Abstract
Virus infections induce a proinflammatory response including expression of cytokines and chemokines. The subsequent leukocyte recruitment and antiviral effector functions contribute to the first line of defense against viruses. The molecular virus-cell interactions initiating these events have been studied intensively, and it appears that viral surface glycoproteins, double-stranded RNA, and intracellular viral proteins all have the capacity to activate signal transduction pathways leading to the expression of cytokines and chemokines. The signaling pathways activated by viral infections include the major proinflammatory pathways, with the transcription factor NF-κB having received special attention. These transcription factors in turn promote the expression of specific inducible host proteins and participate in the expression of some viral genes. Here we review the current knowledge of virus-induced signal transduction by seven human pathogenic viruses and the most widely used experimental models for viral infections. The molecular mechanisms of virus-induced expression of cytokines and chemokines is also analyzed.
A hallmark of a viral infection is an acute reaction by the infected cell. This includes activation of a preexisting antiviral defense machinery, commitment to apoptosis, and production of specific cytokines. These events contribute to the reduction of viral replication and to the limitation of viral spread. By focusing on some important human pathogenic viruses as well as widely used laboratory models for viral infections, this text will review the current knowledge of which viral components are responsible for inducing cytokine production and the mechanisms through which this occurs. The virus-induced cellular signal transduction pathways leading to the host response will also be discussed.
VIRUS-INDUCED SIGNAL TRANSDUCTION AND CYTOKINE EXPRESSION
Immediately following a viral infection, a strong host response is initiated. For a range of viruses it has now been clarified that the mere interaction of viral surface proteins with cellular surface proteins starts a cellular reaction that in many cases leads to the first wave of cytokine production after infection (Table 1 and Fig. 1). In addition, many viral proteins not present in the infectious particle but produced during the course of the viral life cycle are able to affect cellular signaling in a manner leading to cytokine production. Moreover, accumulation of viral RNA and overload of the cellular protein synthesis machinery induces signals that are able to trigger an early host response to the infection. The importance of such alert signals in the clearance of viral infections is illustrated by the fact that many viruses have adopted mechanisms to interfere with these processes (32, 33, 160, 197, 201).
TABLE 1.
Cytokines and chemokines induced by viral proteins
| Virus | Viral protein | Induced cytokines and chemokines | References |
|---|---|---|---|
| HSV | gD | TNF-α, IFN-α/β | 8; Paludan, unpublished |
| CMV | gB | IL-1β, IL-6 | 38, 266 |
| EBV | gp350 | IL-1β, IL-1Ra, IL-6, IL-8, TNF-α, GM-CSF, MIP-1α | 60, 61, 158, 204, 205, 233 |
| LMP-1 | IL-6, IL-8, IL-10 | 68, 175 | |
| EBNA2 | IFN-α/β | 117 | |
| Influenza virus | NA | IL-1α, IL-1β, TGF-β, TNF-α, IFN-α/β | 9, 44, 104, 105, 223 |
| HBV | HBx | IL-6, IL-8, TNF-α | 138, 142, 153 |
| HBsAg | IL-2, IL-10, IFN-γ | 106, 140 | |
| HBc/eAg | IL-10, TNF-α, IFN-γ | 106, 242 | |
| HIV | gp120 | IL-1α, IL-1β, IL-4, IL-6, IL-8, IL-10, IL-13, TNF-α, IFN-α/β, IFN-γ | 5, 7, 36, 73, 190, 220 |
| Tat | IL-1β, IL-2, IL-6, IL-8, TGF-β1, TNF-α, MCP-1 | 39, 100, 145, 177, 216, 252 | |
| Nef | IL-1β, IL-6, IL-10, IL-15, TNF-α, IFN-γ | 29, 63, 199 | |
| Vpr | IL-6, IL-8, IL-10, TNF-α, IFN-γ | 209 | |
| HTLV-1 | Tax | IL-1β, IL-2, IL-3 IL-5, IL-8, IL-15, TGF-β1, TNF-α, IFN-γ, GM-CSF, MIP-1α/β, IP-10, 1309, SCM-1 | 14, 55, 77, 109, 122 |
FIG. 1.
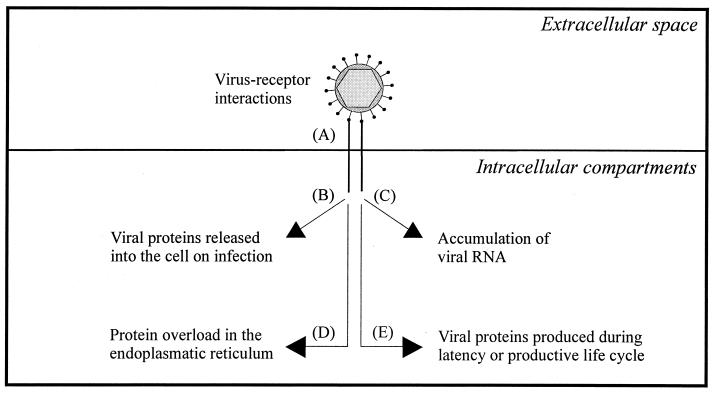
Principles in the activation of cellular signal transduction and gene expression by viruses. (A) Interaction of viral surface proteins with cellular receptors activates intracellular signaling. (B) Virion proteins released into the cytoplasm immediately after infection interact with cellular proteins. (C and D) Production of viral RNA and accumulation of large amounts of viral proteins induces stress signaling in infected cells. (E) Viral proteins produced during viral latency or the productive life cycle stimulate cellular signal transduction and gene expression.
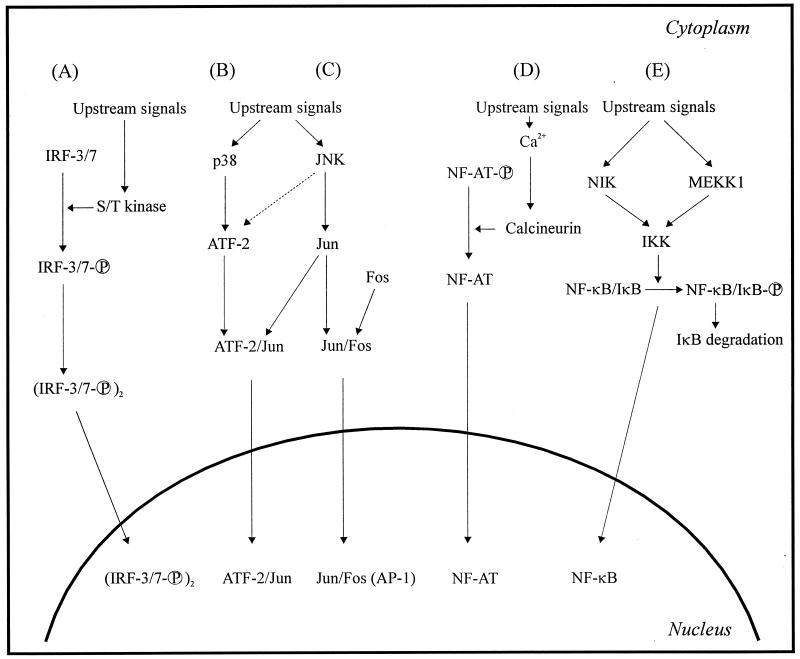
With respect to cytokine induction, some of the most important signal transduction pathways activated by viruses are shown in Fig. 2. Interferon (IFN) regulatory factor 3 (IRF-3) and IRF-7 are recently discovered virus-activated transcription factors that have been ascribed an important role in IFN-α/β expression (155). These transcription factors become activated by serine/threonine phosphorylation (see below). The mitogen-activated protein (MAP) kinases p38 and Jun N-terminal kinase (JNK) are also activated in response to many viruses. Following activation, the serine/threonine kinases phosphorylate their downstream targets, notably activating transcription factor 2 (ATF-2) and Jun, thus promoting their trans-activating potential (reference 97 and references therein). Jun can form homodimers as well as heterodimers with ATF-2 and Fos. The ATF-2/Jun dimer binds to the cyclic AMP response element (CRE), whereas the Jun homodimer and the Jun/Fos heterodimer recognize the TPA-responsive element (253). Another transcription factor activated in response to virus infection is nuclear factor of activated T cells (NF-AT). NF-AT is constitutively present in the cytoplasm in a latent phosphorylated form. Increasing levels of cytoplasmic calcium activate the calmodulin-dependent phosphatase calcineurin, which activates NF-AT by dephosphorylation (56).
FIG. 2.
Major cellular signaling pathways activated by viral infections. Virus-induced expression of cytokines and chemokines is primarily due to stimulation of one or more of the depicted signal transduction cascades, leading to activation of the following transcription factors: IRF-3 and IRF-7 (A), ATF-2/Jun (B), AP-1 (C), NF-AT (D), and NF-κB (E). For more detailed descriptions, see the text. S/T, serine/threonine.
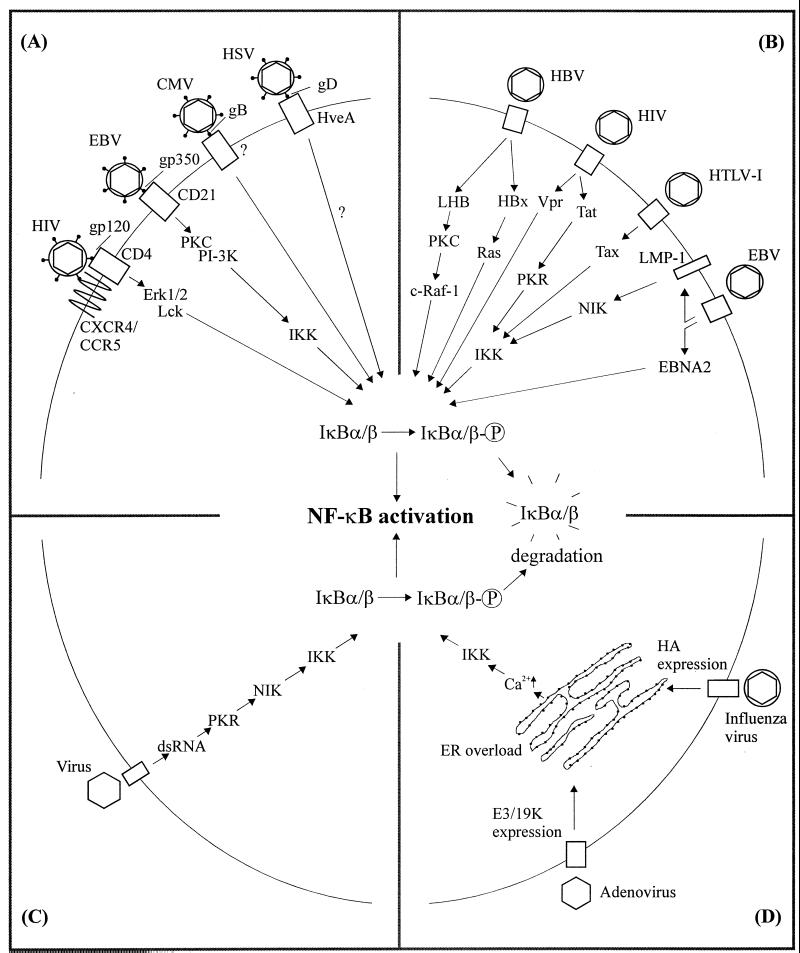
Activation of NF-κB is a hallmark of most infections including viral infections (reviewed in references 91 and 228). This transcription factor is normally found in the cytoplasm complexed with an inhibitory protein, IκB, of which various isoforms exist. Upon infection, signaling events are initiated leading to activation of MAP kinase kinase kinases (MAP3K), which promote the activation of a large kinase complex able to phosphorylate IκB at two specific amino-terminal serine residues. The kinases responsible for IκB phosphorylation are IκB kinase α (IKK α) and IKK β. Phosphorylated IκB is subsequently targeted for degradation through the ubiquitin-dependent 26S proteasome pathway. Degradation of IκB unmasks the nuclear localization signal of NF-κB, which then migrates to the nucleus and activates transcription. Table 2 summarizes how the viruses discussed in this review affect the activity of cellular transcription factors. NF-κB activation by viruses has been the subject of particularly intense investigation, and it appears that a number of different mechanisms are employed (Fig. 3). As will be described below, NF-κB plays a central role in virus-dependent cytokine expression and pathology.
TABLE 2.
Transcription factors activated by viruses
| Virus | Transcription factors |
|---|---|
| HSV | NF-κB, AP-1, ATF/CREB,a Oct-1b |
| CMV | NF-κB, AP-1, ATF/CREB, IRF-3 |
| EBV | NF-κB, AP-1, ATF-2/c-Jun, IRF-7 |
| Influenza virus | NF-κB, AP-1, ATF/CRE |
| HBV | NF-κB, AP-1, AP-2, ATF/CREB, Egr-1, C/EBPα, NF-AT, basal transcription factorsc |
| HIV | NF-κB, AP-1, Sp1,d GR,d C/EBPβ, NF-AT |
| HTLV-1 | NF-κB, AP-1, ATF-2, CREB-2,e C/EBPβe, NF-AT, Spi-1,e GBPe |
The specific nature of the CRE-binding proteins has not been determined.
Oct-1 activates immediate-early HSV promoters through interaction with VP16 and host cell factor.
The HBx protein promotes formation of the transcriptional initiation complex by interacting with the basal transcription factors TFIIB, TFIIIB and TATA-binding protein, as well as RNA polymerase II.
The HIV Vpr protein enhances the activity of Sp1 and GR by physical interaction.
Protein-protein interactions with Tax augment the activity of CREB-2, C/EBPβ, Spi-1, and GBP.
FIG. 3.
Mechanisms of NF-κB activation by viruses. (A) Viral envelope glycoproteins activate signaling through engagement of cellular receptors. (B) Virus-encoded proteins interact with cellular signaling pathways and activate NF-κB. (C) Accumulation of dsRNA activates PKR, which in turn stimulates NF-κB activity. (D) ER overload due to massive viral protein expression can lead to activation of NF-κB.
Herpes Simplex Virus
Herpes simplex virus (HSV) is a herpesvirus belonging to the subgroup Alphaherpesvirinae. This group is characterized by a rapid life cycle and spread, destruction of infected cells, and the ability to establish latency in sensory neurons. HSV causes a number of human diseases including cold sores, eye and genital infections, and encephalitis. Primary HSV infections are generally more severe than secondary infections, which tend to be more localized. On primary HSV infection, the host responds by production of a range of cytokines. These include interleukin-1β (IL-1β), IL-2, IL-6, IL-10, IL-12, IL-13, tumor necrosis factor alpha (TNF-α), IFN-α/β, IFN-γ, and granulocyte-macrophage colony-stimulating factor (GM-CSF) (69, 81, 87, 94, 187, 246). In addition, the chemokines IL-8, macrophage inflammatory protein 1α (MIP-1α) and MIP-1β, monocyte chemoattractant protein 1 (MCP-1), and RANTES are produced during a HSV infection (207, 236).
The HSV genome encodes at least 11 glycoproteins, which alone or in concert play different roles in viral adsorption, entry, cell-to-cell spread, immune evasion etc. (200). Glycoprotein D (gD) is central to viral entry, which is dependent on the interaction of gD with a cellular entry mediator. At present, three entry mediators have been identified, termed HveA, HveB, and HveC (49, 168), and of these at least the TNF receptor family member HveA is known to be a signaling receptor (156). This fact suggests that gD is an attractive candidate to play a role in the induction of the first wave of cytokines, which are insensitive to UV treatment of the virus and include IFN-α/β and TNF-α. Indeed, gD is responsible for induction of IFN-α. Ankel et al. showed that recombinant gD promotes IFN-α secretion whereas 7 other HSV glycoproteins do not (8). Likewise treatment of murine macrophages with recombinant gD triggers expression of TNF-α (S. R. Paludan, unpublished data). In addition to the rapid gD-dependent cytokine induction, HSV infection leads to the production of other cytokines through a different mechanism with slower kinetics. This mechanism is UV sensitive and involves the cytokines IL-6 (116; Paludan, unpublished) and IL-12 (154). The viral components responsible for this second wave of cytokine induction have not been unveiled.
Based on the above, HSV infection seems to trigger at least two cellular signaling pathways: one UV insensitive and dependent on gD, and one UV sensitive and dependent on a functional viral genome. The early gD-dependent signaling is not well described, but HSV-2 infection of murine macrophages induces enhanced DNA-binding activity of NF-κB and ATF-2/c-Jun within one hour post infection (Paludan, unpublished). Although it has not been demonstrated how gD induces signaling, it is interesting that HSV uses HveA as receptor and that HveA signaling leads to activation of NF-κB and activator protein 1 (AP-1) (156). Since the HveA-induced signaling pattern is compatible with the above-described observations in HSV-2-infected macrophages, it is tempting to speculate that the early gD-dependent cellular response proceeds through HveA. In fact, it has been reported that HSV-1 infection of the human cervical carcinoma cell line C33 induces a weak and transient activation of NF-κB and that this can be abrogated with antibodies against gB and gD (189).
At later stages of infection (i.e., 3 to 4 h postinfection), UV-sensitive signaling is initiated, leading to a strong activation of NF-κB and AP-1. The slower kinetics and UV-sensitive nature of the AP-1 activation are explained by the finding that viral protein 16 (VP16)-dependent transcription precedes the activation of AP-1 (159, 269) and that it depends on the immediate-early protein infected-cell polypeptide 0 (ICP0) (113, 269). The upstream kinases responsible for AP-1 activation by HSV are JNK and p38 (269). Likewise, the mechanism of sustained NF-κB activation by HSV is dependent on viral entry and immediate-early gene expression. Specifically, Patel et al. showed that viruses with defects in ICP4 and ICP27 were unable to induce persistent NF-κB activation (189). In the same study it was demonstrated that activation of NF-κB promoted viral replication as evidenced by prevention of replication by overexpression of a nondegradable form of IκBα, thus preventing NF-κB activation. A possible target for NF-κB in the viral genome is the ICP0 promoter, to which NF-κB has been reported to bind (206). Interestingly, activation of NF-κB in macrophages by HSV-2 infection is responsible for the enhanced expression of nitric oxide synthase type 2 following virus infection (186, 188), and it is well documented that nitric oxide is a potent antiviral product (57, 118). This demonstrates the double-edged-sword-like nature of NF-κB activation by HSV and other viruses, since the transcription factor is involved in both viral replication and the protective host response to the infection.
In addition to the effect of HSV infection on cellular signaling pathways, viral proteins with trans-activating potential may directly affect transcription. For instance, VP16 interacts with the host proteins octamer transcription factor 1 (Oct-1) and host cell factor (131) to induce the transcription of the immediate-early viral genes and potentially some host genes. Moreover, ICP0 cooperates with the human immunodeficiency virus (HIV) protein Tat to activate the HIV long terminal repeat (LTR) (219), and it seems very likely that ICP0 or other HSV immediate-early proteins may also modulate the function of cellular transcription factors, although this has not yet been reported.
