Key Points
Question
Is there an association between pretreatment obesity and the safety of immune checkpoint inhibitors?
Findings
In this pooled analysis of 3772 patients with advanced cancer, obesity was associated with an increased incidence of any-grade, but not grade 3 or 4, immune-related adverse events compared with normal weight or underweight body mass index among patients receiving nivolumab monotherapy (n = 2746). This differential likelihood was consistent across all subgroups evaluated except for a higher likelihood of grade 3 or 4 immune-related adverse events among female patients with obesity vs normal weight or underweight body mass index.
Meaning
Obesity may be associated with an increased incidence of mild or moderate immune-related adverse events among patients receiving immune checkpoint inhibitors.
Abstract
Importance
Increased survival with immune checkpoint inhibitors has been reported for patients with obesity vs a normal body mass index (BMI). However, the association of obesity with the safety of immune checkpoint inhibitors warrants study.
Objective
To investigate associations between BMI and immune-related adverse events (irAEs) among patients with advanced cancers treated with nivolumab monotherapy and nivolumab plus ipilimumab combination therapy.
Design, Setting, and Participants
This study was a retrospective pooled analysis of 3772 patients from 14 multicenter CheckMate clinical trials across 8 tumor types. Patients with advanced cancers received nivolumab, 3 mg/kg (n = 2746); nivolumab, 3 mg/kg, plus ipilimumab, 1 mg/kg (n = 713); or nivolumab, 1 mg/kg, plus ipilimumab, 3 mg/kg (n = 313). Baseline BMI was categorized as normal weight or underweight (<25), overweight (25 to <30), or obese (≥30) according to World Health Organization criteria. The studies began patient enrollment between February 9, 2012, and May 21, 2015, and patients were followed up to database lock on May 1, 2019. Data analysis was conducted from May 1 to September 1, 2019.
Interventions
Nivolumab, 3 mg/kg; nivolumab, 3 mg/kg, plus ipilimumab, 1 mg/kg; and nivolumab, 1 mg/kg, plus ipilimumab, 3 mg/kg.
Main Outcomes and Measures
Odds ratios (ORs) and 95% CIs for incidence of any-grade and grade 3 or 4 irAEs were calculated for patients with obesity vs normal weight or underweight BMI in the overall cohort and in subgroups based on patient and tumor characteristics. Analyses for nivolumab plus ipilimumab cohorts were exploratory.
Results
A total of 3772 patients were included, 2600 were male (69%), and median age was 61 years (range, 18-90 years). For patients receiving monotherapy with nivolumab, 3 mg/kg (n = 2746), the incidence of any-grade irAEs was higher in patients with obesity (n = 543) vs those with normal weight or underweight BMI (n = 1266; OR, 1.71; 95% CI, 1.38-2.11). Incidence of grade 3 or 4 irAEs did not differ between patients with obesity and those with normal weight or underweight BMI (OR, 1.21; 95% CI, 0.92-1.61). Risk of any-grade and grade 3 or 4 irAEs appeared consistent with that in the overall population across all subgroups evaluated except for a higher likelihood of grade 3 or 4 irAEs among female patients with obesity vs normal weight or underweight BMI (OR, 1.73; 95% CI, 1.07-2.79). For patients receiving nivolumab plus ipilimumab, the incidence of irAEs appeared consistent across BMI categories.
Conclusions and Relevance
Obesity appeared to be associated with an increased incidence of any-grade irAEs among patients treated with nivolumab monotherapy and with grade 3 or 4 irAEs among female patients only. These findings may inform the monitoring of patients at high risk of developing irAEs.
This pooled analysis investigates the association between pretreatment obesity and the safety of immune checkpoint inhibitors in adult patients with advanced cancer.
Introduction
Obesity, defined by a high body mass index (BMI; calculated as weight in kilograms divided by height in meters squared), is an established risk factor for multiple malignancies. Obesity has been associated with poor patient outcomes across multiple tumor types; however, retrospective analyses among patients with metastatic melanoma, non–small cell lung cancer, or renal cell carcinoma treated with immune checkpoint inhibitors (ICIs) suggest that obesity is paradoxically associated with improved survival in this setting. Investigation of the mechanisms behind improved survival with obesity is ongoing, with hypothesized reasons including immunomodulatory effects of adiposity and increased programmed cell death 1–mediated T-cell dysfunction, resulting in greater tumor susceptibility to ICI treatment.
The programmed cell death 1 inhibitor nivolumab and the cytotoxic T-lymphocyte–associated antigen 4 inhibitor ipilimumab have been approved by the US Food and Drug Administration for the treatment of a range of tumor types, both alone and in combination. Nivolumab monotherapy and nivolumab plus ipilimumab were first approved with weight-based dosing, reflecting the regimens used in registrational trials. However, flat dosing of nivolumab monotherapy was approved by the US Food and Drug Administration in September 2016 based on population pharmacokinetic modeling and exposure-response analyses and has replaced weight-based dosing in all adult monotherapy indications in the United States and Europe. Body weight (BW)–based dosing is still used for the nivolumab plus ipilimumab combination and for pediatric patients with microsatellite instability–high or mismatch repair deficient metastatic colorectal cancer and a BW of less than 40 kg. Population pharmacokinetic studies have shown interindividual variability in nivolumab clearance associated with patient characteristics, including BW and sex; thus, there is a strong rationale to investigate the association between these factors and safety. Nivolumab dose is calculated with actual BW, whereas some oncologists calculate cytotoxic chemotherapy dosing with adjusted BW or body surface area.
Retrospective analyses investigating the association between BMI and risk of immune-related adverse events (irAEs) have been heterogeneous, with some showing higher incidence among patients with overweight or obesity vs normal BMI, whereas others have found no significant association between BMI and incidence of irAEs. This analysis builds on previous studies to further investigate the potential association between BMI and safety among patients receiving ICIs by using existing randomized clinical trial data with rigorous, prospectively collected AE grading and reporting.
For a pooled cohort of patients treated with nivolumab monotherapy in 12 CheckMate trials across 8 tumor types, we reported associations between BMI and irAEs and investigated whether they differed by patient and tumor characteristics. We also performed exploratory analyses to examine these associations among patients treated with nivolumab plus ipilimumab.
