Abstract
The molecule NAD+ is a coenzyme for enzymes catalyzing cellular redox reactions in several metabolic pathways, encompassing glycolysis, TCA cycle, and oxidative phosphorylation, and is a substrate for NAD+-dependent enzymes. In addition to a hydride and electron transfer in redox reactions, NAD+ is a substrate for sirtuins and poly(adenosine diphosphate–ribose) polymerases and even moderate decreases in its cellular concentrations modify signaling of NAD+-consuming enzymes. Age-related reduction in cellular NAD+ concentrations results in metabolic and aging-associated disorders, while the consequences of increased NAD+ production or decreased degradation seem beneficial. This article reviews the NAD+ molecule in the development of aging and the prevention of chronic age-related diseases and discusses the strategies of NAD+ modulation for healthy aging and longevity.
Keywords: NAD+ precursors, NAD+ levels, sirtuin, PARP
1. Introduction
Nicotinamide adenine dinucleotide (NAD+) is composed of an adenosine 5′-phosphate coupled to ribosylnicotinamide 5′-phosphate by the pyrophosphate linkage. NAD+ is necessary for more than 500 enzymatic reactions [1] and is of importance in a large number of evolutionarily conserved signaling pathways regulating DNA repair and genomic signaling, apoptosis, senescence, proliferation and endocrine signaling, mainly through NAD+ regulation of sirtuin deacetylases’ (SIRT) activity that affects metabolism, DNA repair, stress resistance, cell survival, inflammation, mitochondrial function, lipid and glucose homeostasis by targeting transcription factors (FOXO3a, PGC-1α, p53, NF-κB, HIF-1α), and many other cellular targets [2]. Additionally, NAD+ is significant in regulating gene expression required for oxidative stress response, catabolic metabolism and mitochondrial biogenesis. Furthermore, NAD+ influences epigenetics by modulating the acetylation status of histones and other proteins [3]. For example, NAD+ degradation in the aging process increases the consumption of S-adenosylmethionine (SAM) [4], an essential cellular methyl donor. Excessive supplementation with nicotinamide (NAM), nicotinamide riboside (NR) and nicotinamide mononucleotide (NMN) can potentially exacerbate the problem of methylation by increasing NAM levels [5,6,7], as NAM can be methylated to N1-methylnicotinamide (MNAM), which lowers methyl donor levels and increases the risk of vascular disease, neurodegenerative disease and chronic kidney disease by producing more homocysteine [8,9]. Unexpectedly, in the long-term study of nicotinamide riboside chloride (Niagen) [10], no increase in homocysteine was observed in subjects who consumed up to 1000 mg of Niagen.
NAD+’s role in electron transfer throughout oxidation–reduction (redox) reactions is essential for ATP production [11]. Furthermore, as a cosubstrate of enzymes, it is cleaved as a result of increased oxidative stress, senescence, poly-(adenosine diphosphate-ribose) polymerases (PARP) activation by DNA damage and NADases activation during inflammation. Despite specific NAD+ subcellular requirements and the difference in NAD+ levels in various cell parts [12], the NAD+ synthesis in general decreases and NAD+ degradation increases during aging, leading to an overall reduction in the concentration of intracellular NAD+ [13,14,15,16,17,18,19,20,21].
2. NAD+ Usage and Degradation
NAD+ acts as a coenzyme in the production of energy (glycolysis, mitochondrial respiration) and its reduced form (NADH) is used as a means of energy transfer. During redox reactions, NAD+ is converted from reduced (NADH) to oxidized form (NAD+) but is not degraded [22]. Therefore, these reactions do not change the overall amount of NAD+ in cells. NAD+ is degraded as a rate-limiting substrate for many signaling enzymes, including the sirtuins, SIRT1 and SIRT3, the poly(ADP-ribose) polymerase (PARP) proteins, PARP1 and PARP2, a COOH-terminal binding protein (CtBP), the cyclic ADP-ribose (ADPR) synthetases CD38 and CD157, and various other NAD+-dependent enzymes. PARPs, sirtuins, CD157 and SARM1 degrade NAD+ to NAM and ADP-ribose (ADPR) and due to the difference in affinity for NAD+ (Michaelis–Menten constant, Km) for NAD+, they have different potential for degradation of NAD+ (for details, see reference [23]). Briefly, the Km of SIRT1 ranges around 94 to 888 μM, SIRT2, SIRT4 and SIRT6 may have a Km for NAD+ below the physiological range, implying that NAD+ is not necessarily the rate-limiting factor of their activity [24,25,26,27,28]. PARP-1 has Km for NAD+ in the range of 20–97 μM [25,28,29,30,31]. Similarly, CD38 and SARM1 have Km for NAD+ around 15–25 μM [26,32]. Because the Km of PARP1 and CD38 for NAD+ are lower than those of the SIRT1, their activation may limit SIRT1 activation by decreasing the NAD+ content, and inhibition of PARP1 and CD38 can increase the overall availability of NAD+, leading to SIRT1 activation [33]. For example, CD38-deficient mice had 30-fold higher NAD+ levels in their tissues than the wild-type mice [34]. Intracellular NAD+ concentrations are roughly between 0.2 and 0.5 mM; however, these concentrations also differ between the tissue/cell types and cell compartments [28]. The activity of NAD+-consuming enzymes is conditioned by DNA damage (PARPs) and inflammation (CD38, CD157) and is a major contributor to the need to constantly re-synthesize NAD+ [35,36,37]. The NAD+-degrading enzymes CD38, PARPs and sirtuins cause NAD+ depletion in mammalian tissues [21]. NAD+ role in redox reactions affects cellular energy levels and mitochondrial function. NAD+ is an important factor in aging and the development of age-related diseases in the role of a cofactor in redox reactions and a coenzyme in metabolic processes. It also has a decisive role in non-redox reactions, such as cellular signal transduction, when acting as a substrate for sirtuins and PARPs [38]. Sirtuins, PARPs and CD38 all compete for NAD+ in the antagonistic relationship and play different roles regarding aging and life span. Over-activation of PARPs and CD38 negatively influences life span [21,39], while increased sirtuin activation beneficially regulates the organismal life span in several animal models [40,41,42]. Although the exact causes of NAD+ decline with advanced age are not fully understood [21,38], it has been established that NAD+ decline is caused by increased PARP activity due to increased DNA damage. Consequently, the latter lowers cellular NAD+ concentrations up to 80% [43], while a defect in NAMPT-mediated NAD+ biosynthesis due to low-grade chronic inflammation may contribute to this decrease even further [44]. Inflammaging (age-associated inflammation) caused by the cessation of cell division augments NAD+ consumption by activating CD38+ pro-inflammatory macrophages [45]. NAD+ is also phosphorylated by NAD+ kinase to generate NADP+, which in turn is reduced by dehydrogenases to generate NADPH [12].
3. Consequences of Decreased NAD+ Levels
Decreased NAD+ levels manifest at organismic, tissue, cellular and mitochondrial levels. Reduced NAD+ alters mitochondrial activity by elevated NADH/NAD+ ratio related to the increased ROS production [46], decreased oxidative metabolism and mitochondrial biogenesis. Such time-dependent progressive increase in mitochondrial dysfunction and collapse of oxidative phosphorylation (OXPHOS) stimulates a shift in metabolism from using mitochondrially produced ATP to dependence on glycolysis as a consequence of the accumulation of hypoxia-inducible factor 1 alpha (HIF-1α) and decreased activity of respiratory complexes I, III and IV [47], which stimulates the Warburg effect involved in the metabolic syndrome, obesity, type 2 diabetes, the onset of cancer and other degenerative diseases [47,48,49,50,51]. High concentrations of accumulated intracellular NADH may furthermore impede OXPHOS by promoting pyruvate to lactate conversion and permeability reduction of the voltage-gated anion channel in the outer mitochondrial membrane [52]. Additionally, obesity triggers the vicious cycle by decreasing the enzyme nicotinamide phosphoribosyl transferase (NAMPT), which catalyzes the rate-determining step for NAD+ synthesis and NAD+ levels in human tissues. A decrease in NAMPT-mediated NAD+ biosynthesis in adipocytes seriously compromises insulin sensitivity in multiple organs [53]. A reduction in systemic NAD+ biosynthesis also decreases NAD+-dependent deacetylases (sirtuin) activity, which regulates glucose-stimulated insulin secretion [54]. The elevated glucose levels, impaired insulin secretion and insulin resistance interconnected with the development of advanced glycation end products (AGEs) are characteristics of type 2 diabetes [55] and are a contributing determinant of the pathogenesis of cardiovascular diseases (CDs) [56,57]. The mice with a shortage of SIRT3 on a high-fat diet develop symptoms similar to metabolic syndrome in humans, leading to accelerated obesity, insulin resistance, hyperlipidemia, steatohepatitis and chronic inflammation [58]. On the other hand, when the NAD+ level was elevated with NAD+ booster niacin for a period of 4 to 9 months, blood and muscle NAD+ level, OXPHOS function, mitochondrial mass and biogenesis, muscle mass and strength increased, and disease symptoms improved in patients with mitochondrial myopathy, while the percentage of whole-body fat and hepatic/visceral fat decreased by 25 and 50%, respectively, although subcutaneous adipose tissue remained unchanged. In addition, niacin therapy increased HDL, reduced apolipoprotein B particles and did not affect total cholesterol in the treated patient group [59]. Furthermore, increased levels of NAD+ by genetic manipulation (e.g., CD38−/− or PARP1−/−) protected animals from weight gain [60].
NAD+ half-life ranges from 1 to 2 hours in the cytoplasm and nucleus and about 8 hours in the mitochondria [61,62]. According to Zhu and coworkers [63], 3 g of nicotinamide released from NAD+ consumption is necessary for resynthesizing to NAD+ a few times daily in a 75 kg person to achieve NAD+ homeostasis. Intracellular NAD+ concentrations have the range 100–120 µM in the nucleus and 50–100 µM in the cytoplasm [62,64,65]. The total NAD+ levels in mammalian cells appear to be between 200 and 500 μM; higher levels of NAD+ are needed in metabolically active cells like neurons and cardiac myocytes [46]. Intracellular NAD+ concentrations vary between cell compartments, cell types, cellular states, and growth conditions [65].
