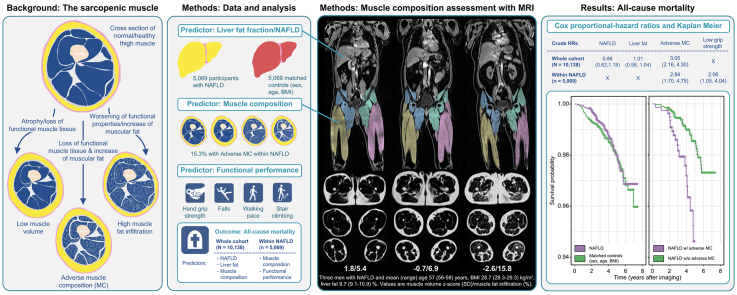
Abstract
Background & Aims
Adverse muscle composition (MC) (i.e., low muscle volume and high muscle fat) has previously been linked to poor functional performance and comorbidities in non-alcoholic fatty liver disease (NAFLD). In this study we aimed to investigate associations of all-cause mortality with liver fat, NAFLD, and MC in the UK Biobank imaging study.
Methods
Magnetic resonance images of 40,174 participants were analyzed for liver proton density fat fraction (PDFF), thigh fat-free muscle volume (FFMV) z-score, and muscle fat infiltration (MFI) using the AMRA® Researcher. Participants with NAFLD were sex-, age-, and BMI-matched to participants without NAFLD with low alcohol consumption. Adverse MC was identified using previously published cut-offs. All-cause mortality was investigated using Cox regression. Models within NAFLD were crude and subsequently adjusted for sex, age, BMI (M1), hand grip strength, physical activity, smoking, alcohol (M2), and previous cancer, coronary heart disease, type 2 diabetes (M3).
Results
A total of 5,069 participants had NAFLD. During a mean (±SD) follow-up of 3.9 (±1.4) years, 150 out of the 10,138 participants (53% men, age 64.4 [±7.6] years, BMI 29.7 [±4.4] kg/m2) died. In the matched dataset, neither NAFLD nor liver PDFF were associated with all-cause mortality, while all MC variables achieved significance. Within NAFLD, adverse MC, MFI and FFMV z-score were significantly associated with all-cause mortality and remained so in M1 and M2 (crude hazard ratios [HRs] 2.84, 95% CI 1.70–4.75, p <0.001; 1.15, 95% CI 1.07–1.24, p <0.001; 0.70, 95% CI 0.55–0.88, p <0.001). In M3, the relationship was attenuated for adverse MC and FFMV z-score (adjusted HRs 1.72, 95% CI 1.00–2.98, p = 0.051; 0.77, 95% CI 0.58–1.02, p = 0.069) but remained significant for MFI (adjusted HR 1.13, 95% CI 1.01–1.26, p = 0.026).
Conclusions
Neither NAFLD nor liver PDFF was predictive of all-cause mortality. Adverse MC was a strong predictor of all-cause mortality in individuals with NAFLD.
Impact and implications
Individuals with fatty liver disease and poor muscle health more often suffer from poor functional performance and comorbidities. This study shows that they are also at a higher risk of dying. The study results indicate that measuring muscle health (the patient's muscle volume and how much fat they have in their muscles) could help in the early detection of high-risk patients and enable targeted preventative care.
Keywords: sarcopenia, Magnetic resonance imaging, Imaging biomarker, survival, Muscle fat infiltration, Myosteatosis, UK Biobank
Abbreviations: FFMV, fat-free muscle volume; HR, hazard ratio; MFI, muscle fat infiltration; NAFLD, non-alcoholic fatty liver disease; PDFF, proton density fat fraction; T2DM, type 2 diabetes mellitus
Graphical abstract
Highlights
-
•
Liver fat and muscle composition were measured in >40,000 UK Biobank participants.
-
•
Neither liver fat nor non-alcoholic fatty liver disease was linked to mortality.
-
•
Adverse muscle composition predicted mortality in non-alcoholic fatty liver disease.
Introduction
The prevalence of non-alcoholic fatty liver disease (NAFLD) is high and rising in parallel with the obesity and type 2 diabetes mellitus (T2DM) epidemics.1 Although NAFLD is highly prevalent, it remains challenging to foresee who will progress towards more advanced liver disease and consequently be at increased risk of liver-related events (i.e., decompensation and/or hepatocellular carcinoma).2 A reason for this unpredictability is the high heterogeneity and wide range of clinical phenotypes observed within NAFLD. Most individuals with NAFLD do not progress to advanced liver disease, and there has been a recent rise in discussions on whether NAFLD should be redefined to include criteria related to obesity and metabolic syndrome (e.g., metabolic [dysfunction]-associated fatty liver disease [MAFLD]).3 Identifying clinically meaningful sub-phenotypes within NAFLD could improve preventative care, aid in the development of effective pharmacological treatments, and reduce healthcare costs by separating high- and low-risk patients in the early stages of liver disease.
Sarcopenia (a muscle disease characterized by progressive loss of muscle mass and function) is intensified in individuals with metabolic disorders and is highly prevalent in end-stage diseases.[4], [5], [6], [7], [8], [9] Although sarcopenia and frailty are clear concerns in later stages of liver disease, the importance of muscle health is commonly overlooked in earlier disease states and more prevalent conditions like obesity, T2DM, or NAFLD.10,11 It is still unclear if sarcopenia accelerates the progression of disease or the other way around, but research has shown that poor muscle health is associated with higher mortality in patients with cirrhosis and may affect the outcome of liver transplantation.[10], [11], [12], [13] Although sarcopenia is recognized as a highly debilitating condition with personal, social, and economic burdens when untreated, there is no consensus on how to diagnose and quantitatively assess the disease.[14], [15], [16], [17] This complicates the implementation of sarcopenia assessment to support treatment decisions in late stages of liver disease and hinders further understanding of whether early knowledge of a patient's muscle status could help guide treatment plans.
Based on a rapid and standardized MRI protocol, muscle composition can be quantified with high accuracy and precision.[18], [19], [20], [21] Measuring both thigh fat-free muscle volume (FFMV) and muscle fat infiltration (MFI) allows for detection of a condition called 'adverse muscle composition’. Adverse muscle composition is prevalent within NAFLD and has been linked to increased comorbidity (coronary heart disease and T2DM) and poor functional performance in individuals with NAFLD, and to all-cause mortality within a general adult population.22,23
This work aimed to investigate the associations of NAFLD, liver fat, and muscle composition with all-cause mortality in the UK Biobank imaging study.
Patients and methods
Participants included in this study were stratified from the first 40,174 individuals scanned in the UK Biobank imaging study. The UK Biobank is a long-term population study following 500,000 volunteers aged 40-69 years recruited between 2006-2010.24 As a sub-study, 100,000 participants are being re-called for a detailed imaging assessment, including a repeat of the baseline assessments.
