar]
- 161. Perusinghe M, Chen KY, McDermott B. Evidence-based management of depression in palliative care: a systematic review. J Palliat Med . 2021;24:767–781. doi: 10.1089/jpm.2020.0659. [DOI] [PubMed] [Google Scholar]
- 162. Rayner L, Price A, Hotopf M, Higginson IJ. The development of evidence-based European guidelines on the management of depression in palliative cancer care. Eur J Cancer . 2011;47:702–712. doi: 10.1016/j.ejca.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 163. Verberkt CA, van den Beuken-van Everdingen MHJ, Schols JMGA, Hameleers N, Wouters EFM, Janssen DJA. Effect of sustained-release morphine for refractory breathlessness in chronic obstructive pulmonary disease on health status: a randomized clinical trial. JAMA Intern Med . 2020;180:1306–1314. doi: 10.1001/jamainternmed.2020.3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Zwerink M, Brusse-Keizer M, van der Valk PD, Zielhuis GA, Monninkhof EM, van der Palen J, et al. Self management for patients with chronic obstructive pulmonary disease. Cochrane Database Syst Rev . 2014;(3):CD002990. doi: 10.1002/14651858.CD002990.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Riegel B, Jaarsma T, Strömberg A. A middle-range theory of self-care of chronic illness. ANS Adv Nurs Sci . 2012;35:194–204. doi: 10.1097/ANS.0b013e318261b1ba. [DOI] [PubMed] [Google Scholar]
- 166. Braido F, Baiardini I, Menoni S, Bagnasco AM, Balbi F, Bocchibianchi S, et al. Disability in COPD and its relationship to clinical and patient-reported outcomes. Curr Med Res Opin . 2011;27:981–986. doi: 10.1185/03007995.2011.563285. [DOI] [PubMed] [Google Scholar]
- 167. Clari M, Matarese M, Ivziku D, De Marinis MG. Self-care of people with chronic obstructive pulmonary disease: a meta-synthesis. Patient . 2017;10:407–427. doi: 10.1007/s40271-017-0218-z. [DOI] [PubMed] [Google Scholar]
- 168. Jalota L, Jain VV. Action plans for COPD: strategies to manage exacerbations and improve outcomes. Int J Chron Obstruct Pulmon Dis . 2016;11:1179–1188. doi: 10.2147/COPD.S76970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Russell AM, Adamali H, Molyneaux PL, Lukey PT, Marshall RP, Renzoni EA, et al. Daily home spirometry: an effective tool for detecting progression in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med . 2016;194:989–997. doi: 10.1164/rccm.201511-2152OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Holm S.Principles of biomedical ethics, 5th ed J Med Ethics 200228332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Brinkman-Stoppelenburg A, Rietjens JA, van der Heide A. The effects of advance care planning on end-of-life care: a systematic review. Palliat Med . 2014;28:1000–1025. doi: 10.1177/0269216314526272. [DOI] [PubMed] [Google Scholar]
- 172. Vranas KC, Plinke W, Bourne D, Kansagara D, Lee RY, Kross EK, et al. The influence of POLST on treatment intensity at the end of life: a systematic review. J Am Geriatr Soc . 2021;69:3661–3674. doi: 10.1111/jgs.17447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Simpson C. Advance care planning in COPD: care versus “code status”. Chron Respir Dis . 2012;9:193–204. doi: 10.1177/1479972312445897. [DOI] [PubMed] [Google Scholar]
