Abstract
Recently, the aging population has increased exponentially around the globe bringing more challenges to improve quality of life in those populations while reducing the economic burden on healthcare systems. Aging is associated with changes in the immune system culminating in detrimental effects such as immune dysfunction, immunosenescence, and chronic inflammation. Age-related decline of immune functions is associated with various pathologies including cardiovascular, autoimmune, neurodegenerative, and infectious diseases to name a few. Conventional treatment addresses the onset of age-related diseases by early detection of risk factors, administration of vaccines as preventive care, immunomodulatory treatment, and other dietary supplements. However, these approaches often come with systemic side-effects, low bioavailability of therapeutic agents, and poor outcomes seen in the elderly. Recent innovations in nanotechnology have led to the development of novel biomaterials/nanomaterials, which explore targeted drug delivery and immunomodulatory interactions in vivo. Current nanotechnology-based immunomodulatory approaches that have the potential to be used as therapeutic interventions for some prominent age-related diseases are discussed here. Finally, we explore challenges and future aspects of nanotechnology in the treatments of age-related disorders to improve quality of life in the elderly.
This article is categorized under:
Therapeutic Approaches and Drug Discovery > Nanomedicine for Cardiovascular Disease
Therapeutic Approaches and Drug Discovery > Nanomedicine for Neurological Disease
Therapeutic Approaches and Drug Discovery > Emerging Technologies
Keywords: age-related diseases, aging, immunomodulation, nanoparticles, nanotechnology
Graphical Abstract
1 |. INTRODUCTION
Humans have long pursued ways to extend life and restore health, as portrayed in the lore’s of the “fountain of youth” through many cultures and times in history. Delayed aging and its associated healthcare spending are expected to be of an economic value of $7.1 trillion over 50 years (Goldman, 2015). The global population of individuals over the age of 65 is more than 600 million and growing at an unprecedented rate and expected to reach 1.6 billion by 2050; nearly 20% of the total population over 65 (Inouye et al., 2021). Typically, aging is discussed in terms of biological and chronological aspects. Biological aging is associated with a decrease in the reparative and regenerative potential in tissues and organs. This reduction manifests as a diminished physiological reserve in response to stress (i.e., homeostenosis). These effects are more significant in the immune system due to aging-associated immune decline. Chronological age, on the other hand, is defined by the number of years of life after birth of an individual and is often used as a prognostic marker for functional impairments, chronic diseases, and mortality. This shows the ambiguity in representation of age because people with disease conditions, where biological aging does not equate with chronological age and the age statutes present to undergo various age related testing are impractical, and use of biological aging measures needs to be adopted by healthcare facilities around the globe (Elliott et al., 2021). For instance, the Medicare age in the United States needs to be lowered to gain access to preventive healthcare in accelerated aging individuals with known risks from hereditary or other health challenges (Elliott et al., 2021). Overall, aging is a major risk factor for many medical conditions, and most older individuals are affected by multiple disease conditions, leading to complex drug treatments and increased risks of adverse drug reactions (ADR) (Brahma et al., 2013). To overcome these challenges, novel strategies like nanotechnology-based drug delivery systems have shown promise to treat age-related diseases more efficiently than conventional medicine.
2 |. THE PROBLEM OF AN AGING IMMUNE SYSTEM
With age, cellular processes become less efficient, ultimately leading to abnormalities in cellular mechanisms including senescence, as reported by various single cell analysis studies (Chintapula et al., 2020). Senescent phenotype in immune cells becomes more prominent with age, resulting in changes of immune system function, an occurrence known as immunosenescence. The immune system plays a major role in protecting against various pathogens in food and the environment and helps in tissue regeneration. Immune response decline with age is associated with alterations in T cell repertoire with a prominent reduction in naïve CD8+ (cluster of differentiation 8) T cells, apoptosis pathways, deregulation of cytokine production, and accumulation of memory and effector immune cells, leading to immunosenescence, often correlated with survival and fragility (Ventura et al., 2017). Age-related reduction in naïve CD8+ T cell populations leads to reduced immunosurveillance and increased risks for diseases, especially infectious diseases. Chronic inflammation arising due to oxidative stress from infections faced through aging can alter arginine transportation in endothelial cells which regulates NO level in response to the stress, leading to endothelial dysfunction (Castellon & Bogdanova, 2016). Considerably, immune system alterations often lead to autoimmune conditions and endothelial dysfunction, which are now considered new cardiovascular risk diseases (Castellon & Bogdanova, 2016). Immune dysfunction, as a result of crosstalk and feedback irregularities among various other age-related processes, affects a string of functional mechanisms and targets within the immune system which justify higher vulnerability to infections, autoimmune diseases, incidence of an inflammatory phenotype and other age-associated pathologies (Fülöp et al., 2007).
Aging induces increased chronic inflammation or inflammaging (low-grade inflammation) with higher concentrations of inflammatory cytokines such as Interleukin-1 β (IL-1β), IL-6, IL-8, and tumor necrosis factor α (TNF-α) together with acute phase proteins seen in the serum (Paudel et al., 2019). Inflammaging is one of the major factors directly linked with neurodegeneration responsible for causing the significant decline of the neuronal functions with aging (Stephenson et al., 2018). The impairment of microglia and macrophages with reduced motility and dysfunctional phagocytosis, respectively, results in an induction of reactive oxygen species (ROS) generation, a precursor for inflammation and aging-associated brain disorders (Koellhoffer et al., 2017). Inflammation and oxidative stress are interrelated in several age-related disorders such as cancer, cardiovascular disease, arthritis, and other neurodegenerative diseases (Paudel et al., 2019). Overall, immunosenescence with its related detrimental inflammatory responses is a prominent phenomenon involved in the progression of age-related diseases. Modulating the immune system to revert to a younger phenotype, inhibit or aid in reducing the effects of immunosenescence may help in preventing and treating underlying conditions of age-related diseases.
3 |. CURRENT TREATMENT LIMITATIONS AND SCOPE OF NANOTECHNOLOGY
Recent medical innovations in the pharmaceutical industry have led to the development of various immunomodulatory drugs to improve the conditions of age-related diseases including both preventative and conventional medicine. These novel drugs address the symptoms but fail to improve the underlying immune decline. Caloric restriction, fasting-based diets, exercise, and other supplements, including antioxidants and senolytics, have gained popularity to improve aging-related immune decline seen in humans (Aiello et al., 2019; Ponnappan & Ponnappan, 2011). Various other interventions, including hormone therapy, cytokine treatment, stem cell delivery, delivery of antibodies, and exercise are discussed as potential interventions to modulate the immune system and reverse the decline seen in the aging population (Aiello et al., 2019). Although free drugs or biological agents administered intravenously or orally show improvement in age associated diseases, they are often associated with side effects (Table 1) hindering their therapeutic efficiencies, especially in chronic conditions of the aging population (Bourke et al., 2016; Ponnappan & Ponnappan, 2011). Consequently, the current drug delivery approaches toward age-related diseases have low bioavailability, off target effects, and importantly, ADR (Khan & Roberts, 2018; Perrie et al., 2012). In addition, age-related changes lead to poor pharmacokinetic profiles from current drug delivery strategies (Mangoni & Jackson, 2004). Keeping in mind the chronic conditions associated in age-related diseases, it is highly desirable to improve the bioavailability of the drug at the target site steering toward higher therapeutic efficacies and reduction in hospitalizations caused by ADR from multiple doses (Brahma et al., 2013).
TABLE 1.
Current treatments of age associated diseases and their limitations
| Disease | Current medications and interventions | Limitations of clinical interventions |
|---|---|---|
| Neurodegenerative diseases | ||
| Alzheimer’s | Cholinesterase inhibitors, memantine, selegiline (Schmitt et al., 2004) | Nausea, muscle weakness, irregular breathing, and heartbeat |
| Parkinson’s | Levodopa, selegiline, COMT inhibitors, dopamine agonists, anticholinergic drugs, amantadine, apomorphine (Jankovic & Aguilar, 2008) | Motor fluctuations, dyskinesias |
| Huntington’s | Serotonin uptake inhibitors, mirtazapine, neuroleptics, methylphenidate, bupropion (Eddy et al., 2016), tetrabenazine and deutetrabenazine (Motor chorea; Potkin & Potkin, 2018) | Nausea, diarrhea, drowsiness, and low blood pressure |
| Amyotrophic lateral sclerosis (ALS) | Glutamate receptor antagonist (Riluzole) (Cheah et al., 2010), Free radical scavenger (Edaravone, 2017) (“Safety and efficacy of edaravone in well-defined patients with amyotrophic lateral sclerosis: a randomized, double-blind, placebo-controlled trial,” 2017) | ALS causes loss of muscle control, which results in swallowing problems of tablets (Riluzole) |
| Glaucoma | Prostaglandin analogs (Xalatan), beta blockers (Timoptic, Betagan) alpha-adrenergic drugs (Iopidine), cholinergic agonists (Pilopine) | Burning sensation, increased iris pigmentation, conjunctival hyperemia (Cuaron et al., 2016) |
| Autoimmune/degenerative diseases | ||
| Osteoarthritis | Acetaminophen, NSAIDs, IA injections of corticosteroids, antioxidants, vitamin D supplements, opioid analgesics (W. Zhang et al., 2019) | Liver related complications, GI tract related complications, post-injection pain, and septic arthritis |
| Osteoporosis | Alendronate, risedronate IR, zoledronic acid (Tu et al., 2018) | Upper GI tract discomfort, jaw osteonecrosis, and atypical femur fractures |
| Rheumatoid arthritis | Etanercept (Enbrel), IL1 inhibitor (Anakinra), JAK inhibitors (Tofacitinib, Baricitinib), IL-6, and its receptor inhibitor (Tocilizumab) (Köhler et al., 2019) | Inflammation at injection site, itching and pain, GI tract perforation, pulmonary embolism, neutropenia, deep vein thrombosis (Gaëtan Gavazzi et al., 2004). |
| Cardiovascular diseases | ||
| Coronary arterial disease | Anticoagulants, betablockers, statin drugs, anti-anginal, and calcium channel blocker (InformedHealth.org [Internet]. Cologne, 2006) | Re-narrowing of arteries, reduces blood clotting, weakness, and exhaustion due to lowered blood pressure (InformedHealth.org [Internet]. Cologne, 2006) |
| Atherosclerosis | Statin drugs (Rosuvastatin), antibodies (targeting upregulated cytokines) | Myotoxicity, hemorrhagic stroke, and type II diabetes (Pinal-Fernandez et al., 2018) |
| Myocardial infarction | Heparin, β-adrenoceptor blockers (Metoprolol, Atenolol), magnesium, and insulin (Maxwell, 1999) | Heart damage and abnormal heart rhythms (Maxwell, 1999) |
| Others | ||
| Type II diabetes | Sulfonylureas (insulin receptors), rosiglitazone (PPAR agonist), and metformin (Marín-Peñalver et al., 2016) | Hypoglycemia, cardiovascular complications—heart failure (Marín-Peñalver et al., 2016) |
| Photoaging/skin atrophy | Sunscreens (UVA and UVB filters), topical retinoids (tretinoin, tazarotene), and cosmeceuticals (Poon et al., 2015) | Skin irritation, erythema, scaling, and skin burning (Poon et al., 2015) |
Apart from all the limitations shown in Table 1 of the current approved drugs for age-associated diseases, many of the pharmacological treatments are based on the reduction of symptoms showing minimal to no therapeutic effect. Current conventional treatment for Alzheimer’s, which consists of cholinesterase inhibitors to increase acetylcholine (ACh) levels, can only mildly improve cognitive symptoms by a short period of time (1–3 years), but treatment fails to slow the progression of the disease or treat the underlying cause (Mauricio et al., 2019). Similarly, anti-inflammatory and pain-relieving agents like acetaminophen, non-steroidal anti-inflammatory drugs (NSAIDS), analgesics, and injections of corticosteroids are used to treat osteoarthritis (OA). These agents only reduce pain and local inflammation associated with OA via administration of multiple doses, but fail to address the causes that lead to joint degeneration and impaired immune function (Hermann et al., 2018). Furthermore, statins, beta blockers, heparin, and calcium channel blockers are utilized for the treatment of age-related cardiovascular diseases. However, these medications only help in the reduction of hepatocyte cholesterol content (Vaughan et al., 2000), reduction of adrenergic system to lower the sympathetic overactivation of the heart caused by coronary heart disease (CAD) (P. H. Lee et al., 2020), and prevention of thrombosis in atherosclerotic plaque, leaving the underlying causes of age-associated cardiovascular pathologies such as immunosenescence and inflammaging untreated.
Nanotechnology has gained significance in the field of drug delivery, in which nanotechnology-driven development of materials is being employed for customizable and controlled delivery of therapeutic drugs in situ. Added to drug delivery, nanotechnology is also being used as a diagnostic tool to image the pathologies of disease and probe disease risks using specific biomarkers (Shi et al., 2010). Nanotechnology offers a large array of tools and techniques for therapeutic delivery and diagnostic imaging for immunomodulatory approaches as described in detail by X. Feng et al. (2019). The majority of nanotechnology-driven medicinal applications involve nanoparticles (<1 μm) loaded with various therapeutic drugs with controlled release in situ with or without stimuli (pH, light, ultrasound, or enzyme). Compared with free drugs, very few nanoparticles (NPs) made of lipids (e.g., liposomes), polymers (e.g., poly[l-lactide-co-glycolic acid], PLGA), or metals (e.g., gold nanoparticles) are approved by the FDA (Figure 1). Advancement in material research is producing more biodegradable and biocompatible materials to aid in diagnostics and drug delivery (Fenton et al., 2018). Various targeting agents such as antibodies, peptides, and environmental stimuli (pH, ROS activity) are employed to decorate these nanoparticles to achieve active targeting in vivo. Here we discuss the benefits of recently reported nanomedicine strategies involving immunomodulation to treat various age-related diseases (Table 2). Persistent inflammation and changes in the immune landscape with age may need a sustained dosage to exert effective immunomodulatory responses to restore tissue homeostasis. This is an area where nanotechnology can play a crucial role in designing drug delivery platforms for targeted drug delivery and controlled drug release. Further sections will probe immunomodulation in age-related pathologies and the contributions of nanotechnology in the treatment of age-associated diseases through immunomodulatory responses.
FIGURE 1.
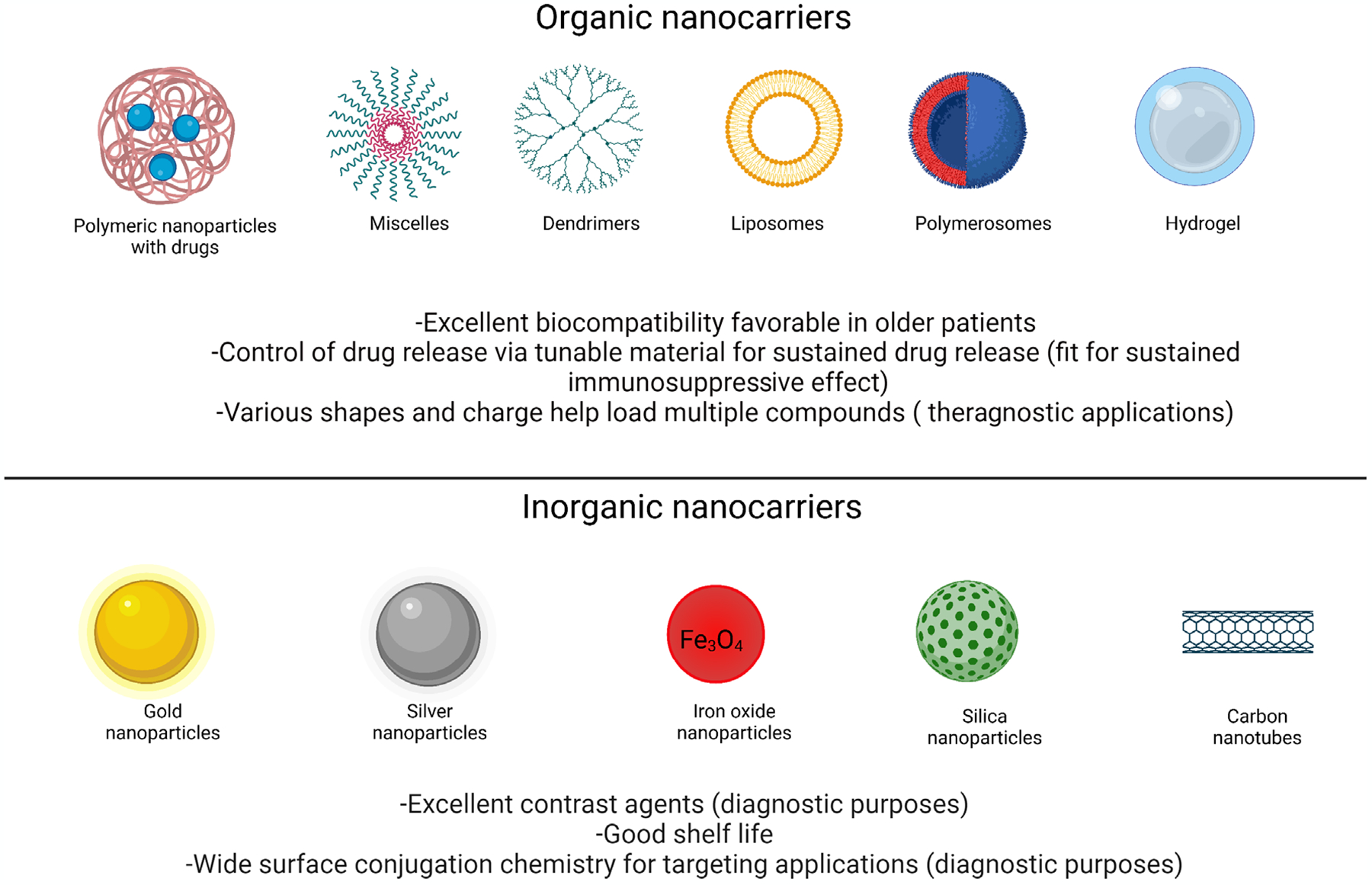
Different types of nanocarriers suitable for immunomodulatory drug delivery for the diagnosis and treatment of age-associated diseases.
TABLE 2.
Nanotechnology-based immunomodulation in age-associated diseases
| Immunomodulatory intervention | |||||
|---|---|---|---|---|---|
| Disease/condition | Nanocarriers | Therapeutic agent | Outcomes | In vivo/in vitro | References |
| Cardiovascular diseases | |||||
| Atherosclerosis | E-selectin antibody conjugated PLGA-PEG High-density lipoprotein nanoparticles (rHDL) |
Dactolisib Statins |
|
In vivo,in vitro | Duivenvoorden et al. (2014); Gholizadeh et al. (2018) |
| Endothelial cell (EC) inflammation | Amino-PEG-PE and N-palmitoyl homocysteine and cRGD as targeting ligand | Rapamycin |
|
In vitro | Nadig et al. (2015) |
| Vascular restenosis | αvβ3-integrin-targeted rapamycin nanoparticles | Rapamycin |
|
In vivo | Cyrus et al. (2008) |
| Arterial restenosis vascular inflammatory diseases | RAP/AOCD NP | Rapamycin |
|
In vivo | R. Zhang et al. (2020) |
| Coronary arterial disease (CAD) | Ac-bCD NP | Rapamycin |
|
In vivo | S. Feng et al. (2016) |
| Acute paradoxical inflammation | Squalene-adenosine NPs | Vitamin E |
|
In vivo | Dormont et al. (2020) |
| Autoimmune diseases | |||||
| Rheumatoid arthritis (RA) | Tuftsin modified-alginate nanoparticles | IL-10 DNA plasmid |
|
In vitro | Shardool Jain and Amiji (2012) |
| Knee osteoarthritis (OA) | PLGA hydrogel | Triamcinolone acetonide |
|
In vivo | Đorđević et al. (2022) |
| Infectious diseases | |||||
| Respiratory syncytial virus (RSV) infection | Liposomal nanoparticles | RSV F protein mRNA antigen |
|
In vivo | Espeseth et al. (2020) |
| Avian leukosis virus | Silica nanoparticles | Gp85 adjuvant |
|
In vivo | Cheng et al. (2017) |
| Seasonal influenza virus | Polyanhydride nanoparticles and pentalock copolymer micelles | Hemagglutinin and nucleoprotein |
|
In vivo | K. Ross et al. (2019) |
| HIV2 split virus | Polymethyl methyl methacrylate (PMMA) nanoparticles | PMMA as a synthetic adjuvant |
|
In vivo | Bettencourt and Almeida (2012) |
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Moderna vaccine |
mRNA-1273 encapsulated lipid nanoparticles | mRNA-1273 |
|
In vivo | Baden et al. (2021) |
4 |. IMMUNOMODULATION IN TREATMENT OF AGE-RELATED NEURODEGENERATION AND NANOTECHNOLOGY INTERVENTIONS
Aging is a predominant factor for most neurodegenerative conditions including Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and amyotrophic lateral sclerosis (ALS) among others (Hou et al., 2019). Chronic immune activation of the microglia is a common feature seen in various neurodegenerative processes leading to loss of neurons and axons (Amor et al., 2010). Activation of glial cells and proinflammatory cytokine release lead to various neuroinflammatory cascades resulting in accumulation of misfolded proteins seen in neurodegenerative diseases. Reducing inflammation may improve these pathological effects, making immunomodulation a potential therapeutic intervention in treating neurodegenerative diseases (Guzman-Martinez et al., 2019). Although some drugs can help reduce inflammation, there are limitations associated with the efficacy of such therapeutic drugs including crossing the blood–brain barrier (BBB) and the blood–CSF barrier as well as targeting the specific neuro-inflammatory biomarkers. High drug dosage through IV administration, poor drug permeation, systemic distribution and clearance, and cytotoxicity from the drug itself pose additional challenges in aging. Various in vitro and in vivo studies report that nanosized carriers are successful in overcoming the challenge of crossing the blood–brain barrier and releasing the drug in a targeted and controlled manner efficiently in both cell cultures and mice models (Goldsmith et al., 2014; Z. Liu et al., 2013; Pahuja et al., 2015; Z. H. Wang et al., 2010). In the sections below, we discuss the role of nanotechnology in treatments of various age-related neurodegenerative diseases (Figure 2).
FIGURE 2.
