Abstract
The state of chromatin (the packaging of DNA in eukaryotes) has long been recognized to have major effects on levels of gene expression, and numerous chromatin-altering strategies—including ATP-dependent remodeling and histone modification—are employed in the cell to bring about transcriptional regulation. Of these, histone acetylation is one of the best characterized, as recent years have seen the identification and further study of many histone acetyltransferase (HAT) proteins and their associated complexes. Interestingly, most of these proteins were previously shown to have coactivator or other transcription-related functions. Confirmed and putative HAT proteins have been identified from various organisms from yeast to humans, and they include Gcn5-related N-acetyltransferase (GNAT) superfamily members Gcn5, PCAF, Elp3, Hpa2, and Hat1: MYST proteins Sas2, Sas3, Esa1, MOF, Tip60, MOZ, MORF, and HBO1; global coactivators p300 and CREB-binding protein; nuclear receptor coactivators SRC-1, ACTR, and TIF2; TATA-binding protein-associated factor TAFII250 and its homologs; and subunits of RNA polymerase III general factor TFIIIC. The acetylation and transcriptional functions of these HATs and the native complexes containing them (such as yeast SAGA, NuA4, and possibly analogous human complexes) are discussed. In addition, some of these HATs are also known to modify certain nonhistone transcription-related proteins, including high-mobility-group chromatin proteins, activators such as p53, coactivators, and general factors. Thus, we also detail these known factor acetyltransferase (FAT) substrates and the demonstrated or potential roles of their acetylation in transcriptional processes.
Eukaryotic transcription is a highly regulated process, and acetylation is now known to play a major role in this regulation. Specifically, acetyltransferase enzymes that act on particular lysine side chains of histones and other proteins are intimately involved in transcriptional activation. By modifying chromatin proteins and transcription-related factors, these acetylases are believed to regulate the transcription of many genes.
Chromatin structure, the way in which DNA is packaged in the eukaryotic cell, is known to have a major impact on levels of transcription. In eukaryotes, DNA typically exists in vivo as a repeating array of nucleosomes (271), in which 146 bp of DNA are wound around a histone octamer (consisting of two each of histone proteins H2A, H2B, H3, and H4). Nucleosomes are the first level of chromatin organization, although they in turn are organized into higher-order structures of increasing complexity (129), an extreme example being the condensed metaphase chromosome during cell division. A number of studies have demonstrated that nucleosomal DNA is generally repressive to transcription (91, 183); thus, nucleosome structure and DNA-histone interactions typically make the DNA of genes and their regulatory regions unavailable for the binding of the transcriptional machinery and other factors involved in activation. The direct connection between chromatin alteration and transcriptional activation has been increasingly demonstrated in recent years.
Certain enzymes and protein complexes are now known to bring about changes in the state of chromatin by numerous mechanisms, with resultant effects on gene expression. One class of complexes alter the DNA packaging (remodel chromatin) in an ATP-dependent manner; these include the Swi-Snf complex and a number of others from various organisms (114, 126). Another class of chromatin-altering factors act by covalently modifying histone proteins. These modifications can include phosphorylation, ubiquitination, ADP-ribosylation, and methylation (25), but the best-characterized mechanism is acetylation, catalyzed by histone acetyltransferase (HAT) enzymes.
HATs function enzymatically by transferring an acetyl group from acetyl-coenzyme A (acetyl-CoA) to the ɛ-amino group of certain lysine side chains within a histone's basic N-terminal tail region (149). Within a histone octamer, these regions extend out from the associated globular domains, and in the context of a nucleosome, they are believed to bind the DNA through charge interactions (positively charged histone tails associated with negatively charged DNA) or mediate interactions between nucleosomes (67, 151). Lysine acetylation, which neutralizes part of a tail region's positive charge, is postulated to weaken histone-DNA (107, 221) or nucleosome-nucleosome interactions (68, 152) and/or signal a conformational change (175), thereby destabilizing nucleosome structure or arrangement and giving other nuclear factors, such as the transcription complex, more access to a genetic locus. In agreement with this is the fact that acetylated chromatin has long been associated with states of transcriptional activation (99, 244). Recently, some of the proteins and complexes that carry out these acetylation functions have been characterized, and they will be discussed in this review. Interestingly, certain HATs have also recently been shown to specifically acetylate lysine residues within transcription-related proteins other than histones; these events and their regulatory potential will be discussed as well.
Finally, histone acetylation is a reversible process, and deacetylases are also integral to cycles of transcription. Acetylation is generally associated with activation, whereas lack of acetylation tends to correlate with repression—two regulatory processes working in harmony to achieve appropriate levels of transcription (135). While outside the scope of this review, it should be noted that a number of deacetylase proteins and complexes have been characterized in the last several years. This has provided a further conceptual linkage between acetylation and transcriptional activity, since some of the histone deacetylases (HDACs) and the proteins with which they associate are previously known DNA-binding repressors or corepressors (reviewed in reference 186).
HISTONE ACETYLTRANSFERASES (HATS)
The phenomenon of histone acetylation in the eukaryotic cell has been known for many years, and since the early 1970s various HAT activities have been isolated and partially characterized. Each of these enzymes generally belongs to one of two categories (30, 74): type A, located in the nucleus, or type B, located in the cytoplasm, although recent evidence indicates that some HAT proteins may function in multiple complexes or locations and thus not precisely fit these historical classifications (200). B-type HATs are believed to have somewhat of a housekeeping role in the cell, acetylating newly synthesized free histones in the cytoplasm for transport into the nucleus, where they may be deacetylated and incorporated into chromatin (4, 199). The A-type HATs, on the other hand, acetylate nucleosomal histones within chromatin in the nucleus; these HATs are potentially linked to transcription and thus are the main focus of this review. A summary of known HAT proteins is presented in Table 1, and these are discussed further in the text.
TABLE 1.
Summary of known and putative HATs
| HAT | Organisms known to contain the HAT | Known transcription-related functions/effects | HAT activity demonstrated in vitrob | Histone specificity of recombinant enzyme in vitroab | Known native HAT complexes and nucleosomal histone specificities in vitro |
|---|---|---|---|---|---|
| GNAT superfamily | |||||
| Hat1 | Various (yeast to humans) | None (histone deposition-related B-type HAT) | Yes | H4 | Yeast HAT-B, HAT-A3 (no nucleosome acetylation) |
| Gcn5 | Various (yeast to humans) | Coactivator (adaptor) | Yes | H3/H4 | Yeast ADA, SAGA (H3/H2B); human GCN5 complex, STAGA, TFTC (H3) |
| PCAF | Humans, mice | Coactivator | Yes | H3/H4 | Human PCAF complex (H3/weak H4) |
| Elp3 | Yeast | Transcript elongation | Yes | ND* | Elongator, polymerase II holoenzyme (H3/weak H4) |
| Hpa2 | Yeast | Unknown | Yes | H3/H4 | |
| MYST family | |||||
| Sas2 | Yeast | Silencing | ND | ||
| Sas3 | Yeast | Silencing | Yes | H3/H4/H2A | NuA3c (H3) |
| Esa1 | Yeast | Cell cycle progression | Yes | H4/H3/H2A | NuA4 (H4/H2A) |
| MOF | Drosophila | Dosage compensation | Yes | H4/H3/H2A | MSL complex (H4) |
| Tip60 | Humans | HIV Tat interaction | Yes | H4/H3/H2A | Tip60 complex |
| MOZ | Humans | Leukemogenesis, upon chromosomal translocation | ND | ||
| MORF | Humans | Unknown (strong homology to MOZ) | Yes | H4/H3/H2A | |
| HBO1 | Humans | ORC interaction | Yes* | ND* | HBO1 complex |
| p300/CBP | Various multicellular | Global coactivator | Yes | H2A/H2B/H3/H4 | |
| Nuclear receptor coactivators | Nuclear receptor coactivators (transcriptional response to hormone signals) | ||||
| SRC-1 | Humans, mice | Yes | H3/H4 | ||
| ACTR | Humans, mice | Yes | H3/H4 | ||
| TIF2 | Humans, mice | ND | |||
| TAFII250 | Various (yeast to humans) | TBP-associated factor | Yes | H3/H4 | TFIID |
| TFIIIC | RNA polymerase III transcription initiation | TFIIIC (H2A/H3/H4) | |||
| TFIIIC220 | Humans | Yes* | ND | ||
| TFIIIC110 | Humans | Yes | ND | ||
| TFIIIC90 | Humans | Yes | H3 |
Histones that are the primary in vitro substrates for a given HAT are bold; other histones listed are acetylated weakly or in a secondary manner.
Asterisks indicate proteins for which HAT activity has been suggested indirectly or demonstrated in an incomplete manner. Elp3 can acetylate all four histones but has only been tested with them individually in in-gel assays. The HAT function of HBO1 has primarily been shown by the in vitro free histone H3/H4-acetylating activity of a purified human complex containing it, although recombinant GST-HBO1 (and the complex) did weakly acetylate nucleosomes. Finally, TFIIIC220 was identified as a HAT only in in-gel assays, and its activity has not been confirmed by recombinant protein studies as of this writing. ND, not determined.
S. John and J. L. Workman, unpublished result.
GNAT Superfamily
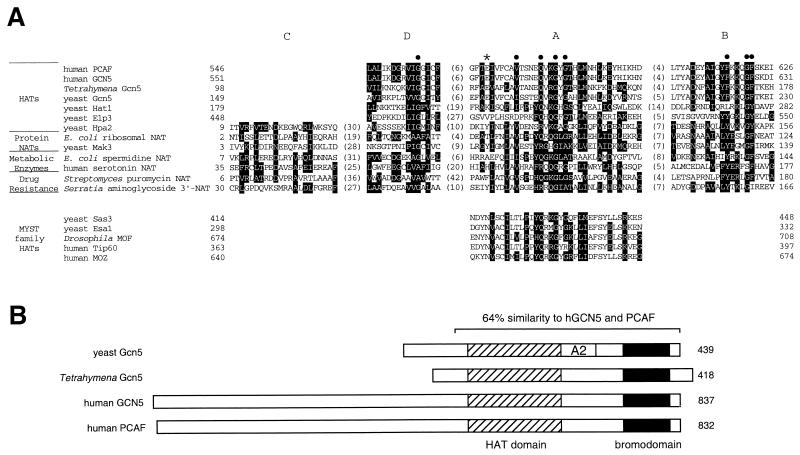
The best-understood set of acetyltransferases is the GNAT (Gcn5-related N-acetyltransferase) superfamily (174), which have been grouped together on the basis of their similarity in several homology regions and acetylation-related motifs (Fig. 1A). This group includes the HAT Gcn5, its close relatives, and at least three more distantly related HATs, Hat1, Elp3, and Hpa2. It also contains a variety of other eukaryotic and prokaryotic acetyltransferases with different substrates, indicating the conservation and wide application of this type of acetylation mechanism throughout evolution. Four sequence motifs whose functions are not yet fully understood—C, D, A, and B, in N-terminal to C-terminal order—define this superfamily. The C motif is found in most of the GNAT family acetyltransferases but not in the majority of known HATs. Motif A is the most highly conserved region, and it is shared with another HAT family, the MYST proteins, described later in this review. Furthermore, it contains an Arg/Gln-X-X-Gly-X-Gly/Ala segment that has been specifically implicated in acetyl-CoA substrate recognition and binding (59, 270).
FIG. 1.
Similarities of GNAT (Gcn5-related N-acetyltransferase) superfamily members. (A) Alignment of GNAT homology motifs A, B, C, and D for HATs and representatives of other types of acetyltransferases. Reversed type indicates consensus sequence residues, as determined by Neuwald and Landsman (174), and solid circles mark residues that are particularly conserved throughout the superfamily. The asterisk indicates the glutamate residue known to be critical for HAT catalysis in yeast Gcn5. At the bottom are several members of another acetyltransferase family, the MYST proteins, that share just the A motif. (B) Alignment of proteins from the Gcn5 subgroup of the GNAT superfamily, showing the location of the HAT domain and bromodomain. Shown at the top is the overall homology region shared by all four proteins, with the similarity between the yeast and human proteins indicated; Tetrahymena Gcn5 has 62% similarity with the three others over this same region. The A2 label designates a region in yeast Gcn5 known to interact with the adaptor protein Ada2 (37). In addition, the N-terminal region of PCAF has been generally defined as its site of interaction with p300/CBP and nuclear receptor coactivator SRC-1 (130, 276); this region and the HAT domain also interact with viral protein E1A (40, 193).
Gcn5.
The first protein identified as an A-type, transcription-related HAT was discovered in the ciliate Tetrahymena thermophila (31). By way of an in-gel assay of nuclear extract chromatographic fractions run on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), a 55-kDa polypeptide (p55) was found to have acetylation activity on free histones (29). Subsequent protein sequencing revealed that it was a homolog of Saccharomyces cerevisiae (yeast) Gcn5 (77), previously identified as a transcriptional adaptor (or coactivator) involved in the interaction between certain activators and the transcription complex (17, 154, 213). Homologs of Gcn5 have more recently been cloned and sequenced from numerous divergent organisms—such as human (36), mouse (276), Schizosaccharomyces pombe, Drosophila melanogaster (215), Arabidopsis thalania, and Toxoplasma gondii (102)—suggesting that its function is highly conserved throughout the eukaryotes.
To date, yeast Gcn5 (general control nonderepressible-5; also referred to as yGcn5) is the best characterized of the HATs, both structurally and functionally and both in vivo and in vitro. Various studies have mapped and characterized the functional domains of yeast Gcn5, shown in Fig. 1B (35, 37). These include a C-terminal bromodomain, an Ada2 interaction domain, and the HAT domain, which by use of truncation mutants was found to be required for adaptor-mediated transcriptional activation in vivo (37). The Gcn5 HAT domain was also functionally analyzed by alanine scan mutagenesis. These analyses identified conserved residues critical to HAT activity and demonstrated the direct correlation of Gcn5 HAT function with cell growth, in vivo transcription, and histone acetylation at the Gcn5-dependent HIS3 promoter in vivo (134, 256). A further study with some of these mutants showed that Gcn5's HAT activity has an effect on chromatin remodeling at the PHO5 promoter in vivo (89).
The substrate specificity of Gcn5 has also been investigated. In vitro, recombinant Gcn5 was found to acetylate histone H3 strongly and H4 weakly in a free histone mixture (although histone H4 was acetylated well individually). Protein sequence analysis of these reaction products revealed that the primary sites of acetylation were lysine 14 on histone H3, as shown in Fig. 2, and lysines 8 and 16 on histone H4 (136). Although recombinant Gcn5 can acetylate free histones efficiently, it is unable to acetylate nucleosomal histones (84, 136, 201), the more physiological substrate, except under special conditions and at high enzyme concentrations (243). Only in the context of multisubunit native complexes such as SAGA and ADA (described later in this review) is Gcn5 able to acetylate nucleosomes effectively, indicating that the influence of other proteins is required to confer this activity.
FIG. 2.
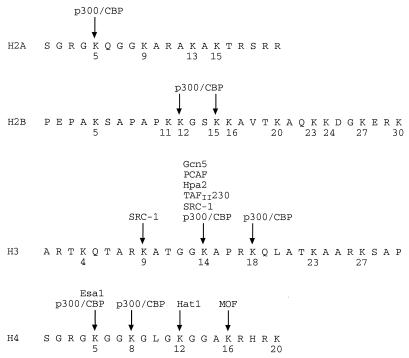
Primary histone acetylation specifities of some of the known HAT proteins in vitro. Shown are the amino acid sequences for the N-terminal tail regions of human histones H2A, H2B, H3, and H4, with lysine residues numbered and arrows indicating the predominant sites used by various HATs in in vitro experiments. TAFII230 is the Drosophila homolog of human TAFII250 used in site specificity determinations. The above H3 and H4 sequences are nearly identical to those of S. cerevisiae and Drosophila. Specific but relatively nonpreferred or minor sites for certain HATs are not indicated, for example, H4 lysine-8 for Gcn5 and PCAF. It should be noted that lysine specificities may be somewhat expanded or restricted with native HAT complexes and/or on nucleosomal substrates.
In mammals (humans and mice), the Gcn5 subclass of acetyltransferases is represented by two closely related proteins, GCN5 and p300/CREB-binding protein-associated factor (PCAF). These proteins share a remarkable degree of homology (about 70% identity and 80% similarity) throughout their sequences, and a distinguishing feature is an approximately 400-residue amino-terminal region not present in yeast Gcn5 (Fig. 1B) (276); such an extension is seen, however, in Drosophila GCN5 (215). The function of human GCN5 (also known as hGCN5) has also been investigated in vitro and in vivo, and it was found to carry out transcriptional adaptor roles analogous to those of yeast Gcn5 (36). Further studies showed that human GCN5 had HAT activity in vitro (279) and that its HAT domain could successfully substitute for that of yeast Gcn5 in vivo, indicating the evolutionary conservation of this HAT function (257).
The HAT domain of human GCN5 is of course indispensable to its acetylation function, but interestingly, two other domains appear to have an influence on its HAT activity and substrate use. Because of the apparent existence of multiple alternatively spliced versions of human GCN5, the original cDNA clones lacked its N-terminal region. While recombinant short-form human GCN5 could acetylate histone H3 (and to a lesser extent H4) only as free histones (257, 279), the full-length forms of human and mouse GCN5 were recently shown to be competent for the acetylation of nucleosomal histones, implicating the N-terminal region in chromatin substrate recognition (276). The C-terminal bromodomain is another region that apparently has an effect on human GCN5 HAT function, interacting with the DNA-dependent protein kinase holoenzyme, which inhibits GCN5's HAT activity by way of phosphorylation (11). Additional functional aspects of the bromodomain are discussed below.
PCAF.
The gene for PCAF (also referred to as P/CAF) was originally identified from a human cDNA database on the basis of its homology to Gcn5. Because of functional similarities between the yeast activator Gcn4 (which interacts with the adaptor complex) and the activator c-Jun in higher eukaryotes (which interacts with coactivators p300 and CREB-binding protein [CBP]), it was postulated that a human counterpart of Gcn5 may participate in p300/CBP-mediated activation. When PCAF was cloned and investigated, in vitro and in vivo studies revealed that it interacts with p300 and CBP (279), hence its name. p300 and CBP are very closely related coactivators that mediate the transcription of many genes and are also HATs, as described below. PCAF HAT activity, like full-length GCN5, in recombinant form acetylates either free histones or nucleosomes (279), primarily on lysine-14 of histone H3, and more weakly on lysine-8 of histone H4 (207).
Relevant to PCAF function is the fact that it binds to the same site on p300/CBP as does adenoviral oncoprotein E1A, and competition between these two proteins was observed (279). Interestingly, transfected PCAF and E1A had opposite effects on cell cycle regulation, suggesting that PCAF has a role in inhibiting cell cycle progression and that E1A's mitogenic activity may occur by disrupting the interaction between PCAF and p300/CBP (279). In addition, E1A and the regulatory protein Twist reduce PCAF-mediated in vivo transcription by binding to PCAF, further identifying this acetyltransferase as a target for regulation. Twist may function by inhibiting PCAF's HAT activity (96); a similar HAT-inhibitory effect was observed for E1A in two studies (40, 96) but not another (193), so it will be important to clarify the generality of HAT inhibition.
The role of PCAF in transcription has been investigated by multiple studies, and its requirement as a HAT and coactivator has been described for myogenesis (192) and nuclear receptor-mediated (21, 130) and growth factor-signaled (275) activation, among other processes. Furthermore, a reporter gene study demonstrated that PCAF could carry out its coactivator function in a HAT-dependent manner and stimulate transcription when bound either to a promoter-proximal site or at a distant enhancer (132). Although PCAF was originally characterized as a HAT, much recent work has focused on its acetylation of various nonhistone transcription-related proteins. These include the chromatin proteins HMG17 and HMG I(Y), activators p53, MyoD, and human immunodeficiency virus (HIV) Tat, and general transcription factors TFIIE and TFIIF. These activities and their potential regulatory significance are described later in this review. At present, it appears likely that both types of activities, HAT and factor acetyltransferase (FAT), are physiologically important for PCAF function.
Finally, there are several noteworthy similarities and differences between PCAF and GCN5. One similarity is that in human cells, each participates in separate SAGA-related multisubunit complexes (described below) whose subunits are otherwise largely identical (177). Also, like PCAF, human and mouse GCN5 bind p300/CBP, suggesting functional similarity, although the precise sites bound may be different for each binding pair (276). A further difference between PCAF and GCN5 is that while both are ubiquitously expressed in the mouse, their comparative levels were very different in many tissues (276). Future studies will be required to determine if PCAF and GCN5 are functionally redundant or distinct.
Hat1, Elp3, Hpa2, and other acetyltransferases.
Gcn5, its homologs, and PCAF have high sequence similarity, but as members of the GNAT superfamily, they are also related by sequence motifs to other HATs and numerous nonhistone acetyltransferases, even prokaryotic ones (174). As shown by the abbreviated list in Fig. 1A, these include the yeast HATs Hat1, Elp3, and Hpa2, protein N-acetyltransferases (which modify N-termini), metabolic enzymes, acetylases involved in drug resistance and detoxification, and a variety of other proteins with unknown specific functions. In addition, GNAT homology is seen in several known transcriptional regulators for which acetylase activity has not yet been described—the yeast Spt10 protein (173), for example, which affects the expression of various genes (172, 278), including certain histone genes (55).
The first HAT protein to be identified was actually yeast Hat1 (127, 185), originally described as a B-type HAT involved in the cytoplasmic acetylation of histones destined for deposition on DNA in the nucleus. Hat1 is responsible for the predominant cytoplasmic HAT activity in S. cerevisiae, although a null mutation of its gene confers no phenotype, suggesting that its function may be redundant with other HATs. Within purified enzyme, Hat1 is associated with a second subunit, Hat2, which is required for strong binding to histone H4 and contributes to substrate specificity (185). Hat2 is a member of a protein family defined by RbAp48, a human retinoblastoma (Rb)-interacting protein that acts as an apparent histone H4 chaperone (198) and is also a subunit of human chromatin assembly factor CAF-1 (249) and histone deacetylase HDAC1 (235). In vitro, Hat1 enzyme can acetylate lysine-12 of the histone H4 N-terminal tail region (127, 185), previously identified as one of the major residues acetylated in newly synthesized histones (45, 218). Although Hat1 is thought to be deposition related, recent evidence suggests that it is not entirely cytoplasmic. Hat1 and Hat2 were found to be part of a nuclear HAT activity on free (but not nucleosomal) histones, indicating its potential involvement in chromatin assembly in a more direct manner, perhaps at replication forks or silenced telomeres (200). Furthermore, a recently characterized HAT complex from human S-phase nuclei contained homologs of Hat1 and Hat2 and had in vitro specificity similar to that of the yeast enzyme, suggesting conservation of its function throughout eukaryotes (248).