There is some evidence about the molecular mechanisms through which HSV-induced cytokine production occurs. Fitzgerald-Bocarsly and associates reported that production of IFN-α was inhibited by the calcium chelator EGTA and was sensitive to inhibitors of protein kinase C (PKC) and tyrosine kinases, whereas inhibitors of the cyclic AMP-dependent protein kinase PKA had no effect (143). We have shown that expression of TNF-α by HSV-2-infected murine macrophages relies on NF-κB and a p38-dependent pathway, possibly ATF-2/Jun (Paludan, unpublished). Treatment of the macrophages with either pyrrolidine dithiocarbamate or SB203580, which inhibit the activation of NF-κB and p38, respectively, prevented HSV-2-induced TNF-α expression. This demonstrates a requirement for both signals in order for HSV-2 infection to bring about TNF-α expression. Finally, there is evidence that the ability of HSV-2 to trigger the expression of the IL-12 subunit p40 is dependent on NF-κB activation (154).
In concert, the cellular response to an HSV infection seems to be biphasic, with an early response which is dependent on viral surface and tegument proteins and a later response involving factors produced during the viral replication cycle. With the growing focus on virus-induced cellular signaling and transcriptional regulation, this field is rapidly accumulating the information required to better explain HSV-induced cytokine expression at the molecular level, which will be an important step in our understanding of the pathology of HSV infections.
Cytomegalovirus
Cytomegalovirus (CMV) is a herpesvirus belonging to the Betaherpesvirinae subgroup. CMV infects most cell types and establishes latency in leukocytes. A CMV infection is normally subclinical but can be fatal in immunocompromised individuals or if the infection is acquired in utero. The cytokine profile of the early phase of CMV infection is typically proinflammatory, with production of IL-1β, IL-6, IL-12, TNF-α, IFN-α/β, and IFN-γ (38, 183, 192, 235, 266). One of the major components of the CMV virion is the glycoprotein gB, and studies have shown that a significant proportion of the virus-host interactions are mediated through gB (27, 267). For instance, induction of IL-1β in monocytes can be blocked by anti-gB (266). Likewise, in studies on the mechanism of IL-6 induction by human CMV, it was shown that UV-inactivated virus retained the ability to induce IL-6 production, which was independent of immediate early virus transcription and could be inhibited by neutralizing antibodies against gB (38). As will be described below, CMV stimulates a large number of typical proinflammatory signaling events, including nuclear translocation of NF-κB (267), and many of these can be mimicked by recombinant gB. Although gB is able to interact with heparan sulfate proteoglycans, which are essential for viral entry (53), these do not appear to be involved in the gB-dependent signaling. The biphasic nature of the Scatchard plot for the cell-gB interaction supports the notion that in addition to heparan sulfate proteoglycans, the viral glycoprotein has a second, as yet unidentified receptor which may be responsible for the signaling (26).
As to the CMV-stimulated cellular signaling, it has long been known that one of the early cellular responses to human CMV infection is production of inositol 1,4,5-triphosphate and 1,2-diacylglycerol (241). The subsequent calcium flow and PKC activation have been suggested to be involved in early activation of transcription factors interacting with DNA motifs, including κB, CRE, and TPA-responsive element (23). In addition, the human CMV particle has been demonstrated to carry phosphatase activity (163) and to activate the membrane-proximal phospholipases C and A2 as well as the MAP kinases extracellular signal-regulated kinases 1 and 2 (ERK1 and ERK2) and p38 immediately following infection (2, 27, 241). The mechanism of activation of NF-κB by CMV has been studied in some detail, and it was shown that the early NF-κB activation can be ascribed to liberation of the transcription factor from the inhibitory subunit IκBα but not IκBβ (266). Moreover, NF-κB activation is mediated by a gB-dependent mechanism. Interestingly, the early activation of NF-κB is amplified by other mechanisms later during infection. This second wave of NF-κB activation relies on an NF-κB-dependent activation of the CMV major immediate-early promoter. Cooperation of IE1–72, IE2–55, and IE2–86 proteins with the cellular transcription factor selective promoter factor 1 (Sp1) up-regulates the promoters for the NF-κB subunits p65 and p105/p50 (211, 268).
Contrasting results have been obtained with regard to activation of CRE-binding activity by CMV. The rapid activation reported for human CMV infection (23) was not seen in murine CMV-infected macrophages (266). Another study showed that ATF/CRE-binding protein (CREB) DNA-binding activity was indeed induced following human CMV infection, but only at late time points after infection (121). These observed discrepancies are probably due to the use of different cell types as well as differences in the way the cells were treated. While some investigators serum-starved cells prior to stimulation, others did not. Moreover, it cannot be excluded that the differences between human and murine CMV may give rise to some nonreconcilable results. Human CMV has also been reported to activate IRF-3 (180), through a rapid de novo protein synthesis-independent mechanism.
The role of CMV-activated signaling in cytokine induction has been clarified to only a limited extent. NF-κB appears to be involved in the expression of both IL-1β and IL-6 (38, 266). In addition, p38 and G proteins are involved in induction of IL-1β and IL-6 production, respectively. Taken together, CMV infection triggers a proinflammatory host response induced at least partly via the interaction of gB with a cellular receptor. A great deal is now known about the signaling events and pattern of transcription factors activated following CMV infection. One of the main challenges is to identify the cellular CMV receptor and to explain molecularly how virus-activated cellular signaling brings about the observed cytokine profile.
Epstein-Barr Virus
The Gammaherpesvirinae family member Epstein-Barr virus (EBV) is a lymphotropic herpesvirus with oncogenic potential. During a primary EBV infection, the virus undergoes lytic replication in epithelial cells and B lymphocytes, where it establishes latency. EBV is the causative agent of infectious mononucleosis and is involved in the development of a variety of human malignancies including Burkitt's lymphoma, Hodgkin's disease, and nasopharyngeal carcinoma (reviewed in references 17 and 107). The acute host response to an EBV infection is normally vigorous, with high fever, swollen glands, and splenomegaly. The first wave of cytokines and chemokines produced during EBV infection includes IL-1β, IL-1 receptor antagonist (IL-1Ra), IL-6, IL-8, IL-18, TNF-α, IFN-α/β, IFN-γ, monokine induced by IFN-γ (Mig), IFN-γ-inducible protein 10 (IP-10) and GM-CSF (61, 139, 150, 158, 204, 205, 226, 233).
As is the case for the above-described herpesviruses, EBV surface proteins are also able to trigger many of the early characteristics of the infection. For example, treatment of human monocytes/macrophages with recombinant EBV glycoprotein gp350 leads to the production of IL-1β and TNF-α (60, 61). Moreover, UV-treated virus can induce production of IL-1Ra, IL-6, IL-8, MIP-1α, and GM-CSF in a variety of cell types, and this effect is neutralized by antibodies against gp350 (158, 204, 205, 233). In addition to gp350, the EBV protein latent membrane protein 1 (LMP-1) is able to trigger the production of some cytokines such as IL-6, IL-8, and IL-10 (68, 175). As described below, LMP-1 gives rise to a strong signaling response, and this suggests that involvement of this protein in the induction of still more cytokines is likely to be revealed as studies progress. Finally, there is evidence that the EBV nuclear antigen 2 (EBNA2), a DNA-binding protein required for B-lymphocyte immortalization, induces IFN-α/β expression in Burkitt's lymphoma cell lines (117).
Parts of the intracellular signaling induced by EBV have similarities to those of HSV and CMV, while others are unique to EBV. The mechanism of action of gp350 is through the cellular receptor complement receptor 2 (CD21), which is known to be a signaling receptor (25, 230). This receptor engagement induces specific tyrosine phosphorylation (24) as well as activation of PKC, phosphatidylinositol 3-kinase (PI-3K), and NF-κB (61, 230). The pathway leading to NF-κB activation seems to include IKK, since it was shown to be sensitive to sodium salicylate (230), which at low millimolar concentrations inhibits IKK (263). Two EBV immediate-early proteins, BZLF-1 and BRLF-1, also activate cellular stress pathways (1). They function as transcriptional activators of EBV early genes and are sufficient for reactivation of latent EBV infections. The two immediate-early proteins activate p38 and JNK and the downstream transcription factors ATF-2 and c-Jun (1). This process is essential for the ability of BZLF-1 and BRLF-1 to promote EBV reactivation. EBNA2 represents yet another EBV-encoded protein with the capacity to modulate cellular signaling. In one study it has been shown that EBNA2 trans activates the HIV-1 LTR and that this function can be ascribed to activation of NF-κB (218).
The mechanism of NF-κB activation by EBV represents a particularly well-documented example of biphasic kinetics of NF-κB activation, as observed for some viruses. First the mere interaction of gp350 with its cognate cellular receptor CD21 induces intracellular signaling from CD21, resulting in rapid activation of NF-κB (61, 230). At later stages of infection, LMP-1 is produced and inserted in the cellular membrane. The cytoplasmic portion of LMP-1 has similarities to the TNF receptor and recruits many of the same adapter proteins and kinases (173). Consequently, like the TNF-α receptor, LMP-1 activates NF-κB through a mechanism common to many stimuli (111, 231), where upstream signals converge at the NF-κB-inducing kinase (NIK) followed by activation of IKK and subsequent nuclear translocation of NF-κB. In line with the similarity to the TNF receptor, LMP-1 also activates AP-1 (82). This activation process occurs through a pathway involving stress-enhancing kinase and JNK (82). Moreover, LMP-1 also activates p38 and the downstream transcription factor ATF-2 (68). Finally, there is now evidence that LMP-1 induces expression of the recently identified IRF-7 (272). Given the reported functions of IRF-7, the discovery that LMP-1 induces IRF-7 expression will probably unveil yet more roles of this viral protein in cell signaling in infected cells.
Among the signaling pathways activated by EBV, some have been specifically demonstrated to be involved in the induction of cytokine production. The ability of gp350 to induce the secretion of IL-6 in B lymphocytes can be abrogated by specific inhibitors of PKC, p38 and tyrosine kinases, implying a role of these factors in the signaling from CD21 to the IL-6 promoter (68, 233). The MAP kinase p38 is also involved in gp350-dependent IL-8 production (68). Finally, there is evidence that the expression of IL-1β and TNF-α by human monocytes/macrophages after exposure to gp350 is dependent on PKC, PI-3K, and NF-κB (60, 61). Of the LMP-1-induced cytokines, production of IL-6 and IL-8 has been found to be dependent on the p38 pathway (68). For IL-8, it was further shown that the AP-1- and NF-κB-binding motifs confer the majority of the LMP-1 responsiveness whereas the CCAAT enhancer-binding protein (C/EBP) motif did not seem to be involved. Participation of other specific signaling pathways in LMP-1-induced cytokine production has not been reported but seems possible, given the extensive signaling from this membrane protein.
Taken together, it seems that gp350 is responsible for the majority of the initial host response to EBV infection whereas later signaling events in the infected cell are heavily influenced by LMP-1. The fact that EBV is endowed with strong NF-κB activation properties may not seem beneficial for the virus, given the pivotal role of this transcription factor in host defense. NF-κB is, however, also involved in antiapoptotic regulation in the cell and is important for cell transformation by EBV (35).
Influenza Virus
Influenza virus, which is the causative agent of influenza, is an orthomyxovirus that can establish infection in the epithelium of the upper and lower respiratory tract after entry through the oral or nasal route. The virus, which has a negative-sense segmented RNA genome, possesses an outer lipid membrane containing the two proteins hemagglutinin (HA) and neuraminidase (NA). HA is responsible for viral cell attachment through interactions with sialic acids on the cell surface, and NA cleaves sialic acids and promotes viral release (221). A typical influenza virus infection results in fever, myalgia, and cough. The symptoms start between 1 and 4 days postinfection and persist for 3 to 5 days. In immunocompromised individuals, influenza virus infection can result in a more severe clinical outcome and may even be fatal. The disease symptoms are due both to immunopathology and cytopathic effects of the virus (18). A whole range of cytokines and chemokines are induced during an influenza virus infection in different cell types. These include IL-1, IL-2, IL-6, IL-8, IL-10, IL-15, IL-18, transforming growth factor β (TGF-β), TNF-α, IFN-α/β, IFN-γ, GM-CSF, MIP-1α/β, and RANTES (71, 93, 96, 101, 114, 128, 167, 194, 212, 213, 223).
In early studies on the mechanism of induction of cytokines by influenza virus, it was noted that different influenza A virus strains differ in their ability to induce IFN-α/β (44). It was further shown that the IFN-inducing potential of a specific strain correlated with the NA activity. Houde and Arora showed that recombinant NA, but not HA, induced the production of IL-1 and TNF-α in murine peritoneal macrophages (9, 104, 105), and another study has shown that NA promotes the conversion of TGF-β from the latent to the active form to an extent sufficient to induce TGF-β-dependent apoptosis (223). The exact mechanism through which NA induces cytokine expression remains obscure yet is very important, given the high immunostimulatory activity of this viral protein.
The cellular signaling induced by influenza virus infection results in activation of the MAP kinases p38 and JNK and the downstream transcription factors NF-κB, AP-1, and CRE-binding factors (34, 101, 128). It has as yet not been shown which upstream signaling pathways are responsible for this response, nor has it been shown if and how the viral envelope proteins initiate the response. It will be interesting to learn if the cytokine-inducing potential of NA is dependent on interaction with a cellular receptor or on the enzymatic activity of NA. The latter is not impossible, since gangliosides (sialic acid-containing glycosphingolipids) have been reported to activate the MAP kinases ERK, JNK, and p38, as well as the transcription factor NF-κB, in rat microglia (198). As to the cellular response following exposure to HA, several studies have shown that overexpression of HA induces activation of NF-κB (16, 72, 184). The mechanism of NF-κB activation has been suggested to include overload of HA in the endoplasmic reticulum (ER), a process which is known to activate NF-κB through the release of calcium (185). Two other influenza virus proteins, matrix protein and nucleoprotein, have also been reported to promote NF-κB activation, although this occurs through an ER-independent mechanism. For all three NF-κB-activating viral proteins, IKKβ is part of the activation pathway (72).
In addition to viral proteins, influenza virus double-stranded RNA (dsRNA) is sensed by the infected cell as an alert signal (90). As will be described below in more detail, dsRNA initiates signaling events through a mechanism dependent on the dsRNA-activated protein kinase (PKR). This PKR-dependent virus-induced cellular stress response is an important part of the first line of defense against virus infections (for reviews, see references 254 and 255). The influenza virus has, however, developed mechanisms to mask the potent PKR-activating ability of its RNA. In fact, influenza virus inhibits the action of PKR by two mechanisms, one involving up-regulation of the cellular PKR inhibitor p58 (160) and one involving the viral nonstructural protein 1 (NS1) (90). Given the well-described role of PKR in virus-induced signal transduction and IFN-α/β expression (130, 259), it is no surprise that NS1 counteracts the activation of NF-κB and induction of IFN-α/β by influenza A virus (249). Despite these mechanisms to down-modulate PKR activity, there is evidence that influenza virus activates the kinase to some extent and that the subsequent cellular signaling triggers apoptosis (232).
Molecular understanding of cytokine and chemokine induction by influenza virus has so far not reached a level comparable to that for some of the other viruses discussed in this review. It is known that influenza virus-induced IL-8 relies on activation of NF-κB (127) and that the ability of influenza virus to regulate the expression of RANTES involves p38 and JNK (128). Given the NF-κB-activating capacity of HA, there is reason to believe that HA induces the production of certain cytokines. Investigation of the molecular mechanisms that govern influenza virus-induced cytokine expression constitutes an important task for this research field and may provide new information about the molecular events that form the basis for the pathogenesis of influenza virus infection.
Hepatitis B Virus
Hepatitis B virus (HBV) is an enveloped DNA virus belonging to the family of hepadnaviruses. HBV has a strict tissue tropism to the liver, causing acute or chronic hepatitis, and is furthermore associated with the development of cirrhosis and hepatocellular carcinoma. Initially, the virus replicates in hepatocytes with few or no cytopathic effects, correlating with the lack of liver damage. The HBV genome is integrated into host DNA, and the infection may remain latent. At later stages, accumulation of filamentous HBV surface antigen (HBsAg) gives rise to the ground-glass hepatocyte pathology characteristic of HBV infection. As is the case in many virus infections, cell-mediated immunity and inflammation, rather than cytopathic effects caused by viral replication, are the main mediators of the hepatic pathology induced by HBV infection (42).
Given that the hepatic damage observed during acute or chronic hepatitis appears to be caused mainly by the intrahepatic inflammatory processes evoked by the immune response to HBV, much effort has been made to determine the cytokine profile of HBV infections as well as the cellular sources of the cytokines produced. Numerous reports have shown that HBV infection is associated with the production of a broad range of proinflammatory cytokines and chemokines such as IL-1β, IL-6, IL-8, IL-12, TNF-α, and IFN-γ (3, 80, 83, 106, 153, 182), as well as the anti-inflammatory cytokine IL-10 (106, 242). While some investigators have shown that proinflammatory cytokine production may be required for viral clearance (80, 208), others report that excessive synthesis of IL-6 may eventually lead to liver cirrhosis and hepatocellular carcinoma (142). Yet other laboratories have demonstrated a predominant Th1 cytokine profile in acute self-limiting HBV infection (191). However, it has been difficult to demonstrate any direct correlation between the type of cytokines produced and the outcome of infection, since a fine balance between viral clearance and extensive hepatocyte necrosis seems to exist. With regard to the cell type responsible for cytokine production, IFN-γ has been shown to be produced predominantly by Th1 cells (106) whereas others have demonstrated TNF-α and IL-6 production by hepatocytes (83, 142).
As to which viral components are responsible for cytokine induction, a number of studies have addressed this question. One of the best-studied proteins is the HBV x protein (HBx), which functions as a transcriptional activator, although it does not bind directly to DNA (95). Instead, HBx plays the role of a dual-specificity activator of transcription, stimulating signal transduction pathways in the cytoplasm as well as acting directly on transcription factors in the nucleus (95). HBx is essential for viral infection and up-regulates a range of cellular and viral genes, even though its precise role in the viral replication cycle is still unknown. Growing evidence links HBx to hepatocarcinogenesis (6), but the exact mechanism initiating intrahepatic inflammatory events remains elusive. However, HBx induces several cytokines and chemokines, including IL-6, IL-8, and TNF-α (138, 142, 153), findings that may at least partly explain the role of HBx in hepatic pathology.
In addition to HBx, the surface antigen (HBsAg) and core antigens (HBcAg and HBeAg) stimulate cytokine production. HBsAg was reported to trigger the production of IL-2, IL-10 and IFN-γ (106, 140) while HBcAg and HBeAg promote the secretion of IL-10, TNF-α, and IFN-γ (106, 242). Since the specific nature of the HBV receptor and the mechanism of HBV entry remain unresolved, it is not certain if the cytokine-inducing capacity of these viral proteins is dependent on interaction with specific cellular receptors or is a secondary effect triggered by lymphocyte activation. Results from one study indicate that HBsAg does have properties as a transcriptional activator (see below), suggesting that at least part of the cytokine-stimulating function of this major HBV surface protein is due to a direct effect on signal transduction (98).