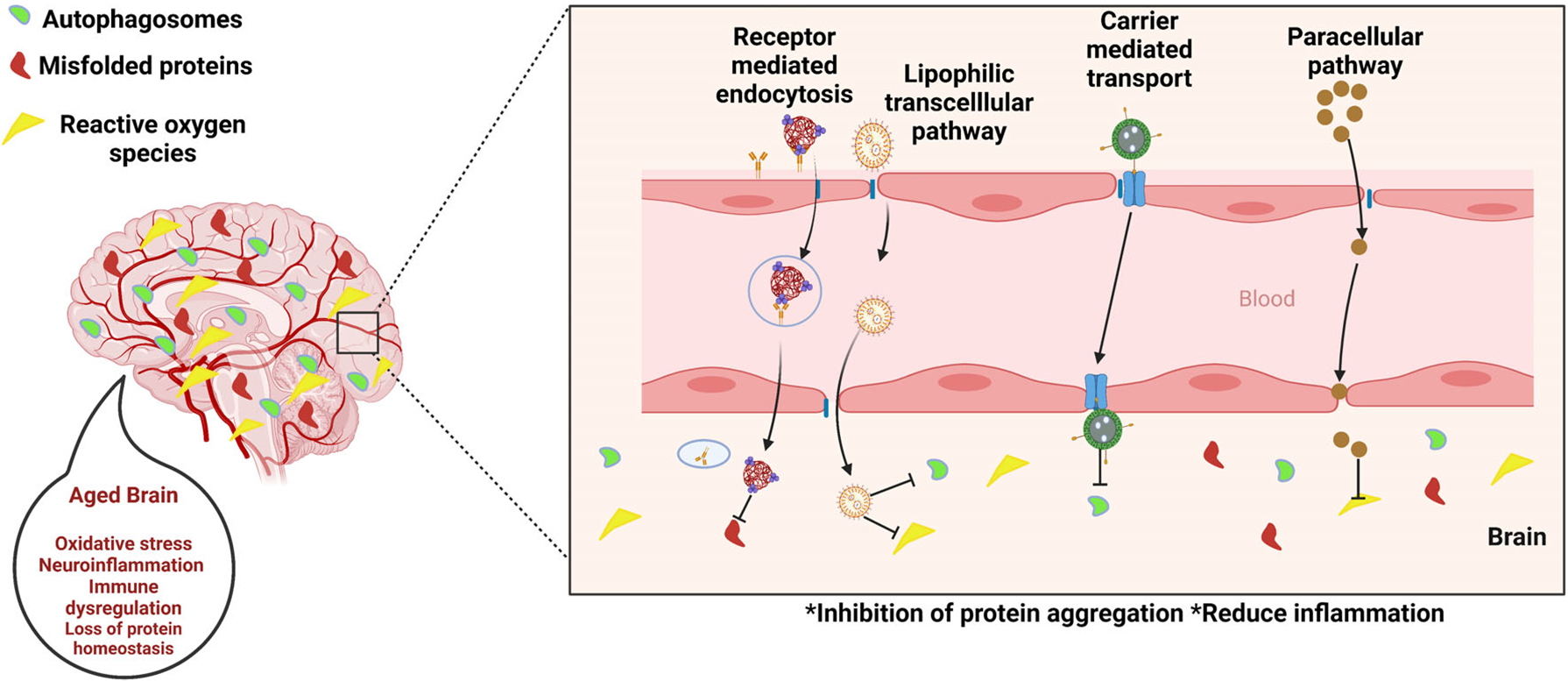
Nanotechnology interventions in age-related neurodegenerative diseases.
4.1 |. Alzheimer’s and Parkinson’s diseases
4.1.1 |. Immunomodulatory mechanisms
Alzheimer’s disease (AD) is characterized by accumulation of tau-proteins, neurofibrillary tangles, senile plaques beta amlyoid (Aβ) depositions, immune cells’ activation in the CNS, astrocytes, and the microglia. Aβ (1–42) has been identified as a stimulating factor in the age progressive AD along with overproduction of proinflammatory cytokines including TNF-α and transforming growth factor-beta 1 (TGF-β1) via induction of cellular oxidative stress (Butterfield & Lauderback, 2002; Drake et al., 2003; Thal et al., 2002; Yatin et al., 1999). Parkinson’s disease (PD) is another prevalent age-related neurodegenerative condition involving the gradual dopaminergic neuronal loss in substantia nigra parts compacta (SNpc), wherein, accumulation of overexpressed protein α-synuclein leads to the formation of “Lewy bodies” in the neuronal body, astrocytes and oligochondrocytes. Several studies analyzing the levels of primary proinflammatory cytokines such as TNF-α, IL-6, and interferon gamma (IFNγ) show elevation with the progression of AD and elevation of TNF-α, IL-2, IL-4, IL-6, and IL-1β with PD progression in the serum of the patients (Alam et al., 2016). The upregulation of the proinflammatory cytokines, chemokines, and its upregulation of immune receptor expression of major histocompatibility complex (MHC II) and Toll-like receptors (TLRs) due to altered intracellular communication, promotes brain damage in AD and PD progression (Hindle, 2010; Rubio-Perez & Morillas-Ruiz, 2012). The excessive release of these toxic proinflammatory cytokines due to altered intracellular signaling cascades in the CNS resulted in reduction of neuroprotective brain-derived neurotrophic factor (BDNF) associated with cellular oxidative stress, neurogenesis, as well as induction of apoptosis (Miller et al., 2009). Similarly, it is being argued that the microglia and associated immune responsive cell phenotype plays an important role in accumulation of amyloid and tau proteins (Leng & Edison, 2021). Recent findings suggest that neuroinflammation is a key player in the progression of AD and PD, and poor pharmacokinetics of conventional drugs in crossing BBB and targeting specific immune cells (e.g., microglia) remain of key concern in treating many neurodegenerative diseases.
4.1.2 |. Nanotechnology interventions
For treating neurodegenerative diseases such as AD and PD with nanotechnology, PLGA NPs are most widely used in drug delivery, due to their properties such as controlled release of drugs, enduring biomedical applications, biocompatibility with tissues and cells, extended residence time, and targeted delivery. PLGA NPs conjugated with a glyco-heptapeptide were used for the transportation of various therapeutic drugs to the brain by efficiently passing through the BBB as their glyco-heptapeptide coating mimics the behavior of opioid peptides; where an absorption-mediated endocytosis mechanism is employed (Tosi et al., 2007; Vergoni et al., 2009). Lactoferrin (Lf), also known as an iron-binding glycoprotein, could be used as a targeting agent on NPs to enhance the effective transport of the immunomodulatory drugs across the BBB by targeting the Lf receptors available on the BBB (Hu et al., 2011). Gold nanoparticles (AuNPs) are another interesting class of drug delivery system which possess the ability to bypass the BBB and have a high permeability in vivo for treatment of neurodegenerative disease conditions. For instance, researchers have established that polyethylene glycol PEGylated AuNPs with anthocyanin could down-regulate Aβ synthesis induced by oxidative stress and neuroinflammation in AD mice model (M. J. Kim et al., 2017). Although the study shows reduction in inflammation and down-regulation of Aβ plaques, a functional outcome of cognitive effects after the treatment is not discussed by the authors, which highlights the need for detailed studies to assess functional restoration of cognition comparable to that of healthy subjects. In another study, ROS-responsive core-cross-linked polysorbate 80 (PS80) nanoparticles have shown to reduce oxidative stress in traumatic brain injury, indicating the potential of NPs for use in reducing neuroinflammation in AD and PD (Yoo et al., 2017). Other strategies like downregulation of proinflammatory factors or microglial phenotype in aging brains using gene delivery and targeting other cells (e.g., astrocytes) involved in the Aβ pathway using NPs capable of crossing the BBB look favorable as potential AD treatments. For instance, Liu et al. developed an “Amyloid-β (Aβ) cleaner”, which consists of zwitterionic poly(carboxybetaine) (PBC)-based nanoparticles (MCPZFS NPs) (R. Liu, Yang, et al., 2020). In their study, the therapeutic effect was analyzed using chimeric mice expressing amyloid precursor protein and a mutant human presenilin 1 (APP/PS1). In vitro and in vivo results, shown in Figure 3, indicated that MCPZFS NPs were able to cross the BBB, (a) decrease the levels of ROS (b, c), IL-1β, interleukin 17A (IL-17A), IL 6, and interferon-γ (INF-γ) (d, e), and overall, modulate the microglia by switching it to an alternative activation phenotype (f). Therefore, these results demonstrate that NPs have a significant potential for treatment of AD via immunomodulatory activity (R. Liu, Yang, et al., 2020).
FIGURE 3.
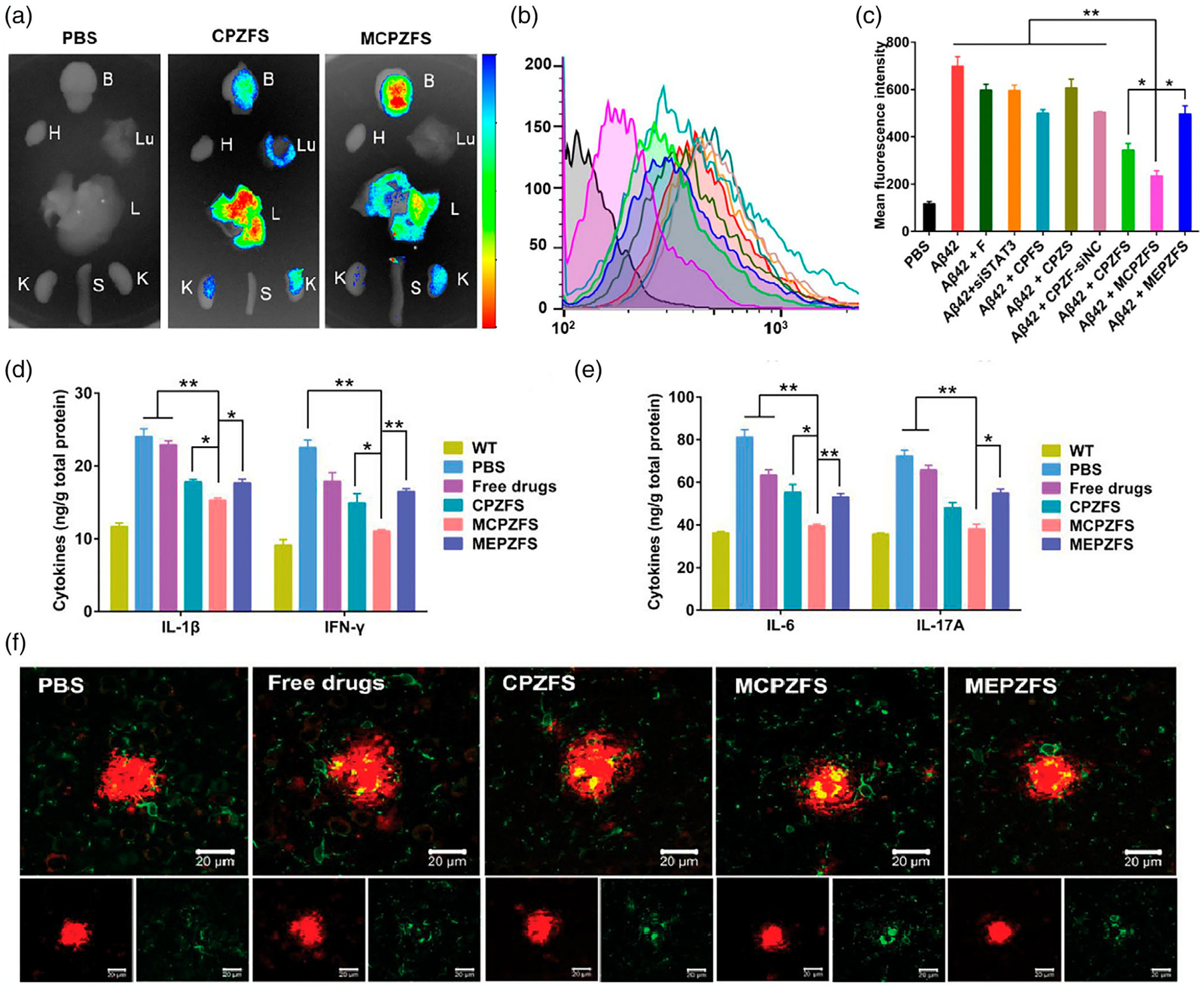
MCPZFs NPs reduction of proinflammatory cytokines and enhancement of microglia phagocytosis in APP/PS1 mice. (a) Ex vivo biodistribution of NPs in PBS, CPZFS, and MCPZFs treated mice after 12 h showing higher numbers of MCPZFs were able to cross the BBB (H: heart, Lu: lung, L: liver, K: kidney, S: spleen, and B: brain), (b) flow cytometry BV2 cells ROS intracellular levels after 12 h, (c) quantified ROS levels, showing MCPZFs better reduced ROS levels in BV2 cells, (d) quantified levels of IFN-γ and IL-1β, (e) quantified levels IL-6 and IL-7 in the brain after treatment, (f) confocal images of interaction Aβ plaque and microglia (green) after 1 week of treatment. MCPZFs NPs increased microglial phagocytosis. Data is presented as the mean ± SD. *p < .005; **p < .001 (R. Liu, Yang, et al., 2020).
Delivery of dopamine or dopamine agonists in PD patients with reduced number of dopaminergic neurons have shown favorable outcomes in the early stages but with associated side effects (Camargo et al., 2014). Liposomes are lipid bi-layer membranes with lower immunogenic profiles compared with other organic/inorganic NPs. PEGylated immunoliposomes carrying dopamine for PD treatment in a PD rat model comprised of adult Wistar rats (PD model of rat developed by transection of medial forebrain bundle) showed an 8-fold increase in the injected dopamine dose per gram of brain tissue in comparison with free dopamine (Kang et al., 2016). Also, in this study, the authors discuss how the brain is targeted with transferrin receptor (TfR) targeting antibody (Ab) OX26 Mab (which is expressed on the abluminal membrane of the capillary endothelium in the brain). Compared with unconjugated immunoliposomes, Ox26Mab conjugated immunoliposomes showed a 3-fold increased uptake in the brain. Similarly, Monge-Fuentes et al. (2021) showed delivery of dopamine across the BBB was achieved using a nanotechnology-based approach via albumin/PLGA nanosystems loaded with dopamine/phthalocyanine. In a 6-OHDA PD mice model, these carriers show significant motor symptom improvement compared with lesioned (no treatment) and l-DOPA (free drug) treatments. Nanotechnology-based treatments like the above mentioned can advance the therapies by providing a targeted delivery platform reducing the side effects associated with conventional therapeutic delivery. There are also many other nanomaterials, including metal nanoparticles (iron oxide, gold nanorods) and quantum dots that are proposed for diagnostic and therapeutic applications in AD and PD where new strategies are developed for efficient drug delivery across the BBB, and modeling of in vitro and in vivo settings to test novel materials are in the works (A. Kumar et al., 2020).
4.2 |. Huntington’s disease
4.2.1 |. Immunomodulatory mechanisms
Huntington’s disease (HD) is an autosomal dominant advancing neurodegenerative condition prompted by a trinucleo-tide (CAG) repeat expansion in the gene comprising the mutant protein Huntingtin (mHTT). Recent findings suggest that more than 11.5% of individuals over 60 may have a late onset of HD, prone for under-diagnosis and requiring effective therapies later on to improve quality of life (Chaganti et al., 2017). Although the primary cause of the progression of HD is overexpression of mHTT in neurons, the mutant protein is also highly expressed in the peripheral immune cells inducing immune activation (Björkqvist et al., 2008; Crotti et al., 2014). The mutant protein HTT is also expressed in the microglia and its cells, mediating the proinflammatory cytokines TNF-α and IL-1β that result in neuroinflammation, neuronal toxicity, and finally cell death (Kreutzberg, 1996). To date, the specific mechanism related to the up regulation of the proinflammatory cytokines and the dysregulated peripheral cells affecting the HD progression in the brain remains ambiguous. Biopsy reports have shown elevated levels of proinflammatory cytokines including IL-6, IL-8, IL-10 in the microglia and IL-4, IL-6, IL-8, TNF-α in the periphery and cerebrospinal fluid (CSF) of patients with HD (Rocha et al., 2016). The interaction of mHTT protein accumulation and the proinflammation cytokines, may serve a potential therapeutic target for HD.
4.2.2 |. Nanotechnology interventions
Supplementing antioxidants or protective molecules via nanoparticles in the brain can attenuate oxidative stress and aggregation of Huntington proteins. In that approach, Cong et al. (2019) show that delivery of selenium using selenium nanoparticles (Nano-Se) in C. elegans exhibited improved neuronal function, cell protection from stress, and inhibited neuronal death (Figure 4). As the concentration of the Nano-Se increased in the muscle of the body wall of C. elegans, the number of polyglutamine (PolyQ) aggregations (representative of Huntington’s aggregates) decreased by 30% at a concentration of 2 μM (a, b); this reduction in aggregates correlated with the reduction in axonal degeneration (c) and ROS levels (d) (Cong et al., 2019).
FIGURE 4.
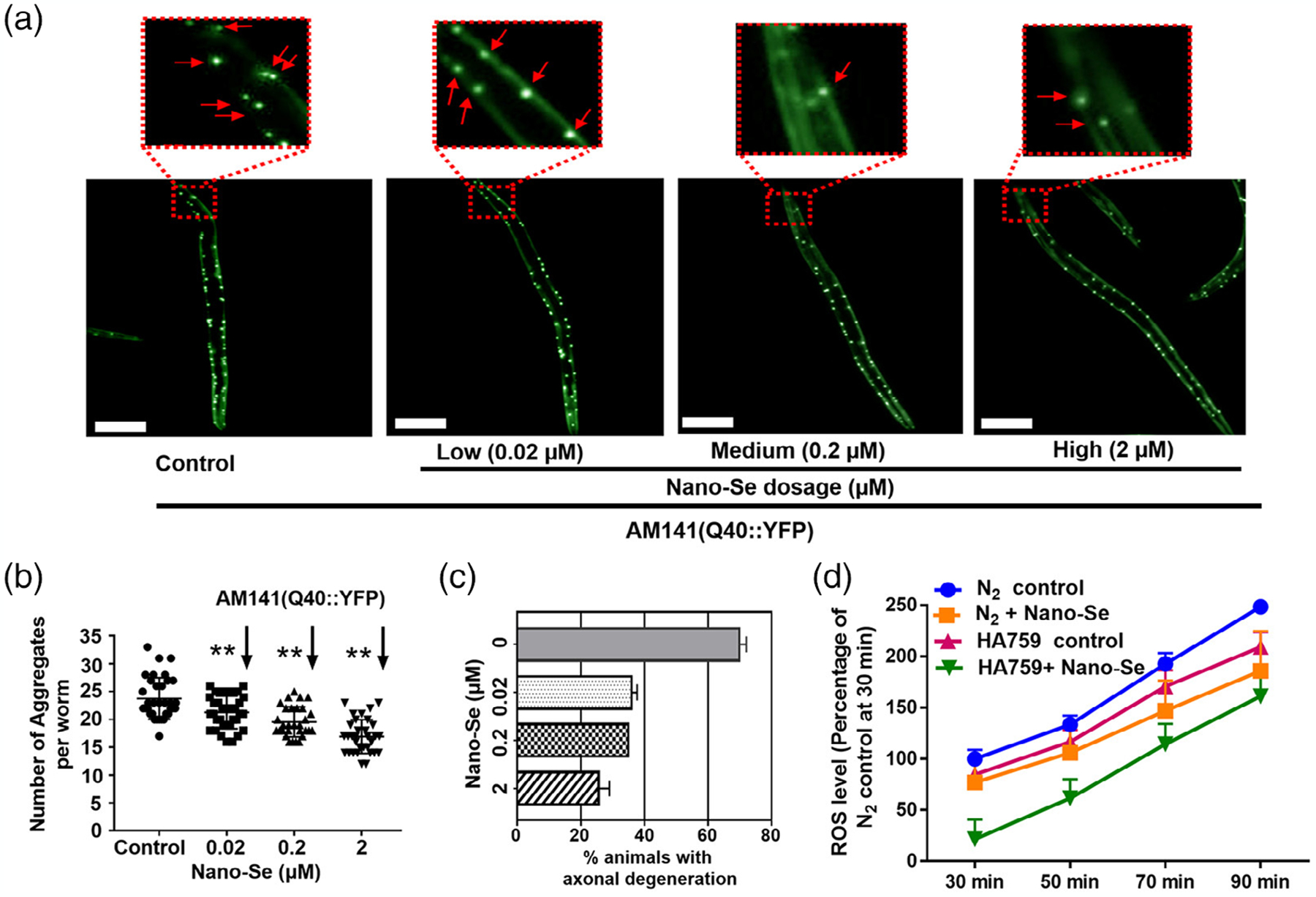
Representative fluorescence microscopic and quantitative images demonstrating Huntington’s aggregates and oxidative stress (ROS) reduction by Nano-Se. (a) Fluorescence microscope shows control versus treated worms. Untreated worms show discontinuous and punctuates signals in the muscle cell of the body wall. (b,c) Quantification of fluorescent aggregates. Results shows higher aggregates and axon degeneration in untreated worms. (d) Quantitative ROS level in Nano-Se treated and control groups. Data show mean value ± standard errors. Double asterisks (**) mean the very significant difference between control and the Nano-Se treated samples. **p < .01. Scale bar represents 100 μm. Reprinted with permission from Cong et al. (2019). Copyright 2019. American Chemical Society.
Selenium is also shown to have protective effects against acute neurotoxicity and is a modulator of redox pathways (Lu et al., 2014). Nanoparticle delivery of selenium is shown to be more effective and have lower toxicity than free drug compounds such as sodium selenite (Huang et al., 2003). Nano-selenium particles with specific cell targeting may be employed for targeting inflamed brain areas of HD. Proteins such as mutant HTT aggregation often lead to cellular toxicity and subsequently neuropathology’s like Huntington’s condition. Debnath et al. (2017) show that using poly(trehalose) nanoparticles with an iron oxide core of 20–30 nm, the delivery of trehalose, an effective inhibitor of protein aggregation, can be achieved in the mouse brain. Delivery of trehalose using these polymeric nanoparticles is shown to have more efficient neutralizing effects on amyloid inhibition and polyglutamine aggregation (a mutant huntingtin protein) compared with molecular trehalose (Debnath et al., 2017). Similarly, many other molecules of interest including polyphenols, peptides, and other new drugs, can be delivered across the BBB as a preventative strategy in the aging population with known hereditary history revealed by the latest techniques like predictive DNA testing. Moreover, the surface of the nanocarriers can be modified to be conjugated with different ligands e.g., transferrin, apolipoprotein E, NG-2 chondroitin sulfate proteoglycan, and other targeting moieties associated with BBB crossing and specific cell targeting to treat neurodegenerative disorders (Jin et al., 2020; Kolhar et al., 2013; C. Zhang et al., 2014).
