Abstract
Early identification of individuals with mild cognitive impairment (MCI) is essential to combat worldwide dementia threats. Physical function indicators might be low-cost early markers for cognitive decline. To establish an early identification tool for MCI by combining physical function indicators (upper and lower limb function) via a clinical prediction modeling strategy. A total of 5393 participants aged 60 or older were included in the model. The variables selected for the model included sociodemographic characteristics, behavioral factors, mental status and chronic conditions, upper limb function (handgrip strength), and lower limb function (self-rated squat ability). Two models were developed to test the predictive value of handgrip strength (Model 1) or self-rated squat ability (Model 2) separately, and Model 3 was developed by combining handgrip strength and self-rated squat ability. The 3 models all yielded good discrimination performance (area under the curve values ranged from 0.719 to 0.732). The estimated net reclassification improvement values were 0.3279 and 0.1862 in Model 3 when comparing Model 3 to Model 1 and Model 2, respectively. The integrated discrimination improvement values were estimated as 0.0139 and 0.0128 when comparing Model 3 with Model 1 and Model 2, respectively. The model that contains both upper and lower limb function has better performance in predicting MCI. The final prediction model is expected to assist health workers in early identification of MCI, thus supporting early interventions to reduce future risk of AD, particularly in socioeconomically deprived communities.
Keywords: clinical prediction model, algorithm, cognitive function, older adults, machine learning
What do we already know about this topic?
Early identification of individuals with mild cognitive impairment (MCI) is essential to combat worldwide dementia threats. Previous studies suggested that upper and lower limb function might be low-cost early markers for cognitive decline.
How does your research contribute to the field?
Studies that simultaneously included upper and lower limb function indicators to predict MCI are rare. Our study suggested that the combination of handgrip strength and self-rated squat ability may have potentials to predict MCI.
What are your research’s implications toward theory, practice, or policy?
The prediction model developed in the current study had a good performance in predicting MCI. The variables included in the model could be easily measured without the requirement of expensive and complicated instruments, so it has the potential to be utilized in socioeconomically deprived communities for screening MCI.
Introduction
Dementia has become a major public health concern worldwide and imposes a very large economic burden on society and families.1 The number of individuals living with dementia worldwide has been projected to reach between 74.4 million and 131.5 million by 2050.2 Due to the high mortality rates associated with dementia and the difficulty of curing dementia, early identification and intervention of cognitive decline have been considered the primary dementia-related strategies. Alzheimer’s disease (AD) is considered the most common type of dementia worldwide, and mild cognitive impairment (MCI), a transition stage from normal cognition to Alzheimer’s disease (AD), has been widely accepted as a key “window of opportunity” to reverse the cognitive deterioration process, as a substantial number of individuals living with MCI may return to a normal cognitive state if early detection and early intervention could be carried out in a timely manner.3 Although a slight decline in the number of people living with dementia could be observed in many developed countries due to the greatly improved social environment (ie, education status), the number of people living with dementia in low- and middle-income countries displays a consistent increasing trend,4 indicating the increasing need to develop an economic, simple, and effective method to identify MCI, especially in low-socioeconomic settings.
Existing evidence has suggested that physical function indicators might be early markers for identifying cognitive decline.5 Many studies have highlighted that handgrip strength might predict cognitive decline among older adults,6,7 although the associations appear to be weak. For example, it has been reported in a longitudinal study that every 5-kilogram increase in handgrip strength was associated with a slightly lower risk for further deterioration of cognitive function among older American adults.8 Another study reported that grip strength was only associated with the attention dimension but was not associated with other dimensions of cognitive function.9 The abovementioned evidence suggests that MCI might be a result of the interaction of multiple factors; therefore, such diseases might not be accurately predicted by models that include handgrip strength only.8-10 Models with higher predictive capacity need to be explored to improve the identification of individuals with MCI. Recently, growing evidence has revealed that a decline in lower limb function might be another indicator related to cognitive function impairment. For example, a study reported that a shorter time (within 12.47 s) to complete the five-time sit-to-stand (FTSS) test was associated with better cognitive function among community-dwelling older Korean individuals.11 As accumulating evidence has shown the significance of lower limb function in the process of cognitive decline, a combined handgrip strength and squat ability modeling strategy might be a better solution for the prediction of MCI.12-17
Due to the potential benefits of using both upper and lower limb function to construct predictive models for the early identification of MCI, the objective of the current study was to establish an early MCI identification tool by combining a set of indicators, including upper limb function (handgrip strength) and lower limb function (squat ability), via a clinical prediction modeling strategy. As it has been widely accepted that early identification and early intervention is the major way to combat the spread of AD, the tool developed in the current study is expected to assist health workers in the early identification of MCI, thus allowing at-risk individuals to receive interventions as early as possible, thereby reducing the risk of AD. Because the disease burden of AD has been reported to be significantly increasing in many developing countries, the current study might provide a new way to predict cognitive decline, especially in a low-socioeconomic context.
Methods
Ethics Statement
The study protocol was approved by Anhui Medical University’s Institutional Review Board (No. 2020H011).
Design and Participants
Data from the Anhui Province Healthy Longevity Survey (AHLS) were extracted for analysis in the current study. Details of the AHLS were reported elsewhere.18 Briefly, the AHLS was designed to investigate the efficacy and feasibility of a behavior modification strategy for controlling major noncommunicable diseases among adults aged 60 years or older dwelling in Anhui Province, China. A multistage sampling strategy was adopted to provide a representative sample. First, 4 cities, that is, Chuzhou, Lu’an, Xuancheng, and Fuyang, were purposefully selected to represent the eastern, western, southern, and northern parts of Anhui Province, respectively. Then, 3 to 5 streets (for urban areas) or villages (for rural areas) were selected in each city. Finally, permanent residents aged 60 years or older were invited to participate in this study. Recruitment stopped when the sample size of each city reached approximately 1500 people, with equal proportions of participants dwelling in urban and rural areas. Verbal informed consent was obtained from each participant prior to data collection, and data from 6211 eligible participants were collected from July to August 2019. The participants were excluded if they (1) had any missing data on cognitive function assessment (n = 203); (2) were not able to complete the grip strength test (n = 98) or did not report their perceived squat ability (n = 5); or (3) had missing data for any covariates (n = 512). Finally, 5393 participants were included in the analysis, resulting in a response rate of 86.83%.
