Summary
Background
It is known that some people age faster than others, some people live into old age disease-free, while others develop age-related chronic diseases. With a rapidly aging population and an emerging chronic diseases epidemic, finding mechanisms and implementing preventive measures that could slow down the aging process has become a new challenge for biomedical research and public health. In mice, lifelong water restriction shortens the lifespan and promotes degenerative changes. Here, we test the hypothesis that optimal hydration may slow down the aging process in humans.
Methods
We performed a cohort analysis of data from the Atherosclerosis Risk in Communities study with middle-age enrollment (45–66 years, n = 15,752) and 25 years follow-up. We used serum sodium, as a proxy for hydration habits. To estimate the relative speed of aging, we calculated the biological age (BA) from age-dependent biomarkers and assessed risks of chronic diseases and premature mortality.
Findings
The analysis showed that middle age serum sodium >142 mmol/l is associated with a 39% increased risk to develop chronic diseases (hazard ratio [HR] = 1.39, 95% confidence interval [CI]:1.18–1.63) and >144 mmol/l with 21% elevated risk of premature mortality (HR = 1.21, 95% CI:1.02–1.45). People with serum sodium >142 mmol/l had up to 50% higher odds to be older than their chronological age (OR = 1.50, 95% CI:1.14–1.96). A higher BA was associated with an increased risk of chronic diseases (HR = 1.70, 95% CI:1.50–1.93) and premature mortality (HR = 1.59, 95% CI 1.39–1.83).
Interpretation
People whose middle-age serum sodium exceeds 142 mmol/l have increased risk to be biologically older, develop chronic diseases and die at younger age. Intervention studies are needed to confirm the link between hydration and aging.
Funding
This work was funded by Intramural Research program of the National Heart, Lung, and Blood Institute (NHLBI). The ARIC study has been funded in whole or in part with federal funds from the NHLBI; the National Institutes of Health (NIH); and the Department of Health and Human Services.
Keywords: Serum sodium, Hydration, Aging, Chronic diseases, Biological age
Research in context.
Evidence before this study
In this study, we aimed to evaluate pro-aging effects of mild subclinical hypohydration that activates water conservation mechanisms leading to the excretion of lower volume of more concentrated urine but does not elevate plasma sodium and osmolality beyond normal ranges. We searched PubMed, and Web of Science, without any language restriction using combinations of the terms “serum sodium,” “hydration,” “aging,” “biological aging,” “chronic diseases,” “mortality.” We focused on finding studies assessing associations between the hydration status of healthy people at middle age or younger with a long-term aging-related outcomes such as future development of chronic diseases or premature mortality. We also were looking for studies estimating biological age in relation to the markers of habitual low hydration such as serum sodium. We did not find studies relating markers of subclinical hypohydration at middle age with the speed of biological aging. Several observational epidemiological studies identified associations of the hydration markers with future development of heart failure, metabolic disease and mortality. Increased risk of mortality after 3–6 years of follow-up was demonstrated among people with serum sodium in the upper end of normal range.
Added value of this study
Current study presents a comprehensive analysis of a large population-based observational study with a long 25-years follow-up. The analysis demonstrated that middle-age serum sodium in the upper part of normal range (135–146 mmol/l) is able to predict faster rate of the biological aging, and an increased burden of chronic diseases later in life, including heart failure, dementia, chronic lung disease, stroke, diabetes, peripheral vascular disease and atrial fibrillation. The analysis identified serum sodium threshold of 142 mmol/l that can be used in clinical practice to identify people at risk.
Implications of all the available evidence
Findings from this and previous studies are consistent with the hypothesis that decreased hydration may accelerate aging. The findings suggest that people whose serum sodium exceeds 142 mmol/l may benefit from a more thorough clinical evaluation of their hydration status, including fluid intake habits and pathological conditions that may predispose to an increased water losses. The results warrant testing possible anti-aging effects of improved hydration in interventional trials, and support addition of recommendations for optimal fluid intake in the prevention guidelines.
Introduction
Finding and implementing preventive measures that can slow down the aging process is currently recognized as a major challenge of preventive medicine to combat the epidemic of age-dependent chronic diseases that is emerging as a result of a rapidly aging world population.1, 2, 3, 4, 5 A new research field of geroscience aims to develop safe, practical, and widely available interventions targeting aging: a common driver of chronic diseases. Accumulating findings suggest that slowing the aging processes and extending healthy life span has a potential to improve quality of life and decrease health care cost to a substantially greater degree than a cure of any single disease.1, 2, 3 Disparities in the pace of biological aging are already detectable at midlife6 indicating that preventive measures that can be applied early in life would be most effective to slow down the aging processes and decrease the burden of chronic diseases.4,7,8
In the current study, we test the hypothesis that optimal hydration may slow down the aging process. Here, we define hypohydration as a state in which water conservation mechanisms, including the secretion of antidiuretic hormone and renal urine concertation, are activated when low water intake or high water loss result in decreased body water content and elevated plasma tonicity.9 This hypothesis was inspired by previous mouse studies in which lifelong water restriction, increasing serum sodium by 5 mmol/l, shortened the mouse lifespan by 6 months which corresponds to about 15 years of human life.10 The shortened life span was accompanied by accelerated degenerative changes within multiple organ systems of the chronically hypohydrated mice. In humans, there is a large variation in daily amounts of fluids consumed and worldwide surveys find that a large proportion of people do not consume the recommended amounts and are hypohydrated.11, 12, 13, 14, 15 In order to test the hypothesis that hydration can affect speed of aging, we analyzed data from Atherosclerosis Risk in Communities (ARIC) study: an ongoing population-based prospective cohort study in which 15,792 45-66 year-old black (African American) and white men and women were enrolled from four US communities in 1987–1989 and followed up for more than 25 years.14 We used serum sodium, that increases when we drink less fluids,9,16,17 as a proxy for the hydration habits of study participants.11, 12, 13, 14, 15 In our previous preliminary assessment of ARIC study participants who lived until old age (70–90 years), we noticed increased prevalence of many chronic diseases among people with middle-age serum sodium in the upper part of the normal reference range.10 Further detailed time-to-event analysis adjusted for major cardiovascular risk factors to exclude possible confounding identified high normal serum sodium, as well as other hydration measures, such as body water deficit and blood tonicity, as independent risk factors for heart failure.18
In healthy people, hypohydration is reflected in increased serum sodium concentration and tonicity.16 Normal serum sodium range, defined as the interval that 95% of reference healthy population fall into, lies between 135 and 146 mmol/l. In a healthy person, free of diseases affecting water and electrolytes balance regulation, there are two major mechanisms that are activated in response to hypohydration: thirst and ADH release.9 Both these mechanisms are controlled by plasma tonicity that depends on the concentration of osmotically active plasma solutes with sodium and glucose being the main contributors. When plasma tonicity increases due to decreased water intake, water conservation mechanisms are activated including release of antidiuretic hormone (ADH) from the posterior pituitary gland,17 that then acts on the kidney resulting in the excretion of a lower volume of more concentrated urine. In the absence of hyperglycemia or renal failure, the sodium concentration is the chief determinant of plasma tonicity representing 96%–98% of its value of 275–295 mosmol/kg. The tonicity threshold that stimulates ADH secretion varies in a narrow range around 285 mosmol/kg that would correspond to approximately 140–142 mmol/l of serum sodium.16,19,20
Here, to test the hypothesis that hydration has a systemic effect on the aging processes, we assessed association of middle-age serum sodium with risk of premature mortality, rate of biological aging and burden of chronic diseases. The analysis showed that people whose serum sodium exceeded 142 mmol/l had increased risk to be biologically older, develop chronic diseases and die at younger age.
Methods
Dataset
We used data from the ARIC study. The data were obtained from the NHLBI Biologic Specimen and Data Repository Information Coordinating Center (BioLINCC). The datasets were redacted to remove personal identifiers to conform to the individual informed consent restrictions. Transfer of datasets was approved by the NIH Office of Human Subjects Research and was excluded from Institutional Review Board review as Not Human Subjects Research, based on the interpretation of 45 CRF 46 under “Research Involving Coded Private Information or Biological Specimens” and Guidance on Engagement of Institutions in Human Subjects Research (October 16, 2008). For current analyses, we used outcome variables, exposure variables and covariates from the data collected and curated by the ARIC study investigators and obtained by us from the BioLINCC data repository.