Inclusion
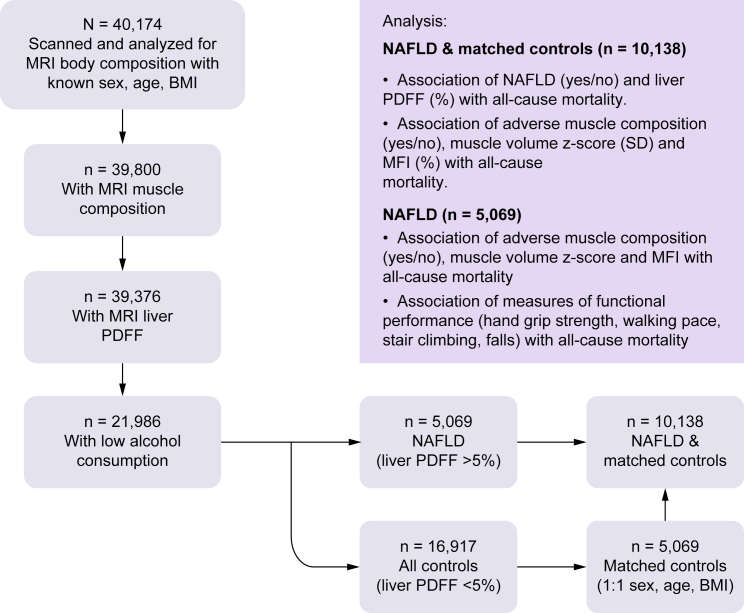
The inclusion of participants and analyses are summarized in Fig. 1. For the muscle composition assessment, participants were required to have known sex, age, weight, height, and a complete description of muscle composition (FFMV and MFI of at least one leg) (n = 374 with missing data). For investigations of NAFLD, participants were also required to have non-missing values for MRI liver proton density fat fraction (PDFF), known alcohol consumption (n = 703 with missing data), and low alcohol consumption (<14/21 units/week [females/males]26) (n = 17,112 excluded), resulting in 21,988 participants.
Fig. 1.
Inclusion of study participants and summary of analyses.
MFI, muscle fat infiltration; MRI, magnetic resonance imaging; NAFLD, non-alcoholic fatty liver disease; PDFF, proton density fat fraction.
Based on the subset of participants with low alcohol consumption, participants with NAFLD (n = 5,069) were matched 1:1 with controls using sex, age, and BMI, creating a matched dataset of 10,138 individuals.
MRI measurements
The participants were scanned in a Siemens MAGNETOM Aera 1.5-T MRI scanner (Siemens Healthineers, Erlangen, Germany) using a 6-10 min dual-echo Dixon Vibe protocol, providing a water and fat separated volumetric neck-to-knee dataset. Body composition analyses were performed using AMRA® Researcher (AMRA Medical AB, Linköping, Sweden).[18], [19], [20], [21] Further details are provided in the supplementary materials and methods. The MRI measurements are publicly available from the UK Biobank (Category 149, Abdominal composition):
-
•
Liver proton density fat fraction (PDFF): The average PDFF of nine regions of interest, placed while avoiding any inhomogeneities, major vessels, and bile ducts.21 Further details are provided in the supplementary materials and methods.
-
•
Fat-free muscle volume (FFMV, referred to as ”muscle volume”): The volume of all voxels with fat fraction <50% (also known as ”viable muscle tissue”) in the thighs.19,20 If data were missing for one leg, the total thigh muscle volume was estimated by multiplying the available measurement by two.
-
•
Fat-free muscle volume z-score (referred to as “muscle volume z-score”): For each participant, a matched virtual control group (VCG) including at least 150 individuals of the same sex and similar BMI was stratified from the study participants with complete muscle composition data. Based on each VCG, a personalized muscle volume z-score was calculated, measuring how many standard deviations each participant was from the mean thigh FFMV/height2 of their VCG.25 This variable is sex-, weight-, and height-invariant and has been associated with poor function and increased hospitalization.22,25
-
•
Muscle fat infiltration (MFI): The mean fat fraction in the “viable muscle tissue” (i.e., FFMV) of the right and left anterior thighs.19,20 If data were missing for one leg, the mean MFI was estimated by the MFI of the other leg. A sex-adjusted MFI was calculated by subtracting the sex-specific population median from each participant's MFI value.
Definitions
NAFLD: Defined by MRI liver PDFF >5% and lack of excess alcohol consumption (<14/21 units/week [females/males]).26,27 NAFLD stratification was made directly from the UK Biobank imaging participants (community volunteers, not selected owing to abnormal liver function tests). No exclusions were made based on rarer forms of liver disease or medications.
High MFI: As the magnitude of MFI differs between female and male participants,18,23 ”high MFI” was defined as >75th percentile of the whole cohort (N = 40,177) for female/male participants separately (>8.82/7.69%).22,23
Low muscle volume z-score: As the muscle volume z-score is sex invariant,25 “low muscle volume z-score” was defined as <25th percentile of the whole cohort (N = 40,174) for both female and male participants (<−0.68 SD).22,23
Adverse muscle composition: Defined by low muscle volume z-score and high MFI according to the previously published definition.22,23
Data collection – UK biobank
Mortality data were obtained through the UK Biobank's linkage to national death registries. Height was recorded using a Seca stadiometer and weight with a Tanita BC418ma. Previous diagnoses of cancer, coronary heart disease, and T2DM were based on electronic healthcare records (accessed September 2021, available from April 1992 to September 2021) and/or self-reported information collected via interviews with trained nurses. Hand grip strength was measured using a Jamar J00105 hydraulic hand dynamometer (protocol provided in supplementary sections 1-3). The data recorded for the dominant hand were used. If information on handedness was missing or a participant reported using both hands, the mean of the right and left hand was used. Low hand grip strength was defined using the sex-specific cut-offs recommended by the European Working Group on Sarcopenia in Older People (EWGSOP2) (16/27 kg for female participants/male participants).16 Information on walking pace, number of falls last year, stairs climbed, smoking, alcohol consumption, and physical activity were acquired through touchscreen questionnaires. The Townsend deprivation index was calculated by the UK Biobank immediately before each participant joined. Further details on variable definitions are provided in the supplementary materials and methods.
Statistical analysis
Population characteristics
Population characteristics were described using mean (SD) for continuous variables and percentages for binary/categorical variables. Statistical testing for differences between groups was linear/logistic regression or Pearson's chi-squared test unadjusted and adjusted for sex, age, and BMI. Due to the skewed distribution of liver PDFF, its distribution was described using the median (IQR).
Associations of NAFLD and muscle composition with all-cause mortality
Investigations were performed using Kaplan-Meier survival curves and Cox regression with years after imaging as the timescale. Both categorical representations (NAFLD=yes/no, 'high MFI’=yes/no, ‘low muscle volume’=yes/no and 'adverse muscle composition’=yes/no) and continuous representations (liver PDFF [%], MFI [%], and muscle volume z-score [SD]) were used as predictors. As sensitivity analyses, models were also implemented using the whole cohort (N = 40,174), the whole cohort excluding participants with a previous cancer diagnosis, in men and women separately, and for younger and older participants (using the median age as the cut-off point), crude and adjusted for sex, age, and BMI.
