Abstract
Artificial intelligence can use real-world data to create models capable of making predictions and medical diagnosis for diabetes and its complications. The aim of this commentary article is to provide a general perspective and present recent advances on how artificial intelligence can be applied to improve the prediction and diagnosis of six significant complications of diabetes including (1) gestational diabetes, (2) hypoglycemia in the hospital, (3) diabetic retinopathy, (4) diabetic foot ulcers, (5) diabetic peripheral neuropathy, and (6) diabetic nephropathy.
Keywords: diabetes, complications, artificial intelligence, machine learning algorithm, risk factors, prediction
Introduction
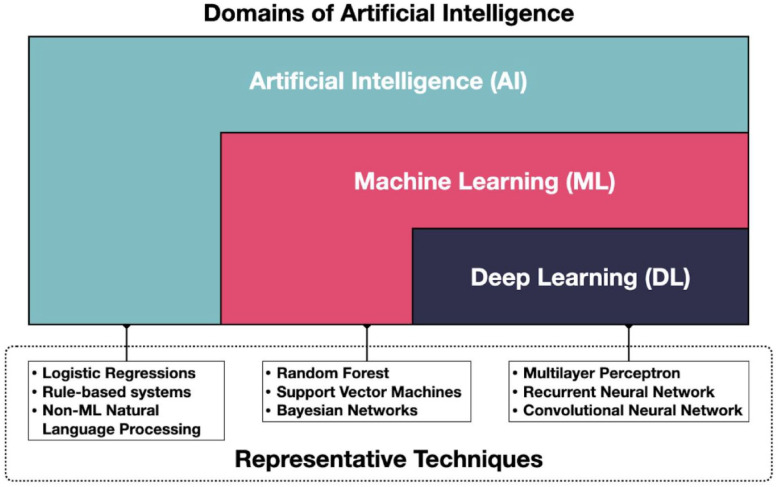
The care of persons with diabetes generates a large amount of data from devices, mobile applications, healthcare encounters, and diagnostic studies. As these tools have proliferated and more and more patients and providers have access to them, interest in applying advanced data science and analytics methods to the data generated has increased. The use of artificial intelligence (AI) in particular has grown exponentially over the past 15 years. 1 Artificial intelligence is an umbrella term that refers to a variety of techniques that enable computers to mimic human intelligence and includes various subdomains (e.g., machine learning and deep learning) and approaches (e.g., logistic regressions and random forest), as shown in Figure 1. 2 Machine learning (ML) is a subset of AI focused on programs that improve over time with experience, and deep learning (DL) is a subset of ML that uses artificial neural networks and large data sets to tackle computationally complex problems.3,4 Depending on the ultimate goal of the model, different types of ML algorithms, 5 such as supervised learning, semisupervised learning, reinforcement learning, and unsupervised learning can be used to achieve optimum outcome for the specific project.
Figure 1.
Conceptual map of AI and representative methods. Figure courtesy of Juan C. Espinoza.
Abbreviations: AI, artificial intelligence; DL, deep leaning; ML, machine learning.
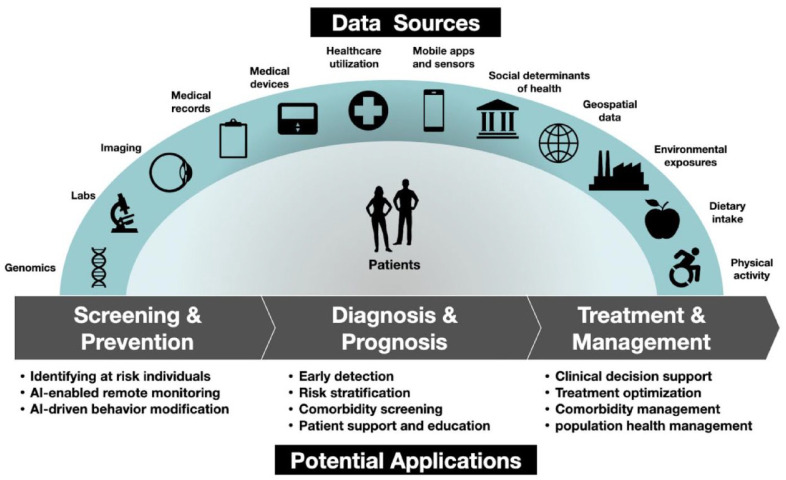
There are several opportunities to use AI to improve or enhance the care of persons with diabetes along the healthcare continuum. Ranging from screening, predicting, and diagnosis to treatment and comorbidity management, researchers around the world are identifying novel applications of AI in diabetes (Figure 2). The use of AI has the potential to improve screening and diagnosis, provide earlier, more targeted therapies, predict complications, reduce morbidity and mortality, improve quality of life, and decrease healthcare costs. Reyna et al 6 recently proposed six suggestions for developers and users of AI-powered algorithms, as listed in Table 1.
Figure 2.
Diagram of data sources and opportunities to apply AI methods to the continuum of care for persons with diabetes. Figure courtesy of Juan C. Espinoza.
Abbreviation: AI, artificial intelligence.
Table 1.
Suggestions for Clinicians Who Are Developing and Using AI for Delivering Care. 6
| 1. Clinicians should not assume that traditional metrics, such as the area under the receiver operating characteristic curve, translate to clinical effects because such performance metrics are usually not optimized or evaluated for specific clinical contexts. |
| 2. Clinicians should be involved in guiding the design of metrics to ensure that the algorithms produce outputs that are clinically useful. |
| 3. Clinicians should prioritize the use of AI tools with well documented and understandable performance metrics to enable informed decisions on how best to use the algorithm. |
| 4. Clinicians should require a prospective evaluation of algorithms in clinical settings to assess their utility for actual clinical outcomes. |
| 5. Adopters of AI tools should require that AI developers make available the full code for an algorithm, including the training data and code. |
| 6. Diagnostic performance metrics should account for differential performance in subgroup populations, where conditions may present differently based on race, ethnicity, or sex. |
Abbreviation: AI, artificial intelligence.
Within the field of endocrinology, diabetes and diabetes-related complications are the conditions for which there is the largest body of evidence for use of AI technologies in disease detection, prediction, and risk assessment. 5 Here we provide an introduction and overview of six key areas of diabetes and how AI is being used. We selected six common and impactful complications of diabetes which account for significant morbidity, mortality, and cost including: (1) gestational diabetes mellitus (GDM), (2) hypoglycemia in the hospital, (3) diabetic retinopathy (DR), (4) diabetic foot ulcers (DFUs), (5) diabetic peripheral neuropathy (DPN), and (6) diabetic nephropathy (DN). For each of these clinical situations, this article will focus on the importance of either predicting a complication before it occurs or making an early diagnosis of a condition that is currently present based on analyzing a complex data set. This article will also address the current status and potential role of AI technology in making predictions and diagnoses, as well as the potential barriers to AI applications. Although data from personal or smart devices collected from patients is expected to be increasingly integrated into clinical information systems and used for personalized health management in the coming decades, this review focuses predominantly on data collected in clinical settings. 7
The Use of AI to Predict Gestational Diabetes
Accurate identification and treatment of GDM is key to reducing the risk of maternal and infant complications.
Artificial intelligence improves the accuracy of predicting GDM than traditional risk factors alone, but the optimal AI algorithm or set of factors to include is uncertain.
Incorporation of glucose data from continuous glucose monitors (CGMs) and data from studies of large, diverse populations may improve the ability of AI to predict GDM development.
Current Status of AI to Predict Gestational Diabetes
In the last decade, AI has been increasingly used to predict development of GDM or the development of impaired glucose intolerance during pregnancy. Gestational diabetes mellitus is one of the most common medical complications affecting 7% to 18% of pregnancies. 8 Gestational diabetes increases the risk of maternal and infant complications, such as pre-eclampsia, cesarean delivery, birth trauma, large-for-gestational age infants, and hypoglycemia at birth. 8 While these risks can be mitigated through diagnosis and treatment in the third trimester, use of AI for early prediction of individuals who will develop GDM offers an opportunity for earlier intervention to prevent these complications.
