Abstract
Background
Despite the availability of extensive literature on the effect of angiotensin‐converting enzyme inhibitors (ACEIs)/angiotensin‐receptor blockers (ARBs) on COVID‐19 outcomes, the evidence is still controversial. We aimed to provide a comprehensive assessment of the effect of ACEIs/ARBs on COVID‐19‐related outcomes by summarising the currently available evidence.
Methods
An umbrella review was conducted using Medline (OVID), Embase, Scopus, Cochrane library and medRxiv from inception to 1 February 2021. Systematic reviews with meta‐analysis that evaluated the effect of ACEIs/ARBs on COVID‐19‐related clinical outcomes were eligible. Studies' quality was appraised using the AMSTAR 2 Critical Appraisal Tool. Data were analysed using the random‐effects modelling including several subgroup analyses. Heterogenicity was assessed using I2 statistic. The study protocol was registered in PROSPERO (CRD42021233398) and reported using PRISMA guidelines.
Results
Overall, 47 reviews were eligible for inclusion. Out of the nine COVID‐19 outcomes evaluated, there was significant associations between ACEIs/ARBs use and each of death (OR = 0.80, 95%CI = 0.75–0.86; I2 = 51.9%), death/ICU admission as composite outcome (OR = 0.86, 95%CI = 0.80–0.92; I2 = 43.9%), severe COVID‐19 (OR = 0.86, 95%CI = 0.78–0.95; I2 = 68%) and hospitalisation (OR = 1.23, 95%CI = 1.04–1.46; I2 = 76.4%). The significant reduction in death/ICU admission, however, was higher among studies which presented adjusted measure of effects (OR = 0.63, 95%CI = 0.47–0.84) and were of moderate quality (OR = 0.74, 95%CI = 0.63–0.85).
Conclusions
Collective evidence from observational studies indicate a good quality evidence on the significant association between ACEIs/ARBs use and reduction in death and death/ICU admission, but poor‐quality evidence on both reducing severe COVID‐19 and increasing hospitalisation. Our findings further support the current recommendations of not discontinuing ACEIs/ARBs therapy in patients with COVID‐19.
Keywords: angiotensin receptor II blockers (ARBs), angiotensin‐converting enzyme inhibitors (ACEIs), COVID‐19, renin–angiotensin–aldosterone system (RAAS) inhibitors, umbrella review
1. INTRODUCTION
Several risk factors linked to poor COVID‐19 outcomes have been identified early on, including cardiovascular diseases such as hypertension. 1 Consequently, the possible impact of renin–angiotensin–aldosterone system (RAAS) inhibitors on COVID‐19‐related outcomes has emerged as a topic of interest 2 and their mechanisms of action– in particular, the potential upregulation of angiotensin‐converting enzyme 2 (ACE2), which is associated with viral entry into bronchial cells. 3 This has resulted in the rapid dissemination of numerous studies, mostly retrospective observational in nature, focussing on the risk of COVID‐19 infection, disease severity and/or disease outcomes in patients being treated with either angiotensin‐converting‐enzyme inhibitors (ACEIs)/angiotensin receptor blockers (ARBs) since early 2020. 4 , 5 , 6
As was the case in most early COVID‐19‐related research, the evidence comprised observational studies with notably small sample sizes and short durations of follow‐up. Resultantly, a number of systematic reviews were swiftly published in an attempt to offer a more substantial view by aggregating findings of these small‐scale studies. These meta‐analyses have offered tentative insights into all three areas of interest with regard to the use of RAAS inhibitors in times of COVID‐19: (i) risk of infection, usually measured as the share of positive PCR tests within a study cohort; (ii) risk of severe COVID‐19, with various underlying definitions ranging from hospitalisation due to the disease to the requirement for mechanical ventilation; and (iii) the risk of mortality. While there were similarities between some of the published results—for example, indicating, in general, no association between RAAS inhibitor use and risk of COVID‐19 infection—other results were more varied and the findings are still controversial/conflicting. 4 , 5 , 6 A logical next step, besides conducting additional systematic reviews/meta‐analyses, is to perform a systematic review of systematic reviews (also known as umbrella review), thereby taking advantage of the availability of high‐level evidence and providing an opportunity to contrast and compare. 7 The aim of this umbrella review and meta‐analysis, therefore, was to assess the effect of ACEIs/ARBs on COVID‐19‐related outcomes by summarising the currently available, aggregate evidence.
2. METHODS
An umbrella literature review and subsequent meta‐analysis was conducted. The protocol was informed by Joanna Briggs Reviewer's Manual for ‘Development of an Umbrella review protocol’ 8 and published on PROSPERO (CRD42021233398).
2.1. Eligibility criteria
Eligible studies were systematic reviews, which conducted a meta‐analysis to explore the effect of ACEIs/ARBs on any COVID‐19‐related clinical outcomes among adults (≥18 years) with COVID‐19 diagnosis.
2.2. Search strategy
The databases Medline, EMBASE, Scopus, Cochrane and medRxiv were searched from January 2019 until February 2021. Furthermore, we have performed a further scoping updated search in September 2022 to identify any potentially eligible studies published after our original search date. The search was limited to the English language and for systematic review articles. Search terms are listed in Supplementary file S1.
2.3. Article selection
Article selection was conducted using Covidence software 9 ; 10% of the articles' titles/abstracts and full texts were randomly selected and screened independently. The percentage of agreement was calculated for all independent validation, with >80% considered adequate. 10
2.4. Data extraction
A data extraction template in Microsoft Excel was piloted with 10% of reviews by NW and agreed for use by all authors. 10% of reviews were randomly selected and underwent independent data extraction; the percentage of agreement was calculated. Again, agreement >80% was considered adequate 10 . Data extracted from the reviews included title; authors; year review published; study design; sample size; setting; population; exposure (e.g. ACEIs/ARBs, ACEIs or ARBs) and outcomes (e.g. death, COVID‐19 infection and hospitalisation).
2.5. Quality assessment
Quality assessment was conducted independently using the AMSTAR 2 tool. 11 Studies were assessed based on the 15 AMSTAR 2 domains. To determine the overall confidence in the results of the review, studies were categorised as having high, moderate, low and critically low confidence in the results. As per AMSTAR 2 guidance, the overall confidence in the results was calculated based on the number of critical and noncritical domains. For this review, there were four critical domains: if there was an explicit statement that the methods were established a priori within a protocol; if a satisfactory technique for assessing the risk of bias was conducted and sufficiently discussed; if the meta‐analysis used appropriate methods; and if publication bias (small study bias) was conducted. If the criteria of a critical domain are not met, then this indicates a critical weakness in the review. The remaining 11 domains were considered noncritical. As per AMSTAR 2 guidance, reviews were classified as having high overall confidence in the results if there was ≤1 noncritical weakness, moderate if there was >1 noncritical weakness, low if there was 1 critical weakness and critically low if there >1 critical weakness.
2.6. Data analysis and synthesis
The random‐effects meta‐analysis model was used to statistically combine the measure of effects for those outcomes that were reported by more than one study, stratified by the three levels of exposure (ACEIs/ARBs, ACEIs and ARBs). In order to explore potential sources of heterogeneity, we conducted several subgroup analyses based on numerous variables including whether the reported measure of effects was crude or adjusted, the study was peer‐reviewed or not, and the study's methodological quality as per the quality assessment. Furthermore, to assess the impact of ACEIs/ARBs among patients with hypertension (the most common indication for ACEIs/ARBs), we also conducted subgroup analysis based on whether the studies had included either patients with hypertension only or at least had hypertension as one of the comorbidities versus those studies which did not record the hypertension status of their study population. In order to account for any possibility of rising in type I error (resulted from the multiple subgroup meta‐analyses), we adopted a lower significance threshold of <0.02 (2%) instead of <0.05 (5%) as a sensitivity analysis. The combined pooled estimates were presented as odds ratios and 95%CI and graphically as forest plots. I2 statistic 12 was used to assess heterogeneity between the studies with I2 of 0% indicating a lack of heterogeneity, whereas 25%, 50% and 75% indicating low, moderate and high heterogeneity, respectively. 12 To evaluate the degree of overlap of the studies within the included reviews, a citation matrix was generated that was used to calculate the Corrected Cover Area (CCA) as suggested by Pieper et al. 13 Publication bias was assessed using funnel plots and Egger's asymmetry test 14 only for those outcomes where >10 studies were included in the analysis as recommended by Cochrane guidelines. 15 Furthermore, we evaluated the influence of individual reviews on the summary pooled estimate for each outcome by conducting influential analyses 16 whereby the pooled meta‐analysis estimates for each outcome were computed by omitting one study at a time. Data were analysed using STATA 12.
2.7. Role of the funding source
None.
3. RESULTS
Out of an initial 157 publications, 66 systematic reviews underwent full‐text screening; after further exclusions based on prespecified criteria, 47 studies were eligible for inclusion (Figure 1). 4 , 5 , 6 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 Through the further scoping search in September 2022, we could identify three additional studies 61 , 62 , 63 of relevance, published after our original search date of 1 February 2021, yet these were not eligible for inclusion due to several reasons. First, the study by Iheanacho CO et al 61 was a systemic review without a meta‐analysis, which makes it ineligible for inclusion in our umbrella review. Second, the other two studies by Laurentius A et al 62 and Singh R et al 63 had a high likelihood of overlap and duplication with the reviews already included in our umbrella review because their search end dates were either earlier or very close to the search end dates of some other reviews included in our umbrella review. Third, the findings of these identified studies offer comparable conclusions to our umbrella review and would have only contributed to the pooled estimates of two out of the nine outcomes analysed in our umbrella review. Therefore, these studies were not considered because we believe it would offer little, if any, further insight into the current landscape of evidence and will have no substantial impact on the conclusions drawn.
FIGURE 1.
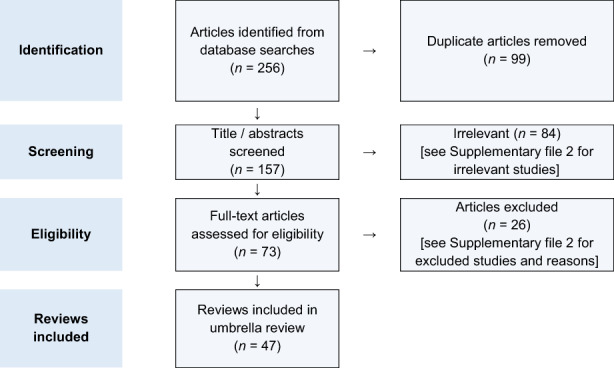
PRISMA flow diagram of the review selection process
3.1. Review characteristics
Forty‐six reviews (97.9%) compared COVID‐19‐related outcomes between ACEI/ARB users vs. nonusers among patients with COVID‐19, 4 , 5 , 6 , 17 , 18 , 19 , 20 , 21 , 22 , 23 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 54 , 55 , 56 , 57 , 58 , 59 , 60 one study (2.12%) compared outcomes between ACEIs/ARBs users in patients with and without COVID‐19 infection 53 , and 16 studies (34.0%) explored both. 6 , 19 , 25 , 26 , 27 , 40 , 41 , 43 , 44 , 48 , 50 , 51 , 54 , 56 , 58 , 60 Definition criteria for COVID‐19 diagnosis were reported by only six (12.8%) reviews as laboratory‐confirmed diagnosis based on a reverse transcriptase–polymerase chain reaction, whereas the remaining 41 (87.2%) reviews did not report any criteria for COVID‐19 diagnosis definition. Most of the included reviews were peer‐reviewed publications (68.1%; n = 32), whereas the remaining 15 (31.9%) reviews were non‐peer‐reviewed publications (i.e. were published in a preprint database). 17 , 18 , 19 , 21 , 22 , 23 , 30 , 32 , 33 , 34 , 36 , 46 , 50 , 54 , 60 The time the searches were conducted ranged from April 2020 to October 2020, with 21 (44.7%) review searches conducted in the month of May 2020 4 , 5 , 6 , 17 , 21 , 23 , 24 , 28 , 30 , 31 , 32 , 35 , 36 , 40 , 41 , 42 , 44 , 46 , 48 , 50 , 54 Preprint articles were included in 28 (59.6%) reviews, 4 , 17 , 19 , 20 , 21 , 22 , 25 , 26 , 30 , 33 , 37 , 41 , 42 , 43 , 44 , 45 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 59 , 60 and 10 (21.3%) reviews adjusted for retracted studies. 4 , 18 , 31 , 40 , 45 , 47 , 48 , 49 , 50 , 56 Full details of the 47 reviews are presented in Supplementary file S3.
