Abstract
Background:
HIV disproportionally affects persons who inject drugs (PWID), but engagement with HIV pre-exposure prophylaxis (PrEP) is low. We describe the rationale and study design for a new study, “Contingency Management and Pre-Exposure Prophylaxis (PrEP) Adherence Support Services (CoMPASS),” a hybrid type 1 effectiveness-implementation trial to promote HIV risk reduction among PWID.
Methods:
In four community-based programs in the northeastern United States, PrEP-eligible PWID (target n=526) are randomized to treatment as usual or Contingency Management (CM) and, as indicated, stepped up to PrEP Adherence Support Services (CoMPASS) over 24 weeks. During CM sessions, participants receive timely tangible rewards for verifiable activities demonstrating 1) PrEP initiation and adherence, and 2) engagement with medications for opioid use disorder (MOUD) and other OUD-related care. Participants who do not have high levels of biomarker-confirmed PrEP adherence at week 12 will be stepped up to receive PrEP Adherence Support Services (PASS) consisting of strengths-based case management over 12 weeks. Interventions are delivered by trained PrEP navigators, staff embedded within the respective sites. The primary outcome is sustained PrEP adherence by dried blood spot testing at 24 weeks. To inform future implementation, we are conducting implementation-focused process evaluations throughout the clinical trial.
Conclusions:
Results from this protocol are anticipated to yield novel findings regarding the impact and scalability of CoMPASS to promote HIV prevention among PWID in partnership with community-based organizations.
ClinicalTrials.gov Identifier:
Keywords: HIV, opioid use disorder, injection drug use, HIV pre-exposure prophylaxis, contingency management, stepped care
1. Introduction
In the United States, persons who inject drugs (PWID) are disproportionately impacted by HIV, accounting for 1 in 10 new cases in 2018.1 To reduce HIV risk, leading international and national organizations, such as the World Health Organization, and Centers for Disease Control and Prevention (CDC), recommend a range of HIV prevention interventions for PWID, including access to syringe service programs (SSP), medications for opioid use disorder (MOUD), and HIV pre-exposure prophylaxis (PrEP).2–4 PWID, however, often do not receive such interventions, likely due to limited knowledge about PrEP, healthcare system mistrust, and competing priorities.5
To address these challenges, there have been efforts to link PWID accessing SSPs to MOUD6 and to integrate HIV testing and counseling with OUD-related care.7 Yet few efforts to date have provided PWID accessing such services comprehensive HIV prevention interventions that include PrEP.5 Further, limited attention has been paid to integrating interventions to enhance motivation to take PrEP, such as contingency management (CM).8 Among PWID, CM improves substance use outcomes,9 prevention and treatment of infectious diseases,10 and medication adherence.11 For some, CM alone may be an insufficient strategy to improve PrEP uptake, necessitating additional interventions, such as strengths-based case management,12,13 to overcome individual and structural barriers. To date, no studies have evaluated the impact of CM on PrEP uptake and adherence nor used a stepped care model to promote HIV prevention among PWID.14
Thus, with the overall goal of promoting HIV risk reduction and improved health among PWID, we are conducting a randomized controlled trial to evaluate the impact of CM with, as indicated, PrEP Adherence Support Services (PASS) (CoMPASS) compared to treatment as usual (TAU) on promoting PrEP initiation and adherence.
2. Methods
2.1. Overall design
Funded by NIDA,15 CoMPASS is a hybrid type 1 effectiveness-implementation trial16 enrolling PWID at one of four participating community-based sites. CoMPASS includes a 24-week intervention period with 12-month follow-up to evaluate the intervention’s impact on PrEP initiation and adherence and OUD-related care and behaviors (Figure 1) among 526 PrEP-eligible PWID. The primary outcome is sustained PrEP adherence at 24 weeks, assessed using dried blood spot (DBS) testing defined as a tenofovir-disoproxil diphosphate (TFV-DP; Truvada®) level >700fmol/punch or emtricitabine/tenofovir disoproxil fumarate (FTC-TDF; Descovy®) level >175fmol/punch, reflecting cumulative dosing over 6-8 weeks and consistent with 4 or more doses per week.17 Secondary outcomes include HIV risk behaviors; engagement in OUD-related care (SSP, MOUD) and extra-medical opioid use; and (exploratory) sexually transmitted infection (STI) and HIV acquisition. Additionally, we will evaluate the impact of the intervention on PrEP adherence based on alternative measures of adherence (i.e., point-of-care urine testing at week 12), self-report, and pharmacy data.
Figure 1.
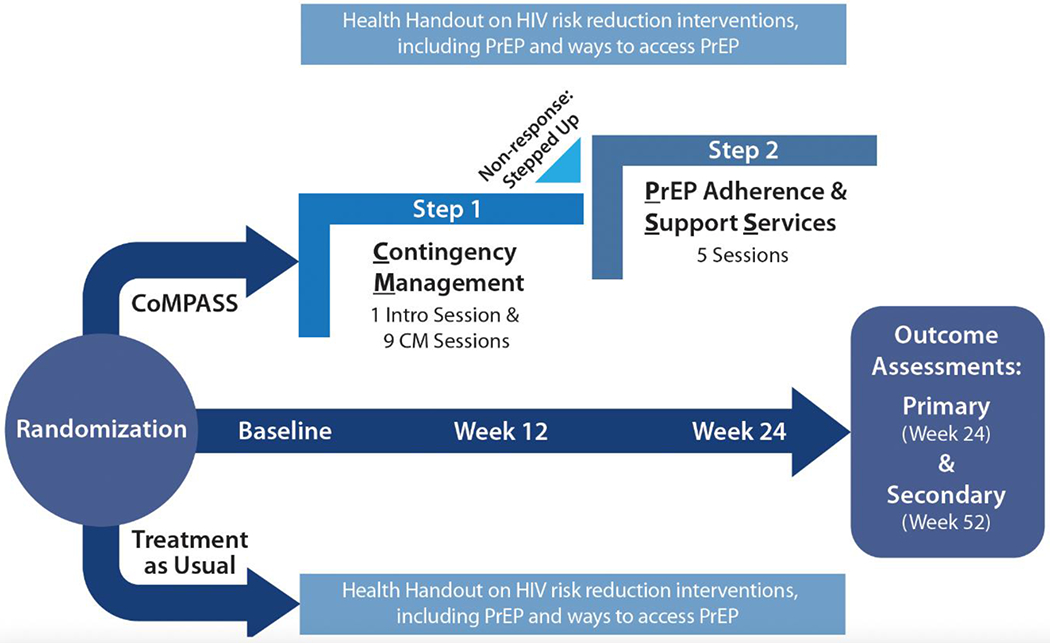
Contingency Management and stepped-up (when indicated) to Pre-Exposure Prophylaxis (PrEP) Adherence and Support Services (CoMPASS) Trial Protocol Overview
We will also generate data regarding factors that impact implementation.18 Consistent with community-engaged research principles,19 all aspects of this project from original concept through design choices and implementation have been conducted with community partners with the goal of optimizing its potential for promoting HIV prevention among PWID in a sustainable fashion. To examine whether treatment effects vary among subgroups, we will conduct exploratory and hypothesis generating analyses. We will study whether treatment effects differ by demographic and clinical characteristics known to be common in this population and impact medication adherence, including housing status and depression. In addition, we will examine whether treatment effects differ by site of recruitment.