Associations between muscle composition and all-cause mortality within NAFLD
Investigations were performed using Kaplan-Meier survival curves and Cox regression with adverse muscle composition as well as continuous representations of MFI (%) and muscle volume z-score (SD) as predictors. An unadjusted model (M0) was implemented, followed by models subsequently adjusted for sex, age, and BMI (M1), hand grip strength, physical activity, alcohol consumption and smoking status (M2), and diagnosis of cancer, coronary heart disease, and T2DM before imaging (M3).
Adverse muscle composition and functional performance within NAFLD
The associations of muscle composition (i.e., adverse muscle composition, low muscle volume z-score and high MFI) and functional performance (i.e., hand grip strength, walking pace, falls and stair climbing) with all-cause mortality within NAFLD were investigated using separate crude and combined Cox regressions (including each muscle composition variable with all measures of functional performance as predictors in the same model).
Adverse muscle composition and liver-related outcomes within NAFLD
Liver-related events were identified through electronic healthcare records (accessed September 2021, available from April 1992 to September 2021) and grouped according to the study by Hagström et al..28 For this specific investigation, participants with NAFLD and other liver diseases at/before imaging, alcohol/drug use disorder at/before imaging, and recorded liver-related events at/before imaging were additionally excluded. Details can be found in Table S1. Logistic regressions of a composite variable including all new liver-related events were performed for sex-adjusted MFI and muscle volume z-score.
This research was conducted using the UK Biobank resource, project ID 6569. The study was approved by the North West Multicenter Research Ethics Committee, UK. Written informed consent was obtained before study entry.
Results
Population characteristics
When adjusted for sex, age, and BMI, participants with NAFLD had lower muscle volume z-scores but similar MFI and prevalence of low functional performance compared to their matched controls. The prevalence of T2DM was higher within NAFLD compared to matched controls (14.7% vs. 6.5%), while the prevalence of coronary heart disease was lower (6.5% vs. 7.7%). Further details on population characteristics are presented in Table 1.
Table 1.
Population characteristics.
| NAFLD | Matched controls | p value | p value (adjusted) | |
|---|---|---|---|---|
| N | 5,069 | 5,069 | — | — |
| Sex (female/male) | 46.9%/53.1% | 46.9%/53.1% | 1.000 | — |
| Age (years) | 64.33 (7.47) | 64.54 (7.69) | 0.152 | — |
| BMI (kg/m2) | 30.26 (4.83) | 29.19 (3.75) | <0.001 | — |
| Waist circumference (cm) | 98.40 (11.92) | 94.48 (10.53) | <0.001 | <0.001 |
| Liver PDFF (%) |
8.42 (6.24-12.30) |
2.70 (1.99-3.62) |
<0.001 |
<0.001 |
| Muscle composition | ||||
| Muscle volume z-score (SD) | -0.06 (0.97) | -0.03 (1.01) | 0.103 | 0.022 |
| FFMV, total thigh (L) | 10.75 (2.56) | 10.69 (2.62) | 0.243 | 0.003 |
| FFMV, left anterior thigh (L) | 1.79 (0.49) | 1.79 (0.51) | 0.744 | <0.001 |
| FFMV, right anterior thigh (L) | 1.80 (0.50) | 1.80 (0.51) | 0.680 | <0.001 |
| FFMV, left posterior thigh (L) | 3.54 (0.80) | 3.52 (0.82) | 0.129 | 0.013 |
| FFMV, right posterior thigh (L) | 3.59 (0.82) | 3.56 (0.83) | 0.087 | 0.022 |
| MFI, mean anterior thigh (%) | 8.03 (2.16) | 7.82 (2.07) | <0.001 | 0.938 |
| MFI, sex-adjusted (%) | 1.02 (2.03) | 0.81 (1.93) | <0.001 | 0.938 |
| MFI, left anterior thigh (%) | 8.16 (2.18) | 7.95 (2.11) | <0.001 | 0.786 |
| MFI, right anterior thigh (%) | 7.91 (2.19) | 7.71 (2.11) | <0.001 | 0.758 |
| MFI, left posterior thigh (%) | 11.94 (2.63) | 11.61 (2.53) | <0.001 | 0.044 |
| MFI, right posterior thigh (%) | 11.74 (2.66) | 11.41 (2.54) | <0.001 | 0.069 |
| Adverse muscle composition (yes/no) | 15.2%/84.8% | 14.7%/85.3% | 0.486 | 0.487 |
| Low muscle volume (yes/no) | 26.2%/73.8% | 26.2%/73.8% | 1.000 | 0.751 |
| High MFI (yes/no) |
38.8%/61.2% |
35.2%/64.8% |
<0.001 |
0.566 |
| Functional performance | ||||
| Hand grip strength (kg) | 31.31 (10.97) | 31.78 (10.95) | 0.032 | <0.001 |
| Low hand grip strength (yes/no/missing) | 8.9%/88.2%/2.9% | 8.0%/89.2%/2.7% | 0.102 | 0.093 |
| Slow walking pace (yes/no/missing) | 9.3%/90.4%/0.3% | 7.8%/92.0%/0.3% | 0.006 | 0.576 |
| >1 fall last year (yes/no/missing) | 5.5%/94.3%/0.2% | 5.8%/94.1%/0.1% | 0.496 | 0.163 |
| No stair climbing (yes/no/missing) |
10.9%/88.6%/0.5% |
10.4%/89.1%/0.6% |
0.373 |
0.702 |
| Comorbidity | ||||
| Cancer (yes/no/missing) | 11.0%/88.5%/0.4% | 10.5%/89.4%/0.1% | 0.311 | 0.227 |
| Type 2 diabetes (yes/no/missing) | 14.7%/84.4%/0.9% | 6.5%/92.9%/0.6% | <0.001 | <0.001 |
| Coronary heart disease (yes/no/missing) |
6.5%/93.1%/0.4% |
7.7%/91.9%/0.4% |
0.030 |
0.010 |
| Lifestyle factors | ||||
| Smoking (no/previous/current/missing) | 65.7%/31.1%/2.8%/0.4% | 64.9%/31.2%/3.5%/0.3% | 0.092 | 0.056 |
| Alcohol consumption (g/day) | 6.83 (6.91) | 7.43 (7.09) | <0.001 | <0.001 |
| Physical activity IPAQ (moderate/low/high/missing) | 45.5%/28.2%/26.1%/0.2% | 43.5%/23.8%/32.6%/0.2% | <0.001 | 0.024 |
| Townsend deprivation index | -1.61 (2.87) | -1.71 (2.77) | 0.074 | 0.581 |
Values are mean (SD) for continuous variables and percentages for binary/categorical variables. p values are from linear/logistic regression or chi-squared test, unadjusted and adjusted for sex, age, BMI. Liver PDFF is given in median (IQR).
FFMV, fat-free muscle volume; IPAQ, international physical activity questionnaire; MFI, muscle fat infiltration; NAFLD, non-alcoholic fatty liver disease; PDFF, proton density fat fraction.