Elp3, a yeast A-type HAT, appears to have a direct role in transcription in that it is part of the RNA polymerase II holoenzyme and is involved in transcriptional elongation. In S. cerevisiae, the three-subunit elongator complex binds tightly to RNA polymerase II and its hyperphosphorylated C-terminal repeat domain (CTD), participating in an elongation-competent form of holoenzyme (182). Elp3, the smallest elongator subunit, was identified by peptide mass spectrometry and found to have GNAT homology (269). Genetic studies showed that an elp3 null mutant was viable but displayed defective phenotypes similar to those of a previous elongator null mutant, elp1 (182): slow activation of certain genes, slow growth adaptation, and salt and temperature sensitivity. Because of its GNAT homology, recombinant Elp3 was produced from insect cells and tested for HAT activity in in-gel assays. Under these conditions, Elp3 was able to acetylate all four core histones when presented with them individually (269). Although the specific function of this HAT activity and its in vivo role remain to be characterized, a clear model emerges, built on insight gained from studies on Gcn5. Since Gcn5's HAT activity is known to cause remodeling of promoter DNA (89) and is thought to assist transcriptional initiation, Elp3 may by analogy facilitate transcript elongation by modifying chromatin within a gene, thereby clearing the way for holoenzyme. Like Gcn5, Elp3 function may be redundant with other mechanisms, since its gene is not essential, but its possible importance is demonstrated by its evolutionary conservation in homologs from various other eukaryotes, including mammals (269).
Hpa2 is the most recently described HAT protein as of this writing, and only limited information has been published about it at this point. As a GNAT superfamily member, this yeast protein was tested in vitro and found to acetylate histones H3 and H4, with a preference for lysine-14 of H3, like the Gcn5 subgroup (5). Interestingly, Hpa2 has a high degree of homology with another yeast GNAT protein, Hpa3, which displayed very poor HAT activity in in vitro assays but did autoacetylate (as did Hpa2). Hpa2 can form a dimer or a tetramer in vitro, and the crystal structure of the tetramer has been determined (5). In vivo, however, Hpa2 has unknown function, as a knockout of the gene conferred no apparent growth phenotype. Further genetic and biochemical studies will be required to determine the roles of this protein and the potential HAT Hpa3 in the cell.
Structure and mechanism.
Along with mutant studies of yeast GCN5, structure determination of several Gcn5-related proteins has added to our knowledge of the mechanisms of acetylation by these enzymes. The first two GNAT superfamily members to have the crystal structures of their acetyltransferase domains solved were yeast Hat1 (59) and Serratia marcescens aminoglycoside N-acetyltransferase (270), a bacterial enzyme that inactivates certain antibiotics by acetylation. In each case, a truncated, catalytically active fragment of the protein bound to CoA or acetyl-CoA substrate was crystallized. Subsequently, HAT domain structures from the Gcn5 subgroup—Tetrahymena (146, 197) and yeast (241) Gcn5 and human PCAF (48)—and HAT protein Hpa2 (5) were also determined.
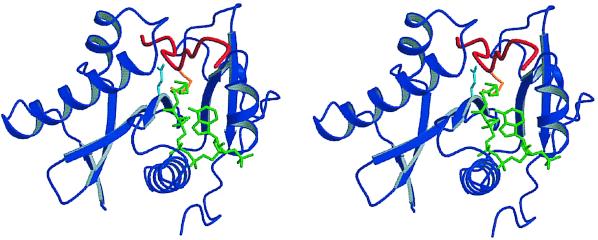
The central regions of these six proteins all have very similar topologies, and together they define a fundamental structure for GNAT acetyltransferases. As shown by the example of the Tetrahymena Gcn5 HAT domain in Fig. 3, the proteins consist of N-terminal and C-terminal domains separated by a deep hydrophobic cleft. A conserved core, formed by a three-stranded β-sheet and an amphipathic α-helix and encompassing GNAT motifs A and D, lies at the bottom of the cleft. The acetyl-CoA substrate binds in part of the cleft and is held between motif A and motif B, which located in the C-terminal domain. One Tetrahymena Gcn5 study in particular (197) provided additional information about HAT function by presenting the structure of a ternary complex containing a histone H3 N-terminal tail peptide as well as the HAT domain and CoA. The histone peptide was shown to occupy the larger part of the cleft, bringing the side chain of acetylatable lysine-14 in proximity to CoA (Fig. 3).
FIG. 3.
Stereo diagram of the structure of a HAT domain bound to its substrates. Shown is the GNAT superfamily protein Tetrahymena Gcn5 (blue) with a histone H3 N-terminal tail peptide (red) and CoA (green) bound to its upper and lower clefts, respectively. At the active site, the glutamate-122 residue (aqua), analogous to yeast Gcn5 glutamate-173, catalyzes the transfer of an acetyl group from acetyl-CoA to the lysine-14 sidechain (orange) of H3 peptide. The N termini of both the Gcn5 protein and the H3 peptide are to the left in diagram, and C-termini are to the right.
The catalytic site and mechanism of histone acetylation by Gcn5 have also been defined as a result of the structure determinations and mutational analyses. Acidic residues within the cleft region of yeast Gcn5 were likely candidates to function as a general base for catalysis; of these, only glutamate-173 was conserved among the Gcn5/PCAF homologs and potentially critical for function, since simultaneous alanine substitution of glutamate-173 and phenylalanine-171 led to major defects (256). The position of Tetrahymena Gcn5's glutamate-122 (analogous to yeast glutamate-173) relative to the substrates is shown in Fig. 3. Further yeast studies used a mutant in which glutamate was replaced with glutamine (which has a similar side chain structure but no acidic group) and found that this mutant was highly defective for HAT activity in vitro (234) and for growth and transcription in vivo (241). It was therefore concluded that the carboxyl moiety of glutamate-173, by deprotonating the lysine substrate, is crucial for the HAT catalytic mechanism and overall function of Gcn5. Altogether, the structural, mutational, and GNAT conservation data were in close agreement for the Gcn5 proteins, also allowing detailed mapping of the substrate-binding determinants (residues critical for acetyl-CoA and histone interaction) (197). With regard to catalysis, however, it should be noted that the critical glutamate residue is only conserved among the direct Gcn5 homologs and PCAF but not the other GNAT HATs or other acetyltransferases. Therefore, in non-Gcn5 acetyltransferases, catalysis may occur through other side chains or by direct nucleophilic attack between the substrates (59).
While the studies described above have focused on GNAT catalytic domains, the bromodomain is another Gcn5 region for which there are structural and functional data suggesting an involvement in HAT function. The bromodomain (whose name is derived from Brahma, the Drosophila protein in which it was first described) (233) is a conserved sequence motif found in PCAF and the Gcn5 homologs as well as a variety of other transcription-related proteins (98). Its precise function is largely unclear, but it has been theorized to be involved in protein-protein interactions (117, 268). In vitro, the bromodomain is not required for recombinant yeast Gcn5 to acetylate free histones (37). However, bromodomain deletion of Gcn5 did cause partial defects in growth and in vivo transcription of certain genes (76, 154) and also resulted in reduced in vitro nucleosome acetylation in the context of a native complex, SAGA (described below) (223), suggesting that the bromodomain does have a HAT-related functional effect. Recent evidence indicates that this effect may involve histone interaction. In vitro binding studies demonstrated that the yeast Gcn5 bromodomain interacts directly with histone H3 and H4 N-terminal tails (181), and a structure determination of the PCAF bromodomain showed that it forms a four-helix bundle with a hydrophobic pocket that binds acetyl-lysine on histone H3 or H4 peptides (53). Together, these results suggest a potential role of HAT bromodomains in contributing to substrate interaction, in addition to possible tethering to chromosomal sites (30) or other protein interactions of a regulatory nature (11, 268).
MYST Family
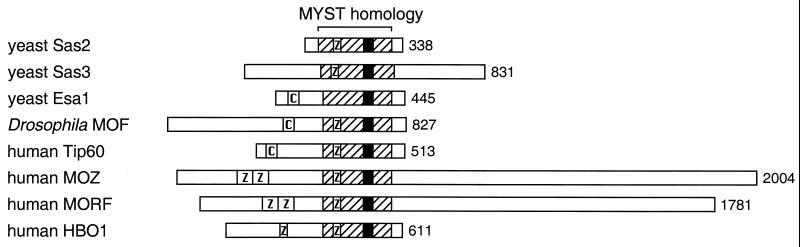
Another group of evolutionarily related proteins that are known or hypothesized to be HATs is the MYST family, named for its founding members: MOZ, Ybf2/Sas3, Sas2, and Tip60 (23). Additional members have more recently been identified, including yeast Esa1, Drosophila MOF, and human HBO1 and MORF. These proteins are grouped together on the basis of their close sequence similarities (Fig. 4) and their possession of a particular acetyltransferase homology region (part of motif A of the GNAT superfamily) (174), as shown in Fig. 1A. Although containing regions similar in sequence, the members of the MYST family are involved in a wide range of regulatory functions in various organisms.
FIG. 4.
Alignment of the MYST family of HATs and putative HAT proteins. The MYST homology region is indicated, with the acetyl-CoA-binding site, corresponding to GNAT family motif A, shown as a black box. Z, zinc finger motifs: an atypical C2HC motif in the MYST region (Esa1 diverges from this motif), a typical C2HC in the N-terminal region of HBO1, and two adjacent C4HC3 (or PHD) fingers in MOZ and MORF. C, chromo-like domain found in Esa1, MOF, and Tip60.
Sas2 and Sas3.
One of the diverse functions mentioned above is transcriptional silencing, which in S. cerevisiae involves at least two MYST proteins, Sas2 and Sas3 (also known as Ybf2). The SAS2 (something about silencing) gene was originally discovered in a screen for defects in epigenetic silencing in a sir1 genetic background (194). sir1 null mutation leads to loss of mating in most cells due to defects in silencing at the HM mating type loci, but a subpopulation of cells remain able to mate. Additional mutation of SAS2, however, led to absence of mating, even though a sas2 single mutant was phenotypically normal. Interestingly, Sas2 seems to have opposite regulatory effects depending on the silenced locus, promoting silencing at HML while inhibiting it at HMR (64). Other tests demonstrated that Sas2 was required for telomeric silencing (194). Sas3 is a second silencing-related yeast MYST protein, identified by its close homology to Sas2. A sas3 single mutant was also phenotypically normal, and subsequent mutant studies showed that Sas3 has overall weaker effects than Sas2: it is involved in silencing at mating loci, since a sas3 mutation (like sas2) restored silencing to a partially defective HMR locus but did not affect silencing at telomeres (194).
Sas3 is a confirmed HAT, as recent in vitro experiments have demonstrated that glutathione-S-transferase (GST)-fused Sas3 can acetylate free histones H3 and H4 strongly and H2A weakly (230). Furthermore, Sas3 is the catalytic subunit of the nucleosomal H3-acetylating complex NuA3, described below (S. John and J. L. Workman, unpublished results). Although HAT activity has not yet been demonstrated for Sas2 in vitro, it may require additional subunits or in vivo modifications in order to function enzymatically.
In vivo, chromatin structure is known to be highly important for transcriptional silencing, which correlates with reduced nucleosome acetylation (28). While negative effects on silencing (such as at the HMR locus) would fit with traditional models of histone acetylation, the positive silencing effects seen with these two potential HATs are suggestive of more complicated regulatory mechanisms. Alternatively, Sas2 or Sas3 may achieve regulation by acetylating substrates other than histones. This is possibly supported by findings that loss of yeast N-terminal acetyltransferase activities leads to silencing defects (7, 170, 264) and by the growing list of known factor acetyltransferases, discussed later in this review. However, discovery of specific silencing mechanisms will require future study.
Esa1.
A third yeast MYST family protein, Esa1, has recently been identified and characterized as an essential HAT required for cell cycle progression. Esa1 was originally identified through its homology with Sas2, Sas3, and other MYST proteins, and a null mutant of its gene was inviable, hence its name (essential Sas family acetyltransferase 1) (216). Esa1 is a HAT, as recombinant protein was able to acetylate free histones H2A, H3, and H4 in vitro, with its strongest activity on histone H4, particularly at lysine-5. It was unable, however, to acetylate nucleosomes in vitro. In vivo, loss of Esa1 led to specific defects in histone acetylation and growth (47). When esa1 temperature-sensitive mutants were grown at the restrictive temperature, the lysine-5-acetylated form of histone H4 was partially lost (extracts were probed with antibody specific to this isoform). Furthermore, flow cytometric and microscopic analyses of these mutants revealed that cells that lose Esa1 exhibit G2/M arrest, blocked in the cell cycle subsequent to DNA replication but prior to mitosis and cell division (47). Taken together, these findings demonstrate the importance of the Esa1 protein in yeast cellular function, and its direct connection to transcription has recently been shown by studies with a native Esa1-containing complex, NuA4 (described below).
MOF.
In Drosophila melanogaster, the MOF protein is a MYST family member with an important role in another transcriptional regulatory process, dosage compensation. Since male fruit flies have only one copy of the X chromosome compared to females' two, dosage compensation occurs in males to cause a twofold increase in the expression of X-linked genes (reviewed in reference 121). Association of a dosage compensation complex (123) with the chromosome is correlated with increased acetylation of histone H4 at a specific residue (lysine-16) (22, 245). The mechanism of this process was elucidated with the characterization of the mof (males absent on the first) mutation, which made male flies inviable. The gene product MOF was found to have MYST homology, and its direct link to histone acetylation was demonstrated by the fact that dying mof mutant males lack the lysine-16-acetylated isoform of histone H4 normally associated with the X chromosome (103). Interestingly, the mutation (mof1) leading to nonfunctional MOF was a single glutamate substitution at a GNAT motif A invariant glycine residue implicated in acetyl-CoA substrate binding.
Recent studies with MOF and a native complex containing it (the MSL complex) have provided confirmation of MOF as a Drosophila HAT of histone H4 (217). In vitro, a recombinant fragment of MOF had an overall histone specificity similar to that of Esa1, acetylating H4 strongly and H2A and H3 weakly. Furthermore, partially purified MSL complex—containing MOF, several dosage compensation-specific proteins, and X chromosome-associated RNA—was able to acetylate nucleosomes specifically on lysine-16 of histone H4 in vitro. This activity was MOF dependent, as immunoprecipitated MSL complex containing mof1-derived protein was essentially inactive (217). Altogether, the data are consistent with MOF's being the HAT responsible for a specific chromatin modification associated with dosage compensation.
Tip60.
The first human MYST protein to be discovered, Tip60, also demonstrated a potential direct relationship between activation and histone acetylation. Tip60 (Tat-interactive protein, 60 kDa) was identified in a yeast two-hybrid/human library screen seeking proteins that interact with the activation domain of the HIV-1 transactivator protein Tat; specific physical interaction was further demonstrated by binding of expressed Tip60 to purified Tat in vitro (120). A recombinant construct of Tip60 lacking the N-terminal 40% but containing the MYST domain homology region was subsequently shown to have in vitro HAT activity, acetylating free histones H2A, H3, and H4 on specific lysines but acetylating nucleosomes poorly (125, 277). The findings of HAT activity and Tat interaction have recently provided insights into the cellular function of Tip60, as the Tat-repressed gene for Mn-dependent superoxide dismutase (262) was tested and found to be positively regulated by Tip60 in vivo. Furthermore, Tat was found to prevent this activation by specifically inhibiting the HAT activity of Tip60, leading to the hypothesis that Tip60 normally activates a set of genes by histone acetylation but that their expression can be opposed by Tat-mediated HAT inhibition (50). More information on the physiological functions of Tip60 may be provided by the very recent identification of a native, nucleosome-acetylating Tip60 complex, described later in this review (Y. Nakatani, unpublished results).
MOZ and MORF.
While Tip60 is apparently associated with the action of HIV, MOZ is a MYST protein involved in another specific human disease process, oncogenic transformation leading to leukemia. When a particular chromosomal translocation in acute myeloid leukemia was characterized, it was found to have resulted in the fusion of two apparent HATs, the novel protein MOZ (monocytic leukemia zinc finger protein) (23) and CBP (described below). This created a chimeric protein consisting of the N-terminal three-quarters of MOZ (including its MYST and zinc finger domains) fused to the C-terminal 90% of CBP, containing its HAT domain and activator interaction regions. Although acetyltransferase activity of MOZ has not been directly demonstrated, it is hypothesized that MOZ-CBP may cause aberrant chromatin acetylation due to mistargeting of specific HAT activities, ultimately leading to leukemogenesis.
MOZ fusion with another transcription-related protein, TIF2, has also recently been reported in certain cases of leukemia (38, 144). These translocations also contained an N-terminal portion of MOZ, in this case fused to the C-terminal part of the nuclear receptor coactivator TIF2 (described further below), including its putative CBP interaction and activation domains. One hypothesis is that this fusion, through TIF2 interaction with CBP, may function similarly to MOZ-CBP, with equivalent aberrant effects. But interestingly, TIF2's own putative HAT domain (42) is part of the fusion, so another misdirection of HAT function may be at work instead. Further characterization of MOZ and TIF2 transcriptional and HAT activities will be required to elucidate their roles in leukemogenic processes.
Another human MYST family member is MORF (MOZ-related factor), which was identified in a database search by its sequence similarity to MOZ and has recently been characterized (41). MORF shows very close homology to MOZ throughout its length, not just in the MYST consensus region. Although MORF mutation has not yet been implicated in cancer, as MOZ has, its in vitro HAT function has been more thoroughly studied, perhaps shedding light on the function of both proteins. Recombinant full-length MORF expressed in insect cells and a bacterially produced MYST domain fragment were both able to acetylate free histones in vitro, with a preference for H3 and H4. Furthermore, the insect-derived protein was also competent for nucleosome acetylation, strongly preferring histone H4. Another finding was that MORF contains an N-terminal repression region (including two zinc fingers), deletion of which led to increased in vitro HAT activity and increased in vivo transcription by Gal4-MORF at a reporter gene. Interestingly, alternative forms of MORF (MORFα and MORFβ) have been observed which have insertions at a site within or near the repression domain, but their impact on MORF function is not yet known. In addition, MORF contains a C-terminal activation domain that is functional in the absence of the HAT domain; the analogous C-terminal region is missing in the MOZ translocations. While MOZ and MORF, like Gcn5 and PCAF, are very closely related in sequence, it remains to be determined how functionally similar they are and in which specific transcriptional processes they participate.
HBO1.
A fourth human MYST protein is HBO1 (histone acetyltransferase bound to ORC), which was discovered in a two-hybrid screen on the basis of its interaction with the ORC1 subunit of the origin recognition complex (ORC) (112). ORC is conserved throughout the eukaryotes and is primarily known to bind DNA replication origins and to be critical for the initiation of replication (13, 60). ORC also has a transcriptional function, however, since it has been demonstrated to be involved in silencing at yeast mating type loci (12, 69, 71) and Drosophila heterochromatin regions (184). In the case of S. cerevisiae, a relationship with the MYST proteins Sas2 and Sas3 is suggested by the fact that ORC binds Sir1 (70, 242) and that Sas2 displays genetic interactions with ORC (SAS2 knockout results in partial suppression of orc2 and orc5 mutant phenotypes) and antagonizes ORC-mediated silencing at the HMR locus (64).
Upon the cloning of HBO1 and discovery of its MYST homology, its HAT function was investigated. Via HBO1-specific antibodies, an HBO1-containing complex was isolated from nuclear extract and found to acetylate free histones H3 and H4 well and nucleosomes weakly. Recombinant HBO1 alone was not observed to acetylate free histones, but it did exhibit some HAT activity, as very weak acetylation of nucleosomal histones was seen (112). Full activity of the HBO1 protein may therefore require other factors or in vivo modifications. The in vivo function of HBO1 and its role in transcriptional silencing remain to be studied, and its relationship to the yeast Sas proteins is still unknown. While a logical hypothesis is that HBO1 may be a functional analog of one of these proteins, none of them (Sas2, Sas3, or Esa1) bound directly to yeast Orc1 in a two-hybrid assay (112).
p300/CBP
After the discovery of histone acetylation by Gcn5 and PCAF, the critical role of acetyltransferases in transcriptional regulation was also demonstrated by the fact that a pair of previously well-characterized coactivators of multicellular eukaryotes, p300 and its close homolog CBP (CREB-binding protein), are themselves HATs (8, 178) and FATs (as described below). The interactions of p300/CBP (p300 and CBP are often referred to as a single entity, since the two proteins are considered structural and functional homologs) with PCAF and GCN5, described above, and with nuclear receptor coactivators, described below, are examples of transcriptional regulatory complexes with multiple acetyltransferase activities.
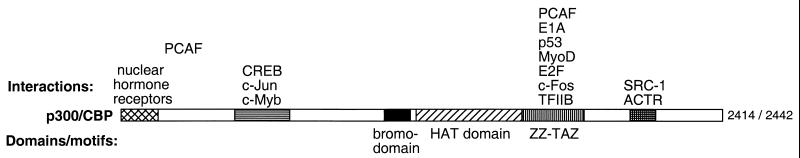
p300/CBP is a ubiquitously expressed, global transcriptional coactivator that has critical roles in a wide variety of cellular processes, including cell cycle control, differentiation, and apoptosis (81, 211), and mutations in p300 and CBP are associated with certain cancers and other human disease processes (80). On the molecular level, p300/CBP stimulates transcription of specific genes by interacting, either directly or through cofactors, with numerous promoter-binding transcription factors such as CREB, nuclear hormone receptors, and oncoprotein-related activators such as c-Fos, c-Jun, and c-Myb. As described above, p300/CBP also binds the HAT PCAF, an interaction with which adenoviral oncoprotein E1A competes (279). p300/CBP is a large protein of about 300 kDa and more than 2,400 residues, and at least four interaction domains with different sets of factors have been characterized throughout its sequence, as shown in Fig. 5. Furthermore, its central region contains a bromodomain motif (98, 117), which is also found in the HATs Gcn5, PCAF, and TAFII250.
FIG. 5.
Domains and interaction regions of the global coactivator HATs p300/CBP. Labeled below the polypeptide diagram are several domains and sequence motifs, including a bromodomain, the HAT domain, and ZZ and TAZ putative zinc fingers (190). Above are indicated some of the proteins demonstrated to interact with p300/CBP at certain regions. PCAF has been shown to interact with two regions of p300/CBP (130).
The HAT activity of p300/CBP was first discovered in an E1A pulldown from HeLa (human) nuclear extract (178) and in direct CBP immunoprecipitations from Cos (primate) cell extracts (8). In vitro studies with recombinant p300 and CBP proteins confirmed that these proteins were indeed HATs, strongly acetylating the amino-terminal tails of all four core histones with little apparent specificity. Unlike other HATs, recombinant p300/CBP was able to acetylate all four histones within nucleosomes as well as in free-histone form. Deletion mutant analysis mapped the HAT domain of p300/CBP to an interior region between the bromodomain and the PCAF/E1A/MyoD/c-Fos interaction region (8, 178). p300/CBP represents a unique class of acetyltransferase, although it may be distantly related to other HATs. Careful sequence analysis identified regions with limited homology to GNAT motifs A, B, and D, in addition to another short motif shared with PCAF and Gcn5 (158). Site-directed mutagenesis demonstrated that all four of these motifs contribute to CBP's HAT function. Furthermore, the connection between p300/CBP's HAT function and transcription in vivo was demonstrated by the fact that a promoter-tethered CBP HAT domain resulted in activation, and HAT-impaired mutant versions showed a direct correlation of acetylation competence with this transcriptional activity (158). p300/CBP's HAT function was also shown to be required for certain types of nuclear receptor-mediated activation in vivo (130).
In addition, the HAT activity of p300/CBP is apparently regulated by other factors. As observed for PCAF, the viral protein E1A and the regulatory protein Twist were shown to bind to p300 and inhibit its HAT activity (40, 96, 187). However, another report indicates that E1A has a HAT-stimulatory effect on CBP (2), suggesting a possible functional difference between p300 and CBP (this study also found that cell cycle-dependent phosphorylation of CBP by Cdk2 increases its HAT activity). Since another study reported no effect of E1A binding on CBP's HAT activity (8), it is possible that these HAT effects are due to experimental discrepancies that need to be resolved.
