This cohort study assesses the association of adherence to 4 healthy eating scores with total and cause-specific mortality among women from the Nurses’ Health Study and men from the Health Professionals Follow-Up Study.
Key Points
Question
Is there an association between Dietary Guidelines for Americans–recommended dietary patterns with total and cause-specific mortality?
Findings
In this cohort study of 75 230 women from the Nurses’ Health Study (1984-2020) and 44 085 men from the Health Professionals Follow-Up Study (1986-2020), greater adherence to several healthy eating patterns was associated with a lower risk of death. These associations were consistent in different racial and ethnic groups, including Hispanic, non-Hispanic Black, and non-Hispanic White individuals.
Meaning
These findings support the recommendations of Dietary Guidelines for Americans that multiple healthy eating patterns can be adapted to individual food traditions and preferences.
Abstract
Importance
The current Dietary Guidelines for Americans recommend multiple healthy eating patterns. However, few studies have examined the associations of adherence to different dietary patterns with long-term risk of total and cause-specific mortality.
Objective
To examine the associations of dietary scores for 4 healthy eating patterns with risk of total and cause-specific mortality.
Design, Setting, and Participants
This prospective cohort study included initially healthy women from the Nurses’ Health Study (NHS; 1984-2020) and men from the Health Professionals Follow-up Study (HPFS; 1986-2020).
Exposures
Healthy Eating Index 2015 (HEI-2015), Alternate Mediterranean Diet (AMED) score, Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI).
Main Outcomes and Measures
The main outcomes were total and cause-specific mortality overall and stratified by race and ethnicity and other potential risk factors.
Results
The final study sample included 75 230 women from the NHS (mean [SD] baseline age, 50.2 [7.2] years) and 44 085 men from the HPFS (mean [SD] baseline age, 53.3 [9.6] years). During a total of 3 559 056 person-years of follow-up, 31 263 women and 22 900 men died. When comparing the highest with the lowest quintiles, the pooled multivariable-adjusted HRs of total mortality were 0.81 (95% CI, 0.79-0.84) for HEI-2015, 0.82 (95% CI, 0.79-0.84) for AMED score, 0.86 (95% CI, 0.83-0.89) for HPDI, and 0.80 (95% CI, 0.77-0.82) for AHEI (P < .001 for trend for all). All dietary scores were significantly inversely associated with death from cardiovascular disease, cancer, and respiratory disease. The AMED score and AHEI were inversely associated with mortality from neurodegenerative disease. The inverse associations between these scores and risk of mortality were consistent in different racial and ethnic groups, including Hispanic, non-Hispanic Black, and non-Hispanic White individuals.
Conclusions and Relevance
In this cohort study of 2 large prospective cohorts with up to 36 years of follow-up, greater adherence to various healthy eating patterns was consistently associated with lower risk of total and cause-specific mortality. These findings support the recommendations of Dietary Guidelines for Americans that multiple healthy eating patterns can be adapted to individual food traditions and preferences.
Introduction
Diet remains a cornerstone for maintaining optimal health. According to the Global Burden of Disease Study 2017, unhealthy diet is estimated as one of the leading causes of death globally. The supportive evidence was largely based on nutrition research focusing on single nutrients or foods in relation to total and cause-specific mortality. However, humans do not consume isolated nutrients or single foods but rather a wide variety of foods with combinations of nutrients and phytochemicals that may have additive and synergistic effects. By accounting for potentially interactive and cumulative effects among different dietary components, overall dietary patterns have been emphasized as a crucial approach when investigating the association between human diet and health outcomes.
Following the evolution in nutritional sciences, the Dietary Guidelines for Americans (DGAs) shifted their focus from individual nutrients to healthy eating patterns in 2010 and have recommended various healthy eating patterns. These recommendations were carried forward to the 2020 to 2025 edition and further highlighted across the lifespan, from birth through older adulthood. However, few long-term prospective studies with repeated dietary measurements have systematically examined whether adherence to various dietary patterns renders similar associations with total and cause-specific mortality. Additionally, these recommended eating patterns were intended for all US individuals, but previous evidence indicated notable differences within dietary patterns by individuals’ characteristics.
Therefore, using 2 large prospective cohorts with data on repeated measures of dietary habits, we derived dietary scores for 4 healthy dietary patterns, including the Healthy Eating Index 2015 (HEI-2015), Alternate Mediterranean Diet (AMED) score, Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI). We then examined their associations with total and cause-specific mortality. We also specifically examined these associations stratified by race and ethnicity and other potential risk factors.
Methods
Study Population
This cohort study used data from the Nurses’ Health Study (NHS) and the Health Professionals Follow-up Study (HPFS). The NHS is a prospective cohort study of 121 700 female registered nurses aged 30 to 55 years from 11 US states that began in 1976. The HPFS is a prospective cohort study of 51 529 male health professionals aged 40 to 75 years at baseline that began in 1986. In both cohorts, information about medical history, lifestyle, and health conditions was collected by self-administered questionnaires at baseline and every 2 years thereafter. Detailed information on the cohorts is described elsewhere. The study protocol was approved by the institutional review boards of the Brigham and Women’s Hospital, Harvard T.H. Chan School of Public Health, and participating registries as required. The return of completed questionnaires was considered to imply informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
For the present analysis, baseline was defined as the year of the first validated food-frequency questionnaire providing enough information to derive the dietary indices in each study—1984 for the NHS and 1986 for the HPFS. We excluded participants who reported cardiovascular disease (CVD), cancer, or diabetes at baseline to reduce the probability of reverse causation because the diagnoses of these conditions might have led to changes in diet. We also excluded participants who did not provide information on diet and those who had daily energy intakes less than 600 kcal or greater than 3500 kcal for women and less than 800 kcal or greater than 4200 kcal for men.
Assessment of Dietary Scores
Dietary information was collected with the use of a validated semiquantitative food frequency questionnaire (FFQ) with over 130 items administered every 2 to 4 years. The reproducibility and validity of the FFQs have been described in detail elsewhere, showing good correlation between nutrients assessed by the FFQs and multiple weeks of food records or biomarkers of diet. Using the food and nutrient components, we calculated the HEI-2015, AMED score, HPDI, and AHEI to measure adherence to the Healthy US-Style Eating Pattern, Alternate Mediterranean Eating Pattern, Healthy Plant-based Eating Pattern, and Alternate Healthy Eating Pattern. The components and scoring criteria for each dietary score are described in detail in eTables 1-4 and eMethods in Supplement 1. Briefly, the HEI-2015 includes 13 components and ranges from 0 to 100, with higher scores indicating higher adherence to DGAs. The AMED score includes 9 components and ranges from 9 to 45, with higher scores indicating a healthier Mediterranean diet. The HPDI includes 18 components and ranges from 18 to 90, with higher scores indicating a healthier plant-based diet. The AHEI, based on DGAs with modification to include factors related to chronic disease risk, includes 10 components and ranges from 0 to 100, with higher scores indicating a healthier diet. All dietary scores have shown moderate to high reproducibility and validity in both men and women.
Ascertainment of Deaths
Deaths were identified from state vital statistics records and the National Death Index or were reported by the participants’ families and the US postal system. Using these methods, 98% of the deaths in each cohort were ascertained. We attempted to obtain the death certificate of each participant who had died and, when appropriate, requested permission from the participant’s next of kin to review medical records. The classification of the cause of death was mostly based on review of medical records. A physician reviewed medical records and death certificates to classify the cause of death according to the International Classification of Diseases, Revision 8, and International Classification of Diseases, Ninth Revision (ICD-9). Deaths were grouped into deaths from CVD (ICD-9: 390-459), cancer (ICD-9: 140-208), neurodegenerative disease (ICD-9: 290, 332, 335, 340, 342, and 348), respiratory disease (ICD-9: 460-519), and all other causes.
Ascertainment of Covariates
Participants reported their race and ethnicity in NHS according to categories (Hispanic, non-Hispanic Black, non-Hispanic White, and other [American Indian, Asian, Hawaiian, and multiracial]) provided by the investigators. The HPFS does not have information on ethnicity; thus, stratified analysis by race and ethnicity was not conducted in this cohort. Every 2 years, participants returned a mailed validated questionnaire that obtained updated information on their lifestyle and other risk factors, including age, body weight, smoking status, physical activity, aspirin use, multivitamin use, menopausal status and postmenopausal hormone use in women, and physician diagnosis of chronic diseases. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared.