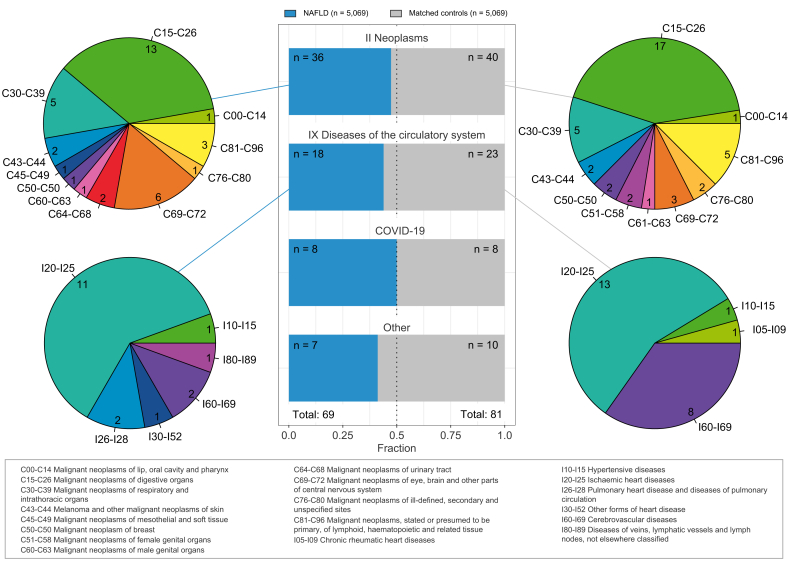
During a mean follow-up of 3.9 (SD ±1.4) years, representing 39,450 person-years at risk, 150 out of the 10,138 participants died. Specific causes of death are visualized in Fig. 2. The most common causes of death were from neoplasms (51%) and diseases of the circulatory system (27%). The numbers of death were similar between NAFLD and matched controls across ICD-10 code chapters, and the breakdown in ICD-10 code blocks also showed similar distributions. A complete breakdown can be found in Table S2-S3.
Fig. 2.
Breakdown of primary causes of death within NAFLD and sex-, age-, and BMI-matched controls.
Middle panel shows distribution of counts between NAFLD and matched controls in ICD-10 chapters with more than 10 deaths recorded. Pie charts show breakdown of ICD-10 blocks within each ICD-10 chapter for NAFLD (left) and matched controls (right) separately. NAFLD, non-alcoholic fatty liver disease.
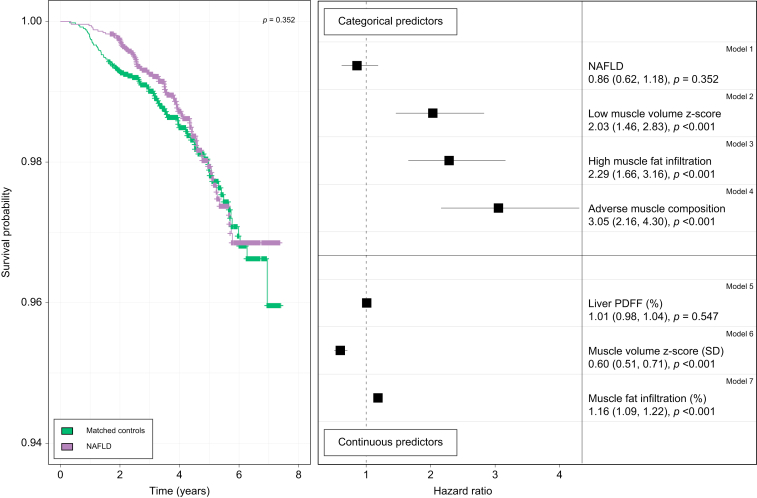
Associations of NAFLD and muscle composition with all-cause mortality
The rate of all-cause mortality was similar comparing NAFLD and matched controls (Fig. 3; number at risk in Fig. S1). Accordingly, the Cox regression showed that neither NAFLD nor liver PDFF were predictive of all-cause mortality (crude hazard ratios [HRs] 0.86, 95% CI 0.62–1.18, p = 0.350 and 1.01, 95% CI 0.98–1.04, p = 0.550, respectively) (Fig. 3). However, a lower muscle volume z-score was significantly associated with all-cause mortality in both categorical (low compared to normal muscle volume) and continuous representation, as was a higher MFI. Adverse muscle composition showed the strongest association with all-cause mortality (crude HR 3.05, 95% CI 2.16–4.30, p <0.001).
Fig. 3.
Associations of all-cause mortality with NAFLD, liver fat, and muscle composition in the matched cohort.
Left: Kaplan-Meier survival curves for all-cause mortality comparing NAFLD (purple) and sex-, age- and BMI-matched controls (green). Right: Unadjusted hazard ratios from Cox regression in the matched cohort (NAFLD and matched controls [n = 10,138]) for categorical (yes/no) variables: NAFLD, low muscle volume z-score, high muscle fat infiltration and adverse muscle, and for continuous variables: liver PDFF (%), muscle volume z-score (SD), and muscle fat infiltration (%). NAFLD, non-alcoholic fatty liver disease; PDFF, proton density fat fraction.
Sensitivity analysis showed similar results: NAFLD had a non-significant relationship with all-cause mortality in all stratified groups (whole cohort, whole cohort without cancer, women only, men only, younger participants, and older participants) both in crude and sex-, age-, and BMI-adjusted models. Liver PDFF was significant in crude modeling for the whole cohort excluding cancer, and in younger participants (crude HR 1.02, 95% CI 1.00–1.05, p = 0.024 and 1.05, 95% CI 1.02-1.08, p = 0.002, respectively), but not in adjusted modeling (adjusted HR 1.01, 95% CI 0.98–1.03, p = 0.559 and 1.03, 95% CI 0.99–1.07, p = 0.140, respectively). All muscle composition variables showed significant associations both in crude and adjusted models except for muscle volume z-score in the adjusted model for younger participants (aHR 0.84, 95% CI 0.70–1.02, p = 0.073). Table S4-5 include full reporting of modeling results.
Associations between muscle composition and all-cause mortality within NAFLD
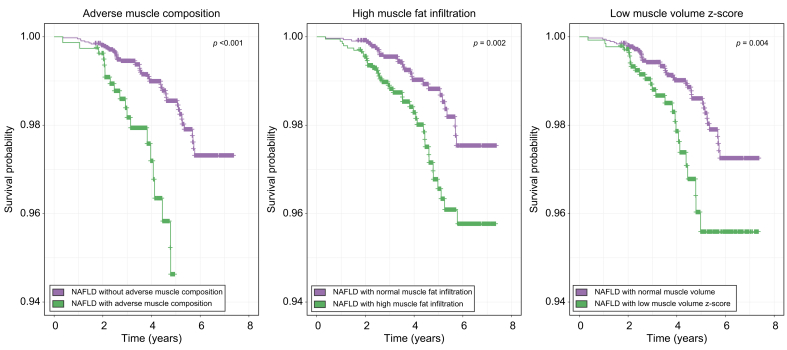
In participants with NAFLD, adverse muscle composition, high MFI, and low muscle volume z-score were significantly associated with all-cause mortality (Fig. 4, Table 2). After adjustment for sex, age, BMI, low hand grip strength, physical activity, smoking status, and alcohol, the association remained significant for all muscle composition variables. When additionally adjusting for relevant comorbidities (previous cancer, coronary heart disease, and T2DM), adverse muscle composition and muscle volume z-score were attenuated to non-significance (p = 0.051 and 0.069, respectively), while MFI remained significantly associated with all-cause mortality (p = 0.026). Table S6-S8 include full reporting of modeling results.
