Supplemental Digital Content is Available in the Text.
Key Words: trial, number needed to treat, systematic review, meta-analysis, statins, ezetimibe, PCSK9-inhibiting monoclonal antibodies, low-density lipoprotein cholesterol
Abstract:
Lipid-modifying agents steadily lower low-density lipoprotein cholesterol (LDL-C) levels with the aim of reducing mortality. A systematic review and meta-analysis were conducted to determine whether all-cause or cardiovascular (CV) mortality effect size for lipid-lowering therapy varied according to the magnitude of LDL-C reduction. Electronic databases were searched, including PubMed and ClinicalTrials.gov, from inception to December 31, 2019. Eligible studies included randomized controlled trials that compared lipid-modifying agents (statins, ezetimibe, and PCSK-9 inhibitors) versus placebo, standard or usual care or intensive versus less-intensive LDL-C–lowering therapy in adults, with or without known history of CV disease with a follow-up of at least 52 weeks. All-cause and CV mortality as primary end points, myocardial infarction, stroke, and non-CV death as secondary end points. Absolute risk differences [ARD (ARDs) expressed as incident events per 1000 person-years], number needed to treat (NNT), and rate ratios (RR) were assessed. Sixty randomized controlled trials totaling 323,950 participants were included. Compared with placebo, usual care or less-intensive therapy, active or more potent lipid-lowering therapy reduced the risk of all-cause death [ARD −1.33 (−1.89 to −0.76); NNT 754 (529–1309); RR 0.92 (0.89–0.96)]. Intensive LDL-C percent lowering was not associated with further reductions in all-cause mortality [ARD −0.27 (−1.24 to 0.71); RR 1.00 (0.94–1.06)]. Intensive LDL-C percent lowering did not further reduce CV mortality [ARD −0.28 (−0.83 to 0.38); RR 1.02 (0.94–1.09)]. Our findings indicate that risk reduction varies across subgroups and that overall NNTs are high. Identifying patient subgroups who benefit the most from LDL-C levels reduction is clinically relevant and necessary.
INTRODUCTION
Atherosclerotic cardiovascular disease (ASCVD), a leading cause of morbidity and mortality, affects 423 million people and is responsible for 32% of all deaths worldwide.1 Although it remains the most common cause of death in North America and Europe, deaths from ASCVD have declined substantially in the past 20 years,2 due to improvements in risk factor management and medical treatment. Statins are considered the cornerstone of cardiovascular (CV) death reduction for both primary and secondary prevention.
Long-term exposure to excessive concentrations of low-density lipoprotein cholesterol (LDL-C) as in familial hypercholesterolemia is linked to premature atherosclerosis and early mortality. Epidemiological studies are conflicting.3–5 After the failure of fibrates to reduce CV events, the 4S trial was the first to demonstrate a significant all-cause mortality reduction with simvastatin in a population of Northern European patients with coronary artery disease (CAD) with high baseline blood cholesterol.6 For almost 30 years, a large number of randomized controlled trials (RCTs) have investigated the benefit of statin therapy and the addition of either ezetimibe or antiproprotein convertase subtilisin/kexin type 9 (PCSK9) monoclonal antibodies to statin therapy. Meta-analyses of LDL-C–lowering therapy found that event reduction correlates with the extent of LDL-C reduction.7,8 Current guidelines recommend at least LDL-C 50% reduction and lower achieved LDL-C levels in high-risk patients.9,10
A comprehensive meta-analysis showed that mortality reduction was observed only in trials with mean baseline LDL-C level higher than 100 mg/dL, suggesting that the benefit from LDL-C–lowering therapy mostly occurs in patients with high baseline LDL-C levels.11 In addition, all-cause mortality was not related to achieved targeted LDL-C levels.11
Because recent guidelines recommend larger LDL-C reduction to prolong life, we conducted an updated systematic review and meta-analysis of RCTs to determine whether the mortality effect size associated with lipid-lowering therapy varied according to the extent of LDL-C reduction. Patients were at risk for various CV conditions and followed for at least 1 year. The RCTs addressed the effectiveness of lipid-lowering therapy regarding absolute rate differences (ARDs) and thereby of the number of subjects needed to treat (NNT) for delaying the outcome beyond the study duration (NNT). The extent of LDL-C lowering for percentage or absolute reduction with the use of statins, ezetimibe, and PCSK-9–inhibiting monoclonal antibodies were extracted along with the reduction in all-cause and CV death risk. We also assessed CV events including myocardial infarction (MI) and stroke as well as non-CV death. Benefits of these therapeutics compared with the baseline and achieved LDL-C levels and also to the CV mortality rate of population studies were additionally evaluated.
METHODS
This systematic review included publicly available, previously published studies, and the meta-analysis was performed according to current guidelines,12 complying with the Preferred Reporting Items for Systematic review and Meta-Analysis statement. The rationale and methods were prespecified in a protocol registered at PROSPERO (CRD42020144859).
Eligibility Criteria
Study Designs
Eligible studies included parallel-group or factorial RCTs. Observational cohort or retrospective case–control studies were not considered. RCTs enrolling more than 100 participants in each comparative group were included in the analysis.
Participants
RCTs enrolling adults with known or unknown ASCVD were included. Trials enrolling patients with left ventricular dysfunction, kidney disease, or aortic stenosis were included, as well as those with rheumatoid arthritis and chronic obstructive pulmonary disease.
Intervention
LDL-C–reduction therapy included statins, ezetimibe, and PCSK-9–inhibiting monoclonal antibodies.
Comparators
Less-intensive LDL-C reduction therapy included placebo of statin, ezetimibe, or PCSK-9–inhibiting monoclonal antibodies, usual care, and less potent or less-intensive statin therapy.
Outcomes
Primary studies were considered for eligibility when they reported at least 1 clinical outcome. Our primary outcomes were all-cause mortality and CV mortality. Secondary outcomes of interest were the number of MI events, stroke events, and non-CV mortality. These outcomes were assessed as ARDs and NNT related to the percent LDL-C reduction as primary analyses. Rate ratios (RR) were also provided as secondary analyses. We also assessed the risk reduction related to the absolute LDL-C–level reduction, baseline LDL-C levels, achieved LDL-C levels in the intervention group, and also according to the annual CV mortality rate in control arms.
Characteristics
Only primary studies with a mean or median follow-up duration of 12 months (ie, 52 weeks) or more were eligible. This time frame was based on the findings of previous trials.13,14 Studies in English or French languages were included. All studies from inception to December 31, 2019 were included.
Information sources, search strategy, study selection, data extraction, data items, risk of bias assessment, and statistical analyses are provided in the Supplemental Digital Content(see, http://links.lww.com/JCVP/A874).
