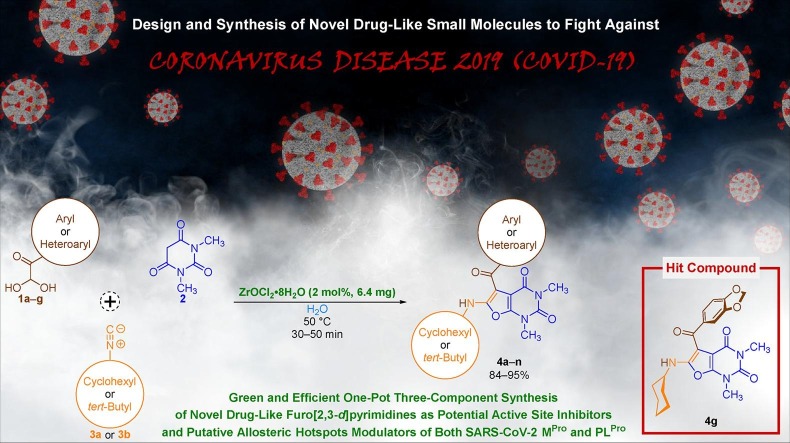
Graphical abstract
Keywords: COVID-19; SARS-CoV-2; Main protease (MPro); Papain-like protease (PLPro); Multi-targeting inhibitor; Molecular docking; ADMET; Green chemistry; One-pot; Multi-component reaction (MCR); Furo[2,3-d]pyrimidine
Abstract
In this paper, an environmentally benign, convenient, and efficient one-pot three-component reaction has been developed for the regioselective synthesis of novel 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) through the sequential condensation of aryl(or heteroaryl)glyoxal monohydrates (1a‒g), 1,3-dimethylbarbituric acid (2), and alkyl(viz. cyclohexyl or tert-butyl)isocyanides (3a or 3b) catalyzed by ultra-low loading ZrOCl2•8H2O (just 2 mol%) in water at 50 ˚C. After synthesis and characterization of the mentioned furo[2,3-d]pyrimidines (4a‒n), their multi-targeting inhibitory properties were investigated against the active site and putative allosteric hotspots of both SARS-CoV-2 main protease (MPro) and papain-like protease (PLPro) based on molecular docking studies and compare the attained results with various medicinal compounds which approximately in three past years were used, introduced, and or repurposed to fight against COVID-19. Furthermore, drug-likeness properties of the mentioned small heterocyclic frameworks (4a‒n) have been explored using in silico ADMET analyses. Interestingly, the molecular docking studies and ADMET-related data revealed that the novel series of furo[2,3-d]pyrimidines (4a‒n), especially 5-(3,4-methylendioxybenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4g) as hit one is potential COVID-19 drug candidate, can subject to further in vitro and in vivo studies. It is worthwhile to note that the protein–ligand-type molecular docking studies on the human body temperature-dependent MPro protein that surprisingly contains zincII (ZnII) ion between His41/Cys145 catalytic dyad in the active site, which undoubtedly can make new plans for designing novel SARS-CoV-2 MPro inhibitors, is performed for the first time in this paper, to the best of our knowledge.
1. Introduction
The coronavirus disease 2019 (COVID-19), which was caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is a life-threatening infectious disease that has posed significant global hazard concerning, including high mortality rate, economic breakdown, life distress, etc. [1]. By end of January 2023, based on World Health Organization (WHO) coronavirus disease situation dashboard and COVID-19 Map-Johns Hopkins Coronavirus Resource Center, over 754 million people had been infected with SARS-CoV-2 worldwide, and more than 6,800,000 reported deaths globally, unfortunately. The SARS-CoV-2 main protease (MPro) [2] and papain-like protease (PLPro) [3] are the most validated antiviral drug targets for combating COVID-19 because the SARS-CoV-2 MPro and PLPro are essential for viral replication, transcription, maintenance, and its life cycle. Therefore, the design and synthesis of small organic molecules that operate simultaneously as inhibitors of both SARS-CoV-2 MPro and PLPro targets are definitely worthwhile in this era. As a matter of fact, despite remarkable efforts in the nearly past three years, the scientific community's understanding about the SARS-CoV-2 phenomenon is still so limited that caused many serious restrictions on the design and preparation of new drugs or vaccines for the combating against the mentioned viral disease, regrettably [4]. Computer-aided drug design (CADD) approaches [5], which in recent years have become an indispensable constituent in medicinal chemistry, can be unquestionably practicable in a full-scale war with COVID-19 [6]. Notably, in the drug design field, computational studies are free from safety and ethical constraints and can increase the speed of a pharmaceutically relevant project and intensely reduce extravagant costs.
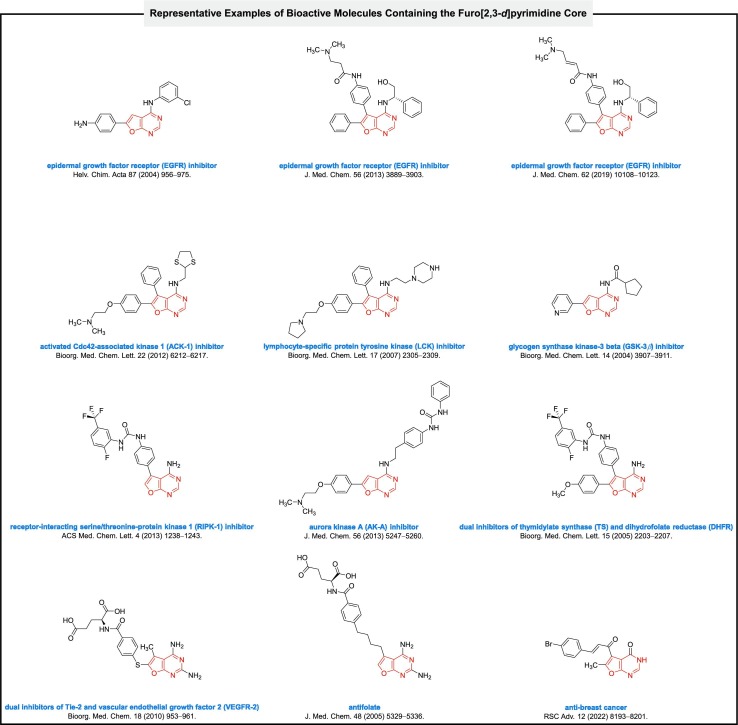
Heterocyclic compounds are valuable organic frameworks in myriad aspects of our life, especially in medicinal chemistry [7]. Furo[2,3-d]pyrimidine heterocyclic fused ring systems are structural analogs of purines and have diverse biological activities. As shown in Fig. 1 , some heterocyclic frameworks with the furo[2,3-d]pyrimidine core reported as inhibitors of epidermal growth factor receptor (EGFR) [8], activated Cdc42-associated kinase 1 (ACK-1) [9], lymphocyte-specific protein tyrosine kinase (LCK) [10], glycogen synthase kinase-3 beta (GSK-3β) [11], receptor-interacting serine/threonine-protein kinase 1 (RIPK-1) [12], and aurora kinase A (AK-A) [13]. Besides, Miyazaki and co-workers reported 1-(4-(4-amino-6-(4-methoxyphenyl)furo[2,3-d]pyrimidin-5-yl)phenyl)-3-(2-fluoro-5-(trifluoromethyl)phenyl)urea compound as dual inhibitors of thymidylate synthase (TS) and dihydrofolate reductase (DHFR) [14]. Also, Gangjee et al. reported (4-((2,4-diamino-5-methylfuro[2,3-d]pyrimidin-6-yl)thio)benzoyl)-l-glutamic acid scaffold as dual inhibitors of Tie-2 and vascular endothelial growth factor 2 (VEGFR-2) [15]. In addition, some of the mentioned compounds were reported as antifolate [16] and potent anti-breast cancer [17] agents. Because of the mentioned significant biological features and many others, which are existed in scientific papers [18], furo[2,3-d]pyrimidines have become an attractive synthetic target for organic and medicinal synthetic groups.
Fig. 1.
Representative examples of bioactive molecules containing the furo[2,3-d]pyrimidine core.
Today, it is imperative to follow green chemistry protocols (GCPs) in designing or modifying a synthetic approach for the preparation of organic compounds (a), [19]. Nowadays, concerning the GCPs in the drug (or drug-like compounds) discovery process is undeniably significant and essential [20]. It is also worth mentioning that pharmaceutical manufacturing, with pioneering works, was one of the first industries to recognize the importance of the GCPs and applied all of (or most of) them as far as possible. In this regard, selecting the reaction solvent, catalyst, and procedure based on GCPs are significant. Among well-known environmentally benign chemical reaction mediums [21], water is the best because it is non-toxic, inexpensive, abundant, sustainable, and in the green chemistry solvent ranking list, holds a top and valuable place among others [22]. Furthermore, in aqueous media, it is unessential to dry co-solvents, substrates, and reagents before use, which cause saving costs and time. Catalysis is another main factor in the GCPs for the design of an environmentally benign organic reaction. ZirconiumIV oxychloride octahydrate (ZrOCl2•8H2O) as an available, low-cost, easy-handling, and moisture-stable catalyst with highly coordinating ability, has attracted the attention in the organic synthetic community [23]. In the past two decades, the mentioned green catalyst has been used frequently in various organic transformations, especially heterocyclic ones, including syntheses of 4H-chromenes [24], pyrimido[4,5-d]pyrimidinones [25], dibenzo[b,i]xanthene-tetraones [26], [1,3]oxazino[5,6-c]quinolin-5-ones [27], 1,8-dioxo-octahydroxanthenes [28], benzopyranopyrimidines [29], 2,4,6-triarylpyridines [30], tetrahydropyrimidine [31], isobenzofuran-1(3H)-ones [32], 1H-imidazoles [33], 2-aryloxazolines [34], dihydroquinolinones [35], 3,4-dihydropyrimidin-2(1H)-ones [36], hexahydroquinolines [37], pyrano[2,3-d:6,5-dʹ]dipyrimidinones [38], pyrimido[4,5-c]pyridazines [39], 5-amino-1-aryl-1H-tetrazoles,[40] and many others. From the green chemistry point of view, one-pot multi-component reactions (MCRs) are amiable, advanced, and innovative strategies in organic synthesis [41]. Rather than the classical sequential pathway approaches, these reactions have attracted expanded attention in combinatorial, synthetic, and pharmaceutical chemistry for their distinct advantages, such as straightforward reaction design, high atom-economy, time-effectiveness, simplified work-up procedures, high overall yields of desired products, and molecular diversity. A literature survey shows that arylglyoxal monohydrate-based [42] and isocyanide-based [43] one-pot multi-component reactions have a unique place in the synthesis of the heterocyclic compounds, each independently. Therefore, the combination of mentioned starting materials in specific one-pot MCRs assuredly leads to the creation of a new avenue in novel heterocyclic scaffolds synthesis.
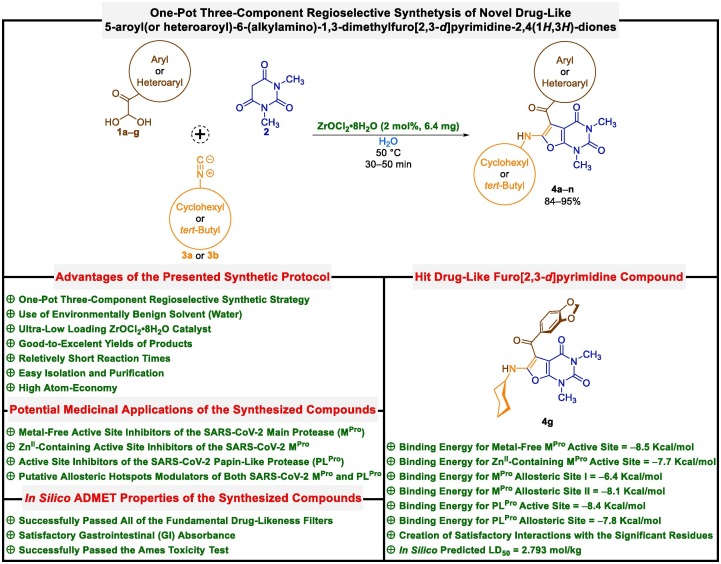
In continuation of our research programs on the synthesis of pharmaceutically interesting heterocyclic frameworks [44], and also due to the importance of introducing new anti-SARS-CoV-2 agents, we wish to report an environmentally benign and efficient one-pot three-component regioselective synthetic strategy for the preparation of novel 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) through the sequential condensation of aryl(or heteroaryl)glyoxal monohydrates (1a‒g), 1,3-dimethylbarbituric acid (2), and alkyl(viz. cyclohexyl or tert-butyl)isocyanides (3a or 3b), which catalyzed by a tremendously small amount of ZrOCl2•8H2O (just 2 mol%), in water at 50 ˚C (Fig. 2 ). Besides, inhibitory activities of the newly synthesized fused heterocyclic frameworks (4a‒n) against the active site and putative allosteric hotspots of both SARS-CoV-2 MPro and PLPro investigated using molecular docking, and the obtained results compared with various medicinal compounds, which used, introduced, and or repurposed to fight against COVID-19 viral disease in nearly past three years. Drug-likeness properties of the mentioned furo[2,3-d]pyrimidines (4a‒n) were also explored by employing in silico ADMET analyses. It is worthwhile to note that 5-(3,4-methylendioxybenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4g) as hit compound is potential COVID-19 drug candidate and can subject to further in vitro and in vivo studies.
Fig. 2.
Green one-pot three-component synthesis of novel drug-like furo[2,3-d]pyrimidines as potential active site inhibitors and putative allosteric hotspots modulators of both SARS-CoV-2 MPro and PLPro. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. Results and discussion
2.1. Synthesis, characterization, and plausible mechanism of 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n)
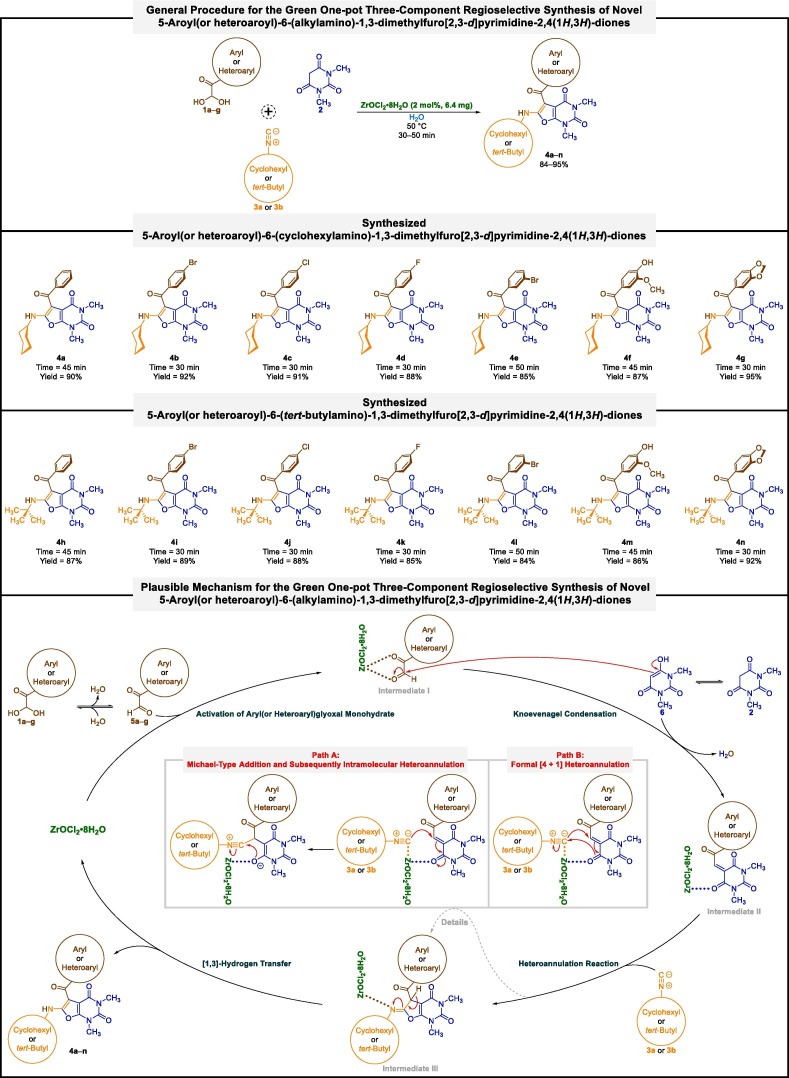
Initially, we commenced our studies with the optimization reaction conditions for the green one-pot regioselective synthesis of 5-benzoyl-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4a) through the tandem three-component condensation of phenylglyoxal monohydrate (1a), 1,3-dimethylbarbituric acid (2), and cyclohexyl isocyanide (also known as isocyanocyclohexane) (3a) as a model reaction (Table 1 ). It is worthwhile to note that in all stages of optimization, we used water as an entirely environmentally benign reaction solvent because one of our main goals was to design a reaction based on green chemistry protocols. Under the catalyst-free conditions at room temperature, just 30% yield of the desired product (4a) was obtained, even after 48 h (Table 1, entry 1). Increasing reaction temperature from room temperature to 50 ˚C and Reflux caused preparation 4a in 56% and 43%, respectively, after 24 h (Table 1, entries 2 and 3). The gained poor results led us to use a catalyst to achieve better and more efficient outcomes. To this purpose, we used 1,5-diazabicyclo[4.3.0]non-5-ene (DBN), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU), and 1,4-diazabicyclo[2.2.2]octane (DABCO) as base organocatalysts, and also zirconiumIV oxychloride octahydrate (ZrOCl2•8H2O) as a green metallic catalyst. When the one-pot three-component reaction was carried out in 2 mol% presence of the mentioned easily accessible catalysts, we observed that the catalytic performance of ZrOCl2•8H2O is better than others at 50 ˚C (Table 1, entry 7). Further investigations showed that the decreasing and even increasing amount of ZrOCl2•8H2O not only did the conditions of the one-pot reaction not improve, but it made the situation worse (Table 1, entries 8 and 9). Once we had the optimized reaction conditions in hand (Table 1, entry 7), we evaluated the scope and limitations of the mentioned green one-pot three-component regioselective synthetic protocol, as shown in Fig. 3 . The one-pot three-component reaction tolerated several aryl(or heteroaryl)glyoxal monohydrates (1a‒g) and alkyl(cyclohexyl or tert-butyl)isocyanides (3a or 3b), affording the corresponding 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) with good-to-excellent yields. Notably, the yields of the desired furo[2,3-d]pyrimidine heterocyclic products containing cyclohexyl isocyanide (3a) were slightly better rather than tert-butyl isocyanide (also known as 2-isocyano-2-methylpropane) (3b), as shown in Fig. 3.
Table 1.
Optimization reaction conditions for the one-pot three-component synthesis of 4a.
 | ||||
|---|---|---|---|---|
| Entry | Catalyst (mol%) | Temperature conditions | Time (h or min) | Yield (%) |
| 1 | Catalyst-free | Room temperature | 48 h | 30 |
| 2 | Catalyst-free | 50 ˚C | 24 h | 56 |
| 3 | Catalyst-free | Reflux | 24 h | 43 |
| 4 | DBN (2 mol%) | 50 ˚C | 1 h | 71 |
| 5 | DBU (2 mol%) | 50 ˚C | 1 h | 75 |
| 6 | DABCO (2 mol%) | 50 ˚C | 1 h | 80 |
| 7 | ZrOCl2•8H2O (2 mol%) | 50 ˚C | 45 min | 90 |
| 8 | ZrOCl2•8H2O (1 mol%) | 50 ˚C | 70 min | 81 |
| 9 | ZrOCl2•8H2O (10 mol%) | 50 ˚C | 45 min | 82 |
Fig. 3.