Most of the work addressing the signal transduction stimulated by HBV has been performed as overexpression studies in cell lines, while results with infectious virus are lacking behind. This is at least partly due to the lack of a cell culture system to grow HBV. Nevertheless, significant advances have been made in recent years. A series of elegant studies conducted in the laboratory of R. J. Schneider have contributed significantly to our knowledge of HBx-stimulated signaling. The emerging picture is that HBx affects the majority of the proinflammatory signaling cascades in a stimulatory fashion. For instance, HBx activates the MAP kinase network through MEKK1, the GTPase Ras, and the serine/threonine kinase Raf (19, 20). This leads to downstream activation of JNK and ERKs, respectively, eventually stimulating transcription factors, most notably AP-1, ATF-2, and NF-κB. As in the case of AP-1, NF-κB activation by HBx has been demonstrated to involve Ras activation (19, 58, 178, 229). HBx appears to activate NF-κB through two distinct cytoplasmic pathways, namely, by inducing phosphorylation and subsequent degradation of IκBα and by reducing the cytoplasmic levels of p105, the inhibitory precursor of p50 (229). Other transcription factors affected by HBx include AP-2, C/EBPα, early growth response factor 1 (Egr-1), and NF-AT (43, 119, 137, 138, 153, 265). However, the exact nature of the signals is still partly unknown, since some data indicate that they are independent of calcium and PKC (19) while other data suggest that PKC may be involved (119).
HBx interacts with cellular functions through a dual mechanism, being able to act both in the cytoplasm by activating signal transduction cascades as described above and in the nucleus by interfering with the basal transcriptional machinery (6). The latter mechanism involves interaction between HBx and transcription factor IIB (TFIIB), TFIIIB, RNA polymerase II (92, 147), and increased levels of TATA-binding protein (247), as well as association with transcription factors such as CREB/ATF (152) and C/EBPα (43). Of particular interest is the interaction between HBx and C/EBPα, which synergistically activates the HBV enhancer II/pregenomic promoter, thereby promoting viral replication (43). The ability of HBx to enhance C/EBP activity also affects IL-8 expression (153). In fact, it was shown that HBx-induced IL-8 production was dependent on cis elements for C/EBP and NF-κB. Another study has shown that TGF-β1 expression is elevated in HBx transgenic mice (265). Moreover, the authors demonstrated that association of HBx with the Egr-1 protein promotes TGF-β1 expression. Finally, the molecular mechanism of HBx-induced TNF-α expression in the hepatocyte-derived cell line HepG2 has been investigated (138). In parallel with what has previously been observed for a number of other TNF-α stimulators, HBx was found to augment TNF-α expression through a mechanism involving NF-AT and AP-1.
As described above, there is evidence that HBsAg is able to activate transcription. Hildt et al. (98) found that the protein resulting from the large translational product of the HBsAg gene (LHB) activates AP-1 and NF-κB. The HBsAg gene can be translated from three in-frame start codons, with LHB containing the pre-S1, pre-S2, and S regions. The middle product (MHB) encompasses the pre-S2 and S regions, while the small product (SHB) contains the S region only. The activating function of HBsAg is dependent on cytoplasmic orientation of the pre-S2 region, as is the case for newly synthesized LHB and a carboxy-terminal truncated version of MHB, termed MHBst. For full-length MHB, however, the pre-S2 region is oriented toward the lumen of the ER, thus preventing it from activating cellular signaling. The pre-S2-induced events rely on direct interaction with PKC, and it was shown that downstream activation of the kinase c-Raf-1 is a prerequisite for LHB-dependent activation of AP-1 and NF-κB. LHB thus provides an elegant example of how viruses with small genomes have evolved mechanisms to compensate for this apparent drawback by endowing the proteins with multiple functions. LHB is expressed on the surface of the HBV virion, is essential for assembly of the virus particle, and also seems to support the activation of the viral promoters.
In summary, virus-host interactions during an HBV infection are attributed mainly to HBx, although HBsAg, HBcAg and HBeAg also contribute. The resulting immune response is known to play a significant role in the pathology of HBV-mediated hepatitis. With the growing information available on the molecular events underlying HBV replication and HBV-induced cellular signaling, the frontiers in this area of research include exploitation of our knowledge to design new strategies for treatment in the clinic.
Human Immunodeficiency Virus
HIV is a retrovirus of the Lentivirus subfamily. It infects CD4 T lymphocytes and monocytes/macrophages through recognition of the CD4 receptor and the coreceptors CXCR4 and CCR5 (181, 243). Following an acute viremia, resulting in a mononucleosis- or influenza-like condition, a state of clinical latency is reached in which CD4 T lymphocyte turnover is vastly increased. However, eventually the immune system (the T lymphocytes in particular) becomes unable to keep pace with the extensive turnover and death of cells, leaving a state of immunoincompetence and AIDS. Although macrophages are not killed by HIV infection, they display dysfunction and serve as a reservoir and source of HIV in the organism. The acute host response to a primary HIV infection is characterized by a Th0 cytokine profile including the proinflammatory cytokines IL-1, IL-2, IL-6, TNF-α, IFN-α/β, and IFN-γ (86, 202, 238), as well as the anti-inflammatory cytokines IL-4, IL-10, and IL-13 (86, 190). At later stages of infection, as full-blown AIDS progresses, the pattern of cytokine production shifts toward a strongly biased Th2-like response (47, 48, 115). The mechanisms of HIV-induced cytokine production have been studied in great detail, and much information is available about the early cytokine expression whereas less is known about what causes the shift in cytokine profile.
The HIV glycoprotein gp120, which interacts with CD4 and the chemokine receptors CXCR4 and CCR5, is able to induce the secretion of many proinflammatory cytokines including IL-1α, IL-1β, IL-6, IL-8, TNF-α, IFN-α/β and IFN-γ (5, 7, 36, 73). Interestingly, gp120 is also able to induce the secretion of IL-4 and IL-13 in basophils (190) and IL-10 in mononuclear cells (220), indicating that the acute Th0-like host response to HIV is explained largely by the interaction of gp120 with cellular receptors. While most gp120-induced functions are explained by its interaction with CD4, the mechanism through which IL-4 and IL-13 are induced appears somewhat different. Recombinant gp120 from various divergent HIV-1 isolates was found to induce secretion of IL-4 and IL-13 in basophils, and this occurred by the action of gp120 as superantigen (190), where gp120 binds to the VH3 region of immunoglobulin E and hence enhances the action of immunoglobulin E on FcɛRI.
Another HIV protein, Tat, is known to stimulate the production of many cytokines. Tat is produced by HIV-infected cells and has pleiotropic effects on viral replication and cell growth (for a recent review, see reference 74). Tat is predominantly cytoplasmic but can be released from infected cells and enter adjacent cells. The protein contains an arginine-rich domain that permits Tat to efficiently cross membranes (224). The cytokines and chemokines induced by Tat include IL-1β, IL-2 IL-6, IL-8, TGF-β1, TNF-α, and MCP-1 (39, 100, 145, 177, 216, 252). A third HIV protein able to induce cytokine production is Nef. This regulatory protein is produced early in the HIV life cycle and is important for viral infectivity and pathogenicity (59). As with Tat, Nef is predominantly cytoplasmic, but soluble Nef as well as anti-Nef antibody can be detected in sera from HIV patients (76). Although cytoplasmic Nef is immunomodulatory (see below), it is not as potent an inducer of cytokine production as is extracellular Nef, which induces the production of IL-1β, IL-6, IL-10, IL-15, TNF-α, and IFN-γ in various human leukocyte populations (29, 63, 199). Finally there is evidence that viral protein R (Vpr) induces the expression of IL-6, IL-8, IL-10, TNF-α, and IFN-γ in a variety of cell types (209). Vpr, which is required for optimal replication of HIV in vivo, is produced late in the HIV life cycle and assembled into the virion (50). Vpr has, furthermore, been suggested to be important for replication of HIV in macrophages (15).
HIV infection affects cellular signaling activity in a very profound manner. At present, signaling properties have been reported for all HIV proteins discussed in this review. gp120 induces signaling mainly through interaction with CD4, but there is also evidence that the chemokine receptors CXCR4 and CCR5 signal following interaction with gp120. The CD4-dependent signaling has been elucidated mainly through studies performed in the laboratory of P. M. Pitha. Binding of gp120 to CD4 results in activation of the tyrosine kinase Lck and the serine/threonine kinase Raf-1 (196). This receptor engagement also induces activation of the MAP kinases ERK1, ERK2, and JNK, as well as the transcription factors NF-κB, AP-1, and C/EBPβ (NF-IL6) (135, 195). Two studies have shown that gp120 triggers signaling from the coreceptors CXCR4 and CCR5, as evidenced by phosphorylation of the protein tyrosine kinase Pyk2 (62, 166). Interestingly, however, whereas the natural CXCR4 ligand stromal cell-derived factor 1α also activates MAP kinase pathways, this was not seen in response to gp120, indicating that gp120-induced chemokine receptor signaling does not fully mimic the response to chemokines.
The Tat protein activates cellular signaling cascades and transcription factors associated with a proinflammatory host response, and, indeed, Tat is a potent inducer of many proinflammatory cytokines. However, given that the HIV LTR is regulated by transcription factors of the NF-κB, NF-AT, Sp1, and C/EBP families (89, 125, 174, 210, 244), this property of Tat is beneficial for viral replication. Activation of NF-κB by Tat proceeds via IKK, which is constitutively active in HIV-infected cells (64). Moreover, the ability of Tat to activate NF-κB also seems to require PKR (66). Interestingly, the involvement of PKR in NF-κB activation is not restricted to Tat, since PKR-deficient cells also display an impaired ability to activate NF-κB in response to TNF-α and dsRNA (270). The specific nature of the upstream kinases regulating IKK activity has not yet been reported. In addition to the IKK pathway, some earlier studies showed that activation of PKC by Tat leads to nuclear translocation of NF-κB (54). At present it is not known if the PKC-dependent pathway represents an alternative mechanism of NF-κB activation by Tat or if Tat activates NF-κB by one sole mechanism involving PKC, PKR, and IKK.
It has been shown that Tat primarily targets IκBα for degradation (65). Tat expression leads to constitutive activation of NF-κB, which is normally associated with degradation of IκBβ rather than of IκBα (237). IκBα degradation and synthesis are subject to autoregulation due to the presence of NF-κB-responsive sites in the IκBα promoter (40). Hence, IκBα-dependent activation of NF-κB is normally self-limiting. One study has addressed this apparent discrepancy and showed that nuclear IκBβ is at least partly responsible for the observed constitutive NF-κB activity in HIV-infected cells (64). Nuclear hypophosphorylated IκBβ is known to maintain NF-κB DNA binding by rendering the protein-DNA complex insensitive to IκBα-mediated dissociation from DNA (10, 193), and DeLuca et al. showed that this mechanism contributes to the sustained NF-κB activity in HIV-infected cells (64). JNK, which is responsible for phosphorylation of c-Jun and, to a lesser extent, ATF-2, is also regulated by Tat. It was demonstrated that Tat activates JNK and AP-1 in the human histocytic lymphoma cell line U937, whereas the effect on ATF-2/c-Jun activity was not examined (129). Similar findings have been achieved in another study, where it was further shown that Tat activates the ERK kinases (165).
Another transcription factor regulated by Tat is Sp1, which is essential for optimal activation of the HIV LTR (89). Sp1 is a ubiquitous transcription factor involved in basal and inducible expression of many genes. Tat enhances Sp1 DNA binding and augments Sp1 phosphorylation, which was shown to be associated with enhanced promoter activity (46, 145). The kinase responsible for phosphorylation of Sp1 in response to Tat remains unknown but may be a DNA-dependent protein kinase, which has been shown to phosphorylate Sp1 on simian virus 40 infection (112). Sendai virus infection has also been reported to augment Sp1 DNA binding to the TNF-α promoter in vivo (70). Of other transcription factors affected by Tat, notably NF-AT and C/EBPβ have received attention, due to their ability to regulate HIV LTR activity (125, 210). NF-AT activation by Tat is cyclosporin A sensitive (252), implying a role of calcineurin in the process. In addition, Tat is able to associate with NF-AT, thus enhancing NF-AT-driven transcription (151). Similarly Tat-dependent DNA binding of C/EBPβ is at least partly attributed to complex formation between the two proteins, which enhances the DNA affinity of C/EBPβ (4).
At present, very little is known about the signaling events induced by extracellular Nef, whereas more extensive knowledge has been gathered about the signaling induced by cytoplasmic Nef. However, given the limited cytokine-inducing function reported for cytoplasmic Nef, this will not be discussed in this review. Extracellular Nef may bind to a cellular receptor and initiate signaling or bind to cation channels leading to cation flow. It has been reported that Nef affects potassium and calcium channel activity in lymphocytes (271). Finally, it should be mentioned that despite the growing body of literature on extracellular Nef, the concept of extracellular Nef is not broadly accepted in the field.
The effects of Vpr on cellular signaling and gene transcription have also been studied, and the data available suggest that Vpr modulates the function of a number of DNA-binding proteins through direct protein-protein interactions. It was first shown that the ability of Vpr to stimulate HIV LTR transcription was mediated mainly through physical interaction between Vpr and Sp1 bound to the HIV LTR (248). Subsequent studies have shown that Vpr also interacts with p53 and that this association antagonizes Vpr/Sp1-driven transcription (215). The glucocorticoid receptor (GR) is also a target for Vpr (124). The interaction between GR and Vpr, which is dependent on the LXXLL motif of Vpr, facilitate the recruitment of general transcription factors to GR. Thus, Vpr functions as a transcriptional coactivator for GR-driven transcription. Finally, a recent report has documented that Vpr enhances the trans-activating function of NF-κB and C/EBPβ (209).
The extensive knowledge about cytokine induction and signal transduction by HIV gp120 is unfortunately not accompanied by a similar in-depth understanding of which signaling pathways are responsible for gp120-induced cytokine synthesis, although it was speculated in one report that activation of AP-1 by gp120 may lead to the expression of IL-3 and GM-CSF (41). For the mechanisms of Tat-induced cytokine production, a substantial amount of knowledge has been gathered. There is evidence that NF-κB is pivotal for expression of IL-2, IL-6, IL-8, TNF-α, and MCP-1 (39, 120, 145, 252) by Tat. The up-regulation of MCP-1 expression by Tat represents a particularly well-documented study. Lim and Garzino-Demo provided evidence that ectopic expression of Tat in the human astrocytoma cell line U-89 triggered MCP-1 expression and that this phenomenon relied on the synergistic action of Sp1, AP-1, and NF-κB (145). Other studies have shown that Tat-supported IL-2 expression relies on the ability of Tat to activate NF-κB and NF-AT, both of which are required to activate the IL-2 promoter (240, 252). Finally, there is evidence that Tat enhances the DNA-binding activity of C/EBPβ and NF-κB to the human IL-6 promoter, which promotes IL-6 expression (4, 120, 217). Together with the knowledge that NF-κB and C/EBPβ interact physically (210), these results indicate that Tat stimulates IL-6 expression both by activating specific transcription factors and by aiding the formation of stable enhanceosome complexes.
For extracellular Nef, it is known that induction of IL-10 secretion is sensitive to the calcium-calmodulin phosphodiesterase inhibitor W7 and the calcium-chelating agent EGTA, suggesting that Nef-induced calcium flux is involved in IL-10 induction (29). The ability of Vpr to stimulate IL-8 expression is dependent on NF-κB and C/EBPβ (209). Moreover, a number of other cytokines controlled by NF-κB- and C/EBPβ-responsive promoters are induced by Vpr (209), indicating that these two transcription factors play a central role in the induction of cytokines and chemokines by Vpr.
In addition to the induction of cytokines by the HIV proteins discussed in the present section, there is accumulating evidence that these proteins contribute to the shift in immune response toward a strongly biased Th2-like response. For instance, Nef decreases the production of IFN-γ and IL-2 (51) and has also been reported to impair the signaling of Th1 cytokines (52). In addition to Nef, Tat supports Th2-cell development by inducing expression of the IL-4 receptor α chain (108) and inhibiting the secretion of IL-12 (110). These functions of the HIV proteins may contribute to the shift in cytokine profile as the infection progresses toward the clinical picture of full-blown AIDS.
Human T-Lymphotropic Virus Type 1
Human T-lymphotropic virus type 1 (HTLV-1) is a retrovirus of the Oncovirinae subfamily. HTLV-1 infection is usually asymptomatic, but after a long latency period (approximately 30 years), it can cause adult T-cell lymphocytic leukemia (ATLL) or the non-oncogenic neurological disease HTLV-1-associated myelopathy/tropical spastic paraparesis (HAM/TSP) (103). These diseases can be attributed to the ability of HTLV-1 to infect Th cells and neurons, since these cell types express an as yet unidentified receptor for the virus. The histopathological picture of ATLL has the appearance of malignant monoclonal Th cells that are pleomorphic and contain lobulated nuclei. Although an elevated white blood cell count is typically seen, a state of immune suppression is associated with HTLV-1 infection in ATLL. On the other hand, immune stimulation appears to be associated with the pathology of demyelinization observed in HAM/TSP. Finally, in some rare cases, HTLV-1 affects other organs, causing dermatitis, pneumonitis, polymyositis, and uveitis (reference 102 and references therein).
During an HTLV-1 infection, a number of cytokines are produced, including IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-15, TGF-β, TNF-α, TNF-β1, IFN-γ, and GM-CSF (13, 22, 31, 55, 85, 99, 122, 161, 170, 172, 176, 227, 239, 258), as well as chemokines such as IL-8, RANTES, and MIP-1α (14, 169). Constitutive expression of the anti-inflammatory cytokine IL-10 has been shown in ATLL cells and in HTLV-1-infected cell lines (171), thus potentially explaining the state of immune suppression in ATLL. However, others have reported that HTLV-1 represses IL-10 expression (222), leaving this question unresolved. TNF-α, which has been associated with other inflammatory diseases of the central nervous system, including multiple sclerosis, encephalopathy secondary to AIDS, bacterial meningitis, and cerebral malaria (133, 164, 245), has been demonstrated to cause direct damage to oligodendrocytes and myelin in vitro (30, 225). Together with the observation that T cells infected by HTLV-1 appear to express high levels of TNF-α constitutively (134), this cytokine seems to have a central position in the neurological pathology of HTLV-1 infections.
For the mechanism of cytokine induction by HTLV-1, numerous studies have shown that the virally encoded Tax protein is by far the prime immune stimulator, regulating not only virus replication but also the expression of cellular genes encoding cytokines, chemokines, and their receptors (14, 55, 77, 109, 122, 234). Moreover, Tax-derived peptides constitute the majority of T-cell-recognized HTLV-1 epitopes (102). Signaling by intracellular Tax is very complex and has been extensively studied. Specific pathways will be described below where detailed knowledge is available.
First, Tax activates NF-κB through the IKK pathway, resulting in constitutive nuclear transcriptionally active NF-κB (79, 144). Activation of IKK by Tax has been characterized and shown to involve IKK-γ and the kinase MEKK-1. Sun and colleges recently showed that a specific interaction between Tax and IKK-γ through leucine zipper domains is necessary for recruitment of Tax to the IKK signalosome (88, 256). This recruitment is a prerequisite for IKK activation by Tax. Other studies have demonstrated a physical interaction between Tax and MEKK1, as well as an essential role of this kinase in activation of the IKK signalosome by Tax (262). One interpretation of these results is that Tax, through interaction with IKK-γ, acts as a docking protein in the IKK signalosome recruiting MEKK1 to the complex. This, in turn, triggers MEKK1-dependent phosphorylation events, leading to activation of IKK and eventually of NF-κB.
