Abstract
Phenobarbital (PB) is a commonly prescribed anti-epileptic drug that can also benefit newborns from hyperbilirubinemia. Being the first drug demonstrating hepatic induction of cytochrome P450 (CYP), PB has since been broadly used as a model compound to study xenobiotic-induced drug metabolism and clearance. Mechanistically, PB-mediated CYP induction is linked to a number of nuclear receptors, such as the constitutive androstane receptor (CAR), pregnane X receptor (PXR), and estrogen receptor α, with CAR being the predominant regulator. Unlike prototypical agonistic ligands, PB-mediated activation of CAR does not involve direct binding with the receptor. Instead, dephosphorylation of threonine 38 in the DNA-binding domain of CAR was delineated as a key signaling event underlying PB-mediated indirect activation of CAR. Further studies revealed that such phosphorylation sites appear to be highly conserved among most human nuclear receptors. Interestingly, while PB is a pan-CAR activator in both animals and humans, PB activates human but not mouse PXR. The species-specific role of PB in gene regulation is a key determinant of its implication in xenobiotic metabolism, drug–drug interactions, energy homeostasis, and cell proliferation. In this review, we summarize the recent progress in our understanding of PB-provoked transactivation of nuclear receptors with a focus on CAR and PXR.
SIGNIFICANCE STATEMENT
Extensive studies using PB as a research tool have significantly advanced our understanding of the molecular basis underlying nuclear receptor-mediated drug metabolism, drug-drug interactions, energy homeostasis, and cell proliferation. In particular, CAR has been established as a cell signaling-regulated nuclear receptor in addition to ligand-dependent functionality. This mini-review highlights the mechanisms by which PB transactivates CAR and PXR.
1. Introduction
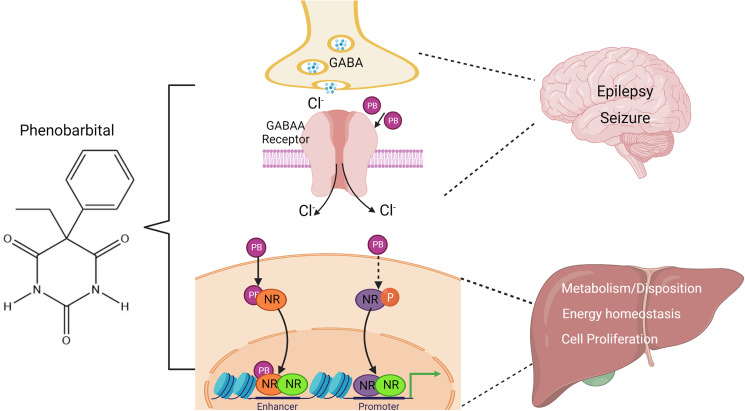
Phenobarbital (PB), known for its sedative and hypnotic properties, is one of the most widely used anti-epileptic drugs (Brodie and Kwan, 2004). Pharmacological studies revealed that PB facilitates γ-aminobutyric acid (GABA)-mediated inhibition via allosteric interaction with the neuronal postsynaptic GABA-A receptor in epilepsy treatment (MacDonald et al., 1989). Beyond the central nervous system, PB is used for the treatment of neonatal jaundice mainly due to its induction of the UDP-glucuronosyltransferase 1A1 (UGT1A1), which facilitates bilirubin conjugation and clearance in the liver (Sugatani et al., 2001; Smith et al., 2005; Kaabneh et al., 2015). In addition to these clinical implementations, accumulating evidence indicates that PB can profoundly influence liver biology involving xenobiotic biotransformation, lipid/glucose metabolism, and cell proliferation by modulating the activity of nuclear receptors (NRs), including the constitutive androstane receptor (CAR, NR1I3), pregnane X receptor (PXR, NR1I2), and estrogen receptor α (ERα, NR3A1) (Fig. 1) as reviewed previously (Sueyoshi and Negishi, 2001; Wang and Negishi, 2003; Elcombe et al., 2014; Negishi et al., 2020; Yoshinari and Shizu, 2022).
Fig. 1.
Effects of PB on the brain and liver. In the brain, PB enhances GABA responses in the neurons by binding to the GABAA-receptor in the postsynaptic membrane, which increases synaptic inhibition and elevates seizure threshold. In the liver, PB transactivates a number of nuclear receptors through both direct and indirect mechanisms to alter the expression of genes associated with drug metabolism and disposition, lipid and glucose metabolism, and cell proliferation.
PB-mediated induction of the cytochrome P450 (CYP) was first observed in liver of treated rats in the early 1960s (Remmer and Merker, 1963; Kato, 1966). CYP2B genes, encoding the major PB-inducible CYP enzymes, were isolated and characterized from rat and human livers approximately twenty years later (Santisteban et al., 1988; Yamano et al., 1989). Thenceforward, PB and the highly inducible CYP2B have been studied extensively as a pair of research tools facilitating our understanding of the inductive expression of drug-metabolizing enzymes (Desrochers et al., 1996; Czekaj, 2000). Significant progress in our understanding of the mechanisms by which PB induces CYP2B expression was achieved when a 163-bp fragment, termed a PB-responsive element which confers PB responsiveness, was identified upstream of the rat CYP2B gene (Trottier et al., 1995). Further characterization narrowed the core sequence to a 51-bp PB-responsive enhancer module (PBREM) in the mouse CYP2B10 promoter (Honkakoski et al., 1998a), and subsequent studies confirmed that the PBREM is evolutionarily conserved across different species (Sueyoshi et al., 1999). The fact that the PBREM contains two nuclear receptor DR4 (direct repeat spaced by 4 nucleotides) binding motifs has triggered the search for nuclear receptors as potential mediators modulating the PB-mediated induction.
In 1998, Negishi and colleagues made seminal findings that mechanistically linked CAR to PB-mediated CYP2B induction (Honkakoski et al., 1998b). Cell-based luciferase reporter and DNA-affinity chromatography experiments indicated that CAR stimulates CYP2B transcription by binding and transactivating the PBREM (Honkakoski et al., 1998b). The definitive role of CAR in PB-mediated induction of CYP2B was further confirmed in CAR knockout (−/−) mice, whereby disruption of CAR fully abolished PB-induced CYP2B10 expression (Wei et al., 2000). Over the years, numerous chemicals have been identified as PB-like inducers that promote CYP2B expression through the activation of CAR (Wang et al., 2012; Lynch et al., 2013; Mackowiak and Wang, 2016; Honkakoski, 2022). Interestingly, while many compounds activate CAR through direct ligand binding, PB and a class of PB-type compounds provoke CAR activation without direct binding to the receptor (Kawamoto et al., 1999; Tzameli et al., 2000; Maglich et al., 2003). The molecular basis underlying this indirect activation of CAR by PB has since been a heightened research topic in the field of drug metabolism and disposition. Without ligand binding, activation of CAR by PB predominantly involves promoting nuclear translocation of CAR from the cytoplasm, with PB-mediated dephosphorylation of CAR as a key signaling event.
In addition to CAR, pregnane X receptor (PXR) is another nuclear receptor that is involved in the regulation of PB-mediated induction of CYP genes. As the closest members in the nuclear receptor superfamily tree, CAR and PXR share numerous common chemical activators as well as co-regulated target genes (Xie et al., 2000; Wang and LeCluyse, 2003). Most recently, Bwayi et al. uncovered that CAR and PXR can mutually inhibit each other’s activity by forming a PXR-CAR heterodimer, further expanding the crosstalk between these two receptors (Bwayi et al., 2022). Interestingly, PB, a selective mouse CAR (mCAR) activator, is a dual activator of both human CAR (hCAR) and human PXR (hPXR). Additional studies found that PB-mediated dephosphorylation of CAR can also affect CAR interaction with other nuclear receptors such as ERα, hepatocyte nuclear factor 4α, RAR-related orphan receptor alpha (RORα) and alter their target gene expression (Miao et al., 2006; Fashe et al., 2018; Yi et al., 2020). This review aims to highlight the recent advances in our understanding of the molecular bases underlying PB-mediated nuclear receptor activation, with the focus on cell signaling control of CAR and PXR.
2. PB-Mediated Activation of CAR
Originally named MB67, CAR is an orphan nuclear receptor cloned by screening a human liver cDNA library (Baes et al., 1994). An initial study demonstrated that CAR forms a heterodimer with the retinoid X receptor (RXR) that can bind to and spontaneously transactivate a DR5 type retinoic acid response element in the absence of retinoic acids or any exogenous ligand (Choi et al., 1997). Due partly to the constitutive activation nature of CAR, the first class of mCAR ligands identified were androstane metabolites, androstanol and androstenol, which function as inverse agonists reversing CAR transactivation (Forman et al., 1998). While this finding led to the name of CAR changing from “constitutive active receptor” to “constitutive androstane receptor,” the concentrations required for the androstanes to antagonize CAR activity significantly exceed their plasma levels, challenging their physiologic relevance. The feature of constitutive activation differentiates CAR from most ligand-dependent nuclear hormone receptors, and accumulating evidence indicates that an endogenous ligand may not be essential for the modulation of CAR-mediated gene transactivation.
