Objective:
ASPP1 (apoptosis stimulating of p53 protein 1) is critical in regulating cell apoptosis as a cofactor of p53 to promote its transcriptional activity in the nucleus. However, whether cytoplasmic ASPP1 affects p53 nuclear trafficking and its role in cardiac diseases remains unknown. This study aims to explore the mechanism by which ASPP1 modulates p53 nuclear trafficking and the subsequent contribution to cardiac ischemia/reperfusion (I/R) injury.
Methods and Results:
The immunofluorescent staining showed that under normal condition ASPP1 and p53 colocalized in the cytoplasm of neonatal mouse ventricular cardiomyocytes, while they were both upregulated and translocated to the nuclei upon hypoxia/reoxygenation treatment. The nuclear translocation of ASPP1 and p53 was interdependent, as knockdown of either ASPP1 or p53 attenuated nuclear translocation of the other one. Inhibition of importin-β1 resulted in the cytoplasmic sequestration of both p53 and ASPP1 in neonatal mouse ventricular cardiomyocytes with hypoxia/reoxygenation stimulation. Overexpression of ASPP1 potentiated, whereas knockdown of ASPP1 inhibited the expression of Bax (Bcl2-associated X), PUMA (p53 upregulated modulator of apoptosis), and Noxa, direct apoptosis-associated targets of p53. ASPP1 was also increased in the I/R myocardium. Cardiomyocyte-specific transgenic overexpression of ASPP1 aggravated I/R injury as indicated by increased infarct size and impaired cardiac function. Conversely, knockout of ASPP1 mitigated cardiac I/R injury. The same qualitative data were observed in neonatal mouse ventricular cardiomyocytes exposed to hypoxia/reoxygenation injury. Furthermore, inhibition of p53 significantly blunted the proapoptotic activity and detrimental effects of ASPP1 both in vitro and in vivo.
Conclusions:
Binding of ASPP1 to p53 triggers their nuclear cotranslocation via importin-β1 that eventually exacerbates cardiac I/R injury. The findings imply that interfering the expression of ASPP1 or the interaction between ASPP1 and p53 to block their nuclear trafficking represents an important therapeutic strategy for cardiac I/R injury.
Keywords: ASPP1, apoptosis, ischemia/reperfusion injury, p53
Novelty and Significance.
What Is Known?
Cardiac ischemia reperfusion (I/R) injury triggers complex pathophysiological processes including cardiomyocyte apoptosis, which contribute to a secondary injury to the heart after myocardial infarction.
ASPP1, a member of ASPP (apoptosis stimulating of p53 protein) family, is emerging as a key regulator of cell apoptosis.
What New Information Does This Article Contribute?
ASPP1 is potently induced by I/R in the heart. Cardiomyocyte-specific overexpression of ASPP1 impairs cardiac function and aggravates I/R injury, whereas knockout of ASPP1 protects the heart against I/R injury.
ASPP1 promotes cardiomyocyte apoptosis by augmenting p53-mediated transcription of apoptosis-related target genes.
Binding of ASPP1 to p53 triggers their nuclear cotranslocation and enhances the transcriptional activation ability of p53.
Cardiac ischemia/reperfusion (I/R) injury is characterized by cardiomyocyte death occurring during restoration of coronary blood flow after myocardial infarction. Here, we report that ASPP1, a member of the ASPP family, is strongly induced by the transcription factor E2F1 upon cardiac I/R. Both gain- and loss-of-functional studies indicated that ASPP1 promotes cardiomyocyte apoptosis and exacerbates myocardial I/R injury by augmenting p53-mediated transcription of apoptosis-related target genes. Binding of ASPP1 to p53 triggers their nuclear cotranslocation via importin-β1, which enhances the transcriptional activation ability of p53, therefore promoting myocardial I/R injury. These findings uncover a previously unrecognized role of ASPP1 in myocardial I/R injury, thereby highlighting a novel pathway with potential therapeutic relevance.
Myocardial infarction poses serious threat to human health with high rates of morbidity and mortality worldwide.1 Cardiac ischemia/reperfusion (I/R) injury often occurs during coronary blood perfusion restoration of myocardial infarction patients. Many complex pathophysiological processes such as oxidative stress, Ca2+ overload and inflammatory response are involved in cardiac I/R injury.2 Although significant efforts have been devoted to prevent cardiac I/R injury, there remains a lack of effective therapeutic drugs and molecular intervention targets. It is still quite necessary to further elucidate the molecular mechanisms and identify effective targets of cardiac I/R injury to improve the therapeutic state.
Cardiomyocyte apoptosis is a form of programmed cell death caused by pathological stimuli, which seriously impairs heart structure and function.3,4 Tumor suppressor p53 is a classical proapoptotic protein, which was shown to be activated in I/R hearts and promote the transcription of proapoptosis target genes.5–7 ASPP1 (apoptosis stimulating protein of p53-1), along with ASPP2 and iASPP, are the members of the ASPP (apoptosis stimulating protein of p53) family. ASPP1 and ASPP2 bind to p53 to promote apoptosis, while iASPP does the opposite.8,9 In a previous study, we proved the antiapoptotic effects of iASPP in cardiomyocytes, which alleviated myocardial ischemia injury by inhibiting p53 expression.10 ASPP1 is crucial in improving the transcriptional activity of p53 in the nucleus.11 Knockdown of ASPP1 blocks the apoptosis of renal clear cell carcinoma by inhibiting the transcriptional activation of p53.12 Similarly, ASPP1 maintains the integrity of hematopoietic stem cells pool and prevents hematological malignancies partly by enhancing the transcriptional activity of p53.13 Currently, the role of ASPP1 in cardiac I/R injury is still unknown.
Nuclear translocation is pivotal for the proapoptotic activity of p53. The classic importin-cargo pathway executes p53 nuclear translocation, in which the nuclear localizing signal (NLS) I domain of p53 binds to importin-α3 and is transited into nucleus by importin-β1.14 An interesting question is that except for ASPP1’s influence on nuclear transcriptional activity of p53, whether it affects nuclear translocation of p53 is unclear. Distinct from that of p53, the other 2 members of ASPP family, ASPP2 and iASPP translocate to the nucleus through an importin-independent pathway. They bind to RanGDP through ankyrin repeat domains and are transported to the nucleus by NTF2 (nuclear transport factor 2).15 To our best knowledge, how ASPP1 translocates to the nucleus remains to be investigated.
In this study, we explored the molecular mechanism of ASPP1 and p53 nuclear translocation and its influence on cardiac I/R injury. We found that the nuclear translocation of ASPP1 and p53 are interdependent in ischemic cardiomyocytes, which remarkably aggravates cardiomyocyte apoptosis. The study highlights the significance of cytoplasmic ASPP1-p53 interaction in cardiac I/R injury.
Methods
Data Availability
Detailed experimental methods and Major Resources Table are available in the Supplemental Material. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Results
The Interdependent Nuclear Translocation of ASPP1 and p53
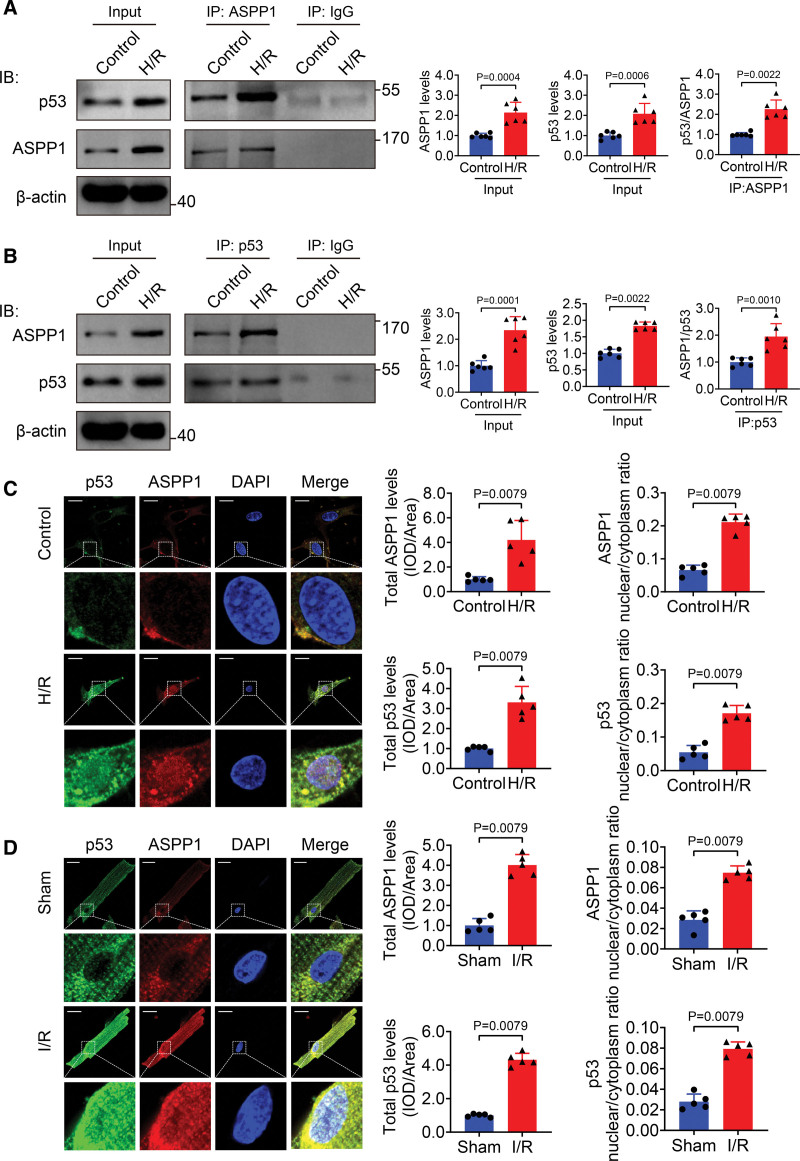
ASPP1 was demonstrated to directly bind and activate p53 in the nucleus to promote apoptosis of cancer cells.8 In this study, we firstly evaluated the interaction between ASPP1 and p53 in cardiomyocyte during hypoxia/reoxygenation (H/R) stimulation. The co-immunoprecipitation assay confirmed the binding between ASPP1 and p53 in cultured neonatal mouse ventricular cardiomyocytes (NMVCs), which was enhanced during H/R stimulation (Figure 1A and 1B). The immunofluorescent staining showed that ASPP1 and p53 colocalized in the cytoplasm under normal condition, and they were both upregulated and translocated to the nuclei after H/R treatment (Figure 1C). The same qualitative data were obtained in isolated cardiomyocytes from sham and I/R injury zone of mice/nonischemic area and ischemic area from I/R mice (Figure 1D and Figure S1).