Interestingly, several distinct NF-κB-inducing pathways may be activated by Tax. For instance, interaction between Tax and certain isoforms of PKC (α, δ and η) results in the phosphorylation of Tax and increased autophosphorylation of PKC. Together with the observation that the PKC inhibitor calphostin C abrogates Tax-induced NF-κB DNA-binding activity, this indicates that Tax-activated PKC is involved in NF-κB activation (149). Whether this pathway involves IKK remains unresolved. Finally, it has been suggested that Tax may activate NF-κB through direct association with NF-κB/Rel members. This hypothesis is based on the finding that Tax can interact with p50, p65, c-Rel, and the precursor p100 under specified conditions (21, 132, 136). The constitutive activation of NF-κB is believed to play a role in HTLV-1-mediated transformation of T cells as seen in ATLL (126).
A second important transcription factor activated by Tax is ATF-2, which, together with the CREB transcription factor family, is involved in the activation of viral transcription through the CRE-like sites in the HTLV-1 LTR (75, 78). ATF-2 is a downstream target for the MAP kinases JNK and p38, and constitutively activated JNK has been found in leukocytes isolated from ATLL patients and associated with HTLV-1-mediated immortalization and tumorigenesis of infected T lymphocytes (257). In agreement with this observation are data showing activation of the transcription factor AP-1, one of the major downstream targets of JNK, in HTLV-1-infected lymphocytes (169).
Third, Tax activates NF-AT. It has long been known that NF-AT is essential for T-lymphocyte activation, and it has become clear that NF-AT also plays a role in HTLV-1 Tax-induced expression of several proteins, including IL-2 and Fas ligand (84, 85, 203), by binding to the CD28-responsive element in the promoters of these genes. While the exact mechanism remains unclear, it has been demonstrated that Tax induces a state of constitutive dephosphorylation and activation of NF-AT, thus implying that Tax may directly or indirectly activate the phosphatase calcineurin, which is the physiological upstream activator of NF-AT. This hypothesis is supported by the finding that the presence of cyclosporin A, a specific inhibitor of calcineurin, reverses the constitutive dephosphorylation and activation of NF-AT induced by Tax in HTLV-1-infected cells (84).
As discussed above, Tax activates several important signal transduction pathways in infected cells, and the downstream targets include numerous well-characterized transcription factors. Activation of transcription factors by Tax does not necessarily involve activation of signal transduction, however. For example, Tax expression has been reported to enhance the DNA binding of CREB-2, C/EBPβ, and the Ets protein Spi-1 (PU.1), presumably through the ability of Tax to engage in protein-protein interaction with these transcription factors (78, 239). In support of this notion, other studies have shown that Tax, together with GATA-binding proteins (GBPs), facilitates DNA recruitment of AP-1 and Ets proteins (22, 258).
Besides activating various intracellular signaling pathways in HTLV-1-infected cells, Tax appears to function as an extracellular cytokine secreted from infected cells and affecting neuronal cells in a paracrine manner (148). Extracellular soluble Tax induces NF-κB activation and expression of immunoglobulin κ light chain, IL-2Rα, TNF-α, TNF-β, and IL-6 (28, 67, 148). The intriguing observation that the presence of soluble Tax for as little as 5 min is sufficient to induce TNF-α production indicates that extracellular Tax may rapidly initiate a signal through a cellular surface receptor (55), whose nature remains unknown.
The current understanding of the molecular mechanisms of cytokine induction by Tax illustrates that a wide range of signaling pathways are involved. For instance, although Tax activates NF-κB and CREB/ATF family members, this is not sufficient to bring about IL-2 expression, which in addition is dependent on the CD28-responsive element of the IL-2 promoter and NF-AT (85). This was supported by another study where a Tax mutant unable to activate NF-κB was shown to retain the capacity to induce the expression of IL-2 and GM-CSF (99). By contrast, expression of IL-15 in response to Tax is highly dependent on NF-κB and a functional κB site in the IL-15 promoter (13). The aberrant IL-5 expression and associated eosinophilia observed in many ATLL patients has been subjected to careful molecular analysis. While one study showed that Tax cooperates with GBPs and AP-1 for expression of IL-5 in ATLL cells (258), another group reported that Tax and GBPs also synergize with Ets transcription factors for stimulation of IL-5 expression in Jurkat cells (22). Tax-induced IL-8 expression has also been studied molecularly and revealed to be regulated by NF-κB and AP-1 (169), which is similar to what has been reported for other stimuli (260). AP-1 is also involved in Tax-induced trans-activation of the TGF-β1 promoter (122). Finally, there is evidence that Tax stimulates IL-1β expression in human THP-1 cells. This was shown to occur through a mechanism where Tax promotes the recruitment of C/EBPβ and Spi-1 to the IL-1β promoter via protein-protein interactions (239).
In conclusion, HTLV-1 Tax-induced signal transduction and transformation have been extensively studied, and many aspects have been clarified. Several questions remain, however, one being the puzzling observation that HTLV-1 infection in some cases seems to result in immune stimulation, as seen in HAM/TSP, while in other cases it causes immune suppression, as observed in ATLL. A better understanding of cytokine production during HTLV-1 infection may help explain why one group of seropositive individuals develop leukemia, another develop a chronic neurologic disease, and yet another, the majority, remain clinically asymptomatic.
EXPERIMENTAL MODELS FOR VIRAL INFECTIONS
Much of our knowledge about virus-cell interactions has been obtained through studies with various models for virus infections. Some of the most widely used viral models have been vesicular stomatitis virus (VSV), Newcastle disease virus (NDV), and Sendai virus. In addition, poly(I-C) has served as a very useful tool in studies of dsRNA accumulation, which is associated with many virus infections. In the following paragraphs, specific advances that have been achieved through the use of these model systems will be described.
IFN-α/β have classically been described as the primary antiviral compounds working in an auto- and paracrine fashion. Consequently, the way in which IFN-α/β is regulated during a virus infection has been studied intensively. A series of studies have addressed the mechanism through which the IFN-β promoter is regulated in response to Sendai virus and NDV infections. Various positive regulatory domains (PRDs) have been identified in the human IFN-β promoter. PRD I and III interact with IRF family members, PRD II encompasses an NF-κB-binding site, while PRD IV binds ATF-2/c-Jun. The pattern that has emerged is that while the promoter is only marginally inducible by either transcription factor alone, a marked degree of synergy is observed when all PRDs are occupied (123). This has led to the enhanceosome theory, which suggests that maximal activation of inducible promoters requires the assembly of a multiprotein complex, which generates a surface with optimal interaction with the transcription initiation machinery (37). This theory is supported by data showing that activation of the IFN-β promoter is dependent on recruitment of the transcriptional coactivators CBP and p300 via a protein surface generated by NF-κB, IRFs, and ATF-2/c-Jun following binding to the promoter (162). In addition to the above-described transcription factors, the architectural protein high-mobility group I(Y) [HMG I(Y)] is part of the IFN-β enhanceosome (123). HMG I(Y) is a DNA-binding protein that recognizes AT-rich sequences. The role of HMG I(Y) in enhanceosome assembly lies in the initial steps, where it facilitates the recruitment of NF-κB and ATF-2/c-Jun to the promoter by inducing allosteric changes in the DNA conformation (261). Sendai virus and NDV rapidly trigger activation of the IFN-β-inducing transcription factors and hence the assembly of the enhanceosome and subsequent high-output IFN-β expression (250).
The IRF family member responsible for virus-induced IFN-β expression was first suggested to be IRF-1. This view was challenged by findings with IRF-1 knockout mice, which showed an apparent unaltered ability to produce IFN-β during NDV infection (157). A series of studies have now shown that IRF-3 and IRF-7 seem to be the IRF family members involved in IFN-α/β expression. Au et al. first showed that IRF-3 was able to interact with IFN stimulation response elements, which per se stimulated transcription only poorly (12). Subsequently it was shown that NDV and Sendai virus infections trigger the trans-activating potential of IRF-3, leading to expression of IFN-α4 and IFN-β (155, 264). The phosphorylation-dependent activation of IRF-3 is largely explained by the interaction with CBP/p300. IRF-3, which prior to phosphorylation is cytoplasmic and unable to interact with CBP/p300, gains this property following phosphorylation and nuclear translocation and hence becomes endowed with a trans-activating potential (214). The virus-induced event that turns on IRF-3 is activation of a serine/threonine kinase that phosphorylates the carboxy-terminal region of IRF-3, allowing the protein to adapt an open conformation and form dimers, which translocate to the nucleus and activate transcription. The kinase responsible for phosphorylation of IRF-3 has not been identified yet, but p38 has been suggested to be involved in the activation of IRF-3 by lipopolysaccharide (179), a component of the cell wall of gram-negative bacteria. Activated IRF-3 stimulates the expression of IFN-β (and IFN-α4), as described above. The resulting IFN in turn stimulates the production of IRF-7, which remains cytoplasmic in uninfected cells and becomes activated in virus-infected cells through a mechanism similar to that used by IRF-3 (11). Activated IRF-3 and IRF-7 form homo- and heterodimers. Following IRF-7 activation, transcription from the IFN-α non-α4 promoters is up-regulated (155).
In addition to a role in IFN-α/β expression, IRF-3 has been reported to stimulate Sendai virus-induced RANTES transcription in the human embryonic kidney cell line 293 (146). Future studies will show if the IRF-3/IRF-7 system plays a broader role in virus-induced expression of cytokines and chemokines. This new field of virus-induced signaling, which may represent a general mechanism in host defense to virus infections, has at present been explored only minimally beyond the model viruses, and it will be interesting to learn how the main human pathogenic viruses affect the IRF-3/IRF-7 system.
Poly(I-C) has been used extensively in experimental models mimicking dsRNA intermediates accumulating during a virus infection. A viral infection initiates a broad range of signals in the host cell, some of which are ascribed to dsRNA accumulation. Our understanding of the molecular events underlying these responses has grown significantly in recent years, although many important questions have yet to be answered. At present, dsRNA is known to activate NF-κB, ATF-2/c-Jun, and IRF-3 (45, 251, 270), with the pathway leading to activation of NF-κB being the best described. Poly(I-C)/dsRNA has been known for several years to activate the serine/threonine protein kinase PKR, which is a major mediator of the antiviral and antiproliferative activities of IFNs (254, 255). Following binding of dsRNA, PKR undergoes autophosphorylation, resulting in activation of PKR kinase activity and subsequent phosphorylation of substrates. The best-studied substrate for PKR is the translational initiation factor eIF-2α, which is sequestered after phosphorylation, eventually leading to inhibition of protein synthesis (141). This renders PKR important in cell cycle regulation, possibly by playing the role of a tumor suppressor. Furthermore, PKR appears to play a broader role in the control of cell proliferation and differentiation, tumor suppression, apoptosis, and transcriptional regulation.
Williams and associates first showed that NF-κB activation by dsRNA is abrogated in PKR-deficient cell lines (130). Recently, Zamanian-Daryoush et al., from the same laboratory, demonstrated that the dsRNA-induced signal is transmitted from PKR to NF-κB through IKKβ (270). The authors further presented data suggesting that PKR may be part of the IKK signalosome and may play a central role in NF-κB activation by stimuli other than dsRNA. This notion is supported by the observation that activation of NF-κB by Tat and TNF-α is impaired in PKR-deficient cells (66, 270). Finally, NIK was identified in the signal transduction pathway to NF-κB between PKR and IKK. Essentially similar results were obtained in a parallel study by Chu et al., who described that NF-κB activation by dsRNA or VSV was abrogated in IKKβ-deficient cell lines (45). This latter study also addressed the role of JNK and p38 in the response to dsRNA or VSV infection. The authors were able to demonstrate involvement of JNK, but not p38, in dsRNA-induced activation of ATF-2/c-Jun. Interestingly, PKR was found not to be involved in VSV- or dsRNA-induced JNK activation. Similarly, IRF-3 activation by dsRNA has also been found to be unaltered in PKR-deficient cells (251). Taken together, these recent data demonstrate in detail the pathway from dsRNA to NF-κB activation, involving the activation of PKR, which signals through NIK and IKK, possibly via physical interaction with the IKK complex, resulting in phosphorylation of the IKKβ subunit. This in turn phosphorylates IκB, eventually leading to the degradation of IκB and to nuclear translocation of NF-κB. Identification of alternative dsRNA-activated pathways, not involving PKR, are interesting new tasks for this area in virus research.
CONCLUDING REMARKS
Cytokines and chemokines play central roles in the host response to viral infections as well as in the immunopathology associated with many viral diseases. Within the last few years, significant advances have been made in our understanding of the molecular mechanisms governing the induction of cytokines and chemokines by viruses. By interacting with specific cellular receptors, many viral glycoproteins stimulate cells directly to secrete cytokines and chemokines. In addition, viral RNA and a number of viral proteins with an intracellular location interfere with cellular signal transduction and transcription factor activity, thus promoting viral replication and expression of proinflammatory proteins. Among the signaling pathways activated by the viruses discussed in this review, NF-κB seems to play a particularly important role as far as expression of cytokines and chemokines is concerned.
Some of the major challenges now facing this field of research are to deepen our understanding of virus-induced signaling and to unveil how this influences viral replication and cellular functions. Ultimately this knowledge could potentially be exploited to design drugs that specifically inhibit viral replication or reduce virus-induced immunopathology with minimal undesired effect for patients. In the past, experimental model systems for viral infections have proven useful and have provided important information that could subsequently be studied with the less manageable yet clinically more relevant viral human pathogens. The interplay between these approaches will also be used in the future as an important tool in studies of virus-host interactions.
ACKNOWLEDGMENTS
This work was supported by grants from the Danish Health Science Research Council (grant 12-1622) and The Leo Research Foundation. T.H.M. was supported by a grant from the Danish Cancer Society.