2.1. Indirect Activation of CAR
Administration of PB in rodents is known to result in rapid liver enlargement, quantitative increase of liver microsomes, and induction of drug-metabolizing enzymes with CYP2B as the representative CYP isoform (Platt and Cockrill, 1967; Honkakoski et al., 1996; Watanabe et al., 2000). Systematic analysis of the upstream regions of CYP2B genes led to the identification of the evolutionarily conserved PBREM at approximately -2k bp upstream from the transcription start site of mouse CYP2B10, rat CYP2B1/2, and human CYP2B6 genes, as well as a xenobiotic-responsive enhancer module located -8k bp in the CYP2B6 upstream region (Trottier et al., 1995; Honkakoski et al., 1998a; Wang et al., 2003). Together, these response elements coordinate the optimal induction of CYP2B genes by xenobiotic inducers, including PB, through their interaction with nuclear receptors, such as CAR.
Within the responsive enhancer modules, the DR4 motifs containing two AGGTCA-like hexamers exhibit the highest binding affinity to the CAR-RXR heterodimer (Honkakoski et al., 1998b). In PB-treated mice, binding of the PBREM-DR4 probe with the hepatic nuclear extracts containing CAR and RXR increased rapidly preceding the elevation of the CYP2B10 mRNA level. Functionally, disruption of the DR4 motifs markedly decreased PB-triggered luciferase reporter activity in transfected primary hepatocytes (Sueyoshi et al., 1999; Wang et al., 2003). In HepG2 cells transfected with mCAR, PBREM-driven luciferase reporter was spontaneously activated without PB treatment, while the activation of mCAR was further boosted by the co-transfected RXR, which is consistent with the constitutive nature of CAR activation and supports the importance of RXR as a functional pattern of CAR (Honkakoski et al., 1998b). In a HepG2 cell line stably expressing mCAR (g2car-3), androstanol-repressed CYP2B6 expression was efficiently restored by PB and 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (Sueyoshi et al., 1999). Notably, unlike the observations in HepG2 cells, PB-mediated activation of PBREM and induction of CYP2B in primary hepatocytes and mouse liver in vivo do not require pre-suppression of CAR by specific inverse agonists. Western blotting and immunochemistry/fluorescence staining analyses revealed that without chemical stimulation, CAR is predominantly restricted in the cytoplasm of primary hepatocytes in vitro and mouse liver in vivo, maintaining a low basal activity, and is translocated into the nucleus upon exposure to PB or other CAR activators (Zelko et al., 2001; Li et al., 2009). On the other hand, CAR is constitutively expressed in the nucleus of HepG2 and other immortalized cell lines with inherent high-basal activities (Baes et al., 1994; Chen et al., 2010). These findings have spawned considerable interest in the identification of cellular factors that sequester CAR in the cytoplasm of hepatocytes maintained under a physiologically relevant microenvironment. It is noteworthy that although activation of CAR is a key mode of action for PB-mediated CYP2B induction, PB had no effect on the steroid receptor coactivator 1 (SRC-1) interaction with mCAR or hCAR, did not compete with [3H]clotrimazole for binding to hCAR even at a concentration (1 mM) that is frequently used for CYP2B induction and CAR activation in primary hepatocytes, and failed to directly bind to hCAR in a surface plasmon resonance binding experiment (Moore et al., 2000; Tzameli et al., 2000; Li et al., 2019). These and other evidence clearly establish PB as a ligand-independent (indirect) CAR activator that functions primarily by provoking its nuclear translocation.
Nuclear translocation from the cytoplasm upon activation is a phenomenon shared by many transcription factors, such as the glucocorticoid receptor and aryl hydrocarbon receptor (AhR) (Yang and DeFranco, 1996; Heid et al., 2000). Before activation, these receptors were retained in the cytoplasm by forming protein complexes with common molecular chaperones including heat shock protein (Hsp) 90 (Hsp90) and Hsp70, as well as specific co-chaperone partners, such as P23 and Ah receptor-associated protein (Carver et al., 1998; Wilson and Bradfield, 2021; Noddings et al., 2022). In the case of CAR, studies initially showed that cytoplasmic CAR interacts with Hsp90, cytoplasmic CAR retention protein (CCRP), and a membrane-associated subunit of protein phosphatase 1β (PPP1R16A) (Kobayashi et al., 2003; Sueyoshi et al., 2008). Overexpression of CCRP in HepG2 cells retains CAR in the cytoplasm by forming the CAR-CCRP-Hsp90 complex (Timsit and Negishi, 2014). Knockout CCRP in mice, however, only moderately affected the intrinsic mCAR localization, while subsequent PB treatment markedly accumulated CAR in the nucleus (Ohno et al., 2014). Utilizing a liver-specific knockout mouse model, Guo et al. found that loss of the peroxisome proliferator-activated receptor binding protein (PBP) abrogates PB-induced CAR nuclear translocation, while adenoviral expression of PBP and EGFP-CAR restored the PB response in PBPliver−/− mice (Guo et al., 2006). GST-pull down experiments further demonstrated that PBP binds to CAR through interaction with the C-terminal AF-2 domain. This finding however appears to be contradictory to Zelko’s findings where deleting the AF-2 domain of hCAR didn’t affect PB-induced nuclear translocation (Zelko et al., 2001). Thus, the role of the AF-2 domain in PBP-mediated nuclear import of CAR in a physiologically relevant system remains unclear.
The membrane subunit of protein phosphatase 1β, PPP1R16A, was another protein identified as a CAR-binding partner that entails CAR nuclear translocation and activation (Sueyoshi et al., 2008). Co-expression of PPP1R16A enhanced CAR nuclear accumulation in mouse liver. PB treatment facilitates PPP1R16A intermolecular interaction and homodimer formation, which is correlated with increased CAR nuclear localization. Of note, while colocalization of CAR and PPP1R16A on the hepatocyte membrane was visualized, whether this membrane-associated subunit of protein phosphatase 1 also contributes to the noncanonical signaling of CAR is yet to be elucidated. Collectively, while these co-chaperone partners, such as CCRP, PBP, and PPP1R16A, are functionally associated with PB-mediated translocation of CAR, none of these individual proteins is indispensable. To this end, the cytoplasmic protein complex of CAR is incomplete. Moreover, signaling molecules such as glutamate receptor-interacting protein 1, protein phosphatase 1β, protein kinase A, protein phosphatase 2 (PP2A), and extracellular signal-regulated protein kinase 1/2 (ERK1/2) were also reported to be associated with PB-induced CAR translocation, further exemplifying the complexity of this process (Miao et al., 2006; Osabe and Negishi, 2011; Shizu et al., 2017).
2.2. Phosphorylation of CAR
The fact that okadaic acid blocks PB-mediated nuclear translocation of CAR in hepatocytes suggests the potential involvement of PP2A and phosphorylation/dephosphorylation of CAR as a key signaling pathway governing cellular localization of CAR (Kawamoto et al., 1999). It has also triggered a systematic search and characterization of various serine (Ser) and threonine (Thr) residues of CAR as phosphorylation sites potentially responsible for CAR translocation. Yoshinari et al. showed that in response to PB treatment CAR forms a complex with Hsp90 in the cytoplasm to recruit PP2A (Yoshinari et al., 2003). Given that a LXXLXXL motif near the C-terminus of CAR is associated with PB-inducible translocation (Zelko et al., 2001), these findings led to the initial speculation that PB may dephosphorylate amino acids in the ligand-binding domain (LBD) for CAR translocation. Sequence alignment analysis revealed four Ser and three Thr residues conserved in the LBD of mCAR, hCAR, and mPXR. Mutation of these residues to aspartic acid (D) as phosphomimetics indicated that only the S202D mutant lost PB-triggered nuclear translocation of CAR in mouse liver (Hosseinpour et al., 2006). Western blotting analysis using an antibody specific to a peptide containing phospho-Ser-202 detected a high molecular weight phosphorylated form of CAR in the wild-type (WT) mCAR transfected HepG2 cells. Notably, the S202A mutant, a dephosphorylated mimic, remains in the cytoplasm of unchallenged hepatocytes in mouse liver and is accumulated in the nucleus after PB treatment, suggesting dephosphorylation of Ser-202 alone is not sufficient to confer nuclear translocation of CAR.
