Abstract
Background
Diabetes is one of the fastest-growing health emergencies of the 21st century, placing a severe economic burden on many countries. Current management approaches have improved diabetic care, but several limitations still exist, such as decreased efficacy, adverse effects, and the high cost of treatment, particularly for developing nations. There is, therefore, a need for more cost-effective therapies for diabetes management. The evidence-based application of phytochemicals from plants in the management of diseases is gaining traction.
Methodology
Various plants and plant parts have been investigated as antidiabetic agents. This review sought to collate and discuss published data on the cellular and molecular effects of medicinal plants and phytochemicals on insulin signaling pathways to better understand the current trend in using plant products in the management of diabetes. Furthermore, we explored available information on medicinal plants that consistently produced hypoglycemic effects from isolated cells to animal studies and clinical trials.
Results
There is substantial literature describing the effects of a range of plant extracts on insulin action and insulin signaling, revealing a depth in knowledge of molecular detail. Our exploration also reveals effective antidiabetic actions in animal studies, and clear translational potential evidenced by clinical trials.
Conclusion
We suggest that this area of research should be further exploited in the search for novel therapeutics for diabetes.
Keywords: Medicinal plants, Insulin action, Diabetes, Glucose transport, Akt, Insulin signaling, Phytochemicals
Introduction
Statement of the problem
Diabetes mellitus is a metabolic disorder characterized by sustained hyperglycemia with numerous macrovascular and microvascular complications (Kharroubi, 2015; Stadlbauer et al., 2021; Zheng, Ley & Hu, 2018). Depending on the etiology, diabetes has been classified broadly into type 1 diabetes mellitus (T1DM), resulting from a deficiency in insulin production, and type 2 diabetes mellitus (T2DM), a defect in insulin action (Kharroubi, 2015). T1DM accounts for around 10% of cases, while T2DM accounts for about 90%. T2DM and its complications have contributed to a significant decrease in life expectancy (Zheng, Ley & Hu, 2018; Ogurtsova et al., 2017). The latest data suggests that 1 in 10 adults are living with diabetes, of which almost half are undiagnosed (Zheng, Ley & Hu, 2018), representing around 537 million citizens, and diabetes contributes to 1 in 9 deaths (Stadlbauer et al., 2021). These figures are projected to continue to rise. It is estimated that most countries devote 5–20% of healthcare expenditure to diabetes (Lin et al., 2020). The global spending to treat diabetes and its complications was US$760 billion in 2019, projected to increase to US$825 billion by 2030 (Stadlbauer et al., 2021; Modi, 2007). The disturbing increase in the prevalence of diabetes is a call for an augmented approach to the management of T2DM (Stadlbauer et al., 2021; Williams et al., 2020).
The conventional management of diabetes involves lifestyle modifications to control contributing factors such as obesity, hypertension and hyperlipidemia, based on the patient’s awareness (Pernicova & Korbonits, 2014; Proks et al., 2018; Farzaei et al., 2017; Soccio, Chen & Lazar, 2014), and the use of hypoglycemic agents by healthcare providers (Hedrington & Davis, 2019; Mogensen, 2007; Hinnen et al., 2006). Approaches to using antihyperglycemic agents to control hyperglycemia in diabetic patients involve different targets. For example, the sulfonylureas (e.g., chlorpropamide) and newer secretagogues (e.g., glipizide) increase insulin output by blocking the K+-ATPase channel of the pancreatic β-cell (Hedrington & Davis, 2019; Wajcberg & Tavaria, 2009; Bailey, 2015). The biguanides (e.g., metformin) act through inhibition of hepatic gluconeogenesis and promoting glycogenesis with increased insulin sensitivity (Hedrington & Davis, 2019; Hinnen et al., 2006; Bailey, 2015). Insulin sensitizers (e.g., thiazolidinediones) potentiate insulin action on muscle, adipocytes, liver and other tissues by selectively binding to peroxisome proliferator-activated receptor gamma (PPARγ) (Hedrington & Davis, 2019; Triggle & Ding, 2014). Others include α-glucosidase inhibitors (e.g., acarbose), which competitively inhibits intestinal α-glucosidase and pancreatic α-amylase with a resulting decrease in postprandial plasma glucose (Hedrington & Davis, 2019; Cetrone, Mele & Tricarico, 2014; Campbell, 2000). Incretin mimetics (e.g., exenatide) control postprandial insulin secretion by binding to the pancreatic glucagon-like peptides-1(GLP-1) receptors leading to increased glucose-dependent insulin secretion from the β-cells (Mogensen, 2007; Campbell, 2000; Harding et al., 2019). Exenatide also restores first-phase insulin secretion in patients with T2DM and promotes β-cells proliferation and islet neogenesis (Harding et al., 2019; Ogurtsova et al., 2017). The use of insulin for immediate glycemic control has been reserved for emergency situations (Mogensen, 2007; Bommer et al., 2017). Undoubtedly, these approaches have improved diabetic care over time, but several limitations still exist, such as decreased efficacy, adverse effects, and high cost of treatment (Michel, Abd Rani & Husain, 2020; Verma, 2014).
Medicinal plants as medicines in diabetes treatment
The global worsening of morbidity and mortality from diabetes (Zheng, Ley & Hu, 2018; Chaudhury et al., 2017; Modi, 2007; Md Sayem et al., 2018; Schreck & Melzig, 2021; Süntar, 2020) justifies the need for more diversified research for new therapies. Throughout human history, medicinal plants have been used for the prevention and treatment of both human and animal diseases (Balunas & Kinghorn, 2005; Majolo et al., 2019; Khazir et al., 2014; Ahmad et al., 2015). Medicinal plants have been recognized as a stable source for drug discovery since ancient times (World Health Organization, 2019; Singhal, Bangar & Naithani, 2012; Mathew & Subramanian, 2014; Martins & Brijesh, 2018) and The World Health Organization has reported an increased patronage of natural and medicinal plant drug products (World Health Organization, 2019). Many modern drugs are obtained from medicinal plants and further purified or optimized using structure-activity relationship-driven drug design and pharmacokinetic parameters (Singhal, Bangar & Naithani, 2012; Setorki, 2020; Sekhon-Loodu & Rupasinghe, 2019).
Evidence-based application of phytochemicals from plants in the management of diseases has received wide acceptability (Chukwuma et al., 2019; Mathew & Subramanian, 2014). For example, several reports of medicinal plants with anticancer activities have been published (Rabiei, Solati & Amini-Khoei, 2019; Sadino, 2018; Khan et al., 2012). Ethnopharmacological surveys of plants and phytochemicals with antihypertensive activities (Joseph & Jini, 2013; Shih, Lin & Lin, 2008; Al-Amin et al., 2006) have been well documented. There is also substantial literature of their utility in treatment of other chronic diseases such as Alzheimer’s (Son, Miura & Yagasaki, 2015; Morakinyo, Akindele & Ahmed, 2011), depressive disorders (Lai et al., 2015; Chien et al., 2009), Parkinson’s disease (Rabiei, Solati & Amini-Khoei, 2019) and diabetes (Bailey, 2015; Ogunbolude et al., 2009; Kanetkar, Singhal & Kamat, 2007).
Various plants and plant parts have been investigated for their hypoglycemic activities as potential medicine in the treatment of diabetes mellitus (Farzaei et al., 2017). By way of examples, phytocompounds from the fruit of Momordica charantia (bitter lemon) have been extensively studies for antidiabetic effects (Tiwari, Mishra & Sangwan, 2014; Kuroda et al., 2003; Shori, 2015). The roots of Zingiber officinale (ginger) exert antidiabetic and hypolipidemic effects on streptozotocin-induced diabetic rats (James, Stöckli & Birnbaum, 2021; Garvey et al., 1998; Ahmad, Choi & Lee, 2020). Bidens pilosa has been shown to reduce fasting blood glucose level and hemoglobin A1c (HbA1c) in clinical trials (Lai et al., 2015); three variants of B. pilosa were shown to possess anti-diabetic properties (Chien et al., 2009). The hydroethanolic extract of the seed of Parinari curatellifolia reduces plasma glucose levels and low-density lipoproteins in diabetic rats (Saini, 2010; Galochkina et al., 2019; Ogbonnia et al., 2009). The blood sugar reducing effects of Gymnema sylvestre popularly known as ‘gurmar’ (‘sugar destroyer’) has been widely studied (Kanetkar, Singhal & Kamat, 2007; Tiwari, Mishra & Sangwan, 2014). Phytochemical constituents of Glycyrriza uralensis (licorice) have been found to exhibit profound antidiabetic properties in experimental animals (Kuroda et al., 2003). While some studies do consider the potential molecular or cellular mechanisms of the antidiabetic effects (Ogurtsova et al., 2017; Vlavcheski et al., 2018), others focus on potential properties such as antioxidant (Ahmad, Choi & Lee, 2020; Galochkina et al., 2019) and anti-obesity (Kadan et al., 2018; Kamatou, Ssemakalu & Shai, 2021) effects without direct discussion of mechanism.
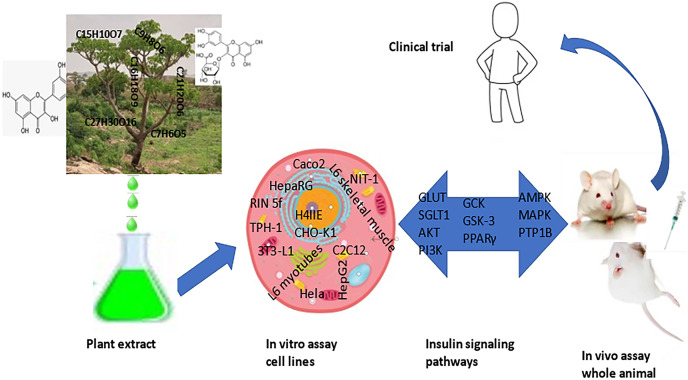
This review aims to collate, discuss, and present published data on the cellular and molecular effects of medicinal plants and phytochemicals on insulin signaling pathways to better understand the current trend in the use of plant products in the management of T2DM (Fig. 1). Furthermore, we have explored available information on the cell-biology of these medicinal plants that consistently produced hypoglycemic effects, with the intention of providing a reference point for the molecular basis of some of the more commonly used anti-diabetic plant extracts. We explored how these plant products might affect known insulin signaling systems and insulin effectors, and then extended our review into known effects on animal models and explored clinical trials of these compounds with the intention of providing a summary-view of related studies and a holistic overview of their use in rodent models or clinical trials. We conclude that plant products should be considered a vital tool in the armory for development of low-cost, effective anti-diabetic therapies.
Figure 1.
Schematic representation of antidiabetic development from plant to clinical trial. The pathway from plant extract to effective therapy involves many steps. Plants, often identified from local knowledge/use, are a source of extract prepared using a range of approaches and the extracts screened using simple cell-based models such as Caco-2 cells or L6 skeletal myotubes for in vitro effects. Signaling pathways and effectors are used as surrogate assays for potential antidiabetic effects (e.g., glucose transport). Further work involves an examination of effects using rodent models of diabetes and clinical trials.
The pathway from plant extract to effective therapy involves many steps. Plants, often identified from local knowledge/use, are a source of extract prepared using a range of approaches and the extracts screened using simple cell-based models such as Caco-2 cells or L6 skeletal myotubes for in vitro effects. Signaling pathways and effectors are used as surrogate assays for potential antidiabetic effects (e.g., glucose transport). Further work involves an examination of effects using rodent models of diabetes and clinical trials.
Methodology
We used a range of search terms to scan Google Scholar, PubMed, Science Direct, NIH National Library of Medicine and Scopus to retrieve published literature on medicinal plants and phytochemical effects on insulin signaling and effector pathways. Search terms focused on known signaling systems involved in propagating insulin signals (e.g., proteinqrynm_32 kinase-B/Akt (hereafter referred to as Akt); phosphoinositide-3 kinase (PI3K); glycogen synthase kinase-3 (GSK-3); AMP-activated protein kinase (AMPK); protein tyrosine phosphatase 1 B (PTP1B)); known effector molecules or processes (e.g., glucose transporters (GLUT) and GLUT4 storage vesicles (GSVs; also known as GSC–GLUT4 storage compartment); glucokinase (GCK)); glucagon secretion; lipolysis; lipogenesis; hepatic glucose output; and other molecules implicated in insulin action of insulin sensitivity, such as peroxisome proliferator-activated receptor gamma (PPARγ). Searches were performed between December 2021 and April 2022. We excluded articles not in English and not freely available via our institution (in this case the University of Strathclyde; <0.2% of articles retrieved) and no time limitation for publication date was employed. Our searches aimed to capture papers which described a potential effect on either signaling systems (e.g., PI3K, Akt etc.) or a biological output (e.g., glucose transport, GLUT4 mRNA). This was subsequently extended into whole animal studies and clinical trials.
Throughout we use the scientific and common names of the medicinal plants, and describe the chemistry used in the extraction process–a key consideration for studies of this type. In all tables, extracts are alphabetized by species unless multiple different species were used in the same study, in which case these are placed arbitrarily at the top of each table for clarity.
Results
Studies using cell lines
Effects of medicinal plants and phytochemicals on glucose transport and glucose transporters
Defective insulin-stimulated glucose transport is hallmark of T2DM (Ogurtsova et al., 2017; Nandabalan, Sujatha & Shanmuganathan, 2010; Drissi et al., 2021; Stadlbauer et al., 2016). Glucose transporters (GLUT) of the facilitative diffusion type are a multi-gene family of proteins which function to move glucose across cell membranes (Ogurtsova et al., 2017; World Health Organization, 2019; Singhal, Bangar & Naithani, 2012). Among the facilitative GLUT isoforms, GLUT4 is particularly important as it is expressed predominantly in skeletal and adipose tissues and accounts for post-prandial glucose disposal in these tissues (World Health Organization, 2019; Nandabalan, Sujatha & Shanmuganathan, 2010). Skeletal muscle contribute largely to a greater part of the total body mass in humans and it regulates several physiological processes including up to 85% of insulin-mediated glucose up-take through GLUT4 (Ahmad, Choi & Lee, 2020). Many studies have utilized this for therapeutic management of diabetes and in particular the role of extracellular matrix (Ahmad, Choi & Lee, 2020). Skeletal muscle contraction during exercise improves GLUT4 translocation to the cell membrane for glucose uptake and insulin-sensitivity (Jiang et al., 2013). Also, altered muscle glycogen synthesis play a major role in insulin resistance, and glycogen synthase, hexokinase, and GLUT4 are the major culprit involved in the skeletal muscle pathogenesis of type 2 dibetes (Petersen & Shulman, 2002; Saini, 2010). GLUT2 and GLUT5 are responsible for intestinal glucose and fructose uptake (Schreck & Melzig, 2021), while GLUT1 is present ubiquitously in all the body tissues (Galochkina et al., 2019). The dominant glucose transporters found in the small intestine are sodium-glucose linked transporter 1 (SGLT1) which accumulates glucose into adsorptive epithelial cells against its concentration gradient and GLUT2 which mediates movement of glucose from the epithelial cells into the blood (Schreck & Melzig, 2021); inhibition reduces the amount of glucose absorbed into the body. The hemodynamic activities of the glucose transporters have been extensively researched (Ogurtsova et al., 2017; World Health Organization, 2019; Singhal, Bangar & Naithani, 2012; Nandabalan, Sujatha & Shanmuganathan, 2010). Medicinal plants’ products and phytochemicals that modify the action of the glucose transporters could significantly contribute to the search for effective drugs in the management of diabetes. Table 1 is a collation of studies of medicinal plants known to modulate glucose transport in cell lines. Some notable highlights of this extensive literature are discussed briefly below.
Table 1. Medicinal plant active on glucose transporters.
Shown are studies in which the indicated plants have been shown to drive a change in glucose transport. Where possible these have been alphabetized, but studies in which multiple plant species or extracts were used in a single study are shown at the top of the table.
