Abstract
The pore-forming hemolysin (HlyA) of Escherichia coli represents a unique class of bacterial toxins that require a posttranslational modification for activity. The inactive protoxin pro-HlyA is activated intracellularly by amide linkage of fatty acids to two internal lysine residues 126 amino acids apart, directed by the cosynthesized HlyC protein with acyl carrier protein as the fatty acid donor. This action distinguishes HlyC from all bacterial acyltransferases such as the lipid A, lux-specific, and nodulation acyltransferases, and from eukaryotic transferases such as N-myristoyl transferases, prenyltransferases, and thioester palmitoyltransferases. Most lipids directly attached to proteins may be classed as N-terminal amide-linked and internal ester-linked acyl groups and C-terminal ether-linked isoprenoid groups. The acylation of HlyA and related toxins does not equate to these but does appear related to a small number of eukaryotic proteins that include inflammatory cytokines and mitogenic and cholinergic receptors. While the location and structure of lipid moieties on proteins vary, there are common effects on membrane affinity and/or protein-protein interactions. Despite being acylated at two residues, HlyA does not possess a “double-anchor” motif and does not have an electrostatic switch, although its dependence on calcium binding for activity suggests that the calcium-myristoyl switch may have relevance. The acyl chains on HlyA may provide anchorage points onto the surface of the host cell lipid bilayer. These could then enhance protein-protein interactions either between HlyA and components of a host signal transduction pathway to influence cytokine production or between HlyA monomers to bring about oligomerization during pore formation.
Protein toxins are prominent virulence factors of many pathogenic bacteria. While toxins of gram-positive bacteria do not generally require activation, many toxins of gram-negative bacteria are translated in an inactive form and require a processing step, most often a proteolytic cleavage, to generate the active form. Enzymatic toxins such as Shiga toxin, cholera toxin, pertussis toxin, diphtheria toxin, and Pseudomonas aeruginosa exotoxin A undergo proteolytic cleavage to produce a catalytic A fragment acting in the eukaryotic target cell (279). Similarly, many nonenzymatic toxins that insert into eukaryotic membranes require proteolytic cleavage to allow oligomerization and pore formation; e.g., Vibrio cholerae El Tor hemolysin is cleaved at its N terminus, while Aeromonas aerolysin, Clostridium septicum alpha-toxin, and P. aeruginosa cytotoxin are cleaved at their C termini (13, 219, 236). The pore-forming hemolysin (HlyA) of Escherichia coli represents a unique class of bacterial toxins that require a posttranslational modification for activity, specifically the covalent amide linkage of fatty acids to internal lysine residues; the suggestion that activation of the Serratia and Proteus hemolysins ShlA and HpmA involves covalent modification has not been supported by subsequent experiments (123). After introducing the hemolysin toxin, this review will focus on the posttranslational mechanism of HlyA modification (maturation) and will examine its relationship to protein modifications involving lipid groups that occur in prokaryotes, lower eukaryotes, and mammalian systems. The functional significance of protein lipidation in these examples will then be reviewed and used as a basis for discussion of the possible role(s) of acylation in the cytokine-inducing and pore-forming functions of the HlyA toxin.
E. coli HlyA and a Family of Bacterial Pore-Forming Toxins
HlyA is an important virulence factor in E. coli extraintestinal infections such as those of the upper urinary tract (81, 107, 124, 318). It is one of a close family of membrane-targeted toxins assumed or proven to be influential not only in urinary tract infections but also hemorrhagic intestinal disease, juvenile periodontitis, pneumonia, whooping cough, and wound infections; these toxins include enterohemorrhagic O157 E. coli hemolysin (257), the leukotoxin of Pasteurella haemolytica (291), the hemolysins and leukotoxins of Actinobacillus spp. (46, 52, 89, 173), the bifunctional adenylate cyclase-hemolysin of Bordetella pertussis (100), and the hemolysins of Proteus vulgaris (167, 319), Morganella morganii (167), and Moraxella bovis (101) (Table 1). These toxins have 30 to 75% sequence identity to E. coli HlyA and share (i) posttranslational maturation, (ii) a C-terminal calcium-binding domain of acidic glycine-rich nonapeptide repeats that has led to the RTX (repeat toxin) family nomenclature, and (iii) export out of the cell by type I secretion systems (34, 59, 129, 171, 320). The posttranslational modification is unique to this toxin family, but Ca2+ binding and type I secretion are both common to other bacterial proteins (18, 34, 86, 221, 301). In HlyA there are between 11 and 17 glycine-rich repeats. When Ca2+ is bound (one calcium ion per repeat), these form short β-strands organized in an unusual “spring-like” structure called a parallel β-barrel or β-superhelix (18). Calcium binding is an absolute requirement for cytotoxic activity (36, 37, 187, 232) and occurs outside bacteria following export, the intracellular level of free calcium in E. coli being very tightly regulated to 0.1 μM (92), a level too low for HlyA activity.
TABLE 1.
The toxins of the RTX exoprotein family
| Bacterium | Toxin | Size (kDa) | HlyA identity (%) |
|---|---|---|---|
| E. coli | HlyA | 110 | |
| EhxA | 107 | 61 | |
| EaggAa | 120b | ? | |
| P. vulgaris | PvxA | 110b | 73c |
| M. morganii | MmxA | 110b | 60c |
| A. pleuropneumoniae | ApxIA | 110 | 59 |
| ApxIIA | 102 | 49 | |
| ApxIIIA | 113 | 56 | |
| A. actinomycetemcomitans | AaltA | 114 | 54 |
| A. suis | AshA | 102 | 49 |
| P. haemolytica | LktA | 102 | 49 |
| P. haemolytica-like | PllktA | 102 | 48 |
| B. pertussis | CyaA | 178 | 31d |
| M. bovis | MbxAa | 110b | ? |
No gene yet identified.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis.
Partial sequence comparison.
Comparison with N-terminal adenylate cyclase-deleted CyaA.
Synthesis and Export of HlyA Toxin
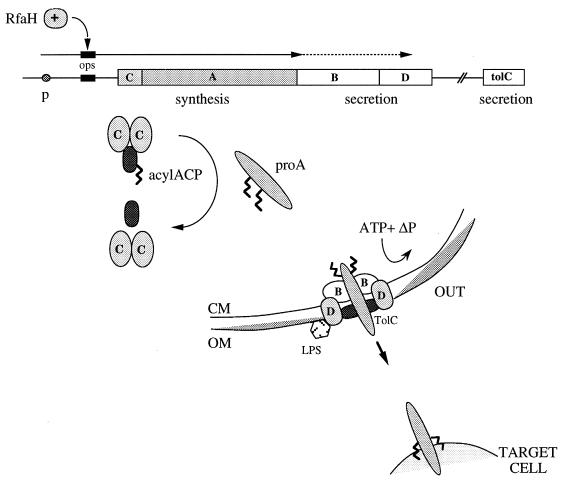
The synthesis, maturation, and secretion of E. coli HlyA are determined by the hlyCABD operon (81, 132, 171, 225) (Fig. 1). The membrane-located export proteins are synthesized at a lower level than the cytosolic HlyC and pro-HlyA, in part due to transcription termination within the hlyCABD operon (81). This termination is suppressed by the elongation protein RfaH and a short 5′ DNA sequence, ops (operon polarity suppressor) (7, 8, 60, 225, 311), that act together to allow the transcription of long operons such as hly, rfa, and tra that encode the synthesis and export of extracellular components important to the virulence and fertility of gram-negative bacteria (7, 9).
FIG. 1.
Hemolysin synthesis, maturation, and export by E. coli. (CM, cytoplasmic membrane; OM, outer membrane; ΔP, total PMF). Expression of the hemolysin operon is governed by components upstream of the hly genes. The hlyA gene encodes inactive prohemolysin, which is activated by HlyC. HlyA is secreted by a type I process. Ca2+ binds to the glycine-rich repeats of the toxin externally before interacting with the mammalian membrane.
The pro-HlyA protoxin is matured in the cytosol to the active form by HlyC-directed fatty acylation (see below). The maturation increases the hydrophobicity of the protein but is not required for export (186). E. coli HlyA and its toxin relatives are all secreted across both membranes by the type I export process employing an uncleaved C-terminal recognition signal (223, 281) but no N-terminal leader peptide (82) or periplasmic intermediate (83, 168). The HlyA secretory apparatus comprises HlyB (an inner membrane traffic ATPase), HlyD (an inner membrane protein that is suggested but not shown to bridge to the outer membrane), and TolC (an outer membrane protein) (260, 313, 315). In E. coli and most other pathogens, TolC is encoded by a gene separated from hlyCABD, but in B. pertussis the toxin locus includes tolC (cyaE). Type I secretion signal sequences have been located within the C-terminal 24 to 80 amino acids (137, 168, 293). The HlyA C terminus is predicted to contain an amphipathic helix (159, 281), and circular dichroism and nuclear magnetic resonance spectroscopy of the HlyA signal has shown that α-helices are formed in a membrane mimetic environment (330). Despite a lack of identity between their primary sequences, the interchangeability of the export genes suggests that higher-order structures in the signals are shared among the extended family of hemolysins, leukotoxins, and proteases (256, 262, 301, 329).
HlyB has an integral membrane domain fused to an ATP-binding cassette (traffic ATPase) cytoplasmic domain, which undergoes conformational change on ATP binding (170) and couples ATP hydrolysis to HlyA export (166). Topological models proposed from fusion data suggest that HlyB inserts in the cytoplasmic membrane via six helical transmembrane segments with both its N and C termini in the cytoplasm. The predicted HlyB cytoplasmic loops are large and positively charged, while the periplasmic loops are small (97). The function of HlyD is less clear, but it is proposed to have a single transmembrane segment with the N terminus in the cytoplasm and a large C-terminal region in the periplasm (260). TolC is a minor outer membrane protein believed to form ion-permeable channels. Electron microscopy of two-dimensional lattices of TolC in phospholipid bilayers has revealed it to be a trimeric porin-like structure with a C-terminal extramembrane domain believed to form a periplasmic bridge to the energized inner membrane components of the translocation complex (172). HlyBD-dependent type I secretion shares with the Sec secretion across the cytoplasmic membrane an early requirement for the total proton motive force (PMF) but also has a late stage that does not require PMF, membrane potential, or the proton gradient. A translocation intermediate identified in the PMF-independent late stage is closely associated with the inner membrane, possibly in a translocation complex spanning both membranes (169). The belief that HlyA interacts with HlyB is compatible with the finding that suppressor mutations in HlyB partially compensate for mutations in the HlyA secretion signal (273), and a view of the assembly of a transport-competent complex occurring in an ordered manner is now being substantiated by cross-linking experiments (34, 300). A consequence for HlyA secretion of the virtual absence of Ca2+ ions in the bacterial cytoplasm is that the glycine-repeat structures should be flexible, allowing translocation of the polypeptide across the bacterial membranes in an unfolded state.
BIOLOGICAL EFFECTS OF HLYA AND RELATED TOXINS
Host Cell Specificities of the Toxin Family
The toxins of the HlyA (RTX) family have been grouped on the basis of their lytic and toxic effects on mammalian host cells: hemolysins exhibit little target cell specificity, while leukotoxins have pronounced species- or cell-specific effects (59, 320) (Tables 2 and 3). E. coli HlyA has a wide spectrum of cytocidal activity, attacking erythrocytes, granulocytes (28, 91), monocytes (29), endothelial cells (294), and renal epithelial cells of mice, ruminants, and primates (155, 212). The 61% identical hemolysin (Ehx) from enterohemorrhagic E. coli O157 lyses sheep and human erythrocytes and kills bovine leukocytes (albeit with a specific activity more than 20-fold lower than that of HlyA), but, unlike HlyA, it has virtually no activity against human leukocytes (16). These contrast with the P. haemolytica and Actinobacillus actinomycetemcomitans leukotoxins which have little erythrolytic activity but a potent cytotoxic activity toward phagocytic cells of particular animals: the P. haemolytica leukotoxin (LktA) lyses leukocytes only from cattle, sheep, and other ruminants (274), while the A. actinomycetemcomitans leukotoxin (AaltA) specifically lyses human and primate polymorphonuclear lymphocytes (328). A. pleuropneumoniae produces hemolysins (ApxIA and ApxIIA) and a leukotoxin (ApxIIIA) (89) (Table 2).
TABLE 2.
Mammalian host cells known to be targeted by the hemolysins and leukotoxins of the HlyA family
| Toxin | Target species | Target cells | Reported effects |
|---|---|---|---|
| HlyA | Human, monkey, pig, rat, mouse, horse, sheep, cow | Erythrocytes, leukocytes, lymphocytes, epithelia, endothelia | Cytolytic, cytotoxic, NO production, cytokine production, cytoskeletal rearrangement, superoxide production, leukotriene production, receptor shedding |
| EhxA | Human, sheep, cow | Erythrocytes, leukocytes | Cytolytic, cytotoxic |
| ApxIA/ApxIIA | Pig, cow, rabbit | Erythrocytes, leukocytes, lymphocytes, macrophages, endothelia | Cytolytic, cytotoxic, superoxide production |
| ApxIIIA | Pig | Leukocytes, macrophages | Cytotoxic |
| AaltA | Human, ape, monkey | Leukocytes, lymphocytes | Cytotoxic |
| AshA | Pig, horse, sheep, cow | Erythrocytes | Cytolytic |
| LktA | Sheep, goat, cow | Leukocytes, lymphocytes, macrophages, platelets | Cytotoxic, superoxide production, leukotriene release, cytokine production, cytoskeletal rearrangement |
| PllktA | Pig, cow | Leukocytes, lymphocytes | Cytotoxic |
| CyaA | Human, sheep | Erythrocytes, leukocytes, lymphocytes, macrophages | cAMP production, cytolytic, cytotoxic, inhibits superoxide production |
| MbxA | Sheep, cow | Erythrocytes, lymphocytes, epithelia | Cytolytic, cytotoxic |
TABLE 3.
Division of toxins into hemolysins and leukotoxins in relation to the amino acids at the conserved KI and KII positions of HlyA
| Toxin | Activity | Position of:
|
Total no. of amino acids | |
|---|---|---|---|---|
| KI | KII | |||
| HlyA | Hemolysin | K564a | K690a | 1,024 |
| EhxA | Enterohemorrhagic hemolysin | K550 | K675 | 998 |
| ApxIA | Hemolysin | K560 | K686 | 1,022 |
| ApxIIA | Hemolysin | K557 | N687 | 956 |
| ApxIIIA | Leukotoxin | K571 | K702 | 1,049 |
| AaltA | Leukotoxin | K562 | K687 | 1,050 |
| AshA | Hemolysin | K557 | N687 | 956 |
| LktA | Leukotoxin | K554 | N684 | 953 |
| PllktA | Leukotoxin | K550 | S680 | 947 |
| CyaA | Adenylate cyclase/hemolysin | K860 | K983a | 1,706 |
Identified as acylated residue in in vivo-expressed toxin.
How such reported host cell specificities arose during the evolution of the toxin family is not clear from phylogenetic trees derived from DNA sequences (320), and the physical basis for cell specificity is unresolved. Studies performed on hybrid toxins created by the exchange of putative domains between HlyA, LktA, ApxIIA, and AaltA have indicated that specificity is unlikely to lie in a single discrete feature. Activity against erythrocytes has been correlated with a central region of HlyA and an N-terminal region of ApxIIA (88). The ability to kill leukocytes has been mapped to C-terminal regions of LktA and ApxIIA but appears less sharply defined in HlyA (88, 202). The critical region of AaltA required for recognition of human target cells spans its glycine-rich repeats (175), whereas the specificity of LktA for ruminants has been linked to a feature at its N terminus (88).
Ambiguity in the experimental definition of the target cell range may contribute to the lack of understanding of targeting by these toxins. Targeting has been defined by the cytotoxic end result (death), but this is the result of a multistage mechanism that might involve receptor recognition, membrane insertion, and protein-protein interaction. There are therefore many reasons why a particular cell line might be insensitive to a particular toxin. The toxin may not interact with the membrane, and this may occur for AaltA with human erythrocytes (298) and for LktA with nonbovine erythrocytes (15, 42). The toxin may adsorb to the membrane but fail to insert into the lipid bilayer, and this may be the behavior of inactive, nonacylated protoxins. Even if the toxin does permeabilize the membrane, the cell line will appear insensitive if it possesses a lesion repair mechanism. It is also possible that the bacterial host will affect the final toxin activity. The B. pertussis CyaA hemolysin synthesized in E. coli has a fourfold-lower hemolytic activity than does the native CyaA (109), and while ApxIIA protein secreted from its native host A. pleuropneumoniae is both hemolytic and cytotoxic, expression of the apxIICA genes in E. coli generates a toxin that is cytotoxic but has little or no hemolytic activity (305). LktA from E. coli carrying the P. haemolytica lktCA genes caused weak lysis of erythrocytes despite reports that LktA has a target cell range limited to leukocytes and platelets (87, 125). Continued biochemical investigation of the action of these toxins should establish whether continued division into hemolysins and leukotoxins is appropriate.
An explanation for the apparent cell specificity of these toxins might be offered by their recognition of specific membrane receptors. The existence of receptors for many bacterial toxins, both enzymatic and pore forming, is well established. Diphtheria toxin enters mammalian cells by using the epidermal growth factor-like growth factor as a receptor (220), and the AB5-type toxins such as cholera toxin, Shiga toxin, and verotoxin bind to cell surface ganglioside lipids (279). The binding of Staphylococcus aureus pore-forming leukotoxins to human polymorphonuclear neutrophils is initiated via a calcium channel or a receptor linked to a calcium channel (280). Aerolysin of Aeromonas hydrophila binds to erythrocytes and T lymphocytes via high-affinity receptors that have been shown to be glycoproteins with common glycosylphosphatidylinositol (GPI) anchors (73); Clostridium perfringens enterotoxin also uses several structurally related proteins as receptors (152); and the receptor for C. difficile toxin A has been identified as sucrase-isomaltase (245). Whether there is a receptor for the E. coli hemolysin is uncertain. Dose-response binding assays performed by one group support an upper limit of 4,000 HlyA binding sites per erythrocyte, implying at least some degree of specificity (15). However, this contrasts with results from others, who estimated up to 50,000 toxin molecules bound per erythrocyte and, using radiolabelled hemolytically active HlyA, subsequently found no evidence for saturation in binding to erythrocytes and leukocytes, indicating that this process is not conditional on specific receptors (31, 76). The absence of a specific receptor could be the reason why HlyA can attack a wide spectrum of target cells; indeed, HlyA can even bind to synthetic planar lipid membranes, suggesting that binding is relatively nonspecific (203). Nonspecific adsorption to the cell surface, rather than high-affinity binding to a surface receptor, has also been proposed to precede membrane insertion of B. pertussis CyaA (133). Inactive (unacylated) precursor toxin does not inhibit cell binding by active, mature toxin for both HlyA and CyaA, compatible with there not being a saturable receptor (15, 133).
It is possible that receptor-ligand interactions do occur with some members of the toxin family, for instance LktA and AaltA, displaying a narrower range of target cell specificities. Competition between inactive LktA mutants and mature LktA has been demonstrated, suggesting that a receptor in the leukocyte membrane is involved in LktA target specificity (61), and LktA is unable to bind to bovine leukocytes pretreated with protease, suggesting the involvement of a proteinaceous component in toxin binding (42). AaltA has been suggested to use a β-integrin as a receptor, since it is able to bind both subunits of the lymphocyte function-associated antigen type 1. HlyA may use the same β2-integrin for recognition of human leukocytes, but no direct binding has been shown (176). Integrins, a large family of αβ heterodimeric transmembrane receptors that are differentially expressed by a variety of cell types, have the potential variability to distinguish both cell type and species (except in erythrocytes) and are used as receptors during Shigella and Yersinia invasion of mammalian cells (84, 85, 130). Protein sequences potentially involved in putative receptor recognition would appear most likely to lie within the nonconserved domains of the toxin molecules (do the differences between E. coli HlyA and Ehx define regions of interaction with a human leukocyte receptor?). A model could be put forward in which toxin action against erythrocytes is an intrinsic property and does not use a receptor while action against leukocytes is defined by specific receptor interactions. The involvement of target cell components such as integrins would be consistent with the lack of receptor involvement in erythrocytes.
Subversion of Signal Transduction and Cytokine Production
Pathogenic bacteria frequently subvert host cell functions such as signal transduction pathways, cytoskeleton rearrangement, and vacuolar trafficking (reviewed in references 84 and 85). For example, enteropathogenic E. coli (EPEC) secretes proteins such as EspA and EspB that stimulate host phospholipase C, inducing inositol triphosphate production, the mobilization of intracellular Ca2+ stores, tyrosine phosphorylation of host proteins, and cytoskeleton rearrangement (251). Cell entry by Yersinia, Listeria, Salmonella, and Shigella also involves the activation of host signalling and a more extensive cytoskeleton rearrangement, which is controlled by small GTP-binding proteins belonging to the Ras family, namely, Rac, Rho, and CDC42. Shigella, Listeria, and Yersinia phosphorylate host proteins, including substrates for the nonreceptor tyrosine kinase Src. Salmonella, Mycobacterium, Chlamydia, and Legionella reside in intracellular membrane-bound vacuoles, and the stability of these vacuoles is believed to result at least in part from the bacteria interacting with vesicular trafficking that is normally mediated by a family of small GTP-binding proteins called Rabs. Proteins of the Ras, Src, and Rab families will be highlighted again in this review because of their possession of fatty acyl groups. There are also many examples of bacterial molecules inducing cytokine synthesis in eukaryotic cells, and although the most widely studied are lipopolysaccharide (LPS), capsular polysaccharides, and peptidoglycan, they also include porins, fimbrial proteins, heat shock proteins, and lipoproteins, as well as extracellular proteins such as proteases and toxins (reviewed in reference 120). Both gram-positive and gram-negative pathogens produce toxins that induce cytokine release; e.g., interleukin-1 (IL-1) and tumor necrosis factor (TNF) are induced in murine macrophages and human monocytes in response to listeriolysin O, pneumolysin, C. difficile toxin B, and Streptococcus pyogenes erythrogenic toxin A. Cholera toxin increases IL-6 synthesis and decreases TNF-α production by rat peritoneal mast cells, pertussis toxin enhances IL-4 production, and verotoxin induces the release of IL-1α, IL-6, and TNF-α in mouse macrophages.
Erythrocytes exposed to E. coli HlyA rapidly undergo cytoskeleton rearrangement, resulting in the formation of teardrop-shaped projections from the surface (147). HlyA also affects cytokine production. At very low, sublytic concentrations, HlyA is a potent trigger of G-protein-dependent generation of inositol triphosphate and diacylglycerol in granulocytes and endothelial cells, stimulating the respiratory burst and the secretion of vesicular constituents (30, 103). HlyA stimulates the release of IL-1β and TNF from human monocytes, the lipoxygenase products leukotriene B4 and 5-hydroxyeicosatetraenoic acid and nitric oxide from endothelial cells, and IL-1β (but not TNF-α) from cultured monocytes, while it inhibits the release of IL-1β, IL-6, and TNF-α from human leukocytes (29, 102, 164, 295). When injected into mice, HlyA produces a cytokine response similar to those seen in vitro, elevating the levels of IL-1 and TNF in serum (200). This ability to affect cytokine release is shared by all the members of the HlyA toxin family; in vitro, LktA and AaltA reduce the lymphocyte response to various stimuli (195, 197), at low concentrations, LktA stimulates the production of IL-1 and TNF by bovine mononuclear phagocytes (287, 326) and potentiates the production of histamine and certain eicosanoids in response to chemokines (1). LktA and ApxII activate bovine and porcine neutrophils, respectively (63, 121, 306). These effects may occur without necrotic cell death, since HlyA and AaltA cause apoptosis of human lymphocytes and LktA causes apoptosis of bovine mononuclear cells and granulocytes (142, 197). As discussed below, some inflammatory cytokines possess a comparable acyl modification to that of HlyA.
E. coli HlyA and other RTX toxins alter the membrane permeability of host cells, causing lysis and death. Lysis of erythrocytes might provide bacteria with iron, and killing nucleated cells may prevent phagocytosis. The benefit to extracellular bacteria as a result of the induction of a host response is not so obvious. A consequence of the release of cytokines may be to induce inflammation, disrupt epithelial cell junctions, and favor translocation of bacteria through the intestinal barrier. The activation of host cell signal transduction pathways may also be advantageous to bacteria if the host responds by presenting a receptor used by them for binding. Such mechanisms are used by B. pertussis, where binding of filamentous hemagglutinin to a monocyte integrin causes the presentation of a second filamentous hemagglutinin-binding site (131), and by EPEC, where adherence to epithelial cells occurs after tyrosine phosphorylation by the host of an inserted bacterial protein, which then associates directly with intimin, an EPEC adhesin (160).