The required sample size for developing predictive models for binary outcomes was estimated according to the principle of at least 10 events for each included predictor.19,20 In the current study, to allow 15 predictors in the final multivariable logistic regression model, we estimated that at least 466 individuals with MCI were needed. In the current study, 1739 individuals with MCI were identified so that the development of more robust models could be expected.
Cognitive Function Assessment
The widely used Mini-Mental State Examination (MMSE)21 was employed as a cognitive function assessment instrument for the participants in the current study. The assessment had no time limit and a maximum score of 30, with a sensitivity and specificity of 79.8 and 81.3, respectively.22 The pilot study showed that the MMSE had acceptable internal consistency (Cronbach’s α = .69).23 A previous study suggested that MMSE performance might be largely influenced by education level; therefore, criteria based on different education levels were applied in the study. Specifically, the participants were classified into the MCI group if they were illiterate and had MMSE scores lower than 18, had 0 to 6 years of education and MMSE scores lower than 21, or had more than 6 years of education and MMSE scores lower than 25.24
Handgrip Strength
Handgrip strength data were obtained by actual measurement in this study and treated as continuous variables in the analysis. Handgrip strength was measured by a handgrip dynamometer by trained investigators. Participants were asked to sit in an upright position, with the measurement side elbow alongside their torso and their other arm relaxed. All participants performed 6 attempts (3 with each hand),25 and the highest value (in kg) was used for the data analysis. The dynamometers were calibrated before starting the study.
Self-Rated Squat Ability (SSA)
The self-rated squat ability of the participants was assessed by asking, “Do you have difficulty performing a squat motion 3 times consecutively?” with the options being “No difficulty,” “With some difficulty,” or “Unable to perform.” The participants were divided into 3 categories according to their responses (without difficulty, with some difficulties, and unable).
Variable Selection
The variables selected in the model included sociodemographic characteristics (age,26 sex,27 residence located in rural or urban areas,28 education level,24 marital status,29 annual income30), known behavioral factors that might be related to cognitive function decline (such as smoking,31 alcohol consumption,32 sleep quality,33 sedentary lifestyle,34 fruit consumption35), mental status (depression36) and chronic conditions37 (overweight, diabetes,38 activities of daily living abilities39).
Age was self-reported by the participants and was included in the model as a continuous variable. Education level was divided into 3 categories (illiterate, 0-6 years and above 6 years). Marital status was classified as married or other (including widowed, divorced, or never married). Participants’ annual income was divided into 3 groups (less than 6500 RMB, 6500-24 000 RMB, and above 24 000 RMB). The activities of daily living abilities of the participants were assessed by the Barthel index of ADL (BADL39), with higher scores indicating better performance of daily living activity. The internal consistency of the BADL was acceptable in the pilot study (Cronbach’s α = .662). Smoking and alcohol consumption status were included as dichotomous variables (current smoker/drinker or current nonsmoker/nondrinker). Sleep quality over the past month was self-rated as “very good,” “good,” “bad” and “very bad.” Sedentary time was included as a continuous variable that reflected the participants’ daily physical activity. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. Weekly fruit consumption frequency was included as a categorical variable (“less than 1 day per week,” “2-3 days per week,” “4-6 days per week” and “everyday”), as it has been reported to be related to cognitive function decline.35
Depression was evaluated by using the Patient Health Questionnaire (PHQ-940), which is composed of 9 questions related to the occurrence of depression symptoms in the past 2 weeks. The total score ranged from 0 to 30, and a higher total score reflected more severe depression symptoms. The internal consistency of the PHQ-9 scale in the current study was appropriate (Cronbach’s α = .89).23
Statistical Analysis
A multivariable binary logistic regression was fitted to model the association of predictor variables with the presence of MCI. A combined modeling approach was established as follows:
Model 1 included sociodemographic variables, behavioral variables and handgrip strength. The model aimed to predict the role of upper limb function in the presence of MCI.
Model 2 included sociodemographic variables, behavioral variables and self-rated squat ability. The model aimed to predict the role of lower limb function in the presence of MCI.
Model 3 included sociodemographic variables, behavioral variables, handgrip strength and self-rated squat ability. The model aimed to predict the joint role of upper and lower limb functions in the presence of MCI.
We divided the original set into a development set and a validation set at a ratio of 7:3 and assessed the performance of the models according to discrimination, calibration, and reclassification. Receiver operating characteristic curves (ROCs) were used to distinguish individuals with MCI from those without MCI. The area under the curve (AUC) value was calculated to represent the discriminatory power of the model, and a larger value indicated a better discriminatory power to differentiate between participants who did and did not have MCI. The models with AUC values of 1 were regarded as “perfect,” and the models with AUC values higher than 0.7 were regarded as “good,” whereas the models with AUCs of 0.5 and below were regarded as “noninformative.” The Hosmer‒Lemeshow test (HL test) was used to test the calibration of the models. A p value greater than 0.05 indicated a good fit between the predicted and actual measurements, that is, the ability of the model to produce unbiased estimation of the likelihood of the risk was good. Conversely, a P value less than .05 indicated a suboptimal fit. Model performance changes were evaluated by net reclassification improvement (NRI) and integrated discrimination improvement (IDI). The NRI is used to evaluate the performance change in the number of individuals who are correctly classified by the new model compared with the old model. The IDI considers the different cut points and reflects the change in the difference between the predicted probabilities of the 2 models. Both the NRI and IDI suggest model performance improvement, and values higher than 0 indicate positive improvement. In addition, decision-curve analysis (DCA) was also conducted to show the net benefits and interventions that were determined by the nomograms of the models. DCA curves were used to assess the applicability of the models.