Study population: overview of recruitment and follow-up
ARIC is an ongoing population-based prospective cohort study in which 15,792 black and white men and women aged 45–66 years were enrolled from four U.S. communities in 1987–1989. A detailed study design description has been published.21 Each community cohort was selected in the ARIC study by probability sampling from lists of persons with driver's licenses or state identification cards, or persons eligible for jury duty. To ensure that all individuals in eligible age group had equal chances of being selected, households were identified after a random selection process was performed. Home interview was administered to each potential cohort member that included items on cardiovascular risk factors, socioeconomic factors, and family medical history. This was followed by an invitation to the clinic examination. Participants were asked to fast for 12 h and bring all medications they used within the last two weeks to the examination.21,22 Detailed analysis of response rates and characteristics of responders and non-responders was performed for this study that allows to estimate the degree of selection bias.22 In summary, among age-eligible individuals, 77% of white people and 72% of black people completed the home interview. 68% of white and only 46% of black eligible individuals also completed the clinical examination and became the study participants.22 There were three subsequent visits at approximately 3-year intervals (Visit 2 in 1990–1992; Visit 3 in 1993–1995; Visit 4 in 1996–1998) followed by visit 5 in 2011–2013 (Fig. 1a). Participants have been contacted semi-annually since baseline, to obtain information about hospitalizations and for additional data collection.
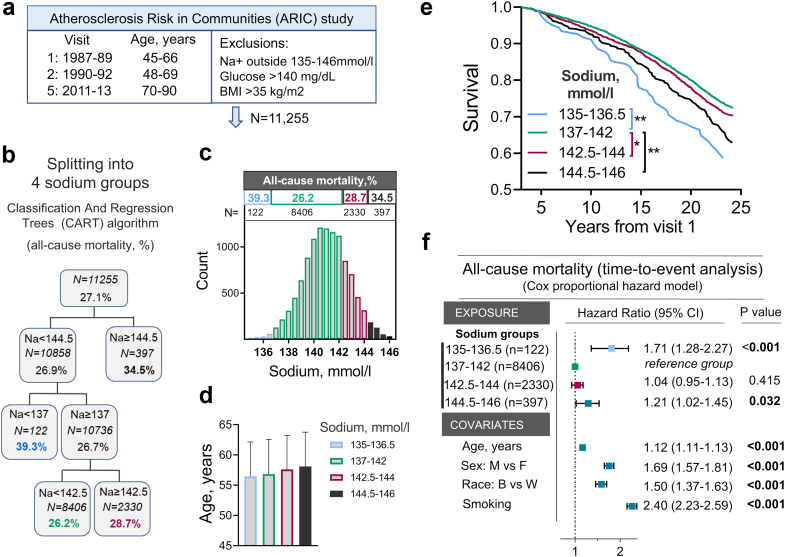
Fig. 1.
Middle-age serum sodium and risk of all-cause mortality in Atherosclerosis Risk in Communities (ARIC) study. a) Overview of ARIC study and exclusion criteria. b, c, d) Splitting study participants into four groups using classification and regression trees (CART) algorithm based on average serum sodium measured at visits 1 and 2 and cumulative mortality by the end of 25 years follow-up. b) Overview of CART algorithm outcome for the group splitting. c) Histograms showing distributions of the study participants according to serum sodium. Groups identified by CART algorithm are shown in different colors. Mortality rate by the end of 25 years follow-up and number of people in each group are shown above histogram. d) Average age does not differ between sodium groups. e, f) Assessment of relative risk for all-cause mortality in four sodium groups. e) Kaplan–Meier Survival Analysis: P < 0.001 (Log–Rank test). All Pairwise Multiple Comparison Procedures): ∗P = 0.04, ∗∗P = 0.001 (Holm-Sidak method). See Table S2 for N at risk at each time point. f) Time-to-event analysis: COX proportional hazard model. People whose middle-age serum sodium exceeds 144 mmol/l or is lower than 137 mmol/l have increased risk of dying at earlier age. See also Table S2 for descriptive statistics and demographic data for these four sodium groups.
Ethics
The ARIC study protocol was approved by the Institutional Review Board of each participating center.21 Written informed consent was obtained from participants at each study visit. Detailed information about participating institutions can be found on the study website: https://sites.cscc.unc.edu/aric/.
Study objectives and overview of the analytical approach
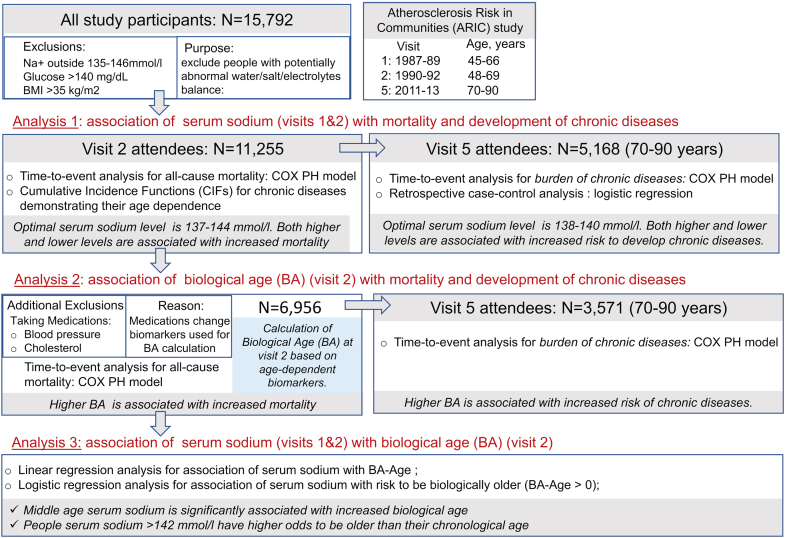
The main goal of the analysis performed in the current study is to find out whether higher serum sodium at middle age is associated with accelerated aging and to identify serum sodium thresholds that can be used in clinical practice to identify people at risk who can potentially benefit from improved hydration. The overview of the study analyses is shown on Fig. S1. To access speed of aging, we used three indicators of faster aging process. Two main age-related outcomes/endpoints were analyzed: 1) age-related chronic diseases and 2) all-cause mortality. We used these aging indicators as outcome variables in the time-to-event analyses with middle age serum sodium as exposure variable (Fig. S1, Analysis 1). Third measure of the aging process that we used in this study was biological age (BA) that was calculated from age-dependent biomarkers. BA have been shown to characterize aging process and predicts mortality better than chronological age.23, 24, 25 We first used BA at baseline as exposure variable in the time-to-event analysis to assess its ability to predict age-dependent outcomes, namely chronic diseases and mortality, in the ARIC cohort (Fig. S1, Analysis 2). To assess a link between serum sodium and BA, we then performed cross-sectional logistic regression analysis using serum sodium as exposure variable and BA as dependent variable (Fig. S1, Analysis 3).
Exclusions
Since the purpose of this analysis was to examine effects of hydration, we aimed to exclude people whose serum sodium could be affected by other factors in addition to the amount of liquids they consume. Therefore, to avoid including people with possible abnormalities of water/salt balance regulation, we excluded people who had an average sodium concentration from Visits 1 and 2 outside normal reference range of 135–146 mmol/l. We also excluded participants with plasma glucose level higher than 140 mg/dL at visits 1 and 2, since hyperglycemia, in spite of causing dehydration, results in decrease of serum sodium concentration.26 Since obesity is known to alter distribution of body fluids and elevates serum sodium, we excluded participants with averaged body mass index (BMI) greater than 35 kg/m2 at visits 1 and 2.27 After these exclusions, 11,255 participants remained in the dataset. For BA calculations, we additionally excluded participants who were taking blood pressure (BP) and cholesterol lowering medications, since systolic BP and total cholesterol were included as biomarkers for these calculations and the medications would change real values for these biomarkers (N = 6956). In each analysis, people with missing data were also excluded.