RESULTS
Study Identification
A flow chart describing the process of publication screening and reasons for exclusion is shown in (see eFigure1, Supplemental Digital Content, http://links.lww.com/JCVP/A874). We identified a total of 60 RCTs including 323,950 individuals. Among these, 162,393 patients were randomized to intensive LDL-C–lowering strategy and 161,557 were randomized to less-intensive strategy. The characteristics of the selected trials are shown in Supplemental Digital Content (see eTables 1 and 2 and commented in the http://links.lww.com/JCVP/A874).
Characteristics of Included Studies
The Supplemental Digital Content (see eTables 1 and 2, http://links.lww.com/JCVP/A874) present the characteristics of the 60 eligible trials involving 323,950 participants (36 statins vs. placebo or usual care, 14 intensive statin vs. less-intensive statin, 5 PCSK9 inhibitors vs. placebo, 3 ezetimibe vs. placebo or usual care and 2 statin plus ezetimibe arm vs. double-placebo arm). Studies that enrolled patients with known and unknown ASCVD were referred to as secondary and primary prevention, respectively. Studies enrolling patients with specific conditions, including kidney disease, left ventricular dysfunction, chronic obstructive pulmonary disease, previous stroke, and rheumatoid arthritis, were included in the meta-analysis. Eight studies used a factorial design, including a comparison between statin and placebo ALLHAT-LLT,15 ASCOT-LLA,16 PREVEND-IT,17 HOPE-3,18 ACAPS,19 Post CABG,20 HPS,21 and GISSI-P.22 In 1 trial, patients received ezetimibe monotherapy (EWTOPIA 75)23; statin and ezetimibe were given in in 4 trials (SEAS,24 IMPROVE-IT,25 HIJ-PROPER,26 and SHARP27), statin and PCSK9-inhibiting monoclonal antibodies in 5 trials (ODYSSEY LONG TERM,28 GLAGOV,29 SPIRE-2,30 FOURIER,13 and ODYSSEY OUTCOME14), and LDL-C with therapeutic targets were compared in 2 trials (EMPATHY31 and TST32). Twenty-four trials fail to reach statistical significance for the primary end point (PATE,33 ALLHAT-LLT,15 CERDIA,34 PREVEND IT,17 St Francis,35 ASPEN,36 SEAS,24 STACOPE,37 EMPATHY,31 TRACE RA,38 GISSI-P,22 FLORIDA,39 A-to-Z,40 IDEAL,41 SAGE,42 SEARCH,5 SATURN,43 HIJ-PROPER,26 CORONA,44 GISSI HF,45 ALERT,46 GDDS,47 AURORA,48 and J-STARS.49) Sample size varied from 250 to 27,564 subjects. Eight trials were specifically conducted in Asia (PATE,33 EMPATHY,31 EWTOPIA,23 MEGA,50 HIJ PROPER,26 J STARS,49 REAL-CAD,51 and Im et al 52), 2 in Italy (GISSI-P22 and GISSI-HF45), and 1 in Greece (GREACE53); most trials were conducted in North America, Northern Europe, and Oceania; of note, South Africa was virtually the only African Investigation site that was involved in 9 trials (ASPEN,36 JUPITER,54 HOPE-3,18 IMPROVE-IT,25 ODYSSEY LONG TERM,28 GLAGOV,29 SPIRE-2,30 FOURIER,13 and SPARCL55). The SEARCH trial had the longest mean follow-up (6.7 years).56 The average follow-up was 3.93 ± 1.56 years. The mean weighted age for participants across primary studies was 63.2 years (range 49.7–80.6 years), and the mean weighted percentage of female participants was 30.1% (range, 0% to 74.5%). The mean weighted prevalence of active or history of cigarette smoking was 22.4% (range, 5% to 44%), hypertension 54.9% (range, 0% to 100%), and diabetes mellitus 24% (range, 0% to 100%). Of note, few black subjects were enrolled in the trials, and whites represented 69 ± 35% of the trial population (information available in 44 trials).
The mean weighted baseline LDL-C level was 121.2 mg/dL (range, 74.2 to 192 mg/dL) at baseline and 79.4 mg/dL (range, 30 to 165 mg/dL) at the end of the follow-up in the intervention group. Compared with the control group, the corresponding absolute and relative mean weighted LDL-C reduction were 36.8 mg/dL (range, 8 to 87 mg/dL) and 31.2% (range, 5 to 65 mg/dL) in the intervention group.
Outcomes
The (see eTables 3–7, Supplemental Digital Content, http://links.lww.com/JCVP/A874) are depicting the numbers of events in each group, which were used for the present meta-analysis. Potential sources of bias are listed in (see eTable8, Supplemental Digital Content, http://links.lww.com/JCVP/A874). Publication bias is shown by (see eFigures 2–5, Supplemental Digital Content, http://links.lww.com/JCVP/A874). The corresponding absolute risk differences, NNTs, and RR pertaining to the extent of LDL-C reduction are shown in (eTables 9–18, Supplemental Digital Content, http://links.lww.com/JCVP/A875). Reassuringly, trends in RR and absolute rate differences were consistent for all outcomes and analyses.
Percentage LDL-C Reduction
All-cause Mortality
Fifty-eight studies reported the incidence of all-cause mortality. Pooled analysis showed that 12,965 of 161,551 patients (8.03%) receiving intensive LDL-C–lowering strategy versus 13,836 of 160,714 (8.61%) receiving less-intensive strategy died during follow-up [ARD −1.33 (−1.89 to −0.76); NNT 754 (529–1309); RR 0.92 (0.89–0.96)].
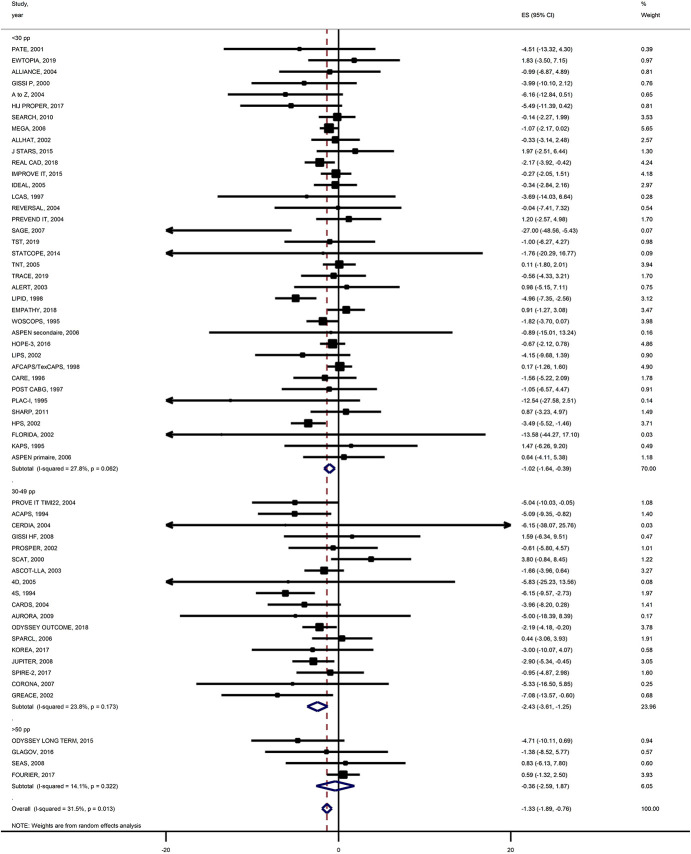
In unadjusted and adjusted meta-regression analyses, we did not find a significant trend toward decrease in both ARDs and RRs for all-cause mortality according to percentage LDL-C reduction (Fig. 1). In the meta-analysis by subgroups of baseline LDL-C level (Fig. 2), both all-cause mortality absolute and relative risks were reduced in the trials with <30% LDL-C reduction and those with 30%–49% LDL-C reduction, although risk reduction was not significant in trials with more than 50% LDL-C reduction [ARD −0.36 (−2.59 to 1.87); RR 0.99 (0.82–1.21) respectively].