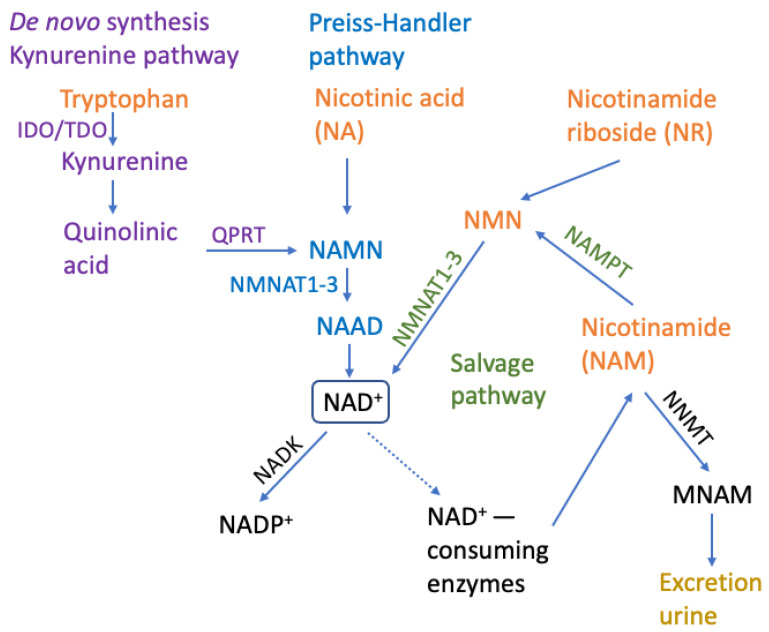
NAD+ may be maintained, restored and re-used via three independent biosynthetic pathways: (1) De Novo Synthesis (from L-tryptophan through the kinurenine pathway), (2) Preiss–Handler Pathway (from nicotinic acid or nicotinic acid ribose to generate NAMN, which is then transformed into NAAD and NAD+) and (3) Salvage Pathway (from niacinamide/nicotinamide, which is generated as a by-product of the enzymatic activities of NAD+-consuming enzymes) (Figure 1) [28,42,66,67]. Nicotinamide salvage pathway, where NAD+ is synthesized from dietary precursors NR, NAM and nicotinic acid (NA), is a dominant pathway enabling stable cellular concentrations of NAD+ in the majority of tissues. The NAMPT functions as the rate-limiting enzyme of the aforementioned pathway [22] generating NMN and pyrophosphate (PP) from NAM and α-D-5-phosphoribosyl-1-pyrophosphate (PRPP). Adenylyltransferases (NMNAT1-3) then form NAD+ from NMN and ATP [68].
Figure 1.
NAD+ metabolism. The two biosynthetic pathways, Kynurenine/De novo synthesis and Preiss–Handler pathway, with crucial steps and enzymes are indicated in violet and blue, respectively, and the salvage pathway is in green. The common NAD+ precursors available from diet are in orange. NAD+ consumption and degradation pathways and enzymes are in black. NAD+-consuming enzymes are PARP1-17, SIRT1-7 and glycohydrases/NADases: CD38, CD157 and SARM1. IDO: indoleamine 2,3-dioxygenase; TDO: tryptophan 2,3-dioxygenase; QPRT: quinolinate phosphoribosyltransferase; NAMN: nicotinamide mononucleotide; NMNAT1-3: nicotinamide mononucleotide adenylyltransferases; NAAD: nicotinic acid adenine dinucleotide; NAD+: nicotinamide adenine dinucleotide; NADK: NAD+ kinase; NADP+: nicotinamide adenine dinucleotide phosphate; NNMT: nicotinamide N-methyltransferase; MNAM: N1-methylnicotinamide; NAMPT: nicotinamide phosphoribosyltransferase; NMN: nicotinamide mononucleotide.
4. Methods to Increase NAD+ Levels
NAD+ levels are reduced during aging due to increased oxidative stress and chronic inflammation, which dysregulate NAD+ metabolism by activating CD38 and PARPs or inhibiting NAMPT [17,69]. The prevention of NAD+ degradation and the increased availability of NAD+ can influence, delay and even somewhat reverse the aging process and age-related diseases [13,14,15,16].
Many studies have documented the genetic or pharmacologic restoration of NAD+ in mice to enhance longevity and healthspan [22,27,38,44,47,70,71,72,73,74,75,76,77,78,79]. NAD+ levels can also be regulated by lifestyle and selected nutritional interventions [80]. Accordingly, NAD+ levels may be elevated in mammals by ingesting NAD+ boosters/intermediates, e.g., nicotinamide mononucleotide, nicotinic acid, nicotinamide, tryptophan and nicotinamide riboside [27,75,81,82,83,84,85,86]. They can also be increased by decreasing NAD+ utilization by PARP enzymes or CD38/CD157 with the use of PARP, CD38 and SAM1 inhibitors [43,83,87,88,89,90,91].
By application of NAD+-replacement therapy with NAD+ precursors NR and NMN supplementation, higher concentrations of NAD+ were observed in mice and humans [27,44,92]. However, it has been reported that CD38 is the central enzyme causing the degradation of the NAD+ precursor nicotinamide mononucleotide (NMN), [21] which can prevent the generation and increase in NAD+ levels from supplementation with NAD+ boosters. Aging is characterized by NAD+ degradation via CD38 as its expression and activity increase with age. Knockout of CD38 inhibits age-related NAD+ degradation, activates sirtuins and mitochondrial function, and prevents age-related metabolic disorders [21]. This may explain why mere supplementation with precursors is insufficient to raise NAD+ levels. There are no observed effects of NR supplementation on the mitochondrial respiration, content or morphology reported in skeletal muscles of pathologically overweight and insulin-resistant males [93]. Similarly, it was reported that daily supplementation with 1000 mg NR for 6 wk did not raise NAD+ levels of skeletal muscles in healthy overweight or obese persons of both genders [94]. NR effectively stimulates NAD+ metabolism and elevates NAD+ [95] in normal-weight disease-free middle-aged and older adults, indicating the potential link between obesity and CD38. One such link could represent circulating lipopolysaccharides (LPS) released by gram-negative bacteria, which are associated with infection and inflammation and are elevated in persons with a BMI of 30 compared to a BMI of 24 [96]. Exposure of macrophage cells to LPS results in CD38 increased gene expression [97]. M1-like macrophages that tend to cause inflammation in visceral white adipose tissue build-ups during aging have significant levels of the NAD+-consuming enzyme CD38. In addition, senescent cells accumulate in visceral adipose tissue during aging and inflammatory cytokines secreted by senescent cells incite macrophages to proliferate and express CD38 [45]. Obesity, LPS and CD38 all increase during aging [17,98,99]. Although NR is resistant to CD38 enzymatic activity in vitro, CD38 can decrease NAD+ levels in vivo due to NR supplementation, as NR is converted to NMN [100,101]. Namely, NMN is either derived from NAM by NAMPT or NR by NR kinase [12].
It can be concluded that the prevention of aging and metabolic disorders can be strongly impacted with the NMN and NR therapies by decreasing LPS, inflammation and CD38 levels with CD38 inhibitors such as flavonoids: epigenin, luteolin, quercetin, kuromanin and thiazoloquin(az)olinones, like the compound 78c [102,103,104,105]; PARP pharmacological inhibitors have also been reported to increase NAD+ levels [60,106]. It is also possible to increase NAD+ levels by activating NAD+-generating enzymes, the most important of which is NAMPT, which converts NAM to NMN, thereby increasing the production of nicotinamide mononucleotide (NMN), which is the major NAD+ precursor in mammalian cells in the salvage pathway (Figure 1) [107]. NAMPT biosynthesis can be modulated by the small synthetic molecule activators P73C and SBI-797812 [107] or a natural compound notoginseng leaf triterpenes and a natural peptide IRW (Ile-Arg-Trp), which also activate Nampt gene expression and increase intracellular NAMPT protein abundance [108,109,110]. Important and well-researched lifestyle approaches to extending lifespan include aerobic exercise, fasting, glucose deprivation and caloric restriction, which increase NAD+ [2,89,90] by activating the enzyme NAMPT, the rate-limiting NAD+ biosynthetic enzyme in mammals [111,112,113,114]. Another approach to affect NAD+ levels can be achieved by inhibiting the enzyme nicotinamide N-methyltransferase (NNMT), which converts nicotinamide to methylnicotinamide, to increase nicotinamide levels, which can then be converted to NAD+ [28,115,116,117,118,119,120,121,122]. Both NR and NMN can increase nicotinamide levels, thereby enhancing NNMT activity due to higher substrate availability [123]. NNMT activity can affect NAD+ biosynthesis, as well as drive epigenetic modifications and influence gene expression by modulating the intracellular methylation index (SAM/SAH ratio) [124] by catalyzing the N-methylation of nicotinamide, using S-adenosyl-L-methionine (SAM) as a methyl donor, resulting in N1-methylnicotinamide (MNAM) and releasing S-adenosyl-L-homocysteine (SAH) [124].
Pharmacological inhibition of CD38, PARPs and NNMT and NAMPT activation can modify NAD+ levels, yet not enough is known about the safety in human (non-oncological) treatments (e.g., in the prevention of aging and amelioration of chronic diseases). Additionally, caloric restriction upregulates SIRT1 in adipocytes and promotes lipolysis and free fatty acid mobilization by suppressing the nuclear hormone receptor PPARγ, which promotes adipogenesis [125]. Aerobic sporting activity adds to the total accumulation of NAD+ induced by the skeletal muscle NAMPT expression [126]. These activities also stimulate the NAD+ salvage pathway through the 5′-AMP-activated protein kinase (AMPK) [111] and thus modify the age-dependent decrease in NAD+ [127]. The application of the exercise mimetics, such as 5-aminoimidazole-4-carboxamide-1-β-D-riboside, resulted in AMPK-induced increase in NAD+, increased sirtuin activity, oxidative mitochondrial activity and improved endurance [85]. Therefore, the optimal approach to increasing NAD+ tissue levels integrates the NAD+ precursors, NAD+ degradation prevention and exercise/caloric restriction.
5. Beneficial Effects of NAD+ Boosting
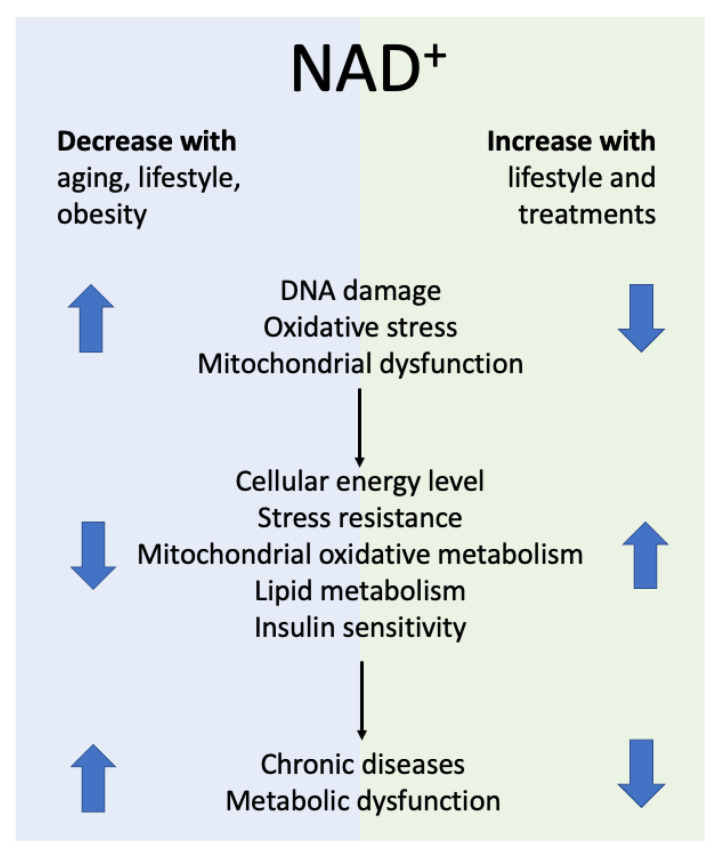
Increasing NAD+ levels restores mitochondrial function and ameliorates mitochondrial disorders [47,75,128,129] and the age-associated disorders observed in various mouse models of human disease (Figure 2). Scientific literature reports that rising NAD+ levels influence several different conditions and illnesses, such as metabolic syndrome, type 2 diabetes and/or insulin sensitivity [27,53,71,86], cancer [2,51,130,131,132], cardiovascular disease [75,133,134,135,136,137], neurodegeneration [138,139,140], renal function [141,142] and Alzheimer’s disease [77,143]. It also reduces inflammation [47,144,145], helps prevent obesity [27,53,146,147,148] and influences life extension [71,144,149,150].
