Abstract
Background
Identifying mechanisms of major depressive disorder that continue into remission is critical, as these mechanisms may contribute to subsequent depressive episodes. Biobehavioral markers related to depressogenic self-referential processing biases have been identified in adults with depression. Thus, we investigated whether these risk factors persisted during remission as well as contributed to the occurrence of stress and depressive symptoms over time.
Methods
At baseline, adults with remitted depression (n = 33) and healthy control subjects (n = 33) were administered diagnostic and stress interviews as well as self-report symptom measures. In addition, participants completed a self-referential encoding task while electroencephalography data were acquired. Stress interviews and self-report symptom measures were readministered at the 6-month follow-up assessment.
Results
Drift diffusion modeling showed that compared with healthy individuals, adults with remitted depression exhibited a slower drift rate to negative stimuli, indicating a slower tendency to reject negative stimuli as self-relevant. At the 6-month follow-up assessment, a slower drift rate to negative stimuli predicted greater interpersonal stress severity among individuals with remitted depression but not healthy individuals while controlling for both baseline depression symptoms and interpersonal stress severity. Highlighting the specificity of this effect, results were nonsignificant when predicting noninterpersonal stress. For self-relevant positive words endorsed, adults with remitted depression exhibited smaller left- than right-hemisphere late positive potential amplitudes; healthy control subjects did not show hemispheric differences.
Conclusions
Self-referential processing deficits persist into remission. In line with the stress generation framework, these biases predicted the occurrence of interpersonal stress, which may provide insight about a potential pathway for the re-emergence of depressive symptoms.
Keywords: Cognitive vulnerability, Late positive potential, Major depressive disorder, Remission, Stress generation
Major depressive disorder (MDD) is a major public health problem associated with significant economic and psychosocial burden (1,2). Although effective treatments for MDD are available (3), approximately 85% of adults who remit from depression experience a recurrence within 15 years (4). Therefore, identifying biobehavioral markers that persist into remission may lead to the development of novel interventions that could prevent future recurrences.
A core feature of MDD is maladaptive self-schemas that often develop from childhood experiences (5,6). Across development, these schemas contribute to depressogenic processing biases whereby information perceived as negative and self-relevant increases depression risk (7,8). Negative self-schemas and depressogenic processing biases emerge in childhood, persist into adulthood, and predict MDD onset and worsening (9, 10, 11, 12, 13, 14, 15, 16, 17). These findings suggest that depressogenic self-referential processing biases emerge early in development and are critical for understanding depression.
Studying individuals with remitted depression is important for establishing whether depressogenic self-referential processing biases are state dependent. Although not currently in episode, these individuals remain vulnerable to recurrences (18,19). Notably, studies of remission cannot determine whether a given deficit reflects a precursor or scar of depression, but they reduce the confounding effects of current symptoms while evaluating vulnerability factors. Presently, it is unclear whether depressogenic self-referential processing biases persist into remission, as meta-analyses have yielded mixed results (20, 21, 22). Some studies reported that individuals with remitted depression and those who are currently depressed exhibit the same bias (23, 24, 25, 26), and yet other studies showed a bias similar to healthy adults (27,28). The predictive validity of self-referential processing biases also is mixed, with some studies showing that negative biases predicted symptom recurrence (29) whereas others did not (30). Given these equivocal findings, research is needed to clarify whether depressogenic self-referential processing biases persist into remission and, accordingly, predict depression recurrence.
Modeling Processing Biases
An informative self-referential processing metric is drift rate (v), which is derived from drift diffusion modeling and integrates responses and reaction time (RT) to estimate the information accumulation rate to make a binary decision (31, 32, 33, 34, 35). Drift diffusion modeling calculates trial-by-trial variability, reducing the influence of outlier trials and baseline group differences (e.g., psychomotor slowing), thereby providing an enhanced measurement of processing speed than the central tendency of RT (35). Drift rate reflects the average slope of RT to make a decision (35,36), where larger absolute values correspond to faster, more consistent responses and smaller values reflect slower, less consistent responses (37). Drift rates to negative stimuli predict depression severity (13,38) and differentiate depressed from nondepressed individuals (36,39). It remains unknown, however, whether these patterns persist into remission.
Neurophysiological Correlates of Self-referential Processing
Several event-related potentials (ERPs) have been implicated during self-referential processing. The P2 is associated with early semantic and emotional monitoring (40,41) and typically peaks between 100 and 200 ms poststimulus in centroparietal regions (42,43). In addition, the late positive potential (LPP) is associated with elaborative processing and memory encoding (42,44). The LPP can be divided into two components: an early LPP (peaking around 300–600 ms in parieto-occipital regions), corresponding to task engagement and motivation, and a late LPP (peaking after 600 ms in frontocentral regions), reflecting longer-term emotional encoding (45,46).
Prior research has shown that while healthy adults exhibit greater P2 amplitudes for positive versus negative self-referential stimuli, currently depressed individuals show the opposite pattern (28,47) or no differentiation (48). Regarding the LPP, adults with depression show greater amplitudes to negative versus positive self-referential stimuli, whereas healthy adults show the opposite pattern or no differences (28,47, 48, 49). Therefore, adults with depression exhibit greater arousal to negative stimuli while healthy individuals show enhanced attendance to positive stimuli. Only one study, to our knowledge, has probed these markers in adults with remitted depression (28). Remitted individuals exhibited larger P2 amplitudes for negative than positive stimuli but showed the opposite pattern for the LPP. Thus, early attentional capture for negative self-referential stimuli may persist into remission, whereas elaborative processing and encoding may not. Given limited power in this study, further research is needed to elucidate whether neurophysiological markers of depressogenic self-referential processing persist into remission and prospectively contribute to symptoms.
Furthermore, the majority of electrophysiology research probing self-referential processing has analyzed all words viewed [e.g., (28,48)]. However, self-referential processing may be best measured by focusing on endorsed words (47,50). For example, healthy adults show greater LPP amplitudes to endorsed versus rejected positive words (50). At the same time, individuals with depression do not differ on early and late ERPs following endorsed versus rejected positive stimuli (47). These findings suggest that endorsement of stimuli may affect ERP amplitudes, but more research is needed.
Hemispheric Laterality of Self-referential Processing
Classic models of MDD feature impairments in self-appraisal, approach mechanisms, and emotional arousal, potentially explained by reduced left hemispheric activation (51,52). Lesion studies suggest that self-referential processes are localized to the right hemisphere (53, 54, 55), while increasing evidence suggests that activation is diffuse across hemispheres with autobiographical information as well as personal and emotional traits processed in the right and left hemispheres, respectively (54,56, 57, 58). Contemporary evidence provides some support for classic models of MDD, suggesting that blunted left hemispheric activation is implicated in depression (59, 60, 61). Only one study, to our knowledge, has probed laterality in remitted adults during self-referential processing (28). Remitted adults showed greater right than left LPP amplitudes, suggesting that blunted left hemisphere activity may be trait-like. Although functional magnetic resonance imaging is not directly analogous with electrophysiology research, compared with healthy adults, individuals with depression exhibited greater left than right dorsolateral prefrontal cortex activation during self-referential processing (62). In addition, adults who received MDD treatment exhibited reduced dorsolateral prefrontal cortex asymmetries, which corresponded to decreased depressive symptoms (63). Thus, asymmetrical dorsolateral prefrontal cortex activation may be an important component of depression symptomology. However, research is needed to determine whether these lateralized effects persist into remission.
Stress Generation Framework
Decades of research have demonstrated a reciprocal relationship between stress and depression, which is more commonly referred to as stress generation (64). Namely, it is now known that depressogenic vulnerability factors may shape the type of stress exposure, particularly among individuals with remitted depression, rendering some individuals more susceptible to experience interpersonal stressors, which over time may lead to the re-emergence of depression (65,66). In line with the stress generation framework, depressogenic self-referential processing biases, which are believed to remain stable among individuals with remitted depression, negatively affect individuals’ views of themselves, the world, and the future (i.e., cognitive triad) (67) and, accordingly, may profoundly affect day-to-day interpersonal interactions, leading to greater interpersonal stress. Over time, interpersonal stress exposure may then serve as a catalyst for the re-emergence of depression symptoms because these stressors frequently precede depressive episodes (65,66) and often hasten their development (68,69).
