Abstract
Pearl of Csaba (PC) is a valuable backbone parent for early-ripening grapevine (Vitis vinifera) breeding, from which many excellent early ripening varieties have been bred. However, the genetic basis of the stable inheritance of its early ripening trait remains largely unknown. Here, the pedigree, consisting of 40 varieties derived from PC, was re-sequenced for an average depth of ∼30×. Combined with the resequencing data of 24 other late-ripening varieties, 5,795,881 high-quality single nucleotide polymorphisms (SNPs) were identified following a strict filtering pipeline. The population genetic analysis showed that these varieties could be distinguished clearly, and the pedigree was characterized by lower nucleotide diversity and stronger linkage disequilibrium than the non-pedigree varieties. The conserved haplotypes (CHs) transmitted in the pedigree were obtained via identity-by-descent analysis. Subsequently, the key genomic segments were identified based on the combination analysis of haplotypes, selective signatures, known ripening-related quantitative trait loci (QTLs), and transcriptomic data. The results demonstrated that varieties with a superior haplotype, H1, significantly (one-way ANOVA, P < 0.001) exhibited early grapevine berry development. Further analyses indicated that H1 encompassed VIT_16s0039g00720 encoding a folate/biopterin transporter protein (VvFBT) with a missense mutation. VvFBT was specifically and highly expressed during grapevine berry development, particularly at veraison. Exogenous folate treatment advanced the veraison of “Kyoho”. This work uncovered core haplotypes and genomic segments related to the early ripening trait of PC and provided an important reference for the molecular breeding of early-ripening grapevine varieties.
Introduction
Grapevine (Vitis vinifera L.) is one of the most widely cultivated fruit crops, which is used as wine, table, juice, and raisins (Massonnet et al., 2017). Berry ripening is an important agronomic trait for grapevine. However, current widely cultivated grapevine varieties are medium- or late-ripening, and the available early-ripening grapevine varieties with excellent berry quality are very few. The lack of early-ripening varieties resulted in a less flexible grapevine supplying marketing period and limited the economic benefits of the planter. Therefore, the exploration of key genes controlling berry ripening is very important for the improvement of grapevine.
Grapevine berry development and ripening is a quantitative trait controlled by multiple genes involving complex physiological and metabolic processes. For example, plant hormones (e.g. abscisic acid, cytokinin, ethylene) (Kuhn et al., 2014), the activity of transcription factors (e.g. MYB: myeloblastosis, NAC: NAM, ATAF1,2, CUC2), and DNA methylation (Dunlevy et al., 2010; Shangguan et al., 2020) could regulate berry size, skin color, berry texture, sugar, acidity, and aroma. Several large-scale and high-resolution transcriptomic studies have been applied to uncover abundant genes that marked the developmental shift from vegetative to reproductive growth (Fasoli et al., 2012; Palumbo et al., 2014), involved in anthocyanin synthesis during berry ripening (Massonnet et al., 2017), and activated berry ripening (Fasoli et al., 2018). These studies have shown that the basic biochemical and molecular trends occurred during grapevine berry ripening, which improved our understanding of grapevine berry ripening. However, the genetic knowledge of early-ripening traits is still unclear.
Quantitative trait locus (QTL) mapping and genome-wide association studies (GWAS) were employed to determine the genetic architecture of grapevine berry ripening (Fechter et al., 2014; Zhao et al., 2016; Guo et al., 2019; Flutre et al., 2022). However, the span of chromosomal regions detected by QTL is often too long due to the limited recombination events (Ott et al., 2011), and the GWAS are sensitive to population structure, leading to both false positive and false negative results (Brachi et al., 2011). Recently, pedigree consisting of backbone parents with superior genotypes and their derived lines have been popularly used to identify genetic loci controlling quantitative traits, which have been successful in identifying genes controlling high-yield in rice (Huang et al., 2018) and maize (Li et al., 2019), early maturity (Li et al., 2021), lint yield, and fiber quality (Lu et al., 2019; Ma et al., 2019; Han et al., 2020) in cotton. A pedigree with one or more fixed phenotypes, high heritability, and recombination events of many generations, could be used more efficiently to target the candidate genes controlling agronomic traits than QTL and GWAS analysis.
“Pearl of Csaba” (PC), a backbone parent for early-ripening grapevine breeding, was developed by crossing the “Muskatellier d’ Hongrie” with “Muscat Ottonel” in 1904, and the 90% varieties selected and bred from PC or PC lines demonstrate the early-ripening trait (Fan et al., 2010). Therefore, we assumed that the excellent early-ripening trait of PC and its lines could be stably passed on to the offspring. In this study, the genomes were re-sequenced with an average depth of ∼30× for 40 varieties from the pedigree of PC to make it possible to follow the transmission of variants with the early-ripening trait and combined previously published data of 24 late-ripening varieties (whole-genome sequences; ∼44×). Using this comprehensive data set, patterns of genome-wide single nucleotide polymorphism (SNP) of all varieties were explored, population genetics of pedigree were characterized, and core haplotypes and genomic segments with fixed natural allelic variation related to the early-ripening trait in PC lines were identified.
Results
Early ripening transmission in the pedigree of “PC”
“PC”, a very early-ripening grapevine variety, is the founder of the pedigree extended to four generations. A rich variety of early-ripening table grapevines had been further developed by crossing the descendants of PC with several medium- and late-ripening varieties. Based on the five distinct stages of the berry development period (BDP): the very early ripening (≤60d), early ripening (61∼80d), medium-ripening (81∼100d), late-ripening (101∼120d), and very late-ripening (≥121d), proposed by Liu et al. (2004), we investigated the BDP of a total of 40 varieties cultivated in Zhengzhou, including PC, 6 hybrid parents, and 33 descendants, and found that 90% of the descendants (30/33) showed early or very early-ripening, only 3 was medium-ripening, and none late-ripening in the pedigree (to easily describe, BDP ≤80 days and >80 days are defined as early-ripening and late-ripening, respectively, in Figure 1; Supplemental Table S1). Although several late-ripening (“Elizabeth”, “Thompsons seedless”, and “Italian”) and medium-ripening (“Muscat Hamburg” and “Hongxiangjiao”) varieties were employed as hybrid parents for the breeding of early-ripening varieties, the descendants still showed early-ripening trait with short BDP (Figure 1; Supplemental Table S1). All of this demonstrated that early ripening was the major breeding target, and it could be highly inherited in this pedigree.
Figure 1.
The four-generation pedigree chart of the “Pearl of Csaba”. The circle, box, and text with background color indicate the founder, descendants, and hybrid parents in pedigree, respectively. Different berry ripening traits are represented by different colors, and varieties with character background colors are sequenced in this study.
To reveal the genetic basis of early ripening transmission in the pedigree, the 40 varieties of the pedigree were sequenced with an average depth of ∼30× (25–36×) and average genome coverage of 90.06%. In order to better explore the population genetic characteristics of pedigree and enhance the resolution of identifying genes related to early ripening under the selection, the high-quality resequencing data with an average depth of 44× (24–60×; Supplemental Table S2) from 5 medium-ripening and 19 late-ripening grapevines were collected as the control. A total of 5,795,881 high-quality SNP was identified from the 64 varieties following a strict filtering pipeline (Materials and Methods), 90.94% of which SNP was consistent with the reported variant loci detected by large-scale grapevine resequencing (Liang et al., 2019). In addition, two selected missense SNPs in genes from seven varieties were examined with Sanger sequencing and verified the validation rate of 100% (Supplemental Table S3), which further supported the confidence in the accuracy of SNPs. Finally, the 64 samples were divided into two groups according to their BDP and genetic background: (1) the early ripening pedigree group (hereafter early pedigree, n = 32) consisting of PC and its 31 derived very early or early ripening varieties, (2) the late-ripening cultivar group (hereafter late-cultivar, n = 32) consisting of 5 late-ripening hybrid parents, and (3) medium-ripening varieties from the pedigree of PC, and 24 non-pedigree medium and late-ripening varieties, which are used for downstream analyses (Supplemental Figure S1; Supplemental Table S1).