Green one-pot three-component regioselective synthesis of 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones catalyzed by ZrOCl2•8H2O. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The structure of all mentioned fused heterocyclic products (4a‒n) was confirmed by Fourier transform infrared spectroscopy (FT-IR), 1H and 13C nuclear magnetic resonance (1H NMR and 13C NMR), and carbon–hydrogen–nitrogen (CHN) analyses. In this regard, the 1H NMR spectrum of 4a, as a simple instance (Figure S1, section A), revealed a doublet peak at δ H 8.61 ppm (J = 8.3 Hz) for the ─NH─ proton. The five aromatic protons of the phenyl ring system appeared at δ H 7.62 ppm (d, J = 6.9 Hz, 2H, Ph─H), 7.51 ppm (t, J = 7.3 Hz, 1H, Ph─H), and 7.40 ppm (t, J = 7.5 Hz, 2H, Ph─H). The proton of the N─CHcyclohexyl has also appeared as a multiplet peak at δ H 3.81–3.70 ppm. The two sharp singlet peaks at δ H 3.58 ppm and 3.29 ppm are related to the two methyl groups of the pyrimidine ring (N─CH3). Also, the four multiplet peaks at δ H 2.11–1.41 ppm showed ten hydrogens related to the five methylene (─CH2─) groups of the cyclohexyl homocyclic ring system. On the other hand, the 1H-decoupled 13C NMR (13C{1H} NMR) spectrum of 4a (Figure S1, section B) showed seventeen peaks in agreement with the represented structure. In this regard, the peak of the benzoyl carbonyl group is revealed at δ C 189.20 ppm, and the peaks of the two amide carbonyl groups of the pyrimidine ring are exposed at δ C 161.09 ppm and 156.56 ppm. It is worth noting that the characteristic peaks at δ C 149.97 ppm and 148.90 ppm are related to the two carbon atoms of the furan ring, which are nearby the oxygen heteroatom (namely C6 and C7a). Also, the other two carbon atoms of the mentioned furan ring, which are far from the furan’s oxygen heteroatom (namely C5 and C4a), are visible at δ C 94.40 ppm and 92.55 ppm. The carbons of the phenyl ring appeared at δ C 140.39 ppm, 131.15 ppm, 128.46 ppm, and 127.33 ppm. Furthermore, the 13C{1H} NMR spectrum of 4a represented one peak at δ C 51.91 ppm for the methine group of the cyclohexyl moiety (N─CHcyclohexyl), and two distinct peaks at δ C 33.43 ppm and 29.69 ppm for the two N─CH3 groups of the pyrimidine ring, and also three peaks at δ C 28.69 ppm, 25.24 ppm, and 24.34 ppm for the cyclohexyl ring methylene groups (─CH2─).
A plausible mechanism for this valuable green one-pot three-component regioselective transformation using ZrOCl2•8H2O catalyst in water at 50 ˚C is also depicted in Fig. 3. In the first step, selected aryl(or heteroaryl)glyoxal monohydrate (1a‒g) was activated by ZrOCl2•8H2O and gave intermediate I. In the second step, a regioselective Knoevenagel condensation reaction between enol form (6) of 1,3-dimethylbarbituric acid (2) with the formyl group of the activated aryl(or heteroaryl)glyoxal (I) led to the formation of 1,3-dimethyl-5-(2-oxo-2-aryl(or heteroaryl)ethylidene)pyrimidine-2,4,6(1H,3H,5H)-trione (intermediate II) along with the elimination of one water molecule. In the third step, a heteroannulation reaction between the activated intermediate II and related alkyl(cyclohexyl or tert-butyl)isocyanide (3a or 3b) caused the formation of (Z)-5-aroyl(or heteroaroyl)-6-(alkylimino)-1,3-dimethyl-5,6-dihydrofuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (intermediate III). It is worth noting that the mentioned step probably proceeds from a simple Michael-type addition and subsequently intramolecular heteroannulation reaction (path A) and or through a formal [4 + 1] heteroannulation process (path B). Finally, the spurred iminolactone (intermediate III) generated desired furo[2,3-d]pyrimidine product (4a‒n) by a [1,3]-hydrogen transfer.
2.2. Molecular docking studies
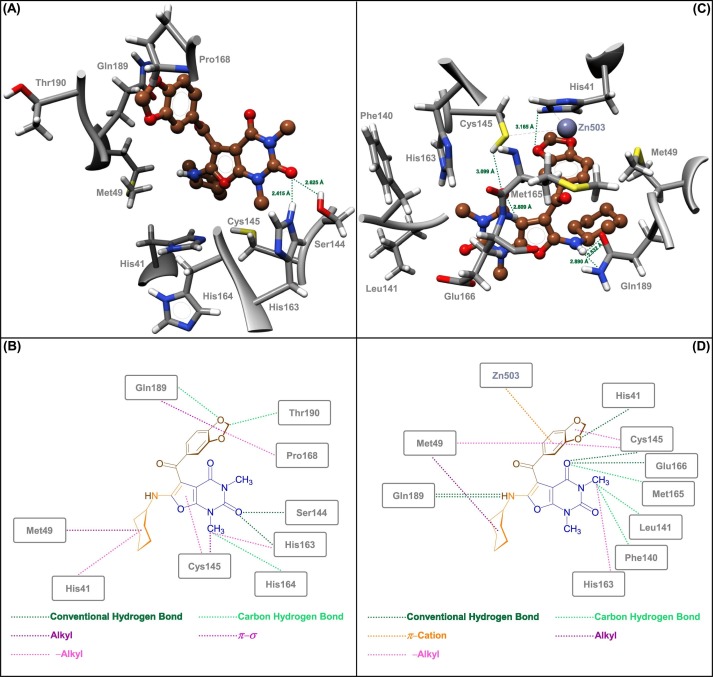
To explore the protein–ligand interactions between the newly synthesized 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) and various pockets of the SARS-CoV-2 MPro and PLPro, in silico molecular docking studies performed using AutoDock Vina as an open-source program for doing molecular docking along with UCSF Chimera as a graphical user interface. First of all, we carried out a molecular docking process for our synthesized furo[2,3-d]pyrimidines (4a‒n) on the active site of MPro protein (PDB ID: 7AEH). It should be noted that MPro of the SARS-CoV-2 contains a highly conserved catalytic dyad comprising amino acid residues His41 and Cys145 that are buried in the active site cavity of the mentioned protein, in which His41 acts as a general acid or base, and on the other site, Cys145 acts as a nucleophile. The obtained binding energies (also known as binding free energies, binding affinities, and or binding scores) of the molecular docking investigations were ranging from −7.2 Kcal/mol to −8.5 Kcal/mol (Table S1). From the binding energy point of view, the attained results demonstrated that 5-(3,4-methylendioxybenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4g) with a binding energy of −8.5 Kcal/mol is slightly better than other synthesized furo[2,3-d]pyrimidine heterocyclic frameworks (4a‒n). As shown in Fig. 4 (sections A and B) and Table S1, the compound 4g was able to form two conventional hydrogen bonds with residues Ser144 (2.625 Å) and His163 (2.415 Å) of the SARS-CoV-2 MPro active site. On the other hand, 4g has some hydrophobic interactions with His41/Cys145 catalytic dyad. In this regard, the compound 4g exhibited π–alkyl interaction with His41 and revealed alkyl and π–alkyl interactions with Cys145. Furthermore, the mentioned fused heterocyclic compound (4g) interacts with residues Met49 (alkyl), His163 (π–alkyl), His164 (carbon hydrogen bond), Pro168 (π–alkyl), Gln189 (carbon hydrogen bond and π–σ), and Thr190 (carbon hydrogen bond), as shown in Fig. 4 (sections A and B) and Table S1. Interestingly, as shown in Table S1, the compound 5-(4-hydroxy-3-methoxybenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4f), with a binding energy of −7.9 Kcal/mol, capable to creation eight hydrogen bonds with the SARS-CoV-2 MPro active site residues, especially Cys145 (2.341 Å). In 2022, Ebrahim and co-workers published a valuable and attention-grabbing paper about the temperature-dependent conformational ensemble of the SARS-CoV-2 MPro [45]. Their studies revealed that in human body temperature (310 K), the mobile zincII (ZnII) ion interleaved between the His41/Cys145 catalytic dyad (Figure S2). The mentioned occurrence can inspire the scientific community to make new and different plans for designing new covalent and non-covalent SARS-CoV-2 MPro inhibitors. To this purpose, we shifted our investigations to the ZnII-containing active site of the SARS-CoV-2 MPro protein (PDB ID: 7MHK). For this stage, the obtained binding energies were ranging from −6.5 Kcal/mol to −7.7 Kcal/mol (Table S2). Gratifyingly, in terms of binding energy we saw that the best heterocyclic compound was 4g (−7.7 Kcal/mol). As shown in Fig. 4 (sections C and D) and Table S2, the compound 4g can form five conventional hydrogen bonds with His41 (3.165 Å), Cys145 (3.099 Å), Glu166 (2.809 Å), and Gln189 (2.532 Å and 2.890 Å). In addition, we observed a π–cation interaction between 4g and ZnII ion (Zn503). Furthermore, the compound 4g interacts with Met49 (alkyl and π–alkyl), Phe140 (carbon hydrogen bond), Leu141 (carbon hydrogen bond), Cys145 (π–alkyl), His163 (π–alkyl), and Met165 (carbon hydrogen bond) (Fig. 4 (sections C and D) and Table S2). It is worth noting that, as shown in Table S2, the compound 4f, with a binding energy of −7.5 Kcal/mol, is capable to create eleven hydrogen bonds with the SARS-CoV-2 MPro active site residues, especially three hydrogen bonds with Cys145 (2.470 Å, 2.830 Å, and 3.128 Å), and also can form a metal–acceptor interaction with Zn503.
Fig. 4.
Close-up views (3D and 2D) of 4g in the metal-free active site (A and B) and ZnII-containing active site (C and D) of the SARS-CoV-2 MPro, respectively.
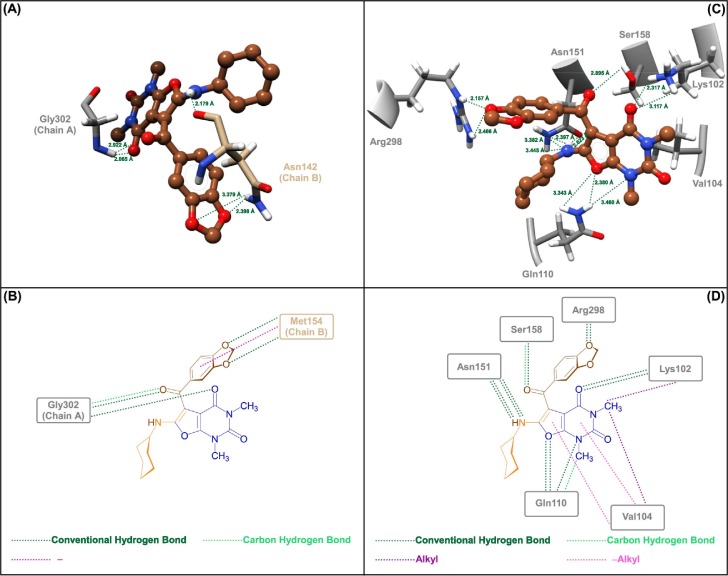
Inhibition of allosteric hotspots is an emerging paradigm in modern pharmacology to the extent that recent years have seen an unprecedented and astonishing level of innovation in the discovery and development of allosteric drugs [46]. Because of the importance of this issue, we investigated the molecular docking process for two putative allosteric pockets of the SARS-CoV-2 MPro, including allosteric site I (known as dimerization site, which could interrupt the dimerization conformation and inactive the MPro) and allosteric site II (Known as cryptic site) [47]. The molecular docking results for the allosteric site I (PDB ID: 7VLP) showed that the binding energies of the investigations were ranging from −5.3 Kcal/mol to −6.4 Kcal/mol (Table S3), which the compound 4g with a binding energy of −6.4 Kcal/mol is somewhat better than others, and as shown in Fig. 5 (sections A and B) and Table S3, it was able to form two conventional hydrogen bonds with residue Gly302A (2.065 Å and 2.922 Å) and three conventional hydrogen bonds with residue Asn142B (2.179 Å, 2.398 Å, and 3.379 Å). Furthermore, we observed a carbon hydrogen bond interaction with Gly302A and a π–σ hydrophobic interaction with Asn142B, as shown in Fig. 5 (sections A and B) and Table S3. On the other hand, the molecular docking outcomes for allosteric site II (PDB: 7MHK) exhibited that the binding energies were ranging from −6.6 Kcal/mol to −8.1 Kcal/mol (Table S4), which again the compound 4g with a binding energy of −8.1 Kcal/mol is slightly better than others. As shown in Fig. 5 (sections C and D) and Table S4, 4g can able to create twelve conventional hydrogen bonds with residues Lys102 (2.317 Å and 3.117 Å), Gln110 (2.380 Å, 3.343 Å, and 3.460 Å), Asn151 (2.397 Å, 2.623 Å, 3.382 Å, and 3.445 Å), Ser158 (2.895 Å), and Arg298 (2.157 Å and 2.486 Å). Besides, 4g has alkyl interaction with Lys102, and alkyl and π–alkyl interactions with Val104 along with carbon hydrogen bond interactions with Gln110 and Ser158, as shown in Fig. 5 (sections C and D) and Table S4.
Fig. 5.
Close-up views (3D and 2D) of 4g in the allosteric site I (A and B) and allosteric site II (C and D) of the SARS-CoV-2 MPro, respectively.
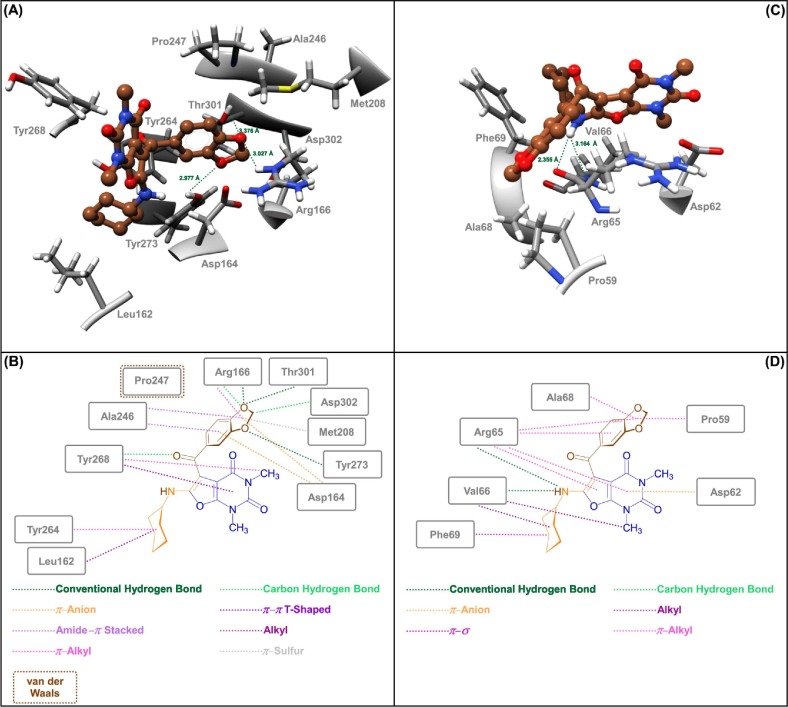
The SARS-CoV-2 PLPro is another essential factor for the COVID-19 proliferation cycle. The mentioned SARS-CoV-2 PLPro active site contains the catalytic triad that is formed by Cys111, His272, and Asp286. In continuation of in silico studies, we investigated another molecular docking process for our synthesized furo[2,3-d]pyrimidines (4a‒n) on the active site of SARS-CoV-2 PLPro protein (PDB ID: 6WX4). The molecular docking investigations showed that obtained binding energies were ranging from −7.5 Kcal/mol to −8.4 Kcal/mol (Table S5). Among the mentioned furo[2,3-d]pyrimidines (4a‒n), the compound 4g with a binding energy of −8.4 Kcal/mol is again slightly better than others. As shown in Fig. 6 (sections A and B) and Table S5, this ligand (4g) formed three conventional hydrogen bonds with the amino acids residues Arg166 (3.027 Å), Tyr273 (2.977 Å), and Thr301 (3.376 Å). In addition, other interactions were observed (Fig. 6 (sections A and B) and Table S5) for this case, including Leu162 (alkyl), Asp164 (π–anion), Arg166 (carbon hydrogen bond and π–alkyl), Met208 (π–sulfur), Ala246 (amide–π stacked), Pro247 (van der Waals), Tyr264 (π–alkyl), Tyr268 (carbon hydrogen bond, π–π T-shaped, and π–alkyl), and Asp302 (carbon hydrogen bond). Remarkably, the SARS-CoV-2 PLPro (PDB ID: 6WX4) allosteric [48] inhibitory properties of our synthesized heterocyclic compounds were also investigated. The binding energies for this case were from −5.8 Kcal/mol to −7.8 Kcal/mol (Table S6). The compound 4g, which is better than others in terms of binding energy (−7.8 Kcal/mol), can able to form two conventional hydrogen bonds with residues Arg65 (2.355 Å) and Val66 (3.164 Å), as shown in Fig. 6 (sections C and D) and Table S6. On the other hand, 4g have hydrophobic interactions with residues Pro59 (π–alkyl), Arg65 (π–alkyl), Val66 (alkyl), Ala68 (π–alkyl), and Phe69 (π–σ), along with an electrostatic interaction with residue Asp62 (π–anion) (Fig. 6 (sections C and D) and Table S6).
Fig. 6.
Close-up views (3D and 2D) of 4g in the active site (A and B) and allosteric site (C and D) of the SARS-CoV-2 PLPro, respectively.
2.3. In silico ADMET prediction
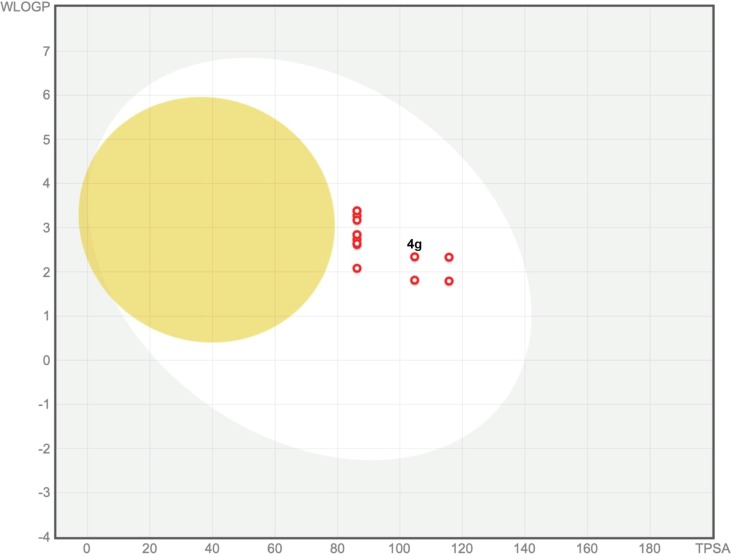
Most of the designed and prepared chemical compounds for medicinal purposes fail and flop in the drug development process due to their poor pharmacokinetics and toxicity problems, which is an undeniable fact worth pondering. Such drawbacks that arise during drug development should be addressed at the early stage in the pipeline of this process to prevent the loss of material and intellectual capital and to achieve the desired goal in a shorter period of time and with low costs. In silico ADMET (absorption, distribution, metabolism, excretion, and toxicity) prediction is a significant analysis to rolling out undesired effects of a proposed drug candidate at the initial step of the drug discovery process [49]. In this regard, in silico ADME analysis of the prepared 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) investigated using free web tool SwissADME, from Swiss Institute of Bioinformatics (http://www.swissadme.ch) [50]. The data related to physicochemical properties and lipophilicity (Table S7), water solubility (Table S8), pharmacokinetics (Table S9), and drug-likeness and medicinal chemistry (Table S10) of the synthesized heterocyclic compounds (4a‒n) have been collected in Supporting Information (SI). Interestingly, the obtained results demonstrated the mentioned furo[2,3-d]pyrimidines (4a‒n) generally possess drug-like behavior because they could successfully be passed fundamental drug-likeness filters, including Lipinski (Pfizer), Ghose (Amgen), Veber (GSK), Egan (Pharmacia), and Muegge (Bayer). Also, the Abbot bioavailability score [51] value for all synthesized compounds (4a‒n) was 0.55 (55%), which indicates the probability of their bioavailability, and it is based on the total charge of compound, topological polar surface area (TPSA), and violation of Lipinski filter. Furthermore, pan assay interference structures (PAINS) and Brenk filters are applied to provide information concerning potentially problematic fragments (putatively toxic, metabolically unstable, or possessing properties responsible for poor pharmacokinetics). Gratifyingly, our heterocyclic frameworks (4a‒n) have no alert for PAINS and Brenk. On the other hand, the BOILED-Egg plot between WLOGP and TPSA was used to predict gastrointestinal absorption and brain penetration of the furo[2,3-d]pyrimidines (4a‒n). As can be seen from the BOILED-Egg plot (Fig. 7 ), all of the mentioned one-pot synthesized compounds (4a‒n) show satisfactory gastrointestinal (GI) absorption and have no blood–brain barrier (BBB) permeability, and the red dots as P-glycoprotein non-substrates (PGP−) demonstrate predictions that our fused heterocyclic compounds (4a‒n) cannot be effluxes from the central nervous system (CNS) by PGP. Overall, from the related BOILED-Egg plot data, especially from the positions of the red dots, we can conclude that the BBB penetration property and PGP effect for our compounds are negative, and the GI absorption property is positive. Besides, the in silico toxicity evaluation was carried out using an online server, pkCSM (https://biosig.lab.uq.edu.au/pkcsm/prediction) [52]. As shown in Table S11, the in silico Ames toxicity test investigation shows that all the newly synthesized fused heterocyclic frameworks (4a‒n) successfully passed the mentioned imperative test and predicted them as non-mutagenic compounds. Also, LD50 (oral rat acute toxicity) amounts for these heterocyclic structures (4a‒n) were from 2.37 mol/kg to 2.793 mol/kg, which highest one related to 4g (Table S11).