Goals of the Present Study
An important next step is to test whether depressogenic biobehavioral markers (15,70) related to self-referential processing persist into remission. First, we hypothesized that compared with healthy adults, adults with remitted depression would exhibit more negative processing biases and slower negative drift rates to negative words. Second, relative to healthy adults, adults with remitted depression would show larger early (P2) and late (LPP) ERP amplitudes to negative than positive words. Given prior research (28,59, 60, 61), we also expected that remitted, but not healthy, adults would show reduced left compared with right LPP amplitudes to endorsed positive words. Finally, as a preliminary test of the stress generation framework, we examined whether depressogenic biobehavioral markers were longitudinal predictors of interpersonal stress and depression symptoms.
Methods and Materials
Participants
Healthy control adults and adults with remitted depression between 20 and 35 years old were recruited from a previously completed parent project that evaluated familial risk for depression and anxiety (71,72). For this study, inclusion criteria included right-handedness and English fluency (see the Supplement for additional criteria). Based on these criteria, 104 of the 745 participants from the parent project were recontacted. Remitted adults reported a past major depressive episode; however, this could not have occurred within the 2 months before the assessment. Healthy control adults reported no lifetime mental disorders. A total of 36 participants were ineligible owing to a current MDD episode (n = 9) or because they declined to participate (n = 27). Two participants completed the study, but their electroencephalography (EEG) data were unusable. The final sample included 33 healthy control adults and 33 adults with remitted depression (mean age = 24.94, SD = 3.28 years). Sample characteristics are summarized in Table 1.
Table 1.
Descriptive Statistics for the Sample Stratified by Group
| Variables | HC | remMDD | t/χ2 (df) | p | d/Δ/φ/V |
|---|---|---|---|---|---|
| Biological Sexa, n (%) | 0.07 (1) | .792 | −0.033 | ||
| Female | 23 (69.70%) | 22 (66.67%) | |||
| Male | 10 (30.30%) | 11 (33.33%) | |||
| Age, Years, Mean (SD) | 24.61 (3.08) | 25.27 (3.49) | 0.82 (64) | .413 | 0.203 |
| Racea, n (%) | 9.50 (5) | .091 | 0.379 | ||
| African American/Black | 3 (9.09%) | 6 (18.18%) | |||
| Asian | 8 (24.24%) | 3 (9.09%) | |||
| Hispanic | 8 (24.24%) | 10 (30.30%) | |||
| Middle Eastern | 3 (9.09%) | 0 (0%) | |||
| Multiple races | 0 (0%) | 3 (9.09%) | |||
| White | 11 (33.33%) | 11 (33.33%) | |||
| Marital Statusa, n (%) | 0.13 (1) | .720 | −0.044 | ||
| Married or partnered | 4 (12.12%) | 5 (15.15%) | |||
| Never married | 29 (87.88%) | 28 (84.85%) | |||
| Highest Educationa, n (%) | 1.05 (2) | .592 | 0.126 | ||
| Some college, trade school, or current student | 5 (15.15%) | 6 (18.18%) | |||
| Two- or 4-year degree | 14 (42.42%) | 17 (51.52%) | |||
| Some graduate/professional school or graduate degree | 14 (42.42%) | 10 (33.30%) | |||
| Employmenta, n (%) | 2.43 (3) | .489 | 0.192 | ||
| Full-time | 14 (42.42%) | 17 (51.52%) | |||
| Part-time | 6 (18.18%) | 6 (18.18%) | |||
| Student | 11 (33.33%) | 6 (18.18%) | |||
| Unemployed | 2 (6.06%) | 4 (12.12%) | |||
| Interpersonal Stress Severity, Mean (SD) | |||||
| Baselinea | 18.94 (18.38) | 34.91 (20.97) | 3.29 (64) | .002 | 0.810 |
| 6 mob | 1.79 (4.81) | 9.45 (10.49) | 3.05 (24.74) | .005 | 1.593 |
| IDAS Depression, Mean (SD) | |||||
| Baselinea | 28.06 (4.96) | 36.70 (8.73) | 4.94 (50.70) | <.001 | 1.743 |
| 6 moc | 31.86 (6.42) | 41.43 (12.24) | 3.39 (31.74) | .002 | 1.493 |
d/Δ/φ/V = effect sizes for t test or χ2; Welch's correction was used where appropriate.
HC, healthy control; IDAS, Inventory of Depression and Anxiety Symptoms; remMDD, remitted major depressive disorder.
HC subjects: n = 33; remitted depressed individuals: n = 33.
HC subjects: n = 28; remitted depressed individuals: n = 20.
HC subjects: n = 28; remitted depressed individuals: n = 23.
Procedure
The University of Illinois at Chicago Institutional Review Board approved study procedures. Data were collected from May 2017 through May 2019. Participants provided informed consent. During the first visit, participants were administered clinical interviews and self-report measures to assess demographic data, current depression symptoms, and medication use. Following this assessment, EEG data were acquired while participants completed a self-referential processing task (see the Supplement for additional EEG task data collected). At the 6-month follow-up, healthy control subjects (n = 29; 87.88%) and remitted adults (n = 25; 75.76%) were readministered a structured stress interview and self-report measures. Baseline depression symptoms and interpersonal stress severity did not differ among completers versus noncompleters (p > .605). Participants were remunerated $120 for the baseline assessment and $45 for the follow-up.
Clinical Interviews
Structured Clinical Interview for DSM-5
Lifetime mental disorders were assessed using the semistructured Structured Clinical Interview for DSM-5 (73). Research has demonstrated strong reliability and validity (74), particularly relating to MDD (75).
Stress and Adversity Inventory
The Stress and Adversity Inventory (76) was administered at the baseline and 6-month follow-up visits to assess stress occurring over the lifetime and past 6 months, respectively. Given prior research focusing on stress generation (65,66), analyses focused on interpersonal stress. To test the specificity of our effects, we estimated models with noninterpersonal stress (see the Supplement for details). The Stress and Adversity Inventory has previously shown excellent test-retest reliability [r = 0.90−0.95 (76,77)].
Self-report Questionnaire
Inventory of Depression and Anxiety Symptoms
The Inventory of Depression and Anxiety Symptoms (78) is a 99-item self-report measure assessing depression and anxiety symptoms over a 2-week period. The Inventory of Depression and Anxiety Symptoms has shown strong psychometric properties (79). Analyses focused on the 20-item general depression subscale (78,79), which demonstrated excellent internal consistency at baseline (α = 0.86) and follow-up (α = 0.90).
Experimental Task
Self-referential Encoding Task
A self-referential encoding task (11,67) was administered using Presentation software (v.18.2, NeuroBehavioral Systems). Participants viewed 40 positive and 40 negative words (see the Supplement for word list). On each trial, a word was presented for 200 ms followed by a fixation cross for 1800 ms and then an untimed prompt, “Does this word describe you?” Participants responded “Yes” or “No” on a button box. Intertrial intervals were jittered between 1575 and 1775 ms. After completing 80 trials, participants counted backward from 50, handwrote words viewed from memory, and completed a recognition task including 80 words from the task and 80 foils.
Behavioral analyses focused on processing bias and drift rate. Processing bias scores were computed by dividing the number of positive or negative words that were endorsed and later recalled by the total number of words endorsed. Hierarchical Drift Diffusion Model for Python [v.0.6.0 (80)] was used to compute drift rate. More positive drift values for positive words (v > 0) reflect more rapid evidence accumulation leading to an endorsement as self-referential. Conversely, more negative values for negative words (v < 0) reflect more rapid evidence accumulation leading to a rejection as self-referential (see the Supplement for details).
EEG Recording, Data Reduction, and Analysis
Continuous EEG data were recorded using a 64-channel elastic cap and the ActiveTwo BioSemi system (BioSemi). Data were sampled at 1024 Hz, using a low-pass fifth-order sync filter with −3-dB cutoff at 208 Hz. The BioSemi ActiveTwo replaces a conventional ground electrode with two electrodes that form a feedback loop, a common mode sense active electrode located between PO3 and POz and a driven right leg electrode located between POz and PO4. Vertical and horizontal eye movement were monitored using electrodes above and below the left eye and near the outer canthi of both eyes, respectively.