As a sensitivity analysis, a k-fold cross-validation method was performed to verify whether the models were overfitted. The data in the development set were randomly split into k = 10 partitions, each of which accounted for 10% of the development set. Nine partitions were included in the training set, and the remaining partition was used as the test set. The procedure was repeated 10 times until each of the partitions had been used as a test set. The method was performed to check model generalizability and to ensure that the model was not overfitted.41-44
All analyses were performed by using Stata 16.0 for Windows (Stata Corp, College Station, TX). The significance level was set at P < .05.
Results
Model Development
The comparison of the distribution of variables between the development set and the validation set is shown in Table 1. The mean age was 71.00 years in the development set and 70.96 years in the validation set. Illiterate individuals accounted for nearly 50% of individuals in both sets (49.37% and 50.53%, respectively). A total of 61.03% of the participants in the development set and 59.75% of the participants in the validation set had annual incomes less than 6500 yuan. The prevalence of MCI in the development set was 32.43% (n = 1232), and the corresponding proportion in the validation set was 31.79% (n = 507). The mean grip strength was 21.32 kg in the development set and 21.27 kg in the validation set. More than half of the participants self-rated no difficulty regarding their squatting ability (54.90% and 54.92% in the development set and validation set, respectively). There was no significant difference in predictors at the individual level between the development set and validation set.
Table 1.
Distribution of Predictor Variables in Development and Validation Set of Model Combined Handgrip Strength and Self-Rated Squat Ability to Predict MCI.
| Development set (n = 3798) | Validation set (n = 1595) | |
|---|---|---|
| Sociodemographic factors | ||
| Age(years) (mean, SD) | 71.00 (7.09) | 70.96 (7.02) |
| Marital | ||
| Married | 2765 (72.80) | 1165 (73.04) |
| Unmarried | 1033 (27.20) | 430 (26.96) |
| Residence located in rural or urban areas (%) | ||
| Urban | 1870 (49.24) | 786 (49.28) |
| Rural | 1928 (50.76) | 809 (50.72) |
| Years of education (%) | ||
| 0 | 1875 (49.37) | 806 (50.53) |
| 0-6 | 1067 (28.09) | 426 (26.71) |
| >6 | 856 (22.54) | 363 (22.76) |
| Individual annual income (%) | ||
| <6500 RMB | 2318 (61.03) | 953 (59.75) |
| 6500-24 000 RMB | 860 (22.64) | 386 (24.20) |
| >24 000 RMB | 620 (16.32) | 256(16.05) |
| BMI (kg/m²) (mean, SD) | 24.19 (3.69) | 24.24 (3.60) |
| Current smoker | ||
| Yes | 796 (20.96) | 346 (21.69) |
| No | 3002 (79.04) | 1249 (78.31) |
| Current drinker | ||
| Yes | 1487 (39.15) | 614 (38.50) |
| No | 2311 (60.85) | 981 (61.50) |
| With diabetes (%) | 582 (15.32) | 246 (15.42) |
| Sedentary time (h/day) (mean, SD) | 4.34 (2.48) | 4.28 (2.49) |
| Sleep quality (%) | ||
| Very well | 806 (21.22) | 309 (19.37) |
| Well | 2074 (54.61) | 901 (56.49) |
| bad | 760 (20.01) | 330 (20.69) |
| Very bad | 158 (4.16) | 55 (3.45) |
| Weekly fruit consumption frequency (%) | ||
| 7 days | 759 (19.98) | 338 (21.19) |
| 4-6 days | 346 (9.11) | 149 (9.34) |
| 2-3 days | 1049 (27.62) | 440 (27.59) |
| Less than 1 day | 1644 (43.29) | 668 (41.88) |
| PHQ-9 score (mean, SD) | 3.69 (4.31) | 3.71 (4.33) |
| BADL score | 87.64 (8.62) | 87.82 (8.44) |
| Self-rated squat ability (%) | ||
| No difficulty | 2085 (54.90) | 876 (54.92) |
| With some difficulty | 1054 (27.75) | 440 (27.59) |
| Unable to perform | 659 (17.35) | 279 (17.49) |
| Handgrip strength (kg) (mean, SD) | 21.32 (9.16) | 21.27 (8.85) |
| Mild cognitive impairment (MCI) | 1232 (32.43) | (31.79) |
Model Discrimination and Calibration
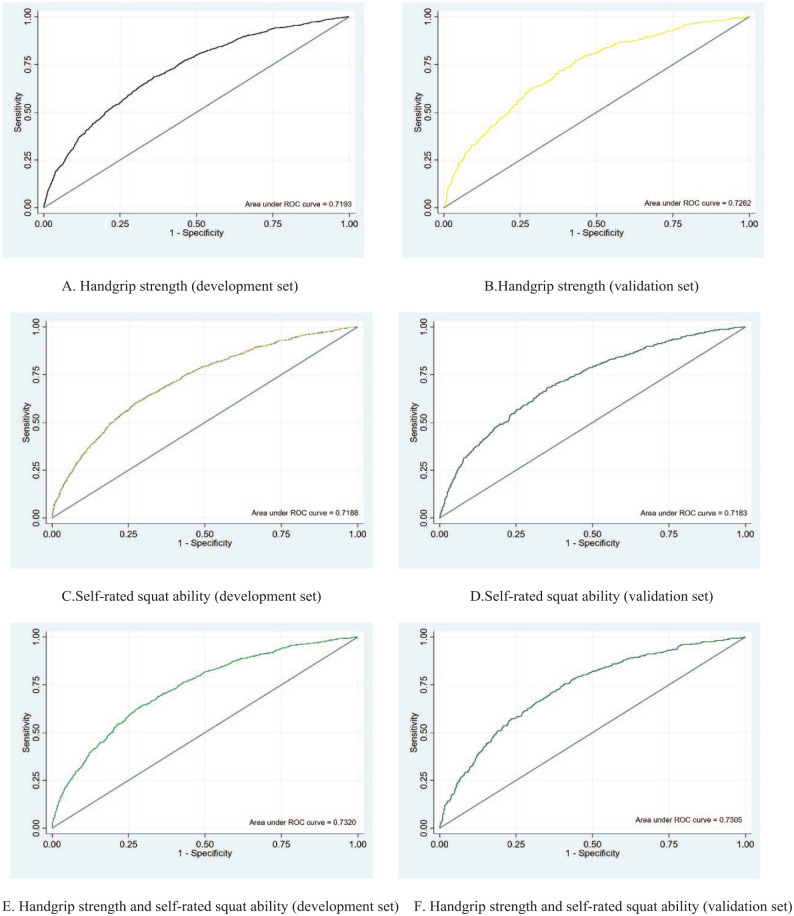
Overall, the 3 models yielded good discrimination performance (AUC = 0.719–0.732). Compared with Model 1 and Model 2, the AUC value was slightly higher in Model 3 (Model 3 AUC values were 0.730 and 0.732 in the development and validation sets, respectively) (Figure 1A-F).