A total of 213 meta‐analyses were conducted by the 47 reviews (Supplementary file S4). In terms of number of COVID‐19‐related outcomes reported in each review, one outcome was reported by 13 reviews (27.7%), 5 , 18 , 20 , 21 , 23 , 24 , 28 , 29 , 38 , 39 , 47 , 52 , 53 two outcomes by 15 reviews (31.9%), 4 , 17 , 26 , 31 , 32 , 34 , 35 , 36 , 37 , 40 , 42 , 49 , 54 , 55 , 58 three outcomes by 11 reviews (23.4%) 6 , 22 , 25 , 27 , 33 , 44 , 45 , 46 , 50 , 56 , 60 and 4–9 outcomes by eight reviews (17%). 19 , 30 , 41 , 43 , 48 , 51 , 57 , 59 Overall, the 47 eligible reviews reported data on 18 unique pooled outcome estimates including death in 36 reviews, 4 , 6 , 17 , 18 , 19 , 22 , 24 , 25 , 27 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 54 , 55 , 56 , 58 , 59 , 60 ICU admission in nine reviews, 27 , 28 , 30 , 41 , 43 , 48 , 51 , 56 , 59 death/ICU admission as a composite outcome in 16 reviews, 4 , 20 , 21 , 23 , 26 , 29 , 31 , 32 , 40 , 41 , 43 , 45 , 51 , 55 , 59 risk of acquiring COVID‐19 infection in 15 reviews, 19 , 25 , 27 , 40 , 41 , 43 , 44 severe COVID‐19 infection in 22 reviews, 6 , 17 , 19 , 22 , 25 , 30 , 33 , 34 , 35 , 36 , 37 , 41 , 42 , 43 , 44 , 45 , 46 , 48 , 59 , 60 hospitalisation in nine reviews, 19 , 30 , 41 , 43 , 48 , 59 length of hospital stay in five reviews, 19 , 22 , 30 , 46 , 59 use of mechanical ventilator in three reviews, 30 , 41 risk of severe acute respiratory syndrome (SARS) in two reviews, 26 , 59 and each of hospital discharge, 30 ICU admission/mechanical ventilator use, 41 risk of COVID‐19 infection/hospitalisation, 53 severe pneumonia, 41 level of serum creatinine, 57 d‐dimer, 57 cough, 57 fever 57 and renal dialysis 59 in one review; accordingly, nine out of these 18 outcomes were included in the meta‐analysis as they were reported by at least two reviews. In terms of the exposure, ACEIs and ARBs were evaluated as one class (ACEIs/ARBs) in all the eligible 47 reviews apart from three, 26 , 53 , 57 and as separate classes in 17 4 , 6 , 23 , 25 , 26 , 27 , 30 , 31 , 38 , 40 , 41 , 43 , 47 , 50 , 53 , 54 , 58 and 16 4 , 6 , 23 , 25 , 26 , 27 , 30 , 31 , 38 , 40 , 41 , 43 , 50 , 53 , 54 , 58 reviews, respectively. The majority of the reviews (66%; n = 31) only evaluated one exposure, mainly ACEIs/ARBs combined as one class (n = 30); whereas one‐third of them (29.8%; n = 14) reported data for the three levels of exposure (ACEIs/ARBs, ACEIs and ARBs).
3.2. Degree of overlap between the 47 included reviews
An analysis of the degree of overlap of the studies within the included 47 reviews was conducted. However, data on the included studies were not fully reported by Zhang G (2020) et al (59), Zhang X (2020) et al (6) and Greco (2020) et al (34). In total, 168 studies were included within the 47 eligible reviews. Of these, the majority of studies (n = 99) were included in three or less reviews, with 71 of these included within only one review. The study included by most reviews was by Li J et al, 64 which was included within 37 of the 47 reviews. An analysis of the degree of overlap across the systematic reviews using a citation matrix and the Corrected Cover Area (CCA) revealed a CCA value of 9.2 indicating a moderate degree of overlap.
3.3. Quality assessment
Overall confidence in the results was ‘moderate’ for 10 (21.3%) reviews, 19 , 25 , 26 , 30 , 37 , 41 , 42 , 43 , 56 , 59 ‘low’ for 15 (30.6%) reviews, 4 , 5 , 20 , 21 , 22 , 27 , 28 , 31 , 34 , 45 , 49 , 50 , 51 , 55 , 60 and ‘critically low’ for 22 (44.9%) reviews 6 , 17 , 18 , 23 , 24 , 29 , 32 , 33 , 35 , 36 , 38 , 39 , 40 , 44 , 46 , 47 , 48 , 52 , 53 , 54 , 57 , 58 (Supplementary file S5). Considering the critical domains, most reviews were considered to have had a satisfactory technique for the statistical combination of results (n = 45, 95.7%) 4 , 5 , 6 , 17 , 18 , 19 , 20 , 21 , 22 , 24 , 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 59 , 60 and for assessing risk of bias (n = 38, 80.1%). 4 , 5 , 6 , 17 , 19 , 20 , 21 , 22 , 23 , 25 , 26 , 27 , 28 , 30 , 31 , 34 , 35 , 36 , 37 , 38 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 48 , 49 , 50 , 51 , 52 , 53 , 55 , 56 , 57 , 59 , 60 Less reviews were favourably considered in terms of accounting for risk of bias when interpreting and discussing the results (n = 32, 68.1%), with appropriate conduct of publication bias (n = 33), 4 , 5 , 6 , 17 , 19 , 20 , 21 , 23 , 24 , 25 , 26 , 27 , 30 , 31 , 32 , 33 , 37 , 38 , 41 , 42 , 43 , 44 , 45 , 47 , 49 , 50 , 51 , 53 , 56 , 57 , 59 , 60 and only 15 (31.9%) reviews referred to the review methods being established a priori. 19 , 22 , 25 , 26 , 28 , 30 , 34 , 37 , 41 , 42 , 43 , 52 , 55 , 56 , 59
3.4. Effect of ACEIs/AEBs (as a one group) on the study outcomes
Overall, the effect of ACEIs/ARBs on nine COVID‐19‐related clinical outcomes was evaluated (Table 1). The combined pooled meta‐analysis estimates indicated that ACEIs/ARBs use was associated with a significant reduction in three clinical outcomes including death (OR = 0.80, 95%CI = 0.75–0.86; I2 = 51.9%) (Figure 2), death/ICU admission as composite outcome (OR = 0.86, 95%CI = 0.80–0.92; I2 = 43.9%) (Figure 3), and severe COVID‐19 infection (OR = 0.86, 95% CI = 0.78–0.95; I2 = 68%) (Figure 4); on the contrary, ACEIs/ARBs was associated with a significant increase in hospitalisation (OR = 1.23, 95%CI = 1.04–1.46; I2 = 76.4%) (Figure 5). However, there was insignificant association with each of ICU admission (Figure 6), risk of acquiring COVID‐19 infection (Figure 7), use of mechanical ventilator (Figure 8), risk of SARS (Figure 9), and risk of severe pneumonia (Figure 10).
TABLE 1.
Meta‐analyses pooled estimates with 95%CI of the effects of ACEIs/ARBs on COVID‐19‐related clinical outcomes
| Outcomes | ACEIs/ARBs | p‐value | ACEIs | p‐value | ARBs | p‐value |
|---|---|---|---|---|---|---|
| Death | 0.80 (0.75, 0.86) | <0.001 | 0.91 (0.89, 1.12) | 0.984 | 1.10 (0.94, 1.25) | 0.263 |
| Number of studies | 47 | 7 | 6 | |||
| I‐squared | 51.9% | 0.001 | 29.1% | 0.206 | 41.5% | 0.129 |
| ICU | 1.03 (0.86, 1.19) | 0.721 | 0.96 (0.87, 1.1) | 0.406 | 1.21 (0.93, 1.47) | 0.312 |
| Number of studies | 10 | 4 | 4 | |||
| I‐squared (p‐value) | 58.7% | 0.01 | 0% | 0.882 | 76.5% | 0.005 |
| Death/ICU | 0.86 (0.80, 0.92) | <0.001 | 0.94 (0.86, 1.03) | 0.167 | 0.98 (0.92, 1.05) | 0.530 |
| Number of studies | 22 | 8 | 8 | |||
| I‐squared (p‐value) | 43.9% | 0.015 | 29.5% | 0.193 | 0% | 0.614 |
| Risk of COVID‐19 | 0.99 (0.97, 1.02) | 0.560 | 0.97 (0.93, 1.01) | 0.058 | 1.01 (0.97, 1.04) | 0.726 |
| Number of studies | 19 | 11 | 10 | |||
| I‐squared (p‐value) | 24.7% | 0.159 | 31.7% | 0.146 | 0% | 0.757 |
| Severe COVID‐19 | 0.86 (0.78, 0.95) | 0.003 | 0.92 (0.81, 1.05) | 0.232 | 0.94 (0.84, 1.05) | 0.281 |
| Number of studies | 28 | 8 | 8 | |||
| I‐squared (p‐value) | 68% | <0.001 | 0% | 0.951 | 53.7% | 0.580 |
| Severe pneumonia | 0.82 (0.22, 3.05) | 0.765 | NA | NA | ||
| Number of studies | 2 | |||||
| I‐squared (p‐value) | 0% | 0.405 | ||||
| Hospitalisation | 1.23 (1.04, 1.46) | 0.019 | 1.18 (1.04, 1.35) | 0.012 | 1.17 (0.84, 1.61) | 0.354 |
| Number of studies | 11 | 5 | 5 | |||
| I‐squared (p‐value) | 76.4% | <0.001 | 6.7% | 0.368 | 86.9% | <0.001 |
| Ventilator use | 1.18 (0.84, 1.66) | 0.347 | 1.01 (0.03, 34.52) | 0.994 | 0.985 (0.084, 11.57) | 0.990 |
| Number of studies | 3 | 1 | 1 | |||
| I‐squared (p‐value) | 53.9% | 0.114 | NA | NA | ||
| Acute SARS infection | 0.71 (0.49, 1.02) | 0.064 | 1.06 (0.84, 1.34) | 0.633 | 1.11 (0.95, 1.29) | 0.493 |
| Number of studies | 1 | 2 | 2 | |||
| I‐squared (p‐value) | NA | 81% | 0.022 | 48.9% | 0.162 |
Abbreviation: NA, not applicable indicating not enough studies to perform meta‐analyses.
FIGURE 2.