2.2. Rationale for study design
The rationale for our study design is guided by several principles. First, given existing data,10,11,20 CM holds promise for promoting PrEP initiation and adherence among PWID.10 Second, the use of PrEP navigators to promote PrEP uptake has largely ignored PWID.21,22 Patient navigation has demonstrated efficacy to help patients with HIV navigate complexities of the medical system and retain them along the HIV care continuum and may also help patients navigate along the PrEP care continuum.23–26 Third, stepped care strategies, the graded implementation of interventions for a given condition based on individual response, serve to optimize resources while being responsive to individual needs; such approaches are especially appropriate when a single intervention approach may not be uniformly adequate and resources are constrained.27,28 Fourth, informed by prior work18 and our own experiences,29,30 implementation science frameworks, including RE-AIM (Reach, Effectiveness, Adoption, Implementation, and Maintenance)31 and Promoting Action on Research Implementation in Health Services (PARiHS), help guide the collection and evaluation of data in the context of ongoing randomized clinical trials to inform future implementation efforts.32 We also use the PARiHS framework in addition to RE-AIM as it is commonly used as a determinant framework, focusing on understanding factors that impact the delivery of an intervention. For example, to assess “implementation” of the intervention, we will track the types of trainings and supports that are needed to enhance fidelity to intervention delivery among PrEP navigators. Similarly, guided by the PARiHS framework, to assess factors that would be necessary to support “maintenance” beyond the research infrastructure, we are evaluating clinician and staff perspectives on the “evidence” for PrEP among individuals with OUD pre- and post-RCT conduct.
2.3. Study aims and hypotheses
Among PrEP-eligible PWID, our study aims to compare effectiveness of CoMPASS vs. TAU on sustained PrEP adherence at 24 weeks. We hypothesize CoMPASS will be associated with a higher proportion of biomarker-confirmed and self-reported PrEP adherence compared to TAU. Second, we will compare the effectiveness of CoMPASS vs. TAU on HIV risk behaviors; engagement in OUD-related care and extra-medical opioid use; and exploratory STI and HIV acquisition. We hypothesize that CoMPASS will be associated with improvements in risk behaviors and OUD-related outcomes. Lastly, we will conduct an implementation-focused process evaluation of CoMPASS among PrEP navigators, front-line providers, staff, and leadership at each site.
2.4. Study context
The study is being conducted in the context of the Yale Center for Interdisciplinary Research on AIDS-supported New England HIV Implementation Science Network.33 The coordinating center is at Yale School of Medicine; the Yale Center for Analytical Sciences coordinates data management and statistical support. The participating organizations include Apex Community Care, Inc., Connecticut Harm Reduction Alliance, Recovery Network of Programs, Inc., and Stanley Street Treatment And Resources, Inc. (Table 1). Diverse in their mission and infrastructure, these organizations serve individuals in areas highly impacted by injection drug use and HIV.34,35
Table 1.
Study site characteristics
| Site characteristics | Study Site | |||
|---|---|---|---|---|
| Site A | Site B | Site C | Site D | |
| Client gender identity (%) | ||||
| Female | 31.5 | 27.7 | 32.6 | 47.9 |
| Male | 67.4 | 70.1 | 67.4 | 51.7 |
| Transgender | 0.2 | 0.2 | -- | 0.2 |
| Nonbinary | 0.4 | -- | -- | 0 |
| Othera | 0.4 | 2.2 | -- | 0.2 |
| Client racial identity (%) | ||||
| American Indian/Native American | 1.8 | 0.1 | 0.2 | 0.6 |
| Asian/Pacific Islander | 1.1 | 1.0 | 0.4 | 2.5 |
| Black/African-American | 10.3 | 24.9 | 32.9 | 6.9 |
| White/Caucasian | 76.6 | 43.2 | 41.6 | 66.9 |
| Otherb | 10.0 | -- | 21.5 | 23.0 |
| Client ethnic identity (%) | ||||
| Hispanic/Latino/Latinx/Latine | 27.4 | 29.1c | 21.5c | 14.7 |
| Number of new clients in 2021 | -- | 952 | 392 | 6081 |
| Number of HIV tests performed | 350 | 20 | 360 | 890 |
| Number of clients with opioid use disorder | 117 | -- | 1133 | 3159 |
| Presence of the following services (yes/no): | ||||
| On site HIV testing | Yes | Yes | Yes | Yes |
| On site PrEPd | Yes | No | No | Yes |
| On site medications to treat opioid use disorder (e.g.e.g., methadone, buprenorphine, naltrexone) | Yes | No | Yes | Yes |
| On site syringe exchange | Yes | Yes | No | Yes |
| On site naloxone provision and overdose education | Yes | Yes | Yes | Yes |
| Outreach services | Yes | Yes | Yes | Yes |
| Number of clinicians on site | 7 | 1 | 4 | 23 |
| Number of counselors/staff | 10 | 5 | 25 | 327 |
Other category includes: not asked, prefer not to disclose, questioning/unsure
Other category includes: do not know, more than one race, declined to answer, not asked, Middle Eastern or North African
Ethnic identity question was combined with racial identity question for this site
PrEP: Pre-exposure Prophylaxis
-- indicates that the data was not available
2.5. Inclusion and exclusion criteria
Individuals are eligible for study entry if they: 1) receive or are willing to receive services at one of the participating sites; 2) have a recent HIV negative test without concern for acute HIV;36 3) are ≥18 years old; 4) report injection drug use in the past 6 months; 5) meet PrEP eligibility criteria by reporting either a) sharing of injection or drug preparation equipment; or b) sexual risk behaviors (i.e. condomless sex or STI) in the past 6 months;3,4 6) meet Diagnostic and Statistical Manual (DSM)-5 criteria for OUD; 7) have a cell phone or access to a cell phone of a household member; and 8) provide written informed consent. Individuals are excluded for: 1) being currently prescribed PrEP; 2) self-report or urine testing confirming pregnancy, are breastfeeding, or trying to conceive; 3) any plans that would preclude study completion (e.g. surgery, major medical treatments such as chemotherapy, incarceration, travel out of state/country); 4) inability to provide at least one collateral contact; 5) non-English speaking (for sites without Spanish-speaking staff); or 6) have kidney disease (as PrEP is contraindicated). While the CDC’s 2021 PrEP guidelines37 highlight the safety of PrEP among pregnant individuals and on the developing fetus, previous guidelines noted the lack of available data on fetal safety and most trials discontinued PrEP use upon discovery of pregnancy. As this study and intervention were developed prior to the release of the 2021 guidelines, we established and maintain pregnancy, intent to become pregnant, and breastfeeding as exclusion criteria.