Figure 1.
Receiver operating characteristic curves for the development set and the validation set of 3 prediction models. These models adjusted age (continuous), residence located in urban or rural areas (urban and rural), marital status (married and unmarried), income (lower 6500 RMB, 6500-24 000 RMB and more than 24 000 RMB), education (illiteracy, 0-6 years and above 6 years), BMI (continuous), drinking (current, noncurrent), smoking (current, noncurrent), diabetes (yes or no), sedentary time (continuous), sleeping quality (very well, well, bad and very bad), weekly fruit consumption frequency (7, 4-6, 2-3 days and less than 1 day), PHQ-9 scores (continuous), BADL (continuous), self-rated squat ability (no difficulty, with some difficulty and unable to perform), handgrip strength (continuous): (A) handgrip strength (development set), (B) handgrip strength (validation set), (C) self-rated squat ability (development set), (D) self-rated squat ability (validation set), (E) handgrip strength and self-rated squat ability (development set), and (F) handgrip strength and self-rated squat ability (validation set).
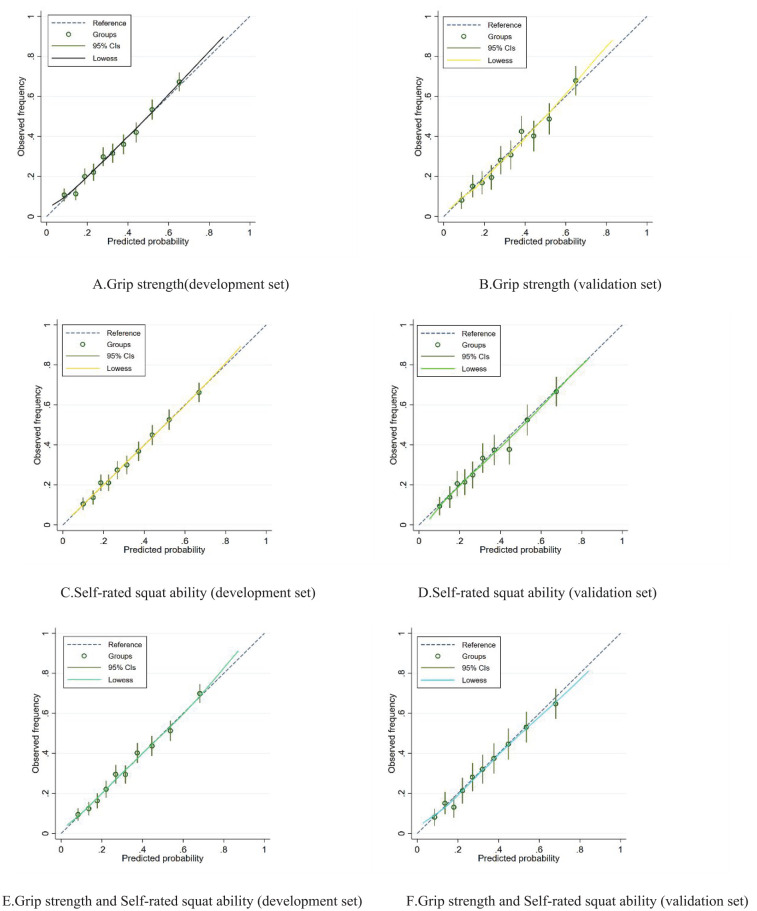
Figure 2 shows the predicted risk of MCI compared to the observed risk. Overall, the results of the Hosmer–Lemeshow goodness-of-fit test showed nonsignificant differences (all P > .05) between the predicted risks and the observed risks in the 3 models, indicating good calibration in all 3 models (Figure 2A-F).
Figure 2.
Calibration curves for the development set and the validation set of 3 prediction models. These models adjusted age (continuous), residence located in urban or rural areas (urban and rural), marital status (married and unmarried), income (lower 6500 RMB, 6500-24 000 RMB and more than 24 000 RMB), education (illiteracy, 0-6 years and above 6 years), BMI (continuous), drinking (current, noncurrent), smoking (current, noncurrent), diabetes (yes or no), sedentary time (continuous), sleeping quality (very well, well, bad, and very bad), weekly fruit consumption frequency (7, 4-6, 2-3 days, and less than 1 day), PHQ-9 scores (continuous), BADL (continuous), self-rated squat ability (no difficulty, with some difficulty and unable to perform), handgrip strength (continuous): (A) grip strength(development set), (B) grip strength (validation set), (C) self-rated squat ability (development set), (D) self-rated squat ability (validation set), (E) grip strength and Self-rated squat ability (development set), and (F) grip strength and Self-rated squat ability (validation set)
In the sensitivity analysis, the results of the 10-fold cross validation indicated that all 3 models had good discrimination without overfitting (Supplemental Figure 1A-C).