| Medicinal plant | Phytochemistry | Key effectors | Summary | References |
|---|---|---|---|---|
| Aronia melanocarpa, Cornus officinalis, Crataegus pinnatifida, Lycium chinense, Vaccinium myrtillus, Brassica oleracea, Juglans regia, Peumus boldus, Adenophora triphylla, Eucommia ulmoides, and Malus domestica | Methanolic extract of the leaves, roots, aqueous extract from the bark, and fruit skin. | SGLT 1 and GLUT2. | Inhibition of intestinal SGLT1 and GLUT2 in Caco-2 cells. | (Schreck & Melzig, 2021) |
| Hoodia, Sapindus mukorossi, Quillaja saponaria, Papaver, Castanea, Bitter orange, Oregon grape, Saposhnikovia divaricata, Sponge gourd, Black radish, Asparagus, Neem, Uzara, Reetha B, Chelidonium majus, Teasel, Tetradium ruticarpum, Southern wax myrtle, Bistort, Indian tobacco, Figwort, Rangoon creeper, Peruvian rhatany, Chinese rhubarb, Poppy capsule and flowers, Ivy, Common daisy leaves and flowers, Rosebay willowherb, and Goldenrod. | Plant extracts. | GLUT4 | Stimulation of GLUT4 translocation in CHO-K1 and 3T3-L1 cells and plasma membrane insertion of GLUT4 in Hela cells. | (Stadlbauer et al., 2021) |
| Trigonella foenumgraecum, Urtica dioica, Atriplex halimus, and Cinnamomum verum | 50% ethanol extract of the various parts. | GLUT4 | Increased translocation of GLUT4 to the plasma membrane in L6-GLUT4myc rat muscle cells. | (Kadan et al., 2013) |
| Rhododendron groenlandicum, Alnus incana, Sarracenia purpurea | Leaf, bark, and whole plant, respectively. | GLUT4 | Increased total membrane expression of GLUT4 and phosphorylation of AKT and AMP in C2C12 and H4IIE cell lines. | (Shang et al., 2015) |
| Strawberry and Apple | Polyphenols, phenolic acid, and tannins. | GLUT2, SGLT1 | Inhibition of GLUT2 and SGLT1 in human intestinal Caco-2 cells. | (Manzano & Williamson, 2010) |
| Annona stenophylla | Aqueous root extract. | GLUT4 | Enhanced GLUT4 and gene expression in C2C12 muscle cell lines. | (Taderera et al., 2019) |
| Apios americana | Glycosides from the leaves. | MAPK and glucose uptake | Restores glucose uptake, glucose consumption, and glycogen content in HepG2 cells via MAPK and Nrf2 pathways. | (Yan et al., 2017) |
| Capparis moonii | Gallotannins from hydro-alcoholic fruit extract. | GLUT4 | Increased phosphorylation of IR-β, IRS-1, and GLUT4, PI3K mRNA expression in L6 myotube cells. | (Kanaujia et al., 2010) |
| Cassia abbreviate | Aqueous leaf, seed, and bark extract. | GLUT4 | Enhanced GLUT4 translocation and gene expression in C2C12 mouse skeletal muscle cells. | (Kamatou, Ssemakalu & Shai, 2021) |
| Cinnamomum burmannii | Water extract and polyphenols. | GLUT4 GLUT1 |
Increased expression of mRNA GLUT4, IR, GLUT1in mouse 3T3- adipocytes. | (Cao, Polansky & Anderson, 2007; Cao, Graves & Anderson, 2010) |
| Cinnamomum cassia | Cinnamic acid from a hydroalcoholic bark extract. | GLUT4 | Increased GLUT4 mRNA and inhibition of PTP1B activity in L6 myotubes. | (Lakshmi et al., 2009) |
| Citrullus colocynthis | Fruit and seed extracts and solvent fractions. | GLUT4 | Enhancement of insulin-induced GLUT4 translocation in adipocytes. | (Drissi et al., 2021) |
| Costus igneus (insulin plant) | Leaf extract | Glucokinase/GLUT2 | Increased glucokinase activity, insulin, and GLUT2 gene expression but inhibition of glucose-6-phosphatase activity in human hematopoietic stem cells (HSCs) showing β-like cells action. C. igneus contained insulin-like proteins (ILP) with hypoglycemic activities in insulin-responsive cell line RIN 5f. |
(Kattaru et al., 2021; Joshi et al., 2013) |
| Dandelion powder | Chloroform extract. | GLUT4 | Increased GLUT4 expression and membrane translocation via the AMPK pathway in L6 cells. | (Zhao et al., 2018b) |
| Folium sennae | Ethanol extract. | GLUT4 | Promotes membrane translocation and mRNA of GLUT4 via AMPK, AKT, and G protein-PLC-PKT pathways and internalization of C2+ in L6 cells. | (Zhao et al., 2018a) |
| Gundelia tournefortii | Hexane and methanol extract of the aerial part. | GLUT4 | Enhanced translocation of GLUT4 to the plasma membrane by the methanol extract than the hexane extract in L6 myotube cells. | (Kadan et al., 2018) |
| Kigelia pinnata | Isolated phytochemicals from ethanol extract of K. pinnata twigs. | GLUT4 | Increased GLUT4 translocation to the skeletal muscle cell surface in skeletal muscle cells. | (Faheem et al., 2012) |
| Mangifera indica | Ethylacetate extract and 3β-taraxerol. | GLUT4 | GLUT4 translocation and glycogen synthesis in 3T3-L1 adipocytes. | (Nandabalan, Sujatha & Shanmuganathan, 2010) |
| Maydis stigma [corn silk] | Extracted polysaccharides. | GLUT4 | Membrane translocation of GLUT4 in rats L6 skeletal muscle and regulation of PI3K/AKT pathways. | (Guo et al., 2019) |
| Mitragyna speciosa | Water, methanol extract, and mitragynine [a principal constituent]. | GLUT1 | Increased GLUT1 content in rat L6 myotubes. | (Purintrapiban et al., 2011) |
| Momordica balsamina | ethanol, ethyl acetate, and n-hexane fruit extract | GLUT2 | Increased GLUT2 gene expression | (Kgopa, Shai & Mogale, 2020) |
| Momordica charantia | Aqueous and chloroform extract of the fruit. | GLUT4 | Increased glucose uptake with GLUT4, PPARγ, and PI3K mRNA gene expression in L6 myotube cells. | (Kumar et al., 2009) |
| Moringa concanensis | Leaf extract | GLUT4 via PPARγ effects | 3T3-L1 adipocytes, enhanced GLUT4 gene expression | (Balakrishnan, Krishnasamy & Choi, 2018) |
| Morus alba | Ethanol leaf extract. | GLUT4 | Stimulation of glucose uptake and GLUT4 translocation to the plasma membrane via activation of PI3K in rat adipocytes. | (Naowaboot et al., 2012) |
| Nymphaea nouchali | Seed extracts | GLUT4 via PPARγ effects | Increased GLUT4 mRNA expression | (Parimala et al., 2015) |
| Ocimum basilicum | Methanol, hexane, and dichloromethane are extracts of the stem, leaf, and flowers. | GLUT4 | Elevated GLUT4 translocation to the plasma membrane HepG2 and rat L6 muscle cells. | (Kadan et al., 2016) |
| Panax ginseng [black ginseng] | Ethanolic extract of black ginseng. | GLUT4 | Increased phosphorylation of AMPK, increased upregulation of GLUT2 in the liver and GLUT4 in the muscle. | (Kang et al., 2017) |
| Pinus pinea [pine] | Bark extract | MAPK and glucose uptake | Activation of p38MAPK, which in turn activates SGLT1 and GLUT2 in Caco-2 cells. | (El-Zein & Kreydiyyeh, 2011) |
| Portulaca oleracea and Coccinia grandis | Plant extract. | GLUT4 | PI3K mediated GLUT4 translocation in insulin-sensitive CHO-K 1 cells and adipocytes. | (Stadlbauer et al., 2016) |
| Rosemary | Carnosol [diterpene] found in Rosemary. | GLUT4 | AMP-dependent increase GLUT4 translocation in L6 skeletal muscle cells. | (Vlavcheski et al., 2018) |
| Salacia oblonga | Hot water extract of the root, stem, and mangiferin, the bioactive compound. | GLUT4 | GLUT4 and concomitant phosphorylation of 5’AMP-activated protein kinase in L6 myotubes and 3T3- adipocytes. | (Giro et al., 2009) |
| Selaginella tamariscina | Selaginellins and bioflavonoids from methanol extract. | PTP1B and glucose uptake | Glucose uptake and inhibition of PTP1B in 3T3-L1 adipocytes | (El-Zein & Kreydiyyeh, 2011; Giro et al., 2009; Nguyen et al., 2015a) |
| Sinocrassula indica Berge | Ethanolic extract | GLUT1, GLUT4 | Increased glucose uptake in L6 myotubes and H4IIE hepatoma cells | (Yin et al., 2009) |
| Gymnema sylvestre | Methanolic leaf extract | GLUT4 | Enhanced glucose uptake in L6 myotubes cells | (Kumar et al., 2016) |
In one of the most comprehensive studies, Schreck & Melzig (2021) used Caco-2 cells exposed to a range of plant extracts to identify potential inhibitors of glucose transport. They reported between 40% to 80% reduction using the methanolic extracts of a range of plants including the fruits of Aronia melanocarpa, Valcheva-Kusmanova et al. (2007) Cornus officinalis, Crataegus pinnatifida, Lycium chinense, and Vaccinium myrtillus; the leaves of Brassica oleracea, Juglans regia, Peumus boldus, and the roots of Adenophora triphylla. The authors also reported 50% to 70% reduction by aqueous extract from the bark of Eucommia ulmoides and fruit skin of Malus domestica. These effects are likely acting via inhibition of GLUT1, the predominant transporter in these cells.
One of the key facets of insulin action is to drive the delivery of GLUT4 molecules from intracellular stores to the surface of fat and muscle cells, a process called ‘translocation’. Stadlbauer et al. (2021) used CHO-K1 cells expressing GLUT4 and total internal reflection microscopy to identify GLUT4 translocation-inducing effects of some thirty plant extracts. Though the taxonomy of some of the plants were not fully defined, they included Hoodia, Sapindus mukorossi, Quillaja saponaria, Papaver, Castanea, Bitter orange (genus and species not specified), Oregon grape (genus and species not specified), Common daisy flowers, Rosebay willowherb leaves and Goldenrod flower as potential compounds that could be exploited as potential anti-hyperglycemic agents in the treatment of T2DM via effects on GLUT4 redistribution.
While the above study used a non-classical insulin target tissue (for good experimental reasons), others have focused upon more physiological cell systems. Through bioassay-guided fractionation, Kanaujia et al. (2010) reported two chebulinic acid derivatives from Capparis moonii with significant stimulatory effects on glucose uptake effects concomitant with increased IR-β, Insulin receptor substrate-1 (IRS-1) phosphorylation, and mRNA expression of GLUT4 and PI3K in L6 muscle cells. Carnosol from rosemary extract stimulated AMPK-dependent GLUT4 translocation with no effect on Akt phosphorylation in L6 myotubes (Vlavcheski et al., 2018). Methanolic extract of Gundelia tournefortii potentiated insulin-stimulated GLUT4 translocation to the plasma membrane in skeletal muscle L6 cells (Kadan et al., 2018). An aqueous extract of Cassia abbreviata induced a two-fold increase in GLUT4 translocation in C2C12 (mouse) skeletal muscle cells probably via activation of the canonical PI3K/Akt pathway (Kamatou, Ssemakalu & Shai, 2021).
Naowaboot and colleagues reported the mechanism of antihyperglycemic effects of Morus alba leaf extract, including increasing glucose uptake via activation of the PI3K pathway and the plasma membrane translocation of GLUT4 in rat adipocytes (Naowaboot et al., 2012). Ethyl acetate extract and 3β-taraxerol isolated from Mangifera indica promoted increased GLUT4 translocation and glycogen synthesis in 3T3-L1 adipocytes (Nandabalan, Sujatha & Shanmuganathan, 2010). The study also noted the effect on glycogen synthesis was due to PI3K-dependent activation of Akt with subsequent inactivation of glycogen synthase kinase 3B (GSK3β) phosphorylation (discussed further below). The fruit of Citrullus colocynthis enhanced insulin-induced GLUT4 translocation and Akt phosphorylation in 3T3-L1 adipocytes (Drissi et al., 2021).
Stadlbauer et al. (2016) screened further natural products as alternatives to insulin through quantitation of GLUT4 translocation in insulin-sensitive CHO-K1 and importantly extended their remarkable study into commercial adipocyte cells. Of the seven medicinal plants tested, Portulaca oleracea and Coccinia grandis were found to induce GLUT4 translocation together with increased glucose concentration uptake likely mediated by PI3K/Akt pathway in adipocytes (Stadlbauer et al., 2016).
Such findings, together with the many others noted in Table 1 and which space preclude detailed discussion of here, suggest natural products can drive re-distribution of GLUT4 to the plasma membrane in an insulin-mimetic manner. However, the effects are not confined to GLUT4. For example, Kang and colleagues reported upregulation of GLUT2 in the liver (and up-regulated GLUT4 in muscle) as the possible mechanism of the antidiabetic effects of Panax ginseng (black ginseng). Manzano & Williamson (2010) investigated the glucose uptake inhibition of polyphenols, phenolic acid, and tannins from strawberry (var. Abion) and apple (var. golden delicious) in Caco-2 intestinal cell monolayers and reported increased inhibition of GLUT2 and SGLT1 and reduced glucose intestinal bilayer transport. Enhancement of glucose uptake by mitragyna speciosa and mitragynine in rat L6 myotubes is associated with increased GLUT1 protein content (Purintrapiban et al., 2011). And the glucose uptake (GLUT4) enhancement activity of G. sylvestre in L6 myotubes alongside the amelioration of insulin resistance in the 3T3-L1 adipocytes cells have been reported (Kumar et al., 2016). One further study of note is the report that the methanol extract of the aerial part of Selaginella tamariscina enhanced glucose uptake in 3T3-L1 adipocytes, possibly by inhibition of PTP1B (El-Zein & Kreydiyyeh, 2011; Giro et al., 2009). Collectively, these studies reveal that medicinal plants have a range of action on glucose transport across multiple tissues of relevance to the treatment of diabetes.
Medicinal plants and phytochemical effects on PI3K and Akt activity
In the canonical insulin signaling pathway controlling glucose homeostasis, insulin receptor substrates are phosphorylated on tyrosine residues which then act as docking sites for downstream signaling molecules. Of particular note, IRS-1 recruits phosphoinositide 3-kinase (PI3K) (Petersen & Shulman, 2018). PI3K phosphorylates phosphatidylinositol 4,5-biphosphate to form phosphatidyl-inositol 3,4,5-trisphosphate that in turn promotes Akt phosphorylation and activation (World Health Organization, 2019; Luna-Vital & De Mejia, 2018). Akt is an important nexus on the insulin signaling cascade because of its multi-substrate activities (Naowaboot et al., 2012; Nandabalan, Sujatha & Shanmuganathan, 2010; Tonks et al., 2013; Huang et al., 2018). Phosphorylation of Akt initiates a cascade of downstream events through many substrates, including phosphorylation of Akt substrate of 160 kDa (AS160), a RabGAP (GTPase activating protein); this in turn leads to GLUT4 translocation in muscle and adipose cells (Matschinsky, 2005; Sharma et al., 2021). Defects in Akt phosphorylation, as seen in impaired insulin activation, are associated with development of muscle and adipose insulin resistance in obesity and T2DM (Naowaboot et al., 2012; Joshi et al., 2013; Luna-Vital & De Mejia, 2018; Kim et al., 1999). The insulin PI3K/Akt pathway has been widely targeted in T2DM pharmacotherapy (World Health Organization, 2019; Naowaboot et al., 2012; Luna-Vital & De Mejia, 2018) and is important in the pathophysiology and therapy of other diseases (Nurcahyanti et al., 2021). Evidence of medicinal plants and phytochemicals modifying PI3K/Akt actions have been documented and are presented in Table 2. Some examples are briefly outlined below.
Table 2. Medicinal plants modifying PI3K/Akt activity.
Shown are studies in which the indicated plants have been shown to drive activation of the canonical insulin signalling molecules PI3K and Akt. Table is constructed in alphabetical order of plant species.
| Medicinal plant | Phytochemistry | Target | Summary | References |
|---|---|---|---|---|
| Anemarrhena asphodeloides Bunge | Monosaccharides | PI3K | Activation of PI3K/Akt, IRS-1 signaling pathway, and inhibition of α-glucosidase activity in HepG2 cells. | (Chen et al., 2022) |
| Broussonetia kazinoki | A flavan [Kazinol B] purified the root. | Akt | Improved insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. | (Lee et al., 2016) |
| Dendrobium officinale | Polysaccharide | PI3K | Increased PI3K/Akt phosphorylation in insulin-resistant HepG2 cells. | (Wang et al., 2018) |
| Folium sennae | Ethanol extract. | Akt | Increased AMPK, Akt, and PKC phosphorylation in L6 rat skeletal muscle. | (Zhao et al., 2018a) |
| Grifola frondosa | Heteropolysaccharide of the fruiting body. | PI3K | Increased mRNA of IRS1/PI3K, and downregulation of JNK1 signaling in HepG2 cells. | (Chen et al., 2018) |
| Juniperus chinensis | α- Methyl artoflavano coumarin from Juniperus chinensis. | Akt | PI3K/Akt and inhibition of PTP1B in HepG2 cells. | (Jung et al., 2017) |
| Mangifera indica | Ethyl acetate extract [EAE] and 3β-taraxerol phytochemistry. | PI3K | Increased PI3K level and GLUT4 translocation in 3T3-L1 adipocytes. | (Nandabalan, Sujatha & Shanmuganathan, 2010) |
| Maydis stigma [corn silk] | Maydis stigma [corn silk] extract. | Akt | A dose-dependent increase in expression of p-Akt/Akt in L6 skeletal muscle myotubes. | (Guo et al., 2019) |
| Nigella glandulifera | Alkaloids from the seeds. | PI3K | Increased PI3K/Akt pathway together with inhibition of PTP1B. | (Tang et al., 2017) |
| Nigella glandulifera | Norditerpenoid alkaloids of the seeds. | Akt | PI3K/Akt pathway, inhibition of PTP1B, increased glycogen synthesis with hexokinase activity in L6 myotubes | (Tang et al., 2017) |
| Sargassum pallidum | Homogeneous polysaccharides. | PI3K | Upregulation of PI3K, GS, and IRS-1 gene expression in insulin-resistant HepG2 cells. | (Cao et al., 2019) |
| Zhenjiang aromatic vinegar | Polyphenol-rich extract. | PI3K | Activation of PI3K/Akt pathway in IR-HepG2 cells. | (Xia et al., 2021) |
An ethyl acetate extract and 3β-taraxerol of Mangifera indica significantly activate GLUT4 translocation via a PI3K dependent pathway in 3T3-L1 adipocytes (Nandabalan, Sujatha & Shanmuganathan, 2010). Polysaccharides from corn silk (Maydis stigma) increased phosphorylation of Akt in a dose-dependent manner in L6 skeletal muscle myotubes (Guo et al., 2019). Similarly, phosphorylation of Akt in response to an extract of Folium sennae was described, together with a significant enhancement of GLUT4 translocation (Zhao et al., 2018a). Antidiabetic effect of alkaloids from the seeds of Nigella glandulifera increased the activity of the PI3K/Akt pathway in L6 myotubes with a concomitant increase in glycogen synthesis and hexokinase activity (Tang et al., 2017). Four C21 steroidal glycosides A-D from G.sylvestre promoted GLUT4 translocation to the plasma membrane in L6 cells via activation of PI3K/AKT (Li et al., 2019a). These and other studies (Table 2) point to a significant number of useful PI3K/Akt modulators within medicinal plants.
Many studies have examined effects of medicinal plants in hepatoma cells, as liver is a key site of post-prandial glucose disposal with a notable emphasis on PI3K/Akt signaling (Table 2). Heteropolysaccharides of Grifola frondosa (edible mushroom) increased IRS1/PI3K mRNA levels and enhanced insulin sensitivity (Chen et al., 2018). Polysaccharides of Dendrobium officinale increased the activity of PI3K and Akt and partially ameliorated symptoms in diabetic mice, pointing to a clear effect in a complex organism rather than simply in cell culture models (Wang et al., 2018). Inhibition of phosphorylated insulin receptor substrate-1(IRS-1), but activation of PI3K/Akt in insulin-resistant HepG2 cells by polyphenol-rich extract of Zhenjiang aromatic vinegar has been documented (Xia et al., 2021). Homogeneous polysaccharides from Sargassum pallidum ameliorate insulin resistance by upregulation of PI3K, Glycogen synthase (GS), and IRS-1 expression in insulin-resistant HepG2 cells (Cao et al., 2019). Monosaccharides from Anemarrhena asphodeloides Bunge exhibited hypoglycemic effects by activating PI3K/Akt, IRS-1signaling pathway, and inhibiting α-glucosidase activities in insulin-resistant-HepG2 cells (Chen et al., 2022). A natural flavonocoumarin (α-Methylartoflavonocoumarin) isolated from Juniperus chinensis was reported to activate PI3K/Akt pathway in insulin-resistant HepG2 cells (Jung et al., 2017). Thus, effects are evident both at the level of gene expression and activity mediated by a range of extracts.
Activity of medicinal plants and phytochemicals on glucokinase
Glucokinase (GCK) is important in the regulation of glucose metabolism in liver and pancreas. GCK is essential for pancreatic insulin secretion and hepatic insulin action via phosphorylation of glucose to glucose 6-phosphate (Naowaboot et al., 2012; Balakrishnan, Krishnasamy & Choi, 2018). Low GCK levels have been observed in T2DM (Haeusler et al., 2015) and could serve as a potential drug target for therapeutic intervention (Kim et al., 2013; Yang, Jang & Hwang, 2012). GCK is currently being targeted as therapeutic for T2DM (Matschinsky, 2009; Matschinsky & Wilson, 2019). Glucokinase activators have been advocated as an alternative approach to restoring and improving glycemic control in T2DM (Zhou et al., 2001; Towler & Hardie, 2007; Toulis et al., 2020). Several medicinal plants and phytochemicals with glucokinase activities have been recognized (Sharma et al., 2021). We have focused on the cellular and molecular glucokinase activities of medicinal plants in tissue culture models (Table 3).
Table 3. Medicinal plants with activities on glucokinase (GCK).
Shown are studies in which the indicated plants have been shown to drive changes in glucokinase activity or expression. Table is constructed in alphabetical order of plant species.
| Medicinal plant | Phytochemistry | Summary | References |
|---|---|---|---|
| Costus igneus (insulin plant) | Leaf extract. | Increased glucokinase activity, insulin, and GLUT2 gene expression but inhibition of glucose-6-phosphatase activity in human hematopoietic stem cells (HSCs) showing β-like cells action. C. igneus contained insulin-like proteins (ILP) with hypoglycemic activities in insulin-responsive cell line RIN 5f. |
(Kattaru et al., 2021; Joshi et al., 2013) |
| Momordica balsamina | Ethanol, ethyl acetate, and n-hexane fruit extract. | Increased GCK and GLUT2 mRNA gene expression in RIN-m5F cells. | (Kgopa, Shai & Mogale, 2020) |
| Zea mays (Purple corn) | Anthocyanins from the pericarp. | Activation of GCK in HepG2 cells decreased glucose uptake in Caco-2 cells and increased glucose-stimulated insulin secretion in iNS-1E in the pancreas. | (Luna-Vital & De Mejia, 2018) |
Glucokinase activation by the leaf extract of Costus igneus (known in India as the ‘insulin plant’ for its purported anti-diabetic action) was examined in differentiated human hematopoietic stem cell (HSCs) as a model of β-cells. The extract increased GCK and inhibited of glucose-6-phosphatase activity by C. igneus, thereby improving glucose sensing, insulin production, and decreased gluconeogenesis (Kattaru et al., 2021). It was also reported that C. igneus profoundly increased insulin receptor and GLUT2 gene expression (Table 1). Insulin-like proteins (ILP) purified from C. igneus also showed hypoglycemic activity in insulin-responsive cell line RIN 5f cells (Joshi et al., 2013). Activation of free fatty acid-receptor1 (FFAR1) and GCK by anthocyanin-rich extract from the pericarp of purple corn was demonstrated in HepG2 cells (Luna-Vital & De Mejia, 2018). Significant elevations in glucokinase gene expression in response to ethanol, ethyl acetate, and n-hexane fruit extract of Momordica balsamina, were reported in RIN-m5F cells (Kgopa, Shai & Mogale, 2020).