Population genetic characteristics in groups of early-pedigree and late-cultivar
To examine population structure, principal component analysis (PCA), phylogenetic tree, and STRUCTURE were applied. The first two principal components (PCs), PC1 and PC2 explained 11.03% and 9.84% of total genetic variance, respectively. Considering both PC1 and PC2, the groups of early-pedigree and late-cultivar could be well distinguished except for the mixture of several varieties (Figure 2A). The neighbor-joining (NJ) tree further explained that the mixing of varieties is mainly due to their breeding lineage. For instance, “Muscat of Alexandria” is one of the hybrid parents of “Muscat Hamburg”, and varieties with the genetic material of Vitis labrusca, such as “Catawba”, “Tamnara”, “Tano Red”, “Chunguang”, “Miguang”, “Zaoheibao”, “Hongbiaowuhe”, and “Wuhezaohong” are clustered together (Figure 2B; Supplemental Table S1). Finally, the STRUCTURE analysis revealed a clearly distinguishable genomic composition between the groups of early-pedigree and late-cultivar of genetic clusters K from 2 to 8 (Supplemental Figure S2A), also identifying admixed individuals and supporting the scenery of PCA and NJ tree.
Figure 2.
Population structure of early-pedigree and late-cultivar samples. A, PCA plot among all varieties of early-pedigree (red) and late-cultivar (blue). B, Unrooted NJ tree across all samples, the unit of tree scale is P distance. C, Genome-wide averaged distance of LD decayed to r2 = 0.2 for the groups of early-pedigree (red), late-cultivar (blue), and all varieties (black). Comparison of levels of D, heterozygosity, E, nucleotide diversity, and F, Hardy–Weinberg equilibrium between the early-pedigree and the late-cultivar. The elements of boxplot are the center line, median; box limits, upper and lower quartiles; whiskers, 1.5 × interquartile range; points, outliers. In heterozygosity, the four outliers in the early group are “Feicui Rose”, “Wuhezaohong”, “Hongbiaowuhe”, and “Chunguang” from low to high; similarly, the five outliers in the late group are “Neijingxiang”, “Tamnara”, “Tano Red”, “Miguang”, and “Catawba”. All outliers belong to Vitis vinifera × Vitis labrusca.
To further understand the population genetic characteristics in the pedigree of PC, we estimated population genetic parameters based on both early-pedigree (n = 32) and late-cultivar (n = 32). Linkage disequilibrium (LD) showed that as r2 dropped to 0.2, the physical distance of early-pedigree was 63 kb longer than that of the late-cultivar at 19 kb (Figure 2C). The early-pedigree group with lower heterozygosity (Hetearly = 0.01564 ± 0.0035; Hetlate = 0.01646 ± 0.0037; Supplemental Table S4), and harbored lower nucleotide diversity (π) than late-cultivar across all sites (mean πearly = 0.01705, mean πlate = 0.01935; Figure 2, D and E). This pattern also occurred in Watterson's θ (Supplemental Figure S2B). Tajima's D value of early-pedigree (D = 0.4550 ± 0.8369) was higher than the late-cultivar (D = −0.07764 ± 0.6371; Supplemental Figure S2C). More SNP loci that significantly break Hardy–Weinberg Equilibrium (HWE) in the early-pedigree group (with 315,787 sites and P-value = 0.0020 ± 0.0025) are observed when compared to the late-cultivar group (with 121,431 sites and P-value = 0.0033 ± 0.0031; Figure 2F). The HWE test, lower π value, and higher LD than late-cultivar indicated that strong artificial selection and nonrandom mating process occurred in the early-pedigree of PC.
Identification of haplotypes inherited from the “PC” to their descendants
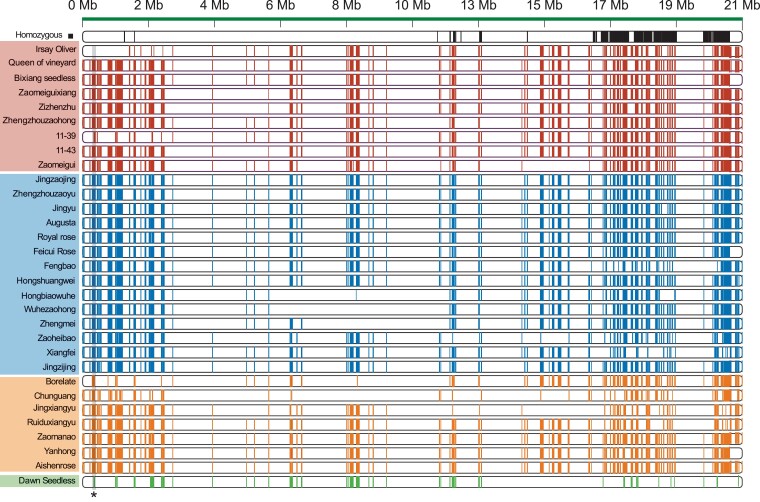
With the pedigree information, haplotypes of PC being passed on to the descendants could be detected (Materials and Methods). In total, 115,467 haplotypes (409.93Mb, 84.30% of genome) of Hap1 and 115,409 haplotypes (412.43 Mb, 84.82% of genome) of Hap2 were shared between the descendants and PC. There were 10,375 haplotypes (62.73 Mb, 12.90% of genome) of Hap1 and 10,552 haplotypes (63.39 Mb, 13.03% of genome) of Hap2 shared in more than 80% (26/33) descendants (Supplemental Table S5). However, 1,463 (31.52 Mb) haplotypes in both Hap1 and Hap2 are derived from the homozygous haplotype, and only 2,232 (31.21 Mb) and 2,297 (31.85 Mb) haplotypes originate from Hap1 and Hap2, respectively (Supplemental Figure S3A). Finally, a total of 5,992 (94.59 Mb) haplotypes encompassing 7,818 genes are retained and further defined as conserved haplotypes (CHs) (Supplemental Figure S3B; Supplemental Table S6); Figure 3 demonstrates the distribution of CHs in chromosome 16. The average length of CHs is around 15 Kb, which is four times the average length of all haplotypes (3.5 Kb) that can be passed on to PC descendants, whereas CHs possess a higher gene density (82.65 genes per Mb) compared with the whole genome (61.67 genes per Mb).
Figure 3.
Transmission pattern of CHs in the “Pearl of Csaba” breeding. The top is the distribution of homozygous haplotypes on chromosomes in the “Pearl of Csaba” and from the top to the bottom is the flow of the pedigree. Different colors represent different generations: F1 (red), F2 (blue), F3 (yellow), and F4 (green). A shadow and asterisks mark chr16 positions of interest (i.e. shown in Figure 5A). The transmission pattern of chromosome 16 is shown.
The genes of CHs were significantly overrepresented (adjusted P < 0.05) in molecular functions focused on nitrilase activity (GO:0000257, GO:0080061, GO:0080109, and GO:0018822) and methyltransferase activity (GO:0030761, GO:0008171, GO:0008757, and GO:0008168), which further involved in the basic biological characteristics of grapevine, such as flavonoid metabolic process (GO:0009812), regulation of anthocyanin biosynthetic process (GO:0031540), and isoflavonoid biosynthesis pathway (ko00943) (Supplemental Table S7). To investigate whether the genes of CHs participated in the berry development process, we examined their expression at the three stages of berry development (pre-veraison, veraison, and harvest-ripe) in two early ripening descendants of PC, “Zaomeiguixiang” and “Xiangfei”, by RNA-seq (Sun et al., 2019). There were 76.34% (5,968/7,818) genes transcribed at both “Zaomeiguixiang” and “Xiangfei” that were considered to be related to the berry development process, of which 47 and 11 differentially expressed genes (DEGs, adjusted P < 0.01 and |log2FoldChange| > 1) were present in the comparison of pre-veraison to veraison and veraison to harvest-ripe, respectively. Functional annotation of DEGs revealed that they were predominantly related to berry ripening including heat shock protein, cytochrome P450, E3 ubiquitin–protein ligase, pectinesterase, glutathione S-transferase, and so on (Supplemental Figure S4).