Technology Needed to Improve the Use of AI to Predict Gestational Diabetes
Compared to the traditional use of clinical risk factors, AI improves the accuracy of predicting GDM (pooled area under the receiver operating characteristic [AUROC] = 0.85).9,10 While the optimal AI algorithm or set of factors to include is not clear, nonlogistic regression models have performed better than clinical factors alone and commonly include such factors as maternal age, family history of diabetes, body mass index, and fasting blood glucose. 9 Previous studies have demonstrated tradeoffs associated with including different types and numbers of factors and different populations, as shown in Table 2.11 -18 For selecting factors in an AI model, one must consider incorporating numerous detailed laboratory and genetic data to improve AI accuracy or fewer data only available with routine prenatal care to improve clinical usability. For different populations, one must decide whether to use data only from high-risk populations to improve accuracy (but sacrifice generalizability) or nonselected populations to improve generalizability (but sacrifice accuracy). Incorporation of novel data from CGM, which is becoming increasingly prevalent, 19 may improve the predictive ability of AI without sacrificing usability. Additional studies are also needed in large multinational, diverse populations to further improve predictive ability and ensure there are no biases by race or other factors.18,20
Table 2.
Key Differences in Select Studies of AI to Predict Development of GDM in Pregnancy.
| Authors | Type of factors included | Number of factors in final model | Population | AI algorithm | AUROC |
|---|---|---|---|---|---|
| Wu et al 11 | Demographic, clinical, and laboratory | 73 | 32,190 pregnant people in China | Deep neural network | 0.80 |
| Wu et al 12 | Demographic, clinical, and laboratory | 15 | 17,005 pregnant people in China | Random forest | 0.746 |
| Xiong et al 13 | Demographic, clinical, and laboratory | 43 | 490 pregnant people in China | Light gradient boosting machine | 0.942 |
| Wang et al 14 | Demographic and clinical only | 7 | 1139 pregnant people in China | Random forest | 0.777 |
| Artzi et al 15 | Medical history and laboratory | 2,355 | 588,622 pregnant people in Israel | Gradient boosting | 0.854 |
| Zheng et al 16 | Demographic, clinical, and laboratory | 4 | 4771 pregnant people in China | Multivariate Bayesian logistic regression | 0.766 |
| Qiu et al 17 | Demographic, clinical, and laboratory | 49 | 33,935 pregnant people in China | Cost-sensitive hybrid model of logistic regression, support vector machine, and CHAID tree | 0.847 |
| Du et al 18 | Demographic, clinical, and laboratory | 5 | 565 overweight or obese pregnant people in Ireland | Support vector machine | 0.792 |
Source: Table adapted from Du et al 18 under a Creative Commons license: https://creativecommons.org/licenses/by/4.0/.
Abbreviations: AI, artificial intelligence; AUROC, area under the receiver operating curve; CHAID, chi-square automatic interaction detection; GDM, gestational diabetes mellitus.
Expected Future Use of AI to Predict Gestational Diabetes
In addition to predicting a diagnosis of GDM, we expect that AI will be integral in predicting an individual’s most effective treatment after GDM is diagnosed, 21 and ultimately in predicting who will develop type 2 diabetes later in life. 22
The Use of AI to Predict Hypoglycemia in the Hospital
Hypoglycemia is one of the most common adverse drug events in hospitalized patients, but this potentially life-threatening outcome can be difficult for clinicians to predict from manual review of various dynamic clinical factors in the electronic health record (EHR).
Machine learning models are being developed to incorporate multiple clinical factors in order to predict hypoglycemia in both intensive care unit (ICU) and non-ICU settings.
Deployment of ML models applied to EHR data to predict hypoglycemia, coupled with AI-derived treatment recommendations for clinicians, is expected to improve patient outcomes in the future.
Current Status of AI to Predict Hypoglycemia in the Hospital
Hypoglycemia due to insulin therapy is one of the most common adverse drug events among hospitalized patients. 23 Iatrogenic hypoglycemia is linked to increased healthcare costs and utilization of nursing resources, patient dissatisfaction, morbidity, and mortality. 23 Many hospitalized patients with diabetes have multiple risk factors for both hyperglycemia (e.g. steroids) and hypoglycemia (e.g. nil per os status, renal dysfunction). It can be difficult for a treating clinician to synthesize these dynamic and sometimes competing factors to identify patients at high risk of iatrogenic hypoglycemia. Accurate early detection systems could allow clinicians to make proactive treatment modifications to avoid such an adverse event.
Technology Needed to Improve the Use of AI to Predict Hypoglycemia in the Hospital
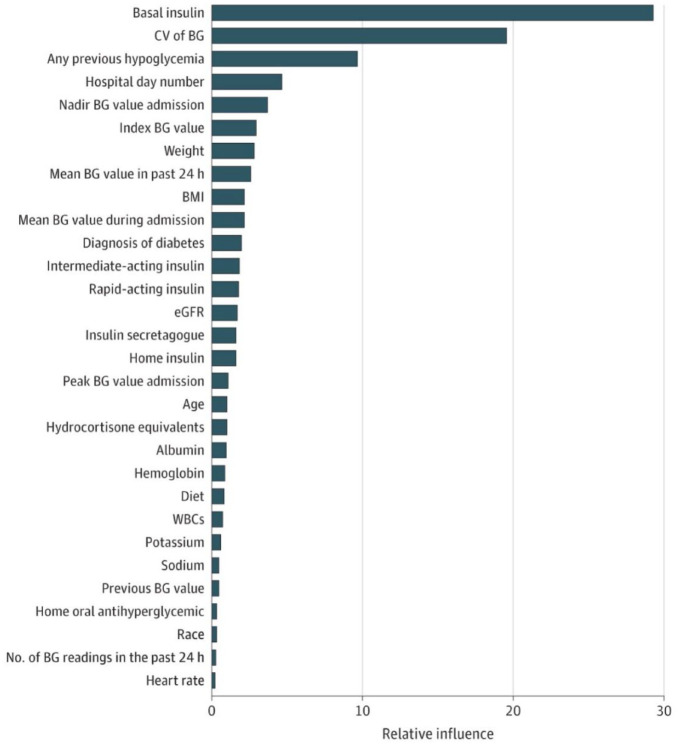
Over the past decade, there has been growing interest in leveraging large EHR data sets to develop prediction models for hypoglycemia using ML algorithms. 24 By including large numbers of predictor variables known to influence glucose homeostasis from very large cohorts of patients, AI technologies can fill an evidence gap by identifying and weighing clinical factors that affect glucose levels in ways that would be difficult for clinicians to recognize from clinical experience alone. Various ML techniques have been used to develop prediction models in both non-ICU and ICU settings, including gradient boosting,25-30 random forest classification, 31 recurrent neural net, 27 and logistic regression.28,29,32-39 For discrimination of hypoglycemia, studies have achieved an AUROC ranging from 0.60 to 0.69, 36 0.70 to 0.79,27,29,33,38,39 0.80 to 0.89,26,28,29,35,37 and ≥0.902.29,31 The top 30 predictor variables of hypoglycemia are shown in Figure 3. Tree-based models (gradient boosting and random forest) seem to outperform other algorithms in predicting this relatively rare outcome. Studies differ with respect to the prediction horizon, ranging from two to seven hours,40-42 the next 24 hours,28,29,35,43 the first few days or another period during an admission, 38 or at any time during hospital/unit stay.25,26,32-34,36,37,39
Figure 3.
Variable importance plot of top 30 predictor variables of hypoglycemia.
Source: Reproduced from Mathioudakis et al. 29
Abbreviations: BG, blood glucose; BMI, body mass index; CV, coefficient of variation; eGFR, estimated glomerular filtration rate; WBC, white blood cell count.
Expected Future Use of AI to Predict Hypoglycemia in the Hospital
Despite increasing predictive accuracy, few AI-based hypoglycemia models have been externally validated, which limits the generalizability of the findings. Many studies used cross-fold validation, which may over-estimate model performance in the real-world because it does not respect the chronological ordering of data and ignores the impact of secular trends. In addition, while the AUROC is often reported as a measure of model performance, the highly imbalanced classification of hypoglycemic outcomes can result in misleading interpretations with this metric, with high overall accuracy but relatively low positive predictive value or positive likelihood ratio. The appropriate trade-off between increasing sensitivity (outcome detection) and false positives (alarm fatigue) is unknown, as ML models have not yet been deployed as clinical decision support tools for predicting hypoglycemia in the EHR. Moreover, given high rates of clinical inertia with overt hypoglycemic episodes, 44 it is unknown whether hospital-based clinicians would even act on early warning information.