Fig. 7.
BOILED-Egg plot of 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones.
2.4. Comparative study
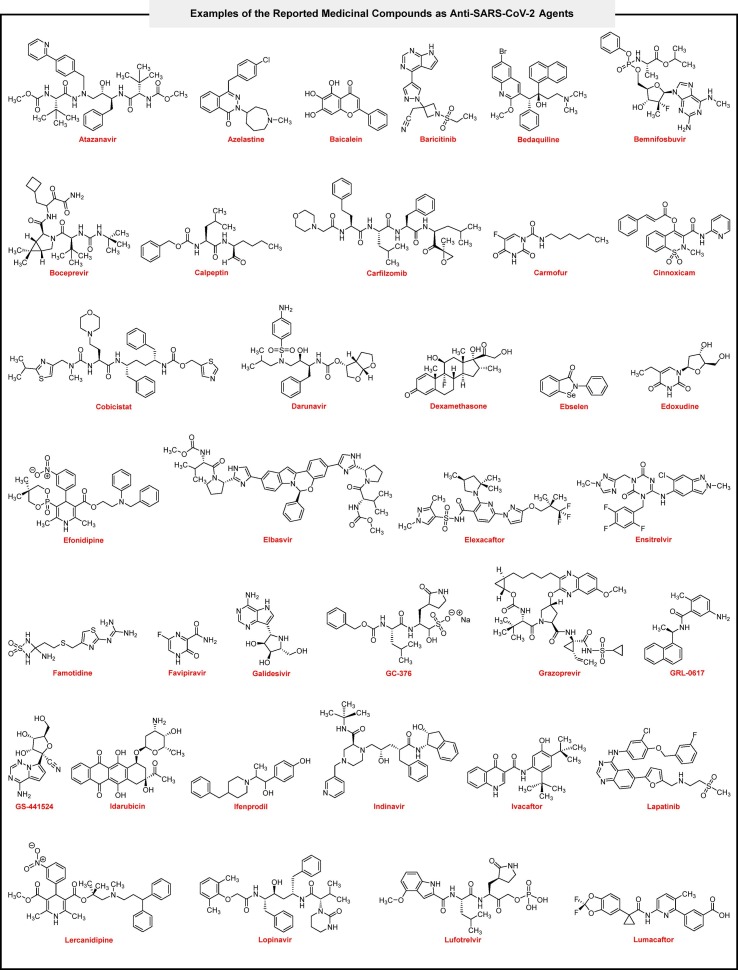
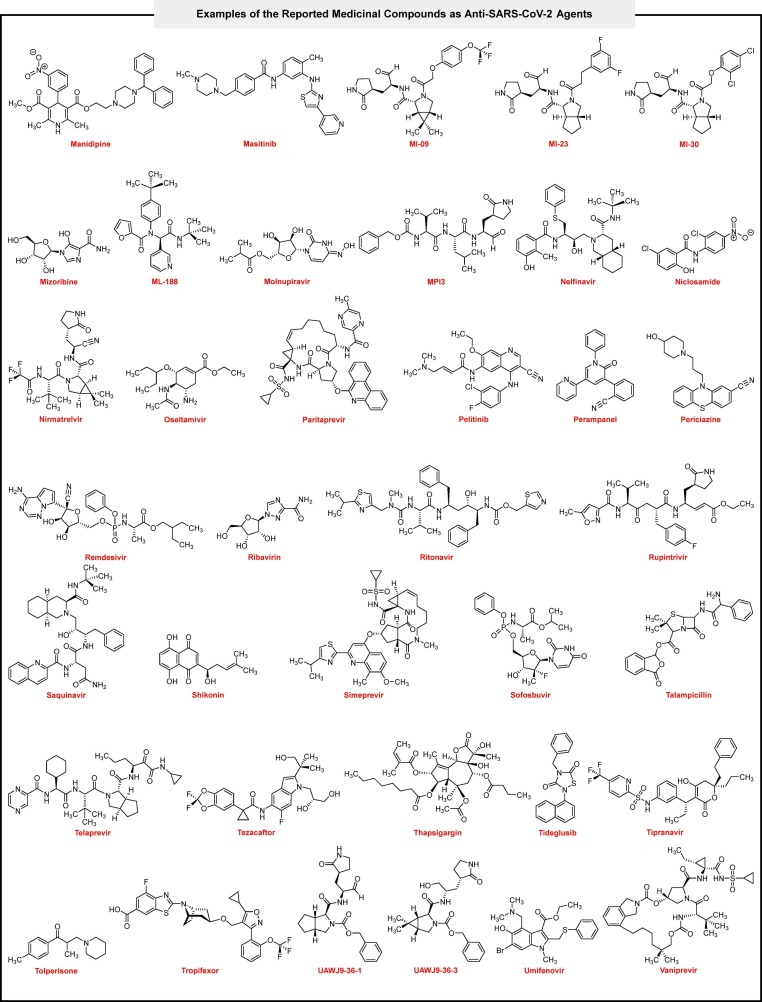
To demonstrate the 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) value and power in fighting against COVID-19 disease, we compared hit one (4g) with seventy-three medicinal compounds (Fig. 8 ) that used, repurposed, investigated, and introduced against SARS-CoV-2 in closely three past years. From the binding energy point of view, the mentioned comparison revealed that 4g could have a unique place in this viral war. As shown in Table 2 , binding energies of the medicinal compounds from the SARS-CoV-2 MPro metal-free active site (PDB ID: 7AEH) showed that only three of them (viz. Elbasvir, Ensitrelvir, and Lumacaftor) are better than 4g, and one of them, namely Idarubicin, has equal binding energy with 4g. Also, the molecular docking results of 4g from the MPro ZnII-containing active site (PDB ID: 7MHK), the allosteric site I (PDB ID: 7VLP), and the allosteric site II (PDB ID: 7MHK) of the SARS-CoV-2 were satisfied and acceptable rather than others. It should be noted that Ensitrelvir (known as S-217622), which is a better inhibitor than 4g in both SARS-CoV-2 MPro metal-free and ZnII-containing active sites and allosteric sites (Table 2, entry 21), was reported in 2022 as the first oral non-covalent and non-peptidic SARS-CoV-2 MPro inhibitor clinical candidate with IC50 value of 13 nM and have EC50 values for wild type (WT) and other strains of SARS-CoV-2 including alpha, betta, gamma, delta, and omicron equal 0.37 μM, 0.33 μM, 0.40 μM, 0.50 μM, 0.41 μM, and 0.29 μM, respectively [53]. Furthermore, a comparison between entries 1, 49, and 57 of Table 2 revealed that 4g has more suitable binding energy than both Nirmatrelvir and Ritonavir as two parts of the oral Pfizer’s Paxlovid SARS-CoV-2 MPro inhibitor drug for COVID-19 [54]. As shown in Table 2 (entries 1 and 44), the compound 4g is also better SARS-CoV-2 MPro inhibitor than ML-188 (with a IC50 of 2.5 ± 0.3 μM) as a non-covalent MPro inhibitor [55]. In the SARS-CoV-2 PLPro active site (PDB ID: 6WX4), Lumacaftor, MI-09, and Tropifexor (Table 2, entries 37, 40, and 70) showed slightly better binding energy than 4g, and also in the SARS-CoV-2 PLPro allosteric site (PDB ID: 6WX4), Lumacaftor and Paritaprevir (Table 2, entries 37 and 51) were somewhat better than 4g, and Simeprevir (Table 2, entry 61) had equal with 4g. Notably, 4g has better binding energy in the SARS-CoV-2 PLPro active and allosteric sites than GRL-0617 (Table 2, entry 27) as a SARS-CoV-2 PLPro inhibitor with IC50 of 1.50 ± 0.08 μM [56]. Also, a comparison between binding energies of 4g and Tropifexor (Table 2, entry 70), which is an efficient repurposed drug for inhibition of SARS-CoV-2 PLPro (with a IC50 of 5.11 ± 1.14 μM and EC50 of 4.3 ± 0.5 μM) [57], the results related to the PLPro active site were so closed and for the PLPro allosteric site, the compound 4g was better one.
Fig. 8.
Examples of the reported medicinal compounds as anti-SARS-CoV-2 agents.
Table 2.
Comparative molecular docking studies between 4g and reported medicinal compounds as anti-SARS-CoV-2 agents.
| Entry | Compound name |
Binding energy (Kcal/mol) |
|||||
|---|---|---|---|---|---|---|---|
|
Main protease (MPro) |
Papain-like protease (PLPro) |
||||||
|
Metal-free active site (PDB ID: 7AEH) |
ZnII-Containing active site (PDB ID: 7MHK) |
Allosteric site I (PDB ID: 7VLP) |
Allosteric site II (PDB ID: 7MHK) |
Active site (PDB ID: 6WX4) |
Allosteric site (PDB ID: 6WX4) |
||
| 1 | 4g | −8.5 | −7.7 | −6.4 | −8.1 | −8.4 | −7.8 |
| 2 | Atazanavir | −7.1 | −6.9 | −5.5 | −7.3 | −6.4 | −6.6 |
| 3 | Azelastine | −7.5 | −7.2 | −6.1 | −7.7 | −7 | −7.1 |
| 4 | Baicalein | −7.3 | −7.1 | −6.3 | −7.3 | −7 | −7.1 |
| 5 | Baricitinib | −7.6 | −7.4 | −5.5 | −7.3 | −7.3 | −6 |
| 6 | Bedaquiline | −7.3 | −7.6 | −5.4 | −7.4 | −7.2 | −7.5 |
| 7 | Bemnifosbuvir | −7.5 | −7.2 | −5.8 | −7.4 | −7.1 | −6.4 |
| 8 | Boceprevir | −7.1 | −7.1 | −5.5 | −7.2 | −7.2 | −5.7 |
| 9 | Calpeptin | −6.1 | −6.3 | −4.8 | −6.5 | −5.8 | −5.5 |
| 10 | Carfilzomib | −6.5 | −7 | −6.2 | −6.3 | −6.3 | −5.8 |
| 11 | Carmofur | −6.2 | −6.2 | −4.9 | −6.4 | −6.4 | −5.7 |
| 12 | Cinnoxicam | −7.9 | −7.8 | −6.5 | −8.4 | −7.2 | −6.7 |
| 13 | Cobicistat | −6.2 | −7.3 | −5.9 | −7.4 | −7.3 | −5.8 |
| 14 | Darunavir | −7.6 | −7 | −5.8 | −7.9 | −7.1 | −6.1 |
| 15 | Dexamethasone | −7.8 | −7.5 | −5.4 | −7.4 | −7.6 | −5.8 |
| 16 | Ebselen | −6.2 | −6.7 | −5.3 | −6.5 | −6.1 | −6.1 |
| 17 | Edoxudine | −6.4 | −6.4 | −5.2 | −6 | −6.1 | −5.6 |
| 18 | Efonidipine | −7.3 | −8.5 | −6.1 | −8.6 | −7.8 | −6.5 |
| 19 | Elbasvir | −8.7 | −7.5 | −6.3 | −8.7 | −7.9 | −7 |
| 20 | Elexacaftor | −7.8 | −7.8 | −6.5 | −8.7 | −7.6 | −6.7 |
| 21 | Ensitrelvir | −9.9 | −8.2 | −6.6 | −8.6 | −7.8 | −7.2 |
| 22 | Famotidine | −6.2 | −6.1 | −4.8 | −5.3 | −6.8 | −5 |
| 23 | Favipiravir | −5.7 | −5.5 | −5.3 | −5.5 | −5.8 | −5.1 |
| 24 | Galidesivir | −6.6 | −6.2 | −6.1 | −6 | −6.5 | −5.8 |
| 25 | GC-376 | −6.9 | −7.5 | −5.6 | −7.8 | −7.2 | −6 |
| 26 | Grazoprevir | −7.4 | −7.2 | −6.4 | −7.8 | −6.6 | −6 |
| 27 | GRL-0617 | −7 | −7.1 | −6.5 | −7.7 | −7.7 | −6.9 |
| 28 | GS-441524 | −6.6 | −6.7 | −5.1 | −6.5 | −6.4 | −6 |
| 29 | Idarubicin | −8.5 | −7.4 | −6.2 | −7.8 | −7.3 | −6.8 |
| 30 | Ifenprodil | −7.6 | −6.9 | −7 | −7.2 | −7 | −6.6 |
| 31 | Indinavir | −7.6 | −7.3 | −6.6 | −7.7 | −7.6 | −7 |
| 32 | Ivacaftor | −7.2 | −7 | −5.9 | −8.4 | −7.1 | −6.6 |
| 33 | Lapatinib | −8.4 | −7.9 | −7 | −8.1 | −7.8 | −7.4 |
| 34 | Lercanidipine | −7.9 | −7.7 | −5.1 | −7.7 | −7.5 | −6.4 |
| 35 | Lopinavir | −7.3 | −7.3 | −5.8 | −7.1 | −7.3 | −6.4 |
| 36 | Lufotrelvir | −7.6 | −7.4 | −5.6 | −7.6 | −7.8 | −5.7 |
| 37 | Lumacaftor | −8.9 | −9.1 | −7.9 | −9.3 | −8.9 | −8.1 |
| 38 | Manidipine | −8.3 | −7.1 | −5.4 | −8.3 | −7.9 | −6.8 |
| 39 | Masitinib | −7.9 | −8.2 | −6.6 | −9.3 | −8.1 | −7.5 |
| 40 | MI-09 | −8.3 | −7.8 | −5.8 | −8.2 | −8.5 | −7.3 |
| 41 | MI-23 | −8.2 | −8.3 | −6.4 | −8.1 | −7.4 | −6.8 |
| 42 | MI-30 | −8.1 | −8 | −6.4 | −8.1 | −7.9 | −6.7 |
| 43 | Mizoribine | −6.7 | −6.7 | −4.5 | −6.4 | −6 | −5.6 |
| 44 | ML-188 | −7.3 | −7 | −5.6 | −6.9 | −7.1 | −5.5 |
| 45 | Molnupiravir | −7.3 | −7 | −5.7 | −7 | −7.4 | −5.9 |
| 46 | MPI3 | −6.7 | −7.1 | −4.5 | −6.7 | −7.3 | −5.4 |
| 47 | Nelfinavir | −8.2 | −7.2 | −6.3 | −8.1 | −6.8 | −6.4 |
| 48 | Niclosamide | −7.3 | −7.1 | −5.4 | −7 | −7 | −6.4 |
| 49 | Nirmatrelvir | −8 | −7.6 | −5.8 | −7 | −8.2 | −6.2 |
| 50 | Oseltamivir | −5.9 | −5.8 | −4.2 | −5.8 | −6.3 | −5.2 |
| 51 | Paritaprevir | −7.7 | −7.9 | −7.6 | −7.8 | −8 | −8.3 |
| 52 | Pelitinib | −7.9 | −7.3 | −6.1 | −7.7 | −7.2 | −6.6 |
| 53 | Perampanel | −8.1 | −7.5 | −5.5 | −7.6 | −8 | −7.3 |
| 54 | Periciazine | −7.7 | −7.8 | −5.7 | −6.8 | −7.4 | −6.4 |
| 55 | Remdesivir | −7.9 | −7.3 | −5.1 | −6.7 | −7.3 | −5.7 |
| 56 | Ribavirin | −6.4 | −6.3 | −5.4 | −6.3 | −6.5 | −5.3 |
| 57 | Ritonavir | −6.9 | −6.7 | −6 | −7.9 | −6.4 | −6.1 |
| 58 | Rupintrivir | −7.6 | −7.3 | −5.6 | −6.9 | −7 | −6 |
| 59 | Saquinavir | −7.8 | −7.9 | −6.5 | −9.9 | −7.9 | −7.1 |
| 60 | Shikonin | −7.3 | −6.8 | −5.8 | −7.2 | −6.9 | −7.6 |
| 61 | Simeprevir | −8 | −7.9 | −6.8 | −9.5 | −7.6 | −7.8 |
| 62 | Sofosbuvir | −7.6 | −7.5 | −5.6 | −7 | −7.3 | −6.4 |
| 63 | Talampicillin | −8.4 | −8.1 | −6.8 | −8.2 | −8.3 | −7.1 |
| 64 | Telaprevir | −7.8 | −7.1 | −6 | −7.8 | −7 | −5.7 |
| 65 | Tezacaftor | −7.7 | −7.6 | −6.7 | −8.5 | −7.4 | −6.9 |
| 66 | Thapsigargin | −6.1 | −5.9 | −4.6 | −6.6 | −6 | −4.3 |
| 67 | Tideglusib | −7.1 | −7.4 | −6.3 | −7.7 | −7.6 | −7.3 |
| 68 | Tipranavir | −7.8 | −6.6 | −6.7 | −8.1 | −7.8 | −7.5 |
| 69 | Tolperisone | −5.8 | −6.1 | −5.2 | −6.7 | −6.3 | −6.1 |
| 70 | Tropifexor | −8.1 | −8.6 | −6.3 | −8.6 | −8.6 | −7.1 |
| 71 | UAWJ9-36-1 | −7.6 | −7.9 | −5 | −7.3 | −6.5 | −6 |
| 72 | UAWJ9-36-3 | −7.5 | −7.7 | −5.9 | −7.5 | −7.1 | −6.3 |
| 73 | Umifenovir | −6.2 | −6.5 | −4.5 | −6 | −6.4 | −5.5 |
| 74 | Vaniprevir | −8.3 | −8.4 | −7.3 | −7 | −7.1 | −6 |
3. Experimental section
3.1. Reagents, samples, and apparatus in the novel furo[2,3-d]pyrimidines (4a‒n) synthesis
1,3-Dimethylbarbituric acid (2), and alkyl(viz. cyclohexyl or tert-butyl)isocyanides (3a or 3b), and catalysts were commercially available (purchased from Merck, Sigma-Aldrich, and Fluka companies) and used directly without further purification. Aryl(or heteroaryl)glyoxal monohydrates (1a‒g) were prepared through the Riley oxidation of related aryl(or heteroaryl) methyl ketones using SeO2 in dioxane:water mixture solvent, in which the amount of water is genuinely trivial compared to dioxane [42b]. Melting points were determined on an Electrothermal 9200 apparatus. Fourier transform infrared (FT-IR) spectra were recorded on a PerkinElmer Spectrum Two FT-IR spectrophotometer, measured as potassium bromide (KBr) disks. 1H and 13C{H} NMR spectra were recorded on a Bruker Avance 300 MHz spectrometer at 300 MHz and 75 MHz, respectively. Chemical shifts were measured in CDCl3 as solvent relative to tetrametylsilane (TMS) as the internal standard. Elemental analyses were performed using a Leco Analyzer 932.
3.2. General procedure for the one-pot three-component synthesis of 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n)
In a round-bottom flask (10 mL) equipped with a magnetic stirrer, a mixture of related aryl(or heteroaryl)glyoxal monohydrate (1 mmol), 1,3-dimethylbarbituric acid (1 mmol), and ZrOCl2•8H2O (2 mol%, 6.4 mg) in the water solvent (5 mL) was prepared and stirred at 50 ˚C for an appropriate time. After the formation of 1,3-dimethyl-5-(2-oxo-2-aryl(or heteroaryl)ethylidene)pyrimidine-2,4,6(1H,3H,5H)-trione intermediate, intended alkylisocyanide (1 mmol) was added to the reaction environment. After compilation of the reaction, the obtained solid product was filtered and washed with hot ethanol (5 mL) to afford the desired pure furo[2,3-d]pyrimidine heterocyclic product.
