Abstract
Despite community vaccination against coronavirus disease 2019 (COVID-19) and reduced mortality, there are still challenges in treatment options for the disease. Due to the continuous mutation of SARS-CoV-2 virus and the emergence of new strains, diversity in the use of existing antiviral drugs to combat the epidemic has become a crucial therapeutic chance. As a broad-spectrum antiparasitic and antiviral drug, ivermectin has traditionally been used to treat many types of disease, including DNA and RNA viral infections. Even so, based on currently available data, it is still controversial that ivermectin can be used as one of the effective antiviral agents to treat severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) or not. The aim of this study was to provide comprehensive information on ivermectin, including its safety and efficacy, as well as its adverse effects in the treatment of COVID-19.
Keywords: COVID-19, Ivermectin, SARS-CoV-2, Favipiravir, Ribavirin, Famotidine
Introduction
Coronavirus Infectious Disease 2019 (COVID-19) is a fatal respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) (Esakandari et al. 2020; Khezri et al. 2022; Khomari et al. 2021; Zhou et al. 2020). COVID-19 began in December 2019 when an unknown cause of pneumonia broke out in Wuhan City, Hubei Province, China. All early reports of the source of the disease came from the Huanan Seafood Wholesale Market, which sells aquatic products, live birds, and wild animals. Fever, headache, cough, and shortness of breath are common symptoms of COVID-19 that can accompany multiple organ failure (Hui et al. 2020). In addition, recent studies have suggested that COVID-19 may lead to the progression of multiple cancers and other diseases (Zalpoor et al. 2022a, 2022b, 2022c, 2022d, 2022e, 2022f, g).
Proteomic and genetic analysis of SARS-CoV-2 showed 94.6% and 79.5% similarity in nonstructural protein amino acid sequence and nucleic acid structure, respectively, between SARS-CoV-2 and other SARS species. SARS-CoV-2 is a positive-stranded RNA virus (+ ssRNA virus) with sequences that translate directly into viral proteins. The five gene segments of the RNA genome are cloning enzymes and four structural proteins, including spike protein (S), membrane protein (M), envelope protein (E), and nucleocapsid protein (N). The replicase gene (ORF1ab gene) encodes 16 nonstructural proteins (NSP 116) that are over 21 kb in size and are translated into pp1ab polyproteins. Replicase (pp1a), an RNA-dependent RNA polymerase (RdRp), is used to synthesize a negative-sense antigenome during genome replication, which serves as a template for generating a novel positive-sense viral genome. The NSP1 protein binds to the 40S subunit of the ribosome in the cell and inhibits translation in the host cell. By inhibiting gene expression in host cells, the NSP1 protein promotes viral gene expression in infected cells and evades the host's immune response. The NSP2 protein is involved in the regulation of cell survival transduction pathways through interactions between host PHB and PHB2 molecules. These two proteins play important roles in stabilizing mitochondrial function and protecting cells from stress. PLPRO, located next to NSP3, has either deubiquitinating or deISGilating activity, which is involved in suppressing the immune response. The NSP2 protein is involved in the regulation of cell survival transduction pathways through interactions between host PHB and PHB2 molecules. These two proteins play important roles in stabilizing mitochondrial function and protecting cells from stress. PLPRO, located next to NSP3, has either deubiquitinating or deISGilating activity, which is involved in suppressing the immune response. This protein, along with the NSP4 protein, is also involved in the formation of membrane vesicles required for viral replication. NSP3 protein suppresses induction of type 1 interferon and innate immunity by blocking phosphorylation, dimerization, and nuclear–cytoplasmic transition. This protein is involved in inhibiting nuclear factor kappa B (NF-κB) message transmission. The spike glycoprotein has two subunits: the S1 protein for binding to the receptor and the S2 protein for the fusion of the viral envelope with the host cell membrane. Viral infection begins with the binding of the S1 protein to the hACE2 receptor (angiotensin converting enzyme 2) and a conformational change of the S glycoprotein, followed by proteolysis of the S glycoprotein by CatB/L (cathepsins B and L) and TMPRRS2 with the S2 fusion peptide and activate the virus. Membrane is integrated into the endosome (Mielech et al. 2014; Payandeh et al. 2021; Wang et al. 2020; Wu et al. 2020).
Ivermectin is a broad-spectrum anthelmintic, antibacterial, and antiviral agent approved by the FD (Campbell and Benz 1984; Crump and Omura 2011). This drug has been shown to be effective against several DNA and RNA viruses, including SARS-CoV-2, in vitro. Ivermectin acts on SARS-CoV-2 by preventing the pathogenic protein/viral genome from entering the nucleus of the host cell (Caly et al. 2020; Lv et al. 2018; Raza et al. 2020; X. Wang et al. 2019).
The current review summarizes the past and present use, mechanisms, and progress of preclinical studies and clinical trials of ivermectin for the treatment of COVID-19. Although there are insufficient data available to explain the in vivo activity of ivermectin on SARS-CoV-2. Results demonstrating the antiviral activity of ivermectin in vitro against SARS-CoV-2 and in vivo effects against other similar viruses provide investigators with sufficient confidence that laboratory and clinical studies of the use of ivermectin for the treatment of COVID-19 are still ongoing.
Ivermectin prevents SARS-CoV-2 entry into the host cells
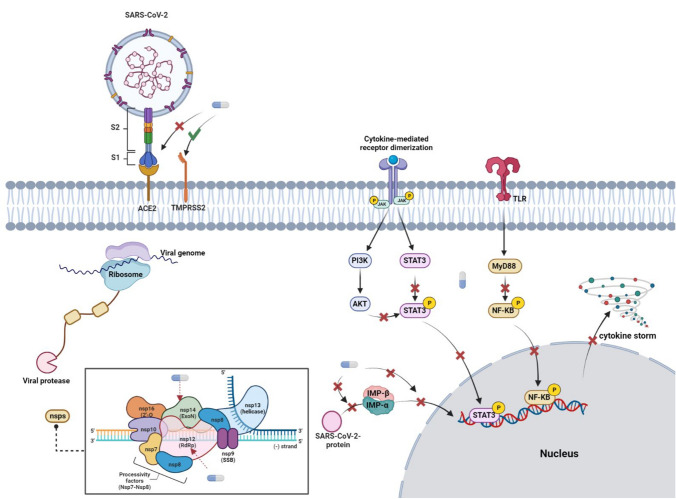
Before SARS-CoV-2 attaches to the host cell, two molecules of ivermectin interact with each other in a “tail” mode and form an ionospheric activated chloride channel, triggering apoptosis and osmotic cell death (Dominguez‑Gomez et al. 2018; Dueñas-González and Juárez-Rodríguez 2021; Rizzo 2020). Additionally, in silico studies of the effects of ivermectin on SARS-CoV-2 have shown that several strategies prevent the virus from entering the host cell. First, ivermectin binds to the leucine 91 region of the S glycoprotein and the histidine 378 region of the host cell ACE2 receptor (the specific receptor of SARS-CoV-2) (Nabi-Afjadi et al. 2022). Moreover, ivermectin showed the highest affinity for S glycoprotein, RdRp, NSP14, and TMPRSS2 active sites with higher H-bond formation compared to ivermectin, chloroquine, favipiravir, remdesivir, and hydroxychloroquine (Choudhury et al. 2021; Eweas et al. 2021; Lehrer and Rheinstein 2020; Zaidi and Dehgani-Mobaraki 2021). By interfering with its ability to bind its relevant ligands, Choudhury et al. demonstrated that ivermectin is also effective against human proteases, replicases, and TMPRSS2 receptors (Choudhury et al. 2021).
Importin alpha (IMP α) is another molecular target of ivermectin for SARS-CoV-2. Ivermectin has been shown to specifically prevent IMP α/β-mediated nuclear transport in HIV-1 replication and Dengue infection. Therefore, by the same mechanism in the mentioned virus, ivermectin was expected to inhibit SARS-CoV-2 (King et al. 2020; Wagstaff et al. 2012). A study by Young et al. confirmed the effect of ivermectin on IMPα in host cells. They showed that ivermectin inhibited the correlation between IMPα and IMPβ, but was also able to dissociate IMPα/β heterodimers. The following study using CD spectroscopy revealed that the armadillo (ARM)-rich domain of IMPα was the specific binding site of ivermectin. In addition, as the concentration of ivermectin increased, the breakage of the alpha helix in ARM significantly increased, but there was no change in the structure of IMPβ. In addition, the effect of ivermectin on IMPα was demonstrated to prevent binding to NLS, including the dengue virus NSP5. Therefore, it can be said that the effect of ivermectin on SARS-CoV-2 is similar to the prevention of N interaction with IMPα (S. N. Yang et al. 2020a, b) (Fig. 1).
Fig. 1.