It is possible that some of the biological effects so far assigned to the HlyA toxin actually reflect cooperative responses to both toxin and LPS. In vivo and in vitro studies indicate that exposure to LPS, or mediators stimulated by LPS, increases the subsequent response to HlyA and LktA (261, 287). One response of host cells attacked by HlyA is the rapid and massive shedding of LPS receptors (CD14), which may then bind to neighboring cells and make them sensitive to LPS. Such a mechanism might underlie the long-range detrimental effects of pore-forming toxins in host organisms (312). The induction of a host response may not be an alternative to cell death but an alternative pathway to death, proceeding via programmed cell death or apoptosis instead of lysis. Many bacterial toxins as well as members of the HlyA family can induce apoptosis (126). Host cell death by apoptosis would occur without leakage of cellular components, without inflammation, and without damage to surrounding cells (4).
Pore Formation in Eukaryotic Membranes
E. coli HlyA appears to have a two-stage interaction with eukaryotic membranes: a reversible adsorption sensitive to electrostatic forces and an irreversible insertion (10, 233). Transition to the inserted form is associated with a change of conformation (211) and is favored by membranes with a fluid state and low cholesterol content, which may explain earlier findings that membranes made from asolectin, a crude lipid mixture from soybean, are very sensitive to HlyA, in contrast to membranes composed of pure lipids such as phosphatidylcholine or phosphatidylserine (21, 231). Once inserted into a membrane, HlyA behaves as an integral protein; it cannot be extracted without the use of a detergent (27). HlyA causes target cell lysis by forming pores which display cation selectivity and voltage and pH dependence, as shown in experiments with whole cells, planar lipid membranes, and liposomes (203, 204). HlyA pores in erythrocytes are thought to be asymmetric, since proteolytic enzymes digest the toxin only when applied to one side of the membrane. Similarly, when monoclonal antibodies raised against HlyA were applied from the same side as the toxin, they could open or close the pore, but they could not do so when applied from the opposite side (204, 205). The physical properties of the transmembrane pore formed by E. coli hemolysin have been determined by using artificial lipid bilayers; it has a diameter of about 1.0 nm, a conductance of about 500 pS in 0.15 M KCl, and a mean lifetime of 2 s at low transmembrane voltages (21). HlyA pores in erythrocytes have been similarly sized, with a predicted physical cutoff of around 2 kDa (27). Patch-clamped human macrophages have been used as targets for HlyA, and this has confirmed that the leukotoxic action of HlyA is also due to the formation of pores with very different properties from the endogenous pores already present in the cell membrane (204). Similar physical properties have been assigned to the pores formed by other members of the HlyA toxin family (22, 55, 194, 203, 258), suggesting that pore formation is a closely conserved step in the action of these toxins.
For many bacterial toxins unrelated to E. coli HlyA, pore formation is synonymous with oligomerization, with their structures varying from trimer to 100-mer. V. cholerae El Tor cytolysin may form 3- to 5-mer oligomeric structures (206). E. coli heat-labile enterotoxin and P. aeruginosa cytotoxin form pentameric pores (228, 307), while aerolysin and S. aureus alpha-toxin oligomerize to produce heptameric pores (236, 278). The two gram-positive toxins streptolysin O and pneumolysin polymerize to form pores with a large and variable oligomeric superstructure of between 25 and 100 monomers (214, 235).
Oligomeric forms of staphylococcal alpha-toxin and streptolysin O are unusually stable and can be extracted intact from membranes with detergent (26, 90). In contrast, HlyA recovered from deoxycholate-solubilized erythrocyte membranes is recovered in a monomeric form, indicating either that oligomerization is not required for pore formation or that oligomers are dissociated in the detergent (27). The lower stability, larger size, and lower solubility of HlyA-related toxins have made elucidation of the accurate subunit stoichiometry of the membrane-inserted pore even more complex and left it currently unresolved. As with streptolysin O and staphylococcal alpha-toxin, membrane insertion of HlyA and CyaA is believed to occur through a monomolecular mechanism (24, 286), with oligomerization, if any, occurring by the subsequent addition of monomers within the membrane. It has been estimated that only one to three HlyA molecules form the pore (21, 203), while CyaA pores in artificial lipid bilayers indicate a functional unit of a trimer or larger (297). The increases in membrane conductance on exposure to HlyA and Apx toxins have been explained by an association between nonconducting monomers and conducting oligomers (21, 194). The complementation of inactive deleted variants of HlyA to produce hemolytic activity and CyaA to produce cytotoxic activity has suggested that two or more toxin molecules aggregate before pore formation and substantiate the view that oligomerization is involved (133, 189). However, in the absence of a defined pore structure, it is worth bearing in mind that membrane disruption may occur through mechanisms other than formation of discrete pores. Examples are provided by the antibacterial peptide cecropin, where a “carpet” of peptide monomers disrupts phospholipid packing (93), and complement-mediated lysis by the formation of “leaky patches” in the lipid bilayer (327). Indeed, the exposure of membranes to toxin at high concentration or for long times may lead to similar lesions through a detergent-like mechanism (210, 231).
A highly conserved region (amino acids [aa] 238 to 410) of HlyA (20, 61) is essential for lysis and is believed to be involved in pore formation (Fig. 2). It spans the only pronounced hydrophobic sequences in the otherwise hydrophilic HlyA protein, and secondary-structure predictions suggest that it comprises four membrane-spanning α-helices, each of 21 aa. The sequences preceding and between these four putative HlyA α-helices have strong amphipathic α-helical properties and possess membrane-binding characteristics. It has been suggested that these repeated hydrophobic and amphipathic helices may insert into the cytoplasmic membrane and form a pore, allowing the influx of extracellular calcium and the escape of potassium (188, 203). Mutations altering the hydrophobicity of this region reduce or abolish the pore-forming activity of the protein on erythrocytes and artificial membranes (186–188).
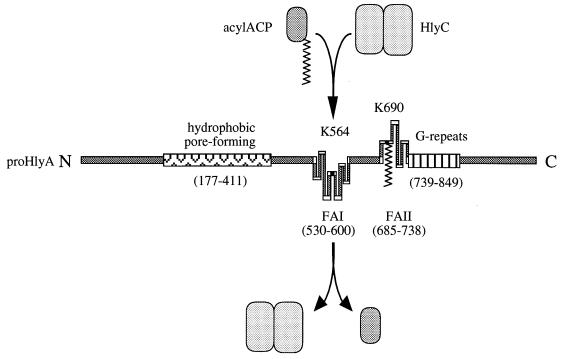
FIG. 2.
Maturation of pro-HlyA to the active toxin by HlyC and acyl-ACP. The positively charged HlyC homodimer associates with the negatively charged ACP. Following binding to the HlyC recognition domains, FAI and FAII, the acyl chain is transferred to the corresponding acyl modification sites, K564 (KI) and K690 (KII). The acylated lysines KI and KII are shown relative to the hydrophobic pore-forming domain and the glycine-rich Ca2+-binding repeats of pro-HlyA.
The conserved glycine-rich repeat domain associated with HlyA Ca2+ binding is required for the hemolysis of erythocytes but not for pore formation in asolectin lipid bilayers, since its deletion leaves the pore-forming ability of HlyA unaffected (187) (Fig. 2). In addition, HlyA shows full channel-forming activity in artificial lipid bilayers even in the presence of 5 mM EDTA (74). This suggests that while Ca2+ binding is critical at some stage of the lytic process such as promoting the irreversible insertion of the toxin into the membrane (11), it does not directly contribute to the pore-forming structure (37, 187). It also suggests that the asolectin lipid bilayer system is not a true reflection of binding and pore formation in real cell membranes, a conclusion supported by the finding that pore formation in asolectin bilayers is also independent of acylation (190). The conformation of the repeat region, which remains on the same side as that from which the toxin approached the membrane, may affect the state of the pore since the binding of a monoclonal antibody to this region led to pore closure (205).
Mutations in the LPS biosynthetic genes affect the expression and/or activity of E. coli HlyA (17, 286, 314). A transposon insertion in rfaP (required for attachment of phosphate-containing substituents to the LPS inner core) reduces the extracellular activity but not the export of HlyA as a consequence of the formation of large extracellular toxin aggregates; biological activity is restored with chaotropic agents (286). Why this occurs is not clear, but LPS possesses calcium-binding sites that may act as outer membrane reservoirs of calcium, and alterations in LPS structure may reduce the availability of calcium, resulting in the secretion of an inactive, unstable HlyA prone to aggregation. Alternatively, direct contact between wild-type LPS and HlyA may prevent toxin aggregation.
None of the dramatic effects of HlyA on eukaryotic host cells outlined above occur in vivo if the toxin is produced in the absence of the cotranslated protein, HlyC. The elucidation of the reason for this strict requirement has revealed a mechanism of bacterial toxin maturation unknown outside the HlyA family.
ESSENTIAL MATURATION OF HLYA: A UNIQUE FATTY ACYLATION
Bacterial Mechanism for Modifying Lysine Residues
HlyA toxin is synthesized as an inactive 1,024-residue protoxin, pro-HlyA, which is activated intracellularly to the mature toxin by the action of the cosynthesized HlyC. It was shown by using an in vitro system that this maturation is a fatty acylation and that HlyC is a homodimeric putative acyltransferase, using acyl-acyl carrier protein (acyl-ACP) as the fatty acid donor (112, 128, 129, 132, 282, 283) (Fig. 2). In in vitro reaction mixtures containing only purified acyl-ACP, HlyC and pro-HlyA, the acquisition of hemolytic activity is directly related to the binding of fatty acid by pro-HlyA. Mass spectrometry and Edman degradation of proteolytic products from mature HlyA toxin activated in vitro by HlyC and [3H]acyl-ACP revealed two fatty-acylated internal lysine residues, K564 (KI) and K690 (KII), and resistance of the acylation to hydroxylamine suggested that the fatty acid is amide linked. Substitution of the two lysines confirmed that they are the only sites of acylation and showed that although each is acylated in the absence of the other, both sites are required for in vivo toxin hemolytic activity (282). The K564 and K690 residues were subsequently confirmed to be modified in in vivo-synthesized and secreted HlyA by peptide mapping and two-dimensional electrophoresis (190). The internal pro-HlyA modification explains the earlier isolation of a monoclonal antibody that recognized only the active form of HlyA, specifically epitope aa627 to 726 (238).
The other HlyA-related toxins secreted by pathogenic gram-negative bacteria all require HlyC-type protoxin activation, and indeed many (PvxA, MmxA, ApxIA, and LktA) have been activated by E. coli HlyC (87, 106, 167). In addition, CyaC, ApxIIC, and AaltC are able to activate pro-LktA (175, 202, 322). CyaA, the adenylate cyclase hemolysin of B. pertussis, requires posttranslational activation both to deliver its catalytic domain into the mammalian target cell (cell-invasive activity) and to form transmembrane channels (hemolytic activity). It has been demonstrated by mass spectrometry that CyaA toxin secreted by B. pertussis is modified by amide-linked palmitoylation on the ɛ-amino group of K983 (analogous to HlyA KII, K690) (108). Only modification sites of HlyA and CyaA have been demonstrated to be acylated, although the loss of activity caused by deletion of the region between aa 358 to 548 of P. haemolytica LktA is compatible with the KI residue, K554, as a potential site of modification (61), and activation of a CyaA-LktA hybrid toxin supports the notion that the region aa 379 to 616 of LktA is important for LktC recognition (322). Substitution of LktA K554 (to T and C) reduced the lytic activity of LktA against bovine lymphocytes by only ca. 40%, but the retention of lytic activity does not rule out K554 as an LktA acylation site, since HlyA mutants with either KI or KII deleted also retained about 50% activity against BL-3 cells despite being inactive to erythrocytes (239, 282). Bovine lymphocytes may be less sensitive to the state of acylation of these toxins than are either erythrocytes or human lymphocytes, but it remains a possibility that LktA contains an acylation site different from the KI and KII sites identified for HlyA and CyaA. No data for the other toxins have been presented.
HlyC, a Novel Acyltransferase
The ability to transfer an acyl group to an internal lysine residue of a target protein distinguishes HlyC from all other bacterial acyltransferases, and hlyA+ hlyC strains are nonhemolytic, indicating that the various constitutive acyltransferases of E. coli are unable to substitute for HlyC to even a small degree. HlyC has no significant sequence homology to known acyltransferases such as the lipid A acyltransferases (3, 54, 56, 158), the Rhizobium Nod factor acyltransferases NodL and NodA (35, 69), or the well characterized acyltransferases such as glycerol-3-phosphate acyltransferases (182) or eukaryotic N-myristoyltransferases (140, 332). HlyC may therefore be an acyltransferase that is structurally and functionally distinct from all other acyltransferases.
Little is known about the biochemical properties of HlyC, the enzyme responsible for protoxin acylation. Bacterial and eukaryote acyltransferases generally accept either acyl coenzyme A (acyl-CoA) or acyl-ACP as an acyl donor, but ACP is a strict requirement for HlyC-directed pro-HlyA acylation (acyl-CoA cannot be used). Myristoyl-ACP gave the highest hemolytic activity of HlyA acylated in vitro with a range of fatty acids (C12 to C18:1) (132), which is consistent with an apparent selection of myristoylation at both the KI and KII sites in vitro. The affinity of KI for myristoyl-ACP (a saturated acyl group of 14 carbons) in vitro is approximately twice that for palmitoyl-ACP (a saturated acyl group of 16 carbons), while the affinity of KII for myristoyl-ACP is approximately five times that for palmitoyl-ACP (285). In vivo, both K564 and K690 appear fully acylated, suggesting that unlike CyaA, HlyA is predominantly myristoylated (190). Not only is HlyC able to discriminate between acyl-ACPs carrying fatty acids of different lengths but also it is presumably able to do this at low substrate concentrations, since E. coli does not have significant pools of acyl-ACPs with fatty acids longer than 3 carbons (249). In vitro measurements of Km for both ACP and protoxin substrates suggest that HlyC does indeed recognize its substrates at extremely low concentrations (∼100 and ∼10 nM, respectively). HlyC is able to bind tightly but, it seems, noncovalently both the acyl chain from acyl-ACP and phosphopantetheine, but the locations of the active site of HlyC and regions that bind its acyl-ACP and pro-HlyA substrates are not known (284). The specificity for acyl-ACP as the acyl donor is shared in E. coli by the acyltransferases involved in lipid A biosynthesis, LpxA, LpxD, HtrB, and MsbB, but the use of a protein as a substrate is unique to HlyC (43, 158, 246). The cytosolic enzyme, LpxA, catalyzes the first step of lipid A biosynthesis, transferring (R)-3-hydroxymyristate from ACP to UDP-N-acetylglucosamine (3). LpxD is also specific for (R)-3-hydroxymyristoyl-ACP (158). The so-called “late” acyltransferases, HtrB and MsbB, can both use lauroyl-ACP and myristoyl-ACP as substrates for generating acyloxyacyl residues in lipid A, although HtrB shows a fivefold preference for lauroyl-ACP (54).
HlyC Recognition Domains on the Protoxin
By using deleted protoxin variants and protoxin peptides as substrates in an in vitro maturation reaction dependent on only HlyC and acyl-ACP, two independent HlyC recognition domains (FAI and FAII) have been identified on the HlyA protoxin, each of which spans one of the target lysine residues, KI or KII, respectively (283) (Fig. 2). Each domain requires 15 to 30 aa for basal (ca. 10%) recognition and 50 to 80 aa for full wild-type acylation. The peptide recognition sequences for HlyC appear to be larger than those of other acyltransferases, since acylation of mutagenized target substrate proteins, chimeric proteins, and substrate peptides has indicated that peptide sequences of 4 to 15 aa are recognized by N-myristoyltransferases and prenyltransferases (198, 240, 302, 303, 331). However, these modification sites are generally at the N and C termini of the substrate proteins, where they may be relatively open and accessible. In contrast, the internal acylation sites of HlyA may require HlyC to recognize a larger topology rather than a linear sequence. While the HlyA FAI domain is symmetrically centered on the modified lysine residue, the FAII domain appears to be asymmetrical, not requiring amino acids N-terminal to the modified residue (283). The two domains are nevertheless functionally indistinguishable and compete with each other for HlyC both in cis and in trans. No other HlyA sequences are required for toxin maturation, including the immediately C-terminal Ca2+-binding repeats (aa 722 to 849). Indeed, in vitro, Ca2+ ions prevent acylation at both the KI and KII sites (283). The extreme sensitivity of the pro-HlyA activation reaction to free Ca2+ supports the view that intracellular Ca2+ levels in E. coli are too low to affect toxin activity (92) and that Ca2+ binding does not occur until the toxin is outside the cell.
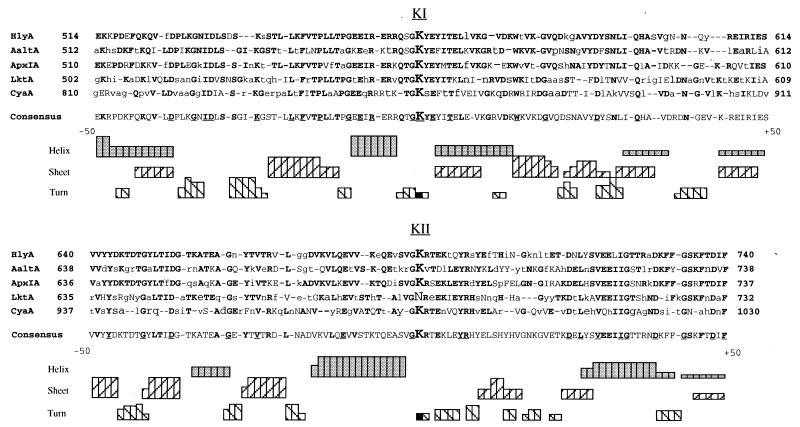
The HlyC recognition sites of E. coli HlyA have been compared with each other and with the corresponding sequences of other members of the toxin family. Although FAI and FAII of HlyA have the same function, there is little primary sequence identity (only 21%), and this lack of homology is also evident within each site among the toxins; only 20% of the FAI residues and 17% of the FAII residues are identical in the five toxins shown (Fig. 3). This divergence may reflect differences in the HlyC-type proteins, the pattern of acylation at the two sites (where present, some toxins of the Pasteurellaceae family lack the KII target lysine), and the preferred target cell range of each toxin. Consensus sequences constructed for each of the two acylation sites have little in common apart from the central “GK” motif, suggesting that similarity between FAI and FAII may be of a higher structural order. Secondary-structure predictions of FAI and FAII indicate that both regions are rich in β-turns, quite regularly spaced approximately every 10 aa, especially in FAI. Other predicted features are not shared by FAI and FAII, except perhaps for a helical region immediately N-terminal to both KI and KII. However, helical-wheel projections do not show amphipathic distributions of charged or hydrophobic residues as related to membrane-associated structures. The lack of identity between FAI and FAII domains in pro-HlyA and corresponding sites on related protoxins currently deters an explanation for the basis of HlyA recognition by HlyC.
FIG. 3.
Alignment of the E. coli HlyA FAI and FAII HlyC recognition domains with corresponding sequences of the related toxins A. actinomycetemcomitans AaltA, A. pleuropneumoniae ApxIA, P. haemolytica LktA, and B. pertussis CyaA. Identical residues are in boldface type; amino acids belonging to the same class are in capitals and non-homologous amino acids are in lowercase. Hyphens represent breaks introduced to maximize identity. Consensus sequences for FAI and FAII indicate residues shared by four toxins (boldface) and by all five toxins (underlined). A cumulative histogram representation of secondary structure predictions by the Chou and Fasman and the Robson and Garnier methods are shown under the primary sequences.
A lack of cross-complementation between protoxin activator C proteins supports the view that C-proteins may be unable to modify a KI or KII site even when it is present. E. coli HlyC is able to activate P. haemolytica LktA, whereas LktC is unable to activate HlyA and CyaA (87, 322). One explanation would be that LktC can acylate only KI even when KII is present. However, this explanation could not extend to A. pleuropneumoniae ApxIIC, which can activate P. haemolytica LktA, whereas LktC is able to activate ApxIIA only marginally, despite both toxins apparently possessing only KI (202) (Table 3). It seems possible that there are additional factors influencing the recognition between protoxin and HlyC-like activator proteins. One such additional factor appears to be the host cell in which the toxin operon is expressed. In contrast to the native CyaA protein from B. pertussis, which is palmitoylated exclusively at K983 (KII), recombinant pro-CyaA protoxin modified by CyaC in E. coli is acylated at K983 and contains an additional acylation site at K860 (analogous to KI) (109). It is perhaps this anomalous acylation in E. coli that allows CyaC to activate LktA (322). The specificity of CyaC peptide recognition may be affected by the host cell ACP, but this presumes that it is a C-ACP complex which recognizes the toxin. Such a mechanism is supported for HlyC by in vitro kinetic data that also indicate the involvement of a ternary HlyA-HlyC-ACP complex during the transfer of fatty acid from acyl-ACP to pro-HlyA (284). An ordered Bi-Bi mechanism would be analogous to that of N-myristoyltransferases, for which it is the binding of acyl-CoA which determines subsequent peptide binding (252, 332). This might explain the earlier failures of LktC to activate the proforms of HlyA, ApxIIA, and CyaA (87, 202, 322) in experiments which were performed in or with extracts from an E. coli background. Alternatively, E. coli may possess an escort protein, similar to that observed with prenylated proteins, which enhances the acylation at KI but which is absent in B. pertussis (269). However, one might expect such an escort protein to have been identified by mutagenesis studies carried out in many laboratories that have successfully identified other accessory proteins, such as TolC and RfaH, required for toxin activity. Although the data available at present are incomplete and address only HlyA and CyaA, it appears that there is selection for the fatty acid attached to the toxin and that E. coli and B. pertussis have evolved hemolysin acyltransferases with different affinities for fatty acid. The E. coli HlyC protein may preferentially acylate HlyA KII with myristic acid, whereas the B. pertussis CyaC protein preferentially acylates CyaA KII with palmitic acid. Similar variations in substrate specificity between related proteins of different species appear between E. coli, Neisseria meningitidis, and P. aeruginosa LpxA, which are specific for (R)-3-hydroxymyristoyl-ACP, 3-hydroxylauroyl-ACP, and 3-hydroxydecanoyl-ACP, respectively (75, 227), and E. coli and Haemophilus influenzae HtrB, which are specific for lauroyl-ACP and myristoyl-ACP, respectively (54, 224). Not only do differential fatty acid affinities require an explanation, but so do cross-species complementation tests with C-proteins and host-specific acylation patterns. Presumably, these explanations will lie in the differences between the structures of the family toxins, activator C-protein acyltransferases, and ACPs.
An insight into the modification of HlyA might be expected from an examination of other examples of protein lipidation. We present an extensive review of these and show that while the different types of modification vary considerably in terms of the location and structure of the lipid moiety, it is possible to define universal themes in terms of their function. Despite the absence of data for acylation events exactly equivalent to that of the prokaryotic toxins, it is still possible to draw conclusions from the more extensively studied examples that are relevant to the roles that toxin acylation may play in determining biological function. Lipidated proteins found in and on eukaryotic membranes are included in this comparison since the eukaryotic cell membrane is the site of action of HlyA and its related toxins. It is also becoming apparent that these acylated proteins are the sites of action of many bacterial effector proteins either directly by modification (e.g., ADP-ribosylation, glucosylation, and deamidation of small G-proteins and heterotrimeric G-protein subunits) or indirectly by interfering with their enzymatic pathways (e.g., protein phosphatases and kinases) (84, 85).