3.2.1. Physicochemical properties and spectroscopic data of the novel furo[2,3-d]pyrimidines (4a‒n)
3.2.1.1. 5-Benzoyl-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4a)
Yellow solid; mp 191–192 ˚C; FT-IR (KBr) ʋ max 3412, 3351, 3285, 3089, 3060, 3027, 2937, 2867, 1712, 1624, 1601, 1504, 1492, 1457, 1352, 1305, 1185, 1058, 963, 808, 736, 591, 517, 456 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.61 (d, J = 8.3 Hz, 1H, ─NH─), 7.62 (d, J = 6.9 Hz, 2H, Ph─H), 7.51 (t, J = 7.3 Hz, 1H, Ph─H), 7.40 (t, J = 7.5 Hz, 2H, Ph─H), 3.81–3.70 (m, 1H, N─CHcyclohexyl), 3.58 (s, 3H, N─CH3), 3.29 (s, 3H, N─CH3), 2.11–2.00 (m, 2H, ─CH2─), 1.87–1.77 (m, 2H, ─CH2─), 1.71–1.58 (m, 2H, ─CH2─), 1.51–1.41 (m, 4H, 2 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 189.20, 161.09, 156.56, 149.97, 148.90, 140.39, 131.15, 128.46, 127.33, 94.40, 92.55, 51.91, 33.43, 29.64, 28.69, 25.24, 24.34; Anal. Calcd for C21H23N3O4: C, 66.13; H, 6.08; N, 11.02. Found: C, 66.18, H, 6.10; N, 11.12.
3.2.1.2. 5-(4-Bromobenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4b)
Yellow solid; mp 180–181 ˚C; FT-IR (KBr) ʋ max 3518, 3269, 2934, 2859, 1707, 1674, 1650, 1624, 1589, 1573, 1509, 1487, 1455, 1300, 1173, 965, 747, 519, 455 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.65 (d, J = 8.3 Hz, 1H, ─NH─), 7.53 (d, J = 8.3 Hz, 2H, Ph─H), 7.49 (d, J = 8.7 Hz, 2H, Ph─H), 3.79–3.71 (m, 1H, N─CHcyclohexyl), 3.58 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 2.11–1.99 (m, 2H, ─CH2─), 1.89–1.77 (m, 2H, ─CH2─), 1.61–1.27 (m, 6H, 3 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.64, 161.31, 156.64, 149.89, 149.01, 139.10, 130.56, 130.17, 125.77, 94.14, 92.28, 51.96, 33.40, 29.69, 28.70, 25.20, 24.33; Anal. Calcd for C21H22BrN3O4: C, 54.79; H, 4.82; N, 9.13. Found: C, 54.83, H, 4.76; N, 9.18.
3.2.1.3. 5-(4-Chlorobenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4c)
Yellow solid; mp 187–188 ˚C; FT-IR (KBr) ʋ max 3686, 3523, 3277, 2930, 2856, 1714, 1671, 1625, 1542, 1506, 1490, 1454, 1308, 1180, 1090, 964, 845, 747, 513, 459 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.65 (d, J = 8.3 Hz, 1H, ─NH─), 7.57 (d, J = 8.2 Hz, 2H, Ph─H), 7.37 (d, J = 7.8 Hz, 2H, Ph─H), 3.79–3.70 (m, 1H, N─CHcyclohexyl), 3.59 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 2.10–2.01 (m, 2H, ─CH2─), 1.89–1.79 (m, 2H, ─CH2─), 1.57–1.30 (m, 6H, 3 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.59, 161.29, 156.65, 149.89, 149.00, 138.66, 137.18, 129.99, 127.64, 94.17, 92.31, 51.96, 33.41, 29.68, 28.69, 25.20, 24.33; Anal. Calcd for C21H22ClN3O4: C, 60.65; H, 5.33; N, 10.10. Found: C, 60.67, H, 5.30; N, 10.14.
3.2.1.4. 5-(4-Fluorobenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4d)
Yellow solid; mp 174–175 ˚C; FT-IR (KBr) ʋ max 3682, 3510, 3293, 3080, 2938, 2867, 1712, 1671, 1626, 1600, 1547, 1507, 1460, 1222, 1152, 967, 845, 747, 619, 517, 464 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.59 (d, J = 8.3 Hz, 1H, ─NH─), 7.69–7.58 (m, 2H, Ph─H), 7.14–7.00 (m, 2H, Ph─H), 3.77–3.69 (m, 1H, N─CHcyclohexyl), 3.59 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 2.11–1.99 (m, 2H, ─CH2─), 1.87–1.77 (m, 2H, ─CH2─), 1.60–1.28 (m, 6H, 3 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.60, 166.22, 161.19, 156.70, 149.90, 149.01, 136.47, 130.87, 114.56, 114.26, 94.25, 92.28, 51.94, 33.42, 29.68, 28.69, 25.22, 24.33; Anal. Calcd for C21H22FN3O4: C, 63.15; H, 5.55; N, 10.52. Found: C, 63.14, H, 5.51; N, 10.55.
3.2.1.5. 5-(3-Bromobenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4e)
Yellow solid; mp 179–180 ˚C; FT-IR (KBr) ʋ max 3421, 3355 3285, 3166, 3064, 2932, 2856, 1717, 1677, 1625, 1569, 1546, 1505, 1477, 1451, 1307, 1259, 1176, 1149, 1047, 964, 894, 754, 716, 605, 521 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.66 (d, J = 8.3 Hz, 1H, ─NH─), 7.73 (s, 1H, Ph─H), 7.61 (d, J = 7.8 Hz, 1H, Ph─H), 7.53 (d, J = 7.8 Hz, 1H, Ph─H), 7.29 (t, J = 7.5 Hz, 1H, Ph─H), 3.83–3.73 (m, 1H, N─CHcyclohexyl), 3.58 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 2.10–2.02 (m, 2H, ─CH2─), 1.84–1.78 (m, 2H, ─CH2─), 1.72–1.63 (m, 2H, ─CH2─), 1.51–1.42 (m, 4H, 2 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.14, 161.30, 156.57, 149.90, 149.02, 142.13, 133.85, 131.14, 128.88, 127.02, 121.49, 94.07, 92.36, 51.97, 33.37, 29.69, 28.70, 25.20, 24.31; Anal. Calcd for C21H22BrN3O4: C, 54.79; H, 4.82; N, 9.13. Found: C, 54.73, H, 4.80; N, 9.10.
3.2.1.6. 5-(4-Hydroxy-3-methoxybenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4f)
Yellow solid; mp 187–189 ˚C; FT-IR (KBr) ʋ max 3392, 3162, 3072, 2928, 2855, 1712, 1669, 1605, 1509, 1488, 1467, 1429, 1386, 1355, 1307, 1299, 1283, 1181, 907, 829, 778, 755, 654, 603, 517, 504 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.40 (d, J = 8.3 Hz, 1H, ─NH─), 7.28 (s, 1H, Ph─H), 7.21 (d, J = 8.1 Hz, 1H, Ph─H), 6.88 (d, J = 8.2 Hz, 1H, Ph─H), 6.23 (bs, 1H, Ph─OH), 3.89 (s, 3H, Ph─O─CH3), 3.77–3.70 (m, 1H, N─CHcyclohexyl), 3.58 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 2.04–1.99 (m, 2H, ─CH2─), 1.83–1.79 (m, 2H, ─CH2─), 1.48–1.40 (m, 6H, 3 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.74, 162.34, 160.97, 156.70, 149.95, 148.96, 145.73, 132.27, 124.09, 113.07, 111.22, 92.20, 90.41, 58.85, 51.88, 33.44, 29.65, 28.67, 25.24, 24.31; Anal. Calcd for C22H25N3O6: C, 61.82; H, 5.90; N, 9.83. Found: C, 61.85, H, 5.87; N, 9.80.
3.2.1.7. 5-(3,4-Methylendioxybenzoyl)-6-(cyclohexylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4g)
Yellow solid; mp 179–180 ˚C; FT-IR (KBr) ʋ max 3678, 3523, 3273, 3166, 3084, 2934, 2855, 2781, 1709, 1675, 1627, 1505, 1491, 1452, 1350, 1307, 1281, 1258, 1246, 1166, 1094, 1040, 967, 931, 856, 821, 767, 748, 669, 521, 494 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.66 (d, J = 8.3 Hz, 1H, ─NH─), 7.73 (s, 1H, Ph─H), 7.61 (d, J = 7.9 Hz, 1H, Ph─H), 7.29 (d, J = 7.9 Hz, 1H, Ph─H), 6.03 (s, 2H, O─CH2─O), 3.81–3.74 (m, 1H, N─CHcyclohexyl), 3.59 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 2.08–2.02 (m, 2H, ─CH2─), 1.85–1.80 (m, 2H, ─CH2─), 1.51–1.39 (m, 6H, 3 × ─CH2─); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.15, 162.33, 161.30, 156.57, 149.90, 149.01, 142.13, 131.51, 128.89, 127.01, 121.49, 103.00, 94.07, 92.36, 51.98, 33.37, 29.69, 28.70, 25.21, 24.31; Anal. Calcd for C22H23N3O6: C, 62.11; H, 5.45; N, 9.88. Found: C, 62.15, H, 5.49; N, 9.90.
3.2.1.8. 5-Benzoyl-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4h)
Yellow solid; mp 179–180 ˚C; FT-IR (KBr) ʋ max 3428, 3363, 3162, 3060, 2962, 2933, 2872, 1718, 1705, 1676, 1660, 1618, 1601, 1490, 1454, 1287, 1226, 1186, 1107, 967, 902, 878, 784, 765, 744, 735, 611, 525, 512, 470 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.85 (s, 1H, ─NH─), 7.68–7.60 (m, 2H, Ph─H), 7.18–6.95 (m, 3H, Ph─H), 3.61 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 1.53 (s, 9H, 3 × CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.70, 161.38, 156.67, 149.86, 149.26, 136.45, 132.14, 131.00, 129.83, 114.58, 114.29, 93.85, 93.21, 53.58, 30.04, 29.72, 28.69; Anal. Calcd for C19H21N3O4: C, 64.21; H, 5.96; N, 11.82. Found: C, 64.20, H, 5.93; N, 11.85.
3.2.1.9. 5-(4-Bromobenzoyl)-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4i)
Yellow solid; mp 176–178 ˚C; FT-IR (KBr) ʋ max 3539, 3363, 3105, 2978, 2933, 2872, 1715, 1664, 1622, 1586, 1574, 1502, 1485, 1454, 1401, 1370, 1227, 1185, 1012, 960, 933, 908, 857, 774, 755, 743, 669, 588, 532, 513, 483 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.91 (s, 1H, ─NH─), 7.52 (d, J = 8.5 Hz, 2H, Ph─H), 7.48 (d, J = 7.8 Hz, 2H, Ph─H), 3.59 (s, 3H, N─CH3), 3.30 (s, 3H, N─CH3), 1.53 (s, 9H, 3 × CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.73, 161.50, 156.61, 149.86, 149.25, 139.08, 130.59, 130.15, 125.82, 93.74, 93.16, 53.63, 30.01, 29.73, 28.69; Anal. Calcd for C19H20BrN3O4: C, 52.55; H, 4.64; N, 9.68. Found: C, 52.53, H, 4.60; N, 9.65.
3.2.1.10. 5-(4-Chlorobenzoyl)-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4j)
Yellow solid; mp 183–184 ˚C; FT-IR (KBr) ʋ max 3539, 3371, 3105, 3080, 2982, 2941, 2870, 1716, 1662, 1622, 1587, 1577, 1502, 1488, 1455, 1402, 1369, 1227, 1184, 1032, 1010, 960, 935, 910, 862, 755, 743, 632, 619, 560, 509, 473 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.91 (s, 1H, ─NH─), 7.63–7.45 (m, 2H, Ph─H), 7.42–7.24 (m, 2H, Ph─H), 3.60 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 1.53 (s, 9H, 3 × CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.67, 161.49, 156.63, 149.86, 149.26, 138.65, 137.23, 130.59, 129.98, 127.66, 93.77, 93.20, 53.63, 30.02, 29.73, 28.70; Anal. Calcd for C19H20ClN3O4: C, 58.54; H, 5.17; N, 10.78. Found: C, 58.51, H, 5.15; N, 10.75.
3.2.1.11. 5-(4-Fluorobenzoyl)-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4k)
Yellow solid; mp 178–179 ˚C; FT-IR (KBr) ʋ max 3419, 3351, 3072, 2978, 2965, 2943, 2880, 1718, 1704, 1675, 1656, 1618, 1602, 1547, 1503, 1461, 1448, 1225, 1186, 1161, 1083, 1054, 973, 921, 849, 778, 758, 745, 619, 517, 478 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.85 (s, 1H, ─NH─), 7.70–7.59 (m, 2H, Ph─H), 7.13–7.01 (m, 2H, Ph─H), 3.61 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 1.52 (s, 9H, 3 × CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.71, 166.26, 161.38, 156.68, 149.87, 149.26, 136.49, 130.96, 114.60, 114.29, 93.86, 93.22, 53.58, 30.04, 29.73, 28.70; Anal. Calcd for C19H20FN3O4: C, 61.12; H, 5.40; N, 11.25. Found: C, 61.14, H, 5.43; N, 11.24.
3.2.1.12. 5-(3-Bromobenzoyl)-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4l)
Yellow solid; mp 179–180 ˚C; FT-IR (KBr) ʋ max 3413, 3351, 3155, 3072, 2982, 2953, 2884, 1718, 1702, 1676, 1659, 1615, 1572, 1546, 1504, 1477, 1451, 1296, 1224, 1185, 1097, 1035, 1001, 986, 941, 849, 753, 740, 700, 651, 632, 608, 525, 512, 472 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.93 (s, 1H, ─NH─), 7.73 (s, 1H, Ph─H), 7.62 (d, J = 7.9 Hz, 1H, Ph─H), 7.53 (d, J = 7.8 Hz, 1H, Ph─H), 7.27 (t, J = 8.1 Hz, 1H, Ph─H), 3.61 (s, 3H, N─CH3), 3.31 (s, 3H, N─CH3), 1.54 (s, 9H, 3 × CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.23, 161.51, 156.56, 149.87, 149.28, 142.11, 133.90, 131.60, 131.53, 127.01, 121.50, 93.68, 93.22, 53.69, 30.01, 29.74, 28.70; Anal. Calcd for C19H20BrN3O4: C, 52.55; H, 4.64; N, 9.68. Found: C, 52.57, H, 4.63; N, 9.70.
3.2.1.13. 5-(4-Hydroxy-3-methoxybenzoyl)-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4m)
Yellow solid; mp 182–183 ˚C; FT-IR (KBr) ʋ max 3191, 3027, 2986, 2953, 2839, 2789, 1718, 1703, 1661, 1615, 1598, 1492, 1400, 1285, 1249, 1215, 1179, 1124, 1099, 1051, 980, 865, 851, 838, 788, 759, 746, 652, 628, 603, 517, 471 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.62 (s, 1H, ─NH─), 7.29 (s, 1H, Ph─H), 7.22 (d, J = 6.2 Hz, 1H, Ph─H), 6.88 (d, J = 8.1 Hz, 1H, Ph─H), 5.84 (bs, 1H, Ph─OH), 3.90 (s, 3H, Ph─O─CH3), 3.61 (s, 3H, N─CH3), 3.33 (s, 3H, N─CH3), 1.51 (s, 9H, 3 × CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.87, 162.33, 156.69, 149.94, 149.26, 149.01, 145.75, 132.26, 124.16, 113.06, 111.19, 94.25, 93.36, 77.45, 76.92, 76.61, 55.84, 53.46, 30.10, 29.71, 28.67; Anal. Calcd for C20H23N3O6: C, 59.84; H, 5.78; N, 10.47. Found: C, 59.86, H, 5.80; N, 10.48.
3.2.1.14. 5-(3,4-Methylendioxybenzoyl)-6-(tert-butylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-dione (4n)
Yellow solid; mp 177–178 ˚C; FT-IR (KBr) ʋ max 3424, 3359, 3093, 2978, 2958, 2912, 2888,2802, 1720, 1660, 1609, 1547, 1503, 1453, 1288, 1258, 1239, 1185, 1038, 923, 891, 865, 781, 756, 743, 663, 610, 589, 569, 517, 490 cm−1; 1H NMR (300 MHz, CDCl3) δ H 8.62 (s, 1H, ─NH─), 7.20 (d, J = 8.0 Hz, 1H, Ph─H), 7.16 (2, 1H, Ph─H), 6.80 (d, J = 7.9 Hz, 1H, Ph─H), 6.02 (s, 2H, O─CH2─O), 3.60 (s, 3H, N─CH3), 3.32 (s, 3H, N─CH3), 1.30 (s, 9H, 3 × N─CH3 ter t-butyl); 13C{1H} NMR (75 MHz, CDCl3) δ C 187.65, 162.34, 161.12, 156.68, 150.31, 149.92, 149.22, 147.03, 134.43, 124.57, 107.12, 101.42, 94.12, 93.36, 53.49, 30.08, 29.70, 28.70; Anal. Calcd for C20H21N3O6: C, 60.14; H, 5.30; N, 10.52. Found: C, 60.15, H, 5.33; N, 10.54.
3.3. In silico molecular docking and ADMET
The molecular docking simulation was carried out using AutoDock Vina (version 1.1.2) as an open-source program incorporating UCSF Chimera (version 1.15) as a graphical user interface on Apple MacBook Pro (Retina, 13-inch, Mid-2014, is equipped with a 2.8 GHz dual-core Intel core i5 processor, 8 GB 1600 MHz DDR3 memory, Intel Iris 1536 MB graphics, and 500 GB Apple SSD SM0512F storage.). All 3D desired crystal structures were downloaded from the Protein Databank (https://www.rcsb.org). Before the molecular docking process, the protein structures were cleaned from the non-standard residues and then prepared and minimized in UCSF Chimera. The 2D structures of the newly synthesized furo[2,3-d]pyrimidines were generated in ChemBioDraw Ultra (version 14.0.0.117). Conversion of the 2D skeletons to the related 3D structures of the ligands and their energy minimization processes (by the MM2 force field calculation method) was carried out by ChemBio3D Ultra (version 14.0.0.117). Also, structure editing steps (including dock prep and minimize structure) for the 3D ligands were repeated in UCSF Chimera. The 3D structures of medicinal compounds, which are existed in Fig. 8, were downloaded from PubChem (https://pubchem.ncbi.nlm.nih.gov) and ChemSpider (http://www.chemspider.com), and for some cases 2D version was generated from ChemBioDraw Ultra and then converted to the desired 3D format using ChemBio3D Ultra. All the 3D structures of the mentioned medicinal compounds underwent the aforementioned structure editing steps in UCSF Chimera. All the protein–ligand interactions were analyzed in UCSF Chimera and BIOVIA Discovery Studio (version v21.1.0.20298). The 3D figures of the protein–ligand interactions were visualized by UCSF Chimera, and the related 2D projects were drawn by ChemBioDraw Ultra. The in silico ADME and toxicity analyses were investigated using SwissADME (http://www.swissadme.ch) and pkCSM (https://biosig.lab.uq.edu.au/pkcsm/prediction), respectively.
