Abstract
Objective:
In retrospective studies, liver stiffness (LS) by vibration-controlled transient elastography (VCTE) is associated with the risk of liver decompensation in patients with non-alcoholic steatohepatitis (NASH), but prospective data in biopsy-confirmed cohorts with advanced fibrosis are limited. We aimed to establish thresholds for LS by VCTE that predict progression to cirrhosis among patients with bridging fibrosis and hepatic decompensation among patients with cirrhosis due to NASH.
Design:
We utilized data from four randomized placebo-controlled trials of selonsertib and simtuzumab in participants with advanced fibrosis (F3-F4). The trials were discontinued due to lack of efficacy. Liver fibrosis was staged centrally at baseline and week 48 (selonsertib study) or week 96 (simtuzumab study). Associations between LS by VCTE with disease progression were determined using Cox-proportional hazards regression analysis.
Results:
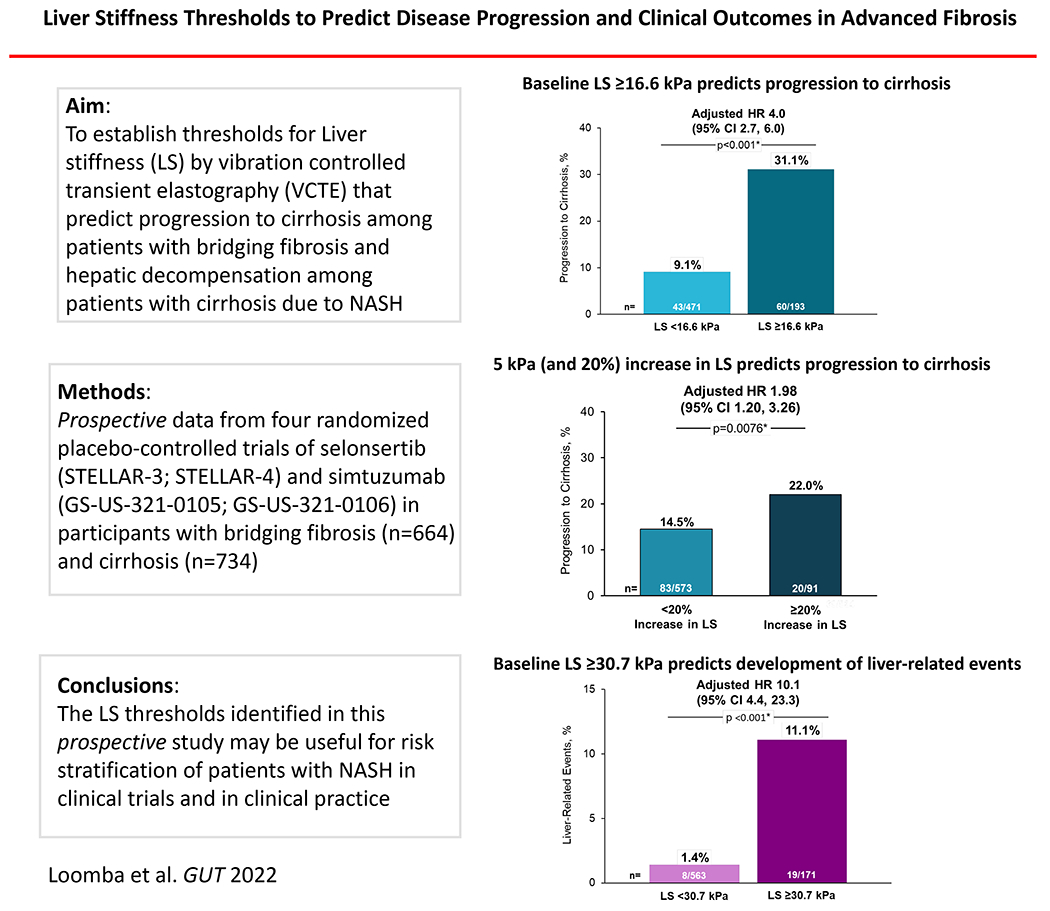
Progression to cirrhosis occurred in 16% (103/664) of participants with bridging fibrosis and adjudicated liver-related events occurred in 4% (27/734) of participants with baseline cirrhosis. The optimal baseline LS thresholds were ≥16.6 kPa for predicting progression to cirrhosis, and ≥30.7 kPa for predicting liver-related events. Baseline LS ≥16.6 kPa (adjusted hazard ratio [HR] 3.99; 95% CI 2.66-5.98, p<.0001) and a ≥5kPa (and ≥20%) increase (adjusted HR 1.98; 95% CI 1.20, 3.26, p=0.008) were independent predictors of progression to cirrhosis in participants with bridging fibrosis, while baseline LS ≥30.7 kPa (adjusted HR 10.13, 95% CI 4.38-23.41, p<.0001) predicted liver-related events in participants with cirrhosis.
Conclusion:
The LS thresholds identified in this study may be useful for risk stratification of NASH patients with advanced fibrosis.
Keywords: NASH, cirrhosis, fibrosis, liver stiffness
INTRODUCTION
Nearly a third of the world’s adult population has non-alcoholic fatty liver disease (NAFLD) 1,2. NAFLD encompasses both non-alcoholic fatty liver and nonalcoholic steatohepatitis (NASH), the inflammatory form of NAFLD that can progress to fibrosis, cirrhosis and subsequent decompensation 3–7. The incidence of NASH cirrhosis and NAFLD-related hepatocellular carcinoma (HCC) are projected to increase rapidly in the next decade 8–12. The risk of liver-related mortality and decompensation in NASH increases in parallel with fibrosis stage 13–16. Although histologic staging of fibrosis is the reference standard, liver biopsy is limited by invasiveness, potential complications and sampling variability 17,18. Noninvasive tests (NITs) of fibrosis, including serum markers such as NAFLD fibrosis score (NFS), fibrosis-4 (FIB-4) index, Enhanced Liver Fibrosis (ELF) test, and liver stiffness (LS) by vibration-controlled transient elastography (VCTE) are not prone to these limitations, and accurately predict histologic fibrosis stage in patients with NASH 19–27. Retrospective studies report that increasing baseline LS by VCTE19,28,29 is associated with the risk of disease progression in patients with NAFLD, but prospective data in well-characterized NASH cohorts with biopsy-confirmed advanced fibrosis are limited. The optimal LS thresholds for prognostication of fibrosis progression and decompensation are unknown, however, a recent consensus report from the Baveno group has proposed incrementally increasing LS thresholds 30.
With these considerations in mind, we analyzed data from two recent phase 3 placebo-controlled trials of selonsertib, a selective inhibitor of apoptosis signal-regulating kinase 1 (ASK1) and two phase 2b placebo-controlled trials of simtuzumab, a humanized monoclonal antibody directed against lysyl oxidase-like 2 31,32. While the studies were discontinued prematurely due to lack of efficacy, the prospectively collected data in these well characterized participants with serial liver biopsies provides a unique opportunity to study the association between baseline LS by VCTE and disease progression. The primary aim of this study was to establish thresholds of LS that prognosticate risk of clinical outcomes in participants with bridging fibrosis and cirrhosis due to NASH. The secondary aims were to examine the association between a change in LS and clinical outcomes, and to compare the prognostic ability of baseline LS versus baseline LS plus a combination of routine clinical parameters (age, sex, AST, ALT, platelets and diabetes status), included within the Agile 3+ and Agile 4 scores, to define risk for fibrosis progression and decompensation 33,34.