Medicinal plants modifying activity of glycogen synthase kinase-3 (GSK-3)
GSK-3 inhibits glycogen synthase activity. Insulin phosphorylates GSK-3 and prevents glycogen synthase inactivation (Nabben & Neumann, 2016). This role of GSK-3 in the insulin signaling pathway provides a mechanistic approach to the use of GSK-3 inhibitors in the treatment of insulin-resistant diabetes. Two studies are worthy of comment. Ethanolic extract of Shilianhua (Sinocrassula indica Berge) was found to induce GSK-3β phosphorylation similarly to insulin in 3T3-L1 preadipocytes and rat skeletal L6 myoblasts, indicating a possible mechanism of antidiabetic activity (Yin et al., 2009). This extract also enhanced insulin-stimulated glucose consumption in L6 myotubes and H4IIE hepatocytes, and insulin-independent glucose uptake in 3T3-L1 adipocytes. The result also showed increased GLUT1 protein expression in 3T3-L1 and GLUT4 protein expression in L6 myotubes cells (Yin et al., 2009). Hot water reduction from the root of Sarcopoterium spinosum increased glycogen synthesis via induction of GSK-3 β phosphorylation in L6 myotubes (Sahuc, 2016). S. spinosum also enhanced basal insulin secretion in the pancreatic β-cells and inhibited isoproterenol-induced lipolysis in 3T3-L1 adipocytes (Sahuc, 2016).
Peroxisome proliferator-activated receptor-gamma (PPARγ) and medicinal plants
Peroxisome proliferator-activated receptor gamma (PPARγ) is a member of the nuclear receptor super-family which play integral roles in glucose and lipid metabolism (Mirza, Althagafi & Shamshad, 2019). These receptors are targets for diabetes therapy and also for the treatment of cardiovascular disease, cancer, and inflammation (Mirza, Althagafi & Shamshad, 2019). We present the effects of various medicinal plants on PPARγ activity and gene expression (Table 4).
Table 4. Medicinal plants modifying activities of peroxisome proliferator-activated receptor-gamma (PPARγ).
Shown are studies in which the indicated plants have been shown to mediate effects probably via PPRγ. Table is constructed in alphabetical order of plant species. Where possible these have been alphabetized, but studies in which multiple plant species or extracts were used in a single study are shown at the top of the table.
| Medicinal plant | Phytochemistry | Summary | References |
|---|---|---|---|
|
Yeongyang korea (Korea red pepper), Capsicum annuum |
Ethanol extract. | Increased PPARγ and AMPK phosphorylation in C2C12 myotubes. | (Yang, Jang & Hwang, 2012) |
| Boehmeria nivea | Ethanol leaf extract. | Increased mRNA levels of PPARγ in C2C12 myotubes cells. | (Kim et al., 2013) |
| Miconia sp. | Ethanol extract of the aerial part. | Increased PPARγ mRNA and GLUT4 in 3T3-L1 adipocytes. | (Ortíz-Martinez et al., 2016) |
| Momordica charantia | Chloroform extract of the fruit. | Increased mRNA gene expression of PPARγ in L6 myotube skeletal muscle cells, as well as GLUT4 and PI3K. | (Kumar et al., 2009) |
| Moringa concanensis | Leaf extract. | Upregulation of mRNA of PPARγ, GLUT4, FAS, Tsrebp, DAG, and Akt signaling in 3T3-L1 adipocytes. | (Balakrishnan, Krishnasamy & Choi, 2018) |
| Nymphaea nouchali | Seed extract. | Increased mRNA of PPARγ and GLUT4 in 3T3-L1 adipocytes. | (Parimala et al., 2015) |
| Punica granatum | Flower aqueous extract and ethyl acetate fraction. | Increased mRNA PPARγ gene and protein expression in TPH-1-derived macrophage cell line. | (Huang et al., 2005) |
Chloroform extract of the fruit of Momordica charantia has been reported to significantly increase PPARγ gene expression 2.8-fold, comparable to the insulin sensitizer rosiglitazone (2.4-fold) in L6 myotube skeletal muscle cells (Kumar et al., 2009). Huang et al. (2005) demonstrated that Punica granatum flower extract and ethyl acetate fractions enhanced PPARγ gene expression and protein levels in a macrophage cell line. Increased mRNA of PPARγ (and GLUT4) by Nymphaea nouchali seed extract in 3T3-L1 adipocytes as the possible mechanism of its anti-hyperglycemic effect was reported (Parimala et al., 2015). Exposure of 3T3-L1 adipocytes to an ethanol extract of Miconia increased mRNA of PPARγ by 1.4-fold and inhibited α-amylase and α-glucosidase. The extract also increased lipid accumulation by around 30% as a possible anti-diabetic mechanism of action (Ortíz-Martinez et al., 2016). Upregulation of PPARγ together with GLUT4, SREBP and FAS expression was observed in 3T3-L1 adipocytes treated with the leaf extract of Moringa concanensis (Balakrishnan, Krishnasamy & Choi, 2018). Effects in muscle models have also been reported: Kim et al. (2013) reported increased transcription activity and mRNA levels of PPARγ in C2C12 myotubes by Boehmeria nivea ethanol leaf extract and Korean red peppers (Yeongyang korea) increased glucose uptake in C2C12 via increased transcriptional activity of PPARγ (Yang, Jang & Hwang, 2012).
AMP-activated protein kinase (AMPK)
AMP-activated protein kinase (AMPK) is a known energy sensor for metabolic homeostasis (Steinberg & Carling, 2019) which plays a central role in regulating lipid and protein metabolism together with fatty acid oxidation and muscle glucose uptake (Sathishsekar & Subramanian, 2005). AMPK plays a crucial role in insulin sensitivity, which explains its place as a potential drug candidate for T2DM therapy (Tasic et al., 2021; Hawkins et al., 2021). AMPK systems have been said to be partly responsible for the health benefits of exercise and AMPK is an important downstream effector of metformin. It has also been proposed as a possible target for novel drugs in managing obesity, type 2 diabetes, and metabolic syndrome (Hu, Zeng & Tomlinson, 2014; Hosseini et al., 2014; Kim et al., 2016). Ethnopharmacological investigators have reported that several medicinal plants modulate the activity of AMPK in cell models, and we have summarized these reports in Table 5, and highlight a few notable studies below.
Table 5. Medicinal plants regulating AMP-activated protein kinase (AMPK).
Shown are studies in which the indicated plants have been shown to mediate effects via AMPK activity. Table is constructed in alphabetical order of plant species.
| Medicinal plant | Phytochemistry | Summary | References |
|---|---|---|---|
| Artemisia dracunculus | Alcoholic extract (PMI-5011). | Increased insulin secretion through AMPK activation in NIT-1 cells. | |
| Artemisia sacrorum | Petroleum ether fraction. | Decreased glucose production via the AMPK-GSK-CREB pathway in HepG2 cells. | (Yuan & Piao, 2011) |
| Aspalathus linearis | 80% ethanol extract. | Amelioration of insulin resistance in C2C12 via activation of AMPK and Akt pathway. | (Mazibuko et al., 2013) |
| Cimicifuga racemosa | Ethanol extract and Phyto-compounds. | Increased AMPK activity in HepaRG cells. | (Moser et al., 2014) |
| Crocus sativus [Saffron] | Methanol extract. | Increased glucose uptake and insulin sensitivity via AMPK phosphorylation in C2C12 mouse myotubes cells. | (Kang et al., 2012) |
| Entada phaseoloides | Total saponin extract. | Suppression of hepatic gluconeogenesis via AMPK and Akt/GSK3β in Primary hepatocytes and HepG2 cells. | (Zheng et al., 2016) |
| Iris sanguinea | Isolated compounds from methanol extract of the seeds. | Increased glucose uptake via activation of ACC and AMPK in mouse C2C12 skeletal myoblast. | (Yang et al., 2017) |
| Malva verticillata | Ethanol extract and compound isolate [β-sitosterol]. | Increased glucose uptake via AMPK phosphorylation in L6 myotubes. | (Jeong & Song, 2011) |
| Momordica charantia | Triterpenoids from the stem. | Overcome insulin resistance via AMPK activation in FL83B and C2C12 cells. | (Cheng et al., 2008) |
| Psidium guajava | Flavonoids from the leaves. | AMPK phosphorylation in rat L6 myotubes and L02 human hepatic cells. | (Li et al., 2019b) |
| Rhodiola crenulata | Methanol extract. | Inhibition of gluconeogenesis in human hepatic HepG2 cell via activation of AMPK. | (Lee et al., 2015) |
| Rosmarinus officinalis | Dichloromethane-methanol extract. | Regulate glucose and lipid metabolism through activation of AMPK and PPAR pathways in HepG2 cells. | (Tu et al., 2013) |
| Sechium edule | Water and polyphenol extract of the shoot. | Inhibition of lipogenesis and stimulation of lipolysis via AMPK activation and decreased lipogenic enzymes in HepG2 cells. | (Wu et al., 2014, 2020) |
| Stauntonia chinensis | Triterpenoid saponins. | Increased glucose uptake in HepG2 insulin-resistant cells via AMPK phosphorylation and IR, IRS-1, PI3K/Akt pathways | (Hu et al., 2014) |
| Toona sinensis | Leaf extract. | Increased glucose uptake in C2C12 myotubes due to AMPK activation. | (Liu et al., 2015) |
| Vigna angularis (Azuki bean) | Extract | Increased phosphorylation of AMPK and Akt in HepG2 cells. | (Sato et al., 2016) |
Ethanolic extract and phytochemical compounds from Cimicifuga racemosa mediate increased AMPK activity in fully differentiated HepaRG cells and is a possible mechanism of antidiabetic activity (Moser et al., 2014). Yuan & Piao (2011) reported activation of AMPK by the petroleum ether fraction of Artemisia sacrorum in HepG2 cells. They showed increased phosphorylation of AMPK (on T172), acetyl-CoA carboxylase (ACC; reside S79), and GSK-3β and reported concomitant downregulation of phosphoenolpyruvate carboxykinase (PEPCK), and glucose-6-phosphatase (G6Pase). Similarly, Zheng et al. (2016) exploring the anti T2DM activity of Entada phaseoloides in primary mouse hepatocytes and HepG2 cells, reported suppression of hepatic gluconeogenesis via activation of the AMPK signaling pathway and Akt/GSK-3β. Extract of Rosmarinus officinalis significantly increased glucose consumption in HepG2 cells via increased phosphorylation of AMPK and ACC and potentially increased liver glycolysis and fatty acid oxidation (Tu et al., 2013). Thus, numerous examples of medicinal plants exerting effects via AMPK in hepatoma cell lines have been described (Table 5).
Effects mediated via AMPK have been reported in other cell types. These include an alcoholic extract of Artemisia dracunculus enhanced insulin release from β-cells isolated from mouse and human islets via activation of AMPK and suppressed LPS/IFNγ-induced inflammation. Effects on glucose transport in muscle lines include an extract of Crocus sativus (saffron) which increased glucose uptake and insulin sensitivity in C2C12 myotubes by increased phosphorylation of AMPK in a dose and time-dependent manner (Kang et al., 2012) and compounds isolated from the seed of Iris sanguinea was reported to be via AMPK and ACC phosphorylation in the same cell type (Yang et al., 2017).
Ethanolic extract and isolated compound (β-sitosterol) from Malva verticillata seed significantly increased activation of AMPK as the molecular mechanism for glucose uptake in L6 myotubes (Jeong & Song, 2011). Triterpenoids from the stem of Momordica charantia have been reported to overcome insulin resistance in FL83B and C2C12 via AMPK activation (Cheng et al., 2008). Hence, the effects of such compounds on AMPK is an active and vigorous area of research.
Studies in animal models
The process of drug development encompasses pre-clinical experimentation (in vitro, in silico, and in vivo) leading ultimately to clinical trials in humans. To understand if there is ongoing vertical research towards developing antidiabetic agents from these medicinal plants, we reviewed their exploitation in experimental animal models as summarized in Table 6. Some highlights are discussed below.
Table 6. Medicinal plants having antidiabetic activity in tissue culture and whole animal biology.
Studies of medicinal plants with demonstrated anti-diabetic properties are listed. Plants are arranged in alphabetical order. The animal model studies are cross-referenced to cellular studies of the same extract/plant wherever possible.
| Medicinal plants | Phytochemistry | Animal model | Summary | Animal study |
Cell study |
|---|---|---|---|---|---|
|
Yeongyang korea (Korea red pepper), Capsicum annuum |
Seed extract. | Mice | Improved glycemic control, decreased hepatic gluconeogenesis, and increased FOXO1 and AMPK phosphorylation. | (Kim et al., 2020) | (Yang, Jang & Hwang, 2012) |
| Anemarrhena asphodeloides | Glycosides | Mice | Inhibition of hepatic gluconeogenesis/glycogenolysis. | (Nakashima et al., 1993) | (Nurcahyanti et al., 2021) |
| Annona stenophylla | Aqueous root extract. | Rats | Decreased glucose level. | (Taderera, Gomo & Shoriwa Chagonda, 2016) | (Taderera et al., 2019) |
| Apios americana | Flower or methanolic extract of the flower. | Mice | Decreased plasma glucose level. | (Kawamura et al., 2015) | (Yan et al., 2017) |
| Aronia melanocarpa | Fruit juice. | Rats | Decreased plasma glucose and triglycerides in diabetic rats. | (Lee et al., 2016; Mazibuko et al., 2013; Mu et al., 2020) | (Schreck & Melzig, 2021) |
| Artemisia dracunculus | Ethanolic extract. | Mice | Lowered glucose and PEPCK concentrations. | (Ribnicky et al., 2006) | |
| Aspalathus linearis | Tea extract. | Mice | Improved impaired glucose tolerance. | (Kawano et al., 2009) | (Mazibuko et al., 2013) |
| Boehmeria nivea | Methanol extract of the root. | Wistar rats | Restore normal glucose, lipids, and antioxidants level. | (Sancheti et al., 2011) | (Kim et al., 2013) |
| Brassica oleracea | Raw sprouts. | Rats | Decreased blood glucose, glycated hemoglobin, and hepatoprotection. | (Sahai & Kumar, 2020) | (Schreck & Melzig, 2021) |
| Cimicifuga racemosa | Rhizomes and root extract. | Mice | Reduced body weight, plasma, glucose, and increased insulin sensitivity. | (Moser et al., 2014) | (Moser et al., 2014) |
| Cinnamomum cassia | Bark extract. | Diabetic mice | Decreased blood glucose and triglycerides levels. | (Kim, Hyun & Choung, 2006) | (Lakshmi et al., 2009) |
| Citrullus colocynthis | Fruit ethanol extract. | Albino rats | Reduced blood glucose and improved pathology. | (Oryan et al., 2014) | (Drissi et al., 2021) |
| Costus igneus (insulin plant) | Powdered leaves. | Rats | Decreased fasting and postprandial glucose level | (Shetty et al., 2010) | (Kattaru et al., 2021) |
| Crataegus pinnatifida | Fruit extract. | Mice | Decreased glucose production and triglyceride synthesis via AMPK phosphorylation. | (Shih et al., 2013) | (Schreck & Melzig, 2021) |
| Crocus sativus | Hydroethanolic extract of aerial parts. | Rats | Reduced blood glucose and improved diabetic complications. | (Ouahhoud et al., 2019) | (Kang et al., 2012) |
| Curcuma longa | Curcuminoids and sesquiterpenoids from rhizome solvent fractions. | Mice | Decreased blood glucose levels and stimulation of adipocyte differentiation. | (Nishiyama et al., 2005) | (Kim et al., 2010) |
| Dendrobium officinale | Stem extract. | Rats | Reduced blood glucose, total cholesterol, triglycerides, and LDLP-C. | (Chen et al., 2020) | (Wang et al., 2018) |
| Entada phaseoloides | Entagenic acid from seed kernel. | Mice | Improved blood glucose, insulin resistance, and changes in pancreatic islets. | (Xiong et al., 2018) | (Zheng et al., 2016) |
| Eucommia ulmoides | Leaves | Rats and Mice | Hypoglycemia and hypolipidemic effects in streptozotocin-induced hyperglycemia. | (Nakashima et al., 1993; Taderera, Gomo & Shoriwa Chagonda, 2016; Park et al., 2006; Lee et al., 2005) | (Schreck & Melzig, 2021) |
| Gundelia tournefortii | Water extract. | Mice | Decreased blood glucose level, body weight, triglycerides, and cholesterol, but increased renal protection. | (Sancheti et al., 2011; Sahai & Kumar, 2020; Mohammadi & Zangeneh, 2018; Azeez & Kheder, 2012) | (Kadan et al., 2018) |
| Juglans regia | Leaves and ridges. | Mice Rats |
Decreased blood glucose, hepatic phosphoenolpyruvate carboxykinase, glycogen phosphorylase activity, glycosylated hemoglobin, LDL, triglycerides, and total cholesterol. | (Kamyab et al., 2010; Liu et al., 2015; Sato et al., 2016) | (Schreck & Melzig, 2021) |
| Juniperus chinensis | Berries ethanol extract. | Rats | Improved blood glucose level and other diabetic parameters. | (Ju et al., 2008) | (Jung et al., 2017) |
| Kigelia pinnata | Methanolic extract of the flower. | Rats | Decreased blood glucose, serum cholesterol, and triglycerides. | (Kumar, Kumar & Prakash, 2012) | (Faheem et al., 2012) |
| Malva verticulata | Tea | Mice | Decreased blood glucose, LDL-C, and total cholesterol and increased HDL-C and leptin. | (Bano & Akhter, 2021) | (Jeong & Song, 2011) |
| Mangifera indica | Aqueous extract of the leaves. | Rats | Decreased fasting blood glucose level. | (Madhuri & Mohanvelu, 2017) | (Nandabalan, Sujatha & Shanmuganathan, 2010) |
| Momordica charantia | Aqueous seed extract. | Rats | Reduced blood glucose, glycosylated hemoglobin, lactate dehydrogenase, glucose-6-phosphatase, fructose-1,6-biphosphatase, and glycogen phosphorylase, but increases the activities of glycogen synthase and hexokinase. | (Sathishsekar & Subramanian, 2005) | (Kumar et al., 2009) |
| Momordica charantia | Saponins | Rats | Decreased fasting blood glucose, triglycerides, total cholesterol, and increased insulin content and sensitivity. | (Jiang et al., 2020) | (Cheng et al., 2008) |
| Morus alba | Polysaccharides from fruit. | Rats | Reduced blood glucose and lipid levels. | (Jiao et al., 2017) | (Naowaboot et al., 2012) |
| Ocimum basilicum | Aerial parts. | Rats | Inhibition of glycogenolysis. | (Ezeani et al., 2017) | (Kadan et al., 2016) |
| Opuntia ficus-indica | Powder or water extract of the stem. | Rats | It inhibits α-glucosidase and reduces blood glucose levels. | (Hwang, Kang & Lim, 2017) | (Leem et al., 2016) |
| Panax ginseng | Ethanol extract of the seed. | Obese diabetic mice | Increased insulin-stimulated glucose disposal, energy expenditure, and reduced cholesterol levels. | (Attele et al., 2002; Shalaby & Hammouda, 2013) | (Kang et al., 2017) |
| Peumus boldus | Boldine alkaloid from the leaves and bark. | Rats | Dose-dependent decrease in oxidative markers and mitochondrial protection | (Jang et al., 2000) | (Schreck & Melzig, 2021) |
| Portulaca oleracea | Aqueous extract. | Male Wistar rats | Decreased Hb A1C, serum glucose level, TNF-α, and IL-6. | (Ramadan, Schaalan & Tolba, 2017) | (Stadlbauer et al., 2016) |
| Psidium guajava | Leaf extract. | Rats | Antidiabetic | (Mazumdar, Akter & Talukder, 2015) | (Li et al., 2019b) |
| Punica granatum | Fruit aqueous extract. | Wistar rats | Reduces fasting blood glucose and lipid levels. | (Gharib & Kouhsari, 2019) | (Huang et al., 2005) |
| Rhodiola crenulata | Methanol root extract. | Mice | Decreased postprandial blood glucose. | (Yue et al., 2022) | (Lee et al., 2015) |
| Rosmarinus officinalis | Water extract. | Rats | Decreased blood sugar level and oxidative stress markers. | (Khalil et al., 2012) | (Vlavcheski et al., 2018) |
| Salacia oblonga | Water extract of the root. | Obese Zucker rats | Improved interstitial and perivascular fibrosis and inhibition of postprandial hyperglycemia. | (Li et al., 2004) | (Giro et al., 2009) |
| Sapindus mukorossi | Fruit | Rats | Decreased glucose and lipid levels. | (Verma et al., 2012) | (Stadlbauer et al., 2021) |
| Sarcopoterium spinosum | Aqueous extract. | Mice | Prevents diabetes progression. | (Smirin et al., 2010) | (Elyasiyan et al., 2017) |
| Sechium edule | Methanol and ethyl acetate fraction. | Rats | Antidiabetic and antioxidant. | (Siahaan et al., 2020) | (Wu et al., 2014) |
| Selaginella tamariscina | Total flavonoids | Rats | Decreased plasma FBG, HbA1c, triglycerides, total cholesterol, FFA with increased insulin, HDL-C, and C-peptides. | (Zheng et al., 2011) | (Nguyen et al., 2015b) |
| Stauntonia chinensis | Total saponins from the stem. | Mice | Hypoglycemic and hypolipidemic. | (Xu et al., 2018) | (Hu et al., 2014) |
| Toona sinensis | Quercetin from the leaves. | Mice | Antidiabetic and antioxidant. | (Zhang et al., 2016) | (Liu et al., 2015) |
| Trigonella foenum-graecum | Seed powder. | Female Albino rats | Reduced elevated fasting blood glucose and enzyme levels. | (Raju et al., 2001) | (Chen et al., 2022) |
| Urtica dioica | Aqueous extract of the aerial parts. | Wistar rats and Swiss mice | Decreased glucose level in oral glucose tolerant test [OGTT]. | (Bnouham et al., 2003) | (Chen et al., 2022) |
| Vaccinium myrtillus | Fruit | Rats | Decreased total cholesterol, LDL-C, VLDL-C, and triglycerides in alloxan-induced hyperglycemic rats. | (Asgary et al., 2016) | (Schreck & Melzig, 2021) |
| Vigna angularis | Hot water extract and polysaccharides from the leaves. | Mice and Rats | Reduced FBG, an triglycerides, but increased HDL-C, and reduction in diabetes progression. | (Zheng et al., 2011; Xu et al., 2018; Itoh et al., 2009) | (Sato et al., 2016) |
| Zea mays (Purple corn) | Extract | Mice | Decreased fasting blood glucose, HbA1c, and PEPCK, increased insulin secretion, AMPK and GLUT4 in diabetic mice. | (Huang et al., 2015) | (Luna-Vital & De Mejia, 2018) |
| Gymnema sylvestre | Phytoconstituents | Rats | Reduced hyperglycemia via through PI3K/AKT activation | (Li et al., 2019a) | (Retz & Glucose, 2021) |
Aronia melanocarpa fruit juice was found to mediate a dose-dependent decrease in plasma glucose and triglyceride levels in streptozotocin-induced hyperglycemic rats (Lee et al., 2016; Mazibuko et al., 2013), corresponding to the observations on glucose transport in Caco-2 cells alluded to above (Table 1) (Schreck & Melzig, 2021). Similarly, the antidiabetic and hyperlipidemic effects of Crataegus pinnatifida were investigated in high fat-fed mice. The results showed decreased glucose production and triglyceride synthesis via induction of AMPK phosphorylation (Shih et al., 2013), compared with inhibition of SGLT1 and GLUT2 in Caco-2 cells (Schreck & Melzig, 2021). These provide a good example of studies in cell lines being translated into animal models.