Fig. 4.
Kaplan-Meier survival curves comparing participants with NAFLD with/without adverse muscle composition, low muscle volume z-score, and high muscle fat infiltration.p values are from log-rank test.
NAFLD, non-alcoholic fatty liver disease.
Table 2.
Cox proportional HRs of all-cause mortality within NAFLD for adverse muscle composition, muscle fat infiltration and muscle volume z-score.
| Crude |
M1 |
M2 |
M3 |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | p value | HR | p value | HR | p value | HR | p value | |
| Adverse muscle composition | 2.84 (1.70–4.75) | <0.001 | 1.83 (1.08–3.12) | 0.0251 | 1.82 (1.06–3.14) | 0.0302 | 1.72 (1.00–2.98) | 0.0514 |
| Muscle fat infiltration | 1.15 (1.07–1.24) | <0.001 | 1.13 (1.03–1.24) | 0.0097 | 1.14 (1.02–1.26) | 0.0171 | 1.13 (1.01–1.26) | 0.0263 |
| Muscle volume z-score | 0.70 (0.55–0.88) | 0.0028 | 0.74 (0.56–0.96) | 0.0248 | 0.75 (0.57–0.99) | 0.0442 | 0.77 (0.58–1.02) | 0.0688 |
HRs for adverse muscle composition (yes/no), muscle fat infiltration (%), and muscle volume z-score (SD) including crude HRs and subsequent adjustments for sex, age, BMI (M1); low hand grip strength, smoking status, alcohol (M2); previous cancer, coronary heart disease, type 2 diabetes diagnosis (M3).
Adverse muscle composition and functional performance within NAFLD
Adverse muscle composition showed the strongest association with all-cause mortality (crude HR 2.84, 95% CI 0.35–4.75, p <0.001), including when compared to measures of functional performance (low hand grip strength, slow walking pace, >1 fall last year, and no stair climbing) (Table 3). The only functional tests that reached significance in the crude modeling were low hand grip strength (p = 0.035) and slow walking pace (p = 0.035). Interestingly, when muscle composition was combined with the functional tests, muscle composition remained significant while associations with the functional tests were attenuated below significance.
Table 3.
Cox proportional HRs of all-cause mortality within NAFLD for categorical variables of muscle composition and functional performance.
| Crude |
M1 |
M2 |
M3 |
|||||
|---|---|---|---|---|---|---|---|---|
| HR | p value | HR | p value | HR | p value | HR | p value | |
| Adverse muscle composition (n = 724 [15%]) | 2.84 (1.70–4.75) | <0.001 | 2.72 (1.60–4.63) | <0.001 | ||||
| High muscle fat infiltration (n = 1,910 [39%]) | 2.10 (1.30–3.38) | 0.002 | 1.83 (1.11–3.00) | 0.017 | ||||
| Low muscle volume z-score (n = 1,289 [26%]) | 2.02 (1.24–3.29) | 0.005 | 1.95 (1.18–3.22) | 0.009 | ||||
| Low hand grip strength (n = 437 [9%]) | 2.06 (1.05–4.04) | 0.035 | 1.67 (0.83–3.33) | 0.148 | 1.79 (0.90–3.56) | 0.097 | 1.69 (0.84–3.38) | 0.141 |
| Slow walking pace (n = 454 [9%]) | 2.00 (1.05–3.82) | 0.035 | 1.55 (0.78–3.09) | 0.208 | 1.54 (0.77–3.06) | 0.22 | 1.68 (0.85–3.33) | 0.136 |
| >1 fall last year (n = 266 [5%]) | 1.57 (0.68–3.62) | 0.294 | 1.22 (0.51–2.90) | 0.660 | 1.28 (0.54–3.05) | 0.576 | 1.26 (0.52–3.02) | 0.607 |
| No stair climbing (n = 540 [11%]) | 1.13 (0.54–2.36) | 0.745 | 0.97 (0.46–2.06) | 0.942 | 1.01 (0.48–2.12) | 0.987 | 1.02 (0.48–2.14) | 0.969 |
HRs for categorical muscle composition variables (adverse muscle composition, high muscle fat infiltration, low muscle volume z-score) and measures of functional performance including crude HRs and results from multivariable modeling (MV1-MV3) that includes each respective muscle composition variable and all measures of functional performance.
Adverse muscle composition and liver-related outcomes within NAFLD
After excluding participants with NAFLD and other liver diseases at/before imaging or alcohol/drug use disorder at/before imaging, 4,923 participants with NAFLD remained. Of the remaining participants, eight had at least one liver-related event at/before imaging and were excluded. Of the 4,915 remaining participants with NAFLD, 13 (0.26%) had at least one liver-related event post imaging (Table S1). Participants with a liver-related event post imaging showed a higher sex-adjusted MFI (1.01 [2.02] vs. 2.14 [2.58] %, p = 0.039). No other significant associations related to muscle composition were observed.
Discussion
In this study, we investigated the relationship of NAFLD and muscle composition with all-cause mortality in the UK Biobank imaging study. The main findings include (1) neither NAFLD nor liver fat was associated with all-cause mortality, and (2) adverse muscle composition was predictive of all-cause mortality in individuals with NAFLD.
Several studies have shown strong associations between fibrosis stage and increased mortality within NAFLD.[29], [30], [31] Similarly, a recent study on >10,000 individuals with biopsy-proven NAFLD showed increased mortality risk by histological stage.32 The study also concluded that simple steatosis significantly increased the risk of death. However, the design of biopsy-based studies is not optimal for investigating if simple steatosis alone drives an increased risk of death as they commonly include controls where there is no indication for biopsy, or where there is no matching on (or adjustment for differences in) overall adiposity or BMI. Although NAFLD was not confirmed by biopsy, but through MRI (liver PDFF) in our study, the results indicate the opposite conclusion. The link between NAFLD (as identified by elevated alanine aminotransferases) and all-cause mortality has also been investigated in the NHANES III study: An early study showed a significant association with all-cause mortality (adjusted HR 1.038, 95% CI 1.036–1.041).33 This was however challenged by another study, on the same dataset, published at about the same time.34 In that study, no significant association was observed (adjusted HR 1.20, 95% CI 0.88–1.60) and the authors claimed that the main difference between their studies was statistical approach.34,35 A more recent study using the NHANES III data also showed a non-significant association with all-cause mortality.36 In fact, there was a trend towards a negative association with all-cause mortality, and a significant negative association with cardiovascular mortality with full adjustment. In the presence of visceral obesity, low liver fat has also recently been shown to increase the risk of cardiovascular disease in both the Dallas Heart Study and UK Biobank.37 These are important facts to consider as an increasing number of pharmacological therapies are aiming to attenuate histopathological surrogates associated with hepatic fibrosis deposition and progression, including the presence of steatosis. Clearly, there are differences in results when investigating the association between NAFLD and all-cause mortality. Several studies show increased mortality30,32,33 while others do not.34,36,38 The differences probably lie in selection bias within the NAFLD population studied. In our study, NAFLD stratification was made directly from the UK Biobank imaging participants (community volunteers, not selected owing to abnormal liver function tests), hence, although unknown, the low prevalence of liver disease in our cohort indicates it mostly constitutes participants with NAFLD without the presence of advanced fibrosis.