Ivermectin effects on SARS-CoV-2 entry to host cells, reproduction, and anti-inflammatory response with targeting JAK/STAT, PI3K/AKT, and NF-κB signaling pathways and transcriptional activity of STAT and NF-κB
Ivermectin inhibits SARS-CoV-2 reproduction
Other viruses that were able to enter the host cell may be affected by other ivermectin schemes. As mentioned above, ivermectin interacts with RdRP (binding energy − 9.7 kcal/mol) located on NSP12 to serve as an important enzyme in the replication and transcription of SARS-CoV-2. Ivermectin also interacts with NSP14 to act as a capping of viral RNA through corrective exoribonuclease and methyltransferase activity. Thus, ivermectin inhibits SARS-CoV-2 replication by interfering with an important protein/replication factor of the virus. In host cells, SARS-CoV-2 RNA is translated into a polyprotein in which specific enzymes are autoproteolytically cleaved, facilitating the separation of the enzyme responsible for viral replication from the polyprotein. Chymotrypsin-like protease (3'cl pro/Mpro) is one of the enzymes that prevents the binding of ivermectin. It also efficiently binds to two proteins, Mpro and, to a lesser extent, SARS-CoV-2 PLpro. Thus, it serves to prevent post-translational processing of viral polyproteins. In addition, ivermectin binds to Mpro and PLpro of the SARS-CoV-2 polyprotein as an important protease in post-translational processing (Eweas et al. 2021; Ma et al. 2015; Mody et al. 2021; Swargiary 2020; V’kovski et al. 2021). It has been suggested that autophagy may promote the invasion and proliferation of SARS-CoV-2 into host cells, which may be exacerbated by ivermectin (Yang and Shen 2020). In addition, studies have shown that ivermectin may induce autophagy via the AKT/mTOR signaling pathway (Dou et al. 2016; Liu et al. 2019). Therefore, these findings suggest that ivermectin may play a double-edged role, and further studies are needed to confirm the positive or negative effects of ivermectin on SARS-CoV-2 invasion and replication in host cells.
Ivermectin role in immune system response and anti-inflammatory effects
Ivermectin acts as an anti-inflammatory agent by inhibiting NF-κB, AKT/mTOR, STAT3, and interferon-dependent pathways, reducing the production of proteins associated with inflammation, significantly reducing the severity and mortality of COVID-19. For example, TNF-α, IL-1ss, IL-4, IL-5, IL-6, and IL-13. Studies have shown that ivermectin regulates the cell-mediated and humoral immune responses of a variety of bacterial, viral, parasitic, and neoplastic diseases (Sajid et al. 2006; Stankiewicz et al. 1995). Ivermectin has been shown in vivo and in vitro to have anti-inflammatory properties by inhibiting the increase in NF-κB activity and inhibiting the production of TNF-α, IL-1 and IL-6 (Zhang et al. 2008). NF-κB is activated by SARS-CoV-2 through pattern recognition receptors (Nabi-Afjadi et al. 2021). When SARS-CoV-2 binds to ACE2, ACE2 decreases on the cell surface, resulting in increased AngII expression. In addition to NF-κB activation by SARS-CoV-2 infection, the type 1 angiotensin receptor axis may play a role in inducing the production of TNF-α and soluble IL-6Ra (sIL-6Ra) via disintegrin and metalloprotease 17 (ADAM17) (Eguchi et al. 2018). In non-immune cells, IL-6 binds to sIL-6R and activates signal transducer and transcriptional activator 3 (STAT3) (Joshi et al. 2021a, b). STAT3 and NF-κB can activate IL-6 enhancers to produce other proinflammatory cytokines and chemokines, including monocyte chemoattractant protein (MCP1), IL-8, and vascular endothelial growth factor (VEGF) (Murakami et al. 2019). When PAK1 binds to both JAK1 and STAT3, the PAK1/STAT3 complex is formed, which activates transcription of the IL-6 gene required for cytokine storm during COVID-19 infection (Kim et al. 2019). Ivermectin has been shown to attenuate the cytokine storm in COVID-19 by inhibiting Akt/mTOR signaling and promoting ubiquitin-mediated PAK1 degradation, thereby interfering with STAT3 activity and IL-6 production (Dou et al. 2016).
When SARS-CoV2 enters the host cell, it intercepts the function of the host cell and inhibits the IFN-mediated antiviral response of the host cell. SARS-CoV-2 proteins, including ORF3a, ORF6, and NSP1, block IFNI signaling (Konno et al. 2020; Yang et al. 2020a, b). Thus, cells surrounding infected cells cannot receive the IFN protective signal, allowing SARS-CoV-2 to freely spread and proliferate. Many IFN-related genes have been shown to be induced by ivermectin, including IFIT1, IFIT2, IF144, ISG20, OASL, and IRF9 (Seth et al. 2016). Collectively, based on the available evidence, in addition to its antiviral effect, ivermectin has the potential to improve protective IFN signaling and has anti-inflammatory properties, which could be effective in reducing the side effects of the cytokine storm and inflammatory response associated with coronavirus. Nevertheless, ivermectin can induce activation of P2X7 receptor and downstream signaling transduction, resulting in inflammatory and pathological effects. Ivermectin has been shown to increase ATP sensitivity and delay the inactivation of current after ATP dissociation at the PX24 receptor. This occurs at the same time as the enhancement of ATP-induced current and Ca2 + signaling at the P2X7 receptor (Juarez et al. 2018). In addition, Zalpoor et al. (Zalpoor et al. 2022c) reported that overactivation of P2X7 receptors may be involved in the pathological and inflammatory responses in SARS-CoV-2 infection. Therefore, more studies are needed to approve the inflammatory or anti-inflammatory effects of ivermectin in patients with COVID-19.
Ivermectin effects in energy metabolism pathways associated with COVID-19
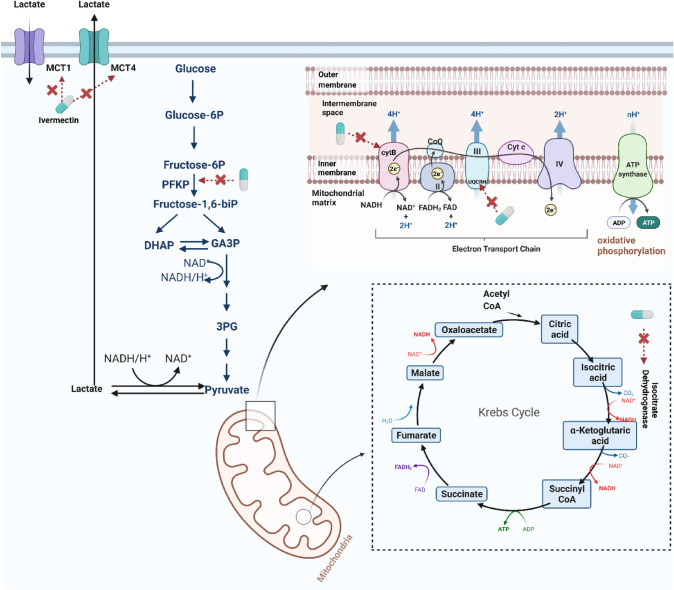
Recent analysis has shown the activation of glycolysis, the TCA cycle, and oxidative phosphorylation chain during COVID-19 (Gardinassi et al. 2020). Codo et al. demonstrated that the expression of genes involved in glycolysis is upregulated in cultures of SARS-CoV-2-infected bronchoalveolar lavage monocytes from COVID-19 patients (Codo et al. 2020). In addition, another similar study showed that SARS-CoV-2 infection stimulated glycolysis specifically in monocytes. For some COVID-19 infections, changes in their pathway may contribute to the pathology and severity of the disease. The Krebs cycle may be considered as the immune-metabolic center of macrophages. The intermediate products of the Krebs cycle, succinate and citric acid, accumulate in proinflammatory macrophages. The non-metabolic signaling roles of these metabolites play an important role in the expression of inflammatory genes (Ryan and O’Neill 2020). Therefore, targeting these metabolic processes could help develop potential treatments to alleviate the inflammatory response and side effects during COVID-19. Ivermectin has recently been shown to play an important role throughout molecular networks by acting on key enzymes in energy metabolism pathways, particularly glycolysis phosphofructokinase, platelet (PFKP), isocitrate dehydrogenase [NADP (+)] 2 (IDH2) and isocitrate dehydrogenase, [NAD(+)] 3-non-catalytic subunit beta (IDH3B), cytochrome b (CYTB), NADH dehydrogenase 2 (ND2), (ND5) and ubiquinol cytochrome c reductase hinge protein (UQCRH) oxidation of the Krebs cycle Phosphorylation, and monocarboxylate transporter 1 (MCT1) and MCT4 in the lactate shuttle(Zhan and Li 2021). Therefore, we hypothesized that ivermectin might have a therapeutic effect on COVID-19 by altering energy metabolic pathways, reducing the inflammatory response, and reducing some of the side effects of COVID-19 (Fig. 2).
Fig. 2.
Ivermectin effects on energy metabolism pathways associated with COVID-19; Krebs cycle, glycolysis, electron transport chain (ETC), and oxidative phosphorylation (OXPHOS)
Ivermectin also plays a role in causing oxidative stress and apoptosis, according to several studies (Atakisi et al. 2009; El-Far 2013). Ivermectin, for example, suppresses cell proliferation in colorectal cancer cells by promoting ROS-mediated mitochondrial apoptosis and causing S-phase arrest, as reported in Shican Zhou et al. (Zhou et al. 2021). Further, antibiotics and anthelmintic drugs which prevent DNA or RNA replication in bacteria act on human cancer cells by targeting mitochondria. In glioblastoma cells and brain endothelial cells, ivermectin increases mitochondrial superoxide levels due to mitochondrial dysfunction. In glioblastoma and endothelial cells, the presence of antioxidants reversed the inhibitory effect of ivermectin, showing that the presence of antioxidants is required to reverse its action (Liu et al. 2016). During the early stages of pregnancy, ivermectin may also interfere with normal placentation processes due to constant exposure and accumulated levels. The authors demonstrated that ivermectin adversely affects pTr and pLE cells, including cell cycle arrest, apoptosis, mitochondrial permeabilization, calcium accumulation, endothelial dysfunction, and disruptions in cellular homeostasis caused by mitochondrial dysfunction. In this way, ivermectin should be investigated more for pregnant women who have been infected with SARS-CoV-2 (Lee et al. 2019).