METHODS
Study population
This analysis utilized data from four large, randomized placebo-controlled trials of selonsertib and simtuzumab in participants with advanced (F3-F4) fibrosis due to NASH 31,32. Pre-planned analysis of the selonsertib studies at 48 weeks and the simtuzumab studies at 96 weeks concluded that these therapies were ineffective, and therefore, the trials were discontinued. At baseline, there were no differences between treatment groups; thus, treatment groups were combined for the present analysis. The primary findings of these studies, as well as the detailed methods, have been reported elsewhere 31,32.
Briefly, the selonsertib STELLAR studies comprised of two 2 randomized, double-blind, placebo-controlled, phase III trials conducted in Europe, North America, South America, Asia, and the Pacific region. Eligible participants were 18 to 70 years of age with a histologic diagnosis of NASH, available data for baseline LS by VCTE and advanced fibrosis (F3-F4). Patients with liver disease of other etiologies, a history of solid organ transplantation, hepatic decompensation, or HCC were excluded. Participants with bridging (F3) fibrosis (n=808; NCT03053050) and compensated cirrhosis (F4) (n=883; NCT03053063) were randomized 2:2:1 to receive selonsertib 18 mg, selonsertib 6 mg, or placebo once daily for 48 weeks. The primary efficacy endpoint was the proportion of participants with ≥1-stage improvement in fibrosis without worsening of NASH at week 48.
The simtuzumab studies consisted of two phase 2b trials in North America and Europe. Eligible participants were 18–65 years of age with a body mass index (BMI) of at least 18 kg/m2 with NASH and bridging fibrosis, NASH cirrhosis or cirrhosis with at least 1 clinical feature suggestive of NASH (such as diabetes, obesity or dyslipidemia). Participants with liver disease of other etiologies, a history of solid organ transplantation, a history of malignancy other than non-melanomatous skin cancer and hepatic decompensation were excluded. Participants with bridging (F3) fibrosis (n=219; NCT01672866) were randomly assigned (1:1:1) to groups given weekly subcutaneous injections of simtuzumab (75 or 125 mg) or placebo for a planned duration of 240 weeks. The primary outcome was a decrease in hepatic collagen content by morphometry. Participants with compensated cirrhosis (F4) (n=258; NCT01672879) were randomly assigned (1:1:1) to receive intravenous infusions of simtuzumab (200 mg or 700 mg) or placebo every other week. The primary outcome was the change in hepatic venous pressure gradient.
Study assessments
Histology:
Liver fibrosis was staged centrally at baseline (all patients), week 48 (all patients), and week 96 (simtuzumab studies only). All biopsies were read by a single central reader (Z.G.) who was blinded to treatment assignment but not biopsy sequence. Histological assessments included the adequacy of the biopsy specimen, confirmation of the diagnosis, fibrosis staged according to the NASH Clinical Research Network (CRN) and modified Ishak fibrosis classifications, and grading of steatosis, lobular inflammation, and hepatocellular ballooning according to the NAFLD activity score (NAS).
Liver stiffness measurements:
LS was measured by VCTE (FibroScan®, Echosens, Paris, France) at baseline by trained operators, with participants in a fasting state and following standard reliability criteria, as previously described 27. Data on type of VCTE probe (M vs. XL) were available in the STELLAR studies only.
Serum markers:
Fasting blood samples were obtained at baseline for clinical laboratory tests, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), platelets, glucose and ELF score (Siemens Healthcare GmbH, Erlangen Germany).
The Agile 3+ and Agile 4 scores are novel non-invasive scores including LSM by VCTE and routine clinical parameters that were developed to identify advanced fibrosis (Agile 3+) and cirrhosis (Agile 4) among patients with NAFLD, and demonstrated better performance compared with FIB-4 and LS by VCTE33,34. The Agile 3+ and Agile 4 scores were calculated as follows: , where x =−3.92+2.30 l n (LS by VCTE)−0.01(platelets)−0.99(1/(AST/ALT))+1.09(diabetes status)−0.39(gender)+0.03(age); , where y =7.50–15.42(1/√ (LS by VCTE))−0.01(platelets)−1.41(1/(AST/ALT))−0.53(gender)+0.42(diabetes status) 33,34.
Objectives and outcome measures
The primary objective of this study was to determine the optimal thresholds of baseline LS by VCTE to predict progression to cirrhosis (F4) in participants with bridging (F3) fibrosis and liver-related clinical events in participants with cirrhosis. The secondary objectives were to determine if a ≥ 5 kPa (and ≥20%)29 increase in LS predicts progression to cirrhosis (F4) in participants with bridging (F3) fibrosis and liver-related clinical events in participants with cirrhosis (F4). In addition, the performance of the Agile 3+ and Agile 4 scores as predictors for progression to cirrhosis and liver-related events were compared with baseline LS by VCTE.
At baseline, cirrhosis (F4) was defined by histology. Progression to cirrhosis from bridging (F3) fibrosis at baseline was defined by cirrhosis (F4) on a post-baseline biopsy, or the development of liver-related events. Liver-related events were adjudicated by a central committee of experts and defined as clinically apparent ascites requiring treatment, Grade ≥2 hepatic encephalopathy according to the West Haven criteria requiring treatment, and portal hypertension-related gastrointestinal bleeding, liver transplantation, qualification for transplantation (Model for End-Stage Liver Disease [MELD] ≥15), or mortality.
Statistical analysis
Baseline demographic and clinical characteristics are presented separately according to the presence of bridging fibrosis (F3) or cirrhosis (F4) at baseline. Associations between LS by VCTE and the Agile scores at baseline, and a ≥ 5 kPa (and ≥20%) increase of LS with disease progression through the end of follow-up were determined using Kaplan-Meier and Cox proportional hazards regression analysis. Discrimination of these measures for disease progression were described by c-statistics which are analogous to the area under a receiver operating characteristic curve estimated for logistic models 35. The optimal baseline LS and Agile score thresholds were determined based on the maximal sum of sensitivity and specificity according to the logistic model, and the operating characteristics (sensitivity, specificity, positive predictive value [PPV], and negative predictive value [NPV]) for disease progression at these thresholds were calculated. Statistical significance was defined as a two-tailed P-value of ⩽ 0.05. All statistical analyses were performed using SAS, version 9.4 (SAS Institute, Cary, North Carolina).
RESULTS
Characteristics of the study population
A total of 664 participants with bridging (F3) fibrosis and 734 participants with cirrhosis (F4) were included in this study (Table 1). Among participants with bridging (F3) fibrosis, 56% were female, 72% were White, 70% had diabetes, and the median (IQR) age and BMI were 58 years (51-64) and 32.7 kg/m2 (29.0-37.0), respectively. At baseline, the median (IQR) LS by VCTE was 12.7 kPa (9.7-17.3). Out of 664 participants with bridging (F3) fibrosis had available data for LS prior to progression to cirrhosis, 14% had a ≥ 5 kPa (and ≥20%) increase in LS.
Table 1.