2.6. Recruitment and randomization
Participants are recruited with a multi-pronged recruitment approach involving: 1) recruitment flyers and self-referral; 2) front-line staff and peer referral and 3) research team outreach. Potentially eligible individuals are asked for verbal consent to complete a brief screener to determine whether they have injected drugs and are PrEP eligible by reporting either a) sharing of injection or drug preparation equipment or b) engaging in sexual risk behaviors in the past 6 months.4 Individuals meeting inclusion/exclusion criteria and providing written informed consent to participate will complete baseline assessments and are randomized 1:1 to either CoMPASS or TAU, stratified by site. To ensure concealment of intervention allocation, a random permuted block sequence has been generated and intervention assignments distributed through Research Electronic Data Capture (REDCap).38,39 Randomized participants receive $50 gift cards upon completion of each of the baseline, week 12, and week 24 assessments.
2.7. Intervention overview
2.7.1. Treatment as Usual (TAU)
Participants randomized to TAU receive a health handout with information on how to access PrEP, MOUD and harm reduction services with options for where such services may be obtained. As none of the study sites are otherwise actively engaging PWID for PrEP receipt, this is in addition to current standard practices at each of the participating sites. All participants may be referred to any additional services as deemed appropriate based on interactions they have with site clinicians and staff; such treatment services are catalogued by self-report as part of the follow-up assessments.
2.7.2. CoMPASS
CoMPASS involves an introductory session with provision of a health handout followed by nine sessions of prize-based CM over 12 weeks to encourage participation in HIV prevention interventions in a flexible manner to promote progress towards engagement in HIV prevention-related care. Participants who do not have evidence of biomarker confirmed PrEP adherence by week 12 (non-responders) will be stepped up to five sessions of PASS.
2.7.2.1. Contingency management
After the introductory session, CM visits are designed to occur weekly for the first 6 weeks and then every other week until week 12 for a total of 9 sessions during which participants are eligible to receive rewards. Since CM interventions are most effective when they involve frequent monitoring, we designed the CM intervention frequency based on the goals of optimizing the balance between: 1) need for regular monitoring to support behavior change; 2) expected timeline during which target behaviors could be reasonably completed; 3) participant burden (e.g., aligning an individual’s visit frequency with that of their routine utilization of services); and 4) organizational resources. Rewards are earned upon verified completion of the planned activities for the week (e.g., attending an appointment with a PrEP prescriber, pick up PrEP prescription) through “draws” of paper slips from a fishbowl with preset probabilities of rewards (Table 2). Rewards are tailored to the preferences of the community-based programs, such as gift cards to local stores and personal care items.8
Table 2.
Fishbowl Rewards for Contingency Managementa
| Reward | Number of Slips | Value |
|---|---|---|
| Non-monetary affirmation (“good job!”) | 250 | $0 |
| Small prize | 109 | $1 |
| Medium prize | 80 | $5 |
| Large prize | 60 | $20 |
| Jumbo prize | 1 | $100 |
| Total | 500 | --- |
Slips are replaced between patients so that fishbowl probabilities stay constant over time.
The multi-target nature of the CM intervention allows for promotion of progression in the two domains of PrEP and MOUD engagement to best support stability over time, while the individualized and flexible approach allows a high level of tailoring to individual patient needs and ability. The reinforcement schedules for each target behavior are independent, promoting participants’ access to reinforcers even if progress is limited to one of the two domains (Tables 3–4). The schedules escalate over time if targeted behaviors are consistently met. A weekly bonus is provided when both activities are completed and verified in a given week. When a target behavior is not completed and verified, zero draws are awarded and the draw schedule resets.
Table 3.
Contingency Management and stepped-up to Pre-exposure Prophylaxis (PrEP) Adherence and Support Services (CoMPASS) Trial: Summary of Assessments and Schedule
| Assessment | Screening | Baseline | Week 12 | Week 24 |
|---|---|---|---|---|
| Demographics | X | X | ||
| HIV Status | X | X | X | |
| HIV Symptom Index | X | X | X | |
| PrEP Eligibility4 | X | |||
| Mini-SCID for Opioid Use Disorder62 | X | |||
| Medical/Psychiatric Comorbidities63* | X | X | X | |
| Housing Status | X | X | X | |
| Criminal Justice Involvement | X | X | X | |
| PrEP Adherence17 | X | X | ||
| PrEP for Health Measures | X | X | X | |
| CTN HIV Risk Behavior Scale64 | X | X | X | |
| Substance Use65,66 | X | X | X | |
| ASSIST-Lite67 | X | X | X | |
| Overdose History and Risk68,69 | X | X | X | |
| Treatment Services Review and Health Services Utilization51* | X | X | X | |
| Treatment Effectiveness Assessment70,71 | X | X | X | |
| Marijuana Assessment | X | X | X | |
| Medical Mistrust and Discrimination72–74* | X | X | X | |
| Transportation Insecurity Index75 | X | X | X | |
| Household Food Insecurity Access Scale76 | X | X | X | |
| PROMIS PROPr52,53 | X | X | X |
PrEP: Pre-exposure Prophylaxis; CTN: Clinical Trials Network; ASSIST: Alcohol, Smoking and Substance Involvement Screening Test; NIDA: National Institute on Drug Abuse; PROMIS: Patient-Report Outcomes Measurement Information System; PROPr: Preference; SCID: Structured Clinical Interview for DSM (Diagnostic and Statistical Manual of Mental Disorders)-5
Modified
Table 4.
Contingency Management Reinforcement Schedule Overviewa
| Metric | |||
|---|---|---|---|
| PrEPb,c | MOUD and Other OUD-related Careb,d | Bonus | |
| Purpose | PrEP initiation and consistent PrEP adherence | Engagement in MOUD and OUD-related care | Reward achievement of both targets given independent health benefits |
| Visits potentially rewarded | Follow-up visits 1-9 | Follow-up visits 1-9 | Follow-up visits 1-9 |
| Examples of potentially rewarded activities | Picked up a PrEP prescription at the pharmacy | Attended narcotics anonymous group meeting | |
| Initial reward | 3 draws | 3 draws | 6 draws |
| Potential increase between visits | 1 draw | 1 draw | N/A |
| Maximum reward (cap) | 8 draws | 8 draws | N/A |
| Total potential rewards if consistently meet target | 57 | 57 | 54 |
PrEP and MOUD activities are reinforced on independent schedules, such that failure to complete the planned MOUD activity does not impact draws for the PrEP planned activity, and vice versa. The bonus is only earned if the participant provides verified evidence of completing both activities in a given week (i.e., completed both the PrEP activity and the MOUD activity as listed on the contract).