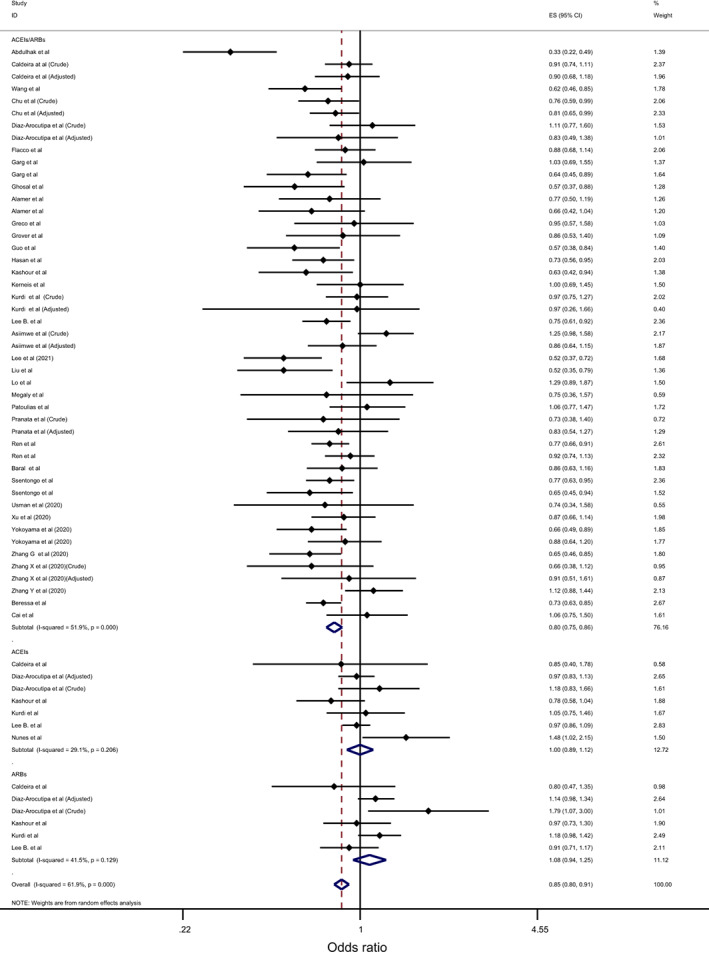
Forest plot depicting pooled estimates for the association between mortality and renin–angiotensin system drugs use
FIGURE 3.
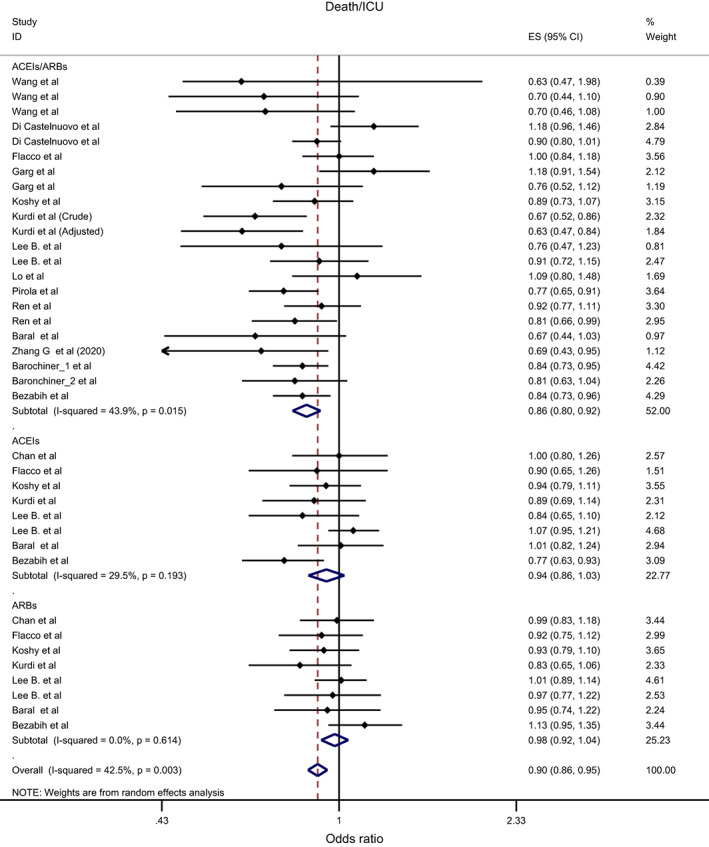
Forest plot depicting pooled estimates for the association between death/Intensive Care Unit (as a composite outcome) and renin–angiotensin system drugs use
FIGURE 4.
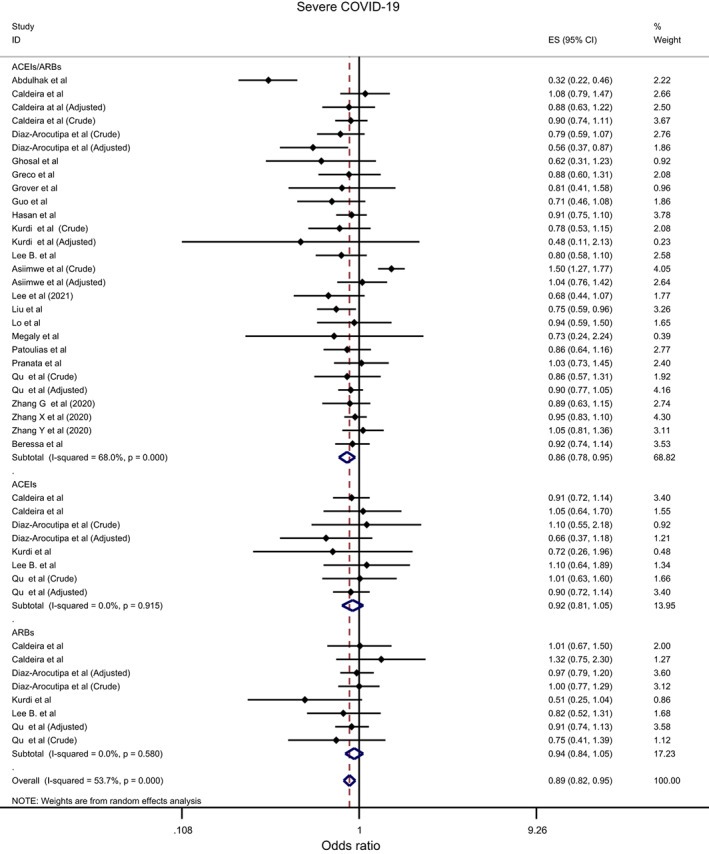
Forest plot depicting pooled estimates for the association between severe COVID‐19 infection and renin–angiotensin system drugs use
FIGURE 5.
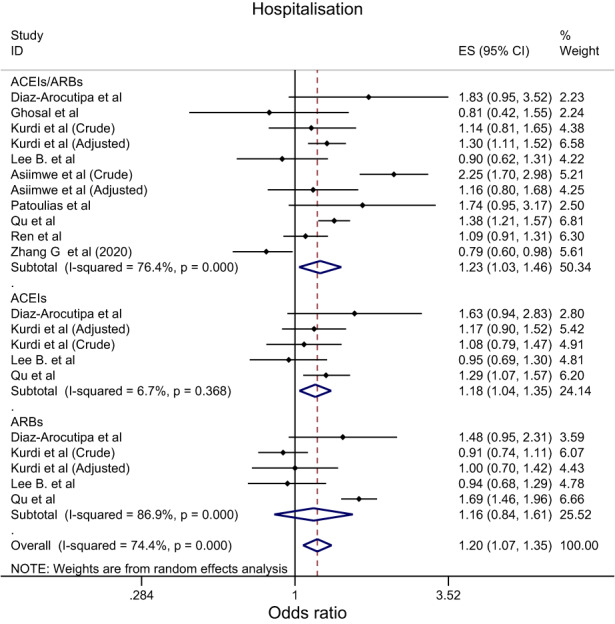
Forest plot depicting pooled estimates for the association between hospitalisation and renin–angiotensin system drugs use
FIGURE 6.
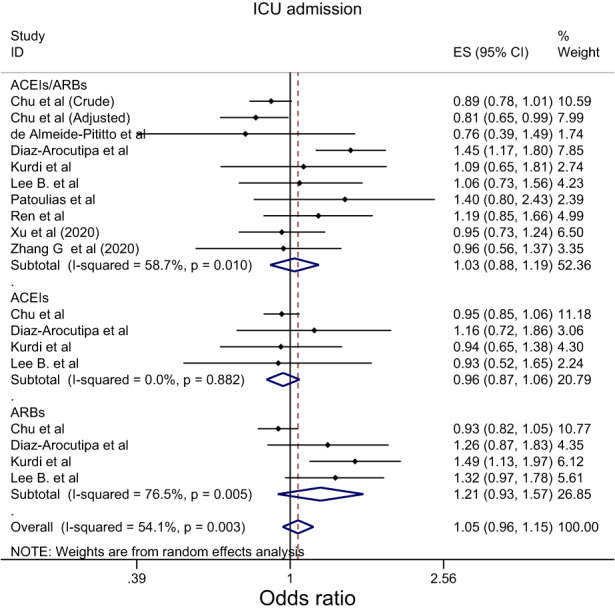
Forest plot depicting pooled estimates for the association between Intensive Care Unit admission and renin–angiotensin system drugs use
FIGURE 7.
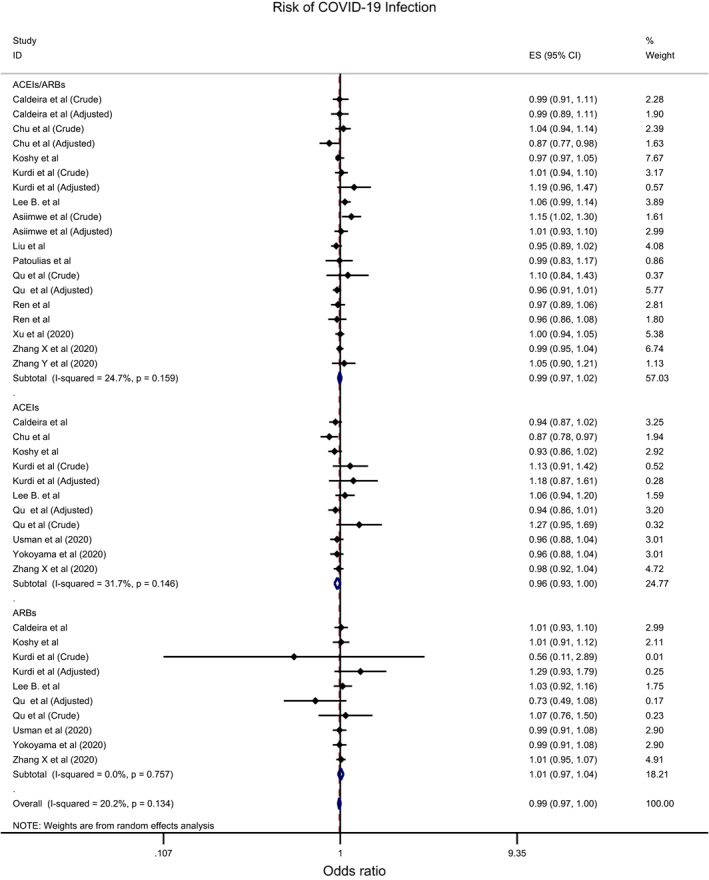
Forest plot depicting pooled estimates for the association between risk of acquiring COVID‐19 infection and renin–angiotensin system drugs use
FIGURE 8.