Methods
Safety data for this pooled analysis were from a total of 3772 patients with advanced cancers treated with weight-based nivolumab monotherapy (n = 2746) or nivolumab plus ipilimumab combination therapy (nivolumab, 3 mg/kg, plus ipilimumab, 1 mg/kg [NIVO3 + IPI1] [n = 713] or nivolumab, 1 mg/kg, plus ipilimumab, 3 mg/kg [NIVO1 + IPI3] [n = 313]) in 14 CheckMate trials across 8 tumor types (ClinicalTrials.gov Identifiers: NCT01472081, NCT01592370, NCT01642004, NCT01658878, NCT01668784, NCT01673867, NCT01721772, NCT01844505, NCT02041533, NCT02060188, NCT02105636, NCT02181738, NCT02231749, and NCT02387996) (eTable 1 in the Supplement). Included studies were the most extensive data sets available with published primary data at the time of analysis. Data from patients receiving NIVO3 were included from 12 trials across 8 tumor types (CheckMate 017, 025, 026, 039, and 040 [all expansion cohorts]; 057, 066, 067, 141, and 142 [all microsatellite instability–high/mismatch repair deficient colorectal cancer per local laboratory; 205 [cohorts A, B, and C]; and 275), data from patients receiving NIVO3 + IPI1 were included from 3 trials across 2 tumor types (CheckMate 016, 142 [all microsatellite instability–high/mismatch repair deficient colorectal cancer], and 214), and data from patients receiving NIVO1 + IPI3 were from 1 trial in 1 tumor type (CheckMate 067) (eTable 1 in the Supplement). Patient enrollment began between February 9, 2012, and May 21, 2015, and patients were followed up to database lock on May 1, 2019. Data analysis was conducted from May 1 to September 1, 2019. Follow-up was defined as the time between randomization date (or first dose date for nonrandomized studies) and last known alive date (for participants who were alive) or death. Instiutional review board approval was not required because the study was a retrospective analysis of data from published clinical trials.
Safety was defined as the incidence of any-grade and grade 3 or 4 irAEs according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events, version 4.0 and the Medical Dictionary for Regulatory Activities, version 21.0 (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) (CheckMate 016, 017, 026, 057, 025, 275, 141, and 205) or version 21.1 (CheckMate 039, 040, 066, 067, 142, and 214) for patients receiving at least 1 dose of nivolumab or nivolumab plus ipilimumab. Immune-related adverse events were defined as specific AEs, including pneumonitis, diarrhea or colitis, hepatitis, nephritis or kidney dysfunction, rash, endocrine AEs, and hypersensitivity, that occurred during the treatment period or within 100 days of the last dose of the study therapy; were considered to be potential irAEs by the investigator; were treated with immune-modulating medication regardless of causality; and had no clear alternative etiology according to investigator assessment. Endocrine AEs, including adrenal insufficiency, hypothyroidism or thyroiditis, hyperthyroidism, diabetes mellitus, and hypophysitis, were considered irAEs regardless of immune-modulating medication because they are often managed without such medications.
Baseline BMI was categorized according to World Health Organization criteria as normal weight or underweight (<25), overweight (25 to <30), or obese (≥30). Because of the small number of patients in the underweight category (n = 160), data were grouped with the normal category; sensitivity analyses showed no differences in safety outcomes between underweight and normal categories. For the CheckMate 016, 025, and 214 trials, the Karnofsky performance status was recalculated to the Eastern Cooperative Oncology Group (ECOG) performance status scale. Cumulative doses of nivolumab and ipilimumab per kilogram of BW from the initiation of study treatment to study drug discontinuation or date of last follow-up were calculated for each BMI category.
Incidence of irAEs was reported by BMI category for each treatment cohort. We focused on the 2 extreme BMI categories and calculated odds ratios (ORs) and 95% CIs for the occurrence of any-grade and grade 3 or 4 irAEs for patients with obesity vs normal weight or underweight BMI. These analyses were descriptive. Breslow-Day testing was conducted to determine data homogeneity. Some variability was identified across the NIVO3 studies, but the data remained homogeneous overall; NIVO3 + IPI1 studies were homogeneous, and NIVO1 + IPI3 comprised a single study, so Breslow-Day testing was not conducted. Formal statistical tests and multivariable analyses were not performed. We further examined the association between BMI category and incidence of irAEs within predefined subgroups (age, ECOG performance status, geographic region, sex, smoking status, and tumor type). Odds ratios were not calculated for subgroups with fewer than 10 patients per treatment group.
Results
A total of 3772 patients were included in the study. There were 2600 men (69%) and 1172 women (31%). Median age was 61 years (range, 18-90 years).
Nivolumab Monotherapy Cohort
The safety analysis included data from 2746 patients treated with NIVO3 (Table). A total of 1881 were male (68%) and 865 were female (32%). Analysis of race was not planned for inclusion in the analysis when it was designed, and these data are not available for the pooled patient population. Patient distribution by BMI category and tumor type in the NIVO3 monotherapy cohort is shown in eFigure 1 in the Supplement. The proportion of patients with obesity was numerically higher in the renal cell carcinoma and melanoma subgroups. Patients received a median of 10 nivolumab doses (range, 1-149 doses), with a higher median number of doses and cumulative dose in patients with overweight and obesity compared with those with normal weight or underweight BMI (eTable 2 in the Supplement).