Overall, p300/CBP is one of the most potent and versatile of the acetyltransferases, consistent with its role as a global coactivator in higher eukaryotes. Like PCAF, p300/CBP is known to acetylate and regulate various transcription-related proteins other than histones. The known FAT substrates of p300/CBP, described later in this review, include HMG I(Y), activators p53, GATA-1, erythroid Krüppel-like factor (EKLF), Drosophila T-cell factor (dTCF), and HIV Tat, nuclear receptor coactivators SRC-1, ACTR, and TIF2, and general factors TFIIE and TFIIF. Another phenomenon relevant to the regulatory activities of p300/CBP is that human chromosomal translocations fusing CBP to either the putative HAT MOZ (23) or the MLL gene (232) can result in leukemogenesis; the mechanisms of these processes, however, and whether they involve HAT or FAT activity remain to be elucidated.
Nuclear Receptor Coactivators
HAT proteins have also been directly implicated in transcriptional activation brought about by hormone signals. The HAT activities of human coactivators ACTR and SRC-1, which interact with nuclear hormone receptors, demonstrate the involvement of acetylation in yet another system of transcriptional regulation and define a unique family of HATs.
SRC-1.
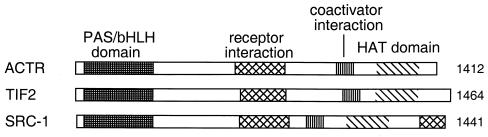
Steroid receptor coactivator-1 (SRC-1), also known as p160 (119) and NCoA-1 in mice (240), is a human nuclear receptor cofactor originally discovered by way of its interaction with the human progesterone receptor (PR) in a yeast two-hybrid screen. In vivo experiments in mammalian cells established the coactivator function of SRC-1, as it was able to stimulate ligand-dependent activation by numerous nuclear receptors, including PR, glucocorticoid receptor (GR), estrogen receptor (ER), thyroid hormone receptor (TR), and retinoid X receptor (RXR) (180). Because of this coactivator function, recombinant SRC-1 was assayed in vitro and found to have HAT activity, acetylating H3 and H4 either as free histones or in mononucleosomes (220). Truncation analysis revealed that the HAT domain is located in the C-terminal region of SRC-1, as diagrammed in Fig. 6. SRC-1 was known to interact with p300/CBP (119, 214, 280), and interestingly, it also interacted with PCAF in vitro and in vivo (220), indicating that multiple HATs are employed to regulate hormone-signaled transcription. In addition, p300/CBP was recently shown to acetylate SRC-1, an event that is likely relevant to its nuclear receptor coactivator function (43).
FIG. 6.
Alignment of the p160 family of mammalian nuclear receptor coactivators. Indicated are the PAS/basic helix-loop-helix homology (bHLH) domain, nuclear receptor interaction regions, and the general area of interaction for coactivators p300/CBP and PCAF. ACTR and SRC-1 each have a HAT domain near their C terminus (42, 220), although the boundaries of these domains have only been approximately defined, and TIF2's domain is inferred by homology.
ACTR.
To identify additional human proteins that interact with nuclear hormone receptors, a yeast one-hybrid screen was employed which used reporter genes with retinoic response elements and a human retinoic acid receptor (RARβ) as bait. Screening with a cDNA library resulted in several known receptor interactors (including SRC-1) and one novel cofactor, termed ACTR (42), also known as RAC3 (142), AIB1 (6), and TRAM-1 (231) in humans and p/CIP in mice (240). Like SRC-1, ACTR was shown to interact with multiple nuclear hormone receptors and stimulate transactivation. Further, it was tested in vitro and also found to be a HAT capable of acetylating free or nucleosomal histones H3 and H4, and its HAT domain similarly mapped to the C-terminal end of the protein (42). In fact, ACTR shows significant sequence similarity to SRC-1 in several regions (Fig. 6): an N-terminal, basic helix-loop-helix/PAS region (236), receptor and coactivator interaction domains, and the C-terminal HAT region, defining, along with TIF2, the p160 (or SRC) family of nuclear receptor coactivators (42, 139, 252).
Further similarities between ACTR and SRC-1 are their interaction with CBP and PCAF and their acetylation by CBP. Acetylation of ACTR has been more thoroughly characterized, and it has distinct functional effects. Specifically, the acetylation occurs in the receptor interaction domain, preventing receptor binding and hence activation by ACTR (43). ACTR is therefore both a HAT and a regulatory target for another acetyltransferase.
TIF2.
A third potential HAT in the human nuclear receptor coactivator family is TIF2 (transcriptional intermediary factor 2) (252), also known as GRIP1 (106) and NCoA-2 (240) in mice. Like SRC-1 and ACTR, TIF2 binds to a number of nuclear hormone receptors, stimulates transcriptional activation (252), and interacts with (251) and is acetylated by (43) CBP. Although its HAT activity has not yet been demonstrated, TIF2 has all of the homology regions shared by SRC-1 and ACTR, including the putative HAT domain (42). Because of the sequence and functional similarities of this protein to the other two coactivators, it stands as a likely HAT candidate whose activity remains to be characterized. Another potentially interesting aspect of TIF2 is its fusion to MOZ in leukemia-associated translocations, as noted above (38, 144). Future studies will be required to determine the mechanism of this oncogenic effect and whether it involves either putative HAT activity.
The three nuclear receptor coactivators discussed above are part of an evolutionarily and functionally related HAT family; all three interact with p300/CBP, and at least two interact with PCAF. However, recent studies have demonstrated that p300/CBP (119) and PCAF (21, 130) can directly interact with nuclear receptors, independent of other factors. Furthermore, the MYST family protein Tip60 was also recently discovered to function as a coactivator with several receptors in a ligand-dependent manner (26). The fact that p300/CBP, PCAF, and Tip60 can also function as nuclear receptor coactivators underscores the importance of acetylation in transcriptional response to hormone signals and demonstrates that in higher eukaryotes, multiple strategies of acetyltransferase recruitment are used for this process.
TBP-Associated Factor TAFII250
Another direct connection between acetylation and activated transcription was demonstrated with the discovery that one of the TAFII (TATA-binding protein [TBP]-associated factor) subunits of the general transcription factor TFIID is itself a HAT. Specifically, homologs of this protein—TAFII250 in humans, TAFII230 in Drosophila, and TafII145/130 in S. cerevisiae—were shown to have HAT activity in vitro (169).
TFIID is one of the general factors required for the assembly of the RNA polymerase II transcription preinitiation complex, along with TFIIA, TFIIB, TFIIE, and TFIIF (32, 97). TFIID is in fact the first factor needed in the stepwise assembly: through its TBP subunit, TFIID binds to specific promoter DNA sequences and allows subsequent formation of the transcription complex. Although TBP without TAFIIs is able to bind promoters and allow basal transcription in vitro, the TAFII subunits promote activated transcription. Furthermore, TAFIIs have been shown to interact with certain activators and initiation-related factors (250).
The potential involvement of acetylation in TAFII function was realized with the discovery that a 250-kDa band from human nuclear extract (in an in-gel assay) and immunoprecipitated human TFIID had HAT activity (169). Further characterization of the TAFII HAT activity was performed with recombinant Drosophila TAFII230, which was found to acetylate H3 (preferentially on lysine-14, like Gcn5) and H4 in a free histone mixture (and H2A as an individual histone). It should be noted that TAFII250 and its homologs, like the p160 nuclear receptor coactivators, have some of the weaker in vitro HAT activities observed—p300/CBP and PCAF, for example, have more potent activities (130, 177; unpublished results). The in vivo significance of these apparent differences in catalytic strength, however, is not yet known.
Truncation studies with yeast and Drosophila TAF mapped the HAT domain to the conserved central region of the protein. This region has little apparent similarity to other known proteins, so TAFII250 may define a unique HAT class. However, a potential acetyl-CoA binding site has been identified within this region; it shares a Gly-X-Gly pattern with Gcn5 and other acetyltransferases, and mutation of these glycines led to reduced HAT activity (58). Like Gcn5, PCAF, and p300/CBP, TAFII250 also has a bromodomain (and Drosophila TAFII230 has two), but truncation studies demonstrated that it is not required for HAT activity (169); this and the fact that the yeast homolog contains no bromodomain argue against a major role for it in TAFII250's HAT function.
The HAT activity of TAFII250 and its homologs suggests a model for the initiation of transcription complex formation at chromatin-packaged promoters. Nucleosomes are known to inhibit binding of TBP to the TATA box (164, 273), and this inhibition is apparently mediated by histone tails (82, 115). As part of TFIID, TAFII250 may well facilitate TBP binding directly by acetylating histones at the TATA box, allowing formation of the preinitiation complex. Also potentially relevant to TAFII250 function is that TFIID is proposed to contain a histone octamer-like structure (104, 274), which may displace nucleosomal histones in concert with TAFII250's HAT activity. Although the widespread involvement of TFIID in initiation (including at TATA-less promoters) is expected to bring TAFII250 to very many genes, recent mutant studies suggest that its HAT activity is required for transcription at only a subset of promoters (e.g., certain cell cycle regulators) (58, 176). The mechanism of this specificity, however, is not yet known.
TFIIIC
Although all of the A-type HATs discussed so far in this review are proposed to be involved with transcription by RNA polymerase II (primarily of mRNA), chromatin structure is expected to affect any kind of transcription, such as the synthesis of rRNA by RNA polymerase I or tRNA precursors by RNA polymerase III. Evidence that histone acetylation is a generally employed mechanism in transcription is the fact that subunits of TFIIIC, a general transcription factor in the RNA polymerase III basal machinery, were also recently identified as HATs (109, 133). The known function of TFIIIC is to initiate transcription complex formation by binding to promoter DNA and recruiting TBP-containing TFIIIB and RNA polymerase III (137). Recent in vitro studies with purified human TFIIIC showed that it harbored HAT activity, acetylating H3, H4, and H2A as free histones and also in nucleosomes. Interestingly, an in-gel assay of TFIIIC revealed that three of its nine subunits have apparent HAT activity. The HAT functions of two of these subunits, TFIIIC110 and TFIIIC90, have been confirmed and further investigated. A bacterially expressed C-terminal fragment of TFIIIC110 had HAT activity in an in-gel assay (133), while recombinant TFIIIC90 was competent for the acetylation of either nucleosomal or free histone H3, with an apparent preference for lysine-14 (like Gcn5 and TAFII230) (109). Future studies should better clarify the function of these HAT activities in this type of transcription, but a logical hypothesis is that it fulfills a role similar to that of TAFII250 in the RNA polymerase II transcription complex. In both cases, a HAT enzyme is intimately associated with the first step in DNA binding of the transcription complex and likely acts to destabilize promoters' nucleosomes to facilitate this process. Furthermore, it is reasonable to predict that RNA polymerase I transcription is also associated with HAT activity, although this has not yet been demonstrated.
NUCLEOSOME-ACETYLATING NATIVE COMPLEXES
To participate in transcription in vivo, the HATs described above have often intricate interactions with various regulatory proteins and/or the transcription apparatus. These interactions can potentiate a HAT enzyme's activity at a particular genetic locus or time (i.e., cell cycle or developmental stage) or modulate substrate specificity—its choice of specific lysine residues in particular histone tails (H2A, H2B, H3, or H4) in a nucleosomal context—to bring about an appropriate transcriptional effect. Some of the native complexes containing HATs have been isolated and studied, and they rae described below.
Yeast HAT Complexes
Most known HATs are able to acetylate free histones in vitro when assayed as a single polypeptide. Many, however, such as Gcn5, are unable to acetylate their probable physiological substrate, nucleosomal histones, under standard conditions in vitro, apparently due to the requirement for other factors to allow this level of substrate specificity. Because of this, a study was performed which sought to identify native yeast complexes capable of acetylating nucleosomal substrates (84). Through fractionation of S. cerevisiae extracts and assays of nucleosomal HAT activity, four distinct complexes were discovered and have been further characterized: SAGA, ADA, NuA4, and NuA3.
SAGA.
After their discovery, the four separable nucleosomal HAT activities were initially analyzed by Western blot and null mutation studies, and it was found that the two nucleosomal histone H3/H2B-specific complexes contained Gcn5 as their HAT catalytic subunit, along with two other transcriptional adaptor proteins, Ada2 and Ada3 (84). Interestingly, one of these complexes also contained several Spt proteins, which were originally identified via another transcription-related genetic screen (suppression of Ty and δ insertions at promoters) (reviewed in reference 267). This complex was therefore named SAGA (Spt-Ada-Gcn5 acetyltransferase) (reviewed in reference 88); the other complex, containing Ada proteins but not Spts, was called ADA (described below).
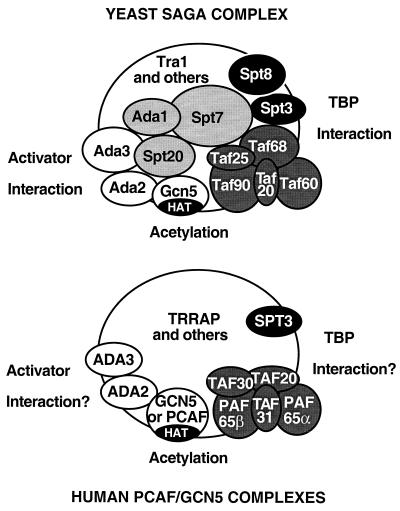
Of the known HAT-containing complexes, yeast SAGA is the best characterized. It is a large complex, approximately 1.8 MDa, as determined by a sizing column. About 15 of its subunits are now known, although it is expected that at least several more remain to be identified. Notably, SAGA brings together in one complex four different groups of previously described transcription-related proteins: the transcriptional adaptors (Ada proteins), a subset of the Spt proteins, a subset of the TafIIs (86), and Tra1 (87, 204), the yeast homolog of the human transcriptional regulatory protein TRRAP. Interestingly, human HAT complexes have also been isolated that contain homologs of each of these groups, as shown in Fig. 7 and discussed below, suggesting evolutionary conservation of SAGA function (177, 247). In addition, yeast SAGA contains the transcriptional regulator Sin4 (282), which is also a component of the Srb/mediator subcomplex of RNA polymerase II holoenzyme (143, 219).
FIG. 7.
Schematic diagram of known subunits and functions of the yeast SAGA and human PCAF or GCN5 HAT complexes. The yeast SAGA complex (top) contains Gcn5 as its HAT catalytic subunit. SAGA has been shown to interact with acidic activation domains, and this function may be mediated by its adaptor components (Ada2, Ada3, and Gcn5) or possibly through Tra1 or other subunits. Another subset of SAGA proteins (Ada1, Spt7, and Spt20/Ada5) are required for its structural integrity and overall function. The Spt3 and Spt8 subunits have been implicated in interaction with TBP, and the TafII group, which also participate in TFIID, may provide TBP-binding function to SAGA as well. The human SAGA-analogous complexes (bottom) contain either GCN5 or PCAF. The PCAF complex has been more thoroughly studied; it has nucleosome acetylation function, and known subunits include TRRAP, hADA2, hADA3, hSPT3, and TAFIIs/PAFs (PCAF-associated factors)—all homologs of proteins found in SAGA. Activator and TBP interaction functions are hypothesized for these human complexes but have not yet been demonstrated.
The adaptors contained in yeast SAGA are Ada1 (107a), Ada2 (17), Ada3 107b(), Ada5, and Gcn5. The Spt proteins it contains are Spt3 (64a), Spt7 (73), Spt8 (64b), and Spt20, previously described as the TBP-related Spt subgroup because of their apparent functional interactions with TBP. Prior to the discovery of SAGA, there was evidence of an Ada-Spt relationship in that ADA5 and SPT20 represent the same gene, which was discovered in independent genetic screens (153, 196). More recently, analysis of SAGA subunit mutant effects on phenotypes and on SAGA composition and function have shown that the Ada and Spt proteins within SAGA can be placed in three categories reflecting their structural and functional roles in the complex (84, 195, 223). Null mutation of the genes encoding Ada1, Spt7, or Spt20/Ada5 leads to disruption of SAGA and severely impaired growth, indicating the requirement of these subunits for SAGA structural integrity and the significant impact of SAGA loss in vivo. Mutations in either of the other two groups, Ada2/Ada3/Gcn5 and Spt3/Spt8, led to largely intact SAGA and moderately impaired yet distinct phenotypes, consistent with their roles as somewhat peripheral subunits that are involved in specific SAGA subfunctions that have been demonstrated—activator interaction (113, 246) and nucleosome acetylation for Ada2/Ada3/Gcn5 and TBP interaction (15, 86, 195, 223) for Spt3/Spt8.
In vivo and in vitro, the SAGA complex and its components have been shown to be critical to certain types of transcription. In vitro, purified SAGA was able to stimulate transcription in various chromatin-template assays by way of its combined HAT activity and interaction with acidic activators (113, 246, 254). The in vivo significance of SAGA has been demonstrated by examination of mutants of its components, which have verified that the complex has an important role in transcriptional activation at a subset of genes, such as GAL1 (57), TRP3, and HIS3 (15), although its regulatory effect may be distinct at different genes. Interestingly, Gcn5/SAGA and the chromatin-remodeling complex Swi-Snf display apparent genetic interactions (189, 195) and complementarity or partial redundancy with each other in the activation of some genes (19, 90, 229). This suggests that both of these complexes may be recruited to certain promoters and contribute to transcriptional activation by altering chromatin, albeit by different mechanisms (14).
Likely relevant to the in vivo chromatin-modifying function of Gcn5 is the fact that its participation in the SAGA complex has distinct consequences for its histone substrate specificity in vitro. SAGA gives Gcn5 the ability to acetylate nucleosomes, with a primary specificity for histone H3 and, to a lesser extent, H2B (84). This capacity to interact with and recognize nucleosomal histones is apparently conferred by other subunits in the complex and may involve Gcn5's bromodomain, deletion of which significantly reduces nucleosome acetylation by SAGA (223). Participation of Gcn5 in the SAGA complex (and ADA) also causes expanded lysine specificity on histone H3, as determined by a recent study (85). SAGA and ADA significantly acetylated other lysine residues in addition lysine-14 both on H3 N-terminal tail peptides and in nucleosomal H3. The patterns of acetylation by these complexes were overlapping yet distinct, further indicating the influence of other subunits on Gcn5's function.
Future studies should further elucidate the roles of various subunits in the structure and transcriptional function of SAGA. It is notable that SAGA does not contain TafII145/130, the TafII shown previously to possess HAT activity (169), but it does contain a histone-related TafII subgroup (TafII20, -25, -60, -68, and -90), which is important for SAGA's acetylation and transcription-stimulation function in vitro (86). These subunits could conceivably provide TBP interaction or histone displacement function, but their specific roles in the context of SAGA remain to be demonstrated. Tra1, the yeast TRRAP homolog, also has implications for SAGA structure and function that require further study. Tra1 is an essential protein (204), and its large size (approximately 400 kDa) suggests that it may be important to the overall structure of SAGA. Functionally, its homolog TRRAP has coactivator function, interacting with the activators c-Myc and E2F (161), which suggests that it may have an activation domain interaction role like Ada2 (10, 213). Finally, recent evidence indicates that SAGA's composition and function may be dynamic, exhibiting changes depending on conditions in the cell. While SAGA produced from rich medium (transcriptionally repressive for HIS3 and other amino acid-biosynthetic genes) has been well described, derepressing conditions gave rise to another form, termed SAGAalt (altered SAGA) (15). SAGAalt lacks the Spt8 subunit and, potentially, its negative regulation of TBP function at HIS3, but this complex and its precise relationship to SAGA await further characterization.
ADA.
The other known Gcn5-containing complex is ADA, which has a size of about 800 kDa. Like SAGA, the ADA complex acetylates nucleosomes primarily on histones H3 and H2B in vitro, and it contains Ada2 and Ada3 but none of the other known subunits of SAGA (84). Recently, peptide analysis revealed a novel subunit unique to ADA, demonstrating that it is a distinct complex and not a subcomplex or artifactual fragment of SAGA (63). This subunit, Ahc1 (ADA HAT complex component 1), is required for the structural integrity of ADA, as a knockout mutation disrupted the complex.
Although ADA does contain Gcn5 and two other adaptors, unlike SAGA it does not seem to participate directly in transcription or have a major functional impact in vivo. Despite its possession of Ada2, a known interactor with acidic activators, ADA could not interact with activation domains in vitro, whereas SAGA could (246). Another functional difference between ADA and SAGA was demonstrated in their histone H3 lysine specificities in vitro; ADA acetylated fewer residues (lysine-14 and -18) than SAGA (lysine-9, -14, -18, and -23) (62, 85). Furthermore, an ahc1Δ mutation had no obvious phenotypic effects; the mutant (lacking the ADA complex) grew as well as wild-type cells on minimal medium and did not display an Ada− phenotype or defects in in vivo transcription of a reporter gene (63). The physiological function of the ADA complex is still unknown, although some connection to histone acetylation in vivo has been suggested by the fact that overexpression of Ahc1 suppresses certain mutations in the gene encoding histone H2A (63).
NuA4.
Another yeast HAT complex identified by Grant et al. (complex 2) was immediately distinguishable from the others in that its nucleosomal substrate was primarily histone H4 (as well as H2A, to a lesser degree) and it did not significantly acetylate histone H3 (84). Further purification and characterization of this 1.3-MDa complex, called NuA4 (nucleosomal acetyltransferase of histone H4), has revealed that its HAT catalytic subunit is the MYST protein Esa1 (3). It also contained Tra1, identified previously as a component of SAGA. Also like SAGA, NuA4 interacted with acidic activation domains in vitro and stimulated transcription in an acetylation-dependent manner in various in vitro assays with chromatin templates (3, 113, 246, 254). Interestingly, extensive acetylation of nucleosomal templates with NuA4 led to transcriptional activation even with other types of activators that do not interact with NuA4, an effect not seen with SAGA (113). This general activation by histone H4/H2A—as opposed to H3/H2B—acetylation shows the potential impact of nucleosomal histone specificity on transcription. NuA4's composition (it contains at least seven additional unknown subunits) and in vivo function remain to be fully characterized, but its possession of two essential transcription-related subunits, including a HAT needed for cell cycle progression (47), suggests that it plays a critical role in the cell.
NuA3.
A fourth yeast HAT complex that has been identified and further investigated is NuA3 (also referred to as complex 3), a 500-kDa complex that exclusively acetylates histone H3 in nucleosomes (84). This is perhaps the least well characterized complex in terms of composition, but its catalytic subunit was recently determined to be Sas3, a MYST protein involved in silencing (S. John and J. L. Workman, unpublished results). Some in vitro studies have been performed with NuA3, and like ADA, it failed to interact with activation domains or to activate transcription in a specific way (246, 254). The function of this complex in vivo—i.e., its role, if any, in transcription or its relationship to silencing—remains to be determined by future studies.
Other complexes.
Several other yeast complexes with HAT subunits and/or activity have also been discovered but await further characterization. For example, four complexes containing Ada2 and Ada3 (and, by inference, Gcn5) were recovered from yeast extracts (203); of these, two approximately 2-MDa complexes were apparently SAGA, SAGAalt, or related complexes (204). Another 900-kDa complex may be ADA, but the composition and function of a 200-kDa complex, and whether it contains Gcn5 and physiological HAT activity, remain to be determined. A separate study also identified three Gcn5-dependent activities that require further characterization (189). Another HAT-containing (Elp3) complex is elongator (269), but its HAT activity in the context of free elongator or RNA polymerase II holoenzyme has not yet been studied. Finally, there are certain remaining yeast HATs and putative HATs, such as Hpa2 and Sas2, for which no native complex has yet been identified. The nature of these unknown complexes, alternative complexes containing other HATs, and their enzymatic and possible transcription-related functions in vitro and in vivo are likely topics for future investigations.