Haplotypes with selective signatures overlaying a group of genes related to early-ripening traits
To identify the molecular basis of early-ripening trait improvement of PC lines, we combined methods of πlate/πearly, FST, and XP-CLR to detect genomic selection signatures using the late-cultivar group as a reference. 1,208, 1,208, and 274 nonoverlapping windows (20 kb) within the top 5% were detected for πlate/πearly, FST, and XP-CLR, respectively. We consider the windows that are shared by all or any two πlate/πearly, FST, and XP-CLR as the selective signatures (Figure 4, B–D). Finally, a total of 6.80 Mb of 229 selected genomic regions located on multiple chromosomes (chr:1–7, 9–13, 15–19, and Un) were identified. Among them, 163 selected regions with a total of 5.4 Mb were located in 252 CHs. These CHs carrying selective signatures are probably under artificial selection during the breeding improvement of early pedigree and diverged between early and late-ripening groups, thus we define them as early ripening selected haplotypes (ESHs) (Figure 4, A–D).
Figure 4.
The identification procedure of candidate genes related to the early-ripening trait. A, Distribution of CHs on 19 chromosomes. The genome-wide selective sweeps detected by B, πlate/πearly, C, Fixation index (FST), and D, Cross-population composite likelihood ratio test (XP-CLR). E, Genome-wide distribution of QTL for flowering, veraison, and ripening in grapevine. The “*” indicates the genomic region under selection where the 47 candidate genes are located. F, The gene name or id of 47 candidate genes related to early-ripening trait, and G, their log2FC value in “Xiangfei” (X) and “Zaomeiguixiang” (Z) compared with “Italia” at three berry developmental stages, and H, the number and distribution of their fixed SNPs (Fisher-exact P-value < 0.0001).
The 252 ESHs encompassed 833 genes, which are also significantly associated with methyltransferase activity (adjusted P < 0.001; GO:0030761, GO:0008171, GO:0008757, and GO:0008168) in agreement with CHs. Notably, these genes are involved in well-known biological processes related to grapevine berry ripening, such as abscisic acid (ABA) response, flavonoid metabolic process, programmed cell death, and DNA methylation (Supplemental Figure S5A). We further annotated these genes in combination with QTL loci for flowering, veraison, and ripening on the grapevine (Delfino et al., 2019), and 480 genes located at both ESHs and QTL loci were retained and considered as candidate early ripening genes (CEGs) (Figure 4, A–E; Supplemental Table S8). The CEGs also participated in the ABA-activated signaling pathway and developmental maturation (Supplemental Figure S5B).
To examine the potential role of CEGs during the early ripening of grapevine berry, we dissected the genetic basis of the early-ripening trait in PC lines by calculating frequencies at each allele-associated SNP in CEGs. Only SNP with Fisher-exact test P-values of both alleles and genotypes between the groups of early pedigree and late-cultivar <0.0001 were considered fixed (Supplemental Table S9). There were 245 genes with fixed variants, which spanned intron, upstream, downstream, 5’ and 3’ UTR, synonymous, missense, and stop gained (Supplemental Table S10). We further compared the transcription of them at pre-veraison, veraison, and harvest-ripe in “Zaomeiguixiang”, “Xiangfei”, and a late-ripening variety “Italian” in PC lines. 76.32% (187/245) genes are expressed and significantly (P < 0.05) overrepresented of genes related to developmental maturation (GO:0010154, GO:0032502, GO:0061458, GO:0021700, and GO:0003006), response to abscisic acid (GO:0071215, GO:0009737) and cytokinin (GO:0009735) (Supplemental Figure S6).
According to gene expression and variation in DNA sequence, 47 of 187 genes with fixed variants in the upstream, which were also differentially expressed (adjusted P < 0.05 and |log2FoldChange| > 1) in the early ripening variety compared with the late-ripening variety or with fixed variants in the coding regions (i.e. missense, stop gained, and frameshift) that could lead to amino acid sequence changes were reserved (Figure 4, F and G; Supplemental Table S11). A series of well-known genes family related to berry ripening including Gly-Asp-Ser-Leu (GDSL) type esterase/lipase (e.g. VvAXY4: ALTERED XYLOGLUCAN 4), nitrate/peptide transporter family (NPF) (e.g. VvNPF4.6: NRT1/PTR FAMILY 4.6), NAC (e.g. VvNAC29: NAC transcription factor 29), MYB (e.g. VvPHL8: Myb family transcription factor PHL8 and VvMYB105: Transcription factor MYB105), Zinc-finger homeodomain (ZF-HD) (e.g. VvZHD8: Zinc-finger homeodomain protein 8), and genes involved with ubiquitination (e.g. VvSKIP11: F-box/kelch-repeat protein SKIP11), methylation (e.g. VvPMT2: probable methyltransferase), and apoptosis (e.g. VvCCAR1: Cell division cycle and apoptosis regulator protein 1) were identified. Both VvNPF4.6 and VvNAC29 up-regulated in two early ripening varieties at three developmental stages catch our attention. The VvNPF4.6 (VIT_01s0026g01570) overlapped with πlate/πearly, FST, and ripening QTL loci and located on Hap1 (chr1: 10,633,815–10,655,618) with fixed SNP in its upstream, was the putative ortholog of Arabidopsis AtNPF4.6, which has been shown to involve ABA transport (Kanno et al., 2012; Kanno et al., 2013). The VvNAC29 (VIT_01s0026g02710) encoding NAC transcription factor 29 located on Hap1 (chr1: 12,389,086–12,527,386) with fixed SNP in its upstream (Supplemental Table S12), was putative ortholog of AtNAP, which could enhance transcription of the abscisic aldehyde oxidase (Yang et al., 2014).
Potential function of VvFBT in advancing grapevine berry ripening
Among 47 candidate genes, the gene VvFBT (VIT_16s0039g00720) encoding a folate/biopterin transporter protein showed multiple SNPs with significantly (Fisher-exact P-value < 0.0001) differentiated allele and genotype frequencies between groups of early-pedigree and late-cultivar. More than 90% (30/32) of varieties in early pedigree shared the haplotype where VvFBT is located. In addition, VvFBT is up-regulated in the early-ripening varieties compared to the late-ripening varieties (Figure 4G; Supplemental Table 11). Folate is indispensable for plant development, and folate transporter protein could affect the folate level in plants, which has been demonstrated in soybean where overexpression of the AtFOLT1 increased its folate levels and broad-spectrum resistance (Kambakam et al., 2021). Therefore, we proposed that VvFBT was possibly related to early-ripening traits in the pedigree.
The VvFBT (chr16:357,505–361,097) is located in a long haplotype (chr16:315,366–374,072) derived from PC Hap2, which contains several genes, but only VvFBT and VvPOLD4 overlap with both flowering QTL loci and selective signatures of FST and XP-CLR (Figure 5A). We annotated 14 fixed SNPs associated with VvFBT, 12 of which are located in the regulatory region, and 2 in the coding region including a missense SNP (chr16:360,203, A-to-G, Gln-to-Arg) and a synonymous SNP (chr16: 360,873, G-to-A) (Figure 5B). In the early-pedigree group, the reference alleles and homozygous reference genotypes of the 14 fixed SNPs showed lower frequencies compared to the late-ripening group, whereas the alternative alleles and alternative genotypes were dominant (Supplemental Table S9). Further characterization of haplotypes of these SNPs could be classified into six haplotypes in 80 samples, including 32 early ripening varieties and 48 late-ripening varieties (Figure 5B; Supplemental Table S13). The varieties with H1 composed of alternative alleles and genotype containing H1 exhibited a significantly (one-way ANOVA P < 0.001; Supplemental Table S14) lower BDP than H3 composed of reference alleles and genotypes containing H3 (Figure 5C; Supplemental Figure S7). To further understand the evolutionary history of the six haplotypes, we investigated the distribution of H1–H6 in 32 early ripening varieties, 48 late-ripening varieties, and 62 wild germplasms (Vitis. vinifera ssp. sylvestris), respectively. H1 is only found and overabundant in the group of early-pedigree; while H3 is excessive in the wild and late-cultivar (Figure 5D). Likewise, the genotype containing the missense allele G on the VvFBT is overrepresented in the early-pedigree group, while it is underrepresented in the late-cultivar group and none in the wild group (Figure 5D). This observation suggested that H1 was selected after grapevine domestication, and the alternative alleles associated with VvFBT may have advantageous effects on the early ripening of grapevine berries.