4. Conclusions
Here, we described a green and efficient one-pot three-component regioselective synthetic strategy for the preparation of novel 5-aroyl(or heteroaroyl)-6-(alkylamino)-1,3-dimethylfuro[2,3-d]pyrimidine-2,4(1H,3H)-diones (4a‒n) in good-to-excellent yields, and then demonstrated their satisfactory multi-targeting inhibitory properties against the active site and putative allosteric hotspots of both SARS-CoV-2 MPro and PLPro based on molecular docking studies, especially in comparison with various medicinal compounds which used or investigated to fight against COVID-19 to yet. Besides, the drug-likeness properties of the synthesized heterocyclic frameworks (4a‒n) were predicted using in silico ADMET analyses. Furthermore, our studies in this paper showed that the novel series of furo[2,3-d]pyrimidines (4a‒n), especially 4g as hit one, can be a potential COVID-19 drug candidate. Notably, research to find and develop new and green synthetic strategies for the pharmaceutically interesting heterocyclic frameworks, especially anti-SARS-CoV-2 agents, is currently underway in our research group.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We are thankful to the Research Council of Urmia University and the Research Council of Payame Noor University for the partial support of this work.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioorg.2023.106390.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- 1.(a) Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Teerawattanananon Y., Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) da Silva S.J.R., do Nascimento J.C.F., Mendes R.P.G., Guarines K.M., da Silva C.T.A., da Silva P.G., de Magalhães J.J.F., Vigar J.R.J., Silva-Júnior A., Kohl A., Pardee K., Pena L. Two years into the COVID-19 pandemic: lessons learned. ACS Infect. Dis. 2022;8:1758–1814. doi: 10.1021/acsinfecdis.2c00204. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shang Z., Chan S.Y., Liu W.J., Li P., Huang W. Recent insights into emerging coronavirus: SARS-CoV-2. ACS Infect. Dis. 2021;7:1369–1388. doi: 10.1021/acsinfecdis.0c00646. [DOI] [PubMed] [Google Scholar]; (d) Nalbandian A., Sehgal K., Gupta A., Madhavan M.V., McGroder C., Stevens J.S., Cook J.R., Nordvig A.S., Shalev D., Sehrawat T.S., Ahluwalia N., Bikdeli B., Dietz D., Der-Nigoghossian C., Liyanage-Don N., Rosner G.F., Bernstein E.J., Mohan S., Beckley A.A., Seres D.S., Choueiri T.K., Uriel N., Ausiello J.C., Accili D., Freedberg D.E., Baldwin M., Schwartz A., Brodie D., Garcia C.K., Elkind M.S.V., Connors J.M., Bilezikian J.P., Landry D.W., Wann E.Y. Post-acute COVID-19 syndrome. Nat. Med. 2021;27:601–615. doi: 10.1038/s41591-021-01283-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Brodin P. Immune determinants of COVID-19 disease presentation and severity. Nat. Med. 2021;27:28–33. doi: 10.1038/s41591-020-01202-8. [DOI] [PubMed] [Google Scholar]; (f) Saville J.W., Berezuk A.M., Srivastava S.S., Subramaniam S. Three-Dimensional visualization of viral structure, entry, and replication underlying the spread of SARS-CoV-2. Chem. Rev. 2022;122:14066–14084. doi: 10.1021/acs.chemrev.1c01062. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ahidjo B.A., Loe M.W.C., Ng Y.L., Mok C.K., Chu J.H. Current perspective of antiviral strategies against COVID-19. ACS Infect. Dis. 2020;6:1624–1634. doi: 10.1021/acsinfecdis.0c00236. [DOI] [PubMed] [Google Scholar]; (h) Rashid H.u., Ahmad N., Abdalla M., Khan K., Martines M.A.U., Shabana S. Molecular docking and dynamic simulations of Cefixime, Etoposide and Nebrodenside A against the pathogenic proteins of SARS-CoV-2. J. Mol. Struct. 2022;1247:131296. doi: 10.1016/j.molstruc.2021.131296. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Kabi A.K., Pal M., Gujjarappa R., Malakar C.C., Roy M. Overview of Hydroxychloroquine and Remdesivir on severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) J. Heterocycl. Chem. 2023;60:165–182. doi: 10.1002/jhet.4541. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Ponnampalli S., Birudukota N.V.S., Kamal A. COVID-19: Vaccines and therapeutics. Bioorg. Med. Chem. Lett. 2022;75:128987. doi: 10.1016/j.bmcl.2022.128987. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Jackson C.B., Farzan M., Chen B., Choe H. Mechanisms of SARS-CoV-2 entry into cells. Nat. Rev. Cell Biol. 2022;23:3–20. doi: 10.1038/s41580-021-00418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Malone B., Urakova N., Snijder E.J., Campbell E.A. Structures and functions of coronavirus replication–transcription complexes and their relevance for SARS-CoV-2 drug design. Nat. Rev. Cell Biol. 2022;23:21–30. doi: 10.1038/s41580-021-00432-z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Cannalire R., Cerchia C., Beccari A.R., Di Leva F.S., Summa V. Targeting SARS-CoV-2 proteases and polymerase for COVID-19 treatment: state of the art and future opportunities. J. Med. Chem. 2022;65:2716–2746. doi: 10.1021/acs.jmedchem.0c01140. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Xiu S., Dick A., Ju H., Mirzaie S., Abdi F., Cocklin S., Zhan P., Liu X. Inhibitors of SARS-CoV-2 entry: current and future opportunities. J. Med. Chem. 2020;63:12256–12274. doi: 10.1021/acs.jmedchem.0c00502. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Chakravarti R., Singh R., Ghosh A., Dey D., Sharma P., Velayutham R., Roy S., Ghosh D. A review on potential of natural products in the management of COVID-19. RSC Adv. 2021;11:16711–16735. doi: 10.1039/d1ra00644d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Al-Wahaibi L.H., Mostafa A., Mostafa Y.A., Abou-Ghadir O.F., Abdelazeem A.H., Gouda A.M., Kutkat O., Shama N.M.A., Shehata M., Gomaa H.A.M., Abdelrahman M.H., Mohamed F.A.M., Gu X., Ali M.A., Trembleau L., Youssif B.G.M. Discovery of novel oxazole-based macrocycles as anti-coronaviral agents targeting SARS-CoV-2 main protease. Bioorg. Chem. 2021;116:105363. doi: 10.1016/j.bioorg.2021.105363. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Liu M., Wang J., Wan X., Li B., Guan M., Ning X., Hu X., Li S., Liu S., Song G. Discovery and structural optimization of 3-O-β-Chacotriosyl betulonic acid saponins as potent fusion inhibitors of Omicron virus infections. Bioorg. Chem. 2023;131:106316. doi: 10.1016/j.bioorg.2022.106316. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Hu W., Zhang X., Liu Y., Liu T., Wen J., Peng X., Xie X., Chen W. Two-stage one-pot synthetic strategy for the key triazone-triazole intermediate of ensitrelvir (S217622), an oral clinical candidate for treating COVID-19. RSC Adv. 2022;12:34808–34814. doi: 10.1039/d2ra06841a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Adel A., Elnaggar M.S., Albohy A., Elrashedy A.A., Mostafa A., Kutkat O., Abdelmohsen U.R., Al-Sayed E., Rabeh M.A. Evaluation of antiviral activity of Carica papaya leaves against SARS-CoV-2 assisted by metabolomic profiling. RSC Adv. 2022;12:32844–32852. doi: 10.1039/d2ra04600h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (t) Santos B.S.A.S., Cunha J.L.R., Carvalho I.C., Costa J.M.C., Longo B.C., Galinari G.C.F., Diniz P.H.S.M., Mendes G.M.M., Fonseca F.G., Abrahão J.S., Mansur A.A.P., Leite M.F., Oréfice R.L., Lobato Z.I.P., Mansur H.S. Nanotechnology meets immunology towards a rapid diagnosis solution: the COVID-19 outbreak challenge. RSC Adv. 2022;12:31711–31728. doi: 10.1039/d2ra05096j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (u) Kerkour R., Chafai N., Moumeni O., Chafa S. Novel α-aminophosphonate derivates synthesis, theoretical calculation, molecular docking, and in silico prediction of potential inhibition of SARS-CoV-2. J. Mol. Struct. 2023;1272:134196. doi: 10.1016/j.molstruc.2022.134196. [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Malik P., Jain S., Jain P., Kumawat J., Dwivedi J., Kishore D. A comprehensive update on the structure and synthesis of potential drug targets for combating the coronavirus pandemic caused by SARS-CoV-2. Arch. Pharm. 2022;355:2100382. doi: 10.1002/ardp.202100382. [DOI] [PMC free article] [PubMed] [Google Scholar]; (w) Souza P.F.N., Mesquita F.P., Amaral J.L., Landim P.G.C., Lima K.R.P., Costa M.B., Farias I.R., Belém M.O., Pinto Y.O., Moreira H.H.T., Magalhaes I.C.L., Castelo-Branco D.S.C.M., Montenegro R.C., de Andrade C.R. The spike glycoprotein of SARS-CoV-2: a review of how mutations of spike glycoproteins have driven the emergence of variants with high transmissibility and immune escape. Int. J. Bio. Macromol. 2022;208:105–125. doi: 10.1016/j.ijbiomac.2022.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; (x) Sacco M.D., Ma C., Lagarias P., Gao A., Townsend J.A., Meng X., Dube P., Zhang X., Hu Y., Kitamura N., Hurst B., Tarbet B., Marty M.T., Kolocouris A., Xiang Y., Chen Y., Wang J. Structure and inhibition of the SARS-CoV-2 main protease reveal strategy for developing dual inhibitors against Mpro and cathepsin L. Sci. Adv. 2020;6:eabe0751. doi: 10.1126/sciadv.abe0751. [DOI] [PMC free article] [PubMed] [Google Scholar]; (y) Ma C., Sacco M.D., Hurst B., Townsend J.A., Hu Y., Szeto T., Zhang X., Tarbet B., Marty M.T., Chen Y., Wang J. Boceprevir, GC-376, and calpain inhibitors II, XII inhibit SARS-CoV-2 viral replication by targeting the viral main protease. Cell Res. 2020;30:678–692. doi: 10.1038/s41422-020-0356-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) La Monica G., Bono A., Lauria A., Martorana A. Targeting SARS-CoV-2 main protease for treatment of COVID-19: covalent inhibitors structure–activity relationship insights and evolution perspectives. J. Med. Chem. 2022;65:12500–12534. doi: 10.1021/acs.jmedchem.2c01005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Huff S., Kummetha I.R., Tiwari S.K., Huant M.B., Clark A.E., Wang S., Bray W., Smith D., Carlin A.F., Endsley M., Rana T.M. Discovery and mechanism of SARS-CoV-2 main protease inhibitors. J. Med. Chem. 2022;65:2866–2879. doi: 10.1021/acs.jmedchem.1c00566. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Gao K., Wang R., Chen J., Tepe J.J., Huang F., Wei G.-W. Perspectives on SARS-CoV-2 main protease inhibitors. J. Med. Chem. 2021;64:16922–16955. doi: 10.1021/acs.jmedchem.1c00409. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hu Q., Xiong Y., Zhu G.-H., Zhang Y.-N., Zhang Y.-W., Huang P., Ge G.-B. The SARS-CoV-2 main protease (MPro): structure, function, and emerging therapies for COVID-19. MedComm. 2022;3:e151. doi: 10.1002/mco2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Antonopoulou I., Sapountazki E., Rova U., Christakopoulos P. Inhibition of the main protease of SARS-CoV-2 (MPro) by repurposing/designing drug-like substances and utilizing nature’s toolbox of bioactive compounds. Compout. Struct. Biotech. J. 2022;20:1306–1344. doi: 10.1016/j.csbj.2022.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Chen R., Gao Y., Liu H., Li H., Chen W., Ma J. Advances in research on 3C-like protease (3CLPro) inhibitors against SARS-CoV-2 since 2020. RSC Med. Chem. 2023;14:9–21. doi: 10.1039/d2md00344a. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Jin Z., Du X., Xu Y., Deng Y., Liu M., Zhao Y., Zhang B., Li X., Zhang L., Peng C., Duan Y., Yu J., Wang L., Yang K., Liu F., Jiang R., Yang X., You T., Liu X., Yang X., Bai F., Liu X., Guddat L.W., Xu W., Xiao G., Qin C., Shi Z., Jiang H., Rao Z., Yang H. Structure of MPro from SARS-CoV-2 and discovery of its inhibitors. Nature. 2020;582:289–293. doi: 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]; (h) Malebari A.M., Ahmed H.E.A., Ihmaid S.K., Omar A.M., Muhammad Y.A., Althagfan S.S., Aljuhani N., El-Sayed A.-A.-A.-A., Halawa A.H., El-Tahir H.M., Turkistani S.A., Almaghrabi M., Aljohani A.K.B., El-Agrody A.M., Abulkhair H.A. Exploring the dual effect of novel 1,4-diarylpyranopyrazoles as antiviral and anti-inflammatory for the management of SARS-CoV-2 and associated inflammatory symptoms. Bioorg. Chem. 2023;130:106255. doi: 10.1016/j.bioorg.2022.106255. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wang R., Zhai G., Zhu G., Wang M., Gong X., Zhang W., Ge G., Chen H., Chen L. Discovery and mechanism of action of Thonzonium bromide from an FDA-approved drug library with potent and broad-spectrum inhibitory activity against main proteases of human coronaviruses. Bioorg. Chem. 2023;130:106264. doi: 10.1016/j.bioorg.2022.106264. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Xu Y.-S., Chigan J.-Z., Li J.-Q., Ding H.-H., Sun L.-Y., Liu L., Hu Z., Yang K.-W. Hydroxamate and thiosemicarbazone: two highly promising scaffolds for the development of SARS-CoV-2 antivirals. Bioorg. Chem. 2022;124:105799. doi: 10.1016/j.bioorg.2022.105799. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Aljuhani A., Ahmed H.E.A., Ihmaid S.K., Omar A.M., Althagfan S.S., Alahmadi Y.M., Ahmad I., Patel H., Ahmed S., Almikhlafi M.A., El-Agrody A.M., Zayed M.F., Turkistani S.A., Abulkhair S.H., Almaghrabi M., Salama S.A., Al-Karmalawy A.A., Abulkhair H.S. In vitro and computational investigations of novel synthetic carboxamide-linked pyridopyrrolopyrimidines with potent activity as SARS-CoV-2-MPro inhibitors. RSC Adv. 2022;12:26895–26907. doi: 10.1039/d2ra04015h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Redjemia R., Berredjem M., Dekir A., Ibrahim-Ouali M., Aissaoui M., Bouacida S., Bouzina A., Bahadi R. A convenient synthesis, in silico study and crystal structure of novel sulfamidophosphonates: interaction with SARS-CoV-2. J. Mol. Struct. 2023;1275:134602. [Google Scholar]; (m) Gao S., Sylvester K., Song L., Claff T., Jing L., Woodson M., Weiße R.H., Cheng Y., Schäkel L., Petry M., Gütschow M., Schiedel A.C., Sträter N., Kang D., Xu S., Toth K., Tavis J., Tollefson A.E., Müller C.E., Liu X., Zhan P. Discovery and crystallographic studies of trisubstituted piperazine derivatives as non-covalent SARS-CoV-2 main protease inhibitors with high target specificity and low toxicity. J. Med. Chem. 2022;65:13343–13364. doi: 10.1021/acs.jmedchem.2c01146. [DOI] [PubMed] [Google Scholar]; (n) Xia Z., Sacco M., Hu Y., Ma C., Meng X., Zhang F., Szeto T., Xiang Y., Chen Y., Wang J. Rational design of hybrid SARS-CoV-2 main protease inhibitors guided by the superimposed cocrystal structures with the peptidomimetic inhibitors GC-376, Telaprevir, and Boceprevir. ACS Pharmacol. Transl. Sci. 2021;4:1408–1421. doi: 10.1021/acsptsci.1c00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.(a) Tan H., Hu Y., Jadhav P., Tan B., Wang J. Progress and challenges in targeting the SARS-CoV-2 papain-like protease. J. Med. Chem. 2022;65:7561–7580. doi: 10.1021/acs.jmedchem.2c00303. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Valipour M. Chalcone-amide, a privileged backbone for the design and development of selective SARS-CoV/SARS-CoV-2 papain-like protease inhibitors. Eur. J. Med. Chem. 2022;240:114572. doi: 10.1016/j.ejmech.2022.114572. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Ullrich S., Nitsche C. SARS-CoV-2 papain-like protease: structure, function and inhibition. ChemBioChem. 2022;23:e202200327. doi: 10.1002/cbic.202200327. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Elseginy S.A., Anwar M.M. In silico analysis of SARS-CoV-2 papain-like protease potential inhibitors. RSC Adv. 2021;11:38616–38631. doi: 10.1039/d1ra07845c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Ton A.-T., Pandey M., Smith J.R., Ban F., Fernandez M., Cherkasov A. Targeting SARS-CoV-2 papain-like protease in the postvaccine era. Trends Pharmacol. Sci. 2022;43:906–919. doi: 10.1016/j.tips.2022.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Perlinska A.P., Stasiulewicz A., Nguyen M.L., Swiderska K., Zmudzinski M., Maksymiuk A.W., Drag M., Sulkowska J.I. Amino acid variants of SARS-CoV-2 papain-like protease have impact on drug binding. Plos Comput. Bio. 2022;18:e1010667. doi: 10.1371/journal.pcbi.1010667. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Sencanski M., Perovic V., Milicevic J., Todorovic T., Prodanovic R., Veljkovic V., Paessler S., Glisic S. Identification of SARS-CoV-2 papain-like protease (PLpro) inhibitors using combined computational approach. ChemistryOpen. 2022;11:e202100248. doi: 10.1002/open.202100248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.(a) Gao K., Wang R., Chen J., Cheng L., Frishcosy J., Huzumi Y., Qiu Y., Schluckbier T., Wei X., Wei G.-W. Methodology-centered review of molecular modeling, simulation, and prediction of SARS-CoV-2. Chem. Rev. 2022;122:11287–11368. doi: 10.1021/acs.chemrev.1c00965. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) De Paris K., Permar S.R. Routine SARS-CoV-2 vaccination for all children. Immun. Rev. 2022;309:90–96. doi: 10.1111/imr.13108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Sabe V.T., Ntombela T., Jhamba L.A., Maguire G.E.M., Govender T., Naicker T., Kruger H.G. Current trends in computer aided drug design and a highlight of drugs discovered via computational techniques: a review. Eur. J. Med. Chem. 2021;224:113705. doi: 10.1016/j.ejmech.2021.113705. [DOI] [PubMed] [Google Scholar]; (b) Cox P.B., Gupta R. Contemporary computational applications and tools in drug discovery. ACS Med. Chem. Lett. 2022;13:1016–1029. doi: 10.1021/acsmedchemlett.1c00662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.