The fruit of Vaccinium myrtillus was reported to significantly reduce serum glucose, total cholesterol, low density lipoprotein cholesterol, and very low density lipoprotein cholesterol, and triglycerides in alloxan-induced hyperglycemic adult male Wistar rats (Asgary et al., 2016).
Juglans regia extracts reduce blood glucose levels in diabetic mice (Kamyab et al., 2010), ameliorated streptozotocin-induced diabetic peripheral neuropathy in rats (Nasiry et al., 2017), and significantly decreased blood glucose, glycosylated hemoglobin, LDL, triglycerides, and total cholesterol in Wistar rats (Mohammadi et al., 2011). An aqueous extract of the seeds of Momordica charantia reduced blood glucose level, glycosylated hemoglobin, lactate dehydrogenase, glucose-6-phosphatase, fructose-1,6-biphosphatase, and glycogen phosphorylase but increased the activities of glycogen synthase and hexokinase in streptozotocin-induced diabetic rats, providing clear evidence of a systematic and programmed action on key metabolic activities (Sathishsekar & Subramanian, 2005). Similarly, polysaccharides of Dendrobium officinale reduced blood glucose level, glycated serum protein, total cholesterol, LDL-C, and increased HDL-C in type 2 diabetic rats (Chen et al., 2020). Cimicifuga racemosa extracts from rhizomes and roots reduced body weight, plasma glucose, improved glucose metabolism, and increased insulin sensitivity in obese diabetic mice (Moser et al., 2014). Tarralin™, an ethanolic extract of Artemisia dracunculus, significantly lowered blood glucose concentrations and PEPCK in diabetic KK-Ay mice (Ribnicky et al., 2006). Li et al. (2019a) described the effects of Gymnemic acid isolated from G. sylvestre on insulin signalling pathways in the type 2 diabetic rats as activation of PI3K/AKT together with AMPK phosphorylation. Such studies exemplify the power of medicinal plants in the amelioration of metabolic disturbances, and Table 5 summarizes the wide array of studies relevant to diabetes research.
Medicinal plants in clinical trials
The process of drug discovery necessitates that a drug molecule or product that has successively passed through the preclinical stage of drug development is carefully tested in clinical trials. We reviewed those plants that progressed to clinical trials and present our findings in Table 7. As this area is particularly important, we have provided some detail of key studies in the sections below.
Table 7. Medicinal plant antidiabetics from cell-biology to clinical trial.
Studies in which the indicated plants were examined in clinical trials.
| Medicinal plants | Phytochemistry Product |
Clinical trial | References |
|---|---|---|---|
| Aronia melanocarpa | Alixir 400 PROTECT® [Standardized extract] |
Prospective open-label trial of 148 patients. | (Tasic et al., 2021) |
| Cinnamomum cassia | Extract and 1,000 mg capsule. | Randomized-placebo control of 70 patients and another study of 19 subjects. | (Hasanzade et al., 2013, Mustafa et al., 2017) |
| Citrullus colocynthis | Fruit capsule. | Randomized clinical trial of 50 T2D patients. | (Jang et al., 2008) |
| Crataegus pinnatifida | Multi-herb | Randomized double-blind, placebo-controlled trial of 40 patients. | (Hu, Zeng & Tomlinson, 2014) |
| Curcuma longa | 500 mg/day | Randomized double blind, placebo-controlled trial of 71 patients | (Neta et al., 2021) |
| Gundelia tournefortii | 250 mg of hydroalcoholic extract of the aerial parts. | Randomized double-blind, placebo-controlled trial of 38 patients. | (Hajizadeh-Sharafabad et al., 2016) |
| Juglans regia | Leaf extract 100 mg twice daily and a hydroalcoholic leaf extract. | Randomized double-blind, placebo-controlled trial of 61 and 50 patients. | (Taghizadeh et al., 2022; López-Romero et al., 2014) |
| Mangifera indica | Low dose [0.5 g/kg] and high dose [1 g/kg] of the leaf extract. | Clinical investigation of 26 T2DM patients. | (Waheed, Miana & Ahmad, 2006) |
| Momordica charantia | 2,000 mg/day of dried powder of fruit. | Randomized, double-blind, placebo-controlled trial of 24 patients. | (Cortez-Navarrete et al., 2018) |
| Morus alba | 300 mg extract. | Randomized clinical trial of 60 type 2 diabetic patient [T2DM]. | (Taghizadeh et al., 2022) |
| Ocimum basilicum | Raw and processed seeds. | 45 days clinical trial using convenient sampling. | (Arivuchudar, Nazni & Uvaraj, 2022) |
| Opuntia ficus-indica | Nopal [Opuntia ficus-indica preparation]. | Clinical study. | (López-Romero et al., 2014) |
| Panax ginseng | Extract of fermented root and Korean red ginseng preparation. | Randomized clinical trial of 42 subjects and double-blind randomized crossover design of 19 subjects. | (Vuksan et al., 2008) |
| Portulaca oleracea | Seeds | Clinical study of 30 patients and randomized trial of 74 subjects. | (El-Sayed, 2011; Darvish Damavandi et al., 2021) |
| Punica granatum | Dried flower mouth wash. | Randomized trial of 80 diabetes patients with gingivitis. | (Sedigh-Rahimabadi et al., 2017) |
| Salacia oblonga | Extract [240, 480 mg/kg] | Randomized double-blind crossover trial of 60 patients. | (Williams et al., 2007) |
| Trigonella foenum-graecum | Seed capsule. | Multicenter randomized, placebo-controlled, double-blind, add-on clinical trial of 154 T2D patients. Another 12 weeks trial of 12 patients. | (Verma et al., 2016; Najdi et al., 2019) |
| Urtica dioica | Ethanolic extract. | Double-blind, randomized trial of 50 diabetic women. | (Amiri Behzadi, Kalalian-Moghaddam & Ahmadi, 2016) |
| Gymnema sylvestre | Leaf water extract | 22 non-insulin dependent diabetic patients | (Baskaran et al., 1990) |
Prospective open-label clinical trials of Alixir 400 PROTECT® (standardized extract of Aronia melanocarpa) in 143 patients demonstrated controlled glycemia, blood pressure improvement, and beneficial effects on LDL-C, triglycerides and total cholesterol, and was of significant (p < 0.05) overall benefit in diabetic hypertensive patients (Tasic et al., 2021). Similarly, a meta-analysis of controlled clinical trials carried out on Aronia melanocarpa daily supplementation revealed significant (p < 0.05) decreases in total cholesterol, blood pressure and a reduction in cardiovascular and diabetic risk factors, clearly supporting a useful role in therapy (Hawkins et al., 2021).
Lipid lowering effects are also a common feature of clinical trials with medicinal plants. Hu, Zeng & Tomlinson (2014) demonstrated the beneficial effects of a multi-herb formula containing Crataegus pinnatifida for dyslipidemia in a randomized double-blind, placebo-controlled trial, reporting decreased plasma lipids, glucose levels, HbA1c, and LDL-C at 95% CI. A randomized, double-blind placebo-controlled trial of Juglans regia leaf extract resulted in a significant decrease (p < 0.05) in fasting blood glucose levels, triglycerides, total cholesterol and HbA1c compared with placebo (Hosseini et al., 2014). Similar beneficial effects were reported in further trials (Rabiei et al., 2018) including beneficial effects in patients with coronary artery disease with significant decreases (p = 0.04) in total cholesterol, BMI, and LDL (Hajizadeh-Sharafabad et al., 2016). A clinical investigation of oral administration of Portulaca oleracea seeds in 30 T2DM subjects revealed a significant decrease (p < 0.001) in serum levels of triglycerides, total cholesterol, LDL-C, and liver enzymes, but increased (p > 0.001) levels of HDL-C and albumin (El-Sayed, 2011). Purslane (Portulaca oleracea capsule) also drove a significant difference (p > 0.01) in the triglycerides, liver enzymesand fasting blood glucose in 74 people with T2DM in a randomized double-blind, placebo-controlled clinical trial. An improvement in both insulin resistance, and LDL-C levels was also reported (Darvish Damavandi et al., 2021).
Decreased blood glucose levels are often used as a key outcome. A 14-day clinical investigation involving 26 people with T2DM on a low (0.5 g/kg) and high (1 g/kg) doses of aqueous and alcoholic extract of the powdered leaves of Mangifera indica showed a significant decrease in blood glucose levels in all groups (Waheed, Miana & Ahmad, 2006). Similarly, both raw and processed seeds of Ocimum basilicum in patients with diabetes and dyslipidemia revealed beneficial effects including decreased blood glucose, a reduction in body mass index, triglycerides, LDL-C, and decreased HDL-C at 5% and 1% levels of significant (Arivuchudar, Nazni & Uvaraj, 2022). A significant decrease (p < 0.05) in the fasting blood glucose and HbA1c levels were observed in people with T2DM after 2 month treatment with the fruit capsule of Citrullus colocynthis (Jang et al., 2008).
In a randomized, double-blind, crossover study of 60 diabetic subjects receiving Salacia oblonga extract, Williams et al. (2007) reported significant decrease (p < 0.05) in glyceamia and insulinemia in patients after high carbohydrate meal. A 4-week randomized double-blind, placebo-controlled clinical trial of fermented red ginseng (Panax ginseng) involving 42 patients with impaired fasting glucose or T2DM also showed significant decrease (p < 0.01) in postprandial glucose levels and increased postprandial insulin levels compared to the placebo group (Oh et al., 2014). A further study supported these conclusions (Vuksan et al., 2008).
Many other studies, highlighted in Table 7, have shown pronounced and beneficial effects.
Not all data are conclusive. The results of a 60-day randomized-placebo clinical trial by Hasanzade and colleagues using Cinnamomum cassia in 70 people with T2D revealed no significant difference (p > 0.05) between the test and placebo (Hasanzade et al., 2013). On the other hand, Hoehn and Stockert in a smaller trial reported significant decrease in blood sugar levels of the patients taking 1,000 mg Cinnamomum cassia capsule for 12 weeks (Hoehn & Stockert, 2012). An aqueous extract of G. sylvestre (GS4) 400 mg/day used over 18 to 20 months supplementation drove significant decreases (p < 0.001) in blood glucose, glycosylated haemoglobin and glycosylated plasma protein in 22 patients (Baskaran et al., 1990). It should also be clearly noted that many of the clinical studies performed to date include relatively small numbers of patients. Larger studies will provide impetus for more work in this area.
Conclusion and future perspectives
Diabetes mellitus-related morbidity and mortality continues to increase globally and necessitates urgent action to identify and drive novel therapies which can be widely used in under-developed economies. Our review reveals that antidiabetic drugs of herbal origin can play a modulatory role in insulin signaling pathways and drive metabolically relevant changes in insulin action, such as elevated glucose transport. Tissue culture systems have provided key insight into the molecular mechanisms of the phytochemicals beneficial to diabetic patients and have contributed both mechanistic insight and facilitated the development of more clinically-facing treatments. Among the plants we reviewed in tissue culture systems, close to half (45%) have been investigated for their antidiabetic activities in mammals (rats, mice, and rabbits) and 4% have been tested in human clinical trials. The positive outcomes reported in these clinical trials should be recognized as providing a new impetus to phytobiology research as an effective treatment for insulin resistance and diabetes. In future, larger-scale clinical trials are clearly warranted given the largely positive effects of many of these natural products. There is a need to screen larger numbers and citizens of different genetic backgrounds to identify potential population-specific benefits. Similarly, the coupling of phytochemical studies to genomic data may offer a powerful means to develop combination therapies and more personalized medicine approaches.
List of Abbreviations
- ACC
Acetyl-CoA carboxylase
- Akt
Protein kinase B
- AMPK
Adenosine monophosphate-activated protein kinase
- AS160
Akt substrate 160 kDa
- ATPase
Adenosine triphosphatase
- CI
Confidence interval
- GCK
Glucokinase
- GLP-1
Glucagon-like peptides-1
- GLUT
Glucose transporter
- GSK-3
Glycogen synthase kinase 3
- GSV
Glucose storage vesicle
- HbA1c
Hemoglobin A1c
- HDL
High-density lipoprotein
- HSC
Hematopoietic stem cell
- IFN
Interferon
- IR
Insulin receptor
- IRS
Insulin receptor substrate
- LDL
Low-density lipoprotein
- LPS
Lipopolysaccharide
- PEPCK
Phosphoenolpyruvate carboxykinase
- PI3K
Phosphoinositide-3 kinase
- PPARγ
Peroxisome proliferator-activated receptor gamma
- PTP1B
Protein tyrosine phosphate 1B
- SGLT1
Sodium-glucose linked transporter1
- T1DM
Type 1 diabetes mellitus
- T2DM
Type 2 diabetes mellitus
Funding Statement
This work was supported by a grant from the African Research Excellence Fund to Simeon Omale and Gwyn Gould (AREF-308-OMALE-F-C0818) and a grant from Diabetes UK (to Gwyn W Gould; 18/0005847). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
Gwyn W. Gould is an Academic Editor for PeerJ.
Author Contributions
Simeon Omale conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Kennedy I. Amagon conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Titilayo O. Johnson conceived and designed the experiments, performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Shaun Kennedy Bremner analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Gwyn W. Gould conceived and designed the experiments, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review.