BrainVision Analyzer 2.1 (Brain Products GmbH) was used to process EEG data offline. Data were re-referenced to the average reference, and offline filters (0.1–30 Hz) were applied. Ocular artifacts were corrected by subtracting the ocular channels’ voltages (81). EEG data were segmented 200 ms before stimulus onset and extended to 1200 ms. Intervals for individual channels were rejected using an automated procedure applying the following criteria: 1) a voltage step >50 μV between sample rates; 2) a voltage difference >100 μV every 200 ms within a trial; 3) a minimum and maximum allowed amplitude of −100 μV and 100 μV, respectively; and 4) a maximum voltage difference of <0.50 μV within a 100-ms interval.
ERPs were time locked to positive and negative words (see Tables S1 and S2 for average number of segments per condition; see the Supplement for Spearman-Brown split-half reliability). Based on maximal amplitudes within topographical maps (Figures S1 and S2), the P2 was scored as the average amplitude over POz during 200 to 280 ms, and the early LPP was scored as the average amplitude over Pz during 300 to 500 ms after stimulus. The late LPP was scored as the average amplitude over FPz and AFz between 500 and 1200 ms after stimulus. These electrodes were selected based on visual inspection of topographical maps and our prior research (11,67). These studies, however, used a different EEG system (HydroCel GSN) with a denser array of electrodes (128 channels) and a higher impedance threshold (below 75 kΩ). Thus, to minimize the potential effect of noise, these prior studies averaged across electrodes.
Additional analyses focused on trials of endorsed positive words. Mean amplitudes from right (FP2/AF4) and left (FP1/AF3) prefrontal electrodes were used to assess laterality of the late LPP for endorsed positive words. Residualized scores were calculated using right electrodes as the predictor variable and left electrodes as the outcome variable in a linear regression to compute standardized residuals. Thus, more positive residuals indicate greater left than right amplitudes, and more negative scores indicate greater right than left amplitudes. Residualized difference scores are preferred because they isolate variance unique to a specific condition (82). Previous work suggests that the LPP is reliable with at least eight trials (83), and thus, 1 healthy control subject was removed from analyses of positive endorsed trials at midline electrodes, and 2 participants (healthy control: n = 1; remitted depressed: n = 1) were removed from laterality analyses. Most participants (n = 47; 71%) endorsed fewer than eight negative words (healthy control: n = 27; remitted depressed: n = 20), preventing the analysis of endorsed negative words.
Data Analysis
Analyses were conducted in SPSS v.27 and R v.3.6.1 (see Table S3 for sample sizes of each group analysis). Pearson correlations were calculated among behavioral, ERP, and symptom severity variables. Bayesian inference (as part of the Hierarchical Drift Diffusion Model package) was used to compare the posterior distribution for drift rate between groups for negative and positive words, with significance defined as <5% overlap (Bayesian q value < .05).
Repeated-measures analyses of covariance tested the group (healthy control, remitted depressed) × valence (positive, negative) interaction for processing bias, P2, early LPP, and late LPP while controlling for current depression symptoms (see the Supplement for endorsement, recall, and recognition). The group × laterality analysis also controlled for baseline depression symptoms.
To determine whether significant biobehavioral indices predicted interpersonal stress and depression symptom severity at the 6-month follow-up, we conducted separate linear regressions controlling for baseline stress and depression severity as well as baseline depression. A square root transformation for 6-month stress severity was used to reduce positive skew, and predictors in these models were mean centered. Reported slopes and standard errors from stress models were squared. To reduce the impact of outliers, 6-month depression scores were winsorized.
Results
Descriptive Analyses
Compared with the healthy adults, adults with remitted depression reported more severe depression symptoms. Drift rate to negative stimuli positively correlated with depression symptoms, suggesting that requiring more time to reject negative words as self-relevant was related to greater symptom severity (Table 2).
Table 2.
Correlations Among Symptoms, Behaviors, and Neurophysiological Components
| Variables | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Baseline depression symptomsa | – | – | – | – | – | – | – | – |
| 2 | 6-month depression symptomsb | 0.556c | – | – | – | – | – | – | – |
| 3 | Baseline interpersonal stress severitya | 0.292d | 0.251 | – | – | – | – | – | – |
| 4 | 6-month interpersonal stress severitye | 0.462c | 0.485c | 0.462c | – | – | – | – | – |
| 5 | Negative drift ratea | 0.511c | 0.374f | 0.153 | 0.426f | – | – | – | – |
| 6 | Positive drift ratea | −0.231 | −0.409f | −0.004 | −0.082 | −0.467c | – | – | – |
| 7 | FP1/AF3 (Positive endorse)g | −0.126 | −0.173 | −0.364f | −0.242 | −0.016 | −0.046 | – | – |
| 8 | FP2/AF4 (Positive endorse)g | −0.027 | 0.041 | −0.218 | −0.090 | −0.119 | −0.127 | 0.744c | – |
| 9 | Residualized LPP (Positive endorse)g | −0.159 | −0.324d | −0.301d | −0.271 | 0.110 | 0.074 | 0.669c | 0.000h |
Depression measured by the IDAS General Depression subscale. Residualized difference wave between FP1/AF3 and FP2/AF4 for endorsed positive stimuli.
IDAS, Inventory of Depression and Anxiety Symptoms; LPP, late positive potential.
Healthy control subjects: n = 33; remitted depressed individuals: n = 33.
Healthy control subjects: n = 28; remitted depressed individuals: n = 23.
p < .001.
p < .05.
Healthy control subjects: n = 28; remitted depressed individuals: n = 20.
p < .01.
Healthy control subjects: n = 31; remitted depressed individuals: n = 32.
In linear regression, the correlation between predictors and the residual is always zero.
Behavioral Phenotypes
Means, standard deviations, and ranges for endorsement, processing bias, and drift rate are summarized in Table S4.
Processing Bias
The main effect of group was nonsignificant (F1,59 = 0.19, p = .668, ηp2 = 0.003). There was a significant main effect of valence (F1,59 = 12.69, p = .001, ηp2 = 0.177). Participants exhibited greater positive than negative processing biases. The group × valence interaction was nonsignificant (F1,59 = 0.43, p = .515, ηp2 = 0.007).
Drift Rate
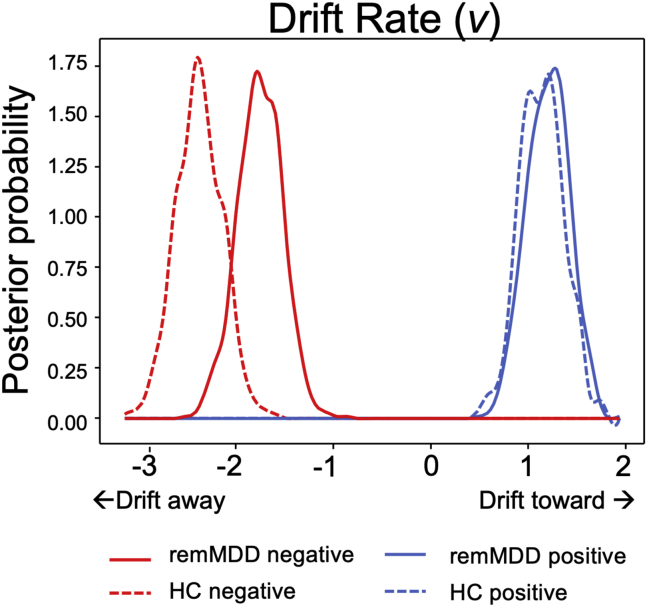
When examining the posterior probability distributions of drift rate (Figure 1), healthy control subjects exhibited significantly greater negative drift rates (mean = −2.34, SD = 0.24, 95% CI = −2.79 to −1.89) than remitted individuals (mean = −1.73, SD = 0.23, 95% CI = −2.19 to −1.29), which corresponded to a between-group overlap of 2.6% (q = .026). Thus, remitted individuals exhibited slower evidence accumulation needed to reject negative words compared with healthy control subjects. Comparatively, healthy control subjects showed a similar positive drift rate (mean = 1.10, SD = 0.22, 95% CI = 0.68 to 1.51) compared with remitted individuals (mean = 1.17, SD = 0.22, 95% CI = 0.75 to 1.57) that did not differ between groups (q = .41), suggesting that similar evidence accumulation rates were needed to endorse positive words.