Model Comparison
The estimated NRI values were 0.3279 and 0.1862 in Model 3 when comparing Model 3 to Model 1 and Model 2, respectively (both P < .01), indicating a significant improvement in the reclassification proportion in the combined model. The IDI values were estimated as 0.0139 and 0.0128 (both P < .001) when comparing Model 3 with Model 1 and Model 2, respectively. The DAC curves demonstrated that Model 3 had the highest net benefit in the prediction probability of any of the 3 prediction models. Interventions guided by Model 3 had a higher net benefit than those guided by Model 1 and Model 2 when the threshold probability was between 0.1 and 0.8 in both datasets. (Supplemental Figure 2A).
Model Selection
In the comparison of the 3 models, Model 3 was found to have the best performance. The final expression of Model 3 is shown as follows:
0.04 * age + 0.26 × residence located in rural or urban area −0.26 × income −0.01 × gender + 0.78 × 10−4 × BADL + 0.31 × 10−4 × BMI + 0.01 × diabetes + 0.02 × PHQ-9 score + 0.08 × fruit consumption frequency + 0.02 × education + 0.11 × marital status −0.17 × 10−4 × drinking −0.02 × smoking −0.17 × 10−4 × sedentary hours + 0.05 × sleeping quality + 0.40 × self-rated squat ability −0.04 × grip strength −4.57
Note: continuous variables: age, BADL score, BMI, handgrip strength, PHQ-9 score, sedentary hours; categorical variables: residence located in rural or urban area (urban = 1*, rural = 2*), income (less than 6500 RMB = 1, 6500-24 000 RMB = 2 and more than 24 000 RMB = 3), gender (male = 1, female = 2), diabetes (yes = 1, no = 0), weekly fruit consumption frequency (7 days = 1, 4-6 days = 2, 2-3 days = 3, less than 1 day = 4), education (illiteracy = 1, 0-6 years = 2, above 6 years = 3), marital status (married = 1, other = 2), drinking (current = 1, noncurrent = 0), smoking (current = 1, noncurrent = 2), sleeping quality (very well = 1, well = 2, bad = 3, very bad = 4), self-rated squat ability (without difficulty = 1, with some difficulties = 2, and unable = 3)
Discussion
In the current study, we developed 3 MCI prediction models for identifying individuals with MCI and validated the performance of the 3 models by using a large and representative sample of older Chinese adults. The results of our current study suggested that models with combined upper and lower limb function predictors had better performance in predicting MCI. The data for the variables that were included in the final model could be easily obtained, and the full risk equation was also reported so that the model could be validated and applied in practice in similar settings. To our knowledge, this study is the first to model upper limb function (handgrip strength) and lower limb function (Self-rated squat ability) to predict MCI.
Many previous studies have indicated correlations between upper and lower limb function and cognitive decline, the conclusions of which are consistent with our findings.5,6,11,45 However, studies that simultaneously included indicators of upper and lower limb function to predict cognitive function decline are still very rare. A study reported correlations between fore-finger tapping and toe-tapping performance with the MMSE score; however, the requirements for specific instruments and trained professionals were relatively high.46 In the current study, we employed handgrip strength and Self-rated squat ability to reflect upper and lower limb function, respectively. The simplicity and economy of data collection give our predictive models more potential to be applied in a low-socioeconomic context.
Among the many metrics that have been developed to assess the discrimination of prediction tools, the AUC is the most traditional and widely applied, as it can address the discrimination ability of the model simply and directly. However, AUC has been criticized because correct or incorrect diagnostic classifications or the absolute number of event cases can hardly be provided. Additionally, the AUC improvement in absolute value is often very small, even for better models.47 Our results showed that the predictive accuracy (assessed by AUC) was not significantly increased in the combined model; however, the NRI and IDI values suggested a significant improvement in the performance of the combined prediction model compared with the models with upper or lower limb function only.
The link between limb function and cognition might be explained by the impact of the central nervous system. Cognition and motor control share some common brain networks; for individuals who are cognitively impaired, their brain networks may be overloaded when facing the complex tasks of cognitive responses and functional mobility.48,49 Thus, impairment of the central nervous system can lead to defects in both motor (eg, limb function) and cognitive functions.45 Another explanation might be the hemodynamic alteration of the blood supply to the brain. People with poor limb function usually have reduced venous return, resulting in insufficient vertebral perfusion and further leading to cognitive function decline.50-52
The present study has many strengths. First, the prediction tool was developed with a representative sample of older Chinese adults. The representativeness of the sample was guaranteed by the multistage sampling strategy and the large sample size. Geographical and urban‒rural differences were considered, and the response rate was high (86.83%). Some risk prediction tools (eg, ANU-ADRI53) for the detection of AD or MCI have been developed, but such tools might not be suitable for older Chinese adults. For example, fish intake has been used as a predictor in ANU-ADRI; however, the intake of fish (especially fatty fish) is generally low among Chinese adults.54 Second, the data of variables included in the model developed in the current study can be obtained without specialized laboratory facilities. Wang et al developed an effective risk prediction model for MCI among older Chinese adults; however, it might not be suitable for population-based screening since it included clinical measures.55 Therefore, the prediction model in the current study is relatively economic and convenient and can be easily applied, especially in economically underdeveloped communities.