References
- Ahmad, Choi & Lee (2020).Ahmad K, Choi I, Lee YH. Implications of skeletal muscle extracellular matrix remodeling in metabolic disorders: diabetes perspective. International Journal of Molecular Sciences. 2020;21(11):3845. doi: 10.3390/ijms21113845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad et al. (2015).Ahmad L, Semotiuk A, Zafar M, Ahmad M, Sultana S, Liu Q-R, Zada MP, Abidin SZU, Yaseen G. Ethnopharmacological documentation of medicinal plants used for hypertension among the local communities of DIR lower, Pakistan. Journal of Ethnopharmacology. 2015;175(3):138–146. doi: 10.1016/j.jep.2015.09.014. [DOI] [PubMed] [Google Scholar]
- Al-Amin et al. (2006).Al-Amin ZM, Thomson M, Al-Qattan KK, Peltonen-Shalaby R, Ali M. Anti-diabetic and hypolipidaemic properties of ginger (Zingiber officinale) in streptozotocin-induced diabetic rats. British Journal of Nutrition. 2006;96(4):660–666. doi: 10.1079/BJN20061849. [DOI] [PubMed] [Google Scholar]
- Amiri Behzadi, Kalalian-Moghaddam & Ahmadi (2016).Amiri Behzadi A, Kalalian-Moghaddam H, Ahmadi AH. Effects of Urtica dioica supplementation on blood lipids, hepatic enzymes and nitric oxide levels in type 2 diabetic patients: a double blind, randomized clinical trial. Avicenna Journal of Phytomedicine. 2016;6(6):686–695. [PMC free article] [PubMed] [Google Scholar]
- Arivuchudar, Nazni & Uvaraj (2022).Arivuchudar R, Nazni P, Uvaraj MG. Experimental investigation on materialistic attributes of Ocimum basilicum seed supplementation on diabetic subjects. Materials Today: Proceedings. 2022;66(81):670–674. doi: 10.1016/j.matpr.2022.03.638. [DOI] [Google Scholar]
- Asgary et al. (2016).Asgary S, Rafieiankopaei M, Sahebkar A, Shamsi F, Goli-malekabadi N. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit) Journal of the Science of Food and Agriculture. 2016;96(3):764–768. doi: 10.1002/jsfa.7144. [DOI] [PubMed] [Google Scholar]
- Attele et al. (2002).Attele AS, Zhou Y-P, Xie J-T, Wu JA, Zhang L, Dey L, Pugh W, Rue PA, Polonsky KS, Yuan C-S. Antidiabetic effects of Panax ginseng berry extract and the identification of an effective component. Diabetes. 2002;51(6):1851–1858. doi: 10.2337/diabetes.51.6.1851. [DOI] [PubMed] [Google Scholar]
- Azeez & Kheder (2012).Azeez OH, Kheder AE. Effect of Gundelia tournefortii on some biochemical parameters in dexamethasone-induced hyperglycemic and hyperlipidemic mice. Iraqi Journal of Veterinary Sciences. 2012;26(2):73–79. doi: 10.33899/ijvs.2012.67458. [DOI] [Google Scholar]
- Bailey (2015).Bailey CJ. The current drug treatment landscape for diabetes and perspectives for the future. Clinical Pharmacology & Therapeutics. 2015;98(2):170–184. doi: 10.1002/cpt.144. [DOI] [PubMed] [Google Scholar]
- Balakrishnan, Krishnasamy & Choi (2018).Balakrishnan BB, Krishnasamy K, Choi KC. Moringa concanensis nimmo ameliorates hyperglycemia in 3T3-L1 adipocytes by upregulating PPAR-γ, C/EBP-α via Akt signaling pathway and STZ-induced diabetic rats. Biomedicine & Pharmacotherapy. 2018;103(February):719–728. doi: 10.1016/j.biopha.2018.04.047. [DOI] [PubMed] [Google Scholar]
- Balunas & Kinghorn (2005).Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sciences. 2005;78(5):431–441. doi: 10.1016/j.lfs.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Bano & Akhter (2021).Bano F, Akhter N. Ginseng malva verticillata tea (GMVT) improve glucose and lipid metabolism by up-regulation of leptin hormone in overweight rats. Lahore Garrison University Journal of Life Sciences. 2021;5(2):89–98. doi: 10.54692/lgujls.2021.0502151. [DOI] [Google Scholar]
- Baskaran et al. (1990).Baskaran K, Ahamath BK, Shanmugasundaram KR, Shanmugasundaram ERB. Antidiabetic effect of a leaf extract from Gymnema sylvestre in non-insulin-dependent diabetes mellitus patients. Journal of Ethnopharmacology. 1990;30(3):295–305. doi: 10.1016/0378-8741(90)90108-6. [DOI] [PubMed] [Google Scholar]
- Bnouham et al. (2003).Bnouham M, Merhfour FZ, Ziyyat A, Mekhfi H, Aziz M, Legssyer A. Antihyperglycemic activity of the aqueous extract of Urtica dioica. Fitoterapia. 2003;74(7–8):677–681. doi: 10.1016/S0367-326X(03)00182-5. [DOI] [PubMed] [Google Scholar]
- Bommer et al. (2017).Bommer C, Heesemann E, Sagalova V, Manne-Goehler J, Atun R, Bärnighausen T, Vollmer S. The global economic burden of diabetes in adults aged 20–79 years: a cost-of-illness study. The Lancet Diabetes & Endocrinology. 2017;5(6):423–430. doi: 10.1016/S2213-8587(17)30097-9. [DOI] [PubMed] [Google Scholar]
- Campbell (2000).Campbell IW. Antidiabetic drugs present and future: will improving insulin resistance benefit cardiovascular risk in type 2 diabetes mellitus? Drugs. 2000;60(5):1017–1028. doi: 10.2165/00003495-200060050-00004. [DOI] [PubMed] [Google Scholar]
- Cao, Graves & Anderson (2010).Cao H, Graves DJ, Anderson RA. Cinnamon extract regulates glucose transporter and insulin-signaling gene expression in mouse adipocytes. Phytomedicine. 2010;17(13):1027–1032. doi: 10.1016/j.phymed.2010.03.023. [DOI] [PubMed] [Google Scholar]
- Cao, Polansky & Anderson (2007).Cao H, Polansky MM, Anderson RA. Cinnamon extract and polyphenols affect the expression of tristetraprolin, insulin receptor, and glucose transporter 4 in mouse 3T3-L1 adipocytes. Archives of Biochemistry and Biophysics. 2007;459(2):214–222. doi: 10.1016/j.abb.2006.12.034. [DOI] [PubMed] [Google Scholar]
- Cao et al. (2019).Cao C, Zhang B, Li C, Huang Q, Fu X, Liu RH. Structure and: in vitro hypoglycemic activity of a homogenous polysaccharide purified from Sargassum pallidum. Food & Function. 2019;10(5):2828–2838. doi: 10.1039/C8FO02525H. [DOI] [PubMed] [Google Scholar]
- Cetrone, Mele & Tricarico (2014).Cetrone M, Mele A, Tricarico D. Effects of the antidiabetic drugs on the age-related atrophy and sarcopenia associated with diabetes type II. Current Diabetes Reviews. 2014;10(4):231–237. doi: 10.2174/1573399810666140918121022. [DOI] [PubMed] [Google Scholar]
- Chaudhury et al. (2017).Chaudhury A, Duvoor C, Reddy Dendi VS, Kraleti S, Chada A, Ravilla R, Marco A, Shekhawat NS, Montales MT, Kuriakose K, Sasapu A, Beebe A, Patil N, Musham CK, Lohani GP, Mirza W. Clinical review of antidiabetic drugs: implications for type 2 diabetes mellitus management. Front Endocrinol (Lausanne) 2017;8(January):6. doi: 10.3389/fendo.2017.00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen et al. (2018).Chen Y, Liu Y, Sarker MMR, Yan X, Yang C, Zhao L, Lv X, Liu B, Zhao C. Structural characterization and antidiabetic potential of a novel heteropolysaccharide from Grifola frondosa via IRS1/PI3K-JNK signaling pathways. Carbohydrate Polymers. 2018;198(15):452–461. doi: 10.1016/j.carbpol.2018.06.077. [DOI] [PubMed] [Google Scholar]
- Chen et al. (2020).Chen H, Nie Q, Hu J, Huang X, Huang W, Nie S. Metabolism amelioration of dendrobium officinale polysaccharide on type II diabetic rats. Food Hydrocolloids. 2020;102(September 2019):105582. doi: 10.1016/j.foodhyd.2019.105582. [DOI] [Google Scholar]
- Chen et al. (2022).Chen J, Wan L, Zheng Q, Lan M, Zhang X, Li Y, Li B, Li L. Structural characterization and in vitro hypoglycaemic activity of glucomannan from Anemarrhena asphodeloides Bunge. Food & Function. 2022;13(4):1797–1807. doi: 10.1039/D1FO03010H. [DOI] [PubMed] [Google Scholar]
- Cheng et al. (2008).Cheng HL, Huang HK, Chang CI, Tsai CP, Chou CH. A cell-based screening identifies compounds from the stem of Momordica charantia that overcome insulin resistance and activate AMP-activated protein kinase. Journal of Agricultural and Food Chemistry. 2008;56(16):6835–6843. doi: 10.1021/jf800801k. [DOI] [PubMed] [Google Scholar]
- Chien et al. (2009).Chien S-C, Young PH, Hsu Y-J, Chen C-H, Tien Y-J, Shiu S-Y, Li T-H, Yang C-W, Marimuthu P, Tsai LF-L, Yang W-C. Anti-diabetic properties of three common Bidens pilosa variants in Taiwan. Phytochemistry. 2009;70(10):1246–1254. doi: 10.1016/j.phytochem.2009.07.011. [DOI] [PubMed] [Google Scholar]
- Chukwuma et al. (2019).Chukwuma CI, Matsabisa MG, Ibrahim MA, Erukainure OL, Chabalala MH, Islam MS. Medicinal plants with concomitant anti-diabetic and anti-hypertensive effects as potential sources of dual acting therapies against diabetes and hypertension: a review. Journal of Ethnopharmacology. 2019;235(october 2018):329–360. doi: 10.1016/j.jep.2019.02.024. [DOI] [PubMed] [Google Scholar]
- Cortez-Navarrete et al. (2018).Cortez-Navarrete M, Martínez-Abundis E, Pérez-Rubio KG, González-Ortiz M, Méndez-Del Villar M. Momordica charantia administration improves insulin secretion in type 2 diabetes mellitus. Journal of Medicinal Food. 2018;21(7):672–677. doi: 10.1089/jmf.2017.0114. [DOI] [PubMed] [Google Scholar]
- Darvish Damavandi et al. (2021).Darvish Damavandi R, Shidfar F, Najafi M, Janani L, Masoodi M, Akbari‐Fakhrabadi M, Dehnad A. Effect of Portulaca oleracea (purslane) extract on liver enzymes, lipid profile, and glycemic status in nonalcoholic fatty liver disease: a randomized, double-blind clinical trial. Phytotherapy Research. 2021;35(6):3145–3156. doi: 10.1002/ptr.6972. [DOI] [PubMed] [Google Scholar]
- Drissi et al. (2021).Drissi F, Lahfa F, Gonzalez T, Peiretti F, Tanti JF, Haddad M, Fabred N, Goversb R. A Citrullus colocynthis fruit extract acutely enhances insulin-induced GLUT4 translocation and glucose uptake in adipocytes by increasing PKB phosphorylation. Journal of Ethnopharmacology. 2021;270(January):113772. doi: 10.1016/j.jep.2020.113772. [DOI] [PubMed] [Google Scholar]
- El-Sayed (2011).El-Sayed MIK. Effects of Portulaca oleracea L. seeds in treatment of type-2 diabetes mellitus patients as adjunctive and alternative therapy. Journal of Ethnopharmacology. 2011;137(1):643–651. doi: 10.1016/j.jep.2011.06.020. [DOI] [PubMed] [Google Scholar]
- El-Zein & Kreydiyyeh (2011).El-Zein O, Kreydiyyeh SI. Pine bark extract inhibits glucose transport in enterocytes via mitogen-activated kinase and phosphoinositol 3-kinase. Nutrition. 2011;27(6):707–712. doi: 10.1016/j.nut.2010.07.001. [DOI] [PubMed] [Google Scholar]
- Elyasiyan et al. (2017).Elyasiyan U, Nudel A, Skalka N, Rozenberg K, Drori E, Oppenheimer R, Kerem Z, Rosenzweig T. Anti-diabetic activity of aerial parts of Sarcopoterium spinosum. BMC Complementary and Alternative Medicine. 2017;17(1):1–12. doi: 10.1186/s12906-017-1860-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezeani et al. (2017).Ezeani C, Ezenyi I, Okoye T, Okoli C. Ocimum basilicum extract exhibits antidiabetic effects via inhibition of hepatic glucose mobilization and carbohydrate metabolizing enzymes. Journal of Intercultural Ethnopharmacology. 2017;6(1):22–28. doi: 10.5455/jice.20161229054825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faheem et al. (2012).Faheem M, Dixit P, Jaiswal N, Kumar A, Kumar A, Maurya R. Fitoterapia chemical constituents of kigelia pinnata twigs and their GLUT4 translocation modulatory effect in skeletal muscle cells. Fitoterapia. 2012;83(1):125–129. doi: 10.1016/j.fitote.2011.10.002. [DOI] [PubMed] [Google Scholar]
- Farzaei et al. (2017).Farzaei F, Morovati MR, Farjadmand F, Farzaei MH. A mechanistic review on medicinal plants used for diabetes mellitus in traditional persian medicine. Journal of Evidence-Based Integrative Medicine. 2017;22(4):944–955. doi: 10.1177/2156587216686461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galochkina et al. (2019).Galochkina T, Ng Fuk Chong M, Challali L, Abbar S, Etchebest C. New insights into GluT1 mechanics during glucose transfer. Scientific Reports. 2019;9(1):1–14. doi: 10.1038/s41598-018-37367-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvey et al. (1998).Garvey WT, Maianu L, Zhu JH, Brechtel-Hook G, Wallace P, Baron AD. Evidence for defects in the trafficking and translocation of GLUT4 glucose transporters in skeletal muscle as a cause of human insulin resistance. Journal of Clinical Investigation. 1998;101(11):2377–2386. doi: 10.1172/JCI1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gharib & Kouhsari (2019).Gharib E, Kouhsari SM. Study of the antidiabetic activity of Punica granatum L. Fruits aqueous extract on the alloxan-diabetic wistar rats. Iranian Journal of Pharmaceutical Research. 2019;18(1):358–368. [PMC free article] [PubMed] [Google Scholar]
- Giro et al. (2009).Giro D, Sevillano N, Salto R, Haidour A, Manzano M, Jiménez ML, Rueda R, López-Pedrosa JM. Salacia oblonga extract increases glucose transporter 4-mediated glucose uptake in L6 rat myotubes: role of mangiferin q. Clinical Nutrition. 2009;28(5):565–574. doi: 10.1016/j.clnu.2009.04.018. [DOI] [PubMed] [Google Scholar]
- Guo et al. (2019).Guo Q, Chen Z, Santhanam RK, Xu L, Gao X, Ma Q, Xue Z, Chen H. Hypoglycemic effects of polysaccharides from corn silk (Maydis stigma) and their beneficial roles via regulating the PI3K/Akt signaling pathway in L6 skeletal muscle myotubes. International Journal of Biological Macromolecules. 2019;121:981–988. doi: 10.1016/j.ijbiomac.2018.10.100. [DOI] [PubMed] [Google Scholar]
- Haeusler et al. (2015).Haeusler RA, Camastra S, Astiarraga B, Nannipieri M, Anselmino M, Ferrannini E. Decreased expression of hepatic glucokinase in type 2 diabetes. Molecular Metabolism. 2015;4(3):222–226. doi: 10.1016/j.molmet.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajizadeh-Sharafabad et al. (2016).Hajizadeh-Sharafabad F, Alizadeh M, Mohammadzadeh MHS, Alizadeh-Salteh S, Kheirouri S. Effect of gundelia tournefortii L. extract on lipid profile and TAC in patients with coronary artery disease: a double-blind randomized placebo controlled clinical trial. Journal of Herbal Medicine. 2016;6(2):59–66. doi: 10.1016/j.hermed.2016.02.001. [DOI] [Google Scholar]
- Harding et al. (2019).Harding JL, Pavkov ME, Magliano DJ, Shaw JE, Gregg EW. Global trends in diabetes complications: a review of current evidence. Diabetologia. 2019;62(1):3–16. doi: 10.1007/s00125-018-4711-2. [DOI] [PubMed] [Google Scholar]
- Hasanzade et al. (2013).Hasanzade F, Toliat M, Emami SA, Emamimoghaadam Z. The effect of cinnamon on glucose of type II diabetes patients. Journal of Traditional and Complementary Medicine. 2013;3(3):171–174. doi: 10.4103/2225-4110.114900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins et al. (2021).Hawkins J, Hires C, Baker C, Keenan L, Bush M. Daily supplementation with aronia melanocarpa (chokeberry) reduces blood pressure and cholesterol: a meta analysis of controlled clinical trials. Journal of Dietary Supplements. 2021;18(5):517–530. doi: 10.1080/19390211.2020.1800887. [DOI] [PubMed] [Google Scholar]
- Hedrington & Davis (2019).Hedrington MS, Davis SN. Considerations when using alpha-glucosidase inhibitors in the treatment of type 2 diabetes. Expert Opinion on Pharmacotherapy. 2019;20(18):2229–2235. doi: 10.1080/14656566.2019.1672660. [DOI] [PubMed] [Google Scholar]
- Hinnen et al. (2006).Hinnen D, Nielsen LL, Waninger A, Kushner P. Incretin mimetics and DPP-IV inhibitors: new paradigms for the treatment of type 2 diabetes. Journal of the American Board of Family Medicine. 2006;19(6):612–620. doi: 10.3122/jabfm.19.6.612. [DOI] [PubMed] [Google Scholar]
- Hoehn & Stockert (2012).Hoehn AN, Stockert AL. The effects of cinnamomum cassia on blood glucose values are greater than those of dietary changes alone. Nutrition and Metabolic Insights. 2012;5:77–83. doi: 10.4137/NMI.S10498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseini et al. (2014).Hosseini S, Jamshidi L, Mehrzadi S, Mohammad K, Najmizadeh AR, Alimoradi H, Huseini HF. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: a randomized double-blind, placebo-controlled clinical trial. Journal of Ethnopharmacology. 2014;152(3):451–456. doi: 10.1016/j.jep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- Hu et al. (2014).Hu X, Wang S, Xu J, Wang DB, Chen Y, Yang GZ. Triterpenoid saponins from Stauntonia chinensis ameliorate insulin resistance via the AMP-activated protein kinase and IR/IRS-1/PI3K/Akt pathways in insulin-resistant HepG2 cells. International Journal of Molecular Sciences. 2014;15(6):10446–10458. doi: 10.3390/ijms150610446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu, Zeng & Tomlinson (2014).Hu M, Zeng W, Tomlinson B. Evaluation of a Crataegus-based multiherb formula for dyslipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evidence-Based Complementary and Alternative Medicine. 2014;2014:365742. doi: 10.1155/2014/365742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2018).Huang X, Liu G, Guo J, Su ZQ. The PI3K/AKT pathway in obesity and type 2 diabetes. International Journal of Biological Sciences. 2018;14(11):1483–1496. doi: 10.7150/ijbs.27173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang et al. (2005).Huang T, Peng G, Kota B, Li G, Yamahara J, Roufogalis B, Li Y. Anti-diabetic action of Punica granatum flower extract: activation of PPAR-γ and identification of an active component. Toxicology and Applied Pharmacology. 2005;207(2):160–169. doi: 10.1016/j.taap.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Huang et al. (2015).Huang B, Wang Z, Park JH, Ryu OH, Choi MK, Lee J-Y, Kang Y-H, Lim SS. Anti-Diabetic effect of purple corn extract on C57BL/KsJ db/db mice. Nutrition Research and Practice. 2015;9(1):17–21. doi: 10.4162/nrp.2015.9.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang, Kang & Lim (2017).Hwang SH, Kang IJ, Lim SS. Antidiabetic effect of fresh Nopal (opuntia ficus-indica) in low-dose streptozotocin-induced diabetic rats fed a high-fat diet. Evidence-Based Complementary and Alternative Medicine. 2017;2017(4):1–8. doi: 10.1155/2017/4380721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh et al. (2009).Itoh T, Kobayashi M, Horio F, Furuichi Y. Hypoglycemic effect of hot-water extract of adzuki (Vigna angularis) in spontaneously diabetic KK-Ay mice. Nutrition. 2009;25(2):134–141. doi: 10.1016/j.nut.2008.08.001. [DOI] [PubMed] [Google Scholar]