Recent literature highlights an increased awareness of the importance of assessing muscle health in earlier stages of liver disease.6,39 Further research within NAFLD (or early metabolic/liver disease) investigating the link between muscle health and disease progression and related outcomes is important in evaluating muscle measurements as potential prognostic biomarkers. Multiple studies have reported an association between sarcopenia and NAFLD.[40], [41], [42] However, it has also been shown that common ways of adjusting muscle mass for body size (i.e., through division by height2, weight or BMI) do not achieve proper normalization, and that different adjustments result in completely different conclusions about the association between NAFLD and sarcopenia.25,43 The use of muscle volume z-score has been shown to effectively remove the association to body size (height, weight, and BMI).25 This is especially important to consider for patient populations, like those with NAFLD, where obesity is more prevalent. In line with our results, a significant association between low muscle mass and all-cause mortality was observed in a recent study investigating the relationship between physical inactivity, NAFLD and sarcopenia.44 Our study showed that muscle composition was predictive of all-cause mortality within NAFLD independent of functional performance (measured by hand grip strength) and physical activity level. It is also important to note that MFI was a strong predictor across all models. A combined assessment of both muscle volume and fat infiltration has been shown to effectively stratify the most vulnerable within a wide range of individuals – from the general population to those with NAFLD, chronic kidney disease, the critically ill, and patients with pancreatic cancer22,23,45,46 – and recent literature has started to indicate the potential of myosteatosis as a prognostic biomarker for NASH and fibrosis progression.47,48 Although too few NAFLD participants in our study had a liver-related event post imaging to draw any conclusions, the significant association with MFI is in line with previous research. Further research, with longer observation times or in higher risk populations, is needed to confirm the potential relationship between MFI and liver disease progression.
The lack of consensus around diagnosis, and how to detect and track sarcopenia, hinders further understanding of how assessment of muscle health could guide preventative care in early liver disease. A wide range of tests for muscle strength and functional performance are used as well as several techniques to measure (and normalize) muscle quantity.16,17,49 Due to the modest performance of muscle mass compared to strength or function in predicting adverse outcomes, the sarcopenia field has moved from definitions based on muscle mass alone towards a more pronounced focus on muscle strength and/or functional performance.16,50 However, use of measures for muscle strength and/or function alone is not sufficient to diagnose sarcopenia as low muscle strength/function can be caused by, or associated with, a variety of factors besides sarcopenia. This study shows that assessing muscle composition could be relevant in the early stages of liver disease and that measures of muscle strength, function and frailty do not attenuate the association between muscle composition and all-cause mortality. Quantitative muscle biomarkers with high precision and a strong link to functional performance and outcomes are important for tracking sarcopenia during therapeutic development and allow for rapid detection of disease progression and shortening of trial durations.
The main strengths of this study are the sample size, enabling analysis of a large number of participants fulfilling the NAFLD criteria with sex-, age-, and BMI-matched controls, and the detailed characterization of muscle composition through volumetric MRI. It is, however, important to point out that the NAFLD population studied is likely not the same as the NAFLD population seen in clinical care. The application of the NAFLD criteria to the population volunteering for the UK Biobank imaging study is like screening for NAFLD in this group – they were not biopsied or imaged due to a referral in clinical care. However, measuring liver PDFF in a large population study like the UK Biobank enables investigation of the independent risk of elevated liver fat content, avoiding confounders associated with the strata of patients in clinical care with an indication for biopsy or imaging. In addition, the participants were not followed for an extensive number of years and although previous diseases (cancer, coronary heart disease and T2DM) were included in the analysis, it is unknown whether these diseases preceded muscle composition changes or the other way around. It is important to further explore interactions between metabolic diseases and muscle composition in the pursuit of validating muscle measurements as potential prognostic biomarkers for liver disease. Additionally, as the serum biomarker panel is not yet released for the UK Biobank imaging visit, such data was not included in this study. The biomarker panel data from the baseline assessment (collected years 2006-2010) for adverse muscle composition in NAFLD has been published before.22 Those data showed that adverse muscle composition was associated with a higher Fibrosis-4 and hemoglobin A1c when adjusted for sex, age, liver PDFF, and BMI. Lastly, although hand grip strength was measured according to gold-standard methods, the other variables describing functional performance were self-reported, and the categories for low function (slow walking pace, no stair climbing and >1 fall last year) stratified relatively few participants. Further research is needed to understand how adverse muscle composition assessed by MRI can be introduced into clinical care and if the workflow could benefit from screening with functional tests.
Conclusion
Neither NAFLD nor liver fat content was predictive of all-cause mortality in the UK Biobank imaging study. Adverse muscle composition (the combination of low muscle volume z-score with high muscle fat infiltration) was a strong predictor of all-cause mortality within NAFLD, independent of functional performance. This research further supports the potential of muscle measurements as prognostic biomarkers for liver disease progression.
Financial support
Funding for image analysis was gratefully received from Pfizer Inc. ALF-grants Region Östergötland.
Authors’ contributions
Study concept and design: JL, ODL, ME; analysis and interpretation of data: JL, PN, ODL, ME; drafting of the manuscript: JL; critical revision of the manuscript for important intellectual content: all authors; statistical analysis: JL.
Data availability statement
This research was conducted using the UK Biobank resource, project ID 6569. UK Biobank data are available through the procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.