Figure 1.
Distribution of drift rate (v) for healthy control (HC) subjects (dashed lines) (n = 33) and individuals with remitted major depressive disorder (remMDD) (solid lines) (n = 33) for positive (blue lines) and negative (red lines) stimuli. These results show that individuals with remMDD exhibited slower drift rate to negative stimuli than did the HC subjects. However, the two groups did not differ in their drift rate to positive stimuli.
Early and Late ERPs
All Words Viewed: P2
When analyzing all positive and negative words, the main effects of group (F1,62 = 0.29, p = .592, ηp2 = 0.005), valence (F1,62 = 0.86, p = .358, ηp2 = 0.014), and group × valence interaction were nonsignificant (F1,62 = 1.12, p = .295, ηp2 = 0.018) (Figure S1).
All Words Viewed: Early and Late LPP
For the early LPP, the main effects of group (F1,63 = 0.13, p = .718, ηp2 = 0.002), valence (F1,63 = 0.39, p = .535, ηp2 = 0.006), and group × valence interaction were nonsignificant (F1,63 = 0.10, p = .752, ηp2 = 0.002) (Figure S2). When probing the late LPP, the main effects of group (F1,63 = 0.10, p = .756, ηp2 = 0.002), valence (F1,63 = 0.66, p = .419, ηp2 = 0.010), and group × valence interaction were nonsignificant (F1,63 = 0.27, p = .604, ηp2 = 0.004) (Figure S3).
Endorsed Positive Words Only: P2
When focusing only on endorsed positive words, the groups did not differ on P2 amplitudes (t62 = 0.60, p = .548, d = 0.151).
Endorsed Positive Words Only: Early and Late LPP
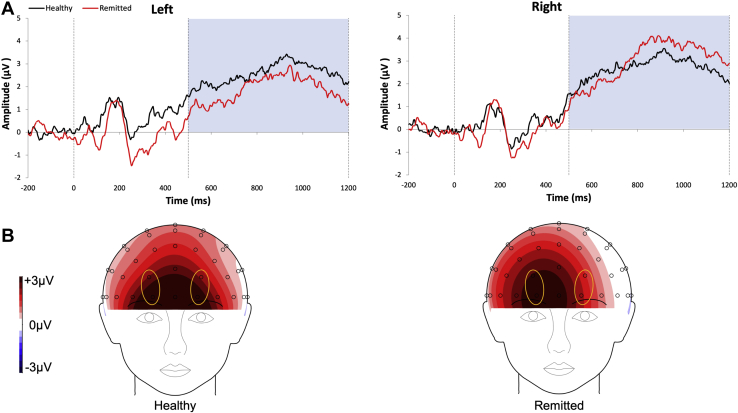
When examining the early LPP following endorsed positive words, the groups did not significantly differ (t63 = 0.75, p = .456, d = 0.186). For late LPP at the midline, no between-group differences emerged (t63 = −0.55, p = .582, d = −0.137). However, given prior research showing asymmetrical frontal activity during self-referential processing (28) and the pronounced laterality effects in the topographical map (Figure 2), we tested for asymmetrical differences in frontal electrodes. After controlling for current depressive symptoms, the group × laterality interaction was significant (F1,60 = 4.01, p = .0496 ηp2 = 0.063). Follow-up comparisons indicated that remitted individuals exhibited a significantly greater right than left LPP (p = .002, ηp2 = 0.154), whereas healthy adults did not (p = .841, ηp2 = 0.001). Similar effects also were found without covarying for depression symptoms (Supplement).
Figure 2.
(A) Waveforms depicting the amplitudes of positive endorsed stimuli in the left (FP1, AF3) and right (FP2, AF4) electrodes with healthy control subjects (black line) (n = 31) and individuals with remitted depression (red line) (n = 32); shaded area is window for the late late positive potential. (B) Topographical maps showing prefrontal electrodes for healthy control subjects (left) and individuals with remitted depression (right). Highlighted in yellow circles are the electrodes of interest: FP1/AF3 and FP2/AF4.
Predicting Stress and Depression Symptom Severity Over Time
Stress Severity
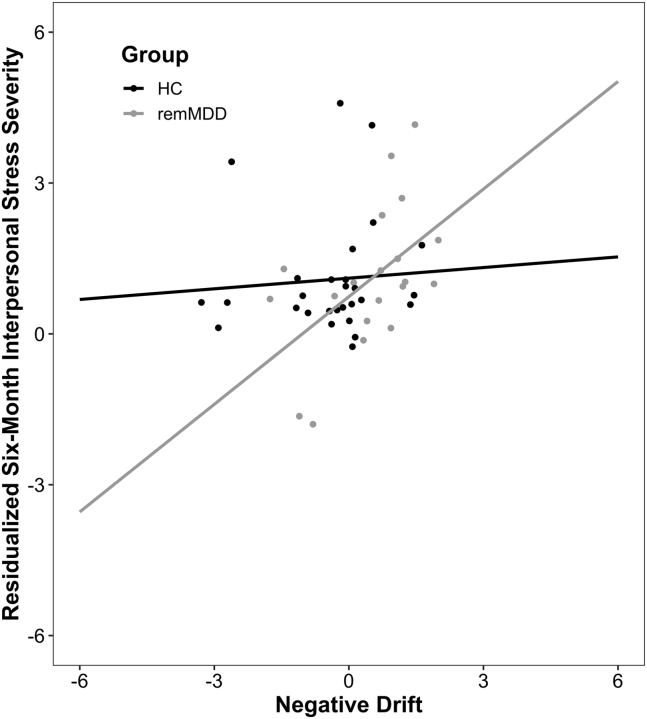
After controlling for baseline depression symptoms and interpersonal stress severity, the group × negative drift interaction predicted interpersonal stress severity at the 6-month follow-up (B = 0.75, SE = 0.12, p = .017, partial R2adj = 0.108). Post hoc analyses revealed a positive association between negative drift and interpersonal stress severity for remitted adults (B = 0.99, SE = 0.09, p = .002) but not healthy adults (p = .495) (Figure 3). Highlighting specificity of this effect, neither the group × negative drift interaction nor main effect for negative drift predicted noninterpersonal stress (ps > .637).
Figure 3.
Plot of the interaction between group (healthy control [HC] subjects, black; individuals with remitted major depressive disorder [remMDD], gray) and negative drift scores predicting squared root residualized interpersonal stress severity at the 6-month follow-up, controlling for baseline depression symptoms and interpersonal stress severity. HC: n = 28; remMDD: n = 20.
A subsequent model testing the group × lateralized late LPP interaction did not reveal a significant effect (B = 0.36, SE = 0.22, p = .210, partial R2adj = 0.015). The lateralized late LPP did not predict interpersonal stress (B = −0.08, SE = 0.06, p = .245, partial R2adj = 0.009).
Depression Symptoms
In separate models controlling for baseline depression symptoms, neither the group × negative drift (β = 0.18, SE = 0.27, p = .520, partial R2adj = −0.012) nor group × lateralized late LPP interaction (β = −0.26, SE = 0.27, p = .342, partial R2adj = −0.002) predicted depression symptoms at the follow-up. Although negative drift was not a main effect predictor of follow-up depression symptoms (β = 0.14, SE = 0.13, p = .294, partial R2adj = 0.003), lateralized late LPP (i.e., reduced LPP in the left vs. right hemisphere) showed a nonsignificant trend for predicting follow-up depression symptom severity (β = −0.24, SE = 0.13, p = .063, partial R2adj = 0.054).