PrEP: Pre-exposure Prophylaxis; MOUD: Medications for Opioid Use Disorder; OUD: Opioid Use Disorder; N/A: Not applicable
If a participant reports no progress towards PrEP initiation or has initiated PrEP, but is non-adherent, no draws will be awarded for that session and the number of draws will reset to the initial value of 3 for the next demonstration of verified activity completion.
If a participant reports no progress towards MOUD or other OUD-related care, no draws will be awarded for that session and the number of draws will reset to the initial value of 3 for the next demonstration of verified activity completion.
Over the course of the nine sessions, participants may earn up to 168 draws equivalent with an average maximum earning of $608.58 The CM counseling is designed to be approximately 20 minutes in duration, manual-guided, and provided by trained PrEP navigators.59
Rewarding PrEP.
The ultimate goal is to move the participant quickly toward high levels of PrEP initiation and adherence (i.e., missing fewer than 3 doses of PrEP in the past week by self-report40,41) with confirmation by point-of-care urine testing for presence of tenofovir metabolites.42 We used these thresholds given high levels of PrEP adherence are particularly important for HIV prevention among PWID43,44 and biomarker testing is an objective and routine method to validate self-reported adherence.
Rewarding engagement in MOUD and other OUD-related care.
Participants are also independently rewarded for objectively verified progress in engaging in OUD-related care, with tailored sessions to the individual with a focus on promoting MOUD (e.g., attending intensive outpatient programs, Opioid Treatment Program intake, pharmacy fill of buprenorphine/naloxone, urine toxicology screen negative for opioids).
2.7.2.2. Determining response for stepped care
Our intention is to have a low threshold to offer additional support when participants do not have evidence of high levels of PrEP adherence.28 Participants who either: 1) did not present for the final CM visit by week 14; 2) have not initiated PrEP by week 12; 3) report ≥3 missed doses of PrEP in the past week;17,40 4) report <3 missed doses of PrEP in the past week but decline point-of-care urine testing; or 5) report <3 missed doses of PrEP in the past week and agrees to point-of-care urine testing but no tenofovir metabolites are detected,42 will be stepped up to PASS (Figure 2).
Figure 2.
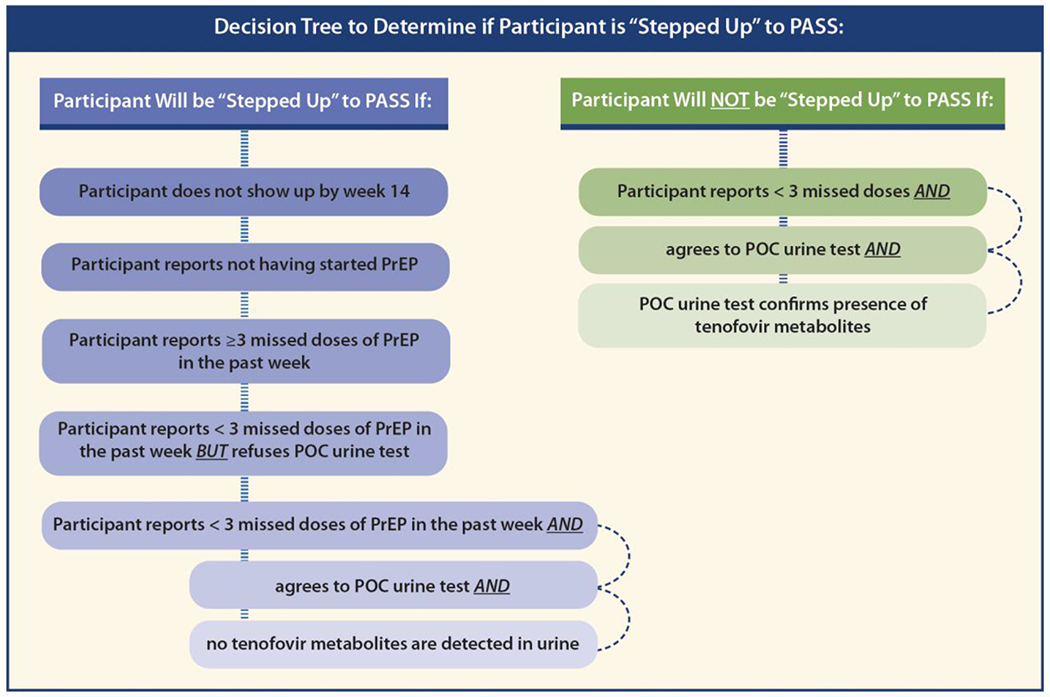
Decision tree to determine if participant is stepped up to Pre-Exposure Prophylaxis (PrEP) Adherence and Support Services (PASS)
POC: Point of Care
2.7.2.3. PrEP Adherence Support Services (PASS)
PASS sessions are informed by the AntiRetroviral Treatment and Access to Service (ARTAS)12 intervention, which demonstrated that 5-session community-based strengths-based case management, informed by Social Cognitive Theory, was successful in linking patients recently diagnosed with HIV to care.12 We integrated ARTAS12 content to be relevant to PrEP using the Project Inform PrEP navigation manual,45 which has been endorsed by the CDC and AIDS Education Training Centers. The core elements of these sessions include: 1) strengthening an effective, working relationship between the PrEP Navigator and the participant; 2) ) assessing the participant’s strengths and encouraging them to use their skills to engage in PrEP and OUD-related care; 3) facilitating the participant’s ability to identify and pursue their own goals and develop a step-by-step plan to accomplish these goals by completing a PASS action plan; and 4) maintaining a participant-driven approach by conducting up to five structured sessions of active, community-based case management.46 Grounded in motivational interviewing principles, these sessions are designed to enhance participant readiness to engage in PrEP and achieve other goals.47 Sessions conclude with completion of the PASS action plan.
2.7.4. Intervention training and monitoring
Designated as “PrEP Navigators,” existing staff (i.e., harm reduction specialist, drug counselor, PrEP navigator) at each site are trained to deliver the intervention. Training in our manualized intervention was led by experts in CM, motivational interviewing, harm reduction, PrEP navigation, and internists certified in HIV and/or Addiction Medicine. Initial training consisted of six hours of content delivered virtually in two sessions with follow-up monthly videoconference and a booster training on the PASS intervention components as the first participants approached 12-week follow-up. Content included CM, motivational interviewing principles, HIV risk reduction with a focus on PrEP as well as MOUD and SSP, harm reduction, and stigma reduction strategies for working with PWID. Facilitated by structured visit forms, the PrEP navigators are trained to monitor and track target behaviors and implement the reward schedule. Following the start of CoMPASS enrollment, subject matter experts on the research team began rating audio recordings of sessions using an adapted CM Competence Scale48 and a corresponding scale developed to assess fidelity to PASS components and provide ongoing feedback.