FIGURE 1.
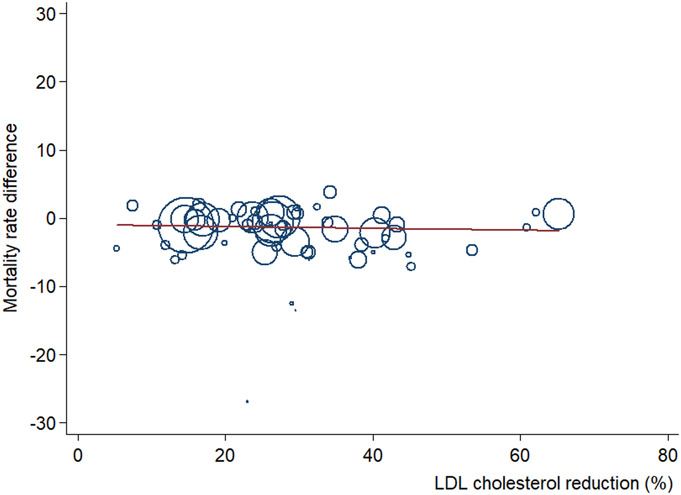
Meta-regression analysis of ARD in all-cause mortality risk by percent LDL-C level reduction. Change in ARD and 95% CIs of more intensive versus less-intensive LDL-C–lowering therapies plotted against percent LDL-C levels reduction. Size of the data markers is proportional to the weight in the meta-regression. The solid line represents the meta-regression slope of the change in ARD for treatment across increasing levels of percent LDL-C reduction.
FIGURE 2.
Meta-analysis of all-cause mortality risk stratified by percent LDL-C level reduction. ARD and 95% CIs of more intensive versus less-intensive LDL-C–lowering therapies and the weight of study data in the meta-analysis.
Cardiovascular Mortality
Fifty-seven studies reported the incidence of CV mortality. Pooled analysis showed that 6631 of 159,494 patients (4.16%) receiving intensive LDL-C–lowering strategy versus 7394 of 158,659 (4.66%) receiving less-intensive strategy died of CV causes during follow-up [ARD −0.97 (−1.32 to −0.62); NNT 1028 (756–1605); RR 0.89 (0.85–0.92)].
In unadjusted and adjusted meta-regression analyses, for each 20% LDL-C reduction, intensive versus less-intensive LDL-C lowering was not associated with a change in ARDs or RRs for CV mortality (see eFigure 7, Supplemental Digital Content, http://links.lww.com/JCVP/A875). Similar to all-cause mortality, CV mortality relative and absolute risks were not reduced in pooled trials with ≥50% LDL-C reduction [ARD −1.03 (−3.23 to 1.17); RR 0.87 (0.63–1.22)] (see eFigure 8, Supplemental Digital Content, http://links.lww.com/JCVP/A875).
Myocardial Infarction
Fifty-five studies reported the incidence of MI. Pooled analysis showed that 7581 of 161,017 patients (4.71%) receiving intensive LDL-C–lowering strategy versus 9564 of 160,172 (5.97%) receiving less-intensive strategy experienced an MI during follow-up [ARD −2.76 (−3.33 to −2.18); NNT 363 (300–459); RR 0.77 (0.73–0.81)].
In both unadjusted and adjusted meta-regression, for each 20% LDL-C reduction, intensive versus less-intensive LDL-C lowering was associated with a change in ARDs (P = 0.001 to 0.002) for MI risk (see eFigure 9, Supplemental Digital Content, http://links.lww.com/JCVP/A875). Only after adjusted meta-regression analysis, including CV mortality rates, associated change was found when MI risk reduction was expressed in RRs (P = 0.028). In meta-analysis by subgroups, MI risk was further reduced in the pooled trials with more than 50% LDL-C reduction compared with those with 30%–49% and <30% reduction. This translated in high ARDs, low NNTs, and RRs in trials with high LDL-C reduction (see eFigure10, Supplemental Digital Content, http://links.lww.com/JCVP/A875).
Stroke
Fifty-three studies reported the incidence of stroke. Pooled analysis showed that 3483 of 160,295 patients (2.17%) receiving intensive LDL-C–lowering strategy versus 4285 of 159,438 (2.69%) receiving less-intensive strategy had a stroke during follow-up [ARD −1.1 (−1.45 to −0.76); NNT 907 (691 to 1319); RR 0.82 (0.77–0.87)].
In both unadjusted and adjusted meta-regression analysis, the ARDs for stroke risk increased significantly (P = 0.003 to 0.005) for each 20% LDL-C percentage reduction; a 9% increase in RRs for stroke was of borderline significance (P = 0.05 and 0.07 respectively) but a significant 13% increase after further adjustment to CV mortality rate (P = 0.003) (see eFigure 11, Supplemental Digital Content, http://links.lww.com/JCVP/A875). Like with mortality, RR and ARD were not significant in the trials with more than 50% LDL-C reduction (see eFigure 12, Supplemental Digital Content, http://links.lww.com/JCVP/A875).
Additional analyses (see Supplemental Digital Content, http://links.lww.com/JCVP/A875) including those for absolute LDL-C reduction (see eTables 9 and 10, Supplemental Digital Content, http://links.lww.com/JCVP/A875), baseline LDL-C levels (see eTables 13, 14 and eFigures 13–16, Supplemental Digital Content, http://links.lww.com/JCVP/A875), and achieved LDL-C levels (see eTables 15, 16 and eFigures 17–22, Supplemental Digital Content, http://links.lww.com/JCVP/A875) as well as for non-CV mortality are shown in the Supplement. The association between CV mortality rates of studied populations and outcomes were also studied (see eTables 17, 18 and eFigures 23–26, Supplemental Digital Content, http://links.lww.com/JCVP/A875).