Human HAT Complexes
Recently, several human protein complexes with known HAT subunits have been isolated from nuclear extracts and partially characterized. Subunit identification has shown that some of these complexes are remarkably analogous in composition to known yeast HAT complexes, and in each case an involvement in transcription is also suggested by subunits besides the HAT protein.
GCN5/PCAF complexes.
One pair of human complexes was identified by way of N-terminal Flag epitope-tagged PCAF and GCN5, which were purified from HeLa nuclear extracts along with their native complexes to near homogeneity with a combination of conventional and antibody affinity chromatographies (177). Interestingly, when both complexes were analyzed on a Coomassie-stained SDS-PAGE gel to visualize all subunits, the patterns of bands were virtually identical, suggesting that the two complexes are very similar with the exception of the identity of the HAT subunit. Two of the GCN5 complex's subunits were confirmed immunochemically to be the same as those in the PCAF complex, further supporting the overall equivalence of the complexes. Since the GCN5 complex purification used the short form of GCN5, lacking the N-terminal 361 amino acids, these results imply that this region is dispensable for complex formation.
Of the two complexes, the PCAF complex has been more thoroughly characterized. It contained more than 20 polypeptides, and a subset of these have been identified by protein sequencing (177, 247). Remarkably, all 11 of the subunits identified so far are apparent homologs of components of yeast SAGA, suggesting strong evolutionary conservation of this type of complex (Fig. 7). Besides the Gcn5 homolog PCAF, the complex contained human adaptor homologs hADA2 and hADA3, Spt protein hSPT3 (281), the transcriptional cofactor TRRAP (161), and a set of five TAFII or TAFII-related proteins. Altogether, the identities of these subunits, like SAGA's, imply a transcriptional role for the PCAF complex (and, by analogy, the GCN5 complex), such as adaptor (through hADA2 and hADA3) and TBP interaction (through hSPT3) function. The c-Myc- and E2F-interacting subunit TRRAP, a member of the ATM superfamily (161, 247), further suggests a coactivator or other transcription-related role for these complexes.
The subunits shared between the PCAF complex and TFIID are TAFII20/15, -30, and -31, homologs of yeast SAGA subunits TafII68, -25, and -17, respectively. Although the PCAF complex did not contain the TFIID-specific human homologs of yeast TafII60 and -90 (the other two TafIIs in SAGA), it did contain two closely related proteins, termed PAF65α and PAF65β (PCAF-associated factors) (177). As in S. cerevisiae, most of these are histone-like TAFIIs, possibly suggesting the formation of a histone octamer-like substructure (274) which could displace nucleosomal histones during remodeling (177). Interestingly, Spt3, its homologs, and related TAFII18 have been found to contain histone fold motifs (20), further suggesting structural parallels between TFIID, the SAGA/PCAF complexes, and the histone octamer (228).
As observed for Gcn5 in yeast SAGA, the participation of PCAF in this multisubunit complex has an effect on its HAT activity and specificity. Although recombinant PCAF can acetylate nucleosomal histones, primarily on histone H3, the PCAF complex acetylates H3 much more strongly (177). Like Gcn5, PCAF seems to require the influence of other subunits in a native complex to bring about its maximal activity on the more physiological substrate.
Two other human complexes, purified on the basis of TAF subunits, are apparently very similar to the human GCN5 complex. One complex, TFTC (TBP-free TAFII-containing complex), was isolated from a nuclear extract by affinity purification with anti-TAFII30 antibodies followed by immunodepletion of TBP (265). Like the PCAF complex, TFTC lacked TBP but contained a subset of the TAFII proteins. Interestingly, this complex was able to support transcription in vitro from both TATA-containing and TATA-less promoters, functionally replacing TFIID despite the absence of TBP. The identity of TFTC as a HAT-containing complex with many of the same subunits as the GCN5 and PCAF complexes was revealed by a subsequent study (27), which demonstrated that TFTC can acetylate free and nucleosomal histones (with a preference for H3) in addition to linker histone H1. A number of TFTC's subunits were identified immunochemically, and it was found to contain the PCAF/GCN5 complex subunits GCN5, hADA3, hSPT3, PAF65β, and, of course, TAFII30. However, TFTC contained several TAFIIs that the PCAF/GCN5 complexes apparently do not—TAFII150, TAFII135, and TAFII100 (substoichiometric in the PCAF complex)—and did not contain human ADA2, suggesting that the complexes are not completely identical. The functional and in vivo significance of these differences is not yet known.
The other TAFII-derived complex is STAGA (SPT3-TAFII31-GCN5-L acetyltransferase), which was purified via antibodies specific for another histone H3-like TAFII, TAFII31 (157). The TAFII31 immunopurified fraction was found by Western blot analysis to possess TBP and TAFII31, as expected, as well as hSPT3 (281), the human homolog of yeast Spt3. TBP-containing species were eliminated by further affinity purification of the fraction with anti-hSPT3 antibodies, and analysis of the resulting complex showed that it also had HAT activity and contained GCN5. To date, hSPT3, TAFII31, and GCN5 are the only known subunits of STAGA, and all three are evidently shared with both TFTC and the GCN5 complex, so further characterization will be required to discover STAGA's structural and functional similarity with each of these two slightly distinct complexes.
Tip60 complex.
Very recently, a human complex containing another type of HAT, the MYST protein Tip60, has been purified and partially characterized (Y. Nakatani, unpublished results). This complex was isolated on the basis of N-terminal epitope-tagged Tip60, and it contained about 12 subunits, ranging from 29 to 400 kDa. While recombinant Tip60 is able to acetylate free but not nucleosomal histones, the Tip60 complex can acetylate either substrate; as observed for Gcn5 and Esa1, participation in a native complex seems to confer chromatin substrate specificity on Tip60. Protein sequencing has now identified a number of subunits in the Tip60 complex, and some of them have known or potential relationships to transcriptional regulation (Y. Nakatani, unpublished results).
Interestingly, the largest subunit of the Tip60 complex is TRRAP, a transcriptional regulatory protein also found in the human GCN5 and PCAF complexes. Tra1, the yeast homolog of TRRAP, as noted above, is also a component of the yeast HAT complexes SAGA and NuA4, which function in transcriptional activation. It is notable that a TRRAP homolog is present in at least two distinct classes of complexes in both S. cerevisiae and humans, and since the PCAF/GCN5 complexes are apparently analogous to SAGA, this leads to the hypothesis that the Tip60 complex may be analogous to NuA4. This possibility is supported by the fact that both catalytic subunits (Esa1 and Tip60) are MYST proteins, but further characterization of NuA4 and the Tip60 complex will be required to determine whether these complexes are otherwise similar, with evolutionarily conserved composition and function. Perhaps relevant to this point, conservation of NuA4 function between S. cerevisiae and ciliates has been suggested by a study that identified a Tetrahymena nuclear HAT activity that resembles NuA4 in its lysine specificities on nucleosomal histones and ability to promote activated transcription (179).
TFIIIC, HBO1, and other complexes.
At least two other complexes have been recovered from human cells and identified as having HAT subunits and activity. One of these, TFIIIC (described earlier), has multiple HAT subunits and the ability to acetylate nucleosomes (109, 133). Purified TFIIIC was able to alleviate chromatin-mediated transcriptional repression in vitro (133), but the direct role of the HAT activities in these assays and in vivo remain to be demonstrated. Another human complex is known to contain MYST protein HBO1 (112) and may interact with ORC, but its other subunits and specific function are still unknown.
In vivo, numerous transcription-related complexes are believed to involve p300/CBP and PCAF (46, 130, 167), bridging interactions between activators and the transcription complex; the same might be said for other coactivator HATs, such as SRC-1, ACTR, and TIF2. However, since some of their interactions may be transient in nature or intimately involve chromatin, many in vivo HAT complexes may be difficult to isolate and characterize in native form in vitro. Finally, the complexes and interacting proteins of several other HATs and putative HATs, such as MOZ and MORF, await identification, as do possible roles of these proteins in transcriptional regulation.
Drosophila MSL Complex
Native HAT complexes from S. cerevisiae and human cells have been best characterized, but an interesting complex from another organism—the Drosophila MSL complex—has also recently been partially purified and characterized. As mentioned above, the MSL complex is involved in dosage compensation, i.e., increased transcription from the X chromosome in male fruit flies. It contains at least five proteins: MSL1 (male-specific lethal), MSL2, MSL3, MLE (maleless, an RNA-DNA helicase), and MOF, the HAT catalytic subunit. These proteins colocalize to many sites throughout the X chromosome, and mutation of any of the genes encoding them leads to male-specific lethality (150). Studies with coexpressed proteins have defined some of the interactions among the MSL components, and MSL1 was found to have a central role in assembly of the complex (210). In addition, two noncoding RNAs, transcribed from the genes roX1 and roX2 (RNA on the X), also colocalize with MSL subunits on the X chromosome (72) and copurify with the MSL complex (166, 217).
Immunoprecipitations from Drosophila nuclear extracts have allowed further characterization of the MSL complex in vitro. In HAT assays, the complex acetylated nucleosomes exclusively on lysine-16 of histone H4 in a MOF-dependent manner (217). Since lysine-16-acetylated histone H4 is a hallmark of dosage-compensated chromatin, it is likely that MOF and the MSL complex carry out this acetylation function in vivo as well. It remains to be determined how the MSL complex specifically targets the X chromosome, but one theory is that it gains a foothold through the roX genetic loci, which are part of the X chromosome (122). Other aspects of dosage compensation (e.g., how the specific acetylation causes or is associated with increased levels of transcription) also require further analysis, but it is clear that the MSL complex and its HAT subunit are critical to the overall process.
Versions of the SAGA complex (and possibly NuA4) seem to be conserved throughout the eukaryotes; its existence also in Drosophila is implied by a recent coimmunoprecipitation of dTAFII24 and dGCN5 (78). The MSL complex, however, may be an example of a specialized HAT complex that is not widely conserved. While dosage compensation in Drosophila apparently involves HAT-mediated activation, it should be noted that other organisms employ different dosage compensation strategies and thus may not possess analogous HAT complexes. In the nematode Caenorhabditis elegans, hermaphrodites experience downregulation of both X chromosomes, and in female mammals, one of the two chromosomes is silenced (165). Instead of an MSL-like complex, these species may therefore use different types of regulatory complexes—which may or may not contain HATs—to control these processes.
FACTOR ACETYLTRANSFERASE (FAT) SUBSTRATES
This review thus far has focused on HATs, but it should be noted that some of these same enzymes are now also known to participate in transcriptional regulation by acetylating proteins other than histones. FAT activities have recently been demonstrated for PCAF, p300/CBP, and TAFII250, with transcription-related substrates ranging from activators and coactivators to basal factors and nonhistone chromosomal proteins. These are summarized in Table 2 and described below.
TABLE 2.
Summary of known FAT substratesa
| FAT substrate | Known function in vivo | Known FAT enzyme in vitro | Functional effect of acetylation | Observed or predicted effect on transcription |
|---|---|---|---|---|
| Nonhistone chromatin proteins | ||||
| HMG1 | Chromatin component | p300/CBP | ND | ND |
| HMG2 | Chromatin component | ND | ND | ND |
| Yeast Sin1 | Transcriptional regulator | Likely Gcn5 | ND | ND |
| HMG14 | Nucleosome binding | p300/CBP | Nucleosome binding weakened | ND |
| HMG17 | Nucleosome binding | PCAF | Nucleosome binding weakened | ND |
| HMG I(Y) | Enhanceosome component | PCAF | Enhanceosome assemblyb | Positiveb |
| p300/CBP | Enhanceosome disruption | Negative | ||
| Transcriptional activators | ||||
| p53 | Tumor suppressor | PCAF, p300/CBP | Increased DNA binding | Positive |
| c-Myb | Cell proliferation, differentiation | p300/CBP, GCN5 | Increased DNA binding | Positive |
| GATA-1 | Blood cell differentiation | p300/CBP | DNA binding may be affected | Positive |
| EKLF | Globin gene expression | p300/CBP | Inhibitory domain modified | Positive |
| MyoD | Muscle differentiation | PCAF | Increased DNA binding | Positive |
| E2F | Cell cycle control | PCAF | Increased DNA binding | Positive |
| dTCF | Developmental regulation | p300/CBP | Coactivator interaction disrupted | Negative |
| HIV Tat | HIV-1 transactivation | PCAF | Increased CDK9 binding | Positive |
| p300/CBP | Release from TAR RNA | Positive | ||
| Nuclear receptor coactivators | Transcriptional response to hormone signals | |||
| ACTR | p300/CBP | Receptor interaction disrupted | Negative | |
| SRC-1 | p300/CBP | ND | ND | |
| TIF2 | p300/CBP | ND | ND | |
| General transcription factors | General transcriptional machinery components | |||
| TFIIE | PCAF, p300/CBP, TAFII250 | ND | ND | |
| TFIIF | PCAF, p300/CBP | ND | ND | |
| Importin-α7, Rch1 | Nuclear import | p300/CBP | Increased importin-β binding | ND (may be unrelated to transcription) |
| α-Tubulin | Microtubule component | ND | ND; stabilized microtubules become acetylated | ND (may be unrelated to transcription) |
The FAT substrates characterized in vitro were human, mouse, or rat versions with the exception of yeast Sin1 and Drosophila TCF. The other substrates (HMG2 and α-tubulin) have unknown FAT enzymes but were observed to contain one or more acetylated internal lysines in various cell extracts. ND, not determined.
D. Thanos, personal communication.
Nonhistone Chromatin Proteins
Histones are by far the best-characterized acetylation substrates within chromatin, but most of the high-mobility-group (HMG) chromatin-associated proteins (reviewed in reference 33) are also known to be acetylated. These proteins participate in transcriptional enhanceosome complexes and higher-order chromatin structure, and recent evidence suggests that HMG acetylation is involved in the regulation of these functions.
HMG1, HMG2, and Sin1.
The acetylation of nonhistone protein factors was first described two decades ago, when it was observed that various HMG proteins isolated from vertebrate nuclei had lysine side chains that were postsynthetically acetylated (226). Two of these, HMG1 and HMG2, are related proteins that belong to one of three HMG families. The HMG1/2 proteins are distinguished by their size (about 23 kDa), two DNA-binding motifs (HMG boxes), and an acidic C-terminal region. A second study the following year found that HMG1 was acetylated in vivo at two sites (225). More recently, in vitro enzymatic experiments with partially purified nuclear HAT found that it could acetylate HMG1 and HMG2 in addition to histones (272), and recombinant CBP was able to acetylate rat HMG1 (9). The in vivo importance of these acetylation events and their effects on transcription remain to be studied.
Interestingly, Gcn5 is an apparent candidate for an analogous FAT role in S. cerevisiae. The yeast protein Sin1 (also known as Spt2), a negative (222) and possibly positive (212) regulator of transcription, has sequence similarity to the HMG1-like proteins of multicellular eukaryotes and is hypothesized to perform a similar function in chromatin (131). When recombinant Sin1 was tested in vitro with partially purified HAT activities from S. cerevisiae, Gcn5-dependent acetylation was observed (189). That this occurs in vivo has yet to be demonstrated, but it will be important to explore the functional significance of these observations and their correspondence to HMG1/2 modification.
HMG14 and HMG17.
Another early biochemical study demonstrated that the members of a second vertebrate HMG family, HMG14 and HMG17, were acetylated at two and three sites, respectively (224). Only recently has an enzymatic source of one of these acetylation events been determined. When tested in vitro, recombinant HMG17 but not HMG14 acted as an acetylation substrate for PCAF (101). PCAF only acetylated HMG17 at one specific site (lysine-2), as determined by mass spectrometry, and physiological relevance was suggested by the fact that this is the same site predominantly acetylated in vivo (224). HMG14 and HMG17 are known to participate in chromatin by binding specifically to nucleosomes via interaction with the N-terminal tails of their histones (51, 155, 205). Acetylation of HMG17 has now been demonstrated to cause reduced interaction with nucleosomes, suggesting a function for this modification (101). Its transcriptional effect, however, remains to be determined. HMG14 and HMG17 have a positive effect on transcription, as they have been shown to enhance transcription from chromatin templates by unfolding higher-order structure (34), so acetylation may regulate this process.
HMG I(Y).
Members of the vertebrate HMG I(Y) family of proteins, comprising HMG I and HMG Y (two isoforms produced from the same gene) and HMG I-C (encoded by a different but closely related gene), have long been known as a type of architectural component of chromosomes (33). In recent years, however, the transcriptional significance of HMG I(Y) has been realized with the finding that it is a component of enhanceosomes (reviewed in reference 39). These are nucleoprotein complexes in which multiple activators and other regulatory proteins interact synergistically at enhancer sequences to bring about activation of specific genes. Perhaps the best characterized of these is formed at the virus-inducible human beta interferon (IFN-β) gene, where two molecules of HMG I(Y) bind enhancer DNA and alter its structure, allowing the recruitment of certain transcription factors (66, 237). This enhanceosome is also known to interact with p300/CBP (167).
Because of the acetylase activities of p300/CBP and its associated PCAF, various IFN-β enhanceosome components were tested in in vitro acetylation assays. Of these factors, only HMG I(Y) was found to be specifically acetylated by both p300/CBP and PCAF (as was HMG I-C, which normally is expressed only in embryonic development but is aberrantly expressed in some cancers) (171). Interestingly, acetylation by p300/CBP, but not PCAF, had negative functional effects in vitro, disrupting the enhanceosome by decreasing HMG I(Y)'s DNA and transcription factor interactions. HMG I(Y) acetylation by p300/CBP was also required for proper IFN-β gene expression in vivo, for both activation and shutoff, while the acetylation function of PCAF was required for full activation but not shutoff.
Transcriptional Activators
Transcriptional activators can be generally defined as proteins that bind to specific sites on promoter DNA and bring about increased transcription of specific genes through interactions with other proteins. As such, they typically contain (i) a DNA-binding domain and (ii) one or more activation domains, which may contact the transcriptional machinery directly or through coactivators and thereby influence transcriptional activity. Acetylation has recently been shown to affect the functions of these domains, either positively or negatively, in a number of activators involved in various cellular and developmental processes.
p53.
In higher eukaryotes, p53 is a tumor suppressor that responds to DNA damage by acting as a transcriptional activator of certain cell death-related genes (83, 128, 140). Because of its direct role in such processes as cell cycle arrest and apoptosis, p53's activity as a transcription factor is tightly regulated (79). The various regulatory and functional domains of the 393-residue human p53 protein include N-terminal activation domains, a central DNA-binding domain, and C-terminal tetramerization and regulatory domains. Posttranslational modifications of the C-terminal regulatory regions are one important mode of p53 regulation (163). For example, phosphorylation results in p53 that is competent for DNA binding (111).
Recently, acetylation of p53's C-terminal regulatory domains by both p300/CBP and PCAF has also been demonstrated to be critical for its regulation. p53 interacts directly with p300/CBP (93, 145, 209) and PCAF (148), and in vitro, two lysines (residues 373 and 382) are acetylated specifically by p300/CBP (92), and one (residue 320) is acetylated specifically by PCAF (147, 202). In vitro, these acetylation events were shown to dramatically increase the sequence-specific DNA-binding activity of p53 (92, 148, 202). The acetylated forms of p53 were also observed in vivo, and interestingly, the levels of acetylation increased under DNA-damaging conditions (UV light or ionizing radiation) (147, 202). These results suggest that by acetylation of specific residues, p300/CBP and PCAF positively regulate the activity of p53 as part of the pathway of DNA damage response.
c-Myb.
Like p53, the vertebrate protooncogene product c-Myb is an activator whose DNA-binding function, and hence transcriptional activity, is controlled by acetylation. While not a tumor suppressor like p53, c-Myb's natural function is to regulate the proliferation and differentiation of certain types of cells, and improper regulation of or by this protein is associated with oncogenic transformation (238, 263). Recently, human c-Myb was found to act as an acetylation substrate for p300 in vitro and in vivo (239). This acetylation correlated with increased DNA binding of c-Myb in vitro, and in transfection experiments p300 caused elevated c-Myb-dependent transcriptional activation from two promoters in vivo. These results suggest that the transcriptional potential of c-Myb is positively regulated by acetylation of domains affecting DNA binding. Relevant to this, point mutations identified c-Myb's C-terminal negative regulatory domain as containing a subset of the target lysines that may be involved in this regulation. Finally, human GCN5 was also able to acetylate c-Myb in vitro, suggesting that alternate or multiple FAT enzymes may act on c-Myb in the context of the cell (239).
GATA-1.
Another acetylation-regulated transcriptional activator important to particular cellular processes of higher eukaryotes is GATA-1, previously known as Eryf1 (65), NF-E1 (253), and GF-1 (156). This transcription factor has a crucial role in the differentiation of certain blood cells, and it binds DNA and interacts with other factors, such as p300/CBP, through a central zinc finger domain. p300 and CBP have recently been shown to acetylate GATA-1 in vitro at two lysine-rich motifs within this central region (24, 110). Furthermore, this acetylation is seen in vivo and serves to increase GATA-1-dependent transcription mediated by p300/CBP, although there are conflicting results as to whether this is caused by augmentation of DNA binding (24, 110).
EKLF.
EKLF is another vertebrate blood cell-specific transcription factor recently shown to be acetylated by p300/CBP as part of its regulation. An activator specific to red blood cells (168), EKLF is involved in the developmental switching between embryonic/fetal and adult globin expression (56). In vivo, EKLF can exist in an acetylated form and has been demonstrated to interact with PCAF as well as with p300 and CBP, but in vitro experiments have shown that it acts as an acetylation substrate only for p300/CBP (283). The acetylatable region was mapped to EKLF's inhibitory domain, shown previously to interfere with the function of the nearby DNA-binding domain (44). Furthermore, unlike PCAF, p300/CBP specifically stimulated transcriptional activation by EKLF at a reporter gene in vivo; this effect was apparently acetylation dependent, since the presence of the deacetylase inhibitor trichostatin A significantly increased the activation (283). The precise mechanism for this activation is not yet known, but besides a potential FAT-mediated increase in DNA binding by EKLF, it may also involve histone acetylation, since the presence of EKLF had an impact on chromatin configuration at a globin locus (266).
MyoD.
MyoD is another tissue-specific, p300/CBP-associated activator regulated by acetylation, but unlike GATA-1 and EKLF, it is acetylated by PCAF instead of p300/CBP. In muscle cell differentiation (myogenesis), MyoD functions to bind regulatory motifs of promoter DNA and stimulate the transcription of cyclin-dependent kinase inhibitor p21 (94, 95) and muscle-specific genes (259). MyoD was shown to interact with both p300/CBP and PCAF and require them for its activation functions (191, 192), but interestingly, it only required the HAT activity of PCAF, suggesting differential roles for these coactivators (192). Recently, the mode of action of PCAF has been found to involve its direct acetylation of MyoD at three evolutionarily conserved lysine residues (206). This acetylation was observed in vivo and in vitro, and its significance was indicated by the fact that mutation of these residues to nonacetylatable arginines resulted in lack of in vivo transcriptional activation and myogenesis. Proper acetylation, however, appeared to increase MyoD's specific DNA-binding ability in vitro, suggesting a mechanism for the positive effect of PCAF.