Baseline demographics and clinical characteristics of patients with bridging fibrosis and cirrhosis due to non-alcoholic steatohepatitis
| Bridging Fibrosis (F3): n=664 | Cirrhosis (F4): n=734 | |||
|---|---|---|---|---|
| Demographics | Age, y | 58 (51, 64) | 59 (53, 65) | |
| Women | 371/664 (56) | 461/734 (63) | ||
| USA | 336/664 (51) | 432/734 (59) | ||
| White | 479/664 (72) | 572/734 (78) | ||
| Hispanic/Latino | 94/664 (14) | 95/734 (13) | ||
| BMI, kg/m2 | 32.7 (29.0, 37.0) | 33.1 (28.7, 38.0) | ||
| Diabetes | 466/664 (70) | 556/734 (76) | ||
| Liver biochemistry | ALT, U/L | 53 (35, 80) | 42 (31, 61) | |
| GGT, U/L | 58 (38, 99) | 81 (47, 144) | ||
| Bilirubin, mg/dL | 0.6 (0.4, 0.8) | 0.6 (0.5, 0.9) | ||
| MELD | 6 (6, 7) | 7 (6, 8) | ||
| Platelets, x103/μL | 206 (166, 257) | 160 (124, 206) | ||
| Liver histology | NAS ≥4 | 628/664 (95) | 691/730 (95) | |
| Steatosis ≥2 | 60/664 (9) | 33/730 (5) | ||
| Lobular inflammation 3 | 332/664 (50) | 396/730 (54) | ||
| Ballooning 2 | 514/664 (77) | 594/730 (81) | ||
| Ishak fibrosis stage | F3 | 378/664 (57) | — | |
| F4 | 286/664 (43) | — | ||
| F5 | — | 281/733 (38) | ||
| F6 | — | 452/733 (62) | ||
| Collagen content, % | 4.4 (2.7, 6.4) | 10.6 (7.4, 14.7) | ||
| αSMA expression, % | 6.0 (3.0, 9.2) | 13.4 (8.9, 19.7) | ||
| Noninvasive tests | ELF | 9.94 (9.34, 10.61) | 10.60 (9.99, 11.26) | |
| FIB-4 | 1.66 (1.23, 2.50) | 2.50 (1.73, 3.51) | ||
| NAFLD fibrosis score | −0.227 (−1.053, 0.484) | 0.615 (−0.215, 1.495) | ||
| LS by VCTE, kPa XL probe, % |
12.7 (9.7, 17.3) 334/620 (54) |
21.1 (14.2, 29.3) 434/694 (63) |
||
Data are median (interquartile range) or n/n (%).
ALT, alanine aminotransferase; αSMA, α-smooth muscle actin; BMI, body mass index; ELF, Enhanced Liver Fibrosis score (Siemens Healthcare GmbH, Erlangen Germany); FIB-4 index, fibrosis-4 index; GGT, γ-glutamyltransferase; NAFLD Activity Score, NAS.
Among 734 participants with cirrhosis, 63% were female, 78% were White, and 76% had diabetes; the median (IQR) age and BMI were 59 years (53-65) and 33.1 kg/m2 (28.7-38.0), respectively (Table 1). The median (IQR) platelet count was 160 x103/μL (124-206) and MELD score was 7 (6-8). At baseline, the median (IQR) LS by VCTE was 21.1 kPa (14.2, 29.3) and 63% (434/694) of participants were scanned with the XL probe. Out of 734 participants with cirrhosis (F4) had available data for LS prior to hepatic decompensation, 22% had a ≥ 5 kPa (and ≥20%) increase in LS.
Progression to cirrhosis among participants with baseline bridging (F3) fibrosis
During a median follow-up of 16.6 months (IQR 15.0-19.4), 16% (103/664) of participants with bridging fibrosis progressed to cirrhosis. In total, 93.2% (n=96) of participants had cirrhosis diagnosed based on post-baseline histology, while 6.8% (n=7) developed liver-related events consistent with cirrhosis.
Optimal baseline LS threshold for predicting progression to cirrhosis
The risk of progression to cirrhosis was greater with higher LS by VCTE at baseline (hazard ratio [HR] per 3-kPa: 1.16; 95% confidence interval [CI] 1.12, 1.20). The optimal LS threshold at baseline was ≥16.6 kPa, with a c-statistic of 0.72 (95% CI 0.66-0.77) (Figure 1). The sensitivity, specificity, PPV, and NPV of this threshold for progression to cirrhosis were 58%, 76%, 31%, and 91%, respectively (Table 2). Progression to cirrhosis occurred in 31.1% (60/193) of participants with baseline LS ≥16.6 kPa compared with 9.1% (43/471) with LS <16.6 kPa (p<0.001; Figures 1 and 2). Baseline LS by VCTE ≥16.6 kPa was associated with a nearly 4-fold risk (HR 3.86, 95% CI 2.61, 5.72) of progression to cirrhosis in participants with bridging (F3) fibrosis. After adjustment for age, gender, ethnicity, and BMI, baseline LS ≥16.6 kPa remained a strong and independent predictor for progression to cirrhosis (adjusted HR 3.99; 95% CI 2.66, 5.98; p<.0001) (Supplemental Table 1).
Figure 1.
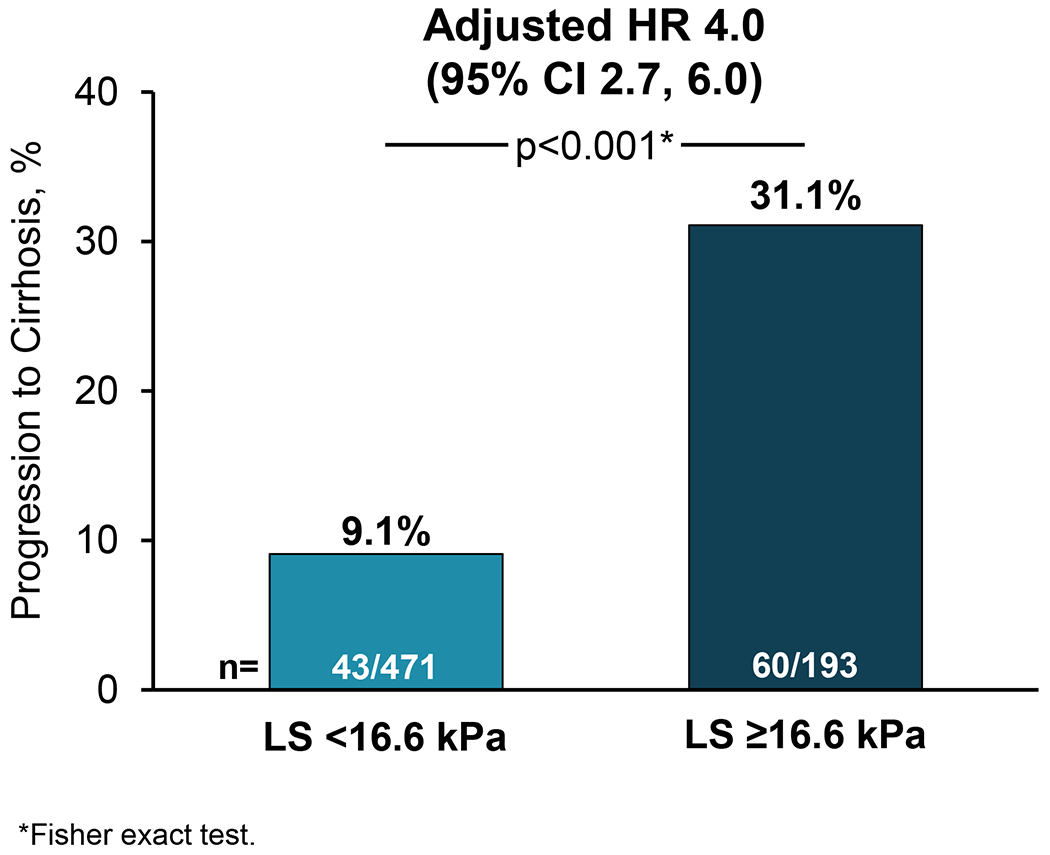
Liver stiffness (LS) by vibration-controlled transient elastography (VCTE) ≥16.6 kPa is associated with increased risk of progression to cirrhosis among patients with baseline bridging (F3) fibrosis secondary to non-alcoholic steatohepatitis
Table 2.