2.8. Data collection protocol
Assessments are collected by research coordinators at baseline, week 12, and week 24, with participant interview and objective measures to assess PrEP adherence (Table 3). Additionally, at month 12, PrEP adherence will be assessed by pharmacy fill/refill data and incident STI and HIV infections will be assessed by surveillance data. These assessments are designed to ensure the participant meets eligibility criteria, assess important predictor variables, and study outcomes. The primary study outcome is sustained PrEP adherence at 24 weeks assessed by dried blood spot (DBS) testing for tenofovir metabolites processed by Molecular Testing Labs.49 For participants not reporting taking PrEP, levels will be assumed to be zero and not confirmed by biomarker testing. Secondary study outcomes, assessed at 12 and 24 weeks, include biomarker-confirmed PrEP adherence by DBS and urine (week 12 only) testing; HIV risk behaviors; engagement in OUD-related care (SSP, MOUD) and opioid use; and exploratory STI and HIV acquisition at 52 weeks. Process outcomes include participation in CM intervention visits, activities completed and rewards earned, proportion who are stepped-up to PASS, participation of PASS visits, and time to initial PrEP fill.
Baseline assessments will facilitate exploratory and hypothesis generating analyses to examine whether the intervention’s effectiveness differs based on baseline sociodemographic and clinical characteristics. The HIV Symptom Index50 will assess bothersome symptoms and study-related and study-unrelated adverse events are recorded. For treatment services, we are tracking meetings with PrEP navigators, substance use and harm-reduction services, and medical services.51 To facilitate future economic analyses, we are measuring health-related quality-of-life using the Patient-Report Outcomes Measurement Information System (PROMIS)-Preference score (PROPr)52,53 and rewards earned from CM. To assess cost, we will track PrEP navigator time associated with delivering the intervention, costs of PrEP adherence monitoring (i.e., urine point-of-care test), and rewards earned per CM session.
2.9. Statistical considerations
2.9.1. Justification of sample size
The primary aim of this study is to compare the effectiveness of CoMPASS versus TAU on sustained PrEP adherence by DBS testing at 14 weeks. Based on prior research54–56 and the vulnerability of the population under study, we hypothesize that 5% of participants randomized to TAU will demonstrate sustained PrEP adherence at 24 weeks. We believe that a 10% absolute increase (5% versus 15%) in sustained PrEP adherence represents a clinically meaningful improvement with CoMPASS over TAU and is consistent with treatment effects observed in studies evaluating the impact of CM.10,11,20 With 90% power and a two-sided type I error rate of 5% using a two-sample test of proportions, we will need 368 total participants (184 per group) to detect the 10% absolute change. To account for participant attrition, we inflated our sample size by 30% and thus aim to recruit a total of 526 participants (263 per arm).
For secondary outcomes, we assume a conservative type I error rate of 1% to account for multiple testing. For self-reported adherence, if we assume a rate in the control group ranging from 5% to 10%, we will have at least 90% power to detect an absolute increase ranging from 14% to 16%, and at least 80% power to detect an absolute increase ranging from 12% to 14%. For HIV risk behavior, extra-medical opioid use, and engagement in OUD-related care, we anticipate being similarly powered. The sample size calculation was performed with PASS 2019 (Kaysville, Utah).
2.9.2. Statistical analyses
Primary outcome.
The primary aim of this analysis is to determine if the proportion of individuals with sustained PrEP adherence differs at week 24 among those randomized to CoMPASS versus TAU. The primary outcome of sustained PrEP adherence is binary and will be modeled using generalized linear mixed models with a logit link with a random intercept adjusting for site. A contrast statement will be used to conduct the 24-week comparison. The use of mixed models allows all participants (even if they do not have the primary endpoint) to contribute information to the final model. Analyses will be conducted using intent-to-treat principles such that all randomized participants will be included in the denominator for calculating the proportion with sustained PrEP adherence except for unavoidable loss to follow-up (i.e., participants known to have died). Since mixed models assume the data are missing at random, we will also conduct sensitivity analyses (assuming those lost are all non-adherent, adherent, or a mixture). We will use multiple imputation techniques for missing data with sensitivity analyses using pattern-mixture and selection models to examine the robustness of the conclusions of the primary analysis to missing data. Additional exploratory and sensitivity analyses will be conducted, including per-protocol analyses and weighting based on adherence. In addition, if any baseline differences are found between treatment arms, we will conduct a sensitivity analysis employing covariate adjustment. We will set statistical significance at p<0.05 and use two-sided tests.
Secondary and exploratory outcomes.
For binary secondary outcomes of interest (e.g., self-reported adherence, extra-medical opioid use, HIV risk behaviors), we will similarly use generalized linear mixed models with a logit link with a random intercept adjusting for site. For continuous outcomes (e.g., tenofovir metabolite levels), we will use a linear mixed model with a random intercept adjusting for site. Methods for multiple comparisons (i.e., Bonferroni correction, false discovery) will be used to adjust for multiple comparisons in the secondary outcomes. We will set statistical significance at p<0.01 and use two-sided tests.
Exploratory analyses will be conducted for STI and HIV incidence by state health department surveillance data and PrEP persistence by pharmacy data will be assessed using a generalized mixed model with month 12 contrast. To examine whether treatment effects vary among subgroups, we will conduct exploratory and hypothesis-generating analyses. We will study whether treatment effects differ by demographic and clinical characteristics known to be common in this population that impact medication adherence by including analysis of treatment with covariate interactions as well as whether treatment effects differ by recruitment site.
2.10. Implementation-focused process evaluation
Our implementation-focused process evaluation of CoMPASS, grounded in RE-AIM31 and PARiHS18,32 frameworks, will include screening logs; minutes and logs from study team meetings; site visits; as well as data from enrolled participants (e.g., participant satisfaction). We will track any needed protocol modifications guided by the Framework for Reporting Adaptations and Modifications-Enhanced (FRAME).57 These data sources are being complemented by a confidential online REDCap-based CoMPASS Implementation Survey within the first six months of launching the clinical trial as well as post-trial of clinicians, staff, and leadership at each site. We will assess the following domains: 1) demographic and practice characteristics; 2) perspectives and experiences regarding HIV prevention with PrEP; 3) potential barriers to promoting PrEP among PWID; 4) beliefs regarding CM; and 5) organizational readiness to implement and refer to CoMPASS (Appendix). The CoMPASS Implementation Survey was developed based on existing literature58 and validated tools,59 reviewed by our multidisciplinary team, and piloted prior to launch. Differences in pre- and post-trial responses will be assessed using appropriate parametric or non-parametric methods.
