Abstract
Converging lines of evidence suggest that aerobic exercise impacts Parkinson’s disease (PD) motor symptoms and might slow it’s progression. We provide an overview of the ongoing randomized clinical trials (RCTs) on aerobic exercise in PD. We found six RCTs with sample sizes between 28 and 370 and a follow-up between 8 weeks and 18 months. PD motor symptoms is mostly used as primary outcome while various secondary outcomes are reported. We need more trials that use both clinical endpoints and markers of neuroplasticity, and provide insight into the optimal exercise mode, duration and intensity.
INTRODUCTION
Exercise is of increasing interest in the treatment of people with Parkinson’s disease (PD), because of both symptomatic and putative disease-modifying effects. Symptomatic effects include a positive influence on balance, gait and other motor symptoms [1–5], as well as beneficial effects on non-motor symptoms such as depression [6, 7] and cognition [8, 9]. In addition, exercise is among only a few available interventions for PD for which there is compelling human and laboratory evidence of a disease-modifying potential [10–12]. Large epidemiological studies of healthy people showed that those who exercise more are less likely to be diagnosed with PD [13]. The same trend is observed in people with clinically manifest PD, where people who are more active experienced a slower deterioration of gait and activities of daily living [14]. Even reduced mortality has been reported [15]. Importantly, complementary bench studies in experimental models of PD showed that rodents that exercise regularly are more resistant to the neurodegeneration characteristic of PD, with evidence for adaptive plasticity in the brain [10, 11]. These converging lines of evidence suggest that exercise might slow the clinical progression of PD.
The mechanisms of action underlying these effects remain largely unknown. Multiple, interdependent pathways have been postulated such as changes in inflammatory factors, anti-oxidative stress factors, brain atrophy and compensatory neuroplasticity [12, 16, 17]. Despite an enormous amount of research on exercise in PD, only a few studies to date have studied the (interrelatedness of) underlying mechanisms of action by assessing imaging or blood-based biomarkers. A recent exploratory analysis in a subgroup of participants of one of the hallmark studies on aerobic exercise (the Park-in-Shape study) [18] showed increased functional connectivity and reduced global brain atrophy in people with PD who performed moderate to high intensity aerobic exercise, three times per week (compared to a stretching control group) [19]. However, this study had a short follow-up duration of 6 months, was not robustly statistically powered for imaging outcomes (a convenience sample of n = 56 was included) and did not include any blood markers to study interrelatedness and relative importance of different mechanisms. Multiple other studies have shown an increase in neurotrophic factors, such as a blood derived marker of neuronal development (BDNF), but did not include imaging outcomes and also mostly included small samples [17].
Moreover, up to now it is unclear what type and dosage of exercise is most effective, as different doses and types of exercise have hardly been compared directly. High-intensity aerobic exercise is thought to have the largest disease modifying potential [1, 4], but recent evidence indicates that intensity may not be the most important driver of neuroplasticity in patient populations [20]. Observational studies in PD show that the volume of exercise matters as well, independent of intensity [15, 21, 22]. Importantly, high-intensity exercise has never been directly compared to high-volume exercise in a randomized controlled trial (RCT). Finally, it is relevant to clearly describe studied interventions in order to translate them in daily clinical practice. In general, little details on exact intensity and delivery mode is given in scientific reports, as well as on adherence rates.
Recent reviews summarized the published studies on aerobic exercise [4, 23]. Therefore, we aim to give an overview of all ongoing registered Randomized Clinical Trials (RCTs) on aerobic exercise in PD.
OVERVIEW OF RANDOMIZED CONTROLLED TRIALS OF AEROBIC EXERCISE IN PD
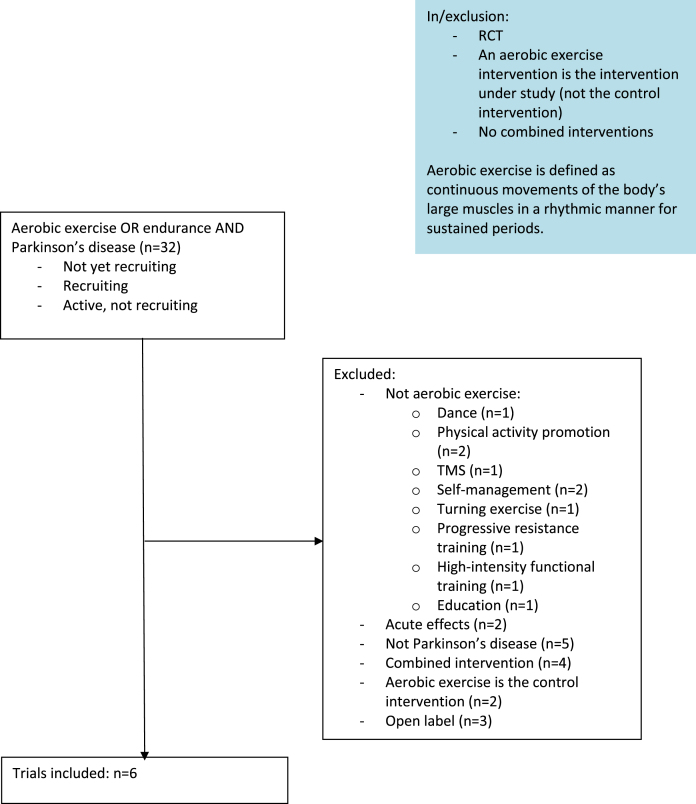
We searched ClinicalTrials.gov for “not yet recruiting”, “recruiting” and “active, not recruiting” interventional studies in Parkinson’s disease with the terms “aerobic exercise OR endurance exercise” (Fig. 1), resulting in the inclusion of six studies. Table 1 summarizes the key elements of the included trials. In the following paragraphs, we will first discuss some general characteristics of the studies. We have evaluated whether the following components were described according to the Consensus on Exercise Reporting Template (CERT): materials, provider, delivery, location, dosage, tailoring, and compliance [24].