Statistical Analysis
To best represent long-term intake and dampen within-person variation in the NHS and HPFS cohorts, we calculated cumulative mean dietary scores up to the start of each 2-year follow-up interval. Person-years were calculated from the date of return of the baseline questionnaire to the date of death or the end of follow-up (June 2020 for both NHS and HPFS), whichever occurred first. We did not censor participants lost to active follow-up because fatal events were included in the outcomes. We stopped updating dietary variables after report of incident diabetes, CVD, or cancer because changes in diet after the development of these conditions may confound the estimates. We used Cox proportional hazards regression models with age as the underlying time scale, with stratification by calendar time, to assess the association between 4 dietary scores and the subsequent risk of total and cause-specific mortality. The proportional-hazards assumption was evaluated with a likelihood-ratio test comparing the model with and without an interaction term between age and dietary scores. In multivariable analysis, we adjusted for age, race and ethnicity, family history of CVD, family history of cancer, and time-dependent confounders, including marital status, living alone or with others, postmenopausal status and hormone use (NHS), smoking status, physical activity, alcohol intake (not for AMED), multivitamin use, aspirin use, total energy intake, and BMI. Tests for linear trends across quintiles were conducted by assigning a median value to each quintile of dietary score, producing a single ordinal variable used in the model. We also used the restricted cubic spline analysis to flexibly model the association between 4 dietary scores and total mortality. Additionally, a 25-percentile difference in each score (25 points for HEI-2015, 9 points for AMED score, 18 points for HPDI, and 25 points for AHEI) was calculated from the range of total dietary scores. Separate analyses were conducted for cause-specific mortality per a 25-percentile difference in each score. All analyses were performed separately for each cohort and then were pooled with the use of fixed-effects meta-analysis with inverse-variance weighting, and the heterogeneity was assessed with the I2 statistic.
We conducted stratified analyses defined a priori by race and ethnicity (NHS) and other potential risk modifiers. The interactions between each of the 4 dietary scores and covariates were examined using the likelihood ratio test. The interactions should be interpreted as exploratory because these findings might be due to chance from multiple testing. We performed several sensitivity analyses to test the robustness of our results. First, we conducted lagged analyses by excluding the first 4 years of follow-up data and adding a 4-year lag period between assessment of dietary intake and each follow-up period to address concern that chronic disease occurrence may influence dietary behavior. Second, to test whether our results were biased by selectively stopping updating diet after an intermediate outcome, we continuously updated diet until the end of follow-up. Third, we analyzed the associations of baseline dietary scores with total mortality. Fourth, given the potential heterogeneity between 2 cohorts, the results were pooled using random effects. Fifth, we applied a competing risk regression model for cause-specific mortality by including dietary scores as exposure and other risk factors as unconstrained covariates, allowing the effects of the covariates to vary across cause-specific mortality. All analyses were performed with the SAS, version 9.4 (SAS Institute). All statistical tests were 2 sided, and P < .05 was considered to indicate statistical significance.
Results
The final study sample included 75 230 women from the NHS (mean [SD] baseline age, 50.2 [7.2] years) and 44 085 men from the HPFS (mean [SD] baseline age, 53.3 [9.6] years). Table 1 shows age and the age-adjusted characteristics of study participants at baseline according to quintiles of the 4 dietary scores. In both cohorts, participants with higher dietary scores tended to be older, less likely to smoke, and more likely to exercise and to have a lower BMI (Table 1). In the NHS at baseline, 597 (1.1%) were Hispanic, 834 participants (0.8%) were non-Hispanic Black, 70 564 (98.0%) were non-Hispanic White, and 3235 (4.3%) were other race and ethnicity. In the HPFS at baseline, 412 participants (0.9%) were Black, 40 099 (91.0%) were White, and 3574 (8.1%) were other race and ethnicity. The correlations between 4 dietary scores ranged from moderately low to moderately high (r = 0.39-0.75).
Table 1. Baseline Characteristics of Participants According to Quintiles of the HEI-2015, AMED Score, HPDI, and AHEI.
| Characteristic | Participantsa | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HEI-2015 | AMED score | HPDI | AHEI | |||||||||
| Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | Quintile 1 | Quintile 3 | Quintile 5 | |
| Nurses’ Health Study (1984) | ||||||||||||
| Participants, No | 15 336 | 15 063 | 14 648 | 16 177 | 14 957 | 13 658 | 16 346 | 15 737 | 15 823 | 15 274 | 15 023 | 14 797 |
| Dietary score, mean (SD) | 51.9 (5.0) | 66.0 (1.4) | 78.5 (3.9) | 19.6 (2.2) | 27.0 (0.8) | 35.4 (2.2) | 44.6 (3.2) | 54.5 (1.1) | 64.6 (3.3) | 29.9 (3.5) | 42.6 (1.5) | 58.4 (5.7) |
| Age, mean (SD), y | 48.2 (7.0) | 50.0 (7.1) | 52.8 (6.8) | 48.9 (7.1) | 50.2 (7.1) | 51.8 (7.0) | 48.2 (7.0) | 50.3 (7.1) | 52.3 (6.8) | 48.5 (7.1) | 50.3 (7.1) | 52.1 (6.8) |
| Race and ethnicity | ||||||||||||
| Hispanic | 90 (0.6) | 133 (0.9) | 125 (0.9) | 123 (0.8) | 112 (0.7) | 120 (0.9) | 76 (0.5) | 135 (0.8) | 168 (1.1) | 78 (0.5) | 115 (0.8) | 161 (1.1) |
| Non-Hispanic Black | 155 (1.0) | 175 (1.2) | 193 (1.3) | 161 (1.0) | 164 (1.1) | 160 (1.1) | 158 (1.0) | 167 (1.1) | 205 (1.3) | 115 (0.8) | 171 (1.1) | 235 (1.6) |
| Non-Hispanic White | 14 419 (94.0) | 14 285 (93.6) | 13 679 (93.4) | 15 171 (93.8) | 14 072 (94.1) | 12 752 (93.4) | 15 487 (94.7) | 14 708 (93.5) | 14 701 (92.9) | 14 515 (95.0) | 14 116 (94.0) | 13 620 (92.0) |
| Otherb | 673 (4.4) | 651 (4.3) | 651 (4.4) | 722 (4.4) | 609 (4.1) | 625 (4.6) | 625 (3.8) | 728 (4.6) | 749 (4.7) | 566 (3.7) | 621 (4.1) | 781 (5.3) |
| Body mass index, mean (SD)c | 25.2 (5.2) | 25.1 (4.6) | 24.3 (4.1) | 25.1 (5.0) | 25.0 (4.6) | 24.6 (4.3) | 25.4 (5.1) | 24.9 (4.6) | 24.3 (4.1) | 25.1 (5.0) | 25.1 (4.7) | 24.5 (4.2) |
| Physical activity, mean (SD), MET-h/w | 9.43 (16.1) | 12.9 (18.0) | 17.3 (24.1) | 9.54 (15.2) | 12.7 (19.1) | 17.8 (23.9) | 10.5 (16.0) | 12.7 (19.2) | 16.6 (23.9) | 9.37 (14.0) | 12.8 (19.3) | 18.0 (25.7) |
| Never smoker | 6179 (40.3) | 6858 (45.5) | 6655 (45.4) | 6587 (40.7) | 6648 (44.4) | 6222 (45.6) | 7438 (45.5) | 7059 (44.9) | 6554 (41.4) | 6708 (43.9) | 6668 (44.4) | 6309 (42.6) |
