Abstract
Neurodegenerative diseases affect over 30 million people worldwide with an ascending trend. Most individuals suffering from these irreversible brain damages belong to the elderly population, with onset between 50 and 60 years. Although the pathophysiology of such diseases is partially known, it remains unclear upon which point a disease turns degenerative. Moreover, current therapeutics can treat some of the symptoms but often have severe side effects and become less effective in long-term treatment. For many neurodegenerative diseases, the involvement of G protein-coupled receptors (GPCRs), which are key players of neuronal transmission and plasticity, has become clearer and holds great promise in elucidating their biological mechanism. With this review, we introduce and summarize class A and class C GPCRs, known to form heterodimers or oligomers to increase their signalling repertoire. Additionally, the examples discussed here were shown to display relevant alterations in brain signalling and had already been associated with the pathophysiology of certain neurodegenerative diseases. Lastly, we classified the heterodimers into two categories of crosstalk, positive or negative, for which there is known evidence.
Keywords: G protein-coupled receptors, dimers, class A, class C, neurodegenerative diseases, brain
1. INTRODUCTION
1.1. Scope of Review
Neurodegenerative diseases, characterized by progressive neuronal dysfunction, toxicity and death [1], are prevalent among the worldwide elderly population [2]. These diseases cause irreversible damage to all types of brain functions and it is estimated that over 30 million individuals suffer from them worldwide [3, 4]. Parkinson’s disease (PD), Alzheimer’s disease (AD), Vascular dementia (VaD), Frontotemporal dementia (FTD), and Huntington’s disease (HD) are the most prevailing ones [5, 6]. Among those, AD and PD have an earlier average onset between 50 and 60 years [5, 7, 8].
Impaired cognitive function, memory loss and negative personality are common traits associated with people suffering from AD [9-11]. The accumulation of amyloid β (Aβ) in amyloid plaques and hyperphosphorylated aggregates of the microtubule-associated protein tau in neurofibrillary tangles, which slowly progress from the frontal and temporal lobes to other areas of the neocortex are the pathological features observed in AD patients [9].
PD is predominantly characterized by motor impairments such as bradykinesia, rigidity, tremor and gait disorder [12]. Also non-motor impairments like cognitive impairment and neuropsychiatric symptoms are observed among PD patients [12]. The pathology of PD has been well-studied over the years. The loss of dopaminergic neurons in the substantia nigra is the major feature observed in PD patients, but also the deposition of Lewy bodies and abnormal aggregates of the α-synuclein protein in several brain regions, such as the substantia nigra and temporal cortex, have been described to play a role in PD [12].
In contrast to AD, VaD has a variable onset age and is the second most common cause of dementia [5]. Disturbance in the frontal executive function and multiple cerebral pathologies, including arteriosclerosis and various forms of arteritis, aneurysms or vessel occlusion, are the characteristic of VaD [13, 14]. Under the age of 65, FTD is known to be the major reason for dementia [5, 15]. FTD patients display neuropsychiatric symptoms and cognitive, motor and behavioural impairments, as well as the abnormal deposition of the three major proteins tau, transactive response DNA-binding protein 43 (TDP-43) and fused in sarcoma (FUS) protein in the brain [16].
As for PD, HD symptoms can be divided into motor and non-motor symptoms such as chorea, bradykinesia, impaired coordination, rigidity, which are motor symptoms, whereas depression and slowed cognitive function are described as non-motor symptoms [17]. The root cause of HD is genetic, unlike the other diseases described here. HD is caused by a CAG trinucleotide repeat expansion in the Huntingtin (Htt) gene [5, 17]. In unaffected individuals, the CAG repeats vary from 6 to 35 nucleotides, while > 36 repeats are present in HD patients [18]. The number of repeats inversely correlates with the age of onset [5, 18]. Consequently, Huntingtin protein (HTT) is deposited in the brain, typically not only in the cerebral cortex, but also in other regions such as striatum, hippocampus, and cerebellum [19].
Some of the structural and biological determinants of neurodegenerative diseases have already been revealed [20-24]. However, the turning point of when a pathological condition becomes chronic and leads to neurodegeneration remains elusive for most of the diseases. In this review, we focus on G protein-coupled receptor (GPCRs) heterodimers, which are known to play significant roles in the brain [25-29].
1.2. G Protein-coupled Receptors
1.2.1. General Mode of Action
GPCRs are the mediators of almost all (patho)physiological responses in the human body and comprise the largest family of membrane proteins [30, 31]. GPCRs share a common architecture of seven transmembrane helices (7TM), connected through 3 intra-and extracellular loops (ICL1-3, ECL1-3) with an extracellular N-terminus and intracellular C-terminus [32, 33]. Around 800 genes encode for GPCRs in the human genome [34, 35] and about 370 of them are non-sensory GPCRs, ~90% of each expressed in the brain [1, 5]. They play important roles in regulating mood, appetite, pain, vision, immune responses, cognition, and synaptic transmissions [5, 30, 33]. Most of these functions are mediated via endocrine and neurological pathways [1, 5, 29, 35].
In the brain, neurotransmitters signal via GPCRs to modulate the activity of muscles and neurons [36, 37]. Dopamine, serotonin, noradrenaline and other derivatives of amino acids and amines, but also oligopeptides like oxytocin or endorphins as well as purines constitute some of known GPCRs ligands [38-45]. Furthermore, an individual small-molecule neurotransmitter might target a dozen different GPCRs. Neurons expressing certain types of receptors are then formed as entire systems. The five main transmission systems are: noradrenaline, dopamine, histamine, serotonin, and the acetylcholine system [46-50]. Strong imbalances or disruption of these systems have been associated with many mental disorders and neurological conditions such as depression, schizophrenia, attention deficit hyperactivity disorder (ADHD), anxiety, memory loss, pain perception as well as dramatic changes in weight and addictions, aside from neurodegenerative diseases [51-59]. Some studies were also able to connect malfunctioning of the dopaminergic system to multiple sclerosis (MS) [60]. Genetics may also play a role [61].
Vertebrate GPCRs were classified through the GRAFS (Glutamate, Rhodophsin, Adhesion, Frizzled/Taste, Smoothened families) system that uses a phylogenetic tree of approximately 800 human GPCR sequences to assign the receptors to a specific family [62-65]. Another system, the A-F system, classifies GPCRs by their amino acid sequences and functional similarities (e.g. fingerprints of the characteristic 7TM domains) [65-67]. Here, GPCRs are categorized into six classes: Class A—rhodopsin-like receptors, Class B—secretin family, Class C—metabotropic glutamate receptors, Class D—fungal mating pheromone receptors (non-vertebrate receptors), Class E—cAMP receptors (non-vertebrate receptors, and Class F—frizzled (FZD) and smoothened (SMO) receptors [68, 69]. The difference between the GRAFS system and the A-F system is the further division of class B from the A-F system into the secretin and adhesion family in the first system based on preliminary findings that these two families evolved distinctly from each other [65].
From these, classes A and C receptor families comprise the relevant members involved in neurodegenerative diseases and neurological pathologies. These receptors also show a higher amount of relevant data regarding alternative signalling pathways through the formation of GPCR dimers.
1.2.2. Dimerization
For a long time, it was believed that the functional entity of GPCRs was monomeric: an extracellular signal, such as the binding of a ligand, would lead to conformational rearrangements within the protein so that the signal was further transmitted intracellularly via heterotrimeric G proteins, arrestin proteins and different downstream signalling cascades [70, 71]. This concept was then extended by findings that the receptors can also function as homo- and/or heterodimers or even higher-order oligomers with relevant biological value [72-74]. It was also reported that GPCRs can also form heterodimers with ionotropic receptors and receptor tyrosine kinases and henceforth modulate their function [76]. In addition, adaptor proteins were described to interact with receptor protomers, modulating their interactions [76]. Consequently, GPCR signalling is not only determined by conformational changes induced by ligand-binding, but also by interaction with other proteins [77], which diversifies and fine-tunes their signalling, rendering it a highly dynamic nature [77-80].
For instance, it was reported that the physiological consequences of GPCR-dimerization result in the modulation of downstream signalling, trafficking, and regulation as well as negative and positive cooperativity on ligand-binding [72, 80, 81]. Furthermore, allosteric dimerization between a monomer and another GPCR can influence ligand recognition by modulation of the orthosteric and allosteric binding sites. This can influence G protein-coupling and selectivity and may cause switching from G protein- to β-arrestin-coupling [80, 82]. Additionally, dimerization may lead to the appearance of novel allosteric sites that can again alter different pharmacological properties [82]. However, the structural basis behind such interactions is not fully understood yet.
While class C GPCRs function as dimers only, there is also evidence for the existence of homodimers, heterodimers, and/or higher-order oligomers in other GPCR classes through a variety of reports describing biophysical studies: single-molecule fluorescence-based approaches, X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, and cryogenic electron microscopy (cryo-EM) - as well as computational studies [83-89]. Furthermore, the knowledge about GPCR-dimers involved in pathological conditions increased in the last few years [72]. Such an impact has been reported for asthma, cardiac failure, preeclampsia, schizophrenia and PD [72]. Several studies have shown that GPCR heterodimers elicit a significant role in various diseases at different stages by regulating the pathological condition towards its progression, or modulating selective downstream signalling cascades [72]. It was already hypothesized that learning and memory occur at a molecular level by the reorganization of homo- and heterodimers in the postsynaptic membrane [76]. According to the authors Borroto-Escuela and Fuxe, disbalances of homo- and heterodimers are linked to diseases and targeting heterodimers represents a novel strategy for the treatment of brain disorders [76, 90].
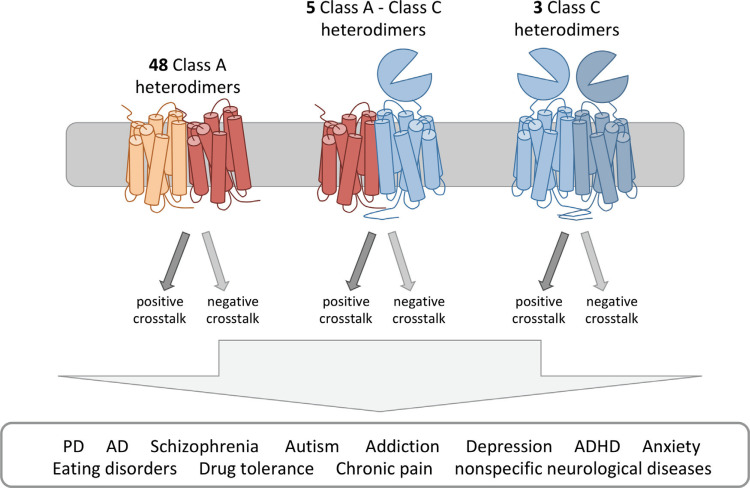
The understanding of the pharmacological and functional properties of GPCR classes A and C dimers can be crucial for the treatment of mental disorders and neurological conditions due to evidence suggesting that these macromolecular structures may play an important role. The large number of GPCRs and their ability to form different complexes, suggests the existence of a high number of possible GPCR heterodimers in the CNS. This also indicates that heterodimers constitute a unique signalling as such that different neurons with different heterodimers may respond differently to the same ligand [91]. Here, we review the latest advances in obtaining and understanding GPCR dimers (classes A and C) structure and function and, consequently, their role in neurodegenerative diseases. Listing of these complexes can be found in Table 1. Until now, 56 dimers were identified as expressed in the brain. Out of these, 48 were from class A-class A dimers, 3 from class C-class C dimers and 5 were class A-class C dimers (Fig. 1).
Table 1.
GPCR Dimers and potential roles in neurodegenerative diseases.
| Heterodimer | GPCR Class | Clinical Relevance | Crosstalk | References |
|---|---|---|---|---|
| DRD1-DRD2 | Class A | PD Schizophrenia Autism Addiction Depression |
Positive crosstalk | [461, 462, 464, 478, 486-488, 492, 495-497, 499, 501, 910, 911] |
| DRD1-DRD3 | Class A | PD | Positive crosstalk | [477, 504-510] |
| DRD2-DRD3 | Class A | PD Schizophrenia Autism ADHD |
Positive crosstalk | [484, 513-517, 910] |
| DRD2-DRD4 | Class A | PD | Positive crosstalk | [476, 521, 522] |
| DRD5-DRD2 | Class A | Depression | Positive crosstalk | [493, 523] |
| A1R-DRD1 | Class A | PD Schizophrenia Addiction |
Negative crosstalk | [459, 524, 525, 527, 529-532, 565, 912-914] |
| A2AR-DRD2 | Class A | PD Schizophrenia Addiction |
Negative crosstalk | [72, 461, 529, 533-542, 915-918] |
| A2AR-DRD3 | Class A | Schizophrenia | Negative crosstalk | [552] |
| DRD1-H3R | Class A | ADHD Schizophrenia Addiction | Positive crosstalk | [554-557] |
| DRD2-H3R | Class A | PD | Negative crosstalk | [558, 559] |
| DRD2-SST5R | Class A | Depression | Positive crosstalk | [561-563, 919] |
| DRD2-NTS1R | Class A | PD Schizophrenia |
Negative crosstalk | [564-569] |
| DRD2-TAA1R | Class A | Schizophrenia | Negative crosstalk | [313, 324, 570-572] |
| DRD2-OTR | Class A | Anxiety Autism |
Positive crosstalk | [573, 574, 910] |
| DRD2-GHS-R1a | Class A | Eating disorders | Negative crosstalk | [582, 920, 921] |
| A1R-A2AR | Class A | Drug tolerance | Negative crosstalk | [458, 560, 583, 585-588] |
| A1R-5-HT2AR | Class A | Schizophrenia Anxiety |
Negative crosstalk | [591, 592] |
| A2AR-H3R | Class A | PD | Negative crosstalk | [593, 922] |
| MOR-DOR | Class A | Chronic pain | Positive crosstalk | [601, 605-614, 923, 924] |
| MOR-KOR | Class A | Chronic pain | Positive crosstalk | [602, 604, 615] |
| MOR-α2AR | Class A | Addiction | Negative crosstalk | [604, 616-619, 925-928] |
| MOR-GPR139 | Class A | Chronic pain | Negative crosstalk | [604, 623] |
| MOR-V1BR | Class A | Chronic pain Morphine tolerance |
Positive crosstalk | [604, 624] |
| MOR-GAL1R | Class A | Chronic pain Addiction |
Positive crosstalk | [604, 625, 626] |
| MOR-CB1R | Class A | Chronic pain | Negative crosstalk | [604, 627, 629-633] |
| MOR-CCKBR | Class A | Chronic pain | Negative crosstalk | [604, 616] |
| MOR-CCR5 | Class A | Chronic pain | Negative crosstalk | [636, 637] |
| MOR-DRD1 | Class A | PD Addiction |
Negative crosstalk | [639] |
| MOR-DRD2 | Class A | Addiction | Negative crosstalk | [640-643] |
| 5-HT1Ar-5-HT2Ar | Class A | Depression | Negative crosstalk | [646-649] |
| 5-HT2A-5-HT2B | Class A | Addiction Depression |
Negative crosstalk | [651, 652, 655] |
| 5-HT2Ar-5-HT2Cr | Class A | Addiction Depression |
Negative crosstalk | [651, 652, 655, 929, 930] |
| 5-HT1Ar-5-HT7r | Class A | Depression Anxiety Schizophrenia Addiction |
Negative crosstalk | [656, 657, 659, 660] |
| 5-HT1AR-DRD2 | Class A | Schizophrenia | Positive crosstalk | [648, 661-665] |
| 5-HT2AR-DRD2 | Class A | Schizophrenia Autism |
Positive crosstalk | [565, 662, 663, 666, 910] |
| 5-HT1AR-GAL1R | Class A | Depression | Negative crosstalk | [668, 670, 672, 674, 675] |
| 5-HT2AR-OTR | Class A | Anxiety Autism Depression |
Negative crosstalk | [676, 910] |
| 5-HT2CR-OTR | Class A | Depression | Negative crosstalk | [681] |
| 5-HT2CR-MT2R | Class A | Depression Anxiety |
Positive crosstalk | [682-687] |
| 5-HT1Ar-MOR | Class A | Chronic pain | Positive crosstalk | [688] |
| CB1R-CB2R | Class A | AD PD |
Positive crosstalk | [687, 698-700] |
| CB1R-DRD1 | Class A | PD | Positive crosstalk | [707-710] |
| CB1R-DRD2 | Class A | PD Schizophrenia Addiction Autism |
Negative crosstalk | [704, 713-716, 718-720, 722, 910, 931] |
| CB1R-A2AR | Class A | Depression | Negative crosstalk | [723, 724, 726] |
| CB1R-5-HT2AR | Class A | Addiction Anxiety |
Positive crosstalk | [731, 738] |
| GAL1R-GAL2R | Class A | Depression Anxiety |
Positive crosstalk | [740, 741] |
| AT1R-AT2R | Class A | PD | Positive crosstalk | [742-744] |
| mGlu1R-mGlu5R | Class C | PD AD Schizophrenia Autism |
Unknow | [35, 826] |
| mGlu2R - mGlu4R | Class C | PD AD Schizophrenia |
Negative crosstalk | [824, 825, 932] |
| GABAB1R- GABAB2R | Class C | Nonspecific neurological diseases | Positive crosstalk | [806-811, 933] |
| DRD1-mGlu5R | Class A and C | PD | Positive crosstalk | [831] |
| A1R-mGlu1R | Class A and C | Schizophrenia | Negative crosstalk | [834-836] |
| 5-HT2AR-mGlu2R | Class A and C | Schizophrenia Autism |
Negative crosstalk | [838, 839, 841, 910, 934, 935] |
| MOR-mGlu5R | Class A and C | Chronic pain Addiction |
Negative crosstalk | [846, 847] |
| A2AR-CB1R-DRD2 | Class A | Schizophrenia | Negative crosstalk | [533, 704, 721, 722, 726, 848, 857-864] |
| A2AR-DRD2-mGlu5R | Class A and C | PD Schizophrenia Addiction Autism |
Negative crosstalk | [865, 866, 868, 910] |
Fig. (1).
Overview of neurodegenerative-relevant GPCR heterodimers of classes A and C. In the next sections, we describe brain-relevant class A GPCRs and known heterodimers followed by class C. A few examples of interclass heterodimers, comprising class A and class C as well as receptor mosaics will also be listed.
2. CLASS A G PROTEIN-COUPLED RECEPTORS
2.1. Class A Receptors in the Brain
The family of class A GPCRs, also referred to as rhodopsin receptors, consists of a very large and diverse group of receptors. They mediate signalling processes in all kinds of physiological actions such as cell communication, the senses of sight, smell and taste, sensory perception, chemotaxis and neurotransmission [71, 92]. In those processes, there is the involvement of a wide array of different ligands including light, peptides, lipids, proteins and small molecules such as biogenic amines, nucleotides and ions [71, 93]. The activation mechanism of class A GPCRs is the prime example for studying how monomeric GPCRs transduce extracellular signals into intracellular ones. All members of class A GPCRs share a sequence identity of more than 20% in their TM domains, so they are expected to have evolved from a common ancestor [94]. Hence, the growing number of structure-function studies and the increase in resolved crystal structures suggest that there are common structural and functional motifs responsible for the activation of this family of GPCRs [71, 95, 96]. In order to make the localization of such structural and functional motifs easy to compare between the different GPCR families, all GPCR residues are usually numbered according to the Ballesteros & Weinstein nomenclature [97]. Hereby, the first digit identifies the TM helix and the second digit identifies the position of the residues in relation to the most conserved residue in the TM helix, which is assigned the index number 50 (numbers decrease towards the N-terminus and increase towards the C-terminus) [71, 97]. As already summarized by Moreira [71] and Zhou et al. [98], the most important motifs are: (i) the interaction of the cytoplasmic “ionic lock” on TM3 with the consensus “(D/E)R(Y/M)” (3.49-3.51) with D/E (6.30) on TM6, which is disrupted when the receptor is activated [99-108]; (ii) the hydrophobic arginine cage around the conserved arginine (R3.50) of the DRY motif, which restrains its conformation in inactive state of the receptor consisting of two hydrophobic amino acids (such as L, V, I or M) on TM3 and TM6 (3.46, 6.37) [109-111]; (iii) the NPxxYxF motif on TM7, responsible for interaction of a tyrosine (7.53) on TM7 with the phenylalanine (7.60) on HX8 together with the side chain and backbone of an arginine on TM2 (2.40) via a water molecule [102, 112-123]; (iv) the Rotamer Toggle Switch, a coordinated change upon ligand coupling of aromatic residues in TM6 around a very conserved tryptophan (6.48) that leads to disruption of the ionic lock [110, 115, 124, 125]; (v) the CWxP motif, the cluster around the conserved tryptophan on TM6, which is part of the Rotamer Toggle Switch and also undergoes a conformational rearrangement upon activation from pointing towards TM7 in the inactive state to pointing towards TM5 in the active state [113, 120-128]; (vi) the PIF motif [97, 102, 129, 130], and (vii) the Na+-pocket [114, 120, 128, 131-137]. It is well established that the outward movement of TM6 upon ligand binding is another common feature of class A GPCR activation. However, at the residue level, the changes that trigger such a movement can be individual for each receptor subfamily as it requires a global rearrangement of residue contacts and water-mediated interactions [98, 108, 138, 139].
2.1.1. 5-Hydroxytryptamine Receptors
Serotonin, also called 5-hydroxytryptamine (5-HT), is an important neurotransmitter responsible for anxiety, aggressive behaviour, stress, blood pressure regulation, peristaltic movements, heart rate, and the coagulation system [140-142]. 5-HT activates the largest subfamily of class A GPCRs [143]. This family comprises many members: 5-HT1AR, 5-HT1BR, 5-HT1DR, 5-HT1eR, 5-HT1FR, 5-HT2AR, 5-HT2BR, 5-HT2CR, 5-HT4R, 5-HT5AR, 5-HT5bR, 5-HT6R, 5-HT7R [144]. Many of them, such as 5-HT1AR, 5-HT1DR, 5-HT1ER are drug targets of numerous disorders [145]. Currently, alterations in the serotoninergic neurotransmission and disturbances in the level of 5-HT have been described to be associated with migraine, epilepsy, PD, MS, ALS, ADHD and autism spectrum disorder (ASD) [140, 141, 146-150]. Especially for migraine, disturbances in the serotoninergic system are the hallmark of this disorder, which affects 11% of adults worldwide [140]. Chronically low 5-HT disposition due to malfunction of its biosynthesis leads to the development of migraine [140].
2.1.2. Adenosine Receptors
Adenosine receptors (AR) are another family of class A GPCRs that are activated by their endogenous ligand, adenosine [151]. The four members, A1R, A2AR, A2BR, A3R, have been considered potential targets for several disorders such as PD, schizophrenia, analgesia, ischemia and cancer [151, 152]. Some studies also reported the effects of adenosine on neuronal protection and neuronal viability as well as in inflammatory processes [153]. Combined effects may lead to considerations for ARs and possible roles in Lesch-Nyhan syndrome, Creutzfeldt-Jakob disease, Huntington's disease, PD and AD and multiple sclerosis, as well as the brain damage associated with stroke [152, 153].
2.1.3. Adrenoceptors
The noradrenergic system in the brain has the global function of neuronal modulation, controlling vigilance, attention, the sleep-wake cycle and to some extent also in learning and memory processes [154-157]. In addition, depression, anxiety and sensory information processing, such as pain or touch, mediated through the sympathetic nervous system, are processes regulated by noradrenaline and the neurohormone epinephrine through the noradrenergic system [154, 156-158]. All these ligands bind to the nine members of the adrenoceptor family, all expressed in the brain: α1AAR, α1BAR, α1DAR, α2AAR, α2BAR, α2CAR, β1-AR, β2-AR and the β3-AR [71, 158, 159]. The adrenoceptors are further classified into three subgroups: the α1 group, which comprises α1AAR, α1BAR and α1DAR since they couple to Gq; the α2 group containing α2AAR, α2BAR and α2CAR, in which all couple to Gi and the β group which consists of the β1-AR, β2-AR and the β3-AR, all able to couple to Gs. However, β2-AR and β3-AR also couple to Gi [160]. Disruption in the noradrenergic system was reported to be connected to a number of neurological diseases such as AD, epilepsy, ADHS, PD, depression, schizophrenia, and posttraumatic stress disorder [154].
2.1.4. Cannabinoid Receptors
The two cannabinoid receptors CB1R and CB2R together with their endogenous ligands, anandamide, 2-arachido- noylglycerol and other endocannabinoids, were discovered in the late 80s and resulted in a major effort in understanding the mechanisms and physiological roles of the endocannabinoid system (ECS) [144, 161]. The ECS regulates a variety of physiological processes such as appetite, mood, memory and pain sensation [162]. This complex system is also believed to play a neuroprotective role during traumatic brain injury, and may be part of a natural compensatory repair mechanism, relevant also during neurodegeneration [163-166]. The modulation of this new neuronal network has been proposed to target many neurological conditions, including epilepsy, cognitive deficits and neurodegenerative diseases [163, 167, 168]. While CB1R is mainly expressed in the brain, CB2R can be found in diverse parts of the immune system and partially in the brain [169-171]. Interestingly, CB1R is a promiscuous protein, able to couple to different G proteins, activate signalling pathways mediated by β-arrestins and signal from intracellular compartments, adding another level of complexity to this system [161, 172, 173]. Therefore, CB1R has an impact in brain disorders including basal ganglia disorders such as AD, MS and HD [168, 174].
The expression pattern of CB2R, contrasting to CB1R, is more defined and increased in microglia and macrophages of the central nervous system (CNS) [171, 175]. The CB2R is mainly associated with inflammation, and due to its selective localization, it is a promising target for AD and other basal ganglia disorders [168, 176-178].
2.1.5. Cholecystokinin Receptors
Cholecystokinin (CCK) is a gastrin-like peptide found in the brain and the gastrointestinal tract [179]. CCK triggers the signalling cascade by activating two GPCRs, CCK1R/CCKAR and CCK2R/CCKBR, also found in similar regions of the human body [144, 180]. The CCK2R has been associated with the neurobiology of anxiety and panic attacks since the 90s [181]. The CCK1R is mainly known as a physiologic mediator of pancreatic enzyme secretion and smooth muscle contraction of the gallbladder and stomach [182]. Yet, at minor levels, CCK1R is also present in different regions of the brain, where it mediates the anorectic action of CCK [183-185]. Besides this function, CCK1R also facilitates dopamine neurotransmission, regulates hypothalamic neurotransmitters, increases the excitability of the cortex and regulates endocrine secretions [182]. For instance, there is accumulating evidence that about 70% of PD patients have experienced diverse non-motor symptoms, most commonly gastrointestinal problems, before the onset of motor dysfunctions [186, 187]. Such findings suggest that neuropeptides derived from the gastrointestinal tract may be related to the onset of PD. This is further supported by the fact that CCK and several other neuropeptides are expressed in dopaminergic neurons of the substantia nigra, and galanin or opioid neuropeptides are also released from the hypothalamic neurons [186, 188, 189]. In PD patients or experimental models, significant changes in brain neuropeptides have already been observed [186].
2.1.6. Dopamine Receptors
The dopamine receptor family consists of five receptors (DRD1-DRD5) [190] which are divided into two subclasses (D1-like and D2-like) based on their coupling to G proteins. DRD1 and DRD5 couple to GS, olf and belong to the D1-like class, while DRD2, DRD3 and DRD4 couple to Gi/o and belong to the D2-like class [190-193]. Additionally, for DRD2 two splicing variants exist, DRD2long and the 29 amino acids shorter DRD2short [190]. While DRD2long is mostly located in the intracellular part, DRD2short is primarily found at the plasma membrane [194]. DRs are associated with many pathological conditions and mental disorders, most prominently PD, schizophrenia, Tourette’s syndrome, depression, bipolar disorder, hypertension, gastroparesis and nausea, as well as others [190, 193, 194].
2.1.7. Galanin Receptors
The neuropeptide galanin is widely found in the human brain and gastrointestinal tract and couples to three GPCRs: GAL1R, GAL2R and GAL3R [195]. In the past, several physiological effects were attributed to galanin signalling including smooth muscle contraction, inhibition of insulin release and stimulation of growth hormone release [196-198]. However, it was revealed that the galanin-like immunoreactivity in the CNS and peripheral nervous system (PNS) leads to the regulation of numerous biological processes such as learning and memory, neurogenesis and neuroprotection, seizure activity, pain threshold, neurotransmitter and hormone release and many more [198-209]. Consequently, the role of galanin in mood disorders has attracted a lot of interest [198, 202, 210]. Neurological disorders have also been linked to galanin signalling such as AD, epilepsy, depression, eating disorders and addiction [205, 211].
2.1.8. Histamine Receptors
The histamine receptor (HR) family comprises four members, H1R, H2R, H3R, H4R [212]. Histamine itself is known to be involved in local immune responses as well as regulating functions in the gastrointestinal tract [213]. For a long time, it has been considered as a local hormone, as it lacks the endocrine glands to secrete it, but it has now been recognized as neurotransmitter [213, 214]. The HRs exert diverse functions in the brain. Whereas H1R promotes wakefulness, nociception, endocrine homeostasis and appetite, the role of H2R has not been established yet, since most known ligands are unable to cross the blood-brain barrier in sufficient concentrations [212, 215-219]. The H3R is described as an “autoreceptor” with constitutive activity and decreases the release of histamine, acetylcholine, serotonin and norepinephrine [212, 220]. Lastly, H4R is not located in the brain, but rather in basophils and in the bone marrow [212]. Especially H1R and H3R orchestrate disparate behaviours and homoeostatic functions [218]. Recent evidence suggested that aberrant neuronal histamine signalling may also be a key factor in degenerative diseases such as PD, AD, sleep disturbance and MS, as well as in addictive behaviours [218, 221-223]. Moreover, the concentration of metabolites of histamine was shown to be increased in the cerebrospinal fluid of schizophrenia patients compared to normal patients [224, 225]. In addition, a decrease in the binding sites of H1R was observed in schizophrenia patients [224, 225].
2.1.9. Opioid Receptors
The oldest and most potent drugs used for the treatment of moderate-severe acute and chronic pain are opioids [226, 227]. Actions of opioids are mediated through opioid receptors (ORs), widely distributed across the skin, digestive tract, spinal cord and in the brain [70, 228-230]. There are four major classes of receptors: delta receptor (DOR), kappa receptor (KOR), mu receptor (MOR) and the NOP receptor [229, 231, 232]. ORs are activated by their endogenous opioid ligands that are released by neurons such as dynorphins, enkephalins, endorphins, endomorphins and nociceptin, but also by exogenous opiate drugs [233-239]. Since the ORs are all coupled to Gi proteins, their activation characteristically inhibits neuronal firing as well as neurotransmitter and hormone release [233, 240-243]. The opioid system plays an important role in hedonic homeostasis, mood and well-being, including a large number of sensory, motivational, emotional and cognitive functions and addictive behaviours [233, 244]. The ORs are also known to regulate peripheral functions, including endocrine, gastrointestinal, immune and respiratory functions and responses to stress [244]. Due to its main role in the control of pain, the opioid system is also associated with multiple adaptations in the nervous, endocrine and immune system which can lead to the development of pathologic, chronic pain [240, 245, 246]. In addition, ORs may play a pivotal role in the development of AD, since ORs are known to regulate the neurotransmitters acetylcholine, GABA, glutamate, norepinephrine and serotonin that have been implicated in the pathogenesis of AD [233].
2.1.10. Somatostatin Receptors
The peptide somatostatin (SST) consists of two bioactive forms, SST-14 and SST-28, produced in neuroendocrine cells in the periphery and in the brain that modulate cell secretion and proliferation as well as neurotransmission [247-250]. Five GPCRs, SST1R, SST2R, SST3R, SST4R and SST5R mediate the actions of SST which are variably expressed in the brain [248, 250, 251]. SST2R, SST3R, SST4R and SST5R undergo rapid endocytosis, induced by the binding of agonists, while SST1R does not internalize but is rather up-regulated when continuously exposed to agonists [252, 253]. The types of active SST isoforms, SST-14 and SST-28 vary in their distribution: SST-14 is more predominant in the CNS, whereas SST-28 is more abundant in peripheral organs [254, 255]. Both bind to the SSTR in nanomolar affinity. However, SST5R has a higher affinity for SST-28 over SST-14, while for the other SSTRs the contrary is true [256]. In the cortex, SST is a protein marker of inhibitory interneurons, as SST is expressed mainly in a subset of GABAergic neurons [254]. SST and SSTRs contribute to cortical processing and in the striatum SST-positive interneurons are able to co-release glutamate and GABA [254]. This co-release generates excitation-inhibition sequences in postsynaptic neurons, which is interpreted as the glutamatergic response and persists for a shorter time than a usual inhibitory response would [254, 257, 258]. The involvement of SSTR in neurodegenerative and neuropsychiatric disorders such as AD, OD, HD, bipolar disorder, schizophrenia and major depressive disorder (MDD) has been linked to a decrease in the amount of expressed SST [254].
2.1.11. Vasopressin and Oxytocin Receptors
Arginine-vasopressin, also known as antidiuretic hormone (ADH), and oxytocin (OT) are hormones derived from neurohypophysis. These are similar nonapeptides that differ only at residues 3 and 8 [259]. ADH is essential for cardiovascular homeostasis (water body balance), key for shock states [259, 260]. OT is also known as the “quick birth” hormone because it facilitates reproduction in vertebrates at several levels due to its uterine-contracting properties. This hormone is the one that responds to sexual activity and during labour where oxytocin controls the highly potent uterotonic activity, induces milk production and additionally induces the first onset of maternal behaviour [259-263]. The actions of ADH are mediated by tissue specific GPCRs and are known as V1 vascular (V1AR), V2 renal (V2R) and V3 pituitary (V1BR, previously known as V3R) [264-266]. The V1AR has been shown to be ubiquitously expressed in the brain [259] and therefore plays a role in many physiological functions including cell contraction and proliferation, platelet aggregation, liver glycogenolysis, vascular smooth muscle, aldosterone secretion by the adrenals and subserve neurotransmitter-like actions of ADH in the CNS [267-271]. Species-typical social behaviours (e.g., affiliative behaviour) in rodents and humans may be associated with the pattern of V1AR expression in the brain [272-275]. The V1BR mediates the release of ADH and beta-endorphin from the anterior pituitary through the mobilization of intracellular calcium by phosphatidylinositol hydrolysis [259, 276]. However, the receptor was also found in other organs including the adrenals, the brain and the pancreas [277-279]. In 2002, SSR149415, a V1BR-antagonist, was developed with antidepressant- and anxiolytic-like properties [259, 280]. Since then, it has been hypothesized that V1BR may play an important role in major depressive disorder (MDD) and chronic stress. In addition it has been shown that a small subset of MDD patients displays an impaired hypothalamus-pituitary-adrenal (HPA) axis function, which was also present in patients with treatment-resistant depression or severe depression [281-290]. This led to the assumption that V1BR-antagonists would improve the treatments of such conditions, and several selective and potent antagonists have been developed and their potential as antidepressants has been verified in animal models [290].
The main endocrine function of ADH, the facilitation of water reabsorption in the kidney through inhibition of the diuresis, is mediated by the V2R [259]. The deployment of ADH analogous (dDADH, desmopressin) as selective V2R-agonists has been successful for the treatment of central diabetes insipidus, patients suffering from hemophilia A and Von Willebrand’s disease, the most frequent congenital bleeding disorders [291-296]. In summary, the key function of the V2R is to regulate fluid homeostasis [297].
The last member of this family, the oxytocin receptor (OTR), is activated by the neurotransmitter oxytocin (OT) which regulates emotional, parental, affiliative and sexual behavioural functions, including mother-infant bonding [259, 298]. The OTR is expressed in the brain and body, especially in reproductive organs [298]. Also, the number of receptors varies in different periods of life such as birth and postpartum [298, 299]. In the brain, OT induces the suppression of GABAergic neurons [300, 301]. It has also been reported that OT has an anti-inflammatory effect, observable in wound healing and pain relief [302, 303]. Besides this function, anti-depressant effects have been described for OT [304, 305]. OT might also have anti-anxiety effects mediated by the HPA axis [306]. Recently, increased methylation levels in the OTR have been linked to obsessive-compulsive disorder (OCD) [307]. Another study demonstrated that substantial loss of hypothalamic oxytocin-producing neurons occurs in amyotrophic lateral sclerosis [308].
2.1.12. Trace Amine-associated Receptors
Trace amine-associated receptors (TAARs) were discovered in 2001 [309, 310] and are activated by a diverse group of aminergic compounds. In mammalian, the nine TAAR members are divided into two sub-families: TAA1-4R and TAA5-9R [311, 312]. In humans, there are six functional TAAR genes (TAA1R, TAA2R, TAA5R, TAA6R, TAA8R and TAA9R) and three pseudogenes (TAA3R, TAA4R and TAA7R) [312]. TAA1R is the most well-characterized member and a potential target for psychiatric disorders, such as schizophrenia [313] and drug abuse [314], as well as for metabolic disorders [315]. The endogenous trace amines p-tyramine, β-phenylethylamine, tryptamine and octopamine bind to TAARs [309, 316, 317], essentially to TAA1R and TAA4R, and they induce effects in CNS. For example, phenylethylamine acts as a postsynaptic neuromodulator of dopamine and noradrenaline neurotransmission [318]. Tryptamine potentiates neural responses to dopamine and causes an increased response to norepinephrine in cortical neurons [319]. Octopamine increases depressive and excitatory responses to norepinephrine in the rat cerebral cortex [320]. 3-Iodothyronamine may have a pro-learning anti-amnesia effect [319]. With the exception of TAA1R, all TAARs have been detected in olfactory sensory neurons [321]. TAA1R is coupled to Gs protein [309, 310], recruits the β-arrestin-2 cascade [322, 323] and increases the opening of inwardly rectifying K+-channels that have the characteristics of G protein-coupled inwardly-rectifying potassium channels (GirK) channels [324, 325]. All the other TAARs within the olfactory epithelium are coupled to Golf to regulate cAMP accumulation [326]. TAA5R is also coupled to Gs cascade [327], Gq/11 cascade and G12/13 dependent MAP kinase pathways [328]. In contrast, TAA8R is Gi-coupled [329]. The signal transduction events of TAA6R and TAA9R are still unknown.
2.1.13. Neurotensin Receptors
The central and peripheral effects of tridecapeptide neurotensin (NT) are mediated through interaction with three identified neurotensin receptors: NTS1, NTS2 and NTS3 (Sortilin 1) [330]. Whereas NTS1 and NTS2 receptors have seven transmembrane helices and are G protein-coupled, the Sortilin 1 receptor is a single transmembrane domain receptor [330]. NTS1R is found in the brain and intestine of rats and humans [331]. In the brain, the NTS1R is mainly found in neurons of the diagonal band of Broca, medial septal nucleus, nucleus basalis magnocellularis, suprachiasmatic nucleus, supramammillary area, substantia nigra and ventral tegmental area, as well as in the small dorsal root ganglion neurons of the spinal cord [332, 333]. NTS2R is mostly expressed in brain [334-336] and mainly localized in the olfactory system, the cerebral and cerebellar cortices, the hippocampal formation and selective hypothalamic nuclei of the mouse [337] and rat [338] brain. NTS1R are Gq-coupled [330, 339, 340], but some other studies demonstrated that NTS1R are also Gi/o and Gs-coupled [330, 341-343]. In contrast, signal transduction of NTS2R receptors is still unclear. The role of neurotensin and its receptors is related to analgesic effects, which could be an alternative to opioids [344-346].
2.1.14. Angiotensin Receptors
The actions of angiotensin II, which is an important peptide hormone in the renin-angiotensin-aldosterone system (RAAS), are mediated through angiotensin receptors AT1R and AT2R [347-349]. The RAAS system involves different peptides and proteins with opposing effects in order to function [350]. On one hand, vasoconstrictive, pro-inflammatory and pro-proliferative are mediated by angiotensin II, AT1R and angiotensin-converting enzyme (ACE), while on the other hand, cardio-protective effects are mediated by Ang(1-7), AT2R and ACE2 [350]. However, angiotensin II displays ubiquitous actions by activation of different pathways by the binding to AT1R and AT2R in order to initiate the RAAS system or to further get cleaved into shorter peptides such as Ang IV, Ang(1-7) and almandine [350-353]. Besides, angiotensin II, angiotensin I and angiotensin III are endogenous ligands of ATRs [347]. The AT1R is clinically relevant as it is targeted by a large class of sartans, AT1R blockers [347]. The AT1R is mainly expressed in the brain, heart, blood vessels, lungs and kidneys [354, 355] and is known to bind to Gq/11, Gi/o proteins, G12 and G13 proteins as well as tyrosine kinases [350, 356]. Functions involving AT1R are cardiac hypertrophy, vasoconstriction, aldosterone synthesis and secretion, increased vasopressin secretion, decreased renal blood flow and renin inhibition, central and peripheral sympathetic nervous system activity and osmocontrol [357]. In the brain, AT1R antagonists were shown to reduce fear memory recall in mice [358, 359].
AT2R was shown in in vitro and in vivo studies to counterbalance the effect of AT1R, however, this is still speculative [349, 350, 352, 360-362]. AT2R are highly expressed in fetus and neonates and induce fetal tissue development, and so, although controversially, it is assumed they are involved in vascular growth [363, 364]. However, some studies could show that AT2R was upregulated after vascular injury, cardiac failure, myocardial infarction or wound healing, suggesting that this possibly reflects the re-activation of this fetal genetic programme [349, 352, 365, 366]. The expression of AT2R in humans is therefore developmentally regulated. In adults, AT2R is expressed in lower density in the adrenal medulla, brain and reproductive tissues [363, 364]. AT2R expression in the cerebellum has been associated with inhibition of cell growth differentiation, neuronal regeneration and ventricular hypertrophy [367]. Also, it was suggested that AT2R-mediated effects in other tissues require the local conversion of angiotensin II to II [368-370]. The downstream signalling transduction of the AT2R is poorly understood. It is known that the receptors possess important structural motifs which are typical for class A GPCR activation; however, several modalities can result in AT2R activation [371-388].
The existence of AT3R and AT4R was also proven, but only AT4R remained to be relevant [389, 390]. AT4R was shown to be the mammalian selective receptor for angiotensin IV (Ang3.8) as well as a receptor for insulin-regulated membrane aminopeptidase [391-394]. It has been proposed that the AT4R may be relevant in the regulation of the extracellular matrix of the CNS and modulation of oxytocin release [391, 395-399].
2.1.15. Growth Hormone Secretagogue Receptors
The Growth Hormone Secretagogue Receptor (GHS-R) is a GPCR that binds growth hormone secretagogues (GHSs), like ghrelin. GHS-R is Gq and Gs-coupled and the binding of ghrelin or synthetic peptidyl and non-peptidyl ghrelin mimetic agents leads to increased intracellular calcium content [400, 401]. GHS-R and its ligand ghrelin have special influence on food intake, gut motility, sleep, memory, behaviour, lipid and glucose metabolism, and cardiovascular effects [402]. GHS-R is expressed by growth hormone-releasing hormone (GHRH) neurons in the pituitary [403], but also in hypothalamus, pancreas, adipose tissue, immune cells and cardiovascular system [404, 405]. GHS-R has two isoforms, GHS-R1a and GHS-R1b, but only GHSR1a transduces ghrelin signalling by binding the active form of ghrelin [406]. GHS-R1a agonist and antagonist revealed to have benefits in cancer, cachexia [407-409], aging related cognitive decline [410, 411], obesity [412] and diabetes [413-415].
2.1.16. Melatonin Receptors
The melatonin receptors MT1R and MT2R are expressed in several areas in the human body such as brain, retina, cardiovascular system, organs or skin are activated by their endogenous ligand melatonin [416-420]. An additional MT3R has been identified in birds and amphibians [420]. MT3R was later identified in humans as a cytoplasmic enzyme, involved in the detoxification by reduction of quinones and also bound with low affinity to melatonin [421, 422]. Melatonin is a hormone mainly produced in a circadian rhythm in the pineal gland, with low levels during the day and high levels at night [418, 423-425]. This circadian secretion was found to be regulated by the suprachiasmatic nucleus (SCN) in a negative feedback-loop by melatonin binding to MT1R and MT2R, which then decreases SCN firing [426]. Melatonin is mainly known as a sleep promoter and regulator of circadian rhythms. Still, more effects such as antioxidants, reproduction-stimulation, analgesic and suppression of tumours have been attributed to it [420, 427].
It has been identified that the sleep-promoting effects of melatonin are mainly regulated by MT1R [428]. MT1R was also shown to be involved in adaptation to the light/dark-circle, phase-shifting activity and prolactin secretion [420, 428]. MT1R and also MT2R exert their signals by binding to Gi/o proteins [429]. However, they are also able to bind to other G proteins such as Gq and soluble guanylate cyclases [418, 428-430]. In contrast to MT1R, MT2R was shown to regulate a variety of functions in the body. It is known that melatonin inhibits through MT2R the Ca2+-dependent release of dopamine in the retina [431] as well as light-dependent phagocytosis and photopigment disc shedding [432]. MT2R was also shown to be expressed in a higher amount on differentiating osteoblasts [433].
In many studies, melatonin improved the treatment of PD, AD, alcoholism, depression or traumatic brain injuries [416, 434, 435]. For instance, addictive behaviours have been associated with an increased MTR-related cAMP concentration in the mesolimbic dopaminergic system [420]. Mostly, melatonin is used as a treatment for different types of insomnia, jet lag or shift work due to its sleep-promoting function [426]. MT1R and MT2R were also found to exist as homo- and heterodimers in vivo and in vitro [419, 436-438]. In mice rod photoreceptors, in vivo melatonin mediated the light sensitivity by formation of heterodimers, which led to heterodimer-specific activation of phospholipase C and protein kinase C [438]. This effect was abolished in MT1R KO mice, MT2R KO mice and in mice overexpressing a non-functional mutant of MT2R that also interfered with the formation of functional heterodimers [438].
2.1.17. Orphan Class A Receptors
The orphan receptor GPR139 was first discovered in 2002 [439], further curated in full-length in 2005 and classified into the class A GPCR family, right next to its closest relative, GPR142 [440-442]. As GPR139 is still considered an orphan receptor, a precise function remains to be determined. However, some reports suggest a role for GPR139 in locomotor activity, metabolism, alcohol addiction and hyperalgesia and phenylketonuria [443]. Lastly, genetic analysis has linked GPR139 to depression, schizophrenia and ADHD [443-448].
2.2. Class A Receptor Heterodimers
While class C GPCRs are obligate dimers, for a long time it was not clear if class A GPCR was able to dimerize, and what was the importance of such macromolecular structures. However, as GPCRs exhibit a high tendency to aggregate, some authors raised the question: What are the criteria for a minimal functional unit? [449, 450]. Indeed, for example, it was found for the 5-HT4R that two monomers were associated with one G protein [451]. In this case, one 5-HT4R was enough to simulate the G protein, but positive receptor crosstalk was observed upon co-activation, leading to the conclusion that 5-HT4R would rather function as homodimers [450]. This was also the case for the DRD2. In a study by Han et al. 2009 [452] it was shown that the maximal activity of the DRD2 was achieved upon agonist-binding to one monomer but was modulated by the constitutive activity of the second monomer, indicating asymmetric functional interaction [450]. Hence, the minimal functional unit of class A receptors, which can either be a monomer or a homodimer, appears to be receptor-dependent [73]. In addition to these findings, heterodimers have been intensively studied using cotransfected cells in biochemical, biophysical and pharmacological experiments with wildtype or often also using mutant receptors [453-455].
Since the family of class A GPCRs comprises many receptor subfamilies such as dopamine, adenosine or serotonin receptors that mediate diverse functions in the human body transduced by only one endogenous ligand, it becomes patent that heterodimerization is indeed also required for this GPCR class [456, 457]. Many prominent examples have been intensively studied, such as the A1R-A2AR complex, which is able to couple to Gi at low concentrations of adenosine and to Gs at high concentrations [458-460]. Another example is the DRD1-DRD2, which couples to Gq, whereas as monomers, the DRD1 and DRD2 couple to Gs or Gi, respectively [461-464]. Lastly, the finding that opioid receptors are also able to form heterodimers resolved many questions about atypical behaviour of targeting drugs, which apparently were selective for such heterodimers [465-472].
However, the idea of dimerization/oligomerization of GPCRs for neurotransmitters was already formulated by Fuxe et al. in the 80s [457, 473-475]. Since then and until 2014 the number of protein-protein interactions between GPCRs was found to be 537, according to Borroto-Escuela et al., indicating that class A GPCR dimers are an important and relevant discovery [476].
2.2.1. Dopamine - Dopamine Receptor Heterodimers
The five members of the dopamine receptor family are known to form dimers among their family and with other class A GPCRs [477-480]. Besides homodimers DRD2-DRD2 [78, 481], DRD3-DRD3 [482], DRD4-DRD4 [480], also many heterodimer combinations were identified such as DRD5-DRD2 [483], DRD1-DRD2 [478], DRD1-DRD3 [477] or DRD2-DRD3 [484]. More combinations were reviewed in Schiedel et al. [80], displaying the dopamine signalling heterogeneity [479, 485].
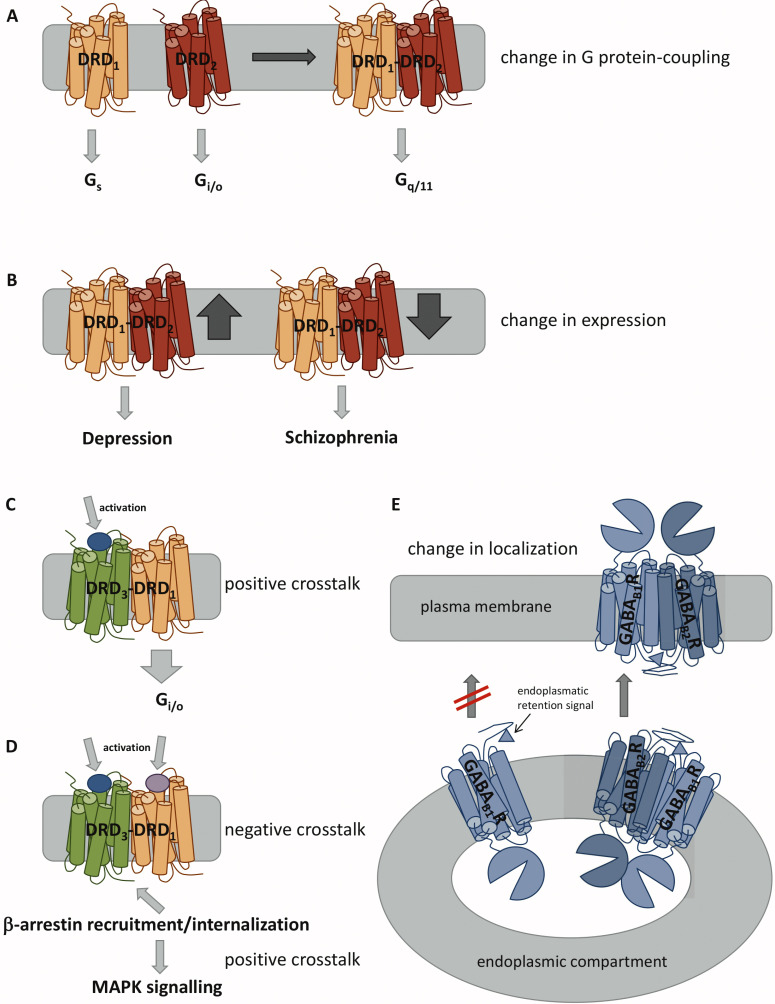
DRD1 and DRD2 receptors are mainly expressed in the dorsal (caudate-putamen) and ventral striatum (nucleus accumbens, NAc) areas [486]. DRD1-DRD2 was discovered using co-immunoprecipitation (Co-IP) and confocal Förster-Resonance-Energy-Transfer (FRET) experiments performed in brain tissues [464, 478, 487] and later by protein complementation studies [488]. More recent studies demonstrated the existence of the heterodimer in the dorsal striatum and NAc of mammalian species, including mouse, rat, nonhuman primate, and human, with a higher extent in the ventral than in the dorsal striatum [489-491]. In 2020, a study showed that the heterodimer is also found in cortical brain regions, such as piriform, medial prefrontal, and orbitofrontal, and claustrum, amygdala, and lateral habenula [492]. Many studies using signalling assays were able to show that the heterodimer formation might induce a change in the pattern of G protein-coupling (Fig. 2A) [461, 487, 491, 493]. Monomeric DRD1 couples to Gs and DRD2 to Gi/o, but DRD1-DRD2 was found to be associated with Gq/11 and activate the phospholipase C cascade in the striatum [464]. However, in order to conduct such actions and subsequent intracellular Ca2+ release, the specific DRD1 agonist SKF83959 had to bind to both receptors: it acted as a full agonist at DRD1 and high-affinity partial agonist for a pertussis toxin-resistant at DRD2 [464]. Furthermore, the intracellular calcium increase was associated with an increase in striatal calcium/calmodulin kinase IIa (CaMKIIa) phosphorylation [494]. The DRD1-DRD2 was reported to be upregulated in individuals suffering from depression [495, 496], while it was diminished in schizophrenia patients (Fig. 2B) [497]. In striatal neurons, the DRD1-DRD2 heterodimer activity resulted in rapid activation of cytosolic and nuclear CaMKII with an increase in brain-derived neurotrophic factor (BDNF) expression, which was the first evidence by then, linking dopamine receptors and endogenous GPCR heterodimers to neuronal maturation [462].
Fig. (2).
Possible modulations upon GPCR dimer formation. (A) Heterodimerization can induce a change of G protein-coupling. (B) Different expression levels of heterodimers are associated with distinct diseases. (C) Activation of one receptor can promote signalling of the other receptor via positive crosstalk. (D) Activation of both receptors can lead to β-arrestin recruitment and internalization via negative crosstalk. This can lead to intracellular signalling via mitogen-activated protein kinase (MAPK). (E) Dimerization can be necessary for plasma membrane localization, e.g., by masking an endoplasmic retention signal, which will prevent the transport to the plasma membrane as monomers.
Regarding the potential interface of the DRD1-DRD2, a comprehensive study by O’Dowd et al. [498] showed that it involves a pair of adjacent glutamic acids in the C-terminus of the DRD1 and a pair of adjacent arginine residues in ICL3 of the DRD2, oppositely charged residues, able to form stable electrostatic interactions [461, 498]. When SKF83959, which apparently is an agonist to the DRD1-DRD2, was administered to rats, activation of the heterodimer generated aversion in conditioned place preference studies, while disruption of it was rather rewarding [461]. Schizophrenia is known to be associated with hyperdopaminergia in subcortical dopamine projections [461]. Compared to globus pallidus tissue from normal subjects, the number of agonist-detected high-affinity state DRD1-DRD2 was found to be increased in globus pallidus tissue of schizophrenia patients [461]. According to George et al., these findings possibly reflect the hyperdopaminergic state associated with schizophrenia, similarly to what was observed upon amphetamine administration [461, 486].
A recent study revealed that genetic variations of DRD2 (Val96Ala, Pro310Ser, and Ser311Cys) affect the heterodimerization between DRD1 and DRD2 [478]. In addition, the Ser311Cys variant seems to be a risk factor in schizophrenia [499] and shows a better response to the schizophrenia treatment [500]. Once this DRD2 variant forms less heterodimeric interactions with DRD1 than DRD2 native, targeting the DRD1-DRD2 heterodimer under excessive dopaminergic firing will result in antipsychotic actions, with minimal side effects [478]. Another recent study showed that DRD1-DRD2 heterodimers play a role in cocaine dependence [501] and repeated cocaine administration in rats increases DRD1-DRD2 heterodimer expression [491]. The cocaine-induced biochemical changes, such as accumulation of ΔFosB, phosphorylation of extracellular signal-regulated kinases (ERK), and phosphorylation of Thr34-DARPP-32 in NAc are blocked by heterodimer activation [501]. Similar to what happens with cocaine, heterodimer expression is also increased after chronic administration of Δ-tetrahydrocannabinol (THC) in rhesus monkeys [491]. Consequently, the DRD1-DRD2 heterodimer would also be a good pharmacological target in cannabis use disorder (CUD) and the THC-induced changes in dopamine signalling are also implicated in behavioural despair disorders [491, 492, 502, 503].