(a) Muratov E.N., Amaro R., Andrade C.H., Brown N., Ekins S., Fourches D., Isayev O., Kozakov D., Medina-Franco J.L., Merz K.M., Oprea T.I., Poroikov V., Schneider G., Todd M.H., Varnek A., Winkler D.A., Zakharov A.V., Cherkasov A., Tropsha A. A critical overview of computational approaches employed for COVID-19 drug discovery. Chem. Soc. Rev. 2021;50:9121–9151. doi: 10.1039/d0cs01065k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Liu Y., Gan J., Wang R., Yang X., Xiao Z., Cao Y. DrugDevCovid19: an atlas of anti-COVID-19 compounds derived by computer-aided drug design. Molecules. 2022;27:683. doi: 10.3390/molecules27030683. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Maghsoudi S., Taghavi Shahraki B., Rameh F., Nazarabi M., Fatahi Y., Akhavan O., Rabiee M., Mostafavi E., Lima E.C., Saeb M.R., Rabiee N. A review on computer-aided chemogenomics and drug repositioning for rational COVID-19 drug discovery. Chem. Biol. Drug Des. 2022;100:699–721. doi: 10.1111/cbdd.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Sharma P.P., Bansal M., Sethi A., Poonam, Pena L., Goel V.K., Grishina M., Chaturvedi S., Kumar D., Rathi B. Computational methods directed towards drug repurposing for COVID-19: advantages and limitations. RSC Adv. 2021;11:36181–36198. doi: 10.1039/d1ra05320e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Tam N.M., Pham M.Q., Ha N.X., Nam P.C., Phung H.T.T. Computational estimation of potential inhibitors from known drugs against the main protease of SARS-CoV-2. RSC Adv. 2021;11:17478–17486. doi: 10.1039/d1ra02529e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Ali F., Alom S., Shakya A., Ghosh S.K., Singh U.P., Bhat H.R. Implication of in silico studies in the search for novel inhibitors against SARS-CoV-2. Arch. Pharm. 2022;355:2100360. doi: 10.1002/ardp.202100360. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Ghahremanpour M.M., Tirado-Rives J., Deshmukh M., Ippolito J.A., Zhang C.-H., de Vaca I.C., Liosi M.-E., Anderson K.S., Jorgensen W.L. Identification of 14 known drugs as inhibitors of the main protease of SARS-CoV-2. ACS Med. Chem. Lett. 2020;11:2526–2533. doi: 10.1021/acsmedchemlett.0c00521. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Mahmoudi S., Mohammadpour Dehkordi M., Asgarshamsi M.H. The effect of various compounds on the COVID mechanisms, from chemical to molecular aspects. Biophys. Chem. 2022;288:106824. doi: 10.1016/j.bpc.2022.106824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.(a) Mousavi H. A comprehensive survey upon diverse and prolific applications of chitosan-based catalytic systems in one-pot multi-component synthesis of heterocyclic rings. Int. J. Bio. Macromol. 2021;186:1003–1166. doi: 10.1016/j.ijbiomac.2021.06.123. [DOI] [PubMed] [Google Scholar]; (b) Callis T.B., Garrett T.R., Montgomery A.P., Danon J.J., Kassiou M. Recent scaffold hopping applications in central nervous system drug discovery. J. Med. Chem. 2022;65:13483–13504. doi: 10.1021/acs.jmedchem.2c00969. [DOI] [PubMed] [Google Scholar]; (c) Hou W., Xu H. Incorporating selenium into heterocycles and natural products─from chemical properties to pharmacological activities. J. Med. Chem. 2022;65:4436–4456. doi: 10.1021/acs.jmedchem.1c01859. [DOI] [PubMed] [Google Scholar]; (d) de Esch I.J.P., Erlanson D.A., Jahnke W., Johnson C.N., Walsh L. Fragment-to-lead medicinal chemistry publications in 2020. J. Med. Chem. 2022;65:84–99. doi: 10.1021/acs.jmedchem.1c01803. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mancini R.S., Barden C.J., Weaver D.F., Reed M.A. Furazans in medicinal chemistry. J. Med. Chem. 2021;64:1786–1815. doi: 10.1021/acs.jmedchem.0c01901. [DOI] [PubMed] [Google Scholar]; (f) Mermer A., Keles T., Sirin Y. Recent studies of nitrogen containing heterocyclic compounds as novel antiviral agents: a review. Bioorg. Chem. 2021;114:105076. doi: 10.1016/j.bioorg.2021.105076. [DOI] [PubMed] [Google Scholar]; (g) Wu, Y.-J. Heterocycles and medicine: a survey of the heterocyclic drugs approved by the U.S. FDA from to present. Prog. Heterocycl. Chem. 2012;24:1–53. [Google Scholar]; (h) dos Santos G.C., Martins L.M., Bregadiolli B.A., Moreno V.F., da Silva-Filho L.C., da Silva B.H.S.T. Heterocyclic compounds as antiviral drugs: synthesis, structure–activity relationship and traditional applications. J. Heterocycl. Chem. 2021;58:2226–2260. [Google Scholar]; (i) Alam M.M. 1,2,3-Triazole hybrids as anticancer agents: a review. Arch. Pharm. 2022;355:2100158. doi: 10.1002/ardp.202100158. [DOI] [PubMed] [Google Scholar]; (j) Dorababu A. Pharmacological report of recently designed multifunctional coumarin and coumarin–heterocycle derivatives. Arch. Pharm. 2022;355:2100345. doi: 10.1002/ardp.202100345. [DOI] [PubMed] [Google Scholar]; (k) Gharat R., Prabhu A., Khambete M.P. Potential of triazines in Alzheimer’s disease: a versatile privileged scaffold. Arch. Pharm. 2022;355:2100388. doi: 10.1002/ardp.202100388. [DOI] [PubMed] [Google Scholar]; (l) Feng L.-S., Su W.-Q., Cheng J.-B., Xiao T., Li H.-Z., Chen D.-A., Zhang Z.-L. Benzimidazole hybrids as anticancer drugs: an updated review on anticancer properties, structure–activity relationship, and mechanisms of action (2019–2021) Arch. Pharm. 2022;355:2200051. doi: 10.1002/ardp.202200051. [DOI] [PubMed] [Google Scholar]; (m) Hendawy O.M. A comprehensive review of recent advances in the biological activities of 1,2,4-oxadiazoles. Arch. Pharm. 2022;355:2200045. doi: 10.1002/ardp.202200045. [DOI] [PubMed] [Google Scholar]; (n) Hassan M.S.A., Ahmed E.M., El-Malah A.A., Kassab A.E. Anti-inflammatory activity of pyridazinones: a review. Arch. Pharm. 2022;355:2200067. doi: 10.1002/ardp.202200067. [DOI] [PubMed] [Google Scholar]; (o) Desai N., Monapara J., Jethawa A., Khedkar A., Shingate B. Oxadiazole: a highly versatile scaffold in drug discovery. Arch. Pharm. 2022;355:2200123. doi: 10.1002/ardp.202200123. [DOI] [PubMed] [Google Scholar]; (p) Yahya S., Haider K., Pathak A., Choudhary A., Hooda P., Shafeeq M., Yar M.S. Strategies in synthetic design and structure–activity relationship studies of novel heterocyclic scaffolds as aldose reductase-2 inhibitors. Arch. Pharm. 2022;355:2200167. doi: 10.1002/ardp.202200167. [DOI] [PubMed] [Google Scholar]; (q) Hong Y., Zhu Y.-Y., He Q., Gu S.-X. Indole derivatives as tubulin polymerization inhibitors for the development of promising anticancer agents. Bioorg. Med. Chem. 2022;55:116597. doi: 10.1016/j.bmc.2021.116597. [DOI] [PubMed] [Google Scholar]; (r) Ourvy G. Recent applications of seven-membered rings in drug design. Bioorg. Med. Chem. 2022;57:116650. doi: 10.1016/j.bmc.2022.116650. [DOI] [PubMed] [Google Scholar]; (s) Sharma V., Das R., Mehta D.K., Gupta S., Venugopala K.N., Mailavaram R., Nair A.B., Shakya A.K., Deb P.K. Recent insight into the biological activities and SAR of quinolone derivatives as multifunctional scaffold. Bioorg. Med. Chem. 2022;59:116674. doi: 10.1016/j.bmc.2022.116674. [DOI] [PubMed] [Google Scholar]; (t) Das B.C., Shareef M.A., Das S., Nandwana N.K., Das Y., Saito M., Weiss L.M. Boron-containing heterocycles as promising pharmacological agents. Bioorg. Med. Chem. 2022;63:116748. doi: 10.1016/j.bmc.2022.116748. [DOI] [PubMed] [Google Scholar]; (u) De S., Aamna B., Sahu R., Perida S., Behera S.K., Dan A.K. Seeking heterocyclic scaffolds as antivirals against dengue virus. Eur. J. Med. Chem. 2022;240:114576. doi: 10.1016/j.ejmech.2022.114576. [DOI] [PMC free article] [PubMed] [Google Scholar]; (v) Finger V., Kufa M., Soukup O., Castagnolo D., Roh J., Korabecny J. Pyrimidine derivatives with antitubercular activity. Eur. J. Med. Chem. 2023;246:114946. doi: 10.1016/j.ejmech.2022.114946. [DOI] [PubMed] [Google Scholar]; (w) Bhat A.A., Singh I., Tandon N., Tandon R. Structure activity relationship (SAR) and anticancer activity of pyrrolidine derivatives: recent developments and future prospects (a review) Eur. J. Med. Chem. 2023;246:114954. doi: 10.1016/j.ejmech.2022.114954. [DOI] [PubMed] [Google Scholar]; (x) Chaurasiya A., Sahu C., Wahan S.K., Chawla P.A. Targeting cancer through recently developed purine clubbed heterocyclic scaffolds: an overview. J. Mol. Struct. 2023;1280:134967. [Google Scholar]; (y) Wu P., Zhao J., Shen X., Liang X., He C., Yin L., Xu F., Li H., Tang H. Research progress on the structure and biological diversities of 2-phenylindole derivatives in recent 20 years. Bioorg. Chem. 2023;132:106342. doi: 10.1016/j.bioorg.2023.106342. [DOI] [PubMed] [Google Scholar]
- 8.(a) Martin-Kohler A., Widmer J., Bold G., Meyer T., Séquin U., Traxler P. Furo[2,3-d]pyrimidines and oxazolo[5,4-d]pyrimidines as inhibitors of receptor tyrosine kinases (RTK) Helv. Chim. Acta. 2004;87:956–975. [Google Scholar]; (b) Peng Y.-H., Shiao H.-Y., Tu C.-H., Liu P.-M., Hsu J.-T.-A., Amancha P.K., Wu J.-S., Coumar M.S., Chen C.-H., Wang S.-Y., Lin W.-H., Sun H.-Y., Chao Y.-S., Lyu P.-C., Hsieh H.-P., Wu S.-Y. Protein kinase inhibitor design by targeting the Asp-Phe-Gly (DFG) motif: the role of the DFG motif in the design of epidermal growth factor receptor inhibitors. J. Med. Chem. 2013;56:3889–3903. doi: 10.1021/jm400072p. [DOI] [PubMed] [Google Scholar]; (c) Lin S.Y., Chang Hsu Y., Peng Y.H., Ke Y.Y., Lin W.H., Sun H.Y., Shiao H.Y., Kuo F.M., Chen P.Y., Lien T.W., Chen C.H., Chu C.Y., Wang S.Y., Yeh K.C., Chen C.P., Hsu T.A., Wu S.Y., Yeh T.K., Chen C.T., Hsieh H.P. Discovery of a furanopyrimidine-based epidermal growth factor receptor inhibitor (DBPR112) as a clinical candidate for the treatment of non-small cell lung cancer. J. Med. Chem. 2019;62:10108–10123. doi: 10.1021/acs.jmedchem.9b00722. [DOI] [PubMed] [Google Scholar]
- 9.Jiao X.Y., Kopecky D.J., Liu J.S., Liu J.Q., Jean J.C., Cardozo M.G., Sharma R., Walker N., Wesche H., Li S., Farrelly E., Xiao S.-H., Wang Z., Kayser F. Synthesis and optimization of substituted furo[2,3-d]-pyrimidin-4-amines and 7H-pyrrolo[2,3-d]pyrimidin-4-amines as ACK1 inhibitors. Bioorg. Med. Chem. Lett. 2012;22:6212–6217. doi: 10.1016/j.bmcl.2012.08.020. [DOI] [PubMed] [Google Scholar]
- 10.DiMauro E.F., Newcomb J., Nunes J.J., Bemis J.E., Boucher C., Buchanan J.L., Buckner W.H., Cheng A., Faust T., Hsieh F., Huang X., Lee J.H., Marshall T.L., Martin M.W., McGowan D.C., Schneider S., Turci S.M., White R.D., Zhu X. Discovery of 4-amino-5,6-biaryl-furo[2,3-d]pyrimidines as inhibitors of Lck: development of an expedient and divergent synthetic route and preliminary SAR. Bioorg. Med. Chem. Lett. 2007;17:2305–2309. doi: 10.1016/j.bmcl.2007.01.057. [DOI] [PubMed] [Google Scholar]
- 11.Maeda Y., Nakano M., Sato H., Miyazaki Y., Schweiker S.L., Smith J.L., Truesdale A.T. 4-Acylamino-6-arylfuro[2,3-d]pyrimidines: potent and selective glycogen synthase kinase-3 inhibitors. Bioorg. Med. Chem. Lett. 2004;14:3907–3911. doi: 10.1016/j.bmcl.2004.05.064. [DOI] [PubMed] [Google Scholar]
- 12.Harris P.A., Bandyopadhyay D., Berger S.B., Campobasso N., Capriotti C.A., Cox J.A., Dare L., Finger J.N., Hoffman S.J., Kahler K.M., Lehr R., Lich J.D., Nagilla R., Nolte R.T., Ouellette M.T., Pao C.S., Schaeffer M.C., Smallwood A., Sun H.H., Swift B.A., Totoritis R.D., Ward P., Marquis R.W., Bertin J., Gough P.J. Discovery of small molecule RIP1 kinase inhibitors for the treatment of pathologies associated with necroptosis. ACS Med. Chem. Lett. 2013;4:1238–1243. doi: 10.1021/ml400382p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shiao H.-Y., Coumar M.S., Chang C.-W., Ke Y.-Y., Chi Y.-H., Chu C.-Y., Sun H.-S., Chen C.-H., Lin W.-H., Fung K.-S., Kuo P.-C., Huang C.-T., Chang K.-Y., Lu C.-T., Hsu J.T.A., Chen C.-T., Jiaang W.-T., Chao Y.-S., Hsieh H.-P. Optimization of ligand and lipophilic efficiency to identify an in vivo active furano-pyrimidine aurora kinase inhibitor. J. Med. Chem. 2013;56:5247–5260. doi: 10.1021/jm4006059. [DOI] [PubMed] [Google Scholar]
- 14.Miyazaki Y., Matsunaga S., Tang J., Maeda Y., Nakano M., Philippe R.J., Shibahara M., Liu W., Sato H., Wang L., Nolte R.T. Novel 4-amino-furo[2,3-d]pyrimidines as Tie-2 and VEGFR2 dual inhibitors. Bioorg. Med. Chem. Lett. 2005;15:2203–2207. doi: 10.1016/j.bmcl.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Gangjee A., Jain H.D., Phan J., Guo X., Queener S.F., Kisliuk R.L. 2,4-Diamino-5- methyl-6-substituted arylthio-furo[2,3-d]pyrimidines as novel classical and nonclassical antifolates as potential dual thymidylate synthase and dihydrofolate reductase inhibitors. Bioorg. Med. Chem. 2010;18:953–961. doi: 10.1016/j.bmc.2009.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gangjee A., Zeng Y., McGuire J.J., Kisliuk R.L. Synthesis of classical, four-carbon bridged 5-substituted furo[2,3-d]pyrimidine and 6-substituted pyrrolo[2,3-d]pyrimidine analogues as antifolates. J. Med. Chem. 2005;48:5329–5336. doi: 10.1021/jm058213s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mansour M.A., Oraby M.A., Muhammad Z.A., Lasheen D.S., Gaber H.M., Abouzid K.A.M. Identification of novel furo[2,3-d]pyrimidine based chalcones as potent anti-breast cancer agents: synthesis, in vitro and in vivo biological evaluation. RSC Adv. 2022;12:8193–8201. doi: 10.1039/d2ra00889k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Aziz M.A., Serya R.A.T., Lasheen D.S., Abouzid K.A.M. Furo[2,3-d]pyrimidine based derivatives as kinase inhibitors and anticancer agents. Future J. Pharm. Sci. 2016;2:1–8. [Google Scholar]; (b) Mansouri A.-E.-E., Oubella A., Dânoun K., Ahmad M., Neyts J., Jochmans D., Snoeck R., Andrei G., Morjani H., Zahouily M., Lazrek H.B. Discovery of novel furo[2,3-d]pyrimidin-2-one–1,3,4-oxadiazole hybrid derivatives as dual antiviral and anticancer agents that induce apoptosis. Arch. Pharm. 2021;354:2100146. doi: 10.1002/ardp.202100146. [DOI] [PubMed] [Google Scholar]; (c) Kumar R.N., Poornachandra Y., Nagender P., Mallareddy G., Kumar N.R., Ranjithreddy P., Kumar C.G., Narsaiah B. Synthesis of novel trifluoromethyl substituted furo[2,3-b]pyridine and pyrido[3ʹ,2ʹ:4,5]furo[3,2-d]pyrimidine derivatives as potential anticancer agents. Eur. J. Med. Chem. 2016;108:68–78. doi: 10.1016/j.ejmech.2015.11.007. [DOI] [PubMed] [Google Scholar]; (d) Hossam M., Lasheen D.S., Ismail N.S.M., Esmat A., Mansour A.M., Singab A.N.B., Abouzid K.A.M. Discovery of anilino-furo[2,3-d]pyrimidine derivatives as dual inhibitors of EGFR/HER2 tyrosine kinase and their anticancer activity. Eur. J. Med. Chem. 2018;144:330–348. doi: 10.1016/j.ejmech.2017.12.022. [DOI] [PubMed] [Google Scholar]; (e) Zhang X., Raghavan S., Ihnat M., Hamel E., Zammiello C., Bastian A., Mooberry S.L., Gangjee A. The design, synthesis and biological evaluation of conformationally restricted 4-substituted-2,6-dimethylfuro[2,3-d]pyrimidines as multi-targeted receptor tyrosine kinase and microtubule inhibitors as potential antitumor agents. Bioorg. Med. Chem. 2015;23:2408–2423. doi: 10.1016/j.bmc.2015.03.061. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Aziz M.A., Serya R.H.T., Lasheen D.S., Abdel-Aziz A.K., Esmat A., Mansour A.M., Singab A.N.B., Abouzid K.A.M. Discovery of potent VEGFR-2 inhibitors based on furopyrimidine and thienopyrimidne scaffolds as cancer targeting agents. Sci. Rep. 2016;6:24460. doi: 10.1038/srep24460. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Sheikhhosseini E. Design and effective synthesis of novel furo[2,3-d]pyrimidine derivatives containing ethylene ether spacers. J. Saudi Chem. Soc. 2018;22:337–342. [Google Scholar]; (h) Zonouzi A., Habibi Rezaei M., Mirzazadeh R., Rezaei Arjomand M. Solvent-free synthesis of halogenated furo[2,3-d]pyrimidines and their cytotoxic activity on the T47D breast cancer cell line. Org. Prep. Proced. Int. 2020;52:374–380. [Google Scholar]
- 19.(a) Zimmerman J.B., Anastas P.T., Erythropel H.C., Leitner W. Designing for a green chemistry future. Science. 2020;367:397–400. doi: 10.1126/science.aay3060. [DOI] [PubMed] [Google Scholar]; (b) Erythropel H.C., Zimmerman J.B., de Winter T.M., Petitjean L., Melnikov F., Lam C.H., Lounsbury A.W., Mellor K.E., Janković N.Z., Tu Q., Pincus L.N., Falinski M.M., Shi W., Coish P., Plata D.L., Anastas P.T. The Green ChemisTREE: 20 years after taking root with the 12 principles. Green Chem. 2018;20:1929–1961. [Google Scholar]; (c) Keijer T., Bakker V., Slootweg J.C. Circular chemistry to enable a circular economy. Nat. Chem. 2019;11:190–195. doi: 10.1038/s41557-019-0226-9. [DOI] [PubMed] [Google Scholar]; (d) Anastas P., Eghbali N. Green Chemistry: principles and practice. Chem. Soc. Rev. 2010;39:301–312. doi: 10.1039/b918763b. [DOI] [PubMed] [Google Scholar]; (e) Hasanpour Galehban M., Zeynizadeh B., Mousavi H. Diverse and efficient catalytic applications of new cockscomb flower-like Fe3O4@SiO2@KCC-1@MPTMS@CuII mesoporous nanocomposite in the environmentally benign reduction and reductive acetylation of nitroarenes and one-pot synthesis of some coumarin compounds. RSC Adv. 2022;12:11164–11189. doi: 10.1039/d1ra08763k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Zeynizadeh B., Sepehraddin F., Mousavi H. Green and highly efficient strategies for the straightforward reduction of carboxylic acids to alcohols using four different and affordable types of hydrogen donors. Ind. Eng. Chem. Res. 2019;58:16379–16388. [Google Scholar]; (g) Mousavi H., Zeynizadeh B., Younesi R., Esmati M. Simple and practical synthesis of various new nickel boride-based nanocomposites and their applications for the green and expeditious reduction of nitroarenes to arylamines under wet-solvent-free mechanochemical grinding. Aust. J. Chem. 2018;71:595–609. [Google Scholar]