REFERENCES
- 1.Adams A L, Darr D, Holley-Guthrie E, Johnson R A, Mauser A, Swenson J, Kenny S. Epstein-Barr virus immediate-early proteins BZLF1 and BRLF1 activate the ATF2 transcription factor by increasing the levels of phosphorylated p38 and c-Jun N-terminal kinases. J Virol. 2000;74:1224–1233. doi: 10.1128/jvi.74.3.1224-1233.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht T, Boldogh I, Fons M, AbuBakar S, Deng C Z. Cell activation signals and the pathogenesis of human cytomegalovirus. Intervirology. 1990;31:68–75. doi: 10.1159/000150140. [DOI] [PubMed] [Google Scholar]
- 3.al-Wabel A, al-Janadi M, Raziuddin S. Cytokine profile of viral and autoimmune chronic active hepatitis. J Allergy Clin Immunol. 1993;92:902–908. doi: 10.1016/0091-6749(93)90068-q. [DOI] [PubMed] [Google Scholar]
- 4.Ambrosino C, Ruocco M R, Chen X, Mallardo M R, Baudi F, Trematerra S, Quinto I, Ventura S, Scala G. HIV-1 Tat induces the expression of the interleukin-6 (IL6) gene by binding to the IL6 leader RNA and by interacting with CAAT enhancer-binding protein β (NF-IL6) transcription factors. J Biol Chem. 1997;272:14883–14892. doi: 10.1074/jbc.272.23.14883. [DOI] [PubMed] [Google Scholar]
- 5.Ameglio F, Capobianchi M R, Castilletti C, Cordiali Fei P, Fais S, Trento E, Dianzani F. Recombinant gp120 induces IL-10 in resting peripheral blood mononuclear cells; correlation with the induction of other cytokines. Clin Exp Immunol. 1994;95:455–458. doi: 10.1111/j.1365-2249.1994.tb07018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Andrisani O M, Barnabas S. The transcriptional function of the hepatitis B virus X protein and its role in hepatocarcinogenesis. Int J Oncol. 1999;15:373–379. doi: 10.3892/ijo.15.2.373. [DOI] [PubMed] [Google Scholar]
- 7.Ankel H, Capobianchi M R, Castilletti C, Dianzani F. Interferon induction by HIV glycoprotein 120: role of the V3 loop. Virology. 1994;205:34–43. doi: 10.1006/viro.1994.1617. [DOI] [PubMed] [Google Scholar]
- 8.Ankel H, Westra D F, Welling-Wester S, Lebon P. Induction of interferon-α by glycoprotein D of herpes simplex virus: a possible role of chemokine receptors. Virology. 1998;251:317–326. doi: 10.1006/viro.1998.9432. [DOI] [PubMed] [Google Scholar]
- 9.Arora D J, Houde M. Modulation of murine macrophage responses stimulated with influenza glycoproteins. Can J Microbiol. 1992;38:188–192. doi: 10.1139/m92-032. [DOI] [PubMed] [Google Scholar]
- 10.Attar R M, Macdonald-Bravo H, Raventos-Suarez C, Durham S K, Bravo R. Expression of constitutively active IκBβ in T cells of transgenic mice: persistent NF-κB activity is required for T-cell immune responses. Mol Cell Biol. 1998;18:477–487. doi: 10.1128/mcb.18.1.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Au W C, Moore P A, LaFleur D W, Tombal B, Pitha P M. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J Biol Chem. 1998;273:29210–29217. doi: 10.1074/jbc.273.44.29210. [DOI] [PubMed] [Google Scholar]
- 12.Au W C, Moore P A, Lowther W, Juang Y T, Pitha P M. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc Natl Acad Sci USA. 1995;92:11657–11661. doi: 10.1073/pnas.92.25.11657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azimi N, Brown K, Bamford R N, Tagaya Y, Siebenlist U, Waldmann T A. Human T cell lymphotropic virus type I Tax protein trans-activates interleukin 15 gene transcription through an NF-κB site. Proc Natl Acad Sci USA. 1998;95:2452–2457. doi: 10.1073/pnas.95.5.2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baba M, Imai T, Yoshida T, Yoshie O. Constitutive expression of various chemokine genes in human T-cell lines infected with human T-cell leukemia virus type 1: role of the viral transactivator Tax. Int J Cancer. 1996;66:124–129. doi: 10.1002/(SICI)1097-0215(19960328)66:1<124::AID-IJC21>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 15.Balotta C, Lusso P, Crowley R, Gallo R C, Franchini G. Antisense phosphorothioate oligodeoxynucleotides targeted to the vpr gene inhibit human immunodeficiency virus type 1 replication in primary human macrophages. J Virol. 1993;67:4409–4414. doi: 10.1128/jvi.67.7.4409-4414.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baumann B, Kistler B, Kirillov A, Bergman Y, Wirth T. The mutant plasmacytoma cell line S107 allows the identification of distinct pathways leading to NF-κB activation. J Biol Chem. 1998;273:11448–11455. doi: 10.1074/jbc.273.19.11448. [DOI] [PubMed] [Google Scholar]
- 17.Baumforth K R, Young L S, Flavell K J, Constandinou C, Murray P G. The Epstein-Barr virus and its association with human cancers. Mol Pathol. 1999;52:307–322. doi: 10.1136/mp.52.6.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bender B S, Small P A., Jr Influenza: pathogenesis and host defense. Semin Respir Infect. 1992;7:38–45. [PubMed] [Google Scholar]
- 19.Benn J, Schneider R J. Hepatitis B virus HBx protein activates Ras-GTP complex formation and establishes a Ras, Raf, MAP kinase signaling cascade. Proc Natl Acad Sci USA. 1994;91:10350–10354. doi: 10.1073/pnas.91.22.10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benn J, Su F, Doria M, Schneider R J. Hepatitis B virus HBx protein induces transcription factor AP-1 by activation of extracellular signal-regulated and c-Jun N-terminal mitogen-activated protein kinases. J Virol. 1996;70:4978–4985. doi: 10.1128/jvi.70.8.4978-4985.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beraud C, Sun S C, Ganchi P, Ballard D W, Greene W C. Human T-cell leukemia virus type 1 Tax associates with and is negatively regulated by the NF-κB2 p100 gene product: implications for viral latency. Mol Cell Biol. 1994;14:1374–1382. doi: 10.1128/mcb.14.2.1374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Blumenthal S G, Aichele G, Wirth T, Czernilofsky A P, Nordheim A, Dittmer J. Regulation of the human interleukin-5 promoter by Ets transcription factors. Ets1 and Ets2, but not Elf-1, cooperate with GATA3 and HTLV-I Tax1. J Biol Chem. 1999;274:12910–12916. doi: 10.1074/jbc.274.18.12910. [DOI] [PubMed] [Google Scholar]
- 23.Boldogh I, Fons M P, Albrecht T. Increased levels of sequence-specific DNA-binding proteins in human cytomegalovirus-infected cells. Biochem Biophys Res Commun. 1993;197:1505–1510. doi: 10.1006/bbrc.1993.2647. [DOI] [PubMed] [Google Scholar]
- 24.Bouillie S, Barel M, Drane P, Cassinat B, Balbo M, Holers V M, Frade R. Signaling through the EBV/C3d receptor (CR2, CD21) in human B lymphocytes: activation of phosphatidylinositol 3-kinase via a CD19-independent pathway. Eur J Immunol. 1995;25:2661–2671. [Google Scholar]
- 25.Bouillie S, Barel M, Frade R. Epstein-Barr virus/C3d receptor (CR2, CD21) activated by its extracellular ligands regulates pp105 phosphorylation through two distinct pathways. J Immunol. 1999;162:136–143. doi: 10.1002/eji.1830250939. [DOI] [PubMed] [Google Scholar]
- 26.Boyle K A, Compton T. Receptor-binding properties of a soluble form of human cytomegalovirus glycoprotein B. J Virol. 1998;72:1826–1833. doi: 10.1128/jvi.72.3.1826-1833.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Boyle K A, Pietropaolo R L, Compton T. Engagement of the cellular receptor for glycoprotein B of human cytomegalovirus activates the interferon-responsive pathway. Mol Cell Biol. 1999;19:3607–3613. doi: 10.1128/mcb.19.5.3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brady J N. Extracellular Tax1 protein stimulates NF-κB and expression of NF-κB-responsive Ig κ and TNF β genes in lymphoid cells. AIDS Res Hum Retroviruses. 1992;8:724–727. [PubMed] [Google Scholar]
- 29.Bribino E, Haraguchi S, Koutsonikolis A, Cianciolo G J, Owens U, Good R A, Day N K. Interleukin 10 is induced by recombinant HIV-1 Nef protein involving the calcium/calmodulin-dependent phosphodiesterase signal transduction pathway Proc. Natl Acad Sci USA. 1997;94:3178–3182. doi: 10.1073/pnas.94.7.3178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brosnan C F, Selmaj K, Raine C S. Hypothesis: a role for tumor necrosis factor in immune-mediated demyelination and its relevance to multiple sclerosis. J Neuroimmunol. 1988;18:87–94. doi: 10.1016/0165-5728(88)90137-3. [DOI] [PubMed] [Google Scholar]
- 31.Brown D A, Nelson F B, Reinherz E L, Diamond D J. The human interferon-γ gene contains an inducible promoter that can be transactivated by tax I and II. Eur J Immunol. 1991;21:1879–1885. doi: 10.1002/eji.1830210815. [DOI] [PubMed] [Google Scholar]
- 32.Burysek L, Yeow W S, Lubyova B, Kellum M, Schafer S L, Huang Y Q, Pitha P M. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J Virol. 1999;73:7334–7342. doi: 10.1128/jvi.73.9.7334-7342.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burysek L, Yeow W S, Pitha P M. Unique properties of a second human herpesvirus 8-encoded interferon regulatory factor (vIRF-2) J Hum Virol. 1999;2:19–32. [PubMed] [Google Scholar]
- 34.Bussfeld D, Bacher M, Moritz A, Gemsa D, Sprenger H. Expression of transcription factor genes after influenza A virus infection. Immunobiology. 1997;198:291–298. doi: 10.1016/S0171-2985(97)80049-6. [DOI] [PubMed] [Google Scholar]
- 35.Cahir McFarland E D, Izumi K M, Mosialos G. Epstein-Barr virus transformation: involvement of latent membrane protein 1-mediated activation of NF-κB. Oncogene. 1999;18:6959–6964. doi: 10.1038/sj.onc.1203217. [DOI] [PubMed] [Google Scholar]
- 36.Capobianchi M R, Barresi C, Borghi P, Gessani S, Fantuzzi G, Ameglio F, Belardelli F, Papadia S, Dianzani F. Human immunodeficiency virus type 1 gp120 stimulates cytomegalovirus replication in monocytes: possible role of endogenous interleukin-8. J Virol. 1997;71:1591–1597. doi: 10.1128/jvi.71.2.1591-1597.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carey M. The enhanceosome and transcriptional synergy. Cell. 1998;92:5–8. doi: 10.1016/s0092-8674(00)80893-4. [DOI] [PubMed] [Google Scholar]
- 38.Carlquist J F, Edelman L, Bennion D W, Anderson J L. Cytomegalovirus induction of interleukin-6 in lung fibroblasts occurs independently of active infection and involves a G protein and the transcription factor, NF-κB. J Infect Dis. 1999;197:1094–1100. doi: 10.1086/314734. [DOI] [PubMed] [Google Scholar]
- 39.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-α production. Implications for HIV-1-associated neurological diseases. J Biol Chem. 1997;272:22385–22388. doi: 10.1074/jbc.272.36.22385. [DOI] [PubMed] [Google Scholar]
- 40.Chiao P J, Miyamoto S, Verma I M. Autoregulation of IκB α activity Proc. Natl Acad Sci USA. 1994;91:28–32. doi: 10.1073/pnas.91.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chirmule N, Goonewardena H, Pahwa S, Pasieka R, Kalyanaraman V S. HIV-1 envelope glycoproteins induce activation of activated protein-1 in CD4+ T cells. J Biol Chem. 1995;270:19364–19369. doi: 10.1074/jbc.270.33.19364. [DOI] [PubMed] [Google Scholar]
- 42.Chisari F V, Ferrari C. Hepatitis B virus immunopathology. Springer Semin Immunopathol. 1995;17:261–281. doi: 10.1007/BF00196169. [DOI] [PubMed] [Google Scholar]
- 43.Choi B H, Park G T, Rho H M. Interaction of hepatitis B viral X protein and CCAAT/ enhancer-binding protein α synergistically activates the hepatitis B viral enhancer II/pregenomic promoter. J Biol Chem. 1999;274:2858–2865. doi: 10.1074/jbc.274.5.2858. [DOI] [PubMed] [Google Scholar]
- 44.Chomik M. Interferon induction by influenza virus: significance of neuraminidase. Arch Immunol Ther Exp. 1981;29:109–114. [PubMed] [Google Scholar]
- 45.Chu W M, Ostertag D, Li Z W, Chang L, Hu Y, Williams B, Perrault J, Karin M. JNK2 and IKKβ are required for activating the innate response to viral infection. Immunity. 1999;11:721–731. doi: 10.1016/s1074-7613(00)80146-6. [DOI] [PubMed] [Google Scholar]
- 46.Chun R F, Semmes O J, Neuveut C, Jeang K T. Modulation of Sp1 phosphorylation by human immunodeficiency virus type 1 Tat. J Virol. 1998;72:2615–2629. doi: 10.1128/jvi.72.4.2615-2629.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clerici M, Hakim F T, Venzon D J, Blatt S, Hendrix C W, Wynn T A, Shearer G M. Changes in interleukin-2 and interleukin-4 production in asymptomatic, human immunodeficiency virus-seropositive individuals. J Clin Investig. 1993;91:759–765. doi: 10.1172/JCI116294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clerici M, Shearer G M. A TH1→TH2 switch is a critical step in the etiology of HIV infection. Immunol Today. 1993;14:107–111. doi: 10.1016/0167-5699(93)90208-3. [DOI] [PubMed] [Google Scholar]
- 49.Cocchi F, Lopez M, Menotti L, Aoubala M, Dubreuil P, Campadelli-Fiume G. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc Natl Acad Sci USA. 1998;95:15700–15705. doi: 10.1073/pnas.95.26.15700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cohen E A, Dehni G, Sodroski J G, Haseltine W A. Human immunodeficiency virus vpr product is a virion-associated regulatory protein. J Virol. 1990;64:3097–3099. doi: 10.1128/jvi.64.6.3097-3099.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Collette Y, Chang H L, Cerdan C, Chambost H, Algarte M, Mawas C, Burny A, Olive D. Specific Th1 cytokine down-regulation associated with primary clinically derived human immunodeficiency virus type 1 Nef gene-induced expression. J Immunol. 1996;156:360–370. [PubMed] [Google Scholar]
- 52.Collette Y, Dutartre H, Benziane A, Olive D. The role of HIV1 Nef in T-cell activation: Nef impairs induction of Th1 cytokines and interacts with the Src family tyrosine kinase Lck. Res Virol. 1997;148:52–58. doi: 10.1016/s0923-2516(97)81914-0. [DOI] [PubMed] [Google Scholar]
- 53.Compton T, Nowlin D M, Cooper N R. Initiation of human cytomegalovirus infection requires initial interaction with cell surface heparan sulfate. Virology. 1993;193:834–841. doi: 10.1006/viro.1993.1192. [DOI] [PubMed] [Google Scholar]
- 54.Conant K, Ma M, Nath A, Major E O. Extracellular human immunodeficiency virus type 1 Tat protein is associated with an increase in both NF-κB binding and protein kinase C activity in primary human astrocytes. J Virol. 1996;70:1384–1389. doi: 10.1128/jvi.70.3.1384-1389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Cowan E P, Alexander R K, Daniel S, Kashanchi F, Brady J N. Induction of tumor necrosis factor α in human neuronal cells by extracellular human T-cell lymphotropic virus type 1 Tax. J Virol. 1997;71:6982–6989. doi: 10.1128/jvi.71.9.6982-6989.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Crabtree G R. Generic signals and specific outcomes: signaling through Ca2+, calcineurin, and NF-AT. Cell. 1999;96:611–614. doi: 10.1016/s0092-8674(00)80571-1. [DOI] [PubMed] [Google Scholar]
- 57.Croen K D. Evidence for an antiviral effect of nitric oxide-inhibition of herpes simplex virus type 1 replication. J Clin Investig. 1993;91:2446–2452. doi: 10.1172/JCI116479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cross J C, Wen P, Rutter W J. Transactivation by hepatitis B virus X protein is promiscuous and dependent on mitogen-activated cellular serine-threonine kinases. Proc Natl Acad Sci USA. 1993;90:8078–8082. doi: 10.1073/pnas.90.17.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cullen B R. The role of Nef in the replication cycle of the human and simian immunodeficiency viruses. Virology. 1994;205:1–6. doi: 10.1006/viro.1994.1613. [DOI] [PubMed] [Google Scholar]
- 60.D'Addario M, Ahmad A, Morgan A, Menezes J. Binding of the Epstein-Barr virus major envelope glycoprotein gp350 results in the upregulation of the TNF-α gene expression in monocytic cells via NF-κB involving PKC, P13-K and tyrosine kinases. J Mol Biol. 2000;298:765–778. doi: 10.1006/jmbi.2000.3717. [DOI] [PubMed] [Google Scholar]
- 61.D'Addario M, Ahmad A, Xu J W, Menezes J. Epstein-Barr virus envelope glycoprotein gp350 induces NF-κB activation and IL-1β synthesis in human monocytes-macrophages involving PKC and P13-K. FASEB J. 1999;13:2203–2213. doi: 10.1096/fasebj.13.15.2203. [DOI] [PubMed] [Google Scholar]
- 62.Davis C B, Dikic I, Unutmaz D, Hill C M, Arthos J, Siani M A, Thompson D A, Schlessinger J, Littman D R. Signal transduction due to HIV-1 envelope interactions with chemokine receptors CXCR4 or CCR5. J Exp Med. 1997;186:1793–1798. doi: 10.1084/jem.186.10.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.De S K, Venkateshan C N, Seth P, Gajdusek D C, Gibbs C J. Adenovirus-mediated human immunodeficiency virus-1 Nef expression in human monocytes/macrophages and effect of Nef on downmodulation of Fcγ receptors and expression of monokines. Blood. 1998;91:2108–2117. [PubMed] [Google Scholar]
- 64.DeLuca C, Petropoulos L, Zmeureanu D, Hiscott J. Nuclear IκBβ maintains persistent NF-κB activation in HIV-1-infected myeloid cells. J Biol Chem. 1999;274:13010–13016. doi: 10.1074/jbc.274.19.13010. [DOI] [PubMed] [Google Scholar]
- 65.Demarchi F, D'Adda di Fagagna F, Falaschi A, Giacca M. Activation of transcription factor NF-κB by the Tat protein of human immunodeficiency virus type 1. J Virol. 1996;70:4427–4437. doi: 10.1128/jvi.70.7.4427-4437.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Demarchi F, Gutierrez M I, Giacca M. Human immunodeficiency virus type 1 tat protein activates transcription factor NF-κB through the cellular interferon-inducible, double-stranded RNA-dependent protein kinase, PKR. J Virol. 1999;73:7080–7086. doi: 10.1128/jvi.73.8.7080-7086.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dhib-Jalbut S, Hoffman P M, Yamabe T, Sun D, Xia J, Eisenberg H, Bergey G, Ruscetti F W. Extracellular human T-cell lymphotropic virus type I Tax protein induces cytokine production in adult human microglial cells. Ann Neurol. 1994;36:787–790. doi: 10.1002/ana.410360516. [DOI] [PubMed] [Google Scholar]
- 68.Eliopoulos A G, Gallagher N J, Blake S M S, Dawson C W, Young L S. Activation of the p38 mitogen-activated protein kinase pathway by Epstein-Barr virus-encoded latent membrane protein 1 coregulates interleukin-6 and interleukin-8 production. J Biol Chem. 1999;274:16085–16096. doi: 10.1074/jbc.274.23.16085. [DOI] [PubMed] [Google Scholar]