Fig. 1.
Flowchart.
Table 1.
Overview of clinical trials on aerobic- or endurance exercise in PD. IPD = Individual Participant Data. NA = Not Applicable
| Sponsor | ID | Exercise mode | Phase | Estimated Enrolment | Estimated completion date | IPD sharing statement |
| University of Aarhus | NCT04379778 | Not specified | NA | 70 | February 2023 | No |
| VA Office of Research and Development | NCT03808675 | Walking | 2 | 100 | June 30 2024 | Yes |
| The Cleveland Clinic | NCT04000360 | Cycle ergometer | 2 and/or 3 | 252 | January 31 2024 | No |
| Saglik Bilimleri Universitesi | NCT05511402 | Not specified | NA | 28 | December 10 2022 | No |
| Pacific Parkinson’s Research Centre | NCT04426786 | Cycling ergometer | NA | 40 | June 1 2023 | No |
| Northwestern University | NCT04284436 | Treadmill walking | 3 | 370 | July 31 2025 | Yes |
Population
The sample sizes vary between 28 and 370. All studies include people with PD in Hoehn and Yahr stage 1 to 3.
Interventions
Only limited information about the interventions is reported in the study registrations. Two studies use indoor cycling as an exercise mode, one study uses treadmill walking and one other uses either outdoor or indoor walking. Two studies do not specify the type of training performed and none of the studies specify the type or amount of supervision or coaching. In one study the intervention is performed in a group, while one other study mentions a home-based protocol. The frequency of exercise varies between two to four times per week with a duration of at least 30 minutes per session. The intensity of the intervention is clarified in all except one study, while it remains unclear how it is offered or adapted to the participant, especially in the beginning of the intervention.
Primary and secondary outcome measures
The MDS-UPDRS motor score (MDS-UPDRS part III) is the most prevalent primary outcome (three studies). Other primary outcomes include effective transverse relaxation rate R2 (MRI), brain energetics and antioxidant status. The LTAE-PD study and the study on the effect on oxidative stress markers report multiple primary outcomes. A wide variety of secondary outcomes such as mobility, balance, cardiorespiratory fitness, gait, non-motor symptoms, cognition, dopaminergic activity and blood biomarkers are assessed.
Markers of neuroplasticity
Only one study includes both imaging and biochemical markers for neuroplasticity: the SPARX3 trial (NCT04284436). They assess BDNF, blood derived markers of inflammation (C-reactive protein levels; CRP) and dopaminergic activity (striatal specific binding ratio). However, no structural or functional MRIs are performed. One study includes oxidative stress markers, and two studies report performing imaging: one structural and functional imaging and one to determine brain energetics (CMRO2, cerebral metabolic rate of oxygen). Two of the included studies only assess functional outcomes.
Follow-up duration
The duration of the interventions varies from 8 weeks to 12 months. SPARX3 assesses participants also at 18 months.
Blinding
Assessors are blinded in all trials, while none of the trials blinds the participants. Blinding of participants is very challenging in exercise studies. The EBEPD study includes an active control group (stretching and/or yoga) with delayed start, but does not mention whether or not the participants are blinded for the randomization options.
Compliance
No information is shared on how compliance is monitored.
IPD sharing statement
Individual participant data (IPD) will be shared by two out of the six studies only. To benefit most from the large effort of aerobic exercise trials, data should be shared.
AARHUS UNIVERSITY - AEROBIC EXERCISE AND BRAIN HEALTH IN PARKINSON’S
Title: Effects of Aerobic Exercise on Brain Health in Parkinson’s Disease.
Phase: NA
Objective: To investigate whether 24 weeks of aerobic exercise (AE) can delay PD progression markers and improve motor/non-motor symptoms in PD.
Status: Recruiting.
Clinicaltrials.gov ID: NCT04379778.
Sponsor: University of Aarhus.
Collaborators: University of Aarhus.
Estimated Enrolment: 70.
Estimated Completion Date: February 2023.