Figure 1.
Nuclear cotranslocation of ASPP1 and p53 in cardiomyocytes during cardiac ischemia reperfusion injury. A, Proteins from cultured neonatal mouse ventricular cardiomyocytes (NMVCs) subjected to control or 12 hours hypoxia followed by 24 hours reoxygenation (H/R) treatment were immunoprecipitated with ASPP1 or IgG antibody, followed by Western blot; n=6. Statistical significance was assessed by Mann-Whitney U test (p53/ASPP1) or Student t test (ASPP1 levels and p53 levels). B, Proteins from NMVCs were immunoprecipitated with p53 or IgG antibody followed by Western blot; n=6. Statistical significance was assessed by Mann-Whitney U test (ASPP1 levels) or Student t test (p53 levels and ASPP1/p53). C and D, Immunostaining assay was used to analyze the colocalization of ASPP1 and p53 in H/R NMVCs and isolated adult cardiomyocytes of I/R (45 minutes ischemia followed by 24 hours reperfusion) mice (Mann-Whitney U test); n=5. Scale bar=20 μm. ASPP1 indicates apoptosis stimulating of p53 protein 1; and DAPI, 4′,6-diamidino-2-phenylindole.
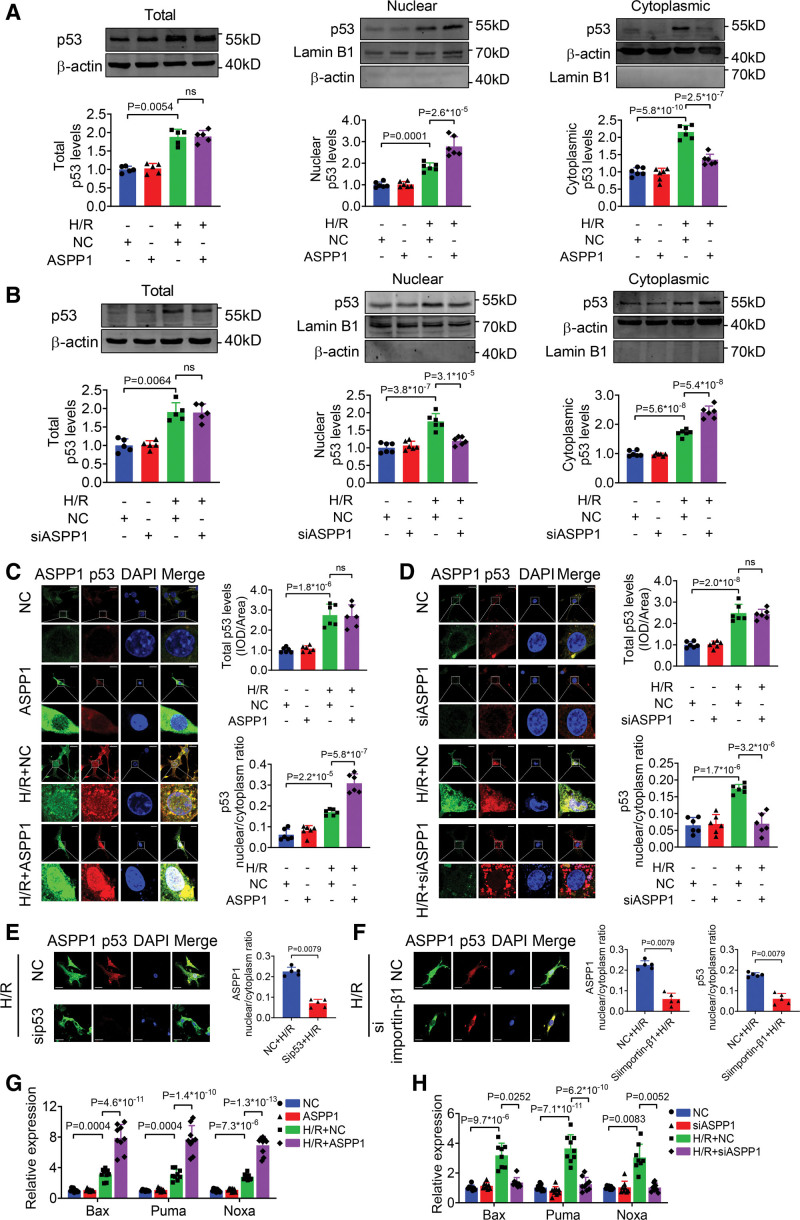
Currently, the influence of ASPP1 on p53 nuclear trafficking remains unknown. We thus explored how ASPP1 altered the nuclear translocation of p53 in NMVCs. We found that overexpression or knockdown of ASPP1 produced no influence on p53 expression and cellular distribution under normal condition (Figure 2A and 2B). The Western blot data showed that upon exposure to H/R insult, overexpression of ASPP1 increased, while knockdown of ASPP1 reduced the nuclear level of p53 in NMVCs (Figure 2A and 2B). The immunofluorescent staining further verified that overexpression of ASPP1 promoted, while knockdown of ASPP1 inhibited the nuclear translocation of p53 in NMVCs under H/R stimulation (Figure 2C and 2D). However, knockdown of ASPP1 produced no influence on subcellular distribution of p63, and p73, 2 other members of p53 family (Figure S2A and S2B). In addition, knockdown of ASPP2 and iASPP produced no effects on subcellular distribution of p53 (Figure S3A and S3B). These results indicated that ASPP1, but not other members of the ASPP family, regulates the nuclear translocation of p53.
Figure 2.
The nuclear translocation of ASPP1 and p53 are interdependent. A and B, Effects of ASPP1 overexpression or knockdown on the total protein level and nuclear distribution of p53 in neonatal mouse ventricular cardiomyocytes (NMVCs) by Western blot; n=5 for total p53 level (Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test); n=6 for nuclear and cytoplasmic p53 levels (1-way ANOVA, followed by Tukey post hoc multicomparisons test); ns, not significant. C and D, Immunofluorescent staining was performed to analyze the effects of ASPP1 overexpression or knockdown on p53 nuclear translocation in NMVCs (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6; ns, not significant. Scale bar=20 μm. E, Effects of p53 knockdown on ASPP1 nuclear translocation in NMVCs (Mann-Whitney U test); n=5. Scale bar=20 μm. F, Effects of importin-β1 knockdown on ASPP1 and p53 nuclear translocation in NMVCs (Mann-Whitney U test); n=5. Scale bar=20 μm. G and H, Effects of ASPP1 overexpression (G) on mRNA levels of Bax (n=9), PUMA (n=9), and Noxa (n=9) or ASPP1 knockdown (H) on mRNA levels of Bax (n=8), PUMA (n=9), and Noxa (n=8) by quantitative real-time PCR (qRT-PCR, 1-way ANOVA, followed by Tukey post hoc multicomparisons test). ASPP1 indicates apoptosis stimulating of p53 protein 1; Bax, Bcl2-associated X; DAPI, 4′,6-diamidino-2-phenylindole; H/R, hypoxia/reoxygenation; and PUMA, p53 upregulated modulator of apoptosis.
ASPP1 does not contain a currently identifiable NLS domain.11,15 We thus speculated that the nuclear translocation of ASPP1 may depend on p53 as a cocargo. Intriguingly, knockdown of p53 resulted in cytoplasmic localization of ASPP1 in NMVCs under H/R insult (Figure 2E). We then interfered the classic importin-cargo pathway for p53 by using the siRNA of importin-β1 (siimportin-β1). Knockdown of importin-β1 resulted in cytoplasmic sequestration of both p53 and ASPP1 in NMVCs with H/R stimulation (Figure 2F). In contrast, knockdown of importin-β1 produced no effects on subcellular distribution of the other 2 members of ASPP family, ASPP2 and iASPP (Figure S3C and S3D). Finally, Bax (Bcl2-associated X), PUMA (p53 upregulated modulator of apoptosis) and Noxa (Latin for damage; also known as Phorbol-12-myristate-13-acetate-induced protein 1 [PMAIP1]), the direct apoptosis associated targets of p53, were further potentiated by ASPP1 overexpression and inhibited by ASPP1 knockdown in NMVCs with H/R treatment (Figure 2G and 2H). These results suggested that nuclear translocation of ASPP1 depends on p53.