In searching for additional phosphorylation sites of CAR, Mutoh et al. identified Thr-38, a protein kinase C (PKC) site of hCAR, as the primary residue responsible for PB-induced nuclear translocation and activation of CAR (Mutoh et al., 2009). Positioned in the DNA-binding domain (DBD) between two zinc finger motifs, the Thr-38 like PKC site is conserved among hCAR, mCAR, human vitamin D receptor, human thyroid hormone receptor-β, human peroxisome proliferator-activated receptor alpha, and hepatocyte nuclear factor 4α. Phosphomimetic mutant T38D not only disrupts PB-induced CAR translocation but also prevents its interaction with the PBREM of CYP2B. Importantly, PB dephosphorylates Thr-38 of endogenous CAR in both mouse and human primary hepatocytes (Mutoh et al., 2009; Yang et al., 2014). Nuclear translocation of CAR is known to be a process modulated by multiple cellular factors. While there might be residues yet to be identified as phosphorylation sites of CAR promoting its cellular localization/translocation, evidence thus far supports the notion that Thr-38 is the primary determinant controlling CAR localization in comparison with other reported residues, such as Ser-202. Thereby, 1) unlike S202A, the non-phospho-T38A is spontaneously accumulated in the nucleus of mouse hepatocytes; 2) PB-induced dephosphorylation of Thr-38 but not Ser-202 of endogenous CAR has been observed in mouse liver and primary hepatocytes; and 3) phosphorylation of Thr-38 caused a conformation change that impairs CAR-PBREM interaction, while the phosphorylation status of Ser-202 has no effect on CAR-DNA binding. Moreover, Thr-38-like phosphorylation sites located within the PKC motif in the DBD have been identified in 41 out of the 48 human nuclear receptors (Negishi et al., 2020), suggesting this residue may have evolutionarily preserved functions in nuclear receptor cellular localization, degradation, and transactivation.
2.3. Epidermal Growth Factor Receptor (EGFR) as Direct Target of PB
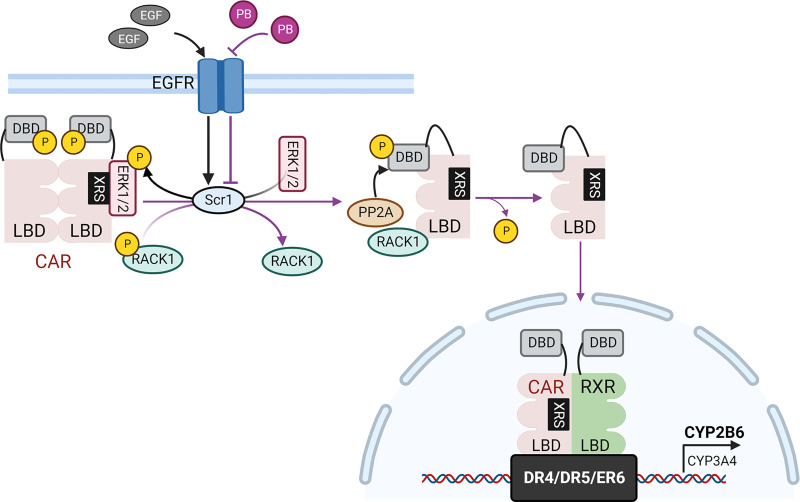
It is now evident that PB can elicit CAR activation by manipulating a series of signaling pathways, leading to dephosphorylation and nuclear translocation of CAR without direct ligand binding. A long-lasting question, however, remains: what is the exact molecular target of PB initiating the cascade resulting in CAR activation? Previous studies have shown that transforming growth factor alpha and epidermal growth factor (EGF) are capable of suppressing hepatic expression of CYP enzymes, such as rat CYP2C11, mouse CYP1A1 and CYP2B10 in cultured hepatocytes (Aubrecht et al., 1995; Ching et al., 1996). More specifically, exposure of EGF in mouse and rat primary hepatocytes suppressed PB-induced CYP2B expression and abolished PB-stimulated PBREM-luciferase activity (Aubrecht et al., 1995; Kietzmann et al., 1999; Bauer et al., 2004). Subsequent studies uncovered that activation of the MEK/ERK1/2 signaling pathway represses nuclear translocation of CAR in mouse hepatocytes, which could be reversed by U0126, an MEK inhibitor that can augment CYP2B induction (Joannard et al., 2006; Koike et al., 2007). Mechanistically, phosphorylated ERK1/2 directly binds to and sequesters phosphorylated CAR in the cytoplasm, and the phosphorylation level of Thr-38 was increased by EGF while decreased by U0126 (Osabe and Negishi, 2011).
Given the contrasting effects of PB and EGF on phosphorylation and activation of CAR, the epidermal growth factor receptor (EGFR) has become an investigation target elucidating mechanisms underlying PB-mediated CAR activation. In 2013, Mutoh et al. demonstrated that through direct binding competition, PB antagonizes EGF-induced activation (phosphorylation) of EGFR, which in turn dephosphorylates the Tyr-52 residue of the receptor for activated C kinase 1 (RACK1) facilitating PP2A dephosphorylation of CAR (Mutoh et al., 2013). Using isothermal titration calorimetry and competitive binding assays, an ED50 value of PB repressing EGF binding to EGFR was estimated at approximately 12 µM. Dynamic computer simulation pointed out that PB can bind to EGFR at multiple sites within or near the EGF binding territory, leading to direct competition between EGF and PB. Yeast two-hybrid and coimmunoprecipitation assays showed that dephosphorylated RACK1 preferentially binds to the LBD of phosphomimetic T38D mutant and recruits PP2A to form a CAR-RACK1-PP2A complex for CAR dephosphorylation (Fig. 2). The importance of RACK1 as a key mediator bridging EGFR, PP2A, and CAR was further confirmed by in vitro dephosphorylation assays demonstrating PP2A dephosphorylates Thr-38 of CAR only in the presence of RACK1; knockdown of RACK1 in mouse primary hepatocytes markedly attenuated PB-induced Thr-38 dephosphorylation and CYP2B10 expression. Moreover, other cell signaling molecules, such as insulin-like growth factor can also stimulate RACK1 phosphorylation and repress PB-mediated CYP induction (Yasujima et al., 2016).
Fig. 2.
Mechanism of PB activation of CAR. PB antagonizes EGFR activity by directly binding to the receptor and competing with EGF, leading to decreased activity of the Scr kinase 1 which in turn reduces the phosphorylation of ERK1/2 and RACK1. This signaling alteration results in dissociation of the nonactive CAR homodimer and release of ERK1/2. The dephosphorylated RACK1 recruits PP2A to the CAR monomer to remove phosphor from Thr-38. Subsequently, the dephosphorylated CAR translocates into the nucleus, forms a heterodimer with RXR, binds to response elements containing DR4, DR5, or ER6, and triggers the expression of target genes, such as CYP2B6 and CYP3A4.
In addition to forming a protein complex with co-chaperones in the cytoplasm, crystal structure analysis suggested CAR could exist as a homodimer through its own helices’ interactions (Shan et al., 2004; Xu et al., 2004). Shizu et al. experimentally demonstrated that CAR constructs a cytoplasmic homodimer in a configuration that seals the RACK1/PP2A binding site within its dimer interface, preventing CAR from dephosphorylation (Shizu et al., 2017). This homodimerization was further strengthened by EGF, while reversed by PB via antagonizing the EGFR signaling, leading to monomerization of CAR and exposure of the RACK1/PP2A binding site for Thr-38 dephosphorylation. Interestingly, both 6-(4-chlorophenyl)imidazo[2,1-b][1,3]thiazole-5-carbaldehyde o-(3,4-dichlorobenzyl)oxime (CITCO, a hCAR agonist) and 1,4-bis-[2-(3,5-dichloropyridyloxy)] benzene (a mCAR agonist) can disrupt the CAR homodimer by directly binding to the LBD to generate CAR monomers, suggesting conversion of the CAR homodimer to a monomer is a key step in CAR activation shared by both direct and indirect activators. More recently, Shizu et al. uncovered that the phosphorylation status of CAR also controls its intramolecular interaction between the DBD and LBD, where Thr-38 phosphorylated DBD interacts with the LBD as an inactive monomer with the homodimer interface masked (Shizu et al., 2018). EGF diminished this internal DBD–LBD interaction favoring the formation of a homodimer. Thus, phosphorylated CAR undergoes homodimer–monomer transition in the cytoplasm and PB treatment recruits RACK1/PP2A to the transiently inactive monomer for subsequent Thr-38 dephosphorylation and CAR activation (Fig. 2).
Collectively, these findings elegantly illustrated the molecular basis of CAR trafficking between the cytoplasm and nucleus upon chemical stimulation. EGFR has been defined as the initial PB binding partner mediating its dephosphorylation and activation of CAR in the liver. This is the first to identify a “phenobarbital-responsive receptor” with direct PB binding after a decades-long quest. It is worth mentioning, however, that although PB binds to EGFR with a Kd value around 12 µM and PB at 10 µM can inhibit EGF-mediated activation of EGFR in mouse primary hepatocytes, maximal induction of CYP2B genes and activation of CAR by PB are often achieved at millimolar concentrations, suggesting the potential involvement of additional mechanisms.
2.4 PB-Mediated CAR-Independent Gene Expression
PB is known to exhibit pleiotropic biologic effects involving xenobiotic metabolism, energy homeostasis, cell proliferation, and allosteric modulation via the regulation of numerous genes in tissue- and species-specific manners. While CAR has been established as a key nuclear receptor regulating PB-mediated gene expression, explicitly profiling CAR-independent gene transcription became possible when CAR−/− mice were generated. Using a cDNA microarray containing 8736 mouse genes/ESTs, Ueda et al. compared hepatic gene expression profiles between WT and CAR−/− mice treated with vehicle control or PB (Ueda et al., 2002). Interestingly, out of a total of 138 genes altered by PB treatment, 37 genes were induced and 23 genes repressed, in a CAR-independent manner. Among others, an important enzyme in heme biosynthesis (aminolevulinate synthase 1) and two cholesterol biosynthesis enzymes (squalene epoxidase and 7-dehydroxycholesterol) were induced by PB in both WT and CAR−/− mice, suggesting the known effects of PB on supplying heme for CYP production and elevating plasma level of cholesterol are independent of CAR.