- James, Stöckli & Birnbaum (2021).James DE, Stöckli J, Birnbaum MJ. The aetiology and molecular landscape of insulin resistance. Nature Reviews Molecular Cell Biology. 2021;22(11):751–771. doi: 10.1038/s41580-021-00390-6. [DOI] [PubMed] [Google Scholar]
- Jang et al. (2008).Jang MH, Piao XL, Kim JM, Kwon SW, Park JH. Inhibition of cholinesterase and amyloid-&bgr; aggregation by resveratrol oligomers from Vitis amurensis. Phytotherapy Research. 2008;22(4):544–549. doi: 10.1002/ptr.2406. [DOI] [PubMed] [Google Scholar]
- Jang et al. (2000).Jang YY, Song JH, Shin YK, Han ES, Lee CS. Protective effect of boldine on oxidative mitochondrial damage in streptozotocin-induced diabetic rats. Pharmacological Research. 2000;42(4):361–371. doi: 10.1006/phrs.2000.0705. [DOI] [PubMed] [Google Scholar]
- Jeong & Song (2011).Jeong YT, Song CH. Antidiabetic activities of extract from Malva verticillata seed via the activation of AMP-activated protein kinase. Journal of Microbiology and Biotechnology. 2011;21(9):921–929. doi: 10.4014/jmb.1104.04015. [DOI] [PubMed] [Google Scholar]
- Jiang et al. (2013).Jiang LQ, Duque-Guimaraes DE, MacHado UF, Zierath JR, Krook A. Altered response of skeletal muscle to IL-6 in type 2 diabetic patients. Diabetes. 2013;62(2):355–361. doi: 10.2337/db11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang et al. (2020).Jiang S, Xu L, Xu Y, Guo Y, Wei L, Li X, Song W. Antidiabetic effect of Momordica charantia saponins in rats induced by high-fat diet combined with STZ. Electronic Journal of Biotechnology. 2020;43(5):41–47. doi: 10.1016/j.ejbt.2019.12.001. [DOI] [Google Scholar]
- Jiao et al. (2017).Jiao Y, Wang X, Jiang X, Kong F, Wang S, Yan C. Antidiabetic effects of Morus alba fruit polysaccharides on high-fat diet- and streptozotocin-induced type 2 diabetes in rats. Journal of Ethnopharmacology. 2017;199(2016)):119–127. doi: 10.1016/j.jep.2017.02.003. [DOI] [PubMed] [Google Scholar]
- Joseph & Jini (2013).Joseph B, Jini D. Antidiabetic effects of Momordica charantia (bitter melon) and its medicinal potency. Asian Pacific Journal of Tropical Disease. 2013;3(2):93–102. doi: 10.1016/S2222-1808(13)60052-3. [DOI] [Google Scholar]
- Joshi et al. (2013).Joshi BN, Munot H, Hardikar M, Kulkarni AA. Orally active hypoglycemic protein from Costus igneus N. E. Br.: an in vitro and in vivo study. Biochemical and Biophysical Research Communications. 2013;436(2):278–282. doi: 10.1016/j.bbrc.2013.05.093. [DOI] [PubMed] [Google Scholar]
- Ju et al. (2008).Ju JB, Kim JS, Choi CW, Lee HK, Oh TK, Kim SC. Comparison between ethanolic and aqueous extracts from Chinese juniper berries for hypoglycaemic and hypolipidemic effects in alloxan-induced diabetic rats. Journal of Ethnopharmacology. 2008;115(1):110–115. doi: 10.1016/j.jep.2007.09.012. [DOI] [PubMed] [Google Scholar]
- Jung et al. (2017).Jung HJ, Seong SH, Ali MY, Min BS, Jung HA, Choi JS. α-Methyl artoflavanocoumarin from Juniperus chinensis exerts anti-diabetic effects by inhibiting PTP1B and activating the PI3K/Akt signaling pathway in insulin-resistant HepG2 cells. Archives of Pharmacal Research. 2017;40(12):1403–1413. doi: 10.1007/s12272-017-0992-0. [DOI] [PubMed] [Google Scholar]
- Kadan et al. (2013).Kadan S, Saad B, Sasson Y, Zaid H. In vitro evaluations of cytotoxicity of eight antidiabetic medicinal plants and their effect on GLUT4 translocation. Evidence-Based Complementary and Alternative Medicine. 2013;2013:549345. doi: 10.1155/2013/549345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadan et al. (2016).Kadan S, Saad B, Sasson Y, Zaid H. In vitro evaluation of anti-diabetic activity and cytotoxicity of chemically analysed Ocimum basilicum extracts. Food Chemistry. 2016;196(3):1066–1074. doi: 10.1016/j.foodchem.2015.10.044. [DOI] [PubMed] [Google Scholar]
- Kadan et al. (2018).Kadan S, Sasson Y, Saad B, Zaid H. Gundelia tournefortii antidiabetic efficacy. Chemical Composition and GLUT4 Translocation. 2018;2018:8294320. doi: 10.1155/2018/8294320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamatou, Ssemakalu & Shai (2021).Kamatou GP, Ssemakalu C, Shai LJ. Cassia abbreviata enhances glucose uptake and glucose transporter 4 translocation in C2C12 mouse skeletal muscle cells. Journal of Evidence-Based Integrative Medicine. 2021;26:1–11. doi: 10.1177/2515690X211006333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamyab et al. (2010).Kamyab H, Hejrati S, Khanavi M, Malihi F, Mohammadirad A, Baeeri M, Esmaily H, Abdollahi M. Hepatic mechanisms of the Walnut antidiabetic effect in mice. Central European Journal of Biology. 2010;5(3):304–309. doi: 10.2478/s11535-010-0019-z. [DOI] [Google Scholar]
- Kanaujia et al. (2010).Kanaujia A, Duggar R, Pannakal ST, Yadav SS, Katiyar CK, Bansal V, Anand S, Sujatha S, Lakshmi BS. Bioorganic & medicinal chemistry Insulinomimetic activity of two new gallotannins from the fruits of Capparis moonii. Bioorganic & Medicinal Chemistry. 2010;18(11):3940–3945. doi: 10.1016/j.bmc.2010.04.032. [DOI] [PubMed] [Google Scholar]
- Kanetkar, Singhal & Kamat (2007).Kanetkar P, Singhal R, Kamat M. Gymnema sylvestre: a memoir. Journal of Clinical Biochemistry and Nutrition. 2007;41(2):77–81. doi: 10.3164/jcbn.2007010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang et al. (2012).Kang C, Lee H, Jung E-S, Seyedian R, Jo MN, Kim J, Kim J-S, Kim E. Saffron (Crocus sativus L.) increases glucose uptake and insulin sensitivity in muscle cells via multipathway mechanisms. Food Chemistry. 2012;135(4):2350–2358. doi: 10.1016/j.foodchem.2012.06.092. [DOI] [PubMed] [Google Scholar]
- Kang et al. (2017).Kang O-H, Shon M-Y, Kong R, Seo Y-S, Zhou T, Kim D-Y, Kim Y-S, Kwon D-Y. Anti-diabetic effect of black ginseng extract by augmentation of AMPK protein activity and upregulation of GLUT2 and GLUT4 expression in db/db mice. BMC Complementary and Alternative Medicine. 2017;17(1):1–11. doi: 10.1186/s12906-017-1839-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattaru et al. (2021).Kattaru S, Manne Mudhu S, Echambadi Loganathan S, Kodavala S, Potukuchi VGKS. Increased insulin and GLUT2 gene expression and elevated glucokinase activity in β-like cells of islets of langerhans differentiated from human haematopoietic stem cells on treatment with Costus igneus leaf extract. Molecular Biology Reports. 2021;48(5):4477–4485. doi: 10.1007/s11033-021-06467-x. [DOI] [PubMed] [Google Scholar]
- Kawamura et al. (2015).Kawamura J, Miura E, Kawakishi K, Kitamura T, Morinaga Y, Norikura T, Matsue H, Iwai K. Investigation of the safety and antihyperglycemic effect of apios americana flower intake as a food material in normal and diabetic mice. Food Science and Technology Research. 2015;21(3):453–462. doi: 10.3136/fstr.21.453. [DOI] [Google Scholar]
- Kawano et al. (2009).Kawano A, Nakamura H, Hata S-I, Minakawa M, Miura Y, Yagasaki K. Hypoglycemic effect of aspalathin, a rooibos tea component from Aspalathus linearis, in type 2 diabetic model db/db mice. Phytomedicine. 2009;16(5):437–443. doi: 10.1016/j.phymed.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Kgopa, Shai & Mogale (2020).Kgopa AH, Shai LJ, Mogale MA. Momordica balsamina fruit extracts enhances selected aspects of the insulin synthesis/secretion pathway. American Journal of Biochemistry and Biotechnology. 2020;16(4):549–560. doi: 10.3844/ajbbsp.2020.549.560. [DOI] [Google Scholar]
- Khalil et al. (2012).Khalil OA, Ramadan K, Danial E, Alnahdi H, Ayaz N. Antidiabetic activity of Rosmarinus officinalis and its relationship with the antioxidant property. African Journal of Pharmacy and Pharmacology. 2012;6(14):1031–1036. doi: 10.5897/AJPP12.162. [DOI] [Google Scholar]
- Khan et al. (2012).Khan V, Najmi AK, Akhtar M, Aqil M, Mujeeb M, Pillai KK. A pharmacological appraisal of medicinal plants with antidiabetic potential. Journal of Pharmacy and Bioallied Sciences. 2012;4(1):27–42. doi: 10.4103/0975-7406.92727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharroubi (2015).Kharroubi AT. Diabetes mellitus: the epidemic of the century. World Journal of Diabetes. 2015;6(6):850. doi: 10.4239/wjd.v6.i6.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazir et al. (2014).Khazir J, Mir BA, Pilcher L, Riley DL. Role of plants in anticancer drug discovery. Phytochemistry Letters. 2014;7(1):173–181. doi: 10.1016/j.phytol.2013.11.010. [DOI] [Google Scholar]
- Kim, Hyun & Choung (2006).Kim SH, Hyun SH, Choung SY. Anti-diabetic effect of cinnamon extract on blood glucose in db/db mice. Journal of Ethnopharmacology. 2006;104(1–2):119–123. doi: 10.1016/j.jep.2005.08.059. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2020).Kim HK, Jeong J, Kang EY, Go GW. Red pepper (Capsicum annuum l.) seed extract improves glycemic control by inhibiting hepatic gluconeogenesis via phosphorylation of foxo1 and ampk in obese diabetic db/db mice. Nutrients. 2020;12(9):1–11. doi: 10.3390/nu12092546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (1999).Kim YB, Nikoulina SE, Ciaraldi TP, Henry RR, Kahn BB. Normal insulin-dependent activation of Akt/protein kinase B, with diminished activation of phosphoinositide 3-kinase, in muscle in type 2 diabetes. Journal of Clinical Investigation. 1999;104(6):733–741. doi: 10.1172/JCI6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2010).Kim JH, Park JM, Kim E-K, Lee JO, Lee SK, Jung JH, You GY, Park SH, Suh P-G, Kim HS. Curcumin stimulates glucose uptake through AMPK-p38 MAPK pathways in L6 myotube cells. Journal of Cellular Physiology. 2010;223(3):771–778. doi: 10.1002/jcp.22093. [DOI] [PubMed] [Google Scholar]
- Kim et al. (2013).Kim SH, Sung MJ, Park JH, Yang HJ, Hwang JT. Boehmeria nivea stimulates glucose uptake by activating peroxisome proliferator-activated receptor gamma in C2C12 cells and improves glucose intolerance in mice fed a high-fat diet. Evidence-Based Complementary and Alternative Medicine. 2013;2013:867893. doi: 10.1155/2013/867893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim et al. (2016).Kim J, Yang G, Kim Y, Kim J, Ha J. AMPK activators: mechanisms of action and physiological activities. Experimental and Molecular Medicine. 2016;48(4):1–12. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2009).Kumar R, Balaji S, Uma TS, Sehgal PK. Fruit extracts of Momordica charantia potentiate glucose uptake and up-regulate Glut-4, PPARγ and PI3K. Journal of Ethnopharmacology. 2009;126(3):533–537. doi: 10.1016/j.jep.2009.08.048. [DOI] [PubMed] [Google Scholar]
- Kumar, Kumar & Prakash (2012).Kumar S, Kumar V, Prakash OM. Antidiabetic and hypolipidemic activities of Kigelia pinnata flowers extract in streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Biomedicine. 2012;2(7):543–546. doi: 10.1016/S2221-1691(12)60093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar et al. (2016).Kumar PM, Venkataranganna MV, Manjunath K, Viswanatha GL, Ashok G. Methanolic leaf extract of Gymnema sylvestre augments glucose uptake and ameliorates insulin resistance by upregulating glucose transporter-4, peroxisome proliferator-activated receptor-gamma, adiponectin, and leptin levels in vitro. Journal of Intercultural Ethnopharmacology. 2016;5(2):146–152. doi: 10.5455/jice.20160224051727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda et al. (2003).Kuroda M, Mimaki Y, Sashida Y, Mae T, Kishida H, Nishiyama T, Tsukagawa M, Konishi E, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Phenolics with PPAR-γ ligand-Binding activity obtained from licorice (Glycyrrhiza uralensis Roots) and ameliorative effects of glycyrin on genetically diabetic KK-Ay mice. Bioorganic & Medicinal Chemistry Letters. 2003;13(24):4267–4272. doi: 10.1016/j.bmcl.2003.09.052. [DOI] [PubMed] [Google Scholar]
- Lai et al. (2015).Lai BY, Chen TY, Huang SH, Kuo TF, Chang TH, Chiang CK, Yang M-T, Chang C-L-T. Bidens pilosa formulation improves blood homeostasis and β-cell function in men: a pilot study. Evidence-Based Complementary and Alternative Medicine. 2015;2015:832314. doi: 10.1155/2015/832314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi et al. (2009).Lakshmi BS, Sujatha S, Anand S, SangeethA KN, Narayanan RB, Katiyar C, Kanaujia A, Duggar R, Singh Y, Srinivas K, Bansal V, Sarin S, Tandon R, Sharma S, Singh S. Cinnamic acid, from the bark of Cinnamomum cassia, regulates glucose transport via activation of GLUT4 on L6 myotubes in a phosphatidylinositol 3-kinase-independent manner. Journal of Diabetes. 2009;1(2):99–106. doi: 10.1111/j.1753-0407.2009.00022.x. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2005).Lee M-K, Kim M-J, Cho S-Y, Park SA, Park K-K, Jung UJ, Park H-M, Choi M-S. Hypoglycemic effect of Du-zhong (Eucommia ulmoides Oliv.) leaves in streptozotocin-induced diabetic rats. Diabetes Research and Clinical Practice. 2005;67(1):22–28. doi: 10.1016/j.diabres.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2015).Lee SY, Lai FY, Shi LS, Chou YC, Yen IC, Chang TC. Rhodiola crenulata extract suppresses hepatic gluconeogenesis via activation of the AMPK pathway. Phytomedicine. 2015;22(4):477–486. doi: 10.1016/j.phymed.2015.01.016. [DOI] [PubMed] [Google Scholar]
- Lee et al. (2016).Lee H, Li H, Jeong JH, Noh M, Ryu JH. Kazinol B from Broussonetia kazinoki improves insulin sensitivity via Akt and AMPK activation in 3T3-L1 adipocytes. Fitoterapia. 2016;112:90–96. doi: 10.1016/j.fitote.2016.05.006. [DOI] [PubMed] [Google Scholar]
- Leem et al. (2016).Leem KH, Kim MG, Hahm YT, Kim HK. Hypoglycemic effect of Opuntia ficus-indica var. saboten is due to enhanced peripheral glucose uptake through activation of AMPK/p38 MAPK pathway. Nutrients. 2016;8(12):800. doi: 10.3390/nu8120800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2019a).Li Y, Liu Y, Liang J, Wang T, Sun M, Zhang Z. Gymnemic Acid ameliorates hyperglycemia through PI3K/AKT- and AMPK-mediated signaling pathways in type 2 diabetes mellitus rats. Journal of Agricultural and Food Chemistry. 2019a;67(47):13051–13060. doi: 10.1021/acs.jafc.9b04931. [DOI] [PubMed] [Google Scholar]
- Li et al. (2004).Li Y, Peng G, Li Q, Wen S, Hsun-Wei Huang T, Roufogalis BD, Yamahara J. Salacia oblonga improves cardiac fibrosis and inhibits postprandial hyperglycemia in obese zucker rats. Life Sciences. 2004;75(14):1735–1746. doi: 10.1016/j.lfs.2004.04.013. [DOI] [PubMed] [Google Scholar]
- Li et al. (2019b).Li J, Zhao Y, Cao L, Zheng Q, Gao J. AMPK Activation of flavonoids from psidium guajava leaves in L6 rat myoblast cells and L02 human hepatic cells. Evidence-Based Complementary and Alternative Medicine. 2019b;2019:1–6. doi: 10.1155/2019/9209043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin et al. (2020).Lin X, Xu Y, Pan X, Xu J, Ding Y, Sun X, Song X, Ren Y, Shan P-F. Global, regional, and national burden and trend of diabetes in 195 countries and territories: an analysis from 1990 to 2025. Scientific Reports. 2020;10(1):1–11. doi: 10.1038/s41598-020-71908-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2015).Liu HW, Huang WC, Yu WJ, Chang SJ. Toona Sinensis ameliorates insulin resistance via AMPK and PPARγ pathways. Food & Function. 2015;6(6):1855–1864. doi: 10.1039/C5FO00056D. [DOI] [PubMed] [Google Scholar]
- Luna-Vital & De Mejia (2018).Luna-Vital DA, De Mejia EG. Anthocyanins from purple corn activate free fatty acid-receptor 1 and glucokinase enhancing in vitro insulin secretion and hepatic glucose uptake. PLOS ONE. 2018;13(7):1–20. doi: 10.1371/journal.pone.0200449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Romero et al. (2014).López-Romero P, Pichardo-Ontiveros E, Avila-Nava A, Vázquez-Manjarrez N, Tovar AR, Pedraza-Chaverri J, Torres N. The effect of nopal (Opuntia Ficus Indica) on postprandial blood glucose, incretins, and antioxidant activity in mexican patients with type 2 diabetes after consumption of two different composition breakfasts. Journal of the Academy of Nutrition and Dietetics. 2014;114(11):1811–1818. doi: 10.1016/j.jand.2014.06.352. [DOI] [PubMed] [Google Scholar]
- Madhuri & Mohanvelu (2017).Madhuri AS, Mohanvelu R. Evaluation of antidiabetic activity of aqueous extract of Mangifera indica leaves in alloxan induced diabetic rats. Biomedical and Pharmacology Journal. 2017;10(2):1029–1035. doi: 10.13005/bpj/1200. [DOI] [Google Scholar]
- Majolo et al. (2019).Majolo F, de Oliveira Becker Delwing LK, Marmitt DJ, Bustamante-Filho IC, Goettert MI. Medicinal plants and bioactive natural compounds for cancer treatment: important advances for drug discovery. Phytochemistry Letters. 2019;31(August 2018):196–207. doi: 10.1016/j.phytol.2019.04.003. [DOI] [Google Scholar]
- Manzano & Williamson (2010).Manzano S, Williamson G. Polyphenols and phenolic acids from strawberry and apple decrease glucose uptake and transport by human intestinal Caco-2 cells. Molecular Nutrition & Food Research. 2010;54(12):1773–1780. doi: 10.1002/mnfr.201000019. [DOI] [PubMed] [Google Scholar]
- Martins & Brijesh (2018).Martins J, Brijesh S. Phytochemistry and pharmacology of anti-depressant medicinal plants: a review. Biomedicine & Pharmacotherapy. 2018;104(January):343–365. doi: 10.1016/j.biopha.2018.05.044. [DOI] [PubMed] [Google Scholar]
- Mathew & Subramanian (2014).Mathew M, Subramanian S. In vitro evaluation of anti-Alzheimer effects of dry ginger (Zingiber officinale Roscoe) extract. Indian Journal of Experimental Biology. 2014;52(6):606–612. [PubMed] [Google Scholar]
- Matschinsky (2005).Matschinsky FM. Glucokinase, glucose homeostasis, and diabetes mellitus. Current Diabetes Reports. 2005;5(3):171–176. doi: 10.1007/s11892-005-0005-4. [DOI] [PubMed] [Google Scholar]
- Matschinsky (2009).Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nature Reviews Drug Discovery. 2009;8(5):399–416. doi: 10.1038/nrd2850. [DOI] [PubMed] [Google Scholar]