We anticipate the next phase of investigation in this area will involve deployment of ML models within EHR systems and prospective evaluation of impact on clinical outcomes. Two large EHR vendors in the United States (US) have platforms to directly embed ML models within their systems (Epic cognitive computing platform and Cerner Apollo), but there are no guiding procedures or framework for translating developed ML algorithms into an informatics alert using these platforms. Furthermore, investigators would need approval at institutional levels before deploying such models in their EHR systems. Hypoglycemia informatics alerts are likely to have the greatest impact when coupled with actionable treatment recommendations for clinicians, 45 another potential area where AI technology can contribute to evidence gaps. Given recent interest in using CGMs in hospitalized patients in a telemetry-based care model,46,47 we anticipate that CGM data will eventually be another source of glucose data for ML prediction models. Inclusion of a larger number of glucose data points from CGM, coupled with clinical data from the EHR, is likely to yield even greater predictive accuracy. As multiple data sources are incorporated into ML models, widespread dissemination will require careful mapping of data elements across different EHR systems.
The Use of AI to Diagnose Diabetic Retinopathy
Artificial intelligence for diagnosing DR has greatly advanced in the past decades as numerous algorithms are being developed from the publicly available Kaggle data set containing 100,000 retinal images.
Commercial algorithms for diagnosing DR include: the IDx-DR (Digital Diagnostics, Coralville, Iowa) and the EyeArt (Eyenuk, Inc, Woodland Hills, California).
Adapting the workflow for diagnosing DR in healthcare systems is expected to greatly improve screening results and ultimately patient outcome.
Current Status of AI to Diagnose Diabetic Retinopathy
Diabetic retinopathy continues to be the main cause of irreversible blindness among working-age adults in the world. 48 Artificial intelligence promises to facilitate prevention of blindness from DR with instantaneous point-of-care detection in diverse settings, such as primary care, endocrinology, and diabetes clinics, as well as pharmacies, hospitals, and community centers. 49 Development of AI for DR began more than 20 years ago and greatly accelerated during the past decade with increased computer power, the application of convolutional neural networks, and other DL techniques.
Kaggle is an online community of data scientists. The Kaggle data science competition in 2015 50 generated thousands of different algorithms that outperformed human grading of a retinal image data set consisting of 100,000 retinal images from primary-care screenings. This public data set is still available today, assisting many research groups worldwide to develop novel algorithms. 51
Technology Needed to Improve the Use of AI to Diagnose Diabetic Retinopathy
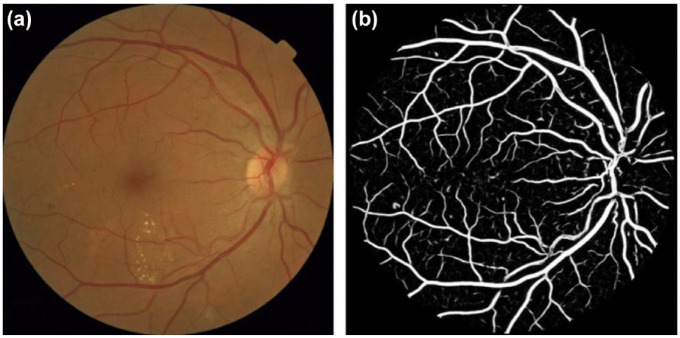
In 2018, the US Food and Drug Administration (FDA) granted clearance to IDx, an AI diagnostic system that autonomously diagnoses patients with DR using DL. This was the first system in any field of medicine to receive FDA authorization for an autonomous AI. In 2020, the EyeArt AI diagnostic system was also granted FDA clearance for autonomous diagnosis of DR. Clinical trials of various commercial programs, including IDx and EyeArt as well as the Automated Retinal Disease Assessment (ARDA) (Google Health Palo Alto, California), the AEYE AI algorithm (AEYE Health, New York, New York), and others have shown high sensitivities and specificities above 90% for the detection of referable DR (defined as greater than mild DR).52,53 Recent studies, however, have shown poorer performance of these algorithms in real-world settings. 54 Researchers have also expressed reservations because of a lack of (1) detail in grading, 55 (2) grading of nondiabetic retinal conditions and glaucoma, and (3) an explanation of how the algorithm determines the retinal grade, which often incorporates multiple features from fundus imaging. This technique produces a two-dimensional image that represents the three-dimensional structure of the retina. Multiple features of a fundus image can aid an algorithm to reach a diagnosis of DR. One such feature can be a model of the vasculature of a retina which can be recognized by the AI diagnostic system, as shown in Figure 4. However, because of the black box nature of DL algorithms, it can be difficult to understand which specific features of a fundus image contributed to the determination of a retinal grade. In addition, concerns over health equity have emerged because of a lack of representation of some populations in the ground truth data that is used to train the AI. 56
Figure 4.
Examples of the outputs of the proposed computer-aided diagnosis system. (a) An original fundus image from the Messidor database (filename: 20051020 57566 0100 PP.tif), kindly provided by the Messidor program partners (https://www.adcis.net/en/third-party/messidor/). The quality-verification module automatically assigned a probability of 0.98 that the image would have good quality. (b) Output of the automatic vessel segmentation module. The image shows the obtained pixel probability map indicating the likelihood of the pixel to belong to a vessel. White: higher probability.
Source: Reproduced with permission from Sánchez et al. 57
Active research is being conducted to address these concerns, such as explainable AI programs that annotate the lesions and regions of interest 58 and AI programs that detect comorbidities from retinal images, such as cardiovascular disease, nephropathy, and neuropathy. 59 Artificial intelligence that predicts likelihood of disease progression 40 is also being developed for risk stratification that can be used in patient care and in clinical trial recruitment.
Expected Future Use of AI to Diagnose Diabetic Retinopathy
Health systems will need to adapt workflows to maximize the benefit of this new tool. For example, immediate triage of patients with sight-threatening DR has demonstrated substantial increases in adherence to follow-up care. 60 Ultimately, the greatest measure of success of this new technology will not be the number of patients screened, but rather the number of patients who avert blindness.
The Use of AI to Diagnose Diabetic Foot Ulcers
Current ML algorithms can (1) detect whether images contain diabetic foot wounds, (2) localize the region of the image containing the wound, and (3) segment wound borders.
The ultimate goal is for ML algorithms to predict whether a patient will develop a wound before clinical signs are present.
Larger, clinically annotated data sets and improved algorithms are necessary to achieve this goal.
Current Status of AI to Diagnose Diabetic Foot Ulcers
In the last decade, ML algorithms have been applied to DFU management. 61 Currently, advanced ML algorithms are able to detect, localize, and segment DFUs in images. 41 Yap et al 42 created an app that allows users to determine whether an image contains a DFU, which is an especially useful tool for clinicians in regions where little training for DFU management exists or for use by vision-impaired patients. Stefanopoulos et al 62 used ML to retrospectively determine which patients in the Nationwide Inpatient Sample (a database which contains approximately 20% of all US hospital admissions) with active DFUs were noted to have a variety of risk factors. Using six risk factors incorporating both physical parameters and demographic information (cellulitis, Charcot joint, peripheral arterial disease, uncontrolled diabetes mellitus, peripheral vascular disease, and male gender), they developed an algorithm that can predict the likelihood of developing a DFU with 79.8% accuracy. The performance evaluation of this algorithm is shown in Figure 5.
Figure 5.

The receiver operating characteristic (ROC) curve for the six-variable model of predicting DFU is shown in blue. The area under the curve (AUC) is shown at the bottom right.
Source: Reproduced from Stephanopoulos et al. 62
Abbreviations: AUC, area under the curve; DFU, diabetic foot ulcer; ROC, receiver operating characteristic.
Technology Needed to Improve the Use of AI to Diagnose Diabetic Foot Ulcers
Although we can determine which patient images currently show DFUs, the ultimate goal is to identify patients who will develop DFUs before clinical signs, such as skin breakdown, are present. This type of advanced warning has been made possible with other technologies, like thermography and multispectral imaging.63,64 However, to be able to achieve accurate prediction while imaging in the visible light spectrum, a massive, clinically annotated data set will be required to train a new algorithm.