PROTEIN LIPIDATION IN PROKARYOTES AND EUKARYOTES
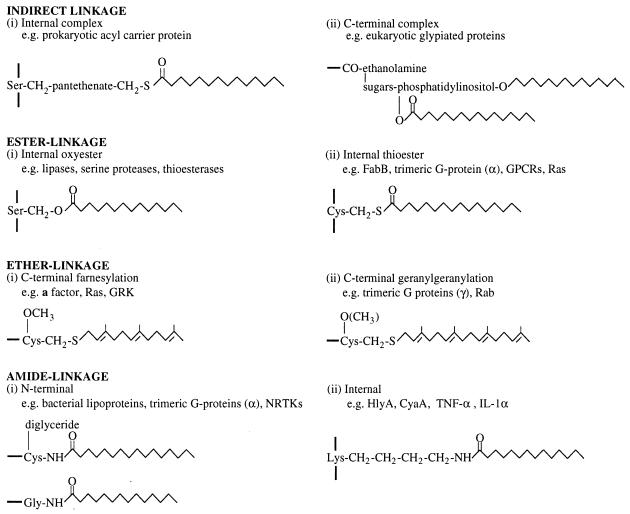
Lipidation is involved in the maturation of many proteins in both prokaryotic and eukaryotic cells, including viral oncogene products, but it is achieved by various mechanisms which differ according to the fatty acid transferred, the amino acid modified, and the fatty acyl donor. Myristic and palmitic acids are the most common fatty acids cross-linked to proteins. Proteins sorted to the bacterial outer membrane or eukaryotic plasma membrane undergo processing in which an acyl group is attached to the N-terminal amino acid; enzymes with acyltransferase, lipase, or esterase activity use catalytic mechanisms involving ester-linked acyl groups attached to serine and cysteine residues; and eukaryotic proteins use ester-linked palmitoylation and ether-linked prenylation of cysteine residues for membrane sorting and protein-protein interaction. As can be seen below, the acylation of pro-HlyA does not equate to any of these but instead appears to be limited to its related toxins and perhaps a handful of eukaryotic proteins (Fig. 4).
FIG. 4.
Major classes of lipidated proteins in prokaryotes and eukaryotes. The structure of the lipid and their attachment to the protein peptide backbone are shown, except for the complex indirect linkages of pantetheinylated and glypiated proteins. For heterotrimeric G-proteins, the particular modified subunit is indicated in parentheses. The placement of the methyl group of geranylated proteins in parentheses indicates that carboxymethylation is not universal.
Lipid Groups Indirectly Linked to Their Peptide Backbone
Bacterial phosphopantetheinylated proteins.
The inclusion of a phosphopantetheine cofactor increases the number of thiol groups on a protein and introduces a tether to increase the flexibility of acyl chains between various active sites. As one of the most abundant proteins in E. coli, constituting 0.25% of the total soluble protein, ACP is an important example of a protein carrying an indirectly linked acyl group. In addition, the central involvement of ACP in the acylation of bacterial toxins warrants an extended account of this small but multifaceted protein. ACP is required throughout fatty acid biosynthesis, carrying fatty acids as thioester intermediates attached to the terminal sulfhydryl of a 4′-phosphopantetheine group (4′-PP), which is in turn attached to a serine residue (Ser36) via a phosphodiester linkage (192).
E. coli ACP is a 9-kDa acidic protein (pI 4.1). The acpP structural gene has been cloned and overexpressed (143), and the ACP solution structure has been defined (127). Sequences between aa 31 and 71 are thought to be involved in fatty acid binding, with the acyl chain held in a pocket (144, 201). The 4′-PP sulfhydryl is the only thiol group in E. coli ACP, and without it the apoprotein is inactive. Holo-ACP synthase (encoded by acpS, formerly dpj) transfers the 4′-PP moiety from CoA to apo-ACP to produce holo-ACP (178). In normally growing cells, virtually all of the ACP is maintained in the active, holo-form (134, 156). During logarithmic growth, there is a significant pool of unacylated holo-ACP together with acetyl-ACP and malonyl-ACP, but there is no acyl-ACPs of four carbons or longer (249).
As well as being a component of the fatty acid synthase and acting as an acyl donor for the HlyA family of protein toxins (132), E. coli ACP functions in the transfer of long-chain fatty acids to phospholipids (58, 249) and to the lipid A component of LPS (3). In E. coli (and mitochondria), ACP is used by lipoate transferases as a lipoate donor (146). In addition, ACP is tightly associated with MukB, a protein required for chromosome partitioning (226), and it may also be involved in the initiation of the transposition of Tn3 (191). In a role known not to require the 4′-PP group, ACP is an essential component of a UDP-glucose-requiring transglucosylase system that catalyzes the synthesis of the β-1,2 backbone of membrane-derived oligosaccharides (299). Homologs of E. coli ACP occur as integral domains of large multifunctional enzymes such as in the eukaryotic fatty acid synthases and in multiprotein complexes as discrete proteins. Apart from playing a role in fatty acid synthesis, ACP or ACP-like proteins are needed in the synthesis of polyketide (e.g., Streptomyces glaucescens 9-kDa TcmM [268]), nonribosomally synthesized peptide (e.g., S. clavuligerus 300-kDa ACV synthetase [12]), and depsipeptide (e.g., Fusarium scirpi 347-kDa enniatin synthetase [241]), cell host signalling (e.g., Rhizobium 10-kDa NodF [95]), and cell wall synthesis in gram-positive bacteria (e.g., Lactobacillus casei 6-kDa Dcp [118]). In bioluminescent bacteria such as Vibrio harveyi, ACP is also involved in the synthesis of the myristaldehyde substrate used by luciferase (47).
Only one ACP has been found in E. coli, and this appears sufficient for all ACP-dependent roles. In contrast, Rhizobium species make not only a constitutive ACP but also the inducible NodF, which participates in the transacylation of oligosaccharides for nodulation (243, 267), and AcpXL, which donates 27-hydroxyoctacosanoic acid to a lipid A precursor (44). Three ACPs have also been identified in S. coelicolor; one is presumed to be the fatty acid synthase ACP, while the other two are involved in the synthesis of aromatic polyketides (248). The anonymous gene iacP of Salmonella typhimurium SPI-1 (Salmonella pathogenicity island 1) encodes an ACP that may be specific for bacterial invasion, with an as yet unidentified ACP being responsible for the synthesis of essential phospholipids, as in E. coli (151). The importance of these specialized bacterial ACPs may be to allow the synthesis of unusual metabolites without interfering with the synthesis of essential cellular lipids. In a typical bacterial cell, as many as a dozen different enzymes, with overlapping acyl chain specificities, compete for the pool of acyl-ACP. Consequently, variations in the structure of ACP could have a large influence on the metabolic fate of acyl groups, including their transfer to acylated bacterial toxins.
Apart from ACP, E. coli possesses at least two other proteins that contain a 4′-PP group and so potentially have indirectly linked acyl groups. EntB is a 33-kDa protein that serves as an aryl carrier protein, and EntF is a 142-kDa enzyme that is responsible for serine activation during the biosynthesis of enterobactin (94, 253). Gramicidin S synthetase, a Bacillus enzyme with homology to EntF, carries 4′-PP on a serine residue within a conserved LGGDSI motif (310). Three phosphopantetheinyl transferases have been identified in E. coli. ACP synthase is specific for ACP, EntD modifies EntB and EntF, and the substrate for the third is unknown (94, 177).
Eukaryotic glypiated proteins.
Fatty acyl groups are linked indirectly to many eukaryotic proteins by the GPI moiety (Fig. 5). This contains an entire phospholipid, in which the lipid moiety is variable, possessing C14 to C24 acyl groups, and is associated with sugars and ethanolamine (77). In many instances, the inositol ring contains an additional lipid modification in the form of an ester-linked palmitic acid. The complete GPI anchor is transferred to proteins by GPI-transamidases at a specific C-terminal recognition sequence which is cleaved in the process. The GPI signal sequence is extremely degenerate but commonly features a run of 12 to 20 hydrophobic residues. GPI-anchored proteins are abundant on the cell surfaces of lower eukaryotes such as protozoa and yeasts and have a wide variety of functions such as nutrient uptake and membrane-signalling events; they include hydrolytic enzymes, receptors, cell adhesion molecules, complement inhibitors, and antigens of unknown function (77, 79, 98).
FIG. 5.
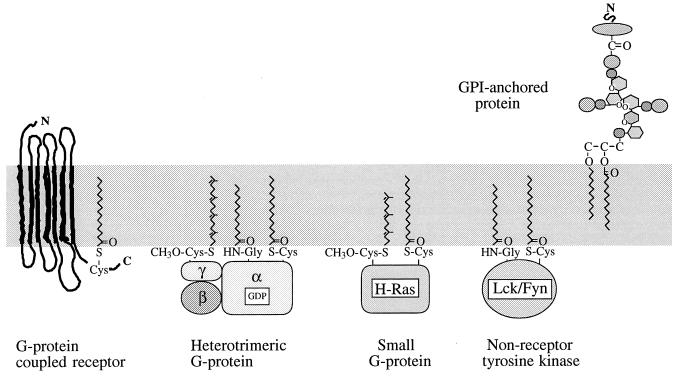
Membrane localisation of lipidated proteins on the plasma membrane of mammalian cells targeted by HlyA. Typical proteins from the major types involved in cell signalling are represented. The lipid groups are shown inserted directly into the bilayer, although other protein interactions may be involved. Many G-protein coupled receptors possess internal cysteine residues that may act as palmitoylation sites as shown, and some have been shown to be modified. The heterotrimeric G-protein α subunit, Ras and NRTK proteins shown on the inner face of the plasma membrane possess two lipids, although other isotypes may have only one. The G-protein α-subunit and Src-related NRTK contain both a myristoyl group linked to the N terminus and a thioester-linked palmitoyl group. The G-protein γ subunit contains a C-terminal thioether-linked geranylgeranyl isoprenoid. The Ras-related small G-protein contains a C-terminal thioether-linked farnesyl isoprenoid and a thioester-linked palmitoyl group. The structure of a typical GPI-anchored protein with its constituent inositol, glucosamine, mannose, and ethanolamine groups is shown. An additional palmitoyl chain may be attached to the inositol ring.
Lipid Groups Directly Linked to the Protein Peptide Backbone
Ester-linked acylation.
The attachment of lipid groups to proteins through ester bonds provides a labile connection suitable for a transient role such as in catalytic mechanisms. Some enzymes in the biosynthetic pathways of fatty acids, phospholipids, and LPS bind acyl groups tightly but do not necessarily form covalent acyl-enzyme intermediates, e.g., E. coli acyl-ACP synthase (136) and β-hydroxy-decanoyl-ACP dehydrase (180). However, other enzymes, particularly acyltransferases but also lipases and esterases, do use mechanisms involving oxy- and thioester acyl-enzyme intermediates (45, 66, 80, 138, 179, 181, 192, 193). In addition to these enzymes, a class of acylated proteins found in eukaryotes but apparently absent in prokaryotes carry ester-linked acyl groups not for a catalytic purpose but for structural reasons. The nature of this modification is outlined below, and its influence on protein structure and function is examined for lessons that may be applied to the acylation of HlyA.
The covalent modification of eukaryotic proteins with palmitate occurs posttranslationally at the thiol group of cysteine residues. A number of proteins involved in intracellular signalling are palmitoylated (208, 209, 216, 247). A wide variety of cell responses to signals such as growth factors, neurotransmitters and hormones is mediated by GTP-binding (G)-protein-coupled receptors (GPCRs), which are integral heptahelical proteins located on the plasma membrane. On ligand binding, these activate heterotrimeric G-proteins on the inner face of the membrane through direct interaction. Heterotrimeric G-proteins comprise three subunits, α, β, and γ, the last two forming a very tight complex (317). Many GPCRs, including the pigment rhodopsin (39, 321), some heterotrimeric G-protein α-subunits (216), receptor tyrosine kinases (RTKs) (290), small G-proteins (222), and non-receptor tyrosine kinases (NRTKs) of the Src family (247, 272) are palmitoylated (Fig. 5).
The labile nature of the thioester bond opens the possibility of acylation being reversible, and there is evidence for this in enzymes that catalyze both attachment and removal of lipid, which could regulate the palmitoylation state of a target protein (216). So far, these enzymes are largely uncharacterized. However, an assay for the palmitoyl acyltransferase that palmitoylates members of the Src family has been developed (23), enzymes that transfer palmitoyl groups to H-Ras and spectrin have been purified (67, 184), and a thioesterase that removes palmitate from H-Ras and Gα subunits has been cloned (50).
In contrast to the labile connection of ester-linked acyl groups, the ether linkage of isoprenoid groups and the amide linkage of acyl groups provide proteins with a stably attached lipid. Such irreversible modifications are potentially more relevant to the amide-linked maturation of toxins, although, again, the responsible transferases are distinct from HlyC.
Ether-linked prenylation.
Several eukaryotic intracellular proteins are post-translationally modified by farnesyl (C15) or, more commonly, geranylgeranyl (C20) unsaturated isoprenoid lipids, attached through thioether bonds to cysteine residues at or near their C terminus (Fig. 5). This constitutive process results in a stably modified protein. Three protein prenyltransferases are responsible for the modification of separate substrate groups. Farnesyltransferase and geranylgeranyltransferase I recognize proteins with a -CaaX C-terminal motif. Following prenylation, the three C-terminal residues are removed from most CaaX-type proteins and the prenylcysteine residues is methylated at its exposed carboxyl group. Geranylgeranyltransferase II recognizes C-terminal double cysteine motifs such as -CC or -CxC (198, 331).
Isoprenylated proteins include fungal mating factors, nuclear lamins, several vesicular transport proteins, the oncogene product Ras and Ras-related GTP-binding proteins, the subunits of trimeric G-proteins, and protein kinases (48, 331). For a variety of fungi, mating is initiated by peptide pheromones, several of which have been suggested to be lipopeptides. In Saccharomyces cerevisiae, the a-factor is isoprenylated with a farnesyl group. Members of the Ras family of monomeric GTP-binding proteins are modified by isoprenoid lipids (198), and Ras itself is farnesylated. Geranylgeranyl and geranylgeranyl/farnesyl modifications are reported for mammalian Rab proteins and yeast Ypt proteins. The digeranylgeranylation of Rab proteins is a complex process, requiring a Rab geranylgeranyltransferase and an escort protein (REP). Each acylation is an independent reaction, but mono-GG-Rab remains bound to REP, ensuring the efficient double modification of Rab (269). Heterotrimeric G-protein γ subunits are prenylated (217). The geranylgeranyl moiety is found on all γ subunits except the retinal-specific form, which is farnesylated. G-protein-coupled receptor kinases may be modified with a farnesyl group (e.g., retinal G-protein-coupled receptor kinase) (234).
Amide-linked acylation.
(i) N-terminally acylated bacterial lipoproteins.
Lipoproteins exported to the periplasm or outer membrane of gram-negative bacteria are modified with diacylglycerol, via thioether linkage to cysteine, and with an amide-linked fatty acid (65% palmitate) on the N terminus (116). The precursor protein undergoes three reactions directed by enzymes in the inner membrane (i.e., phosphatidylglycerol diacylglyceryl transferase, signal peptidase II, and apolipoprotein N-acyl transferase), resulting in the generation of N-acyl diacylglycerylcysteine as the N-terminal amino acid (254). The first known example of bacterial lipoprotein, E. coli murein (also called Braun’s lipoprotein), occurs as a free form and a bound form covalently attached to the peptidoglycan layer by a peptide linkage via the ɛ-NH2 group of the C-terminal lysine. The fatty acids transferred to the prolipoprotein are taken from the phospholipid pool, with the major source being phosphatidylethanolamine (104, 135). Prolipoprotein acylation produces lysophospholipids that are reacylated by the inner membrane 2-acyl-glycerophosphoethanolamine acyltransferase. This acyltransferase can use acyl-ACPs from fatty acid biosynthesis or can convert fatty acid to acyl-ACP in the presence of ATP-Mg2+ (58).
Bacterial proteins with a broad range of functions have been found to be lipidated. More than 130 direct homologs of Braun’s lipoprotein have been identified in gram-negative bacteria, including extracellular enzymes (e.g., β-lactamases, chitobiase, and β-1,4-endoglycanase); TraT, a surface-exposed lipoprotein that blocks conjugative transfer to cells carrying related plasmids (113); PulA, the pullulanase of Klebsiella oxytoca, and proteins for its secretion (65); flagellar proteins such as Salmonella typhimurium FlgH that forms the outer membrane L ring of the flagellar basal body (259); and LamB, which facilitates the uptake of maltose and maltodextrins across the bacterial outer membrane and acts as a general porin for small molecules (162). Many proteins in gram-positive bacteria have been suggested to be lipidated due to the presence of the consensus cleavage site (LAGC), and in some cases this has been confirmed by incorporation of radiolabelled fatty acid or interference by the antibiotic globomycin that inhibits processing by signal peptidase II (292). As in gram-negative bacteria, these lipoproteins are involved in diverse processes such as transport, adhesion, protein secretion, and conjugation. Examples include an Alicyclobacillus 40-kDa protein with similarity to enterobacterial maltose-binding proteins (122), and NisI, which affords Lactococcus lactis immunity to the antimicrobial peptide nisin (174).
(ii) N-myristoylated eukaryotic proteins.
Another N-terminal lipidation is undergone by several eukaryotic proteins in which a myristoyl group is attached to the α-NH2 group of the N-terminal glycine (position 2) following removal of the initiator methionine (140). The enzyme responsible, myristoyl-CoA:protein N-myristoyl transferase, is ubiquitous in eukaryotic cells. It was first isolated from Saccharomyces cerevisiae and has been extensively investigated (140, 302, 303, 332). It is almost exclusively specific for myristic acid and has stringent sequence requirements with respect to the 5 aa immediately distal to the glycine residue (140). An example of a myristoyl-CoA:protein N-myristoyl transferase using an acyl substrate other than myristoyl-CoA has been characterized in the retina (141, 163). Protein myristoylation is cotranslational and constitutive, and the product is usually a stably modified protein, although lipid removal from a mature protein has been reported (196).
N-myristoylation has been reported for more than 100 eukaryotic cellular and viral proteins, including a number of proteins involved in cell signalling that are also palmitoylated. Nearly all G-protein α-subunits are modified by an N-terminal myristoyl group (215), as are all members of the Src family of NRTKs. Other protein tyrosine kinases, while not lipidated themselves, signal through members of the Ras family of monomeric (“small”) G proteins that are lipidated (Fig. 5), e.g., the Arf family involved in vesicular transport (38). Other N-myristoylated proteins include MARCKS (myristoylated alanine-rich C kinase substrate) (296); human immunodeficiency virus type 1 matrix protein (333); the calcium-binding sensor of photoreceptor cells, recoverin (2); and the hisactophilins of Dictyostelium discoideum (111).
(iii) Internal amide-linked acylation.
As we have shown above, protein acylation is generally divided into labile internal modifications and stable modifications at the N and C termini. The mechanism of the stable acylation of HlyA at internal lysines via amide bonds is unique to the HlyA toxin family; no other prokaryotic proteins are known to be modified in this way. However, there are eukaryotic proteins that possibly have similarities to the HlyA acylation mechanism, but there has been little detailed characterization since their identification in the 1980s. Internal acylated lysines have been defined in four eukaryotic proteins, while another three proteins may possess hydroxylamine-resistant acyl groups not present at the N terminus. None of the associated acyltransferase activities has been characterized (Table 4).
TABLE 4.
Prokaryotic and eukaryotic proteins known or believed to possess internal amide-linked acyl groups
| Protein | Total no. of amino acids | Acyl donor | Modified residue | Acyl group |
|---|---|---|---|---|
| Prokaryotic | ||||
| Pore-forming toxins | ||||
| E. coli HlyA | 1,024 | Acyl-ACP | K564 | ?a |
| K690 | ?a | |||
| B. pertussis CyaA | 1,706 | Acyl-ACP? | K860b | C16b |
| K983 | C14b, C16 | |||
| Eukaryotic | ||||
| Inflammatory cytokines | ||||
| TNF-α | 233 | Acyl-CoA | K19 | C14 |
| K20 | C14 | |||
| IL-1α | 271 | Acyl-CoA | K82c | C14 |
| K83c | C14 | |||
| Mitogenic receptors | ||||
| Insulin receptor (α and β) | 1,382 | ? | ? | C14, C16 |
| Immunoglobulin | ||||
| μm | 593 | ? | ? | C14 |
| μs | 572 | ? | ? | C14 |
| Cholinergic receptors | ||||
| Nicotinic acetylcholine receptor | ||||
| α | 457 | ? | ? | C16 |
| β | 501 | ? | ? | C16 |
| Membrane complexes | ||||
| cytochrome c oxidase (subunit 1) | 557 | Acyl-ACP? | K324 | C14 |
C14 preferred substrate in vitro.
Acylated in E. coli only.
Monoacylated preferred product.
Three inflammatory cytokines, TNF-α, IL-1α, and IL-1β, possess myristoyl groups attached through amide bonds to internal lysines. They are translated as 26- and 31-kDa precursors and are subsequently processed to produce extracellularly active, C-terminal 17-kDa mature proteins (117). The myristoylated residues actually lie within their cleaved propieces, and so acylation is not connected to the activity of secreted cytokines. Full-length TNF-α is myristoylated at lysine residues K19 and K20, and IL-α precursor is myristoylated at K82 and K83. A similar sequence in the IL-1β propiece is myristoylated but at a lower efficiency (288, 289). Lysyl N-ɛ-NH2-myristoyl transferases that recognize small peptides (14 aa) as substrate and myristoyl-CoA as an acyl donor presumably exist for both TNF-α and IL-α, but these have not yet been identified (288, 289). However, even from the preliminary characterizations of peptide substrate and acyl donor, the acyltransferases are clearly different from HlyC.
Nicotinic acetylcholine receptor and insulin receptor are integral membrane glycoproteins composed of five and two subunits, respectively (57, 62). The presence of covalently bound fatty acids on these receptors has been explored by metabolic labelling of cultured cell lines with [3H]myristic and [3H]palmitic acids. The α- and β-subunits of both receptors have an amide-linked fatty acid that is thought to be attached to internal free amino groups (119, 229). The site(s) of fatty acid attachment and the chemical nature of the lipid linkage have not been identified, but insensitivity to hydroxylamine indicates an amide bond, and the N-terminal residues are ruled out as sites of acylation since amino acid sequencing was not blocked. Membrane immunoglobulins are the recognition components of B-lymphocyte antigen receptors. Membrane immunoglobulin heavy chain (μm) reaches the cell surface and acts with light chain (L) as an antigen receptor. Following activation by antigen and the appropriate lymphokines and the synthesis of J chains, μm is secreted as a covalent pentamer [(μs)2L2]5J. Metabolic labelling with [3H]myristic acid revealed that μm, μs, and light chains are covalently acylated probably by amide linkage to a lysine side chain since the myristate moiety is resistant to hydroxylamine (242).
Cytochrome c oxidase is a multisubunit enzyme complex of the inner mitochondrial membrane, catalyzing the terminal electron transfer and proton translocation steps of the mitochondrial electron transport system (6). Subunit 1 of cytochrome c oxidase of Neurospora crassa cells is myristoylated through an amide linkage at a lysine residue (K324) within one of its transmembrane domains (309).
From this section, one can see that there are many examples of processing leading to the addition of lipid groups to proteins in both prokaryotes and eukaryotes but that there are no prokaryotic mechanisms analogous to toxin maturation and only the possibility of a related mechanism in eukaryotes. The next section attempts to set out what is known about the roles of these different lipidations in biological function and how they are affected by other features possessed by the lipidated proteins; later, we will relate these to the effects produced by HlyA.
ROLES OF LIPIDATION IN PROTEIN FUNCTION
Increasing Affinity of Proteins for Biological Membranes
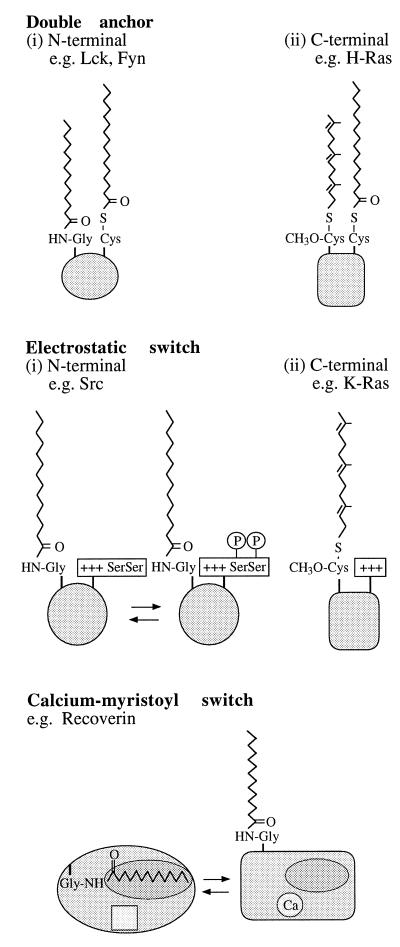
The increase in hydrophobicity resulting from the attachment of a fatty acid chain increases the association of many proteins with membranes. In gram-negative bacteria, N-terminal acylation localizes lipoproteins to the outer membrane, e.g., FlgH involved in membrane anchoring of the flagellar basal body, and PulS, promoting the correct localization of other membrane proteins involved in type III secretion of pullulanase (65, 259). In gram-positive organisms, many surface proteins are anchored by their C termini, but N-terminal lipidation may offer an additional membrane anchor generating a topology similar to that for Braun’s lipoprotein (292). A similar function can be attributed to the acyl group of N-myristoylated proteins, many of which are cytosolic when unmyristoylated but acquire biological activity when bound to membranes through simple insertion of their myristate chain into the hydrophobic interior of the lipid bilayer (40, 140, 163, 265, 277, 296). Many thiopalmitoylated proteins are either integral membrane proteins or additionally N-myristoylated and do not require palmitoyl groups for membrane association. An exception appears to be the Gα-interacting protein (72). Membrane interaction is also promoted by prenylation, e.g., a-factor (53), G-protein γ subunits (217), and RTKs (64, 234, 290). Many eukaryotic cell surface proteins are anchored to the membrane by GPI, which in terms of stability is comparable to a hydrophobic peptide domain. If the GPI anchor is not attached, the protein is not localized on the cell surface but is instead retained in the cell (98).