E2F.
The function of cell cycle-related activator E2F is regulated by acetylation in a manner similar to that observed for MyoD. E2F, typically a heterodimer of an E2F family member and the DP1 protein (138), is known to regulate S-phase-specific genes and thus be required for cell cycle progression (reviewed in reference 1). Interestingly, one of the E2F family members, E2F1, was recently demonstrated to be acetylated by PCAF, and this acetylation has several positive effects on E2F function (159). These modifications were found to occur at three specific lysine residues near the DNA-binding domain of E2F1, and they have the effect of increasing specific DNA binding in vitro and stimulating E2F-mediated transcription in vivo. Furthermore, acetylation seems to stabilize the E2F1 protein and increase its half-life, likely also contributing to the in vivo effectiveness of E2F as an activator (159). However, it should be noted that E2F can also act as a transcriptional repressor when complexed with the Rb protein (260), and a further experiment demonstrated that this interaction may also negatively regulate the FAT modification of E2F1. Specifically, the HDAC associated with Rb was able to deacetylate E2F1 (159), demonstrating that HDACs and HATs may act antagonistically on their nonhistone substrates, as they do on histones. Finally, the regulatory effect of PCAF may occur with other forms of E2F as well, since the three acetylatable lysine residues are conserved in two other E2F family proteins, E2F2 and E2F3, but this remains to be demonstrated.
dTCF.
For the transcription factor substrates described above, acetylation has the net effect of enhancing transcriptional activation. However, an example of a FAT activity participating in transcriptional repression has been demonstrated in the relationship between TCF and CBP in Drosophila. As a result of developmental signaling, Drosophila TCF (dTCF) normally interacts with its coactivator Armadillo (the ortholog of vertebrate β-catenin), binds DNA at certain enhancer elements, and activates Wnt/Wingless-specific genes (reviewed in references 49 and 61). Interestingly, genetic interactions between CBP and this pathway were recently demonstrated, and moreover, CBP was shown to interact physically with TCF (255). Furthermore, CBP was found to acetylate TCF at lysine-25 within its Armadillo-binding domain, thereby weakening the TCF-Armadillo interaction. The physiological significance of this effect was suggested by a large in vivo increase in TCF-responsive gene expression in a nonacetylatable (Lys-25-Ala) TCF mutant (255). Overall, this study suggests that CBP negatively regulates TCF-mediated transcription by disrupting the activator-coactivator interaction through acetylation. While these experiments were performed with Drosophila proteins and embryos, analogous regulation may occur in more complex eukaryotes with regard to the TCF/β-catenin interaction; future studies should reveal whether this particular FAT function is evolutionarily conserved.
HIV Tat.
Tat, a transcriptional activator encoded by HIV-1, is another protein whose activation-related functions are affected by acetylation. Unlike conventional activators, this vital protein acts by binding to a region of leader RNA (called TAR) instead of to DNA (18, 54). Tat is known to interact with the human MYST protein Tip60, as mentioned above, but recent studies have shown that it interacts with three other HATs as well: TAFII250, p300/CBP, and PCAF (16, 108, 160, 261). Interestingly, the latter two HATs have recently been demonstrated to acetylate Tat at specific, functionally distinct sites (124): PCAF acetylated lysine-28 in the activation domain, whereas p300 acetylated lysine-50 in the TAR RNA-binding domain. In vivo transcription with nonacetylatable alanine substitution mutants and in vitro interaction studies then demonstrated the specific roles of each of these FAT events in Tat-mediated transcription. PCAF-acetylated Tat was found to have an increased affinity (124) for the CDK9/P-TEFb CTD kinase complex (118, 258, 284), suggesting that this acetylation event enhances transcriptional elongation by bringing about hyperphosphorylation of the RNA polymerase II CTD. Acetylation by p300, however, was discovered to decrease the affinity between Tat and the TAR RNA, implicating this modification in the cycled release of Tat from TAR, another important step that apparently allows elongation to proceed (124). Therefore, two acetyltransferases play critical roles in two different steps that promote elongation of Tat-specific transcripts, indicating that HIV-1 has taken advantage of the unique FAT specificities of PCAF and p300 for the regulation of its own gene expression.
Nuclear Receptor Coactivators ACTR, SRC-1, and TIF2
As described earlier, nuclear receptor coactivator ACTR is a HAT that interacts with both p300/CBP and PCAF and activates transcription in response to hormone signals. Remarkably, acetylation of ACTR by p300/CBP has recently been demonstrated to regulate ACTR's activation potential—the first example of one acetylase regulating another by FAT activity (43). By truncation and in vitro acetylation studies, the site of acetylation was identified as the central receptor interaction domain. Amino acid substitution experiments and peptide analysis implicated several lysine residues, all adjacent to an LXXLL motif (100), shown previously to be structurally crucial in the interaction of other proteins with hormone-bound receptors (52). Further experiments showed that acetylation by p300 prevented ACTR from binding receptors, apparently by disturbing key interaction surfaces, ultimately leading to loss of ACTR's activation function (43).
The two other members of the p160 family of nuclear receptor coactivators, SRC-1 and TIF2, can also be efficiently acetylated by p300/CBP in vitro (43). Although the consequences of these acetylation events have not yet been studied, the structural and functional parallels of the three coactivator proteins suggest that they may have a similar mode of regulation. Future investigation should resolve this question.
General Transcription Factors TFIIE and TFIIF
Most of the known targets of FAT activities are activators or coactivators of specific sets of genes, but one study has reported that certain general transcription factors can be acetylated as well. When a number of recombinant human general factors (TFIIA, TFIIB, TBP, TFIIE, and TFIIF) were tested in in vitro assays with PCAF, p300/CBP, and TAFII250, it was found that certain components of TFIIE and TFIIF could be acetylated (116). Specifically, the RAP74 and RAP30 subunits of TFIIF were acetylated by PCAF and p300/CBP, and the β subunit of TFIIE was acetylated by these enzymes as well as by TAFII250. While these basal factor acetylation events are intriguing, their functions remain to be demonstrated. In vitro transcription studies so far have found no significant effect of TFIIE or TFIIF acetylation on transcription at various promoters (75, 116). Future in vivo studies will likely be required to determine if these factor acetylation events occur physiologically and what, if any, regulatory effects they may have on transcription.
Self-Acetylation and Transcription-Unrelated Substrates
Two other issues that relate to FAT activity but about which relatively little is known are self-acetylation by HATs/FATs and the acetylation of apparently transcription-unrelated substrates, such as α-tubulin and nuclear import factors. Several HATs have been observed to self-acetylate in vitro, including PCAF, p300, Tip60, MORF, Hpa2, and Hpa3. However, it is unknown whether these events have physiological relevance as self-regulation or whether they are merely in vitro artifacts. Further examination of the functional effects of these self-modifications and their occurrence in vivo will be required to resolve this question for each acetylase.
The substrates apparently unrelated to transcription include the microtubule component α-tubulin, known for years to be acetylated in vivo, and two human nuclear import factors, recently demonstrated to be acetylated in vitro by CBP (9). In the case of α-tubulin, lysine-40-acetylated protein has been identified in various organisms (141, 188). This acetylation is known to occur on stabilized microtubules (208), although its actual functional effect is unclear. Since these acetylated microtubules are cytoplasmic, a direct connection to transcription seems unlikely. Very recently, an in vitro screen for potential acetylation substrates of CBP identified Rch1 and importin-α7 (9), two nuclear import factors that function by binding both to nuclear localization signals on various proteins and to importin-β, which mediates import. For Rch1, acetylation was found to have the effect of enhancing the Rch1/importin-β interaction. This result presents the possibility that this modification could promote nuclear import and is another potential example of acetylation regulating cellular processes other than transcription.
Finally, for all of the FAT substrates described above, it should be noted that acetylation is only one type of modification important for the regulation of some these various factors. For example, p53 is also regulated by phosphorylation, and HMG I(Y) is known to receive various types of modifications. Future studies on the functions of FAT substrates must ultimately address the effects of all relevant modifications in order to provide a complete picture of these proteins' regulation.
CONCLUSIONS AND PERSPECTIVES
As demonstrated by the preceding descriptions of nuclear HATs, their in vivo and in vitro functions, and their transcription-related substrates, acetylation is intimately involved with transcriptional regulation on many levels. For many years, it had been known that there was a correlation between histone-acetylated chromatin and activated transcription; HAT and FAT activities were also recognized and partially purified from various organisms in the last several decades. However, only within the last 5 years have HAT catalytic proteins been identified at a molecular level. The revolutionary finding that a transcriptional adaptor protein, Gcn5, was actually a nuclear HAT—followed quickly by similar discoveries with the well-known coactivator p300/CBP, TFIID subunit TAFII250, and other coactivators—established histone acetylation as an apparently ubiquitous mechanism in transcription. This has led to an explosion in HAT-related research, and the lists of HAT proteins, complexes, activities, and substrates continue to grow rapidly.
In many ways, recent advances in molecular biology methods, technology, and informatics have been and will be responsible for the identification and functional characterization of transcriptionally important acetyltransferases. For example, development of the in-gel HAT assay (29) led to the discovery of Tetrahymena Gcn5, and the now routine use of protein microsequencing made possible the identification of this and various other HATs. Genome determination and the proliferation of sequence databases are another factor in the discovery of HATs; for example, yeast Esa1 and human PCAF and MORF were originally noticed as database sequences with HAT homology. The imminent completion of human genome sequencing should lead to the identification of additional human HATs. It should be noted that some HATs (e.g., TAFII250 and nuclear receptor coactivators) have no recognizable homology to known acetyltransferase motifs and will not be discovered in this way, but the recognition of histone acetylation as a major regulatory mechanism has led to the now widespread use of HAT assays in the characterization of transcription-related proteins, resulting in perhaps unanticipated findings of certain HAT activities (e.g., TFIIIC). This trend is expected to continue in the future.
As the body of information about transcriptional regulation grows and cellular processes previously considered distinct are found to be intricately linked, HAT functional studies are benefiting from multiple, unified scientific approaches. Biochemical and molecular biology techniques are being used to purify HAT complexes and characterize thoroughly their activities and subunits, genetics are providing information about in vivo function, and structural studies, such as the recent GNAT determinations, are giving insights into mechanisms and interactions. These types of investigations will continue, and two recently developed techniques in particular also show promise for future functional determinations of identified HATs and other proteins. Chromatin immunoprecipitation (ChIP) is a way in which chromatin can be retrieved from cells and analyzed for acetylation state or transcriptional proteins at specific genes, providing a wealth of information about in vivo HAT functions and complexes. For a wider functional view, whole-genome analyses with oligonucleotide microarrays have been and will be used to analyze cells' RNA and assess the impact of HAT mutations on the expression of all genes for a given organism or cell type. This has already been performed for Gcn5 and other interesting transcriptional proteins in S. cerevisiae (105). In the future, such expression studies will likely be carried out with other HATs in S. cerevisiae and also in more complex eukaryotes as the technology and genomic data advance. Thus, in complementary ways, chromatin immunoprecipitation assays and microarrays may provide detailed data about HAT action and other transcriptional regulation under various conditions.
In addition, it should be remembered that histones and regulatory proteins receive a variety of functionally important covalent modifications in vivo, not just acetylation (227). Although this review has focused rather narrowly on chromatin- and transcription-related acetylation, future studies must increasingly address the interplay of multiple modifications with one another, with other activities such as ATP-dependent chromatin remodeling, and with the mechanisms that reverse or antagonize these processes (e.g., deacetylation and chromatin assembly). Recent investigations have established that there are close relationships among all of these functions, but the nature of these must be better defined by future research. Another relevant issue is that the state of chromatin also influences other significant nuclear processes besides transcription, such as DNA replication, recombination, and repair. For example, a recent study revealed that V(D)J recombination of antibody genes is tightly correlated with histone H3 acetylation (162); the connection between such processes and various modes of chromatin alteration will require further investigation. Finally, a largely unexplored frontier in this field is the topic of higher-order chromatin structure, whose effects on transcription must be addressed along with those of nucleosomes in future studies, contributing to the eventual goal of a detailed, overall understanding of the regulation of gene expression in the eukaryotic cell.
ACKNOWLEDGMENTS
We thank Y. Nakatani, T. Kouzarides, D. Thanos, S. John, and J. L. Workman for sharing unpublished results; J. R. Rojas, R. C. Trievel, and R. Marmorstein for providing the Tetrahymena Gcn5 structural figure; and B. Stillman, L. Pillus, and C. D. Allis for helpful discussions.
Research support to S.L.B. is provided by the National Institutes for Health/General Medicinal Sciences and National Cancer Institute, the National Science Foundation, and the American Cancer Society, and D.E.S. is supported by an NRSA fellowship from the National Institutes of Health.
ADDENDUM IN PROOF
The in vitro HAT activity of recombinant full-length MOF has recently been demonstrated by Akhtar and Becker (Mol. Cell 5:367–375, 2000). Also, a study by Bergel et al. (J. Biol. Chem. 257:11514–11520, 2000) has shown that p300 acetylates HMG14 and weakens its interaction with nucleosomes, as observed for PCAF and HMG17. In addition, Marzio et al. (J. Biol. Chem. 275:10887–10892, 2000) have demonstrated that E2F2 and E2F3, in addition to E2F1, are acetylated by p300/CBP. Finally, Suzuki et al. (Genes Cells 5:29–41, 2000) have shown that p300 can acetylate Sp1, an activator with which it interacts.
REFERENCES
- 1.Adams P D, Kaelin W G J. Transcriptional control by E2F. Semin Cancer Biol. 1995;6:99–108. doi: 10.1006/scbi.1995.0013. [DOI] [PubMed] [Google Scholar]
- 2.Ait-Si-Ali S, Ramirez S, Barre F X, Dkhissi F, Magnaghi-Jaulin L, Girault J A, Robin P, Knibiehler M, Pritchard L L, Ducommun B, Trouche D, Harel-Bellan A. Histone acetyltransferase activity of CBP is controlled by cycle-dependent kinases and oncoprotein E1A. Nature. 1998;396:184–186. doi: 10.1038/24190. [DOI] [PubMed] [Google Scholar]
- 3.Allard S, Utley R T, Savard J, Clarke A, Grant P, Brandl C J, Pillus L, Workman J L, Côté J. NuA4, an essential transcription adaptor/histone H4 acetyltransferase complex containing Esa1p and the ATM-related cofactor Tra1p. EMBO J. 1999;18:5108–5119. doi: 10.1093/emboj/18.18.5108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allis C D, Chicoine L G, Richman R, Schulman I G. Deposition-related histone acetylation in micronuclei of conjugating Tetrahymena. Proc Natl Acad Sci USA. 1985;82:8048–8052. doi: 10.1073/pnas.82.23.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Angus-Hill M L, Dutnall R N, Tafrov S T, Sternglanz R, Ramakrishnan V. Crystal structure of the histone acetyltransferase Hpa2: a tetrameric member of the Gcn5-related N-acetyltransferase superfamily. J Mol Biol. 1999;294:1311–1325. doi: 10.1006/jmbi.1999.3338. [DOI] [PubMed] [Google Scholar]
- 6.Anzick S L, Kononen J, Walker R L, Azorsa D O, Tanner M M, Guan X Y, Sauter G, Kallioniemi O P, Trent J M, Meltzer P S. AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science. 1997;277:965–968. doi: 10.1126/science.277.5328.965. [DOI] [PubMed] [Google Scholar]
- 7.Aparicio O M, Billington B L, Gottschling D E. Modifiers of position effect are shared between telomeric and silent mating-type loci in S. cerevisiae. Cell. 1991;66:1279–1287. doi: 10.1016/0092-8674(91)90049-5. [DOI] [PubMed] [Google Scholar]
- 8.Bannister A J, Kouzarides T. The CBP co-activator is a histone acetyltransferase. Nature. 1996;384:641–643. doi: 10.1038/384641a0. [DOI] [PubMed] [Google Scholar]
- 9.Bannister A J, Miska E A, Görlich D, Kouzarides T. Acetylation of importin-α nuclear import factors by CBP/p300. Curr Biol. 2000;10:467–470. doi: 10.1016/s0960-9822(00)00445-0. [DOI] [PubMed] [Google Scholar]
- 10.Barlev N A, Candau R, Wang L, Darpino P, Silverman N, Berger S L. Characterization of physical interactions of the putative transcriptional adaptor, ADA2, with acidic activation domains and TATA-binding protein. J Biol Chem. 1995;270:19337–19344. doi: 10.1074/jbc.270.33.19337. [DOI] [PubMed] [Google Scholar]
- 11.Barlev N A, Poltoratsky V, Owen-Hughes T, Ying C, Liu L, Carter T, Workman J L, Berger S L. Repression of GCN5 histone acetyltransferase activity via bromodomain-mediated binding and phosphorylation by the Ku/DNA-PKcs complex. Mol Cell Biol. 1998;18:1349–1358. doi: 10.1128/mcb.18.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bell S P, Kobayashi R, Stillman B. Yeast origin recognition complex functions in transcription silencing and DNA replication. Science. 1993;262:1844–1849. doi: 10.1126/science.8266072. [DOI] [PubMed] [Google Scholar]
- 13.Bell S P, Stillman B. ATP-dependent recognition of eukaryotic origins of DNA replication by a multiprotein complex. Nature. 1992;357:128–134. doi: 10.1038/357128a0. [DOI] [PubMed] [Google Scholar]
- 14.Belotserkovskaya R, Berger S L. Interplay between chromatin modifying and remodeling complexes in transcriptional regulation. Crit Rev Eukaryot Gene Expression. 1999;9:221–230. doi: 10.1615/critreveukargeneexpr.v9.i3-4.70. [DOI] [PubMed] [Google Scholar]
- 15.Belotserkovskaya R, Sterner D E, Deng M, Sayre M H, Lieberman P M, Berger S L. Inhibition of TATA-binding protein function by SAGA subunits Spt3 and Spt8 at Gcn4-activated promoters. Mol Cell Biol. 2000;20:634–647. doi: 10.1128/mcb.20.2.634-647.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benkirane M, Chun R F, Xiao H, Ogryzko V V, Howard B H, Nakatani Y, Jeang K T. Activation of integrated provirus requires histone acetyltransferase. p300 and P/CAF are coactivators for HIV-1 Tat. J Biol Chem. 1998;273:24898–24905. doi: 10.1074/jbc.273.38.24898. [DOI] [PubMed] [Google Scholar]
- 17.Berger S L, Piña B, Silverman N, Marcus G A, Agapite J, Regier J L, Triezenberg S J, Guarente L. Genetic isolation of ADA2: a potential transcriptional adaptor required for function of certain acidic activation domains. Cell. 1992;70:251–265. doi: 10.1016/0092-8674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- 18.Berkhout B, Silverman R H, Jeang K T. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell. 1989;59:273–282. doi: 10.1016/0092-8674(89)90289-4. [DOI] [PubMed] [Google Scholar]
- 19.Biggar S R, Crabtree G R. Continuous and widespread roles for the Swi-Snf complex in transcription. EMBO J. 1999;18:2254–2264. doi: 10.1093/emboj/18.8.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birck C, Poch O, Romier C, Ruff M, Mengus G, Lavigne A-C, Davidson I, Moras D. Human TAFII28 and TAFII18 interact through a histone fold encoded by atypical evolutionary conserved motifs also found in the SPT3 family. Cell. 1998;94:239–249. doi: 10.1016/s0092-8674(00)81423-3. [DOI] [PubMed] [Google Scholar]