Clinical trials of ivermectin
Due to insufficient human clinical evidence for ivermectin, more clinical trials are required to determine its efficacy. While some researchers have shown ivermectin to be helpful in treating COVID-19, others have reported it to be ineffective. For example, Rajter et al. studied 280 patients with COVID-19 at four Broward Health Florida hospitals from March 15 to May 11, 2020. In this study, patients were divided into two groups. 173 patients received ivermectin and 107 received controls. Hydroxychloroquine, azithromycin, or both drugs were administered together to control and intervention patients. The results showed that ivermectin could significantly reduce mortality in both general and severe lung disease patients compared to controls. There was no significant difference in length of hospitalization between the two groups (Rajter et al. 2021). However, this outcome for hospitalization differs from a randomized double-blind trial by Shahbaznejad et al. A single dose of ivermectin was studied in Mazandaran, Iran, in 69 patients with COVID-19. This study showed that mean dyspnea, persistent cough, hospitalization, and incidence of lymphopenia were significantly reduced in patients treated with ivermectin (Shahbaznejad et al. 2021b). To investigate the rate and safety of viral load reduction of ivermectin (Ahmed et al.). A randomized, double-blind, placebo-controlled trial was conducted in 72 hospitalized patients in Dhaka, Bangladesh. In this study, patients were divided into three groups. The first group received 12 mg ivermectin once daily for 5 days, the second group received a single dose of ivermectin (12 mg) plus 200 mg doxycycline on the first day, and the next day 100 mg every 12 h only doxycycline was administered. As a result, the safety and effectiveness of ivermectin were confirmed as well as the effect of ivermectin on the virus clearance rate and improvement of clinical symptoms in the treatment of mild COVID-19 in adult patients (Ahmed et al. 2021). Okumus et al. aimed to find genetic mutations that affect ivermectin metabolism and its toxic effects in patients with severe COVID-19 pneumonia, as well as to evaluate the efficacy and safety of ivermectin in unmutated patients. Their study was a prospective, randomized, and single-blind trial, included both study and control groups. They reported that ivermectin could improve clinical recovery, improve prognostic test indicators, and reduce mortality in patients with severe COVID-19. They suggested that ivermectin should be explored as an alternative drug to treat COVID-19 disease or as a complement to an existing protocol (Okumuş et al. 2021).
Despite these results, Mohan et al. refute the beneficial effect of ivermectin in reducing viral load, through a search of randomized, double-blind controlled trials in 125 hospitalized patients with mild-to-moderate COVID-19. In this study, patients were randomly divided into two groups: recipients of ivermectin (24 or 12 mg daily) and placebo. After 5 days, the reverse transcriptase-polymerase chain reaction (RT-PCR) test was negative for 47.5% in the ivermectin 24 mg group, 35.0% in the ivermectin 12 mg group, and 31.1% in the placebo group. The difference between groups was not statistically significant, p value = 0.30. Compared with placebo, oral doses of ivermectin increased negative RT-PCR tests or decreased viral loads in patients receiving COVID-19, but these differences between groups were not statistically significant (18). Another double-blind, randomized, placebo-controlled trial in Indian patients with mild-to-moderate disease COVID-19 was conducted by Ravikirti et al. Patients in the intervention group received 12 mg of ivermectin on days 1 and 2, while patients in the control group received a placebo. On day 6, approximately one-quarter (23.6%) of patients in the intervention group and one-third (31.6%) of patients in the placebo group tested negative for SARS-CoV-2 by RT-PCR. Although the differences were not statistically significant. Patients in the ivermectin group were all successfully discharged, while the placebo group had a 93% success rate. Mohan. et al. reported that the inclusion of ivermectin in the treatment regimens of patients with COVID-19 could not be definitively confirmed based on the results of this trial; because, aside from the slight advantage of successful patient discharge, no other advantages were observed (Mohan et al. 2021). Chee Loon Lim et al. designed another open-label, randomized clinical trial of 490 COVID-19 patients in various public hospitals in Malaysia public hospitals to investigate the effectiveness of ivermectin in preventing the progression of severe disease in high-risk COVID-19 patients. The study patients received oral ivermectin (0.4 mg/kg body weight daily) for 5 days in addition to standard of care. The results showed that there was no significant difference between the groups. They reported that 21.6% of the ivermectin group progressed to serious disease and 17.3% of the control group (standard of care only) progressed to serious disease. Therefore, treatment with ivermectin in the early stages of COVID-19 could not prevent the disease from progressing to more serious stages (Lim et al. 2022a). Vallejos et al. reported the same results of their study, conducted on 501 people who were not hospitalized with COVID-19 in Corrientes, Argentina, between August 19, 2020 and February 22, 2021 (Vallejos et al. 2021).
A randomized, double-blind, single-point trial was conducted by LópezMedina et al. in Cali, Colombia to see the efficacy of ivermectin therapy in patients with moderate COVID-19. The participants were randomly selected from the electronic database of symptomatic, laboratory-identified patients with COVID-19 from the state health agency (July 15th to November 30th, 2020). Study participants received 300 g/kg.B.W of oral ivermectin in solution or an equivalent amount of placebo for 5 days. In the ivermectin group, the median symptom relief period was 10 days compared to 12 days in the placebo group. By day 21, 82% of patients taking ivermectin and 79% of patients taking placebo had no symptoms. The most commonly reported side effect is headache, which was reported by 52% of patients taking ivermectin and 56% of patients taking placebo. They concluded that using ivermectin for 5 days did not significantly improve symptom remission in people with moderate COVID-19. However, further trials may be needed to understand the effects of ivermectin on other clinically relevant outcomes (López-Medina et al. 2021). Samaha et al. in a randomized controlled study of 100 asymptomatic Lebanese COVID-19 patients investigated the beneficial effect of ivermectin in reducing the SARS-CoV-2 viral load. The difference between the participants’ Ct values was not significant (p = 0.06) prior to ivermectin administration, suggesting that the participants’ viral loads were the same. They were divided into two groups and one group took ivermectin. 72 h after the start of the diet, Ct values were significantly elevated in the ivermectin supplement group compared with the control group. In addition, the control group had more patients who developed clinical symptoms. They concluded that ivermectin appeared to provide therapeutic benefit, leading to fewer symptoms, lower viral loads, and fewer hospitalizations (Samaha et al. 2021). The mentioned clinical trials are summarized in Table 1.
Table 1.
Clinical trials investigated the anti-COVID-19 effects of ivermectin
| Number of patients | Country of study | Ivermectin dose | Description/outcome | References | |
|---|---|---|---|---|---|
| Positive responded studies | 280 | United States | 200 mg/kg/day |
1) Reduced mortality in both general and severe lung disease patients compared to the control 2) No significant difference in length of hospitalization relative to the control |
Rajter et al. (2021) |
| 69 | Iran | 0.2 mg/kg/day | Significantly reduced mean dyspnea, persistent cough, hospitalization, and incidence of lymphopenia | Shahbaznejad et al. (2021a) | |
| 72 | Bangladesh | 12 mg | The safety and effectiveness of Ivermectin were confirmed as well as the effect of Ivermectin on the virus clearance rate and improvement of clinical symptoms | Ahmed et al. (2021) | |
| 66 | Turkey | 200 µg/kg/day | Improved clinical recovery, improve prognostic test indicators, and reduce mortality | Okumuş et al. (2021) | |
| Negative responded studies | 125 | India | 12 and 24 mg/day | Increased negative RT-PCR tests or decreased viral loads in patients receiving Covid19, but no statistically significant difference compared with the placebo group | Mohan et al. (2021) |
| 490 | Malaysia | 0.4 mg/kg/day | No significant difference compared to the control, so treatment with Ivermectin in the early stages of COVID-19 could not prevent the disease from progressing to more serious stages | Lim et al. (2022b) | |
| 501 | Argentina | 12 and 18 mg/day | No significant difference compared to the control | Vallejos et al. (2021) | |
| 476 | Colombia | 300 g/kg/day | No significant improved symptom remission of COVID-19 | López-Medina et al. (2021) | |
| 100 | Lebanon | 9 mg, 12 mg, and 150 µg/kg |
1) Significantly elevated Ct values compared with the control group 2) Fewer symptoms, lower viral loads, and fewer hospitalizations compared with the control group |
Samaha et al. (2021) |
Ivermectin potential prophylactic effects against COVID-19
In 18 randomized controlled trials of ivermectin for COVID-19, the drug was reported to play a statistically significant role in reducing viral clearance time, clinical recovery time, and mortality. Additionally, several controlled prophylaxis trials have shown that ivermectin can significantly reduce the risk of contracting COVID-19 (Kory et al. 2021). A recent study by Behera et al. found that oral administration of ivermectin in two doses (300 mcg/kg every 72 h) to healthcare workers as a chemopreventive agent reduced the risk of contracting COVID-19 by 83% over the next month (Behera et al. 2021). Therefore, it may be hypothesized that ivermectin can be used not only for the treatment of COVID-19, but also as a chemopreventive agent for high-risk occupational groups such as healthcare workers and high-risk groups for COVID-19 severity, such as patients with primary diseases such as immunity. Reduce your risk of contracting COVID-19 by eliminating deficiencies and malignancies. In a study by Okumush and colleagues, ivermectin could be an alternative or complementary option to current treatment protocols available to treat COVID-19. This could improve predictive laboratory parameters, accelerate clinical recovery, and reduce mortality even in severe COVID-19 patients.