Performance of liver stiffness by vibration-controlled transient elastography for predicting progression to cirrhosis among patients with baseline bridging (F3) fibrosis and liver-related clinical events among patients with cirrhosis (F4)
| Progression to Cirrhosis (F4) in Patients with Bridging Fibrosis (F3) (n=664) | Liver-Related Clinical Events Among Patients with Cirrhosis (F4) (n=734) | |
|---|---|---|
| c-statistic (95% CI) | 0.72 (0.66, 0.77) | 0.77 (0.67, 0.87) |
| Optimal threshold | ≥ 16.6 kPa | ≥ 30.7 kPa |
| Sensitivity (95% CI) | 58% (48, 68) (60/103) | 70% (50, 86) (19/27) |
| Specificity (95% CI) | 76% (73, 80) (428/561) | 79% (75, 81) (555/707) |
| PPV (95% CI) | 31% (25, 38) (60/193) | 11% (7, 17) (19/171) |
| NPV (95% CI) | 91% (88, 93) (428/471) | 99% (97, 99) (555/563) |
NPV, negative predictive value; PPV, positive predictive value.
95% CI for sensitivity, specificity, PPV, and NPV are based on exact limits.
Figure 2.
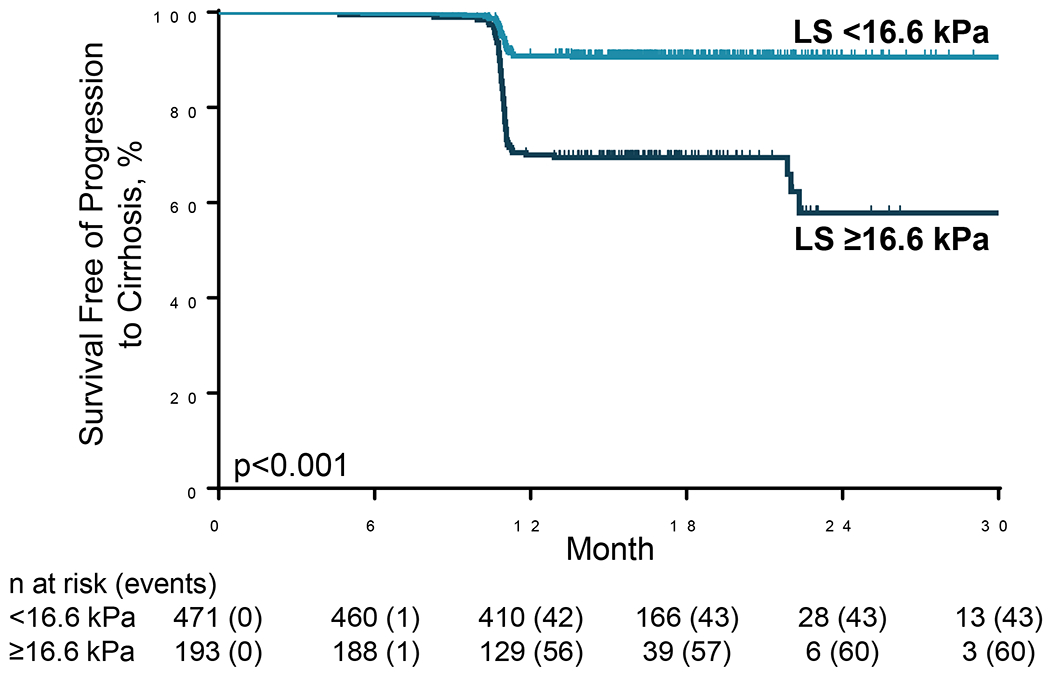
Progression to cirrhosis in patients with bridging (F3) fibrosis secondary to non-alcoholic steatohepatitis, stratified by liver stiffness (LS) by vibration-controlled transient elastography (VCTE) ≥16.6 kPa versus <16.6 kPa
≥5kPA (and ≥20%) increase in LS as a predictor of progression to cirrhosis
Progression to cirrhosis occurred in 22% (20/91) of participants with a ≥5kPA (and ≥20%) increase in LS compared with 14% (83/573) with <5kPa (and ≥20%) increase in LS (p=0.051; Supplemental Figures 1 and 2). A ≥5kPA (and ≥20%) increase in LS was associated with a nearly 1.6-fold risk (HR 1.59, 95% CI 0.97, 2.59) of progression to cirrhosis in participants with bridging (F3) fibrosis. After adjustment for baseline LS, age, gender, ethnicity, and BMI, a ≥ 5kPa (and ≥20%) increase in LS remained a strong and independent predictor for progression to cirrhosis (adjusted HR 1.98; 95% CI 1.20, 3.26; p=0.008) (Supplemental Table 2).
Optimal Agile 3+ threshold for predicting progression to cirrhosis
A subgroup of 629 participants with bridging (F3) fibrosis (95%) had complete data for calculation of the Agile 3+ score (Supplemental Table 3). At baseline, the median (IQR) Agile 3+ score was 0.76 (0.53-0.89). In this subgroup, 15% (95/629) progressed to cirrhosis (89 on histology and 6 with clinical events) during a median follow-up of 16.6 months (IQR 15.0-19.4). The risk of disease progression was greater with higher baseline Agile 3+ score (HR per 0.1-units: 1.34; 95% CI 1.19, 1.52; p<0.001). The median (IQR) baseline Agile 3+ score in participants with versus without progression to cirrhosis was 0.94 (0.82, 0.98) vs 0.88 (0.68, 0.97), respectively (p=0.001). The optimal threshold for baseline Agile 3+ score to predict progression to cirrhosis was ≥ 0.90 (Supplemental Figure 3). After adjustment for age, gender, ethnicity and BMI, baseline Agile 3+ ≥0.90 was an independent predictor for progression to cirrhosis (adjusted HR 4.75, 95% CI 3.07, 7.34; p<.0001) (Supplemental Table 4)
Comparison of LS versus Agile 3+ score for predicting progression to cirrhosis
Among participants with sufficient data (n=629) for calculation of the Agile 3+ score, the performance of LS by VCTE and Agile 3+ for predicting progression to cirrhosis were similar (c-statistic 0.71 [0.65-0.76] vs 0.70 [0.64, 0.76], p=0.88) (Supplemental Table 5).
Liver-related events among participants with baseline cirrhosis (F4)
During a median follow-up of 16.2 months (IQR 13.9-18.7), 4% (27/734) of participants with cirrhosis at baseline had liver-related events: ascites (n=15), hepatic encephalopathy (n=5), portal hypertension-related upper gastrointestinal bleeding (n=3), qualification for liver transplantation (n=2), liver transplantation (n=1), and death (n=1).
Optimal baseline LS threshold for predicting liver-related events among participants with cirrhosis
The risk of liver-related events was greater with higher LS by VCTE at baseline (HR per 5-kPa: 1.29; 95% CI 1.18, 1.41). The optimal LS threshold at baseline was ≥30.7 kPa, which had a c-statistic of 0.77 (95% CI 0.67-0.87) (Figure 3). The sensitivity, specificity, PPV, and NPV of this threshold for liver-related events were 70%, 79%, 11%, and 99%, respectively (Table 2). Liver-related events occurred in 11.1% (19/171) of cirrhotic participants with baseline LS ≥30.7 kPa compared with 1.4% (8/563) of participants with baseline LS <30.7 kPA (p<0.001; Figures 3 and 4). Baseline LS by VCTE ≥30.7 kPa was associated with an 8-fold risk (HR 8.24; 95% CI 3.61, 18.82) of clinical events in participants with cirrhosis (F4). LS by VCTE ≥30.7 kPa remained a strong and independent predictor of liver-related events after adjustment for age, gender, ethnicity and BMI (adjusted HR 10.13; 95% CI 4.38, 23.41; p<.0001) (Supplemental Table 6), with a similar finding in another model incorporating weight changes (Supplemental Table 7). A sensitivity analysis for classic clinical events related to portal hypertension (development of ascites, hepatic encephalopathy and portal hypertension-related bleeding) determined similar findings (Supplemental Table 8).