However, the current study still has limitations. First, our model can only predict the presence of MCI due to the cross-sectional design. Longitudinal studies should be conducted to verify the prediction ability of such variables for incident MCI in the future. Second, in the current study, lower limb function was assessed by self-rated squat ability, and it might be over- or underestimated. Actual measurements are usually considered more accurate; however, they might not be precisely measured without a trained health worker and instruments. For example, for measuring FTSS, some researchers have even suggested using smartphones and body-worn inertial sensors to enable precise and objective movement measurements.56,57 Additionally, actual measurements might not be appropriate for all individuals. For example, those who are suffering chronic pain, loss of physical function or mental disability tend to be excluded from the measurements. As an alternative, individuals’ perception of lower limb function had moderate to high consistency with the actual performance.58-62 For example, a previous study suggested that a single-item self-rated question of physical function could be a useful tool to identify variation in measured fitness,63 indicating the validity of the Self-rated squat ability, which was used for this study. Finally, some factors (eg, genetic and biochemical indicators) that may influence MCI were not included in the prediction model. However, specialized instruments are needed for those tests so that their practical application might be limited. Despite the fact that those factors were not included, the general performance of the prediction model was good, so the ability of the predictive model to specify individuals with and without MCI can be guaranteed.
Conclusions
In summary, in the current study, we established an early MCI identification tool by combining a set of indicators, including upper limb function (handgrip strength) and lower limb function (self-rated squat ability), via a clinical prediction modeling strategy. This prediction model included variables that could be easily measured without the requirement of expensive and complicated instruments, so it has the potential to be utilized in socioeconomically deprived communities for screening MCI. Since early identification and intervention of MCI is essential to combat the spreading of AD, the present study suggests the utilization of the aforementioned model to identify individuals with MCI as early as possible, although this model should be further verified in some other population, especially in some appropriate longitudinal cohorts.
Supplemental Material
Supplemental material, sj-docx-1-inq-10.1177_00469580231155295 for The Value of Handgrip Strength and Self-Rated Squat Ability in Predicting Mild Cognitive Impairment: Development and Validation of a Prediction Model by Han Xiao, Hou Fangfang, Wang Qiong, Zhou Shuai, Zhang Jingya, Lou Xu, Shen Guodong and Zhang Yan in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Supplemental material, sj-docx-2-inq-10.1177_00469580231155295 for The Value of Handgrip Strength and Self-Rated Squat Ability in Predicting Mild Cognitive Impairment: Development and Validation of a Prediction Model by Han Xiao, Hou Fangfang, Wang Qiong, Zhou Shuai, Zhang Jingya, Lou Xu, Shen Guodong and Zhang Yan in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Acknowledgments
The authors thank all participants in the AHLS for providing their personal data. We also thank all colleagues involved in the study for their cooperation and efforts in data collection and management.
Footnotes
Author Contributions: All authors meet the criteria for authorship according to the COI form and their contributions to the manuscript. H.X. drafted the manuscript. L.X., S.G., and Z.Y. framed the concept and designed the study. The data collection and material preparation were conducted by Z.S., W.Q., and Z.J., and the data analysis was performed by H.F. and H.X.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was funded by the National Natural Science Foundation of China, Grant Number 72004003 to YZ, the Key Projects of Science and the Key Project of Science and Technology of Anhui Province, Gant Number 202004b11020019 to GS, and the Hefei Municipal Natural Science Foundation, Grant Number 2021005 to GS.
ORCID iD: Zhang Yan  https://orcid.org/0000-0003-3367-5832
https://orcid.org/0000-0003-3367-5832
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Wu YT, Beiser AS, Breteler MMB, et al. The changing prevalence and incidence of dementia over time - current evidence. Nat Rev Neurol. 2017;13(6):327-339. [DOI] [PubMed] [Google Scholar]
- 2. Prince M. The global impact of dementia: an analysis of prevalence, incidence, cost and trends; 2015. [Google Scholar]
- 3. Jia L, Du Y, Chu L, et al. Prevalence, risk factors, and management of dementia and mild cognitive impairment in adults aged 60 years or older in China: a cross-sectional study. Lancet Public Health. 2020;5(12):e661-e671. [DOI] [PubMed] [Google Scholar]
- 4. Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer Dement J Alzheimer Assoc. 2013;9(1):63-75. [DOI] [PubMed] [Google Scholar]
- 5. Chou MY, Nishita Y, Nakagawa T, et al. Role of gait speed and grip strength in predicting 10-year cognitive decline among community-dwelling older people. BMC Geriatr. 2019; 19(1):186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bohannon RW. Grip strength: an indispensable biomarker for older adults. Clin Interv Aging. 2019;14:1681-1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zammit AR, Robitaille A, Piccinin AM, Muniz-Terrera G, Hofer SM. Associations between aging-related changes in grip strength and cognitive function in older adults: a systematic review. J Gerontol A Biol Sci Med Sci. 2019;74(4):519-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McGrath R, Vincent BM, Hackney KJ, Robinson-Lane SG, Downer B, Clark BC. The longitudinal associations of handgrip strength and cognitive function in aging Americans. J Am Med Dir Assoc. 