Table. Patient Demographic and Baseline Characteristics.
| Characteristic | NIVO3 | NIVO3 + IPI1 | NIVO1 + IPI3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All patients (n = 2746)a | BMI category | All patients (n = 713) | BMI category | All patients (n = 313)a | BMI category | |||||||
| <25 (n = 1266)b | 25 to <30 (n = 881) | ≥30 (n = 543) | <25 (n = 232)b | 25 to <30 (n = 277) | ≥30 (n = 200) | <25 (n = 94)b | 25 to <30 (n = 108) | ≥30 (n = 104) | ||||
| BMI, median (range) | 25.3 (14.7-67.5) | 22.2 (14.7-24.9) | 27.0 (25.0-29.9) | 32.9 (30.0-67.5) | 27.0 (15.1-54.9) | 22.5 (15.1-24.9) | 27.3 (25.0-29.9) | 33.3 (30.0-54.9) | 27.7 (12.6-53.4) | 23.0 (12.6-24.9) | 27.5 (25.0-29.8) | 33.2 (30.0-53.4) |
| Sex, No. (%) | ||||||||||||
| Male | 1881 (68.5) | 797 (63.0) | 662 (75.1) | 392 (72.2) | 514 (72.1) | 147 (63.4) | 218 (78.7) | 147 (73.5) | 205 (65.5) | 54 (57.4) | 77 (71.3) | 70 (67.3) |
| Female | 865 (31.5) | 469 (37.0) | 219 (24.9) | 151 (27.8) | 199 (27.9) | 85 (36.6) | 59 (21.3) | 53 (26.5) | 108 (34.5) | 40 (42.6) | 31 (28.7) | 34 (32.7) |
| Age, median (range), y | 61 (18-90) | 60 (18-90) | 62 (19-90) | 61 (19-87) | 61 (21-88) | 59 (21-88) | 61 (35-85) | 61 (30-85) | 61 (18-87) | 58 (18-78) | 62 (22-85) | 61 (31-87) |
| Follow-up, median (range), moc | 17.8 (0.1-72.2) | 14.2 (0.1-84.2) | 19.6 (0.2-83.9) | 22.7 (0.2-85.3) | 35.2 (0.1-63.1) | 49.4 (0.1-74.3) | 49.1 (0.6-74.1) | 47.3 (0.1-73.0) | 55.5 (0.1-68.7) | 37.9 (0.5-85.4) | 66.6 (0.1-85.5) | 66.2 (0.4-86.4) |
| Tumor type, No. (%) | ||||||||||||
| Non–small cell lung cancer | 685 (24.9) | 351 (27.7) | 224 (25.4) | 96 (17.7) | NA | NA | NA | NA | NA | NA | NA | NA |
| Metastatic melanoma | 519 (18.9) | 169 (13.3) | 199 (22.6) | 140 (25.8) | NA | NA | NA | NA | 313 (100) | 94 (100) | 108 (100) | 104 (100) |
| Renal cell carcinoma | 406 (14.8) | 135 (10.7) | 145 (16.5) | 111 (20.4) | 594 (83.3) | 167 (72.0) | 243 (87.7) | 180 (90.0) | NA | NA | NA | NA |
| Hodgkin lymphoma | 342 (12.5) | 164 (13.0) | 95 (10.8) | 79 (14.5) | NA | NA | NA | NA | NA | NA | NA | NA |
| Metastatic urothelial carcinoma | 270 (9.8) | 123 (9.7) | 85 (9.6) | 58 (10.7) | NA | NA | NA | NA | NA | NA | NA | NA |
| Squamous cell carcinoma of the head and neck | 236 (8.6) | 168 (13.3) | 52 (5.9) | 13 (2.4) | NA | NA | NA | NA | NA | NA | NA | NA |
| Hepatocellular carcinoma | 214 (7.8) | 116 (9.2) | 63 (7.2) | 32 (5.9) | NA | NA | NA | NA | NA | NA | NA | NA |
| MSI-H/dMMR metastatic colorectal cancer | 74 (2.7) | 40 (3.2) | 18 (2.0) | 14 (2.6) | 119 (16.7) | 65 (28.0) | 34 (12.3) | 20 (10.0) | NA | NA | NA | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); dMMR, mismatch repair deficient; IPI1, ipilimumab, 1 mg/kg; IPI3, ipilimumab, 3 mg/kg; MSI-H, microsatellite instability–high; NA, not applicable; NIVO1, nivolumab, 1 mg/kg; NIVO3, nivolumab, 3 mg/kg.
Includes 56 patients (2.0%) in the NIVO3 group, 4 (0.6%) in the NIVO3 + IPI1 group, and 7 (2.2%) in the NIVO1 + IPI3 group with no reported BMI.
Includes 146 patients (11.5%) in the NIVO3 group, 9 (1.3%) in the NIVO3 + IPI1 group, and 5 (1.6%) in the NIVO1 + IPI3 group with an underweight BMI (<18.5).
For randomized studies, follow-up was defined as the time between randomization date and last known alive date (for patients who were alive) or death. For nonrandomized studies, follow-up was defined as the time between first dose of the study drug and last known alive date (for patients who were alive) or death.
Patients with obesity (n = 543) and overweight (n = 881) who were treated with NIVO3 had a higher incidence of any-grade and grade 3 or 4 irAEs vs those with normal weight or underweight BMI (n = 1266). The incidences of irAEs for patients with obesity vs overweight vs normal weight or underweight BMI were 70.0% (95% CI, 65.9%-73.8%), 63.6% (95% CI, 60.3%-66.7%), and 57.7% (95% CI, 55.0%-60.5%), respectively, for any grade and were 15.8% (95% CI, 12.9%-19.2%), 14.9% (95% CI, 12.6%-17.4%), and 13.4% (95% CI, 11.6%-15.4%), respectively, for grade 3 or 4 (eFigure 2 in the Supplement).
Odds ratios for any-grade and grade 3 or 4 irAEs for patients with obesity vs normal weight or underweight BMI in the overall NIVO3 cohort and across various patient subgroups are shown in Figure 1. The likelihood of any-grade irAEs was higher among patients with obesity vs normal weight or underweight BMI (OR, 1.71; 95% CI, 1.38-2.11), which was consistent across subgroups of age, sex, smoking status, and ECOG performance status. The likelihood of grade 3 or 4 irAEs was not higher among patients with obesity vs normal weight or underweight BMI (OR, 1.21; 95% CI, 0.92-1.61); this finding was consistent across all subgroups except for a higher likelihood of grade 3 or 4 irAEs among female patients with obesity vs normal weight or underweight BMI (OR, 1.73; 95% CI, 1.07-2.79). Across geographic region subgroups, ORs for any-grade and grade 3 or 4 irAEs remained consistent with that of the overall population except for a higher likelihood of grade 3 or 4 irAEs among patients from Europe with obesity vs normal weight or underweight BMI (OR, 1.56; 95% CI, 1.01-2.40). Similarly, ORs for any-grade and grade 3 or 4 irAEs among patients with obesity vs normal weight or underweight BMI were consistent across tumor type subgroups.
Figure 1. Odds Ratios (ORs) for the Occurrence of Immune-Related Adverse Events (irAEs) for Patients With Obesity vs Normal Weight or Underweight Body Mass Index Who Received Nivolumab, 3 mg/kg.