Figure 3.
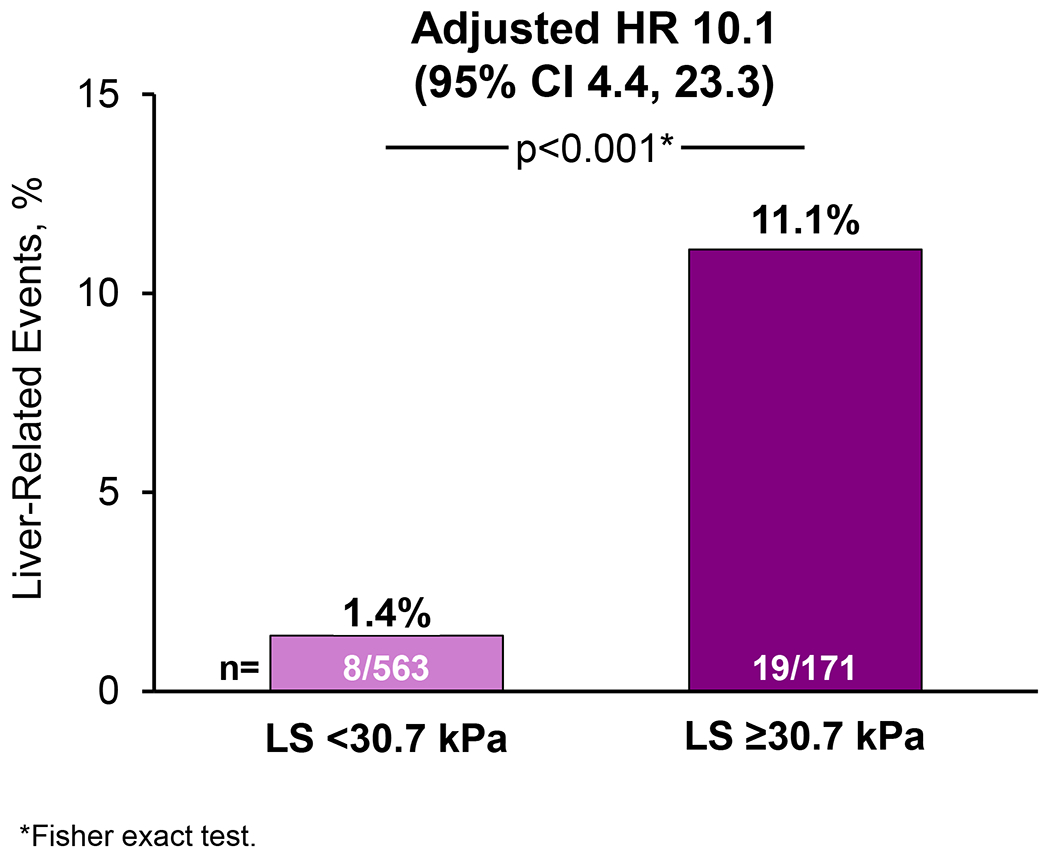
Liver stiffness (LS) by vibration-controlled transient elastography (VCTE) ≥30.7 kPa is associated with increased risk of liver-related events among patients with baseline cirrhosis (F4) secondary to non-alcoholic steatohepatitis
Figure 4.
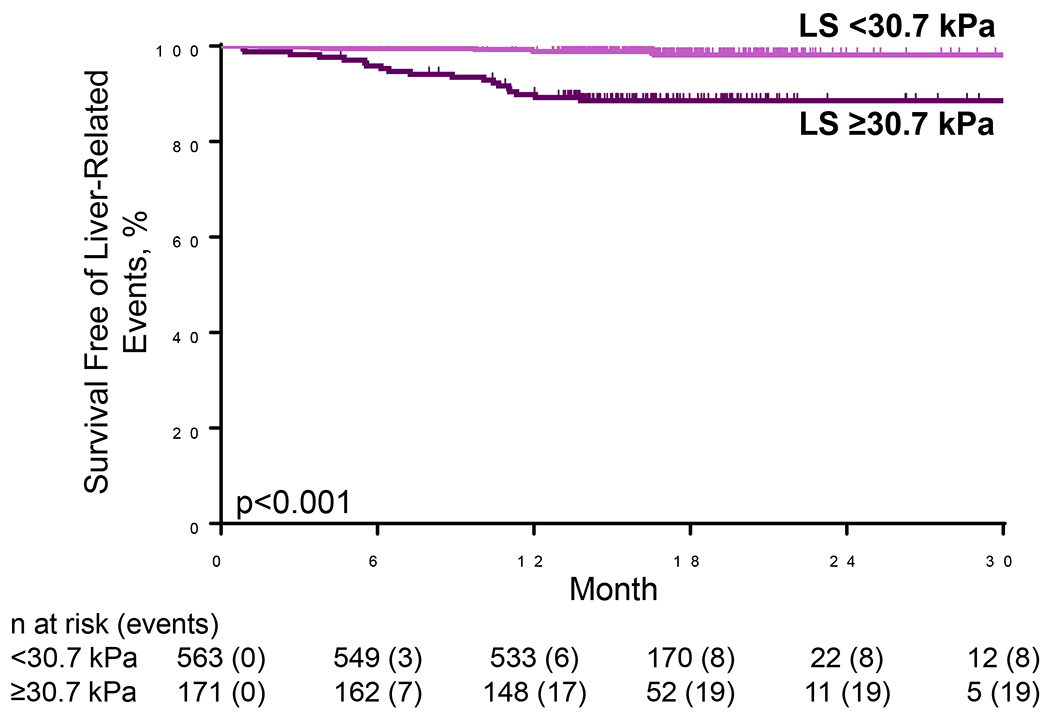
Liver-related events in patients with cirrhosis (F4) secondary to non-alcoholic steatohepatitis, stratified by liver stiffness (LS) by vibration-controlled elastography (VCTE) ≥30.7 kPa versus <30.7 kPa
≥5kPA (and ≥20%) increase in LS as a predictor of liver-related events among participants with cirrhosis
Liver-related events occurred in 3% (4/160) of participants with a ≥ 5kPa (and ≥20%) increase in LS compared with 4% (23/574) with <5kPA (and ≥20%) increase in LS (p=0.36; Supplemental Figures 4 and 5). Among participants with cirrhosis, a ≥5kPa (and ≥20%) increase in LS was not associated with liver-related events in univariable (HR 0.61, 95% CI 0.21, 1.76; p=0.36) and multivariable analysis (adjusted HR 0.75; 95% CI 0.26, 2.18; p=0.60) (Supplemental Table 9).
Optimal Agile 4 threshold for predicting liver-related events among participants with baseline cirrhosis (F4)
A subgroup of 701 participants (96%) had data sufficient for calculation of the Agile 4 score (Supplemental Table 1). The median (IQR) Agile 4 score at baseline was 0.63 (0.34-0.80). In this subgroup, 3% (23/701) had liver-related events during a median follow-up of 16.3 months (IQR 13.9-18.7). The risk of disease progression was greater with higher baseline Agile 4 score (HR per 0.1-units: 1.91; 95% CI 1.40, 2.61; p<0.001). The median baseline Agile 4 scores in participants with versus without liver-related events were 0.53 (IQR 0.23, 0.75) versus 0.37 (IQR 0.13, 0.69), respectively (p=0.002). The optimal Agile 4 threshold for predicting liver-related events was ≥0.72 (Supplemental Figure 6). After adjustment for age, gender, ethnicity and BMI, baseline Agile 4 ≥0.72 was an independent predictor for liver-related events (adjusted HR 11.84; 95% CI 3.51, 39.99; p<.0001) (Supplemental Table 10).