4.3 |. Amyotrophic lateral sclerosis
4.3.1 |. Immunomodulatory mechanisms
Amyotrophic lateral sclerosis (ALS) is a progressive age-associated neurodegenerative disorder leading to the degeneration of the brain and the motor neuron. The dysfunction of mitochondria of the motor neuron is the prime contributing factor for the pathology of ALS (Van Damme et al., 2017). The branching and distribution of the microglia is affected by the altered communication of the immune cells as well as the microglia, leading to the accumulation of the toxic proinflammatory cytokines such as TNF-α, IL 1β, IL 2, and IL 6 in the CNS (Lana et al., 2019; C. A. Lewis et al., 2012). In recent findings, inflammatory cytokines including IL-2, IL-6, IL-10, IFN-gamma, and TNF-alpha were significantly increased in the plasma of ALS patients compared with healthy controls; further confirming the role of inflammation in ALS. Like other neurodegenerative conditions, excessive protein aggregation plays a vital role in the pathogenesis of disease progression, as the aggregation of the mutant superoxide dismutase 1 (SOD1) is found in the motor neurons in ALS (Proctor et al., 2016). SOD1 along with TAR DNA-binding protein (TDP-43) aggregates are majorly responsible for the pathogenesis of ALS. Mutant protein SOD1 is also associated with the hallmarks of aging along with inflammation and telomere shortening (Pandya & Patani, 2019). Neuronal inhibition of NFκB signaling via expression of NFκB inhibitor IκBalpha showed reduced molecular and behavioral hallmarks of ALS in TDP-43 mutant mice (Dutta et al., 2020). Polarization of microglia via fractalkine signaling to an M2 neuroprotective phenotype is reduced in ALS patients in relation to reduction in fractalkine cytokine levels (Harland et al., 2020). With the involvement of NFκB signaling and cytokine (e.g., fractalkine) regulation of microglial response, immunomodulatory drugs can intervene in these processes to inhibit protein aggregation and progression of ALS.
4.3.2 |. Nanotechnology interventions
Nano-carriers with surface modifications can bridge the gap in brain targeting via bypassing the selectively permeable membrane of the blood–brain barrier (BBB) and delivering protein inhibitors or immune modulators. One research group aimed to administer minocycline encapsulated liposomes surface modified with lipopolysaccharide (LPS) targeting the Toll like receptors 4 (TLR-4) (Wiley et al., 2012). This approach significantly downregulated the levels of proinflammatory cytokines via 29% increase in uptake of drugs in the microglial cells when compared with non-targeted liposomes. Moreover, the targeted nano-drug delivery system when administered intracerebroventricularly delayed the onset of ALS in the SOD1 murine model (Wiley et al., 2012). The rotarod apparatus was used to measure motor performance in this study with mouse correcting from falling and the age at that stage recorded for motor performance. Mean age for rotarod failure in untreated control was 103 days while the targeted liposome group was 111 days showing an 8% delay in onset of ALS behaviors. Maintaining the microglial population as an M2 phenotype via sustained delivery of immunomodulatory drugs such as minocycline may inhibit the progression of ALS and improve therapeutic outcomes. Lipofuscin aggregates accumulation is being reported in aged animals and in human motor neurons in aged subjects (Moreno-García et al., 2019). Targeting lipofuscin aggregates via surface modified liposomes as a lipofuscin cleaner and inhibiting them may reduce inflammation and prevent the aging population developing ALS related pathologies. Hence, there are promising approaches for nanotechnology-based strategies, targeting protein aggregates and neuronal inflammation in ALS treatments.
5 |. IMMUNOMODULATION IN AGE-RELATED AUTOIMMUNE DISEASES AND NANOTECHNOLOGY INTERVENTIONS
5.1 |. Rheumatoid arthritis
5.1.1 |. Immunomodulatory mechanisms
Rheumatoid arthritis (RA) is an age-associated autoimmune chronic inflammatory bone disorder progressively affecting the integrity of the synovial joints. Although RA affects all age groups, it is more prevalent in aged populations as reported in several studies (Laiho et al., 2001; Symmons et al., 1994). The age-associated disease condition can be vividly characterized molecularly by its accelerated aging of the immune system, tapered range of the T-cell repertoire, impaired generation of T-cells and elevated frequency of telomerase shortened CD28-cells (Weyand et al., 2014). Regulatory T-cell (Treg) suppressive effects on proinflammatory cytokines such as TNF-α is critical during the period of RA pathogenesis (Farrugia & Baron, 2016). Another cytokine, such as IL-6 production, elevated by immune cells such as T-cells, monocytes, macrophages, and synovial fibroblasts, results in joint damage (Firestein, 2003; Robak et al., 1998; Van Snick, 1990). Telomere shortening, a characteristic of aging, is observed in hematopoietic progenitor cells from RA patients reflecting premature immunosenescence driven by inflammation, and it was hypothesized that telomere shortening leads to excessive loss of T cells in individuals (Colmegna & Weyand, 2011; Schönland et al., 2003). Studies demonstrated that elevation in DNA damage provoking events such as cellular oxidative stress as well as dysregulated protein levels are probable causes of DNA damage accumulation and perpetual growth arrest of RA-associated T-cells (Y. Li et al., 2018).
RA is also accompanied with a buildup of senescent cells such as the synovial fibroblasts (p16INK4a+) showing an altered inflammatory phenotype (Del Rey et al., 2019). Continual DNA damage and inflammaging are associated with functional changes occurring from cellular senescence and lead to the upregulation of senescence associated secretory phenotype (SASP). However, activated p53 may aid in suppression of the secreted senescent linked proinflammatory cytokines such as IL-6 and IL-8 (Mijit et al., 2020). The persistent inflammation caused by the proinflammatory cytokines could also be involved with cognitive impairments during healthy aging as the upregulation of the late-stage differentiated CD28 T cells were negatively associated with the intellectual functioning of RA patients (Bauer, 2020). Altered nutrient sensing pathways in RA are related to elevated inflammaging, which is linked to the local inflammatory process in RA (Bauer, 2020).
Several researchers addressed the effect and efficacy of anti-inflammatory treatment on reversing the condition of the early aging of T-cells. In vitro studies demonstrated that usage of anti-TNF-α inhibitors like adalimumab and etanercept as well as IL-6 inhibitors such as Toclizumab partly restored the loss of CD 28 for slowing the progression of the disease condition (Ceeraz et al., 2013; Nerurkar et al., 2019). Disease-modifying anti-rheumatic drug (DMARD) in combination with phytochemicals treatment applied to in vitro cell line models and patients of RA displayed reduction of glucocorticoid (GC) mediated SASP, IL-6, and IL-8 (Cutolo et al., 2014; Gerards et al., 2003; Kour et al., 2021). Although, there are drugs showing positive effects in the treatment of RA, their targeting is poor requiring multiple doses which lead to side effects in elderly populations (Gaëtan Gavazzi et al., 2004). Hence, there is a need for alternative treatment strategies to reduce dosage and improve efficacy.
5.1.2 |. Nanotechnology interventions
Advances in nanotechnology interventions can aid to limit the multiple administration/injections of drugs in elderly patients for treating RA. In the following study, researchers have used non-condensing alginate (natural polymer) NPs to encapsulate the anti-inflammatory (IL-10) cytokine encoding plasmid DNA and then alter the surface of the NPs with tuftsin peptide to dynamically target macrophages (S. Jain et al., 2015). This strategy allowed nano drugs to move across the diseased synovium to transport the therapeutic drug to macrophages and efficaciously revert the macrophage phenotype from M1 to M2 (from proinflammatory to anti-inflammatory). It also helped by reducing levels of proinflammatory cytokines (IL-6, IL-1β, and TNF-α) in systemic and joint tissues and subsequently preventing the development of inflammation and joint impairment in rheumatic arthritic rat models (S. Jain et al., 2015). Other studies use siRNA-mediated knockdown of upregulated proinflammatory cytokines like TNF-α at the mRNA level for alleviation of inflammation (Howard et al., 2009). In another context, for delivery of pain-alleviating drugs like triamcinolone acetonide, PLGA hydrogels were employed which showed reduction of pain for 3 months in more than 70% of patients in clinical trials (Pietrosimone, 2020). Efficacy of therapeutic nucleic acids depends on the in situ delivery and bioavailability where nanocarriers can be of critical importance. Other researchers have used chitosan/siRNA NPs for administration of the TNF-α inhibiting siRNA, displaying effective anti-inflammatory outcomes in arthritic murine model (Howard et al., 2009). A CIA mice model with B-cell and T-cell dependent arthritis was chosen for the prophylactic study involving collagen immunization. Mice administered intraperitoneally with siRNA chitosan NPs exhibited lower arthritic scores over a 14-day period in comparison with control siRNA nanoparticles (Howard et al., 2009). Duan and Li (2018) found similar results by loading siRNA/methotrexate (MTX) into calcium phosphate liposomes (f-siRMI) and using folate as a targeting ligand. The results affirmed that the f-siRML could effectively decrease the expression of proinflammatory cytokines IL-1β and TNF-α, and inhibited the NF-kB pathway. The f-siRML treated animal group showed an overall reduction in the arthritis score and paw thickness (Figure 5) (Duan & Li, 2018). Prophylactic interventions like the above approaches can be employed to the at-risk older population to inhibit RA progression and improve quality of life ahead.
FIGURE 5.
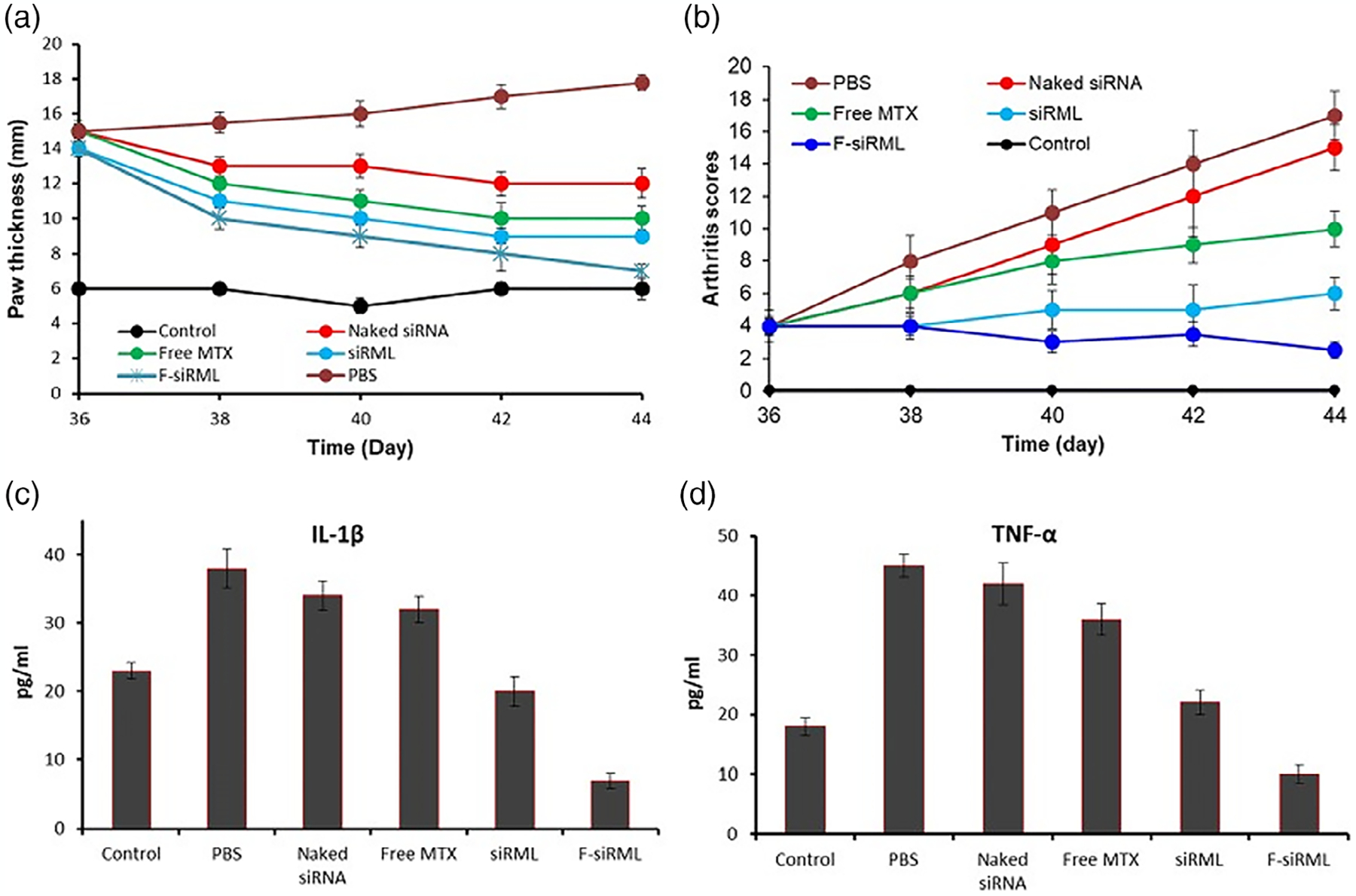
Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards an effective treatment in rheumatoid arthritis. (a,b) Therapeutic efficacy of siRML and F-siRML by measuring paw thickness and arthritic scores, respectively. (c,d) Analysis of proinflammatory cytokines IL-1β and TNF-α levels after administration of treatment at day 44. Results show how F-siRML reduced their levels more efficiently (Duan & Li, 2018).
In addition, the use of stimuli-responsive NPs encapsulated with immunomodulatory therapeutics that could be administered to the synovium and trigger drug release through using an external stimuli source (photo, electricity, or ultrasound) can provide “on-demand” drug release and avoid multiple administrations of drugs seen in conventional treatment strategies. In a recent study, a group of investigators used ultrasound targeted microbubble destruction (UTMD) as the externally triggered stimuli to control the drug release of dexamethasone encapsulated in PEGylated-folate conjugated liposomes (Xu et al., 2020). The folate conjugated liposomes targeted the activated macrophages resulting in higher cellular uptake in vitro, and the augmentation of ultrasound triggered the release of drugs providing synergistic effects to the pathological tissues (Xu et al., 2020). The UTMD approach with the dexamethasone liposomes showed no recurrence of disease, while the free dexamethasone had slightly increased arthritic scores. In addition, TNF-alpha and IL-1beta levels were suppressed from dexamethasone liposome treatment while free dexamethasone was not sufficient to suppress the cytokine levels. An external stimulus such as low intensity ultrasound has also been proven to be advantageous in alleviation of inflammation and promotion of tissue repair in RA disease models. Efficient use of potent steroids like triamcinolone, methyl prednisone, and cortisone via externally triggerable stimuli using nanocarriers may also help reduce the side effects from conventional treatment strategies involving multiple dosage regimens.
5.2 |. Inflammatory bowel disease
5.2.1 |. Immunomodulatory mechanisms
Inflammatory bowel disease (IBD) comprising of colitis and Crohn’s disease is rising as an effect of inappropriate and persistent stimulation of the mucosal immune system regulated by the presence of normal flora of the gut microbiota (H. J. Wu & Wu, 2012). This chronic inflammatory disease is frequent among the advanced aged population as evident from the spiking rate of prevalence in the elder population with over 25% of hospitalized patients aged above 65 years (Nimmons & Limdi, 2016). IBD hospitalization in older adults is more frequent than younger patients with a mortality risk from disease-related hypercoagulability, reduced mobility, and dehydration during hospitalization (Arnott et al., 2018). Various factors including genetics, smoking, high fat, and processed food diets play a key role in increased risks of IBD (Ramos & Papadakis, 2019). Primary B cells, T cells, and other cells of the immune system are affected by the onset of IBD. The aging immune system of the gut microbiome faces a drop in quantity of naïve T cell precursors and a reduced capability of memory T cells resulting in elderly inflammations, leading to IBD (N. Lee & Kim, 2017; A. Liu, Lv, et al., 2020). The impact of the aging gut microbiota and its equilibrium with the immune system could be an aspect accompanying the progression of various disease conditions seen in the gut of elderly populations. Precise delivery of anti-inflammatory drugs and modulation of the microbiome might show promise in treatment of complex IBDs.
5.2.2 |. Nanotechnology interventions
The toxic side effects and increase in allergies due to the administration of free drugs such as corticosteroids and other immunomodulators have prompted the need for better therapeutics such as drug encapsulated nanocarriers for enhancing therapeutic efficacy and improving bioavailability of drugs via targeting drug delivery to the colon (Katz & Feldstein, 2008). Immune-suppressant drugs used in severe cases have less patient compliance due to its adverse effects, including leukopenia, infections, decreased clearance, and other toxicities (Katz & Feldstein, 2008; Taleban et al., 2015). Various nanoparticles have been designed for the delivery of therapeutics downregulating the levels of inflammatory cytokines and modulating the gut immune system (Giron et al., 2019). For instance, to minimize the adverse drug toxic effects, polymeric nano and microcarrier systems such as, polymer Eudragit S100 which is a pH-sensitive material, have been used for protecting nanocarriers from acidic pH and degrading at neutral (to alkaline pH) in the colon (Barea et al., 2010). Poly-d,l-lactic acid (PLA) microspheres (FDA approved), PLGA (FDA approved GRAS material), G-5 PAMAM dendrimers (anti-inflammatory properties from the carrier itself), which carry the immunomodulatory and anti-inflammatory payloads for the suppression of proinflammatory cytokines while ameliorating the mucosal damage, are employed (Yang et al., 2020). Detailed nanocarrier formulations targeted for IBD can be found in recent review by Yang et al. (2020). Highly acidic pH to neutral and basic pH are encountered by many IBD treatment drugs including sensitive biologicals such as antibodies, peptides, and proteins. Nanocarriers such as chitosan coated NPs, eudragit polymer coated NPs, and many other pH-responsive materials can be employed for achieving targeted delivery in the gastrointestinal tract (De Leo et al., 2018; J. Kumar & Newton, 2017).
Other challenges of conventional treatment are the penetration of the gut mucosal layer for drug delivery. Some studies have used CD98 overexpressing inflamed colon epithelial cells as a target to deliver nanoparticles for treatment of colitis (Xiao, Yang, et al., 2014). Use of small interfering RNA (siRNA) loaded single chain CD98 antibody functionalized PEGylated NPs to treat colitis in mice showed a reduction in the severity of colitis along with a reduced expression of inflammatory markers such as CD98 (Xiao, Laroui, et al., 2014). Use of nanomedicine with biodegradable materials can help target specific inflamed cell populations and deliver across colonic mucosa reducing multiple drug doses used to treat IBD in the elderly.
Modulation of the microbiome to alleviate IBD conditions is cutting-edge research still being pursued in a lab setting. With respect to modulation, a nanoparticle curcumin (colloidal curcumin dispersion-Theracurmin®) was employed to treat ulcerative colitis, where the challenges of low oral bioavailability due to low solubility was addressed (Ohno et al., 2017). In the study, the nanoparticle curcumin was able to reduce dextran sulfate sodium induced experimental colitis, along with the increased expansion of CD4+Foxp3+ regulatory T cells and CD103+CD8α− regulatory dendritic cells in colonic mucosa, leading to improved anti-inflammatory effects from the Tregs induction. Interestingly, the nanoparticle curcumin increased butyrate-producing bacteria and butyrate level in feces, showing a potential for improved efficacy in mucosal delivery using nano formulation-based drug delivery (Ohno et al., 2017). Another approach that has been studied for the treatment of IBD is the use of nanocarriers to enhance the retention of current modulatory drugs. Zhao et al. (2021) developed a core shell nanoparticle fluid coacervate as a drug carrier and bioadhesive to coat the gastric tract (Figure 6). In their study, dexamethasone sodium phosphate (Dex-P) (corticosteroid) was used as model drug; rats treated with the Dex-P NPA2 coacervate showed suppressed M1 polarization, reducing proinflammatory levels, and higher polarization to M2 macrophages in the intestine, increasing the levels of anti-inflammatory molecules (Figure 6a,b). Hence, the results indicated that the NPA2 coacervate could moderate the sustained release of Dex-P as well as other biological agents (Figure 6f,g) and prolong its retention (Figure 6d), which results in an enhanced therapeutic effect by regulating the immune responses and restoring gut microbiota (Figure 6c–g) essential for treating IBD (Zhao et al., 2021).
FIGURE 6.