| Never drinker | 5592 (36.5) | 4390 (29.1) | 4261 (29.1) | 6915 (42.7) | 4422 (29.6) | 2760 (20.2) | 5111 (31.3) | 4778 (30.4) | 4839 (30.6) | 5302 (34.7) | 4450 (29.6) | 4383 (29.6) |
| Premenopausal | 6308 (41.1) | 6335 (42.1) | 5961 (40.7) | 6666 (41.2) | 6208 (41.5) | 5642 (41.3) | 6987 (42.8) | 6502 (41.3) | 6357 (40.2) | 6437 (42.1) | 6261 (41.7) | 6043 (40.8) |
| Married | 9950 (64.9) | 10 495 (69.7) | 10 265 (70.1) | 10 481 (64.8) | 10 402 (69.6) | 9721 (71.2) | 11 060 (67.7) | 10 845 (68.9) | 11 023 (69.7) | 10 498 (68.7) | 10 314 (68.6) | 10 130 (68.5) |
| Live alone | 1502 (9.8) | 1424 (9.4) | 1534 (10.5) | 1695 (10.5) | 1434 (9.6) | 1334 (9.8) | 1585 (9.7) | 1503 (9.6) | 1657 (10.5) | 1307 (8.6) | 1435 (9.6) | 1678 (11.3) |
| Total calorie intake, mean (SD), kcal/d | 2016 (568) | 1736 (497) | 1491 (427) | 1524 (489) | 1727 (506) | 2019 (526) | 2077 (505) | 1716 (497) | 1458 (439) | 1932 (492) | 1732 (536) | 1581 (504) |
| Multivitamin supplement use | 4440 (29.0) | 5620 (37.3) | 6717 (45.9) | 4924 (30.4) | 5377 (36.0) | 6171 (45.2) | 5286 (32.3) | 5853 (37.2) | 6668 (42.1) | 4687 (30.7) | 5433 (36.2) | 6676 (45.1) |
| Aspirin use | 10 923 (71.2) | 10 846 (72.0) | 9990 (68.2) | 11 321 (70.0) | 10 675 (71.4) | 9708 (71.1) | 11 897 (72.8) | 11 230 (71.4) | 10 860 (68.6) | 11 092 (72.6) | 10 766 (71.2) | 10 091 (68.2) |
| Family history | ||||||||||||
| Diabetes | 4397 (28.7) | 4426 (29.4) | 4001 (27.3) | 4707 (29.1) | 4253 (28.4) | 3850 (28.2) | 4666 (28.5) | 4490 (28.5) | 4510 (28.5) | 4407 (28.8) | 4398 (29.3) | 4197 (28.4) |
| Myocardial infarction | 3891 (25.4) | 3718 (24.7) | 3717 (25.4) | 4110 (25.4) | 3764 (25.2) | 3564 (26.1) | 3966 (24.3) | 3897 (24.8) | 4147 (26.2) | 3816 (25.0) | 3802 (25.3) | 3835 (25.9) |
| Cancer | 2075 (13.5) | 2254 (15.0) | 2094 (14.3) | 2169 (13.4) | 2128 (14.2) | 2017 (14.8) | 2340 (14.3) | 2312 (14.7) | 2271 (14.4) | 2180 (14.3) | 2126 (14.2) | 2152 (14.5) |
| Past diagnoses | ||||||||||||
| Hypertension | 2967 (19.4) | 3048 (20.2) | 2929 (20.0) | 3396 (21.0) | 3061 (20.5) | 2661 (19.5) | 3461 (21.2) | 3137 (19.9) | 3040 (19.2) | 3177 (20.8) | 3155 (21.0) | 2903 (19.6) |
| High cholesterol level | 228 (1.5) | 257 (1.7) | 381 (2.6) | 264 (1.6) | 290 (1.9) | 338 (2.5) | 247 (1.5) | 297 (1.9) | 394 (2.5) | 230 (1.5) | 306 (2.0) | 349 (2.4) |
| Health Professionals Follow-up Study (1986) | ||||||||||||
| Participants, No. | 9116 | 8842 | 8411 | 9714 | 10816 | 9005 | 9540 | 9063 | 7949 | 9121 | 8858 | 8460 |
| Dietary score, mean (SD) | 53.6 (5.3) | 69.0 (1.6) | 82.7 (3.7) | 19.3 (2.4) | 27.5 (1.1) | 35.7 (2.5) | 44.5 (3.2) | 54.5(1.1) | 65.6 (3.3) | 32.2 (4.2) | 46.7 (1.6) | 62.7 (5.2) |
| Age, mean (SD), y | 51.6 (9.3) | 53.5 (9.7) | 55.2 (9.6) | 51.7 (9.4) | 53.5 (9.6) | 54.8 (9.6) | 51.0 (9.3) | 53.4 (9.6) | 55.6 (9.4) | 51.4 (9.3) | 53.4 (9.7) | 55.3 (9.5) |
| Whited | 8265 (90.7) | 8054 (91.1) | 7692 (91.5) | 8824 (90.8) | 6875 (90.4) | 8261 (91.7) | 8795 (92.2) | 8204 (90.5) | 7200 (90.6) | 8397 (92.1) | 8019 (90.5) | 7714 (91.2) |
| Body mass index, mean (SD)c | 25.8 (3.5) | 25.7 (3.4) | 24.8 (3.0) | 25.8 (3.4) | 25.5 (3.2) | 25.0 (3.3) | 25.6 (3.4) | 25.6 (3.3) | 25.1 (3.2) | 25.8 (3.4) | 25.6 (3.3) | 25.0 (3.2) |
| Physical activity, mean (SD), MET-h/w | 15.9 (22.2) | 20.6 (24.2) | 27.2 (29.0) | 15.1 (20.8) | 20.4 (23.8) | 28.1 (28.8) | 17.9 (22.7) | 20.0 (23.7) | 25.8 (28.5) | 15.5 (21.0) | 20.3 (23.6) | 27.3 (28.7) |
| Never smoker | 4054 (44.5) | 4394 (49.7) | 4639 (55.2) | 4454 (45.9) | 5404 (50.0) | 4733 (52.6) | 4865 (51.0) | 4514 (49.8) | 3793 (47.7) | 4220 (46.3) | 4500 (50.8) | 4401 (52.0) |
| Never drinker | 2440 (26.7) | 1892 (21.4) | 1918 (22.8) | 3131 (32.2) | 2355 (21.8) | 1336 (14.8) | 2197 (23.0) | 2039 (22.5) | 1903 (23.9) | 2266 (24.8) | 1987 (22.4) | 1875 (22.2) |
| Married | 8060 (88.4) | 8077 (91.3) | 7585 (90.2) | 8601 (88.5) | 9795 (90.6) | 8195 (91.0) | 8544 (89.6) | 8197 (90.5) | 7201 (90.6) | 8197 (89.9) | 8005 (90.4) | 7658 (90.5) |
| Live alone | 643 (7.1) | 472 (5.3) | 530 (6.3) | 696 (7.2) | 625 (5.8) | 497 (5.5) | 621 (6.5) | 533 (5.9) | 480 (6.0) | 577 (6.3) | 531 (6.0) | 476 (5.6) |
| Total calorie intake, mean (SD), kcal/d | 2283 (678) | 1975 (580) | 1724 (498) | 1768 (569) | 1980 (602) | 2265 (618) | 2351 (616) | 1944 (580) | 1703 (517) | 2120 (595) | 1979 (636) | 1893 (589) |
| Multivitamin supplement use | 3151 (34.6) | 3577 (40.5) | 4357 (51.8) | 3434 (35.4) | 4501 (41.6) | 4526 (50.3) | 3432 (36.0) | 3770 (41.6) | 3848 (48.4) | 3230 (35.4) | 3673 (41.5) | 4215 (49.8) |
| Aspirin use | 2515 (27.6) | 2436 (27.6) | 2295 (27.3) | 2501 (25.7) | 3096 (28.6) | 2641 (29.3) | 2637 (27.6) | 2489 (27.5) | 2295 (28.9) | 2537 (27.8) | 2410 (27.2) | 2304 (27.2) |
| Family history | ||||||||||||
| Diabetes | 1808 (19.8) | 1787 (20.2) | 1700 (20.2) | 1996 (20.6) | 2153 (19.9) | 1863 (20.7) | 1933 (20.3) | 1885 (20.8) | 1674 (21.1) | 1766 (19.4) | 1828 (20.6) | 1778 (21.0) |
| Myocardial infarction | 2740 (30.1) | 2937 (33.2) | 2954 (35.1) | 2932 (30.2) | 3534 (32.7) | 3122 (34.7) | 2925 (30.7) | 2935 (32.4) | 2821 (35.5) | 2764 (30.3) | 2887 (32.6) | 2999(35.5) |
| Cancer | 3076 (33.8) | 3052 (34.5) | 2910 (34.6) | 3209 (33.0) | 3748 (34.7) | 3141 (34.9) | 3357 (35.2) | 3069 (33.9) | 2671 (33.6) | 3084 (33.8) | 3040 (34.3) | 2925 (34.6) |
| Past diagnoses | ||||||||||||
| Hypertension | 1856 (20.4) | 2058 (23.3) | 2078 (24.7) | 2177 (22.4) | 2482 (23.0) | 2084 (23.1) | 1969 (20.6) | 2093 (23.1) | 1967 (24.8) | 1895 (20.8) | 2084 (23.5) | 2047 (24.2) |
| High cholesterol level | 754 (8.3) | 907 (10.3) | 1348 (16.0) | 823 (8.5) | 1124 (10.4) | 1383 (15.4) | 757 (7.9) | 931 (10.3) | 1234 (15.5) | 750 (8.2) | 961 (10.9) | 1234 (14.6) |
Abbreviations: AHEI, Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015; HPDI, Healthful Plant-based Diet Index; MET, metabolic equivalent.
Data are presented as number (percentage) of participants unless otherwise indicated. All variables except age are age-standardized.
Includes American Indian, Asian, Hawaiian, and multiracial.
Calculated as weight in kilograms divided by height in meters squared.
No other detailed racial and ethnic group information was available in the Health Professionals Follow-up Study.
In the NHS, during up to 36 years of follow-up (2 343 144 person-years), we documented 31 263 deaths, including 6128 deaths from CVD and 8733 deaths from cancer; in the HPFS during 34 years of follow-up (1 215 912 person-years), we documented 22 900 deaths, including 6641 deaths from CVD and 5710 deaths from cancer. After adjustment for potential confounders, when comparing the highest with the lowest quintiles, the pooled HRs of all-cause mortality were 0.81 (95% CI, 0.79-0.84) for HEI-2015, 0.82 (95% CI, 0.79-0.84) for AMED score, 0.86 (95% CI, 0.83-0.89) for HPDI, and 0.80 (95% CI, 0.77-0.82) for AHEI (P < .001 for trend for all) (Table 2 and eFigure 1 in Supplement 1).