- 174. Heyland DK, Dodek P, Rocker G, Groll D, Gafni A, Pichora D, et al. Canadian Researchers End-of-Life Network(CARENET) What matters most in end-of-life care: perceptions of seriously ill patients and their family members. CMAJ . 2006;174:627–633. doi: 10.1503/cmaj.050626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Goodridge D, Duggleby W, Gjevre J, Rennie D. Caring for critically ill patients with advanced COPD at the end of life: a qualitative study. Intensive Crit Care Nurs . 2008;24:162–170. doi: 10.1016/j.iccn.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 176. Sudore RL, Fried TR. Redefining the “planning” in advance care planning: preparing for end-of-life decision making. Ann Intern Med . 2010;153:256–261. doi: 10.1059/0003-4819-153-4-201008170-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. Hsieh HF, Shannon SE, Curtis JR. Contradictions and communication strategies during end-of-life decision-making in the intensive care unit. J Crit Care . 2006;21:294–304. doi: 10.1016/j.jcrc.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 178. Nevins ML, Epstein SK. Predictors of outcome for patients with COPD requiring invasive mechanical ventilation. Chest . 2001;119:1840–1849. doi: 10.1378/chest.119.6.1840. [DOI] [PubMed] [Google Scholar]
- 179. Khalaila R, Zbidat W, Anwar K, Bayya A, Linton DM, Sviri S. Communication difficulties and psychoemotional distress in patients receiving mechanical ventilation. Am J Crit Care . 2011;20:470–479. doi: 10.4037/ajcc2011989. [DOI] [PubMed] [Google Scholar]
- 180. Claessens MT, Lynn J, Zhong Z, Desbiens NA, Phillips RS, Wu AW, et al. Study to understand prognoses and preferences for outcomes and risks of treatments Dying with lung cancer or chronic obstructive pulmonary disease: insights from SUPPORT. J Am Geriatr Soc . 2000;48:S146–S153. doi: 10.1111/j.1532-5415.2000.tb03124.x. [DOI] [PubMed] [Google Scholar]
- 181. Kirchhoff KT, Hammes BJ, Kehl KA, Briggs LA, Brown RL. Effect of a disease-specific planning intervention on surrogate understanding of patient goals for future medical treatment. J Am Geriatr Soc . 2010;58:1233–1240. doi: 10.1111/j.1532-5415.2010.02760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Perkins HS. Controlling death: the false promise of advance directives. Ann Intern Med . 2007;147:51–57. doi: 10.7326/0003-4819-147-1-200707030-00008. [DOI] [PubMed] [Google Scholar]
- 183. Fagerlin A, Schneider CE. Enough. The failure of the living will. Hastings Cent Rep . 2004;34:30–42. [PubMed] [Google Scholar]
- 184. Hickman SE, Hammes BJ, Moss AH, Tolle SW. Hope for the future: achieving the original intent of advance directives. Hastings Cent Rep . 2005;Spec No:S26–S30. doi: 10.1353/hcr.2005.0093. [DOI] [PubMed] [Google Scholar]
- 185. Dying in America: improving quality and honoring individual preferences near the end of life. Mil Med . 2015;180:365–367. doi: 10.7205/MILMED-D-15-00005. [DOI] [PubMed] [Google Scholar]
- 186. Gardiner C, Gott M, Small N, Payne S, Seamark D, Barnes S, et al. Living with advanced chronic obstructive pulmonary disease: patients concerns regarding death and dying. Palliat Med . 2009;23:691–697. doi: 10.1177/0269216309107003. [DOI] [PubMed] [Google Scholar]
- 187. Heffner JE. Advance care planning in chronic obstructive pulmonary disease: barriers and opportunities. Curr Opin Pulm Med . 2011;17:103–109. doi: 10.1097/mcp.0b013e328341ce80. [DOI] [PubMed] [Google Scholar]
- 188. Fleuren N, Depla MFIA, Janssen DJA, Huisman M, Hertogh CMPM. Underlying goals of advance care planning (ACP): a qualitative analysis of the literature. BMC Palliat Care . 2020;19:27. doi: 10.1186/s12904-020-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. MacKenzie MA, Smith-Howell E, Bomba PA, Meghani SH. Respecting choices and related models of advance care planning: a systematic review of published evidence. Am J Hosp Palliat Care . 2018;35:897–907. doi: 10.1177/1049909117745789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Sean Morrison R. Advance directives/care planning: clear, simple, and wrong. J Palliat Med . 2020;23:878–879. doi: 10.1089/jpm.2020.0272. [DOI] [PubMed] [Google Scholar]
- 191. Halpern SD. Goal-concordant care - searching for the holy grail. N Engl J Med . 2019;381:1603–1606. doi: 10.1056/NEJMp1908153. [DOI] [PubMed] [Google Scholar]