- 21.Blanco J C, Minucci S, Lu J, Yang X J, Walker K K, Chen H, Evans R M, Nakatani Y, Ozato K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998;12:1638–1651. doi: 10.1101/gad.12.11.1638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bone J R, Lavender J, Richman R, Palmer M J, Turner B M, Kuroda M I. Acetylated histone H4 on the male X chromosome is associated with dosage compensation in Drosophila. Genes Dev. 1994;8:96–104. doi: 10.1101/gad.8.1.96. [DOI] [PubMed] [Google Scholar]
- 23.Borrow J, Stanton V P, Jr, Andresen J M, Becher R, Behm F G, Chaganti R S, Civin C I, Disteche C, Dube I, Frischauf A M, Horsman D, Mitelman F, Volinia S, Watmore A E, Housman D E. The translocation t(8;16)(p11;p13) of acute myeloid leukaemia fuses a putative acetyltransferase to the CREB-binding protein. Nat Genet. 1996;14:33–41. doi: 10.1038/ng0996-33. [DOI] [PubMed] [Google Scholar]
- 24.Boyes J, Byfield P, Nakatani Y, Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 25.Bradbury E M. Reversible histone modifications and the chromosome cell cycle. Bioessays. 1992;14:9–16. doi: 10.1002/bies.950140103. [DOI] [PubMed] [Google Scholar]
- 26.Brady M E, Ozanne D M, Gaughan L, Waite I, Cook S, Neal D E, Robson C N. Tip60 is a nuclear hormone receptor coactivator. J Biol Chem. 1999;274:17599–17604. doi: 10.1074/jbc.274.25.17599. [DOI] [PubMed] [Google Scholar]
- 27.Brand M, Yamamoto K, Staub A, Tora L. Identification of TATA-binding protein-free TAFII-containing complex subunits suggests a role in nucleosome acetylation and signal transduction. J Biol Chem. 1999;274:18285–18289. doi: 10.1074/jbc.274.26.18285. [DOI] [PubMed] [Google Scholar]
- 28.Braunstein M, Rose A B, Holmes S G, Allis C D, Broach J R. Transcriptional silencing in yeast is associated with reduced nucleosome acetylation. Genes Dev. 1993;7:592–604. doi: 10.1101/gad.7.4.592. [DOI] [PubMed] [Google Scholar]
- 29.Brownell J E, Allis C D. An activity gel assay detects a single, catalytically active histone acetyltransferase subunit in Tetrahymena macronuclei. Proc Natl Acad Sci USA. 1995;92:6364–6368. doi: 10.1073/pnas.92.14.6364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brownell J E, Allis C D. Special HATs for special occasions: linking histone acetylation to chromatin assembly and gene activation. Curr Opin Genet Dev. 1996;6:176–184. doi: 10.1016/s0959-437x(96)80048-7. [DOI] [PubMed] [Google Scholar]
- 31.Brownell J E, Zhou J, Ranalli T, Kobayashi R, Edmondson D G, Roth S Y, Allis C D. Tetrahymena histone acetyltransferase A: a transcriptional co-activator linking gene expression to histone acetylation. Cell. 1996;84:843–851. doi: 10.1016/s0092-8674(00)81063-6. [DOI] [PubMed] [Google Scholar]
- 32.Burley S K, Roeder R G. Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem. 1996;65:769–799. doi: 10.1146/annurev.bi.65.070196.004005. [DOI] [PubMed] [Google Scholar]
- 33.Bustin M, Reeves R. High-mobility-group chromosomal proteins: architectural components that facilitate chromatin function. Prog Nucleic Acids Res Mol Biol. 1996;54:35–100. doi: 10.1016/s0079-6603(08)60360-8. [DOI] [PubMed] [Google Scholar]
- 34.Bustin M, Trieschmann L, Postnikov Y V. The HMG-14/-17 chromosomal protein family: architectural elements that enhance transcription from chromatin templates. Semin Cell Biol. 1995;6:247–255. doi: 10.1006/scel.1995.0033. [DOI] [PubMed] [Google Scholar]
- 35.Candau R, Berger S L. Structural and functional analysis of yeast putative adaptors: evidence for an adaptor complex in vivo. J Biol Chem. 1996;271:5237–5345. doi: 10.1074/jbc.271.9.5237. [DOI] [PubMed] [Google Scholar]
- 36.Candau R, Moore P A, Wang L, Barlev N, Ying C Y, Rosen C A, Berger S L. Identification of human proteins functionally conserved with the yeast putative adaptors ADA2 and GCN5. Mol Cell Biol. 1996;16:593–602. doi: 10.1128/mcb.16.2.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Candau R, Zhou J, Allis C D, Berger S L. Histone acetyltransferase activity and interaction with ADA2 are critical for GCN5 function in vivo. EMBO J. 1997;16:555–565. doi: 10.1093/emboj/16.3.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carapeti M, Aguiar R C, Goldman J M, Cross N C. A novel fusion between MOZ and the nuclear receptor coactivator TIF2 in acute myeloid leukemia. Blood. 1998;91:3127–3133. [PubMed] [Google Scholar]
- 39.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 40.Chakravarti D, Ogryzko V, Kao H Y, Nash A, Chen H, Nakatani Y, Evans R M. A viral mechanism for inhibition of p300 and PCAF acetyltransferase activity. Cell. 1999;96:393–403. doi: 10.1016/s0092-8674(00)80552-8. [DOI] [PubMed] [Google Scholar]
- 41.Champagne N, Bertos N R, Pelletier N, Wang A H, Vezmar M, Yang Y, Heng H H, Yang X J. Identification of a human histone acetyltransferase related to monocytic leukemia zinc finger protein. J Biol Chem. 1999;274:28528–28536. doi: 10.1074/jbc.274.40.28528. [DOI] [PubMed] [Google Scholar]
- 42.Chen H, Lin R J, Schiltz R L, Chakravarti D, Nash A, Nagy L, Privalsky M L, Nakatani Y, Evans R M. Nuclear receptor coactivator ACTR is a novel histone acetyltransferase and forms a multimeric activation complex with PCAF and CBP/p300. Cell. 1997;90:569–580. doi: 10.1016/s0092-8674(00)80516-4. [DOI] [PubMed] [Google Scholar]
- 43.Chen H, Lin R J, Xie W, Wilpitz D, Evans R M. Regulation of hormone-induced histone hyperacetylation and gene activation via acetylation of acetylase. Cell. 1999;98:675–686. doi: 10.1016/s0092-8674(00)80054-9. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Bieker J J. Erythroid Krüppel-like factor (EKLF) contains a multifunctional transcriptional activation domain important for inter- and intramolecular interactions. EMBO J. 1996;15:5888–5896. [PMC free article] [PubMed] [Google Scholar]
- 45.Chicoine L G, Schulman I G, Richman R, Cook R G, Allis C D. Nonrandom utilization of acetylation sites in histones isolated from Tetrahymena: evidence for functionally distinct H4 acetylation sites. J Biol Chem. 1986;261:1071–1076. [PubMed] [Google Scholar]
- 46.Cho H, Orphanides G, Sun X, Yang X J, Ogryzko V, Lees E, Nakatani Y, Reinberg D. A human RNA polymerase II complex containing factors that modify chromatin structure. Mol Cell Biol. 1998;18:5355–5363. doi: 10.1128/mcb.18.9.5355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clarke A S, Lowell J E, Jacobson S J, Pillus L. Esa1p is an essential histone acetyltransferase required for cell cycle progression. Mol Cell Biol. 1999;19:2515–2526. doi: 10.1128/mcb.19.4.2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clements A, Rojas J R, Trievel R C, Wang L, Berger S L, Marmorstein R. Crystal structure of the histone acetyltransferase domain of the human PCAF transcriptional regulator bound to coenzyme A. EMBO J. 1999;18:3521–3532. doi: 10.1093/emboj/18.13.3521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Clevers H, van de Wetering M. TCF/LEF factor earn their wings. Trends Genet. 1997;13:485–489. doi: 10.1016/s0168-9525(97)01305-x. [DOI] [PubMed] [Google Scholar]
- 50.Creaven M, Hans F, Mutskov V, Col E, Caron C, Dimitrov S, Khochbin S. Control of the histone-acetyltransferase activity of Tip60 by the HIV-1 transactivator protein. Tat Biochemistry. 1999;38:8826–8830. doi: 10.1021/bi9907274. [DOI] [PubMed] [Google Scholar]
- 51.Crippa M P, Alfonso P J, Bustin M. Nucleosome core binding region of chromosomal protein HMG-17 acts as an independent functional domain. J Mol Biol. 1992;228:442–449. doi: 10.1016/0022-2836(92)90833-6. [DOI] [PubMed] [Google Scholar]
- 52.Darimont B D, Wagner R L, Apriletti J W, Stallcup M R, Kushner P J, Baxter J D, Fletterick R J, Yamamoto K R. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 1998;12:3343–3356. doi: 10.1101/gad.12.21.3343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dhalluin C, Carlson J E, Zeng L, He C, Aggarwal A K, Zhou M M. Structure and ligand of a histone acetyltransferase bromodomain. Nature. 1999;399:491–496. doi: 10.1038/20974. [DOI] [PubMed] [Google Scholar]
- 54.Dingwall C, Ernberg I, Gait M J, Green S M, Heaphy S, Karn J, Lowe A D, Singh M, Skinner M A, Valerio R. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci USA. 1989;86:6925–6929. doi: 10.1073/pnas.86.18.6925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dollard C, Ricupero-Hovasse S L, Natsoulis G, Boeke J D, Winston F. SPT10 and SPT21 are required for transcription of particular histone genes in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:5223–5228. doi: 10.1128/mcb.14.8.5223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Donze D, Townes T M, Bieker J J. Role of erythroid Krüppel-like factor in human gamma- to beta-globin gene switching. J Biol Chem. 1995;270:1955–1959. doi: 10.1074/jbc.270.4.1955. [DOI] [PubMed] [Google Scholar]
- 57.Dudley A M, Rougeulle C, Winston F. The Spt components of SAGA facilitate TBP binding to a promoter at a post-activator-binding step in vivo. Genes Dev. 1999;13:2940–2945. doi: 10.1101/gad.13.22.2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dunphy E L, Johnson T, Auerbach S S, Wang E H. Requirement for TAFII250 acetyltransferase activity in cell cycle progression. Mol Cell Biol. 2000;20:1134–1139. doi: 10.1128/mcb.20.4.1134-1139.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dutnall R N, Tafrov S T, Sternglanz R, Ramakrishnan V. Structure of the histone acetyltransferase Hat1: a paradigm for the GCN5-related N-acetyltransferase superfamily. Cell. 1998;94:427–438. doi: 10.1016/s0092-8674(00)81584-6. [DOI] [PubMed] [Google Scholar]
- 60.Dutta A, Bell S P. Initiation of DNA replication in eukaryotic cells. Annu Rev Cell Dev Biol. 1997;13:293–332. doi: 10.1146/annurev.cellbio.13.1.293. [DOI] [PubMed] [Google Scholar]
- 61.Eastman Q, Grosschedl R. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr Opin Cell Biol. 1999;11:233–240. doi: 10.1016/s0955-0674(99)80031-3. [DOI] [PubMed] [Google Scholar]
- 62.Eberharter A, John S, Grant P A, Utley R T, Workman J L. Identification and analysis of yeast nucleosomal histone acetyltransferase complexes. Methods (Orlando) 1998;15:315–321. doi: 10.1006/meth.1998.0635. [DOI] [PubMed] [Google Scholar]
- 63.Eberharter A, Sterner D E, Schieltz D, Hassan A, Yates J R 3, Berger S L, Workman J L. The ADA complex is a distinct histone acetyltransferase complex in Saccharomyces cerevisiae. Mol Cell Biol. 1999;19:6621–6631. doi: 10.1128/mcb.19.10.6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ehrenhofer-Murray A E, Rivier D H, Rine J. The role of Sas2, an acetyltransferase homologue of Saccharomyces cerevisiae, in silencing and ORC function. Genetics. 1997;145:923–934. doi: 10.1093/genetics/145.4.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64a.Eisenmann D M, Arndt K M, Ricupero S L, Rooney J W, Winston F. SPT3 interacts with TFIID to allow normal transcription in Saccharomyces cerevisiae. Genes Dev. 1992;6:1319–1331. doi: 10.1101/gad.6.7.1319. [DOI] [PubMed] [Google Scholar]
- 64b.Eisenmann D M, Chapon C, Roberts S M, Dollard C, Winston F. The Saccharomyces cerevisiae SPT8 gene encodes a very acidic protein that is functionally related to SPT3 and TATA-binding protein. Genetics. 1994;137:647–657. doi: 10.1093/genetics/137.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Evans T, Reitman M, Felsenfeld G. An erythrocyte-specific DNA-binding factor recognizes a regulatory sequence common to all chicken globin genes. Proc Natl Acad Sci USA. 1988;85:5976–5980. doi: 10.1073/pnas.85.16.5976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Falvo J V, Thanos D, Maniatis T. Reversal of intrinsic DNA bends in the IFN-β gene enhancer by transcription factors and the architectural protein HMG I(Y) Cell. 1995;83:1101–1111. doi: 10.1016/0092-8674(95)90137-x. [DOI] [PubMed] [Google Scholar]
- 67.Fletcher T M, Hansen J C. Core histone tail domains mediate oligonucleosome folding and nucleosomal DNA organization through distinct molecular mechanisms. J Biol Chem. 1995;270:25359–25362. doi: 10.1074/jbc.270.43.25359. [DOI] [PubMed] [Google Scholar]
- 68.Fletcher T M, Hansen J C. The nucleosomal array: structure/function relationships. Crit Rev Eukaryot Gene Expression. 1996;6:149–188. doi: 10.1615/critreveukargeneexpr.v6.i2-3.40. [DOI] [PubMed] [Google Scholar]
- 69.Foss M, McNally F J, Laurenson P, Rine J. Origin recognition complex (ORC) in transcriptional silencing and DNA replication in S. cerevisiae. Science. 1993;262:1838–1844. doi: 10.1126/science.8266071. [DOI] [PubMed] [Google Scholar]
- 70.Fox C A, Ehrenhofer-Murray A E, Loo S, Rine J. The origin recognition complex, SIR1, and the S phase requirement for silencing. Science. 1997;276:1547–1551. doi: 10.1126/science.276.5318.1547. [DOI] [PubMed] [Google Scholar]
- 71.Fox C A, Loo S, Dillin A, Rine J. The origin recognition complex has essential functions in transcriptional silencing and chromosomal replication. Genes Dev. 1995;9:911–924. doi: 10.1101/gad.9.8.911. [DOI] [PubMed] [Google Scholar]
- 72.Franke A, Baker B S. The roX1 and roX2 RNAs are essential components of the compensasome, which mediates dosage compensation in Drosophila. Mol Cell. 1999;4:117–122. doi: 10.1016/s1097-2765(00)80193-8. [DOI] [PubMed] [Google Scholar]
- 73.Gansheroff L J, Dollard C, Tan P, Winston F. The Saccharomyces cerevisiae SPT7 gene encodes a very acidic protein important for transcription in vivo. Genetics. 1995;139:523–536. doi: 10.1093/genetics/139.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Garcea R L, Alberts B M. Comparative studies of histone acetylation in nucleosomes, nuclei, and intact cells: evidence for special factors which modify acetylase action. J Biol Chem. 1980;255:11454–11463. [PubMed] [Google Scholar]
- 75.Ge H, Martinez E, Chiang C M, Roeder R G. Activator-dependent transcription by mammalian RNA polymerase II: in vitro reconstitution with general transcription factors and cofactors. Methods Enzymol. 1996;274:57–71. doi: 10.1016/s0076-6879(96)74008-9. [DOI] [PubMed] [Google Scholar]
- 76.Georgakopoulos T, Gounalaki N, Thireos G. Genetic evidence for the interaction of the yeast transcriptional co-activator proteins GCN5 and ADA2. Mol Gen Genet. 1995;246:723–728. doi: 10.1007/BF00290718. [DOI] [PubMed] [Google Scholar]
- 77.Georgakopoulos T, Thireos G. Two distinct yeast transcriptional activators require the function of the GCN5 protein to promote normal levels of transcription. EMBO J. 1992;11:4145–4152. doi: 10.1002/j.1460-2075.1992.tb05507.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Georgieva S, Kirschner D B, Jagla T, Nabirochkina E, Hanke S, Schenkel H, de Lorenzo C, Sinha P, Jagla K, Mechler B, Tora L. Two novel Drosophila TAFIIs have homology with human TAFII30 and are differentially regulated during development. Mol Cell Biol. 2000;20:1639–1648. doi: 10.1128/mcb.20.5.1639-1648.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Giaccia A J, Kastan M B. The complexity of p53 modulation: emerging patterns from divergent signals. Genes Dev. 1998;12:2973–2983. doi: 10.1101/gad.12.19.2973. [DOI] [PubMed] [Google Scholar]
- 80.Giles R H, Peters D J, Breuning M H. Conjunction dysfunction: CBP/p300 in human disease. Trends Genet. 1998;14:178–183. doi: 10.1016/s0168-9525(98)01438-3. [DOI] [PubMed] [Google Scholar]
- 81.Giordano A, Avantaggiati M L. p300 and CBP: partners for life and death. J Cell Physiol. 1999;181:218–230. doi: 10.1002/(SICI)1097-4652(199911)181:2<218::AID-JCP4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 82.Godde J S, Nakatani Y, Wolffe A P. The amino-terminal tails of the core histones and the translational position of the TATA box determine TBP/TFIIA association with nucleosomal DNA. Nucleic Acids Res. 1995;23:4557–4564. doi: 10.1093/nar/23.22.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gottlieb T M, Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996;1287:77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- 84.Grant P A, Duggan L, Côté J, Roberts S M, Brownell J E, Candau R, Ohba R, Owen-Hughes T, Allis C D, Winston F, Berger S L, Workman J L. Yeast Gcn5 functions in two multisubunit complexes to acetylate nucleosomal histones: characterization of an Ada complex and the SAGA (Spt/Ada) complex. Genes Dev. 1997;11:1640–1650. doi: 10.1101/gad.11.13.1640. [DOI] [PubMed] [Google Scholar]
- 85.Grant P A, Eberharter A, John S, Cook R G, Turner B M, Workman J L. Expanded lysine acetylation specificity of Gcn5 in native complexes. J Biol Chem. 1999;274:5895–5900. doi: 10.1074/jbc.274.9.5895. [DOI] [PubMed] [Google Scholar]
- 86.Grant P A, Schieltz D, Pray-Grant M G, Steger D J, Reese J C, Yates III J R, Workman J L. A subset of TAFIIs are integral components of the SAGA complex required for nucleosome acetylation and transcription stimulation. Cell. 1998;94:45–53. doi: 10.1016/s0092-8674(00)81220-9. [DOI] [PubMed] [Google Scholar]
- 87.Grant P A, Schieltz D, Pray-Grant M G, Yates III J R, Workman J L. The ATM-related cofactor Tra1 is a component of the purified SAGA complex. Mol Cell. 1998;2:863–867. doi: 10.1016/s1097-2765(00)80300-7. [DOI] [PubMed] [Google Scholar]
- 88.Grant P A, Sterner D E, Duggan L J, Workman J L, Berger S L. The SAGA unfolds: convergence of transcription regulators in chromatin-modifying complexes. Trends Cell Biol. 1998;8:193–197. doi: 10.1016/s0962-8924(98)01263-x. [DOI] [PubMed] [Google Scholar]
- 89.Gregory P D, Schmid A, Zavari M, Liu L, Berger S L, Hörz W. Absence of Gcn5 HAT activity defines a novel state in the opening of chromatin at the PHO5 promoter in yeast. Mol Cell. 1998;1:495–505. doi: 10.1016/s1097-2765(00)80050-7. [DOI] [PubMed] [Google Scholar]
- 90.Gregory P D, Schmid A, Zavari M, Münsterkötter M, Hörz W. Chromatin remodelling at the PHO8 promoter requires SWI-SNF and SAGA at a step subsequent to activator binding. EMBO J. 1999;18:6407–6414. doi: 10.1093/emboj/18.22.6407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grunstein M. Nucleosomes: regulators of transcription. Trends Genet. 1990;6:395–400. doi: 10.1016/0168-9525(90)90299-l. [DOI] [PubMed] [Google Scholar]
- 92.Gu W, Roeder R. Activation of p53 sequence-specific DNA binding by acetylation of the p53 C-terminal domain. Cell. 1997;90:595–606. doi: 10.1016/s0092-8674(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 93.Gu W, Shi X L, Roeder R G. Synergistic activation of transcription by CBP and p53. Nature. 1997;387:819–823. doi: 10.1038/42972. [DOI] [PubMed] [Google Scholar]
- 94.Guo K, Wang J, Andres V, Smith R C, Walsh K. MyoD-induced expression of p21 inhibits cyclin-dependent kinase activity upon myocyte terminal differentiation. Mol Cell Biol. 1995;15:3823–3829. doi: 10.1128/mcb.15.7.3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Halevy O, Novitch B G, Spicer D B, Skapek S X, Rhee J, Hannon G J, Beach D, Lassar A B. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science. 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- 96.Hamamori Y, Sartorelli V, Ogryzko V, Puri P L, Wu H Y, Wang J Y, Nakatani Y, Kedes L. Regulation of histone acetyltransferases p300 and PCAF by the bHLH protein twist and adenoviral oncoprotein E1A. Cell. 1999;96:405–413. doi: 10.1016/s0092-8674(00)80553-x. [DOI] [PubMed] [Google Scholar]
- 97.Hampsey M. Molecular genetics of the RNA polymerase II general transcriptional machinery. Microbiol Mol Biol Rev. 1998;62:465–503. doi: 10.1128/mmbr.62.2.465-503.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Haynes S R, Dollard C, Winston F, Beck S, Trowsdale J, Dawid I B. The bromodomain: a conserved sequence found in human, Drosophila and yeast proteins. Nucleic Acids Res. 1992;20:2603. doi: 10.1093/nar/20.10.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hebbes T R, Thorne A W, Crane-Robinson C. A direct link between core histone acetylation and transcriptionally active chromatin. EMBO J. 1988;7:1395–1402. doi: 10.1002/j.1460-2075.1988.tb02956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heery D M, Kalkhoven E, Hoare S, Parker M G. A signature motif in transcriptional co-activators mediates binding to nuclear receptors. Nature. 1997;387:733–736. doi: 10.1038/42750. [DOI] [PubMed] [Google Scholar]
- 101.Herrera J E, Sakaguchi K, Bergel M, Trieschmann L, Nakatani Y, Bustin M. Specific acetylation of chromosomal protein HMG-17 by PCAF alters its interaction with nucleosomes. Mol Cell Biol. 1999;19:3466–3473. doi: 10.1128/mcb.19.5.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hettmann C, Soldati D. Cloning and analysis of a Toxoplasma gondii histone acetyltransferase: a novel chromatin remodelling factor in Apicomplexan parasites. Nucleic Acids Res. 1999;27:4344–4352. doi: 10.1093/nar/27.22.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hilfiker A, Hilfiker-Kleiner D, Pannuti A, Lucchesi J C. mof, a putative acetyl transferase gene related to the Tip60 and MOZ human genes and to the SAS genes of yeast, is required for dosage compensation in Drosophila. EMBO J. 1997;16:2054–2060. doi: 10.1093/emboj/16.8.2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hoffmann A, Chiang C-M, Oelgeschläger T, Xie X, Burley S K, Nakatani Y, Roeder R G. A histone octamer-like structure within TFIID. Nature. 1996;380:356–359. doi: 10.1038/380356a0. [DOI] [PubMed] [Google Scholar]
- 105.Holstege F C, Jennings E G, Wyrick J J, Lee T I, Hengartner C J, Green M R, Golub T R, Lander E S, Young R A. Dissecting the regulatory circuitry of a eukaryotic genome. Cell. 1998;95:717–728. doi: 10.1016/s0092-8674(00)81641-4. [DOI] [PubMed] [Google Scholar]
- 106.Hong H, Kohli K, Garabedian M J, Stallcup M R. GRIP1, a transcriptional coactivator for the AF-2 transactivation domain of steroid, thyroid, retinoid, and vitamin D receptors. Mol Cell Biol. 1997;17:2735–2744. doi: 10.1128/mcb.17.5.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Hong L, Schroth G P, Matthews H R, Yau P, Bradbury E M. Studies of the DNA binding properties of histone H4 amino terminus: thermal denaturation studies reveal that acetylation markedly reduces the binding constant of the H4 “tail” to DNA. J Biol Chem. 1993;268:305–314. [PubMed] [Google Scholar]