Discussion
Identifying biobehavioral markers among individuals with remitted depression is essential to clarify risk factors for MDD. Several important findings emerged. First, drift diffusion modeling showed that remitted adults exhibited slower drift rates to negative stimuli (i.e., slower to reject negative words) than healthy adults. Second, slower negative drift rates were cross-sectionally associated with greater depressive symptom severity (Table 2). Third, contrary to our hypotheses, there were no group differences when comparing early or late ERPs for all seen words. However, analyses focusing on LPP amplitudes for endorsed positive words indicated that relative to healthy adults, remitted individuals exhibited reduced LPP amplitudes in the left versus right hemisphere. Last, in line with the stress generation framework, drift rate to negative stimuli predicted interpersonal, but not noninterpersonal, stress severity among remitted individuals over time.
Self-referential Decision Making
Compared with healthy adults, adults with remitted depression exhibited slower negative drift rates, suggesting that they required more evidence to reject negative stimuli as self-relevant. Relative to a central tendency RT approach, drift rate may more precisely probe self-referential decision making, as it is less susceptible to outlier trials and sensitive to biases that persist in remission (33, 34, 35,84,85). Drift rate reflects the rate at which an individual accumulates information to derive meaning of a stimulus and respond consistently (31,32,35). Therefore, rejecting negative stimuli may be more challenging for remitted than healthy individuals (86). As prior research found that slower negative drift rates distinguished adults with depression symptoms from healthy individuals (36,38,39,87), these results extend findings and suggest that this impairment persists during remission.
Neurophysiological Markers of Depressogenic Self-referential Processing
Prior self-referential processing research has consistently demonstrated blunted ERP amplitudes to positive versus negative stimuli (28,47, 48, 49). This research, however, has not explored whether this effect is being driven by blunted ERP amplitudes for endorsed positive words, which may better capture self-reference attributes. Relative to healthy adults, remitted individuals showed reduced late LPP amplitudes in the left versus right hemisphere to positive endorsed words. Prior results suggest that positive self-referential processing is reflected in left frontal LPPs (47,48,88,89) and potentially mediated by emotional arousal (57,90, 91, 92). Indeed, eliciting emotional arousal is associated with left prefrontal hemispheric activation (93, 94, 95, 96), and depressed individuals show impaired emotional arousal and reduced left prefrontal activity (51,52,60,94,97). Moreover, following neurostimulation to left prefrontal regions, individuals with current MDD symptoms show increased emotional arousal and positive self-referential processing compared with baseline (98,99). Therefore, attenuated left late LPP amplitudes in remitted individuals may reflect blunted emotional arousal while processing positive self-referential stimuli. Notably, reduced emotional arousal during positive self-referential processing may impede reinforcement of positive self-schemas, which may increase vulnerability for relapse (100,101). An important next step is to establish whether left frontal LPP amplitudes longitudinally predict MDD recurrence.
Interpersonal Stressful Exposure
Negative drift rate at baseline predicted worsening interpersonal, but not noninterpersonal, stress severity over time for remitted adults. Several studies have shown that negative cognitive biases can promote stress generation, particularly for interpersonal stress (65,102,103). Our findings extend this work by showing that a slower tendency to reject negative words as self-relevant may increase interpersonal stress susceptibility among remitted individuals. Interestingly, as these effects were not found for depression symptoms, cognitive mechanisms for future depressive symptoms may be distinguishable from those involved in experiencing stressors (29). Insomuch as interpersonal stressors may precede relapse (65,66,104), future studies using longer follow-up periods are needed to further test the predictive validity of self-referential processing biases.
Limitations
There are several noteworthy limitations. First, given the study design, it is unclear if biobehavioral markers in remitted individuals are a cause or consequence of depression. Second, we did not analyze the count, duration, severity, or recency of previous depressive episodes, which may influence these findings. For example, in remitted adults, more severe episodes and shorter remission periods predict greater depressogenic self-referential processing biases (30,105). Third, participants endorsed an insufficient number of negative word trials to analyze ERPs in this condition. We observed blunted LPP amplitudes to endorsed positive words, but we could not test ERP-related effects for endorsed negative words. Fourth, although the Stress and Adversity Inventory has many strengths, it cannot tease apart whether stressors were dependent or independent. Last, our sample size was modest, and thus, replicating effects in a larger sample is essential.
Conclusions
Biobehavioral markers of depressogenic self-referential processing persist into symptom remission. Compared with healthy adults, adults with remitted depression showed LPP alterations and greater difficulty rejecting negative stimuli, the latter of which predicted worsening interpersonal stress. It is, therefore, critical for future research to determine whether residual depressogenic self-referential processing biases longitudinally predict MDD recurrence.
Acknowledgments and Disclosures
This project was partially supported by funds from the National Institute of Mental Health (Grant Nos. R21 MH112330 and R01 MH119771 [to RPA and SAS] and Grant No. K08 MH103443 [to GMS]), the Society in Science-Branco Weiss Fellowship (to GMS), and the NARSAD Young Investigator Grant from the Brain & Behavior Research Foundation (Grant No. 23958 [to GMS]).
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
RPA and SAS developed the study concept. GOA, RAK, RPA, SAS, DP, and GMS drafted the paper. VC, RAK, and KLA assisted with data processing and data collection. DP and GOA performed data analysis and interpretation under the supervision of RPA and SAS. All authors approved the final version of the paper submission.
RPA serves as an unpaid scientific adviser for Ksana Health and the Research Grants Committee of the American Foundation for Suicide Prevention. All other authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary material cited in this article is available online at https://doi.org/10.1016/j.bpsgos.2021.12.005.
Supplementary Material
References
- 1.Greenberg P.E., Fournier A.A., Sisitsky T., Pike C.T., Kessler R.C. The economic burden of adults with major depressive disorder in the United States (2005 and 2010) J Clin Psychiatry. 2015;76:155–162. doi: 10.4088/JCP.14m09298. [DOI] [PubMed] [Google Scholar]
- 2.Steel Z., Marnane C., Iranpour C., Chey T., Jackson J.W., Patel V., Silove D. The global prevalence of common mental disorders: A systematic review and meta-analysis 1980–2013. Int J Epidemiol. 2014;43:476–493. doi: 10.1093/ije/dyu038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cuijpers P., Berking M., Andersson G., Quigley L., Kleiboer A., Dobson K.S. A meta-analysis of cognitive-behavioural therapy for adult depression, alone and in comparison with other treatments. Can J Psychiatry. 2013;58:376–385. doi: 10.1177/070674371305800702. [DOI] [PubMed] [Google Scholar]
- 4.Hardeveld F., Spijker J., De Graaf R., Nolen W.A., Beekman A.T.F. Prevalence and predictors of recurrence of major depressive disorder in the adult population. Acta Psychiatr Scand. 2010;122:184–191. doi: 10.1111/j.1600-0447.2009.01519.x. [DOI] [PubMed] [Google Scholar]
- 5.Beck A.T. Harper & Row; New York: 1967. Depression: Clinical, Experimental, and Theoretical Aspects. [Google Scholar]
- 6.Beck A.T. Cognitive models of depression. J Cogn Psychother. 1987;1:5–37. [Google Scholar]