- 20.(a) Kar S., Sanderson H., Roy K., Benfenati E., Leszczynski J. Green chemistry in the synthesis of pharmaceuticals. Chem. Rev. 2022;122:3637–3710. doi: 10.1021/acs.chemrev.1c00631. [DOI] [PubMed] [Google Scholar]; (b) Becker J., Manske C., Randl S. Green chemistry and sustainability metrics in the pharmaceutical manufacturing sector. Curr. Opin. Green Sustainable Chem. 2022;33:100562. [Google Scholar]; (c) Jimenez-Gonzalez C., Lund C. Green metrics in pharmaceutical development. Curr. Opin. Green Sustainable Chem. 2022;33:100564. [Google Scholar]; (d) Koenig S.G., Bee C., Borovika A., Briddell C., Colberg J., Humphery G.R., Kopach M.E., Martinez I., Nambiar S., Plummer S.V., Ribe S.D., Roschangar F., Scott J.P., Sneddon H.F. Green chemistry continuum for a robust and sustainable active pharmaceutical ingredient supply chain. ACS Sustainable Chem. Eng. 2019;7:16937–16951. [Google Scholar]; (e) Bryan M.C., Dillon B., Hamann L.G., Hughes G.J., Kopach M.E., Peterson E.A., Pourashraf M., Raheem I., Richardson P., Richter D., Sneddon H.F. Sustainable practices in medicinal chemistry: current state and future directions. J. Med. Chem. 2013;56:6007–6021. doi: 10.1021/jm400250p. [DOI] [PubMed] [Google Scholar]; (f) de Marco B.A., Rechelo B.S., Tótoli E.G., Kogawa A.C., Salgado H.R.N. Evolution of green chemistry and its multidimensional impacts: a review. Saudi Pharm. J. 2019;27:1–8. doi: 10.1016/j.jsps.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Bryan M.C., Dunn P.J., Entwistle D., Gallou F., Koenig S.G., Hayler J.D., Hickey M.R., Hughes S., Kopach M.E., Moine G., Richardson P., Roschangar F., Steven A., Weiberth F.J. Key Green Chemistry research areas from a pharmaceutical manufacturers’ perspective revisited. Green Chem. 2018;20:5082–5103. [Google Scholar]; (h) Koenig S.G., Leahy D.K., Wells A.S. Evaluating the impact of a decade of funding from the green chemistry institute pharmaceutical roundtable. Org. Process Res. Dev. 2018;22:1344–1359. [Google Scholar]
- 21.(a) Capello C., Fischer U., Hungerbühler K. What is a green solvent? a comprehensive framework for the environmental assessment of solvents. Green Chem. 2007;9:927–934. [Google Scholar]; (b) Prat D., Hayler J., Wells A. A survey of solvent selection guides. Green Chem. 2014;16:4546–4551. [Google Scholar]; (c) Hessel V., Tran N.N., Asrami M.R., Tran Q.D., Long N.V.C., Escribà-Gelonch M., Tejada J.O., Linke S., Sunmacher K. Sustainability of green solvents–review and perspective. Green Chem. 2022;24:410–437. [Google Scholar]; (d) Clarke C.J., Tu W.-C., Levers O., Bröhl A., Hallett J.P. Green and sustainable solvents in chemical processes. Chem. Rev. 2018;118:747–800. doi: 10.1021/acs.chemrev.7b00571. [DOI] [PubMed] [Google Scholar]; (e) Byrne F.P., Jin S., Paggiola G., Petchey T.H.M., Clarck J.H., Farmer T.J., Hunt A.J., McElory C.R., Sherwood J. Tools and techniques for solvent selection: green solvent selection guides. Sustainable Chem. Process. 2016;4:7. [Google Scholar]
- 22.(a) Butler R.N., Coyne A.C. Water: nature’s reaction enforcer—comparative effects for organic synthesis “in-water” and “on-water”. Chem. Rev. 2010;110:6302–6337. doi: 10.1021/cr100162c. [DOI] [PubMed] [Google Scholar]; (b) Chanda A., Fokin V.V. Organic synthesis “on water”. Chem. Rev. 2009;109:725–748. doi: 10.1021/cr800448q. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Simon M.-O., Li C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012;41:1415–1427. doi: 10.1039/c1cs15222j. [DOI] [PubMed] [Google Scholar]; (d) Zeynizadeh B., Mousavi H., Mohammad Aminzadeh F. A hassle-free and cost-effective transfer hydrogenation strategy for the chemoselective reduction of arylnitriles to primary amines through in situ-generated nickelII dihydride intermediate in water. J. Mol. Struct. 2022;1255:131926. [Google Scholar]; (e) Zeynizadeh B., Mohammad Aminzadeh F., Mousavi H. Chemoselective reduction of nitroarenes, N-acetylation of arylamines, and one-pot reductive acetylation of nitroarenes using carbon-supported palladium catalytic system in water. Res. Chem. Intermed. 2021;47:3289–3312. [Google Scholar]; (f) Zeynizadeh B., Mohammad Aminzadeh F., Mousavi H. Green and convenient protocols for the efficient reduction of nitriles and nitro compounds to corresponding amines with NaBH4 in water catalyzed by magnetically retrievable CuFe2O4 nanoparticles. Res. Chem. Intermed. 2019;45:3329–3357. [Google Scholar]; (g) Zeynizadeh B., Mohammad Aminzadeh F., Mousavi H. Two different facile and efficient approaches for the synthesis of various N-arylacetamides via N-acetylation of arylamines and straightforward one-pot reductive acetylation of nitroarenes promoted by recyclable CuFe2O4 nanoparticles in water. Green Process. Synth. 2019;8:742–755. [Google Scholar]
- 23.Nikoofar K., Khademi Z. A review on green Lewis acids: zirconium(IV) oxydichloride octahydrate (ZrOCl2·8H2O) and zirconium(IV) tetrachloride (ZrCl4) in organic chemistry. Res. Chem. Intermed. 2016;42:3929–3977. [Google Scholar]
- 24.Ziyaei Halimehjani A., Keshavarzi N. One-pot three-component route for the synthesis of functionalized 4H-chromenes catalyzed by ZrOCl2·8H2O in water. J. Heterocycl. Chem. 2018;55:522–529. [Google Scholar]
- 25.Rimaz M., Khalafy J., Mousavi H., Bohlooli S., Khalili B. Two different green catalytic systems for one-pot regioselective and chemoselective synthesis of some pyrimido[4,5-d]pyrimidinone derivatives in water. J. Heterocycl. Chem. 2017;54:3174–3186. [Google Scholar]
- 26.Tavakoli H.R., Moosavi S.M., Bazgir A. ZrOCl2·8H2O as an efficient catalyst for the synthesis of dibenzo [b,i]xanthene-tetraones and fluorescent hydroxyl naphthalene-1,4-diones. Res. Chem. Intermed. 2015;41:3041–3046. [Google Scholar]
- 27.Han L., Zhou Z. ZrOCl2·8H2O as an efficient and recyclable catalyst for the one-pot multicomponent synthesis of novel [1,3]oxazino[5,6-c]quinolin-5-one derivatives. Appl. Organomet. Chem. 2019;33:e4755. [Google Scholar]
- 28.Lü H.-Y., Li J.-J., Zhang Z.-H. ZrOCl2·8H2O: a highly efficient catalyst for the synthesis of 1,8-dioxo-octahydroxanthene derivatives under solvent-free conditions. Appl. Organomet. Chem. 2009;23:165–169. [Google Scholar]
- 29.Tavakoli H.R., Moosavi S.M., Bazgir A. ZrOCl2·8H2O as an efficient catalyst for the pseudo four-component synthesis of benzopyranopyrimidines. J. Korean Chem. Soc. 2013;57:260–263. [Google Scholar]
- 30.Moosavi-Zare A.R., Zolfigol M.A., Farahmand S., Zare A., Pourali A.R., Ayazi-Nasrabadi R. Synthesis of 2,4,6-triarylpyridines using ZrOCl2 under solvent-free conditions. Synlett. 2014;25:193–196. [Google Scholar]
- 31.Darandale S.N., Pansare D.N., Mulla N.A., Shinde D.B. Green synthesis of tetrahydropyrimidine analogues and evaluation of their antimicrobial activity. Bioorg. Med. Chem. Lett. 2013;23:2632–2635. doi: 10.1016/j.bmcl.2013.02.099. [DOI] [PubMed] [Google Scholar]
- 32.Sangshetti J.N., Ansari S.A.M.K., Shinde D.B. ZrOCl2·8H2O catalyzed solvent-free synthesis of isobenzofuran-1(3H)-ones. Chinese Chem. Lett. 2011;22:163–166. [Google Scholar]
- 33.Sangshetti J.N., Kokare N.D., Kotharkar S.A., Shinde D.B. ZrOCl2·8H2O catalyzed one-pot synthesis of 2,4,5-triaryl-1H-imidazoles and substituted 1,4-di(4,5-diphenylimidazol-yl)benzene. Chinese Chem. Lett. 2008;19:762–766. [Google Scholar]
- 34.Mohammadpoor-Baltork I., Khosropour A.R., Hojati S.F. ZrOCl2·8H2O as an environmentally friendly and recyclable catalyst for the chemoselective synthesis of 2-aryloxazolines and bis-oxazolines under thermal conditions and microwave irradiation. Catal. Commun. 2007;8:200–204. [Google Scholar]
- 35.Azarifar D., Sheikh D. ZrOCl2·8H2O: an efficient, ecofriendly, and recyclable catalyst for ultrasound-accelerated, one-pot, solvent-free synthesis of 8-aryl-7,8-dihydro-[1,3]dioxolo[4,5-g]quinolin-6-(5H)-one and 4-aryl-3,4-duhydroquinolin-2(1H)-one derivatives. Synth. Commun. 2013;43:2517–2526. [Google Scholar]
- 36.Shirini F., Zolfigol M.A., Mollarazi E. ZrOCl2·8H2O as an efficient reagent for the solvent-free synthesis of 3,4-dihydropyrimidin-2-(1H)-ones. Synth. Commun. 2006;36:2307–2310. [Google Scholar]
- 37.Tasqeeruddin S., Asiri Y.I., Shaheen S. Zirconium(IV) oxychloride octahydrate (ZrOCl2·8H2O): an efficient catalyst for the one-pot multicomponent synthesis of hexahydroquinoline derivatives under conventional heating and microwave irradiation. Russian J. Org. Chem. 2022;58:1008–1014. [Google Scholar]
- 38.Rimaz M., Mousavi H., Behnam M., Khalili B. A green chemoselective one-pot protocol for expeditious synthesis of symmetric pyranodipyrimidine derivatives using ZrOCl2·8H2O. Curr. Chem. Lett. 2016;5:145–154. [Google Scholar]
- 39.Rimaz M., Mousavi H., Keshavarz P., Khalili B. ZrOCl2·8H2O as a green and efficient catalyst for the expeditious synthesis of substituted 3-arylpyrimido[4,5-c]pyridazines in water. Curr. Chem. Lett. 2015;4:159–168. [Google Scholar]
- 40.Khalili B., Sadeghzadeh Darabi F., Eftekhari-sis B., Rimaz M. Green chemistry: ZrOCl2·8H2O catalyzed regioselective synthesis of 5-amino-1-aryl-1H-tetrazoles from secondary arylcyanamides in water. Monatsh. Chem. 2013;144:1569–1572. [Google Scholar]
- 41.(a) Rostein B.H., Zaretsky S., Rai V., Yudin A.K. Small heterocycles in multicomponent reactions. Chem. Rev. 2014;114:8323–8359. doi: 10.1021/cr400615v. [DOI] [PubMed] [Google Scholar]; (b) Dömling A., Wang W., Wang K. Chemistry and biology of multicomponent reactions. Chem. Rev. 2012;112:3083–3135. doi: 10.1021/cr100233r. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Sutanto F., Shaabani S., Neochoritis C.G., Zarganes-Tzitzikas T., Patil P., Ghonchepour E., Dömling A. Multicomponent reaction–derived covalent inhibitor space. Sci. Adv. 2021;7:eabd9307. doi: 10.1126/sciadv.abd9307. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Hurtado-Rodríguez D., Salinas-Torres A., Rojas H., Becerra D., Castillo J.-C. Bioactive 2-pyridone-containing heterocycle syntheses using multicomponent reactions. RSC Adv. 2022;12:35158–35176. doi: 10.1039/d2ra07056a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.(a) Eftekhari-Sis B., Zirak M., Akbari A. Arylglyoxals in synthesis of heterocyclic compounds. Chem. Rev. 2013;113:2958–3043. doi: 10.1021/cr300176g. [DOI] [PubMed] [Google Scholar]; (b) Mousavi H. A concise and focused overview upon arylglyoxal monohydrates-based one-pot multi-component synthesis of fascinating potentially biologically active pyridazines. J. Mol. Struct. 2022;1251:131742. [Google Scholar]; (c) Jana A., Ali D., Bhaumick P., Choudhury L.H. Sc(OTf)3-Mediated one-pot synthesis of coumarin-fused furans: a thiol-dependent reaction for the easy access of 2-phenyl-4H-furo[3,2-c]chromen-4-ones. J. Org. Chem. 2022;87:7763–7777. doi: 10.1021/acs.joc.2c00353. [DOI] [PubMed] [Google Scholar]; (d) Gapanenok D., Makhmet A., Peshkov A.A., Smirnova D., Amire N., Peshkov V.A., Spiridonova D., Dar'in D., Balalaie S., Krasavin M. Multicomponent assembly of trisubstituted imidazoles and their photochemical cyclization into fused polyheterocyclic scaffolds. J. Org. Chem. 2022;87:7838–7851. doi: 10.1021/acs.joc.2c00475. [DOI] [PubMed] [Google Scholar]; (e) Bhaumick P., Kumar R., Acharya S.S., Parvin T., Choudhury L.H. Multicomponent synthesis of fluorescent thiazole–indole hybrids and thiazole-based novel polymers. J. Org. Chem. 2022;87:11399–11413. doi: 10.1021/acs.joc.2c00922. [DOI] [PubMed] [Google Scholar]; (f) Yaragorla S., Arun D. Arylation and aryne insertion into C-acylimines: a simple, flexible, and divergent synthesis of C2-aryl indoles. J. Org. Chem. 2022;87:14250–14263. doi: 10.1021/acs.joc.2c01753. [DOI] [PubMed] [Google Scholar]; (g) Zi Q.-X., Yang C.-L., Li K., Luo Q., Lin J., Yan S.-J. Multicomponent cascade reaction by metal-free aerobic oxidation for synthesis of highly functionalized 2-amino-4-coumarinyl-5-arylpyrroles. J. Org. Chem. 2020;85:327–338. doi: 10.1021/acs.joc.9b02063. [DOI] [PubMed] [Google Scholar]; (h) Brahmachari G., Nayek N., Karmakar I., Nurjamal K., Chandra S.K., Bhowmick A. Series of functionalized 5-(2-arylimidazo[1,2-a]pyridin-3-yl)pyrimidine-2,4(1H,3H)-diones: a water-mediated three-component catalyst-free protocol revisited. J. Org. Chem. 2020;85:8405–8414. doi: 10.1021/acs.joc.0c00732. [DOI] [PubMed] [Google Scholar]; (i) Shtamburg V.G., Shtamburg V.V., Anishchenko A.A., Mazepa A.V., Rusanov E.B. 3-Alkoxy-1,5-bis(aryl)imidazolidine-2,4-diones, synthesis and structure. J. Mol. Struct. 2022;1264:133259. [Google Scholar]; (j) Jalani H.B., Jeong J.-H. p-Toluenesulfonic acid catalyzed, isocyanide-free, Groebke– Blackburn-Bienayme (GBB) type multicomponent synthesis of 3-anilino-imdazo[1,2-a]pyridines. J. Heterocycl. Chem. 2022;59:1266–1271. [Google Scholar]; (k) El-Araby M.E., Omar A.M.E. A single-step synthesis of 1,3,4,6-tetraaryl-5-aryliminopiperazin-2-one. J. Heterocycl. Chem. 2021;58:442–449. [Google Scholar]; (l) Asadi S., Zebarjad M., Masoudi H., Mehrabi H. Survey reactivity of 2-aminopyridine and Meldrum’s acid in the presence of aryl glyoxals or or aryl aldehydes; ethyl 2-(3-aryl imidazo[1,2-a]pyridin-2-yl)acetates versus ethyl 3-aryl-3-(pyridin-2-ylamino)propanoates. Res. Chem. Intermed. 2022;48:251–265. [Google Scholar]; (m) Yadav M.B., Jeong Y.T. Construction of novel penta cyclic indolo-furo[3,2-c]quinoline and dihydrochromeno-furo[2,3-b]indol via sequential annulation strategy. Tetrahedron Lett. 2022;109:154158. [Google Scholar]; (n) Rimaz M., Mousavi H., Behnam M., Sarvari L., Khalili B. Fast and convenient synthesis of new symmetric pyrano[2,3-d:6,5-d’]dipyrimidinones by an organocatalyzed annulation reaction. Curr. Chem. Lett. 2017;6:55–68. [Google Scholar]
- 43.(a) Xiong J., He H.-T., Yang H.-Y., Zeng Z.-G., Zhong C.-R., Shi H., Ouyang M.-L., Tao Y.-Y., Pang Y.-L., Zhang Y.-H., Hu B., Fu Z.-X., Miao X.-L., Zhu H.-L., Yao G. Synthesis of 4-tetrazolyl-substituted 3,4-dihydroquinazoline derivatives with anticancer activity via a one-pot sequential Ugi-azide/palladium-catalyzed azide-isocyanide cross-coupling/cyclization reaction. J. Org. Chem. 2022;87:9488–9496. doi: 10.1021/acs.joc.2c00382. [DOI] [PubMed] [Google Scholar]; (b) Frippiat S., Sarre C., Baudequin C., Hoarau C., Bischoff L. Insights in the synthesis of imidazolones from aldehydes, isocyanides, or oxazolines. J. Org. Chem. 2022;87:7464–7473. doi: 10.1021/acs.joc.1c02454. [DOI] [PubMed] [Google Scholar]; (c) Zhang B., Kurpiewska K., Dömling A. Highly stereoselective Ugi/Pictet–Spengler sequence. J. Org. Chem. 2022;87:7085–7096. doi: 10.1021/acs.joc.2c00244. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Il’in M.V., Sysoeva A.A., Novikov A.S., Bolotin D.S. Diaryliodoniums as hybrid hydrogen-and halogen-bond-donating organocatalysts for the Groebke–Blackburn–Bienaymé reaction. J. Org. Chem. 2022;87:4569–4579. doi: 10.1021/acs.joc.1c02885. [DOI] [PubMed] [Google Scholar]; (e) Il’in M.V., Sysoeva A.A., Novikov A.S., Bolotin D.S. Sulfonium and selenonium salts as noncovalent organocatalysts for the multicomponent Groebke–Blackburn–Bienaymé reaction. J. Org. Chem. 2022;87:10199–10207. doi: 10.1021/acs.joc.2c01141. [DOI] [PubMed] [Google Scholar]; (f) Sajko J.S., Jerić I. Synthesis of Nβ-substituted 1,2-diazetidin-3-ones by the Ugi reaction comprising chiral α-hydrazino acids. J. Org. Chem. 2022;87:7076–7084. doi: 10.1021/acs.joc.2c00238. [DOI] [PubMed] [Google Scholar]; (g) González-Saiz B., Carreira-Barral I., Pertejo P., Gómez-Ayuso J., Quesada R., García-Valverde M. One-pot diastereoselective synthesis of pyrrolopiperazine-2,6-diones by a Ugi/nucleophilic substitution/N-acylation sequence. J. Org. Chem. 2022;87:9391–9398. doi: 10.1021/acs.joc.2c00694. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Li X., Wang Q., Zheng Q., Kurpiewska K., Kalinowska-Tluscik J., Dömling A. Access to isoquinolin-2(1H)-yl-acetamides and isoindolin-2-yl-acetamides from a common MCR precursor. J. Org. Chem. 2022;87:14463–14475. doi: 10.1021/acs.joc.2c01905. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Xu R., Wang Z., Zheng Q., Patil P., Dömling A. A bifurcated multicomponent synthesis approach to polycyclic quinazolinones. J. Org. Chem. 2022;87:13023–13033. doi: 10.1021/acs.joc.2c01561. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Shahriari A., Amiri K., Nikbakht A., Rominger F., Bijanzadeh H.R., Balalaie S. Synthesis of pyrrolidin-5-one-2-carboxamides through cyclization of N-substituted-2-alleneamides. J. Org. Chem. 2022;87:7778–7785. doi: 10.1021/acs.joc.2c00387. [DOI] [PubMed] [Google Scholar]; (k) Ge S., Zhu Y.-M., Xu X.-P., Ji S.-J. [4 + 1 + 1] Tandem cyclization reaction involving isocyanides: access to 2-(trifluoromethyl)quinazolin-4(3H)-imines. J. Org. Chem. 2022;87:3422–3432. doi: 10.1021/acs.joc.1c03008. [DOI] [PubMed] [Google Scholar]; (l) Li Y., Xu J., He L.-J., Luo Y.-F., Meng J.-P., Tang D.-Y., Li H.-y., Chen Z.-Z., Xu Z.-G. Dieckmann condensation of Ugi N-acylamino amide product: facile access to functionalized 2,2-disubstituted indolin-3-ones. J. Org. Chem. 2022;87:823–834. doi: 10.1021/acs.joc.1c02501. [DOI] [PubMed] [Google Scholar]; (m) Meng X.-H., Xu X.-C., Wang Z., Liang Y.-X., Zhao Y.-L. NaN(SiMe3)2/CsTFA copromoted aminobenzylation/cyclization of 2-isocyanobenzaldehydes with toluene derivatives or benzyl compounds: one-pot access to dihydroquinazolines and quinazolines. J. Org. Chem. 2022;87:3156–3166. doi: 10.1021/acs.joc.1c02890. [DOI] [PubMed] [Google Scholar]; (n) Lv Y., Chen L., Li K., Yun X.-H., Yan S.-J. Multicomponent cascade reaction of 3-cyanochromones: highly site-selective synthesis of 2-(1H-imidazol-1-yl)-4H-chromen-4-one derivatives. J. Org. Chem. 2022;87:15187–15196. doi: 10.1021/acs.joc.2c01719. [DOI] [PubMed] [Google Scholar]; (o) Liu L.-Q., Fang Y.-L., Lin J.-X., Wang Y.-C. Aerobic copper-catalyzed four-component reaction of O-phenylenediamines, isocyanides, and selenium powder for the assembly of benzo[4,5]imidazo[2,1-c][1,2,4]selenadiazol-3-imine derivatives. J. Org. Chem. 2022;87:15120–15128. doi: 10.1021/acs.joc.2c01622. [DOI] [PubMed] [Google Scholar]; (p) Bao L., Liu Y., Peng J., Wang Y., Dong J., Xu X. Chemoselective trimerization of isocyanides: de novo synthesis of 2-indole-substituted quinolines and pyridines. Org. Lett. 2022;24:105–109. doi: 10.1021/acs.orglett.1c03693. [DOI] [PubMed] [Google Scholar]; (q) Zheng Q., Li X., Kurpiewska K., Dömling A. Synthesis of tunable fluorescent imidazole-fused heterocycle dimers. Org. Lett. 2022;24:5014–5017. doi: 10.1021/acs.orglett.2c01642. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Mohlala R.L., Coyanis E.M., Fernandes M.A., Bode M.L. Catalyst-free synthesis of novel 1,5-benzodiazepines and 3,4-dihydroquinoxalines using isocyanide-based one-pot, three- and four-component reactions. RSC Adv. 2021;11:24466–24473. doi: 10.1039/d1ra04444c. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Nazeri M.T., Shaabani A. Synthesis of polysubstituted pyrroles via isocyanide-based multicomponent reactions as an effecient synthesis tool. New J. Chem. 2021;45:21967–22011. [Google Scholar]; (t) Khodadadi M., Ghandi M., Abbasi A. One-pot synthesis of novel spirocyclic-dihydropyrazine-2-(1H)ones through a Ugi 4-CR/deprotection. J. Heterocycl. Chem. 2022;59:686–694. [Google Scholar]; (u) Singh R., Kumar R., Kaur M., Patil M.T., Sahoo S.C., Salunke D.B. Groebke–Blackburn–Bienaymé multicomponent reaction coupled with unconventional Pictet-Spengler cyclization for the synthesis of imidazo[4,5-b]pyridine fused polycyclic heterocycles. J. Heterocycl. Chem. 2022;59:1007–1015. [Google Scholar]; (v) Saeedi S., Rahmati A., Chavoshpour-Natanzi Z. Synthesis of pyrazolo[5ʹ,1ʹ: 2,3]imidazo[1,5-c]quinazolin-6(5H)-ones and molecular docking study of their affinity against the COVID-19 main protease. RSC Adv. 2022;12:19579–19589. doi: 10.1039/d2ra03179e. [DOI] [PMC free article] [PubMed] [Google Scholar]; (w) Chen T., Ye F., Hu R., Tang B.Z. Multicomponent polymerizations of isocyanides, aldehydes, and 2-aminopyridine toward imidazo[1,2-a]pyridine-containing fused heterocyclic polymers. Macromolecules. 2022;55:8590–8598. [Google Scholar]