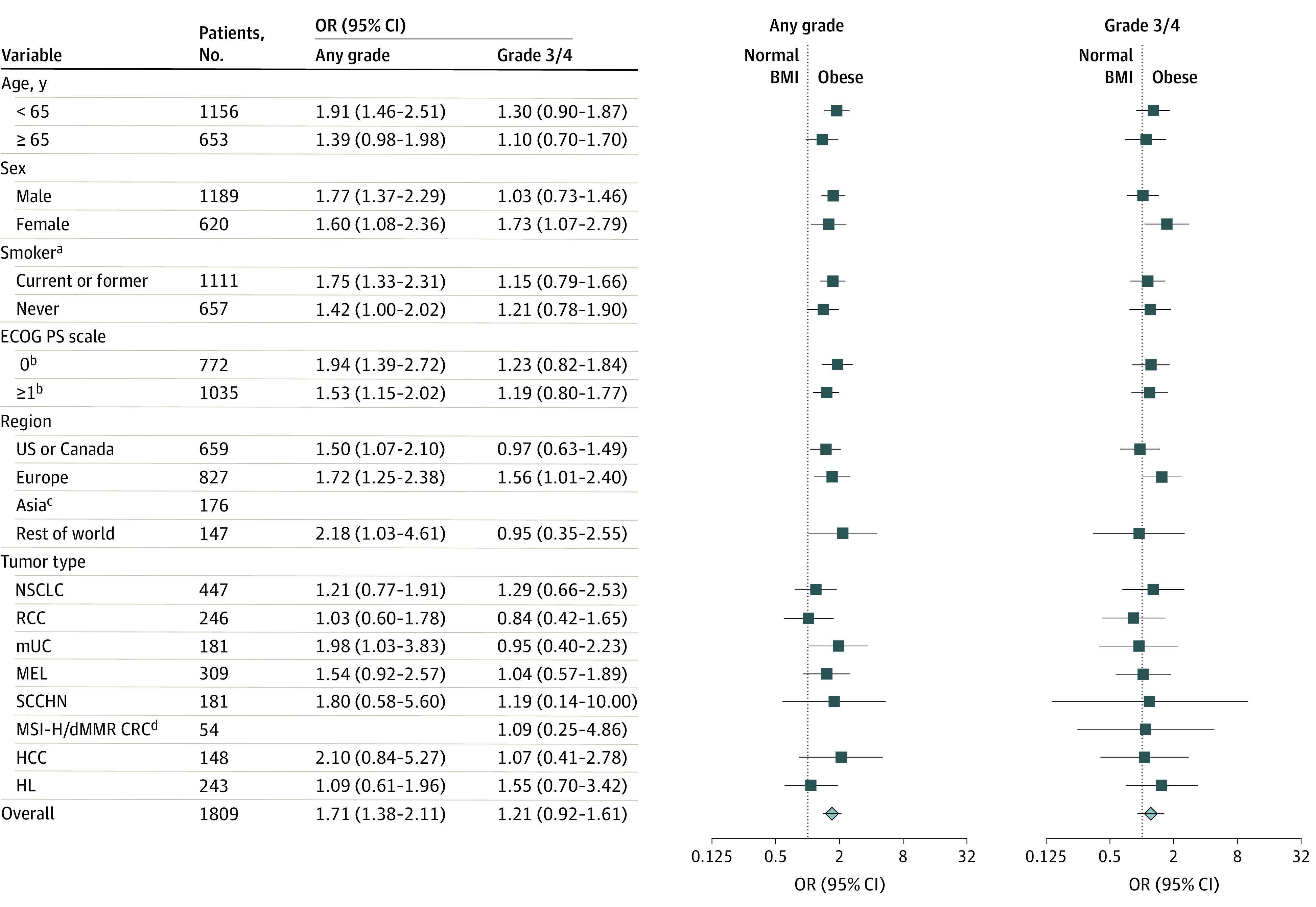
Symbols indicate the point estimates of the ORs, and horizontal lines indicate the 95% CIs. BMI indicates body mass index; CRC, colorectal cancer; dMMR, mismatch repair deficient; ECOG PS, Eastern Cooperative Oncology Group performance status; HCC, hepatocellular carcinoma; HL, Hodgkin lymphoma; MEL, melanoma; MSI-H, microsatellite instability–high; mUC, metastatic urothelial carcinoma; NSCLC, non–small cell lung cancer; RCC, renal cell carcinoma; and SCCHN, squamous cell carcinoma of the head and neck.
aForty-one patients had unknown smoking status.
bBaseline ECOG PS was not available for 2 patients.
cOdds ratios for occurrence of any-grade and grade 3 or 4 irAEs were not calculated for 176 patients in the Asia subgroup because fewer than 10 patients were categorized as having obesity. Asia was not included in the “rest of world” analysis.
dAn OR could not be calculated for occurrence of any-grade irAEs in the CRC subgroup because all patients categorized as having obesity had at least 1 any-grade irAE.
Higher incidence of some individual any-grade irAEs among male and female patients appeared to be associated with increasing BMI, mirroring the trend for overall incidence of irAEs (eFigure 3 in the Supplement). Evaluation of the frequency of individual occurrences of any-grade irAEs by irAE and BMI categories showed that the increasing frequency of diarrhea or colitis, dermatitis or immune-related skin reactions, and nephritis or kidney disorders appeared to be associated with increasing BMI in the overall NIVO3 cohort (eFigure 4 in the Supplement).
Nivolumab and Ipilimumab Combination Therapy Cohorts
The safety analysis included 713 patients treated with NIVO3 + IPI1 and 313 patients treated with NIVO1 + IPI3. Most patients who received combination therapy were male (Table). Median age was 61 years (range, 21-88 years) for the NIVO3 + IPI1 cohort and 61 years (range, 18-87 years) for the NIVO1 + IPI3 cohort. Patients received a median of 16 nivolumab doses (range, 1-131 doses) in the NIVO3 + IPI1 group, 4 nivolumab doses (range, 1-137 doses) in the NIVO1 + IPI3 group, and a median of 4 ipilimumab doses (range, 1-4 doses) in both the NIVO3 + IPI1 and NIVO1 + IPI3 groups. The cumulative dose of both nivolumab and ipilimumab was similar for patients with obesity vs normal weight or underweight BMI (eTable 2 in the Supplement).
For the NIVO3 + IPI1 cohort, exploratory analyses showed that an upward trend in any-grade irAE incidence was associated with increasing BMI, from 82.3% (95% CI, 76.8%-87.0%) for patients with normal weight or underweight BMI to 85.2% (95% CI, 80.5%-89.2%) for those with overweight and 88.0% (95% CI, 82.7%-92.2%) for those with obesity, with no trend observed for grade 3 or 4 irAEs (eFigure 5 in the Supplement; Figure 2), similar to the NIVO3 cohort. The likelihood of irAEs in the NIVO1 + IPI3 cohort did not increase with increasing BMI (eFigures 6 and 7 in the Supplement). Although the female subgroup sample size was small, there was a numerically higher incidence of grade 3 or 4 irAEs among female patients with overweight vs normal weight or obesity who received NIVO3 + IPI1 and an association of increased likelihood of grade 3 or 4 irAEs with increasing BMI among female patients who received NIVO1 + IPI3. No clear trends were observed in the incidence of individual any-grade irAEs and frequency of individual irAEs as presented in eFigures 8, 9, 10, and 11 in the Supplement.
Figure 2. Odds Ratios (ORs) for the Occurrence of Immune-Related Adverse Events for Patients With Obesity vs Normal Weight or Underweight Body Mass Index Who Received Nivolumab, 3 mg/kg, Plus Ipilimumab, 1 mg/kg.