- 192. Meehan E, Foley T, Kelly C, Burgess Kelleher A, Sweeney C, Hally RM, et al. Advance care planning for individuals with chronic obstructive pulmonary disease: a scoping review of the literature. J Pain Symptom Manage . 2020;59:1344–1361. doi: 10.1016/j.jpainsymman.2019.12.010. [DOI] [PubMed] [Google Scholar]
- 193. Gott M, Gardiner C, Small N, Payne S, Seamark D, Barnes S, et al. Barriers to advance care planning in chronic obstructive pulmonary disease. Palliat Med . 2009;23:642–648. doi: 10.1177/0269216309106790. [DOI] [PubMed] [Google Scholar]
- 194. Fulmer T, Escobedo M, Berman A, Koren MJ, Hernández S, Hult A. Physicians’ views on advance care planning and end-of-life care conversations. J Am Geriatr Soc . 2018;66:1201–1205. doi: 10.1111/jgs.15374. [DOI] [PubMed] [Google Scholar]
- 195. Jabbarian LJ, Zwakman M, van der Heide A, Kars MC, Janssen DJA, van Delden JJ, et al. Advance care planning for patients with chronic respiratory diseases: a systematic review of preferences and practices. Thorax . 2018;73:222–230. doi: 10.1136/thoraxjnl-2016-209806. [DOI] [PubMed] [Google Scholar]
- 196. Chan CWH, Ng NHY, Chan HYL, Wong MMH, Chow KM. A systematic review of the effects of advance care planning facilitators training programs. BMC Health Serv Res . 2019;19:362. doi: 10.1186/s12913-019-4192-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Fessler HE, Addrizzo-Harris D, Beck JM, Buckley JD, Pastores SM, Piquette CA, et al. Entrustable professional activities and curricular milestones for fellowship training in pulmonary and critical care medicine: report of a multisociety working group. Chest . 2014;146:813–834. doi: 10.1378/chest.14-0710. [DOI] [PubMed] [Google Scholar]
- 198. Morrison RS, Meier DE, Arnold RM. What’s wrong with advance care planning? JAMA . 2021;326:1575–1576. doi: 10.1001/jama.2021.16430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Farquhar M, Bausewein C, Currow DC, Johnson MJ.Supporting informal carersPalliative care in respiratory disease: ERS monograph. Lausanne, Switzerland: European Respiratory Society; 2016258. [Google Scholar]
- 200. Anderson EW, White KM. “It Has Changed My Life”: an exploration of caregiver experiences in serious illness. Am J Hosp Palliat Care . 2018;35:266–274. doi: 10.1177/1049909117701895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Farquhar M. Assessing carer needs in chronic obstructive pulmonary disease. Chron Respir Dis . 2018;15:26–35. doi: 10.1177/1479972317719086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Klein S, Logan A, Lindell KO. A scoping review of unmet needs of caregivers of patients with pulmonary fibrosis. Curr Opin Support Palliat Care . 2021;15:226–232. doi: 10.1097/SPC.0000000000000571. [DOI] [PubMed] [Google Scholar]
- 203. Graney BA, Wamboldt FS, Baird S, Churney T, Fier K, Korn M, et al. Informal caregivers experience of supplemental oxygen in pulmonary fibrosis. Health Qual Life Outcomes . 2017;15:133. doi: 10.1186/s12955-017-0710-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Lindell KO, Collins EG, Catanzarite L, Garvey CM, Hernandez C, Mclaughlin S, et al. Equipment, access and worry about running short of oxygen: key concerns in the ATS patient supplemental oxygen survey. Heart Lung . 2019;48:245–249. doi: 10.1016/j.hrtlng.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 205. Micklewright K, Farquhar M. Does the carer support needs assessment tool cover the established support needs of carers of patients with chronic obstructive pulmonary disease? A systematic literature search and narrative review. Palliat Med . 2020;34:1305–1315. doi: 10.1177/0269216320939243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Micklewright K, Farquhar M. Face and content validity of the carer support needs assessment tool (CSNAT), and feasibility of the CSNAT intervention for carers of patients with chronic obstructive pulmonary disease. Chronic Illn . 2021 doi: 10.1177/1742395321999433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Caring Bridge. 2021. https://www.caringbridge.org/