Expected Future Use of AI to Diagnose Diabetic Foot Ulcers
A central DFU repository does not yet exist. Even the largest repository, publicly available from Manchester Metropolitan University in the United Kingdom (UK), consists of 11,000 images with ground truth labeling of DFUs, and is, at present, at least an order of magnitude too small to achieve the goal of predicting DFUs before clinical signs are present. 65 In addition, most existing DFU images are taken during healthcare visits and are thus sequestered in medical records, although new tools are making it easier for patients to image their feet themselves. 66 This type of technology could allow them to opt in to share their images for research purposes. Finally, improved algorithms will be needed, because even the best algorithms today suffer from significant false-positive results and difficulty discriminating between DFUs and other skin conditions. 67
The Use of AI to Diagnose Diabetic Peripheral Neuropathy
Current diagnostic tests for DPN are largely inefficient because of their time-consuming nature and lack of specificity.
Various emerging technologies that use ML algorithms can diagnose DPN noninvasively and quickly with great accuracy and specificity.
Corneal confocal microscopy (CCM) is expected to be used as a screening tool for early detection of DPN at the population level in the near future.
Current Status of AI to Diagnose Diabetic Peripheral Neuropathy
The diagnosis of DPN, a major long-term complication of diabetes mellitus, has become the target of ML algorithms. Diabetic peripheral neuropathy can be painful or nonpainful. 68 It is important to make this diagnose early to begin intensive metabolic management for preventing progression of this disease. However, current diagnostic tests, such as a nerve conduction velocity test, a quantitative sensory test, and a skin biopsy are not only time consuming and laborious but are also lacking in specificity. 69 As a result, large-scale screening of DPN is difficult to achieve, which results in poor patient outcomes because an early diagnosis cannot be accurately made.69,70 In order to improve the accuracy and efficiency of diagnosing DPN, several ML algorithms have been developed, including one that incorporates phenotypic variables from the Michigan Neuropathy Screening Instrument. 71 However, these early studies were only internally validated or contained a small sample size.71-73
Technology Needed to Improve the Use of AI to Diagnose Diabetic Peripheral Neuropathy
Three types of tests can use AI to diagnose DPN: qualitative, quantitative physiological, and anatomical. To better distinguish subsets of DPN, an ML algorithm has been developed that uses major clinical factors, such as the EuroQol- 5 Dimension (EQ5D) health-related quality of life test which is a qualitative test to classify painful DPN and nonpainful DPN. 68 There is a need for a standardized scoring scale of nerve function tests to classify the severity of disease. Artificial intelligence methods can be used to quantify physiological responses for severity stratification across multiple anatomical sites and across time for the same site. 74 For example, vibration perception threshold (VPT), a quantitative physiological variable, is operator-dependent. However, a novel operator-independent system, which connects a VPT sensor to an ML algorithm, is currently being developed. 71 Besides quantitative data, anatomical data such as magnetic resonance imaging scans, are collected as biomarkers to diagnose painful DPN based on functional connectivity 75 and blood flow 76 in the central nervous system. Similarly, ultrasound images of the peripheral nerves can be utilized as anatomical inputs to a convolutional neural network capable of identifying peripheral nerve patterns indicative of DPN. When the images are processed by an AI algorithm, the diagnostic accuracy for peripheral neuropathy improves.69,77 However, these AI systems have low throughput compared to another approach utilizing CCM. Loss of corneal nerves, which can be detected by CCM, can predict the onset of diabetic neuropathy and correlates with the severity of neuropathy.69,77 Corneal confocal microscopy is a rapid and noninvasive imaging technique that uses DL algorithms to automatically classify nerve fiber images of the cornea (where nerves are not covered by skin) to diagnose DPN. 69 Corneal confocal microscopy images of healthy patients and patients with DPN are shown in Figure 6. Preliminary clinical trials with CCM are promising and demonstrate higher sensitivity, faster results, and less invasiveness, compared to a conventional skin biopsy.69,77 These advantages of CCM demonstrate its promise for population-scale screening, which can lead to early detection and intervention for DPN.
Figure 6.
(a-b) Example CCM images from healthy individuals. (c-d) Example CCM images from individuals with diabetic neuropathy. (e) An example of a CCM image. (f) Manual annotation of the previous image in 5e with red lines representing manual tracing of the nerve. (g) Manual annotation of 5e indicating branch and terminal points with green triangles denoting tail points and blue squares denoting branching points.
Source: Reproduced from Williams et al 69 under a Creative Commons license: http://creativecommons.org/licenses/by/4.0/.
Abbreviation: CCM, corneal confocal microscopy.
Expected Future Use of AI to Diagnose Diabetic Peripheral Neuropathy
Machine learning algorithms will provide numerous benefits in the diagnosis of DPN. Ensembles of diverse data sets and algorithms can result in the optimal accuracy and outcome. 68 Continued development of these AI-based algorithms is predicted to play a central role in DPN diagnosis as clinical diagnostic aids for clinicians or as an online risk or diagnosis calculator for patients. 68 The data input to algorithms are also expected to diversify and include increasingly specific qualitative biomarkers and quantitative data from high-definition imaging to avoid qualitative fallacies (where incorrect decisions are made based exclusively on quantitative metrics without regard for qualitative factors). 78 In addition, rapid CCM is expected to precede clinical diagnoses and become widely adopted as a screening tool to enable early intervention for DPN.
The Use of AI to Diagnose Diabetic Nephropathy
Untreated and long-term diabetes can lead to DN, which can lead to renal failure and eventually to dialysis, renal transplantation, or death; early intervention and new predictive models are crucial to improving patient outcomes.
Artificial intelligence combines information from demographic data, vital signs, and laboratory tests to make predictions about DN risk that have outperformed other algorithms.
Because the risk and rate of DN progression is higher in some populations than others, ML algorithms must be trained using data that are representative of the recipients of the AI-derived treatment.
Current Status of AI to Diagnose Diabetic Nephropathy
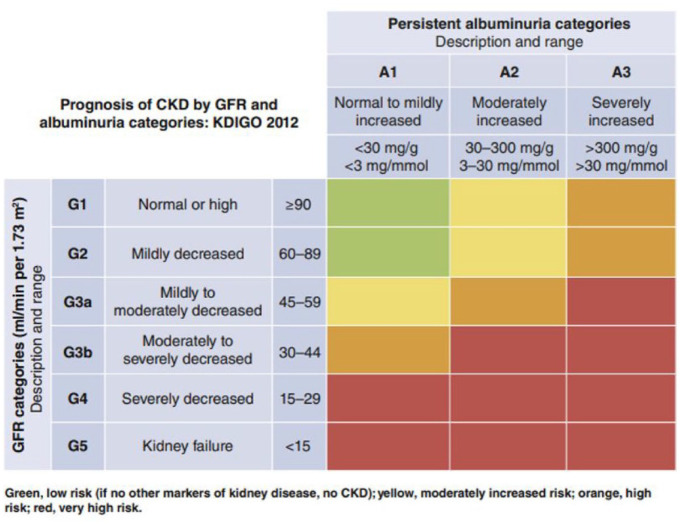
Diabetic nephropathy is a clinical syndrome characterized by albuminuria and a progressive decline in renal function. Kidney complications of diabetes are the leading cause of end-stage renal disease (ESRD) 79 and account for approximately 50% of ESRD cases in developed countries. 80 Diabetic nephropathy, when resulting in ESRD, can progress to dialysis, renal transplantation, or death. Diabetic nephropathy occurs in up to 50% of persons with diabetes and is associated with increased cardiovascular morbidity and mortality. 81 Early detection of DN can prompt protective interventions to prevent its progression and improve outcomes. Traditional risk scores for DN have been established using demographic information about pre-existing conditions and clinical data. For example, a widely used risk matrix, developed by Kidney Disease: Improving Global Outcomes (KDIGO), 82 is presented in Figure 7. While traditional risk scores may be useful, there is still an opportunity and a need to identify patients who are at high risk of having DN based on their clinical features. Since many patients with diabetes do not undergo regular urinary albumin screening, 83 models that can make predictions without this piece of information would allow for early intervention and improvement of patient outcomes.