Figure 5.
A superior haplotype is associated with the early-ripening trait in the pedigree. A, Two selective signatures, FST: fixation index (black line) and XP-CLR: Cross-population composite likelihood ratio test (red line), were detected simultaneously in the gray shade (chr16: 353.37- 365.97 kb) of a long haplotype carrying multiple genes. The black dashed line and the red dashed line indicate the top 5% values of FST and XP-CLR, respectively. B, The two genes contained in the gray shade and their six haplotypes consisting of 14 SNPs associated with VvFBT inferred from 80 varieties (32 early ripening and 48 late-ripening varieties). C, Box plot of berry development period (BDP; in days) for the genotype of 80 varieties carrying H1–H6 haplotypes. The significant difference between test methods and post hoc tests are one-way ANOVA (P < 0.001) and LSD, respectively. D, The number and proportion of genotype of a missense SNP (chr16:360203) located on VvFBT, and number and proportion of H1–H6 haplotypes in early-ripening, late-ripening, and wild grapevine groups. E, The phenotype of control, and exogenous 0.1 mmol•L−1, 0.5 mmol•L−1 and 1 mmol•L−1 folate treatment (y axis) of “Kyoho” at different development stages (x axis). A row represents the variation of the same bunch of “Kyoho” at 35, 55, 67, 72, and 79 days after flowering (DAF; in x axis). Images have been digitally extracted for comparison.
We investigated the expression profile of VvFBT in various tissues of grapevine and found that it is highly expressed during berry ripening (particularly for veraison) (Supplemental Figure S8, A and B), meanwhile being higher than other putative BT1 family members (Supplemental Figure S9A). Moreover, the best-hit putative orthologues (i.e. the lowest E-value) of VvFBT in climatic and non-climatic fruits also expressed in ripening, especially at the onset of fruit ripening or veraison (Supplemental Figure S9B; Supplemental Table S15). Furthermore, exogenous folate solutions treatment of “Kyoho” grape berry significantly (independent sample t-test, P < 0.05) advances its veraison compared with the control (Figure 5E; Supplemental Figure S10). These results indicated that VvFBT possibly played an important role in initiating grapevine berry ripening.
Discussion
Early ripening is a desirable trait of grapevine breeding. The PC lines with excellent, stably inheritable, early ripening trait provide valuable germplasm resources for the molecular breeding of early ripening grapevine varieties. In this study, we collected and deeply re-sequenced a four-generation pedigree consisting of 40 varieties derived from PC and integrated 24 late-ripening varieties with high-depth sequencing from public data to assist us in more comprehensively dissecting the possible genetic basis responsible for the early-ripening trait in PC lines (Figure 1; Supplemental Figure S1; Supplemental Table S1; Supplemental Table S2). High-quality SNPs were identified, part of which was validated by Sanger sequencing (Supplemental Table S3).
In the breeding process, the pedigree constructed from varieties with founding genotypes tends to exhibit more consistent genetic composition and stable genetic traits after multiple rounds of directional selection (Ott et al., 2011). This characteristic could well explain why the early-pedigree group shows a more distinct genetic composition (Figure 2, A and B; Supplemental Figure S2), stronger LD (Figure 2C), and lower nucleotide diversity (Figure 2E) than the non-pedigree cultivar in grapevine, which is a similar observation with the pedigree of Upland cotton (Ma et al., 2018; Ma et al., 2019; Han et al., 2020). In addition, when r2 decreased to 0.2, our estimated physical distance of LD disequilibrium in the late-cultivar group was at 19 Kb, which was almost consistent with the 20 Kb estimated in grapevines by Zhou et al., (2017), and the D value was also similar to those of them. This further increases the reliability of our conclusions. Based on the genealogical information, the haplotype of each variety could be inferred more accurately (Chen et al., 2013), thus making it possible to trace the DNA segments that control one or several major traits in the pedigree.
A widely used method to trace haplotypes of the founder in the pedigree is identity-by-descent (IBD), which has been applied to crops such as maize (Li et al., 2019), rice (Huang et al., 2018), cotton (Lu et al., 2019; Han et al., 2020), and apple (Minamikawa et al., 2021) over the past few years, and further combined with the methods of QTL loci, selective sweeps or GWAS to successfully identify a set of genes associated with the target traits, particularly quantitative traits. On the basis of these studies, we developed a more robust method for analyzing the pedigree of PC (Figure 4). The first step is to infer haplotypes in the pedigree using genealogical information, and then access CHs in PCs that could be stably passed on to the offspring using IBD analysis (Figure 4A); The second step is an improved one compared to the existing studies (Zhou et al., 2016; Li et al., 2019; Ma et al., 2019; Han et al., 2020) and consists of two main aspects: (1) using an equal number of varieties with the opposite trait (i.e. late-ripening) to build up a group as a reference, and (2) employing three methods of inter-population selection sweeps (i.e. πlate/πearly, FST, and XP-CLR) to explore selection signatures with high confidence in the early pedigree group; In the third step, CHs carrying selection signatures are intersected with ripening-related QTL to obtain possible candidate haplotypes (Figure 4, B–E). Compared with studies that only kept the overlapping regions (Ma et al., 2019), we considered more the impact of genetic hitchhiking on the results. We further used the Fisher-exact test to account for the differences in the frequencies of alleles and genotypes for SNPs, between the groups of early-pedigree and late-cultivar, and set the threshold (i.e. P < 0.0001) to retain those haplotypes or genes with a fixed variant, which is unprecedented in the studies of pedigree (Zhou et al., 2016; Huang et al., 2018; Li et al., 2018; Ma et al., 2019; Zhang et al., 2020). Moreover, the annotation of these fixed variants helped us to understand the possible effects of these mutations on genes (Figure 4H), and mutation and transcription were linked by combining the expression of genes during berry ripening in late and early ripening varieties within the early pedigree group (Figure 4, G and H). Finally, 47 genes including well-known genes family related to berry ripening were identified as possible genes controlling early ripening traits in PC lines (Figure 4, F and G; Supplemental Table S11), which are consistent with the existing studies, increasing our confidence in this analytical method.
In terms of genome-wide gene expression profile, the transition to mature growth predominantly involves the suppression of vegetative pathways rather than the activation of mature pathways in the grapevine (Fasoli et al., 2012), which is further supported by the fact that most of the DEGs are down-regulated during berry ripening (Massonnet et al., 2017). A substantial proportion of genes whose functions are not yet known are involved in the process of grapevine berry ripening (Fasoli et al., 2012; Massonnet et al., 2017; Fasoli et al., 2018). In our results, 36% (17/47) of candidate genes were no hit or unknown function, but more than 80% (39/47) of candidate genes with fixed variants were up-regulated during berry ripening in early-ripening varieties (Figure 4, F and G). This implies that early berry ripening in the PC line is likely to be achieved by activating the expression of the genes with a variation.
Among all the candidate haplotypes, we finally focused on H1 (chr16: 353,369- 365,973) composed of 14 alternative alleles, which encompasses a gene encoding folate transport protein (Figure 5, A and B). As an essential vitamin, folate (also known as vitamin B9) being effective at low concentrations, is indispensable for plant development and closely linked with a myriad of physiological processes, including photorespiration (Li et al., 2003), plastid biogenesis (Van Wilder et al., 2009), epigenetic regulation of gene expression, and immunity (González and Vera, 2019). There are several facts such as varieties carrying the H1 haplotype significantly exhibiting short BDP (Figure 5C; Supplemental Figure S7), H1 haplotype, and missense SNP on VvFBT are overrepresented in the early-pedigree group (Figure 5D; Supplemental Figure S7B), and VvFBT is specifically and highly expressed in veraison of grapevine berry (Supplemental Figure S8; Supplemental Figure S9A) that build a strong relationship between VvFBT and the early ripening trait in the pedigree group. Meanwhile, the best-hit putative orthologues of VvFBT in climatic and non-climatic fruits are highly expressed at the onset of fruit ripening or veraison (Supplemental Figure S9B; Supplemental Table S14), which suggests a role of putative orthologues of VvFBT in initiating other fruit development processes. Several studies using exogenous folate treatment were found to promote the yield and seed quality of peas (Farouk and Qados, 2018) and barley (Stakhova et al., 2000). In this study, exogenous folate solutions treatment of “Kyoho” grape berry advanced its veraison (Figure 5E; Supplemental Figure S10), which provides insight into the possible role of folate in grapevine berry ripening. However, how VvFBT in grapevine mediates the transport of folate and affects its content in the berry, and the mechanisms by which folate regulates grapevine berry development need to be further investigated.