Administration of ivermectin to patients without the MDR1/ABCB1 and/or CYP3A4 mutations is safe and not expected to cause serious side effects and has the potential to alleviate side effects with appropriate treatment (Okumuş et al. 2021). As one of the most widely distributed drugs in the body, ivermectin is rapidly absorbed by mouth, metabolized in the liver (cytochrome P450 system), and in healthy individuals binds strongly to plasma proteins such as albumin. In contrast, the severity of lung injury is associated with hypoalbuminemia in COVID-19 patients. Therefore, it was hypothesized that this observation could increase the availability of the free fraction of free plasma ivermectin. As one of the most widely distributed drugs in the body, ivermectin is rapidly absorbed by mouth, metabolized in the liver (cytochrome P450 system), and in healthy individuals binds strongly to plasma proteins such as albumin. In contrast, the severity of lung injury is associated with hypoalbuminemia in COVID-19 patients. Therefore, it was hypothesized that this observation could increase the availability of the free fraction of free plasma ivermectin (Canga et al. 2008; Klotz et al. 1990; Wu et al. 2021). Co-administration of ivermectin with antibiotics, antivirals, and corticosteroids may enhance effectiveness, avoid the need for high doses, and reduce the risk of toxicity and side effects in a dose-dependent manner. Therefore, ivermectin could be a promising safe treatment for COVID-19; ivermectin mechanisms against SARS-CoV-2 virus as a multifunction medication.
Ivermectin is a drug with a wide range of biological activities. Initially used for multi-purpose veterinary applications, it has been used successfully to treat parasitic infections in humans for over 30 years (Omura 2008). Originally proposed in veterinary medicine and medicine for the treatment of onchocerciasis (Fodjo et al. 2019; Hopkins 2005; Otabil et al. 2019), strongyloidiasis (Henriquez‐Camacho et al. 2016; Igual-Adell et al. 2004), lymphatic filariasis (Beng et al. 2020; Brown et al. 2000; Kazura 1993), and scabies (Anderson and Strowd 2017; Rosumeck et al. 2018). It has now been demonstrated that ivermectin can be used to limit a variety of ailments, including orbital myopathy, trichinosis, malaria, leishmaniasis, African trypanosomiasis, asthma, epilepsy, neurological disorders, certain cancers, and a wide range of diseases caused by viruses (Li et al. 2021). There is also research information on the beneficial effects of ivermectin on a wide range of RNA and DNA viruses, including positive single-stranded RNA viruses such as ZIKV, Dengue virus, and Venezuelan Equine Encephalitis Virus (VEEV). Similarly, inhibition of nuclear entry of other viral proteins/genomes into host cells (Heidary and Gharebaghi 2020) stimulates the introduction of SARS-CoV-2 with similar genomic properties. In this regard, the antiviral effect of ivermectin on SARS-CoV-2 has now been published (Caly et al.). It has been shown that a single dose of ivermectin can reduce viral RNA approximately 5000-fold in Vero/hSLAM cells affected by SARS-CoV-2 48 h after treatment (Caly et al. 2020). However, these results are attracting considerable attention from researchers around the world. This conclusion should be drawn with caution as this study only investigated the antiviral effect of ivermectin on SARS-CoV-2 in vitro (Schmith et al. 2020).
One of the first in vivo studies to examine the effects of ivermectin on COVID-19 was Sabeena (Ahmed et al.). In this study, they gave patients ivermectin for 5 days and found that treated patients had significantly higher rates of viral clearance and significantly reduced disease severity indicators (CRP and LDH) compared to placebo (Kazura 1993). A meta-analysis also showed that ivermectin can reduce inpatient mortality by 68% (Chen et al. 2020). Given the antiviral effect of ivermectin, it has been suggested that this drug may directly or indirectly affect the pathogenesis of COVID-19 through inactivation of extracellular viral particles, interfering with the pathways of virus entry into the host cells, and viral replication, protein production, and post-translational changes and other probable pathways. The exact mechanism of action of this drug against COVID-19 disease has not yet been elucidated, but some mechanisms have been previously mentioned and are briefly described below.
Ivermectin in combination with other drugs
There are many published studies explaining the effectiveness of ivermectin in combination with other drugs. Previous studies have shown that the combination of ivermectin and doxycycline may reduce the recovery period and the proportion of patients who progress to advanced stages of the disease. In addition, this combination was negative for a COVID-19 test using RT-PCR on day 14 (Mahmud et al. 2021). Hashim et al. also concluded that treatment with the combination of ivermectin and doxycycline could reduce mortality in severely ill patients (Hashim et al. 2020). Butters’ study found that the antiviral activity of ivermectin could be improved when combined with a zinc supplement. It has been suggested that increasing zinc levels may help fight infection in people with COVID-19 and speed up the recovery process (Butters and Whitehouse 2021).
Elalfy et al. explored the effects of a unique combination of ivermectin in mild-to-moderate cases of COVID-19 receiving treatment at home. Results showed that the combination of ivermectin with nitazoxanide, ribavirin, and zinc reduced the COVID-19 viral load in nasopharyngeal swabs. The synergistic effect of zinc in combination with antiviral agents has been demonstrated in other viral infections including hepatitis C virus, pediatric viral diarrhea, human papillomavirus, and human immunodeficiency virus. This study showed that 88% of patients treated with this combination had negative RT-PCR results on 15th of infection (Elalfy et al. 2021).
Clinical studies have shown that azithromycin inhibits the release of cytokines, attenuates the inflammatory response, and enhances the immunoglobulin response. Depending on its antiviral and anti-inflammatory properties, azithromycin alone may be an effective treatment for early COVID-19 (Andreani et al. 2020). The antiviral activity of azithromycin is attributed to several mechanisms, including structural and functional lysosomal damage to infected cells, inhibition of lysosomal proteases that promote the binding of SARS-CoV-2 to receptors such as ACE2, and viral entry into host cells (Al-Kuraishy et al. 2020). There was no clear interaction between ivermectin and azithromycin and no torsadogenic effect was observed (Al-Kuraishy et al. 2020). Therefore, the combination of azithromycin and ivermectin can be considered effective in COVID-19 patients.
Combinations of antiviral drugs with drugs that act on cellular targets or use a different mechanism of action also help to minimize drug resistance and toxicity during antiviral therapy (Day and Siu 2016). Several effective drug combinations are available for the treatment of HIV-1 and hepatitis C viruses (Ghany et al. 2019; Organization 2018). Remdesivir and ivermectin are two repurposed drugs that have received significant interest in the treatment of COVID-19. Remdesivir is a prodrug, a nucleotide analog, which acts against RNA viruses and inhibits RNA polymerase. It showed effective inhibitory activity against SARS-CoV-1 and Middle East Respiratory Syndrome (MERS-CoV) in vitro (Agostini et al. 2018; Sheahan et al. 2017). Additionally, Remdesivir was demonstrated to have antiviral efficacy against SARS-CoV-2 in vitro early in the epidemic and was subsequently clinically evaluated in humans (Pizzorno et al. 2020). Jeffreys et al. (Jeffreys et al. 2020) reported increased antiviral activity against SARS-CoV-2 in vitro through a synergistic interaction between remdesivir and ivermectin. The combination of remdesivir and ivermectin may enhance the antiviral properties of remdesivir as a viral RNA polymerase inhibitor while facilitating the use of ivermectin’s anti-inflammatory and/or immunomodulatory properties. However, more research is needed to evaluate the combined effects of ivermectin and remdesivir on COVID-19.
Favipiravir, an antiviral drug approved for use during the 2014 influenza pandemic in Japan, showed potent antiviral activity against SARS-CoV-2 in vitro. This drug has shown a wide range of therapeutic safety. According to the COVID-19 clinical study, it clears the viral infection faster than lopinavir/ritonavir (LPV/RTV) and recovers faster than umifenovir. In the end, favipiravir was proven effective in clinical trials in China, Russia, and Japan. In addition, clinical trials are underway in several countries, including the United States, United Kingdom, and India (Joshi et al. 2021a, b). Recent studies have identified favipiravir and ivermectin as a promising drug combination for clinical trial testing for the treatment of COVID-19 due to their synergistic effects, a fairly high safety profile, and easy availability (Jitobaom et al. 2021).
Ribavirin, an analog of guanosine, has significant antiviral activity against DNA and RNA viruses. Although the exact mechanism of action of ribavirin has not been elucidated, one of the possible mechanisms is the inhibition of mRNA capping and the induction of mutations during viral replication. Such mechanisms can limit viral proliferation (Crotty et al. 2001; Graci and Cameron 2006). Researchers have investigated ribavirin as a potentially effective antiviral agent against SARS-CoV2 infection based on previous clinical experience with SARS-CoV-2 and Middle East Respiratory Syndrome (MERS) coronaviruses (Al-Tawfiq et al. 2014; Booth et al. 2003; Elalfy et al. 2021). After ribavirin was recommended by the Chinese government for the treatment of SARS-CoV-2 infection, numerous clinical trials have been conducted to evaluate its effectiveness in treating SARS-CoV-2 infection (Hung et al. 2020). Studies have shown that ribavirin is more effective in treating COVID-19 when combined with interferon-α or lopinavir/ritonavir (Yousefi et al. 2020; Zhong et al. 2020). However, in studies using only ribavirin, its efficacy was reduced compared to the control drug (Elalfy et al. 2021). As a result, ribavirin monotherapy has a limited therapeutic effect on COVID-19, and the dose must be increased to enhance the effect. On the other hand, the increase in dose may lead to side effects such as hepatotoxicity and Hematological problems (Sanders et al. 2020).