DISCUSSION
The purpose of this systematic review and meta-analysis was to determine whether all-cause or CV mortality effect size for lipid-lowering therapy varied according to the magnitude of LDL-C reduction. Our findings indicate that all-cause and CV mortality reduction was not related to LDL-C relative or absolute reduction (RR and ARD) or to baseline and achieved LDL-C levels in meta-regression models. In trials with LDL-C lowering above 50% and in those with baseline LDL-C levels below 130 mg/dL, all-cause and CV mortality benefits were not consistent and clinically relevant both for absolute and relative risk reduction. Overall, MI risk was reduced but mostly in trials with LDL-C reduction above 50% or 65 mg/dL and in trials with high baseline LDL-C levels. The relationship between achieved LDL-C levels and MI risk was not apparent from our findings. The benefit on stroke risk was not evident with percentage reduction above 50% or when absolute LDL-C reduction was more than 65 mg/dL. The annual NNTs were overall high. The lowest annual NNTs (129–187) were found in trials with high relative or absolute LDL-C reduction and those with the highest baseline LDL-C levels for the risk of MI. Furthermore, our findings do not support a clear relationship between lowering LDL-C achieved levels and events reduction. Achieving LDL-C level below 55 mg/dL did not translate in further significant all-cause and CV mortality benefit.
Several meta-analyses have found consistent event reduction with lipid-lowering therapies. The CTT collaboration reported that a 1.0-mmol/L (38.7-mg/dL) LDL-C reduction was associated with a 10% reduction [RR 0·90, 95% confidence interval (CI), 0.87–0.93; P < 0.0001] and significant mortality reductions from CAD (RR 0.80, 99% CI, 0.74–0.87; P < 0.0001) and other cardiac causes (RR 0·89, 99% CI, 0.81–0.98; P = 0.002)8; Silverman et al7 found that the relative risk reduction for major vascular events per absolute 1-mmol/L (38.7-mg/dL) reduction in LDL-C level was 0.77 (95% CI, 0.71–0.84; P < 0.001) for statins.
The latest European Society of Cardiology guidelines recommend LDL-C reduction of ≥50% from baseline and lower LDL-C goal below 55 mg/dL in extremely high-risk patients whether for primary or secondary prevention and below 40 mg/dL in those with a second vascular event within 2 years, while taking maximally tolerated statins.9 A LDL-C reduction of ≥50% from baseline and a LDL-C goal of <70 mg/dL are recommended in patients at high CV risk. A LDL-C target of <100 mg/dL is recommended in patients at moderate CV risk, whereas a target of <116 mg/dL should be targeted for low-risk individuals. However, the benefits of these lower LDL-C goals have not been formally demonstrated in patients previously treated with lipid-lowering agents and are derived from meta-analyses and from trials, including PCSK-9 inhibition added to statin and ezetimibe, occurring in the past 30 years. Indeed, past meta-analyses mixed a variety of populations treated differently. In the 4S RCT, for instance, 37% of randomized Scandinavians received aspirin and only 7% had not benefited from revascularization therapy,6 whereas the use of secondary preventive therapies was utmost in patients enrolled in the FOURIER RCT.13 When considering high-risk patients with mean baseline LDL-C levels of 190 mg/dL enrolled in 4S6 or WOSCOPS57 trials, the target of 55 or even 40 mg/dL requires lipid-lowering therapy to reduce LDL-C by 70%–80% (135–150-mg/dL in absolute reduction). This strategy needs to be prospectively demonstrated with adequately powered trials.
All-Cause and Cardiovascular Mortality End points
All-cause mortality is the less equivocal end point; only 2 early seminal trials (4S6 & HPS21) were individually positive for mortality as primary end point and a limited number of trials, including LIPID,58 GREACE,53 JUPITER,54 REAL CAD,51 and ODYSSEY OUTCOME,14 showed mortality reduction with intensive lipid-lowering therapy in a secondary end point analysis. Adjudication of CV mortality may be controversial even in prospective clinical trials; however, our findings on CV mortality were consistent with those of all-cause mortality and the association was not present in patients who experience high LDL-C relative reduction. Aggressive management of CV events currently may account for the lack of mortality benefit and even more so when LDL-C percentage reduction was greater than 50%. The short duration of RCTs and the limited number of RCTs showing the highest LDL-C reductions may also explain wider CIs and lack of mortality benefit.13 The present meta-analysis also concurs with main findings of Navarese et al.11 All-cause and CV mortality risks were not substantially lowered when baseline LDL-C levels were below 130 mg/dL for ARDs and RRs. For both end points, mean NNTs were high, suggesting a questionable individual mortality benefit. Despite the limited value of information from observational epidemiological studies about treatment effects, it is worthy to note that in the Cooper Center Longitudinal Study, only LDL-C categories of ≥160 mg/dL remained independently associated with CV mortality.59 By contrast, risk of all-cause mortality did not increase substantially between LDL-C categories <100 mg/dL, 100 to 129 mg/dL, 130 to 159 mg/dL, 160 to 189 mg/dL, and ≥190 mg/dL.59
Myocardial Infarction and Stroke End points
These end points are less robust for meta-analyses because the definition of MI has evolved with the continuous development of high-sensitivity cardiac injury biomarkers. In the seminal 4S trial, “major coronary events” comprised coronary deaths, definite or probable hospital-verified nonfatal acute MI, resuscitated cardiac arrest, and definite silent MI verified by major Q-wave pattern changes on electrocardiogram.6 Three decades ago, creatine kinase and its MB isoenzyme (CK-MB) were commonly used for the diagnosis of MI. In the ODYSSEY Outcomes trial, MI was defined in accordance with ACC/AHA/ESC universal definition of MI with the use of cardiac troponin I or T.60 As a result, MI prevalence in the 4S trial will differ with the use of high-sensitivity cardiac troponin. The lowest NNTs (high therapeutic impact) to postpone MI were observed with highly intensive LDL-C relative or absolute reduction and high baseline LDL-C levels; however, mean annual NNT numbers still remained above 100. Of note, Navarese et al 11 reported similar findings with an RR of 0.84 when baseline LDL-C levels were less than 100 mg/dL and 0.64 for baseline LDL-C levels greater than 160 mg/dL.