2020;21(5):634-639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McGough EL, Cochrane BB, Pike KC, Logsdon RG, McCurry SM, Teri L. Dimensions of physical frailty and cognitive function in older adults with amnestic mild cognitive impairment. Ann Phys Rehabil Med. 2013;56(5):329-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Adamo DE, Anderson T, Koochaki M, Fritz NE. Declines in grip strength may indicate early changes in cognition in healthy middle-aged adults. PLoS One. 2020;15(4):e0232021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim GM, Kim BK, Kim DR, Liao Y, Park JH, Park H. An association between lower extremity function and cognitive frailty: a sample population from the KFACS study. Int J Environ Res Public Health. 2021;18(3):eng1012384551661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Balsalobre-Fernández C, Cordón A, Unquiles N, Muñoz-García D. Movement velocity in the chair squat is associated with measures of functional capacity and cognition in elderly people at low risk of fall. PeerJ. 2018;6:e4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Eggermont LH, Gavett BE, Volkers KM, et al. Lower-extremity function in cognitively healthy aging, mild cognitive impairment, and Alzheimer’s disease. Arch Phys Med Rehabil. 2010;91(4):584-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Boyle PA, Wilson RS, Buchman AS, et al. Lower extremity motor function and disability in mild cognitive impairment. Exp Aging Res. 2007;33(3):355-371. [DOI] [PubMed] [Google Scholar]
- 15. Alcazar J, Losa-Reyna J, Rodriguez-Lopez C, et al. The sit-to-stand muscle power test: an easy, inexpensive and portable procedure to assess muscle power in older people. Exp Gerontol. 2018;112:38-43. [DOI] [PubMed] [Google Scholar]
- 16. Teruya SL, Dimino C, Silverman KD, Mielenz T. Poor lower extremity functioning is associated with modest increased incidence of probable dementia. Geriatrics. 2021;6(3): eng1017040192308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frith E, Loprinzi PD. The association between lower extremity muscular strength and cognitive function in a national sample of older adults. J Lifestyle Med. 2018;8(2):99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fangfang H, Xiao H, Shuai Z, et al. Living Environment, built environment and cognitive function among older Chinese adults: results from a cross-sectional study. J Prev Alzheimer Dis. 2022;9(1):126-135. [DOI] [PubMed] [Google Scholar]
- 19. Concato J. The risk of determining risk with multivariable models. Ann Intern Med. 1993;118(3):201-210. [DOI] [PubMed] [Google Scholar]
- 20. Riley RD, Ensor J, Snell KIE, et al. Calculating the sample size required for developing a clinical prediction model. BMJ. 2020;368:m441. [DOI] [PubMed] [Google Scholar]
- 21. Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 22. Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411-431. [DOI] [PubMed] [Google Scholar]
- 23. Zhou S, Wang Q, Zhang J, et al. Depressive symptoms and cognitive decline among Chinese rural elderly individuals: a longitudinal study with 2-year follow-up. Front Public Health. 2022;10:939150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. De Silva R, Disanayaka S, De Zoysa N, et al. Norms for the mini-mental state examination from a sample of Sri Lankan older people. Int J Geriatr Psychiatry. 2009;24(7):666-670. [DOI] [PubMed] [Google Scholar]
- 25. Coldham F, Lewis J, Lee H., et al. The reliability of one vs. three grip trials in symptomatic and asymptomatic subjects. J Hand Ther. 2006;19(3):318-326. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira N, Owen A, Mohan A, Corbett A, Ballard C. Associations between cognitively stimulating leisure activities, cognitive function and age-related cognitive decline. Int J Geriatr Psychiatry. 2015;30(4):422-430. [DOI] [PubMed] [Google Scholar]
- 27. Guo Y, Yang M, Yan Y, Wang L, Gong J. Sex differentials in relationships between functional fitness and cognitive performance in older adults: a canonical correlation analysis. Sci Rep. 2018;8:4146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nakamura K, Kitamura K, Watanabe Y, Shinoda H, Sato H, Someya T. Rural-urban differences in the prevalence of cognitive impairment in independent community-dwelling elderly residents of Ojiya city, Niigata prefecture, Japan. Environ Health Prev Med. 2016;21(6):422-429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhang Z, Liu H, Zhang Y. Marital loss and cognitive function: does timing matter? J Gerontol B Psychol Sci Soc Sci. 2022;77(10):1916-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Rodriguez FS, Hofbauer LM, Röhr S. The role of education and income for cognitive functioning in old age: a cross-country comparison. Int J Geriatr Psychiatry. 2021;36(12):1908-1921. [DOI] [PubMed] [Google Scholar]
- 31. Campos MW, Serebrisky D, Castaldelli-Maia JM. Smoking and cognition. Curr Drug Abuse Rev. 2016;9(2):76-79. [DOI] [PubMed] [Google Scholar]
- 32. Topiwala A, Ebmeier KP. Effects of drinking on late-life brain and cognition. Evid Based Ment Health. 2018;21(1):12-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dzierzewski JM, Dautovich N, Ravyts S. Sleep and cognition in older adults. Sleep Med Clin. 2018;13(1):93-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Chandrasekaran B, Pesola AJ, Rao CR, Arumugam A. Does breaking up prolonged sitting improve cognitive functions in sedentary adults? A mapping review and hypothesis formulation on the potential physiological mechanisms. BMC Musculoskelet Disord. 2021;22(1):274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Román GC, Jackson RE, Gadhia R, Román AN, Reis J. Mediterranean diet: the role of long-chain ω-3 fatty acids in fish; polyphenols in fruits, vegetables, cereals, coffee, tea, cacao and wine; probiotics and vitamins in prevention of stroke, age-related cognitive decline, and Alzheimer disease. Rev Neurol (Paris). 2019;175(10):724-741. [DOI] [PubMed] [Google Scholar]
- 36. Dehn LB, Beblo T. [Depressed, biased, forgetful: the interaction of emotional and cognitive dysfunctions in depression]. Neuropsychiatr. 2019;33(3):123-130. [DOI] [PubMed] [Google Scholar]
- 37. Dye L, Boyle NB, Champ C, Lawton C. The relationship between obesity and cognitive health and decline. Proc Nutr Soc. 2017;76(4):443-454. [DOI] [PubMed] [Google Scholar]