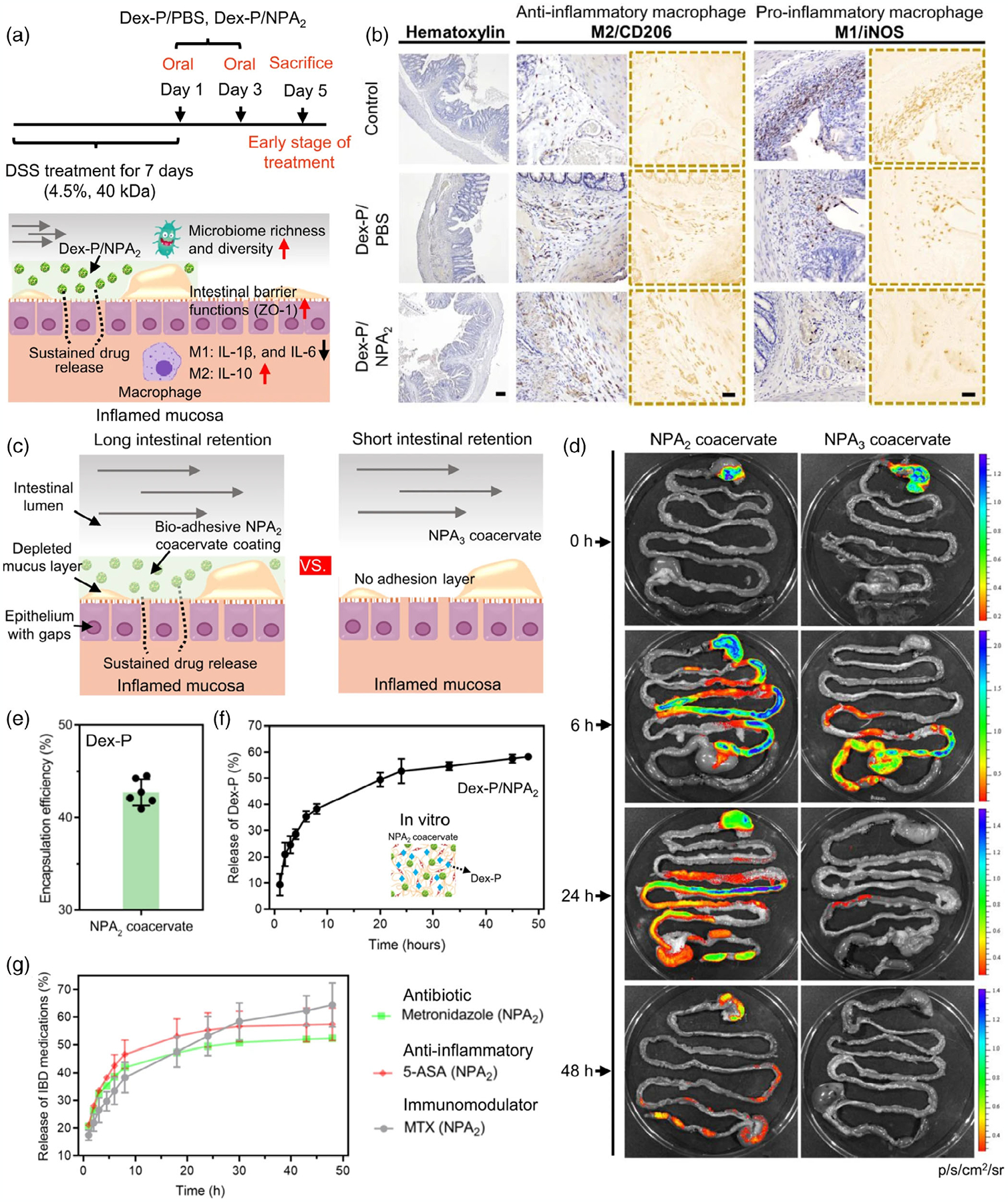
Polarization of M1 and M2 macrophages and drug release and retention efficiency of NPA 2 coacervate to treat IBM. (a,b) Immunohistochemical staining M1/M2 macrophages polarization markers iNOS/CD206 showing decrease of proinflammatory macrophages (M1) and increase of anti-inflammatory macrophages (M2). (c,d) Adhesiveness analysis: Bioadhesive NPA 2 adhered to GI tract ~2 days, control non-adhesive NPA 3 had short retention. (e,f) Encapsulation efficiency and drug release analysis showed NPA 2 has a high Dex-P encapsulation efficiency. g) Analysis of NPA 2 sustained release of different water soluble small-molecular drugs (metronidazole, anti-inflammatory 5-aminosalicylic acid [5-ASA], immunoregulatory methotrexate disodium salt [MTX]). The results showed the increased catechol oxidation potential facilitated sustained release, (f) the data were presented as mean ± SD. *p < .05; **p < .01; ***p < .001 (two-tailed Student’s t-test; Zhao et al., 2021).
6 |. IMMUNOMODULATION IN AGE-RELATED VASCULAR DISEASES AND NANOTECHNOLOGY INTERVENTIONS
Vascular diseases are a group of ailments of the heart and blood vessels which include coronary heart disease, peripheral arterial disease (PAD), myocardial infarction, cardiomyopathy, atherosclerosis, heart fibrosis, hypertrophy, and stroke. Age is a major risk factor for vascular diseases, which is a leading cause of death for the population over 65 years of age (North & Sinclair, 2012). Aging leads to progressive deterioration in the structure and function of the heart and vasculature that likely contributes to the development of cardiovascular disease (CVD; Elísio Costa et al., 2015). Markers of inflammation have been associated with cardiovascular diseases and proposed as other cardiovascular risk factors (Cesari et al., 2003). Elevated levels of proinflammatory cytokines that contribute to the inflammaging process, augment the probability of endothelial damage, vascular remodeling impairment, atherosclerosis, and others in age related cardiovascular dysfunction (Kritchevsky et al., 2005). Nanotechnological interventions in cardiovascular diseases have evolved to address various pathological conditions in vascular diseases and will play a critical role in developing preventive strategies (Figure 7). Major cardiovascular disease immunomodulatory mechanisms in aging groups and nanotechnology interventions are discussed below.
FIGURE 7.
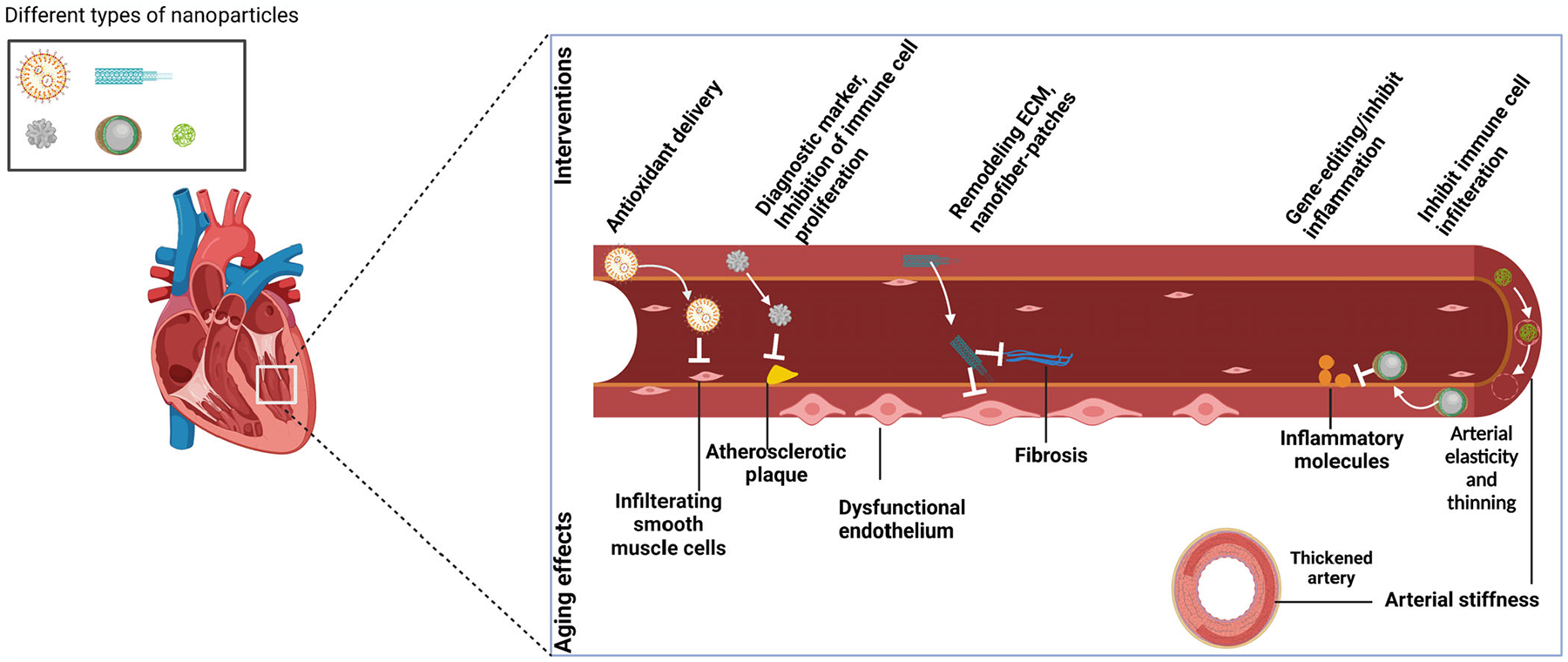
Nanotechnology interventions in age-related cardiovascular disorders.
6.1 |. Cardiovascular disease
6.1.1 |. Immunomodulatory mechanisms
Aging is accompanied by the buildup of pathogenic senescent cells in the cardiovascular system, which mediate vascular remodeling. Venous endothelial cells from older adults showed increased IL-6, TNF-alpha, and chemokine (C–C motif) ligand 2 (CCL2) proinflammatory molecules which can be attributed to an increase of age-associated arterial cell senescence (Morgan et al., 2013; R. Ross, 1999). Various other factors including C-reactive protein (CRP), IL-6, and soluble Intracellular adhesive molecule (ICAM1) circulating concentrations in older adults show effects of inflammation on arterial functions in aging (Trott & Fadel, 2019). Elevated levels of proinflammatory cytokines, which induce activation of cell adhesion molecules in the endothelial cells as well as increased oxidative stress, lower nitric oxide bioavailability, and contribute to endothelial dysfunction along with accumulation of vascular smooth muscle cells (VSMCs) in the vessel wall, reducing vascular permeability (Barbe-Tuana et al., 2020). Endothelial dysfunction may also cause higher systemic vascular resistance, which leads to the development of hypertension in arteries (Sun et al., 2020). The proinflammatory molecules accumulate in the arterial walls causing arterial cell senescence, extracellular matrix (ECM) degradation, increased intimal thickening, and elevated blood pressure that ultimately leads to arterial aging (M. Wang et al., 2018). Arterial stiffening as a result of collagen deposition and elastin degradation elevates hypertension in the aged population (J. Wu et al., 2014). With age, elevated oxidative stress and inflammation in perivascular adipose tissue (PVAT) contribute to changes, including structural and functional modifications, remodeling of the extracellular matrix, and dysfunction of VSMCs and endothelial cells (ECs) that lead to hypertension and other age-related cardiovascular diseases (Nosalski & Guzik, 2017).
Inflammaging directs atherosclerosis where cytokines and inflammatory cells participate, and atherosclerotic plaques contain cells, which frequently demonstrate senescent phenotype (Ferrucci & Fabbri, 2018). DAMPs and crystals of cholesterol in the atherosclerotic lesion stimulate the NLR Family Pyrin Domain containing three (NLRP3) inflammasome in macrophages and increase the production of the proinflammatory cytokines: IL-1beta and IL-18 (Rajamäki et al., 2010). T lymphocytes also contribute to the development of inflammation that promotes damage of prevailing collagen in susceptible plaques (Hansson & Libby, 2006; Spagnoli et al., 2007). All these factors ultimately lead to formation of atherosclerotic plaques, a major event seen in the progression of age-associated cardiovascular disease. Treating the inflamed endothelium and inhibiting progression of atherosclerosis is vital in addressing age-related cardiovascular disease.
6.1.2 |. Nanotechnology interventions
Targeting inflammation in experimental models has been advantageous in reducing myocardial and arterial damage, decreased disease progression, and fostering healing, but clinical translation has been discouraging. Innovative nanoparticle-based therapies that directly target inflammation and deliver bioactive combinations with anti-inflammatory characteristics to the site of action, have been extensively explored. Ma et al. (2016) utilized atheroprotective and anti-inflammatory microRNA(miR) such as miR-146a and miR-181b loaded porous silicon multistage nanoparticles. These nanoparticles were coated with thioaptamer, targeting E-selectin markers that are upregulated in inflamed endothelium to reduce atherosclerosis. This study showed that miRs were able to effectively enhance the anti-inflammatory and atheroprotective effects, while reducing chemokine molecules CCL2, CCL5, CCL8, and chemokine (C–X–C motif) ligand 9 (CXCL9) responsible for inflammation in TNF-α treated C57BL/6J mouse aortas. The results effectively demonstrated the delivery of atheroprotective agents to the inflamed endothelium and reduction in plaque size displaying enhancement in endothelial functions in ApoE−/− mice.
Similarly, Gholizadeh et al. (2018) employed anti-E-selectin immunoliposomes to target activated endothelial cells in the inflamed endothelium. Other studies also target E-selectin or αvβ3-integrin expressed either in inflamed endothelial cells or vascular restenosis, respectively, to deliver rapamycin for reducing inflammation and subsequent plaque formation (Cyrus et al., 2008; Nadig et al., 2015). In addition, strategies targeting atherosclerosis involve delivery of therapeutic agents including rapamycin, statis, and vitamin E to inhibit neointimal hyperplasia, inflammation and even disrupting the crosstalk between oxidative stress and inflammation progression, respectively (Dormont et al., 2020; Duivenvoorden et al., 2014; R. Zhang et al., 2020). These immunoliposomes were loaded with Rapamycin, which showed efficient inhibition of proliferation and migration of E-selectin over-expressing tumor necrosis factor-α (TNF-α) activated endothelial cells along with considerable downregulation of inflammatory genes such as VCAM-1, E-Selectin, VEGF, and TIE2.
Various immunotherapies, which involve treatment with anti-interleukin-1β or CD-47 antibodies, have shown side effects including anemia and recurrent cardiovascular events. In an immunotherapeutic approach, Flores et al. (2020) have used single-walled carbon nanotubes (SWNT) loaded with antiphagocytic CD47-signal regulatory protein α (CD47-SIRPα) to target macrophages in plaque. With the use of SWNT–SHP1 in both an accelerated inflammation model and chronic atherosclerosis murine models, results from this study showed accumulation of carbon nanotubes in plaque and significant plaque reduction by disrupting downstream signaling of antiphagocytic CD-SIRPα. Besides carbon nanotubes, metallic nanoparticles such as gadolinium (Gd) chelates/nanoparticles (Kotb et al., 2016), super-paramagnetic iron oxide nanoparticles (SPIONs) 8F (Herranz et al., 2014), methacrylate copolymers (Y. Liu et al., 2019), gold nanoparticles (Cormode et al., 2010), and others have been used in the diagnosis of atherosclerosis as such or with surface modifications in combination with modalities including MRI, PET/SPECT, CT, and optical near infrared fluoroscopy (NIRF). Nanoparticles have the ability to target specific cells or mechanisms in the atherosclerotic lesions involving macrophage scavenger receptors, macrophage phagocytosis, reactive oxygen species (ROS), proteases, annexin V for apoptosis, αvβ3 for neoangiogenesis, and adhesion molecules among others (Flores et al., 2019). These studies show the potential of nanoparticles in both detection and therapeutic purposes to treat cardiovascular pathologies in contrast with the free drug, which lacks specificity and bioavailability (Ponnappan & Ponnappan, 2011).
Since ROS generation and subsequent oxidative drive pathology and CVD (Senoner & Dichtl, 2019), nanoparticle-based drug delivery of antioxidants and antioxidant donor combinations may be advantageous as nanoparticles can be targeted to specific sites and administration of nanoparticles in the older population can reduce the side effects occurring from surgical interventions. Nano formulations of resveratrol, curcumin, and quercetin have shown to be anti-inflammatory, ROS scavenging, and cardioprotective (Vaiserman et al., 2019). Hardy et al. showed that simultaneous delivery of antioxidant curcumin and peptide against alpha-interacting domain of L-type Ca(2+) channel (LTCC) loaded into multifunctional poly(glycidyl methacrylate) (PGMA) nanoparticles could attenuate L-type Ca(2+) channel activity and decrease superoxide production in cardiomyocytes following LTCC agonist. In their study, rat heart muscle damage and oxidative stress were reduced as seen by increase in GSH/GSSG ratio (normal (GSH)/oxidized glutathione (GSSH)) and a decrease in superoxide production in cardiac myocytes. Here, low soluble curcumin was delivered to ischemic regions of the heart with organic polymer PGMA nanoparticles, displaying the advantages of nanotechnology in delivery of antioxidants and cardio protection against ischemic-reperfusion injuries. Nano-antioxidants comprised of non-organic nanoparticles such as metallic nanoparticles display intrinsic antioxidant properties along with nanoparticles functionalized with antioxidant enzymes or natural antioxidants. D. Lee et al. (2013) have used polyoxalate containing vanillyl alcohol (PVAX), an H2O2 responsive antioxidant polymer, to scavenge H2O2 and deliver vanillyl alcohol, which would exert highly potent anti-inflammatory and antiapoptotic for ischemia/reperfusion (I/R) injury (D. Lee et al., 2013). Similarly, Nethi et al. (2017) functionalized cerium oxide particles with (6-{2-[2-(2-methoxy-ethoxy)-ethoxy]-ethoxy-hexyl) triethoxysilane for the reduction of ROS in endothelial cells. In another study, S. Feng et al. (2016) designed pH- and ROS-responsive NPs laden with rapamycin that efficiently weakened neointimal hyperplasia in an arterial stenosis rat model (Cartaya et al., 2019). Several other nanoparticle mediated antioxidant delivery methods explored reducing oxidative stress in cardiovascular diseases have been reported elsewhere (K. S. Kim et al., 2019).
Besides antioxidant delivery, nanocarriers have also be used for facilitating autophagy as the loss of autophagy includes oxidative stress-mediated cellular senescence, contributing to endothelial dysfunction and the emergence of cardiovascular diseases (Jiang, 2016; Nussenzweig et al., 2015). For instance, Ke et al. (2018) designed molybdenum disulfide nanoparticles (MoS2 NPs), which inhibited H2O2-induced endothelial senescence and enhanced endothelial functions in vitro by silencing cellular senescence via activating autophagy and defying impaired autophagic flux.
Biomimetic nanoparticles designed with cell membranes are also considered as a medium to achieve targeted drug delivery by regulating the nano-biointerface in the immunomodulation of diseases. Cell membrane-based biomimetic NPs mimic various cell membranes for these applications, such as platelets, leukocytes, and protein complexes that are ideal for targeting CVD pathologies (Sushnitha et al., 2020). Boada et al. (2020) fabricated leukocyte-based NPs encapsulated with rapamycin that exhibited selective accumulation in atherosclerotic plaque in a mouse model, restricting local inflammation by reducing macrophage proliferation. Moreover, diminished plaque burden was observed in the vessels with the release of rapamycin from these particles (Boada et al., 2020). With the scope of designing various drug delivery platforms targeting various pathways inhibiting atherosclerotic plaques, nanotechnology can help improve patient outcomes by reducing invasiveness seen in current treatments, and subsequently lessen side effects from surgeries to improve quality of life in elderly.
6.2 |. Peripheral arterial disease
6.2.1 |. Immunomodulatory mechanisms
Peripheral artery disease (PAD) affects close to 13% of the Western population, who are over the age of 50 years. It is most commonly due to atherosclerosis, where an atherosclerotic plaque causes arterial stenosis or occlusion, leading to a reduction of blood flow to the lower limbs. Most patients are asymptomatic, but many experience intermittent claudication especially pain on walking or lack of ability to walk long distances (Morley et al., 2018). Critical limb ischemia occurs when the reduction in blood flow is so severe that it causes pain even at rest (Conte & Vale, 2017). The prevalence of PAD increases with age; there was an increase from 1% of 40- to 49-year-old to 15% of those aged over 70 (Selvin & Erlinger Thomas, 2004). Surgical interventions such as balloon angioplasty and revascularization techniques seem promising but may not be enough for superficial femoral arteries in a clinical setting, especially in elderly patients with low compliance to invasive procedures. Recently, atherosclerosis has been linked to inflammation with the discovery of various antiendothelial cell antibodies, proatherogenic oxidized low-density lipoprotein/β2-glycoprotein I complex, and elevated levels of antiphospholipid antibodies where various immunosuppressive drugs can be employed to address the inflammation (Berger et al., 2014; Gavier et al., 2016; Moses et al., 2003; Varela et al., 2011). Although there are various immunomodulatory drugs, including cytokines and chemokines and their associated antagonists, the drug delivery method is still vascular delivery, which is invasive and relies on passive accumulation of drugs at a diseased site.
6.2.2 |. Nanotechnology interventions
Nanoparticles loaded with immunomodulatory drugs such as Rapamycin and other drugs targeting various biomarkers in PAD ischemic sites can help change the immune environment, leading to reduced restenosis and providing the overall clinical improvement in elderly patients. Toward that approach, Gomes et al. (2019) have loaded cilostazol, a vasodilator, and antiplatelet agent, into polymeric nanocapsules made from PCL–PEG blend. PCL–PEG loaded cilostazol reduced platelet aggregation in the blood of Wistar rats compared with the cilostazol drug alone when administered orally. Nanotechnology platforms like these can be used for targeting PAD ischemic sites for inhibiting platelet aggregation and progression of atherosclerotic plaque in PAD while its applications to other plaque locations is yet to investigated. In a novel study of atherosclerotic therapeutics, sugar-based amphiphilic macromolecule (AMs) serum-stable core/shell nanoparticles (SM nanoparticles) were targeted to MSR1 and CD36 overexpressing atherogenic macrophages to inhibit their progression to form cells and counteract inflammatory vascular diseases (D. R. Lewis et al., 2015). The SM nanoparticles not only accumulated in the atherosclerotic plaque lesion, but also downregulated the macrophage scavenger receptor 1 (MSR1) or CD36 as well as reduced oxLDL uptake in atherogenic macrophages, which usually leads to form cell formation and further inflammation (Figure 8) (D. R. Lewis et al., 2015). These results indicate that nanotechnology shows promise in non-invasive treatment strategies targeting vascular inflammation and immunomodulation at ischemic sites. Non-invasive strategies such as these are ideal for older patients who are ineligible for surgeries and reducing the adverse side effects with efficient targeting. As a preventive strategy, patients with known risks factors including age, can be screened regularly for plaque using an imaging approach where the targeted nanocarrier loaded with contrast agents can be employed to find any developments in early stages that can be controlled with change in food habits, exercise, or less potent drugs.
FIGURE 8.