Overexpression of ASPP1 Aggravates Cardiac I/R Injury
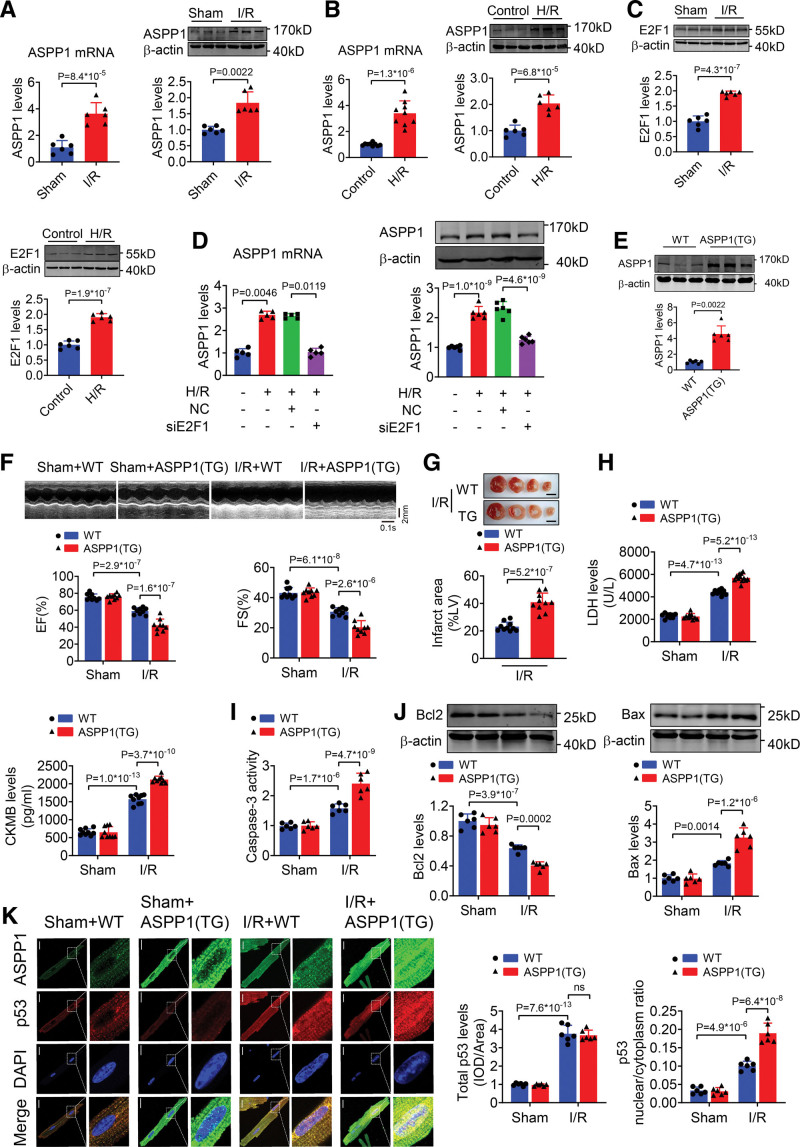
To understand the functional role of ASPP1 in cardiac I/R injury, we examined its expression change in I/R hearts. Both the mRNA and protein levels of ASPP1 were significantly increased in I/R hearts (Figure 3A) and in cultured NMVCs exposed to H/R stimulation (Figure 3B). Transcription factor E2F1 was demonstrated to positively regulate the expression of ASPP1 in U2OS human osteosarcoma cells and Saos-2 cells.15,16 We found that the expression level of E2F1 was elevated in I/R hearts and NMVCs subjected to H/R stimulation (Figure 3C). We then analyzed whether E2F1 determines the expression of ASPP1 during cardiac injury, and found that knockdown of E2F1 led to a significant decrease in ASPP1 mRNA and protein levels of NMVCs under H/R stimulation (Figure 3D). These data indicated that upregulation of ASPP1 during cardiac I/R injury was driven by E2F1.
Figure 3.
Cardiomyocyte-specific overexpression of ASPP1 aggravates cardiac I/R injury. A, The mRNA (Student t test) and protein (Mann-Whitney U test) levels of ASPP1 in cardiac tissues of mice subjected to 45 minutes ischemia followed by 24 hours reperfusion (I/R); n=6. B, The mRNA and protein levels of ASPP1 in neonatal mouse ventricular cardiomyocytes (NMVCs) subjected to H/R (Student t test); n=9 for mRNA level; n=6 for protein level. C, The protein levels of E2F1 in I/R hearts and NMVCs treated with H/R stimulation by Western blot (Student t test); n=6. D, Transfection of E2F1 siRNA inhibited the mRNA (Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test) and protein (1-way ANOVA, followed by Tukey post hoc multicomparisons test) expression of ASPP1 in NMCMs exposed to H/R by quantitative real-time PCR (qRT-PCR) and Western blot assay; n=5 for mRNA level; n=6 for protein level. E, The protein levels of ASPP1 in wild type (WT) and ASPP1 transgenic mice by Western blot (Mann-Whitney U test); n=6. F, Cardiac function of wild type (WT) and ASPP1 transgene mice after I/R injury by echocardiography (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=9. G, Infarct area of I/R mice by 2,3,5-triphenyltetrazolium chloride (TTC) staining (Student t test); n=10. Scale bar=2 mm. H, Serum LDH and creatine kinase isoenzyme MB (CKMB) levels (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=10 (WT+Sham and ASPP1 transgene+Sham); n=12 (WT+IR and ASPP1 transgene+I/R) for LDH assay; n=9 for CKMB assay. I, Caspase-3 activity detected by ELISA assay (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=9. J, The protein levels of Bcl2 and Bax detected by Western blot (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. K, Immunofluorescent staining was performed to analyze the effect of ASPP1 transgene on p53 nuclear translocation in isolated adult cardiomyocytes after cardiac I/R injury (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. ns, not significant. Scale bar=20 μm. ASPP1 indicates apoptosis stimulating of p53 protein 1; DAPI, 4′,6-diamidino-2-phenylindole; EF, ejection fraction; FS, fractional shortening; H/R, hypoxia/reoxygenation; and I/R, ischemia/reperfusion.
To explore the effects of ASPP1 on cardiac I/R injury, we generated an ASPP1 transgenic overexpressing mouse model driven by α-myosin heavy chain promoter (Figure S4A). The expression of ASPP1 was significantly increased in heart tissues of ASPP1 transgenic mice than negative littermate controls (wild type, WT; Figure 3E). Echocardiographic and base line heart weight, body weight results showed no significant difference in cardiac function and the ratio of heart weight/body weight between WT and ASPP1 transgenic mice until 8 weeks (Figure S4B and S4C). The echocardiographic examination showed that overexpression of ASPP1 prominently impaired cardiac function under cardiac I/R injury, as manifested by further declination of ejection fraction and fractional shortening compared to WT mice (Figure 3F). Histological staining with 2,3,5-triphenyltetrazolium chloride revealed that cardiomyocyte-specific overexpression of ASPP1 significantly increased infarct size (Figure 3G). Consistently, serum lactate dehydrogenase (LDH) and creatine kinase isoenzyme MB were further elevated during cardiac I/R injury in ASPP1 transgenic mice compared to WT controls (Figure 3H). In addition, I/R-elicited caspase-3 activation in heart tissue was further promoted by ASPP1 overexpression (Figure 3I). ASPP1 overexpression inhibited the expression of Bcl2 (B-cell lymphoma 2), whilst promoted the expression of Bax in I/R hearts as compared with WT mice (Figure 3J). Consistently, upon I/R injury the nuclear distribution of p53 was further increased in cardiomyocytes of ASPP1 transgenic mice than WT controls (Figure 3K).
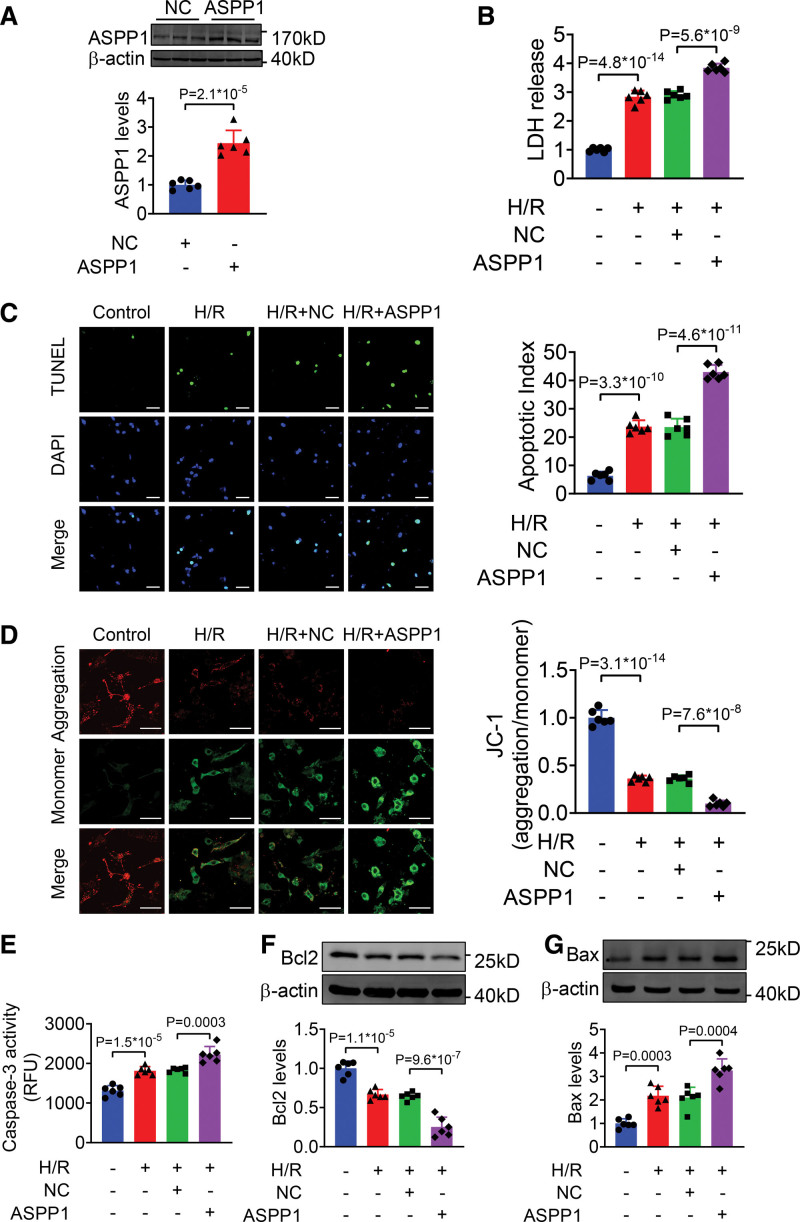
We then examined the effects of ASPP1 overexpression on NMVCs subjected to H/R injury in vitro. The level of ASPP1 was increased in NMVCs after transfection of ASPP1-overexpressing plasmid (Figure 4A). Overexpression of ASPP1 significantly increased LDH release and TUNEL (TdT-mediated dUTP nick end labeling)-positive apoptotic cells of NMVCs treated with H/R (Figure 4B and 4C). JC-1 assay revealed that ASPP1 aggravates the reduction of mitochondrial membrane potential in NMVCs after H/R stimulation (Figure 4D). Consistently, caspase-3 activity was increased in the ASPP1 overexpressing cells than NC group after H/R stimulation (Figure 4E). Meanwhile, Bcl2 level was further decreased in ASPP1 overexpression cells as compared with NC group after H/R treatment, while Bax level was significantly upregulated after ASPP1 overexpression (Figure 4F and 4G).