Similarly, lipid-lowering therapy aims at reducing atherothrombotic nonhemorrhagic stroke, whereas stroke may include embolic, nonembolic, and cryptogenic ischemic stroke as well as hemorrhagic stroke unrelated to cerebral infarction. Over time, the increasing use of brain magnetic resonance imaging, CT angiography, transesophageal echocardiogram with bubble study, or ambulatory electrocardiogram also refined the diagnosis of atherosclerotic strokes. The number of unclassified strokes was substantial in the 4S trial.6 These specific issues may explain why the present findings are at variance with previous reports. Nevertheless, the RR of 0.82 reported by Navarese et al11 equals that of the present investigation. In addition, the benefit for stroke did not vary with baseline LDL-C levels in both studies and became nonsignificant within the trials with the highest LDL-C reductions (more than 50% or 65 mg/dL reduction). The high mean NNTs were overall noted for end point stroke risk reduction. It is worthy to note that in contrast to the relationship between MI risk and blood cholesterol, the association between spontaneous cholesterol levels and stroke risk was found weak or nonexistent in large epidemiologic studies.61,62 Indeed, the 2 large trials aimed at demonstrating LDL-C lowering after atherosclerotic stroke, SPARCL55 and TST32 showed small absolute benefits and high NNTs; the Japanese J-STARS49 trial was neutral. Of note, a 20% and 60% (atorvastatin 80 mg) increase in hemorrhagic stroke per mmol/L LDL-C reduction was reported in the CTT meta-analysis8 and SPARCL trial,55 respectively.
General Comments
The NNT offers a better appreciation of the clinical relevance of treatment benefit and thereby helps improved clinical decision making at the individual level. The NNT derives directly from the absolute benefit, which is the difference in the observed rates of events in the treated and control groups over a fixed period. The greater the absolute benefit, the smaller the NNT. Although relative risk or rate reduction remains constant or increases in low-risk patients, absolute benefit drops to small numbers and NNTs raise to large numbers. Like hazard ratios, RR may overstate the benefits in populations with low event rates while the NNT rises. For instance, previous meta-analyses found a relative risk reduction of 38% (RR 0.62) in patients with 5-year event risk of <5% and a 21% risk reduction in those with a 5-year risk of >30%, although absolute risk reductions were opposed (0.18% in low-risk and 1.18% in high-risk patients).63 Such data presentation may mislead clinicians. Similarly, Wang et al64 found that risk reduction of major vascular events is independent of the starting LDL-C; however, the ARD was 0.5% in studies with baseline LDL-C less than 100 mg/dL but 1.2% in studies with baseline LDL-C more than 158 mg/dL. Recently, Yourman et al 65 reported that treating 100 adults aged >55 years without known CV disease with a statin for 2.5 years prevented 1 CV event in 1 adult without mortality benefit.
Risk-based intervention strategies recommended by guidelines suggest that the higher baseline CV risk, the greater the absolute reduction in risk.10 Only CV mortality absolute risk reduction was tightly related to CV mortality rates, whereas for both MI and stroke end points, annual CV mortality rates did not relate to the extent of benefits in absolute risk reduction. Of note, significant meta-regressions between percent LDL-C reduction and MI or stroke absolute risk reduction (see eTable 8, Supplemental Digital Content, http://links.lww.com/JCVP/A874) or between absolute LDL-C reduction and MI or stroke relative risk reduction (see eTable 10, Supplemental Digital Content, http://links.lww.com/JCVP/A875) emerged only after adjustment on CV mortality rates.
Large NNTs suggest that the “one size fits all” approach is not recommended for the future of precision medicine and may not be adapted to a constraint health care economy.65 Benefits found with LDL-C–lowering therapies are indeed modest when compared with the benefits of timely myocardial revascularization in acute MI, antithrombotic therapy, therapies aimed at left ventricular dysfunction, smoking cessation, regular exercise, and obesity prevention.66–69
Short duration or early termination in recent trials including ASCOT-LLA,16 CARDS,70 JUPITER,54 or FOURIER13 may account for the lack of mortality benefit despite large randomized populations. Short follow-up duration might also account for the discrepancy between MI benefit and mortality benefit in trials with more than 50% LDL-C reduction or low baseline LDL-C levels. Alternatively, premature trial termination may also have overestimated benefits of the lipid-lowering strategy.71
Although the choice of major adverse cardiac events as an end point reduced the NNTs, they were nevertheless 67 (147 per year) in FOURIER13 and 49 (137 per year) in ODYSSEY OUTCOME trial.14
Limitations
The study did not solely consist of published trials that had prespecified clinical outcomes. Other data besides primary and secondary end points may not have been rigorously and consistently recorded. Some outcomes, such as stroke and MI, resulted in imprecise estimates of effects and low certainty evidence. The present meta-analysis only considered trials with the required data; thus, it made it challenging to compare trials for specific differences. We did not address the specific impact of respective lipid-lowering drugs. Limitations include heterogeneity in clinical settings of the trials. Lipid lowering is indeed prescribed in a vast proportion of the population to prevent ASCVD. Our findings might be confounded by various comorbid conditions, such as rheumatoid arthritis. Yet they were reported inconsistently and could not be extracted from primary study reports. In addition, mixing trials that investigated lipid-lowering strategies for primary and secondary prevention may somewhat weaken the present meta-analysis. However, vascular benefits of statin therapy were demonstrated in large meta-analyses mixing primary and secondary prevention trials.8,64,72 Conversely, an unknown proportion of patients who were enrolled in primary prevention trials had silent ASCVD, significant plaque on coronary or carotid artery or target organ damage, and may be viewed today as being at a higher risk. Recent LDL-C goals along with CV risk categories released by the latest guidelines illustrate this strategy.9
LDL-C may not be the best risk predictor of CV mortality/events as small dense LDL-C phenotype or as non-HDL-C.9,73 However, these data were not consistently available from all trials. Moreover, guidelines largely focus on the simple LDL-C parameter, whereas ESC guidelines state that there is “compelling evidence that LDL-C is causally associated with the risk of ASCVD, and that lowering LDL-C reduces the risk of ASCVD proportionally to the absolute achieved reduction in LDL-C.”
The ARD and NNT are influenced by the baseline risk for disease and amount of time at risk, although the RR is theoretically not affected. Nevertheless, impressive RRs may mask low ARDs especially in control groups with low event rates, as previously discussed.
Benefits of LDL-C–lowering therapy may accrue over time, causing the NNT to become much lower over longer periods. By contrast, mortality may become similar after trial's termination.74
Empirical evidence suggests that meta-analysis of difference measures (rate and risk differences) are prone to heterogeneity, although this observation may relate to statistical power favoring heterogeneity tests on difference versus relative effect measure scale.75,76
CONCLUSIONS
The present meta-analysis confirms that LDL-C–lowering therapy statistically reduces mortality. The meta-regressions and meta-analyses by subgroups indicate that LDL-C lowering may not be beneficial for all-cause and CV mortality end points in trials with more than 50% LDL-C reduction and in trials with low baseline LDL-C levels. By contrast, the reduction in MI risk was consistent across all analyses. However, annual NNTs were overall relatively high, and trials enrolling patients with high baseline LDL-C levels reported the most benefit from LDL-C–lowering therapy especially for MI. Achieving lower LDL-C goals did not further increase risk reduction consistently. Uncovering subgroups of patients who derive the most benefits from LDL-C levels reduction remains clinically relevant.