Figure 2.
Association of NAD+ bioavailability and healthspan.
Clinical trials with small participant numbers indicate that NAD+ boosters could elevate NAD+ levels in volunteers and are relatively safe for humans [59,81,95,151,152,153]. For example, orally administered NR increased NAD+ 2.7-fold in human blood in 12 subjects with only one dose of 1000 mg applied per os [81]. Similarly, Airhart and coworkers [152] documented that the NAD+ amount was doubled by oral ingestion of NR (250–1000 mg/d) after nine days in 140 healthy volunteers. In the recent study by Fahy et al. [154] the human epigenetic clock, predicting their biological age, was for the first time turned back by 2.5 years with a combination of growth hormone, metformin and dehydroepiandrosterone, through the thymus and immune system regeneration and a decrease in the CD38 enzyme activity, which conserves NAD+.
6. The Effects of NAD+ on Cancer and Inflammation
The increase in cancer risk due to impaired genome stability is related to NAD+ depletion in aging because NAD+ levels regulate cellular energy production, cellular DNA repair, signal transduction and genome stability. This topic has been presented in detail in [2]. In short, sufficient NAD+ levels could prevent or reverse the phenotype of malignant cells at early stages by inducing cellular repair and adaptive response to stress and regulating cell cycle arrest and apoptotic clearance of damaged cells. Thus, adequate amounts of NAD+ have a protective role in genomic stability, mutation formation and cancer prevention. In contrast, during cancer promotion, progression and treatment, elevated NAD+ levels could have deleterious effects on the malignant process due to growth benefits, increased resistance and prolonged cell survival as NAD+ deficiency regulates oncogene-induced DNA damage and tumor development [155].
Sirtuins are involved in carcinogenesis and cancer prevention as they regulate genes involved in the process of DNA repair and maintenance [156]. For example, the activity of mammalian SIRT1 depends on the NAD+/NADH ratio. By increasing cellular NAD+ levels, AMPK increases SIRT1 activity, leading to deacetylation and modulation of the activity of downstream SIRT1 targets. In contrast, decreased levels of NAD+ reduce the efficacy of sirtuins (SIRT1) that deacetylate tumor suppressor proteins such as p53 [157], which are involved in cell cycle arrest, apoptosis and autophagy [156,158]. It has been observed that low NAM levels acting as NAD+ precursors are beneficial for SIRT1 activity, whereas the opposite, NAM accumulation, could be detrimental by inhibiting SIRT1 [159,160,161].
The tumor suppression ability has also been reported for SIRT2 and SIRT3. SIRT3 acts as a tumor suppressor by suppressing ROS through the activation of antioxidant defense by the manganese superoxide dismutase (MnSOD), an essential mitochondrial antioxidant enzyme, and regulating HIF-1 [162,163,164,165]. SIRT2 acts as a tumor suppressor by preventing chromosomal instability during mitosis [166], by regulating the microtubule network [167], by increasing FOXO DNA binding and enhancing the expression of FOXO target genes [168], and preventing oxidative stress-induced death [169] by increasing the expression of antioxidant enzymes such as MnSOD, glutathione peroxidase and catalase [170]. The involvement of NAD+ in apoptosis remains controversial [171], as it could be both pro- and antitumorogenic. Although sirtuins play an important role in tumorigenesis, their role in various aspects of the carcinogenesis process remains debatable. For example, SIRT1 has been observed to both promote and suppress tumor growth [172]. It appears that sirtuins may have a cell-protective function during stress, preventing cells from developing damage. In contrast, they could prevent apoptotic death, stimulate proliferation and facilitate acquired resistance, thus promoting cancer stem cell survival [173]. Sirtuins could give cancer cells a growth advantage [174] by preventing cell loss through apoptosis and senescence-like growth arrest. Elevated NAD+ levels could also increase resistance to radio- and chemotherapy, promote inflammation [2,175] and stimulate angiogenesis [176].
Elevated NAD+ levels may also play an opposing role at different stages of sepsis. For example, activation of SIRT1 has been reported to have a beneficial effect in the initial (proinflammatory) phase [177], whereas SIRT1 expression should be reduced in the later stages of sepsis [178]. NAD+-mediated suppression of AMPK kinase leads to suppression of p53-mediated inhibition of p38 mitogen-activated protein kinase (MAPK) and enhanced NF-κB activity. It affects inflammatory signaling of senescent cells in vivo through higher mobility group A (HMGA) proteins and NAMPT expression, which promotes the proinflammatory senescence-associated secretory phenotype (SASP) [179], thereby exacerbating SASP-associated inflammation [180].
Caution should be exercised in the use of NAD+ and its precursors if aging is a defense mechanism against cancer. Despite these concerns, no evidence has been found that treatment with NR or NMN for a prolonged period of time stimulates tumor development in animals [71,75].
7. Side Effects of NAD+ Precursors Observed in Human Trials
Although several small human clinical trials have been conducted and the results imply that increased NAD+ levels by NAD+ boosters are safe in humans (reviewed in [181]), long-term safety studies are lacking to determine the proper dose of NAD+ boosters and treatment duration for aging prevention and as chronic disease therapy. During administration of NAM, NR and NMN, few minor and relatively infrequent side effects were reported in clinical studies, including diarrhea, nausea, rashes, skin flushing, calf cramps, thrombocytopenia, erythema pruritis, skin burning, fatigue, abdominal discomfort and headache [182,183]. NA in high doses can cause hot flashes and elevated blood glucose as well as elevated homocysteine levels.
Nicotinamide (NAM) overdose can cause hepatotoxicity in rare cases [184]. NA and NR decreased physical performance in young rats [185] and the capacity for high-intensity exercise in humans [186]. In contrast, cardiopulmonary performance improved after 6 weeks of NMN supplementation in 48 recreationally trained runners [187], and chronic oral NMN supplementation for 12 weeks significantly improved muscle strength and performance in 10 athletes [188].
8. The Future Research Directions and Strategies
Currently, uncertainties remain regarding the pharmacokinetics and pharmacodynamics, tissue specificity of NAD+ boosters and dosing, safety and side effects of chronically elevated NAD+ levels by supplementation with NAD+ precursors or by inhibitors of NAD+-consuming enzymes. Since all NAD+ precursors (NMN, NAM, NR, nicotinic acid and tryptophan) increase the availability of NAD+, the question arises as to which precursor is better and more suitable for human consumption. All of them are naturally present in different foods, thus it may not be a priority to invest money in large human trials. The precursors differ in their potency with respect to increasing NAD+ synthesis and in the number of steps required to form NAD+ in the biochemical pathways. Observed differences also result from the expression of NAD+ biosynthetic enzymes and the preference for specific NAD+ precursors [189]. Subcellular distribution and needs, tissue specificity and efficacy of NAD+ boosters in increasing NAD+ levels in humans should be studied in more detail. To determine which age-related degenerative diseases (e.g., cardiovascular, metabolic, inflammatory, neurodegenerative, muscle-damaging, mitochondrial and cancer) can be ameliorated, prevented or merely delayed with which precursor, further studies using different precursors in the same trial are needed. In addition, to investigate NAD+’s role in promoting health and longevity, studies should also be conducted in healthy populations.
9. Conclusions
Although it is difficult to prove causality experimentally, the studies presented support the hypothesis that the decline of NAD+ significantly contributes to aging, chronic diseases and metabolic dysfunction. Metabolic syndrome (e.g., increased blood sugar, decreased insulin sensitivity/insulin resistance), decreased ATP synthesis, reduced mitochondrial function, increased visceral adipose tissue, diabetes, cancer, atherosclerosis, etc., may result also from NAD+ decrease with age. NAD+ is related to aging and the occurrence of age-associated chronic diseases because NAD+-driven processes enable cells and organisms to maintain their high organization, while preserving a lower entropy state [190], which leads to a better health span and life expectancy [113]. NAD+ steady-state levels depend on the ratio between NAD+ synthesis and utilization [12] and can be replenished by three strategies, including (i) reduction of NAD+ degradation by NAD+ consumers (e.g., CD 38 and CD 157) through their inhibitors, such as naturally occurring flavonoids apigenin and luteolinidin [103,113,191], (ii) increasing NAD+ supply through NAD+ precursors (e.g., nicotinamide, niacin, NMN and NR) and (iii) activation of NAD+ generating enzymes, such as NAMPT, as well as converting NAM to NMN in the salvage pathway by NAD+ biosynthesis modulators [107,192].
The extensive research on animals confirmed the interdependence of NAD+ and the organism’s maintenance of full function. Nevertheless, several additional studies on the molecule NAD+ are desirable to elucidate the comprehensive role of NAD+ decay in the causation of aging and human age-associated chronic diseases. Current knowledge of the NAD+ beneficial impact on aging and healthspan is grounded primarily on the research on cell cultures and model organisms and tentatively on the beneficial anti-aging effects of NAD+ in human beings—including increased vitality, reduction in all-cause mortality and an extended healthspan. The latter requires additional corroboration by further extensive research and clinical trials.
There are no long-term safety studies on NAD+ boosters aimed at determining the optimal effective dose, treatment period, bioavailability, metabolism and tissue specificity. The long-term consequences of increased NAD+ levels and the best approaches and combinations to increase the NAD+ levels also need to be elucidated. Further research will answer these crucial questions.
Acknowledgments
We would like to thank Karen Thiebes for assistance in figure preparation.
Author Contributions
Conceptualization, B.P. and I.M.; methodology, B.P., S.Š. and I.M.; resources, V.K. and I.M.; writing. B.P., V.K., S.Š. and I.M.; visualization, V.K.; supervision, B.P. and I.M. All authors have read and agreed to the published version of the manuscript.