Table 2. Hazard Ratios of Death From Any Cause According to Quintiles of the HEI-2015, AMED score, HPDI, and AHEI.
| Quintile 1 | Quintile 2 | Quintile 3 | Quintile 4 | Quintile 5 | P value for trend | |
|---|---|---|---|---|---|---|
| HEI-2015 | ||||||
| NHS | ||||||
| Score, median (IQR) | 56 (52-59) | 64 (62-65) | 69 (67-70) | 73 (72-74) | 79 (77-82) | NA |
| Cases/PYs | 6291/439 732 | 6100/462 519 | 6195/475 859 | 6197/484 161 | 6480/480 873 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.80 (0.77-0.83) | 0.72 (0.70-0.75) | 0.65 (0.63-0.67) | 0.57 (0.55-0.59) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.89 (0.86-0.93) | 0.87 (0.84-0.90) | 0.82 (0.79-0.85) | 0.76 (0.73-0.79) | <.001 |
| HPFS | ||||||
| Score, median (IQR) | 57 (53-60) | 65 (63-66) | 70 (69-72) | 75 (74-77) | 82 (80-85) | NA |
| Cases/PYs | 4560/237 489 | 4532/245 889 | 4566/246 767 | 4553/249 794 | 4689/235 973 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.85 (0.82-0.89) | 0.78 (0.75-0.82) | 0.72 (0.69-0.75) | 0.68 (0.66-0.71) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.97 (0.93-1.01) | 0.94 (0.90-0.98) | 0.89 (0.85-0.93) | 0.90 (0.86-0.94) | <.001 |
| Pooled | ||||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.82 (0.80-0.84) | 0.75 (0.73-0.77) | 0.68 (0.66-0.70) | 0.62 (0.60-0.63) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.92 (0.90-0.95) | 0.90 (0.87-0.92) | 0.85 (0.83-0.87) | 0.81 (0.79-0.84) | <.001 |
| AMED score | ||||||
| NHS | ||||||
| Score, median (IQR) | 20 (19-22) | 24 (23-25) | 27 (26-28) | 30 (29-31) | 34 (32-35) | NA |
| Cases/PYs | 6373/443 032 | 6660/477 671 | 6373/468 492 | 6078/492 732 | 5779/461 217 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.83 (0.80-0.86) | 0.76 (0.73-0.78) | 0.69 (0.66-0.71) | 0.65 (0.63-0.67) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.90 (0.87-0.94) | 0.86 (0.83-0.89) | 0.80 (0.77-0.83) | 0.77 (0.74-0.81) | <.001 |
| HPFS | ||||||
| Score, median (IQR) | 20 (18-22) | 24 (23-25) | 27 (27-28) | 31 (30-31) | 35 (33-37) | NA |
| Cases/PYs | 4434/243 269 | 4629/237 941 | 4725/258 396 | 4595/239 235 | 4517/237 070 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.89 (0.85-0.92) | 0.81 (0.78-0.84) | 0.76 (0.73-0.79) | 0.72 (0.69-0.75) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.97 (0.93-1.01) | 0.92 (0.88-0.96) | 0.88 (0.85-0.91) | 0.87 (0.84-0.90) | <.001 |
| Pooled | ||||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.85 (0.83-0.87) | 0.78 (0.76-0.80) | 0.72 (0.70-0.74) | 0.68 (0.66-0.70) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.93 (0.90-0.95) | 0.89 (0.86-0.91) | 0.83 (0.81-0.86) | 0.82 (0.79-0.84) | <.001 |
| HPDI | ||||||
| NHS | ||||||
| Score, median (IQR) | 46 (44-48) | 51 (50-52) | 55 (54-56) | 58 (57-59) | 63 (62-66) | NA |
| Cases/PYs | 5889/459 000 | 6298/480 456 | 6384/484 214 | 6535/468 647 | 6157/450 827 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.89 (0.86-0.92) | 0.82 (0.79-0.85) | 0.80 (0.77-0.83) | 0.72 (0.70-0.75) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.93 (0.90-0.97) | 0.88 (0.85-0.92) | 0.87 (0.84-0.90) | 0.80 (0.77-0.83) | <.001 |
| HPFS | ||||||
| Score, median (IQR) | 46 (44-48) | 51 (50-52) | 55 (54-56) | 59 (58-60) | 64 (62-67) | NA |
| Cases/PYs | 4316/251 063 | 4658/253 138 | 4716/245 702 | 4757/247 849 | 4453/218 159 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.95 (0.91-0.99) | 0.89 (0.85-0.93) | 0.85 (0.82-0.89) | 0.84 (0.80-0.87) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 1.00 (0.96-1.04) | 0.97 (0.93-1.01) | 0.96 (0.92,1.00) | 0.95 (0.91-0.99) | .006 |
| Pooled | ||||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.91 (0.89-0.94) | 0.85 (0.83-0.87) | 0.82 (0.80-0.84) | 0.77 (0.75-0.79) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.96 (0.93-0.99) | 0.92 (0.89-0.95) | 0.91 (0.88-0.93) | 0.86 (0.83-0.89) | <.001 |
| AHEI | ||||||
| NHS | ||||||
| Score, median (IQR) | 34 (31-36) | 41 (39-42) | 46 (44-47) | 51 (49-52) | 59 (56-62) | NA |
| Cases/PYs | 6285/443 704 | 6533/477 209 | 6512/482 114 | 6240/481 477 | 5693/458 639 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.83 (0.80-0.86) | 0.75 (0.73-0.78) | 0.68 (0.66-0.71) | 0.61 (0.59-0.63) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.90 (0.87-0.94) | 0.86 (0.83-0.89) | 0.81 (0.79-0.84) | 0.75 (0.72-0.77) | <.001 |
| HPFS | ||||||
| Score, median (IQR) | 35 (32-37) | 42 (41-44) | 48 (46-49) | 53 (52-55) | 61 (59-65) | NA |
| Cases/PYs | 4443/239 791 | 4710/246 148 | 4617/250 070 | 4576/245 755 | 4554/234 148 | NA |
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.88 (0.85-0.92) | 0.79 (0.76-0.83) | 0.73 (0.70-0.76) | 0.69 (0.67-0.72) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.98 (0.94-1.02) | 0.91 (0.87-0.95) | 0.88 (0.84-0.92) | 0.87 (0.83-0.91) | <.001 |
| Pooled | ||||||
| Age-adjusted HR (95% CI) | 1 [Reference] | 0.85 (0.83-0.87) | 0.77 (0.75-0.79)b | 0.70 (0.68-0.72) | 0.64 (0.63-0.66) | <.001 |
| Multivariable adjusted HR (95% CI)a | 1 [Reference] | 0.93 (0.91-0.96) | 0.88 (0.86-0.91) | 0.84 (0.82-0.86) | 0.80 (0.77-0.82) | <.001 |
Abbreviations: AHEI, Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015; HPDI, Healthful Plant-based Diet Index; HPFS, Health Professionals Follow-Up Study; HR, hazard ratio; NA, not applicable; NHS, Nurses’ Health Study; PY, person-years.
Multivariable analysis was adjusted for age, calendar year, race and ethnicity (NHS: Hispanic, non-Hispanic Black, non-Hispanic White, or other; HPFS, Black, White, or other), marriage status (married; divorced, separated, or single; or widowed), living status (alone or not alone), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), family history of cancer (yes or no), menopausal status (pre- or postmenopausal [never, past, or current menopausal hormone use]; NHS only), multivitamin use (yes or no), aspirin use (yes or no), total energy intake (quintile), smoking status (never, former, or current smoker [1-14, 15-24, or ≥25 cigarettes/d), alcohol drinking (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), physical activity (quintile), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), and body mass index (<21, 21-24.9, 25-29.9, 30-34.9, or ≥35 [calculated as weight in kilograms divided by height in meters squared]).
Represents I2 less than 75%.
In multivariate analyses, the 4 dietary scores were inversely associated with the risk of most major causes of death. In the pooled analysis of women and men, significant inverse associations were observed between 4 dietary scores and deaths due to CVD (HEI-2015: HR, 0.87 [95% CI, 0.83-0.92]; AMED score: HR, 0.94 [95% CI, 0.91-0.97]; HPDI: HR, 0.94 [95% CI, 0.89-0.99]; and AHEI: HR, 0.88 [95% CI, 0.84-0.92]), cancer (HEI-2015: HR, 0.82 [95% CI, 0.78-0.86]; AMED score: HR, 0.93 [95% CI, 0.90-0.96]; HPDI: HR, 0.90 [95% CI, 0.86-0.95]; and AHEI: HR, 0.84 [95% CI, 0.81-0.88]), and respiratory disease (HEI-2015: HR, 0.54 [95% CI, 0.49-0.59]; AMED score: HR, 0.65 [95% CI, 0.61-0.69]; HPDI: HR, 0.63 [95% CI, 0.58-0.70]; and AHEI: HR, 0.56 [95% CI, 0.52-0.61]) (Table 3 and eTables 5-8 in Supplement 1). The AMED score and AHEI were associated with lower risk of mortality caused by neurodegenerative disease. For deaths from the top 3 cancers among men and women, consistent inverse associations were observed between the 4 dietary scores and death from lung cancer in both men and women (eTable 9 in Supplement 1).