- 69.Ellermann-Eriksen S. Autocrine secretion of interferon-α/β and tumour necrosis factor-α synergistically activates mouse macrophages after infection with herpes simplex virus type 2. J Gen Virol. 1993;74:2191–2199. doi: 10.1099/0022-1317-74-10-2191. [DOI] [PubMed] [Google Scholar]
- 70.Falvo J V, Uglialoro A D, Brinkman B M N, Merika M, Parekh B S, Tsai E Y, King H C, Morielli A D, Peralta E G, Maniatis T, Thanos D, Goldfeld A E. Stimulus-specific assembly of enhancer complexes on the tumor necrosis factor α gene promoter. Mol Cell Biol. 2000;20:2239–2247. doi: 10.1128/mcb.20.6.2239-2247.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fawaz L M, Sharif-Askari E, Menezes J. Up-regulation of NK cytotoxic activity via IL-15 induction by different viruses: a comparative study. J Immunol. 1999;163:4473–4480. [PubMed] [Google Scholar]
- 72.Flory E, Kunz M, Scheller C, Jassoy C, Stauber R, Rapp U R, Ludwig S. Influenza virus-induced NF-κB-dependent gene expression is mediated by overexpression of viral proteins and involves oxidative radicals and activation of IκB kinase. J Biol Chem. 2000;275:8307–8314. doi: 10.1074/jbc.275.12.8307. [DOI] [PubMed] [Google Scholar]
- 73.Francis M L, Meltzer M S. Induction of IFN-α by HIV-1 in monocyte-enriched PBMC requires gp120-CD4 interaction but not virus replication. J Immunol. 1993;151:2208–2216. [PubMed] [Google Scholar]
- 74.Frankel A D, Young J A. HIV-1: fifteen proteins and an RNA. Annu Rev Biochem. 1998;67:1–25. doi: 10.1146/annurev.biochem.67.1.1. [DOI] [PubMed] [Google Scholar]
- 75.Franklin A A, Kubik M F, Uittenbogaard M N, Brauweiler A, Utaisincharoen P, Matthews M A, Dynan W S, Hoeffler J P, Nyborg J K. Transactivation by the human T-cell leukemia virus Tax protein is mediated through enhanced binding of activating transcription factor-2 (ATF-2) ATF-2 response and cAMP element-binding protein (CREB) J Biol Chem. 1993;268:21225–21231. [PubMed] [Google Scholar]
- 76.Fujii Y, Otake K, Tashiro M, Adachi A. Soluble Nef antigen of HIV-1 is cytotoxic for human CD4+ T cells. FEBS Lett. 1996;393:93–96. doi: 10.1016/0014-5793(96)00859-9. [DOI] [PubMed] [Google Scholar]
- 77.Fujisawa J, Seiki M, Kiyokawa T, Yoshida M. Functional activation of the long terminal repeat of human T-cell leukemia virus type I by a trans-acting factor. Proc Natl Acad Sci USA. 1985;82:2277–2281. doi: 10.1073/pnas.82.8.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gachon F, Thebault S, Peleraux A, Devaux C, Mesnard J M. Molecular interactions involved in the transactivation of the human T-cell leukemia virus type 1 promoter mediated by Tax and CREB-2 (ATF-4) Mol Cell Biol. 2000;20:3470–3481. doi: 10.1128/mcb.20.10.3470-3481.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Geleziunas R, Ferrell S, Lin X, Mu Y, Cunningham E T, Grant M, Connelly M A, Hambor J E, Marcu K B, Greene W C. Human T-cell leukemia virus type 1 Tax induction of NF-κB involves activation of the IκB kinase α (IKKα) and IKKβ cellular kinases. Mol Cell Biol. 1998;18:5157–5165. doi: 10.1128/mcb.18.9.5157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Geneva-Popova M, Murdjeva M. Study on proinflammatory cytokines (IL-1 β, IL-6, TNF-α) and IL-2 in patients with acute hepatitis B. Folia Med. 1999;41:78–81. [PubMed] [Google Scholar]
- 81.Ghanekar S, Zheng L, Logar A, Navratil J, Borowski L, Gupta P, Rinaldo C. Cytokine expression by human peripheral blood dendritic cells stimulated in vitro with HIV-1 and herpes simplex virus. J Immunol. 1996;157:4028–4036. [PubMed] [Google Scholar]
- 82.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Gonzalez-Amaro R, Garcia-Monzon C, Garcia-Buey L, Moreno-Otero R, Alonso J L, Yague E, Pivel J P, Lopez-Cabrera M, Fernandez-Ruiz E, Sanchez-Madrid F. Induction of tumor necrosis factor α production by human hepatocytes in chronic viral hepatitis. J Exp Med. 1994;179:841–848. doi: 10.1084/jem.179.3.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Good L, Maggirwar S B, Harhaj E W, Sun S C. Constitutive dephosphorylation and activation of a member of the nuclear factor of activated T cells, NF-AT1, in Tax-expressing and type I human T-cell leukemia virus-infected human T cells. J Biol Chem. 1997;272:1425–1428. doi: 10.1074/jbc.272.3.1425. [DOI] [PubMed] [Google Scholar]
- 85.Good L, Maggirwar S B, Sun S C. Activation of the IL-2 gene promoter by HTLV-I tax involves induction of NF-AT complexes bound to the CD28-responsive element. EMBO J. 1996;15:3744–3750. [PMC free article] [PubMed] [Google Scholar]
- 86.Graziosi C, Gantt K R, Vaccarezza M, Demarest J F, Daucher M, Saag M S, Shaw G M, Quinn T C, Cohen O J, Welbon C C, Pantaleo G, Fauci A S. Kinetics of cytokine expression during primary human immunodeficiency virus type 1 infection. Proc Natl Acad Sci USA. 1996;93:4386–4391. doi: 10.1073/pnas.93.9.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Halford W P, Gebhardt B M, Carr D J. Persistent cytokine expression in trigeminal ganglion latently infected with herpes simplex virus type 1. J Immunol. 1996;157:3542–3549. [PubMed] [Google Scholar]
- 88.Harhaj E W, Sun S C. IKKγ serves as a docking subunit of the IκB kinase (IKK) and mediates interaction of IKK with the human T-cell leukemia virus Tax protein. J Biol Chem. 1999;274:22911–22914. doi: 10.1074/jbc.274.33.22911. [DOI] [PubMed] [Google Scholar]
- 89.Harrich D, Garcia J, Wu F, Mitsuyasu R, Gonazalez J, Gaynor R. Role of Sp1-binding domains in in vivo transcriptional regulation of the human immunodeficiency virus type 1 long terminal repeat. J Virol. 1989;63:2585–2591. doi: 10.1128/jvi.63.6.2585-2591.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hatada E, Saito S, Fukuda R. Mutant influenza viruses with a defective NS1 protein cannot block the activation of PKR in infected cells. J Virol. 1999;73:2425–2433. doi: 10.1128/jvi.73.3.2425-2433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hatada E N, Krappmann D, Scheidereit C. NF-κB and the innate immune response. Curr Opin Immunol. 2000;12:52–58. doi: 10.1016/s0952-7915(99)00050-3. [DOI] [PubMed] [Google Scholar]
- 92.Haviv I, Vaizel D, Shaul Y. pX, the HBV-encoded coactivator, interacts with components of the transcription machinery and stimulates transcription in a TAF-independent manner. EMBO J. 1996;15:3413–3420. [PMC free article] [PubMed] [Google Scholar]
- 93.Hayden F G, Fritz R S, Lobo M C, Alvord W G, Strober W, Straus S E. Local and systemic cytokine responses during experimental human influenza A virus infection. Relation to symptom formation and host defense. J Clin Investig. 1998;101:643–649. doi: 10.1172/JCI1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.He J, Ichimura H, Iida T, Minami M, Kobayashi K, Kita M, Sotozono C, Tagawa Y I, Iwakura Y, Imanishi J. Kinetics of cytokine production in the cornea and trigeminal ganglion of C57BL/6 mice after corneal HSV-1 infection. J Interferon Cytokine Res. 1999;19:609–615. doi: 10.1089/107999099313749. [DOI] [PubMed] [Google Scholar]
- 95.Henkler F F, Koshy R. Hepatitis B virus transcriptional activators: mechanisms and possible role in oncogenesis. J Viral Hepatol. 1996;3:109–121. doi: 10.1111/j.1365-2893.1996.tb00001.x. [DOI] [PubMed] [Google Scholar]
- 96.Hennet T, Ziltener H J, Frei K, Peterhans E. A kinetic study of immune mediators in the lungs of mice infected with influenza A virus. J Immunol. 1992;149:932–939. [PubMed] [Google Scholar]
- 97.Herlaar E, Brown Z. p38 MAPK signalling cascades in inflammatory disease. Mol Med Today. 1999;5:439–447. doi: 10.1016/s1357-4310(99)01544-0. [DOI] [PubMed] [Google Scholar]
- 98.Hildt E, Saher G, Bruss V, Hofschneider P H. The hepatitis B virus large surface protein (LHBs) is a transcriptional activator. Virology. 1996;225:235–239. doi: 10.1006/viro.1996.0594. [DOI] [PubMed] [Google Scholar]
- 99.Himes S R, Katsikeros R, Shannon M F. Costimulation of cytokine gene expression in T cells by the human T leukemia/lymphotropic virus type 1 trans activator Tax. J Virol. 1996;70:4001–4008. doi: 10.1128/jvi.70.6.4001-4008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hofman F M, Chen P, Incardona F, Zidovetzki R, Hinton D R. HIV-1 tat protein induces the production of interleukin-8 by human brain-derived endothelial cells. J Neuroimmunol. 1999;94:28–39. doi: 10.1016/s0165-5728(98)00198-2. [DOI] [PubMed] [Google Scholar]
- 101.Hofmann P, Sprenger H, Kaufmann A, Bender A, Hasse C, Nain M, Gemsa D. Susceptibility of mononuclear phagocytes to influenza A virus infection and possible role in the antiviral response. J Leukoc Biol. 1997;61:408–414. doi: 10.1002/jlb.61.4.408. [DOI] [PubMed] [Google Scholar]
- 102.Hollsberg P. Mechanisms of T-cell activation by human T-cell lymphotropic virus type I. Microbiol Mol Biol Rev. 1999;63:308–333. doi: 10.1128/mmbr.63.2.308-333.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hollsberg P, Hafler D A. Pathogenesis of diseases induced by human lymphotropic virus type I infection. N Engl J Med. 1993;328:1173–1182. doi: 10.1056/NEJM199304223281608. [DOI] [PubMed] [Google Scholar]
- 104.Houde M, Arora D J. Human influenza virus neuraminidase, but not hemagglutinin, induces murine macrophage interleukin 1 in vivo and in vitro. Immunol Lett. 1989;22:41–46. doi: 10.1016/0165-2478(89)90140-5. [DOI] [PubMed] [Google Scholar]
- 105.Houde M, Arora D J. Stimulation of tumor necrosis factor secretion by purified influenza virus neuraminidase. Cell Immunol. 1990;129:104–111. doi: 10.1016/0008-8749(90)90190-3. [DOI] [PubMed] [Google Scholar]
- 106.Hsu H Y, Chang M H, Ni Y H, Lee P I. Cytokine release of peripheral blood mononuclear cells in children with chronic hepatitis B virus infection. J Pediatr Gastroenterol Nutr. 1999;29:540–545. doi: 10.1097/00005176-199911000-00013. [DOI] [PubMed] [Google Scholar]
- 107.Hsu J L, Glasser S L. Epstein-Barr virus-associated malignancies: epidemiologic patterns and etiologic implications. Crit Rev Oncol Hematol. 2000;43:27–53. doi: 10.1016/s1040-8428(00)00046-9. [DOI] [PubMed] [Google Scholar]
- 108.Husain S R, Leland P, Aggarwal B B, Puri R K. Transcriptional up-regulation of interleukin 4 receptors by human immunodeficiency virus type 1 tat gene. AIDS Res Hum Retroviruses. 1996;12:1349–1359. doi: 10.1089/aid.1996.12.1349. [DOI] [PubMed] [Google Scholar]
- 109.Inoue J, Seiki M, Taniguchi T, Tsuru S, Yoshida M. Induction of interleukin 2 receptor gene expression by p40x encoded by human T-cell leukemia virus type 1. EMBO J. 1986;5:2883–2888. doi: 10.1002/j.1460-2075.1986.tb04583.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ito M, Ishida T, He L, Tanabe F, Rongge Y, Miyakawa Y, Terunuma H. HIV type 1 Tat protein inhibits interleukin 12 production by human peripheral blood mononuclear cells. AIDS Res Hum Retroviruses. 1998;14:845–849. doi: 10.1089/aid.1998.14.845. [DOI] [PubMed] [Google Scholar]
- 111.Izumi K, Kieff E. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-κB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Jackson S P, MacDonald J J, Lees-Miller S, Tjian R. GC box binding induces phosphorylation of Sp1 by a DNA-dependent protein kinase. Cell. 1990;63:155–165. doi: 10.1016/0092-8674(90)90296-q. [DOI] [PubMed] [Google Scholar]
- 113.Jang K L, Pulverer B, Woodgett J R, Latchman D S. Activation of the cellular transcription factor AP-1 in herpes simplex virus infected cells is dependent on the viral immediate-early protein ICP0. Nucleic Acids Res. 1991;19:4879–4883. doi: 10.1093/nar/19.18.4879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kallas E G, Reynolds K, Andrews J, Treanor J J, Evans T G. Production of influenza-stimulated tumor necrosis factor-α by monocytes following acute influenza infection in humans. J Interferon Cytokine Res. 1999;19:751–755. doi: 10.1089/107999099313596. [DOI] [PubMed] [Google Scholar]
- 115.Kanagawa O, Vaupel B A, Gayama S, Koehler G, Kopf M. Resistance of mice deficient in IL-4 to retrovirus-induced immunodeficiency syndrome (MAIDS) Science. 1993;262:240–242. doi: 10.1126/science.8211142. [DOI] [PubMed] [Google Scholar]
- 116.Kanangat S, Babu J S, Knipe D M, Rouse B T. HSV-1-mediated modulation of cytokine gene expression in a permissive cell line: selective upregulation of IL-6 gene expression. Virology. 1996;219:295–300. doi: 10.1006/viro.1996.0250. [DOI] [PubMed] [Google Scholar]
- 117.Kanda K, Kempkes B, Bornkamm G W, von Gabain A, Decker T. The Epstein-Barr virus nuclear antigen 2 (EBNA2), a protein required for B lymphocyte immortalization, induces the synthesis of type I interferon in Burkitt's lymphoma cell lines. Biol Chem. 1999;380:213–221. doi: 10.1515/BC.1999.029. [DOI] [PubMed] [Google Scholar]
- 118.Karupiah G, Xie Q, Buller R M L, Nathan C, Duate C, MacMicking J. Inhibition of viral replication by interferon-γ-induced nitric oxide synthase. Science. 1993;261:1445–1448. doi: 10.1126/science.7690156. [DOI] [PubMed] [Google Scholar]
- 119.Kekule A S, Lauer U, Weiss L, Luber B, Hofschneider P H. Hepatitis B virus transactivator HBx uses a tumor promoter signalling pathway. Nature. 1993;361:742–745. doi: 10.1038/361742a0. [DOI] [PubMed] [Google Scholar]
- 120.Kelly G D, Ensoli B, Gunthel C J, Offermann M K. Purified Tat induces inflammatory response genes in Kaposi's sarcoma cells. AIDS. 1998;12:1753–1761. doi: 10.1097/00002030-199814000-00006. [DOI] [PubMed] [Google Scholar]
- 121.Kerry J A, Priddy M A, Staley T L, Jones T R, Stenberg R M. The role of ATF in regulating the human cytomegalovirus DNA polymerase (UL54) promoter during viral infection. J Virol. 1997;71:2120–2126. doi: 10.1128/jvi.71.3.2120-2126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim S J, Kehrl J H, Burton J, Tendler C L, Jeang K T, Danielpour D, Thevenin C, Kim K Y, Sporn M B, Roberts A B. Transactivation of the transforming growth factor β 1 (TGF-β 1) gene by human T lymphotropic virus type 1 tax: a potential mechanism for the increased production of TGF-β 1 in adult T cell leukemia. J Exp Med. 1990;172:121–129. doi: 10.1084/jem.172.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim T K, Maniatis T. The mechanism of transcriptional synergy of an in vitro assembled interferon-β enhanceosome. Mol Cell. 1998;1:119–129. doi: 10.1016/s1097-2765(00)80013-1. [DOI] [PubMed] [Google Scholar]
- 124.Kino T, Gragerov A, Kopp J P, Stauber R H, Pavlakis G N, Chrousos G P. The HIV-1 virion-associated protein vpr is a coactivator of the human glucocorticoid receptor. J Exp Med. 1999;189:51–62. doi: 10.1084/jem.189.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kinoshita S, Su L, Amano M, Timmerman L A, Kaneshima H, Nolan G P. The T cell activation factor NF-ATc positively regulates HIV-1 replication and gene expression in T cells. Immunity. 1997;6:235–244. doi: 10.1016/s1074-7613(00)80326-x. [DOI] [PubMed] [Google Scholar]
- 126.Kitajima I, Shinohara T, Bilakovics J, Brown D A, Nerenberg M. Ablation of transplanted HTLV-I Tax-transformed tumors in mice by antisense inhibition of NF-κB. Science. 1992;258:1792–1795. doi: 10.1126/science.1299224. [DOI] [PubMed] [Google Scholar]
- 127.Knobil K, Choi A M, Weigand G W, Jacoby D B. Role of oxidants in influenza virus-induced gene expression. Am J Physiol. 1998;274:134–142. doi: 10.1152/ajplung.1998.274.1.L134. [DOI] [PubMed] [Google Scholar]
- 128.Kujime K, Hashimoto S, Gon Y, Shimizu K, Horie T. p38 mitogen-activated protein kinase and c-jun-NH2-terminal kinase regulate RANTES production by influenza virus-infected human bronchial epithelial cells. J Immunol. 2000;164:3222–3228. doi: 10.4049/jimmunol.164.6.3222. [DOI] [PubMed] [Google Scholar]
- 129.Kumar A, Manna S K, Dhawan S, Aggarwal B B. HIV-Tat protein activates c-Jun N-terminal kinase and activator protein-1. J Immunol. 1998;161:776–781. [PubMed] [Google Scholar]
- 130.Kumar A, Yang Y L, Flati V, Der S, Kadereit S, Deb A, Haque J, Reis L, Weissmann C, Williams B R. Deficient cytokine signaling in mouse embryo fibroblasts with a targeted deletion in the PKR gene: role of IRF-1 and NF-κB. EMBO J. 1997;16:406–416. doi: 10.1093/emboj/16.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.LaBoissiere S, O'Hare P. Analysis of HCF, the cellular cofactor of VP16, in herpes simplex virus-infected cells. J Virol. 2000;74:99–109. doi: 10.1128/jvi.74.1.99-109.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lacoste J, Lanoix J, Pepin N, Hiscott J. Interactions between HTLV-I Tax and NF-κB/Rel proteins in T cells. Leukemia. 1994;8:71–76. [PubMed] [Google Scholar]
- 133.Lahdevirta J, Maury C P, Teppo A M, Repo H. Elevated levels of circulating cachectin/tumor necrosis factor in patients with acquired immunodeficiency syndrome. Am J Med. 1998;85:289–291. doi: 10.1016/0002-9343(88)90576-1. [DOI] [PubMed] [Google Scholar]
- 134.Lal R B, Rudolph D L. Constitutive production of interleukin-6 and tumor necrosis factor-α from spontaneously proliferating T cells in patients with human T-cell lymphotropic virus type-I/II. Blood. 1991;78:571–574. [PubMed] [Google Scholar]
- 135.Lannuzel A, Barnier J V, Hery C, Huynh V T, Guibert B, Gray F, Vincent J D, Tardieu M. Human immunodeficiency virus type 1 and its coat protein gp120 induce apoptosis and activate JNK and ERK mitogen-activated protein kinases in human neurons. Ann Neurol. 1997;42:847–856. doi: 10.1002/ana.410420605. [DOI] [PubMed] [Google Scholar]
- 136.Lanoix J, Lacoste J, Pepin N, Rice N, Hiscott J. Overproduction of NFKB2 (lyt-10) and c-Rel: a mechanism for HTLV-I Tax-mediated trans-activation via the NF-κB signalling pathway. Oncogene. 1994;9:841–852. [PubMed] [Google Scholar]
- 137.Lara-Pezza E, Armesilla A L, Majano P L, Redondo M J, Lopez-Cabrera M. The hepatitis B virus X protein activates nuclear factor of activated T cells (NF-AT) by a cyclosporin A-sensitive pathway. EMBO J. 1998;17:7066–7077. doi: 10.1093/emboj/17.23.7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Lara-Pezza E, Majano P L, Gomez-Gonzalo M, Garcia-Monzon C, Moreno-Otero R, Levrero M, Lopez-Cabrera M. The hepatitis B virus X protein up-regulates tumor necrosis factor α gene expression in hepatocytes. Hepatology. 2000;28:1013–1021. doi: 10.1002/hep.510280416. [DOI] [PubMed] [Google Scholar]