Calculation of biological age
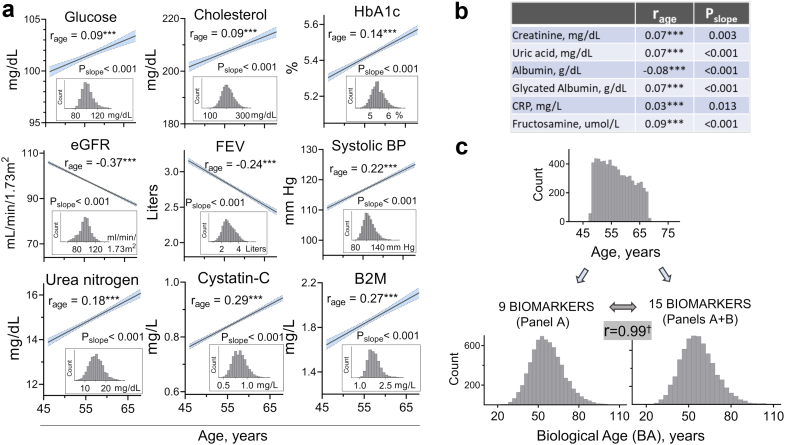
We calculated BA of the ARIC study participants at Visit 2, that was used as the study baseline, using the Klemera and Doubal method. BA estimated by this method have been shown to be a reliable predictor of mortality performing superior to chronological age23 and also a good predictor of aging rates in young adults.24 We selected biomarkers for BA calculation based on knowledge about their age dependency, role in aging process, good performance in previous BA calculations23, 24, 25,28 and availability in ARIC study. We selected 15 biomarkers characterizing performance of multiple organ systems and processes: cardiovascular (systolic blood pressure), renal (eGFR, cystatin-C, urea nitrogen, creatinine, uric acid), respiratory (FEV), metabolic (glucose, cholesterol, HbA1c, glycated albumin, fructosamine), immune/inflammatory (CRP, albumin, beta 2-microglobulin). We assessed age-dependence of the biomarkers by Pearson correlation analysis with age and selected 9 biomarkers with strongest correlation for the BA calculations. For sensitivity analysis, we also calculated BA using all 15 biomarkers. The results are presented on Fig. 3.
Fig. 3.
Calculation of biological age (BA) based on age-dependent biomarkers. Fifteen biomarkers were selected based on prior knowledge of their age-dependence and availability in ARIC study. a, b) Verification of age-dependence for 15 biomarkers measured in ARIC study participants at visit 2 (n = 6,956, see methods and Fig. S1 for exclusions criteria). a) Linear regression lines for nine biomarkers that showed significant correlation with age and were used for BA calculations. 95% CI for the regression lines are shown in blue. rage denotes Pearson Coefficient for correlation of each biomarker with age (∗∗∗P < 0.0001). Pslope denotes P-value for the slope deviation from zero. The panel inserts show distribution of the study participants based on the corresponding biomarkers. b) rage and Pslope for six additional biomarkers with lower correlation coefficients that were included for BA calculation based on 15 biomarkers. c) Distributions of the study participants based on their chronological age (Age) and BA calculated from 9 to 15 biomarkers using Klemera and Doubal's method (see methods section for details). Distributions of BA from nine and 15 biomarkers are almost identical with Pearson correlation r = 0.99 (†P < 0.0001) indicating that they gave similar results.
To calculate BA from selected 9 and 15 biomarkers, we used Python implementation of the Klemera and Doubal method.23,28 The implementation was based on a source code provided by Elisa Warner on GitHub repository (https://github.com/elisawarner/Biological-Age-Project).
Exposure variables
Serum sodium at baseline
The primary exposure variable was average serum sodium from visits 1 and 2 that occurred 3 years apart (Fig. 1a). Serum sodium was used as a measure of hydration habits of the study participants. Participants were fasting for 8–12 h before the blood draw. We performed the analysis with the assumption that average serum sodium measured at visits 1 and 2 three years apart represent hydration habits of each individual (see “Serum sodium and hydration habits” section of the results for details). To identify clinically usable thresholds for increased risks, we categorized serum sodium exposure variable. We applied Classification and regression trees (CART) algorithm in order to split continuous ranges of sodium into maximally distinct groups based on cumulative mortality or chronic diseases rates by the end of follow-up.29 Since these outcomes may have different underlying etiology, separate categorization of sodium as exposure variable was performed for the mortality (Fig. 1b) and for chronic diseases (Fig. 2c).
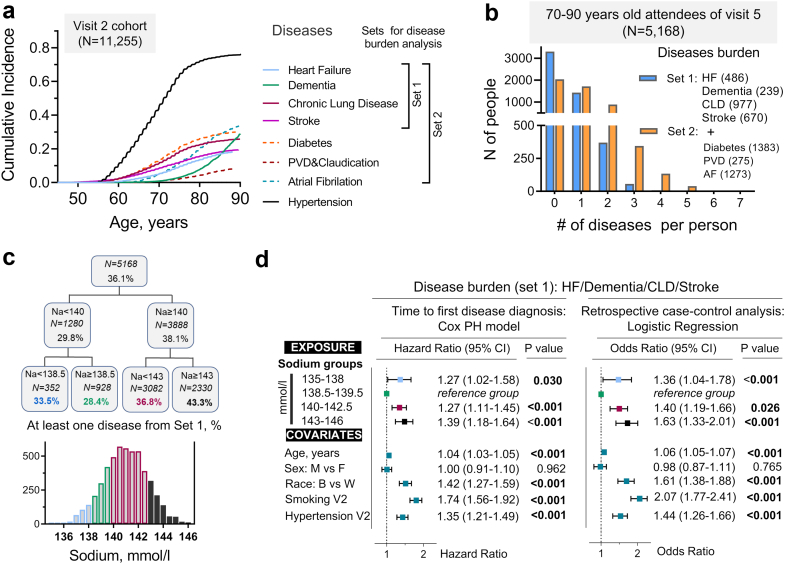
Fig. 2.
Middle-age serum sodium and risk to develop age-related chronic diseases. a) Age dependence of chronic diseases. Cumulative incidence curves (CIFs) accounting for competing mortality for eight age-dependent chronic diseases in ARIC study participants (N = 11,255). Right panel shows how diseases are combined into two sets containing four and seven diseases for following analysis of chronic diseases burden. b-d) Analysis of chronic diseases burden in 5168 ARIC study participants aged 70–90 years who attended visit 5 at the end of 25 years follow-up. b) Distribution of the study participants based on number of diseases from Set 1 (blue) and Set 2 (orange) that they are diagnosed with. c, d) Analysis of association between middle-age serum sodium and risk to develop chronic diseases from Set 1. c) Splitting study participants into four groups using classification and regression trees (CART) algorithm based on average serum sodium measured at visits 1 and 2. Percent of people diagnosed with at least one out of four diseases from Set 1 by the end of follow-up was used as outcome variable for the splitting algorithm. d) Assessment of relative risk to develop at least one disease from Set 1 in four sodium groups. Participants are divided into 2 groups: 0 – disease-free at the end of follow-up; 1 – have at least one disease. Left panel: Time-to-event analysis: Cox proportional hazards model. Right panel: Retrospective case–control analysis: multivariable logistic regression. Participants with serum sodium 138-140 mmol/l have lowest risk to develop chronic diseases. Both lower and higher sodium concentrations are associated with increased risk. See also Fig. S3 for analysis of Set 2 diseases.
Age and biological age at baseline
Age and BA were used as exposure variables in time-to-event analyses assessing association between BA at middle age with development of chronic diseases and mortality. They were categorized into four quartiles to split the study participants to four equal groups.
Outcomes: mortality and burden of chronic diseases
All-cause-mortality
In ARIC study, participants were contacted semi-annually and date of death was recorded based on these interviews with participants or family members and also based on information from death certificates.
Age-related chronic diseases
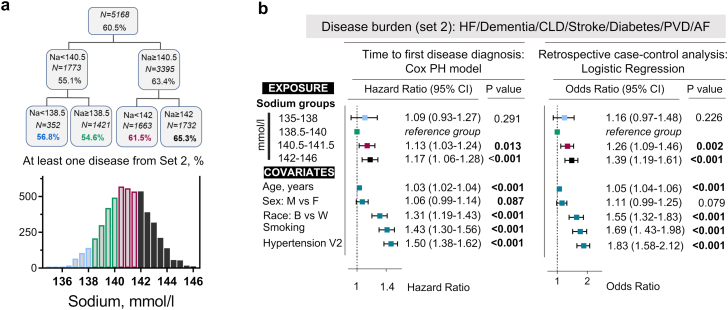
For analysis of chronic diseases, we created new outcome variables (disease burden (set 1) and disease burden (set 2)) based of information about diagnosis of eight chronic diseases in ARIC study participants (Fig. 2a): heart failure (HF), dementia, chronic lung disease (CLD), stroke, diabetes, peripheral vascular disease (PVD) and claudication, atrial fibrillation (AF), and hypertension. Consistent with chronic degenerative nature of these diseases, incidence of the diseases started to increase at approximate ages of about 55–60 years in ARIC study participants (Fig. 2a). To analyze burden of chronic diseases, we created two dichotomous variables (“1”-developed at least 1 disease; “0”-no diseases) based on either four major degenerative diseases (Set 1: HF, dementia, CLD, stroke) or based on seven diseases (Set 2: additionally added diabetes, PVD and AF). These outcome variables divide study participants into two groups: participants who developed at least one of these diseases (from set 1 or set 2) and those who were disease-free at the end of follow-up. Since initial age-dependence analysis showed that hypertension developed much earlier than all other diseases (Fig. 2a), we did not include it in the disease sets for assessment of burden of chronic diseases. However, in order to account for the role of hypertension in the risk of chronic diseases, we adjusted all the models for hypertension diagnosis at baseline visit 2 examination.