Study Design: The study is a single blind (assessor), randomized clinical trial. Participants (N = 70) are randomly assigned (parallel assignment 1 : 1) to 24 weeks (60 sessions) of supervised AE at moderate to high intensity, or standard care. Assessments are performed at baseline, after the intervention (24 weeks) and after 48 weeks. The trial is recruiting people diagnosed with PD in the last 5 years, age between 40 and 100 years, Hoehn and Yahr 3 or lower and who are able to transport themselves to the exercise location. People who report alcohol abuse, depression, have a pacemaker, have comorbidities that hamper participation in the intervention, are pregnant, have metallic implants that prevent MRI, expect exercise adherence below 85% of all sessions or are already participating in moderate to high level of aerobic exercise more than twice per week will be excluded.
Intervention: Moderate to high intensity aerobic exercise, 24 weeks, 60 sessions. Control group: standard care.
Outcome Measures: Primary: Effective transverse relaxation rate (R2*) MRI change [Time Frame: 0, 24 and 48 wks]. Secondary: -MRI (QSM, volumetry, neuromelanin, DKI), blood markers (alpha-synuclein), levodopa equivalent dose, motor symptoms (MDS-UPDRS), VO2max, mobility and balance (Timed Up and Go (TUG), six minute walk test (6MWT), MiniBESTest), cognition (Montreal Cognitive Assessment, MoCa), depression (Beck Depression Scale), non-motor symptoms (NMS), quality life (Parkinson’s Disease Questionnaire-39 (PDQ-39)).
Comments: The trial is focused on understanding the possible neuroprotective effects of aerobic exercise. The type of aerobic exercise is not further specified. Participants are followed up directly after termination of the intervention (24 weeks) and again 24 weeks after the intervention (48 weeks). This is a strength of the study, which also allows for studying effects after wash-out. This trial will provide important insights into the impact of aerobic exercise on PD symptoms and potentially on disease progression. The duration of the trial and the sample size may, however not be sufficient to find evidence for slowed disease progression.
Results: The estimated primary completion date passed in July 2022, the study is expected to be completed in February 2023.
References: -
VA OFFICE OF R&D –AEROBIC EXERCISE IN PARKINSON’S DISEASE (LTAE-PD)
Title: Long Term Aerobic Exercise to Slow Progression in Parkinson’s Disease.
Phase: 2
Objective: To fill the translational gap by determining the biological, clinical, and functional effects of long-term aerobic exercise (LTAE) in PD.
Status: Recruiting
Clinicaltrials.gov ID: NCT03808675
Sponsor: VA Office of Research and Development
Collaborators: University of Iowa
Estimated Enrolment: 100
Estimated Completion Date: June 30 2024
Study Design: The study is a single blind (assessor) randomized clinical trial. Participants (N = 100) are randomly assigned (parallel assignment 1 : 1) to one year of self-administered moderate aerobic exercise (continuous walking at 40-59% of heart rate reserve or 64-77% of heart rate at gas exchange threshold, 3 times/week, 50 mins/session) or usual care with health education. Assessments are performed at baseline and after one year. The trial is recruiting people diagnosed with Parkinson’s Disease, age 40 years or older, Hoehn and Yahr 1-3, on stable dopaminergic treatment for 4 weeks or longer and VO2max below “very good” fitness level for their age and gender at baseline, no dementia and have to be a currently active driver. People with secondary parkinsonism, history of brain surgery for PD, corrected visual acuity less than 20/50, contra-indications for exercise, confounding acute or unstable medical, psychiatric or orthopedic condition, clinically significant traumatic brain injury (TBI) or post-traumatic stress disorder (PTSD), Beck Depression inventory > 15, history of exposure to (a)typical antipsychotics or other dopamine blocking agents within 6 months prior to baseline, use of investigational drugs within 30 days prior to screening, not on stable regimen of central nervous system acting medications within 30 days prior to screening or have a contraindication for brain MRI are excluded.
Intervention: Moderate intensity exercise (walking outdoor), 3 sessions per week, 50 minutes per session, 40-59% heart rate reserve (HRR). Control group: usual care+health education (freely available videos).
Outcome Measures: Primary: motor symptoms (MDS-UPDRS motor score OFF medication), cognitive function (Eriksen’s Flanker Test), driving (driving safety test), brain tissue integrity (DTI), non-motor symptoms (MDS-UPDRS part I), quality of life (PDQ-39). Secondary: Motor symptoms (MDS-UPDRS motor score ON medication), dexterity (9 hole peg board), cognitive function (COGSTAT, MMSE), brain tissue integrity (radial diffusivity on diffusion imaging tractography), depression (Geriatric Depression Scale), motor experiences of daily living score, anxiety (Beck Anxiety Inventory), sleep (Parkinson’s disease Sleep Scale), fatigue (Fatigue Severity Scale), walking speed, finger tapping, endurance (6MWT), activities of daily life (Schwab & England), vision (Pelli-Robson contrast sensitivity, Early Treatment Diabetic Retinopathy Study (ETDRS Acuity Log Score), EEG.
Comments: This trial will provide important insights into the effect of one-year moderate aerobic exercise on brain function and symptoms. Five primary outcome measures are reported, which is unusual, in addition to many secondary outcomes. Some new, but interesting outcomes (i.e. driving and vision) are used. It is not clear whether the trial is powered, and if so, based on what outcome measure power calculations were performed. The aerobic intervention is self-administered, which is a risk of compliance. Information on how compliance to the intervention is determined is lacking. In their previous trial the authors used electronic heart rate and walking speed monitors and exercise diaries [25].