Considering the difference of CAR biology and PB response between species, Li et al. carried out an RNA-seq analysis of the global transcriptomes in PB and CITCO treated WT and hCAR−/− HepaRG cells (Li et al., 2015a), a functional surrogate of human primary hepatocytes (Jackson et al., 2016). Besides CAR-dependent gene manipulation, PB upregulated the expression of 72 genes while down regulated 22 genes in both WT and hCAR−/− HepaRG cell lines, which include many genes encoding CYP enzymes (i.e., CYP1A1, CYP2C9, CYP2B6, CYP3A4), transport proteins (ABCB1, ABCC2, SLC5A12), and genes associated with skeletal system development and cell growth. Notably, unlike that of CITCO, a selective hCAR agonist, PB robustly induced the expression of CYP2B6 in hCAR−/− HepaRG cells; this also differs from the lack of Cyp2b10 induction by PB in mCAR−/− mice. Further analyses of the differential expression profiles revealed that among a total of 118 genes induced by CITCO, only 13 (10%) genes were upregulated in both WT and hCAR−/− HepaRG cells independent of CAR, while 72 (40%) out of 190 genes induced by PB fell into this category. These results are consistent with the promiscuous role of PB in gene regulation which involves other transcription factors beyond CAR.
3. PB-Mediated Activation of PXR
Highly expressed in the liver and small intestines containing a bulky LBD, PXR was initially defined as a promiscuous xenobiotic sensor that can be activated by numerous clinical drugs, environmental chemicals, and steroid metabolites (Jones et al., 2000; Watkins et al., 2001; Noble et al., 2006). The PXR–RXR heterodimer can recognize and bind to the relatively flexible PXR-DNA response elements containing DR3, DR4, DR5, ER6 (everted repeat spaced by six nucleotides), ER8, or IR0 (inverted repeat spaced by zero nucleotides) in the non-coding regions of its target genes. Thus, activation of PXR is known to alter the transcription of a significant number of genes encoding phase I (e.g., CYP3A4, CYP2B6, and CYP2Cs), phase II drug-metabolizing enzymes (e.g., UGT1A1, SULT2A1, and GSTA2), and uptake and efflux transporters (e.g., OATPs, MRPs, and SLC13A5) with CYP3A as its signature target genes (Lehmann et al., 1998; Xie et al., 2000; Goodwin et al., 2001; Duanmu et al., 2002; Li et al., 2015b). Mounting evidence also pointed out an endobiotic function of PXR impacting lipid and glucose homeostasis, cell proliferation, and immune response (Mackowiak et al., 2018; Cai et al., 2021; Bautista-Olivier and Elizondo, 2022). Of note, many of these target genes of PXR overlap with that of CAR, suggesting that together, CAR and PXR coordinate a liver-centered gene network with potential pharmacological and clinical impact.
While the large and flexible ligand-binding cavity of PXR can accommodate a large number of structurally diverse ligands, the less conserved LBD sequences between rodents and humans also dictate species-dependent ligand specificity of PXR (LeCluyse, 2001; Zhou et al., 2009). For instance, the antibiotic rifampicin is a specific activator of human and rabbit PXRs without activating mouse and rat PXRs; pregnenolone-16α-carbonitrile, on the other hand, activates mouse and rat but not human and rabbit PXRs (Jones et al., 2000; Ostberg et al., 2002). Interestingly, accumulating evidence reveals that PB selectively activates mCAR but not mPXR, while it activates both their human counterparts, which may contribute to the observed differential biologic functions of PB in rodents and humans.
3.1. PB Activates hPXR but Not mPXR
Both CAR–RXR and PXR–RXR heterodimers can bind to and transactivate xenobiotic response elements containing DR3, DR4, DR5, or ER8 motifs with varying affinity and efficacy. Through functional crosstalk, CAR and PXR modulate the expression of a spectrum of distinct and overlapping target genes, whereby activation of one of the two receptors is often sufficient to induce both CYP2B and CYP3A genes (Xie et al., 2000; Faucette et al., 2006; Faucette et al., 2007). Notably, PB-mediated induction of CYP2B10 and CYP3A11 genes in WT mice was completely eliminated in the CAR−/− mice, while the response to dexamethasone, an mPXR activator, was retained, indicating that PB activates mCAR but not mPXR (Wei et al., 2002). This conclusion, however, is rather controversial with regard to activation of hCAR and hPXR.
In human primary hepatocytes, CITCO, a selective hCAR agonist, preferentially induces the expression of CYP2B6 over CYP3A4, while rifampicin, a known hPXR activator, exhibits selectivity for CYP3A4 over CYP2B6, reflecting the asymmetric crosstalk between hCAR and hPXR (Faucette et al., 2007). In contrast, PB robustly induces both CYP2B6 and CYP3A4 in human primary hepatocytes with less discernible differences, a pattern that not only differs from that of CITCO and rifampicin, but also varies from that in rats and mice where PB treatment favors the induction of CYP2B over CYP3A (Jones et al., 1992; Ariyoshi et al., 2001). Specifically, lentivirus shRNA knockdown and pharmacological inhibition (SPA70, a selective hPXR deactivator) of hPXR in human primary hepatocytes significantly suppressed PB-mediated induction of CYP3A4 (Lin et al., 2017). Moreover, in hCAR−/− HepaRG cells, PB markedly induced the expression of CYP2B6 and CYP3A4, while CITCO induction of CYP2B6 was significantly attenuated at both mRNA and protein levels (Li et al., 2019).
Further inhibition of hPXR by SPA70 in hCAR−/− HepaRG cells fully eliminated PB-mediated CYP2B6/CYP3A4 induction. Recently, Li et al. showed that PB markedly elevates the expression of solute carrier family 13 number 5 (SLC13A5), a citrate uptake transporter, through activation of PXR but not CAR (Li et al., 2021). Pharmacological inhibition and genetic modification of hCAR and hPXR revealed that disruption of hPXR but not hCAR signaling dramatically repressed PB-mediated induction of SLC13A5. Notably, disruption of hCAR (hCAR−/−) further enhanced PB-mediated induction of SLC13A5 in comparison with that in WT HepaRG cells, an observation that could be associated with the removal of CAR from competing with PXR in DNA binding and gene transactivation. Most recently, Bwayi et al. revealed a molecular basis of the inhibitory crosstalk between hPXR and hCAR, where they form a novel PXR-CAR heterodimer preventing each other’s functional interaction with RXR (Bwayi et al., 2022). Together, these findings suggest that hPXR plays a pivotal role in governing PB-mediated induction.
3.2. Mechanisms of PB Activation of hPXR
Unlike CAR, PXR was generally considered a ligand-activated transcription factor requiring direct ligand binding and coactivator recruitment to stimulate gene expression. Indeed, the majority of currently known PXR activators are agonistic ligands. This perception, however, was challenged when multiple putative phosphorylation sites in PXR were shown to influence its transcriptional activity without direct ligand binding (Negishi et al., 2020). Notably, while hPXR is one of only five human nuclear receptors (out of a total of 48) that does not preserve a Thr-38-like phosphorylation motif in the DBD, several phosphorylation residues, including Ser-8, Thr-57, Ser-114, Ser-208, Ser-350, Thr-408, and Thr-422 have been identified, with many of them located within the LBD (Lin et al., 2008; Pondugula et al., 2009; Elias et al., 2014; Hu et al., 2020). Studies have shown that these residues could be phosphorylated by kinases, such as cyclin-dependent kinase 2, protein kinase A, PKC, and vaccinia-related kinase 1, and phosphomimetic mutation of T57D and S350D markedly impaired hPXR-mediated CYP3A4 transactivation (Lin et al., 2008; Gotoh et al., 2017; Bulutoglu et al., 2019). Together, these results characterize PXR as another nuclear receptor that is regulated through both phosphorylation signaling and traditional ligand binding.