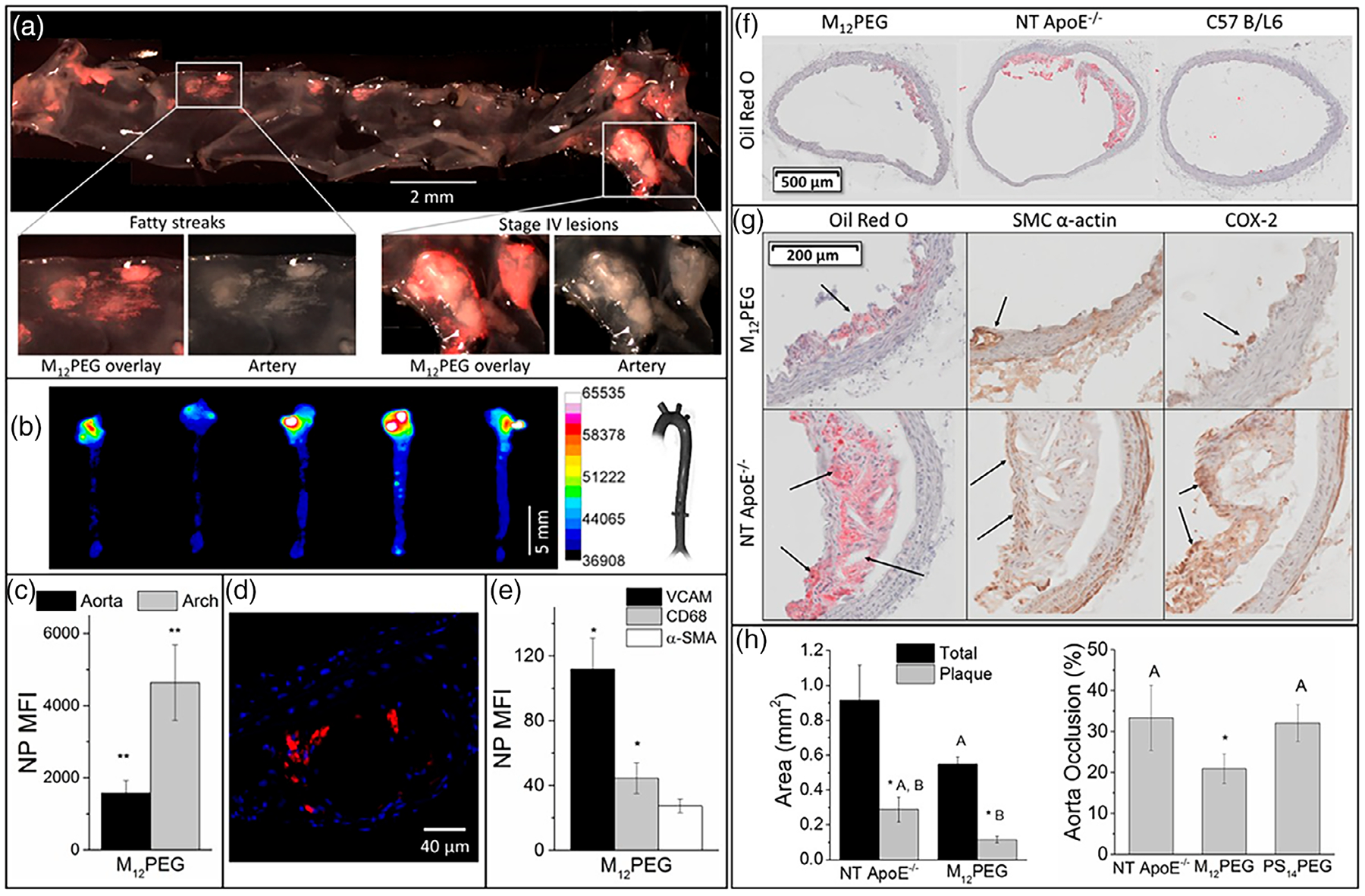
Sugar-based amphiphilic NPs for the treatment of atherosclerosis in vivo. (a) Fluorescence images of aorta show M12PEG efficiently targeted atherosclerotic plaque. (b,c) Ex vivo fluorescence images demonstrated sugar-based NPs can remain in the aortic arch up to 60 days after injection. (d) Accumulation of NPs in atherosclerotic plaque shown by confocal microscopy. (e) Flow cytometry results showing NPs target VCAM 1 and CD68 expressing cells. (f) In vivo aortic regions showed significant reduction of lipid content and plaque after treatment with NPs. (g) M12PEG lowered lesion severity (arrows Oil Red O images), neointimal hyperplasia (arrows in SMC α-actin images), and inflammation (arrows in COX-2 images). (h) Treatment with M12PEG resulted in 37% occlusion decrease in aorta. Error bars represent SEM and n = 5. *p < .05 from the control (NT), treatments with the same letter are statistically indistinguishable (D. R. Lewis et al., 2015).
7 |. IMMUNE MODULATION IN AGE-RELATED INFECTIOUS DISEASES AND NANOTECHNOLOGY INTERVENTIONS
The elderly are prone to infections more than the younger population due to immune dysfunction, progressive homeostatic dysfunction in various organs and other environmental factors including closed institutions such as nursing homes, frequent hospitalization and urban migration to closed spaces (Yoshikawa, 2000). Various pathogens including urinary tract pathogens, tuberculosis-related pathogens, tropical infections, and other infectious agents are prevalent in the aging population (El Chakhtoura et al., 2017; Gaëtan Gavazzi et al., 2004; Steens et al., 2014; Yoshikawa, 2000). Increasing rates of antimicrobial resistance in the aging population need further advanced treatment strategies. Administration of antibiotics such as β-lactamines, Cefepime, Carboxypenicillin, Trimethoprim, Rifampicin, Vancomycin, and other antibiotics is still the primary mode of treatment for various bacterial infections in the elderly, especially via intravenous and oral drug delivery routes (G. Gavazzi & Krause, 2002). These modes of antibiotic administration have adverse effects with respect to the elderly population, including gastrointestinal side-effects of nausea and diarrhea, and renal failure, which has increased in prevalence due to drug interactions (G. Gavazzi & Krause, 2002). Age-related changes in the immune landscape pose a challenge for current drug administration to treat infectious diseases. Here we explore how nanotechnology can aid in improving drug efficacy to treat bacterial infections, which are considered a global threat due to emerging antibiotic resistance.
7.1 |. Bacterial infections
7.1.1 |. Immunomodulatory mechanisms
As the immune system declines in functionality through age, protection against various pathogens is reduced. Bacterial infections and viral infections are most relevant, which increase morbidity and mortality in the elderly. Aging is associated with telomere shortening in immune cell population, increased DNA breakage and other metabolic reprograming of CD4+ T cells such as reduced ATP production, enhanced shunting into the pentose phosphate pathway and excessive reductive elements (Weyand & Goronzy, 2016). All of these add to the reduced functionality of immune cells in protecting against the infectious agents. Tuberculosis is a respiratory bacterial infection largely seen in the elderly, which is conventionally protected by the immune system involving cell mediated immunity with phagocytic activity from macrophages and T cell responses (Thomas & Rajagopalan, 2001). Tuberculosis caused by mycobacterium tuberculosis is the most lethal infection seen in the elderly with recent reports showing around 70% of infectious pathogens found in hospitals are resistant to one or more antibiotics with approximately 400,000 multidrug-resistant cases seen in the world according to the World Health Organization (Rajagopalan, 2016). Late diagnosis, poor responses to vaccines, antibiotics, and poor tolerance of intravenous treatments in the elderly pose a challenge in drug delivery. T cell immune senescence and reduced T cell production by the thymus in the elderly lead to reduced T cell numbers and related signaling molecules. Ex vivo stimulation of elderly PBMCs with Mycobacterium tuberculosis (Mtb) antigens showed reduced IFN-gamma responses and similarly, older mice showed increased susceptibility to Mtb infections (Bodnár et al., 1997; Orme, 1987). With intracellular infection of the tuberculosis pathogen, macrophages infected are suppressed from their antibacterial responses of releasing proinflammatory cytokines such as IL-12 and IFN-γ (Giacomini et al., 2001). In the older population, there are many other complications associated with clinical management of bacterial infections including co-morbidities, chest tube duration, secondary spontaneous pneumothorax, and other surgical issues (Byng-Maddick & Noursadeghi, 2016; S. C. Lee & Lee, 2016; Madansein et al., 2015). With the slow progression of antibiotic development, multidrug resistant variants, and other toxic effects associated with current drugs, there is an eminent need for novel drug delivery methods to treat bacterial infections.
7.1.2 |. Nanotechnology interventions
Various first line drugs including Isoniazid, Rifampicin, and Pyrazinamide, and second-line drugs including Ethambutol, Streptomycin, Kanamycin and others have adverse effects such as hepatitis, burning sensations, loss of memory, bleeding, pain in joints, hepatotoxicity, hypokalemia, and a few others as described by S. C. Lee & Lee (2016). Nanoparticle surface engineering facilitates incorporation of various targeting moieties, which can enable site-specific delivery of payloads, including antibiotics and other drugs, to inhibit pathogens. With high loading, relatively higher bioavailability of drugs, specific targeting, and stability, nanoparticles provide a superior drug delivery platform than free drugs to aid in tuberculosis interventions in older patients who have reduced compliance to drugs due to their toxicity and adverse reactions from multiple dosages (Nasiruddin et al., 2017; Pandey & Khuller, 2005; Tăbăran et al., 2020). In addition, Pandey and Khuller (2005) showed that seven doses of Rifampicin loaded solid lipid particles (SLP) were sufficient to remove tubercle bacilli in Guinea pigs’ lungs/spleen compared with 46 daily oral doses, therefore avoiding toxicity arising from multiple doses. These strategies with pathogen and organ specific modifications are translatable to clinics, eventually reducing drug doses in older patients.
Silver nanoparticles possess antibacterial properties, and when combined with antibiotics, show synergistic effects on various drug resistant gram-positive and gram-negative bacteria (Tăbăran et al., 2020). Silver nanoparticles increase the expression of inducible nitric oxide synthetase (iNOS) and enhancement of phagolysosomal activity along with macrophage and neutrophil activation that are crucial in interventions of infectious diseases such as Multidrug resistant tuberculosis (MDR-tb), Methicillin resistant staphylococcus aureus (MRSA) and other drug resistant bacteria, whose current drug treatments are lengthy and often lead to co-morbidities in the elderly. Nasal administration of N-trimethyl chitosan chloride, chitosan glutamate, chitosan chloride, and carboxymethyl pullan nanoformulations incorporated with antigen BSA were shown to be uptaken by macrophages inducing immune responses against bacteria (Cevher et al., 2015). Similarly, alginate-polyethylenimine nanogels were used for releasing antigens inside dendritic cells to enhance MHC class-1 and class-II mediated antigen presentation, which is known to decline with age (Chougnet et al., 2015; P. Li et al., 2013; Wong & Goldstein, 2013). Aptamers, which are polymeric oligonucleotides, have a high affinity toward targets, including proteins/peptides and vitamins. Aptamers can also be employed to combine with drugs to improve antibacterial activity toward β-lactamase resistance in gram-positive and gram-negative bacteria (Schlesinger et al., 2011). These nanoformulations can serve as improved therapeutics in elderly patients, with reduced immune responses. With the capability of surface functionalization, nanoparticles with targeting moieties toward infected cells and sustained release of antibiotics reducing multiple doses of immunomodulatory agents and other drugs for treatment of infectious diseases, nanotechnology has come a long way to be capable of addressing the adverse drug reactions issues in elderly patients.
7.2 |. Other infection-related diseases
Urinary tract infections (UTI) are commonly seen in the elderly due to hospitalization or immunocompromised conditions (Finucane, 2017). Renal function declines with age, and free drugs such as antimicrobials prescribed cause more renal damage and related toxicity in older patients with UTI. Also, identification of pathogens in the infection is a laborious and time-consuming process, for example, urinalysis and lab culture of pathogens (Han, 2013). Another disease issue are ADEs, which are common in the elderly due to various physiological factors and poor diagnosis of the underlying illness, leading to improper dosage prescription (Natkin, 1998). Phytochemical inspired Ag NPs showed effective antimicrobial activity with a higher zone of inhibition and robust MIC values against multidrug resistant uropathogen bacteria compared with last resort antibiotics such a Ciprofloxacin (Rahuman et al., 2021). With slow wound healing times, infection increases leading to the formation of bacterial bio-films and subsequently, antibiotic resistance stemming from inefficient conventional therapies (Negut et al., 2018). Due to aging, the blood supply is reduced when compared with young subjects, and it has been shown that diabetic patients with reduced renal functionality can hinder tissue repair and cannot combat ulcer infections (Huo et al., 2016; Zubair et al., 2011). Novel nanotechnology platforms have shown their benefits with respect to overcoming antibiotic resistance stemming from bio-film formations, controlled drug delivery to alter immune responses steering toward wound healing and improved antimicrobial devices in healthcare of the elderly (Ramasamy & Lee, 2016). Reduced releases of growth factors and increased oxidative stress and persistent proinflammatory cytokines such as IL-6 and TNF-alpha are commonly seen in the elderly and diabetic wounds. Nanocomposite dressings developed by Singla et al. (2017), consisting of silver NPs incorporated inside plant-based cellulose nanocrystals showed accelerated wound healing in both acute and diabetic mice of a young age (6–8 weeks old) of Swiss albino type. Although this study was done in young mice, the material possesses therapeutic potential in vitro and may be used in older subjects for wound healing, especially toward chronic wounds seen in the elderly with diabetes. In this study, the anti-inflammatory properties of Ag NPs showed inhibition of proinflammatory cytokines IL-6 and TNF-alpha responses, leading to accelerated wound healing in both acute and diabetic wounds, along with their well-established antibacterial effects. In a similar immunomodulatory approach, delivery of α-gal epitope expressing nanoparticles in mice models of diabetic wounds were able to induce anti-Gal antibody-mediated recruitment of macrophages for accelerated wound healing (Figure 9) (Kaymakcalan et al., 2020). In mice treated with nanoparticles, complete wound healing was observed at post-operative day (POD) 12 compared with only 33% wounds healed in untreated mice. Additionally, antibacterial biomaterials such as silver, gold, copper, gelatin, alginate, chitosan, and others can be used to deliver immunomodulatory molecules such as growth factors, immune stimulants, and ECM precursors to aid in wound healing of both diabetic and chronic wounds. Considering all these factors, treatment of infectious diseases needs a change in drug delivery with more targeted drugs to reduce ADE and improve compliance in the elderly. Various key nanotechnological interventions in dealing with infectious diseases and their treatment side effects are illustrated in Figure 10.
FIGURE 9.
α-Gal epitope expressing nanoparticles for the recruitment of macrophages for wound healing treatment. (a,b) H&E longitudinal hemi section of wounds showing macrophage density within wounds (a: Treated vs. b: Untreated). (c) Flow cytometry data. Closed column: PMN, gray columns: Medium size macrophages, open columns: Large size macrophages. Most macrophages are M2. (d) Macrophage density in wound bed. Greater density was observed in AGN (α-gel)-treated wounds. (e) Healing rate showing 100% healing by POD12 versus 33% in PBS (untreated) wounds (Kaymakcalan et al., 2020).
FIGURE 10.
Nanotechnology interventions in age-related infectious diseases. NPs, nanoparticles.
Viral infections such as respiratory syncytial virus, seasonal influenza virus, HIV2 split virus and the recent pandemic capable SARS-CoV-2 were treated with nanotechnology consisting of loading viral mRNA proteins in liposomes, PMMA for vaccines; whereas nanocarriers, including silica NPs, were employed to tag adjuvants to improve adjuvant efficacy (Baden et al., 2021; Bettencourt & Almeida, 2012; Cheng et al., 2017). Capabilities of nanotechnology to generate vaccines approved by the FDA for the recent pandemic of COVID-19 show their potential in developing preventative medicine for age-associated infections to improve the quality of life and reduce the economic burden on healthcare. Overall, we believe nanotechnology has entered the main stage of clinical translations.
8 |. CHALLENGES OF NANOCARRIERS FOR CLINICAL TRANSLATION
Despite the significant potential of nanocarriers to overcome many limitations of the conventional treatment with free drugs, multiple factors remain which slow progression toward clinical translation. Currently, the FDA regulatory guidelines for the translation of nanotechnology are limited, with nanocarriers and nanotherapeutics still being categorized as drugs themselves, despite their complexity in synthesis and rapid clearance of NPs by the spleen and liver seen in vivo (Hua et al., 2018; Y.-N. Zhang et al., 2016). In addition, long-term toxicological effects on the human health of nanocarriers are not completely understood (Missaoui et al., 2018). The standardized methods for testing cytotoxicity of NPs by American Safety for Testing and Materials uses nonhematological cells such as porcine kidney cells and human hepatocarcinoma cells which make it difficult to translate to cells exposed in various modes of delivery i.e. intravenous, intramuscular, and so on, owing to their different phenotype, especially in age-associated pathologies (Saha et al., 2020). Toxicity and safety studies with relevant cell lines and animal models need to be performed in the preclinical phase to make nanocarriers compatible for clinical translation. Currently, fewer than 100 nanomedicine-related drugs are approved by the FDA, EMA or other regulatory bodies around the world, for example, lipid-based Tozinameran® nanocarriers for COVID-19 vaccine and Zilretta®—a PLGA based hydrogel for knee osteoarthritis (Đorđević et al., 2022) showing the need for developing specific tools and techniques.
Nanocarriers are more complex than conventional drug formulations, creating a need to develop preclinical bioas-says which mimic in vivo conditions in elderly patients. Specific considerations include the use of alternate administration routes to reduce stress on organs such as kidneys from intravenous delivery, investigation of dosage regimen in age-associated models to avoid adverse drug reactions, and analyzing nanocarriers biocompatibility, biodegradability and overall pharmaceutical stability (Hua et al., 2018). Furthermore, drug deposition changes with age, contributing to variation in the absorption, permeability, distribution, cell uptake, metabolism, and clearance of drugs. These variations can result in modifications in NPs functionality causing reduced degradation, potential increase in NPs toxicity, reduced distribution, reduced cellular uptake, and prolonged circulation (Hunt et al., 2022). Although the nanotechnology characterization laboratory (NCL) has been established to prepare nanocarriers to meet regulatory requirements for investigational new drug (IND) or investigational device exemption (IDE), new assays involving age-related cell lines, pathologically significant phenotypes and relevant pharmacodynamic landscapes still need to be integrated for more comprehensive characterization. With the recent development in microfluidics and bioreactors, synthesis of organoid and spheroids has become feasible. Organoids and spheroids developed directly from disease significant cells can mimic in vivo conditions more accurately than 2D cultures or in vitro lab models, presenting a robust tool to assess nanocarrier toxicity and their drug delivery efficacies improving the compliance and making an easier transition to the clinical phase (Prasad et al., 2021).
Currently, the major limitation for the translation of nanocarriers is their inability to transform the pharmacokinetics of the encapsulated drug to produce efficient therapeutic effects because of their rapid clearance. For others, like cationic nanoparticles, it is the induced-toxicity due to their increased surface area to size ratio which can cause oxidative stress, epigenetic modifications, activation of inflammatory pathways and the accumulation of nanocarriers deeply into tissues and cells which can result in tissue damage, organ dysfunction and overall cell death. Hence, a more comprehensive and detailed understanding of the effects of nanocarriers physiochemical and structural properties in the aging population and the long-term effects is needed for nanotechnology advancement in the clinical setting (Missaoui et al., 2018).
9 |. LIMITATIONS OF NANOTECHNOLOGY FOR IMMUNOMODULATION
With new biomaterials and improved formulation methods, nanotechnology has come a long way in improving drug delivery for various disease treatments. Although nanomedicine stands out as a potential drug delivery strategy for treating age-related disorders, its few limitations are yet to be addressed for smooth clinical translations. Limitations including profiling immune responses of various nanomaterial immunotoxicity in immune declined elderly patients, cost efficiency, scale-up feasibility, and stability are still under investigation. Challenges of immunogenicity from protein corona formation around nanoparticles include their degradation products and metabolic pathways involved as well as cellular toxicity from cellular reorganization with respect to nanoparticle uptake need to be addressed. Tian et al. (2015) showed that zinc oxide nanoparticles exposure via intraperitoneal injection had more adverse effects in aged C57BL/6J mice than young ones. A synergistic effect between aging and zinc oxide exposure was seen by increases in proinflammatory cytokines, demanding a more holistic approach for designing drug carriers (Tian et al., 2015).
Aging is a factor often not considered while performing preclinical studies to test various nanoparticle-based treatments. Smith et al. (2002) showed that primary cells from aged donors tend to have a low proliferative potential, which may impact the studies such as migration assays and cytocompatibility studies, which rely on the proliferation rate of the cell. Foroozandeh et al. (2019) showed that toxicity from PEGylated quantum dots is increased in senescent cells compared with young cells showing the differences in bio-responses with respect to cell passage and donor. Successful translation of preclinical research including animal (rodent) studies is highly dependent on the experimental design. A recent survey among researchers shows that most studies chose the age of 6- to 20-week-old rodents for the studies due to cost and maintenance (Jackson et al., 2017), which is non-representative of aging physiology. Nanotechnology-based treatments have a greater impact in diseases related to the aged population due to their ability to reduce side effects. Organ functions of the liver and kidneys to filter and breakdown the biomaterials used in nanoparticle formulations deteriorate with age (Alexis et al., 2008). In that regard, age of animals for in vivo studies and mammalian cells for in vitro testing should be considered appropriately in the experimental designs for testing nanotechnology-based drugs, to improve their translational potential for age-specific design of treatments. Age-specific nanoparticles can be tailored by considering the disease prevalence age and choosing relevant aged experimental models.
Biomimetic nanoparticles have gained attention in this decade to address some of the immunotoxicity of nanoparticles. This technology involves coating of the nanoparticle with cell membranes to avoid phagocytosis and reduce other determinantal immune responses (Yaman et al., 2020). Although this technology is still in the nascent stage, keeping in mind declined immune responses in the elderly, care should be taken in assessing cell membrane mediated immune responses in nanomedicine for drug delivery. Efficient models such as zebra fish can be employed for faster screening of toxicity from nano-formulations before entering large animal models and eventually making way to clinical trials (Haque & Ward, 2018; Messerschmidt et al., 2022). Overall, most of the recent nanotechnology for treating age-associated diseases as discussed in this review are largely restricted to a laboratory setting with very few entering clinical trials. This shows the need for more established guidelines for testing the novel nano-formulations in a clinical setting.
10 |. CONCLUSIONS AND FUTURE ASPECTS
An increase in life expectancy is the result of public health initiatives and successful infection control interventions. Reports indicate that 8 million people are being added to the world’s elderly population each year, and this increase will reach 24 million per year by 2030. At the molecular scale, aging is a result of collective damage to the biomolecular systems over a time span that could lead to various organ injuries/failures and subsequently, contribute to age-related diseases, imposing high costs on the medical system. Therefore, efforts to achieve effective treatment and medication methods that can prevent the disorders by targeting aging pathways are highly desired moving ahead in this century.