Results: The estimated completion date is June 30 2024.
References: -
CLEVELAND CLINIC –PRAGMATIC CYCLICAL LOWER EXTREMITY EXERCISE TRIAL FOR PARKINSON’S DISEASE
Title: Pragmatic Cyclical Lower Extremity Exercise Trial for Parkinson’s Disease.
Phase: 2
Objective: Study the disease-altering capabilities of a long-term, high-intensity aerobic exercise intervention.
Status: Active, not recruiting.
Clinicaltrials.gov ID: NCT04000360.
Sponsor: The Cleveland Clinic.
Collaborators: National Institutes of Health (NIH).
Estimated Enrolment: 252.
Estimated Completion Date: January 31 2024.
Study Design: The study is a multi-site, pragmatic, single blind (assessor) randomized clinical trial. Participants (N = 252) will be randomly assigned to 12 months home-based aerobic exercise (cycling at 60-80% of heart rate reserve at a target cadence of 80-90 revolutions/minute, 3 times/week, 45 mins/session) or usual care. Participants in the aerobic exercise group receive a stationary bicycle at home and a heart rate monitor strap. The progression of each participant in the aerobic exercise group is monitored by a physical therapist. Participants in both groups receive activity monitors to monitor their daily activity levels. Assessments are performed at baseline, 6 and 12 months. The trial is recruiting people diagnosed with Parkinson’s Disease, age 18 years or older, Hoehn and Yahr 1-3, able to safely (dis)mount a stationary bicycle and having in-home wireless network. People participating in pharmaceutical or behavioral disease modifying PD clinical trials, diagnosed with dementia or cognitive impairment that hampers ability to provide informed consent, have implanted deep brain stimulated electrodes, do not get medical clearance from their health care provider, have any musculoskeletal issues that might limit one’s ability to engage in exercise or have any other neurological disease than PD are excluded.
Intervention: Home based indoor cycling, 3 times per week, 12 months, 60-80% HRR. Control group: usual care.
Outcome Measures: Primary: motor symptoms (MDS-UPDRS III motor score OFF medication). Secondary: dexterity (nine hole peg test), processing speed time (Processing Speed Test Score), disease symptoms (MDS-UPDRS part I, II and IV), mobility, balance and cardiovascular fitness (TUG, 10MWT, 6MWT), cognition (Trail Making Test, Visual memory test, processing speed test), postural stability (sway area and volume), self-reported falls, quality of life (Neuro-QoL).
Comments: This trial will provide important insights into the effect of one-year aerobic exercise on PD symptoms in a large sample. The sample size is, however, not justified. In addition, while the objective is to study the disease altering effects of aerobic exercise, no biomarkers of neuroplasticity (either imaging or neurochemical) were included. Moreover, the effect of physical fitness as a possible mediator is not assessed.
Results: The estimated study completion date is April 30 2024.
References: Alberts JL, Rosenfeldt AB, Lopez-Lennon C, et al. Effectiveness of a Long-Term, Home-Based Aerobic Exercise Intervention on Slowing the Progression of Parkinson Disease: Design of the Cyclical Lower Extremity Exercise for Parkinson Disease II (CYCLE-II) Study. Physical therapy 2021; 101(11).
PACIFIC PARKINSON’S RESEARCH CENTRE - EXERCISE & BRAIN ENERGETICS IN PD (EBEPD)
Title: The Effect of Exercise on Brain Energetics in Parkinson’s Disease.
Phase: NA.
Objective: Study whether exercise has a positive impact on brain energetics using fMRI and PET (positron emission tomography) brain scanning in PD. The second objective is to study the effects of the intervention: while this will be a pilot study, involving limited exercise regimens, any knowledge gained about the impact of exercise on brain energetics will have a tremendous impact on the design of neuroprotective therapies and personalized treatment.
Status: Recruiting.
Clinicaltrials.gov ID: NCT04426786.
Sponsor: Pacific Parkinson’s Research Centre.
Collaborators: Natural Sciences and Engineering Research Council, Canada.
Estimated Enrolment: 40.
Estimated Completion Date: June 1 2023.
Study Design: The study is a single blind (assessor) randomized clinical trial. Thirty non-exercisers are randomly assigned to six months of immediate- or delayed supervised aerobic exercise (group cycling on ergometer, intensity based on baseline VO2max, 3 times/week; stretching and/or yoga in groups, 3 times/week, during the delay). Ten exercisers are only assessed at baseline. Assessments are performed at baseline and after six months. The trial recruits people diagnosed with Parkinson’s disease, age 40 to 80, Hoehn and Yahr 1-3, exercising less than 120 minutes per week (non-exercisers). People with atypical parkinsonism, significant osteoporosis or arthritis, having a history of cancer within 5 years prior to study participation, not able to tolerate being off PD medication for 24 hours are excluded. An extensive set of exclusion criteria, i.e. having other significant diseases or related to MRI, is formulated.