Informed Consent Statement
The study did not require ethical approval.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Slovenian Research Agency, grant numbers P3-0388 and P3-0019.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hong W., Mo F., Zhang Z., Huang M., Wei X. Nicotinamide Mononucleotide: A Promising Molecule for Therapy of Diverse Diseases by Targeting NAD+ Metabolism. Front. Cell Dev. Biol. 2020;8:246. doi: 10.3389/fcell.2020.00246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Poljsak B. NAD+ in Cancer Prevention and Treatment: Pros and Cons. J. Clin. Exp. Oncol. 2016;5:4. doi: 10.4172/2324-9110.1000165. [DOI] [Google Scholar]
- 3.Imai S.I., Guarente L. Ten years of NAD-dependent SIR2 family deacetylases: Implications for metabolic diseases. Trends Pharmacol. Sci. 2010;31:212–220. doi: 10.1016/j.tips.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kraus D., Yang Q., Kong D., Banks A.S., Zhang L., Rodgers J.T., Pirinen E., Pulinilkunnil T.C., Gong F., Wang Y.C., et al. Nicotinamide N-methyltransferase knockdown protects against diet-induced obesity. Nature. 2014;508:258–262. doi: 10.1038/nature13198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schmeisser K., Mansfeld J., Kuhlow D., Weimer S., Priebe S., Heiland I., Birringer M., Groth M., Segref A., Kanfi Y., et al. Role of sirtuins in lifespan regulation is linked to methylation of nicotinamide. Nat. Chem. Biol. 2013;9:693–700. doi: 10.1038/nchembio.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lavery G.G., Oakey L.A., Fletcher R.S., Elhassan Y.S., Cartwright D.M., Doig C.L., Garten A., Thakker A., Maddocks O.D.K., Zhang T., et al. Metabolic tracing reveals novel adaptations to skeletal muscle cell energy production pathways in response to NAD + depletion. Wellcome Open Res. 2019;3:147. doi: 10.12688/WELLCOMEOPENRES.14898.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gallo C.M., Daniel L., Smith J., Smith J.S. Nicotinamide Clearance by Pnc1 Directly Regulates Sir2-Mediated Silencing and Longevity. Mol. Cell. Biol. 2004;24:1301. doi: 10.1128/MCB.24.3.1301-1312.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun W.P., Li D., Lun Y.Z., Gong X.J., Sun S.X., Guo M., Jing L.X., Zhang L.B., Xiao F.C., Zhou S.S. Excess nicotinamide inhibits methylation-mediated degradation of catecholamines in normotensives and hypertensives. Hypertens. Res. 2012;35:180–185. doi: 10.1038/hr.2011.151. [DOI] [PubMed] [Google Scholar]
- 9.Ostrakhovitch E.A., Tabibzadeh S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2019;49:144–164. doi: 10.1016/J.ARR.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 10.Conze D., Brenner C., Kruger C.L. Safety and Metabolism of Long-term Administration of NIAGEN (Nicotinamide Riboside Chloride) in a Randomized, Double-Blind, Placebo-controlled Clinical Trial of Healthy Overweight Adults. Sci. Rep. 2019;9:9772. doi: 10.1038/s41598-019-46120-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi Y., Sauve A.A. Nicotinamide riboside, a trace nutrient in foods, is a Vitamin B3 with effects on energy metabolism and neuroprotection. Curr. Opin. Clin. Nutr. Metab. Care. 2013;16:657–661. doi: 10.1097/MCO.0b013e32836510c0. [DOI] [PubMed] [Google Scholar]
- 12.Cambronne X., Kraus W. Location, Location, Location: Compartmentalization of NAD + Synthesis and Functions in Mammalian Cells. Trends Biochem. Sci. 2020;45:858–873. doi: 10.1016/j.tibs.2020.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai S.I. The NAD world: A new systemic regulatory network for metabolism and aaging-Sirt1, systemic NAD biosynthesis, and their importance. Cell Biochem. Biophys. 2009;53:65–74. doi: 10.1007/s12013-008-9041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houtkooper R.H., Cantó C., Wanders R.J., Auwerx J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr. Rev. 2010;31:194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khan J.A., Forouhar F., Tao X., Tong L. Nicotinamide adenine dinucleotide metabolism as an attractive target for drug discovery. Expert Opin. Ther. Targets. 2007;11:695–705. doi: 10.1517/14728222.11.5.695. [DOI] [PubMed] [Google Scholar]
- 16.Ying W. Therapeutic potential of NAD+ for neurological diseases. Future Neurol. 2007;2:129–132. doi: 10.2217/14796708.2.2.129. [DOI] [Google Scholar]
- 17.Chini C.C.S., Tarragó M.G., Chini E.N. NAD and the aging process: Role in life, death and everything in between. Mol. Cell. Endocrinol. 2017;455:62–74. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harman D. Role of free radicals in aging and disease. Ann. N. Y. Acad. Sci. 1992;673:126–141. doi: 10.1111/j.1749-6632.1992.tb27444.x. [DOI] [PubMed] [Google Scholar]
- 19.Beckman K.B., Ames B.N. The free radical theory of aging matures. Physiol. Rev. 1998;78:547–581. doi: 10.1152/physrev.1998.78.2.547. [DOI] [PubMed] [Google Scholar]
- 20.Schultz M.B., Sinclair D.A. Why NAD + Declines during Aging: It’s Destroyed. Cell Metab. 2016;23:965–966. doi: 10.1016/j.cmet.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Camacho-Pereira J., Tarragó M.G., Chini C.C.S., Nin V., Escande C., Warner G.M., Puranik A.S., Schoon R.A., Reid J.M., Galina A., et al. CD38 Dictates Age-Related NAD Decline and Mitochondrial Dysfunction through an SIRT3-Dependent Mechanism. Cell Metab. 2016;23:1127–1139. doi: 10.1016/j.cmet.2016.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Verdin E. NAD+ in aging, metabolism, and neurodegeneration. Science. 2015;350:1208–1213. doi: 10.1126/science.aac4854. [DOI] [PubMed] [Google Scholar]
- 23.Xie N., Zhang L., Gao W., Huang C., Huber P.E., Zhou X., Li C., Shen G., Zou B. NAD+ metabolism: Pathophysiologic mechanisms and therapeutic potential. Signal Transduct. Target. Ther. 2020;5:227. doi: 10.1038/s41392-020-00311-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pacholec M., Bleasdale J.E., Chrunyk B., Cunningham D., Flynn D., Garofalo R.S., Griffith D., Griffor M., Loulakis P., Pabst B., et al. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J. Biol. Chem. 2010;285:8340–8351. doi: 10.1074/jbc.M109.088682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Madsen A.S., Andersen C., Daoud M., Anderson K.A., Laursen J.S., Chakladar S., Huynh F.K., Colaço A.R., Backos D.S., Fristrup P., et al. Investigating the Sensitivity of NAD+-dependent Sirtuin Deacylation Activities to NADH. J. Biol. Chem. 2016;291:7128–7141. doi: 10.1074/jbc.M115.668699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith B.C., Hallows W.C., Denu J.M. A continuous microplate assay for sirtuins and nicotinamide-producing enzymes. Anal. Biochem. 2009;394:101–109. doi: 10.1016/j.ab.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cantó C., Houtkooper R.H., Pirinen E., Youn D.Y., Oosterveer M.H., Cen Y., Fernandez-Marcos P.J., Yamamoto H., Andreux P.A., Cettour-Rose P., et al. The NAD+ precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell Metab. 2012;15:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cantó C., Menzies K.J.J., Auwerx J. NAD+ Metabolism and the Control of Energy Homeostasis: A Balancing Act between Mitochondria and the Nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bai P., Cantó C. The role of PARP-1 and PARP-2 enzymes in metabolic regulation and disease. Cell Metab. 2012;16:290–295. doi: 10.1016/j.cmet.2012.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Amé J.C., Rolli V., Schreiber V., Niedergang C., Apiou F., Decker P., Muller S., Höger T., Ménissier-de Murcia J., De Murcia G. PARP-2, A novel mammalian DNA damage-dependent poly(ADP-ribose) polymerase. J. Biol. Chem. 1999;274:17860–17868. doi: 10.1074/jbc.274.25.17860. [DOI] [PubMed] [Google Scholar]
- 31.Alvarez-Gonzalez R., Mendoza-Alvarez H. Dissection of ADP-ribose polymer synthesis into individual steps of initiation, elongation, and branching. Biochimie. 1995;77:403–407. doi: 10.1016/0300-9084(96)88153-3. [DOI] [PubMed] [Google Scholar]
- 32.Cakir-Kiefer C., Muller-Steffner H., Oppenheimer N., Schuber F. Kinetic competence of the cADP-ribose-CD38 complex as an intermediate in the CD38/NAD+ glycohydrolase-catalysed reactions: Implication for CD38 signalling. Biochem. J. 2001;358:399–406. doi: 10.1042/bj3580399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pehar M., Harlan B.A., Killoy K.M., Vargas M.R. Nicotinamide Adenine Dinucleotide Metabolism and Neurodegeneration. Antioxid. Redox Signal. 2018;28:1652–1668. doi: 10.1089/ars.2017.7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aksoy P., White T.A., Thompson M., Chini E.N. Regulation of intracellular levels of NAD: A novel role for CD38. Biochem. Biophys. Res. Commun. 2006;345:1386–1392. doi: 10.1016/j.bbrc.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 35.Bogan K.L., Brenner C. Nicotinic Acid, Nicotinamide, and Nicotinamide Riboside: A Molecular Evaluation of NAD + Precursor Vitamins in Human Nutrition. Annu. Rev. Nutr. 2008;28:115–130. doi: 10.1146/annurev.nutr.28.061807.155443. [DOI] [PubMed] [Google Scholar]
- 36.Altmeyer M., Hottiger M.O. Poly(ADP-ribose) polymerase 1 at the crossroad of metabolic stress and inflammation in aging. Aging. 2009;1:458–469. doi: 10.18632/aging.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulikova V., Shabalin K., Nerinovski K., Dölle C., Niere M., Yakimov A., Redpath P., Khodorkovskiy M., Migaud M.E., Ziegler M., et al. Generation, release, and uptake of the NAD precursor nicotinic acid riboside by human cells. J. Biol. Chem. 2015;290:27124–27137. doi: 10.1074/jbc.M115.664458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poljsak B., Milisav I. NAD+ as the Link between Oxidative Stress, Inflammation, Caloric Restriction, Exercise, DNA Repair, Longevity, and Health Span. Rejuvenation Res. 2016;19:406–413. doi: 10.1089/rej.2015.1767. [DOI] [PubMed] [Google Scholar]