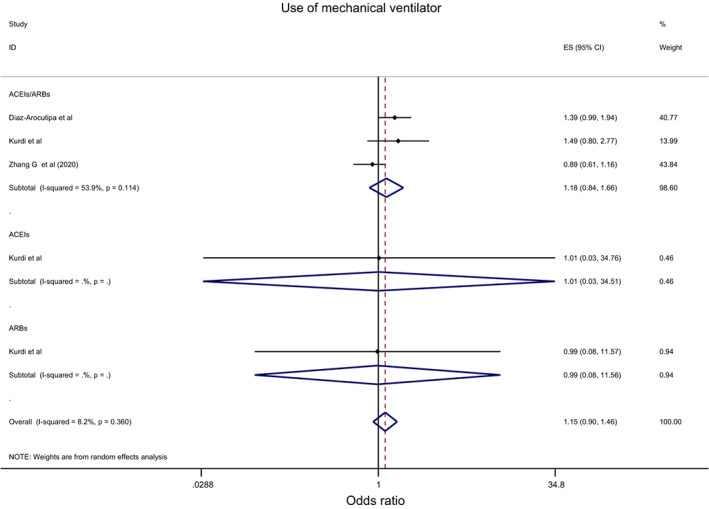
Forest plot depicting pooled estimate for the association between use of mechanical ventilator and renin–angiotensin system drugs use
FIGURE 9.
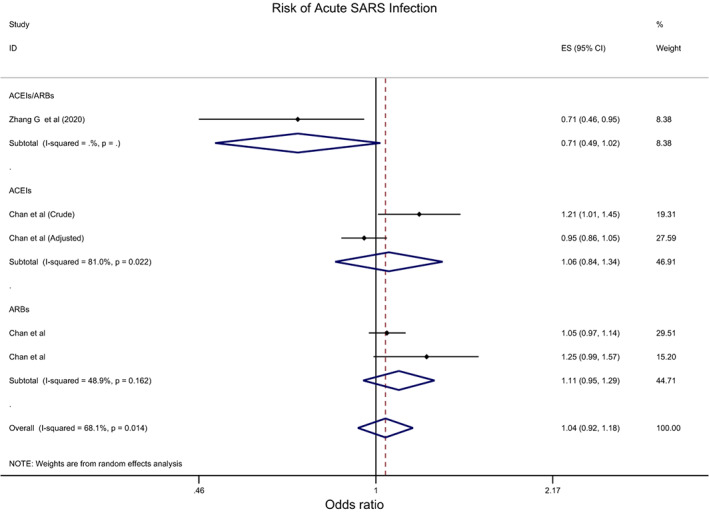
Forest plot depicting pooled estimates for the association between risk of severe acute respiratory syndrome (SARS) and renin–angiotensin system drugs use
FIGURE 10.
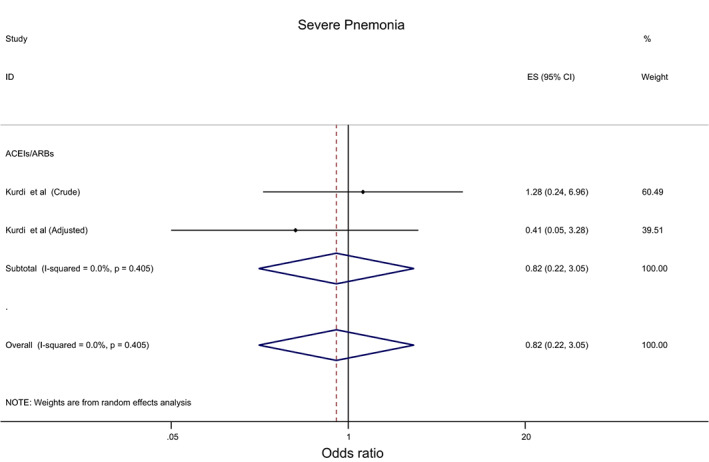
Forest plot depicting pooled estimates for the association between severe pneumonia and renin–angiotensin system drugs use
However, the subgroup analyses indicated different results for some of the outcomes (Table 2). Firstly, despite the consistent significant reduction in death in association with ACEIs/ARBs use regardless of studies' crude/adjusted measure of effects, peer‐review status and hypertension use status, there was a trend towards lower protective effective of ACEIs/ARBs on death as the quality of the studies enhanced from critically low (OR = 0.75, 95%CI = 0.66–0.85; I2 = 60.4%) to moderate (OR = 0.85, 95%CI = 0.75–0.96; I2 = 53.4%) (Supplementary file S6A; Table 2). Similarly, the significant reduction in death/ICU admission associated with ACEIs/ARBs appeared to be higher among the studies, which presented adjusted measure of effects (adjusted: OR = 0.63, 95%CI = 0.47–0.84 vs. crude: OR = 0.87, 95%CI = 0.81–0.93); and the pooled estimates for association ranged from insignificant association among the critically low‐quality studies (OR = 0.94, 95%CI = 0.84–1.06; I2 = 57.4%) to a significantly higher reduction among the moderate‐quality studies (OR = 0.74, 95%CI = 0.63–0.85; I2 = 18.9%); (Supplementary file S7A; Table 2). Besides, the significant protective impact of ACEIs/ARBs on death/ICU admission was observed only among peer‐reviewed studies (peer‐reviewed: OR = 0.85, 95%CI = 0.79–0.92 vs. non‐peer‐reviewed: OR = 0.89, 95%CI = 0.75–1.10) and studies including hypertensive patients (OR = 0.85, 95%CI = 0.80–0.90) Supplementary file S7A; Table 2).
TABLE 2.
Subgroup meta‐analyses pooled estimates with 95%CI of the effects of ACEIs/ARBs on COVID‐19‐related clinical outcomes
| ACEIs/ARBs | ACEIs | ARBs | |
|---|---|---|---|
| Death (n = 60) | |||
| Adjusted outcome measure | |||
| Adjusted OR | 0.80 (0.74, 0.91) | 0.90 (0.89, 1.12) | 1.1 (0.96, 1.26) |
| Crude OR | 0.80 (0.73, 0.86) | 1.10 (0.92, 1.25) | 1.1 (0.85, 1.42) |
| Number of studies | 10 vs. 37 | 2 vs. 5 | 2 vs. 4 |
| I‐squared (p‐value) | 0.0% (0.947) vs. 61% (<0.001) | 40.3% (0.196) vs. 26.7% (0.244) | 0.0% (0.335) vs. 60.6% (0.055) |
| Peer‐reviewed article? | |||
| Yes | 0.80 (0.76, 0.85) | 1.0 (0.83, 1.2) | 1.02 (0.87, 1.19) |
| No | 0.79 (0.66, 0.95) | 1.0 (0.87, 1.16) | 1.33 (0.88, 2.03) |
| Number of studies | 33 vs. 14 | 5 vs. 2 | 4 vs. 2 |
| I‐squared (p‐value) | 25.3% (0.095) vs. 75.3% (>0.001) | 45.7% (0.117) vs. 2.5% (0.331) | 27.2% (0.249) vs. 62.9% (0.101) |
| Study's quality | |||
| Critically low | 0.75 (0.66, 0.85) | 1.06 (0.57, 1.99) | 0.97 (0.37, 1.29) |
| Low | 0.81 (.075, 0.88) | NA | NA |
| Moderate | 0.85 (0.75, 0.96) | 0.99 (0.90, 1.10) | 1.11 (0.94, 1.30) |
| Number of studies | 21 vs. 12 vs. 14 | 2 vs. 0 vs. 5 | 1 vs. 0 vs. 5 |
| I‐squared (p‐value) | 60.4% (>0.001) vs. 18.8% (0.259) vs. 53.4% (0.009) | 85.8% (0.008) vs. NA vs. 29.1% (0.206) | NA vs. NA vs. 48.4% (0.101) |
| Hypertension use status | |||
| Hypertensive patients | 0.74 (0.69, 0.79) | 0.97 (0.86, 1.09) | 0.91 (0.71, 1.17) |
| Not recorded | 0.84 (0.77, 0.92) | 1.02 (0.87, 1.21) | 1.13 (0.98, 1.31) |
| Number of studies | 15 vs. 32 | 1 vs. 6 | 1 vs. 5 |
| I‐squared (p‐value) | 0.0% (0.617) vs. 57.3% (>0.001) | NA vs. 39.9% (0.140) | NA vs. 33.5% (0.129) |
| ICU admission (n = 18) | |||
| Adjusted outcome measure | |||
| Adjusted OR | 0.86 (0.73, 1.02) | NA | NA |
| Crude OR | 1.09 (0.91, 1.32) | 0.96 (0.87, 1.06)* | 1.21 (0.93, 1.57)* |
| Number of studies | 2 vs. 8 | 0 vs. 4 | 0 vs. 4 |
| I‐squared (p‐value) | 0.0% (0.356) vs. 59.8% (0.015) | NA vs. 0.0% (0.882) | NA vs. 76.5% (0.005) |
| Peer‐reviewed article? | |||
| Yes | 0.93 (0.85, 1.01) | 0.95 (0.86, 1.05) | 1.20 (0.87, 1.66) |
| No | 1.45 (1.17, 1.80) | 1.16 (0.72, 1.86) | 1.26 (0.87, 1.83) |
| Number of studies | 9 vs. 1 | 3 vs. 1 | 3 vs. 1 |
| I‐squared (p‐value) | 0.0% (0.488) vs. NA | 0.0% (0.997) vs. NA | 83.1% (0.003) vs. NA |
| Study's quality | |||
| Critically low | 1.40 (0.80, 2.44) | NA | NA |
| Low | 0.90 (0.78, 1.03) | 0.95 (0.85, 1.06) | 0.93 (0.82, 1.05) |
| Moderate | 1.12 (0.92, 1.37) | 1.0 (0.77, 1.30) | 1.37 (1.15, 1.64) |
| Number of studies | 1 vs. 4 vs. 5 | 0 vs. 1 vs. 3 | 0 vs. 1 vs. 3 |
| I‐squared (p‐value) | NA vs. 22.6% (0.275) vs. 45% (0.122) | NA vs. NA vs. 0.0% (0.770) | NA vs. NA vs. 0.0% (0.742) |
| Hypertension use status | |||
| Hypertensive patients | 0.97 (0.75, 1.27) | 0.93 (0.52, 1.66) | 1.32 (0.97, 1.79) |
| Not recorded | 1.05, 0.87, 1.27) | 0.96 (0.87, 1.06) | 1.18 (0.85, 1.64) |
| Number of studies | 3 vs. 7 | 1 vs. 3 | 1 vs. 3 |
| I‐squared (p‐value) | 0.0% (0.697) vs. 71.5% (0.002) | NA vs. 0.0% (0.722) | NA vs. 80.8% (0.006) |
| Death/ICU admission (n = 38) | |||
| Adjusted outcome measure | |||
| Adjusted OR | 0.63 (0.47, 0.84) | 1.0 (0.80, 1.26) | 1.0 (0.83, 1.18) |
| Crude OR | 0.87 (0.81, 0.93) | 0.93 (0.85, 1.03) | 0.98 (0.91, 1.05) |
| Number of studies | 1 vs. 21 | 1 vs. 7 | 1 vs. 7 |
| I‐squared (p‐value) | NA vs. 38.9% (0.036) | NA vs. 38.5% (0.135) | NA vs. 0.0% (0.498) |
| Peer‐reviewed article? | |||
| Yes | 0.85 (0.79, 0.92) | 0.99 (0.92, 1.10) | 0.96 (0.89, 1.03) |
| No | 0.89 (0.75, 1.10) | 0.77 (0.63, 0.94) | 1.13 (0.95, 1.34) |
| Number of studies | 18 vs. 4 | 7 vs. 1 | 7 vs. 1 |
| I‐squared (p‐value) | 45.5% (0.019) vs. 51.5% (0.103) | 0.0% (0.605) vs. NA | 0.0% (0.874) vs. NA |
| Study's quality | |||
| Critically low | 0.94 (0.84, 1.06) | 0.86 (0.70, 1.04) | 1.02 (0.85, 1.24) |
| Low | 0.85 (0.79, 0.92) | 0.98 (0.82, 1.16) | 0.93 (0.80, 1.10) |
| Moderate | 0.74 (0.63, 0.85) | 0.99 90.88, 1.10) | 0.98 (0.89, 1.06) |
| Number of studies | 6 vs. 11. vs. 5 | 2 vs. 2 vs. 4 | 2 vs. 2 vs. 4 |
| I‐squared (p‐value) | 57.4% (0.038) vs. 15.8% (0.293) vs. 18.9% (0.294) | 56.3% (0.130) vs. 0.0% (0.568) vs. 20.7% (0.286) | 60% (0.114) vs. 0.0% (0.865) vs. 0.0% (0.572) |
| Hypertension use status | |||
| Hypertensive patients | 0.85 (0.80, 0.9) | 0.9 (0.75, 1.08) | 1.01 (0.93, 1.10) |
| Not recorded | 0.88 (0.76, 1.03) | 0.96 (0.87, 1.06) | 0.93 (0.85, 1.03) |
| Number of studies | 13 vs. 9 | 4 vs. 4 | 4 vs. 4 |
| I‐squared (p‐value) | 0.0% (0.595) vs. 69% (0.001) | 67.1% (0.028) vs. 0.0% (0.852) | 0.0% (0.473) vs. 0.0% (0.723) |
| Risk of COVID‐19 infection (n = 40) | |||
| Adjusted outcome measure | |||
| Adjusted OR | 0.98 (0.94, 1.03) | 1.0 (0.82, 1.2) | 0.98 (0.56, 1.7) |