- 7.Bower G.H. Mood and memory. Am Psychol. 1981;36:129–148. doi: 10.1037//0003-066x.36.2.129. [DOI] [PubMed] [Google Scholar]
- 8.Teasdale J.D. Cognitive vulnerability to persistent depression. Cogn Emot. 1988;2:247–274. [Google Scholar]
- 9.Alloy L.B., Abramson L.Y., Whitehouse W.G., Hogan M.E., Panzarella C., Rose D.T. Prospective incidence of first onsets and recurrences of depression in individuals at high and low cognitive risk for depression. J Abnorm Psychol. 2006;115:145–156. doi: 10.1037/0021-843X.115.1.145. [DOI] [PubMed] [Google Scholar]
- 10.Alloy L.B., Black S.K., Young M.E., Goldstein K.E., Shapero B.G., Stange J.P., et al. Cognitive vulnerabilities and depression versus other psychopathology symptoms and diagnoses in early adolescence. J Clin Child Adolesc Psychol. 2012;41:539–560. doi: 10.1080/15374416.2012.703123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Auerbach R.P., Bondy E., Stanton C.H., Webb C.A., Shankman S.A., Pizzagalli D.A. Self-referential processing in adolescents: Stability of behavioral and ERP markers. Psychophysiology. 2016;53:1398–1406. doi: 10.1111/psyp.12686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Connolly S.L., Abramson L.Y., Alloy L.B. Information processing biases concurrently and prospectively predict depressive symptoms in adolescents: Evidence from a self-referent encoding task. Cogn Emot. 2016;30:550–560. doi: 10.1080/02699931.2015.1010488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Disner S.G., Shumake J.D., Beevers C.G. Self-referential schemas and attentional bias predict severity and naturalistic course of depression symptoms. Cogn Emot. 2017;31:632–644. doi: 10.1080/02699931.2016.1146123. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein B.L., Hayden E.P., Klein D.N. Stability of self-referent encoding task performance and associations with change in depressive symptoms from early to middle childhood. Cogn Emot. 2015;29:1445–1455. doi: 10.1080/02699931.2014.990358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LeMoult J., Gotlib I.H. Depression: A cognitive perspective. Clin Psychol Rev. 2019;69:51–66. doi: 10.1016/j.cpr.2018.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Leppert K.A., Wasserbach M.C., Dougherty L.R. Cognitive styles in preschool-age children: Associations with depression risk and evidence of stability. J Psychopathol Behav Assess. 2019;41:612–626. [Google Scholar]
- 17.McGrath E.P., Repetti R.L. A longitudinal study of children’s depressive symptoms, self-perceptions, and cognitive distortions about the self. J Abnorm Psychol. 2002;111:77–87. doi: 10.1037//0021-843x.111.1.77. [DOI] [PubMed] [Google Scholar]
- 18.Klein D.N., Kotov R., Bufferd S.J. Personality and depression: Explanatory models and review of the evidence. Annu Rev Clin Psychol. 2011;7:269–295. doi: 10.1146/annurev-clinpsy-032210-104540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Weinberg A., Shankman S.A. Blunted reward processing in remitted melancholic depression. Clin Psychol Sci. 2017;5:14–25. doi: 10.1177/2167702616633158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Everaert J., Podina I.R., Koster E.H.W. A comprehensive meta-analysis of interpretation biases in depression. Clin Psychol Rev. 2017;58:33–48. doi: 10.1016/j.cpr.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 21.Miskowiak K.W., Carvalho A.F. ‘Hot’ cognition in major depressive disorder: A systematic review. CNS Neurol Disord Drug Targets. 2014;13:1787–1803. doi: 10.2174/1871527313666141130205713. [DOI] [PubMed] [Google Scholar]
- 22.Phillips W.J., Hine D.W., Thorsteinsson E.B. Implicit cognition and depression: A meta-analysis. Clin Psychol Rev. 2010;30:691–709. doi: 10.1016/j.cpr.2010.05.002. [DOI] [PubMed] [Google Scholar]
- 23.Dobson K.S., Shaw B.F. Specificity and stability of self-referent encoding in clinical depression. J Abnorm Psychol. 1987;96:34–40. doi: 10.1037//0021-843x.96.1.34. [DOI] [PubMed] [Google Scholar]
- 24.Dozois D.J.A. Stability of negative self-structures: A longitudinal comparison of depressed, remitted, and nonpsychiatric controls. J Clin Psychol. 2007;63:319–338. doi: 10.1002/jclp.20349. [DOI] [PubMed] [Google Scholar]
- 25.Fritzsche A., Dahme B., Gotlib I.H., Joormann J., Magnussen H., Watz H., et al. Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychol Med. 2010;40:815–826. doi: 10.1017/S0033291709990948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gemar M.C., Segal Z.V., Sagrati S., Kennedy S.J. Mood-induced changes on the Implicit Association Test in recovered depressed patients. J Abnorm Psychol. 2001;110:282–289. doi: 10.1037//0021-843x.110.2.282. [DOI] [PubMed] [Google Scholar]
- 27.Romero N., Sanchez A., Vazquez C. Memory biases in remitted depression: The role of negative cognitions at explicit and automatic processing levels. J Behav Ther Exp Psychiatry. 2014;45:128–135. doi: 10.1016/j.jbtep.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 28.Shestyuk A.Y., Deldin P.J. Automatic and strategic representation of the self in major depression: Trait and state abnormalities. Am J Psychiatry. 2010;167:536–544. doi: 10.1176/appi.ajp.2009.06091444. [DOI] [PubMed] [Google Scholar]
- 29.LeMoult J., Kircanski K., Prasad G., Gotlib I.H. Negative self-referential processing predicts the recurrence of major depressive episodes. Clin Psychol Sci. 2017;5:174–181. doi: 10.1177/2167702616654898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruhe H.G., Mocking R.J.T., Figueroa C.A., Seeverens P.W.J., Ikani N., Tyborowska A., et al. Emotional biases and recurrence in major depressive disorder. Results of 2.5 years follow-up of drug-free cohort vulnerable for recurrence. Front Psychiatry. 2019;10:145. doi: 10.3389/fpsyt.2019.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Milosavljevic M., Malmaud J., Huth A., Koch C., Rangel A. The drift diffusion model can account for the accuracy and reaction time of value-based choices under high and low time pressure. Judg Decis Mak. 2010;5:437–449. [Google Scholar]
- 32.Mulder M.J., Wagenmakers E.J., Ratcliff R., Boekel W., Forstmann B.U. Bias in the brain: A diffusion model analysis of prior probability and potential payoff. J Neurosci. 2012;32:2335–2343. doi: 10.1523/JNEUROSCI.4156-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ratcliff R., McKoon G. The diffusion decision model: Theory and data for two-choice decision tasks. Neural Comput. 2008;20:873–922. doi: 10.1162/neco.2008.12-06-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratcliff R., Smith P.L., Brown S.D., McKoon G. Diffusion decision model: Current issues and history. Trends Cogn Sci. 2016;20:260–281. doi: 10.1016/j.tics.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Voss A., Nagler M., Lerche V. Diffusion models in experimental psychology: A practical introduction. Exp Psychol. 2013;60:385–402. doi: 10.1027/1618-3169/a000218. [DOI] [PubMed] [Google Scholar]
- 36.Dainer-Best J., Lee H.Y., Shumake J.D., Yeager D.S., Beevers C.G. Determining optimal parameters of the self-referent encoding task: A large-scale examination of self-referent cognition and depression. Psychol Assess. 2018;30:1527–1540. doi: 10.1037/pas0000602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lerche V., Voss A., Nagler M. How many trials are required for parameter estimation in diffusion modeling? A comparison of different optimization criteria. Behav Res Methods. 2017;49:513–537. doi: 10.3758/s13428-016-0740-2. [DOI] [PubMed] [Google Scholar]