- Matschinsky & Wilson (2019).Matschinsky FM, Wilson DF. The central role of glucokinase in glucose homeostasis: a perspective 50 years after demonstrating the presence of the enzyme in islets of Langerhans. Frontiers in Physiology. 2019;10(MAR):389. doi: 10.3389/fphys.2019.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazibuko et al. (2013).Mazibuko SE, Muller CJF, Joubert E, de Beer D, Johnson R, Opoku AR, Louw J. Amelioration of palmitate-induced insulin resistance in C2C12 muscle cells by rooibos (Aspalathus linearis) Phytomedicine. 2013;20(10):813–819. doi: 10.1016/j.phymed.2013.03.018. [DOI] [PubMed] [Google Scholar]
- Mazumdar, Akter & Talukder (2015).Mazumdar S, Akter R, Talukder D. Antidiabetic and antidiarrhoeal effects on ethanolic extract of Psidium guajava (L.) Bat. leaves in Wister rats. Asian Pacific Journal of Tropical Biomedicine. 2015;5(1):10–14. doi: 10.1016/S2221-1691(15)30163-5. [DOI] [Google Scholar]
- Md Sayem et al. (2018).Md Sayem AS, Arya A, Karimian H, Krishnasamy N, Hasamnis AA, Hossain CF. Action of phytochemicals on insulin signaling pathways accelerating glucose transporter (GLUT4) protein translocation. Molecules. 2018;23(2):258. doi: 10.3390/molecules23020258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel, Abd Rani & Husain (2020).Michel J, Abd Rani NZ, Husain K. A review on the potential use of medicinal plants from asteraceae and lamiaceae plant family in cardiovascular diseases. Frontiers in Pharmacology. 2020;11(June):1–26. doi: 10.3389/fphar.2020.00852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza, Althagafi & Shamshad (2019).Mirza AZ, Althagafi II, Shamshad H. Role of PPAR receptor in different diseases and their ligands: physiological importance and clinical implications. European Journal of Medicinal Chemistry. 2019;166:502–513. doi: 10.1016/j.ejmech.2019.01.067. [DOI] [PubMed] [Google Scholar]
- Modi (2007).Modi P. Diabetes beyond insulin: review of new drugs for treatment of diabetes mellitus. Current Drug Discovery Technologies. 2007;4(1):39–47. doi: 10.2174/157016307781115476. [DOI] [PubMed] [Google Scholar]
- Mogensen (2007).Mogensen CE. Pharmacotherapy of diabetes: new developments: improving life and prognosis for diabetic patients. Berlin: Springer; 2007. pp. 277–281. [Google Scholar]
- Mohammadi et al. (2011).Mohammadi J, Saadipour K, Delaviz H, Mohammadi A. Anti-diabetic effects of an alcoholic extract of Juglans regia in an animal model. Turkish Journal of Medical Sciences. 2011;41(4):685–691. doi: 10.3906/sag-1004-802. [DOI] [Google Scholar]
- Mohammadi & Zangeneh (2018).Mohammadi G, Zangeneh MM. Evaluation of nephroprotective and antidiabetic effects of gundelia tournefortii aqueous extract on diabetic nephropathy in male mice. Research Journal of Pharmacognosy. 2018;5(July):65–73. doi: 10.22127/RJP.2018.69223. [DOI] [Google Scholar]
- Morakinyo, Akindele & Ahmed (2011).Morakinyo AO, Akindele AJ, Ahmed Z. Modulation of antioxidant enzymes and inflammatory cytokines: possible mechanism of anti-diabetic effect of ginger extracts. African Journal of Biomedical Research. 2011;14(3):195–202. [Google Scholar]
- Moser et al. (2014).Moser C, Vickers SP, Brammer R, Cheetham SC, Drewe J. Antidiabetic effects of the Cimicifuga racemosa extract Ze 450 in vitro and in vivo in ob/ob mice. Phytomedicine. 2014;21(11):1382–1389. doi: 10.1016/j.phymed.2014.06.002. [DOI] [PubMed] [Google Scholar]
- Mu et al. (2020).Mu J, Xin G, Zhang B, Wang Y, Ning C, Meng X. Beneficial effects of Aronia melanocarpa berry extract on hepatic insulin resistance in type 2 diabetes mellitus rats. Journal of Food Science. 2020;85(4):1307–1318. doi: 10.1111/1750-3841.15109. [DOI] [PubMed] [Google Scholar]
- Mustafa et al. (2017).Mustafa G, Arif R, Atta A, Sharif S, Jamil A. Bioactive compounds from medicinal plants and their importance in drug discovery in Pakistan. Matrix Science Pharma. 2017;1(1):17–26. doi: 10.26480/msp.01.2017.17.26. [DOI] [Google Scholar]
- Nabben & Neumann (2016).Nabben M, Neumann D. GSK-3 inhibitors: anti-diabetic treatment associated with cardiac risk?: editorial to: the impact of chronic glycogen synthase kinase-3 inhibition on remodeling of normal and pre-diabetic rat hearts. by Barbara Huisamen et al. Cardiovascular Drugs and Therapy. 2016;30(3):233–235. doi: 10.1007/s10557-016-6669-y. [DOI] [PubMed] [Google Scholar]
- Najdi et al. (2019).Najdi RA, Hagras MM, Kamel FO, Magadmi RM. A randomized controlled clinical trial evaluating the effect of Trigonella foenum-graecum (fenugreek) versus glibenclamide in patients with diabetes. African Health Sciences. 2019;19(1):1594–1601. doi: 10.4314/ahs.v19i1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima et al. (1993).Nakashima N, Kimura I, Kimura M, Matsuura H. Isolation of pseudoprototimosaponin AIII from rhizomes of anemarrhena asphodeloides and its hypoglycemic activity in streptozotocin-induced diabetic mice. Journal of Natural Products. 1993;56(3):345–350. doi: 10.1021/np50093a006. [DOI] [PubMed] [Google Scholar]
- Nandabalan, Sujatha & Shanmuganathan (2010).Nandabalan K, Sujatha S, Shanmuganathan V. Biochimica et Biophysica Acta 3 β-taraxerol of Mangifera indica, a PI3K dependent dual activator of glucose transport and glycogen synthesis in 3T3-L1 adipocytes. Biochimica et Biophysica Acta (BBA) - General Subjects. 2010;1800(3):359–366. doi: 10.1016/j.bbagen.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Naowaboot et al. (2012).Naowaboot J, Pannangpetch P, Kukongviriyapan V, Prawan A, Kukongviriyapan U, Itharat A. Extract stimulates glucose uptake and GLUT4 translocation in rat adipocytes. The American Journal of Chinese Medicine. 2012;40(1):163–175. doi: 10.1142/S0192415X12500139. [DOI] [PubMed] [Google Scholar]
- Nasiry et al. (2017).Nasiry D, khalatbary AR, Ahmadvand H, Talebpour Amiri F, Akbari E. Protective effects of methanolic extract of Juglans regia L. leaf on streptozotocin-induced diabetic peripheral neuropathy in rats. BMC Complementary and Alternative Medicine. 2017;17(1):1–11. doi: 10.1186/s12906-017-1983-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neta et al. (2021).Neta JFDF, Veras VS, Sousa DF, Cunha MCSO, Queiroz MVO, Neto JCGL, Damasceno MMC, Araújo MFM, Freitas RWJF. Effectiveness of the piperine-supplemented Curcuma longa L. in metabolic control of patients with type 2 diabetes: a randomised double-blind placebo-controlled clinical trial. International Journal of Food Sciences and Nutrition. 2021;72(7):968–977. doi: 10.1080/09637486.2021.1885015. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2015a).Nguyen P-H, Ji D-J, Han Y-R, Choi J-S, Rhyu D-Y, Min B-S, Woo M-H. Selaginellin and biflavonoids as protein tyrosine phosphatase 1B inhibitors from Selaginella tamariscina and their glucose uptake stimulatory effects. Bioorganic & Medicinal Chemistry. 2015a;23(13):3730–3737. doi: 10.1016/j.bmc.2015.04.007. [DOI] [PubMed] [Google Scholar]
- Nguyen et al. (2015b).Nguyen P-H, Zhao B-T, Ali MY, Choi J-S, Rhyu D-Y, Min B-S, Woo M-H. Insulin-mimetic selaginellins from selaginella tamariscina with protein tyrosine phosphatase 1B (PTP1B) inhibitory activity. Journal of Natural Products. 2015b;78(1):34–42. doi: 10.1021/np5005856. [DOI] [PubMed] [Google Scholar]
- Nishiyama et al. (2005).Nishiyama T, Mae T, Kishida H, Tsukagawa M, Mimaki Y, Kuroda M, Sashida Y, Takahashi K, Kawada T, Nakagawa K, Kitahara M. Curcuminoids and sesquiterpenoids in turmeric (Curcuma longa L.) Suppress an increase in blood glucose level in type 2 diabetic KK-Aγ mice. Journal of Agricultural and Food Chemistry. 2005;53(4):959–963. doi: 10.1021/jf0483873. [DOI] [PubMed] [Google Scholar]
- Nurcahyanti et al. (2021).Nurcahyanti ADR, Jap A, Lady J, Prismawan D, Sharopov F, Daoud R, Wink M, Sobeh M. Function of selected natural antidiabetic compounds with potential against cancer via modulation of the PI3K/AKT/mTOR cascade. Biomedicine & Pharmacotherapy. 2021;144(November):112138. doi: 10.1016/j.biopha.2021.112138. [DOI] [PubMed] [Google Scholar]
- Ogbonnia et al. (2009).Ogbonnia S, Mbaka G, Anyika E, Lediju O, Ota D. Evaluation of the effects of Parinari curatellifolia seed and Anthocleista vogelli root extracts individually and in combination on postprandial and alloxan-induced diabetes in animals. Planta Medica. 2009;77(12):146–162. doi: 10.1055/s-0031-1282433. [DOI] [Google Scholar]
- Ogunbolude et al. (2009).Ogunbolude Y, Ajayi MA, Ajagbawa TM, Igbakin AP, Rocha JBT, Kade IJ. Ethanolic extracts of seeds of Parinari curatellifolia exhibit potent antioxidant properties: a possible mechanism of its antidiabetic action. Journal of Pharmacognosy and Phytochemistry. 2009;1(6):67–75. [Google Scholar]
- Ogurtsova et al. (2017).Ogurtsova K, da Rocha Fernandes JD, Huang Y, Linnenkamp U, Guariguata L, Cho NH, Cavan D, Shaw JE, Makaroff LE. IDF diabetes atlas: global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Research and Clinical Practice. 2017;128:40–50. doi: 10.1016/j.diabres.2017.03.024. [DOI] [PubMed] [Google Scholar]
- Oh et al. (2014).Oh MR, Park SH, Kim SY, Back HI, Kim MG, Jeon JY, Ha KC, Na WT, Cha YS, Park BH, Park TS, Chae SW. Postprandial glucose-lowering effects of fermented red ginseng in subjects with impaired fasting glucose or type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. BMC Complementary and Alternative Medicine. 2014;14:1–7. doi: 10.1186/1472-6882-14-237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortíz-Martinez et al. (2016).Ortíz-Martinez DM, Rivas-Morales C, de la Garza-Ramos MA, Verde-Star MJ, Nuñez-Gonzalez MA, Leos-Rivas C. Miconia sp. Increases mRNA levels of PPAR gamma and inhibits alpha amylase and alpha glucosidase. Evidence-Based Complementary and Alternative Medicine. 2016;2016:1–6. doi: 10.1155/2016/5123519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oryan et al. (2014).Oryan A, Hashemnia M, Hamidi AR, Mohammadalipour A. Effects of hydro-ethanol extract of Citrullus colocynthis on blood glucose levels and pathology of organs in alloxan-induced diabetic rats. Asian Pacific Journal of Tropical Disease. 2014;4(2):125–130. doi: 10.1016/S2222-1808(14)60328-5. [DOI] [Google Scholar]
- Ouahhoud et al. (2019).Ouahhoud S, Lahmass I, Bouhrim M, Khoulati A, Sabouni A, Benabbes R, Asehraou A, Choukri M, Bnouham M, Saalaoui E. Antidiabetic effect of hydroethanolic extract of Crocus sativus stigmas, tepals and leaves in streptozotocin-induced diabetic rats. Physiol Pharmacol. 2019;23(1):9–20. [Google Scholar]
- Parimala et al. (2015).Parimala M, Debjani M, Vasanthi HR, Shoba FG. Nymphaea nouchali Burm. f. hydroalcoholic seed extract increases glucose consumption in 3T3-L1 adipocytes through activation of peroxisome proliferator-activated receptor gamma and insulin sensitization. Journal of Advanced Pharmaceutical Technology and Research. 2015;6(4):183–189. doi: 10.4103/2231-4040.165013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park et al. (2006).Park SA, Choi M-S, Kim M-J, Jung UJ, Kim H-J, Park K-K, Noh HJ, Park H-M, Park YB, Lee J-S, Lee M-K. Hypoglycemic and hypolipidemic action of Du-zhong (Eucommia ulmoides Oliver) leaves water extract in C57BL/KsJ-db/db mice. Journal of Ethnopharmacology. 2006;107(3):412–417. doi: 10.1016/j.jep.2006.03.034. [DOI] [PubMed] [Google Scholar]
- Pernicova & Korbonits (2014).Pernicova I, Korbonits M. Metformin-mode of action and clinical implications for diabetes and cancer. Nature Reviews Endocrinology. 2014;10(3):143–156. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- Petersen & Shulman (2002).Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. American Journal of Cardiology. 2002;90(5 SUPPL):11–18. doi: 10.1016/S0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- Petersen & Shulman (2018).Petersen MC, Shulman GI. Mechanisms of insulin action and insulin resistance. Physiological Reviews. 2018;98(4):2133–2223. doi: 10.1152/physrev.00063.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks et al. (2018).Proks P, Kramer H, Haythorne E, Ashcroft FM. Binding of sulphonylureas to plasma proteins–A KATP channel perspective. PLOS ONE. 2018;13(5):1–16. doi: 10.1371/journal.pone.0197634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purintrapiban et al. (2011).Purintrapiban J, Keawpradub N, Kansenalak S, Chittrakarn S, Janchawee B, Sawangjaroen K. Study on glucose transport in muscle cells by extracts from Mitragyna speciosa (Korth) and mitragynine. Natural Product Research. 2011;25(15):1379–1387. doi: 10.1080/14786410802267627. [DOI] [PubMed] [Google Scholar]
- Rabiei et al. (2018).Rabiei K, Ebrahimzadeh MA, Saeedi M, Bahar A, Akha O, Kashi Z. Effects of a hydroalcoholic extract of Juglans regia (walnut) leaves on blood glucose and major cardiovascular risk factors in type 2 diabetic patients: a double-blind, placebo-controlled clinical trial. BMC Complementary and Alternative Medicine. 2018;18(1):1–7. doi: 10.1186/s12906-018-2268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabiei, Solati & Amini-Khoei (2019).Rabiei Z, Solati K, Amini-Khoei H. Phytotherapy in treatment of Parkinson’s disease: a review. Pharmaceutical Biology. 2019;57(1):355–362. doi: 10.1080/13880209.2019.1618344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju et al. (2001).Raju J, Gupta D, Rao AR, Yadava PK, Baquer NZ. Trigonella foenum graecum (fenugreek) seed powder improves glucose homeostasis in alloxan diabetic rat tissues by reversing the altered glycolytic, gluconeogenic and lipogenic enzymes. Molecular and Cellular Biochemistry. 2001;224(1–2):45–51. doi: 10.1023/a:1011974630828. [DOI] [PubMed] [Google Scholar]
- Ramadan, Schaalan & Tolba (2017).Ramadan BK, Schaalan MF, Tolba AM. Hypoglycemic and pancreatic protective effects of Portulaca oleracea extract in alloxan induced diabetic rats. BMC Complementary and Alternative Medicine. 2017;17(1):1–10. doi: 10.1186/s12906-016-1530-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retz & Glucose (2021).Retz G, Glucose T. Identification of C 21 steroidal glycosides from uptake activities. Molecules. 2021;26(21):1–14. doi: 10.3390/molecules26216549. [DOI] [Google Scholar]
- Ribnicky et al. (2006).Ribnicky DM, Poulev A, Watford M, Cefalu WT, Raskin I. Antihyperglycemic activity of TarralinTM, an ethanolic extract of Artemisia dracunculus L. Phytomedicine. 2006;13(8):550–557. doi: 10.1016/j.phymed.2005.09.007. [DOI] [PubMed] [Google Scholar]
- Sadino (2018).Sadino A. A review on medicinal plants with antidiabetic activity from rubiaceae family. International Research Journal of Pharmacy. 2018;9(7):36–41. doi: 10.7897/2230-8407.097122. [DOI] [Google Scholar]
- Sahai & Kumar (2020).Sahai V, Kumar V. Anti-diabetic, hepatoprotective and antioxidant potential of Brassica oleracea sprouts. Biocatalysis and Agricultural Biotechnology. 2020;25(April):101623. doi: 10.1016/j.bcab.2020.101623. [DOI] [Google Scholar]
- Sahuc (2016).Sahuc J. Evaluating the macroeconomic effects of the ECB’s unconventional monetary policies. Journal of Money, Credit and Banking. 2016;55(4):1–33. doi: 10.1111/jmcb.12628. [DOI] [Google Scholar]
- Saini (2010).Saini V. Molecular mechanisms of insulin resistance in type 2 diabetes mellitus. World Journal of Diabetes. 2010;1(3):68. doi: 10.4239/wjd.v1.i3.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancheti et al. (2011).Sancheti S, Sancheti S, Bafna M, Kim HR, You YH, Seo SY. Evaluation of antidiabetic, antihyperlipidemic and antioxidant effects of Boehmeria nivea root extract in streptozotocin-induced diabetic rats. Revista Brasileira De Farmacognosia-Brazilian Journal of Pharmacognosy. 2011;21(1):146–154. doi: 10.1590/S0102-695X2011005000021. [DOI] [Google Scholar]
- Sathishsekar & Subramanian (2005).Sathishsekar D, Subramanian S. Antioxidant properties of Momordica Charantia (bitter gourd) seeds on Streptozotocin induced diabetic rats. Asia Pacific Journal of Clinical Nutrition. 2005;14(2):153–158. [PubMed] [Google Scholar]
- Sato et al. (2016).Sato S, Mukai Y, Kataoka S, Kurasaki M. Azuki bean (Vigna angularis) extract stimulates the phosphorylation of AMP-activated protein kinase in HepG2 cells and diabetic rat liver. Journal of the Science of Food and Agriculture. 2016;96(7):2312–2318. doi: 10.1002/jsfa.7346. [DOI] [PubMed] [Google Scholar]
- Schreck & Melzig (2021).Schreck K, Melzig MF. Traditionally used plants in the treatment of diabetes mellitus: screening for uptake inhibition of glucose and fructose in the Caco2-cell model. Frontiers in Pharmacology. 2021;12(August):1–12. doi: 10.3389/fphar.2021.692566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedigh-Rahimabadi et al. (2017).Sedigh-Rahimabadi M, Fani M, Rostami-chijan M, Zarshenas MM, Shams M. A traditional mouthwash (Punica granatum var pleniflora) for controlling gingivitis of diabetic patients: a double-blind randomized controlled clinical trial. Journal of Evidence-Based Complementary and Alternative Medicine. 2017;22(1):59–67. doi: 10.1177/2156587216633370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon-Loodu & Rupasinghe (2019).Sekhon-Loodu S, Rupasinghe HPV. Evaluation of antioxidant, antidiabetic and antiobesity potential of selected traditional medicinal plants. Frontiers in Nutrition. 2019;6(April):1–11. doi: 10.3389/fnut.2019.00053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setorki (2020).Setorki M. Medicinal herbs with anti-depressant effects. Journal of Herbmed Pharmacology. 2020;9(4):309–317. doi: 10.34172/jhp.2020.39. [DOI] [Google Scholar]
- Shalaby & Hammouda (2013).Shalaby M, Hammouda A. Antiobesity, antioxidant and antidiabetic activities of red Ginseng plant extract in obese diabetic rats. Journal of Intercultural Ethnopharmacology. 2013;2(3):165. doi: 10.5455/jice.20130910051230. [DOI] [Google Scholar]