Elalfy et al. found that co-administration of ivermectin, ribavirin, and nitazoxanide with zinc supplementation cleared SARS-CoV-2 in the nasopharynx significantly faster than symptomatic therapy (Elalfy et al. 2021). Therefore, it can be concluded that the combination therapy of ivermectin and ribavirin can be an effective treatment for COVID-19 with a synergistic effect and a lower risk of dose-dependent side effects compared to ivermectin monotherapy. At the same time, further studies are needed to confirm the combined effects of these drugs.
Famotidine, as a histamine-2 receptor antagonist, inhibits the action of histamine in parietal cells, ultimately inhibiting gastric acid secretion (Freedberg et al. 2020). It was previously reported to inhibit HIV.1 replication in vitro (Bourinbaiar and Fruhstorfer 1996). A recent study in the United States reported that famotidine helped improve the clinical outcome of 1620 inpatients with COVID-19 and reduce the risk of death (Freedberg et al. 2020). Based on the data of Sen Gupta et al. (Sen Gupta and Rana 2020), it was suggested that the combination of famotidine and ivermectin may have a synergistic effect. As a first-level barrier, famotidine inhibits viral entry. Ivermectin then acts as a second barrier, preventing virus replication in the host cell, thereby completely inhibiting the virus (Sen Gupta and Rana 2020). In addition, these drugs are more attractive because of their safety, availability, and cost effectiveness. However, it is suggested that additional studies are needed to confirm the synergistic effect of ivermectin and famotidine combination therapy as a promising treatment for COVID-19.
Ivermectin against future variants
Because the SARS-CoV-2 vaccine targets the biology of the spike protein, there is growing concern about the recently documented “hard-to-vaccine strain”. In these circumstances, ivermectin may work with the strategies mentioned for these new strains that can evade immunity with a vaccine (Zaidi and Dehgani-Mobaraki 2021), such as Delta, the newly emerged Omicron variant, and possibly future super-variants. However, new studies are needed to evaluate the efficacy of ivermectin against emerging variants that bind to the SARS-CoV-2 spike protein and block its binding to established host cell receptors including ACE2, CD147, neuropilin-1, etc. (Zalpoor et al. 2022c).
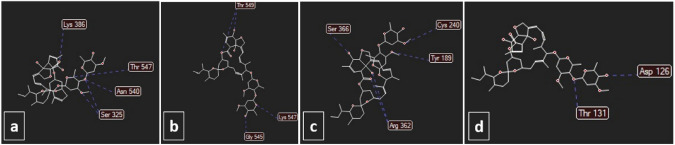
Omicron contains a variety of mutations. There are at least 32 mutations within its spike protein alone in comparison to the Delta variant. In addition to these newly discovered mutations, the Omicron variant is dependent on NSP12 for viral replication and NSP14 for methyltransferase and exoribonuclease activity. Therefore, they can have a positive effect on viral RNA replication. It is thought that the Omicron variant is at least three times more infectious in comparison to the original SARS‐CoV‐2 and possibly even more infectious than the delta variant (Raj 2021; Saxena et al. 2022). On the one hand, ivermectin binds to the predicted active site of NSP14 and RNA-dependent RNA polymerase (RdRp) with a high affinity (Zaidi and Dehgani-Mobaraki 2021) which are highly required for viral replication. On the other hand, we postulate that ivermectin possibly may bind to the Spike protein of Omicron, as seen in previous variants. For Assessment of these effects of ivermectin, we used molegro virtual docker to simulate ivermectin potential for binding to the active site of NSP14, RdRp, TMPRSS2, Delta, and Omicron variant spike protein, which we found that not only ivermectin can bind to these proteins with high affinity but also it binds to Omicron spike protein with higher affinity than Delta spike protein (Fig. 3).
Fig. 3.
Docking of a spike Delta (score: 120), b spike Omicron (score: 124), c RdRp (score: 137), and d NSP14 (score: 147) with ivermectin. The unit of scoring function is moldockscore. The energies were obtained using molegro virtual docker
Conclusion and future directions
Ivermectin alone or in combination with other drugs appears to have beneficial effects on SARS-CoV-2 infection. In addition, ivermectin is known to inhibit the entry and replication of viral RNA into host cells through a variety of strategies/pathways. However, we hypothesize that ivermectin is capable of increasing the replication capacity of SARS-CoV-2 by enhancing autophagy. Although ivermectin has anti-inflammatory properties, it can induce pathological complications and inflammatory responses during COVID-19 treatment by increasing stimulation of the P2X7 receptor and its downstream signaling pathways. Furthermore, despite its role in altering metabolic processes, it may induce ROS and it may be one of the other side effects of ivermectin in the treatment of COVID-19. It appears that the combined treatment of ivermectin with other drugs may reduce side effects and demonstrate its therapeutic efficacy in the treatment of COVID-19.
Acknowledgements
This study was approval from Ethics Committee (Ethical code: IR.ASAUMS.REC.1400.011) and supported by a Research Grant from Asadabad School of Medical Science (Grant No. 70). The authors have no financial conflicts of interest to declare.
Abbreviations
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- COVID-19
Coronavirus disease 2019
- RdRp
RNA-dependent RNA polymerase
- NSP
Nonstructural protein
- IMPα
Importin alpha
- NF-κB
Nuclear factor kappa B
- VEGF
Vascular endothelial growth factor
- ETC
Electron transport chain
- OXPHOS
Oxidative phosphorylation
- MERS-CoV
Middle East Respiratory Syndrome
Author contributions
The core idea of this study came from MNA. MNA, FM, HZ, FA, AA, HE, HMS, and AM. M and HE wrote the manuscript. MNA, HZ, HMS, and EB edited the manuscript. FM created the figures. FA created the docking. All authors approved the manuscript and they have declared that no conflict of interest exists.
Funding
This study was supported by a Research Grant from Asadabad School of Medical Science (Grant No. 70).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All other authors declare no conflict of interest.
Ethical approval
There is no involvement of human or animal in this study.
Consent for publication
All other authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Mohsen Nabi Afjadi and Fatemeh Mohebi have contributed equally to this work.
Contributor Information
Mohsen Nabi-Afjadi, Email: Mohsennabi66@gmail.com.
Hamidreza Zalpoor, Email: hamidreza.zlpr1998@gmail.com.
Hemen Moradi-Sardareh, Email: hemen.moradi@yahoo.com.
Elham Bahreini, Email: elhambahreini@yahoo.com.
Amir Mansour Moeini, Email: am.moeini.md@gmail.com.