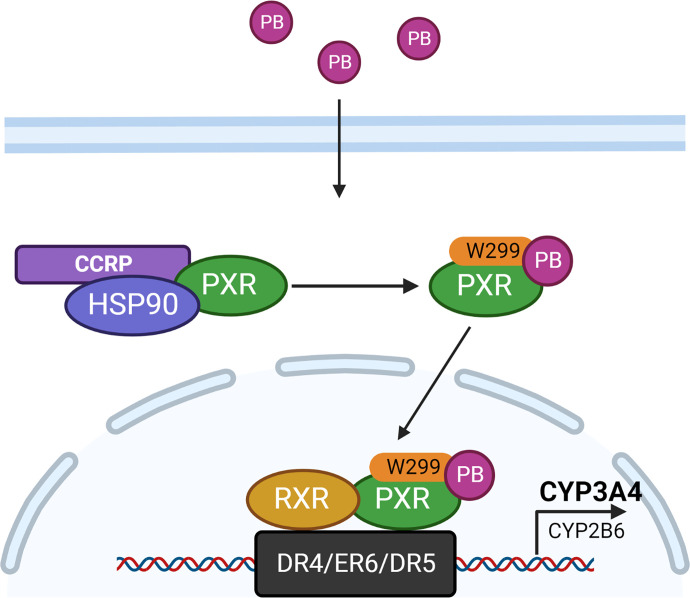
In the case of PB, luciferase reporter assays showed that PB selectively activates hPXR but not mouse and rat PXRs in transfected HepG2 and CV-1 cells (Jones et al., 2000; Li et al., 2019). Mammalian two-hybrid assays demonstrated that PB significantly enhances the recruitment of SRC-1 to the DNA-protein complex of hPXR, while not to mPXR, suggesting PB may function as an agonist of hPXR. This conception was further confirmed by a surface plasmon resonance binding assay in which PB exhibited efficient binding to hPXR with a KD value of 1.42 × 10−5 while a lack of hCAR binding is as expected (Li et al., 2019). Subsequently, computational modeling was used to gain insights into the structural basis of PB interaction with hPXR. Docking of PB into the LBD of hPXR revealed that PB is strongly linked with W299, a key amino acid that involves hydrophobic interaction with various ligands and hPXR transactivation (Li et al., 2019). Notably, mutation of W299 to a negatively charged aspartic acid or a neutral residue alanine completely abolished PB-mediated activation of hPXR.
Collectively, these findings provide convincing evidence demonstrating that PB, an indirect activator of CAR, stimulates the activation of hPXR but not mPXR through direct ligand binding (Fig. 3). Given that CAR and PXR can exhibit different even opposite biologic functions impacting lipogenesis, gluconeogenesis, and cell proliferation, dual activation of hCAR and hPXR by PB may contribute to its pharmacological discrepancy observed between rodents and humans.
Fig. 3.
Mechanism of PB activation of PXR. PB can directly bind to hPXR through close interaction with the W299 residue within the ligand-binding pocket of hPXR. This interaction facilitates the recruitment of SRC-1 to the hPXR–RXR heterodimer and induces target gene expression (This figure was adopted with minor modification from Li et al., 2019, Mol. Pharmocol).
4. Conclusion
Over the past 25 years, remarkable advances have been achieved in our understanding of the molecular basis by which PB modulates drug metabolism, drug–drug interactions, energy homeostasis, and cell proliferation in the liver. It is now evident that CAR and PXR are two key xenobiotic sensors that confer PB-mediated gene transcription. As a “universal” CAR activator, PB transactivates CAR signaling across different species through a ligand-independent mechanism triggering a cascade of cell signaling molecules, resulting in CAR dephosphorylation, nuclear translocation, and subsequent activation. On the other hand, PB activation of PXR exhibits clear species specificity where PB directly binds to the LBD of PXR and recruits transcriptional coactivators, such as SRC-1 for target gene transcription in humans but not in mice. The interspecies difference in CAR and PXR regulation by PB would be beneficial in explaining varying pharmacological effects of PB among humans and rodents and calls for further studies using human-relevant models. The mechanistic studies of PB-mediated indirect activation of CAR led to the realization of the highly conserved Thr-38 like phosphorylation sites existing in the majority of human nuclear receptors. Functional analysis reveals that altering the phosphorylation status of PXR (Thr-57, Ser-350), ERα (Ser-216, Ser-236), FXR (Ser-154), RXR (Thr-167), and RORα (Ser-100) can also affect transactivation of these receptors, suggesting phosphorylation/dephosphorylation signaling might be a fundamental function essential for the nuclear receptor superfamily (Pondugula et al., 2009; Hashiguchi et al., 2016; Fashe et al., 2020; Hu et al., 2020; Yi et al., 2022). Thus, in addition to the canonical ligand-based activation, future investigation on the phosphorylation-related “indirect activation” for a broad spectrum of nuclear receptors is warranted.
In addition to CAR and PXR, PB can influence the activity of several other nuclear receptors affecting homeostasis of estrogen and bile acids. Investigation of the mechanisms underlying PB-mediated induction of SULT1E1, an estrogen sulfotransferase, revealed that PB-activated CAR recruits ERα onto the SULT1E1 promoter as an ERα-ERα-CAR-RXR tetramer promoting transcription (Yi et al., 2020). This induction by PB was markedly suppressed in the liver of ERα non-phosphoryl S216A knock-in mice. Of note, PB-mediated ERα phosphorylation and SULT1E1 induction were lost in CAR−/− mice (Fashe et al., 2020). Likewise, Fashe et al. showed that PB can also cause the phosphorylation RORα via the activated CAR and constructs a RORα-CAR-RXR complex to induce SULT1E1 expression (Fashe et al., 2018). These findings imply that PB can alter the activity of other receptors indirectly through CAR-mediated protein-protein and protein-DNA interactions. It should be noted that PB is slowly converted to 5-p-hydroxyphenly-5-ethyl-barbituric acid in the liver, with an elimination half-life around 90 hours (Viswanathan et al., 1978). In comparison with PB, administration of barbituric acid only moderately increased CYP expression in rat liver (Lake et al., 1998). Thus, we speculate that PB-mediated nuclear receptor activation and enzyme CYP induction relies predominantly on the parent drug rather than its metabolites.
Collectively, using PB as a research tool, the mechanism of indirect CAR activation is well-appreciated now, which further establishes CAR as a cell signaling-governed nuclear receptor in addition to its ligand-dependent functionality. The highly conserved multiple phosphorylation motifs in most human nuclear receptors suggest that cell signaling-mediated regulation may represent a broadly adopted yet understudied mechanism for nuclear receptor activation. Undoubtedly, further insights into the nuclear receptor biology elicited by PB will eventually improve our understanding of its pleotropic effects on drug disposition, energy homeostasis, and cell proliferation.
Acknowledgments
The authors thank Sydney Stern, Ritika Kurian, and other members from the Wang Laboratory for critical reading of and comments on the manuscript. We apologize to the scientists who made contributions to the field but have not been cited because of space limitations.
Abbreviations
- CAR
constitutive androstane receptor
- CCRP
cytoplasmic CAR retention protein
- CITCO
6-(4-chlorophenyl)imidazo[2, 1-b][1, 3]thiazole-5-carbaldehyde o-(3, 4-dichlorobenzyl)oxime
- CYP
cytochrome P450
- DBD
DNA-binding domain
- EGF
epidermal growth factor
- EGFR
epidermal growth factor receptor
- ERα
estrogen receptor α
- ERK1/2
extracellular signal-regulated protein kinase
- GABA
γ-aminobutyric acid
- hCAR
Hsp, heat shock protein
- human constitutive androstane receptor
- hPXR
human pregnane X receptor
- LBD
ligand-binding domain
- mCAR
mouse constitutive androstane receptor
- NR
nuclear receptor
- PB
phenobarbital
- PBREM
PB-responsive enhancer module
- PBP
peroxisome proliferator-activated receptor binding protein
- PKC
protein kinase C
- PPP1R16A
membrane-associated subunit of protein phosphatase 1β
- PP2A
protein phosphatase 2
- PXR
pregnane X receptor
- RACK1
receptor for activated C kinase 1
- RORα
RAR-related orphan receptor alpha
- RXR
retinoic x receptor
- ser
serine
- SLC13A5
solute carrier family 13 member 5
- SRC-1
steroid receptor coactivator 1
- Thr
threonine
- WT
wild type
Authorship Contributions
Participated in research design: Men, Wang.
Wrote or contributed to the writing of the manuscript: Men, Wang.
Footnotes
This work was partially supported by the National Institutes of Health National Institute of General Medical Sciences [Grant R01GM121550], National Cancer Institute [Grant R01CA262084], and National Institute on Alcohol Abuse and Alcoholism [Grant R21AA028521].
The authors declare there are no actual or perceived conflicts of interest with the contents of this article.