WHAT IS ALREADY KNOWN ON THIS TOPIC
A large number of meta-analyses have found indiscriminate benefits of LDL-C reduction on mortality and CV events.
WHAT THIS STUDY ADDS
Trials reducing LDL-C more than 50% did not consistently further decrease all-cause and CV mortality; the reduction in MI risk was consistent across all analyses.
Mortality benefits were found within trials with baseline LDL-C levels more than 130 mg/dL.
Achieved LDL-C levels were not related to mortality reductions.
Overall annual NNTs were relatively high, thereby uncovering subgroups of patients who benefit the most from LDL-C levels reduction is clinically relevant.
Supplementary Material
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.jcvp.org).
P. V. Ennezat had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Acquisition, analysis, or interpretation of data: P. V. Ennezat, R. -A. Guerbaai, P. François. Drafting of the manuscript: P. V. Ennezat, R. -A. Guerbaai, P. François. Critical revision of the manuscript for important intellectual content: S. Maréchaux, T. H. Le Jemtel. Statistical analysis: P. François.
Study Registration: PROSPERO registration number CRD42020144859.
The authors affirm that the manuscript is an honest, accurate, and transparent account of the study being reported, and that no important aspects of the study have been omitted; the protocol was registered in advance.
Data availability statement: Data are from published research and therefore are mostly in the public domain. Extracted data and analyses are fully available on request from the corresponding author
Contributor Information
Raphaëlle-Ashley Guerbaai, Email: raphaelleashley.guerbaai@unibas.ch.
Sylvestre Maréchaux, Email: marechaux.sylvestre@ghicl.net.
Thierry H. Le Jemtel, Email: lejemtel@tulane.edu.
Patrice François, Email: pfrancois@chu-grenoble.fr.
REFERENCES
- 1.Roth GA, Huffman MD, Moran AE, et al. Global and regional patterns in cardiovascular mortality from 1990 to 2013. Circulation. 2015;132:1667–1678. [DOI] [PubMed] [Google Scholar]
- 2.Sidney S, Quesenberry CP, Jr, Jaffe MG, et al. Recent trends in cardiovascular mortality in the United States and public health goals. JAMA Cardiol. 2016;1:594–599. [DOI] [PubMed] [Google Scholar]
- 3.Stamler J, Daviglus ML, Garside DB, et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;284:311–318. [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Vittinghoff E, Pletcher MJ, et al. Associations of blood pressure and cholesterol levels during young adulthood with later cardiovascular events. J Am Coll Cardiol. 2019;74:330–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johannesen CDL, Langsted A, Mortensen MB, Nordestgaard BG. Association between low density lipoprotein and all cause and cause specific mortality in Denmark: prospective cohort study. BMJ. 2020;371:m4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 7.Silverman MG, Ference BA, Im K, et al. Association between lowering ldl-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. [DOI] [PubMed] [Google Scholar]
- 8.Cholesterol Treatment Trialists’ CTT Collaboration; Baigent C, Blackwell L, Holland LE, et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170, 000 participants in 26 randomised trials. Lancet. 2010;376:1670–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mach F, Baigent C, Catapano AL, et al. ; ESC Scientific Document Group. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J. 2020;41:111–188. [DOI] [PubMed] [Google Scholar]
- 10.Grundy SM, Stone NJ, Bailey AL, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: executive summary: a report of the American college of Cardiology/American heart association task force on clinical practice guidelines. Circulation. 2019;139:e1046–e1081. [DOI] [PubMed] [Google Scholar]
- 11.Navarese EP, Robinson JG, Kowalewski M, et al. Association between baseline ldl-C level and total and cardiovascular mortality after LDL-C lowering: a systematic review and meta-analysis. JAMA. 2018;319:1566–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol. 2009;62:1006–1012. [DOI] [PubMed] [Google Scholar]
- 13.Sabatine MS, Giugliano RP, Keech AC, et al. ; FOURIER Steering Committee and Investigators. Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376:1713–1722. [DOI] [PubMed] [Google Scholar]
- 14.Schwartz GG, Steg PG, Szarek M, et al. ; ODYSSEY OUTCOMES Committees and Investigators. Alirocumab and cardiovascular outcomes after acute coronary syndrome. N Engl J Med. 2018;379:2097–2107. [DOI] [PubMed] [Google Scholar]
- 15.ALLHAT Officers and Coordinators for the ALLHAT Collaborative Research Group The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). JAMA. 2002;288:2998–3007. [DOI] [PubMed] [Google Scholar]
- 16.Sever PS, Dahlof B, Poulter NR, et al. ; ASCOT investigators. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial--Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet. 2003;361:1149–1158. [DOI] [PubMed] [Google Scholar]
- 17.Asselbergs FW, Diercks GFH, Hillege HL, et al. ; Prevention of Renal and Vascular Endstage Disease Intervention Trial PREVEND IT Investigators. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbuminuria. Circulation. 2004;110:2809–2816. [DOI] [PubMed] [Google Scholar]
- 18.Yusuf S, Bosch J, Dagenais G, et al. ; HOPE-3 Investigators. Cholesterol lowering in intermediate-risk persons without cardiovascular disease. N Engl J Med. 2016;374:2021–2031. [DOI] [PubMed] [Google Scholar]
- 19.Furberg CD, Adams HP, Jr, Applegate WB, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic carotid artery progression study (ACAPS) research group. Circulation. 1994;90:1679–1687. [DOI] [PubMed] [Google Scholar]
- 20.Post Coronary Artery Bypass Graft Trial Investigators. The effect of aggressive lowering of low-density lipoprotein cholesterol levels and low-dose anticoagulation on obstructive changes in saphenous-vein coronary-artery bypass grafts. N Engl J Med. 1997;336:153–162. [DOI] [PubMed] [Google Scholar]
- 21.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20, 536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22.12114036 [Google Scholar]
- 22.Results of the low-dose (20 mg) pravastatin GISSI Prevenzione trial in 4271 patients with recent myocardial infarction: do stopped trials contribute to overall knowledge? GISSI Prevenzione Investigators (Gruppo Italiano per lo Studio della Sopravvivenza nell'Infarto Miocardico). Ital Heart J. 2000;1:810–820. [PubMed] [Google Scholar]