References
- Agostini ML, Andres EL, Sims AC, Graham RL, Sheahan TP, Lu X, Jordan R. Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. Mbio. 2018;9(2):e00221–e1218. doi: 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S, Karim MM, Ross AG, Hossain MS, Clemens JD, Sumiya MK, Somani J. A five-day course of ivermectin for the treatment of COVID-19 may reduce the duration of illness. Int J Infect Dis. 2021;103:214–216. doi: 10.1016/j.ijid.2020.11.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Kuraishy HM, Hussien NR, Al-Naimi MS, Al-Buhadily AK, Al-Gareeb AI, Lungnier C. Is ivermectin–Azithromycin combination the next step for COVID-19? Biomed Biotechnol Res J (BBRJ) 2020;4(5):101. [Google Scholar]
- Al-Tawfiq JA, Momattin H, Dib J, Memish ZA. Ribavirin and interferon therapy in patients infected with the Middle East respiratory syndrome coronavirus: an observational study. Int J Infect Dis. 2014;20:42–46. doi: 10.1016/j.ijid.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson KL, Strowd LC. Epidemiology, diagnosis, and treatment of scabies in a dermatology office. J Am Board Family Med. 2017;30(1):78–84. doi: 10.3122/jabfm.2017.01.160190. [DOI] [PubMed] [Google Scholar]
- Andreani J, Le Bideau M, Duflot I, Jardot P, Rolland C, Boxberger M, La Scola B. In vitro testing of combined hydroxychloroquine and azithromycin on SARS-CoV-2 shows synergistic effect. Microb Pathog. 2020;145:104228. doi: 10.1016/j.micpath.2020.104228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atakisi E, Atakisi O, Topcu B, Uzun M. Effects of therapeutic dose of ivermectin on plasma nitric oxide and total antioxidant capacity in rabbits. Eur Rev Med Pharmacol Sci. 2009;13(6):425–429. [PubMed] [Google Scholar]
- Behera P, Patro BK, Padhy BM, Mohapatra PR, Bal S K, Chandanshive PD, Pentapati SSK (2021) Prophylactic role of ivermectin in SARS-CoV-2 infection among healthcare workers [DOI] [PMC free article] [PubMed]
- Beng AA, Esum ME, Deribe K, Njouendou AJ, Ndongmo PW, Abong RA, Amambo G. Mapping lymphatic filariasis in Loa loa endemic health districts naïve for ivermectin mass administration and situated in the forested zone of Cameroon. BMC Infect Dis. 2020;20(1):1–11. doi: 10.1186/s12879-020-05009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth CM, Matukas LM, Tomlinson GA, Rachlis AR, Rose DB, Dwosh HA, Derkach P. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA. 2003;289(21):2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Bourinbaiar AS, Fruhstorfer EC. The effect of histamine type 2 receptor antagonists on human immunodeficiency virus (HIV) replication: identification of a new class of antiviral agents. Life Sci. 1996;59(23):PL365–PL370. doi: 10.1016/S0024-3205(96)00553-X. [DOI] [PubMed] [Google Scholar]
- Brown K, Ricci F, Ottesen E. Ivermectin: effectiveness in lymphatic filariasis. Parasitology. 2000;121(S1):S133–S146. doi: 10.1017/S0031182000006570. [DOI] [PubMed] [Google Scholar]
- Butters D, Whitehouse M. COVID-19 and nutriceutical therapies, especially using zinc to supplement antimicrobials. Inflammopharmacology. 2021;29(1):101–105. doi: 10.1007/s10787-020-00774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178:104787. doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell W, Benz G. Ivermectin: a review of efficacy and safety. J Vet Pharmacol Ther. 1984;7(1):1–16. doi: 10.1111/j.1365-2885.1984.tb00872.x. [DOI] [PubMed] [Google Scholar]
- Canga AG, Prieto AMS, Liébana MJD, Martínez NF, Vega MS, Vieitez JJG. The pharmacokinetics and interactions of ivermectin in humans—a mini-review. AAPS J. 2008;10(1):42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Wei Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury A, Das NC, Patra R, Bhattacharya M, Ghosh P, Patra BC, Mukherjee S. Exploring the binding efficacy of ivermectin against the key proteins of SARS-CoV-2 pathogenesis: an in silico approach. Futur Virol. 2021;16(4):277–291. doi: 10.2217/fvl-2020-0342. [DOI] [Google Scholar]
- Codo AC, Davanzo GG, de Brito Monteiro L, de Souza GF, Muraro SP, Virgilio-da-Silva JV, Crunfli F. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32(3):437–446. e435. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotty S, Cameron CE, Andino R. RNA virus error catastrophe: direct molecular test by using ribavirin. Proc Natl Acad Sci. 2001;98(12):6895–6900. doi: 10.1073/pnas.111085598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crump A, Omura S. Ivermectin, ‘wonder drug’from Japan: the human use perspective. Proc Jpn Acad Ser B. 2011;87(2):13–28. doi: 10.2183/pjab.87.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D, Siu LL. Approaches to modernize the combination drug development paradigm. Genome Med. 2016;8(1):1–14. doi: 10.1186/s13073-016-0369-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez-Gomez G, Chavez-Blanco A, Medina-Franco JL, Saldivar-Gonzalez F, Flores-Torrontegui Y, Juarez M, Dueñas-González A. Ivermectin as an inhibitor of cancer stem-like cells. Mol Med Rep. 2018;17(2):3397–3403. doi: 10.3892/mmr.2017.8231. [DOI] [PubMed] [Google Scholar]
- Dou Q, Chen H-N, Wang K, Yuan K, Lei Y, Li K, Xie N. Ivermectin induces cytostatic autophagy by blocking the PAK1/Akt axis in breast cancer. Can Res. 2016;76(15):4457–4469. doi: 10.1158/0008-5472.CAN-15-2887. [DOI] [PubMed] [Google Scholar]
- Dueñas-González A, Juárez-Rodríguez M. Ivermectin: potential repurposing of a versatile antiparasitic as a novel anticancer repurposed drugs for cancer. IntechOpen; 2021. [Google Scholar]
- Eguchi S, Kawai T, Scalia R, Rizzo V. Understanding angiotensin II type 1 receptor signaling in vascular pathophysiology. Hypertension. 2018;71(5):804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elalfy H, Besheer T, El-Mesery A, El-Gilany AH, Soliman MAA, Alhawarey A, Zaghloul H. Effect of a combination of nitazoxanide, ribavirin, and ivermectin plus zinc supplement (MANS. NRIZ study) on the clearance of mild COVID-19. J Med Virol. 2021;93(5):3176–3183. doi: 10.1002/jmv.26880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Far AH. Effect of therapeutic and double therapeutic doses of ivermectin on oxidative status and reproductive hormones in male rabbits. Am J Anim Vet Sci. 2013;8(3):128–133. doi: 10.3844/ajavsp.2013.128.133. [DOI] [Google Scholar]
- Esakandari H, Nabi-Afjadi M, Fakkari-Afjadi J, Farahmandian N, Miresmaeili S-M, Bahreini E. A comprehensive review of COVID-19 characteristics. Biol Proced Online. 2020;22:1–10. doi: 10.1186/s12575-020-00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eweas AF, Alhossary AA, Abdel-Moneim AS. Molecular docking reveals Ivermectin and Remdesivir as potential repurposed drugs against SARS-CoV-2. Front Microbiol. 2021;11:3602. doi: 10.3389/fmicb.2020.592908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fodjo JNS, Kugler M, Hotterbeekx A, Hendy A, Van Geertruyden J-P, Colebunders R. Would ivermectin for malaria control be beneficial in onchocerciasis-endemic regions? Infect Dis Poverty. 2019;8(1):1–4. doi: 10.1186/s40249-019-0588-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg DE, Conigliaro J, Wang TC, Tracey KJ, Callahan MV, Abrams JA, O’Donnell MR. Famotidine use is associated with improved clinical outcomes in hospitalized COVID-19 patients: a propensity score matched retrospective cohort study. Gastroenterology. 2020;159(3):1129–1131. e1123. doi: 10.1053/j.gastro.2020.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardinassi LG, Souza CO, Sales-Campos H, Fonseca SG. Immune and metabolic signatures of COVID-19 revealed by transcriptomics data reuse. Front Immunol. 2020;11:1636. doi: 10.3389/fimmu.2020.01636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghany MG, Marks KM, Morgan TR, Wyles DL, Aronsohn AI, Bhattacharya D, Gordon SC. Hepatitis C guidance 2019 update: AASLD-IDSA recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2019;71(2):686–721. doi: 10.1002/hep.31060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graci JD, Cameron CE. Mechanisms of action of ribavirin against distinct viruses. Rev Med Virol. 2006;16(1):37–48. doi: 10.1002/rmv.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashim HA, Maulood MF, Rasheed AM, Fatak DF, Kabah KK, Abdulamir AS. Controlled randomized clinical trial on using Ivermectin with Doxycycline for treating COVID-19 patients in Baghdad, Iraq. MedRxiv. 2020 doi: 10.1101/2020.10.26.20219345. [DOI] [Google Scholar]
- Heidary F, Gharebaghi R. Ivermectin: a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot. 2020;73(9):593–602. doi: 10.1038/s41429-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriquez-Camacho C, Gotuzzo E, Echevarria J, White AC, Jr, Terashima A, Samalvides F, Plana MN. Ivermectin versus albendazole or thiabendazole for Strongyloides stercoralis infection. Cochrane Database Syst Rev. 2016 doi: 10.1002/14651858.CD007745.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins A. Ivermectin and onchocerciasis: is it all solved? Eye. 2005;19(10):1057–1066. doi: 10.1038/sj.eye.6701962. [DOI] [PubMed] [Google Scholar]
- Hui DS, Azhar EI, Madani TA, Ntoumi F, Kock R, Dar O, Drosten C. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—the latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IF-N, Lung K-C, Tso EY-K, Liu R, Chung TW-H, Chu M-Y, Tam AR. Triple combination of interferon beta-1b, lopinavir–ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395(10238):1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igual-Adell R, Oltra-Alcaraz C, Soler-Company E, Sánchez-Sánchez P, Matogo-Oyana J, Rodríguez-Calabuig D. Efficacy and safety of ivermectin and thiabendazole in the treatment of strongyloidiasis. Expert Opin Pharmacother. 2004;5(12):2615–2619. doi: 10.1517/14656566.5.12.2615. [DOI] [PubMed] [Google Scholar]