Variables for the disease status and time of the diagnosis
Except for dementia, numeric variables that we used in the current study for presence of the diseases (1-Yes; 0 – No) were created based on self-report of the incident disease diagnosis. Date for the onset of the diseases was recorded as date the first time a participant self-reported incident disease. HF diagnosis was additionally ascertained by evaluation of medical records and the physician heart failure survey. Dementia diagnosis was based on the telephone interviews for cognitive status, proxy interviews, dementia hospitalization discharge codes, diagnostic codes from death certificates, and neurophysiological assessment during visit 5 exam. Date of the diagnosis was recorded as earliest dementia indication from these examinations.
We used BA as outcome variable for analysis of its cross-sectional association with serum sodium at visit 2. See “Calculation of biological age” method section for details.
Covariates and other variables used in the study
Several covariates were used for adjustment of statistical models and for exclusions. Sex (“1”: male and “0”: female) and race (“0”-white and “1”-black (African American) were self-reported by study participants. Smoking status was self-reported (“1”-smoker or “0”-not-smoker at visit 2). BP was measured three times after 5 min of rest and recorded as average of the two last measurements. High BP was defined as Systolic BP greater than 140 mm Hg or Diastolic BP greater than 90 mm Hg or if participant took BP medications. Taking BP medications were ascertained based on current medications that participants were asked to bring to the visit or based on self-report. Plasma glucose (mg/dL, visits 1 and 2) and Body Mass Index (BMI) (kg/m2, visit 1 and 2) were used for exclusions.
Detailed information about analytic measurements in blood samples, and about collection of information for numerical variables and their derivations for final databases is also available at ARIC study website (https://www2.cscc.unc.edu/aric/cohort-manuals).
Statistics
Overview of the analyses performed in the current study is given on Fig. S1.
Analysis 1: association of serum sodium with mortality and chronic diseases
Cox proportional hazard models were used in the time-to-event analyses. Time from baseline examination to death or to diagnosis of first chronic disease was used as time variable. In absence of the outcome event, participants were censored at the time of last follow-up. For mortality analysis, all the participants who are left after exclusions were included (n = 11,255). For the analysis of chronic diseases, only attendees of visit 5 evaluation were included (n = 5,168). To control for possible confounding, the models were adjusted for age, sex, race, current smoking status and hypertension at visit 2. Visit 2 was used as a base for all analyses to prevent possibility of reverse causation due to using averaged values from visit 1 and visit 2 for serum sodium.
For a cohort of 70–90 years old attendees of visit 5, as a sensitivity analysis, we additionally performed retrospective case–control analysis by running multiple logistic regression with burden of chronic diseases as outcome variable and serum sodium groups as exposure variable.30 Cases were people who developed at least one disease from Set 1 or Set 2 and controls were people who did not develop any of these diseases. Follow-up time was time from baseline to visit 5 examination for all participants included in this analysis (N = 5,168).
Analysis 2: association of biological age with mortality and chronic diseases
Similar Cox proportional hazard models were used as for the Analysis 1 but with BA as exposure variable and age at death or diagnosis of first disease as time variable. Changing the time variable to age was chosen to better demonstrate how age of outcomes is affected by BA at baseline.
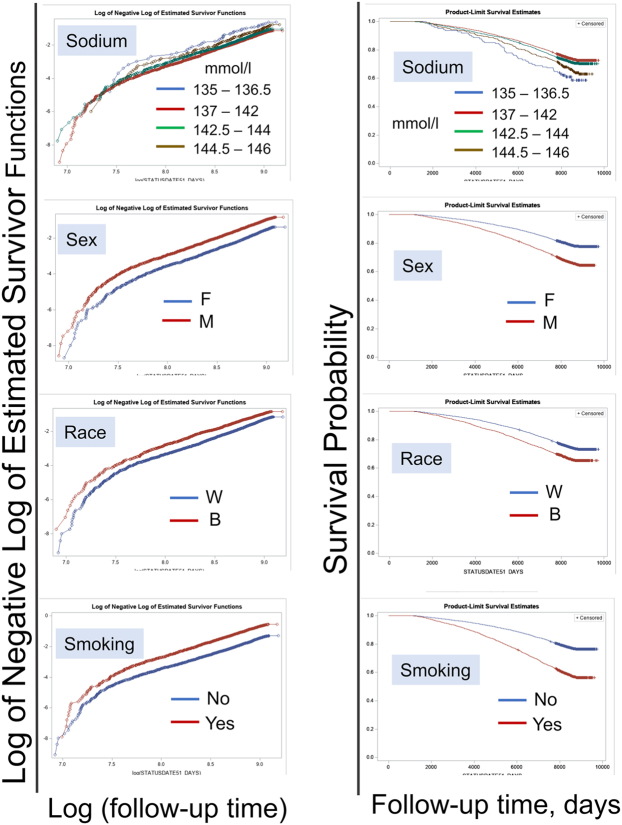
We validated the proportional hazard assumption in the COX models by drawing the survival probability versus time and the log (-log (survival)) versus log of survival time graphs for the models covariates. The analyses resulted in the graphs with approximately parallel curves indicating that covariates satisfied the proportional hazard assumptions (Fig. S2). The results of the time-to-event analyses are presented as hazard ratios (HR) with a 95% confidence intervals (CI). P values were assessed based on Chi-square test.
Analysis 3: cross-sectional association of serum sodium with biological age
We used two approaches for this analysis. In multivariable linear regression model, BA and serum sodium were used as continuous variables (Fig. 5b). In cross-sectional multivariable logistic regression analysis, serum sodium was categorized into 4 groups and BA was categorized into 2 groups: being older (“1”) or not (“0”) than one's chronological age (Fig. 5c). Both models were adjusted for age, sex, race, and smoking status.
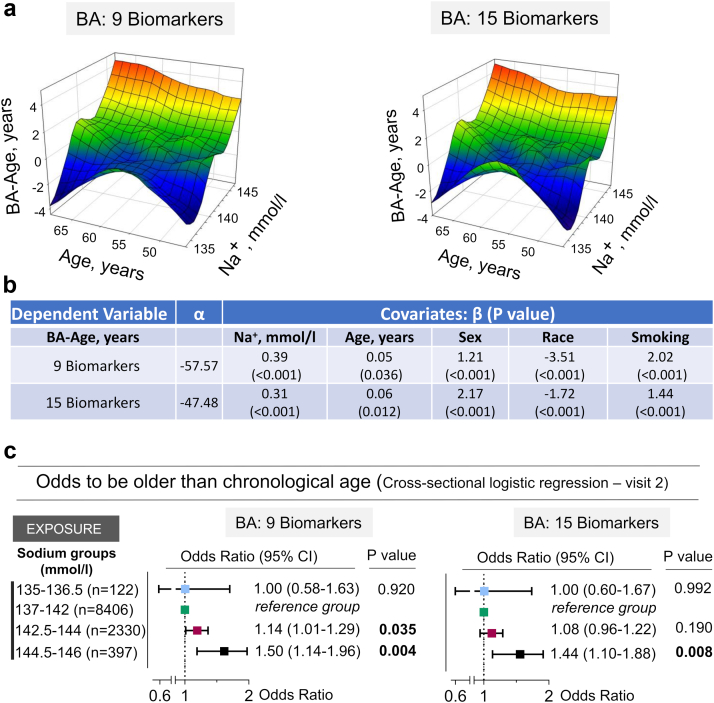
Fig. 5.
ARIC study participants with middle age serum sodium in upper part of normal range have increased odds to be older than their chronological age. a) 3D Mesh Plots, visualizing difference between biological and chronological age (BA-Age) calculated for visit 2 as functions of serum sodium concentration and age. Participants with serum sodium in the upper half of the normal range have higher BA. b) Serum sodium is positively associated with BA-Age in multivariable linear regression model adjusted for age, sex, race and smoking status. c) Study participants were divided into two groups based on having BA higher or lower than chronological age. In cross-sectional multivariable logistic regression analysis adjusted for age, sex, race, and smoking status, odds to have higher BA are increased by ∼10–15% for serum sodium exceeding 142 mmol/l and by ∼50% for serum sodium exceeding 144 mmol/l. See Table S6 for full models results.