Another dopamine receptor heterodimer, DRD1-DRD3 was also found to be expressed in the ventromedial striatum by FRET and bioluminescence resonance energy transfer (BRET) techniques [477, 504-507]. One of the first studies about DRD1-DRD3 heterodimer’s mechanism, reported in 2008 that DRD3 activation amplified DRD1-mediated AC signalling in the DRD1-DRD3 heterodimer (Fig. 2C) [507]. However, in 2014, Ferré and co-workers reported that co-activation of both receptors had antagonistic effects at the level of the AC, due to DRD3-mediated inhibition [504]. Therefore, co-activation of both receptors led to the canonical negative interaction at the level of AC signalling, the recruitment of β-arrestin-1 and selective activation of MAPK signalling, which was mediated by a G protein-independent mechanism (Fig. 2D) [504, 505]. Furthermore, this positive crosstalk through β-arrestin-1 recruitment and MAPK signalling, induced by DRD3 and DRD1 agonists, respectively, was counteracted by DRD1 and DRD3 antagonists. Moreover, the DRD1-DRD3 heterodimer was implicated in L-DOPA-induced dyskinesia [504, 508-510]. Some studies reported that DRD1 super-sensitivity during L-Dopa induced dyskinesia was accompanied by DRD3 up-regulation [508-510], and mice with DRD3 knockout displayed reduced L-Dopa-induced dyskinesia [510, 511]. In vitro studies performed by Cortés and colleagues using transfected human embryonic kidney 293 (HEK293) cells [504] and in vivo studies conducted by Bishop and colleagues (using hemi-parkinsonian rats) [509] demonstrated that DRD1-DRD3 heterodimers influenced the cooperative effect of both receptors in L-Dopa-induced dyskinesia. The co-activation with the DRD1 and DRD3 agonists SKF38393 and PD128907, respectively, generated an exacerbated dyskinetic effect, and an increase of downstream signalling of ERK phosphorylation, which is specific to dyskinesia as general locomotor effects or pERK were not observed in non-responders [509].
In 2001, evidence based on Co-IP studies at cultured cells pointed DRD2 and DRD3 heterodimerization [484]. DRD2 and DRD3 were found to colocalize on dopaminergic neurons as autoreceptors and at postsynaptic loci to dopaminergic projections in the globus pallidus, nucleus accumbens and in the frontal cortex on pyramidal cells and/or GABAergic interneurons [512, 513]. In a study by Maggio and colleagues, [514], it was shown that some antiparkinsonian agents (pramipexole and ropinirole) with a preference for DRD3, displayed amplified potency at DRD2-DRD3 heterodimers. In COS-7 cells cotransfected with DRD2 and DRD3, together with a chimeric AC AC-V/VI, these same agents were able to suppress forskolin (FK)-stimulated cAMP production with higher potencies as compared to cells only transfected with DRD2 or DRD3 receptors and without exposure to the ligands [514, 515]. Furthermore, the binding of this heterodimer may be responsible for the antipsychotic actions of DRD2 partial agonists and DRD3 agonists, such as aripiprazole and N-desmethylclozapine [514, 516]. The characterization of the pharmacological properties of the DRD2-DRD3 heterodimer by Novi and co-workers [516] showed that the agonist quinpirole potently suppresses FK-induced cAMP accumulation in recombinant cell lines transfected with DRD2 receptors and AC-V/VI, while the partial agonists aripiprazole, S33592, bifeprunox, NDMC, and preclamol less strongly reduce FK-stimulated cAMP accumulation. On the other hand, all these compounds failed to modify FK-induced cAMP accumulation in cells transfected with DRD3 and the chimeric DRD3-insensitive AC-V/VI [516]. However, in cells transfected with DRD2 and an excess of DRD3, together with AC-V/VI, quinpirole diminished FK-induced cAMP accumulation with a potency and efficacy comparable to cells transfected solely with DRD2, and the partial agonists were inactive [516]. These results suggest that an excess of DRD3 receptors can modify the functional status of DRD2 receptors, since partial agonists of DRD2 are transformed into antagonists at the DRD2-DRD3 heterodimers [514, 517]. Thus, this could justify the low incidence of extrapyramidal side effects of the partial agonists, as the extent of the DRD2-DRD3 heterodimer formation is low in the dorsal striatum [514, 517]. Knowledge about the structure and action mechanism of this heterodimer provides insights into cellular processes associated with diseases such as schizophrenia, PD, and ADHD.
DRD4 is also expressed in the brain, but its expression is lower than other types of dopamine receptors [518, 519]. However, human DRD4 has polymorphic variants [520] that are more abundant: DRD4.2, DRD4.4 and DRD4.7 [476]. DRD2 and DRD4 receptors partially co-distribute in the dorsal striatum and appear to play a fundamental role in complex behaviours and motor function. In 2011, based on BRET and in situ proximity ligation assay (PLA) in cotransfected HEK293T cells showed the coupling between DRD2 and DRD4 [476]. Specifically, they showed that the long form of human DRD2 (DRD2long) was able to interact and form heterodimers with the three human DRD4 isoforms, with the DRD4.7 variant being the least effective [476]. Upon co-activation by the DRD4 agonist PD168077, DRD2 agonist-induced ERK phosphorylation was enhanced in cells co-expressing DRD2 with DRD4.2 and DRD4.4, but not in cells co-expressing DRD2long with DRD4.7 [476]. The DRD4.7 variant showed reduced ability to form a heterodimer with DRD2long, as no additive effect was observed after combined treatment with DRD2 and DRD4 agonists (quinerolane and PD, respectively) on MAPK activity when these receptors were expressed together [476]. Furthermore, the short form of DRD2 (DRD2short) was reported to form heterodimer complexes with DRD4.2 and DRD4.4, while the DRD4.7 failed to interact with DRD2short in BRET studies, using cotransfected HEK293T cells [476]. So, the biochemical crosstalk between DRD2short and cotransfected DRD4 variants potentiates DRD4-mediated MAPK activation and ERK phosphorylation by DRD2 and not the inverse [521]. This biochemical crosstalk was not observed in striatal slices taken from gene knock-in mice carrying the human DRD4.7, confirming that DRD2 and DRD4.7 do not form heterodimers [521]. Solely DRD2-DRD4.2 and DRD2-DRD4.4 heterodimers exist in the striatum and they may be a potential target for antiparkinsonian drugs [522].
Finally, O’Dowd and colleagues also demonstrated the existence of the DRD5-DRD2 heterodimer in HEK293T cells co-expressing both receptors, through FRET analyses [493]. The authors reported that co-activation of both receptors of the DRD5-DRD2 heterodimer resulted in the generation of a calcium signal [493]. DRD5 was able to activate a strong calcium signal when it was expressed alone. These calcium signals resulting from activation of DRD5 alone or within a heterodimer require Gq/11 and PLC activity and the presence of extracellular calcium [493]. However, DRD5 and DRD2 heterodimerization negatively modified the functional unit of calcium signalling, attenuating the ability of the DRD5 receptor to trigger a calcium signal. DRD5 and DRD2 receptors have been shown to cooperate functionally to facilitate motor activity and striatal long-term depression [523].
2.2.2. Dopamine - Adenosine Receptor Heterodimers
Besides neuronal dopaminergic transmission regulation through different heterodimers compositions, dopamine can also be regulated by adenosine. According to George et al., two mechanisms of adenosine receptor-mediated neuromodulation of dopamine exist in cells: (i) adenosine counteracts cyclic adenosine monophosphate (cAMP) levels, which are modulated by dopamine; (ii) adenosine-dopamine receptor dimers exert a different signal then when they are activated as monomers [461].
Co-expression of adenosine and dopamine receptors in different basal ganglia pathways and pathways that control motor behaviour, underlined that different heterodimers exist in neuronal subpopulations [461]. In 2000, Gines and co-workers showed the existence of A1R-DRD1 heterodimer, using Co-IP in cotransfected fibroblast cells and cortical neurons in culture [524]. The expression of the A1R-DRD1 heterodimer in the brain was demonstrated by Franco and co-workers using FRET and BRET techniques [525]. A1R and DRD1 were found to colocalize in soma and dendritic regions of cortical neurons [526, 527]. One of the first pieces of evidence found was that A1R agonists can reduce oral dyskinesias induced by levodopa in rabbits [528]. Adenosine agonists inhibited the motor responses of dopamine in basal ganglia and vice-versa, suggesting their functional antagonist action [529]. While DRD1 is predominantly coupled to Gs protein, which in turns stimulates AC, A1R is coupled to Gi/o protein, which has inhibitory effects [528]. A1R antagonist 1, 3-dipropyl-8-cyclopentylxanthine leads to an increase in the DRD1-induced cAMP response, which can be related to their regulation of G proteins having offsetting activities [530]. Thus, co-activation of A1R-DRD1 heterodimer induces a decrease in the affinity of DRD1 for agonist and, consequently, decrease of the DRD1-induced cAMP accumulation [524, 530]. Kalivas and co-workers demonstrated that A1R-DRD1 heterodimer can also be involved in the pathophysiology of addiction [531]. They reported that cocaine, a potent stimulant of the CNS, targets the A1R-DRD1 heterodimer in rat nucleus accumbens, inhibiting the physical interaction between A1R and DRD1 [531]. This evidence emphasizes the therapeutic relevance of this heterodimer for cocaine addiction. Moreover, a recent study demonstrated the existence of A1R-DRD1 heterodimers in the spinal motoneuron, using PLA experiments and that adenosine tonically inhibited DRD1-mediated signalling in the spinal motoneuron [532]. Given the importance of controlling motoneuron excitability, the A1R-DRD1 heterodimer may also be a potential target for the treatment of spinal cord injury, motor aging-associated disorders, and restless legs syndrome.
A2AR-DRD2 heterodimer was among the first heterodimers reported, involving two different neurotransmitters [461, 533, 534]. The existence of A2AR-DRD2 was proven by Co-IP, BRET and FRET analyses [535, 536]. Later on, PLA studies located the A2AR-DRD2 in the mice striatum [537, 538]. A functional association between A2AR and DRD2 was also reported to exhibit a negative allosteric cooperativity in which the activation of the A2AR by CGS21680 (A2AR agonist) leads to a decrease of DRD2 of dopamine binding affinity [533, 539-541]. Furthermore, the activation of A2AR was shown to decrease the coupling of DRD2 to its Gi/o protein and stimulation of DRD2 was shown to decrease the coupling of A2AR to its Gs protein [534, 542]. The effect of the A2AR-DRD2 heterodimer on ligand binding of the monomers and G protein-coupling was also associated with cross-desensitization mechanisms, which function via agonist-induced coaggregation and co-internalization of both receptors [534]. The A2AR-DRD2 is also a promising candidate target for the treatment of PD, schizophrenia and addiction [529, 535, 542, 543]. For instance, the A2AR-DRD2 has been considered a potential target to reduce L-DOPA-induced dyskinesia in PD treatment [72, 544]. Behavioural and microdialysis experiments in mouse, rat, dog and human models suggested a mechanism that involves a co-expression of A2AR-DRD2 in striatopallidal GABAergic neurons and nucleus accumbens [545-548]. Consequently, selective and potent A2AR antagonists are able to reduce DRD2-dependent signalling in these areas and enhance therapeutic effects, as was demonstrated in animal models of PD [540, 549-551].
Indications that DRD3 can heterodimerize with A2AR emerged in 2005, based on confocal microscopy and FRET studies using transiently cotransfected HeLa cells [552]. Results from confocal microscopy showed that A2AR and DRD3 colocalize in the plasma membrane, and results from FRET experiments showed that A2AR and DRD3 receptors could form heterodimers in the transiently cotransfected HeLa cells [552]. Also, saturation analysis of [3H]dopamine binding in the A2AR-DRD3, a CHO cell line was generated, indicating that A2AR agonist CGS-21680 is able to significantly reduce the affinity of the high affinity binding state of the DRD3 receptors for dopamine [552]. Moreover, A2A and DRD3 receptors seem to interact at the G protein coupling level since the CGS-21680 A2AR agonist fully counteracted the dopamine mediated strong inhibition of forskolin-induced cAMP accumulation. So, when both receptors are co-expressed in the same cells, the antagonistic interaction of A2AR-DRD3 is verified, that is, A2A receptors antagonistically modulate both, the affinity and signalling of DRD3 receptors [552]. Since DRD3 is involved in the treatment of schizophrenia, the DRD3-AA2R receptor interactions could provide an alternative antischizophrenic treatment.
2.2.3. Dopamine Receptor and Other GPCR Heterodimers
Besides the intensive relationship between dopamine and adenosine receptors, DRs may also form heterodimers with GPCRs from other families. For instance, H3R is found in striatal medium spiny neuron that expresses post-synaptic DRD1 and obtains histaminergic input from hypothalamic asynaptic varicosities [553]. The receptors were then shown to form DRD1-H3R heterodimers by BRET and binding assays in transiently transfected human embryonic cells [554], Co-IP experiments in rats [555] and PLA studies in mice striatum [556]. Upon DRD1 and H3R receptors activation by their respective agonists (SKF 38393 and (R)-α-methylhistamine (RAMH)), DRD1 and H3R lead to the coupling to the Gi/o protein and MAPK cascades, respectively [554]. The unique biochemical function of this heterodimer is supported by the fact that, when each receptor is activated alone, DRD1 leads to the coupling to the Gs/olf protein, while H3R does not signal through the MAPK pathway, and they are unable to induce ERK1/2 phosphorylation in mice with either receptor knockout [554, 555, 557]. In addition, DRD1 and H3R antagonists, such as SCH 23390 and thioperamide, can block the distinct signalling mediated by the heterodimer [554]. An antagonist of one of the receptor units in the DRD1-H3R heterodimer is able to induce conformational changes in the other receptor and block specific signals originating in the heterodimer [554]. One of the last studies on this heterodimer, performed in rats and mice, reported that cocaine inhibited the bidirectional cross antagonism and the inhibitory effect of the DRD1 and H3R signalling [556]. McCormick and co-workers reported that 𝞂1R binds DRD1-H3R heterodimers in transfected cells and in mouse and rat striatum. Authors also postulated that cocaine, a 𝞂1R agonist, modifies the structure and counteract the biochemical properties of the DRD1-H3R heterodimer, such as heterodimer signalling through Gi protein, the ability of H3R activation to signal through MAPK, and the ability of H3R ligands to inhibit the effects of DRD1-mediated signalling, including cell death [556]. They also reported that blockade of H3R-mediated inhibition of DRD1 function in the 𝞂1R-DRD1-H3R complexes plays a key role in the effects of cocaine [556]. So, 𝞂1R-DRD1-H3R may be a new target for the treatment of cocaine abuse.
Besides the DRD1, also DRD2 was found to form a heterodimer with H3R, which was discovered by Ferrada and co-workers in 2008 using BRET in cotransfected HEK293 cells [558]. Heterodimerization of DRD2 and H3R was also demonstrated in vivo by Moreno and co-workers, using Co-IP studies in rat striatal tissues [555]. DRD2 and H3R can colocalize in GABAergic striatal efferent neurons and in specific DRD2-expressing GABAergic enkephalinergic neurons [559]. The study by Ferrada and co-workers reported the existence of behaviourally significant antagonistic postsynaptic interactions [560] between H3R and DRD2 receptors in reserpinized mouse model [558]. Whereby the stimulation of the H3R significantly decreased the ability of agonists to bind to the DRD2, while antagonists were unaffected [558]. Thus, this heterodimer may play a role in the function of the GABAergic enkephalinergic neuron [558]. Beyond Parkinson's disease, a therapeutic approach based on H3R receptor-mediated negative modulation of DRD2 receptor function may emerge and play a role in disorders involving the cortico-striatal-thalamo-cortical circuits, such as Huntington’s disease, Tourette syndrome, obsessive-compulsive disorder, schizophrenia and addiction [558].
DRD2 was also found to colocalize with SST5R in transfected HEK293 cells, using FRET [561]. The heterodimerization of both receptors was promoted by application of antidepressant drugs (desipramine and citalopram) [561]. The physical evidence of DRD2-SST5R heterodimer was then proven with PLA studies in the striata of mice and striatal neuronal cultures [562]. It was suggested that the DRD2-SST5R may be a potential mediator of antidepressant effects since the heterodimerization of these receptors appeared to occur in native brain tissue and in primary striatal neuronal cultures [562]. Furthermore, prolactin is a neurotransmitter regulated by those two receptors and its excessive excretion was reported in cases of depression [563]. In addition, a study by Szafran-Pilch et al. suggested that the stimulation of DRD2-SST5R may enhance the inhibition of this prolactin [562]. Proceeding with the promiscuous DRD2, another interaction partner was reported in a BRET study using transfected HEK293T cells: NTS1R [564]. Recently, Friedland et al. reported the existence of DRD2-NTS1R heteroreceptor complexes in the accumbens core and shell, especially in the dorsal striatum, using PLA assays [565]. The NTS1R was shown to negatively modulate DRD2 signalling through immediate receptor-receptor crosstalkbased on CRE luciferase gene assay, NTS1R activation generates a blockade of the DRD2 induced inhibition of the AC-PKA-CREB pathway [566-568]. Also, the NTS1R agonist NT(8-13) reduces the Gαq-mediated calcium signal in the DRD2-NTS1R heterodimer compared to the NTS1R monomer, which can also be reversed by DRD2 antagonists [565]. The heterodimer activation by CS148, an NTS1R agonist and also DRD2 antagonist, increases the calcium response, depending on the effect of the monovalent ligands indicating an allosteric DRD2-mediated modulation [565]. This provides the evidential basis for functional association of DRD2 and NTS1R in brain areas that are closely linked to the pathophysiology of schizophrenia [565, 569].
Another partner for DRD2 is the TAA1R, a member of class A GPCRs, not yet well investigated. The DRD2-TAA1R heterodimer was found in dopaminergic innervated areas and provided a mechanism for dopamine neurotransmission modulation via TAA1R [570, 571]. Different studies could show that TAA1R may affect the DRD2 function and firing rate of dopaminergic neurons [324, 570, 572]. The DRD2- TAA1R heterodimer exerts its effect through the cAMP pathway, and haloperidol was found to promote cAMP-mediated TAA1R signalling [570]. With haloperidol as a known antipsychotic, the DRD2- TAA1R may have a role in the treatment of schizophrenia [313]. The DRD2-OTR was identified in cotransfected HEK293 cells using PLA [573, 574]. Further studies on the DRD2-OTR suggested the existence of allosteric reciprocal interactions endowed with the ability to enhance signalling of DRD2-OTR. The heterodimer is excreted upon OT activation, facilitating DRD2 signalling via allosteric receptor-receptor interactions [574]. It was also reported that the dysfunction of the DRD2-OTR in the central amygdala might lead to anxiety development [574]. Therefore, restoration of its activity may be a new therapeutic approach to treat anxiety [574].
Another DRD2 interaction partner represents the growth hormone secretagogue receptors (GHS-R), also known as Ghrelin receptors [575, 576]. GHS-R1a is a transcript variant of GHS-R and encodes the functional protein, which defines a neuroendocrine pathway for growth hormone release [577]. GHS-R signals via Gαq/11 cascade to mobilize calcium from intracellular stores [578] and plays a role in the regulation of feeding behaviour [579]. Similarly, DRD2 is also known to control physiological functions like food consumption [580, 581]. Henceforth, it was very likely that a DRD2-GHS-R1a exists, which was eventually discovered by Smith and co-workers using immunofluorescence and time-resolved FRET experiments in hypothalamic neurons of rodents [582]. Within the DRD2-GHS-R1a, the apo-ghrelin (unliganded) GHS-R1a was reported to modulate DRD2 signalling from the normal Gαi/o subunit mediated inhibition of cAMP to Gβγ subunit mediated PLC-IP3 cascade [582]. Also, in the absence of ghrelin, the endogenous ligand of GHS-R, dopamine and/or DRD2 agonists were able to activate this biased Gβγ subunit mediated PLC-IP3 signalling, suggesting that apo-GHS-R1a acts as an allosteric modulator on DRD2 [582]. In order to assess if the allosteric interaction between DRD2 and GHS-R1a could be pharmacologically targeted, the selective GHS-R1a antagonist JMV2959 was applied in treated mice with the highly selective neutral GHS-R1a antagonist JMV2959 prior to cabergoline treatment. It was shown that cabergoline-induced anorexia (selective DRD2 agonist) was blocked upon binding to the DRD2-GHS-R1a [582]. Targeting heterodimers represents a therapeutic advantage for the treatment of eating disorders.
2.2.4. Adenosine - Adenosine Receptor Heterodimers
The endogenous purine nucleoside adenosine is obtained by the breakdown of adenosine triphosphate (ATP) and consists of ribose sugar and adenine attached by a glycosidic linkage [583]. The importance of ATP and its metabolites is further underlined as they are the main energetic molecules in living organisms. The actions of adenosine through the four specific ARs are found in every single mammalian cell [583]. The first heterodimer consisting of A1R and A2AR was found in 2006 by a study using Co-IP, BRET and time-resolved FRET techniques from Ciruela et al. [458]. It was shown that A1R-A2AR exists in striatal glutamate neurotransmission at the presynaptic level [458]. Interestingly, both monomers are known to couple to different G proteins, A1R couples to Gi/o whereas A2AR couples to Gs [584]. Ciruela and co-workers were able to demonstrate that depending on the concentration of adenosine, the regulation of glutamate release by cortical glutamatergic terminals would be opposite [458, 583]. A1R-A2AR was shown to regulate the GABA uptake through adenosine in astrocytes. Hence, it was suggested that A1R-A2AR acts as a sensor of adenosine concentration as consequent fine-tuning modulation of striatal glutamatergic neurotransmission, in a manner, that there is either A1R or A2AR-mediated signalling [583]. Elevated extracellular levels of adenosine activate the A2AR protomer in this complex, producing an antagonist allosteric receptor-receptor interaction inhibiting A1R protomer signalling. Thus, activation of the A2AR in A1R-A2AR heterodimers produces an increase in glutamate release, while the activation of A1R leads to the opposite effect [458, 560, 585]. Upon G protein-coupling to the heterodimer, the long C-terminus of A2AR is the key region that determines the dominant A2AR-mediated signalling [583, 586]. A1R-A2AR heterodimers may exist in glutamate projections that regulate GABA striatal pallidal neurons, mediating motor inhibition. In the case of this heterodimer, A2AR-induced glutamate release should neutralize movement inhibition, making it a therapeutic target for neurological diseases associated with motor activity [587]. Other studies determined caffeine as a new ligand for A1R-A2AR, which when chronically applied, led to strong tolerance to the psychomotor effects of caffeine mediated by A1R-A2AR [588].
2.2.5. Adenosine Receptor and Other GPCR Heterodimers
The frequency of ‘spontaneous’ (non-electrically evoked) excitatory postsynaptic currents (EPSCs) in layer V pyramidal neurons increases after 5-HT2A receptor activation [589] and leads to an increase in late components of EPSCs evoked by electrical stimulation [590]. Since A1R and 5-HT2AR receptors are both localized in the prefrontal cortex, a study on how A1R receptor modulates 5-HT2A-enhanced ‘spontaneous’ and electrically evoked excitatory postsynaptic currents in layer V pyramidal neurons in the medial prefrontal cortex was conducted by Aghajanian and co-workers [591]. They showed that A1R agonist (N6-cyclopentyladenosine) suppressed the frequency of EPSCs generated via 5-HT2A receptor-induced glutamate release in the medial prefrontal cortex. As it did not generate large postsynaptic currents, the suppression mechanism was thought to be predominantly presynaptic [591]. Also, in 2009, Marek studied the effects of the A1R receptor agonist N6-cyclohexyladenosine on phenethylamine hallucinogen DOI-induced head shakes in order to examine a behaviour induced by activation of 5-HT2A receptors in the rat prefrontal cortex [592]. The results showed that while N6-cyclopentyladenosine suppressed head shakes, induced by activation of 5-HT2A receptors with the DOI, an A1R receptor antagonist (DPCPX) enhanced DOI-induced head shakes and blocked the suppressant action of an A1R agonist on DOI-induced head shakes [592]. This mechanism of action of A1R agonists on the 5-HT2A receptor suggests a novel therapeutic approach for schizophrenia as well as psychosis and anxiety disorders [591, 592].
In 2018, an A2AR-H3R heterodimer was discovered for the first time in recombinant cell systems and in rat striatal nerve terminals, based on functional complementation and Co-IP assays in HEK293T cells [593]. A2AR and H3R were found to be co-expressed in the cortico-striatal glutamatergic afferents and the GABAergic medium-sized spiny neurons that originate from the indirect pathway of the basal ganglia [593]. Therefore, both monomers can regulate the striatal GABAergic and glutamatergic transmission. It was reported that the co-activation of A2AR and H3R leads to enhancement of A2AR signalling and decrease of H3R functionality via their coupled G proteins [593]. As a protomer, H3R is coupled to Gi/o proteins and, consequently, inhibits AC activity. When RAMH, a H3R agonist, activates the H3R receptor, it leads to a decrease in cAMP formation. However, the expression of A2AR leads to an increase in the H3R-mediated cAMP formation [593]. In addition, the endogenous ligand histamine cannot signal through the heterodimer, unlike the exogenous agonist RAMH, suggesting that RAMH can lead to conformational changes in the H3R, allowing heterodimerization. On the other hand, the histamine-induced changes may not be sufficient to signal the heterodimer [593]. Based on binding studies with striatal membranes and histamine, it was demonstrated that H3R activation by histamine increased the binding affinity of the A2AR for its agonist CGS-21680, while RAMH resulted in a decrease of the binding affinity, indicating that histamine and RAMH lock the H3R in different conformational states that affect its interaction with the A2AR [593]. It is possible that the H3R-A2AR heterodimer plays a role with key physiological implications.
2.2.6. Opioid - Opioid Receptor Heterodimers
As referred, the most effective analgesics in clinical pain management are opioids such as morphine, codeine, hydrocodone, oxycodone, fentanyl, and tramadol [594]. However, they are also commonly prescribed and frequently abused [595, 596]. Among the intricacy of opioid receptor pharmacology, opioid receptor heterodimers represent another important layer of signalling complexity and provide an opportunity for the development of analgesics with fewer side effects [597]. Dimerization was already reported for homodimers MOR-MOR [598] and heterodimers containing only opioid receptors such as MOR-DOR [599-601], MOR-KOR [602] and DOR-KOR heterodimer [603], were proven to exist both in vitro and in vivo. OR heterodimers are often expressed in limited and specific brain regions and are involved in adverse effects induced by chronic opioid therapy, underlining the importance to develop therapeutic strategies to target these heterodimers [109, 604].
Selective agonists and antagonists were developed to target MOR-DOR [605]. Devi and Rozenfeld reported that the MOR agonist Tyr-D-Ala-Gly-N-Me-Phe-Gly-ol (DAMGO) activates Gi/o-mediated signalling in MOR-expressing cells as well as β-arrestin-2-mediated signalling for changing the dynamics of ERK-mediated signalling in MOR-DOR heterodimer-expressing cells [606]. In MOR-DOR expressing cells, using a chimeric G protein-mediated calcium fluorescence assay, it was shown that the DOR selective agonist SNC80 induces intracellular Ca2+ release [607]. Another example is CYM51010, a selective MOR-DOR agonist, able to induce the recruitment of β-arrestin-2 and GTPγS binding, which could then be blocked by a MOR-DOR selective antibody (mAb) [608]. Here, the DOR was shown to have an antagonistic allosteric influence on MOR activity within the heterodimer [604]. The DOR peptide antagonist TIPPΨ was shown to enhance the binding of morphine to MOR, Gi/o coupling and inhibition of cAMP levels [601]. Furthermore, the MOR-DOR may also have specific intracellular trafficking. According to studies by Milan-Lobo and Whistler, MOR and DOR are only able to dimerize when both are present in the plasma membrane [609]. Controversy, Hasbi et al. stated that MOR-DOR are located in the endoplasmic reticulum, where they recruit the Gaz protein [610]. Another study by Décaillot et al. reported that the agonists DAMGO, Deltorphin (Delt) II, SNC80 and methadone could induce MOR-DOR endocytosis, but others such as DADPE were not able to do so [611]. In the same study, Décaillot and co-workers identified RTP4, a Golgi chaperone as an important regulator of MOR-DOR levels at the cell surface [611]. This was found to be in concordance with a study by He et al., where the application of DOR-selective agonists Delt I, Delt II and SNC80 induced endocytosis and further procession of degradation of DOR and MOR, resulting in a reduced MOR surface expression in double-transfected HEK293 cells [612]. This effect was also achieved when DAMGO, a selective MOR agonist, was applied [612]. The effect was then only diminished when an interfering peptide D-Phe-Cys-Tyr-D-Trp-Om-Thr-Pen-Thr-NH2 (CTOP) or the antagonist naloxone was added [612]. To perform a whole-brain dual receptor mapping study, RedMOR/ greenDOR double knock-in mice were generated. MOR and DOR were colocalized in subcortical neuronal networks, responsible for eating actions, sexual behaviours or response to aversive stimuli [613]. In 2018, Wang et al. found that the co-expression of MOR and DOR is restricted to small populations of spinal cord neurons and yet is rare in the parabrachial, amygdala and cortical regions of the brain for pain processing [614]. In another study Gomes et al. showed, using tail-flick assays in mice, that the CYM51010 ligand for the heterodimer MOR-DOR has analgesic activity identical to the one from morphine [608]. Also, CYM51010-induced analgesia was abrogated in MOR knockout mice and still persisted in morphine-tolerant mice [608]. The evidence of these heterodimers in CNS pain circuits suggests that MOR-DOR heterodimers cellular interactions are important for the development of novel opioid analgesics.
The MOR-KOR heterodimer was discovered in 2010 by Chakrabarti and co-workers [602]. The results of their study showed that MOR-KOR was more prevalent in the spinal cord of proestrus (with high estrogen receptor levels) vs. diestrus females and vs. males [602, 615]. It was then concluded that dynorphin would serve a potential female-specific KOR-ligand within the MOR-KOR. Furthermore, gender- and ovarian steroid-dependent recruitment of MOR-KOR was seen as a way to balance the actions of anti-nociceptive and pro-nociceptive functions of the dynorphin/KOR opioid system in the spinal cord. Lastly, various types of chronic pain states that are significantly more common in women than men, could be the result of the impaired formation of MOR-KOR and therefore, this holds promise for the development of a special ligand to target the MOR-KOR [604].
2.2.7. Opioid Receptor and Other GPCR Heterodimers
The main goal of studying OR heterodimers is to understand their existence and physiological function from the perspective of pain transmission. Once resolved, potent analgesics with fewer side effects could be developed. Studies by Vilardaga et al. and Yang et al. showed that a conformational antagonistic crosstalk exists between MOR and α2AAR [616, 617]. FRET microscopy studies showed that MOR and α2AAR communicate via a switch of conformations within the monomers that leads to inhibition of one monomer by the other [616]. Morphine binding to MOR within the MOR-α2AAR was reported to induce a conformational change in the norepinephrine-bound α2AAR, which then inhibits Gαi signalling and the downstream MAP kinase responses [616]. Hence, MOR activation mediates the rapid inactivation of its coupled partner α2AAR. Already in the 90s it was reported that combined agonists acting on MOR and α2AAR would act synergistically when co-administered into the spinal cord and would have an analgesic effect [618]. Furthermore, it was also reported that norepinephrine or clonidine, which are both agonists of α2AAR, were able to reduce significantly the release of glutamate, substance P and calcitonin gene related peptides from spinal cord synapses [619, 620]. MOR and α2AAR were both located in the superficial layers of the dorsal horn of the spinal cord, and in the rat spinal cord, α2AAR was located on the terminals of capsaicin-sensitive, SP-containing primary afferent fibers in immunostaining studies [619]. Both receptors were reported to affect the nociceptive system and are particularly involved in the depression of neurotransmitter release in the spinal cord [621, 622]. However, the synergy of MOR and α2AAR agonists in the MOR-α2AAR in analgesia remains unclear [604].
In 2019, Wang and co-workers identified the MOR-GPR139 heterodimer in which the orphan GPR139 negatively regulates the opioid receptor function, signalling and trafficking [604, 623]. By using C. elegans as a model organism, it was shown that mammalian MOR was expressed in the nervous system of nematodes (tgMOR), and that application of morphine and fentanyl leads to a decrease in locomotion in nematodes expressing tgMOR [604, 623]. By applying a large-scale genetic screening and whole genome sequencing, Wang et al. identified the orphan receptor FRPR-13, the homolog of the human GPR139, as a negative regulator of MOR in vivo [604, 623]. The functional relationship between MOR and GPR139 was further investigated in MOR and GPR139 transfected HEK293 cells, where MOR activation was shown to cause an opening of G protein-coupled inwardly rectifying potassium channels (GIRKs). This leads to hyperpolarization of membrane potential, which can be inhibited by GPR139 expression [604, 623]. In addition, Wang et al. were able to show that MOR and GP139 could be co-immunoprecipitated. They also showed that when GPR139 was highly overexpressed, the cell surface expression of MOR was reduced, suggesting that GPR139 is able to regulate MOR trafficking to the plasma membrane or internalization [604]. Furthermore, GPR139 was found to bind directly to MOR in vitro, promote the recruitment of β-arrestin-2 and inhibit GIRK and G protein activation [604]. More evidence for MOR-GPR139 was generated in in situ hybridization experiments, where MOR and GPR139 are co-expressed in similar brain regions [604]. Wang et al. also provided electrophysiological evidence, where in cultured brain slices GPR139 deficiency reduced the basal firing rate and increased opioid sensitivity in neurons [604]. Lastly, Wang et al. investigated the relationship between MOR and GPR139 in in vivo animal studies in mice. GPR139 knockout (KO) mice had normal baseline learning, nociception, locomotor activities, and motor coordination but showed sensitivity to morphine-induced analgesia and reward effects [604, 623]. When JNJ-63533054, an GPR139-agonist, was administered, morphine-induced analgesia and rewards were inhibited in mice [604, 623]. Also, GPR139 KO mice did not show explicit opioid withdrawal reactions [604, 623]. Hence, GPR139 was identified as a novel anti-opioid system in the brain.
In 2018, Koshimizu and co-workers identified the MOR-V1BR heterodimer [624]. The endogenous ligand of V1BR, ADH (or also AVP) was reported to regulate morphine tolerance and sensitivity [604]. Koshimizu et al. revealed that in V1BR KO mice, the nociceptive thresholds and morphine sensitivity are enhanced. Also, the development of analgesic tolerance to morphine was significantly delayed in these mice when V1BR-subtype-selective antagonist SSR149415 was administrated [624]. Furthermore, application of SSR149415, a selective V1BR-antagonist but not a V1AR-antagonist, into the lateral ventricle of the mice also reduced the development of morphine tolerance [604, 624]. In in situ hybridization experiments, Koshimizu et al. discovered that MOR and V1BR colocalized in the rostral ventromedial medulla [604, 624]. By using cotransfected HEK293 cells, a functional interaction between MOR and V1BR was observed as well as in single cells BRET analysis (close proximity of both receptors <10 nm) [604, 624]. In another experiment, using a radioligand binding assay and cyclic AMP assay, morphine binding to MOR was shown to be significantly influenced by MOR-V1BR formation [604, 624]. Also, ADH-enhanced morphine-induced super activation of the AC triggered by the MOR-V1BR, was indicated to be dependent on β-arrestin-2 and ERK phosphorylation [604, 624]. Koshimizu and co-workers also discovered that a leucine-rich segment in the C-terminal tail of the V1BR is responsible for binding of β-arrestin-2, which when deleted through genome editing, increased morphine analgesia and reduced ADH-mediated AC super activation increased [604, 624]. Taking all findings together, it was suggested that the MOR-V1BR is indeed another mechanism to alter opioid receptor function such that morphine-induced analgesia could be potentiated, and morphine tolerance could be delayed.
The formation of a MOR-GAL1R was identified by Moreno et al., in transfected cells and in neurons in the rat ventral tegmental area (VTA) [625]. Previous in vivo studies showed that behavioural effects of MOR agonists were counteracted by galanin [604]. According to Moreno et al., the MOR-GAL1R mediates antagonist interactions between MOR- and GAL1R-selective ligands and is a key player in the functioning of dopaminergic neurons [625]. In another study by Cai et al., it was discovered that methadone potency for stimulating dopamine release and euphoria was reduced through MOR-GAL1R heterodimers in the rat VTA [604, 626]. Such alterations of opioid receptor functions in opioid-induced rewarding were not observed for other opioids such as morphine and fentanyl [604, 626]. These data suggest that MOR-GAL1R mediates dopaminergic effects of opioids and that pharmacological differences between methadone and other opioids may provide a way to dissect the euphoric from therapeutic effects of methadone-like compounds [604, 626]. Consequently, novel methadone-like compounds with reduced potency, able to activate MOR-GAL1R may be a possibility to develop safer opioid analgesics [604, 626].
Early studies in 2001 identified MOR and CB1R co-localization in lamina II neurons in the spinal cord [627]. Synergistic interactions between the opioid and the cannabinoid system in analgesia were already known, as the CB1R is also present in the brain on primary sensory neurons in the DRGs, spinal cord, and some brain regions related to pain processing [604, 628, 629]. Rios et al. were able to show MOR-CB1R heterodimers in transfected HEK293 cells using biophysical methods, such as BRET [630]. Additionally, they demonstrated that co-activation of MOR-CB1R would lead to antagonistic allosteric interactions, which was determined by cross-inhibition of neurite outgrowth involving inhibition of the Src-STAT3 pathway [604, 630]. In 2016, Manduca et al. identified MOR-CB1R heterodimers in rodents nucleus accumbens core (NAcC) and studied the importance of MOR-CB1R heterodimers to control social behaviour in adolescent rodents [631]. They studied, in particular, the role of the endocannabinoid 2-arachidonoylglycerol (2-AG) in social play [631]. 2-AG is released in the brain of adolescent rats during social play [632] and 2-AG levels are high in the NAc of socially stimulated mice [633]. Systemic administration of the JZL184 (a 2-AG hydrolysis inhibitor) or morphine (MOR agonist) increased social play behaviour in adolescent rats [631]. However, these social play-enhancing effects were blocked by direct infusion of SR141716 (CB1R antagonist) and naloxone (MOR antagonist) into the NAcC [631]. Neuronal plasticity and socioemotional behaviours could be modulated by MOR-CB1R.
Already in the 90s it was discovered that the cholecystokinin octapeptide (CCK8) antagonises opioid analgesia [634]. Furthermore, using L-365, 260, a CCK2R/CCKBR-selective antagonist, it was shown that CCK-8 inhibited opioid analgesia through CCKBR [635]. In 2018, Yang et al. identified the MOR-CCKBR heterodimer, which they believed may underlie the CCK8-antagonism of opioid analgesia [604, 616]. Co-localization studies using double-labelling immunofluorescence staining showed that MOR and CCKBR colocalize in neurons in spinal cord dorsal horn and DRGs. Using Co-IP and fluorescence lifetime-imaging-microscopy-based fluorescence resonance energy transfer (FLIM-FRET) assays, Yang et al. showed heterodimerization of MOR and CCKBR in HEK293 cells [604, 616]. They also validated that the TM3 of MOR plays a key role in the formation of MOR-CCKBR [604, 616]. The MOR-CCKBR functions include a decrease in MOR affinity for ligands and reduction of agonist-mediated phosphorylation of ERK1/2 in transfected HEK293 cells [604, 616]. In their study Yang and co-workers developed a cell-penetrating interfering peptide by adding the TAT sequence (RKKRRQRRR) to the C terminal of the entire TM3 (TM3MOR-TAT), which disrupted the MOR-CCKBR [604, 616]. In transfected cells TM3MOR-TAT was shown to enhance MOR signalling and in rats it prevented CCK8-induced antagonism against morphine analgesia, rendering TM3MOR-TAT as a promising target for increasing morphine analgesia without applying increasing amounts of morphine [604, 616].
Suzuki et al. demonstrated that MOR and CCR5 can also form heterodimers in the cell membrane of lymphocytes, using Co-IP and chemical crosslinking experiments [636]. In this study, the authors demonstrated that the MOR-CCR5 heterodimer is functional, since the co-activation of receptors with morphine (MOR agonist) and MIP-1beta (CCR5 agonist) suppresses the inhibitory effect of MIP-1beta and increases the stimulatory effect of morphine on CCR5 expression [636]. Also in 2002, based on behavioural test in rats’ PAG (the brain area that is the focus of opioid analgesic actions), Szabo et al. found the ability of CCR5 receptors to desensitize MOR receptors [637]. They demonstrated that chemokine ligands for CCR5 (CCL5) can inactivate the normal neuronal signalling pathway involved in reducing the sensation of pain [637]. Thus, activation of MOR-CCR5 increased nociception.
In 2008, immunohistochemistry experiments by Juhasz et al. demonstrated that MOR and DRD1 colocalized in neurons of the cortex and caudate nucleus and in living cells [638]. They showed within the cellular nuclear translocation pathway that MOR-DRD1 formation resulted in a significantly enhanced surface expression of MOR [638]. Tao et al. performed Co-IP, BRET and cross-antagonism assays and confirmed the existence of MOR-DRD1 [639]. Furthermore, they showed that SCH23390, a DRD1-selective antagonist, was able to inhibit the agonist-induced activation of MOR and downstream signalling in transfected cells and in striatal tissues from wild-type but not DRD1 KO mice [639]. Similarly, to what has been described for heterodimers so far, antagonizing one monomer within the dimer also inhibits the signalling of the partnered monomer, although the latter was activated by its own ligand [639]. In addition, the MOR-DRD1 was identified in vivo through biochemical and biophysical assays [639]. Here it was shown that by destruction of the dopaminergic projection from the ventral tegmental area to the striatum, dopamine release was abolished, and SCH23390 was still able to significantly inhibit agonist-induced MOR behavioural responses in rats [639]. Lastly, Tao et al. demonstrated that MOR or DRD1 KO mice were not able to show locomotor sensitization to morphine because they were unable to form MOR-DRD1 [639]. Hence, MOR-DRD1 may be involved in the dopamine-independent expression of locomotor sensitization to opiates [639].
MOR and DRD2 receptors are colocalized in the spinal cords of mice, confirmed by Co-IP assays [640]. In 2019, Stove and co-workers proved the existence of MOR-DRD2 heterodimers using HEK293T and HeLa cells, both cotransfected, by Co-IP, BRET, FRET and functional complementation of split luciferase techniques [641]. MOR activation by its agonists (DAMGO and fentanyl) resulted in recruitment of β-arrestin to the receptor and, consequently, caused internalization of the receptor [642, 643]. This β-arrestin recruitment is associated with the unwanted effects of opioids [644, 645]. Based on time-lapse imaging technique, the effect of heterodimerization of MOR-DRD2long on the internalization characteristics of MOR indicated a decrease in the internalization of MOR-YFP (MOR associated with Yellow fluorescent protein) with the co-expression of DRD2long, when stimulated upon addition of DAMGO [641]. This suggests that the heterodimer may be a potential therapeutic target associated with diseases such as addiction.
2.2.8. Serotonin - Serotonin Receptor Heterodimers
5-HT1A and 5-HT2A receptors, which have inhibitory actions via Gi/o and excitatory actions via Gq/11, respectively, are the two major known 5-HT receptors in the brain [646]. The evidence that 5-HT1A and 5-HT2A receptors can form a heterodimer was given by Borroto-Escuela et al. in the dorsal hippocampus and the anterior cingulate cortex using in situ PLA assay and BRET saturation assay in cotransfected HEK293T cells [647]. Based on a 5-HT1A radioligand binding assay, Borroto-Escuela et al. showed that TCB2 (5-HT2A agonist) reduced the binding affinity of the 5-HT1A agonist ipsapirone in membranes of the frontal lobe of the cortex [647]. However, this action seems to be blocked by ketanserin, a 5-HT2A antagonist. These results suggest that 5-HT1A-5-HT2A heterodimers perform inhibitory interactions of the allosteric type, with a dominant effect of 5-HT2A over 5-HT1A protomer [647]. In 2018, another study with this heterodimer was performed to understand how antipsychotic drugs, such as clozapine, ketamine and haloperidol affect the formation of the heterodimer [648]. Clozapine and ketamine showed an impact on heterodimer formation, whereas ketamine exhibited high affinity only for 5-HT2A, clozapine only had an effect on heterodimers in low dosage [648]. Since both receptors are known to be involved in depression [649], this heterodimer may play a role in this disease.
5-HT2A, 5-HT2B and 5-HT2C receptors are both Gq/11-coupled receptors, which mediate excitatory neurotransmission [650]. These receptors are co-expressed in GABAergic interneurons and in a subpopulation of pyramidal neurons of the prefrontal cortex (PFC) [651, 652] and in dopaminergic neurons of the ventral tegmental area [653, 654]. Using Co-IP and BRET techniques, Moutkine and co-workers demonstrated that 5-HT2A-5-HT2B and 5-HT2A-5-HT2C heterodimers can be formed when co-expressed in heterologous expression systems [655]. In 5-HT2C-containing heterodimers, ligands bind and activate only the 5-HT2C protomer. The same authors also demonstrated that 5-HT2A-5-HT2B and 5-HT2A-5-HT2C heterodimers exhibit an asymmetry in Gq-protein coupling, and that signalling from 5-HT2A and 5-HT2B protomers is blunted, as only the 5-HT2C protomer is able to activate the Gq protein [655]. Thus, there is a dominance of 5-HT2C on 5-HT2A and 5-HT2B receptor binding. Also, this dominant effect was validated in vivo (observed in neurons), which resulted in an exogenous expression of an inactive form of the 5-HT2C receptor in the locus ceruleus associated with a decreased 5-HT2A-dependent noradrenergic transmission [655]. As such, these heterodimers must be considered for depression and addiction conditions.
Heterodimerization between 5-HT1A and 5-HT7 receptors was demonstrated by Ponimaskin and colleagues using Co-IP and immunoblotting techniques and FRET assays in cotransfected neuroblastoma N1E-115 cells [656]. The 5-HT1A receptor is Gi/o-coupled, which induces inhibition of AC and decrease in intracellular cAMP [657, 658], and the 5-HT7 receptor is Gβγ-coupled, which activates K+ channels and MAPK Erk2 [657]. 5-HT1A-5-HT7 heterodimerization decreases the 5-HT1A-receptor-mediated activation of Gi protein without affecting 5-HT7-receptor-mediated Gs protein activation. Also, authors discovered that 5-HT1A-5-HT7 heterodimers reduce the ability of 5-HT1A receptors to activate GIRK channels, an effect mediated through the Gβγ subunits of inhibitory G proteins [657]. This phenomenon may result from 5-HT7 interacting with and directly modulating 5-HT1A. In addition, MAP kinases ERK1/2 phosphorylation is induced by 5-HT1A agonists, and this signal is enhanced when 5-HT7 receptors are co-expressed suggesting that heterodimerization favors activation of 5-HT1A-receptor-mediated ERK signalling whereas it prevents 5-HT1A-mediated activation of Gi/o-GIRK channel activity [657]. The differences in desensitization patterns between pre- and postsynaptic 5-HT1A receptors can be explained by the differences in the relative concentration of 5-HT1A-5-HT7 heterodimers on presynaptic serotonergic neurons and postsynaptic neurons. Besides, a regulated and balanced ratio of homo- and heterodimerization on pre- and postsynaptic neurons may be involved in both the onset and the response to the treatment of neurological conditions, such as depression, anxiety, schizophrenia and drug abuse [659, 660].
2.2.9. Serotonin Receptor and Other GPCR Heterodimers
It is well established that the dopaminergic and the serotonin system play an important role in neurotransmission, and thus their malfunctioning is suggested to be linked to the development of psychiatric disorders such as schizophrenia [648]. Łukasiewicz and co-workers identified in HEK293 cells the presence of 5-HT1AR-DRD2 heterodimers [661]. The heterodimerization was shown to be mainly enhanced by exposure of clozapine but also by other antipsychotics such as olanzapine, aripiprazole, and lurasidone [661]. Functional assays like cAMP and IP1 and ERK activation, indicated that the different antipsychotics exhibited diverse effects on the 5-HT1AR-DRD2 [661]. For instance, Łukasiewicz et al. demonstrated that clozapine and 8-OH-DPAT potentiated postsynaptic effects, especially ERK activation [661]. Furthermore, 5-HT1AR activation by 8-OH-DPAT along with the DRD2-blockade by clozapine led to a conformal change within the heterodimer and consequently changed their signalling via Gαq/11-mediated activation of ERK1/2 [661]. In 2018, a study by Szlachta et al. investigated the role of well-known antipsychotic drugs, clozapine and haloperidol, in the formation of 5-HT1AR-DRD2 heterodimers in mouse cortex [648]. By using PLA, in in vitro and ex vivo experiments, co-localization of 5-HT1AR and DRD2 was confirmed [648]. Also, Szlacht and co-workers demonstrated that low-dose administration of clozapine increased the levels of 5-HT1AR-DRD2, while administration of haloperidol decreased their level in mouse cortices [648]. Different studies located the 5-HT1AR-DRD2 in the dorsal and ventral striatum using in situ PLA and FRET as well as in cellular models using BRET [662-664]. The 5-HT1AR-DRD2 has been developed as an important therapeutic target due to a well-documented serotonin-dopamine interaction and its relevance to schizophrenia [665].
A study by Albizu et al., using radioligand-binding and inositol phosphate production assays, identified functional crosstalk between 5-HT2AR and DRD2 in the mouse brain and in HEK293 cells [663]. They were able to show that DRD2 activation increases the hallucinogenic agonist affinity for 5-HT2AR and decreases the 5-HT2AR induced inositol phosphate production [663]. Albizu and co-workers demonstrated that the inhibition of MK-801-induced locomotor activity by DRD2 antagonist haloperidol requires the 5-HT2AR expression [663]. MK-801, a potent and selective non-competitive NMDA receptor antagonist also known as dizocilpine, serves as a pharmacological model for schizophrenia in mice [666]. It was reported that MK-801 increases the locomotor activity of mice, a behaviour that is suppressed by the DR-antagonist haloperidol [667]. In Co-IP studies, Albizu et al. showed that 5-HT2AR and DRD2 are able to interact physically in HEK293 cells [663]. Lastly, they suggested that depending on the treatment combination, different actions could be achieved by application of DRD2-ligands such as quinpirole or butaclamol and 5-HT2AR-ligands such as DOI and ketanserin [663]. DRD2 expression was shown to increase the efficacy of DOI to activate the 5-HT2AR-induced phosphoinositol Gq/11 signalling pathway [663]. Only the hallucinogenic partial agonist DOI seemed to promote this effect on 5-HT2AR signalling [663].
The 5-HT1AR and GAL1R are both known to couple to Gi/o proteins and transduce their signals mainly by inhibitions of the AC, calcium channel activity and neurotransmitter release [657, 668, 669]. In 2010, Borroto-Escuela et al. discovered 5-HT1AR-GAL1R heterodimers in double-transfected mammalian cells with PLA and FRET techniques [668]. The presence of 5-HT1AR-GAL1R, induced either MAPK or AC signalling pathways, indicating an allosteric cross-inhibition mechanism in order to block the excessive activation of Gi/o and an exaggerated inhibition of AC or stimulation of MAPK activity [668]. By using reporter gene assays, CRE-luciferase and SRE-luciferase assays, it was possible for Borroto-Escuela et al. to further assess possible antagonistic allosteric receptor-receptor interactions 5-HT1AR-GAL1R [668]. In the brain, previous biochemical, cardiovascular and behavioural work has given additional proof for the existence of antagonistic 5-HT1AR-GAL1R interactions [670-675].
Only recently, in 2019, Chruścicka et al. discovered the existence of 5-HT2AR-OTR heterodimers in vitro in living cells using a flow cytometry-based FRET approach and confocal microscopy [676]. The 5-HT2AR and OTR were found to be expressed in similar brain regions modulating central pathways critical for social and cognition-related behaviours [677-680]. Therefore, Chruścicka et al. applied the PLA technique ex vivo in order to observe the formation and location of the 5-HT2AR-OTR, which were found in limbic regions such as hippocampus, cingulate cortex and nucleus accumbens [676]. These were identified as key regions associated with cognition and social-related behaviours [676]. Functional crosstalk was observed in 5-HT2AR-OTR using cellular-based assays, when a reduction in potency and efficacy of oxytocin, carbetocin and WAY267464 (synthetic OTR-agonists) was observed on OTR-mediated Gαq signalling [676]. Likewise, 5HT-induced activation of 5-HT2AR also revealed attenuation in Gαq-mediated signalling. According to Chruścicka et al. co-trafficking of 5-HT2AR and OTR within the cell was also demonstrated [676].
Chruścicka et al. pointed toward the existence of 5-HT2C-OTR heterodimer, based on FRET and confocal microscopy in vitro in a heterologous cell expression system and further using PLA assays in the rat brain [681]. 5-HT2CR and OTR co-expression resulted in an attenuation of OTR-mediated Gq-signalling and significant changes in receptor trafficking. This attenuation was specifically caused by 5-HT2CR protomer activation [681]. It seems likely that 5-HT2AR-OTR and 5-HT2C-OTR heterodimers can be involved in the development of depression and other types of psychiatric diseases involving disturbances in social behaviours.
To date, a functional link between the serotoninergic and melatoninergic systems has only been sparsely reported. In a study by Prosser et al., functional crosstalk between those two systems was reported, revealing that melatonin inhibits the ability of 5-HT to phase shift the suprachiasmatic circadian clock [682]. In addition, melatonin is synthetically derived from 5-HT, and therefore a close relationship is probable [683]. Furthermore, the clinically proven antidepressant agomelatine, the first non-monoaminergic therapeutic, was shown to act as an agonist at MT1R and MT2R, which are coupled to Gi proteins, while it is a neutral antagonist at the Gq/11-coupled 5-HT2CR system [684, 685]. According to Racagni, the affinity of agomelatine was reported to be substantially lower at 5-HT2CR compared to MT1R and MT2R in vitro, suggesting that it may exert its actions “synergistically” [686]. It was also discovered that 5-HT2CR, MT1R and MT2R are necessary for the expression of the antidepressant actions of agomelatine, which cannot be reproduced either by melatonin or by selective 5-HT2CR antagonists alone [683, 686]. However, Kamal et al. presented evidence that 5-HT2CR and MT2R are able to form a heterodimer, by using Co-IP, BRET and pharmacological techniques in transfected cells and in human cortex and hippocampus [683]. The 5-HT2CR-MT2R was also discovered in the mouse brain [687]. The 5-HT2CR-MT2R was reported to be composed of Gi-coupled melatonin MT2R and Gq-coupled serotonin 5-HT2CR [683, 687]. The activation of 5-HT2CR-MT2R was shown to amplify the activation of 5-HT-mediated Gq/phospholipase C response and trigger melatonin-induced unidirectional transactivation of the 5-HT2CR [683, 687]. According to Kamal et al., agomelatine (antidepressant) has a distinctive profile on 5-HT2CR-MT2R. Whereas melatonin is able to activate both Gi and Gq pathways, agomelatine tends to activate the Gi/cAMP pathway and has an allosteric antagonistic effect on 5-HT-induced Gq pathway activation [683]. Lastly, a beneficial involvement of agomelatine in 5-HT2CR-MT2R heterodimer was suggested for the treatment of major depression and generalized anxiety disorder [683].
MOR and 5-HT1A receptors are co-expressed in discrete areas of brain, such as, dorsal raphe nucleus, periaqueductal grey neuron, dorsal horn of the spinal cord, amygdala and primary afferent nociceptive fibers [688-690]. Also, both receptors are coupled to Gi/o protein, which induces the inhibition of AC, the opening of K+ channels, the closing of Ca2+ channels and the stimulation MAPK ERK1/2 pathways [691]. 5-HT1A-MOR heterodimers were detected by Cussac et al. using Co-IP and by BRETmax determination in transiently cotransfected COS7, HEK293 or CHO-K1 cells [692]. To demonstrate the functional transactivation in GPCR heterodimers, they used receptor-Gα-protein fusions, consisting of the application of fusion proteins of protomers with a subtype of Gα protein, and that it is only activated by protomers if they are not in a free form [693]. As a result, by co-expressing the MOR and 5-HT1A-Gαo fusion protein as well as MOR and 5-HT1A-Gα15 fusion protein, they demonstrated that both receptors could induce transactivation of the Gα protein fused to its partner protomer in membrane preparations and in live cells, respectively [692]. In addition, MOR and 5-HT1A receptors can co-exert control in the ERK1/2 pathway. However, the MOR receptor-induced EKR1/2 phosphorylation was selectively desensitized by prolonged stimulation and activation of 5-HT1A receptor with 8-OH-DPAT agonist [692]. This heterodimer could have interesting therapeutic influences since MOR and 5-HT1A are involved in pain control.