- 39.Johnson S., Imai S. NAD+ biosynthesis, aging, and disease. F1000Research. 2018;7:132. doi: 10.12688/f1000research.12120.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee S., Lee J., Lee H., Min K. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019;52:24–34. doi: 10.5483/BMBRep.2019.52.1.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grabowska W., Sikora E., Bielak-Zmijewska A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017;18:447. doi: 10.1007/S10522-017-9685-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Imai S.I., Guarente L. It takes two to tango: Nad+ and sirtuins in aging/longevity control. NPJ Aging Mech. Dis. 2016;2:16017. doi: 10.1038/npjamd.2016.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Braidy N., Guillemin G.J., Mansour H., Chan-Ling T., Poljak A., Grant R. Age Related Changes in NAD+ Metabolism Oxidative Stress and Sirt1 Activity in Wistar Rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Imai S., Guarente L. NAD+ and sirtuins in aging and disease. Trends Cell Biol. 2014;24:464–471. doi: 10.1016/j.tcb.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Covarrubias A.J., Kale A., Perrone R., Lopez-Dominguez J.A., Pisco A.O., Kasler H.G., Schmidt M.S., Heckenbach I., Kwok R., Wiley C.D., et al. Senescent cells promote tissue NAD+ decline during ageing via the activation of CD38+ macrophages. Nat. Metab. 2020;2:1265–1283. doi: 10.1038/s42255-020-00305-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang Y., Sauve A.A. NAD+ metabolism: Bioenergetics, signaling and manipulation for therapy. Biochim. Biophys. Acta Proteins Proteomics. 2016;1864:1787–1800. doi: 10.1016/j.bbapap.2016.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gomes A.P., Price N.L., Ling A.J.Y., Moslehi J.J., Montgomery M.K., Rajman L., White J.P., Teodoro J.S., Wrann C.D., Hubbard B.P., et al. Declining NAD+ Induces a Pseudohypoxic State Disrupting Nuclear-Mitochondrial Communication during Aging. Cell. 2013;155:1624–1638. doi: 10.1016/j.cell.2013.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.De Grey A.D.N.J. A mechanism proposed to explain the rise in oxidative stress during aging. J. Anti. Aging. Med. 1998;1:53–66. doi: 10.1089/rej.1.1998.1.53. [DOI] [Google Scholar]
- 49.Brewer G.J. Epigenetic oxidative redox shift (EORS) theory of aging unifies the free radical and insulin signaling theories. Exp. Gerontol. 2010;45:173–179. doi: 10.1016/j.exger.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shigenaga M.K., Hagen T.M., Ames B.N. Oxidative damage and mitochondrial decay in aging. Proc. Natl. Acad. Sci. USA. 1994;91:10771–10778. doi: 10.1073/pnas.91.23.10771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Poljsak B., Kovac V., Dahmane R., Levec T., Starc A. Volume 2019. Hindawi Limited; London, UK: 2019. Cancer Etiology: A Metabolic Disease Originating from Life’s Major Evolutionary Transition? [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ying W. NAD+ and NADH in cellular functions and cell death. Front. Biosci. 2006;11:3129–3148. doi: 10.2741/2038. [DOI] [PubMed] [Google Scholar]
- 53.Yamaguchi S., Yoshino J. Adipose tissue NAD+ biology in obesity and insulin resistance: From mechanism to therapy. BioEssays. 2017;39 doi: 10.1002/bies.201600227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramsey K.M., Mills K.F., Satoh A., Imai S.I. Age-associated loss of Sirt1-mediated enhancement of glucose-stimulated insulin secretion in beta cell-specific Sirt1-overexpressing (BESTO) mice. Aging Cell. 2008;7:78–88. doi: 10.1111/j.1474-9726.2007.00355.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Unoki H., Yamagishi S. Advanced Glycation End Products and Insulin Resistance. Curr. Pharm. Des. 2008;14:987–989. doi: 10.2174/138161208784139747. [DOI] [PubMed] [Google Scholar]
- 56.Rasool M., Malik A., Butt T.T., Ashraf M.A.B., Rasool R., Zahid A., Waquar S., Asif M., Zaheer A., Jabbar A., et al. Implications of advanced oxidation protein products (AOPPs), advanced glycation end products (AGEs) and other biomarkers in the development of cardiovascular diseases. Saudi J. Biol. Sci. 2019;26:334–339. doi: 10.1016/j.sjbs.2018.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang P., Feng J., Peng Q., Liu X., Fan Z., Luca M. Advanced Glycation End Products: Potential Mechanism and Therapeutic Target in Cardiovascular Complications under Diabetes. Oxid. Med. Cell. Longev. 2019;2019:9570616. doi: 10.1155/2019/9570616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hirschey M.D., Shimazu T., Jing H., Grueter C., Collins A., Aouizerat B., Stančáková A., Goetzman E., Lam N., Schwer B., et al. SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell. 2011;44:177–190. doi: 10.1016/j.molcel.2011.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pirinen E., Auranen M., Khan N.A., Brilhante V., Urho N., Pessia A., Hakkarainen A., Kuula J., Heinonen U., Schmidt M.S., et al. Niacin Cures Systemic NAD+ Deficiency and Improves Muscle Performance in Adult-Onset Mitochondrial Myopathy. Cell Metab. 2020;31:1078–1090.e5. doi: 10.1016/j.cmet.2020.04.008. [DOI] [PubMed] [Google Scholar]
- 60.Bai P., Cantó C., Oudart H., Brunyánszki A., Cen Y., Thomas C., Yamamoto H., Huber A., Kiss B., Houtkooper R.H., et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011;13:461–468. doi: 10.1016/j.cmet.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rechsteiner M., Hillyard D., Olivera B.M. Turnover of nicotinamide adenine dinucleotide in cultures of human cells. J. Cell. Physiol. 1976;88:207–217. doi: 10.1002/jcp.1040880210. [DOI] [PubMed] [Google Scholar]
- 62.Cambronne X.A., Stewart M.L., Kim D., Jones-Brunette A.M., Morgan R.K., Farrens D.L., Cohen M.S., Goodman R.H. Biosensor reveals multiple sources for mitochondrial NAD+ Science. 2016;352:1474–1477. doi: 10.1126/science.aad5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zhu X.H., Lu M., Lee B.Y., Ugurbil K., Chen W. In vivo NAD assay reveals the intracellular NAD contents and redox state in healthy human brain and their age dependences. Proc. Natl. Acad. Sci. USA. 2015;112:2876–2881. doi: 10.1073/pnas.1417921112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ryu K., Nandu T., Kim J., Challa S., DeBerardinis R., Kraus W. Metabolic regulation of transcription through compartmentalized NAD + biosynthesis. Science. 2018;360:eaan5780. doi: 10.1126/science.aan5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sallin O., Reymond L., Gondrand C., Raith F., Koch B., Johnsson K. Semisynthetic biosensors for mapping cellular concentrations of nicotinamide adenine dinucleotides. eLife. 2018;7:e32638. doi: 10.7554/eLife.32638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Belenky P., Bogan K.L., Brenner C. NAD+ Metabolism in Health and Disease. Volume 32. Elsevier Ltd.; Amsterdam, The Netherlands: 2007. pp. 12–19. [DOI] [PubMed] [Google Scholar]
- 67.Stein L.R., Imai S.I. The dynamic regulation of NAD metabolism in mitochondria. Trends Endocrinol. Metab. 2012;23:420–428. doi: 10.1016/j.tem.2012.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Magni G., Amici A., Emanuelli M., Orsomando G., Raffaelli N., Ruggieri S. Enzymology of NAD+ homeostasis in man. Cell. Mol. Life Sci. 2004;61:19–34. doi: 10.1007/s00018-003-3161-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aksoy P., Escande C., White T.A., Thompson M., Soares S., Benech J.C., Chini E.N. Regulation of SIRT 1 mediated NAD dependent deacetylation: A novel role for the multifunctional enzyme CD38. Biochem. Biophys. Res. Commun. 2006;349:353–359. doi: 10.1016/j.bbrc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 70.Zhang H., Ryu D., Wu Y., Gariani K., Wang X., Luan P., D’Amico D., Ropelle E.R., Lutolf M.P., Aebersold R., et al. NAD+ repletion improves mitochondrial and stem cell function and enhances life span in mice. Science. 2016;352:1436–1443. doi: 10.1126/science.aaf2693. [DOI] [PubMed] [Google Scholar]
- 71.Trammell S.A.J., Weidemann B.J., Chadda A., Yorek M.S.M.A.M.S., Holmes A., Coppey L.J., Obrosov A., Kardon R.H., Yorek M.S.M.A.M.S., Brenner C. Nicotinamide riboside opposes type 2 diabetes and neuropathy in mice. Sci. Rep. 2016;6:1–7. doi: 10.1038/srep26933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gong B., Pan Y., Vempati P., Zhao W., Knable L., Ho L., Wang J., Sastre M., Ono K., Sauve A.A., et al. Nicotinamide riboside restores cognition through an upregulation of proliferator-activated receptor-γ coactivator 1α regulated β-secretase 1 degradation and mitochondrial gene expression in Alzheimer’s mouse models. Neurobiol. Aging. 2013;34:1581–1588. doi: 10.1016/j.neurobiolaging.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Frederick D.W.W., Loro E., Liu L., Davila A., Chellappa K., Silverman I.M.M., Quinn W.J.J., Gosai S.J.J., Tichy E.D.D., Davis J.G.G., et al. Loss of NAD Homeostasis Leads to Progressive and Reversible Degeneration of Skeletal Muscle. Cell Metab. 2016;24:269–282. doi: 10.1016/j.cmet.2016.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mendelsohn A.R., Larrick J.W. Partial reversal of skeletal muscle aging by restoration of normal NAD + levels. Rejuvenation Res. 2014;17:62–69. doi: 10.1089/rej.2014.1546. [DOI] [PubMed] [Google Scholar]
- 75.Wu L.E., Sinclair D.A. Restoring stem cells-all you need is NAD+ Cell Res. 2016;26:971–972. doi: 10.1038/cr.2016.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mills K.F., Yoshida S., Stein L.R., Grozio A., Kubota S., Sasaki Y., Redpath P., Migaud M.E., Apte R.S., Uchida K., et al. Long-Term Administration of Nicotinamide Mononucleotide Mitigates Age-Associated Physiological Decline in Mice. Cell Metab. 2016;24:795–806. doi: 10.1016/j.cmet.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nóbrega-Pereira S., Fernandez-Marcos P.J., Brioche T., Gomez-Cabrera M.C., Salvador-Pascual A., Flores J.M., Viña J., Serrano M. G6PD protects from oxidative damage and improves healthspan in mice. Nat. Commun. 2016;7:10894. doi: 10.1038/ncomms10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gariani K., Menzies K.J., Ryu D., Wegner C.J., Wang X., Ropelle E.R., Moullan N., Zhang H., Perino A., Lemos V., et al. Eliciting the mitochondrial unfolded protein response by nicotinamide adenine dinucleotide repletion reverses fatty liver disease in mice. Hepatology. 2016;63:1190–1204. doi: 10.1002/hep.28245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brown K.D., Maqsood S., Huang J.Y., Pan Y., Harkcom W., Li W., Sauve A., Verdin E., Jaffrey S.R. Activation of SIRT3 by the NAD+ precursor nicotinamide riboside protects from noise-induced hearing loss. Cell Metab. 2014;20:1059–1068. doi: 10.1016/j.cmet.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Poljsak B., Kovač V., Milisav I. Healthy Lifestyle Recommendations: Do the Beneficial Effects Originate from NAD+Amount at the Cellular Level? Oxid. Med. Cell. Longev. 2020;2020:8819627. doi: 10.1155/2020/8819627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trammell S.A.J., Schmidt M.S., Weidemann B.J., Redpath P., Jaksch F., Dellinger R.W., Li Z., Abel E.D., Migaud M.E., Brenner C. Nicotinamide riboside is uniquely and orally bioavailable in mice and humans. Nat. Commun. 2016;7:1–14. doi: 10.1038/ncomms12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yiasemides E., Sivapirabu G., Halliday G.M., Park J., Damian D.L. Oral nicotinamide protects against ultraviolet radiation-induced immunosuppression in humans. Carcinogenesis. 2009;30:101–105. doi: 10.1093/carcin/bgn248. [DOI] [PubMed] [Google Scholar]