References
- Ariyoshi N, Imaoka S, Nakayama K, Takahashi Y, Fujita K, Funae Y, Kamataki T (2001) Comparison of the levels of enzymes involved in drug metabolism between transgenic or gene-knockout and the parental mice. Toxicol Pathol 29 (Suppl):161–172. [DOI] [PubMed] [Google Scholar]
- Aubrecht J, Hirsch-Ernst KI, Becker-Rabbenstein V, Kahl GF, Taniguchi H, Höhne MW (1995) Induction of cytochrome P-4502B1-related mouse cytochrome P-450 and regulation of its expression by epidermal growth factor/transforming growth factor α in primary hepatocyte culture. Biochem Pharmacol 50:781–785. [DOI] [PubMed] [Google Scholar]
- Baes M, Gulick T, Choi HS, Martinoli MG, Simha D, Moore DD (1994) A new orphan member of the nuclear hormone receptor superfamily that interacts with a subset of retinoic acid response elements. Mol Cell Biol 14:1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer D, Wolfram N, Kahl GF, Hirsch-Ernst KI (2004) Transcriptional regulation of CYP2B1 induction in primary rat hepatocyte cultures: repression by epidermal growth factor is mediated via a distal enhancer region. Mol Pharmacol 65:172–180. [DOI] [PubMed] [Google Scholar]
- Bautista-Olivier CD, Elizondo G (2022) PXR as the tipping point between innate immune response, microbial infections, and drug metabolism. Biochem Pharmacol 202:115147. [DOI] [PubMed] [Google Scholar]
- Brodie MJ, Kwan P (2004) Phenobarbital: a drug for the 21st century? Epilepsy Behav 5:802–803. [DOI] [PubMed] [Google Scholar]
- Bulutoglu B, Mert S, Rey-Bedón C, Deng SL, Yarmush ML, Usta OB (2019) Rapid maturation of the hepatic cell line Huh7 via CDK inhibition for PXR dependent CYP450 metabolism and induction. Sci Rep 9:15848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bwayi MN, Garcia-Maldonado E, Chai SC, Xie B, Chodankar S, Huber AD, Wu J, Annu K, Wright WC, Lee HM, et al. (2022) Molecular basis of crosstalk in nuclear receptors: heterodimerization between PXR and CAR and the implication in gene regulation. Nucleic Acids Res 50:3254–3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Young GM, Xie W (2021) The xenobiotic receptors PXR and CAR in liver physiology, an update. Biochim Biophys Acta Mol Basis Dis 1867:166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver LA, LaPres JJ, Jain S, Dunham EE, Bradfield CA (1998) Characterization of the Ah receptor-associated protein, ARA9. J Biol Chem 273:33580–33587. [DOI] [PubMed] [Google Scholar]
- Chen T, Tompkins LM, Li L, Li H, Kim G, Zheng Y, Wang H (2010) A single amino acid controls the functional switch of human constitutive androstane receptor (CAR) 1 to the xenobiotic-sensitive splicing variant CAR3. J Pharmacol Exp Ther 332:106–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ching KZ, Tenney KA, Chen J, Morgan ET (1996) Suppression of constitutive cytochrome P450 gene expression by epidermal growth factor receptor ligands in cultured rat hepatocytes. Drug Metab Dispos 24:542–546. [PubMed] [Google Scholar]
- Choi HS, Chung M, Tzameli I, Simha D, Lee YK, Seol W, Moore DD (1997) Differential transactivation by two isoforms of the orphan nuclear hormone receptor CAR. J Biol Chem 272:23565–23571. [DOI] [PubMed] [Google Scholar]
- Czekaj P (2000) Phenobarbital-induced expression of cytochrome P450 genes. Acta Biochim Pol 47:1093–1105. [PubMed] [Google Scholar]
- Desrochers M, Christou M, Jefcoate C, Belzil A, Anderson A (1996) New proteins in the rat CYP2B subfamily: presence in liver microsomes of the constitutive CYP2B3 protein and the phenobarbital-inducible protein product of alternatively spliced CYP2B2 mRNA. Biochem Pharmacol 52:1311–1319. [DOI] [PubMed] [Google Scholar]
- Duanmu Z, Locke D, Smigelski J, Wu W, Dahn MS, Falany CN, Kocarek TA, Runge-Morris M (2002) Effects of dexamethasone on aryl (SULT1A1)- and hydroxysteroid (SULT2A1)-sulfotransferase gene expression in primary cultured human hepatocytes. Drug Metab Dispos 30:997–1004. [DOI] [PubMed] [Google Scholar]
- Elcombe CR, Peffer RC, Wolf DC, Bailey J, Bars R, Bell D, Cattley RC, Ferguson SS, Geter D, Goetz A, et al. (2014) Mode of action and human relevance analysis for nuclear receptor-mediated liver toxicity: A case study with phenobarbital as a model constitutive androstane receptor (CAR) activator. Crit Rev Toxicol 44:64–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elias A, High AA, Mishra A, Ong SS, Wu J, Peng J, Chen T (2014) Identification and characterization of phosphorylation sites within the pregnane X receptor protein. Biochem Pharmacol 87:360–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fashe M, Hashiguchi T, Negishi M, Sueyoshi T (2020) Ser100-Phosphorylated RORα Orchestrates CAR and HNF4α to Form Active Chromatin Complex in Response to Phenobarbital to Regulate Induction of CYP2B6. Mol Pharmacol 97:191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fashe M, Hashiguchi T, Yi M, Moore R, Negishi M (2018) Phenobarbital-induced phosphorylation converts nuclear receptor RORα from a repressor to an activator of the estrogen sulfotransferase gene Sult1e1 in mouse livers. FEBS Lett 592:2760–2768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faucette SR, Sueyoshi T, Smith CM, Negishi M, Lecluyse EL, Wang H (2006) Differential regulation of hepatic CYP2B6 and CYP3A4 genes by constitutive androstane receptor but not pregnane X receptor. J Pharmacol Exp Ther 317:1200–1209. [DOI] [PubMed] [Google Scholar]
- Faucette SR, Zhang TC, Moore R, Sueyoshi T, Omiecinski CJ, LeCluyse EL, Negishi M, Wang H (2007) Relative activation of human pregnane X receptor versus constitutive androstane receptor defines distinct classes of CYP2B6 and CYP3A4 inducers. J Pharmacol Exp Ther 320:72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman BM, Tzameli I, Choi HS, Chen J, Simha D, Seol W, Evans RM, Moore DD (1998) Androstane metabolites bind to and deactivate the nuclear receptor CAR-beta. Nature 395:612–615. [DOI] [PubMed] [Google Scholar]
- Goodwin B, Moore LB, Stoltz CM, McKee DD, Kliewer SA (2001) Regulation of the human CYP2B6 gene by the nuclear pregnane X receptor. Mol Pharmacol 60:427–431. [PubMed] [Google Scholar]
- Gotoh S, Miyauchi Y, Moore R, Negishi M (2017) Glucose elicits serine/threonine kinase VRK1 to phosphorylate nuclear pregnane X receptor as a novel hepatic gluconeogenic signal. Cell Signal 40:200–209. [DOI] [PubMed] [Google Scholar]
- Guo D, Sarkar J, Ahmed MR, Viswakarma N, Jia Y, Yu S, Sambasiva Rao M, Reddy JK (2006) Peroxisome proliferator-activated receptor (PPAR)-binding protein (PBP) but not PPAR-interacting protein (PRIP) is required for nuclear translocation of constitutive androstane receptor in mouse liver. Biochem Biophys Res Commun 347:485–495. [DOI] [PubMed] [Google Scholar]
- Hashiguchi T, Arakawa S, Takahashi S, Gonzalez FJ, Sueyoshi T, Negishi M (2016) Phosphorylation of Farnesoid X Receptor at Serine 154 Links Ligand Activation With Degradation. Mol Endocrinol 30:1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heid SE, Pollenz RS, Swanson HI (2000) Role of heat shock protein 90 dissociation in mediating agonist-induced activation of the aryl hydrocarbon receptor. Mol Pharmacol 57:82–92. [PubMed] [Google Scholar]
- Honkakoski P (2022) Searching for Constitutive Androstane Receptor Modulators. Drug Metab Dispos 50:1002–1009. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Moore R, Gynther J, Negishi M (1996) Characterization of phenobarbital-inducible mouse Cyp2b10 gene transcription in primary hepatocytes. J Biol Chem 271:9746–9753. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Moore R, Washburn KA, Negishi M (1998a) Activation by diverse xenochemicals of the 51-base pair phenobarbital-responsive enhancer module in the CYP2B10 gene. Mol Pharmacol 53:597–601. [DOI] [PubMed] [Google Scholar]
- Honkakoski P, Zelko I, Sueyoshi T, Negishi M (1998b) The nuclear orphan receptor CAR-retinoid X receptor heterodimer activates the phenobarbital-responsive enhancer module of the CYP2B gene. Mol Cell Biol 18:5652–5658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosseinpour F, Moore R, Negishi M, Sueyoshi T (2006) Serine 202 regulates the nuclear translocation of constitutive active/androstane receptor. Mol Pharmacol 69:1095–1102. [DOI] [PubMed] [Google Scholar]
- Hu H, Yokobori K, Negishi M (2020) PXR phosphorylated at Ser350 transduces a glucose signal to repress the estrogen sulfotransferase gene in human liver cells and fasting signal in mouse livers. Biochem Pharmacol 180:114197. [DOI] [PubMed] [Google Scholar]