2.2.10. Cannabinoid - Cannabinoid Receptor Heterodimers
One of the most important inhibitory regulation mechanisms acting in the CNS is the cannabinoid system [694, 695]. The two cannabinoid receptors, CB1R and CB2R, share around 44% sequence similarity [696, 697]. Until 2012 it was not clear if cannabinoid receptors were able to form heterodimers, despite the fact that CB1R and CB2R have overlapping expression tissues and that they have been shown to regulate similar cellular processes [698]. Heteromerization of CB1R and CB2R was then demonstrated in a large study by Callén et al. [698]. In this study, the receptors were investigated using cotransfected cells and in a variety of brain tissues, including pineal gland, nucleus accumbens, and globus pallidus and BRET technique and in situ PLA [698]. Another study by Sierra and co-workers identified the first CB1R-CB2R heterodimers in pallidothalamic projection neurons in the monkey, using PLA [699]. Both, CB1R and CB2R are coupled to Gi proteins, which is particularly interesting as within the CB1R-CB2R heterodimer the CB1R-antagonist AM251 was reported to block the effect of the CB2R-agonist JWH133 and vice versa, the CB2R-antagonist AM630 was reported to inhibit the effect of the CB1R-agonist ACEA [698, 700]. Furthermore, agonist co-activation by ACEA and JWH133 of the CB1R-CB2R heterodimer was shown to lead to negative crosstalk in Akt phosphorylation and neurite outgrowth [698, 700]. Recently, a study by Narvarro et al. showed that CB1R-CB2R heterodimers are expressed in LPS/IFN-γ-activated microglia [701]. When compared to resting cells, it was visible that CB2R became robustly coupled to Gi in activated cells if CB1R and CB2R were also present, suggesting a potentiation effect by CB1R-CB2R [701]. In addition, an upregulated expression of CB1R-CB2R was observed in primary microglia cultures from the hippocampus of mutant β-amyloid precursor protein (APPSw, Ind) mice, a transgenic AD model [701]. Lastly, Navarro and co-workers identified a correlation between the increased expression of CB1R-CB2R in the striatum of in 6-hydroxy-DA-lesioned rat model for PD and dyskinesias by chronic levodopa treatment [701].
2.2.11. Cannabinoid Receptor and Other GPCR Heterodimers
The cannabinoid and the dopaminergic system are known to display complex interactions within the basal ganglia [702-704]. The CB1R was shown to be co-expressed with DRD2 in the soma and dendrites of the ventral striatopallidal GABAergic neurons [705]. Meschler et al. also reported interactions between CB1R and DRD2 (and DRD1) in the rat and monkey striatum [706].
CB1R and DRD1 receptors colocalize in the basal ganglia circuitry, sharing the same G protein transduction pathway and playing a main role in the control of motor activity in both systems [707, 708]. In 2011, Tersian and colleagues provided the first evidence for physiological crosstalk between CB1R and DRD1 receptors in the modulation of depression-like behaviour, social skills, and fear conditioning [709]. In this study, the authors revealed that conditional CB1R knockout mice lacking CB1Rs in neurons expressing DRD1 exhibited significantly increased contextual and auditory-cued fear. This suggested that a specific reduction of endocannabinoid signalling in neurons that express simultaneously dopamine DRD1 is indeed able to affect acute fear adaptation [709]. Serrani et al. studied the role of DRD1 receptors in the behavioural responses induced by acute and repeated stimulation of cannabinoid CB1R receptors, including the development of physical dependence, using female dopamine DRD1 receptor-deficient mice and wild-type littermates treated with HU-210 (a synthetic cannabinoid agonist) [710]. The results of the study showed that the mutant mice, compared to wild-type females, exhibited an enhanced response to the acute motor and hypothermic effects of HU-210 [710]. Administration of SR141716A (CB1R antagonist) precipitated a cannabinoid withdrawal syndrome in HU-210 tolerant female mice. Furthermore, the severity of the cannabinoid withdrawal syndrome was potentiated in female mice with DRD1 deficiency [710]. Therefore, there is involvement in DRD1 in the acute effects induced by HU-210, as well as in the somatic expression of cannabinoid withdrawal, supporting the functional interaction between the cannabinoid and dopaminergic systems in the development of cannabinoid dependence [710].
Some studies pointed out that CB1R and DRD2 receptors are colocalized in the basal ganglia, mainly in the striatopallidal GABAergic neurons and in the cortico-striatal glutamate neurons [711-713]. The first author to provide evidence of CB1R-DRD2 heterodimerization was Kearn et al., based on Co-IP studies in KEK293 cells [704]. Then, other studies confirmed this evidence in globus-pallidus and striatum of rodents and primates, using BRET, PLA and Co-IP assays [713-718]. In the study of Kearn et al., it was demonstrated that stable expression of CB1R and DRD2 in HEK293 cells resulted in a pertussis toxin-insensitive component to CB1R activation of ERK 1/2 and a stimulation of AC activity after simultaneous activation of both receptors by the agonists quinpirole (DR-agonist) and CP55940 (CB1R-agonist) [704]. Furthermore, the study showed and confirmed previous results [719, 720] that DRD2-activation together with the activation of CB1R resulted in the complex coupling Gs instead of its preferred G-protein, Gi/o, which was observed in an increase in cAMP levels instead of a synergistic inhibition of AC activity [704, 719, 721, 722]. In addition, recent studies revealed that CB1R-DRD2 heterodimerization requires the bidirectional allosteric interaction of the two receptors, as the expected effect was not observed when only one receptor was activated [715, 716]. A recent study from Bagher et al. revealed that CB1R-DRD2 heterodimer formation in C57BL/6J mice is reduced when treated with the typical antipsychotic haloperidol [714]. In addition, the abundance of the heterodimer increased when treated with the nonselective cannabinoid receptor agonist (CP55, 940), whereas the atypical antipsychotic olanzapine treatment had no effect [714]. These results suggest that this heterodimer has an influence on dopamine and cannabinoid-related disorders.
The expression of CB1R and A2AR in corticostriatal glutamatergic terminals suggests an interaction potential between those two receptors [723, 724]. Indeed, it has been demonstrated that the ability of WIN 55212-2, a CB1R-agonist, to increase DARPP32 phosphorylation and inhibit motor activity requires the presence and the activation of A2AR, which then functions as a heterodimer [725, 726]. The study of Carriba et al. also demonstrated, through Co-IP and BRET experiments in living cells and in rat striata, that CB1R-A2AR heterodimers are functional since they were shown to mediate the cannabinoid-induced motor effects [723, 726]. Another study by Tebano et al. using SH-SY5Y neuroglioblastoma cells in biochemical and cellular signalling assays as well as behavioural tests using wildtype and A2AR KO mice indicated that striatal CB1R activation-induced synaptic effects depend on A2AR activation [724]. Indeed, CB1R-agonist WIN55, 212-2-induced motor depressant effects are inhibited by the A2AR-antagonist ZM241385 [726]. Furthermore, Tebano and co-workers demonstrated that the blockade of A2AR reduces WIN55, 212-2-induced depression of synaptic transmission in corticostriatal slices and that the synaptic effects of WIN 55212-2 are reduced in slices from A2AR KO mice. According to Tebano et al., this suggests the occurrence of a permissive role of A2ARs towards CB1R effects [726]. In addition, this permissive role of the A2AR was reported to occur in postsynaptic effects [726].
The main psychoactive compound in Cannabis sativa, THC, a ligand of cannabinoid receptors, is known to induce a variety of behavioural responses and undesirable effects such as dependence, anti-anxiety effects and memory impairments [727-731]. Different studies have shown that THC and other cannabinoid-induced behaviours are typically mediated by 5-HT2AR [732-734]. CB1R, which typically couples to Gi and Gq 5-HT2AR, coupled to Gq, was found to be colocalized in brain structures involved in regulating emotions, learning, and memory, including the amygdala, cerebral cortex, and hippocampus [735-737]. For the first time in 2015, it was discovered that the anxiolytic and amnestic effects of THC, a CB1R-agonist, require the presence of 5-HT2AR [731]. Behavioural studies in 5-HT2AR KO mice, BRET, cAMP and calcium signalling assays using cotransfected HEK293T cells and in situ PLA using mouse brain slices, determined a remarkable 5-HT2AR-dependent dissociation in the beneficial antinociceptive effects of THC and its detrimental amnesic properties, mediated by CB1R-5-HT2AR [731]. Furthermore, their study showed that CB1R and 5-HT2AR are expressed and functional in specific brain regions involved in memory impairment [731]. Moreover, it was shown that in CB1R-5-HT2AR co-stimulation of both receptors by agonists WIN 55212-2 and DOI reduces cell signalling, antagonist binding to one receptor (either rimonabant or MDL 100907) blocks signalling of the interacting receptor, and heterodimer formation leads to a switch in G-protein coupling for 5-HT2AR from Gq to Gi [731]. Heterodimerization was shown to be disrupted in vivo by ICV infusion of synthetic peptides with the sequence of TM5 and TM6 of CB1R, leading to blunted amnesic and anxiolytic, but not antinociceptive, effects of THC selectively in wild-type mice [731]. Later Galindo et al. presented more evidence that CB1R-5-HT2AR exists in ex-vivo primary cultures of human olfactory epithelial cells [738]. Furthermore, they observed a positive correlation between CB1R-5-HT2AR heterodimer expression, and the amount of cannabis consumed. A negative correlation was observed between heterodimer expression levels and attention and working memory performance in cannabis users [738]. Galindo and co-workers also observed negative crosstalk between CB1R and 5-HT2AR within the heterodimers in human olfactory epithelial cells when co-stimulated with WIN 55212-2 and DOI, which would lead to reduced activation of ERK1/2 signalling [738]. Furthermore, rimonabant and MDL 100907 blocked the effects induced by WIN 55212-2 and DOI, suggesting that CB1R-5-HT2AR in control subjects and in cannabis users display bidirectional cross antagonism [738].
2.2.12. Diverse GPCR Heterodimers
Besides the more common families described above, other class A GPCRs can form heterodimers. One example is the GAL1R-GAL2R heterodimer, identified in HEK293T cells using BRET and in the midbrain raphe-dorsal hippocampal pathways of rodents using in situ PLA [739]. In this study by Borroto-Escuela et al., the hypothesis was formulated that the N-terminal galanin fragments preferring binding sites on galanin receptors are formed through the formation of GAL1R-GAL2R heterodimers. The galanin 1-15 fragment was shown to induce a disbalance in GAL1R-GAL2R signalling, where enhanced activation of Gi/o-mediated signalling via GAL1R was observed, while no significant effects were induced by Gq/11-mediated signalling of GAL2R [739]. By comparing the results of the study between the two galanin fragments, galanin (1-15) and galanin (1-29), it was suggested that the orthosteric agonist binding site of GAL1R may have an increased affinity for the galanin (1-15) vs galanin (1-29), leading to its demonstrated increase in potency to inhibit CREB vs galanin (1-29) in CRE luciferase reporter gene assays [739]. Furthermore, Borroto-Escuela and co-workers demonstrated that NFAT reporter gene assays galanin (1-29) shows a higher efficacy than galanin (1-15) in increasing Gq/11-mediated signalling over GAL2R of GAL1R-GAL2R heterodimers [739]. The reported galanin(1-15)-mediated disbalance may contribute to depression and anxiety-related behaviours [740, 741].
In 2020, Rivas-Santisteban et al. discovered the existence of AT1R and AT2R heterodimer expression in hemilesioned 6-OH-DA rat model of PD [742]. AT1R and AT2R, which are part of the angiotensin-peptide producing RAS, and their endogenous ligand angiotensin are important regulators of motor control, have been suggested to be promising targets for PD and related conditions such as levodopa (L-DOPA)-induced dyskinesias [742-744]. In their study, Rivas-Santisteban and co-workers demonstrated that co-activation of AT1R and AT2R by Ang II and CGP-42112A within the AT1R-AT2R heterodimer was known to reduce the downstream signalling of angiotensin II [742]. However, a cross-potentiation was observed, as the application of AT1R-antagonist candesartan increased the effect of the selective AT2R-agonist CGP-42112A [742]. Regarding their relevance for PD, it was demonstrated that microglial AT1R-AT2R heterodimers are upregulated in parkinsonian conditions and in L-DOPA-induced dyskinesias and their activation was observed to exert neuroprotective effects [742]. Lastly, Rivas-Santisteban et al. suggested that the opposite action of AT1R and AT2R by AT1R-antagonist-mediated cross-potentiation of AT2R actions and the upregulation of AT1R-AT2R heterodimers in microglia may be beneficial to treat PD through AT2R by this heterodimer signalling mechanism [742].
3. CLASS C G PROTEIN-COUPLED RECEPTORS
3.1. Class C Receptors in the Brain
The class C receptor family in humans is composed of γ-aminobutyric acid B receptors (GABAB1R and GABAB2R receptors), calcium-sensing receptor (CaSR), metabotropic glutamate receptors (mGlu1-8R), sweet and amino acid taste receptors and several orphan receptors (GPR156, GPR158, GPR179, GPRC5A, GPRC5B, GPRC5C, GPRC5D, GPRC6) [144, 443, 745]. Among them, CaSR, GABABR and mGluR are highly expressed in the brain and represent an important class of drug targets for neurological diseases and calcium homeostasis [746-748].
mGluR and GABABR receptors are particularly relevant as they constitute a comprehensive model for the allosteric regulation and cooperativity of receptor protomers, which can be tendentially transferred to other GPCR classes, such as class A receptors [89]. Even though their sequences and overcall structures differ significantly from other classes, some structural similarities have been reported between classes A and C receptors. The most significant similarities were found in the TM domains. In class A receptors, the “ionic lock” is defined by a salt bridge between a conserved Arg3.50 and Glu(Asp)6.30, while this motif occurs via Lys3.50 and Glu6.35 in class C [749].
Aside from the common architecture of GPCRs, the class C receptors possess an extracellular domain that contains a Venus flytrap (VFT) module and a cysteine rich domain (CRD, except in the GABABR) [748]. This exceptionally large extracellular domain contains the orthosteric binding site for ligands, while in the 7TM region only allosteric binding sites are found [748]. Moreover, the C-terminus is highly variable and plays a role in scaffolding and signalling protein coupling [745]. Another unique characteristic of the class C receptor family is the fact that they only function as homodimers (mGluR and CaSR) or heterodimers (GABABR) [748]. The structure of the VFT was first solved for the mGlu1R (PDB-id: 1EWV, 1EWT, 1EWK) [750] and it revealed that the VFT consists of a bilobed domain being separated by a cleft in which endogenous ligands are able to bind [750-752]. In the absence of a ligand, the VFT oscillates between an open and closed conformation [748]. Agonists interact with lobe 1 in the open form of the VFT and stabilize the closed conformation through additional contacts with lobe 2, while antagonists inhibit the VFT closure [748, 753]. Due to the necessary dimerization of class C receptors, the VFTs consequently interact with each other by forming constitutive dimers. Different studies found that a hydrophobic interaction between lobe 1 of each monomer is the driving force for VFT dimerization [754, 755]. An additional disulphide bond linking the two VFTs was reported to further stabilize the dimer [755-757]. The CRD, which is a segment of 80 amino acids, containing 9 conserved cysteines, connects the VFT and the 7TM domains [745, 748]. Crystallography data shows that the CRD physically separates the VFT and 7TM modules (PDB-id: 2E4U, 2E4V, 2E4W, 2E4X, 2E4Y, 2E4Z) [758]. Especially for mGluR, a conserved disulphide bond between the VFT and the CRD is necessary for receptor activation through allosteric interaction between VFT and 7TM [759]. CRD is also a mediator of receptor activation for CaSR [748, 760].
3.1.1. Calcium-sensing Receptor
CaSR is a unique receptor, highly sensitive to calcium ions (Ca2+) and their concentration change in the extracellular space [748]. CaSR ensures calcium homeostasis and can consequently be activated by calcium itself without the cooperation of other ligands [746, 761]. Pathological conditions involving CaSR are hyperparathyroidism, osteoporosis and different forms of hypocalcemia [761-763]. CaSR is pharmacologically targeted by positive allosteric modulators (PAMs), i.e., cinacalcet, evocalcet and etelcalcetide, for the treatment of secondary hyperparathyroidism and familial hypocalciuric hypercalcemia (FHH1). CaSR negative allosteric modulators (NAMs) act as calcilytics and are currently in phase II clinical trials for the treatment of Autosomal-Dominant Hypocalcemia Type 1 (ADH1) [764, 765].
Although CaSR is mainly expressed in the parathyroid gland and in the renal tubules of the kidney, there is also a significant expression in the brain [766, 767]. Calcium is one of the most abundant second messengers in the brain [768]. In the extracellular space calcium levels are maintained constant (between 1.1 and 1.4 mM), whereas in resting neurons calcium levels are strictly maintained around 100 nM [769, 770]. Without a substantial calcium gradient, neuronal functions, such as gene transcription, synaptic transmission, memory encoding, apoptosis, and many others, may not be conducted [768, 769]. The inability to maintain required calcium levels has been brought into context with neurodegenerative diseases such as PD, AD, HD, where this neuronal calcium dysregulation contributes to motor and/or cognitive dysfunctions [769, 771-775].
3.1.2. γ-aminobutyric Acid B Receptors
γ-aminobutyric acid (GABA) is the major neurotransmitter for inhibitory signals in the mammalian CNS [748]. GABABR, which responds to GABA, regulates synaptic plasticity, learning, and memory in the dentate gyrus [1], mediating a slow and prolonged synaptic inhibition [776]. It only functions as obligate heterodimers of the two subtypes, GABAB1R and GABAB2R [777-782] with two distinct features: GABAB1R contains the GABA binding site [783], whereas GABAB2R activates the Gi/o protein [784]. GABAB receptors are responsible for neuronal excitability and plasticity [748]. For instance, in a VaD rat hippocampus, a reduction was observed in GABABR expression, resulting in spatial learning and memory deficits [1, 785]. However, under certain conditions, they may promote neuron survival such as metabolic stress, ischemia and apoptosis [748, 786-788]. Consequently, these receptors are considered promising targets for the treatment of many diseases, including spasticity, neuropathic pain, drug addiction, schizophrenia, anxiety, depression or epilepsy [789-792].
3.1.3. Metabotropic Glutamate Receptors
L-Glutamate is the major neurotransmitter for most of the excitatory synapses in the mammalian CNS [8]. As L-glutamate is the endogenous ligand for mGluR, they participate in the neuronal excitability and modulation of synaptic transmission in the CNS [793, 794]. The mGluR family comprises eight members, which are further classified based on their G protein coupling and sequence homology. The first group (Group I) consists of mGlu1R and mGlu5R, which are coupled to Gq/G11 [1, 795]. The second group consists of mGlu2R and mGlu3R (Group II) and the third group (Group III) of mGlu4R, mGlu6R, mGlu7R and mGlu8R, of which all are coupled to Gi/Go [793, 795]. As such, mGlu receptors negatively regulated the adenylyl cyclase (AC) and were also reported to activate MAP kinase and PI-3-kinase pathways [793, 795]. The mGlu5R has been reported to be involved in several neurodegenerative disorders [793]. Since mGlu5R is highly expressed in astrocytes, glial cells and neurons of the forebrain and hippocampus, several lines of evidence suggest a significant role of mGlu5R in developmental and neurodegenerative disorders such as Down Syndrome and AD [796, 797].
Over the last years, several mGluR agonists, antagonists, PAMs and NAMs have been developed and studied in vivo animal models [1]. Comprehensive work from Chen et al. showed that LY341495 (Group I/II mGluR antagonist) was able to block amyloid β-enhanced long-term depression and improve synaptic plasticity [798]. In addition, the authors also showed that pre-treatment with mGlu1/5R agonist, DHPG, decreased amyloid β-enhanced long-term depression [798]. Another study by Renner et al. demonstrated that SIB1757, a non-competitive antagonist of mGlu5R, prevented amyloid β oligomer-induced synaptic N-Methyl-D-aspartic acid receptor NMDAR reduction [799]. Moreover, Caraci and co-workers demonstrated that the mGlu2R PAM LY566332 amplified amyloid β-induced neurodegeneration, while treatment with the antagonist LY341495 of mGlu2/3R prevented this effect [800]. In a similar manner, the dual mGlu2/3R agonist LY379268 exhibited neuroprotection by a paracrine mechanism mediated by transforming growth factor-β1 [800]. Consequently, negative modulation of the mGlu5R could be a promising strategy for the treatment of PD and AD. Moreover, dual activation of Group II receptors, mGlu2R and mGlu3R could be a strategy for providing neuroprotection against amyloid β-induced toxicity [800].
3.2. Class C Receptor Heterodimers
In 1998, Marshall and colleagues discovered that heterodimerization formation was crucial for a functional GABABR [76, 801]. Since then, the concept of GPCR dimerization has been widely accepted for class C receptors. The receptor-receptor cooperation has been found to be positive and negative and vital for signal transduction [89, 802-805]. Class C GPCRs act as obligate dimers, since the VFTs of the single receptors have to interact with each other [754-757]. Therefore, homo- and heterodimerization is a common event among class C GPCRs.
In a recombinant system, it was found that GABAB1R cannot reach the cell surface without the presence of GABAB2R, as GABAB1R contains a C-terminal endoplasmic retention motif, only masked when the heterodimer is formed (Fig. 2E) [806, 807]. Unexpectedly, all orthosteric agonists and antagonists rather bind to the VFT of the GABAB1R. This coupling leads to the necessary conformational change in GABAB1R, which crosstalks to GABAB2R leading to an active conformer able to bind to G protein and promoting functional physiological responses [808-811]. Also, additional GPCRs which can bind to GABABR were identified: e.g., GABAAR, mGlu1R, NMDA, IGF-1, and TrkBR. It also has been shown that these GPCRs are able to form multi-complexes such as tetramers [812, 813]. Such tetramers were described to exhibit negative cooperativity between the GABABR-heterodimers by decreasing the coupling efficiency towards Gi proteins [802, 814]. Despite the large therapeutic potential and the development of many PAMs and NAMs which could help investigate the relationship between the monomers of the GABABR, only Baclofen (Lioresal), a selective GABABR agonist is available on the market [815, 816].
Furthermore, the well-known GABABR, the eight members of the mGluR family, are key modulators of glutamatergic synaptic transmission of excitatory and inhibitory responses in the brain [794, 817, 818]. The structure of the mGluR contains special features such as a large cysteine enriched domain, which is linked to the transmembrane domain, and a large extracellular domain involving the VTF, where glutamate binds involving the VFT, which is also linked to the transmembrane domain, binding pocket to glutamate [76]. Many GPCRs are known to interact and regulate the mGluR subgroups, such as the neuronal Ca2+ binding protein 2 that forms a co-assembly and coupling with activated Ca2+-activated K+-channels; and the contactin-associated protein 1, which appear to be important for the function of mGlu5R to control memory formation in the hippocampus [76, 819-823].
For instance, the mGlu2R-mGlu4R heterodimer was already discovered in 2005 by Doumazane and co-workers using a technique to study plasma membrane receptor complexes and FRET [824]. Later, Kammermeier and co-workers elucidated that mGlu2R-mGlu4R complexes are functional in neurons, only using both, mGlu2R specific and mGlu4R specific agonists [825]. Each individual receptor has two NAM binding sites and one PAM binding site. The activation of each receptor by NAMs was able to reverse the signalling of this heterodimer. Moreover, only one PAM per complex was needed for full enhancement of heterodimer complex activity [824].
The mGlu1R-mGlu5R heterodimer in mice, was identified by Pandya and co-workers in 2016 [826]. The mGlu1R-mGlu5R was expressed in the cerebral cortex, hippocampus and hippocampal neurons using an interaction proteomics strategy and super resolution microscopy [826]. The exact receptor complex composition is still unclear, but there is the indication that scaffolding proteins, phosphatases and kinases are involved in the process [827]. In synaptic and extra-synaptic locations, mGlu1R-mGlu5R also appears to be in balance with the corresponding homodimers mGlu1R-mGlu1R and mGlu5R-mGlu5R [828]. The mGlu1R-mGlu5R heterodimer may be a potential therapeutic target in autism spectrum disorders [826].
Although class C GPCRs, and especially mGluRs, are functional as constitutive dimers, the importance of dimerization remains unclear [818]. A study by El Moustaine et al. also demonstrated that the dimer formation is not required for G protein coupling, but rather for agonist activation and for limiting the agonist activity of PAMs [810, 818]. This asymmetrical activation is also consistent with the asymmetric functioning reported for class A GPCR dimers [452, 829, 830].
4. HETERODIMERS CLASS A-CLASS C
GPCR heterodimers of the same classes such as class A - class A or class C - class C appear to be physiologically conclusive as they have the same activation mechanism despite the different ligands and G protein-coupling state. Also, their physiological functions appear coherently, notwithstanding that the partnered proteins often belong to different families. However, the existence of GPCR heterodimers of different classes such as class A - class C heterodimers, which will be described here, add another perspective to the complexity of GPCR signalling.
In 2020, Sebastianutto et al. discovered the DRD1-mGlu5R heterodimer using BRET and bimolecular fluorescence complementation (BiFC) techniques at the plasma membrane in HEK293 cells, primary hippocampal neurons and in 6-OHDA lesion in mice and rats, which were used as PD models [831]. The dopaminergic and glutamatergic systems are known to signal to the striatum where their crucial inputs control action selection and behavioural plasticity [832, 833]. Hence, these basal-ganglia circuits represent an important target of L-DOPA-based therapy in PD [831]. Sebastianutto and co-workers demonstrated that the DRD1-mGlu5R synergistically activates PLC signalling and intracellular calcium release in response to either glutamate or dopamine [831]. In addition, PLC signalling was seen to be responsible for a considerable proportion of striatal ERK1/2 activation in PD-model rodents which were treated with DRD1-agonists SKF38393 or quinpirole [831]. Moreover, in the PD-model rodents, DRD1-mGlu5R complexes were found to be strongly upregulated in the dopamine-denervated striatum [831]. DRD1-mGlu5R-dependant PLC signalling was also linked to enhanced activation of extracellular signal-regulated kinases in striatal neurons, leading to dyskinesia in animals treated with L-DOPA or DRD1-agonists SKF38393 or quinpirole [831]. It was concluded that DRD1 appeared to engage in preferential crosstalk with mGlu5R- and Gq-related signalling components in dopamine-denervated striatal neurons [831].
Another example, A1R-mGlu1R, was discovered by Ciruela et al. in 2001 using Co-IP, immunohistochemistry and ligand-binding experiments in HEK293 and rat cerebellum synaptosomes [834]. Furthermore, they showed that activation of A1R and mGlu1R would lead to a synergistic neuroprotection effect, since preincubation with quisqualic acid (mGlu1R-agonist) and adenosine was much more effective than pre-treatment with any of the compounds used in their study. Later, more studies based on an analysis of non-neuronal cells using Co-IP and FRET by Kamikubo et al. supported the existence of A1R-mGlu1R [835]. In a previous study, it was described that, in cerebellar Purkinje cells, the activation of A1R attenuates neuronal responses to glutamate, as mediated by mGlu1R [835, 836]. The mGlu1R is also known to regulate responses such as long-term depression of postsynaptic response to glutamate, which is a cellular basis for cerebellar motor learning [835]. Furthermore, Kamikubo and co-workers demonstrated that the activation of mGlu1R through glutamate inhibits A1R signalling, which was measured in elevated cAMP signalling, since the A1R is known to couple Gi/o-proteins [835, 837]. Kamikubo et al. concluded from their findings that mGlu1R-mediated inhibition of A1R signalling, which should activate PKA and CREB may play a role in mGlu1R-dependent cerebellar long-term depression and motor learning [835].
In 2008, González-Maeso et al. identified a physical and functional interaction between 5-HT2AR and mGlu2R in cortical pyramidal neurons using Co-IP, BRET and FRET in HEK293 cells and brain cortices from mice and humans [838, 839]. Competition binding experiments showed that the mGlu2R-agonist LY379268 was able to increase the affinity of hallucinogenic drugs such as DOI, DOM or for the 5-HT2AR-binding site [839]. However, it was also shown that the 5-HT2AR-agonist DOI decreased the affinity for mGlu2R-agonists LY379268, DCG-IV, and L-CCG-I [839]. Hence, within the 5-HT2AR-mGlu2R, unique cellular responses are mediated when targeted by hallucinogenic drugs and activation of mGlu2R was shown to abolish hallucinogen-specific signalling and behavioural responses. González-Maeso et al. further supported those findings by showing that hallucinogens, including mescaline, psilocybin, and lysergic acid diethylamide (LSD) which profoundly affect perception, cognition, and mood and are known to activate 5-HT2AR, but not all excerpt hallucinogenic behaviours [840]. It was shown that hallucinogenic and non-hallucinogenic 5-HT2AR-agonists both regulate signalling in the same 5-HT2AR-expressing cortical neurons. However, different agonists were found to either regulate phospholipase C via coupling to Gq/11 proteins and/or bind to Gi/o proteins and Src [840]. Fribourg et al. demonstrated that the signalling of the endogenous ligand on the associated protomer is suppressed or potentiated by an agonist or an inverse agonist of one protomer, respectively [841]. Therefore, the 5-HT2AR-mGlu2R heterodimer establishes an optimal Gi/o-Gq balance in response to serotonergic and glutamatergic drugs binding. The hallucinogenic agonists LY341495 (mGlu2R inverse agonist) and DOI (5-HT2AR receptor agonist) promote a decrease in Gi/o and a strong increase in Gq. The opposite happens with the antipsychotics LY379268 (mGlu2R receptor agonist) and clozapine (inverse 5-HT2AR receptor agonist), which produce the opposite effect on Gi/o-Gq balance. Lastly, González-Maeso and co-workers identified that mGlu2R interacts via TM4 and TM5 with 5-HT2AR [839].
In 2009, Schröder et al. identified the MOR-mGlu5R heterodimer using Co-IP in HEK293 cells [842]. It was long hypothesized that opioid analgesia and tolerance could be modulated by metabotropic glutamate receptors [842-845]. Studies by Gabra et al. and Lee et al. were able to show that the mGlu5R-antagonist MPEP inhibits hyperalgesia, nociceptive behaviour and inflammation. Moreover, when co-administered with morphine, the morphine-induced antinociception development was suppressed [846, 847]. The treatment of the cotransfected MOR and mGlu5R cells with DAMGO, a selective MOR-agonist, showed that co-expression of mGlu5R had no significant effect on the agonist binding sites and functional coupling of the MOR towards DAMGO, as DAMGO-induced inhibition of intracellular cAMP level was still observed [842]. However, when MPEP was co-administered, DAMGO-induced MOR phosphorylation, internalization, and desensitization were decreased, whereas non-selective competitive mGlu5R-antagonists or -agonists had no effects [842]. According to Schröder et al., this allosteric modulation of mGlu5R on MOR displayed a mechanistic basis as to how the MOR-mGlu5R functions, further supported by DAMGO-induced co-internalization of MOR and mGlu5R and the increase of MPEP bindings sites and a change of binding affinity of mGlu5R after the co-expression of MOR [842].
5. HETERORECEPTOR MOSAICS
The term “receptor mosaics” stands for assemblies of more than two receptors and was already introduced in the 80s to underline the role of topology in the highly dynamic life cycles of GPCRs [848-851]. Such mosaics may be the result of engrams of short-term memory, which are stored as a state of a molecular circuit. They further suggested that these mosaics may be the representations of engrams of ultra-short memory in transient receptor mosaic formed in kiss-and-run encounters [850, 852-854]. There are now many indications that heteroreceptor mosaics exist in nerve cells and throughout the brain [850, 853, 855].
The A2AR-CB1R-DRD2 mosaic is one of the few examples where more than two receptors exhibit protein-protein interactions [533, 726, 856]. It also underlines the relevance of adenosine, dopamine and cannabinoid signalling and their pivotal contribution to various signalling mechanisms. The A2AR-CB1R-DRD2 heterooligomer was identified for the first time in 2008 [857] using a method combining BiFC and BRET techniques [858-864]. In 2009, the A2AR-DRD2-mGlu5R was discovered in HEK293 cells, using BiFC and BRET approaches [865]. In addition to adenosine and dopamine transmission, glutamate transmission also plays an important role in the function of striatal GABAergic efferent neurons originating in the nucleus accumbens. In 2001, Popli et al. discovered the DRD2-mGlu5R heterodimer and its association with A2AR receptor [866]. Authors used 6-OH-DA-lesioned rats as PD models to conduct behavioural and microdialysis experiments. In 6-OH-DA rats, the selective mGlu5R-agonist (RS)-2-Cholro-5-Hydroxyphenylglycine (CHPG) was shown to inhibit the contralateral turning induced by quinpirole, a DR-agonist and less pronounced by the DR-agonist SKF 38393 [866]. The effect of CHPG on quinpirole-induced turning was significantly potentiated by CGS 21680, an A2AR-agonist and attenuated by SCH 58261, an A2AR-antagonist [866]. CHPG was shown to reduce the affinity of the high-affinity state of DRD2 for quinpirole and this effect was again enhanced by CGS 21680 in rat striatal membranes [866]. A2AR and mGlu5R agonists (CGS 21680 and CHPG, respectively) synergistically increase ventral pallidal extracellular level of GABA in the nucleus accumbens promoting potential stability of the inhibitory dopaminergic DRD2 effects on the striatopallidal GABA pathway [867]. In PD, where the dopaminergic nerve terminals are degenerated, the DRD2 on the glutamate nerve terminals can no longer appropriately inhibit glutamate release. Here, A2AR and mGlu5R antagonists could be successful to inhibit parkinsonian symptoms considering their increasing dominance, since the inhibitory DRD2 lose their function [868]. Consequently, extracellular levels of adenosine and glutamate may increase, leading to a higher probability of formation of A2AR-DRD2, DRD2-mGlu5R and A2AR-DRD2-mGlu5R that leads to further inhibition of PD symptoms. Lastly, A2AR-CB1R-DRD2 and A2AR-DRD2-mGlu5R mosaics have recently been demonstrated in living cells using fluorescent techniques [861, 865].
6. GPCR INTERACTING PROTEINS
Besides the binding of GPCRs to G proteins, β-arrestins and kinases, there exists a large number of GPCR interacting proteins (GIPs) [823, 869-872]. GIPs can be other cytoplasmic or transmembrane proteins such as heat-shock proteins, PSD-95/Discs-large/ZO-1 (PDZ) domain-containing proteins or GPCR-associated sorting proteins (GASPs) [873-875], among many others. They excerpt multiple effects on GPCRs: interact with GPCRs in a more receptor-selective manner and can additionally mediate downstream signalling directly through binding to GPCRs, organize GPCR signalling through G proteins, promote receptor trafficking or anchor the receptors in certain subcellular areas [823, 869, 872, 876]. In contrast to GPCRs, GIPs are capable of clustering various proteins and coordinating different types of signals such as positive and negative feedback signals, graded or digital signals, and transient or oscillatory signalling [823, 877, 878].
In terms of GPCR dimerization, it is known that the receptors influence each other through protein-protein interactions (PPIs) and subsequent conformational rearrangements upon the dimerization event. These can also influence the affinity for the binding of G proteins, and alterations in ligand binding affinity, among many other effects [72, 80-82]. It also raised the question, how could dimerization affect the coupling to GIPs [871]? For instance, it was reported that MT1R directly and constitutively bind to Gi proteins and RGS20, forming the MT1R homodimers-RGS20-Gi protein complex [879]. Regulators of G-protein signalling (RGS) proteins bind to the activated form of G𝛼 proteins and accelerate their GTPase activity [880, 881]. By using BRET probed inserted at multiple sites of the complex and by homology modelling experiments, Maurice et al. suggested a model, where the Gi protein can bind to one MT1R, while the RGS20 binds to the other MT1R [879]. Similar observations were made for MT1R/MT2R-RGS20-Gi protein-complex, which was previously not known to bind to RGS20 [879]. Hence, it was concluded that the formation of asymmetric quaternary complexes involving GIP-binding and non-GIP-binding receptors may lead to sensitivity for GIPs, which is only present upon formation of such complexes [871, 879]. Another example was recently discovered involving mGluRs, known to be obligate dimers. The constitutive active mGluR1 and mGluR5, in the absence of glutamate, were reported to form an interaction with Homer1a. This protein, part of the scaffolding protein family Homer, lacks dimerization capacity [882-885]. Usually, mGlu1/5R dimers are functionally physically linked to NMDA receptors via scaffolding proteins, which are then disrupted through the binding of Homer1a [882, 886, 887]. The mGlu1/5R-Homer1a-complex has been associated with several functions in synaptic plasticity of the visual system, in rewarded synapses, in chronically overactivated synaptic networks and sleep cycle [882].
Hence, the binding of GIPs to GPCR dimers adds another level of signalling complexity towards downstream signalling and indicates that its fine-tuning can also be context-dependent [886, 888].
SUMMARY AND CONCLUDING REMARKS
It has been widely accepted by now that GPCRs are able to couple to other GPCRs to alter their partner’s signalling and/or their own, which furthermore diversifies and fine-tunes their physiological responses. Many studies have demonstrated that the nature of crosstalk within the heterodimer or oligomer can be either positive or negative. Hence, when GPCRs form a heterodimer, it was shown that this leads to the enhancement of each other’s natural signalling pathways or inhibition of downstream signalling of either one receptor or both. Among all heterodimers described in this review, there was a clear balance between examples, which promoted either positive or negative crosstalk. In addition, there are still more options of alternate signalling by heterodimers to be investigated. Especially, when it comes to formation of oligomers, the signalling repertoire is even further increased.
The concept of GPCR dimers, which carry out physiological and pathophysiological actions in the brain, adds a new dimension to molecular signalling in the nervous system.
To be able to target both monomers within the dimer at the same time, new concepts in drug design have been explored. Already in 1982, the concept of bivalent ligand was discovered and introduced for opioid heteromers by Philip Portoghese [889, 890]. Many have been developed over the years [891, 892] to target other disease-relevant dimers. By definition, bivalent ligands consist of two distinct pharmacophores that are connected by a linker/spacer; hence they are able to target two GPCRs simultaneously [893]. They can be further classified into either homobivalent ligands with two identical pharmacophores or heterobivalent ligands with two different pharmacophores [891].
The advantage of targeting both monomers of the dimer simultaneously provides insight into enhanced physiological responses and may help to understand the dynamic interactions of the two proteins, as usually, either one of them is targeted by their ligands resulting in positive or negative crosstalk. Moreover, bivalent ligands can be designed to either consisting of two agonists, two antagonists or cross-overs which make them a helpful tool to understand the dynamics of dimerization and subsequent downstream signalling [891]. Considering the recent developments of bivalent ligands, most of them target class A heteromers, including opioid receptors, dopamine receptors, serotonin receptors and cannabinoid receptors [891]. However, one ligand was developed for a heterodimer between class A and class C, MQ-22a, which targets the DRD2-mGlu5R heteromer [894]. Interestingly, an allosteric modulator 3-[(2-methyl-4-thiazolyl)ethynyl]pyridine (MTEP) was chosen for mGlu5R, while for DRD2 ligands targeting the orthosteric binding pocket were selected, the DRD2- and DRD4-agonist 5-hydroxy-2-(dipropylamino)tetralin (DPAT) and the DRD2-antagonist 1, 4-disubstituted aromatic piperazine (DAP) [894]. Consequently, also the level of binding property of the selected pharmacophores provides an additional chemical space to be explored in terms of dimerization dynamics as it has been shown for monomers that the physiological response also varies if ligands bind either to orthosteric or to the allosteric binding pockets [895-897]. Other bivalent ligands were also proposed to induce dimerization, as shown for the gastrin-releasing peptide receptor GRPR within 20-30Å [898].
Together with the growing numbers of GPCR crystal structures available and the improvement in computational techniques such as homology modelling, ligand docking and molecular dynamics, bivalent ligands are additional pharmacological tools for investigating the dimerization process and dynamics. Still, there is room for improvement as none of the bivalent ligands has made it to clinical trials yet, mostly due to their large size (> 700 kDa) and consequent unfavourable pharmaco-chemical properties, selectivity profiles and lacking in vivo studies [891].
Aside from bivalent ligands, nanobodies, which are mostly derived from antibody fragments from the heavy chain-only antibodies of camelids, have emerged as promising alternatives due to their high target specificity [899, 900]. Like bivalent ligands, nanobodies were also discovered in the 1980s but their utility was for long not recognized [901]. Nanobodies can be fused to fluorescent tags, radioisotopes or other biosensors to monitor cellular processes in living cells [900]. More recently, fluorescently labelled conformation-specific nanobodies were utilized to monitor the activation of GPCRs upon ligand binding or rapid state transformation in living cells [900, 902-905]. In another more recent study. nanobodies were used to modulate the activity of mGlu4R in the brain but not Glu4R heteromers with other GluRs, indicating that therapy of PD or pain could be improved through subtype-selective and blood-brain barrier permeable nanobodies [899]. While only biparatopic nanobody targeting different binding sites of the chemokine receptor CXCR2 entered phase 1 studies as potential new therapeutic for inflammation [906, 907], nanobodies specifically targeting GPCR dimers (homo- and heterodimers) will be for sure a promising new therapeutic approach. Ernumab, a monoclonal antibody, was recently approved for preventive treatment of chronic migraine as it binds to the calcitonin gene-related peptide receptor dimers [906, 908, 909].
All in all, future studies should be directed to identifying the dimer interface to design and develop interface-interfering molecules, able specifically disrupt the dimer. This strategy will help determine the functional role of the dimer as well as the allosteric receptor-receptor interaction within the dimer.
Herein, we provided a collection of neurodegenerative-relevant GPCR heterodimers of classes A and C, which appear to be promiscuous in their signalling. A detailed structural and functional characterization of these macromolecular machineries will be key to the development of new and improved drugs to treat neurodegenerative diseases.
ACKNOWLEDGEMENTS
Declared none.