Figure 7.
Current chronic kidney disease (CKD) risk factors used by KDIGO: CKD is defined as abnormalities of kidney structure or function, present for at least 3 months. CKD prognosis is currently classified into risk categories by a combination of clinical features, such as persistent albuminuria category (A1-A3) and glomerular filtration rate (GFR) category (G1-G5). Green = low or no risk; Yellow = moderately increased risk; Orange = high risk; Red = very high risk.
Source: Reproduced with permission from de Boer et al. 82
Abbreviations: CKD, chronic kidney disease; GFR, glomerular filtration rate; KDIGO, Kidney Disease: Improving Global Outcomes risk matrix.
Technology Needed to Improve the Use of AI to Diagnose Diabetic Nephropathy
AI has been applied to ten populations of patients with type 2 diabetes to predict development of DN using various combinations of demographics, vital signs, and laboratory tests.83-92 The predictions were compared with modified databases in a sensitivity analysis comparison in two of these reports84,87 and outperformed algorithms derived exclusively from clinical data in four of these reports.83,85,86,91 In addition to standard data extraction, one study used natural language processing to identify data from the EHR. 87
Besides laboratory tests, historical information, and a chart review, another way to diagnose DN in the presence of microscopic hematuria or a rapid decline in renal function is through a renal biopsy. This procedure can distinguish DN from other types of glomerular diseases. In one series, immunofluorescent images revealed no characteristic findings, but AI was able to diagnose DN from these types of images, which pathologists rarely examine. 93
Expected Future Use of AI to Diagnose Diabetic Nephropathy
More research is needed in two areas that incorporate AI into making DN diagnoses. First, these algorithms must be relevant to the individuals for whom they are to be used. This is because the risk and rate of DN progression is higher in some populations than others, and the predictions, which determine further actions, must be derived from data representing the recipients of the AI-based treatment. 94 Second, it will be necessary for clinicians to trust a decision from an AI model, which is often referred to as a black box. They must identify the inputs and outputs and reinforce the accuracy of these long-term predictions with additional outcomes data. 95 Data used for the ML algorithm will eventually be routinely extracted from the EHR to enable improved identification, followed by the use of modern renoprotective medications for patients at high risk of DN. 96
Discussion
It is highly evident that AI can be incorporated into the process of predicting and diagnosing the progression of major complications associated with diabetes. Furthermore, advances in AI technology can be integrated into the personalized management of diabetes and its complications, leading to better treatment plans and improved patient outcomes. An important input for any AI software intended to predict, diagnose, treat, or prevent virtually every complication of diabetes will be CGM data. Activity monitors and apps to track nutrition may also prove to be useful. 97 Donated data from the OpenAPS Data Commons, 98 which include hundreds of individuals and thousands of days of data, have enabled more accurate blood glucose forecasting. 99 Challenges to creating trustworthy AI for diagnosis and treatment include the need for standardized aggregation of clinical data, maintenance of patient privacy, de-emphasis of outlier data and noise, and the use of advanced statistical learning methods and ML algorithms. The value of an AI model would be degraded if contributing data sets had different sample sizes or dissimilar methods for feature extraction. Furthermore, data set can be modeled with AI in a variety of configurations.
Artificial intelligence can support a precision medicine paradigm for diabetes. This will be possible if multiple types of genetic, genomic, physiological, biomarker, environmental, and behavioral data can be collected, assembled, and analyzed with methods that ordinarily require human intelligence (artificial intelligence) or with methods that can identify patterns without being specifically programmed to find them (machine learning).
Six complications of diabetes are particularly common, debilitating, and costly for society, including (1) gestational diabetes, (2) hypoglycemia in the hospital, (3) retinopathy, (4) foot ulcers, (5) neuropathy, and (6) nephropathy. The use of larger data sets from real world data sources like EHRs will help improve the predictive accuracy of AI-powered software. The next decade promises to be one of great advances in precision medicine for predicting and diagnosing complications of diabetes powered by AI.
Conclusion
Artificial intelligence can be applied to predict and diagnose complications of diabetes, including (1) gestational diabetes, (2) hypoglycemia in the hospital, (3) retinopathy, (4) foot ulcers, (5) neuropathy, and (6) nephropathy. As data sets containing risk factor and outcomes data become larger and more detailed, the accuracy of these predictive programs is expected to improve. Artificial intelligence–powered algorithms for predicting and diagnosing various diabetic complications are expected to eventually be widely applied.
Acknowledgments
The authors thank Annamarie Sucher-Jones for her expert editorial assistance.
Footnotes
Abbreviations: AI, Artificial Intelligence; ARDA, automated retinal disease assessment; AUC, area under the curve; AUROC, area under the Receiver Operator Characteristic Curve; BG, blood glucose; BMI, body mass index; CCM, corneal confocal microscopy; CGM, continuous glucose monitor; CHAID, chi-square automatic interaction detection; CKD, chronic kidney disease; CV, coefficient of variation; DFU, diabetic foot ulcer; DL, deep learning; DN, diabetic nephropathy; DPN, diabetic peripheral neuropathy; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; EHR, electronic health record; ESRD, end-stage renal disease; EQ5D, EuroQol- 5 Dimension; FDA, Food and Drug Administration; GDM, gestational diabetes mellitus; GFR, glomerular filtration rate; ICU, intensive care unit; KDIGO, Kidney Disease: Improving Global Outcomes risk matrix; ML, machine learning; ROC, receiver operating characteristic; UK, United Kingdom; US, United States; VPT, vibration perception threshold; WBC, white blood cell count.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.C. is the CEO of EyePACS, Inc. J.C.E.’s time is supported in part by the Food and Drug Administration under award number P50FD006425 for The West Coast Consortium for Technology & Innovation in Pediatrics (PI: Espinoza). D.C.K. is a consultant to EOFlow, Fractyl Health, Integrity, Lifecare, Rockley Photonics, and Thirdwayv. The remaining authors have nothing to disclose.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jingtong Huang  https://orcid.org/0000-0002-3119-9361
https://orcid.org/0000-0002-3119-9361
Andrea M. Yeung  https://orcid.org/0000-0002-5592-453X
https://orcid.org/0000-0002-5592-453X
David G. Armstrong  https://orcid.org/0000-0003-1887-9175
https://orcid.org/0000-0003-1887-9175
Ashley N. Battarbee  https://orcid.org/0000-0002-4837-8059
https://orcid.org/0000-0002-4837-8059
Jorge Cuadros  https://orcid.org/0000-0002-7804-5386
https://orcid.org/0000-0002-7804-5386
Juan C. Espinoza  https://orcid.org/0000-0003-0513-588X
https://orcid.org/0000-0003-0513-588X
Samantha Kleinberg  https://orcid.org/0000-0001-6964-3272
https://orcid.org/0000-0001-6964-3272
Nestoras Mathioudakis  https://orcid.org/0000-0002-0210-655X
https://orcid.org/0000-0002-0210-655X
Mark A. Swerdlow  https://orcid.org/0000-0003-3311-6408
https://orcid.org/0000-0003-3311-6408
David C. Klonoff  https://orcid.org/0000-0001-6394-6862
https://orcid.org/0000-0001-6394-6862
References