- 107a.Horiuchi J, Silverman N, Piña B, Marcus G A, Guarente L. ADA1, a novel component of the ADA/GCN5 complex, has broader effects than GCN5, ADA2, or ADA3. Mol Cell Biol. 1997;17:3220–3228. doi: 10.1128/mcb.17.6.3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107b.Horiuchi J, Silverman N, Marcus G A, Guarente L. ADA3, a putative transcriptional adaptor, consists of two separable domains and interacts with ADA2 and GCN5 in a trimeric complex. Mol Cell Biol. 1995;15:1203–1209. doi: 10.1128/mcb.15.3.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hottiger M O, Nabel G J. Interaction of human immunodeficiency virus type 1 Tat with the transcriptional coactivators p300 and CREB binding protein. J Virol. 1998;72:8252–8256. doi: 10.1128/jvi.72.10.8252-8256.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hsieh Y J, Kundu T K, Wang Z, Kovelman R, Roeder R G. The TFIIIC90 subunit of TFIIIC interacts with multiple components of the RNA polymerase III machinery and contains a histone-specific acetyltransferase activity. Mol Cell Biol. 1999;19:7697–7704. doi: 10.1128/mcb.19.11.7697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hung H L, Lau J, Kim A Y, Weiss M J, Blobel G A. CREB-binding protein acetylates hematopoietic transcription factor GATA-1 at functionally important sites. Mol Cell Biol. 1999;19:3496–3505. doi: 10.1128/mcb.19.5.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hupp T R, Meek D W, Midgley C A, Lane D P. Regulation of the specific DNA binding function of p53. Cell. 1992;71:875–886. doi: 10.1016/0092-8674(92)90562-q. [DOI] [PubMed] [Google Scholar]
- 112.Iizuka M, Stillman B. Histone acetyltransferase HBO1 interacts with the ORC1 subunit of the human initiator protein. J Biol Chem. 1999;274:23027–23034. doi: 10.1074/jbc.274.33.23027. [DOI] [PubMed] [Google Scholar]
- 113.Ikeda K, Steger D J, Eberharter A, Workman J L. Activation domain-specific and general transcription stimulation by native histone acetyltransferase complexes. Mol Cell Biol. 1999;19:855–863. doi: 10.1128/mcb.19.1.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Imbalzano A N. Energy-dependent chromatin remodelers: complex complexes and their components. Crit Rev Eukaryot Gene Expression. 1998;8:225–255. doi: 10.1615/critreveukargeneexpr.v8.i3-4.10. [DOI] [PubMed] [Google Scholar]
- 115.Imbalzano A N, Kwon H, Green M R, Kingston R E. Facilitated binding of TATA-binding protein to nucleosomal DNA. Nature. 1994;370:481–485. doi: 10.1038/370481a0. [DOI] [PubMed] [Google Scholar]
- 116.Imhof A, Yang X J, Ogryzko V V, Nakatani Y, Wolffe A P, Ge H. Acetylation of general transcription factors by histone acetyltransferases. Curr Biol. 1997;7:689–692. doi: 10.1016/s0960-9822(06)00296-x. [DOI] [PubMed] [Google Scholar]
- 117.Jeanmougin F, Wurtz J-M, Le Douarin B, Chambon P, Losson R. The bromodomain revisited. Trends Biochem Sci. 1997;22:151–153. doi: 10.1016/s0968-0004(97)01042-6. [DOI] [PubMed] [Google Scholar]
- 118.Jones K A. Taking a new TAK on tat transactivation. Genes Dev. 1997;11:2593–2599. doi: 10.1101/gad.11.20.2593. [DOI] [PubMed] [Google Scholar]
- 119.Kamei Y, Xu L, Heinzel T, Torchia J, Kurokawa R, Gloss B, Lin S C, Heyman R A, Rose D W, Glass C K, Rosenfeld M G. A CBP integrator complex mediates transcriptional activation and AP-1 inhibition by nuclear receptors. Cell. 1996;85:403–414. doi: 10.1016/s0092-8674(00)81118-6. [DOI] [PubMed] [Google Scholar]
- 120.Kamine J, Elangovan B, Subramanian T, Coleman D, Chinnadurai G. Identification of a cellular protein that specifically interacts with the essential cysteine region of the HIV-1 Tat transactivator. Virology. 1996;216:357–366. doi: 10.1006/viro.1996.0071. [DOI] [PubMed] [Google Scholar]
- 121.Kelley R L, Kuroda M I. Equality for X chromosomes. Science. 1995;270:1607–1610. doi: 10.1126/science.270.5242.1607. [DOI] [PubMed] [Google Scholar]
- 122.Kelley R L, Meller V H, Gordadze P R, Roman G, Davis R L, Kuroda M I. Epigenetic spreading of the Drosophila dosage compensation complex from roX RNA genes into flanking chromatin. Cell. 1999;98:513–522. doi: 10.1016/s0092-8674(00)81979-0. [DOI] [PubMed] [Google Scholar]
- 123.Kelley R L, Solovyeva I, Lyman L M, Richman R, Solovyev V, Kuroda M I. Expression of msl-2 causes assembly of dosage compensation regulators on the X chromosomes and female lethality in Drosophila. Cell. 1995;81:867–877. doi: 10.1016/0092-8674(95)90007-1. [DOI] [PubMed] [Google Scholar]
- 124.Kiernan R E, Vanhulle C, Schiltz L, Adam E, Xiao H, Maudoux F, Calomme C, Burny A, Nakatani Y, Jeang K T, Benkirane M, Van Lint C. HIV-1 Tat transcriptional activity is regulated by acetylation. EMBO J. 1999;18:6106–6118. doi: 10.1093/emboj/18.21.6106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kimura A, Horikoshi M. Tip60 acetylates six lysines of a specific class in core histones in vitro. Genes Cells. 1998;3:789–800. doi: 10.1046/j.1365-2443.1998.00229.x. [DOI] [PubMed] [Google Scholar]
- 126.Kingston R E, Bunker C A, Imbalzano A N. Repression and activation by multiprotein complexes that alter chromatin structure. Genes Dev. 1996;10:905–920. doi: 10.1101/gad.10.8.905. [DOI] [PubMed] [Google Scholar]
- 127.Kleff S, Andrulis E D, Anderson C W, Sternglanz R. Identification of a gene encoding a yeast histone H4 acetyltransferase. J Biol Chem. 1995;270:24674–24677. doi: 10.1074/jbc.270.42.24674. [DOI] [PubMed] [Google Scholar]
- 128.Ko L J, Prives C. p53: puzzle and paradigm. Genes Dev. 1996;10:1054–1072. doi: 10.1101/gad.10.9.1054. [DOI] [PubMed] [Google Scholar]
- 129.Kornberg R D, Lorch Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell. 1999;98:285–294. doi: 10.1016/s0092-8674(00)81958-3. [DOI] [PubMed] [Google Scholar]
- 130.Korzus E, Torchia J, Rose D W, Xu L, Kurokawa R, McInerney E M, Mullen T M, Glass C K, Rosenfeld M G. Transcription factor-specific requirements for coactivators and their acetyltransferase functions. Science. 1998;279:703–707. doi: 10.1126/science.279.5351.703. [DOI] [PubMed] [Google Scholar]
- 131.Kruger W, Herskowitz I. A negative regulator of HO transcription, SIN1 (SPT2), is a nonspecific DNA-binding protein related to HMG1. Mol Cell Biol. 1991;11:4135–4146. doi: 10.1128/mcb.11.8.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Krumm A, Madisen L, Yang X J, Goodman R, Nakatani Y, Groudine M. Long-distance transcriptional enhancement by the histone acetyltransferase PCAF. Proc Natl Acad Sci USA. 1998;95:13501–13506. doi: 10.1073/pnas.95.23.13501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Kundu T K, Wang Z, Roeder R G. Human TFIIIC relieves chromatin-mediated repression of RNA polymerase III transcription and contains an intrinsic histone acetyltransferase activity. Mol Cell Biol. 1999;19:1605–1615. doi: 10.1128/mcb.19.2.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kuo M-H, Zhou J, Jambeck P, Churchill M E A, Allis C D. Histone acetyltransferase activity of yeast Gcn5p is required for the activation of target genes in vivo. Genes Dev. 1998;12:627–639. doi: 10.1101/gad.12.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kuo M-H, Allis C D. Roles of histone acetyltransferases and deacetylases in gene regulation. Bioessays. 1998;20:615–626. doi: 10.1002/(SICI)1521-1878(199808)20:8<615::AID-BIES4>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 136.Kuo M-H, Brownell J E, Sobel R E, Ranalli T A, Cook R G, Edmonson D G, Roth S Y, Allis C D. GCN5p, a yeast nuclear histone acetyltransferase, acetylates specific lysines in histone H3 and H4 that differ from deposition-related acetylation sites. Nature. 1996;383:269–272. doi: 10.1038/383269a0. [DOI] [PubMed] [Google Scholar]
- 137.Lassar A B, Martin P L, Roeder R G. Transcription of class III genes: formation of preinitiation complexes. Science. 1983;222:740–748. doi: 10.1126/science.6356356. [DOI] [PubMed] [Google Scholar]
- 138.La Thangue N B. DP and E2F proteins: components of a heterodimeric transcription factor implicated in cell cycle control. Curr Opin Cell Biol. 1994;6:443–450. doi: 10.1016/0955-0674(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 139.Leo C, Chen J D. The SRC family of nuclear receptor coactivators. Gene. 2000;245:1–11. doi: 10.1016/s0378-1119(00)00024-x. [DOI] [PubMed] [Google Scholar]
- 140.Levine A J. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 141.L'Hernault S W, Rosenbaum J L. Chlamydomonas alpha-tubulin is posttranslationally modified by acetylation on the epsilon-amino group of a lysine. Biochemistry. 1985;24:473–478. doi: 10.1021/bi00323a034. [DOI] [PubMed] [Google Scholar]
- 142.Li H, Gomes P J, Chen J D. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc Natl Acad Sci USA. 1997;94:8479–8484. doi: 10.1073/pnas.94.16.8479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li Y, Bjorklund S, Jiang Y W, Kim Y J, Lane W S, Stillman D J, Kornberg R D. Yeast global transcriptional regulators Sin4 and Rgr1 are components of mediator complex/RNA polymerase II holoenzyme. Proc Natl Acad Sci USA. 1995;92:10864–10868. doi: 10.1073/pnas.92.24.10864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liang J, Prouty L, Williams B J, Dayton M A, Blanchard K L. Acute mixed lineage leukemia with an inv(8)(p11q13) resulting in fusion of the genes for MOZ and TIF2. Blood. 1998;92:2118–2122. [PubMed] [Google Scholar]
- 145.Lill N L, Grossman S R, Ginsberg D, DeCaprio J, Livingston D M. Binding and modulation of p53 by p300/CBP coactivators. Nature. 1997;387:823–827. doi: 10.1038/42981. [DOI] [PubMed] [Google Scholar]
- 146.Lin Y, Fletcher C M, Zhou J, Allis C D, Wagner G. Solution structure of the catalytic domain of GCN5 histone acetyltransferase bound to coenzyme A. Nature. 1999;400:86–89. doi: 10.1038/21922. [DOI] [PubMed] [Google Scholar]
- 147.Liu D, Ishima R, Tong K I, Bagby S, Kokubo T, Muhandiram D R, Kay L E, Nakatani Y, Ikura M. Solution structure of a TBP-TAFII230 complex: protein mimicry of the minor groove surface of the TATA box unwound by TBP. Cell. 1998;94:573–583. doi: 10.1016/s0092-8674(00)81599-8. [DOI] [PubMed] [Google Scholar]
- 148.Liu L, Scolnick D M, Trievel R C, Zhang H B, Marmorstein R, Halazonetis T D, Berger S L. p53 sites acetylated in vitro by PCAF and p300 are acetylated in vivo in response to DNA damage. Mol Cell Biol. 1999;19:1202–1209. doi: 10.1128/mcb.19.2.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Loidl P. Histone acetylation: facts and questions. Chromosoma. 1994;103:441–449. doi: 10.1007/BF00337382. [DOI] [PubMed] [Google Scholar]
- 150.Lucchesi J C. Dosage compensation in flies and worms: the ups and downs of X-chromosome regulation. Curr Opin Genet Dev. 1998;8:179–184. doi: 10.1016/s0959-437x(98)80139-1. [DOI] [PubMed] [Google Scholar]
- 151.Luger K, Mader A W, Richmond R K, Sargent D F, Richmond T J. Crystal structure of the nucleosome core particle at 2.8 Å resolution. Nature. 1997;389:251–260. doi: 10.1038/38444. [DOI] [PubMed] [Google Scholar]
- 152.Luger K, Richmond T J. The histone tails of the nucleosome. Curr Opin Genet Dev. 1998;8:140–146. doi: 10.1016/s0959-437x(98)80134-2. [DOI] [PubMed] [Google Scholar]
- 153.Marcus G A, Horiuchi J, Silverman N, Guarente L. ADA5/SPT20 links the ADA and SPT genes, which are involved in yeast transcription. Mol Cell Biol. 1996;16:3197–3205. doi: 10.1128/mcb.16.6.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marcus G A, Silverman N, Berger S L, Horiuchi J, Guarente L. Functional similarity and physical association between GCN5 and ADA2: putative transcriptional adaptors. EMBO J. 1994;13:4807–4815. doi: 10.1002/j.1460-2075.1994.tb06806.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mardian J K, Paton A E, Bunick G J, Olins D E. Nucleosome cores have two specific binding sites for nonhistone chromosomal proteins HMG 14 and HMG 17. Science. 1980;209:1534–1536. doi: 10.1126/science.7433974. [DOI] [PubMed] [Google Scholar]
- 156.Martin D I, Tsai S F, Orkin S H. Increased gamma-globin expression in a nondeletion HPFH mediated by an erythroid-specific DNA-binding factor. Nature. 1989;338:435–438. doi: 10.1038/338435a0. [DOI] [PubMed] [Google Scholar]
- 157.Martinez E, Kundu T K, Fu J, Roeder R G. A human SPT3-TAFII31-GCN5-L acetylase complex distinct from transcription factor IID. J Biol Chem. 1998;273:23781–23785. doi: 10.1074/jbc.273.37.23781. [DOI] [PubMed] [Google Scholar]
- 158.Martínez-Balbás M A, Bannister A J, Martin K, Haus-Seuffert P, Meisterernst M, Kouzarides T. The acetyltransferase activity of CBP stimulates transcription. EMBO J. 1998;17:2886–2893. doi: 10.1093/emboj/17.10.2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Martínez-Balbás M A, Bauer U-M, Nielsen S J, Brehm A, Kouzarides T. Regulation of E2F1 activity by acetylation. EMBO J. 2000;19:662–671. doi: 10.1093/emboj/19.4.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Marzio G, Tyagi M, Gutierrez M I, Giacca M. HIV-1 tat transactivator recruits p300 and CREB-binding protein histone acetyltransferases to the viral promoter. Proc Natl Acad Sci USA. 1998;95:13519–13524. doi: 10.1073/pnas.95.23.13519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McMahon S B, Van Buskirk H A, Dugan K A, Copeland T D, Cole M D. The novel ATM-related protein TRRAP is an essential cofactor for the c-Myc and E2F oncoproteins. Cell. 1998;94:363–374. doi: 10.1016/s0092-8674(00)81479-8. [DOI] [PubMed] [Google Scholar]
- 162.McMurry M T, Krangel M S. A role for histone acetylation in the developmental regulation of V(D)J recombination. Science. 2000;287:495–498. [PubMed] [Google Scholar]
- 163.Meek D W. Post-translational modification of p53. Semin Cancer Biol. 1994;5:203–210. [PubMed] [Google Scholar]
- 164.Meisterernst M, Horikoshi M, Roeder R G. Recombinant yeast TFIID, a general transcription factor, mediates activation by the gene-specific factor USF in a chromatin assembly assay. Proc Natl Acad Sci USA. 1990;87:9153–9157. doi: 10.1073/pnas.87.23.9153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Meller V H. Dosage compensation: making 1X equal 2X. Trends Cell Biol. 2000;10:54–59. doi: 10.1016/s0962-8924(99)01693-1. [DOI] [PubMed] [Google Scholar]
- 166.Meller V H, Gordadze P R, Park Y, Chu X, Stuckenholz C, Kelley R L, Kuroda M I. Ordered assembly of roX RNAs into MSL complexes on the dosage-compensated X chromosome in Drosophila. Curr Biol. 2000;10:136–143. doi: 10.1016/s0960-9822(00)00311-0. [DOI] [PubMed] [Google Scholar]
- 167.Merika M, Williams A J, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN-β enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 168.Miller I J, Bieker J J. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol. 1993;13:2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Mizzen C A, Yang X-J, Kokubo T, Brownell J E, Bannister A J, Owen-Hughes T, Workman J, Wang L, Berger S L, Kouzarides T, Nakatani Y, Allis C D. The TAFII250 subunit of TFIID has histone acetyltransferase activity. Cell. 1996;87:1261–1270. doi: 10.1016/s0092-8674(00)81821-8. [DOI] [PubMed] [Google Scholar]
- 170.Mullen J R, Kayne P S, Moerschell R P, Tsunasawa S, Gribskov M, Colavito-Shepanski M, Grunstein M, Sherman F, Sternglanz R. Identification and characterization of genes and mutants for an N-terminal acetyltransferase from yeast. EMBO J. 1989;8:2067–2075. doi: 10.1002/j.1460-2075.1989.tb03615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Munshi N, Merika M, Yie J, Senger K, Chen G, Thanos D. Acetylation of HMG I(Y) by CBP turns off IFN beta expression by disrupting the enhanceosome. Mol Cell. 1998;2:457–467. doi: 10.1016/s1097-2765(00)80145-8. [DOI] [PubMed] [Google Scholar]
- 172.Natsoulis G, Dollard C, Winston F, Boeke J D. The products of the SPT10 and SPT21 genes of Saccharomyces cerevisiae increase the amplitude of transcriptional regulation at a large number of unlinked loci. New Biol. 1991;3:1249–1259. [PubMed] [Google Scholar]
- 173.Natsoulis G, Winston F, Boeke J D. The SPT10 and SPT21 genes of Saccharomyces cerevisiae. Genetics. 1994;136:93–105. doi: 10.1093/genetics/136.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Neuwald A F, Landsman D. GCN5-related histone N-acetyltransferases belong to a diverse superfamily that includes the yeast SPT10 protein. Trends Biochem Sci. 1997;22:154–155. doi: 10.1016/s0968-0004(97)01034-7. [DOI] [PubMed] [Google Scholar]
- 175.Norton V G, Imai B S, Yau P, Bradbury E M. Histone acetylation reduces nucleosome core particle linking number change. Cell. 1989;57:449–457. doi: 10.1016/0092-8674(89)90920-3. [DOI] [PubMed] [Google Scholar]
- 176.O'Brien T, Tjian R. Different functional domains of TAFII250 modulate expression of distinct subsets of mammalian genes. Proc Natl Acad Sci USA. 2000;97:2456–2461. doi: 10.1073/pnas.97.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ogryzko V V, Kotani T, Zhang X, Schiltz R L, Howard T, Yang X-J, Howard B H, Qin J, Nakatani Y. Histone-like TAFs within the PCAF histone acetylase complex. Cell. 1998;94:35–44. doi: 10.1016/s0092-8674(00)81219-2. [DOI] [PubMed] [Google Scholar]
- 178.Ogryzko V V, Schlitz R L, Russanova V, Howard B H, Nakatani Y. The transcriptional coactivators p300 and CBP are histone acetyltransferases. Cell. 1996;87:953–959. doi: 10.1016/s0092-8674(00)82001-2. [DOI] [PubMed] [Google Scholar]
- 179.Ohba R, Steger D J, Brownell J E, Mizzen C A, Cook R G, Côté J, Workman J L, Allis C D. A novel H2A/H4 nucleosomal histone acetyltransferase in Tetrahymena thermophila. Mol Cell Biol. 1999;19:2061–2068. doi: 10.1128/mcb.19.3.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Oñate S A, Tsai S Y, Tsai M J, O'Malley B W. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- 181.Ornaghi P, Ballario P, Lena A M, Gonzalez A, Filetici P. The bromodomain of Gcn5p interacts in vitro with specific residues in the N terminus of histone H4. J Mol Biol. 1999;287:1–7. doi: 10.1006/jmbi.1999.2577. [DOI] [PubMed] [Google Scholar]
- 182.Otero G, Fellows J, Li Y, de Bizemont T, Dirac A M, Gustafsson C M, Erdjument-Bromage H, Tempst P, Svejstrup J Q. Elongator, a multisubunit component of a novel RNA polymerase II holoenzyme for transcriptional elongation. Mol Cell. 1999;3:109–118. doi: 10.1016/s1097-2765(00)80179-3. [DOI] [PubMed] [Google Scholar]
- 183.Owen-Hughes T, Workman J L. Experimental analysis of chromatin function in transcription control. Crit Rev Eukarot Gene Expression. 1994;4:403–441. [PubMed] [Google Scholar]
- 184.Pak D T, Pflumm M, Chesnokov I, Huang D W, Kellum R, Marr J, Romanowski P, Botchan M R. Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell. 1997;91:311–323. doi: 10.1016/s0092-8674(00)80415-8. [DOI] [PubMed] [Google Scholar]
- 185.Parthun M R, Widom J, Gottschling D E. The major cytoplasmic histone acetyltransferase in yeast: links to chromatin replication and histone metabolism. Cell. 1996;87:85–94. doi: 10.1016/s0092-8674(00)81325-2. [DOI] [PubMed] [Google Scholar]
- 186.Pazin M, Kadonaga J. What's up and down with histone deacetylation and transcription? Cell. 1997;89:325–328. doi: 10.1016/s0092-8674(00)80211-1. [DOI] [PubMed] [Google Scholar]
- 187.Perissi V, Dasen J S, Kurokawa R, Wang Z, Korzus E, Rose D W, Glass C K, Rosenfeld M G. Factor-specific modulation of CREB-binding protein acetyltransferase activity. Proc Natl Acad Sci USA. 1999;96:3652–3657. doi: 10.1073/pnas.96.7.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Piperno G, Fuller M T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985;101:2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Pollard K J, Peterson C L. Role for ADA/GCN5 products in antagonizing chromatin-mediated transcriptional repression. Mol Cell Biol. 1997;17:6212–6222. doi: 10.1128/mcb.17.11.6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ponting C P, Blake D J, Davies K E, Kendrick-Jones J, Winder S J. ZZ and TAZ: new putative zinc fingers in dystrophin and other proteins. Trends Biochem Sci. 1996;21:11–13. [PubMed] [Google Scholar]
- 191.Puri P L, Avantaggiati M L, Balsano C, Sang N, Graessmann A, Giordano A, Levrero M. p300 is required for MyoD-dependent cell cycle arrest and muscle-specific gene transcription. EMBO J. 1997;16:369–383. doi: 10.1093/emboj/16.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Puri P L, Sartorelli V, Yang X J, Hamamori Y, Ogryzko V V, Howard B H, Kedes L, Wang J Y, Graessmann A, Nakatani Y, Levrero M. Differential roles of p300 and PCAF acetyltransferases in muscle differentiation. Mol Cell. 1997;1:35–45. doi: 10.1016/s1097-2765(00)80005-2. [DOI] [PubMed] [Google Scholar]
- 193.Reid J L, Bannister A J, Zegerman P, Martinez-Balbas M A, Kouzarides T. E1A directly binds and regulates the P/CAF acetyltransferase. EMBO J. 1998;17:4469–4477. doi: 10.1093/emboj/17.15.4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Reifsnyder C, Lowell J, Clarke A, Pillus L. Yeast SAS silencing genes and human genes associated with AML and HIV-1 Tat interactions are homologous with acetyltransferases. Nat Genet. 1996;14:42–49. doi: 10.1038/ng0996-42. [DOI] [PubMed] [Google Scholar]
- 195.Roberts S M, Winston F. Essential functional interactions of SAGA, a Saccharomyces cerevisiae complex of Spt, Ada, and Gcn5 proteins, with the Snf/Swi and Srb/mediator complexes. Genetics. 1997;147:451–465. doi: 10.1093/genetics/147.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Roberts S M, Winston F. SPT20/ADA5 encodes a novel protein functionally related to the TATA-binding protein and important for transcription in Saccharomyces cerevisiae. Mol Cell Biol. 1996;16:3206–3213. doi: 10.1128/mcb.16.6.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rojas J R, Trievel R C, Zhou J, Mo Y, Li X, Berger S L, Allis C D, Marmorstein R. Structure of Tetrahymena GCN5 bound to coenzyme A and a histone H3 peptide. Nature. 1999;401:93–98. doi: 10.1038/43487. [DOI] [PubMed] [Google Scholar]