- 139.Lay J D, Tsao C J, Chen J Y, Kadin M E, Su I J. Upregulation of tumor necrosis factor-α gene by Epstein-Barr virus and activation of macrophages in Epstein-Barr virus-infected T cells in the pathogenesis of hemophagocytic syndrome. J Clin Investig. 1997;100:1969–1979. doi: 10.1172/JCI119728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lee M, Lee S K, Son M, Cho S W, Park S, Kim H I. Expression of Th1 and Th2 type cytokines responding to HBsAg and HBxAg in chronic hepatitis B patients. J Korean Med Sci. 1999;14:175–181. doi: 10.3346/jkms.1999.14.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee S B, Melkova Z, Yan W, Williams B R, Hovanessian A G, Esteban M. The interferon-induced double-stranded RNA-activated human p68 protein kinase potently inhibits protein synthesis in cultured cells. Virology. 1993;192:380–385. doi: 10.1006/viro.1993.1048. [DOI] [PubMed] [Google Scholar]
- 142.Lee Y, Park U S, Choi I, Yoon S K, Park Y M, Lee Y I. Human interleukin 6 gene is activated by hepatitis B virus-X protein in human hepatoma cells. Clin Cancer Res. 1998;4:1711–1717. [PubMed] [Google Scholar]
- 143.Li Q, Feldman M, Harmon C, Fitzgerald-Bocarsly P. Role of tyrorine kinases, protein kinase C, and protein kinase A in the regulation of interferon-α production induced by herpes simplex virus type 1. J Interferon Cytokine Res. 1996;16:109–118. doi: 10.1089/jir.1996.16.109. [DOI] [PubMed] [Google Scholar]
- 144.Li X H, Murphy K M, Palka K T, Surabhi M, Gaynor R B. The human T-cell leukemia virus type-1 Tax protein regulates the activity of the IκB kinase complex. J Biol Chem. 1999;274:34417–34424. doi: 10.1074/jbc.274.48.34417. [DOI] [PubMed] [Google Scholar]
- 145.Lim S P, Garzino-Demo A. The human immunodeficiency virus type 1 Tat protein up-regulates the promoter activity of the β-chemokine monocyte chemoattractant protein 1 in the human astrocytoma cell line U-87 MG: role of SP-1, AP-1, and NF-κB consensus sites. J Virol. 2000;74:1632–1640. doi: 10.1128/jvi.74.4.1632-1640.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin R, Heylbroeck C, Genin P, Pitha P M, Hiscott J. Essential role of interferon regulatory factor 3 in direct activation of RANTES chemokine transcription. Mol Cell Biol. 1999;19:959–966. doi: 10.1128/mcb.19.2.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Lin Y, Nomura T, Cheong J, Dorjsuren D, Iida K, Murakami S. Hepatitis B virus X protein is a transcriptional modulator that communicates with transcription factor IIB and the RNA polymerase II subunit 5. J Biol Chem. 1997;272:7132–7139. doi: 10.1074/jbc.272.11.7132. [DOI] [PubMed] [Google Scholar]
- 148.Lindholm P F, Marriott S J, Gitlin S D, Bohan C A, Brady J N. Induction of nuclear NF-κB DNA binding activity after exposure of lymphoid cells to soluble tax1 protein. New Biol. 1990;2:1034–1043. [PubMed] [Google Scholar]
- 149.Lindholm P F, Tamami M, Makowski J, Brady J N. Human T-cell lymphotropic virus type 1 Tax1 activation of NF-κB: involvement of the protein kinase C pathway. J Virol. 1996;70:2525–2532. doi: 10.1128/jvi.70.4.2525-2532.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lotz M, Tsoukas C D, Fong S, Dinarello C A, Carson D A, Vaughan J H. Release of lymphokines after Epstein Barr virus infection in vitro. I. Sources of and kinetics of production of interferons and interleukins in normal humans. J Immunol. 1996;136:3636–3642. [PubMed] [Google Scholar]
- 151.Macian F, Rao A. Reciprocal modulatory interaction between human immunodeficiency virus type 1 Tat and transcription factor NFAT1. Mol Cell Biol. 1999;19:3645–3653. doi: 10.1128/mcb.19.5.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Maguire H F, Hoeffler J P, Siddiqui A. HBV X protein alters the DNA binding specificity of CREB and ATF-2 by protein-protein interactions. Science. 1991;252:842–844. doi: 10.1126/science.1827531. [DOI] [PubMed] [Google Scholar]
- 153.Mahe Y, Mukaida N, Kuno K, Akiyama M, Ikeda N, Matsushima K, Murakami S. Hepatitis B virus X protein transactivates human interleukin-8 gene through acting on nuclear factor κB and CCAAT/enhancer-binding protein-like cis-elements. J Biol Chem. 1991;266:13759–13763. [PubMed] [Google Scholar]
- 154.Malmgaard L, Paludan S R, Mogensen S C, Ellermann-Eriksen S. Herpes simplex virus type 2 induces interleukin-12 by macrophages through a mechanism involving NF-κB. J Gen Virol. 2000;81:3011–3020. doi: 10.1099/0022-1317-81-12-3011. [DOI] [PubMed] [Google Scholar]
- 155.Marie I, Durbin J E, Levy D E. Differential viral induction of distinct interferon-α genes by positive feedback through interferon regulatory factor-7. EMBO J. 1998;17:6660–6669. doi: 10.1093/emboj/17.22.6660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Marsters S A, Ayres T M, Skubatch M, Gray C L, Rothe M, Ashkenazi A. Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-κB and AP-1. J Biol Chem. 1997;272:14029–14032. doi: 10.1074/jbc.272.22.14029. [DOI] [PubMed] [Google Scholar]
- 157.Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kundin T M, Amakawa R, Kishihara K, Wakeham A, Potter J, Furlonger C L, Narendran A, Suzuki H, Ohashi P S, Paige C J, Taniguchi T, Mak T W. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- 158.McColl S R, Roberge C J, Larochelle B, Gosselin J. EBV induces the production and release of IL-8 and macrophage inflammatory protein-1α in human neutrophils. J Immunol. 1997;159:6164–6168. [PubMed] [Google Scholar]
- 159.McLean T I, Bachenheimer S L. Activation of cJUN N-terminal kinase by herpes simplex virus type 1 enhances viral replication. J Virol. 1999;73:8415–8426. doi: 10.1128/jvi.73.10.8415-8426.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Melville M W, Tan S L, Wambach M, Song J, Morimoto R, Katze M G. The cellular inhibitor of the PKR protein kinase, P58(IPK), is an influenza virus-activated co-chaperone that modulates heat shock protein 70 activity. J Biol Chem. 1999;274:3797–3803. doi: 10.1074/jbc.274.6.3797. [DOI] [PubMed] [Google Scholar]
- 161.Mendez E, Kawanishi T, Clemens K, Siomi H, Soldan S, Calabresi P, Brady J, Jacobsen S. Astrocyte-specific expression of human T-cell lymphotropic virus type 1 (HTLV-1) Tax: induction of tumor necrosis factor alpha and susceptibility to lysis by CD8+ HTLV-1 specific cytotoxic T cells. J Virol. 1997;71:9143–9149. doi: 10.1128/jvi.71.12.9143-9149.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Merika M, Williams A J, Chen G, Collins T, Thanos D. Recruitment of CBP/p300 by the IFN β enhanceosome is required for synergistic activation of transcription. Mol Cell. 1998;1:277–287. doi: 10.1016/s1097-2765(00)80028-3. [DOI] [PubMed] [Google Scholar]
- 163.Michelson S, Turowski P, Picard L, Goris J, Landini M P, Topilko A, Hemmings B, Bessia C, Carcia A, Virelizier J L. Human cytomegalovirus carries serine/threonine protein phosphatases PP1 and a host-cell derived PP2A. J Virol. 1996;70:1415–1423. doi: 10.1128/jvi.70.3.1415-1423.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mintz M, Rapaport R, Oleske J M, Connor J M, Koenigsberger M R, Denny T, Epstein L G. Elevated serum levels of tumor necrosis factor are associated with progressive encephalopathy in children with acquired immunodeficiency syndrome. Am J Dis Child. 1989;143:771–774. doi: 10.1001/archpedi.1989.02150190021012. [DOI] [PubMed] [Google Scholar]
- 165.Mischiati C, Pironi F, Milani D, Giacca M, Mirandola P, Capitani S, Zauli G. Extracellular HIV-1 Tat protein differentially activates the JNK and ERK/MAPK pathways in CD4 T cells. AIDS. 1999;13:1637–1645. doi: 10.1097/00002030-199909100-00006. [DOI] [PubMed] [Google Scholar]
- 166.Misse D, Cerutti M, Noraz N, Jourdan P, Favero J, Devauchelle G, Yssel H, Taylor N, Veas F. A CD4-independent interaction of human immunodeficiency virus-1 gp120 with CXCR4 induces their cointernalization, cell signaling, and T-cell chemotaxis. Blood. 1999;93:2454–2462. [PubMed] [Google Scholar]
- 167.Monteiro J M, Harvey C, Trinchieri G. Role of interleukin-12 in primary influenza virus infection. J Virol. 1998;72:4825–4831. doi: 10.1128/jvi.72.6.4825-4831.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Montgomery R I, Warner M S, Lum B J, Spear P G. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 169.Mori N, Mukaida N, Ballard D W, Matsushima K, Yamamoto N. Human T-cell leukemia virus type I Tax transactivates human interleukin 8 gene through acting concurrently on AP-1 and nuclear factor-κB-like sites. Cancer Res. 1998;58:3993–4000. [PubMed] [Google Scholar]
- 170.Mori N, Prager D. Transactivation of the interleukin-1α promoter by human T-cell leukemia virus type I and type II Tax proteins. Blood. 1996;87:3410–3417. [PubMed] [Google Scholar]
- 171.Mori N, Prager D. Interleukin-10 gene expression and adult T-cell leukemia. Leuk Lymphoma. 1998;29:239–248. doi: 10.3109/10428199809068561. [DOI] [PubMed] [Google Scholar]
- 172.Mori N, Shirakawa F, Abe M, Kamo Y, Koyama Y, Murakami S, Shimizu H. Human T-cell leukemia virus type I tax transactivates the interleukin-6 gene in human rheumatoid synovial cells. J Rheumatol. 1995;22:2049–2054. [PubMed] [Google Scholar]
- 173.Mosialos G, Birkenbach M, Yalamanchili R, VanArsdale T, Ware C, Kieff E. The Epstein-Barr virus transforming protein LMP1 engages signaling proteins for the tumor necrosis factor receptor family. Cell. 1995;80:389–399. doi: 10.1016/0092-8674(95)90489-1. [DOI] [PubMed] [Google Scholar]
- 174.Nabel G, Baltimore D. An inducible transcription factor activates expression of human immunodeficiency virus in T cells. Nature. 1987;326:771–773. doi: 10.1038/326711a0. [DOI] [PubMed] [Google Scholar]
- 175.Nakagomi H, Dolcetti R, Bejarano M T, Pisa P, Kiessling R, Masucci M G. The Epstein-Barr virus latent membrane protein-1 (LMP1) induces interleukin-10 production in Burkitt lymphoma lines. Int J Cancer. 1994;57:240–244. doi: 10.1002/ijc.2910570218. [DOI] [PubMed] [Google Scholar]
- 176.Nakajima T, Kitamura K, Yamashita N, Sakane T, Mizushima Y, Delespesse G, Lal R B. Constitutive expression and production of tumor necrosis factor-β in T-cell lines infected with HTLV-I and HTLV-II. Biochem Biophys Res Commun. 1993;191:371–377. doi: 10.1006/bbrc.1993.1227. [DOI] [PubMed] [Google Scholar]
- 177.Nath A, Conant K, Chen P, Scott C, Major E O. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem. 1999;274:17098–17102. doi: 10.1074/jbc.274.24.17098. [DOI] [PubMed] [Google Scholar]
- 178.Natoli G, Avantaggiati M L, Chirillo P, Puri P L, Ianni A, Balsano C, Levrero M. Ras- and Raf-dependent activation of c-jun transcriptional activity by the hepatitis B virus transactivator pX. Oncogene. 1994;9:2837–2843. [PubMed] [Google Scholar]
- 179.Navarro L, David M. p38-dependent activation of interferon regulatory factor 3 by lipopolysaccharide. J Biol Chem. 1999;274:35535–35538. doi: 10.1074/jbc.274.50.35535. [DOI] [PubMed] [Google Scholar]
- 180.Navarro L, Mowen K, Rodems S, Weaver B, Reich N, Spector D, David M. Cytomegalovirus activates interferon immediate-early response gene expression and an interferon regulatory factor 3-containing interferon-stimulated response element-binding complex. Mol Cell Biol. 1998;18:3796–3802. doi: 10.1128/mcb.18.7.3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Oberlin E, Amara A, Bachelerie F, Bessia C, Virelizier J L, Arenzana-Seisdedos F, Schwartz O, Heard J M, Clark-Lewis I, Legler D F, Loetscher M, Baggiolini M, Moser B. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature. 1996;382:833–835. doi: 10.1038/382833a0. [DOI] [PubMed] [Google Scholar]
- 182.Oquendo J, Karray S, Galanaud P, Petit M A. Effect of hepatitis B virus on tumour necrosis factor (TNF α) gene expression in human THP-1 monocytic and Namalwa B cell lines. Res Immunol. 1997;148:399–409. doi: 10.1016/s0923-2494(97)82873-8. [DOI] [PubMed] [Google Scholar]
- 183.Orange J S, Biron C A. An absolute and restricted requirement for IL-12 in natural killer cell IFN-γ production and antiviral defense. Studies of natural killer and T cell responses in contrasting viral infections. J Immunol. 1996;156:1138–1142. [PubMed] [Google Scholar]
- 184.Pahl H L, Baeuerle P A. Expression of influenza virus hemagglutinin activates transcription factor NF-κB. J Virol. 1995;69:1480–1484. doi: 10.1128/jvi.69.3.1480-1484.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Pahl H L, Baeuerle P A. The ER overload response: activation of NF-κB. Trends Biochem Sci. 1997;22:63–67. doi: 10.1016/s0968-0004(96)10073-6. [DOI] [PubMed] [Google Scholar]
- 186.Paludan S R, Ellermann-Eriksen S, Mogensen S C. NF-κB activation is responsible for the synergistic effect of herpes simplex virus type 2 infection on interferon-γ-induced nitric oxide production. J Gen Virol. 1998;79:2785–2793. doi: 10.1099/0022-1317-79-11-2785. [DOI] [PubMed] [Google Scholar]
- 187.Paludan S R, Ellermann-Eriksen S, Malmgaard L, Mogensen S C. Inhibition of NO production in macrophages by IL-13 is counteracted by HSV infection through TNF-α-induced activation of NF-κB. Eur Cytokine Netw. 2000;11:275–282. [PubMed] [Google Scholar]
- 188.Paludan, S. R., L. Malmgaard, S. Ellermann-Eriksen, L. Bosca, and S. C. Mogensen. Interferon (IFN)-γ and herpes simplex virus/tumor necrosis factor-α synergistically induce nitric oxide synthase 2 in macrophages through cooperative action of nuclear factor-κB and IFN regulatory factor-1. Eur. Cytokine Netw., in press. [PubMed]
- 189.Patel A, Hanson J, McLean T I, Olgiate J, Hilton M, Miller W E, Bachenheimer S L. Herpes simplex type 1 induction of persistent NF-κB nuclear translocation increases the efficiency of virus replication. Virology. 1998;247:212–222. doi: 10.1006/viro.1998.9243. [DOI] [PubMed] [Google Scholar]
- 190.Patella V, Florio G, Petraroli A, Marone G. HIV-1 gp120 induces IL-4 and IL-13 release from human FcɛRI+ cells through interaction with the VH3 region of IgE. J Immunol. 2000;164:589–595. doi: 10.4049/jimmunol.164.2.589. [DOI] [PubMed] [Google Scholar]
- 191.Penna A, Del Prete G, Cavalli A, Bertoletti A, D'Elios M M, Sorrentino R, D'Amato M, Boni C, Pilli M, Fiaccadori F, Ferrari C. Predominant T-helper 1 cytokine profile of hepatitis B virus nucleocapsid-specific T cells in acute self-limited hepatitis B. Hepatology. 1997;25:1022–1027. doi: 10.1002/hep.510250438. [DOI] [PubMed] [Google Scholar]
- 192.Peterson P K, Gekker G, Chao C C, Hu S X, Edelman L, Balfour H H J, Verhoef J. Human cytomegalovirus-stimulated peripheral blood mononuclear cells induce HIV-1 replication via a tumor necrosis factor-α-mediated mechanism. J Clin Investig. 1992;89:574–580. doi: 10.1172/JCI115623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Phillips R J, Ghosh S. Regulation of IκB β in WEHI 231 mature B cells. Mol Cell Biol. 1997;17:4390–4396. doi: 10.1128/mcb.17.8.4390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Pirhonon J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. [PubMed] [Google Scholar]
- 195.Popik W, Hesselgesser J, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 and CXCR4 receptors differentially regulates expression of inflammatory genes and activates the MEK/ERK signaling pathway. J Virol. 1998;72:6406–6413. doi: 10.1128/jvi.72.8.6406-6413.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Popik W, Pitha P M. Binding of human immunodeficiency virus type 1 to CD4 induces association of Lck and Raf-1 and activates Raf-1 by a Ras-independent pathway. Mol Cell Biol. 1996;16:6532–6541. doi: 10.1128/mcb.16.11.6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Powell P P, Dixon L K, Parkhouse R M E. An IκB homolog encoded by African swine fever virus provides a novel mechanism for downregulation of proinflammatory cytokine responses in host macrophages. J Biol Chem. 1996;70:8527–8533. doi: 10.1128/jvi.70.12.8527-8533.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Pyo H, Joe E H, Jung S, Lee S H, Jou I. Gangliosides activate cultured rat brain microglia. J Biol Chem. 1999;274:34584–34589. doi: 10.1074/jbc.274.49.34584. [DOI] [PubMed] [Google Scholar]
- 199.Quaranta M G, Camponeschi B, Straface E, Malorni W, Viora M. Induction of interleukin-15 production by HIV-1 Nef protein: a role in the proliferation of uninfected cells. Exp Cell Res. 1999;250:112–121. doi: 10.1006/excr.1999.4494. [DOI] [PubMed] [Google Scholar]
- 200.Rajcani J, Vojvodova A. The role of herpes simplex virus glycoproteins in the virus replication cycle. Acta Virol. 1998;42:103–118. [PubMed] [Google Scholar]
- 201.Revilla Y, Callejo M, Rodriguez J M, Culebras E, Nogal M L, Salas M L, Vinuela E, Fresno M. Inhibition of nuclear factor κB activation by a virus-encoded IκB-like protein. J Biol Chem. 1998;273:5405–5411. doi: 10.1074/jbc.273.9.5405. [DOI] [PubMed] [Google Scholar]
- 202.Rinaldo C R, Jr, Armstrong J A, Kingsley L A, Zhou S, Ho M. Relation of α and γ interferon levels to development of AIDS in homosexual men. J Exp Pathol. 1990;5:127–132. [PubMed] [Google Scholar]
- 203.Rivera I, Harhaj E W, Sun S C. Involvement of NF-AT in type I human T-cell leukemia virus Tax-mediated Fas ligand promoter transactivation. J Biol Chem. 1998;273:22382–22388. doi: 10.1074/jbc.273.35.22382. [DOI] [PubMed] [Google Scholar]
- 204.Roberge C J, Larochelle B, Rola-Pleszczynski M, Gosselin J. Epstein-Barr virus induces GM-CSF synthesis by monocytes: effect on EBV-induced IL-1 and IL-1 receptor antagonist production in neutrophils. Virology. 1997;238:344–352. doi: 10.1006/viro.1997.8852. [DOI] [PubMed] [Google Scholar]
- 205.Roberge C J, Poubelle P E, Beaulieu A D, Heitz D, Gosselin J. The IL-1 and IL-1 receptor antagonist (IL-1Ra) response of human neutrophils to EBV stimulation. Preponderance of IL-Ra detection. J Immunol. 1996;156:4884–4891. [PubMed] [Google Scholar]
- 206.Rong B L, Libermann T A, Kogawa K, Ghosh S, Cao L X, Pavan-Langston D, Dunkel E C. HSV-1-inducible proteins bind to NF-κB-like sites in the HSV-1 genome. Virology. 1992;189:750–756. doi: 10.1016/0042-6822(92)90599-k. [DOI] [PubMed] [Google Scholar]