Intervention: The intervention group exercises on a cycle ergometer in groups, three times per week for six months. The exercise intensity is regulated by % VO2max. The control group performs stretching/yoga in groups, three times per week for six months.
Outcome Measures: Primary: CMRO2 (cerebral metabolic rate of oxygen) will be computed from MRI data and CMRGlu (cerebral metabolic rate of glucose) from PET data; their ratio will be used to assess brain energetics. Secondary outcomes: motor symptoms (MDS-UPDRS part III), cognition (MoCa).
Comments: This trial will provide insights into the effect of six months aerobic exercise on brain energetics. However, the exercise duration and intensity are not further specified. Moreover, aerobic capacity is not assessed, as a potential mediator of the effect. The sample size is relatively small and not supported by a sample size calculation. While the research team indicates that this is a double-blind study, patients are not blinded, which makes this a single blind study.
Results: The estimated study completion date is June 1 2023.
References:-
SAGLIK BILIMLERI UNIVERSITESI –EFFECT OF AEROBIC TRAINING ON OXIDATIVE STRESS MARKERS IN PATIENTS WITH PARKINSON’S DISEASE
Title: Effect of aerobic training on oxidative stress markers in patients with Parkinson’s disease
Phase: N.A.
Objective: To study the effect of aerobic exercise training on total antioxidant status, total oxidant status, glutathione, oxidative glutathione, and zonulin levels; and to study the relationship between change in motor symptoms and disease severity and change of total antioxidant status, total oxidant status, glutathione, oxidative glutathione, and zonulin levels.
Status: Not yet recruiting
Clinicaltrials.gov ID: NCT05511402
Sponsor: Saglik Bilimleri Universitesi
Collaborators: Gazi University
Estimated Enrolment: 28
Estimated Completion Date: December 10 2022
Study Design: This Is a single blinded randomized clinical trial with a follow-up duration of 8 weeks. Participants are randomly assigned to either an intervention group receiving aerobic exercise and conventional physiotherapy or a control group who will have only conventional physiotherapy. Assessments are performed by a blinded researcher at baseline and 8 weeks follow-up. People with PD in Hoehn & Yahr stage 1 to 3, who are at least 40 years of age, can be included. Participants will be excluded when they have any other neurological condition, or a cardiovascular, orthopedic, vestibular or rheumatic condition that hampers exercise training. Participants with visual, auditory or perception problems are also excluded.
Intervention: Aerobic exercise for 8 weeks, 3 times per week, 30 minutes per session combined with conventional physiotherapy (3 times per week, 45 minutes per session). The control group receives conventional physiotherapy for 8 weeks, 3 times per week, 45 minutes per session.
Outcome Measures: Primary outcome: total antioxidant status, total oxidant status, glutathione, oxidative glutathione, zonulin. Secondary outcomes: disease severity (MDS-UPDRS).
Comments: Of the included studies, this is the study with the smallest sample size and with the shortest duration. The outcomes related to oxidative stress are innovative and relevant for future hypothesis driven studies. The exercise intervention, however, is not well described.
Results: The estimated completion date is December 10 2022.
References:-
PHASE 3 STUDY IN FOCUS
NORTHWESTERN UNIVERSITY – STUDY IN PARKINSON DISEASE OF EXERCISE (SPARX3)
Title: Study in Parkinson Disease of Exercise Phase 3 Clinical Trial: SPARX3.
Phase: 3.
Objective: Study whether the progression of the signs of Parkinson’s disease is attenuated at 12 months when performing high-intensity endurance treadmill exercise in persons who have not initiated medication for PD.
Status: Recruiting.
Clinicaltrials.gov ID: NCT04284436.
Sponsor: Northwestern University.
Collaborators: University of Pittsburgh, The Parkinson Study Group.
Estimated Enrolment: 370.
Estimated Completion Date: July 31 2025.
Study Design: The study is a multi-site, single blind (assessor), randomized clinical trial. Participants (N = 370) are randomized across the active comparator (60-65% HRmax) or experimental group (80-85% HRmax). Both groups perform treadmill walking 4 times/week, 30 mins/session in the target heart rate for 12 months. Assessments are performed at baseline, after 12 and 18 months. The trial recruits people diagnosed with Parkinson’s disease within the last three years, age 40 to 80 years, Hoehn and Yahr less than 3, positive DaTscanTM SPECT by quantitative readout for idiopathic Parkinson disease. People treated with PD medications or expected to require PD medication within 6 months, use of dopamine receptor blockers, known cardiovascular, metabolic or renal disease, uncontrolled hypertension, orthostatic hypotension and standing systolic blood pressure below 100, MoCA score lower than 24, BDI score greater than 28, exercising at greater than moderate intensity (60-65% HRmax) for 120 minutes or more over the last six months, allergy to iodinated products, hypersensitivity to DaTscanTM SPECT, actively breast-feeding and/or pregnant or experiencing other conditions that might interfere with the ability to perform endurance exercises are excluded.
Intervention: High-intensity exercise, four times per week, 80-85% HR max for 12 months. The control group performs moderate intensity exercise, four times per week, 60-65% HRmax for 12 months.