Table 3. Hazard Ratios for Death From Specific Causes According to 4 Healthy Eating Scoresa.
| Cause of death | Cases, No. | Hazard ratio (95% CI) | |||
|---|---|---|---|---|---|
| HEI-2015 | AMED score | HPDI | AHEI | ||
| NHS | |||||
| Cardiovascular disease | 6128 | 0.77 (0.72-0.83) | 0.86 (0.82-0.91) | 0.85 (0.78-0.92) | 0.77 (0.71-0.83) |
| Heart disease | 4330 | 0.74 (0.68-0.81) | 0.85 (0.80-0.90) | 0.81 (0.74-0.89) | 0.74 (0.68-0.81) |
| Stroke | 1798 | 0.85 (0.74-0.98) | 0.90 (0.82-0.99) | 0.94 (0.81-1.09) | 0.85 (0.74-0.97) |
| Cancer | 8733 | 0.82 (0.76-0.87) | 0.95 (0.91-0.99) | 0.91 (0.85-0.98) | 0.83 (0.78-0.88) |
| Respiratory disease | 2491 | 0.44 (0.39-0.49) | 0.57 (0.52-0.61) | 0.50 (0.44-0.57) | 0.45 (0.40-0.51) |
| Neurodegenerative disease | 5004 | 1.04 (0.96-1.13) | 0.96 (0.90-1.01) | 0.96 (0.88-1.05) | 0.94 (0.87-1.01) |
| HPFS | |||||
| Cardiovascular disease | 6641 | 0.97 (0.90-1.04) | 1.00 (0.96-1.05) | 1.02 (0.95-1.10) | 0.97 (0.91-1.04) |
| Heart disease | 5386 | 0.94 (0.87-1.02) | 1.00 (0.95-1.05) | 0.99 (0.92-1.08) | 0.94 (0.87-1.01) |
| Stroke | 1255 | 1.11 (0.94-1.31) | 1.02 (0.92-1.13) | 1.13 (0.96-1.33) | 1.12 (0.97-1.29) |
| Cancer | 5710 | 0.82 (0.76-0.89) | 0.91 (0.87-0.96) | 0.89 (0.82-0.96) | 0.86 (0.81-0.92) |
| Respiratory disease | 1738 | 0.71 (0.62-0.81) | 0.76 (0.70-0.83) | 0.84 (0.73-0.96) | 0.72 (0.63-0.81) |
| Neurodegenerative disease | 2101 | 0.98 (0.86-1.11) | 0.92 (0.85-0.99) | 0.94 (0.83-1.06) | 0.92 (0.82-1.03) |
| Pooled | |||||
| Cardiovascular disease | 12 769 | 0.87 (0.83-0.92) | 0.94 (0.91-0.97) | 0.94 (0.89-0.99) | 0.88 (0.84-0.92) |
| Heart disease | 9716 | 0.85 (0.80-0.90) | 0.94 (0.90-0.97) | 0.92 (0.86-0.97) | 0.85 (0.81-0.90) |
| Stroke | 3053 | 0.96 (0.86-1.06) | 0.95 (0.89-1.02)b | 1.02 (0.92-1.14)b | 0.96 (0.87-1.06) |
| Cancer | 14 443 | 0.82 (0.78-0.86)b | 0.93 (0.90-0.96)b | 0.90 (0.86-0.95)b | 0.84 (0.81-0.88)b |
| Respiratory disease | 4229 | 0.54 (0.49-0.59) | 0.65 (0.61-0.69) | 0.63 (0.58-0.70) | 0.56 (0.52-0.61) |
| Neurodegenerative disease | 7105 | 1.02 (0.95-1.09)b | 0.94 (0.90-0.99)b | 0.95 (0.89-1.02)b | 0.93 (0.87-0.99)b |
Abbreviations: AHEI, Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015; HPDI, Healthful Plant-based Diet Index; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Calculated per 25-percentile increment in 4 healthy eating scores (25 points for HEI-2015, 9 points for AMED score, 18 points for HPDI, and 25 points for AHEI-2010). Multivariable analysis was adjusted for age, calendar year, race and ethnicity (NHS: Hispanic, non-Hispanic Black, non-Hispanic White, or other; HPFS, Black, White, or other), marriage status (married; divorced, separated, or single; or widowed), living status (alone or not alone), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), family history of cancer (yes or no), menopausal status (pre- or postmenopausal [never, past, or current menopausal hormone use]; NHS only), multivitamin use (yes or no), aspirin use (yes or no), total energy intake (quintile), smoking status (never, former, or current smoker [1-14, 15-24, or ≥25 cigarettes/d), alcohol drinking (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), physical activity (quintile), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), and body mass index (<21, 21-24.9, 25-29.9, 30-34.9, or ≥35 [calculated as weight in kilograms divided by height in meters squared]).
Represents I2 less than 75%.
The baseline characteristics, including the dietary scores, were similar across different racial and ethnic groups in the NHS (eTable 10 in Supplement 1). The associations between dietary patterns and total mortality did not differ significantly by race and ethnicity; the HRs of total mortality per 25-percentile difference in HEI-2015 were 0.55 (95% CI, 0.33-0.89; P = .21 for interaction) in Hispanic women, 0.59 (95% CI, 0.41-0.84; P = .19 for interaction) in non-Hispanic Black women, 0.75 (95% CI, 0.72-0.78) in non-Hispanic White women, and 0.72 (95% CI, 0.62-0.85; P = .65 for interaction) in other racial and ethnic minority groups (Figure). Similar results were found for AMED score, HPDI, and AHEI. In subgroup analyses by other potential risk factors for death, the inverse association between dietary scores and total mortality persisted in all subgroups (Table 4). Significant interactions were detected between 4 dietary scores and total mortality by sex and smoking status; HRs were higher among women than among men and among current and ever smokers than among never smokers. The significant inverse associations between dietary scores and total mortality remained largely unchanged when pack-years of smoking were further adjusted (eTable 11 in Supplement 1), the baseline and simple updated dietary data were used (eTables 12 and 13 in Supplement 1), the diet was continuously updated until the end of follow-up (eTable 14 in Supplement 1), and the random-effects model was used (eTables 15 and 16 in Supplement 1). When applying a competing risk regression model for cause-specific mortality, the results remained consistent with those from the primary analysis (eTable 17 in Supplement 1).
Figure. Hazard Ratios of Death From Any Cause According to 4 Healthy Eating Scores Across Racial and Ethnic Groups in the Nurses’ Health Study.
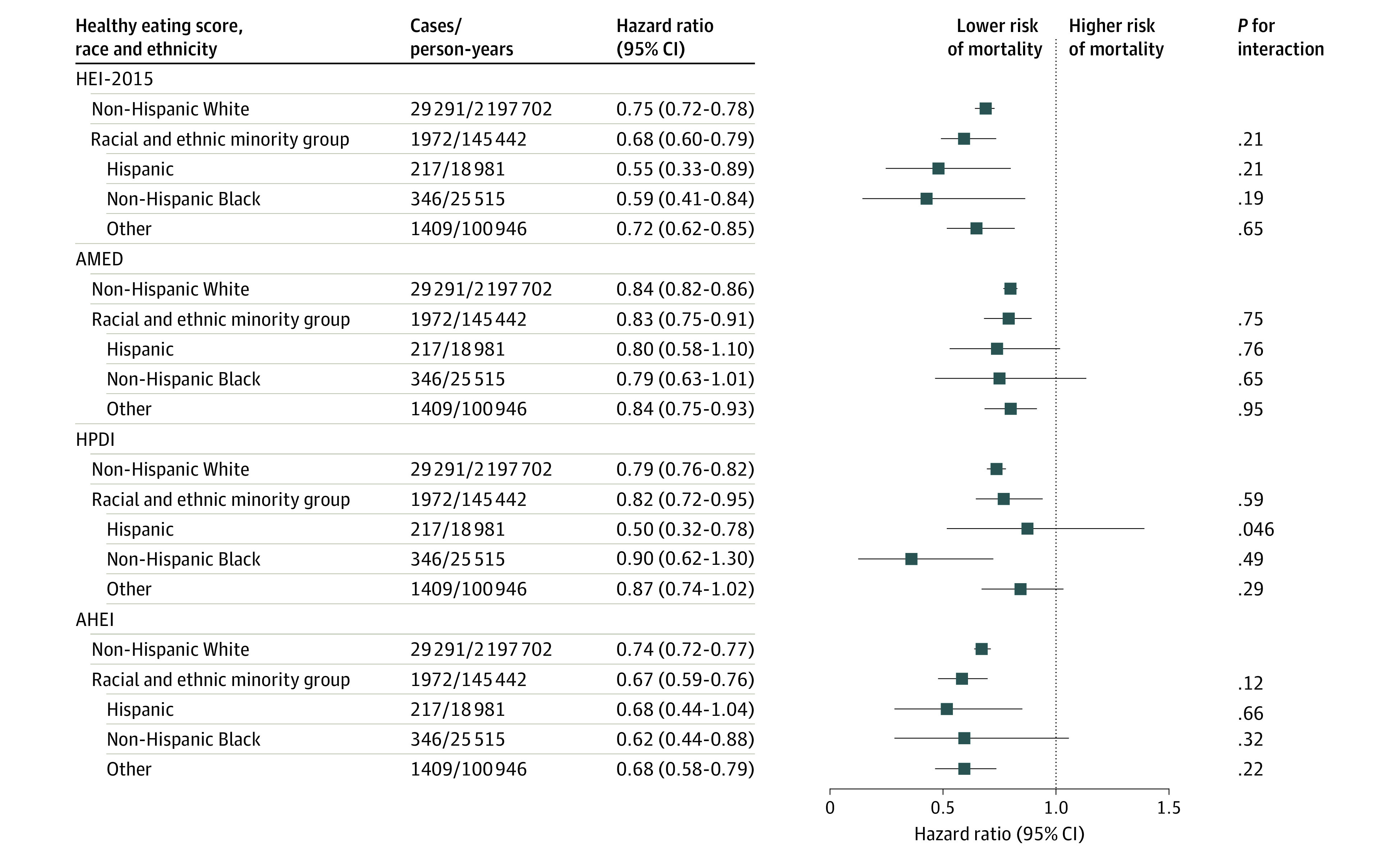
Multivariable analysis was adjusted for age, calendar year, marriage status (married; divorced, separated, or single; or widowed), living status (alone or not alone), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), family history of cancer (yes or no), menopausal status (pre- or postmenopausal [never, past, or current menopausal hormone use]; Nurses’ Health Study only), multivitamin use (yes or no), aspirin use (yes or no), total energy intake (quintile), smoking status (never, former, or current smoker [1-14, 15-24, or ≥25 cigarettes/d]), alcohol drinking (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), physical activity (quintile), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), and body mass index (<21, 21-24.9, 25-29.9, 30-34.9, or ≥35 [calculated as weight in kilograms divided by height in meters squared]). AHEI indicates Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet; HEI-2015, Healthy Eating Index 2015; HPDI, Healthful Plant-based Diet Index.