It is easy to assume that the addition of a fatty acid with a long hydrophobic chain would be sufficient to convert a protein into a membrane-associated form, but the thermodynamics are not so unequivocal. It has been calculated from experiments with acylated peptides mixed with phospholipid vesicles that binding energy increases by 0.8 kcal/mol per -CH2 group along an acyl chain (237, 266). The hydrophobic energy supplied by myristate (C14) may not therefore be sufficient to attach a protein firmly to a membrane. This has been confirmed experimentally, since myristoylated proteins are anchored at a membrane surface but not embedded within it (161), and even a palmitoyl (C16) chain cannot force a hydrophilic peptide sequence into a hydrophobic environment. Therefore, while palmitoylation next to a transmembrane segment may increase the partitioning of this hydrophobic segment into the membrane, the sole presence of a fatty acid moiety may not be sufficient for membrane binding, especially in hydrophilic proteins (148). However, the effort invested in the incorporation of fatty acids suggests that there is a benefit, especially where the lipid donor is a minor component of the substrate pool. This advantage may be that the low hydrophobicity of single lipid groups allows a reversible association with membranes (302).
Where single lipophilic groups determine low membrane binding, a simple means of increasing the membrane affinity of a protein is to incorporate a second fatty acid. The incorporation of fluorescently labelled peptides bearing double-lipid modifications (C20 + C20, C14 + C16, C16 + C15, and C16 + C16) into phospholipid vesicles has been used to monitor the effect of double acylation on membrane affinity (266). Lipopeptides with “double-anchor” motifs do indeed have a greater membrane affinity than that of monoacylated or monoprenylated peptides (78). Surprisingly, peptides possessing palmitoyl (C16) groups associate with lipid bilayers even more than do geranygeranylated (C20) peptides, and double palmitoylation may be the most hydrophobic lipid modification currently known in intracellular proteins. This view is supported by the finding that a MARCKS derivative bearing two N-terminal palmitic acids in place of the wild-type single myristoyl moiety is no longer released from the membrane (265).
Many membrane-associated proteins use a double anchor. Among the intracellular proteins that are covalently modified with acyl or isoprenyl groups, a significant number carry more than one hydrophobic group. In some N-myristoylated proteins such as those of the Src family (e.g., Lck and Fyn but not Src and Blk) and α-subunits of heterotrimeric G proteins, membrane binding is reinforced with additional palmitoylation of nearby cysteine residues (209, 247, 272, 308, 317, 334). The isoprenoid group binds to membranes with about the same affinity as myristate (266), and so membrane-bound prenylated proteins are also commonly doubly acylated. For instance, H-Ras and N-Ras which are farnesylated on their C-terminal cysteine residue are also reversibly S-palmitoylated on one or more nearby cysteines (198) (Fig. 6). The combined hydrophobic interactions of the two lipid moieties are necessary to anchor these proteins to the plasma membrane, as shown by the inability of prenylated, nonpalmitoylated H-Ras mutants to bind to plasma membranes (323). Other double-lipid substitutions, including double geranylgeranyl and geranylgeranyl-farnesyl modifications, have been suggested to occur on mammalian Rab proteins and yeast Ypt (71, 99, 269).
FIG. 6.
Mechanisms to enhance the effects of single lipid groups. Double anchors increase hydrophobicity over single lipid groups. Members of the Src family of nonreceptor tyrosine kinases such as Lck or Fyn possess both an N-myristoylated glycine residue and a thioester-linked palmitoyl group. Small G-proteins such as H-Ras contain a C-terminal farnesyl group and a thioester-linked palmitoyl group. Electrostatic switches modulate the penetration of acyl chains into the membrane through the interaction of positively charged amino acids with the polar head groups of lipids. The myristoyl group of Src inserts into the bilayer when a basic domain interacts with acidic lipids, but phosphorylation of serine residues within this domain reduces the interaction. The hydrophobic interaction of the C-terminal farnesyl moiety of K-Ras with the bilayer is enhanced by an adjacent polybasic domain. The calcium-myristoyl switch of recoverin determines the accessibility of its lipid group. In the Ca2+-free state, the myristoyl group occupies a hydrophobic cluster (shaded oval), while in the Ca2+-bound state, the myristoyl group leaves the hydrophobic cluster and is exposed to interact with membranes or other proteins.
The membrane affinity of single acyl chains is not only enhanced by additional fatty acids. Both myristoylated and prenylated proteins use electrostatic interaction with the negative phospholipid head groups in the lipid bilayer to increase and decrease association with the membrane (218). The myristoyl groups of human immunodeficiency virus type 1 matrix protein cooperate with basic residues to interact with the acidic phospholipids in the membrane (333), and similar cooperative interactions between a C-terminal farnesyl moiety and an adjacent hexalysine domain have been observed for K-Ras (198, 222) (Fig. 6). Further subtlety arises from this additional electrostatic interaction, since in some proteins the phosphorylation of serine residues within basic sequences reduces the attraction for acidic lipids, providing an “electrostatic switch” for reversible binding, e.g., MARCKS and Src (161, 247, 296). N-myristoylated hisactophilins possess an electrostatic binding site containing both basic and acidic residues, so that below pH 7 electrostatic attraction increases interaction with a lipid bilayer while above pH 7 it determines electrostatic repulsion (111). Other myristoyl switch mechanisms are responsible for the reversible membrane-binding of ADP-ribosylation factor and recoverin. ADP-ribosylation factor requires the binding of GTP first to expose an otherwise hidden myristoyl group and a patch of basic residues that then promote membrane insertion, while in the case of recoverin it is the binding of Ca2+ that exposes a myristoyl moiety masked within a hydrophobic groove, allowing it to interact with a lipid bilayer (2, 5, 38) (Fig. 6).
Enhancement of Protein-Protein Interactions
Lipid attached to a protein acts not only as a mediator of membrane association but also as a determinant of specific protein-protein interactions and the assembly of membrane protein complexes. One obvious reason why membrane binding aids the interaction between proteins is that it reduces diffusion from three to two dimensions and increases their effective concentration approximately 1,000-fold. It may also expedite the lateral movement of membrane proteins, which may be required for rapid cross-linking and oligomerization. Nevertheless, very little is known about how acyl groups alter protein structure, especially during the change from the monomeric non-membrane-associated protein to the multimeric membrane-associated protein. Signal transduction is one area in which the contact of proteins with other proteins is crucial, and acylation plays a central role in these processes. The palmitoylation of GPCRs, the myristoylation, palmitoylation and prenylation of heterotrimeric G-proteins, the prenylation of Ras-related small G-proteins, the myristoylation and palmitoylation of Src family tyrosine kinases, and the glypiation of GPI-linked membrane proteins are all critical at different stages of signal transduction for reasons other than influencing membrane affinity (110, 199, 222, 247, 317, 331) (Fig. 5).
Approximately 1,000 different GPCRs exist in mammals, each member interacting with a limited number of closely related G-protein heterotrimers, and palmitoylation may play a role in this interaction since it is necessary for coupling to β2-adrenoceptors although it is not essential for other receptors (115, 139, 153). The proximity of sites for palmitoylation, G-protein binding, and RTK-mediated phosphorylation on GPCRs (321) allows an interplay such that the state of palmitoylation influences RTK recognition of the agonist-occupied receptor and plays a role in signal desensitization through the removal of the GPCR from the cell surface (213, 234). As well as associating with GPCRs, G-protein α-subunits interact with βγ-subunit complexes and with effector molecules such as adenylyl cyclase. The N-terminal myristoyl group on Gα subunits specifically contributes to Gβγ binding (19, 183, 317). There may not be direct contact between the fatty acid on α and the protein in βγ (32), but the isoprenoid group attached to the γ polypeptide does contact Gα in the αβγ trimer (217, 317, 325, 331). In addition, nearly all Gα subunits are reversibly palmitoylated, especially when the α subunit is not complexed with βγ (145, 317).
The role of the farnesylation of the Saccharomyces pheromone a-factor is not restricted to increasing hydrophobicity but may be important in binding the a-factor receptor (68). Lipidation also affects the interaction of many small G proteins such as Rho and Rab with their regulatory proteins, e.g., GDP-dissociation inhibitors (GDIs) and GDI displacement factor (198). The prenyl groups of small G proteins may bind to pockets in GDIs to form soluble complexes (150, 157, 324).
GPI-anchored proteins are cell surface proteins that are involved in signal transduction; e.g., GPI-anchored glycoproteins of Trypanosoma cruzi induce proinflammatory cytokine synthesis by macrophages (49). To generate intracellular signals, GPI-anchored proteins such as Thy-1 that do not extend into the cytoplasm must interact with proteins on the inner face of the membrane such as Gα subunits and the Src family of nonreceptor tyrosine kinases. These interactions are influenced by the presence of saturated acyl chains; e.g., Src family members need to be N myristoylated and palmitoylated to interact with GPI-linked proteins (271), but this influence may be nonspecific and caused by concentration of lipidated proteins within detergent-resistant membrane domains of eukaryotic cells such as caveolae (41, 276). Dual palmitoylation may have additional effects more subtle than an increase in membrane affinity such as preferential targeting to the plasma membrane or Golgi apparatus (33, 149). Recognition between Src kinases and their substrates is also affected by their state of palmitoylation (255).
INTERNAL AMIDE-LINKED ACYLATION AND HLYA TOXIN FUNCTION
Despite the differences in the structure of the lipid groups attached at or near the N and C termini of proteins, as we have shown above, their consequences can be discussed together in terms of affecting membrane affinity and/or protein-protein interactions. These functions are also applicable to the modification of internal lysine residues with long-chain fatty acids. Since the E. coli HlyA fatty acylation appears to be most similar to the internal amide-linked acylation found on a small number of eukaryotic proteins, we now discuss separately the consequences of acylation proposed specifically for these proteins.
Eukaryotic Internal Amide-Linked Acylation
Lipopeptides with long-chain fatty acids attached to the side group of lysine residues have been used to investigate the biological effect of the unusual lipidation involving internal amide-linked acylation. A 14-aa hydrophilic peptide with a palmitoylated lysine at either the N- or C-terminal end is able to enter intact human cells, whereas the unmodified peptide is not (185). This system used synthetically modified peptides, but biological peptides do contain internal Nɛ-palmitoyl-lysine residues. An isoform of the 35-residue surfactant protein SP-C possesses two thioester-linked palmitoylated cysteine residues as well as a palmitoyl group linked to the ɛ-amino group of Lys11. The origin of this third modification appears to be nonenzymatic, and its biological significance is unclear (105). The acylation of phospholipase A2 at the ɛ-amino groups of lysine residues converts the soluble enzyme into a membrane-penetrating form with an increased interaction with phospholipid monolayers (270). While this modification followed chemical treatment with short (C8) acyl chains and may not be physiologically relevant, the findings nevertheless appear relevant to the other eukaryotic proteins (e.g., TNF-α, IL-1, and cytochrome c oxidase) known or suggested to carry internal amide-linked fatty acids (Table 4).
The acylated lysine residues of TNF-α are located immediately downstream from a hydrophobic, membrane-spanning segment of the propiece, and so acylation may facilitate the membrane localization or insertion of the precursor molecule by enhancing overall hydrophobicity. Such a role for the acylation of cytokine propieces is supported by the fact that both the TNF-α and IL-α precursor proteins are active as plasma membrane-associated proteins whereas the poorly myristoylated IL-1β precursor is active only as the processed secreted form (25, 70). Dansylated myristoyl acceptor peptides derived from IL-α have demonstrated the specific binding of the myristoylated form to the inner leaflet of erythrocyte ghosts, suggesting that acylation does indeed facilitate membrane binding (289). The unmodified subunit 1 of cytochrome c oxidase is already extremely hydrophobic, with several predicted transmembrane α-helices, and so it seems unlikely that acylation is required for integration of the protein into the lipid bilayer of the inner mitochondrial membrane. It is possible that myristoylation of subunit 1 encourages protein-protein interactions, contributing to assembly with subunit 2 and perhaps other subunits of this enzyme complex (309). The role of covalently bound fatty acids in the insulin receptor is unclear, but they may have important implications in terms of receptor stability, life cycle, or function (119). Immunoglobulin heavy chain (μm) is initially synthesized as a relatively hydrophilic protein, which is nonetheless stably anchored in the endoplasmic reticulum membrane. Myristoylation of μm correlates with its transport to the cell surface and its conversion to a relatively hydrophobic form. However, myristoylation does not result in detergent solubility, suggesting that its effect on μm may be an indirect one through mediating intermolecular interactions during its assembly and transport to the cell surface (242). Similarly, acylation of the nicotinic acetylcholine receptor, an integral membrane protein, appears to have a greater influence on protein-protein interaction rather than membrane affinity. These interactions may not be restricted to the receptor subunits but may include molecular chaperones such as calnexin, which forms complexes with nascent α subunits (96). Subunit assembly of nicotinic acetylcholine receptor is relatively slow and begins with the interaction of α and δ subunits in the endoplasmic reticulum to form a heterodimer. This process requires the tethering of the α subunit to the membrane, but whether this is provided by a lipid group is not known (316). Nevertheless, in the absence of acylation, subunit assembly is impaired, possibly because nonacylated subunits are degraded before they can be transported to the plasma membrane (207).
The roles of acylation at internal lysine residues thus appear to be the same as those of other lipid modifications. Eukaryotic proteins with internal amide-linked acyl groups form active membrane-associated complexes. Can this be linked to HlyA toxin function in eukaryotic membranes? Is it a coincidence that six of the seven eukaryotic proteins with internal amide-linked acyl groups are involved in signalling events either as cytokines or as receptors?
Host Cell Targeting by the HlyA Toxin Family
We have set out how fatty acylation of proteins can be central to anchoring to membranes, signal transduction, and oligomerization. The functions of the HlyA toxin suggest that its acylation could contribute in all three of these areas. It is tempting to try and explain the differences in the cell type and host species attacked by the toxins of the HlyA family in terms of what might be differences in their posttranslational modification. One interpretation of domain exchanges between hemolysins and leukotoxins and between leukotoxins that attack cells from different species could be that separate sequences account for erythrocyte specificity (∼aa 450 to 700 on HlyA) and leukocyte specificity (∼aa 700 to 850) (88, 175, 202). Although these regions approximate to the acylation sites of HlyA, examination of KI and KII within the toxin family does not allow a simple view that their acylation might individually determine erythrocyte and leukocyte specificity (Table 3). For instance, ApxIIA and LktA are cytolytic but do not possess KII, while AaltA and LktA are not hemolytic but do possess KI. The number of acylation sites also appears not to be decisive, since ApxIIA, a hemolysin, and LktA, a leukotoxin, both have one site whereas HlyA, a hemolysin, and AaltA, a leukotoxin, both have two sites. More subtle models are also not substantiated experimentally. Of the two (KI and KII) acylated lysines responsible for the hemolytic activity of HlyA, only KI is conserved in the strictly leukotoxic LktA (282). A simple hypothesis would be that the acylation of KI determines targeting to leukocytes while acylation at both KI and KII is required for erythrolysis. Although simple, this hypothesis has proved wrong, since HlyA with a substituted KII lost not only hemolytic activity but also its leukotoxic activity against human polymorphonuclear leukocytes (31, 239), and an N684K substitution, placing a lysine residue at the prospective KII site of LktA, failed to confer activity against erythrocytes (239). As previously indicated by studies with hybrid gene constructions (88, 175, 202), the structural basis of target cell specificity is unlikely to lie in single features of the toxins. In addition, the contribution made by putative receptors is unknown. Identification of all the acylation sites of the family of toxins will not be sufficient to explain cell targeting but will provide a further understanding of the mode of action of individual toxins at the molecular level.
Toxin Association with Eukaryotic Cell Membranes
A discussion of cell targeting in terms of toxin acylation may be more revealing if one considers the separate processes of membrane insertion, host cell signalling, and pore formation. The influence of fatty acylation on HlyA membrane binding has been investigated, but the results are contradictory. Several research groups have concluded that unmodified, inactive pro-HlyA is unable to bind to erythrocyte membranes (37, 187) while the acylation of CyaA has been proposed to be essential for tight association with target cells (109). However, other work has suggested that acylation does not increase the affinity of toxin either to artificial liposomes or to real mammalian target membranes (15, 76, 210, 275). An explanation of these apparently opposing conclusions may lie in small differences in the preparation of the toxin samples. Fully modified toxin preparations lose their activity after dilution, possibly through self-aggregation and an inability to bind, and it appears that this tendency may be greater for unacylated than for acylated toxin and for calcium-bound rather than calcium-free toxin (11, 285). In addition, toxin at least partially denatured by the action of chaotropic agents appears to acquire the potential to bind lipid bilayers in an acylation-dependent manner (285). Under certain conditions, HlyA binding to erythrocytes is effective in the absence of acylation, Ca2+ binding, and the N-terminal hydrophobic region (15); there may therefore be no single factor that is decisive for in vivo membrane insertion. Whether the primary interaction and binding of HlyA to target membranes is dependent on fatty acylation is therefore unresolved and will probably require a more sophisticated approach than that allowed by in vivo systems.
Theoretically, by increasing the hydrophobicity of HlyA, the influence of fatty acylation would be to increase membrane binding. However, an alternative view is that the N-terminal hydrophobic domain of HlyA, possessing eight transmembrane helices, is sufficient for at least initial membrane binding and that before insertion is complete, the acyl chains are separated from the aqueous environment, perhaps hidden within hydrophobic tunnels. The transition from a membrane-bound to a membrane-inserted state could involve a conformational change that may at least partially release the acyl chains to the lipid bilayer. This transition could lie behind the temperature sensitivity of the lytic process; binding of HlyA to a membrane is slower at 4°C than at 37°C, but the amount eventually bound is constant. However, at the lower temperature, no cell lysis results despite the binding (15, 76, 210).
An essential step in toxin action, in addition to acylation, is the binding of calcium at the glycine-rich repeats of the toxin. Here the “Ca2+-myristoyl switch” of recoverin may be useful as a functional model (Fig. 6) (2), with the binding of Ca2+ being required to expose the acyl groups and make them available to the lipid bilayer. Certainly, Ca2+ binding by HlyA does appear to increase its overall surface hydrophobicity (11). It is possible that the binding of calcium affects the conformation of the toxin not only at KII adjacent to the glycine-rich repeats but also at KI more than 170 aa away, since in the presence of Ca2+ both KI and KII are no longer recognized by HlyC in vitro (283). While a large conformational change has not been detected by circular dichroism for HlyA on calcium binding, it has been shown for CyaA (11, 250). Why should the doubly acylated HlyA require an additional mechanism to increase membrane affinity when double acylations are among the strongest modifications known? The answer may lie in the HlyA acylation sites being 126 aa apart whereas in doubly modified N-myristoylated and prenylated proteins the two lipid groups are close together. It is more correct to view HlyA not as a doubly anchored protein but as being monoacylated in two places, a view corroborated by the existence of a sole palmitoyl group on native CyaA. The highly charged nature of the residues either side of KI and KII (30% of the flanking 20 residues are DEKR, with no overall positive charge) further increases the instability of any potential membrane association. The acylated lysines of HlyA do not have neighboring clusters of hydrophobic or positively charged residues as for K-Ras whether considered as a linear sequence, a helix, or a β-sheet (Fig. 3). Without these, single myristic or even palmitic side chains are unlikely to provide sufficient hydrophobic interaction to stabilize a transmembrane region even if there are additional contributions by the lysine side groups. Rather, the acyl groups on HlyA may act at most as anchorage points to the surface of the lipid bilayer.
Effects of HlyA Toxin on Host Cell Signalling and Cytokine Production
The hydrophobicity of fatty acylation does not appear to be a simple key to HlyA targeting to mammalian cell membranes, but it may be significant in mediating specific protein-protein and/or protein-lipid interactions. Investigations into the effect of acylation on the ability of sublytic concentrations of HlyA to stimulate a host cell response have been complicated, perhaps significantly, by the presence of LPS in toxin preparations. At least the major component of HlyA binding to granulocytes appears to be non-receptor mediated, with subsequent triggering of the respiratory burst presumably not through the occupancy of a receptor but through a short-circuiting of the classical signal transduction pathway (103, 165). Acylation may therefore enhance protein-protein interactions between HlyA and components of a signal transduction pathway, perhaps by targeting to detergent-resistant domains (Fig. 5). With acylation sites C-terminal to a multiple transmembrane helical domain, HlyA is at least superficially similar to the GPCRs, but unlike the labile thioester-linked palmitoylation of GPCRs, the amide-linked modification of HlyA would not be reversible. Effects of acyl groups on cytokine induction, however, are not restricted to a model incorporating complex protein-protein interactions. A synthetic lipopeptide of the N terminus of a bacterial lipoprotein stimulates the synthesis of IL-1, IL-6, and TNF-α by murine macrophages (114). Lipoteichoic acids from Streptococcus faecalis stimulate murine mononuclear cells to release both IL-1 and TNF, but deacylation stops the cytokine-stimulating activity (304), and deacylation of lipoarabinomannan of Mycobacterium spp. almost totally inhibits its capacity to induce cytokine synthesis (14). Intriguingly, another response of monocytes exposed to lipoteichoic acid is to increase the expression of CD11a/CD18, a putative receptor that may be recognized by RTX toxins (51, 176).
HlyA Pore Formation in Host Cell Membranes
As well as being a pseudo-chemokine, HlyA is cytotoxic as a result of its ability to form pores. If monomers of the toxin must combine within the membrane to form pores, perhaps the acyl groups are involved in protein-protein interactions to bring about oligomerization. By providing anchorage points in the membrane, acyl groups may prevent essential domains from looping away from the membrane surface and may enhance lateral diffusion to accelerate the contact between them. The assembly of the acylated subunit 1 into the cytochrome oxidase complex and the interaction of the acyl groups involved in contact between the α and γ subunits of heterotrimeric G-proteins may provide useful analogies to this process (Fig. 5; Table 4). The most dramatic effect of the absence of fatty acylation on the action of HlyA indeed appears to be at the pore-forming step. Both acyl modifications must be present for HlyA to form channels. Loss of either acyl group results in the virtual abolition (>99.5%) of cytolytic activity (282). This is at least true for erythrocytes and human polymorphonuclear leukocytes. Monoacylated HlyA derivatives lacking either KI or KII do retain a reduced ability to form pores in bovine lymphocytes (BL-3 cells) (239). Why pore formation in BL-3 cells should be less dependent on toxin acylation is unknown, but the impression of systems involving bovine lymphocytes and the specific P. haemolytica leukotoxin LktA is that they represent a special case.
An influence of acylation on pore formation has also been demonstrated for CyaA. After expression in E. coli, CyaA carries an acyl group on K860 in addition to the K983 acylated in the native host. This overacylated toxin is impaired in the formation of the CyaA channels, being about 20% as hemolytically active as the wild-type CyaA (109). The hemolytic activity of HlyA absolutely requires double acylation, while monoacylation is sufficient for the hemolytic activity of CyaA. However, while both HlyA and CyaA are described as hemolysins, there is a dramatic difference in their erythrolytic activity, such that CyaA hemolytic assays are performed at nanomolar toxin concentrations but HlyA assays require only picomolar toxin concentrations. Monoacylated HlyA derivatives retaining only <0.5% of the wild-type activity are correctly defined as nonhemolytic but are still more hemolytic than the wild-type CyaA toxin. Nevertheless, whereas acylation at KI is essential for pore formation by HlyA, acylation at KI actually seems to impede the hemolytic activity of CyaA. The primary sequences 50 aa either side of KI are only 30% identical between HlyA and CyaA (283), so perhaps a reason for this difference will be confined to individual structures. It might be significant that KI is the acylation site closest to the toxin hydrophobic regions, with the hydrophobic region of CyaA possibly having an intrinsically stable topology while that of HlyA requires its C-terminal side to be stabilized by an anchorage to the membrane (Fig. 2). Evidence that the acyl chains attached to HlyA may provide membrane anchors and that their stability in the membrane is influential in pore formation is provided by the observation that doubly palmitoylated HlyA has a specific hemolytic activity at least fivefold higher than that of doubly myristoylated HlyA (285).