Outcome Measures: Primary: Change from baseline in the MDS-UPDRS motor score (Part III) to 12 months follow-up. Secondary outcomes: Change in dopaminergic activity (striatal specific binding ratio) –12 months, change in motor symptoms of PD -18 months, change in walking capacity (6MWT)- 12 months, change in walking capacity (6MWT) - 18 months, change in activity (number of steps) –12 months, change in activity (number of steps) 18 months, change in cognitive function (MoCA) –12 months, change in cognitive function (MoCa) –18 months, change in fitness (VO2max) –12 months, change in fitness (VO2max) –18 months, change in Quality of life (PDQ-39) 12 months, change in Quality of life (PDS-39) –18 months, initiation of dopaminergic therapy (12 months), change in blood derived markers of inflammation (CRP) –12 months, change in blood derived marker of neuronal development (BDNF) –12 months, change in blood derived marker of neuronal development (BDNF) –18 months, change in stride length –12 months, change in stride length –18 months, change in turning velocity –12 months, change in turning velocity –18 months
Comments: This will be the first phase 3 trial on aerobic exercise in PD. The trial will provide important insights into the effects of twelve months of aerobic exercise on disease progression. The trial has a large sample size. Even though the sample size is not justified on clinicaltrials.gov and a design paper is not (yet) available, it is plausible that the sample size is based on earlier work of the research group [27, 28]. An extensive set of outcomes is applied assessing the impact on motor symptoms, cognition, daily walking activity, cardiorespiratory fitness and, importantly, neuroplasticity as assessed by striatal specific binding ratio and blood-derived biomarkers. A subset of the outcome measures is assessed at 18 months, which makes it possible to study wash out effects. The study does not contain a no intervention control group, but only a comparison between different exercise intensities.
Results: The estimated study completion date is June 1 2023.
References: Schenkman M, Moore CG, Kohrt WM, et al. Effect of High-Intensity Treadmill Exercise on Motor Symptoms in Patients With De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA neurology 2018; 75(2): 219-26.
Moore CG, Schenkman M, Kohrt WM, Delitto A, Hall DA, Corcos D. Study in Parkinson disease of exercise (SPARX): Translating high-intensity exercise from animals to humans. Contemp Clin Trials 2013; 36(1): 90-8.
[29] Patterson CG, Joslin E, Gil AB, Spigle W, Nemet T, Chahine L, Christiansen CL, Melanson E, Kohrt WM, Mancini M, Josbeno D, Balfany K, Griffith G, Dunlap MK, Lamotte G, Suttman E, Larson D, Branson C, McKee KE, Goelz L, Poon C, Tilley B, Kang UJ, Tansey MG, Luthra N, Tanner CM, Haus JM, Fantuzzi G, McFarland NR, Gonzalez-Latapi P, Foroud T, Motl R, Schwarzschild MA, Simuni T, Marek K, Naito A, Lungu C, Corcos DM; SPARX3-PSG Investigators. Study in Parkinson’s disease of exercise phase 3 (SPARX3): study protocol for a randomized controlled trial. Trials. 2022 Oct 6;23(1):855.
DISCUSSION/COMMENT/REFLECTION
This overview gives a valuable summary of the evidence on the effectiveness of aerobic exercise that can be expected in the (near) future. The expectations of the impact of exercise on both clinical features as well as brain function and structure are high because of the converging lines of evidence for its potential disease modifying effect [1, 4, 15, 19, 29, 30]. The studies that are currently performed will help to move the field forward. The SPARX3 trial in particular, which is well designed and adequately powered, will inform us on both the clinical and the potential disease modifying effects of high intensity aerobic exercise (compared to moderate intensity aerobic exercise). Unfortunately, most studies will not be able to obtain a comprehensive picture of the disease modifying potential, because of (inadvertent) limitations in the available set of outcomes. In addition, most studies are not based on a formal sample size calculation, which will make it difficult to draw conclusions regarding the statistical robustness of their results. This review also highlights that there is still some room for improvement in reporting exercise intervention according to a standard template like the CERT standard [24]. For instance, the trial protocols would benefit from more information on exercise supervision, coaching or guidance, on where the exercise intervention is performed and on how adherence is monitored. Most studies do report the frequency, duration and intensity of the exercise intervention(s).
While the number of exercise interventions as well as the expectations of their effects are increasing, the evidence, especially on its disease modifying effect remains relatively low. Many different interventions are sequentially being evaluated in small studies with a short duration and never pass the pilot testing phase. Different sets of outcome measures are used, making it impossible to compare different types of interventions. To push the current field forward there is a clear need for 1) robust, but flexible and efficient trials, for example using innovative designs such as the multi arm multi stage platform trial; 2) trials using a standard set of outcome measures focused on both clinical endpoints and markers for neuroplasticity and disease modification, that also share their data with the research community; 3) trials comparing different types of interventions or intervention doses.
In conclusion, while the current evidence emphasizes the potential of aerobic exercise, several key questions on its disease-modifying properties are therefore yet to be answered. Consequently, there is an urgent need for adequately designed and powered RCTs to unravel the disease-modifying effects of exercise and their underlying mechanisms in humans with PD.