- 1. Contreras I, Vehi J. Artificial intelligence for diabetes management and decision support: literature review. J Med Internet Res. 2018;20(5):e10775. doi: 10.2196/10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jiang F, Jiang Y, Zhi H, et al. Artificial intelligence in healthcare: past, present and future. Stroke Vasc Neurol. 2017;2(4):230-243. doi: 10.1136/svn-2017-000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tran KA, Kondrashova O, Bradley A, Williams ED, Pearson JV, Waddell N. Deep learning in cancer diagnosis, prognosis and treatment selection. Genome Med. 2021;13:152. doi: 10.1186/s13073-021-00968-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LeCun Y, Bengio Y, Hinton G. Deep learning. Nature. 2015;521(7553):436-444. doi: 10.1038/nature14539. [DOI] [PubMed] [Google Scholar]
- 5. Hong N, Park H, Rhee Y. Machine learning applications in endocrinology and metabolism research: an overview. Endocrinol Metab (Seoul). 2020;35(1):71-84. doi: 10.3803/EnM.2020.35.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reyna MA, Nsoesie EO, Clifford GD. Rethinking algorithm performance metrics for artificial intelligence in diagnostic medicine. JAMA. 2022;328:329-330. doi: 10.1001/jama.2022.10561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Goecks J, Jalili V, Heiser LM, Gray JW. How machine learning will transform biomedicine. Cell. 2020;181(1):92-101. doi: 10.1016/j.cell.2020.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. ACOG practice bulletin no.190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(2):e49-e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 9. Zhang Z, Yang L, Han W, et al. Machine learning prediction models for gestational diabetes mellitus: meta-analysis. J Med Inter Res. 2022;24(3):e26634. Accessed June 28, 2022. https://www.jmir.org/2022/3/e26634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ye Y, Xiong Y, Zhou Q, Wu J, Li X, Xiao X. Comparison of machine learning methods and conventional logistic regressions for predicting gestational diabetes using routine clinical data: a retrospective cohort study. J Diabetes Res. 2020;2020:4168340. doi: 10.1155/2020/4168340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu YT, Zhang CJ, Mol BW, et al. Early prediction of gestational diabetes mellitus in the Chinese population via advanced machine learning. J Clin Endocrinol Metab. 2021;106(3):e1191-e1205. doi: 10.1210/clinem/dgaa899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wu Y, Ma S, Wang Y, et al. A risk prediction model of gestational diabetes mellitus before 16 gestational weeks in Chinese pregnant women. Diabetes Res Clin Pract. 2021;179:109001. doi: 10.1016/j.diabres.2021.109001. [DOI] [PubMed] [Google Scholar]
- 13. Xiong Y, Lin L, Chen Y, et al. Prediction of gestational diabetes mellitus in the first 19 weeks of pregnancy using machine learning techniques. J Matern Fetal Neonatal Med. 2022;35(13):2457-2463. doi: 10.1080/14767058.2020.1786517. [DOI] [PubMed] [Google Scholar]
- 14. Wang J, Lv B, Chen X, et al. An early model to predict the risk of gestational diabetes mellitus in the absence of blood examination indexes: application in primary health care centres. BMC Pregn Childb. 2021;21(1):814. doi: 10.1186/s12884-021-04295-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Artzi NS, Shilo S, Hadar E, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med. 2020;26(1):71-76. doi: 10.1038/s41591-019-0724-8. [DOI] [PubMed] [Google Scholar]
- 16. Zheng T, Ye W, Wang X, et al. A simple model to predict risk of gestational diabetes mellitus from 8 to 20 weeks of gestation in Chinese women. BMC Pregn Childb. 2019;19(1):252. doi: 10.1186/s12884-019-2374-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Qiu H, Yu HY, Wang LY, et al. Electronic health record driven prediction for gestational diabetes mellitus in early pregnancy. Sci Rep. 2017;7(1):16417. doi: 10.1038/s41598-017-16665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Du Y, Rafferty AR, McAuliffe FM, Wei L, Mooney C. An explainable machine learning-based clinical decision support system for prediction of gestational diabetes mellitus. Sci Rep. 2022;12(1):1170. doi: 10.1038/s41598-022-05112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wyckoff JA, Brown FM. Time in range in pregnancy: is there a role. Diabetes Spectr. 2021;34(2):119-132. doi: 10.2337/ds20-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iftikhar P, Kuijpers MV, Khayyat A, Iftikhar A, DeGouvia De, Sa M. Artificial intelligence: a new paradigm in obstetrics and gynecology research and clinical practice. Cureus. 2020;12(2):e7124. doi: 10.7759/cureus.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Velardo C, Clifton D, Hamblin S, Khan R, Tarassenko L, Mackillop L. Toward a multivariate prediction model of pharmacological treatment for women with gestational diabetes mellitus: algorithm development and validation. J Med Internet Res. 2021;23(3):e21435. doi: 10.2196/21435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ilari L, Piersanti A, Göbl C, et al. Unraveling the factors determining development of type 2 diabetes in women with a history of gestational diabetes mellitus through machine-learning techniques. Front Physiol. 2022;13:789219. doi: 10.3389/fphys.2022.789219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kulasa K, Juang P. How low can you go? Reducing rates of hypoglycemia in the non-critical care hospital setting. Curr Diab Rep. 2017;17(9):74. doi: 10.1007/s11892-017-0902-3. [DOI] [PubMed] [Google Scholar]
- 24. Zale A, Mathioudakis N. Machine learning models for inpatient glucose prediction. Curr Diab Rep. 2022;22(8):353-364. doi: 10.1007/s11892-022-01477-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ruan Y, Bellot A, Moysova Z, et al. Predicting the risk of inpatient hypoglycemia with machine learning using electronic health records. Diabetes Care. 2020;43(7):1504-1511. doi: 10.2337/dc19-1743. [DOI] [PubMed] [Google Scholar]
- 26. Mantena S, Arévalo AR, Maley JH, et al. Predicting hypoglycemia in critically Ill patients using machine learning and electronic health records [published online ahead of print October 4, 2021]. J Clin Monit Comput. doi: 10.1007/s10877-021-00760-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Witte H, Nakas CT, Bally L, Leichtle AB. Machine-learning prediction of hypo- and hyperglycemia from electronic health records: algorithm development and validation. JMIR Form Res. 2022;6:e36176. doi: 10.2196/36176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fralick M, Dai D, Pou-Prom C, Verma AA, Mamdani M. Using machine learning to predict severe hypoglycaemia in hospital. Diabetes Obes Metab. 2021;23(10):2311-2319. doi: 10.1111/dom.14472. [DOI] [PubMed] [Google Scholar]
- 29. Mathioudakis NN, Abusamaan MS, Shakarchi AF, et al. Development and validation of a machine learning model to predict near-term risk of iatrogenic hypoglycemia in hospitalized patients. JAMA Netw Open. 2021;4(1):e2030913. doi: 10.1001/jamanetworkopen.2020.30913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fitzgerald O, Perez-Concha O, Gallego B, et al. Incorporating real-world evidence into the development of patient blood glucose prediction algorithms for the ICU. J Am Med Inform Assoc. 2021;28(8):1642-1650. doi: 10.1093/jamia/ocab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zale AD, Abusamaan MS, McGready J, Mathioudakis N. Development and validation of a machine learning model for classification of next glucose measurement in hospitalized patients. Eclinicalmedicine. 2022;44:101290. doi: 10.1016/j.eclinm.2022.101290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Elliott MB, Schafers SJ, McGill JB, Tobin GS. Prediction and prevention of treatment-related inpatient hypoglycemia. J Diabetes Sci Technol. 2012;6(2):302-309. doi: 10.1177/193229681200600213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Stuart K, Adderley NJ, Marshall T, et al. Predicting inpatient hypoglycaemia in hospitalized patients with diabetes: a retrospective analysis of 9584 admissions with diabetes. Diabet Med. 2017;34(10):1385-1391. doi: 10.1111/dme.13409. [DOI] [PubMed] [Google Scholar]
- 34. Ena J, Gaviria AZ, Romero-Sánchez M, et al. Derivation and validation model for hospital hypoglycemia. Eur J Intern Med. 2018;47:43-48. doi: 10.1016/j.ejim.2017.08.024. [DOI] [PubMed] [Google Scholar]
- 35. Winterstein AG, Jeon N, Staley B, Xu D, Henriksen C, Lipori GP. Development and validation of an automated algorithm for identifying patients at high risk for drug-induced hypoglycemia. Am J Health Syst Pharm. 2018;75(21):1714-1728. doi: 10.2146/ajhp180071. [DOI] [PubMed] [Google Scholar]