| Crude OR | 1.0 (0.97, 1.02) | 0.97 (0.93, 1.01) | 1.0 (0.97, 1.04) |
| Number of studies | 6 vs. 13 | 2 vs. 9 | 2 vs. 8 |
| I‐squared (p‐value) | 41.7% (0.127) vs. 18.7% (0.255) | 49% (0.161) vs. 36.6% (0.125) | 78.9% (0.03) vs. 0.0% (0.993) |
| Peer‐reviewed article? | |||
| Yes | 0.99 (0.97, 1.01) | 0.96 (0.92, 1.01) | 1.01 (0.98, 1.05) |
| No | 1.03 (0.96, 1.10) | 0.97 (0.89, 1.10) | 0.97 (0.85, 1.11) |
| Number of studies | 14 vs. 5 | 8 vs. 3 | 7 vs. 3 |
| I‐squared (p‐value) | 14.6% (0.294) vs. 52.5% (0.077) | 34.8% (0.150) vs. 48.6% (0.143) | 0.0% (0.814) vs. 18.1% (0.295) |
| Study's quality | |||
| Critically low | 0.97 (0.95, 1.0) | 0.96 (0.93, 0.99) | 1.0 (0.96, 1.04) |
| Low | 0.97 (0.93, 1.01) | 0.95 (0.84, 1.09) | 0.90 (0.62, 1.30) |
| Moderate | 1.03 (0.99, 1.06) | 1.03 (0.93, 1.14) | 1.03 (0.96, 1.10) |
| Number of studies | 4 vs. 7 vs. 8 | 4 vs. 3 vs. 4 | 4 vs. 2 vs. 4 |
| I‐squared (p‐value) | 0.0% (0.780) vs. 17.5% (0.296) vs. 12.7% (0.331) | 0.0% (0.811) vs. 66.7% (0.050) vs. 45.3% (0.140) | 0.0% (0.970) vs. 51.6% (0.151) vs. 0.0% (0.467) |
| Hypertension use status | |||
| Hypertensive patients | 1.02 (0.93, 1.11) | 1.0 (0.91, 1.11) | 1.0 (0.94, 1.08) |
| Not recorded | 0.99 (0.97, 1.01) | 0.96 (0.92, 0.99) | 1.0 (0.97, 1.05) |
| Number of studies | 2 vs. 17 | 2 vs. 9 | 2 vs. 8 |
| I‐squared (p‐value) | 58.3% (0.122) vs. 19.7% (0.224) | 42.0% (0.189) vs. 33.5% (0.150) | 0.0% (0.590) vs. 0.0% (0.595) |
| Severe COVID‐19 (n = 44) | |||
| Adjusted outcome measure | |||
| Adjusted OR | 0.88 (0.78, 0.99) | 0.86 (0.70, 1.07) | 0.94 (0.81, 1.10) |
| Crude OR | 0.86 (0.75, 0.97) | 0.96 (0.81, 1.14) | 0.93 (0.78, 1.13) |
| Number of studies | 6 vs. 22 | 2 vs. 6 | 2 vs. 6 |
| I‐squared (p‐value) | 19.3% (0.287) vs. 73% (>0.001) | 0.0% (0.330) vs. 0.0% (0.954) | 0.0% (0.674) vs. 8.8% (0.360) |
| Peer‐reviewed article? | |||
| Yes | 0.89 (0.83, 0.96) | 0.94 (0.78, 1.14) | 0.91 (0.66, 1.25) |
| No | 0.82 (0.66, 1.01) | 0.9 (0.75, 1.10) | 0.95 (0.83, 1.10) |
| Number of studies | 15 vs. 13 | 4 vs. 4 | 4 vs. 4 |
| I‐squared (p‐value) | 0.0% (0885) vs. 84% (>0.001) | 0.0% (0.832) vs. 0.0% (0.646) | 36.3% (0.194) vs. 0.0% (0.821) |
| Study's quality | |||
| Critically low | 0.69 (0.53, 0.92) | NA | NA |
| Low | 0.93 (0.85, 1.03) | 0.92 (0.75, 1.31) | 0.89 (0.73, 1.09) |
| Moderate | 0.89 (0.77, 1.04) | 0.92 (0.78, 1.10) | 0.96 (0.84, 1.10) |
| Number of studies | 7 vs. 7 vs. 14 | 0 vs. 2 vs. 6 | 0 vs. 2 vs. 6 |
| I‐squared (p‐value) | 80.5% (>0.001) vs. 0.0% (0.954) vs. 69.8% (>0.001) | NA vs. 0.0% (0.664) vs. 0.0% (0.782) | NA vs. 0.0% (0.557) vs. 0.0% (0.426) |
| Hypertension use status | |||
| Hypertensive patients | 0.89 (0.77, 1.01) | 1.10 (0.64, 1.89) | 0.82 (0.52, 1.30) |
| Not recorded | 0.85 (0.758, 0.96) | 0.91 (0.79, 1.10) | 0.95 (0.84, 1.10) |
| Number of studies | 5 vs. 23 | 1 vs. 7 | 1 vs. 7 |
| I‐squared (p‐value) | 0.0% (0.684) vs. 73.1% (>0.001) | NA vs. 0.0% (0.899) | Na vs. 0.0% (0.506) |
| Hospitalisation (n = 21) | |||
| Adjusted outcome measure | |||
| Adjusted OR | 1.33 (1.21, 1.47) | 1.25 (1.10, 1.46) | 1.33 (0.80, 2.23) |
| Crude OR | 1.21 (0.91, 1.61) | 1.10 (0.86, 1.41) | 1.02 (0.79, 1.31) |
| Number of studies | 3 vs. 8 | 2 vs. 3 | 2 vs. 3 |
| I‐squared (p‐value) | 0.0% (0.634) vs. 81.5% (>0.001) | 0.0% (0.556) vs. 27.9% (0.250) | 86.1% (0.007) vs. 49% (0.141) |
| Peer‐reviewed article? | |||
| Yes | 1.11 (0.90, 1.31) | 1.11 (0.91, 1.27) | 0.93 (0.80, 1.10) |
| No | 1.45 (1.10, 2.0) | 1.32 (1.10, 1.59) | 1.67 (1.45, 1.92) |
| Number of studies | 6 vs. 5 | 3 vs. 2 | 3 vs. 2 |
| I‐squared (p‐value) | 66.2% (0.011) vs. 73.1% (0.005) | 0.0% (0.611) vs. 0.0% (0.432) | 0.0% (894) vs. 0.0% (0.578) |
| Study's quality | |||
| Critically low | 1.20 (0.57, 2.54) | NA | NA |
| Low | 1.24 (0.98, 1.56) | 1.29 (1.07, 1.56) | 1.69 (1.46, 1.96) |
| Moderate | 1.24 (0.94, 1.63) | 1.12 (0.95, 1.31) | 0.99 (0.94, 1.19) |
| Number of studies | 2 vs. 2 vs. 7 | 0 vs. 1 vs. 4 | 0 vs. 1 vs. 4 |
| I‐squared (p‐value) | 64.8% (0.092) vs. 76.5% (0.039) vs. 82.9% (>0.001) | NA vs. NA vs. 0.0% (0.368) | NA vs. NA vs. 23.9% (0.268) |
| Hypertension use status | |||
| Hypertensive patients | 0.82 (0.67, 1.01) | 0.95 (0.69, 1.30) | 0.94 (0.68, 1.31) |
| Not recorded | 1.35 (1.15, 1.58) | 1.23 (1.10, 1.41) | 1.23 (0.84, 1.78) |
| Number of studies | 2 vs. 9 | 1 vs. 4 | 1 vs. 4 |
| I‐squared (p‐value) | 0.0% (0.568) vs. 66% (0.003) | NA vs. 0.0% (0.553) | NA vs. 88.7% (>0.001) |
| Ventilator use (n = 5) | |||
| Adjusted outcome measure | |||
| Adjusted OR | NA | NA | NA |
| Crude OR | 1.18 (0.84, 1.66)* | 1.01 (0.03, 34.52)* | 0.985 (0.084, 11.57)* |
| Number of studies | 0 vs. 3 | 0 vs. 1 | 0 vs. 1 |
| I‐squared (p‐value) | NA vs. 53.4% (0.114) | NA | NA |
| Peer‐reviewed article? | |||
| Yes | 1.10 (0.66, 1.75) | 1.01 (0.03, 34.52)* | 0.985 (0.084, 11.57)* |
| No | 1.39 (0.99, 1.95) | NA | NA |
| Number of studies | 2 vs. 1 | 1 vs. 0 | 1 vs. 0 |
| I‐squared (p‐value) | 52.6% (0.146) vs. NA | NA | NA |
| Study's quality | |||
| Critically low | NA | NA | NA |
| Low | NA | NA | NA |
| Moderate | 1.18 (0.84, 1.66)* | 1.01 (0.03, 34.52)* | 0.985 (0.084, 11.57)* |
| Number of studies | 0 vs. 0 vs. 3 | 0 vs. 0 vs. 1 | 0 vs. 0 vs. 1 |
| I‐squared (p‐value) | NA vs. NA vs. 53.4% (0.114) | NA | NA |
| Hypertension use status | |||
| Hypertensive patients | 0.89 (0.65, 1.23) | NA | NA |
| Not recorded | 1.41 (1.10, 1.90) | 1.014 (0.030, 34.758)* | 0.985 (0.084, 11.570)* |
| Number of studies | 1 vs. 2 | 0 vs. 1 | 0 vs. 1 |
| I‐squared (p‐value) | NA vs. 0.0% (0.844) | NA | NA |
| Acute SARS (n = 5) | |||
| Adjusted outcome measure | |||
| Adjusted OR | NA | 0.95 (0.86, 1.05) | 1.05 (0.97, 1.14) |
| Crude OR | 0.71 (0.49, 1.02) | 1.21 (1.01, 1.45) | 1.25 (0.99, 1.57) |
| Number of studies | 0 vs. 1 | 1 vs. 1 | 1 vs. 1 |
| I‐squared (p‐value) | NA | NA | NA |
| Peer‐reviewed article? | |||
| Yes | 0.71 (0.49, 1.02)* | 1.06 (0.84, 1.34)* | 1.11 (0.95, 1.29)* |
| No | NA | NA | NA |
| Number of studies | 1 vs. 0 | 2 vs. 0 | 2 vs. 0 |
| I‐squared (p‐value) | NA | 81% (0.022) vs. NA | 48.9% (0.162) vs. NA |
| Study's quality | |||
| Critically low | |||
| Low | NA | NA | NA |
| Moderate | NA | NA | NA |
| Number of studies | 0.71 (0.49, 1.02) | 1.06 (0.84, 1.34)* | 1.11 (0.95, 1.29)* |
| I‐squared (p‐value) | 0 vs. 0 vs. 1 | 0 vs. 0 vs. 2 | 0 vs. 0 vs. 2 |
| Hypertension use status | NA vs. NA. vs. 81% (0.022) | NA vs. NA. vs. 48.9% (0.162) | |
| Hypertensive patients | 0.71 (0.49, 1.02) | NA | NA |
| Not recorded | NA | 1.06 (0.84, 1.34) | 1.11 (0.95, 1.29) |
| Number of studies | 1 vs. 0 | 0 vs. 2 | 0 vs. 2 |
| I‐squared (p‐value) | NA | NA vs. 81% (0.022) | NA vs. 48.9% (0.162) |
Note: *Indicates that the pooled estimate is the same as the overall analyses because all the studies were in one group.