REFERENCES
- [1]. Schootemeijer S, van der Kolk NM, Bloem BR, de Vries NM (2020) Current Perspectives on Aerobic Exercise in People with Parkinson’sDisease. Neurotherapeutics 17, 1418–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Radder DLM, Lígia Silva de Lima A, Domingos J, Keus SHJ, vanNimwegen M, Bloem BR, de Vries NM (2020) Physiotherapy inParkinson’s Disease: A Meta-Analysis of Present TreatmentModalities. Neurorehabil Neural Repair 34, 871–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Bloem BR, de Vries NM, Ebersbach G (2015) Nonpharmacological treatments for patients with Parkinson’s disease. Mov Disord 30, 1504–1520. [DOI] [PubMed] [Google Scholar]
- [4]. Gamborg M, Hvid LG, Dalgas U, Langeskov-Christensen M (2022) Parkinson’s disease and intensive exercise therapy –An updated systematic review and meta-analysis. Acta Neurol Scand 145, 504–528. [DOI] [PubMed] [Google Scholar]
- [5]. Mak MK, Wong-Yu IS, Shen X, Chung CL (2017) Long-term effects of exercise and physical therapy in people with Parkinson disease. Nat Rev Neurol 13, 689–703. [DOI] [PubMed] [Google Scholar]
- [6]. Feller D, Fox I, Gozzer P, Trentin F, Papola D (2022) Exercise for depressive symptoms in Parkinson’s disease: A systematic review and meta – analysis of randomized controlled trials. Arch Phys Med Rehabil 10.1016/j.apmr.2022.07.021. [DOI] [PubMed] [Google Scholar]
- [7]. Tian J, Kang Y, Liu P, Yu H (2022) Effect of Physical Activity on Depression in Patients with Parkinson’s Disease: A Systematic Review and Meta-Analysis. Int J Environ Res Public Health 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Farì G, Lunetti P, Pignatelli G, Raele MV, Cera A, Mintrone G, Ranieri M, Megna M, Capobianco L (2021) The Effect of PhysicalExercise on Cognitive Impairment in Neurodegenerative Disease: FromPathophysiology to Clinical and Rehabilitative Aspects. Int JMol Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9]. Intzandt B, Beck EN, Silveira CRA (2018) The effects of exercise on cognition and gait in Parkinson’s disease: A scoping review. Neurosci Biobehav Rev 95, 136–169. [DOI] [PubMed] [Google Scholar]
- [10]. Jang Y, Koo JH, Kwon I, Kang EB, Um HS, Soya H, Lee Y, Cho JY (2017) Neuroprotective effects of endurance exercise against neuroinflammation in MPTP-induced Parkinson’s disease mice. Brain Res 1655, 186–193. [DOI] [PubMed] [Google Scholar]
- [11]. Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW (2010) Enhancing neuroplasticity in the basalganglia: The role of exercise in Parkinson’s disease. MovDisord 25Suppl 1, S141–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Sujkowski A, Hong L, Wessells RJ, Todi SV (2022) The protective role of exercise against age-related neurodegeneration. Ageing Res Rev 74, 101543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Rafferty MR, Schmidt PN, Luo ST, Li K, Marras C, Davis TL, Guttman M, Cubillos F, Simuni T (2017) Regular Exercise, Quality of Life, and Mobility in Parkinson’s Disease: A Longitudinal Analysis of National Parkinson Foundation Quality Improvement Initiative Data. J Parkinsons Dis 7, 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Tsukita K, Sakamaki-Tsukita H, Takahashi R (2022) Long-term Effect of Regular Physical Activity and Exercise Habits in Patients With Early Parkinson Disease. Neurology 98, e859–e871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. Yoon SY, Suh JH, Yang SN, Han K, Kim YW (2021) Association of physical activity with all-cause mortality in Parkinson’s disease: Importance of total amount and maintenance. JAMA Neurol 78(12), 1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Darweesh SKL, De Vries NM, Helmich RC, Verbeek MM, Schwarzschild MA, Bloem BR (2022) Inhibition of Neuroinflammation May Mediate the Disease-Modifying Effects of Exercise: Implications for Parkinson’s Disease. J Parkinsons Dis 12, 1419–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17]. Ruiz-González D, Hernández-Martínez A, Valenzuela PL, Morales JS, Soriano-Maldonado A (2021) Effects of physical exerciseon plasma brain-derived neurotrophic factor in neurodegenerativedisorders: A systematic review and meta-analysis of randomizedcontrolled trials. Neurosci Biobehav Rev 128, 394–405. [DOI] [PubMed] [Google Scholar]
- [18]. van der Kolk NM, de Vries NM, Kessels RPC, Joosten H, Zwinderman AH, Post B, Bloem BR (2019) Effectiveness of home-based and remotely supervised aerobic exercise in Parkinson’s disease: A double-blind, randomised controlled trial. Lancet Neurol 18(11), 998–1008. [DOI] [PubMed] [Google Scholar]