- 23.Ouchi Y, Sasaki J, Arai H, et al. Ezetimibe lipid-lowering trial on prevention of atherosclerotic cardiovascular disease in 75 or older (EWTOPIA 75): a randomized, controlled trial. Circulation. 2019;140:992–1003. [DOI] [PubMed] [Google Scholar]
- 24.Rossebo AB, Pedersen TR, Boman K, et al. ; SEAS Investigators. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359:1343–1356. [DOI] [PubMed] [Google Scholar]
- 25.Cannon CP, Blazing MA, Giugliano RP, et al. Ezetimibe added to statin therapy after acute coronary syndromes. N Engl J Med. 2015;372:2387–2397. [DOI] [PubMed] [Google Scholar]
- 26.Hagiwara N, Kawada-Watanabe E, Koyanagi R, et al. Low-density lipoprotein cholesterol targeting with pitavastatin + ezetimibe for patients with acute coronary syndrome and dyslipidaemia: the HIJ-PROPER study, a prospective, open-label, randomized trial. Eur Heart J. 2017;38:2264–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baigent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo-controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robinson JG, Farnier M, Krempf M, et al. ; ODYSSEY LONG TERM Investigators. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. N Engl J Med. 2015;372:1489–1499. [DOI] [PubMed] [Google Scholar]
- 29.Nicholls SJ, Puri R, Anderson T, et al. Effect of evolocumab on progression of coronary disease in statin-treated patients: the GLAGOV randomized clinical trial. JAMA. 2016;316:2373–2384. [DOI] [PubMed] [Google Scholar]
- 30.Ridker PM, Revkin J, Amarenco P, et al. ; SPIRE Cardiovascular Outcome Investigators. Cardiovascular efficacy and safety of bococizumab in high-risk patients. N Engl J Med. 2017;376:1527–1539. [DOI] [PubMed] [Google Scholar]
- 31.Itoh H, Komuro I, Takeuchi M, et al. ; EMPATHY Investigators. Intensive treat-to-target statin therapy in high-risk Japanese patients with hypercholesterolemia and diabetic retinopathy: report of a randomized study. Diabetes Care. 2018;41:1275–1284. [DOI] [PubMed] [Google Scholar]
- 32.Amarenco P, Kim JS, Labreuche J, et al. ; Treat Stroke to Target Investigators. A comparison of two LDL cholesterol targets after ischemic stroke. N Engl J Med. 2020;382:9. [DOI] [PubMed] [Google Scholar]
- 33.Ito H, Ouchi Y, Ohashi Y, et al. A comparison of low versus standard dose pravastatin therapy for the prevention of cardiovascular events in the elderly: the pravastatin anti-atherosclerosis trial in the elderly (PATE). J Atheroscler Thromb. 2001;8:33–44. [DOI] [PubMed] [Google Scholar]
- 34.Beishuizen ED, van de Ree MA, Jukema JW, et al. Two-year statin therapy does not alter the progression of intima-media thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care. 2004;27:2887–2892. [DOI] [PubMed] [Google Scholar]
- 35.Arad Y, Spadaro LA, Roth M, et al. Treatment of asymptomatic adults with elevated coronary calcium scores with atorvastatin, vitamin C, and vitamin E: the St. Francis Heart Study randomized clinical trial. J Am Coll Cardiol. 2005;46:166–172. [DOI] [PubMed] [Google Scholar]
- 36.Knopp RH, d'Emden M, Smilde JG, Pocock SJ. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care. 2006;29:1478–1485. [DOI] [PubMed] [Google Scholar]
- 37.Criner GJ, Connett JE, Aaron SD, et al. ; COPD Clinical Research Network, Canadian Institutes of Health Research. Simvastatin for the prevention of exacerbations in moderate-to-severe COPD. N Engl J Med. 2014;370:2201–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kitas GD, Nightingale P, Armitage J, et al. ; TRACE RA Consortium. A multicenter, randomized, placebo-controlled trial of atorvastatin for the primary prevention of cardiovascular events in patients with rheumatoid arthritis. Arthritis Rheumatol. 2019;71:1437–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liem AH, van Boven AJ, Veeger NJGM, et al. ; FLuvastatin On Risk Diminishment after Acute myocardial infarction study group. Effect of fluvastatin on ischaemia following acute myocardial infarction: a randomized trial. Eur Heart J. 2002;23:1931–1937. [DOI] [PubMed] [Google Scholar]
- 40.de Lemos JA, Blazing MA, Wiviott SD, et al. ; Investigators. Early intensive vs a delayed conservative simvastatin strategy in patients with acute coronary syndromes: phase Z of the A to Z trial. JAMA. 2004;292:1307–1316. [DOI] [PubMed] [Google Scholar]
- 41.Pedersen TR, Faergeman O, Kastelein JJP, et al. ; Incremental Decrease in End Points Through Aggressive Lipid Lowering IDEAL Study Group. High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial. JAMA. 2005;294:2437–2445. [DOI] [PubMed] [Google Scholar]
- 42.Deedwania P, Stone PH, Bairey Merz CN, et al. Effects of intensive versus moderate lipid-lowering therapy on myocardial ischemia in older patients with coronary heart disease: results of the Study Assessing Goals in the Elderly (SAGE). Circulation. 2007;115:700–707. [DOI] [PubMed] [Google Scholar]
- 43.Nicholls SJ, Ballantyne CM, Barter PJ, et al. Effect of two intensive statin regimens on progression of coronary disease. N Engl J Med. 2011;365:2078–2087. [DOI] [PubMed] [Google Scholar]
- 44.Kjekshus J, Apetrei E, Barrios V, et al. ; CORONA Group. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–2261. [DOI] [PubMed] [Google Scholar]
- 45.Tavazzi L, Maggioni AP, Marchioli R, et al. ; Gissi-HF Investigators. Effect of rosuvastatin in patients with chronic heart failure (the GISSI-HF trial): a randomised, double-blind, placebo-controlled trial. Lancet. 2008;372:1231–1239. [DOI] [PubMed] [Google Scholar]
- 46.Holdaas H, Fellstrom B, Jardine AG, et al. ; Assessment of LEscol in Renal Transplantation ALERT Study Investigators. Effect of fluvastatin on cardiac outcomes in renal transplant recipients: a multicentre, randomised, placebo-controlled trial. Lancet. 2003;361:2024–2031. [DOI] [PubMed] [Google Scholar]
- 47.Wanner C, Krane V, Marz W, et al. ; German Diabetes and Dialysis Study Investigators. Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 48.Fellstrom BC, Jardine AG, Schmieder RE, et al. ; AURORA Study Group. Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 49.Hosomi N, Nagai Y, Kohriyama T, et al. ; J-STARS Collaborators. The Japan statin treatment against recurrent stroke (J-STARS): a multicenter, randomized, open-label, parallel-group study. EBioMedicine. 2015;2:1071–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nakamura H, Arakawa K, Itakura H, et al. ; MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet. 2006;368:1155–1163. [DOI] [PubMed] [Google Scholar]