Table 4. Pooled Hazard Ratios of Death From Any Cause According to 4 Healthy Eating Scores Across Subgroupsa.
| Subgroup | HEI-2015 | P value for interaction | AMED score | P value for interaction | HPDI | P value for interaction | AHEI | P value for interaction |
|---|---|---|---|---|---|---|---|---|
| Age, y | ||||||||
| <65 | 0.80 (0.75-0.87) | .87 | 0.84 (0.80-0.88) | .055 | 0.82 (0.76-0.89) | .15 | 0.77 (0.72-0.83) | .19 |
| ≥65 | 0.81 (0.79-0.83) | 0.88 (0.87-0.90) | 0.87 (0.85-0.90) | 0.81 (0.79-0.83) | ||||
| Sex | ||||||||
| Female | 0.76 (0.73-0.78) | <.001 | 0.85 (0.83-0.87) | <.001 | 0.80 (0.77-0.83) | <.001 | 0.75 (0.73-0.77) | <.001 |
| Male | 0.88 (0.84-0.91) | 0.91 (0.89-0.94) | 0.94 (0.90-0.98) | 0.88 (0.85-0.91) | ||||
| Raceb | ||||||||
| Racial minority groupsc | 0.80 (0.72-0.90) | .93 | 0.90 (0.84-0.97) | .48 | 0.90 (0.81-1.01) | .41 | 0.81 (0.73-0.90) | .98 |
| White | 0.81 (0.79-0.83) | 0.88 (0.86-0.89) | 0.86 (0.84-0.88) | 0.81 (0.79-0.83) | ||||
| Smoking status | ||||||||
| Never | 0.92 (0.88-0.96) | <.001 | 0.95 (0.92-0.98) | <.001 | 0.95 (0.91-1.00) | <.001 | 0.90 (0.86-0.93) | <.001 |
| Past | 0.76 (0.73-0.78) | 0.85 (0.83-0.86) | 0.81 (0.78-0.84) | 0.76 (0.74-0.78) | ||||
| Current | 0.78 (0.72-0.84) | 0.85 (0.81-0.90) | 0.86 (0.79-0.94) | 0.77 (0.71-0.84) | ||||
| Alcohol consumption | ||||||||
| Ever | 0.80 (0.77-0.83) | .48 | 0.88 (0.86-0.90) | <.001 | 0.87 (0.84-0.90) | .36 | 0.81 (0.78-0.84) | .91 |
| Never | 0.82 (0.79-0.85) | 0.93 (0.91-0.96) | 0.85 (0.82-0.89) | 0.81 (0.78-0.84) | ||||
| Physical activity | ||||||||
| Inactive | 0.86 (0.83-0.88) | .11 | 0.91 (0.89-0.93) | .15 | 0.90 (0.87-0.93) | .61 | 0.85 (0.82-0.88) | .17 |
| Active | 0.82 (0.79-0.86) | 0.89 (0.86-0.91) | 0.89 (0.85-0.92) | 0.82 (0.79-0.85) | ||||
| BMI | ||||||||
| <25 | 0.78 (0.75-0.81) | .01 | 0.86 (0.84-0.88) | .002 | 0.83 (0.79-0.86) | .001 | 0.78 (0.75-0.80) | .003 |
| 25-29.9 | 0.84 (0.81-0.88) | 0.89 (0.86-0.91) | 0.92 (0.88-0.96) | 0.85 (0.82-0.88) | ||||
| ≥30 | 0.83 (0.77-0.89) | 0.94 (0.90-0.99) | 0.85 (0.79-0.92) | 0.81 (0.75-0.87) | ||||
| Multivitamin use | ||||||||
| No | 0.85 (0.82-0.88) | <.001 | 0.90 (0.88-0.92) | .005 | 0.89 (0.86-0.92) | .005 | 0.83 (0.81-0.86) | .003 |
| Yes | 0.76 (0.73-0.79) | 0.85 (0.83-0.88) | 0.82 (0.79-0.86) | 0.77 (0.74-0.80) | ||||
| Aspirin use | ||||||||
| No | 0.80 (0.77-0.83) | .53 | 0.86 (0.84-0.88) | .02 | 0.83 (0.80-0.86) | .006 | 0.78 (0.76-0.81) | .04 |
| Yes | 0.81 (0.78-0.84) | 0.89 (0.87-0.92) | 0.89 (0.86-0.92) | 0.82 (0.80-0.85) | ||||
| Family history of myocardial infarction | ||||||||
| No | 0.82 (0.79-0.84) | .37 | 0.88 (0.86-0.90) | .34 | 0.86 (0.83-0.88) | .65 | 0.81 (0.79-0.83) | .89 |
| Yes | 0.78 (0.75-0.83) | 0.88 (0.85-0.91) | 0.88 (0.84-0.93) | 0.80 (0.76-0.84) | ||||
| Family history of diabetes | ||||||||
| No | 0.80 (0.78-0.83) | .17 | 0.87 (0.86-0.89) | .79 | 0.86 (0.83-0.89) | .39 | 0.80 (0.78-0.83) | .74 |
| Yes | 0.82 (0.78-0.86) | 0.89 (0.86-0.92) | 0.87 (0.83-0.91) | 0.81 (0.77-0.84) | ||||
| Family history of cancer | ||||||||
| No | 0.81 (0.78-0.83) | .73 | 0.87 (0.85-0.89) | .06 | 0.86 (0.84-0.89) | .78 | 0.80 (0.78-0.82) | .34 |
| Yes | 0.81 (0.77-0.86) | 0.90 (0.87-0.94) | 0.87 (0.82-0.92) | 0.82 (0.78-0.86) |
Abbreviations: AHEI, Alternate Healthy Eating Index; AMED, Alternate Mediterranean Diet; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HEI-2015, Healthy Eating Index 2015; HPDI, Healthful Plant-based Diet Index; HPFS, Health Professionals Follow-Up Study; NHS, Nurses’ Health Study.
Calculated per 25-percentile increment in 4 healthy eating scores (25 points for HEI-2015, 9 points for AMED, 18 points for HPDI, and 25 points for AHEI-2010). Multivariable analysis was adjusted for age, calendar year, race and ethnicity (NHS: Hispanic, non-Hispanic Black, non-Hispanic White, or other; HPFS, Black, White, or other), marriage status (married; divorced, separated, or single; or widowed), living status (alone or not alone), family history of myocardial infarction (yes or no), family history of diabetes (yes or no), family history of cancer (yes or no), menopausal status (pre- or postmenopausal [never, past, or current menopausal hormone use]; NHS only), multivitamin use (yes or no), aspirin use (yes or no), total energy intake (quintile), smoking status (never, former, or current smoker [1-14, 15-24, or ≥25 cigarettes/d), alcohol drinking (0, 0.1-4.9, 5.0-14.9, 15.0-19.9, 20.0-29.9, or ≥30 g/d), physical activity (quintile), history of hypertension (yes or no), history of hypercholesterolemia (yes or no), and body mass index (<21, 21-24.9, 25-29.9, 30-34.9, or ≥35 [calculated as weight in kilograms divided by height in meters squared]), except the corresponding subgroup variates.