LIST OF ABBREVIATIONS
- 5-HT
5-hydroxytryptamine
- ACE
Angiotensin-Converting Enzyme
- AD
Alzheimer’s Disease
- ADH
Antidiuretic Hormone
- ADH1
Autosomal-Dominant Hypocalcemia Type 1
- ADHD
Attention Deficit Hyperactivity Disorder
- ALS
Amyotrophic Lateral Sclerosis
- ASD
Autism Spectrum Disorder
- AR
Adenosine Receptors
- Aβ
Amyloid β
- BDNF
Brain-Derived Neurotrophic Factor
- BiFC
Bimolecular Fluorescence Complementation
- BRET
Bioluminescence Resonance Energy Transfer
- CaMKIIa
Calcium/Calmodulin Kinase IIa
- cAMP
Cyclic Adenosine Monophosphate
- CaSR
Calcium-Sensing Receptor
- CCK
Cholecystokinin
- CCK8
Cholecystokinin Octapeptide
- CCL5
Chemokine Ligands for CCR5
- Cryo-EM
Cryogenic Electron Microscopy
- CNS
Central Nervous System
- Co-IP
co-immunoprecipitation
- CUD
Cannabis Use Disorder
- DOR
Delta Receptor
- ECS
Endocannabinoid System
- ERK
Extracellular Signal-regulated Kinases
- EPSCs
Excitatory Postsynaptic Currents
- FHH1
Familial Hypocalciuric Hypercalcemia
- FRET
Förster-Resonance-Energy-Transfer
- FK
Forskolin
- FTD
Frontotemporal Dementia
- FUS
Fused in Sarcoma
- FZD
Class F-frizzled
- GABA
γ-aminobutyric Acid
- GASPs
GPCR-associated Sorting Proteins
- GHS-R
Growth Hormone Secretagogue Receptor
- GHRH
Growth Hormone-Releasing Hormone
- GIPs
GPCR Interacting Proteins
- GIRKs
G Protein-Coupled Inwardly Rectifying Potassium Channels
- GPCR
G Protein-Coupled Receptor
- HD
Huntington’s Disease
- HEK293
Transfected Human Embryonic Kidney 293
- HR
Histamine Receptor
- HTT
Huntingtin
- KO
Knockout
- KOR
Kappa Receptor
- LSD
Lysergic Acid Diethylamide
- MAPK
Mitogen-activated Protein Kinase
- MDD
Major Depressive Disorder
- MS
Multiple Sclerosis
- MOR
Mu Receptor
- NAcC
Nucleus Accumbens Core
- NAMs
Negative Allosteric Modulators
- NMR
Nuclear Magnetic Resonance
- NT
Neurotensin
- OCD
Obsessive-compulsive Disorder
- OT
Oxytocin
- OTR
Oxytocin Receptor
- OR
Opioid Receptor
- PAMs
Positive Allosteric Modulators
- PD
Parkinson’s Disease
- PFC
Prefrontal Cortex
- PNS
Peripheral Nervous System
- PPIs
Protein-Protein Interactions
- RAAS
Renin-Angiotensin-Aldosterone System
- RAMH
(R)-α-Methylhistamine
- RGS
Regulators of G-protein Signalling
- SCN
Suprachiasmatic Nucleus
- SMO
Smoothened
- SST
Somatostatin
- TAARs
Trace Amine-Associated Receptors
- THC
Δ-Tetrahydrocannabinol
- TDP-43
DNA-Binding Protein 43
- VaD
Vascular Dementia
- VFT
Venus Flytrap
- VTA
Ventral Tegmental Area
AUTHORS’ CONTRIBUTION
All authors contributed equally to the preparation of this manuscript.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
This work was funded by COMPETE 2020 - Operational Programme for Competitiveness and Internationalization and Portuguese national funds via FCT - Fundação para a Ciência e a Tecnologia, under projects LA/P/0058/2020, UIDB/ 04539/2020, UIDP/04539/2020, PTDC/QUI-OUT/32243/ 2017. B.B. was supported by FCT through PhD-scholarship SFRH/BD/149709/2019.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Azam S., Haque M.E., Jakaria M., Jo S.H., Kim I.S., Choi D.K. G-Protein-Coupled Receptors in CNS: A Potential Therapeutic Target for Intervention in Neurodegenerative Disorders and Associated Cognitive Deficits. Cells. 2020;9(2):506. doi: 10.3390/cells9020506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Przedborski S., Vila M., Jackson-Lewis V. Neurodegeneration: what is it and where are we? J. Clin. Invest. 2003;111(1):3–10. doi: 10.1172/JCI200317522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakaria M., Azam S., Cho D.Y., Haque M.E., Kim I.S., Choi D.K. The methanol extract of Allium cepa L. Protects inflammatory markers in LPS-induced BV-2 microglial cells and upregulates the antiapoptotic gene and antioxidant enzymes in N27-A cells. Antioxidants. 2019;8(9):348. doi: 10.3390/antiox8090348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jakaria M., Azam S., Jo S.H., Kim I.S., Dash R., Choi D.K. Potential therapeutic targets of quercetin and its derivatives: its role in the therapy of cognitive impairment. J. Clin. Med. 2019;8(11):1789. doi: 10.3390/jcm8111789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y., Todd N., Thathiah A. The role of GPCRs in neurodegenerative diseases: Avenues for therapeutic intervention. Curr. Opin. Pharmacol. 2017;32:96–110. doi: 10.1016/j.coph.2017.02.001. [DOI] [PubMed] [Google Scholar]
- 6.Arlt S. Non-Alzheimer’s disease-related memory impairment and dementia. Dialogues Clin. Neurosci. 2013;15(4):465–473. doi: 10.31887/DCNS.2013.15.4/sarlt. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertram L., Tanzi R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Invest. 2005;115(6):1449–1457. doi: 10.1172/JCI24761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lemos A., Melo R., Preto A.J., Almeida J.G., Moreira I.S., Dias Soeiro Cordeiro M.N. In silico studies targeting G-protein coupled receptors for drug research against Parkinson’s disease. . Curr. Neuropharmacol. 2018;16(6):786–848. doi: 10.2174/1570159X16666180308161642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Serrano-Pozo A., Frosch M.P., Masliah E., Hyman B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011;1(1):a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lopez OL. The growing burden of Alzheimer’s disease. Am J Manag Care. 2011;17(Suppl 1) [PubMed] [Google Scholar]
- 11.Dickerson B.C., Bakkour A., Salat D.H., Feczko E., Pacheco J., Greve D.N., Grodstein F., Wright C.I., Blacker D., Rosas H.D., Sperling R.A., Atri A., Growdon J.H., Hyman B.T., Morris J.C., Fischl B., Buckner R.L. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex. 2009;19(3):497–510. doi: 10.1093/cercor/bhn113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kalia L.V., Lang A.E. Parkinson’s disease. Lancet. 2015;386(9996):896–912. doi: 10.1016/S0140-6736(14)61393-3. [DOI] [PubMed] [Google Scholar]
- 13.Yassi N., Desmond P.M., Masters C.L. Magnetic resonance imaging of vascular contributions to cognitive impairment and dementia. J. Mol. Neurosci. 2016;60(3):349–353. doi: 10.1007/s12031-016-0799-3. [DOI] [PubMed] [Google Scholar]
- 14.Kalaria R.N. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016;131(5):659–685. doi: 10.1007/s00401-016-1571-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warren J.D., Rohrer J.D. Rossor, MN Frontotemporal dementia. BMJ. 2013;347(123):f4827. doi: 10.1136/bmj.f4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jicha G.A., Nelson P.T. Management of frontotemporal dementia: targeting symptom management in such a heterogeneous disease requires a wide range of therapeutic options. Neurodegener. Dis. Manag. 2011;1(2):141–156. doi: 10.2217/nmt.11.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ross C.A., Aylward E.H., Wild E.J., Langbehn D.R., Long J.D., Warner J.H., Scahill R.I., Leavitt B.R., Stout J.C., Paulsen J.S., Reilmann R., Unschuld P.G., Wexler A., Margolis R.L., Tabrizi S.J. Huntington disease: natural history, biomarkers and prospects for therapeutics. Nat. Rev. Neurol. 2014;10(4):204–216. doi: 10.1038/nrneurol.2014.24. [DOI] [PubMed] [Google Scholar]
- 18.Andrew S.E., Goldberg Y.P., Kremer B., Telenius H., Theilmann J., Adam S., Starr E., Squitieri F., Lin B., Kalchman M.A. The relationship between trinucleotide (CAG) repeat length and clinical features of Huntington’s disease. Nat. Genet. 1993;4(4):398–403. doi: 10.1038/ng0893-398. [DOI] [PubMed] [Google Scholar]
- 19.Gutekunst C.A., Li S.H., Yi H., Mulroy J.S., Kuemmerle S., Jones R., Rye D., Ferrante R.J., Hersch S.M., Li X.J. Nuclear and neuropil aggregates in Huntington’s disease: relationship to neuropathology. J. Neurosci. 1999;19(7):2522–2534. doi: 10.1523/JNEUROSCI.19-07-02522.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hinz F.I., Geschwind D.H. Molecular genetics of neurodegenerative dementias. Cold Spring Harb. Perspect. Biol. 2017;9(4):a023705. doi: 10.1101/cshperspect.a023705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dickson D.W., Ahmed Z., Algom A.A., Tsuboi Y., Josephs K.A. Neuropathology of variants of progressive supranuclear palsy. Curr. Opin. Neurol. 2010;23(4):394–400. doi: 10.1097/WCO.0b013e32833be924. [DOI] [PubMed] [Google Scholar]
- 22.Braak H., Thal D.R., Ghebremedhin E., Del Tredici K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011;70(11):960–969. doi: 10.1097/NEN.0b013e318232a379. [DOI] [PubMed] [Google Scholar]
- 23.Dugger B.N., Hentz J.G., Adler C.H., Sabbagh M.N., Shill H.A., Jacobson S., Caviness J.N., Belden C., Driver-Dunckley E., Davis K.J., Sue L.I., Beach T.G. Clinicopathological outcomes of prospectively followed normal elderly brain bank volunteers. J. Neuropathol. Exp. Neurol. 2014;73(3):244–252. doi: 10.1097/NEN.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dugger B.N., Dickson D.W. Pathology of neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2017;9(7):a028035. doi: 10.1101/cshperspect.a028035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jabeen A., Ranganathan S. Applications of machine learning in GPCR bioactive ligand discovery. Curr. Opin. Struct. Biol. 2019;55:66–76. doi: 10.1016/j.sbi.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 26.Saikia S., Bordoloi M., Sarmah R. Established and in-trial GPCR families in clinical trials: A review for target selection. Curr. Drug Targets. 2019;20(5):522–539. doi: 10.2174/1389450120666181105152439. [DOI] [PubMed] [Google Scholar]
- 27.Sensoy O., Almeida J.G., Shabbir J., Moreira I.S., Morra G. Methods in Cell Biology. Academic Press; 2017. Computational studies of G protein-coupled receptor complexes: Structure and dynamics. In: pp. 205–245. [DOI] [PubMed] [Google Scholar]
- 28.Guerram M., Zhang L.Y., Jiang Z.Z. G-protein coupled receptors as therapeutic targets for neurodegenerative and cerebrovascular diseases. Neurochem. Int. 2016;101:1–14. doi: 10.1016/j.neuint.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Heng B.C., Aubel D., Fussenegger M. An overview of the diverse roles of G-protein coupled receptors (GPCRs) in the pathophysiology of various human diseases. Biotechnol. Adv. 2013;31(8):1676–1694. doi: 10.1016/j.biotechadv.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 30.Rosenbaum D.M., Rasmussen S.G.F., Kobilka B.K. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245):356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fredriksson R., Lagerström M.C., Lundin L.G., Schiöth H.B. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol. Pharmacol. 2003;63(6):1256–1272. doi: 10.1124/mol.63.6.1256. [DOI] [PubMed] [Google Scholar]
- 32.Jabeen A., Vijayram R., Ranganathan S. BIO-GATS: A tool for automated GPCR template selection through a biophysical approach for homology modeling. Front. Mol. Biosci. 2021;8:617176. doi: 10.3389/fmolb.2021.617176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyagi H., Asada H., Suzuki M., Takahashi Y., Yasunaga M., Suno C., Iwata S., Saito J.I. The discovery of a new antibody for BRIL-fused GPCR structure determination. Sci. Rep. 2020;10(1):11669. doi: 10.1038/s41598-020-68355-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang Y., DeVries M.E., Skolnick J. Correction: Structure modeling of all identified G protein-coupled receptors in the human genome. PLOS Comput. Biol. 2006;2(3):e29. doi: 10.1371/journal.pcbi.0020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hauser A.S., Attwood M.M., Rask-Andersen M., Schiöth H.B., Gloriam D.E. Trends in GPCR drug discovery: new agents, targets and indications. Nat. Rev. Drug Discov. 2017;16(12):829–842. doi: 10.1038/nrd.2017.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y., Thathiah A. Regulation of neuronal communication by G protein-coupled receptors. FEBS Lett. 2015;589(14):1607–1619. doi: 10.1016/j.febslet.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 37.Betke K.M., Wells C.A., Hamm H.E. GPCR mediated regulation of synaptic transmission. Prog. Neurobiol. 2012;96(3):304–321. doi: 10.1016/j.pneurobio.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder S.H., Innis R.B. Peptide neurotransmitters. Annu. Rev. Biochem. 1979;48:755–782. doi: 10.1146/annurev.bi.48.070179.003543. [DOI] [PubMed] [Google Scholar]
- 39.Lodish H., Berk A., Zipursky S.L.E.A. 2000. Neurotransmitters, synapses, and impulse transmission. In: Mol Cell Biol, 4th Ed; Neurotransmitters, Synapses, and Imp. [Google Scholar]
- 40.Hall R.A. β-adrenergic receptors and their interacting proteins. Semin. Cell Dev. Biol. 2004;15(3):281–288. doi: 10.1016/j.semcdb.2003.12.017. [DOI] [PubMed] [Google Scholar]
- 41.Pytliak M., Vargová V., Mechírová V., Felšöci M. Serotonin receptors - from molecular biology to clinical applications. Physiol. Res. 2011;60(1):15–25. doi: 10.33549/physiolres.931903. [DOI] [PubMed] [Google Scholar]
- 42.Hoyer D., Bartfai T. Neuropeptides and neuropeptide receptors: drug targets, and peptide and non-peptide ligands: A tribute to Prof. Dieter Seebach. Chem. Biodivers. 2012;9(11):2367–2387. doi: 10.1002/cbdv.201200288. [DOI] [PubMed] [Google Scholar]
- 43.Kruse A.C., Kobilka B.K., Gautam D., Sexton P.M., Christopoulos A., Wess J. Muscarinic acetylcholine receptors: novel opportunities for drug development. Nat. Rev. Drug Discov. 2014;13(7):549–560. doi: 10.1038/nrd4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vaidya A., Jain S., Jain A.K., Agrawal A., Kashaw S.K., Jain S.K., Agrawal R.K. Metabotropic glutamate receptors: A review on prospectives and therapeutic aspects. Mini Rev. Med. Chem. 2013;13(13):1967–1981. doi: 10.2174/1389557511313130010. [DOI] [PubMed] [Google Scholar]
- 45.Beaulieu J.M., Gainetdinov R.R. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol. Rev. 2011;63(1):182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 46.Emerson G.M. Emerson studies on growth hormone effects in the Norway rat. Ala. J. Med. Sci. 1973;10(4):410–416. [PubMed] [Google Scholar]
- 47.Marston O.J., Garfield A.S., Heisler L.K. Role of central serotonin and melanocortin systems in the control of energy balance. Eur. J. Pharmacol. 2011;660(1):70–79. doi: 10.1016/j.ejphar.2010.12.024. [DOI] [PubMed] [Google Scholar]
- 48.Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010;35(2):129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rinaman L. Hindbrain noradrenergic A2 neurons: diverse roles in autonomic, endocrine, cognitive, and behavioral functions. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011;300(2):R222–R235. doi: 10.1152/ajpregu.00556.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iwańczuk W., Guźniczak P. Neurophysiological foundations of sleep, arousal, awareness and consciousness phenomena. Part 1. Anaesthesiol. Intensive Ther. 2015;47(2):162–167. doi: 10.5603/AIT.2015.0015. [DOI] [PubMed] [Google Scholar]
- 51.Trofimova I., Robbins T.W. Temperament and arousal systems: A new synthesis of differential psychology and functional neurochemistry. Neurosci. Biobehav. Rev. 2016;64:382–402. doi: 10.1016/j.neubiorev.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 52.Bittigau P., Ikonomidou C. Glutamate in neurologic diseases. J. Child Neurol. 1997;12(8):471–485. doi: 10.1177/088307389701200802. [DOI] [PubMed] [Google Scholar]
- 53.Werner F.M., Coveñas R. Classical neurotransmitters and neuropeptides involved in major depression: A review. Int. J. Neurosci. 2010;120(7):455–470. doi: 10.3109/00207454.2010.483651. [DOI] [PubMed] [Google Scholar]
- 54.Mehta T.R., Monegro A., Nene Y., Fayyaz M., Bollu P.C. Current Developmental Disorders Reports. Springer; 2019. Neurobiology of ADHD: A Review. In: p. 6. [Google Scholar]
- 55.Khalifeh S., Pour M.S., Ghermezian A., Behvarmanesh A., Moghtadaei M., Ashabi G. Introduction to neurocircuitry and neurobiology of anxiety. Arch Adv Biosci. 2021;12(1):45–51. [Google Scholar]
- 56.Meister B. Neurotransmitters in key neurons of the hypothalamus that regulate feeding behavior and body weight. Physiol. Behav. 2007;92(1-2):263–271. doi: 10.1016/j.physbeh.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 57.Li Y., Wang X. Ge, S.N.; Wang, X.L. Alterations in neurotransmitters targeted metabolomics from the key nuclei of brain reward circuits in cocaine-induced behavioral sensitization for selfadministering rats. Res Sq; 2021. [Google Scholar]
- 58.Palkovits M. The brain and the pain: neurotransmitters and neuronal pathways of pain perception and response. Orv. Hetil. 2000;141(41):2231–2239. [PubMed] [Google Scholar]
- 59.Shetty D.N., Pathak S.S. Correlation between plasma neurotransmitters and memory loss in pregnancy. J. Reprod. Med. 2002;47(6):494–496. [PubMed] [Google Scholar]
- 60.Dobryakova E., Genova H.M., DeLuca J., Wylie G.R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front. Neurol. 2015;6(MAR):52. doi: 10.3389/fneur.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fernández M.V., Kim J.H., Budde J.P., Black K., Medvedeva A., Saef B., Deming Y., Del-Aguila J., Ibañez L., Dube U., Harari O., Norton J., Chasse R., Morris J.C., Goate A., Cruchaga C. Analysis of neurodegenerative Mendelian genes in clinically diagnosed Alzheimer Disease. PLoS Genet. 2017;13(11):e1007045. doi: 10.1371/journal.pgen.1007045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schiöth H.B., Fredriksson R. In: General and Comparative Endocrinology. Gen Comp Endocrinol; 2005. The GRAFS classification system of G-protein coupled receptors in comparative perspective. pp. 94–101. [DOI] [PubMed] [Google Scholar]
- 63.Alexander . SPH G protein-coupled receptors. IUPHAR/BPS Guide to Pharmacology; 2019. [Google Scholar]
- 64.Alexander S.P.H., Christopoulos A., Davenport A.P., Kelly E., Mathie A., Peters J.A., Veale E.L., Armstrong J.F., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L., Southan C., Davies J.A. The concise guide to pharmacology 2019/20: g protein-coupled receptors. Br. J. Pharmacol. 2019;176(S1) Suppl. 1:S21–S141. doi: 10.1111/bph.14748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hu G.M., Mai T.L., Chen C.M. Visualizing the GPCR network: classification and evolution. Sci. Rep. 2017;7(1):15495. doi: 10.1038/s41598-017-15707-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Attwood T.K., Findlay J.B.C. Fingerprinting G-protein-coupled receptors. Protein Eng. 1994;7(2):195–203. doi: 10.1093/protein/7.2.195. [DOI] [PubMed] [Google Scholar]
- 67.Kolakowski L.F., Jr GCRDb: A G-protein-coupled receptor database. Receptors Channels. 1994;2(1):1–7. [PubMed] [Google Scholar]
- 68.Lee Y., Basith S., Choi S. Recent advances in structure-based drug design targeting class A G protein-coupled receptors utilizing crystal structures and computational simulations. J. Med. Chem. 2018;61(1):1–46. doi: 10.1021/acs.jmedchem.6b01453. [DOI] [PubMed] [Google Scholar]
- 69.Basith S., Cui M., Macalino S.J.Y., Park J., Clavio N.A.B., Kang S., Choi S. Exploring G protein-coupled receptors (GPCRs) ligand space via cheminformatics approaches: Impact on rational drug design. . Front. Pharmacol. 2018;9:128. doi: 10.3389/fphar.2018.00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zöllner C., Stein C. Opioids. Handb. Exp. Pharmacol. 2007;177(177):31–63. doi: 10.1007/978-3-540-33823-9_2. [DOI] [PubMed] [Google Scholar]
- 71.Moreira I.S. Structural features of the G-protein/GPCR interactions. Biochim. Biophys. Acta. 2014;1840(1):16–33. doi: 10.1016/j.bbagen.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 72.Somvanshi R.K., Kumar U. Pathophysiology of GPCR homo- and heterodimerization: special emphasis on somatostatin receptors. Pharmaceuticals (Basel) 2012;5(5):417–446. doi: 10.3390/ph5050417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferré S., Casadó V., Devi L.A., Filizola M., Jockers R., Lohse M.J., Milligan G., Pin J.P., Guitart X. G protein-coupled receptor oligomerization revisited: functional and pharmacological perspectives. Pharmacol. Rev. 2014;66(2):413–434. doi: 10.1124/pr.113.008052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mondal S., Khelashvili G., Johner N., Weinstein H. How the dynamic properties and functional mechanisms of GPCRs are modulated by their coupling to the membrane environment. 2014. p. 55-74. [DOI] [PubMed]
- 75.Filizola M., Weinstein H. The study of G-protein coupled receptor oligomerization with computational modeling and bioinformatics. FEBS J. 2005;272(12):2926–2938. doi: 10.1111/j.1742-4658.2005.04730.x. [DOI] [PubMed] [Google Scholar]
- 76.Borroto-Escuela D.O., Fuxe K. Oligomeric receptor complexes and their allosteric receptor-receptor interactions in the plasma membrane represent a new biological principle for integration of signals in the CNS. Front. Mol. Neurosci. 2019;12:230. doi: 10.3389/fnmol.2019.00230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Borroto-Escuela D.O., Rodriguez D., Romero-Fernandez W., Kapla J., Jaiteh M., Ranganathan A., Lazarova T., Fuxe K., Carlsson J. Mapping the interface of a GPCR Dimer: A structural model of the A2A Adenosine and D2 dopamine receptor heteromer. Front. Pharmacol. 2018;9:829. doi: 10.3389/fphar.2018.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wouters E., Marín A.R., Dalton J.A.R., Giraldo J., Stove C. Distinct dopamine D2 receptor antagonists differentially impact D2 receptor oligomerization. Int. J. Mol. Sci. 2019;20(7):1686. doi: 10.3390/ijms20071686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Farran B. An update on the physiological and therapeutic relevance of GPCR oligomers. Pharmacol. Res. 2017;117:303–327. doi: 10.1016/j.phrs.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 80.Schiedel A.C., Köse M., Barreto C., Bueschbell B., Morra G., Sensoy O., Moreira I.S. Prediction and targeting of interaction interfaces in g-protein coupled receptor oligomers. Curr. Top. Med. Chem. 2018;18(8):714–746. doi: 10.2174/1568026618666180604082610. [DOI] [PubMed] [Google Scholar]
- 81.Guo H., An S., Ward R., Yang Y., Liu Y., Guo X-X., Hao Q., Xu T.R. Methods used to study the oligomeric structure of G-protein-coupled receptors. Biosci. Rep. 2017;37(2):BSR20160547. doi: 10.1042/BSR20160547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuxe K., Borroto-Escuela D.O., Marcellino D., Romero-Fernandez W., Frankowska M., Guidolin D., Filip M., Ferraro L., Woods A.S., Tarakanov A., Ciruela F., Agnati L.F., Tanganelli S. GPCR heteromers and their allosteric receptor-receptor interactions. Curr. Med. Chem. 2012;19(3):356–363. doi: 10.2174/092986712803414259. [DOI] [PubMed] [Google Scholar]
- 83.Yang J., Gong Z., Lu Y.B., Xu C.J., Wei T.F., Yang M.S., Zhan T.W., Yang Y.H., Lin L., Liu J., Tang C., Zhang W.P. FLIM-FRET-based structural characterization of a class-A GPCR dimer in the cell membrane. J. Mol. Biol. 2020;432(16):4596–4611. doi: 10.1016/j.jmb.2020.06.009. [DOI] [PubMed] [Google Scholar]
- 84.Townsend-Nicholson A., Altwaijry N., Potterton A., Morao I., Heifetz A. Computational prediction of GPCR oligomerization. Curr. Opin. Struct. Biol. 2019;55:178–184. doi: 10.1016/j.sbi.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 85.Pin J-P., Bettler B. Organization and functions of mGlu and GABAB receptor complexes. Nature. 2016;540(7631):60–68. doi: 10.1038/nature20566. [DOI] [PubMed] [Google Scholar]
- 86.Møller T.C., Moreno-Delgado D., Pin J-P., Kniazeff J. Class C G protein-coupled receptors: reviving old couples with new partners. Biophys. Rep. 2017;3(4):57–63. doi: 10.1007/s41048-017-0036-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Möller J., Isbilir A., Sungkaworn T., Osberg B., Karathanasis C., Sunkara V., Grushevskyi E.O., Bock A., Annibale P., Heilemann M., Schütte C., Lohse M.J. Single-molecule analysis reveals agonist-specific dimer formation of µ-opioid receptors. Nat. Chem. Biol. 2020;16(9):946–954. doi: 10.1038/s41589-020-0566-1. [DOI] [PubMed] [Google Scholar]
- 88.Kasai R.S., Ito S.V., Awane R.M., Fujiwara T.K., Kusumi A. The class-A GPCR dopamine D2 receptor forms transient dimers stabilized by agonists: Detection by single-molecule tracking. Cell Biochem. Biophys. 2018;76(1-2):29–37. doi: 10.1007/s12013-017-0829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazim R., Suh D., Lee J.W., Vu T.N.L., Yoon S., Choi S. Structural characterization of receptor-receptor interactions in the allosteric modulation of G protein-coupled receptor (Gpcr) dimers. Int. J. Mol. Sci. 2021;22(6):1–20. doi: 10.3390/ijms22063241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zoli M., Agnati L.F., Hedlund P.B., Li X.M., Ferré S., Fuxe K. Receptor-receptor interactions as an integrative mechanism in nerve cells. Mol. Neurobiol. 1993;7(3-4):293–334. doi: 10.1007/BF02769180. [DOI] [PubMed] [Google Scholar]
- 91.Ferré S., Baler R., Bouvier M., Caron M.G., Devi L.A., Durroux T., Fuxe K., George S.R., Javitch J.A., Lohse M.J., Mackie K., Milligan G., Pfleger K.D., Pin J.P., Volkow N.D., Waldhoer M., Woods A.S., Franco R. Building a new conceptual framework for receptor heteromers. Nat. Chem. Biol. 2009;5(3):131–134. doi: 10.1038/nchembio0309-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tuteja N. Signaling through G protein coupled receptors. Plant Signal. Behav. 2009;4(10):942–947. doi: 10.4161/psb.4.10.9530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreira I.S., Shi L., Freyberg Z., Ericksen S.S., Weinstein H., Javitch J.A. In: The Dopamine Receptors. Totowa, NJ: Humana Press; 2010. Structural basis of dopamine receptor activation. pp. 47–73. [DOI] [Google Scholar]
- 94.Vauquelin G., Van Liefde I. G protein-coupled receptors: A count of 1001 conformations. In: Fundamental and Clinical Pharmacology; John Wiley & Sons, Ltd, 2005; 19, pp. 45-56. doi: 10.1111/j.1472-8206.2005.00319.x. [DOI] [PubMed] [Google Scholar]
- 95.Latek D., Pasznik P., Carlomagno T., Filipek S. Towards improved quality of GPCR models by usage of multiple templates and profile-profile comparison. PLoS One. 2013;8(2):e56742. doi: 10.1371/journal.pone.0056742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Isberg V., de Graaf C., Bortolato A., Cherezov V., Katritch V., Marshall F.H., Mordalski S., Pin J.P., Stevens R.C., Vriend G., Gloriam D.E. Generic GPCR residue numbers - aligning topology maps while minding the gaps. Trends Pharmacol. Sci. 2015;36(1):22–31. doi: 10.1016/j.tips.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ballesteros J.A., Weinstein H. Integrated methods for the construction of three-dimensional models and computational probing of structure-function relations in G protein-coupled receptors. Methods Neurosci. 1995;25(C):366–428. doi: 10.1016/S1043-9471(05)80049-7. [DOI] [Google Scholar]
- 98.Zhou Q., Yang D., Wu M., Guo Y., Guo W., Zhong L., Cai X., Dai A., Jang W., Shakhnovich E.I., Liu Z.J., Stevens R.C., Lambert N.A., Babu M.M., Wang M.W., Zhao S. Common activation mechanism of class A GPCRs. eLife. 2019;8:8. doi: 10.7554/eLife.50279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ballesteros J., Kitanovic S., Guarnieri F., Davies P., Fromme B.J., Konvicka K., Chi L., Millar R.P., Davidson J.S., Weinstein H., Sealfon S.C. Functional microdomains in G-protein-coupled receptors. The conserved arginine-cage motif in the gonadotropin-releasing hormone receptor. J. Biol. Chem. 1998;273(17):10445–10453. doi: 10.1074/jbc.273.17.10445. [DOI] [PubMed] [Google Scholar]
- 100.Schneider E.H., Schnell D., Strasser A., Dove S., Seifert R. Impact of the DRY motif and the missing “ionic lock” on constitutive activity and G-protein coupling of the human histamine H4 receptor. J. Pharmacol. Exp. Ther. 2010;333(2):382–392. doi: 10.1124/jpet.109.163220. [DOI] [PubMed] [Google Scholar]
- 101.Ballesteros J.A., Jensen A.D., Liapakis G., Rasmussen S.G.F., Shi L., Gether U., Javitch J.A. Activation of the β 2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 2001;276(31):29171–29177. doi: 10.1074/jbc.M103747200. [DOI] [PubMed] [Google Scholar]
- 102.Schönegge A.M., Gallion J., Picard L.P., Wilkins A.D., Le Gouill C., Audet M., Stallaert W., Lohse M.J., Kimmel M., Lichtarge O., Bouvier M. Evolutionary action and structural basis of the allosteric switch controlling β2AR functional selectivity. Nat. Commun. 2017;8(1):2169. doi: 10.1038/s41467-017-02257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alhadeff R., Vorobyov I., Yoon H.W., Warshel A. Exploring the free-energy landscape of GPCR activation. Proc. Natl. Acad. Sci. USA. 2018;115(41):10327–10332. doi: 10.1073/pnas.1810316115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Jacobson K.A., Costanzi S., Paoletta S. Computational studies to predict or explain G protein coupled receptor polypharmacology. Trends Pharmacol. Sci. 2014;35(12):658–663. doi: 10.1016/j.tips.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Feng X., Ambia J., Chen K.M., Young M., Barth P. Computational design of ligand-binding membrane receptors with high selectivity. Nat. Chem. Biol. 2017;13(7):715–723. doi: 10.1038/nchembio.2371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roth B.L., Irwin J.J., Shoichet B.K. Discovery of new GPCR ligands to illuminate new biology. Nat. Chem. Biol. 2017;13(11):1143–1151. doi: 10.1038/nchembio.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shihoya W., Nishizawa T., Yamashita K., Inoue A., Hirata K., Kadji F.M.N., Okuta A., Tani K., Aoki J., Fujiyoshi Y., Doi T., Nureki O. X-ray structures of endothelin ETB receptor bound to clinical antagonist bosentan and its analog. Nat. Struct. Mol. Biol. 2017;24(9):758–764. doi: 10.1038/nsmb.3450. [DOI] [PubMed] [Google Scholar]
- 108.Yuan S., Filipek S., Palczewski K., Vogel H. Activation of G-protein-coupled receptors correlates with the formation of a continuous internal water pathway. Nat. Commun. 2014;5:4733. doi: 10.1038/ncomms5733. [DOI] [PubMed] [Google Scholar]
- 109.Filizola M., Weinstein H. Structural models for dimerization of G-protein coupled receptors: The opioid receptor homodimers. In: Biopolymers - Peptide Science Section; Biopolymers, 2002:pp. 317-325. doi: 10.1002/bip.10311. [DOI] [PubMed]
- 110.Weinstein H. Hallucinogen actions on 5-HT receptors reveal distinct mechanisms of activation and signaling by G protein-coupled receptors. AAPS J. 2006;7(4):E871–E884. doi: 10.1208/aapsj070485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Visiers I., Ballesteros J.A., Weinstein H. Three-dimensional representations of G protein-coupled receptor structures and mechanisms. Methods Enzymol. 2002;343:329–371. doi: 10.1016/S0076-6879(02)43145-X. [DOI] [PubMed] [Google Scholar]
- 112.Fritze O., Filipek S., Kuksa V., Palczewski K., Hofmann K.P., Ernst O.P. Role of the conserved NPxxY(x)5,6F motif in the rhodopsin ground state and during activation. Proc. Natl. Acad. Sci. USA. 2003;100(5):2290–2295. doi: 10.1073/pnas.0435715100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Trzaskowski B., Latek D., Yuan S., Ghoshdastider U., Debinski A., Filipek S. Action of molecular switches in GPCRs--theoretical and experimental studies. Curr. Med. Chem. 2012;19(8):1090–1109. doi: 10.2174/092986712799320556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chen S., Lu M., Liu D., Yang L., Yi C., Ma L., Zhang H., Liu Q., Frimurer T.M., Wang M.W., Schwartz T.W., Stevens R.C., Wu B., Wüthrich K., Zhao Q. Human substance P receptor binding mode of the antagonist drug aprepitant by NMR and crystallography. Nat. Commun. 2019;10(1):638. doi: 10.1038/s41467-019-08568-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Venkatakrishnan A.J., Deupi X., Lebon G., Heydenreich F.M., Flock T., Miljus T., Balaji S., Bouvier M., Veprintsev D.B., Tate C.G., Schertler G.F., Babu M.M. Diverse activation pathways in class A GPCRs converge near the G-protein-coupling region. Nature. 2016;536(7617):484–487. doi: 10.1038/nature19107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Angel T.E., Chance M.R., Palczewski K. Conserved waters mediate structural and functional activation of family A (rhodopsin-like) G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2009;106(21):8555–8560. doi: 10.1073/pnas.0903545106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Prioleau C., Visiers I., Ebersole B.J., Weinstein H., Sealfon S.C. Conserved helix 7 tyrosine acts as a multistate conformational switch in the 5HT2C receptor. Identification of a novel “locked-on” phenotype and double revertant mutations. J. Biol. Chem. 2002;277(39):36577–36584. doi: 10.1074/jbc.M206223200. [DOI] [PubMed] [Google Scholar]
- 118.Angel T.E., Gupta S., Jastrzebska B., Palczewski K., Chance M.R. Structural waters define a functional channel mediating activation of the GPCR, rhodopsin. Proc. Natl. Acad. Sci. USA. 2009;106(34):14367–14372. doi: 10.1073/pnas.0901074106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rasmussen S.G.F., DeVree B.T., Zou Y., Kruse A.C., Chung K.Y., Kobilka T.S., Thian F.S., Chae P.S., Pardon E., Calinski D., Mathiesen J.M., Shah S.T., Lyons J.A., Caffrey M., Gellman S.H., Steyaert J., Skiniotis G., Weis W.I., Sunahara R.K., Kobilka B.K. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature. 2011;477(7366):549–555. doi: 10.1038/nature10361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Filipek S. Molecular switches in GPCRs. Curr. Opin. Struct. Biol. 2019;55:114–120. doi: 10.1016/j.sbi.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 121.Wescott M.P., Kufareva I., Paes C., Goodman J.R., Thaker Y., Puffer B.A., Berdougo E., Rucker J.B., Handel T.M., Doranz B.J. Signal transmission through the CXC chemokine receptor 4 (CXCR4) transmembrane helices. Proc. Natl. Acad. Sci. USA. 2016;113(35):9928–9933. doi: 10.1073/pnas.1601278113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Nygaard R., Frimurer T.M., Holst B., Rosenkilde M.M., Schwartz T.W. Ligand binding and micro-switches in 7TM receptor structures. Trends Pharmacol. Sci. 2009;30(5):249–259. doi: 10.1016/j.tips.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 123.Hofmann K.P., Scheerer P., Hildebrand P.W., Choe H.W., Park J.H., Heck M., Ernst O.P.A. G protein-coupled receptor at work: the rhodopsin model. Trends Biochem. Sci. 2009;34(11):540–552. doi: 10.1016/j.tibs.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 124.Kaiser A., Hempel C., Wanka L., Schubert M., Hamm H.E., Beck-Sickinger A.G. G protein preassembly rescues efficacy of W 6.48 toggle mutations in neuropeptide Y 2 receptor. Mol. Pharmacol. 2018;93(4):387–401. doi: 10.1124/mol.117.110544. [DOI] [PubMed] [Google Scholar]
- 125.Holst B., Nygaard R., Valentin-Hansen L., Bach A., Engelstoft M.S., Petersen P.S., Frimurer T.M., Schwartz T.W. A conserved aromatic lock for the tryptophan rotameric switch in TM-VI of seven-transmembrane receptors. J. Biol. Chem. 2010;285(6):3973–3985. doi: 10.1074/jbc.M109.064725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhang X.C., Zhou Y., Cao C. Proton transfer during class-A GPCR activation: do the CWxP motif and the membrane potential act in concert? Biophys. Rep. 2018;4(3):115–122. doi: 10.1007/s41048-018-0056-0. [DOI] [Google Scholar]
- 127.Tehan B.G., Bortolato A., Blaney F.E., Weir M.P., Mason J.S. Unifying family A GPCR theories of activation. Pharmacol. Ther. 2014;143(1):51–60. doi: 10.1016/j.pharmthera.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 128.Eddy M.T., Lee M.Y., Gao Z.G., White K.L., Didenko T., Horst R., Audet M., Stanczak P., McClary K.M., Han G.W., Jacobson K.A., Stevens R.C., Wüthrich K. Allosteric Coupling of Drug Binding and Intracellular Signaling in the A2A Adenosine Receptor. Cell. 2018;172(1-2):68–80.e12. doi: 10.1016/j.cell.2017.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ishchenko A., Wacker D., Kapoor M., Zhang A., Han G.W., Basu S., Patel N., Messerschmidt M., Weierstall U., Liu W., Katritch V., Roth B.L., Stevens R.C., Cherezov V. Structural insights into the extracellular recognition of the human serotonin 2B receptor by an antibody. Proc. Natl. Acad. Sci. USA. 2017;114(31):8223–8228. doi: 10.1073/pnas.1700891114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kato H.E., Zhang Y., Hu H., Suomivuori C.M., Kadji F.M.N., Aoki J., Krishna Kumar K., Fonseca R., Hilger D., Huang W., Latorraca N.R., Inoue A., Dror R.O., Kobilka B.K., Skiniotis G. Conformational transitions of a neurotensin receptor 1-Gi1 complex. Nature. 2019;572(7767):80–85. doi: 10.1038/s41586-019-1337-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu W., Chun E., Thompson A.A., Chubukov P., Xu F., Katritch V. Structural basis for allosteric regulation of GPCRS by sodium ions. Science (80) 2012;337((6091)):232-236. doi: 10.1126/science.1219218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yuan S., Vogel H., Filipek S. The role of water and sodium ions in the activation of the μ-opioid receptor. Angew. Chem. Int. Ed. Engl. 2013;52(38):10112–10115. doi: 10.1002/anie.201302244. [DOI] [PubMed] [Google Scholar]
- 133.Fenalti G., Giguere P.M., Katritch V., Huang X.P., Thompson A.A., Cherezov V., Roth B.L., Stevens R.C. Molecular control of δ-opioid receptor signalling. Nature. 2014;506(7487):191–196. doi: 10.1038/nature12944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Vickery O.N., Carvalheda C.A., Zaidi S.A., Pisliakov A.V., Katritch V., Zachariae U. Intracellular transfer of Na+ in an active-state G-protein-coupled receptor. Structure. 2018;26(1):171–180.e2. doi: 10.1016/j.str.2017.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Katritch V., Fenalti G., Abola E.E., Roth B.L., Cherezov V., Stevens R.C. Allosteric sodium in class A GPCR signaling. Trends Biochem. Sci. 2014;39(5):233–244. doi: 10.1016/j.tibs.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.White K.L., Eddy M.T., Gao Z.G., Han G.W., Lian T., Deary A., Patel N., Jacobson K.A., Katritch V., Stevens R.C. Structural connection between activation microswitch and allosteric sodium site in GPCR signaling. Structure. 2018;26(2):259–269.e5. doi: 10.1016/j.str.2017.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ye L., Neale C., Sljoka A., Lyda B., Pichugin D., Tsuchimura N., Larda S.T., Pomès R., García A.E., Ernst O.P., Sunahara R.K., Prosser R.S. Mechanistic insights into allosteric regulation of the A2A adenosine G protein-coupled receptor by physiological cations. Nat. Commun. 2018;9(1):1372. doi: 10.1038/s41467-018-03314-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Yuan S., Filipek S., Vogel H. A gating mechanism of the serotonin 5-HT3 receptor. Structure. 2016;24(5):816–825. doi: 10.1016/j.str.2016.03.019. [DOI] [PubMed] [Google Scholar]
- 139.Venkatakrishnan A.J., Ma A.K., Fonseca R., Latorraca N.R., Kelly B., Betz R.M., Asawa C., Kobilka B.K., Dror R.O. Diverse GPCRs exhibit conserved water networks for stabilization and activation. Proc. Natl. Acad. Sci. USA. 2019;116(8):3288–3293. doi: 10.1073/pnas.1809251116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Dorszewska J., Florczak-Wyspianska J., Kowalska M., Stanski M., Kowalewska A., Kozubski W. Serotonin - A Chemical Messenger Between All Types of Living Cells; IntechOpen. 2017. Serotonin in neurological diseases. [DOI] [Google Scholar]
- 141.Dorszewska J., Prendecki M., Oczkowska A., Rozycka A., Lianeri M., Kozubski W. Polymorphism of the COMT, MAO, DAT, NET and 5-HTT genes, and biogenic amines in Parkinson’s disease. Curr. Genomics. 2013;14(8):518–533. doi: 10.2174/1389202914666131210210241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Mohammad-Zadeh L.F., Moses L., Gwaltney-Brant S.M. Serotonin: A review. J. Vet. Pharmacol. Ther. 2008;31(3):187–199. doi: 10.1111/j.1365-2885.2008.00944.x. [DOI] [PubMed] [Google Scholar]
- 143.Hannon J., Hoyer D. Molecular biology of 5-HT receptors. Behav. Brain Res. 2008;195(1):198–213. doi: 10.1016/j.bbr.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 144.Armstrong J.F., Faccenda E., Harding S.D., Pawson A.J., Southan C., Sharman J.L., Campo B., Cavanagh D.R., Alexander S.P.H., Davenport A.P., Spedding M., Davies J.A. The IUPHAR/ BPS Guide to PHARMACOLOGY in 2020: extending immunopharmacology content and introducing the IUPHAR/MMV Guide to malaria pharmacology. Nucleic Acids Res. 2020;48(D1):D1006–D1021. doi: 10.1093/nar/gkz951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xu P., Huang S., Zhang H., Mao C., Zhou X.E., Cheng X., Simon I.A., Shen D.D., Yen H.Y., Robinson C.V., Harpsøe K., Svensson B., Guo J., Jiang H., Gloriam D.E., Melcher K., Jiang Y., Zhang Y., Xu H.E. Structural insights into the lipid and ligand regulation of serotonin receptors. Nature. 2021;592(7854):469–473. doi: 10.1038/s41586-021-03376-8. [DOI] [PubMed] [Google Scholar]
- 146.Kowalska M., Prendecki M., Kozubski W., Lianeri M., Dorszewska J. Molecular factors in migraine. Oncotarget. 2016;7(31):50708–50718. doi: 10.18632/oncotarget.9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Theodore W.H. Does serotonin play a role in epilepsy? Epilepsy Curr. 2003;3(5):173–177. doi: 10.1046/j.1535-7597.2003.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hercigonja Novkovic V., Rudan V., Pivac N., Nedic G., Muck-Seler D. Platelet serotonin concentration in children with attention-deficit/hyperactivity disorder. Neuropsychobiology. 2009;59(1):17–22. doi: 10.1159/000202825. [DOI] [PubMed] [Google Scholar]
- 149.Whitney M.S., Shemery A.M., Yaw A.M., Donovan L.J., Glass J.D., Deneris E.S. Adult brain serotonin deficiency causes hyperactivity, circadian disruption, and elimination of siestas. J. Neurosci. 2016;36(38):9828–9842. doi: 10.1523/JNEUROSCI.1469-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sandyk R. Serotonergic mechanisms in amyotrophic lateral sclerosis. Int. J. Neurosci. 2006;116(7):775–826. doi: 10.1080/00207450600754087. [DOI] [PubMed] [Google Scholar]
- 151.Yang X., Heitman L.H., IJzerman A.P., van der Es D. Molecular probes for the human adenosine receptors. Purinergic Signal. 2021;17(1):85–108. doi: 10.1007/s11302-020-09753-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jacobson K.A., Gao Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006;5(3):247–264. doi: 10.1038/nrd1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Stone T.W., Ceruti S., Abbracchio M.P. Adenosine receptors and neurological disease: neuroprotection and neurodegeneration. Handb. Exp. Pharmacol. 2009;193(193):535–587. doi: 10.1007/978-3-540-89615-9_17. [DOI] [PubMed] [Google Scholar]
- 154.Ordway G.A., Schwartz M.A., Frazer A. Brain norepinephrine: neurobiology and therapeutics. Cambridge University Press; 2007. pp. 1–642. [Google Scholar]
- 155.Klimek V., Rajkowska G., Luker S.N., Dilley G., Meltzer H.Y., Overholser J.C., Stockmeier C.A., Ordway G.A. Brain noradrenergic receptors in major depression and schizophrenia. Neuropsychopharmacology. 1999;21(1):69–81. doi: 10.1016/S0893-133X(98)00134-1. [DOI] [PubMed] [Google Scholar]
- 156.Gupta M.K., Papay R.S., Jurgens C.W.D., Gaivin R.J., Shi T., Doze V.A., Perez D.M. α1-Adrenergic receptors regulate neurogenesis and gliogenesis. Mol. Pharmacol. 2009;76(2):314–326. doi: 10.1124/mol.109.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nguyen P.V., Connor S.A. Noradrenergic regulation of hippocampus-dependent memory. Cent. Nerv. Syst. Agents Med. Chem. 2019;19(3):187–196. doi: 10.2174/1871524919666190719163632. [DOI] [PubMed] [Google Scholar]
- 158.Perez D.M. α1-adrenergic receptors in neurotransmission, synaptic plasticity, and cognition. Vol. 11, Frontiers in Pharmacology. Front. Pharmacol. 2020 doi: 10.3389/fphar.2020.581098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Hertz L., Lovatt D., Goldman S.A., Nedergaard M. Adrenoceptors in brain: cellular gene expression and effects on astrocytic metabolism and [Ca(2+)]i. Neurochem. Int. 2010;57(4):411–420. doi: 10.1016/j.neuint.2010.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Xiao R-P. The Adrenergic Receptors in the 21st Century. In: Circulation; Springer. 2006:113(18), pp. 129-134. [Google Scholar]
- 161.Kendall D.A., Yudowski G.A. Cannabinoid receptors in the central nervous system: Their signaling and roles in disease. Front. Cell. Neurosci. 2017;10:294. doi: 10.3389/fncel.2016.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Aizpurua-Olaizola O., Elezgarai I., Rico-Barrio I., Zarandona I., Etxebarria N., Usobiaga A. Targeting the endocannabinoid system: future therapeutic strategies. Drug Discov. Today. 2017;22(1):105–110. doi: 10.1016/j.drudis.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 163.Pryce G., Ahmed Z., Hankey D.J.R., Jackson S.J., Croxford J.L., Pocock J.M., Ledent C., Petzold A., Thompson A.J., Giovannoni G., Cuzner M.L., Baker D. Cannabinoids inhibit neurodegeneration in models of multiple sclerosis. Brain. 2003;126(Pt 10):2191–2202. doi: 10.1093/brain/awg224. [DOI] [PubMed] [Google Scholar]
- 164.Klein T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005;5(5):400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 165.Campbell V.A., Gowran A. Alzheimer’s disease; taking the edge off with cannabinoids? Br. J. Pharmacol. 2007;152(5):655–662. doi: 10.1038/sj.bjp.0707446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bilkei-Gorzo A. The endocannabinoid system in normal and pathological brain ageing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012;367(1607):3326–3341. doi: 10.1098/rstb.2011.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Scotter E.L., Abood M.E., Glass M. The endocannabinoid system as a target for the treatment of neurodegenerative disease. Br. J. Pharmacol. 2010;160(3):480–498. doi: 10.1111/j.1476-5381.2010.00735.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Fernández-Ruiz J., Moreno-Martet M., Rodríguez-Cueto C., Palomo-Garo C., Gómez-Cañas M., Valdeolivas S., Guaza C., Romero J., Guzmán M., Mechoulam R., Ramos J.A. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 2011;163(7):1365–1378. doi: 10.1111/j.1476-5381.2011.01365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Marsicano G., Kuner R. In: Cannabinoids and the Brain. Boston, MA: Springer; 2008. Anatomical distribution of receptors, ligands and enzymes in the brain and in the spinal cord: Circuitries and neurochemistry. pp. 161–201. [DOI] [Google Scholar]
- 170.Jordan C.J., Xi Z.X. Progress in brain cannabinoid CB2 receptor research: From genes to behavior. Neurosci. Biobehav. Rev. 2019;98:208–220. doi: 10.1016/j.neubiorev.2018.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mackie K. Cannabinoid receptors: Where they are and what they do. J. Neuroendocrinol. 2008:10–14. doi: 10.1111/j.1365-2826.2008.01671.x. [DOI] [PubMed] [Google Scholar]
- 172.Bosier B., Muccioli G.G., Hermans E., Lambert D.M. Functionally selective cannabinoid receptor signalling: therapeutic implications and opportunities. Biochem. Pharmacol. 2010;80(1):1–12. doi: 10.1016/j.bcp.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 173.Nogueras-Ortiz C., Yudowski G.A. The multiple waves of cannabinoid 1 receptor signaling. Mol. Pharmacol. 2016;90(5):620–626. doi: 10.1124/mol.116.104539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Di Marzo V., Stella N., Zimmer A. Endocannabinoid signalling and the deteriorating brain. Nat. Rev. Neurosci. 2015;16(1):30–42. doi: 10.1038/nrn3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Palazuelos J., Aguado T., Pazos M.R., Julien B., Carrasco C., Resel E., Sagredo O., Benito C., Romero J., Azcoitia I., Fernández-Ruiz J., Guzmán M., Galve-Roperh I. Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain. 2009;132(Pt 11):3152–3164. doi: 10.1093/brain/awp239. [DOI] [PubMed] [Google Scholar]
- 176.Yeh F.L., Wang Y., Tom I., Gonzalez L.C., Sheng M. TREM2 binds to apolipoproteins, including APOE and CLU/APOJ, and thereby facilitates uptake of amyloid-beta by microglia. Neuron. 2016;91(2):328–340. doi: 10.1016/j.neuron.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 177.Sagredo O., García-Arencibia M., de Lago E., Finetti S., Decio A., Fernández-Ruiz J. Cannabinoids and neuroprotection in basal ganglia disorders. Mol. Neurobiol. 2007;36(1):82–91. doi: 10.1007/s12035-007-0004-3. [DOI] [PubMed] [Google Scholar]
- 178.Ramírez B.G., Blázquez C., Gómez del Pulgar T., Guzmán M., de Ceballos M.L. Prevention of Alzheimer’s disease pathology by cannabinoids: neuroprotection mediated by blockade of microglial activation. J. Neurosci. 2005;25(8):1904–1913. doi: 10.1523/JNEUROSCI.4540-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dockray G.J. Cholecystokinins in rat cerebral cortex: identification, purification and characterization by immunochemical methods. Brain Res. 1980;188(1):155–165. doi: 10.1016/0006-8993(80)90564-8. [DOI] [PubMed] [Google Scholar]
- 180.Innis R.B., Snyder S.H. Distinct cholecystokinin receptors in brain and pancreas. Proc. Natl. Acad. Sci. USA. 1980;77(11):6917–6921. doi: 10.1073/pnas.77.11.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bradwejn J., Koszycki D., Meterissian G. Cholecystokinin-tetrapeptide induces panic attacks in patients with panic disorder. Can. J. Psychiatry. 1990;35(1):83–85. doi: 10.1177/070674379003500115. [DOI] [PubMed] [Google Scholar]
- 182.Ballaz S. The unappreciated roles of the cholecystokinin receptor CCK(1) in brain functioning. Rev. Neurosci. 2017;28(6):573–585. doi: 10.1515/revneuro-2016-0088. [DOI] [PubMed] [Google Scholar]
- 183.Beglinger C., Degen L., Matzinger D., D’Amato M., Drewe J. Loxiglumide, a CCK-A receptor antagonist, stimulates calorie intake and hunger feelings in humans. Am. J. Physiol. – Regul. Integr. Comp. Physiol. 2001;280(4):49–54. doi: 10.1152/ajpregu.2001.280.4.R1149. [DOI] [PubMed] [Google Scholar]
- 184.Dockray G.J. Cholecystokinin. Curr. Opin. Endocrinol. Diabetes Obes. 2012;19(1):8–12. doi: 10.1097/MED.0b013e32834eb77d. [DOI] [PubMed] [Google Scholar]
- 185.Beglinger C. Overview. Cholecystokinin and eating. Curr. Opin. Investig. Drugs. 2002;3(4):587–588. [PubMed] [Google Scholar]
- 186.Choi J.G., Jeong M., Joo B.R., Ahn J.H., Woo J.H., Kim D.H., Oh M.S., Choi J.H. Reduced levels of intestinal neuropeptides and neurotrophins in neurotoxin-induced Parkinson disease mouse models. J. Neuropathol. Exp. Neurol. 2021;80(1):15–20. doi: 10.1093/jnen/nlaa113. [DOI] [PubMed] [Google Scholar]
- 187.Fasano A., Visanji N.P., Liu L.W.C., Lang A.E., Pfeiffer R.F. Gastrointestinal dysfunction in Parkinson’s disease. Lancet Neurol. 2015;14(6):625–639. doi: 10.1016/S1474-4422(15)00007-1. [DOI] [PubMed] [Google Scholar]
- 188.Everitt B.J., Meister B., Hökfelt T., Melander T., Terenius L., Rökaeus A., Theodorsson-Norheim E., Dockray G., Edwardson J., Cuello C. The hypothalamic arcuate nucleus-median eminence complex: immunohistochemistry of transmitters, peptides and DARPP-32 with special reference to coexistence in dopamine neurons. Brain Res. 1986;396(2):97–155. doi: 10.1016/0165-0173(86)90001-9. [DOI] [PubMed] [Google Scholar]
- 189.Hökfelt T., Skirboll L., Rehfeld J.F., Goldstein M., Markey K., Dann O. A subpopulation of mesencephalic dopamine neurons projecting to limbic areas contains a cholecystokinin-like peptide: evidence from immunohistochemistry combined with retrograde tracing. Neuroscience. 1980;5(12):2093–2124. doi: 10.1016/0306-4522(80)90127-X. [DOI] [PubMed] [Google Scholar]
- 190.Beaulieu J.M., Espinoza S., Gainetdinov R.R. Dopamine receptors - IUPHAR Review 13. Br. J. Pharmacol. 2015;172(1):1–23. doi: 10.1111/bph.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Kebabian J.W. Multiple classes of dopamine receptors in mammalian central nervous system: the involvement of dopamine-sensitive adenylyl cyclase. Life Sci. 1978;23(5):479–483. doi: 10.1016/0024-3205(78)90157-1. [DOI] [PubMed] [Google Scholar]
- 192.Spano P.F., Govoni S., Trabucchi M. Studies on the pharmacological properties of dopamine receptors in various areas of the central nervous system. Adv. Biochem. Psychopharmacol. 1978;19:155–165. [PubMed] [Google Scholar]
- 193.Bueschbell B., Barreto C.A.V., Preto A.J., Schiedel A.C., Moreira I.S. A complete assessment of dopamine receptor-ligand interactions through computational methods. Molecules. 2019;24(7):E1196. doi: 10.3390/molecules24071196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Cokan K.B., Mavri M., Rutland C.S., Glišić S., Senćanski M., Vrecl M. Critical impact of different conserved endoplasmic retention motifs and dopamine receptor interacting proteins (Drips) on intracellular localization and trafficking of the d2 dopamine receptor (D2-r) isoforms. Biomolecules Biomolecules; 2020. pp. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mitsukawa K., Lu X., Bartfai T. Galanin, galanin receptors and drug targets. Cell. Mol. Life Sci. 2008;65(12):1796–1805. doi: 10.1007/s00018-008-8153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Tatemoto K., Rökaeus A., Jörnvall H., McDonald T.J., Mutt V. Galanin - a novel biologically active peptide from porcine intestine. FEBS Lett. 1983;164(1):124–128. doi: 10.1016/0014-5793(83)80033-7. [DOI] [PubMed] [Google Scholar]
- 197.Ottlecz A., Samson W.K., McCann S.M. Galanin: evidence for a hypothalamic site of action to release growth hormone. Peptides. 1986;7(1):51–53. doi: 10.1016/0196-9781(86)90060-4. [DOI] [PubMed] [Google Scholar]
- 198.Lu X., Sharkey L., Bartfai T. The brain galanin receptors: targets for novel antidepressant drugs. CNS Neurol. Disord. Drug Targets. 2007;6(3):183–192. doi: 10.2174/187152707780619335. [DOI] [PubMed] [Google Scholar]
- 199.Hua X.Y., Salgado K.F., Gu G., Fitzsimmons B., Kondo I., Bartfai T. Mechanisms of antinociception of spinal galanin: How does galanin inhibit spinal sensitization?. Neuropeptides. 2005:211–216. doi: 10.1016/j.npep.2004.12.024. [DOI] [PubMed] [Google Scholar]
- 200.Nordström O., Melander T., Hökfelt T., Bartfai T., Goldstein M. Evidence for an inhibitory effect of the peptide galanin on dopamine release from the rat median eminence. Neurosci. Lett. 1987;73(1):21–26. doi: 10.1016/0304-3940(87)90024-3. [DOI] [PubMed] [Google Scholar]
- 201.Liu H.X., Hökfelt T. The participation of galanin in pain processing at the spinal level. Trends Pharmacol. Sci. 2002;23(10):468–474. doi: 10.1016/S0165-6147(02)02074-6. [DOI] [PubMed] [Google Scholar]
- 202.Wrenn C.C., Crawley J.N. Pharmacological evidence supporting a role for galanin in cognition and affect. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2001;25(1):283–299. doi: 10.1016/S0278-5846(00)00156-1. [DOI] [PubMed] [Google Scholar]
- 203.Hökfelt T., Wiesenfeld-Hallin Z., Villar M., Melander T. Increase of galanin-like immunoreactivity in rat dorsal root ganglion cells after peripheral axotomy. Neurosci. Lett. 1987;83(3):217–220. doi: 10.1016/0304-3940(87)90088-7. [DOI] [PubMed] [Google Scholar]
- 204.Elliott-Hunt C.R., Marsh B., Bacon A., Pope R., Vanderplank P., Wynick D. Galanin acts as a neuroprotective factor to the hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101(14):5105–5110. doi: 10.1073/pnas.0304823101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Counts S.E., Perez S.E., Ginsberg S.D., De Lacalle S., Mufson E.J. Galanin in Alzheimer disease. Mol. Interv. 2003;3(3):137–156. doi: 10.1124/mi.3.3.137. [DOI] [PubMed] [Google Scholar]
- 206.Mazarati A., Lu X., Kilk K., Langel U., Wasterlain C., Bartfai T. Galanin type 2 receptors regulate neuronal survival, susceptibility to seizures and seizure-induced neurogenesis in the dentate gyrus. Eur. J. Neurosci. 2004;19(12):3235–3244. doi: 10.1111/j.0953-816X.2004.03449.x. [DOI] [PubMed] [Google Scholar]
- 207.Hökfelt T., Bartfai T., Bloom F. Neuropeptides: opportunities for drug discovery. Lancet Neurol. 2003;2(8):463–472. doi: 10.1016/S1474-4422(03)00482-4. [DOI] [PubMed] [Google Scholar]
- 208.Mazarati A.M. Galanin and galanin receptors in epilepsy. Neuropeptides. 2004;38(6):331–343. doi: 10.1016/j.npep.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 209.Wiesenfeld-Hallin Z., Xu X.J., Crawley J.N., Hökfelt T. Galanin and spinal nociceptive mechanisms: Recent results from transgenic and knock-out models. Neuropeptides. 2005:207–210. doi: 10.1016/j.npep.2004.12.017. [DOI] [PubMed] [Google Scholar]
- 210.Wang P., Li H., Barde S., Zhang M.D., Sun J., Wang T., Zhang P., Luo H., Wang Y., Yang Y., Wang C., Svenningsson P., Theodorsson E., Hökfelt T.G., Xu Z.Q. Depression-like behavior in rat: Involvement of galanin receptor subtype 1 in the ventral periaqueductal gray. Proc. Natl. Acad. Sci. USA. 2016;113(32):E4726–E4735. doi: 10.1073/pnas.1609198113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Lundström L., Elmquist A., Bartfai T., Langel U. Galanin and its receptors in neurological disorders. Neuromol. Med. 2005;7(1-2):157–180. doi: 10.1385/NMM:7:1-2:157. [DOI] [PubMed] [Google Scholar]
- 212.Panula P., Chazot P.L., Cowart M., Gutzmer R., Leurs R., Liu W.L.S., Stark H., Thurmond R.L., Haas H.L. International union of basic and clinical pharmacology. XCVIII. Histamine receptors. Pharmacol. Rev. 2015;67(3):601–655. doi: 10.1124/pr.114.010249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nieto-Alamilla G., Márquez-Gómez R., García-Gálvez A-M., Morales-Figueroa G-E., Arias-Montaño J-A. The histamine H3 receptor: Structure, pharmacology, and function. Mol. Pharmacol. 2016;90(5):649–673. doi: 10.1124/mol.116.104752. [DOI] [PubMed] [Google Scholar]
- 214.Keppel H.J.M. The terms ‘autacoid’, ‘hormone’ and ‘chalone’ and how they have shifted with time. Auton. Autacoid Pharmacol. 2015;35(4):51–58. doi: 10.1111/aap.12037. [DOI] [PubMed] [Google Scholar]
- 215.Wouters M.M., Vicario M., Santos J. The role of mast cells in functional GI disorders. Gut. 2016;65(1):155–168. doi: 10.1136/gutjnl-2015-309151. [DOI] [PubMed] [Google Scholar]
- 216.Blandina P., Provensi G., Munari L., Passani M.B. Histamine neurons in the tuberomamillary nucleus: A whole center or distinct subpopulations? Front. Syst. Neurosci. 2012;6:33. doi: 10.3389/fnsys.2012.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Stromberga Z., Chess-Williams R., Moro C. Histamine modulation of urinary bladder urothelium, lamina propria and detrusor contractile activity via H1 and H2 receptors. . Sci. Rep. 2019;9(1):3899. doi: 10.1038/s41598-019-40384-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Passani M.B., Panula P., Lin J-S. Histamine in the brain. Front. Syst. Neurosci. 2014;8:64. doi: 10.3389/fnsys.2014.00064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Chazot P., Cowart M., Fukui H., Ganellin C.R., Gutzmer R., Haas H.L. Histamine receptors (version 2019.4) in the IUPHAR/ BPS guide to pharmacology database. IUPHAR/BPS Guid to Pharmacol CITE. 2019:4. [Google Scholar]
- 220.Bond R.A., Ijzerman A.P. Recent developments in constitutive receptor activity and inverse agonism, and their potential for GPCR drug discovery. Trends Pharmacol. Sci. 2006;27(2):92–96. doi: 10.1016/j.tips.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 221.Baronio D., Gonchoroski T., Castro K., Zanatta G., Gottfried C., Riesgo R. Histaminergic system in brain disorders: lessons from the translational approach and future perspectives. Ann. Gen. Psychiatry. 2014;13(1):34. doi: 10.1186/s12991-014-0034-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Jadidi-Niaragh F., Mirshafiey A. Histamine and histamine receptors in pathogenesis and treatment of multiple sclerosis. Neuropharmacology. 2010;59(3):180–189. doi: 10.1016/j.neuropharm.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 223.Naddafi F., Mirshafiey A. The neglected role of histamine in Alzheimer’s disease. Am. J. Alzheimers Dis. Other Demen. 2013;28(4):327–336. doi: 10.1177/1533317513488925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Ito C. The role of the central histaminergic system on schizophrenia. Drug News Perspect. 2004;17(6):383–387. doi: 10.1358/dnp.2004.17.6.829029. [DOI] [PubMed] [Google Scholar]
- 225.Mahmood D. Histamine H3 receptors and its antagonism as a novel mechanism for antipsychotic effect: A current preclinical & clinical perspective. Int. J. Health Sci. (Qassim) 2016;10(4):564–575. doi: 10.12816/0048906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Liu Q., Fan W., He H., Huang F. The role of peripheral opioid receptors in orofacial pain. Oral Dis. 2021;27(5):1106–1114. doi: 10.1111/odi.13435. [DOI] [PubMed] [Google Scholar]