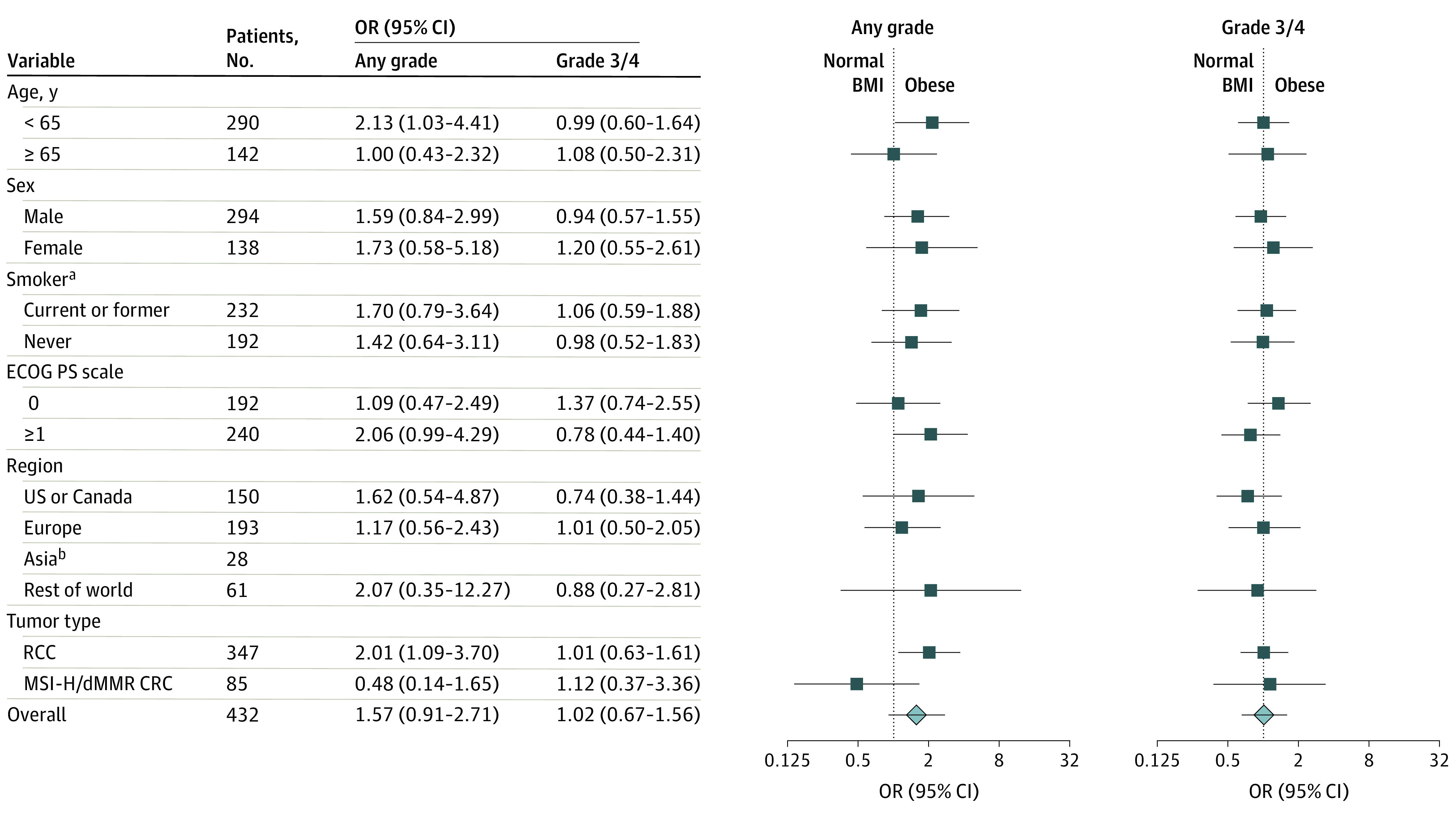
Symbols indicate the point estimates of the ORs, and horizontal lines indicate the 95% CIs. BMI indicates body mass index; CRC, colorectal cancer; dMMR, mismatch repair deficient; ECOG PS, Eastern Cooperative Oncology Group performance status; MSI-H, microsatellite instability–high; and RCC, renal cell carcinoma.
aSmoking status was unknown for 8 patients.
bOdds ratios for occurrence of any-grade and grade 3 or 4 immune-related adverse events were not calculated for 28 patients in the Asia subgroup because fewer than 10 patients were categorized as having obesity. Asia was not included in the “rest of world” analysis.
Discussion
Obesity, a risk factor for developing many tumor types, has paradoxically been associated with improved outcomes for patients receiving ICIs. Studies on the BMI-safety association using clinical trial data have shown heterogeneous results, with some studies indicating no association and others suggesting an increased risk. Similarly, meta-analyses have demonstrated heterogeneous results. Our study analyzed primary trial data in which irAEs were uniformly defined across the trials and included 2746 patients receiving nivolumab monotherapy and 1026 patients treated with combination therapy. We focused our analysis on the most extreme BMI categories of obese vs normal weight or underweight BMI because BMI is less likely to misclassify adiposity in these categories than in patients with overweight BMI. In our primary analysis of NIVO3 monotherapy, a higher incidence of any-grade irAEs, but not grade 3 or 4 irAEs, was observed among patients with obesity vs normal weight or underweight BMI. Although a formal comparison of irAE likelihood was not conducted for the groups with overweight vs normal weight or underweight BMI, we observed a trend for increasing incidence of any-grade irAEs with increasing BMI category, supporting a biological association between BMI and irAEs.
Results of subgroup analyses of the NIVO3 monotherapy group, including age, sex, ECOG performance status, smoking status, geographic region, and tumor type, were generally consistent with that of the overall population in that there was an association between increasing incidence of any-grade irAEs in patients with obesity vs normal weight or underweight BMI. There was no increase in the likelihood of grade 3 or 4 irAEs, consistent with the overall population, except for higher incidence observed among female patients with obesity. However, given the post hoc design of this analysis, these results should be interpreted with caution. Limitations when multiple subgroup analyses are performed are well known, and the probability of a false-positive finding can be substantial.
A systematic review found that higher BMI was associated with increased incidence of irAEs among patients receiving ipilimumab monotherapy or nivolumab plus ipilimumab. Our current analysis of patients treated with this combination indicated that the incidence of irAEs trended upward with BMI in the NIVO3 + IPI1 cohort but not in the NIVO1 + IPI3 cohort. Further evaluation of BMI and safety associations among patients receiving combination therapy is needed to draw more definitive conclusions. It is also possible that any increase in the overall incidence of irAEs attributable to BMI was masked by the high overall incidence of irAEs in the combination group.
In contrast to the steep dose-response relationship with chemotherapy, ICIs have a wide therapeutic window, with BW having a minimal association with clinical efficacy and pharmacokinetics. Although pharmacokinetic modeling identified BW as contributing to interindividual variability in nivolumab pharmacokinetics, steady-state exposure was comparable across evaluated BWs (34.1-168.2 kg), suggesting that the effect of BW was unlikely to be clinically relevant. Subsequent exposure-response modeling studies provided further evidence that nivolumab exposure is not a significant predictor for AEs, suggesting a relatively flat exposure-response relationship. In addition, many cytotoxic agents are associated with cumulative toxicities after repeated dosing, which has not been observed among patients receiving ICIs. These factors suggest that the association between BMI and irAEs in this study may not have been due to differences in ICI exposure.