Abbreviation: NA, not applicable indicating that no studies were available to perform meta‐analyses for these outcomes.
Likewise, the protective effect of ACEIs/ARBs use on severe COVID‐19 infection was observed only among: peer‐reviewed studies (peer‐reviewed: OR = 0.89, 95%CI = 0.83–0.96 vs. non‐peer‐reviewed: OR = 0.82, 95%CI = 0.66–1.01), studies that did not record the hypertension status of their patients (OR = 0.85, 95%CI = 0.76–0.96), and critically low‐quality studies (OR = 0.69, 95%CI = 0.53–0.92); and in fact the protective effect disappeared completely as the quality of the studies improved since insignificant association was observed among both low‐ and moderate‐quality studies (OR = 0.93, 95%CI = 0.85–1.03; OR = 0.89, 95%CI = 0.77–1.04, respectively) (Supplementary file S8A; Table 2 ). In terms of ACEIs/ARBs' increasing impact on hospitalisation, this impact was demonstrated only among the studies which presented adjusted measure of effects (adjusted: OR = 1.33, 95%CI = 1.21–1.47 vs. crude: OR = 1.21, 95%CI = 0.91–1.61), were not peer‐reviewed (OR = 1.45, 95%CI = 1.10–10.20 vs. peer‐reviewed: OR = 1.11, 95%CI = 0.90–1.31), and did not record the hypertension status of their patients (OR = 1.35, 95%CI = 1.15–1.58) (Supplementary file S9A; Table 2).
3.5. Effect of ACEIs and AEBs (as a separate group) on the study outcomes
Overall, the effect of ACEIs and ARBs on seven COVID‐19‐related clinical outcomes (death, ICU admission, death/ICU admission, risk of acquiring COVID‐19 infection, severe COVID‐19 infection, hospitalisation and acute SARS) was evaluated. Neither ACEIs nor ARBs had any significant impact on any of the seven studied outcomes (Figures 2, 3, 4, 5, 6, 7, 8, 9, 10, Table 1) except for hospitalisation whereby ACEIs use was associated with a significant increase in COVID‐19‐related hospitalisation (OR = 1.18, 95%CI = 1.04–1.35; I2 = 6.7%) (Figure 5; Table 1). These results were mostly consistent across all the subgroup analyses (Supplementary file S6B,C, 7B,C, 8B,C; Table 2) except for the increasing effect of ACEIs on hospitalisation, which was only observed among those studies which did not record the hypertension status of their patients (OR = 1.23, 95%CI = 1.10–1.41) (Supplementary file S9B,C; Table 2). Results from the sensitivity analysis of adopting a lower significance threshold of <0.02 were consistent with those obtained from using the <0.05 significance threshold, indicating no effect of type I error on our pooled estimates' significance level.
3.6. Publication bias
Results from the funnel plots and Egger's asymmetry tests for the six outcomes that were reported by at least 10 studies indicated no evidence of significant publication bias in all of them except for death/ICU admission and severe COVID‐19 infection (p‐value = 0.022 and 0.019, respectively) (Supplementary file S10).
3.7. Influential analyses
The results from the influential analyses indicated that none of the combined pooled meta‐analysis estimates for the nine outcomes were dominated/influenced by an individual review/meta‐analysis since the omission of any of these individual reviews/meta‐analyses one at a time made no difference to the pooled meta‐analysis estimate because all of pooled meta‐analysis estimates were overlapping (Supplementary file S11). It is worth mentioning that it might be possible that one or few of the original primary studies influenced some of the 47 review/meta‐analyses included in our umbrella review but it was not possible for us to assess this information because these data were not reported by the included review/meta‐analyses.
4. DISCUSSION
This umbrella review for the first time combined all the available evidence so far from observational studies on the impact of ACEIs/ARBs on COVID‐19 clinical outcomes (47 systematic review studies which reported 213 meta‐analyses) into one pooled estimate. The collective, combined pooled estimates indicated evidence of statistically significant reduction in mortality, death/ICU admission, and severe COVID‐19 infection in association with ACEIs/ARBs use, but significant increase in the risk of hospitalisation (Table 1). Interestingly, there was no evidence of any significant association between ACEIs or ARBs and any of the nine COVID‐19‐related clinical outcomes analysed in our study.
Although the magnitude of observed impact of ACEIs/ARBs use on reducing mortality was decreasing as the quality of studies improved (Table 2), the evidence was overall mostly consistent across all the subgroup analyses including a greater impact among studies that included hypertensive patients compared with studies that did not record the hypertension status of their study population (Table 2). In terms of death/ICU admission, the quality of the evidence was even better because the impact of ACEIs/ARBs use was greater and significant only among moderate‐quality studies, peer‐reviewed studies and studies with hypertensive patients; however, the impact was significant regardless of whether the measure of effects was crude or adjusted, even though the impact was greater among studies with adjusted measure of effects compared those studies with crude measure of effects (Table 2). By contrast, the quality of the evidence for the impact of ACEIs/ARBs use on severe COVID‐19 was low since the significant reduction was only observed among critically low‐quality studies and, in fact, the significant association disappeared as the quality of the studied enhanced from critically low quality to either low or moderate quality (Table 2).
In terms of the impact of ACEIs/ARBs on hospitalisation, the quality of the evidence was low because the significant association was not apparent when the data were analysed by the quality of the studies, even though the magnitude of the effect was almost consistent across the various quality of the studies; besides, the significant increase in hospitalisation was observed only among studies that reported adjusted measure of effects, non‐peer‐reviewed studies and studies that did not record the hypertensive status of their study population (Table 2).
The subgroup analyses demonstrated low‐quality evidence regarding the different impact of ACEIs and ARBs (as separate groups) (Table 2). This observed difference has been suggested to be due to the increased level of angiotensin II, which occurs following ARBs treatment but not ACEIs, which in turn imposes an increased substrate load on ACE2 enzyme requiring its upregulation, 65 hence facilitating COVID‐19 virus cell entry and its subsequent infectivity/pathogenicity. 66 Furthermore, the increase in ACE2 activity demonstrated in patients with hypertension, either due to the pathophysiology of hypertension itself 67 or the administration of ACEIs/ARBs as antihypertensive medications, 68 could at least partially explain some of our study findings as why ACEIs/ARBs had significant greater impact on certain COVID‐19 clinical outcomes (i.e. mortality and death/ICU admission) only among studies that included patient with hypertension.
Several hypotheses (related to the pathophysiology of COVID‐19 infection and functions of ACE2) can explain the observed impact of ACEIs/ARBs in our current study. The adverse negative effects of ACEIs/ARBs could be due to ACEIs/ARBs ability to cause upregulation of ACE2 expression (the cell entry point for COVID‐19), hence facilitating and enhance COVID‐19 viral binding and cell entry, 68 whereas the positive protective effects could be through ACEIs/ARBs blockage of the harmful angiotensin II‐ AT1R axis and their effects on angiotensin II expression leading to subsequent increase in the level of the protective angiotensin 1–7 and 1–9 which have anti‐inflammatory and vasodilatory effects (i.e. the corresponding increase in Ang I and Ang II in response to ACEIs and ARBs, respectively, use would activate and increase the protective Ang 1–7/1–9 axis via MasR and AT2R receptor resulting in their antioxidant, anti‐inflammatory, vasodilation and antifibrosis effects; also the protective Ang 1–7 level can be generated by neutral endopeptidase), hence potentially attenuating the cardiac and pulmonary damages of COVID‐19, 2 which could potentially explain why discontinuation of ACEIs/ARBs might increase the risk of mortality due to the loss of this protective Ang 1–7/1–9 axis via MasR and AT2R pathway. Interestingly, a recent study has demonstrated that these benefits from ACEIs/ARBs antihypertensive drugs on in‐hospital mortality could be observed/extended with/to any first‐line antihypertensive drug treatment (OR: 0.25, 95%CI: 0.2–0.3) 69 ; this is maybe due to the fact that almost all antihypertensive drugs are protective for the endothelium, an arterial layer targeted by COVID‐19. Genetic ACE2 polymorphism among some individuals has been also suggested as potential factor explaining, at least partially, the harmful effects on ACEIs/ARBs on COVID‐19 outcomes. 70
It is worth to highlight that our study findings are still important despite the recently published randomised clinical trial (RCT) 71 which found insignificant differences in the mean number of days alive/out of the hospital between those assigned to discontinue vs continue ACEIs or ARBs. This is because of certain points that are related to the findings from this RCT. First, this RCT was designed to evaluate the impact of continuing ACEIs or ARBs vs. their discontinuation after contracting COVID‐19 rather than evaluating ACEIs/ARBs use vs. nonuse of these medications which was the focus of most of the observational studies involved in our current study. Secondly, the RCT included only patients with mild or moderate COVID‐19 with more than half of the participants (57%; n = 376) having mild COVID‐19 and evaluated only two COVID‐19‐related clinical outcomes, namely days alive (mortality) and out‐of‐hospital days, hence leaving a big gap in the evidence around ACEIs/ARBs' impact on other important COVID‐19 clinical outcomes as well as limiting generalisability to patients with severe COVID‐19. Furthermore, although the RCT's participants were all hypertensive patients, about one‐third (~31%) and ~ 1% had diabetes and heart failure, respectively, which further limits the generalisability of the RCT's findings to these conditions for which ACEIs/ARBs are commonly indicated. Moreover, the RCT's participants were all from Brazil and hence extending the findings to other races or ethnicities will be limited; this is particularly important because there are evidence demonstrating that there are potential genetic variants of renin, angiotensinogen, ACE, angiotensin II and ACE2 among various populations that influence the function of the renin–angiotensin–aldosterone system, hence affecting someone’s response to the COVID‐19 infection. 72 On the contrary, another more recent RCT 73 exploring the effect of ACEIs/ARBs discontinuation vs. their continuation among 659 patients found that continuing ACEIs/ARBs in comparison with their discontinuation resulted in lower rates of in‐hospital and 30‐day mortality, hospitalisation stay and COVID‐19 disease progression; however, this positive effect was only seen among those with moderate COVID‐19 at baseline and not those with mild COVID‐19, suggesting that ACEIs/ARBs should be continued in patients with moderate COVID‐19 disease severity, especially as ACEIs/ARBs are well known to have substantial benefits for patients with hypertension and heart failure and thus stopping them would be deleterious; it is worth noting that because about 80% of patients were on ARBs, the observed benefits might not be extended to ACEIs.