- 227.Wiffen P.J., Wee B., Derry S., Bell R.F., Moore R.A. Opioids for cancer pain - an overview of Cochrane reviews. Cochrane Database Syst. Rev. 2017;7(7):CD012592. doi: 10.1002/14651858.CD012592.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Barber A. μ- and κ-opioid receptor agonists produce peripheral inhibition of neurogenic plasma extravasation in rat skin. Eur. J. Pharmacol. 1993;236(1):113–120. doi: 10.1016/0014-2999(93)90233-8. [DOI] [PubMed] [Google Scholar]
- 229.Earl J.R., Grootveld M.C., Blake D.R., Morris C.J. Effect of μ, δ and κ opioid receptor agonists on a reactive oxygen species mediated model of skin inflammation. Skin Pharmacol. 1996;9(4):250–258. doi: 10.1159/000211422. [DOI] [PubMed] [Google Scholar]
- 230.Stein C., Machelska H. Modulation of peripheral sensory neurons by the immune system: implications for pain therapy. Pharmacol. Rev. 2011;63(4):860–881. doi: 10.1124/pr.110.003145. [DOI] [PubMed] [Google Scholar]
- 231.Corbett A.D., Henderson G., McKnight A.T., Paterson S.J. 75 years of opioid research: the exciting but vain quest for the Holy Grail. Br. J. Pharmacol. 2006;147(Suppl. 1):S153–S162. doi: 10.1038/sj.bjp.0706435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Israel Y., Kandov Y., Khaimova E., Kest A., Lewis S.R., Pasternak G.W., Pan Y.X., Rossi G.C., Bodnar R.J. NPY-induced feeding: pharmacological characterization using selective opioid antagonists and antisense probes in rats. Peptides. 2005;26(7):1167–1175. doi: 10.1016/j.peptides.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 233.Cai Z., Ratka A. Opioid system and Alzheimer’s disease. Neuromolecular Med. 2012;14(2):91–111. doi: 10.1007/s12017-012-8180-3. [DOI] [PubMed] [Google Scholar]
- 234.Nissen J.B., Kragballe K. Enkephalins modulate differentiation of normal human keratinocytes in vitro . . Exp. Dermatol. 1997;6(5):222–229. doi: 10.1111/j.1600-0625.1997.tb00166.x. [DOI] [PubMed] [Google Scholar]
- 235.Hadjiconstantinou M., Neff N.H. Nicotine and endogenous opioids: neurochemical and pharmacological evidence. Neuropharmacology. 2011;60(7-8):1209–1220. doi: 10.1016/j.neuropharm.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 236.Jeftinija S. Enkephalins modulate excitatory synaptic transmission in the superficial dorsal horn by acting at μ-opioid receptor sites. Brain Res. 1988;460(2):260–268. doi: 10.1016/0006-8993(88)90371-X. [DOI] [PubMed] [Google Scholar]
- 237.Kong H., Raynor K., Yano H., Takeda J., Bell G.I., Reisine T. Agonists and antagonists bind to different domains of the cloned κ opioid receptor. Proc. Natl. Acad. Sci. USA. 1994;91(17):8042–8046. doi: 10.1073/pnas.91.17.8042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Maggi R., Pimpinelli F., Martini L., Piva F. Inhibition of luteinizing hormone-releasing hormone secretion by delta-opioid agonists in GT1-1 neuronal cells. Endocrinology. 1995;136(11):5177–5181. doi: 10.1210/endo.136.11.7588256. [DOI] [PubMed] [Google Scholar]
- 239.Meucci E., Delay-Goyet P., Roques B.P., Zajac J.M. Binding in vivo of selective μ and δ opioid receptor agonists: opioid receptor occupancy by endogenous enkephalins. . Eur. J. Pharmacol. 1989;171(2-3):167–178. doi: 10.1016/0014-2999(89)90105-2. [DOI] [PubMed] [Google Scholar]
- 240.Stein C. Opioid Receptors. Annu. Rev. Med. 2016;67:433–451. doi: 10.1146/annurev-med-062613-093100. [DOI] [PubMed] [Google Scholar]
- 241.Alfaras-Melainis K. Modulation of opioid receptor function by protein-protein interactions. Front. Biosci. 2009;(14):3594. doi: 10.2741/3474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Simonds W.F. The molecular basis of opioid receptor function. Endocr. Rev. 1988;9(2):200–212. doi: 10.1210/edrv-9-2-200. [DOI] [PubMed] [Google Scholar]
- 243.Barreto C.A.V., Baptista S.J., Preto A.J., Silvério D., Melo R., Moreira I.S. Decoding partner specificity of opioid receptor family. Front Mol Biosci. p. 812. [DOI] [PMC free article] [PubMed]
- 244.Chu S.C.P., Kieffer B.L. Delta opioid receptors in brain function and diseases. Pharmacol. Ther. 2013;140(1):112–120. doi: 10.1016/j.pharmthera.2013.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Stein C. Opioids, sensory systems and chronic pain. Eur. J. Pharmacol. 2013;716(1-3):179–187. doi: 10.1016/j.ejphar.2013.01.076. [DOI] [PubMed] [Google Scholar]
- 246.Rittner H.L., Brack A., Stein C. Pain and the immune system. Br. J. Anaesth. 2008;101(1):40–44. doi: 10.1093/bja/aen078. [DOI] [PubMed] [Google Scholar]
- 247.Epelbaum J., Dournaud P., Fodor M., Viollet C. The neurobiology of somatostatin. Crit. Rev. Neurobiol. 1994;8(1-2):25–44. [PubMed] [Google Scholar]
- 248.Patel Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999;20(3):157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 249.Somatostatin R.S. N. Engl. J. Med. 1983;309(24):1495–1501. doi: 10.1056/NEJM198312153092406. [DOI] [PubMed] [Google Scholar]
- 250.Ramírez J.L., Mouchantaf R., Kumar U., Otero Corchon V., Rubinstein M., Low M.J., Patel Y.C. Brain somatostatin receptors are up-regulated in somatostatin-deficient mice. Mol. Endocrinol. 2002;16(8):1951–1963. doi: 10.1210/me.2002-0068. [DOI] [PubMed] [Google Scholar]
- 251.Reisine T., Bell G.I. Molecular biology of somatostatin receptors. Endocr. Rev. 1995;16(4):427–442. doi: 10.1210/edrv-16-4-427. [DOI] [PubMed] [Google Scholar]
- 252.Hukovic N., Rocheville M., Kumar U., Sasi R., Khare S., Patel Y.C. Agonist-dependent up-regulation of human somatostatin receptor type 1 requires molecular signals in the cytoplasmic C-tail. J. Biol. Chem. 1999;274(35):24550–24558. doi: 10.1074/jbc.274.35.24550. [DOI] [PubMed] [Google Scholar]
- 253.Hukovic N., Panetta R., Kumar U., Patel Y.C. Agonist-dependent regulation of cloned human somatostatin receptor types 1-5 (hSSTR1-5): subtype selective internalization or upregulation. Endocrinology. 1996;137(9):4046–4049. doi: 10.1210/endo.137.9.8756582. [DOI] [PubMed] [Google Scholar]
- 254.Song Y-H., Yoon J., Lee S-H. The role of neuropeptide somatostatin in the brain and its application in treating neurological disorders. Exp. Mol. Med. 2021;53(3):328–338. doi: 10.1038/s12276-021-00580-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Francis B.H., Baskin D.G., Saunders D.R., Ensinck J.W. Distribution of somatostatin-14 and somatostatin-28 gastrointestinal-pancreatic cells of rats and humans. Gastroenterology. 1990;99(5):1283–1291. doi: 10.1016/0016-5085(90)91151-U. [DOI] [PubMed] [Google Scholar]
- 256.Abdel-Rahman O., Lamarca A., Valle J.W., Hubner R.A. Somatostatin receptor expression in hepatocellular carcinoma: prognostic and therapeutic considerations. Endocr. Relat. Cancer. 2014;21(6):R485–R493. doi: 10.1530/ERC-14-0389. [DOI] [PubMed] [Google Scholar]
- 257.Liguz-Lecznar M., Urban-Ciecko J., Kossut M. Somatostatin and somatostatin-containing neurons in shaping neuronal activity and plasticity. Front. Neural Circuits. 2016;10:48. doi: 10.3389/fncir.2016.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Baraban S.C., Tallent M.K. Interneuron Diversity series: Interneuronal neuropeptides--endogenous regulators of neuronal excitability. Trends Neurosci. 2004;27(3):135–142. doi: 10.1016/j.tins.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 259.Bichet D., Bouvier M., Chini B., Gimpl G., Guillon G., Kimura T. Vasopressin and oxytocin receptors (version 2019.4) in the IUPHAR/ BPS Guide to Pharmacology Database. IUPHAR/BPS Guid to Pharmacol CITE. 2019:4. [Google Scholar]
- 260.Holmes C.L., Landry D.W., Granton J.T. Science review: Vasopressin and the cardiovascular system part 1--receptor physiology. Crit. Care. 2003;7(6):427–434. doi: 10.1186/cc2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fineberg S.K., Ross D.A. Oxytocin and the Social Brain. Biol. Psychiatry. 2017;81(3):e19–e21. doi: 10.1016/j.biopsych.2016.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Lee H.J., Macbeth A.H., Pagani J.H., Young W.S., III Oxytocin: the great facilitator of life. Prog. Neurobiol. 2009;88(2):127–151. doi: 10.1016/j.pneurobio.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Yang H-P., Wang L., Han L., Wang S.C. Nonsocial functions of hypothalamic oxytocin. ISRN Neurosci. 2013;2013:179272. doi: 10.1155/2013/179272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Rousseau-Merck M.F., René P., Derré J., Bienvenu T., Berger R., de Keyzer Y. Chromosomal localization of the human V3 pituitary vasopressin receptor gene (AVPR3) to 1q32. Genomics. 1995;30(2):405–406. [PubMed] [Google Scholar]
- 265.Thibonnier M., Preston J.A., Dulin N., Wilkins P.L., Berti-Mattera L.N., Mattera R. The human V3 pituitary vasopressin receptor: ligand binding profile and density-dependent signaling pathways. Endocrinology. 1997;138(10):4109–4122. doi: 10.1210/endo.138.10.5432. [DOI] [PubMed] [Google Scholar]
- 266.Thibonnier M., Conarty D.M., Preston J.A., Wilkins P.L., Berti-Mattera L.N., Mattera R. Molecular pharmacology of human vasopressin receptors. Adv. Exp. Med. Biol. 1998;449:251–276. doi: 10.1007/978-1-4615-4871-3_34. [DOI] [PubMed] [Google Scholar]
- 267.Koshimizu T.A., Nasa Y., Tanoue A., Oikawa R., Kawahara Y., Kiyono Y., Adachi T., Tanaka T., Kuwaki T., Mori T., Takeo S., Okamura H., Tsujimoto G. V1a vasopressin receptors maintain normal blood pressure by regulating circulating blood volume and baroreflex sensitivity. Proc. Natl. Acad. Sci. USA. 2006;103(20):7807–7812. doi: 10.1073/pnas.0600875103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Aoyagi T., Birumachi J., Hiroyama M., Fujiwara Y., Sanbe A., Yamauchi J., Tanoue A. Alteration of glucose homeostasis in V1a vasopressin receptor-deficient mice. Endocrinology. 2007;148(5):2075–2084. doi: 10.1210/en.2006-1315. [DOI] [PubMed] [Google Scholar]
- 269.Birumachi J., Hiroyama M., Fujiwara Y., Aoyagi T., Sanbe A., Tanoue A. Impaired arginine-vasopressin-induced aldosterone release from adrenal gland cells in mice lacking the vasopressin V1A receptor. Eur. J. Pharmacol. 2007;566(1-3):226–230. doi: 10.1016/j.ejphar.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 270.Briley E.M., Lolait S.J., Axelrod J., Felder C.C. The cloned vasopressin V1a receptor stimulates phospholipase A2, phospholipase C, and phospholipase D through activation of receptor-operated calcium channels. Neuropeptides. 1994;27(1):63–74. doi: 10.1016/0143-4179(94)90017-5. [DOI] [PubMed] [Google Scholar]
- 271.Chandrashekhar Y., Prahash A.J., Sen S., Gupta S., Roy S., Anand I.S. The role of arginine vasopressin and its receptors in the normal and failing rat heart. J. Mol. Cell. Cardiol. 2003;35(5):495–504. doi: 10.1016/S0022-2828(03)00053-1. [DOI] [PubMed] [Google Scholar]
- 272.Yirmiya N., Rosenberg C., Levi S., Salomon S., Shulman C., Nemanov L., Dina C., Ebstein R.P. Association between the arginine vasopressin 1a receptor (AVPR1a) gene and autism in a family-based study: mediation by socialization skills. Mol. Psychiatry. 2006;11(5):488–494. doi: 10.1038/sj.mp.4001812. [DOI] [PubMed] [Google Scholar]
- 273.Young L.J., Nilsen R., Waymire K.G., MacGregor G.R., Insel T.R. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature. 1999;400(6746):766–768. doi: 10.1038/23475. [DOI] [PubMed] [Google Scholar]
- 274.Bielsky I.F., Hu S.B., Szegda K.L., Westphal H., Young L.J. Profound impairment in social recognition and reduction in anxiety-like behavior in vasopressin V1a receptor knockout mice. Neuropsychopharmacology. 2004;29(3):483–493. doi: 10.1038/sj.npp.1300360. [DOI] [PubMed] [Google Scholar]
- 275.Lim M.M., Wang Z., Olazábal D.E., Ren X., Terwilliger E.F., Young L.J. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature. 2004;429(6993):754–757. doi: 10.1038/nature02539. [DOI] [PubMed] [Google Scholar]
- 276.Gaillard R.C., Schoenenberg P., Favrod-Coune C.A., Muller A.F., Marie J., Bockaert J., Jard S. Properties of rat anterior pituitary vasopressin receptors: relation to adenylate cyclase and the effect of corticotropin-releasing factor. Proc. Natl. Acad. Sci. USA. 1984;81(9):2907–2911. doi: 10.1073/pnas.81.9.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lolait S.J., O’Carroll A.M., Mahan L.C., Felder C.C., Button D.C., Young W.S., III, Mezey E., Brownstein M.J. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc. Natl. Acad. Sci. USA. 1995;92(15):6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Lolait S.J., Stewart L.Q., Jessop D.S., Young W.S., III, O’Carroll A.M. The hypothalamic-pituitary-adrenal axis response to stress in mice lacking functional vasopressin V1b receptors. Endocrinology. 2007;148(2):849–856. doi: 10.1210/en.2006-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.René P., Lenne F., Ventura M.A., Bertagna X., de Keyzer Y. Nucleotide sequence and structural organization of the human vasopressin pituitary receptor (V3) gene. Gene. 2000;241(1):57–64. doi: 10.1016/S0378-1119(99)00468-0. [DOI] [PubMed] [Google Scholar]
- 280.Griebel G., Simiand J., Serradeil-Le Gal C., Wagnon J., Pascal M., Scatton B., Maffrand J.P., Soubrie P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl. Acad. Sci. USA. 2002;99(9):6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Nikkheslat N., McLaughlin A.P., Hastings C., Zajkowska Z., Nettis M.A., Mariani N., Enache D., Lombardo G., Pointon L., Cowen P.J., Cavanagh J., Harrison N.A., Bullmore E.T., Pariante C.M., Mondelli V. Childhood trauma, HPA axis activity and antidepressant response in patients with depression. Brain Behav. Immun. 2020;87:229–237. doi: 10.1016/j.bbi.2019.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Rosenblat J.D., McIntyre R.S., Alves G.S., Fountoulakis K.N., Carvalho A.F. Beyond monoamines-novel targets for treatment-resistant depression: A comprehensive review. Curr. Neuropharmacol. 2015;13(5):636–655. doi: 10.2174/1570159X13666150630175044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Juruena M.F., Pariante C.M., Papadopoulos A.S., Poon L., Lightman S., Cleare A.J. Prednisolone suppression test in depression: prospective study of the role of HPA axis dysfunction in treatment resistance. Br. J. Psychiatry. 2009;194(4):342–349. doi: 10.1192/bjp.bp.108.050278. [DOI] [PubMed] [Google Scholar]
- 284.Stetler C., Miller G.E. Depression and hypothalamic-pituitary-adrenal activation: A quantitative summary of four decades of research. Psychosom. Med. 2011;73(2):114–126. doi: 10.1097/PSY.0b013e31820ad12b. [DOI] [PubMed] [Google Scholar]
- 285.Dinan T.G., Scott L.V. Anatomy of melancholia: focus on hypothalamic-pituitary-adrenal axis overactivity and the role of vasopressin. J. Anat. 2005;207(3):259–264. doi: 10.1111/j.1469-7580.2005.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Meynen G., Unmehopa U.A., van Heerikhuize J.J., Hofman M.A., Swaab D.F., Hoogendijk W.J.G. Increased arginine vasopressin mRNA expression in the human hypothalamus in depression: A preliminary report. Biol. Psychiatry. 2006;60(8):892–895. doi: 10.1016/j.biopsych.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 287.Zhou J-N., Riemersma R.F., Unmehopa U.A., Hoogendijk W.J.G., van Heerikhuize J.J., Hofman M.A., Swaab D.F. Alterations in arginine vasopressin neurons in the suprachiasmatic nucleus in depression. Arch. Gen. Psychiatry. 2001;58(7):655–662. doi: 10.1001/archpsyc.58.7.655. [DOI] [PubMed] [Google Scholar]
- 288.van Londen L., Goekoop J.G., van Kempen G.M.J., Frankhuijzen-Sierevogel A.C., Wiegant V.M., van der Velde E.A., De Wied D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17(4):284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 289.Purba J.S., Hoogendijk W.J.G., Hofman M.A., Swaab D.F. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch. Gen. Psychiatry. 1996;53(2):137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- 290.Chaki S. Vasopressin V1B receptor antagonists as potential antidepressants. Int. J. Neuropsychopharmacol. 2021;24(6):450–463. doi: 10.1093/ijnp/pyab013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Fatima S., Muhammad H., Arif A. Nephrogenic diabetes insipidus. Pak. Pediatr. J. 2011;35(3):169–170. [Google Scholar]
- 292.Ala Y., Morin D., Mouillac B., Sabatier N., Vargas R., Cotte N., Déchaux M., Antignac C., Arthus M.F., Lonergan M., Turner M.S., Balestre M.N., Alonso G., Hibert M., Barberis C., Hendy G.N., Bichet D.G., Jard S. Functional studies of twelve mutant V2 vasopressin receptors related to nephrogenic diabetes insipidus: molecular basis of a mild clinical phenotype. J. Am. Soc. Nephrol. 1998;9(10):1861–1872. doi: 10.1681/ASN.V9101861. [DOI] [PubMed] [Google Scholar]
- 293.Sato K., Fukuno H., Taniguchi T., Sawada S., Fukui T., Kinoshita M. A novel mutation in the vasopressin V2 receptor gene in a woman with congenital nephrogenic diabetes insipidus. Intern. Med. 1999;38(10):808–812. doi: 10.2169/internalmedicine.38.808. [DOI] [PubMed] [Google Scholar]
- 294.Schöneberg T., Kostenis E., Liu J., Gudermann T., Wess J. Molecular aspects of vasopressin receptor function. Adv. Exp. Med. Biol. 1998;449:347–358. doi: 10.1007/978-1-4615-4871-3_44. [DOI] [PubMed] [Google Scholar]
- 295.Weig H.J., Laugwitz K.L., Moretti A., Kronsbein K., Städele C., Brüning S., Seyfarth M., Brill T., Schömig A., Ungerer M. Enhanced cardiac contractility after gene transfer of V2 vasopressin receptors in vivo by ultrasound-guided injection or transcoronary delivery. . Circulation. 2000;101(13):1578–1585. doi: 10.1161/01.CIR.101.13.1578. [DOI] [PubMed] [Google Scholar]
- 296.Åkerlund M., Bossmar T., Brouard R., Kostrzewska A., Laudanski T., Lemancewicz A., Serradeil-Le Gal C., Steinwall M. Receptor binding of oxytocin and vasopressin antagonists and inhibitory effects on isolated myometrium from preterm and term pregnant women. Br. J. Obstet. Gynaecol. 1999;106(10):1047–1053. doi: 10.1111/j.1471-0528.1999.tb08112.x. [DOI] [PubMed] [Google Scholar]
- 297.Juul KV, Bichet DG, Nielsen S, Nørgaard JP. The physiological and pathophysiological functions of renal and extrarenal vasopressin V2 receptors. 2014;306(9):931-940. doi: 10.1152/ajprenal.00604.2013. [DOI] [PubMed] [Google Scholar]
- 298.Yayla MA, Arda B. Peptide hormones and neurodegenerative diseases. J Exp Basic Med Sci. 2021;2(1):062-75. [Google Scholar]
- 299.Buisman-Pijlman F.T.A., Sumracki N.M., Gordon J.J., Hull P.R., Carter C.S., Tops M. Individual differences underlying susceptibility to addiction: Role for the endogenous oxytocin system. Pharmacol. Biochem. Behav. 2014;119:22–38. doi: 10.1016/j.pbb.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 300.Viviani D., Stoop R. Opposite effects of oxytocin and vasopressin on the emotional expression of the fear response. Prog. Brain Res. 2008:170, 207-218. doi: 10.1016/S0079-6123(08)00418-4. [DOI] [PubMed] [Google Scholar]
- 301.Kirsch P., Esslinger C., Chen Q., Mier D., Lis S., Siddhanti S., Gruppe H., Mattay V.S., Gallhofer B., Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J. Neurosci. 2005;25(49):11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Petersson M., Lundeberg T., Sohlström A., Wiberg U., Uvnäs-Moberg K. Oxytocin increases the survival of musculocutaneous flaps. Naunyn Schmiedebergs Arch. Pharmacol. 1998;357(6):701–704. doi: 10.1007/PL00005227. [DOI] [PubMed] [Google Scholar]
- 303.Grewen K.M., Light K.C., Mechlin B., Girdler S.S. Ethnicity is associated with alterations in oxytocin relationships to pain sensitivity in women. Ethn. Health. 2008;13(3):219–241. doi: 10.1080/13557850701837310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Neumann I.D., Landgraf R. Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci. 2012;35(11):649–659. doi: 10.1016/j.tins.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 305.McQuaid R.J., McInnis O.A., Abizaid A., Anisman H. Making room for oxytocin in understanding depression. Neurosci. Biobehav. Rev. 2014;45:305–322. doi: 10.1016/j.neubiorev.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 306.Wulsin A.C., Herman J.P., Solomon M.B. Mifepristone decreases depression-like behavior and modulates neuroendocrine and central hypothalamic-pituitary-adrenocortical axis responsiveness to stress. Psychoneuroendocrinology. 2010;35(7):1100–1112. doi: 10.1016/j.psyneuen.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Bey K., Campos-Martin R., Klawohn J., Reuter B., Grützmann R., Riesel A., Wagner M., Ramirez A., Kathmann N. Hypermethylation of the oxytocin receptor gene (OXTR) in obsessive-compulsive disorder: further evidence for a biomarker of disease and treatment response. Epigenetics. 2021:1–11. doi: 10.1080/15592294.2021.1943864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Gabery S., Ahmed R.M., Caga J., Kiernan M.C., Halliday G.M., Petersén Å. Loss of the metabolism and sleep regulating neuronal populations expressing orexin and oxytocin in the hypothalamus in amyotrophic lateral sclerosis. Neuropathol. Appl. Neurobiol. 2021;47(7):979–989. doi: 10.1111/nan.12709. [DOI] [PubMed] [Google Scholar]
- 309.Bunzow J.R., Sonders M.S., Arttamangkul S., Harrison L.M., Zhang G., Quigley D.I., Darland T., Suchland K.L., Pasumamula S., Kennedy J.L., Olson S.B., Magenis R.E., Amara S.G., Grandy D.K. Amphetamine, 3,4-methylenedioxymethamphe-tamine, lysergic acid diethylamide, and metabolites of the catecholamine neurotransmitters are agonists of a rat trace amine receptor. Mol. Pharmacol. 2001;60(6):1181–1188. doi: 10.1124/mol.60.6.1181. [DOI] [PubMed] [Google Scholar]
- 310.Borowsky B., Adham N., Jones K.A., Raddatz R., Artymyshyn R., Ogozalek K.L., Durkin M.M., Lakhlani P.P., Bonini J.A., Pathirana S., Boyle N., Pu X., Kouranova E., Lichtblau H., Ochoa F.Y., Branchek T.A., Gerald C. Trace amines: identification of a family of mammalian G protein-coupled receptors. Proc. Natl. Acad. Sci. USA. 2001;98(16):8966–8971. doi: 10.1073/pnas.151105198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311.Eyun S-I., Moriyama H., Hoffmann F.G., Moriyama E.N. Molecular evolution and functional divergence of trace amine-associated receptors. PLoS One. 2016;11(3):e0151023. doi: 10.1371/journal.pone.0151023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Lindemann L., Ebeling M., Kratochwil N.A., Bunzow J.R., Grandy D.K., Hoener M.C. Trace amine-associated receptors form structurally and functionally distinct subfamilies of novel G protein-coupled receptors. Genomics. 2005;85(3):372–385. doi: 10.1016/j.ygeno.2004.11.010. [DOI] [PubMed] [Google Scholar]
- 313.Revel F.G., Moreau J.L., Pouzet B., Mory R., Bradaia A., Buchy D., Metzler V., Chaboz S., Groebke Z.K., Galley G., Norcross R.D., Tuerck D., Bruns A., Morairty S.R., Kilduff T.S., Wallace T.L., Risterucci C., Wettstein J.G., Hoener M.C. A new perspective for schizophrenia: TAAR1 agonists reveal antipsychotic- and antidepressant-like activity, improve cognition and control body weight. Mol. Psychiatry. 2013;18(5):543–556. doi: 10.1038/mp.2012.57. [DOI] [PubMed] [Google Scholar]
- 314.Pei Y., Asif-Malik A., Canales J.J. Trace amines and the trace amine-associated receptor 1: Pharmacology, neurochemistry, and clinical implications. Front. Neurosci. 2016;10:148. doi: 10.3389/fnins.2016.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Raab S., Wang H., Uhles S., Cole N., Alvarez-Sanchez R., Künnecke B., Ullmer C., Matile H., Bedoucha M., Norcross R.D., Ottaway-Parker N., Perez-Tilve D., Conde Knape K., Tschöp M.H., Hoener M.C., Sewing S. Incretin-like effects of small molecule trace amine-associated receptor 1 agonists. Mol. Metab. 2015;5(1):47–56. doi: 10.1016/j.molmet.2015.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Zucchi R., Chiellini G., Scanlan T.S., Grandy D.K. Trace amine-associated receptors and their ligands. Br. J. Pharmacol. 2006;149(8):967–978. doi: 10.1038/sj.bjp.0706948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Gainetdinov R.R., Hoener M.C., Berry M.D. Trace amines and their receptors. Pharmacol. Rev. 2018;70(3):549–620. doi: 10.1124/pr.117.015305. [DOI] [PubMed] [Google Scholar]
- 318.Boulton A.A. Phenylethylaminergic modulation of catecholaminergic neurotransmission. Prog. Neuropsychopharmacol. Biol. Psychiatry. 1991;15(2):139–156. doi: 10.1016/0278-5846(91)90076-D. [DOI] [PubMed] [Google Scholar]
- 319.Rutigliano G., Accorroni A., Zucchi R. The case for TAAR1 as a modulator of central nervous system function. Front. Pharmacol. 2018;8(JAN):987. doi: 10.3389/fphar.2017.00987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Jones R.S.G. Noradrenaline-octopamine interactions on cortical neurones in the rat. Eur. J. Pharmacol. 1982;77(2-3):159–162. doi: 10.1016/0014-2999(82)90012-7. [DOI] [PubMed] [Google Scholar]
- 321.Liberles S.D. Trace amine-associated receptors: ligands, neural circuits, and behaviors. Curr. Opin. Neurobiol. 2015;34:1–7. doi: 10.1016/j.conb.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Espinoza S., Ghisi V., Emanuele M., Leo D., Sukhanov I., Sotnikova T.D., Chieregatti E., Gainetdinov R.R. Postsynaptic D2 dopamine receptor supersensitivity in the striatum of mice lacking TAAR1. Neuropharmacology. 2015;93:308–313. doi: 10.1016/j.neuropharm.2015.02.010. [DOI] [PubMed] [Google Scholar]
- 323.Harmeier A., Obermueller S., Meyer C.A., Revel F.G., Buchy D., Chaboz S., Dernick G., Wettstein J.G., Iglesias A., Rolink A., Bettler B., Hoener M.C. Trace amine-associated receptor 1 activation silences GSK3β signaling of TAAR1 and D2R heteromers. Eur. Neuropsychopharmacol. 2015;25(11):2049–2061. doi: 10.1016/j.euroneuro.2015.08.011. [DOI] [PubMed] [Google Scholar]
- 324.Bradaia A., Trube G., Stalder H., Norcross R.D., Ozmen L., Wettstein J.G., Pinard A., Buchy D., Gassmann M., Hoener M.C., Bettler B. The selective antagonist EPPTB reveals TAAR1-mediated regulatory mechanisms in dopaminergic neurons of the mesolimbic system. Proc. Natl. Acad. Sci. USA. 2009;106(47):20081–20086. doi: 10.1073/pnas.0906522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Revel F.G., Moreau J-L., Gainetdinov R.R., Bradaia A., Sotnikova T.D., Mory R., Durkin S., Zbinden K.G., Norcross R., Meyer C.A., Metzler V., Chaboz S., Ozmen L., Trube G., Pouzet B., Bettler B., Caron M.G., Wettstein J.G., Hoener M.C. TAAR1 activation modulates monoaminergic neurotransmission, preventing hyperdopaminergic and hypoglutamatergic activity. Proc. Natl. Acad. Sci. USA. 2011;108(20):8485–8490. doi: 10.1073/pnas.1103029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326.Liberles S.D., Buck L.B. A second class of chemosensory receptors in the olfactory epithelium. Nature. 2006;442(7103):645–650. doi: 10.1038/nature05066. [DOI] [PubMed] [Google Scholar]
- 327.Wallrabenstein I., Kuklan J., Weber L., Zborala S., Werner M., Altmüller J., Becker C., Schmidt A., Hatt H., Hummel T., Gisselmann G. Human trace amine-associated receptor TAAR5 can be activated by trimethylamine. PLoS One. 2013;8(2):e54950. doi: 10.1371/journal.pone.0054950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Dinter J., Mühlhaus J., Wienchol C.L., Yi C.X., Nürnberg D., Morin S., Grüters A., Köhrle J., Schöneberg T., Tschöp M., Krude H., Kleinau G., Biebermann H. Inverse agonistic action of 3-iodothyronamine at the human trace amine-associated receptor 5. PLoS One. 2015;10(2):e0117774. doi: 10.1371/journal.pone.0117774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Mühlhaus J., Dinter J., Nürnberg D., Rehders M., Depke M., Golchert J., Homuth G., Yi C.X., Morin S., Köhrle J., Brix K., Tschöp M., Kleinau G., Biebermann H. Analysis of human TAAR8 and murine Taar8b mediated signaling pathways and expression profile. Int. J. Mol. Sci. 2014;15(11):20638–20655. doi: 10.3390/ijms151120638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Mazella J., Sarret P., Vincent J-P. Neurotensin receptors (version 2019.4) in the IUPHAR/BPS Guide to Pharmacology Database. IUPHAR/BPS Guid to Pharmacol CITE. 2019:4. [Google Scholar]
- 331.Vita N., Laurent P., Lefort S., Chalon P., Dumont X., Kaghad M., Gully D., Le Fur G., Ferrara P., Caput D. Cloning and expression of a complementary DNA encoding a high affinity human neurotensin receptor. FEBS Lett. 1993;317(1-2):139–142. doi: 10.1016/0014-5793(93)81509-X. [DOI] [PubMed] [Google Scholar]
- 332.Zhang X., Xu Z.Q., Bao L., Dagerlind A., Hökfelt T. Complementary distribution of receptors for neurotensin and NPY in small neurons in rat lumbar DRGs and regulation of the receptors and peptides after peripheral axotomy. J. Neurosci. 1995;15(4):2733–2747. doi: 10.1523/JNEUROSCI.15-04-02733.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Elde R., Schalling M., Ceccatelli S., Nakanishi S., Hökfelt T. Localization of neuropeptide receptor mRNA in rat brain: initial observations using probes for neurotensin and substance P receptors. Neurosci. Lett. 1990;120(1):134–138. doi: 10.1016/0304-3940(90)90187-E. [DOI] [PubMed] [Google Scholar]
- 334.Chalon P., Vita N., Kaghad M., Guillemot M., Bonnin J., Delpech B., Le Fur G., Ferrara P., Caput D. Molecular cloning of a levocabastine-sensitive neurotensin binding site. FEBS Lett. 1996;386(2-3):91–94. doi: 10.1016/0014-5793(96)00397-3. [DOI] [PubMed] [Google Scholar]
- 335.Mazella J., Botto J.M., Guillemare E., Coppola T., Sarret P., Vincent J.P. Structure, functional expression, and cerebral localization of the levocabastine-sensitive neurotensin/neuromedin N receptor from mouse brain. J. Neurosci. 1996;16(18):5613–5620. doi: 10.1523/JNEUROSCI.16-18-05613.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336.Vita N., Oury-Donat F., Chalon P., Guillemot M., Kaghad M., Bachy A., Thurneyssen O., Garcia S., Poinot-Chazel C., Casellas P., Keane P., Le Fur G., Maffrand J.P., Soubrie P., Caput D., Ferrara P. Neurotensin is an antagonist of the human neurotensin NT2 receptor expressed in Chinese hamster ovary cells. Eur. J. Pharmacol. 1998;360(2-3):265–272. doi: 10.1016/S0014-2999(98)00678-5. [DOI] [PubMed] [Google Scholar]
- 337.Sarret P., Beaudet A., Vincent J.P., Mazella J. Regional and cellular distribution of low affinity neurotensin receptor mRNA in adult and developing mouse brain. J. Comp. Neurol. 1998;394(3):344–356. doi: 10.1002/(SICI)1096-9861(19980511)394:3<344:AID-CNE6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 338.Walker N., Lepee-Lorgeoux I., Fournier J., Betancur C., Rostene W., Ferrara P., Caput D. Tissue distribution and cellular localization of the levocabastine-sensitive neurotensin receptor mRNA in adult rat brain. Brain Res. Mol. Brain Res. 1998;57(2):193–200. doi: 10.1016/S0169-328X(98)00074-6. [DOI] [PubMed] [Google Scholar]
- 339.Amar S., Kitabgi P., Vincent J.P. Activation of phosphatidylinositol turnover by neurotensin receptors in the human colonic adenocarcinoma cell line HT29. FEBS Lett. 1986;201(1):31–36. doi: 10.1016/0014-5793(86)80565-8. [DOI] [PubMed] [Google Scholar]
- 340.Amar S., Kitabgi P., Vincent J-P. Stimulation of inositol phosphate production by neurotensin in neuroblastoma N1E115 cells: implication of GTP-binding proteins and relationship with the cyclic GMP response. J. Neurochem. 1987;49(4):999–1006. doi: 10.1111/j.1471-4159.1987.tb09986.x. [DOI] [PubMed] [Google Scholar]
- 341.Gailly P., Najimi M., Hermans E. Evidence for the dual coupling of the rat neurotensin receptor with pertussis toxin-sensitive and insensitive G-proteins. FEBS Lett. 2000;483(2-3):109–113. doi: 10.1016/S0014-5793(00)02095-0. [DOI] [PubMed] [Google Scholar]
- 342.Amar S., Mazella J., Checler F., Kitabgi P., Vincent J.P. Regulation of cyclic GMP levels by neurotensin in neuroblastoma clone N1E115. Biochem. Biophys. Res. Commun. 1985;129(1):117–125. doi: 10.1016/0006-291X(85)91411-1. [DOI] [PubMed] [Google Scholar]
- 343.Bozou J.C., Amar S., Vincent J.P., Kitabgi P. Neurotensinmediated inhibition of cyclic AMP formation in neuroblastoma N1E115 cells: involvement of the inhibitory GTP-binding component of adenylate cyclase. Mol. Pharmacol. 1986;29(5):489–496. [PubMed] [Google Scholar]
- 344.Clineschmidt B.V., McGuffin J.C. Neurotensin administered intracisternally inhibits responsiveness of mice to noxious stimuli. Eur. J. Pharmacol. 1977;46(4):395–396. doi: 10.1016/0014-2999(77)90236-9. [DOI] [PubMed] [Google Scholar]
- 345.Furuta S., Kisara K., Sakurada S., Sakurada T., Sasaki Y., Suzuki K. Structure-antinociceptive activity studies with neurotensin. Br. J. Pharmacol. 1984;83(1):43–48. doi: 10.1111/j.1476-5381.1984.tb10117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Kleczkowska P., Lipkowski A.W. Neurotensin and neurotensin receptors: characteristic, structure-activity relationship and pain modulation--a review. Eur. J. Pharmacol. 2013;716(1-3):54–60. doi: 10.1016/j.ejphar.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 347.Alexander W, Bernstein KE, Catt KJ, Gasparo M. de, ; Singh, KD; Eguchi, S Angiotensin receptors (version 2019.4) in the IUPHAR/ BPS Guide to Pharmacology Database. IUPHAR/BPS Guid to Pharmacol CITE, 2019, 4.
- 348.Karnik S.S., Unal H., Kemp J.R., Tirupula K.C., Eguchi S., Vanderheyden P.M.L., Thomas W.G. International union of basic and clinical pharmacology. XCIX. Angiotensin receptors: interpreters of pathophysiological angiotensinergic stimuli. [corrected]. Pharmacol. Rev. 2015;67(4):754–819. doi: 10.1124/pr.114.010454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.de Gasparo M., Catt K.J., Inagami T., Wright J.W., Unger T. International union of pharmacology. XXIII. The angiotensin II receptors. Pharmacol. Rev. 2000;52(3):415–472. [PubMed] [Google Scholar]
- 350.Verma K., Pant M., Paliwal S., Dwivedi J., Sharma S. An insight on multicentric signaling of angiotensin II in cardiovascular system: A recent update. Front. Pharmacol. 2021;12:734917. doi: 10.3389/fphar.2021.734917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Adamcova M., Kawano I., Simko F. The impact of microRNAs in renin-angiotensin-system-induced cardiac remodelling. Int. J. Mol. Sci. 2021;22(9):4762. doi: 10.3390/ijms22094762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Matsubara H. Pathophysiological role of angiotensin II type 2 receptor in cardiovascular and renal diseases. Circ. Res. 1998;83(12):1182–1191. doi: 10.1161/01.RES.83.12.1182. [DOI] [PubMed] [Google Scholar]
- 353.Kawai T., Forrester S.J., O’Brien S., Baggett A., Rizzo V., Eguchi S. AT1 receptor signaling pathways in the cardiovascular system. Pharmacol. Res. 2017;125((Pt A)):4-13. doi: 10.1016/j.phrs.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Allen A.M., MacGregor D.P., McKinley M.J., Mendelsohn F.A.O. Angiotensin II receptors in the human brain. Regul. Pept. 1999;79(1):1–7. doi: 10.1016/S0167-0115(98)00138-4. [DOI] [PubMed] [Google Scholar]
- 355.Li Y., Li X.H., Yuan H. Angiotensin II type-2 receptor-specific effects on the cardiovascular system. Cardiovasc. Diagn. Ther. 2012;2(1):56–62. doi: 10.3978/j.issn.2223-3652.2012.02.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Higuchi S., Ohtsu H., Suzuki H., Shirai H., Frank G.D., Eguchi S. Angiotensin II signal transduction through the AT1 receptor: novel insights into mechanisms and pathophysiology. Clin. Sci. (Lond.) 2007;112(8):417–428. doi: 10.1042/CS20060342. [DOI] [PubMed] [Google Scholar]
- 357.Catt K.J., Mendelsohn F.A., Millan M.A., Aguilera G. The role of angiotensin II receptors in vascular regulation. J. Cardiovasc. Pharmacol. 1984;6(Suppl. 4):S575–S586. doi: 10.1097/00005344-198406004-00004. [DOI] [PubMed] [Google Scholar]
- 358.Hurt R.C., Garrett J.C., Keifer O.P., Jr, Linares A., Couling L., Speth R.C., Ressler K.J., Marvar P.J. Angiotensin type 1a receptors on corticotropin-releasing factor neurons contribute to the expression of conditioned fear. Genes Brain Behav. 2015;14(7):526–533. doi: 10.1111/gbb.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Marvar P.J., Goodman J., Fuchs S., Choi D.C., Banerjee S., Ressler K.J. Angiotensin type 1 receptor inhibition enhances the extinction of fear memory. Biol. Psychiatry. 2014;75(11):864–872. doi: 10.1016/j.biopsych.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Nazzaro P., Manzari M., Merlo M., Triggiani R., Scarano A., Ciancio L., Pirrelli A. Distinct and combined vascular effects of ACE blockade and HMG-CoA reductase inhibition in hypertensive subjects. Hypertension. 1999;33(2):719–725. doi: 10.1161/01.HYP.33.2.719. [DOI] [PubMed] [Google Scholar]
- 361.Nakajima M., Hutchinson H.G., Fujinaga M., Hayashida W., Morishita R., Zhang L., Horiuchi M., Pratt R.E., Dzau V.J. The angiotensin II type 2 (AT2) receptor antagonizes the growth effects of the AT1 receptor: gain-of-function study using gene transfer. Proc. Natl. Acad. Sci. USA. 1995;92(23):10663–10667. doi: 10.1073/pnas.92.23.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Millatt L.J., Abdel-Rahman E.M., Siragy H.M. Angiotensin II and nitric oxide: A question of balance. Regul. Pept. 1999;81(1-3):1–10. doi: 10.1016/S0167-0115(99)00027-0. [DOI] [PubMed] [Google Scholar]
- 363.Griendling K.K., Lassègue B., Alexander R.W. Angiotensin receptors and their therapeutic implications. Annu. Rev. Pharmacol. Toxicol. 1996;36:281–306. doi: 10.1146/annurev.pa.36.040196.001433. [DOI] [PubMed] [Google Scholar]
- 364.Laragh J.H., Brenner B.M. Characteristics of angiotensin II receptors and their role in cell and organ physiology.Hypertension : pathophysiology, diagnosis, and management. Raven Press; 1995. pp. 1695–1720. [Google Scholar]
- 365.Horiuchi M., Akishita M., Dzau V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension. 1999;33(2):613–621. doi: 10.1161/01.HYP.33.2.613. [DOI] [PubMed] [Google Scholar]
- 366.de Gasparo M., Siragy H.M. The AT2 receptor: fact, fancy and fantasy. Regul. Pept. 1999;81(1-3):11–24. doi: 10.1016/S0167-0115(99)00023-3. [DOI] [PubMed] [Google Scholar]
- 367.D’Amore A., Black M.J., Thomas W.G. The angiotensin II type 2 receptor causes constitutive growth of cardiomyocytes and does not antagonize angiotensin II type 1 receptor-mediated hypertrophy. Hypertension. 2005;46(6):1347–1354. doi: 10.1161/01.HYP.0000193504.51489.cf. [DOI] [PubMed] [Google Scholar]
- 368.Gold S., Haran I., Attias J., Shapira I., Shahar A. Biochemical and cardiovascular measures in subjects with noise-induced hearing loss. J. Occup. Med. 1989;31(11):933–937. doi: 10.1097/00043764-198911000-00018. [DOI] [PubMed] [Google Scholar]
- 369.Padia S.H., Kemp B.A., Howell N.L., Fournie-Zaluski M-C., Roques B.P., Carey R.M. Conversion of renal angiotensin II to angiotensin III is critical for AT2 receptor-mediated natriuresis in rats. Hypertension. 2008;51(2):460–465. doi: 10.1161/HYPERTENSIONAHA.107.103242. [DOI] [PubMed] [Google Scholar]
- 370.Kemp B.A., Bell J.F., Rottkamp D.M., Howell N.L., Shao W., Navar L.G., Padia S.H., Carey R.M. Intrarenal angiotensin III is the predominant agonist for proximal tubule angiotensin type 2 receptors. Hypertension. 2012;60(2):387–395. doi: 10.1161/HYPERTENSIONAHA.112.191403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Barber M.N., Sampey D.B., Widdop R.E. AT(2) receptor stimulation enhances antihypertensive effect of AT(1) receptor antagonist in hypertensive rats. Hypertension. 1999;34(5):1112–1116. doi: 10.1161/01.HYP.34.5.1112. [DOI] [PubMed] [Google Scholar]
- 372.Buisson B., Laflamme L., Bottari S.P., de Gasparo M., Gallo-Payet N., Payet M.D.A.A. G protein is involved in the angiotensin AT2 receptor inhibition of the T-type calcium current in non-differentiated NG108-15 cells. J. Biol. Chem. 1995;270(4):1670–1674. doi: 10.1074/jbc.270.4.1670. [DOI] [PubMed] [Google Scholar]
- 373.Stroth U., Blume A., Mielke K., Unger T. Angiotensin AT(2) receptor stimulates ERK1 and ERK2 in quiescent but inhibits ERK in NGF-stimulated PC12W cells. Brain Res. Mol. Brain Res. 2000;78(1-2):175–180. doi: 10.1016/S0169-328X(00)00093-0. [DOI] [PubMed] [Google Scholar]
- 374.Tsutsumi Y., Matsubara H., Masaki H., Kurihara H., Murasawa S., Takai S., Miyazaki M., Nozawa Y., Ozono R., Nakagawa K., Miwa T., Kawada N., Mori Y., Shibasaki Y., Tanaka Y., Fujiyama S., Koyama Y., Fujiyama A., Takahashi H., Iwasaka T. Angiotensin II type 2 receptor overexpression activates the vascular kinin system and causes vasodilation. J. Clin. Invest. 1999;104(7):925–935. doi: 10.1172/JCI7886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Wu L., Iwai M., Nakagami H., Chen R., Suzuki J., Akishita M., de Gasparo M., Horiuchi M. Effect of angiotensin II type 1 receptor blockade on cardiac remodeling in angiotensin II type 2 receptor null mice. Arterioscler. Thromb. Vasc. Biol. 2002;22(1):49–54. doi: 10.1161/hq0102.102277. [DOI] [PubMed] [Google Scholar]
- 376.Wu L., Iwai M., Nakagami H., Li Z., Chen R., Suzuki J., Akishita M., de Gasparo M., Horiuchi M. Roles of angiotensin II type 2 receptor stimulation associated with selective angiotensin II type 1 receptor blockade with valsartan in the improvement of inflammation-induced vascular injury. Circulation. 2001;104(22):2716–2721. doi: 10.1161/hc4601.099404. [DOI] [PubMed] [Google Scholar]
- 377.Yamada T., Horiuchi M., Dzau V.J. Angiotensin II type 2 receptor mediates programmed cell death. Proc. Natl. Acad. Sci. USA. 1996;93(1):156–160. doi: 10.1073/pnas.93.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Zimpelmann J., Burns K.D. Angiotensin II AT2 receptors inhibit growth responses in proximal tubule cells. Am. J. Physiol. Renal Physiol. 2001;281(2):50–52. doi: 10.1152/ajprenal.2001.281.2.F300. [DOI] [PubMed] [Google Scholar]
- 379.Hansen J.L., Servant G., Baranski T.J., Fujita T., Iiri T., Sheikh S.P. Functional reconstitution of the angiotensin II type 2 receptor and G(i) activation. Circ. Res. 2000;87(9):753–759. doi: 10.1161/01.RES.87.9.753. [DOI] [PubMed] [Google Scholar]
- 380.Zhang J., Pratt R.E. The AT2 receptor selectively associates with Gialpha2 and Gialpha3 in the rat fetus. J. Biol. Chem. 1996;271(25):15026–15033. doi: 10.1074/jbc.271.25.15026. [DOI] [PubMed] [Google Scholar]
- 381.Cui T., Nakagami H., Iwai M., Takeda Y., Shiuchi T., Daviet L., Nahmias C., Horiuchi M. Pivotal role of tyrosine phosphatase SHP-1 in AT2 receptor-mediated apoptosis in rat fetal vascular smooth muscle cell. Cardiovasc. Res. 2001;49(4):863–871. doi: 10.1016/S0008-6363(00)00299-6. [DOI] [PubMed] [Google Scholar]
- 382.Dimitropoulou C., White R.E., Fuchs L., Zhang H., Catravas J.D., Carrier G.O. Angiotensin II relaxes microvessels via the AT2 receptor and Ca2+-activated K+ (BKCa) channels. Hypertension. 2001;37(21):301-307. doi: 10.1161/01.hyp.37.2.301. [DOI] [PubMed] [Google Scholar]
- 383.Fischer T.A., Singh K., O’Hara D.S., Kaye D.M., Kelly R.A. Role of AT1 and AT2 receptors in regulation of MAPKS and MKP-1 by ANG II in adult cardiac myocytes. Am. J. Physiol. Heart Circ. Physiol. 1998;275(3):44–53. doi: 10.1152/ajpheart.1998.275.3.H906. [DOI] [PubMed] [Google Scholar]
- 384.Gohlke P., Pees C., Unger T. AT2 receptor stimulation increases aortic cyclic GMP in SHRSP by a kinin-dependent mechanism. Hypertension. 1998;31(1 Pt 2):349–355. doi: 10.1161/01.HYP.31.1.349. [DOI] [PubMed] [Google Scholar]
- 385.Kang J., Richards E.M., Posner P., Sumners C. Modulation of the delayed rectifier K+ current in neurons by an angiotensin II type 2 receptor fragment. Am. J. Physiol. Cell Physiol. 1995;268(1):37. doi: 10.1152/ajpcell.1995.268.1.C278. [DOI] [PubMed] [Google Scholar]
- 386.Rueckschloss U., Quinn M.T., Holtz J., Morawietz H. Dose-dependent regulation of NAD(P)H oxidase expression by angiotensin II in human endothelial cells: protective effect of angiotensin II type 1 receptor blockade in patients with coronary artery disease. Arterioscler. Thromb. Vasc. Biol. 2002;22(11):1845–1851. doi: 10.1161/01.ATV.0000035392.38687.65. [DOI] [PubMed] [Google Scholar]
- 387.Silvestre J.S., Tamarat R., Senbonmatsu T., Icchiki T., Ebrahimian T., Iglarz M., Besnard S., Duriez M., Inagami T., Lévy B.I. Antiangiogenic effect of angiotensin II type 2 receptor in ischemia-induced angiogenesis in mice hindlimb. Circ. Res. 2002;90(10):1072–1079. doi: 10.1161/01.RES.0000019892.41157.24. [DOI] [PubMed] [Google Scholar]
- 388.Sohn H.Y., Raff U., Hoffmann A., Gloe T., Heermeier K., Galle J., Pohl U. Differential role of angiotensin II receptor subtypes on endothelial superoxide formation. Br. J. Pharmacol. 2000;131(4):667–672. doi: 10.1038/sj.bjp.0703566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Touyz R.M., Berry C. Recent advances in angiotensin II signaling. Braz. J. Med. Biol. Res. 2002;35(9):1001–1015. doi: 10.1590/S0100-879X2002000900001. [DOI] [PubMed] [Google Scholar]
- 390.Inagami T., Iwai N., Sasaki K., Guo D.F., Furuta H., Yamano Y., Bardhan S., Chaki S., Makito N., Badr K. Angiotensin II receptors: cloning and regulation. Arzneimittelforschung. 1993;43(2A):226–228. [PubMed] [Google Scholar]
- 391.Albiston A.L., Mustafa T., McDowall S.G., Mendelsohn F.A., Lee J., Chai S.Y. AT4 receptor is insulin-regulated membrane aminopeptidase: potential mechanisms of memory enhancement. Trends Endocrinol. Metab. 2003;14(2):72–77. doi: 10.1016/S1043-2760(02)00037-1. [DOI] [PubMed] [Google Scholar]
- 392.Chaki S., Inagami T. A newly found angiotensin II receptor subtype mediates cyclic GMP formation in differentiated Neuro-2A cells. Eur. J. Pharmacol. 1992;225(4):355–356. doi: 10.1016/0922-4106(92)90111-8. [DOI] [PubMed] [Google Scholar]
- 393.Hallberg M. Targeting the insulin-regulated aminopeptidase/AT4 receptor for cognitive disorders. Drug News Perspect. 2009;22(3):133–139. doi: 10.1358/dnp.2009.22.3.1325032. [DOI] [PubMed] [Google Scholar]
- 394.Harding J.W., Cook V.I., Miller-Wing A.V., Hanesworth J.M., Sardinia M.F., Hall K.L., Stobb J.W., Swanson G.N., Coleman J.K., Wright J.W. Identification of an AII(3-8) [AIV] binding site in guinea pig hippocampus. Brain Res. 1992;583(1-2):340–343. doi: 10.1016/S0006-8993(10)80047-2. [DOI] [PubMed] [Google Scholar]
- 395.Benoist C.C., Wright J.W., Zhu M., Appleyard S.M., Wayman G.A., Harding J.W. Facilitation of hippocampal synaptogenesis and spatial memory by C-terminal truncated Nle1-angiotensin IV analogs. J. Pharmacol. Exp. Ther. 2011;339(1):35–44. doi: 10.1124/jpet.111.182220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Wright J.W., Harding J.W. Brain renin-angiotensin--a new look at an old system. Prog. Neurobiol. 2011;95(1):49–67. doi: 10.1016/j.pneurobio.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 397.Beyer C.E., Dwyer J.M., Platt B.J., Neal S., Luo B., Ling H.P., Lin Q., Mark R.J., Rosenzweig-Lipson S., Schechter L.E. Angiotensin IV elevates oxytocin levels in the rat amygdala and produces anxiolytic-like activity through subsequent oxytocin receptor activation. Psychopharmacology (Berl.) 2010;209(4):303–311. doi: 10.1007/s00213-010-1791-1. [DOI] [PubMed] [Google Scholar]
- 398.Davis C.J., Kramár E.A., De A., Meighan P.C., Simasko S.M., Wright J.W., Harding J.W. AT4 receptor activation increases intracellular calcium influx and induces a non-N-methyl-D-aspartate dependent form of long-term potentiation. Neuroscience. 2006;137(4):1369–1379. doi: 10.1016/j.neuroscience.2005.10.051. [DOI] [PubMed] [Google Scholar]
- 399.Chai S.Y., Fernando R., Peck G., Ye S-Y., Mendelsohn F.A.O., Jenkins T.A., Albiston A.L. The angiotensin IV/AT4 receptor. Cell. Mol. Life Sci. 2004;61(21):2728–2737. doi: 10.1007/s00018-004-4246-1. [DOI] [PubMed] [Google Scholar]
- 400.Laviano A., Molfino A., Rianda S., Rossi Fanelli F. The growth hormone secretagogue receptor (Ghs-R). Curr. Pharm. Des. 2012;18(31):4749–4754. doi: 10.2174/138161212803216906. [DOI] [PubMed] [Google Scholar]
- 401.Soares J-B., Roncon-Albuquerque R., Jr, Leite-Moreira A. Ghrelin and ghrelin receptor inhibitors: Agents in the treatment of obesity. Expert Opin. Ther. Targets. 2008;12(9):1177–1189. doi: 10.1517/14728222.12.9.1177. [DOI] [PubMed] [Google Scholar]
- 402.Pusztai P., Sarman B., Ruzicska E., Toke J., Racz K., Somogyi A., Tulassay Z. Ghrelin: A new peptide regulating the neurohormonal system, energy homeostasis and glucose metabolism. Diabetes Metab. Res. Rev. 2008;24(5):343–352. doi: 10.1002/dmrr.830. [DOI] [PubMed] [Google Scholar]
- 403.Guan X.M., Yu H., Palyha O.C., McKee K.K., Feighner S.D., Sirinathsinghji D.J.S., Smith R.G., Van der Ploeg L.H., Howard A.D. Distribution of mRNA encoding the growth hormone secretagogue receptor in brain and peripheral tissues. Brain Res. Mol. Brain Res. 1997;48(1):23–29. doi: 10.1016/S0169-328X(97)00071-5. [DOI] [PubMed] [Google Scholar]
- 404.Schellekens H., Dinan T.G., Cryan J.F. Lean mean fat reducing “ghrelin” machine: hypothalamic ghrelin and ghrelin receptors as therapeutic targets in obesity. Neuropharmacology. 2010;58(1):2–16. doi: 10.1016/j.neuropharm.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 405.Gnanapavan S., Kola B., Bustin S.A., Morris D.G., McGee P., Fairclough P., Bhattacharya S., Carpenter R., Grossman A.B., Korbonits M. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002;87(6):2988–2991. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 406.Gómez R., Lago F., Gómez-Reino J.J., Gualillo O. Novel factors as therapeutic targets to treat diabetes. Focus on leptin and ghrelin. Expert Opin. Ther. Targets. 2009;13(5):583–591. doi: 10.1517/14728220902914834. [DOI] [PubMed] [Google Scholar]
- 407.Broglio F., Gianotti L., Destefanis S., Fassino S., Abbate D.G., Mondelli V., Lanfranco F., Gottero C., Gauna C., Hofland L., Van der Lely A.J., Ghigo E. The endocrine response to acute ghrelin administration is blunted in patients with anorexia nervosa, a ghrelin hypersecretory state. Clin. Endocrinol. (Oxf.) 2004;60(5):592–599. doi: 10.1111/j.1365-2265.2004.02011.x. [DOI] [PubMed] [Google Scholar]
- 408.Müller T.D., Tschöp M.H., Jarick I., Ehrlich S., Scherag S., Herpertz-Dahlmann B., Zipfel S., Herzog W., de Zwaan M., Burghardt R., Fleischhaker C., Klampfl K., Wewetzer C., Herpertz S., Zeeck A., Tagay S., Burgmer M., Pfluger P.T., Scherag A., Hebebrand J., Hinney A. Genetic variation of the ghrelin activator gene ghrelin O-acyltransferase (GOAT) is associated with anorexia nervosa. J. Psychiatr. Res. 2011;45(5):706–711. doi: 10.1016/j.jpsychires.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 409.Laviano A., Meguid M.M., Inui A., Muscaritoli M., Rossi-Fanelli F. Therapy insight: Cancer anorexia-cachexia syndrome--when all you can eat is yourself. Nat. Clin. Pract. Oncol. 2005;2(3):158–165. doi: 10.1038/ncponc0112. [DOI] [PubMed] [Google Scholar]
- 410.Ma X., Lin L., Qin G., Lu X., Fiorotto M., Dixit V.D., Sun Y. Ablations of ghrelin and ghrelin receptor exhibit differential metabolic phenotypes and thermogenic capacity during aging. PLoS One. 2011;6(1):e16391. doi: 10.1371/journal.pone.0016391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 411.Nagaya N., Kangawa K. Ghrelin, a novel growth hormone-releasing peptide, in the treatment of chronic heart failure. Regul. Pept. 2003;114(2-3):71–77. doi: 10.1016/S0167-0115(03)00117-4. [DOI] [PubMed] [Google Scholar]
- 412.Gjesing A.P., Larsen L.H., Torekov S.S., Hainerová I.A., Kapur R., Johansen A., Albrechtsen A., Boj S., Holst B., Harper A., Urhammer S.A., Borch-Johnsen K., Pisinger C., Echwald S.M., Eiberg H., Astrup A., Lebl J., Ferrer J., Schwartz T.W., Hansen T., Pedersen O. Family and population-based studies of variation within the ghrelin receptor locus in relation to measures of obesity. PLoS One. 2010;5(4):e10084. doi: 10.1371/journal.pone.0010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Kamegai J., Tamura H., Shimizu T., Ishii S., Sugihara H., Oikawa S. Insulin-like growth factor-I down-regulates ghrelin receptor (growth hormone secretagogue receptor) expression in the rat pituitary. Regul. Pept. 2005;127(1-3):203–206. doi: 10.1016/j.regpep.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 414.Yada T., Dezaki K., Sone H., Koizumi M., Damdindorj B., Nakata M., Kakei M. Ghrelin regulates insulin release and glycemia: physiological role and therapeutic potential. Curr. Diabetes Rev. 2008;4(1):18–23. doi: 10.2174/157339908783502352. [DOI] [PubMed] [Google Scholar]
- 415.Dezaki K., Sone H., Yada T. Ghrelin is a physiological regulator of insulin release in pancreatic islets and glucose homeostasis. Pharmacol. Ther. 2008;118(2):239–249. doi: 10.1016/j.pharmthera.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 416.Zlotos D.P., Jockers R., Cecon E., Rivara S., Witt-Enderby P.A. MT1 and MT2 melatonin receptors: ligands, models, oligomers, and therapeutic potential. J. Med. Chem. 2014;57(8):3161–3185. doi: 10.1021/jm401343c. [DOI] [PubMed] [Google Scholar]
- 417.Reppert S.M., Weaver D.R., Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13(5):1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 418.Dubocovich M.L., Delagrange P., Krause D.N., Sugden D., Cardinali D.P., Olcese J. International Union of Basic and Clinical Pharmacology. LXXV. Nomenclature, classification, and pharmacology of G protein-coupled melatonin receptors. Pharmacol. Rev. 2010;62(3):343–380. doi: 10.1124/pr.110.002832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 419.Cardinali D.P., Delagrange P., Dubocovich M.L., Jockers R., Krause D.N., Markus R.P. Melatonin receptors (version 2019.4) in the IUPHAR/BPS guide to pharmacology database. 4 IUPHAR/BPS Guid to Pharmacol CITE; 2019. [Google Scholar]
- 420.Emet M., Ozcan H., Ozel L., Yayla M., Halici Z., Hacimuftuoglu A. A Review of Melatonin, Its Receptors and Drugs. Eurasian J. Med. 2016;48(2):135–141. doi: 10.5152/eurasianjmed.2015.0267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Boutin J.A., Ferry G. Is There Sufficient Evidence that the Melatonin Binding Site MT3 Is Quinone Reductase 2? . J. Pharmacol. Exp. Ther. 2019;368(1):59–65. doi: 10.1124/jpet.118.253260. [DOI] [PubMed] [Google Scholar]
- 422.Nosjean O., Ferro M., Cogé F., Beauverger P., Henlin J.M., Lefoulon F., Fauchere J.L., Delagrange P., Canet E., Boutin J.A. Identification of the melatonin-binding site MT3 as the quinone reductase 2. J. Biol. Chem. 2000;275(40):31311–31317. doi: 10.1074/jbc.M005141200. [DOI] [PubMed] [Google Scholar]
- 423.Tosini G., Owino S., Guillaume J.L., Jockers R. Understanding melatonin receptor pharmacology: latest insights from mouse models, and their relevance to human disease. BioEssays. 2014;36(8):778–787. doi: 10.1002/bies.201400017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 424.Markus R.P., Cecon E., Pires-Lapa M.A. Immune-pineal axis: nuclear factor κB (NF-kB) mediates the shift in the melatonin source from pinealocytes to immune competent cells. Int. J. Mol. Sci. 2013;14(6):10979–10997. doi: 10.3390/ijms140610979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Jockers R., Maurice P., Boutin J.A., Delagrange P. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br. J. Pharmacol. 2008;154(6):1182–1195. doi: 10.1038/bjp.2008.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 426.Doghramji K. Melatonin and its receptors: A new class of sleep-promoting agents. J. Clin. Sleep Med. 2007;3(5) Suppl.:S17–S23. doi: 10.5664/jcsm.26932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 427.Wongprayoon P., Govitrapong P. Melatonin Receptor as a Drug Target for Neuroprotection. Curr. Mol. Pharmacol. 2021;14(2):150–164. doi: 10.2174/1874467213666200421160835. [DOI] [PubMed] [Google Scholar]
- 428.Dubocovich M.L. Melatonin receptors: role on sleep and circadian rhythm regulation. Sleep Med. 2007;8(Suppl. 3):34–42. doi: 10.1016/j.sleep.2007.10.007. [DOI] [PubMed] [Google Scholar]
- 429.Lépinay J., Taragnat C., Dubois J.P., Chesneau D., Jockers R. Delagrange, P Negative regulation of melatonin secretion by melatonin receptors in ovine pinealocytes. PLoS One. 2021;16:e0255249. doi: 10.1371/journal.pone.0255249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 430.Jockers R., Delagrange P., Dubocovich M.L., Markus R.P., Renault N., Tosini G., Cecon E., Zlotos D.P. Update on melatonin receptors: IUPHAR Review 20. Br. J. Pharmacol. 2016;173(18):2702–2725. doi: 10.1111/bph.13536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Reppert S.M., Godson C., Mahle C.D., Weaver D.R., Slaugenhaupt S.A., Gusella J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA. 1995;92(19):8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Besharse JC, Dunis DA. Methoxyindoles and photoreceptor metabolism: Activation of rod shedding. Science (80) 1983;219((4590)):1341-1343. doi: 10.1126/science.6828862. [DOI] [PubMed] [Google Scholar]
- 433.Sharan K., Lewis K., Furukawa T., Yadav V.K. Regulation of bone mass through pineal-derived melatonin-MT2 receptor pathway. J. Pineal Res. 2017;63(2):e12423. doi: 10.1111/jpi.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Rui T., Wang H., Li Q., Cheng Y., Gao Y., Fang X., Ma X., Chen G., Gao C., Gu Z., Song S., Zhang J., Wang C., Wang Z., Wang T., Zhang M., Min J., Chen X., Tao L., Wang F., Luo C. Deletion of ferritin H in neurons counteracts the protective effect of melatonin against traumatic brain injury-induced ferroptosis. J. Pineal Res. 2021;70(2):e12704. doi: 10.1111/jpi.12704. [DOI] [PubMed] [Google Scholar]
- 435.Noseda A.C.D., Rodrigues L.S., Targa A.D.S., Ilkiw J.L., Fagotti J., Dos Santos P.D., Cecon E., Markus R.P., Solimena M., Jockers R., Lima M.M.S. MT2 melatonin receptors expressed in the olfactory bulb modulate depressive-like behavior and olfaction in the 6-OHDA model of Parkinson’s disease. Eur. J. Pharmacol. 2021;891:173722. doi: 10.1016/j.ejphar.2020.173722. [DOI] [PubMed] [Google Scholar]
- 436.Ayoub M.A., Couturier C., Lucas-Meunier E., Angers S., Fossier P., Bouvier M., Jockers R. Monitoring of ligand-independent dimerization and ligand-induced conformational changes of melatonin receptors in living cells by bioluminescence resonance energy transfer. J. Biol. Chem. 2002;277(24):21522–21528. doi: 10.1074/jbc.M200729200. [DOI] [PubMed] [Google Scholar]
- 437.Ayoub M.A., Levoye A., Delagrange P., Jockers R. Preferential formation of MT1/MT2 melatonin receptor heterodimers with distinct ligand interaction properties compared with MT2 homodimers. Mol. Pharmacol. 2004;66(2):312–321. doi: 10.1124/mol.104.000398. [DOI] [PubMed] [Google Scholar]
- 438.Baba K., Benleulmi-Chaachoua A., Journé A.S., Kamal M., Guillaume J.L., Dussaud S., Gbahou F., Yettou K., Liu C., Contreras-Alcantara S., Jockers R., Tosini G. Heteromeric MT1/MT2 melatonin receptors modulate photoreceptor function. Sci. Signal. 2013;6(296):ra89. doi: 10.1126/scisignal.2004302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Takeda S., Kadowaki S., Haga T., Takaesu H., Mitaku S. Identification of G protein-coupled receptor genes from the human genome sequence. FEBS Lett. 2002;520(1-3):97–101. doi: 10.1016/S0014-5793(02)02775-8. [DOI] [PubMed] [Google Scholar]
- 440.Alexander S.P., Christopoulos A., Davenport A.P., Kelly E., Marrion N.V., Peters J.A., Faccenda E., Harding S.D., Pawson A.J., Sharman J.L., Southan C., Davies J.A. The concise guide to pharmacology 2017/18: G protein-coupled receptors. Br. J. Pharmacol. 2017;174(Suppl. 1):S17–S129. doi: 10.1111/bph.13878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 441.Davenport A.P., Alexander S.P.H., Sharman J.L., Pawson A.J., Benson H.E., Monaghan A.E., Liew W.C., Mpamhanga C.P., Bonner T.I., Neubig R.R., Pin J.P., Spedding M., Harmar A.J. International Union of Basic and Clinical Pharmacology. LXXXVIII. G protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol. Rev. 2013;65(3):967–986. doi: 10.1124/pr.112.007179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 442.Gloriam D.E.I., Schiöth H.B., Fredriksson R. Nine new human Rhodopsin family G-protein coupled receptors: identification, sequence characterisation and evolutionary relationship. Biochim. Biophys. Acta. 2005;1722(3):235–246. doi: 10.1016/j.bbagen.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 443.Vedel L., Nøhr A.C., Gloriam D.E., Bräuner-Osborne H. Pharmacology and function of the orphan GPR139 G protein-coupled receptor. Basic Clin. Pharmacol. Toxicol. 2020;126(S6) Suppl. 6:35–46. doi: 10.1111/bcpt.13263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Kononoff J., Kallupi M., Kimbrough A., Conlisk D., de Guglielmo G., George O. Systemic and intra-habenular activation of the orphan G protein-coupled receptor GPR139 decreases compulsive-like alcohol drinking and hyperalgesia in alcohol-dependent rats. eNeuro. 2018;5(3):153–171. doi: 10.1523/ENEURO.0153-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 445.Hitchcock S, Lam B, Monenschein H, Reichard H. 4-oxo-3,4- dihydro-1,2,3-benzotriazine modulators of GPR139. 2015.