Materials and methods
Samples and sequencing
We collected grapevine (Vitis vinifera) leaf tissue for a total of 40 individuals from the pedigree of PC from the Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences (ZFRI, CAAS) in Henan, China (Supplemental Table S1). For each sample, genomic DNA was extracted from leaf samples with a modified cetyltrimethylammonium bromide (CTAB protocol) method (Borges et al., 2009). The sequencing is carried out according to the standard paired-end protocol provided by Illumina company in Biomarker Technologies Corporation (Beijing, China). Paired-end sequencing libraries were constructed with an insert size of 300 bp, which were then sequenced using the Illumina HiSeq 2500 platform with 2 × 150 bp paired reads to a target depth of 30×. The Illumina raw pair-end reads of 24 non-pedigree cultivars were downloaded from the SRA at NCBI (Supplemental Table S2). The Vitis International Variety Catalogue (https://www.vivc.de/) was used to search parent–offspring relationships for samples of the late-ripening group.
Reads cleaning, variant calling, and annotation
FastQC v0.11.9 (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) was used to check the quality of both raw and filtered reads. Raw paired reads removed adapter sequences and trimmed bases when average quality per base dropped below 30 in 4 bp windows and reserved reads when they were >50 base length and paired after trimming using Trimmomatic v0.39 (Bolger et al., 2014). Clean paired reads were mapped to the Vitis vinifera Pinot Noir (PN40024) reference genome (12×) (Jaillon et al., 2007) downloaded from Ensemble Plants (release 51) using BWA-MEM algorithm with default parameters (Li and Durbin, 2009). SAMtools v1.14 (Li et al., 2009) was used to sort and filter out non- and multiply mapped reads and reads with a mapping quality of <30. PCR-duplicated reads were removed using Picard's MarkDuplicates v2.25.0 (https://github.com/broadinstitute/picard). Finally, the reads with high base quality and mapping quality were kept for downstream analysis.
We used HaplotypeCaller, GenomicsDBImport, and GenotypeGVCFs implemented in GATK v4.2.0.0 for SNPs, indel, and genotype calling across all samples (McKenna et al., 2010). We applied expression “QD < 2.0 || FS > 60.0 || MQ < 40.0 || SOR > 3.0 || MQRankSum < −12.5 || ReadPosRankSum < −8.0” to filter SNPs. To further reduce the false positives of detected SNPs and ensure higher quality calls, we also removed (1) SNPs with more than two alleles in all samples; (2) SNPs with less than covered 10 reads and more than 70 reads to avoid including low-quality calls and repeated regions in the analysis; (3) SNPs with more than 10% missing genotypes across all samples; (4) SNPs with minor allele frequency (MAF) <0.1 across all samples by using VCFtools v0.1.16 (Danecek et al., 2011). Finally, the effects of high-quality SNPs were annotated using VEP v104 (McLaren et al., 2016), and the indexed cache of Vitis vinefera was download from http://ftp.ebi.ac.uk/ensemblgenomes/pub/plants/release-51/variation/indexed_vep_cache/.
Population genetic analysis
The NJ tree was constructed based on a P-distance matrix estimated using VCF2Dis v1.46 (https://github.com/BGI-shenzhen/VCF2Dis) and the analyses of LD decay performed with PopLDdecay v3.41 (Zhang et al., 2019). We used ANGSD v0.935 (Korneliussen et al., 2014) to infer the population structure. SAMtools model implemented in the ANGSD was used to generate a BEAGLE file from bam files of all samples using only sites with SNP P-value < 1e-6 (Kim et al., 2011), containing < 10% of missing data, a minimal quality score of 30, a minimal mapping quality of 30, and depth within 10×∼70×. Then we performed PCAs and population structure with PCAngsd v1.03 (Meisner and Albrechtsen, 2018) and NGSadmix (Skotte et al., 2013), respectively, using sites with MAF > 0.1 to consider genotype likelihoods.
To measure genome-wide genetic diversity, we estimated population genetic statistics for the pedigree and the cultivar, respectively, using the SAMtools genotype likelihoods model implemented in ANGSD without calling SNPs. Only unique and proper pair reads with a mapping quality > 30 and bases with a quality score > 30 and sites with missing < 10% were employed to calculate the site allele frequency likelihoods by using −doSaf. The PN40024 reference genome was set as the ancestral state. Then we used realSFS to obtain a maximum likelihood estimate of the folded site frequency spectrum (SFS). Watterson's θw (Watterson, 1975), pairwise nucleotide differences (π), and Tajima's D (Tajima, 1989) were calculated by doing saf2theta and thetaStat. The unique and proper pair reads with a mapping quality > 30 and bases with a quality score > 30 of each sample were selected to estimate the global heterozygosity of individuals by using realSFS. We applied the HWE test defined by Wigginton et al. (2005) to report the P-value for SNPs in the VCF of early-pedigree and late-cultivar, respectively.
Haplotype inference and inheritance analysis
Pedigree phasing (especially for parent–offspring trios) could provide the most accurate haplotype information (Chen et al., 2013). Therefore, we used the Beagle v4.0 (Browning and Browning, 2007) to phase genotypes of pedigree by modeling the parent–offspring relationships. The gtgl parameter was set to consider both genotype and genotype likelihoods, and the window and step size were set to 400 and 200 SNPs, respectively. We further improved the accuracy of the phase by increasing the number of iterations of phase and burning to 10. After that, we employed hap-ibd v1.0 (Zhou et al., 2020) to detect pairwise identical-by-descent (IBD) segments between samples in the pedigree. Only identical-by-state (IBS) segments with at least 50 SNPs and longer than 5 kb were used as seeds, each of which was used to extend itself to the nearest distance (<10 kb) IBS segments with a minimum length of >2.5 kb.
To obtain possible haplotypes inherited from the PC to their descendants, we preserved from the hap-ibd results only the IBD segments of the descendants identical to the PC. Furthermore, IBD segments identical to the PC in descendants were used to trace the genetic pathway of haplotype from the PC by using bedtools v2.30.0 (Quinlan and Hall, 2010) multiinter sub-command. Given that the recombination events of two sets of haplotypes (Hap1 and Hap2) of the PC in the process of inheritance, we considered the genetic pathway of Hap1 and Hap2 of the PC to its descendants, respectively. Stably passed haplotypes of the same type (i.e. Hap1 or Hap2) with no gaps among them were concatenated as one longer haplotype. Meanwhile, it is very difficult to correctly distinguish the origin of Hap1 and Hap2, which overlap with the homozygous region in the ancestral genome, we also combined the stably passed Hap1 and Hap2 with the same homozygous region of the PC into one to remove redundancy.
The phasing accuracy of the groups of late-ripening and wild (V. vinifera ssp. sylvestris) is limited because these varieties are not in the same pedigree. We used HapCUT2 v1.3.1 (Edge et al., 2017) to assemble reads localized to the gene of interest after identifying fixed differential loci, while using the phasing result of Beagle v5.2 and IGV to manually check for possible errors to infer accurate haplotype information. Rare haplotype (number < 3) in all materials is filtered.
GO and KEGG analysis
The eggnog-mapper v2 (Cantalapiedra et al., 2021) was used to perform GO and KEGG annotation of grapevine genome-wide genes. Then, TBtools v1.09873 (Chen et al., 2020) was used to extract annotation results and perform GO and KEGG enrichment analysis. To remove redundant terms from GO enrichment results, we used Revigo (Supek et al., 2011) to calculate the semantic similarity from the GO terms list with default parameters.