- 44.(a) Rimaz M., Mousavi H. A one-pot strategy for regioselective synthesis of 6-aryl-3-oxo-2,3-dihydropyridazine-4-carbohydrazides. Turk. J. Chem. 2013;37:252–261. [Google Scholar]; (b) Rimaz M., Jalalian Z., Mousavi H., Prager R.H. Base organocatalyst mediated annulation of arylglyoxalmonohydrates with 2,4-dihydroxyquinoline to form new pyranodiquinolinones. Tetrahedron Lett. 2016;57:105–109. [Google Scholar]; (c) Rimaz M., Khalafy J., Mousavi H. A green organocatalyzed one-pot protocol for efficient synthesis of new substituted pyrimido[4,5-d]pyrimidinones using a Biginelli-like reaction. Res. Chem. Intermed. 2016;42:8185–8200. [Google Scholar]; (d) Rimaz M., Mousavi H., Nikpey L., Khalili B. Novel and convenient one-pot strategy for regioselective synthesis of new 5-aryl-3-methyl-1-phenyl-1,2-dihydro-7aH-pyrazolo[3,4-c]pyridazin-7a-ol derivatives. Res. Chem. Intermed. 2017;43:3925–3937. [Google Scholar]; (e) Rimaz M., Khalili B., Khatyal G., Mousavi H., Aali F. A simple and efficient diversity-oriented synthesis of new substituted 3-(arylamino)-6,7-dihydro-1H-indazol-4(5H)-ones by a KOH-assisted one-pot reaction. Aust. J. Chem. 2017;70:1274–1284. [Google Scholar]; (f) Rimaz M., Mousavi H., Khalili B., Aali F. A green and practical one-pot two-step strategy for the synthesis of symmetric 3,6-diarylpyridazines. J. Chinese Chem. Soc. 2018;65:1389–1397. [Google Scholar]; (g) Rimaz M., Mousavi H., Khalili B., Sarvari L. One-pot pseudo three-component condensation reaction of arylglyoxal monohydrates with 1-ethyl-2-thioxodihydropyrimidine-4,6(1H,5H)-dione for the synthesis of new pyrano[2,3-d:6,5-d’]dipyrimidines as HIV integrase inhibitor-like frameworks using two different environmentally benign catalytic systems. J. Iran. Chem. Soc. 2019;16:1687–1701. [Google Scholar]; (h) Rimaz M., Mousavi H., Ozzar L., Khalili B. Facile, capable, atom-economical one-pot multicomponent strategy for the direct regioselective synthesis of novel isoxazolo[5,4-d]pyrimidines. Res. Chem. Intermed. 2019;45:2673–2694. [Google Scholar]; (i) Bakhshi R., Zeynizadeh B., Mousavi H. Green, rapid, and highly efficient syntheses of α,α′-bis[(aryl or allyl)idene]cycloalkanones and 2-[(aryl or allyl)idene]-1-indanones as potentially biologic compounds via solvent-free microwave-assisted Claisen-Schmidt condensation catalyzed by MoCl5. J. Chinese Chem. Soc. 2020;67:623–637. [Google Scholar]; (j) Hasanpour Galehban M., Zeynizadeh B., Mousavi H. NiII NPs entrapped within a matrix of L-glutamic acid cross-linked chitosan supported on magnetic carboxylic acid-functionalized multi-walled carbon nanotube: a new and efficient multi-task catalytic system for the green one-pot synthesis of diverse heterocyclic frameworks. RSC Adv. 2022;12:16454–16478. doi: 10.1039/d1ra08454b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Hasanpour Galehban M., Zeynizadeh B., Mousavi H. Introducing Fe3O4@SiO2@KCC-1@MPTMS@CuII catalytic applications for the green one-pot syntheses of 2-aryl(or heteroaryl)-2,3-dihydroquinazolin-4(1H)-ones and 9-aryl-3,3,6,6- tetramethyl-3,4,5,6,7,9-hexahydro-1H-xanthene-1,8(2H)-diones. J. Mol. Struct. 2023;1271:134017. [Google Scholar]
- 45.Ebrahim A., Riley B.T., Kumaran D., Andi B., Fuches M.R., McSweeney S., Keedy D.A. The temperature-dependent conformational ensemble of SARS-CoV-2 main protease (Mpro) IUCrJ. 2022;9:682–694. doi: 10.1107/S2052252522007497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.(a) Fan J., Liu Y., Kong R., Ni D., Yu Z., Lu S., Zhang J. Harnessing reversed allosteric communication: a novel strategy for allosteric drug discovery. J. Med. Chem. 2021;64:17728–17743. doi: 10.1021/acs.jmedchem.1c01695. [DOI] [PubMed] [Google Scholar]; (b) Han B., Salituro F.G., Blanco M.-J. Impact of allosteric modulation in drug discovery: innovation in emerging chemical modalities. ACS Med. Chem. Lett. 2020;11:1810–1819. doi: 10.1021/acsmedchemlett.9b00655. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Abdel-Magid A.F. Allosteric modulators: an emerging concept in drug discovery. ACS Med. Chem. Lett. 2015;6:104–107. doi: 10.1021/ml5005365. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Ni D., Liu Y., Kong R., Yu Z., Lu S., Zhang J. Computational elucidation of allosteric communication in proteins for allosteric drug design. Drug. Discov. Today. 2022;27:2226–2234. doi: 10.1016/j.drudis.2022.03.012. [DOI] [PubMed] [Google Scholar]; (e) Ni D., Wei J., He X., Rehman A.U., Li X., Qui Y., Pu J., Lu S., Zhang J. Discovery of cryptic allosteric sites using reversed allosteric communication by a combined computational and experimental strategy. Chem. Sci. 2021;12:464–476. doi: 10.1039/d0sc05131d. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Tan Z.W., Tee W.-V., Samsudin F., Guarnera E., Bond P.J., Berezovsky I.N. Allosteric perspective on the mutability and druggability of the SARS-CoV-2 Spike protein. Structure. 2022;30:590–607.e4. doi: 10.1016/j.str.2021.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Guarnera E., Berezovsky I.N. Allosteric drugs and mutations: chances, challenges, and necessity. Curr. Opin. Struct. Biol. 2020;62:149–157. doi: 10.1016/j.sbi.2020.01.010. [DOI] [PubMed] [Google Scholar]; (h) Ni D., Chai Z., Wang Y., Li M., Yu Z., Liu Y., Lu S., Zhang J. Along the allostery stream: recent advances in computational methods for allosteric drug discovery. WIREs Comput. Mol. Sci. 2022;12:e1585. [Google Scholar]; (i) Kalhor H.R., Taghikhani E. Probe into the molecular mechanism of ibuprofen interaction with warfarin bound to human serum albumin in comparison to ascorbic and salicylic acids: allosteric inhibition of anticoagulant release. J. Chem. Inf. Model. 2021;61:4045–4057. doi: 10.1021/acs.jcim.1c00352. [DOI] [PubMed] [Google Scholar]; (j) Kremer D.M., Lyssiotis C.A. Targeting allosteric regulation of cancer metabolism. Nat. Chem. Biol. 2022;18:441–450. doi: 10.1038/s41589-022-00997-6. [DOI] [PubMed] [Google Scholar]; (k) Bishop Ö.T., Musyoka T.M., Barozi V. Allostery and missense mutations as intermittently linked promising aspects of modern computational drug discovery. J. Mol. Biol. 2022;434:167610. doi: 10.1016/j.jmb.2022.167610. [DOI] [PubMed] [Google Scholar]; (l) Faure A.J., Domingo J., Schmiedel J.M., Hidalgo-Carcedo C., Diss G., Lehner B. Mapping the energetic and allosteric landscapes of protein binding domains. Nature. 2022;604:175–183. doi: 10.1038/s41586-022-04586-4. [DOI] [PubMed] [Google Scholar]
- 47.(a) Günther S., Reinke P.Y.A., Fernández-García Y., Lieske J., Lane T.J., Ginn H.M., Koua F.H.M., Ehrt C., Ewert W., Oberthuer D., Yefanov O., Meier S., Lorenzen K., Krichel B., Kopicki J.-D., Gelisio L., Brehm W., Dunkel I., Seychell B., Gieseler H., Norton-Baker B., Escudero-Pérez B., Domaracky M., Saounane S., Tolstikova A., White T.A., Hänle A., Groessler M., Fleckenstein H., Trost F., Galchenkova M., Gevorkov Y., Li C., Awel S., Peck A., Barthelmess M., Schlünzen F., Xavier P.L., Werner N., Andaleeb H., Ullah N., Falke S., Srinivasan V., França B.A., Schwinzer M., Brognaro H., Rogers C., Melo D., Zaitseva-Doyle J.J., Knoska J., Peña-Murillo G.E., Rahmani Mashhour A., Hennicke V., Fischer P., Hakanpää J., Meyer J., Gribbon P., Ellinger B., Kuzikov M., Wolf M., Beccari A.R., Bourenkov G., von Stetten D., Pompidor G., Bento I., Panneerselvam S., Karpics I., Schneider T.R., Garcia-Alai M.M., Niebling S., Günther C., Schmidt C., Schubert R., Han H., Boger J., Monteiro D.C.F., Zhang L., Sun X., Pletzer-Zelgert J., Wollenhaupt J., Feiler C.G., Weiss M.S., Schulz E.-C., Mehrabi P., Karničar K., Usenik A., Loboda J., Tidow H., Chari A., Hilgenfeld R., Uetrecht C., Cox R., Zaliani A., Beck T., Rarey M., Günther S., Turk D., Hinrichs W., Chapman H.N., Pearson A.A., Betzel C., Meents A. X-ray screening identifies active site and allosteric inhibitors of SARS-CoV-2 main protease. Science. 2021;372:642–646. doi: 10.1126/science.abf7945. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Amamuddy O.S., Boateng R.A., Barozi V., Nyamai D.W., Bishop Ö. Novel dynamic residue network analysis approaches to study allosteric modulation: SARS-CoV-2 MPro and its evolutionary mutations as a case study. Comput. Struct. Biotech. J. 2021;19:6431–6455. doi: 10.1016/j.csbj.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Alzyoud L., Ghattas M.A., Atatreh N. Allosteric binding sites of the SARS-CoV-2 main protease: potential targets for broad-spectrum anti-coronavirus agents. Drug Des. Devel. Ther. 2022;16:2463–2478. doi: 10.2147/DDDT.S370574. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Samrat S.K., Xu J., Xie X., Gianti E., Chen H., Zou J., Pattis J.G., Elokely K., Lee H., Li Z., Klein M.L., Shi P.-Y., Zhou J., Li H. Allosteric inhibitors of the main protease of SARS-CoV-2. Antiviral Res. 2022;205:105381. doi: 10.1016/j.antiviral.2022.105381. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Strömich L., Wu N., Barahona M., Yaliraki S.N. Allosteric hotspots in the main protease of SARS-CoV-2. J. Mol. Biol. 2022;434:167748. doi: 10.1016/j.jmb.2022.167748. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Jiménez-Avalos G., Vargas-Ruiz A.P., Delgado-Pease N.E., Olivos-Ramirez G.E., Sheen P., Fernández-Díaz M., Quiliano M., Zimic M. Comprehensive virtual screening of 4.8 k flavonoids reveals novel insights into allosteric inhibition of SARS-CoV-2 MPro. Sci. Rep. 2021;11:15452. doi: 10.1038/s41598-021-94951-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Bram Y., Duan X., Nilsson-Payant B.E., Chandar V., Wu H., Shore D., Fajardo A., Sinha S., Hassan N., Weinstein H., TenOever B.R., Chen S., Schwartz R.E. Dual-reporter system for real-time monitoring of SARS-CoV-2 main protease activity in live cells enables identification of an allosteric inhibition path. ACS Bio Med Chem Au. 2022;2:627–641. doi: 10.1021/acsbiomedchemau.2c00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Srinivasan V., Brognaro H., Prabhu P.R., de Souza E.E., Günther S., Reinke P.Y.A., Lane T.J., Ginn H., Han H., Ewert W., Sprenger J., Koua F.H.M., Falke S., Werner N., Andaleeb H., Ullah N., Franca B.A., Wang M., Barra A.L.C., Perbandt M., Schwinzer M., Schmidt C., Brings L., Lorenzen K., Schubert R., Machado R.R.G., Candido E.D., Oliveira D.B.L., Durigon E.L., Niebling S., Garcia S., Yefanov O., Lieske J., Gelisio L., Domaracky M., Middendorf P., Groessler M., Trost F., Galchenkova M., Rahmani Mashhour A., Saouane S., Hakanpää J., Wolf M., Alai M.G., Turk D., Pearson A.R., Chapman H.N., Hinrichs W., Wrenger C., Meents A., Betzel C. Antiviral activity of natural phenolic compounds in complex at an allosteric site of SARS-CoV-2 papain-like protease. Commun. Biol. 2022;5:805. doi: 10.1038/s42003-022-03737-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.(a) van de Waterbeemd H., Gifford E. ADMET in silico modelling: towards prediction paradise? Nat. Rev. Drug. Discov. 2003;2:192–204. doi: 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]; (b) Feinberg E.V., Joshi E., Pande V.S., Cheng A.C. Improvement in ADMET prediction with multitask deep featurization. J. Med. Chem. 2020;63:8835–8848. doi: 10.1021/acs.jmedchem.9b02187. [DOI] [PubMed] [Google Scholar]; (c) Jia C.-Y., Hao G.-F., Yang G.-F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today. 2020;25:248–258. doi: 10.1016/j.drudis.2019.10.014. [DOI] [PubMed] [Google Scholar]; (d) Ferreira L.L.G., Andricopulo A.D. ADMET modeling approaches in drug discovery. Drug Discov. Today. 2019;24:1157–1165. doi: 10.1016/j.drudis.2019.03.015. [DOI] [PubMed] [Google Scholar]; (e) Guan L., Yang H., Cai Y., Sun L., Di P., Li W., Liu G., Tang Y. ADMET-score – a comprehensive scoring function for evaluation of chemical drug-likeness. Med. Chem. Commun. 2019;10:148–157. doi: 10.1039/c8md00472b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Pantaleão S.Q., Fernandes P.O., Gonçalves J.E., Maltarollo V.G., Honorio K.M. Recent advances in the prediction of pharmacokinetics properties in drug design studies: a review. ChemMedChem. 2022;17:e202100542. doi: 10.1002/cmdc.202100542. [DOI] [PubMed] [Google Scholar]
- 50.(a) Daina A., Michielin O., Zoete V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Bakchi B., Krishna A.D., Sreecharan E., Ganesh V.B.J., Niharika M., Maharshi S., Puttagunta S.B., Sigalapalli D.K., Bhandare R.R., Shaik A.B. An overview on applications of SwissADME web tool in the design and development of anticancer, antitubercular and antimicrobial agents: a medicinal chemist's perspective. J. Mol. Struct. 2022;1259:132712. [Google Scholar]
- 51.Martin Y.C. A bioavailability score. J. Med. Chem. 2005;48:3164–3170. doi: 10.1021/jm0492002. [DOI] [PubMed] [Google Scholar]
- 52.Pires D.E.V., Blundell T.L., Ascher D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015;58:4066–4072. doi: 10.1021/acs.jmedchem.5b00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Unoh Y., Uehara S., Nakahara K., Nobori H., Yamatsu Y., Yamamoto S., Maruyama Y., Taoda Y., Kasamatsu K., Suto T., Kouki K., Nakahashi A., Kawashima S., Sanaki T., Toba S., Uemura K., Mizutare T., Ando S., Sasaki M., Orba Y., Sawa H., Sato A., Sato T., Kato T., Tachibana Y. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 2022;65:6499–6512. doi: 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.(a) Halford B. The path to Paxlovid. ACS Cent. Sci. 2022;8:405–407. doi: 10.1021/acscentsci.2c00369. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Lamb Y.N. Nirmatrelvir plus Ritonavir: first approval. Drugs. 2022;82:585–591. doi: 10.1007/s40265-022-01692-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Kincaid J.R.A., Caravez J.C., Iyer K.S., Kavthe R.D., Fleck N., Aue D.H., Lipshutz B.H. A sustainable synthesis of the SARS-CoV-2 Mpro inhibitor nirmatrelvir, the active ingredient in Paxlovid. Commun. Chem. 2022;4:156. doi: 10.1038/s42004-022-00758-5. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Cotrim B.A., Barros J.C. Development and patent synthesis of nirmatrelvir – the main component of the first oral drug against SARS-CoV-2 Paxlovid®. Aust. J. Chem. 2022;75:487–491. [Google Scholar]
- 55.Lockbaum G.J., Reyes A.C., Lee J.M., Tilvawala R., Nalivaika E.A., Ali A., Yilmaz K.N., Thompson P.R., Schiffer C.A. Crystal structure of SARS-CoV-2 main protease in complex with the non-covalent inhibitor ML188. Viruses. 2021;13:174. doi: 10.3390/v13020174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., Geurink P.P., Wilhelm A., van der Heden van Noort G.J., Ovaa H., Müller S., Knobeloch K.P., Rajalingam K., Schulman B.A., Cinatl J., Hummer G., Ciesek S., Dikic I. Papain-like protease regulates SARS-CoV-2 viral spread and innate immunity. Nature. 2020;587:657–662. doi: 10.1038/s41586-020-2601-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma C., Hu Y., Wang Y., Choza J., Wang J. Drug-repurposing screening identified Tropifexor as a SARS-CoV-2 papain-like protease inhibitor. ACS Infect. Dis. 2022;8:1022–1030. doi: 10.1021/acsinfecdis.1c00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.