- 51.Taguchi I, Iimuro S, Iwata H, et al. High-Dose versus low-dose pitavastatin in Japanese patients with stable coronary artery disease (REAL-CAD): a randomized superiority trial. Circulation. 2018;137:1997–2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Im E, Cho YH, Suh Y, et al. High-intensity statin treatments in clinically stable patients on aspirin monotherapy 12 Months after drug-eluting stent implantation: a randomized study. Rev Esp Cardiol (Engl Ed). 2018;71:423–431. [DOI] [PubMed] [Google Scholar]
- 53.Athyros VG, Papageorgiou AA, Mercouris BR, et al. Treatment with atorvastatin to the National Cholesterol Educational Program goal versus 'usual' care in secondary coronary heart disease prevention. The GREek Atorvastatin and Coronary-heart-disease Evaluation (GREACE) study. Curr Med Res Opin. 2002;18:220–228. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM, Danielson E, Fonseca FAH, et al. ; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. [DOI] [PubMed] [Google Scholar]
- 55.Amarenco P, Bogousslavsky J, Callahan A, III, et al. ; Stroke Prevention by Aggressive Reduction in Cholesterol Levels SPARCL Investigators. High-dose atorvastatin after stroke or transient ischemic attack. N Engl J Med. 2006;355:549–559. [DOI] [PubMed] [Google Scholar]
- 56.Study of the Effectiveness of Additional Reductions in C, Homocysteine Collaborative G, Bowman L, Wallendszus K, Bulbulia R, et al. Armitage JIntensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12, 064 survivors of myocardial infarction: a double-blind randomised trial. Lancet. 2010;376:1658–1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shepherd J, Cobbe SM, Ford I, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. [DOI] [PubMed] [Google Scholar]
- 58.Long-Term Intervention with Pravastatin in Ischaemic Disease Study G. Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels. N Engl J Med. 1998;339:1349–1357. [DOI] [PubMed] [Google Scholar]
- 59.Abdullah SM, Defina LF, Leonard D, et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation. 2018;138:2315–2325. [DOI] [PubMed] [Google Scholar]
- 60.Thygesen K, Alpert JS, White HD, Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction, Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole-Wilson PA, Gurfinkel EP, Lopez-Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez-Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio-Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck-Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez-Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al-Attar N. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 61.Gu X, Li Y, Chen S, et al. Association of lipids with ischemic and hemorrhagic stroke: a prospective cohort study among 267 500 Chinese. Stroke. 2019;50:3376–3384. [DOI] [PubMed] [Google Scholar]
- 62.Shahar E, Chambless LE, Rosamond WD, et al. ; Atherosclerosis Risk in Communities Study. Plasma lipid profile and incident ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2003;34:623–631. [DOI] [PubMed] [Google Scholar]
- 63.Ridker PM. LDL cholesterol: controversies and future therapeutic directions. Lancet. 2014;384:607–617. [DOI] [PubMed] [Google Scholar]
- 64.Wang N, Fulcher J, Abeysuriya N, et al. Intensive LDL cholesterol-lowering treatment beyond current recommendations for the prevention of major vascular events: a systematic review and meta-analysis of randomised trials including 327 037 participants. Lancet Diabetes Endocrinol. 2020;8:36–49. [DOI] [PubMed] [Google Scholar]
- 65.Yourman LC, Cenzer IS, Boscardin WJ, et al. Evaluation of time to benefit of statins for the primary prevention of cardiovascular events in adults aged 50 to 75 Years: a meta-analysis. JAMA Intern Med. 2021;181:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ennezat PV, Le Jemtel T, Cosgrove S, et al. Outcome postponement as a potential patient centred measure of therapeutic benefit: examples in cardiovascular medicine. Acta Cardiol. 2020;75:10–19. [DOI] [PubMed] [Google Scholar]
- 67.Renteria E, Jha P, Forman D, Soerjomataram I. The impact of cigarette smoking on life expectancy between 1980 and 2010: a global perspective. Tob Control. 2016;25:551–557. [DOI] [PubMed] [Google Scholar]
- 68.Moore SC, Patel AV, Matthews CE, et al. Leisure time physical activity of moderate to vigorous intensity and mortality: a large pooled cohort analysis. PLoS Med. 2012;9:e1001335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Rimm EB, Stampfer MJ. Diet, lifestyle, and longevity--the next steps? JAMA. 2004;292:1490–1492. [DOI] [PubMed] [Google Scholar]
- 70.Colhoun HM, Betteridge DJ, Durrington PN, et al. ; CARDS investigators. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet. 2004;364:685–696. [DOI] [PubMed] [Google Scholar]
- 71.Bassler D, Briel M, Montori VM, Lane M, Glasziou P, Zhou Q, Heels-Ansdell D, Walter SD, Guyatt GH, STOPIT-2 Study Group, Flynn DN, Elamin MB, Murad MH, Abu Elnour NO, Lampropulos JF, Sood A, Mullan RJ, Erwin PJ, Bankhead CR, Perera R, Ruiz Culebro C, You JJ, Mulla SM, Kaur J, Nerenberg KA, Schunemann H, Cook DJ, Lutz K, Ribic CM, Vale N, Malaga G, Akl EA, Ferreira-Gonzalez I, Alonso-Coello P, Urrutia G, Kunz R, Bucher HC, Nordmann AJ, Raatz H, da Silva SA, Tuche F, Strahm B, Djulbegovic B, Adhikari NKJ, Mills EJ, Gwadry-Sridhar F, Kirpalani H, Soares HP, Karanicolas PJ, Burns KEA, Vandvik PO, Coto-Yglesias F, Chrispim PPM, Ramsay T. Stopping randomized trials early for benefit and estimation of treatment effects: systematic review and meta-regression analysis. JAMA. 2010;303:1180–1187. [DOI] [PubMed] [Google Scholar]
- 72.Baigent C, Keech A, Kearney PM, et al. ; Cholesterol Treatment Trialists' CTT Collaborators. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90, 056 participants in 14 randomised trials of statins. Lancet. 2005;366:1267–1278. [DOI] [PubMed] [Google Scholar]
- 73.Ikezaki H, Lim E, Cupples LA, et al. Small dense low-density lipoprotein cholesterol is the most atherogenic lipoprotein parameter in the prospective framingham offspring study. J Am Heart Assoc. 2021;10:e019140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Heart Protection Study Collaborative G. Effects on 11-year mortality and morbidity of lowering LDL cholesterol with simvastatin for about 5 years in 20, 536 high-risk individuals: a randomised controlled trial. Lancet. 2011;378:2013–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spiegelman D, VanderWeele TJ. Evaluating public health interventions: 6. Modeling ratios or differences? Let the data tell us. Am J Public Health. 2017;107:1087–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Poole C, Shrier I, VanderWeele TJ. Is the risk difference really a more heterogeneous measure? Epidemiology. 2015;26:714–718. [DOI] [PubMed] [Google Scholar]