Comparison of LS versus Agile 4 score and other NITs for predicting liver-related events
Among participants with sufficient data for analysis of the baseline Agile 4 score (n=701), the performance of LS by VCTE and Agile 4 for predicting liver-related events were similar (c-statistic 0.81 [0.72-0.90] versus 0.82 [0.74-0.90], p=0.97) (Supplemental Table 5). Baseline LS by VCTE had a similar performance compared to baseline NFS, ELF and FIB-4 for predicting liver-related events among those with baseline cirrhosis (F4) (Supplemental Table 11)
Optimal baseline LS threshold for predicting liver-related events among participants with advanced (F3-F4) fibrosis
Among 664 participants with baseline advanced fibrosis (F3-F4), there were 34 incident liver-related events (7 from participants with baseline F3 and 27 from participants with baseline F4). The optimal LS threshold at baseline for predicting liver-related events remained ≥30.7 kPa, which had a c-statistic of 0.77 (95% CI 0.68-0.86). The sensitivity, specificity, PPV, and NPV of this threshold for liver-related events were 62%, 87%, 10%, and 99%, respectively. Baseline LS by VCTE ≥30.7 kPa was associated with an 11-fold risk (adjusted HR 10.52; 95% CI 5.15, 21.48; p<.0001) of liver-related events in participants with advanced fibrosis (F3-F4) after adjustment for age, gender, ethnicity, treatment arm and BMI (Supplemental Table 12).
DISCUSSION
Main findings
In this analysis of four large, randomized, placebo-controlled trials of selonsertib and simtuzumab in participants with NASH and biopsy-confirmed advanced fibrosis (F3-F4), baseline LS by VCTE was a strong and independent predictor of disease progression (Graphical summary enclosed as Figure 5). Among participants with bridging fibrosis (F3), the optimal LS threshold at baseline LS to predict progression to cirrhosis was ≥16.6 kPa, which had a c-statistic of 0.72, consistent with good prognostic performance. Overall, 31% of participants with bridging fibrosis (F3) and LS ≥16.6 kPa at baseline progressed to cirrhosis, compared with only 9% with LS ≥16.6 kPa. After adjustment for age, gender, ethnicity and BMI, baseline LS ≥16.6 kPa was associated with a four-fold higher risk of progression to cirrhosis during follow-up. A ≥5kPa (and ≥20%) increase in LS by VCTE was associated with an increased risk of progression to cirrhosis among participants with baseline bridging (F3) fibrosis.
Figure 5.
Graphical summary
Similar findings were observed among participants with cirrhosis (F4) at baseline, although a higher threshold for identifying patients at risk of liver-related complications was observed, as expected. Specifically, the optimal threshold of LS at baseline to predict liver-related events was ≥30.7 kPa, which had a c-statistic of 0.77. A total of 11% of participants with cirrhosis and baseline LS ≥30.7 kPa developed liver-related events versus only 1% of those with baseline LS <30.7 kPa. After adjustment for demographic factors and BMI, baseline LS by VCTE ≥30.7 kPa was associated with a ten-fold higher risk of liver-related events. In a sensitivity analysis of the F3 and F4 patient populations, the prognostic performance of VCTE did not differ based on the type of VCTE probe (M versus XL) used to measure LS (data not shown). In addition, we determined that the optimal LS threshold by VCTE (≥30.7 kPa) to predict development of liver-related events among participants with advanced fibrosis (F3-F4) was the same as the threshold for participants with cirrhosis (F4).
The Agile 3+ and Agile 4 scores — which include LS by VCTE plus other clinical and demographic factors — and were designed to identify patients with bridging fibrosis and cirrhosis, respectively, were also significant predictors of disease progression in this cohort. However, their diagnostic performance was similar to LS measurement alone, suggesting that these additional parameters do not improve upon the prognostic utility of LS by VCTE. Further studies are required to define the relationship between serum-based NITs and LS by transient elastography for the prediction of future clinical outcomes.
In context with current literature
Multiple studies have demonstrated a correlation between LS by VCTE with liver-related complications and mortality among patients with liver disease of various etiologies, but prospective data in patients with NASH and advanced fibrosis are limited 36,37. A retrospective analysis of 1,039 participants (53% with biopsy) with NAFLD and advanced fibrosis (F3 or F4) or with LS by VCTE >10 kPa, demonstrated that baseline LS was associated with liver-related events and mortality 29. However, this study did not provide data for progression from bridging fibrosis to cirrhosis. Another study of 2,251 patients with NAFLD demonstrated that baseline LS by VCTE was an independent predictor of survival, and liver-related and cardiovascular events, however, this study did not evaluate the utility of baseline LS for patients with F3 or F4 fibrosis due to the relatively small numbers with advanced fibrosis (13% had baseline LS >12 kPa) 28. The current prospective study of patients with biopsy-confirmed, advanced fibrosis (F3 or F4) provides clinical validation that baseline LS by VCTE can be used as a prognostic tool for progression to cirrhosis and development of liver-related events. In addition, the LS thresholds identified in this study may be useful for risk stratification of patients with NASH in clinical trials and in clinical practice to identify patients at increased risk of disease progression 38. For example, high-risk patients could be offered increased clinical surveillance or targeted for enrollment in clinical trials of novel therapies. Finally, LS by VCTE, along with platelet count, may be useful to predict the development of clinically significant portal hypertension in patients with NASH and compensated advanced chronic liver disease, as suggested in a recent Baveno consensus report 30,39. In this regard, the LS thresholds recommended by this group (e.g., 15 and 30 kPa) are close to those of the optimal thresholds identified in our dataset (16.7 and 30.7 kPa) and have similar prognostic utility (Supplemental Table 13).
Strengths and limitations
The novelty of this study includes its prospective design, well-phenotyped participants with serial, centrally-read liver biopsies, adjudication of liver-related events by a committee of experts, and the establishment of thresholds for baseline LS by VCTE that predict disease progression in NAFLD with F3-F4 fibrosis. However, this study is not without limitations. Firstly, all included participants were selected for clinical trials and it is unclear whether these data are generalizable to the broader population of NASH patients with advanced fibrosis. Therefore, these data require validation in clinical practice. Secondly, the histologic definition of progression to cirrhosis in patients classified as having bridging fibrosis (F3) at baseline is susceptible to misclassification due to sampling variability of liver biopsy 17. Third, the median follow-up duration was relatively short (~16-17 months) given the slow rate of disease progression in NASH, potentially contributing to a relatively low rate of clinical liver-related events; therefore, prospective studies with longer follow-up duration are required 5. All liver biopsies were evaluated by a single pathologist, which may introduce an element of interpretation bias. Finally, although VCTEs were performed by trained operators, there was no quality control of the VCTE measurements, and further data are required to examine the variability of VCTE in NASH and advanced fibrosis. Nevertheless, our data demonstrate that LS by VCTE provides highly discriminant prognostic information even when performed under standards of usual clinical practice and lend further justification for the use of non-invasive surrogates in prognosticating clinically meaningful outcomes beyond ordinal histologic staging of fibrosis alone.
Conclusion
In this analysis of four large, randomized placebo-controlled trials of participants with NASH and biopsy-proven advanced fibrosis (F3-F4), clinical disease progression was associated with higher LS by VCTE at baseline. The optimal LS thresholds for predicting progression to cirrhosis among patients with bridging fibrosis (F3) and development of liver-related events among patients with cirrhosis were ≥16.6 kPa and ≥30.7 kPa, respectively. A ≥5kPA (and ≥20%) increase in LS by VCTE was associated with an increased risk of progression to cirrhosis among participants with baseline bridging (F3) fibrosis. The LS thresholds identified in this study may be useful for risk stratification of patients with NASH in clinical trials and in clinical practice and lend further support to the use of non-invasive surrogates rather than liver histology to predict the risk of clinically meaningful outcomes.