- 208. Davidson PM, Phillips JL, Dennison-Himmelfarb C, Thompson SC, Luckett T, Currow DC. Providing palliative care for cardiovascular disease from a perspective of sociocultural diversity: a global view. Curr Opin Support Palliat Care . 2016;10:11–17. doi: 10.1097/SPC.0000000000000188. [DOI] [PubMed] [Google Scholar]
- 209. Hawley P. Barriers to access to palliative care. Palliat Care . 2017;10:1178224216688887. doi: 10.1177/1178224216688887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210. Aldridge MD, Hasselaar J, Garralda E, van der Eerden M, Stevenson D, McKendrick K, et al. Education, implementation, and policy barriers to greater integration of palliative care: a literature review. Palliat Med . 2016;30:224–239. doi: 10.1177/0269216315606645. [DOI] [PubMed] [Google Scholar]
- 211. Johnson KS. Racial and ethnic disparities in palliative care. J Palliat Med . 2013;16:1329–1334. doi: 10.1089/jpm.2013.9468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212. Hoerger M, Perry LM, Korotkin BD, Walsh LE, Kazan AS, Rogers JL, et al. Statewide differences in personality associated with geographic disparities in access to palliative care: findings on openness. J Palliat Med . 2019;22:628–634. doi: 10.1089/jpm.2018.0206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Friedman J, Kim D, Schneberk T, Bourgois P, Shin M, Celious A, et al. Assessment of racial/ethnic and income disparities in the prescription of opioids and other controlled medications in California. JAMA Intern Med . 2019;179:469–476. doi: 10.1001/jamainternmed.2018.6721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Enzinger AC, Ghosh K, Keating NL, Cutler DM, Landrum MB, Wright AA. US trends and racial/ethnic disparities in opioid access among patients with poor prognosis cancer at the end of life (EOL) J Clin Oncol . 2020;38:7005. [Google Scholar]
- 215. Seya MJ, Gelders SF, Achara OU, Milani B, Scholten WK. A first comparison between the consumption of and the need for opioid analgesics at country, regional, and global levels. J Pain Palliat Care Pharmacother . 2011;25:6–18. doi: 10.3109/15360288.2010.536307. [DOI] [PubMed] [Google Scholar]
- 216. De Lima L, Sweeney C, Palmer JL, Bruera E. Potent analgesics are more expensive for patients in developing countries: a comparative study. J Pain Palliat Care Pharmacother . 2004;18:59–70. [PubMed] [Google Scholar]
- 217. De Lima L. Opioid availability in Latin America as a global problem: a new strategy with regional and national effects. J Palliat Med . 2004;7:97–103. doi: 10.1089/109662104322737368. [DOI] [PubMed] [Google Scholar]
- 218. Cherny NI, Cleary J, Scholten W, Radbruch L, Torode J. The Global Opioid Policy Initiative (GOPI) project to evaluate the availability and accessibility of opioids for the management of cancer pain in Africa, Asia, Latin America, and the Caribbean, and the Middle East: introduction and methodology. Ann Oncol . 2013;24:xi7–xi13. doi: 10.1093/annonc/mdt498. [DOI] [PubMed] [Google Scholar]
- 219. Cintron A, Morrison RS. Pain and ethnicity in the United States: a systematic review. J Palliat Med . 2006;9:1454–1473. doi: 10.1089/jpm.2006.9.1454. [DOI] [PubMed] [Google Scholar]
- 220. Morrison RS, Wallenstein S, Natale DK, Senzel RS, Huang LL. “We don’t carry that”--failure of pharmacies in predominantly nonwhite neighborhoods to stock opioid analgesics. N Engl J Med . 2000;342:1023–1026. doi: 10.1056/NEJM200004063421406. [DOI] [PubMed] [Google Scholar]