The potential mechanisms through which obesity affects outcomes with ICI therapies are only partially understood. Studies to date have largely focused on improvements in ICI efficacy for patients with obesity vs normal BMI, whereas few studies have investigated the associations between obesity and safety. Obesity may be associated with response to ICI through increased chronic inflammation, increased programmed cell death 1 expression on CD8+ T cells, decreased T-cell function, and increased frequency of exhausted T cells. A study of patients with renal cell carcinoma also theorized that peritumoral fat may serve as a reservoir of activated immune cells that are mobilized after ICI administration. It is unclear whether some or all of the suggested mechanisms involved in the increased effectiveness of ICIs in patients with obesity may contribute to increased incidence of irAEs. Results from a number of studies indicate a possible association between irAEs and improved ICI efficacy, with suggested mechanisms including immune responses against antigens common to both tumor cells and healthy tissue or off-target immune responses resulting from preexisting inflammation in healthy tissue. Male-female differences in immune function have also been identified and are attributed to various factors, including diet, sex hormones, and other physiologic differences, which could explain our observation of increased incidence of grade 3 or 4 irAEs associated with obesity in female patients. Alterations to the gut microbiome have been associated with obesity, response to ICIs, and irAE occurrence. Because potential roles of the microbiome in these areas have been only newly discovered and are rapidly evolving, further studies to determine clinical relevance are warranted.
Limitations
This analysis is subject to several limitations because of its retrospective pooled-data design, the use of descriptive univariable analyses, and the heterogeneity of the included studies in terms of study designs, tumor types, treatment settings, and patient demographic and clinical characteristics. However, homogeneity testing showed that the overall populations were homogeneous, and associations between BMI and incidence of irAEs were similar across all subgroups except for increased incidence of grade 3 or 4 irAEs with obesity among female patients. The irAE incidence data used for this analysis were limited to prospectively collected data for specific irAEs included in the case report form used during the conduct of each study, as described in the Methods. Because knowledge of ICI-related irAEs has continued to evolve since these studies were designed, data were not available for some irAEs that were identified more recently. Furthermore, because increased median follow-up duration and cumulative nivolumab exposure were associated with increased BMI, our analysis is subject to immortal time bias. Investigation of associations between BMI-associated exposure and effectiveness outcomes using this pooled data set was not possible because of the heterogeneous tumor types, lines of therapy, and patient populations. The finding of an apparent association between obesity and an increased incidence of mild but not severe irAEs has potential clinical relevance. Additional studies are needed to confirm this result and further elucidate the associated mechanisms, especially because this finding could be related to statistical power given that grade 3 or 4 events account for only a small proportion of irAEs. The association between BMI and ICI efficacy remains an area of interest, with retrospective studies in specific tumor types identifying possible relationships. Finally, the value of BMI as a surrogate measure of body composition is subject to considerable interindividual differences in body composition and fat distribution.
Conclusions
In this pooled analysis of patients treated with weight-based nivolumab monotherapy or combination therapy across 14 CheckMate trials and 8 tumor types, an increased incidence of any-grade irAEs, but not grade 3 or 4 irAEs, was observed for patients with obesity vs normal weight or underweight BMI who were treated with NIVO3 monotherapy. The likelihood of any-grade irAEs among patients with obesity vs normal weight or underweight BMI who received NIVO3 appeared consistent with that of the overall population across subgroups according to patient demographic characteristics, baseline characteristics, and tumor type. Similarly, the likelihood of grade 3 or 4 irAEs across all evaluated subgroups appeared consistent with that of the overall population except for a higher likelihood of grade 3 or 4 irAEs among female patients with obesity vs normal weight or underweight BMI. The results of this analysis highlight the importance of BMI as a clinical covariate for patients receiving ICIs and support further evaluation in prospective studies of associations between body composition and patient outcomes during ICI treatment.
eTable 1. Included Clinical Trials
eTable 2. Cumulative Dose of Nivolumab by BMI
eFigure 1. Distribution of Patients Into BMI Categories by Tumor Type in the NIVO3 Cohort
eFigure 2. Incidence of irAEs by Sex and BMI in the NIVO3 Cohort
eFigure 3. Incidence of Any-Grade (Top) and Grade 3 or 4 (Bottom) irAEs in (A) Male and (B) Female Patients in the NIVO3 Cohort
eFigure 4. Frequency of Individual irAEs of Any Grade by BMI in the Overall NIVO3 Cohort
eFigure 5. Incidence of irAEs by Sex and BMI in the NIVO3 + IPI1 Cohort
eFigure 6. Incidence of Individual Any-Grade (Top) and Grade 3 or 4 irAEs (Bottom) by BMI in (A) Male and (B) Female Patients in the NIVO3 + IPI1 Cohort
eFigure 7. Frequency of Individual Any-Grade irAEs by BMI in the NIVO3 + IPI1 Cohort
eFigure 8. Incidence of irAEs by Sex and BMI in the NIVO1 + IPI3 Cohort
eFigure 9. ORs for irAE Occurrence in Obese vs Normal or Underweight Patients in Subgroups Based on Patient Characteristics in the NIVO1 + IPI3 Cohort
eFigure 10. Incidence of Individual Any-Grade (Top) and Grade 3 or 4 irAEs (Bottom) by BMI in (A) Male and (B) Female Patients in the NIVO1 + IPI3 Cohort
eFigure 11. Frequency of Individual Any-Grade irAEs by BMI in the NIVO1 + IPI3 Cohort
eReferences.
References
- 1.Arnold M, Pandeya N, Byrnes G, et al. Global burden of cancer attributable to high body-mass index in 2012: a population-based study. Lancet Oncol. 2015;16(1):36-46. doi: 10.1016/S1470-2045(14)71123-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ng M, Fleming T, Robinson M, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384(9945):766-781. doi: 10.1016/S0140-6736(14)60460-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McQuade JL, Daniel CR, Hess KR, et al. Association of body-mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: a retrospective, multicohort analysis. Lancet Oncol. 2018;19(3):310-322. doi: 10.1016/S1470-2045(18)30078-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Cao D, He A, Ge W. The prognostic role of obesity is independent of sex in cancer patients treated with immune checkpoint inhibitors: a pooled analysis of 4090 cancer patients. Int Immunopharmacol. 2019;74:105745. doi: 10.1016/j.intimp.2019.105745 [DOI] [PubMed] [Google Scholar]
- 5.Wang Z, Aguilar EG, Luna JI, et al. Paradoxical effects of obesity on T cell function during tumor progression and PD-1 checkpoint blockade. Nat Med. 2019;25(1):141-151. doi: 10.1038/s41591-018-0221-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cortellini A, Bersanelli M, Buti S, et al. A multicenter study of body mass index in cancer patients treated with anti-PD-1/PD-L1 immune checkpoint inhibitors: when overweight becomes favorable. J Immunother Cancer. 2019;7(1):57. doi: 10.1186/s40425-019-0527-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kichenadasse G, Miners JO, Mangoni AA, Rowland A, Hopkins AM, Sorich MJ. Association between body mass index and overall survival with immune checkpoint inhibitor therapy for advanced non–small cell lung cancer. JAMA Oncol. 2020;6(4):512-518. doi: 10.1001/jamaoncol.2019.5241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Labadie BW, Liu P, Bao R, et al. BMI, irAE, and gene expression signatures associate with resistance to immune-checkpoint inhibition and outcomes in renal cell carcinoma. J Transl Med. 2019;17(1):386. doi: 10.1186/s12967-019-02144-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An Y, Wu Z, Wang N, et al. Association between body mass index and survival outcomes for cancer patients treated with immune checkpoint inhibitors: a systematic review and meta-analysis. J Transl Med. 2020;18(1):235. doi: 10.1186/s12967-020-02404-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Giorgi U, Procopio G, Giannarelli D, et al. Association of systemic inflammation index and body mass index with survival in patients with renal cell cancer treated with nivolumab. Clin Cancer Res. 2019;25(13):3839-3846. doi: 10.1158/1078-0432.CCR-18-3661 [DOI] [PubMed] [Google Scholar]
- 11.Richtig G, Hoeller C, Wolf M, et al. Body mass index may predict the response to ipilimumab in metastatic melanoma: an observational multi-centre study. PLoS One. 2018;13(10):e0204729. doi: 10.1371/journal.pone.0204729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H, Wang D, Zhong Q, Tao Y, Zhou Y, Shi Y. Pretreatment body mass index and clinical outcomes in cancer patients following immune checkpoint inhibitors: a systematic review and meta-analysis. Cancer Immunol Immunother. 2020;69(12):2413-2424. doi: 10.1007/s00262-020-02680-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Opdivo. Package insert. Bristol Myers Squibb ; 2014.