To grasp a richer understanding of the experiences and attitudes regarding HIV PrEP and CM, we will conduct a content analysis of open-ended responses in the participant and staff surveys.
2.11. Protection of participants
This HIPAA-compliant study is approved by the Yale School of Medicine Human Investigation Committee, the institutional review board of record for all participating sites. The study is also approved by the Connecticut Department of Public Health and the Massachusetts Department of Public Health. The Data Safety and Monitoring Board reviews study progress, experiences and adverse events, biannually.
2.12. Current status of CoMPASS
After completion of planning meetings, site visits (in-person and virtual as needed due to COVID-19), and trainings, CoMPASS opened for enrollment on October 4, 2021. Recruitment and study implementation has been hindered by the COVID-19 pandemic with decreased client volume at the sites, study staff illness and turnover, but is ongoing. To enhance safety of all individuals involved, we have ensured study procedures focus on minimizing risk of COVID-19 transmission, optimize social distancing, and maintain flexibility given uncertainty with the circumstances and variable access to technology among potential participants.
The web-based survey of site clinicians, staff, and leadership was launched on April 7, 2022, and data collection is complete and analyses underway.
3. Discussion
CoMPASS will evaluate the impact of a novel, multi-targeted CM intervention with stepped care to structured PrEP navigation to promote HIV risk reduction and engagement in OUD care among PWID accessing community-based services. Several aspects make our study innovative. First, it uses CM to promote PrEP with point-of-care testing to assess past-48-hour PrEP adherence to temporally link behaviors and rewards.60 Second, it will be the first time a stepped care design is used to sequentially add PrEP navigation to adaptively promote sustained PrEP adherence among PWID and to incorporate multi-target CM design in a package of HIV prevention services for PWID.61 Third, inclusion of diverse sites will allow for exploratory examination of whether site characteristics impact outcomes. Fourth, by triangulating participant-reported data with pharmacy fill/refill data and health department surveillance data, we will have robust measures of key outcomes. Fifth, consistent with a hybrid type 1 effectiveness-implementation design,16 we will collect data from key stakeholders pre- and post-trial to inform future implementation efforts of CoMPASS. Lastly, the FRAME will guide systematic tracking of any modifications needed to enhance the intervention evaluation given the unpredictable circumstances and its consequences created by the COVID-19 pandemic. Studies focused on promoting PrEP among pregnant women with OUD should be conducted in the future given growing safety data.
3.1. Limitations
First, while the COVID-19 pandemic has improved and the participating study sites are functioning at full capacity, the unpredictable nature of the pandemic may impact future participant recruitment and protocol adherence. Second, this is a non-blinded study, research coordinators and PrEP navigators participating in data collection and CM and PASS delivery will not be blinded to treatment assignment of participants. However, our primary outcome is an objective biomarker-based testing of PrEP adherence. Third, all sites are located in the northeast US potentially limiting generalizability to other settings where resources may be different.
3.2. Conclusion
CoMPASS will generate data on the impact of a novel adaptive intervention employing CM and structured PrEP navigation components to promote linkage to PrEP and OUD-related care to reduce HIV transmission among PWID. Findings generated from this study will be directly relevant for informing delivery of these HIV prevention interventions in a variety of organizational settings.
Supplementary Material
Supplement. CoMPASS Implementation Survey
Highlights.
Few persons who inject drugs (PWID) are taking HIV pre-exposure prophylaxis (PrEP).
Contingency management with stepped care may promote sustained PrEP adherence.
Point-of-care testing may be useful for verifying self-reported PrEP adherence among PWID.
PWID may be reachable for HIV prevention in opioid treatment and harm reduction programs.
Funding sources and role of the funding source:
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award Number R01DA051871 and the Yale Clinical and Translational Science Award UL1TR001863. ML Sung was supported by the Department of Veterans Affairs (VA), Veterans Health Administration, VISN 1 Career Development Award. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or VA. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health and VA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Handanagic S, Finlayson T, Burnett JC, Broz D, Wejnert C. HIV Infection and HIV-Associated Behaviors Among Persons Who Inject Drugs - 23 Metropolitan Statistical Areas, United States, 2018. MMWR Morb Mortal Wkly Rep. 2021;70(42):1459–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Office of National AIDS Policy. The National HIV/AIDS Strategy: Updated to 2020 the White House; July 2015. 2015. [Google Scholar]
- 3.U. S. Preventive Services Task Force, Owens DK, Davidson KW, et al. Preexposure Prophylaxis for the Prevention of HIV Infection: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321(22):2203–2213. [DOI] [PubMed] [Google Scholar]
- 4.Service UPH. Pre-exposure Prophylaxis for the Prevention of HIV Infection in the United States - 2021 Update: A Clinical Practice Guideline. CDC;2021. [Google Scholar]
- 5.Biello KB, Bazzi AR, Mimiaga MJ, et al. Perspectives on HIV pre-exposure prophylaxis (PrEP) utilization and related intervention needs among people who inject drugs. Harm Reduct J. 2018;15(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fox AD, Sohler NL, Frost T, Lopez C, Cunningham CO. Development and evaluation of a community-based buprenorphine treatment intervention. Harm Reduct J. 2017;14(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oldfield BJ, Munoz N, McGovern MP, et al. Integration of care for HIV and opioid use disorder: a systematic review of interventions in clinical and community-based settings. AIDS. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petry N Contingency Management for Substance Abuse Treatment: A guide to implementing this evidence-based practice New York, New York: Routledge; 2012. [Google Scholar]
- 9.Davis DR, Kurti AN, Skelly JM, Redner R, White TJ, Higgins ST. A review of the literature on contingency management in the treatment of substance use disorders, 2009-2014. Prev Med. 2016;92:36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herrmann ES, Matusiewicz AK, Stitzer ML, Higgins ST, Sigmon SC, Heil SH. Contingency Management Interventions for HIV, Tuberculosis, and Hepatitis Control Among Individuals With Substance Use Disorders: A Systematized Review. J Subst Abuse Treat. 2017;72:117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petry NM, Rash CJ, Byrne S, Ashraf S, White WB. Financial reinforcers for improving medication adherence: findings from a meta-analysis. Am J Med. 2012;125(9):888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gardner LI, Metsch LR, Anderson-Mahoney P, et al. Efficacy of a brief case management intervention to link recently diagnosed HIV-infected persons to care. AIDS. 2005;19(4):423–431. [DOI] [PubMed] [Google Scholar]
- 13.Kushel MB, Colfax G, Ragland K, Heineman A, Palacio H, Bangsberg DR. Case management is associated with improved antiretroviral adherence and CD4+ cell counts in homeless and marginally housed individuals with HIV infection. Clin Infect Dis. 2006;43(2):234–242. [DOI] [PubMed] [Google Scholar]
- 14.Stitzer ML, Hammond AS, Matheson T, et al. Enhancing Patient Navigation with Contingent Incentives to Improve Healthcare Behaviors and Viral Load Suppression of Persons with HIV and Substance Use. AIDS Patient Care STDS. 2018;32(7):288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Services DoHaH. PrEP for HIV Prevention among Substance Using Populations (R01 - Clinical Trial Optional). NIH. https://grants.nih.gov/grants/guide/rfa-files/rfa-da-21-024.html. Published 2020. Accessed. [Google Scholar]
- 16.Curran GM, Bauer M, Mittman B, Pyne JM, Stetler C. Effectiveness-implementation hybrid designs: combining elements of clinical effectiveness and implementation research to enhance public health impact. Med Care. 2012;50(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brooks KM, Anderson PL. Pharmacologic-Based Methods of Adherence Assessment in HIV Prevention. Clin Pharmacol Ther. 2018;104(6):1056–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hagedorn HJ, Stetler CB, Bangerter A, Noorbaloochi S, Stitzer ML, Kivlahan D. An implementation-focused process evaluation of an incentive intervention effectiveness trial in substance use disorders clinics at two Veterans Health Administration medical centers. Addiction science & clinical practice. 2014;9:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Israel BA, Schulz AJ, Parker EA, Becker AB. Review of community-based research: assessing partnership approaches to improve public health. Annu Rev Public Health. 1998;19:173–202. [DOI] [PubMed] [Google Scholar]
- 20.Benishek LA, Dugosh KL, Kirby KC, et al. Prize-based contingency management for the treatment of substance abusers: a meta-analysis. Addiction. 2014;109(9):1426–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NIH Research Portfoli Online Reporting Tools (RePORT). A MULTI-SITE MULTI-SETTING RCT OF INTEGRATED HIV PREVENTION AND HCV CARE FOR PWID.