- 221. Adegunsoye A, Vela M, Saunders M. Racial disparities in pulmonary fibrosis and the impact on the Black population. Arch Bronconeumol . 2021 doi: 10.1016/j.arbres.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Schraufnagel DE, Blasi F, Kraft M, Gaga M, Finn PW, Rabe KF, ATS/ERS Committee on Disparities in Respiratory Health An official American Thoracic Society/European Respiratory Society policy statement: disparities in respiratory health. Am J Respir Crit Care Med . 2013;188:865–871. doi: 10.1164/rccm.201308-1509ST. [DOI] [PubMed] [Google Scholar]
- 223. Welch LC, Teno JM, Mor V. End-of-life care in Black and White: race matters for medical care of dying patients and their families. J Am Geriatr Soc . 2005;53:1145–1153. doi: 10.1111/j.1532-5415.2005.53357.x. [DOI] [PubMed] [Google Scholar]
- 224. Gordon HS, Street RL, Sharf BF, Souchek J. Racial differences in doctors’ information-giving and patients’ participation. Cancer . 2006;107:1313–1320. doi: 10.1002/cncr.22122. [DOI] [PubMed] [Google Scholar]
- 225. Gordon HS, Street RL, Jr, Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol . 2006;24:904–909. doi: 10.1200/JCO.2005.03.1955. [DOI] [PubMed] [Google Scholar]
- 226. Yaqoob ZJ, Al-Kindi SG, Zein JG. Trends and disparities in hospice use among patients dying of COPD in the United States. Chest . 2017;151:1183–1184. doi: 10.1016/j.chest.2017.02.030. [DOI] [PubMed] [Google Scholar]
- 227. Brown CE, Engelberg RA, Sharma R, Downey L, Fausto JA, Sibley J, et al. Race/ethnicity, socioeconomic status, and healthcare intensity at the end of life. J Palliat Med . 2018;21:1308–1316. doi: 10.1089/jpm.2018.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Kwak J, Salmon JR. Attitudes and preferences of Korean-American older adults and caregivers on end-of-life care. J Am Geriatr Soc . 2007;55:1867–1872. doi: 10.1111/j.1532-5415.2007.01394.x. [DOI] [PubMed] [Google Scholar]
- 229. Kelley AS, Wenger NS, Sarkisian CA. Opiniones: end-of-life care preferences and planning of older Latinos. J Am Geriatr Soc . 2010;58:1109–1116. doi: 10.1111/j.1532-5415.2010.02853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Sanders JJ, Robinson MT, Block SD. Factors impacting advance care planning among African Americans: results of a systematic, integrated review. J Palliat Med . 2016;19:202–227. doi: 10.1089/jpm.2015.0325. [DOI] [PubMed] [Google Scholar]
- 231. Bazargan M, Bazargan-Hejazi S. Disparities in palliative and hospice care and completion of advance care planning and directives among non-Hispanic Blacks: a scoping review of recent literature. Am J Hosp Palliat Care . 2021;38:688–718. doi: 10.1177/1049909120966585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Portanova J, Ailshire J, Perez C, Rahman A, Enguidanos S. Ethnic differences in advance directive completion and care preferences: what has changed in a decade? J Am Geriatr Soc . 2017;65:1352–1357. doi: 10.1111/jgs.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. LoPresti MA, Dement F, Gold HT. End-of-life care for people with cancer from ethnic minority groups: a systematic review. Am J Hosp Palliat Care . 2016;33:291–305. doi: 10.1177/1049909114565658. [DOI] [PubMed] [Google Scholar]