- 38.Beevers C.G., Mullarkey M.C., Dainer-Best J., Stewart R.A., Labrada J., Allen J.J.B., et al. Association between negative cognitive bias and depression: A symptom-level approach. J Abnorm Psychol. 2019;128:212–227. doi: 10.1037/abn0000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.White C., Ratcliff R., Vasey M., McKoon G. Dysphoria and memory for emotional material: A diffusion-model analysis. Cogn Emot. 2009;23:181–205. doi: 10.1080/02699930801976770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Crowley K.E., Colrain I.M. A review of the evidence for P2 being an independent component process: Age, sleep and modality. Clin Neurophysiol. 2004;115:732–744. doi: 10.1016/j.clinph.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 41.Lei Y., Dou H., Liu Q., Zhang W., Zhang Z., Li H. Automatic processing of emotional words in the absence of awareness: The critical role of P2. Front Psychol. 2017;8:592. doi: 10.3389/fpsyg.2017.00592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Citron F.M.M. Neural correlates of written emotion word processing: A review of recent electrophysiological and hemodynamic neuroimaging studies. Brain Lang. 2012;122:211–226. doi: 10.1016/j.bandl.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 43.Xu K., Li S., Ren D., Xia R., Xue H., Zhou A., Xu Y. Importance modulates the temporal features of self-referential processing: An event-related potential study. Front Hum Neurosci. 2017;11:470. doi: 10.3389/fnhum.2017.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kissler J., Assadollahi R., Herbert C. Emotional and semantic networks in visual word processing: Insights from ERP studies. Prog Brain Res. 2006;156:147–183. doi: 10.1016/S0079-6123(06)56008-X. [DOI] [PubMed] [Google Scholar]
- 45.Foti D., Hajcak G., Dien J. Differentiating neural responses to emotional pictures: Evidence from temporal-spatial PCA. Psychophysiology. 2009;46:521–530. doi: 10.1111/j.1469-8986.2009.00796.x. [DOI] [PubMed] [Google Scholar]
- 46.Nowparast Rostami H., Ouyang G., Bayer M., Schacht A., Zhou C., Sommer W. Dissociating the influence of affective word content and cognitive processing demands on the late positive potential. Brain Topogr. 2016;29:82–93. doi: 10.1007/s10548-015-0438-2. [DOI] [PubMed] [Google Scholar]
- 47.Poulsen C., Luu P., Crane S.M., Quiring J., Tucker D.M. Frontolimbic activity and cognitive bias in major depression. J Abnorm Psychol. 2009;118:494–506. doi: 10.1037/a0015920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dainer-Best J., Trujillo L.T., Schnyer D.M., Beevers C.G. Sustained engagement of attention is associated with increased negative self-referent processing in major depressive disorder. Biol Psychol. 2017;129:231–241. doi: 10.1016/j.biopsycho.2017.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Waters A.C., Tucker D.M. Principal components of electrocortical activity during self-evaluation indicate depressive symptom severity. Soc Cogn Affect Neurosci. 2016;11:1335–1343. doi: 10.1093/scan/nsw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tucker D.M., Luu P., Desmond R.E., Jr., Hartry-Speiser A., Davey C., Flaisch T. Corticolimbic mechanisms in emotional decisions. Emotion. 2003;3:127–149. doi: 10.1037/1528-3542.3.2.127. [DOI] [PubMed] [Google Scholar]
- 51.Crews W.D., Jr., Harrison D.W. The neuropsychology of depression and its implications for cognitive therapy. Neuropsychol Rev. 1995;5:81–123. doi: 10.1007/BF02208437. [DOI] [PubMed] [Google Scholar]
- 52.Shenal B.V., Harrison D.W., Demaree H.A. The neuropsychology of depression: A literature review and preliminary model. Neuropsychol Rev. 2003;13:33–42. doi: 10.1023/a:1022300622902. [DOI] [PubMed] [Google Scholar]
- 53.Coffey C.E. Cerebral laterality and emotion: The neurology of depression. Compr Psychiatry. 1987;28:197–219. doi: 10.1016/0010-440x(87)90027-7. [DOI] [PubMed] [Google Scholar]
- 54.Gillihan S.J., Farah M.J. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychol Bull. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- 55.Morin A. Self-awareness and the left hemisphere: The dark side of selectively reviewing the literature. Cortex. 2007;43:1068–1073. doi: 10.1016/s0010-9452(08)70704-4. discussion 1074–1082. [DOI] [PubMed] [Google Scholar]
- 56.Gallagher I. Philosophical conceptions of the self: Implications for cognitive science. Trends Cogn Sci. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- 57.Sui J., Humphreys G.W. The integrative self: How self-reference integrates perception and memory. Trends Cogn Sci. 2015;19:719–728. doi: 10.1016/j.tics.2015.08.015. [DOI] [PubMed] [Google Scholar]
- 58.Turk D.J., Heatherton T.F., Macrae C.N., Kelley W.M., Gazzaniga M.S. Out of contact, out of mind: The distributed nature of the self. Ann N Y Acad Sci. 2003;1001:65–78. doi: 10.1196/annals.1279.005. [DOI] [PubMed] [Google Scholar]
- 59.Demaree H.A., Everhart D.E., Youngstrom E.A., Harrison D.W. Brain lateralization of emotional processing: Historical roots and a future incorporating “dominance.”. Behav Cogn Neurosci Rev. 2005;4:3–20. doi: 10.1177/1534582305276837. [DOI] [PubMed] [Google Scholar]
- 60.Helm K., Viol K., Weiger T.M., Tass P.A., Grefkes C., Del Monte D., Schiepek G. Neuronal connectivity in major depressive disorder: A systematic review. Neuropsychiatr Dis Treat. 2018;14:2715–2737. doi: 10.2147/NDT.S170989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Klumpp H., Deldin P. Review of brain functioning in depression for semantic processing and verbal fluency. Int J Psychophysiol. 2010;75:77–85. doi: 10.1016/j.ijpsycho.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 62.Lemogne C., le Bastard G., Mayberg H., Volle E., Bergouignan L., Lehéricy S., et al. In search of the depressive self: Extended medial prefrontal network during self-referential processing in major depression. Soc Cogn Affect Neurosci. 2009;4:305–312. doi: 10.1093/scan/nsp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lemogne C., Mayberg H., Bergouignan L., Volle E., Delaveau P., Lehéricy S., et al. Self-referential processing and the prefrontal cortex over the course of depression: A pilot study. J Affect Disord. 2010;124:196–201. doi: 10.1016/j.jad.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 64.Hammen C. Generation of stress in the course of unipolar depression. J Abnorm Psychol. 1991;100:555–561. doi: 10.1037//0021-843x.100.4.555. [DOI] [PubMed] [Google Scholar]
- 65.Hammen C. Stress generation in depression: Reflections on origins, research, and future directions. J Clin Psychol. 2006;62:1065–1082. doi: 10.1002/jclp.20293. [DOI] [PubMed] [Google Scholar]
- 66.Slavich G.M., Auerbach R.P. In: Butcher J.N., Hooley J.M., editors. American Psychological Association; Washington, DC: 2018. Stress and its sequelae: Depression, suicide, inflammation, and physical illness; pp. 375–402. (APA Handbook of Psychopathology: Psychopathology: Understanding, Assessing, and Treating Adult Mental Disorders, vol. 1). [Google Scholar]
- 67.Auerbach R.P., Stanton C.H., Proudfit G.H., Pizzagalli D.A. Self-referential processing in depressed adolescents: A high-density event-related potential study. J Abnorm Psychol. 2015;124:233–245. doi: 10.1037/abn0000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Slavich G.M., O’Donovan A., Epel E.S., Kemeny M.E. Black sheep get the blues: A psychobiological model of social rejection and depression. Neurosci Biobehav Rev. 2010;35:39–45. doi: 10.1016/j.neubiorev.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Slavich G.M., Thornton T., Torres L.D., Monroe S.M., Gotlib I.H. Targeted rejection predicts hastened onset of major depression. J Soc Clin Psychol. 2009;28:223–243. doi: 10.1521/jscp.2009.28.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goldstein B.L., Klein D.N. A review of selected candidate endophenotypes for depression. Clin Psychol Rev. 2014;34:417–427. doi: 10.1016/j.cpr.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorka S.M., Lieberman L., Shankman S.A., Phan K.L. Startle potentiation to uncertain threat as a psychophysiological indicator of fear-based psychopathology: An examination across multiple internalizing disorders. J Abnorm Psychol. 2017;126:8–18. doi: 10.1037/abn0000233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weinberg A., Liu H., Shankman S.A. Blunted neural response to errors as a trait marker of melancholic depression. Biol Psychol. 2016;113:100–107. doi: 10.1016/j.biopsycho.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.First M.B., Williams J.B., Karg R.S., Spitzer R.L. American Psychiatric Association; Arlington, VA: 2015. Structured clinical interview for DSM-5 – Research version (SCID-5-RV) [Google Scholar]
- 74.Shankman S.A., Funkhouser C.J., Klein D.N., Davila J., Lerner D., Hee D. Reliability and validity of severity dimensions of psychopathology assessed using the Structured Clinical Interview for DSM-5 (SCID) Int J Methods Psychiatr Res. 2018;27 doi: 10.1002/mpr.1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Osório F.L., Loureiro S.R., Hallak J.E.C., Machado-de-Sousa J.P., Ushirohira J.M., Baes C.V.W., et al. Clinical validity and intrarater and test–retest reliability of the Structured Clinical Interview for DSM-5–Clinician Version (SCID-5-CV) Psychiatry Clin Neurosci. 2019;73:754–760. doi: 10.1111/pcn.12931. [DOI] [PubMed] [Google Scholar]