- 446.Ebejer J.L., Duffy D.L., van der Werf J., Wright M.J., Montgomery G., Gillespie N.A., Hickie I.B., Martin N.G., Medland S.E. Genome-wide association study of inattention and hyperactivity-impulsivity measured as quantitative traits. Twin Res. Hum. Genet. 2013;16(2):560–574. doi: 10.1017/thg.2013.12. [DOI] [PubMed] [Google Scholar]
- 447.Castellani C.A., Awamleh Z., Melka M.G., O’Reilly R.L., Singh S.M. Copy number variation distribution in six monozygotic twin pairs discordant for schizophrenia. Twin Res. Hum. Genet. 2014;17(2):108–120. doi: 10.1017/thg.2014.6. [DOI] [PubMed] [Google Scholar]
- 448.Al Hafid N., Christodoulou J. Phenylketonuria: A review of current and future treatments. Transl. Pediatr. 2015;4(4):304–317. doi: 10.3978/j.issn.2224-4336.2015.10.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 449.Salim K., Fenton T., Bacha J., Urien-Rodriguez H., Bonnert T., Skynner H.A., Watts E., Kerby J., Heald A., Beer M., McAllister G., Guest P.C. Oligomerization of G-protein-coupled receptors shown by selective co-immunoprecipitation. J. Biol. Chem. 2002;277(18):15482–15485. doi: 10.1074/jbc.M201539200. [DOI] [PubMed] [Google Scholar]
- 450.Derouiche L., Massotte D. G protein-coupled receptor heteromers are key players in substance use disorder. Neurosci. Biobehav. Rev. 2019;106:73–90. doi: 10.1016/j.neubiorev.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 451.Pellissier L.P., Barthet G., Gaven F., Cassier E., Trinquet E., Pin J.P., Marin P., Dumuis A., Bockaert J., Banères J.L., Claeysen S. G protein activation by serotonin type 4 receptor dimers: evidence that turning on two protomers is more efficient. J. Biol. Chem. 2011;286(12):9985–9997. doi: 10.1074/jbc.M110.201939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 452.Han Y., Moreira I.S., Urizar E., Weinstein H., Javitch J.A. Allosteric communication between protomers of dopamine class A GPCR dimers modulates activation. Nat. Chem. Biol. 2009;5(9):688–695. doi: 10.1038/nchembio.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Maggio R., Rocchi C., Scarselli M. Experimental strategies for studying G protein-coupled receptor homo- and heteromerization with radioligand binding and signal transduction methods. Methods Enzymol. 2013;521:295–310. doi: 10.1016/B978-0-12-391862-8.00016-8. [DOI] [PubMed] [Google Scholar]
- 454.Borroto-Escuela D.O., Hagman B., Woolfenden M., Pinton L., Jiménez-Beristain A., Oflijan J. In situ proximity ligation assay to study and understand the distribution and balance of GPCR homo- and heteroreceptor complexes in the brain. . Neuromethods. 2016;110:109–124. doi: 10.1007/978-1-4939-3064-7_9. [DOI] [Google Scholar]
- 455.Vischer H.F., Castro M., Pin J.P. G protein-coupled receptor multimers: A question still open despite the use of novel approaches. Mol. Pharmacol. 2015;88(3):561–571. doi: 10.1124/mol.115.099440. [DOI] [PubMed] [Google Scholar]
- 456.Franco R. G-protein-coupled receptor heteromers or how neurons can display differently flavoured patterns in response to the same neurotransmitter. Br. J. Pharmacol. 2009;158(1):23–31. doi: 10.1111/j.1476-5381.2009.00181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Franco R., Martínez-Pinilla E., Lanciego J.L., Navarro G. Basic pharmacological and structural evidence for class A G-protein-coupled receptor heteromerization. Front. Pharmacol. 2016;7(MAR):76. doi: 10.3389/fphar.2016.00076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 458.Ciruela F., Casadó V., Rodrigues R.J., Luján R., Burgueño J., Canals M., Borycz J., Rebola N., Goldberg S.R., Mallol J., Cortés A., Canela E.I., López-Giménez J.F., Milligan G., Lluis C., Cunha R.A., Ferré S., Franco R. Presynaptic control of striatal glutamatergic neurotransmission by adenosine A1-A2A receptor heteromers. J. Neurosci. 2006;26(7):2080–2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Ferré S., Ciruela F., Quiroz C., Luján R., Popoli P., Cunha R.A., Agnati L.F., Fuxe K., Woods A.S., Lluis C., Franco R. Adenosine receptor heteromers and their integrative role in striatal function. Sci. World J. 2007;7(Suppl. 2):74–85. doi: 10.1100/tsw.2007.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 460.Cristóvão-Ferreira S., Navarro G., Brugarolas M., Pérez-Capote K., Vaz S.H., Fattorini G., Conti F., Lluis C., Ribeiro J.A., McCormick P.J., Casadó V., Franco R., Sebastião A.M. A1R-A2AR heteromers coupled to Gs and G i/0 proteins modulate GABA transport into astrocytes. Purinergic Signal. 2013;9(3):433–449. doi: 10.1007/s11302-013-9364-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.George S.R., Kern A., Smith R.G., Franco R. Dopamine receptor heteromeric complexes and their emerging functions. Prog. Brain Res. 2014:211, 183-200. doi: 10.1016/B978-0-444-63425-2.00008-8. [DOI] [PubMed]
- 462.Hasbi A., Fan T., Alijaniaram M., Nguyen T., Perreault M.L., O’Dowd B.F., George S.R. Calcium signaling cascade links dopamine D1-D2 receptor heteromer to striatal BDNF production and neuronal growth. Proc. Natl. Acad. Sci. USA. 2009;106(50):21377–21382. doi: 10.1073/pnas.0903676106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.So C.H., Verma V., O’Dowd B.F., George S.R. Desensitization of the dopamine D1 and D2 receptor hetero-oligomer mediated calcium signal by agonist occupancy of either receptor. Mol. Pharmacol. 2007;72(2):450–462. doi: 10.1124/mol.107.034884. [DOI] [PubMed] [Google Scholar]
- 464.Rashid A.J., So C.H., Kong M.M.C., Furtak T., El-Ghundi M., Cheng R., O’Dowd B.F., George S.R. D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum. Proc. Natl. Acad. Sci. USA. 2007;104(2):654–659. doi: 10.1073/pnas.0604049104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 465.Portoghese P.S., Lunzer M.M. Identity of the putative δ1-opioid receptor as a δ-κ heteromer in the mouse spinal cord. Eur. J. Pharmacol. 2003;467(1-3):233–234. doi: 10.1016/S0014-2999(03)01599-1. [DOI] [PubMed] [Google Scholar]
- 466.Gomes I., Jordan B.A., Gupta A., Trapaidze N., Nagy V., Devi L.A. Heterodimerization of μ and δ opioid receptors: A role in opiate synergy. J. Neurosci. 2000;20(22):RC110–RC110. doi: 10.1523/JNEUROSCI.20-22-j0007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 467.Gupta A., Mulder J., Gomes I., Rozenfeld R., Bushlin I., Ong E., Lim M., Maillet E., Junek M., Cahill C.M., Harkany T., Devi L.A. Increased abundance of opioid receptor heteromers after chronic morphine administration. Sci. Signal. 2010;3(131):ra54. doi: 10.1126/scisignal.2000807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 468.Yekkirala A.S., Kalyuzhny A.E., Portoghese P.S. Standard opioid agonists activate heteromeric opioid receptors: evidence for morphine and [d-Ala(2)-MePhe(4)-Glyol(5)]enkephalin as selective μ-δ agonists. ACS Chem. Neurosci. 2010;1(2):146–154. doi: 10.1021/cn9000236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 469.van Rijn R.M., Whistler J.L., Waldhoer M. Opioid-receptor-heteromer-specific trafficking and pharmacology. Curr. Opin. Pharmacol. 2010;10(1):73–79. doi: 10.1016/j.coph.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 470.Waldhoer M., Fong J., Jones R.M., Lunzer M.M., Sharma S.K., Kostenis E., Portoghese P.S., Whistler J.L. A heterodimer-selective agonist shows in vivo relevance of G protein-coupled receptor dimers. . Proc. Natl. Acad. Sci. USA. 2005;102(25):9050–9055. doi: 10.1073/pnas.0501112102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 471.Daniels D.J., Kulkarni A., Xie Z., Bhushan R.G., Portoghese P.S. A bivalent ligand (KDAN-18) containing δ-antagonist and κ-agonist pharmacophores bridges δ2 and κ1 opioid receptor phenotypes. J. Med. Chem. 2005;48(6):1713–1716. doi: 10.1021/jm034234f. [DOI] [PubMed] [Google Scholar]
- 472.Bhushan R.G., Sharma S.K., Xie Z., Daniels D.J., Portoghese P.S. A bivalent ligand (KDN-21) reveals spinal δ and κ opioid receptors are organized as heterodimers that give rise to δ(1) and κ(2) phenotypes. Selective targeting of δ-κ heterodimers. J. Med. Chem. 2004;47(12):2969–2972. doi: 10.1021/jm0342358. [DOI] [PubMed] [Google Scholar]
- 473.Fuxe K., Harfstrand A., Agnati L.F., Kalia M., Fredholm B., Svensson T. Central catecholamine-neuropeptide Y interactions at the pre- and postsynaptic level in cardiovascular centers. J Cardiovasc Pharmacol. 1987. p. 10(Suppl. 12). [PubMed]
- 474.Fuxe K., Agnati L.F. Receptor-receptor interactions in the central nervous system. A new integrative mechanism in synapses. Med. Res. Rev. 1985;5(4):441–482. doi: 10.1002/med.2610050404. [DOI] [PubMed] [Google Scholar]
- 475.Fuxe K., Agnati L.F., Benfenati F., Celani M., Zini I., Zoli M., Mutt V. Evidence for the existence of receptor--receptor interactions in the central nervous system. Studies on the regulation of monoamine receptors by neuropeptides. J. Neural Transm. Suppl. 1983;18:165–179. [PubMed] [Google Scholar]
- 476.Borroto-Escuela D.O., Van Craenenbroeck K., Romero-Fernandez W., Guidolin D., Woods A.S., Rivera A., Haegeman G., Agnati L.F., Tarakanov A.O., Fuxe K. Dopamine D2 and D4 receptor heteromerization and its allosteric receptor-receptor interactions. Biochem. Biophys. Res. Commun. 2011;404(4):928–934. doi: 10.1016/j.bbrc.2010.12.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 477.Fiorentini C., Busi C., Spano P., Missale C. Dimerization of dopamine D1 and D3 receptors in the regulation of striatal function. Curr. Opin. Pharmacol. 2010;10(1):87–92. doi: 10.1016/j.coph.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 478.Błasiak E., Łukasiewicz S., Szafran-Pilch K., Dziedzicka-Wasylewska M. Genetic variants of dopamine D2 receptor impact heterodimerization with dopamine D1 receptor. Pharmacol. Rep. 2017;69(2):235–241. doi: 10.1016/j.pharep.2016.10.016. [DOI] [PubMed] [Google Scholar]
- 479.Martel J.C. Gatti, McArthur, S. Dopamine receptor subtypes, physiology and pharmacology: New ligands and concepts in schizophrenia. Front. Pharmacol. 2020;11:1003. doi: 10.3389/fphar.2020.01003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 480.Van Craenenbroeck K., Borroto-Escuela D.O., Skieterska K., Duchou J., Romero-Fernandez W., Fuxe K. Role of dimerization in dopamine D(4) receptor biogenesis. Curr. Protein Pept. Sci. 2014;15(7):659–665. doi: 10.2174/1389203715666140901110256. [DOI] [PubMed] [Google Scholar]
- 481.Ng G.Y.K., O’Dowd B.F., Lee S.P., Chung H.T., Brann M.R., Seeman P., George S.R. Dopamine D2 receptor dimers and receptor-blocking peptides. Biochem. Biophys. Res. Commun. 1996;227(1):200–204. doi: 10.1006/bbrc.1996.1489. [DOI] [PubMed] [Google Scholar]
- 482.Karpa K.D., Lin R., Kabbani N., Levenson R. The dopamine D3 receptor interacts with itself and the truncated D3 splice variant d3nf: D3-D3nf interaction causes mislocalization of D3 receptors. Mol. Pharmacol. 2000;58(4):677–683. doi: 10.1124/mol.58.4.677. [DOI] [PubMed] [Google Scholar]
- 483.O’Dowd B.F., Nguyen T., Ji X., George S.R. D5 dopamine receptor carboxyl tail involved in D5-D2 heteromer formation. Biochem. Biophys. Res. Commun. 2013;431(3):586–589. doi: 10.1016/j.bbrc.2012.12.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 484.Scarselli M., Novi F., Schallmach E., Lin R., Baragli A., Colzi A., Griffon N., Corsini G.U., Sokoloff P., Levenson R., Vogel Z., Maggio R. D2/D3 dopamine receptor heterodimers exhibit unique functional properties. J. Biol. Chem. 2001;276(32):30308–30314. doi: 10.1074/jbc.M102297200. [DOI] [PubMed] [Google Scholar]
- 485.Guo W., Shi L., Filizola M., Weinstein H., Javitch J.A. Crosstalk in G protein-coupled receptors: changes at the transmembrane homodimer interface determine activation. Proc. Natl. Acad. Sci. USA. 2005;102(48):17495–17500. doi: 10.1073/pnas.0508950102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 486.Perreault M.L., Hasbi A., Alijaniaram M., Fan T., Varghese G., Fletcher P.J., Seeman P., O’Dowd B.F., George S.R. The dopamine D1-D2 receptor heteromer localizes in dynorphin/enkephalin neurons: increased high affinity state following amphetamine and in schizophrenia. J. Biol. Chem. 2010;285(47):36625–36634. doi: 10.1074/jbc.M110.159954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 487.Lee S.P., So C.H., Rashid A.J., Varghese G., Cheng R., Lança A.J., O’Dowd B.F., George S.R. Dopamine D1 and D2 receptor Co-activation generates a novel phospholipase C-mediated calcium signal. J. Biol. Chem. 2004;279(34):35671–35678. doi: 10.1074/jbc.M401923200. [DOI] [PubMed] [Google Scholar]
- 488.Urizar E., Yano H., Kolster R., Galés C., Lambert N., Javitch J.A. CODA-RET reveals functional selectivity as a result of GPCR heteromerization. Nat. Chem. Biol. 2011;7(9):624–630. doi: 10.1038/nchembio.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 489.Perreault M.L., Hasbi A., Shen M.Y.F., Fan T., Navarro G., Fletcher P.J., Franco R., Lanciego J.L., George S.R. Disruption of a dopamine receptor complex amplifies the actions of cocaine. Eur. Neuropsychopharmacol. 2016;26(9):1366–1377. doi: 10.1016/j.euroneuro.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 490.Rico A.J., Dopeso-Reyes I.G., Martínez-Pinilla E., Sucunza D., Pignataro D., Roda E., Marín-Ramos D., Labandeira-García J.L., George S.R., Franco R., Lanciego J.L. Neurochemical evidence supporting dopamine D1-D2 receptor heteromers in the striatum of the long-tailed macaque: changes following dopaminergic manipulation. Brain Struct. Funct. 2017;222(4):1767–1784. doi: 10.1007/s00429-016-1306-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 491.Hasbi A., Madras B.K., Bergman J., Kohut S., Lin Z., Withey S.L., George S.R. Δ-tetrahydrocannabinol increases dopamine D1-D2 receptor heteromer and elicits phenotypic reprogramming in adult primate striatal neurons. iScience. 2020;23(1):100794. doi: 10.1016/j.isci.2019.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 492.Hasbi A., Sivasubramanian M., Milenkovic M., Komarek K., Madras B.K., George S.R. Dopamine D1-D2 receptor heteromer expression in key brain regions of rat and higher species: Upregulation in rat striatum after cocaine administration. Neurobiol. Dis. 2020;143:105017. doi: 10.1016/j.nbd.2020.105017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 493.So C.H., Verma V., Alijaniaram M., Cheng R., Rashid A.J., O’Dowd B.F., George S.R. Calcium signaling by dopamine D5 receptor and D5-D2 receptor hetero-oligomers occurs by a mechanism distinct from that for dopamine D1-D2 receptor hetero-oligomers. Mol. Pharmacol. 2009;75(4):843–854. doi: 10.1124/mol.108.051805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 494.Ng J., Rashid A.J., So C.H., O’Dowd B.F., George S.R. Activation of calcium/calmodulin-dependent protein kinase IIalpha in the striatum by the heteromeric D1-D2 dopamine receptor complex. Neuroscience. 2010;165(2):535–541. doi: 10.1016/j.neuroscience.2009.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 495.Nestler E.J., Carlezon W.A., Jr The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 496.Tye K.M., Mirzabekov J.J., Warden M.R., Ferenczi E.A., Tsai H.C., Finkelstein J., Kim S.Y., Adhikari A., Thompson K.R., Andalman A.S., Gunaydin L.A., Witten I.B., Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493(7433):537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 497.Seeman P., Van Tol H.H.M. Dopamine receptor pharmacology. Trends Pharmacol. Sci. 1994;15(7):264–270. doi: 10.1016/0165-6147(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 498.O’Dowd B.F., Ji X., Nguyen T., George S.R. Two amino acids in each of D1 and D2 dopamine receptor cytoplasmic regions are involved in D1-D2 heteromer formation. Biochem. Biophys. Res. Commun. 2012;417(1):23–28. doi: 10.1016/j.bbrc.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 499.Glatt S.J., Faraone S.V., Lasky-Su J.A., Kanazawa T., Hwu H.G., Tsuang M.T. Family-based association testing strongly implicates DRD2 as a risk gene for schizophrenia in Han Chinese from Taiwan. Mol. Psychiatry. 2009;14(9):885–893. doi: 10.1038/mp.2008.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 500.Lane H.Y., Lee C.C., Chang Y.C., Lu C.T., Huang C.H., Chang W.H. Effects of dopamine D2 receptor Ser311Cys polymorphism and clinical factors on risperidone efficacy for positive and negative symptoms and social function. Int. J. Neuropsychopharmacol. 2004;7(4):461–470. doi: 10.1017/S1461145704004389. [DOI] [PubMed] [Google Scholar]
- 501.Hasbi A., Perreault M.L., Shen M.Y.F., Fan T., Nguyen T., Alijaniaram M., Banasikowski T.J., Grace A.A., O’Dowd B.F., Fletcher P.J., George S.R. Activation of dopamine D1-D2 receptor complex attenuates cocaine reward and reinstatement of cocaine-seeking through inhibition of DARPP-32, ERK, and ΔFosB. Front. Pharmacol. 2018;8:924. doi: 10.3389/fphar.2017.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 502.Pei L., Li S., Wang M., Diwan M., Anisman H., Fletcher P.J., Nobrega J.N., Liu F. Uncoupling the dopamine D1-D2 receptor complex exerts antidepressant-like effects. Nat. Med. 2010;16(12):1393–1395. doi: 10.1038/nm.2263. [DOI] [PubMed] [Google Scholar]
- 503.Hasbi A., Perreault M.L., Shen M.Y.F., Zhang L., To R., Fan T., Nguyen T., Ji X., O’Dowd B.F., George S.R. A peptide targeting an interaction interface disrupts the dopamine D1-D2 receptor heteromer to block signaling and function in vitro and in vivo : effective selective antagonism. . FASEB J. 2014;28(11):4806–4820. doi: 10.1096/fj.14-254037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 504.Guitart X., Navarro G., Moreno E., Yano H., Cai N.S., Sánchez-Soto M., Kumar-Barodia S., Naidu Y.T., Mallol J., Cortés A., Lluís C., Canela E.I., Casadó V., McCormick P.J., Ferré S. Functional selectivity of allosteric interactions within G protein-coupled receptor oligomers: the dopamine D1-D3 receptor heterotetramer. Mol. Pharmacol. 2014;86(4):417–429. doi: 10.1124/mol.114.093096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 505.Guitart X., Moreno E., Rea W., Sánchez-Soto M., Cai N.S., Quiroz C., Kumar V., Bourque L., Cortés A., Canela E.I., Bishop C., Newman A.H., Casadó V., Ferré S., Biased G. Protein-independent signaling of dopamine D1-D3 receptor heteromers in the nucleus accumbens. Mol. Neurobiol. 2019;56(10):6756–6769. doi: 10.1007/s12035-019-1564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 506.Fiorentini C., Busi C., Gorruso E., Gotti C., Spano P., Missale C. Reciprocal regulation of dopamine D1 and D3 receptor function and trafficking by heterodimerization. Mol. Pharmacol. 2008;74(1):59–69. doi: 10.1124/mol.107.043885. [DOI] [PubMed] [Google Scholar]
- 507.Marcellino D., Ferré S., Casadó V., Cortés A., Le Foll B., Mazzola C., Drago F., Saur O., Stark H., Soriano A., Barnes C., Goldberg S.R., Lluis C., Fuxe K., Franco R. Identification of dopamine D1-D3 receptor heteromers. Indications for a role of synergistic D1-D3 receptor interactions in the striatum. J. Biol. Chem. 2008;283(38):26016–26025. doi: 10.1074/jbc.M710349200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 508.Farré D., Muñoz A., Moreno E., Reyes-Resina I., Canet-Pons J., Dopeso-Reyes I.G., Rico A.J., Lluís C., Mallol J., Navarro G., Canela E.I., Cortés A., Labandeira-García J.L., Casadó V., Lanciego J.L., Franco R. Stronger dopamine D1 receptor-mediated neurotransmission in dyskinesia. Mol. Neurobiol. 2015;52(3):1408–1420. doi: 10.1007/s12035-014-8936-x. [DOI] [PubMed] [Google Scholar]
- 509.Lanza K., Meadows S.M., Chambers N.E., Nuss E., Deak M.M., Ferré S., Bishop C. Behavioral and cellular dopamine D1 and D3 receptor-mediated synergy: Implications for L-DOPA-induced dyskinesia. Neuropharmacology. 2018;138:304–314. doi: 10.1016/j.neuropharm.2018.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 510.Solís O., Garcia-Montes J.R., González-Granillo A., Xu M., Moratalla R. Dopamine D3 receptor modulates l-DOPA-induced dyskinesia by targeting D1 receptor-mediated striatal signaling. Cereb. Cortex. 2017;27(1):435–446. doi: 10.1093/cercor/bhv231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 511.Cote S.R., Chitravanshi V.C., Bleickardt C., Sapru H.N., Kuzhikandathil E.V. Overexpression of the dopamine D3 receptor in the rat dorsal striatum induces dyskinetic behaviors. Behav. Brain Res. 2014;263:46–50. doi: 10.1016/j.bbr.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 512.Surmeier D.J., Song W.J., Yan Z. Coordinated expression of dopamine receptors in neostriatal medium spiny neurons. J. Neurosci. 1996;16(20):6579–6591. doi: 10.1523/JNEUROSCI.16-20-06579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 513.Joyce J.N. Dopamine D3 receptor as a therapeutic target for antipsychotic and antiparkinsonian drugs. Pharmacol. Ther. 2001;90(2-3):231–259. doi: 10.1016/S0163-7258(01)00139-5. [DOI] [PubMed] [Google Scholar]
- 514.Maggio R., Millan M.J. Dopamine D2-D3 receptor heteromers: pharmacological properties and therapeutic significance. Curr. Opin. Pharmacol. 2010;10(1):100–107. doi: 10.1016/j.coph.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 515.Maggio R., Scarselli M., Novi F., Millan M.J., Corsini G.U. Potent activation of dopamine D3/D2 heterodimers by the antiparkinsonian agents, S32504, pramipexole and ropinirole. J. Neurochem. 2003;87(3):631–641. doi: 10.1046/j.1471-4159.2003.02038.x. [DOI] [PubMed] [Google Scholar]
- 516.Novi F., Millan M.J., Corsini G.U., Maggio R. Partial agonist actions of aripiprazole and the candidate antipsychotics S33592, bifeprunox, N-desmethylclozapine and preclamol at dopamine D(2L) receptors are modified by co-transfection of D(3) receptors: potential role of heterodimer formation. J. Neurochem. 2007;102(4):1410–1424. doi: 10.1111/j.1471-4159.2007.04660.x. [DOI] [PubMed] [Google Scholar]
- 517.Maggio R., Scarselli M., Capannolo M., Millan M.J. Novel dimensions of D3 receptor function: Focus on heterodimerisation, transactivation and allosteric modulation. Eur. Neuropsychopharmacol. 2015;25(9):1470–1479. doi: 10.1016/j.euroneuro.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 518.Missale C., Nash S.R., Robinson S.W., Jaber M., Caron M.G. Dopamine receptors: from structure to function. Physiol. Rev. 1998;78(1):189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 519.Rondou P., Haegeman G., Van Craenenbroeck K. The dopamine D4 receptor: biochemical and signalling properties. Cell. Mol. Life Sci. 2010;67(12):1971–1986. doi: 10.1007/s00018-010-0293-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 520.Van Tol H.H.M., Bunzow J.R., Guan H.C., Sunahara R.K., Seeman P., Niznik H.B., Civelli O. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 521.González S., Rangel-Barajas C., Peper M., Lorenzo R., Moreno E., Ciruela F., Borycz J., Ortiz J., Lluís C., Franco R., McCormick P.J., Volkow N.D., Rubinstein M., Floran B., Ferré S. Dopamine D4 receptor, but not the ADHD-associated D4.7 variant, forms functional heteromers with the dopamine D2S receptor in the brain. Mol. Psychiatry. 2012;17(6):650–662. doi: 10.1038/mp.2011.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 522.Fuxe K., Guidolin D., Agnati L.F., Borroto-Escuela D.O. Dopamine heteroreceptor complexes as therapeutic targets in Parkinson’s disease. Expert Opin. Ther. Targets. 2015;19(3):377–398. doi: 10.1517/14728222.2014.981529. [DOI] [PubMed] [Google Scholar]
- 523.Centonze D., Grande C., Usiello A., Gubellini P., Erbs E., Martín A.B., Pisani A., Tognazzi N., Bernardi G., Moratalla R., Borrelli E., Calabresi P. Receptor subtypes involved in the presynaptic and postsynaptic actions of dopamine on striatal interneurons. J. Neurosci. 2003;23(15):6245–6254. doi: 10.1523/JNEUROSCI.23-15-06245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 524.Ginés S., Hillion J., Torvinen M., Le Crom S., Casadó V., Canela E.I., Rondin S., Lew J.Y., Watson S., Zoli M., Agnati L.F., Verniera P., Lluis C., Ferré S., Fuxe K., Franco R. Dopamine D1 and adenosine A1 receptors form functionally interacting heteromeric complexes. Proc. Natl. Acad. Sci. USA. 2000;97(15):8606–8611. doi: 10.1073/pnas.150241097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 525.Franco R., Lluis C., Canela E.I., Mallol J., Agnati L., Casadó V., Ciruela F., Ferré S., Fuxe K. Receptor-receptor interactions involving adenosine A1 or dopamine D1 receptors and accessory proteins. J. Neural Transm. (Vienna) 2007;114(1):93–104. doi: 10.1007/s00702-006-0566-7. [DOI] [PubMed] [Google Scholar]
- 526.Caillé I., Dumartin B., Bloch B. Ultrastructural localization of D1 dopamine receptor immunoreactivity in rat striatonigral neurons and its relation with dopaminergic innervation. Brain Res. 1996;730(1-2):17–31. doi: 10.1016/0006-8993(96)00424-6. [DOI] [PubMed] [Google Scholar]
- 527.Rivkees S.A., Price S.L., Zhou F.C. Immunohistochemical detection of A1 adenosine receptors in rat brain with emphasis on localization in the hippocampal formation, cerebral cortex, cerebellum, and basal ganglia. Brain Res. 1995;677(2):193–203. doi: 10.1016/0006-8993(95)00062-U. [DOI] [PubMed] [Google Scholar]
- 528.Ferré S., Popoli P., Giménez-Llort L., Finnman U.B., Martínez E., Scotti de Carolis A., Fuxe K. Postsynaptic antagonistic interaction between adenosine A1 and dopamine D1 receptors. Neuroreport. 1994;6(1):73–76. doi: 10.1097/00001756-199412300-00020. [DOI] [PubMed] [Google Scholar]
- 529.Ferré S., Fredholm B.B., Morelli M., Popoli P., Fuxe K. Adenosine-dopamine receptor-receptor interactions as an integrative mechanism in the basal ganglia. Trends Neurosci. 1997;20(10):482–487. doi: 10.1016/S0166-2236(97)01096-5. [DOI] [PubMed] [Google Scholar]
- 530.Ferré S., Torvinen M., Antoniou K., Irenius E., Civelli O., Arenas E., Fredholm B.B., Fuxe K. Adenosine A1 receptor-mediated modulation of dopamine D1 receptors in stably cotransfected fibroblast cells. J. Biol. Chem. 1998;273(8):4718–4724. doi: 10.1074/jbc.273.8.4718. [DOI] [PubMed] [Google Scholar]
- 531.Toda S., Alguacil L.F., Kalivas P.W. Repeated cocaine administration changes the function and subcellular distribution of adenosine A1 receptor in the rat nucleus accumbens. J. Neurochem. 2003;87(6):1478–1484. doi: 10.1046/j.1471-4159.2003.02121.x. [DOI] [PubMed] [Google Scholar]
- 532.Rivera-Oliver M., Moreno E., Álvarez-Bagnarol Y., Ayala-Santiago C., Cruz-Reyes N., Molina-Castro G.C., Clemens S., Canela E.I., Ferré S., Casadó V., Díaz-Ríos M. Adenosine A1-dopamine D1 receptor heteromers control the excitability of the spinal motoneuron. Mol. Neurobiol. 2019;56(2):797–811. doi: 10.1007/s12035-018-1120-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 533.Canals M., Marcellino D., Fanelli F., Ciruela F., de Benedetti P., Goldberg S.R., Neve K., Fuxe K., Agnati L.F., Woods A.S., Ferré S., Lluis C., Bouvier M., Franco R. Adenosine A2A-dopamine D2 receptor-receptor heteromerization: qualitative and quantitative assessment by fluorescence and bioluminescence energy transfer. J. Biol. Chem. 2003;278(47):46741–46749. doi: 10.1074/jbc.M306451200. [DOI] [PubMed] [Google Scholar]
- 534.Hillion J., Canals M., Torvinen M., Casadó V., Scott R., Terasmaa A., Hansson A., Watson S., Olah M.E., Mallol J., Canela E.I., Zoli M., Agnati L.F., Ibanez C.F., Lluis C., Franco R., Ferre S., Fuxe K. Coaggregation, cointernalization, and codesensitization of adenosine A2A receptors and dopamine D2 receptors. J. Biol. Chem. 2002;277(20):18091–18097. doi: 10.1074/jbc.M107731200. [DOI] [PubMed] [Google Scholar]
- 535.Fuxe K., Agnati L.F., Jacobsen K., Hillion J., Canals M., Torvinen M., Tinner-Staines B., Staines W., Rosin D., Terasmaa A., Popoli P., Leo G., Vergoni V., Lluis C., Ciruela F., Franco R., Ferré S. Receptor heteromerization in adenosine A2A receptor signaling: relevance for striatal function and Parkinson’s disease. Neurology. 2003;61(11) Suppl. 6:S19–S23. doi: 10.1212/01.WNL.0000095206.44418.5C. [DOI] [PubMed] [Google Scholar]
- 536.Kamiya T., Saitoh O., Yoshioka K., Nakata H. Oligomerization of adenosine A2A and dopamine D2 receptors in living cells. Biochem. Biophys. Res. Commun. 2003;306(2):544–549. doi: 10.1016/S0006-291X(03)00991-4. [DOI] [PubMed] [Google Scholar]
- 537.Trifilieff P., Rives M.L., Urizar E., Piskorowski R.A., Vishwasrao H.D., Castrillon J., Schmauss C., Slättman M., Gullberg M., Javitch J.A. Detection of antigen interactions ex vivo by proximity ligation assay: endogenous dopamine D2-adenosine A2A receptor complexes in the striatum. Biotechniques. 2011;51(2):111–118. doi: 10.2144/000113719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 538.Borroto-Escuela D.O., Romero-Fernandez W., Garriga P., Ciruela F., Narvaez M., Tarakanov A.O., Palkovits M., Agnati L.F., Fuxe K. G protein-coupled receptor heterodimerization in the brain. Methods Enzymol. 2013;521:281–294. doi: 10.1016/B978-0-12-391862-8.00015-6. [DOI] [PubMed] [Google Scholar]
- 539.Fuxe K., Marcellino D., Genedani S., Agnati L. Adenosine A(2A) receptors, dopamine D(2) receptors and their interactions in Parkinson’s disease. Mov. Disord. 2007;22(14):1990–2017. doi: 10.1002/mds.21440. [DOI] [PubMed] [Google Scholar]
- 540.Ferre S., von Euler G., Johansson B., Fredholm B.B., Fuxe K. Stimulation of high-affinity adenosine A2 receptors decreases the affinity of dopamine D2 receptors in rat striatal membranes. Proc. Natl. Acad. Sci. USA. 1991;88(16):7238–7241. doi: 10.1073/pnas.88.16.7238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 541.Ferré S., Fuxe K. Dopamine denervation leads to an increase in the intramembrane interaction between adenosine A2 and dopamine D2 receptors in the neostriatum. Brain Res. 1992;594(1):124–130. doi: 10.1016/0006-8993(92)91036-E. [DOI] [PubMed] [Google Scholar]
- 542.Ferré S., Quiroz C., Woods A.S., Cunha R., Popoli P., Ciruela F., Lluis C., Franco R., Azdad K., Schiffmann S.N. An update on adenosine A2A-dopamine D2 receptor interactions: implications for the function of G protein-coupled receptors. Curr. Pharm. Des. 2008;14(15):1468–1474. doi: 10.2174/138161208784480108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 543.Ballesteros-Yáñez I., Castillo C.A., Merighi S., Gessi S. The Role of Adenosine Receptors in Psychostimulant Addiction. Front. Pharmacol. 2018;8(JAN):985. doi: 10.3389/fphar.2017.00985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 544.Dalrymple M.B., Pfleger K.D.G., Eidne K.A. G protein-coupled receptor dimers: functional consequences, disease states and drug targets. Pharmacol. Ther. 2008;118(3):359–371. doi: 10.1016/j.pharmthera.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 545.Rosin D.L., Hettinger B.D., Lee A., Linden J. Anatomy of adenosine A2A receptors in brain: morphological substrates for integration of striatal function. Neurology. 2003;61(11) Suppl. 6:S12–S18. doi: 10.1212/01.WNL.0000095205.33940.99. [DOI] [PubMed] [Google Scholar]
- 546.Fuxe K., Ferré S., Genedani S., Franco R., Agnati L.F. Adenosine receptor-dopamine receptor interactions in the basal ganglia and their relevance for brain function. Physiol. Behav. 2007;92(1-2):210–217. doi: 10.1016/j.physbeh.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 547.Gluck M.R., Santana L.A., Granson H., Yahr M.D. Novel dopamine releasing response of an anti-convulsant agent with possible anti-Parkinson’s activity. J. Neural Transm. (Vienna) 2004;111(6):713–724. doi: 10.1007/s00702-004-0107-1. [DOI] [PubMed] [Google Scholar]
- 548.Schiffmann S.N., Fisone G., Moresco R., Cunha R.A., Ferré S. Adenosine A2A receptors and basal ganglia physiology. Prog. Neurobiol. 2007;83(5):277–292. doi: 10.1016/j.pneurobio.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 549.Shen H.Y., Coelho J.E., Ohtsuka N., Canas P.M., Day Y.J., Huang Q.Y., Rebola N., Yu L., Boison D., Cunha R.A., Linden J., Tsien J.Z., Chen J.F. A critical role of the adenosine A2A receptor in extrastriatal neurons in modulating psychomotor activity as revealed by opposite phenotypes of striatum and forebrain A2A receptor knock-outs. J. Neurosci. 2008;28(12):2970–2975. doi: 10.1523/JNEUROSCI.5255-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 550.Kim D.S., Palmiter R.D. Adenosine receptor blockade reverses hypophagia and enhances locomotor activity of dopamine-deficient mice. Proc. Natl. Acad. Sci. USA. 2003;100(3):1346–1351. doi: 10.1073/pnas.252753799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 551.Chase T.N., Bibbiani F., Bara-Jimenez W., Dimitrova T., Oh-Lee J.D. Translating A2A antagonist KW6002 from animal models to parkinsonian patients. Neurology. 2003;61(11) Suppl. 6:S107–S111. doi: 10.1212/01.WNL.0000095223.08711.48. [DOI] [PubMed] [Google Scholar]
- 552.Torvinen M., Marcellino D., Canals M., Agnati L.F., Lluis C., Franco R., Fuxe K. Adenosine A2A receptor and dopamine D3 receptor interactions: evidence of functional A2A/D3 heteromeric complexes. Mol. Pharmacol. 2005;67(2):400–407. doi: 10.1124/mol.104.003376. [DOI] [PubMed] [Google Scholar]
- 553.Takagi H., Morishima Y., Matsuyama T., Hayashi H., Watanabe T., Wada H. Histaminergic axons in the neostriatum and cerebral cortex of the rat: A correlated light and electron microscopic immunocytochemical study using histidine decarboxylase as a marker. Brain Res. 1986;364(1):114–123. doi: 10.1016/0006-8993(86)90992-3. [DOI] [PubMed] [Google Scholar]
- 554.Ferrada C., Moreno E., Casadó V., Bongers G., Cortés A., Mallol J., Canela E.I., Leurs R., Ferré S., Lluís C., Franco R. Marked changes in signal transduction upon heteromerization of dopamine D1 and histamine H3 receptors. Br. J. Pharmacol. 2009;157(1):64–75. doi: 10.1111/j.1476-5381.2009.00152.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 555.Moreno E., Hoffmann H., Gonzalez-Sepúlveda M., Navarro G., Casadó V., Cortés A., Mallol J., Vignes M., McCormick P.J., Canela E.I., Lluís C., Moratalla R., Ferré S., Ortiz J., Franco R. Dopamine D1-histamine H3 receptor heteromers provide a selective link to MAPK signaling in GABAergic neurons of the direct striatal pathway. J. Biol. Chem. 2011;286(7):5846–5854. doi: 10.1074/jbc.M110.161489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 556.Moreno E., Moreno-Delgado D., Navarro G., Hoffmann H.M., Fuentes S., Rosell-Vilar S., Gasperini P., Rodríguez-Ruiz M., Medrano M., Mallol J., Cortés A., Casadó V., Lluís C., Ferré S., Ortiz J., Canela E., McCormick P.J. Cocaine disrupts histamine H3 receptor modulation of dopamine D1 receptor signaling: σ1-D1-H3 receptor complexes as key targets for reducing cocaine’s effects. J. Neurosci. 2014;34(10):3545–3558. doi: 10.1523/JNEUROSCI.4147-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 557.Kononoff Vanhanen J., Nuutinen S., Tuominen M., Panula P. Histamine H3 receptor regulates sensorimotor gating and dopaminergic signaling in the striatum. J. Pharmacol. Exp. Ther. 2016;357(2):264–272. doi: 10.1124/jpet.115.230771. [DOI] [PubMed] [Google Scholar]
- 558.Ferrada C., Ferré S., Casadó V., Cortés A., Justinova Z., Barnes C., Canela E.I., Goldberg S.R., Leurs R., Lluis C., Franco R. Interactions between histamine H3 and dopamine D2 receptors and the implications for striatal function. Neuropharmacology. 2008;55(2):190–197. doi: 10.1016/j.neuropharm.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 559.Pillot C., Heron A., Cochois V., Tardivel-Lacombe J., Ligneau X., Schwartz J.C., Arrang J.M. A detailed mapping of the histamine H(3) receptor and its gene transcripts in rat brain. Neuroscience. 2002;114(1):173–193. doi: 10.1016/S0306-4522(02)00135-5. [DOI] [PubMed] [Google Scholar]
- 560.Ferré S., Ciruela F., Woods A.S., Lluis C., Franco R. Functional relevance of neurotransmitter receptor heteromers in the central nervous system. Trends Neurosci. 2007;30(9):440–446. doi: 10.1016/j.tins.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 561.Szafran K., Łukasiewicz S., Faron-Górecka A., Kolasa M., Kuśmider M., Solich J., Dziedzicka-Wasylewska M. Antidepressant drugs promote the heterodimerization of the dopamine D2 and somatostatin Sst5 receptors--fluorescence in vitro studies. . Pharmacol. Rep. 2012;64(5):1253–1258. doi: 10.1016/S1734-1140(12)70921-0. [DOI] [PubMed] [Google Scholar]
- 562.Szafran-Pilch K., Faron-Górecka A., Kolasa M., Żurawek D., Szlachta M., Solich J., Kuśmider M., Dziedzicka-Wasylewska M. Antidepressants promote formation of heterocomplexes of dopamine D2 and somatostatin subtype 5 receptors in the mouse striatum. Brain Res. Bull. 2017;135:92–97. doi: 10.1016/j.brainresbull.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 563.Faron-Górecka A., Kuśmider M., Solich J., Kolasa M., Szafran K., Zurawek D., Pabian P., Dziedzicka-Wasylewska M. Involvement of prolactin and somatostatin in depression and the mechanism of action of antidepressant drugs. Pharmacol. Rep. 2013;65(6):1640–1646. doi: 10.1016/S1734-1140(13)71525-1. [DOI] [PubMed] [Google Scholar]
- 564.Borroto-Escuela D.O., Ravani A., Tarakanov A.O., Brito I., Narvaez M., Romero-Fernandez W., Corrales F., Agnati L.F., Tanganelli S., Ferraro L., Fuxe K. Dopamine D2 receptor signaling dynamics of dopamine D2-neurotensin 1 receptor heteromers. Biochem. Biophys. Res. Commun. 2013;435(1):140–146. doi: 10.1016/j.bbrc.2013.04.058. [DOI] [PubMed] [Google Scholar]
- 565.Plach M., Schäfer T., Borroto-Escuela D.O., Weikert D., Gmeiner P., Fuxe K., Friedland K. Differential allosteric modulation within dopamine D2R - neurotensin NTS1R and D2R - serotonin 5-HT2AR receptor complexes gives bias to intracellular calcium signalling. Sci. Rep. 2019;9(1):16312. doi: 10.1038/s41598-019-52540-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 566.Ferraro L., Tomasini M.C., Mazza R., Fuxe K., Fournier J., Tanganelli S., Antonelli T. Neurotensin receptors as modulators of glutamatergic transmission. Brain Res. Brain Res. Rev. 2008;58(2):365–373. doi: 10.1016/j.brainresrev.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 567.Koschatzky S., Tschammer N., Gmeiner P. Cross-receptor interactions between dopamine D2L and neurotensin NTS1 receptors modulate binding affinities of dopaminergics. ACS Chem. Neurosci. 2011;2(6):308–316. doi: 10.1021/cn200020y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 568.Antonelli T., Tomasini M.C., Fuxe K., Agnati L.F., Tanganelli S., Ferraro L. Focus on NTR/D2 interactions in the basal ganglia. J. Neural. Trans. 2007. p. 105-113. [DOI] [PubMed]
- 569.Tanganelli S., Antonelli T., Tomasini M.C., Beggiato S., Fuxe K., Ferraro L. Relevance of dopamine D(2)/neurotensin NTS1 and NMDA/neurotensin NTS1 receptor interaction in psychiatric and neurodegenerative disorders. Curr. Med. Chem. 2012;19(3):304–316. doi: 10.2174/092986712803414268. [DOI] [PubMed] [Google Scholar]
- 570.Espinoza S., Salahpour A., Masri B., Sotnikova T.D., Messa M., Barak L.S., Caron M.G., Gainetdinov R.R. Functional interaction between trace amine-associated receptor 1 and dopamine D2 receptor. Mol. Pharmacol. 2011;80(3):416–425. doi: 10.1124/mol.111.073304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 571.Espinoza S., Masri B., Salahpour A., Gainetdinov R.R. BRET approaches to characterize dopamine and TAAR1 receptor pharmacology and signaling. Methods Mol. Biol. 2013;964:107–122. doi: 10.1007/978-1-62703-251-3_8. [DOI] [PubMed] [Google Scholar]
- 572.Lindemann L., Meyer C.A., Jeanneau K., Bradaia A., Ozmen L., Bluethmann H., Bettler B., Wettstein J.G., Borroni E., Moreau J.L., Hoener M.C. Trace amine-associated receptor 1 modulates dopaminergic activity. J. Pharmacol. Exp. Ther. 2008;324(3):948–956. doi: 10.1124/jpet.107.132647. [DOI] [PubMed] [Google Scholar]
- 573.Romero-Fernandez W., Borroto-Escuela D.O., Agnati L.F., Fuxe K. Evidence for the existence of dopamine D2-oxytocin receptor heteromers in the ventral and dorsal striatum with facilitatory receptor-receptor interactions. Mol. Psychiatry. 2013;18(8):849–850. doi: 10.1038/mp.2012.103. [DOI] [PubMed] [Google Scholar]
- 574.de la Mora M.P., Pérez-Carrera D., Crespo-Ramírez M., Tarakanov A., Fuxe K., Borroto-Escuela D.O. Signaling in dopamine D2 receptor-oxytocin receptor heterocomplexes and its relevance for the anxiolytic effects of dopamine and oxytocin interactions in the amygdala of the rat. Biochim. Biophys. Acta. 2016;1862(11):2075–2085. doi: 10.1016/j.bbadis.2016.07.004. [DOI] [PubMed] [Google Scholar]
- 575.Pradhan G., Samson S.L., Sun Y. Ghrelin: much more than a hunger hormone. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16(6):619–624. doi: 10.1097/MCO.0b013e328365b9be. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 576.Davenport A.P., Bonner T.I., Foord S.M., Harmar A.J., Neubig R.R., Pin J-P., Spedding M., Kojima M., Kangawa K. International Union of Pharmacology. LVI. Ghrelin receptor nomenclature, distribution, and function. Pharmacol. Rev. 2005;57(4):541–546. doi: 10.1124/pr.57.4.1. [DOI] [PubMed] [Google Scholar]
- 577.GHSR growth hormone secretagogue receptor [Homo sapiens (human)] Gene - NCBI. 2005.