The analyses were performed using SAS (SAS Institute Inc., Cary, NC, USA), SigmaPlot 14.0 (Systat Software, San Jose, CA) and GraphPad Prism version 9.0.2 for Windows (GraphPad Software, San Diego, California USA). The 3D mesh graphs were constructed using SigmaPlot 14.0 software with LOESS smoothing and rejection of outliers.
Role of funders
The funders had no role in study design, data collection, data analyses, interpretation, patient recruitment, or writing of this manuscript.
Results
Overview of the study analyses and main conclusions is presented in Fig. S1.
Study population
We performed analysis on 11,255 participants who remained after exclusions of people whose serum sodium concentration was outside the normal range (135–146 mmol/l) or could be shifted by factors affecting water balance regulation and therefore would not correctly represent level of hydration such as diabetes and obesity (Fig. 1a, methods and Fig. S1). The average age at visit 2 (baseline) was 57 years. The visit 2 cohort consisted of 53% female and 47% male, 80% white and 20% black participants (Table S1). Average age at visit 5 was 76 years. The visit 5 cohort consisted of 56% female and 44% male, 82% white and 18% black (African American) participants (Table S1). There were no major differences between visit 2 and visits 5 cohorts in baseline cholesterol, glucose, BMI, eGFR, serum sodium and average age at visit 2. However, visit 5 cohort contained smaller proportion of people who were smoking and already had high blood pressure at visit 2 (Table S1)
Serum sodium and hydration habits
We used serum sodium as a proxy for habitual hydration status because it increases as we drink less fluids.9,16 As levels of body fluids decreases, elevating blood tonicity, for which sodium represents 95%, activates neurohumoral mechanisms for water conservation resulting in excretion of lower volumes of more concentrated urine.9,17 We performed the analysis with the assumption that average serum sodium measured at visits 1 and 2, 3 years apart represent hydration habits of each individual. We made this assumption based on reported analysis of clinical records showing little individual variations of serum sodium over 10 year period.31 In addition, our previous analysis of serum sodium in ARIC study have demonstrated that it was stable within 2–3 mmol/l interval between visits 1 and 2, 3 years apart.32 The stability of serum sodium is also confirmed in the current study by identical sodium concentrations between visits 1 and 2 within groups created based on serum sodium (Table S2, Table S4). The existence of individual hydration habits is supported by worldwide surveys of fluid consumption.11, 12, 13, 14, 15 The surveys found large individual differences within populations and even between countries suggesting that local and family traditions may play a role in creating these habits for amount of fluids people tend to drink.11
Middle age serum sodium and risk of all-cause mortality and age-related chronic diseases
Aging is associated with the increasing risk of mortality.33 Therefore, to assess associations between serum sodium and the aging process in ARIC study participants, we performed time-to-event analysis for all-cause mortality (Fig. 1). We first applied classification and regression trees (CART) algorithm to split the study participants into four groups based on a cumulative all-cause mortality by the end of follow-up (Figs. 1b and c). There was substantial difference in mortality rates between groups in spite of similar average age at baseline (Fig. 1d). Lowest mortality rate was among people with 137–142 mmol/l serum sodium (26.2%, n = 8604), with increased mortality in 135–136.5 mmol/l (39.3%, n = 122) and 144.5–146 mmol/l (34.5%, n = 397) groups. Kaplan–Meier survival analysis gave similar results showing increased mortality rates among people with serum sodium less than 137 mmol/l and greater than 142 mmol/l (Fig. 1e). In Cox proportional hazard time-to-event analysis adjusted for age, sex, race and smoking, serum sodium 135–136.5 mmol/l was associated with 71% increased risk of all-cause mortality, and 144.5–146 mmol/l increased risk of premature mortality by 21% in comparison to the 137–142 mmol/l group (Fig. 1f).
Aging is associated with the development of chronic diseases.34 Consistent with the age-dependence, incidence of the chronic diseases for ARIC study participants was increasing with age (Fig. 2a). We assessed a burden of chronic diseases in 5,168 participants aged 70–90 years who lived until old age and attended visit 5 evaluation (Fig. 2b–d). We used four major degenerative disease in main analysis: heart failure, dementia, chronic lung disease, and stroke (Set 1) (Figs. 2a and b). For sensitivity analysis, we additionally included diabetes, PVD/claudication, and atrial fibrillation (Figs. 2a and b). Among visit 5 attendees, 36.1% were diagnosed with at least one disease from Set 1 (Fig. 2c), 60.5% with at least one disease from Set 2 (Fig. S3a), and some participants developed multiple diseases (Fig. 2b). In order to assess association of middle age serum sodium with risk to develop chronic diseases, we divided study participants into four groups by CART algorithm based on % of people who developed at least one disease (Fig. 2c, Fig. S3a). We then estimated relative risk to develop chronic diseases for these 4 groups by Cox time-to-event and by retrospective case–control logistic regression analyses (Fig. 2d, Fig. S3b). The analysis showed that people with serum sodium 138–140 mmol/l had the lowest risk of developing chronic diseases. Higher serum sodium was associated with an increased risk, of up to 39% to develop chronic diseases in time-to-event analysis (Fig. 2d). In logistic regression analysis, serum sodium above 140 mmol/l was associated with up to 63% increased odds to develop chronic diseases in comparison to the 138–140 mmol/l group (Fig. 2d, and Fig. S3b).
Biological age estimations of ARIC study participants at middle age
Association of serum sodium with mortality and the burden of chronic diseases at higher sodium concentrations provided initial support for our hypothesis that hypohydration may be associated with accelerated aging. Biological age calculated based on age-dependent biomarkers, represents an emerging measure of speed of aging process.23,28 To directly assess association of serum sodium with aging process, we next analyzed the biological age of ARIC study participants (Fig. S1). For BA estimation, we selected 15 age-dependent biomarkers that have been shown to predict aging and mortality23,24 and were measured in ARIC study participants at visit 2 (Fig. 3a and b). All biomarkers showed significant age-dependence (Fig. 3a and b). We calculated the BA based on all 15 biomarkers and on nine biomarkers that showed strongest age-dependence. These calculations gave almost identical results (Fig. 3c).
Biological age and risk of all-cause mortality and age-related chronic diseases
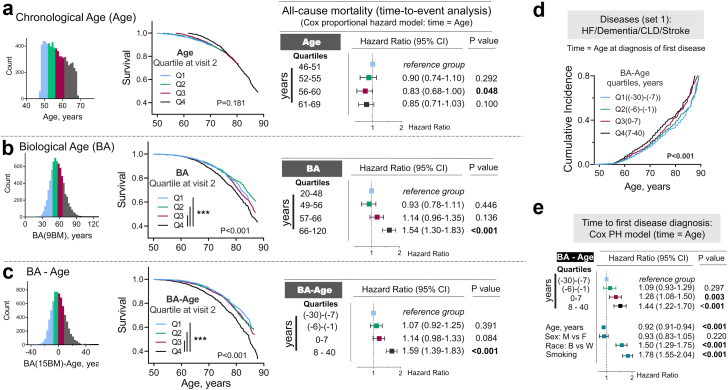
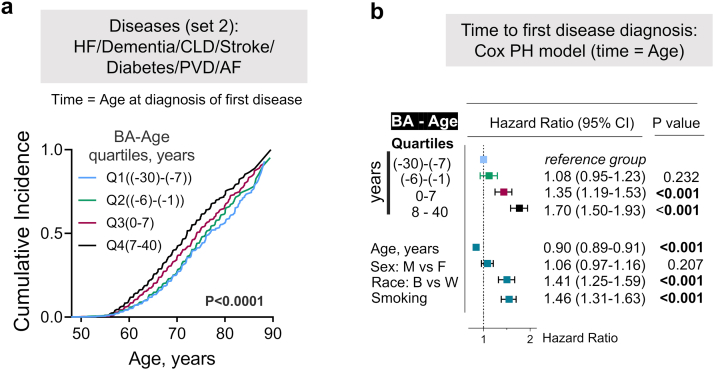
Due to its better prediction of mortality and degenerative changes, biological aging is considered as a measure of speed of aging.23,24 We next tested the ability of BA calculated for ARIC study participants at visit 2 from nine and 15 biomarkers to predict risk of premature mortality and development of chronic diseases (Fig. 4). We divided study participants into four quartiles based on their chronological age (Age) (Fig. 4a), BA (Fig. 4b) and the difference between BA and Age (BA-Age) (Fig. 4c). When chronological age was used as predictor variable, there were no differences in survival curves between four quartiles groups (Fig. 4a). On the other hand, biological age was able to predict future premature mortality (Fig. 4b and c). Thus, in non-adjusted Kaplan–Meier survival analysis, people in highest quartile of BA and BA-Age (Q4 group) had higher probability to die at younger age (Fig. 4b and c, middle panels). In Cox PH time-to-event analysis adjusted for age, sex, race and smoking, risk to die at younger age was 54% or 59% higher for people in Q4 group of BA and BA-Age in comparison to people in Q1 group (Fig. 4b and c, Table S5). In addition, the analysis of 70–90 years old participants who attended visit 5 evaluation showed that participants who were biologically older at baseline than their chronological age (Q3 and Q4 groups of BA-Age) had up to 44% higher risk than people in Q1 group to develop chronic diseases (Fig. 4d and e, Fig. 4a and b).