Early detection can drastically improve therapeutic outcomes in age-related pathologies. Health checks to detect disease and assess risk factors are not beneficial in reducing disease and mortality rates, as shown in a recent study (Krogsbøll et al., 2019). Hence, there is need for better screening technologies for risk assessment, especially in the elderly. With growing research in nanomedicine, nanoparticles have undergone substantial improvement in targeting and as a carrier of contrast agents demonstrating their application as a diagnostic tool. For instance, atherosclerosis is a progressive condition leading to many cardiac diseases in the elderly, where efficient screening can prevent severe cases and further complications including thrombus formations, ischemic events, and death. For instance, Hyafil et al. (2007) showed that the use of iodized particles as a contrast agent in combination with CT could image atherosclerotic lesions in balloon-injured rabbit animal models. The uptake of particles by macrophages can enhance and show critical plaques with macrophage infiltration, which was not visible using conventional contrast agents in CT scans. Weissleder et al. (2014) also discussed the potential of imaging macrophages involved in various pathologies with the use of nanoparticles. Similarly, various studies have shown the use of super magnetic iron oxide nanoparticles to detect AB plaque in Alzheimer’s disease (Ulanova et al., 2020). Nanotechnologies like these can be employed as in vivo imaging probes for detection of risk factors including, atherosclerotic plaque, ischemic sites, gliomas, detection of biomarkers in neurodegenerative diseases, infectious pathogens, and other biomarkers of age-related diseases.
Cellular senescence leads to various age-related pathologies and is an emerging opportunity for medical interventions in age-associated diseases (Di Micco et al., 2021). Use of nanotechnology to target and treat senescent cells may improve aging effects and have an influence on longevity. To that context, Agostini et al. (2012) showed effective targeting of senescent-associated β-galactosidase using galacto-oligosaccharide (GOS) substrate coated mesoporous silica nanoparticles with release of cargo in senescent cells specifically. Similarly, CD9 receptors overexpressed in senescent cells were targeted using CD9 antibody conjugated calcium carbonate nanoparticles loaded with Rapamycin. These nanoparticles exhibited a high uptake and increased drug delivery in senescent cells, evident by improved proliferation abilities and reduction in senescence associated secretory phenotypes, including IL-6 and IL-1 β. Targeting senescent cells for their modulation or removal using nano-senolytics is a new direction of research with respect to reverse of aging effects, and nanotechnology, in particular, shows potential to steer treatments toward aging-associated diseases.
Nanocarriers, combined with chemical drugs and, in some cases, drugs of natural origin, have become very popular over the past decade for targeted therapeutic research. Nanomedicine could act by directly modulating age progression by various signaling pathways and functions as anti-inflammatory, antiapoptotic, autophagy regulation, and genomic stabilizer compounds. Nevertheless, it is evident that the evaluation of the antiaging potential of nano-based compounds must include some pivotal assessments to mitigate concerns on toxicity, modes of functions, interacting targets/pathways, optimum dosage, clearance from the body, and finally, reproducibility and predictability of the interactions.
ACKNOWLEDGMENTS
All graphic illustrations were created by Biorender.com
FUNDING INFORMATION
Research reported in this manuscript was supported by the National Institute of Health under NIH R15 HL156076 and NIH R01 HL 158204. Additionally, this work was supported by DWT through R01 (AG060395) and K01 (AG061271) funding.
National Institutes of Health, Grant/Award Numbers: K01/AG061271, R01/AG060395, R01/HL158204, R15/HL156076
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Agostini A, Mondragón L, Bernardos A, Martínez-Máñez R, Marcos MD, Sancenón F, Soto J, Costero A, Manguan-García C, Perona R, Moreno-Torres M, Aparicio-Sanchis R, & Murguía JR (2012). Targeted cargo delivery in senescent cells using capped mesoporous silica nanoparticles. Angewandte Chemie International Edition, 51(42), 10556–10560. 10.1002/anie.201204663 [DOI] [PubMed] [Google Scholar]
- Aiello A, Farzaneh F, Candore G, Caruso C, Davinelli S, Gambino CM, Ligotti ME, Zareian N, & Accardi G (2019). Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Frontiers in Immunology, 10, 2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam Q, Alam MZ, Mushtaq G, Damanhouri GA, Rasool M, Kamal MA, & Haque A (2016). Inflammatory process in Alzheimer’s and Parkinson’s diseases: Central role of cytokines. Current Pharmaceutical Design, 22(5), 541–548. 10.2174/1381612822666151125000300 [DOI] [PubMed] [Google Scholar]
- Alexis F, Pridgen E, Molnar LK, & Farokhzad OC (2008). Factors affecting the clearance and biodistribution of polymeric nanoparticles. Molecular Pharmaceutics, 5(4), 505–515. 10.1021/mp800051m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amor S, Puentes F, Baker D, & van der Valk P (2010). Inflammation in neurodegenerative diseases. Immunology, 129(2), 154–169. 10.1111/j.1365-2567.2009.03225.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnott I, Rogler G, & Halfvarson J (2018). The management of inflammatory bowel disease in elderly: Current evidence and future perspectives. Inflammatory Intestinal Diseases, 2(4), 189–199. 10.1159/000490053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, Diemert D, Spector SA, Rouphael N, Creech CB, McGettigan J, Khetan S, Segall N, Solis J, Brosz A, Fierro C, Schwartz H, Neuzil K, Corey L, … for the COVE Study Group. (2021). Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. The New England Journal of Medicine, 384(5), 403–416. 10.1056/NEJMoa2035389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbe-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, & Bauer ME (2020). The interplay between immunosenescence and age-related diseases. Seminars in Immunopathology, 42, 545–557. 10.1007/s00281-020-00806-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barea MJ, Jenkins MJ, Gaber MH, & Bridson RH (2010). Evaluation of liposomes coated with a pH responsive polymer. International Journal of Pharmaceutics, 402(1–2), 89–94. 10.1016/j.ijpharm.2010.09.028 [DOI] [PubMed] [Google Scholar]
- Bauer ME (2020). Accelerated immunosenescence in rheumatoid arthritis: Impact on clinical progression. Immunity & Ageing, 17(1), 6. 10.1186/s12979-020-00178-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger JS, Rockman CB, Guyer KE, & Lopez LR (2014). Proatherogenic oxidized low-density lipoprotein/β2-glycoprotein I complexes in arterial and venous disease. Journal of Immunology Research, 2014, 234316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettencourt A, & Almeida AJ (2012). Poly(methyl methacrylate) particulate carriers in drug delivery. Journal of Microencapsulation, 29(4), 353–367. 10.3109/02652048.2011.651500 [DOI] [PubMed] [Google Scholar]
- Björkqvist M, Wild EJ, Thiele J, Silvestroni A, Andre R, Lahiri N, Raibon E, Lee RV, Benn CL, Soulet D, Magnusson A, Woodman B, Landles C, Pouladi MA, Hayden MR, Khalili-Shirazi A, Lowdell MW, Brundin P, Bates GP, … Tabrizi SJ (2008). A novel pathogenic pathway of immune activation detectable before clinical onset in Huntington’s disease. The Journal of Experimental Medicine, 205(8), 1869–1877. 10.1084/jem.20080178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boada C, Zinger A, Tsao C, Zhao P, Martinez JO, Hartman K, Naoi T, Sukhoveshin R, Sushnitha M, Molinaro R, Trachtenberg B, Cooke JP, & Tasciotti E (2020). Rapamycin-loaded biomimetic nanoparticles reverse vascular inflammation. Circulation Research, 126(1), 25–37. 10.1161/CIRCRESAHA.119.315185 [DOI] [PubMed] [Google Scholar]
- Bodnár Z, Steger MM, Saurwein-Teissl M, Maczek C, & Grubeck-Loebenstein B (1997). Cytokine production in response to stimulation with tetanus toxoid, mycobacterium tuberculosis and influenza antigens in peripheral blood mononuclear cells and T cell lines from healthy elderlies. International Archives of Allergy and Immunology, 112(4), 323–330. [DOI] [PubMed] [Google Scholar]
- Bourke CD, Berkley JA, & Prendergast AJ (2016). Immune dysfunction as a cause and consequence of malnutrition. Trends in Immunology, 37(6), 386–398. 10.1016/j.it.2016.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahma DK, Wahlang JB, Marak MD, & Ch Sangma M (2013). Adverse drug reactions in the elderly. Journal of Pharmacology & Pharmacotherapeutics, 4(2), 91–94. 10.4103/0976-500X.110872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butterfield DA, & Lauderback CM (2002). Lipid peroxidation and protein oxidation in Alzheimer’s disease brain: Potential causes and consequences involving amyloid beta-peptide-associated free radical oxidative stress. Free Radical Biology & Medicine, 32(11), 1050–1060. 10.1016/s0891-5849(02)00794-3 [DOI] [PubMed] [Google Scholar]
- Byng-Maddick R, & Noursadeghi M (2016). Does tuberculosis threaten our ageing populations? BMC Infectious Diseases, 16, 119. 10.1186/s12879-016-1451-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo SM, Vuille-dit-Bille RN, Mariotta L, Ramadan T, Huggel K, Singer D, Götze O, & Verrey F (2014). The molecular mechanism of intestinal levodopa absorption and its possible implications for the treatment of Parkinson’s disease. The Journal of Pharmacology and Experimental Therapeutics, 351(1), 114–123. 10.1124/jpet.114.216317 [DOI] [PubMed] [Google Scholar]
- Cartaya A, Maiocchi S, & Bahnson EM (2019). Nanotherapies for treatment of cardiovascular disease: A case for antioxidant targeted delivery. Current Pathobiology Reports, 7(3), 47–60. 10.1007/s40139-019-00196-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellon X, & Bogdanova V (2016). Chronic inflammatory diseases and endothelial dysfunction. Aging and Disease, 7(1), 81–89. 10.14336/AD.2015.0803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceeraz S, Hall C, Choy EH, Spencer J, & Corrigall VM (2013). Defective CD8+CD28+ regulatory T cell suppressor function in rheumatoid arthritis is restored by tumour necrosis factor inhibitor therapy. Clinical and Experimental Immunology, 174(1), 18–26. 10.1111/cei.12161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, & Pahor M (2003). Inflammatory markers and cardiovascular disease (the health, aging and body composition [health ABC] study). The American Journal of Cardiology, 92(5), 522–528. 10.1016/s0002-9149(03)00718-5 [DOI] [PubMed] [Google Scholar]
- Cevher E, Salomon SK, Makrakis A, Li XW, Brocchini S, & Alpar HO (2015). Development of chitosan–pullulan composite nanoparticles for nasal delivery of vaccines: Optimisation and cellular studies. Journal of Microencapsulation, 32(8), 755–768. 10.3109/02652048.2015.1073392 [DOI] [PubMed] [Google Scholar]
- Chaganti SS, McCusker EA, & Loy CT (2017). What do we know about late onset Huntington’s disease? Journal of Huntington’s Disease, 6(2), 95–103. 10.3233/JHD-170247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheah BC, Vucic S, Krishnan AV, & Kiernan MC (2010). Riluzole, neuroprotection and amyotrophic lateral sclerosis. Current Medicinal Chemistry, 17(18), 1942–1199. 10.2174/092986710791163939 [DOI] [PubMed] [Google Scholar]
- Cheng J, Wen S, Wang S, Hao P, Cheng Z, Liu Y, Zhao P, & Liu J (2017). gp85 protein vaccine adjuvanted with silica nanoparticles against ALV-J in chickens. Vaccine, 35(2), 293–298. [DOI] [PubMed] [Google Scholar]
- Chintapula U, Iqbal SM, & Kim Y-T (2020). A compendium of single cell analysis in aging and disease. AIMS Molecular Science, 7(1), 49–69. 10.3934/molsci.2020004 [DOI] [Google Scholar]
- Chougnet CA, Thacker RI, Shehata HM, Hennies CM, Lehn MA, Lages CS, & Janssen EM (2015). Loss of phagocytic and antigen cross-presenting capacity in aging dendritic cells is associated with mitochondrial dysfunction. Journal of Immunology, 195(6), 2624–2632. 10.4049/jimmunol.1501006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colmegna I, & Weyand CM (2011). Haematopoietic stem and progenitor cells in rheumatoid arthritis. Rheumatology (Oxford, England), 50(2), 252–260. 10.1093/rheumatology/keq298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cong W, Bai R, Li Y-F, Wang L, & Chen C (2019). Selenium nanoparticles as an efficient nanomedicine for the therapy of Huntington’s disease. ACS Applied Materials & Interfaces, 11(38), 34725–34735. 10.1021/acsami.9b12319 [DOI] [PubMed] [Google Scholar]
- Conte SM, & Vale PR (2017). Peripheral arterial disease. Heart, Lung and Circulation, 27(4), 427–432. [DOI] [PubMed] [Google Scholar]
- Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, Fuster V, Fisher EA, Mulder WJ, Proksa R, & Fayad ZA (2010). Atherosclerotic plaque composition: Analysis with multicolor CT and targeted gold nanoparticles. Radiology, 256(3), 774–782. 10.1148/radiol.10092473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotti A, Benner C, Kerman BE, Gosselin D, Lagier-Tourenne C, Zuccato C, Cattaneo E, Gage FH, Cleveland DW, & Glass CK (2014). Mutant huntingtin promotes autonomous microglia activation via myeloid lineage-determining factors. Nature Neuroscience, 17(4), 513–521. 10.1038/nn.3668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuaron J, Gburcik V, & Prabhakar B (2016). PharmaPoint: Type 2 diabetes—Global drug forecast and market analysis to 2025. GlobalData. [Google Scholar]
- Cutolo M, Spies CM, Buttgereit F, Paolino S, & Pizzorni C (2014). The supplementary therapeutic DMARD role of low-dose glucocorticoids in rheumatoid arthritis. Arthritis Research & Therapy, 16(2), S1. 10.1186/ar4685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyrus T, Zhang H, Allen JS, Williams TA, Hu G, Caruthers SD, Wickline SA, & Lanza GM (2008). Intramural delivery of rapamycin with alphavbeta3-targeted paramagnetic nanoparticles inhibits stenosis after balloon injury. Arteriosclerosis, Thrombosis, and Vascular Biology, 28(5), 820–826. 10.1161/ATVBAHA.107.156281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Leo V, Milano F, Mancini E, Comparelli R, Giotta L, Nacci A, Longobardi F, Garbetta A, Agostiano A, & Catucci L (2018). Encapsulation of curcumin-loaded liposomes for colonic drug delivery in a pH-responsive polymer cluster using a pH-driven and organic solvent-free process. Molecules, 23(4), 739. 10.3390/molecules23040739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debnath K, Pradhan N, Singh BK, Jana NR, & Jana NR (2017). Poly(trehalose) nanoparticles prevent amyloid aggregation and suppress polyglutamine aggregation in a Huntington’s disease model mouse. ACS Applied Materials & Interfaces, 9(28), 24126–24139. 10.1021/acsami.7b06510 [DOI] [PubMed] [Google Scholar]
- Del Rey MJ, Valín Á, Usategui A, Ergueta S, Martín E, Municio C, Cañete JD, Blanco FJ, Criado G, & Pablos JL (2019). Senescent synovial fibroblasts accumulate prematurely in rheumatoid arthritis tissues and display an enhanced inflammatory phenotype. Immunity & Ageing, 16, 29. 10.1186/s12979-019-0169-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Micco R, Krizhanovsky V, Baker D, & d’Adda di Fagagna F (2021). Cellular senescence in ageing: From mechanisms to therapeutic opportunities. Nature Reviews Molecular Cell Biology, 22(2), 75–95. 10.1038/s41580-020-00314-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Đorđevic S, Gonzalez MM, Conejos-Sánchez I, Carreira B, Pozzi S, Acúrcio RC, Satchi-Fainaro R, Florindo HF, & Vicent MJ (2022). Current hurdles to the translation of nanomedicines from bench to the clinic. Drug Delivery and Translational Research, 12(3), 500–525. 10.1007/s13346-021-01024-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormont F, Brusini R, Cailleau C, Reynaud F, Peramo A, Gendron A, Mougin J, Gaudin F, Varna M, & Couvreur P (2020). Squalene-based multidrug nanoparticles for improved mitigation of uncontrolled inflammation in rodents. Science Advances, 6(23), eaaz5466. 10.1126/sciadv.aaz5466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake J, Link CD, & Butterfield DA (2003). Oxidative stress precedes fibrillar deposition of Alzheimer’s disease amyloid beta-peptide (1–42) in a transgenic Caenorhabditis elegans model. Neurobiology of Aging, 24(3), 415–420. 10.1016/s0197-4580(02)00225-7 [DOI] [PubMed] [Google Scholar]
- Duan W, & Li H (2018). Combination of NF-kB targeted siRNA and methotrexate in a hybrid nanocarrier towards the effective treatment in rheumatoid arthritis. Journal of Nanobiotechnology, 16(1), 58. 10.1186/s12951-018-0382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duivenvoorden R, Tang J, Cormode DP, Mieszawska AJ, Izquierdo-Garcia D, Ozcan C, Otten MJ, Zaidi N, Lobatto ME, van Rijs SM, Priem B, Kuan EL, Martel C, Hewing B, Sager H, Nahrendorf M, Randolph GJ, Stroes ES, Fuster V, … Mulder WJ (2014). A statin-loaded reconstituted high-density lipoprotein nanoparticle inhibits atherosclerotic plaque inflammation. Nature Communications, 5, 3065. 10.1038/ncomms4065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta K, Thammisetty SS, Boutej H, Bareil C, & Julien J-P (2020). Mitigation of ALS pathology by neuron-specific inhibition of nuclear factor kappa B signaling. The Journal of Neuroscience, 40(26), 5137–5154. 10.1523/JNEUROSCI.0536-20.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddy CM, Parkinson EG, & Rickards HE (2016). Changes in mental state and behaviour in Huntington’s disease. Lancet Psychiatry, 3(11), 1079–1086. 10.1016/s2215-0366(16)30144-4 [DOI] [PubMed] [Google Scholar]
- El Chakhtoura NG, Bonomo RA, & Jump RLP (2017). Influence of aging and environment on presentation of infection in older adults. Infectious Disease Clinics of North America, 31(4), 593–608. 10.1016/j.idc.2017.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elísio Costa AS-S, Paúl C, & Gallego JG (2015). Aging and cardiovascular risk. BioMed Research International, 2015, 871656. 10.1155/2015/871656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott ML, Caspi A, Houts RM, Ambler A, Broadbent JM, Hancox RJ, Harrington H, Hogan S, Keenan R, Knodt A, Leung JH, Melzer TR, Purdy SC, Ramrakha S, Richmond-Rakerd LS, Righarts A, Sugden K, Thomson WM, Thorne PR, … Moffitt TE (2021). Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nature Aging, 1(3), 295–308. 10.1038/s43587-021-00044-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espeseth AS, Cejas PJ, Citron MP, Wang D, DiStefano DJ, Callahan C, Donnell GO, Galli JD, Swoyer R, Touch S, Wen Z, Antonello J, Zhang L, Flynn JA, Cox KS, Freed DC, Vora KA, Bahl K, Latham AH, … Bett AJ (2020). Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. npj Vaccines, 5(1), 16. 10.1038/s41541-020-0163-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrugia M, & Baron B (2016). The role of TNF-α in rheumatoid arthritis: A focus on regulatory T cells. Journal of Clinical and Translational Research, 2(3), 84–90. [PMC free article] [PubMed] [Google Scholar]
- Feng S, Hu Y, Peng S, Han S, Tao H, Zhang Q, Xu X, Zhang J, & Hu H-Y (2016). Nanoparticles responsive to the inflammatory microenvironment for targeted treatment of arterial restenosis. Biomaterials, 105, 167–184. 10.1016/j.biomaterials.2016.08.003 [DOI] [PubMed] [Google Scholar]
- Feng X, Xu W, Li Z, Song W, Ding J, & Chen X (2019). Immunomodulatory nanosystems. Advanced Science, 6(17), 1900101. 10.1002/advs.201900101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton OS, Olafson KN, Pillai PS, Mitchell MJ, & Langer R (2018). Advances in biomaterials for drug delivery. Advanced Materials, 30, e1705328. 10.1002/adma.201705328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrucci L, & Fabbri E (2018). Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nature Reviews. Cardiology, 15(9), 505–522. 10.1038/s41569-018-0064-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane TE (2017). “Urinary tract infection”-requiem for a heavyweight. Journal of the American Geriatrics Society, 65(8), 1650–1655. [DOI] [PubMed] [Google Scholar]
- Firestein GS (2003). Evolving concepts of rheumatoid arthritis. Nature, 423(6937), 356–361. 10.1038/nature01661 [DOI] [PubMed] [Google Scholar]
- Flores AM, Hosseini-Nassab N, Jarr K-U, Ye J, Zhu X, Wirka R, Koh AL, Tsantilas P, Wang Y, Nanda V, Kojima Y, Zeng Y, Lotfi M, Sinclair R, Weissman IL, Ingelsson E, Smith BR, & Leeper NJ (2020). Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nature Nanotechnology, 15(2), 154–161. 10.1038/s41565-019-0619-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores AM, Ye J, Jarr K-U, Hosseini-Nassab N, Smith BR, & Leeper NJ (2019). Nanoparticle therapy for vascular diseases. Arteriosclerosis, Thrombosis, and Vascular Biology, 39(4), 635–646. 10.1161/ATVBAHA.118.311569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foroozandeh P, Aziz AA, & Mahmoudi M (2019). Effect of cell age on uptake and toxicity of nanoparticles: The overlooked factor at the nanobiointerface. ACS Applied Materials & Interfaces, 11(43), 39672–39687. 10.1021/acsami.9b15533 [DOI] [PubMed] [Google Scholar]