Figure 4.
Overexpression of ASPP1 aggravates H/R-induced damage in neonatal mouse ventricular cardiomyocytes (NMVCs). A, The efficiency of ASPP1 overexpression plasmid in NMVCs by Western blot (Student t test); n=6. B, LDH release (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. C, Apoptosis of NMVCs detected by TUNEL (TdT-mediated dUTP nick end labeling) assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. Scale bar=50 μm. Green, TUNEL -positive cells; blue, DAPI. D, Mitochondrial membrane potential of NMVCs by JC-1 assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. Scale bar=50 μm. E, Caspase-3 activity in NMVCs by ELISA assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. F and G, The protein levels of Bcl2 (F) and Bax (G) detected by Western blot (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. ASPP1 indicates apoptosis stimulating of p53 protein 1; Bax, Bcl2-associated X; DAPI, 4′,6-diamidino-2-phenylindole; H/R, hypoxia/reoxygenation; and LDH, lactate dehydrogenase.
The findings from both in vivo and in vitro studies indicate that overexpression of ASPP1 promoted cardiac I/R injury.
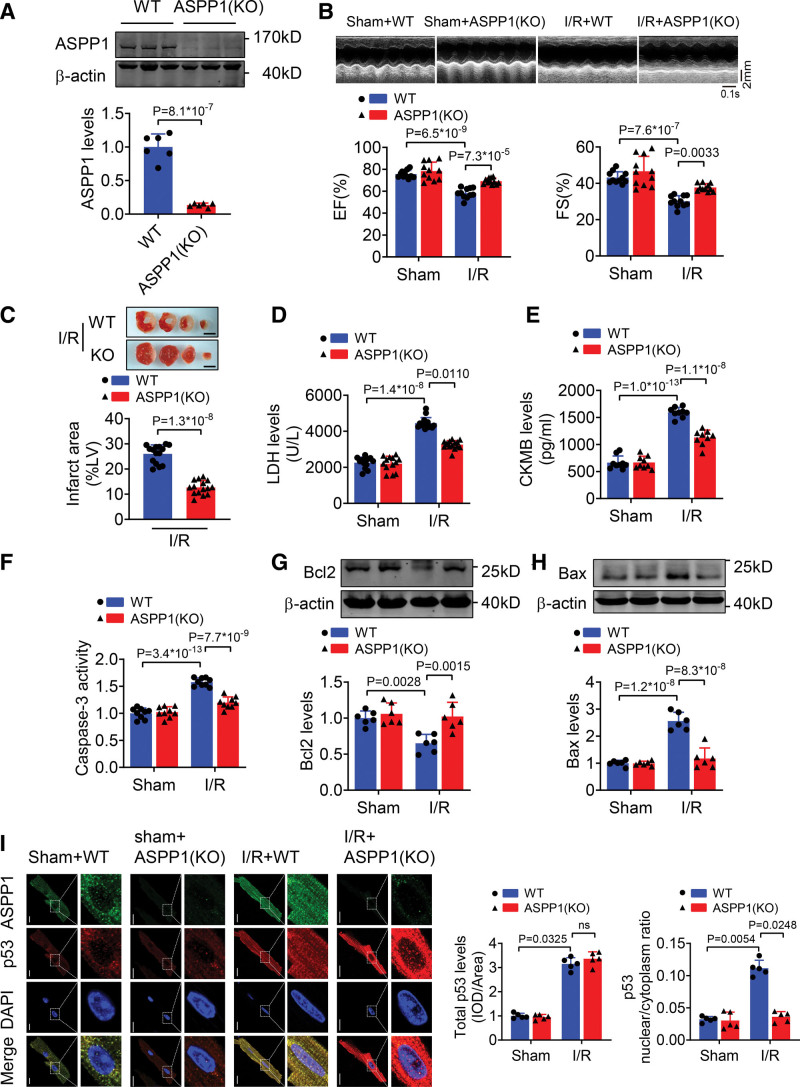
Suppression of ASPP1 Improves Myocardial I/R Injury
To further determine the specific function of ASPP1 in I/R injury, we engineered an ASPP1 conventional knockout mouse model in which the second exon region of ASPP1 was deleted by clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeat–associated 9 technique (Figure S5A). The successful deletion of ASPP1 was confirmed by Western blot with heart tissue of homozygous conventional knockout mice (Figure 5A). Echocardiographic and base line heart weight, body weight results showed no significant difference in cardiac function and the ratio of heart weight/body weight between WT and ASPP1 knockout mice until 8 weeks (Figure S5B and S5C). Adult ASPP1 knockout mice were subjected to I/R injury. Knockout of ASPP1 prominently improved cardiac function under cardiac I/R injury, as manifested by elevation of ejection fraction and fractional shortening compared to WT mice (Figure 5B). Knockout of ASPP1 significantly reduced infarct size (Figure 5C). Consistently, serum LDH and creatine kinase isoenzyme MB were decreased in ASPP1 knockout mice during cardiac I/R injury (Figure 5D and 5E). In addition, I/R-elicited caspase-3 activation was decreased by ASPP1 knockout in heart tissue (Figure 5F). Furthermore, ASPP1 knockout promoted the expression of Bcl2, whilst inhibited the expression of Bax in I/R hearts as compared with WT mice (Figure 5G and 5H). Upon I/R injury, the nuclear distribution of p53 was decreased in cardiomyocytes of ASPP1 knockout mice than WT controls (Figure 5I).
Figure 5.
Knockout of ASPP1 improves cardiac I/R injury. A, Verification of ASPP1 conventional knockout in mice by Western blot (Student t test); n=6. B, Cardiac function of wild type (WT) and ASPP1 knockout (KO) mice after I/R injury by echocardiography (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=11. C, Infarct area of I/R mice by 2,3,5-triphenyltetrazolium chloride (TTC) staining (Mann-Whitney U test); n=15. Scale bar=2 mm. D and E, Serum LDH (Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test) and creatine kinase isoenzyme MB (CKMB; 2-way ANOVA, followed by Tukey post hoc multicomparisons test) levels; n=13 (WT+Sham and ASPP1[KO]+Sham); n=15 (WT+I/R and ASPP1[KO]+I/R) for LDH assay; n=9 for CKMB assay. F, Caspase-3 activity detected by ELISA assay (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=9. G and H, The protein levels of Bcl2 and Bax detected by Western blot (2-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. I, Immunofluorescent staining was performed to analyze the effects of ASPP1 knockout on p53 nuclear translocation in isolated adult cardiomyocytes after cardiac I/R injury (Kruskal-Wallis, followed by FDR method of Benjamini and Hochberg test); n=5; ns, not significant. Scale bar=20 μm. ASPP1 indicates apoptosis stimulating of p53 protein 1; DAPI, 4′,6-diamidino-2-phenylindole; I/R, ischemia/reperfusion; and LDH, lactate dehydrogenase.
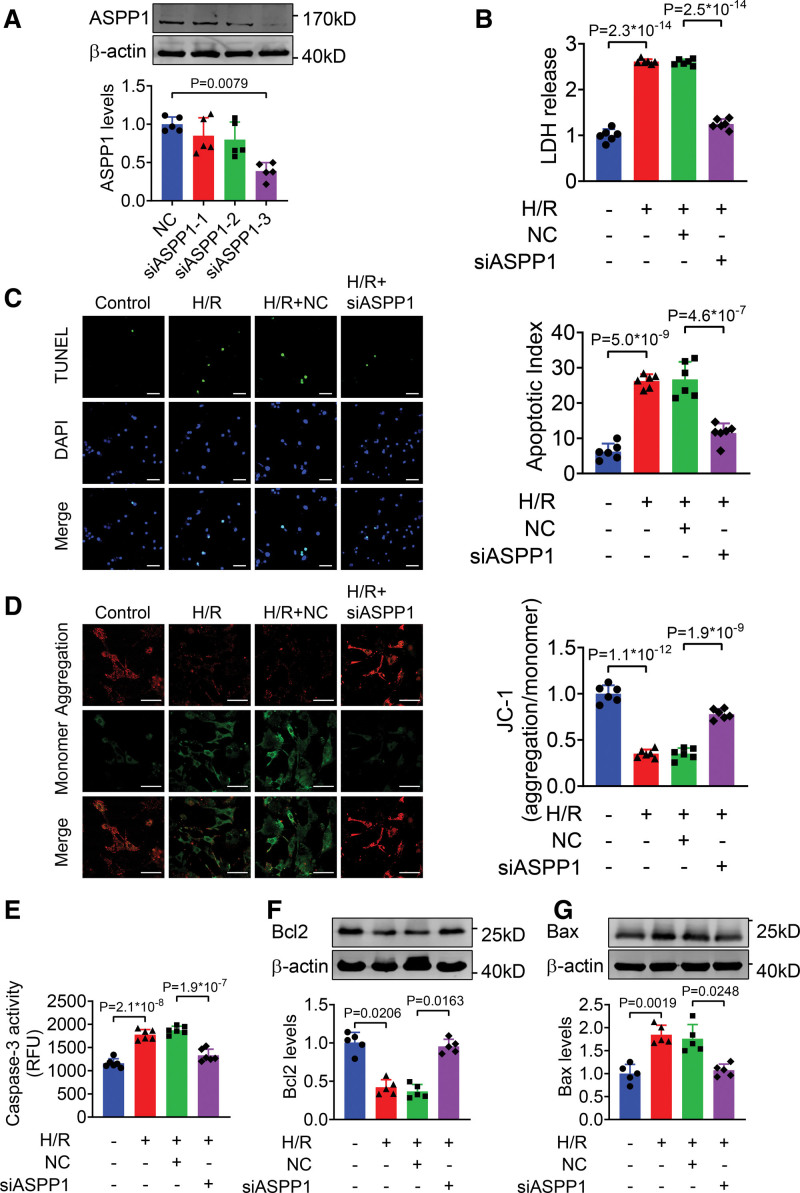
We also employed siRNAs to knockdown ASPP1 expression in NMVCs. Western blot assay showed that siRNA-3 caused about 61.3% decrease of ASPP1 protein (Figure 6A). H/R stimulation led to increased LDH release in NMVCs, which was markedly attenuated with ASPP1 knockdown (Figure 6B). The TUNEL-positive apoptotic cells were significantly reduced in ASPP1 knockdown cells compared to NC group under H/R stimulation (Figure 6C). JC-1 assay revealed that the reduction of mitochondrial membrane potential was notably preserved after knockdown of ASPP1 (Figure 6D). Furthermore, ASPP1 knockdown remarkably decreased caspase-3 activity under H/R treatment (Figure 6E). ASPP1 knockdown increased Bcl2 level and decreased Bax level (Figure 6F and 6G). These findings suggest that inhibition of ASPP1 attenuates cardiac I/R injury.