Supplementary Material
What is already known on this topic
Retrospective studies report that increasing liver stiffness as assessed by vibration-controlled transient elastography is associated with the increased risk of liver disease progression in patients with non-alcoholic fatty liver disease (NAFLD). However, there are limited prospective data in well-characterized patients with biopsy-confirmed non-alcoholic steatohepatitis (NASH) with bridging fibrosis and cirrhosis regarding the optimal cut-points associated with higher risk of progression to cirrhosis, and hepatic decompensation among patients with cirrhosis.
What this study adds
In this analysis of four large, prospective, international, multicenter, randomized placebo-controlled trials of participants with NASH and biopsy-proven advanced fibrosis (F3-F4), clinical disease progression was associated with higher liver stiffness by vibration-controlled transient elastography at baseline. We identified optimal liver stiffness thresholds for predicting progression to cirrhosis among patients with bridging fibrosis, and development of liver-related events among patients with compensated cirrhosis. In addition, we determined that a ≥5kPa (and ≥20%) increase in liver stiffness was associated with progression to cirrhosis, among participants with bridging fibrosis at baseline.
How this study might affect research, practice or policy
The liver stiffness thresholds identified in this study will be useful for risk stratification of patients with NASH in clinical trials and in clinical practice. High-risk patients identified using these thresholds could be offered increased clinical surveillance or targeted for enrolment in clinical trials for treatment of NASH related fibrosis and cirrhosis. These data further support the use of non-invasive biomarkers as a surrogate to predict the risk of clinically meaningful outcomes.
Acknowledgments:
Editorial assistance was provided by Sandra Chen of Gilead Sciences.
Conflict of interest disclosure:
R.L. receives funding support from NIAAA (U01AA029019), NIEHS (5P42ES010337), NCATS (5UL1TR001442), NIDDK (U01DK130190, U01DK061734, R01DK106419, P30DK120515, R01DK121378, R01DK124318), NHLBI (P01HL147835), and DOD PRCRP (W81XWH-18-2-0026). RL serves as a consultant to Aardvark Therapeutics, Altimmune, Anylam/Regeneron, Amgen, Arrowhead Pharmaceuticals, AstraZeneca, Bristol-Myer Squibb, CohBar, Eli Lilly, Galmed, Gilead, Glympse bio, Hightide, Inipharma, Intercept, Inventiva, Ionis, Janssen Inc., Madrigal, Metacrine, Inc., NGM Biopharmaceuticals, Novartis, Novo Nordisk, Merck, Pfizer, Sagimet, Theratechnologies, 89 bio, Terns Pharmaceuticals and Viking Therapeutics. In addition his institutions received research grants from Arrowhead Pharmaceuticals, Astrazeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Eli Lilly, Galectin Therapeutics, Galmed Pharmaceuticals, Gilead, Intercept, Hanmi, Intercept, Inventiva, Ionis, Janssen, Madrigal Pharmaceuticals, Merck, NGM Biopharmaceuticals, Novo Nordisk, Merck, Pfizer, Sonic Incytes and Terns Pharmaceuticals. Co-founder of LipoNexus Inc.
D.H. receives funding support from Singapore Ministry of Health’s National Medical Research Council under its NMRC Research Training Fellowship (MOH-000595-01). In addition, he has served as an advisory board member for Eisai.
M.F.A. receives funding support from NIDDK (U01DK130177, U01DK061713, R01DK12137), NCI (UG1CA242643) and DOD (W81XWH2010514). MFA serves as a consultant/advisor to Bristol-Myer Squibb, CohBar, Inventiva, Madrigal, NGM Biopharmaceuticals, Novo Nordisk, Theratechnologies, 89 Bio, Somologics, and Hanmi Pharmaceuticals. In addition, her institutions received research grants from Allergan, Boehringer-Ingelheim, Bristol-Myers Squibb, Enyo, Enanta, Durect, Galectin Therapeutics, Gilead, Galmed, Intercept, Hanmi, Inventiva, Madrigal Pharmaceuticals, NGM Biopharmaceuticals, Novartis, Novo Nordisk, Poxel, TARGET PharmaSolutions, Celgene, Genentech. and Viking Pharmaceuticals.
Q.M.A. is funded by the LITMUS (Liver Investigation: Testing Marker Utility in Steatohepatitis) consortium funded by the Innovative Medicines Initiative (IMI2) Program of the European Union under Grant Agreement 777377; this Joint Undertaking receives support from the European Union’s Horizon 2020 research and innovation programme and EFPIA. He reports Research Grant Funding from Allergan/Tobira, AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Glympse Bio, Intercept, Novartis Pharma AG, Pfizer Ltd; Consultancy from 89Bio, Abbvie/Allergan, Altimentiv, Altimmune, AstraZeneca, Axcella, Blade, BMS, BNN Cardio, Boehringer Ingelheim, Cirius, CymaBay, EcoR1, E3Bio, Eli Lilly & Company Ltd., Galmed, Genentech, Genfit SA, Gilead, Grunthal, Histolndex, Indalo, Intercept Pharma Europe Ltd., Inventiva, IQVIA, Janssen, Johnson & Johnson, Madrigal, MedImmune, Medpace, Merck, Metacrine, NGMBio, North Sea Therapeutics, Novartis, Novo Nordisk A/S, PathAI, Pfizer Ltd., Poxel, ProSciento, Raptor Pharma, Roche, Servier, Shionogi, Terns, The Medicines Company, Viking Therapeutics; Speaker fees from Abbott Laboratories, Allergan/Tobira, BMS, Clinical Care Options, Falk, Fishawack, Genfit SA, Gilead, Integritas Communications, Kenes, Medscape; and Royalties from Elsevier Ltd.
D.D., L.M., C.J., A.B., R.S.H., C.C., and R.P.M are employees and shareholders of Gilead Sciences, Inc.
V.W. served as a consultant or advisory board member for AbbVie, Boehringer Ingelheim, Echosens, Gilead Sciences, Inventiva, Merck, Novo Nordisk, Pfizer, ProSciento, Sagimet Biosciences, and TARGET PharmaSolutions; and a speaker for Abbott, AbbVie, Echosens, Gilead Sciences, Novo Nordisk. He has received a research grant from Gilead Sciences, and was a co-founder of Illuminatio Medical Technology Limited.
J.B. was a consultant for Gilead Sciences, Actelion, and Surrozen.
Funding statement:
This study was funded by Gilead Sciences, Inc. (NCT03053050; NCT03053063; NCT01672866; NCT01672879)
Abbreviations:
- NAFLD
non-alcoholic fatty liver disease
- LS
liver stiffness
- VCTE
vibration controlled transient elastography
- BMI
body mass index
Footnotes
Ethics approval statement: All study centers had ethics approval from their respective institutions.
Patient consent statement: Consent was obtained from each patient.
Permission to reproduce material from other sources: No material was used from external sources.