4.1. Strengths and limitations
This review presents the most comprehensive systematic overview on the impact of RAAS inhibitors on COVID‐19‐related clinical outcomes, with a wide range of sensitivity (subgroup) analyses to assess the robustness of the evidence. None of the pooled meta‐analysis estimates for the nine studied outcomes was affected/dominated by an individual study. Although most of the included studies were classified as ‘low’ or ‘critically low’ quality using the AMSTAR 2 tool, it is widely acknowledged that the AMSTAR 2 tool has a high standard with most reviews rated as ‘critically low’. 74 , 75 The AMSTAR 2 tool is also prone to subjective biases, 76 and assessment results are at the discretion of the reviewers regarding what is a ‘comprehensive’ literature search or ‘satisfactory’ explanation of heterogeneity or risk of bias assessment 76 ; therefore, quality assessment was conducted fully independently in this review. Alternative tools to AMSTAR 2 exist, such as the ROBIS tool; however, the measurement categories are found to be broadly similar to the AMSTAR 2 tool which considered more reliable. 76 An assessment of the degree of overlap among the 47 included reviews revealed a moderate degree of overlap of original studies with a calculated CCA of 9.2, with the CCA typically ranging from 1.5–11.4 for umbrella reviews (13). However, as three reviews did not provide full details of the included studies, this may be a slight underestimation. The overlap of primary studies within umbrella reviews is a well‐established limitation of this type of review and meta‐analysis, 77 and we wish to stress to readers that this umbrella review provides a summary overview to offer insight into the current landscape of evidence (77). The moderate overlap identified highlights the unnecessary duplication of reviews conducted within this topic in 2020, which strongly relates to our observation that most of the eligible reviews did not prospectively register their protocol. Furthermore, this degree of overlap should be interpreted with caution because although a primary study might have been included in more than one review for different exposure–outcome relationships (hence, technically seen as an overlap), it might be included to assess different outcomes. It is plausible that data on different outcomes of a primary study could be extracted and included in two reviews. This is of particular relevance because many COVID‐19‐related clinical outcomes have been assessed (e.g. mortality, severity and hospitalisation) and not all the eligible reviews assessed the same outcomes. Therefore, a primary study might have been included in several reviews for different outcomes with no real overlap in data but misleadingly declared as an overlap (13). We have retained all the overlapping reviews as recommended by Okoth et al 78 who suggested to retain the overlapping reviews when there is slight to moderate degree of overlap (CCA ≤10). Moreover, we do acknowledge that we might have missed potential review(s) that have been published after our search date, but we do believe that our umbrella review is the first study to comprehensively summarise the available evidence on the impact of ACEIs/ARBs on COVID‐19 clinical outcomes.
5. CONCLUSION
Collective evidence so far from observational studies indicates a good quality evidence on the significant association between ACEIs/ARBs use and reduction in death and death/ICU admission (as a composite outcome). Additionally, ACEIs/ARBs use was found to be associated with a significant reduction in severe COVID‐19 but a significant increase in hospitalisation; however, the evidence for these two outcomes was of poor quality; hence, cautious interpretation of these findings is required. Interestingly, findings for some of the clinical outcomes were dependent on whether the included patients had hypertension or not. Overall, our study findings further support the current recommendations of not discontinuing ACEIs/ARBs therapy in patients with COVID‐19 due to the lack of good quality evidence on their harm but rather it could be beneficial to patients.
AUTHOR CONTRIBUTORS
NW and TM involved in data collection and management; AK involved in data analysis and interpretation; all authors involved in study conception and design, manuscript writing and drafting, manuscript reviewing and revising as well as providing constrictive criticism and final approval.
FUNDING INFORMATION
None.
CONFLICT OF INTEREST
Nothing to declare.
Supporting information
Supplementary file S1
Supplementary file S2
Supplementary file S3
Supplementary file S4
Supplementary file S5
Supplementary file S6
Supplementary file S6A
Supplementary file S6B
Supplementary file S7
Supplementary file S7A
Supplementary file S7B
Supplementary file S8
Supplementary file S8A
Supplementary file S8B
Supplementary file S9
Supplementary file S9A
Supplementary file S9B
Supplementary file S10
Supplementary file S11
Kurdi A, Mueller T, Weir N. An umbrella review and meta‐analysis of renin–angiotensin system drugs use and COVID‐19 outcomes. Eur J Clin Invest. 2023;53:e13888. doi: 10.1111/eci.13888
REFERENCES
- 1. Clift AK, Coupland CAC, Keogh RH, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin–angiotensin–aldosterone system inhibitors in patients with Covid‐19. N Engl J Med. 2020;382(17):1653‐1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Akhtar S, Benter IF, Danjuma MI, Doi SAR, Hasan SS, Habib AM. Pharmacotherapy in COVID‐19 patients: a review of ACE2‐raising drugs and their clinical safety. J Drug Target. 2020;28(7–8):683‐699. [DOI] [PubMed] [Google Scholar]
- 4. Baral R, White M, Vassiliou VS. Effect of renin‐angiotensin‐aldosterone system inhibitors in patients with COVID‐19: a systematic review and meta‐analysis of 28,872 patients. Curr Atheroscler Rep. 2020;22(10):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pirola CJ, Sookoian S. Estimation of renin‐angiotensin‐aldosterone‐system (RAAS)‐inhibitor effect on COVID‐19 outcome: a meta‐analysis. J Infect. 2020;81(2):276‐281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang X, Yu J, Pan L‐Y, Jiang H‐Y. ACEI/ARB use and risk of infection or severity or mortality of COVID‐19: a systematic review and meta‐analysis. Pharmacol Res. 2020;158:104927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Aromataris E, Fernandez R, Godfrey CM, Holly C, Khalil H, Tungpunkom P. Summarizing systematic reviews: methodological development, conduct and reporting of an umbrella review approach. JBI Evidence Implementation. 2015;13(3):132‐140. [DOI] [PubMed] [Google Scholar]
- 8. Aromataris E, Fernandez R, Godfrey C, Holly C, Khalil H, Tungpunkom P. In: Aromataris E, Munn Z, eds. JBI Manual for Evidence Synthesis., in JBI Manual for Evidence Synthesis. JBI; 2020. [Google Scholar]
- 9. Covidence . Better systematic review management 2022; Available from: https://www.covidence.org/home.
- 10. House AE, House BJ, Campbell MB. Measures of interobserver agreement: calculation formulas and distribution effects. J Behav Assess. 1981;3(1):37‐57. [Google Scholar]
- 11. Shea BJ, Reeves BC, Wells G, et al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non‐randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. Br Med J. 2003;327(7414):557‐560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pieper D, Antoine S‐L, Mathes T, Neugebauer EA, Eikermann M. Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol. 2014;67(4):368‐375. [DOI] [PubMed] [Google Scholar]
- 14. Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins, J.P. and Green S.. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1. 0 [updated March 2011]. The Cochrane Collaboration. 2011. [cited 2020 13th September ]; Available from: https://handbook‐5‐1.cochrane.org/ [Google Scholar]
- 16. Mander A, Clayton D. Assessing the influence of a single study in meta‐analysis. Stata Tech Bull Reprints. 1999;8:108‐110. [Google Scholar]
- 17. Abdulhak AAB, Kashour T, Noman A, et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and outcome of COVID‐19: a systematic review and meta‐analysis. medRxiv. 2020;2020.05.06.20093260. [Google Scholar]
- 18. Alamer A, Abraham I. Mortality in COVID‐19 patients treated with ACEIs/ARBs: re‐estimated meta‐analysis results following the Mehra Abraham I. retraction. Pharmacol Res. 2020;160:105053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Asiimwe IG, Pushpakom S, Turner RM, Kolamunnage‐Dona R, Jorgensen AL, Pirmohamed M. Cardiovascular drugs and COVID‐19 clinical outcomes: a living systematic review and meta‐analysis. Br J Clin Pharmacol. 2021;87:4534‐4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Barochiner J, Martínez R. Use of inhibitors of the renin‐angiotensin system in hypertensive patients and COVID‐19 severity: a systematic review and meta‐analysis. J Clin Pharm Ther. 2020;45(6):1244‐1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Barochiner J, Martínez R. Use of inhibitors of the renin angiotensin system and COVID‐19 prognosis: a systematic review and meta‐analysis. medRxiv. 2020;2020.05.19.20106799. [Google Scholar]
- 22. Beressa TB, Sahilu T, Deyno S. Effect of renin‐angiotensin‐aldosterone system inhibitors on outcomes of COVID‐19 patients with hypertension: systematic review and meta‐analysis. medRxiv. 2020;2020.09.03.20187393. [Google Scholar]
- 23. Bezabih YM, Bezabih A, Aalamneh E, Peterson GM, Bezabhe WM. Comparison of renin–angiotensin–aldosterone system inhibitors with other antihypertensives in association with coronavirus disease‐19 clinical outcomes: systematic review and meta‐analysis. medRxiv. 2020;2020.05.21.20108993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cai XJ, Tay JCK, Kui SL, Tin AS, Tan VH. Impact of angiotensin‐converting enzyme inhibitors and angiotensin receptor blockers on in‐hospital mortality in COVID‐19 patients: a systematic review and meta‐analysis. Singapore Med J. 2020;1:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Caldeira D, Alves M, e Melo RG, et al. Angiotensin‐converting enzyme inhibitors and angiotensin‐receptor blockers and the risk of COVID‐19 infection or severe disease: systematic review and meta‐analysis. IJC Heart Vasc. 2020;31:100627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chan C‐K, Huang Y‐S, Liao H‐W, et al. Renin‐angiotensin‐aldosterone system inhibitors and risks of severe acute respiratory syndrome coronavirus 2 infection: a systematic review and meta‐analysis. Hypertension. 2020;76(5):1563‐1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chu C, Zeng S, Hasan AA, Hocher CF, Krämer BK, Hocher B. Comparison of infection risks and clinical outcomes in patients with and without SARS‐CoV‐2 lung infection under renin–angiotensin–aldosterone system blockade: systematic review and meta‐analysis. Br J Clin Pharmacol. 2021;87(6):2475‐2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. de Almeida‐Pititto B, Dualib PM, Zajdenverg L, et al. Severity and mortality of COVID 19 in patients with diabetes, hypertension and cardiovascular disease: a meta‐analysis. Diabetol Metab Syndr. 2020;12(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Di Castelnuovo A, Costanzo S, Antinori A, et al. RAAS inhibitors are not associated with mortality in COVID‐19 patients: findings from an observational multicenter study in Italy and a meta‐analysis of 19 studies. Vascul Pharmacol. 2020;135:106805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Diaz‐Arocutipa C, Saucedo‐Chinchay J, Hernandez AV. Association between ACEIs or ARBs use and clinical outcomes in COVID‐19 patients: a systematic review and meta‐analysis. medRxiv. 2020. [Google Scholar]
- 31. Flacco ME, Martellucci CA, Bravi F, et al. Treatment with ACE inhibitors or ARBs and risk of severe/lethal COVID‐19: a meta‐analysis. Heart. 2020;106(19):1519‐1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Garg A, Rout A, Sharma A, Fiorello B, Kostis JB. Association of renin angiotensin system blockers with outcomes in patients with Covid‐19: a systematic review and meta‐analysis. medRxiv. 2020;2020.05.23.20111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ghosal S, Mukherjee JJ, Sinha B, Gangopadhyay K. The effect of angiotensin converting enzyme inhibitors and angiotensin receptor blockers on death and severity of disease in patients with coronavirus disease 2019 (COVID‐19): a meta‐analysis. medRxiv. 2020;2020.04.23.20076661. [Google Scholar]