Figure 6.
Knockdown of ASPP1 alleviates H/R-induced damage in cultured neonatal mouse ventricular cardiomyocytes (NMVCs). A, Efficiency of small interfering RNA (siRNA) for ASPP1 in NMVCs by Western blot (Mann-Whitney U test); n=5. B, LDH release (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. C, Apoptosis of NMVCs detected by TUNEL (TdT-mediated dUTP nick end labeling) assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. Scale bar=50 μm. Green, TUNEL - positive cells; blue, DAPI. D, Mitochondrial membrane potential of NMVCs by JC-1 assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. Scale bar=50 μm. E, Caspase-3 activity in NMVCs by ELISA assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. F and G, The protein levels of Bcl2 and Bax detected by Western blot (Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test); n=5. ASPP1 indicates apoptosis stimulating of p53 protein 1; Bax, Bcl2-associated X; DAPI, 4′,6-diamidino-2-phenylindole; H/R, hypoxia/reoxygenation; and LDH, lactate dehydrogenase.
p53 Mediates the Proapoptotic Effects of ASPP1
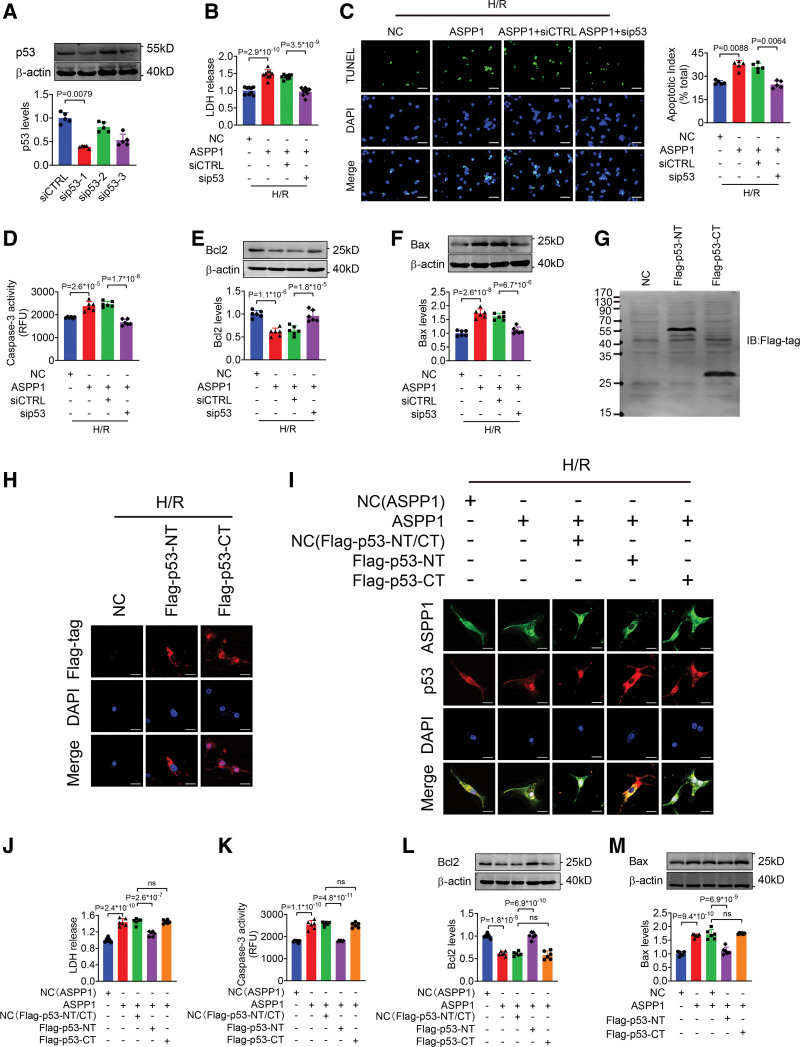
Next, we experimentally verified the role of p53 in ASPP1 induced cardiomyocyte injury. The siRNAs were used to knockdown p53 expression and the efficiency of siRNA-1 was about 60.2% (Figure 7A), which was used in the following experiments. Overexpression of ASPP1 increased LDH level, cell apoptosis and caspase-3 activity under H/R stimulation, which were markedly inhibited by cotransfection of p53 siRNA (Figure 7B through 7D). Further detection confirmed that p53 knockdown significantly reversed the reduction of Bcl2 and increase of Bax induced by ASPP1 overexpression (Figure 7E and 7F). Furthermore, overexpression of p53 (Figure S6A) had no significant effects on the protective role of ASPP1 knockdown in NMVCs under H/R stimulation, as evidenced by the fact that LDH release, caspase-3 activity, Bcl2 level and Bax level did not change in p53 and ASPP1 siRNA cotransfection group over ASPP1 siRNA alone group (Figure S6B through S6E). Knockdown of p63 or p73 (Figure S7A and S7F) produced no significant influence on ASPP1 overexpression promoted cell apoptosis under H/R stimulation (Figure S7B through S7E and S7G through S7J).
Figure 7.
p53 mediates the proapoptotic effects of ASPP1. A, The efficiency of small interfering RNA (siRNA) of p53 in neonatal mouse ventricular cardiomyocytes (NMVCs) by Western blot (Mann-Whitney U test); n=5. B, LDH release after cotransfection of siRNA of p53 and ASPP1 plasmid (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=9. C, Apoptosis index of NMVCs detected by TUNEL (TdT-mediated dUTP nick end labeling) assay (Kruskal-Wallis, followed by false discovery rate [FDR] method of Benjamini and Hochberg test); n=5. Scale bar=50 μm. Green, TUNEL- positive cells; blue, DAPI. D, Caspase-3 activity in NMVCs by ELISA assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. E and F, The protein levels of Bcl2 and Bax detected by Western blot (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. G, Validation of p53-NT and p53-CT by Western blot. Flag-p53-NT, N-terminal fragment containing binding domain for ASPP1 without nuclear localizing signal (NLS, 1–288 aa) and Flag-p53-CT, C-terminal fragment containing NLS of p53, which is responsible for its nuclear localization (310-381 aa); n=5. H, Celluar distribution of Flag-p53-NT and Flag-p53-CT by immunofluorescent staining; n=5. Scale bar=20 μm. I, Effects of Flag-p53-NT and Flag-p53-CT on ASPP1 and p53 nuclear translocation in NMVCs; n=5. Scale bar=20 μm. J, LDH level in culture medium after treated with Flag-p53-NT or Flag-p53-CT (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. K, Caspase-3 activity in NMVCs after cotransfection of Flag-p53-NT or Flag-p53-CT and ASPP1 plasmid by ELISA assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. L and M, The protein levels of Bcl2 (L) and Bax (M) after cotransfection of Flag-p53-NT or Flag-p53-CT and ASPP1 plasmid detected by Western blot (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. ASPP1 indicates apoptosis stimulating of p53 protein 1; Bax, Bcl2-associated X; DAPI, 4′,6-diamidino-2-phenylindole; H/R, hypoxia/reoxygenation; and LDH, lactate dehydrogenase.
To further determine the specific role of ASPP1 and p53 interaction on cell apoptosis, we explored whether the fragment of p53 containing ASPP1 binding domain can compete with the endogenous p53 to exert protective effects. We constructed 2 Flag-p53 truncated fragments, N-terminal fragment (p53-NT containing binding domain for ASPP1 without NLS, 1-288 aa) and C-terminal fragment (p53-CT containing NLS of p53 responsible for nuclear localization, 310-381 aa). The Western blot for Flag successfully detected the protein expression of Flag-p53-NT and Flag-p53-CT (Figure 7G). The immunofluorescent staining showed that Flag-p53-NT distributed in the cytoplasm, while the Flag-p53-CT in the nucleus of NMVCs exposed to H/R injury (Figure 7H). The 2 p53 fragments were then cotransfected with ASPP1 to NMVCs, respectively. The data showed that Flag-p53-NT inhibited the nuclear translocation of endogenous p53 by competitively binding with ASPP1 (Figure 7I), and alleviated NMVCs H/R injury as evidenced by the decreased LDH release and caspase-3 activity, increased Bcl2 level and decreased Bax level. In contrast, Flag-p53-CT had no effects on the proapoptotic role of ASPP1 overexpression in NMVCs under H/R stimulation (Figure 7J through 7M). These data suggested that ASPP1 exerted its detrimental effects on H/R-induced injury by binding to p53.