- Jackson JP, Li L, Chamberlain ED, Wang H, Ferguson SS (2016) Contextualizing Hepatocyte Functionality of Cryopreserved HepaRG Cell Cultures. Drug Metab Dispos 44:1463–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joannard F, Rissel M, Gilot D, Anderson A, Orfila-Lefeuvre L, Guillouzo A, Atfi A, Lagadic-Gossmann D (2006) Role for mitogen-activated protein kinases in phenobarbital-induced expression of cytochrome P450 2B in primary cultures of rat hepatocytes. Toxicol Lett 161:61–72. [DOI] [PubMed] [Google Scholar]
- Jones CR, Guengerich FP, Rice JM, Lubet RA (1992) Induction of various cytochromes CYP2B, CYP2C and CYP3A by phenobarbitone in non-human primates. Pharmacogenetics 2:160–172. [DOI] [PubMed] [Google Scholar]
- Jones SA, Moore LB, Shenk JL, Wisely GB, Hamilton GA, McKee DD, Tomkinson NC, LeCluyse EL, Lambert MH, Willson TM, et al. (2000) The pregnane X receptor: a promiscuous xenobiotic receptor that has diverged during evolution. Mol Endocrinol 14:27–39. [DOI] [PubMed] [Google Scholar]
- Kaabneh MA, Salama GS, Shakkoury AG, Al-Abdallah IM, Alshamari A, Halaseh RA (2015) Phenobarbital and Phototherapy Combination Enhances Decline of Total Serum Bilirubin and May Decrease the Need for Blood Exchange Transfusion in Newborns with Isoimmune Hemolytic Disease. Clin Med Insights Pediatr 9:67–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato R (1966) Possible role of P-450 in the oxidation of drugs in liver microsomes. J Biochem 59:574–583. [DOI] [PubMed] [Google Scholar]
- Kawamoto T, Sueyoshi T, Zelko I, Moore R, Washburn K, Negishi M (1999) Phenobarbital-responsive nuclear translocation of the receptor CAR in induction of the CYP2B gene. Mol Cell Biol 19:6318–6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kietzmann T, Hirsch-Ernst KI, Kahl GF, Jungermann K (1999) Mimicry in primary rat hepatocyte cultures of the in vivo perivenous induction by phenobarbital of cytochrome P-450 2B1 mRNA: role of epidermal growth factor and perivenous oxygen tension. Mol Pharmacol 56:46–53. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Sueyoshi T, Inoue K, Moore R, Negishi M (2003) Cytoplasmic accumulation of the nuclear receptor CAR by a tetratricopeptide repeat protein in HepG2 cells. Mol Pharmacol 64:1069–1075. [DOI] [PubMed] [Google Scholar]
- Koike C, Moore R, Negishi M (2007) Extracellular signal-regulated kinase is an endogenous signal retaining the nuclear constitutive active/androstane receptor (CAR) in the cytoplasm of mouse primary hepatocytes. Mol Pharmacol 71:1217–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lake BG, Renwick AB, Cunninghame ME, Price RJ, Surry D, Evans DC (1998) Comparison of the effects of some CYP3A and other enzyme inducers on replicative DNA synthesis and cytochrome P450 isoforms in rat liver. Toxicology 131:9–20. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL (2001) Pregnane X receptor: molecular basis for species differences in CYP3A induction by xenobiotics. Chem Biol Interact 134:283–289. [DOI] [PubMed] [Google Scholar]
- Lehmann JM, McKee DD, Watson MA, Willson TM, Moore JT, Kliewer SA (1998) The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J Clin Invest 102:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Mackowiak B, Brayman TG, Mitchell M, Zhang L, Huang SM, Wang H (2015a) Genome-wide analysis of human constitutive androstane receptor (CAR) transcriptome in wild-type and CAR-knockout HepaRG cells. Biochem Pharmacol 98:190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Chen T, Cottrell J, Wang H (2009) Nuclear translocation of adenoviral-enhanced yellow fluorescent protein-tagged-human constitutive androstane receptor (hCAR): a novel tool for screening hCAR activators in human primary hepatocytes. Drug Metab Dispos 37:1098–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Li H, Garzel B, Yang H, Sueyoshi T, Li Q, Shu Y, Zhang J, Hu B, Heyward S, et al. (2015b) SLC13A5 is a novel transcriptional target of the pregnane X receptor and sensitizes drug-induced steatosis in human liver. Mol Pharmacol 87:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Welch MA, Li Z, Mackowiak B, Heyward S, Swaan PW, Wang H (2019) Mechanistic Insights of Phenobarbital-Mediated Activation of Human but Not Mouse Pregnane X Receptor. Mol Pharmacol 96:345–354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Li L, Heyward S, Men S, Xu M, Sueyoshi T, Wang H (2021) Phenobarbital Induces SLC13A5 Expression through Activation of PXR but Not CAR in Human Primary Hepatocytes. Cells 10:3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wang YM, Chai SC, Lv L, Zheng J, Wu J, Zhang Q, Wang YD, Griffin PR, Chen T (2017) SPA70 is a potent antagonist of human pregnane X receptor. Nat Commun 8:741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, Wu J, Dong H, Bouck D, Zeng FY, Chen T (2008) Cyclin-dependent kinase 2 negatively regulates human pregnane X receptor-mediated CYP3A4 gene expression in HepG2 liver carcinoma cells. J Biol Chem 283:30650–30657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch C, Pan Y, Li L, Ferguson SS, Xia M, Swaan PW, Wang H (2013) Identification of novel activators of constitutive androstane receptor from FDA-approved drugs by integrated computational and biological approaches. Pharm Res 30:489–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald RL, Rogers CJ, Twyman RE (1989) Barbiturate regulation of kinetic properties of the GABAA receptor channel of mouse spinal neurones in culture. J Physiol 417:483–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak B, Hodge J, Stern S, Wang H (2018) The Roles of Xenobiotic Receptors: Beyond Chemical Disposition. Drug Metab Dispos 46:1361–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackowiak B, Wang H (2016) Mechanisms of xenobiotic receptor activation: Direct vs. indirect. Biochim Biophys Acta 1859:1130–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglich JM, Parks DJ, Moore LB, Collins JL, Goodwin B, Billin AN, Stoltz CA, Kliewer SA, Lambert MH, Willson TM, et al. (2003) Identification of a novel human constitutive androstane receptor (CAR) agonist and its use in the identification of CAR target genes. J Biol Chem 278:17277–17283. [DOI] [PubMed] [Google Scholar]
- Miao J, Fang S, Bae Y, Kemper JK (2006) Functional inhibitory cross-talk between constitutive androstane receptor and hepatic nuclear factor-4 in hepatic lipid/glucose metabolism is mediated by competition for binding to the DR1 motif and to the common coactivators, GRIP-1 and PGC-1alpha. J Biol Chem 281:14537–14546. [DOI] [PubMed] [Google Scholar]
- Moore LB, Parks DJ, Jones SA, Bledsoe RK, Consler TG, Stimmel JB, Goodwin B, Liddle C, Blanchard SG, Willson TM, et al. (2000) Orphan nuclear receptors constitutive androstane receptor and pregnane X receptor share xenobiotic and steroid ligands. J Biol Chem 275:15122–15127. [DOI] [PubMed] [Google Scholar]
- Mutoh S, Osabe M, Inoue K, Moore R, Pedersen L, Perera L, Rebolloso Y, Sueyoshi T, Negishi M (2009) Dephosphorylation of threonine 38 is required for nuclear translocation and activation of human xenobiotic receptor CAR (NR1I3). J Biol Chem 284:34785–34792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutoh S, Sobhany M, Moore R, Perera L, Pedersen L, Sueyoshi T, Negishi M (2013) Phenobarbital indirectly activates the constitutive active androstane receptor (CAR) by inhibition of epidermal growth factor receptor signaling. Sci Signal 6:ra31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negishi M, Kobayashi K, Sakuma T, Sueyoshi T (2020) Nuclear receptor phosphorylation in xenobiotic signal transduction. J Biol Chem 295:15210–15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble SM, Carnahan VE, Moore LB, Luntz T, Wang H, Ittoop OR, Stimmel JB, Davis-Searles PR, Watkins RE, Wisely GB, et al. (2006) Human PXR forms a tryptophan zipper-mediated homodimer. Biochemistry 45:8579–8589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noddings CM, Wang RY, Johnson JL, Agard DA (2022) Structure of Hsp90-p23-GR reveals the Hsp90 client-remodelling mechanism. Nature 601:465–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno M, Kanayama T, Moore R, Ray M, Negishi M (2014) The roles of co-chaperone CCRP/DNAJC7 in Cyp2b10 gene activation and steatosis development in mouse livers. PLoS One 9:e115663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osabe M, Negishi M (2011) Active ERK1/2 protein interacts with the phosphorylated nuclear constitutive active/androstane receptor (CAR; NR1I3), repressing dephosphorylation and sequestering CAR in the cytoplasm. J Biol Chem 286:35763–35769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostberg T, Bertilsson G, Jendeberg L, Berkenstam A, Uppenberg J (2002) Identification of residues in the PXR ligand binding domain critical for species specific and constitutive activation. Eur J Biochem 269:4896–4904. [DOI] [PubMed] [Google Scholar]
- Platt DS, Cockrill BL (1967) Liver enlargement and hepatoxicity: an investigation into the effects of several agents on rat liver enzyme activities. Biochem Pharmacol 16:2257–2270. [DOI] [PubMed] [Google Scholar]