- 38. Low S, Ng TP, Lim CL, et al. Association between lower extremity skeletal muscle mass and impaired cognitive function in type 2 diabetes. Sci Rep. 2020;10(1):2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rajan KB, Hebert LE, Scherr P, et al. Cognitive and physical functions as determinants of delayed age at onset and progression of disability. J Gerontol A Biol Sci Med Sci. 2012;67(12):1419-1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Levis B, Benedetti A, Thombs BD, DEPRESsion Screening Data (DEPRESSD) Collaboration. Accuracy of patient health questionnaire-9 (PHQ-9) for screening to detect major depression: individual participant data meta-analysis. BMJ. 2019;365:l1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Dankers F, Traverso A, Wee L, van Kuijk SMJ. Prediction modeling methodology. In: Kubben P, Dumontier M, Dekker A. eds. Fundamentals of Clinical Data Science. Springer; 2019:101-120. [PubMed] [Google Scholar]
- 42. Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12(2):132-139. [DOI] [PubMed] [Google Scholar]
- 43. Linden A. KFOLDCLASS: stata module for generating classification statistics of k-fold cross-validation for binary outcomes; 2017. [Google Scholar]
- 44. Witten IH. Data Mining: Practical Machine Learning Tools and Techniques, 3rd ed. Elsevier Press; 2011. [Google Scholar]
- 45. Ishii H, Makizako H, Doi T, Tsutsumimoto K, Shimada H. Associations of skeletal muscle mass, lower-extremity functioning, and cognitive impairment in community-dwelling older people in Japan. J Nutr Health Aging. 2019;23(1):35-41. [DOI] [PubMed] [Google Scholar]
- 46. Mancioppi G, Fiorini L, Rovini E, et al. Innovative motor and cognitive dual-task approaches combining upper and lower limbs may improve dementia early detection. Sci Rep. 2021;11(1):7449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moons KGM, de Groot JAH, Linnet K, Reitsma JB, Bossuyt PMM. Quantifying the added value of a diagnostic test or marker. Clin Chem. 2012;58(10):1408-1417. [DOI] [PubMed] [Google Scholar]
- 48. Rosano C, Studenski SA, Aizenstein HJ, Boudreau RM, Longstreth Wt Jr, Newman AB. Slower gait, slower information processing and smaller prefrontal area in older adults. Age Ageing. 2012;41(1):58-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Annweiler C, Beauchet O, Celle S, et al. Contribution of brain imaging to the understanding of gait disorders in Alzheimer’s disease: a systematic review. Am J Alzheimers Dis Other Demen. 2012;27(6):371-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McLeod KJ, Jain T. Postural hypotension and cognitive function in older adults. Gerontol Geriatr Med. 2017;3:2333721417733216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Wolters FJ, Zonneveld HI, Hofman A, et al. Cerebral perfusion and the risk of dementia: a population-based study. Circulation. 2017;136(8):719-728. [DOI] [PubMed] [Google Scholar]
- 52. McLeod KJ, Stromhaug A. Reversal of cognitive impairment in a hypotensive elderly population using a passive exercise intervention. Clin Interv Aging. 2017;12:1859-1866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Anstey KJ, Cherbuin N, Herath PM. Development of a new method for assessing global risk of Alzheimer’s disease for use in population health approaches to prevention. Prev Sci. 2013;14(4):411-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Jin X, Xiong S, Yuan C, et al. Apolipoprotein E genotype, meat, fish, and egg intake in relation to mortality among older adults: a longitudinal analysis in China. Front Med (Lausanne). 2021;8:697389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wang B, Shen T, Mao L, Xie L, Fang QL, Wang X. Establishment of a risk prediction model for mild cognitive impairment among elderly Chinese. J Nutr Health Aging. 2020;24(3):255-261. [DOI] [PubMed] [Google Scholar]
- 56. Ejupi A, Brodie M, Lord SR, Annegarn J, Redmond SJ, Delbaere K. Wavelet-based sit-to-stand detection and assessment of fall risk in older people using a wearable pendant device. IEEE Trans Biomed Eng. 2017;64(7):1602-1607. [DOI] [PubMed] [Google Scholar]
- 57. Pham MH, Warmerdam E, Elshehabi M, et al. Validation of a lower back “wearable”-based sit-to-stand and stand-to-sit algorithm for patients with Parkinson’s disease and older adults in a home-like environment. Front Neurol. 2018;9:652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Kluft N, van Dieën JH, Pijnappels M. The degree of misjudgment between perceived and actual gait ability in older adults. Gait Posture. 2017;51:275-280. [DOI] [PubMed] [Google Scholar]
- 59. Siltanen S, Portegijs E, Saajanaho M, et al. The combined effect of lower extremity function and cognitive performance on perceived walking ability among older people: a 2-year follow-up study. J Gerontol A Biol Sci Med Sci. 2018;73(11):1568-1573. [DOI] [PubMed] [Google Scholar]
- 60. Weijer RHA, Hoozemans MJM, van Dieën JH, Pijnappels M. Consistency and test-retest reliability of stepping tests designed to measure self-perceived and actual physical stepping ability in older adults. Aging Clin Exp Res. 2019;31(12):1765-1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Ryckewaert G, Luyat M, Rambour M, et al. Self-perceived and actual ability in the functional reach test in patients with Parkinson’s disease. Neurosci Lett. 2015;589:181-184. [DOI] [PubMed] [Google Scholar]
- 62. Nicholas ML, Burch K, Mitchell JR, Fox AB, Baum CM, Connor LT. Self-perception of physical function contributes to participation in cognitively- and physically-demanding activities after stroke. Front Neurol. 2020;11:474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Petersen CB, Eriksen L, Dahl-Petersen IK, Aadahl M, Tolstrup JS. Self-rated physical fitness and measured cardiorespiratory fitness, muscular strength, and body composition. Scand J Med Sci Sports. 2021;31(5):1086-1095. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-inq-10.1177_00469580231155295 for The Value of Handgrip Strength and Self-Rated Squat Ability in Predicting Mild Cognitive Impairment: Development and Validation of a Prediction Model by Han Xiao, Hou Fangfang, Wang Qiong, Zhou Shuai, Zhang Jingya, Lou Xu, Shen Guodong and Zhang Yan in INQUIRY: The Journal of Health Care Organization, Provision, and Financing
Supplemental material, sj-docx-2-inq-10.1177_00469580231155295 for The Value of Handgrip Strength and Self-Rated Squat Ability in Predicting Mild Cognitive Impairment: Development and Validation of a Prediction Model by Han Xiao, Hou Fangfang, Wang Qiong, Zhou Shuai, Zhang Jingya, Lou Xu, Shen Guodong and Zhang Yan in INQUIRY: The Journal of Health Care Organization, Provision, and Financing