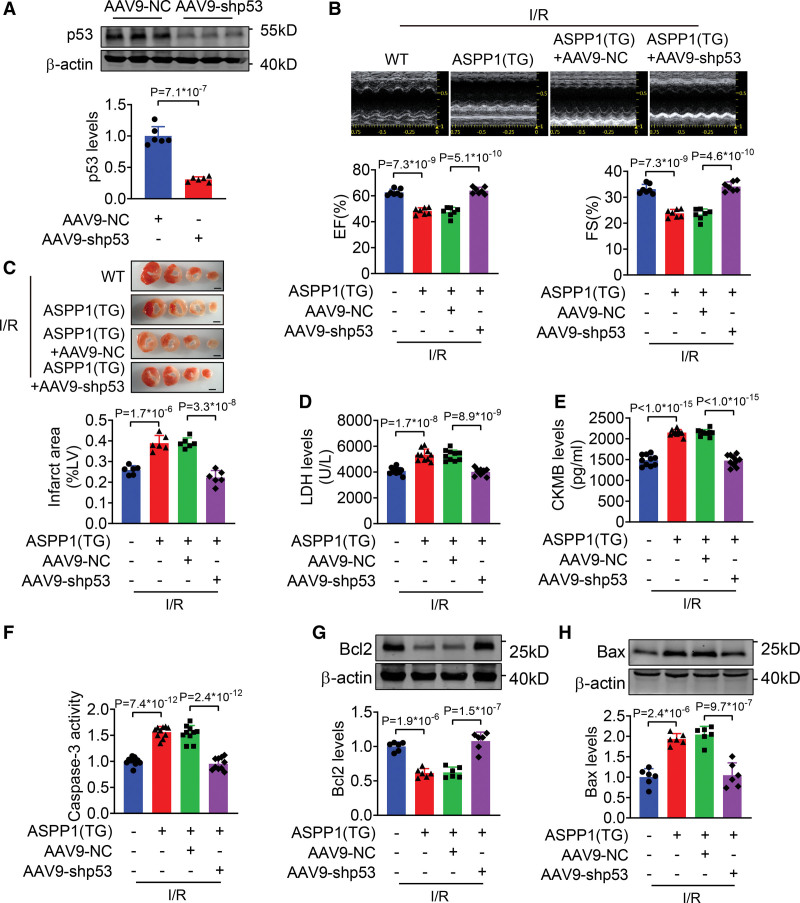
We then constructed the adeno-associated virus 9 (AAV9) carrying p53 shRNA (AAV9-shp53) to knockdown p53 in vivo (Figure 8A). Administration of AAV9-shp53 restored ejection fraction and fractional shortening, and reduced infarct size of ASPP1 transgenic mice after I/R injury (Figure 8B and 8C). Consistently, AAV9-shp53 abolished the increase of serum LDH and creatine kinase isoenzyme MB levels in ASPP1 transgenic mice after I/R injury (Figure 8D and 8E). The increased caspase-3 activity in ASPP1 transgenic mice with I/R injury was attenuated by AAV9-shp53 (Figure 8F). Furthermore, the downregulation of Bcl2 and upregulation of Bax in I/R hearts of ASPP1 transgenic mice were reversed by AAV9-shp53 (Figure 8G and 8H). These findings suggest that knockdown of p53 rescues cardiac I/R injury in ASPP1 transgenic mice.
Figure 8.
AAV9-shp53 rescues the cardiac injury mediated by the transgenic overexpression of ASPP1. A, The efficiency of AAC9-shp53 in cardiac tissues by Western blot (Student t test); n=6. B, Echocardiographic measurement of cardiac function (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=7. C, Infarct area of I/R mice by 2,3,5-triphenyltetrazolium chloride (TTC) staining (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. Scale bar=2 mm. D and E, Serum LDH and creatine kinase isoenzyme MB (CKMB) levels (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=10 for LDH assay and CKMB assay. F, Caspase-3 activity detected by ELISA assay (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=10. G and H, The protein levels of Bcl2 and Bax detected by Western blot (1-way ANOVA, followed by Tukey post hoc multicomparisons test); n=6. AAV9 indicates adeno-associated virus serotype 9; ASPP1, apoptosis stimulating of p53 protein 1; Bax, Bcl2-associated X; Bcl2, B-cell lymphoma 2; EF, ejection fraction; FS, fractional shortening; I/R, ischemia/reperfusion; LDH, lactate dehydrogenase; and TG, transgenic.
Discussion
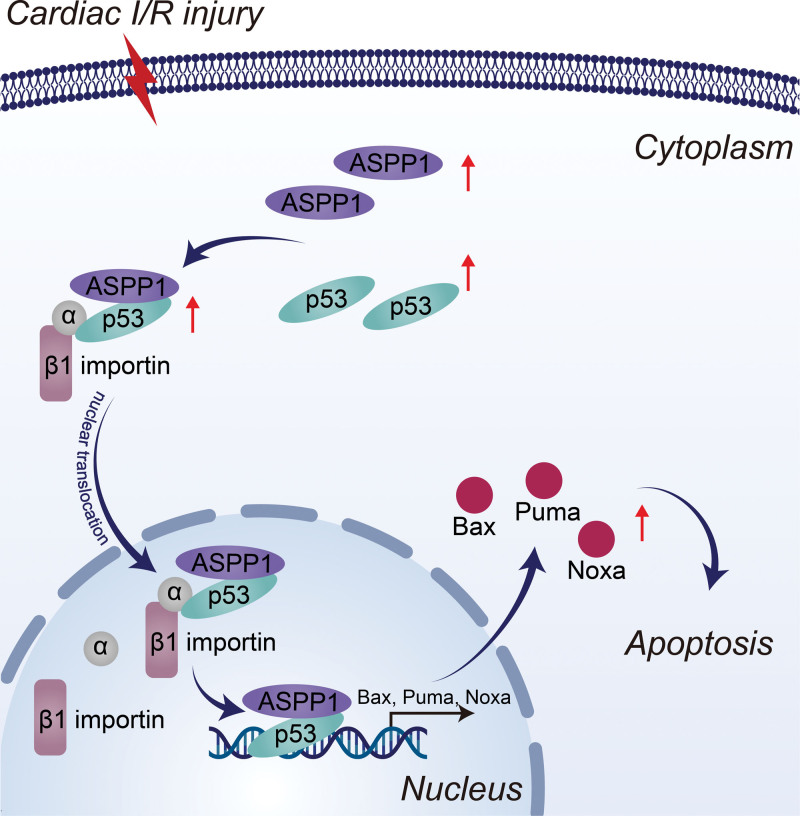
In this study, we demonstrated that under ischemic state binding of ASPP1 to p53 promotes their nuclear translocation, and the nuclear trafficking of ASPP1 and p53 are interdependent. ASPP1 was robustly increased in I/R hearts and H/R primary cardiomyocytes of mice under the control of transcription factor E2F1. Both gain- and loss-of-function studies indicated that ASPP1 was detrimental to cardiac I/R injury. The damaging effects of ASPP1 was mediated by p53 in cardiomyocytes. Inhibition of ASPP1 alleviated cardiac I/R injury (Figure 9). The findings highlight the distinct mechanism for the nuclear trafficking of ASPP1 and p53, and imply the therapeutic potential of ASPP1 as a novel target for cardiac I/R injury.
Figure 9.
The diagram of the regulation of ASPP1 on cardiac I/R injury. ASPP1 indicates apoptosis stimulating of p53 protein 1; Bax, Bcl2-associated X; I/R, ischemia/reperfusion; and PUMA, p53 upregulated modulator of apoptosis.
ASPP1 is a crucial member of the apoptosis stimulating of p53 protein (ASPP) family that promotes cell apoptosis. In a variety of cancer cells, the decreased level of ASPP1 was closely related to tumor progression.16–18 Overexpression of ASPP1 promoted apoptosis, whereas knockdown of ASPP1 inhibited apoptosis and induced 5-FU resistance of clear cell renal cell carcinoma.12 Knockdown of ASPP1 inhibited apoptosis and drove tumor growth in liver cancer.19 In this study, we found that the expression of ASPP1 was upregulated in both mRNA and protein levels in cardiac I/R mice and H/R NMVCs. Cardiomyocytes specific overexpression of ASPP1 activated cell apoptosis and aggravated cardiac I/R injury. Inversely, knockout of ASPP1 mitigated apoptosis of cardiomyocytes and improved cardiac function in I/R injury. These findings indicate that ASPP1 is also a key regulator of cardiomyocyte apoptosis.
The transcription factor p53 activates apoptosis by regulating the expression of a host of target genes.20,21 p53 was upregulated and thereby aggravated cardiac I/R injury by promoting the transcription of apoptosis-related genes such as Bax, PUMA, Noxa.22 The inhibitor of p53 transcription, PIFITHRIN-α decreased cardiomyocyte apoptosis and improved cardiac function of rats after cardiac I/R injury.23 ERK (extracellular signal-regulated kinase)/GSK-3β (glycogen synthase kinase-3 beta) pathway activated the apoptosis process and participated in the cardiac I/R injury by enhancing the activity of p53.24 As a crucial cofactor of p53, ASPP1 binds to p53 and specifically enhances the transcriptional ability of p53 to activate apoptotic targets such as Bax and PUMA in the nucleus, thereby promoting apoptosis.8 The proapoptotic activity of ASPP1 in cardiomyocytes was related to the activation of p53. We found that the binding between ASPP1 and p53 was increased in cardiomyocytes after H/R injury, which led to increased transcription of p53 targets. Overexpression of ASPP1 increased, while knockdown of ASPP1 inhibits the expression of apoptosis-related p53 targets such as Bax, PUMA, and Noxa.