RNA-seq analysis
The RNA-seq raw data of “Italian”, “Xiangfei”, and “Zaomeiguixiang” was downloaded from the NCBI under accessions SRP184152 (Sun et al., 2019) for berry samples at three developmental stages (pre-veraison, veraison, and harvest-ripe) with three independent biological replicates. The FastQC and Trimmomatic were used to check and trim data with the same options as above. We used STAR v2.7.9a (Dobin et al., 2013) to map trimmed pair reads onto the PN40024 reference genome. Furthermore, DESeq2 was employed for differential gene expression analysis. The read counts file was used to caculate TPM value with R v3.6.0 software. The genes in each variety for which the sum of TPM was >1 and the frequency of samples with an expression of 0 was <0.5 were screened for the following analysis.
Detection of selective signatures
Based on the results of LD decay, genomic regions in 20 kb nonoverlapping sliding windows were scanned across genomes. We used ANGSD to obtain the site allele frequency likelihoods for the early and late-ripening groups by unique and proper pair reads with a mapping quality > 30 and bases with a quality score > 30 and sites with missing < 10%, without calling SNP. The PN40024 reference genome was set as the ancestral state and π was calculated as above. For FST, the realSFS implemented in ANGSD was used to estimate 2D folded SFS, which was further used to calculate the average FST value via the fst index and fst stats2 sub-command. The XP-CLR test (Chen et al., 2010) was also employed to detect selective sweeps in the early pedigree using the late-cultivar group as a reference. We estimated XP-CLR scores across nonoverlapping 20 kb windows and the number of SNPs involved in the calculation ranged from 20 to 200. The genetic distance between SNPs was derived from Zhu et al. (2018) to use as an input for XP-CLR. The continuous selection regions with no gap were merged into one.
The analysis of the expression profile of VvFBT
The variation in VvFBT expression in different grapevine tissues was retrieved from Grape eFP Browser (http://bar.utoronto.ca/efp_grape/cgi-bin/efpWeb.cgi). The BT1 family members were identified using the Hidden Markov Model (HMM) profile corresponding to the BT1 domain (PF03092), and their transcript levels during berry ripening in 10 grapevine varieties were obtained from Massonnet et al. (2017). To investigate the expression profile of VvFBT during fruit ripening in climacteric and non-climacteric, we used BLASTp v2.12.0 + to align the amino acid sequences of VvFBT with the whole-genome protein sequences of climacteric (tomato and peach) and non-climacteric fruits (citrus, pepper, strawberry, and grape), respectively. We retained only the top three genes with the smallest E-value (i.e. E-value: Top1 < Top2 < Top3) for each species as putative orthologues of VvFBT. The data sources for the expression of these genes are detailed in Supplemental Table S15.
Folate solution treatment of “Kyoho” berry
Seven-year-old “Kyoho” cultivated in a hedgerow trellis on an experimental farm of the Henan University of Science and Technology was used in this study. Folate is a weak acid and easily soluble in an alkaline solution. We prepared 0.1 mmol•L−1, 0.5 mmol•L−1, and 1 mmol•L−1 of folate solutions using PBS buffer, and Silwet-77 (Solarbio, Beijing, China) surfactant was added to the folate solutions at a final concentration of 0.03% (v/v), all of which have a pH value around 7. Then, we selected “Kyoho” with the same growth potential and cultivation direction and used three concentrations of folate solution and PBS buffer with 0.03% (v/v) Silwet-77 (control) to dip the uniformly shaped and positioned berry of “Kyoho” for 10 min at June 7, 2021 (35 days after flowering, DAF), with three replicates per group. We measured the percentage in color-changing berry per bunch (named color-changing rate) over time of three folate concentrations treated “Kyoho” vs control at first color change was observed in the berry (i.e. DAF 72). The berry with a clearly observable purplish red color on the surface is considered to be color-changing. An independent sample t-test (one-sided) was used to compare the difference of color-changing rate between folate-treated and control using stats (R package). The Shapiro test function and the F-test were used to check whether the data meet the normal distribution and the homogeneity of the variance, respectively. The color-changing rate of 0.5 mmol•L−1 at DAF72 and 1 mmol•L−1 at DAF79 did not meet homogeneity of the variance, which used Mann–Whitney instead of the t-test.
Statistical analysis
The Fisher-exact test and one-way ANOVA were performed using the stats (R package) of R v3.6.0 and SPSS v20, respectively.
Accession numbers
The raw sequencing data in this study for the pedigree of the “PC” have been deposited in the Short Read Archive at NCBI under BioProject ID: PRJNA876640. The resequencing data of 62 wild and an additional 16 late-ripening cultivars reported in (Liang et al., 2019) were retrieved from NCBI under BioProject ID: PRJNA393611. Transcriptome data of “Xiangfei”, “Zaomeiguixiang”, and “Italian” were downloaded from PRJNA521100 (Sun et al., 2019). The expression data of 10 varieties of grapevine berries were downloaded from GSE62744 and GSE62745 (Massonnet et al., 2017).
Supplementary Material
Acknowledgments
We thank the Zhengzhou Fruit Research Institute, Chinese Academy of Agricultural Sciences (ZFRI, CAAS) for providing the grapevine germplasm resource.
Contributor Information
Guang-Qi He, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Xi-Xi Huang, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China.
Mao-Song Pei, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Hui-Ying Jin, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Yi-Zhe Cheng, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Tong-Lu Wei, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Hai-Nan Liu, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Yi-He Yu, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Da-Long Guo, College of Horticulture and Plant Protection, Henan University of Science and Technology, Luoyang 471023, China; Henan Engineering Technology Research Center of Quality Regulation of Horticultural Plants, Henan University of Science and Technology, Luoyang 471023, China.
Code availability
The data analysis scripts associated with this project have been deposited into GitHub (https://github.com/Guangqi-He/pedigree).
Supplemental data
Supplemental Figure S1 . Berry development period (BDP; in days) of 64 grapevine varieties investigated in Zhengzhou, China.
Supplemental Figure S2 . The population structure of early-pedigree and late-cultivar.
Supplemental Figure S3 . The distribution of genome-wide conserved haplotypes.
Supplemental Figure S4 . 47 and 11 differentially expressed genes (DEGs) in both “Xiangfei” and “Zaomeiguixiang” at the pre-veraison (PV) to veraison (V), and veraison (V) to harvest ripe (R), respectively.
Supplemental Figure S5 . The Gene Ontology (GO) enrichment analysis of genes related to early ripening.
Supplemental Figure S6 . The Gene Ontology enrichment of candidate early ripening genes (CEGs) with fixed single nucleotide polymorphism (SNPs) between the groups of early pedigree and late-cultivar, expressed during berry ripening.
Supplemental Figure S7 . Association of H1–H6 haplotypes with grapevine berry ripening.
Supplemental Figure S8 . Characterization of VvFBT expression during grapevine development.
Supplemental Figure S9 . The effect of folate on grapevine berry ripening.
Supplemental Figure S10 . The percentage of color-changing berries per bunch (named color-changing rate) of folate-treated “Kyoho” vs control at 72 days after flowering (DAF) and 79 DAF.
Supplemental Table S1 . Information about the varieties used in this study.
Supplemental Table S2 . Summarized statistics of sequenced samples.
Supplemental Table S3 . Validation of SNP calling by Sanger sequencing.
Supplemental Table S4 . Estimated heterozygosity of varieties from the early and late groups.
Supplemental Table S5 . Haplotypes stably passed from the Pearl of Csaba to the descendants.
Supplemental Table S6 . Information about conserved haplotypes (CHs).
Supplemental Table S7 . GO and KEGG enrichment of genes in CHs (padj < 0.05).
Supplemental Table S8 . The list of candidate CEGs detected by conserved haplotypes (CHs), sweeps (πlate/πearly, FST, XP-CLR), and QTLs (flowering, ripening, veraison).
Supplemental Table S9 . Information about fixed SNPs between early pedigree and late-cultivar.
Supplemental Table S10 . The distribution and number of fixed SNPs in 245 genes.
Supplemental Table S11 . Information about 47 genes with fixed variants in the upstream that were also differentially expressed (adjusted P < 0.05 and |log2FoldChange| > 1) in the early ripening variety compared to the late-ripening variety or with fixed variants in the coding regions.
Supplemental Table S12 . Number of alleles and genotypes with fixed SNPs derived from VvNPF4.6 and VvNAC29 in the early pedigree and late-cultivar groups.