REFERENCES
- 1.Younossi ZM, Koenig AB, Abdelatif D, et al. Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84. [DOI] [PubMed] [Google Scholar]
- 2.Le MH, Yeo YH, Li X, et al. 2019 global NAFLD prevalence: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2021;2021:2. doi: 10.1016/j.cgh.2021.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10:686–90. [DOI] [PubMed] [Google Scholar]
- 4.Loomba R, Friedman SL, Shulman GI. Mechanisms and disease consequences of nonalcoholic fatty liver disease. Cell 2021;184:2537–64. [DOI] [PubMed] [Google Scholar]
- 5.Singh S, Allen AM, Wang Z, et al. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired-biopsy studies. Clin Gastroenterol Hepatol 2015;13:643–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dufour J-F, Anstee QM, Bugianesi E, et al. Current therapies and new developments in NASH. Gut 2022:326874. doi: 10.1136/gutjnl-2021-326874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anstee QM, Castera L, Loomba R. Impact of non-invasive biomarkers on hepatology practice: past, present and future. J Hepatol 2022;76:1362–78. [DOI] [PubMed] [Google Scholar]
- 8.Estes C, Razavi H, Loomba R, et al. Modeling the epidemic of nonalcoholic fatty liver disease demonstrates an exponential increase in burden of disease. Hepatology 2018;67:123–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016-2030. J Hepatol 2018;69:896–904. [DOI] [PubMed] [Google Scholar]
- 10.Tan DJH, Ng CH, Lin SY, et al. Clinical characteristics, surveillance, treatment allocation, and outcomes of non-alcoholic fatty liver disease-related hepatocellular carcinoma: a systematic review and meta-analysis. Lancet Oncol 2022;23:521–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol 2021;18:223–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang DQ, Singal AG, Kono Y. Changing global epidemiology of liver cancer from 2010 to 2019: NASH is the fastest growing cause of liver cancer. Cell Metab;34:969–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tan DJH, Setiawan VW, Ng CH, DJH T, CH N, et al. Global burden of liver cancer in males and females: changing etiological basis and the growing contribution of NASH. Hepatology 2022. doi: 10.1002/hep.32758. [Epub ahead of print: 29 Aug 2022]. [DOI] [PubMed] [Google Scholar]
- 14.Dulai PS, Singh S, Patel J, et al. Increased risk of mortality by fibrosis stage in nonalcoholic fatty liver disease: systematic review and meta-analysis. Hepatology 2017;65:1557–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanyal AJ, Van Natta ML, Clark J, et al. Prospective study of outcomes in adults with nonalcoholic fatty liver disease. N Engl J Med 2021;385:1559–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and metaanalysis. Gastroenterology 2020;158:1611–25. [DOI] [PubMed] [Google Scholar]
- 17.Ng CH, Lim WH, Hui Lim GE, et al. Mortality outcomes by fibrosis stage innonalcoholic fatty liver disease: a systematic review and meta-analysis. Clin Gastroenterol Hepatol 2022:14. doi: 10.1016/j.cgh.2022.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ratziu V, Charlotte F, Heurtier A, et al. Sampling variability of liver biopsy in nonalcoholic fatty liver disease. Gastroenterology 2005;128:1898–906. [DOI] [PubMed] [Google Scholar]
- 19.Rockey DC, Caldwell SH, Goodman ZD, et al. Liver biopsy. Hepatology 2009;49:1017–44. [DOI] [PubMed] [Google Scholar]
- 20.Boursier J, Vergniol J, Guillet A, et al. Diagnostic accuracy and prognostic significance of blood fibrosis tests and liver stiffness measurement by FibroScan in non-alcoholic fatty liver disease. J Hepatol 2016;65:570–8. [DOI] [PubMed] [Google Scholar]
- 21.Eddowes PJ, Sasso M, Allison M, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology 2019;156:1717–30. [DOI] [PubMed] [Google Scholar]
- 22.Wong VW-S, Vergniol J, Wong GL-H, et al. Diagnosis of fibrosis and cirrhosis using liver stiffness measurement in nonalcoholic fatty liver disease. Hepatology 2010;51:454–62. [DOI] [PubMed] [Google Scholar]
- 23.Jung J, Loomba RR, Imajo K, et al. MRE combined with FIB-4 (MEFIB) index in detection of candidates for pharmacological treatment of NASH-related fibrosis. Gut 2021;70:1946–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Angulo P, Bugianesi E, Bjornsson ES, et al. Simple noninvasive systems predict longterm outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology 2013;145:782–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R, Adams LA. Advances in non-invasive assessment of hepatic fibrosis. Gut 2020;69:1343–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tamaki N, Ajmera V, Loomba R. Non-invasive methods for imaging hepatic steatosis and their clinical importance in NAFLD. Nat Rev Endocrinol 2022;18:55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamaki N, Imajo K, Sharpton S. MRE plus FIB-4 (MEFIB) versus FAST in detection of candidates for pharmacological treatment of NASH-related fibrosis. Hepatology 2021. [Google Scholar]
- 28.Anstee QM, Lawitz EJ, Alkhouri N, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the stellar trials. Hepatology 2019;70:1521–30. [DOI] [PubMed] [Google Scholar]
- 29.Shili-Masmoudi S, Wong GL-H, Hiriart J-B, et al. Liver stiffness measurement predicts long-term survival and complications in non-alcoholic fatty liver disease. Liver Int 2020;40:581–9. [DOI] [PubMed] [Google Scholar]
- 30.Petta S, Sebastiani G, Viganò M, et al. Monitoring occurrence of liver-related events and survival by transient elastography in patients with nonalcoholic fatty liver disease and compensated advanced chronic liver disease. Clin Gastroenterol Hepatol 2021;19:806–15. [DOI] [PubMed] [Google Scholar]
- 31.de Franchis R, Bosch J, Garcia-Tsao G. BAVENO VII - renewing consensus in portal hypertension: report of the Baveno VII consensus workshop: personalized care in portal hypertension. J Hepatol;76:959–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harrison SA, Wong VW-S, Okanoue T, et al. Selonsertib for patients with bridging fibrosis or compensated cirrhosis due to NASH: Results from randomized phase III STELLAR trials. J Hepatol 2020;73:26–39. [DOI] [PubMed] [Google Scholar]
- 33.Harrison SA, Abdelmalek MF, Caldwell S, et al. Simtuzumab is ineffective for patients with bridging fibrosis or compensated cirrhosis caused by nonalcoholic steatohepatitis. Gastroenterology 2018;155:1140–53. [DOI] [PubMed] [Google Scholar]
- 34.Younossi ZS, Newsome PN, Chan W-K. Development and validation of Agile 3+: novel FibroScan based score for the diagnosis of advanced fibrosis in patients with nonalcoholic fatty liver disease. J Hepatol 2021;75:S205–93. [Google Scholar]
- 35.Newsome PN, Sasso M, Deeks JJ, et al. Independent validation of Agile 4: novel FibroScan based score for the diagnosis of cirrhosis in patients with non-alcoholic fatty liver disease. J Hepatol 2021;75:S205–93. [Google Scholar]
- 36.Heagerty PJ, Zheng Y. Survival model predictive accuracy and ROC curves. Biometrics 2005;61:92–105. [DOI] [PubMed] [Google Scholar]
- 37.Pang JXQ, Zimmer S, Niu S, et al. Liver stiffness by transient elastography predicts liver-related complications and mortality in patients with chronic liver disease. PLoS One 2014;9:e95776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vergniol J, Foucher J, Terrebonne E, et al. Noninvasive tests for fibrosis and liver stiffness predict 5-year outcomes of patients with chronic hepatitis C. Gastroenterology 2011;140:9.e1–3.: 1970–9. [DOI] [PubMed] [Google Scholar]
- 39.Manns MP, Burra P, Sargent J, et al. The Lancet–EASL Commission on liver diseases in Europe: overcoming unmet needs, stigma, and inequities. The Lancet 2018;392:621–2. [DOI] [PubMed] [Google Scholar]
- 40.Hsieh Y-C, Lee K-C, Wang Y-W. Correlation between noninvasive markers of fibrosis and hepatic venous pressure gradient in patients with compensated cirrhosis due to nonalcoholic steatohepatitis. Hepatology 2015;62: 10.1371/journal.pone.0208903:33A–92. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