- 234. Evans MK, Rosenbaum L, Malina D, Morrissey S, Rubin EJ. Diagnosing and treating systemic racism. N Engl J Med . 2020;383:274–276. doi: 10.1056/NEJMe2021693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Ferrell B, Connor SR, Cordes A, Dahlin CM, Fine PG, Hutton N, et al. National Consensus Project for Quality Palliative Care Task Force Members The national agenda for quality palliative care: the National Consensus Project and the national quality forum. J Pain Symptom Manage . 2007;33:737–744. doi: 10.1016/j.jpainsymman.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 236. Enguidanos S, Kogan AC, Lorenz K, Taylor G. Use of role model stories to overcome barriers to hospice among African Americans. J Palliat Med . 2011;14:161–168. doi: 10.1089/jpm.2010.0380. [DOI] [PubMed] [Google Scholar]
- 237. Volandes AE, Ariza M, Abbo ED, Paasche-Orlow M. Overcoming educational barriers for advance care planning in Latinos with video images. J Palliat Med . 2008;11:700–706. doi: 10.1089/jpm.2007.0172. [DOI] [PubMed] [Google Scholar]
- 238. Volandes AE, Paasche-Orlow M, Gillick MR, Cook EF, Shaykevich S, Abbo ED, et al. Health literacy not race predicts end-of-life care preferences. J Palliat Med . 2008;11:754–762. doi: 10.1089/jpm.2007.0224. [DOI] [PubMed] [Google Scholar]
- 239. Fischer SM, Sauaia A, Kutner JS. Patient navigation: a culturally competent strategy to address disparities in palliative care. J Palliat Med . 2007;10:1023–1028. doi: 10.1089/jpm.2007.0070. [DOI] [PubMed] [Google Scholar]
- 240. Morrison RS, Zayas LH, Mulvihill M, Baskin SA, Meier DE. Barriers to completion of healthcare proxy forms: a qualitative analysis of ethnic differences. J Clin Ethics . 1998;9:118–126. [PubMed] [Google Scholar]
- 241. Liu JM, Lin WC, Chen YM, Wu HW, Yao NS, Chen LT, et al. The status of the do-not-resuscitate order in Chinese clinical trial patients in a cancer centre. J Med Ethics . 1999;25:309–314. doi: 10.1136/jme.25.4.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242. Garrido MM, Harrington ST, Prigerson HG. End-of-life treatment preferences: a key to reducing ethnic/racial disparities in advance care planning? Cancer . 2014;120:3981–3986. doi: 10.1002/cncr.28970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Cain CL, Surbone A, Elk R, Kagawa-Singer M. Culture and palliative care: preferences, communication, meaning, and mutual decision making. J Pain Symptom Manage . 2018;55:1408–1419. doi: 10.1016/j.jpainsymman.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 244. Wanko Keutchafo EL, Kerr J, Jarvis MA. Evidence of nonverbal communication between nurses and older adults: a scoping review. BMC Nurs . 2020;19:53. doi: 10.1186/s12912-020-00443-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Stepanikova I, Zhang Q, Wieland D, Eleazer GP, Stewart T. Non-verbal communication between primary care physicians and older patients: how does race matter? J Gen Intern Med . 2012;27:576–581. doi: 10.1007/s11606-011-1934-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Elliott AM, Alexander SC, Mescher CA, Mohan D, Barnato AE. Differences in physicians’ verbal and nonverbal communication with Black and White patients at the end of life. J Pain Symptom Manage . 2016;51:1–8. doi: 10.1016/j.jpainsymman.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Pham K, Thornton JD, Engelberg RA, Jackson JC, Curtis JR. Alterations during medical interpretation of ICU family conferences that interfere with or enhance communication. Chest . 2008;134:109–116. doi: 10.1378/chest.07-2852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Thornton JD, Pham K, Engelberg RA, Jackson JC, Curtis JR. Families with limited English proficiency receive less information and support in interpreted intensive care unit family conferences. Crit Care Med . 2009;37:89–95. doi: 10.1097/CCM.0b013e3181926430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Smith AK, Sudore RL, Perez-Stable EJ. Palliative care for Latino patients and their families: whenever we prayed, she wept. JAMA . 2009;301:1047–1057, E1. doi: 10.1001/jama.2009.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Kagawa-Singer M, Blackhall LJ. Negotiating cross-cultural issues at the end of life: “You got to go where he lives”. JAMA . 2001;286:2993–3001. doi: 10.1001/jama.286.23.2993. [DOI] [PubMed] [Google Scholar]