Fig. 4.
Higher biological age is associated with increased risk of premature dying and/or developing age-related chronic diseases. a-c) Comparison of predictive value of chronological and biological age for all-cause-mortality: people from 4th quartile of BA, but not chronological age, as well as people whose BA age is more than 7 years higher than their age (4th quartile) have more that 50% increased risk of premature dying. The ARIC study participants were divided in four quartile groups based on a) Age; b) Biological age (BA) calculated using nine biomarkers and on c) Difference between BA and Age (BA-Age). Left panels: Distributions of the study participants based on Age, BA and BA-Age with four quartile groups shown by different colors. Middle panels: Kaplan–Meier Survival Analysis. P values for difference between survival curves are shown (log-rank statistical analysis). People in 4th quartile of BA and BA-Age have higher mortality rate (pairwise multiple comparison of survival curves: ∗∗∗P < 0.0001 (Holm-Sidak method); . Right panels: Time-to-event analysis: COX proportional hazard models for all-cause mortality as outcome and four quartiles of Age, BA and BA-Age as exposure variables. The models are adjusted for age, sex, race and smoking status. See Table S5 for full models results. BA predicts mortality better than chronological age: age is no longer significant when BA is considered.d, e) Analysis of risk for chronic diseases in relation to BA: people whose BA is higher than chronological age (3rd and 4th quartiles) have up to 70% higher risk to develop chronic diseases.d) CIFs for diagnosis of first out of 4 diseases from set 1 constructed separately for four quartiles of BA-Age calculated from nine biomarkers. e) Time-to-event analysis: COX proportional hazard models for diagnosis of first disease as outcome and four quartiles of BA-Age as exposure variable. See also Fig. S4 for the same analysis performed for seven chronic diseases (set 2).
Middle-age serum sodium and risk to be biologically older than chronological age
Having shown that higher sodium similar to higher BA are associated with increased risk to develop chronic diseases and of premature mortality, we next analyzed the BA of ARIC study participants at visit two in relation to serum sodium (Fig. S1 and Fig. 5). 3D Mesh Plots visualizing BA-Age as function of serum sodium and Age showed that participants with serum sodium in upper part of normal range have higher BA than chronological age (Fig. 5a). Consistently, serum sodium was positively associated with BA-Age in multivariable linear regression model adjusted for age, sex, race and smoking status (Fig. 5b). To estimate approximate serum sodium threshold for elevated risk to be biologically older, we divided study participants into two groups for which BA-Age is greater than 0 or BA-Age is less than or equal to 0. In logistic regression analysis adjusted for age, sex, race, and smoking status, odds to be biologically older that one's age were increased by approximately 10–15% for serum sodium exceeding 142 mmol/l and by approximately 50% for serum sodium exceeding 144 mmol/l as compared to participants with serum sodium 137–142 mmol/l (Fig. 5c and Table S6).
Discussion
In this study, we report on serum sodium in the upper part of the normal range being a risk factor for accelerated aging. In the ARIC study, odds to be biologically older than chronological age was increased in the study participants whose serum sodium exceeded 142 mmol/l reaching 50% increased odds at sodium levels exceeding 144 mmol/l (Fig. 5). Such elevated BA at middle age (47–68 years) translates into an approximate 20% increased risk of premature mortality at sodium levels greater than 144 mmol/l (Fig. 1) and increased risk to develop chronic diseases, that was already evident at sodium concentrations greater than 140 mmol/l and increased to approximately 40% higher risk in 143–146 mmol/l group (Fig. 2). In addition to elevated risk of mortality and chronic diseases at higher end of normal serum sodium range, increased risk was also evident at lower end of normal sodium range (Fig. 1, Fig. 2). This is consistent with previous reports of increased mortality and cardiovascular disease (CVD) incidence in community subjects with low normal sodium (135–137 mmol/l) that is attributed to diseases causing electrolyte disregulations.35,36 Our results indicate that serum sodium range 138–142 mmol/l is associated with lowest risk of chronic diseases and/or premature mortality. This range corresponds to more conservative definition of normal range proposed by Kumar and Berl.37 Since decreased body water is the most common reason for increasing sodium concentration,9,16,17 these results suggest that for people whose serum sodium exceeds 142 mmol/l, consistently maintaining optimal hydration may slow down aging process.
Accumulating evidence from the field of geroscience indicate that aging-related degenerative changes promote development of majority of chronic diseases.3 Frequent appearance of multiple chronic diseases in one individual highlights integrative nature of human physiology and supports notion that targeting systemic aging to delay chronic diseases rather than treating individual diseases can give better health outcomes.1,2 Results of our study support the hypothesis that optimal hydration can potentially be such systemic preventive approach that is able to prolong diseases-free lifespan. Our data are consistent with previous reports from epidemiological and interventional studies that link hypohydration biomarkers including higher serum sodium and copeptin as well as low fluid intake with adverse health effects and increased risk of mortality.38, 39, 40, 41, 42, 43, 44 In agreement with the ARIC data from four U.S. communities that were used in current study, similar sodium levels, >144 mmol/l, were found to be associated with increased risk of all-cause and chronic disease associated mortality within 3–6 years for U.S. adults aged 51–70 years in 2009–2012 National Health and Nutrition Examination Survey (NHANES).45 Owing to long 25 years follow-up period, our analysis demonstrated that serum sodium is able to predict faster rate of the biological aging and increased risk of future development of chronic diseases well before the age at which the chronic diseases start appearing at high rate in general population. Our analysis also identified serum sodium threshold of 142 mmol/l that can be used to identify people at risk who may benefit from a more thorough clinical evaluation.
The main limitation of our study is its observational nature resulting in the possibility of residual confounding. This common limitation of the observational studies is reduced to some degree in our case, because the idea for this analysis originated from a well-controlled mouse study in which lifelong water restriction shortened life span and promoted degenerative changes in multiple organ systems.10 Residual confounding that can potentially affect results of this study may come from inability to fully restrict analyzed cohort to healthy people, lack of data for hormonal effects, such as aldosterone, as well as medications that could affect serum sodium. Also, endogeneity could be present in the analyses of the associations between serum sodium and BA due to potential effects of the hydration on some of the biological aging biomarkers, such as creatinine, urea nitrogen and albumin. Potential selection bias at recruitment and due to loss to follow-up could also affect the study results. Due to such limitations, the causative relationships of serum sodium and hydration with accelerated aging, mortality and chronic diseases in humans would need to be confirmed in interventional randomized controlled clinical trials.