- 83.Baur J.A., Pearson K.J., Price N.L., Jamieson H.A., Lerin C., Kalra A., Prabhu V.V., Allard J.S., Lopez-Lluch G., Lewis K., et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444:337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cantó C., Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Curr. Opin. Lipidol. 2009;20:98–105. doi: 10.1097/MOL.0b013e328328d0a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cantó C., Gerhart-Hines Z., Feige J.N., Lagouge M., Noriega L., Milne J.C., Elliott P.J., Puigserver P., Auwerx J. AMPK regulates energy expenditure by modulating NAD + metabolism and SIRT1 activity. Nature. 2009;458:1056–1060. doi: 10.1038/nature07813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yoshino J., Mills K.F., Yoon M.J., Imai S. Nicotinamide Mononucleotide, a Key NAD+ Intermediate, Treats the Pathophysiology of Diet- and Age-Induced Diabetes in Mice. Cell Metab. 2011;14:528–536. doi: 10.1016/j.cmet.2011.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Barbosa M.T.P., Soares S.M., Novak C.M., Sinclair D., Levine J.A., Aksoy P., Chini E.N. The enzyme CD38 (a NAD glycohydrolase, EC 3.2.2.5) is necessary for the development of diet-induced obesity. FASEB J. 2007;21:3629–3639. doi: 10.1096/fj.07-8290com. [DOI] [PubMed] [Google Scholar]
- 88.Hipkiss A.R. Energy metabolism, altered proteins, sirtuins and ageing: Converging mechanisms? Biogerontology. 2008;9:49–55. doi: 10.1007/s10522-007-9110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Morris K.C., Lin H.W., Thompson J.W., Perez-Pinzon M.A. Pathways for ischemic cytoprotection: Role of sirtuins in caloric restriction, resveratrol, and ischemic preconditioning. J. Cereb. Blood Flow Metab. 2011;31:1003–1019. doi: 10.1038/jcbfm.2010.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Morselli E., Maiuri M.C., Markaki M., Megalou E., Pasparaki A., Palikaras K., Criollo A., Galluzzi L., Malik S.A., Vitale I., et al. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis. 2010;1:10. doi: 10.1038/cddis.2009.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Curtin N. PARP inhibitors for anticancer therapy. Biochem. Soc. Trans. 2014;42:82–88. doi: 10.1042/BST20130187. [DOI] [PubMed] [Google Scholar]
- 92.Prolla T.A., Denu J.M. NAD+ deficiency in age-related mitochondrial dysfunction. Cell Metab. 2014;19:178–180. doi: 10.1016/j.cmet.2014.01.005. [DOI] [PubMed] [Google Scholar]
- 93.Dollerup O., Chubanava S., Agerholm M., Søndergård S., Altıntaş A., Møller A., Høyer K., Ringgaard S., Stødkilde-Jørgensen H., Lavery G., et al. Nicotinamide riboside does not alter mitochondrial respiration, content or morphology in skeletal muscle from obese and insulin-resistant men. J. Physiol. 2020;598:731–754. doi: 10.1113/JP278752. [DOI] [PubMed] [Google Scholar]
- 94.Remie C.M.E., Roumans K.H.M., Moonen M.P.B., Connell N.J., Havekes B., Mevenkamp J., Lindeboom L., Wit D., Van De Weijer T., Arts S., et al. Nicotinamide riboside supplementation alters body composition and skeletal muscle acetylcarnitine concentrations in healthy obese humans. Am. J. Clin. Nutr. 2020;112:413–426. doi: 10.1093/ajcn/nqaa072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Martens C.R., Denman B.A., Mazzo M.R., Armstrong M.L., Reisdorph N., McQueen M.B., Chonchol M., Seals D.R. Chronic nicotinamide riboside supplementation is well-Tolerated and elevates NAD+ in healthy middle-Aged and older adults. Nat. Commun. 2018;9:1286. doi: 10.1038/s41467-018-03421-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kallio K., Hätönen K., Lehto M., Salomaa V., Männistö S., Pussinen P. Endotoxemia, nutrition, and cardiometabolic disorders. Acta Diabetol. 2015;52:395–404. doi: 10.1007/s00592-014-0662-3. [DOI] [PubMed] [Google Scholar]
- 97.Lee C., Song E., Yoo C., Kwak Y., Han M. Lipopolysaccharide induces CD38 expression and solubilization in J774 macrophage cells. Mol. Cells. 2012;34:573–576. doi: 10.1007/s10059-012-0263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kim K.A., Jeong J.J., Yoo S.Y., Kim D.H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016;16:9. doi: 10.1186/s12866-016-0625-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ghosh S., Lertwattanarak R., Garduño Jde J., Galeana J., Li J., Zamarripa F., Lancaster J., Mohan S., Hussey S., Musi N. Elevated muscle TLR4 expression and metabolic endotoxemia in human aging. J. Gerontol. A. Biol. Sci. Med. Sci. 2015;70:232–246. doi: 10.1093/gerona/glu067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bieganowski P., Brenner C. Discoveries of nicotinamide riboside as a nutrient and conserved NRK genes establish a Preiss-Handler independent route to NAD+ in fungi and humans. Cell. 2004;117:495–502. doi: 10.1016/S0092-8674(04)00416-7. [DOI] [PubMed] [Google Scholar]
- 101.Grozio A., Sociali G., Sturla I., Caffa I., Soncini D., Salis A., Raffaelli N., De Flora A., Nencioni A., Bruzzone S. CD73 protein as a source of extracellular precursors for sustained NAD+ biosynthesis in FK866-treated tumor cells. J. Biol. Chem. 2013;288:25938–25949. doi: 10.1074/jbc.M113.470435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kellenberger E., Kuhn I., Schuber F., Muller-Steffner H. Flavonoids as inhibitors of human CD38. Bioorg. Med. Chem. Lett. 2011;21:3939–3942. doi: 10.1016/j.bmcl.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 103.Escande C., Nin V., Price N.L., Capellini V., Gomes A.P., Barbosa M.T., O’Neil L., White T.A., Sinclair D.A., Chini E.N. Flavonoid apigenin is an inhibitor of the NAD+ase CD38: Implications for cellular NAD+ metabolism, protein acetylation, and treatment of metabolic syndrome. Diabetes. 2013;62:1084–1093. doi: 10.2337/db12-1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Haffner C.D., Becherer J.D., Boros E.E., Cadilla R., Carpenter T., Cowan D., Deaton D.N., Guo Y., Harrington W., Henke B.R., et al. Discovery, synthesis, and biological evaluation of thiazoloquin(az)olin(on)es as potent CD38 inhibitors. J. Med. Chem. 2015;58:3548–3571. doi: 10.1021/jm502009h. [DOI] [PubMed] [Google Scholar]
- 105.Chillemi A., Zaccarello G., Quarona V., Ferracin M., Ghimenti C., Massaia M., Horenstein A., Malavasi F. Anti-CD38 antibody therapy: Windows of opportunity yielded by the functional characteristics of the target molecule. Mol. Med. 2013;19:99–108. doi: 10.2119/molmed.2013.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pirinen E., Cantó C., Jo Y.S., Morato L., Zhang H., Menzies K.J., Williams E.G., Mouchiroud L., Moullan N., Hagberg C., et al. Pharmacological inhibition of poly(ADP-ribose) polymerases improves fitness and mitochondrial function in skeletal muscle. Cell Metab. 2014;19:1034–1041. doi: 10.1016/j.cmet.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gardell S.J., Hopf M., Khan A., Dispagna M., Hampton Sessions E., Falter R., Kapoor N., Brooks J., Culver J., Petucci C., et al. Boosting NAD+ with a small molecule that activates NAMPT. Nat. Commun. 2019;10:3421. doi: 10.1038/s41467-019-11078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Xie W., Zhu T., Zhou P., Xu H., Meng X., Ding T., Nan F., Sun G., Sun X. Notoginseng Leaf Triterpenes Ameliorates OGD/R-Induced Neuronal Injury via SIRT1/2/3-Foxo3a-MnSOD/PGC-1 α Signaling Pathways Mediated by the NAMPT-NAD Pathway. Oxid. Med. Cell. Longev. 2020;2020:7308386. doi: 10.1155/2020/7308386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie W., Zhu T., Zhou P., Xu H., Meng X., Ding T., Nan F., Sun G., Sun X. Notoginseng leaf triterpenes ameliorates mitochondrial oxidative injury via the NAMPT-SIRT1/2/3 signaling pathways in cerebral ischemic model rats. J. Ginseng Res. 2020 doi: 10.1016/j.jgr.2020.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhullar K.S., Son M., Kerek E., Cromwell C.R., Wingert B.M., Wu K., Jovel J., Camacho C.J., Hubbard B.P., Wu J. Tripeptide IRW Upregulates NAMPT Protein Levels in Cells and Obese C57BL/6J Mice. J. Agric. Food Chem. 2021;69:1555–1566. doi: 10.1021/acs.jafc.0c07831. [DOI] [PubMed] [Google Scholar]
- 111.Fulco M., Cen Y., Zhao P., Hoffman E.P., McBurney M.W., Sauve A.A., Sartorelli V. Glucose Restriction Inhibits Skeletal Myoblast Differentiation by Activating SIRT1 through AMPK-Mediated Regulation of Nampt. Dev. Cell. 2008;14:661–673. doi: 10.1016/j.devcel.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang Y., Cimen H., Han M.J., Shi T., Deng J.H., Koc H., Palacios O.M., Montier L., Bai Y., Tong Q., et al. NAD+-dependent deacetylase SIRT3 regulates mitochondrial protein synthesis by deacetylation of the ribosomal protein MRPL10. J. Biol. Chem. 2010;285:7417–7429. doi: 10.1074/jbc.M109.053421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rajman L., Chwalek K., Sinclair D.A. Therapeutic Potential of NAD-Boosting Molecules: The In Vivo Evidence. Cell Metab. 2018;27:529–547. doi: 10.1016/j.cmet.2018.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Costford S.R., Bajpeyi S., Pasarica M., Albarado D.C., Thomas S.C., Xie H., Church T.S., Jubrias S.A., Conley K.E., Smith S.R. Skeletal muscle NAMPT is induced by exercise in humans. Am. J. Physiol. Endocrinol. Metab. 2010;298:E117. doi: 10.1152/ajpendo.00318.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Van Haren M.J., Zhang Y., Thijssen V., Buijs N., Gao Y., Mateuszuk L., Fedak F.A., Kij A., Campagna R., Sartini D., et al. Macrocyclic peptides as allosteric inhibitors of nicotinamide N-methyltransferase (NNMT) RSC Chem. Biol. 2021;2:1546–1555. doi: 10.1039/D1CB00134E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee H.Y., Suciu R.M., Horning B.D., Vinogradova E.V., Ulanovskaya O.A., Cravatt B.F. Covalent inhibitors of nicotinamide N-methyltransferase (NNMT) provide evidence for target engagement challenges in situ. Bioorg. Med. Chem. Lett. 2018;28:2682–2687. doi: 10.1016/j.bmcl.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 117.Policarpo R.L., Decultot L., May E., Kuzmič P., Carlson S., Huang D., Chu V., Wright B.A., Dhakshinamoorthy S., Kannt A., et al. High-Affinity Alkynyl Bisubstrate Inhibitors of Nicotinamide N-Methyltransferase (NNMT) J. Med. Chem. 2019;62:9837–9873. doi: 10.1021/acs.jmedchem.9b01238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Gao Y., Van Haren M.J., Moret E.E., Rood J.J.M., Sartini D., Salvucci A., Emanuelli M., Craveur P., Babault N., Jin J., et al. Bisubstrate inhibitors of nicotinamide N-methyltransferase (NNMT) with enhanced activity. J. Med. Chem. 2019;62:6597–6614. doi: 10.1021/acs.jmedchem.9b00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gao Y., Van Haren M.J., Buijs N., Innocenti P., Zhang Y., Sartini D., Campagna R., Emanuelli M., Parsons R.B., Jespers W., et al. Potent Inhibition of Nicotinamide N-Methyltransferase by Alkene-Linked Bisubstrate Mimics Bearing Electron Deficient Aromatics. J. Med. Chem. 2021;64:12938–12963. doi: 10.1021/acs.jmedchem.1c01094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.van Haren M.J., Gao Y., Buijs N., Campagna R., Sartini D., Emanuelli M., Mateuszuk L., Kij A., Chlopicki S., de Castilla P.E.M., et al. Esterase-Sensitive Prodrugs of a Potent Bisubstrate Inhibitor of Nicotinamide N-Methyltransferase (NNMT) Display Cellular Activity. Biomolecules. 2021;11:1357. doi: 10.3390/biom11091357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lu X.M., Long H. Nicotinamide N-methyltransferase as a potential marker for cancer. Neoplasma. 2018;65:656–663. doi: 10.4149/neo_2018_171024N680. [DOI] [PubMed] [Google Scholar]