- 578.Smith R.G., Van der Ploeg L.H.T., Howard A.D., Feighner S.D., Cheng K., Hickey G.J., Wyvratt M.J., Jr, Fisher M.H., Nargund R.P., Patchett A.A. Peptidomimetic regulation of growth hormone secretion. Endocr. Rev. 1997;18(5):621–645. doi: 10.1210/edrv.18.5.0316. [DOI] [PubMed] [Google Scholar]
- 579.Adriaenssens A.E., Svendsen B., Lam B.Y.H., Yeo G.S.H., Holst J.J., Reimann F., Gribble F.M. Transcriptomic profiling of pancreatic alpha, beta and delta cell populations identifies delta cells as a principal target for ghrelin in mouse islets. Diabetologia. 2016;59(10):2156–2165. doi: 10.1007/s00125-016-4033-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 580.Meguid M.M., Fetissov S.O., Varma M., Sato T., Zhang L., Laviano A., Rossi-Fanelli F. Hypothalamic dopamine and serotonin in the regulation of food intake. Nutrition. 2000;16(10):843–857. doi: 10.1016/S0899-9007(00)00449-4. [DOI] [PubMed] [Google Scholar]
- 581.Vucetic Z., Reyes T.M. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2(5):577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- 582.Kern A., Albarran-Zeckler R., Walsh H.E., Smith R.G. Apo-ghrelin receptor forms heteromers with DRD2 in hypothalamic neurons and is essential for anorexigenic effects of DRD2 agonism. Neuron. 2012;73(2):317–332. doi: 10.1016/j.neuron.2011.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 583.Franco R., Cordomí A., Llinas Del Torrent C., Lillo A., Serrano-Marín J., Navarro G., Pardo L. Structure and function of adenosine receptor heteromers. Cell. Mol. Life Sci. 2021;78(8):3957–3968. doi: 10.1007/s00018-021-03761-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 584.Gao Z.G., Jacobson K.A. Emerging adenosine receptor agonists. Expert Opin. Emerg. Drugs. 2007;12(3):479–492. doi: 10.1517/14728214.12.3.479. [DOI] [PubMed] [Google Scholar]
- 585.Fredholm B.B., Irenius E., Kull B., Schulte G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001;61(4):443–448. doi: 10.1016/S0006-2952(00)00570-0. [DOI] [PubMed] [Google Scholar]
- 586.Navarro G., Cordomí A., Brugarolas M., Moreno E., Aguinaga D., Pérez-Benito L., Ferre S., Cortés A., Casadó V., Mallol J., Canela E.I., Lluís C., Pardo L., McCormick P.J., Franco R. Cross-communication between Gi and Gs in a G-protein-coupled receptor heterotetramer guided by a receptor C-terminal domain. BMC Biol. 2018;16(1):24. doi: 10.1186/s12915-018-0491-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 587.Borroto-Escuela D.O., Fuxe K. Adenosine heteroreceptor complexes in the basal ganglia are implicated in Parkinson’s disease and its treatment. J. Neural Transm. (Vienna) 2019;126(4):455–471. doi: 10.1007/s00702-019-01969-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 588.Ferre S., Ciruela F., Borycz J., Solinas M., Quarta D., Antoniou K., Quiroz C., Justinova Z., Lluis C., Franco R., Goldberg S.R. Adenosine A1-A2A receptor heteromers: new targets for caffeine in the brain. Front. Biosci. 2008;13(6):2391–2399. doi: 10.2741/2852. [DOI] [PubMed] [Google Scholar]
- 589.Aghajanian G.K., Marek G.J. Serotonin induces excitatory postsynaptic potentials in apical dendrites of neocortical pyramidal cells. Neuropharmacology. 1997;36(4-5):589–599. doi: 10.1016/S0028-3908(97)00051-8. [DOI] [PubMed] [Google Scholar]
- 590.Aghajanian G.K., Marek G.J. Serotonin-glutamate interactions:A new target for antipsychotic drugs. Neuropsychopharmacology. 1999;21(6):S122–S133. doi: 10.1016/S0893-133X(99)00106-2. [DOI] [Google Scholar]
- 591.Stutzmann G.E., Marek G.J., Aghajanian G.K. Adenosine preferentially suppresses serotonin2A receptor-enhanced excitatory postsynaptic currents in layer V neurons of the rat medial prefrontal cortex. Neuroscience. 2001;105(1):55–69. doi: 10.1016/S0306-4522(01)00170-1. [DOI] [PubMed] [Google Scholar]
- 592.Marek G.J. Activation of adenosine(1) (A(1)) receptors suppresses head shakes induced by a serotonergic hallucinogen in rats. Neuropharmacology. 2009;56(8):1082–1087. doi: 10.1016/j.neuropharm.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 593.Márquez-Gómez R., Robins M.T., Gutiérrez-Rodelo C., Arias J.M., Olivares-Reyes J.A., van Rijn R.M., Arias-Montaño J.A. Functional histamine H3 and adenosine A2A receptor heteromers in recombinant cells and rat striatum. Pharmacol. Res. 2018;129:515–525. doi: 10.1016/j.phrs.2017.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 594.Ballantyne J.C., Mao J. Opioid therapy for chronic pain. N. Engl. J. Med. 2003;349(20):1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 595.Skolnick P. The Opioid Epidemic: Crisis and Solutions. Annu. Rev. Pharmacol. Toxicol. 2018;58:143–159. doi: 10.1146/annurev-pharmtox-010617-052534. [DOI] [PubMed] [Google Scholar]
- 596.Dart R.C., Surratt H.L., Cicero T.J., Parrino M.W., Severtson S.G., Bucher-Bartelson B., Green J.L. Trends in opioid analgesic abuse and mortality in the United States. N. Engl. J. Med. 2015;372(3):241–248. doi: 10.1056/NEJMsa1406143. [DOI] [PubMed] [Google Scholar]
- 597.Machelska H., Celik M.Ö. Advances in Achieving Opioid Analgesia Without Side Effects. Front. Pharmacol. 2018;9(NOV):1388. doi: 10.3389/fphar.2018.01388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 598.Li-Wei C., Can G., De-He Z., Qiang W., Xue-Jun X., Jie C., Zhi-Qiang C. Homodimerization of human mu-opioid receptor overexpressed in Sf9 insect cells. Protein Pept. Lett. 2002;9(2):145–152. doi: 10.2174/0929866023408850. [DOI] [PubMed] [Google Scholar]
- 599.Yekkirala A.S., Banks M.L., Lunzer M.M., Negus S.S., Rice K.C., Portoghese P.S. Clinically employed opioid analgesics produce antinociception via μ-δ opioid receptor heteromers in Rhesus monkeys. . ACS Chem. Neurosci. 2012;3(9):720–727. doi: 10.1021/cn300049m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 600.Costantino C.M., Gomes I., Stockton S.D., Lim M.P., Devi L.A. Opioid receptor heteromers in analgesia. Expert Rev. Mol. Med. 2012;14:e9. doi: 10.1017/erm.2012.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 601.Gomes I., Gupta A., Filipovska J., Szeto H.H., Pintar J.E., Devi L.A. A role for heterodimerization of mu and delta opiate receptors in enhancing morphine analgesia. Proc. Natl. Acad. Sci. USA. 2004;101(14):5135–5139. doi: 10.1073/pnas.0307601101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 602.Chakrabarti S., Liu N-J., Gintzler A.R. Formation of mu-/kappa-opioid receptor heterodimer is sex-dependent and mediates female-specific opioid analgesia. Proc. Natl. Acad. Sci. USA. 2010;107(46):20115–20119. doi: 10.1073/pnas.1009923107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 603.Jordan B.A., Devi L.A. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399(6737):697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 604.Zhang L., Zhang J-T., Hang L., Liu T. Mu opioid receptor heterodimers emerge as novel therapeutic targets: Recent progress and future perspective. Front. Pharmacol. 2020;11:1078. doi: 10.3389/fphar.2020.01078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 605.Olson K.M., Keresztes A., Tashiro J.K., Daconta L.V., Hruby V.J., Streicher J.M. Synthesis and evaluation of a novel bivalent selective antagonist for the mu-delta opioid receptor heterodimer that reduces morphine withdrawal in mice. J. Med. Chem. 2018;61(14):6075–6086. doi: 10.1021/acs.jmedchem.8b00403. [DOI] [PubMed] [Google Scholar]
- 606.Rozenfeld R., Devi L.A. Receptor heterodimerization leads to a switch in signaling: β-arrestin2-mediated ERK activation by μ-δ opioid receptor heterodimers. FASEB J. 2007;21(10):2455–2465. doi: 10.1096/fj.06-7793com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 607.Metcalf M.D., Yekkirala A.S., Powers M.D., Kitto K.F., Fairbanks C.A., Wilcox G.L., Portoghese P.S. The δ opioid receptor agonist SNC80 selectively activates heteromeric μ-δ opioid receptors. ACS Chem. Neurosci. 2012;3(7):505–509. doi: 10.1021/cn3000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 608.Gomes I., Fujita W., Gupta A., Saldanha S.A., Negri A., Pinello C.E., Eberhart C., Roberts E., Filizola M., Hodder P., Devi L.A. Identification of a μ-δ opioid receptor heteromer-biased agonist with antinociceptive activity. Proc. Natl. Acad. Sci. USA. 2013;110(29):12072–12077. doi: 10.1073/pnas.1222044110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 609.Milan-Lobo L., Whistler J.L. Heteromerization of the μ- and δ-opioid receptors produces ligand-biased antagonism and alters μ-receptor trafficking. J. Pharmacol. Exp. Ther. 2011;337(3):868–875. doi: 10.1124/jpet.111.179093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 610.Hasbi A., Nguyen T., Fan T., Cheng R., Rashid A., Alijaniaram M., Rasenick M.M., O’Dowd B.F., George S.R. Trafficking of preassembled opioid μ-δ heterooligomer-Gz signaling complexes to the plasma membrane: coregulation by agonists. Biochemistry. 2007;46(45):12997–13009. doi: 10.1021/bi701436w. [DOI] [PubMed] [Google Scholar]
- 611.Décaillot F.M., Rozenfeld R., Gupta A., Devi L.A. Cell surface targeting of μ-δ opioid receptor heterodimers by RTP4. Proc. Natl. Acad. Sci. USA. 2008;105(41):16045–16050. doi: 10.1073/pnas.0804106105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 612.He S.Q., Zhang Z.N., Guan J.S., Liu H.R., Zhao B., Wang H.B., Li Q., Yang H., Luo J., Li Z.Y., Wang Q., Lu Y.J., Bao L., Zhang X. Facilitation of μ-opioid receptor activity by preventing δ-opioid receptor-mediated codegradation. Neuron. 2011;69(1):120–131. doi: 10.1016/j.neuron.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 613.Erbs E., Faget L., Scherrer G., Matifas A., Filliol D., Vonesch J.L., Koch M., Kessler P., Hentsch D., Birling M.C., Koutsourakis M., Vasseur L., Veinante P., Kieffer B.L., Massotte D. A mu-delta opioid receptor brain atlas reveals neuronal co-occurrence in subcortical networks. Brain Struct. Funct. 2015;220(2):677–702. doi: 10.1007/s00429-014-0717-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 614.Wang D., Tawfik V.L., Corder G., Low S.A., François A., Basbaum A.I., Scherrer G. Functional divergence of delta and mu opioid receptor organization in CNS pain circuits. Neuron. 2018;98(1):90–108.e5. doi: 10.1016/j.neuron.2018.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 615.Liu N.J., Chakrabarti S., Schnell S., Wessendorf M., Gintzler A.R. Spinal synthesis of estrogen and concomitant signaling by membrane estrogen receptors regulate spinal κ- and μ-opioid receptor heterodimerization and female-specific spinal morphine antinociception. J. Neurosci. 2011;31(33):11836–11845. doi: 10.1523/JNEUROSCI.1901-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 616.Yang Y., Li Q., He Q.H., Han J.S., Su L., Wan Y. Heteromerization of μ-opioid receptor and cholecystokinin B receptor through the third transmembrane domain of the μ-opioid receptor contributes to the anti-opioid effects of cholecystokinin octapeptide. Exp. Mol. Med. 2018;50(5):1–16. doi: 10.1038/s12276-018-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 617.Vilardaga J.P., Nikolaev V.O., Lorenz K., Ferrandon S., Zhuang Z., Lohse M.J. Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signaling. Nat. Chem. Biol. 2008;4(2):126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- 618.Stone L.S., MacMillan L.B., Kitto K.F., Limbird L.E., Wilcox G.L. The α2a adrenergic receptor subtype mediates spinal analgesia evoked by α2 agonists and is necessary for spinal adrenergic-opioid synergy. J. Neurosci. 1997;17(18):7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 619.Bourgoin S., Pohl M., Mauborgne A., Benoliel J.J., Collin E., Hamon M., Cesselin F. Monoaminergic control of the release of calcitonin gene-related peptide- and substance P-like materials from rat spinal cord slices. Neuropharmacology. 1993;32(7):633–640. doi: 10.1016/0028-3908(93)90076-F. [DOI] [PubMed] [Google Scholar]
- 620.Kamisaki Y., Hamada T., Maeda K., Ishimura M., Itoh T. Presynaptic α2 Adrenoceptors Inhibit Glutamate Release from Rat Spinal Cord Synaptosomes. John Wiley & Sons, Ltd; 1993. pp. 522–526. [DOI] [PubMed] [Google Scholar]
- 621.Jordan B., Devi L.A. Molecular mechanisms of opioid receptor signal transduction. Br. J. Anaesth. 1998;81(1):12–19. doi: 10.1093/bja/81.1.12. [DOI] [PubMed] [Google Scholar]
- 622.Richman J.G., Regan J.W. α2-adrenergic receptors increase cell migration and decrease F-actin labeling in rat aortic smooth muscle cells. Am. J. Physiol. Cell Physiol. 1998;274(3):43. doi: 10.1152/ajpcell.1998.274.3.C654. [DOI] [PubMed] [Google Scholar]
- 623.Wang D., Stoveken H.M., Zucca S., Dao M., Orlandi C., Song C. Genetic behavioral screen identifies an orphan anti-opioid system. Science (80-) 2019;365((6459)):1267-1273. doi: 10.1126/science.aau2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 624.Koshimizu T.A., Honda K., Nagaoka-Uozumi S., Ichimura A., Kimura I., Nakaya M., Sakai N., Shibata K., Ushijima K., Fujimura A., Hirasawa A., Kurose H., Tsujimoto G., Tanoue A., Takano Y. Complex formation between the vasopressin 1b receptor, β-arrestin-2, and the μ-opioid receptor underlies morphine tolerance. Nat. Neurosci. 2018;21(6):820–833. doi: 10.1038/s41593-018-0144-y. [DOI] [PubMed] [Google Scholar]
- 625.Moreno E., Quiroz C., Rea W., Cai N.S., Mallol J., Cortés A., Lluís C., Canela E.I., Casadó V., Ferré S. Functional µ-Opioid-galanin receptor heteromers in the ventral tegmental area. J. Neurosci. 2017;37(5):1176–1186. doi: 10.1523/JNEUROSCI.2442-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 626.Cai N.S., Quiroz C., Bonaventura J., Bonifazi A., Cole T.O., Purks J., Billing A.S., Massey E., Wagner M., Wish E.D., Guitart X., Rea W., Lam S., Moreno E., Casadó-Anguera V., Greenblatt A.D., Jacobson A.E., Rice K.C., Casadó V., Newman A.H., Winkelman J.W., Michaelides M., Weintraub E., Volkow N.D., Belcher A.M., Ferré S. Opioid-galanin receptor heteromers mediate the dopaminergic effects of opioids. J. Clin. Invest. 2019;129(7):2730–2744. doi: 10.1172/JCI126912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 627.Salio C., Fischer J., Franzoni M.F., Mackie K., Kaneko T., Conrath M. CB1-cannabinoid and μ-opioid receptor co-localization on postsynaptic target in the rat dorsal horn. Neuroreport. 2001;12(17):3689–3692. doi: 10.1097/00001756-200112040-00017. [DOI] [PubMed] [Google Scholar]
- 628.Raehal K.M., Bohn L.M. β-arrestins: regulatory role and therapeutic potential in opioid and cannabinoid receptor-mediated analgesia. Handb. Exp. Pharmacol. 2014;219:427–443. doi: 10.1007/978-3-642-41199-1_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 629.Bouchet C.A., Ingram S.L. Cannabinoids in the descending pain modulatory circuit: Role in inflammation. Pharmacol. Ther. 2020;209:107495. doi: 10.1016/j.pharmthera.2020.107495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 630.Christie M.J. Opioid and cannabinoid receptors: friends with benefits or just close friends? Br. J. Pharmacol. 2006;148(4):385–386. doi: 10.1038/sj.bjp.0706756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 631.Manduca A., Lassalle O., Sepers M., Campolongo P., Cuomo V., Marsicano G., Kieffer B., Vanderschuren L.J., Trezza V., Manzoni O.J. Interacting cannabinoid and opioid receptors in the nucleus accumbens core control adolescent social play. Front. Behav. Neurosci. 2016;10(NOV):211. doi: 10.3389/fnbeh.2016.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 632.Manduca A., Morena M., Campolongo P., Servadio M., Palmery M., Trabace L., Hill M.N., Vanderschuren L.J., Cuomo V., Trezza V. Distinct roles of the endocannabinoids anandamide and 2-arachidonoylglycerol in social behavior and emotionality at different developmental ages in rats. Eur. Neuropsychopharmacol. 2015;25(8):1362–1374. doi: 10.1016/j.euroneuro.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 633.Wei D., Lee D., Li D., Daglian J., Jung K.M., Piomelli D. A role for the endocannabinoid 2-arachidonoyl-sn-glycerol for social and high-fat food reward in male mice. Psychopharmacology (Berl.) 2016;233(10):1911–1919. doi: 10.1007/s00213-016-4222-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 634.Pu S.F., Zhuang H.X., Han J.S. Cholecystokinin octapeptide (CCK-8) antagonizes morphine analgesia in nucleus accumbens of the rat via the CCK-B receptor. . Brain Res. 1994;657(1-2):159–164. doi: 10.1016/0006-8993(94)90963-6. [DOI] [PubMed] [Google Scholar]
- 635.Dourish C.T., O’Neill M.F., Coughlan J., Kitchener S.J., Hawley D., Iversen S.D. The selective CCK-B receptor antagonist L-365,260 enhances morphine analgesia and prevents morphine tolerance in the rat. Eur. J. Pharmacol. 1990;176(1):35–44. doi: 10.1016/0014-2999(90)90129-T. [DOI] [PubMed] [Google Scholar]
- 636.Suzuki S., Chuang L.F., Yau P., Doi R.H., Chuang R.Y. Interactions of opioid and chemokine receptors: oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp. Cell Res. 2002;280(2):192–200. doi: 10.1006/excr.2002.5638. [DOI] [PubMed] [Google Scholar]
- 637.Szabo I., Chen X-H., Xin L., Adler M.W., Howard O.M.Z., Oppenheim J.J., Rogers T.J. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc. Natl. Acad. Sci. USA. 2002;99(16):10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 638.Juhasz J.R., Hasbi A., Rashid A.J., So C.H., George S.R., O’Dowd B.F. Mu-opioid receptor heterooligomer formation with the dopamine D1 receptor as directly visualized in living cells. Eur. J. Pharmacol. 2008;581(3):235–243. doi: 10.1016/j.ejphar.2007.11.060. [DOI] [PubMed] [Google Scholar]
- 639.Tao Y.M., Yu C., Wang W.S., Hou Y.Y., Xu X.J., Chi Z.Q., Ding Y.Q., Wang Y.J., Liu J.G. Heteromers of μ opioid and dopamine D1 receptors modulate opioid-induced locomotor sensitization in a dopamine-independent manner. Br. J. Pharmacol. 2017;174(17):2842–2861. doi: 10.1111/bph.13908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 640.Dai W.L., Xiong F., Yan B., Cao Z.Y., Liu W.T., Liu J.H., Yu B.Y. Blockade of neuronal dopamine D2 receptor attenuates morphine tolerance in mice spinal cord. Sci. Rep. 2016;6(1):38746. doi: 10.1038/srep38746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 641.Vasudevan L., Borroto-Escuela D.O., Huysentruyt J., Fuxe K., Saini D.K., Stove C. Heterodimerization of MU opioid receptor protomer with dopamine D2 receptor modulates agonist- induced internalization of MU opioid receptor. Biomolecules. 2019;9(8):368. doi: 10.3390/biom9080368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 642.Grecksch G., Just S., Pierstorff C., Imhof A.K., Glück L., Doll C., Lupp A., Becker A., Koch T., Stumm R., Höllt V., Schulz S. Analgesic tolerance to high-efficacy agonists but not to morphine is diminished in phosphorylation-deficient S375A μ-opioid receptor knock-in mice. J. Neurosci. 2011;31(39):13890–13896. doi: 10.1523/JNEUROSCI.2304-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 643.McPherson J., Rivero G., Baptist M., Llorente J., Al-Sabah S., Krasel C., Dewey W.L., Bailey C.P., Rosethorne E.M., Charlton S.J., Henderson G., Kelly E. μ-opioid receptors: correlation of agonist efficacy for signalling with ability to activate internalization. Mol. Pharmacol. 2010;78(4):756–766. doi: 10.1124/mol.110.066613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 644.Whistler J.L., von Zastrow M. Morphine-activated opioid receptors elude desensitization by beta-arrestin. Proc. Natl. Acad. Sci. USA. 1998;95(17):9914–9919. doi: 10.1073/pnas.95.17.9914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 645.Bohn LM, Gainetdinov RR, Caron MG. G. Protein-coupled receptor kinase/β-arrestin systems and drugs of abuse: Psychostimulant and opiate studies in knockout mice. Neuro. Mol. Med. 2004. p. 5(1), 041-50. [DOI] [PubMed]
- 646.Artigas F. Serotonin receptors involved in antidepressant effects. Pharmacol. Ther. 2013;137(1):119–131. doi: 10.1016/j.pharmthera.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 647.Borroto-Escuela D.O., Li X., Tarakanov A.O., Savelli D., Narváez M., Shumilov K., Andrade-Talavera Y., Jimenez-Beristain A., Pomierny B., Díaz-Cabiale Z., Cuppini R., Ambrogini P., Lindskog M., Fuxe K. Existence of brain 5-HT1A-5-HT2A isoreceptor complexes with antagonistic allosteric receptor-receptor interactions regulating 5-HT1A receptor recognition. ACS Omega. 2017;2(8):4779–4789. doi: 10.1021/acsomega.7b00629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 648.Szlachta M., Kuśmider M., Pabian P., Solich J., Kolasa M., Żurawek D., Dziedzicka-Wasylewska M., Faron-Górecka A. Repeated clozapine increases the level of serotonin 5-HT1A R heterodimerization with 5-HT2A or dopamine D2 receptors in the mouse cortex. Front. Mol. Neurosci. 2018;11:40. doi: 10.3389/fnmol.2018.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 649.Celada P., Bortolozzi A., Artigas F. Serotonin 5-HT1A receptors as targets for agents to treat psychiatric disorders: rationale and current status of research. CNS Drugs. 2013;27(9):703–716. doi: 10.1007/s40263-013-0071-0. [DOI] [PubMed] [Google Scholar]
- 650.Millan M.J., Marin P., Bockaert J., Mannoury la Cour C. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol. Sci. 2008;29(9):454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 651.Carr D.B., Cooper D.C., Ulrich S.L., Spruston N., Surmeier D.J. Serotonin receptor activation inhibits sodium current and dendritic excitability in prefrontal cortex via a protein kinase C-dependent mechanism. . J. Neurosci. 2002;22(16):6846–6855. doi: 10.1523/JNEUROSCI.22-16-06846.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 652.Anastasio N.C., Stutz S.J., Fink L.H.L., Swinford-Jackson S.E., Sears R.M., DiLeone R.J., Rice K.C., Moeller F.G., Cunningham K.A. Serotonin (5-HT) 5-HT2A Receptor (5-HT2AR):5-HT2CR Imbalance in Medial Prefrontal Cortex Associates with Motor Impulsivity. ACS Chem. Neurosci. 2015;6(7):1248–1258. doi: 10.1021/acschemneuro.5b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 653.Bubar M.J., Stutz S.J., Cunningham K.A. 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS One. 2011;6(6):e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 654.Esposito E. Serotonin-dopamine interaction as a focus of novel antidepressant drugs. Curr. Drug Targets. 2006;7(2):177–185. doi: 10.2174/138945006775515455. [DOI] [PubMed] [Google Scholar]
- 655.Moutkine I., Quentin E., Guiard B.P., Maroteaux L., Doly S. Heterodimers of serotonin receptor subtypes 2 are driven by 5-HT2C protomers. J. Biol. Chem. 2017;292(15):6352–6368. doi: 10.1074/jbc.M117.779041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 656.Renner U., Zeug A., Woehler A., Niebert M., Dityatev A., Dityateva G., Gorinski N., Guseva D., Abdel-Galil D., Fröhlich M., Döring F., Wischmeyer E., Richter D.W., Neher E., Ponimaskin E.G. Heterodimerization of serotonin receptors 5-HT1A and 5-HT7 differentially regulates receptor signalling and trafficking. J. Cell Sci. 2012;125(Pt 10):2486–2499. doi: 10.1242/jcs.101337. [DOI] [PubMed] [Google Scholar]
- 657.Barnes N.M., Sharp T. A review of central 5-HT receptors and their function. Neuropharmacology. 1999;38(8):1083–1152. doi: 10.1016/S0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- 658.Raymond J.R., Mukhin Y.V., Gettys T.W., Garnovskaya M.N. The recombinant 5-HT1A receptor: G protein coupling and signalling pathways. Br. J. Pharmacol. 1999;127(8):1751–1764. doi: 10.1038/sj.bjp.0702723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 659.Li Y.H., Xiang K., Xu X., Zhao X., Li Y., Zheng L., Wang J. Co-activation of both 5-HT1A and 5-HT7 receptors induced attenuation of glutamatergic synaptic transmission in the rat visual cortex. Neurosci. Lett. 2018;686:122–126. doi: 10.1016/j.neulet.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 660.Naumenko V.S., Popova N.K., Lacivita E., Leopoldo M., Ponimaskin E.G. Interplay between serotonin 5-HT1A and 5-HT7 receptors in depressive disorders. CNS Neurosci. Ther. 2014;20(7):582–590. doi: 10.1111/cns.12247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 661.Łukasiewicz S., Błasiak E., Szafran-Pilch K., Dziedzicka-Wasylewska M. Dopamine D2 and serotonin 5-HT1A receptor interaction in the context of the effects of antipsychotics - in vitro studies. J. Neurochem. 2016;137(4):549–560. doi: 10.1111/jnc.13582. [DOI] [PubMed] [Google Scholar]
- 662.Łukasiewicz S., Polit A., Kędracka-Krok S., Wędzony K., Maćkowiak M., Dziedzicka-Wasylewska M. Hetero-dimerization of serotonin 5-HT(2A) and dopamine D(2) receptors. Biochim. Biophys. Acta. 2010;1803(12):1347–1358. doi: 10.1016/j.bbamcr.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 663.Albizu L., Holloway T., González-Maeso J., Sealfon S.C. Functional crosstalk and heteromerization of serotonin 5-HT2A and dopamine D2 receptors. Neuropharmacology. 2011;61(4):770–777. doi: 10.1016/j.neuropharm.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 664.Borroto-Escuela D.O., Romero-Fernandez W., Tarakanov A.O., Marcellino D., Ciruela F., Agnati L.F., Fuxe K. Dopamine D2 and 5-hydroxytryptamine 5-HT(2A) receptors assemble into functionally interacting heteromers. Biochem. Biophys. Res. Commun. 2010;401(4):605–610. doi: 10.1016/j.bbrc.2010.09.110. [DOI] [PubMed] [Google Scholar]
- 665.Glennon J., Wadman W., McCreary A., Werkman T. Dopamine Receptor Pharmacology: Interactions with Serotonin Receptors and Significance for the Aetiology and Treatment of Schizophrenia. CNS Neurol. Disord. Drug Targets. 2008;5(1):3–23. doi: 10.2174/187152706784111614. [DOI] [PubMed] [Google Scholar]
- 666.Reimherr F.W., Wood D.R., Wender P.H. The use of MK-801, a novel sympathomimetic, in adults with attention deficit disorder, residual type. Psychopharmacol. Bull. 1986;22(1):237–242. [PubMed] [Google Scholar]
- 667.Gattaz W.F., Schummer B., Behrens S. Effects of zotepine, haloperidol and clozapine on MK-801-induced stereotypy and locomotion in rats. J. Neural Transm. (Vienna) 1994;96(3):227–232. doi: 10.1007/BF01294789. [DOI] [PubMed] [Google Scholar]
- 668.Borroto-Escuela D.O., Narvaez M., Marcellino D., Parrado C., Narvaez J.A., Tarakanov A.O., Agnati L.F., Díaz-Cabiale Z., Fuxe K. Galanin receptor-1 modulates 5-hydroxtryptamine-1A signaling via heterodimerization. . Biochem. Biophys. Res. Commun. 2010;393(4):767–772. doi: 10.1016/j.bbrc.2010.02.078. [DOI] [PubMed] [Google Scholar]
- 669.Branchek T., Smith K.E., Walker M.W. Molecular biology and pharmacology of galanin receptors. Ann. N. Y. Acad. Sci. 1998;863(1):94–107. doi: 10.1111/j.1749-6632.1998.tb10687.x. [DOI] [PubMed] [Google Scholar]
- 670.Fuxe K., Hedlund P., von Euler G., Lundgren K., Martire M., Ögren S.O. Galanin/5-HT interactions in the rat central nervous system. Relevance for depression. Galanin; 1991. pp. 221–235. [DOI] [Google Scholar]
- 671.Razani H., Díaz-Cabiale Z., Misane I., Wang F.H., Fuxe K., Ögren S.O. Prolonged effects of intraventricular galanin on a 5-hydroxytryptamine(1A) receptor mediated function in the rat. Neurosci. Lett. 2001;299(1-2):145–149. doi: 10.1016/S0304-3940(00)01788-2. [DOI] [PubMed] [Google Scholar]
- 672.Fuxe K., von Euler G., Agnati L.F., Ögren S.O. Galanin selectively modulates 5-hydroxytryptamine 1A receptors in the rat ventral limbic cortex. Neurosci. Lett. 1988;85(1):163–167. doi: 10.1016/0304-3940(88)90448-X. [DOI] [PubMed] [Google Scholar]
- 673.Razani H., Diaz-Cabiale Z., Fuxe K., Ögren S.O. Intraventricular galanin produces a time-dependent modulation of 5-HT1A receptors in the dorsal raphe of the rat. Neuroreport. 2000;11(18):3943–3948. doi: 10.1097/00001756-200012180-00008. [DOI] [PubMed] [Google Scholar]
- 674.Fuxe K., Ögren S.O., Jansson A., Cintra A., Härfstrand A., Agnati L.F. Intraventricular injections of galanin reduces 5-HT metabolism in the ventral limbic cortex, the hippocampal formation and the fronto-parietal cortex of the male rat. Acta Physiol. Scand. 1988;133(4):579–581. doi: 10.1111/j.1748-1716.1988.tb08444.x. [DOI] [PubMed] [Google Scholar]
- 675.Kehr J., Yoshitake T., Wang F.H., Razani H., Gimenez-Llort L., Jansson A., Yamaguchi M., Ogren S.O. Galanin is a potent in vivo modulator of mesencephalic serotonergic neurotransmission. . Neuropsychopharmacology. 2002;27(3):341–356. doi: 10.1016/S0893-133X(02)00309-3. [DOI] [PubMed] [Google Scholar]
- 676.Chruścicka B., Wallace Fitzsimons S.E., Borroto-Escuela D.O., Druelle C., Stamou P., Nally K., Dinan T.G., Cryan J.F., Fuxe K., Schellekens H. Attenuation of oxytocin and serotonin 2A receptor signaling through novel heteroreceptor formation. ACS Chem. Neurosci. 2019;10(7):3225–3240. doi: 10.1021/acschemneuro.8b00665. [DOI] [PubMed] [Google Scholar]
- 677.Eaton J.L., Roache L., Nguyen K.N., Cushing B.S., Troyer E., Papademetriou E., Raghanti M.A. Organizational effects of oxytocin on serotonin innervation. Dev. Psychobiol. 2012;54(1):92–97. doi: 10.1002/dev.20566. [DOI] [PubMed] [Google Scholar]
- 678.Lefevre A., Richard N., Jazayeri M., Beuriat P.A., Fieux S., Zimmer L., Duhamel J.R., Sirigu A. Oxytocin and serotonin brain mechanisms in the nonhuman primate. J. Neurosci. 2017;37(28):6741–6750. doi: 10.1523/JNEUROSCI.0659-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 679.Dölen G., Darvishzadeh A., Huang K.W., Malenka R.C. Social reward requires coordinated activity of nucleus accumbens oxytocin and serotonin. Nature. 2013;501(7466):179–184. doi: 10.1038/nature12518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 680.Yoshida M., Takayanagi Y., Inoue K., Kimura T., Young L.J., Onaka T., Nishimori K. Evidence that oxytocin exerts anxiolytic effects via oxytocin receptor expressed in serotonergic neurons in mice. . J. Neurosci. 2009;29(7):2259–2271. doi: 10.1523/JNEUROSCI.5593-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 681.Chruścicka B., Cowan C.S.M., Wallace Fitzsimons S.E., Borroto-Escuela D.O., Druelle C.M., Stamou P., Bergmann C.A., Dinan T.G., Slattery D.A., Fuxe K., Cryan J.F., Schellekens H. Molecular, biochemical and behavioural evidence for a novel oxytocin receptor and serotonin 2C receptor heterocomplex. Neuropharmacology. 2021;183:108394. doi: 10.1016/j.neuropharm.2020.108394. [DOI] [PubMed] [Google Scholar]
- 682.Prosser R.A. Melatonin inhibits in vitro serotonergic phase shifts of the suprachiasmatic circadian clock. . Brain Res. 1999;818(2):408–413. doi: 10.1016/S0006-8993(98)01295-5. [DOI] [PubMed] [Google Scholar]
- 683.Kamal M., Gbahou F., Guillaume J.L., Daulat A.M., Benleulmi-Chaachoua A., Luka M., Chen P., Kalbasi Anaraki D., Baroncini M., Mannoury la Cour C., Millan M.J., Prevot V., Delagrange P., Jockers R. Convergence of melatonin and serotonin (5-HT) signaling at MT2/5-HT2C receptor heteromers. J. Biol. Chem. 2015;290(18):11537–11546. doi: 10.1074/jbc.M114.559542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 684.Millan M.J., Gobert A., Lejeune F., Dekeyne A., Newman-Tancredi A., Pasteau V., Rivet J.M., Cussac D. The novel melatonin agonist agomelatine (S20098) is an antagonist at 5-hydroxytryptamine2C receptors, blockade of which enhances the activity of frontocortical dopaminergic and adrenergic pathways. J. Pharmacol. Exp. Ther. 2003;306(3):954–964. doi: 10.1124/jpet.103.051797. [DOI] [PubMed] [Google Scholar]
- 685.Audinot V., Bonnaud A., Grandcolas L., Rodriguez M., Nagel N., Galizzi J.P., Balik A., Messager S., Hazlerigg D.G., Barrett P., Delagrange P., Boutin J.A. Molecular cloning and pharmacological characterization of rat melatonin MT1 and MT2 receptors. Biochem. Pharmacol. 2008;75(10):2007–2019. doi: 10.1016/j.bcp.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 686.Racagni G., Riva M.A., Molteni R., Musazzi L., Calabrese F., Popoli M., Tardito D. Mode of action of agomelatine: synergy between melatonergic and 5-HT2C receptors. World J. Biol. Psychiatry. 2011;12(8):574–587. doi: 10.3109/15622975.2011.595823. [DOI] [PubMed] [Google Scholar]
- 687.Gerbier R., Ndiaye-Lobry D., Martinez de Morentin P.B., Cecon E., Heisler L.K., Delagrange P., Gbahou F., Jockers R. Pharmacological evidence for transactivation within melatonin MT2 and serotonin 5-HT2C receptor heteromers in mouse brain. FASEB J. 2021;35(1):e21161. doi: 10.1096/fj.202000305R. [DOI] [PubMed] [Google Scholar]
- 688.Kishimoto K., Koyama S., Akaike N. Synergistic μ-opioid and 5-HT1A presynaptic inhibition of GABA release in rat periaqueductal gray neurons. Neuropharmacology. 2001;41(5):529–538. doi: 10.1016/S0028-3908(01)00100-9. [DOI] [PubMed] [Google Scholar]
- 689.Daval G., Vergé D., Basbaum A.I., Bourgoin S., Hamon M. Autoradiographic evidence of serotonin1 binding sites on primary afferent fibres in the dorsal horn of the rat spinal cord. Neurosci. Lett. 1987;83(1-2):71–76. doi: 10.1016/0304-3940(87)90218-7. [DOI] [PubMed] [Google Scholar]
- 690.Pompeiano M., Palacios J.M., Mengod G. Distribution and cellular localization of mRNA coding for 5-HT1A receptor in the rat brain: correlation with receptor binding. J. Neurosci. 1992;12(2):440–453. doi: 10.1523/JNEUROSCI.12-02-00440.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 691.Law P-Y., Wong Y.H., Loh H.H. Molecular mechanisms and regulation of opioid receptor signaling. Annu. Rev. Pharmacol. Toxicol. 2000;40(1):389–430. doi: 10.1146/annurev.pharmtox.40.1.389. [DOI] [PubMed] [Google Scholar]
- 692.Cussac D., Rauly-Lestienne I., Heusler P., Finana F., Cathala C., Bernois S., De Vries L. μ-Opioid and 5-HT1A receptors heterodimerize and show signalling crosstalk via G protein and MAP-kinase pathways. . Cell. Signal. 2012;24(8):1648–1657. doi: 10.1016/j.cellsig.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 693.Milligan G. Insights into ligand pharmacology using receptor-G-protein fusion proteins. Trends Pharmacol. Sci. 2000;21(1):24–28. doi: 10.1016/S0165-6147(99)01404-2. [DOI] [PubMed] [Google Scholar]
- 694.Ashton J.C., Appleton I., Darlington C.L., Smith P.F. Immunohistochemical localization of cerebrovascular cannabinoid CB1 receptor protein. J. Cardiovasc. Pharmacol. 2004;44(5):517–519. doi: 10.1097/00005344-200411000-00001. [DOI] [PubMed] [Google Scholar]
- 695.Smith T.H., Sim-Selley L.J., Selley D.E. Cannabinoid CB1 receptor-interacting proteins: novel targets for central nervous system drug discovery? Br. J. Pharmacol. 2010;160(3):454–466. doi: 10.1111/j.1476-5381.2010.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 696.Munro S., Thomas K.L., Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365(6441):61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 697.Latek D., Kolinski M., Ghoshdastider U., Debinski A., Bombolewski R., Plazinska A. Modeling of ligand binding to G protein coupled receptors: Cannabinoid CB 1, CB 2 and adrenergic β 2AR. J. Mol. Mod. 2011. p. 2353-2366. [DOI] [PubMed]
- 698.Callén L., Moreno E., Barroso-Chinea P., Moreno-Delgado D., Cortés A., Mallol J., Casadó V., Lanciego J.L., Franco R., Lluis C., Canela E.I., McCormick P.J. Cannabinoid receptors CB1 and CB2 form functional heteromers in brain. J. Biol. Chem. 2012;287(25):20851–20865. doi: 10.1074/jbc.M111.335273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 699.Sierra S., Luquin N., Rico A.J., Gómez-Bautista V., Roda E., Dopeso-Reyes I.G., Vázquez A., Martínez-Pinilla E., Labandeira-García J.L., Franco R., Lanciego J.L. Detection of cannabinoid receptors CB1 and CB2 within basal ganglia output neurons in macaques: changes following experimental parkinsonism. Brain Struct. Funct. 2015;220(5):2721–2738. doi: 10.1007/s00429-014-0823-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 700.Pertwee R.G. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol. Ther. 1997;74(2):129–180. doi: 10.1016/S0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 701.Navarro G., Borroto-Escuela D., Angelats E., Etayo Í., Reyes-Resina I., Pulido-Salgado M., Rodríguez-Pérez A.I., Canela E.I., Saura J., Lanciego J.L., Labandeira-García J.L., Saura C.A., Fuxe K., Franco R. Receptor-heteromer mediated regulation of endocannabinoid signaling in activated microglia. Role of CB1 and CB2 receptors and relevance for Alzheimer’s disease and levodopa-induced dyskinesia. Brain Behav. Immun. 2018;67:139–151. doi: 10.1016/j.bbi.2017.08.015. [DOI] [PubMed] [Google Scholar]
- 702.Beltramo M., de Fonseca F.R., Navarro M., Calignano A., Gorriti M.A., Grammatikopoulos G., Sadile A.G., Giuffrida A., Piomelli D. Reversal of dopamine D(2) receptor responses by an anandamide transport inhibitor. J. Neurosci. 2000;20(9):3401–3407. doi: 10.1523/JNEUROSCI.20-09-03401.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 703.Giuffrida A., Parsons L.H., Kerr T.M., Rodríguez de Fonseca F., Navarro M., Piomelli D. Dopamine activation of endogenous cannabinoid signaling in dorsal striatum. Nat. Neurosci. 1999;2(4):358–363. doi: 10.1038/7268. [DOI] [PubMed] [Google Scholar]
- 704.Kearn C.S., Blake-Palmer K., Daniel E., Mackie K., Glass M. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors enhances heterodimer formation: A mechanism for receptor cross-talk? Mol. Pharmacol. 2005;67(5):1697–1704. doi: 10.1124/mol.104.006882. [DOI] [PubMed] [Google Scholar]
- 705.Herkenham M., Lynn A.B., de Costa B.R., Richfield E.K. Neuronal localization of cannabinoid receptors in the basal ganglia of the rat. Brain Res. 1991;547(2):267–274. doi: 10.1016/0006-8993(91)90970-7. [DOI] [PubMed] [Google Scholar]
- 706.Meschler J.P., Howlett A.C. Signal transduction interactions between CB1 cannabinoid and dopamine receptors in the rat and monkey striatum. Neuropharmacology. 2001;40(7):918–926. doi: 10.1016/S0028-3908(01)00012-0. [DOI] [PubMed] [Google Scholar]
- 707.Van Der Stelt M., Di Marzo V. In: European J. Pharmacol; 2003. The endocannabinoid system in the basal ganglia and in the mesolimbic reward system: Implications for neurological and psychiatric disorders. pp. 133–150. [DOI] [PubMed] [Google Scholar]
- 708.Julian M.D., Martin A.B., Cuellar B. Rodriguez, De Fonseca, F.; Navarro, M.; Moratalla, R.; Garcia-Segura, L.M. Neuroanatomical relationship between type 1 cannabinoid receptors and dopaminergic systems in the rat basal ganglia. Neuroscience. 2003;119(1):309–318. doi: 10.1016/S0306-4522(03)00070-8. [DOI] [PubMed] [Google Scholar]
- 709.Terzian A.L., Drago F., Wotjak C.T., Micale V. The dopamine and cannabinoid interaction in the modulation of emotions and cognition: Assessing the role of cannabinoid CB1 receptor in neurons expressing dopamine D1 receptors. Front. Behav. Neurosci. 2011;5:49. doi: 10.3389/fnbeh.2011.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 710.Serrano A., Vadas E., Ferrer B., Bilbao A., Granado N., Suárez J., Pavon F.J., Moratalla R., Rodríguez de Fonseca F. Genetic deletion of dopamine D1 receptors increases the sensitivity to cannabinoid CB1 receptor antagonist-precipitated withdrawal when compared with wild-type littermates: studies in female mice repeatedly exposed to the Spice cannabinoid HU-210. Psychopharmacology (Berl.) 2021;238(2):551–557. doi: 10.1007/s00213-020-05704-8. [DOI] [PubMed] [Google Scholar]
- 711.Egertová M., Elphick M.R. Localisation of cannabinoid receptors in the rat brain using antibodies to the intracellular C-terminal tail of CB. J. Comp. Neurol. 2000;422(2):159–171. doi: 10.1002/(SICI)1096-9861(20000626)422:2<159:AID-CNE1>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 712.Yin H.H., Lovinger D.M. Frequency-specific and D2 receptor-mediated inhibition of glutamate release by retrograde endocannabinoid signaling. Proc. Natl. Acad. Sci. USA. 2006;103(21):8251–8256. doi: 10.1073/pnas.0510797103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 713.Pickel V.M., Chan J., Kearn C.S., Mackie K. Targeting dopamine D2 and cannabinoid-1 (CB1) receptors in rat nucleus accumbens. J. Comp. Neurol. 2006;495(3):299–313. doi: 10.1002/cne.20881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 714.Bagher A.M., Young A.P., Laprairie R.B., Toguri J.T., Kelly M.E.M., Denovan-Wright E.M. Heteromer formation between cannabinoid type 1 and dopamine type 2 receptors is altered by combination cannabinoid and antipsychotic treatments. J. Neurosci. Res. 2020;98(12):2496–2509. doi: 10.1002/jnr.24716. [DOI] [PubMed] [Google Scholar]
- 715.Bagher A.M., Laprairie R.B., Toguri J.T., Kelly M.E.M., Denovan-Wright E.M. Bidirectional allosteric interactions between cannabinoid receptor 1 (CB1) and dopamine receptor 2 long (D2L) heterotetramers. Eur. J. Pharmacol. 2017;813:66–83. doi: 10.1016/j.ejphar.2017.07.034. [DOI] [PubMed] [Google Scholar]
- 716.Bagher A.M., Laprairie R.B., Kelly M.E.M., Denovan-Wright E.M. Antagonism of dopamine receptor 2 long affects cannabinoid receptor 1 signaling in a cell culture model of striatal medium spiny projection neurons. Mol. Pharmacol. 2016;89(6):652–666. doi: 10.1124/mol.116.103465. [DOI] [PubMed] [Google Scholar]
- 717.Pinna A., Bonaventura J., Farré D., Sánchez M., Simola N., Mallol J., Lluís C., Costa G., Baqi Y., Müller C.E., Cortés A., McCormick P., Canela E.I., Martínez-Pinilla E., Lanciego J.L., Casadó V., Armentero M.T., Franco R. L-DOPA disrupts adenosine A(2A)-cannabinoid CB(1)-dopamine D(2) receptor heteromer cross-talk in the striatum of hemiparkinsonian rats: biochemical and behavioral studies. Exp. Neurol. 2014;253:180–191. doi: 10.1016/j.expneurol.2013.12.021. [DOI] [PubMed] [Google Scholar]
- 718.Bonaventura J., Rico A.J., Moreno E., Sierra S., Sánchez M., Luquin N., Farré D., Müller C.E., Martínez-Pinilla E., Cortés A., Mallol J., Armentero M.T., Pinna A., Canela E.I., Lluís C., McCormick P.J., Lanciego J.L., Casadó V., Franco R. L-DOPA-treatment in primates disrupts the expression of A(2A) adenosine-CB(1) cannabinoid-D(2) dopamine receptor heteromers in the caudate nucleus. Neuropharmacology. 2014;79:90–100. doi: 10.1016/j.neuropharm.2013.10.036. [DOI] [PubMed] [Google Scholar]
- 719.Jarrahian A., Watts V.J., Barker E.L. D2 dopamine receptors modulate Galpha-subunit coupling of the CB1 cannabinoid receptor. J. Pharmacol. Exp. Ther. 2004;308(3):880–886. doi: 10.1124/jpet.103.057620. [DOI] [PubMed] [Google Scholar]
- 720.Glass M., Felder C.C. Concurrent stimulation of cannabinoid CB1 and dopamine D2 receptors augments cAMP accumulation in striatal neurons: evidence for a Gs linkage to the CB1 receptor. J. Neurosci. 1997;17(14):5327–5333. doi: 10.1523/JNEUROSCI.17-14-05327.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 721.Marcellino D., Carriba P., Filip M., Borgkvist A., Frankowska M., Bellido I., Tanganelli S., Müller C.E., Fisone G., Lluis C., Agnati L.F., Franco R., Fuxe K. Antagonistic cannabinoid CB1/dopamine D2 receptor interactions in striatal CB1/D2 heteromers. A combined neurochemical and behavioral analysis. Neuropharmacology. 2008;54(5):815–823. doi: 10.1016/j.neuropharm.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 722.Ferré S., Goldberg S.R., Lluis C., Franco R. Looking for the role of cannabinoid receptor heteromers in striatal function. Neuropharmacology. 2009;56(Suppl. 1):226–234. doi: 10.1016/j.neuropharm.2008.06.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 723.Soria G., Castañé A., Berrendero F., Ledent C., Parmentier M., Maldonado R., Valverde O. Adenosine A2A receptors are involved in physical dependence and place conditioning induced by THC. Eur. J. Neurosci. 2004;20(8):2203–2213. doi: 10.1111/j.1460-9568.2004.03682.x. [DOI] [PubMed] [Google Scholar]
- 724.Tebano M.T., Martire A., Chiodi V., Pepponi R., Ferrante A., Domenici M.R., Frank C., Chen J.F., Ledent C., Popoli P. Adenosine A2A receptors enable the synaptic effects of cannabinoid CB1 receptors in the rodent striatum. J. Neurochem. 2009;110(6):1921–1930. doi: 10.1111/j.1471-4159.2009.06282.x. [DOI] [PubMed] [Google Scholar]
- 725.Anderson W.W., Collingridge G.L. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J. Neurosci. Methods. 2001;108(1):71–83. doi: 10.1016/S0165-0270(01)00374-0. [DOI] [PubMed] [Google Scholar]
- 726.Carriba P., Ortiz O., Patkar K., Justinova Z., Stroik J., Themann A., Müller C., Woods A.S., Hope B.T., Ciruela F., Casadó V., Canela E.I., Lluis C., Goldberg S.R., Moratalla R., Franco R., Ferré S. Striatal adenosine A2A and cannabinoid CB1 receptors form functional heteromeric complexes that mediate the motor effects of cannabinoids. Neuropsychopharmacology. 2007;32(11):2249–2259. doi: 10.1038/sj.npp.1301375. [DOI] [PubMed] [Google Scholar]
- 727.Rodríguez de Fonseca F., Rubio P., Menzaghi F., Merlo-Pich E., Rivier J., Koob G.F., Navarro M. Corticotropin-releasing factor (CRF) antagonist [D-Phe12,Nle21,38,C alpha MeLeu37]CRF attenuates the acute actions of the highly potent cannabinoid receptor agonist HU-210 on defensive-withdrawal behavior in rats. J. Pharmacol. Exp. Ther. 1996;276(1):56–64. [PubMed] [Google Scholar]
- 728.Castellano C., Rossi-Arnaud C., Cestari V., Costanzi M. Cannabinoids and memory: Animal studies. Curr. Drug Targets CNS Neurol. Disord. 2003;2(6):389–402. doi: 10.2174/1568007033482670. [DOI] [PubMed] [Google Scholar]
- 729.Moreira F.A., Lutz B. The endocannabinoid system: emotion, learning and addiction. Addict. Biol. 2008;13(2):196–212. doi: 10.1111/j.1369-1600.2008.00104.x. [DOI] [PubMed] [Google Scholar]
- 730.Maldonado R., Berrendero F., Ozaita A., Robledo P. Neurochemical basis of cannabis addiction. Neuroscience. 2011;181:1–17. doi: 10.1016/j.neuroscience.2011.02.035. [DOI] [PubMed] [Google Scholar]
- 731.Viñals X., Moreno E., Lanfumey L., Cordomí A., Pastor A., de La Torre R., Gasperini P., Navarro G., Howell L.A., Pardo L., Lluís C., Canela E.I., McCormick P.J., Maldonado R., Robledo P. Cognitive impairment induced by delta9- tetrahydrocannabinol occurs through heteromers between cannabinoid CB1 and serotonin 5-HT2A receptors. PLoS Biol. 2015;13(7):e1002194. doi: 10.1371/journal.pbio.1002194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 732.Gorzalka B.B., Hill M.N., Sun J.C. Functional role of the endocannabinoid system and AMPA/kainate receptors in 5-HT2A receptor-mediated wet dog shakes. Eur. J. Pharmacol. 2005;516(1):28–33. doi: 10.1016/j.ejphar.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 733.Darmani N.A. Cannabinoids of diverse structure inhibit two DOI-induced 5-HT(2A) receptor-mediated behaviors in mice. Pharmacol. Biochem. Behav. 2001;68(2):311–317. doi: 10.1016/S0091-3057(00)00477-9. [DOI] [PubMed] [Google Scholar]
- 734.Cheer J.F., Cadogan A.K., Marsden C.A., Fone K.C.F., Kendall D.A. Modification of 5-HT2 receptor mediated behaviour in the rat by oleamide and the role of cannabinoid receptors. Neuropharmacology. 1999;38(4):533–541. doi: 10.1016/S0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]
- 735.de Almeida J., Mengod G. Quantitative analysis of glutamatergic and GABAergic neurons expressing 5-HT(2A) receptors in human and monkey prefrontal cortex. J. Neurochem. 2007;103(2):475–486. doi: 10.1111/j.1471-4159.2007.04768.x. [DOI] [PubMed] [Google Scholar]
- 736.Mechoulam R., Parker L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013;64(1):21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 737.Bombardi C., Di Giovanni G. Functional anatomy of 5-HT2A receptors in the amygdala and hippocampal complex: relevance to memory functions. Exp. Brain Res. 2013;230(4):427–439. doi: 10.1007/s00221-013-3512-6. [DOI] [PubMed] [Google Scholar]
- 738.Galindo L., Moreno E., López-Armenta F., Guinart D., Cuenca-Royo A., Izquierdo-Serra M., Xicota L., Fernandez C., Menoyo E., Fernández-Fernández J.M., Benítez-King G., Canela E.I., Casadó V., Pérez V., de la Torre R., Robledo P. Cannabis users show enhanced expression of CB1-5HT2A receptor heteromers in olfactory neuroepithelium cells. Mol. Neurobiol. 2018;55(8):6347–6361. doi: 10.1007/s12035-017-0833-7. [DOI] [PubMed] [Google Scholar]
- 739.Borroto-Escuela D.O., Narvaez M., Di Palma M., Calvo F., Rodriguez D., Millon C., Carlsson J., Agnati L.F., Garriga P., Díaz-Cabiale Z., Fuxe K. Preferential activation by galanin 1-15 fragment of the GalR1 protomer of a GalR1-GalR2 heteroreceptor complex. Biochem. Biophys. Res. Commun. 2014;452(3):347–353. doi: 10.1016/j.bbrc.2014.08.061. [DOI] [PubMed] [Google Scholar]
- 740.Millón C., Flores-Burgess A., Narváez M., Borroto-Escuela D.O., Santín L., Parrado C. A role for galanin N-terminal fragment (1-15) in anxiety-and depression-related behaviors in Ra. Int. J. Neuropsychopharmacol. 2015;18(3):1–13. doi: 10.1093/ijnp/pyu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 741.Fuxe K., Borroto-Escuela D.O., Romero-Fernandez W., Tarakanov A.O., Calvo F., Garriga P., Tena M., Narvaez M., Millón C., Parrado C., Ciruela F., Agnati L.F., Narvaez J.A., Díaz-Cabiale Z. On the existence and function of galanin receptor heteromers in the central nervous system. Front. Endocrinol. (Lausanne) 2012;3(OCT):127. doi: 10.3389/fendo.2012.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 742.Rivas-Santisteban R., Rodriguez-Perez A.I., Muñoz A., Reyes-Resina I., Labandeira-García J.L., Navarro G. Angiotensin AT1and AT2receptor heteromer expression in the hemilesioned rat model of Parkinson’s disease that increases with levodopa-induced dyskinesia. J. Neuroinflammation. 2020;17(1):1–16. doi: 10.1186/s12974-020-01908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 743.Perez-Lloret S., Otero-Losada M., Toblli J.E., Capani F. Renin-angiotensin system as a potential target for new therapeutic approaches in Parkinson’s disease. Expert Opin. Investig. Drugs. 2017;26(10):1163–1173. doi: 10.1080/13543784.2017.1371133. [DOI] [PubMed] [Google Scholar]
- 744.Muñoz A., Garrido-Gil P., Dominguez-Meijide A., Labandeira-Garcia J.L. Angiotensin type 1 receptor blockage reduces l-dopa-induced dyskinesia in the 6-OHDA model of Parkinson’s disease. Involvement of vascular endothelial growth factor and interleukin-1β. Exp. Neurol. 2014;261:720–732. doi: 10.1016/j.expneurol.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 745.Pin J.P., Galvez T., Prézeau L. Evolution, structure, and activation mechanism of family 3/C G-protein-coupled receptors. Pharmacol. Ther. 2003;98(3):325–354. doi: 10.1016/S0163-7258(03)00038-X. [DOI] [PubMed] [Google Scholar]
- 746.Rondard P., Goudet C., Kniazeff J., Pin J.P., Prézeau L. The complexity of their activation mechanism opens new possibilities for the modulation of mGlu and GABAB class C G protein-coupled receptors. Neuropharmacology. 2011;60(1):82–92. doi: 10.1016/j.neuropharm.2010.08.009. [DOI] [PubMed] [Google Scholar]
- 747.Urwyler S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol. Rev. 2011;63(1):59–126. doi: 10.1124/pr.109.002501. [DOI] [PubMed] [Google Scholar]
- 748.Chun L., Zhang W.H., Liu J.F. Structure and ligand recognition of class C GPCRs. Acta Pharmacol. Sin. 2012;33(3):312–323. doi: 10.1038/aps.2011.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 749.Binet V., Duthey B., Lecaillon J., Vol C., Quoyer J., Labesse G., Pin J.P., Prézeau L. Common structural requirements for heptahelical domain function in class A and class C G protein-coupled receptors. J. Biol. Chem. 2007;282(16):12154–12163. doi: 10.1074/jbc.M611071200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 750.Kunishima N., Shimada Y., Tsuji Y., Sato T., Yamamoto M., Kumasaka T., Nakanishi S., Jingami H., Morikawa K. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407(6807):971–977. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 751.Tsuchiya D., Kunishima N., Kamiya N., Jingami H., Morikawa K. Structural views of the ligand-binding cores of a metabotropic glutamate receptor complexed with an antagonist and both glutamate and Gd3+. Proc. Natl. Acad. Sci. USA. 2002;99(5):2660–2665. doi: 10.1073/pnas.052708599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 752.Conn P.J., Lindsley C.W., Jones C.K. Activation of metabotropic glutamate receptors as a novel approach for the treatment of schizophrenia. Trends Pharmacol. Sci. 2009;30(1):25–31. doi: 10.1016/j.tips.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 753.Bessis A-S., Rondard P., Gaven F., Brabet I., Triballeau N., Prezeau L., Acher F., Pin J.P. Closure of the Venus flytrap module of mGlu8 receptor and the activation process: Insights from mutations converting antagonists into agonists. Proc. Natl. Acad. Sci. USA. 2002;99(17):11097–11102. doi: 10.1073/pnas.162138699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 754.Romano C., Miller J.K., Hyrc K., Dikranian S., Mennerick S., Takeuchi Y., Goldberg M.P., O’Malley K.L. Covalent and noncovalent interactions mediate metabotropic glutamate receptor mGlu5 dimerization. Mol. Pharmacol. 2001;59(1):46–53. doi: 10.1124/mol.59.1.46. [DOI] [PubMed] [Google Scholar]
- 755.Tsuji Y., Shimada Y., Takeshita T., Kajimura N., Nomura S., Sekiyama N., Otomo J., Usukura J., Nakanishi S., Jingami H. Cryptic dimer interface and domain organization of the extracellular region of metabotropic glutamate receptor subtype 1. J. Biol. Chem. 2000;275(36):28144–28151. doi: 10.1074/jbc.M003226200. [DOI] [PubMed] [Google Scholar]