- Pondugula SR, Brimer-Cline C, Wu J, Schuetz EG, Tyagi RK, Chen T (2009) A phosphomimetic mutation at threonine-57 abolishes transactivation activity and alters nuclear localization pattern of human pregnane x receptor. Drug Metab Dispos 37:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmer H, Merker HJ (1963)DRUG-INDUCED CHANGES IN THE LIVER ENDOPLASMIC RETICULUM: ASSOCIATION WITH DRUG-METABOLIZING ENZYMES. Science 142:1657–1658. [DOI] [PubMed] [Google Scholar]
- Santisteban I, Povey S, Shephard EA, Phillips IR (1988) The major phenobarbital-inducible cytochrome P-450 gene subfamily (P450IIB) mapped to the long arm of human chromosome 19. Ann Hum Genet 52:129–135. [DOI] [PubMed] [Google Scholar]
- Shan L, Vincent J, Brunzelle JS, Dussault I, Lin M, Ianculescu I, Sherman MA, Forman BM, Fernandez EJ (2004) Structure of the murine constitutive androstane receptor complexed to androstenol: a molecular basis for inverse agonism. Mol Cell 16:907–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizu R, Min J, Sobhany M, Pedersen LC, Mutoh S, Negishi M (2018) Interaction of the phosphorylated DNA-binding domain in nuclear receptor CAR with its ligand-binding domain regulates CAR activation. J Biol Chem 293:333–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shizu R, Osabe M, Perera L, Moore R, Sueyoshi T, Negishi M (2017) Phosphorylated Nuclear Receptor CAR Forms a Homodimer To Repress Its Constitutive Activity for Ligand Activation. Mol Cell Biol 37: e00649-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CM, Faucette SR, Wang H, LeCluyse EL (2005) Modulation of UDP-glucuronosyltransferase 1A1 in primary human hepatocytes by prototypical inducers. J Biochem Mol Toxicol 19:96–108. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Kawamoto T, Zelko I, Honkakoski P, Negishi M (1999) The repressed nuclear receptor CAR responds to phenobarbital in activating the human CYP2B6 gene. J Biol Chem 274:6043–6046. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Moore R, Sugatani J, Matsumura Y, Negishi M (2008) PPP1R16A, the membrane subunit of protein phosphatase 1beta, signals nuclear translocation of the nuclear receptor constitutive active/androstane receptor. Mol Pharmacol 73:1113–1121. [DOI] [PubMed] [Google Scholar]
- Sueyoshi T, Negishi M (2001) Phenobarbital response elements of cytochrome P450 genes and nuclear receptors. Annu Rev Pharmacol Toxicol 41:123–143. [DOI] [PubMed] [Google Scholar]
- Sugatani J, Kojima H, Ueda A, Kakizaki S, Yoshinari K, Gong QH, Owens IS, Negishi M, Sueyoshi T (2001) The phenobarbital response enhancer module in the human bilirubin UDP-glucuronosyltransferase UGT1A1 gene and regulation by the nuclear receptor CAR. Hepatology 33:1232–1238. [DOI] [PubMed] [Google Scholar]
- Timsit YE, Negishi M (2014) Coordinated regulation of nuclear receptor CAR by CCRP/DNAJC7, HSP70 and the ubiquitin-proteasome system. PLoS One 9:e96092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trottier E, Belzil A, Stoltz C, Anderson A (1995) Localization of a phenobarbital-responsive element (PBRE) in the 5′-flanking region of the rat CYP2B2 gene. Gene 158:263–268. [DOI] [PubMed] [Google Scholar]
- Tzameli I, Pissios P, Schuetz EG, Moore DD (2000) The xenobiotic compound 1,4-bis[2-(3,5-dichloropyridyloxy)]benzene is an agonist ligand for the nuclear receptor CAR. Mol Cell Biol 20:2951–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda A, Hamadeh HK, Webb HK, Yamamoto Y, Sueyoshi T, Afshari CA, Lehmann JM, Negishi M (2002) Diverse roles of the nuclear orphan receptor CAR in regulating hepatic genes in response to phenobarbital. Mol Pharmacol 61:1–6. [DOI] [PubMed] [Google Scholar]
- Viswanathan CT, Booker HE, Welling PG (1978) Bioavailability of oral and intramuscular phenobarbital. J Clin Pharmacol 18:100–105. [DOI] [PubMed] [Google Scholar]
- Wang H, Faucette S, Sueyoshi T, Moore R, Ferguson S, Negishi M, LeCluyse EL (2003) A novel distal enhancer module regulated by pregnane X receptor/constitutive androstane receptor is essential for the maximal induction of CYP2B6 gene expression. J Biol Chem 278:14146–14152. [DOI] [PubMed] [Google Scholar]
- Wang H, LeCluyse EL (2003) Role of orphan nuclear receptors in the regulation of drug-metabolising enzymes. Clin Pharmacokinet 42:1331–1357. [DOI] [PubMed] [Google Scholar]
- Wang H, Negishi M (2003) Transcriptional regulation of cytochrome p450 2B genes by nuclear receptors. Curr Drug Metab 4:515–525. [DOI] [PubMed] [Google Scholar]
- Wang YM, Ong SS, Chai SC, Chen T (2012) Role of CAR and PXR in xenobiotic sensing and metabolism. Expert Opin Drug Metab Toxicol 8:803–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe J, Mondo H, Takamori Y, Takeda K, Kanamura S (2000) Effect of phenobarbital on intralobular expression of CYP2B1/2 in livers of rats: difference in the expression between single and repetitive administrations. Biochem Pharmacol 60:285–291. [DOI] [PubMed] [Google Scholar]
- Watkins RE, Wisely GB, Moore LB, Collins JL, Lambert MH, Williams SP, Willson TM, Kliewer SA, Redinbo MR (2001) The human nuclear xenobiotic receptor PXR: structural determinants of directed promiscuity. Science 292:2329–2333. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Dowhan DH, Han Y, Moore DD (2002) Specific and overlapping functions of the nuclear hormone receptors CAR and PXR in xenobiotic response. Pharmacogenomics J 2:117–126. [DOI] [PubMed] [Google Scholar]
- Wei P, Zhang J, Egan-Hafley M, Liang S, Moore DD (2000) The nuclear receptor CAR mediates specific xenobiotic induction of drug metabolism. Nature 407:920–923. [DOI] [PubMed] [Google Scholar]
- Wilson RH, Bradfield CA (2021) Rodent genetic models of Ah receptor signaling. Drug Metab Rev 53:350–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Barwick JL, Simon CM, Pierce AM, Safe S, Blumberg B, Guzelian PS, Evans RM (2000) Reciprocal activation of xenobiotic response genes by nuclear receptors SXR/PXR and CAR. Genes Dev 14:3014–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu RX, Lambert MH, Wisely BB, Warren EN, Weinert EE, Waitt GM, Williams JD, Collins JL, Moore LB, Willson TM, et al. (2004) A structural basis for constitutive activity in the human CAR/RXRalpha heterodimer. Mol Cell 16:919–928. [DOI] [PubMed] [Google Scholar]
- Yamano S, Nhamburo PT, Aoyama T, Meyer UA, Inaba T, Kalow W, Gelboin HV, McBride OW, Gonzalez FJ (1989) cDNA cloning and sequence and cDNA-directed expression of human P450 IIB1: identification of a normal and two variant cDNAs derived from the CYP2B locus on chromosome 19 and differential expression of the IIB mRNAs in human liver. Biochemistry 28:7340–7348. [DOI] [PubMed] [Google Scholar]
- Yang H, Garzel B, Heyward S, Moeller T, Shapiro P, Wang H (2014) Metformin represses drug-induced expression of CYP2B6 by modulating the constitutive androstane receptor signaling. Mol Pharmacol 85:249–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, DeFranco DB (1996) Assessment of glucocorticoid receptor-heat shock protein 90 interactions in vivo during nucleocytoplasmic trafficking. Mol Endocrinol 10:3–13. [DOI] [PubMed] [Google Scholar]
- Yasujima T, Saito K, Moore R, Negishi M (2016) Phenobarbital and Insulin Reciprocate Activation of the Nuclear Receptor Constitutive Androstane Receptor through the Insulin Receptor. J Pharmacol Exp Ther 357:367–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Fashe M, Arakawa S, Moore R, Sueyoshi T, Negishi M (2020) Nuclear receptor CAR-ERα signaling regulates the estrogen sulfotransferase gene in the liver. Sci Rep 10:5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi M, Shindo S, Negishi M (2022) Immunoprecipitation Analyses of Estrogen Receptor α Phosphorylated at Serine 216 in the Mouse Liver. Methods Mol Biol 2418:41–51. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Kobayashi K, Moore R, Kawamoto T, Negishi M (2003) Identification of the nuclear receptor CAR:HSP90 complex in mouse liver and recruitment of protein phosphatase 2A in response to phenobarbital. FEBS Lett 548:17–20. [DOI] [PubMed] [Google Scholar]
- Yoshinari K, Shizu R (2022) Distinct Roles of the Sister Nuclear Receptors PXR and CAR in Liver Cancer Development. Drug Metab Dispos 50:1019–1026. [DOI] [PubMed] [Google Scholar]
- Zelko I, Sueyoshi T, Kawamoto T, Moore R, Negishi M (2001) The peptide near the C terminus regulates receptor CAR nuclear translocation induced by xenochemicals in mouse liver. Mol Cell Biol 21:2838–2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Verma S, Blumberg B (2009) The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl Recept Signal 7:e001. [DOI] [PMC free article] [PubMed] [Google Scholar]