PERSPECTIVE
The pore-forming HlyA of E. coli represents a remarkable class of bacterial toxins that requires a posttranslational modification for activity. The maturation of HlyA by the covalent amide linkage of fatty acids to internal lysine residues is determined by an apparently unique, virulence-related lysyl-acyltransferase activity unlike any other in prokaryotes. Analogous activities have been identified in a few eukaryotic systems, but these have not been isolated. The mechanism underlying this activity may have implications for the value of acyltransferases as therapeutic targets, as realized for the enzymes in the LPS biosynthetic pathway (230) and the farnesyltransferase of oncogenic Ras (263).
Predictions can be made about the possible roles that the HlyA acylation plays in endowing biological activity. One general consequence of lipidation is an increase in the membrane affinity of a protein, but this may not be relevant to HlyA. Protein lipidation is also important in the regulation of protein-protein interactions. High concentrations of HlyA produce cell lysis, possibly through the formation of oligomeric pores, a process that is absolutely dependent on acylation. The association of acylated eukaryotic proteins into active membrane-bound complexes, as exemplified by internally acylated cytochrome oxidase or the heterotrimeric G-proteins, may provide useful analogies for the design of a model for these bacterial toxins.
In eukaryotic cells, mitogenic receptor activation is linked through tyrosine and serine/threonine kinase pathways to the activation of phospholipase C and the liberation of diacylglycerol (199, 264). Sublytic concentrations of HlyA also trigger the generation of diacylglycerol (28, 30), but where it interacts with the host signalling pathway is unknown. It may be significant that both membrane immunoglobulin and insulin receptors appear to possess identical amide-linked, internal fatty acylation, as found on HlyA. In addition, the Ras and Src components of these signalling pathways, and the heterotrimeric G-proteins and their coupled receptors, all have acylated residues. A role for the HlyA acyl groups in signalling would not necessarily require them to cross into the inner lipid layer of the eukaryotic membrane, since GPI-anchored proteins remain on the outside of the cell and are believed to signal through Lck and Fyn NRTKs (271). However, the delivery of hydrophilic peptides containing Nɛ-palmitoyl-lysine residues into the cytoplasm of intact cells suggests that the cell membrane may not be an impervious barrier to the acylated domains of HlyA (185). HlyA also affects cytokine production, such as stimulating the release of IL-1β and TNF-α from human monocytes. It has been suggested that virulent E. coli has receptors for IL-1 and responds to its binding by proliferation (244), so the HlyA-mediated release of IL-1 might act as an environmental signal. In addition, it is known that IL-1β increases the cytotoxicity of Shiga toxin toward human vascular epithelial cells by increasing the synthesis of its receptor, globotriaosylceramide (154). There is therefore the possibility of a similar link between the action of HlyA and Shiga toxin in E. coli pathogenesis. A relationship between the acylation of HlyA and the acylation of lysine residues in the precursor, membrane-bound forms of IL-1 and TNF-α would support speculation that cytokine-inducing proteins produced by bacteria are evolutionary precursors of mammalian cytokines (120). If the posttranslational acylation of HlyA does indeed allow it to mimic a eukaryotic cell counterpart, it will be a further example of interaction between prokaryotic and eukaryotic cells.
ACKNOWLEDGMENT
Our work is supported by a Programme grant from the U.K. Medical Research Council.
REFERENCES
- 1.Adusu T E, Conlon P D, Shewen P E, Black W D. Pasteurella haemolytica leukotoxin induces histamine release from bovine pulmonary mast cells. Can J Vet Res. 1994;58:1–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Ames J B, Ishima R, Tanaka T, Gordon J I, Stryer L, Ikura M. Molecular mechanics of calcium-myristoyl switches. Nature. 1997;389:198–202. doi: 10.1038/38310. [DOI] [PubMed] [Google Scholar]
- 3.Anderson M S, Raetz C R H. Biosynthesis of lipid A precursors in Escherichia coli—a cytoplasmic acyltransferase that converts UDP-N-acetylglucosamine to UDP-3-O-(R-3-hydroxymyristoyl)-N-acetylglucosamine. J Biol Chem. 1987;262:5159–5169. [PubMed] [Google Scholar]
- 4.Anderson P. Kinase cascades regulating entry into apoptosis. Microbiol Mol Biol Rev. 1997;61:33–46. doi: 10.1128/mmbr.61.1.33-46.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Antonny B, Beraud-Dufour S, Chardin P, Chabre M. N-terminal hydrophobic residues of the G-protein ADP-ribosylation factor-1 insert into membrane phospholipids upon GDP to GTP exchange. Biochemistry. 1997;36:4675–4684. doi: 10.1021/bi962252b. [DOI] [PubMed] [Google Scholar]
- 6.Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- 7.Bailey M J A, Koronakis V, Schmoll T, Hughes C. Escherichia coli HlyT protein, a transcriptional activator of hemolysin synthesis and secretion, is encoded by the rfaH (sfrB) locus required for expression of sex factor and lipopolysaccharide genes. Mol Microbiol. 1992;6:1003–1012. doi: 10.1111/j.1365-2958.1992.tb02166.x. [DOI] [PubMed] [Google Scholar]
- 8.Bailey M J A, Hughes C, Koronakis V. Increased distal gene-transcription by the elongation-factor RfaH, a specialized homolog of NusG. Mol Microbiol. 1996;22:729–737. doi: 10.1046/j.1365-2958.1996.d01-1726.x. [DOI] [PubMed] [Google Scholar]
- 9.Bailey M J A, Hughes C, Koronakis V. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol Microbiol. 1997;26:845–851. doi: 10.1046/j.1365-2958.1997.6432014.x. [DOI] [PubMed] [Google Scholar]
- 10.Bakas L, Ostolaza H, Vaz W L C, Goni F M. Reversible adsorption and nonreversible insertion of Escherichia coli α-hemolysin into lipid bilayers. Biophys J. 1996;71:1869–1876. doi: 10.1016/S0006-3495(96)79386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bakas L, Veiga M P, Soloaga A, Ostolaza H, Goni F M. Calcium-dependent conformation of E. coli α-haemolysin. Implications for the mechanism of membrane insertion and lysis. Biochim Biophys Acta Biomembr. 1998;1368:225–234. doi: 10.1016/s0005-2736(97)00181-8. [DOI] [PubMed] [Google Scholar]
- 12.Baldwin J E, Bird J W, Field R A, O’Callaghan N M, Schofield C J, Willis A C. Isolation and partial characterization of ACV synthetase from Cephalosporium acremonium and Streptomyces clavuligerus—evidence for the presence of phosphopantothenate in ACV synthetase. J Antibiot. 1991;44:241–248. doi: 10.7164/antibiotics.44.241. [DOI] [PubMed] [Google Scholar]
- 13.Ballard J, Sokolov Y, Yuan W L, Kagan B L, Tweten R K. Activation and mechanism of Clostridium septicum α-toxin. Mol Microbiol. 1993;10:627–634. doi: 10.1111/j.1365-2958.1993.tb00934.x. [DOI] [PubMed] [Google Scholar]
- 14.Barnes P F, Chatterjee D, Abrams J S, Lu S H, Wang E, Yamamura M, Brennan P J, Modlin R L. Cytokine production induced by Mycobacterium tuberculosis lipoarabinomannan—relationship to chemical structure. J Immunol. 1992;149:541–547. [PubMed] [Google Scholar]
- 15.Bauer M E, Welch R A. Association of RTX toxins with erythrocytes. Infect Immun. 1996;64:4665–4672. doi: 10.1128/iai.64.11.4665-4672.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bauer M E, Welch R A. Characterization of an RTX toxin from enterohemorrhagic Escherichia coli O157:H7. Infect Immun. 1996;64:167–175. doi: 10.1128/iai.64.1.167-175.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bauer M E, Welch R A. Pleiotropic effects of a mutation in rfaC on Escherichia coli hemolysin. Infect Immun. 1997;65:2218–2224. doi: 10.1128/iai.65.6.2218-2224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baumann U, Wu S, Flaherty K M, McKay D B. Three-dimensional structure of the alkaline protease of Pseudomonas aeruginosa: a two-domain protein with a calcium binding parallel beta roll motif. EMBO J. 1993;12:3357–3364. doi: 10.1002/j.1460-2075.1993.tb06009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beck H I, Chan J S C, Wong Y H. Receptor-induced βγ release from fatty acylation-deficient mutants of Gαz. Neuroreport. 1997;8:937–940. doi: 10.1097/00001756-199703030-00024. [DOI] [PubMed] [Google Scholar]
- 20.Bellalou J, Sakamoto H, Ladant D, Geoffroy C, Ullmann A. Deletions affecting hemolytic and toxin activities of Bordetella pertussis adenylate cyclase. Infect Immun. 1990;58:3242–3247. doi: 10.1128/iai.58.10.3242-3247.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Benz R, Schmid A, Wagner W, Goebel W. Pore formation by the Escherichia coli hemolysin: evidence for an association-dissociation equilibrium of the pore-forming aggregates. Infect Immun. 1989;57:887–895. doi: 10.1128/iai.57.3.887-895.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Benz R, Hardie K R, Hughes C. Pore formation in artificial membranes by the secreted hemolysins of Proteus vulgaris and Morganella morganii. Eur J Biochem. 1994;220:339–347. doi: 10.1111/j.1432-1033.1994.tb18630.x. [DOI] [PubMed] [Google Scholar]
- 23.Berthiaume L, Resh M D. Biochemical characterization of a palmitoyl acyltransferase activity that palmitoylates myristoylated proteins. J Biol Chem. 1995;270:22399–22405. doi: 10.1074/jbc.270.38.22399. [DOI] [PubMed] [Google Scholar]
- 24.Betsou F, Sebo P, Guiso N. CyaC mediated activation is important not only for toxic but also for protective activities of Bordetella pertussis adenylate cyclase-hemolysin. Infect Immun. 1993;61:3583–3589. doi: 10.1128/iai.61.9.3583-3589.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beuscher H U, Fallon R J, Colten H R. Macrophage membrane interleukin 1 regulates the expression of acute phase proteins in human hepatoma Hep 3B cells. J Immunol. 1987;139:1896–1901. [PubMed] [Google Scholar]
- 26.Bhakdi S, Tranum-Jensen J, Sziegoleit A. Mechanism of membrane damage by streptolysin O. Infect Immun. 1985;47:52–60. doi: 10.1128/iai.47.1.52-60.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhakdi S, Mackman N, Nicaud J M, Holland I B. Escherichia coli hemolysin may damage target cell membranes by generating transmembrane pores. Infect Immun. 1986;52:63–69. doi: 10.1128/iai.52.1.63-69.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhakdi S, Greulich S, Muhly M, Eberspacher B, Becker H, Thiele A, Hugo F. Potent leukocidal action of Escherichia coli hemolysin mediated by permeabilization of target cell membranes. J Exp Med. 1989;169:737–754. doi: 10.1084/jem.169.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bhakdi S, Muhly M, Korom S, Schmidt G. Effects of Escherichia coli hemolysin on human monocytes: cytocidal action and stimulation of interleukin 1 release. J Clin Invest. 1990;85:1746–1753. doi: 10.1172/JCI114631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bhakdi S, Martin E. Superoxide generation by human neutrophils induced by low doses of Escherichia coli hemolysin. Infect Immun. 1991;59:2955–2962. doi: 10.1128/iai.59.9.2955-2962.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bhakdi, S. Unpublished data.
- 32.Bigay J, Faurobert E, Franco M, Chabre M. Roles of lipid modifications of transducin subunits in their GDP-dependent association and membrane binding. Biochemistry. 1994;33:14081–14090. doi: 10.1021/bi00251a017. [DOI] [PubMed] [Google Scholar]
- 33.Bijlmakers M J E, Isobe-Nakamura M, Ruddock L J, Marsh M. Intrinsic signals in the unique domain target p56lck to the plasma membrane independently of CD4. J Cell Biol. 1997;137:1029–1040. doi: 10.1083/jcb.137.5.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Binet R, Letoffe S, Ghigo J M, Delepelaire P, Wandersman C. Protein secretion by Gram-negative bacterial ABC exporters—a review. Gene. 1997;192:7–11. doi: 10.1016/s0378-1119(96)00829-3. [DOI] [PubMed] [Google Scholar]
- 35.Bloemberg G V, Lagas R M, Van Leeuwen S, Van der Marel G A, Van Boom J H, Lugtenberg B J J, Spaink H P. Substrate specificity and kinetic studies of nodulation protein NodL of Rhizobium leguminosarum. Biochemistry. 1995;34:12712–12720. doi: 10.1021/bi00039a030. [DOI] [PubMed] [Google Scholar]
- 36.Boehm D F, Welch R A, Snyder I S. Calcium is required for binding of Escherichia coli hemolysin (HlyA) to erythrocyte membranes. Infect Immun. 1990;58:1951–1958. doi: 10.1128/iai.58.6.1951-1958.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Boehm D F, Welch R A, Snyder I S. Domains of Escherichia coli hemolysin (HlyA) involved in binding of calcium and erythrocyte membranes. Infect Immun. 1990;58:1959–1964. doi: 10.1128/iai.58.6.1959-1964.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boman A L, Kahn R A. Arf proteins—the membrane traffic police. Trends Biochem Sci. 1995;20:147–150. doi: 10.1016/s0968-0004(00)88991-4. [DOI] [PubMed] [Google Scholar]
- 39.Bouvier M, Chidiac P, Hebert T E, Loisel T P, Moffett S, Mouillac B. Dynamic palmitoylation of G-protein coupled receptors in eukaryotic cells. Methods Enzymol. 1995;250:300–314. doi: 10.1016/0076-6879(95)50080-4. [DOI] [PubMed] [Google Scholar]
- 40.Brand S H, Holtzman E J, Scher D A, Ausiello D A, Stow J L. Role of myristoylation in membrane attachment and function of Gαi-3 on Golgi membranes. Am J Physiol. 1996;39:C1362–C1369. doi: 10.1152/ajpcell.1996.270.5.C1362. [DOI] [PubMed] [Google Scholar]
- 41.Brown D A, London E. Structure of detergent-resistant membrane domains: does phase separation occur in biological membranes? Biochem Biophys Res Commun. 1997;240:1–7. doi: 10.1006/bbrc.1997.7575. [DOI] [PubMed] [Google Scholar]
- 42.Brown J F, Leite F, Czuprynski C J. Binding of Pasteurella haemolytica leukotoxin to bovine leukocytes. Infect Immun. 1997;65:3719–3724. doi: 10.1128/iai.65.9.3719-3724.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brozek K A, Raetz C R H. Biosynthesis of lipid A in Escherichia coli: acyl carrier protein-dependent incorporation of laurate and myristate. J Biol Chem. 1990;265:15410–15417. [PubMed] [Google Scholar]
- 44.Brozek K A, Carlson R W, Raetz C R H. A special acyl carrier protein for transferring long hydroxylated fatty acids to lipid A in Rhizobium. J Biol Chem. 1996;271:32126–32136. doi: 10.1074/jbc.271.50.32126. [DOI] [PubMed] [Google Scholar]
- 45.Brumlik M J, Buckley J T. Identification of the catalytic triad of the lipase/acyltransferase from Aeromonas hydrophila. J Bacteriol. 1996;178:2060–2064. doi: 10.1128/jb.178.7.2060-2064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Burrows L L, Lo R Y C. Molecular characterization of an RTX toxin determinant from Actinobacillus suis. Infect Immun. 1992;60:2166–2173. doi: 10.1128/iai.60.6.2166-2173.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Byers D M, Meighen E A. Acyl-acyl carrier protein as a source of fatty acids for bacterial bioluminescence. Proc Natl Acad Sci USA. 1985;82:6085–6089. doi: 10.1073/pnas.82.18.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caldwell G A, Naider F, Becker J M. Fungal lipopeptide mating pheromones: a model system for the study of protein prenylation. Microbiol Rev. 1995;59:406–422. doi: 10.1128/mr.59.3.406-422.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camargo M M, Almeida I C, Pereira M E S, Ferguson M A J, Travassos L R, Gazzinelli R T. Glycosylphosphatidylinositol-anchored mucin-like glycoproteins isolated from Trypanosoma cruzi trypomastigotes initiate the synthesis of proinflammatory cytokines by macrophages. J Immunol. 1997;158:5890–5901. [PubMed] [Google Scholar]
- 50.Camp L A, Verkruyse L A, Afendis S J, Slaughter C A, Hofmann S L. Molecular cloning and expression of palmitoyl-protein thioesterase. J Biol Chem. 1994;269:23212–23219. [PubMed] [Google Scholar]
- 51.Carratelli C R, Nuzzo I, Bentivoglio C, Galdiero M. CD11a/CD18 and CD11b/CD18 modulation by lipoteichoic acid, N-acetyl-muramyl-alpha-alanyl-d-isoglutamine, muramic acid and protein A from Staphylococcus aureus. FEMS Immunol Med Microbiol. 1996;16:309–315. doi: 10.1111/j.1574-695X.1996.tb00150.x. [DOI] [PubMed] [Google Scholar]
- 52.Chang Y F, Young R Y, Struck D K. Cloning and characterization of a hemolysin gene from Actinobacillus (Hemophilus) pleuropneumoniae. DNA J Mol Cell Biol. 1989;8:635–647. doi: 10.1089/dna.1.1989.8.635. [DOI] [PubMed] [Google Scholar]
- 53.Chen P, Sapperstein S K, Choi J D, Michaelis S. Biogenesis of the Saccharomyces cerevisiae mating pheromone a-factor. J Cell Biol. 1997;136:251–269. doi: 10.1083/jcb.136.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Clementz T, Zhou Z M, Raetz C R H. Function of the Escherichia coli msbB gene, a multicopy suppressor of htrB knockouts, in the acylation of lipid A: acylation by MsbB follows laurate incorporation by HtrB. J Biol Chem. 1997;272:10353–10360. doi: 10.1074/jbc.272.16.10353. [DOI] [PubMed] [Google Scholar]
- 55.Clinkenbeard K D, Thiessen A E. Mechanism of action of Moraxella bovis hemolysin. Infect Immun. 1991;59:1148–1152. doi: 10.1128/iai.59.3.1148-1152.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Coleman J, Raetz C R H. First committed step of lipid A biosynthesis in Escherichia coli: sequence of the lpxA gene. J Bacteriol. 1988;170:1268–1274. doi: 10.1128/jb.170.3.1268-1274.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Conti-Tronconi B M, Raftery M A. The nicotinic cholinergic receptor: correlation of molecular structure with functional properties. Annu Rev Biochem. 1982;51:491–530. doi: 10.1146/annurev.bi.51.070182.002423. [DOI] [PubMed] [Google Scholar]
- 58.Cooper C L, Hsu L, Jackowski S, Rock C O. 2-acylglycerolphosphoethanolamine acyltransferase acyl-acyl carrier protein-binding protein. J Biol Chem. 1989;264:7384–7389. [PubMed] [Google Scholar]
- 59.Coote J G. Structural and functional relationships among the RTX toxin determinants of gram-negative bacteria. FEMS Microbiol Rev. 1992;88:137–162. doi: 10.1111/j.1574-6968.1992.tb04961.x. [DOI] [PubMed] [Google Scholar]
- 60.Cross M A, Koronakis V, Stanley P L D, Hughes C. HlyB dependent secretion of hemolysin by uropathogenic Escherichia coli requires conserved sequences flanking the chromosomal hly determinant. J Bacteriol. 1990;172:1217–1224. doi: 10.1128/jb.172.3.1217-1224.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cruz W T, Young R, Chang Y F, Struck D K. Deletion analysis resolves cell binding and lytic domains of the Pasteurella leukotoxin. Mol Microbiol. 1990;4:1933–1939. doi: 10.1111/j.1365-2958.1990.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 62.Czech M P. The nature and regulation of the insulin receptor: structure and function. Annu Rev Physiol. 1985;47:357–381. doi: 10.1146/annurev.ph.47.030185.002041. [DOI] [PubMed] [Google Scholar]
- 63.Czuprynski C J, Noel E J, Ortizcarranza O, Srikumaran S. Activation of bovine neutrophils by partially purified Pasteurella haemolytica leukotoxin. Infect Immun. 1991;59:3126–3133. doi: 10.1128/iai.59.9.3126-3133.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Daaka Y, Pitcher J A, Richardson M, Stoffel R H, Robishaw J D, Lefkowitz R J. Receptor and Gβγ isoform-specific interactions with G protein-coupled receptor kinases. Proc Natl Acad Sci USA. 1997;94:2180–2185. doi: 10.1073/pnas.94.6.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Daefler S, Guilvout I, Hardie K R, Pugsley A P, Russel M. The C-terminal domain of the secretin PulD contains the binding site for its cognate chaperone, PulS, and confers PulS dependence on plV(f1) function. Mol Microbiol. 1997;24:465–475. doi: 10.1046/j.1365-2958.1997.3531727.x. [DOI] [PubMed] [Google Scholar]
- 66.Damblon C, Raquet X, Lian L Y, Lamotte-Brasseur J, Fonze E, Charlier P, Roberts G C K, Frere J M. The catalytic mechanism of β-lactamases—NMR titration of an active site lysine residue of the TEM-1 enzyme. Proc Natl Acad Sci USA. 1996;93:1747–1752. doi: 10.1073/pnas.93.5.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Das A K, Dasgupta B, Bhattacharya R, Basu J. Purification and biochemical characterization of a protein-palmitoyl acyltransferase from human erythrocytes. J Biol Chem. 1997;272:11021–11025. doi: 10.1074/jbc.272.17.11021. [DOI] [PubMed] [Google Scholar]
- 68.Dawe A L, Becker J M, Jiang Y, Naider F, Eummer J T, Mu Y Q, Gibbs R A. Novel modifications to the farnesyl moiety of the a-factor lipopeptide pheromone from Saccharomyces cerevisiae: a role for isoprene modifications in ligand presentation. Biochemistry. 1997;36:12036–12044. doi: 10.1021/bi9709755. [DOI] [PubMed] [Google Scholar]
- 69.Debelle F, Plazanet C, Roche P, Pujol C, Savagnac A, Rosenberg C, Prome J C, Denarie J. The NodA proteins of Rhizobium meliloti and Rhizobium tropici specify the N-acylation of Nod factors by different fatty acids. Mol Microbiol. 1996;22:303–314. doi: 10.1046/j.1365-2958.1996.00069.x. [DOI] [PubMed] [Google Scholar]
- 70.Decker T, Lohmann-Matthes M L, Gifford G E. Cell associated tumor necrosis factor (TNF) as a killing mechanism of activated cytotoxic macrophages. J Immunol. 1987;138:957–962. [PubMed] [Google Scholar]
- 71.Desnoyers L, Anant J S, Seabra M C. Geranylgeranylation of Rab proteins. Biochem Soc Trans. 1996;24:699–703. doi: 10.1042/bst0240699. [DOI] [PubMed] [Google Scholar]
- 72.DeVries L, Elenko E, Hubler L, Jones T L Z, Farquhar M G. GAIP is membrane-anchored by palmitoylation and interacts with the activated (GTP-bound) form of Gαi subunits. Proc Natl Acad Sci USA. 1996;93:15203–15208. doi: 10.1073/pnas.93.26.15203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Diep D B, Nelson K L, Raja S M, Pleshak E N, Buckley J T. Glycosylphosphatidylinositol anchors of membrane glycoproteins are binding determinants for the channel-forming toxin aerolysin. J Biol Chem. 1998;273:2355–2360. doi: 10.1074/jbc.273.4.2355. [DOI] [PubMed] [Google Scholar]