- 14.Yervoy. Package insert. Bristol Myers Squibb ; 2011.
- 15.US Food and Drug Administration . Modification of the dosage regimen for nivolumab. Accessed July 20, 2020. https://www.fda.gov/drugs/resources-information-approved-drugs/modification-dosage-regimen-nivolumab
- 16.Bajaj G, Wang X, Agrawal S, Gupta M, Roy A, Feng Y. Model-based population pharmacokinetic analysis of nivolumab in patients with solid tumors. CPT Pharmacometrics Syst Pharmacol. 2017;6(1):58-66. doi: 10.1002/psp4.12143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sanghavi K, Zhang J, Zhao X, et al. Population pharmacokinetics of ipilimumab in combination with nivolumab in patients with advanced solid tumors. CPT Pharmacometrics Syst Pharmacol. 2020;9(1):29-39. doi: 10.1002/psp4.12477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Sanghavi K, Shen J, et al. Population pharmacokinetics of nivolumab in combination with ipilimumab in patients with advanced malignancies. CPT Pharmacometrics Syst Pharmacol. 2019;8(12):962-970. doi: 10.1002/psp4.12476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Griggs JJ, Mangu PB, Anderson H, et al. ; American Society of Clinical Oncology . Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553-1561. doi: 10.1200/JCO.2011.39.9436 [DOI] [PubMed] [Google Scholar]
- 20.Cortellini A, Bersanelli M, Santini D, et al. Another side of the association between body mass index (BMI) and clinical outcomes of cancer patients receiving programmed cell death protein-1 (PD-1)/programmed cell death-ligand 1 (PD-L1) checkpoint inhibitors: a multicentre analysis of immune-related adverse events. Eur J Cancer. 2020;128:17-26. doi: 10.1016/j.ejca.2019.12.031 [DOI] [PubMed] [Google Scholar]
- 21.Guzman-Prado Y, Ben Shimol J, Samson O. Body mass index and immune-related adverse events in patients on immune checkpoint inhibitor therapies: a systematic review and meta-analysis. Cancer Immunol Immunother. 2021;70(1):89-100. doi: 10.1007/s00262-020-02663-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gülave B, Hew MN, de Groot JS, Rodwell L, Teerenstra S, Fabriek BO. High body mass index and pre-existing autoimmune disease are associated with an increased risk of immune-related adverse events in cancer patients treated with PD-(L)1 inhibitors across different solid tumors. ESMO Open. 2021;6(3):100107. doi: 10.1016/j.esmoop.2021.100107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability–high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182-1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2017;18(3):312-322. doi: 10.1016/S1470-2045(17)30065-7 [DOI] [PubMed] [Google Scholar]
- 25.Robert C, Long GV, Brady B, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320-330. doi: 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 26.Larkin J, Chiarion-Sileni V, Gonzalez R, et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373(1):23-34. doi: 10.1056/NEJMoa1504030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375(19):1856-1867. doi: 10.1056/NEJMoa1602252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non–small-cell lung cancer. N Engl J Med. 2015;373(17):1627-1639. doi: 10.1056/NEJMoa1507643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med. 2015;372(4):311-319. doi: 10.1056/NEJMoa1411087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carbone DP, Reck M, Paz-Ares L, et al. ; CheckMate 026 Investigators . First-line nivolumab in stage IV or recurrent non–small-cell lung cancer. N Engl J Med. 2017;376(25):2415-2426. doi: 10.1056/NEJMoa1613493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Khoueiry AB, Sangro B, Yau T, et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet. 2017;389(10088):2492-2502. doi: 10.1016/S0140-6736(17)31046-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Younes A, Santoro A, Shipp M, et al. Nivolumab for classical Hodgkin’s lymphoma after failure of both autologous stem-cell transplantation and brentuximab vedotin: a multicentre, multicohort, single-arm phase 2 trial. Lancet Oncol. 2016;17(9):1283-1294. doi: 10.1016/S1470-2045(16)30167-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non–small-cell lung cancer. N Engl J Med. 2015;373(2):123-135. doi: 10.1056/NEJMoa1504627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Motzer RJ, Escudier B, McDermott DF, et al. ; CheckMate 025 Investigators . Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803-1813. doi: 10.1056/NEJMoa1510665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hammers HJ, Plimack ER, Infante JR, et al. Safety and efficacy of nivolumab in combination with ipilimumab in metastatic renal cell carcinoma: the CheckMate 016 study. J Clin Oncol. 2017;35(34):3851-3858. doi: 10.1200/JCO.2016.72.1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Motzer RJ, Tannir NM, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277-1290. doi: 10.1056/NEJMoa1712126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Overman MJ, Lonardi S, Wong KYM, et al. Durable clinical benefit with nivolumab plus ipilimumab in DNA mismatch repair-deficient/microsatellite instability–high metastatic colorectal cancer. J Clin Oncol. 2018;36(8):773-779. doi: 10.1200/JCO.2017.76.9901 [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization . A healthy lifestyle—WHO recommendations. Accessed April 17, 2020. http://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi
- 39.Islami F, Goding Sauer A, Gapstur SM, Jemal A. Proportion of cancer cases attributable to excess body weight by US state, 2011-2015. JAMA Oncol. 2019;5(3):384-392. doi: 10.1001/jamaoncol.2018.5639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Caan BJ, Meyerhardt JA, Kroenke CH, et al. Explaining the obesity paradox: the association between body composition and colorectal cancer survival (C-SCANS Study). Cancer Epidemiol Biomarkers Prev. 2017;26(7):1008-1015. doi: 10.1158/1055-9965.EPI-17-0200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lagakos SW. The challenge of subgroup analyses—reporting without distorting. N Engl J Med. 2006;354(16):1667-1669. doi: 10.1056/NEJMp068070 [DOI] [PubMed] [Google Scholar]