- 122.Neelakantan H., Vance V., Wetzel M.D., Wang H.Y.L., McHardy S.F., Finnerty C.C., Hommel J.D., Watowich S.J. Selective and membrane-permeable small molecule inhibitors of nicotinamide N-methyltransferase reverse high fat diet-induced obesity in mice. Biochem. Pharmacol. 2018;147:141–152. doi: 10.1016/j.bcp.2017.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Campagna R., Pozzi V., Sartini D., Salvolini E., Brisigotti V., Molinelli E., Campanati A., Offidani A., Emanuelli M. Beyond Nicotinamide Metabolism: Potential Role of Nicotinamide N-Methyltransferase as a Biomarker in Skin Cancers. Cancers. 2021;13:4943. doi: 10.3390/cancers13194943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aksoy S., Szumlanski C.L., Weinshilboums R.M. Human Liver Nicotinamide N-Methyltransferase. J. Biol. Chem. 1994;269:14835–14840. doi: 10.1016/S0021-9258(17)36700-5. [DOI] [PubMed] [Google Scholar]
- 125.Picard F., Kurtev M., Chung N., Topark-Ngarm A., Senawong T., De Oliveira R.M., Leid M., McBurney M.W., Guarente L. Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-γ. Nature. 2004;429:771–776. doi: 10.1038/nature02583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Brandauer J., Vienberg S.G., Andersen M.A., Ringholm S., Risis S., Larsen P.S., Kristensen J.M., Frøsig C., Leick L., Fentz J., et al. AMP-activated protein kinase regulates nicotinamide phosphoribosyl transferase expression in skeletal muscle. J. Physiol. 2013;591:5207–5220. doi: 10.1113/jphysiol.2013.259515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.de Guia R.M., Agerholm M., Nielsen T.S., Consitt L.A., Søgaard D., Helge J.W., Larsen S., Brandauer J., Houmard J.A., Treebak J.T. Aerobic and resistance exercise training reverses age-dependent decline in NAD+ salvage capacity in human skeletal muscle. Physiol. Rep. 2019;7:e14139. doi: 10.14814/phy2.14139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Khan N.A., Auranen M., Paetau I., Pirinen E., Euro L., Forsström S., Pasila L., Velagapudi V., Carroll C.J., Auwerx J., et al. Effective treatment of mitochondrial myopathy by nicotinamide riboside, a vitamin B3. EMBO Mol. Med. 2014;6:721–731. doi: 10.1002/emmm.201403943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Srivastava S. Emerging therapeutic roles for NAD + metabolism in mitochondrial and age-related disorders. Clin. Transl. Med. 2016;5:25. doi: 10.1186/s40169-016-0104-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wu L.E., Gomes A.P., Sinclair D.A. Geroncogenesis: Metabolic changes during aging as a driver of tumorigenesis. Cancer Cell. 2014;25:12–19. doi: 10.1016/j.ccr.2013.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tummala K.S., Gomes A.L., Yilmaz M., Graña O., Bakiri L., Ruppen I., Ximénez-Embún P., Sheshappanavar V., Rodriguez-Justo M., Pisano D.G., et al. Inhibition of De Novo NAD+ Synthesis by Oncogenic URI Causes Liver Tumorigenesis through DNA Damage. Cancer Cell. 2014;26:826–839. doi: 10.1016/j.ccell.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 132.Santidrian A.F., Matsuno-Yagi A., Ritland M., Seo B.B., LeBoeuf S.E., Gay L.J., Yagi T., Felding-Habermann B. Mitochondrial complex I activity and NAD+/NADH balance regulate breast cancer progression. J. Clin. Investig. 2013;123:1068–1081. doi: 10.1172/JCI64264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Matasic D.S., Brenner C., London B. Emerging potential benefits of modulating NAD+ metabolism in cardiovascular disease. Am. J. Physiol. Hear. Circ. Physiol. 2018;314:H839–H852. doi: 10.1152/ajpheart.00409.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Mericskay M. Nicotinamide adenine dinucleotide homeostasis and signalling in heart disease: Pathophysiological implications and therapeutic potential. Arch. Cardiovasc. Dis. 2016;109:207–215. doi: 10.1016/j.acvd.2015.10.004. [DOI] [PubMed] [Google Scholar]
- 135.Martin A.S., Abraham D.M., Hershberger K.A., Bhatt D.P., Mao L., Cui H., Liu J., Liu X., Muehlbauer M.J., Grimsrud P.A., et al. Nicotinamide mononucleotide requires SIRT3 to improve cardiac function and bioenergetics in a Friedreich’s ataxia cardiomyopathy model. JCI Insight. 2017;2:e93885. doi: 10.1172/jci.insight.93885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.de Picciotto N.E., Gano L.B., Johnson L.C., Martens C.R., Sindler A.L., Mills K.F., ichiro Imai S., Seals D.R. Nicotinamide mononucleotide supplementation reverses vascular dysfunction and oxidative stress with aging in mice. Aging Cell. 2016;15:522–530. doi: 10.1111/acel.12461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Csiszar A., Tarantini S., Yabluchanskiy A., Balasubramanian P., Kiss T., Farkas E., Baur J.A., Ungvari Z. Role of endothelial NAD + deficiency in age-related vascular dysfunction. Am. J. Physiol. Heart Circ. Physiol. 2019;316:H1253–H1266. doi: 10.1152/ajpheart.00039.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ying W. NAD+ and NADH in brain functions, brain diseases and brain aging. Front. Biosci. 2007;12:1863–1888. doi: 10.2741/2194. [DOI] [PubMed] [Google Scholar]
- 139.Hikosaka K., Yaku K., Okabe K., Nakagawa T. Implications of NAD metabolism in pathophysiology and therapeutics for neurodegenerative diseases. Nutr. Neurosci. 2021;24:371–383. doi: 10.1080/1028415X.2019.1637504. [DOI] [PubMed] [Google Scholar]
- 140.Klaidman L., Morales M., Kem S., Yang J., Chang M.L., Adams J.D. Nicotinamide offers multiple protective mechanisms in stroke as a precursor for NAD+, as a PARP inhibitor and by partial restoration of mitochondrial function. Pharmacology. 2003;69:150–157. doi: 10.1159/000072668. [DOI] [PubMed] [Google Scholar]
- 141.Ugur S., Ulu R., Dogukan A., Gurel A., Yigit I.P., Gozel N., Aygen B., Ilhan N. The renoprotective effect of curcumin in cisplatin-induced nephrotoxicity. Ren. Fail. 2015;37:332–336. doi: 10.3109/0886022X.2014.986005. [DOI] [PubMed] [Google Scholar]
- 142.Zhuo L., Fu B., Bai X., Zhang B., Wu L., Cui J., Cui S., Wei R., Chen X., Cai G. NAD blocks high glucose induced mesangial hypertrophy via activation of the sirtuins-AMPK-mTOR pathway. Cell. Physiol. Biochem. 2011;27:681–690. doi: 10.1159/000330077. [DOI] [PubMed] [Google Scholar]
- 143.Hou Y., Lautrup S., Cordonnier S., Wang Y., Croteau D.L., Zavala E., Zhang Y., Moritoh K., O’Connell J.F., Baptiste B.A., et al. NAD+ supplementation normalizes key Alzheimer’s features and DNA damage responses in a new AD mouse model with introduced DNA repair deficiency. Proc. Natl. Acad. Sci. USA. 2018;115:E1876–E1885. doi: 10.1073/pnas.1718819115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fang E.F., Kassahun H., Croteau D.L., Scheibye-Knudsen M., Marosi K., Lu H., Shamanna R.A., Kalyanasundaram S., Bollineni R.C., Wilson M.A., et al. NAD+ Replenishment Improves Lifespan and Healthspan in Ataxia Telangiectasia Models via Mitophagy and DNA Repair. Cell Metab. 2016;24:566–581. doi: 10.1016/j.cmet.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Chen A.C., Damian D.L. Nicotinamide and the skin. Australas. J. Dermatol. 2014;55:169–175. doi: 10.1111/ajd.12163. [DOI] [PubMed] [Google Scholar]
- 146.Jukarainen S., Heinonen S., Rämö J.T., Rinnankoski-Tuikka R., Rappou E., Tummers M., Muniandy M., Hakkarainen A., Lundbom J., Lundbom N., et al. Obesity is associated with low nad+/sirt pathway expression in adipose tissue of BMI-discordant monozygotic twins. J. Clin. Endocrinol. Metab. 2016;101:275–283. doi: 10.1210/jc.2015-3095. [DOI] [PubMed] [Google Scholar]
- 147.Drew J.E., Farquharson A.J., Horgan G.W., Williams L.M. Tissue-specific regulation of sirtuin and nicotinamide adenine dinucleotide biosynthetic pathways identified in C57Bl/6 mice in response to high-fat feeding. J. Nutr. Biochem. 2016;37:20–29. doi: 10.1016/j.jnutbio.2016.07.013. [DOI] [PubMed] [Google Scholar]
- 148.Nielsen K.N., Peics J., Ma T., Karavaeva I., Dall M., Chubanava S., Basse A.L., Dmytriyeva O., Treebak J.T., Gerhart-Hines Z. NAMPT-mediated NAD + biosynthesis is indispensable for adipose tissue plasticity and development of obesity. Mol. Metab. 2018;11:178–188. doi: 10.1016/j.molmet.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mouchiroud L., Houtkooper R.H., Moullan N., Katsyuba E., Ryu D., Cantó C., Mottis A., Jo Y.S., Viswanathan M., Schoonjans K., et al. The NAD+/sirtuin pathway modulates longevity through activation of mitochondrial UPR and FOXO signaling. Cell. 2013;154:430. doi: 10.1016/j.cell.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.North B.J., Rosenberg M.A., Jeganathan K.B., Hafner A.V., Michan S., Dai J., Baker D.J., Cen Y., Wu L.E., Sauve A.A., et al. SIRT 2 induces the checkpoint kinase BubR1 to increase lifespan. EMBO J. 2014;33:1438–1453. doi: 10.15252/embj.201386907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Irie J., Inagaki E., Fujita M., Nakaya H., Mitsuishi M., Yamaguchi S., Yamashita K., Shigaki S., Ono T., Yukioka H., et al. Effect of oral administration of nicotinamide mononucleotide on clinical parameters and nicotinamide metabolite levels in healthy Japanese men. Endocr. J. 2020;67:153–160. doi: 10.1507/endocrj.EJ19-0313. [DOI] [PubMed] [Google Scholar]