- 251. Hern HE, Jr, Koenig BA, Moore LJ, Marshall PA. The difference that culture can make in end-of-life decision-making. Camb Q Healthc Ethics . 1998;7:27–40. doi: 10.1017/s0963180198701045. [DOI] [PubMed] [Google Scholar]
- 252. Carrese JA, Rhodes LA. Western bioethics on the Navajo reservation. Benefit or harm? JAMA . 1995;274:826–829. [PubMed] [Google Scholar]
- 253. Anderson RJ, Stone PC, Low JTS, Bloch S. Managing uncertainty and references to time in prognostic conversations with family members at the end of life: a conversation analytic study. Palliat Med . 2020;34:896–905. doi: 10.1177/0269216320910934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Krawczyk M, Gallagher R. Communicating prognostic uncertainty in potential end-of-life contexts: experiences of family members. BMC Palliat Care . 2016;15:59. doi: 10.1186/s12904-016-0133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.2009 UNISDR terminology and disaster risk reduction. Geneva, Switzerland: United Nations Office for Disaster Risk Reduction; 2009. [Google Scholar]
- 256. Wilkinson A. The potential role for palliative care in mass casualty events. J Palliat Care Med . 2012;2:1000e112. [Google Scholar]
- 257. Kelly M, Mitchell I, Walker I, Mears J, Scholz B. End-of-life care in natural disasters including epidemics and pandemics: a systematic review. BMJ Support Palliat Care . 2021 doi: 10.1136/bmjspcare-2021-002973. [DOI] [PubMed] [Google Scholar]
- 258. Arya A, Buchman S, Gagnon B, Downar J. Pandemic palliative care: beyond ventilators and saving lives. CMAJ . 2020;192:E400–E404. doi: 10.1503/cmaj.200465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Matzo M, Wilkinson A, Lynn J, Gatto M, Phillips S. Palliative care considerations in mass casualty events with scarce resources. Biosecur Bioterror . 2009;7:199–210. doi: 10.1089/bsp.2009.0017. [DOI] [PubMed] [Google Scholar]
- 260.Phillips S, Knebel A, Mass medical care with scarce resources: a community planning guide 2007https://www.calhospitalprepare.org/sites/main/files/resources/Mass%20Medical%20Care%20with%20Scarce%20Resources.pdf.
- 261. Zalenski RJ, Jones SS, Courage C, Waselewsky DR, Kostaroff AS, Kaufman D, et al. Impact of palliative care screening and consultation in the ICU: a multihospital quality improvement project. J Pain Symptom Manage . 2017;53:5–12.e3. doi: 10.1016/j.jpainsymman.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 262. Quinn KL, Shurrab M, Gitau K, Kavalieratos D, Isenberg SR, Stall NM, et al. Association of receipt of palliative care interventions with health care use, quality of life, and symptom burden among adults with chronic noncancer illness: a systematic review and meta-analysis. JAMA . 2020;324:1439–1450. doi: 10.1001/jama.2020.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. de Wolf-Linder S, Dawkins M, Wicks F, Pask S, Eagar K, Evans CJ, et al. Which outcome domains are important in palliative care and when? An international expert consensus workshop using the nominal group technique. Palliat Med . 2019;33:1058–1068. doi: 10.1177/0269216319854154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. Aslakson RA, Reinke LF, Cox C, Kross EK, Benzo RP, Curtis JR. Developing a research agenda for integrating palliative care into critical care and pulmonary practice to improve patient and family outcomes. J Palliat Med . 2017;20:329–343. doi: 10.1089/jpm.2016.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Collins LM, Murphy SA, Strecher V. The multiphase optimization strategy (MOST) and the sequential multiple assignment randomized trial (SMART): new methods for more potent eHealth interventions. Am J Prev Med . 2007;32(5, Suppl):S112–S118. doi: 10.1016/j.amepre.2007.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Kamal AH, Wolf SP, Troy J, Leff V, Dahlin C, Rotella JD, et al. Policy changes key to promoting sustainability and growth of the specialty palliative care workforce. Health Aff (Millwood) . 2019;38:910–918. doi: 10.1377/hlthaff.2019.00018. [DOI] [PubMed] [Google Scholar]