- 36. Shah BR, Walji S, Kiss A, James JE, Lowe JM. Derivation and validation of a risk-prediction tool for hypoglycemia in hospitalized adults with diabetes: the hypoglycemia during hospitalization (HyDHo) score. Can J Diabetes. 2019;43(4):278-282. doi: 10.1016/j.jcjd.2018.08.061. [DOI] [PubMed] [Google Scholar]
- 37. Kyi M, Gorelik A, Reid J, et al. Clinical prediction tool to identify adults with type 2 diabetes at risk for persistent adverse glycemia in hospital. Can J Diabetes. 2021;45(2):114-121. doi: 10.1016/j.jcjd.2020.06.006. [DOI] [PubMed] [Google Scholar]
- 38. Elbaz M, Nashashibi J, Kushnir S, Leibovici L. Predicting hypoglycemia in hospitalized patients with diabetes: a derivation and validation study. Diabetes Res Clin Pract. 2021;171:108611. doi: 10.1016/j.diabres.2020.108611. [DOI] [PubMed] [Google Scholar]
- 39. Horton WB, Barros AJ, Andris RT, Clark MT, Moorman JR. Pathophysiologic signature of impending ICU hypoglycemia in bedside monitoring and electronic health record data: model development and external validation. Crit Care Med. 2022;50(3):e221-e230. doi: 10.1097/CCM.0000000000005171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bhatt A. Artificial intelligence in managing clinical trial design and conduct: man and machine still on the learning curve. Perspect Clin Res. 2021;12(1):1-3. doi: 10.4103/picr.PICR_312_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Goyal M, Reeves ND, Rajbhandari S, Yap MH. Robust methods for real-time diabetic foot ulcer detection and localization on mobile devices. IEEE J Biomed Health Inform. 2019;23(4):1730-1741. doi: 10.1109/JBHI.2018.2868656. [DOI] [PubMed] [Google Scholar]
- 42. Yap MH, Chatwin KE, Ng CC, et al. A new mobile application for standardizing diabetic foot images. J Diabetes Sci Technol. 2018;12(1):169-173. doi: 10.1177/1932296817713761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Mathioudakis NN, Everett E, Routh S, et al. Development and validation of a prediction model for insulin-associated hypoglycemia in non-critically ill hospitalized adults. BMJ Open Diabetes Res Care. 2018;6(1):e000499. doi: 10.1136/bmjdrc-2017-000499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mathioudakis N, Everett E, Golden SH. Prevention and management of insulin-associated hypoglycemia in hospitalized patients. Endocr Pract. 2016;22(8):959-969. doi: 10.4158/EP151119.OR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mathioudakis N, Aboabdo M, Abusamaan MS, et al. Stakeholder perspectives on an inpatient hypoglycemia informatics alert: mixed-methods study. JMIR Hum Fact. 2021;8:e31214. Accessed June 28, 2022. https://humanfactors.jmir.org/2021/4/e31214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Singh LG, Satyarengga M, Marcano I, et al. Reducing inpatient hypoglycemia in the general wards using real-time continuous glucose monitoring: the glucose telemetry system, a randomized clinical trial. Diabetes Care. 2020;43(11):2736-2743. doi: 10.2337/dc20-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Spanakis EK, Levitt DL, Siddiqui T, et al. The effect of continuous glucose monitoring in preventing inpatient hypoglycemia in general wards: the glucose telemetry system. J Diabetes Sci Technol. 2018;12(1):20-25. doi: 10.1177/1932296817748964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Barth T, Helbig H. [Diabetic retinopathy]. Klin Monbl Augenheilkd. 2021;238(10):1143-1159. doi: 10.1055/a-1545-9927. [DOI] [PubMed] [Google Scholar]
- 49. Campbell JP, Mathenge C, Cherwek H, et al. Artificial intelligence to reduce ocular health disparities: moving from concept to implementation. Transl Vis Sci Technol. 2021;10(3):19. doi: 10.1167/tvst.10.3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Diabetic retinopathy detection. Accessed June 29, 2022. https://kaggle.com/competitions/diabetic-retinopathy-detection.
- 51. Kaggle: your machine learning and data science community. Accessed July 22, 2022. https://www.kaggle.com/.
- 52. Ipp E, Liljenquist D, Bode B, et al. Pivotal evaluation of an artificial intelligence system for autonomous detection of referrable and vision-threatening diabetic retinopathy. JAMA Netw Open. 2021;4(11):e2134254. doi: 10.1001/jamanetworkopen.2021.34254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dutt S, Sivaraman A, Savoy F, Rajalakshmi R. Insights into the growing popularity of artificial intelligence in ophthalmology. Indian J Ophthalmol. 2020;68(7):1339-1346. doi: 10.4103/ijo.IJO_1754_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lee AY, Yanagihara RT, Lee CS, et al. Multicenter, head-to-head, real-world validation study of seven automated artificial intelligence diabetic retinopathy screening systems. Diabetes Care. 2021;44(5):1168-1175. doi: 10.2337/dc20-1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Shi D, Tan Z, et al. Screening referable diabetic retinopathy using a semi-automated deep learning algorithm assisted approach. Front Med (Lausanne). 2021;8:740987. doi: 10.3389/fmed.2021.740987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Burlina P, Joshi N, Paul W, Pacheco KD, Bressler NM. Addressing artificial intelligence bias in retinal diagnostics. Transl Vis Sci Technol. 2021;10(2):13. doi: 10.1167/tvst.10.2.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sánchez CI, Niemeijer M, Dumitrescu AV, Suttorp-Schulten MSA, Abràmoff MD, van Ginneken B. Evaluation of a computer-aided diagnosis system for diabetic retinopathy screening on public data. Invest Ophthalmol Visual Sci. 2011;52(7):4866-4871. doi: 10.1167/iovs.10-6633. [DOI] [PubMed] [Google Scholar]
- 58. Jin D, Harrison AP, Zhang L, et al. Artificial intelligence in radiology. In: Xing L, Giger ML, Min JK, eds. Artificial Intelligence in Medicine. Amsterdam, the Netherlands: Elsevier, 2021:265-289. doi: 10.1016/B978-0-12-821259-2.00014-4. [DOI] [Google Scholar]
- 59. Dankwa-Mullan I, Rivo M, Sepulveda M, Park Y, Snowdon J, Rhee K. Transforming diabetes care through artificial intelligence: the future is here. Popul Health Manag. 2019;22(3):229-242. doi: 10.1089/pop.2018.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Pedersen ER, Cuadros J, Khan M, et al. Redesigning clinical pathways for immediate diabetic retinopathy screening results. NEJM Catalyst. 2021;2:96. Accessed June 29, 2022. https://catalyst.nejm.org/doi/full/10.1056/CAT.21.0096. [Google Scholar]
- 61. Xie P, Li Y, Deng B, et al. An explainable machine learning model for predicting in-hospital amputation rate of patients with diabetic foot ulcer. Int Wound J. 2022;19(4):910-918. doi: 10.1111/iwj.13691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stefanopoulos S, Ayoub S, Qiu Q, et al. Machine learning prediction of diabetic foot ulcers in the inpatient population [published online ahead of print August 30, 2021]. Vascular. doi: 10.1177/17085381211040984. [DOI] [PubMed] [Google Scholar]
- 63. Kaabouch N, Hu WC, Chen Y, Anderson JW, Ames F, Paulson R. Predicting neuropathic ulceration: analysis of static temperature distributions in thermal images. J Biomed Opt. 2010;15(6):061715. doi: 10.1117/1.3524233. [DOI] [PubMed] [Google Scholar]
- 64. Yudovsky D, Nouvong A, Schomacker K, Pilon L. Assessing diabetic foot ulcer development risk with hyperspectral tissue oximetry. J Biomed Opt. 2011;16(2):026009. doi: 10.1117/1.3535592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Cassidy B, Kendrick C, Reeves ND, et al. Diabetic foot ulcer grand challenge 2021: evaluation and summary. In: Yap MH, Cassidy B, Kendrick C, eds. Diabetic Foot Ulcers Grand Challenge, vol. 13183. Amsterdam, the Netherlands: Elsevier, 2022:90-105. doi: 10.1007/978-3-030-94907-5_7. [DOI] [Google Scholar]
- 66. Swerdlow M, Shin L, D’Huyvetter K, Mack WJ, Armstrong DG. Initial clinical experience with a simple, home system for early detection and monitoring of diabetic foot ulcers: the foot selfie [published online ahead of print October 31, 2021]. J Diabetes Sci Technol. doi: 10.1177/19322968211053348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yap MH, Hachiuma R, Alavi A, et al. Deep learning in diabetic foot ulcers detection: a comprehensive evaluation. Comput Biol Med. 2021;135:104596. doi: 10.1016/j.compbiomed.2021.104596. [DOI] [PubMed] [Google Scholar]