- 42.Lyman GH. Weight-based chemotherapy dosing in obese patients with cancer: back to the future. J Oncol Pract. 2012;8(4):e62-e64. doi: 10.1200/JOP.2012.000606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hendrikx JJMA, Haanen JBAG, Voest EE, Schellens JHM, Huitema ADR, Beijnen JH. Fixed dosing of monoclonal antibodies in oncology. Oncologist. 2017;22(10):1212-1221. doi: 10.1634/theoncologist.2017-0167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Y, Wang X, Bajaj G, et al. Nivolumab exposure-response analyses of efficacy and safety in previously treated squamous or nonsquamous non–small cell lung cancer. Clin Cancer Res. 2017;23(18):5394-5405. doi: 10.1158/1078-0432.CCR-16-2842 [DOI] [PubMed] [Google Scholar]
- 45.Zhao X, Shen J, Ivaturi V, et al. Model-based evaluation of the efficacy and safety of nivolumab once every 4 weeks across multiple tumor types. Ann Oncol. 2020;31(2):302-309. doi: 10.1016/j.annonc.2019.10.015 [DOI] [PubMed] [Google Scholar]
- 46.Velasco R, Bruna J. Taxane-induced peripheral neurotoxicity. Toxics. 2015;3(2):152-169. doi: 10.3390/toxics3020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caglar K, Kinalp C, Arpaci F, et al. Cumulative prior dose of cisplatin as a cause of the nephrotoxicity of high-dose chemotherapy followed by autologous stem-cell transplantation. Nephrol Dial Transplant. 2002;17(11):1931-1935. doi: 10.1093/ndt/17.11.1931 [DOI] [PubMed] [Google Scholar]
- 48.McGowan JV, Chung R, Maulik A, Piotrowska I, Walker JM, Yellon DM. Anthracycline chemotherapy and cardiotoxicity. Cardiovasc Drugs Ther. 2017;31(1):63-75. doi: 10.1007/s10557-016-6711-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Motzer RJ, Escudier B, George S, et al. Nivolumab versus everolimus in patients with advanced renal cell carcinoma: updated results with long-term follow-up of the randomized, open-label, phase 3 CheckMate 025 trial. Cancer. 2020;126(18):4156-4167. doi: 10.1002/cncr.33033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Motzer RJ, Rini BI, McDermott DF, et al. ; CheckMate 214 Investigators . Nivolumab plus ipilimumab versus sunitinib in first-line treatment for advanced renal cell carcinoma: extended follow-up of efficacy and safety results from a randomised, controlled, phase 3 trial. Lancet Oncol. 2019;20(10):1370-1385. doi: 10.1016/S1470-2045(19)30413-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Duma N, Abdel-Ghani A, Yadav S, et al. Sex differences in tolerability to anti-programmed cell death protein 1 therapy in patients with metastatic melanoma and non–small cell lung cancer: are we all equal? Oncologist. 2019;24(11):e1148-e1155. doi: 10.1634/theoncologist.2019-0094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woodall MJ, Neumann S, Campbell K, Pattison ST, Young SL. The effects of obesity on anti-cancer immunity and cancer immunotherapy. Cancers (Basel). 2020;12(5):1230. doi: 10.3390/cancers12051230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez A, Furberg H, Kuo F, et al. Transcriptomic signatures related to the obesity paradox in patients with clear cell renal cell carcinoma: a cohort study. Lancet Oncol. 2020;21(2):283-293. doi: 10.1016/S1470-2045(19)30797-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Das S, Johnson DB. Immune-related adverse events and anti-tumor efficacy of immune checkpoint inhibitors. J Immunother Cancer. 2019;7(1):306. doi: 10.1186/s40425-019-0805-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim YS, Unno T, Kim BY, Park MS. Sex differences in gut microbiota. World J Mens Health. 2020;38(1):48-60. doi: 10.5534/wjmh.190009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol. 2016;16(10):626-638. doi: 10.1038/nri.2016.90 [DOI] [PubMed] [Google Scholar]
- 57.Crovesy L, Masterson D, Rosado EL. Profile of the gut microbiota of adults with obesity: a systematic review. Eur J Clin Nutr. 2020;74(9):1251-1262. doi: 10.1038/s41430-020-0607-6 [DOI] [PubMed] [Google Scholar]
- 58.Zheng Y, Wang T, Tu X, et al. Gut microbiome affects the response to anti–PD-1 immunotherapy in patients with hepatocellular carcinoma. J Immunother Cancer. 2019;7(1):193. doi: 10.1186/s40425-019-0650-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andrews MC, Duong CPM, Gopalakrishnan V, et al. Gut microbiota signatures are associated with toxicity to combined CTLA-4 and PD-1 blockade. Nat Med. 2021;27(8):1432-1441. doi: 10.1038/s41591-021-01406-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Buss J. Limitations of body mass index to assess body fat. Workplace Health Saf. 2014;62(6):264. doi: 10.1177/216507991406200608 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Included Clinical Trials
eTable 2. Cumulative Dose of Nivolumab by BMI
eFigure 1. Distribution of Patients Into BMI Categories by Tumor Type in the NIVO3 Cohort
eFigure 2. Incidence of irAEs by Sex and BMI in the NIVO3 Cohort
eFigure 3. Incidence of Any-Grade (Top) and Grade 3 or 4 (Bottom) irAEs in (A) Male and (B) Female Patients in the NIVO3 Cohort
eFigure 4. Frequency of Individual irAEs of Any Grade by BMI in the Overall NIVO3 Cohort
eFigure 5. Incidence of irAEs by Sex and BMI in the NIVO3 + IPI1 Cohort
eFigure 6. Incidence of Individual Any-Grade (Top) and Grade 3 or 4 irAEs (Bottom) by BMI in (A) Male and (B) Female Patients in the NIVO3 + IPI1 Cohort
eFigure 7. Frequency of Individual Any-Grade irAEs by BMI in the NIVO3 + IPI1 Cohort
eFigure 8. Incidence of irAEs by Sex and BMI in the NIVO1 + IPI3 Cohort
eFigure 9. ORs for irAE Occurrence in Obese vs Normal or Underweight Patients in Subgroups Based on Patient Characteristics in the NIVO1 + IPI3 Cohort
eFigure 10. Incidence of Individual Any-Grade (Top) and Grade 3 or 4 irAEs (Bottom) by BMI in (A) Male and (B) Female Patients in the NIVO1 + IPI3 Cohort
eFigure 11. Frequency of Individual Any-Grade irAEs by BMI in the NIVO1 + IPI3 Cohort
eReferences.