An important strength of the ARIC study is a very long follow-up from middle age to 70–90 years including comprehensive standardized clinical evaluations. The main difficulty with performing interventional studies for the assessments of mortality and chronic diseases is requirement for a very long follow up period. However, BA calculated from the age-dependent biomarkers (Fig. 3) is more sensitive to anti-aging intervention and can potentially be used in future studies for testing the ability of improved hydration to slow down biological aging. Thus, in the National Institute on Aging CALERIE trial, the effect of caloric restriction on biological aging was already detectable after 24 months that is feasible time frame for randomized trials.46
In addition to the direct effect of life-long water restriction on life span and degenerative changes in the mouse model, pro-aging effects of hypohydration is also supported by other results from previous basic research studies. In those studies, increased sodium in cell culture models as well as water restriction in mouse model triggered the same changes that have been identified as underlying factors for accelerated aging and are currently considered as targets for anti-aging interventions.3,47, 48, 49, 50, 51 These processes include pro-inflammatory and pro-coagulation changes within vascular endothelial cells,52, 53, 54, 55 DNA damage,56,57 protein oxidation,58 increased energy expenditure due to metabolic remodeling towards metabolic water production10 and cellular scenescence.59
In summary, our study shows that people whose fasting serum sodium exceeds 142 mmol/l have increased risk to be biologically older, develop chronic diseases, and die at a younger age. This threshold can be used in clinical practice to identify people at risk. Since decreased hydration is one of the main factors that elevates serum sodium, the results are consistent with hypothesis that decreased hydration may accelerate aging. However, interventional trials are needed to prove this link. World-wide surveys find that more than 50% of people do not drink the recommended amounts of fluids.11, 12, 13, 14, 15 Therefore, results of our study provide additional reasons for reinforcing already existent recommendations for optimal fluid intake.60,61 A strategy was recently proposed for developing personal recommendations regarding optimal fluid intake depending on health status.62,63
Contributors
NID conceptualized the project, designed the analysis, interpreted the data and wrote the manuscript. NID and DL accessed, verified, and analyzed the data. COW and DL consulted on the data analysis, interpreted the data and edited the manuscript. AG adapted the code and performed biological age calculations. MB interpreted the data, edited the manuscript, acquired funding, and supervised the project. NID, DL, COW and MB were responsible for the decision to submit the manuscript. All authors read and approved the final version of the manuscript.
Data sharing statement
Anonymized data from the ARIC study are available through the National Heart, Lung, and Blood Institute Biologic Specimen and Data Repository Information Coordinating Center. Interested researchers may additionally contact the ARIC study Coordinating Center to access the study data.
Declaration of interests
The authors declare that there is no conflict of interest.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions we also thank Yolanda L. Jones, NIH Library, for manuscript editing assistance. Data from the ARIC study were obtained from the National Heart, Lung, and Blood Institute's Biologic Specimen and Data Repository Information Coordinating Center. This work was supported by Intramural Research program of the National Heart, Lung, and Blood Institute (NHLBI): the National Institutes of Health grant ZIA-HL006077-10. The ARIC study has been funded in whole or in part with federal funds from the NHLBI; the National Institutes of Health (NIH); and the Department of Health and Human Services, under contract numbers HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104404.
Appendix A. Supplementary data
Fig. S1.
Fig. S2.
Fig. S3.
Fig. S4.
References
- 1.Fontana L., Kennedy B.K., Longo V.D., Seals D., Melov S. Medical research: Treat ageing. Nature. 2014;511(7510):405–407. doi: 10.1038/511405a. [DOI] [PubMed] [Google Scholar]
- 2.Kaeberlein M., Rabinovitch P.S., Martin G.M. Healthy aging: The ultimate preventative medicine. Science. 2015;350(6265):1191–1193. doi: 10.1126/science.aad3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy B.K., Berger S.L., Brunet A., et al. Geroscience: linking aging to chronic disease. Cell. 2014;159(4):709–713. doi: 10.1016/j.cell.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Prince M.J., Wu F., Guo Y.F., et al. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–562. doi: 10.1016/S0140-6736(14)61347-7. [DOI] [PubMed] [Google Scholar]
- 5.Holman H.R. The relation of the chronic disease epidemic to the health care crisis. ACR Open Rheumatol. 2020;2(3):167–173. doi: 10.1002/acr2.11114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elliott M.L., Caspi A., Houts R.M., et al. Disparities in the pace of biological aging among midlife adults of the same chronological age have implications for future frailty risk and policy. Nat Aging. 2021;1(3):295–308. doi: 10.1038/s43587-021-00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vos T., Flaxman A.D., Naghavi M., et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bauer U.E., Briss P.A., Goodman R.A., Bowman B.A. Prevention of chronic disease in the 21st century: elimination of the leading preventable causes of premature death and disability in the USA. Lancet. 2014;384(9937):45–52. doi: 10.1016/S0140-6736(14)60648-6. [DOI] [PubMed] [Google Scholar]
- 9.Thornton S.N. Thirst and hydration: physiology and consequences of dysfunction. Physiol Behav. 2010;100(1):15–21. doi: 10.1016/j.physbeh.2010.02.026. [DOI] [PubMed] [Google Scholar]
- 10.Allen M.D., Springer D.A., Burg M.B., Boehm M., Dmitrieva N.I. Suboptimal hydration remodels metabolism, promotes degenerative diseases, and shortens life. JCI Insight. 2019;4(17) doi: 10.1172/jci.insight.130949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferreira-Pego C., Guelinckx I., Moreno L.A., et al. Total fluid intake and its determinants: cross-sectional surveys among adults in 13 countries worldwide. Eur J Nutr. 2015;54(Suppl 2):35–43. doi: 10.1007/s00394-015-0943-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Malisova O., Athanasatou A., Pepa A., et al. Water intake and hydration indices in healthy European adults: The European hydration research study (EHRS) Nutrients. 2016;8(4):204. doi: 10.3390/nu8040204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Drewnowski A., Rehm C.D., Constant F. Water and beverage consumption among adults in the United States: cross-sectional study using data from NHANES 2005-2010. BMC Publ Health. 2013;13 doi: 10.1186/1471-2458-13-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heen E., Yassin A.A., Madar A.A., Romøren M. Estimates of fluid intake, urine output and hydration-levels in women from Somaliland: a cross-sectional study. J Nutr Sci. 2021;10:e66. doi: 10.1017/jns.2021.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sui Z., Zheng M., Zhang M., Rangan A. Water and beverage consumption: analysis of the Australian 2011-2012 national nutrition and physical activity survey. Nutrients. 2016;8(11) doi: 10.3390/nu8110678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ackerman G.L. In: Clinical Methods: The History, Physical, and Laboratory Examinations. 3rd ed. Walker H.K., Hall W.D., Hurst J.W., editors. Butterworths; Boston: 1990. Chapter 194. Serum sodium; pp. 878–883. [PubMed] [Google Scholar]
- 17.Verbalis J.G. How does the brain sense osmolality? J Am Soc Nephrol. 2007;18(12):3056–3059. doi: 10.1681/ASN.2007070825. [DOI] [PubMed] [Google Scholar]
- 18.Dmitrieva N.I., Liu D., Wu C.O., Boehm M. Middle age serum sodium levels in the upper part of normal range and risk of heart failure. Eur Heart J. 2022;43(35):3335–3348. doi: 10.1093/eurheartj/ehac138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robertson G.L., Shelton R.L., Athar S. Osmoregulation of vasopressin. Kidney Int. 1976;10(1):25–37. doi: 10.1038/ki.1976.76. [DOI] [PubMed] [Google Scholar]
- 20.Thompson C.J., Bland J., Burd J., Baylis P.H. The osmotic thresholds for thirst and vasopressin release are similar in healthy man. Clin Sci (Lond) 1986;71(6):651–656. doi: 10.1042/cs0710651. [DOI] [PubMed] [Google Scholar]
- 21.The Atherosclerosis Risk in Communities (ARIC) study - design and objectives. ARIC investigators. Am J Epidemiol. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 22.Jackson R., Chambless L.E., Yang K., et al. Differences between respondents and nonrespondents in a multicenter community-based study vary by gender and ethnicity. J Clin Epidemiol. 1996;49(12):1441–1446. doi: 10.1016/0895-4356(95)00047-x. [DOI] [PubMed] [Google Scholar]