- Jeffreys L, Pennington SH, Duggan J, Breen A, Jinks J, Ardrey A, Hong WD. Remdesivir-Ivermectin combination displays synergistic interaction with improved in vitro antiviral activity against SARS-CoV-2. BioRxiv. 2020 doi: 10.1101/2020.12.23.424232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jitobaom K, Boonarkart C, Manopwisedjaroen S, Punyadee N, Borwornpinyo S, Thitithanyanont A, Auewarakul P (2021) Favipiravir and ivermectin showed in vitro synergistic antiviral activity against SARS-CoV-2
- Joshi N, Hajizadeh F, Dezfouli EA, Zekiy AO, Afjadi MN, Mousavi SM, Hassannia H. Silencing STAT3 enhances sensitivity of cancer cells to doxorubicin and inhibits tumor progression. Life Sci. 2021;275:119369. doi: 10.1016/j.lfs.2021.119369. [DOI] [PubMed] [Google Scholar]
- Joshi S, Parkar J, Ansari A, Vora A, Talwar D, Tiwaskar M, Barkate H. Role of favipiravir in the treatment of COVID-19. Int J Infect Dis. 2021;102:501–508. doi: 10.1016/j.ijid.2020.10.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juarez M, Schcolnik-Cabrera A, Dueñas-Gonzalez A. The multitargeted drug ivermectin: from an antiparasitic agent to a repositioned cancer drug. Am J Cancer Res. 2018;8(2):317. [PMC free article] [PubMed] [Google Scholar]
- Kazura JW. Ivermectin and human lymphatic filariasis. Microb Pathog. 1993;14(5):337–342. doi: 10.1006/mpat.1993.1033. [DOI] [PubMed] [Google Scholar]
- Khezri MR, Varzandeh R, Ghasemnejad-Berenji M. The probable role and therapeutic potential of the PI3K/AKT signaling pathway in SARS-CoV-2 induced coagulopathy. Cell Mol Biol Lett. 2022;27(1):1–10. doi: 10.1186/s11658-022-00308-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khomari F, Nabi-Afjadi M, Yarahmadi S, Eskandari H, Bahreini E. Effects of cell proteostasis network on the survival of SARS-CoV-2. Biol Proced Online. 2021;23(1):1–10. doi: 10.1186/s12575-021-00145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J-H, Choi HS, Kim S-L, Lee D-S. The PAK1-Stat3 signaling pathway activates IL-6 gene transcription and human breast cancer stem cell formation. Cancers. 2019;11(10):1527. doi: 10.3390/cancers11101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King CR, Tessier TM, Dodge MJ, Weinberg JB, Mymryk JS. Inhibition of human adenovirus replication by the importin α/β1 nuclear import inhibitor ivermectin. J Virol. 2020;94(18):e00710–00720. doi: 10.1128/JVI.00710-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz U, Ogbuokiri J, Okonkwo P. Ivermectin binds avidly to plasma proteins. Eur J Clin Pharmacol. 1990;39(6):607–608. doi: 10.1007/BF00316107. [DOI] [PubMed] [Google Scholar]
- Konno Y, Kimura I, Uriu K, Fukushi M, Irie T, Koyanagi Y, Sato K. USFQ-COVID19 consortium SARS-CoV-2 ORF3b is a potent interferon antagonist whose activity is increased by a naturally occurring elongation variant. Cell Rep. 2020;32:108185. doi: 10.1016/j.celrep.2020.108185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kory P, Meduri GU, Varon J, Iglesias J, Marik PE. Review of the emerging evidence demonstrating the efficacy of ivermectin in the prophylaxis and treatment of COVID-19. Am J Ther. 2021;28(3):e299. doi: 10.1097/MJT.0000000000001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-Y, Lim W, Ham J, Kim J, You S, Song G. Ivermectin induces apoptosis of porcine trophectoderm and uterine luminal epithelial cells through loss of mitochondrial membrane potential, mitochondrial calcium ion overload, and reactive oxygen species generation. Pestic Biochem Physiol. 2019;159:144–153. doi: 10.1016/j.pestbp.2019.06.009. [DOI] [PubMed] [Google Scholar]
- Lehrer S, Rheinstein PH. Ivermectin docks to the SARS-CoV-2 spike receptor-binding domain attached to ACE2. In Vivo. 2020;34(5):3023–3026. doi: 10.21873/invivo.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Zhao L, Zhan X. Quantitative proteomics reveals a broad-spectrum antiviral property of ivermectin, benefiting for COVID-19 treatment. J Cell Physiol. 2021;236(4):2959–2975. doi: 10.1002/jcp.30055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SCL, Hor CP, Tay KH, Jelani AM, Tan WH, Ker HB, Lim HH. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022 doi: 10.1001/jamainternmed.2022.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SCL, Hor CP, Tay KH, Jelani AM, Tan WH, Ker HB, Lim HH. Efficacy of ivermectin treatment on disease progression among adults with mild to moderate COVID-19 and comorbidities: the I-TECH randomized clinical trial. JAMA Intern Med. 2022;182(4):426–435. doi: 10.1001/jamainternmed.2022.0189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Fang S, Sun Q, Liu B. Anthelmintic drug ivermectin inhibits angiogenesis, growth and survival of glioblastoma through inducing mitochondrial dysfunction and oxidative stress. Biochem Biophys Res Commun. 2016;480(3):415–421. doi: 10.1016/j.bbrc.2016.10.064. [DOI] [PubMed] [Google Scholar]
- Liu J, Liang H, Chen C, Wang X, Qu F, Wang H, Meng J (2019) Ivermectin induces autophagy-mediated cell death through the AKT/mTOR signaling pathway in glioma cells. Biosci Rep 39(12). 10.1042/bsr20192489 [DOI] [PMC free article] [PubMed]
- López-Medina E, López P, Hurtado IC, Dávalos DM, Ramirez O, Martínez E, Herrera S. Effect of ivermectin on time to resolution of symptoms among adults with mild COVID-19: a randomized clinical trial. JAMA. 2021;325(14):1426–1435. doi: 10.1001/jama.2021.3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv C, Liu W, Wang B, Dang R, Qiu L, Ren J, Wang X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018;159:55–62. doi: 10.1016/j.antiviral.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Ma Y, Wu L, Shaw N, Gao Y, Wang J, Sun Y, Rao Z. Structural basis and functional analysis of the SARS coronavirus nsp14–nsp10 complex. Proc Natl Acad Sci. 2015;112(30):9436–9441. doi: 10.1073/pnas.1508686112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmud R, Rahman MM, Alam I, Ahmed KGU, Kabir AH, Sayeed SJB, Islam MM. Ivermectin in combination with doxycycline for treating COVID-19 symptoms: a randomized trial. J Int Med Res. 2021;49(5):03000605211013550. doi: 10.1177/03000605211013550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielech AM, Kilianski A, Baez-Santos YM, Mesecar AD, Baker SC. MERS-CoV papain-like protease has deISGylating and deubiquitinating activities. Virology. 2014;450:64–70. doi: 10.1016/j.virol.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mody V, Ho J, Wills S, Mawri A, Lawson L, Ebert MC, Taval S. Identification of 3-chymotrypsin like protease (3CLPro) inhibitors as potential anti-SARS-CoV-2 agents. Commun Biol. 2021;4(1):1–10. doi: 10.1038/s42003-020-01577-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan A, Tiwari P, Suri T, Mittal S, Patel A, Jain A, Pandey R (2021) Ivermectin in mild and moderate COVID-19 (RIVET-COV): a randomized, placebo-controlled trial [DOI] [PMC free article] [PubMed]
- Murakami M, Kamimura D, Hirano T. Pleiotropy and specificity: insights from the interleukin 6 family of cytokines. Immunity. 2019;50(4):812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- Nabi-Afjadi M, Karami H, Goudarzi K, Alipourfard I, Bahreini E. The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection. Clini Mol Allergy. 2021;19(1):1–10. doi: 10.1186/s12948-021-00161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi-Afjadi M, Heydari M, Zalpoor H, Arman I, Sadoughi A, Sahami P, Aghazadeh S. Lectins and lectibodies: potential promising antiviral agents. Cell Mol Biol Lett. 2022;27(1):1–25. doi: 10.1186/s11658-022-00338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okumuş N, Demirtürk N, Çetinkaya RA, Güner R, Avcı İY, Orhan S, Yamanel L. Evaluation of the effectiveness and safety of adding ivermectin to treatment in severe COVID-19 patients. BMC Infect Dis. 2021;21(1):1–11. doi: 10.1186/s12879-021-06104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omura S. Ivermectin: 25 years and still going strong. Int J Antimicrob Agents. 2008;31(2):91–98. doi: 10.1016/j.ijantimicag.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Organization WH (2018) Updated recommendations on first-line and second-line antiretroviral regimens and post-exposure prophylaxis and recommendations on early infant diagnosis of HIV: interim guidelines: supplement to the 2016 consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. Retrieved from
- Otabil KB, Gyasi SF, Awuah E, Obeng-Ofori D, Atta-Nyarko RJ, Andoh D, Ankrah CB. Prevalence of onchocerciasis and associated clinical manifestations in selected hypoendemic communities in Ghana following long-term administration of ivermectin. BMC Infect Dis. 2019;19(1):1–7. doi: 10.1186/s12879-019-4076-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payandeh Z, Mohammadkhani N, Nabi Afjadi M, Khalili S, Rajabibazl M, Houjaghani Z, Dadkhah M. The immunology of SARS-CoV-2 infection, the potential antibody based treatments and vaccination strategies. Expert Rev Anti-Infect Ther. 2021;19(7):899–910. doi: 10.1080/14787210.2020.1863144. [DOI] [PubMed] [Google Scholar]
- Pizzorno A, Padey B, Dubois J, Julien T, Traversier A, Dulière V, Terrier O. In vitro evaluation of antiviral activity of single and combined repurposable drugs against SARS-CoV-2. Antiviral Res. 2020;181:104878. doi: 10.1016/j.antiviral.2020.104878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raj R. Analysis of non-structural proteins, NSPs of SARS-CoV-2 as targets for computational drug designing. Biochem Biophys Rep. 2021;25:100847. doi: 10.1016/j.bbrep.2020.100847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter J-J. Use of ivermectin is associated with lower mortality in hospitalized patients with coronavirus disease 2019: the ivermectin in COVID nineteen study. Chest. 2021;159(1):85–92. doi: 10.1016/j.chest.2020.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza S, Shahin F, Zhai W, Li H, Alvisi G, Yang K, Hu C. Ivermectin inhibits bovine herpesvirus 1 DNA polymerase nuclear import and interferes with viral replication. Microorganisms. 2020;8(3):409. doi: 10.3390/microorganisms8030409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo E. Ivermectin, antiviral properties and COVID-19: a possible new mechanism of action. Naunyn Schmiedebergs Arch Pharmacol. 2020;393:1153–1156. doi: 10.1007/s00210-020-01902-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosumeck S, Nast A, Dressler C. Ivermectin and permethrin for treating scabies. Cochrane Database Syst Rev. 2018 doi: 10.1002/14651858.CD012994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan DG, O'Neill LA. Krebs cycle reborn in macrophage immunometabolism. Annu Rev Immunol. 2020;38:289–313. doi: 10.1146/annurev-immunol-081619-104850. [DOI] [PubMed] [Google Scholar]