Conflict of interest
JL is an employee of AMRA Medical AB and reports other from BioMarin and Eli Lilly. AJS reports grants from Intercept, during the conduct of the study; other from Sanyal Bio, Genfit, Indalo, Tiziana, Durect, Exhalenz, Galmed, second genome, Cymabay, Prosciento, Labcorp, Medimmune, Astra Zeneca, Albireo; grants from Merck, Bristol Myers, Boehringer Ingelhiem, Immuron, Malinkrodt, Cumberland, Sequana; grants and personal fees from Novartis, Gilead, Conatus, Echosens; personal fees from Pfizer, Lilly, Novo Nordisk, Sanofi, Tern, Hemoshear, Glympse, Birdrock, Blade, Teva, Artham, Salix, NASH pharmaceuticals, outside the submitted work. ODL is an employee of and shareholder in AMRA Medical AB and reports other from Eli Lilly. ME is a member of the advisory board at AMRA Medical AB.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhepr.2022.100663.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Kechagias S., Nasr P., Blondahl J., Ekstedt M. Established and emerging factors affecting the progression of nonalcoholic fatty liver disease. Metabolism. 2020;111S doi: 10.1016/j.metabol.2020.154183. [DOI] [PubMed] [Google Scholar]
- 2.Ekstedt M., Nasr P., Kechagias S. Natural history of NAFLD/NASH. Curr Hepatol Rep. 2017;16(4):391–397. doi: 10.1007/s11901-017-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eslam M., Sanyal A.J., George J. International consensus panel. International consensus panel. MAFLD: a consensus-driven proposed nomenclature for metabolic associated fatty liver disease. Gastroenterology. 2020;158(7):1999–2014.e1. doi: 10.1053/j.gastro.2019.11.312. [DOI] [PubMed] [Google Scholar]
- 4.Volpi E., Nazemi R., Fujita S. Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care. 2004;7(4):405–410. doi: 10.1097/01.mco.0000134362.76653.b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mesinovic J., Zengin A., De Courten B., Ebeling P.R., Scott D. Sarcopenia and type 2 diabetes mellitus: a bidirectional relationship. Diabetes Metab Syndr Obes. 2019;12:1057–1072. doi: 10.2147/DMSO.S186600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chakravarthy M.V., Siddiqui M.S., Forsgren M.F., Sanyal A.J. Harnessing muscle–liver crosstalk to treat nonalcoholic steatohepatitis. Front Endocrinol (Lausanne) 2020 doi: 10.3389/fendo.2020.592373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fearon K., Strasser S., Anker S.D., Bosaeus I., Bruera E., Fainsinger R.L., et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12(5):489–495. doi: 10.1016/S1470-2045(10)70218-7. [DOI] [PubMed] [Google Scholar]
- 8.Dasarthy S. Etiology and management of muscle wasting in chronic liver disease. Curr Opin Gastroentrol. 2016;32(3):159–165. doi: 10.1097/MOG.0000000000000261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mak R.H., Ikizler A.T., Kovesdy C.P., Raj D.S., Stenvinkel P., Kalantar-Zadeh K. Wasting in chronic kidney disease. J Cachexia Sarcopenia Muscle. 2011;2(1):9–25. doi: 10.1007/s13539-011-0019-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Montano-Loza A.J., Angulo P., Meza-Junco J., Prado C.M.M., Sawyer M.B., Beaumont C., et al. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J Cachexia Sarcopenia Muscle. 2016;7(2):126–135. doi: 10.1002/jcsm.12039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carey E.J., Lai J.C., Sonnenday C., Tapper E.B., Tandon P., Duarte-Rojo A., et al. A North American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70(5):1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebadi M., Bhanji R.A., Mazurak V.C., Montano-Loza A.J. Sarcopenia in cirrhosis: from pathogenesis to interventions. J Gastroenterol. 2019;54(10):845–859. doi: 10.1007/s00535-019-01605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Englesbe M.J., Patel S.P., He K., Lynch R.J., Schaubel D.E., Harbaugh C., et al. Sarcopenia and mortality after liver transplantation. J Am Coll Surg. 2010;211(2):271–278. doi: 10.1016/j.jamcollsurg.2010.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mijnarends D.M., Luiking Y.C., Halfens R.J.G., Evers E.L.A., Lenaerts E.L.A., Verlaan S., et al. Muscle, health and costs: a glance at their relationship. J Nutr Health Aging. 2018;22:766–773. doi: 10.1007/s12603-018-1058-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cawthon P.M., Lui L.Y., Taylor B.C., McCulloch C.E., Cauley J.A., Lapidus J., et al. Clinical definitions of sarcopenia and risk of hospitalization in community-dwelling older men: the osteoporotic fractures in men study. J Gerontol A Biol Sci Med Sci. 2017;72:1383–1389. doi: 10.1093/gerona/glw327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cruz-Jentoft A.J., Bahat G., Bauer J., Boirie Y., Bruyére O., Cederholm C.T., et al. Writing group for the European working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:601. doi: 10.1093/ageing/afz046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Studenski S.A., Peters K.W., Alley D.E., Cawthon P.M., McLean R.R., Harris T.B., et al. The FNIH sarcopenia project: rationale, study description, conference recommendations, and final estimates. J Gerontol A Biol Sci Med Sci. 2014;69:547–558. doi: 10.1093/gerona/glu010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linge J., Borga M., West J., Tuthill T., Miller M.R., Dumitriu A., et al. Body composition profiling in the UK Biobank imaging study. Obesity (Silver Spring) 2018;26:1785–1795. doi: 10.1002/oby.22210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Karlsson A., Rosander J., Romu T., Tallberg J., Grönqvist A., Borga M., et al. Automatic and quantitative assessment of regional muscle volume by multi-atlas segmentation using whole-body water-fat MRI. J Magn Reson Imaging. 2015;41:1558–1569. doi: 10.1002/jmri.24726. [DOI] [PubMed] [Google Scholar]
- 20.West J., Romu T., Thorell S., Lindblom H., Berin E., Spetz Holm A.C., et al. Precision of MRI-based body composition measurements of postmenopausal women. PLoS One. 2018;13 doi: 10.1371/journal.pone.0192495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Borga M., Ahlgren A., Romu T., Widholm P., Dahlqvist Leinhard O., West J. Reproducibility and repeatability of MRI-based body composition analysis. Magn Reson Med. 2020;84:3146–3156. doi: 10.1002/mrm.28360. [DOI] [PubMed] [Google Scholar]
- 22.Linge J., Ekstedt M., Dahlqvist Leinhard O. Adverse muscle composition is linked to poor functional performance and metabolic comorbidities in NAFLD. JHEP Rep. 2020;3 doi: 10.1016/j.jhepr.2020.100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linge J., Petersson M., Forsgren M.F., Sanyal A.J., Dahlqvist Leinhard O. Adverse muscle composition predicts all-cause mortality in the UK Biobank imaging study. J Cachexia Sarcopenia Muscle. 2021 doi: 10.1002/jcsm.12834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Biobank imaging study. Accessed April 22, 2022. URL: https://www.ukbiobank.ac.uk/explore-your-participation/contribute-further/imaging-study.