- 68. Baskozos G, Themistocleous AC, Hebert HL, et al. Classification of painful or painless diabetic peripheral neuropathy and identification of the most powerful predictors using machine learning models in large cross-sectional cohorts. BMC Med Inform Decis Mak. 2022;22(1):144. doi: 10.1186/s12911-022-01890-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Williams BM, Borroni D, Liu R, et al. An artificial intelligence-based deep learning algorithm for the diagnosis of diabetic neuropathy using corneal confocal microscopy: a development and validation study. Diabetologia. 2020;63(2):419-430. doi: 10.1007/s00125-019-05023-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu X, Zhou H, Wang Z, et al. Fully convolutional neural network deep learning model fully in patients with type 2 diabetes complicated with peripheral neuropathy by high-frequency ultrasound image. Comput Math Methods Med. 2022;2022:5466173-5466178. doi: 10.1155/2022/5466173. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 71. Dubey VN, Dave JM, Beavis J, Coppini DV. Predicting diabetic neuropathy risk level using artificial neural network and clinical parameters of subjects with diabetes. J Diabetes Sci Technol. 2022;16(2):275-281. doi: 10.1177/1932296820965583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Haque F, Bin Ibne Reaz M, Chowdhury MEH, et al. Performance analysis of conventional machine learning algorithms for diabetic sensorimotor polyneuropathy severity classification. Diagnostics. 2021;11(5):801. doi: 10.3390/diagnostics11050801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kazemi M, Moghimbeigi A, Kiani J, Mahjub H, Faradmal J. Diabetic peripheral neuropathy class prediction by multicategory support vector machine model: a cross-sectional study. Epidemiol Health. 2016;38:e2016011. doi: 10.4178/epih.e2016011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Dagliati A, Marini S, Sacchi L, et al. Machine learning methods to predict diabetes complications. J Diabetes Sci Technol. 2018;12(2):295-302. doi: 10.1177/1932296817706375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Teh K, Wilkinson ID, Heiberg-Gibbons F, et al. Somatosensory network functional connectivity differentiates clinical pain phenotypes in diabetic neuropathy. Diabetologia. 2021;64(6):1412-1421. doi: 10.1007/s00125-021-05416-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chitneni A, Rupp A, Ghorayeb J, Abd-Elsayed A. Early detection of diabetic peripheral neuropathy by fMRI: an evidence-based review. Brain Sci. 2022;12(5):557. doi: 10.3390/brainsci12050557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Preston FG, Meng Y, Burgess J, et al. Artificial intelligence utilising corneal confocal microscopy for the diagnosis of peripheral neuropathy in diabetes mellitus and prediabetes. Diabetologia. 2022;65(3):457-466. doi: 10.1007/s00125-021-05617-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kerr D, Klonoff DC. Digital diabetes data and artificial intelligence: a time for humility not hubris. J Diabetes Sci Technol. 2019;13(1):123-127. doi: 10.1177/1932296818796508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hsu C, yuan Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342-350. doi: 10.1001/archinternmed.2008.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Tuttle KR, Bakris GL, Bilous RW, et al. Diabetic kidney disease: a report from an ADA Consensus Conference. Diabetes Care. 2014;37(10):2864-2883. doi: 10.2337/dc14-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Selby NM, Taal MW. An updated overview of diabetic nephropathy: diagnosis, prognosis, treatment goals and latest guidelines. Diabetes Obes Metab. 2020;22Suppl1:3-15. doi: 10.1111/dom.14007. [DOI] [PubMed] [Google Scholar]
- 82. Kidney Disease:Improving Global Outcomes (KDIGO) Diabetes Work Group. KDIGO 2020 clinical practice guideline for diabetes management in chronic kidney disease. Kidney Int. 2020;98(4S):S1-S115. doi: 10.1016/j.kint.2020.06.019. [DOI] [PubMed] [Google Scholar]
- 83. Allen A, Iqbal Z, Green-Saxena A, et al. Prediction of diabetic kidney disease with machine learning algorithms, upon the initial diagnosis of type 2 diabetes mellitus. BMJ Open Diab Res Care. 2022;10:e002560. Accessed June 28, 2022. https://drc.bmj.com/content/10/1/e002560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Song X, Waitman LR, Hu Y, Yu ASL, Robbins DC, Liu M. Robust clinical marker identification for diabetic kidney disease with ensemble feature selection. J Am Med Inform Assoc. 2019;26(3):242-253. doi: 10.1093/jamia/ocy165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ravizza S, Huschto T, Adamov A, et al. Predicting the early risk of chronic kidney disease in patients with diabetes using real-world data. Nat Med. 2019;25(1):57-59. doi: 10.1038/s41591-018-0239-8. [DOI] [PubMed] [Google Scholar]
- 86. Chan L, Nadkarni GN, Fleming F, et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia. 2021;64(7):1504-1515. doi: 10.1007/s00125-021-05444-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Makino M, Yoshimoto R, Ono M, et al. Artificial intelligence predicts the progression of diabetic kidney disease using big data machine learning. Sci Rep. 2019;9(1):11862. doi: 10.1038/s41598-019-48263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. David SK, Rafiullah M, Siddiqui K. Comparison of different machine learning techniques to predict diabetic kidney disease. J Healthc Eng. 2022;2022:7378307. doi: 10.1155/2022/7378307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Zou Y, Zhao L, Zhang J, et al. Development and internal validation of machine learning algorithms for end-stage renal disease risk prediction model of people with type 2 diabetes mellitus and diabetic kidney disease. Ren Fail. 2022;44(1):562-570. doi: 10.1080/0886022X.2022.2056053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Khitan Z, Nath T, Santhanam P. Machine learning approach to predicting albuminuria in persons with type 2 diabetes: an analysis of the LOOK AHEAD Cohort. J Clin Hypertens (Greenwich). 2021;23(12):2137-2145. doi: 10.1111/jch.14397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Belur Nagaraj S, Pena MJ, Ju W, Heerspink HL, BEAt-DKD Consortium. Machine-learning-based early prediction of end-stage renal disease in patients with diabetic kidney disease using clinical trials data. Diabetes Obes Metab. 2020;22(12):2479-2486. doi: 10.1111/dom.14178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Rodriguez-Romero V, Bergstrom RF, Decker BS, Lahu G, Vakilynejad M, Bies RR. Prediction of nephropathy in type 2 diabetes: an analysis of the ACCORD trial applying machine learning techniques. Clin Transl Sci. 2019;12(5):519-528. doi: 10.1111/cts.12647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Kitamura S, Takahashi K, Sang Y, Fukushima K, Tsuji K, Wada J. Deep learning could diagnose diabetic nephropathy with renal pathological immunofluorescent images. Diagnostics. 2020;10(7):466. doi: 10.3390/diagnostics10070466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Loftus TJ, Shickel B, Ozrazgat-Baslanti T, et al. Artificial intelligence-enabled decision support in nephrology. Nat Rev Nephrol. 2022;18(7):452-465. doi: 10.1038/s41581-022-00562-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Savage N. Breaking into the black box of artificial intelligence [published online ahead of print March 29, 2022]. Nature. doi: 10.1038/d41586-022-00858-1. [DOI] [PubMed] [Google Scholar]
- 96. Tsai JL, Chen CH, Wu MJ, Tsai SF. New Approaches to diabetic nephropathy from bed to bench. Biomedicines. 2022;10(4):876. doi: 10.3390/biomedicines10040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Heintzman ND. A digital ecosystem of diabetes data technology: services, systems, and tools enabled by wearables, sensors, and apps. J Diab Sci Technol. 2016;10:35-41. Accessed June 28, 2022. https://journals.sagepub.com/doi/full/10.1177/1932296815622453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Data commons —OpenAPS.org. Accessed June 28, 2022. https://openaps.org/outcomes/data-commons/.
- 99. Hameed H, Kleinberg S. Comparing machine learning techniques for blood glucose forecasting using free-living and patient generated data. PMLR. 2020;126:871-894. Accessed June 28, 2022. https://proceedings.mlr.press/v126/hameed20a.html. [PMC free article] [PubMed] [Google Scholar]