- 207.Rosler A, Pohl M, Braune H J, Oertel W H, Gemsa D, Sprenger H. Time course of chemokines in the cerebrospinal fluid and serum during herpes simplex type 1 encephalitis. J Neurol Sci. 1998;157:82–89. doi: 10.1016/s0022-510x(98)00061-6. [DOI] [PubMed] [Google Scholar]
- 208.Rossol S, Marinos G, Carucci P, Singer M V, Williams R, Naoumov N V. Interleukin-12 induction of Th1 cytokines is important for viral clearance in chronic hepatitis B. J Clin Investig. 1997;99:3025–3033. doi: 10.1172/JCI119498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roux P, Alfieri C, Hrimech M, Cohen E A, Tanner J E. Activation of transcription factors NF-κB and NF-IL-6 by human immunodeficiency virus type 1 protein R (Vpr) induces interleukin-8 expression. J Virol. 2000;74:4658–4665. doi: 10.1128/jvi.74.10.4658-4665.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ruocco M R, Chen X, Ambrosino C, Dragonetti E, Liu W, Mallardo M, De Falco G, Palmieri C, Franzoso G, Quinto I, Ventura S, Scala G. Regulation of HIV-1 long terminal repeats by interaction of C/ EBP(NF-IL6) and NF-κB/Rel transcription factors. J Biol Chem. 1996;271:22479–22486. doi: 10.1074/jbc.271.37.22479. [DOI] [PubMed] [Google Scholar]
- 211.Sambucetti L C, Cherrington J M, Wilkinson G W, Mocarski E S. NF-κB activation of the cytomegalovirus enhancer is mediated by a viral transactivator and by T cell stimulation. EMBO J. 1989;8:4251–4258. doi: 10.1002/j.1460-2075.1989.tb08610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Sarawar S R, Doherty P C. Concurrent production of interleukin-2, interleukin-10, and γ interferon in the regional lymph nodes of mice with influenza pneumonia. J Virol. 1994;68:3112–3119. doi: 10.1128/jvi.68.5.3112-3119.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Sareneva T, Matikainen S, Kurimoto M, Julkunen I. Influenza A virus-induced IFN-α/β and IL-18 synergistically enhance IFN-γ gene expression in human T cells. J Immunol. 1998;160:6032–6038. [PubMed] [Google Scholar]
- 214.Sato M, Tanaka N, Hata N, Oda E, Taniguchi T. Involvement of the IRF family transcription factor IRF-3 in virus-induced activation of the IFN-β gene. FEBS Lett. 1998;425:112–116. doi: 10.1016/s0014-5793(98)00210-5. [DOI] [PubMed] [Google Scholar]
- 215.Sawaya B E, Khalili K, Mercer W E, Denisova L, Amini S. Cooperative actions of HIV-1 Vpr and p53 modulate viral gene transcription. J Biol Chem. 1998;273:20052–20057. doi: 10.1074/jbc.273.32.20052. [DOI] [PubMed] [Google Scholar]
- 216.Sawaya B E, Thatikunta P, Denisova L, Brady J, Khalili K, Amini S. Regulation of TNFα and TGFβ-1 gene transcription by HIV-1 Tat in CNS cells. J Neuroimmunol. 1998;87:33–42. doi: 10.1016/s0165-5728(98)00044-7. [DOI] [PubMed] [Google Scholar]
- 217.Scala G, Ruocco M R, Ambrosino C, Mallardo M, Giordano V, Baldassarre F, Dragonetti E, Quinto I, Ventura S. The expression of the interleukin 6 gene is induced by the human immunodeficiency virus 1 tat protein. J Exp Med. 1994;179:961–971. doi: 10.1084/jem.179.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Scala S, Quinto I, Ruocco M R, Mallardo M R, Ambrosino C, Squitieri B, Tassone P, Venuta S. Epstein-Barr virus nuclear antigen 2 transactivates the long terminal repeat of human immunodeficiency virus type 1. J Virol. 1993;67:2853–2861. doi: 10.1128/jvi.67.5.2853-2861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Schafer S L, Vlach J, Pitha P M. Cooperation between herpes simplex virus type 1-encoded ICP0 and Tat to support transcription of human immunodeficiency virus type 1 long terminal repeat in vivo can occur in the absence of the TAR binding site. J Virol. 1996;70:6937–6946. doi: 10.1128/jvi.70.10.6937-6946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Schols D, de Clercq E. Human immunodeficiency virus type 1 gp120 induces anergy in human peripheral blood lymphocytes by inducing interleukin-10 production. J Virol. 1996;70:4953–4960. doi: 10.1128/jvi.70.8.4953-4960.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Scholtissek C. Synthesis and function of influenza A virus glycoproteins. Behring Inst Mitt. 1991;89:46–53. [PubMed] [Google Scholar]
- 222.Scholz C, Hafler D A, Hollsberg P. Downregulation of IL-10 secretion and enhanced antigen-presenting abilities following HTLV-I infection of T cells. J Neurosci Res. 1996;45:786–794. doi: 10.1002/(SICI)1097-4547(19960915)45:6<786::AID-JNR15>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 223.Schultz-Cherry S, Hinshaw V S. Influenza virus neuraminidase activates latent transforming growth factor β. J Virol. 1996;70:8624–8629. doi: 10.1128/jvi.70.12.8624-8629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Schwarze S R, Ho A, Vocero-Akbani A, Dowdy S F. In vivo transduction: delivery of a biologically active protein into the mouse. Science. 1999;285:1569–1572. doi: 10.1126/science.285.5433.1569. [DOI] [PubMed] [Google Scholar]
- 225.Selmaj K W, Raine C S. Tumor necrosis factor mediates myelin and oligodendrocyte damage in vitro. Ann Neurol. 1988;23:339–346. doi: 10.1002/ana.410230405. [DOI] [PubMed] [Google Scholar]
- 226.Setsuda J, Teruya-Feldstein J, Harris N L, Ferry J A, Sorbara L, Gupta G, Jaffe E S, Tosato G. Interleukin-18, interferon-γ, IP-10, and Mig expression in Epstein-Barr virus-induced infectious mononucleosis and posttransplant lymphoproliferative disease. Am J Pathol. 1999;155:257–265. doi: 10.1016/s0002-9440(10)65119-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sharma V, May C C. Human T-cell lymphotrophic virus type-I tax gene induces secretion of human macrophage inflammatory protein-1α. Biochem Biophys Res Commun. 1999;262:429–432. doi: 10.1006/bbrc.1999.1242. [DOI] [PubMed] [Google Scholar]
- 228.Siebenlist U, Franzoso G, Brown K. Structure, regulation and function of NF-κB. Annu Rev Cell Biol. 1994;10:405–455. doi: 10.1146/annurev.cb.10.110194.002201. [DOI] [PubMed] [Google Scholar]
- 229.Su F, Schneider R J. Hepatitis B virus HBx protein activates transcription factor NF-κB by acting on multiple cytoplasmic inhibitors of rel-related proteins. J Virol. 1996;70:4558–4566. doi: 10.1128/jvi.70.7.4558-4566.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sugano N, Chen W, Roberts M L. Epstein-Barr virus binding to CD21 activates the initial viral promoter via NF-κB induction. J Exp Med. 1997;186:731–737. doi: 10.1084/jem.186.5.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Sylla B S, Hung S C, Davidson D M, Hatzivassiliou E, Malinin N L, Wallach D, Gilmore T D, Kieff E, Mosialos G. Epstein-Barr virus-transforming protein latent infection membrane protein 1 activates transcription factor NF-κB through a pathway that includes the NF-κB-inducing kinase and the IκB kinases IKKα and IKKβ. Proc Natl Acad Sci USA. 1998;95:10106–10111. doi: 10.1073/pnas.95.17.10106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Takizawa T, Ohashi K, Nakanishi Y. Possible involvement of double-stranded RNA-activated protein kinase in cell death by influenza virus infection. J Virol. 1996;70:8128–8132. doi: 10.1128/jvi.70.11.8128-8132.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Tanner J E, Alfieri C, Chatila T A, Diaz-Mitoma F. Induction of interleukin-6 after stimulation of human B-cell CD21 by Epstein-Barr virus glycoproteins gp350 and gp220. J Virol. 1996;70:570–575. doi: 10.1128/jvi.70.1.570-575.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tatewaki M, Yamaguchi K, Matsuoka M, Ishii T, Miyasaka M, Mori S, Takatsuki K, Watanabe T. Constitutive overexpression of the L-selectin gene in fresh leukemic cells of adult T-cell leukemia that can be transactivated by human T-cell lymphotropic virus type 1 Tax. Blood. 1995;86:3109–3117. [PubMed] [Google Scholar]
- 235.Tay C H, Welsh R M. Distinct organ-dependent machanism for the control of murine cytomegalovirus infection by natural killer cells. J Virol. 1997;71:267–275. doi: 10.1128/jvi.71.1.267-275.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Thomas J, Kanangat S, Rouse B T. Herpes simplex virus replication-induced expression of chemokines and proimflammatory cytokines in the eye: implications in herpetic stromal keratitis. J Interferon Cytokine Res. 1998;18:681–690. doi: 10.1089/jir.1998.18.681. [DOI] [PubMed] [Google Scholar]
- 237.Thompson J E, Phillips R J, Erdjument Bromage H, Tempst P, Ghosh S. IκB-β regulates the persistent response in a biphasic activation of NF-κB. Cell. 1995;80:573–582. doi: 10.1016/0092-8674(95)90511-1. [DOI] [PubMed] [Google Scholar]
- 238.Toso J F, Ferbas J J, Rappocciolo G, Armstrong J A, Ho M, Rinaldo C R., Jr Stimulation of IFN-α production in HLA-DR+, light density peripheral blood mononuclear cells by human immunodeficiency virus. J Exp Pathol. 1990;5:123–125. [PubMed] [Google Scholar]
- 239.Tsukada J, Misago M, Serino Y, Ogawa R, Murakami S, Nakanishi M, Tonai S, Kominato Y, Morimoto I, Auron P E, Eto S. Human T-cell leukemia virus type I Tax transactivates the promoter of human prointerleukin-1β gene through association with two transcription factors, nuclear factor-interleukin-6 and Spi-1. Blood. 1997;90:3142–3153. [PubMed] [Google Scholar]
- 240.Vacca A, Farina M, Maroder M, Alesse E, Screpanti I, Frati L, Gulino A. Human immunodeficiency virus type-1 tat enhances interleukin-2 promoter activity through synergism with phorbol ester and calcium-mediated activation of the NF-AT cis-regulatory motif. Biochem Biophys Res Commun. 1994;30:467–474. doi: 10.1006/bbrc.1994.2689. [DOI] [PubMed] [Google Scholar]
- 241.Valyi-Nagy T, Bandi Z, Boldogh I, Albrecht T. Hydrolysis of inositol lipids: an early signal of human cytomegalovirus infection. Arch Virol. 1988;101:199–207. doi: 10.1007/BF01311001. [DOI] [PubMed] [Google Scholar]
- 242.Vingerhoets J, Michielsen P, Vanham G, Bosmans E, Paulij W, Ramon A, Pelckmans P, Kestens L, Leroux-Roels G. HBV-specific lymphoproliferative and cytokine responses in patients with chronic hepatitis B. J Hepatol. 1998;28:8–16. doi: 10.1016/s0168-8278(98)80196-7. [DOI] [PubMed] [Google Scholar]
- 243.Virelizier J L. Blocking HIV co-receptors by chemokines. Dev Biol Stand. 1999;97:105–109. [PubMed] [Google Scholar]
- 244.Vlach J, Garcia A, Jacque J M, Rodrigues M S, Michelson S, Virelizier J L. Induction of Sp1 phosphorylation and NF-κB-independent HIV promoter domain activity in T lymphocytes stimulated by okadaic acid. Virology. 1995;208:753–761. doi: 10.1006/viro.1995.1207. [DOI] [PubMed] [Google Scholar]
- 245.Waage A, Halstensen A, Shalaby R, Brandtzaeg P, Kierulf P, Espevik T. Local production of tumor necrosis factor α, interleukin 1, and interleukin 6 in meningococcal meningitis. Relation to the inflammatory response. J Exp Med. 1989;170:1859–1867. doi: 10.1084/jem.170.6.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Walev I, Dienes H P, Bohl J, Podlech J, Falke D. Correlation of virus replication, cytokine (TNF-α and IL-1) producing cells, neuronal necrosis and inflammation after intranasal infection of mice with herpes simplex virus strains of different virulence. Arch Virol. 1995;140:1957–1967. doi: 10.1007/BF01322685. [DOI] [PubMed] [Google Scholar]
- 247.Wand H D, Yuh C H, Dang C V, Johnson D L. The hepatitis B virus X protein increases the cellular level of TATA-binding protein, which mediates transactivation of RNA polymerase III genes. Mol Cell Biol. 1995;15:6720–6728. doi: 10.1128/mcb.15.12.6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Wang L, Mukherjee S, Jia F, Narayan O, Zhao L J. Interaction of virion protein Vpr of human immunodeficiency virus type 1 with cellular transcription factor Sp1 and trans-activation of viral long terminal repeat. J Biol Chem. 1995;270:25564–25569. doi: 10.1074/jbc.270.43.25564. [DOI] [PubMed] [Google Scholar]
- 249.Wang X, Li M, Zheng H, Muster T, Palese P, Beg A A, Garcia-Sastre A. Influenza A virus NS1 protein prevents activation of NF-κB and induction of alpha/beta interferon. J Virol. 2000;74:11566–11573. doi: 10.1128/jvi.74.24.11566-11573.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Wathelet M G, Lin C H, Parekh B S, Ronco L V, Howley P M, Maniatis T. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-β enhancer in vivo. Mol Cell. 1998;1:507–518. doi: 10.1016/s1097-2765(00)80051-9. [DOI] [PubMed] [Google Scholar]
- 251.Weaver B K, Kumar K P, Reich N C. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol Cell Biol. 1998;18:1359–1368. doi: 10.1128/mcb.18.3.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Westendorp M O, Li Weber M, Frank R W, Krammer P H. Human immunodeficiency virus type 1 Tat upregulates interleukin-2 secretion in activated T cells. J Virol. 1994;68:4177–4185. doi: 10.1128/jvi.68.7.4177-4185.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Whitmarsh A J, Davis R J. Transcription factor AP-1 regulation by mitogen-activated protein kinase signal transduction pathways. J Mol Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- 254.Williams B R. The role of the dsRNA-activated protein kinase, PKR, in signal transduction. Semin Virol. 1995;6:191–202. [Google Scholar]
- 255.Williams B R. Role of the dsRNA-activated protein kinase (PKR) in cell regulation. Biochem Soc Trans. 1997;25:509–513. doi: 10.1042/bst0250509. [DOI] [PubMed] [Google Scholar]
- 256.Xiao G, Harhaj E W, Sun S C. Domain-specific interaction with the IκB kinase (IKK) regulatory subunit IKK-γ as an essential step in tax-mediated activation of IKK. J Biol Chem. 2000;275:34060–34067. doi: 10.1074/jbc.M002970200. [DOI] [PubMed] [Google Scholar]
- 257.Xu X, Heidenreich O, Kitajima I, McGuire K, Li Q, Su B, Nerenberg M. Constitutively activated JNK is associated with HTLV-1 mediated tumorigenesis. Oncogene. 1996;13:135–142. [PubMed] [Google Scholar]
- 258.Yamagata T, Mitani K, Ueno H, Kanda Y, Yazaki Y, Hirai H. Triple synergism of human T-lymphotropic virus type 1-encoded tax, GATA-binding protein, and AP-1 is required for constitutive expression of the interleukin-5 gene in adult T-cell leukemia cells. Mol Cell Biol. 1997;17:4272–4281. doi: 10.1128/mcb.17.8.4272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Yang Y L, Reis L F, Pavlovic J, Aguzzi A, Schafer R, Kumar A, Williams B R, Aguet M, Weissmann C. Deficient signaling in mice devoid of double-stranded RNA-dependent protein kinase. EMBO J. 1995;14:6095–6106. doi: 10.1002/j.1460-2075.1995.tb00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Yasumoto K, Okamoto S, Mukaida N, Murakami S, Mai M, Matsushima K. Tumor necrosis factor α and interferon γ synergistically induce interleukin 8 production in a human gastric cancer cell line through acting concurrently on AP-1 and NF-κB-like binding sites of the interleukin 8 gene. J Biol Chem. 1992;267:22506–22511. [PubMed] [Google Scholar]
- 261.Yie J, Merika M, Munshi N, Chen G, Thanos D. The role of HMG I(Y) in the assembly and function of the IFN-β enhanceosome. EMBO J. 1999;18:3074–3089. doi: 10.1093/emboj/18.11.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Yin M J, Christerson L B, Yamamoto Y, Kwak Y T, Xu S, Mercurio F, Barbosa M, Cobb M H, Gaynor R B. HTLV-I Tax protein binds to MEKK1 to stimulate IκB kinase activity and NF-κB activation. Cell. 1998;93:875–884. doi: 10.1016/s0092-8674(00)81447-6. [DOI] [PubMed] [Google Scholar]
- 263.Yin M J, Yamamoto Y, Gaynor R B. The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(κ)B kinase-β. Nature. 1998;396:77–80. doi: 10.1038/23948. [DOI] [PubMed] [Google Scholar]
- 264.Yoneyama M, Suhara W, Fukuhara Y, Fukuda M, Nishida E, Fujita T. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 1998;17:1087–1095. doi: 10.1093/emboj/17.4.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Yoo Y D, Ueda H, Park K, Flanders K C, Lee Y I, Jay G, Kim S J. Regulation of transforming growth factor-β 1 expression by the hepatitis B virus (HBV) X transactivator. Role in HBV pathogenesis. J Clin Investig. 1996;97:388–395. doi: 10.1172/JCI118427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Yurochko A D, Huang E S. Human cytomegalovirus binding to human monocytes induces immunoregulatory gene expression. J Immunol. 1999;162:4806–4816. [PubMed] [Google Scholar]
- 267.Yurochko A D, Hwang E S, Rasmussen L, Keay S, Pereira L, Huang E S. The human cytomegalovirus UL55 (gB) and UL75 (gH) glycoprotein ligands initiate the rapid activation of Sp1 and NF-κB during infection. J Virol. 1997;71:5051–5059. doi: 10.1128/jvi.71.7.5051-5059.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yurochko A D, Mayo L D, Poma E E, Baldwin A S, Jr, Huang E S. Induction of the transcription factor Sp1 during human cytomegalovirus infection mediates upregulation of the p65 and p105/p50 NF-κB promoters. J Virol. 1997;71:4638–4648. doi: 10.1128/jvi.71.6.4638-4648.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Zachos G, Clements B, Conner J. Herpes simplex virus type 1 infection stimulates p38/c-Jun N-terminal mitogen-activated protein kinase pathways and activates transcription factor AP-1. J Biol Chem. 1999;274:5097–5103. doi: 10.1074/jbc.274.8.5097. [DOI] [PubMed] [Google Scholar]
- 270.Zamanian-Daryoush M, Mogensen T H, Didonato J A, Williams B R. NF-κB activation by double-stranded-RNA-activated protein kinase (PKR) is mediated through NF-κB-inducing kinase and IκB kinase. Mol Cell Biol. 2000;20:1278–1290. doi: 10.1128/mcb.20.4.1278-1290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Zegarra-Moran O, Rasola A, Rugolo M, Porcelli A M, Rossi B, Galietta L J. HIV-1 nef expression inhibits the activity of a Ca2+-dependent K+ channel involved in the control of the resting potential in CEM lymphocytes. J Immunol. 1999;162:5359–5366. [PubMed] [Google Scholar]
- 272.Zhang L, Pagano J S. Interferon regulatory factor 7 is induced by Epstein-Barr virus latent membrane protein 1. J Virol. 2000;74:1061–1068. doi: 10.1128/jvi.74.3.1061-1068.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]