- 198.Roth S Y, Allis C D. Histone acetylation and chromatin assembly: a single escort, multiple dances? Cell. 1996;87:5–8. doi: 10.1016/s0092-8674(00)81316-1. [DOI] [PubMed] [Google Scholar]
- 199.Ruiz-Carrillo A, Wangh L J, Allfrey V G. Processing of newly synthesized histone molecules. Science. 1975;190:117–128. doi: 10.1126/science.1166303. [DOI] [PubMed] [Google Scholar]
- 200.Ruiz-García A B, Sendra R, Galiana M, Pamblanco M, Perez-Ortin J E, Tordera V. HAT1 and HAT2 proteins are components of a yeast nuclear histone acetyltransferase enzyme specific for free histone H4. J Biol Chem. 1998;273:12599–12605. doi: 10.1074/jbc.273.20.12599. [DOI] [PubMed] [Google Scholar]
- 201.Ruiz-García A B, Sendra R, Pamblanco M, Tordera V. Gcn5p is involved in the acetylation of histone H3 in nucleosomes. FEBS Lett. 1997;403:186–190. doi: 10.1016/s0014-5793(97)00049-5. [DOI] [PubMed] [Google Scholar]
- 202.Sakaguchi K, Herrera J E, Saito S, Miki T, Bustin M, Vassilev A, Anderson C W, Appella E. DNA damage activates p53 through a phosphorylation-acetylation cascade. Genes Dev. 1998;12:2831–2841. doi: 10.1101/gad.12.18.2831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Saleh A, Lang V, Cook R, Brandl C J. Identification of native complexes containing the yeast coactivator/repressor proteins NGG1/ADA3 and ADA2. J Biol Chem. 1997;272:5571–5578. doi: 10.1074/jbc.272.9.5571. [DOI] [PubMed] [Google Scholar]
- 204.Saleh A, Schieltz D, Ting N, McMahon S B, Litchfield D W, Yates III J R, Lees-Miller S P, Cole M D, Brandl C J. Tra1p is a component of the yeast Ada-Spt transcriptional regulatory complexes. J Biol Chem. 1998;273:26559–26565. doi: 10.1074/jbc.273.41.26559. [DOI] [PubMed] [Google Scholar]
- 205.Sandeen G, Wood W I, Felsenfeld G. The interaction of high mobility proteins HMG14 and 17 with nucleosomes. Nucleic Acids Res. 1980;8:3757–3778. doi: 10.1093/nar/8.17.3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sartorelli V, Puri P L, Hamamori Y, Ogryzko V, Chung G, Nakatani Y, Wang J Y J, Kedes L. Acetylation of MyoD directed by PCAF is necessary for the execution of the muscle program. Mol Cell. 1999;4:725–734. doi: 10.1016/s1097-2765(00)80383-4. [DOI] [PubMed] [Google Scholar]
- 207.Schiltz R L, Mizzen C A, Vassilev A, Cook R G, Allis C D, Nakatani Y. Overlapping but distinct patterns of histone acetylation by the human coactivators p300 and PCAF within nucleosomal substrates. J Biol Chem. 1999;274:1189–1192. doi: 10.1074/jbc.274.3.1189. [DOI] [PubMed] [Google Scholar]
- 208.Schulze E, Asai D J, Bulinski J C, Kirschner M. Posttranslational modification and microtubule stability. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Scolnick D M, Chehab N H, Stavridi E S, Lien M C, Caruso L, Moran E, Berger S L, Halazonetis T D. CREB-binding protein and p300/CBP-associated factor are transcriptional coactivators of the p53 tumor suppressor protein. Cancer Res. 1997;57:3693–3696. [PubMed] [Google Scholar]
- 210.Scott M J, Pan L L, Cleland S B, Knox A L, Heinrich J. MSL1 plays a central role in assembly of the MSL complex, essential for dosage compensation in Drosophila. EMBO J. 2000;19:144–155. doi: 10.1093/emboj/19.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Shikama N, Lyon J, La Thangue N B. The p300/CBP family: integrating signals with transcription factors and chromatin. Trends Cell Biol. 1997;7:230–236. doi: 10.1016/S0962-8924(97)01048-9. [DOI] [PubMed] [Google Scholar]
- 212.Shpungin S, Liberzon A, Bangio H, Yona E, Katcoff D J. Association of yeast SIN1 with the tetratrico peptide repeats of CDC23. Proc Natl Acad Sci USA. 1996;93:8274–8277. doi: 10.1073/pnas.93.16.8274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Silverman N, Agapite J, Guarente L. Yeast ADA2 protein binds to the VP16 protein activation domain and activates transcription. Proc Natl Acad Sci USA. 1994;91:11665–11668. doi: 10.1073/pnas.91.24.11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Smith C L, Onate S A, Tsai M J, O'Malley B W. CREB binding protein acts synergistically with steroid receptor coactivator-1 to enhance steroid receptor-dependent transcription. Proc Natl Acad Sci USA. 1996;93:8884–8888. doi: 10.1073/pnas.93.17.8884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Smith E R, Belote J M, Schiltz R L, Yang X J, Moore P A, Berger S L, Nakatani Y, Allis C D. Cloning of Drosophila GCN5: conserved features among metazoan GCN5 family members. Nucleic Acids Res. 1998;26:2948–2954. doi: 10.1093/nar/26.12.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Smith E R, Eisen A, Gu W, Sattah M, Pannuti A, Zhou J, Cook R G, Lucchesi J C, Allis C D. ESA1 is a histone acetyltransferase that is essential for growth in yeast. Proc Natl Acad Sci USA. 1998;95:3561–3565. doi: 10.1073/pnas.95.7.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Smith E R, Pannuti A, Gu W, Steurnagel A, Cook R G, Allis C D, Lucchesi J C. The Drosophila MSL complex acetylates histone H4 at lysine 16, a chromatin modification linked to dosage compensation. Mol Cell Biol. 2000;20:312–318. doi: 10.1128/mcb.20.1.312-318.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Sobel R E, Cook R G, Perry C A, Annunziato A T, Allis C D. Conservation of deposition-related acetylation sites in newly synthesized histones H3 and H4. Proc Natl Acad Sci USA. 1995;92:1237–1241. doi: 10.1073/pnas.92.4.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Song W, Treich I, Qian N, Kuchin S, Carlson M. SSN genes that affect transcriptional repression in Saccharomyces cerevisiae encode SIN4, ROX3, and SRB proteins associated with RNA polymerase II. Mol Cell Biol. 1996;16:115–120. doi: 10.1128/mcb.16.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Spencer T E, Jenster G, Burcin M M, Allis C D, Zhou J, Mizzen C A, McKenna N J, Onate S A, Tsai S Y, Tsai M J, O'Malley B W. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- 221.Steger D J, Workman J L. Remodeling chromatin structures for transcription: what happens to the histones? BioEssays. 1996;18:875–884. doi: 10.1002/bies.950181106. [DOI] [PubMed] [Google Scholar]
- 222.Sternberg P W, Stern M J, Clark I, Herskowitz I. Activation of the yeast HO gene by release from multiple negative controls. Cell. 1987;48:567–577. doi: 10.1016/0092-8674(87)90235-2. [DOI] [PubMed] [Google Scholar]
- 223.Sterner D E, Grant P A, Roberts S M, Duggan L J, Belotserkovskaya R, Pacella L A, Winston F, Workman J L, Berger S L. Functional organization of the yeast SAGA complex: distinct components involved in structural integrity, nucleosome acetylation, and TATA-binding protein interaction. Mol Cell Biol. 1999;19:86–98. doi: 10.1128/mcb.19.1.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sterner R, Vidali G, Allfrey V G. Studies of acetylation and deacetylation in high mobility group proteins: identification of the sites of acetylation in high mobility group proteins 14 and 17. J Biol Chem. 1981;256:8892–8895. [PubMed] [Google Scholar]
- 225.Sterner R, Vidali G, Allfrey V G. Studies of acetylation and deacetylation in high mobility group proteins: identification of the sites of acetylation in HMG-1. J Biol Chem. 1979;254:11577–11583. [PubMed] [Google Scholar]
- 226.Sterner R, Vidali G, Heinrikson R L, Allfrey V G. Postsynthetic modification of high mobility group proteins: evidence that high mobility group proteins are acetylated. J Biol Chem. 1978;253:7601–7604. [PubMed] [Google Scholar]
- 227.Strahl B D, Allis C D. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 228.Struhl K, Moqtaderi Z. The TAFs in the HAT. Cell. 1998;94:1–4. doi: 10.1016/s0092-8674(00)81213-1. [DOI] [PubMed] [Google Scholar]
- 229.Sudarsanam P, Cao Y, Wu L, Laurent B C, Winston F. The nucleosome remodeling complex, Snf/Swi, is required for the maintenance of transcription in vivo and is partially redundant with the histone acetyltransferase, Gcn5. EMBO J. 1999;18:3101–3106. doi: 10.1093/emboj/18.11.3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Takechi S, Nakayama T. Sas3 is a histone acetyltransferase and requires a zinc finger motif. Biochem Biophys Res Commun. 1999;266:405–410. doi: 10.1006/bbrc.1999.1836. [DOI] [PubMed] [Google Scholar]
- 231.Takeshita A, Cardona G R, Koibuchi N, Suen C S, Chin W W. TRAM-1, a novel 160-kDa thyroid hormone receptor activator molecule, exhibits distinct properties from steroid receptor coactivatorr-1. J Biol Chem. 1997;272:27629–27634. doi: 10.1074/jbc.272.44.27629. [DOI] [PubMed] [Google Scholar]
- 232.Taki T, Sako M, Tsuchida M, Hayashi Y. The t(11;16)(q23;p13) translocation in myelodysplastic syndrome fuses the MLL gene to the CBP gene. Blood. 1997;89:3945–3950. [PubMed] [Google Scholar]
- 233.Tamkun J W, Deuring R, Scott M P, Kissinger M, Pattatucci A M, Kaufman T C, Kennison J A. brahma: a regulator of Drosophila homeotic genes structurally related to the yeast transcriptional activator SNF2/SWI2. Cell. 1992;68:561–572. doi: 10.1016/0092-8674(92)90191-e. [DOI] [PubMed] [Google Scholar]
- 234.Tanner K G, Trievel R C, Kuo M H, Howard R M, Berger S L, Allis C D, Marmorstein R, Denu J M. Catalytic mechanism and function of invariant glutamic acid 173 from the histone acetyltransferase GCN5 transcriptional coactivator. J Biol Chem. 1999;274:18157–18160. doi: 10.1074/jbc.274.26.18157. [DOI] [PubMed] [Google Scholar]
- 235.Taunton J, Hassig C A, Schreiber S L. A mammalian histone deacetylase related to the yeast transcription regulator Rpd3p. Science. 1996;272:408–411. doi: 10.1126/science.272.5260.408. [DOI] [PubMed] [Google Scholar]
- 236.Taylor B L, Zhulin I B. PAS domains: internal sensors of oxygen, redox potential, and light. Microbiol Mol Biol Rev. 1999;63:479–506. doi: 10.1128/mmbr.63.2.479-506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Thanos D, Maniatis T. Virus induction of human IFN-β gene expression requires the assembly of an enhanceosome. Cell. 1995;83:1091–1100. doi: 10.1016/0092-8674(95)90136-1. [DOI] [PubMed] [Google Scholar]
- 238.Thompson M A, Ramsay R G. Myb: an old oncoprotein with new roles. Bioessays. 1995;17:341–350. doi: 10.1002/bies.950170410. [DOI] [PubMed] [Google Scholar]
- 239.Tomita A, Towatari M, Tsuzuki S, Hayakawa F, Kosugi H, Tamai K, Miyazaki T, Kinoshita T, Saito H. c-Myb acetylation at the carboxyl-terminal conserved domain by transcriptional co-activator p300. Oncogene. 2000;19:444–451. doi: 10.1038/sj.onc.1203329. [DOI] [PubMed] [Google Scholar]
- 240.Torchia J, Rose D W, Inostroza J, Kamei Y, Westin S, Glass C K, Rosenfeld M G. The transcriptional co-activator p/CIP binds CBP and mediates nuclear-receptor function. Nature. 1997;387:677–684. doi: 10.1038/42652. [DOI] [PubMed] [Google Scholar]
- 241.Trievel R C, Rojas J R, Sterner D E, Venkataramani R, Wang L, Zhou J, Allis C D, Berger S L, Marmorstein R. Crystal structure and mechanism of histone acetylation of the yeast GCN5 transcriptional coactivator. Proc Natl Acad Sci USA. 1999;96:8931–8936. doi: 10.1073/pnas.96.16.8931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Triolo T, Sternglanz R. Role of interactions between the origin recognition complex and SIR1 in transcriptional silencing. Nature. 1996;381:251–253. doi: 10.1038/381251a0. [DOI] [PubMed] [Google Scholar]
- 243.Tse C, Georgieva E I, Ruiz-García A B, Sendra R, Hansen J C. Gcn5p, a transcription-related histone acetyltransferase, acetylates nucleosomes and folded nucleosomal arrays in the absence of other protein subunits. J Biol Chem. 1998;273:32388–32392. doi: 10.1074/jbc.273.49.32388. [DOI] [PubMed] [Google Scholar]
- 244.Turner B M. Decoding the nucleosome. Cell. 1993;75:5–8. [PubMed] [Google Scholar]
- 245.Turner B M, Birley A J, Lavender J. Histone H4 isoforms acetylated at specific lysine residues define individual chromosomes and chromatin domains in Drosophila polytene nuclei. Cell. 1992;69:375–384. doi: 10.1016/0092-8674(92)90417-b. [DOI] [PubMed] [Google Scholar]
- 246.Utley R T, Ikeda K, Grant P A, Côté J, Steger D J, Eberharter A, John S, Workman J L. Transcriptional activators direct histone acetyltransferase complexes to nucleosomes. Nature. 1998;394:498–502. doi: 10.1038/28886. [DOI] [PubMed] [Google Scholar]
- 247.Vassilev A, Yamauchi J, Kotani T, Prives C, Avantaggiati M L, Qin J, Nakatani Y. The 400 kDa subunit of the PCAF histone acetylase complex belongs to the ATM superfamily. Mol Cell. 1998;2:869–875. doi: 10.1016/s1097-2765(00)80301-9. [DOI] [PubMed] [Google Scholar]
- 248.Verreault A, Kaufman P D, Kobayashi R, Stillman B. Nucleosomal DNA regulates the core-histone-binding subunit of the human Hat1 acetyltransferase. Curr Biol. 1998;8:96–108. doi: 10.1016/s0960-9822(98)70040-5. [DOI] [PubMed] [Google Scholar]
- 249.Verreault A, Kaufman P D, Kobayashi R, Stillman B. Nucleosome assembly by a complex of CAF-1 and acetylated histones H3/H4. Cell. 1996;87:95–104. doi: 10.1016/s0092-8674(00)81326-4. [DOI] [PubMed] [Google Scholar]
- 250.Verrijzer C, Tjian R. TAFs mediate transcriptional activation and promoter selectivity. Trends Biochem Sci. 1996;21:338–342. [PubMed] [Google Scholar]
- 251.Voegel J J, Heine M J, Tini M, Vivat V, Chambon P, Gronemeyer H. The coactivator TIF2 contains three nuclear receptor-binding motifs and mediates transactivation through CBP binding-dependent and -independent pathways. EMBO J. 1998;17:507–519. doi: 10.1093/emboj/17.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Voegel J J, Heine M J, Zechel C, Chambon P, Gronemeyer H. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF-2 of nuclear receptors. EMBO J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- 253.Wall L, deBoer E, Grosveld F. The human beta-globin gene 3′ enhancer contains multiple binding sites for an erythroid-specific protein. Genes Dev. 1988;2:1089–1100. doi: 10.1101/gad.2.9.1089. [DOI] [PubMed] [Google Scholar]
- 254.Wallberg A E, Neely K E, Gustafsson J A, Workman J L, Wright A P, Grant P A. Histone acetyltransferase complexes can mediate transcriptional activation by the major glucocorticoid receptor activation domain. Mol Cell Biol. 1999;19:5952–5959. doi: 10.1128/mcb.19.9.5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Waltzer L, Bienz M. Drosophila CBP represses the transcription factor TCF to antagonize Wingless signalling. Nature. 1998;395:521–525. doi: 10.1038/26785. [DOI] [PubMed] [Google Scholar]
- 256.Wang L, Liu L, Berger S L. Critical residues for histone acetylation by GCN5, functioning in Ada and SAGA complexes, are also required for transcriptional function in vivo. Genes Dev. 1998;12:640–653. doi: 10.1101/gad.12.5.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Wang L, Mizzen C, Ying C, Candau R, Barlev N, Brownell J, Allis C D, Berger S. Histone acetyltransferase activity is conserved between yeast and human GCN5 and required for complementation of growth and transcriptional activation. Mol Cell Biol. 1997;17:519–527. doi: 10.1128/mcb.17.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Wei P, Garber M E, Fang S M, Fischer W H, Jones K A. A novel CDK9-associated C-type cyclin interacts directly with HIV-1 Tat and mediates its high-affinity, loop-specific binding to TAR RNA. Cell. 1998;92:451–462. doi: 10.1016/s0092-8674(00)80939-3. [DOI] [PubMed] [Google Scholar]
- 259.Weintraub H, Dwarki V J, Verma I, Davis R, Hollenberg S, Snider L, Lassar A, Tapscott S J. Muscle-specific transcriptional activation by MyoD. Genes Dev. 1991;5:1377–1386. doi: 10.1101/gad.5.8.1377. [DOI] [PubMed] [Google Scholar]
- 260.Weintraub S J, Chow K N, Luo R X, Zhang S H, He S, Dean D C. Mechanism of active transcriptional repression by the retinoblastoma protein. Nature. 1995;375:812–815. doi: 10.1038/375812a0. [DOI] [PubMed] [Google Scholar]
- 261.Weissman J D, Brown J A, Howcroft T K, Hwang J, Chawla A, Roche P A, Schiltz L, Nakatani Y, Singer D S. HIV-1 Tat binds TAFII250 and represses TAFII250-dependent transcription of major histocompatibility class I genes. Proc Natl Acad Sci USA. 1998;95:11601–11606. doi: 10.1073/pnas.95.20.11601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Westendorp M O, Shatrov V A, Schulze-Osthoff K, Frank R, Kraft M, Los M, Krammer PH, Droge W, Lehmann V. HIV-1 Tat potentiates TNF-induced NF-kappa B activation and cytotoxicity by altering the cellular redox state. EMBO J. 1995;14:546–554. doi: 10.1002/j.1460-2075.1995.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Weston K. Reassessing the role of C-MYB in tumorigenesis. Oncogene. 1999;18:3034–3038. doi: 10.1038/sj.onc.1202728. [DOI] [PubMed] [Google Scholar]
- 264.Whiteway M, Freedman R, Van Arsdell S, Szostak J W, Thorner J. The yeast ARD1 gene product is required for repression of cryptic mating-type information at the HML locus. Mol Cell Biol. 1987;7:3713–3722. doi: 10.1128/mcb.7.10.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wieczorek E, Brand M, Jacq X, Tora L. Function of TAFII-containing complex without TBP in transcription by RNA polymerase II. Nature. 1998;393:187–191. doi: 10.1038/30283. [DOI] [PubMed] [Google Scholar]
- 266.Wijgerde M, Gribnau J, Trimborn T, Nuez B, Philipsen S, Grosveld F, Fraser P. The role of EKLF in human beta-globin gene competition. Genes Dev. 1996;10:2894–2902. doi: 10.1101/gad.10.22.2894. [DOI] [PubMed] [Google Scholar]
- 267.Winston F. Analysis of SPT genes: a genetic approach toward analysis of TFIID, histones, and other transcription factors of yeast. In: McKnight S L, Yamamoto K R, editors. Transcriptional regulation. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1992. pp. 1271–1293. [Google Scholar]
- 268.Winston F, Allis C D. The bromodomain: a chromatin-targeting module? Nat Struct Biol. 1999;6:601–604. doi: 10.1038/10640. [DOI] [PubMed] [Google Scholar]
- 269.Wittschieben B Ø, Otero G, de Bizemont T, Fellows J, Erdjument-Bromage H, Ohba R, Li Y, Allis C D, Tempst P, Svejstrup J Q. A novel histone acetyltransferase is an integral subunit of elongating RNA polymerase II holoenzyme. Mol Cell. 1999;4:123–128. doi: 10.1016/s1097-2765(00)80194-x. [DOI] [PubMed] [Google Scholar]
- 270.Wolf E, Vassilev A, Makino Y, Sali A, Nakatani Y, Burley S K. Crystal structure of a GCN5-related N-acetyltransferase: Serratia marcescens aminoglycoside 3-N-acetyltransferase. Cell. 1998;94:439–449. doi: 10.1016/s0092-8674(00)81585-8. [DOI] [PubMed] [Google Scholar]
- 271.Wolffe A P. Chromatin: structure and function. London, England: Academic Press; 1992. [Google Scholar]
- 272.Wong L C, Sharpe D J, Wong S S. High-mobility group and other nonhistone substrates for nuclear histone N-acetyltransferase. Biochem Genet. 1991;29:461–475. doi: 10.1007/BF02399688. [DOI] [PubMed] [Google Scholar]
- 273.Workman J L, Roeder R G. Binding of transcription factor TFIID to the major late promoter during in vitro nucleosome assembly potentiates subsequent initiation by RNA polymerase II. Cell. 1987;51:613–622. doi: 10.1016/0092-8674(87)90130-9. [DOI] [PubMed] [Google Scholar]
- 274.Xie X, Kokubo T, Cohen S L, Mirza U A, Hoffmann A, Chait B T, Roeder R G, Nakatani Y, Burley S K. Structural similarity between TAFs and the heterotetrameric core of the histone octamer. Nature. 1996;380:316–322. doi: 10.1038/380316a0. [DOI] [PubMed] [Google Scholar]
- 275.Xu L, Lavinsky R M, Dasen J S, Flynn S E, McInerney E M, Mullen T M, Heinzel T, Szeto D, Korzus E, Kurokawa R, Aggarwal A K, Rose D W, Glass C K, Rosenfeld M G. Signal-specific co-activator domain requirements for Pit-1 activation. Nature. 1998;395:301–306. doi: 10.1038/26270. [DOI] [PubMed] [Google Scholar]
- 276.Xu W, Edmondson D G, Roth S Y. Mammalian GCN5 and P/CAF acetyltransferases have homologous amino-terminal domains important for recognition of nucleosomal substrates. Mol Cell Biol. 1998;18:5659–5669. doi: 10.1128/mcb.18.10.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Yamamoto T, Horikoshi M. Novel substrate specificity of the histone acetyltransferase activity of HIV-1-Tat interactive protein Tip60. J Biol Chem. 1997;272:30595–30598. doi: 10.1074/jbc.272.49.30595. [DOI] [PubMed] [Google Scholar]
- 278.Yamashita I. Isolation and characterization of the SUD1 gene, which encodes a global repressor of core promoter activity in Saccharomyces cerevisiae. Mol Gen Genet. 1993;241:616–626. doi: 10.1007/BF00279904. [DOI] [PubMed] [Google Scholar]
- 279.Yang X-J, Ogryzko V V, Nishikawa J, Howard B H, Nakatani Y. A p300/CBP-associated factor that competes with the adenoviral E1A oncoprotein. Nature. 1996;382:319–324. doi: 10.1038/382319a0. [DOI] [PubMed] [Google Scholar]
- 280.Yao T P, Ku G, Zhou N, Scully R, Livingston D M. The nuclear hormone receptor coactivator SRC-1 is a specific target of p300. Proc Natl Acad Sci USA. 1996;93:10626–10631. doi: 10.1073/pnas.93.20.10626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Yu J, Madison J M, Mundlos S, Winston F, Olsen B R. Characterization of a human homologue of the Saccharomyces cerevisiae transcription factor Spt3 (SUPT3H) Genomics. 1998;53:90–96. doi: 10.1006/geno.1998.5500. [DOI] [PubMed] [Google Scholar]
- 282.Yu Y, Eriksson P, Stillman D J. Architectural transcription factors and the SAGA complex function in parallel pathways to activate transcription. Mol Cell Biol. 2000;20:2350–2357. doi: 10.1128/mcb.20.7.2350-2357.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Zhang W, Bieker J J. Acetylation and modulation of erythroid Krüppel-like factor (EKLF) activity by interaction with histone acetyltransferases. Proc Natl Acad Sci USA. 1998;95:9855–9860. doi: 10.1073/pnas.95.17.9855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zhu Y, Pe'ery T, Peng J, Ramanathan Y, Marshall N, Marshall T, Amendt B, Mathews M B, Price D H. Transcription elongation factor P-TEFb is required for HIV-1 tat transactivation in vitro. Genes Dev. 1997;11:2622–2632. doi: 10.1101/gad.11.20.2622. [DOI] [PMC free article] [PubMed] [Google Scholar]