- 76.Slavich G.M., Shields G.S. Assessing lifetime stress exposure using the Stress and Adversity Inventory for Adults (Adult STRAIN): An overview and initial validation. Psychosom Med. 2018;80:17–27. doi: 10.1097/PSY.0000000000000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cazassa M.J., Oliveira MdaS., Spahr C.M., Shields G.S., Slavich G.M. The Stress and Adversity Inventory for Adults (Adult STRAIN) in Brazilian Portuguese: Initial validation and links with executive function, sleep, and mental and physical health. Front Psychol. 2020;10:3083. doi: 10.3389/fpsyg.2019.03083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Watson D., O’Hara M.W., Simms L.J., Kotov R., Chmielewski M., McDade-Montez E.A., et al. Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS) Psychol Assess. 2007;19:253–268. doi: 10.1037/1040-3590.19.3.253. [DOI] [PubMed] [Google Scholar]
- 79.Watson D., O’Hara M.W., Chmielewski M., McDade-Montez E.A., Koffel E., Naragon K., Stuart S. Further validation of the IDAS: Evidence of convergent, discriminant, criterion, and incremental validity. Psychol Assess. 2008;20:248–259. doi: 10.1037/a0012570. [DOI] [PubMed] [Google Scholar]
- 80.Wiecki T.V., Sofer I., Frank M.J. HDDM: Hierarchical Bayesian estimation of the Drift-Diffusion Model in Python. Front Neuroinform. 2013;7:14. doi: 10.3389/fninf.2013.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gratton G., Coles M.G., Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 82.Meyer A., Lerner M.D., De Los Reyes A., Laird R.D., Hajcak G. Considering ERP difference scores as individual difference measures: Issues with subtraction and alternative approaches. Psychophysiology. 2017;54:114–122. doi: 10.1111/psyp.12664. [DOI] [PubMed] [Google Scholar]
- 83.Moran T.P., Jendrusina A.A., Moser J.S. The psychometric properties of the late positive potential during emotion processing and regulation. Brain Res. 2013;1516:66–75. doi: 10.1016/j.brainres.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 84.Faurholt-Jepsen M., Brage S., Vinberg M., Christensen E.M., Knorr U., Jensen H.M., Kessing L.V. Differences in psychomotor activity in patients suffering from unipolar and bipolar affective disorder in the remitted or mild/moderate depressive state. J Affect Disord. 2012;141:457–463. doi: 10.1016/j.jad.2012.02.020. [DOI] [PubMed] [Google Scholar]
- 85.Paradiso S., Lamberty G.J., Garvey M.J., Robinson R.G. Cognitive impairment in the euthymic phase of chronic unipolar depression. J Nerv Ment Dis. 1997;185:748–754. doi: 10.1097/00005053-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 86.van der Maas H.L.J., Molenaar D., Maris G., Kievit R.A., Borsboom D. Cognitive psychology meets psychometric theory: On the relation between process models for decision making and latent variable models for individual differences. Psychol Rev. 2011;118:339–356. doi: 10.1037/a0022749. [DOI] [PubMed] [Google Scholar]
- 87.Lawlor V.M., Webb C.A., Wiecki T.V., Frank M.J., Trivedi M., Pizzagalli D.A., Dillon D.G. Dissecting the impact of depression on decision-making. Psychol Med. 2020;50:1613–1622. doi: 10.1017/S0033291719001570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Faehling F., Plewnia C. Controlling the emotional bias: Performance, late positive potentials, and the effect of anodal transcranial direct current stimulation (tDCS) Front Cell Neurosci. 2016;10:159. doi: 10.3389/fncel.2016.00159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kopf J., Dresler T., Reicherts P., Herrmann M.J., Reif A. The effect of emotional content on brain activation and the late positive potential in a word n-back task. PLoS One. 2013;8 doi: 10.1371/journal.pone.0075598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Northoff G., Heinzel A., de Greck M., Bermpohl F., Dobrowolny H., Panksepp J. Self-referential processing in our brain—A meta-analysis of imaging studies on the self. Neuroimage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 91.Sui J., Humphreys G.W. The interaction between self-bias and reward: Evidence for common and distinct processes. Q J Exp Psychol (Hove) 2015;68:1952–1964. doi: 10.1080/17470218.2015.1023207. [DOI] [PubMed] [Google Scholar]
- 92.Tamir D.I., Mitchell J.P. Disclosing information about the self is intrinsically rewarding. Proc Natl Acad Sci U S A. 2012;109:8038–8043. doi: 10.1073/pnas.1202129109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dolcos F., LaBar K.S., Cabeza R. Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: An event-related fMRI study. Neuroimage. 2004;23:64–74. doi: 10.1016/j.neuroimage.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 94.Frewen P., Schroeter M.L., Riva G., Cipresso P., Fairfield B., Padulo C., et al. Neuroimaging the consciousness of self: Review, and conceptual-methodological framework. Neurosci Biobehav Rev. 2020;112:164–212. doi: 10.1016/j.neubiorev.2020.01.023. [DOI] [PubMed] [Google Scholar]
- 95.Keil A., Müller M.M., Gruber T., Wienbruch C., Stolarova M., Elbert T. Effects of emotional arousal in the cerebral hemispheres: A study of oscillatory brain activity and event-related potentials. Clin Neurophysiol. 2001;112:2057–2068. doi: 10.1016/s1388-2457(01)00654-x. [DOI] [PubMed] [Google Scholar]
- 96.O’Hare A.J., Atchley R.A., Young K.M. Valence and arousal influence the late positive potential during central and lateralized presentation of images. Laterality. 2017;22:541–559. doi: 10.1080/1357650X.2016.1241257. [DOI] [PubMed] [Google Scholar]
- 97.Katz A.C., Sarapas C., Bishop J.R., Patel S.R., Shankman S.A. The mediating effect of prefrontal asymmetry on the relationship between the COMT Val(158)Met SNP and trait consummatory positive affect. Cogn Emot. 2015;29:867–881. doi: 10.1080/02699931.2014.951030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Baeken C., Remue J., Vanderhasselt M.A., Brunoni A.R., De Witte S., Duprat R., et al. Increased left prefrontal brain perfusion after MRI compatible tDCS attenuates momentary ruminative self-referential thoughts. Brain Stimul. 2017;10:1088–1095. doi: 10.1016/j.brs.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 99.Dedoncker J., Vanderhasselt M.A., Remue J., De Witte S., Wu G.R., Hooley J.M., et al. Prefrontal TDCS attenuates medial prefrontal connectivity upon being criticized in individuals scoring high on perceived criticism. Brain Imaging Behav. 2019;13:1060–1070. doi: 10.1007/s11682-018-9927-8. [DOI] [PubMed] [Google Scholar]
- 100.Mennin D.S., Fresco D.M. What, me worry and ruminate about DSM-5 and RDoC? The importance of targeting negative self-referential processing. Clin Psychol (New York) 2013;20:258–267. doi: 10.1111/cpsp.12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Paulus M.P., Stein M.B. Interoception in anxiety and depression. Brain Struct Funct. 2010;214:451–463. doi: 10.1007/s00429-010-0258-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bos E.H., Bouhuys A.L., Geerts E., Van Os T.W.D.P., Ormel J. Lack of association between conversation partners’ nonverbal behavior predicts recurrence of depression, independently of personality. Psychiatry Res. 2006;142:79–88. doi: 10.1016/j.psychres.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 103.Łosiak W., Blaut A., Kłosowska J., Łosiak-Pilch J. Stressful life events, cognitive biases, and symptoms of depression in young adults. Front Psychol. 2019;10:2165. doi: 10.3389/fpsyg.2019.02165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Buckman J.E.J., Underwood A., Clarke K., Saunders R., Hollon S.D., Fearon P., Pilling S. Risk factors for relapse and recurrence of depression in adults and how they operate: A four-phase systematic review and meta-synthesis. Clin Psychol Rev. 2018;64:13–38. doi: 10.1016/j.cpr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Timm C., Ubl B., Zamoscik V., Ebner-Priemer U., Reinhard I., Huffziger S., et al. Cognitive and affective trait and state factors influencing the long-term symptom course in remitted depressed patients. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178759. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.