- 23.Levine M.E. Modeling the rate of senescence: can estimated biological age predict mortality more accurately than chronological age? J Gerontol A Biol Sci Med Sci. 2013;68(6):667–674. doi: 10.1093/gerona/gls233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Belsky D.W., Caspi A., Houts R., et al. Quantification of biological aging in young adults. Proc Natl Acad Sci USA. 2015;112(30):E4104–E4110. doi: 10.1073/pnas.1506264112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crimmins E., Vasunilashorn S., Kim J.K., Alley D. Biomarkers related to aging in human populations. Adv Clin Chem. 2008;46:161–216. doi: 10.1016/s0065-2423(08)00405-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Katz M.A. Hyperglycemia-induced hyponatremia--calculation of expected serum sodium depression. N Engl J Med. 1973;289(16):843–844. doi: 10.1056/NEJM197310182891607. [DOI] [PubMed] [Google Scholar]
- 27.Stookey J.D., Barclay D., Arieff A., Popkin B.M. The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr. 2007;61(2):190–199. doi: 10.1038/sj.ejcn.1602521. [DOI] [PubMed] [Google Scholar]
- 28.Klemera P., Doubal S. A new approach to the concept and computation of biological age. Mech Ageing Dev. 2006;127(3):240–248. doi: 10.1016/j.mad.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Krzywinski M., Altman N. Classification and regression trees. Nat Methods. 2017;14(8):755–756. [Google Scholar]
- 30.Breslow N. Design and analysis of case-control studies. Annu Rev Publ Health. 1982;3:29–54. doi: 10.1146/annurev.pu.03.050182.000333. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z., Duckart J., Slatore C.G., et al. Individuality of the plasma sodium concentration. Am J Physiol Ren Physiol. 2014;306(12):F1534–F1543. doi: 10.1152/ajprenal.00585.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gao S.G., Cui X.Q., Wang X.J., Burg M.B., Dmitrieva N.I. Cross-sectional positive association of serum lipids and blood pressure with serum sodium within the normal reference range of 135-145 mmol/L. Arterioscler Thromb Vasc Biol. 2017;37(3):598–+. doi: 10.1161/ATVBAHA.116.308413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collaborators GBDCoD Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang A.Y., Skirbekk V.F., Tyrovolas S., Kassebaum N.J., Dieleman J.L. Measuring population ageing: an analysis of the global burden of disease study 2017. Lancet Public Health. 2019;4(3):e159–e167. doi: 10.1016/S2468-2667(19)30019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sajadieh A., Binici Z., Mouridsen M.R., Nielsen O.W., Hansen J.F., Haugaard S.B. Mild hyponatremia carries a poor prognosis in community subjects. Am J Med. 2009;122(7):679–686. doi: 10.1016/j.amjmed.2008.11.033. [DOI] [PubMed] [Google Scholar]
- 36.Wannamethee S.G., Shaper A.G., Lennon L., Papacosta O., Whincup P. Mild hyponatremia, hypernatremia and incident cardiovascular disease and mortality in older men: a population-based cohort study. Nutr Metabol Cardiovasc Dis. 2016;26(1):12–19. doi: 10.1016/j.numecd.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar S., Berl T. Sodium. Lancet. 1998;352(9123):220–228. doi: 10.1016/S0140-6736(97)12169-9. [DOI] [PubMed] [Google Scholar]
- 38.Oh S.W., Baek S.H., An J.N., et al. Small increases in plasma sodium are associated with higher risk of mortality in a healthy population. J Kor Med Sci. 2013;28(7):1034–1040. doi: 10.3346/jkms.2013.28.7.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roussel R., Matallah N., Bouby N., et al. Plasma copeptin and decline in renal function in a cohort from the community: The prospective D.E.S.I.R. Study. Am J Nephrol. 2015;42(2):107–114. doi: 10.1159/000439061. [DOI] [PubMed] [Google Scholar]
- 40.Enhorning S., Tasevska I., Roussel R., et al. Effects of hydration on plasma copeptin, glycemia and gluco-regulatory hormones: a water intervention in humans. Eur J Nutr. 2019;58(1):315–324. doi: 10.1007/s00394-017-1595-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson R.J., Garcia-Arroyo F.E., Gonzaga-Sanchez G., et al. Current hydration habits: The disregarded factor for the development of renal and cardiometabolic diseases. Nutrients. 2022;14(10) doi: 10.3390/nu14102070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perrier E.T., Armstrong L.E., Bottin J.H., et al. Hydration for health hypothesis: a narrative review of supporting evidence. Eur J Nutr. 2021;60(3):1167–1180. doi: 10.1007/s00394-020-02296-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Enhorning S., Wang T.J., Nilsson P.M., et al. Plasma copeptin and the risk of diabetes mellitus. Circulation. 2010;121(19):2102–2108. doi: 10.1161/CIRCULATIONAHA.109.909663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schill F., Timpka S., Nilsson P.M., Melander O., Enhorning S. Copeptin as a predictive marker of incident heart failure. ESC Heart Fail. 2021;8(4):3180–3188. doi: 10.1002/ehf2.13439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stookey J.D., Kavouras S., Suh H., Lang F. Underhydration is associated with obesity, chronic diseases, and death within 3 to 6 Years in the U.S. Population aged 51-70 Years. Nutrients. 2020;12(4) doi: 10.3390/nu12040905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belsky D.W., Huffman K.M., Pieper C.F., Shalev I., Kraus W.E. Change in the rate of biological aging in response to caloric restriction: CALERIE biobank analysis. J Gerontol A Biol Sci Med Sci. 2017;73(1):4–10. doi: 10.1093/gerona/glx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrucci L., Gonzalez-Freire M., Fabbri E., et al. Measuring biological aging in humans: a quest. Aging Cell. 2020;19(2) doi: 10.1111/acel.13080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferrucci L., Fabbri E. Inflammageing: chronic inflammation in ageing, cardiovascular disease, and frailty. Nat Rev Cardiol. 2018;15(9):505–522. doi: 10.1038/s41569-018-0064-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jones D.P. Redox theory of aging. Redox Biol. 2015;5:71–79. doi: 10.1016/j.redox.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Redman L.M., Smith S.R., Burton J.H., Martin C.K., Il'yasova D., Ravussin E. Metabolic slowing and reduced oxidative damage with sustained caloric restriction support the rate of living and oxidative damage theories of aging. Cell Metabol. 2018;27(4):805–+. doi: 10.1016/j.cmet.2018.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.de Cabo R., Carmona-Gutierrez D., Bernier M., Hall M.N., Madeo F. The search for antiaging interventions: From elixirs to Fasting regimens. Cell. 2014;157(7):1515–1526. doi: 10.1016/j.cell.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dmitrieva N.I., Burg M.B. Elevated sodium and dehydration stimulate inflammatory signaling in endothelial cells and promote Atherosclerosis. PLoS One. 2015;10(6) doi: 10.1371/journal.pone.0128870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dmitrieva N.I., Burg M.B. Secretion of von Willebrand factor by endothelial cells links sodium to hypercoagulability and thrombosis. Proc Natl Acad Sci U S A. 2014;111(17):6485–6490. doi: 10.1073/pnas.1404809111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oberleithner H., Riethmueller C., Schillers H., MacGregor G.A., de Wardener H.E., Hausberg M. Plasma sodium stiffens vascular endothelium and reduces nitric oxide release. Proc Natl Acad Sci U S A. 2007;104(41):16281–16286. doi: 10.1073/pnas.0707791104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wild J., Soehnlein O., Dietel B., Urschel K., Garlichs C.D., Cicha I. Rubbing salt into wounded endothelium: sodium potentiates proatherogenic effects of TNF-alpha under non-uniform shear stress. Thromb Haemostasis. 2014;112(1):183–195. doi: 10.1160/TH13-11-0908. [DOI] [PubMed] [Google Scholar]
- 56.Dmitrieva N.I., Cai Q., Burg M.B. Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci U S A. 2004;101(8) doi: 10.1073/pnas.0308463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dmitrieva N.I., Cui K., Kitchaev D.A., Zhao K., Burg M.B. DNA double-strand breaks induced by high NaCl occur predominantly in gene deserts. Proc Natl Acad Sci U S A. 2011;108(51) doi: 10.1073/pnas.1114677108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Z., Dmitrieva N.I., Park J.H., Levine R.L., Burg M.B. High urea and NaCl carbonylate proteins in renal cells in culture and in vivo, and high urea causes 8-oxoguanine lesions in their DNA. Proc Natl Acad Sci U S A. 2004;101(25) doi: 10.1073/pnas.0402961101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dmitrieva N.I., Burg M.B. High NaCl promotes cellular senescence. Cell Cycle. 2007;6(24) doi: 10.4161/cc.6.24.5084. [DOI] [PubMed] [Google Scholar]
- 60.Agostoni C. European food safety association: EFSA panel on dietetic products, nutrition, and allergies (NDA); Scientific opinion on dietary reference values for water. EFSA J. 2010;8(3):1459. [Google Scholar]
- 61.US Institute of medicine . THE NATIONAL ACADEMIES PRESS; Washington, DC: 2005. Dietary reference intakes for water, potassium, sodium, chloride, and sulfate. [Google Scholar]
- 62.Dmitrieva N.I., Rosing D.R., Boehm M. Making decision about fluid intake: increase or not increase. Eur Heart J. 2022;43(41):4438–4439. doi: 10.1093/eurheartj/ehac368. [DOI] [PubMed] [Google Scholar]
- 63.Sarafidis P., Ferro C.J., Ortiz A. Hypernatremia and subclinical chronic kidney disease. Eur Heart J. 2022;43(41):4436–4437. doi: 10.1093/eurheartj/ehac367. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.