- 756.Ray K., Hauschild B.C. Cys-140 is critical for metabotropic glutamate receptor-1 dimerization. J. Biol. Chem. 2000;275(44):34245–34251. doi: 10.1074/jbc.M005581200. [DOI] [PubMed] [Google Scholar]
- 757.Ray K., Hauschild B.C., Steinbach P.J., Goldsmith P.K., Hauache O., Spiegel A.M. Identification of the cysteine residues in the amino-terminal extracellular domain of the human Ca(2+) receptor critical for dimerization. Implications for function of monomeric Ca(2+) receptor. J. Biol. Chem. 1999;274(39):27642–27650. doi: 10.1074/jbc.274.39.27642. [DOI] [PubMed] [Google Scholar]
- 758.Muto T., Tsuchiya D., Morikawa K., Jingami H. Structures of the extracellular regions of the group II/III metabotropic glutamate receptors. Proc. Natl. Acad. Sci. USA. 2007;104(10):3759–3764. doi: 10.1073/pnas.0611577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 759.Rondard P., Liu J., Huang S., Malhaire F., Vol C., Pinault A., Labesse G., Pin J.P. Coupling of agonist binding to effector domain activation in metabotropic glutamate-like receptors. J. Biol. Chem. 2006;281(34):24653–24661. doi: 10.1074/jbc.M602277200. [DOI] [PubMed] [Google Scholar]
- 760.Hu J., Hauache O., Spiegel A.M. Human Ca2+ receptor cysteine-rich domain. Analysis of function of mutant and chimeric receptors. J. Biol. Chem. 2000;275(21):16382–16389. doi: 10.1074/jbc.M000277200. [DOI] [PubMed] [Google Scholar]
- 761.Brown E.M. Clinical lessons from the calcium-sensing receptor. Nat. Clin. Pract. Endocrinol. Metab. 2007;3(2):122–133. doi: 10.1038/ncpendmet0388. [DOI] [PubMed] [Google Scholar]
- 762.Deal C. Future therapeutic targets in osteoporosis. Curr. Opin. Rheumatol. 2009;21(4):380–385. doi: 10.1097/BOR.0b013e32832cbc2a. [DOI] [PubMed] [Google Scholar]
- 763.Brown E.M. Anti-parathyroid and anti-calcium sensing receptor antibodies in autoimmune hypoparathyroidism. Endocrinol. Metab. Clin. North Am. 2009;38(2):437–445. doi: 10.1016/j.ecl.2009.01.001. x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 764.Burger A. Progress in Medicinal Chemistry. J. Med. Chem. 1963;6(6):827. [Google Scholar]
- 765.Gao Y., Robertson M.J., Rahman S.N., Seven A.B., Zhang C., Meyerowitz J.G., Panova O., Hannan F.M., Thakker R.V., Bräuner-Osborne H., Mathiesen J.M., Skiniotis G. Asymmetric activation of the calcium-sensing receptor homodimer. Nature. 2021;595(7867):455–459. doi: 10.1038/s41586-021-03691-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 766.Yano S., Brown E.M., Chattopadhyay N. Calcium-sensing receptor in the brain. Churchill Livingstone; 2004. pp. 257–264. [DOI] [PubMed] [Google Scholar]
- 767.Giudice M.L., Mihalik B., Dinnyés A., Kobolák J. The nervous system relevance of the calcium sensing receptor in health and disease. Molecules. 2019;24(14):2546. doi: 10.3390/molecules24142546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 768.Berridge M.J. Neuronal calcium signaling. Neuron. 1998;21(1):13–26. doi: 10.1016/S0896-6273(00)80510-3. [DOI] [PubMed] [Google Scholar]
- 769.Schrank S., Barrington N., Stutzmann G.E. Calcium-handling defects and neurodegenerative disease. Cold Spring Harb. Perspect. Biol. 2020;12(7):1–25. doi: 10.1101/cshperspect.a035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 770.Kawamoto E.M., Vivar C., Camandola S. Physiology and pathology of calcium signaling in the brain. Front. Pharmacol. 2012;3:61. doi: 10.3389/fphar.2012.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 771.Khachaturian Z.S. Hypothesis on the regulation of cytosol calcium concentration and the aging brain. Neurobiol. Aging. 1987;8(4):345–346. doi: 10.1016/0197-4580(87)90073-X. [DOI] [PubMed] [Google Scholar]
- 772.Mattson M.P., Pedersen W.A., Duan W., Culmsee C., Camandola S. Cellular and molecular mechanisms underlying perturbed energy metabolism and neuronal degeneration in Alzheimer’s and Parkinson’s diseases. In: Annals of the New York Academy of Sciences, 1999:pp. 154-175. doi: 10.1111/j.1749-6632.1999.tb07824.x. [DOI] [PubMed] [Google Scholar]
- 773.Surmeier D.J., Schumacker P.T., Guzman J.D., Ilijic E., Yang B., Zampese E. Calcium and Parkinson’s disease. Biochem. Biophys. Res. Commun. 2017;483(4):1013–1019. doi: 10.1016/j.bbrc.2016.08.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 774.Stutzmann G.E., Smith I., Caccamo A., Oddo S., Laferla F.M., Parker I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006;26(19):5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 775.Pchitskaya E., Popugaeva E., Bezprozvanny I. Calcium signaling and molecular mechanisms underlying neurodegenerative diseases. Cell Calcium. 2018;70:87–94. doi: 10.1016/j.ceca.2017.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 776.Bettler B., Tiao J.Y.H. Molecular diversity, trafficking and subcellular localization of GABAB receptors. Pharmacol. Ther. 2006;110(3):533–543. doi: 10.1016/j.pharmthera.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 777.Sakamaki K., Nomura M., Hatakenaka S., Miyakubo H., Tanaka J. GABAergic modulation of noradrenaline release in the median preoptic nucleus area in the rat. Neurosci. Lett. 2003;342(1-2):77–80. doi: 10.1016/S0304-3940(03)00242-8. [DOI] [PubMed] [Google Scholar]
- 778.Waldmeier P.C., Kaupmann K., Urwyler S. Roles of GABAB receptor subtypes in presynaptic auto- and heteroreceptor function regulating GABA and glutamate release. J. Neural Transm. (Vienna) 2008;115(10):1401–1411. doi: 10.1007/s00702-008-0095-7. [DOI] [PubMed] [Google Scholar]
- 779.Jones K.A., Borowsky B., Tamm J.A., Craig D.A., Durkin M.M., Dai M., Yao W.J., Johnson M., Gunwaldsen C., Huang L.Y., Tang C., Shen Q., Salon J.A., Morse K., Laz T., Smith K.E., Nagarathnam D., Noble S.A., Branchek T.A., Gerald C. GABA(B) receptors function as a heteromeric assembly of the subunits GABA(B)R1 and GABA(B)R2. Nature. 1998;396(6712):674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 780.Kaupmann K., Malitschek B., Schuler V., Heid J., Froestl W., Beck P., Mosbacher J., Bischoff S., Kulik A., Shigemoto R., Karschin A., Bettler B. GABA(B)-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998;396(6712):683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 781.Fatemi S.H., Folsom T.D., Thuras P.D. Deficits in GABA(B) receptor system in schizophrenia and mood disorders: A postmortem study. Schizophr. Res. 2011;128(1-3):37–43. doi: 10.1016/j.schres.2010.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 782.Nyitrai G., Kékesi K.A., Emri Z., Szárics E., Juhász G., Kardos J. GABA(B) receptor antagonist CGP-36742 enhances somatostatin release in the rat hippocampus in vivo and in vitro . . Eur. J. Pharmacol. 2003;478(2-3):111–119. doi: 10.1016/j.ejphar.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 783.Galvez T., Parmentier M.L., Joly C., Malitschek B., Kaupmann K., Kuhn R., Bittiger H., Froestl W., Bettler B., Pin J.P. Mutagenesis and modeling of the GABAB receptor extracellular domain support a venus flytrap mechanism for ligand binding. J. Biol. Chem. 1999;274(19):13362–13369. doi: 10.1074/jbc.274.19.13362. [DOI] [PubMed] [Google Scholar]
- 784.Margeta-Mitrovic M., Jan Y.N., Jan L.Y. Function of GB1 and GB2 subunits in G protein coupling of GABA(B) receptors. Proc. Natl. Acad. Sci. USA. 2001;98(25):14649–14654. doi: 10.1073/pnas.251554498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 785.Li C jun, Lu Y, Zhou M, Zong X gang, Li C, Xu X lin. Activation of GABAB receptors ameliorates cognitive impairment via restoring the balance of HCN1/HCN2 surface expression in the hippocampal CA1 area in rats with chronic cerebral hypoperfusion. Mol. Neurobiol. 2014;50(2):704–720. doi: 10.1007/s12035-014-8736-3. [DOI] [PubMed] [Google Scholar]
- 786.Kuramoto N., Wilkins M.E., Fairfax B.P., Revilla-Sanchez R., Terunuma M., Tamaki K., Iemata M., Warren N., Couve A., Calver A., Horvath Z., Freeman K., Carling D., Huang L., Gonzales C., Cooper E., Smart T.G., Pangalos M.N., Moss S.J. Phospho-dependent functional modulation of GABA(B) receptors by the metabolic sensor AMP-dependent protein kinase. Neuron. 2007;53(2):233–247. doi: 10.1016/j.neuron.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 787.Dave K.R., Lange-Asschenfeldt C., Raval A.P., Prado R., Busto R., Saul I., Pérez-Pinzón M.A. Ischemic preconditioning ameliorates excitotoxicity by shifting glutamate/γ-aminobutyric acid release and biosynthesis. J. Neurosci. Res. 2005;82(5):665–673. doi: 10.1002/jnr.20674. [DOI] [PubMed] [Google Scholar]
- 788.Tu H., Xu C., Zhang W., Liu Q., Rondard P., Pin J.P., Liu J. GABAB receptor activation protects neurons from apoptosis via IGF-1 receptor transactivation. . J. Neurosci. 2010;30(2):749–759. doi: 10.1523/JNEUROSCI.2343-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 789.Cryan J.F., Kaupmann K. Don’t worry ‘B’ happy!: A role for GABA(B) receptors in anxiety and depression. Trends Pharmacol. Sci. 2005;26(1):36–43. doi: 10.1016/j.tips.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 790.Bowery N.G. GABAB receptor: A site of therapeutic benefit. Curr. Opin. Pharmacol. 2006;6(1):37–43. doi: 10.1016/j.coph.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 791.Goudet C., Magnaghi V., Landry M., Nagy F., Gereau R.W., IV, Pin J.P. Metabotropic receptors for glutamate and GABA in pain. Brain Res. Brain Res. Rev. 2009;60(1):43–56. doi: 10.1016/j.brainresrev.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 792.Boczek T., Mackiewicz J., Sobolczyk M., Wawrzyniak J., Lisek M., Ferenc B., Guo F., Zylinska L. The role of G protein-coupled receptors (GPCRs) and calcium signaling in schizophrenia. focus on GPCRs activated by neurotransmitters and chemokines. Cells. 2021;10(5):1228. doi: 10.3390/cells10051228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 793.Niswender C.M., Conn P.J. Metabotropic glutamate receptors: physiology, pharmacology, and disease. Annu. Rev. Pharmacol. Toxicol. 2010;50(1):295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 794.Conn P.J., Pin J-P. Pharmacology and functions of metabotropic glutamate receptors. Annu. Rev. Pharmacol. Toxicol. 1997;37(1):205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- 795.Nicoletti F., Bockaert J., Collingridge G.L., Conn P.J., Ferraguti F., Schoepp D.D., Wroblewski J.T., Pin J.P. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60(7-8):1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 796.Iyer A.M., van Scheppingen J., Milenkovic I., Anink J.J., Lim D., Genazzani A.A., Adle-Biassette H., Kovacs G.G., Aronica E. Metabotropic glutamate receptor 5 in Down’s syndrome hippocampus during development: increased expression in astrocytes. Curr. Alzheimer Res. 2014;11(7):694–705. doi: 10.2174/1567205011666140812115423. [DOI] [PubMed] [Google Scholar]
- 797.Spampinato S.F., Copani A., Nicoletti F., Sortino M.A., Caraci F. Metabotropic glutamate receptors in glial cells: A new potential target for neuroprotection? Front. Mol. Neurosci. 2018;11:414. doi: 10.3389/fnmol.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 798.Chen X., Lin R., Chang L., Xu S., Wei X., Zhang J., Wang C., Anwyl R., Wang Q. Enhancement of long-term depression by soluble amyloid β protein in rat hippocampus is mediated by metabotropic glutamate receptor and involves activation of p38MAPK, STEP and caspase-3. Neuroscience. 2013;253:435–443. doi: 10.1016/j.neuroscience.2013.08.054. [DOI] [PubMed] [Google Scholar]
- 799.Renner M., Lacor P.N., Velasco P.T., Xu J., Contractor A., Klein W.L., Triller A. Deleterious effects of amyloid β oligomers acting as an extracellular scaffold for mGluR5. Neuron. 2010;66(5):739–754. doi: 10.1016/j.neuron.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 800.Caraci F., Molinaro G., Battaglia G., Giuffrida M.L., Riozzi B., Traficante A., Bruno V., Cannella M., Merlo S., Wang X., Heinz B.A., Nisenbaum E.S., Britton T.C., Drago F., Sortino M.A., Copani A., Nicoletti F. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: selective activation of mGlu2 receptors amplifies β-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol. Pharmacol. 2011;79(3):618–626. doi: 10.1124/mol.110.067488. [DOI] [PubMed] [Google Scholar]
- 801.White J.H., Wise A., Main M.J., Green A., Fraser N.J., Disney G.H., Barnes A.A., Emson P., Foord S.M., Marshall F.H. Heterodimerization is required for the formation of a functional GABA(B) receptor. Nature. 1998;396(6712):679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 802.Stewart G.D., Comps-Agrar L., Nørskov-Lauritsen L.B., Pin J.P., Kniazeff J. Allosteric interactions between GABAB1 subunits control orthosteric binding sites occupancy within GABAB oligomers. Neuropharmacology. 2018;136((Pt A)):92-101. doi: 10.1016/j.neuropharm.2017.12.042. [DOI] [PubMed] [Google Scholar]
- 803.Pin J.P., Kniazeff J., Prézeau L., Liu J.F., Rondard P. GPCR interaction as a possible way for allosteric control between receptors. Mol. Cell. Endocrinol. 2019;486:89–95. doi: 10.1016/j.mce.2019.02.019. [DOI] [PubMed] [Google Scholar]
- 804.Koehl A., Hu H., Feng D., Sun B., Zhang Y., Robertson M.J., Chu M., Kobilka T.S., Laeremans T., Steyaert J., Tarrasch J., Dutta S., Fonseca R., Weis W.I., Mathiesen J.M., Skiniotis G., Kobilka B.K. Structural insights into the activation of metabotropic glutamate receptors. Nature. 2019;566(7742):79–84. doi: 10.1038/s41586-019-0881-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 805.Ellaithy A., Gonzalez-Maeso J., Logothetis D.A., Levitz J. Structural and biophysical mechanisms of class C G protein-coupled receptor function. Trends Biochem. Sci. 2020;45(12):1049–1064. doi: 10.1016/j.tibs.2020.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 806.Pagano A., Rovelli G., Mosbacher J., Lohmann T., Duthey B., Stauffer D., Ristig D., Schuler V., Meigel I., Lampert C., Stein T., Prezeau L., Blahos J., Pin J., Froestl W., Kuhn R., Heid J., Kaupmann K., Bettler B. C-terminal interaction is essential for surface trafficking but not for heteromeric assembly of GABA(b) receptors. J. Neurosci. 2001;21(4):1189–1202. doi: 10.1523/JNEUROSCI.21-04-01189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 807.Couve A., Filippov A.K., Connolly C.N., Bettler B., Brown D.A., Moss S.J. Intracellular retention of recombinant GABAB receptors. J. Biol. Chem. 1998;273(41):26361–26367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- 808.Duthey B., Caudron S., Perroy J., Bettler B., Fagni L., Pin J.P., Prézeau L. A single subunit (GB2) is required for G-protein activation by the heterodimeric GABA(B) receptor. J. Biol. Chem. 2002;277(5):3236–3241. doi: 10.1074/jbc.M108900200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 809.Robbins M.J., Calver A.R., Filippov A.K., Hirst W.D., Russell R.B., Wood M.D., Nasir S., Couve A., Brown D.A., Moss S.J., Pangalos M.N. GABA(B2) is essential for g-protein coupling of the GABA(B) receptor heterodimer. J. Neurosci. 2001;21(20):8043–8052. doi: 10.1523/JNEUROSCI.21-20-08043.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 810.Galvez T., Duthey B., Kniazeff J., Blahos J., Rovelli G., Bettler B., Prézeau L., Pin J.P. Allosteric interactions between GB1 and GB2 subunits are required for optimal GABA(B) receptor function. EMBO J. 2001;20(9):2152–2159. doi: 10.1093/emboj/20.9.2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 811.Margeta-Mitrovic M., Jan Y.N., Jan L.Y. Ligand-induced signal transduction within heterodimeric GABA(B) receptor. Proc. Natl. Acad. Sci. USA. 2001;98(25):14643–14648. doi: 10.1073/pnas.251554798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 812.Fritzius T., Bettler B. The organizing principle of GABAB receptor complexes: Physiological and pharmacological implications. Basic Clin. Pharmacol. Toxicol. 2020;126(S6) Suppl. 6:25–34. doi: 10.1111/bcpt.13241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 813.Benke D. GABAB receptor trafficking and interacting proteins: targets for the development of highly specific therapeutic strategies to treat neurological disorders? Biochem. Pharmacol. 2013;86(11):1525–1530. doi: 10.1016/j.bcp.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 814.Calebiro D., Rieken F., Wagner J., Sungkaworn T., Zabel U., Borzi A., Cocucci E., Zürn A., Lohse M.J. Single-molecule analysis of fluorescently labeled G-protein-coupled receptors reveals complexes with distinct dynamics and organization. Proc. Natl. Acad. Sci. USA. 2013;110(2):743–748. doi: 10.1073/pnas.1205798110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 815.Kent C.N., Park C., Lindsley C.W. Classics in Chemical Neuroscience: Baclofen. ACS Chem. Neurosci. 2020;11(12):1740–1755. doi: 10.1021/acschemneuro.0c00254. [DOI] [PubMed] [Google Scholar]
- 816.Agabio R., Sinclair J.M., Addolorato G., Aubin H-J., Beraha E.M., Caputo F., Chick J.D., de La Selle P., Franchitto N., Garbutt J.C., Haber P.S., Heydtmann M., Jaury P., Lingford-Hughes A.R., Morley K.C., Müller C.A., Owens L., Pastor A., Paterson L.M., Pélissier F., Rolland B., Stafford A., Thompson A., van den Brink W., de Beaurepaire R., Leggio L. Baclofen for the treatment of alcohol use disorder: the Cagliari Statement. Lancet Psychiatry. 2018;5(12):957–960. doi: 10.1016/S2215-0366(18)30303-1. [DOI] [PubMed] [Google Scholar]
- 817.Pin J-P., Kniazeff J., Liu J., Binet V., Goudet C., Rondard P., Prézeau L. Allosteric functioning of dimeric class C G-protein-coupled receptors. FEBS J. 2005;272(12):2947–2955. doi: 10.1111/j.1742-4658.2005.04728.x. [DOI] [PubMed] [Google Scholar]
- 818.El Moustaine D., Granier S., Doumazane E., Scholler P., Rahmeh R., Bron P., Mouillac B., Banères J.L., Rondard P., Pin J.P. Distinct roles of metabotropic glutamate receptor dimerization in agonist activation and G-protein coupling. Proc. Natl. Acad. Sci. USA. 2012;109(40):16342–16347. doi: 10.1073/pnas.1205838109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 819.Morató X., Luján R., Gonçalves N., Watanabe M., Altafaj X., Carvalho A.L., Fernández-Dueñas V., Cunha R.A., Ciruela F. Metabotropic glutamate type 5 receptor requires contactin-associated protein 1 to control memory formation. Hum. Mol. Genet. 2018;27(20):3528–3541. doi: 10.1093/hmg/ddy264. [DOI] [PubMed] [Google Scholar]
- 820.García-Negredo G., Soto D., Llorente J., Morató X., Galenkamp K.M.O., Gómez-Soler M., Fernández-Dueñas V., Watanabe M., Adelman J.P., Shigemoto R., Fukazawa Y., Luján R., Ciruela F. Coassembly and coupling of SK2 channels and mGlu5 receptors. J. Neurosci. 2014;34(44):14793–14802. doi: 10.1523/JNEUROSCI.2038-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 821.Fagni L., Chavis P., Ango F., Bockaert J. Complex interactions between mGluRs, intracellular Ca2+ stores and ion channels in neurons. Trends Neurosci. 2000;23(2):80–88. doi: 10.1016/S0166-2236(99)01492-7. [DOI] [PubMed] [Google Scholar]
- 822.Canela L., Fernández-Dueñas V., Albergaria C., Watanabe M., Lluís C., Mallol J., Canela E.I., Franco R., Luján R., Ciruela F. The association of metabotropic glutamate receptor type 5 with the neuronal Ca2+-binding protein 2 modulates receptor function. J. Neurochem. 2009;111(2):555–567. doi: 10.1111/j.1471-4159.2009.06348.x. [DOI] [PubMed] [Google Scholar]
- 823.Bockaert J., Perroy J., Bécamel C., Marin P., Fagni L. GPCR interacting proteins (GIPs) in the nervous system: Roles in physiology and pathologies. Annu. Rev. Pharmacol. Toxicol. 2010;50(1):89–109. doi: 10.1146/annurev.pharmtox.010909.105705. [DOI] [PubMed] [Google Scholar]
- 824.Goudet C., Kniazeff J., Hlavackova V., Malhaire F., Maurel D., Acher F., Blahos J., Prézeau L., Pin J.P. Asymmetric functioning of dimeric metabotropic glutamate receptors disclosed by positive allosteric modulators. J. Biol. Chem. 2005;280(26):24380–24385. doi: 10.1074/jbc.M502642200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 825.Kammermeier P.J. Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol. Pharmacol. 2012;82(3):438–447. doi: 10.1124/mol.112.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 826.Pandya N.J., Klaassen R.V., van der Schors R.C., Slotman J.A., Houtsmuller A., Smit A.B., Li K.W. Group 1 metabotropic glutamate receptors 1 and 5 form a protein complex in mouse hippocampus and cortex. Proteomics. 2016;16(20):2698–2705. doi: 10.1002/pmic.201500400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 827.Hayashi M.K., Tang C., Verpelli C., Narayanan R., Stearns M.H., Xu R.M., Li H., Sala C., Hayashi Y. The postsynaptic density proteins Homer and Shank form a polymeric network structure. Cell. 2009;137(1):159–171. doi: 10.1016/j.cell.2009.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 828.Jong Y-J.I., Sergin I., Purgert C.A., O’Malley K.L. Location-dependent signaling of the group 1 metabotropic glutamate receptor mGlu5. Mol. Pharmacol. 2014;86(6):774–785. doi: 10.1124/mol.114.094763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 829.Damian M., Martin A., Mesnier D., Pin J.P., Banères J.L. Asymmetric conformational changes in a GPCR dimer controlled by G-proteins. EMBO J. 2006;25(24):5693–5702. doi: 10.1038/sj.emboj.7601449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 830.Albizu L., Cottet M., Kralikova M., Stoev S., Seyer R., Brabet I., Roux T., Bazin H., Bourrier E., Lamarque L., Breton C., Rives M.L., Newman A., Javitch J., Trinquet E., Manning M., Pin J.P., Mouillac B., Durroux T. Time-resolved FRET between GPCR ligands reveals oligomers in native tissues. Nat. Chem. Biol. 2010;6(8):587–594. doi: 10.1038/nchembio.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 831.Sebastianutto I., Goyet E., Andreoli L., Font-Ingles J., Moreno-Delgado D., Bouquier N., Jahannault-Talignani C., Moutin E., Di Menna L., Maslava N., Pin J.P., Fagni L., Nicoletti F., Ango F., Cenci M.A., Perroy J. D1-mGlu5 heteromers mediate noncanonical dopamine signaling in Parkinson’s disease. J. Clin. Invest. 2020;130(3):1168–1184. doi: 10.1172/JCI126361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 832.Surmeier D.J., Graves S.M., Shen W. Dopaminergic modulation of striatal networks in health and Parkinson’s disease. Curr. Opin. Neurobiol. 2014;29:109–117. doi: 10.1016/j.conb.2014.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 833.Bagetta V., Ghiglieri V., Sgobio C., Calabresi P., Picconi B. Synaptic dysfunction in Parkinson’s disease. Biochem. Soc. Trans. 2010;38(2):493–497. doi: 10.1042/BST0380493. [DOI] [PubMed] [Google Scholar]
- 834.Ciruela F., Escriche M., Burgueño J., Angulo E., Casadó V., Soloviev M.M., Canela E.I., Mallol J., Chan W.Y., Lluis C., McIlhinney R.A., Franco R. Metabotropic glutamate 1α and adenosine A1 receptors assemble into functionally interacting complexes. J. Biol. Chem. 2001;276(21):18345–18351. doi: 10.1074/jbc.M006960200. [DOI] [PubMed] [Google Scholar]
- 835.Kamikubo Y., Tabata T., Sakairi H., Hashimoto Y., Sakurai T. Complex formation and functional interaction between adenosine A1 receptor and type-1 metabotropic glutamate receptor. J. Pharmacol. Sci. 2015;128(3):125–130. doi: 10.1016/j.jphs.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 836.Kamikubo Y., Shimomura T., Fujita Y., Tabata T., Kashiyama T., Sakurai T., Fukurotani K., Kano M. Functional cooperation of metabotropic adenosine and glutamate receptors regulates postsynaptic plasticity in the cerebellum. J. Neurosci. 2013;33(47):18661–18671. doi: 10.1523/JNEUROSCI.5567-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 837.Klinger M., Freissmuth M., Nanoff C. Adenosine receptors: G protein-mediated signalling and the role of accessory proteins. Cell. Signal. 2002;14(2):99–108. doi: 10.1016/S0898-6568(01)00235-2. [DOI] [PubMed] [Google Scholar]
- 838.Moreno J.L., Muguruza C., Umali A., Mortillo S., Holloway T., Pilar-Cuéllar F., Mocci G., Seto J., Callado L.F., Neve R.L., Milligan G., Sealfon S.C., López-Giménez J.F., Meana J.J., Benson D.L., González-Maeso J. Identification of three residues essential for 5-hydroxytryptamine 2A-metabotropic glutamate 2 (5-HT2A·mGlu2) receptor heteromerization and its psychoactive behavioral function. J. Biol. Chem. 2012;287(53):44301–44319. doi: 10.1074/jbc.M112.413161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 839.González-Maeso J., Ang R.L., Yuen T., Chan P., Weisstaub N.V., López-Giménez J.F., Zhou M., Okawa Y., Callado L.F., Milligan G., Gingrich J.A., Filizola M., Meana J.J., Sealfon S.C. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452(7183):93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 840.González-Maeso J., Weisstaub N.V., Zhou M., Chan P., Ivic L., Ang R., Lira A., Bradley-Moore M., Ge Y., Zhou Q., Sealfon S.C., Gingrich J.A. Hallucinogens recruit specific cortical 5-HT(2A) receptor-mediated signaling pathways to affect behavior. Neuron. 2007;53(3):439–452. doi: 10.1016/j.neuron.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 841.Fribourg M., Moreno J.L., Holloway T., Provasi D., Baki L., Mahajan R., Park G., Adney S.K., Hatcher C., Eltit J.M., Ruta J.D., Albizu L., Li Z., Umali A., Shim J., Fabiato A., MacKerell A.D., Jr, Brezina V., Sealfon S.C., Filizola M., González-Maeso J., Logothetis D.E. Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell. 2011;147(5):1011–1023. doi: 10.1016/j.cell.2011.09.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 842.Schröder H., Wu D.F., Seifert A., Rankovic M., Schulz S., Höllt V., Koch T. Allosteric modulation of metabotropic glutamate receptor 5 affects phosphorylation, internalization, and desensitization of the micro-opioid receptor. Neuropharmacology. 2009;56(4):768–778. doi: 10.1016/j.neuropharm.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 843.Neugebauer V., Li W., Bird G.C., Han J.S. The amygdala and persistent pain. Neuroscientist. 2004;10(3):221–234. doi: 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- 844.Spooren W.P.J.M., Gasparini F., Salt T.E., Kuhn R. Novel allosteric antagonists shed light on mglu(5) receptors and CNS disorders. Trends Pharmacol. Sci. 2001;22(7):331–337. doi: 10.1016/S0165-6147(00)01694-1. [DOI] [PubMed] [Google Scholar]
- 845.Dickenson A.H. Central acute pain mechanisms. Ann. Med. 1995;27(2):223–227. doi: 10.3109/07853899509031963. [DOI] [PubMed] [Google Scholar]
- 846.Lee H.J., Choi H.S., Ju J.S., Bae Y.C., Kim S.K., Yoon Y.W., Ahn D.K. Peripheral mGluR5 antagonist attenuated craniofacial muscle pain and inflammation but not mGluR1 antagonist in lightly anesthetized rats. Brain Res. Bull. 2006;70(4-6):378–385. doi: 10.1016/j.brainresbull.2005.09.021. [DOI] [PubMed] [Google Scholar]
- 847.Gabra B.H., Smith F.L., Navarro H.A., Carroll F.I., Dewey W.L. mGluR5 antagonists that block calcium mobilization in vitro also reverse (S)-3,5-DHPG-induced hyperalgesia and morphine antinociceptive tolerance in vivo . . Brain Res. 2008;1187(1):58–66. doi: 10.1016/j.brainres.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 848.Fuxe K., Marcellino D., Borroto-Escuela D.O., Frankowska M., Ferraro L., Guidolin D., Ciruela F., Agnati L.F. The changing world of G protein-coupled receptors: from monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. J. Recept. Signal Transduct. 2010;30(5):272–283. doi: 10.3109/10799893.2010.506191. [DOI] [PubMed] [Google Scholar]
- 849.Agnati L.F., Guidolin D., Albertin G., Trivello E., Ciruela F., Genedani S., Tarakanov A., Fuxe K. An integrated view on the role of receptor mosaics at perisynaptic level: focus on adenosine A(2A), dopamine D(2), cannabinoid CB(1), and metabotropic glutamate mGlu(5) receptors. J. Recept. Signal Transduct. 2010;30(5):355–369. doi: 10.3109/10799893.2010.487492. [DOI] [PubMed] [Google Scholar]
- 850.Agnati L.F., Guidolin D., Vilardaga J.P., Ciruela F., Fuxe K. On the expanding terminology in the GPCR field: the meaning of receptor mosaics and receptor heteromers. J Recept Signal Transduct. 2010;30(5):287–303. doi: 10.3109/10799891003786226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 851.Agnati L.F., Fuxe K., Zoli M., Rondanini C., Ogren S.O. New vistas on synaptic plasticity: the receptor mosaic hypothesis of the engram. Med. Biol. 1982;60(4):183–190. [PubMed] [Google Scholar]
- 852.Fuxe K., Canals M., Torvinen M., Marcellino D., Terasmaa A., Genedani S., Leo G., Guidolin D., Diaz-Cabiale Z., Rivera A., Lundstrom L., Langel U., Narvaez J., Tanganelli S., Lluis C., Ferré S., Woods A., Franco R., Agnati L.F. Intramembrane receptor-receptor interactions: A novel principle in molecular medicine. J. Neural Transm. (Vienna) 2007;114(1):49–75. doi: 10.1007/s00702-006-0589-0. [DOI] [PubMed] [Google Scholar]
- 853.Agnati L.F., Guidolin D., Leo G., Carone C., Genedani S., Fuxe K. Receptor-receptor interactions: A novel concept in brain integration. Prog. Neurobiol. 2010;90(2):157–175. doi: 10.1016/j.pneurobio.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 854.Agnati L.F., Franzen O., Ferré S., Leo G., Franco R., Fuxe K. Possible role of intramembrane receptor-receptor interactions in memory and learning via formation of long-lived heteromeric complexes: Focus on motor learning in the basal ganglia. In: J. Neural Transmission, Supplement, 2003:pp. 1-28. doi: 10.1007/978-3-7091-0643-3_1. [DOI] [PubMed] [Google Scholar]
- 855.Denning D.W., Follansbee S.E., Scolaro M., Norris S., Edelstein H., Stevens D.A. Pulmonary aspergillosis in the acquired immunodeficiency syndrome. N. Engl. J. Med. 1991;324(10):654–662. doi: 10.1056/NEJM199103073241003. [DOI] [PubMed] [Google Scholar]
- 856.Fuxe K., Marcellino D., Borroto-Escuela D.O., Frankowska M., Ferraro L., Guidolin D., Ciruela F., Agnati L.F. The changing world of G protein-coupled receptors: from monomers to dimers and receptor mosaics with allosteric receptor-receptor interactions. J. Recept. Signal Transduct. Res. 2010;30(5):272–283. doi: 10.3109/10799893.2010.506191. [DOI] [PubMed] [Google Scholar]
- 857.Navarro G., Carriba P., Gandía J., Ciruela F., Casadó V., Cortés A., Mallol J., Canela E.I., Lluis C., Franco R. Detection of heteromers formed by cannabinoid CB1, dopamine D2, and adenosine A2A G-protein-coupled receptors by combining bimolecular fluorescence complementation and bioluminescence energy transfer. Sci. World J. 2008;8:1088–1097. doi: 10.1100/tsw.2008.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 858.Dean B., Sundram S., Bradbury R., Scarr E., Copolov D. Studies on [3H]CP-55940 binding in the human central nervous system: regional specific changes in density of cannabinoid-1 receptors associated with schizophrenia and cannabis use. Neuroscience. 2001;103(1):9–15. doi: 10.1016/S0306-4522(00)00552-2. [DOI] [PubMed] [Google Scholar]
- 859.Guillin O., Abi-Dargham A., Laruelle M. Neurobiology of dopamine in schizophrenia. Int. Rev. Neurobiol. 2007;78:1–39. doi: 10.1016/S0074-7742(06)78001-1. [DOI] [PubMed] [Google Scholar]
- 860.Kerppola T.K. Bimolecular fluorescence complementation: visualization of molecular interactions in living cells. Methods Cell Biol. 2008;85:431–470. doi: 10.1016/S0091-679X(08)85019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 861.Carriba P., Navarro G., Ciruela F., Ferré S., Casadó V., Agnati L., Cortés A., Mallol J., Fuxe K., Canela E.I., Lluís C., Franco R. Detection of heteromerization of more than two proteins by sequential BRET-FRET. Nat. Methods. 2008;5(8):727–733. doi: 10.1038/nmeth.1229. [DOI] [PubMed] [Google Scholar]
- 862.Deckert J., Brenner M., Durany N., Zöchling R., Paulus W., Ransmayr G., Tatschner T., Danielczyk W., Jellinger K., Riederer P. Up-regulation of striatal adenosine A(2A) receptors in schizophrenia. Neuroreport. 2003;14(3):313–316. doi: 10.1097/00001756-200303030-00003. [DOI] [PubMed] [Google Scholar]
- 863.Sundram S., Copolov D., Dean B. Clozapine decreases [3H] CP 55940 binding to the cannabinoid 1 receptor in the rat nucleus accumbens. Naunyn Schmiedebergs Arch. Pharmacol. 2005;371(5):428–433. doi: 10.1007/s00210-005-1074-2. [DOI] [PubMed] [Google Scholar]
- 864.Urigüen L., García-Fuster M.J., Callado L.F., Morentin B., La Harpe R., Casadó V., Lluis C., Franco R., García-Sevilla J.A., Meana J.J. Immunodensity and mRNA expression of A2A adenosine, D2 dopamine, and CB1 cannabinoid receptors in postmortem frontal cortex of subjects with schizophrenia: effect of antipsychotic treatment. Psychopharmacology (Berl.) 2009;206(2):313–324. doi: 10.1007/s00213-009-1608-2. [DOI] [PubMed] [Google Scholar]
- 865.Cabello N., Gandía J., Bertarelli D.C.G., Watanabe M., Lluís C., Franco R., Ferré S., Luján R., Ciruela F. Metabotropic glutamate type 5, dopamine D2 and adenosine A2a receptors form higher-order oligomers in living cells. J. Neurochem. 2009;109(5):1497–1507. doi: 10.1111/j.1471-4159.2009.06078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 866.Popoli P., Pèzzola A., Torvinen M., Reggio R., Pintor A., Scarchilli L., Fuxe K., Ferré S. The selective mGlu(5) receptor agonist CHPG inhibits quinpirole-induced turning in 6-hydroxydopamine-lesioned rats and modulates the binding characteristics of dopamine D(2) receptors in the rat striatum: interactions with adenosine A(2a) receptors. Neuropsychopharmacology. 2001;25(4):505–513. doi: 10.1016/S0893-133X(01)00256-1. [DOI] [PubMed] [Google Scholar]
- 867.Díaz-Cabiale Z., Vivó M., Del Arco A., O’Connor W.T., Harte M.K., Müller C.E., Martínez E., Popoli P., Fuxe K., Ferré S. Metabotropic glutamate mGlu5 receptor-mediated modulation of the ventral striopallidal GABA pathway in rats. Interactions with adenosine A(2A) and dopamine D(2) receptors. Neurosci. Lett. 2002;324(2):154–158. doi: 10.1016/S0304-3940(02)00179-9. [DOI] [PubMed] [Google Scholar]
- 868.Schwarzschild M.A., Agnati L., Fuxe K., Chen J.F., Morelli M. Targeting adenosine A2A receptors in Parkinson’s disease. Trends Neurosci. 2006;29(11):647–654. doi: 10.1016/j.tins.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 869.Bockaert J., Fagni L., Dumuis A., Marin P. GPCR interacting proteins (GIP). Pharmacol. Ther. 2004;103(3):203–221. doi: 10.1016/j.pharmthera.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 870.Fagni L., Ango F., Perroy J., Bockaert J. Identification and functional roles of metabotropic glutamate receptor-interacting proteins. Semin. Cell Dev. Biol. 2004;15(3):289–298. doi: 10.1016/j.semcdb.2003.12.018. [DOI] [PubMed] [Google Scholar]
- 871.Kamal M., Maurice P., Jockers R. Expanding the concept of G protein-coupled receptor (GPCR) dimer asymmetry towards GPCR-interacting proteins. Pharm. 2011;4(2):273–284. [Google Scholar]
- 872.Bockaert J., Dumuis A., Fagni L., Marin P. GPCR-GIP networks: A first step in the discovery of new therapeutic drugs?. Curr. Opin. Drug Discov. Devel. 2004;7(5):649–657. [PubMed] [Google Scholar]
- 873.Kowalsman N., Niv M.Y., Kowalsman N. Niv • M Y, Filizola M. GPCR & Company: Databases and Servers for GPCRs and Interacting Partners. Adv. Exp. Med. Biol. 2014;796:185–204. doi: 10.1007/978-94-007-7423-0_9. [DOI] [PubMed] [Google Scholar]
- 874.Maurice P., Guillaume J.L., Benleulmi-Chaachoua A., Daulat A.M., Kamal M., Jockers R. GPCR-interacting proteins, major players of GPCR function. Adv. Pharmacol. 2011;62:349–380. doi: 10.1016/B978-0-12-385952-5.00001-4. [DOI] [PubMed] [Google Scholar]
- 875.Magalhaes A.C., Dunn H., Ferguson S.S.G. Regulation of GPCR activity, trafficking and localization by GPCR-interacting proteins. Br. J. Pharmacol. 2012;165(6):1717–1736. doi: 10.1111/j.1476-5381.2011.01552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 876.Ritter S.L., Hall R.A. Fine-tuning of GPCR activity by receptor-interacting proteins. Nat. Rev. Mol. Cell Biol. 2009;10(12):819–830. doi: 10.1038/nrm2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 877.Shaw A.S., Filbert E.L. Scaffold proteins and immune-cell signalling. Nat. Rev. Immunol. 2009;9(1):47–56. doi: 10.1038/nri2473. [DOI] [PubMed] [Google Scholar]
- 878.Wong W., Scott J.D. AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 2004;5(12):959–970. doi: 10.1038/nrm1527. [DOI] [PubMed] [Google Scholar]
- 879.Maurice P., Daulat A.M., Turecek R., Ivankova-Susankova K., Zamponi F., Kamal M., Clement N., Guillaume J.L., Bettler B., Galès C., Delagrange P., Jockers R. Molecular organization and dynamics of the melatonin MT1 receptor/RGS20/G(i) protein complex reveal asymmetry of receptor dimers for RGS and G(i) coupling. EMBO J. 2010;29(21):3646–3659. doi: 10.1038/emboj.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 880.Neitzel K.L., Hepler J.R. Cellular mechanisms that determine selective RGS protein regulation of G protein-coupled receptor signaling. Semin. Cell Dev. Biol. 2006;17(3):383–389. doi: 10.1016/j.semcdb.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 881.Xie G.X., Palmer P.P. How regulators of G protein signaling achieve selective regulation. J. Mol. Biol. 2007;366(2):349–365. doi: 10.1016/j.jmb.2006.11.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 882.Bockaert J., Perroy J., Ango F. The complex formed by group i metabotropic glutamate receptor (mGluR) and homer1a plays a central role in metaplasticity and homeostatic synaptic scaling. J. Neurosci. 2021;41(26):5567–5578. doi: 10.1523/JNEUROSCI.0026-21.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 883.Ango F., Prézeau L., Muller T., Tu J.C., Xiao B., Worley P.F. Agonist-independent activation of metabotropic glutamate receptors by the intracellular protein. Homer. . Nat. 2001;411(6840):962–965. doi: 10.1038/35082096. [DOI] [PubMed] [Google Scholar]
- 884.Kammermeier P.J., Xiao B., Tu J.C., Worley P.F., Ikeda S.R. Homer proteins regulate coupling of group I metabotropic glutamate receptors to N-type calcium and M-type potassium channels. J. Neurosci. 2000;20(19):7238–7245. doi: 10.1523/JNEUROSCI.20-19-07238.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 885.Xiao B., Tu J.C., Worley P.F. Homer: A link between neural activity and glutamate receptor function. Curr. Opin. Neurobiol. 2000;10(3):370–374. doi: 10.1016/S0959-4388(00)00087-8. [DOI] [PubMed] [Google Scholar]
- 886.Ehlers M.D. Synapse structure: glutamate receptors connected by the shanks. Curr. Biol. 1999;9(22):R848–R850. doi: 10.1016/S0960-9822(00)80043-3. [DOI] [PubMed] [Google Scholar]
- 887.Bockaert J., Fagni L., Perroy J. In: G Protein-Coupled Receptors, 2011. Functional crosstalk between group I metabotropic glutamate receptors and ionotropic glutamate receptors controls synaptic transmission. ; p. pp. 269-283. [DOI] [Google Scholar]
- 888.Ferré S., Ciruela F., Dessauer C.W., González-Maeso J., Hébert T.E., Jockers R., Logothetis D.E., Pardo L. G protein-coupled receptor-effector macromolecular membrane assemblies (GEMMAs). Pharmacol. Ther. 2022;231(Sep):107977. doi: 10.1016/j.pharmthera.2021.107977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 889.Erez M., Takemori A.E., Portoghese P.S. Narcotic antagonistic potency of bivalent ligands which contain beta-naltrexamine. Evidence for bridging between proximal recognition sites. J. Med. Chem. 1982;25(7):847–849. doi: 10.1021/jm00349a016. [DOI] [PubMed] [Google Scholar]
- 890.Portoghese P.S., Ronsisvalle G., Larson D.L., Yim C.B., Sayre L.M., Takemori A.E. Opioid agonist and antagonist bivalent ligands as receptor probes. Life Sci. 1982;31(12-13):1283–1286. doi: 10.1016/0024-3205(82)90362-9. [DOI] [PubMed] [Google Scholar]
- 891.Huang B., St Onge C.M., Ma H., Zhang Y. Design of bivalent ligands targeting putative GPCR dimers. Drug Discov. Today. 2021;26(1):189–199. doi: 10.1016/j.drudis.2020.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 892.Shonberg J., Scammells P.J., Capuano B. Design strategies for bivalent ligands targeting GPCRs. ChemMedChem. 2011;6(6):963–974. doi: 10.1002/cmdc.201100101. [DOI] [PubMed] [Google Scholar]
- 893.Budzinski J., Maschauer S., Kobayashi H., Couvineau P., Vogt H., Gmeiner P., Roggenhofer A., Prante O., Bouvier M., Weikert D. Bivalent ligands promote endosomal trafficking of the dopamine D3 receptor-neurotensin receptor 1 heterodimer. Commun. Biol. 2021;4(1):1062. doi: 10.1038/s42003-021-02574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 894.Qian M., Wouters E., Dalton J.A.R., Risseeuw M.D.P., Crans R.A.J., Stove C., Giraldo J., Van Craenenbroeck K., Van Calenbergh S. Synthesis toward Bivalent Ligands for the Dopamine D2 and Metabotropic Glutamate 5 Receptors. J. Med. Chem. 2018;61(18):8212–8225. doi: 10.1021/acs.jmedchem.8b00671. [DOI] [PubMed] [Google Scholar]
- 895.Nasrallah C., Cannone G., Briot J., Rottier K., Berizzi A.E., Huang C.Y., Quast R.B., Hoh F., Banères J.L., Malhaire F., Berto L., Dumazer A., Font-Ingles J., Gómez-Santacana X., Catena J., Kniazeff J., Goudet C., Llebaria A., Pin J.P., Vinothkumar K.R., Lebon G. Agonists and allosteric modulators promote signaling from different metabotropic glutamate receptor 5 conformations. Cell Rep. 2021;36(9):109648. doi: 10.1016/j.celrep.2021.109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 896.Bock A., Bermudez M. Allosteric coupling and biased agonism in G protein-coupled receptors. FEBS J. 2021;288(8):2513–2528. doi: 10.1111/febs.15783. [DOI] [PubMed] [Google Scholar]
- 897.Ma N., Nivedha A.K., Vaidehi N. Allosteric communication regulates ligand-specific GPCR activity. FEBS J. 2021;288(8):2502–2512. doi: 10.1111/febs.15826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 898.Romantini N., Alam S., Dobitz S., Spillmann M., De Foresta M., Schibli R., Schertler G.F.X., Wennemers H., Deupi X., Behe M., Berger P. Exploring the signaling space of a GPCR using bivalent ligands with a rigid oligoproline backbone. Proc. Natl. Acad. Sci. USA. 2021;118(48):e2108776118. doi: 10.1073/pnas.2108776118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 899.Haubrich J., Font J., Quast R.B., Goupil-Lamy A., Scholler P., Nevoltris D., Acher F., Chames P., Rondard P., Prézeau L., Pin J.P. A nanobody activating metabotropic glutamate receptor 4 discriminates between homo- and heterodimers. Proc. Natl. Acad. Sci. USA. 2021;118(33):e2105848118. doi: 10.1073/pnas.2105848118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 900.Wagner T.R., Rothbauer U. Nanobodies - Little helpers unravelling intracellular signaling. Free Radic. Biol. Med. 2021;176:46–61. doi: 10.1016/j.freeradbiomed.2021.09.005. [DOI] [PubMed] [Google Scholar]
- 901.Hamers-Casterman C., Atarhouch T., Muyldermans S., Robinson G., Hammers C., Songa E.B. Naturally occurring antibodies devoid of light chains. Nat. 1993;363(6428):446–448. doi: 10.1038/363446a0. [DOI] [PubMed] [Google Scholar]
- 902.Che T., English J., Krumm B.E., Kim K., Pardon E., Olsen R.H.J., Wang S., Zhang S., Diberto J.F., Sciaky N., Carroll F.I., Steyaert J., Wacker D., Roth B.L. Nanobody-enabled monitoring of kappa opioid receptor states. Nat. Commun. 2020;11(1):1145. doi: 10.1038/s41467-020-14889-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 903.Stoeber M., Jullié D., Li J., Chakraborty S., Majumdar S., Lambert N.A., Manglik A., von Zastrow M. Agonist-selective recruitment of engineered protein probes and of GRK2 by opioid receptors in living cells. eLife. 2020;9:9. doi: 10.7554/eLife.54208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 904.Stoeber M., Jullié D., Lobingier B.T., Laeremans T., Steyaert J., Schiller P.W., Manglik A., von Zastrow M. A genetically encoded biosensor reveals location bias of opioid drug action. Neuron. 2018;98(5):963–976.e5. doi: 10.1016/j.neuron.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 905.Che T., Majumdar S., Zaidi S.A., Ondachi P., McCorvy J.D., Wang S., Mosier P.D., Uprety R., Vardy E., Krumm B.E., Han G.W., Lee M.Y., Pardon E., Steyaert J., Huang X.P., Strachan R.T., Tribo A.R., Pasternak G.W., Carroll F.I., Stevens R.C., Cherezov V., Katritch V., Wacker D., Roth B.L. Structure of a nanobody-stabilized active state of the kappa opioid receptor. Cell. 2018;172(1-2):55–67.e15. doi: 10.1016/j.cell.2017.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 906.Johnson G.P., Agwuegbo U., Jonas K.C. New insights into the functional impact of G protein-coupled receptor oligomerization. Curr. Opin. Endocr. Metab. Res. 2021;16:43–50. doi: 10.1016/j.coemr.2020.08.005. [DOI] [Google Scholar]
- 907.De Groof T.W.M., Bobkov V., Heukers R., Smit M.J. Nanobodies: New avenues for imaging, stabilizing and modulating GPCRs. Mol. Cell. Endocrinol. 2019;484:15–24. doi: 10.1016/j.mce.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 908.Dolgin E. First GPCR-directed antibody passes approval milestone. Nat. Rev. Drug Discov. 2018;17(7):457–459. doi: 10.1038/nrd.2018.103. [DOI] [PubMed] [Google Scholar]
- 909.Han L., Liu Y., Xiong H., Hong P. CGRP monoclonal antibody for preventive treatment of chronic migraine: An update of meta-analysis. Brain Behav. 2019;9(2):e01215. doi: 10.1002/brb3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 910.DelaCuesta-Barrutia J., Peñagarikano O., Erdozain A.M.G.G. Protein-coupled receptor heteromers as putative pharmacotherapeutic targets in autism. Front. Cell. Neurosci. 2020;14:588662. doi: 10.3389/fncel.2020.588662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 911.So C.H., Varghese G., Curley K.J., Kong M.M.C., Alijaniaram M., Ji X., Nguyen T., O’dowd B.F., George S.R. D1 and D2 dopamine receptors form heterooligomers and cointernalize after selective activation of either receptor. Mol. Pharmacol. 2005;68(3):568–578. doi: 10.1124/mol.105.012229. [DOI] [PubMed] [Google Scholar]
- 912.Franco R., Ferré S., Agnati L., Torvinen M., Ginés S., Hillion J., Casadó V., Lledó P., Zoli M., Lluis C., Fuxe K. Evidence for adenosine/dopamine receptor interactions: indications for heteromerization. Neuropsychopharmacology. 2000;23(4) Suppl.:S50–S59. doi: 10.1016/S0893-133X(00)00144-5. [DOI] [PubMed] [Google Scholar]
- 913.Torvinen M., Ginés S., Hillion J., Latini S., Canals M., Ciruela F., Bordoni F., Staines W., Pedata F., Agnati L.F., Lluis C., Franco R., Ferré S., Fuxe K. Interactions among adenosine deaminase, adenosine A(1) receptors and dopamine D(1) receptors in stably cotransfected fibroblast cells and neurons. Neuroscience. 2002;113(3):709–719. doi: 10.1016/S0306-4522(02)00058-1. [DOI] [PubMed] [Google Scholar]
- 914.Cao Y., Xie K.Q., Zhu X.Z. The enhancement of dopamine D1 receptor desensitization by adenosine A1 receptor activation. Eur. J. Pharmacol. 2007;562(1-2):34–38. doi: 10.1016/j.ejphar.2007.01.090. [DOI] [PubMed] [Google Scholar]
- 915.Soriano A., Ventura R., Molero A., Hoen R., Casadó V., Cortés A., Fanelli F., Albericio F., Lluís C., Franco R., Royo M. Adenosine A2A receptor-antagonist/dopamine D2 receptor-agonist bivalent ligands as pharmacological tools to detect A2A-D2 receptor heteromers. J. Med. Chem. 2009;52(18):5590–5602. doi: 10.1021/jm900298c. [DOI] [PubMed] [Google Scholar]
- 916.Fuxe K., Ferré S., Canals M., Torvinen M., Terasmaa A., Marcellino D., Goldberg S.R., Staines W., Jacobsen K.X., Lluis C., Woods A.S., Agnati L.F., Franco R. Adenosine A2A and dopamine D2 heteromeric receptor complexes and their function. J. Mol. Neurosci. 2005;26(2-3):209–220. doi: 10.1385/JMN:26:2-3:209. [DOI] [PubMed] [Google Scholar]
- 917.Ciruela F., Burgueño F., Casadó V., Canals M., Marcellino D., Goldberg S.R. Combining mass spectrometry and pull-down techniques for the study of receptor heteromerization. Direct epitope−epitope electrostatic interactions between adenosine A2A and dopamine D2 receptors. Anal. Chem. 2004;76(18):5354–5363. doi: 10.1021/ac049295f. [DOI] [PubMed] [Google Scholar]
- 918.Bara-Jimenez W., Sherzai A., Dimitrova T., Favit A., Bibbiani F., Gillespie M., Morris M.J., Mouradian M.M., Chase T.N. Adenosine A(2A) receptor antagonist treatment of Parkinson’s disease. Neurology. 2003;61(3):293–296. doi: 10.1212/01.WNL.0000073136.00548.D4. [DOI] [PubMed] [Google Scholar]
- 919.Rocheville M., Lange D.C., Kumar U., Patel S.C., Patel R.C., Patel Y.C. Receptors for dopamine and somatostatin: Formation of hetero-oligomers with enhanced functional activity. Science (80) 2000. p. 288(5463), 154-157. [DOI] [PubMed]
- 920.Damian M., Pons V., Renault P., M’Kadmi C., Delort B., Hartmann L., Kaya A.I., Louet M., Gagne D., Ben Haj Salah K., Denoyelle S., Ferry G., Boutin J.A., Wagner R., Fehrentz J.A., Martinez J., Marie J., Floquet N., Galès C., Mary S., Hamm H.E., Banères J.L. GHSR-D2R heteromerization modulates dopamine signaling through an effect on G protein conformation. Proc. Natl. Acad. Sci. USA. 2018;115(17):4501–4506. doi: 10.1073/pnas.1712725115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 921.Cordisco G.S., Mustafá E.R., Rodriguez S.S., Perello M., Raingo J. Dopamine receptor type 2 and ghrelin receptor coexpression alters CaV2.2 modulation by G protein signaling cascades. ACS Chem. Neurosci. 2020;11(1):3–13. doi: 10.1021/acschemneuro.9b00426. [DOI] [PubMed] [Google Scholar]
- 922.Morales-Figueroa G.E., Rivera-Ramírez N., González-Pantoja R., Escamilla-Sánchez J., García-Hernández U., Galván E.J., Arias-Montaño J.A. Adenosine A2A and histamine H3 receptors interact at the cAMP/PKA pathway to modulate depolarization-evoked [3H]-GABA release from rat striato-pallidal terminals. Purinergic Signal. 2019;15(1):85–93. doi: 10.1007/s11302-018-9638-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 923.Lenard N.R., Daniels D.J., Portoghese P.S., Roerig S.C. Absence of conditioned place preference or reinstatement with bivalent ligands containing mu-opioid receptor agonist and delta-opioid receptor antagonist pharmacophores. Eur. J. Pharmacol. 2007;566(1-3):75–82. doi: 10.1016/j.ejphar.2007.02.040. [DOI] [PubMed] [Google Scholar]
- 924.Daniels D.J., Lenard N.R., Etienne C.L., Law P-Y., Roerig S.C., Portoghese P.S. Opioid-induced tolerance and dependence in mice is modulated by the distance between pharmacophores in a bivalent ligand series. Proc. Natl. Acad. Sci. USA. 2005;102(52):19208–19213. doi: 10.1073/pnas.0506627102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 925.Zhang Y.Q., Limbird L.E. Hetero-oligomers of α2A-adrenergic and μ-opioid receptors do not lead to transactivation of G-proteins or altered endocytosis profiles. Biochem. Soc. Trans. 2004;•••:856–860. doi: 10.1042/BST0320856. [DOI] [PubMed] [Google Scholar]
- 926.Jordan B.A., Gomes I., Rios C., Filipovska J., Devi L.A. Functional interactions between μ opioid and α 2A-adrenergic receptors. Mol. Pharmacol. 2003;64(6):1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- 927.Glass M.J., Pickel V.M. α(2A)-adrenergic receptors are present in μ-opioid receptor containing neurons in rat medial nucleus tractus solitarius. Synapse. 2002;43(3):208–218. doi: 10.1002/syn.10036. [DOI] [PubMed] [Google Scholar]
- 928.Gabilondo A.M., Meana J.J., Barturen F., Sastre M., García-Sevilla J.A. μ-Opioid receptor and α 2-adrenoceptor agonist binding sites in the postmortem brain of heroin addicts. Psychopharmacology (Berl.) 1994;115(1-2):135–140. doi: 10.1007/BF02244763. [DOI] [PubMed] [Google Scholar]
- 929.Fongang B., Cunningham K.A., Rowicka M. Kudlicki, A protein co-evolution strategies detect predicted functional interaction between the serotonin 5-HT2A and 5-HT2C receptors. bioRxiv. 2019:512558. doi: 10.1101/512558. [DOI]
- 930.Cunningham K.A., Anastasio N.C., Fox R.G., Stutz S.J., Bubar M.J., Swinford S.E., Watson C.S., Gilbertson S.R., Rice K.C., Rosenzweig-Lipson S., Moeller F.G. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chem. Neurosci. 2013;4(1):110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 931.Martín A.B., Fernandez-Espejo E., Ferrer B., Gorriti M.A., Bilbao A., Navarro M., Rodriguez de Fonseca F., Moratalla R. Expression and function of CB1 receptor in the rat striatum: localization and effects on D1 and D2 dopamine receptor-mediated motor behaviors. Neuropsychopharmacology. 2008;33(7):1667–1679. doi: 10.1038/sj.npp.1301558. [DOI] [PubMed] [Google Scholar]
- 932.Doumazane E., Scholler P., Zwier J.M., Trinquet E., Rondard P., Pin J-P. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- 933.Nieto A., Bailey T., Kaczanowska K., McDonald P. GABAB receptor chemistry and pharmacology: Agonists, antagonists, and allosteric modulators. Curr. Top. Behav. Neurosci. 2021 doi: 10.1007/7854_2021_232. [DOI] [PubMed] [Google Scholar]
- 934.Delille H.K., Becker J.M., Burkhardt S., Bleher B., Terstappen G.C., Schmidt M., Meyer A.H., Unger L., Marek G.J., Mezler M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62(7):2184–2191. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 935.Shah U.H., González-Maeso J. Serotonin and glutamate interactions in preclinical schizophrenia models. ACS Chem. Neurosci. 2019;10(7):3068–3077. doi: 10.1021/acschemneuro.9b00044. [DOI] [PubMed] [Google Scholar]