- Fülöp T, Larbi A, Hirokawa K, Mocchegiani E, Lesourds B, Castle S, Wikby A, Franceschi C, & Pawelec G (2007). Immunosupportive therapies in aging. Clinical Interventions in Aging, 2(1), 33–54. 10.2147/ciia.2007.2.1.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavazzi G, Herrmann F, & Krause K-H (2004). Aging and infectious diseases in the developing world. Clinical Infectious Diseases, 39(1), 83–91. 10.1086/421559 [DOI] [PubMed] [Google Scholar]
- Gavazzi G, & Krause KH (2002). Ageing and infection. The Lancet Infectious Diseases, 2(11), 659–666. 10.1016/s1473-3099(02)00437-1 [DOI] [PubMed] [Google Scholar]
- Gavier B, Vazquez F, & Gandara E (2016). Antiphospholipid antibodies and lower extremity peripheral arterial disease: A systematic review and meta-analysis. Vasa, 45(4), 325–330. [DOI] [PubMed] [Google Scholar]
- Gerards AH, de Lathouder S, de Groot ER, Dijkmans BAC, & Aarden LA (2003). Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology, 42(10), 1189–1196. 10.1093/rheumatology/keg323 [DOI] [PubMed] [Google Scholar]
- Gholizadeh S, Kamps JAAM, Hennink WE, & Kok RJ (2018). PLGA-PEG nanoparticles for targeted delivery of the mTOR/PI3kinase inhibitor dactolisib to inflamed endothelium. International Journal of Pharmaceutics, 548(2), 747–758. 10.1016/j.ijpharm.2017.10.032 [DOI] [PubMed] [Google Scholar]
- Giacomini E, Ferroni L, Miettinen M, Fattorini L, Orefici G, Julkunen I, & Coccia EM (2001). Infection of human macrophages and dendritic cells with Mycobacterium tuberculosis induces a differential cytokine gene expression that modulates T cell response. Journal of Immunology, 166(12), 7033–7041. [DOI] [PubMed] [Google Scholar]
- Giron F, Pastó A, Tasciotti E, & Abraham BP (2019). Nanotechnology in the treatment of inflammatory bowel disease. Inflammatory Bowel Diseases, 25(12), 1871–1880. 10.1093/ibd/izz205 [DOI] [PubMed] [Google Scholar]
- Goldman D (2015). The economic promise of delayed aging. Cold Spring Harbor Perspectives in Medicine, 6(2), a025072. 10.1101/cshperspect.a025072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith M, Abramovitz L, & Peer D (2014). Precision nanomedicine in neurodegenerative diseases. ACS Nano, 8(3), 1958–1965. 10.1021/nn501292z [DOI] [PubMed] [Google Scholar]
- Gomes MLS, da Silva Nascimento N, Borsato DM, Pretes AP, Nadal JM, Novatski A, Gomes RZ, Fernandes D, Farago PV, & Zanin SMW (2019). Long-lasting anti-platelet activity of cilostazol from poly(ε-caprolactone)-poly(ethylene glycol) blend nanocapsules. Materials for Biological Applications. Materials Science and Engineering: C, 94, 694–702. [DOI] [PubMed] [Google Scholar]
- Guzman-Martinez L, Maccioni RB, Andrade V, Navarrete LP, Pastor MG, & Ramos-Escobar N (2019). Neuroinflammation as a common feature of neurodegenerative disorders. Frontiers in Pharmacology, 10(1008). 10.3389/fphar.2019.01008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TH (2013). Urinalysis: The usefulness and limitations of urine dipstick testing. Childhood Kidney Diseases, 17(2), 42–48. 10.3339/jkspn.2013.17.2.42 [DOI] [Google Scholar]
- Hansson GK, & Libby P (2006). The immune response in atherosclerosis: A double-edged sword. Nature Reviews. Immunology, 6(7), 508–519. 10.1038/nri1882 [DOI] [PubMed] [Google Scholar]
- Haque E, & Ward AC (2018). Zebrafish as a model to evaluate nanoparticle toxicity. Nanomaterials (Basel, Switzerland), 8(7), 561. 10.3390/nano8070561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harland M, Torres S, Liu J, & Wang X (2020). Neuronal mitochondria modulation of LPS-induced Neuroinflammation. The Journal of Neuroscience, 40(8), 1756–1765. 10.1523/JNEUROSCI.2324-19.2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann W, Lambova S, & Muller-Ladner U (2018). Current treatment options for osteoarthritis. Current Rheumatology Reviews, 14(2), 108–116. 10.2174/1573397113666170829155149 [DOI] [PubMed] [Google Scholar]
- Herranz F, Salinas B, Groult H, Pellico J, Lechuga-Vieco AV, Bhavesh R, & Ruiz-Cabello J (2014). Superparamagnetic nanoparticles for atherosclerosis imaging. Nanomaterials, 4(2), 408–438. 10.3390/nano4020408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hindle JV (2010). Ageing, neurodegeneration and Parkinson’s disease. Age and Ageing, 39(2), 156–161. 10.1093/ageing/afp223 [DOI] [PubMed] [Google Scholar]
- Hou Y, Dan X, Babbar M, Wei Y, Hasselbalch SG, Croteau DL, & Bohr VA (2019). Ageing as a risk factor for neurodegenerative disease. Nature Reviews. Neurology, 15(10), 565–581. 10.1038/s41582-019-0244-7 [DOI] [PubMed] [Google Scholar]
- Howard KA, Paludan SR, Behlke MA, Besenbacher F, Deleuran B, & Kjems J (2009). Chitosan/siRNA nanoparticle-mediated TNF-alpha knockdown in peritoneal macrophages for anti-inflammatory treatment in a murine arthritis model. Molecular Therapy, 17(1), 162–168. 10.1038/mt.2008.220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu K, Shi Y, Jiang W, Han J, Huang S, & Jiang X (2011). Lactoferrin conjugated PEG-PLGA nanoparticles for brain delivery: Preparation, characterization and efficacy in Parkinson’s disease. International Journal of Pharmaceutics, 415(1–2), 273–283. 10.1016/j.ijpharm.2011.05.062 [DOI] [PubMed] [Google Scholar]
- Hua S, de Matos MBC, Metselaar JM, & Storm G (2018). Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Frontiers in Pharmacology, 9, 790. 10.3389/fphar.2018.00790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Zhang J, Hou J, & Chen C (2003). Free radical scavenging efficiency of Nano-Se in vitro. Free Radical Biology and Medicine, 35(7), 805–813. [DOI] [PubMed] [Google Scholar]
- Hunt NJ, McCourt PAG, Kuncic Z, Le Couteur DG, & Cogger VC (2022). Opportunities and challenges for nanotherapeutics for the aging population. Frontiers in Nanotechnology, 10. 10.3389/fnano.2022.832524 [DOI] [Google Scholar]
- Huo X, Gao L, Guo L, Xu W, Wang W, Zhi X, Li L, Ren Y, Qi X, Sun Z, Li W, Ji Q, Ran X, Su B, Hao C, Lu J, Guo X, Zhuo H, Zhang D, … Ji L (2016). Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: A cross-sectional study. Lancet Diabetes & Endocrinology, 4(2), 115–124. [DOI] [PubMed] [Google Scholar]
- Hyafil F, Feig JE, Gordon R, Vucic E, Amirbekian V, Fisher EA, & Fayad ZA (2007). Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nature Medicine, 13(5), 636–641. [DOI] [PubMed] [Google Scholar]
- InformedHealth.org. (2006). Medication for the long-term treatment of coronary artery disease. Institute for Quality and Efficiency in health care (IQWiG). [Google Scholar]
- Inouye SK, Ganguli I, & Jacobs EA (2021). Enhancing aging and ending ageism: JAMA network open call for papers. JAMA Network Open, 4(6), e2117621. 10.1001/jamanetworkopen.2021.17621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson SJ, Andrews N, Ball D, Bellantuono I, Gray J, Hachoumi L, Holmes A, Latcham J, Petrie A, Potter P, Rice A, Ritchie A, Stewart M, Strepka C, Yeoman M, & Chapman K (2017). Does age matter? The impact of rodent age on study outcomes. Laboratory Animals, 51(2), 160–169. 10.1177/0023677216653984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S, & Amiji M (2012). Tuftsin-modified alginate nanoparticles as a noncondensing macrophage-targeted DNA delivery system. Biomacromolecules, 13(4), 1074–1085. 10.1021/bm2017993 [DOI] [PubMed] [Google Scholar]
- Jain S, Tran TH, & Amiji M (2015). Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials, 61, 162–177. 10.1016/j.biomaterials.2015.05.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankovic J, & Aguilar LG (2008). Current approaches to the treatment of Parkinson’s disease. Neuropsychiatric Disease and Treatment, 4(4), 743–757. 10.2147/ndt.s2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F (2016). Autophagy in vascular endothelial cells. Clinical and Experimental Pharmacology & Physiology, 43(11), 1021–1028. 10.1111/1440-1681.12649 [DOI] [PubMed] [Google Scholar]
- Jin G-Z, Chakraborty A, Lee J-H, Knowles JC, & Kim H-W (2020). Targeting with nanoparticles for the therapeutic treatment of brain diseases. Journal of Tissue Engineering, 11. 10.1177/2041731419897460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang YS, Jung HJ, Oh JS, & Song DY (2016). Use of PEGylated immunoliposomes to deliver dopamine across the blood-brain barrier in a rat model of Parkinson’s disease. CNS Neuroscience & Therapeutics, 22(10), 817–823. 10.1111/cns.12580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz S, & Feldstein R (2008). Inflammatory bowel disease of the elderly: A wake-up call. Gastroenterology & Hepatology, 4(5), 337–347. [PMC free article] [PubMed] [Google Scholar]
- Kaymakcalan OE, Abadeer A, Goldufsky JW, Galili U, Karinja SJ, Dong X, Jin JL, Samadi A, & Spector JA (2020). Topical α-gal nanoparticles accelerate diabetic wound healing. Experimental Dermatology, 29(4), 404–413. 10.1111/exd.14084 [DOI] [PubMed] [Google Scholar]
- Ke S, Lai Y, Zhou T, Li L, Wang Y, Ren L, & Ye S (2018). Molybdenum disulfide nanoparticles resist oxidative stress-mediated impairment of autophagic flux and mitigate endothelial cell senescence and angiogenic dysfunctions. ACS Biomaterials Science & Engineering, 4(2), 663–674. 10.1021/acsbiomaterials.7b00714 [DOI] [PubMed] [Google Scholar]
- Khan MS, & Roberts MS (2018). Challenges and innovations of drug delivery in older age. Advanced Drug Delivery Reviews, 135, 3–38. 10.1016/j.addr.2018.09.003 [DOI] [PubMed] [Google Scholar]
- Kim KS, Song CG, & Kang PM (2019). Targeting oxidative stress using nanoparticles as a theranostic strategy for cardiovascular diseases. Antioxidants & Redox Signaling, 30(5), 733–746. 10.1089/ars.2017.7428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Rehman SU, Amin FU, & Kim MO (2017). Enhanced neuroprotection of anthocyanin-loaded PEG-gold nanoparticles against Aβ(1–42)-induced neuroinflammation and neurodegeneration via the NF-(K)B /JNK/GSK3β signaling pathway. Nanomedicine, 13(8), 2533–2544. 10.1016/j.nano.2017.06.022 [DOI] [PubMed] [Google Scholar]
- Koellhoffer EC, McCullough LD, & Ritzel RM (2017). Old maids: Aging and its impact on microglia function. International Journal of Molecular Sciences, 18(4), 769. 10.3390/ijms18040769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler BM, Günther J, Kaudewitz D, & Lorenz HM (2019). Current therapeutic options in the treatment of rheumatoid arthritis. Journal of Clinical Medicine, 8(7), 938. 10.3390/jcm8070938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolhar P, Anselmo AC, Gupta V, Pant K, Prabhakarpandian B, Ruoslahti E, & Mitragotri S (2013). Using shape effects to target antibody-coated nanoparticles to lung and brain endothelium. Proceedings of the National Academy of Sciences of the United States of America, 110(26), 10753–10758. 10.1073/pnas.1308345110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotb S, Piraquive J, Lamberton F, Lux F, Verset M, Di Cataldo V, Contamin H, Tillement O, Canet-Soulas E, & Sancey L (2016). Safety evaluation and imaging properties of gadolinium-based nanoparticles in nonhuman primates. Scientific Reports, 6(1), 35053. 10.1038/srep35053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kour G, Haq SA, Bajaj BK, Gupta PN, & Ahmed Z (2021). Phytochemical add-on therapy to DMARDs therapy in rheumatoid arthritis: In vitro and in vivo bases, clinical evidence and future trends. Pharmacological Research, 169, 105618. 10.1016/j.phrs.2021.105618 [DOI] [PubMed] [Google Scholar]
- Kreutzberg GW (1996). Microglia: A sensor for pathological events in the CNS. Trends in Neurosciences, 19(8), 312–318. 10.1016/0166-2236(96)10049-7 [DOI] [PubMed] [Google Scholar]
- Kritchevsky SB, Cesari M, & Pahor M (2005). Inflammatory markers and cardiovascular health in older adults. Cardiovascular Research, 66(2), 265–275. 10.1016/j.cardiores.2004.12.026 [DOI] [PubMed] [Google Scholar]
- Krogsbøll LT, Jørgensen KJ, & Gøtzsche PC (2019). General health checks in adults for reducing morbidity and mortality from disease. The Cochrane Database of Systematic Reviews, 1(1), CD009009. 10.1002/14651858.CD009009.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Chaudhary RK, Singh R, Singh SP, Wang S-Y, Hoe Z-Y, Pan CT, Shiue YL, Wei DQ, Kaushik AC, & Dai X (2020). Nanotheranostic applications for detection and targeting neurodegenerative diseases. Frontiers in Neuroscience, 14(305). 10.3389/fnins.2020.00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar J, & Newton AMJ (2017). Rifaximin–chitosan nanoparticles for inflammatory bowel disease (IBD). Recent Patents on Inflammation & Allergy Drug Discovery, 11(1), 41–52. 10.2174/1872213x10666161230111226 [DOI] [PubMed] [Google Scholar]
- Laiho K, Tuomilehto J, & Tilvis R (2001). Prevalence of rheumatoid arthritis and musculoskeletal diseases in the elderly population. Rheumatology International, 20(3), 85–87. 10.1007/s002960000087 [DOI] [PubMed] [Google Scholar]
- Lana D, Ugolini F, Wenk GL, Giovannini MG, Zecchi-Orlandini S, & Nosi D (2019). Microglial distribution, branching, and clearance activity in aged rat hippocampus are affected by astrocyte meshwork integrity: Evidence of a novel cell-cell interglial interaction. The FASEB Journal, 33(3), 4007–4020. 10.1096/fj.201801539R [DOI] [PubMed] [Google Scholar]
- Lee D, Bae S, Hong D, Lim H, Yoon JH, Hwang O, … Kang PM (2013). H2O2-responsive molecularly engineered polymer nanoparticles as ischemia/reperfusion-targeted nanotherapeutic agents. Scientific Reports, 3, 2233. 10.1038/srep02233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N, & Kim WU (2017). Microbiota in T-cell homeostasis and inflammatory diseases. Experimental & Molecular Medicine, 49(5), e340. 10.1038/emm.2017.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PH, Park G-M, Han S, Kim Y-G, Lee J-Y, Roh J-H, Lee JH, Kim YH, & Lee S-W (2020). Beta-blockers provide a differential survival benefit in patients with coronary artery disease undergoing contemporary post-percutaneous coronary intervention management. Scientific Reports, 10(1), 22121. 10.1038/s41598-020-79214-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, & Lee DH (2016). Influence of old pulmonary tuberculosis on the management of secondary spontaneous pneumothorax in patients over the age of 70 years. Journal of Thoracic Disease, 8(10), 2903–2910. 10.21037/jtd.2016.10.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leng F, & Edison P (2021). Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nature Reviews Neurology, 17(3), 157–172. 10.1038/s41582-020-00435-y [DOI] [PubMed] [Google Scholar]
- Lewis CA, Manning J, Rossi F, & Krieger C (2012). The neuroinflammatory response in ALS: The roles of microglia and T cells. Neurology Research International, 2012, 803701. 10.1155/2012/803701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Petersen LK, York AW, Zablocki KR, Joseph LB, Kholodovych V, Prud’homme RK, Uhrich KE, & Moghe PV (2015). Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proceedings of the National Academy of Sciences of the United States of America, 112(9), 2693–2698. 10.1073/pnas.1424594112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P, Luo Z, Liu P, Gao N, Zhang Y, Pan H, Liu L, Wang C, Cai L, & Ma Y (2013). Bioreducible alginate-poly(ethylenimine) nanogels as an antigen-delivery system robustly enhance vaccine-elicited humoral and cellular immune responses. Journal of Controlled Release, 168(3), 271–279. 10.1016/j.jconrel.2013.03.025 [DOI] [PubMed] [Google Scholar]
- Li Y, Goronzy JJ, & Weyand CM (2018). DNA damage, metabolism and aging in pro-inflammatory T cells: Rheumatoid arthritis as a model system. Experimental Gerontology, 105, 118–127. 10.1016/j.exger.2017.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Lv H, Wang H, Yang H, Li Y, & Qian J (2020). Aging increases the severity of colitis and the related changes to the gut barrier and gut microbiota in humans and mice. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(7), 1284–1292. 10.1093/gerona/glz263 [DOI] [PubMed] [Google Scholar]
- Liu R, Yang J, Liu L, Lu Z, Shi Z, Ji W, Shen J, & Zhang X (2020). An “amyloid-β cleaner” for the treatment of Alzheimer’s disease by normalizing microglial dysfunction. Advanced Science, 7(2), 1901555. 10.1002/advs.201901555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Luehmann HP, Detering L, Pressly ED, McGrath AJ, Sultan D, Nguyen A, Grathwohl S, Shokeen M, Zayed M, Gropler RJ, Abendschein D, Hawker CJ, & Woodard PK (2019). Assessment of targeted nanoparticle assemblies for atherosclerosis imaging with positron emission tomography and potential for clinical translation. ACS Applied Materials & Interfaces, 11(17), 15316–15321. 10.1021/acsami.9b02750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z, Gao X, Kang T, Jiang M, Miao D, Gu G, Hu Q, Song Q, Yao L, Tu Y, Chen H, Jiang X, & Chen J (2013). B6 peptide-modified PEG-PLA nanoparticles for enhanced brain delivery of neuroprotective peptide. Bioconjugate Chemistry, 24(6), 997–1007. 10.1021/bc400055h [DOI] [PubMed] [Google Scholar]
- Lu Z, Marks E, Chen J, Moline J, Barrows L, Raisbeck M, Volitakis I, Cherny RA, Chopra V, Bush AI, Hersch S, & Fox JH (2014). Altered selenium status in Huntington’s disease: Neuroprotection by selenite in the N171–82Q mouse model. Neurobiology of Disease, 71, 34–42. [DOI] [PubMed] [Google Scholar]
- Ma S, Tian XY, Zhang Y, Mu C, Shen H, Bismuth J, Pownall HJ, Huang Y, & Wong WT (2016). E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Scientific Reports, 6(1), 22910. 10.1038/srep22910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madansein R, Parida S, Padayatchi N, Singh N, Master I, Naidu K, Zumla A, & Maeurer M (2015). Surgical treatment of complications of pulmonary tuberculosis, including drug-resistant tuberculosis. International Journal of Infectious Diseases, 32, 61–67. 10.1016/j.ijid.2015.01.019 [DOI] [PubMed] [Google Scholar]
- Mangoni AA, & Jackson SHD (2004). Age-related changes in pharmacokinetics and pharmacodynamics: Basic principles and practical applications. British Journal of Clinical Pharmacology, 57(1), 6–14. 10.1046/j.1365-2125.2003.02007.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marín-Peñalver JJ, Martín-Timón I, Sevillano-Collantes C, & Del Cañizo-Gómez FJ (2016). Update on the treatment of type 2 diabetes mellitus. World Journal of Diabetes, 7(17), 354–395. 10.4239/wjd.v7.i17.354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauricio R, Benn C, Davis J, Dawson G, Dawson LA, Evans A, Fox N, Gallacher J, Hutton M, Isaac J, Jones DNC, Jones L, Lalli G, Libri V, Lovestone S, Moody C, Noble W, Perry H, Pickett J, … Therapeutics for Dementia Consortium. (2019). Tackling gaps in developing life-changing treatments for dementia. Alzheimer’s & Dementia, 5, 241–253. 10.1016/j.trci.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxwell S (1999). Emergency management of acute myocardial infarction. British Journal of Clinical Pharmacology, 48(3), 284–298. 10.1046/j.1365-2125.1999.00998.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messerschmidt VL, Chintapula U, Bonetesta F, Laboy-Segarra S, Naderi A, Nguyen KT, Cao H, Mager E, & Lee J (2022). In vivo evaluation of non-viral NICD plasmid-loaded PLGA nanoparticles in developing zebrafish to improve cardiac functions. Frontiers in Physiology, 13. 10.3389/fphys.2022.819767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijit M, Caracciolo V, Melillo A, Amicarelli F, & Giordano A (2020). Role of p53 in the regulation of cellular senescence. Biomolecules, 10(3), 420. 10.3390/biom10030420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AH, Maletic V, & Raison CL (2009). Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biological Psychiatry, 65(9), 732–741. 10.1016/j.biopsych.2008.11.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missaoui WN, Arnold RD, & Cummings BS (2018). Toxicological status of nanoparticles: What we know and what we don’t know. Chemico-Biological Interactions, 295, 1–12. 10.1016/j.cbi.2018.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monge-Fuentes V, Biolchi Mayer A, Lima MR, Geraldes LR, Zanotto LN, Moreira KG, Martins OP, Piva HL, MSS F, Amaral AC, Bocca AL, Tedesco AC, & Mortari MR (2021). Dopamine-loaded nanoparticle systems circumvent the blood–brain barrier restoring motor function in mouse model for Parkinson’s disease. Scientific Reports, 11(1), 15185. 10.1038/s41598-021-94175-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-García A, Kun A, Calero O, Medina M, & Calero M (2019). An overview of the role of lipofuscin in age-related neurodegeneration. Frontiers in Neuroscience, 12, 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RG, Ives SJ, Lesniewski LA, Cawthon RM, Andtbacka RH, Noyes RD, Richardson RS, & Donato AJ (2013). Age-related telomere uncapping is associated with cellular senescence and inflammation independent of telomere shortening in human arteries. American Journal of Physiology. Heart and Circulatory Physiology, 305(2), H251–H258. 10.1152/ajpheart.00197.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley RL, Sharma A, Horsch AD, & Hinchliffe RJ (2018). Peripheral artery disease. BMJ, 360, j5842. [DOI] [PubMed] [Google Scholar]
- Moses JW, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, & Kuntz RE (2003). Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. New England Journal of Medicine, 349(14), 1315–1323. [DOI] [PubMed] [Google Scholar]
- Nadig SN, Dixit SK, Levey N, Esckilsen S, Miller K, Dennis W, Atkinson C, & Broome AM (2015). Immunosuppressive nanotherapeutic micelles downregulate endothelial cell inflammation and immunogenicity. RSC Advances, 5(54), 43552–43562. 10.1039/c5ra04057d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasiruddin M, Neyaz MK, & Das S (2017). Nanotechnology-based approach in tuberculosis treatment. Tuberculosis Research and Treatment, 2017, 4920209. 10.1155/2017/4920209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natkin JA (1998). Practical ambulatory geriatrics. Journal of Family Practice, 47(6), 466. [Google Scholar]