- [19]. Johansson ME, Cameron IGM, Van der Kolk NM, de Vries NM, Klimars E, Toni I, Bloem BR, Helmich RC (2022) Aerobic Exercise Alters Brain Function and Structure in Parkinson’s Disease: A Randomized Controlled Trial. Ann Neurol 91, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20]. Hortobágyi T, Vetrovsky T, Balbim GM, Sorte Silva NCB, Manca A, Deriu F, Kolmos M, Kruuse C, Liu-Ambrose T, Radák Z, Váczi M, Johansson H, Dos Santos PCR, Franzén E, Granacher U (2022) The impact of aerobic and resistance training intensity on markersof neuroplasticity in health and disease. Ageing Res Rev 80, 101698. [DOI] [PubMed] [Google Scholar]
- [21]. de Vries NM, Darweesh SKL, Bloem BR (2021) Citius, Fortius, Altius-Understanding Which Components Drive Exercise Benefits in Parkinson Disease. JAMA Neurol 78(12), 1443–1445. [DOI] [PubMed] [Google Scholar]
- [22]. Paul KC, Chuang YH, Shih IF, Keener A, Bordelon Y, Bronstein JM, Ritz B (2019) The association between lifestyle factors and Parkinson’s disease progression and mortality. Mov Disord 34, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Aburub A, Ledger SJ, Sim J, Hunter SM (2020) Cardiopulmonary Function and Aerobic Exercise in Parkinson’s: A Systematic Review of the Literature. Mov Disord Clin Pract 7, 599–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24]. Slade SC, Dionne CE, Underwood M, Buchbinder R, Beck B, Bennell K, Brosseau L, Costa L, Cramp F, Cup E, Feehan L, Ferreira M, Forbes S, Glasziou P, Habets B, Harris S, Hay-Smith J, Hillier S, Hinman R, Holland A, Hondras M, Kelly G, Kent P, Lauret GJ, Long A, Maher C, Morso L, Osteras N, Peterson T, Quinlivan R, Rees K, Regnaux JP, Rietberg M, Saunders D, Skoetz N, Sogaard K, Takken T, van Tulder M, Voet N, Ward L, White C (2016) Consensus on Exercise Reporting Template (CERT): Modified Delphi Study. Phys Ther 96, 1514–1524. [DOI] [PubMed] [Google Scholar]
- [25]. Uc EY, Doerschug KC, Magnotta V, Dawson JD, Thomsen TR, Kline JN, Rizzo M, Newman SR, Mehta S, Grabowski TJ, Bruss J, Blanchette DR, Anderson SW, Voss MW, Kramer AF, Darling WG (2014) Phase I/II randomized trial of aerobic exercise in Parkinson disease in a community setting. Neurology 83, 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Alberts JL, Rosenfeldt AB, Lopez-Lennon C, Suttman E, Jansen AE, Imrey PB, Dibble LE (2021) Effectiveness of a Long-Term, Home-Based Aerobic Exercise Intervention on Slowing the Progression of Parkinson Disease: Design of the Cyclical Lower Extremity Exercise for Parkinson Disease II (CYCLE-II) Study. Phys Ther 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27]. Schenkman M, Moore CG, Kohrt WM, Hall DA, Delitto A, Comella CL, Josbeno DA, Christiansen CL, Berman BD, Kluger BM, Melanson EL, Jain S, Robichaud JA, Poon C, Corcos DM (2018) Effectof High-Intensity Treadmill Exercise on Motor Symptoms in PatientsWith De Novo Parkinson Disease: A Phase 2 Randomized Clinical Trial. JAMA Neurol 75, 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Moore CG, Schenkman M, Kohrt WM, Delitto A, Hall DA, Corcos D (2013) Study in Parkinson disease of exercise (SPARX): Translating high-intensity exercise from animals to humans. Contemp Clin Trials 36, 90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Patterson CG, Joslin E, Gil AB, Spigle W, Nemet T, Chahine L, Christiansen CL, Melanson E, Kohrt WM, Mancini M, Josbeno D, Balfany K, Griffith G, Dunlap MK, Lamotte G, Suttman E, Larson D, Branson C, McKee KE, Goelz L, Poon C, Tilley B, Kang UJ, Tansey MG, Luthra N, Tanner CM, Haus JM, Fantuzzi G, McFarland NR, Gonzalez-Latapi P, Foroud T, Motl R, Schwarzschild MA, Simuni T, Marek K, Naito A, Lungu C, Corcos DM; SPARX3-PSG Investigators (2022) Study in Parkinson’s disease of exercise phase 3 (SPARX3): study protocol for a randomized controlled trial. Trials 23(1), 855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30]. Petzinger GM, Fisher BE, McEwen S, Beeler JA, Walsh JP, Jakowec MW (2013) Exercise-enhanced neuroplasticity targeting motor and cognitive circuitry in Parkinson’s disease. Lancet Neurol 12, 716–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Toy WA, Petzinger GM, Leyshon BJ, Akopian GK, Walsh JP, Hoffman MV, Vuckovic MG, Jakowec MW (2014) Treadmill exercise reverses dendritic spine loss in direct and indirect striatal medium spiny neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of Parkinson’s disease. Neurobiol Dis 63, 201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]