- Sajid M, Iqbal Z, Muhammad G, Iqbal M. Immunomodulatory effect of various anti-parasitics: a review. Parasitology. 2006;132(3):301–313. doi: 10.1017/S0031182005009108. [DOI] [PubMed] [Google Scholar]
- Samaha AA, Mouawia H, Fawaz M, Hassan H, Salami A, Bazzal AA, Chouman M. Effects of a single dose of ivermectin on viral and clinical outcomes in asymptomatic SARS-CoV-2 infected subjects: a pilot clinical trial in Lebanon. Viruses. 2021;13(6):989. doi: 10.3390/v13060989. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323(18):1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Saxena SK, Kumar S, Ansari S, Paweska JT, Maurya VK, Tripathi AK, Abdel-Moneim AS. Characterization of the novel SARS-CoV-2 Omicron (B. 1.1 529) variant of concern and its global perspective. J Med Virol. 2022;94(4):1738–1744. doi: 10.1002/jmv.27524. [DOI] [PubMed] [Google Scholar]
- Schmith VD, Zhou J, Lohmer LR. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther. 2020;108(4):762–765. doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen Gupta PS, Rana MK. Ivermectin, famotidine, and doxycycline: a suggested combinatorial therapeutic for the treatment of COVID-19. ACS Pharmacol Transl Sci. 2020;3(5):1037–1038. doi: 10.1021/acsptsci.0c00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth C, Mas C, Conod A, Mueller J, Siems K, Kuciak M, RuizIAltaba A. Long-lasting WNT-TCF response blocking and epigenetic modifying activities of Withanolide F in human cancer cells. PLoS ONE. 2016;11(12):e0168170. doi: 10.1371/journal.pone.0168170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, Rezai MS. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021;43(6):1007–1019. doi: 10.1016/j.clinthera.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahbaznejad L, Davoudi A, Eslami G, Markowitz JS, Navaeifar MR, Hosseinzadeh F, Rezai MS. Effects of ivermectin in patients with COVID-19: a multicenter, double-blind, randomized, controlled clinical trial. Clin Ther. 2021 doi: 10.1016/j.clinthera.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheahan TP, Sims AC, Graham RL, Menachery VD, Gralinski LE, Case JB, Trantcheva I. Broad-spectrum antiviral GS-5734 inhibits both epidemic and zoonotic coronaviruses. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aal3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stankiewicz M, Cabaj W, Jonas W, Moore L, Millar K, Chie WN. Influence of ivermectin on cellular and humoral immune responses of lambs. Vet Immunol Immunopathol. 1995;44(3–4):347–358. doi: 10.1016/0165-2427(94)05308-F. [DOI] [PubMed] [Google Scholar]
- Swargiary A (2020) Ivermectin as a promising RNA-dependent RNA polymerase inhibitor and a therapeutic drug against SARS-CoV2: evidence from in silico studies
- V’kovski P, Kratzel A, Steiner S, Stalder H, Thiel V. Coronavirus biology and replication: implications for SARS-CoV-2. Nat Rev Microbiol. 2021;19(3):155–170. doi: 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallejos J, Zoni R, Bangher M, Villamandos S, Bobadilla A, Plano F, Achinelli F. Ivermectin to prevent hospitalizations in patients with COVID-19 (IVERCOR-COVID19) a randomized, double-blind, placebo-controlled trial. BMC Infect Dis. 2021;21(1):1–11. doi: 10.1186/s12879-021-06348-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff KM, Sivakumaran H, Heaton SM, Harrich D, Jans DA. Ivermectin is a specific inhibitor of importin α/β-mediated nuclear import able to inhibit replication of HIV-1 and dengue virus. Biochem J. 2012;443(3):851–856. doi: 10.1042/BJ20120150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Lv C, Ji X, Wang B, Qiu L, Yang Z. Ivermectin treatment inhibits the replication of Porcine circovirus 2 (PCV2) in vitro and mitigates the impact of viral infection in piglets. Virus Res. 2019;263:80–86. doi: 10.1016/j.virusres.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang Y, Wu L, Niu S, Song C, Zhang Z, Yuen K-Y. Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell. 2020;181(4):894–904.e899. doi: 10.1016/j.cell.2020.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A, Peng Y, Huang B, Ding X, Wang X, Niu P, Wang J. Genome composition and divergence of the novel coronavirus (2019-nCoV) originating in China. Cell Host Microbe. 2020;27(3):325–328. doi: 10.1016/j.chom.2020.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Fossali T, Pandolfi L, Carsana L, Ottolina D, Frangipane V, Agarossi A. Hypoalbuminemia in COVID-19: assessing the hypothesis for underlying pulmonary capillary leakage. J Int Med. 2021 doi: 10.1111/joim.13208. [DOI] [PubMed] [Google Scholar]
- Yang N, Shen H-M. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci. 2020;16(10):1724. doi: 10.7150/ijbs.45498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D, Chu H, Hou Y, Chai Y, Shuai H, Lee AC-Y, Huang X. Attenuated interferon and proinflammatory response in SARS-CoV-2–infected human dendritic cells is associated with viral antagonism of STAT1 phosphorylation. J Infect Dis. 2020;222(5):734–745. doi: 10.1093/infdis/jiaa356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang SN, Atkinson SC, Wang C, Lee A, Bogoyevitch MA, Borg NA, Jans DA. The broad spectrum antiviral ivermectin targets the host nuclear transport importin α/β1 heterodimer. Antiviral Res. 2020;177:104760. doi: 10.1016/j.antiviral.2020.104760. [DOI] [PubMed] [Google Scholar]
- Yousefi B, Valizadeh S, Ghaffari H, Vahedi A, Karbalaei M, Eslami M. A global treatments for coronaviruses including COVID-19. J Cell Physiol. 2020;235(12):9133–9142. doi: 10.1002/jcp.29785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi AK, Dehgani-Mobaraki P. The mechanisms of action of ivermectin against SARS-CoV-2—an extensive review. J Antibiot. 2021;75:60–71. doi: 10.1038/s41429-021-00491-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Akbari A, Nabi-Afjadi M, Forghaniesfidvajani R, Tavakol C, Barzegar Z, Farrokhi MR. Hypoxia-inducible factor 1 alpha (HIF-1α) stimulated and P2X7 receptor activated by COVID-19, as a potential therapeutic target and risk factor for epilepsy. Hum Cell. 2022 doi: 10.1007/s13577-022-00747-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Akbari A, Nayerain Jazi N, Liaghat M, Bakhtiyari M. Possible role of autophagy induced by COVID-19 in cancer progression, chemo-resistance, and tumor recurrence. Infect Agents Cancer. 2022;17(1):1–4. doi: 10.1186/s13027-022-00450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Akbari A, Samei A, Forghaniesfidvajani R, Kamali M, Afzalnia A, Khoshmirsafa M. The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies. Cell Mol Biol Lett. 2022;27(1):1–21. doi: 10.1186/s11658-022-00311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Bakhtiyari M, Liaghat M, Nabi-Afjadi M, Ganjalikhani-Hakemi M. Quercetin potential effects against SARS-CoV-2 infection and COVID-19-associated cancer progression by inhibiting mTOR and hypoxia-inducible factor-1α (HIF-1α) Phytother Res. 2022 doi: 10.1002/ptr.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Bakhtiyari M, Shapourian H, Rostampour P, Tavakol C, Nabi-Afjadi M. Hesperetin as an anti-SARS-CoV-2 agent can inhibit COVID-19-associated cancer progression by suppressing intracellular signaling pathways. Inflammopharmacology. 2022 doi: 10.1007/s10787-022-01054-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Rezaei M, Yahyazadeh S, Ganjalikhani-Hakemi M. Flt3-ITD mutated acute myeloid leukemia patients and COVID-19: potential roles of autophagy and HIF-1α in leukemia progression and mortality. Hum Cell. 2022 doi: 10.1007/s13577-022-00718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalpoor H, Shapourian H, Akbari A, Shahveh S, Haghshenas L. Increased neuropilin-1 expression by COVID-19: a possible cause of long-term neurological complications and progression of primary brain tumors. Hum Cell. 2022 doi: 10.1007/s13577-022-00716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X, Li N. The anti-cancer effects of anti-parasite drug ivermectin in ovarian cancer ovarian cancer-updates in tumour biology and therapeutics. IntechOpen; 2021. [Google Scholar]
- Zhang X, Song Y, Ci X, An N, Ju Y, Li H, Deng X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57(11):524–529. doi: 10.1007/s00011-008-8007-8. [DOI] [PubMed] [Google Scholar]
- Zhong H, Wang Y, Zhang Z-L, Liu Y-X, Le K-J, Cui M, Lin H-W. Efficacy and safety of current therapeutic options for COVID-19-lessons to be learnt from SARS and MERS epidemic: a systematic review and meta-analysis. Pharmacol Res. 2020;157:104872. doi: 10.1016/j.phrs.2020.104872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Yang X-L, Wang X-G, Hu B, Zhang L, Zhang W, Huang C-L. Discovery of a novel coronavirus associated with the recent pneumonia outbreak in humans and its potential bat origin. BioRxiv. 2020 doi: 10.1101/2020.01.22.914952. [DOI] [Google Scholar]
- Zhou S, Wu H, Ning W, Wu X, Xu X, Ma Y, Wang J. Ivermectin has new application in inhibiting colorectal cancer cell growth. Front Pharmacol. 2021 doi: 10.3389/fphar.2021.717529. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.