- 74.Dobereiner A, Schmid A, Ludwig A, Goebel W, Benz R. The effects of calcium and other polyvalent cations on channel formation by Escherichia coli α-hemolysin in red blood cells and lipid bilayer membranes. Eur J Biochem. 1996;240:454–460. doi: 10.1111/j.1432-1033.1996.0454h.x. [DOI] [PubMed] [Google Scholar]
- 75.Dotson G D, Kaltashov I A, Cotter R J, Raetz C R H. Expression and cloning of a Pseudomonas gene encoding a hydroxydecanoyl-acyl carrier protein-dependent UDP-GlcNAc acyltransferase. J Bacteriol. 1998;180:330–337. doi: 10.1128/jb.180.2.330-337.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Eberspacher B, Hugo F, Bhakdi S. Quantitative study of the binding and hemolytic efficiency of Escherichia coli hemolysin. Infect Immun. 1989;57:983–988. doi: 10.1128/iai.57.3.983-988.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Englund P T. The structure and biosynthesis of glycosyl phosphatidylinositol protein anchors. Annu Rev Biochem. 1993;62:121–138. doi: 10.1146/annurev.bi.62.070193.001005. [DOI] [PubMed] [Google Scholar]
- 78.Epand R M. Biophysical studies of lipopeptide-membrane interactions. Biopolymers. 1997;43:15–24. doi: 10.1002/(SICI)1097-0282(1997)43:1<15::AID-BIP3>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 79.Fankhauser C, Homans S W, Thomasoates J E, McConville M J, Desponds C, Conzelmann A, Ferguson M A J. Structures of glycosylphosphatidylinositol membrane anchors from Saccharomyces cerevisiae. J Biol Chem. 1993;268:26365–26374. [PubMed] [Google Scholar]
- 80.Farooqui J Z, Wohl R C, Kezdy F J, Scanu A M. Identification of the active site serine in human lecithin-cholesterol acyltransferase. Arch Biochem Biophys. 1988;261:330–335. doi: 10.1016/0003-9861(88)90348-7. [DOI] [PubMed] [Google Scholar]
- 81.Felmlee T, Pellett S, Welch R A. Nucleotide sequence of an Escherichia coli chromosomal hemolysin. J Bacteriol. 1985;163:94–105. doi: 10.1128/jb.163.1.94-105.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Felmlee T, Pellett S, Lee E Y, Welch R A. Escherichia coli hemolysin is released extracellularly without cleavage of a signal peptide. J Bacteriol. 1985;163:88–93. doi: 10.1128/jb.163.1.88-93.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Felmlee T, Welch R A. Alterations of amino acid repeats in the Escherichia coli hemolysin affect cytolytic activity and secretion. Proc Natl Acad Sci USA. 1988;85:5269–5273. doi: 10.1073/pnas.85.14.5269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Finlay B B, Cossart P. Exploitation of mammalian host cell functions by bacterial pathogens. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 86.Finnie C, Hartley N M, Findlay K C, Downie J A. The Rhizobium leguminosarum prsDE genes are required for secretion of several proteins, some of which influence nodulation, symbiotic nitrogen fixation and exopolysaccharide modification. Mol Microbiol. 1997;25:135–146. doi: 10.1046/j.1365-2958.1997.4471803.x. [DOI] [PubMed] [Google Scholar]
- 87.Forestier C, Welch R A. Nonreciprocal complementation of the hlyC and lktC genes of the Escherichia coli hemolysin and Pasteurella haemolytica leukotoxin determinants. Infect Immun. 1990;58:828–832. doi: 10.1128/iai.58.3.828-832.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Forestier C, Welch R A. Identification of RTX toxin target cell specificity domains by use of hybrid genes. Infect Immun. 1991;59:4212–4220. doi: 10.1128/iai.59.11.4212-4220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Frey J, Bosse J T, Chang Y F, Cullen J M, Fenwick B, Gerlach G F, Gygi D, Haesebrouck F, Inzana T J, Jansen R, Kamp E M, MacDonald J, MacInnes J I, Mittal K R, Nicolet J, Rycroft A N, Segers R P A M, Smits M A, Stenbaek E, Struck D K, Van den Bosch J F, Willson P J, Young R. Actinobacillus pleuropneumoniae RTX toxins—uniform designation of hemolysins, cytolysins, pleurotoxin and their genes. J Gen Microbiol. 1993;139:1723–1728. doi: 10.1099/00221287-139-8-1723. [DOI] [PubMed] [Google Scholar]
- 90.Fussle R, Bhakdi S, Sziegoleit A, Tranum-Jensen J, Kranz T, Wellensiek H-J. On the mechanism of membrane damage by Staphylococcus aureus α-toxin. J Cell Biol. 1981;91:83–94. doi: 10.1083/jcb.91.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gadeberg O V, Orskov I. In vitro cytotoxic effect of α-hemolytic Escherichia coli on human blood granulocytes. Infect Immun. 1984;45:255–260. doi: 10.1128/iai.45.1.255-260.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gangola P, Rosen B P. Maintenance of intracellular calcium in Escherichia coli. J Biol Chem. 1987;262:12570–12574. [PubMed] [Google Scholar]
- 93.Gazit E, Miller I R, Biggin P C, Sansom M S P, Shai Y. Structure and orientation of the mammalian antibacterial peptide cecropin P1 within phospholipid membranes. J Mol Biol. 1996;258:860–870. doi: 10.1006/jmbi.1996.0293. [DOI] [PubMed] [Google Scholar]
- 94.Gehring A M, Bradley K A, Walsh C T. Enterobactin biosynthesis in Escherichia coli: isochorismate lyase (EntB) is a bifunctional enzyme that is phosphopantetheinylated by EntD and then acylated by EntE using ATP and 2,3-dihydroxybenzoate. Biochemistry. 1997;36:8495–8503. doi: 10.1021/bi970453p. [DOI] [PubMed] [Google Scholar]
- 95.Geiger O, Spaink H P, Kennedy E P. Isolation of the Rhizobium leguminosarum NodF nodulation protein—NodF carries a 4′-phosphopantetheine prosthetic group. J Bacteriol. 1991;173:2872–2878. doi: 10.1128/jb.173.9.2872-2878.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gelman M S, Prives J M. Arrest of subunit folding and assembly of nicotinic acetylcholine receptors in cultured muscle cells by dithiothreitol. J Biol Chem. 1996;271:10709–10714. doi: 10.1074/jbc.271.18.10709. [DOI] [PubMed] [Google Scholar]
- 97.Gentschev I, Goebel W. Topological and functional studies on HlyB of Escherichia coli. Mol Gen Genet. 1992;232:40–48. doi: 10.1007/BF00299135. [DOI] [PubMed] [Google Scholar]
- 98.Gerold P, Eckert V, Schwarz R T. GPI anchors: an overview. Trends Glycosci Glycotechnol. 1996;8:265–277. [Google Scholar]
- 99.Giannakouros T, Newman C M H, Craighead M W, Armstrong J, Magee A I. Post-translational processing of Schizosaccharomyces pombe YPT5 protein: in vitro and in vivo analysis of processing mutants. J Biol Chem. 1993;268:24467–24474. [PubMed] [Google Scholar]
- 100.Glaser P, Sakamoto H, Bellalou J, Ullmann A, Danchin A. Secretion of cyclolysin, the calmodulin sensitive adenylate cyclase-hemolysin bifunctional protein of Bordetella pertussis. EMBO J. 1988;7:3997–4004. doi: 10.1002/j.1460-2075.1988.tb03288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gray J T, Fedorkacray P J, Rogers D G. Partial characterization of a Moraxella bovis cytolysin. Vet Microbiol. 1995;43:183–196. doi: 10.1016/0378-1135(94)00084-a. [DOI] [PubMed] [Google Scholar]
- 102.Grimminger F, Walmrath D, Birkemeyer R G, Bhakdi S, Seeger W. Leukotriene and hydroxyeicosatetraenoic acid generation elicited by low doses of Escherichia coli hemolysin in rabbit lungs. Infect Immun. 1990;58:2659–2663. doi: 10.1128/iai.58.8.2659-2663.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Grimminger F, Rose F, Sibelius U, Meinhardt M, Potzsch B, Spriestersbach R, Bhakdi S, Suttorp N, Seeger W. Human endothelial cell activation and mediator release in response to the bacterial exotoxins Escherichia coli hemolysin and staphylococcal α-toxin. J Immunol. 1997;159:1909–1916. [PubMed] [Google Scholar]
- 104.Gupta S D, Dowhan W, Wu H C. Phosphatidylethanolamine is not essential for the N-acylation of apolipoprotein in Escherichia coli. J Biol Chem. 1991;266:9983–9986. [PubMed] [Google Scholar]
- 105.Gustafsson M, Curstedt T, Jornvall H, Johansson J. Detection of a surfactant protein C isoform containing Nɛ-palmitoyl-lysine. Biochem J. 1997;326:799–806. doi: 10.1042/bj3260799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gygi D, Nicolet J, Frey J, Cross M, Koronakis V, Hughes C. Isolation of the Actinobacillus pleuropneumoniae hemolysin gene and the activation and secretion of the prohaemolysin by the HlyC, HlyB and HlyD proteins of Escherichia coli. Mol Microbiol. 1990;4:123–128. doi: 10.1111/j.1365-2958.1990.tb02021.x. [DOI] [PubMed] [Google Scholar]
- 107.Hacker J, Hughes C, Hof H, Goebel W. Cloned hemolysin genes from Escherichia coli that cause urinary tract infection determine different levels of toxicity in mice. Infect Immun. 1983;42:57–63. doi: 10.1128/iai.42.1.57-63.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hackett M, Guo L, Shabanowitz J, Hunt D F, Hewlett E L. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science. 1994;266:433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- 109.Hackett M, Walker C B, Guo L, Gray M C, van Cuyk S, Ullmann A, Shabanowitz J, Hunt D F, Hewlett E L, Sebo P. Hemolytic, but not cell-invasive activity, of adenylate-cyclase toxin is selectively affected by differential fatty acylation in Escherichia coli. J Biol Chem. 1995;270:20250–20253. doi: 10.1074/jbc.270.35.20250. [DOI] [PubMed] [Google Scholar]
- 110.Hallak H, Brass L F, Manning D R. Failure to myristoylate the α subunit of GZ is correlated with an inhibition of palmitoylation and membrane attachment, but has no affect on phosphorylation by protein-kinase C. J Biol Chem. 1994;269:4571–4576. [PubMed] [Google Scholar]
- 111.Hanakam F, Gerisch G, Lotz S, Alt T, Seelig A. Binding of hisactophilin I and hisactophilin II to lipid membranes is controlled by a pH dependent myristoyl-histidine switch. Biochemistry. 1996;35:11036–11044. doi: 10.1021/bi960789j. [DOI] [PubMed] [Google Scholar]
- 112.Hardie K R, Issartel J P, Koronakis E, Hughes C, Koronakis V. In vitro activation of Escherichia coli prohaemolysin to the mature membrane-targeted toxin requires HlyC and a low molecular weight cytosolic polypeptide. Mol Microbiol. 1991;5:1669–1679. doi: 10.1111/j.1365-2958.1991.tb01914.x. [DOI] [PubMed] [Google Scholar]
- 113.Harrison J L, Taylor I M, Platt K, O’Connor C D. Surface exclusion specificity of the TraT lipoprotein is determined by single alterations in a five amino acid region of the protein. Mol Microbiol. 1992;6:2825–2832. doi: 10.1111/j.1365-2958.1992.tb01462.x. [DOI] [PubMed] [Google Scholar]
- 114.Hauschildt S, Hoffmann P, Beuscher H U, Dufhues G, Heinrich P, Wiesmuller K H, Jung G, Bessler W G. Activation of bone marrow derived mouse macrophages by bacterial lipopeptide—cytokine production, phagocytosis and IgA expression. Eur J Immunol. 1990;20:63–68. doi: 10.1002/eji.1830200110. [DOI] [PubMed] [Google Scholar]
- 115.Hayashi M K, Haga T. Palmitoylation of muscarinic acetylcholine receptor m2 subtypes: reduction in their ability to activate G proteins by mutation of a putative palmitoylation site, cysteine 457, in the carboxyl-terminal tail. Arch Biochem Biophys. 1997;340:376–382. doi: 10.1006/abbi.1997.9906. [DOI] [PubMed] [Google Scholar]
- 116.Hayashi S, Wu H C. Lipoproteins in bacteria. J Bioenerg Biomemb. 1990;22:451–471. doi: 10.1007/BF00763177. [DOI] [PubMed] [Google Scholar]
- 117.Hazuda D J, Strickler J, Simon P, Young P R. Structure-function mapping of interleukin-1 precursors: cleavage leads to a conformational change in the mature protein. J Biol Chem. 1991;266:7081–7086. [PubMed] [Google Scholar]
- 118.Heaton M P, Neuhaus F C. Role of the d-alanyl carrier protein in the biosynthesis of d-alanyl-lipoteichoic acid. J Bacteriol. 1994;176:681–690. doi: 10.1128/jb.176.3.681-690.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hedo J A, Collier E, Watkinson A. Myristyl and palmityl acylation of the insulin receptor. J Biol Chem. 1987;262:954–957. [PubMed] [Google Scholar]
- 120.Henderson B, Poole S, Wilson M. Bacterial modulins—a novel class of virulence factors which cause host tissue pathology by inducing cytokine synthesis. Microbiol Rev. 1996;60:316–341. doi: 10.1128/mr.60.2.316-341.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Henricks P A J, Binkhorst G J, Drijver A A, Nijkamp F P. Pasteurella haemolytica leukotoxin enhances production of leukotriene-B4 and 5-hydroxyeicosatetraenoic acid by bovine polymorphonuclear leukocytes. Infect Immun. 1992;60:3238–3243. doi: 10.1128/iai.60.8.3238-3243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Herrmann A, Schlosser A, Schmid R, Schneider E. Biochemical identification of a lipoprotein with maltose binding activity in the thermoacidophilic Gram-positive bacterium Alicyclobacillus acidocaldarius. Res Microbiol. 1996;147:733–737. doi: 10.1016/s0923-2508(97)85120-0. [DOI] [PubMed] [Google Scholar]
- 123.Hertle R, Brutsche S, Groeger W, Hobbie S, Koch W, Konninger U, Braun V. Specific phosphatidylethanolamine dependence of Serratia marcescens cytotoxin activity. Mol Microbiol. 1997;26:853–865. doi: 10.1046/j.1365-2958.1997.6031978.x. [DOI] [PubMed] [Google Scholar]
- 124.Hess J, Wels W, Vogel M, Goebel W. Nucleotide sequence of a plasmid encoded hemolysin determinant and its comparison with a corresponding chromosomal hemolysin sequence. FEMS Microbiol Lett. 1986;34:1–11. [Google Scholar]
- 125.Highlander S K, Engler M J, Weinstock G M. Secretion and expression of the Pasteurella haemolytica leukotoxin. J Bacteriol. 1990;172:2343–2350. doi: 10.1128/jb.172.5.2343-2350.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Hofer M F, Newell K, Duke R C, Schlievert P M, Freed J H, Leung D Y M. Differential effects of staphylococcal toxic shock syndrome toxin I on B cell apoptosis. Proc Natl Acad Sci USA. 1996;93:5425–5430. doi: 10.1073/pnas.93.11.5425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Holak T A, Nilges M, Prestegard J H, Gronenborn A M, Clore G M. Three dimensional structure of acyl carrier protein in solution determined by nuclear magnetic resonance and the combined use of dynamical simulated annealing and distance geometry. Eur J Biochem. 1988;175:9–15. doi: 10.1111/j.1432-1033.1988.tb14159.x. [DOI] [PubMed] [Google Scholar]
- 128.Hughes C, Issartel J P, Hardie K, Stanley P, Koronakis E, Koronakis V. Activation of Escherichia coli prohemolysin to the membrane targeted toxin by HlyC-directed ACP-dependent fatty acylation. FEMS Microbiol Immunol. 1992;105:37–44. doi: 10.1111/j.1574-6968.1992.tb05884.x. [DOI] [PubMed] [Google Scholar]
- 129.Hughes C, Stanley P, Koronakis V. Escherichia coli hemolysin interactions with prokaryotic and eukaryotic cell membranes. Bioessays. 1992;14:519–525. doi: 10.1002/bies.950140804. [DOI] [PubMed] [Google Scholar]
- 130.Isberg R R, Leong J M. Multiple β1-chain integrins are receptors for invasin, a protein that promotes bacterial penetration into mammalian cells. Cell. 1990;60:861–871. doi: 10.1016/0092-8674(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 131.Ishibashi Y, Claus S, Relman D A. Bordetella pertussis filamentous hemagglutinin interacts with a leukocyte signal transduction complex and stimulates bacterial adherence to monocyte CR3 (CD11B/CD18) J Exp Med. 1994;180:1225–1233. doi: 10.1084/jem.180.4.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Issartel J P, Koronakis V, Hughes C. Activation of Escherichia coli prohaemolysin to the mature toxin by acyl carrier protein-dependent fatty acylation. Nature. 1991;351:759–761. doi: 10.1038/351759a0. [DOI] [PubMed] [Google Scholar]
- 133.Iwaki M, Ullmann A, Sebo P. Identification by in vitro complementation of regions required for cell-invasive activity of Bordetella pertussis adenylate cyclase toxin. Mol Microbiol. 1995;17:1015–1024. doi: 10.1111/j.1365-2958.1995.mmi_17061015.x. [DOI] [PubMed] [Google Scholar]
- 134.Jackowski S, Rock C O. Ratio of active to inactive forms of acyl carrier protein in Escherichia coli. J Biol Chem. 1983;258:5186–5191. [PubMed] [Google Scholar]
- 135.Jackowski S, Rock C O. Transfer of fatty acids from the 1-position of phosphatidylethanolamine to the major outer membrane lipoprotein of Escherichia coli. J Biol Chem. 1986;261:1328–1333. [PubMed] [Google Scholar]
- 136.Jackowski S, Jackson P D, Rock C O. Sequence and function of the aas gene in Escherichia coli. J Biol Chem. 1994;269:2921–2928. [PubMed] [Google Scholar]
- 137.Jarchau T, Chakraborty T, Garcia F, Goebel W. Selection for transport competence of C-terminal polypeptides derived from Escherichia coli hemolysin—the shortest peptide capable of autonomous HlyB/HlyD-dependent secretion comprises the C-terminal 62 amino acids of HlyA. Mol Gen Genet. 1994;245:53–60. doi: 10.1007/BF00279750. [DOI] [PubMed] [Google Scholar]
- 138.Jauhiainen M, Stevenson K J, Dolphin P J. Human plasma lecithin-cholesterol acyltransferase—the vicinal nature of cysteine 31 and cysteine 184 in the catalytic site. J Biol Chem. 1988;263:6525–6533. [PubMed] [Google Scholar]
- 139.Jin H, Zastawny R, George S R, O’Dowd B F. Elimination of palmitoylation sites in the human dopamine D1 receptor does not affect receptor-G protein interaction. Eur J Pharmacol. 1997;324:109–116. doi: 10.1016/s0014-2999(97)00059-9. [DOI] [PubMed] [Google Scholar]
- 140.Johnson D R, Bhatnagar R S, Knoll L J, Gordon J I. Genetic and biochemical studies of protein N-myristoylation. Annu Rev Biochem. 1994;63:869–914. doi: 10.1146/annurev.bi.63.070194.004253. [DOI] [PubMed] [Google Scholar]
- 141.Johnson R S, Ohguro H, Palczewski K, Hurley J B, Walsh K A, Neubert T A. Heterogeneous N-acylation is a tissue specific and species specific posttranslational modification. J Biol Chem. 1994;269:21067–21071. [PubMed] [Google Scholar]
- 142.Jonas D, Schultheis B, Klas C, Krammer P H, Bhakdi S. Cytocidal effects of Escherichia coli hemolysin on human T lymphocytes. Infect Immun. 1993;61:1715–1721. doi: 10.1128/iai.61.5.1715-1721.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Jones A L, Kille P, Dancer J E, Harwood J L. The cloning and overexpression of Escherichia coli acyl carrier protein. Grasas Aceites. 1993;44:116–117. doi: 10.1042/bst021202s. [DOI] [PubMed] [Google Scholar]
- 144.Jones P J, Cioffi E A, Prestegard J H. [19F]-1H heteronuclear Overhauser effect studies of the acyl chain-binding site of acyl carrier protein. J Biol Chem. 1987;262:8963–8965. [PubMed] [Google Scholar]
- 145.Jones T L Z, Degtyarev M Y, Backlund P S. The stoichiometry of GαS palmitoylation in its basal and activated states. Biochemistry. 1997;36:7185–7191. doi: 10.1021/bi9628376. [DOI] [PubMed] [Google Scholar]
- 146.Jordan S W, Cronan J E. A new metabolic link: The acyl carrier protein of lipid synthesis donates lipoic acid to the pyruvate dehydrogenase complex in Escherichia coli and mitochondria. J Biol Chem. 1997;272:17903–17906. doi: 10.1074/jbc.272.29.17903. [DOI] [PubMed] [Google Scholar]
- 147.Jorgensen S E, Hammer R F, Wu G K. Effects of a single hit from the α-hemolysin produced by Escherichia coli on the morphology of sheep erythrocytes. Infect Immun. 1980;27:988–994. doi: 10.1128/iai.27.3.988-994.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Joseph M, Nagaraj R. Interaction of peptides corresponding to fatty acylation sites in proteins with model membranes. J Biol Chem. 1995;270:16749–16755. doi: 10.1074/jbc.270.28.16749. [DOI] [PubMed] [Google Scholar]
- 149.Kabouridis P S, Magee A I, Ley S C. S-acylation of LCK protein tyrosine kinase is essential for its signalling function in T lymphocytes. EMBO J. 1997;16:4983–4998. doi: 10.1093/emboj/16.16.4983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kahn R A, Yucel J K, Malhotra V. ARF signaling: a potential role for phospholipase D in membrane traffic. Cell. 1993;75:1045–1048. doi: 10.1016/0092-8674(93)90314-g. [DOI] [PubMed] [Google Scholar]
- 151.Kaniga K, Trollinger D, Galan J E. Identification of two targets of the type III protein secretion system encoded by the inv and spa loci of Salmonella typhimurium that have homology to the Shigella IpaD and IpaA proteins. J Bacteriol. 1995;177:7078–7085. doi: 10.1128/jb.177.24.7078-7085.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Katahira J, Sugiyama H, Inoue N, Horiguchi Y, Matsuda M, Sugimoto N. Clostridium perfringens enterotoxin utilizes two structurally related membrane proteins as functional receptors in vivo. J Biol Chem. 1997;272:26652–26658. doi: 10.1074/jbc.272.42.26652. [DOI] [PubMed] [Google Scholar]
- 153.Kawate N, Peegel H, Menon K M J. Role of palmitoylation of conserved cysteine residues of luteinizing hormone human choriogonadotropin receptors in receptor down-regulation. Mol Cell Endocrinol. 1997;127:211–219. doi: 10.1016/s0303-7207(97)04010-0. [DOI] [PubMed] [Google Scholar]
- 154.Kaye S A, Louise C B, Boyd B, Lingwood C A, Obrig T G. Shiga toxin-associated hemolytic uremic syndrome: interleukin-1β enhancement of Shiga toxin cytotoxicity toward human vascular endothelial cells in vitro. Infect Immun. 1993;61:3886–3891. doi: 10.1128/iai.61.9.3886-3891.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keane W F, Welch R, Gekker G, Peterson P K. Mechanism of Escherichia coli α-hemolysin induced injury to isolated renal tubular cells. Am J Pathol. 1987;126:350–357. [PMC free article] [PubMed] [Google Scholar]
- 156.Keating D H, Zhang Y, Cronan J E. The apparent coupling between synthesis and post-translational modification of Escherichia coli acyl carrier protein is due to inhibition of amino acid biosynthesis. J Bacteriol. 1996;178:2662–2667. doi: 10.1128/jb.178.9.2662-2667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Keep N H, Barnes M, Barsukov I, Badii R, Lian L Y, Segal A W, Moody P C E, Roberts G C K. A modulator of rho family G proteins, rhoGDI, binds these G proteins via an immunoglobulin-like domain and a flexible N-terminal arm. Structure. 1997;5:623–633. doi: 10.1016/s0969-2126(97)00218-9. [DOI] [PubMed] [Google Scholar]
- 158.Kelly T M, Stachula S A, Raetz C R H, Anderson M S. The firA gene of Escherichia coli encodes UDP-3-O-(R-3-hydroxymyristoyl)-glucosamine N-acyltransferase: the third step of endotoxin biosynthesis. J Biol Chem. 1993;268:19866–19874. [PubMed] [Google Scholar]
- 159.Kenny B, Taylor S, Holland I B. Identification of individual amino acids required for secretion within the hemolysin (HlyA) C-terminal targeting region. Mol Microbiol. 1992;6:1477–1489. doi: 10.1111/j.1365-2958.1992.tb00868.x. [DOI] [PubMed] [Google Scholar]