- 25.Linge J., Heymsfield S.B., Dahlqvist Leinhard O. On the definition of sarcopenia in the presence of aging and obesity—initial results from UK Biobank. J Gerontol A Biol Med Sci. 2020;75:1309–1316. doi: 10.1093/gerona/glz229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leoni S., Tovoli F., Napoli L., Serio I., Ferri S., Bolondi L. Current guidelines for the management of non-alcoholic fatty liver disease: a systematic review with comparative analysis. World J Gastroentorol. 2018;24:3361–3373. doi: 10.3748/wjg.v24.i30.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Szczepaniak L.S., Nurenberg P., Leonard D., Browning J.D., Reingold J.S., Grundy S., et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 28.Hagström H., Adams L.A., Allen A.M., Byrne C.D., Chang Y., Grønbaek H., et al. Administrative coding in electronic health care record-based research of NAFLD: an expert panel consensus statement. Hepatology. 2021;74(1):474–482. doi: 10.1002/hep.31726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dulai P.S., Singh S., Patel J., Soni M., Prokop L.J., Younossi Z., et al. Increased risk of mortality by fibrosis stage in non-alcoholic fatty liver disease: systematic review and meta-analysis. Hepatology. 2017;65(5):1557–1565. doi: 10.1002/hep.29085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ekstedt M., Hagström H., Nasr P., Fredrikson M., Stål P., Kechagias S., et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61(5):1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 31.Angulo P., Kleiner D.E., Dam-Larsen S., Adams L.A., Bjornsson E.S., Charatcharoenwitthaya P., et al. Liver fibrosis, but No other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149(2):389–397.e10. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simon T.G., Roelstraete B., Khalili H., Hagström H., Ludvigsson J.F. Mortality in biopsy-confirmed non-alcoholic fatty liver disease: results from a nationwide cohort. Gut. 2021;70(7):1375–1382. doi: 10.1136/gutjnl-2020-322786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ong J.P., Pitts A., Younossi Z.B. Increased overall mortality and liver-related mortality in non-alcoholic fatty liver disease. J Hepatol. 2008 Oct;49(4):608–612. doi: 10.1016/j.jhep.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 34.Ruhl C.E., Everhart J.E. Elevated serum alanine aminotransferase and gamma-glutamyltransferase and mortality in the United States population. Gastroenterology. 2009 Feb;136(2):477–485.e11. doi: 10.1053/j.gastro.2008.10.052. [DOI] [PubMed] [Google Scholar]
- 35.Ruhl C.E., Everhart J.E. Non-alcoholic fatty liver disease (NAFLD) and mortality. J Hepatol. 2009 Sep;51(3):593. doi: 10.1016/j.jhep.2009.05.010. author reply 594-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Q., Zou X., Wen X., Zhou X., Ji L. NAFLD or MAFLD: which has closer association with all-cause and cause-specific mortality?-results from NHANES III. Front Med (Lausanne) 2021;8 doi: 10.3389/fmed.2021.693507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tejani S., McCoy C., Ayers C.R., Powell-Wiley T.M., Després J.P., Linge J., et al. Cardiometabolic health outcomes associated with discordant visceral and liver fat phenotypes: insights from the Dallas heart study and UK Biobank. Mayo Clin Proc. 2022 Feb;97(2):225–237. doi: 10.1016/j.mayocp.2021.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hagström H., Nasr P., Ekstedt M., Hammar U., Stål P., Hultcrantz R., et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017 Dec;67(6):1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 39.Nachit M., Leclercq I.A. Emerging awareness on the importance of skeletal muscle in liver diseases: time to dig deeper into mechanisms! Clin Sci (Lond) 2019;133(3):465–481. doi: 10.1042/CS20180421. 12. [DOI] [PubMed] [Google Scholar]
- 40.De Fré C.H., De Fré M.A., Kwanten W.J., Op de Beeck B.J., Van Gaal L.F., Francque S.M. Sarcopenia in patients with non-alcoholic fatty liver disease: is it a clinically significant entity? Obes Rev. 2019 Feb;20(2):353–363. doi: 10.1111/obr.12776. [DOI] [PubMed] [Google Scholar]
- 41.Lee Y.H., Kim S.U., Song K., Park J.Y., Kim D.Y., Ahn S.H., et al. Sarcopenia is associated with significant liver fibrosis independently of obesity and insulin resistance in nonalcoholic fatty liver disease: nationwide surveys (KNHANES 2008-2011) Hepatology. 2016 Mar;63(3):776–786. doi: 10.1002/hep.28376. [DOI] [PubMed] [Google Scholar]
- 42.Yu R., Shi Q., Liu L., Chen L. Relationship of sarcopenia with steatohepatitis and advanced liver fibrosis in non-alcoholic fatty liver disease: a meta-analysis. BMC Gastroenterol. 2018 Apr 19;18(1):51. doi: 10.1186/s12876-018-0776-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng T.C., Wu L.W., Chen W.L., Liaw F.Y., Chang Y.W., Kao T.W. Nonalcoholic fatty liver disease and sarcopenia in a Western population (NHANES III): the importance of sarcopenia definition. Clin Nutr. 2019 Feb;38(1):422–428. doi: 10.1016/j.clnu.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Golabi P., Berber L., Paik J.M., Deshpande R., de Avila L., Younossi Z.M. Contribution of sarcopenia and physical inactivity to mortality in people with non-alcoholic fatty liver disease. JHEP Rep. 2020 Aug 15;2(6) doi: 10.1016/j.jhepr.2020.100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loosen S.H., Schulze-Hagen M., Püngel T., Bündgens L., Wirtz T., Kather J.N., et al. Skeletal muscle composition predicts outcome in critically ill patients. Crit Care Explor. 2020;2(8) doi: 10.1097/CCE.0000000000000171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stretch C., Aubin J.M., Mickiewicz B., Leugner D., Al-Manasra T., Tobola E., et al. Sarcopenia and myosteatosis are accompanied by distinct biological profiles in patients with pancreatic and periampullary adenocarcinomas. PLoS One. 2018;13(5) doi: 10.1371/journal.pone.0196235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nachit M., Kwanten W.J., Thissen J.P., Op de Beeck B., Van Gaal L., Vonghia L., et al. Muscle fat content is strongly associated with NASH: a longitudinal study in patients with morbid obesity. J Hepatol. 2021;75(2):292–301. doi: 10.1016/j.jhep.2021.02.037. [DOI] [PubMed] [Google Scholar]
- 48.Hsieh Y.C., Joo S.K., Koo B.K., Lin H.C., Lee D.H., Chang M.S., et al. Innovative Target Exploration of NAFLD (ITEN) Consortium. Myosteatosis, but not sarcopenia, predisposes NAFLD subjects to early steatohepatitis and fibrosis progression. Clin Gastroenterol Hepatol. 2022 doi: 10.1016/j.cgh.2022.01.020. S1542-S3565(22)74-X. [DOI] [PubMed] [Google Scholar]
- 49.Chen L.K., Woo J., Assantachai P., Auyeung T.W., Chou M.Y., Iijima K., et al. Asian working group for sarcopenia: 2019 consensus update on sarcopenia diagnosis and treatment. J Am Med Dir Assoc. 2020;21(3):300–307.e2. doi: 10.1016/j.jamda.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 50.Newman A.B., Kupelian V., Visser M., Simonsick E.M., Goodpaster B.H., Kritchevsky S.B., et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–77. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
This research was conducted using the UK Biobank resource, project ID 6569. UK Biobank data are available through the procedure described at http://www.ukbiobank.ac.uk/using-the-resource/.