Nuclear transport of certain proteins is an essential step for them to exert their biological functions, including p53.25,26 Most nuclear proteins contain the NLS domain and traffic into the nucleus via the classic nuclear transport protein importins.27 In this classical pathway, importin-α proteins recognized NLS domains and transited into nucleus by binding with importin-β1 through their cargo.28 In terms of nuclear translocation of p53, importin-α3 recognized the NLS I domain of p53 and transited it into nucleus by importin-β1.14 In this study, we found that ASPP1 enhanced the nuclear translocation and accumulation of p53, which indicates that except for the activation of p53 in the nucleus as a cofactor, ASPP1 is also a key regulator of p53 trafficking. Intriguingly, we found that nuclear import of ASPP1 is dependent on p53, as knockdown of p53 significantly reduced the nuclear localization of ASPP1 under H/R stimulation in NMVCs. Furthermore, knockdown of importin-β1 significantly reduced the nuclear localization of both p53 and ASPP1 under H/R stimulation in NMVCs. As ASPP1 does not contain a currently identifiable NLS domain, it may be a cocargo of p53 nuclear trafficking pathway to accomplish nuclear translocation. These data imply that the nuclear translocation of ASPP1 and p53 are interdependent. p63 and p73 are 2 p53 homologs, which also participates in the induction of cell apoptosis29,30 In this study, we found ASPP1 did not colocalized with p63 or p73, and knockdown of ASPP1 did not affect the subcellular distribution of p63 and p73 in cardiomyocytes under H/R stimulation, indicating that the detrimental effects of ASPP1 on cardiomyocytes during ischemia injury was not mediated by p63 and p73. There are also studies showing that p53 was not very important in cardiac I/R injury.31,32 It would be quite necessary to dissect how the discrepancy of p53’s role in cardiac I/R injury happened. The settlement of this issue will definitively progress our understanding of the unique mechanism of p53-mediated cardiac I/R injury and facilitate the development of novel therapeutic strategy.
Lu et al15 has reported that the other 2 ASPP family members, ASPP2 and iASPP contain ankyrin repeat domains, which can bind to RanGDP and be translocated into the nucleus by nuclear transport factor 2. In this study, we found that knockdown of iASPP increases p53 expression, and knockdown of ASPP2 or iASPP produced no effects on subcellular distribution of p53. These findings highlight the special role of ASPP1 of the ASPP family proteins in regulating p53 nuclear translocation, which is different from the original understanding of ASPP1 as a nuclear cofactor of p53 in the nucleus. Consistent with the different influence of iASPP on p53 expression and distribution than ASPP1, knockdown of iASPP aggravates cardiac ischemic damage.10
The proapoptotic effect of ASPP1 depends on its nuclear localization. Arnaud et al33 reported that unlike in the nucleus, cytoplasmic ASPP1 competed with YAP (Yes-associated protein) for LATS2 (large tumor suppressor homolog 2) and inhibited the phosphorylation of YAP, thereby exerting an antiapoptotic effect. Consistently, we found that ASPP1 localized predominantly in the cytoplasm of unstressed cardiomyocytes, while significant nuclear translocation and accumulation occurred after cardiac I/R injury and NMVCs H/R stimulation.
E2F1 is a pivotal transcription factor in promoting apoptosis and suppressing proliferation.34 During cardiac I/R injury, E2F1 was upregulated and aggravated cardiac I/R injury by regulating the expression of multiple genes, including promoting apoptosis by enhancing the expression of FoxO-1a and promoting necrosis by inhibiting expression of miR-30b.35,36 E2F1 was shown to be the transcription factor for ASPP1.36–38 Consistently, E2F1 was robustly increased in I/R hearts and H/R primary cardiomyocytes, and knockdown of E2F1 inhibited the upregulation of ASPP1 in H/R NMVCs.
In conclusion, the study demonstrated that ASPP1 aggravates cardiac I/R injury by activating the p53 proapoptotic pathway. This detrimental effect stems from the unique interdependent nuclear translocation of p53 and ASPP1. The findings indicate that modulation of ASPP1 and p53 nuclear translocation represents a novel strategy in treating cardiac I/R injury.
Article Information
Author Contributions
Z. Pan, B. Yang, Y. Lu, and Y. Yang designed the research. Y. Yang and Y. Zhang supervised all aspects of the research. M. Zhang, Y. Jiang, X. Liu, S. Li, Z. Li, Y. Guo, L. Zhao, H. Bao, J. Song, L. Xuan, and G. Yang performed cellular experiments. J. Yang, T. Tian, G. Xue, X. Li, X. Zhang, X. Huang, and Z. Zhou conducted animal experiments. Y. Yang, H. Shan, Z. Zhang., Y. Lu, and Z. Pan wrote and finalized the article. All persons have made contributions to this work.
Sources of Funding
This work was supported by National Natural Science Foundation of China (82070283 to Y. Lu and 82070344 to Z. Pan), Heilongjiang Touyan Innovation Team Program and CAMS Innovation Fund for Medical Sciences (CIFMS, 2019-I2M-5-078), and HMU Marshal Initiative Funding (HMUMIF-21017 to Z. Pan).
Disclosures
None.
Supplemental Material
Expanded Materials and Methods
Figures S1–S9
Tables S1–S8
Supplementary Material
Nonstandard Abbreviations and Acronyms
- AAV9
- adeno-associated virus serotype 9
- ASPP1
- apoptosis stimulating of p53 protein 1
- Bax
- Bcl2-associated X
- Bcl2
- B-cell lymphoma 2
- H/R
- hypoxia/reoxygenation
- I/R
- ischemia/reperfusion
- LDH
- lactate dehydrogenase
- NLS
- nuclear localizing signal
- NMVCs
- neonatal mouse ventricular cardiomyocytes
- PUMA
- p53 upregulated modulator of apoptosis
- WT
- wild type
Y. Yang and Y. Zhang contributed equally.
For Sources of Funding and Disclosures, see page 221.
Supplemental Material is available at https://www.ahajournals.org/doi/suppl/10.1161/CIRCRESAHA.122.321153.
References
- 1.Yellon DM, Hausenloy DJ. Myocardial reperfusion injury. N Engl J Med. 2007;357:1121–1135. doi: 10.1056/NEJMra071667 [DOI] [PubMed] [Google Scholar]
- 2.Heusch G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat Rev Cardiol. 2020;17:773–789. doi: 10.1038/s41569-020-0403-y [DOI] [PubMed] [Google Scholar]
- 3.Jose Corbalan J, Vatner DE, Vatner SF. Myocardial apoptosis in heart disease: does the emperor have clothes?. Basic Res Cardiol. 2016;111:31. doi: 10.1007/s00395-016-0549-2 [DOI] [PubMed] [Google Scholar]
- 4.Scarabelli TM, Gottlieb RA. Functional and clinical repercussions of myocyte apoptosis in the multifaceted damage by ischemia/reperfusion injury: old and new concepts after 10 years of contributions. Cell Death Differ. 2004;11:S144–S152. doi: 10.1038/sj.cdd.4401544 [DOI] [PubMed] [Google Scholar]
- 5.Wang X, Ha T, Zou J, Ren D, Liu L, Zhang X, Kalbfleisch J, Gao X, Williams D, Li C. MicroRNA-125b protects against myocardial ischaemia/reperfusion injury via targeting p53-mediated apoptotic signalling and TRAF6. Cardiovasc Res. 2014;102:385–395. doi: 10.1093/cvr/cvu044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buerke M, Pruefer D, Sankat D, Carter JM, Buerke U, Russ M, Schlitt A, Friedrich I, Borgermann J, Vahl CF, et al. Effects of aprotinin on gene expression and protein synthesis after ischemia and reperfusion in rats. Circulation.2007;116:I121–I126. doi: 10.1161/CIRCULATIONAHA.106.680249 [DOI] [PubMed] [Google Scholar]
- 7.Su Q, Lv XW, Xu YL, Cai RP, Dai RX, Yang XH, Zhao WK, Kong BH. Exosomal LINC00174 derived from vascular endothelial cells attenuates myocardial I/R injury via p53-mediated autophagy and apoptosis. Mol Ther Nucleic Acids. 2021;23:1304–1322. doi: 10.1016/j.omtn.2021.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Samuels-Lev Y, O’Connor DJ, Bergamaschi D, Trigiante G, Hsieh JK, Zhong S, Campargue I, Naumovski L, Crook T, Lu X. ASPP proteins specifically stimulate the apoptotic function of p53. Mol Cell. 2001;8:781–794. doi: 10.1016/s1097-2765(01)00367-7 [DOI] [PubMed] [Google Scholar]
- 9.Bergamaschi D, Samuels Y, O’Neil NJ, Trigiante G, Crook T, Hsieh JK, O’Connor DJ, Zhong S, Campargue I, Tomlinson ML, et al. iASPP oncoprotein is a key inhibitor of p53 conserved from worm to human. Nat Genet. 2003;33:162–167. doi: 10.1038/ng1070 [DOI] [PubMed] [Google Scholar]
- 10.Yagudin T, Zhao Y, Gao H, Zhang Y, Yang Y, Zhang X, Ma W, Daba TM, Ishmetov V, Kang K, et al. iASPP protects the heart from ischemia injury by inhibiting p53 expression and cardiomyocyte apoptosis. Acta Biochim Biophys Sin (Shanghai).2021;53:102–111. doi: 10.1093/abbs/gmaa104 [DOI] [PubMed] [Google Scholar]