Supplemental Table S13 . Haplotypes of 14 fixed SNPs associated with VvFBT in 142 samples.
Supplemental Table S14 . The P-values of one-way ANOVA in Figure 5C.
Supplemental Table S15 . Expression profile of VvFBT putative orthologues in climacteric and non-climacteric fruit during ripening.
Funding
This work was financially supported by the National Key Research and Development Program of China (2018YFD1000105), the Natural Science Foundation of China (NSFC: U1904113), and Program for Innovative Research Team (in Science and Technology) in the University of Henan Province (21IRTSTHN021).
Author Contributions
D.L.G. conceptualized the research program, designed the experiments, and coordinated the project. M.S.P. extracted the high-quality DNA. X.X.H. and H.Y.J. did the PCR amplification test. G.Q.H. and Y.Z.C. treated grapes with folate. Y.H.Y., T.L.W., H.N.L., and D.L.G. gave advice for this study and helped to revise the manuscript. G.Q.H. performed the genotyping and bioinformatics analyses, analyzed all the data, and wrote the manuscript. All authors discussed the results and commented on the manuscript. All authors read and approved the final manuscript.
References
- Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics 30(15): 2114–2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borges A, Rosa MS, Recchia GH, Queiroz-Silva JRD, Bressan EDA, Veasey EA (2009) CTAB Methods for DNA extraction of sweetpotato for microsatellite analysis. Sci agric 66(4): 529–534 [Google Scholar]
- Brachi B, Morris GP, Borevitz JO (2011) Genome-wide association studies in plants: the missing heritability is in the field. Genome Biol 12(10): 232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning SR, Browning BL (2007) Rapid and accurate haplotype phasing and missing-data inference for whole-genome association studies by use of localized haplotype clustering. Am J Hum Genet 81(5): 1084–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantalapiedra CP, Hernández-Plaza A, Letunic I, Bork P, Huerta-Cepas J (2021) eggNOG-mapper v2: functional annotation, orthology assignments, and domain prediction at the metagenomic scale. Mol Biol Evol 38(12): 5825–5829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Chen H, Zhang Y, Thomas HR, Frank MH, He Y, Xia R (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13(8): 1194–1202 [DOI] [PubMed] [Google Scholar]
- Chen W, Li B, Zeng Z, Sanna S, Sidore C, Busonero F, Kang HM, Li Y, Abecasis GR (2013) Genotype calling and haplotyping in parent-offspring trios. Genome Res 23(1): 142–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Patterson N, Reich D (2010) Population differentiation as a test for selective sweeps. Genome Res 20(3): 393–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danecek P, Auton A, Abecasis G, Albers CA, Banks E, DePristo MA, Handsaker RE, Lunter G, Marth GT, Sherry ST, et al. (2011) The variant call format and VCFtools. Bioinformatics 27(15): 2156–2158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino P, Zenoni S, Imanifard Z, Tornielli GB, Bellin D (2019) Selection of candidate genes controlling veraison time in grapevine through integration of meta-QTL and transcriptomic data. BMC Genomics 20(1): 739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobin A, Davis CA, Schlesinger F, Drenkow J, Zaleski C, Jha S, Batut P, Chaisson M, Gingeras TR (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29(1): 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlevy JD, Soole KL, Perkins MV, Dennis EG, Keyzers RA, Kalua CM, Boss PK (2010) Two O-methyltransferases involved in the biosynthesis of methoxypyrazines: grape-derived aroma compounds important to wine flavour. Plant Mol Biol 74(1–2): 77–89 [DOI] [PubMed] [Google Scholar]
- Edge P, Bafna V, Bansal V (2017) HapCUT2: robust and accurate haplotype assembly for diverse sequencing technologies. Genome Res 27(5): 801–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Liu C, Sun H, Li M (2010) The evolution and genetic diversity analysis of pearl of csaba and its derived varieties [J]. J Plant Genet Resour 11(5): 625–628 [Google Scholar]
- Farouk S, Qados AMA (2018) Enhancing seed quality and productivity as well as physio-anatomical responses of pea plants by folic acid and/or hydrogen peroxide application. Sci Hortic 240: 29–37 [Google Scholar]
- Fasoli M, Dal Santo S, Zenoni S, Tornielli GB, Farina L, Zamboni A, Porceddu A, Venturini L, Bicego M, Murino V, et al. (2012) The grapevine expression atlas reveals a deep transcriptome shift driving the entire plant into a maturation program. Plant Cell 24(9): 3489–3505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasoli M, Richter CL, Zenoni S, Bertini E, Vitulo N, Dal Santo S, Dokoozlian N, Pezzotti M, Tornielli GB (2018) Timing and order of the molecular events marking the onset of berry ripening in grapevine. Plant Physiol 178(3): 1187–1206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fechter I, Hausmann L, Zyprian E, Daum M, Holtgräwe D, Weisshaar B, Töpfer R (2014) QTL Analysis of flowering time and ripening traits suggests an impact of a genomic region on linkage group 1 in Vitis. Theoretical and Applied Genetics 127(9): 1857–1872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flutre T, Le Cunff L, Fodor A, Launay A, Romieu C, Berger G, Bertrand Y, Terrier N, Beccavin I, Bouckenooghe V, et al. (2022) A genome-wide association and prediction study in grapevine deciphers the genetic architecture of multiple traits and identifies genes under many new QTLs. G3 (Bethesda) 12(7): jkac103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González B, Vera P (2019) Folate metabolism interferes with plant immunity through 1c methionine synthase-directed genome-wide dna methylation enhancement. Mol Plant 12(9): 1227–1242 [DOI] [PubMed] [Google Scholar]
- Guo D, Zhao H, Li Q, Zhang G, Jiang J, Liu C, Yu Y (2019) Genome-wide association study of berry-related traits in grape [Vitis vinifera L.] based on genotyping-by-sequencing markers. Hortic Res 6(11): 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Hu Y, Tian Q, Cao Y, Si A, Si Z, Zang Y, Xu C, Shen W, Dai F, et al. (2020) Genomic signatures and candidate genes of lint yield and fibre quality improvement in upland cotton in Xinjiang. Plant Biotechnol J 18(10): 2002–2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J, Li J, Zhou J, Wang L, Yang S, Hurst LD, Li WH, Tian D (2018) Identifying a large number of high-yield genes in rice by pedigree analysis, whole-genome sequencing, and CRISPR-Cas9 gene knockout. Proc Natl Acad Sci U S A 115(32): E7559–E7567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaillon O, Aury JM, Noel B, Policriti A, Clepet C, Casagrande A, Choisne N, Aubourg S, Vitulo N, Jubin C, et al. (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449(7161): 463–467 [DOI] [PubMed] [Google Scholar]
- Kambakam S, Ngaki MN, Sahu BB, Kandel DR, Singh P, Sumit R, Swaminathan S, Muliyar-Krishna R, Bhattacharyya MK (2021) Arabidopsis non-host resistance PSS30 gene enhances broad-spectrum disease resistance in the soybean cultivar williams 82. Plant J 107(5): 1432–1446 [DOI] [PubMed] [Google Scholar]
- Kanno Y, Hanada A, Chiba Y, Ichikawa T, Nakazawa M, Matsui M, Koshiba T, Kamiya Y, Seo M (2012) Identification of an abscisic acid transporter by functional screening using the receptor complex as a sensor. Proc Natl Acad Sci U S A 109(24): 9653–9658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanno Y, Kamiya Y, Seo M (2013) Nitrate does not compete with abscisic acid as a substrate of AtNPF4.6/NRT1.2/AIT1 in Arabidopsis. Plant Signal Behav 8(12): e26624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SY, Lohmueller KE, Albrechtsen A, Li Y, Korneliussen T, Tian G, Grarup N, Jiang T, Andersen G, Witte D, et al. (2011) Estimation of allele frequency and association mapping using next-generation sequencing data. BMC Bioinformatics 12(1): 231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korneliussen TS, Albrechtsen A, Nielsen R (2014) ANGSD: analysis of next generation sequencing data. BMC Bioinformatics 15(1): 356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn N, Guan L, Dai ZW, Wu BH, Lauvergeat V, Gomès E, Li SH, Godoy F, Arce-Johnson P, Delrot S (2014) Berry ripening: recently heard through the grapevine. J Exp Bot 65(16): 4543–4559 [DOI] [PubMed] [Google Scholar]