- Negut I, Grumezescu V, & Grumezescu AM (2018). Treatment strategies for infected wounds. Molecules, 23(9), 2392. 10.3390/molecules23092392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nerurkar L, Siebert S, McInnes IB, & Cavanagh J (2019). Rheumatoid arthritis and depression: An inflammatory perspective. Lancet Psychiatry, 6(2), 164–173. 10.1016/s2215-0366(18)30255-4 [DOI] [PubMed] [Google Scholar]
- Nethi SK, Nanda HS, Steele TWJ, & Patra CR (2017). Functionalized nanoceria exhibit improved angiogenic properties. Journal of Materials Chemistry B, 5(47), 9371–9383. 10.1039/c7tb01957b [DOI] [PubMed] [Google Scholar]
- Nimmons D, & Limdi JK (2016). Elderly patients and inflammatory bowel disease. World Journal of Gastrointestinal Pharmacology and Therapeutics, 7(1), 51–65. 10.4292/wjgpt.v7.i1.51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North BJ, & Sinclair DA (2012). The intersection between aging and cardiovascular disease. Circulation Research, 110(8), 1097–1108. 10.1161/circresaha.111.246876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nosalski R, & Guzik TJ (2017). Perivascular adipose tissue inflammation in vascular disease. British Journal of Pharmacology, 174(20), 3496–3513. 10.1111/bph.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nussenzweig SC, Verma S, & Finkel T (2015). The role of autophagy in vascular biology. Circulation Research, 116(3), 480–488. 10.1161/CIRCRESAHA.116.303805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Nishida A, Sugitani Y, Nishino K, Inatomi O, Sugimoto M, Kawahara M, & Andoh A (2017). Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells. PLoS One, 12(10), e0185999. 10.1371/journal.pone.0185999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orme IM (1987). Aging and immunity to tuberculosis: Increased susceptibility of old mice reflects a decreased capacity to generate mediator T lymphocytes. Journal of Immunology, 138(12), 4414–4418. [PubMed] [Google Scholar]
- Pahuja R, Seth K, Shukla A, Shukla RK, Bhatnagar P, Chauhan LK, Saxena PN, Arun J, Chaudhari BP, Patel DK, Singh SP, Shukla R, Khanna VK, Kumar P, Chaturvedi RK, & Gupta KC (2015). Trans-blood brain barrier delivery of dopamine-loaded nanoparticles reverses functional deficits in parkinsonian rats. ACS Nano, 9(5), 4850–4871. 10.1021/nn506408v [DOI] [PubMed] [Google Scholar]
- Pandey R, & Khuller GK (2005). Solid lipid particle-based inhalable sustained drug delivery system against experimental tuberculosis. Tuberculosis (Edinburgh, Scotland), 85(4), 227–234. 10.1016/j.tube.2004.11.003 [DOI] [PubMed] [Google Scholar]
- Pandya VA, & Patani R (2019). Decoding the relationship between ageing and amyotrophic lateral sclerosis: A cellular perspective. Brain, 143(4), 1057–1072. 10.1093/brain/awz360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paudel S, Sharma P, & Puri N (2019). Immunosenescence, Inflammaging, and their implications for cancer and anemia. Springer. [Google Scholar]
- Perrie Y, Badhan R, Kirby D, Lowry D, Mohammed A, & Ouyang D (2012). The impact of ageing on the barriers to drug delivery. Journal of Controlled Release, 161, 389–398. 10.1016/j.jconrel.2012.01.020 [DOI] [PubMed] [Google Scholar]
- Pietrosimone B (2020). Effect of ZILRETTA injection on neuromuscular function, gait biomechanics and physical performance. https://clinicaltrials.gov/ct2/show/record/NCT04261049
- Pinal-Fernandez I, Casal-Dominguez M, & Mammen AL (2018). Statins: Pros and cons. Medicina Clínica (Barcelona), 150(10), 398–402. 10.1016/j.medcli.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnappan S, & Ponnappan U (2011). Aging and immune function: Molecular mechanisms to interventions. Antioxidants & Redox Signaling, 14(8), 1551–1585. 10.1089/ars.2010.3228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon F, Kang S, & Chien AL (2015). Mechanisms and treatments of photoaging. Photodermatology, Photoimmunology & Photomedicine, 31(2), 65–74. [DOI] [PubMed] [Google Scholar]
- Potkin KT, & Potkin SG (2018). New directions in therapeutics for Huntington disease. Future Neurology, 13(2), 101–121. 10.2217/fnl-2017-0035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad M, Kumar R, Buragohain L, Kumari A, & Ghosh M (2021). Organoid technology: A reliable developmental biology tool for organ-specific nanotoxicity evaluation. Frontiers in Cell and Developmental Biology, 9. 10.3389/fcell.2021.696668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor EA, Fee L, Tao Y, Redler RL, Fay JM, Zhang Y, Lv Z, Mercer I, Deshmukh M, Lyubchenko Y, & Dokholyan NV (2016). Nonnative SOD1 trimer is toxic to motor neurons in a model of amyotrophic lateral sclerosis. Proceedings of the National Academy of Sciences, 113(3), 614–619. 10.1073/pnas.1516725113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahuman HBH, Dhandapani R, Palanivel V, Thangavelu S, Paramasivam R, & Muthupandian S (2021). Bioengineered phytomolecules-capped silver nanoparticles using Carissa carandas leaf extract to embed on to urinary catheter to combat UTI pathogens. PLoS One, 16(9), e0256748. 10.1371/journal.pone.0256748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S (2016). Tuberculosis in older adults. Clinics in Geriatric Medicine, 32(3), 479–491. 10.1016/j.cger.2016.02.006 [DOI] [PubMed] [Google Scholar]
- Rajamäki K, Lappalainen J, Oörni K, Välimäki E, Matikainen S, Kovanen PT, & Eklund KK (2010). Cholesterol crystals activate the NLRP3 inflammasome in human macrophages: A novel link between cholesterol metabolism and inflammation. PLoS One, 5(7), e11765. 10.1371/journal.pone.0011765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M, & Lee J (2016). Recent nanotechnology approaches for prevention and treatment of biofilm-associated infections on medical devices. BioMed Research International, 2016, 1851242. 10.1155/2016/1851242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos GP, & Papadakis KA (2019). Mechanisms of disease: Inflammatory bowel diseases. Mayo Clinic Proceedings, 94(1), 155–165. 10.1016/j.mayocp.2018.09.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robak T, Gladalska A, Stepień H, & Robak E (1998). Serum levels of interleukin-6 type cytokines and soluble interleukin-6 receptor in patients with rheumatoid arthritis. Mediators of Inflammation, 7(5), 347–353. 10.1080/09629359890875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocha NP, Ribeiro FM, Furr-Stimming E, & Teixeira AL (2016). Neuroimmunology of Huntington’s disease: Revisiting evidence from human studies. Mediators of Inflammation, 2016, 8653132. 10.1155/2016/8653132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross K, Alley J, Darling R, Goodman J, Jefferson M, Uz M, & Narasimhan B (2019). Single dose combination nanovaccine provides protection against influenza A virus in young and aged mice. Biomaterials Science, 7, 809–821. [DOI] [PubMed] [Google Scholar]
- Ross R (1999). Atherosclerosis: An inflammatory disease. The New England Journal of Medicine, 340(2), 115–126. 10.1056/nejm199901143400207 [DOI] [PubMed] [Google Scholar]
- Rubio-Perez JM, & Morillas-Ruiz JM (2012). A review: Inflammatory process in Alzheimer’s disease, role of cytokines. Scientific World Journal, 2012, 756357. 10.1100/2012/756357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha AK, Zhen MS, Erogbogbo F, & Ramasubramanian AK (2020). Design considerations and assays for hemocompatibility of FDA-approved nanoparticles. Seminars in Thrombosis and Hemostasis, 46(5), 637–652. 10.1055/s-0039-1688491 [DOI] [PubMed] [Google Scholar]
- Schlesinger S, Lahousse M, Foster T, & Kim S-K (2011). Metallo-β-lactamases and aptamer-based inhibition. Pharmaceuticals, 4, 419–428. 10.3390/ph4020419 [DOI] [Google Scholar]
- Schmitt B, Bernhardt T, Moeller HJ, Heuser I, & Frölich L (2004). Combination therapy in Alzheimer’s disease: A review of current evidence. CNS Drugs, 18(13), 827–844. 10.2165/00023210-200418130-00001 [DOI] [PubMed] [Google Scholar]
- Schönland SO, Lopez C, Widmann T, Zimmer J, Bryl E, Goronzy JJ, & Weyand CM (2003). Premature telomeric loss in rheumatoid arthritis is genetically determined and involves both myeloid and lymphoid cell lineages. Proceedings of the National Academy of Sciences of the United States of America, 100(23), 13471–13476. 10.1073/pnas.2233561100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvin E, & Erlinger Thomas P (2004). Prevalence of and risk factors for peripheral arterial disease in the United States. Circulation, 110(6), 738–743. 10.1161/01.CIR.0000137913.26087.F0 [DOI] [PubMed] [Google Scholar]
- Senoner T, & Dichtl W (2019). Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients, 11(9), 2090. 10.3390/nu11092090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J, Votruba AR, Farokhzad OC, & Langer R (2010). Nanotechnology in drug delivery and tissue engineering: From discovery to applications. Nano Letters, 10(9), 3223–3230. 10.1021/nl102184c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singla R, Soni S, Patial V, Kulurkar PM, Kumari A, Mahesh S, Padwad YS, & Yadav SK (2017). Cytocompatible anti-microbial dressings of Syzygium cumini cellulose nanocrystals decorated with silver nanoparticles accelerate acute and diabetic wound healing. Scientific Reports, 7(1), 10457. 10.1038/s41598-017-08897-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Venable S, Roberts TW, Metter EJ, Monticone R, & Schneider EL (2002). Relationship between in vivo age and in vitro aging: Assessment of 669 cell cultures derived from members of the Baltimore Longitudinal Study of Aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 57(6), B239–B246. 10.1093/gerona/57.6.b239 [DOI] [PubMed] [Google Scholar]
- Spagnoli LG, Bonanno E, Sangiorgi G, & Mauriello A (2007). Role of inflammation in atherosclerosis. Journal of Nuclear Medicine, 48(11), 1800–1815. 10.2967/jnumed.107.038661 [DOI] [PubMed] [Google Scholar]
- Steens A, Eriksen H-M, & Blystad H (2014). What are the most important infectious diseases among those ≥65 years: A comprehensive analysis on notifiable diseases, Norway, 1993–2011. BMC Infectious Diseases, 14, 57. 10.1186/1471-2334-14-57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson J, Nutma E, van der Valk P, & Amor S (2018). Inflammation in CNS neurodegenerative diseases. Immunology, 154(2), 204–219. 10.1111/imm.12922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H-J, Wu Z-Y, Nie X-W, & Bian J-S (2020). Role of endothelial dysfunction in cardiovascular diseases: The link between inflammation and hydrogen sulfide. Frontiers in Pharmacology, 10, 1568. 10.3389/fphar.2019.01568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sushnitha M, Evangelopoulos M, Tasciotti E, & Taraballi F (2020). Cell membrane-based biomimetic nanoparticles and the immune system: Immunomodulatory interactions to therapeutic applications. Frontiers in Bioengineering and Biotechnology, 8, 627. 10.3389/fbioe.2020.00627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symmons DP, Barrett EM, Bankhead CR, Scott DG, & Silman AJ (1994). The incidence of rheumatoid arthritis in the United Kingdom: Results from the Norfolk Arthritis Register. British Journal of Rheumatology, 33(8), 735–739. 10.1093/rheumatology/33.8.735 [DOI] [PubMed] [Google Scholar]
- Tăbăran A-F, Matea CT, Mocan T, Tăbăran A, Mihaiu M, Iancu C, & Mocan L (2020). Silver nanoparticles for the therapy of tuberculosis. International Journal of Nanomedicine, 15, 2231–2258. 10.2147/IJN.S241183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taleban S, Colombel J-F, Mohler MJ, & Fain MJ (2015). Inflammatory bowel disease and the elderly: A review. Journal of Crohn’s and Colitis, 9(6), 507–515. 10.1093/ecco-jcc/jjv059 [DOI] [PubMed] [Google Scholar]
- Thal DR, Rüb U, Orantes M, & Braak H (2002). Phases of a beta-deposition in the human brain and its relevance for the development of AD. Neurology, 58(12), 1791–1800. 10.1212/wnl.58.12.1791 [DOI] [PubMed] [Google Scholar]
- Thomas TY, & Rajagopalan S (2001). Tuberculosis and aging: A global health problem. Clinical Infectious Diseases, 33(7), 1034–1039. 10.1086/322671 [DOI] [PubMed] [Google Scholar]
- Tian L, Lin B, Wu L, Li K, Liu H, Yan J, Liu X, & Xi Z (2015). Neurotoxicity induced by zinc oxide nanoparticles: Age-related differences and interaction. Scientific Reports, 5(1), 16117. 10.1038/srep16117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tosi G, Costantino L, Rivasi F, Ruozi B, Leo E, Vergoni AV, Tacchi R, Bertolini A, Vandelli MA, & Forni F (2007). Targeting the central nervous system: In vivo experiments with peptide-derivatized nanoparticles loaded with Loperamide and Rhodamine-123. Journal of Controlled Release, 122(1), 1–9. [DOI] [PubMed] [Google Scholar]
- Trott DW, & Fadel PJ (2019). Inflammation as a mediator of arterial ageing. Experimental Physiology, 104(10), 1455–1471. 10.1113/EP087499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu KN, Lie JD, Wan CKV, Cameron M, Austel AG, Nguyen JK, Van K, & Hyun D (2018). Osteoporosis: A review of treatment options. P T, 43(2), 92–104. [PMC free article] [PubMed] [Google Scholar]
- Ulanova M, Poljak A, Wen W, Bongers A, Gloag L, Gooding J, Tilley R, Sachdev P, & Braidy N (2020). Nanoparticles as contrast agents for the diagnosis of Alzheimer’s disease: A systematic review. Nanomedicine, 15(7), 725–743. 10.2217/nnm-2019-0316 [DOI] [PubMed] [Google Scholar]
- Vaiserman A, Koliada A, Zayachkivska A, & Lushchak O (2019). Nanodelivery of natural antioxidants: An anti-aging perspective. Frontiers in Bioengineering and Biotechnology, 7, 447. 10.3389/fbioe.2019.00447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Damme P, Robberecht W, & Van Den Bosch L (2017). Modelling amyotrophic lateral sclerosis: Progress and possibilities. Disease Models & Mechanisms, 10(5), 537–549. 10.1242/dmm.029058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Snick J (1990). Interleukin-6: an overview. Annual Review of Immunology, 8, 253–278. 10.1146/annurev.iy.08.040190.001345 [DOI] [PubMed] [Google Scholar]
- Varela C, Bleda S, Esparza L, de Maturana IL, & Acin F (2011). Anti-endothelial cell antibodies are associated with peripheral arterial disease and markers of endothelial dysfunction and inflammation. Interactive CardioVascular and Thoracic Surgery, 13, 463–467. [DOI] [PubMed] [Google Scholar]
- Vaughan CJ, Gotto AM, & Basson CT (2000). The evolving role of statins in the management of atherosclerosis. Journal of the American College of Cardiology, 35(1), 1–10. 10.1016/S0735-1097(99)00525-2 [DOI] [PubMed] [Google Scholar]
- Ventura MT, Casciaro M, Gangemi S, & Buquicchio R (2017). Immunosenescence in aging: Between immune cells depletion and cytokines up-regulation. Clinical and Molecular Allergy, 15, 21. 10.1186/s12948-017-0077-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergoni AV, Tosi G, Tacchi R, Vandelli MA, Bertolini A, & Costantino L (2009). Nanoparticles as drug delivery agents specific for CNS: In vivo biodistribution. Nanomedicine: Nanotechnology, Biology and Medicine, 5(4), 369–377. [DOI] [PubMed] [Google Scholar]
- Wang M, Monticone RE, & McGraw KR (2018). Proinflammatory arterial stiffness syndrome: A signature of large arterial aging. Journal of Vascular Research, 55(4), 210–223. 10.1159/000490244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZH, Wang ZY, Sun CS, Wang CY, Jiang TY, & Wang SL (2010). Trimethylated chitosan-conjugated PLGA nanoparticles for the delivery of drugs to the brain. Biomaterials, 31(5), 908–915. 10.1016/j.biomaterials.2009.09.104 [DOI] [PubMed] [Google Scholar]
- Weissleder R, Nahrendorf M, & Pittet MJ (2014). Imaging macrophages with nanoparticles. Nature Materials, 13(2), 125–138. 10.1038/nmat3780 [DOI] [PubMed] [Google Scholar]
- Weyand CM, & Goronzy JJ (2016). Aging of the immune system. Mechanisms and therapeutic targets. Annals of the American Thoracic Society, 13, S422–S428. 10.1513/AnnalsATS.201602-095AW [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand CM, Yang Z, & Goronzy JJ (2014). T-cell aging in rheumatoid arthritis. Current Opinion in Rheumatology, 26(1), 93–100. 10.1097/BOR.0000000000000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley NJ, Madhankumar A, Mitchell RM, Neely EB, Rizk E, Douds GL, Simmons Z, & Connor JR (2012). Lipopolysaccharide modified liposomes for amyotropic lateral sclerosis therapy: Efficacy in SOD1 mouse model. Advances in Nanoparticles, 1, 25169. [Google Scholar]
- Wong C, & Goldstein DR (2013). Impact of aging on antigen presentation cell function of dendritic cells. Current Opinion in Immunology, 25(4), 535–541. 10.1016/j.coi.2013.05.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Writing Group, & Edaravone (MCI-186) ALS 19 Study Group. (2017). Safety and efficacy of edaravone in well defined patients with amyotrophic lateral sclerosis: A randomised, double-blind, placebo-controlled trial. Lancet Neurology, 16(7), 505–512. 10.1016/s1474-4422(17)30115-1 [DOI] [PubMed] [Google Scholar]
- Wu HJ, & Wu E (2012). The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes, 3(1), 4–14. 10.4161/gmic.19320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Thabet SR, Kirabo A, Trott DW, Saleh MA, Xiao L, Madhur MS, Chen W, & Harrison DG (2014). Inflammation and mechanical stretch promote aortic stiffening in hypertension through activation of p38 mitogen-activated protein kinase. Circulation Research, 114(4), 616–625. 10.1161/CIRCRESAHA.114.302157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Laroui H, Viennois E, Ayyadurai S, Charania MA, Zhang Y, Zhang Z, Baker MT, Zhang B, Gewirtz AT, & Merlin D (2014). Nanoparticles with surface antibody against CD98 and carrying CD98 small interfering RNA reduce colitis in mice. Gastroenterology, 146(5), 1289–1300. 10.1053/j.gastro.2014.01.056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B, Yang Y, Viennois E, Zhang Y, Ayyadurai S, Baker M, Laroui H, & Merlin D (2014). Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticle. Journal of Materials Chemistry B, 2(11), 1499–1508. 10.1039/c3tb21564d [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhou J, Zhong Y, Zhang Y, Ye M, Hou J, Wang Z, Ran H, Liu J, & Guo D (2020). EWVDV-mediated platelet-targeting nanoparticles for the multimodal imaging of thrombi at different blood flow velocities. International Journal of Nanomedicine, 15, 1759–1770. 10.2147/IJN.S233968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaman S, Chintapula U, Rodriguez E, Ramachandramoorthy H, & Nguyen KT (2020). Cell-mediated and cell membrane-coated nanoparticles for drug delivery and cancer therapy. Cancer Drug Resistance, 3, 879–911. 10.20517/cdr.2020.55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M, Zhang Y, Ma Y, Yan X, Gong L, Zhang M, & Zhang B (2020). Nanoparticle-based therapeutics of inflammatory bowel diseases: A narrative review of the current state and prospects. Journal of Bio-X Research, 3(4), 157–173. [Google Scholar]
- Yatin SM, Yatin M, Aulick T, Ain KB, & Butterfield DA (1999). Alzheimer’s amyloid beta-peptide associated free radicals increase rat embryonic neuronal polyamine uptake and ornithine decarboxylase activity: Protective effect of vitamin E. Neuroscience Letters, 263(1), 17–20. 10.1016/s0304-3940(99)00101-9 [DOI] [PubMed] [Google Scholar]
- Yoo YJ, Kim H, Park SR, & Yoon YJ (2017). An overview of rapamycin: From discovery to future perspectives. Journal of Industrial Microbiology & Biotechnology, 44(4–5), 537–553. 10.1007/s10295-016-1834-7 [DOI] [PubMed] [Google Scholar]
- Yoshikawa TT (2000). Epidemiology and unique aspects of aging and infectious diseases. Clinical Infectious Diseases, 30(6), 931–933. 10.1086/313792 [DOI] [PubMed] [Google Scholar]
- Zhang C, Wan X, Zheng X, Shao X, Liu Q, Zhang Q, & Qian Y (2014). Dual-functional nanoparticles targeting amyloid plaques in the brains of Alzheimer’s disease mice. Biomaterials, 35(1), 456–465. 10.1016/j.biomaterials.2013.09.063 [DOI] [PubMed] [Google Scholar]
- Zhang R, Liu R, Liu C, Pan L, Qi Y, Cheng J, Guo J, Jia Y, Ding J, Zhang J, & Hu H (2020). A pH/ROS dual-responsive and targeting nanotherapy for vascular inflammatory diseases. Biomaterials, 230, 119605. 10.1016/j.biomaterials.2019.119605 [DOI] [PubMed] [Google Scholar]
- Zhang W, Robertson WB, Zhao J, Chen W, & Xu J (2019). Emerging trend in the pharmacotherapy of osteoarthritis. Frontiers in Endocrinology, 10, 431. 10.3389/fendo.2019.00431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y-N, Poon W, Tavares AJ, McGilvray ID, & Chan WCW (2016). Nanoparticle–liver interactions: Cellular uptake and hepatobiliary elimination. Journal of Controlled Release, 240, 332–348. 10.1016/j.jconrel.2016.01.020 [DOI] [PubMed] [Google Scholar]
- Zhao P, Xia X, Xu X, Leung KKC, Rai A, Deng Y, Yang B, Lai H, Peng X, Shi P, Zhang H, Chiu PWY, & Bian L (2021). Nanoparticle-assembled bioadhesive coacervate coating with prolonged gastrointestinal retention for inflammatory bowel disease therapy. Nature Communications, 12(1), 7162. 10.1038/s41467-021-27463-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zubair M, Malik A, & Ahmad J (2011). The impact of creatinine clearance on the outcome of diabetic foot ulcers in north Indian tertiary care hospital. Diabetes & Metabolic Syndrome, 5, 120–125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