Due to relatively small numbers, racial and ethnic minority groups were combined in the stratified analyses.
Includes American Indian, Asian, Black, Hawaiian, and multiracial.
Discussion
In this cohort study of 2 large prospective cohorts, we found a significant dose-dependent inverse association between adherence to various dietary patterns and total mortality after adjusting for potential confounders. The inverse associations were observed for mortality from CVD, cancer, and respiratory diseases and persisted across different racial and ethnic groups and other subgroups.
Our results are generally consistent with previous studies that reported inverse associations between individual dietary scores and all-cause mortality. The Atherosclerosis Risk in Communities (ARIC) study, the Dietary Patterns Methods Project, and the Women's Health Initiative Observational Study found similar inverse associations in direction and magnitude for HEI-2015, AHEI, and AMED. When comparing the associations between 4 dietary scores of healthy eating patterns, the HRs of mortality associated with HEI-2015 and AHEI were higher than those for AMED and HPDI. These results are partly in line with previous findings that relative risk of death over 12 years was lower when diet quality was assessed by the AHEI than the AMED score. However, in the ARIC study, the relative risks were lower for the AMED than for HEI-2015 and AHEI. The reason for the similarity in the associations between the diet quality scores and mortality is probably that these dietary patterns share several components, such as whole grains, fruits, vegetables, nuts, and legumes. However, there are also some distinct components for each dietary score; for example, the AMED score encourages fish intake, but the HPDI discourages all animal foods. Accordingly, the correlations between 4 dietary scores ranged from moderately low to moderately high (r = 0.39-0.75). Our findings support the recommendations of the current DGAs to achieve long-term health benefits by adherence to various healthy eating patterns that can be adopted based on individuals’ health needs, food preferences, and cultural traditions, although all these diet patterns encourage high consumption of healthy plant-based foods.
Our study provided additional data supporting inverse associations of heathy eating patterns with cause-specific mortality. The deaths in our study were mainly attributable to major chronic diseases, including CVD, cancer, respiratory disease, and neurodegenerative disease. Consistent with previous studies on CVD incidence and mortality, we found an inverse association between each healthy eating score and CVD mortality. Our results of lower HRs for the AHEI and HEI-2015 than for the AMED score were expected given that the AHEI and HEI-2015 are mostly based on current knowledge of dietary factors contributing to cardiovascular disease. The current evidence on healthy eating patterns and cancer mortality remains controversial. Our data support that healthy eating patterns may be associated with reduced mortality from all cancers, but the presence of associations varied for mortality due to specific cancer, which were consistent with some previous findings for incidence of these specific cancers. Previous data on healthy eating patterns and mortality attributable to respiratory disease are sparse. The Singapore Chinese Health Study found similar results between various dietary patterns and respiratory disease mortality. In addition, a previous study by some of us found that a healthy dietary pattern was associated with a lower risk of chronic obstructive pulmonary disease. In the present study, we found an association of the AMED score and AHEI with reduced neurodegenerative disease mortality, which may suggest benefits of some unique dietary components in 2 dietary patterns, such as nuts and monounsaturated fat. However, these findings require confirmation in further studies.
Our study observed consistent inverse associations between individual dietary scores and total mortality across different subgroups, supporting the protective association of various healthy eating patterns with mortality among individuals with diverse cultural food traditions. Consistent inverse associations between individual dietary scores and risk of total mortality across racial and ethnic groups were found among women, and no significant interaction was detected, which was generally in line with previous findings on the HEI, AHEI, and AMED score in relation to mortality from the Multiethnic Cohort and the ARIC study. Considering the same profession of all participants and the relatively limited sample size of racial and ethic minority individuals in the present study, further studies are warranted to confirm these findings.
Strengths and Limitations
Strengths of the present study include a direct comparison of multiple dietary patterns, long-term and repeated measures of diet, multiple racial and ethnic groups, and observed associations between dietary quality scores and respiratory and neurological disease mortality. However, the study has certain limitations. First, because dietary intake information was self-reported, some measurement error was inevitable. However, the FFQs used in the present study were extensively validated against diet records and biomarkers. Second, the possibility of residual and unmeasured confounding could not be completely ruled out because of the observational nature of the study. Third, we did not examine the association between each dietary component and mortality because we considered diet as a combination of multiple components that act synergistically. Fourth, generalizability may be limited because participants were mostly health professionals.
Conclusions
In this cohort study, greater adherence to various healthy eating patterns was consistently associated with a lower risk of death. Our findings support the recommendations of DGAs for multiple healthy eating patterns for all US individuals with diverse cultural and personal food traditions and preferences.
eMethods. Assessment of dietary scores
eTable 1. Healthy Eating Index 2015 components and criteria for scoring
eTable 2. Alternate Mediterranean Diet Score components and criteria for scoring
eTable 3. Healthful Plant-based Diet Index components and criteria for scoring
eTable 4. Alternate Healthy Eating Index components and criteria for scoring
eTable 5. Hazard ratios for death from specific causes according to quintiles of the Healthy Eating Index-2015 (HEI-2015)
eTable 6. Hazard ratios for death from specific causes according to quintiles of the Alternate Mediterranean Diet score (AMED)
eTable 7. Hazard ratios for death from specific causes according to quintiles of the Healthful Plant-based Diet Index (HPDI)
eTable 8. Hazard ratios for death from specific causes according to quintiles of the Alternate Healthy Eating Index (AHEI)
eTable 9. Hazard ratios for death from top three cancers among men and women according to four healthy eating scores
eTable 10. Baseline characteristics of participants according to racial/ethnic groups in NHS
eTable 11 Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI), further adjusting for pack-years of smoking
eTable 12. Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI) based on baseline diet assessment
eTable 13. Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI) based on simple updated diet assessment
eTable 14. Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI) by updating diet after diagnosis of chronic disease
Data Sharing Statement
References
- 1.Downer S, Berkowitz SA, Harlan TS, Olstad DL, Mozaffarian D. Food is medicine: actions to integrate food and nutrition into healthcare. BMJ. 2020;369:m2482. doi: 10.1136/bmj.m2482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2017 Diet Collaborators . Health effects of dietary risks in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393(10184):1958-1972. doi: 10.1016/S0140-6736(19)30041-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Keum N, Giovannucci E, et al. Whole grain consumption and risk of cardiovascular disease, cancer, and all cause and cause specific mortality: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2016;353:i2716. doi: 10.1136/bmj.i2716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang DD, Li Y, Bhupathiraju SN, et al. Fruit and vegetable intake and mortality: results from 2 prospective cohort studies of us men and women and a meta-analysis of 26 cohort studies. Circulation. 2021;143(17):1642-1654. doi: 10.1161/CIRCULATIONAHA.120.048996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Naghshi S, Sadeghi O, Willett WC, Esmaillzadeh A. Dietary intake of total, animal, and plant proteins and risk of all cause, cardiovascular, and cancer mortality: systematic review and dose-response meta-analysis of prospective cohort studies. BMJ. 2020;370:m2412. doi: 10.1136/bmj.m2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y, Li Y, Satija A, et al. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ. 2019;365:l2110. doi: 10.1136/bmj.l2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dehghan M, Mente A, Zhang X, et al. ; Prospective Urban Rural Epidemiology (PURE) study investigators . Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet. 2017;390(10107):2050-2062. doi: 10.1016/S0140-6736(17)32252-3 [DOI] [PubMed] [Google Scholar]
- 8.Hu FB. Dietary pattern analysis: a new direction in nutritional epidemiology. Curr Opin Lipidol. 2002;13(1):3-9. doi: 10.1097/00041433-200202000-00002 [DOI] [PubMed] [Google Scholar]