- 11.Aylon Y, Ofir-Rosenfeld Y, Yabuta N, Lapi E, Nojima H, Lu X, Oren M. The Lats2 tumor suppressor augments p53-mediated apoptosis by promoting the nuclear proapoptotic function of ASPP1. Genes Dev. 2010;24:2420–2429. doi: 10.1101/gad.1954410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X, Cheng Y, Zhu Y, Li H, Ge W, Wu X, Zhao K, Yuan J, Li Z, Jiang S, et al. Epigenetic silencing of ASPP1 confers 5-FU resistance in clear cell renal cell carcinoma by preventing p53 activation. Int J Cancer. 2017;141:1422–1433 doi: 10.1002/ijc.30852 [DOI] [PubMed] [Google Scholar]
- 13.Yamashita M, Nitta E, Suda T. Aspp1 preserves hematopoietic stem cell pool integrity and prevents malignant transformation. Cell Stem Cell.2015;17:23–34. doi: 10.1016/j.stem.2015.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Marchenko ND, Hanel W, Li D, Becker K, Reich N, Moll UM. Stress-mediated nuclear stabilization of p53 is regulated by ubiquitination and importin-alpha3 binding. Cell Death Differ. 2010;17:255–267. doi: 10.1038/cdd.2009.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu M, Zak J, Chen S, Sanchez-Pulido L, Severson DT, Endicott J, Ponting CP, Schofield CJ, Lu X. A code for RanGDP binding in ankyrin repeats defines a nuclear import pathway. Cell.2014;157:1130–1145. doi: 10.1016/j.cell.2014.05.006 [DOI] [PubMed] [Google Scholar]
- 16.Mak VC, Lee L, Siu MK, Wong OG, Lu X, Ngan HY, Wong ES, Cheung AN. Downregulation of ASPP1 in gestational trophoblastic disease: correlation with hypermethylation, apoptotic activity and clinical outcome. Mod Pathol. 2011;24:522–532. doi: 10.1038/modpathol.2010.216 [DOI] [PubMed] [Google Scholar]
- 17.Agirre X, Roman-Gomez J, Jimenez-Velasco A, Garate L, Montiel-Duarte C, Navarro G, Vazquez I, Zalacain M, Calasanz MJ, Heiniger A, et al. ASPP1, a common activator of TP53, is inactivated by aberrant methylation of its promoter in acute lymphoblastic leukemia. Oncogene.2006;25:1862–1870. doi: 10.1038/sj.onc.1209236 [DOI] [PubMed] [Google Scholar]
- 18.Liu D, Ertay A, Hill C, Zhou Y, Li J, Zou Y, Qiu H, Yuan X, Ewing RM, Lu X, et al. ASPP1 deficiency promotes epithelial-mesenchymal transition, invasion and metastasis in colorectal cancer. Cell Death Dis. 2020;11:224. doi: 10.1038/s41419-020-2415-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao J, Wu G, Bu F, Lu B, Liang A, Cao L, Tong X, Lu X, Wu M, Guo Y. Epigenetic silence of ankyrin-repeat-containing, SH3-domain-containing, and proline-rich-region- containing protein 1 (ASPP1) and ASPP2 genes promotes tumor growth in hepatitis B virus-positive hepatocellular carcinoma. Hepatology.2010;51:142–153. doi: 10.1002/hep.23247 [DOI] [PubMed] [Google Scholar]
- 20.Brady CA, Jiang D, Mello SS, Johnson TM, Jarvis LA, Kozak MM, Kenzelmann Broz D, Basak S, Park EJ, McLaughlin ME, et al. Distinct p53 transcriptional programs dictate acute DNA-damage responses and tumor suppression. Cell.2011;145:571–583. doi: 10.1016/j.cell.2011.03.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yonish-Rouach E, Resnitzky D, Lotem J, Sachs L, Kimchi A, Oren M. Wild-type p53 induces apoptosis of myeloid leukaemic cells that is inhibited by interleukin-6. Nature.1991;352:345–347. doi: 10.1038/352345a0 [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Liu X, She ZG, Jiang DS, Wan N, Xia H, Zhu XH, Wei X, Zhang XD, Li H. Interferon regulatory factor 9 is an essential mediator of heart dysfunction and cell death following myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2014;109:434. doi: 10.1007/s00395-014-0434-9 [DOI] [PubMed] [Google Scholar]
- 23.Liu P, Xu B, Cavalieri TA, Hock CE. Inhibition of p53 by pifithrin-alpha reduces myocyte apoptosis and leukocyte transmigration in aged rat hearts following 24 hours of reperfusion. Shock.2008;30:545–551. doi: 10.1097/SHK.0b013e31816a192d [DOI] [PubMed] [Google Scholar]
- 24.Lin CL, Tseng HC, Chen RF, Chen WP, Su MJ, Fang KM, Wu ML. Intracellular zinc release-activated ERK-dependent GSK-3beta-p53 and Noxa-Mcl-1 signaling are both involved in cardiac ischemic-reperfusion injury. Cell Death Differ. 2011;18:1651–1663. doi: 10.1038/cdd.2011.80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Faustino RS, Nelson TJ, Terzic A, Perez-Terzic C. Nuclear transport: target for therapy. Clin Pharmacol Ther. 2007;81:880–886. doi: 10.1038/sj.clpt.6100141 [DOI] [PubMed] [Google Scholar]
- 26.Ferrando-May E. Nucleocytoplasmic transport in apoptosis. Cell Death Differ. 2005;12:1263–1276. doi: 10.1038/sj.cdd.4401626 [DOI] [PubMed] [Google Scholar]
- 27.Stewart M. Molecular mechanism of the nuclear protein import cycle. Nat Rev Mol Cell Biol. 2007;8:195–208. doi: 10.1038/nrm2114 [DOI] [PubMed] [Google Scholar]
- 28.Vetter IR, Arndt A, Kutay U, Gorlich D, Wittinghofer A. Structural view of the Ran-Importin beta interaction at 2.3 A resolution. Cell.1999;97:635–646. doi: 10.1016/s0092-8674(00)80774-6 [DOI] [PubMed] [Google Scholar]
- 29.Yang A, McKeon F. P63 and P73: P53 mimics, menaces and more. Nat Rev Mol Cell Biol. 2000;1:199–207. doi: 10.1038/35043127 [DOI] [PubMed] [Google Scholar]
- 30.Flores ER, Tsai KY, Crowley D, Sengupta S, Yang A, McKeon F, Jacks T. p63 and p73 are required for p53-dependent apoptosis in response to DNA damage. Nature.2002;416:560–564. doi: 10.1038/416560a [DOI] [PubMed] [Google Scholar]
- 31.Webster KA, Discher DJ, Kaiser S, Hernandez O, Sato B, Bishopric NH. Hypoxia-activated apoptosis of cardiac myocytes requires reoxygenation or a pH shift and is independent of p53. J Clin Invest. 1999;104:239–252. doi: 10.1172/JCI5871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bialik S, Geenen DL, Sasson IE, Cheng R, Horner JW, Evans SM, Lord EM, Koch CJ, Kitsis RN. Myocyte apoptosis during acute myocardial infarction in the mouse localizes to hypoxic regions but occurs independently of p53. J Clin Invest. 1997;100:1363–1372. doi: 10.1172/JCI119656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vigneron AM, Ludwig RL, Vousden KH. Cytoplasmic ASPP1 inhibits apoptosis through the control of YAP. Genes Dev. 2010;24:2430–2439. doi: 10.1101/gad.1954310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Field SJ, Tsai FY, Kuo F, Zubiaga AM, Kaelin WG, Jr, Livingston DM, Orkin SH, Greenberg ME. E2F-1 functions in mice to promote apoptosis and suppress proliferation. Cell.1996;85:549–561. doi: 10.1016/s0092-8674(00)81255-6 [DOI] [PubMed] [Google Scholar]
- 35.Angelis E, Zhao P, Zhang R, Goldhaber JI, Maclellan WR. The role of E2F-1 and downstream target genes in mediating ischemia/reperfusion injury in vivo. J Mol Cell Cardiol. 2011;51:919–926. doi: 10.1016/j.yjmcc.2011.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang K, An T, Zhou LY, Liu CY, Zhang XJ, Feng C, Li PF. E2F1-regulated miR-30b suppresses Cyclophilin D and protects heart from ischemia/reperfusion injury and necrotic cell death. Cell Death Differ. 2015;22:743–754. doi: 10.1038/cdd.2014.165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hershko T, Chaussepied M, Oren M, Ginsberg D. Novel link between E2F and p53: proapoptotic cofactors of p53 are transcriptionally upregulated by E2F. Cell Death Differ. 2005;12:377–383. doi: 10.1038/sj.cdd.4401575 [DOI] [PubMed] [Google Scholar]
- 38.Fogal V, Kartasheva NN, Trigiante G, Llanos S, Yap D, Vousden KH, Lu X. ASPP1 and ASPP2 are new transcriptional targets of E2F. Cell Death Differ. 2005;12:369–376. doi: 10.1038/sj.cdd.4401562 [DOI] [PubMed] [Google Scholar]
- 39.Puglisi R, Mattia G, Carè A, Marano G, Malorni W, Matarrese P. Non-genomic effects of estrogen on cell homeostasis and remodeling with special focus on cardiac ischemia/reperfusion injury. Front Endocrinol. 2019;10:733. doi: 10.3389/fendo.2019.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang C, Dai D, Xie H, Zhu Z, Hu J, Su M, Liu M, Lu L, Shen W, Ning G, et al. Lgr4 governs a pro-inflammatory program in macrophages to antagonize post-infarction cardiac repair. Circ Res. 2020;127:953–973. doi: 10.1161/CIRCRESAHA.119.315807 [DOI] [PubMed] [Google Scholar]
- 41.Gu S, Tan J, Li Q, Liu S, Ma J, Zheng Y, Liu J, Bi W, Sha P, Li X, et al. Downregulation of LAPTM4B contributes to the impairment of the autophagic flux via unopposed activation of mTORC1 signaling during myocardial ischemia/reperfusion injury. Circ Res. 2020;127:e148–e165. doi: 10.1161/CIRCRESAHA.119.316388 [DOI] [PubMed] [Google Scholar]
- 42.Li X, Luo S, Zhang J, Yuan Y, Jiang W, Zhu H, Ding X, Zhan L, Wu H, Xie Y, et al. lncRNA H19 alleviated myocardial I/RI via suppressing miR-877-3p/Bcl-2-mediated mitochondrial apoptosis. Mol Ther Nucleic Acids. 2019;17:297–309. doi: 10.1016/j.omtn.2019.05.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Detailed experimental methods and Major Resources Table are available in the Supplemental Material. The data that support the findings of this study are available from the corresponding author upon reasonable request.