- 160.Kenny B, DeVinney R, Stein M, Reinscheid D J, Frey E A, Finlay B B. Enteropathogenic E. coli (EPEC) transfers its receptor for intimate adherence into mammalian cells. Cell. 1997;91:511–520. doi: 10.1016/s0092-8674(00)80437-7. [DOI] [PubMed] [Google Scholar]
- 161.Kim J Y, Shishido T, Jiang X L, Aderem A, McLaughlin S. Phosphorylation, high ionic strength, and calmodulin reverse the binding of MARCKS to phospholipid vesicles. J Biol Chem. 1994;269:28214–28219. [PubMed] [Google Scholar]
- 162.Klebba P E, Hofnung M, Charbit A. A model of maltodextrin transport through the sugar specific porin, LamB, based on deletion analysis. EMBO J. 1994;13:4670–4675. doi: 10.1002/j.1460-2075.1994.tb06790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kokame K, Fukada Y, Yoshizawa T, Takao T, Shimonishi Y. Lipid modification at the N-terminus of photoreceptor G-protein α-subunit. Nature. 1992;359:749–752. doi: 10.1038/359749a0. [DOI] [PubMed] [Google Scholar]
- 164.Konig B, Ludwig A, Goebel W, Konig W. Pore formation by the Escherichia coli alpha-hemolysin: role for mediator release from human inflammatory cells. Infect Immun. 1994;62:4611–4617. doi: 10.1128/iai.62.10.4611-4617.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Konig B, Konig W. Effect of growth factors on Escherichia coli alpha-hemolysin induced mediator release from inflammatory cells: involvement of the signal transduction pathway. Infect Immun. 1994;62:2085–2093. doi: 10.1128/iai.62.5.2085-2093.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Koronakis E, Hughes C, Milisav I, Koronakis V. Protein exporter function and in vitro ATPase activity are correlated in ABC-domain mutants of HlyB. Mol Microbiol. 1995;16:87–96. doi: 10.1111/j.1365-2958.1995.tb02394.x. [DOI] [PubMed] [Google Scholar]
- 167.Koronakis V, Cross M, Senior B, Koronakis E, Hughes C. The secreted hemolysins of Proteus mirabilis, Proteus vulgaris, and Morganella morganii are genetically related to each other and to the alpha-hemolysin of Escherichia coli. J Bacteriol. 1987;169:1509–1515. doi: 10.1128/jb.169.4.1509-1515.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Koronakis V, Koronakis E, Hughes C. Isolation and analysis of the C-terminal signal directing export of Escherichia coli hemolysin protein across both bacterial membranes. EMBO J. 1989;8:595–605. doi: 10.1002/j.1460-2075.1989.tb03414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Koronakis V, Hughes C, Koronakis E. Energetically distinct early and late stages of HlyB/HlyD-dependent secretion across both Escherichia coli membranes. EMBO J. 1991;10:3263–3272. doi: 10.1002/j.1460-2075.1991.tb04890.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Koronakis V, Hughes C, Koronakis E. ATPase activity and ATP/ADP-induced conformational change in the soluble domain of the bacterial protein translocator HlyB. Mol Microbiol. 1993;8:1163–1175. doi: 10.1111/j.1365-2958.1993.tb01661.x. [DOI] [PubMed] [Google Scholar]
- 171.Koronakis V, Hughes C. Synthesis, maturation and export of the Escherichia coli hemolysin. Med Microbiol Immunol. 1996;185:65–71. doi: 10.1007/s004300050016. [DOI] [PubMed] [Google Scholar]
- 172.Koronakis V, Li J, Koronakis E, Stauffer K. Structure of TolC, the outer membrane component of the bacterial type I efflux system, derived from two-dimensional crystals. Mol Microbiol. 1997;23:617–626. doi: 10.1046/j.1365-2958.1997.d01-1880.x. [DOI] [PubMed] [Google Scholar]
- 173.Kraig E, Dailey T, Kolodrubetz D. Nucleotide sequence of the leukotoxin gene from Actinobacillus actinomycetemcomitans—homology to the alpha-hemolysin leukotoxin gene family. Infect Immun. 1990;58:920–929. doi: 10.1128/iai.58.4.920-929.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kuipers O P, Beerthuyzen M M, Siezen R J, deVos W M. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis: requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem. 1993;216:281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- 175.Lally E T, Golub E E, Kieba I R. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J Biol Chem. 1994;269:31289–31295. [PubMed] [Google Scholar]
- 176.Lally E T, Kieba I R, Sato A, Green C L, Rosenbloom J, Korostoff J, Wang J F, Shenker B J, Ortlepp S, Robinson M K, Billings P C. RTX toxins recognize a β2 integrin on the surface of human target cells. J Biol Chem. 1997;272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 177.Lambalot R H, Gehring A M, Flugel R S, Zuber P, Lacelle M, Marahiel M A, Reid R, Khosla C, Walsh C T. A new enzyme superfamily: the phosphopantetheinyl transferases. Chem Biol. 1996;3:923–936. doi: 10.1016/s1074-5521(96)90181-7. [DOI] [PubMed] [Google Scholar]
- 178.Lambalot R H, Walsh C T. Holo-[acyl-carrier-protein] synthase of Escherichia coli. Methods Enzymol. 1997;279:254–262. doi: 10.1016/s0076-6879(97)79029-3. [DOI] [PubMed] [Google Scholar]
- 179.Lee C Y, Meighen E A. Cysteine-286 as the site of acylation of the Lux-specific fatty acyl-CoA reductase. Biochim Biophys Acta. 1997;1338:215–222. doi: 10.1016/s0167-4838(96)00203-8. [DOI] [PubMed] [Google Scholar]
- 180.Leesong M, Henderson B S, Gillig J R, Schwab J M, Smith J L. Structure of a dehydratase-isomerase from the bacterial pathway for biosynthesis of unsaturated fatty acids: two catalytic activities in one active site. Structure. 1996;4:253–264. doi: 10.1016/s0969-2126(96)00030-5. [DOI] [PubMed] [Google Scholar]
- 181.Li J, Szittner R, Derewenda Z S, Meighen E A. Conversion of serine-114 to cysteine-114 and the role of the active site nucleophile in acyl transfer by myristoyl-ACP thioesterase from Vibrio harveyi. Biochemistry. 1996;35:9967–9973. doi: 10.1021/bi9605292. [DOI] [PubMed] [Google Scholar]
- 182.Lightner V A, Bell R M, Modrich P. The DNA sequences encoding plsB and dgk loci of Escherichia coli. J Biol Chem. 1983;258:856–861. [PubMed] [Google Scholar]
- 183.Linder M E, Pang I H, Duronio R J, Gordon J I, Sternweis P C, Gilman A G. Lipid modifications of G-protein subunits: myristoylation of Gα increases its affinity for βγ. J Biol Chem. 1991;266:4654–4659. [PubMed] [Google Scholar]
- 184.Liu L, Dudler T, Gelb M H. Purification of a protein palmitoyltransferase that acts on H-ras protein and on a C-terminal N-ras peptide. J Biol Chem. 1996;271:23269–23276. doi: 10.1074/jbc.271.38.23269. [DOI] [PubMed] [Google Scholar]
- 185.Loing E, Delanoye A, Sergheraert C, Tartar A, Gras-Masse H. Assessing delivery of lipopeptides into the cytoplasm of intact cells by a functional assay based on PKC inhibition. 1. The Jurkat model. Peptide Res. 1996;9:229–232. [PubMed] [Google Scholar]
- 186.Ludwig A, Vogel M, Goebel W. Mutations affecting activity and transport of hemolysin in Escherichia coli. Mol Gen Genet. 1987;206:238–245. doi: 10.1007/BF00333579. [DOI] [PubMed] [Google Scholar]
- 187.Ludwig A, Jarchau T, Benz R, Goebel W. The repeat domain of Escherichia coli hemolysin (HlyA) is responsible for its Ca2+-dependent binding to erythrocytes. Mol Gen Genet. 1988;214:553–561. doi: 10.1007/BF00330494. [DOI] [PubMed] [Google Scholar]
- 188.Ludwig A, Schmid A, Benz R, Goebel W. Mutations affecting pore formation by hemolysin from Escherichia coli. Mol Gen Genet. 1991;226:198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- 189.Ludwig A, Benz R, Goebel W. Oligomerization of Escherichia coli hemolysin (HlyA) is involved in pore formation. Mol Gen Genet. 1993;241:89–96. doi: 10.1007/BF00280205. [DOI] [PubMed] [Google Scholar]
- 190.Ludwig A, Garcia F, Bauer S, Jarchau T, Benz R, Hoppe J, Goebel W. Analysis of the in vivo activation of hemolysin (HlyA) from Escherichia coli. J Bacteriol. 1996;178:5422–5430. doi: 10.1128/jb.178.18.5422-5430.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Maekawa T, Yanagihara K, Ohtsubo E. Specific nicking at the 3′ ends of the terminal inverted repeat sequences in transposon Tn3 by transposase and an E. coli protein ACP. Genes Cells. 1996;1:1017–1030. doi: 10.1046/j.1365-2443.1996.d01-221.x. [DOI] [PubMed] [Google Scholar]
- 192.Magnuson K, Jackowski S, Rock C O, Cronan J E. Regulation of fatty acid biosynthesis in Escherichia coli. Microbiol Rev. 1993;57:522–542. doi: 10.1128/mr.57.3.522-542.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Magnuson K, Carey M R, Cronan J E. The putative fabJ gene of Escherichia coli fatty acid synthesis is the fabF gene. J Bacteriol. 1995;177:3593–3595. doi: 10.1128/jb.177.12.3593-3595.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Maier E, Reinhard N, Benz R, Frey J. Channel forming activity and channel size of the RTX toxins ApxI, ApxII, and ApxIII of Actinobacillus pleuropneumoniae. Infect Immun. 1996;64:4415–4423. doi: 10.1128/iai.64.11.4415-4423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Majury A L, Shewen P E. Preliminary investigation of the mechanism of inhibition of bovine lymphocyte proliferation by Pasteurella haemolytica A1 leukotoxin. Vet Immunol Immunopathol. 1991;29:57–68. doi: 10.1016/0165-2427(91)90052-e. [DOI] [PubMed] [Google Scholar]
- 196.Manenti S, Sorokine O, vanDorsselaer A, Taniguchi H. Demyristoylation of the major substrate of protein kinase C (MARCKS) by the cytoplasmic fraction of brain synaptosomes. J Biol Chem. 1994;269:8309–8313. [PubMed] [Google Scholar]
- 197.Mangan D F, Taichman N S, Lally E T, Wahl S M. Lethal effects of Actinobacillus actinomycetemcomitans leukotoxin on human lymphocytes. Infect Immun. 1991;59:3267–3272. doi: 10.1128/iai.59.9.3267-3272.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Marshall C J. Protein prenylation: a mediator of protein-protein interactions. Science. 1993;259:1865–1866. doi: 10.1126/science.8456312. [DOI] [PubMed] [Google Scholar]
- 199.Marshall M. Ras target proteins in eukaryotic cells. FASEB J. 1995;9:1311–1318. doi: 10.1096/fasebj.9.13.7557021. [DOI] [PubMed] [Google Scholar]
- 200.May A K, Sawyer R G, Gleason T, Whitworth A, Pruett T L. In vivo cytokine response to Escherichia coli alpha-hemolysin determined with genetically engineered hemolytic and nonhemolytic Escherichia coli variants. Infect Immun. 1996;64:2167–2171. doi: 10.1128/iai.64.6.2167-2171.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Mayo K H, Prestegard J H. Acyl carrier protein from Escherichia coli: structural characterization of short chain acylated acyl carrier proteins by NMR. Biochemistry. 1985;24:7834–7838. doi: 10.1021/bi00347a049. [DOI] [PubMed] [Google Scholar]
- 202.McWhinney D R, Chang Y F, Young R, Struck D K. Separable domains define target cell specificities of an RTX hemolysin from Actinobacillus pleuropneumoniae. J Bacteriol. 1992;174:291–297. doi: 10.1128/jb.174.1.291-297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Menestrina G, Moser C, Pellett S, Welch R. Pore formation by Escherichia coli hemolysin (HlyA) and other members of the RTX toxins family. Toxicology. 1994;87:249–267. doi: 10.1016/0300-483x(94)90254-2. [DOI] [PubMed] [Google Scholar]
- 204.Menestrina G, DallaSerra M, Pederzolli C, Bregante M, Gambale F. Bacterial hemolysins and leukotoxins affect target cells by forming large exogenous pores into their plasma membrane: Escherichia coli hemolysin A as a case example. Biosci Rep. 1995;15:543–551. doi: 10.1007/BF01204356. [DOI] [PubMed] [Google Scholar]
- 205.Menestrina G, Ropele M, Dalla Serra M, Pederzolli C, Hugo F, Pellett S, Welch R A. Binding of antibodies to functional epitopes on the pore formed by Escherichia coli hemolysin in cells and model membranes. Biochim Biophys Acta. 1995;1238:72–80. doi: 10.1016/0005-2736(95)00113-h. [DOI] [PubMed] [Google Scholar]
- 206.Menzl K, Maier E, Chakraborty T, Benz R. HlyA hemolysin of Vibrio cholerae O1 biotype El Tor: identification of the hemolytic complex and evidence for the formation of anion selective ion-permeable channels. Eur J Biochem. 1996;240:646–654. doi: 10.1111/j.1432-1033.1996.0646h.x. [DOI] [PubMed] [Google Scholar]
- 207.Merlie J P, Lindstrom J. Assembly in vivo of mouse muscle acetylcholine receptor—identification of an α-subunit species that may be an assembly intermediate. Cell. 1983;34:747–757. doi: 10.1016/0092-8674(83)90531-7. [DOI] [PubMed] [Google Scholar]
- 208.Milligan G, Parenti M, Magee A I. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- 209.Milligan G, Grassie M A, Wise A, Macewan D J, Magee A I, Parenti M. G-protein palmitoylation: regulation and functional significance. Biochem Soc Trans. 1995;23:583–587. doi: 10.1042/bst0230583. [DOI] [PubMed] [Google Scholar]
- 210.Moayeri M, Welch R A. Effects of temperature, time, and toxin concentration on lesion formation by the Escherichia coli hemolysin. Infect Immun. 1994;62:4124–4134. doi: 10.1128/iai.62.10.4124-4134.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moayeri M, Welch R A. Prelytic and lytic conformations of erythrocyte-associated Escherichia coli hemolysin. Infect Immun. 1997;65:2233–2239. doi: 10.1128/iai.65.6.2233-2239.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Mobley H L T, Green D M, Trifillis A L, Johnson D E, Chippendale G R, Lockatell C V, Jones B D, Warren J W. Pyelonephritogenic Escherichia coli and killing of cultured human renal proximal tubular epithelial cells: role of hemolysin in some strains. Infect Immun. 1990;58:1281–1289. doi: 10.1128/iai.58.5.1281-1289.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Moffett S, Mouillac B, Bonin H, Bouvier M. Altered phosphorylation and desensitization patterns of a human β2-adrenergic receptor lacking the palmitoylated Cys341. EMBO J. 1993;12:349–356. doi: 10.1002/j.1460-2075.1993.tb05663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Morgan P J, Hyman S C, Rowe A J, Mitchell T J, Andrew P W, Saibil H R. Subunit organization and symmetry of pore-forming, oligomeric pneumolysin. FEBS Lett. 1995;371:77–80. doi: 10.1016/0014-5793(95)00887-f. [DOI] [PubMed] [Google Scholar]
- 215.Mumby S M, Heukeroth R O, Gordon J I, Gilman A G. G-protein α-subunit expression, myristoylation, and membrane association in Cos cells. Proc Natl Acad Sci USA. 1990;87:728–732. doi: 10.1073/pnas.87.2.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Mumby S M. Reversible palmitoylation of signaling proteins. Curr Opin Cell Biol. 1997;9:148–154. doi: 10.1016/s0955-0674(97)80056-7. [DOI] [PubMed] [Google Scholar]
- 217.Muntz K H, Sternweis P C, Gilman A G, Mumby S M. Influence of γ-subunit prenylation on association of guanine nucleotide binding regulatory proteins with membranes. Mol Biol Cell. 1992;3:49–61. doi: 10.1091/mbc.3.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Murray D, Ben Tal N, Honig B, McLaughlin S. Electrostatic interaction of myristoylated proteins with membranes: simple physics, complicated biology. Structure. 1997;5:985–989. doi: 10.1016/s0969-2126(97)00251-7. [DOI] [PubMed] [Google Scholar]
- 219.Nagamune K, Yamamoto K, Naka A, Matsuyama J, Miwatani T, Honda T. In vitro proteolytic processing and activation of the recombinant precursor of El Tor cytolysin/hemolysin (proHlyA) of Vibrio cholerae by soluble hemagglutinin/protease of Vibrio cholerae, trypsin, and other proteases. Infect Immun. 1996;64:4655–4658. doi: 10.1128/iai.64.11.4655-4658.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Naglich J G, Metherall J E, Russell D W, Eidels L. Expression cloning of a diphtheria toxin receptor: identity with a heparin binding EGF-like growth factor precursor. Cell. 1992;69:1051–1061. doi: 10.1016/0092-8674(92)90623-k. [DOI] [PubMed] [Google Scholar]
- 221.Nakahama K, Yoshimura K, Marumoto R, Kikuchi M, Lee I S, Hase T, Matsubara H. Cloning and sequencing of Serratia protease gene. Nucleic Acid Res. 1986;14:5843–5855. doi: 10.1093/nar/14.14.5843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Newman C M H, Magee A I. Posttranslational processing of the Ras superfamily of small GTP-binding proteins. Biochim Biophys Acta. 1993;1155:79–96. doi: 10.1016/0304-419x(93)90023-6. [DOI] [PubMed] [Google Scholar]
- 223.Nicaud J M, Mackman N, Gray L, Holland I B. The C-terminal, 23kDa peptide of Escherichia coli hemolysin 2001 contains all the information necessary for its secretion by the hemolysin (Hly) export machinery. FEBS Lett. 1986;204:331–335. doi: 10.1016/0014-5793(86)80838-9. [DOI] [PubMed] [Google Scholar]
- 224.Nichols W A, Raetz C R H, Clementz T, Smith A L, Hanson J A, Ketterer M R, Sunshine M, Apicella M A. htrB of Haemophilus influenzae—determination of biochemical activity and effects on virulence and lipooligosaccharide toxicity. J Endotoxin Res. 1997;4:163–172. [Google Scholar]
- 225.Nieto J M, Bailey M J A, Hughes C, Koronakis V. Suppression of transcription polarity in the Escherichia coli hemolysin operon by a short upstream element shared by polysaccharide and DNA transfer determinants. Mol Microbiol. 1996;19:705–713. doi: 10.1046/j.1365-2958.1996.446951.x. [DOI] [PubMed] [Google Scholar]
- 226.Niki H, Imamura R, Kitaoka M, Yamanaka K, Ogura T, Hiraga S. Escherichia coli MukB protein involved in chromosome partition forms a homodimer with a rod-and-hinge structure having DNA binding and ATP/GTP binding activities. EMBO J. 1992;11:5101–5109. doi: 10.1002/j.1460-2075.1992.tb05617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Odegaard T J, Kaltashov I A, Cotter R J, Steeghs L, van der Ley P, Khan S, Maskell D J, Raetz C R H. Shortened hydroxyacyl chains on lipid A of Escherichia coli cells expressing a foreign UDP-N-acetylglucosamine O-acyltransferase. J Biol Chem. 1997;272:19688–19696. doi: 10.1074/jbc.272.32.19688. [DOI] [PubMed] [Google Scholar]
- 228.Ohnishi M, Hayashi T, Terawaki Y. Purification and characterization of procytotoxin of Pseudomonas aeruginosa—dimer to monomer conversion of protoxin by proteolytic activation. J Biol Chem. 1998;273:453–458. doi: 10.1074/jbc.273.1.453. [DOI] [PubMed] [Google Scholar]
- 229.Olson E N, Glaser L, Merlie J P. α and β subunits of the nicotinic acetylcholine receptor contain covalently bound lipid. J Biol Chem. 1984;259:5364–5367. [PubMed] [Google Scholar]
- 230.Onishi H R, Pelak B A, Gerckens L S, Silver L L, Kahan F M, Chen M H, Patchett A A, Galloway S M, Hyland S A, Anderson M S, Raetz C R H. Antibacterial agents that inhibit lipid A biosynthesis. Science. 1996;274:980–982. doi: 10.1126/science.274.5289.980. [DOI] [PubMed] [Google Scholar]
- 231.Ostolaza H, Bartolome B, Ortiz de Zarate I, de la Cruz F, Goni F M. Release of lipid vesicle contents by the bacterial protein toxin α-hemolysin. Biochim Biophys Acta. 1993;1147:81–88. doi: 10.1016/0005-2736(93)90318-t. [DOI] [PubMed] [Google Scholar]
- 232.Ostolaza H, Soloaga A, Goni F M. The binding of divalent cations to Escherichia coli α-hemolysin. Eur J Biochem. 1995;228:39–44. [PubMed] [Google Scholar]
- 233.Ostolaza H, Bakas L, Goni F M. Balance of electrostatic and hydrophobic interactions in the lysis of model membranes by E. coli α-haemolysin. J Membr Biol. 1997;158:137–145. doi: 10.1007/s002329900251. [DOI] [PubMed] [Google Scholar]
- 234.Palczewski K. GTP-binding-protein-coupled receptor kinases: two mechanistic models. Eur J Biochem. 1997;248:261–269. doi: 10.1111/j.1432-1033.1997.00261.x. [DOI] [PubMed] [Google Scholar]
- 235.Palmer M, Saweljew P, Vulicevic I, Valeva A, Kehoe M, Bhakdi S. Membrane penetrating domain of streptolysin O identified by cysteine scanning mutagenesis. J Biol Chem. 1996;271:26664–26667. doi: 10.1074/jbc.271.43.26664. [DOI] [PubMed] [Google Scholar]
- 236.Parker M W, VanderGoot F G, Buckley J T. Aerolysin: the ins and outs of a model channel forming toxin. Mol Microbiol. 1996;19:205–212. doi: 10.1046/j.1365-2958.1996.355887.x. [DOI] [PubMed] [Google Scholar]
- 237.Peitzsch R M, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipid vesicles: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- 238.Pellett S, Boehm D F, Snyder I S, Rowe G, Welch R A. Characterization of monoclonal antibodies against the Escherichia coli hemolysin. Infect Immun. 1990;58:822–827. doi: 10.1128/iai.58.3.822-827.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Pellett S, Welch R A. Escherichia coli hemolysin mutants with altered target cell specificity. Infect Immun. 1996;64:3081–3087. doi: 10.1128/iai.64.8.3081-3087.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Pellman D, Garber E A, Cross F R, Hanafusa H. An N-terminal peptide from p60Src can direct myristylation and plasma membrane localization when fused to heterologous proteins. Nature. 1985;314:374–377. doi: 10.1038/314374a0. [DOI] [PubMed] [Google Scholar]
- 241.Pieper R, Haese A, Schroder W, Zocher R. Arrangement of catalytic sites in the multifunctional enzyme enniatin synthetase. Eur J Biochem. 1995;230:119–126. doi: 10.1111/j.1432-1033.1995.0119i.x. [DOI] [PubMed] [Google Scholar]
- 242.Pillai S, Baltimore D. Myristoylation and the post-translational acquisition of hydrophobicity by the membrane immunoglobulin heavy chain polypeptide in B lymphocytes. Proc Natl Acad Sci USA. 1987;84:7654–7658. doi: 10.1073/pnas.84.21.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Platt M W, Miller K J, Lane W S, Kennedy E P. Isolation and characterization of the constitutive acyl carrier protein from Rhizobium meliloti. J Bacteriol. 1990;172:5440–5444. doi: 10.1128/jb.172.9.5440-5444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Porat R, Clark B D, Wolff S M, Dinarello C A. Enhancement of the growth of virulent strains of E. coli by interleukin 1. Science. 1991;254:430–432. doi: 10.1126/science.1833820. [DOI] [PubMed] [Google Scholar]
- 245.Pothoulakis C, Gilbert R J, Cladaras C, Castagliuolo I, Semenza G, Hitti Y, Montcrief J S, Linevsky J, Kelly C P, Nikulasson S, Desai H P, Wilkins T D, Lamont J T. Rabbit sucrase-isomaltase contains a functional intestinal receptor for Clostridium difficile toxin A. J Clin Invest. 1996;98:641–649. doi: 10.1172/JCI118835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Raetz C R H, Roderick S L. A left-handed parallel β helix in the structure of UDP-N-acetylglucosamine acyltransferase. Science. 1995;270:997–1000. doi: 10.1126/science.270.5238.997. [DOI] [PubMed] [Google Scholar]