- 22.NIH Research Portfoli Online Reporting Tools (RePORT). BUILDING ON NEEDLE EXCHANGE TO OPTIMIZE HIV PREVENTION/TREATMENT. [Google Scholar]
- 23.Mizuno Y, Higa DH, Leighton CA, Roland KB, Deluca JB, Koenig LJ. Is HIV patient navigation associated with HIV care continuum outcomes? AIDS. 2018;32(17):2557–2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roland KB, Higa DH, Leighton CA, Mizuno Y, DeLuca JB, Koenig LJ. Client Perspectives and Experiences With HIV Patient Navigation in the United States: A Qualitative Meta-Synthesis. Health Promot Pract. 2019:1524839919875727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelley CF, Kahle E, Siegler A, et al. Applying a PrEP Continuum of Care for Men Who Have Sex With Men in Atlanta, Georgia. Clin Infect Dis. 2015;61(10):1590–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nunn AS, Brinkley-Rubinstein L, Oldenburg CE, et al. Defining the HIV pre-exposure prophylaxis care continuum. AIDS. 2017;31(5):731–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Straten A, Hill J, Richards DA, Cuijpers P. Stepped care treatment delivery for depression: a systematic review and meta-analysis. Psychol Med. 2015;45(2):231–246. [DOI] [PubMed] [Google Scholar]
- 28.Edelman EJ, Maisto SA, Hansen NB, et al. Integrated stepped alcohol treatment for patients with HIV and alcohol use disorder: a randomised controlled trial. Lancet HIV. 2019;6(8):e509–e517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.(RePORT) NRPORT. Consortium to improve OutcoMes in hiv/Aids, Alcohol, Aging, and multi-Substance use (COMpAAAS U01): Intervention Study. NIAAA. https://reporter.nih.gov/search/ASIrDOxO9Eyj6LR5vfCitw/project-details/10003118. Published 2020. Accessed. [Google Scholar]
- 30.Edelman EJ, Dziura J, Deng Y, et al. A SMARTTT approach to Treating Tobacco use disorder in persons with HIV (SMARTTT): Rationale and design for a hybrid type 1 effectiveness-implementation study. Contemp Clin Trials. 2021;110:106379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gaglio B, Shoup JA, Glasgow RE. The RE-AIM framework: a systematic review of use over time. Am J Public Health. 2013;103(6):e38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvey G, Kitson A. PARIHS revisited: from heuristic to integrated framework for the successful implementation of knowledge into practice. Implement Sci. 2016;11(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van den Berg JJ, O’Keefe E, Davidson D, et al. The development and evaluation of an HIV implementation science network in New England: lessons learned. Implement Sci Commun. 2021;2(1):64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massachusetts Deparrtment of Public Health Bureau of Infectious Disease and Laboratory Sciences: 2018 Masschusetts HIV/AIDS Epidemiologic Profile. [Google Scholar]
- 35.Newly diagnosed HIV infection by residence at diagnosis, Connecticut, 2017. https://portal.ct.gov/-/media/Departments-and-Agencies/DPH/AIDS--Chronic-Diseases/Surveillance/statewide/map_hiv_dx.pdf?la=en. Accessed. [Google Scholar]
- 36.Taylor JL, Walley AY, Bazzi AR. Stuck in the window with you: HIV exposure prophylaxis in the highest risk people who inject drugs. Subst Abus. 2019:1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention. HIV Prevention Progress Report, 2019. [Google Scholar]
- 38.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cressey TR, Siriprakaisil O, Klinbuayaem V, et al. A randomized clinical pharmacokinetic trial of Tenofovir in blood, plasma and urine in adults with perfect, moderate and low PrEP adherence: the TARGET study. BMC Infect Dis. 2017;17(1):496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Crane HM, Nance RM, Delaney JA, et al. A Comparison of Adherence Timeframes Using Missed Dose Items and Their Associations with Viral Load in Routine Clinical Care: Is Longer Better? AIDS Behav. 2017;21(2):470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Daughtride GH S; Fischl M; Hashim J; Kahn-Woods E; Kardos K Development and validation of a point-of-care, urine assay to measure adherence to PrEP and ART. Paper presented at: IAS2019; Mexico City, Mexico. [Google Scholar]
- 43.Grant RM, Anderson PL, McMahan V, et al. Uptake of pre-exposure prophylaxis, sexual practices, and HIV incidence in men and transgender women who have sex with men: a cohort study. Lancet Infect Dis. 2014;14(9):820–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Martin M, Vanichseni S, Suntharasamai P, et al. The impact of adherence to preexposure prophylaxis on the risk of HIV infection among people who inject drugs. AIDS. 2015;29(7):819– 824. [DOI] [PubMed] [Google Scholar]
- 45.Inform Project. Helping people access pre-exposure prophylaxis. [Google Scholar]
- 46.Centers for Disease Control and Prevention. ARTAS Core Elements and Fact Sheet [Google Scholar]
- 47.Miller WaR S Motivational interviewing: Helping people change New York: Guilford Press; 2013. [Google Scholar]
- 48.Petry NM, Alessi SM, Ledgerwood DM, Sierra S. Psychometric properties of the contingency management competence scale. Drug Alcohol Depend. 2010;109(1-3): 167–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Molecular Testing Labs. https://moleculartestinglabs.com/. Published 2022. Accessed. [Google Scholar]
- 50.Justice AC, Holmes W, Gifford AL, et al. Development and validation of a self-completed HIV symptom index. J Clin Epidemiol. 2001;54 Suppl 1:S77–90. [DOI] [PubMed] [Google Scholar]
- 51.McLellan AT, Alterman AI, Cacciola J, Metzger D, O’Brien CP. A new measure of substance abuse treatment. Initial studies of the treatment services review. J Nerv Ment Dis. 1992;180(2):101–110. [DOI] [PubMed] [Google Scholar]