- 267. Kamal AH, Docherty SL, Reeve BB, Samsa GP, Bosworth HB, Pollak KI. Helping the demand find the supply: messaging the value of specialty palliative care directly to those with serious illnesses. J Pain Symptom Manage . 2019;57:e6–e7. doi: 10.1016/j.jpainsymman.2019.02.026. [DOI] [PubMed] [Google Scholar]
- 268. Wysham NG, Hua M, Hough CL, Gundel S, Docherty SL, Jones DM, et al. Improving ICU-based palliative care delivery: a multicenter, multidisciplinary survey of critical care clinician attitudes and beliefs. Crit Care Med . 2017;45:e372–e378. doi: 10.1097/CCM.0000000000002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Aslakson RA, Ast K, AAHPM Research Committee Writing Group Introduction to a new special series for the journal of pain and symptom management-science in action: evidence and opportunities for palliative care across diverse populations and care settings. J Pain Symptom Manage . 2019;58:134–136. doi: 10.1016/j.jpainsymman.2019.02.005. [DOI] [PubMed] [Google Scholar]
- 270. King JD, Eickhoff J, Traynor A, Campbell TC. Integrated onco-palliative care associated with prolonged survival compared to standard care for patients with advanced lung cancer: a retrospective review. J Pain Symptom Manage . 2016;51:1027–1032. doi: 10.1016/j.jpainsymman.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 271. Bajwah S, Oluyase AO, Yi D, Gao W, Evans CJ, Grande G, et al. The effectiveness and cost-effectiveness of hospital-based specialist palliative care for adults with advanced illness and their caregivers. Cochrane Database Syst Rev . 2020;9:CD012780. doi: 10.1002/14651858.CD012780.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Eisenstadt M, Liverpool S, Infanti E, Ciuvat RM, Carlsson C. Mobile apps that promote emotion regulation, positive mental health, and well-being in the general population: systematic review and meta-analysis. JMIR Ment Health . 2021;8:e31170. doi: 10.2196/31170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Pinto S, Caldeira S, Martins JC. e-Health in palliative care: review of literature, Google Play and App Store. Int J Palliat Nurs . 2017;23:394–401. doi: 10.12968/ijpn.2017.23.8.394. [DOI] [PubMed] [Google Scholar]
- 274. Putranto D, Rochmawati E. Mobile applications for managing symptoms of patients with cancer at home: a scoping review. Int J Nurs Pract . 2020;26:e12842. doi: 10.1111/ijn.12842. [DOI] [PubMed] [Google Scholar]
- 275. Turnbull AE, Hartog CS. Goal-concordant care in the ICU: a conceptual framework for future research. Intensive Care Med . 2017;43:1847–1849. doi: 10.1007/s00134-017-4873-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Sanders JJ, Miller K, Desai M, Geerse OP, Paladino J, Kavanagh J, et al. Measuring goal-concordant care: results and reflections from secondary analysis of a trial to improve serious illness communication. J Pain Symptom Manage . 2020;60:889–897.e2. doi: 10.1016/j.jpainsymman.2020.06.023. [DOI] [PubMed] [Google Scholar]
- 277. Campbell ML, Donesky D, Sarkozy A, Reinke LF. Treatment of dyspnea in advanced disease and at the end of life. J Hosp Palliat Nurs . 2021;23:406–420. doi: 10.1097/NJH.0000000000000766. [DOI] [PubMed] [Google Scholar]