- Li H, Durbin R (2009) Fast and accurate short read alignment with burrows-wheeler transform. Bioinformatics 25(14): 1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R (2009) The sequence alignment/map format and SAMtools. Bioinformatics 25(16): 2078–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li R, Moore M, King J (2003) Investigating the regulation of one-carbon metabolism in Arabidopsis thaliana. Plant Cell Physiol 44(3): 233–241 [DOI] [PubMed] [Google Scholar]
- Li C, Song W, Luo Y, Gao S, Zhang R, Shi Z, Wang X, Wang R, Wang F, Wang J, et al. (2019) The HuangZaoSi maize genome provides insights into genomic variation and improvement history of maize. Mol Plant 12(3): 402–409 [DOI] [PubMed] [Google Scholar]
- Li X, Xu J, Duan S, Bian C, Hu J, Shen H, Li G, Jin L (2018) Pedigree-based deciphering of genome-wide conserved patterns in an elite potato parental line. Front Plant Sci 9(690): 1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhang C, Huang J, Liu Q, Wei H, Wang H, Liu G, Gu L, Yu S (2021) Genomic analyses reveal the genetic basis of early maturity and identification of loci and candidate genes in upland cotton (Gossypium hirsutum L. Plant Biotechnol J 19(1): 109–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Z, Duan S, Sheng J, Zhu S, Ni X, Shao J, Liu C, Nick P, Du F, Fan P, et al. (2019) Whole-genome resequencing of 472 Vitis accessions for grapevine diversity and demographic history analyses. Nat Commun 10(1): 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Pan X, Guo J, Fan X, Kong Q (2004) Evaluation on the diversity of maturity time of grape cultivars and its classification [J]. J Fruit Sci 21(6): 535-539 [Google Scholar]
- Lu X, Fu X, Wang D, Wang J, Chen X, Hao M, Wang J, Gervers KA, Guo L, Wang S, et al. (2019) Resequencing of cv CRI-12 family reveals haplotype block inheritance and recombination of agronomically important genes in artificial selection. Plant Biotechnol J 17(5): 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Z, He S, Wang X, Sun J, Zhang Y, Zhang G, Wu L, Li Z, Liu Z, Sun G, et al. (2018) Resequencing a core collection of upland cotton identifies genomic variation and loci influencing fiber quality and yield. Nat Genet 50(6): 803–813 [DOI] [PubMed] [Google Scholar]
- Ma X, Wang Z, Li W, Zhang Y, Zhou X, Liu Y, Ren Z, Pei X, Zhou K, Zhang W, et al. (2019) Resequencing core accessions of a pedigree identifies derivation of genomic segments and key agronomic trait loci during cotton improvement. Plant Biotechnol J 17(4): 762–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massonnet M, Fasoli M, Tornielli GB, Altieri M, Sandri M, Zuccolotto P, Paci P, Gardiman M, Zenoni S, Pezzotti M (2017) Ripening transcriptomic program in red and white grapevine varieties correlates with berry skin anthocyanin accumulation. Plant Physiol 174(4): 2376–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, et al. (2010) The genome analysis toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20(9): 1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaren W, Gil L, Hunt SE, Riat HS, Ritchie GR, Thormann A, Flicek P, Cunningham F (2016) The ensembl variant effect predictor. Genome Biol 17(1): 122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner J, Albrechtsen A (2018) Inferring population structure and admixture proportions in low-depth ngs data. Genetics 210(2): 719–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamikawa MF, Kunihisa M, Noshita K, Moriya S, Abe K, Hayashi T, Katayose Y, Matsumoto T, Nishitani C, Terakami S, et al. (2021) Tracing founder haplotypes of Japanese apple varieties: application in genomic prediction and genome-wide association study. Hortic Res 8(1): 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott J, Kamatani Y, Lathrop M (2011) Family-based designs for genome-wide association studies. Nat Rev Genet 12(7): 465–474 [DOI] [PubMed] [Google Scholar]
- Palumbo MC, Zenoni S, Fasoli M, Massonnet M, Farina L, Castiglione F, Pezzotti M, Paci P (2014) Integrated network analysis identifies fight-club nodes as a class of hubs encompassing key putative switch genes that induce major transcriptome reprogramming during grapevine development. Plant Cell 26(12): 4617–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan AR, Hall IM (2010) BEDTools: a flexible suite of utilities for comparing genomic features. Bioinformatics 26(6): 841–842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shangguan L, Fang X, Jia H, Chen M, Zhang K, Fang J (2020) Characterization of DNA methylation variations during fruit development and ripening of Vitis vinifera (cv. ‘fujiminori’. . Physiol Mol Biol Plants 26(4): 617–637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skotte L, Korneliussen TS, Albrechtsen A (2013) Estimating individual admixture proportions from next generation sequencing data. Genetics 195(3): 693–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stakhova LN, Stakhov LF, Ladygin VG (2000) Effect of exogenic folic acid on the yield and amino acid composition of the seeds of Pisum sativum L. And Hordeum vulgare L. Prikl Biokhim Mikrobiol 36(1): 98–103 [PubMed] [Google Scholar]
- Sun L, Zhu B, Zhang X, Zhang G, Yan A, Wang H, Wang X, Xu H (2019) Transcriptome profiles of three Muscat table grape cultivars to dissect the mechanism of terpene biosynthesis. Sci Data 6(1): 89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supek F, Bošnjak M, Škunca N, Šmuc T (2011) REVIGO Summarizes and visualizes long lists of gene ontology terms. PLoS One 6(7): e21800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123(3): 585–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Wilder V, De Brouwer V, Loizeau K, Gambonnet B, Albrieux C, Van Der Straeten D, Lambert WE, Douce R, Block MA, Rebeille F, et al. (2009) C1 metabolism and chlorophyll synthesis: the mg-protoporphyrin IX methyltransferase activity is dependent on the folate status. New Phytol 182(1): 137–145 [DOI] [PubMed] [Google Scholar]
- Watterson GA (1975) On the number of segregating sites in genetical models without recombination. Theor Popul Biol 7(2): 256–276 [DOI] [PubMed] [Google Scholar]
- Wigginton JE, Cutler DJ, Abecasis GR (2005) A note on exact tests of hardy-weinberg equilibrium. Am J Hum Genet 76(5): 887–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Worley E, Udvardi M (2014) A NAP-AAO3 regulatory module promotes chlorophyll degradation via ABA biosynthesis in Arabidopsis leaves. Plant Cell 26(12): 4862–4874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Dong SS, Xu JY, He WM, Yang TL (2019) PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 35(10): 1786–1788 [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhang Y, Qiu H, Guo Y, Wan H, Zhang X, Scossa F, Alseekh S, Zhang Q, Wang P, et al. (2020) Genome assembly of wild tea tree DASZ reveals pedigree and selection history of tea varieties. Nat Commun 11(1): 3719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Su K, Guo Y, Ma H, Guo X (2016) Molecular genetic map construction and QTL analysis of fruit maturation period in grapevine. Genet Mol Res 15(2): gmr.15028040. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Browning SR, Browning BL (2020) A fast and simple method for detecting identity-by-descent segments in large-scale data. Am J Hum Genet 106(4): 426–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D, Chen W, Lin Z, Chen H, Wang C, Li H, Yu R, Zhang F, Zhen G, Yi J, et al. (2016) Pedigree-based analysis of derivation of genome segments of an elite rice reveals key regions during its breeding. Plant Biotechnol J 14(2): 638–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Massonnet M, Sanjak JS, Cantu D, Gaut BS (2017) Evolutionary genomics of grape (Vitis vinifera ssp. vinifera) domestication. Proc Natl Acad Sci U S A 114(44): 11715–11720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Guo Y, Su K, Liu Z, Ren Z, Li K, Guo X (2018) Construction of a highly saturated genetic map for vitis by next-generation restriction site-associated dna sequencing. BMC Plant Biol 18(1): 347. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.