- 52.Hanmer J, Dewitt B, Yu L, et al. Cross-sectional validation of the PROMIS-Preference scoring system. PloS one. 2018;13(7):e0201093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dewitt B, Feeny D, Fischhoff B, et al. Estimation of a Preference-Based Summary Score for the Patient-Reported Outcomes Measurement Information System: The PROMIS(®)-Preference (PROPr) Scoring System. Medical decision making : an international journal of the Society for Medical Decision Making. 2018;38(6):683–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Doblecki-Lewis S, Butts S, Botero V, Klose K, Cardenas G, Feaster D. A Randomized Study of Passive versus Active PrEP Patient Navigation for a Heterogeneous Population at Risk for HIV in South Florida. J Int Assoc Provid AIDS Care. 2019;18:2325958219848848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stein M, Thurmond P, Bailey G. Willingness to use HIV pre-exposure prophylaxis among opiate users. AIDS Behav. 2014;18(9):1694–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bazzi AR, Biancarelli DL, Childs E, et al. Limited knowledge and mixed interest in pre-exposure prophylaxis for HIV prevention among people who inject drugs. (Special Issue: HIV pre-exposure prophylaxis (PrEP).). AIDS Patient Care and STDs. 2018;32(12):529–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wiltsey Stirman S, Baumann AA, Miller CJ. The FRAME: an expanded framework for reporting adaptations and modifications to evidence-based interventions. Implement Sci. 2019;14( 1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Blackstock OJ, Moore BA, Berkenblit GV, et al. A Cross-Sectional Online Survey of HIV Pre-Exposure Prophylaxis Adoption Among Primary Care Physicians. J Gen Intern Med. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rash CJ, Petry NM, Kirby KC, Martino S, Roll J, Stitzer ML. Identifying provider beliefs related to contingency management adoption using the contingency management beliefs questionnaire. Drug Alcohol Depend. 2012;121(3):205–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gandhi M, Wang G, King R, et al. Development and Validation of the First Point-of-Care Assay to Objectively Monitor Adherence to HIV Treatment and Prevention in Real-Time in Routine Settings. AIDS. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mayer KH, Allan-Blitz LT. PrEP 1.0 and Beyond: Optimizing a Biobehavioral Intervention. J Acquir Immune Defic Syndr. 2019;82 Suppl 2:S113–S117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.First MB, Williams JB, Karg RS, Spitzer RL. Structured clinical interview for DSM-5—Research version (SCID-5 for DSM-5, research version; SCID-5-RV). Arlington, VA: American Psychiatric Association. 2015:1–94. [Google Scholar]
- 63.Sangha O, Stucki G, Liang MH, Fossel AH, Katz JN. The Self-Administered Comorbidity Questionnaire: a new method to assess comorbidity for clinical and health services research. Arthritis Rheum. 2003;49(2):156–163. [DOI] [PubMed] [Google Scholar]
- 64.Darke S, Hall W, Heather N, Ward J, Wodak A. The reliability and validity of a scale to measure HIV risk-taking behaviour among intravenous drug users. Aids. 1991;5(2):181–185. [DOI] [PubMed] [Google Scholar]
- 65.Weatherby NL, Needle R, Cesari H, et al. Validity of self-reported drug use among injection drug users and crack cocaine users recruited through street outreach. Evaluation and Program Planning. 1994;17(4):347–355. [Google Scholar]
- 66.Needle R, Fisher DG, Weatherby N, et al. Reliability of self-reported HIV risk behaviors of drug users. Psychology of Addictive Behaviors. 1995;9(4):242–250. [Google Scholar]
- 67.Ali R, Meena S, Eastwood B, Richards I, Marsden J. Ultra-rapid screening for substance-use disorders: the Alcohol, Smoking and Substance Involvement Screening Test (ASSIST-Lite). Drug Alcohol Depend. 2013;132(1-2):352–361. [DOI] [PubMed] [Google Scholar]
- 68.Pouget ER, Bennett AS, Elliott L, et al. Development of an opioid-related Overdose Risk Behavior Scale (ORBS). SubstAbus. 2017;38(3):239–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pouget ER, Bennett AS, Elliott L, Rosenblum A, Britton PC. Recent Overdose Experiences in a Community Sample of Military Veterans Who Use Opioids. J Drug Issues. 2017;47(3):479–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ling W, Nadipelli VR, Solem CT, et al. Measuring recovery in opioid use disorder: clinical utility and psychometric properties of the Treatment Effectiveness Assessment. Subst Abuse Rehabil. 2019;10:13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ling W, Farabee D, Liepa D, Wu L-T. The Treatment Effectiveness Assessment (TEA): an efficient, patient-centered instrument for evaluating progress in recovery from addiction. Subst Abuse Rehabil. 2012;3:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Thompson HS, Valdimarsdottir HB, Winkel G, Jandorf L, Redd W. The Group-Based Medical Mistrust Scale: psychometric properties and association with breast cancer screening. Prev Med. 2004;38(2):209–218. [DOI] [PubMed] [Google Scholar]
- 73.Schneider J, Kaplan SH, Greenfield S, Li W, Wilson IB. Better physician-patient relationships are associated with higher reported adherence to antiretroviral therapy in patients with HIV infection. J Gen Intern Med. 2004;19(11):1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Brincks AM, Shiu-Yee K, Metsch LR, et al. Physician Mistrust, Medical System Mistrust, and Perceived Discrimination: Associations with HIV Care Engagement and Viral Load. AIDS Behav. 2019;23(10):2859–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gould-Werth A, Griffin J, Murphy AK. Developing a New Measure of Transportation Insecurity: An Exploratory Factor Analysis. Survey Practice. 2018;11(2):1–28. [Google Scholar]
- 76.Coates J, Swindale A, Bilinsky P. Household Food Insecurity Access Scale (HFIAS) for measurement of food access: indicator guide: version 3. 2007. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement. CoMPASS Implementation Survey