- 247.Resh M D. Regulation of cellular signaling by fatty acid acylation and prenylation of signal transduction proteins. Cell Signalling. 1996;8:403–412. doi: 10.1016/s0898-6568(96)00088-5. [DOI] [PubMed] [Google Scholar]
- 248.Revill W P, Bibb M J, Hopwood D A. Relationships between fatty acid and polyketide synthases from Streptomyces coelicolor A3(2): characterization of the fatty acid synthase acyl carrier protein. J Bacteriol. 1996;178:5660–5667. doi: 10.1128/jb.178.19.5660-5667.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Rock C O, Jackowski S. Regulation of phospholipid synthesis in Escherichia coli: composition of the acyl-acyl carrier protein pool in vivo. J Biol Chem. 1982;257:759–765. [PubMed] [Google Scholar]
- 250.Rose T, Sebo P, Bellalou J, Ladant D. Interaction of calcium with Bordetella pertussis adenylate-cyclase toxin. J Biol Chem. 1995;270:26370–26376. doi: 10.1074/jbc.270.44.26370. [DOI] [PubMed] [Google Scholar]
- 251.Rosenshine I, Ruschkowski S, Stein M, Reinscheid D J, Mills S D, Finlay B B. A pathogenic bacterium triggers epithelial signals to form a functional bacterial receptor that mediates actin pseudopod formation. EMBO J. 1996;15:2613–2624. [PMC free article] [PubMed] [Google Scholar]
- 252.Rudnick D A, Rocque W J, McWherter C A, Toth M V, Jackson-Machelski E, Gordon J I. Use of photoactivatable peptide substrates of Saccharomyces cerevisiae myristoyl-CoA protein N-myristoyltransferase (Nmt1p) to characterize a myristoylCoA-Nmt1p-peptide ternary complex and to provide evidence for an ordered reaction mechanism. Proc Natl Acad Sci USA. 1993;90:1087–1091. doi: 10.1073/pnas.90.3.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Rusnak F, Sakaitani M, Drueckhammer D, Reichert J, Walsh C Y. Biosynthesis of the Escherichia coli siderophore enterobactin—sequence of the entF gene, expression and purification of EntF, and analysis of covalent phosphopantetheine. Biochemistry. 1991;30:2916–2927. doi: 10.1021/bi00225a027. [DOI] [PubMed] [Google Scholar]
- 254.Sankaran K, Wu H C. Lipid modification of bacterial prolipoprotein: transfer of diacylglyceryl moiety from phosphatidylglycerol. J Biol Chem. 1994;269:19701–19706. [PubMed] [Google Scholar]
- 255.Saouaf S J, Wolven A, Resh M D, Bolen J B. Palmitylation of Src family tyrosine kinases regulates functional interaction with a B cell substrate. Biochem Biophys Res Commun. 1997;234:325–329. doi: 10.1006/bbrc.1997.6638. [DOI] [PubMed] [Google Scholar]
- 256.Scheu A K, Economou A, Hong G F, Ghelani S, Johnston A W B, Downie J A. Secretion of the Rhizobium leguminosarum nodulation protein NodO by hemolysin-type systems. Mol Microbiol. 1992;6:231–238. doi: 10.1111/j.1365-2958.1992.tb02004.x. [DOI] [PubMed] [Google Scholar]
- 257.Schmidt H, Kernbach C, Karch H. Analysis of the EHEC hly operon and its location in the physical map of the large plasmid of enterohemorrhagic Escherichia coli O157-H7. Microbiology. 1996;142:907–914. doi: 10.1099/00221287-142-4-907. [DOI] [PubMed] [Google Scholar]
- 258.Schmidt H, Maier E, Karch H, Benz R. Pore-forming properties of the plasmid encoded hemolysin of enterohemorrhagic Escherichia coli O157-H7. Eur J Biochem. 1996;241:594–601. doi: 10.1111/j.1432-1033.1996.00594.x. [DOI] [PubMed] [Google Scholar]
- 259.Schoenhals G J, MacNab R M. Physiological and biochemical analyses of FlgH, a lipoprotein forming the outer membrane L-ring of the flagellar basal body of Salmonella typhimurium. J Bacteriol. 1996;178:4200–4207. doi: 10.1128/jb.178.14.4200-4207.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Schulein R, Gentschev I, Mollenkopf H J, Goebel W. A topological model for the hemolysin translocator protein HlyD. Mol Gen Genet. 1992;234:155–163. doi: 10.1007/BF00272357. [DOI] [PubMed] [Google Scholar]
- 261.Schutte H, Rosseau S, Czymek R, Ermert L, Walmrath D, Kramer H J, Seeger W, Grimminger F. Synergism between endotoxin priming and exotoxin. Am J Respir Crit Care Med. 1997;156:819–824. doi: 10.1164/ajrccm.156.3.9611010. [DOI] [PubMed] [Google Scholar]
- 262.Sebo P, Ladant D. Repeat sequences in the Bordetella pertussis adenylate-cyclase toxin can be recognized as alternative carboxy-proximal secretion signals by the Escherichia coli α-hemolysin translocator. Mol Microbiol. 1993;9:999–1009. doi: 10.1111/j.1365-2958.1993.tb01229.x. [DOI] [PubMed] [Google Scholar]
- 263.Sebti S M, Hamilton A D. New approaches to anticancer drug design based on the inhibition of farnesyltransferase. Drug Discov Today. 1998;3:26–33. [Google Scholar]
- 264.Seger R, Krebs E. The MAPK signaling cascade. FASEB J. 1995;9:726–735. [PubMed] [Google Scholar]
- 265.Seykora J T, Myat M M, Allen L A H, Ravetch J V, Aderem A. Molecular determinants of the myristoyl-electrostatic switch of MARCKS. J Biol Chem. 1996;271:18797–18802. doi: 10.1074/jbc.271.31.18797. [DOI] [PubMed] [Google Scholar]
- 266.Shahinian S, Silvius J R. Doubly-lipid-modified protein sequence motifs exhibit long-lived anchorage to lipid bilayer membranes. Biochemistry. 1995;34:3813–3822. doi: 10.1021/bi00011a039. [DOI] [PubMed] [Google Scholar]
- 267.Shearman C A, Rossen L, Johnston A W B, Downie J A. The Rhizobium leguminosarum nodulation gene nodF encodes a polypeptide similar to acyl carrier protein and is regulated by NodD plus a factor in pea root exudate. EMBO J. 1986;5:647–652. doi: 10.1002/j.1460-2075.1986.tb04262.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Shen B, Summers R G, Gramajo H, Bibb M J, Hutchinson C R. Purification and characterization of the acyl carrier protein of the Streptomyces glaucescens tetracenomycin C polyketide synthase. J Bacteriol. 1992;174:3818–3821. doi: 10.1128/jb.174.11.3818-3821.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Shen F, Seabra M C. Mechanism of digeranylgeranylation of Rab proteins: formation of a complex between monogeranylgeranyl-Rab and Rab escort protein. J Biol Chem. 1996;271:3692–3698. doi: 10.1074/jbc.271.7.3692. [DOI] [PubMed] [Google Scholar]
- 270.Shen Z, Wu S K, Cho W H. Effects of specific fatty acid acylation of phospholipase A2 on its interfacial binding and catalysis. Biochemistry. 1994;33:11598–11607. doi: 10.1021/bi00204a022. [DOI] [PubMed] [Google Scholar]
- 271.Shenoy-Scaria A M, Gauen L K T, Kwong J, Shaw A S, Lublin D M. Palmitylation of an amino-terminal cysteine motif of protein tyrosine kinases p56lck and p59fyn mediates interaction with glycosylphosphatidylinositol-anchored proteins. Mol Cell Biol. 1993;13:6385–6392. doi: 10.1128/mcb.13.10.6385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Shenoy-Scaria A M, Dietzen D J, Kwong J, Link D C, Lublin D M. Cysteine(3) of Src family protein tyrosine kinases determines palmitoylation and localization in caveolae. J Cell Biol. 1994;126:353–363. doi: 10.1083/jcb.126.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Sheps J A, Cheung I, Ling V. Hemolysin transport in Escherichia coli—point mutants in HlyB compensate for a deletion in the predicted amphiphilic helix region of the HlyA signal. J Biol Chem. 1995;270:14829–14834. doi: 10.1074/jbc.270.24.14829. [DOI] [PubMed] [Google Scholar]
- 274.Shewen P E, Wilkie B N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Soloaga A, Ostolaza H, Goni F M, DelaCruz F. Purification of Escherichia coli prohemolysin, and a comparison with the properties of mature α-hemolysin. Eur J Biochem. 1996;238:418–422. doi: 10.1111/j.1432-1033.1996.0418z.x. [DOI] [PubMed] [Google Scholar]
- 276.Solomon K R, Rudd C E, Finberg R W. The association between glycosylphosphatidylinositol-anchored proteins and heterotrimeric G-protein α-subunits in lymphocytes. Proc Natl Acad Sci USA. 1996;93:6053–6058. doi: 10.1073/pnas.93.12.6053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Song K S, Sargiacomo M, Galbiati F, Parenti M, Lisanti M P. Targeting of a Gα subunit (Gαi-1) and c-Src tyrosine kinase to caveolae membranes: clarifying the role of N-myristoylation. Cell Mol Biol. 1997;43:293–303. [PubMed] [Google Scholar]
- 278.Song L Z, Hobaugh M R, Shustak C, Cheley S, Bayley H, Gouaux J E. Structure of staphylococcal α-hemolysin, a heptameric transmembrane pore. Science. 1996;274:1859–1866. doi: 10.1126/science.274.5294.1859. [DOI] [PubMed] [Google Scholar]
- 279.Spangler B D. Structure and function of cholera toxin and the related Escherichia coli heat-labile enterotoxin. Microbiol Rev. 1992;56:622–647. doi: 10.1128/mr.56.4.622-647.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Staali L, Monteil H, Colin D A. Is the membrane target of the pore-forming leucotoxins from S. aureus a calcium channel? Med Microbiol Immunol. 1996;185:119. [Google Scholar]
- 281.Stanley P, Koronakis V, Hughes C. Mutational analysis supports a role for multiple structural features in the C-terminal secretion signal of Escherichia coli hemolysin. Mol Microbiol. 1991;5:2391–2403. doi: 10.1111/j.1365-2958.1991.tb02085.x. [DOI] [PubMed] [Google Scholar]
- 282.Stanley P, Packman L C, Koronakis V, Hughes C. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science. 1994;266:1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- 283.Stanley P, Koronakis V, Hardie K, Hughes C. Independent interaction of the acyltransferase HlyC with two maturation domains of the Escherichia coli toxin HlyA. Mol Microbiol. 1996;20:813–822. doi: 10.1111/j.1365-2958.1996.tb02519.x. [DOI] [PubMed] [Google Scholar]
- 284.Stanley, P., V. Koronakis, and C. Hughes. Binding of fatty acid and phosphopantetheine by the acyltransferase HlyC required for the maturation of the E. coli toxin HlyA. Unpublished data.
- 285.Stanley, P. Unpublished data.
- 286.Stanley P L D, Diaz P, Bailey M J A, Gygi D, Juarez A, Hughes C. Loss of activity in the secreted form of Escherichia coli hemolysin caused by an rfaP lesion in core lipopolysaccharide assembly. Mol Microbiol. 1993;10:781–787. doi: 10.1111/j.1365-2958.1993.tb00948.x. [DOI] [PubMed] [Google Scholar]
- 287.Stevens P, Czuprynski C. Dissociation of cytolysis and monokine release by bovine mononuclear phagocytes incubated with Pasteurella haemolytica partially purified leukotoxin and lipopolysaccharide. Can J Vet Res. 1995;59:110–117. [PMC free article] [PubMed] [Google Scholar]
- 288.Stevenson F T, Bursten S L, Locksley R M, Lovett D H. Myristyl acylation of the tumor necrosis factor α precursor on specific lysine residues. J Exp Med. 1992;176:1053–1062. doi: 10.1084/jem.176.4.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Stevenson F T, Bursten S L, Fanton C, Locksley R M, Lovett D H. The 31kDa precursor of interleukin-1α is myristoylated on specific lysines within the 16kDa N-terminal propiece. Proc Natl Acad Sci USA. 1993;90:7245–7249. doi: 10.1073/pnas.90.15.7245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Stoffel R H, Randall R R, Premont R T, Lefkowitz R J, Inglese J. Palmitoylation of G-protein coupled receptor kinase, Grk6—lipid modification diversity in the Grk family. J Biol Chem. 1994;269:27791–27794. [PubMed] [Google Scholar]
- 291.Strathdee C A, Lo R Y C. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987;55:3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sutcliffe I C, Russell R R B. Lipoproteins of gram-positive bacteria. J Bacteriol. 1995;177:1123–1128. doi: 10.1128/jb.177.5.1123-1128.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Sutton J M, Peart J, Dean G, Downie J A. Analysis of the C-terminal secretion signal of the Rhizobium leguminosarum nodulation protein NodO: a potential system for the secretion of heterologous proteins during nodule invasion. Mol Plant-Microbe Interact. 1996;9:671–680. doi: 10.1094/mpmi-9-0671. [DOI] [PubMed] [Google Scholar]
- 294.Suttorp N, Floer B, Schnittler H, Seeger W, Bhakdi S. Effects of Escherichia coli hemolysin on endothelial cell function. Infect Immun. 1990;58:3796–3801. doi: 10.1128/iai.58.11.3796-3801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Suttorp N, Fuhrmann M, Tannertotto S, Grimminger F, Bhakdi S. Pore-forming bacterial toxins potently induce release of nitric oxide in porcine endothelial cells. J Exp Med. 1993;178:337–341. doi: 10.1084/jem.178.1.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Swierczynski S L, Blackshear P J. Myristoylation-dependent and electrostatic interactions exert independent effects on the membrane association of the myristoylated alanine rich protein kinase C substrate protein in intact cells. J Biol Chem. 1996;271:23424–23430. doi: 10.1074/jbc.271.38.23424. [DOI] [PubMed] [Google Scholar]
- 297.Szabo G, Gray M C, Hewlett E L. Adenylate cyclase toxin from Bordetella pertussis produces ion conductance across artificial lipid bilayers in a calcium dependent and polarity dependent manner. J Biol Chem. 1994;269:22496–22499. [PubMed] [Google Scholar]
- 298.Taichman N S, Iwase M, Lally E T, Shattil S J, Cunningham M E, Korchak H M. Early changes in cytosolic calcium and membrane potential induced by Actinobacillus actinomycetemcomitans leukotoxin in susceptible and resistant target cells. J Immunol. 1991;147:3587–3594. [PubMed] [Google Scholar]
- 299.Tang L J, Weissborn A C, Kennedy E P. Domains of Escherichia coli acyl carrier protein important for membrane-derived-oligosaccharide biosynthesis. J Bacteriol. 1997;179:3697–3705. doi: 10.1128/jb.179.11.3697-3705.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Thanabalu, T., E. Koronakis, C. Hughes, and V. Koronakis. Dynamic interaction between inner and outer membrane components of Escherichia coli hemolysin secretion machinery. Unpublished data.
- 301.Thompson S A, Sparling P F. The RTX cytotoxin related FrpA protein of Neisseria meningitidis is secreted extracellularly by meningococci and by HlyBD+Escherichia coli. Infect Immun. 1993;61:2906–2911. doi: 10.1128/iai.61.7.2906-2911.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Towler D A, Gordon J I, Adams S P, Glaser L. The biology and enzymology of eukaryotic protein acylation. Annu Rev Biochem. 1988;57:69–99. doi: 10.1146/annurev.bi.57.070188.000441. [DOI] [PubMed] [Google Scholar]
- 303.Towler D A, Adams S P, Eubanks S R, Towery D S, Jackson-Machelski E, Glaser L, Gordon J I. Myristoyl CoA-protein N-myristoyltransferase activities from rat liver and yeast possess overlapping yet distinct peptide substrate specificities. J Biol Chem. 1988;263:1784–1790. [PubMed] [Google Scholar]
- 304.Tsutsui O, Kokeguchi S, Matsumura T, Kato K. Relationship of the chemical structure and immunobiological activities of lipoteichoic acid from Streptococcus faecilis (Enterococcus hirae) ATCC 9790. FEMS Microbiol Immunol. 1991;76:211–218. doi: 10.1111/j.1574-6968.1991.tb04217.x. [DOI] [PubMed] [Google Scholar]
- 305.Tu A H T, Hausler C, Young R, Struck D K. Differential expression of the cytotoxic and hemolytic activities of the ApxIIA toxin from Actinobacillus pleuropneumoniae. Infect Immun. 1994;62:2119–2121. doi: 10.1128/iai.62.5.2119-2121.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Udeze F A, Kadis S. Effects of Actinobacillus pleuropneumoniae hemolysin on porcine neutrophil function. Infect Immun. 1992;60:1558–1567. doi: 10.1128/iai.60.4.1558-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.vandenAkker F, Sarfaty S, Twiddy E M, Connell T D, Holmes R K, Hol W G. Crystal structure of a new heat labile enterotoxin, LT-IIB. Structure. 1996;4:665–678. doi: 10.1016/s0969-2126(96)00073-1. [DOI] [PubMed] [Google Scholar]
- 308.van’tHof W, Resh M D. Rapid plasma membrane anchoring of newly synthesized p59fyn: selective requirement for NH2-terminal myristoylation and palmitoylation at cysteine-3. J Cell Biol. 1997;136:1023–1035. doi: 10.1083/jcb.136.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Vassilev A O, Plesofsky-Vig N, Brambl R. Cytochrome c oxidase in Neurospora crassa contains myristic acid covalently linked to subunit 1. Proc Natl Acad Sci USA. 1995;92:8680–8684. doi: 10.1073/pnas.92.19.8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Vater J, Stein T, Vollenbroich D, Kruft V, Wittmann-Liebold B, Franke P, Liu L, Zuber P. The modular organization of multifunctional peptide synthetases. J Protein Chem. 1997;16:557–564. doi: 10.1023/a:1026386100259. [DOI] [PubMed] [Google Scholar]
- 311.Vogel M, Hess J, Then I, Juarez A, Goebel W. Characterization of a sequence (hlyR) which enhances synthesis and secretion of hemolysin in Escherichia coli. Mol Gen Genet. 1988;212:76–84. doi: 10.1007/BF00322447. [DOI] [PubMed] [Google Scholar]
- 312.Walev I, Vollmer P, Palmer M, Bhakdi S, Rosejohn S. Pore-forming toxins trigger shedding of receptors for interleukin-6 and lipopolysaccharide. Proc Natl Acad Sci USA. 1996;93:7882–7887. doi: 10.1073/pnas.93.15.7882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 313.Wandersman C, Delepelaire P. TolC, an Escherichia coli outer membrane protein required for hemolysin secretion. Proc Natl Acad Sci USA. 1990;87:4776–4780. doi: 10.1073/pnas.87.12.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314.Wandersman C, Letoffe S. Involvement of lipopolysaccharide in the secretion of Escherichia coli α-hemolysin and Erwinia chrysanthemi proteases. Mol Microbiol. 1993;7:141–150. doi: 10.1111/j.1365-2958.1993.tb01105.x. [DOI] [PubMed] [Google Scholar]
- 315.Wang R C, Seror S J, Blight M, Pratt J M, Broomesmith J K, Holland I B. Analysis of the membrane organization of an Escherichia coli protein translocator, HlyB, a member of a large family of prokaryote and eukaryote surface transport proteins. J Mol Biol. 1991;217:441–454. doi: 10.1016/0022-2836(91)90748-u. [DOI] [PubMed] [Google Scholar]
- 316.Wang Z Z, Hardy S F, Hall Z W. Membrane tethering enables an extracellular domain of the acetylcholine receptor A subunit to form a heterodimeric ligand binding site. J Cell Biol. 1996;135:809–817. doi: 10.1083/jcb.135.3.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wedegaertner P B, Wilson P T, Bourne H R. Lipid modifications of trimeric G-proteins. J Biol Chem. 1995;270:503–506. doi: 10.1074/jbc.270.2.503. [DOI] [PubMed] [Google Scholar]
- 318.Welch R A, Dellinger E P, Minshew B, Falkow S. Hemolysin contributes to virulence of extraintestinal Escherichia coli infections. Nature. 1981;294:665–667. doi: 10.1038/294665a0. [DOI] [PubMed] [Google Scholar]
- 319.Welch R A. Identification of two different hemolysin determinants in uropathogenic Proteus isolates. Infect Immun. 1987;55:2183–2190. doi: 10.1128/iai.55.9.2183-2190.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Welch R A. Pore-forming cytolysins of gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 321.Wess J. G-protein-coupled receptors: molecular mechanisms involved in receptor activation and selectivity of G-protein recognition. FASEB J. 1997;11:346–354. [PubMed] [Google Scholar]
- 322.Westrop G, Hormozi K, da Costa N, Parton R, Coote J. Structure-function studies of the adenylate cyclase toxin of Bordetella pertussis and the leukotoxin of Pasteurella haemolytica by heterologous C protein activation and construction of hybrid proteins. J Bacteriol. 1997;179:871–879. doi: 10.1128/jb.179.3.871-879.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Willumsen B M, Cox A D, Solski P A, Der C J, Buss J E. Novel determinants of H-ras plasma membrane localization and transformation. Oncogene. 1996;13:1901–1909. [PubMed] [Google Scholar]
- 324.Wu S K, Zeng K, Wilson I A, Balch W E. Structural insights into the function of the Rab-GDI superfamily. Trends Biochem Sci. 1996;21:472–476. doi: 10.1016/s0968-0004(96)10062-1. [DOI] [PubMed] [Google Scholar]
- 325.Yasuda H, Lindorfer M A, Woodfork K A, Fletcher J E, Garrison J C. Role of the prenyl group on the G-protein γ-subunit in coupling trimeric G-proteins to A1 adenosine receptors. J Biol Chem. 1996;271:18588–18595. doi: 10.1074/jbc.271.31.18588. [DOI] [PubMed] [Google Scholar]
- 326.Yoo H S, Rajagopal B S, Maheswaran S K, Ames T R. Purified Pasteurella haemolytica leukotoxin induces expression of inflammatory cytokines from bovine alveolar macrophages. Microb Pathog. 1995;18:237–252. doi: 10.1016/s0882-4010(05)80001-4. [DOI] [PubMed] [Google Scholar]
- 327.Zalman L S, Muller-Eberhard H J. Comparison of channels formed by poly C9, C5b-8 and the membrane attack complex of complement. Mol Immunol. 1990;27:533–537. doi: 10.1016/0161-5890(90)90072-8. [DOI] [PubMed] [Google Scholar]
- 328.Zambon J J, Deluca C, Slots J, Genco R J. Studies of leukotoxin from Actinobacillus actinomycetemcomitans by using the promyelocytic HL-60 cell line. Infect Immun. 1983;40:205–212. doi: 10.1128/iai.40.1.205-212.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Zhang F, Greig D I, Ling V. Functional replacement of the hemolysin A transport signal by a different primary sequence. Proc Natl Acad Sci USA. 1993;90:4211–4215. doi: 10.1073/pnas.90.9.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Zhang F, Yin Y, Arrowsmith C H, Ling V. Secretion and circular dichroism analysis of the C-terminal signal peptides of HlyA and LktA. Biochemistry. 1995;34:4193–4201. doi: 10.1021/bi00013a007. [DOI] [PubMed] [Google Scholar]
- 331.Zhang F L, Casey P J. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269. doi: 10.1146/annurev.bi.65.070196.001325. [DOI] [PubMed] [Google Scholar]
- 332.Zhang L T, Jackson Machelski E, Gordon J I. Biochemical studies of Saccharomyces cerevisiae myristoyl-coenzyme A: protein N-myristoyltransferase mutants. J Biol Chem. 1996;271:33131–33140. doi: 10.1074/jbc.271.51.33131. [DOI] [PubMed] [Google Scholar]
- 333.Zhou W J, Parent L J, Wills J W, Resh M D. Identification of a membrane binding domain within the amino-terminal region of human immunodeficiency virus type-1 Gag protein which interacts with acidic phospholipids. J Virol. 1994;68:2556–2569. doi: 10.1128/jvi.68.4.2556-2569.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Zlatkine P, Mehul B, Magee A I. Retargeting of cytosolic proteins to the plasma membrane by the Lck protein tyrosine kinase dual acylation motif. J Cell Sci. 1997;110:673–679. doi: 10.1242/jcs.110.5.673. [DOI] [PubMed] [Google Scholar]