- Shang et al. (2015).Shang N, Saleem A, Musallam L, Walshe-Roussel B, Badawi A, Cuerrier A, Arnason JT, Haddad PS. Novel approach to identify potential bioactive plant metabolites: pharmacological and metabolomics analyses of ethanol and hot water extracts of several Canadian medicinal plants of the cree of eeyou istchee. PLOS ONE. 2015;10(8):1–15. doi: 10.1371/journal.pone.0135721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma et al. (2021).Sharma S, Wadhwa K, Choudhary M, Budhwar V. Ethnopharmacological perspectives of glucokinase activators in the treatment of diabetes mellitus. Natural Product Research. 2021;36(11):1–15. doi: 10.1080/14786419.2021.1931187. [DOI] [PubMed] [Google Scholar]
- Shetty et al. (2010).Shetty AJ, Choudhury D, Rejeesh, Nair V, Kuruvilla M, Kotian S. Effect of the insulin plant (Costus igneus) leaves on dexamethasone-induced hyperglycemia. International Journal of Ayurveda Research. 2010;1(2):100. doi: 10.4103/0974-7788.64396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih, Lin & Lin (2008).Shih CC, Lin CH, Lin WL. Effects of Momordica charantia on insulin resistance and visceral obesity in mice on high-fat diet. Diabetes Research and Clinical Practice. 2008;81(2):134–143. doi: 10.1016/j.diabres.2008.04.023. [DOI] [PubMed] [Google Scholar]
- Shih et al. (2013).Shih CC, Lin CH, Lin YJ, Bin Wu J. Validation of the antidiabetic and hypolipidemic effects of hawthorn by assessment of gluconeogenesis and lipogenesis related genes and AMP-activated protein kinase phosphorylation. Evidence-Based Complementary and Alternative Medicine. 2013;2013(4, part 2):1–12. doi: 10.1155/2013/597067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shori (2015).Shori AB. Screening of antidiabetic and antioxidant activities of medicinal plants. Journal of Integrative Medicine. 2015;13(5):297–305. doi: 10.1016/S2095-4964(15)60193-5. [DOI] [PubMed] [Google Scholar]
- Siahaan et al. (2020).Siahaan JM, Illyas S, Lindarto D, Nainggolan M. The effect of ethanol and ethyl acetate fraction of chayote fruit (Sechium edule jacq. swartz) on the oxidative stress and insulin resistance of male white rat model type 2 diabetes mellitus. Open Access Macedonian Journal of Medical Sciences. 2020;8(A):962–969. doi: 10.3889/oamjms.2020.4517. [DOI] [Google Scholar]
- Singhal, Bangar & Naithani (2012).Singhal A, Bangar O, Naithani V. Medicinal plants with a potential to treat Alzheimer and associated symptoms. International Journal of Nutrition, Pharmacology, Neurological Diseases. 2012;2(2):84. doi: 10.4103/2231-0738.95927. [DOI] [Google Scholar]
- Smirin et al. (2010).Smirin P, Taler D, Abitbol G, Brutman-Barazani T, Kerem Z, Sampson SR, Rosenzweig T. Sarcopoterium spinosum extract as an antidiabetic agent: in vitro and in vivo study. Journal of Ethnopharmacology. 2010;129(1):10–17. doi: 10.1016/j.jep.2010.02.021. [DOI] [PubMed] [Google Scholar]
- Soccio, Chen & Lazar (2014).Soccio RE, Chen ER, Lazar MA. Thiazolidinediones and the promise of insulin sensitization in type 2 diabetes. Cell Metabolism. 2014;20(4):573–591. doi: 10.1016/j.cmet.2014.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son, Miura & Yagasaki (2015).Son MJ, Miura Y, Yagasaki K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology. 2015;67(4):641–652. doi: 10.1007/s10616-014-9730-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer et al. (2016).Stadlbauer V, Haselgrübler R, Lanzerstorfer P, Plochberger B, Höglinger O, Weghuber J. Biomolecular characterization of putative antidiabetic herbal extracts. PLOS ONE. 2016;11(1):1–20. doi: 10.1371/journal.pone.0148109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer et al. (2021).Stadlbauer V, Neuhauser C, Aumiller T, Stallinger A, Iken M, Weghuber J. Identification of insulin-mimetic plant extracts: from an in vitro high-content screen to blood glucose reduction in live animals. Molecules. 2021;26(14):4346. doi: 10.3390/molecules26144346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg & Carling (2019).Steinberg GR, Carling D. AMP-activated protein kinase: the current landscape for drug development. Nature Reviews Drug Discovery. 2019;18(7):527–551. doi: 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- Süntar (2020).Süntar I. Importance of ethnopharmacological studies in drug discovery: role of medicinal plants. Phytochemistry Reviews. 2020;19(5):1199–1209. doi: 10.1007/s11101-019-09629-9. [DOI] [Google Scholar]
- Taderera et al. (2019).Taderera T, Chagonda LS, Gomo E, Katerere D, Shai LJ. Annona stenophylla aqueous extract stimulate glucose uptake in established C2Cl2 muscle cell lines. African Health Sciences. 2019;19(2):2219–2229. doi: 10.4314/ahs.v19i2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taderera, Gomo & Shoriwa Chagonda (2016).Taderera T, Gomo E, Shoriwa Chagonda L. The antidiabetic activity of an aqueous root extract of annona stenophylla engl. and diels in non-diabetic control and alloxan-induced diabetic rats. Journal of Biologically Active Products from Nature. 2016;6(4):315–322. doi: 10.1080/22311866.2016.1234412. [DOI] [Google Scholar]
- Taghizadeh et al. (2022).Taghizadeh M, Mohammad Zadeh A, Asemi Z, Farrokhnezhad AH, Memarzadeh MR, Banikazemi Z, Shariat M, Shafabakhsh R. Morus Alba leaf extract affects metabolic profiles, biomarkers inflammation and oxidative stress in patients with type 2 diabetes mellitus: a double-blind clinical trial. Clinical Nutrition ESPEN. 2022;49:68–73. doi: 10.1016/j.clnesp.2022.03.027. [DOI] [PubMed] [Google Scholar]
- Tang et al. (2017).Tang D, Chen Q-B, Xin X-L, Aisa H-A. Anti-diabetic effect of three new norditerpenoid alkaloids in vitro and potential mechanism via PI3K/Akt signaling pathway. Biomedicine & Pharmacotherapy. 2017;87:145–152. doi: 10.1016/j.biopha.2016.12.058. [DOI] [PubMed] [Google Scholar]
- Tasic et al. (2021).Tasic N, Jakovljevic VLJ, Mitrovic M, Djindjic B, Tasic D, Dragisic D, Citakovic Z, Kovacevic Z, Radoman K, Zivkovic V, Bolevich S, Turnic TN. Black chokeberry Aronia melanocarpa extract reduces blood pressure, glycemia and lipid profile in patients with metabolic syndrome: a prospective controlled trial. Molecular and Cellular Biochemistry. 2021;476(7):2663–2673. doi: 10.1007/s11010-021-04106-4. [DOI] [PubMed] [Google Scholar]
- Tiwari, Mishra & Sangwan (2014).Tiwari P, Mishra BN, Sangwan NS. Phytochemical and pharmacological properties of Gymnema sylvestre: an important medicinal plant. Biomed Research International. 2014;2014:830285. doi: 10.1155/2014/830285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonks et al. (2013).Tonks KT, Ng Y, Miller S, Coster ACF, Samocha-Bonet D, Iseli TJ, Xu A, Patrick E, Yang JYH, Junutula JR, Modrusan Z, Kolumam G, Stöckli J, Chisholm DJ, James DE, Greenfield JR. Impaired Akt phosphorylation in insulin-resistant human muscle is accompanied by selective and heterogeneous downstream defects. Diabetologia. 2013;56(4):875–885. doi: 10.1007/s00125-012-2811-y. [DOI] [PubMed] [Google Scholar]
- Toulis et al. (2020).Toulis KA, Nirantharakumar K, Pourzitaki C, Barnett AH, Tahrani AA. Glucokinase activators for type 2 diabetes: challenges and future developments. Drugs. 2020;80(5):467–475. doi: 10.1007/s40265-020-01278-z. [DOI] [PubMed] [Google Scholar]
- Towler & Hardie (2007).Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circulation Research. 2007;100(3):328–341. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- Triggle & Ding (2014).Triggle CR, Ding H. Cardiovascular impact of drugs used in the treatment of diabetes. Therapeutic Advances in Chronic Disease. 2014;5(6):245–268. doi: 10.1177/2040622314546125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu et al. (2013).Tu Z, Moss-Pierce T, Ford P, Jiang TA. Rosemary (Rosmarinus officinalis L.) extract regulates glucose and lipid metabolism by activating AMPK and PPAR pathways in HepG2 cells. Journal of Agricultural and Food Chemistry. 2013;61(11):2803–2810. doi: 10.1021/jf400298c. [DOI] [PubMed] [Google Scholar]
- Valcheva-Kusmanova et al. (2007).Valcheva-Kusmanova S, Kuzmanov K, Tancheva S, Belcheva A. Hypoglycemic and hypolipidemic effects of Aronia melanocarpa fruit juice in streptozotocin-induced diabetic rats. Methods and Findings in Experimental and Clinical Pharmacology. 2007;29(2):101–105. doi: 10.1358/mf.2007.29.2.1075349. [DOI] [PubMed] [Google Scholar]
- Verma (2014).Verma RK. An ethnobotanical study of plants used for the treatment of livestock diseases in Tikamgarh District of Bundelkhand, Central India. Asian Pacific Journal of Tropical Biomedicine. 2014;4(Suppl 1):S460–S467. doi: 10.12980/APJTB.4.2014C1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma et al. (2012).Verma N, Amresh G, Sahu PK, Mishra N, Singh AP, Rao CV. Antihyperglycemic activity, antihyperlipedemic activity, haematological effects and histopathological analysis of Sapindus mukorossi Gaerten fruits in streptozotocin induced diabetic rats. Asian Pacific Journal of Tropical Medicine. 2012;5(7):518–522. doi: 10.1016/S1995-7645(12)60091-1. [DOI] [PubMed] [Google Scholar]
- Verma et al. (2016).Verma N, Usman K, Patel N, Jain A, Dhakre S, Swaroop A, Bagchi M, Kumar P, Preuss HG, Bagchi D. A multicenter clinical study to determine the efficacy of a novel fenugreek seed (Trigonella foenum-graecum) extract (FenfuroTM) in patients with type 2 diabetes. Food & Nutrition Research. 2016;60(1):32382. doi: 10.3402/fnr.v60.32382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlavcheski et al. (2018).Vlavcheski F, Baron D, Vlachogiannis IA, Macpherson REK, Tsiani E. Carnosol increases skeletal muscle cell glucose uptake via AMPK-dependent GLUT4 glucose transporter translocation. International Journal of Molecular Sciences. 2018;19(5):1321. doi: 10.3390/ijms19051321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuksan et al. (2008).Vuksan V, Sung MK, Sievenpiper JL, Stavro PM, Jenkins AL, Di Buono M. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutrition, Metabolism and Cardiovascular Diseases. 2008;18(1):46–56. doi: 10.1016/j.numecd.2006.04.003. [DOI] [PubMed] [Google Scholar]
- Waheed, Miana & Ahmad (2006).Waheed A, Miana GA, Ahmad SI. Clinical investigation of hypoglycemic effect of seeds of Azadirachta-inidca in type-2 (NIDDM) diabetes mellitus. Pakistan Journal of Pharmaceutical Sciences. 2006;19(4):322–325. [PubMed] [Google Scholar]
- Wajcberg & Tavaria (2009).Wajcberg E, Tavaria A. Exenatide: clinical aspects of the first incretin-mimetic for the treatment of type 2 diabetes mellitus. Expert Opinion on Pharmacotherapy. 2009;10(1):135–142. doi: 10.1517/14656560802611832. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2018).Wang K, Wang H, Liu Y, Shui W, Wang J, Cao P, Wang H, You R, Zhang Y. Dendrobium officinale polysaccharide attenuates type 2 diabetes mellitus via the regulation of PI3K/Akt-mediated glycogen synthesis and glucose metabolism. Journal of Functional Foods. 2018;40(November 2017):261–271. doi: 10.1016/j.jff.2017.11.004. [DOI] [Google Scholar]
- Williams et al. (2007).Williams JA, Choe YS, Noss MJ, Baumgartner CJ, Mustad VA. Extract of Salacia oblonga lowers acute glycemia in patients with type 2 diabetes. American Journal of Clinical Nutrition. 2007;86(1):124–130. doi: 10.1093/ajcn/86.1.124. [DOI] [PubMed] [Google Scholar]
- Williams et al. (2020).Williams R, Karuranga S, Malanda B, Saeedi P, Basit A, Besançon S, Bommer C, Esteghamati A, Ogurtsova K, Zhang P, Colagiuri S. Global and regional estimates and projections of diabetes-related health expenditure: results from the international diabetes federation diabetes atlas, 9th edition. Diabetes Research and Clinical Practice. 2020;162:108072. doi: 10.1016/j.diabres.2020.108072. [DOI] [PubMed] [Google Scholar]
- World Health Organization (2019).World Health Organization WHO global report on traditional and complementary medicine 2019. World Health Organization. 1–228. 2019. https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?ua=1 https://apps.who.int/iris/bitstream/handle/10665/312342/9789241515436-eng.pdf?ua=1
- Wu et al. (2020).Wu G, Bai Z, Wan Y, Shi H, Huang X, Nie S. Antidiabetic effects of polysaccharide from azuki bean (Vigna angularis) in type 2 diabetic rats via insulin/PI3K/AKT signaling pathway. Food Hydrocolloids. 2020;101(September 2019):105456. doi: 10.1016/j.foodhyd.2019.105456. [DOI] [Google Scholar]
- Wu et al. (2014).Wu CH, Ou TT, Chang CH, Chang XZ, Yang MY, Wang CJ. The polyphenol extract from sechium edule shoots inhibits lipogenesis and stimulates lipolysis via activation of AMPK signals in HepG2 cells. Journal of Agricultural and Food Chemistry. 2014;62(3):750–759. doi: 10.1021/jf404611a. [DOI] [PubMed] [Google Scholar]
- Xia et al. (2021).Xia T, Duan W, Zhang Z, Fang B, Zhang B, Xu B, de la Cruz CBV, El-Seedi H, Simal-Gandara J, Wang S, Wang M, Xiao J. Polyphenol-rich extract of Zhenjiang aromatic vinegar ameliorates high glucose-induced insulin resistance by regulating JNK-IRS-1 and PI3K/Akt signaling pathways. Food Chemistry. 2021;335(October 2019):127513. doi: 10.1016/j.foodchem.2020.127513. [DOI] [PubMed] [Google Scholar]
- Xiong et al. (2018).Xiong H, Zhang S, Zhao Z, Zhao P, Chen L, Mei Z. Antidiabetic activities of entagenic acid in type 2 diabetic db/db mice and L6 myotubes via AMPK/GLUT4 pathway. J Ethnopharmacol. 2018;211(August 2017):366–374. doi: 10.1016/j.jep.2017.10.004. [DOI] [PubMed] [Google Scholar]
- Xu et al. (2018).Xu J, Wang S, Feng T, Chen Y, Yang G. Hypoglycemic and hypolipidemic effects of total saponins from Stauntonia chinensis in diabetic db/db mice. Journal of Cellular and Molecular Medicine. 2018;22(12):6026–6038. doi: 10.1111/jcmm.13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan et al. (2017).Yan F, Yang Y, Yu L, Zheng X. Effects of C-Glycosides from apios americana leaves against oxidative stress during hyperglycemia through regulating mitogen-activated protein kinases and nuclear factor erythroid 2-related factor 2. Journal of Agricultural and Food Chemistry. 2017;65(34):7457–7466. doi: 10.1021/acs.jafc.7b03163. [DOI] [PubMed] [Google Scholar]
- Yang et al. (2017).Yang JL, Ha TKQ, Lee BW, Kim J, Oh WK. PTP1B inhibitors from the seeds of Iris sanguinea and their insulin mimetic activities via AMPK and ACC phosphorylation. Bioorganic & Medicinal Chemistry Letters. 2017;27(22):5076–5081. doi: 10.1016/j.bmcl.2017.09.031. [DOI] [PubMed] [Google Scholar]
- Yang, Jang & Hwang (2012).Yang HJ, Jang DJ, Hwang JT. Anti-diabetic effects of Korean red pepper via AMPK and PPAR-γ activation in C2C12 myotubes. Journal of Functional Foods. 2012;4(2):552–558. doi: 10.1016/j.jff.2012.02.016. [DOI] [Google Scholar]
- Yin et al. (2009).Yin J, Zuberi A, Gao Z, Liu D, Liu Z, Ye J. Shilianhua extract inhibits GSK-3β and promotes glucose metabolism. American Journal Of Physiology-Endocrinology And Metabolism. 2009;296(6):1275–1280. doi: 10.1152/ajpendo.00092.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan & Piao (2011).Yuan HD, Piao GC. An active part of Artemisia sacrorum Ledeb. suppresses gluconeogenesis through AMPK mediated GSK3β and CREB phosphorylation in human HepG2 cells. Bioscience, Biotechnology, and Biochemistry. 2011;75(6):1079–1084. doi: 10.1271/bbb.100881. [DOI] [PubMed] [Google Scholar]
- Yue et al. (2022).Yue H, Wang L, Jiang S, Banma C, Jia W, Tao Y, Zhao X. Hypoglycemic effects of Rhodiola crenulata (HK. f. et. Thoms) H. Ohba in vitro and in vivo and its ingredient identification by UPLC-triple-TOF/MS. Food & Function. 2022;13(3):1659–1667. doi: 10.1039/D1FO03436G. [DOI] [PubMed] [Google Scholar]
- Zhang et al. (2016).Zhang Y, Dong H, Wang M, Zhang J. Quercetin isolated from toona sinensis leaves attenuates hyperglycemia and protects hepatocytes in high-carbohydrate/high-fat diet and alloxan induced experimental diabetic mice. Journal of Diabetes Research. 2016;2016(4, article e000361):1–10. doi: 10.1155/2016/8492780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2018a).Zhao P, Ming Q, Qiu J, Tian D, Liu J, Shen J, Liu Q-H, Yang X. Ethanolic extract of folium sennae mediates the glucose uptake of L6 cells by GLUT4 and Ca2+ Molecules. 2018a;23(11):1–20. doi: 10.3390/molecules23112934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao et al. (2018b).Zhao P, Ming Q, Xiong M, Song G, Tan L, Tian D, Liu J, Huang Z, Ma J, Shen J, Liu Q-H, Yang X. Dandelion chloroform extract promotes glucose uptake via the AMPK/GLUT4 pathway in L6 cells. Evidence-Based Complementary and Alternative Medicine. 2018b;2018(12):1–10. doi: 10.1155/2018/1709587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng et al. (2016).Zheng T, Hao X, Wang Q, Chen L, Jin S, Bian F. Entada phaseoloides extract suppresses hepatic gluconeogenesis via activation of the AMPK signaling pathway. Journal of Ethnopharmacology. 2016;193(Suppl 1):691–699. doi: 10.1016/j.jep.2016.10.039. [DOI] [PubMed] [Google Scholar]
- Zheng, Ley & Hu (2018).Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nature Reviews Endocrinology. 2018;14(2):88–98. doi: 10.1038/nrendo.2017.151. [DOI] [PubMed] [Google Scholar]
- Zheng et al. (2011).Zheng XK, Zhang L, Wang WW, Wu YY, Zhang QB, Feng WS. Anti-diabetic activity and potential mechanism of total flavonoids of Selaginella tamariscina (Beauv.) Spring in rats induced by high fat diet and low dose STZ. Journal of Ethnopharmacology. 2011;137(1):662–668. doi: 10.1016/j.jep.2011.06.018. [DOI] [PubMed] [Google Scholar]
- Zhou et al. (2001).Zhou G, Myers R, Li Y, Chen Y, Shen X, Fenyk-Melody J, Wu M, Ventre J, Doebber T, Fujii N, Musi N, Hirshman MF, Goodyear LJ, Moller DE. Role of AMP-activated protein kinase in mechanism of metformin action. Journal of Clinical Investigation. 2001;108(8):1167–1174. doi: 10.1172/JCI13505. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review.