- 34. Greco A, Buccheri S, D'Arrigo P, et al. Outcomes of renin–angiotensin–aldosterone system blockers in patients with COVID‐19: a systematic review and meta‐analysis. Eur Heart J Cardiovasc Pharmacother. 2020;6(5):335‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Grover A, Oberoi M. A systematic review and meta‐analysis to evaluate the clinical outcomes in COVID‐19 patients on angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers. European Heart Journal‐Cardiovascular Pharmacotherapy. 2021;7(2):148‐157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Guo X, Zhu Y, Hong Y. Decreased mortality of COVID‐19 with renin‐angiotensin‐aldosterone system inhibitors therapy in patients with hypertension: a meta‐analysis. Hypertension. 2020;76(2):e13‐e14. [DOI] [PubMed] [Google Scholar]
- 37. Hasan SS, Kow CS, Hadi MA, Zaidi STR, Merchant HA. Mortality and disease severity among COVID‐19 patients receiving renin‐angiotensin system inhibitors: a systematic review and meta‐analysis. Am J Cardiovasc Drugs. 2020;20(6):571‐590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kashour T, Bin Abdulhak AA, Tlayjeh H, et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and mortality among COVID‐19 patients: a systematic review and meta‐analysis. Am J Ther. 2020; doi: 10.1097/MJT.0000000000001281. Online Ahead of Print. [DOI] [PubMed] [Google Scholar]
- 39. Kerneis M, Ferrante A, Guedeney P, Vicaut E, Montalescot G. Severe acute respiratory syndrome coronavirus 2 and renin‐angiotensin system blockers: a review and pooled analysis. Arch Cardiovasc Dis. 2020;113:797‐810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koshy AN, Murphy AC, Farouque O, Ramchand J, Burrell LM, Yudi MB. Renin–angiotensin system inhibition and risk of infection and mortality in COVID‐19: a systematic review and meta‐analysis. Intern Med J. 2020;50(12):1468‐1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kurdi A, Abutheraa N, Akil L, Godman B. A systematic review and meta‐analysis of the use of renin‐angiotensin system drugs and COVID‐19 clinical outcomes: what is the evidence so far? Pharmacol Res Perspect. 2020;8(6):e00666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lee HW, Yoon C‐H, Jang EJ, Lee C‐H. Renin‐angiotensin system blocker and outcomes of COVID‐19: a systematic review and meta‐analysis. Thorax. 2021;76(5):479‐486. [DOI] [PubMed] [Google Scholar]
- 43. Lee MM, Docherty KF, Sattar N, et al. Renin–angiotensin system blockers, risk of SARS‐CoV‐2 infection and outcomes from CoViD‐19: systematic review and meta‐analysis. European heart journal—cardiovascular. Pharmacotherapy. 2020;8(2):165‐178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Liu X, Long C, Xiong Q, et al. Association of angiotensin converting enzyme inhibitors and angiotensin II receptor blockers with risk of COVID‐19, inflammation level, severity, and death in patients with COVID‐19: a rapid systematic review and meta‐analysis. Clin Cardiol. 2020; doi: 10.1002/clc.23421. Online Ahead of Print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lo KB, Bhargav R, Salacup G, et al. Angiotensin converting enzyme inhibitors and angiotensin II receptor blockers and outcomes in patients with COVID‐19: a systematic review and meta‐analysis. Expert Rev Cardiovasc Ther. 2020;18(12):919‐930. [DOI] [PubMed] [Google Scholar]
- 46. Megaly M, Glogoza M. Renin‐angiotensin system antagonists are associated with lower mortality in hypertensive patients with COVID‐19. Scott Med J. 2020;65(4):123‐126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Nunes JPL. Mortality and use of angiotensin‐converting enzyme inhibitors in COVID 19 disease: a systematic review. Porto Biomed J. 2020;5(6):e085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Patoulias D, Katsimardou A, Stavropoulos K, Imprialos K, Kalogirou M‐S, Doumas M. Renin‐angiotensin system inhibitors and COVID‐19: a systematic review and meta‐analysis. Evidence for significant geographical disparities. Curr Hypertens Rep. 2020;22(11):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Pranata R, Permana H, Huang I, et al. The use of renin angiotensin system inhibitor on mortality in patients with coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. Diabetes Metab Syndr Clin Res Rev. 2020;14(5):983‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Qu G, Shu L, Song EJ, et al. Association between angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers use and the risk of infection and clinical outcome of COVID‐19: a comprehensive systematic review and meta‐analysis. medRxiv. 20200;2020.07.02.20144717. [Google Scholar]
- 51. Ren L, Yu S, Xu W, Overton JL, Chiamvimonvat N, Thai PN. Lack of association of antihypertensive drugs with the risk and severity of COVID‐19: a meta‐analysis. J Cardiol. 2021;77(5):482‐491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ssentongo AE, Ssentongo P, Heilbrunn ES, et al. Renin–angiotensin–aldosterone system inhibitors and the risk of mortality in patients with hypertension hospitalised for COVID‐19: systematic review and meta‐analysis. Open Heart. 2020;7(2):e001353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tleyjeh IM, Bin Abdulhak AA, Tlayjeh H, et al. Angiotensin converting enzyme inhibitors and angiotensin receptor blockers and the risk of SARS‐CoV‐2 infection or hospitalization with COVID‐19 disease: a systematic review and meta‐analysis. Am J Therapeut. 2022;29(1):e74‐e84. [DOI] [PubMed] [Google Scholar]
- 54. Usman MS, Siddiqi TJ, Khan MS, et al. A meta‐analysis of the relationship between renin‐angiotensin‐aldosterone system inhibitors and COVID‐19. Am J Cardiol. 2020;130:159‐161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang Y, Chen B, Li Y, et al. The use of renin–angiotensin–aldosterone system (RAAS) inhibitors is associated with a lower risk of mortality in hypertensive COVID‐19 patients: a systematic review and meta‐analysis. J Med Virol. 2021;93(3):1370‐1377. [DOI] [PubMed] [Google Scholar]
- 56. Xu J, Teng Y, Shang L, et al. The effect of prior angiotensin‐converting enzyme inhibitor and angiotensin receptor blocker treatment on coronavirus disease 2019 (COVID‐19) susceptibility and outcome: a systematic review and meta‐analysis. Clin Infect Dis. 2021;72(11):e901‐e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Xue Y, Sun S, Cai J, et al. Effects of ACEI and ARB on COVID‐19 patients: a meta‐analysis. J Renin Angiotensin Aldosterone Sys. 2020;21(4):1470320320981321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yokoyama Y, Aikawa T, Takagi H, Briasoulis A, Kuno T. Association of renin‐angiotensin‐aldosterone system inhibitors with mortality and testing positive of COVID‐19: meta‐analysis. J Med Virol. 2021;93(4):2084‐2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang G, Wu Y, Xu R, Du X. Effects of renin‐angiotensin‐aldosterone system inhibitors on disease severity and mortality in patients with COVID‐19: a meta‐analysis. J Med Virol. 2021;93(4):2287‐2300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhang Y, Yu S, Xu Y, Williams B. Renin angiotensin system inhibition and susceptibility and outcomes from COVID‐19: a systematic review and meta‐analysis of 69,200 COVID‐19 patients. medRxiv. 2020. [Google Scholar]
- 61. Iheanacho CO, Odili VU, Eze UI. Risk of SARS‐CoV‐2 infection and COVID‐19 prognosis with the use of renin–angiotensin–aldosterone system (RAAS) inhibitors: a systematic review. Futur J Pharm Sci. 2021;7(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Laurentius A, Mendel B, Prakoso R. Clinical outcome of renin‐angiotensin‐aldosterone system blockers in treatment of hypertensive patients with COVID‐19: a systematic review and meta‐analysis. Egypt Heart J. 2021;73(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh R, Rathore SS, Khan H, et al. Mortality and severity in COVID‐19 patients on ACEIs & ARBs‐A systematic review, meta‐analysis and meta‐regression analysis. Front Med. 2021;8:2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Li J, Wang X, Chen J, Zhang H, Deng A. Association of renin‐angiotensin system inhibitors with severity or risk of death in patients with hypertension hospitalized for coronavirus disease 2019 (COVID‐19) infection in Wuhan, China. JAMA Cardiol. 2020;5(7):825‐830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Esler M, Esler D. Can angiotensin receptor‐blocking drugs perhaps be harmful in the COVID‐19 pandemic? J Hypertens. 2020;38(5):781‐782. [DOI] [PubMed] [Google Scholar]
- 66. Rico‐Mesa JS, White A, Anderson AS. Outcomes in patients with COVID‐19 infection taking ACEI/ARB. Curr Cardiol Rep. 2020;22:1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Warner FJ, Guy JL, Lambert DW, Hooper NM, Turner AJ. Angiotensin converting enzyme‐2 (ACE2) and its possible roles in hypertension, diabetes and cardiac function. Lett Pept Sci. 2003;10(5):377‐385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Soler MJ, Barrios C, Oliva R, Batlle D. Pharmacologic modulation of ACE2 expression. Curr Hypertens Rep. 2008;10(5):410‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wojciechowska W, Terlecki M, Klocek M, et al. Impact of arterial hypertension and use of antihypertensive pharmacotherapy on mortality in patients hospitalized due to COVID‐19: the CRACoV‐HHS study. Hypertension. 2022;40:101161hypertensionaha12219575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Fang L, Karakiulakis G, Roth M. Are patients with hypertension and diabetes mellitus at increased risk for COVID‐19 infection? Lancet Respir Med. 2020;8(4):e21‐e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Lopes RD, Macedo AVS, de Barros ESPGM, et al. Effect of discontinuing vs continuing angiotensin‐converting enzyme inhibitors and angiotensin II receptor blockers on days alive and out of the Hospital in Patients Admitted with COVID‐19: a randomized clinical trial. Jama. 2021;325(3):254‐264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Snyder EM, Johnson BD. ACE2 and COVID‐19: using antihypertensive medications and pharmacogenetic considerations. Pharmacogenomics. 2020;21(10):695‐703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Macedo AVS, de Barros e Silva PGM, de Paula TC, et al. Discontinuing vs continuing ACEIs and ARBs in hospitalized patients with COVID‐19 according to disease severity: insights from the BRACE CORONA trial. Am Heart J. 2022;249:86‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Leclercq V, Beaudart C, Tirelli E, Bruyère O. Psychometric measurements of AMSTAR 2 in a sample of meta‐analyses indexed in PsycINFO. J Clin Epidemiol. 2020;119:144‐145. [DOI] [PubMed] [Google Scholar]
- 75. Pieper D, Lorenz RC, Rombey T, et al. Authors should clearly report how they derived the overall rating when applying AMSTAR 2—a cross‐sectional study. J Clin Epidemiol. 2021;129:97‐103. [DOI] [PubMed] [Google Scholar]
- 76. Pieper D, Puljak L, González‐Lorenzo M, Minozzi S. Minor differences were found between AMSTAR 2 and ROBIS in the assessment of systematic reviews including both randomized and nonrandomized studies. J Clin Epidemiol. 2019;108:26‐33. [DOI] [PubMed] [Google Scholar]
- 77. Gianfredi V, Nucci D, Amerio A, Signorelli C, Odone A, Dinu M. What can we expect from an umbrella review? Adv Nutr. 2022;13(2):684‐685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file S1
Supplementary file S2
Supplementary file S3
Supplementary file S4
Supplementary file S5
Supplementary file S6
Supplementary file S6A
Supplementary file S6B
Supplementary file S7
Supplementary file S7A
Supplementary file S7B
Supplementary file S8
Supplementary file S8A
Supplementary file S8B
Supplementary file S9
Supplementary file S9A
Supplementary file S9B
Supplementary file S10
Supplementary file S11