- 152.Airhart S.E., Shireman L.M., Risler L.J., Anderson G.D., Gowda G.A.N., Raftery D., Tian R., Shen D.D., O’Brien K.D. An open-label, non-randomized study of the pharmacokinetics of the nutritional supplement nicotinamide riboside (NR) and its effects on blood NAD+ levels in healthy volunteers. PLoS ONE. 2017;12:e0186459. doi: 10.1371/journal.pone.0186459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Poljsak B., Milisav I. Vitamin B3 forms as precursors to NAD+: Are they safe? Trends Food Sci. Technol. 2018;79:198–203. doi: 10.1016/j.tifs.2018.07.020. [DOI] [Google Scholar]
- 154.Fahy G.M., Brooke R.T., Watson J.P., Good Z., Vasanawala S.S., Maecker H., Leipold M.D., Lin D.T.S., Kobor M.S., Horvath S. Reversal of epigenetic aging and immunosenescent trends in humans. Aging Cell. 2019;18:e13028. doi: 10.1111/acel.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Tummala K.S., Djouder N. Oncogene-induced NAD(+) depletion in tumorigenesis. Oncoscience. 2015;2:318–319. doi: 10.18632/oncoscience.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Jacobson E.L., Dame A.J., Pyrek J.S., Jacobson M.K. Evaluating the role of niacin in human carcinogenesis. Biochimie. 1995;77:394–398. doi: 10.1016/0300-9084(96)88152-1. [DOI] [PubMed] [Google Scholar]
- 157.Smith J.S. Human Sir2 and the “silencing” of p53 activity. Trends Cell Biol. 2002;12:404–406. doi: 10.1016/S0962-8924(02)02342-5. [DOI] [PubMed] [Google Scholar]
- 158.Tucci P. Caloric restriction: Is mammalian life extension linked to p53? Aging. 2012;4:525–534. doi: 10.18632/aging.100481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Olsson A.R., Sheng Y., Pero R.W., Chaplin D.J., Horsman M.R. DNA damage and repair in tumour and non-tumour tissues of mice induced by nicotinamide. Br. J. Cancer. 1996;74:368. doi: 10.1038/bjc.1996.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yang T., Sauve A.A. NAD metabolism and sirtuins: Metabolic regulation of protein deacetylation in stress and toxicity. AAPS J. 2006;8:E632. doi: 10.1208/aapsj080472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Cantó C., Auwerx J. Interference between PARPs and SIRT1: A novel approach to healthy ageing? Aging. 2011;3:543–547. doi: 10.18632/aging.100326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Chen Y., Zhang J., Lin Y., Lei Q., Guan K.L., Zhao S., Xiong Y. Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 2011;12:534–541. doi: 10.1038/embor.2011.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tao R., Coleman M.C., Pennington J.D., Ozden O., Park S.H., Jiang H., Kim H.S., Flynn C.R., Hill S., McDonald W.H., et al. Sirt3-mediated deacetylation of evolutionarily conserved lysine 122 regulates MnSOD activity in response to stress. Mol. Cell. 2010;40:893–904. doi: 10.1016/j.molcel.2010.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Qiu X., Brown K., Hirschey M.D., Verdin E., Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 165.Kim H.S., Patel K., Muldoon-Jacobs K., Bisht K.S., Aykin-Burns N., Pennington J.D., van der Meer R., Nguyen P., Savage J., Owens K.M., et al. SIRT3 is a mitochondria-localized tumor suppressor required for maintenance of mitochondrial integrity and metabolism during stress. Cancer Cell. 2010;17:41–52. doi: 10.1016/j.ccr.2009.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kim H.S., Vassilopoulos A., Wang R.H., Lahusen T., Xiao Z., Xu X., Li C., Veenstra T.D., Li B., Yu H., et al. SIRT2 maintains genome integrity and suppresses tumorigenesis through regulating APC/C activity. Cancer Cell. 2011;20:487–499. doi: 10.1016/j.ccr.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hiratsuka M., Inoue T., Toda T., Kimura N., Shirayoshi Y., Kamitani H., Watanabe T., Ohama E., Tahimic C.G.T., Kurimasa A., et al. Proteomics-based identification of differentially expressed genes in human gliomas: Down-regulation of SIRT2 gene. Biochem. Biophys. Res. Commun. 2003;309:558–566. doi: 10.1016/j.bbrc.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 168.Wang F., Nguyen M., Qin F.X.F., Tong Q. SIRT2 deacetylates FOXO3a in response to oxidative stress and caloric restriction. Aging Cell. 2007;6:505–514. doi: 10.1111/j.1474-9726.2007.00304.x. [DOI] [PubMed] [Google Scholar]
- 169.Kim M.J., Kim D.W., Park J.H., Kim S.J., Lee C.H., Yong J.I., Ryu E.J., Cho S.B., Yeo H.J., Hyeon J., et al. PEP-1-SIRT2 inhibits inflammatory response and oxidative stress-induced cell death via expression of antioxidant enzymes in murine macrophages. Free Radic. Biol. Med. 2013;63:432–445. doi: 10.1016/j.freeradbiomed.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 170.Rawling J.M., Jackson T.M., Driscoll E.R., Kirkland J.B. Dietary niacin deficiency lowers tissue poly(ADP-ribose) and NAD+ concentrations in Fischer-344 rats. J. Nutr. 1994;124:1597–1603. doi: 10.1093/jn/124.9.1597. [DOI] [PubMed] [Google Scholar]
- 171.Wright S.C., Wei Q.S., Kinder D.H., Larrick J.W. Biochemical pathways of apoptosis: Nicotinamide adenine dinucleotide-deficient cells are resistant to tumor necrosis factor or ultraviolet light activation of the 24-kD apoptotic protease and DNA fragmentation. J. Exp. Med. 1996;183:463–471. doi: 10.1084/jem.183.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Roth M., Chen W.Y. Sorting out functions of sirtuins in cancer. Oncogene. 2014;33:1609–1620. doi: 10.1038/onc.2013.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Wang Z., Chen W.Y. Emerging Roles of SIRT1 in Cancer Drug Resistance. Genes Cancer. 2013;4:82–90. doi: 10.1177/1947601912473826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Brooks C.L., Gu W. How does SIRT1 affect metabolism, senescence and cancer? Nat. Rev. Cancer. 2009;9:123–128. doi: 10.1038/nrc2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Poljsak B., Milisav I. The Role of Antioxidants in Cancer, Friends or Foes? Curr. Pharm. Des. 2019;24:5234–5244. doi: 10.2174/1381612825666190123112647. [DOI] [PubMed] [Google Scholar]
- 176.Das A., Huang G.X., Bonkowski M.S., Longchamp A., Li C., Schultz M.B., Kim L.J., Osborne B., Joshi S., Lu Y., et al. Impairment of an Endothelial NAD+-H2S Signaling Network Is a Reversible Cause of Vascular Aging. Cell. 2018;173:74–89. doi: 10.1016/j.cell.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu T.F., Brown C.M., El Gazzar M., McPhail L., Millet P., Rao A., Vachharajani V.T., Yoza B.K., McCall C.E. Fueling the flame: Bioenergy couples metabolism and inflammation. J. Leukoc. Biol. 2012;92:499. doi: 10.1189/jlb.0212078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Li L., Chen Z., Fu W., Cai S., Zeng Z. Emerging evidence concerning the role of sirtuins in sepsis. Crit. Care Res. Pract. 2018;2018:8. doi: 10.1155/2018/5489571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nacarelli T., Lau L., Fukumoto T., Zundell J., Fatkhutdinov N., Wu S., Aird K.M., Iwasaki O., Kossenkov A.V., Schultz D., et al. NAD+ metabolism governs the proinflammatory senescence-associated secretome. Nat. Cell Biol. 2019;21:397–407. doi: 10.1038/s41556-019-0287-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chini C., Hogan K.A., Warner G.M., Tarragó M.G., Peclat T.R., Tchkonia T., Kirkland J.L., Chini E. The NADase CD38 is induced by factors secreted from senescent cells providing a potential link between senescence and age-related cellular NAD + decline. Biochem. Biophys. Res. Commun. 2019;513:486–493. doi: 10.1016/j.bbrc.2019.03.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Poljšak B., Kovač V., Milisav I. Current Uncertainties and Future Challenges Regarding NAD+ Boosting Strategies. Antioxidants. 2022;11:1637. doi: 10.3390/antiox11091637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Braidy N., Liu Y. NAD+ therapy in age-related degenerative disorders: A benefit/risk analysis. Exp. Gerontol. 2020;132:110831. doi: 10.1016/j.exger.2020.110831. [DOI] [PubMed] [Google Scholar]
- 183.Dragovic J., Kim S.H., Brown S.L., Kim J.H. Nicotinamide pharmacokinetics in patients. Radiother. Oncol. 1995;36:225–228. doi: 10.1016/0167-8140(95)01581-Z. [DOI] [PubMed] [Google Scholar]
- 184.Knip M., Douek I.F., Moore W.P.T., Gillmor H.A., McLean A.E.M., Bingley P.J., Gale E.A.M. Safety of high-dose nicotinamide: A review. Diabetologia. 2000;43:1337–1345. doi: 10.1007/s001250051536. [DOI] [PubMed] [Google Scholar]
- 185.Kourtzidis I.A., Stoupas A.T., Gioris I.S., Veskoukis A.S., Margaritelis N.V., Tsantarliotou M., Taitzoglou I., Vrabas I.S., Paschalis V., Kyparos A., et al. The NAD+ precursor nicotinamide riboside decreases exercise performance in rats. J. Int. Soc. Sports Nutr. 2016;13:1–4. doi: 10.1186/s12970-016-0143-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Murray R., Bartoli W.P., Eddy D.E., Horn M.K. Physiological and performance responses to nicotinic-acid ingestion during exercise. Med. Sci. Sports Exerc. 1995;27:1057–1062. doi: 10.1249/00005768-199507000-00015. [DOI] [PubMed] [Google Scholar]
- 187.Liao B., Zhao Y., Wang D., Zhang X., Hao X., Hu M. Nicotinamide mononucleotide supplementation enhances aerobic capacity in amateur runners: A randomized, double-blind study. J. Int. Soc. Sports Nutr. 2021;18:54. doi: 10.1186/s12970-021-00442-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Igarashi M., Nakagawa-Nagahama Y., Miura M., Kashiwabara K., Yaku K., Sawada M., Sekine R., Fukamizu Y., Sato T., Sakurai T., et al. Chronic nicotinamide mononucleotide supplementation elevates blood nicotinamide adenine dinucleotide levels and alters muscle function in healthy older men. NPJ Aging. 2022;8:1–11. doi: 10.1038/s41514-022-00084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liu L., Su X., Quinn W.J., Hui S., Krukenberg K., Frederick D.W., Redpath P., Zhan L., Chellappa K., White E., et al. Quantitative Analysis of NAD Synthesis-Breakdown Fluxes. Cell Metab. 2018;27:1067–1080. doi: 10.1016/j.cmet.2018.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Poljsak B., Milisav I. Restoring NAD(+) Levels with NAD(+) intermediates, the second law of thermodynamics, and aging delay. Rejuvenation Res. 2018;21:506–509. doi: 10.1089/rej.2017.2037. [DOI] [PubMed] [Google Scholar]
- 191.Ogura Y., Kitada M., Xu J., Monno I., Koya D. CD38 inhibition by apigenin ameliorates mitochondrial oxidative stress through restoration of the intracellular NAD+/NADH ratio and Sirt3 activity in renal tubular cells in diabetic rats. Aging. 2020;12:11325–11336. doi: 10.18632/aging.103410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Khaidizar F.D., Bessho Y., Nakahata Y. Nicotinamide phosphoribosyltransferase as a key molecule of the aging/senescence process. Int. J. Mol. Sci. 2021;22:3709. doi: 10.3390/ijms22073709. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.