- 9.Schulze MB, Martínez-González MA, Fung TT, Lichtenstein AH, Forouhi NG. Food based dietary patterns and chronic disease prevention. BMJ. 2018;361:k2396. doi: 10.1136/bmj.k2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mozaffarian D, Rosenberg I, Uauy R. History of modern nutrition science-implications for current research, dietary guidelines, and food policy. BMJ. 2018;361:k2392. doi: 10.1136/bmj.k2392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dietary Guidelines Advisory Committee. Scientific Report of the 2015 Dietary Guidelines Advisory Committee: Advisory Report to the Secretary of Health and Human Services and the Secretary of Agriculture. US Department of Agriculture,Agricultural Research Service, Washington, DC. Published 2015. Accessed December 5, 2022. https://health.gov/our-work/nutrition-physical-activity/dietary-guidelines/previous-dietary-guidelines/2015/advisory-report
- 12.Lichtenstein AH, Appel LJ, Vadiveloo M, et al. 2021 Dietary guidance to improve cardiovascular health: a scientific statement from the American Heart Association. Circulation. 2021;144(23):e472-e487. doi: 10.1161/CIR.0000000000001031 [DOI] [PubMed] [Google Scholar]
- 13.Harmon BE, Boushey CJ, Shvetsov YB, et al. Associations of key diet-quality indexes with mortality in the Multiethnic Cohort: the Dietary Patterns Methods Project. Am J Clin Nutr. 2015;101(3):587-597. doi: 10.3945/ajcn.114.090688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hu EA, Steffen LM, Coresh J, Appel LJ, Rebholz CM. Adherence to the Healthy Eating Index-2015 and other dietary patterns may reduce risk of cardiovascular disease, cardiovascular mortality, and all-cause mortality. J Nutr. 2020;150(2):312-321. doi: 10.1093/jn/nxz218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.English LK, Ard JD, Bailey RL, et al. Evaluation of dietary patterns and all-cause mortality: a systematic review. JAMA Netw Open. 2021;4(8):e2122277. doi: 10.1001/jamanetworkopen.2021.22277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shan Z, Li Y, Baden MY, et al. Association between healthy eating patterns and risk of cardiovascular disease. JAMA Intern Med. 2020;180(8):1090-1100. doi: 10.1001/jamainternmed.2020.2176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan Z, Rehm CD, Rogers G, et al. Trends in dietary carbohydrate, protein, and fat intake and diet quality among US adults, 1999-2016. JAMA. 2019;322(12):1178-1187. doi: 10.1001/jama.2019.13771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bao Y, Bertoia ML, Lenart EB, et al. Origin, methods, and evolution of the Three Nurses’ Health Studies. Am J Public Health. 2016;106(9):1573-1581. doi: 10.2105/AJPH.2016.303338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338(8765):464-468. doi: 10.1016/0140-6736(91)90542-W [DOI] [PubMed] [Google Scholar]
- 20.Willett WC, Sampson L, Stampfer MJ, et al. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122(1):51-65. doi: 10.1093/oxfordjournals.aje.a114086 [DOI] [PubMed] [Google Scholar]
- 21.Yuan C, Spiegelman D, Rimm EB, et al. Validity of a dietary questionnaire assessed by comparison with multiple weighed dietary records or 24-hour recalls. Am J Epidemiol. 2017;185(7):570-584. doi: 10.1093/aje/kww104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yuan C, Spiegelman D, Rimm EB, et al. Relative validity of nutrient intakes assessed by questionnaire, 24-hour recalls, and diet records as compared with urinary recovery and plasma concentration biomarkers: findings for women. Am J Epidemiol. 2018;187(5):1051-1063. doi: 10.1093/aje/kwx328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yue Y, Yuan C, Wang DD, et al. Reproducibility and validity of diet quality scores derived from food-frequency questionnaires. Am J Clin Nutr. 2022;115(3):843-853. doi: 10.1093/ajcn/nqab368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. Am J Epidemiol. 1984;119(5):837-839. doi: 10.1093/oxfordjournals.aje.a113804 [DOI] [PubMed] [Google Scholar]
- 25.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364(25):2392-2404. doi: 10.1056/NEJMoa1014296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang M, Spiegelman D, Kuchiba A, et al. Statistical methods for studying disease subtype heterogeneity. Stat Med. 2016;35(5):782-800. doi: 10.1002/sim.6793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.George SM, Ballard-Barbash R, Manson JE, et al. Comparing indices of diet quality with chronic disease mortality risk in postmenopausal women in the Women’s Health Initiative Observational Study: evidence to inform national dietary guidance. Am J Epidemiol. 2014;180(6):616-625. doi: 10.1093/aje/kwu173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sotos-Prieto M, Bhupathiraju SN, Hu FB. Changes in diet quality and total and cause-specific mortality. N Engl J Med. 2017;377(13):1304-1305. doi: 10.1056/NEJMoa1613502 [DOI] [PubMed] [Google Scholar]
- 29.Weston LJ, Kim H, Talegawkar SA, Tucker KL, Correa A, Rebholz CM. Plant-based diets and incident cardiovascular disease and all-cause mortality in African Americans: a cohort study. PLoS Med. 2022;19(1):e1003863. doi: 10.1371/journal.pmed.1003863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reedy J, Lerman JL, Krebs-Smith SM, et al. Evaluation of the Healthy Eating Index-2015. J Acad Nutr Diet. 2018;118(9):1622-1633. doi: 10.1016/j.jand.2018.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142(6):1009-1018. doi: 10.3945/jn.111.157222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Steck SE, Murphy EA. Dietary patterns and cancer risk. Nat Rev Cancer. 2020;20(2):125-138. doi: 10.1038/s41568-019-0227-4 [DOI] [PubMed] [Google Scholar]
- 33.Rock CL, Thomson C, Gansler T, et al. American Cancer Society guideline for diet and physical activity for cancer prevention. CA Cancer J Clin. 2020;70(4):245-271. doi: 10.3322/caac.21591 [DOI] [PubMed] [Google Scholar]
- 34.Bahrami A, Khalesi S, Makiabadi E, Alibeyk S, Hajigholam-Saryazdi M, Hejazi E. Adherence to the Mediterranean diet and the risk of lung cancer: a systematic review and dose-response meta-analysis of observational studies. Nutr Rev. 2022;80(5):1118-1128. doi: 10.1093/nutrit/nuab117 [DOI] [PubMed] [Google Scholar]
- 35.Grosso G, Bella F, Godos J, et al. Possible role of diet in cancer: systematic review and multiple meta-analyses of dietary patterns, lifestyle factors, and cancer risk. Nutr Rev. 2017;75(6):405-419. doi: 10.1093/nutrit/nux012 [DOI] [PubMed] [Google Scholar]
- 36.Du M, Liu SH, Mitchell C, Fung TT. Associations between diet quality scores and risk of postmenopausal estrogen receptor-negative breast cancer: a systematic review. J Nutr. 2018;148(1):100-108. doi: 10.1093/jn/nxx015 [DOI] [PubMed] [Google Scholar]
- 37.Bosire C, Stampfer MJ, Subar AF, et al. Index-based dietary patterns and the risk of prostate cancer in the NIH-AARP diet and health study. Am J Epidemiol. 2013;177(6):504-513. doi: 10.1093/aje/kws261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loeb S, Fu BC, Bauer SR, et al. Association of plant-based diet index with prostate cancer risk. Am J Clin Nutr. 2022;115(3):662-670. doi: 10.1093/ajcn/nqab365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neelakantan N, Koh WP, Yuan JM, van Dam RM. Diet-quality indexes are associated with a lower risk of cardiovascular, respiratory, and all-cause mortality among chinese adults. J Nutr. 2018;148(8):1323-1332. doi: 10.1093/jn/nxy094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Varraso R, Chiuve SE, Fung TT, et al. Alternate Healthy Eating Index 2010 and risk of chronic obstructive pulmonary disease among US women and men: prospective study. BMJ. 2015;350:h286. doi: 10.1136/bmj.h286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bao Y, Han J, Hu FB, et al. Association of nut consumption with total and cause-specific mortality. N Engl J Med. 2013;369(21):2001-2011. doi: 10.1056/NEJMoa1307352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guasch-Ferré M, Li Y, Willett WC, et al. Consumption of olive oil and risk of total and cause-specific mortality among US adults. J Am Coll Cardiol. 2022;79(2):101-112. doi: 10.1016/j.jacc.2021.10.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Assessment of dietary scores
eTable 1. Healthy Eating Index 2015 components and criteria for scoring
eTable 2. Alternate Mediterranean Diet Score components and criteria for scoring
eTable 3. Healthful Plant-based Diet Index components and criteria for scoring
eTable 4. Alternate Healthy Eating Index components and criteria for scoring
eTable 5. Hazard ratios for death from specific causes according to quintiles of the Healthy Eating Index-2015 (HEI-2015)
eTable 6. Hazard ratios for death from specific causes according to quintiles of the Alternate Mediterranean Diet score (AMED)
eTable 7. Hazard ratios for death from specific causes according to quintiles of the Healthful Plant-based Diet Index (HPDI)
eTable 8. Hazard ratios for death from specific causes according to quintiles of the Alternate Healthy Eating Index (AHEI)
eTable 9. Hazard ratios for death from top three cancers among men and women according to four healthy eating scores
eTable 10. Baseline characteristics of participants according to racial/ethnic groups in NHS
eTable 11 Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI), further adjusting for pack-years of smoking
eTable 12. Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI) based on baseline diet assessment
eTable 13. Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI) based on simple updated diet assessment
eTable 14. Hazard ratios of death from any cause according to quintiles of the Healthy Eating Index-2015 (HEI-2015), Alternate Mediterranean Diet score (AMED), Healthful Plant-based Diet Index (HPDI), and Alternate Healthy Eating Index (AHEI) by updating diet after diagnosis of chronic disease
Data Sharing Statement


